Abstract
Aims
Alcohol use disorder (AUD) is linked to hyperactivity of brain stress systems, leading to withdrawal states which drive relapse. AUD differs among the sexes, as men are more likely to have AUD than women, but women progress from casual use to binge and heavy alcohol use more quickly and are more likely to relapse into repetitive episodes of heavy drinking. In alcohol dependence animal models of AUD, the central amygdala (CeA) functions as a hub of stress and anxiety processing and gamma-Aminobutyric acid (GABA)ergic signaling within the CeA is involved in dependence-induced increases in alcohol consumption. We have shown dysregulation of CeA GABAergic synaptic signaling in alcohol dependence animal models, but previous studies have exclusively used males.
Methods
Here, we used whole-cell patch clamp electrophysiology to examine basal CeA GABAergic spontaneous inhibitory postsynaptic currents (sIPSC) and the effects of acute alcohol in both naïve and alcohol dependent rats of both sexes.
Results
We found that sIPSC kinetics differ between females and males, as well as between naïve and alcohol-dependent animals, with naïve females having the fastest current kinetics. Additionally, we find differences in baseline current kinetics across estrous cycle stages. In contrast to the increase in sIPSC frequency routinely found in males, acute alcohol (11–88 mM) had no effect on sIPSCs in naïve females, however the highest concentration of alcohol increased sIPSC frequency in dependent females.
Conclusion
These results provide important insight into sex differences in CeA neuronal function and dysregulation with alcohol dependence and highlight the need for sex-specific considerations in the development of effective AUD treatment.
Short Summary: Female central amygdala (CeA) GABAA receptor current kinetics are faster than males and depend on estrous cycle, with alcohol dependent animals having slower kinetics. Acute alcohol increases presynaptic GABA release in male CeA but has no effect in naïve female CeA. High concentrations of alcohol increase GABA release in dependent female CeA.
INTRODUCTION
Alcohol use disorder (AUD; APA, 2013), is a chronically relapsing disease characterized by a loss of control over seeking and consumption of alcohol. Lifetime prevalence of AUD is high (29.1%) but affects greater numbers of men (36% prevalence) than women (22.7% prevalence; Grant et al., 2015). Sex differences in prevalence are also seen with other psychiatric disorders, including stress disorders and anxiety disorders (Kushner et al., 2000). Specifically, women are more likely to have a stress or anxiety disorder than men, and men are more likely to have AUD than women (Cover et al., 2014). However, even at lower levels of consumption, women have a higher likelihood of adverse health consequences from alcohol, such as liver disease, cardiomyopathy, brain damage and breast cancer (Erol and Karpyak, 2015; Agabio et al., 2016; Roerecke et al., 2019). Additionally, alcohol use by women has been increasing recently, especially among younger individuals, and as some studies suggest women may progress to binge drinking and heavy alcohol use more rapidly, the sex difference in AUD incidence rates is shrinking (Diehl et al., 2007; Keyes et al., 2011; Dawson et al., 2015; White et al., 2015; Slade et al., 2016).
Alcohol dependence is inexorably linked to stress and altered functioning of brain stress systems. Chronic alcohol use recruits these systems, eventually leading to withdrawal symptoms when abstinent from alcohol. The relief of these withdrawal symptoms drives further drinking and is characteristic of dependence (Koob and Le Moal, 2005). One brain region that functions as a hub of stress and anxiety processing is the central nucleus of the amygdala (CeA), and gamma-Aminobutyric acid (GABA)ergic signaling within the CeA is involved in the regulation of alcohol consumption (Roberto et al., 2021). In male animals, we have found CeA dysregulation is a hallmark of alcohol dependence across species (Roberto et al., 2004; Nie et al., 2009; Roberto et al., 2010; Herman et al., 2016; Augier et al., 2018; Jimenez et al., 2019; Tunstall et al., 2019; Khom et al., 2020a; Khom et al., 2020b). CeA GABA transmission is enhanced in alcohol dependence, and we have previously shown the importance of this elevated GABA transmission in dependence-induced alcohol drinking (Roberto et al., 2003, 2004, 2010). However, sex differences exist in response to both alcohol and stress (Shansky, 2015; Wellman et al., 2018; Steinman et al., 2020), and to our knowledge no one has investigated CeA alterations induced by alcohol dependence in female animals.
In this study, we used whole-cell patch clamp electrophysiology in an ex vivo CeA slice preparation to compare basal GABAergic spontaneous inhibitory postsynaptic currents (sIPSC) in naïve and alcohol dependent rats of both sexes. Further, we examined the effects of acutely applied alcohol in naïve and alcohol dependent female CeA slices, and compared them with the enhanced GABA transmission seen in the CeA of naïve and dependent male rats.
