Abstract
Background
Disorders of calcium and phosphorus metabolism have been reported to be associated with all-cause and cardiovascular mortality in patients requiring long-term dialysis therapy. However, its role in disease progression is not well established in patients without dialysis, especially in immunoglobulin A (IgA) nephropathy. We aim to evaluate the association of serum phosphorus and calcium and progression of IgA nephropathy.
Methods
We assessed 2567 patients with IgA nephropathy at the First Affiliated Hospital, College of Medicine, Zhejiang University. Serum phosphorus and calcium were collected at the time of kidney biopsy and at each visit. The associations of serum phosphorus and serum calcium with composite kidney disease progression events, defined as 50% estimated glomerular filtration rate (eGFR) decline and kidney failure, were examined using Cox models and restricted cubic splines.
Results
During a median follow-up of 31.9 months, 248 (10%) patients reached composite kidney disease progression events. A linear relationship was observed between serum phosphorus and composite kidney disease progression events. With higher levels of phosphorus, the risk of kidney disease progression events increased {hazard ratio [HR] 3.54 [95% confidence interval (CI) 1.37–9.12]; P = 0.009}. Compared with the first quartile group, the HR of kidney disease progression events was 1.66 (95% CI 0.91–301) for the second quartile, 1.67 (95% CI 0.91–3.08) for the third and 2.62 (95% CI 1.44–4.77) for the fourth (P for trend = 0.002). The association between serum phosphorus and kidney disease progression was detectable [HR 8.94 (95% CI 2.33–34.21); P = 0.001] within the subgroup with eGFR <60 mL/min/1.73 m2 but not among patients with eGFR ≥60 mL/min/1.73 m2 [HR 0.87 (95% CI 0.17–4.44); P = 0.87]. After adjustment for traditional risk factors, a higher level of serum calcium was not associated with kidney disease progression events [HR 0.33 (95% CI 0.10–1.09)].
Conclusions
Higher serum phosphorus rather than serum calcium was independently associated with kidney disease progression in IgA nephropathy.
Keywords: IgA nephropathy, kidney disease progression, serum calcium, serum phosphorus
INTRODUCTION
Immunoglobulin (IgA) nephropathy is the most common primary glomerulonephritis and the major cause of kidney failure requiring kidney replacement therapy (KRT) [1–3]. IgA nephropathy is characterized by a highly variable clinical course ranging from a benign incidental condition to rapidly progressive kidney failure. Impaired renal function, persistent proteinuria, hypertension, hematuria and the MEST-C [mesangial (M) and endocapillary (E) hypercellularity, segmental sclerosis (S), interstitial fibrosis/tubular atrophy (T) and crescents (C)] score have been established as risk factors for long-term outcomes in patients with IgA nephropathy [4–11]. In addition to these traditional predictors, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that new biomarkers are still needed to further improve the prediction of renal outcomes in patients with IgA nephropathy [12].
Disorders of calcium and phosphorus metabolism are the common complications in patients with advanced chronic kidney disease (CKD) and associated with mortality and cardiovascular events in patients requiring long-term dialysis therapy [13–17]. However, the roles of serum calcium and phosphorus in kidney disease progression are still not well established, especially in patients who are not undergoing dialysis. Even the KDIGO guidelines recommend correcting for disorders of calcium and phosphorus metabolism when making predictions regarding death or cardiovascular events rather than renal outcomes [18]. IgA nephropathy is one of the most common causes of CKD and calcium and phosphorus metabolism disorders are commonly observed in patients with IgA nephropathy. However, their roles in long-term outcomes are unclear. In this study we aimed to evaluate the effects of serum phosphorus and calcium levels on kidney disease progression in patients with IgA nephropathy.
MATERIALS AND METHODS
Study participants
A total of 2567 patients diagnosed between 2002 and 2019 with IgA nephropathy in the First Affiliated Hospital, Zhejiang University School of Medicine were enrolled in this study. The diagnosis of IgA nephropathy was based on the dominant deposition of IgA in the mesangial area as observed with immunofluorescence; patients with secondary IgA nephropathy, such as IgA vasculitis, systemic lupus erythematosis and rheumatic disease, were excluded. All patients were followed up regularly every 3−12 months.
The study was approved by the local ethics committees and conducted in accordance with the principles of the Declaration of Helsinki.
