Abstract
Immune checkpoint inhibitors (ICIs), immunomodulatory antibodies that are used to enhance the immune system, have substantially improved the prognosis of patients with advanced malignancy. As the use of ICI therapy becomes increasingly widespread across different types of cancer, their use in patients receiving dialysis is likely to increase. In this review we summarize the current literature on the use of ICIs in end-stage kidney disease (ESKD) patients and provide aggregate data from reported cases and series. Based on available pharmacological information, ICIs require no dosing adjustment in ESKD patients. Analysis of the reported cases in the literature demonstrates a similar incidence of immune-related adverse events in patients with ESKD receiving dialysis as compared with the general population (49%). Severe reactions graded as 3 and 4 have been seen in 15 patients (16%). As such, it is important that these patients are monitored very closely for immune-related adverse events; however, the risk of these adverse events should not preclude patients on dialysis from receiving these therapies. Cancer remission (complete and partial) was seen in close to 30% of patients, stable disease was seen in 28% and progression of disease in ∼36%. One-third of the patients died. Urothelial and renal cell cancer represented approximately half of all treated cancers and accounted for ∼50% of all deaths reported. Additional data in the dialysis population with the use of ICIs and involvement in prospective studies are needed to better assess outcomes, particularly within specific cancer types.
Keywords: cancer, dialysis, ESKD, immunotherapy, pembrolizumab, nivolumab
INTRODUCTION
Immune checkpoint inhibitors (ICIs), immunomodulatory antibodies that are used to enhance the immune system, have substantially improved the prognosis of patients with advanced malignancy. Although there is no renal clearance of these agents, the data on the use of ICIs in end-stage kidney disease (ESKD) patients on hemodialysis (HD) as well as peritoneal dialysis (PD) patients is sparse [1]. Initial reports of the use of ICI therapy in dialysis patients were limited to patients who had rejected a transplanted kidney and were resumed on dialysis with continued ICI therapy [2, 3]. In the last year, several single-center case reports and series from around the world have emerged suggesting their safe use in both HD and PD patients [4–6]. In this review we summarize the pharmacology of ICI therapy and then systematically review all published cases of the use of ICI therapy in patients on dialysis (both incident dialysis patients due to allograft rejection as well as prevalent dialysis patients).
Pharmacology of ICIs
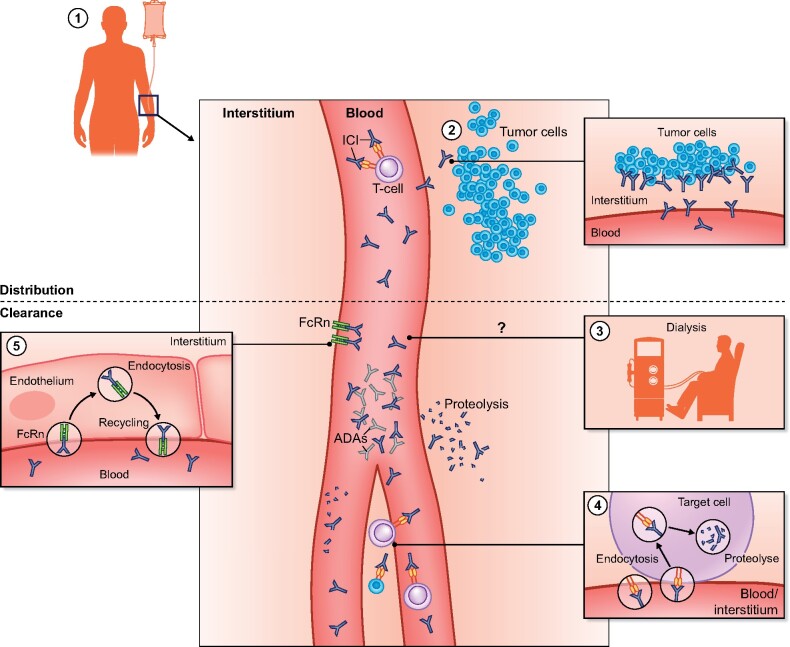
ICIs are humanized or human immunoglobulin (Ig) antibodies of the IgG1 isotype (except nivolumab, which is of the IgG4 isotype) with pharmacokinetic properties similar to other therapeutic monoclonal antibodies (mAbs) [1, 7]. They have a small volume of distribution following intravenous administration and are for the most part confined to the vascular space [1, 7]. Their distribution is determined by rates of extravasation out of the vascular space through convective transport and transcytosis, distribution in the interstitial space via diffusion, convection and antibody binding, followed by intracellular degradation or recycling by the neonatal Fc receptor (FcRn) [8, 9]. Removal of ICIs from the interstitial space is dependent on convection into the lymph. The time to reach drug steady state varies for each ICI and ranges from 4 to 18 weeks [1, 7]. mAbs are too large to be eliminated by the kidneys, except possibly in the setting of high-grade non-selective proteinuria [10]. Hepatic elimination of these agents is negligible. Intracellular catabolism by lysosomal degradation following pinocytosis or receptor-mediated endocytosis is the main route of elimination. Receptor-mediated endocytosis of IgG molecules occurs after binding of their Fc domain to cell surface receptors or after binding of their Fab domains to their target antigen, so-called target-mediated drug disposition (TMDD). Catabolic degradation of IgG following pinocytotic uptake occurs throughout the body, particularly in organs and tissues that are rich in endothelial cells.
The rate of elimination through TMDD is dependent on the availability of the target antigen (tumor type and burden), the affinity of the ICI for the antigen, the dose of the ICI, the rate of internalization and the rate of catabolism within the target cell. The elimination of ICI is time dependent (changing clearance over time after the start of treatment) and this is probably due to a change in the availability of their tumor antigen during the disease process [11–13]: reduced tumor burden results in a lower availability of available antigens and lower TMDD. The elimination of ipilimumab is not time dependent, possibly because of the small amount of target cells in comparison with other ICIs. To prevent excessive degradation of IgG after pinocytosis, there is a salvage pathway through binding to the FcRn. The FcRn is present in the endosome and binds IgG. FcRn–IgG complexes are returned to the cell surface and IgG is released back into the extracellular space. It is estimated that two-thirds of ICIs are recycled in this manner [14], resulting in extended half-lives of ICIs (6–27 days) [9]. Further highlighting the importance of the FcRn salvage pathway is the observation that a genetic variant in the FCGRT gene encoding for FcRn results in decreased expression of FcRn and is associated with increased mAb clearance and decreased systemic exposure [15, 16].
