Abstract
Background
Bariatric surgery (BS) has been postulated as the most effective measure for weight reduction. Weight loss improves metabolic parameters and exerts changes in renal function that lead to the amelioration of absolute or relative glomerular hyperfiltration, a condition that may be renoprotective in the long term. However, few studies have demonstrated the influence of BS in patients with severe obesity and chronic kidney disease (CKD). Our objective was to analyse the evolution of renal function, adipose tissue–derived molecules and inflammatory parameters in patients with CKD after BS.
Methods
This is an observational and prospective study. Thirty patients were screened and 12 were included between January 2016 and January 2018 with a 24-month follow-up. Glomerular filtration rate (GFR) was determined by plasma iohexol clearance. Adipokines, cytokines, circulating hormones and fibrotic parameters were evaluated before and 12 months after BS using the Bioplex system.
Results
The mean age was 50.6 years and 58.3% were males. Seven patients had a body mass index >40 kg/m2 and 66.7% were diabetic. Twenty-four months following BS there was a significant decrease in body weight (36.4%). Proteinuria decreased by 63.7 ± 28.2%. Measured GFR significantly diminished from before surgery to Month 24 after surgery (94 ± 44 to 79 ± 44 mL/min, P = 0.03). There was a significant decrease in adipocyte-derived molecules (leptin and vifastin) as well as in pro-inflammatory cytokines [interleukin (IL)-1β, tumour necrosis factor α, IL-6 and monocyte chemoattractant protein-1] and other circulating factors (vascular endothelial growth factor and transforming growth factor β isoforms).
Conclusions
BS is an effective option to prevent kidney damage in obese subjects with CKD due to the improvement of glomerular hyperfiltration, adipocyte cytokines metabolic and inflammatory parameters.
Keywords: adipocytokines, bariatric surgery, chronic kidney disease, hyperfiltration, obesity, pro-inflammatory cytokines, proteinuria, triglycerides
INTRODUCTION
Obesity is a proven risk factor for the onset and progression of chronic kidney disease (CKD) and both CKD and obesity have experienced an alarming increase worldwide [1–3]. Obesity can induce renal injury independent of diabetes and hypertension, which are often associated with obesity [4]. There are several renal effects of obesity, including the development of obesity-related glomerulopathy, albuminuria and a progressive increase in both renal blood flow and glomerular filtration rate (GFR), so-called hyperfiltration. Taken together, these changes can induce long-term progressive renal function loss and CKD. Glomerular hyperfiltration has been classically defined by an increase in GFR over a threshold (i.e. 120 or 140 mL/min). However, more recent studies indicate that patients with established CKD may also hyperfiltrate, what has been defined as relative hyperfiltration [5]. Therefore the persistent glomerular hyperfiltration, independent of a threshold used to define this condition, may determine glomerular damage and GFR decline in the long term. The involvement of adipose tissue and its derived molecules in the pathogenesis of obesity-related kidney disease is gaining importance. Moreover, obesity is considered a chronic low-grade inflammatory disease, the infiltration of adipose tissue with pro-inflammatory immune cells, mainly macrophages that release pro-inflammatory molecules, further fuelling this pro-inflammatory status. Some studies support that the adipose tissue secretion pattern is altered in obese patients and that these factors could affect kidney function [6].
Prospective controlled studies have shown a beneficial effect of bariatric surgery (BS) in the control of hypertension and diabetes in obese subjects [7, 8]. Also, drastic weight loss that follows BS associates with favourable effects in patients with CKD, mainly through a marked reduction in proteinuria and stabilization of GFR [9–12]. However, most of these studies are retrospective and use estimated GFR (eGFR), which is problematic in morbidly obese patients. The improvement in kidney outcomes is usually attributed to a reduction in glomerular hyperfiltration that accompanies obesity and that induces persistent and progressive haemodynamically mediated damage on glomerular structures [13–15]. Scarce information is available about the influence of BS on the production of pro-inflammatory and pro-fibrotic adipokines and cytokines in patients with established CKD. This issue is relevant because recent data suggest that fat deposition in kidney parenchyma and obesity-related chronic inflammation are other pathogenic mechanisms likely involved in obesity-related kidney disease [16–18]. Likewise, few granular studies exist on the impact of BS on the lipid profile and liver fat content of CKD patients.
We designed a prospective study to evaluate the impact of BS in renal function changes, proteinuria, pro-inflammatory and pro-fibrotic cytokines and adipokines, as well as the metabolic and glycaemic profile, in a group of obese patients with established CKD.
MATERIALS AND METHODS
Design
This is an observational, prospective, single-centre study designed to evaluate the effect of weight loss in obese patients with CKD who underwent BS (trial registration NCT02644928). Patients on the waiting list for BS were selected according to the following criteria: (i) body mass index (BMI) >35 kg/m2 plus GFR 30–60 mL/min and proteinuria >1 g/24 h or GFR >60 mL/min and proteinuria >2.5 g/24 h despite receiving maximally tolerated doses of renin–angiotensin–aldosterone system (RAAS) blocker and (ii) BMI >40 kg/m2 with a GFR >30 mL/min and proteinuria >0.5 g/24 h despite receiving maximally tolerated doses of RAAS blocker. For the renal function criterion, eGFR was used. The follow-up time was 24 months. The exclusion criteria were (i) subjects who were participating or have participated in another clinical trial and/or who are taking or have taken an experimental drug (not registered) in the last 28 days; (ii) dialysis or renal transplantation; (iii) poorly controlled blood pressure [BP; systolic BP (SBP) >170 mmHg or diastolic BP (DBP) >110 mmHg]; (iv) cardiovascular events (stroke and ischaemic heart disease) in the past 6 months; (v) treatment with steroids or other immunosuppressants; (vi) renovascular disease, obstructive uropathy, autoimmune diseases, cancer and drug use; (vii) pregnancy or lactation; or (viii) patients who did not sign the informed consent.
The patients underwent two types of BS according to the criteria of the specialized obesity surgery team: Roux-en-Y gastric bypass or sleeve gastrectomy. Patients were evaluated 6 and 3 months before BS, on the day of surgery and after BS at Months 3, 6, 12 and 24 (Supplementary data, Figure S1A).
Body measurements and biochemical tests
During the study, different procedures were performed and the following data were obtained: epidemiological and a physical examination including height, body weight, BMI, waist and hip circumference, measurement of BP and heart rate. The control target for SBP was <140/90 mmHg. This measurement was made in triplicate after 5 min of rest with the patient seated, using automated equipment (adapted for obese patients; OMRON, Kyoto, Japan). Mean BP was calculated as the sum of one-third of the SBP and two-thirds of the DBP.