Data collection and patient follow-up
Clinical data, including age, sex, 24-h urine protein excretion, systolic and diastolic blood pressure, serum creatine level, serum albumin level at the time of kidney biopsy and serum phosphorus and calcium levels at the time of kidney biopsy and at each visit were collected. Histopathologic elements were evaluated according to the Oxford classification. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [19]. Mean arterial pressure (MAP) was calculated as the sum of one-third of pulse pressure and the diastolic blood pressure.
Baseline was defined as the time of kidney biopsy. In addition to baseline serum phosphorus and calcium levels, we also included time-varying phosphorus and time-varying calcium levels in the analysis. A 12-month interval was used for the time-varying method analysis. The average serum phosphorus level was calculated every 12 months during follow-up for each patient and then the average values during the entire follow-up period were used in the time-varying method analysis. For the analysis of the associations between serum phosphorus and serum calcium levels and the composite kidney disease progression outcome, patients were divided into four groups according to the quartiles of baseline serum calcium levels, time-varying calcium levels, serum phosphorus levels and time-varying phosphorus levels. End-stage kidney disease (ESKD) was defined as an eGFR <15 mL/min/1.73 m2 or the need for KRT (including hemodialysis, peritoneal dialysis or kidney transplantation). For the survival analysis, the composite endpoint was defined as a 50% decrease in the eGFR or ESKD.
Statistical analyses
Quantitative variables are presented as means ± standard devaitions (SDs) and were compared using a t-test for normally distributed data. Non-normally distributed data are summarized as medians and interquartile ranges (IQRs) and were compared using the Mann–Whitney test. Categorical data are expressed as percentages or frequencies and were assessed with the chi-squared test. To determine the associations of serum phosphorus and calcium levels with kidney disease progression, serum phosphorus and serum calcium levels were modeled using restricted cubic splines after multivariable adjustment. Furthermore, Cox proportional hazards models were used to evaluate the relationships between the levels of serum phosphorus and serum calcium and the risk of kidney disease progression events. Traditional risk factors (age, sex, proteinuria, MAP, basal eGFR and MEST-C scores) were adjusted in multivariable Cox models. SPSS 24.0 (IBM, Armonk, NY, USA) and Stata 14.0 (StataCorp, College Station, TX, USA) were used for the statistical analysis and P-values <0.05 were considered statistically significant.
RESULTS
Clinical data
This study included 2567 patients with IgA nephropathy. The clinical characteristics are summarized in Table 1. There were 1226 (48%) men with a mean age of 38.14 ± 12.25 years. The initial proteinuria level was 1.07 g/day (IQR 0.53–2.23) and the eGFR was 84.83 ± 29.71 mL/min/1.73 m2. The serum phosphorus and calcium levels were 1.21 ± 0.20 and 2.21 ± 0.14 mmol/L, respectively. After a median follow-up of 31.90 months (IQR 14.77–65.00), 248 (10%) patients reached composite kidney outcomes, including 222 (9%) patients with a 50% decrease in the eGFR and 184 (7%) with kidney failure.
Table 1.
Clinical characteristics of patients with IgA nephropathy
| Characteristics | Value (N = 2567) |
|---|---|
| Baseline | |
| Male, n (%) | 1226 (48) |
| Age (years), mean ± SD | 38.14 ± 12.25 |
| MAP (mmHg), mean ± SD | 95.26 ± 14.27 |
| Proteinuria (g/day), median (IQR) | 1.07 (0.53–2.23) |
| eGFR (mL/min/1.73 m2), mean ± SD | 84.83 ± 29.71 |
| Serum calcium (mmol/L), mean ± SD | 2.21 ± 0.14 |
| Serum phosphorus (mmol/L), mean ± SD | 1.21 ± 0.20 |
| Serum PTH (pg/mL), median (IQR) | 36.40 (23.90–53.30) |
| Oxford classification, n (%) | |
| M1 | 543 (21.15) |
| E1 | 274 (10.67) |
| S1 | 1660 (64.67) |
| T1–T2 | 272 (10.60) |
| C1–C2 | 1252 (48.77) |
| CKD stages, n (%) | |
| 1 | 1221 (47.57) |
| 2 | 734 (28.59) |
| 3 | 538 (20.96) |
| 4 | 74 (2.88) |
| Follow-up and outcome | |
| Follow-up duration (months), median (IQR) | 31.90 (14.77–65.00) |
| 50% eGFR decline, n (%) | 222 (9) |
| Kidney failure, n (%) | 184 (7) |
| Composite outcome, n (%) | 248 (10) |
The composite outcome was defined as a 50% decrease in the eGFR or kidney failure. M, mesangial hypercellularity; E1, endocapillary hypercellularity; S, segmental glomerulosclerosis/adhesion; T, severity of tubular atrophy/interstitial fibrosis; C, presence of crescent.
