Abstract
Carbon fractions under different bamboo species viz., Bambusa balcooa, Bambusa bambos, Bambusa nutans, Dendrocalamus hamiltonii, Dendrocalamus asper and Dendrocalamus strictus were evaluated to understand the potential of these different bamboo species in soil rehabilitation in Himalayan foothills. The highest accumulation of the different carbon fractions likes very labile (6.12 mg g−1), less labile (2.55 mg g−1) and non-labile (11.40 mg g−1) was observed under D. hamiltonii, while highest labile fraction (3.17 mg g−1) was recorded under D. strictus. The highest active (8.85 mg g−1) and passive pool (13.95 mg g−1) were recorded under D. hamiltonii. Higher carbon management index (CMI) was obtained under D. hamiltonii (186.04) which was comparable with D. strictus (182.66) and B. nutans (179.24). Among all the six species, D. hamiltonii had the highest buildup of active and passive pool in both the soil depths. Bamboo plantations irrespective of the different species helped in enhancing the SOC fraction and enhanced C buildup in the soil in comparison to the open fallow land and holds potential in combating the problems of land degradation and soil rehabilitation.
Keywords: Active pool, Carbon management index, Labile pool, Non-labile pool, Passive pool
Active pool, Carbon management index, Labile pool, Non-labile pool, Passive pool
1. Introduction
Approximately 31.10 billion tons of net world carbon dioxide (CO2) emissions have increased by the year 2010 and it is estimated to set a new record in 2019 reaching an all-time high of 43.1 billion tons. This increased atmospheric CO2 concentration not only aggravates the climate change phenomenon (IPCC, 2007) but also poses a serious environmental threat in the form of land degradation. Agriculture sector (including intensive cropping, forestry, land-use changes and improper farm management) contributes a whopping 24 per cent to the total global CO2 emissions (IPCC, 2014). Therefore, there is an urgent need to develop methods for reducing carbon emissions and sequestering them in the soil (Sanderman and Baldock, 2010). The rerouting of atmospheric C into the soil system is the underlying principle for achieving Land Degradation Neutrality (LDN) as the soil organic C (SOC) holds the key to deter and reverse the process of land degradation https://www.iamrenew.com/environment/manage-soil-organic-carbon-to-pursue-land-degradation-neutrality-report/. Since, accretion of soil organic C and its fractions and the increment in the Net Primary Productivity (NPP) are the two key indicators or proxies for rehabilitation of degraded lands (Chappell et al., 2019). Therefore, any management practice which helps to achieve both would be the key to attain success.
In this direction, International Bamboo and Rattan Organization (INBAR) and many researchers (Sohel et al., 2015; Kaushal et al., 2020a) have underlined the significance of bamboo for reclamation of degraded lands across the globe. The fast-growing nature of bamboo species makes it capable to sequester more carbon, resulting in substantial “carbon-gain” through increased primary productivity as well as enhancing the SOC (inbar.int) and potentially take part in global climate change mitigation. Worldwide, there are approximately 1500 species of bamboo from 87 genera (Ohrnberger, 1999) of which India has around 136 species belonging to 23 different genera (IFSR, 2017). India stands second after China in terms of world bamboo genetic resources. The total estimated bamboo area of India is 15.69 m ha with a standing stock of 189 m tons. The scientific community is taking a keen interest in studying the role of bamboo in carbon storage (INBAR, 2010) and making it a prospective net C sink (Kleinhenz and Midmore, 2001). High litterfall and fine roots of bamboo help in improving the quality of degraded soil and sequestering soil C (Nath et al., 2015a, b). Bamboo produces phytolith occluded carbon (PhytOC) from decomposing vegetation that stays long in the soil (Huang et al., 2014). According to Parr et al. (2010) sequestration of PhytOC in bamboo corresponds to 11 per cent of the current CO2 increase.
The dynamics of soil carbon is critical and contributes significantly to carbon cycling and sustainability of terrestrial ecosystems (Chen et al., 2004). Labile or active pool and passive pool are two broad groups of SOC fractions; the labile pool comprises of the microbial biomass C and other fractions which are subjected to rapid decomposition and cycling within the soil system while the passive pool encompasses the more complex carbonaceous materials which are resistant to decomposition. Soil quality and mineralization pattern is determined by the relative proportion of different soil carbon fractions (Ghosh et al., 2012) and thorough knowledge about these fractions in any land-use system will help to determine the soil quality and sustainability. Further, indices derived from the various SOC pools like the Lability index (LI) and Carbon Management Index (CMI) helps to determine the suitability of land use for sustainable development. Though the C sequestration potential of bamboo is well established (Nath et al., 2009; Nath and Das, 2011; Kaushal et al., 2016), however, there is no study that reflects the changes in the SOC pools under bamboo plantations which are significant from the C storage point of view, since a minute change in the SOC pools could change the atmospheric CO2concentration (Guo and Gifford, 2002). Thus, the current study was initiated to answer these specific questions (a) How the bamboo plantations impact the dynamics of different fractions of SOC and is the impact species-specific (b) How does Lability Index of C and the CMI vary across the different bamboo species and (c) Identifying the best bamboo species suited for rehabilitation of degraded land, particularly with the focus on the North-West Himalayan region. This study would be a step forward towards the achievement of the goals of LDN in India.
