Abstract
Background
High uptake of hepatitis B virus (HBV) tests and antiviral therapy are required to improve the clinical outcomes of patients with chronic hepatitis B (CHB) at the population level. In the current study, we used the Basic Medical Care Insurance for Employees (BMCIE) to investigate the changes of clinical care cascade of CHB in Beijing, China.
Methods
Records for medical service of CHB patients from January 1, 2010 to December 31, 2018 were retrieved from the BMCIE database. The annual and cumulative rates of CHB patients in care, receiving HBV tests and on antiviral therapy were calculated. The trends of annual percentage changes (APCs) were estimated using Joinpoint regression model.
Findings
Among estimated HBsAg positive employees, the rate of CHB patients in care increased from 4•77% in 2010 to 18•61% in 2018 (APC=17•3, 95%CI: 14•4-20•4). The rate of HBV tests increased from 4•41% in 2010 to 16•39% in 2018. Among the estimated eligible employees for treatment, the rate of antiviral therapy increased from 3•92% in 2010 to 30•88% in 2018. The proportion of hospital visits for HBV≥4 times per year had increased from 47•07% in 2010 to 65•31% in 2018. By 2018, entecavir (65•07%) and tenofovir (12•98%) had become the predominantly prescribed antiviral agents.
Interpretation
The rates of CHB patients in care, receiving HBV tests and on antiviral therapy substantially increased in Beijing, China. However, more efforts are still needed to increase the uptake of HBV tests and treatment for achieving the goal of HBV elimination by 2030.
Funding
Beijing Municipal Science and Technology Commission (No.D161100002716003), National Science and Technology Major Special Project for Infectious Diseases (No.Z191100007619037, No.2018ZX10302204), and Digestive Medical Coordinated Development Center of Beijing Municipal Administration of Hospitals (No. XXX 0104).
Key words: Chronic Hepatitis B, Diagnosis, Antiviral therapy, Clinical care cascade
Research in context.
Evidence before this study
WHO sets targets of 90% of hepatitis B virus (HBV)-infected people diagnosed and 80% of those eligible treated with antiviral therapy by 2030. In the past decades, China has made great progress in controlling HBV disease. Assessment of clinical care cascade of patients with chronic hepatitis B (CHB) will facilitate evidence-based decision-making.
Added value of this study
This study quantified the current status of clinical care cascade of CHB in Beijing, China. The rates of CHB cases in care, receiving HBV tests and on antiviral therapy substantial increased from 4•77% to 18•61%, 4•41% to 16•39%, and 3•92% in 2010 to 30•88% in 2018 in Beijing, China. The proportion of hospital visit for HBV ≥4 times per year increased from 47•07% in 2010 to 65•31% in 2018, while the proportion of first-line therapy recommended by the international guidelines–entecavir and tenofovir had increased to 78•05% of the total antiviral agents in 2018.
Implications of all the available evidence
This study suggested that the use of administrative database such as health insurance could be a useful approach to estimate the clinical care cascade of certain disease. Further efforts to scale-up the testing, linkage to care and treatment of CHB are still needed in Beijing as well as whole China to achieve the goal of elimination HBV by 2030.
Alt-text: Unlabelled box
1. Introduction
Chronic hepatitis B virus (HBV) infection is a major public health issue worldwide [1], [2], [3]. Up to 40% of patients with chronic hepatitis B (CHB) will develop liver-related complications and there are nearly 1 million HBV-related deaths each year 4. Owing to these drastic clinical consequences of CHB, global efforts, including universal vaccination and high coverage of potent antiviral therapy for HBV, are continuously made aiming to reduce new infections and to improve the outcomes in the existing pool of CHB patients 5. Up to now, the global HBsAg positive rate has significantly decreased to 3•5% over the past decades 6. However, there are still approximately 257 million people living with chronic HBV infection in the world, with majority of them born before the era of universal HBV vaccination 6.
Timely diagnosis and effective treatment of eligible individuals living with CHB have been shown to substantially reduce the risk of developing decompensated liver cirrhosis or hepatocellular carcinoma (HCC) and to be a cost-effective intervention 7. Current first-line antiviral therapies for CHB are highly potent, well-tolerated and confer very low resistance profile, but require long-term treatment 8. To maximize this benefit, countries in the Western Pacific have endorsed a regional action plan for viral hepatitis which established 2020 targets that 30% of people living with HBV would be diagnosed, and 50% of eligible people received treatment 9.