Association of serum phosphorus levels with kidney disease progression in patients with IgA nephropathy
The characteristics of patients stratified according to quartiles of serum phosphorus are summarized in Supplementary data, Table S1 (first quartile: 0.35–1.07; second quartile: 1.08–1.19; third quartile: 1.20–1.32; fourth quartile: 1.33–2.24 mmol/L). The levels of proteinuria and the incidence rate of renal outcome were higher in patients with higher levels of serum phosphorus.
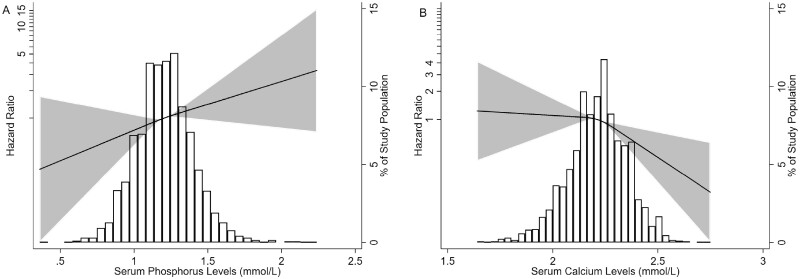
To evaluate the association between serum phosphorus levels and kidney disease progression, we first modeled baseline serum phosphorus levels as a continuous variable using restricted cubic splines. A linear relationship was observed and patients with higher serum phosphorus levels had poor renal outcomes (Figure 1A). In the Cox proportional hazards model, serum phosphorus was an independent risk factor for the composite kidney disease progression outcome {hazard ratio [HR] 3.54 [95% confidence interval (CI) 1.37–9.12]; P = 0.009} after adjustment for sex, age, proteinuria, initial MAP, eGFR and MEST-C scores (Table 2). Using the first quartile of serum phosphorus levels as the reference, the risk of composite kidney disease progression events was higher in patients with higher serum phosphorus levels: the HRs were 1.66 (95% CI 0.91–3.01) in the second quartile, 1.67 (95% CI 0.91–3.08) in the third quartile and 2.62 (95% CI 1.44–4.77) in the fourth quartile (P-value for trend = 0.002) (Table 2).
FIGURE 1:
Association of (A) serum phosphorusand (B) serum calciumlevels with the composite kidney disease progression outcome. Three knots at the 25th, 50th and 75th percentiles were used to model restricted cubic splines. Composite kidney disease progression events were defined as 50% eGFR decline or ESKD. The solid line represents the estimated HR, the shaded area represents the 95% CI, the histogram represents the distribution of serum phosphorus and serum calcium. Models were adjusted for age, sex, proteinuria, hypertension, eGFR and Oxford classification (MEST-C scores).
Table 2.