2. Material and methods
2.1. Study site
The present study was undertaken at the Agroforestry Research Centre of G.B. Pant University of Agriculture and Technology, Pantnagar, Distt. Udham Singh Nagar, Uttarakhand, India. The experimental site is located at 29° N latitude, 79.3° E longitude at an altitude of 243 m above mean sea level in the Himalayan foothills (Figure 1). The study site is humid sub-tropical with cold winters and hot summers. The maximum daily temperature in summer reaches up to 42 °C and the minimum temperature goes down to 0.5 °C in winter. The monsoon sets in the second/third week of June and remains by the end of September. The mean annual rainfall is about 1450 mm, of which 80–90 per cent is received during the monsoon. The soils of tarai region are developed from alluvium, medium to moderately coarse textured materials under predominant influence of tall vegetation and moderate to well drain conditions. The soils are weakly developed with mollic epipedons and horizons and are classified as Mollisols.
Figure 1.
Location map of study site.
2.2. Experimental setup
Six bamboo species viz., Bambusa balcooa, Bambusa bambos, Bambusa nutans, Dendrocalamus asper, Dendrocalamus hamiltonii, and Dendrocalamus strictus were planted at a spacing of 5 m × 5 m in the year 2005 in Completely Randomized Block Design (CRBD) in three replications. Nine plants were planted in each plot for each species covering an area of 225 m2. In total there were 18 plots (6 species × 3 replications) covering an area of 4050 m2.
These bamboo species were chosen from the priority list of the International Network on Bamboo and Rattan (INBAR) and National Bamboo Mission, India. The experimental field was intercropped with cowpea and mustard for the first 4 years. After the fourth year, mounding operation (heaping of soil near the base of clump) was carried out annually to provide support to the new culms. Regular weeding and cleaning were done to manage the experiment. Regular pruning was also done in all the species to manage the culms. The bamboo culms were harvested regularly after the 6th year.
2.3. Soil sampling
Bulk soil samples were collected from each replication of the six bamboo species as well as the open plot (fallow) without bamboo during the year 2019 (fourteen years after the establishment of the bamboo plantation). Soil samples were collected from two soil depths 0–15 cm and 15–30 cm with the help of an auger as majority of roots (fine root and coarse roots) in bamboo species are confined to 0–30 cm soil depth (Kaushal et al., 2020b). For each plot, rhizosphere soil samples were collected from six random locations under the canopy and mixed to obtain a composite sample of about 1000 g. The soil samples were air-dried in shade, ground and sieved for the analysis of various C fractions. A total of 42 soil samples for the six bamboo species and open fallow comprising of two soil depths were analyzed for the various soil carbon fractions. The open fallow was a site adjoining the bamboo experiment which was devoid of anthropogenic management and free from canopy interferences. However, natural vegetation existed at the site and it served as the control plot or reference for comparison of the impact of the different bamboo species on the soil C fractions.
2.4. Analysis of different soil organic carbon fractions
The different Soil Organic Carbon (SOC) fractions were determined by the Modified Walkley and Black Method using 5, 10 and 20 ml of concentrated sulphuric acid (H2SO4) which corresponds to three acid aqueous solution ratios of 0.5:1, 1:1 and 2:1 which correspond to 12 N, 18 N and 24 N H2SO4, respectively (Chan et al., 2001; Ghosh et al., 2010). The oxidation of soil organic C with varying strengths of acid allows the total soil organic C to be separated into four distinct fractions of decreasing oxidisability which are given in Table 1. These four fractions together correspond to the total organic C present in the surface and sub-surface layer (Chan et al., 2001).
Table 1.
Methods used for calculating various C fractions and Carbon management Index (CMI).
| Fraction 1 (VLP) - Very labile | Organic C oxidizable under 12 N H2SO4 |
|---|---|
| Fraction 2 (LP) -Labile | Difference in oxidizable organic C extracted between 18 N and 12 N H2SO4 (18 N–12 N H2SO4) |
| Fraction 3 (LLP) - Less labile | Difference in oxidizable organic C extracted between 24 N and 18 N H2SO4(18 N–24 N H2SO4) |
| Fraction 4 (NLP) – Non labile | Difference in organic C extracted with 24 N H2SO4 and TOC determined by CHN analyzer (TOC–24 N H2SO4). |
| Active pool (AP) | VLP + LP (unstable/labile), |
| Passive pool (PP) | LLP + NLP (stable/non -labile). |
| Lability index for the organic carbon (LI) | [(Cfrac1/TOC) × 3 + (Cfrac2/TOC) × 2 + (Cfrac3/TOC) × 1]. |
| Carbon pool index (CPI) | Sample total C (mg/kg)/reference total C (mg/kg), where reference total carbon is the total carbon content (mg/kg) of control plots |
| Carbon management index (CMI) (Blair et al., 1995) | CPI × LI × 100. |
The separation of SOC into different fractions based on their oxidisability allows grouping SOC into two distinct groups, the easily oxidisable or labile fraction (corresponding to Fraction 1 and 2) and the stable or resistance fraction (Fraction 3 and Fraction 4). SOC fractions with different stability serve as a good indicator for monitoring the soil quality and deciphering the capacity of the soil to sequester C (Blair et al., 1995; Chan et al., 2001; Barreto et al., 2011). The easily oxidisable or labile fractions (Fraction 1 and 2) are more sensitive to changes in management practices and exhibits greater variations among treatments while the stable fractions (Fraction 3 and 4) are least affected by management practices and do not exhibit much variation. Thus, studying these four fractions in any ecosystem would fairly indicate the capacity of the ecosystem to store and sequester C in soil and about the long term sustainability of the ecosystem.
The active carbon pool (ACP) and passive carbon pool (PCP) is calculated from the four different fractions and is related to the oxidisability of the C in soil (Table 1). The ACP is the summation of Fraction 1 and Fraction 2 which is the easily oxidisable/labile/active part as the name suggests while the passive C pool is the stable/recalcitrant/less reactive pool which is oxidised and extracted with more difficulty (Fraction 3 and 4). Distribution of C either in the active or passive pool have a direct bearing on various soil physical, chemical and biological properties and have implications concerning atmospheric C retention.