MATERIALS AND METHODS
Animals
Female (n = 44) and male (n = 32) Sprague Dawley rats (Charles River, Raleigh, NC) weighed on average 263 ± 5.6 g and 424 ± 15.3 g, respectively, at time of sacrifice. Estrous cycle was not selected for and determined by vaginal lavage only at time of sacrifice to limit the stressful or reproductive effects of vaginocervical stimulation (Logrip et al., 2016; Kirson et al., 2018). All rats were housed in a temperature- and humidity-controlled room on a 12-h light/dark cycle with food and water available ad libitum. Alcohol dependent rats (average blood alcohol level 213.8 ± 20.1 mg/dl at time of sacrifice) were generated by exposure to alcohol vapor daily (14-h alcohol vapor; 10-h air vapor) for 5–7 weeks (Gilpin et al., 2008; Gilpin et al., 2011). All procedures and care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Electrophysiology
Preparation of acute brain slices and electrophysiological recordings were performed as previously described (Roberto et al., 2010; Steinman et al., 2020). Briefly, coronal CeA slices (300 μm) were prepared from anesthetized rats in an ice-cold high-sucrose cutting solution (sucrose 206 mM; KCl 2.5 mM; CaCl2 0.5 mM; MgCl2 7 mM; NaH2PO4 1.2 mM; NaHCO3 26 mM; glucose 5 mM and HEPES 5 mM). Slices were incubated in superfused (flow rate of 2–4 ml/min) 95% O2/5% CO2 equilibrated artificial cerebrospinal fluid (aCSF; NaCl 130 mM; KCl 3.5 mM; NaH2PO4 1.25 mM; MgSO4·7H2O 1.5 mM; CaCl2 2.0 mM; NaHCO3 24 mM and glucose 10 mM). Whole-cell patch-clamp recordings of GABAergic sIPSCs were performed in neurons from the medial subdivision of the CeA clamped at −60 mV (n = 169 neurons). Patch pipettes (3–6 MΩ) were filled with an internal solution composed of the following (in mM): 145 KCl, 0.5 EGTA, 2 MgCl2, 10 HEPES, 2 Mg-ATP and 0.2 Na-GTP. For animal variability, each experimental group contained neurons from a minimum of 3–4 rats. GABAergic activity was pharmacologically isolated with 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM), DL-2-amino-5-phosphonovalerate (DL-AP5, 30 μM) and CGP 55845A (1 μM). In all experiments, cells with a series resistance > 25 MΩ were excluded from analysis, and series resistance was continuously monitored during gap-free recording with a 10 mV pulse. Cells in which series resistance changed >25% during the experiment were excluded from analysis. Data were analyzed using Mini Analysis (Synaptosoft Inc., Fort Lee, NJ) with 3-min bins of gap-free recording. All drugs were applied by bath superfusion.
Drugs
CGP 55845A, DL-AP5 and DNQX were obtained from Tocris (Ellisville, MO). Ethanol was purchased from Remet (La Mirada, CA). Drugs were added to the aCSF from stock solutions to obtain known concentrations in the superfusate.
Statistical analysis
Data are presented as mean ± SEM, and n refers to the number of cells. The criterion for statistical significance was P < 0.05. Statistical analyses were performed in Prism 7 (GraphPad Software, La Jolla, CA). Data were analyzed with two-way analysis of variance (ANOVA; Treatment Group × Sex or Treatment Group × Cycle) followed by Bonferroni post hoc comparisons or one-sample t-test as appropriate.
