Abstract
Introduction
Weaning from cardiopulmonary bypass (CPB) is a critical step of any cardiac surgical procedure and often requires pharmacologic intervention. Calcium ions are pivotal elements for the excitation-contraction coupling process of cardiac myocytes. Thus, calcium administration might be helpful during weaning from CPB.
Methods
We describe a multicenter, placebo-controlled, double blind randomized clinical trial to assess the effect of calcium chloride on the need for inotropic support among adult patients during weaning from CPB. The experimental group (409 patients) will receive 15 mg/kg of calcium chloride. The control group (409 patients) will receive an equivalent volume of 0.9% sodium chloride. Both drugs will be administered intravenously as a bolus at the beginning of weaning from CPB.
Results
The primary outcome will be the need for inotropic support between termination of CPB and completion of surgery. Secondary outcomes will be: duration of inotropic support, vasoactive-inotropic score 30 min after transfer to intensive care unit and on postoperative day 1, plasma alpha-amylase on postoperative day 1, plasma Ca2+ concentration immediately before and 10–15 min after calcium chloride administration, non-fatal myocardial infarction, blood loss on postoperative day 1, need for transfusion of red blood cells, signs of myocardial ischemia on electrocardiogram after arrival to intensive care unit, all-cause mortality at 30 days or during hospital stay if this is longer than 30 days.
Discussion
This trial is designed to assess whether intravenous calcium chloride administration could reduce the need for inotropic support after cardiopulmonary bypass weaning among adults undergoing cardiac surgery.
Keywords: Cardiac surgery, Cardiopulmonary bypass, Calcium chloride, Hypocalcemia, Inotropic support
1. Background
Weaning from cardiopulmonary bypass (CPB) is a crucial step of cardiac surgical procedure. Data suggest that difficult weaning from CPB occurs in 10–45% of cases and those patients who fail to successfully wean from CPB at first attempt has high risk of postoperative complications and mortality [1,2].
The use of inotropic agents in patients undergoing cardiac surgery is associated with reduced mortality [3]. This may be mediated by achieving the hemodynamic stability necessary for successful weaning from CPB. Different drugs are commonly used to improve hemodynamics in cardiac surgery, including catecholamines, phosphodiesterase inhibitors, levosimendan, and calcium salts [4]. Classic inotropic agents (epinephrine, norepinephrine, dobutamine) are well known for their beneficial as well as harmful effects [5]. Thus, the search for the optimal drug for hemodynamic support in this setting remains the subject for clinical research.
Calcium ions play a pivotal role in excitation-contraction coupling process of cardiomyocytes [6,7]. Increase in intracellular Ca2+ concentration causes a conformational change in cardiac troponin C, the tropomyosin complex disconnects and uncovers the myosin binding site on the actin molecule, which starts muscle contraction [7]. Several studies have shown that hypocalcemia might be responsible for the development of hypotension and heart failure [8,9]. Considering these data, calcium salts (calcium chloride, calcium gluconate) may facilitate CPB weaning.
According to an international survey, calcium salts are widely used (in 71% of centers) to improve hemodynamics during CPB termination [10]. The rationale of calcium administration are derived from several studies that reported transient improvement of cardiac output and mean arterial pressure [11,12]. Shapira et al. showed that either bolus (10 mg/kg) or same bolus plus continuous infusion (1.5 mg/kg/min for 10 min) of CaCl2 immediately after the end of CPB increased cardiac index, stroke volume and mean blood pressure for 3–6 min [11]. Similarly, Urban et al. demonstrated that CaCl2 injected at the same doses immediately after CPB weaning led to a transient increase in cardiac output and right ventricular ejection fraction [12]. In contrast, further research failed to show hemodynamic improvement as a result of CaCl2 administration after CPB [13].