In China, the new infection rate of HBV becomes very low and the overall prevalence has been dramatically declined from 9.75% to approximately 6% 10,11. Nevertheless, China still has the highest burden of CHB, where inhabits nearly 25% of the total number of persons with chronic HBV infection in the world 3. The availability and affordability to first line antiviral agents for HBV has also greatly improved 6,12,13. However, modeling studies demonstrated that the current rates of diagnosis and treatment of CHB in China are still low 14.Therefore, advocacy to increase the uptake of HBV diagnosis and treatment at the population level is still of utmost importance to invert the premature deaths caused by CHB 15. In this sense, regular analysis of the clinical care cascade of CHB will assist in assessing the progress toward HBV elimination goals.
Therefore, in the present study we investigate the dynamic changes of clinical care cascade of CHB patients in Beijing, China, by using the database of the Basic Medical Care Insurance for Employees (BMCIE) from 2010 to 2018.
2. Methods
2.1. Database description
The BMCIE (both current and retired) is a mandatory social insurance program, which covers nearly 80% of the total population in Beijing. This database includes records for health care service performed in both inpatient and outpatient settings, as well as the demographic profiles for covered individuals. In the present study, all retrieved individual data were de-identified.
2.2. Identification of patients with CHB
For the present study, CHB patients were identified from the BMCIE database of Beijing from 1 January 2010 to 31 December 2018. Records of hospital visit due to CHB were screened electronically using the International Classification of Diseases (ICD)-10 codes of B18.0 and B18.1, in combination of diagnostic term of CHB.
2.3. Ascertainment of total number of HBsAg positive employees in Beijing
To estimate the total number of HBsAg positive employees in Beijing, the sero-prevalence rate of HBsAg in Beijing was multiplied to the number of employees covered by the Basic Medical Care Insurance over the time period of interest.
The sero-prevalence rate of HBsAg (around 3•12% for individuals aged≥20 year old) was generated by a multistage randomized cluster sampling survey of Beijing general population conducted by Beijing Municipal Center for Disease Control and Prevention from August 2013 to February 2014 16.
The overall number of employees covered by Basic Medical Care Insurance in Beijing was derived from the Beijing Statistical Yearbook published by Beijing Municipal Bureau Statistics. The number of age- and gender- specific employees covered by Basic Medical Care Insurance was estimated based on the age proportion and sex ratio in the whole population reported in the Beijing Statistical Yearbook of the same year (available at http://tjj.beijing.gov.cn/).
The estimated number of CHB patients eligible for antiviral therapy was calculated by the total number of HBsAg positive employees times the proportion of persons eligible for treatment among all HBsAg positive subjects (37•57%) as previous study reported 2.
2.4. Definitions for CHB patients in care, receiving HBV tests and on antiviral therapy
CHB patients annually in care were defined as having at least one hospital visit due to CHB (encounter), combined with HBV tests or antiviral therapy for HBV at a given year. CHB patients ever in care were defined as having ever received CHB related care till a given year. To calculate the rate of CHB patients in care, we divided the CHB patients in care among estimated HBsAg positive employees. We also calculated the age-and-gender specified rate per 100 persons by age group (<30, 30-39, 40-49, 50-59, and ≥60 years) and gender group (male and female).
CHB patients received HBV tests were defined as CHB patients having received any serology or virology tests for HBV, including HBsAg, Anti-HBs, HBeAg, Anti-HBe, Anti-HBc, and HBV-DNA quantification at least one time for a given year. CHB patients ever received HBV tests were defined as CHB patients having ever received HBV tests till a given year. The rate of HBV tests was calculated by dividing CHB patients received HBV tests by the estimated HBsAg positive employees.
Yearly treated CHB patients were defined as having been treated with lamivudine, adefovir dipivoxil, telbivudine, entecavir, tenofovir, and/or interferon at least one time for a given year. Ever treated CHB patients were defined as having ever received antiviral agents after their initial diagnosis.
The rate of treatment was calculated in two dimensions, one is dividing the antiviral treated patients by estimated eligible CHB patients for treatment (population level), the other is dividing the antiviral treated patients by CHB patients in care (hospital level).
The times of hospital visits for HBV were summarized, and proportion of hospital visits for HBV≥4 times per year was also calculated. In addition, the proportion of hospitalization for CHB and its complications (compensated/decompensated cirrhosis, or HCC) among those in care was calculated.
2.5. Statistical Analysis
For descriptive purpose, baseline characteristics were described as percent for categorical variables, and mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables.