RESULTS
We recorded GABAergic sIPSCs from neurons (n = 175) in the medial subdivision of the CeA of both female and male naïve and alcohol dependent rats, using the whole-cell patch clamp configuration. We first examined baseline sIPSC group differences (Fig. 1) where in general, changes in the frequency of currents reflect altered presynaptic neurotransmitter release, changes in current kinetics reflect alterations in postsynaptic GABAA receptor sensitivity and changes in current amplitudes can be a mix of pre- and postsynaptic changes (De Koninck and Mody, 1994; Otis et al., 1994). We did not find a significant main effect of sex or alcohol treatment group for baseline sIPSC frequency (Fig. 1C), but there was a Sex X Group interaction effect (two-way ANOVA; F1,171 = 6.62, P < 0.05). This effect was driven by a significant elevation in sIPSC frequency in dependent males compared with naïve males (Bonferroni; t171 = 2.30, P < 0.05), indicating increased presynaptic GABA release in dependent males but not females. We found no significant differences between female and male, naive and dependent CeA neurons in baseline sIPSC amplitude (Fig. 1D). Females had significantly faster sIPSC rise time (two-way ANOVA; Sex: F1,171 = 9.72, P < 0.01; Fig. 1E) than males, and naïve animals had significantly faster sIPSC rise time (two-way ANOVA; Group: F1,171 = 10.64, P < 0.01; Fig. 1E) than dependent animals. Additionally, a significant Sex X Group interaction effect (F1,171 = 7.09, P < 0.01) found that naïve females had significantly faster sIPSC rise time than dependent females (Bonferroni; t171 = 4.41, P = 0.0001), and naïve (Bonferroni; t171 = 3.95, P < 0.001) and dependent males (Bonferroni; t171 = 4.75, P < 0.0001). Females also had significantly faster decay times (two-way ANOVA; Sex: F1,171 = 8.01, P < 0.01; Fig. 1F) than males, and naïve animals had significantly faster decay times (two-way ANOVA; Group: F1,171 = 7.37, P < 0.01; Fig. 1F) than dependent animals. These sIPSC kinetic differences indicate CeA postsynaptic GABAA receptor sex differences as well as changes induced by alcohol dependence.
Fig. 1.
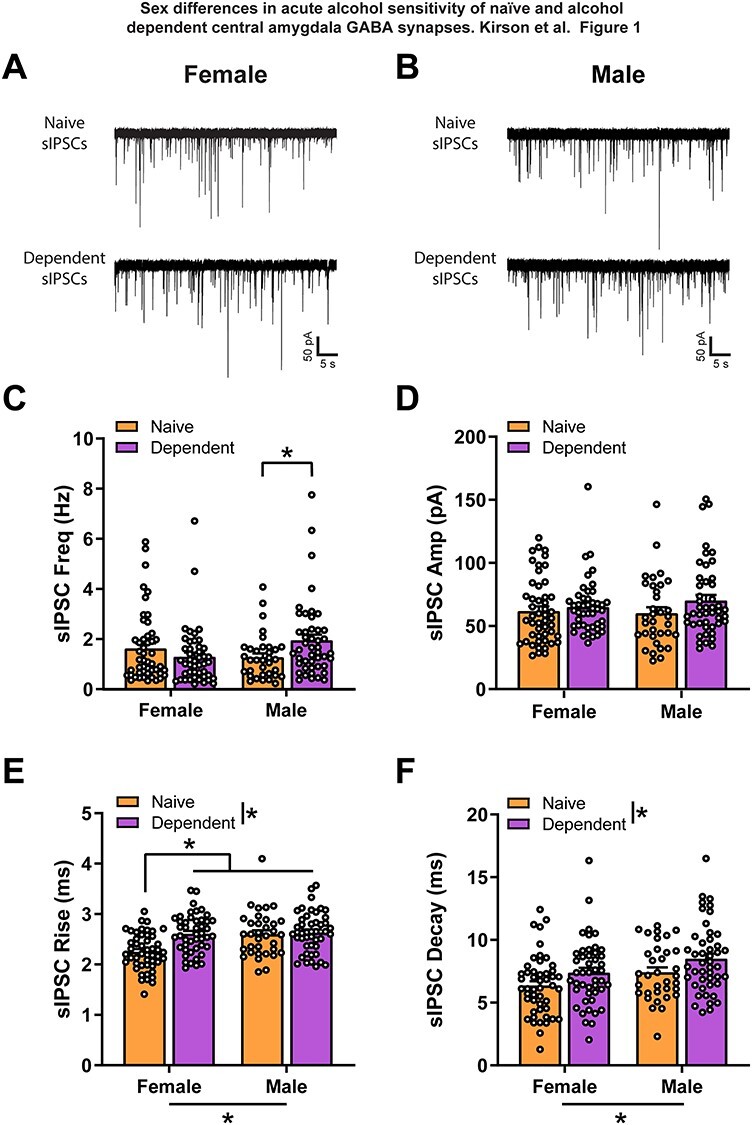
Female CeA spontaneous GABAergic transmission has faster postsynaptic kinetics. (A, B) Representative GABAA-mediated sIPSCs of CeA neurons from naive and dependent female (A) and male (B) rats. (C) There is no main sex difference and no significant difference between female naïve and dependent CeA baseline sIPSC frequency. However, dependent male baseline sIPSC frequency (1.95 ± 0.22 Hz) is significantly higher than naïve male (1.28 ± 0.15 Hz). (D) There are no significant differences between female and male naïve and dependent CeA baseline sIPSC amplitude. (E) Female naïve CeA baseline sIPSC rise time (2.25 ± 0.05 ms) is significantly lower than male naïve (2.60 ± 0.08 ms) and female (2.61 ± 0.06 ms) and male dependent (2.64 ± 0.06 ms) rise times. (F) Females have faster sIPSC decay time (naïve, 6.37 ± 0.34 ms; dependent, 7.39 ± 0.39 ms) than males (naïve, 7.43 ± 0.38 ms; dependent, 8.51 ± 0.41 ms) and naïve animals have faster decay time than alcohol dependent animals. n = 34–49 neurons per group. *Significant difference (P < 0.05).