The available evidence is limited by being out of date, using a small sample size and its inherent lack of statistical power to assess clinically relevant outcomes. Moreover, considering the possible side effect of calcium salts, e.g. the “stone heart” phenomenon, pancreatic injury, and inhibition of the vasoactive-inotropic effect of catecholamines, the use of intravenous calcium might be dangerous in cardiac surgical patients. Thus, currently, no high quality evidence exists regarding the clinical efficacy and safety of calcium salts administration during weaning from CPB.
The aim of this multicenter, randomized, double-blind, placebo-controlled clinical trial is to test the hypothesis that administration of CaCl2 during separation from CPB reduces the need for inotropic support, expressed as number of patients requiring such support, at the end of surgery.
2. Methods
2.1. Study approval and registration
The study was approved by local Ethics Committees of all participating centers, the copies of approval protocols are held by the coordinating center (E. Meshalkin National Medical Research Centre, Novosibirsk, Russia). The trial will be conducted in compliance with the principles of the Declaration of Helsinki. The study was registered with the US National Library of Medicine (www.clinicaltrials.gov), registration number NCT03772990, date of registration 12 December 2018.
2.2. Settings
At least 12 centers in Russia, Bahrein and Saudi Arabia will take part in the study. On October 26th, 2020, the study randomized 291 patients at 9 centers in 2 countries, and further 3 centers are getting ready to start enrollment.
2.3. Population
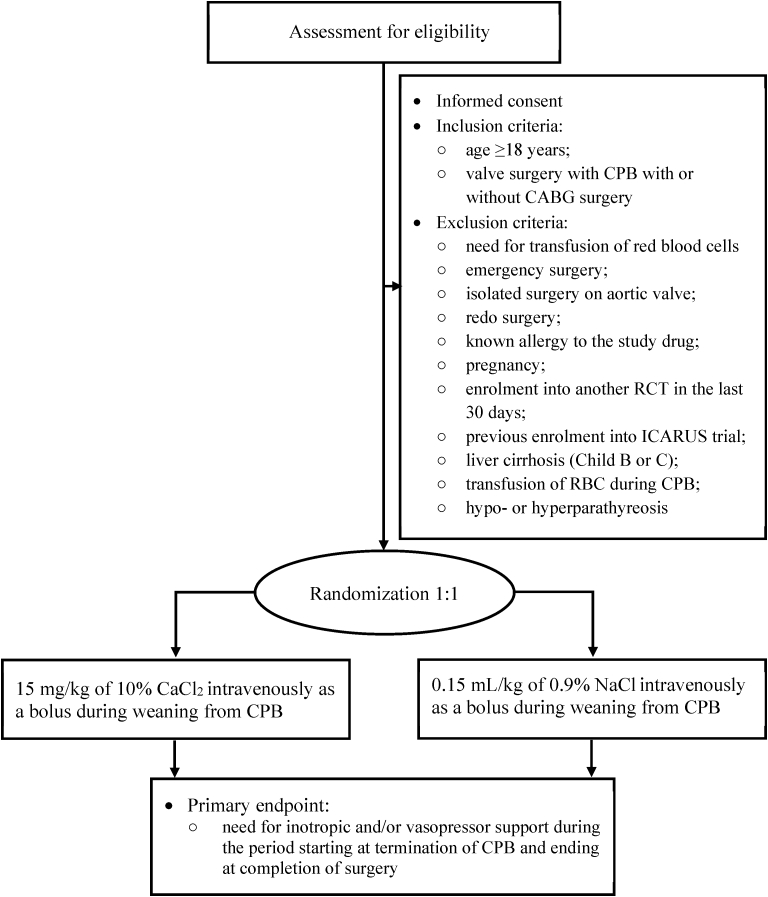
Adult patients (age >18 years), scheduled for elective cardiac surgery, including valve surgery or valve surgery combined with coronary artery bypass graft (CABG) surgery under CPB, will be screened against inclusion and exclusion criteria, as summarized in Fig. 1.
Fig. 1.
Study flow chart. CPB, cardiopulmonary bypass; CABG, coronary artery bypass graft; RCT, randomized controlled trial; RBC, red blood cells.