Joinpoint regression program version 4.7.0.0 (National Cancer Institute, Bethesda, MD) 17, was used to investigate temporal changes. This program used a piecewise linear regression approach to examine whether a single segment or multiple linear segments best explain the rate during the study period. We provided each trend segment by annual percent change (APC) and the trend for the entire study period by the average APC, which determined the year when the trend in rate changed significantly and estimated the magnitude of the change 18. The trends were considered significant if the 95% confidence interval (CI) of APC did not include zero.
2.6. Ethical standards
This study was approved by the Institutional Review Board of Beijing Friendship Hospital, Capital Medical University (approval number 2016-P2-024-01), with the requirement for patients’ informed consent waived.
2.7. Role of the funding source
The funding source of this study had no role in the study design, data collection, data analysis, data interpretation, or drafting of the manuscript. The corresponding authors had full access to all study data and are responsible for the decision to submit for publication.
3. Results
3.1. Demographic and baseline characteristics of CHB patients
Totally, 216,667 CHB patients in care were identified. The patients were predominantly male (57•99%), with a mean age of approximate 42•87 years. The proportion of cirrhosis decreased from 27•80% in 2010 to 21•78% in 2018, while the proportion of HCC decreased from 13•76% to 6•12%. During the study period, the proportion of patients with dual infection (HCV, HDV or HIV), co-liver disease and other co-morbidities decreased gradually (Table S1 and Table S2).
3.2. Changes in clinical care cascade of CHB patients
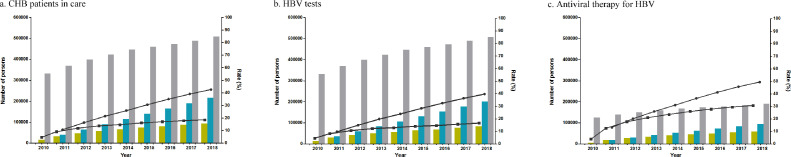
Among the estimated total number of HBsAg positive employees, the number of patients in care each year increased from 15,810 (4•77%) in 2010 to 94,495 (18•61%) in 2018 (Table 1, Figure 1). The average APC was 17•3%/year (95%CI, 14•4 to 20•4) for CHB patients in care. Joinpoint analysis demonstrated the change in rate of CHB patients in care went through two segments: the APC was 55•6%/year (95%CI, 35•4 to 78•9) in the rapid increase phase of year 2010-2012, and 6•8%/year (95%CI, 5•4 to 8•1) in the slow increase phase starting in year 2012 (Table 2, Figure S1).
Table 1.
Cascade of patients with chronic hepatitis B in care, receiving HBV tests and on antiviral therapy, 2010-2018
| Year | |||||||||
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Estimated total number of HBsAg(+) persons | 331,590 | 370,339 | 398,925 | 422,336 | 446,183 | 460,024 | 473,086 | 489,171 | 507,782 |
| Estimated eligible CHB patients for treatment | 124,579 | 139,136 | 149,876 | 158,672 | 167,631 | 172,831 | 177,738 | 183,782 | 190,774 |
| No. of CHB patients in care | 15,810 | 33,926 | 47,570 | 58,122 | 65,947 | 74,352 | 80,565 | 87,833 | 94,495 |
| % per 100 estimated HBsAg(+) persons | 4•77% | 9•16% | 11•92% | 13•76% | 14•78% | 16•16% | 17•03% | 17•96% | 18•61% |
| No. of CHB patients receiving HBV tests | 14,629 | 29,821 | 41,876 | 51,018 | 57,113 | 63,850 | 68,472 | 76,156 | 83,240 |
| % per 100 estimated HBsAg(+) persons | 4•41% | 8•05% | 10•50% | 12•08% | 12•80% | 13•88% | 14•47% | 15•57% | 16•39% |
| No. of CHB patients receiving antiviral therapy | 4,886 | 17,556 | 26,798 | 33,584 | 39,737 | 45,086 | 49,798 | 54,487 | 58,908 |
| % per 100 estimated eligible CHB patients for treatment | 3•92% | 12•62% | 17•88% | 21•17% | 23•71% | 26•09% | 28•02% | 29•65% | 30•88% |
| % per 100 CHB patients in care | 30•90% | 51•75% | 56•33% | 57•78% | 60•26% | 60•64% | 61•81% | 62•03% | 62•34% |
| No. of CHB patients ever in care | - | 40,128 | 65,229 | 90,630 | 115,507 | 141,026 | 165,836 | 191,475 | 216,667 |
| % per 100 estimated HBsAg(+) persons | - | 10•84% | 16•35% | 21•46% | 25•89% | 30•66% | 35•05% | 39•14% | 42•67% |
| No. of CHB patients ever receiving HBV tests | - | 36,284 | 59,764 | 83,584 | 106,718 | 130,407 | 153,450 | 177,714 | 201,681 |
| % per 100 estimated HBsAg(+) persons | - | 9•80% | 14•98% | 19•79% | 23•92% | 28•35% | 32•44% | 36•33% | 39•72% |
| No. of CHB patients ever receiving antiviral therapy | - | 18,492 | 30,163 | 41,131 | 52,085 | 62,967 | 73,467 | 84,063 | 94,390 |
| % per 100 estimated eligible CHB patients for treatment | - | 13•29% | 20•13% | 25•92% | 31•07% | 36•43% | 41•33% | 45•74% | 49•48% |
| % per 100 CHB patients ever in care | - | 46•08% | 46•24% | 45•38% | 45•09% | 44•65% | 44•30% | 43•90% | 43•56% |
CHB, chronic hepatitis B.
