ABSTRACT
Long-term effective use of antiretroviral therapy (ART) among people with HIV (PWH) has significantly reduced the burden of disease, yet a cure for HIV has not been universally achieved, likely due to the persistence of an HIV reservoir. The central nervous system (CNS) is an understudied HIV sanctuary. Importantly, due to viral persistence in the brain, cognitive disturbances persist to various degrees at high rates in PWH despite suppressive ART. Given the complexity and accessibility of the CNS compartment and that it is a physiologically and anatomically unique immune site, human studies to reveal molecular mechanisms of viral entry, reservoir establishment, and the cellular and structural interactions leading to viral persistence and brain injury to advance a cure and either prevent or limit cognitive impairments in PWH remain challenging. Recent advances in human brain organoids show that they can mimic the intercellular dynamics of the human brain and may recapitulate many of the events involved in HIV infection of the brain (neuroHIV). Human brain organoids can be produced, spontaneously or with addition of growth factors and at immature or mature states, and have become stronger models to study neurovirulent viral infections of the CNS. While organoids provide opportunities to study neuroHIV, obstacles such as the need to incorporate microglia need to be overcome to fully utilize this model. Here, we review the current achievements in brain organoid biology and their relevance to neuroHIV research efforts.
KEYWORDS: cerebral organoids, HIV reservoirs, HIV cure, neuropathology, cure, HIV, reservoirs
INTRODUCTION
Although antiretroviral therapy (ART) has been effective in suppressing viremia and decreasing mortality and morbidity, human immunodeficiency virus (HIV) remains a chronic disease requiring lifelong treatment due to the persistence of viral reservoirs. Thus, a cure for HIV remains a crucial public health need. Cessation of ART in people with HIV (PWH) ultimately results in the resurgence of virus in the periphery (1, 2), with evidence showing anatomical compartments, such as lymphoid organs, gut-associated lymphoid tissue, and the brain, being likely sources of viral recrudescence (3–6). These tissue sites of viral persistence have hindered current efforts in the eradication of HIV. Evidence suggest that long-lasting viral reservoirs are established in the central nervous system (CNS), likely due to poor drug penetration and reduced immune surveillance, among other factors (7, 8). The CNS compartment should thus be taken into consideration in designing and implementing effective HIV curative strategies permitting ART-free remission. Furthermore, PWH continue to experience high rates (>50%) of cognitive abnormalities even when HIV replication is suppressed by ART (9–14), despite variations in prevalence by cohort and means of cognitive assessments (15–18). These neurocognitive deficits interfere with psychomotor speed and coordination and diminish memory and executive functions, reducing quality of life (19, 20), and current ART regimens have had variable impacts in reducing these deficits (21).
HIV penetration into the CNS is thought to occur early during acute infection, likely through the trafficking of infected lymphocytes and/or myeloid cells during heightened systemic immune activation across the blood-brain barrier (BBB) (22–30). Once within the brain parenchyma, HIV can infect and integrate into the genome of permissive resident cells, such as microglia and perivascular macrophages (19). Evidence exists that microglial cells and perivascular macrophages constitute the major cellular HIV brain reservoirs, with astrocytes being potentially another cellular reservoir (31–37). HIV RNA and associated viral protein expression in human brain tissues has been reported (38–40), with recent studies showing macrophage-tropic HIV type 1 (HIV-1) replication in myeloid cells in the CNS of individuals with HIV-associated dementia (41). In humans, these studies have mostly been limited to the examination of postmortem brain tissue. Addressing viral persistence and neuropathological mechanisms in the human brain remains a challenge, supporting alternate model systems to advance research on HIV infection of the brain (neuroHIV). Large- and small-animal models have been widely used to address these issues (42, 43). Evidence from simian immunodeficiency virus (SIV) nonhuman primate (NHP) studies have shown that CNS infection occurs within days of exposure, viral DNA can be detected in the brain during plasma viral suppression, and replication-competent virus resides in both perivascular macrophages and microglia (44–46). NHP models can simulate HIV disease in the CNS better than in vitro assays; however, these costly and time-consuming experiments still do not faithfully predict treatment outcomes and have not fully shed light on the underlying mechanisms of HIV-induced CNS disease (47–49). Genetically modified mouse models have been a useful tool for the understanding of molecular and cellular mechanisms and development of therapeutics for neuropathological infections (50). More recent studies using HIV-infected humanized mice and conventional mice infected with chimeric HIV (EcoHIV) have furthered the understanding of viral reservoirs in the brain and the impact of HIV on cognition (51–53). While animal models provide insight into retroviral dynamics within a host, there are still limitations in recapitulating the pathology, transcriptional phenotypes, and functionality to in comparison to that of human CNS infection.
Evaluations of mechanistic outcomes of HIV and human-specific CNS cell interactions have been predominately relegated to two-dimensional (2D) tissue culture models. Immortalized microglial cell lines, such as HMC3 and C20, have been heavily used as HIV infection models (48, 54), while generated latently HIV-infected microglia models, immortalized human microglia carrying a single round HIV construct, have elucidated potential mechanisms for HIV reactivation from latency (48, 55, 56). While peripheral blood monocyte-derived microglia (MMG) have also been used and shown to be productively infected with HIV infection (47), recent technological advancements in human induced pluripotent stem cells (hiPSCs) allowed the generation of microglia that more accurately represent the phenotype of primary microglia and are permissive to HIV (57). Human astrocyte cultures have shown that cellular entry of HIV is not through the classic CD4/gp120 fusion but rather by cell-to-cell contact with infected cells, the engulfment of infected cells, or the internalization of HIV through endocytosis (58–60). Several studies indicate that a small fraction of astrocytes can be transiently productive and latently infected by HIV in vitro (36, 61). However, the detection of virions and components within astrocytes from the engulfment of entire infected cells and through the endocytic cycle of HIV internalization and release can be mistaken for productive infection and integration (60).
More recent coculture 2D models that incorporate multiple CNS resident cells have demonstrated the importance of intercellular dynamics in regulating neuroinflammatory responses and HIV kinetics. Healthy neurons have been shown to mediate HIV transcriptional silencing in microglia when cocultured (49). Tricultures, containing human iPSC-derived neurons, astrocytes, and microglia, demonstrate that microglia are the predominate cells that are productively infected as well as that cellular cross talk elicits distinct microglial functional and proinflammatory signatures. Furthermore, infected and HIV-exposed microglia, in these tricultures, show impairments in cell cycle regulation, phagocytoses of synapses, and DNA repair mechanisms (62). While essential for neuroHIV research, these 2D cultures lack the 3D complexity and the complete cellular composition of the brain and, thus, do not represent the actual cellular environments or fully recapitulate human disease or treatment effectiveness.
