Abstract
This cohort study characterizes changes in breast cancer screening costs as digital breast tomosynthesis has been adopted at both the patient and population levels.
Over the past decade, screening mammography in the US has undergone notable technological change, as digital breast tomosynthesis (DBT) has replaced 2-dimensional (2D) mammography.1 Although DBT is more expensive than 2D mammography, DBT may generate downstream savings by reducing the number of women called back for additional imaging.2 The goal of this cohort study was to characterize changes in breast cancer screening costs as DBT has been adopted at both the patient and population levels.
Methods
We used deidentified insurance claims from the Blue Cross Blue Shield Axis database. This study was considered exempt by the Yale Institutional Review Board because the study used previously collected, deidentified data. We included women aged 40 to 64 years who underwent screening mammography (2D or DBT) from January 1, 2013, through June 30, 2019. We calculated the mean cost of the initial screening test, mean cost of subsequent testing (diagnostic mammography, magnetic resonance imaging, ultrasonography, and biopsy), and mean cost of the screening episode (initial screen plus any subsequent testing) at 6-month intervals (eMethods and eTable 1 in the Supplement). We used generalized estimating equations to adjust for age, state, and use of screening ultrasonography. To project national costs, we multiplied adjusted mean episode costs by the number of privately insured women aged 40 to 64 years screened in each 6-month period in the US (eMethods and eTable 2 in the Supplement). We used Stata 16.0 SE (StataCorp LLC) and Microsoft Excel, version 16.50 (Microsoft Corporation) for statistical analysis and R, version 4.0.4 (R Foundation for Statistical Computing) to create figures.
Results
Our sample included 8 432 037 women who underwent 15 669 701 screening mammograms. The proportion of mammograms billed with DBT rose from 13% in early 2015 to 70% in early 2019 (Table). The proportion of women who underwent subsequent testing remained stable, from 12% in early 2013 to 13% in early 2019.
Table. Estimates of Population Screening Costs.
| Year | Period | Screened population sizea | Proportion screened with DBT, %b | Population episode cost, total, $ | Population episode cost if all women had been screened with 2D, total, $ | Incremental population episode cost for DBT, $c |
|---|---|---|---|---|---|---|
| 2013 | Jan-Jun | 6 082 174 | NA | 3 876 939 840 | 3 876 939 840 | 0 |
| Jul-Dec | 6 082 174 | NA | ||||
| 2014 | Jan-Jun | 6 273 671 | NA | 4 152 791 722 | 4 152 791 722 | 0 |
| Jul-Dec | 6 273 671 | NA | ||||
| 2015 | Jan-Jun | 6 441 883 | 13 | 4 385 130 618 | 4 249 005 262 | 136 125 355 |
| Jul-Dec | 6 441 883 | 18 | ||||
| 2016 | Jan-Jun | 6 433 818 | 26 | 4 551 542 116 | 4 285 222 585 | 266 319 531 |
| Jul-Dec | 6 433 818 | 31 | ||||
| 2017 | Jan-Jun | 6 527 144 | 38 | 4 677 127 492 | 4 226 304 185 | 450 823 307 |
| Jul-Dec | 6 527 144 | 45 | ||||
| 2018 | Jan-Jun | 6 515 177 | 55 | 4 983 751 119 | 4 307 065 983 | 676 685 137 |
| Jul-Dec | 6 515 177 | 63 | ||||
| 2019 | Jan-Jun | 6 497 495 | 70 | 5 151 922 633 | 4 346 435 917 | 805 486 716 |
| Jul-Decd | 6 497 495 | 70 | ||||
| Projectede | Jan-Jun | 6 497 495 | 100 | 5 425 851 844 | 4 346 435 917 | 1 079 415 927 |
| Jul-Dec | 6 497 495 | 100 |
Abbreviations: DBT, digital breast tomosynthesis; NA, not applicable; 2D, 2-dimensional mammography.
Population size was estimated from US Census data, accounting for the proportion of privately insured US women screened in a 6-month period.
Screening DBT billing codes were introduced in 2015.
Incremental population cost is equal to the population episode cost minus population episode cost if all women had been screened with 2D mammography only.
Cost data were carried forward from the first half of 2019 to the second half to allow for calculation of annual costs. This assumes no increase in the growth of DBT use or episode cost.
