Highlights
-
•
The T1w/T2w ratio can be used to distinguish between patients with depressive disorders and controls.
-
•
Selected regions are important for reward and executive functioning, as well as sensory processing.
-
•
Patterns of cortical myelin may be a biomarker for depressive disorders.
Keywords: Depression, Cortical myelin, MRI, Machine learning, T1w/T2w ratio, Elastic net, LDA
Abstract
The association between depressive disorders and measures reflecting myelin content is underexplored, despite growing evidence of associations with white matter tract integrity. We characterized the T1w/T2w ratio using the Glasser atlas in 39 UD and 47 HC participants (ages = 19–44, 75% female). A logistic elastic net regularized regression with nested cross-validation and a subsequent linear discriminant analysis conducted on held-out samples were used to select brain regions and classify patients vs. healthy controls (HC). True-label model performance was compared against permuted-label model performance. The T1w/T2w ratio distinguished patients from HC with 68% accuracy (p < 0.001; sensitivity = 63.8%, specificity = 71.5%). Brain regions contributing to this classification performance were located in the orbitofrontal cortex, anterior cingulate, extended visual, and auditory cortices, and showed statistically significant differences in the T1w/T2w ratio for patients vs. HC. As the T1w/T2w ratio is thought to characterize cortical myelin, patterns of cortical myelin in these regions may be a biomarker distinguishing individuals with depressive disorders from HC.
1. Introduction
Depressive disorders, also collectively referred as unipolar depression (UD), is a leading cause of disability worldwide (World Health Organisation, 2017), with an economic burden of $210 billion dollars in the United States alone (Greenberg et al., 2015). Despite its impact, treatments for the disorder remain ineffective for many patients (Cuijpers et al., 2020). Thus, there is a pressing need to understand the neurobiological etiology of UD to facilitate the development of improved treatments and prevention strategies.
Unipolar depression is characterized by dysfunctional affective and cognitive processing (Gotlib and Joormann, 2010), including reduced executive functioning (Snyder, 2013), biased emotional processing (Suslow et al., 2020), and impaired reward processing (Halahakoon et al., 2020). Correspondingly, individuals with UD show aberrant activation during tasks which recruit these processes, including activation in the striatum, hippocampus, amygdala, orbitofrontal cortex, prefrontal cortex, insula, cingulate, and occipital cortex (Hamilton et al., 2012, Ng et al., 2019, Stuhrmann et al., 2011, Wang et al., 2015). In addition, a growing body of literature has reported structural abnormalities associated with depression in many of these same regions described above, both in grey matter (Ho et al., 2020, Schmaal et al., 2017, Schmaal et al., 2016) and in white matter (Manelis et al., 2021, Shen et al., 2017, van Velzen et al., 2020) Meta-analyses of diffusion weighted imaging (DWI) studies have repeatedly found evidence for lower fractional anisotropy (FA) in depressed populations (Liao et al., 2013, Murphy and Frodl, 2011, Wise et al., 2016). More recently, studies using very large samples (i.e., the UK Biobank), as well as meta-analyses combining both published and unpublished data (i.e., the ENIGMA consortium), have observed widespread and replicable reductions in FA (Shen et al., 2017, van Velzen et al., 2020). Notably, white matter integrity in identified regions has been shown to correlate with the cognitive processes disrupted in depression, including processing speed (Chopra et al., 2018, Penke et al., 2010), emotion regulation (Eden et al., 2015, Welton et al., 2020), and reward learning (de Boer et al., 2020).
Emerging evidence suggests that cortical myelin may be impacted in individuals with UD and that it may partially mediate some of the cognitive processes that are impaired in affected individuals. For example, studies of post mortem brain tissue from donors with UD have observed reduced myelination, a reduction in the number of oligodendrocytes and other glia (cells whose functions include generating and maintaining myelin (Simons and Nave, 2016)), and reduced expression of oligodendrocyte lineage genes (Boda, 2021, Tham et al., 2011). A study of individuals with treatment resistant depression revealed a reduced magnetization transfer ratio (MTR), which is thought to reflect lower myelin levels, in the cingulate cortex and insula (Zhang et al., 2009). A recent study using R1 (1/T1) as a measure of myelination observed reduced whole-brain myelin, but no significant difference in cortical myelin in a handful of a priori bilateral regions between individuals with depression and healthy controls (HC) (Sacchet and Gotlib, 2017).
Developments in magnetic resonance imaging (MRI) methodology permit the examination of cortical myelin via the T1w/T2w ratio (Glasser and Van Essen, 2011, Shafee et al., 2015). While the T1w/T2w ratio does not solely reflect cortical myelin (Fukutomi et al., 2018, Hagiwara et al., 2018, Tardif et al., 2017, Yasuno et al., 2017), it has been shown to correlate with several white matter metrics (Ganzetti et al., 2014, Glasser and Van Essen, 2011, Ritchie et al., 2018, Shams et al., 2019), indicating that it is sensitive to cortical myelin. Studies in population-based samples using this metric have found that lower myelin in the cingulate, orbitofrontal cortex, and middle temporal cortex correlated with poor sleep quality (Toschi et al., 2020), lower frontal-pole myelin and greater myelin in the occipital cortex correlated with neuroticism (Toschi and Passamonti, 2019), and lower myelin in the motor and higher myelin in the insular, cingulate, prefrontal, and superior parietal cortices correlated with trait anxiety (Norbom et al., 2019). While poor sleep, high neuroticism, and trait anxiety might represent concurrent symptoms of depression, prior studies have not systematically examined cortical myelin in participants with depression as compared to HC.
The goals of the present study were (1) to ascertain whether the T1w/T2w ratio is predictive of UD, and (2) to characterize the brain regions that are predictive of case/control status. Based on the prior studies mentioned above, we hypothesized that the T1w/T2w ratio would distinguish individuals with UD from HC and that these differences will be especially pronounced in the prefrontal cortical (PFC), cingulate, parietal and occipital regions that support reward and emotional processing, which are dysregulated in UD (Dalili et al., 2015, Ng et al., 2019).
2. Methods
2.1. Participants
The study was approved by the University of Pittsburgh Institutional Review Board. Participants were recruited from the community, universities, and counseling and medical centers. They gave written informed consent, were right-handed, fluent in English, and were matched on age and sex. Individuals with unipolar depression (UD) met DSM-5 criteria for major depressive or persistent depressive disorders. Healthy controls (HC) had no personal or family history of psychiatric disorders. Exclusion criteria included a history of head injury, metal in the body, pregnancy, claustrophobia, neurodevelopmental disorders, systemic medical illness, premorbid IQ < 85 per the National Adult Reading Test (Nelson, 1982), current alcohol/drug abuse, Young Mania Rating Scale scores > 10 (YMRS (Young et al., 1978)) at scan, or meeting criteria for any psychotic-spectrum disorder. Data were drawn from an ongoing longitudinal study that includes neuroimaging sessions at baseline and 6-month follow-up and clinical evaluations at baseline, 6-months, and 12-months. The present report includes baseline data available for 55 HC and 50 UD. Participants were excluded from analyses due to (1) previously undetected brain abnormalities of potential clinical relevance: 2 UD, (2) diagnosis conversion during the course of the study: 1 HC was diagnosed with major depressive disorder, and 1 UD was diagnosed with bipolar disorder; (3) scanner or movement-related artifacts in MRI data (4 HC, 7 UD), and (4) poor-quality myelin maps (see Section 2.4.2.1 Subject-level processing; 3 HC, 1 UD). The final sample included 47 HC and 39 UD.
2.2. Clinical assessment
All diagnoses were made by a trained clinician and confirmed by a psychiatrist according to DSM-5 criteria using SCID-5 (First, 2015). Additional information collected included illness onset and duration, number of current episodes, comorbid psychiatric disorders, current depression symptoms using the Hamilton Rating Scale for Depression (HRSD-25) (Hamilton, 1960), current mania symptoms using the Young Mania Rating Scale (YMRS) (Young et al., 1978), and lifetime depression and hypo/mania spectrum symptomatology using the Mood Spectrum Self Report (MOODS-SR) (Dell’Osso et al., 2002). A total psychotropic medication load was calculated for each participant with UD, with greater numbers and doses of medications corresponding to a greater medication load (Hassel et al., 2008, Manelis et al., 2016).