Figure 1.
Cascade of patients with chronic hepatitis B in care, receiving HBV tests and on antiviral therapy, 2010-2018. The bars represent the annual number of CHB patients (yellow bar), the cumulative number of CHB patients (blue bar), the estimated total number of HBsAg(+) persons (gray bar) in a and b, and the estimated eligible CHB patients for treatment (gray bar) in c. The dots represent the annual rate (square) and the cumulative rate (circle). CHB, chronic hepatitis B.
Table 2.
Annual percentage change in the annual rates of CHB patients in care, receiving HBV tests and on antiviral therapy
| Overall | Rapid increase phase | Slow increase phase | ||||
| Year | Average APC (95%CI) | Year | APC (95%CI) | Year | APC (95%CI) | |
| In care | 2010-2018 | 17•3%(14•4 to 20•4) | 2010-2012 | 55•6%(35•4 to 78•9) | 2012-2018 | 6•8%(5•4 to 8•1) |
| HBV tests | 2010-2018 | 16•6%(15•3 to 18•0) | 2010-2012 | 52•0%(42•7 to 61•9) | 2012-2018 | 6•8%(6•2 to 7•4) |
| Antiviral therapy | ||||||
| Among estimated eligible patients for treatment | 2010-2018 | 24•6%(18•7 to 30•8) | 2010-2012 | 90•3%(45•2 to 149•4) | 2012-2018 | 8•2%(6•2 to 10•3) |
| Among CHB patients in care | 2010-2018 | 6•7%(4•6 to 8•9) | 2010-2012 | 24•9%(11•7 to 39•6) | 2012-2018 | 1•3%(0•5 to 2•1) |
CHB: chronic hepatitis B; APC: annual percent change; HBV: hepatitis B virus.
Among the estimated total number of HBsAg positve employees, the number of patients receiving HBV tests also increased, from 14,629 (4•41%) in 2010 to 83,240 (16•39%) in 2018. The average APC was 16•6%/year (95%CI, 15•3 to 18•0) for HBV tests. Meanwhile, the APC of CHB patients receiving HBV tests was 52•0%/year (95%CI, 42•7 to 61•9) and 6•8%/year (95%CI, 6•2 to 7•4) in the rapid increase phase and slow increase phase, respectively.
Among the estimated eligible patients for treatment, the number of patients receiving antiviral therapy also increased, from 4,886 (3•92%) in 2010 to 58,908 (30•88%) in 2018. The average APC was 24•6%/year (95%CI, 18•7 to 30•8) for antiviral therapy. The APC of CHB patients receiving antiviral therapy was 90•3%/year (95%CI, 45•2 to 149•4) and 8•2%/year (95%CI, 6•2 to 10•3) in the rapid increase phase and slow increase phase, respectively.
Among CHB patients in care, the rate of patients receiving antiviral therapy also increased, from 30•90% in 2010 to 62•34% in 2018. The average APC was 6•7%/year (95%CI, 4•6 to 8•9) for antiviral therapy. The APC of CHB patients receiving antiviral therapy was 24•9%/year (95%CI, 11•7 to 39•6) and 1•3%/year (95%CI, 0•5 to 2•1) in the rapid increase phase and slow increase phase, respectively.
