Abstract
This study compares the immune responses to the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) COVID-19 vaccines in health care workers in Belgium.
The SARS-CoV-2 messenger RNA (mRNA) vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) have each shown more than 90% efficacy in preventing COVID-19 illness1,2 but, to our knowledge, humoral immune responses have not been compared directly.
Methods
Health care workers at a tertiary care center (Ziekenhuis Oost-Limburg, Belgium) who were scheduled for vaccination with 2 doses of either mRNA-1273 or BNT162b2 were invited to participate in this prospective cohort. Serologic testing was performed prior to vaccination as well as 6 to 10 weeks after the second dose (between April 27 and May 20, 2021). Total immunoglobulin levels to the receptor-binding domain of the SARS-CoV-2 spike protein were measured with an anti–SARS-CoV-2 S enzyme immunoassay (Elecsys, Roche Diagnostics International Ltd). After vaccination, antibodies against the SARS-CoV-2 nucleocapsid protein were determined. Previous infection was defined as anti-nucleocapsid positivity at any point, anti-spike positivity before vaccination, and/or a history of positive polymerase chain reaction results on nasopharyngeal swab.
Antibody levels were compared after the second dose of each vaccine for the entire cohort; for those previously infected vs uninfected; and by age group (<35, 35-55, and >55 years) among previously uninfected individuals, using the t test after log10 transformation. Correlation between age and log10-transformed antibody levels was assessed with Pearson correlation. To adjust for confounding, a multiple linear regression was fitted with inclusion of age, sex, previous infection, and time between vaccination and serologic testing. All tests were 2-sided with statistical significance set at α = .05. Analyses were performed using RStudio (version 1.2.1335). This study was approved by the local institutional review board; participants provided written informed consent.
Results
Of 2499 health care workers who received 2 doses of SARS-CoV-2 mRNA vaccines, 1647 participated in this study. A total of 688 were vaccinated with mRNA-1273 (mean age, 43.2 years; 76.7% women; 21.8% previously infected with SARS-CoV-2), and 959 with BNT162b2 (mean age, 44.7 years; 84.9% women; 13.2% previously infected).
Higher antibody titers were observed in participants vaccinated with 2 doses of mRNA-1273 compared with those vaccinated with BNT162b2 (geometric mean titer [GMT], 3836 U/mL [95% CI, 3586-4104] vs 1444 U/mL [95% CI, 1350-1544]; P < .001) (Figure, A).
Figure. Humoral Immune Response Following SARS-CoV-2 mRNA Vaccination.
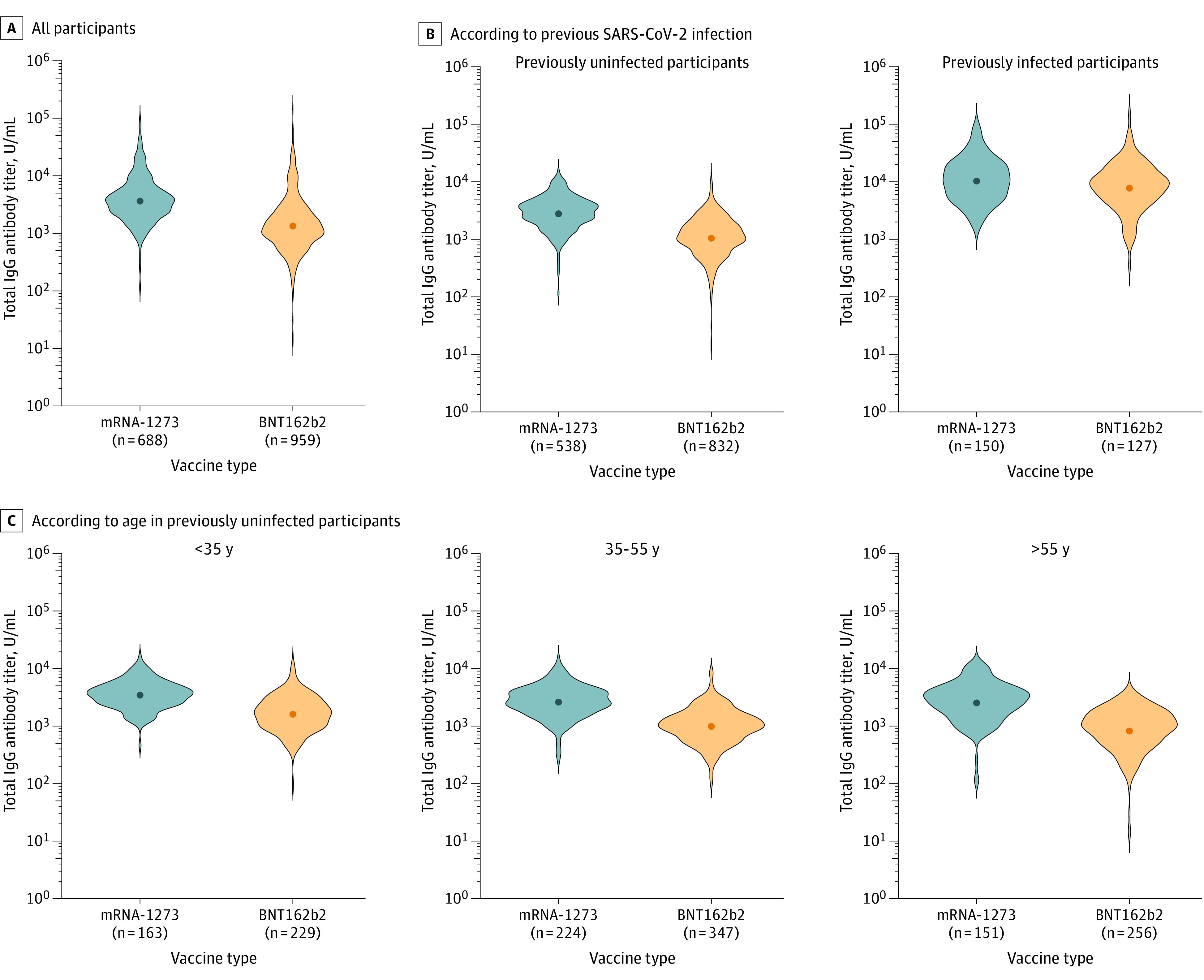
Violin plots of circulating SARS-CoV-2 anti–spike protein receptor-binding domain antibodies in serum samples obtained from participants after they received 2 doses of an mRNA vaccine. Inside each violin plot, the geometric mean is depicted as a point. A, Difference between participants vaccinated with mRNA-1273 (Moderna) vs those with BNT162b2 (Pfizer-BioNTech). B, Difference according to previous SARS-CoV-2 infection and the type of mRNA vaccine. C, Difference according to age and the type of mRNA vaccine in previously uninfected participants. All comparisons were significant at P < .001 except previously infected participants (panel B), which was significant at P = .01.