2.4. Study procedures
2.4.1. Consent
Patients will be approached by a dedicated member of the research team at each center at least 24 h prior to their planned surgery. After providing oral and written information about the study and discussing ethical considerations, including the right to withdraw at any time without negative consequences, patients will be asked to sign written informed consent to participate in the study. The number of patients refusing participation in the study will be collected.
2.4.2. Randomization
Patients who consented and were deemed eligible will be randomly allocated to receive either 15 mg/kg of CaCl2 (active group) or placebo (control group). The randomization will be performed using an online randomization service (https://sealedenvelope.com/), utilizing a permuted-block technique with varying block size, concealed from investigators. Treatment allocation will be stratified by center. Opaque sealed envelopes containing information on treatment allocation will be opened 10–15 min before the end of CPB. At this time, the anesthetist will check the patient against the last exclusion criterion (i.e. need for transfusion of red blood cells). The randomization performed at the last available moment (i.e. before end of CPB), along with the double-blind nature of the study, should reduce most biases.
2.4.3. Allocation concealment and blinding
The randomization will be performed by an independent biostatistician not otherwise involved in the study. Similarly, a nurse not otherwise involved in the study will open the envelope and prepare the study drug. The study drugs, identical in color, appearance and volume, will be delivered to the operation room and handed to the anesthetist who will be aware of the trial but blinded as to the content of the syringe. The marking on the syringe will read “ICARUS trial”. All care providers (i.e. surgeons, anesthetists, intensivists, nurses), outcome assessors and patients will be blinded to treatment allocation. All statistical analyses will be blinded and performed by a biostatistician not participating in treatment allocation.
2.4.4. Unblinding
The only reason for breaking randomization code will be the need for transfusion of red blood cells during the period starting at randomization and ending at arrival to intensive care unit (ICU). Open label CaCl2 administration will be permitted after randomization code break. All events of code breaks will be communicated to the coordinating center within 24 h.
2.4.5. Interventions
The active group will receive 15 mg/kg of CaCl2. Based on available evidence, this dose was effective in improving hemodynamics in patients after cardiopulmonary bypass [11,14,15]. The control group will receive an equivolume of 0.9% NaCl. Both drugs will be administered intravenously as a bolus (delivered over several seconds) at the beginning of weaning from CPB. We decided to use this timepoint in order to standardize the intervention across procedures, as the time between end of CPB and protamine administration or aortic de-cannulation may fluctuate due to various reasons (slow fluid shift from CPB to patient, bleeding that must be stopped before protamine administration, etc.). Additionally, the study hypothesis is that calcium administration improves myocardial contractility and helps avoid use of inotropes. Therefore, to assess the effect of calcium (or lack thereof) it's important to increase its concentration before the heart gets loaded with volume.
2.4.6. Standard care
Open label use of calcium salts will not be permitted until patient's arrival to ICU, except in the event of randomization code break. Apart from calcium salts, there will be no limitation to use of any drugs. The patients will receive the best available care according to local protocols at each center. Beyond standard care, the study protocol will require measurement of plasma Ca2+ concentration before and within 10 min after drug administration and plasma alpha-amylase level on postoperative day 1 (POD 1).