The Lability Index (LI), Carbon Pool Index (CPI) and the Carbon Management Index (CMI) were calculated as per standard protocol outlined by Blair et al. (1995) (Table 1). The CMI is a derivative of the LI and CPI. The Lability Index mainly throws light on the nature or oxidisability of the SOC fractions, while CPI as an independent index is not popularly used IN SOC studies. The CMI is widely accepted as an indicator for soil degradation or improvement in response to land use changes (Sainepo et al., 2018). It takes into account both the quality and quantity of SOC and effectively monitors short term changes in soil C pools (Blair et al., 1995; Sodhi et al., 2009).
2.5. Statistical analysis
The impact of different bamboo species on SOC fractions and various indices such as LI, CMI was compared using two-way analysis of variance (ANOVA), and the means comparisons in posthoc analysis were performed using Tukey's Honest Significant Difference (HSD) test for factorial randomized complete block design (RCBD). Correlation analysis between the different soil C fractions, LI and CMI was also carried out using Pearson's correlation coefficient method. Experimental data with significant skewness were transformed prior to statistical testing, wherelog10(x) transformation were done for positively skewed data (Frctn.2, Frctn.3, Frctn.4, PP, CPI, CMI), and log10 [max (x+1)-x] transformation were done for negatively skewed data (Frctn.1, AP, LI). All statistical analyses were done using the R-studio (R version 4.0.2) statistical software.
3. Results
3.1. Variation in different SOC fractions
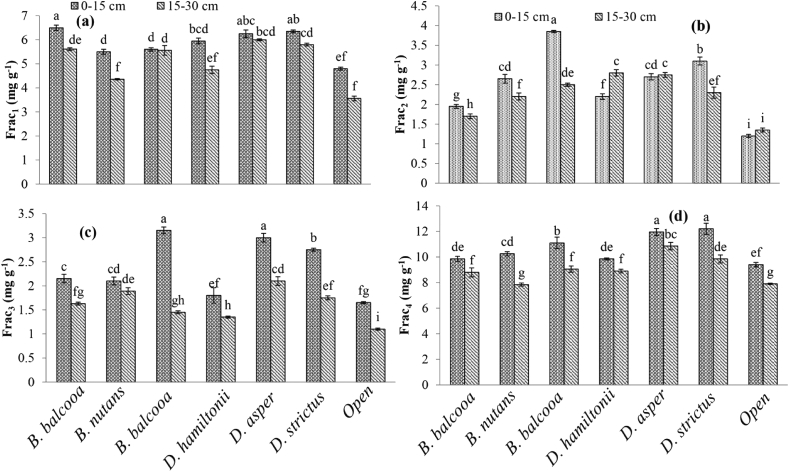
SOC fractions differed significantly between various bamboo species at both soil depths. The very labile carbon fraction (frac1) was significantly (p < 0.0001) higher at 0–15 cm depth (5.85 mg g−1) than 15–30 cm soil depth (5.09 mg g−1) (Table 2). Among the different bamboo species (Table 3), frac1 significantly (p < 0.0001) increased under D. hamiltonii (46.4%), D. strictus (45.2%), B. balcooa (44.74%), B. nutans (33.5%), D. asper (28.0%) and B. bambos (17.9%) as compared to control (open). The interaction between soil depth and bamboo species (Figure 2a) was also found significant (p < 0.0001). The significantly (p < 0.0001) higher labile carbon (frac2) (Table 2) was recorded at 0–15 cm soil depth (2.52 mg g−1) as compared to 15–30 cm (2.22 mg g−1). Under different bamboo species, significantly (p < 0.0001) higher (Table 3) frac2 was observed under B. nutans (3.17 mg g−1) and decreased under D. hamiltonii (2.72 mg g−1) which was at par with D. strictus (2.70 mg g−1). The labile carbon increased under B. balcooa (43.3%), D. strictus (45.2%), D. hamiltonii (114.2%), D. asper (96.8%), B. nutans (149.6%) and B. bambos (90.5%) over the control. The interaction between soil depth and species (Figure 2b) on frac2 was found significant (p < 0.0001).
Table 2.
SOC fraction, active, passive pools and carbon management index at different soil depths.
| Parameters | Soil depth (cm) |
|
|---|---|---|
| 0–15 | 15–30 | |
| Frac1 | 5.85a | 5.09b |
| Frac2 | 2.52a | 2.22b |
| Frac3 | 2.37a | 1.61b |
| Frac4 | 10.65a | 9.02b |
| AP | 8.37a | 7.32b |
| PP | 13.02a | 10.63b |
| LI | 1.16b | 1.18a |
| CMI | 173.25a | 153.61b |
Table 3.
SOC fraction, active, passive pools and carbon management index under different bamboo species.
| Species | Frac1 | Frac2 | Frac3 | Frac4 | AP | PP | LI | CMI |
|---|---|---|---|---|---|---|---|---|
| B. balcooa | 6.05a | 1.82d | 1.89c | 9.32c | 7.88b | 11.21d | 1.24a | 167.57a |
| B. bambos | 4.93c | 2.42c | 1.99c | 9.04cd | 7.35c | 11.04d | 1.17bc | 152.52ab |
| B. nutans | 5.58b | 3.17a | 2.30b | 10.07b | 8.75a | 12.37c | 1.20b | 179.24a |
| D. asper | 5.35b | 2.50c | 1.57d | 9.37c | 7.85b | 10.95d | 1.20b | 159.72ab |
| D. hamiltonii | 6.12a | 2.72b | 2.55a | 11.40a | 8.85a | 13.95a | 1.15c | 186.04a |
| D. strictus | 6.07a | 2.70b | 2.25b | 11.02a | 8.77a | 13.27b | 1.17bc | 182.66a |
| Open | 4.18d | 1.27e | 1.37e | 8.65d | 5.45d | 10.02e | 1.06d | 116.26b |
Figure 2.
