Abstract
Background
Chagas disease (caused by Trypanosoma cruzi infection) evolves to chronic chagasic cardiomyopathy (CCC) affecting 1.8 million people worldwide. This is the first randomized, placebo-controlled, double-blinded, clinical trial designed to estimate efficacy and safety of selenium (Se) treatment in CCC.
Methods
66 patients with CCC stages B1 (left ventricular ejection fraction [LVEF] > 45% and no heart failure; n = 54) or B2 (LVEF < 45% and no heart failure; n = 12) were randomly assigned to receive 100 mcg/day sodium selenite (Se, n = 32) or placebo (Pla, n = 34) for one year (study period: May 2014-September 2018). LVEF changes over time and adverse effects were investigated. Trial registration number: NCT00875173 (clinicaltrials.gov).
Findings
No significant differences between the two groups were observed for the primary outcome: mean LVEF after 6 (β= +1.1 p = 0.51 for Se vs Pla) and 12 months (β= +2.1; p = 0.23). In a subgroup analysis, statistically significant longitudinal changes were observed for mean LVEF in the stage B2 subgroup (β= +10.1; p = 0.02 for Se [n = 4] vs Pla [n = 8]). Se treatment was safe for CCC patients, and the few adverse effects observed were similarly distributed across the two groups.
Interpretation
Se treatment did not improve cardiac function (evaluated from LVEF) in CCC. However, in the subgroup of patients at B2 stage, a potential beneficial influence of Se was observed. Complementary studies are necessary to explore diverse Se dose and/or associations in different CCC stages (B2 and C), as well as in A and B1 stages with longer follow-up.
Funding
Brazilian Ministry of Health, Fiocruz, CNPq, FAPERJ.
Keywords: Selenium, Trypanosoma cruzi, Chagas disease, cardiopathy, biomarkers
Research in context.
Evidence before this study
The role of selenium (Se) salts or organic compounds in the treatment of heart disease is still under study for different clinical conditions. In Chagas disease (CD) myocardiopathy, due to infection by Trypanosoma cruzi, we have previously shown that Se levels in patients’ sera decrease in severe cases.
Added value of this study
This clinical trial adds to the propaedeutic and therapeutics of Se in chronic chagasic cardiomyopathy (CCC), showing an adjuvant and complementary approach that improves treatment of patients with CCC. We showed a potential beneficial effect of Se treatment in a subset of patients with CCC with ventricular dysfunction (LVEF <45%). Despite trial limitations, this is a novel therapeutic option for mild CCC. Additionally, Se may decrease the speed of worsening cardiac function and/or may even improve cardiac function in patients with CCC stage B2.
Implications of all the available evidence
Considering the potential beneficial influence of Se treatment in a subset of CCC patients, and that Se supplementation to CCC patients is safe, Se might be prescribed as an antioxidant complementary therapeutic approach for patients with CCC B2 stage. New clinical trials with a longer follow-up are needed to investigate the effects of Se in the mild (stages A and B) or severe (stages C and D) CCC, and in the asymptomatic indeterminate clinical form.
Alt-text: Unlabelled box
Introduction
Chagas disease (CD) affects 6–7 million people worldwide and is considered one of the most neglected tropical diseases [1,2]. CD spread to other continents because of enhanced global travelers and migration to and from Latin America [3]. Anti-parasite treatment is recommended to prevent or reduce disease progression in both the acute and chronic phases [4,5].
CD present three clinical forms in the chronic phase [6], [7], [8], all requiring follow-up and/or symptomatic treatment: (i) indeterminate, in 70% of seropositive cases, a silent subclinical infection without symptoms and with normal electrocardiogram (ECG); (ii) chronic chagasic cardiopathy (CCC), in ∼30% of chronically infected people, with typical ECG alterations associated or not with clinical symptoms; (iii) digestive in ∼10% of seropositive cases; mixed forms may also occur [6]. Echocardiography (ECHO) and ECG changes can further categorize CCC into stages from A to D7, a classification of CD cardiac form proposed in 2005 and adopted in 2015 in the 2nd Brazilian Consensus on Chagas Disease [8]. Left ventricular ejection fraction (LVEF) is the most powerful predictor of mortality in CCC when associated to the presence of segmental wall motion abnormalities [6]. Patients in stage A present typical CD ECG changes but not ECHO abnormalities; stage B patients present wall motion abnormalities and according to LVEF can be divided in B1, LVEF ≥ 45%, and B2 <45%; stage C patients have LVEF <45% and heart failure (HF) symptoms and, in case of end-stage HF, the patient is reclassified as stage D. Each stage is associated with different prognosis and progressive increase in mortality: the 5-year mortality of D patients is 98%, 91% in C patients, 45% in B patients and only 13% in patients on stage A [7,8]. The search for innovative therapies to slow and decrease CCC progression or mortality would be of real benefit to CD patients, and this is the rational for testing selenium (Se) treatment in early CCC (stage B) in the present trial.
In the natural course of CD [9], especially in CCC, a chronic inflammation increases the oxidative status of the infected person, as indicated by biomarkers such as glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase, glutathione reductase, glutathione S-transferase, reduced glutathione, vitamin E, thiobarbituric acid reactive substances and protein carbonyl [10,11]. Heart disease progression in CD is also followed by a decrease in Se status [12]. Se-dependent enzymes and proteins turn Se into a crucial microelement for many adaptive functions, such as thyroid hormone metabolism, antioxidant defense systems, adaptive and acquired immune function, prevention of certain cancers and optimal functioning of the cardiovascular system [13,14]. It is a consensus that Se deficiency (<40 mcg/L) may increase the risk of coronary artery disease, although the effect of Se supplementation is still controversial on ischemic heart disease according to numerous randomized controlled trials reviewed in 201,513. Se supplementation in Swedish elderly people with low Se levels showed a decrease in cardiovascular mortality [15,16] and improvement in the quality of life [17]. A 2017 meta-analysis, including 43.998 participants, found a decrease of serum C-reactive protein and an increase of GPx levels after Se supplementation, suggesting a positive effect on reducing oxidative stress and inflammation, despite the absent of direct effect on reduction of cardiovascular mortality [14]. Plasma Se status varies according to Se in diet, decreasing in those who are frail, poorly nourished, and unwell [18], as is the case for most of the population affected by CD. Se status depends on the availability of Se in the soils [19] and extremely low or high plasma Se concentrations lead to different effects on health. Data on Se levels are known for US, Canada, Europe, and Middle East [20] but lack for Latin American countries.
Due to the known involvement of chronic inflammation, oxidative stress, and fibrosis in the physiopathology mechanism of dilated myocardiopathies, including those related to infectious diseases [4,6], we studied Se levels in CD patients providing evidence that lower Se levels were observed for those with severe cardiac form of CD in comparison to healthy individuals and patients with T. cruzi infection without cardiac disease [12]. In the geographic region of Minas Gerais we found low Se levels even in control non infected adults, unsatisfactory for selenoprotein function [12]. Further pre-clinical studies in T. cruzi-infected mice showed that treatment with Se prevented heart dilatation and reversed acute and chronic cardiomyopathy [21] and prevented reduction of intestinal motility [22], introducing the concept that selenium treatment and/or supplementation could aid in therapy for CD [23]. We present here the results of the clinical trial named “Selenium Treatment and Chagasic Cardiopathy (STCC)” [24,25], the first randomized trial aiming to estimate the effect of Selenium (Se) versus Placebo (Pla) treatment on prevention of cardiac disease progression in chronic adult patients in early stages of CCC (B1 and B2). Contrasting to the long time necessary to follow-up clinical outcomes on indeterminate or CCC stage A patients, the choice of stage B patients allowed a shorter time of follow-up considering outcomes such as the continuous variable of “LVEF trajectory”[25] and the categorical variable of “rate of CD progression”[24] thus facilitating any further recommendation of Se association to CCC therapy guidelines. A very recent review from an independent group reinforced the hypothesis that Se could be an interesting option for the treatment of CD [26].
Methods
Study design
STCC is a single-center, prospective, double-blinded, placebo-controlled, phase 3, superiority randomized clinical trial aiming to estimate the effect of Se treatment on prevention of cardiac disease progression in patients with CD cardiac form. The full protocol is described elsewhere [24,25]. The intervention protocol comprised 100 mcg of sodium selenite (Se) once per day, during a meal, for 365 consecutive days, compared to Placebo (Pla). According to ICH Q7 Good Manufacturing Practices for Active Pharmaceutical Ingredients guidelines Se and Pla were produced for STCC in GMP/API conditions (Catalent Pharm. Co., Brazil). The study protocol was updated and fully adhered in 2018 [25], considering the primary outcome as a significant difference in LVEF values between Se and Pla groups over time. The sample size was calculated based on the improvement in LVEF of 5.3 ± 6.2% after micronutrient supplementation in patients with non-infective HF [27], considering a beta error of 20%, an alpha error of 5% and anticipating for 30% of losses to follow-up: 62 patients would be necessary for the study, 31 in each group [25]. Secondary outcomes [24] were disease progression indicated by the following parameters: (a) decrease in CCC progression stages (b) a change in 10 absolute percentual points from initial to final LVEF and/or (c) rate of adverse outcomes. The study was registered at clinicaltrials.gov (NCT00875173). Quality of Life (QoL) data was included as a secondary outcome in the original protocol [24] but was beyond the scope of the present study, as proposed in the updated protocol [25]. The Brazilian Medical Product Agency (ANVISA) authorized the study protocol (Special Announcement on Clinical Research 1244/2009). The following authors had full access to data, for management and analysis: TCdA-J, MTdH, MFFM, VBP, AMH-M, PEAAdB and ACCC.