Finally, ICI clearance can also occur through the formation of antidrug antibodies, which facilitate endocytic degradation of these drugs [1, 7]. The percentage of patients developing antidrug antibodies, as well as its impact on clearance, is considered limited for most ICIs, as they are either human or humanized antibodies [17, 18]. Interestingly, the development of antidrug antibodies might be more frequent in patients receiving ICI combination therapy (e.g. ipilimumab + nivolumab) [19]. Figure 1 summarizes the pharmacokinetics of ICIs. No registry or large database exists on the use of ICIs in ESKD patients. Hence we performed a detailed literature review to assess the use of these agents in ESKD patients from published case reports and case series.
FIGURE 1:
Pharmacology of ICIs. (1) After intravenous administration, ICIs are distributed in the vascular space and the interstitial space. (2) Distribution in the interstitial space is determined via diffusion, convection and antibody binding followed by intracellular degradation. Receptor-mediated endocytosis of IgG occurs after binding of their Fc domain to cell surface receptors or by TMDD, which is dependent on the availability of the target antigen (tumor type and burden). (3) mAbs are too large to be eliminated during dialysis, although adherence to the dialysis filter is a theoretical possibility. (4) Intracellular catabolism by lysosomal degradation following pinocytosis or receptor-mediated endocytosis is the main route of elimination. Endocytic degradation can be facilitated by antidrug antibodies. (5) To prevent excessive degradation of IgG after pinocytosis, there is a salvage pathway through binding to the FcRn.
Literature search
We performed a descriptive literature review of ICI use in ESKD patients receiving HD or PD. Case series and reports with at least one ESKD patient receiving ICIs were included. Manuscripts were required to report individual-level data for patients. A similar descriptive study design as previously used for outcomes of myeloma patients with kidney transplants was utilized [20].
Literature search methodology
We performed a structured search (developed by a health information specialist) of the MEDLINE and Embase databases from inception to February 2021. We sought to identify case reports, case series, observational studies and clinical trials that described the use of ICI therapy for cancer in patients receiving dialysis (either HD or PD). We included cases in which kidney transplant recipients received ICI, provided that they continued on ICI therapy following graft failure and received dialysis. The search strategy and terms can be found in the Supplementary Appendix. Citations retrieved from the search underwent title and abstract review for inclusion. Articles identified from the search were then reviewed in full for relevance and inclusion. We then reviewed the references of included articles for additional relevant citations not identified by the search strategy. All authors were involved in data abstraction of clinical information from the search strategy.
Data abstraction and descriptive statistical analysis
For each included study we performed a standardized patient-level data abstraction using prespecified parameters of interest: patient demographics, cancer diagnosis, ICI treatment characteristics, dialysis modality, immune-related adverse events (irAEs), cancer outcomes and survival. We reported descriptive statistics related to the prespecified parameters of interest. We expressed continuous variables as the mean [standard deviation (SD)] or median (25th–75th percentile) and categorical variables as a percentage.
The structured search yielded 136 citations for title and abstract screening. Of these, 31 articles were reviewed in full and 28 articles met inclusion criteria. Review of the reference lists identified 5 additional articles, resulting in a total of 33 articles for inclusion [2–6, 21–49]. From these 33 articles, 98 cases with patient-level data were included. A summary of each included citation is shown in Table 1. Aggregate data summarizing patient characteristics (stratified by previous kidney transplant status) are shown in Table 2. Tables 3 and 4 report these characteristics among recipients of nivolumab and pembrolizumab (the two most commonly used ICIs in the cohort) and those with urothelial cancers and renal cell carcinoma (RCC; the two most common cancer diagnoses in the cohort), respectively.
Table 1.
Published case reports and series of ICI therapy in patients with ESKD on dialysis
| References | Patients (n) | Cancer(s) | ICI(s) | Grades 3/4 irAEs (nonrenal) | Summary of cancer outcomes |
|---|---|---|---|---|---|
| Cavalcante et al. [40] | 2 | Melanoma | Ipilimumab | Pemphigoid rash and bullous lesions | PR (1), CR (1) |
| Alhamad et al. [44] | 1 | Melanoma | Ipilimumab, pembrolizumab | None | PDS |
| Boils et al. [3] | 1 | Lung | Nivolumab | None | N/A |
| Carlo and Feldman [41] | 1 | RCC | Nivolumab | None | PR |
| Chang and Shirai [39] | 1 | Melanoma | Pembrolizumab | None | CR |
| Jose et al. [54] | 1 | Melanoma | Ipilimumab | None | PDS |
| Lipson et al. [2] | 1 | Skin SCC | Pembrolizumab | None | PR |
| Ong et al. [28] | 1 | Melanoma | Nivolumab | None | PR |
| Spain et al. [48] | 1 | Melanoma | Ipilimumab, nivolumab | None | SD |
| Boyle et al. [42] | 1 | Multiple myeloma | Nivolumab | None | PR |
| Park | 4 | RCC (2), skin SCC (2) | Nivolumab (2), pembrolizumab (2) | Grade 3 penumonitis, Grade 4 encephalitis/death (1) | PDS (1), PR (2), death (1) |
| Tabei et al. [24] | 1 | RCC | Nivolumab | None | PR |
| Akturk et al. [45] | 1 | Melanoma | Pembrolizumab, nivolumab | Hypothyroidism | PR |
| Ansari et al. [55] | 1 | RCC | Nivolumab | None | PR |
| Ishizuka et al. [37] | 1 | Lung | Pembrolizumab | None | PR |
| Cheun et al. [6] | 3 | RCC (2), urothelial (1) | Nivolumab (2), atezolizumab (1) | None | PR (1), SD (1), PDS (1), death (1) |
| Fernandez-Diaz et al. [38] | 1 | Melanoma | Nivolumab | None | SD |
| Ito et al. [47] | 1 | RCC | Nivolumab | None | CR |
| Morinaga et al. [30] | 1 | RCC | Nivolumab | None | PDS |
| Osa et al. [27] | 1 | Lung | Pembrolizumab | None | SD |
| Parisi et al. [25] | 1 | Urothelial | Atezolizumab | None | PDS |
| Tachibana et al. [23] | 7 | RCC | Nivolumab | Grade 3 fatigue | PR (1), SD (4), PDS (2), death (2) |
| Vitale et al. [21] | 8 | RCC | Nivolumab | Grade 3 diarrhea, Grade 3 asthenia, Grade 3 anorexia | PR (1), SD (5), PDS (2), death (3) |
| Hirsch et al. [4] | 8 | Cholangiocarcinoma (1), hepatocellular carcinoma (1), Hodgkin’s lymphoma (1), NET (1), RCC (1), urothelial (3) | Atezolizumab (1), nivolumab (2), ipilimumab + nivolumab (1), pembrolizumab (4) | Dermatitis (grade unspecified) | SD (3), PDS (5), death (4) |
| Jain et al. [35] | 8 | Angiosarcoma (1), lung (1), melanoma (2), retroperitoneal sarcoma (2), RCC (1), urothelial (1) | Nivolumab (3), pembrolizumab (5) | Pneumonitis (grade unspecified) | SD (3), PDS (5), death (2) |
| Kuo et al. [33] | 11 | Urothelial | Atezolizumab (3), nivolumab (2), pembrolizumab (6) | Grade 3 anemia (3), Grade 4 anemia (1), Grade 4 neutropenia (1), Grade 4 TEN (1) | PR (6), SD (1), PDS (4), death (3) |
| Osmán-García et al. [26] | 3 | RCC | Nivolumab | None | PR (2), PDS (1) |
| Mejia et al. [31] | 1 | RCC | Ipilimumab + nivolumab | None | PR |
| Murakami et al. [46] | 3 | Skin SCC (2), melanoma | Cemiplimab, pembrolizumab, iplimumab + nivolumab | None | PR (1), SD (2) |
| Strohbehn et al. [1] | 19 | GU (6), melanoma (4), Merkle cell (3), head and neck (3), lung (2), GI (1) | Avelumab (1), atezolizumab (2), ipilimumab (1), ipilimumab/nivolumab (2), nivolumab (5), pembrolizumab (8) | Grades 3–4 myocarditis (1), pneumonitis (1) | SD (4), PDS (9), death (13) |
| Duni et al. [49] | 1 | RCC | Nivolumab | None | SD |
| Tan et al. [22] | 1 | Melanoma | Nivolumab | None | CR |
NET, neuroendocrine tumor; SCC, squamous cell carcinoma; PDS, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
Table 2.
