Abstract
Background
In patients with end-stage kidney disease (ESKD), home dialysis offers socio-economic and health benefits compared with in-centre dialysis but is generally underutilized. We hypothesized that the pre-dialysis course and institutional factors affect the choice of dialysis modality after dialysis initiation (DI).
Methods
The Peridialysis study is a multinational, multicentre prospective observational study assessing the causes and timing of DI and consequences of suboptimal DI. Clinical and biochemical data, details of the pre-dialytic course, reasons for DI and causes of the choice of dialysis modality were registered.
Results
Among 1587 included patients, 516 (32.5%) were judged unsuitable for home dialysis due to contraindications [384 ( 24.2%)] or no assessment [106 (6.7%); mainly due to late referral and/or suboptimal DI] or death [26 (1.6%)]. Older age, comorbidity, late referral, suboptimal DI, acute illness and rapid loss of renal function associated with unsuitability. Of the remaining 1071 patients, 700 (65.4%) chose peritoneal dialysis (61.7%) or home haemodialysis (HD; 3.6%), while 371 (34.6%) chose in-centre HD. Somatic differences between patients choosing home dialysis and in-centre dialysis were minor; factors linked to the choice of in-centre dialysis were late referral, suboptimal DI, acute illness and absence of a ‘home dialysis first’ institutional policy.
Conclusions
Given a personal choice with shared decision making, 65.4% of ESKD patients choose home dialysis. Our data indicate that the incidence of home dialysis potentially could be further increased to reduce the incidence of late referral and unplanned DI and, in acutely ill patients, by implementing an educational programme after improvement of their clinical condition.
Keywords: glomerular filtration rate, haemodialysis, peritoneal dialysis, pre-dialysis, uraemia
INTRODUCTION
Home dialysis modalities, peritoneal dialysis (PD) and home haemodialysis (HD) are generally considered to be beneficial as compared with in-centre HD. PD does not require blood access and results in steady, low metabolite levels and is characterized by lower cost, less time for dialysis, less disturbance of social life and markedly improved travel opportunities compared with in-centre HD. Furthermore, mortality was reported to be lower with PD during the first 2–3 years of dialysis [1, 2], although other studies do not confirm this finding [3–5]. It was suggested that differences are due to analytic difficulties and insufficient correction for differences between PD and in-centre patients [3, 5]. However, the subject is controversial [6].
More frequent dialysis as offered by home HD has been reported to be associated with improved electrolyte control, reduced left ventricular hypertrophy and other signs of heart disease, better quality of life and reduced mortality [7–10]. The Frequent Hemodialysis Network randomized study showed a 46% reduction in mortality compared with in-centre HD [11]. Home HD requires more training than PD, is more time-consuming and offers fewer travel opportunities, and is generally less utilized. In one study [12], most physicians and health workers considered home dialysis modalities to be preferable to facility modalities.
While home dialysis incidence, including that of PD, varies considerably throughout the world, it remains low in most countries [13, 14]. There are many possible causes, recently reviewed by Van et al. [15] and Chan et al. [16]. Economic considerations play a major role in many countries [17] and PD is actively promoted in some countries as a cheaper modality [18]. Nevertheless, PD prevalence has been falling in many low- and middle-income countries (LMICs). Possible factors include the introduction of private–public partnerships for end-stage kidney disease (ESKD) care, where increased HD capacity leads to an increase in in-centre HD utilization. The absence of well-designed health economic studies perpetuates the myth of lower cost of in-centre HD in LMICs.
Despite medical and socio-economic advantages, PD and home HD remain underutilized dialysis modalities worldwide. As non-medical barriers are thought to play an important role [19, 20], many countries have introduced programmes to increase the use of home dialysis by general stimulatory measures [17]. Recently, the USA introduced the Advancing American Kidney Health programme, aimed at reaching the target of having 80% of incident patients with ESKD begin care with a pre-emptive kidney transplant or home dialysis by 2025 [21]. The successful implementation of such programmes will require an improved understanding of factors influencing patient pathways during the course of pre-dialysis care, especially within renal care management models that are built on a patient-centric approach with shared decision-making involving patients, nurses, physicians and other caregivers.
The effects of pre-dialysis care and early dialysis education on subsequent outcomes of dialysis treatment have been studied extensively. In addition to important effects such as a lower incidence of suboptimal dialysis initiation (DI) and reduced morbidity and mortality, the presence of programmes for pre-dialysis care and early dialysis education is generally associated with a higher incidence of home modalities, particularly PD [22–26]. A meta-analysis of studies in this area concluded that a pre-dialysis education programme was associated with a doubling of PD incidence [27].
The Peridialysis study is a prospective, multicentre, international observation study of the relevance of pre-dialysis renal care on the causes and timing of DI, modality choice and clinical outcomes [28, 29]. This study focuses on modality choice and its aim was to explore factors during the pre-dialysis course, including patient-related factors and organizational institutional factors, some of which could be potentially modifiable, that affect the choice of dialysis modality before and after DI.
MATERIALS AND METHODS
This observational multinational multicentre prospective study comprised 1588 ESKD patients who started dialysis over a 3-year period at 15 nephrology departments from seven Nordic and Baltic countries. The methodology of the Peridialysis project has been previously described [28, 29]. All centres delivered both PD and HD. They were publicly financed, with no dialysis costs to the patient, but with varying financial support for medicine costs. One centre reported suboptimal availability of HD stations, but otherwise there were no restrictions of access to dialysis care. They all had a developed and working multidisciplinary pre-dialysis care structure, including specialist physician and nursing treatment. Thirteen of 15 centres also had a dietician and 5 of 15 had a clinical social worker.
The most common method of assessing residual renal function and guiding clinical treatment was estimated glomerular filtration rate (eGFR) as measured by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [30].
Patients
Patients included in this study were consecutive patients starting chronic dialysis therapy for ESKD at the participating centres between 1 January 2015 and 31 December 2017. Five centres had a shorter recruiting period.
A patient was considered to have ESKD at first dialysis if the patient was diagnosed as having ESKD according to the treating physician (in practice, this was the most commonly used definition), the patient received dialysis treatment for >90 days and if the doctor was in doubt whether the patient had acute or chronic renal failure, the patient was included retrospectively as soon as there was no doubt that the patient had chronic renal failure and ESKD .
The study protocol was approved by the ethical review boards in centres located in countries where according to the country’s regulations such approval was required. The study was approved by the Swedish Ethical Review Authority ( 2017/7). However, in Denmark, due to the observational, non-interventional design of the study using anonymized patient data, the study protocol was not considered to be eligible for ethical review. Informed consent, either written or verbal depending on the regulations in the different countries, was obtained from participants in all centres, including those in Denmark, with the exception of Lithuania, where patient permission was waived by the ethics board (P2-BE-2-9/2014). The study is registered with ClinicalTrials.gov (NCT02488200).
Outcome: modality choice
Modality choice was decided before DI or shortly thereafter. Modality choice was categorized as either patient modality choice (between PD, home HD or in-centre HD), usually in the context of a shared decision-making process with the doctors and nurses; unsuitability for patient choice or no information given about home dialysis modalities. Unsuitability was further classified as HD not possible, physical contraindication to PD, mental contraindication to PD and abdominal contraindication to PD. Changes in modality during the first year after DI were registered. No information given was assessed by a retrospective review of patient notes.
