ABSTRACT
Invasive infections with emerging yeasts such as Geotrichum, Saprochaete/Magnusiomyces, Trichosporon, and other species are associated with high morbidity and mortality rates. Due to the rarity and heterogeneity of these yeasts, medical mycology has lacked guidance in critical areas affecting patient management. Now, physicians and life scientists from multiple disciplines and all world regions have united their expertise to create the “Global guideline for the diagnosis and management of rare yeast infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology.” Recommendations are stratified for high- and low-resource settings and are therefore applicable worldwide. The advantages and disadvantages of various diagnostic methods and treatment options are outlined. This guideline reflects the current best-practice management for invasive rare yeast infections in a range of settings, with the intent of establishing a global standard of care for laboratorians and clinicians alike.
KEYWORDS: Malassezia, Pseudozyma, Rhodotorula, antifungal therapy, invasive fungal infection, invasive microorganisms, yeasts, ECMM, ISHAM, ASM
COMMENTARY
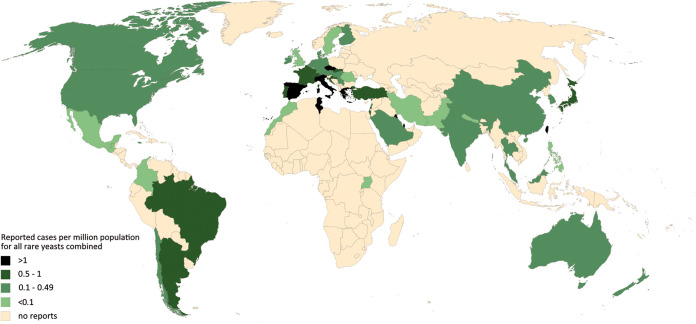
The German surgeon Bernhard von Langenbeck was probably the first to directly link yeasts as an etiological agent of oropharyngo-esophageal thrush, publishing the first case of esophageal candidiasis in a patient who died of typhoid fever in 1839 (1, 2). It took another 22 years, however, to discover that yeasts also lead to disseminated disease, when the pathologist Friedrich Albert Zenker described a case of disseminated yeast infection as metastatic brain lesions in 1861 (3). Today, Candida species are major pathogens in hospitalized and immunocompromised patients and the third most dominant cause of nosocomial bloodstream infections (4). However, uncommon yeasts other than Candida and Cryptococcus spp. have emerged as significant pathogens during the last 2 decades (5, 6). These fungi are commonly encountered in the environment and frequent colonizers of human skin and mucosal surfaces (7–9). As such, they may be inadvertently dismissed as innocent bystanders or as contaminants. Particularly in the setting of immunosuppression or other immune compromise, they are increasingly reported to cause life-threatening invasive infections (5, 10). This may be a result of common and prolonged exposure to antifungal agents, use of indwelling catheters, new anticancer treatments, immunosuppressants, and increased overall survival of multimorbid patients (11, 12). In addition, it is hypothesized that the emergence of fungal pathogens with higher virulence for endothermic organisms may be related to their thermal adaptation in response to climate change (13). Invasive diseases with rare yeasts are of particular concern because they are associated with unacceptably high mortality rates (14–17). Unfortunately, the epidemiology of many of these rare and emerging infections is still not well studied, with little or no epidemiological surveillance on mycoses conducted in public health agencies of most countries (18). The worldwide distribution and incidence of rare yeast infections are therefore unclear. Efforts are made to extrapolate the distribution in geographical regions from reported cases, despite confounding factors such as availability of diagnostic approaches or awareness (Fig. 1).
FIG 1.
Worldwide distribution of reported rare yeast infections. Numbers of reported cases of severe fungal infections caused by fungi of the genera Geotrichum, Kodamaea, Malassezia, Pseudozyma (now Moesziomyces/Dirkmeia), Rhodotorula, Saccharomyces, Saprochaete/Magnusiomyces, Sporobolomyces, and Trichosporon in humans as provided for each pathogen separately in the Rare Yeast Global Guideline are presented in a concatenated format for a general overview of the worldwide distribution. The map provides a current view on published cases that is likely related to the medical infrastructure and economic resources in some countries. Numbers are not supposed to predict incidences per country.