Participants and clinical center: STCC was conducted at the cardiology center of Evandro Chagas National Institute of Infectious Diseases (INI-Fiocruz) in Rio de Janeiro, Brazil. INI is a national reference center for treatment and research in infectious diseases and tropical medicine in Brazil. Most patients with suspected CD in the state of Rio de Janeiro are referred to INI outpatient clinic for diagnostic investigation and integral health care. Regular diagnostic and therapeutic interventions followed the recommendations of the specific guidelines for diagnosis, monitoring and treating chronic CCC, updated by the II Brazilian Consensus on CD [8].
Randomization and masking: Randomization, allocation concealment and blinding were performed as described in the published protocol [24,25]. Briefly, a sequence was computer-generated to randomly allocate the patients into 2 groups in a 1:1 ratio. This sequence was generated in blocks of 4, 8 and 12 using the “blockrand” extension of the R Project software package. This sequence was available only to a pharmacist not involved in volunteer recruitment. This pharmacist decided which group would be the placebo group and which would be the intervention group by flipping a coin. Next, opaque boxes were filled either with placebo or sodium selenite capsules and later sealed and numbered to correspond to the computer-generated sequence. As patients underwent volunteer recruitment by the medical staff, they were assigned a number sequentially corresponding to a treatment box. Therefore, a strategy of numbered boxes was used for sequence concealment. All the patients, the health-care providers and the staff involved in outcome assessment were blinded to treatment. The blinding was conducted using the same strategy of allocation concealment by numbered boxes. Therefore, numbers were assigned to volunteers’ treatments, but only one pharmacist not involved in these tasks was aware of what was in each numbered box.
Recruitment and follow-up: Recruitment occurred from May 2014 (first inclusion) to August 2017 (last inclusion) among patients regularly followed at the outpatient clinic of INI-Fiocruz. The final visit of the last patient on follow-up occurred in September 2018. One of the difficulties for recruiting was the age limit and some comorbidities of the aging cohort of chronic CD patients [28,29]. The final study population included male and female seropositive cases for T. cruzi infection, from 18 to 75 years old, with typical CCC ECG findings, and presenting B1 or B2 CCC: normal, mild, or moderate LV systolic dysfunction, respectively LVEF >55%, between 54 and 45% or 44 and 35%. Exclusion criteria were: severe digestive form of CD (with dysphagia with evidence of food residues in the esophagus and/or with weight loss higher than 15% of usual weight in the previous four months), non-chagasic heart disease, pregnancy or breastfeeding, tobacco use, alcohol abuse, depression, other medical prescription of vitamins or supplements, residence close to mineral deposits, metal industries or places with radioactive exposure, participation as a volunteer in other clinical trials with any intervention, any condition that may result in low protocol adherence (residence far from the health center, frequent travel trips).
The participants were invited to 10 visits (V1 to V10) and received the proper financial support to cover transport costs in all appointments. Eligibility was detected when (a) inclusion criteria were fit in regular follow-up at the outpatient clinic, and (b) informed signed consent was obtained (V1=inclusion). Then interviews and tests were scheduled for baseline data collection (V2). These included questionnaires to collect (i) sociodemographic data, (ii) medical history, (iii) detailed information for a nutritional profile including Se intake in their diet [29], as well as (iv) clinical and cardiovascular exam (ECG and ECHO), and (v) biochemical and hematological laboratory measurements. In sequential visits (V3=21, V4=42, V5=60, V6= 120, V7=180, V8= 240, V9= 300 and V10=360 days) blood and/or urine collection were performed. Visits V3 to V9 were designed to detect and follow possible adverse effects, since this was the first Se supplementation trial in CD patients with CCC and a metanalysis study reported conflicting results on beneficial/deleterious/absent effects of Se supplementation in different heart disease conditions [14]. At V7 and V10 sequential ECG and ECHO were recorded. Participants received their GMP identical Se or Pla softgel capsules from the STCC professional pharmaceutic, each with their blinded codes, and were oriented to ingest one capsule/day during one meal.
Nutrition profile: Patients were oriented to do not change their common/regular diet and to do not eat Brazil nut (possible additional source of diet Se). Food intake was estimated by a blinded to randomization single-trained nutritionist using the mean of 24-h dietary recalls assessed in V2 and V10) using a previously validated food frequency questionnaire [30], to evaluate if the recommended dietary allowance (RDA) for adults is 55 mcg/day [14] was supplied by the participant´s regular diet. This dietary recall is a structured interview intended to capture detailed information about all foods and beverages consumed by the respondent in the past 24 h from midnight to midnight of the previous day. The dietitians entered dietary data directly into a nutrition analysis software (diet Win Plus software) to obtain a Se estimate of ingestion, the amount of carbohydrates, proteins, lipids, fiber, cholesterol, fatty acids (monounsaturated, polyunsaturated, saturated) and total calories intake. The consumption of each nutrient was adjusted using the nutrient residual model, that remove the variation caused by the total energy intake [30]. Anthropometric evaluation consisted of measurements of height, body weight, waist, and hip circumferences, tricipital skinfold thickness and arm circumference. Height and body weight were assessed with minimal clothes and without shoes using a calibrated digital scale coupled with a stadiometer. The body mass index (BMI) was calculated to determine the nutritional status [31]. Randomization was performed without any previous nutritional data collected and/or analyzed.
Blood samples and T. cruzi DNA analysis: Venous blood samples were obtained while the participants were at rest in a supine position. Different vials were used to collect serum, plasma, or whole blood for future immunological and biochemical studies. For Se measurements special trace elements-free vials were used. They were centrifuged at 3000g at 4°C and were frozen at −20 °C. No sample was thawed more than twice. For T. cruzi DNA analysis by polymerase chain reaction (PCR), 10 mL whole blood samples were collected, immediately mixed with an equal volume of 6 M Guanidine-HCl/0.2 M EDTA solution (GEB) and then split in two identical aliquots. GEB samples were coded and blinded delivered to perform the molecular tests. At the laboratory, GEB samples were boiled for 15 min and stored at 4°C until use. DNA extraction from two aliquots (300 µL) of the GEB sample was performed with commercial kit (High Pure PCR Template preparation kit, Roche Applied Science) according to the manufacturer's protocol. DNA solution was stored at −20 °C until use. Blood of individuals without T. cruzi infection (negative serology) from nonendemic CD areas were included as negative controls for DNA extraction and molecular assays. One negative control was used per DNA extraction of 11 GEB samples and tested in parallel with the DNA samples in all qPCR-based assays, as described elsewhere [29,32].
Selenium analysis: Se in plasma samples was determined by high-performance liquid chromatography inductively coupled to plasma mass spectrometry (ICP–MS) with element- specific detectors in a local clinical laboratory (Diagnósticos da América, Rio de Janeiro). The method was proven valuable for determining trace elements at µg levels [33]. Se content was determined after a 50-fold dilution with 0.16 M HNO3 and quantified by using addition calibration and gallium as an internal standard. The method accuracy was evaluated by analysis of a human serum certified reference material (Seronorm Serum level II, Sero A/S, Norway).
Echocardiography: ECHO imaging was conducted with Vivid 7 or Vivid I ultrasound systems (GE Medical Systems, Milwaukee, WI, USA) equipped with a four-matrix transducer from 2 to 4 MHz. LVEF and end-systolic and end-diastolic LV volumes were estimated according to Simpson's rule [34]. Normal systolic function was defined as LVEF ≥ 55%, mild dysfunction as LVEF between 54 and 45% and moderate dysfunction as LVEF between 44 and 35% and severe dysfunction as LVEF<35%.
Data analysis and statistics: Data study were performed using SAS and Stata softwares. A p value ≤0.05 gave support against the null hypothesis for all analyses. Baseline characteristics were expressed as absolute and percentages values for categorical variables and as mean ± standard deviation for continuous variables. We checked all continuous variables for normality and those that demonstrated a skewed distribution were presented as median (IQR 25%−75%). To evaluate differences in LVEF longitudinal changes between groups, intention-to-treat analyses were performed using linear mixed effect models. This type of analysis assesses the rate of change of the outcomes by the time X intervention group interaction term, considering the correlations between repeated measures over time and missing data. All models were fitted using random intercept and were assumed as an unstructured variance-covariance pattern. Models were adjusted for baseline LVEF. Residual plots of all models were examined and did not show major deviations from regression assumptions. For categorical variables, additional analysis was performed using generalized estimated equations with log link and binomial distribution to evaluate changes in the proportion of individuals between groups. Comparison between groups for the proportion of combined occurrence and severity of all adverse events were performed using the Fisher`s exact test.