Patient clinical data
The following patient data were registered at DI: age, sex, height, weight, body mass index (BMI) and renal diagnosis. The presence of the following comorbidities was registered: previous myocardial infarction, heart failure, cardiac atherosclerosis, cerebrovascular disease, diabetes, peripheral atherosclerosis, previous cancer (except basocellular), chronic pulmonary disease, chronic liver disease, psychiatric disease, previous renal transplantation and other chronic conditions.
Dialysis access at first dialysis was registered. DI was classified as optimal if the access was an arteriovenous fistula (AVF) or graft (AVG); the access was a tunnelled catheter as the patient’s permanent access due to inability to place an AVF/AVG or the access was a PD catheter and PD was started >6 days after placement.
DI was suboptimal if the access was a temporary vascular catheter; the access was a tunnelled catheter, but a later AVF/AVG was planned or the access was a PD catheter and PD was started <6 days after placement.
The period between PD catheter placement and the start of PD should preferably be 2–3 weeks in order to avoid leaks and hernias. Early peritoneal DI was therefore judged suboptimal. The choice of <6 days was derived from the Danish Society of Nephrology treatment quality indicators. Late referral was defined as referral to the specialist nephrology unit ˂3 months before DI. As many pre-emptive transplants were often assessed and treated at other departments, they were excluded from the study.
Clinical data for this population have previously been published [28]. For the purposes of this study, clinical symptoms were classified as present or not present and as primary cause of DI or not primary cause of DI. Life-threatening conditions were defined as the presence of pulmonary stasis, dyspnoea, cardiac symptoms, pericarditis, acidosis or hyperkalaemia (as assessed by the treating physician) and similarly classified.
Patient biochemical data
The following biochemical data prior to or in conjunction with the first dialysis were registered: blood haemoglobin, plasma concentrations of urea, creatinine, potassium, hydrogen carbonate (bicarbonate), albumin, C-reactive protein (CRP), total or ionized calcium and phosphate. Most centres measured ionized calcium, for other centres, ionized calcium was assumed to be 50% of total calcium.
Whenever available, plasma creatinine concentration and date of measurement were registered at the following time points: referral to the nephrology department, 6 and 3 months before DI and at the time of information about dialysis, dialysis access prescription, dialysis access placement and first dialysis. The time of information was defined as initiation of personalized dialysis information with the purpose of choosing a dialysis modality. If data on creatinine were not available on the same date, the value closest in time to this date was chosen. eGFR was calculated using the CKD-EPI formula [30]. Patients had often received general information about dialysis at a very early point; information about dialysis is here defined as the initiation of detailed information in order to make a final choice of modality and plan for surgical placement of access and would be expected to be completed within 1 month.
Physician DI motivation questionnaire
Physicians gave details in an English-language questionnaire of their reasons for prescribing chronic dialysis at DI. They could choose between several pre-stated clinical and/or biochemical reasons. Details of these have already been published [28]. For the purposes of this study, reasons were defined as primarily clinical or primarily biochemical.
Physician data
Participating physicians were requested to provide the following anonymized personal data: age, sex, specialist qualification, duration of physician experience and duration of specialist nephrology experience.
Centre data
Centres supplied estimates of incidence rates of HD and PD. The number of nephrology specialists at the centre was noted. Centre policies concerning anaemia treatment, dialysis planning, access placement and DI were registered. The existence of a ‘home dialysis first’ policy was noted. This was defined as a belief by the clinic that, for one or more reasons, home dialysis is the preferred modality in the absence of contraindications and that this belief was communicated to the patient.
Statistics
Data are presented as mean ± standard deviation for normally distributed variables, median (interquartile range) for non-normally distributed variables or frequencies and percentages for categorical variables. All variables used in the analyses were categorical and were compared using chi-squared tests. Odds ratios (ORs) were calculated according to Altman [31]. A significance level of <0.05 was considered significant. All variables that were significant on univariate analyses were included in a forward stepwise regression analysis in order to identify independent associations with modality choice. Significance values were expressed as P < 0.05, P < 0.01, P < 0.001.
RESULTS
A total of 1587 patients were included in the study. This was 19% below the expected total incidence, suggesting that some patients were not included. The number of non-included patients was not registered, nor was the cause of their non-inclusion. Seventeen (1%) patients were not included due to a pre-emptive transplantation. This was known to be an underestimate of the true incidence of pre-emptive transplantation, since these patients were often assessed at other clinics. The median number of included patients per centre was 67 (range 16–198). No data concerning non-included patients were registered. The mean age of the 1587 patients was 63.8 ± 15.3 years and 35.9% were female. Their renal diagnoses were glomerulonephritis [283 (17.8%)], chronic interstitial nephropathy [185 (11.7%)], polycystic renal disease [106 (6.7%)], diabetic nephropathy [388 (24.4%)], hypertensive nephropathy [303 (19.1%)] and other [179 (11.3%)] or unknown [143 (9.0%)] aetiology. The vast majority were Caucasian.
Treatment modality and causes of choice
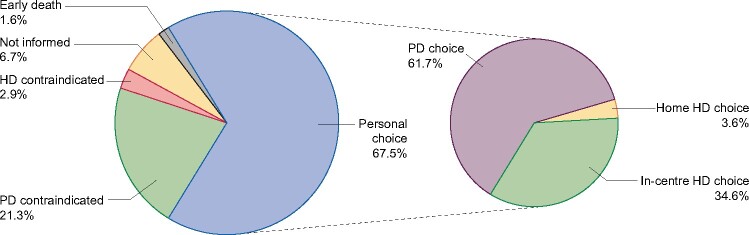
The distribution of stated causes of modality choice is shown in Figure 1. Of 1587 included patients, 516 (32.5%) patients were judged not suitable for a personal choice. Of these, PD was contraindicated in 338 (21.3%) patients for physical [142 (8.9%)], mental [80 (5.0%)] or abdominal [116 (7.3%)] reasons. HD was contraindicated in 46 (2.9%) patients. A total of 106 (6.7%) patients were not informed about home dialysis modalities and deaths before modality choice occurred in 26 (1.6%).
FIGURE 1:
Distribution of stated causes of modality choice.
Among the remaining 1071 patients (representing 67.5% of the 1587 patients) with a personal choice of modality, 700 (65.4%) chose home dialysis, either PD [661 (61.7%)] or home HD [39 (3.6%)], while 371 patients chose in-centre HD. The overall incidence of PD in the 15 centres varied between 19% and 57%, with one outlier (4%).
Among the 661 patients who chose PD, some had to start dialysis in a suboptimal way. Fifty-eight (8.8%) of these started on suboptimal PD and 128 (19.4%) started on suboptimal HD. Of the latter, 81 (63%) switched subsequently to PD, while 40 (31%) remained on HD, 3 (2%) changed to home HD and 4 (3%) regained renal function. There were some differences between the centres in the ability to fulfil these patients’ wishes for PD. In seven centres, all patients who started on dialysis using suboptimal HD subsequently switched to PD, while in three centres the figure was <70%. A further 18 patients (1% of total) switched to PD despite PD not being their original choice. Initially, six of these had chosen HD, six had not been informed about PD and six were previously thought to be unsuited to PD. In all, 632 (39.8% of total) patients were treated with PD and 39 (2.5% of total) patients initially chose home HD. None of these received home HD as the initial modality. Only 19 (49%) later received home HD, while 5 (13%) received a renal transplant, 14 (36%) remained on in-centre HD and 1 regained renal function. However, a further 23 patients (1.4% of all patients) were also later treated with home HD, their initial modality choice being PD [8 patients (35%)], in-centre HD [1 (4%)], PD not possible [13 (57%)] or home dialysis not offered [1 (4%)]. In all, 42 (2.6% of total) were treated with home HD.