At each visit, blood samples were collected in a fasting state in the early morning. Lab analysis included blood count with leukocyte formula, serum creatinine, glucose, glycated haemoglobin, sodium, potassium, calcium, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoportein (LDL) cholesterol (Friedewald formula), triglycerides, uric acid, aspartate aminotransferase and alanine aminotransferase. Twenty-four-hour urine collection was required to measure proteinuria, sodium, potassium, urea and creatinine. A sample of the first-morning urine was collected to measure the urinary albumin:creatinine ratio.
Evaluation of renal function
Measured GFR (mGFR) and eGFR were determined at all the prespecified visits (−6 and −3 months, baseline and Months 3, 6, 12 and 24). Repeated measurements before surgery were planned a priori to calculate a mean value to avoid the regression towards the mean effect.
Measurement of GFR
The plasma clearance of iohexol, a gold standard method, was used to measure GFR. In brief, 5 mL of iohexol (Omnipaque 300, GE Healthcare, Chicago, IL, USA) was injected intravenously over 2 min. Afterward, venous samples were obtained at 120, 180, 240, 300, 360, 420 and 480 min for patients with eGFR ≤40 mL/min or at 120, 150, 180, 210 and 240 min for those with eGFR >40 mL/min. The concentrations of iohexol were determined in plasma. Plasma clearance of iohexol was calculated according to a one-compartment model adjusted by the Bröchner–Mortensen equation [19].
Estimation of GFR by formulas
Two creatinine-based equations, Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), were used to evaluate renal function. Both formulas are adjusted for body surface area (BSA), a limitation for the assessment of renal function in obesity [19] and conditions associated with drastic weight change. Thus we reversed the adjustment of the result by applying the following formula (GFR adjusted = GFR unadjusted/BSA × 1.73). BSA was calculated by the DuBois and DuBois formula (BSA = 0.007184 × Weight 0.425 × Height 0.725) [20].
Glomerular hyperfiltration
There is no consensus-based definition of hyperfiltration. Several studies used a threshold of GFR (i.e. 120 and 130 mL/min or higher) to define those patients with glomerular hyperfiltration. However, other studies have indicated that patients with diverse degrees of renal function, even with CKD, may have hyperfiltration [5], a phenomenon called ‘relative hyperfiltration’. Thus we did not use a threshold to define hyperfiltration. We a priori assumed that a decrease in GFR after weight reduction—after the exclusion of AKI—indicated hyperfiltration before BS.
Analysis of circulating adipokines, cytokines and fibrotic factors
Circulating levels of adipokines, cytokines and transforming growth factor β (TGF-β) isoforms were analysed using the high-sensitivity Bio-Plex ProHuman system (Bio-Rad, Hercules, CA, USA). Serum samples obtained before and after BS were used to measure three different multiplex panels (Diabetes 10-Plex and Diabetes 2-Plex Assay, Cytokine 27-Plex Assay and TGF-β 3-Plex Assay; Bio-Rad) according to the manufacturer’s protocol.
Body densitometry
Measurement of body composition, fat distribution and an estimation of the fat loss by dual-energy X-ray absorptiometry (DXA) was made with an QDR 4500 system(Hologic, Waltham, MA, USA). The mean coefficients of variation for the repeated DXA analyses were provided by the company and were total body mineral density 0.84%, fat mass 2.20% and fat-free mass 0.86%.
Non-alcoholic fatty liver disease
Ultrasound criteria and the diagnosis were used as the non-alcoholic fatty liver disease (NAFLD) fibrosis score with an online calculator [21].
Outcome measures
The outcomes were the changes at 3, 6, 12 and 24 months after BS in mGFR and 24-h proteinuria; BP, glycaemic control and lipid profile; adipokines and pro-inflammatory and pro-fibrotic cytokines levels; and fatty liver content and estimation of liver fibrosis.
Statistical analyses
Variables were shown as mean ± standard deviation (SD) or median and 25th and 75th percentiles and interquartile range (IQR), as appropriate. Baseline comparisons between groups were performed using the Student’s t-test and Mann–Whitney test. For within-group analysis we used the Student’s t-test for paired comparisons or Wilcoxon’s test for matched pairs. Correlations were evaluated by Pearson’s r. Differences between categorical variables were analysed using the χ2 test and Fisher’s exact test. An analysis of variance with repeated measures was used to compare three or more group means where the participants are the same in each group. For other outcomes, P ˂ 0.05 was considered statistically significant. Statistics were calculated using SPSS version 15.0 (SPSS, Chicago, IL, USA).
RESULTS
Patient’s characteristics
Thirty patients were screened and 12 were included in the study (Supplementary data, Figure S1B). Baseline characteristics are shown in Table 1. The mean age was 50.6 years (range 26–70), 91.6% were hypertensive, 66.7% were diabetic and 83.3% had dyslipidaemia. The aetiological diagnosis of CKD was made according to clinical criteria, being the most frequent obesity-associated kidney disease (58.3%). Two patients had diabetic nephropathy and an immunoglobulin A mesangial nephropathy diagnosed by renal biopsy (RB). The average BMI was 41.1 kg/m2. About 70% of the patients presented ultrasound criteria compatible with fatty liver. Nine patients underwent a Roux-en-Y gastric bypass and three a sleeve gastrectomy.
Table 1.
Baseline characteristic of patients
| Age (years), mean ± SD (range) | 50.6 ± 16 (26–70) |
| Gender (male/female), n (%) | 7 (58.3)/5 (41.7) |
| Hypertension, n (%) | 9 (91.6) |
| Diabetes mellitus, n (%) | 8 (66.7) |
| Dyslipidaemia, n (%) | 10 (83.3) |
| Diagnosis CKD, n (%)a (two cases RB) | |
| Obesity | 7 (58.3) |
| Diabetic nephropathy | 4 (33.3)a |
| Chronic glomerular disease | 1 (8.3)a |
| Fatty liver, n (%) | 8 (66.7) |
| Body weight (kg), mean ± SD (range) | 116 ± 23.2 (89.7–173.5) |
| BMI (kg/m2), mean ± SD (range) | 41.1 ± 4.4 (35.1–48.1) |
| Body fat (%), mean ± SD | 42.5 ± 7.5 |
| Waist circumference (cm), mean ± SD (range) | 127 ± 11.4 (111–148) |
| Hip circumference (cm), mean ± SD (range) | 128.3 ± 11.9 (105–145) |
| eGFR (MDRD; mL/min), mean ± SD | 96.8 ± 49.8 |
| Proteinuria (g/24 h), mean ± SD | 2.6 ± 2.9 |
| Median (IQR) | 1.6 (1–2.7) |
| Concomitant medication, n (%) | |
| RAAS blocker | 12 (100) |
| Insulin | 5 (41.7) |
| Oral anti-diabetics | 5 (41.7) |
| Statins | 7 (58.3) |
| Fibrates | 3 (25) |
Patients with renal biopsy; one patient with diabetic nephropathy and the other with chronic glomerular disease.