We next re-examined the female naïve and dependent baseline sIPSC characteristics grouped according to estrous cycle stage of the animal on the day of recording. A chi-square test of independence showed no significant association between alcohol treatment and estrous cycle stage, χ2 (3, N = 40) = 5.01, P > 0.05. For examination of sIPSC characteristics, we combined metestrus and diestrus neurons into a single group (Kirson et al., 2018). We found no significant differences between estrous cycle stage of naïve and dependent females for sIPSC frequency (Fig. 2A), amplitude (Fig. 2B) or decay time (Fig. 2D). However, sIPSC rise time was significantly different between estrous cycle stages (two-way ANOVA; Cycle: F2,86 = 3.30, P < 0.05; Fig. 2C) and naive and dependent (two-way ANOVA; Group: F1,86 = 7.50, P < 0.01; Fig. 2C) female rats. Additionally, a significant Estrous Cycle X Group interaction effect (F2,86 = 3.46, P < 0.05) found that estrus females had significantly slower sIPSC rise time than proestrus (Bonferroni; t86 = 3.34, P < 0.05) or metestrus/diestrus females (Bonferroni; t86 = 3.37, P < 0.05), and diestrus dependent females had significantly slower sIPSC rise time than diestrus naïve females (Bonferroni; t86 = 5.25, P < 0.0001).
Fig. 2.
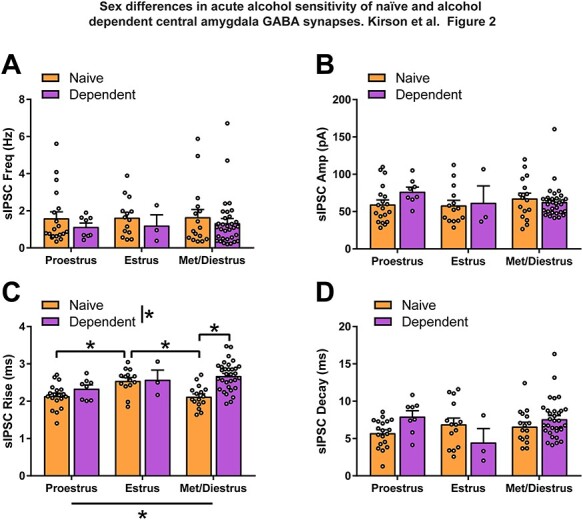
CeA GABAergic transmission postsynaptic kinetics are estrous cycle dependent and affected by alcohol dependence. There are no significant differences in naïve and dependent female baseline sIPSC frequency (A), amplitude (B) or decay time (D) regardless of estrous cycle. (C) Female CeA baseline sIPSC rise time is dependent on estrous cycle and alcohol dependence. Estrus naïve female CeA sIPSC rise time (2.55 ± 0.09 ms) is significantly slower than proestrus (2.14 ± 0.08 ms) or metestrus/diestrus (2.12 ± 0.08 ms) naïve females. Alcohol dependence induces a slower sIPSC rise time in metestrus/diestrus females (2.68 ± 0.07 ms) compared with naïve females in the same stage of the estrous cycle. n = 3–32 neurons per group. *Significant difference (P < 0.05).
We then investigated the effects of acute alcohol on sIPSCs in female and male naïve and dependent CeA neurons. For male animals, acute ethanol application increases CeA GABA signaling (Roberto et al., 2003, 2010; Varodayan et al., 2017; Tunstall et al., 2019; Khom et al., 2020a). Thus, as expected, acute alcohol (44 mM) significantly increased sIPSC frequency to 135.3 ± 4.5% of baseline in naïve (t9 = 7.79, P < 0.0001) and 133.2 ± 10.7% of baseline in dependent (t12 = 3.11, P < 0.01) males, indicating increased presynaptic GABA release (Fig. 3B and C). However, acute alcohol had no effect on sIPSC frequency in either naïve (96.9 ± 8.9% of baseline) or dependent (104 ± 6.9% of baseline) females (Fig. 3A and C). A two-way ANOVA of Sex and Treatment Group found a significant difference in the effect of acute alcohol between males and females (Sex: F1,42 = 15.73, P < 0.001; Fig. 3C), but no effect of alcohol treatment or interaction. As expected, acute 44-mM alcohol had no effect on sIPSC amplitude (Fig. 3D), rise time (Fig. 3E) or decay time (Fig. 3F) of female or male, naïve and dependent rats.