Association of serum phosphorus levels with the composite kidney disease progression outcome
| Groups |
|
|||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |
| Composite kidney disease progression outcome according to the level of serum phosphorus, HR (95% CI) | 3.67 (2.04–6.63) | 4.51 (2.45–8.28) | 2.58 (1.17–5.68) | 3.54 (1.37–9.12) |
| P-value | <0.001 | <0.001 | 0.02 | 0.009 |
| Phosphorus quartiles (mmol/L, range), HR (95% CI) | ||||
| First (0.35 − 1.07) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Second (1.08–1.19) | 1.66 (1.14–2.44) | 1.72 (1.17–2.53) | 1.57 (0.95–2.57) | 1.66 (0.91–3.01) |
| P-value | 0.009 | 0.006 | 0.08 | 0.098 |
| Third (1.20–1.32 | 1.54 (1.04–2.27) | 1.70 (1.15–2.52) | 1.48 (0.90–2.43) | 1.67 (0.91–3.08) |
| P-value | 0.03 | 0.009 | 0.13 | 0.10 |
| Fourth (1.33–2.24) | 1.94 (1.33–2.83) | 2.18 (1.48–3.19) | 1.95 (1.20–3.16) | 2.62 (1.44–4.77) |
| P-value | 0.001 | <0.001 | 0.001 | <0.002 |
| P-value for trend | 0.002 | <0.001 | 0.01 | 0.002 |
CKD progression events were a 50% decrease in the eGFR or kidney failure. Model 1 was adjusted for sex and age, and sex was expressed as a dichotomous variable. Model 3 was adjusted for covariates in Model 2 and Oxford classification (MEST-C scores)
Next, we evaluated the role of baseline serum phosphorus in kidney disease progression events according to renal function. Patients were divided into two groups: eGFR <60 mL/min/1.73 m2 and eGFR ≥60 mL/min/1.73 m2. A total of 151 (24.35%) patients in the eGFR <60 mL/min/1.73 m2 group and 97 (4.98%) patients in the eGFR ≥60 mL/min/1.73 m2 group reached the composite kidney disease progression outcome (P < 0.001). After adjustment for traditional risk factors, including basal eGFR, age, sex, proteinuria, MAP and MEST-C scores, the serum phosphorus level was an independent risk factor for the composite kidney disease progression outcome [HR 8.94 (95% CI 2.33–34.21); P = 0.001] in patients with eGFR <60 mL/min/1.73 m2 and was not associated with a poor renal outcome [HR 0.87 (95% CI 0.17–4.44); P = 0.87] in the eGFR ≥60 mL/min/1.73 m2 group.
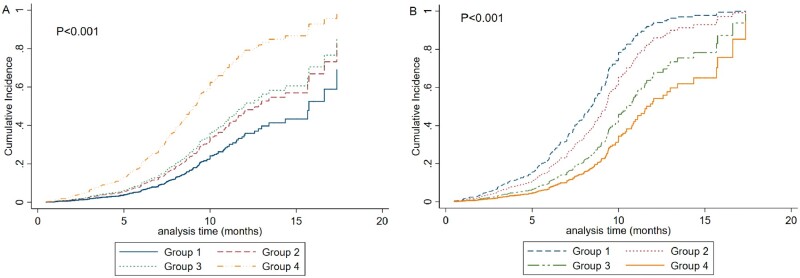
In addition to baseline serum phosphorus levels, we also included time-varying phosphorus levels in the analysis. As shown in Figure 2A, patients in the fourth quartile of time-varying phosphorus levels had the highest incidence of kidney disease progression events, followed by those in the third quartile and the second quartile. The patients in the first quartile had the lowest incidence of the composite kidney disease progression outcome (P < 0.001).
FIGURE 2:
Cumulative incidence of the composite kidney disease progression outcome in patients according to the quartiles of (A) time-varying phosphorusand (B) time-varying calciumlevels. (A) Group 1: first quartile, time-varying phosphorus <1.01; Group 2: second quartile, time-varying phosphorus ≥1.01–≤1.12; Group 3: third quartile, time-varying phosphorus >1.12–≤1.23; Group 4: fourth quartile, time-varying phosphorus >1.23. (B) Group 1: first quartile, time-varying calcium ≤2.12; Group 2: second quartile, time-varying calcium >2.12–≤2.21; Group 3: third quartile, time-varying phosphorus >2.21–≤ 2.3; Group 4: fourth quartile, time-varying phosphorus >2.31.
Association of serum calcium levels with kidney disease progression in patients with IgA nephropathy
Compared with lower levels of serum calcium, patients in the high group showed lower proteinuria and higher eGFR (Supplementary Table S2) (first quartile: 1.64–2.12, second quartile: 2.13–2.21, third quartile: 2.22–2.30 and fourth quartile: 2.31–2.75 mmol/L). As shown in Figure 1B, after adjustment for age, sex, initial proteinuria, MAP, eGFR and MEST-C scores, the risk of the composite kidney disease progression outcome was lower in patients with higher levels of serum calcium. In the Cox regression model, serum calcium was first analyzed as a continuous variable, and we found that a higher level of serum calcium was not associated with poor renal outcomes after adjustment for traditional risk factors [HR 0.33 (95% CI 0.10–1.09)] (Table 3). Furthermore, patients were divided into four groups according to the quartiles of serum calcium level. Compared with the patients with levels in the first quartile, the risk of the composite kidney disease outcome was 1.34 for those with levels in the second quartile, 1.03 for those with levels in the third quartile and 0.80 for those with levels in the fourth quartile of serum calcium levels after adjustment for age, sex, proteinuria, MAP and eGFR (P-value for trend = 0.27) (Table 3).