Ethical considerations: The STCC trial was approved by the INI Ethics Committee (IRB 0043.0.009.000–04, on April 11th, 2005; the first Ethical Agreement dates from 2008 but regulatory issues, trial team composition and changes in the selenium manufacturer caused an important delay in the project and we started the recruitment in 2014; the final modified protocol was reevaluated on February 26th, 2018 and renumbered IRB 88,797,318.7.0000.5248 on June 14th, 2018) on date and conformed to standards currently applied by the Brazilian National Committee for Research Ethics (CONEP). An external data safety monitoring board (DSMB) followed the trial to guarantee the quality of the study.
Role of the funding source: The sponsors of STCC had no role in the study design, data collection, data analysis, data interpretation or the writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility of the decision to submit the report for publication.
Results
Baseline characteristics of the participants
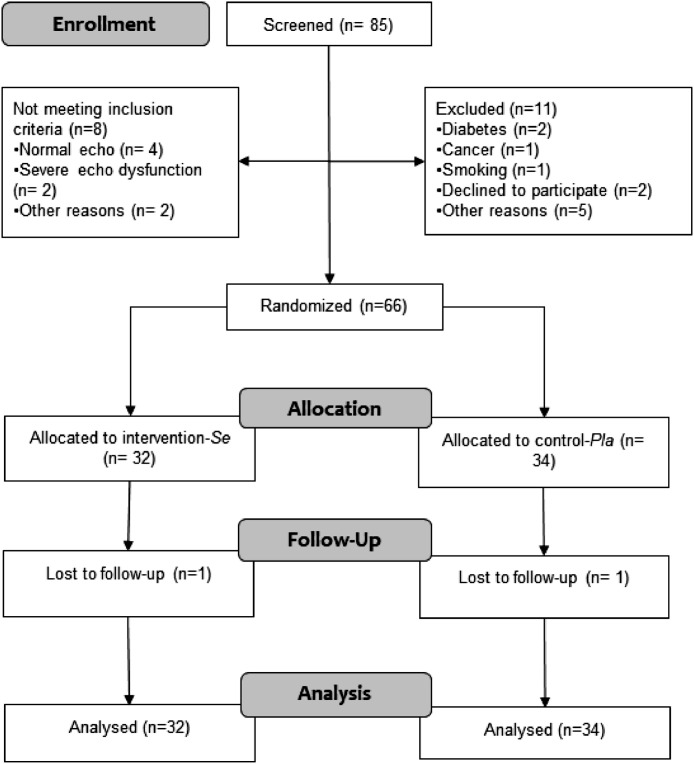
STCC study CONSORT flow diagram is shown in Fig. 1. We screened 85 patients and randomized 66 patients. They were randomly allocated to intervention (n = 32) or control (n = 34) groups and only one discontinued treatment in each group (no adverse effect was reported for these participants). The characteristics of the patients at baseline did not show any major differences between groups (Table 1).
Fig. 1.
CONSORT 2010 flowchart of study participants indicating the two groups: Selenium (active treatment) and Placebo.
Table 1.
Characteristics of the study patients at baseline.
| Variables | All (n = 66) | Placebo (n = 34) | Selenium (n = 32) |
|---|---|---|---|
| Mean (+SD) or Median (IQR 25%−75%) Frequency (%) |
|||
| Age (Years) | 64.6 ± 10.0 | 65.1 ± 8.7 | 64.0 ± 11.4 |
| Sex | |||
| Male | 29 (43.9) | 16 (47.1) | 13 (40.6) |
| Female | 37 (56.1) | 18 (52.9) | 19 (59.4) |
| Schooling | |||
| Complete Primary | 22 (33.3) | 9 (26.5) | 13 (40.6) |
| Incomplete Primary | 21 (31.8) | 9 (26.5) | 12 (37.5) |
| Complete Secondary | 15 (22.7) | 10 (29.4) | 5 (15.7) |
| Incomplete Secondary | 1 (1.5) | 1 (2.9) | 0 (0.00) |
| Tertiary | 3 (4.6) | 2 (5.9) | 1 (3.1) |
| Illiterate | 4 (6.1) | 3 (8.8) | 1 (3.1) |
| Clinical Characteristics | |||
| Simpson LVEF (%) | 54.8 ± 10.4 | 53.2 ± 11.4 | 56.4 ± 9.1 |
| CCC Classification | |||
| B1 | 54 (81.8) | 26 (76.5) | 28 (87.5) |
| B2 | 12 (18.2) | 8 (23.5) | 4 (12.5) |
| Associated digestive form | |||
| Yes | 7 (10.6) | 4 (11.8) | 3 (9.4) |
| No | 59 (89.4) | 30 (88.2) | 29 (90.6) |
| Previous Benznidazol Treatment | |||
| Yes | 3 (4.5) | 0 (0.00) | 3 (9.4) |
| No | 60 (90.9) | 33 (97.1) | 27 (84.4) |
| Ignored | 3 (4.6) | 1 (2.9) | 2 (6.2) |
| Cardiac Device (pacemaker or ICD) | |||
| Yes | 4 (6.1) | 2 (5.9) | 2 (6.3) |
| No | 62 (93.9) | 32 (94.1) | 30 (93.7) |
| Atrial Fibrilation | |||
| Yes | 1 (1.5) | 0 (0.00) | 1 (3.1) |
| No | 65 (98.5) | 34 (100.00) | 31 (96.9) |
| Previous Embolic Stroke | |||
| Yes | 7 (10.6) | 4 (11.7) | 3 (9.4) |
| No | 59 (89.4) | 30 (88.3) | 29 (90.6) |
| Hypertension | |||
| Yes | 24 (36.4) | 10 (29.4) | 14 (43.7) |
| No | 42 (63.6) | 24 (70.6) | 18 (56.3) |
| ECG abnormalities | |||
| CD-related abnormalities | 60 (90.9) | 32 (94.1) | 28 (87.5) |
| Other | 6 (9.1) | 2 (5.9) | 4 (12.5) |
| Diastolic Dysfunction | |||
| None | 16 (24.3) | 10 (29.4) | 6 (18.8) |
| Grade I (impaired relaxation) | 39 (59.1) | 16 (47.1) | 23 (71.9) |
| Grade II (pseudonormalization) | 9 (13.6) | 8 (23.5) | 1 (3.1) |
| Grade III (restrictive) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-classifiable | 1 (1.5) | 0 (0.0) | 1 (3.1) |
| Ignored | 1 (1.5) | 0 (0.0) | 1 (3.1) |
| Blood Tests | |||
| Positive conventional PCR | 22 (39.3) | 10 (34.5) | 12 (44.4) |
| TSH (microU/mL) | 1.8 (1.1–2.6) | 1.9 (1.2–2.6) | 1.6 (1.1–2.6) |
| T3 (mmol/L) | 1.7 (1.4–2.2) | 1.7 (1.2–2.3) | 1.7 (1.5–2.1) |
| Free T4 (ng/dL) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (1.0–1.3) |
| Leukocyte Count (cel/mm3) | 6088 ± 1420 | 6071 ± 1200 | 6105 ± 1641 |
| Neutrophils (cel/mm3) | 3285 ± 1112 | 3263 ± 1047 | 3308 ± 1194 |
| Haemoglobin (g/dL) | 13.8 (13.3–14.7) | 13.8 (13.3–15.0) | 13.7 (13.3–14.7) |
| Platelets (cel/mm3) | 220,500 (194,000–253,000) | 217,000 (176,000–238,000) | 229,000 (207,500–256,500) |
| ALT (U/L) | 32.0 (25.0–39.0) | 33.0 (25.0–41.0) | 30.0 (26.0–38.0) |
| AST (U/L) | 25.0 (21.0–30) | 27.0 (21.0–31.0) | 24.0 (21.0–29.0) |
| Total Bilirubin (mg/dL) | 054 (0.45–0.66) | 0.58 (0.50–0.74) | 0.50 (0.39–0.57) |
| Direct Bilirubin (mg/dL) | 0.13 (0.10–0.16) | 0.13 (0.10–0.17) | 0.12 (0.10–0.16) |
| Alkaline Phosphatase (U/L) | 77.62 ± 17.31 | 79.31 ± 14.79 | 75.87 ± 19.7 |
| Creatinine (mg/dL) | 0.90 (0.82–1.08) | 0.90 (0.80–1.11) | 0.90 (0.85–1.08) |
| Urea (mg/dL) | 34.54 ± 8.5 | 33.38 ± 5.8 | 35.81 ± 10.7 |
| Glucose (mg/dL) | 95.5 (87.0–103.0) | 92.0 (87.0–103.0) | 97.0 (91.0–103.0) |
| Albumin (g/dL) | 4.00 ± 0.3 | 4.03 ± 0.2 | 3.96 ± 0.3 |
| Iron Binding Capacity (microg/dL) | 323.63 ± 47.9 | 327.23 ± 49.9 | 320.03 ± 46.4 |
| Iron (microg/dL) | 101 (72–117) | 101 (81–123) | 100.5 (68.0–113.0) |
| Troponin (mg/L) | 0.02 (0.0–0.06) | 0.04 (0.0–0.1) | 0.01 (0.0–0.04) |
| Serum Selenium (mcg/L) | 95.4 ± 21.3 | 94.2 ± 23.4 | 96.6 ± 19.1 |
| Nutritional Characteristics | |||
| Weight (Kg) | 70.4 ± 12.9 | 71.21 ± 12.9 | 69.61 ± 12.9 |
| Height (meters) | 1.60 ± 0.1 | 1.61 ± 0.1 | 1.59 ± 0.1 |
| BMI (Kg/m2) | 27.4 ± 4.3 | 27.6 ± 4.3 | 27.3 ± 4.2 |
| Caloric Intake (Kcal) | 1617.2 (1283.9–1880.2) | 1741.9 (1447.8–2074.9) | 1475.3 (1274.5–1707.3) |
| Adjusted Protein Intake (g) | 68.6 (54.3–89.4) | 71.4 (52.1–99.1) | 67.9 (57.5–78.4) |
| Adjusted Carbohydrate Intake (g) | 223.8 (181.8–264.9) | 210.4 (183.5–264.0) | 239.7 (174.1–265.8) |
| Adjusted Lipid Intake (g) | 45.9 (36.7–59.9) | 48.7 (36.0–60.0) | 44.1 (37.6–55.5) |
| Adjusted Selenium Intake (microgram) | 72.5 (50.4–104.8) | 78.4 (53.6–111.9) | 60.0 (48.1–91.1) |
| Insufficient diary Se ingestion (<55mcg/day) | 28 (46.7) | 10 (35.7) | 18 (63.3) |
| Adequate diary Se ingestion (≥55mcg/day) | 32 (53.3) | 20 (62.5) | 12 (37.5) |
| Medication | |||
| Beta-blockers | 27 (41.5) | 14 (42.4) | 13 (40.6) |
| Angiotensin-converting enzyme inhibitors/Angiotensin II receptor blockers | 48 (72.7) | 27 (79.4) | 21 (65.6) |
| Aldosterone antagonist | 5 (7.7) | 4 (12.1) | 1 (3.1) |
| Amiodarone | 7 (10.6) | 4 (11.7) | 3 (9.4) |
| Warfarin | 14 (21.2) | 7 (20.6) | 7 (21.9) |
Data are mean ± standard deviation or n (%).