Interaction between species and soil depth on different carbon fractions.
The less labile carbon fraction (frac3) at 0–15 cm depth (Table 2) was significantly (p < 0.0001) higher (2.37 mg g−1) than 15–30 (1.61 mg g−1). However, among the different bamboo species (Table 3) the significantly (p < 0.0001) higher frac3 was recorded under D. hamiltonii (2.55 mg g−1), and lower under D. asper (1.57 mg g−1). The percentage increase for frac3 under different bamboo species was as D. asper (14.6%), B. bambos (45.3%), B. balcooa (38.0%), D. strictus (64.2%), D. hamiltonii (86.1%) and B. nutans (67.9%) over the control. The interaction effect between soil depth and species (Figure 2c) on frac3 was significant (p < 0.0001). The higher frac3 was observed at 0–15 cm soil depth under B. nutans (3.15 mg g−1) and lowest at 15–30 cm in open (1.10 mg g−1).
The significantly (p < 0.0001) higher non-labile carbon fraction (frac4) was observed at 0–15 cm soil depth (10.65 mg g−1) than 15–30 cm (9.02 mg g−1) (Table 2). The significantly (p < 0.0001) higher (Table 2) non-labile carbon fraction (frac4) was observed under D. hamiltonii (11.40 mg g−1) and lower under B. bambos (9.04 mg g−1). The non-labile carbon pool increased under B. balcooa (7.7 %), D. strictus (27.4%), D. hamiltonii (31.8%), D. asper (8.3%), B. nutans (16.4%) and B. bamboos (4.5%) over the control. The interaction for frac4 between soil depth and species was also found to be significant (p < 0.0001). The highest frac4 (Figure 2d) was recorded at 0–15 cm soil depth under D. strictus (12.2 mg g−1) and lowest at 15–30 cm in open (7.9 mg g−1).
3.2. Active and passive carbon pools
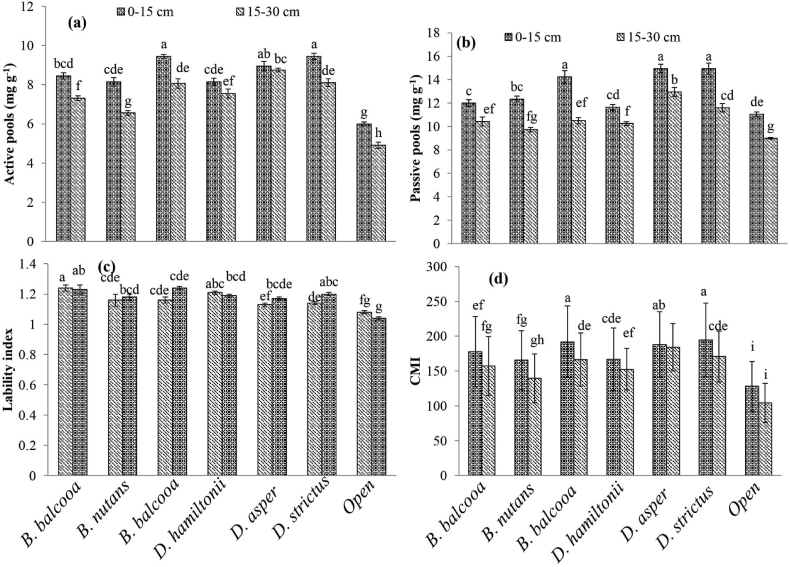
Soiil depth 0–15 cm soil depth had a significantly (p < 0.0001) higher ACP (8.37 mg g−1) as compared to 15–30 cm (7.32 mg g−1) soil depth. Among the different bamboo species (Table 3), the significantly higher AP was recorded under D. hamiltonii (8.85 mg g−1) which was statistically at par with D. strictus (8.77 mg g−1) and B. nutans (8.75 mg g−1). The interaction (Figure 3a) between soil depth and species was also found to be significant (p < 0.0001). The highest ACP was recorded at 0–15 cm soil depth under D. hamiltonii (10.85 mg g−1) and lowest in open (6.0 mg g−1). The ACP was increased by 44.0%, 34.9%, 44.5%, 62.4%, 60.5% and 60.9% under D. asper, B. bambos B. balcooa, D. hamiltonii, B. nutans and D. strictus, respectively over the control. Similarly, PCP were significantly (p < 0.0001) higher at 0–15 cm soil depth (13.02 mg g−1) than 15–30 cm (10.63 mg g−1) (Table 2). Among the different bamboo species (Table 3), the significantly higher PCP was observed under D. hamiltonii (13.95 mg g−1) followed by D. strictus (13.27 mg g−1), B. nutans (12.37 mg g−1) and B. balcooa (11.21 mg g−1). PCP were increased under B. balcooa (11.18%), D. strictus (32.4%), D. hamiltonii (39.2%), D. asper (9.3%), B. nutans (23.4%) and B. bambos (10.2%) over the control. The interaction effect on PCP (Figure 3b) between soil depths and species was also significant (p < 0.0001). The highest PCP was recorded at 0–15 cm soil depth (14.9 mg g−1) under D. strictus and lowest at 15–30 cm (9.0 mg g−1) in open/fallow.