Previous guidelines on invasive rare yeast infections were either region specific, required updating, were limited to single pathogens, or were missing altogether for many of the uncommon, but emerging, pathogenic yeasts (12, 19, 20). Now, international societies of medical mycology and microbiology have created the “Global guideline for the diagnosis and management of rare yeast infections: an initiative of the European Confederation of Medical Mycology (ECMM) in cooperation with the International Society for Human and Animal Mycology (ISHAM) and the American Society for Microbiology (ASM)” (21) to facilitate best-practice multidisciplinary care for patients with invasive rare yeast infections. In alignment with the “One World–One Guideline” initiative, physicians and scientists from multiple disciplines and all parts of the world involved in managing uncommon yeast infections were invited to contribute to the guideline development (22). The guidance document was reviewed and endorsed by 45 scientific societies, including national societies from 31 countries and several international societies. From the ASM, five reviewers conducted outstanding detailed reviews that contributed significantly to improving the guideline. Systemic infections caused by the basidiomycetous yeasts Trichosporon, Malassezia, Pseudozyma (now Moesziomyces/Dirkmeia), Rhodotorula, Sporobolomyces, and the ascomycetous yeasts Geotrichum, Kodamaea, Saccharomyces, and Saprochaete/Magnusiomyces are covered.
The present recommendations comprise the third guidance document of the “One World–One Guideline” initiative, after the mucormycosis guideline, published in 2019, and the rare mold guideline, published in 2021 (23, 24). The awareness and knowledge about rare diseases is a strong factor for the accurate diagnosis and timely treatment of these conditions (25). Emerging yeasts present multiple challenges in diagnosis and management. In the laboratory, these pathogens require personnel with a high level of specific mycology training, as they can be difficult to culture, e.g., Malassezia, and are easily misidentified by classical phenotypic methods (20, 26). Specific diagnostic surrogate markers are not available for these pathogens, and culture-based methods, including blood culture, remain central to diagnosis despite being insensitive and time-consuming (27, 28). Identification is increasingly enabled by the application of modern molecular or proteomic tools, though the availability is resource dependent (27). Further, outbreaks, often health care related, have been reported for most of the uncommon yeasts (29–31).
Timely administration of targeted antifungal therapy is a critical component affecting disease outcomes (32). Therefore, a high index of clinical suspicion is necessary, and clinicians should be familiar with predisposing factors and signs and symptoms of invasive fungal disease. Patients with underlying hematological diseases, but also HIV, uncontrolled diabetes mellitus, and soft tissue trauma, are prone to infections with Geotrichum spp. (17, 33–35). Geotrichosis frequently manifests systemically with positive blood cultures and often presents with skin lesions and pulmonary infection (33, 35, 36). The overall mortality for Geotrichum candidum infections is highest in oncological patients, at >60% (17). Hematological malignancy is also a major risk factor for Saprochaete/Magnusiomyces species infection (37). Patients with fungemia often present with hepatosplenic abscesses, metastatic skin lesion, brain abscesses, or osteomyelitis (37–39). However, these yeasts also cause disease in immunocompetent individuals (40). Trichosporon asahii is the major etiological agent of invasive trichosporonosis (5). Invasive disease has been diagnosed mostly in immunocompromised patients, especially in patients with prolonged neutropenia, indwelling catheters, and previous antifungal exposure (41). The most common manifestation is fungemia with/without metastatic skin lesions, pneumonia, and splenic and liver abscesses (42–44). Mortality ranges from 30% to 90% (16, 42).
To reduce mortality in susceptible hosts, appropriate antifungal prophylaxis and treatment are important tools. However, both are complicated by the fact that several rare yeasts are intrinsically resistant to one or more classes of antifungals. For example, Trichosporon spp., Rhodotorula spp., and Magnusiomyces capitatus are considered intrinsically resistant to the echinocandins, and Rhodotorula spp. show resistance to some azoles (45, 46), with episodes of breakthrough infections described previously (25, 47–49). Due to the absence of clinical breakpoints, antifungal susceptibility profiles of these yeasts may be difficult to interpret. Tailored treatment that takes into account accurate classification at the species level, susceptibility profiles, and clinical management pathways is imperative.
The endorsement of the ASM is of particular importance to increase the awareness of rare yeast infections among physicians and laboratory scientists. The guideline’s target is to provide guidance on the correct utilization and application of established and new diagnostic and therapeutic options and to be of substantial help to clinicians dealing with rare yeast infections worldwide. Simultaneously, the document provides an overview of areas of uncertainty for invasive yeast infections and new directions of future research.