Evolution of weight and BP
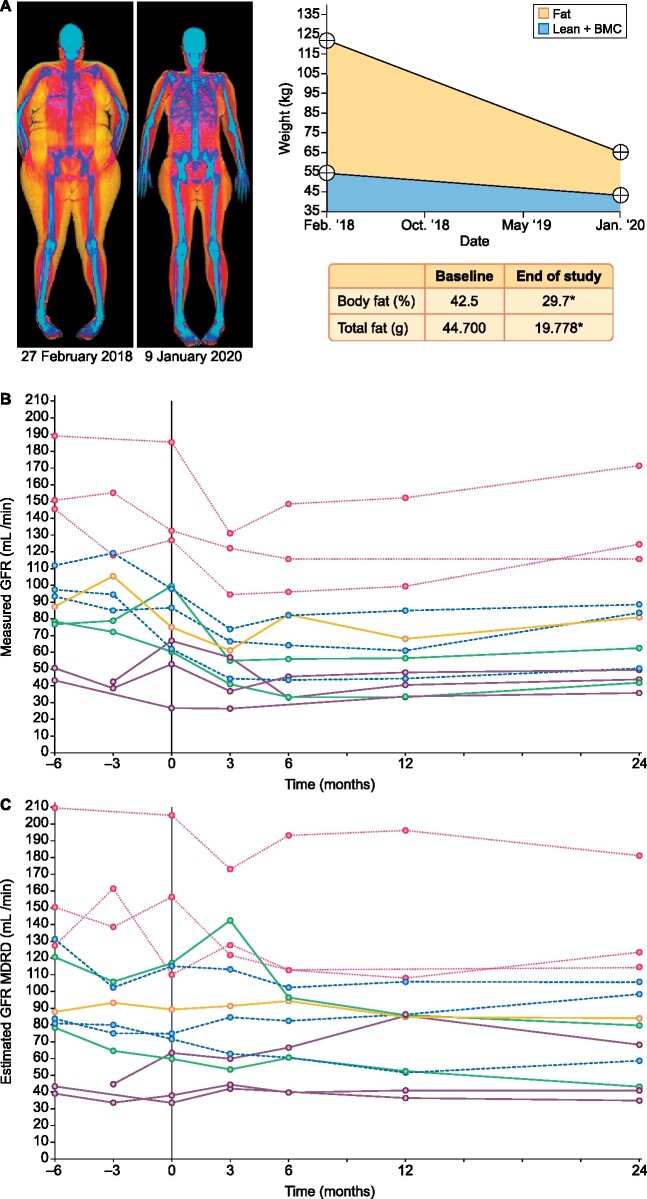
After BS, weight was reduced by 19.9 ± 3.7% at 3 months, 34.2 ± 7.7% at 12 months and 36.4 ± 7.3% at 24 months (Table 2). Accordingly, BMI decreased from 41.6 to 26.4 kg/m2 (P < 0.001) and waist and hip circumferences decreased at 24 months from 128.5 to 104.9 cm and 130.1 to 108.8 cm, respectively (P < 0.001). Body fat densitometry imaging showed a decrease in the fat composition of all patients (42.5–29.7%, P < 0.001) (Figure 1A).
Table 2.
Effects of BS on weight and BP
| Variables | 6 months pre-BS | 3 months pre-BS | Baseline | Month 3 | Month 6 | Month 12 | Month 24 |
|---|---|---|---|---|---|---|---|
| Body weight (kg) | 116.0 ± 23.2 | 117.7 ± 24.8 | 117.5 ± 25.4 | 94.6 ± 24* | 83.8 ± 21.1* | 77.4 ± 20.1* | 74.8 ± 18.9 |
| Weight reduction (%) | 19.9 ± 3.7 | 28.8 ± 6.8 | 34.2 ± 7.7 | 36.4 ± 7.3 | |||
| BMI (kg/m2) | 41.1 ± 4.4 | 41.7 ± 4.7 | 41.6 ± 5.4 | 33.4 ± 5.5* | 29.7 ± 5.6* | 27.4 ± 5.2* | 26.4 ± 4.6* |
| Waist circumference (cm) | 127.0 ± 11.4 | 127.9 ± 11.9 | 128.5 ± 11.9 | 117.8 ± 13.7* | 109.6 ± 12.7* | 105.6 ± 12.7* | 104.9 ± 12.6* |
| Body fat (%) | – | – | 42.5 ± 7.5 | – | – | – | 29.7 ± 8.1* |
|
Total fat (g), median (IQR) |
– | – |
44.700 (37.302–54.967) |
– | – | – |
19.778* (12.405–27.676) |
|
Total lean mass (g), median (IQR) |
– | – |
62.672 (50.315–67.206) |
– | – | – |
47 234* (38 010–54 626) |
| Hip circumference (cm) | 128.3 ± 11.9 | 129.4 ± 12.8 | 130.1 ± 11.5 | 121.1 ± 10.4* | 116.3 ± 11.5* | 112.6 ± 10.5* | 108.8 ± 12.2* |
| SBP (mmHg) | 141.6 ± 19.8 | 141.1 ± 12.8 | 139.2 ± 8.8 | 133.4 ± 9.2 | 130.9 ± 12.0* | 129.1 ± 11.9* | 127.2 ± 11.5* |
| DBP (mmHg) | 83.5 ± 11.6 | 83.2 ± 9.3 | 7.0 ± 9.1 | 75.1 ± 8.4 | 73.6 ± 5.2* | 71.8 ± 14.9 | 74.4 ± 7.1 |
| Hypertension (%) | 91.6 | 91.6 | 91.6 | 58.3 | 58.3 | 58.3 | 58.3 |
| BP drugs (n), mean ± SD (range) |
2.1 ± 1.2 (1–4) |
2.1 ± 1.2 (1–4) |
2.1 ± 1.2 (1–4) |
0.9 ± 0.8 (0–2) |
0.7 ± 0.7 (0–2) |
0.8 ± 0.8 (0–2) |
0.9 ± 0.9 (0–2) |
| Renin (μL/U/mL), mean ± SD (range) | 57.6 ± 61.8 (8.9–192.1) |
52.7 ± 52.4 (8–154) |
46.4 ± 49.1 (8.3–183.9) |
14.8 ± 7.9* (1.7–27) |
15.1 ± 8.2* (2.4–33) |
20.2 ± 14.3 (3.8–48) |
26.1 ± 17.5 (3.7–55.9) |
| Aldosterone (ng/dL), mean ± SD (range) |
12.3 ± 10.3 (2–38) |
13.1 ± 10.6 (1–40) |
12.8 ± 11.9 (1.6–40) |
9.8 ± 8.1 (2.5–23) |
9.7 ± 6.5 (0.9–19.9) |
9.3 ± 6.1 (2.4–20) |
9.1 ± 8.1 (1.6–29) |
Values are presented as mean ± SD unless stated otherwise.P < 0.05 versus baseline.