3. Outcome measurements
A large propensity score matched study suggested that use of inotropes in operating room is associated with increased risk of poor outcome (2.5-fold for 1-year mortality, 7-fold for renal replacement therapy, 2-fold for myocardial infarction [MI] and stroke during hospitalization [16]. However, it is difficult to relate those data to our study due to the differing timing of administration and range of inotropic agents used. Therefore, the primary endpoint will be the need for inotropic and/or vasopressor support using any of the following drugs: epinephrine, norepinephrine, dopamine, dobutamine, vasopressin, phenylephrine, isoproterenol started at any dose during the period starting at termination of CPB and ending at completion of surgery. Secondary endpoints will be: total duration of inotropic support, starting at the beginning of weaning from CPB (with cut-off of 30 min, i.e. duration of less than 30 min will be rounded down to the nearest hour, values equal to or greater than 30 min will be rounded up to the nearest hour); vasoactive-inotropic score 30 min after transfer to ICU and on POD 1, calculated as: Dobutamine dose (in mcg/kg/min) + Dopamine dose (in mcg/kg/min) + Enoximone dose (in mcg/kg/min) + [Epinephrine dose (in mcg/kg/min) x 100] + [Norepinephrine dose (in mcg/kg/min) x 100] [17]; plasma alpha-amylase on POD 1; plasma Ca2+ concentration immediately before and 10–15 min after CaCl2 administration; non-fatal MI, defined as an elevation of cardiac troponin (cTn) values > 10 times of the 99th percentile upper range limit ≤48 h after the index procedure in patients with normal baseline values. Patients with elevated pre-procedural cTn values, in whom the pre-procedural cTn level are stable (≤20% variation) or falling, must meet the criteria for a >10-fold increase and manifest a change from the baseline value of >20% in addition with at least one of the following: development of new pathological Q waves, imaging evidence of loss of viable myocardium that is presumed to be new and in a pattern consistent with an ischemic etiology, angiographic findings consistent with a procedural flow-limiting complication such as coronary dissection, occlusion of a major epicardial artery or graft, side-branch occlusion-thrombus, disruption of collateral flow or distal embolization, isolated development of new pathological Q waves meets the type MI criteria with if cTn values are elevated and rising but less than the pre-specified threshold [18]; blood loss on the morning of POD 1 (ml/kg); need for transfusion of red blood cells postoperatively, based on local protocol; signs of myocardial ischemia on electrocardiogram after arrival to ICU; all-cause mortality at 30 days or during hospital stay if this is longer than 30 days.
We will also collect and report perioperative characteristics (internal mammary artery vascular resistance measured by transit time flowmetry, duration of CPB, mechanical ventilation, etc.), complications (rhythm disturbances, neurologic events, etc.) and administrative data (readmission to ICU, hospital stay, etc.).
The patients will be followed-up to 30 days after enrolment or hospital discharge, if this is longer than 30 days. The follow-up contact with patient will be made via telephone call.
4. Statistical analysis and sample size estimation
4.1. Sample size
Approximately 60% patients undergoing cardiac surgery need inotropic support in operating room [[19], [20], [21], [22]]. Assuming a 10% reduction (i.e. from 60% to 50%) in the need for inotropic support, we estimated that a sample of 774 (387 patients per group) would be needed to detect this effect with probability of 80% at an alpha level of 0.05. The expected duration of the study is 36 months. One unblinded interim analysis using the Hwang-Shih-DeCani alpha spending function will be carried out after 50% of the patients have been enrolled. For the interim analysis, the significance level of 0.003 for a one-sided test will be used to stop the trial early for efficacy. Additionally, we will use the “gsProbability” function of the “gsDesign” package for R to estimate the upper boundary crossing probability for the final analysis. If this will be lower than 0.2, the trial will be stopped for futility. For the final analysis, the significance level for efficacy will be set at 0.0238 for a one-sided test. Thus, the overall alpha level of 0.5 (two-sided) will be preserved. To compensate for incomplete observations and account for one interim analysis, the estimated sample size was increased to 818 (409 patients in each group).
4.2. Data collection and storage
Baseline and clinical data will be collected prospectively and entered into the electronic Case Report Form (eCRF) by a dedicated research team member at each center. After obtaining follow-up information, the data will be anonymized. Security measures (storage in a secure electronic environment, restricted access) will be applied to the eCRFs to protect patient privacy and data integrity. At each center, the following information about numbers of patients will be recorded: potentially eligible (i.e. before formal assessment), ineligible, eligible and not consenting, eligible and consenting, randomized, randomized and excluded due to meeting an exclusion criterion, withdrawing consent, lost to follow-up, analyzed. Completed eCRFs will be sent to the coordinating center monthly, where the data will be transferred into a secure electronic database. Baseline and clinical data will be collected for randomized patients only. At the coordinating center, a dedicated research team member not otherwise involved in the study will be responsible for data entry to the electronic database. Blinded data will be cross-checked for consistency by the biostatistician on a monthly basis. Access to the database will be restricted to the two aforementioned persons. All changes in the database will be tracked. After the study completion, the participating centers will have 30 days to respond to data queries before database lock, after which no further changes to the database will be possible.