The 2019 population size and DBT episode cost were used to project population episode costs if 100% of the population were screened with DBT.
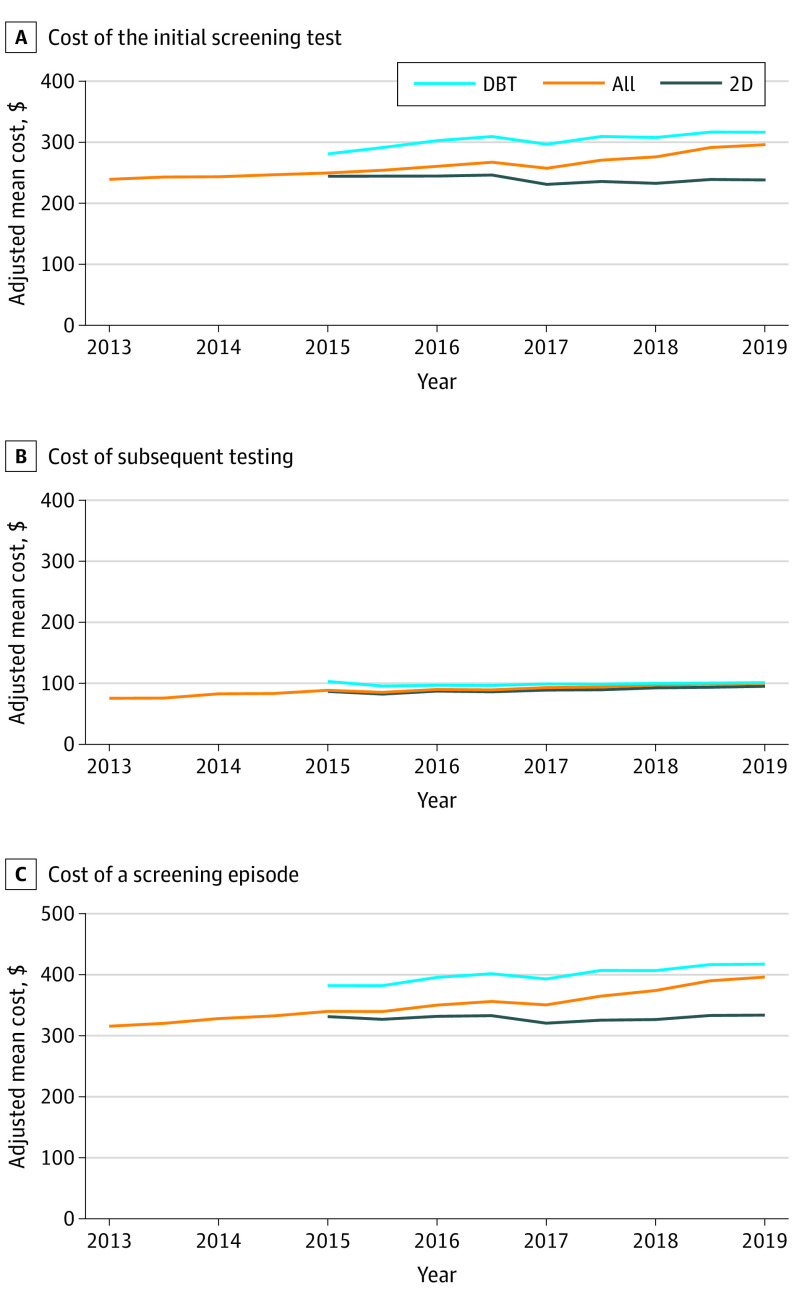
The adjusted mean cost of a screening mammogram (with or without DBT) rose from $239.8 (95% CI, $239.7-$239.9) in early 2013 to $295.6 (95% CI, $296.4-$295.7) in early 2019, an increase of $55.8, or 23% (Figure, A). The cost of 2D mammography remained stable, from $239.8 (95% CI, $239.7-$239.9) in 2013 to $238.8 (95% CI, $238.6-$239.0) in 2019. The cost of DBT was higher than 2D mammography at every time point and rose over time, from $280.7 (95% CI, $280.3-$281.1) in 2015 to $315.6 (95% CI, 315.3-315.8) in 2019.