Summary data from published cases of ICI therapy in patients with ESKD on dialysis stratified by prior kidney transplant statusa
| Variable | Total reported cases | ESKD without prior kidney transplant | ESKD with prior kidney transplant |
|---|---|---|---|
| Patients, n | 98 (100) | 80 (81.6) | 18 (18.4) |
| Age at ICI initiation (years), mean (SD) | 64.2 (12.1) | 65.6 (12.1) | 59.4 (11.1) |
| Age at ICI initiation (years), median (IQR) | 66 (58–73) | 67 (63–73) | 60.5 (53.3–67.5) |
| Male, n (%) | 69 (70.4) | 60 (75.0) | 9 (64.3) |
| Female, n (%) | 29 (29.6) | 20 (25.0) | 5 (35.7) |
| Cancer site, n (%) | |||
| RCC | 32 (32.6) | 30 (37.5) | 2 (11.1) |
| Melanoma | 16 (17.3) | 9 (11.3) | 8 (44.4) |
| Urothelial/GU NOS | 23 (23.5) | 23 (28.8) | 0 (0.0) |
| Lung | 6 (6.1) | 5 (6.3) | 1 (7.1) |
| Skin SCC | 5 (5.1) | 3 (3.8) | 2 (11.1) |
| Head and neck | 3 (3.1) | 2 (2.5) | 1 (5.6) |
| Merkle cell | 3 (3.1) | 3 (3.8) | 0 (0.0) |
| Otherb | 9 (9.6) | 5 (6.3) | 4 (22.2) |
| ICI initial therapy, n (%) | |||
| Atezolizumab | 8 (8.2) | 8 (10.0) | 0 (0.0) |
| Avelumab | 1 (1.0) | 1 (1.3) | 0 (0.0) |
| Cemiplimab | 1 (1.0) | 0 (0.0) | 1 (5.6) |
| Ipilimumab | 6 (6.1) | 3 (3.8) | 3 (16.7) |
| Nivolumab | 45 (45.9) | 38 (47.5) | 7 (38.9) |
| Pembrolizumab | 31 (31.6) | 26 (32.5) | 5 (27.8) |
| Combination (ipilimumab + nivolumab | 6 (6.1) | 4 (5.0) | 2 (11.1) |
| Dialysis modality, n (%) | |||
| Hemodialysis | 89 (90.8) | 76 (95.0) | 13 (72.2) |
| Peritoneal dialysis | 9 (9.2) | 4 (5.0) | 5 (27.8) |
| Treatment duration (months), median (IQR) | 6.1 (4.4–9.7) | 6 (2.8–9.0) | 4.2 (1.0–6.0) |
| irAEs (nonrenal), n (%) | |||
| irAEs | 76 | – | – |
| Grade 1/2 irAEs | 53 | – | – |
| Grade 3/4 irAEs | 23 | – | – |
| Patients experiencing any irAE | 48 (49.0) | 45 (56.3) | 3 (16.7) |
| Patients experiencing Grade 1/2 | 33 (33.7) | 30 (37.5) | 3 (16.7) |
| Patients experiencing Grade 3/4 | 15 (15.3) | 15 (18.8) | 0 (0.0) |
| irAEs by type, n | |||
| Cutaneous | 8 | – | – |
| Encephalitis | 1 | – | – |
| Endocrine | 4 | – | – |
| GI/diarrhea | 2 | – | – |
| Hematologic | 12 | – | – |
| Hepatitis | 3 | – | – |
| Myocarditis | 1 | – | – |
| Pneumonitis | 5 | – | – |
| Cancer outcomes, n (%) | |||
| Complete remission | 4 (4.1) | 3 (3.8) | 1 (5.6) |
| Partial remission | 25 (25.5) | 19 (23.8) | 5 (33.3) |
| Stable disease | 27 (27.5) | 23 (28.8) | 4 (22.2) |
| Progressive disease | 35 (35.7) | 30 (37.5) | 5 (27.8) |
| Not available | 7 (7.1) | 5 (6.3) | 2 (11.1) |
| Death | 30 (30.6) | 27 (33.8) | 3 (16.7) |
Values and proportions reported for each characteristic among those patients in whom individual (patient-level) data were available.
Sarcoma (3), GI (1), cholangiocarcinoma (1), hepatocellular (1) Hodgkin’s lymphoma (1), multiple myeloma (1), neuroendocrine (1).
SCC, squamous cell cancer.
Table 3.