4.3. Analysis
Categorical variables will be reported as absolute numbers and percentages. Continuous variables will be reported as mean ± standard deviation or median and interquartile range. Primary endpoint analysis will be done using Fisher's exact test. Additionally, absolute risk difference and associated 95% confidence interval (CI) will be estimated. The same approach will be used for analysis of binary secondary endpoints, whereas for continuous secondary endpoints mean difference and associated 95% CIs will be estimated. For between-group comparisons of variables that are not primary endpoints, Fisher's exact test, unpaired t-test or Wilcoxon signed rank test will be used as appropriate. For post-hoc exploratory analyses, mixed effects regression models (i.e. linear, logistic or Cox) with center as fixed effect and clinically important variables as random effects will be used.
In addition to the main analysis, the primary endpoint will be analyzed across the following subgroups: sex (male vs female), serum Ca2+ before weaning (low vs normal, cut-off defined as per local laboratory reference range), duration of CPB (<120 min vs ≥ 120 min).
All analyses will be according to the intention-to-treat principle. Missing data will not be imputed. Statistical significance will be set at the two tailed 0.05 level. All the analyses will be done using R software (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.).
4.4. Study monitoring
At the coordinating center, a Data Monitoring Committee (DMC) will be formed, consisting of an anesthetist, an intensive care specialist and a cardiac surgeon not involved in the study, supported by the study biostatistician. The DMC will consider blinded data on a quarterly basis to assess recruitment rate, data quality and safety issues (e.g. adverse events, unexpectedly high complication rates, etc.). Midway through the study, the DMC will consider results of the interim analysis and will decide whether the trial should continue or be stopped for efficacy or futility. Additionally, the DMC will have a right to demand randomization code break and to recommend termination of the study due to a safety concerns.
5. Discussion
Cardiopulmonary bypass is an essential component of cardiac surgery, and successful weaning from CPB remains a critical step in the intraoperative course. Pharmacologic agents are usually necessary to support hemodynamics during the transition period from mechanical pump-assisted circulation to spontaneous systemic circulation. The choice of agents depends on the underlying pathophysiology of hemodynamic instability: systemic vasodilation, left ventricular dysfunction, pulmonary hypertension and associated right heart failure [23,24].
Routine use of calcium salts during weaning from CPB is a common practice among cardiac anesthetists, although the evidence supporting this routine is lacking [25,26]. At the same time, the rationale for calcium treatment may be justified by the transient hypocalcemia seen in patients after CPB [27] and by the inotropic properties of calcium salts [28].
Low quality evidence supports the notion that the beneficial effects of calcium administration (increase in arterial blood pressure, systemic vascular resistance, cardiac index, stroke volume, and coronary perfusion pressure) might be helpful in cases of moderate contractility reduction or vasoplegia, with no risk of undesirable effects [11]. Administration of CaCl2 at a dose of 5–15 mg/kg appears safe and is associated with transient improvement in hemodynamic parameters. On the other hand, possible side effects of such calcium use include temporary impairment of mammary artery graft flow [14], acute pancreatitis [29], the “stone heart” phenomenon [30] and inhibition of the inotropic effects of catecholamines in the postoperative period [15,31]. However, none of the existing small trials have been powered to detect differences in clinical outcomes. Despite this fact a recent survey on perioperative calcium use in cardiac surgery showed a high rate use during CPB weaning in daily practice worldwide [10].