The reprogrammable nature of human pluripotent stem cells (hPSCs) and innovations in stem cell technology have facilitated the generation of organoid technologies, 3D culture systems that undergo some level of self-organization and resemble in vivo organs (63–65). Brain organoids offer the possibility to investigate cellular development and intercellular interactions within a 3D human brain microenvironment. Brain organoids, which maintain the genotype of the original cell or tissue source, are more heterogeneous and complex than 2D models and have provided useful insights into human brain development and a variety of neurological disorders and neurotropic infections (64, 66, 67). Given the limitations of current in vitro models, the accessibility of primary human CNS cells, the restriction of infecting rodent CNS models only with the substantial manipulation of the host or HIV, and the expense and expertise required to examine nonhuman primates, many HIV investigators are turning to human brain organoids in hopes of having an affordable, physiological, and reproducible model to study HIV disease mechanisms and predictors of treatment efficacy in the CNS.
ADVANCES IN HUMAN BRAIN ORGANOID TECHNOLOGY
Brain organoid development, advantages, and limitations.
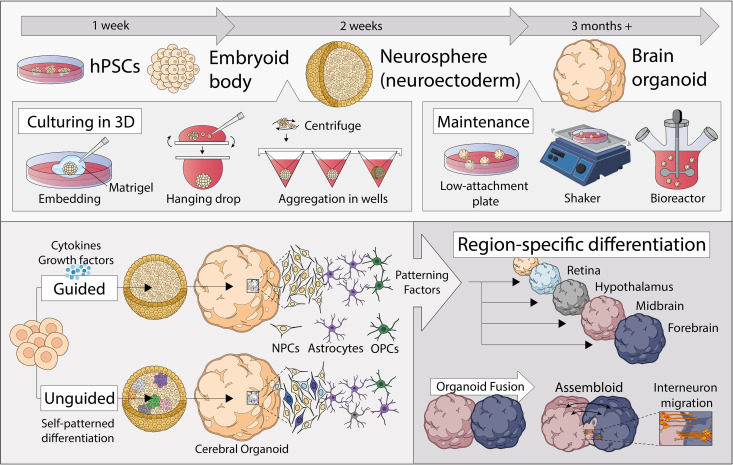
hPSCs, including human embryonic stem cells (hESCs) and hiPSCs, are able to differentiate into any cell or tissue type, under specific cues and favorable conditions (68). hPSCs are initially differentiated into embryoid bodies, further toward the neuroectodermal lineage as simple clusters/spheroids, and then to a more complex organization resembling the intricacy of the human brain (Fig. 1). Typical methods of achieving a three-dimensional structure by culturing cells under low-adhesion conditions include the hanging-drop method, cell aggregation in U- or V-bottom well plates, and embedding cells in extracellular matrices (64, 69, 70). Continued growth and maintenance of brain organoids generated can be done in low-attachment plates spun in a bioreactor (a culture system that allows nutrients to be supplied while cells are agitated) or shaken in tissue culture plates. The generation of brain organoids, using these methods, can be under undirected (or unguided) (64, 71) or directed (or guided) conditions (72–74). Undirected organoids are generated in the absence of inductive cues and rely on the intrinsic signaling and self-organization capacities of hPSCs, allowing them to stochastically give rise to cells resembling those found in multiple brain regions, ranging from the retina to hindbrain (63, 75, 76). Alternatively, specific combinations and timing of exogenously applied signaling molecules and growth factors (i.e., Hedgehog signaling) can “direct” brain organoid development to generate specific regions of the brain (64, 72, 73, 77–85). Unguided organoids are more suitable for exploring cell type diversity during whole-brain development, while brain region-specific organoids better recapitulate the brain cytoarchitecture with less heterogeneity that allows for better investigation between specific brain regions with more consistent molecular and functional characterization (63, 75, 76).
FIG 1.
Advances in human brain organoid development, including three-dimensional brain organoids from human pluripotent stem cells (hPSCs) and various means of their production and maintenance. Organoids can be generated under undirected conditions through self-patterned differentiation or directed methods using patterning factors to produce brain-specific regions, which can be subsequently fused to form “assembloids.”
Brain organoids recapitulate several key features of in vivo organogenesis, which allows the interrogation of a working structural human brain in vitro (64). Brain organoids also possess neurons that are functionally capable of electrical excitation and exhibit neuronal transcriptomes that parallel those found in the human brain (86). However, the cellular diversity and structural complexity of brain organoids highly depend on its state of maturity or length of culturing. At the morphological level, immature brain organoids (∼3 months of culture) present a rosette-like structure, containing neuroepithelial stem cells and ventricular radial glial cells that divide at the apical surface and form a ventricular-like zone (65). These present, however, less cell type diversity than those that are more mature. Mature organoids (≥6 months of culture), however, have multiple progenitor zones, including a ventricular-like zone and an outer subventricular-like zone formed by outer radial glial cells that express specific cortical layer and glial cell markers (65, 73). These mature brain organoids also resemble human cortical development at the gene expression level and allow in-depth analysis of neural networks, cell behavior, drug screening, and disease modeling (87). Single-cell mRNA analysis of hPSC-derived brain organoids revealed a multitude of distinct cell populations in mature organoids, including astrocytes, neuroepithelial progenitors, oligodendrocyte precursor cells, neuronal-lineage cells, cells enriched for forebrain markers, and cells expressing retina-specific genes and displayed an enrichment of genes associated with neuronal and glial maturation (71). Although the majority of brain organoid generation protocols aim at modeling cortical development, upon differing culture “guided” conditions they can also form a variety of brain regions, including the cerebrum, midbrain, retina, choroid plexus, and hypothalamus, that all mimic the signaling patterns of their respective brain structures (64, 72, 73, 77–84). While brain region composition varies in organoids from different iPSC lines, regional human-specific gene expression patterns remain largely reproducible across individuals (88). Therefore, brain organoids recapitulate the characteristics of the human brain not only at the cellular level but also in the architecture and developmental trajectories (63).
The 3-dimensionality of brain organoids offer several advantages over 2-dimensional models. Brain organoids are more physiologically relevant, as they can more accurately recapitulate the spatiotemporal organization of the human brain, grow in a more realistic way like cells and organs in vivo, and reproduce key aspects of human brain function. Cells within organoids also display their natural cell shape, exhibit gene and protein expression levels resembling levels observed in cells in vivo, form multiple layers, have realistic proliferation rates, and can retain homeostasis for longer periods (89–91). Brain organoids can model how different cell types interact and respond to flow differentiation and metabolic adaptation, allow efficient cell-to-cell communication (as cell junctions are highly abundant), and more accurately represent responses to mechanical stimuli of cells (89–92). Brain organoids also provide an advantage over other organ-like 3D tissue culture systems, such as neurospheres, that are 3D aggregates of CNS cell types derived from neuronal progenitor cells without a cytoarchitecture (93) or “organ-on-a-chip” technology composed of neural cells artificially assembled in culture chambers and biomaterial scaffolds (94).