Summary data from published cases of nivolumab and pembrolizumab in patients with ESKD on dialysisa
| Variable | Total reported cases | Nivolumab | Pembrolizumab |
|---|---|---|---|
| Patients, n (%) | 76 (100) | 45 (59.2) | 31 (40.8) |
| Age at ICI initiation (years), mean (SD) | 64.2 (12.1) | 65.8 (9.2) | 59.4 (11.1) |
| Age at ICI initiation (years), median (IQR) | 66 (58–73) | 67 (63–73) | 60.5 (53.3–67.5) |
| Male, n (%) | 42 (76.4) | 29 (76.3) | 13 (76.5) |
| Female, n (%) | 13 (29.6) | 9 (23.7) | 4 (23.5 |
| Cancer site, n (%) | |||
| RCC | 30 (39.5) | 29 (64.4) | 1 (3.2) |
| Melanoma | 10 (13.2) | 5 (11.1) | 5 (16.1) |
| Urothelial/GU NOS | 15 (19.7) | 5 (11.1) | 10 (32.3) |
| Lung | 5 (6.5) | 2 (4.4) | 3 (9.7) |
| Skin SCC | 4 (5.2) | 0 (0.0) | 4 (12.9) |
| Head and neck | 3 (3.9) | 0 (0.0) | 3 (9.7) |
| Merkle cell | 1 (1.3) | 1 (2.2) | 0 (0.0) |
| Other | 8 (10.5) | 3 (6.7) | 5 (16.1) |
| Dialysis modality, n (%) | |||
| Hemodialysis | 73 (96.1) | 43 (95.6) | 30 (96.8) |
| Peritoneal dialysis | 3 (3.9) | 2 (4.4) | 1 (3.2) |
| Treatment duration (months), median (IQR) | 6.1 (4.4–9.7) | 8.0 (4.6–14.0) | 6.5 (2.9–7.7) |
| irAE (non-renal), n (%) | |||
| irAEs | 47 | – | – |
| Grade 1/2 irAEs | 38 | – | – |
| Grade 3/4 irAEs | 14 | – | – |
| Patients experiencing any irAE | 40 (52.6) | 21 (46.7) | 19 (61.3) |
| Patients experiencing Grade 1/2 | 26 (34.2) | 14 (31.1) | 12 (38.7) |
| Patients experiencing Grade 3/4 | 14 (18.4) | 7 (15.6) | 7 (22.5) |
| irAEs by type, n | |||
| Cutaneous | 5 | – | – |
| Encephalitis | 1 | – | – |
| Endocrine | 4 | – | – |
| GI/diarrhea | 0 | – | – |
| Hematologic | 9 | – | – |
| Hepatitis | 2 | – | – |
| Myocarditis | 1 | – | – |
| Pneumonitis | 3 | – | – |
| Cancer outcomes, n (%) | |||
| Complete remission | 3 (3.9) | 2 (4.4) | 1 (3.2) |
| Partial remission | 20 (26.3) | 13 (28.9) | 7 (22.5) |
| Stable disease | 22 (28.9) | 15 (33.3) | 7 (22.5) |
| Progressive disease | 26 (34.2) | 12 (26.7) | 14 (45.2) |
| Not available | 5 (6.6) | 3 (6.7) | 2 (6.4) |
| Death | 21 (27.6) | 12 (26.7) | 9 (29.0) |
Values and proportions reported for each characteristic among those patient in whom individual (patient-level) data were available.
SCC, squamous cell cancer.
Table 4.
Summary data from published cases of ICI therapy in patients with urothelial cancers and RCC and ESKD on dialysisa
| Variable | Total reported cases | Urothelial cancers | RCC |
|---|---|---|---|
| Patients, n (%) | 55 (100) | 23 (41.8) | 32 (58.1) |
| Age at ICI initiation (years), mean (SD) | 65.3 (11.2) | 62.3 (13.4) | 62.3 (11.1) |
| Age at ICI initiation (years), median (IQR) | 67 (63–73) | 67 (61–75) | 67 (64–73) |
| Male, n (%) | 36 (65.5) | 10 (58.8) | 26 (81.3 |
| Female, n (%) | 13 (23.6) | 7 (41.2) | 6 (18.8) |
| ICI (initial therapy), n (%) | |||
| Atezolizumab | 7 (12.3) | 7 (30.4) | 0 (0.0) |
| Avelumab | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cemiplimab | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ipilimumab | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nivolumab | 33 (60.0) | 5 (21.7) | 28 (87.5) |
| Pembrolizumab | 12 (21.8) | 10 (43.5) | 2 (6.3) |
| Combination (ipilimumab + nivolumab) | 3 (5.4) | 1 (4.3) | 2 (6.3) |
| Dialysis modality, n (%) | |||
| Hemodialysis | 44 (90.8 | 17 (73.9) | 27 (84.3) |
| Peritoneal dialysis | 3 (9.2) | 0 (0.0) | 3 (9.4) |
| Unavailable | 7 (12.7) | 6 (26.0) | 1 (3.1) |
| Treatment duration (months), median (IQR) | 6.0 (2.1–8.3) | 2.1 (1.7–3.5) | 6.1 (3.2–8.3) |
| irAE (non-renal), n (%) | |||
| irAEs | 50 | – | – |
| Grade 1/2 irAEs | 36 | – | – |
| Grade 3/4 irAEs | 16 | – | – |
| Patients experiencing any irAE | 35 (63.6) | 19 (82.6) | 26 (81.3 |
| Patients experiencing Grade 1/2 | 21 (38.2) | 10 (43.5 | 21 (65.6) |
| Patients experiencing Grade 3/4 | 14 (25.5) | 9 (39.1) | 5 (15.6) |
| irAEs by type, n (%) | |||
| Cutaneous | 5 | – | – |
| Encephalitis | 0 | – | – |
| Endocrine | 0 | – | – |
| GI/diarrhea | 0 | – | – |
| Hematologic | 11 | – | – |
| Hepatitis | 3 | – | – |
| Myocarditis | 1 | – | – |
| Pneumonitis | 3 | – | – |
| Cancer outcomes, n (%) | |||
| Complete remission | 1 (1.8) | 0 (0.0) | 1 (3.1) |
| Partial remission | 17 (30.9) | 6 (26.1) | 11 (34.4) |
| Stable disease | 17 (30.9) | 6 (26.1) | 11 (34.4) |
| Progressive disease | 20 (36.4) | 11 (47.8) | 9 (28.1) |
| Not available | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Death | 17 (30.9) | 9 (39.1) | 8 (25.0) |
Values and proportions reported for each characteristic among those patients in whom individual (patient-level) data were available.
SCC, squamous cell cancer.
Demographics of ESKD patients receiving ICI
Among the 98 patients with ESKD receiving ICI, 80 patients had a diagnosis of ESKD preceding the administration of ICI and an additional 18 were kidney transplant patients who rejected their allograft with the use of ICI and initiated dialysis with continued use of these drugs. The median age at ICI initiation was 66 years [interquartile range (IQR) 58–73]. The most frequent cancers reported were RCC (33%), genitourinary (GU; 24%) and melanoma (17%). It should be noted that ICIs were initially approved for melanoma; however, over the years they have shown considerable promise in the treatment for various other cancers [50]. In our patient population, more than half of the patients (56%) treated with these agents had RCC or GU cancer. This is likely reflective of the increased incidence of these types of cancers seen in the ESKD population as reported previously in the literature [51, 52]. Nivolumab and pembrolizumab were the most common ICI agents used, representing 46% and 32% of initial therapy, respectively. HD recipients comprised 91% of reported dialysis patients.