ACKNOWLEDGMENTS
R.S. drafted the initial version of the manuscript and reviewed and approved the final version of the manuscript. O.A.C. conceived the idea and reviewed and approved the final version of the manuscript. S.C.-A.C. contributed to manuscript preparation and reviewed and approved the final version of the manuscript. D.S. prepared the figure and figure legend and reviewed and approved the final version of the manuscript. A.N.S. and S.X.Z. contributed to manuscript preparation and reviewed and approved the final version of the manuscript.
R.S., D.S., and A.N.S. have nothing to disclose. O.A.C. reports grants and personal fees from Actelion, personal fees from Allecra Therapeutics, personal fees from Al-Jazeera Pharmaceuticals, grants and personal fees from Amplyx, grants and personal fees from Astellas, grants and personal fees from Basilea, personal fees from Biosys, grants and personal fees from Cidara, grants and personal fees from Da Volterra, personal fees from Entasis, grants and personal fees from F2G, grants and personal fees from Gilead, personal fees from Grupo Biotoscana, personal fees from IQVIA, grants from Janssen, personal fees from Matinas, grants from Medicines Company, grants and personal fees from MedPace, grants from Melinta Therapeutics, personal fees from Menarini, grants and personal fees from Merck/MSD, personal fees from Mylan, personal fees from Nabriva, personal fees from Noxxon, personal fees from Octapharma, personal fees from Paratek, grants and personal fees from Pfizer, personal fees from PSI, personal fees from Roche Diagnostics, grants and personal fees from Scynexis, personal fees from Shionogi, grants from DFG, German Research Foundation, grants from German Federal Ministry of Research and Education, and grants from Immunic, outside the submitted work. S.X.Z. reports research grants from IMMY Diagnostics and Vela Diagnostics. S.C.-A.C. reports untied educational grants from MSD Australia and F2G Ltd.
We received no specific funding for this work.
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
Footnotes
Citation Sprute R, Cornely OA, Chen SC-A, Seidel D, Schuetz AN, Zhang SX. 2021. All you need to know and more about the diagnosis and management of rare yeast infections. mBio 12:e01594-21. https://doi.org/10.1128/mBio.01594-21.
REFERENCES
- 1.von Langenbeck B. 1839. Auffindung von Pilzen auf der Schleimhaut der Speiseröhre einer Typhusleiche. Neue Notizen Aus Dem Gebiete Der Natur- Und Heilkunde 12:146–147. [Google Scholar]
- 2.Knoke M, Bernhardt H. 2006. The first description of an oesophageal candidosis by Bernhard von Langenbeck in 1839. Mycoses 49:283–287. doi: 10.1111/j.1439-0507.2006.01237.x. [DOI] [PubMed] [Google Scholar]
- 3.Zenker FA. 1861. Encephalitis mit Pilzentwicklungen im Gehirn. Jahresb D Gesellsch F Nat U Heik in Dresd 62:51. [Google Scholar]
- 4.de Jong AW, Hagen F. 2019. Attack, defend and persist: how the fungal pathogen Candida auris was able to emerge globally in healthcare environments. Mycopathologia 184:353–365. doi: 10.1007/s11046-019-00351-w. [DOI] [PubMed] [Google Scholar]
- 5.Miceli MH, Díaz JA, Lee SA. 2011. Emerging opportunistic yeast infections. Lancet Infect Dis 11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 6.Xiao M, Chen SC-A, Kong F, Fan X, Cheng J, Hou X, Zhou M, Wang H, Xu Y-C. 2018. Five-year China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study of invasive fungal infections caused by noncandidal yeasts: species distribution and azole susceptibility. Infect Drug Resist 11:1659–1667. doi: 10.2147/IDR.S173805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho O, Matsukura M, Sugita T. 2015. Molecular evidence that the opportunistic fungal pathogen Trichosporon asahii is part of the normal fungal microbiota of the human gut based on rRNA genotyping. Int J Infect Dis 39:87–88. doi: 10.1016/j.ijid.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Nunes JM, Bizerra FC, Ferreira RC, Colombo AL. 2013. Molecular identification, antifungal susceptibility profile, and biofilm formation of clinical and environmental Rhodotorula species isolates. Antimicrob Agents Chemother 57:382–389. doi: 10.1128/AAC.01647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. 