Abstract
This study examines 1-year outcomes of critical care patients in the UK after COVID-19 multisystem inflammatory syndrome in children.
Medium- to long-term outcomes of the novel pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS)1 or multisystem inflammatory syndrome in children (MIS-C) are unknown. Short-term, 40-day, and 6-month outcomes have been published previously.2,3,4,5
Methods
We collected follow-up data for the previously published multicenter national critical care cohort admitted to the hospital prior to May 10, 2020.6 Data from critical care readmissions and outpatient follow-up clinics up to April 2021 (1 year postadmission) were collected. Monitoring frequency was at the discretion of each unit. The project was classified as a service evaluation by the Nottingham Research and Innovation team (reference 20-235C), and ethics approval and informed consent were not required as per the UK Health Research Authority. The study team analyzed routinely collected deidentified data submitted by clinicians from the participating centers. Definitions of cardiological normal values were locally defined. Descriptive statistics were used.
Results
Data were available from 68 of 76 patients (89%) of the initial surviving cohort. Six-month outcomes of 18 of these patients have been published previously.5 There were no deaths, and 2 patients (3%) had critical care readmission. Both readmissions were unrelated to complications of PIMS-TS or immunomodulatory therapy. Median length of hospital stay was 10 days (interquartile range [IQR], 7 to 14 days) and none needed respiratory support postdischarge.
Outcome data are presented in the Table. It is possible that for many patients whose blood test results did not normalize, lack of repeated tests, rather than failure to normalize, may be contributory. Only 2 of 65 test results (3%) for C-reactive protein, 2 of 59 test results (3%) for D-dimer, and 1 of 60 test results (2%) for troponin were abnormal when tested more than 50 days postadmission. All results for blood tests performed for levels of lymphocytes, neutrophils, platelets, creatinine, ferritin, and alanine transaminase more than 50 days postadmission were normal. Although resolution to normality for D-dimer, ferritin, and troponin was demonstrated in fewer patients, fewer patients had serial monitoring of these parameters.
Table. Resolution of Echocardiographic and Laboratory Parameters in Patients Admitted to UK Pediatric Intensive Care Units With Post–SARS-CoV-2 Inflammatory Shock.
| Normal within 24 h of admission, No./total No. (%) | Postadmission day by which | No./total No. (%) | |||
|---|---|---|---|---|---|
| 50% Normalized | 75% Normalized | Abnormal when tested >50 d after admission | Resolution to normal not demonstrated | ||
| Echocardiography | |||||
| All patients | 11/68 (16) | 10 | 40 | 6/68 (9) | 6/68 (9) |
| Aneurysms at admission | 1/19 (5) | 40 | 254 | 5/19 (26) | 5/19 (26) |
| Bright coronaries at admission | 1/10 (10) | 10 | 16 | 1/19 (10) | 1/10 (10) |
| Functional problems only | 9/39 (23) | 7 | 21 | 0 | 0 |
| Lymphocytes (>1500/µL) | 11/66 (16) | 3 | 6 | 0 | 1/66 (2) |
| Neutrophils (<7500/µL) | 12/65 (18) | 8 | 16 | 0 | 1/65 (2) |
| CRP (<1 mg/dL) | 2/65 (3) | 11 | 14 | 2/65 (3) | 7/65 (11) |
| Platelet count (>150 ×103/µL) | 32/66 (48) | 2 | 6 | 0 | 1/66 (2) |
| Creatinine (<0.11 mg/dL) | 44/65 (68) | 0 | 2 | 0 | 1/65 (2) |
| D-dimer (<0.5 µg/mL) | 0/59 (0) | 22 | 57 | 2/59 (3) | 15/59 (25) |
| Ferritin (<200 ng/mL) | 1/64 (2) | 23 | 53 | 0 | 11/64 (17) |
| Troponin (<0.017 ng/mL) | 4/60 (7) | 14 | 21 | 1/60 (2) | 10 (17) |
| ALT (<50 U/L) | 27/65(42) | 5 | 21 | 0 | 3 (5) |
Abbreviations: ALT, alanine aminotransferase; CRP, C-reactive protein.
SI conversion factors: To convert ALT to µkat/L, multiply by 0.0167; CRP to mg/L, multiply by 10; creatinine to µmol/L, multiply by 88.4; D-dimer to nmol/L, multiply by 5.476; ferritin to μg/L, multiply by 1; lymphocytes to ×109/L, multiply by 0.001; neutrophils to ×109/L, multiply by 0.001; platelet count to ×109/L, multiply by 1; and troponin to µg/L, multiply by 1.