CCC = Chagas Chronic Cardiopathy; ICD = implantable cardiac defibrillator; BMI=body mass index; CD=Chagas disease.
Number of observations for variables with missing information.
Control group (n = 34): LVEF (n = 32); TSH (n = 29); T3 (n = 31); Free T4 (n = 31); Alkaline Phosphatase (n = 32); Iron (n = 33); Protein Intake (n = 30); Carbohydrate Intake (n = 30); Lipid Intake (n = 30); Selenium Intake (n = 30); Simpson LVEF (n = 32); Total Bilirubin (n = 33); Creatinine (n = 31); Albumin (n = 33); Iron Binding Capacity (n = 31); Caloric Intake (n = 30); Troponin (n = 31); Beta-blockers (n = 33); Aldosterone Antagonist (n = 33); Serum Selenium (n = 31); Selenium ingestion estimates (n = 30).
Intervention group (n = 32): LVEF (n = 30); TSH (n = 29); T3 (n = 28); Free T4 (n = 28); Haemoglobin (n = 31); Alkaline Phosphatase (n = 31); Iron (n = 30); Protein Intake (n = 30); Carbohydrate Intake (n = 30); Lipid Intake (n = 30); Selenium Intake (n = 30); Simpson LVEF (n = 30); Urea (n = 31); Creatinine (n = 30); Iron Binding Capacity (n = 31); Direct Bilirubin (n = 30); Caloric Intake (n = 30); Troponin (n = 30); Serum Selenium (n = 30); Selenium ingestion estimates (n = 30).
Age ranged from 34 to 72 years, with a mean of 64 years and a slight predominance of women (56%, Table 1). All participants have immigrated to Rio de Janeiro from different Brazilian states, 80% came from six states of the Northeast region (data not shown) and vector transmission at their endemic birth places accounted for 84% of their epidemiology histories. Only 27% reported regular labor occupations and ¾ of the participants were unemployed, retired or dealt with homework (data not shown). Despite currently living in urban areas in Rio de Janeiro in regular houses (98%) after leaving their original poor endemic areas for more than 43 years (mean), their education status remained low: 70% were illiterate or have only completed Elementary School (Table 1). From all the included participants, 81.8% were in B1 stage (n = 54) and 18.2% in the B2 stage (n = 12), with similar proportions in the groups that were allocated for Pla and Se treatments. An associated digestive form was observed in about 10% of the participants and more than 90% had no history of previous benznidazole (Bz) treatment. Other relevant clinical characteristics were a small percentage of patients using pacemaker or ICD devices (6%); most patients showed CD related ECG abnormalities (91%). Most participants (60.7%) had negative T. cruzi DNA PCR blood tests. PCR positivity and nutritional Se estimated levels did not account for exclusion or inclusion criteria, nor were related to primary or secondary expected STCC outcomes; however, it was relevant to follow them for further comparisons after the intervention, especially to search for correlation with possible improvement or worsening on CCC participants. Mean laboratory parameters analyzed were in the normal range and did not differ between the two groups (Table 1). The patients used some medications at baseline according to clinical and cardiological prescriptions recommended for the profile of these early stages of CCC (Table 1).
Adherence to protocol was checked by quantifying the number and the percentage of ingested capsules and showed no difference between the two groups: Pla= 364.1 + 5.1 vs Se= 360.9 + 16.2; 99.8% vs 98.9% of the capsules were ingested respectively for Pla and Se (p-value= 0.27).
Longitudinal analysis
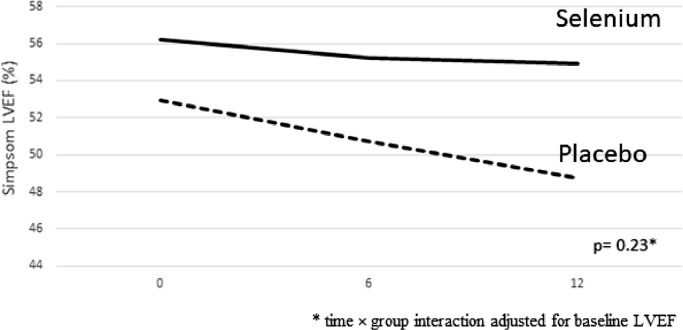
The participants were treated for one year with 100 mcg/day sodium selenite (Se) or placebo (Pla) in a double-blinded way and LVEF changes (primary outcome) were followed (Fig. 1, Table 2, Fig. 2). Only two participants were lost to follow-up after one-year of treatment (Fig. 1) due to absence from the scheduled appointment at 12 months for travel reasons (they were recovered for future analysis after 5 years). The longitudinal changes for LVEF during the follow-up (overall and stratified by LVEF> and < 45%) are demonstrated in Table 2. LVEF had a slight decrease during the follow-up (Fig. 2), but no significant differences were observed for LVEF changes between groups after 6 (β= +1.1; p = 0.51 for Se vs Pla) and 12 months (β= +2.1; p = 0.23 for Se vs Pla). A subgroup analysis considering LVEF ≥ 45% and <45% at baseline (Table 2) showed that in the subgroup of LVEF > 45%, the LVEF trajectories remained stable in both Pla and Se groups, with no significant differences between groups after one year of follow-up (Pla= 54.5 ± 9.2 vs Se = 56.1 ± 11.2, β=+0.70, p = 0.73; Table 2). However, in the subgroup with LVEF <45% in the beginning of the study (characterized as moderate LV systolic dysfunction), a significant difference (β= +10.1; p = 0.02) was found for the trajectories between groups (Se vs Pla) after one year (Table 2). We performed sensitivity analysis considering different LVEF cut-off points (>50% and > 55%) and no longitudinal changes were observed for the proportion of patients with LVEF > and < 50% (p = 0.64) and LVEF > and < 55% (p = 0.27) after 1-yr of follow-up. The per-protocol analysis including only those that completed the study follow-up demonstrated similar results, with no significant difference for the selenium group in comparison to placebo after 6 (β=+1.1; p = 0.54) and 12 months (β=+2.4; p = 0.20) of follow-up.
Table 2.