Fig. 3.
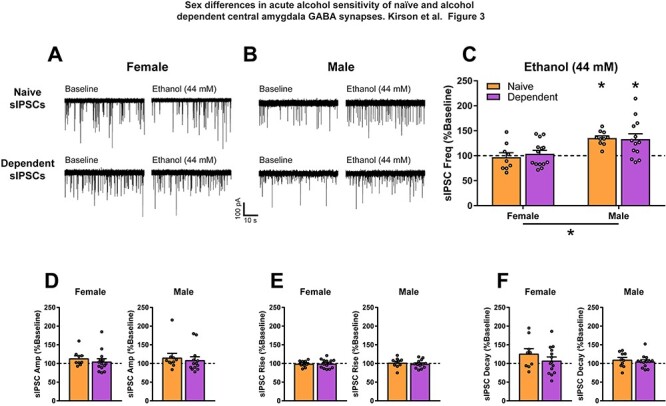
Female CeA spontaneous GABAergic transmission is insensitive to an effective concentration of acute alcohol in males. (A, B) Representative GABAA-mediated sIPSCs of CeA neurons from naive and dependent female (A) and male (B) rats at baseline, and with subsequent acute application of alcohol (ethanol; 44 mM). (C) Acute alcohol has different effects on female and male CeA sIPSC frequency regardless of alcohol treatment history. As previously shown, acute alcohol increases CeA sIPSC frequency in naïve (135.3 ± 4.5% of baseline) and dependent (133.2 ± 10.7% of baseline) males. However, acute alcohol has no effect on sIPSC frequency in naïve (96.9 ± 8.9% of baseline) or dependent (104 ± 6.9% of baseline) females. There are no significant effects of acute alcohol on female and male naïve and dependent CeA sIPSC amplitude (D), rise time (E) or decay time (F). n = 9–14 neurons per group. *Significant difference (P < 0.05).
Finally, we examined increasing concentrations of acute alcohol to determine whether the lack of effect on presynaptic GABA release in females was due to a shift in alcohol sensitivity. In males, we found 44 and 66-mM alcohol significantly increased sIPSC frequency in both naïve (44: t9 = 7.79, P < 0.0001; 66: t4 = 3.42, P < 0.05) and dependent (44: t12 = 3.11, P < 0.01; 66: t4 = 3.31, P < 0.05) animals, whereas 88-mM alcohol only increased sIPSC frequency in naïve (t5 = 6.27, P < 0.01) males (Fig. 4A and C). Lower concentrations of alcohol (11 and 22 mM) were ineffective in naïve and dependent males (Fig. 4C). Alcohol did not affect sIPSC frequency at any tested concentration in naïve females (Fig. 4B), however, 88-mM alcohol (the highest tested concentration) significantly increased sIPSC frequency in female dependents (t7 = 3.22, P < 0.05; Fig. 4A and B). These data suggest that dependence generates a mild sensitivity to acute alcohol induced GABA release in the CeA of females.
Fig. 4.
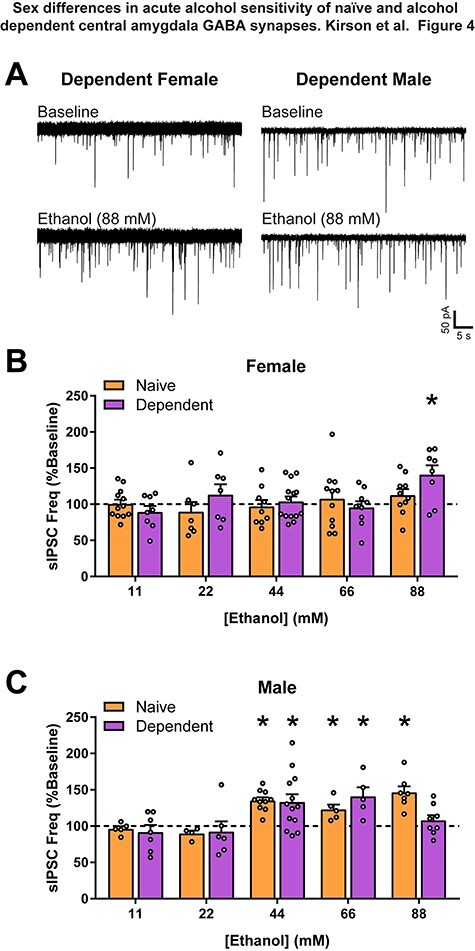
Alcohol dependent female CeA GABAergic transmission is only sensitive to high concentrations of alcohol. (A) Representative GABAA-mediated sIPSCs of CeA neurons from dependent female and male rats at baseline, and with subsequent acute application of a high concentration of alcohol (ethanol, 88 mM). (B) Acute alcohol at varying concentrations (ethanol; 11, 22, 44, 66 and 88 mM) has no effect on naïve female CeA sIPSC frequency. However, in dependent females, 88-mM alcohol increased sIPSC frequency (141 ± 12.74% of baseline). (C) Moderate acute alcohol concentrations increased CeA sIPSC frequency in both naïve (44: 135.3 ± 4.52% of baseline; 66: 122.9 ± 6.7% of baseline) and dependent (44: 133.2 ± 10.67% of baseline; 66: 141 ± 12.4% of baseline) males. However, 88-mM alcohol only increased sIPSC frequency in naïve males (150.9 ± 8.1% of baseline), with a lack of effect in dependent males (107.6 ± 7.3% of baseline). Lower concentrations of alcohol (11 and 22 mM) had no effect on sIPSC frequency in naïve or dependent males. n = 4–14 neurons per group. *Significant difference (P < 0.05).