FIGURE 1:
Body fat densitometry and evolution of GFR before and after BS. (A) Measurement of body composition, fat distribution and an estimation of the fat loss were made by DXA. GFR was measured by (B) the plasma clearance of iohexol and (C) estimated by the MDRD equation, both unadjusted by body surface area. *P < 0.05.
Both SBP (139.2 ± 8.8–127.2 ± 11.5 mmHg, P < 0.05) and DBP (79 ± 9.1–74.4 ± 7.1 mmHg, P < 0.05) and the number of hypotensive drugs per patient (from 2.1 to 0.9) decreased during follow-up. Finally, renin and aldosterone values tended to decrease but were not statistically significant (Table 2).
Evolution of renal function and proteinuria
The mean reduction of proteinuria 3 months after surgery was 49.3%, reaching 60.7% at 12 months and 63.7% at the end of the follow-up (Table 3). The ACR decreased from 1912 to 479.7 mg/g (P < 0.05) at the end of the follow-up. mGFR significantly decreased from before surgery to Month 24 after surgery (94 ± 44 to 79 ± 44 mL/min, P=0.03, respectively) (Figure 1B). Most of this change was attributed to an acute decrease at Month 3 after surgery (97 ± 44 to 67 ± 33 mL/min, P=0.003, which was maintained until the study end (Figure 1B). eGFR by the MDRD equation also decreased from before surgery to Month 24, although with borderline significance (97 ± 50 to 81 ± 44 mL/min, P=0.06 (Figure 1C). However, eGFR did not detect the acute change between baseline and 3 months after surgery (97 ± 50 to 93 ± 42 mL/min, P = 0.63) (Figure 1C). Similar results were observed with the CKD-EPI equation (data not shown).
Table 3.
Effects of BS on renal function and proteinuria
| Variables | 6 months pre-BS | 3 months pre-BS | Baseline | Month 3 | Month 6 | Month 12 | Month 24 |
|---|---|---|---|---|---|---|---|
| Serum creatinine ( mg/dL) | 1 ± 0.4 | 1.1 ± 0.5 | 1.1 ± 0.4 | 1 ± 0.4 | 1 ± 0.4* | 1 ± 0.4 | 1 ± 0.4 |
| eGFR (MDRD, mL/min) | 104.8 ± 49.6 | 89.9 ± 39.5 | 96.8 ± 49.8 | 93 ± 42.5 | 92.9 ± 40.8 | 84.9 ± 44.5 | 81.2 ± 44.7 |
| eGFR (CKD-EPI, mL/min) | 108.9 ± 48.5 | 95.2 ± 41.5 | 97.3 ± 47.8 | 96.4 ± 43.2 | 95.9 ± 37.9 | 86.6 ± 40.2 | 80.5 ± 40.1 |
| mGFR (iohexol, mL/min) | 102.23 ± 44.2 | 90.9 ± 35.8 | 94 ± 43.7 | 67.5 ± 32.9* | 72.7 ± 36.6* | 65.6 ± 35.5* | 79.6 ± 44.5* |
| Proteinuria (g/24 h) | 2.1 ± 1.51 | 2.3 ± 1.6 | 2.6 ± 2.9 | 1.1 ± 0.9* | 0.9 ± 0.6* | 0.9 ± 0.9* | 0.6 ± 0.4* |
| Median (IQR) | 1.6 (0.9–3.7) | 1.7 (1–3.6) | 1.6 (1–2.7) | 0.9 (0.3–1.8) | 0.7 (0.4–1.2) | 0.6 (0.3–0.9) | 0.5 (0.3–0.9) |
| Proteinuria reduction (%) | – | – | – | 49.3 ± 27.2 | 54.9 ± 26.4 | 60.7 ± 16.8 | 63.7 ± 28.2 |
| UACR (mg/g) | 1140 ± 1096 | 1095 ± 859 | 1912 ± 2233 | 650.3 ± 871* | 478.8 ± 623* | 582.4 ± 884* | 479.7 ± 540* |
| Median (IQR) | 601 (411–1863) | 790 (317–1882) | 1004 (554–2078) | 296 (139–599) | 266 (97–636) | 194 (81–671) | 220 (87–860) |
| UACR reduction (%) | – | – | – | 61.7 ± 25.5 | 69.3 ± 23.2 | 70.6 ± 23.8 | mean ± SD |
Values are presented as mean ± SD unless stated otherwise.P < 0.05 versus baseline.
UACR: urinary albumin:creatinine ratio.
Evolution of diabetes and nutritional sate
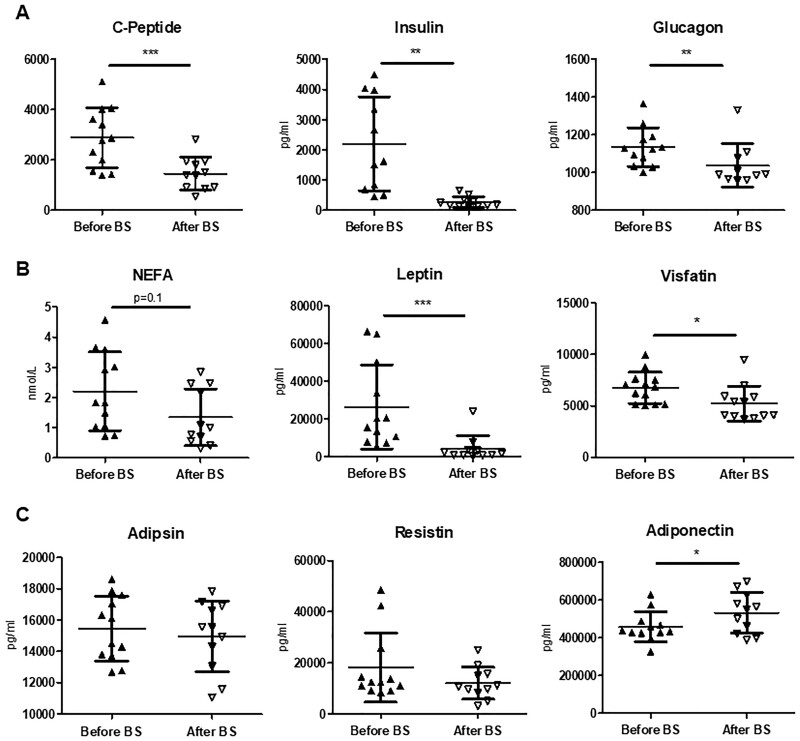
There was a significant reduction in blood glucose levels in all patients, and especially in diabetic patients (66.7%) (Table 4). Glycated haemoglobin was reduced from baseline values of 7.3–5.4% (P < 0.01) at the end of follow-up, allowing a reduction in insulin and oral anti-diabetic needs. At 24 months, all diabetic subjects except one had stopped anti-diabetic medications. The levels of C-peptide, insulin and glucagon decreased significantly (P < 0.01) after BS (Figure 2A). Albumin, pre-albumin, vitamin D and homocysteine did not change during the follow-up (Table 4).