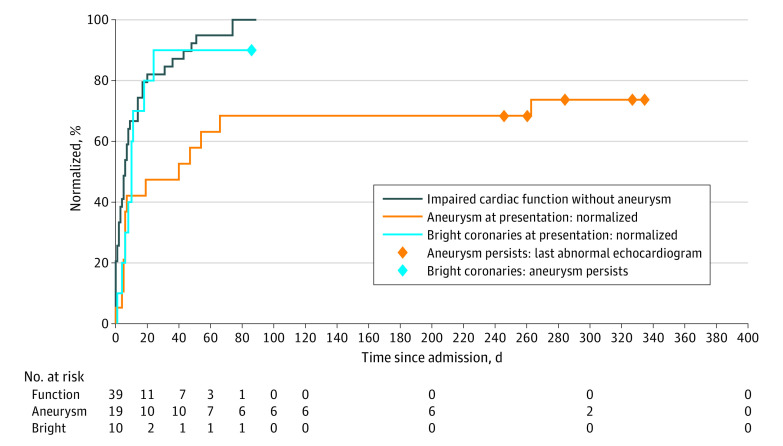
Echocardiographic outcomes are shown in the Table and Figure. For abnormal data, the date of the last test is shown as a diamond in the Figure. Of those patients who presented with aneurysms, 14 of 19 had resolution, and of those who presented with subjectively “bright” coronary arteries, 9 of 10 had resolution and 1 patient progressed to having unresolved coronary artery aneurysms (albeit the latest follow-up echocardiography was 86 days postadmission). All patients who presented with impaired function without aneurysm recovered by day 74.
Figure. Time to First Evidence of Normalization .
For UK patients with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 or multisystem inflammatory syndrome in children presenting with echocardiographic evidence of functional problems, aneurysms, or subjectively bright coronaries. Patients with no evidence of normalization are denoted by a diamond at the time of their last abnormal echocardiogram.
All 6 patients (9%) with ongoing echocardiographic abnormalities had aneurysmal changes, with latest echocardiograms between days 86 and 336 postadmission. At presentation, these 6 patients had a median (interquartile range [IQR]) C-reactive protein level of 22.1 mg/dL (6.6-36.9 mg/dL), compared with 25.8 mg/dL (18.2-32.3 mg/dL) (to convert to milligrams per liter, multiply by 10) for those without aneurysmal changes at follow-up, a lymphocyte count of 1100/µL (600-3300/µL) vs 900/µL (500-1400/µL) ( to convert to ×109/L, multiply by 0.001), and platelet count of 217 ×103/µL (128-368 ×103/µL) vs 151 ×103/µL (85-198×103/µL) (to convert to ×109/L, multiply by 1). Surprisingly, median troponin levels were significantly lower in the group with aneurysm (0.06 ng/mL [0.02-0.418 ng/mL] vs 0.157 ng/mL [0.033-0.81 ng/mL] [to convert to micrograms per liter, multiply by 1]; P = .02). All 6 received intravenous immunoglobulin, compared with 46 of 62 of the other patients; 5 of 6 received steroids vs 46 of 62; and 1 of 6 received a biologic agent vs 13 of 62. According to the case reports, 5 of the 6 were Afro-Caribbean boys and 1 was a White girl, with an age range of 0 to 13 years (median, 8.75 years).
Discussion
Interpretation is limited by the small numbers, lack of consistent follow-up protocol, and hospital-wide readmission data, but is strengthened by a nationwide data set. While the majority of the units have established a consistent multidisciplinary follow-up protocol, this analysis is restricted to one of the earliest reported cohorts of patients worldwide, when the presence of such an entity was becoming apparent.
Although our data identify a group of patients with a risk of significant long-term morbidity, it is reassuring that the majority of patients had good outcomes with no significant medium- or long-term sequelae.
References
- 1.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia . Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259-269. doi: 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies P, Lillie J, Prayle A, et al. Association between treatments and short-term biochemical improvements and clinical outcomes in post-severe acute respiratory syndrome coronavirus-2 inflammatory syndrome. Pediatr Crit Care Med. 2021;22(5):e285-e293. doi: 10.1097/PCC.0000000000002728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrams JY, Oster ME, Godfred-Cato SE, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323-331. doi: 10.1016/S2352-4642(21)00050-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators . Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074-1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penner J, Abdel-Mannan O, Grant K, et al. ; GOSH PIMS-TS MDT Group . 6-Month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health. 2021;5(7):473-482. doi: 10.1016/S2352-4642(21)00138-3 [DOI] [PubMed] [Google Scholar]
- 6.Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4(9):669-677. doi: 10.1016/S2352-4642(20)30215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]