DISCUSSION
The goal of this study was to characterize sex differences in CeA inhibitory synaptic transmission of naïve and alcohol dependent rats, and any sexually dimorphic effects of acute alcohol on inhibitory control of these CeA neurons. Here, we report similar presynaptic GABAergic input on CeA neurons between naïve female and male rats, but faster postsynaptic GABAA receptor current kinetics (rise and decay time) in female rats. Additionally, alcohol dependence induces increased GABAergic input on CeA neurons of male but not female rats, and a slowing of postsynaptic GABAA receptor current kinetics in CeA neurons of both sexes. We examined the female data for any differences based on estrous cycle and found that in naïve females, sIPSC rise time is higher in CeA neurons from estrus females. This indicates the faster rise time in naïve females is primarily due to those in proestrus and metestrus/diestrus, and the increased rise time with alcohol dependence is due to alterations in GABAA receptor functioning in these estrous cycle stages. As we did not select for estrous cycle and pooled metestrus and diestrus data, group size appears heavily shifted to a higher occurrence of low hormone estrous cycle stages in dependent females (n = 8 cells, proestrus; n = 3 cells, estrus; n = 32 cells and metestrus/diestrus), but we do not find a significant difference in the distribution of estrous cycle stage on day of recording between naïve and dependent females. Acute and chronic alcohol administration disrupts the normal estrous cycling of rats, resulting in low levels of hormones consistent with a persistent diestrus-like state (Eskay et al., 1981; Rettori et al., 1987; Alfonso et al., 1993; LaPaglia et al., 1997; Emanuele et al., 2002). However, the sIPSC rise times in dependent females are dissimilar to naïve females in both the lowest hormone state (metestrus/diestrus) and the highest hormone state (proestrus).
Importantly, the progesterone derivative allopregnanolone is a potent positive allosteric modulator of the GABAA receptor, and changing progesterone levels could play a role in the estrous cycle dependent GABAA receptor kinetics seen here (Callachan et al., 1987; Smith et al., 2007). During proestrus, peak estradiol levels lead to increasing levels of progesterone, and subsequently allopregnanolone, that fall to low levels during estrus and remain low throughout metestrus and diestrus (Staley and Scharfman, 2005). One effect of the low levels of these hormones during late diestrus is upregulation of the allopregnanolone sensitive α4 and δ GABAA receptor subunits in multiple brain regions (Smith et al., 2007; Lovick, 2012). However, GABAA receptors containing these subunits are extrasynaptic and responsible for tonic inhibition (Smith et al., 2007; Herman et al., 2016), and unlikely to be responsible for the estrous cycle dependent kinetics of the fast phasic GABA transmission examined here. Future studies may need to monitor estrous cycle more closely throughout the chronic alcohol exposure to fully characterize estrous cycling dysregulation and identify changes to GABAergic signaling at any inflection points.