Table 4.
Effects of BS on diabetes mellitus, nutrition and lipids
| Variables | 6 months pre-BS | 3 months pre-BS | Baseline | Month 3 | Month 6 | Month 12 | Month 24 |
|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | 143.1 ± 58.1 | 158.2 ± 65 | 164 ± 68.5 | 98 ± 21.1* | 91.6 ± 18.4* | 88.1 ± 19.1* | 86.7 ± 14* |
| Glycated haemoglobin (%) | 7.2 ± 1.7 | 7.6 ± 2.1 | 7.3 ± 1.8 | 5.7 ± 0.8* | 5.4 ± 0.7* | 5.4 ± 0.8* | 5.4 ± 0.7* |
| Diabetes, % | 66.7 | 66.7 | 66.7 | 0* | 0* | 0* | 8.3* |
| Insulin, % | 62.5 | 62.5 | 62.5 | 0* | 0* | 0* | 0* |
| Oral anti-diabetics, % | 62.5 | 62.5 | 62.5 | 0* | 0* | 0* | 8.3* |
| Pre-albumin (mg/dL) | 28.2 ± 5.3 | 26.4 ± 4.2 | 26.8 ± 4.7 | 20.9 ± 6* | 22.3 ± 5.3* | 22.9 ± 4.6* | 24.1 ± 4.3 |
| Albumin (mg/dL) | 4.2 ± 0.3 | 4.2 ± 0.2 | 4.2 ± 0.5 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.1 ± 0.4 |
| Homocysteine (μmol/L) | 13.4 ± 6.7 | 13.5 ± 6.3 | 15 ± 8.3 | 16.8 ± 6.4 | 17.5 ± 6.8 | 16.7 ± 6.4 | 15.3 ± 5.9 |
| Vitamin D (ng/mL) | 10.8 ± 4.8 | 13.3 ± 4.6 | 14.1 ± 6.1 | 13.7 ± 4.4 | 16.9 ± 8.6 | 19.4 ± 9.2 | 19.2 ± 10.5 |
| Cholesterol (mg/dL) | 185.7 ± 34.6 | 181.6 ± 41.6 | 198.1 ± 49.3 | 167.5 ± 33.4* | 167.6 ± 34.6* | 164.8 ± 42.6* | 163.3 ± 29.7* |
| HDL cholesterol (mg/dL) | 36.6 ± 7.3 | 35.5 ± 6.7 | 35.9 ± 8.9 | 41.1 ± 10 | 42.3 ± 9.4 | 48.5 ± 12.2* | 55.6 ± 17.6* |
| LDL cholesterol (mg/dL) | 104.2 ± 41.3 | 101.7 ± 38.3 | 110.6 ± 43.1 | 93.6 ± 33.7 | 95.8 ± 35.9 | 93.8 ± 40 | 88.4 ± 32.9 |
| Triglycerides (mg/dL) | 301.2 ± 138.6 | 293.6 ± 169.2 | 302.2 ± 109.9 | 164.9 ± 48.7* | 128.4 ± 45.8* | 121.2 ± 47.8* | 112.2 ± 43.7* |
| Hyperlipidaemia, % | 83.3 | 83.3 | 83.3 | 16.7 | 16.7 | 16.7 | 16.7 |
| Statins, % | 58.3 | 58.3 | 58.3 | 16.7 | 16.7 | 16.7 | 16.7 |
| Fibrates, % | 25 | 25 | 25 | 0 | 0 | 0 | 0 |
Values are presented as mean ± SD unless stated otherwise.P < 0.05 versus baseline.
FIGURE 2:
Analysis of the circulating levels of C-peptide, insulin, glucagon and adipose tissue–derived molecules before and after BS. Levels of (A) C-peptide, insulin and glucagon;(B) non-sterified fatty acids and the adipokines leptin and visfatin; and(C) adipsin, resistin and adiponectinwere determined in serum samples of patients before and 12 months after BS. Data are shown as mean ± SD (n = 10–11). Statistical analysis was performed using the Student’s t‐test for paired samples or Wilcoxon matched-pairs test. *P < 0.05, **P < 0.01, ***P < 0.001.
Changes in the lipid profile after BS
The levels of total cholesterol (198.1–163.3 mg/dL, P < 0.05) and triglycerides (302.2–112.2 mg/dL, P < 0.001) were reduced after surgery, leading to a lower need of statins and fibrates (Table 4). The levels of HDL cholesterol increased (35.9–55.6 mg/dL, P < 0.05), whereas no significant changes were observed in LDL cholesterol.
Evolution of adipose tissue–derived molecules and inflammatory parameters
The levels of non-esterified fatty acids showed a trend towards reduction. Regarding adipokine levels, leptin and visfatin decreased significantly after surgery (Figure 2B). However, the levels of adipsin and resistin showed a tendency towards a decrease. Conversely, circulating levels of adiponectin increased significantly after BS (Figure 2C).