Factors associated with unsuitability for patient modality choice
Significant univariate and multivariable associations to unsuitability for modality choice are shown in Table 1 and Figure 2. Unsurprisingly, advanced age and most comorbid diseases were associated with increased unsuitability. Hepatic and cardiac disease, cardiac failure in particular, were major factors in unsuitability for HD. Sixteen (26.2%) patients with psychiatric disease had mental contraindications to PD. Cancer and obesity were associated with abdominal contraindication to PD. PD was considered contraindicated in 12 of 24 (50%) patients with a BMI >40 kg/m2. High levels of urea and CRP and low plasma albumin were associated to unsuitability, while eGFR showed no relationship.
Table 1.
Clinical, biochemical and organizational factors linked to unsuitability for patient modality choice
| Unsuitable for patient modality choice |
||||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Patient choice | Mental | Abdominal | Physical | HD not possible | No choice (combined) | OR (95%CI) | |
| No. of patients | 1455 | 1071 (73.6) | 80 (5.5) | 116 (8.0) | 142 (9.8) | 46 (3.2) | 384 (26.4) | |
| Patient age (years) | <60 | 400 (79.3) | 25 (5.0) | 31 (6.2) | 35 (7.0) | 13 (2.6) | 104 (20.7) | |
| ≥60 | 671 (70.5) | 55 (5.8) | 85 (9.0)a | 107 (11.3)b | 33 (3.5) | 280 (29.5)c | 1.60 (1.24–2.07)c | |
| BMI (kg/m2) | <30 | 733 (75.1) | 53 (5.4) | 69 (7.1) | 89 (9.1) | 32 (3.3) | 243 (24.9) | |
| ≥29 | 196 (68.3) | 13 (4.5) | 34 (11.9)b | 31 (10.8) | 13 (4.5) | 91 (31.7)a | 1.40 (1.05–1.87)a | |
| Comorbidity | ||||||||
| Any comorbidity | No | 331 (84.0) | 14 (3.6) | 19 (4.8) | 24 (6.1) | 6 (1.5) | 63 (16.0) | |
| Yes | 740 (69.8) | 66 (6.2)a | 97 (9.1)b | 118 (11.1)c | 40 (3.8)c | 321 (30.2)c | 2.27 (1.69–3.08)c | |
| Myocardial infarction | No | 960 (74.2) | 72 (5.6) | 107 (8.3) | 116 (9.0) | 39 (3.0) | 334 (25.8) | |
| Yes | 111 (68.9) | 8 (5.0) | 9 (5.6) | 26 (16.1)b | 7 (4.4) | 50 (31.1)a | 1.29 (0.91–1.85)a | |
| Cardiac failure | No | 913 (75.1) | 67 (5.5) | 101 (8.3) | 109 (9.0) | 25 (2.1) | 302 (24.9) | |
| Yes | 158 (65.8) | 13 (5.4) | 15 (6.3) | 33 (13.8) | 21 (8.8)c | 82 (34.2)b | 1.57 (1.17–2.11)b | |
| All cardiac | No | 731 (76.2) | 46 (4.8) | 84 (8.8) | 78 (8.1) | 20 (2.1) | 228 (23.8) | |
| Yes | 340 (68.5) | 34 (6.9)a | 32 (6.6) | 64 (12.9)b | 26 (5.2)c | 156 (31.5)b | 1.47 (1.116–1.87)b | |
| Cerebrovascular | No | 961 (75.3) | 70 (5.5) | 103 (8.1) | 106 (8.3) | 37 (2.9) | 316 (24.7) | |
| Yes | 110 (61.8) | 10 (5.6) | 13 (7.3) | 36 (20.2)c | 9 (5.1)a | 68 (38.2)c | 1.88 (1.35–2.61)c,C | |
| DM | No | 703 (74.8) | 52 (5.5) | 76 (8.1) | 88 (9.4) | 21 (2.2) | 237 (25.2) | |
| Yes | 368 (71.5) | 28 (5.4) | 40 (7.8) | 54 (10.5) | 25 (4.9)b | 147 (28.5) | 1.18 (0.93–1.51) | |
| Peripheral vascular | No | 955 (74.6) | 72 (5.6) | 97 (7.6) | 120 (9.4) | 36 (2.8) | 325 (25.4) | |
| Yes | 116 (66.3) | 8 (4.6) | 19 (10.9) | 22 (12.6) | 10 (5.7)a | 59 (33.7)a | 1.49 (1.07–2.10)a | |
| Cancer | No | 923 (75.4) | 67 (5.5) | 78 (6.4) | 115 (9.4) | 41 (3.3) | 301 (24.6) | |
| Yes | 148 (64.1) | 13 (5.6) | 38 (16.5)c | 27 (11.7) | 5 (2.2) | 83 (35.9)c | 1.72 (1.28–2.32)c,A | |
| Pulmonary | No | 985 (74.7) | 71 (5.4) | 101 (7.7) | 123 (9.3) | 39 (3.0) | 334 (25.3) | |
| Yes | 86 (63.2) | 9 (6.6) | 15 (11.0) | 19 (14.0)a | 7 (5.1) | 50 (36.8)b | 1.71 (1.18–2.48)b,A | |
| Hepatic | No | 1037 (74.0) | 78 (5.6) | 112 (8.0) | 137 (9.8) | 38 (2.7) | 365 (26.0) | |
| Yes | 34 (64.2) | 2 (3.8) | 4 (7.5) | 5 (9.4) | 8 (15.1)c | 19 (35.8) | 1.59 (0.89–2.82) | |
| Previous transplant | No | 1019 (73.9) | 76 (5.5) | 107 (7.8) | 135 (9.8) | 42 (3.0) | 360 (26.1) | |
| Yes | 52 (68.4) | 4 (5.3) | 9 (11.8) | 7 (9.2) | 4 (5.3) | 24 (31.6) | 1.31 (0.79–2.15) | |
| Psychiatric | No | 1036 (74.3) | 64 (4.6) | 114 (8.2) | 134 (9.6) | 46 (3.3) | 358 (25.7) | |
| Yes | 35 (57.4) | 16 (26.2)c | 2 (3.3) | 8 (13.1) | 0 (0) | 26 (42.6)b | 2.15 (1.28–3.62)b,B | |
| Comorbidity number | 0 | 481 (78.6) | 32 (5.2) | 44 (7.2) | 45 (7.4) | 10 (1.6) | 131 (21.4) | 1.00 (Referent) |
| Subgroups | 1 | 324 (73.1) | 24 (5.4) | 42 (9.5) | 43 (9.7) | 10 (2.3) | 119 (26.9)a | 1.35 (1.01–1.79)a |
| >1 | 266 (66.5) | 24 (6.0) | 30 (7.5) | 54 (13.5)c | 26 (6.5)c | 134 (33.5)c | 1.85 (1.39–2.46)c | |
| Pre-dialysis course | ||||||||
| Late referral (<3 months before DI) | No | 843 (75.9) | 57 (5.1) | 81 (7.3) | 99 (8.9) | 31 (2.8) | 268 (24.1) | |
| Yes | 153 (62.7) | 19 (7.8) | 25 (10.2)a | 35 (14.3)b | 12 (4.9)a | 91 (37.3)c | 1.87 (1.39–2.51)c,A | |
| Suboptimal DI | No | 724 (80.2) | 35 (3.9) | 62 (6.9) | 61 (6.8) | 21 (2.3) | 179 (19.8) | |