Safety data and irAEs
As patients with CKD and ESKD have been largely excluded from trials of cancer therapy, including ICI [53], estimating the risk of safety events and irAEs is challenging. From our literature search we observed that there were 75 irAEs reported among 98 patients. In total, 49% of patients (48/98) experienced any irAE. The majority of the adverse events were Grades 1 and 2, seen in 33 patients (34%). Fifteen patients experienced Grades 3 and 4 adverse events (15%). The most common adverse events reported were hematologic, seen in 12 patients. These manifested as anemia, thrombocytopenia and neutropenia. The next most common adverse effect was dermatologic (seen in eight patients), including pruritus, dermatitis, toxic epidermal necrolysis and pemphigoid rash. Gastrointestinal (GI) toxicity was seen in five patients, presenting as hepatitis, ascites, ileus, abdominal pain and diarrhea. Pulmonary toxicity presenting as pneumonitis was seen in four patients. Tuberculosis reactivation was described in one patient. Other irAEs described in dialysis patients but occuring at a low frequency include asthenia, anorexia, fatigue and endocrine (thyroid dysfunction).
With respect to severe (i.e. Grade 4) irAEs, notable reported events included one death in the context of encephalitis, one (nonfatal) episode of myocarditis and two (nonfatal) pneumonitis events [5]. The death associated with encephalitis was the only reported fatal event attributed to therapy.
The frequency of irAEs has been estimated to be in the range of 56% in a large meta-analysis of multiple cancer sites [31, 56]. Other large analyses assessing individual cancer types have estimated the incidence of irAEs to range between 39% and 59%, depending on the malignancy and ICI [29, 57, 58]. As such, the observed frequency of irAEs among patients receiving dialysis does not appear substantially different from that of the nondialysis general population, although definitive conclusions cannot be drawn from a review of reported literature alone. As in the general population, the skin and GI systems were among the most commonly observed sites for irAEs [56, 59]. Regarding the severe irAEs noted in this review, including encephalitis and myocarditis, these events are similar and occur infrequently in the general population, with incidence estimates of 1.3% [60, 61] and <1% [60], respectively. Given these findings, along with the nonrenal excretion and metabolism of ICI described above, it is plausible that the overall risk for irAEs and severe events is unlikely to differ from that of nondialysis patients. Of note, no particular elevated risk for irAEs has been noted among elderly patients, and drug tolerance appears similar [62]. Also, there were no apparent major differences in overall rates of irAEs between nivolumab and pembrolizumab.
It should be noted that the relatively high frequency of hematologic adverse events among reported patients with ESKD differs from the general population, in which hematologic adverse events are comparatively infrequent [63]. Of the 12 cases of hematologic adverse events noted in this review, 11 were reported in the series by Kuo et al. [33] and predominantly reflective of anemia. In their report, immune-mediated anemia was not specifically distinguished from other potential causes of low hemoglobin, including malignancy and ESRD-associated anemia. As anemia and other hematologic abnormalities may be multifactorial in patients receiving dialysis, careful monitoring of these parameters (with appropriate investigations as to etiology) is warranted during ICI therapy in this population.
Lastly, management of irAEs among patients receiving dialysis was broadly reported to be similar as in nondialysis patients. The mainstays of treatment remain corticosteroids for most irAEs and endocrine replacement therapy, in keeping with American Society of Clinical Oncology and European Society of Medical Oncology guidelines [64, 65]. None of the reported cases describe the use of other forms of immunosuppression for management of irAEs. However, given the predilection of patients with ESKD for other potentially steroid-adverse medical comorbidities, including diabetes mellitus, heart failure, etc., there should be consideration for limiting corticosteroid exposure in this population (where required and permitted by the indicating irAE). Since immune senescence is known to correlate with dialysis vintage, the relationship between dialysis vintage and irAEs is a subject for future study [66, 67].
Dialysis patients with prior kidney transplants
Eighteen of the reported dialysis patients had prior kidney transplants. Of these, 11 (61%) initiated dialysis after ICI-related rejection of their kidney allografts [31]. Unfortunately, data on specific modifications of immunosuppressive regimens were often not reported. Caution is warranted when prescribing ICIs in a patient on dialysis with a failed kidney allograft. Hirsch et al. [4] described a series of eight patients on dialysis receiving ICIs in which one of the patients who had a previous history of kidney allograft failure on HD for 3 years developed hepatocellular cancer and received nivolumab. He subsequently experienced acute rejection of his allograft immediately after starting ICI therapy and also suffered from cancer progression. Likewise, Mejia et al. [31] recently reported the case of a 66-year-old patient with a failed kidney allograft undergoing HD who received combination immunotherapy with anti-PD-1 (nivolumab) and anti-CTLA-4 (ipilimumab) for metastatic papillary renal and urothelial cancer. The patient’s clinical course was complicated by development of gross hematuria and pain over the allograft, necessitating removal of the allograft. Histopathological evaluation following transplant nephrectomy revealed chronic active T-cell-mediated rejection. Another similar report by Duni et al. [49] describes a single case of kidney graft rejection of a long-term nonfunctioning graft 14 days after the first cycle of therapy with PD-1 inhibitor (nivolumab) in a patient receiving PD for >3 years. On the other hand, a report of 19 dialysis patients who received ICI therapy between 2013 and 2019 showed that although 32% of the patients experienced diverse irAEs, none of the 4 patients with prior failed kidney allografts included in the study demonstrated clinical evidence of rejection [5]. Rejection of the old allograft can occur with initiation of ICI in dialysis patients. One might consider a ‘mini-pulse’ steroid protocol in the first 6–10 weeks of initiation of ICIs to prevent this occurrence [46, 68]. A recent multicenter retrospective cohort study of kidney transplant patients with cancer receiving ICIs showed that 42% of patients developed acute graft rejection, of which 65.5% progressed to ESKD requiring dialysis [46]. Continuing ICI therapy in patients who recently failed their allograft has been done safely without dose alterations, as noted in some of the cases reviewed above in our analysis.
Cancer and patient outcomes
Survival of patients receiving ICI is largely dependent on the tumor type. Real-world outcomes seen with these agents are different from those in clinical trials. This is likely due to patients with a lower comorbidity index being enrolled in these studies [69–71]. In a large Veterans Affairs study with 11 888 (non-ESKD) patients with different types of cancer receiving ICI, the overall survival ranged between 6.7 and 25.5 months [72]. This was largely dependent on the tumor type, with the longest survival seen in melanoma patients (25 months) and the shortest in patients with urothelial cancer receiving second-line ICI (6 months). Although this study reported outcomes worse than those seen in clinical trials, these patients still did better than the historical controls. There are also data that show that development of irAEs is associated with treatment response and improved survival in patients with cancer treated with ICI [73]
Mortality on dialysis remains unacceptably high in various situations, including cardiac surgery [74], influenza [75] or severe acute respiratory syndrome coronavirus 2 infection [76]. In a broad population-based cohort study in Canada, men treated with dialysis had worse adjusted 5-year survival than men with prostate or colorectal cancer and women on dialysis had worse adjusted 5-year survival than women with breast or colorectal cancer [77]. The 5-year cumulative incidence of any cancer in ESKD patients is ~9.5%, much higher than the incidence expected in the general population. The risk for kidney cancer is four times higher than the risk of bladder cancer [51, 78]. The mortality risk is also high when patients receiving dialysis are diagnosed with cancer [79]. Patient and cancer outcomes of immunotherapy use in cancer patients on dialysis are not well known.