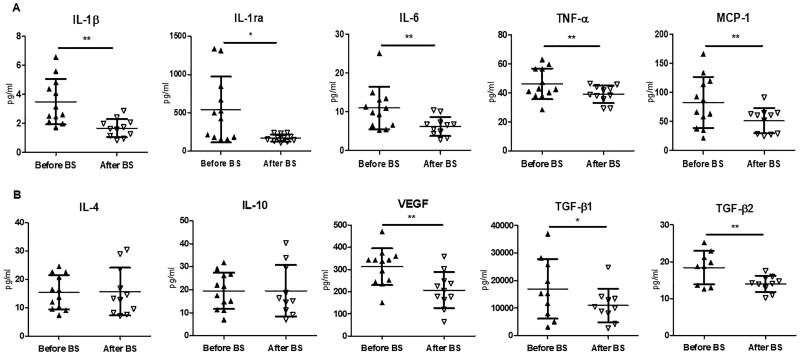
There was a significant decrease in pro-inflammatory cytokines, including IL-1β, IL-1ra, TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1) (Figure 3A). In contrast, no differences were found in the levels of cytokines considered to be anti-inflammatory, such as IL-4 and IL-10. The angiogenic factor, vascular endothelial growth factor (VEGF) and pro-fibrotic factors TGF-β1 and TGF-β2 decreased 1 year after BS (Figure 3B). A positive correlation between the fold change of proteinuria and the fold change of the inflammatory parameters IL-1β and VEGF as well as a positive correlation between both inflammatory parameters themselves (Supplementary data, Figure S2A) was observed. All the variations in metabolic, inflammatory and specific renal parameters prior to and after BS are represented in a heatmap (Supplementary data, Figure S2B).
FIGURE 3:
Circulating levels of cytokines and growth factors before and after BS. Levels of (A) the pro-inflammatory cytokines IL-1β, IL-1ra, IL-6, TNF-α and MCP-1 and (B) the anti-inflammatory cytokines IL-4 and IL-10, as well as levels of VEGF and the isoforms 1 and 2 of the TGF-β, TGF-β1 and TGF-β2, were analysed in the patients serum before and 12 months after BS. Data are shown as mean ± SD (n = 10–11) and statistical analysis was performed using Student’s t‐test for paired samples or Wilcoxon matched-pairs test. *P < 0.05, **P < 0.01.
Evolution of NAFLD’s score
At baseline, the composition of the different stages of liver fibrosis was F3–F4 fibrosis (>0.675), four patients; indeterminate score (<−1.455–<0.675), five patients; and F0–F2 (<−1.455), three patients. At the end of the study the change in the score was F3–F4, one patient; indeterminate score, four patients; and F0–F2, seven patients. There was a reduction in fibrosis values from −0.3 to −1.4 (P < 0.001).
Complications of BS
Two patients who underwent sleeve gastrectomy required hospitalization and reoperation—one patient due to stenosis of the surgical anastomosis and the other as a result of gastrointestinal haemorrhage of the anastomotic area. There were no episodes of infection or acute kidney failure after BS.
DISCUSSION
Our major finding was that patients with morbid obesity and CKD undergoing BS showed a significant decrease in proteinuria and renal function, which may indicate a correction of glomerular hyperfiltration as well as excellent control of metabolic parameters and a reduction in adipose tissue–derived molecules and inflammatory parameters. Thus drastic weight reduction through BS would be an excellent ‘multitarget’ therapy in extremely obese patients with CKD.
As in chronic proteinuric nephropathies, a significant reduction in proteinuria is assumed to have a nephroprotective effect in obese patients with CKD. RAAS blockade and weight reduction through diet or BS are the most studied therapeutic options in obese patients [16, 22, 23]. Several clinical trials have demonstrated a beneficial effect of BS on cardiometabolic parameters (hypertension and diabetes mellitus) in obese patients without renal disease [7, 8]. On the other hand, some small studies that included patients with early CKD stages reported stabilization of the GFR and a reduction in microalbuminuria after BS [24, 25]. These beneficial renal effects were accompanied by significant improvements in BP and glycaemic control and favourable changes in metabolic and inflammatory markers that persisted for 1–5 years after surgery [16].
We found a significant 40% reduction in proteinuria in the first month after BS, when body weight has decreased only 13% with respect to baseline values. At the end of the study (24 months), proteinuria reduction reached 60% when weight loss represented 36% of the baseline values. Similar changes were found in a previous study of our group that evaluated the effect of low-calorie diets in obese patients with CKD [14].
DXA can accurately measure body composition with high-precision, low X-ray exposure and short scanning time [26]. Through measurement of body composition, we were able to verify the significant reduction of lean tissue mass (23%) and total fat mass (53%), both in the percentage of fat lost and in the absolute values, after BS.
Most of the studies in which the impact of BS on renal function has been analysed have used formulas based on serum creatinine to estimate GFR [11, 12]. Several studies have pointed out the inaccuracies of these formulas that are even more evident in obese subjects [27–29]. A systematic underestimation of the true GFR has been reported in obese subjects when these formulas are used to evaluate eGFR [30–32]. This is particularly relevant considering the hyperfiltration that typically accompanies obesity. The use of body surface–corrected GFR has been discouraged in obese people [27, 33]. On the other hand, the loss of muscle mass that accompanies BS-induced weight loss may lead to GFR overestimation [11, 12]. To avoid these inaccuracies, we use a gold standard procedure, iohexol, to measure GFR. This technique allowed us to observe a rapid and significant GFR decrease in the first months after BS and a subsequent stabilization. This sequence of changes coincides with the data provided by the few studies that have used techniques to measure true GFR after BS and can be interpreted as rapid abolition of obesity-related hyperfiltration [34, 35]. Of note, we did not use a threshold to define glomerular hyperfiltration. However, mGFR decreased in all subjects with the exception of one. This may indicate that in these patients obesity was inducing glomerular hyperfiltration. Importantly, no patient showed an episode of AKI. The pathogenic mechanisms leading to GFR decrease after weight loss, induced by BS or low-calorie diets, have not been fully elucidated. Previous studies showed that tight control of hyperglycaemia and BP and an increase in insulin sensitivity was associated with a reduction of GFR in diabetic subjects with hyperfiltration [16, 28]. Similar changes were observed in our study. Accordingly, it may be plausible to speculate that the reduction in GFR was due to improvement in the metabolic milieu induced by weight reduction.
Interestingly, the estimation of GFR by formulas did not show significant changes after BS in our study, again illustrating the limitations of these measurements.
Obesity increases the risk of having hypertension and diabetes mellitus, two main causes of CKD. Previous studies have demonstrated that BS reduces the risk of diabetes, hypertension and hyperlipidaemia in the long term [36, 37]. The better glycaemic control and significant reduction of BP in our patients are in line with these reports. Only one of our patients continued to require oral anti-diabetic agents 24 months after surgery, while insulin and anti-diabetic agents were discontinued in the remaining diabetic patients. Accordingly, circulating levels of C-peptide and insulin levels showed a significant decrease after BS, indicating the improvement of insulin resistance induced by weight loss. Serum triglycerides showed a drastic decrease after BS, in parallel with the decrease in proteinuria and the reduction in glomerular hyperfiltration.