Table 3.
Association of serum calcium levels with the composite kidney disease progression outcome
| Groups |
|
|||
|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | |
| Composite kidney disease progression outcome according to the level of serum calcium, HR (95% CI) | 0.18 (0.08–0.39) | 0.22 (0.10–0.49) | 0.22 (0.08–0.61) | 0.33 (0.10–1.09) |
| P-value | <0.001 | <0.001 | 0.004 | 0.068 |
| Calcium quartiles (mmol/L, range), HR (95% CI) | ||||
| First (1.64–2.12) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Second (2.13–2.21) | 0.77 (0.54–1.10) | 0.80 (0.56–1.15) | 1.18 (0.77–1.82) | 1.34 (0.79–2.25) |
| P-value | 0.16 | 0.23 | 0.45 | 0.28 |
| Third (2.22–2.30) | 0.59 (0.41–0.83) | 0.64 (0.44–0.91) | 0.89 (0.57–1.40) | 1.03 (0.59–1.80) |
| P-value | 0.003 | 0.01 | 0.61 | 0.91 |
| Fourth (2.31–2.75) | 0.46 (0.32–0.64) | 0.50 (0.35–0.71) | 0.63 (0.39–1.01) | 0.80 (0.47–1.37) |
| P-value | <0.001 | <0.001 | 0.05 | 0.42 |
| P-value for trend | <0.001 | <0.001 | 0.03 | 0.27 |
CKD progression events were a 50% decrease in the eGFR or kidney failure. Model 1 was adjusted for sex and age, and sex was expressed as a dichotomous variable. Model 2 was adjusted for the covariates in Model 1 and mean arterial pressure, log-transformed proteinuria and eGFR. Model 3 was adjusted for covariates in Model 2 and Oxford classification (MEST-C scores).
We also evaluated the role of serum calcium in kidney disease progression in the subgroups of patients with eGFR <60 mL/min/1.73 m2 and eGFR ≥60 mL/min/1.73 m2 and found that after adjustment for traditional risk factors including sex, age, proteinuria, MAP, basal eGFR, parathyroid hormone and MEST-C scores, serum calcium was not associated with the composite kidney disease progression outcome in the group with eGFR <60 mL/min/1.73 m2 [HR 0.99 (95% CI 0.981–1.001); P = 0.07] and eGFR ≥60 mL/min/1.73 m2 [HR 1.002 (95% CI 0.995–1.010); P = 0.529].
DISCUSSION
Calcium and phosphorus metabolism disorders are typical in patients with CKD and are associated with mortality in patients undergoing maintenance dialysis [14, 20–22]. IgA nephropathy is the major cause of kidney failure requiring KRT [10, 23–25]. However, the roles of calcium and phosphorus metabolism disorders in kidney disease progression in patients with IgA nephropathy who are not undergoing dialysis are still not well established. In this large-sample study with 2567 nondialysis-dependent IgA nephropathy patients and 248 patients who experienced the composite kidney disease progression outcome, we found that high levels of serum phosphorus were strongly associated with kidney disease progression events in patients with IgA nephropathy, especially in those with eGFR <60 mL/min/1.73 m2. Serum calcium was not associated with kidney disease progression events. These results suggest the potential importance of therapy targeting serum phosphorus in nondialysis-dependent IgA nephropathy patients.
Calcium and phosphorus metabolism disorders are hallmarks of CKD [26]. In multiple studies, the serum phosphorus level was established as an important risk factor for cardiovascular events and all-cause mortality in patients with CKD. In an American cohort of 35 114 patients undergoing hemodialysis with a median follow-up of 1.3 years, among the patients with higher residual renal urea clearance there was an elevated mortality risk in patients with higher serum phosphorus levels [20]. However, in a cohort of 10 672 CKD subjects with a median follow-up of 2.3 years, the authors found no significant association between baseline serum phosphorus levels and all-cause mortality or the development of ESKD [27]. Rivara et al. [14, 28] found an inverse association between serum phosphorus levels and mortality in 129 076 patients undergoing maintenance dialysis. Based on these conflicting results, whether a dose–response relationship exists in the association between the serum phosphorus level and kidney disease outcomes in patients, especially in those not undergoing dialysis, is unclear. Current evidence does not show that there is a benefit to maintaining normal serum phosphate levels in IgA nephropathy patients who are not receiving dialysis [18]. A high serum phosphorus level is not included in the risk evaluation system for IgA nephropathy in the KDIGO guidelines due to the lack of robust evidence [12].