Figure 3.
Interaction between species and soil depth on SOC pools, lability and carbon management index.
3.3. Lability Index (LI) and Carbon Management Index (CMI)
The significantly (p < 0.003) higher (Table 2) lability Index (LI) was recorded at 15–30 cm soil depth (1.18) as compared to 0–15 cm (1.16). It increased (Table 3) under B. balcooa (17.0%), D. strictus (10.4%), D. hamiltonii (8.5%), D. asper (13.2%), B. nutans (13.2%) and B. bamboos (10.4%) over the control. The interaction between soil depth and species (Figure 3c) was found significant (p < 0.0001). The highest LI was recorded at 0–15 cm soil depth (1.24) under B. balcooa and lowest at 15–30 cm (1.04) in open.
However, the significantly (p < 0.02) highest (Table 2) CMI was also recorded at 0–15 cm soil depth (173.2) as compared to 15–30 cm (153.6). Among the different bamboo species (Table 3), significantly (p < 0.0001) higher CMI was observed under D. hamiltonii (186.0) which was at par with D. strictus (182. 7), B. nutans (179.2) and B. balcooa (167.6) than decreased under D. asper (159.7), B. bambos (152.5) and control (116.3). It increased under B. balcooa (44.0%), D. strictus (57.1%), D. hamiltonii (60.0%), D. asper (37.4%), B. nutans (54.2%) and B. bamboos (31.2%) over the control. The interaction between soil depth and species (Figure 3d) was also found significant (p < 0.0001). The highest CMI was recorded at 0–15 cm soil depth under D. strictus (194. 5) and lowest at 15–30 cm in open (104.3).
3.4. Correlation analysis
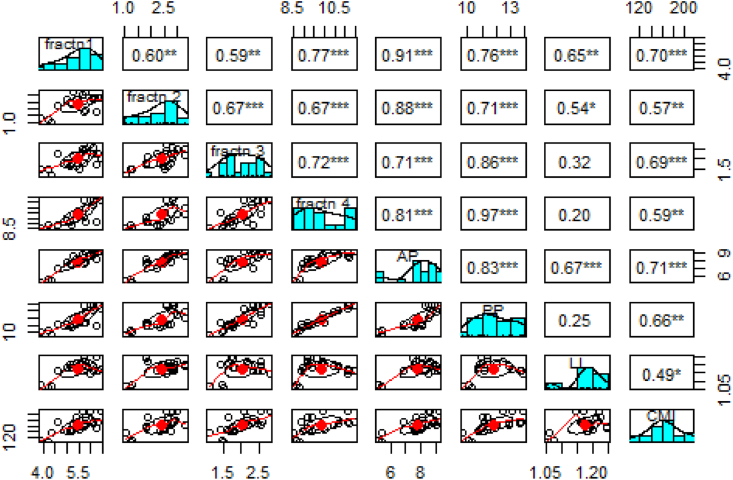
The correlation analysis graph (Figure 4) shows that fraction 1 is significantly positively correlated with fractions 2, 3 and 4 as well as with ACP, PCP, lability and CMI. Likewise, fraction 2 is also significant and positively correlated with fractions 3 and 4 as well as active, passive carbon pools, lability and carbon management index. Fraction 3 is significantly positively correlated with fraction 4, active, passive carbon pools and carbon management index but non-significantly with the lability index. Fraction 4 is also non-significantly positively correlated with lability index. Active carbon pools are also significantly and positively correlated with passive pools, LI and CMI. Likewise, passive pool is significantly positively correlated with CMI but non-significantly with LI. The lability index and carbon management index are also significantly positively correlated with each other.
Figure 4.
Correlation between carbon fractions, LI and CMI under different bamboo species.
4. Discussion
4.1. Labile C fractions or active C pool
It is now well accepted that bamboo species have the efficiency to control soil erosion and reduce the risk of land degradation by conserving the natural resources (Tardio et al., 2018; Singh et al., 2014, Kurothe et al., 2012). Bamboo due to fine mesh-like root system helps in binding the soil aggregates together and preventing soil erosion. The results of th e study revealed the ability of bamboo species to effectively build up organic carbon. The current study also showed that significantly low carbon content was recorded in an open condition as compared to different bamboo species in both surface (0–15 cm) and sub-surface (15–30 cm) after fourteen years of plantation. This shows the positive influence of bamboo species in the building of soil carbon in both soil depths. Bamboos complete their growth cycle within a short period (120–150 days) due to their vigorous growth rate. This characteristic of bamboo makes it a highly effective species for sequestration of soil C (Nath et al., 2015a, Nath et al., 2015b). The higher carbon pools under various species of bamboo may be due to the continuous litter addition and the development of ample fine root biomass by the different species of bamboos (Kaushal et al., 2020a,b). The greater percentage of C in the upper soil layer (0–15 cm) could be attributed to the presence of litter debris in this layer; which facilitates the availability and supplying of mineralizable and easily hydrolysable carbon leading to higher activity and population of microbes (Kaur et al., 2008; Benbi et al., 2015). Kumar et al. (2020a) also observed that tree-based agroforestry systems have significant labile carbon pool as compared to open land. Other researchers have reported that tree species contribute to the enhancement of the soil's organic carbon content due to the continuous addition of huge quantities of litter and higher concentrations of fine roots (Munoz and Beer, 2001). Growing trees, shrubs and herbs with a flourishing rooting system and litter supplement may also increase soil carbon storage, which has been confirmed by a positive correlation between soil organic carbon and litterfall (Singh, 2005). Our results indicated that the highest active pools were contributed by the D. hamiltonii amongst all bamboo species in both the soil depths. This could be attributed to higher coarse and fine root biomass incorporated by D. hamiltonii. Contrary to this, the lowest active carbon pool was observed under D. asper, which probably contributed to the lowest biomass of coarse and fine roots (Kaushal et al., 2020b; Kumar et al., 2020b). D. hamiltonii is a species of the sub-tropical to subtemperate region growing up to an elevation of 1500 m and prefer medium-textured slightly acidic soil (pH 5–6). This favorable agro-climatic condition for the species could have triggered better stocking of C in the soil system compared to other bamboo species.