| Yes | 346 (62.8) | 45 (8.2)c | 54 (9.8)b | 81 (14.7)c | 25 (4.5)b | 205 (37.2)c | 2.40 (1.89–3.04)c | |
| eGFR decrease 3–0 months prior to dialysis (mL/min/1.73 m2/month) |
<1 >1 |
541 (80.1) 313 (66.9) |
23 (3.4) 38 (8.1)c |
47 (7.0) 40 (8.5) |
47 (7.0) 62 (13.2)c |
15 (3.2) |
134 (19.9) 155 (33.1)c |
2.00 (1.53–2.62)c,A |
|
eGFR at DI (mL/min/1.73 m2) |
<6 | 125 (67.6) | 13 (7.0) | 15 (8.1) | 24 (13.0) | 8 (4.3) | 60 (32.4) | 1.00 (Referent) |
| 6–12 | 535 (74.8) | 39 (5.5) | 52 (7.3) | 70 (9.8) | 19 (2.7) | 180 (25.2)a | 0.70 (0.49–1.00)a | |
| >12 | 328 (73.9) | 23 (5.2) | 42 (9.5) | 33 (7.4)a | 18 (4.1) | 116 (26.1) | 0.74 (0.51–1.07) | |
| Late dialysis information (<3 months before DI) |
No Yes |
635 (79.7) 367 (65.1) |
32 (4.0) 43 (7.6) |
55 (6.9) 55 (9.8) |
57 (7.2) 72 (12.8) |
18 (2.3) 27 (4.8) |
162 (20.3) 197 (34.9) |
2.08 (1.45–2.70)c |
| Biochemistry | ||||||||
| Urea (mM) | <20 | 103 (76.9) | 6 (4.5) | 13 (9.7) | 10 (7.5) | 2 (1.5) | 31 (23.1) | 1.00 (Referent) |
| 20–29.9 | 379 (77.8) | 24 (4.9) | 38 (7.8) | 29 (6.0) | 17 (3.5) | 108 (22.2) | 0.95 (0.60–1.49) | |
| ≥30 | 552 (70.1) | 50 (6.4) | 61 (7.8) | 97 (12.3)c | 27 (3.4) | 235 (29.9) | 1.41 (0.92–2.17) | |
| Albumin (g/L) | <30 | 281 (65.0) | 34 (7.9)a | 45 (10.4)b | 51 (11.8)b | 21 (4.9)b | 151 (35.0)c | 2.15 (1.60–2.90)c,B |
| 30–34.9 | 282 (74.4) | 16 (4.2) | 31 (8.2) | 42 (11.1)a | 8 (2.1) | 97 (25.6)a | 1.38 (1.00–1.90)a | |
| ≥35 | 393 (80.0) | 27 (5.5) | 28 (5.7) | 36 (7.3) | 7 (1.4) | 98 (20.0) | 1.00 (Referent) | |
| CRP (mg/L) | <50 | 809 (77.3) | 52 (5.0) | 80 (7.6) | 74 (7.1) | 32 (3.1) | 238 (22.7) | |
| ≥50 | 153 (56.9) | 22 (8.2)b | 27 (10.0)a | 56 (20.8)c | 11 (4.1) | 116 (43.1)c | 2.58 (1.95–3.41)c,C | |
| Life-threatening cause | Not primary | 889 (77.1) | 58 (5.0) | 81 (7.0) | 96 (8.3) | 29 (2.5) | 264 (22.9) | |
| Primary | 156 (59.1) | 19 (7.2) | 31 (11.7)c | 41 (15.5)c | 17 (6.4)c | 108 (40.9)c | 2.33 (1.76–3.09)c | |
| Hospital | ||||||||
| University hospital | No | 205 (79.5) | 11 (4.3) | 19 (7.4) | 22 (8.5) | 1 (0.4) | 53 (20.5) | |
| Yes | 866 (72.3) | 69 (5.8) | 97 (8.1) | 120 (10.0) | 45 (3.8)b | 331 (27.7) | 1.48 (1.07–2.05)a | |
| Home dialysis first policy | No | 208 (73.2) | 14 (4.9) | 21 (8.1) | 38 (13.4) | 3 (1.1) | 76 (26.8) | |
| Yes | 863 (73.7) | 66 (5.6) | 95 (8.1) | 104 (8.9)a | 43 (3.7)a | 308 (26.4) | 0.98 (0.73–1.31) | |
| Physician age | <50 | 332 (77.2) | 21 (4.9) | 25 (5.8) | 34 (7.9) | 18 (4.2) | 98 (22.8) | |
| ≥50 | 304 (71.4) | 27 (6.3) | 36 (8.5) | 46 (10.8) | 13 (3.1) | 122 (28.6) | 1.36 (0.99–1.85)0.05 | |
| Physician sex | Male | 329 (76.3) | 23 (5.3) | 32 (7.4) | 39 (9.0) | 8 (1.9) | 102 (23.7) | |
| Female | 307 (72.2) | 25 (5.9) | 29 (6.8) | 41 (9.6) | 23 (5.4)b | 118 (27.8) | 1.24 (0.91–1.69) | |
Significant factors only. OR shown for no choice (combined) versus patient choice. Row percentages in brackets. Bold type: independent significant factors on multivariable analysis; 132 patients were excluded (106 were not assessed and 26 patients died).
Univariate analysis: aP < 0.05, bP < 0.01, cP < 0.001. Multivariable analysis: AP < 0.05, BP < 0.01, CP < 0.001. Only variables significant in the univariate analysis were included in the multivariable analysis.
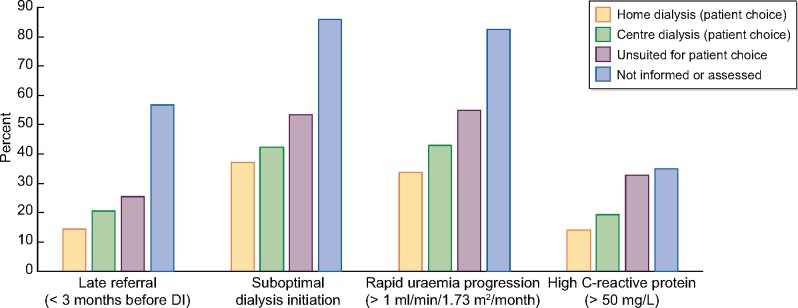
FIGURE 2:
Relationship of pre-dialysis factors to choice of modality. The percentage figures refer to the percentage of patients in each modality choice group with the relevant factor.
Many factors during the pre-dialytic course were associated with unsuitability. Dialysis information given in conjunction with a low eGFR, rapid uraemia progression, late referral, suboptimal DI and life-threatening DI cause were all associated with increased unsuitability. Rapid uraemia progression and late referral were significant factors even after correction for comorbidity.
Organizational and physician-related factors were minor factors and were not significant on a multivariable analysis.
Factors associated with not receiving modality choice information
Significant univariate and multivariable associations to not receiving modality choice information are shown in Table 2 and Figure 2. The main factors were late referral and suboptimal DI. Patients with late referral were 5.75 [95% confidence interval (CI) 3.77–8.79] times more likely not to receive information, while those receiving suboptimal DI were 9.63 (95% CI 5.52–16.8) times more likely not to receive information. Patients not receiving information were characterized by a low eGFR and low plasma albumin at DI and elevated CRP. University departments and departments without a ‘home dialysis first’ policy were less likely to give modality choice information.