Crude means and beta coefficients for LVEF trajectories during the follow-up (overall and stratified according to baseline LVEF≥45%).
| OVERALL | Baseline (Pla n = 32; Se n = 30) |
6 months (Pla n = 31; Se n = 30) |
β | p-value | 12 months (Pla n = 32; Se n = 29) |
β | p-value | |
| Pla | 53.2 ± 11.4 | 50.9 ± 12.3 | +1.14 | 0.51 | 49.9 ± 12.5 | +2.13 | 0.23 | |
| Se | 56.4 ± 9.2 | 55.0 ± 9.9 | 55.3 ± 10.8 | |||||
| LVEF ≥45% | Baseline (Pla n = 24; Se n = 26) |
6 months (Pla n = 21; Se n = 25) |
β | p-value | 12 months (Pla n = 22; Se n = 25) |
β | p-value | |
| Pla | 57.6 ± 9.6 | 55.5 ± 8.8 | +0.47 | 0.81 | 54.5 ± 9.2 | +0.70 | 0.73 | |
| Se | 58.6 ± 7.8 | 56.6 ± 9.5 | 56.1 ± 11.2 | |||||
| LVEF <45% | Baseline (Pla n = 8; Se n = 4) |
6 months (Pla n = 8; Se n = 4 |
β | p-value | 12 months (Pla n = 8; Se n = 3) |
β | p-value | |
| Pla | 40.0 ± 2.7 | 36.4 ± 8.8 | +4.13 | 0.30 | 34.0 ± 6.3 | +10.11 | 0.02 | |
| Se | 42.5 ± 3.0 | 43.0 ± 2.4 | 47.3 ± 4.1 | |||||
Data are mean ± standard deviation.
β = linear mixed-effect model including time, treatment, and time*treatment adjusted by baseline LVEF (Se vs Pla).
Fig. 2.
Adjusted trajectories of LVEF during the follow-up of the two trial groups: Placebo (dashed line) and Selenium (solid line).
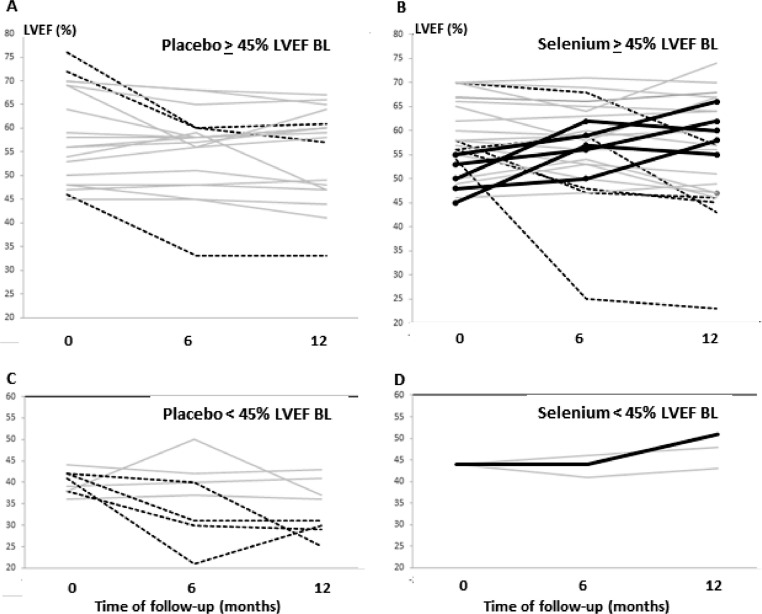
Individual crude trajectories of participants showing the LVEF evolution during follow-up in the two subgroups of the Pla and Se are depicted in Fig. 3. In all the subgroups some trajectories that did not change over 5 absolute percentual points were observed (shown in gray in Fig. 3). Trajectories with progressive decrease in LVEF (shown in black dotted lines in Fig. 3A, 3B, 3C) were seen in all the three subgroups except those with <45% in the baseline and treated with selenium (Fig. 3D). Only in the Se subgroups we observed growing trajectories (Fig. 3B, n = 5, in the subgroup ≥ 45%; and Fig. 3D, n = 1, in the subgroup <45%) indicating potential improvements in LVEF during the one year of follow-up. However, the percentage of patients with LVEF ≥45% did not change during the follow-up (Pla 74.2% vs Se 86.7%, p = 0.86 for 6 months and Pla 62.5% vs Se 89.7%, p = 0.10 for 1-year). An additional analysis to explore the influence of baseline serum Se levels on the LVEF responses to Se treatment did not demonstrate any differential effect (p-value for interaction time X group X baseline serum selenium =0.82). A sensitivity analysis adjusting the models for baseline Selenium consumption did not change the results.
Fig. 3.
Crude individual LVEF trajectories of patients treated with Placebo (A, C) or Selenium (B, D) during one year of follow-up, shown in the two different subgroups presenting baseline (BL) LVEF ≥ 45% (A, B) or <45% (C, D). Solid black lines highlight patients with rising trajectories, increasing > 5 absolute percentual points in LVEF. Dotted lines show cases with falling trajectories, decreasing > 10 absolute percentual points. Gray lines indicate patients varying < 5 percentual points in LFEV during 12 months of follow-up. Note that all the 6 patients showing an increase in LVEF (solid black lines) are only in the Selenium group (B, D).
Secondary outcomes concerning CCC stages and their changes during the one-year of follow-up were investigated (Table 3). After one year, most of the cases remained in the same CCC stage, both for Pla (75.8%) and Se (87.1%). Nine cases of worsening in CCC stages were observed, (n = 7 [21.2%] in Pla and n = 2 [6.5%] in Se) and three cases of improvement (n = 1 [3.0%] in the Pla and n = 2 [6.4%] in Se, Table 3) were observed with no significant differences between Pla and Se groups.
Table 3.
Chagas Chronic Cardiopathy classification during the follow-up.
| OVERALL | Baseline |
6 months |
p-value | 12 months |
p-value | |||
|---|---|---|---|---|---|---|---|---|
|
Pla n = 34 (%) |
Se n = 32 (%) |
Pla n = 33 (%) |
Se n = 32 (%) |
Pla n = 33 (%) |
Se n = 31 (%) |
|||
| Stage A | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 | 1 (3.03) | 0 (0.0) | 0.25 |
| Stage B1 | 26 (76.5) | 28 (87.5) | 25 (75.7) | 28 (87.5) | 20 (60.6) | 28 (90.3) | ||
| Stage B2 | 8 (23.5) | 4 (12.5) | 7 (21.2) | 3 (9.4) | 9 (27.3) | 2 (6.4) | ||
| Stage C | 0 (0.0) | 0 (0.0) | 1 (3.0) | 1 (3.1) | 3 (9.1) | 1 (3.2) | ||
| CCC worsening, (B1 to B2/C or B2 to C) |
– | – | 2 (6.1) | 1 (3.1) | 0.70 | 7 (21.2) | 2 (6.5) | 0.09 |
| CCC improvement, (B2 to B1 or B1 to A) |
– | – | 1 (3.0) | 1 (3.1) | 1 (3.0) | 2 (6.4) | ||
| CCC maintenance | – | – | 30 (90.9) | 30 (93.8) | 25 (75.8) | 27 (87.1) | ||
* Multilevel mixed effect generalised linear model with logit link and ordinal distribution.
Safety and side effects
Concerning the safety and adverse drug reactions (ADR) to Se treatment, we compared clinical and laboratory records of patients in Se group (n = 287 records) and Pla group (n = 285 records) and did not found any typical Se-related ADR (irritability, pain, tremor, nausea, vomiting, alopecia, hair/nail dryness). The most common ADR recorded (in about 3% of the patients in each group) was mild leucopenia/ neutropenia/thrombocytopenia (Table 4), but the distribution of ADR was similar in both groups (p = 0.84). Other minor reactions recorded were rare. No differences for severity of ADR were observed between groups (p = 0.82).
Table 4.
Adverse events by intervention group during the follow-up.
| Adverse drug reactions |
Placebo (n = 302) |
Selenium (n = 310) |
p-value* |
|---|---|---|---|
| Cardiac Arrhythmia | 1 (100.0) | 0 (0.0) | 0.84 |
| Arthralgia/Arthritis | 3 (75.0) | 1 (25.0) | |
| Tiredness | 0 (0.0) | 1 (100.0) | |
| Headache/Migraine | 0 (0.0) | 1 (100.0) | |
| Palmoplantar Desquamation | 0 (0.0) | 1 (100.0) | |
| Abdominal Pain | 2 (40.0) | 3 (60.0) | |
| Elevated Alkaline Phosphatase | 0 (0.0) | 1 (100.0) | |
| Elevated Serum Bilirubin | 0 (0.0) | 1 (100.0) | |
| Perianal Fistula | 1 (100.0) | 0 (0.0) | |
| Leukopenia/Neutropenia | 10 (47.6) | 11 (52.4) | |
| Low Back Pain | 1 (100.0) | 0 (0.0) | |
| Altered Levels of Thyroid Hormones | 2 (66.7) | 1 (33.3) | |
| Upper Limb Paresis | 1 (100.0) | 0 (0.0) | |
| Urticaria Plates in Trunk and Limbs | 0 (0.0) | 1 (100.0) | |
| Low Platelet Count | 3 (75.0) | 1 (25.0) | |
| Pruritus | 1 (100.0) | 0 (0.0) | |
| Lower Limb Peripheral Polyneuropathy | 0 (0.0) | 1 (100.0) | |
| Others | 18 (54.5) | 15 (45.5) | |
| No Events | 259 (48.9) | 271 (51.1) | |
| Severity of ADR | |||
| Severe | 2 (50.0) | 2 (50.0) | 0.81 |
| Moderate | 6 (42.9) | 8 (57.1) | |
| Low | 14 (60.9) | 9 (39.1) | |
| No Events | 259 (48.9) | 271 (51.1) | |
| No severity information | 21 (51.2) | 20 (48.8) |
Data are n (%); * Fisher`s exact test.