Previously infected participants had higher antibody titers (GMT, 9461 U/mL [95% CI, 8494-10 539]) compared with previously uninfected participants (GMT, 1613 U/mL [95% CI, 1539-1690]) (P < .001). In both groups, those vaccinated with mRNA-1273 had higher antibody titers compared with those vaccinated with BNT162b2 (previously uninfected: GMT, 2881 U/mL [95% CI, 2721-3051] vs 1108 U/mL [95% CI, 1049-1170]; P < .001; previously infected: GMT, 10 708 U/mL [95% CI, 9311-12 315] vs 8174 U/mL [95% CI, 6923-9649]; P = .01). The difference in antibody levels according to previous infection was higher than the difference between the 2 mRNA vaccines (Figure, B, and Table).
Table. Multivariable Linear Regression Model of log10-Transformed Antibody Levels After Second Dose of an mRNA COVID-19 Vaccine.
| Regression coefficient (95% CI) | P value | |
|---|---|---|
| Vaccine type | ||
| BNT162b2 | [Reference] | <.001 |
| mRNA-1273 | 0.359 (0.326 to 0.392) | |
| Previous infection with SARS-CoV-2 | ||
| Uninfected | [Reference] | |
| Infected | 0.692 (0.649 to 0.736) | <.001 |
| Age, per year (starting at 21 y) | –0.006 (–0.007 to –0.004) | <.001 |
| Sex | ||
| Male | [Reference] | |
| Female | 0.047 (0.005 to 0.089) | .03 |
| Time between vaccination and testing, per day | –0.005 (–0.006 to –0.003) | <.001 |
Antibody levels negatively correlated with age in previously uninfected participants (correlation coefficient, −0.22; P < .001), being highest among those younger than 35 years. Across all age categories, previously uninfected participants vaccinated with mRNA-1273 had higher antibody titers compared with those vaccinated with BNT162b2 (P < .001 for all comparisons; Figure, C).
The type of mRNA vaccine remained independently associated with the log-transformed antibody titer in a multiple linear regression (P < .001; Table).
Discussion
This study demonstrated a significantly higher humoral immunogenicity of the SARS-CoV-2 mRNA-1273 vaccine (Moderna) compared with the BNT162b2 vaccine (Pfizer-BioNTech), in infected as well as uninfected participants, and across age categories. The higher mRNA content in mRNA-1273 compared with BNT162b2 and the longer interval between priming and boosting for mRNA-12733 (4 weeks vs 3 weeks for BNT162b2) might explain this difference.
A relationship between neutralization level after SARS-CoV-2 vaccination and protection against COVID-19 has been demonstrated by several studies.4 As such, the height of the humoral response after vaccination, which correlates with neutralizing antibody titers,5 might be clinically relevant.
Limitations of this study include the lack of data on cellular immunity and on neutralizing antibodies, as well as the specific focus on health care workers. Whether the observed difference in antibody level translates to a difference in the duration of protection,4 the protection against variants of concern, and the risk of transmission6 needs further investigation. Future research should also address the relevance for patients with reduced antibody response after vaccination.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Costa Clemens SA, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Acero R, Castelletti N, Fingerle V, et al. ; KoCo19 study team . In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infect Dis Ther. 2021;10(3):1505-1518. doi: 10.1007/s40121-021-00475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine-Tiefenbrun M, Yelin I, Katz R, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27(5):790-792. doi: 10.1038/s41591-021-01316-7 [DOI] [PubMed] [Google Scholar]