2.3. Neuroimaging data acquisition
The neuroimaging data were collected at the University of Pittsburgh/UPMC Magnetic Resonance Research Center using a 3 T Siemens Prisma scanner with a 64-channel receiver head coil and named according to the ReproIn convention (Visconti di Oleggio Castello et al., 2020). The DICOM images were converted to BIDS dataset using heudiconv (Halchenko et al., 2019) and dcm2niix (Li et al., 2016). High-resolution T1w images were collected using the MPRAGE sequence with TR = 2400 ms, resolution = 0.8x0.8x0.8 mm, 208 slices, FOV = 256, TE = 2.22 ms, flip angle = 8°. High-resolution T2w images were collected using TR = 3200 ms, resolution = 0.8x0.8x0.8 mm, 208 slices, FOV = 256, TE = 563 ms. Field maps were collected in the AP and PA directions using the spin echo sequence (TR = 8000, resolution = 2x2x2mm, FOV = 210, TE = 66 ms, flip angle = 90°, 72 slices).
2.4. Data analyses
2.4.1. Clinical data analysis
HC and UD groups were compared on demographic and clinical variables using t-tests and chi-square tests. All analyses were conducted in R (https://www.r-project.org/).
2.4.2. Neuroimaging data processing
2.4.2.1. Subject-level preprocessing
Data quality was examined using mriqc version 0.15.1 (Esteban et al., 2017) and visually inspected (Supplemental Methods). Each participant’s cortical myelin was characterized with the T1w/T2w ratio (Glasser et al., 2016, Glasser et al., 2013, Glasser and Van Essen, 2011) using the PreFreeSurfer, FreeSurfer, and PostFreeSurfer minimal preprocessing pipelines for the human connectome project (Glasser et al., 2013). Workbench v1.4.2 and HCPpipelines-4.1.3 were installed system-wide on a workstation with GNU/Linux Debian 10 operating system. The spin echo field maps collected in AP and PA phase encoding directions were used for bias field correction in PreFreeSurfer. Registration to standard space was achieved via MSMSulc (Robinson et al., 2018) in PostFreeSurfer. If FreeSurfer images and myelin maps had artifacts and gross errors (e.g., large regions of apparent low myelin in the occipital cortex due to the transverse sinus interfering with accurate identification of the pial surface), the data were removed from analyses. The resulting myelin maps were parcellated in Workbench using the 360 region Glasser Atlas (Glasser et al., 2016). Further data quality assurance resulted in the removal of 11 outlier parcels (Supplemental Methods), leaving 349 parcels.
2.4.3. Neuroimaging data analysis
2.4.3.1. Elastic net and linear discriminant analysis
Neuroimaging data decoding studies can capitalize on complex relationships between variables, but their large numbers can present a challenge in deriving a generalizable model. The elastic net approach has emerged as a flexible tool for use with neuroimaging data (Jollans et al., 2019) as it is able to reduce the influence of overly large coefficients and reducing the number of variables while generating multivariate models predictive of complex behaviors (Acuff et al., 2019, Bertocci et al., 2016, Manelis et al., 2020). Elastic net is a regularized regression which combines lasso and ridge regression (i.e., L1- and L2- norm regularization) (Zou and Hastie, 2005). Ridge regression penalizes overly large coefficients, while lasso regression removes variables with small coefficients.
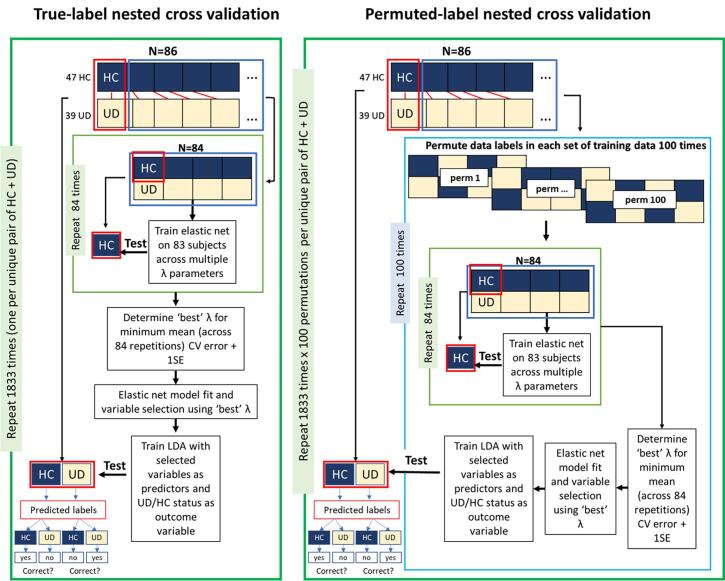
We used logistic elastic net regularized regression (Friedman et al., 2010) to select variables (brain parcels) that were most predictive of case/control status. Elastic net has two parameters: alpha (α) controls the balance between the ridge and lasso regularizations, and lambda (λ) controls the strength of regularization. To provide equal contribution of each penalty to the loss function, we used α = 0.5. To avoid model overfitting and bias, we implemented nested cross-validation to identify the optimal λ parameter. A linear discriminant analysis (LDA) (Venables and Ripley, 2002) model was subsequently trained using selected variables to make out-of-sample prediction on held-out participants. This strategy is illustrated in Fig. 1A and described in detail in Supplemental Methods. For each repetition of the nested cross-validation loop, two participants (1 UD and 1 HC) were held out. The rest of the sample was used to identify the optimal λ parameter which were then used to fit the elastic net model and select variables whose myelin levels were predictive of UD/HC status. These variables were then used to train an LDA model, which was tested on held-out participants. Results (model fit, variable selection, prediction accuracy) were evaluated against results from a permuted-label analysis (Fig. 1B and Supplemental Methods). To contribute to the ongoing discussion regarding best-practices for nested cross-validation with neuroimaging data sets (Vabalas et al., 2019, Varoquaux, 2018), post-hoc analyses systematically varied cross-validation parameters (i.e., the size of the held-out sample and the number of folds used to fit the elastic net model) and examined their impact on prediction accuracy and variable selection.
Fig. 1.
Diagram of analysis steps. Conceptual depiction of analysis steps including: (1) a unique pair of one UD and one HC participant is held-out; (2) an elastic net regression is used to select variables; (3) the retained variables are used an LDA model predicting case/control status; (4) the LDA model is tested on the held-out sample; (5) this process is repeated for each of the n = 1833 pairs of subjects; (6) for each held-out pair, the training procedure is repeated with 100 unique permutations.
2.4.3.2. Post-hoc analyses
2.4.3.2.1. The relationship between true-label and permuted-label sample demographics
To ensure that the permuted results were not due to changes in the internal structure of the permuted samples in terms of demographic variables (i.e., age, sex and IQ), we compared the age, sex and IQ values in the permuted samples with that in the true-label samples.
2.4.3.2.2. Association of LDA accuracy and cortical myelin with demographic and clinical characteristics
To further characterize the parcels selected by the logistic elastic net regression, we compared cortical myelin in UD vs. HC while controlling for age, sex, and IQ. To assess the potential influence of confounding variables on model fit, we tested whether demographic or clinical characteristics were predictive of classification accuracy. Regressions tested whether the joint effect of group, the variable in question, or their interaction, was associated with participant-wise accuracy (Dinga et al., 2020). Additional models tested whether clinical characteristics of UD participants were associated with model accuracy. To assess the influence of confounding variables on variable selection, regressions similarly tested the association of demographic and clinical variables with cortical myelin, controlling for age, sex, and IQ. Results for each variable were separately corrected for multiple comparisons using false discovery rate (FDR).
2.4.3.3. Exploratory analysis of a HC participant who was diagnosed with major depressive disorder 12 months from the baseline scan
One participant entered the study as a HC but was diagnosed with major depressive disorder sometime between 6 and 12 months after study onset. At the study visit at 12 months the participant had mild depressive symptoms. While this participant was excluded from all primary analyses described above, exploratory analyses investigated whether the myelin was predictive of the participant’s conversion from HC to UD. This analysis used the primary 86 participants and the variables selected in primary analyses (cortical myelin in 33 parcels and IQ) to train an LDA model. UD/HC status was then predicted at study onset and at the 6-month follow-up (both time points were prior to conversion).