Table 2.
Clinical, biochemical and organizational factors associated with lack of information concerning home dialysis modalities
| Factor | Number (%) | OR (95% CI) | |
|---|---|---|---|
| Patients | 106 (6.7) | ||
| Diabetic nephropathy | No | 90 (7.7) | |
| Yes | 16 (4.1) | 0.53 (0.31–0.91)a | |
| Comorbidity | |||
| DM | No | 78 (7.7) | |
| Yes | 28 (5.2) | 0.66 (0.42–1.03)0.06 | |
| Cancer | No | 80 (6.1) | |
| Yes | 26 (10.1) | 1.70 (1.07–2.70)a | |
| Comorbidity number | 0 | 54 (8.0) | 1.00 (Referent) |
| Subgroups | 1 | 23 (4.9) | 0.59 (0.36–0.98)a |
| >1 | 29 (6.6) | 0.82 (0.51–1.31) | |
| Pre-dialysis course | |||
| Late referral (<3 months) | No | 42 (3.6) | |
| Yes | 55 (18.4) | 5.75 (3.77–8.79)c,C | |
| Suboptimal DI | No | 15 (1.6) | |
| Yes | 91 (14.2) | 9.63 (5.52–16.8)c,C | |
|
eGFR decrease 3–0 months prior to dialysis (mL/min/1.73 m2/month) |
<1 >1 |
10 (1.5) 47 (9.1) |
6.70 (3.35–13.8)c |
| Biochemistry | |||
| eGFR (mL/min/1.73 m2) | ≥7 | 32 (4.5) | |
| <7 | 73 (8.8) | 2.04 (1.33–3.14)c | |
| Albumin (g/L) | <30 | 45 (9.4) | 2.37 (1.39–4.05)b |
| 30–34.9 | 24 (6.0) | 1.47 (0.80–2.68) | |
| ≥35 | 21 (4.1)b | 1.00 (Referent) | |
| CRP (mg/L) | <50 | 67 (6.0) | |
| ≥50 | 36 (11.8) | 2.04 (1.33–3.13)c | |
| Initial dialysis | |||
| Dialysis cause | Clinical | 44 (4.7) | |
| Biochemical | 59 (10.2)c | 2.31 (1.54–3.47)c | |
| Life-threatening cause | Not primary | 75 (6.1) | |
| Primary | 29 (9.9)a | 1.66 (1.06–2.58)a | |
| Hospital | |||
| University hospital | No | 5 (1.9) | |
| Yes | 101 (7.8)c | 4.32 (1.74–10.7)b,B | |
| Home dialysis first policy | No | 45 (13.4) | |
| Yes | 61 (4.9) | 0.33 (0.22.0.50)c,C | |
| Physician age | <50 | 43 (9.0) | |
| ≥50 | 22 (4.8) | 0.51 (0.30–0.87)a | |
| Nephrologist vintage | <10 | 18 (4.5) | |
| ≥10 | 47 (8.8) | 2.03 (1.16–3.55)a |
Univariate analysis: aP < 0.05, bP < 0.01, cP < 0.001. Multivariable analysis: AP < 0.05, BP < 0.01, CP < 0.001. Only variables significant in the univariate analysis were included in the multivariable analysis. Comparison to all other patients. Significant factors only. Bold type: independent significant factors on multivariable analysis.
Factors associated with patient modality choice
Significant univariate and multivariable associations to modality choice for patients offered a personal choice are shown in Table 3 and Figure 2. For statistical purposes, in-centre HD and home HD were compared individually with PD and home dialysis (home HD and PD combined) with in-centre HD.
Table 3.
Clinical, biochemical and organizational factors affecting choice of dialysis modality in 1072 patients offered a personal choice of modality
| Variable | Value | In-centre HD, n (%) | PD, n (%) | Home HD, n (%) | OR for home dialysis versus in-centre HD (95% CI) |
|---|---|---|---|---|---|
| Patients | 1071 | 371 (34.6) | 661 (61.7) | 39 (3.6) | |
| Patient age (years) | <60 | 133 (33.3) | 244 (60.9) | 23 (5.8) | |
| ≥60 | 238 (35.5) | 417 (62.1) | 16 (2.4)b | 0.92 (0.71–1.20) | |
| Sex | Male | 250 (36.1) | 423 (61.1) | 19 (2.8) | |
| Female | 121 (31.9) | 238 (62.8) | 20 (5.3)0.06 | 1.21 (0.92–1.57) | |
| BMI (kg/m2) | <30 | 253 (34.5) | 459 (62.6) | 21 (2.9) | |
| ≥30 | 85 (43.4)b | 98 (50.0) | 13 (6.6) | 0.69 (0.50–0.95)a,B | |
| Polycystic disease | No | 357 (35.8) | 611 (61.3) | 29 (2.9) | |
| Yes | 14 (18.9)a | 50 (67.6) | 10 (13.5)c | 2.39 (1.32–4.34)b,A | |
| Comorbidity | |||||
| Any comorbidity | No | 101 (30.5) | 213 (64.4) | 17 (5.1) | |
| Yes | 270 (36.5) | 448 (60.5) | 22 (3.0) | 0.76 (0.58–1.01)0.06 | |
| Previous transplant | No | 349 (34.3) | 636 (62.4) | 34 (3.3) | |
| Yes | 22 (42.3) | 25 (48.1) | 5 (9.6)b | 0.71 (0.40–1.25) | |
| Pre-dialysis course | |||||
| Late referral (<3 months) | No | 274 (32.4) | 537 (63.6) | 34 (4.0) | |
| Yes | 71 (46.4)b | 78 (51.0) | 4 (2.6) | 0.55 (0.39–0.79)c,B | |
| Suboptimal DI | No | 214 (29.6) | 485 (67.0) | 25 (3.5) | |
| Yes | 157 (45.4)c | 175 (50.6) | 14 (4.1) | 0.51 (0.39–0.66)c,C | |
|
eGFR decrease 3–0 months prior to dialysis (mL/min/1.73 m2/month) |
<1 >1 |
154 (28.5) 115 (36.7)b |
368 (68.0) 182 (58.2) |
19 (3.5) 16 (5.1) |
0.69 (0.51–0.92)a |
|
eGFR at DI (mL/min/1.73 m2) |
<6 6–12 |
58 (46.4) 183 (34.2) |
64 (51.2) 331 (61.9) |
3 (2.4) 21 (3.9) |
1.00 (Referent) 1.67 (1.12–2.47)a |
| ≥12 | 88 (26.8)c | 226 (68.9) | 14 (4.3) | 2.36 (1.54–3.62)c | |
| Late dialysis information (<3 months before DI) |
No Yes |
199 (31.3) 135 (36.8) |
413 (65.0) 217 (59.1) |
23 (3.6) 15 (4.1) |
0.78 (0.60–1.03) |
| Biochemistry | |||||
| eGFR (mL/min/1.73 m2) | ≥7 | 151 (30.0) | 326 (64.7) | 27 (5.4) | |
| <7 | 217 (39.2)b | 324 (58.6) | 12 (2.2)a | 1.51 (1.17–1.95)b | |
| Urea (mM) | <20 | 23 (22.3) | 74 (71.8) | 6 (5.8) | |
| 20–29.9 | 104 (27.4) | 255 (67.3) | 20 (5.3) | 0.76 (0.45–1.27) | |
| ≥30 | 237 (42.9)c | 302 (54.7) | 13 (2.4) | 0.38 (0.23–0.63)c,C | |
| CRP (mg/L) | <50 | 266 (32.9) | 512 (63.3) | 31 (3.8) | |
| ≥50 | 64 (41.8)a | 83 (54.3) | 6 (3.9) | 0.68 (0.48–0.97)a | |
| Initial dialysis cause | |||||
| Symptoms | None | 44 (25.9) | 119 (70.0) | 7 (4.1) | |
| Some | 318 (36.5)b | 524 (60.1) | 30 (3.4) | 0.61 (0.42–0.88)b,C | |
| Life-threatening cause | Not primary | 288 (32.4) | 567 (63.8) | 34 (3.8) | |
| Primary | 74 (47.4)c | 79 (50.6) | 3 (1.9) | 0.53 (0.38–0.75)c | |
| Hospital | |||||
| Home dialysis first policy | No | 52 (60.5) | 31 (36.0) | 3 (3.5) | |
| Yes | 319 (32.4 )c | 630 (64.0) | 36 (3.7) | 3.19 (2.03–5.02)c | |
| Physician | |||||
| Nephrologist vintage, years | <10 | 79 (28.6) | 185 (67.0) | 12 (4.3) | |
| ≥10 | 143 (39.7)b | 202 (56.1) | 15 (4.2) | 0.61 (0.44–0.85)b,A |
Significant factors only. In-centre HD and home HD each compared with PD. Bold type: independent significant factors on multivariable analysis. OR shown for home dialysis (combined home HD and PD) versus in-centre HD. In-centre HD and home HD compared with PD. Univariate analysis: aP < 0.05, bP < 0.01, cP < 0.001. Multivariable analysis: AP < 0.05, BP < 0.01, CP < 0.001. Only variables significant in the univariate analysis were included in the multivariable analysis.