Despite the advantages of brain organoids as a model system to study brain pathology, gas and nutrient exchange within organoids, organoid heterogeneity between batches, recapitulating structural components of the human CNS, and increasing cellular diversity need to be addressed to advance the physiological relevance of these organoid models in neuroHIV pathology. To address nutrient and gas exchange, organoids can be grown in bioreactors, in orbital-shaking incubators, or at the liquid-air interface; however, apoptotic cell death within center regions of the organoid due to lack of oxygen with long-term culture could remain a problem (95, 96). Major consideration should be given to batch effect when choosing the ideal organoid generation method, as the stochastic nature of differentiation in unguided organoids coupled with the intrinsic differentiation propensities of individual hPSCs results in high interorganoid variability (71). Transcriptomics data show that bioreactors have helped decrease organoid heterogeneity between batches, as organoids grown in the same bioreactor tend to have more similar cell types, suggesting that organoids secrete signaling factors that influence sister organoids (71, 97). To improve structural organization, some groups have begun fusing individual region-specific organoids into larger “assembloids” that generate cortical interneurons to allow modeling interconnectivity between multiple brain regions (76). Furthermore, as brain organoids derive from a neural lineage cell population, they lack other CNS cells like microglia and vascular cells, which play essential roles in proper brain function. However, there have been recent exciting studies directed at the interplay of these CNS resident nonectodermal cell types in organoids (63, 75, 76), which allow a greater degree of cellular and network maturity.
Cerebral organoids, a tool for the investigation of neurotropic viruses.
While human brain organoids have been essential in elucidating mechanisms of neurodevelopment and neurodegenerative disorders in the past decade (64, 98–100), they have also recently been used as viable alternatives to animal models to enhance investigation of neurotropic viruses, including the study of host-pathogen interactions and associated neuropathological outcomes. Brain organoids have been used to study neurotropic viruses associated with diseases of major public health concern worldwide in which the pathogenesis is still poorly understood, including Zika virus (ZIKV), herpes simplex virus (HSV), and human cytomegalovirus (HCMV), and in recent studies evaluating the ramifications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on the CNS. While in vitro stem cell-based and in vivo animal models helped elucidate cell specificity and brain development with CNS viral infection (101–103), these can lack brain complexity and differ in viral entry receptors (104–106), which brain organoids can address.
ZIKV, declared a public health emergency in 2016, has been largely studied in brain organoids. The strong relationship between newborns with microcephaly and ZIKV infection propelled the use of iPSC-derived organoids, immature and many guided as forebrain region specific, to model the effect ZIKV has on the developing brain (107). Brain organoids revealed that ZIKV infection causes neuronal cell death at the early stages of brain development, the dysregulation of neurogenesis, and the premature differentiation of infected neural progenitor cells (73, 101, 108–111). Although no specific therapeutic antiviral has yet been developed, substantial knowledge on the effects of ZIKV infection on fetal brain development was gained, including that ZIKV-associated microcephaly could potentially be due to the abrogation of neurogenesis and cortical thinning from neural progenitor cell NPC depletion induced by ZIKV (73, 108, 110).
Neonatal infection with herpes simplex virus (HSV) occurs in 1 out of every 3,200 to 10,000 live births, resulting in high mortality or permanent neurological sequelae (112–114). The use of brain organoids has shown to be valuable in the attempt to elucidate the primary molecular mechanisms of HSV-1-driven neuropathology that previously were difficult to study. In immature brain organoids, HSV-1 efficiently infected the complex laminar structure, was transported from the periphery to the central layers, and was able to be effectively reactivated from latency (115). HSV-1 infection also impaired neurogenesis and dysregulated the cortical layer along with brain regionalization during the active period of proliferation of the neuroepithelium (15 days), while in the later stages of infection (45 days), neuronal differentiation was compromised, and numerous inflammatory factors were markedly released (116). Furthermore, much like ZIKV, HSV-1 impaired brain organoid development and induced distinct morphological changes (117).
HCMV neuropathogenesis is the most common cause of infectious-related birth defects. While animal models have elucidated many aspects of brain abnormalities and neuropathogenic mechanisms induced by CMV congenital infection, the main caveat lies in the strict host specificity of CMVs (118, 119). To illuminate human-specific effects of HCMV infection, iPSC-derived human brain organoids were infected with the widely used strain TB40/E, which resulted in reduced brain organoid growth, impaired formation of cortical layers, and abnormal calcium signaling and neural network activity (120–122). Additionally, the use of organoids to model CMV infection confirmed both epidermal growth factor receptor and platelet-derived growth factor receptor A in facilitating viral entry into human CNS resident cells, as well as the efficacy for the use of monoclonal antibody therapeutics in the human brain, specifically those that target the envelope pentamer glycoprotein of HCMV (120).
Individuals with severe coronavirus disease 2019 (COVID-19) have experienced severe neurological symptoms, such as encephalopathy, Guillain-Barré syndrome, and Miller Fisher syndrome (123–125), and more recently, iPSC-derived human brain organoids have been used to investigate the impact of SARS-CoV-2 infection on the CNS. Robust antiviral antibody titers in the cerebrospinal fluid (CSF) were observed in COVID-19 patients and human autopsy samples demonstrate the presence of SARS-CoV-2 RNA transcripts in brain tissues as well as viral proteins in olfactory bulb endothelial cells (126–128). Furthermore, autopsy data indicate that neurons are not directly infected, rather vascular and immune cells with indications of microglia and astrocyte activation (129, 130). To investigate the ramifications of COVID-19 in the human brain, mature iPSC-derived human cortical organoids were infected with SARS-CoV-2 (131–133). Brain organoid models demonstrated that infection was mediated through the ACE2 receptor, resulting in the active release of progeny virions. This contrasted with in vivo data, which showed entry into cortical neurons and neuronal progenitor cells (132, 133). Furthermore, cellular pathways related to cell division, organelle fission, and metabolic processes were upregulated in neurons, with SARS‐CoV‐2‐positive neurons exhibiting an altered distribution of Tau from axons to soma, hyperphosphorylation, and ultimately cell death within 2 days postinfection (131). Exposure at different developmental stages of the organoid showed a preferred tropism for mature neurons (131, 133); however, another study showed that the choroid plexuses are primarily infected, with only large amounts of virus leading to neuronal and glial infection subsequently (134).