Therefore, we plan to conduct a large multicenter randomized double-blind placebo-controlled trial to test the hypothesis that intraoperative administration of CaCl2 will reduce the need for inotropic support (expressed as number of patients requiring such support) before transfer to intensive care after cardiac surgery. The study is designed as a pragmatic trial, thus reflecting routine clinical practice and maximizing feasibility and external validity. We focus on complex cardiac surgery and exclude patients undergoing isolated coronary artery bypass graft surgery because in our experience the latter cohort rarely requires use of inotropes, thus diminishing relevance of our intervention in this particular group.
6. Conclusion
The ICARUS trial will be the first study powered to assess the effect of intravenous calcium chloride administration on need for inotropic support in adult patients undergoing cardiac surgery under CPB. The findings of this study are likely to affect global practice.
Trial registration
Clinical Trials Primary Registry: U.S. National Library of Medicine.
Clinical Trial Registration Number: NCT03772990.
Date of Registration: December 12, 2018.
Trial Sponsor: E. Meshalkin National Medical Research Centre, Novosibirsk 630055, Russian Federation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kloner R.A., Przyklenk K., Kay G.L. Clinical evidence for stunned myocardium after coronary artery bypass surgery. J. Card. Surg. 1994;9(3 Suppl):397–402. doi: 10.1111/jocs.1994.9.3s.397. [DOI] [PubMed] [Google Scholar]
- 2.Licker M. Clinical review: management of weaning from cardiopulmonary bypass after cardiac surgery. Ann. Card Anaesth. 2012;15(3):206–223. doi: 10.4103/0971-9784.97977. [DOI] [PubMed] [Google Scholar]
- 3.Belletti A. The effect of inotropes and vasopressors on mortality: a meta-analysis of randomized clinical trials. Br. J. Anaesth. 2015;115(5):656–675. doi: 10.1093/bja/aev284. [DOI] [PubMed] [Google Scholar]
- 4.Lomivorotov V.V. Low-cardiac-output syndrome after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2017;31(1):291–308. doi: 10.1053/j.jvca.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Gillies M. Bench-to-bedside review: inotropic drug therapy after adult cardiac surgery -- a systematic literature review. Crit. Care. 2005;9(3):266–279. doi: 10.1186/cc3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sejersted O.M. Calcium controls cardiac function--by all means! J. Physiol. 2011;589(Pt 12):2919–2920. doi: 10.1113/jphysiol.2011.210989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisner D.A. Calcium and excitation-contraction coupling in the Heart. Circ. Res. 2017;121(2):181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llach F. Effect of acute and long-standing hypocalcemia on blood pressure and plasma renin activity in man. J. Clin. Endocrinol. Metab. 1974;38(5):841–847. doi: 10.1210/jcem-38-5-841. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth J.F.S., Zaloga G.P. Hemodynamic actions and drug interactions of calcium and magnesium. Probl. Crit. Care. 1990:402–415. R. A. [Google Scholar]
- 10.Lomivorotov V.V. Current practice of calcium use during cardiopulmonary bypass weaning: results of an international survey. J. Cardiothorac. Vasc. Anesth. 2020;34(8):2111–2115. doi: 10.1053/j.jvca.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Shapira N. Hemodynamic effects of calcium chloride injection following cardiopulmonary bypass: response to bolus injection and continuous infusion. Ann. Thorac. Surg. 1984;37(2):133–140. doi: 10.1016/s0003-4975(10)60300-1. [DOI] [PubMed] [Google Scholar]
- 12.Urban M.K., Hines R. The effect of calcium on pulmonary vascular resistance and right ventricular function. J. Thorac. Cardiovasc. Surg. 1992;104(2):327–332. [PubMed] [Google Scholar]
- 13.Johnston W.E. Is calcium or ephedrine superior to placebo for emergence from cardiopulmonary bypass? J. Cardiothorac. Vasc. Anesth. 1992;6(5):528–534. doi: 10.1016/1053-0770(92)90094-n. [DOI] [PubMed] [Google Scholar]