The subgroup of patients choosing home HD was relatively small, permitting only limited analysis. Patients with younger age [<60 years; OR 2.56 (95% CI 1.30–5.01), P < 0.01], female sex [OR 2.17 (95% CI 1.12–4.23), P < 0.05], polycystic disease [OR 8.79 (95% CI 3.59–21.5), P < 0.001], a higher eGFR at DI [≥7 mM; OR 3.23 (95% CI 1.59–6.58), P < 0.01] and a low plasma urea at DI [<20 mM; OR 2.70 (95% CI 1.03–7.09), P < 0.05] were more likely to choose home HD than in-centre HD.
Patients choosing in-centre HD had a similar age and sex as those choosing PD and did not differ significantly in terms of comorbidity. Obese patients were less likely to choose PD and patients with polycystic renal disease more likely to choose PD. Important factors for the choice of in-centre HD were late information concerning dialysis modalities, late referral, suboptimal DI and severe symptomatic and biochemical uraemia at DI. Patients treated by physicians with lengthy nephrology experience (>9 years) were less likely to choose PD. There was an insignificant tendency for physicians with greater experience to prescribe home HD (<10 years, 1.9%; 10–19 years, 3.9%; >19 years, 5.3%; P = 0.07). Physician age, physician sex, specialist qualification and number of specialists per patient had no effect on modality choice.
Although 11 of the 15 centres were able to provide subacute PD, PD was relatively uncommon as the initial modality for patients with suboptimal DI. Seventy-two patients (4.5% of all 1588 patients, 10.9% of PD starts) received subacute PD, with the incidence varying between 1% and 33% for different centres. More frequent use of subacute PD seemed to be associated with a higher incidence of PD choice: in four centres that did not provide subacute PD, PD incidence was 31.2%; in centres with little use of subacute PD (2–9%, 7 centres), it was 40.7%; and in centres with high use of subacute PD (13–27%, 4 centres), it was 51.6%.
Ten of 15 centres provided assisted PD, defined as professional help in the home. This was not associated with the incidence of PD choice (PD incidence in centres with and without assisted PD was 40.0 and 40.5%, respectively).
DISCUSSION
This study, comprising 1587 ESKD patients starting dialysis in centres in the Nordic and Baltic countries, shows that among patients who had a personal choice of dialysis modality, 65.3% chose home dialysis using PD (61.7%) or in some cases home HD (3.6%). Whereas most ESKD patients given a personal choice chose home dialysis, many patients did not receive timely information about dialysis modality options due to potentially modifiable factors such as late referral and suboptimal DI. Based on our data, it seems likely that the incidence of DI using home dialysis (44.1% of all 1587 patients) could potentially have been further increased by efforts to reduce the incidence of late referral and unplanned DI. Moreover, our data may imply that acutely ill patients starting suboptimal DI, in most cases using subacute HD, could probably benefit from an educational programme (possibly repeated) after their clinical condition has improved and this could potentially increase the incidence of DI using home dialysis.
The centres involved in this study were similar in many respects. The incidence of DI using home dialysis was, from an international perspective, relatively high in most of the participating centres. The choice of modality was independent of economic considerations since all modalities were available and free of charge to patients. All centres offered a pre-dialysis education programme to patients with timely referral, although the quality of these programmes was not assessed in this study. Most departments had a ‘home dialysis first’ policy. These factors may explain the generally high incidence of home dialysis in this population.
Reasons for (perceived) contraindications to home dialysis were classified as physical (medical conditions preventing practical performance of home dialysis), mental (e.g. psychiatric disease and dementia) and PD and abdominal problems. Contraindications to PD were noted in 21.2% of all 1588 patients and contraindications to HD in 3% of the patients. These findings are similar to previously reported data from Europe and the USA [26, 32, 33].
Unsurprisingly, obesity, older age, hypoalbuminaemia and comorbidity were associated with the stated presence of contraindications. Additional risk factors included late referral, suboptimal DI, particularly due to life-threatening conditions, rapid uraemia progression and a high CRP (Figure 2). This is perhaps surprising, since these contraindications are in principle temporary problems. These observations suggest that delaying decisions on modality choice until the patient is healthy, or repeating dialysis information after 2–3 months, might increase the incidence of home HD. A recent study [34] demonstrated that an education programme initiated after unplanned DI can increase the incidence of home dialysis.
A minority of patients (7%) were not assessed for home dialysis. The clinical differences between these patients and other patients were minor, the main risk factors being late referral and suboptimal DI (Figure 2). This suggests again that the establishment of a formalized evaluation and education programme for these patients after DI should be an integrated element in departmental practice.
Most patients (67.5%) were offered a personal, informed choice of dialysis modality, regardless of the existence of a ‘home dialysis first’ policy. The term ‘personal’ should not be interpreted literally; most patients will have participated in a shared decision-making process, with input from doctors and nurses. For instance, obese patients were apparently often advised to choose HD. Among patients with a personal choice, 61.7% chose PD, a somewhat higher figure than described in the literature [35]. This seemed to be related to the high prevalence of ‘home dialysis first’ policies at the participating centres. Another factor may be that all the centres in this study provided home dialysis. The results may not have been the same if centres had been obliged to transfer patients to other centres to perform dialysis at home, as is the case in certain other countries. Other well-known non-medical factors affecting patient choice (level of education, revenue, housing, familial status, etc.) were not assessed in this study.
All centres offered home HD, but most patients chose PD. This finding is similar to international results. However, the low incidence of home HD may have been related to physician bias, in that some centres, e.g. in New Zealand, exhibit almost similar uptake for both techniques. Further data concerning this question were not available.