In our literature review, cancer outcomes in these patients were divided into three categories: remission (complete and partial), stable disease and progressive disease. No data were available for seven patients. Remission (complete and partial) was seen in 29 patients (29.6%). Of note, partial remission was more common than complete remission (25.5% versus 4.1%). Stable disease was seen in 27 patients (27.5%). There was progression of disease in a total of 35 patients (35.7%). Of the 98 patients, there were 30 deaths recorded (31%). Urothelial and renal cell cancer accounted for 56% of cases and 50% of all deaths. When comparing urothelial and renal cell cancers, outcomes appeared similar overall within the categories assessed.
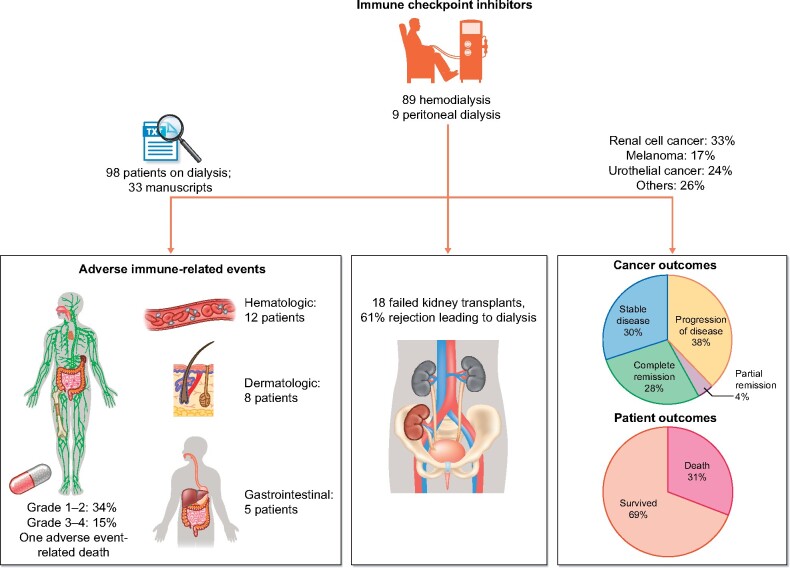
Of all the patients studied, more than half (57%) had evidence of remission or stable disease. We are unable to comment on the overall and progression-free survival of these patients as all our studies were limited to case reports and case series not reporting this specific outcome. Most trials reporting on the efficacy of these agents are limited to their use in specific cancer types [69–71]. In our analysis, different cancer types were included, making it impossible to directly compare to non-ESKD patients and comment on these outcomes within individual cancer types. Figure 2 summarizes our findings.
FIGURE 2:
Summary of review of ICI use in dialysis patients, immune related Adverse Event(irAE) rate and patient outcomes. Figure created using biorender.com.
Future directions
Most clinical trials exclude patients with advanced kidney disease on dialysis. Due to limited data, these patients are excluded from potential lifesaving drugs. On reviewing the literature, it appears that ICI is reasonably well tolerated in this population, with modest survival benefit. Although our analysis has several limitations, it is the largest review to date looking at the outcomes of these patients. The safety signal of these drugs and the modest survival benefit seen should encourage oncologists to use these drugs in dialysis patients with close monitoring. Additional larger studies enrolling dialysis patients in early clinical trials are needed to define the incidence and outcomes of the irAEs.
CONCLUSIONS
As ICI therapy becomes increasingly widespread across the spectrum of cancer, its use in patients receiving dialysis is likely to increase. Based on available pharmacological information, ICIs require no dosing adjustment in ESKD patients. Our review demonstrates a similar incidence of irAEs in patients with ESKD receiving dialysis as compared with the general population (49%). Severe reactions graded as 3 and 4 were seen in 15 patients (16%). As such, it is important that these patients are monitored very closely for irAEs; however, the risk of these adverse events should not preclude patients on dialysis from receiving these therapies. The three most common groups of organ toxicities reported were hematologic, dermatologic and GI. Caution is warranted when prescribing ICIs in a patient on dialysis with a failed renal allograft, as there is a heightened risk for rejection and need for possible transplant nephrectomy. Cancer remission (complete and partial) was seen in close to 30% of patients. Stable disease was seen in 26% and progression of disease in ∼36% of patients. One-third of the patients died. Urothelial and renal cell cancer represented approximately half of all cases and accounted for ∼50% of the deaths reported. At present, comparison with general population outcomes on mortality and cancer progression cannot be definitively made. Additional data in the dialysis population and involvement in prospective studies are needed to better assess outcomes, particularly within specific cancer types.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ani Orchanian-Cheff, Medical Information Specialist (University Health Network), for assistance with the structured literature search.
CONFLICT OF INTEREST STATEMENT
K.D.J. is a consultant for Astex Pharmaceuticals, GlaxoSmithKline, ChemoCentryx, Chinook and Natera. K.D.J. receives honorarium from the International Society of Nephrology and American Society of Nephrology and is a paid contributor to UptoDate.com. M.Y. received grants from Kyowa Kirin, Chugai, Tanabe Mitsubishi Pharma and Boehringer Ingelheim. B.S. is a senior clinical investigator of the Research Foundation Flanders (1842919N) and received funding from the Foundation Against Cancer (Stichting tegen Kanker; C/2020/1380).