3.3. Subgroup analysis
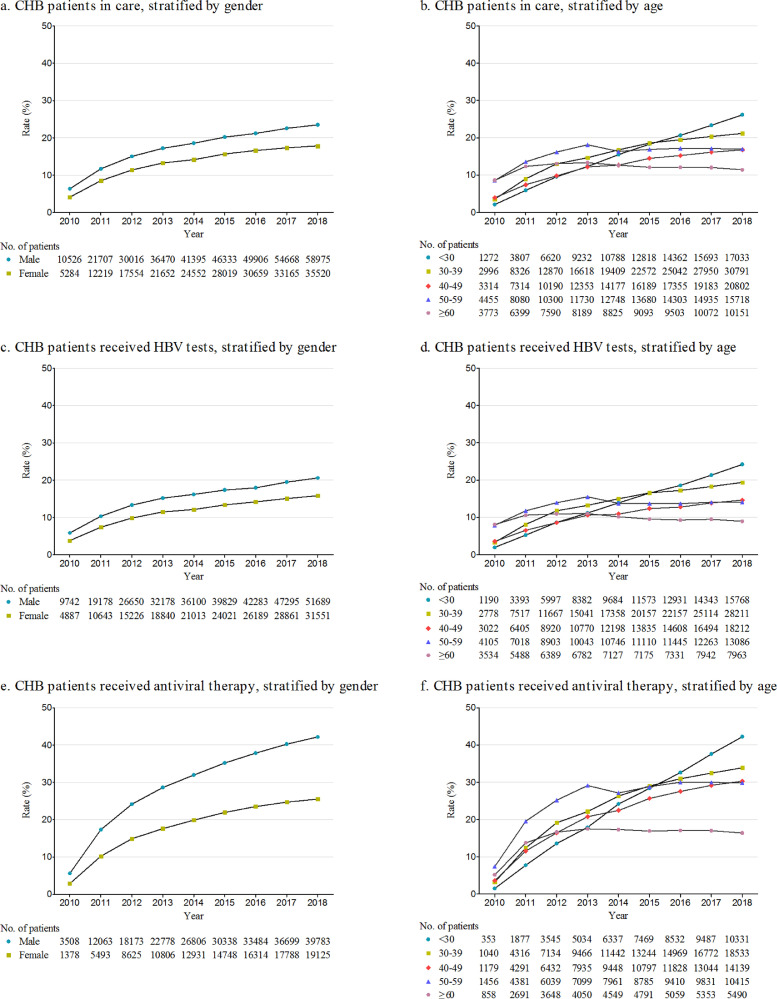
Age- and gender-specific rates of CHB patients in care among estimated total number of HBsAg positive employees were calculated (Figure 2). The annual rate of CHB patients in care increased from 6•33% to 23•49% for males (avearge APC: 16•7%), and from 4•12% to 17•82% for females (average APC: 18•7%). From 2010 to 2018, the average APC in annual rate of CHB patients in care was 34•8% for CHB patients aged ≤30 years old, followed by 22•5%, 19•1%, 8•0%, and 2•6% for patients aged 30-39, 40-49, 50-59, and ≥60 years old, respectively. In 2018, 26•16% of estimated HBsAg positive employees aged ≤30 years old were in care, reaching the highest across all age groups, which indicated that younger CHB patients were more active in care. Similar trends were observed for the age- and gender-specific cumulative rates of CHB patients in care, as showed in Figure S2.
Figure 2.
Annual rates of patients with chronic hepatitis B in care, receiving HBV tests and on antiviral therapy stratified by gender and age. a and b are the rates of CHB patients in care among estimated HBsAg(+) population; c and d are the rates of CHB patients receiving HBV tests among estimated HBsAg(+) population; e and f are the rates of CHB patients on antiviral therapy among estimated eligible CHB patients for treatment. CHB, chronic hepatitis B; HBV, hepatitis B virus.
Age- and gender-specific rates of HBV tests among estimated total number of HBsAg positive employees were calculated (Figure 2). The annual rate of HBV tests increased from 5•85% to 20•59% for males (average APC: 15•9%), and from 3•81% to 15•83% for females (average APC: 18•1%). From 2010 to 2018, the average APC in annual rate of HBV tests was 34•9% for CHB patients aged ≤30 years old, followed by 22•3%, 18•4%, 6•5%, and 0•4% for patients aged 30-39, 40-49, 50-59, and ≥60 years old, respectively. In 2018, 24•22% of estimated HBsAg positive employees aged ≤30 years old have received HBV tests, reaching the highest across all age groups, which indicated that younger CHB patients were more active in HBV monitoring. Similar trends were observed for the age- and gender-specific cumulative rates of CHB patients receiving HBV tests, as showed in Figure S2.