3. Results
3.1. Sample demographics
Individuals with UD did not differ by age or sex but had higher IQ and current and lifetime depression severity compared to HC (Table 1).
Table 1.
Demographic and clinical characteristics.
| HC (mean/sd or count/percent) | UD (mean/sd or count/percent) | t-test or chi-squared test HC vs. UD | |
|---|---|---|---|
| N | 47 (54.7%) | 39 (45.3%) | |
| Gender (number females) | 36 (76.6%) | 29 (74.6%) | χ2(1) = 0, p = 1 |
| UD diagnoses (MDD/PDD) | na | 26/13 | na |
| Age (years) | 28.55 (6.15) | 29.07 (6.88) | t(84) = -0.37, p = 0.71 |
| IQ (NART) | 106.72 (6.24) | 110.2 (7.49) | t(84) = -2.35, p = 0.02 |
| Illness Onset (year of age) | na | 15.05 (5.02) | na |
| Lifetime episodes of depression | na | 3.25 (1.5) | na |
| Current depression severity (HRSD-25) | 1.74 (2.16) | 12.69 (6.77) | t(84) = −10.48, p < 0.01 |
| Lifetime depression (MOODS-SR) | 2.15 (2.27) | 18.41 (4.36) | t(84) = −22.21, p < 0.01 |
| Number taking Antidepressants | na | 22 (56.4%) | na |
| Number taking Mood stabilizers | na | 3 (7.7%) | na |
| Number taking Antipsychotics | na | 1 (2.6%) | na |
| Number taking Benzodiazepines | na | 5 (12.8%) | na |
| Number taking Stimulants | na | 3 (7.7 %) | na |
| Mean number of psychotropic medications | na | 1.05 (1.12) | na |
| Mean total medication load | na | 1.33 (1.53) | na |
| Number with comorbid diagnoses | na | 27 (69.2%) | na |
Note: Comparison of demographic and clinical characteristics between healthy controls (HC) and individuals with Unipolar Depression (UD). Tests were run as t-tests or chi-squared tests, as specified. Bold = p < 0.05. na = not applicable.
3.2. UD vs. HC LDA nested cross-validation classification accuracy
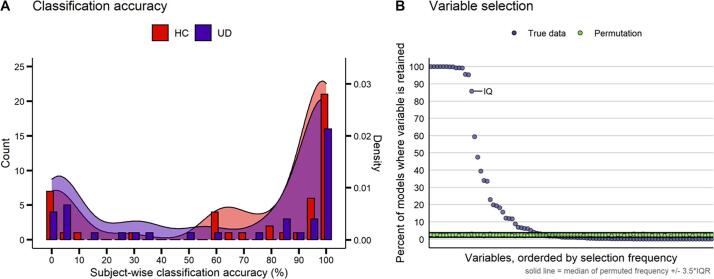
The T1w/T2w ratio and IQ distinguished UD from HC in subjects held-out during cross-validation, with an average accuracy of 68% (Fig. 2A; sensitivity (UD): 63.8%, specificity (HC): 71.5%). The mean participant-wise accuracy across 84 classification loops ranged from 0% to 100%, and 84% of participants were classified either quite accurately (>80% n = 53) or inaccurately (<20% n = 19). When demographic variables were excluded from the LDA classification, nested cross-validation achieved 69% accuracy (UD: 65.2%, HC: 73%).While the mean classification accuracy was higher in the HC group, this difference was not statistically significant (t = 0.88, p = 0.38). Notably, post-hoc analyses found that accuracy, sensitivity, and specificity were always significantly above chance across all cross-validation schemes examined (i.e., varying the size of the held-out sample and the number of folds used to fit the elastic net model) (Supplemental Fig. 3).
Fig. 2.
Classification accuracy and variable selection. A) Average subject-wise nested cross-validation classification accuracy in both healthy controls (HC) and participants with unipolar depression (UD). B) Variable selection frequency (percent of models where a given parcel was retained) in the true data (light blue) and permutations (green). The n = 90 parcels which were retained at least once are shown. IQ, the only demographic variable selected, is labeled. Solid black lines represent the median of the variable selection frequency for all n = 350 variables +/- 3.5 × the interquartile range (IQR). See Supplemental Table 2 for the full list of variable selection frequencies. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In permutation analyses, no variable was selected in 66.9% of 183,300 models. Within each participant, the proportion of models that did not select any variables ranged from 65.4% to 68.7%. Excluding instances when no variables were selected, the average participant-wise LDA accuracy in permutation analyses ranged from 41.2% to 59.2%, with an average accuracy of 50.5% (i.e., chance level). The test of whether age, sex and IQ in permuted samples differed from that in the sample with true labels showed that the demographics of the permuted groups differed (p < 0.05, uncorrected) from the true-label groups only in 1.7% of cases.
3.3. Elastic net variable selection
True-label nested cross-validated elastic net models predicting diagnostic status (UD vs. HC) with 349 parcels, age, sex and IQ, selected 90 myelin parcels (Supplemental Table 1) and IQ in at least one model (Fig. 2B). Each model selected between 9 and 68 variables, with a median of 17 variables. Permuted-label nested cross-validated elastic net models selected all predictor variables at least once, but no variable was selected by>3% of the models. In addition, 66.9% of models with permuted labels did not select any variable at all. Within permuted-label models where at least one variable was selected, the number of selected variables ranged from 1 to 113, with a median of 17 variables selected.
Given that the variable selection frequency in the models with permuted labels likely represents noise, we applied the criterion of the median + 3.5*IQR across all permuted variables (3.77%) as the cutoff value to separate the potential noise variables from ‘signal’ variables in the nested cross-validated elastic net with true labels. This latter analysis identified 33 out of 90 parcels, plus IQ, across the true-label nested cross-validated elastic net models that were above the cutoff line (Table 2, Fig. 2B). These 33 parcels included multiple regions in the orbitofrontal cortex, insula, cingulate, and frontal operculum, as well as regions in the auditory and visual cortices (Supplemental Fig. 3). No model retained the age and sex variables. Notably, post-hoc analyses which tested different cross-validation schemes found that these 34 variables were selected across all parameter variations (Supplemental Fig. 4).
Table 2.
The comparison of the T1w/T2w ratio in selected parcels in individuals with UD vs. HC.