In this much larger study with 2567 IgA nephropathy patients, we confirmed that higher levels of serum phosphorus were strongly associated with the development of kidney disease progression, especially in those with eGFR ≤60 mL/min/1.73 m2. Among those with eGFR >60 mL/min/1.73 m2, a higher level of serum phosphorus was not a risk factor for kidney disease progression. To exclude the bias of serum phosphorus detection at baseline, we also included time-varying serum phosphorus during the entire follow-up period in the analysis and found consistent results. These results highlight the importance of reducing serum phosphorus levels in patients with IgA nephropathy with CKD Stages G3a–G5D.
The role of serum calcium levels in kidney disease progression is less clear. Foley et al. [29] demonstrated that a low serum calcium was independently associated with morbidity and mortality in a cohort of 433 ESKD patients during an average of 41 months of follow-up. New data support associations between higher calcium concentrations and increased mortality and nonfatal cardiovascular events in patients with CKD [18, 30, 31]. In a large Japanese CKD Stage 5D case–cohort study, Fukagawa et al. [32] indicated that the serum calcium level was a predictor of mortality in hemodialysis patients with secondary hyperparathyroidism. Until now there has been a lack of robust evidence to confirm the role of serum calcium in kidney disease progression in patients with all stages of CKD. In our study with 2567 IgA nephropathy patients with all stages of CKD, we found that the serum calcium level was not associated with kidney disease progression events in patients with eGFR ≥60 mL/min/1.73 m2 and those with eGFR <60 mL/min/1.73 m2. Given these findings, an individualized approach should be used for the correction of abnormal serum calcium levels.
In some studies, the albumin-corrected calcium level was used in the analysis. We also included albumin-corrected calcium in our research and found that after correction for albumin, the proportion of patients with hypercalcemia was ∼12%, which was high and inconsistent with the clinical situation, thus these data are not shown in our study.
The strengths of this study include the large sample size and a large number of kidney disease progression events. To the best of our knowledge, this is the first study to evaluate the role of disorders of calcium and phosphorus metabolism in kidney disease progression in patients with IgA nephropathy who were not undergoing dialysis; our study included patients with all stages of CKD rather than only patients with CKD Stages 3–5. All serum phosphorus and calcium values during follow-up rather than just the baseline values were included in the analysis, and the findings remained consistent. However, this study also has some limitations. First, the data came from a single center and the follow-up time was relatively short. Second, >90% of patients received renin–angiotensin–aldosterone system inhibitors (RAASis), steroids and other immunosuppressant therapy in our study, therefore we did not include the use of RAASis and immunosuppressives in the analysis. Third, since <25% patients were in CKD Stages 3–4, vitamin D was not tested routinely. Phosphate binders were not used routinely. The findings still need further confirmation in other populations.
In conclusion, in our large-sample study, we demonstrated that higher levels of serum phosphorus were associated with kidney disease progression in IgA nephropathy patients, especially in those with eGFR ≤60 mL/min/1.73 m2. Furthermore, serum calcium was not associated with kidney disease progression events in IgA nephropathy patients who were not undergoing dialysis.
FUNDING
This work was supported by grants LY19H050007 and LQ19H050009 from the Zhejiang Provincial Natural Science Foundation of China and grant 2016KYA087 from the Zhejiang Medical and Health Science and Technology Project.
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no other relevant financial interests.