Age- and gender-specific rates of antiviral therapy among estimated total number of HBsAg positive employees eligible for treatment were calculated (Figure 2). The annual rate of antiviral therapy increased from 5•61% to 42•18% for males (average APC: 24•1%), and from 2•86% to 25•54% for females (average APC: 25•9%). From 2010 to 2018, the average APC in annual rate of antiviral therapy was 42•9% for CHB patients aged ≤30 years old, followed by 28•1%, 25•8%, 15•2%, and 11•1% for patients aged 30-39, 40-49, 50-59, and ≥60 years old, respectively. Compared with patients aged<60 years old, the rate of antiviral therapy increased relatively slow for CHB patients aged ≥60 years old. Similar trends were observed for the age- and gender-specific cumulative rates of CHB patients reveiving antiviral therapy, as showed in Figure S2.
3.4. Rates of antiviral therapy in different disease stages of CHB
The annual rate of antiviral therapy stably increased among patients with non-cirrhotic CHB, cirrhotic CHB and HCC. The rate of antiviral therapy increased with calendar time, particularly in 2011, and it reached its highest value of 57•76% for non-cirrhotic CHB, 74•05% for cirrhotic CHB, and 74•68% for HCC patients in 2018 (Table 3). The average APC in the rate of antiviral therapy is 5•3% for non-cirrhotic CHB patients, 8•3% for cirrhotic CHB patients, and 11•6% for HCC patients. Similar trends were observed for the age- and gender-specific annual rates of CHB patients receiving antiviral therapy (Figure S3), while the cumulative rates were basically stable (Figure S4).
Table 3.
Antiviral therapy for patients with chronic hepatitis B at different disease stages, 2010-2018
| Disease stages | Year | ||||||||
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Non-cirrhotic CHB | |||||||||
| No. of CHB patients annually in care | 9,239 | 22,674 | 33,003 | 40,896 | 46,445 | 52,787 | 57,360 | 62,754 | 68,130 |
| No. of patients on antiviral therapy | 2,817 | 11,395 | 17,682 | 22,218 | 26,225 | 29,792 | 32,948 | 36,177 | 39,349 |
| % per 100 non-cirrhotic CHB patients | 30•49% | 50•26% | 53•58% | 54•33% | 56•46% | 56•44% | 57•44% | 57•65% | 57•76% |
| Cirrhosis | |||||||||
| No. of cirrhotic patients annually in care | 4,395 | 8,249 | 10,912 | 13,040 | 14,912 | 16,719 | 18,071 | 20,280 | 20,578 |
| No. of patients on antiviral therapy | 1,431 | 4,757 | 7,012 | 8,773 | 10,429 | 11,923 | 13,152 | 14,821 | 15,237 |
| % per 100 cirrhotic patients | 32•56% | 57•67% | 64•26% | 67•28% | 69•94% | 71•31% | 72•78% | 73•08% | 74•05% |
| HCC | |||||||||
| No. of HCC patients annually in care | 2,176 | 3,003 | 3,655 | 4,186 | 4,590 | 4,846 | 5,134 | 4,799 | 5,787 |
| No. of patients on antiviral therapy | 638 | 1,404 | 2,104 | 2,593 | 3,083 | 3,371 | 3,698 | 3,489 | 4,322 |
| % per 100 HCC patients | 29•32% | 46•75% | 57•56% | 61•94% | 67•17% | 69•56% | 72•03% | 72•70% | 74•68% |
CHB, chronic hepatitis B; HCC, hepatocellular carcinoma
3.5. Changes of medical care utility and prescription patterns of antiviral agents
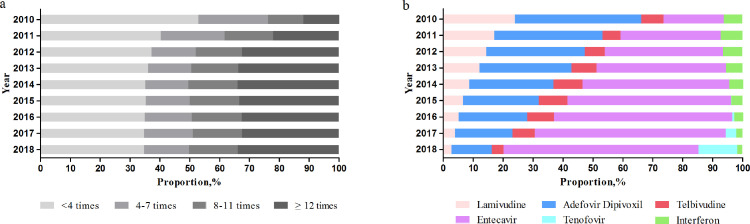
The annual times of hospital visits for HBV increased, with a median of 3 times in 2010 and 8 times in 2018 (Table S3). The proportion of hospital visits for HBV≥4 times per year increased from 47•07% to 65•31% (Figure 3). In contrast, the proportion of hospitalization for CHB and its complications decreased from 50•57% in 2010 to 10•34% in 2018 (Table S4).