2012. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev 25:106–141. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pande A, Non LR, Romee R, Santos CA. 2017. Pseudozyma and other non-Candida opportunistic yeast bloodstream infections in a large stem cell transplant center. Transpl Infect Dis 19:e12664. doi: 10.1111/tid.12664. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Guo M, Wang C, Li Y, Fernandez AM, Ferraro TN, Yang R, Chen Y. 2020. Epidemiological study of Trichosporon asahii infections over the past 23 years. Epidemiol Infect 148:e169. doi: 10.1017/S0950268820001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, European Confederation of Medical Mycology . 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 20(Suppl 3):76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall A, Kontoyiannis DP, Robert V. 2021. Environmental Candida auris and the global warming emergence hypothesis. mBio 12:e00360-21. doi: 10.1128/mBio.00360-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potenza L, Chitasombat MN, Klimko N, Bettelli F, Dragonetti G, Del Principe MI, Nucci M, Busca A, Fracchiolla N, Sciumè M, Spolzino A, Delia M, Mancini V, Nadali GP, Dargenio M, Shadrivova O, Bacchelli F, Aversa F, Sanguinetti M, Luppi M, Kontoyiannis DP, Pagano L. 2019. Rhodotorula infection in haematological patient: risk factors and outcome. Mycoses 62:223–229. doi: 10.1111/myc.12875. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti A, Rudramurthy SM, Kale P, Hariprasath P, Dhaliwal M, Singhi S, Rao KLN. 2014. Epidemiological study of a large cluster of fungaemia cases due to Kodamaea ohmeri in an Indian tertiary care centre. Clin Microbiol Infect 20:O83–O89. doi: 10.1111/1469-0691.12337. [DOI] [PubMed] [Google Scholar]
- 16.Chagas-Neto TC, Chaves GM, Melo AS, Colombo AL. 2009. Bloodstream infections due to Trichosporon spp.: species distribution, Trichosporon asahii genotypes determined on the basis of ribosomal DNA intergenic spacer 1 sequencing, and antifungal susceptibility testing. J Clin Microbiol 47:1074–1081. doi: 10.1128/JCM.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durán Graeff L, Seidel D, Vehreschild MJGT, Hamprecht A, Kindo A, Racil Z, Demeter J, De Hoog S, Aurbach U, Ziegler M, Wisplinghoff H, Cornely OA, FungiScope Group . 2017. Invasive infections due to Saprochaete and Geotrichum species: report of 23 cases from the FungiScope Registry. Mycoses 60:273–279. doi: 10.1111/myc.12595. [DOI] [PubMed] [Google Scholar]
- 18.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 19.Cornely OA, Cuenca-Estrella M, Meis JF, Ullmann AJ. 2014. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) and European Confederation of Medical Mycology (ECMM) 2013 joint guidelines on diagnosis and management of rare and emerging fungal diseases. Clin Microbiol Infect 20(Suppl 3):1–4. doi: 10.1111/1469-0691.12569. [DOI] [PubMed] [Google Scholar]
- 20.Iatta R, Battista M, Miragliotta G, Boekhout T, Otranto D, Cafarchia C. 2018. Blood culture procedures and diagnosis of Malassezia furfur bloodstream infections: strength and weakness. Med Mycol 56:828–833. doi: 10.1093/mmy/myx122. [DOI] [PubMed] [Google Scholar]
- 21.Chen SC-A, Perfect J, Colombo AL, Cornely OA, Groll AH, Seidel D, Albus K, de Almedia JN, Jr, Garcia-Effron G, Gilroy N, Lass-Flörl C, Ostrosky-Zeichner L, Pagano L, Papp T, Rautemaa-Richardson R, Salmanton-García J, Spec A, Steinmann J, Arikan-Akdagli S, Arenz DE, Sprute R, Duran-Graeff L, Freiberger T, Girmenia C, Harris M, Kanj SS, Roudbary M, Lortholary O, Meletiadis J, Segal E, Tuon FF, Wiederhold N, Bicanic T, Chander J, Chen Y-C, Hsueh P-R, Ip M, Munoz P, Spriet I, Temfack E, Thompson L, Tortorano AM, Velegraki A, Govender NP. Global guideline for the diagnosis and management of rare yeast infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis, in press. [DOI] [PubMed] [Google Scholar]