Laboratory findings
T. cruzi PCR: We were able to isolate and amplify DNA in 59 out of the 66 patients starting the protocol (data not shown). At baseline, the T. cruzi PCR positivity was observed in 10 out of 31 (32%) and 13 out of 28 (46%) in the Pla and Se groups, respectively. After one year the two groups were still similar despite the slightly lower percentage of PCR positivity (Pla 26.1% vs Se 39.1%; p = 0.60) and the lower parasite load in positive samples in the Se group, which changed from 2.1 ± 1.4 to 0.9 ± 0.7 parasite equivalents/mL from baseline to one-year after treatment (data not shown). However, the small number of positive samples in which the study of parasite load could be performed was a limitation for a conclusion on this point. We also studied if higher PCR positivity was associated with the 12 cases that progressed to a worse outcome in one-year follow up (Table 3) but did not find any association: in the Pla group, 4 out of 8 cases were PCR negative, 3 were positive and 1 was missing; in the Se group, 2 out of 4 cases were positive, 1 was negative and another was missing. In the case of B1 patients that remained in this stage during follow up (Table 3), the percentage of positive PCR after 1 year was 7 out of 23 cases (30%) for the Pla group and 9 out of 28 cases (32%) for the Se group.
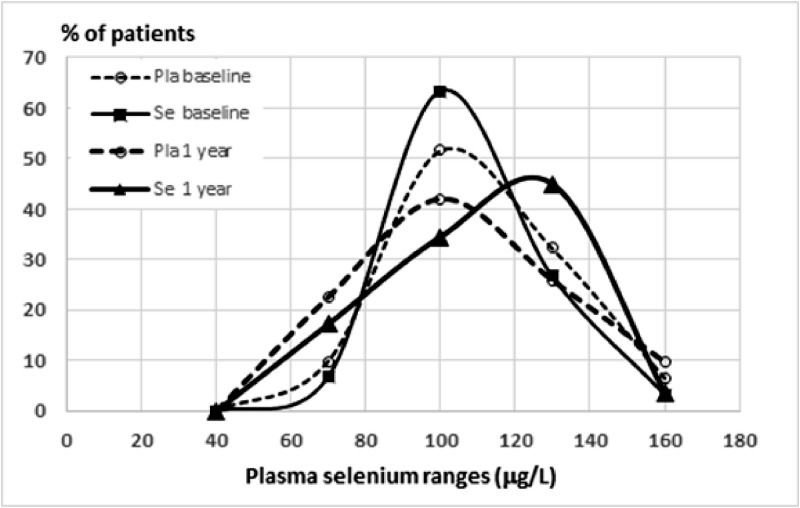
Selenium levels: The mean plasma Se levels measured by ICP–MS were similar between the two groups at baseline (mean of 96.6 and 94.2 mcg/L respectively for Se and Pla), indicating that the estimated difference in Se ingestion (Table 1) was not relevant. No difficulty was reported by the participants concerning the ingestion of Pla or Se capsules. Se plasma levels vary in CD patients [12] and recent literature allocate blood circulating Se in three ranges: inadequate low (< 64 mcg/L), sufficient (>64 and <100) and ideal (>100) Se levels. Since low plasma Se levels are known to be inadequate for the function of selenoenzymes[13,14,18,19] we stratified the patients in ranges of Se levels (Fig. 4) and found that: (i) at the beginning of the clinical trial most of the patients were in the sufficient or ideal range of Se status (not Se deficient); (ii) the frequency distribution of Se range curve differed after treatment (Fig. 4) but a large area of overlapping remained, indicating that the dose chosen for the trial might have been lower than necessary. A clear shift to the right was detected in the frequency curve of Se- group (Fig. 4, solid thick line), contrasting with a sinus-like normal distribution on the three other conditions (Fig. 4). Abnormal low (< 65 mcg/L) was detected in 10 out of 66 patients at baseline; (iii) comparing the samples of the Se group before and after treatment we observed an increase in the number of patients in the “ideal” range with a concomitant decrease in the number of patients in the “sufficient” range (Fig. 4); (iv) the absence of significant differences in Se status estimated through diet in the Se or the Pla groups (data not shown) confirmed that regular patient´s diet did not influence trial follow-up. An unexpected finding was that serum Se levels were not significantly different between studied groups after one-year treatment (p = 0.97), even when participants were stratified by initial LVEF ≤45% (p = 0.60) or > 45% (p = 0.27). The same was found when nutritionally estimated Se intake was compared between the two groups during follow-up, in an overall (p = 0.14) or a stratified analysis by initial LVEF <45% (p = 0.57) or > 45% (p = 0.09). Nutritional data that were collected and analyzed were also similar between the two groups during follow-up.
Fig. 4.
Frequency distribution of patients (percentage) in different ranges of selenium plasma levels measured by ICP-MS, comparing baseline (thin lines) with one-year follow-up (thick lines) in participants treated with Placebo (dashed lines) or Selenium (Solid lines). Note that curves do differ only after treatment with selenium. A shift to the right was detected in the frequency curve of selenium-treated participants, contrasting with the three other conditions. Abnormal low (< 64 mcg/L) was detected only in 10 out of 66 patients at baseline.
Discussion
The hypothesis of Se supplementation for improvement of CCC has been formulated long ago [21,23], but has never been clinically tested, although the subject has recently been revisited in the literature [26]. This is the first clinical trial of Se treatment in patients with CD, specifically designed to detect an effect in early progression of heart disease. We found Se treatment in CD patients was safe, as did other Se clinical trials [35,36]. However, in STCC conditions (100 mcg sodium selenite/day for one year, in stage B patients) the expected primary outcome was not confirmed. A benefit of Se treatment was observed only by subgroup analysis on CCC patients classified as stage B2, with LVEF <45%. Considering the limitations of the small number of B2 patients included in STCC, this result indicates the need of a new trial specifically designed to consider Se effect in middle/advanced CCC stages. However, as Se supplementation to CCC patients is safe, it might be prescribed in the diet and/or as specific formulations (at least in concentrations such as 100 mcg/day) as an antioxidant complementary therapeutic approach in patients at the CCC B2 stage. This is a first implication of the evidence presented here that may add value to CD treatment, associated with the evidence already shown in cardiac patients and elder people, a sociodemographic profile like CCC. Besides, this is the first report of a non-specific cardiovascular or trypanocide drug with a potential benefit on cardiac function in a subgroup of CCC patients and this result brings a small hope for chronic CD patients who, presently, do not know if they will evolve to severe CCC. A surrogate prognostic biomarker of CCC progression is still lacking. Additionally, these results suggest that questions concerning nutritional profile and Se-rich food in the patients´ diet might be included in the propaedeutics /anamnesis of CD cases.
In most countries Se is considered a supplement instead of a drug. Between 2009 and 2018, Brazil adopted a definition of Se 100 mcg/day as a drug and not as a supplement, and the regulation conditions changed twice during STCC assay. This fact introduced a difficulty element for running the trial, contributing to its slow recruitment rate. This dose (100 mcg/day) and Se source (sodium selenite) were chosen due to its known benefits to Keshan cardiopathy prevention, to a reduction on ECG changes in patients nourished by the parenteral route and to reduce reinfarction and cardiac death due to acute myocardial infarction [23]. This same dose was used in the Danish clinical trial called SELGEN study[35] and in a recently published trial on pregnant women [36]. Was 100 mcg/day of inorganic selenium adequate for the expected anti-oxidant effect? The slight difference in the curves of plasma Se frequency in patients from the two groups (Fig. 4) apparently indicates that Se administration in STCC was not sufficient to sustain high plasma levels. Moreover, in the Se group there was a higher percentage of participants with a lower dietary Se intake, and possibly consuming more rapidly the Se provided by treatment capsules. Studies with long-term Se supply [35,37] showed that best results were obtained using 200 mcg/day of organic selenium, mainly when associated with coQ10 [15,16,37].
A promising association of drugs on hand to treat CD is anti-oxidants plus benznidazol, the most used trypanocide drug in Brazil [38]. Previous work showed that administration of Bz (5 mg / kg / day; 2 months) in chronic CD patients followed by antioxidant therapy (Vitamins E and C; 6 months) helped the recovery of blood oxidative stress caused by Bz [38]. In STCC, Bz was not prescribed, and only three participants had a previous story of Bz treatment, many years before the trial.