The role of hepatic lipotoxicity in CKD and its influence on the progression of renal disease have been previously described [38–41]. At the end of our study there was a reduction in the NAFLD score. Other studies indicate that fat in the renal sinus is a possible mediator of kidney damage in obesity, through the release of pro-inflammatory cytokines and increased levels of fetuin A [42]. Visceral rather than subcutaneous adipose tissue is associated with metabolic alterations and a chronic low-grade pro-inflammatory state and an altered secretion pattern of adipose-derived molecules. BS induces a decrease in adipokines like leptin and visfatin related with obesity-induced metabolic disorders [43]. Importantly, circulating levels of adiponectin, an adipokine with protective effects on kidney function, increased 12 months after BS [44]. Adiponectin is downregulated in obesity [45] and numerous experimental studies have reported anti-inflammatory, anti-fibrotic and antioxidant properties of this adipokine, together with a reduction in albuminuria [46–49]. The increased levels of adiponectin that we have found after BS may constitute an additional pathway to explain the protective influences of BS on renal function.
The role of inflammation in obesity-related renal disease is well established [50]. We found a drastic decrease in many pro-inflammatory mediators after BS, with a significant correlation with the reduction in proteinuria. Interestingly, only pro-inflammatory cytokines decreased after surgery, while no significant changes were observed in the levels of anti-inflammatory cytokines. In addition, we explored the circulating levels of the pro-fibrotic TGF-β isoforms. TGF-β1 is a cytokine known to participate in several processes related to the development of CKD [51], and associations between the serum level of TGF-β1 and progression of renal disease have been reported. Interestingly, TGF-β1 is increased in the adipose tissue of obese subjects and TGF-β1 circulating levels have been independently associated with increased BMI and with circulating leptin in hypertensive patients [52]. Accordingly, we found a parallel significant decrease in circulating leptin levels and in the levels of both TGF-β1 and β2 isoforms after BS. To the best of our knowledge, this is the first study reporting a decrease in circulating TGF-β1 and TGF-β2 levels after BS in patients with CKD.
Our study has several limitations, such as the small number of patients, the lack of a control group and the lack of kidney histology in most of the patients, although the diagnosis could be assumed on clinical findings. On the other hand, it has important strengths, like the global analysis of changes occurring in renal, metabolic, inflammatory and lipotoxic parameters after BS and the prospective design of the study.
In conclusion, BS is a safe and effective technique to obtain a drastic weight reduction in obese CKD patients, with a significant decrease in proteinuria and improvement in glomerular hyperfiltration. The assessment of mGFR by iohexol is needed to know exactly the changes in GFR occurring after surgery. Weight loss after BS induces a decrease in triglycerides in parallel with a decrease in adipokines and pro-inflammatory and pro-fibrotic parameters. The study results are considered current clinical practice or existing evidence in the field. For this reason, the results of this study could affect future research. Prospective randomized trials with a larger number of patients to draw definitive conclusions regarding BS and hard renal outcomes are necessary.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
This clinical study is part of the research work of the European group ERA-EDTA Diabesity. A.E.R. is working in the Fundación General de la ULL. S.L.L. has a Juan Rodés Contract (grant JR18/00027) from the Instituto de Salud Carlos III (Spain) and is working at the Department of Nephrology and Hypertension, IIS-Fundación Jimenez Díaz, Madrid, Spain. E.P. is a researcher in the Programme Ramón y Cajal (RYC-2014-16573). Thanks to the Institute Carlos III for grant PI16/01814 (to E.L.P.). Thanks to Federico González Rinne for preparation of the figures. The authors acknowledge the valuable contributions of Julian Segura, Angel Sevillano and Natalia Polanco in patient recruitment.
FUNDING
This study was supported by grants from the Beca de Investigación de la Fundación de la Sociedad Española de Nefrología (2015/0117), Ministerio de Economía y Competitividad de España (BFU2016-78951-R, BFU2017-90578-REDT), Comunidad de Madrid (Spain) (B2017/BMD-3684) and Karolinska Institutet (Sweden).
AUTHORS’ CONTRIBUTIONS
E.M., M.P., G.M. and E.P. designed the study. E.P., M.M., S.L., R.V., I.G., L.T., B.L., A.R., M.M. and G.M. carried out experiments. E.M., M.P., E.P. and G.M. analysed the data. E.M., E.P., G.M., M.M. and R.V. made the figures. E.M., M.P., E.P., G.M., M.M. and R.V. drafted and revised the manuscript. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Kambham N, Markowitz GS, Valeri AM. et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59: 1498–1509 [DOI] [PubMed] [Google Scholar]
- 2.McClellan WM, Plantinga LC.. A public health perspective on CKD and obesity. Nephrol Dial Transplant 2013; 28: iv37–iv42 [DOI] [PubMed] [Google Scholar]
- 3.Câmara NOS, Iseki K, Kramer H. et al. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol 2017; 13: 181–190 [DOI] [PubMed] [Google Scholar]
- 4.Kiortsis DN, Christou MA.. Management of obesity-induced kidney disease: a critical review of the literature. Obes Facts 2012; 5: 821–832 [DOI] [PubMed] [Google Scholar]
- 5.Premaratne E, Macisaac RJ, Tsalamandris C. et al. Renal hyperfiltration in type 2 diabetes: effect of age-related decline in glomerular filtration rate. Diabetologia 2005; 48: 2486–2493 [DOI] [PubMed] [Google Scholar]
- 6.Stadler K, Goldberg IJ, Susztak K.. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep 2015; 15: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams TD, Davidson LE, Litwin SE. et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 2017; 377: 1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman AN, Wang J, Wahed AS. et al. The association between kidney disease and diabetes remission in bariatric surgery patients with type 2 diabetes. Am J Kidney Dis 2019; 74: 761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Praga M, Morales E.. The fatty kidney: obesity and renal disease. Nephron 2017; 136: 273–276 [DOI] [PubMed] [Google Scholar]
- 10.Chagnac A, Weinstein T, Herman M. et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 2003; 14: 1480–1486 [DOI] [PubMed] [Google Scholar]
- 11.Afshinnia F, Wilt TJ, Duval S. et al. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 2010; 25: 1173–1183 [DOI] [PubMed] [Google Scholar]