Supplementary Material
REFERENCES
- 1.Chen P, Yu G, Zhang X. et al. Plasma galactose-deficient IgA1 and C3 and CKD progression in IgA nephropathy. Clin J Am Soc Nephrol 2019; 14: 1458–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy M, Berger J.. Worldwide perspective of IgA nephropathy. Am J Kidney Dis 1988; 12: 340–347 [DOI] [PubMed] [Google Scholar]
- 3.McGrogan A, Franssen CF, de Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011; 26: 414–430 [DOI] [PubMed] [Google Scholar]
- 4.Zhao YF, Zhu L, Liu LJ. et al. Measures of urinary protein and albumin in the prediction of progression of IgA nephropathy. Clin J Am Soc Nephrol 2016; 11: 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimarchi H, Barratt J, Cattran DC. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 6.Berthoux F, Mohey H, Laurent B. et al. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 2011; 22: 752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour SJ, Reich HN.. Risk stratification of patients with IgA nephropathy. Am J Kidney Dis 2012; 59: 865–873 [DOI] [PubMed] [Google Scholar]
- 8.Goto M, Wakai K, Kawamura T. et al. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant 2009; 24: 3068–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour SJ, Espino-Hernandez G, Reich HN. et al. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int 2016; 89: 167–175 [DOI] [PubMed] [Google Scholar]
- 10.Yu GZ, Guo L, Dong JF. et al. Persistent hematuria and kidney disease progression in IgA nephropathy: a cohort study. Am J Kidney Dis 2020; 76: 90–99 [DOI] [PubMed] [Google Scholar]
- 11.Sevillano AM, Gutiérrez E, Yuste C. et al. Remission of hematuria improves renal survival in IgA nephropathy. J Am Soc Nephrol 2017; 28: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floege J, Barbour SJ, Cattran DC. et al. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 268–280 [DOI] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Kuwae N, Regidor DL. et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006; 70: 771–780 [DOI] [PubMed] [Google Scholar]
- 14.Rivara MB, Ravel V, Kalantar-Zadeh K. et al. Uncorrected and albumin-corrected calcium, phosphorus, and mortality in patients undergoing maintenance dialysis. J Am Soc Nephrol 2015; 26: 1671–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wald R, Sarnak MJ, Tighiouart H. et al. Disordered mineral metabolism in hemodialysis patients: an analysis of cumulative effects in the Hemodialysis (HEMO) Study. Am J Kidney Dis 2008; 52: 531–540 [DOI] [PubMed] [Google Scholar]
- 16.Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 17.Young EW, Akiba T, Albert JM. et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44: 34–38 [DOI] [PubMed] [Google Scholar]
- 18.Ketteler M, Block GA, Evenepoel P. et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the Kidney Disease: Improving Global Outcomes 2017 clinical practice guideline update. Ann Intern Med 2018; 168: 422–430 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Obi Y, Streja E. et al. Association of parameters of mineral bone disorder with mortality in patients on hemodialysis according to level of residual kidney function. Clin J Am Soc Nephrol 2017; 12: 1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tentori F, Blayney MJ, Albert JM. et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 22.Kimata N, Akiba T, Pisoni RL. et al. Mineral metabolism and haemoglobin concentration among haemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2005; 20: 927–935 [DOI] [PubMed] [Google Scholar]
- 23.Le W, Liang S, Hu Y. et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 2012; 27: 1479–1485 [DOI] [PubMed] [Google Scholar]
- 24.D'Amico G.Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 2004; 24: 179–196 [DOI] [PubMed] [Google Scholar]
- 25.Nair R, Walker PD.. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int 2006; 69: 1455–1458 [DOI] [PubMed] [Google Scholar]
- 26.Da J, Xie X, Wolf M. et al. Serum phosphorus and progression of CKD and mortality: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 66: 258–265 [DOI] [PubMed] [Google Scholar]
- 27.Mehrotra R, Peralta CA, Chen SC. et al. No independent association of serum phosphorus with risk for death or progression to end-stage renal disease in a large screen for chronic kidney disease. Kidney Int 2013; 84: 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haynes R, Wheeler DC.. Chronic kidney disease: does serum phosphate predict death and ESRD in CKD patients? Nat Rev Nephrol 2013; 9: 438–439 [DOI] [PubMed] [Google Scholar]
- 29.Foley RN, Parfrey PS, Harnett JD. et al. Hypocalcemia, morbidity, and mortality in end-stage renal disease. Nat Rev Nephrol 2013; 9: 438–439 [DOI] [PubMed] [Google Scholar]
- 30.Nakano C, Hamano T, Fujii N. et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol 2012; 7: 810–819 [DOI] [PubMed] [Google Scholar]
- 31.Gallieni M, Caputo F, Filippini A. et al. Prevalence and progression of cardiovascular calcifications in peritoneal dialysis patients: a prospective study. Bone 2012; 51: 332–337 [DOI] [PubMed] [Google Scholar]
- 32.Fukagawa M, Kido R, Komaba H. et al. Abnormal mineral metabolism and mortality in hemodialysis patients with secondary hyperparathyroidism: evidence from marginal structural models used to adjust for time-dependent confounding. Am J Kidney Dis 2014; 63: 979–987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.