Figure 3.
Number of hospital visits (a) and antiviral prescription (b) among patients with chronic hepatitis B, 2010-2018.
The prescription patterns of antiviral agents greatly changed (Figure 3). In 2010, adefovir dipivoxil and lamivudine were predominant. In contract, the prescription of entecavir increased from 20•17% in 2010 to 65•07% in 2018. Furthermore, tenofovir dipivoxil increased to 12•98% after its aproval in 2015 and inclusion in the reimbursement list in 2017 in China. Consquently, entecavir (65•07%) and tenofovir dipivoxil (12•98%) were the predominantly used antiviral agents in 2018.
4. Discussion
This study quantified the current status of clinical care cascade of CHB in Beijing, China using BMCIE database, which covers 80% of the residents in Beijing. The rates of CHB patients in care, receiving HBV tests and on antiviral therapy substantially increased from 4•77% to 18•61%, 4•41% to 16•39%, and 3•92% in 2010 to 30•88% in 2018 in Beijing, China.
In our study, we found that the increase pattern was biphasic: a rapid increase phase (2010-2012) followed by a slow increase phase (2012-2018). The major contributor for this overall increase might be the progress by adopting a comprehensive strategy to increase awareness of CHB and affordability of antiviral therapy in Beijing as well as whole China. Specifically, the antiviral agents for HBV have included in the reimbursement list of Basic Medical Care Insurance in Beijing since July 1, 2011, thereby leading to a rapid increase around year 2011 5,19.
Our study also revealed different patterns of clinical care cascade among patients with different age and gender. The rate of CHB patients in care was higher in younger patients. Similarly, the rates of antiviral therapy among estimated CHB patients eligible for treatment varied widely in persons with different ages, ranging from 16•43% (≥60 years old) to 42•24% (<30 years old) in 2018. One of the explanations might be that younger patients have higher awareness of and more active attitude towards CHB disease20.
An interesting finding was that the proportion of hospital visits for HBV≥4 times per year increased from 47•07% to 65•31%, whereas the proportion of hospitalization for CHB and its complications decreased from 50•57% in 2010 to 10•34% in 2018. This kind of changes of clinical care utility may reflect the fact that effective antiviral therapy reduced the disease progression and complication of CHB, therefore decreased the need for hospitalization 5. Indeed, health economic study showed antiviral therapy for CHB is highly cost effective, all even cost saving 15. Furthermore, the proportion of first-line therapy recommended by the international guidelines–entecavir and tenofovir had increased to 78•05% of the total antiviral agents in 2018. This improvement was mainly due to massive price reduction of antiviral agents and their inclusion to the reimbursement list 5, 19.
Finally, the rate of antiviral therapy among CHB patients eligible for treatment in Beijing (31%) seemed higher than the estimates of whole China (16%) and the whole Western Pacific Region (5%), but lower than that in Korea (51%) 14. Obviously, a huge gap still existed between the current status and the WHO strategy targets of 90% of HBV-infected people are diagnosed and 80% of those eligible for treatment are treated by 2030 6. Therefore, further efforts to scale-up the testing, linkage to care and treatment was still needed in Beijing as well as whole China.
Overall, our study suggested that the use of administrative database such as health insurance could be an important way to estimate the clinical care cascade of certain disease. This approach may facilitate the continuous monitoring of CHB disease burden and clinical care cascade, thereby supporting evidence-based decision-making for clinical and public health issues.
However, the interpretation of our results required caution due to the following limitations. Firstly, the administrative database does not contain the information of laboratory and radiology results therefor preclude accurate assessment of clinical diagnosis and therapeutic effectiveness. Secondly, the number of HBsAg positive employees is estimated using the rate derived from a population based survey reported in 2016, and change of HBsAg positive rate over the study period might affect the accuracy of this estimation. However, the amplitude of change in adults would be very small during the studied period because the rate of new infection and the rate of spontaneous or treatment-induced HBsAg loss are very low. Thirdly, due to the availability, we could only analyze nine time points of the clinical care cascade of CHB patients (2010 to 2018). Fourthly, the results were derived from employees covered by Basic Medical Care Insurance, the core population of CHB management in Beijing. However it may not be extrapolated to the whole population, because this BMCIE system covers only about 80% of the total population in Beijing, and the left 20% mainly consists of children/adolescents and residents who are not currently in employment, which are covered by the database of Basic Medical Care Insurance for Residences.