- 14.Janelle G.M. Effects of calcium chloride on grafted internal mammary artery flow after cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2000;14(1):4–8. doi: 10.1016/s1053-0770(00)90046-6. [DOI] [PubMed] [Google Scholar]
- 15.Zaloga G.P. Calcium attenuates epinephrine's beta-adrenergic effects in postoperative heart surgery patients. Circulation. 1990;81(1):196–200. doi: 10.1161/01.cir.81.1.196. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen D.V. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120(5):1098–1108. doi: 10.1097/ALN.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 17.Belletti A. Vasoactive-inotropic score: evolution, clinical utility, and pitfalls. J. Cardiothorac. Vasc. Anesth. 2020;(S1053–0770(20)31035–1.) doi: 10.1053/j.jvca.2020.09.117. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):E618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 19.Ellenberger C. Risk factors of postcardiotomy ventricular dysfunction in moderate-to-high risk patients undergoing open-heart surgery. Ann. Card Anaesth. 2017;20(3):287–296. doi: 10.4103/aca.ACA_60_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellenberger C. Myocardial protection by glucose-insulin-potassium in moderate- to high-risk patients undergoing elective on-pump cardiac surgery: a randomized controlled trial. Anesth. Analg. 2018;126(4):1133–1141. doi: 10.1213/ANE.0000000000002777. [DOI] [PubMed] [Google Scholar]
- 21.Groban L. Intraoperative insulin therapy does not reduce the need for inotropic or antiarrhythmic therapy after cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2002;16(4):405–412. doi: 10.1053/jcan.2002.125152. [DOI] [PubMed] [Google Scholar]
- 22.Kumbhani D.J. Intraoperative regional myocardial acidosis predicts the need for inotropic support in cardiac surgery. Am. J. Surg. 2004;188(5):474–480. doi: 10.1016/j.amjsurg.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Cui W.W., Ramsay J.G. Pharmacologic approaches to weaning from cardiopulmonary bypass and extracorporeal membrane oxygenation. Best Pract. Res. Clin. Anaesthesiol. 2015;29(2):257–270. doi: 10.1016/j.bpa.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Monaco F. Management of challenging cardiopulmonary bypass separation. J. Cardiothorac. Vasc. Anesth. 2020;34(6):1622–1635. doi: 10.1053/j.jvca.2020.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Dinardo J.A. Pro: calcium is routinely indicated during separation from cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 1997;11(7):905–907. doi: 10.1016/s1053-0770(97)90132-4. [DOI] [PubMed] [Google Scholar]
- 26.Prielipp R., Butterworth J. Con: calcium is not routinely indicated during separation from cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 1997;11(7):908–912. doi: 10.1016/s1053-0770(97)90133-6. [DOI] [PubMed] [Google Scholar]
- 27.Bartel B. Variations of total and ionized calcium levels during cardiopulmonary bypass. J. Extra Corpor. Technol. 1982;20:593–602. [Google Scholar]
- 28.Royster R.L. A randomized, blinded, placebo-controlled evaluation of calcium chloride and epinephrine for inotropic support after emergence from cardiopulmonary bypass. Anesth. Analg. 1992;74(1):3–13. doi: 10.1213/00000539-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-del Castillo C. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N. Engl. J. Med. 1991;325(6):382–387. doi: 10.1056/NEJM199108083250602. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S. Myocardial stunning is associated with impaired calcium uptake by sarcoplasmic reticulum. Biochem. Biophys. Res. Commun. 2009;387(1):77–82. doi: 10.1016/j.bbrc.2009.06.115. [DOI] [PubMed] [Google Scholar]
- 31.Butterworth J.F. Calcium inhibits the cardiac stimulating properties of dobutamine but not of amrinone. Chest. 1992;101(1):174–180. doi: 10.1378/chest.101.1.174. [DOI] [PubMed] [Google Scholar]