From all laboratory parameters studied in the present work, neither PCR nor plasma Se levels were considered for inclusion of patients in the trial. However, we followed both biomarkers to interpret the results. At baseline, the PCR positivity (Pla=32% and Se=46%) and parasite load observed agreed with recent studies with chronic patients from Brazil [29,38,39]. Patients in the Se group did not significantly change the parasite load, although a slight decrease in the PCR positivity and T. cruzi DNA levels in blood was observed, in contrast to an increase detected in the Pla group. The small number of samples in which parasite load could be studied was a limiting factor for any conclusion. Though, two points need to be discussed: (a) the frequency of 30–40% positivity discourages using T. cruzi PCR as a baseline biomarker in clinical trials of chronic patients except when the drug tested has a clear trypanocide effect; in the present study ECG and ECHO variables were the best indicators of CCC progression; (b) T. cruzi PCR would certainly be helpful in future assays combining Se and trypanocide drugs. Selenium does not present a direct trypanocide effect [40] and the benefits for B2 patients observed in this present trial could not be attributed to changes in parasite load or proliferation in chronic patients. However, this hypothesis still should be explored in a study with a higher number of patients per group.
Improvement in the quality of life after treatment with Se was reported in the Swedish studies [17]. Our original protocol included QoL data at one year follow up as a secondary outcome [24]. However, as the focus of the present study was to examine the influence of Selenium supplementation on clinical outcomes, including cardiac function (evaluated by LVEF) and adverse effects, QoL data was beyond this scope in the updated protocol [25] and will be more appropriately explored (including overall and QoL domains) in future studies.
We still don´t have a clear picture of the multiple pathophysiology mechanisms of CCC progression, as well as the possible roles of Se in this complex scenario. Presently, results from STCC open more questions than bring answers: (1) Would Se-treated CCC patients benefit from concurrent Bz administration? (2) Could basal Se status be a biomarker for CCC progression [41]? Plasma Se levels below 70 mcg/L turns more vulnerable patients that confront cardiovascular pathologies [14,18]. (3) High-quality maps of soil and plasma selenium levels are available in Europe, North America, and Asia, but data for Latin America is lacking. Geographic differences in Se in Latin America regions may be associated with CD progression? (4) Would hard CCC outcomes such as cardiovascular mortality be impacted by Se treatment as shown in other studies [15,16,37] ? (5) What are the most relevant selenoproteins in CCC progression [19] and would genetic polymorphism [42] be implicated in the different clinical evolution of CCC patients?
STCC was designed in 2005, when pre-clinical data fostered the proof-of-concept [24,25]. This led to four important choices of the trial: (i) the Se dose, (ii) the exclusion criteria for CD patients in indeterminate clinical form, the A stage of CCC and the severe CCC stages C and D, (iii) the option for do not use a hard outcome as cardiovascular mortality and (iv) the choice of LVEF values as the primary outcome. New studies are needed to address questions concerning other CD stages and outcomes, and the administration of Se associated with coQ10. Clinical trials using Se started in the 70s [43] and a search in clinical trials platform (clinicaltrials.gov; access: 2021/03/13) retrieved 329 studies with Se, 71 with CD but STCC is the single addressing Se in CD patients.
The severity of CCC progression was clearly observed in the placebo group: in just one year, seven patients out of 33 (21%) on the Pla group progressed to stages B2 and C. Notwithstanding this severity, one year is not sufficient time for a large natural progression: a high number of patients remained in the starting CCC stage. However, only in the Se-treated group we observed individual trajectories of improvement in LVEF. This finding, as well as the borderline difference between the two groups in CCC stage progression converge to the idea that a higher Se dose and a longer period of follow-up could clarify this effect. Four Se treated patients that improved LVEF, showed an increase in ≥10 percentual points between final and initial LVEF. The two major limitations of this study, the short time of one-year follow-up and the small number of patients with LVEF <45% in the baseline do not allow any further conclusion and reinforce the need for more studies to clarify those questions. A recent review further highlighted the role of selenium in cell survival and its correlation with protective effects against cardiovascular disease [44].
Another limitation of STCC was the excessive time required to start the study (2005 to 2014). During this time, many new knowledge and interesting results added concepts that could not be included in the trial design, such as (i) the organic Se as best for cardiovascular effects [18,35], (ii) the better performance of Se in trials when coenzyme Q10 is synergistically administered as the active substance [37] and (iii) genetic and epigenetic polymorphisms that could interfere with Se intake and expression of selenoproteins [44]. Self-reported information of nutritional consumption, including dietary Selenium intake, should also acknowledged as a limitation and we cannot exclude the possibility of other factors that we did not control could influence the results obtained so far.
In conclusion, this study demonstrated that 100 mcg/day of Se treatment for one year in patients with early CCC was safe and showed a potential beneficial effect in the subset of patients that had already attained stage B2. This new pharmaceutical/ nutritional approach deserves further studies to clarify its potential use as an adjuvant therapy in CCC, specially designed using a larger number of patients, coming from different geographic regions.
Author contributions
Conceptualization and trial design: TCdA-J, AMH-M, MTdH, MFFM, SXX, PEAAdB, LHCS and RMS
Sample size estimation: CSdAC, TCdA-J, MFFM and PEAAdB
Patient orientation and supervision: ERM, FMF, FSP-S, LOP, MJA, MOM and LRG
Patient evaluations and acquisition of the data: MTdH, RMS, GMSdS, SSX, HHV, MFFM, ASdS, PSdS, LHCS, ERM, FMC and BMSG
Data acquisition and procedure standardization: ERM, FMC, MTdH, MFFM and GMSdS
Nutritional data acquisition and analysis: PSdS, MFFM, TCdA-J
Laboratory data acquisition and evaluations: EHR, ACCC, ALdS, BMSG, RRF, CB, OCM, AGF, CMS, JC, MdGB-A and LRG
Data management and analysis: TCdA-J, MTdH, MFFM, VBP, AMH-M, PEAAdB and ACCC
Project development: MTdH, MFFM, AMH-M, PEAAdB and TCdA-J
Validation: MTdH, MFFM, TCdA-J, AMH-M,
Manuscript drafting: TCdA-J, MTdH, MFFM, AMH-M, ACCC,
Manuscript review: MTdH, RMS, GMSdS, SSX, HHV, MFFM, MdGB-A, ASdS, PSdS, LHCS, FSP-S, RRF, LOP and LRG
Regulatory issues. TCdA-J, AMH-M and ERM
Preclinical data gathering and proof of concept: TCdA-J and AMH-M
Acquisition of funding and project administration: TCdA-J
Writing ± original draft: TCdA-J, MTdH, MFFM, AMH-M, ACCC, RFF, LRG, LHCS, RMS, GMSdS, SSX, HHV, ASdS, PSdS, EHR, ALdS, BMSG, CB, OCM, MdGB-A, FSP-S, LOP, MJA, MOM and LRG
All authors had full access to the data, and reviewed, revised, and gave final approval of the article before submission.
Declaration of Competing Interest
The authors have nothing to disclose.
Acknowledgments
Funding
This clinical trial was supported by the Department of the Industrial Complex and Innovation in Health from the Brazilian Health Ministry (IOC Fiotec IOC-001-LIV-11-2-1; 25380.001603/2017-89), the governmental agencies CNPq (306184/2010-9; 309545/2014-5;313011/2018-4), FAPERJ (E26/151.506/99; Protocol 33); and the Innovation and the Research Vice-Presidencies of Fiocruz. CB and OM are researcher fellows of CNPq and FAPERJ (CNE, JCNE). Students who participated in the project received fellowships from Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES; www.capes.gov.br) related to their respective Fiocruz post-graduation courses. The funders had no role in study design, data collection and analysis.
Data sharing statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
This is a collaborative long-term study between two Fiocruz Institutes, INI and IOC, engaging staff professionals, post-graduating students and post-doctoral fellowships. We thank all the former members of the Selenium project staff, as well as the designers that prepared communication materials used in the recruiting and sensibilization stages of the project.
The authors thank Catalent Brasil Ltda for supplying free-of-charge the sodium selenite and placebo for the study.
We would like to dedicate this work to all the patients who participate in this study, some of them contributing to creation of the Rio Chagas Association, and to Prof. Alejandro Luquetti who helped us in 1997 giving the first five reference Chagas disease serum samples to start proofing the concept of lower Se levels according to CCC severity. We thank our data safety monitoring board who reviewed the study data and provided valuable and timely responses and advice. Fiocruz is a member of the Ibero-American New Tools for the Diagnosis and Evaluation of Chagas Disease (NHEPACHA) network.