The contribution of coarse and fine roots and their decomposition control the addition of carbon into the soil. However, a higher proportion of fine roots would indicate a higher rate of decomposition, leading to a lesser buildup of soil C in comparison to the coarse roots which would decompose at a slower rate. This plays a dynamic role in the building up of organic carbon over time and plays a significant role in C turnover as well as in the long-term productivity of any ecosystem (Raz-Yaseef et al., 2013; Mao et al., 2011; Langley and Hungate, 2003). A key measure of soil quality is the accumulation of active carbon reservoirs in the soil which is very prone to changes in land-use and makes up a fraction of total organic carbon in soil (Sahoo et al., 2019). It is apparent from the published findings that, when open barren land is brought under perennial vegetation like bamboo, a significant buildup of C takes place (Zhang et al., 2013). Change in active or labile carbon pool is most important as this is the pool exposed to rapid changes owing to any alteration or perturbation in the system. Sahoo et al. (2019) recorded a higher active or labile carbon pool in various bamboo species than open land which was in line with the observations recorded in the present study.
4.2. No-labile fractions and passive C pool
Of the total organic carbon in sub-surface (15–30 cm) soil, the major portion was contributed by the passive carbon pools (less labile and non-labile SOC fractions). PCP contribute about ~60 per cent of total organic carbon in the soil system. PCP is the recalcitrant fraction of organic carbon and it is not easily influenced by the alterations in land use management practices (Sainepo et al., 2018). These carbon fractions are strongly bound to the soil mineral matrix to form mineral-humus complexes of and thus, are shielded from the microbial action and least decomposed (Dwivedi et al., 2019). Besides this, the bamboo also produces Phytolith occluded carbon (PhytOC) that is highly constant and remains in the soil for long time (Parr et al., 2010; Huang et al., 2014). The open land had significantly lower values of passive carbon pools when compared to the different bamboo species in both surface and sub-surface soil layers. Huang et al. (2014) reported that stable PhytOC concentration in 0–40 cm soil layer increased by 217 Mg C ha−1after conversion of paddy field to bamboo plantation after 20 years. The PhytOC accumulated at 79 kg C ha−1 yr−1under bamboo which was much higher than the global mean long term soil C accumulation rate (24 kg C ha−1 yr−1). The increased passive pool may be attributed to the accumulation of PhytOC by bamboo plants, though it was not analyzed in the present study.
Erosion of soil triggered by water and wind is the most persuading reason for land degradation. Plantation of bamboo in these eroded sites performed better in terms of storage of carbon and buildup of SOC fractions compared to the open land which would eventually help to reduce soil erosion and prevent land degradation. The open barren lands are more prone to erosion as well as losses of carbon from the surface soil due to the lack of vegetation. So, extensive plantation of bamboo species on open fallows would reduce the risk of soil erosion and help in the buildup of soil organic carbon.
4.3. Carbon Management Index
The CMI is used to quantify soil C restoration; higher values indicate the rehabilitation of soil C and lower values reflect the degradation of the system (Blair et al., 1995). So it is critical how different carbon pools and CMI are influenced under different bamboo species with respect to open land. Bamboo plays a significant role in sequestering carbon and mitigating the climate change impacts (INBAR, 2010; Nath et al., 2015a, Nath et al., 2015b). It acts as a potential tool to develop management practices for increasing the storage of carbon in the soil (Sodhi et al., 2009). Among different bamboo species evaluated in the present study, all exhibited higher CMI than open systems which indicate that open system had significantly lower rates of soil C rehabilitation than under bamboo plantation. The highest CMI was observed in D. hamiltonii followed by D. strictus which is related to the TOC accumulated in the soil. B. bambos had the lower CMI among all the bamboo species along with open fallow which was again a reflection of poor C input as evident from lower root biomass and litterfall for B. bambos and minimum organic input in case of the open fallow. The higher the CMI values, the more is the potential for storing soil C and reduce the losses consequent upon the improvement of soil quality (Blair, 2000; Kalambukattu et al., 2013). Additionally, a higher CMI under bamboo is indicative of the high labile fraction C assimilated in soil which is essential for improving the various physical and chemical properties and microbial dynamics in the soil (Kalambukattu et al., 2013).
5. Conclusion
The fourteen year old bamboo plantation irrespective of the species helped in enhancing the fraction of SOC and enhanced C buildup in the soil in comparison to the open fallow. The impact of Bamboo on the active and the passive C pools was positive. Thus, in response to the research questions raised at the beginning of the study, we can conclude that (a) All the six species of bamboo had a significant positive impact on the different SOC fractions. The magnitude of increase in various fractions was, however, driven by the amount of root biomass and litter input added by each species. Hence, among all the six species, D. hamiltonii had the highest buildup of active and passive pool in both the soil depths. (b) The CMI was highest for D. hamiltonii and D. strictus, and (c) Amongst all the six species, D. hamiltonii, D. strictus and B. nutans emerged as the most promising species for rehabilitation of degraded land and could be used to monitor soil erosion and land degradation problems in the foothills of north-west Himalayas and pave the way towards achieving land degradation neutrality in the Indian context.