REFERENCES
- 1.Centanni M, Moes DJAR, Trocóniz IF. et al. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 2019; 58: 835–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipson EJ, Bagnasco SM, Moore J Jr. et al. Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med 2016; 374: 896–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boils CL, Aljadir DN, Cantafio AW.. Use of the PD-1 pathway inhibitor nivolumab in a renal transplant patient with malignancy. Am J Transplant 2016; 16: 2496–2497 [DOI] [PubMed] [Google Scholar]
- 4.Hirsch JS, Wanchoo R, Ng JH. et al. Use of immune checkpoint inhibitors in end stage kidney disease patients, single center experience and review of the literature. Kidney360 2020; 1: 399–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strohbehn IA, Lee M, Seethapathy H. et al. Safety and efficacy of immune checkpoint inhibitors in patients on dialysis: a retrospective case series. Am J Kidney Dis 2020; 76: 299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheun H, Kim M, Lee H. et al. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: a retrospective case series and literature review. Invest New Drugs 2019; 37: 579–583 [DOI] [PubMed] [Google Scholar]
- 7.Ryman JT, Meibohm B.. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol 2017; 6: 576–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Wang EQ, Balthasar JP.. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008; 84: 548–558 [DOI] [PubMed] [Google Scholar]
- 9.Keizer RJ, Huitema ADR, Schellens JHM. et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharm 2010; 49: 493–507 [DOI] [PubMed] [Google Scholar]
- 10.Counsilman CE, Jol–van der C, Stevens J. et al. Pharmacokinetics of rituximab in a pediatric patient with therapy-resistant nephrotic syndrome. Pediatr Nephrol 2015; 30: 1367–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacFarlane AW, Jillab M, Plimack ER. et al. PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer Immunol Res 2014; 2: 320–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glassman PM, Balthasar JP.. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med 2014; 11: 20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waki K, Yamada T, Yoshiyama K. et al. PD‐1 expression on peripheral blood T‐cell subsets correlates with prognosis in non‐small cell lung cancer. Cancer Sci 2014; 105: 1229–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Hayton WL, Robinson JM. et al. Kinetics of FcRn-mediated recycling of IgG and albumin in human: pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin Immunol 2007; 122: 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billiet T, Dreesen E, Cleynen I. et al. A genetic variation in the neonatal Fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am J Gastroenterol 2016; 111: 1438–1445 [DOI] [PubMed] [Google Scholar]
- 16.Sachs UJH, Socher I, Braeunlich CG. et al. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor α-chain promoter. Immunology 2006; 119: 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Masson E, Dai D. et al. Model-based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma: clinical pharmacology profiling of ipilimumab in advanced melanoma. Br J Clin Pharmacol 2014; 78: 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroh M, Winter H, Marchand M. et al. Clinical pharmacokinetics and pharmacodynamics of atezolizumab in metastatic urothelial carcinoma. Clin Pharmacol Ther 2017; 102: 305–312 [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, Suryawanshi S, Hruska M. et al. Assessment of nivolumab benefit–risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol 2017; 28: 2002–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitty DW, Hartley-Brown MA, Abate M. et al. Kidney transplantation in patients with multiple myeloma: narrative analysis and review of the last 2 decades. Nephrol Dial Transplant 2020; doi: 10.1093/ndt/gfaa361 [DOI] [PubMed] [Google Scholar]
- 21.Vitale MG, Baldessari C, Milella M. et al. Immunotherapy in dialysis-dependent cancer patients: our experience in patients with metastatic renal cell carcinoma and a review of the literature. Clin Genitourin Cancer 2019; 17: e903–e908 [DOI] [PubMed] [Google Scholar]
- 22.Tan B, Baxter M, Casasola R.. Acute renal transplant rejection following nivolumab therapy for metastatic melanoma. BMJ Case Rep 2021; 14: e238037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tachibana H, Kondo T, Ishihara H. et al. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma and end-stage renal disease at 2 centers. Clin Genitourin Cancer 2019; 17: e772–e778 [DOI] [PubMed] [Google Scholar]
- 24.Tabei T, Natsume I, Kobayashi K.. Successful treatment of metastatic clear cell carcinoma with nivolumab in a patient receiving dialysis treatment. Int J Urol 2017; 24: 708–710 [DOI] [PubMed] [Google Scholar]
- 25.Parisi A, Cortellini A, Cannita K. et al. Safe administration of anti-PD-L1 atezolizumab in a patient with metastatic urothelial cell carcinoma and end-stage renal disease on dialysis. Case Rep Oncol Med 2019; 2019: 3452762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osmán-García I, Congregado-Ruiz CB, Lendínez-Cano G. et al. Outcomes and safety of biweekly and monthly nivolumab in patients with metastatic renal cell carcinoma and dialysis: three case reports and literature review. Urol Int 2020; 104: 323–326 [DOI] [PubMed] [Google Scholar]
- 27.Osa A, Uenami T, Naito Y. et al. Monitoring antibody binding to T cells in a pembrolizumab‐treated patient with lung adenocarcinoma on hemodialysis. Thorac Cancer 2019; 10: 2183–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong M, Ibrahim AM, Bourassa-Blanchette S. et al. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer 2016; 4: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mroue A, Moujaess E, Kourie HR. et al. Exploring the knowledge gap of immune checkpoint inhibitors in chronic renal failure: a systematic review of the literature. Critic Rev Oncol Hematol 2021: 157: 103169. [DOI] [PubMed] [Google Scholar]
- 30.Morinaga R, Kawahara T, Miyoshi Y. et al. Longer control of nivolumab in metastatic renal cell carcinoma patients with end-stage kidney disease on dialysis. Case Rep Oncol 2019; 12: 608–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mejia CD, Frank AM, Singh P. et al. Immune checkpoint inhibitor therapy-associated graft intolerance syndrome in a failed kidney transplant recipient. Am J Transplant 2021; 21: 1322–1325 [DOI] [PubMed] [Google Scholar]
- 32.Lipson EJ, Naqvi FF, Loss MJ. et al. Kidney retransplantation after anti-programmed cell death-1 (PD-1)-related allograft rejection. Am J Transplant 2020; 20: 2264–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo MC, Su PJ, Huang CC. et al. Safety and efficacy of immune checkpoint inhibitors for patients with metastatic urothelial carcinoma and end-stage renal disease: experiences from real-world practice. Front Oncol 2020; 10: 584834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klajer E, Garnier L, Goujon M. et al. Targeted and immune therapies among patients with metastatic renal carcinoma undergoing hemodialysis: a systematic review. Semin Oncol 2020; 47: 103–116 [DOI] [PubMed] [Google Scholar]
- 35.Jain J, Stein J, Garje R.. Evaluation of checkpoint inhibitors in cancer patients with end-stage renal disease on hemodialysis: case series and review of the literature. J Immunother 2020; 43: 244–249 [DOI] [PubMed] [Google Scholar]
- 36.Iwaki T, Niimi A, Kano M. et al. Safe administration of ipilimumab plus nivolumab to a dialysis patient with renal cell carcinoma. IJU Case Rep 2021; 4: 32–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishizuka S, Sakata S, Yoshida C. et al. Successful treatment by pembrolizumab in a patient with end-stage renal disease with advanced non-small cell lung cancer and high PD-L1 expression. Respir Investig 2018; 56: 361–364 [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Diaz AB, Cunquero-Tomas AJ, Garcia-Medina A. et al. Are patients in haemodialysis good candidates for immunotherapy treatment? Melan Res 2019; 29: 553–555 [DOI] [PubMed] [Google Scholar]