| Parcel | t | p | p-FDR | Selection Frequency | Extended Description |
|---|---|---|---|---|---|
| Left 11 l | −3.056 | 0.003 | 0.032 | 100.00 | BA 11 (orbital and polar frontal) |
| Left 7PC | 3.081 | 0.003 | 0.032 | 100.00 | BA 7 (superior parietal cortex) |
| Left MST | 2.892 | 0.005 | 0.032 | 100.00 | Medial superior temporal area |
| Left p10p | −2.536 | 0.013 | 0.038 | 100.00 | BA 10 (frontopolar margin of orbital prefrontal cortex) |
| Right FOP2 | −2.574 | 0.012 | 0.038 | 100.00 | Frontal Opercular area 2 (posterior opercular cortex) |
| Right FFC | −2.534 | 0.013 | 0.038 | 100.00 | Fusiform face complex |
| Left STSdp | −2.036 | 0.045 | 0.071 | 99.95 | Auditory association cortex |
| Right TE2p | 2.928 | 0.004 | 0.032 | 99.89 | Lateral temporal complex |
| Left LBelt | −2.713 | 0.008 | 0.038 | 99.35 | Lateral belt complex (auditory) |
| Left a24pr | −3.003 | 0.004 | 0.032 | 99.29 | Ventral anterior cingulate cortex. |
| Right 7AL | −2.512 | 0.014 | 0.038 | 99.18 | BA 7 (superior parietal cortex) |
| Left d23ab | −2.157 | 0.034 | 0.060 | 95.53 | Ventral posterior cingulate cortex |
| Left 6a | −2.291 | 0.025 | 0.054 | 95.25 | BA 6 (premotor subdivisions) |
| Left 24dv | −2.556 | 0.012 | 0.038 | 59.36 | Ventral anterior cingulate cortex. |
| Right V3A | 1.795 | 0.076 | 0.097 | 47.52 | Visual area V3A |
| Left V4t | −1.650 | 0.103 | 0.117 | 39.44 | Visual area V4t |
| Right LBelt | −2.122 | 0.037 | 0.061 | 33.99 | Lateral belt complex (auditory) |
| Left p32 | 2.179 | 0.032 | 0.060 | 33.66 | BA 32 (pregenual anterior cingulate) |
| Right PH | 2.152 | 0.034 | 0.060 | 22.97 | Posterior temporal visual region |
| Right POS2 | 1.921 | 0.058 | 0.082 | 19.97 | Parieto-occipital sulcus area 2 |
| Right FOP1 | −1.816 | 0.073 | 0.096 | 19.31 | Frontal Opercular area 1 (posterior opercular cortex) |
| Right RI | 1.559 | 0.123 | 0.135 | 18.22 | RetroInsular cortex |
| Left 47 m | −1.735 | 0.086 | 0.102 | 15.77 | BA 47 (orbital part of inferior frontal gyrus) |
| Left s32 | −2.442 | 0.017 | 0.040 | 12.22 | BA 32 (subgenual anterior cingulate) |
| Left MT | 2.176 | 0.033 | 0.060 | 11.95 | Middle temporal area |
| Left RI | −2.520 | 0.014 | 0.038 | 11.78 | RetroInsular cortex |
| Right OP1 | −1.237 | 0.220 | 0.220 | 8.89 | Parietal operculum (secondary somatosensory cortex) |
| Left PeEc | −1.429 | 0.157 | 0.162 | 6.98 | Perirhinal ectorhinal cortex |
| Left FOP4 | −2.489 | 0.015 | 0.038 | 6.66 | Frontal Opercular area 4 (posterior opercular cortex) |
| Right RSC | −2.009 | 0.048 | 0.072 | 6.22 | RetroSplenial complex |
| Right 52 | 1.493 | 0.139 | 0.148 | 6.06 | BA 52 (parainsular) |
| Right 7Pm | 1.913 | 0.059 | 0.082 | 5.18 | BA 7 (superior medial parietal cortex) |
| Right 5 m | −1.777 | 0.079 | 0.097 | 3.98 | BA5 (paracentral lobule) |
The association of UD with the T1w/T2w ratio in the n = 33 selected variables. Tests were run as linear regressions, testing the association of case/control status with the T1w/T2w ratio, controlling for age, sex, and IQ. Positive t-values indicate greater T1w/T2w ratio (and, consequently, greater cortical myelin level) in UD vs. HC. We report both uncorrected and FDR-corrected p-values.
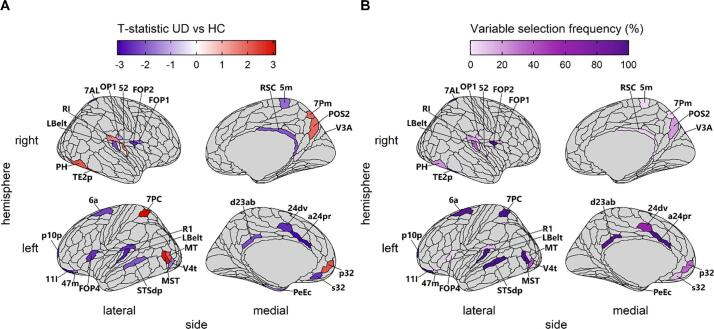
Post-hoc analyses tested the association of diagnostic status with the T1w/T2w ratio in the 33 selected parcels (Table 2, Fig. 3). These parcels include both regions where UD participants have a lower mean T1w/T2w ratio than HC and regions where UD participants have a greater mean T1w/T2w ratio, as well as regions where the two groups do not differ. After FDR-correction for multiple comparisons, fourteen of the parcels showed evidence of significant differences between UD and HC participants (Table 2). An additional 8 parcels showed nominally significant differences between UD and HC participants (Table 2; p < 0.05 uncorrected). Parcels that showed a greater absolute mean difference between the groups were selected more frequently in the nested cross-validation analysis (r = 0.7, p = 4 × 10−6).
Fig. 3.
Association of diagnostic status with the T1w/T2w ratio in parcels selected by elastic net. A) The t-statistic for the association of unipolar depression (UD) with T1w/T2w ratio in the n = 33 selected parcels. Regions where individuals with UD had a greater average T1w/T2w ratio than HC are in red, and regions where HC had a greater average T1w/T2w ratio than individuals with UD are in blue. B) The percent of models in which each parcel was retained. Regions are individually labeled (note that FFC is not visible, as it lies on the ventral surface). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The 33 selected parcels included 13 parcels in regions important for executive functioning and cognitive control, located in the anterior cingulate (left a24pr, left 24dv, left p32, and left s32), orbitofrontal cortex (left 11 l, left p10p, and left 47 m), posterior cingulate (right RSC, and left d23ab), frontal operculum (right FOP1 and left FOP4), and parietal cortex (right POS2 and right 7Pm). UD was largely associated with a reduced T1w/T2w ratio in these regions, except in one region of the anterior cingulate (left p32), and the two regions in the parietal cortex (right POS2 and right 7Pm), where it was associated with an increased T1w/T2w ratio. An additional 19 of the identified parcels play roles in visual, somatomotor, and auditory processing. Visual processing regions included extrastriate regions (right V3A, left V4t, and left MT), ventral stream regions (right FFC and right TE2p), and dorsal stream regions (left MST, right PH, left 7PC, right 7AL). Auditory processing regions included regions in the auditory cortex (left LBelt, right LBelt, left RI, and right RI) and regions implicated in language (left STSdp, left FOP2, and right 52). Somatomotor regions included regions implicated in somatosensation (right 5 m and right OP1) and a region in the premotor cortex (left 6a). UD was largely associated with a reduced T1w/T2w ratio in auditory and somatomotor regions, except for two auditory regions (right 52 and right RI) where it was associated with an increased T1w/T2w ratio. In contrast, participants with UD had a greater T1w/T2w ratio in 6 visual regions and had a reduced T1w/T2w ratio in 3 regions (left V4t, left FFC, and left 7AL).
3.4. Post-hoc analyses
3.4.1. Association of the T1w/T2w ratio with demographic and clinical variables
The association of the T1w/T2w ratio in the 33 selected parcels with demographic and clinical variables (see Table 1) was not statistically significant (Supplemental Table 4). Across all participants neither sex, IQ, nor MOODs-SR score were correlated with cortical myelin in selected parcels, nor did they interact with group (HC/UD) to predict cortical myelin. There was evidence for associations between age and the T1w/T2w ratio in two regions (p-fdr < 0.05), driven by a positive correlation in right LBelt, and a negative correlation in left a24pr. In individuals with UD, no clinical variable was correlated with the T1w/T2w ratio in selected parcels. However, taking antidepressants was nominally associated (p < 0.05 uncorrected) with an increased T1w/T2w ratio in right OP1, left 24dv, and left p32, and a decreased T1w/T2w ratio in right LBelt (Supplemental Table 4, Supplemental Figure 5).
3.4.2. Association of LDA accuracy with demographic and clinical variables
Analyses found that no demographic or clinical variable was predictive of classification accuracy (Table 3). Similarly, within the UD participant group, no clinical or medication variable was associated with classification accuracy (Table 3).
Table 3.
Association of demographic and clinical variables with classification accuracy.
| Sample | Variable | F | p |
|---|---|---|---|
| All | IQ × Group | 0.5 | 0.68 |
| All | Age × Group | 1.65 | 0.18 |
| All | Sex × Group | 2.44 | 0.07 |
| All | HRSD-25 × Group | 0.73 | 0.54 |
| All | MOODS-SR × Group | 1.13 | 0.34 |
| UD Only | Antidepressants | 0.17 | 0.69 |
| UD Only | Age of illness onset | 0.39 | 0.53 |
| UD Only | Illness duration | 0.43 | 0.52 |
| UD Only | Lifetime episodes of depression | 1.67 | 0.2 |
| UD Only | Number of comorbid diagnoses | 0.13 | 0.72 |
| UD Only | UD Diagnosis (MDD/PDD) | 2.04 | 0.16 |
| UD Only | Medication load | 0.46 | 0.5 |
Joint regressions were run as Group × Variable + Group + Variable, predicting classification accuracy. No test was significant (all ps > 0.05). Medications other than antidepressants were not examined, as too few participants were taking them (Table 1).