Clinical differences between the three modality groups for patients given a personal choice were minor, although patients choosing in-centre HD had a marginally higher morbidity. This contrasts with the Netherlands Cooperative Study on the Adequacy of Dialysis [36], where older patients were more likely to choose HD, and with the Offering Patients Therapy Options in Unplanned Start study [37], where patients with a high comorbidity index were more likely to choose PD. Patients with polycystic kidney disease were significantly more likely to choose home dialysis, particularly home HD, independent of other clinical factors. Again, circumstances occurring during the pre-dialysis course, up to DI, seemed to affect patient choice. Patients with late referral, rapid loss of renal function, suboptimal DI, life-threatening disease or symptomatic uraemia; those having a high urea and CRP concentration; and those with low plasma albumin at DI were more likely to choose in-centre HD. This is in accordance with the literature [38–42]. One possible explanation is that, at the time of modality choice, these patients had not fully recovered from their poor clinical condition at DI and felt unable to cope with the responsibility for home dialysis.
These findings may have particular implications for countries where PD is used very little. It is important to stress that based on a patient-centric approach with shared decision-making, each patient should have the right to be informed about home dialysis options.
Previous renal transplantation increased home HD choice, as found previously [43]. The cause of this is unknown. One possibility is that these patients have a long ESKD history and may be better informed about the benefits of home dialysis. Some may no longer be suitable for transplantation and home HD may represent their best chance of long-term survival.
The influence of diabetes on modality choice is controversial, with one study showing an increased uptake of PD [34] and another reduced [36]. We found little effect of diabetes on modality choice. The influence may be conflicting: while diabetics may have a reduced possibility of home dialysis due to high morbidity, they are usually referred early to clinics, with the consequent possibility of optimal planning.
As previously noted [37], not all patients opting for home dialysis after suboptimal DI actually received this therapy. This may have been due to the patient changing his/her mind or a reassessment of patient suitability, but the considerable differences between centres suggest that suboptimal institutional planning after DI is a factor in some centres.
Institutional factors seemed to play a role in modality choice. University hospitals were more likely to judge patients unsuitable for home dialysis and less likely to assess patients for home dialysis. This could be related, at least in part, to a difference in patient characteristics since these hospitals are more likely to treat sicker patients. One might hypothesize that the larger number of trainees at these departments could have contributed to this difference. More importantly, the presence of a ‘home dialysis first’ policy at a department was a highly significant factor for predicting both timely assessment and information concerning home dialysis, and subsequent patient choice of home dialysis. The departments seemed to be following shared decision-making principles, such that the physicians’ opinions affected patient choice. However, the patient:nephrologist ratio was not a factor, in accordance with previous findings for Europe [44]. Patients treated by physicians with lengthy specialist experience were—perhaps surprisingly—less likely to be informed of and less likely to choose home dialysis. The causes of this relationship are unclear.
The use of PD for suboptimal DI has been practised in some centres for many years [45, 46]. It is more economical [47] and may increase the incidence of PD. Most centres in this study provided urgent-start PD, but only three centres commonly used it. These centres had a higher PD incidence (52% of all DI). Whether urgent-start PD per se increases PD incidence or is just a marker of institutional dedication to home dialysis is debatable.
Being an observational study, any causal explanations of these findings must be purely speculative. Some relations are obviously causative, e.g. high morbidity resulting in unsuitability for home dialysis, while other causative relationships are difficult to elucidate. In particular, rapid loss of renal function, late referral, unplanned DI, acute infection and severe uraemia form a conglomerate of clinical problems collectively associated with reduced choice of home dialysis modalities. A summary of putative causal relationships is shown in Figure 3.
FIGURE 3:
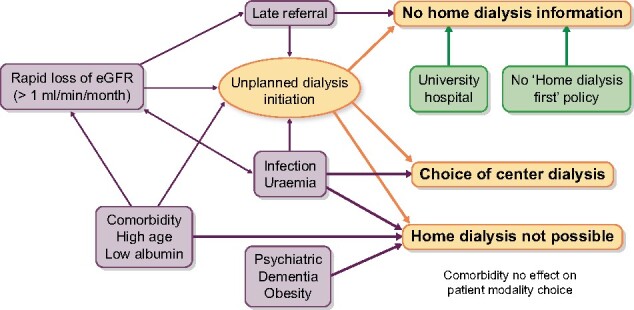
Putative causal mechanisms affecting modality choice. Uraemia: high plasma urea.
The use of assisted PD, defined as professional assistance in the home, has been suggested to increase PD prevalence [32, 48]. However, we were unable to confirm this association.
There are several limitations of this study. As in all observational studies, correlations do not prove causality. As compared with the overall expected incidence of DI at the participating centres, an estimated 19% of patients starting dialysis were not included in the study. Non-included patients were not quantified or characterized and may have differed from the included population. Social problems (e.g. poor dwelling conditions, social isolation, distance from dialysis facility and illiteracy) were not quantified and would probably have increased the proportion of patients choosing in-centre HD. Registration was incomplete for many variables, in particular variables concerning the pre-dialysis course. This may have reduced the accuracy of the results. With these caveats in mind, it should be noted as a strength of our study that we were able to prospectively document detailed information on causes and timing of DI and factors associated with the choice of initial dialysis modality in a relatively large number of patients.
In summary, this study shows that given a personal choice, most patients (65%) choose home dialysis. The results of the study suggest that the incidence of home dialysis could potentially be further increased if a number of organizational changes are initiated, such as the establishment of a formal departmental ‘home dialysis first’ policy, steps to reduce late referral and suboptimal DI, formalized post-DI education programmes for late referrals with suboptimal DI and, for acutely ill patients, the postponement (or repetition) of suitability assessment and modality choice until their condition has improved.
ACKNOWLEDGEMENTS
We thank all the physicians and other staff members who participated in this study. Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet. Thanks to Sara Denguir for data collection assistance.
FUNDING
The project was supported by an unrestricted grant (05253284) from Baxter Healthcare, Deerfield, IL, USA. The funder had no role in the study design; collection, analysis and interpretation of data; writing the report or the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
B.L. is employed by Baxter Healthcare at Baxter Novum, Karolinska Institutet. None of the other authors declare any conflicts of interest. The results presented in this article have not been published previously in whole or part, except in abstract format.
DATA AVAILABILITY STATEMENT
The data used in this study are available on the Open Source Project (OSP) data repository [49].