Contrasting results in the SARS-CoV-2-infected brain organoid illustrates the need to improve methodologies to address accuracy, robustness, and reproducibility between laboratories. Furthermore, the lack of CNS resident cells not belonging to the neural lineage, such as vascular cells and microglia, could be a major impediment in accurately assessing the impact of this neuroviral infection. For example, in SARS-CoV-2 infection, endothelial cells are suggested to be important in the progression of COVID-19 (135); thus, brain organoids without a vascular system could be missing key elements in COVID-19-related CNS complications. While current human brain organoids have facilitated breakthroughs in neurotropic viral infections, their continuous development is still needed to allow standardization and their use in high-throughput screenings for compound discovery as well as to address HIV-specific outcomes.
OPPORTUNITIES TO STUDY HIV IN HUMAN BRAIN ORGANOIDS
Persistent or latent HIV infection of specific brain regions may induce chronic inflammatory changes which play pivotal roles in the pathophysiology of cognitive disorders in PWH on ART. While it is inconclusive that HIV directly infects neurons (136), the presence of HIV RNA transcripts, proteins (137), and proviral DNA (138) has been detected in neurons. Astrocytes, the most abundant cell type in the CNS (20 to 55%), also play a major role in HIV CNS disease, as they are shown to be permissive to HIV, although with limited replication, and produce robust expression of viral proteins (60). Many HIV proteins are known to activate CNS resident macrophages and glial cells and induce them to produce factors that mediate neuronal injury and apoptosis, such as proinflammatory cytokines and reactive oxygen species. As functional neurons and astrocytes are present in cerebral organoids, these processes could be easily modeled in a 3D environment with intercellular networks. However, evidence shows that HIV remains persistent in myeloid and microglial cells, and the identification and quantification of these reservoirs in virally suppressed patients present a major roadblock to HIV cure research (139–141).
Microglia, the CNS resident tissue macrophages, can comprise up to 17% of all cells in the adult brain depending on region and play multiple roles in health and disease. Microglia shape neuronal plasticity through pruning and stripping synapses and participating in bidirectional signaling with closely intertwined neurons to promote efficient neuronal circuits (142). Microglia can regulate the number and diversity of neurons by triggering apoptosis and phagocytizing dead neurons (143, 144). Microglia also secrete a wide array of factors that impact neuronal function, including those that promote neurogenesis (145, 146). Beyond microglia serving as a viral reservoir, they are shown to exhibit excessive or unchecked activation and an altered proteome with HIV infection that contributes to associated cognitive impairments (147). Therefore, to fully recapitulate events of HIV infection and persistence in the human brain, microglia incorporation into organoids would be highly beneficial to study neuroHIV and to develop comprehensive strategies to prevent or treat CNS complications in HIV.
A recent study showed microglia-like cells can innately develop within iPSC-derived cerebral organoids and exhibit characteristic morphology and gene and protein expression, as well as function like adult human microglia. However, these innate “microglia” are not shown to occur in high numbers (148). Several studies recently reported the incorporation of microglia and myeloid cells as immune mediators in human brain organoid models; however, the source of these cells is crucial in mimicking the phenotype of microglia and their infectibility by HIV-1 (57). The sources of microglia and myeloid cells in published models include primary cells, immortalized cell lines, and stem cell-derived microglia. Incorporation of primary microglia is ideal for modeling HIV-1 disease in the CNS, as these cells are readily infected by HIV-1 and retain phenotypic markers (i.e., IBA-1, high CX3CR1, P2RY12, and TMEM119) (149, 150). However, despite their physiological relevance, primary microglia must be harvested from postmortem biopsy specimens or derived from fetal brain tissue, which limits their availability. In addition, since they are terminally differentiated, they do not proliferate substantially and have a limited life span in culture, making them less useful for HIV studies using organoid models.
To overcome this barrier, groups have added immortalized microglial cell lines or iPSC-derived microglia to organoids to mimic primary microglia in the CNS. A recent study utilizing the HMC3 microglial cell line showed that both uninfected and HIV-infected microglial cells migrated into NPC-derived spheroids, resulting in productive viral infection, and exhibited augmented inflammatory responses (151). However, commonly used microglia cell lines, such as HMC3 and C20, are reported to express low levels of CD4, and their infection is limited primarily to pseudotyped virus (48, 57). They are also limited by their relatively poor expression of genes relevant to HIV replication (i.e., SAMHD1, BST2/tetherin, and APOBEC3G genes) and retain little, if any, of the surface markers displayed on human microglia (CD11b, CD45, and SIGLEC1) (54, 57). Distinct transcriptional profiles are also observed between human immortalized microglial cell lines and primary microglia and MMG, making these cell lines less relevant for studying HIV (57). Both MMG and monocyte-derived macrophages (MDMs) express CD4 and can be productively infected with R5-tropic HIV, providing justification for their use in brain organoid models. However, infection of MMG and MDMs results in lower viral production and apoptosis than infection of primary microglia, which may be due to differing culture conditions and viruses used for infection (57, 152). While viral production occurs for a longer period in primary cells, these terminally differentiated cells exhibit low levels of cell proliferation and have a limited life span in culture.
Recently developed iPSC-derived microglia retain CD4, support ongoing HIV replication, and have been incorporated into brain organoid models (57, 140, 153–155). Several studies show that primary and iPSC-derived microglia dramatically alter their gene expression and phenotype when cultured in vitro under different conditions (140, 155–160). However, a recent study shows that neurons and astrocytes drive specific microglial identity and regulate their phagocytic function and inflammatory responses in vitro (161), indicating that signaling from other CNS resident cells may be required for these microglia to mimic in vivo counterparts. In a study in which iPSC-derived microglia were aggregated with predifferentiated neural cultures at a postmitotic and gliogenesis stage, NPC-derived spheroids were formed with embedded microglia that were highly branched (140). Similarly, migration and ramification of iPSC-derived microglia in cortical organoids were observed when these were cultured for more than 100 days (153), and iPSC-derived microglia have been incorporated into a 2-month-old cerebral organoid model of Alzheimer’s disease (154). Additionally, iPSC-derived microglia that were incorporated into cerebral organoids resembled microglia in vivo and responded to injury stimulus by migrating to the injury site and becoming activated (155).