- 22.Hoenigl M, Gangneux J-P, Segal E, Alanio A, Chakrabarti A, Chen SC-A, Govender N, Hagen F, Klimko N, Meis JF, Pasqualotto AC, Seidel D, Walsh TJ, Lagrou K, Lass-Flörl C, Cornely OA, European Confederation of Medical Mycology (ECMM) . 2018. Global guidelines and initiatives from the European Confederation of Medical Mycology to improve patient care and research worldwide: new leadership is about working together. Mycoses 61:885–894. doi: 10.1111/myc.12836. [DOI] [PubMed] [Google Scholar]
- 23.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE, Mellinghoff SC, Mer M, Pana ZD, Seidel D, Sheppard DC, Wahba R, Akova M, Alanio A, Al-Hatmi AMS, Arikan-Akdagli S, Badali H, Ben-Ami R, Bonifaz A, Bretagne S, Castagnola E, Chayakulkeeree M, Colombo AL, Corzo-León DE, Drgona L, Groll AH, Guinea J, Heussel C-P, Ibrahim AS, Kanj SS, Klimko N, Lackner M, Lamoth F, Lanternier F, Lass-Floerl C, Lee D-G, Lehrnbecher T, Lmimouni BE, Mares M, Maschmeyer G, Meis JF, Meletiadis J, Morrissey CO, Nucci M, Oladele R, Pagano L, et al. 2019. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoenigl M, Salmanton-García J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, Lackner M, Sprute R, Al-Hatmi AMS, Bassetti M, Carlesse F, Freiberger T, Koehler P, Lehrnbecher T, Kumar A, Prattes J, Richardson M, Revankar S, Slavin MA, Stemler J, Spiess B, Taj-Aldeen SJ, Warris A, Woo PCY, Young JH, Albus K, Arenz D, Arsic-Arsenijevic V, Bouchara JP, Chinniah TR, Chowdhary A, de Hoog GS, Dimopoulos G, Duarte RF, Hamal P, Meis JF, Mfinanga S, Queiroz-Telles F, Patterson TF, Rahav G, Rogers TR, Rotstein C, Wahyuningsih R, Seidel D, Cornely OA. 16 February 2021. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 21(8):E246–E257. 10.1016/S1473-3099(20)30784-2. [Erratum, 21(4):E81, 2021, .] [DOI] [PubMed] [Google Scholar]
- 25.Liao Y, Hartmann T, Zheng T, Yang RY, Ao JH, Wang WL. 2012. Breakthrough trichosporonosis in patients receiving echinocandins: case report and literature review. Chin Med J (Engl) 125:2632–2635. [PubMed] [Google Scholar]
- 26.Barnes RA. 2008. Early diagnosis of fungal infection in immunocompromised patients. J Antimicrob Chemother 61(Suppl 1):i3–6. doi: 10.1093/jac/dkm424. [DOI] [PubMed] [Google Scholar]
- 27.de Almeida JN, Sztajnbok J, da Silva AR, Vieira VA, Galastri AL, Bissoli L, Litvinov N, Del Negro GMB, Motta AL, Rossi F, Benard G. 2016. Rapid identification of moulds and arthroconidial yeasts from positive blood cultures by MALDI-TOF mass spectrometry. Med Mycol 54:885–889. doi: 10.1093/mmy/myw044. [DOI] [PubMed] [Google Scholar]
- 28.Kozel TR, Wickes B. 2014. Fungal diagnostics. Cold Spring Harb Perspect Med 4:a019299. doi: 10.1101/cshperspect.a019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanzani M, Cricca M, Sassi C, Sutto E, De Cicco G, Bonifazi F, Bertuzzi C, Bacci F, Paolini S, Cavo M, Lewis RE. 2019. Saprochaete clavata infections in patients undergoing treatment for haematological malignancies: a report of a monocentric outbreak and review of the literature. Mycoses 62:1100–1107. doi: 10.1111/myc.12978. [DOI] [PubMed] [Google Scholar]
- 30.Otag F, Kuyucu N, Erturan Z, Sen S, Emekdas G, Sugita T. 2005. An outbreak of Pichia ohmeri infection in the paediatric intensive care unit: case reports and review of the literature. Mycoses 48:265–269. doi: 10.1111/j.1439-0507.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- 31.Ersoz G, Otag F, Erturan Z, Aslan G, Kaya A, Emekdas G, Sugita T. 2004. An outbreak of Dipodascus capitatus infection in the ICU: three case reports and review of the literature. Jpn J Infect Dis 57:248–252. [PubMed] [Google Scholar]
- 32.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 33.Ng KP, Soo-Hoo TS, Koh MT, Kwan PW. 1994. Disseminated Geotrichum infection. Med J Malaysia 49:424–426. [PubMed] [Google Scholar]
- 34.Heinic GS, Greenspan D, MacPhail LA, Greenspan JS. 1992. Oral Geotrichum candidum infection associated with HIV infection. A case report. Oral Surg Oral Med Oral Pathol 73:726–728. doi: 10.1016/0030-4220(92)90019-M. [DOI] [PubMed] [Google Scholar]