REFERENCES
- 1.Heaf JG, Wehberg S.. Relative survival of peritoneal dialysis and haemodialysis patients: effect of cohort and mode of dialysis initiation. PLoS One 2014; 9: e90119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinhandl ED, Foley RN, Gilbertson DT. et al. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 2010; 21: 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn RR, Hux JE, Oliver MJ. et al. Selection bias explains apparent differential mortality between dialysis modalities. J Am Soc Nephrol 2011; 22: 1534–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrotra R, Chiu YW, Kalantar-Zadeh K. et al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171: 110–118 [DOI] [PubMed] [Google Scholar]
- 5.Noordzij M, Jager KJ.. Survival comparisons between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant 2012; 27: 3385–3387 [DOI] [PubMed] [Google Scholar]
- 6.Marshall MR.The benefit of early survival on PD versus HD–why this is (still) very important. Perit Dial Int 2020; 40: 405–418 [DOI] [PubMed] [Google Scholar]
- 7.Ok E, Duman S, Asci G. et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dial Transplant 2011; 26: 1287–1296 [DOI] [PubMed] [Google Scholar]
- 8.Marshall MR, Hawley CM, Kerr PG. et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis 2011; 58: 782–793 [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Levin NW, Beck GJ. et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hladunewich MA, Hou S, Odutayo A. et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol 2014; 25: 1103–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chertow GM, Levin NW, Beck GJ. et al. Long-term effects of frequent in-center hemodialysis. J Am Soc Nephrol 2016; 27: 1830–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledebo I, Ronco C.. The best dialysis therapy? Results from an international survey among nephrology professionals. NDT Plus 2008; 1: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 14.Jain AK, Blake P, Cordy P. et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012; 23: 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van BW, Jha V, Abu-Alfa AK. et al. Considerations on equity in management of end-stage kidney disease in low- and middle-income countries. Kidney Int Suppl (2011) 2020; 10: e63–e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CT, Blankestijn PJ, Dember LM. et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 96: 37–47 [DOI] [PubMed] [Google Scholar]
- 17.Li PK, Chow KM, van de Luijtgaarden MW. et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol 2017; 13: 90–103 [DOI] [PubMed] [Google Scholar]
- 18.Neil N, Walker DR, Sesso R. et al. Gaining efficiencies: resources and demand for dialysis around the globe. Value Health 2009; 12: 73–79 [DOI] [PubMed] [Google Scholar]
- 19.de Jong RW, Stel VS, Heaf JG. et al. Non-medical barriers reported by nephrologists when providing renal replacement therapy or comprehensive conservative management to end-stage kidney disease patients: a systematic review. Nephrol Dial Transplant 2020; doi: 10.1093/ndt/gfz271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies SJ.Peritoneal dialysis—current status and future challenges. Nat Rev Nephrol 2013; 9: 399–408 [DOI] [PubMed] [Google Scholar]
- 21.Abra G, Schiller B.. Public policy and programs – missing links in growing home dialysis in the United States. Semin Dial 2020; 33: 75–82 [DOI] [PubMed] [Google Scholar]
- 22.Manns BJ, Taub K, Vanderstraeten C. et al. The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005; 68: 1777–1783 [DOI] [PubMed] [Google Scholar]
- 23.Lacson E Jr, Wang W, DeVries C. et al. Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis 2011; 58: 235–242 [DOI] [PubMed] [Google Scholar]
- 24.Little J, Irwin A, Marshall T. et al. Predicting a patient’s choice of dialysis modality: experience in a United Kingdom renal department. Am J Kidney Dis 2001; 37: 981–986 [DOI] [PubMed] [Google Scholar]
- 25.Ribitsch W, Haditsch B, Otto R. et al. Effects of a pre-dialysis patient education program on the relative frequencies of dialysis modalities. Perit Dial Int 2013; 33: 367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goovaerts T, Jadoul M, Goffin E.. Influence of a pre-dialysis education programme (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant 2005; 20: 1842–1847 [DOI] [PubMed] [Google Scholar]
- 27.Devoe DJ, Wong B, James MT. et al. Patient education and peritoneal dialysis modality selection: a systematic review and meta-analysis. Am J Kidney Dis 2016; 68: 422–433 [DOI] [PubMed] [Google Scholar]
- 28.Heaf J, Petersons A, Vernere B. et al. Why do physicians prescribe dialysis? A prospective questionnaire study. PLoS One 2017; 12: e0188309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaf J, Heiro M, Petersons A. et al. Suboptimal dialysis initiation is associated with comorbidities and uraemia progression rate but not with estimated glomerular filtration rate. Clin Kidney J 2020; 13: sfaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG.. Statistics notes. The odds ratio. BMJ 2000; 320: 1468–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliver MJ, Quinn RR, Richardson EP. et al. Home care assistance and the utilization of peritoneal dialysis. Kidney Int 2007; 71: 673–678 [DOI] [PubMed] [Google Scholar]
- 33.Mendelssohn DC, Mujais SK, Soroka SD. et al. A prospective evaluation of renal replacement therapy modality eligibility. Nephrol Dial Transplant 2008; 24: 555–561 [DOI] [PubMed] [Google Scholar]
- 34.Machowska A, Alscher MD, Vanga SR. et al. Offering Patients Therapy Options in Unplanned Start (OPTiONS): implementation of an educational program is feasible and effective. BMC Nephrol 2017; 18: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blake PG, Quinn RR, Oliver MJ.. Peritoneal dialysis and the process of modality selection. Perit Dial Int 2013; 33: 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jager KJ, Korevaar JC, Dekker FW. et al. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am J Kidney Dis 2004; 43: 891–899 [DOI] [PubMed] [Google Scholar]
- 37.Machowska A, Alscher MD, Reddy VS. et al. Factors influencing access to education, decision making, and receipt of preferred dialysis modality in unplanned dialysis start patients. Patient Prefer Adherence 2016; 10: 2229–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddux DW, Usvyat LA, Blanchard T. et al. Transition period clinical trajectories for PD versus HD starters. Perit Dial Int 2019; 39: 42–45 [DOI] [PubMed] [Google Scholar]
- 39.Oliver MJ, Garg AX, Blake PG. et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant 2010; 25: 2737–2744 [DOI] [PubMed] [Google Scholar]
- 40.Smart NA, Titus TT.. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 2011; 124: 1073–1080 [DOI] [PubMed] [Google Scholar]
- 41.Kazmi WH, Obrador GT, Khan SS. et al. Late nephrology referral and mortality among patients with end-stage renal disease: a propensity score analysis. Nephrol Dial Transplant 2004; 19: 1808–1814 [DOI] [PubMed] [Google Scholar]
- 42.Winkelmayer WC, Owen WF Jr, Levin R. et al. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol 2003; 14: 486–492 [DOI] [PubMed] [Google Scholar]
- 43.Rioux JP, Cheema H, Bargman JM. et al. Effect of an in-hospital chronic kidney disease education program among patients with unplanned urgent-start dialysis. Clin J Am Soc Nephrol 2011; 6: 799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machowska A, Rutherford P.. Peritoneal dialysis use within the context of the population and healthcare systems of Europe – differences, trends and future challenges. Int J Artif Organs 2016; 39: 211–219 [DOI] [PubMed] [Google Scholar]
- 45.Ivarsen P, Povlsen JV.. Can peritoneal dialysis be applied for unplanned initiation of chronic dialysis? Nephrol Dial Transplant 2014; 29: 2201–2206 [DOI] [PubMed] [Google Scholar]
- 46.Povlsen JV, Sorensen AB, Ivarsen P.. Unplanned start on peritoneal dialysis right after PD catheter implantation for older people with end-stage renal disease. Perit Dial Int 2015; 35: 622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu FX, Ghaffari A, Dhatt H. et al. Economic evaluation of urgent-start peritoneal dialysis versus urgent-start hemodialysis in the United States. Medicine (Baltimore) 2014; 93: e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Povlsen JV, Ivarsen P.. Assisted automated peritoneal dialysis (AAPD) for the functionally dependent and elderly patient. Perit Dial Int 2005; 25(Suppl 3): S60–S63 [PubMed] [Google Scholar]
- 49.Heaf J, Heiro M, Peterson A et al. .Dialysis Modality Choice [dataset]. www.Osf.io/qgr6c (date last accessed, 23 December 2020)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available on the Open Source Project (OSP) data repository [49].