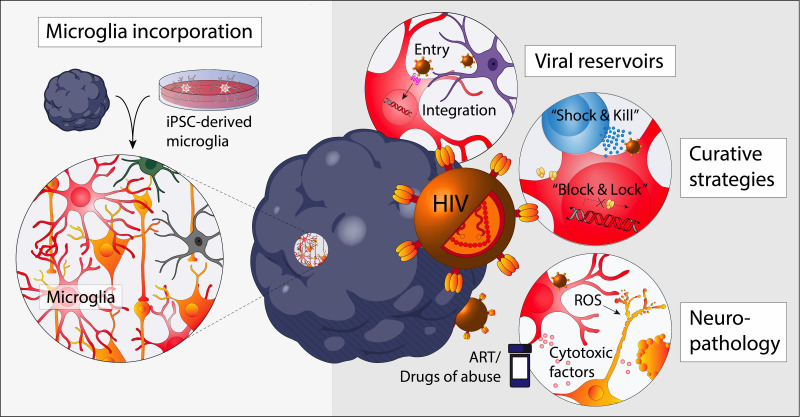
Although brain organoid usage is not yet commonplace in the HIV research field, recent advances in organoid development and composition open the opportunity to model the impact of various aspects of neuroHIV, including viral reservoir establishment, curative strategies, and HIV-induced neuroinflammation and neuropathology, as well as the effects of antiretroviral toxicity and additive pathologies following substance abuse (Fig. 2). Advances in brain organoid have led to efforts to investigate HIV, but many ongoing studies are limited by the lack of microglia in these models. Attempts to overcome this issue are currently being made, specifically by coculturing two doxycycline-inducible iPSC sublines that can differentiate into either microglia or excitatory neurons, incorporating iPSC-derived microglia into already formed brain organoids, or the simple coculture of iPSC-derived microglia and brain organoids.
FIG 2.
Opportunities to study neuroHIV using brain organoids. Brain organoids with the incorporation of microglia allow the study of neuroHIV, including cellular reservoirs, curative strategies, and neuropathological outcomes due to HIV infection, microglial activation (reactive oxygen species [ROS] and proinflammatory cytokine secretion), and toxicities from antiretroviral therapy or drugs of abuse.
Reservoir studies are attempting to identify key intrinsic signals mediating viral silencing and reactivation. Brain organoids could be particularly efficacious in elucidating the efficacy of agents for HIV curative measures that either silence HIV transcription (the “block-and-lock” approach) or promote latency reversal from cellular reservoirs for the subsequent killing by effector immune cells (the “shock-and-kill” approach) (162–164). Autologous immune cells, including HIV-specific cytotoxic T lymphocytes (CTLs) and natural killer cells, can be added to autologous induced progenitor cell-derived CNS organoids to evaluate effectiveness of various cell therapy cure strategies in eliminating productively and latently HIV-infected cells, a method found effective in NHP models and lymph node organoid cultures (165). CTL-mediated “killing” methods are often paired with latency-activating agents, such as histone deacetylase inhibitors and Toll-like receptor agonists, that “shock” these latently infected cells to express HIV (166, 167). Studies under way are also evaluating the alternative block-and-lock approach to silence HIV long term, specifically using CRISPR interference by promoting H3K9me3 and DNA methylation or using didehydro-cortistatin A (dCA), an inhibitor of the HIV transactivator of transcription (Tat) protein (168–171). The use of brain organoids also opens avenues for testing other potential curative approaches for the CNS compartment, including using CRISPR to mutate or excise the HIV-1 genome, neutralizing the activity of HIV-1 resistant strains using broadly neutralizing antibodies, and ART intensification (172–175).
Importantly, microglial activation and neuronal damage associated with HIV infection are being investigated, which both have been linked to poor cognitive performance in ART-suppressed PWH (176–178). Current research is aimed at determining how HIV infection alters microglia-intrinsic pathways, neurodegeneration induced by HIV-infected microglia, along with the effects on organoid structure, neural network health, and signaling. Studies are under way to investigate how individual viral proteins cause neurological damage, namely, Tat and gp120, which are shown to be highly toxic to neurons and glia cells (179, 180). Brain organoid studies may also have translational potential, through corroborating with gene expression findings for microglia and neurons obtained from postmortem tissues of donors that exhibited HIV-associated cognitive impairment. This opens research avenues into novel therapies, namely, pharmacological agents against microglial receptors and/or mediators of inflammation, to potentially alleviate neuropathological outcomes and/or to delay the development of cognitive complications.
Substance abuse and addiction are highly prevalent in PWH, and many drugs of abuse (DOA), including opioids, methamphetamine (meth), and cocaine, are thought to exacerbate the effects of HIV-associated cognitive impairment (181). Glial cells are thought to be an important cellular site for drug-HIV-1 interactions (182). Morphine is shown to increase HIV replication in infected macrophages and microglia in vitro (183–185). Meth potentiates gp120-induced microglial neurotoxic activity in vitro (186) and increases the proportion of infected microglia/macrophages in NHPs (187). BBB in vitro models have shown that DOA impact the integrity of the BBB and promote the transmigration of monocytes across this layer (188), factors that are prevalent with HIV infection and that contribute to neuropathogenesis (189, 190). The adverse effects of several DOA alone have already been investigated with brain organoids, including methadone and cocaine (189, 191). Methadone has been shown to influence neuronal growth and function, including suppressing neural network activity and synaptic transmission (192), while cocaine limits neuronal differentiation and neural tissue development (193). However, the effect of HIV and drug coexposure on the CNS is not fully understood, and brain organoids would provide an efficient model of these processes. Current proposed studies to investigate these processes in brain organoids are now under way, aiming to unravel the impact of DOA on neuron-microglia signaling and microglial inflammatory responses. These novel approaches using organoids overall will likely result in a better understanding of the cell and molecular mechanisms underlying the additive effects of HIV and DOA on the brain.
As organoid models containing microglial and myeloid cells advance to study HIV disease within the CNS, parallel attention needs to be placed on methods of assessing HIV-induced neuropathology. Current qualitative and quantitative assessments of CNS pathology in organoid models evaluate cell subset viability and their infectibility, proinflammatory cytokine fluctuations, and synaptic integrity. In brain organoids, cytotoxicity can be measured by fluorescent dyes (i.e., calcein AM and ethidium homodimer) paired with microscopy or flow cytometry and specific cell subsets using subset-specific markers for neurons (βIII-tubulin and MAP2), astrocytes (GFAP), and microglia (IBA-1) (148, 194). Proinflammatory cytokines (i.e., tumor necrosis factor alpha [TNF-α] and interleukin 1β [IL-1β]) can be measured as surrogate markers of virus-induced neuroinflammation (195). The use of fluorescent or luciferase reporter viruses will help assess cell-specific infection by HIV, while viral antigen (p24) and viral RNA production could be used as readouts for productive HIV infection (196, 197). Furthermore, with the growing ability and sophistication in mapping intracellular neuronal-neuronal, glial-glial, and neuronal-glial electric signaling networks (i.e., optogenetics, multiphoton microscopy with genetic calcium transient reporters, and matrix electrical grids) (198–200), HIV disruption of normal cerebral physiology can be investigated by mapping “normal” electrical signaling in brain organoids with uninfected microglial/macrophages compared to that in individuals infected with HIV.