3.4.3. Exploratory analysis of classification in a participant who converted from HC to UD
The LDA trained on the whole sample of 86 participants, with IQ and the 33 parcels identified in the previous analyses as predictors classified this participant as ‘UD’ both times: 12 months and 6 months before illness onset.
4. Discussion
The T1w/T2w ratio distinguished healthy control participants (HC) from individuals with unipolar depression (UD) with 68% accuracy. The elastic net regression selected regions implicated in executive function and reward processing, as well as those involved in visual, auditory, and somatomotor processing. The pattern of T1w/T2w ratio distinguishing UD from HC was associated with a lower T1w/T2w ratio in some regions and a higher T1w/T2w ratio in other regions. As the T1w/T2w ratio partially reflects cortical myelin, these results demonstrate that aberrant levels of cortical myelin may be a biomarker of depressive disorders.
The 33 selected parcels included 13 parcels in regions important for executive functioning, cognitive control, and reward processing, including the anterior and posterior cingulate, orbitofrontal cortex, frontal operculum, and parietal cortex. There is abundant evidence that these processes are disrupted in depression (Ng et al., 2019, Snyder, 2013). The present results converge with mounting evidence of disrupted myelination (Zhang et al., 2009), thickness (Schmaal et al., 2017), connectivity (Weiskopf et al., 2006), and activation (Ng et al., 2019) of these regions in those with depression. In addition to regions traditionally reported in neuroimaging studies of depression, we found that parcels located along the dorsal and ventral visual pathways, somatosensory and auditory processing regions and regions implicated in language also contributed to UD vs. HC classification. While depression is not typically considered a disorder of dysfunctional sensory processing, some studies report visual (Grange and Rydon-Grange, 2020) and auditory (Zweerings et al., 2019) processing deficits in depression. In addition, somatosensory symptoms (e.g., motor retardation) are among the diagnostic criteria for this disorder and have been well described (Simon et al., 1999). Our results add to a growing body of literature documenting associations of depression with altered structure (Schmaal et al., 2017) and functional connectivity (Chen et al., 2019, Korgaonkar et al., 2020) in sensory regions. Broadly, our results contribute to the consensus that the neurobiology of regions that play important roles in cognitive processes and information transfer are disrupted in depression, which may contribute to the etiology of the disorder.
It is notable that in contrast to studies of the major white matter tracts, where depression is associated with lower integrity (van Velzen et al., 2020), we observed both a decreased and an increased T1w/T2w ratio in individuals with UD relative to HC. Additionally, the anterior cingulate, the superior parietal cortex, the retroinsular cortex, and visual processing regions included a combination of proximal regions with T1w/T2w ratio decreases and increases. While the relationship between the T1w/T2w ratio and functional activation or connectivity remains under-explored, these observations suggest that cortical myelin imbalance, rather than a uniform reduction, could drive some of the observed functional differences in depression, such as disrupted network integration (Chen et al., 2019, Zheng et al., 2018) and patterns of both hypo- and hyper- connectivity (Kaiser et al., 2015, Yu et al., 2019).
Intriguingly, while classification accuracy was bimodal, in permutations participants were classified at chance (50%). This suggests that participants who were consistently misclassified differed from those who were correctly classified in their demographic or clinical features. However, post-hoc analyses did not reveal any associations between classification accuracy and demographic or clinical variables, thus suggesting that misclassification could reflect the presence of environmental or genetic risk factors that are not captured by clinical measures. These factors could alter the myelin content in selected regions thus creating predisposition to depression at the brain level. As a preliminary exploration of this idea, we explored the T1w/T2w ratio in a participant who experienced the onset of UD during the course of the study and was not used in primary analyses. This participant completed two MRI scans: at baseline and 6 months follow-up with both time points occurring prior to UD onset. Remarkably, this participant was classified as UD on both scans, despite not yet meeting criteria for a UD diagnosis at the time of scan. This preliminary result suggests that the T1w/T2w ratio in frontal, sensorimotor and extended visual cortices may be a biological risk marker predictive of UD diagnosis in the future. Further longitudinal studies are needed to test this hypothesis.
While the present results demonstrate that the T1w/T2w ratio is disrupted in UD, the cause of the disruption remains unknown. Post-hoc analyses suggest the T1w/T2w ratio is not correlated with medication use, lifetime depression severity, or illness duration. However, the T1w/T2w ratio may reflect other risk factors for UD. For instance, sleep disturbance, which is a well-established risk factor and symptom of UD (Franzen and Buysse, 2008), was recently shown to correlate with cortical myelin in several of the same regions found in the present study, including the cingulate and middle temporal cortex (Toschi et al., 2020). Stress is also associated with reduced white matter integrity in depression (Ho et al., 2017, van Velzen et al., 2020, Ziegler et al., 2020). After stress, remyelination can occur (Bonnefil et al., 2019) thus resulting into altered patterns of myelination across the cortex (Orthmann-Murphy et al., 2020). This ‘remyelination’ hypothesis could potentially explain our observations of both decreased and increased cortical myelin in unipolar depression.
Limitations of this work includes the need to replicate our findings in an independent sample. To partially address this limitation, our analyses used robust machine learning methods involving model testing using held-out samples. The major strength of this approach is that it helps to reduce model bias, which occurs when the same participants are used to train and test a model (Davatzikos, 2019). It has been suggested that model performance with nested cross-validation is close to the accuracy that would be achieved on fully independent data (Varma and Simon, 2006). The second limitation concerns measurement noise due to susceptibility artifacts. Several regions with documented relevance to depression, including the bilateral hippocampus, entorhinal cortex, and posterior orbitofrontal cortex complex, were not included in the present analyses, as these regions showed an excess of between-person variability (see Supplemental Methods). Future research should explore the association of the T1w/T2w ratio in these regions with unipolar depression.
In summary, the T1w/T2w ratio can distinguish participants with UD from HC, even when clinical and demographic variables are not included in analyses. Regions that were most important for this classification include several that play key roles in reward and emotion processing as well as a host of regions important for sensory processing. This result highlights that the association of UD with sensory processing bears further investigation. Notably, UD was associated with both a decreased and an increased T1w/T2w ratio, suggesting that observations of reduced integrity of major white matter tracts in UD may not fully extend to the cortex. These results suggest that cortical myelin holds promise as a biomarker of unipolar depression and may be an early predictor of risk for this disorder.
CRediT authorship contribution statement
David A.A. Baranger: Formal analysis, Data curation, Visualization, Writing – original draft. Yaroslav O. Halchenko: Data curation, Formal analysis, Writing - review & editing. Skye Satz: Investigation, Data curation, Visualization, Project administration, Writing - review & editing. Rachel Ragozzino: Investigation, Data curation, Project administration, Writing - review & editing. Satish Iyengar: Formal analysis, Writing - review & editing. Holly A. Swartz: Project administration, Writing - review & editing. Anna Manelis: Conceptualization, Funding acquisition, Project administration, Supervision, Investigation, Data curation, Formal analysis, Writing – original draft.
Acknowledgments
Acknowledgments
Funding Acknowledgements: This work was supported by a grant from the National Institute of Health R01MH114870 to A.M., and Y.O.H was supported by P41EB019936 to the Center for Reproducible Neuroimaging Computation (PI: Kennedy).
D.A.A.B, Y.O.H., S.S., R.R, S.I., and A.M.: declare no conflict of interest.
H.A.S: receives royalties from Wolters Kluwer, royalties and an editorial stipend from APA Press, and honorarium from Novus Medical Education.
The authors thank participants for taking part in this research study. We also thank Dr. Mary L. Phillips for fruitful discussions of the study design.
Data availability
All neuroimaging data are available in BIDS format in OpenNeuro repository under the accession number ds003653 at https://doi.org/10.18112/openneuro.ds003653.v1.0.0.
Authors contribution
D.B. – data quality assurance, analysis, visualization, and interpretation; drafted and critically evaluated the manuscript
Y.O.H. – curated data organization and analyses, drafted and critically evaluated the manuscript
S.S., R.R. – data acquisition and quality assurance; drafted and critically evaluated the manuscript
S.I. – curated data analyses, interpreted the data, critically evaluated the manuscript
H.A.S. – curated participants’ recruitment, interpreted the data, critically evaluated the manuscript.