References
- 1.World Health Organization. Chagas disease (American trypanosomiasis). Fact sheet updated March 11th, 2020. Last accessed on March 07 2021,, https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
- 2.Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2017;(17):31612–31614. doi: 10.1016/S0140-6736(17)31612-4. S0140-6736. [DOI] [Google Scholar]
- 3.Schmunis G.A., Yadon Z.E. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Viotti R., Alarcón de Noya B., Araujo-Jorge T.C. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother. 2014;58:635. doi: 10.1128/AAC.01662-13R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasslocher-Moreno A.M., Saraiva R.M., Sangenis L.H.C. Benznidazole decreases the risk of chronic Chagas disease progression and cardiovascular events: a long-term follow up study. EClin Med. 2021:31. doi: 10.1016/j.eclinm.2020.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rassi A., Jr, Rassi A., Rassi S.G. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 7.Xavier S., Sousa A., Hasslocher-Moreno A. Application of the new classification of cardiac insufficiency (ACC/AHA) in chronic Chagas cardiopathy: a critical analysis of the survival curves. Rev SOCERJ. 2005;18:227. http://sociedades.cardiol.br/socerj/revista/2005_03/a2005_v18_n03_art06.pdf Available: Accessed: March 13 2021, [Google Scholar]
- 8.Dias J.C.P., Ramos Jr N.A., Gontijo E.D. 2nd Brazilian consensus on chagas disease, 2015. Rev Soc Bras Med Trop. 2016;49(supl. 1):1. doi: 10.1590/0037-8682-0505-2016. [DOI] [PubMed] [Google Scholar]
- 9.Lidani K.C.F., Bavia L., Ambrosio A.R. Messias-Reason IJ. The complement system: a prey of Trypanosoma cruzi. Front Microbiol. 2017;8:607. doi: 10.3389/fmicb.2017.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa J.L., Thiers C.A., Pereira B.B. Impact of the use of benznidazole followed by antioxidant supplementation in the prevalence of ventricular arrhythmias in patients with chronic Chagas disease: pilot study. Am J Ther. 2016;23:e1474. doi: 10.1097/MJT.0000000000000137.8. [DOI] [PubMed] [Google Scholar]
- 11.Wen J.J., Yachelini P.C., Sembaj A., Manzur R.E., Garg N.J. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med. 2006;41:270. doi: 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Rivera M.T., Souza A.P., Hasslocher-Moreno A. Progressive Chagas’ cardiomyopathy is associated with low selenium levels. Am J Trop Med Hyg. 2002;66:706. doi: 10.4269/ajtmh.2002.66.706. [DOI] [PubMed] [Google Scholar]
- 13.Benstoem C., Goetzenich A., Kraemer S. Selenium and its supplementation in cardiovascular disease: what do we know? Nutrients. 2015;7:3094. doi: 10.3390/nu7053094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju W., Li X., Li Z., Wu G.R., Fu X.F., Yang X.M., Zhang X.Q., Gao X.B. The effect of selenium supplementation on coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol. 2017;44:8–16. doi: 10.1016/j.jtemb.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Alehagen U., Aaseth J., Johansson P. Reduced cardiovascular mortality 10 years after supplementation with selenium and coenzyme Q10 for four years: follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly citizens. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0141641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alehagen U., Alexander J., Aaseth J. Supplementation with selenium and coenzyme Q10 reduces cardiovascular mortality in elderly with low selenium status. A secondary analysis of a randomized clinical trial. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson P., Dahlström Ö., Dahlström U., Alehagen U. Improved health-related quality of life, and more days out of hospital with supplementation with selenium and coenzyme Q10 combined results from a double blind, placebo-controlled prospective study. J Nutr Health Aging. 2015;19:870. doi: 10.1007/s12603-015-0509-9. [DOI] [PubMed] [Google Scholar]
- 18.Rayman M.P. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 19.Avery J.C., Hoffmann P.R. Selenium, Selenoproteins, and Immunity. Nutrients. 2018;10:1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoffaneller R., Morse N.L. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients. 2015;7:1494. doi: 10.3390/nu7031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souza A.P., Jelicks L.A., Tanowitz H.B. The benefits of using selenium in the treatment of Chagas disease: prevention of right ventricle chamber dilatation and reversion of Trypanosoma cruzi-induced acute and chronic cardiomyopathy in mice. Mem. Inst. Oswaldo Cruz. 2010;105:746. doi: 10.1590/S0074-02762010000600003. [DOI] [PubMed] [Google Scholar]
- 22.Souza A.P., Sieberg R., Li H. The role of selenium in intestinal motility and morphology in a murine model of Trypanosoma cruzi infection. Parasitol Res. 2010;106:1293. doi: 10.1007/s00436-010-1794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelicks L.A., Souza A.P., Araujo-Jorge T.C., Tanowitz H.B. Would selenium supplementation aid in therapy for Chagas disease? Trends Parasitol. 2011;27:102. doi: 10.1016/j.pt.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasil P.E.A.A., Souza A.P., Hasslocher-Moreno A.M. Selenium Treatment and Chagasic Cardiopathy (STCC): study protocol for a double-blind randomized controlled trial. Trials. 2014;15:388. doi: 10.1186/1745-6215-15-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holanda M.T., Mediano M.F.F., Hasslocher-Moreno A.M. A protocol update for the Selenium Treatment and Chagasic Cardiomyopathy (STCC) trial. Trials. 2018;19:507. doi: 10.1186/s13063-018-2889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcolea V., Pérez-Silanes S. Selenium as an interesting option for the treatment of Chagas disease: a review. Eur J Med Chem. 2020;206 doi: 10.1016/j.ejmech.2020.112673. [DOI] [PubMed] [Google Scholar]
- 27.Witte K.K.A., Nikitin N.P., Parker A.C. The effect of micronutrient supplementation on quality-of life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 28.Vizzoni A.G., Varela M.C., Sangenis L.H.C. Ageing with Chagas disease: an overview of a urban Brazilian cohort in Rio de Janeiro. Parasit Vectors. 2018;11:354. doi: 10.1186/s13071-018-2929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigues-dos-Santos I., Melo M.F., Castro L. Exploring the parasite load and molecular diversity of Trypanosoma cruzi in patients with chronic Chagas disease from different regions of Brazil. PloS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willett W.C., Howe G.R., Kushi L.H. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 31.Slater B., Marchioni D.L., Fisberg R.M. Estimating prevalence of inadequate nutrient intake. Rev Saúde Pública. 2004;38:599. doi: 10.1590/S0034-89102004000400019. [DOI] [PubMed] [Google Scholar]
- 32.Schijman A.G., Bisio M., Orellana L. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PloS Negl Trop Dis. 2011;5(1):e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stürup S., Hayes R.B., Peters U. Development and application of a simple routine method for the determination of selenium in serum by octopole reaction system ICPMS. Anal Bioanal Chem. 2005;381:686. doi: 10.1007/s00216-004-2946-x. [DOI] [PubMed] [Google Scholar]
- 34.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79e108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Rayman M.P., Wintherb K.H., Pastor-Barriusoc R. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomized controlled trial. Free Rad Biol Med. 2018;127:46. doi: 10.1016/j.freeradbiomed.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Najib F.S., Poordast T., Nia M.R., Dabbaghmanesh M.H. Effects of selenium supplementation on glucose homeostasis in women with gestational diabetes mellitus: a randomized, controlled trial. Int J Reproductive BioMedicine. 2020;18(1):57–64. doi: 10.18502/ijrm.v18i1.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alehagen U., Johansson P., Bjornstedt M., Rosén A., Dahlstrom U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. 2013;167:1860. doi: 10.1016/j.ijcard.2012.04.156. [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro C.M., Budni P., Pedrosa R.C. Antioxidant therapy attenuates oxidative insult caused by benzonidazole in chronic Chagas' heart disease. Int J Cardiol. 2010;145:27–33. doi: 10.1016/j.ijcard.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Melo M.F., Moreira O.C., Tenório P., Lorena V., Lorena-Rezende I., Júnior W.O., Gomes Y., Britto C. Usefulness of real time PCR to quantify parasite load in serum samples from chronic Chagas disease patients. Parasit Vectors. 2015;8:154. doi: 10.1186/s13071-015-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souza A.P., Oliveira G.M., Neve J. Trypanosoma cruzi: host selenium deficiency leads to higher mortality but similar parasitemia in mice. Exp Parasitol. 2002;101:193. doi: 10.1016/s0014-4894(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 41.Combs G.F., Jr. Biomarkers of Selenium Status. Nutrients. 2015;7:2209. doi: 10.3390/nu7042209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopp T.I., Outzen M., Olsen A., Vogel U., Ravn-Haren G. Genetic polymorphism in selenoprotein P modifies the response to selenium-rich foods on blood levels of selenium and selenoprotein P in a randomized dietary intervention study in Danes. Genes Nutr. 2018;13:20. doi: 10.1186/s12263-018-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson M.F., Rea H.M., Friend G.M. On supplementing the selenium intake of New Zealanders.2. Prolonged metabolic experiments with daily supplements of selenomethionine, selenite and fish. Br J Nutr. 1978;39:589. doi: 10.1079/BJN19780074. [DOI] [PubMed] [Google Scholar]
- 44.Shalihat A., Hasanah A.N., Mutakin The role of selenium in cell survival and its correlation with protective effects against cardiovascular disease: a literature review. Biomed Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111125. [DOI] [PubMed] [Google Scholar]