FUTURE PERSPECTIVES
The research field of neuroHIV has made significant strides in elucidating the mechanism of viral persistence and the onset of CNS dysfunction through 2D in vitro models and animal surrogates; however, these have limitations related to their cost and to not fully recapitulating the complex and dynamic interactions of HIV and CNS resident cells. The development of brain organoid technology has facilitated the study of the neuropathogenicity of neurotropic viruses at different stages of brain maturity. The use of human brain organoids opens research avenues in investigating novel insights into the pathogenesis of HIV-associated cognitive impairment and neurotoxicity, as well as adding exciting findings on HIV-1 latency in the brain. While microglia are still lacking in most brain organoid models, their incorporation has been successful and significant efforts for their further incorporation are ongoing given their relevance in CNS HIV infection. Developing a model that incorporates microglia and/or perivascular macrophages is essential for neuroHIV research, as it would support robust HIV-1 infection, allow the testing of curative strategies, and facilitate the study of HIV-associated neuropathology. However, as the understanding of viral reservoir composition and activity in the CNS is still evolving, including the roles of both astrocytes and microglia, brain organoids could address the true nature of the cellular and frequency of latent HIV.
Recent brain organoid models developed to include a vascularized system addresses the size limitation of organoids but also allow investigation of endothelial dysfunction and BBB integrity. HIV, viral proteins, and inflammatory mediators induce structural and functional damage to the BBB and alter its permeability (201–203). This increased BBB permeability occurs early in acute HIV infection and can persist in PWH up to a year after ART initiation (191, 204–206). While in vitro BBB models have been essential in investigating HIV-associated endothelial dysfunction and BBB disruption, HIV research would benefit from insight into the interplay of neural and endothelial cells and the effects of infection on vascular integrity in a 3D system. Recent models of transplantation of human brain organoids into mouse brains show that the vascularization of brain organoids is an important aspect to facilitate progressive neuronal differentiation and maturation and gliogenesis (207, 208). The incorporation of human E26 transformation-specific variant 2-expressing cells or homologous iPSC-derived human endothelial cells show that organoids become more complex with prolonged culture to form a vascular-like network with functional openings and a BBB-like phenotype that is perfusable and facilitates the diffusion of oxygen (209, 210).
HIV is also thought to exacerbate age-associated cognitive decline. Cognitive deficits are twice as prevalent in PWH over 40 years of age than in their uninfected counterparts, and the risk of developing these deficiencies is disproportionately increased with age, of further importance in the current era, in which the HIV population is drifting progressively toward an older demographic (211–214). Aged human organoids could elucidate this collective effect of aging and HIV on the brain and associated neuropathological mechanisms that may occur. Previous work investigating the role of HIV infection in cellular aging and dysfunction can be easily implemented in a brain organoid model, such as assessing telomere length, toxic protein aggregate formation (i.e., amyloid-β and α-synuclein), and characterization of dysfunctional autophagy or senescence-activated secretory phenotype development (215–218). However, aged brain organoids require long-term maintenance and a stable and growth-arrested state. To overcome these issues, several studies attempted to “speed up the aging process” by obtaining cells from individuals affected with progeroid syndrome for iPSC generation or exposing brain organoids to reactive oxygen species, inflammation, or radiation to mimic stress-associated aging (219–222). Furthermore, as immature brain organoids can reflect the early processes of neurogenesis, the effect of HIV on early neurodevelopment could be evaluated, as vertical transmission persists (223, 224).
Although the use of brain organoid models to study neuroHIV is still in its infancy, rapid advancements highlight their utility in understanding mechanisms of neuropathology and viral persistence as well as testing of curative and neuropathological therapeutic approaches to ameliorate brain injury in PWH. Numerous HIV-focused research laboratories are actively pursuing methodological routes to determine the physiological relevance and predictive capability of brain organoids, and these ongoing efforts are expected to add value to current organoid technology to better explore the complexities of HIV within the CNS.
ACKNOWLEDGMENTS
The figures were created with BioRender.com and Adobe Illustrator v25.2.
This work resulted in part from research supported by National Institute on Drug Abuse (NIDA) award R01-DA052027 to L.C.N., S.T.V., and D.F.N.
Footnotes
Citation Premeaux TA, Mediouni S, Leda A, Furler RL, Valente ST, Fine HA, Nixon DF, Ndhlovu LC. 2021. Next-generation human cerebral organoids as powerful tools to advance neuroHIV research. mBio 12:e00680-21. https://doi.org/10.1128/mBio.00680-21.
Contributor Information
Lishomwa C. Ndhlovu, Email: lndhlovu@med.cornell.edu.
Tricia H. Burdo, Temple University
Vinayaka R. Prasad, Albert Einstein College of Medicine
REFERENCES
- 1.Butler KM, Gavin P, Coughlan S, Rochford A, Mc Donagh S, Cunningham O, Poulsom H, Watters SA, Klein N. 2015. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. Pediatr Infect Dis J 34:e48–e51. doi: 10.1097/INF.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 2.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee T-H, Robles YP, Davis BT, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR. 2014. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 161:319–327. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jambo KC, Banda DH, Kankwatira AM, Sukumar N, Allain TJ, Heyderman RS, Russell DG, Mwandumba HC. 2014. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol 7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Y, Sugimoto C, Arainga M, Midkiff CC, Liu DX, Alvarez X, Lackner AA, Kim W-K, Didier ES, Kuroda MJ. 2015. Preferential destruction of interstitial macrophages over alveolar macrophages as a cause of pulmonary disease in simian immunodeficiency virus-infected rhesus macaques. J Immunol 195:4884–4891. doi: 10.4049/jimmunol.1501194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damouche A, Lazure T, Avettand-Fènoël V, Huot N, Dejucq-Rainsford N, Satie A-P, Mélard A, David L, Gommet C, Ghosn J, Noel N, Pourcher G, Martinez V, Benoist S, Béréziat V, Cosma A, Favier B, Vaslin B, Rouzioux C, Capeau J, Müller-Trutwin M, Dereuddre-Bosquet N, Le Grand R, Lambotte O, Bourgeois C. 2015. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 11:e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mzingwane ML, Tiemessen CT. 2017. Mechanisms of HIV persistence in HIV reservoirs. Rev Med Virol 27:e1924. doi: 10.1002/rmv.1924. [DOI] [PubMed] [Google Scholar]