Declarations
Author contribution statement
Rajesh Kaushal, Salil Tewari: Conceived and designed the experiments; Wrote the paper.
Shanker Dutt Thapliyal, Amit Kumar: Performed the experiments; Wrote the paper.
Trisha Roy, Sadikul Islam: Analyzed and interpreted the data.
STS. Lepcha, Jayaraman Durai: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by National Mission on Bamboo Application (NMBA), Department of Science and Technology (2005–2009), National Bamboo Mission (2010–2012), State Forest Department, Uttarakhand, India and International Bamboo and Rattan Organisation (INBAR) China from 2016–21.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to the Jt. Director Agroforestry Research Centre (AFRC) for providing necessary facilities to conduct the work. The authors are also highly thankful to Mr. Ramesh Kumar for the field support.
References
- Barreto P.A., Gama-Rodrigues E.F., Gama-Rodrigues A.C., Fontes A.G., Polidoro J.C., Moço M.K.S., Baligar V.C. Distribution of oxidizable organic C fractions in soils under cacao agroforestry systems in Southern Bahia, Brazil. Agrofor. Syst. 2011;81(3):213–220. [Google Scholar]
- Benbi D.K., Brar K., Toor A.S., Singh P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma. 2015;237:149–158. [Google Scholar]
- Blair G.J., Lefroy R.D., Lisle L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995;46(7):1459–1466. [Google Scholar]
- Blair N. Impact of cultivation and sugar-cane green trash management on carbon fractions and aggregate stability for a Chromic Luvisol in Queensland, Australia. Soil Tillage Res. 2000;55:183–191. [Google Scholar]
- Chan K.Y., Bowman A., Oates A. Oxidizible organic carbon fractions and soil quality changes in an oxicpaleustalf under different pasture leys. Soil Sci. 2001;166(1):61–67. [Google Scholar]
- Chappell A., Webb N.P., Leys J.F., Waters C.M., Orgill S., Eyres M.J. Minimising soil organic carbon erosion by wind is critical for land degradation neutrality. Environ. Sci. Pol. 2019;93:43–52. [Google Scholar]
- Chen C.R., Xu Z.H., Mathers N.J. Soil carbon pools in adjacent natural and plantation forests of subtropical Australia. Soil Sci. Soc. Am. J. 2004;68:282–291. [Google Scholar]
- Dwivedi D., Tang J., Bouskill N., Georgiou K., Chacon S.S., Riley W.J. Abiotic and biotic controls on soil organo–mineral interactions: developing model structures to analyze why soil organic matter persists. Rev. Mineral. Geochem. 2019;85(1):329–348. [Google Scholar]
- Ghosh P.K., Venkatesh M.S., Hazraand K.K., Kumar Narendra. Long-term effect of pulses and nutrient management on soil organic carbon dynamics and sustainability on an Inceptisol of Indo-gangetic plains of India. Exp. Agric. 2012;48(4):473–487. [Google Scholar]
- Ghosh S., Wilson B.R., Mandal B., Ghoshal S.K., Growns I. Changes in soil organic carbon pool in three long-term fertility experiments with different cropping systems and inorganic and organic soil amendments in the eastern cereal belt of India. Soil Res. 2010;48(5):413–420. [Google Scholar]
- Guo L.B., Gifford R.M. Soil carbon stocks and land use change. Global Change Biol. 2002;8:345–360. https://www.iamrenew.com/environment/manage-soil-organic-carbon-to-pursue-land-degradation-neutrality-report [Google Scholar]
- Huang Z.-T., Li Y.-F., Jiang P.-K., Chang S.X., Song Z.-L., Liu J., Zhou G.-M. Long-term intensive management increased carbon occluded in phytolith(PhytOC) in bamboo forest soils. Sci. Rep. 2014;4:3602. doi: 10.1038/srep03602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IFSR 2017. http://fsi.nic.in/forest-report-2017
- INBAR . International Network for bamboo and Rattan (INBAR); Beijing, China: 2010. 2010 Bamboo and Climate Change Mitigation: a Comparative Analysis of Carbon Sequestration; p. 47. Technical Report No. 32. [Google Scholar]
- IPCC . IPCC; 2014. Inter-Governmental Panel on Climate Change- Assessment Report 5.https://www.ipcc.ch/report/ar5/wg2 [Google Scholar]
- IPCC C.C. 2007. The Physical Science Basis. [Google Scholar]
- Kalambukattu J.G., Singh R., Patra A.K., Arunkumar K. Soil carbon pools and carbon management index under different land use systems in the Central Himalayan region. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013;63(3):200–205. [Google Scholar]
- Kaur T., Brar B.S., Dhillon N.S. Soil organic matter dynamics as affected by long-term use of organic and inorganic fertilizers under maize-wheat cropping system. Nutr. Cycl. Agroecosyst. 2008;81:159–180. [Google Scholar]
- Kaushal R., Subbulaksmi V., Tomar J.M.S., Alam N.M., Jayaprakash J., Mehta H., Chaturvedi O.P.C. Predictive models for biomass and carbon stock estimation in Male bamboo (DendrocalamusstrictusL) in Doon valley, India. Acta Ecol. Sin. 2016;36:469–476. [Google Scholar]