Figure. Mean Adjusted Screening Costs per Screened Patient Over Time by Modality and Overall.
A, Adjusted mean cost of the initial screening test by modality and overall in 6-month intervals. Billing codes for DBT were introduced in 2015. B, Adjusted mean cost of subsequent testing among screened women by modality and overall by 6-month intervals. Subsequent testing includes any diagnostic mammography (including diagnostic DBT), breast ultrasonography, breast magnetic resonance imaging, or biopsy performed within 4 months of the initial screening test. C, Adjusted mean cost of a screening episode in 6-month intervals. A screening episode includes the cost of the initial screening test plus the cost of any subsequent testing. All costs are inflation-adjusted to 2017 dollars. DBT indicates digital breast tomosynthesis; 2D, 2-dimensional mammography.
Subsequent testing costs rose from $75.3 (95% CI, $74.5-$76.1) in early 2013 to $99.3 (95% CI, $98.4-$100.3) in early 2019 (Figure, B). The cost of a screening episode rose from $316.4 (95% CI, $315.6-$317.2) in early 2013 to $396.5 (95% CI, $395.5-$397.4) in early 2019, an increase of $80.1, or 25% (Figure, C).
Total annual screening expenditures for privately insured women aged 40 to 64 years in the US increased from an estimated $3.9 billion in 2013 to $5.2 billion in 2019. Assuming 2019 costs, if DBT were used for screening all women in this population, the total cost would be $5.4 billion per year, compared with $4.2 billion for screening with 2D, a difference of $1.1 billion (Table).
Discussion
From 2013 through 2019, national expenditures for breast cancer screening rose considerably. This rise appears to be driven by the widespread adoption of DBT, a more expensive screening test. Our results differ from some studies that suggested DBT may be cost-saving,3,4 largely because we did not find that the higher cost of DBT was offset by lower costs of downstream testing. Higher screening costs are acceptable if they result in sufficient improvements in health or quality of life. However, a 2020 study5 suggested that although screening with DBT may be associated with small gains in quality of life, it is generally not cost-effective. Our findings highlight how shifting to DBT has been associated with substantial national expenditures, despite questions about value.
This work has limitations. This is an observational study, and women screened with DBT may be higher risk and may have higher follow-up costs. However, we specifically evaluated changes in costs for all women screened, a strategy robust to selection bias. The findings also may not apply to other payer populations, though privately insured women comprise about two-thirds of women screened in the US.6 In summary, breast cancer screening costs have risen considerably as DBT has become the predominant screening mode in the US.
eMethods.
eTable 1. HCPCS and ICD9 and 10 definitions
eTable 2. Values use to calculate screened population size
References
- 1.Richman IB, Hoag JR, Xu X, et al. Adoption of digital breast tomosynthesis in clinical practice. JAMA Intern Med. 2019;179(9):1292-1295. doi: 10.1001/jamainternmed.2019.1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marinovich ML, Hunter KE, Macaskill P, Houssami N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J Natl Cancer Inst. 2018;110(9):942-949. doi: 10.1093/jnci/djy121 [DOI] [PubMed] [Google Scholar]
- 3.Miller JD, Bonafede MM, Herschorn SD, Pohlman SK, Troeger KA, Fajardo LL. Value analysis of digital breast tomosynthesis for breast cancer screening in a US Medicaid population. J Am Coll Radiol. 2017;14(4):467-474.e5. doi: 10.1016/j.jacr.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 4.Bonafede MM, Kalra VB, Miller JD, Fajardo LL. Value analysis of digital breast tomosynthesis for breast cancer screening in a commercially-insured US population. Clinicoecon Outcomes Res. 2015;7:53-63. doi: 10.2147/CEOR.S76167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowry KP, Trentham-Dietz A, Schechter CB, et al. Long-term outcomes and cost-effectiveness of breast cancer screening with digital breast tomosynthesis in the United States. J Natl Cancer Inst. 2020;112(6):582-589. doi: 10.1093/jnci/djz184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blewett LA, Rivera Drew JA, King ML, Williams KC. IPUMS Health Surveys: National Health Interview Survey, version 6.4 [data set]. doi: 10.18128/D070.V6.4 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. HCPCS and ICD9 and 10 definitions
eTable 2. Values use to calculate screened population size