- 7.Osborne O, Peyravian N, Nair M, Daunert S, Toborek M. 2020. The paradox of HIV blood-brain barrier penetrance and antiretroviral drug delivery deficiencies. Trends Neurosci 43:695–708. doi: 10.1016/j.tins.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell BI, Laws EI, Ndhlovu LC. 2019. Impact of myeloid reservoirs in HIV cure trials. Curr HIV/AIDS Rep 16:129–140. doi: 10.1007/s11904-019-00438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group . 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. 2007. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 11.Gascón MRP, Vidal JE, Mazzaro YM, Smid J, Marcusso RMN, Capitão CG, Coutinho EM, Benute GRG, De Lucia MCS, de Oliveira ACP. 2018. Neuropsychological assessment of 412 HIV-infected individuals in São Paulo, Brazil. AIDS Patient Care STDS 32:1–8. doi: 10.1089/apc.2017.0202. [DOI] [PubMed] [Google Scholar]
- 12.Simioni S, Cavassini M, Annoni J-M, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave J-P, Giacobini E, Hirschel B, Du Pasquier RA. 2010. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 13.Ian E, Gwen CL, Soo CT, Melissa C, Chun-Kai H, Eosu K, Hyo-Youl K, Asad K, Scott L, Chung-Ki LP, Anekthananon T, Jordan TG, Han-Ting W, Wing-Wai W. 2016. The burden of HIV-associated neurocognitive disorder (HAND) in the Asia-Pacific region and recommendations for screening. Asian J Psychiatr 22:182–189. doi: 10.1016/j.ajp.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Debalkie Animut M, Sorrie MB, Birhanu YW, Teshale MY. 2019. High prevalence of neurocognitive disorders observed among adult people living with HIV/AIDS in Southern Ethiopia: a cross-sectional study. PLoS One 14:e0204636. doi: 10.1371/journal.pone.0204636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. 2007. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK, HNRC Group . 2004. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 17.Gisslen M, Price RW, Nilsson S. 2011. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 11:356. doi: 10.1186/1471-2334-11-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Underwood J, De Francesco D, Leech R, Sabin CA, Winston A, Pharmacokinetic and Clinical Observations in PeoPle Over fiftY (POPPY) study . 2018. Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV-associated cognitive impairment. PLoS One 13:e0194760. doi: 10.1371/journal.pone.0194760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spudich S, Gonzalez-Scarano F. 2012. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med 2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, HNRC Group . 2004. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 21.INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM, Molina JM, Munderi P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN, Neaton JD. 2015. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J. RV254/SEARCH 010 Study Group. 2012. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusao I, Shiramizu B, Liang C-Y, Grove J, Agsalda M, Troelstrup D, Velasco V-N, Marshall A, Whitenack N, Shikuma C, Valcour V. 2012. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci 24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Scarano F, Martin-Garcia J. 2005. The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 25.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K. 2004. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology 63:2084–2090. doi: 10.1212/01.WNL.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 26.Cassol E, Misra V, Morgello S, Gabuzda D. 2013. Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J Neuroimmune Pharmacol 8:1087–1097. doi: 10.1007/s11481-013-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thames AD, Briones MS, Magpantay LI, Martinez-Maza O, Singer EJ, Hinkin CH, Morgello S, Gelman BB, Moore DJ, Heizerling K, Levine AJ. 2015. The role of chemokine C-C motif ligand 2 genotype and cerebrospinal fluid chemokine C-C motif ligand 2 in neurocognition among HIV-infected patients. AIDS 29:1483–1491. doi: 10.1097/QAD.0000000000000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gartner S. 2000. HIV infection and dementia. Science 287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 29.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. 2005. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 30.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. 2010. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog 6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallet C, De Rovere M, Van Assche J, Daouad F, De Wit S, Gautier V, Mallon PWG, Marcello A, Van Lint C, Rohr O, Schwartz C. 2019. Microglial cells: the main HIV-1 reservoir in the brain. Front Cell Infect Microbiol 9:362. doi: 10.3389/fcimb.2019.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Harti L, Joseph J, Nath A. 2018. Astrocytes as an HIV CNS reservoir: highlights and reflections of an NIMH-sponsored symposium. J Neurovirol 24:665–669. doi: 10.1007/s13365-018-0691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards MH, Narasipura SD, Kim S, Seaton MS, Lutgen V, Al-Harthi L. 2015. Dynamic interaction between astrocytes and infiltrating PBMCs in context of neuroAIDS. Glia 63:441–451. doi: 10.1002/glia.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abreu C, Shirk EN, Queen SE, Beck SE, Mangus LM, Pate KAM, Mankowski JL, Gama L, Clements JE. 2019. Brain macrophages harbor latent, infectious simian immunodeficiency virus. AIDS 33(Suppl 2):S181–S188. doi: 10.1097/QAD.0000000000002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barat C, Proust A, Deshiere A, Leboeuf M, Drouin J, Tremblay MJ. 2018. Astrocytes sustain long-term productive HIV-1 infection without establishment of reactivable viral latency. Glia 66:1363–1381. doi: 10.1002/glia.23310. [DOI] [PubMed] [Google Scholar]
- 36.Li GH, Maric D, Major EO, Nath A. 2020. Productive HIV infection in astrocytes can be established via a nonclassical mechanism. AIDS 34:963–978. doi: 10.1097/QAD.0000000000002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutgen V, Narasipura SD, Barbian HJ, Richards M, Wallace J, Razmpour R, Buzhdygan T, Ramirez SH, Prevedel L, Eugenin EA, Al-Harthi L. 2020. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog 16:e1008381. doi: 10.1371/journal.ppat.1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell JE. 1998. The neuropathology of adult HIV infection. Rev Neurol (Paris) 154:816–829. [PubMed] [Google Scholar]
- 39.Cosenza MA, Zhao ML, Si Q, Lee SC. 2002. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol 12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kure K, Llena JF, Lyman WD, Soeiro R, Weidenheim KM, Hirano A, Dickson DW. 1991. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol 22:700–710. doi: 10.1016/0046-8177(91)90293-X. [DOI] [PubMed] [Google Scholar]
- 41.Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. 2004. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol 61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- 42.Joseph J. 2018. Optimizing animal models for HIV-associated CNS dysfunction and CNS reservoir research. J Neurovirol 24:137–140. doi: 10.1007/s13365-018-0631-7. [DOI] [PubMed] [Google Scholar]