- 12.Navaneethan SD, Yehnert H, Moustarah F. et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2009; 4: 1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIsaac M, Kaban G, Clay A. et al. Long-term impact of bariatric surgery on renal outcomes at a community-based publicly funded bariatric program: the Regina bariatric study. Can J Kidney Health Dis 2019; 6: 205435811988490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales E, Valero MA, León M. et al. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis 2003; 41: 319–327 [DOI] [PubMed] [Google Scholar]
- 15.Bolignano D, Zoccali C.. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant 2013; 28: iv82–iv98 [DOI] [PubMed] [Google Scholar]
- 16.D’Agati VD, Chagnac A, de Vries AP. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 2016; 12: 453–471 [DOI] [PubMed] [Google Scholar]
- 17.MacLaughlin HL, Hall WL, Patel AG. et al. Weight loss, adipokines, and quality of life after sleeve gastrectomy in obese patients with stages 3-4 CKD: a randomized controlled pilot study. Am J Kidney Dis 2014; 64: 660–663 [DOI] [PubMed] [Google Scholar]
- 18.Chang AR, Grams ME, Navaneethan SD.. Bariatric surgery and kidney-related outcomes. Kidney Int Rep 2017; 2: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bröchner-Mortensen J.A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Investig 1972; 30: 271–274 [DOI] [PubMed] [Google Scholar]
- 20.Du Bois D, Du Bois EF.. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5: 303–311 [PubMed] [Google Scholar]
- 21.Angulo P, Hui JM, Marchesini G. et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45: 846–854 [DOI] [PubMed] [Google Scholar]
- 22.Praga M, Morales E.. Obesity-related renal damage: changing diet to avoid progression. Kidney Int 2010; 78: 633–635 [DOI] [PubMed] [Google Scholar]
- 23.Morales E, Gutiérrez E, Caro J. et al. Beneficial long-term effect of aldosterone antagonist added to a traditional blockade of the renin-angiotensin-aldosterone system among patients with obesity and proteinuria. Nefrologia 2015; 35: 554–561 [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Díaz M, Serra A, Romero R. et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 2006; 17: S213–S217 [DOI] [PubMed] [Google Scholar]
- 25.Serra A, Esteve A, Navarro-Díaz M. et al. Long-term normal renal function after drastic weight reduction in patients with obesity-related glomerulopathy. Obes Facts 2015; 8: 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaul S, Rothney MP, Peters DM. et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012; 20: 1313–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Martínez M, Luis-Lima S, Morales E. et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: a dreadful combination of two errors. Int J Obes 2020; 44: 1129–1140 [DOI] [PubMed] [Google Scholar]
- 28.Ruggenenti P, Porrini EL, Gaspari F. et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012; 35: 2061–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang SHS, Sharma AP, Yasin A. et al. Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol 2011; 6: 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porrini E, Ruggenenti P, Luis-Lima S. et al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 2019; 15: 177–190 [DOI] [PubMed] [Google Scholar]
- 31.Imam TH, Fischer H, Jing B. et al. Estimated GFR before and after bariatric surgery in CKD. Am J Kidney Dis 2017; 69: 380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang A, Greene TH, Wang X. et al. The effects of weight change on glomerular filtration rate. Nephrol Dial Transplant 2015; 30: 1870–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang AR, Chen Y, Still C. et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int 2016; 90: 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruggenenti P, Abbate M, Ruggiero B. et al. Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: a randomized controlled trial. Diabetes 2017; 66: 75–86 [DOI] [PubMed] [Google Scholar]
- 35.Gaspari F, Ruggenenti P, Porrini E. et al. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 2013; 84: 164–173 [DOI] [PubMed] [Google Scholar]
- 36.Navaneethan SD, Malin SK, Arrigain S. et al. Bariatric surgery, kidney function, insulin resistance, and adipokines in patients with decreased GFR: a cohort study. Am J Kidney Dis 2015; 65: 345–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.English WJ, Williams DB.. Metabolic and bariatric surgery: an effective treatment option for obesity and cardiovascular disease. Prog Cardiovasc Dis 2018; 61: 253–269 [DOI] [PubMed] [Google Scholar]
- 38.Escasany E, Izquierdo-Lahuerta A, Medina-Gomez G.. Underlying mechanisms of renal lipotoxicity in obesity. Nephron 2019; 143: 28–32 [DOI] [PubMed] [Google Scholar]
- 39.de Vries AP, Ruggenenti P, Ruan XZ. et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol 2014; 2: 417–426 [DOI] [PubMed] [Google Scholar]
- 40.Byrne CD, Targher G.. NAFLD as a driver of chronic kidney disease. J Hepatol 2020; 72: 785–801 [DOI] [PubMed] [Google Scholar]
- 41.Musso G, Gambino R, Tabibian JH. et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014; 11: e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner R, Machann J, Guthoff M. et al. The protective effect of human renal sinus fat on glomerular cells is reversed by the hepatokine fetuin-A. Sci Rep 2017; 7: 2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosseinzadeh-Attar MJ, Golpaie A, Janani L. et al. Effect of weight reduction following bariatric surgery on serum visfatin and adiponectin levels in morbidly obese subjects. Obes Facts 2013; 6: 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salman AA, Sultan AAEA, Abdallah A. et al. Effect of weight loss induced by laparoscopic sleeve gastrectomy on liver histology and serum adipokine levels. J Gastroenterol Hepatol 2020; 35: 1769–1773 [DOI] [PubMed] [Google Scholar]
- 45.Hotta K, Funahashi T, Arita Y. et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000; 20: 1595–1599 [DOI] [PubMed] [Google Scholar]
- 46.Ohashi K, Iwatani H, Kihara S. et al. Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol 2007; 27: 1910–1917 [DOI] [PubMed] [Google Scholar]
- 47.Sharma K, Ramachandrarao S, Qiu G. et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 2008; 118: 1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamaki S, Satoh H, Kudoh A. et al. Adiponectin reduces proteinuria in streptozotocin-induced diabetic Wistar rats. Exp Biol Med (Maywood) 2011; 236: 614–620 [DOI] [PubMed] [Google Scholar]
- 49.Christou GA, Kiortsis DN.. The role of adiponectin in renal physiology and development of albuminuria. J Endocrinol 2014; 221: R49–R61 [DOI] [PubMed] [Google Scholar]
- 50.Park S, Kim YJ, Choi CY. et al. Bariatric surgery can reduce albuminuria in patients with severe obesity and normal kidney function by reducing systemic inflammation. Obes Surg 2018; 28: 831–837 [DOI] [PubMed] [Google Scholar]
- 51.Meng XM, Nikolic-Paterson DJ, Lan HY.. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 2016; 12: 325–338 [DOI] [PubMed] [Google Scholar]
- 52.Porreca E, Di Febbo C, Vitacolonna E. et al. Transforming growth factor-β1 levels in hypertensive patients: association with body mass index and leptin. Am J Hypertens 2002; 15: 759–765 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.