In this study, acute alcohol increases presynaptic GABA release on naïve and alcohol dependent male CeA neurons, an effect we consistently see in male rodents (Roberto et al., 2003, 2004; Gilpin et al., 2011; Varodayan et al., 2017; Tunstall et al., 2019). However, acute alcohol had no effect on presynaptic GABA release in naïve female CeA, and in dependent females, only increased GABA at the highest alcohol concentration tested (88 mM), approximately equivalent to a 0.4% blood alcohol level. Sex differences in stress-related signaling may underlie the lack of effect of alcohol in female CeA neurons. Corticotropin releasing factor (CRF), a major stress-related neuromodulator, is released in response to stress, and CRF increases GABA release in the CeA of male rats (Roberto et al., 2010). CeA CRF signaling is upregulated in male alcohol dependent rats, and the acute alcohol induced increase in CeA GABA release seen in these rats is mediated by the CRF system (Roberto et al., 2010; Varodayan et al., 2017). In rodents, sex differences in response to CRF have been identified at the neuron, brain region and circuit levels (Bangasser et al., 2010; Salvatore et al., 2018; Wellman et al., 2018), and a recent study found sex differences in response to exogenous CRF and a lack of effect of alcohol in females in CRF receptor 1 expressing neurons in the CeA of mice (Agoglia et al., 2020). In addition, we have previously reported on sex differences in the interactions of alcohol and other stress-related neuromodulator systems on synaptic transmission at CeA glutamatergic synapses (Logrip et al., 2016; Kirson et al., 2018). Although stress-related systems and alcohol dependence are linked, it is unclear what adaptation has occurred in dependent females to induce the presynaptic GABA release seen with acute alcohol, or why the sensitivity to alcohol is so vastly different from the dependent males. Interestingly, 88-mM alcohol did not increase GABA release in dependent males. We have previously found that acute alcohol has an inverted U-shaped concentration response curve on CeA GABA (Roberto et al., 2003, 2004), so the most parsimonious explanation of this lack of effect is a left shift in this curve due to the already elevated GABA with alcohol dependence. Specific estrous cycle stage may also play a role in the disparate effect of acute alcohol between the sexes. However, in this study we did not have adequate representation of all cycle stages for each experiment. Future studies into whether the CRF system or other stress-related or sex steroid hormone-related mechanisms are involved in the effects of alcohol in female dependents is warranted.
To our knowledge, this is the first electrophysiological study to examine sex differences in CeA GABAergic activity and alcohol effects in alcohol dependent rats. In general, GABAergic signaling in female CeA was largely insensitive to alcohol, perhaps suggesting an alternative circuit may be primarily responsible for sex differences in drinking behaviors compared with males. However, the increased sensitivity of CeA to alcohol in the dependent females suggests that some of the adverse neural consequences of AUD in women may derive from this now compromised GABAergic circuitry, similar to the hallmark of alcohol dependence we observe in male animal models. Due to the intersection of stress- and alcohol dependence-related signaling, the CeA is a continuing region of interest for understanding sex differences in AUD and stress related disorders. Examination of other aspects of CeA GABA signaling, such as tonic conductances, as well as further identification of specific modulatory systems involved in these sex and estrous cycle differences will be important for the development of more targeted treatments for AUD.
ACKNOWLEDGMENTS
This is manuscript number 30069 from The Scripps Research Institute.
Contributor Information
Dean Kirson, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Sophia Khom, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Larry Rodriguez, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Sarah A Wolfe, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Florence P Varodayan, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Pauravi J Gandhi, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Reesha R Patel, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Roman Vlkolinsky, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Michal Bajo, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
Marisa Roberto, The Scripps Research Institute, Department of Molecular Medicine, 10550 N Torrey Pines Rd, La Jolla, CA 92037, USA.
FUNDING
This work was supported by National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (grants AA026638, AA027700, AA015566, AA021491, AA017447, AA006420, AA013498, AA026765, AA007456 and AA025408); and The Pearson Center for Alcoholism and Addiction Research.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflict of interest.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- Agabio R, Campesi I, Pisanu C, et al. (2016) Sex differences in substance use disorders: focus on side effects. Addict Biol 21:1030–42. [DOI] [PubMed] [Google Scholar]
- Agoglia AE, Tella J, Herman MA. (2020) Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons. Neuropharmacology 180:108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso M, Durán R, Marcó J. (1993) Ethanol-induced alterations in gonadotrophins secretion during the estrous cycle of rats. Alcohol Alcohol 28:667–74. [PubMed] [Google Scholar]
- APA . (2013) Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association. [Google Scholar]
- Augier E, Barbier E, Dulman RS, et al. (2018) A molecular mechanism for choosing alcohol over an alternative reward. Science 360:1321–6. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, et al. (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:96–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, et al. (1987) Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci 231:359–69. [DOI] [PubMed] [Google Scholar]
- Cover KK, Maeng LY, Lebrón-Milad K, et al. (2014) Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl Psychiatry 4:e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Saha TD, et al. (2015) Changes in alcohol consumption: United States, 2001-2002 to 2012-2013. Drug Alcohol Depend 148:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. (1994) Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol 71:1318–35. [DOI] [PubMed] [Google Scholar]