A.M. – obtained funding; designed the study; data acquisition, quality assurance, analysis and interpretation; drafted and critically evaluated the manuscript
All authors have approved the final version of the manuscript and agreed to be accountable for all aspects of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102790.
Contributor Information
David A.A. Baranger, Email: dbaranger@pitt.edu.
Anna Manelis, Email: anna.manelis@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Acuff H.E., Versace A., Bertocci M.A., Ladouceur C.D., Hanford L.C., Manelis A., Monk K., Bonar L., McCaffrey A., Goldstein B.I., Goldstein T.R., Sakolsky D., Axelson D., Birmaher B., Phillips M.L. Baseline and follow-up activity and functional connectivity in reward neural circuitries in offspring at risk for bipolar disorder. Neuropsychopharmacology. 2019;44(9):1570–1578. doi: 10.1038/s41386-019-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci M.A., Bebko G., Versace A., Fournier J.C., Iyengar S., Olino T., Bonar L., Almeida J.R.C., Perlman S.B., Schirda C., Travis M.J., Gill M.K., Diwadkar V.A., Forbes E.E., Sunshine J.L., Holland S.K., Kowatch R.A., Birmaher B., Axelson D., Horwitz S.M., Frazier T.W., Arnold L.E., Fristad M.A., Youngstrom E.A., Findling R.L., Phillips M.L. Predicting clinical outcome from reward circuitry function and white matter structure in behaviorally and emotionally dysregulated youth. Mol. Psychiatry. 2016;21(9):1194–1201. doi: 10.1038/mp.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda E. Myelin and oligodendrocyte lineage cell dysfunctions: New players in the etiology and treatment of depression and stress-related disorders. Eur. J. Neurosci. 2021;53(1):281–297. doi: 10.1111/ejn.v53.110.1111/ejn.14621. [DOI] [PubMed] [Google Scholar]
- Bonnefil, V., Dietz, K., Amatruda, M., Wentling, M., Aubry, A. V., Dupree, J.L., Temple, G., Park, H.J., Burghardt, N.S., Casaccia, P., Liu, J., 2019. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. eLife 8. doi:10.7554/eLife.40855. [DOI] [PMC free article] [PubMed]
- Chen H., Liu K., Zhang B., Zhang J., Xue X., Lin Y., Zou D., Chen M., Kong Y., Wen G., Yan J., Deng Y. More optimal but less regulated dorsal and ventral visual networks in patients with major depressive disorder. J. Psychiatr. Res. 2019;110:172–178. doi: 10.1016/j.jpsychires.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Chopra S., Shaw M., Shaw T., Sachdev P.S., Anstey K.J., Cherbuin N. More highly myelinated white matter tracts are associated with faster processing speed in healthy adults. NeuroImage. 2018;171:332–340. doi: 10.1016/j.neuroimage.2017.12.069. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., Stringaris A., Wolpert M. Treatment outcomes for depression: challenges and opportunities. The Lancet Psychiatry. 2020;7(11):925–927. doi: 10.1016/S2215-0366(20)30036-5. [DOI] [PubMed] [Google Scholar]
- Dalili M.N., Penton-Voak I.S., Harmer C.J., Munafò M.R. Meta-analysis of emotion recognition deficits in major depressive disorder. Psychol. Med. 2015;45(6):1135–1144. doi: 10.1017/S0033291714002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C. Machine learning in neuroimaging: Progress and challenges. NeuroImage. 2019;197:652–656. doi: 10.1016/j.neuroimage.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer, L., Garzón, B., Axelsson, J., Riklund, K., Nyberg, L., Bäckman, L., Guitart-Masip, M., 2020. Corticostriatal white matter integrity and dopamine D1 receptor availability predict age differences in prefrontal value signaling during reward learning. Cerebral Cortex. doi:10.1093/cercor/bhaa104. [DOI] [PMC free article] [PubMed]
- Dell'Osso L., Armani A., Rucci P., Frank E., Fagiolini A., Corretti G., Shear M.K., Grochocinski V.J., Maser J.D., Endicott J., Cassano G.B. Measuring mood spectrum: Comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Compr. Psychiatry. 2002;43(1):69–73. doi: 10.1053/comp.2002.29852. [DOI] [PubMed] [Google Scholar]
- Dinga, R., Schmaal, L., Penninx, B.W.J.H., Veltman, D.J., Marquand, A.F., 2020. Controlling for Effects of Confounding Variables on Machine Learning Predictions. bioRxiv.
- Eden A.S., Schreiber J., Anwander A., Keuper K., Laeger I., Zwanzger P., Zwitserlood P., Kugel H., Dobel C. Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J. Neurosci. 2015;35(15):6020–6027. doi: 10.1523/JNEUROSCI.3659-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Birman D., Schaer M., Koyejo O.O., Poldrack R.A., Gorgolewski K.J., Bernhardt B.C. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS ONE. 2017;12(9):e0184661. doi: 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M.B., 2015. Structured Clinical Interview for the DSM (SCID) , in: The Encyclopedia of Clinical Psychology. doi:10.1002/9781118625392.wbecp351.
- Franzen P.L., Buysse D.J. Sleep disturbances and depression: Risk relationships for subsequent depression and therapeutic implications. Dialogues Clin. Neurosci. 2008;10:473–481. doi: 10.31887/dcns.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33(1) doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi H., Glasser M.F., Zhang H., Autio J.A., Coalson T.S., Okada T., Togashi K., Van Essen D.C., Hayashi T. Neurite imaging reveals microstructural variations in human cerebral cortical gray matter. NeuroImage. 2018;182:488–499. doi: 10.1016/j.neuroimage.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzetti M., Wenderoth N., Mantini D. Whole brain myelin mapping using T1- and T2-weighted MR imaging data. Front. Hum. Neurosci. 2014;8:671. doi: 10.3389/fnhum.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Ugurbil K., Andersson J., Beckmann C.F., Jenkinson M., Smith S.M., Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Van Essen D.C. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 2011;31(32):11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. Cognition and depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2010;6(1):285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange J.A., Rydon-Grange M. Computational modelling of attentional selectivity in depression reveals perceptual deficits. Psychol. Med. 2020 doi: 10.1017/S0033291720002652. [DOI] [PubMed] [Google Scholar]
- Greenberg P.E., Fournier A.-A., Sisitsky T., Pike C.T., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J. Clin. Psychiatry. 2015;76(02):155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Hagiwara A., Hori M., Kamagata K., Warntjes M., Matsuyoshi D., Nakazawa M., Ueda R., Andica C., Koshino S., Maekawa T., Irie R., Takamura T., Kumamaru K.K., Abe O., Aoki S. Myelin Measurement: Comparison between Simultaneous Tissue Relaxometry, Magnetization Transfer Saturation Index, and T1w/T2w Ratio Methods. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-28852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halahakoon D.C., Kieslich K., O’Driscoll C., Nair A., Lewis G., Roiser J.P. Reward-Processing Behavior in Depressed Participants Relative to Healthy Volunteers: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2020;77(12):1286. doi: 10.1001/jamapsychiatry.2020.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halchenko, Y., Goncalves, M., Castello, M.V. di O., Ghosh, S., Hanke, M., Dae, Amlien, I., Brett, M., Salo, T., Gorgolewski, C., pvelasco, Stadler, J., Kaczmarzyk, J., lee, john, Lurie, D., Pellman, J., Melo, B., Poldrack, B., Nielson, D., Schiffler, B., Szczepanik, M., Carlin, J., Feingold, F., 2019. nipy/heudiconv: v0.5.4 [0.5.4] - 2019-04-29. doi:10.5281/ZENODO.2653784.
