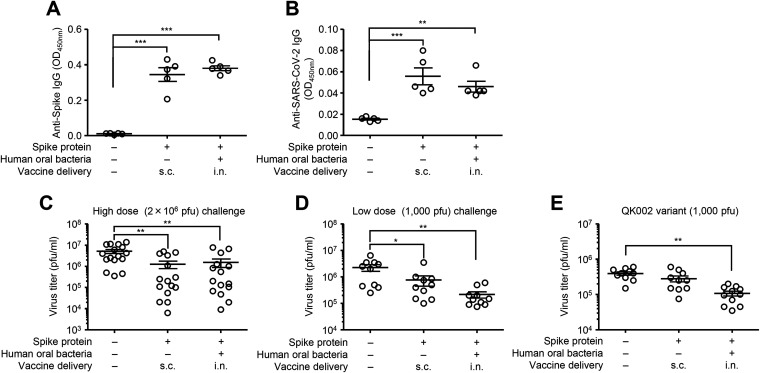
FIG 8.
Protective effects of oral bacteria-adjuvanted intranasal vaccine against SARS-CoV-2 infection. (A to E) Hamsters were immunized intranasally with the spike protein of SARS-CoV-2 with cultured oral bacteria from a healthy volunteer twice in a 3-week interval. As controls, hamsters were left unimmunized or subcutaneously immunized with the spike protein alone twice in a 3-week interval. Two weeks after the last vaccination, hamsters were challenged with 2 × 106 (A to C) or 1,000 PFU (D and E) of SARS-CoV-2. (A and B) Sera were collected at 3 days postinfection. The recombinant spike protein- (A) or formalin-inactivated SARS-CoV-2 virion- (B) specific serum IgG antibody titers were determined by ELISA. (C to E) Two weeks after the last vaccination, hamsters were challenged with 2 × 106 (C) or 1,000 PFU (D and E) of SARS-CoV-2 (C and D) or its QK002 variant (E). The lung wash of SARS-CoV-2-infected hamsters was collected at 3 days postinfection, and viral titers were determined by plaque assay. Open circles indicate values for individual hamsters. The data are from two or three independent experiments (mean ± SEM). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (one-way ANOVA and Tukey’s test).