- Diehl A, Croissant B, Batra A, et al. (2007) Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci 257:344–51. [DOI] [PubMed] [Google Scholar]
- Emanuele MA, Wezeman F, Emanuele NV. (2002) Alcohol's effects on female reproductive function. Alcohol Res Health 26:274–81. [PMC free article] [PubMed] [Google Scholar]
- Erol A, Karpyak VM. (2015) Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend 156:1–13. [DOI] [PubMed] [Google Scholar]
- Eskay RL, Ryback RS, Goldman M, et al. (1981) Effect of chronic ethanol administration on plasma levels of LH and the estrous cycle in the female rat. Alcohol Clin Exp Res 5:204–6. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, et al. (2011) Neuropeptide Y opposes alcohol effects on gamma-aminobutyric acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry 69:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, et al. (2008) Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, et al. (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on alcohol and related conditions III. JAMA Psychiat 72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Roberto M. (2016) A functional switch in tonic GABA currents alters the output of central amygdala corticotropin releasing factor receptor-1 neurons following chronic ethanol exposure. J Neurosci 36:10729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez VA, Herman MA, Cuzon Carlson VC, et al. (2019) Synaptic adaptations in the central amygdala and hypothalamic paraventricular nucleus associated with protracted ethanol abstinence in male rhesus monkeys. Neuropsychopharmacology 44:982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS. (2011) Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol Clin Exp Res 35:2101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S, Steinkellner T, Hnasko TS, et al. (2020a) Alcohol dependence potentiates substance P/neurokinin-1 receptor signaling in the rat central nucleus of amygdala. Sci Adv 6:eaaz1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khom S, Wolfe SA, Patel RR, et al. (2020b) Alcohol dependence and withdrawal impair serotonergic regulation of GABA transmission in the rat central nucleus of the amygdala. J Neurosci 40:6842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Oleata CS, Parsons LH, et al. (2018) CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msP and Wistar rats. Addict Biol 23:676–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Le Moal M. (2005) Neurocircuitry theories of addiction-executive function. In Press A (ed). Neurobiology of Addiction. London: Elsevier, 378–416. [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. (2000) The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev 20:149–71. [DOI] [PubMed] [Google Scholar]
- LaPaglia N, Steiner J, Kirsteins L, et al. (1997) The impact of acute ethanol on reproductive hormone synthesis, processing, and secretion in female rats at proestrous. Alcohol Clin Exp Res 21:1567–72. [PubMed] [Google Scholar]
- Logrip ML, Oleata C, Roberto M. (2016) Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology 114:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA. (2012) Estrous cycle and stress: influence of progesterone on the female brain. Braz J Med Biol Res 45:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, et al. (2009) Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. Scientific World Journal 9:68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. (1994) Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type a receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A 91:7698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V, Skelley CW, McCann SM, et al. (1987) Detrimental effects of short-term ethanol exposure on reproductive function in the female rat. Biol Reprod 37:1089–96. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, et al. (2010) Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry 67:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Kirson D, Khom S. (2021) The role of the central amygdala in alcohol dependence. Cold Spring Harb Perspect Med 11:a039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, et al. (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A 100:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, et al. (2004) Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24:10159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, Vafaei A, Hasan OSM, et al. (2019) Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol 114:1574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore M, Wiersielis KR, Luz S, et al. (2018) Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav 97:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM. (2015) Sex differences in PTSD resilience and susceptibility: challenges for animal models of fear learning. Neurobiol Stress 1:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade T, Chapman C, Swift W, et al. (2016) Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: systematic review and metaregression. BMJ Open 6:e011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, et al. (2007) Neurosteroid regulation of GABA(A) receptors: focus on the alpha4 and delta subunits. Pharmacol Ther 116:58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K, Scharfman H. (2005) A woman's prerogative. Nat Neurosci 8:697–9. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Kirson D, Wolfe SA, et al. (2020) Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders. Mol Psychiatry. doi: 10.1038/s41380-020-00920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Kirson D, Zallar LJ, et al. (2019) Oxytocin blocks enhanced motivation for alcohol in alcohol dependence and blocks alcohol effects on GABAergic transmission in the central amygdala. PLoS Biol 17:e2006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, de Guglielmo G, Logrip ML, et al. (2017) Alcohol dependence disrupts Amygdalar L-type voltage-gated calcium channel mechanisms. J Neurosci 37:4593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Bangasser DA, Bollinger JL, et al. (2018) Sex differences in risk and resilience: stress effects on the neural substrates of emotion and motivation. J Neurosci 38:9423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Castle IJ, Chen CM, et al. (2015) Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012. Alcohol Clin Exp Res 39:1712–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