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. Functional neuroimaging of major depressive disorder: A meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S., Almeida J.R., Kerr N., Nau S., Ladouceur C.D., Fissell K., Kupfer D.J., Phillips M.L. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., Gutman B., Pozzi E., Grabe H.J., Hosten N., Wittfeld K., Völzke H., Baune B., Dannlowski U., Förster K., Grotegerd D., Redlich R., Jansen A., Kircher T., Krug A., Meinert S., Nenadic I., Opel N., Dinga R., Veltman D.J., Schnell K., Veer I., Walter H., Gotlib I.H., Sacchet M.D., Aleman A., Groenewold N.A., Stein D.J., Li M., Walter M., Ching C.R.K., Jahanshad N., Ragothaman A., Isaev D., Zavaliangos-Petropulu A., Thompson P.M., Sämann P.G., Schmaal L. Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Hum. Brain Mapp. 2020 doi: 10.1002/hbm.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, T.C., King, L.S., Leong, J.K., Colich, N.L., Humphreys, K.L., Ordaz, S.J., Gotlib, I.H., 2017. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Social Cognitive and Affective Neuroscience. doi:10.1093/scan/nsx065. [DOI] [PMC free article] [PubMed]
- Jollans L., Boyle R., Artiges E., Banaschewski T., Desrivières S., Grigis A., Martinot J.-L., Paus T., Smolka M.N., Walter H., Schumann G., Garavan H., Whelan R. Quantifying performance of machine learning methods for neuroimaging data. NeuroImage. 2019;199:351–365. doi: 10.1016/j.neuroimage.2019.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Goldstein-Piekarski A.N., Fornito A., Williams L.M. Intrinsic connectomes are a predictive biomarker of remission in major depressive disorder. Mol. Psychiatry. 2020;25(7):1537–1549. doi: 10.1038/s41380-019-0574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Morgan P.S., Ashburner J., Smith J., Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods. 2016;264:47–56. doi: 10.1016/j.jneumeth.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Liao Y.i., Huang X., Wu Q., Yang C., Kuang W., Du M., Lui S.u., Yue Q., Chan R., Kemp G., Gong Q. Is depression a disconnection syndrome? Meta- analysis of diffusion tensor imaging studies in patients with MDD. J. Psychiatry Neurosci. 2013;38(1):49–56. doi: 10.1503/jpn10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A., Almeida J.R.C., Stiffler R., Lockovich J.C., Aslam H.A., Phillips M.L. Anticipation-related brain connectivity in bipolar and unipolar depression: A graph theory approach. Brain. 2016;139(9):2554–2566. doi: 10.1093/brain/aww157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A., Iyengar S., Swartz H.A., Phillips M.L. Prefrontal cortical activation during working memory task anticipation contributes to discrimination between bipolar and unipolar depression. Neuropsychopharmacology. 2020;45(6):956–963. doi: 10.1038/s41386-020-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A., Soehner A., Halchenko Y.O., Satz S., Ragozzino R., Lucero M., Swartz H.A., Phillips M.L., Versace A. White matter abnormalities in adults with bipolar disorder type-II and unipolar depression. Sci. Rep. 2021;11:7541. doi: 10.1038/s41598-021-87069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.L., Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biology of Mood & Anxiety Disorders. 2011;1(1) doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, H.E., 1982. National Adult Reading Test (NART): For the Assessment of Premorbid Intelligence in Patients with Dementia: Test Manual, 1982. Nfer-Nelson.
- Ng T.H., Alloy L.B., Smith D.V. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl. Psychiatry. 2019;9(1) doi: 10.1038/s41398-019-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbom L.B., Doan N.T., Alnæs D., Kaufmann T., Moberget T., Rokicki J., Andreassen O.A., Westlye L.T., Tamnes C.K. Probing Brain Developmental Patterns of Myelination and Associations With Psychopathology in Youths Using Gray/White Matter Contrast. Biol. Psychiatry. 2019;85(5):389–398. doi: 10.1016/j.biopsych.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Orthmann-Murphy J., Call C.L., Molina-Castro G.C., Hsieh Y.C., Rasband M.N., Calabresi P.A., Bergles D.E. Remyelination alters the pattern of myelin in the cerebral cortex. eLife. 2020;9:1–61. doi: 10.7554/eLife.56621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L., Maniega S.M., Murray C., Gow A.J., Valdes Hernandez M.C., Clayden J.D., Starr J.M., Wardlaw J.M., Bastin M.E., Deary I.J. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J. Neurosci. 2010;30(22):7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J., Pantazatos S.P., French L. Transcriptomic characterization of MRI contrast with focus on the T1-w/T2-w ratio in the cerebral cortex. NeuroImage. 2018;174:504–517. doi: 10.1016/j.neuroimage.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E.C., Garcia K., Glasser M.F., Chen Z., Coalson T.S., Makropoulos A., Bozek J., Wright R., Schuh A., Webster M., Hutter J., Price A., Cordero Grande L., Hughes E., Tusor N., Bayly P.V., Van Essen D.C., Smith S.M., Edwards A.D., Hajnal J., Jenkinson M., Glocker B., Rueckert D. Multimodal surface matching with higher-order smoothness constraints. NeuroImage. 2018;167:453–465. doi: 10.1016/j.neuroimage.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet M.D., Gotlib I.H. Myelination of the brain in major depressive disorder: An in vivo quantitative magnetic resonance imaging study. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-02062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Hibar D.P., Sämann P.G., Hall G.B., Baune B.T., Jahanshad N., Cheung J.W., van Erp T.G.M., Bos D., Ikram M.A., Vernooij M.W., Niessen W.J., Tiemeier H., Hofman A., Wittfeld K., Grabe H.J., Janowitz D., Bülow R., Selonke M., Völzke H., Grotegerd D., Dannlowski U., Arolt V., Opel N., Heindel W., Kugel H., Hoehn D., Czisch M., Couvy-Duchesne B., Rentería M.E., Strike L.T., Wright M.J., Mills N.T., de Zubicaray G.I., McMahon K.L., Medland S.E., Martin N.G., Gillespie N.A., Goya-Maldonado R., Gruber O., Krämer B., Hatton S.N., Lagopoulos J., Hickie I.B., Frodl T., Carballedo A., Frey E.M., van Velzen L.S., Penninx B.W.J.H., van Tol M.-J., van der Wee N.J., Davey C.G., Harrison B.J., Mwangi B., Cao B., Soares J.C., Veer I.M., Walter H., Schoepf D., Zurowski B., Konrad C., Schramm E., Normann C., Schnell K., Sacchet M.D., Gotlib I.H., MacQueen G.M., Godlewska B.R., Nickson T., McIntosh A.M., Papmeyer M., Whalley H.C., Hall J., Sussmann J.E., Li M., Walter M., Aftanas L., Brack I., Bokhan N.A., Thompson P.M., Veltman D.J. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry. 2017;22(6):900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Veltman D.J., van Erp T.G.M., Sämann P.G., Frodl T., Jahanshad N., Loehrer E., Tiemeier H., Hofman A., Niessen W.J., Vernooij M.W., Ikram M.A., Wittfeld K., Grabe H.J., Block A., Hegenscheid K., Völzke H., Hoehn D., Czisch M., Lagopoulos J., Hatton S.N., Hickie I.B., Goya-Maldonado R., Krämer B., Gruber O., Couvy-Duchesne B., Rentería M.E., Strike L.T., Mills N.T., de Zubicaray G.I., McMahon K.L., Medland S.E., Martin N.G., Gillespie N.A., Wright M.J., Hall G.B., MacQueen G.M., Frey E.M., Carballedo A., van Velzen L.S., van Tol M.J., van der Wee N.J., Veer I.M., Walter H., Schnell K., Schramm E., Normann C., Schoepf D., Konrad C., Zurowski B., Nickson T., McIntosh A.M., Papmeyer M., Whalley H.C., Sussmann J.E., Godlewska B.R., Cowen P.J., Fischer F.H., Rose M., Penninx B.W.J.H., Thompson P.M., Hibar D.P. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry. 2016;21(6):806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee R., Buckner R.L., Fischl B. Gray matter myelination of 1555 human brains using partial volume corrected MRI images. NeuroImage. 2015;105:473–485. doi: 10.1016/j.neuroimage.