- Kaushal R., Singh I., Thapliyal S.D., Gupta A.K., Mandal D., Tomar J.M.S., Kumar A., Alam N.M., Kadam D., Singh D.V., Mehta H., Dogra P., Ojasvi P.R., Reza S., Durai J. Rooting behaviour and soil properties in different bamboo species of Western Himalayan Foothills, India. Sci. Rep. 2020;10:4966. doi: 10.1038/s41598-020-61418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal R., Tewari S., Banik R.L., Thapliyal S.D., Singh I., Reza S., Durai J. Root distribution and soil properties under 12-year old sympodial bamboo plantation in Central Himalayan Tarai Region, India. Agrofor. Syst. 2020;94:917–932. [Google Scholar]
- Kleinhenz V., Midmore D. Aspects of bamboo agronomy. Adv. Agron. 2001;94:99–144. [Google Scholar]
- Kumar A., Dwivedi G.K., Tewari S., Sah V.K., Singh H., Kumar P., Kumar N., Kaushal R. Soil organic carbon pools under Terminalia chebula Retz. based agroforestry system in Himalayan foothills, India. Curr. Sci. 2020;118(7) [Google Scholar]
- Kumar A., Dwivedi G.K., Tewari S., Paul J., Anand R., Kumar N., Kumar P., Singh H., Kaushal R. Carbon mineralization and inorganic nitrogen pools under Terminalia chebula Retz.-based agroforestry system in Himalayan foothills, India. For. Sci. 2020:1–10. [Google Scholar]
- Kurothe R.S., Gaur M.L., Rao B.K., Parandiyal A.K., Singh A.K. CSWCRTI; 2012. Conservation and Production Potentials of Bamboo in Ravine Lands; pp. 978–981. [Google Scholar]
- Langley J.A., Hungate B.A. Mycorrhizal controls on belowground litter quality. Ecology. 2003;84:2302–2312. [Google Scholar]
- Mao R., Zeng D.H., Li L.J. Fresh root decomposition pattern of two contrasting tree species from temperate agroforestry systems, effects of root diameter and nitrogen enrichment of soil. Plant Soil. 2011;347:115–124. [Google Scholar]
- Munoz F., Beer J. Fine root dynamics of shaded cacao plantations in Costa Rica. Agrofor. Syst. 2001;51:119–130. [Google Scholar]
- Nath A.J., Das G., Das A.K. Above ground standing biomass and carbon storage in village bamboos in North East India. Biomass Bioenergy. 2009;33:1188–1196. [Google Scholar]
- Nath A.J., Lal R., Das A.K. Ethnopedology and soil quality of bamboo (Bambusa sp.) based agroforestry system. Sci. Total Environ. 2015;521–522:372–379. doi: 10.1016/j.scitotenv.2015.03.059. [DOI] [PubMed] [Google Scholar]
- Nath A.J., Lal R., Das A.K. Managing woody bamboos for carbon farming and carbon trading. Glob. Ecol. Conserv. 2015;3:653–663. [Google Scholar]
- Nath A.J., Das A.K. Carbon storage and sequestration in bamboo based small holder homegardens of BarakValley.Assam. Curr. Sci. 2011;100:229–233. [Google Scholar]
- Ohrnberger D. Elsevier; 1999. The Bamboos of the World: Annotated Nomenclature and Literature of the Species and the Higher and Lower Taxa. [Google Scholar]
- Parr J., Sullivan L., Chen B., Ye G., Zheng W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Global Change Biol. 2010;16:2661–2667. [Google Scholar]
- Raz-Yaseef N., Koteen L., Baldocchi D.D. Coarse root distribution of a semi-arid oak savanna estimated with ground penetrating radar. J. Geophys. Res. 2013;118:135–147. [Google Scholar]
- Sahoo U.K., Singh S.L., Gogoi A., Kenye A., Sahoo S.S. Active and passive soil organic carbon pools as affected by different land use types in Mizoram, Northeast India. PloS One. 2019;14(7) doi: 10.1371/journal.pone.0219969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainepo B.M., Gachene C.K., Karuma A. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Bal. Manag. 2018;13(1):4. doi: 10.1186/s13021-018-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderman J., Baldock J.A. Accounting for soil carbon sequestration in national inventories: a soil scientist’s perspective. Environ. Res. Lett. 2010;5(3) [Google Scholar]
- Singh A.K., Kala S., Dubey S.K., Rao B.K., Gaur M.L., Mohapatra K.P., Prasad B. Evaluation of bamboo based conservation measures for rehabilitation of degraded Yamuna ravines. Ind. J. Soil. Conserv. 2014;42(1):80–84. [Google Scholar]
- Singh G. Carbon sequestration under an agri-silvicultural system in the arid region. Indian For. 2005;147:543–552. [Google Scholar]
- Sodhi G.P.S., Beri V., Benbi D.K. Using carbon management index to assess the impact of compost application on changes in soil carbon after ten years of rice–wheat cropping. Commun. Soil Sci. Plant Anal. 2009;40(21-22):3491–3502. [Google Scholar]
- Sohel M.S.I., Alamgir M., Akhter S., Rahman M. Carbon storage in a bamboo (Bambusa vulgaris) plantation in the degraded tropical forests: implications for policy development. Land Use Pol. 2015;49:142–151. [Google Scholar]
- Tardio G., Mickovski S.B., Rauch H.P., Fernandes J.P., Acharya M.S. The use of bamboo for erosion control and slope stabilization: soil bioengineering works. Bamboo: Curr. Future Prosp. 2018;105 [Google Scholar]
- Zhang T., Li Y., Chang S.X., Jiang P., Zhou G., Liu J., Lin L. Converting paddy fields to Lei bamboo (Phyllostachys praecox) stands affected soil nutrient concentrations, labile organic carbon pools, and organic carbon chemical compositions. Plant Soil. 2013;367(1-2):249–261. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.