5. Conclusion
This study demonstrated that the rates of CHB patients in care, receiving HBV tests and on antiviral therapy steadily increased in Beijing, China. The use of administrative database could be a useful approach to estimate the clinical care cascade of CHB. Further efforts to scale-up the testing, linkage to care and treatment of CHB are still needed in Beijing as well as whole China to achieve the goal of elimination HBV by 2030.
6. Contributors
Study concept and design: Jidong Jia, Yuanyuan Kong. Statistical analysis and verify the underlying data: Min Li, Lianhui Zhao. Drafting of the manuscript: Min Li. Critical revision of the manuscript for important intellectual content: Jialing Zhou, Yameng Sun, Xiaoning Wu, Xiaojuan Ou, Hong You. All authors approved the final version of the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgements
This work was supported by Beijing Municipal Science and Technology Commission (No.D161100002716003), National Science and Technology Major Special Project for Infectious Diseases (No.Z191100007619037, No.2018ZX10302204), and Digestive Medical Coordinated Development Center of Beijing Municipal Administration of Hospitals (No. XXX 0104).
Data sharing
Additional data are available on request from the corresponding author at jia_jd@ccmu.edu.cn; kongyy@ccmu.edu.cn.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100249.
Contributor Information
Yuanyuan Kong, Email: kongyy@ccmu.edu.cn.
Jidong Jia, Email: jia_jd@ccmu.edu.cn.
Appendix. Supplementary materials
Reference
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Vol. 390. Lancet; 2016. pp. 1211–1259. (Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383–403. [DOI] [PubMed]
- 3.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.Nayagam S, Thursz M, Sicuri E, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16:1399–1408. doi: 10.1016/S1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Kong YY, Wu SS, et al. Impact of reimbursement program on liver-related mortality in patients with chronic hepatitis B in Beijing, China. J Dig Dis. 2019;20:467–475. doi: 10.1111/1751-2980.12794. [DOI] [PubMed] [Google Scholar]
- 6.WHO. 2017. Global hepatitis report. Accessed on Jun 20, 2021 https://apps.who.int/iris/discover/export?format=refman&singleItemid=258639&handle=10665/255016. [Google Scholar]
- 7.Lok AS, McMahon BJ, Jr Brown RS, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 8.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO . 2021. Implementation progress of the regional action plan for viral hepatitis in the Western Pacific.https://www.who.int/westernpacific/health-topics/hepatitis/implementation-progress-of-the-regional-action-plan-for-viral-hepatitis-in-the-western-pacific-2016-2020/regional-action-on-viral-hepatitis 2016-2020. Accessed on Jun 20. [Google Scholar]
- 10.Cui F, Shen L, Li L, et al. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg Infect Dis. 2017;23:765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease. China. Bull World Health Organ. 2019;97:230–238. doi: 10.2471/BLT.18.219469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarin SK, Kumar M, Eslam M, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167–228. doi: 10.1016/S2468-1253(19)30342-5. Erratum in: Lancet Gastroenterol Hepatol 2020;5:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke GS, Andrieux-Meyer I, Applegate TL, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135–184. doi: 10.1016/S2468-1253(18)30270-X. Erratum in: Lancet Gastroenterol Hepatol 2019;4:e4. [DOI] [PubMed] [Google Scholar]
- 14.CDA Foundation . 2021. Preliminary data, Polaris Observatory. https://cdafound.org/dashboard/polaris/dashboard.html Accessed on Jun 20. [Google Scholar]
- 15.Nayagam S, Chan P, Zhao K, et al. Investment Case for a Comprehensive Package of Interventions Against Hepatitis B in China: Applied Modeling to Help National Strategy Planning. Clin Infect Dis. 2021;72:743–752. doi: 10.1093/cid/ciaa134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao P, Wang H, Chen WX, et al. A sero-epidemiological study of hepatitis B among general population in Beijing. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37:658–662. doi: 10.3760/cma.j.issn.0254-6450.2016.05.014. [In Chinese.] [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu Q, Duan XW, Li Y, et al. Impact of partial reimbursement on hepatitis B antiviral utilization and adherence. World J Gastroenterol. 2015;21:9588–9597. doi: 10.3748/wjg.v21.i32.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Zhou H, Zhang L, et al. Prevalence of chronic hepatitis B and status of HBV care among rural women who planned to conceive in China. Sci Rep. 2017;7:12090. doi: 10.1038/s41598-017-12005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.