- 35.Girmenia C, Pagano L, Martino B, D'Antonio D, Fanci R, Specchia G, Melillo L, Buelli M, Pizzarelli G, Venditti M, Martino P, GIMEMA Infection Program . 2005. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol 43:1818–1828. doi: 10.1128/JCM.43.4.1818-1828.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henrich TJ, Marty FM, Milner DA, Jr, Thorner AR. 2009. Disseminated Geotrichum candidum infection in a patient with relapsed acute myelogenous leukemia following allogeneic stem cell transplantation and review of the literature. Transpl Infect Dis 11:458–462. doi: 10.1111/j.1399-3062.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- 37.Del Principe MI, Sarmati L, Cefalo M, Fontana C, De Santis G, Buccisano F, Maurillo L, De Bellis E, Postorino M, Sconocchia G, Del Poeta G, Sanguinetti M, Amadori S, Pagano L, Venditti A. 2016. A cluster of Geotrichum clavatum (Saprochaete clavata) infection in haematological patients: a first Italian report and review of literature. Mycoses 59:594–601. doi: 10.1111/myc.12508. [DOI] [PubMed] [Google Scholar]
- 38.Gadea I, Cuenca-Estrella M, Prieto E, Diaz-Guerra TM, Garcia-Cia JI, Mellado E, Tomas JF, Rodriguez-Tudela JL. 2004. Genotyping and antifungal susceptibility profile of Dipodascus capitatus isolates causing disseminated infection in seven hematological patients of a tertiary hospital. J Clin Microbiol 42:1832–1836. doi: 10.1128/JCM.42.4.1832-1836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulu-Kilic A, Atalay MA, Metan G, Cevahir F, Koç N, Eser B, Çetin M, Kaynar L, Alp E. 2015. Saprochaete capitata as an emerging fungus among patients with haematological malignencies. Mycoses 58:491–497. doi: 10.1111/myc.12347. [DOI] [PubMed] [Google Scholar]
- 40.Tanabe MB, Patel SA. 2018. Blastoschizomyces capitatus pulmonary infections in immunocompetent patients: case report, case series and literature review. Epidemiol Infect 146:58–64. doi: 10.1017/S0950268817002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo AL, Padovan AC, Chaves GM. 2011. Current knowledge of Trichosporon spp. and trichosporonosis. Clin Microbiol Rev 24:682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Almeida Júnior JN, Hennequin C. 2016. Invasive Trichosporon Infection: a systematic review on a re-emerging fungal pathogen. Front Microbiol 7:1629. doi: 10.3389/fmicb.2016.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yong AMY, Yang SS, Tan KB, Ho SA. 2017. Disseminated cutaneous trichosporonosis in an adult bone marrow transplant patient. Indian Dermatol Online J 8:192–194. doi: 10.4103/idoj.IDOJ_92_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kontoyiannis DP, Torres HA, Chagua M, Hachem R, Tarrand JJ, Bodey GP, Raad II. 2004. Trichosporonosis in a tertiary care cancer center: risk factors, changing spectrum and determinants of outcome. Scand J Infect Dis 36:564–569. doi: 10.1080/00365540410017563. [DOI] [PubMed] [Google Scholar]
- 45.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diekema DJ, Petroelje B, Messer SA, Hollis RJ, Pfaller MA. 2005. Activities of available and investigational antifungal agents against rhodotorula species. J Clin Microbiol 43:476–478. doi: 10.1128/JCM.43.1.476-478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuermans C, van Bergen M, Coorevits L, Verhaegen J, Lagrou K, Surmont I, Jeurissen A. 2011. Breakthrough Saprochaete capitata infections in patients receiving echinocandins: case report and review of the literature. Med Mycol 49:414–418. doi: 10.3109/13693786.2010.535179. [DOI] [PubMed] [Google Scholar]
- 48.Mori T, Nakamura Y, Kato J, Sugita K, Murata M, Kamei K, Okamoto S. 2012. Fungemia due to Rhodotorula mucilaginosa after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 14:91–94. doi: 10.1111/j.1399-3062.2011.00647.x. [DOI] [PubMed] [Google Scholar]
- 49.Cornely OA, Hoenigl M, Lass-Flörl C, Chen SC-A, Kontoyiannis DP, Morrissey CO, Thompson GR, Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM) . 2019. Defining breakthrough invasive fungal infection—position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 62:716–729. doi: 10.1111/myc.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]