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams Z., Norris D.G., Marques J.P., Lundberg P. A comparison of in vivo MRI based cortical myelin mapping using T1w/T2w and R1 mapping at 3T. PLoS ONE. 2019;14(7):e0218089. doi: 10.1371/journal.pone.0218089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Reus L.M., Cox S.R., Adams M.J., Liewald D.C., Bastin M.E., Smith D.J., Deary I.J., Whalley H.C., McIntosh A.M. Subcortical volume and white matter integrity abnormalities in major depressive disorder: Findings from UK Biobank imaging data. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-05507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G.E., VonKorff M., Piccinelli M., Fullerton C., Ormel J. An International Study of the Relation between Somatic Symptoms and Depression. N. Engl. J. Med. 1999;341(18):1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- Simons M., Nave K.A. Oligodendrocytes: Myelination and axonal support. Cold Spring Harbor Perspect. Biol. 2016;8:1–16. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol. Bull. 2013;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A., Suslow T., Dannlowski U. Facial emotion processing in major depression: A systematic review of neuroimaging findings. Biol. Mood Anxiety Disorders. 2011;1(1):10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T., Hußlack A., Kersting A., Bodenschatz C.M. Attentional biases to emotional information in clinical depression: A systematic and meta-analytic review of eye tracking findings. J. Affect. Disord. 2020;274:632–642. doi: 10.1016/j.jad.2020.05.140. [DOI] [PubMed] [Google Scholar]
- Tardif C.L., Steele C.J., Lampe L., Bazin P.L., Ragert P., Villringer A., Gauthier C.J. Investigation of the confounding effects of vasculature and metabolism on computational anatomy studies. NeuroImage. 2017;149:233–243. doi: 10.1016/j.neuroimage.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Tham M.W., Woon P.S., Sum M.Y., Lee T.-S., Sim K. White matter abnormalities in major depression: Evidence from post-mortem, neuroimaging and genetic studies. J. Affect. Disord. 2011;132(1-2):26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Toschi N., Passamonti L. Intra-cortical myelin mediates personality differences. J. Pers. 2019;87(4):889–902. doi: 10.1111/jopy.v87.410.1111/jopy.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi, N., Passamonti, L., Bellesi, M., 2020. Sleep quality relates to emotional reactivity via intracortical myelination. Sleep. doi:10.1093/sleep/zsaa146. [DOI] [PMC free article] [PubMed]
- Vabalas A., Gowen E., Poliakoff E., Casson A.J., Hernandez-Lemus E. Machine learning algorithm validation with a limited sample size. PLoS ONE. 2019;14(11):e0224365. doi: 10.1371/journal.pone.0224365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen L.S., Kelly S., Isaev D., Aleman A., Aftanas L.I., Bauer J., Baune B.T., Brak I.V., Carballedo A., Connolly C.G., Couvy-Duchesne B., Cullen K.R., Danilenko K.V., Dannlowski U., Enneking V., Filimonova E., Förster K., Frodl T., Gotlib I.H., Groenewold N.A., Grotegerd D., Harris M.A., Hatton S.N., Hawkins E.L., Hickie I.B., Ho T.C., Jansen A., Kircher T., Klimes-Dougan B., Kochunov P., Krug A., Lagopoulos J., Lee R., Lett T.A., Li M., MacMaster F.P., Martin N.G., McIntosh A.M., McLellan Q., Meinert S., Nenadić I., Osipov E., Penninx B.W.J.H., Portella M.J., Repple J., Roos A., Sacchet M.D., Sämann P.G., Schnell K., Shen X., Sim K., Stein D.J., van Tol M.-J., Tomyshev A.S., Tozzi L., Veer I.M., Vermeiren R., Vives-Gilabert Y., Walter H., Walter M., van der Wee N.J.A., van der Werff S.J.A., Schreiner M.W., Whalley H.C., Wright M.J., Yang T.T., Zhu A., Veltman D.J., Thompson P.M., Jahanshad N., Schmaal L. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry. 2020;25(7):1511–1525. doi: 10.1038/s41380-019-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S., Simon R. Bias in error estimation when using cross-validation for model selection. BMC Bioinf. 2006;7:1–8. doi: 10.1186/1471-2105-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoquaux G. Cross-validation failure: Small sample sizes lead to large error bars. NeuroImage. 2018;180:68–77. doi: 10.1016/j.neuroimage.2017.06.061. [DOI] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. ed. Springer; 2002. Modern Applied Statistics with S, Fourth edi. [Google Scholar]
- Visconti di Oleggio Castello, M., Dobson, J.E., Sackett, T., Kodiweera, C., Haxby, J. V., Goncalves, M., Ghosh, S., Halchenko, Y.O., 2020. ReproNim/reproin 0.6.0. doi:10.5281/ZENODO.3625000.
- Wang X.-L., Du M.-Y., Chen T.-L., Chen Z.-Q., Huang X.-Q., Luo Y.a., Zhao Y.-J., Kumar P., Gong Q.-Y. Neural correlates during working memory processing in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;56:101–108. doi: 10.1016/j.pnpbp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Weiskopf N., Hutton C., Josephs O., Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: A whole-brain analysis at 3 T and 1.5 T. NeuroImage. 2006;33(2):493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Welton T., Indja B.E., Maller J.J., Fanning J.P., Vallely M.P., Grieve S.M. Replicable brain signatures of emotional bias and memory based on diffusion kurtosis imaging of white matter tracts. Hum. Brain Mapp. 2020;41(5):1274–1285. doi: 10.1002/hbm.v41.510.1002/hbm.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., Radua J., Nortje G., Cleare A.J., Young A.H., Arnone D. Voxel-based meta-Analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol. Psychiatry. 2016;79(4):293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- World Health Organisation, 2017. Depression and other common mental disorders: global health estimates. World Health Organization.
- Yasuno F., Kazui H., Morita N., Kajimoto K., Ihara M., Taguchi A., Yamamoto A., Matsuoka K., Takahashi M., Nakagawara J., Iida H., Kishimoto T., Nagatsuka K. Use of T1-weighted/T2-weighted magnetic resonance ratio to elucidate changes due to amyloid β accumulation in cognitively normal subjects. NeuroImage: Clinical. 2017;13:209–214. doi: 10.1016/j.nicl.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yu M., Linn K.A., Shinohara R.T., Oathes D.J., Cook P.A., Duprat R., Moore T.M., Oquendo M.A., Phillips M.L., McInnis M., Fava M., Trivedi M.H., McGrath P., Parsey R., Weissman M.M., Sheline Y.I. Childhood trauma history is linked to abnormal brain connectivity in major depression. PNAS. 2019;116(17):8582–8590. doi: 10.1073/pnas.1900801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.J., Wu Q.Z., Huang X.Q., Sun X.L., Zou K., Lui S., Liu F., Hu J.M., Kuang W.H., Li D.M., Li F., Chen H.F., Chan R.C.K., Mechelli A., Gong Q.Y. Magnetization transfer imaging reveals the brain deficit in patients with treatment-refractory depression. J. Affect. Disord. 2009;117(3):157–161. doi: 10.1016/j.jad.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Zheng H., Li F., Bo Q., Li X., Yao L., Yao Z., Wang C., Wu X. The dynamic characteristics of the anterior cingulate cortex in resting-state fMRI of patients with depression. J. Affect. Disord. 2018;227:391–397. doi: 10.1016/j.jad.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Ziegler G., Moutoussis M., Hauser T.U., Fearon P., Bullmore E.T., Goodyer I.M., Fonagy P., Jones P.B., Lindenberger U., Dolan R.J. Childhood socio-economic disadvantage predicts reduced myelin growth across adolescence and young adulthood. Hum. Brain Mapp. 2020;41(12):3392–3402. doi: 10.1002/hbm.v41.1210.1002/hbm.25024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Hastie T. Regularization and variable selection via the elastic net. J. Royal Statistical Soc. Series B: Statistical Methodology. 2005;67(2):301–320. doi: 10.1111/rssb:2005.67.issue-210.1111/j.1467-9868.2005.00503.x. [DOI] [Google Scholar]
- Zweerings J., Zvyagintsev M., Turetsky B.I., Klasen M., König A.A., Roecher E., Gaebler A.J., Mathiak K. Fronto-parietal and temporal brain dysfunction in depression: A fMRI investigation of auditory mismatch processing. Hum. Brain Mapp. 2019;40:hbm.24623. doi: 10.1002/hbm.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All neuroimaging data are available in BIDS format in OpenNeuro repository under the accession number ds003653 at https://doi.org/10.18112/openneuro.ds003653.v1.0.0.