- 39.Chang R, Shirai K.. Safety and efficacy of pembrolizumab in a patient with advanced melanoma on haemodialysis. BMJ Case Rep 2016; 2016: bcr2016216426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavalcante L, Amin A, Lutzky J.. Ipilimumab was safe and effective in two patients with metastatic melanoma and end-stage renal disease. Cancer Manag Res 2015; 7: 47–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlo MI, Feldman DR.. Response to nivolumab in a patient with metastatic clear cell renal cell carcinoma and end-stage renal disease on dialysis. Eur Urol 2016; 70: 1082–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyle SM, Ali N, Olszanski AJ. et al. Donor-derived metastatic melanoma and checkpoint inhibition. Transplant Proc 2017; 49: 1551–1554 [DOI] [PubMed] [Google Scholar]
- 43.Venkatachalam K, Malone AF, Heady B. et al. Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients. Transplantation 2019; 104: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 44.Alhamad T, Venkatachalam K, Linette GP. et al. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant 2016; 16: 1332–1333 [DOI] [PubMed] [Google Scholar]
- 45.Akturk HK, Alkanani A, Zhao Z. et al. PD-1 inhibitor immune-related adverse events in patients with preexisting endocrine autoimmunity. J Clin Endocrinol Metab 2018; 103: 3589–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami N, Mulvaney P, Danesh M. et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int 2021; 100: 196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito A, Hisano M, Takahashi M. et al. Complete response to nivolumab for metastatic renal cell carcinoma on hemodialysis patients; a case report. Urol Case Rep 2019; 28: 101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spain L, Higgins R, Gopalakrishnan K. et al. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol 2016; 27: 1135–1137 [DOI] [PubMed] [Google Scholar]
- 49.Duni A, Kitsos A, Liapis G. et al. Acute kidney transplant rejection after administration of nivolumab in a dialysis patient with a failed graft. Kidney Int Rep 2021; 6: 1459–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haslam A, Prasad V.. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019; 2: e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butler AM, Olshan AF, Kshirsagar AV. et al. Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996–2009. Am J Kidney Dis 2015; 65: 763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maisonneuve P, Agodoa L, Gellert R. et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999; 354: 93–99 [DOI] [PubMed] [Google Scholar]
- 53.Kitchlu A, Shapiro J, Amir E. et al. Representation of patients with chronic kidney disease in trials of cancer therapy. JAMA 2018; 319: 2437–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jose A, Yiannoullou P, Bhutani S. et al. Renal allograft failure after ipilimumab therapy for metastatic melanoma: a case report and review of the literature. Transplant Proc 2016; 48: 3137–3141 [DOI] [PubMed] [Google Scholar]
- 55.Ansari J, Ali M, Farrag A , Ali AM, Alhamad A. Efficacy of Nivolumab in a patient with metastatic renal cell carcinoma and end-stage renal disease on dialysis: case report and literature review. Case Reports in Immunology 2018; 10.1155/2018/1623957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramos-Casals M, Brahmer JR, Callahan MK. et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020; 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Indini A, Di Guardo L, Cimminiello C. et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019; 145: 511–521 [DOI] [PubMed] [Google Scholar]
- 58.Grangeon M, Tomasini P, Chaleat S. et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer 2019; 20: 201–207 [DOI] [PubMed] [Google Scholar]
- 59.Postow MA, Sidlow R, Hellmann MD.. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168 [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Jones-O’Connor M, Awadalla M. et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Treat Options Cardiovasc Med 2019; 21: 32. [DOI] [PubMed] [Google Scholar]
- 61.Cuzzubbo S, Javeri F, Tissier M. et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer 2017; 73: 1–8 [DOI] [PubMed] [Google Scholar]
- 62.Daste A, Domblides C, Gross-Goupil M. et al. Immune checkpoint inhibitors and elderly people: a review. Eur J Cancer 2017; 82: 155–166 [DOI] [PubMed] [Google Scholar]
- 63.Sui JD, Wang Y, Wan Y. et al. Risk of hematologic toxicities with programmed cell death-1 inhibitors in cancer patients: a meta-analysis of current studies. Drug Des Devel Ther 2018; 12: 1645–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brahmer JR, Lacchetti C, Schneider BJ. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018; 36: 1714–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haanen JBAG, Carbonnel F, Robert C. et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: iv119–iv142 [DOI] [PubMed] [Google Scholar]
- 66.Petrescu L, Stancu S, Tardei G. et al. Tuberculin skin test, interferon-gamma assay, and T cells subpopulations in hemodialysis patients. J Renal Nutr 2010; 20(5 Suppl): S109–S117 [DOI] [PubMed] [Google Scholar]
- 67.Chiu YL, Shu KH, Yang FJ. et al. A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: the iESRD study. Immun Ageing 2018; 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnett R, Barta VS, Jhaveri KD.. Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med 2017; 376: 191–192 [DOI] [PubMed] [Google Scholar]
- 69.Lavacchi D, Pellegrini E, Palmieri VE. et al. Immune checkpoint inhibitors in the treatment of renal cancer: current state and future perspective. Int J Mol Sci 2020; 21: 4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrscher H, Robert C.. Immune checkpoint inhibitors in melanoma in the metastatic, neoadjuvant, and adjuvant setting. Curr Opin Oncol 2020; 32: 106–113 [DOI] [PubMed] [Google Scholar]
- 71.Massarelli E, Papadimitrakopoulou V, Welsh J. et al. Immunotherapy in lung cancer. Trans Lung Cancer Res 2014; 3: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.La J, Cheng D, Brophy MT. et al. Real-world outcomes for patients treated with immune checkpoint inhibitors in the veterans affairs system. JCO Clin Cancer Inform 2020; 4: 918–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fiala O, Sorejs O, Sustr J. et al. Immune-related adverse effects and outcome of patients with cancer treated with immune checkpoint inhibitors. Anticancer Res 2020; 40: 1219–1227 [DOI] [PubMed] [Google Scholar]
- 74.Pang PYK, Teow CKJ, Huang MJ. et al. Long-term prognosis in patients with end-stage renal disease after coronary artery bypass grafting. J Thorac Dis 2020; 12: 6722–6730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilbertson DT, Rothman KJ, Chertow GM. et al. Excess deaths attributable to influenza-like illness in the ESRD population. J Am Soc Nephrol 2019; 30: 346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ng JH, Hirsch JS, Wanchoo R. et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int 2020; 98: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naylor KL, Kim SJ, McArthur E. et al. Mortality in incident maintenance dialysis patients versus incident solid organ cancer patients: a population-based cohort. Am J Kidney Dis 2019; 73: 765–776 [DOI] [PubMed] [Google Scholar]
- 78.Yanik EL, Clarke CA, Snyder JJ. et al. Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals. J Am Soc Nephrol 2016; 27: 1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan GCW, Chan SK, Cheun et al. Cancer incidence and mortality in chronic dialysis population: a multicenter cohort study. Am J Neuroradiol 2016; 43: 153–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.