- 43.Mallard J, Williams KC. 2018. Animal models of HIV-associated disease of the central nervous system. Handb Clin Neurol 152:41–53. doi: 10.1016/B978-0-444-63849-6.00004-9. [DOI] [PubMed] [Google Scholar]
- 44.Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, Engle EL, Forsyth E, Bullock BT, Mac Gabhann F, Wietgrefe SW, Haase AT, Zink MC, Mankowski JL, Clements JE, Gama L. 2017. Brain macrophages in simian immunodeficiency virus-infected, antiretroviral-suppressed macaques: a functional latent reservoir mBio 8:e01186-17. doi: 10.1128/mBio.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gama L, Abreu C, Shirk EN, Queen SE, Beck SE, Metcalf Pate KA, Bullock BT, Zink MC, Mankowski JL, Clements JE. 2018. SIV latency in macrophages in the CNS. Curr Top Microbiol Immunol 417:111–130. doi: 10.1007/82_2018_89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams KC, Hickey WF. 2002. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci 25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- 47.Rawat P, Spector SA. 2017. Development and characterization of a human microglia cell model of HIV-1 infection. J Neurovirol 23:33–46. doi: 10.1007/s13365-016-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Mesa Y, Jay TR, Checkley MA, Luttge B, Dobrowolski C, Valadkhan S, Landreth GE, Karn J, Alvarez-Carbonell D. 2017. Immortalization of primary microglia: a new platform to study HIV regulation in the central nervous system. J Neurovirol 23:47–66. doi: 10.1007/s13365-016-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez-Carbonell D, Ye F, Ramanath N, Garcia-Mesa Y, Knapp PE, Hauser KF, Karn J. 2019. Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog 15:e1008249. doi: 10.1371/journal.ppat.1008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Johansen N, Penn O, Yao Z, Eggermont J, Höllt T, Levi BP, Shehata SI, Aevermann B, Beller A, Bertagnolli D, Brouner K, Casper T, Cobbs C, Dalley R, Dee N, Ding S-L, Ellenbogen RG, Fong O, Garren E, Goldy J, Gwinn RP, Hirschstein D, Keene CD, Keshk M, Ko AL, Lathia K, Mahfouz A, Maltzer Z, McGraw M, Nguyen TN, Nyhus J, Ojemann JG, Oldre A, Parry S, Reynolds S, Rimorin C, Shapovalova NV, Somasundaram S, Szafer A, Thomsen ER, Tieu M, Quon G, Scheuermann RH, Yuste R, Sunkin SM, Lelieveldt B, Feng D, Ng L, Bernard A, Hawrylycz M, Phillips JW, Tasic B, Zeng H, Jones AR, Koch C, Lein ES. 2019. Conserved cell types with divergent features in human versus mouse cortex. Nature 573:61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honeycutt JB, Garcia JV. 2018. Humanized mice: models for evaluating NeuroHIV and cure strategies. J Neurovirol 24:185–191. doi: 10.1007/s13365-017-0567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu C-J, Borjabad A, Hadas E, Kelschenbach J, Kim B-H, Chao W, Arancio O, Suh J, Polsky B, McMillan J, Edagwa B, Gendelman HE, Potash MJ, Volsky DJ. 2018. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog 14:e1007061. doi: 10.1371/journal.ppat.1007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelschenbach J, He H, Kim B-H, Borjabad A, Gu C-J, Chao W, Do M, Sharer LR, Zhang H, Arancio O, Potash MJ, Volsky DJ. 2019. Efficient expression of HIV in immunocompetent mouse brain reveals a novel nonneurotoxic viral function in hippocampal synaptodendritic injury and memory impairment. mBio 10:e00591-19. doi: 10.1128/mBio.00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dello Russo C, Cappoli N, Coletta I, Mezzogori D, Paciello F, Pozzoli G, Navarra P, Battaglia A. 2018. The human microglial HMC3 cell line: where do we stand? A systematic literature review. J Neuroinflammation 15:259. doi: 10.1186/s12974-018-1288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez-Carbonell D, Garcia-Mesa Y, Milne S, Das B, Dobrowolski C, Rojas R, Karn J. 2017. Toll-like receptor 3 activation selectively reverses HIV latency in microglial cells. Retrovirology 14:9. doi: 10.1186/s12977-017-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvarez-Carbonell D, Ye F, Ramanath N, Dobrowolski C, Karn J. 2019. The glucocorticoid receptor is a critical regulator of HIV latency in human microglial cells. J Neuroimmune Pharmacol 14:94–109. doi: 10.1007/s11481-018-9798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rai MA, Hammonds J, Pujato M, Mayhew C, Roskin K, Spearman P. 2020. Comparative analysis of human microglial models for studies of HIV replication and pathogenesis. Retrovirology 17:35. doi: 10.1186/s12977-020-00544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo X, He JJ. 2015. Cell-cell contact viral transfer contributes to HIV infection and persistence in astrocytes. J Neurovirol 21:66–80. doi: 10.1007/s13365-014-0304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chauhan A, Mehla R, Vijayakumar TS, Handy I. 2014. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology 456–457:1–19. doi: 10.1016/j.virol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell RA, Chojnacki J, Jones DM, Johnson E, Do T, Eggeling C, Padilla-Parra S, Sattentau QJ. 2017. Astrocytes resist HIV-1 fusion but engulf infected macrophage material. Cell Rep 18:1473–1483. doi: 10.1016/j.celrep.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narasipura SD, Kim S, Al-Harthi L. 2014. Epigenetic regulation of HIV-1 latency in astrocytes. J Virol 88:3031–3038. doi: 10.1128/JVI.03333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryan SK, Gonzalez MV, Garifallou JP, Bennett FC, Williams KS, Sotuyo NP, Mironets E, Cook K, Hakonarson H, Anderson SA, Jordan-Sciutto KL. 2020. Neuroinflammation and EIF2 signaling persist despite antiretroviral treatment in an hiPSC tri-culture model of HIV infection. Stem Cell Rep 14:703–716. doi: 10.1016/j.stemcr.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qian X, Song H, Ming GL. 2019. Brain organoids: advances, applications and challenges. Development 146:dev166074. doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. 2013. Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Lullo E, Kriegstein AR. 2017. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O’Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Paşca SP. 2017. Assembly of functionally integrated human forebrain spheroids. Nature 545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dutta D, Heo I, Clevers H. 2017. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med 23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Tiscornia G, Vivas EL, Izpisua Belmonte JC. 2011. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat Med 17:1570–1576. doi: 10.1038/nm.2504. [DOI] [PubMed] [Google Scholar]
- 69.Saheli M, Sepantafar M, Pournasr B, Farzaneh Z, Vosough M, Piryaei A, Baharvand H. 2018. Three-dimensional liver-derived extracellular matrix hydrogel promotes liver organoids function. J Cell Biochem 119:4320–4333. doi: 10.1002/jcb.26622. [DOI] [PubMed] [Google Scholar]
- 70.Hoarau-Vechot J, Rafii A, Touboul C, Pasquier J. 2018. Halfway between 2D and animal models: are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int J Mol Sci 19:181. doi: 10.3390/ijms19010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P. 2017. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park J-Y, O’Rourke NA, Nguyen KD, Smith SJ, Huguenard JR, Geschwind DH, Barres BA, Paşca SP. 2015. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon K-J, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho C-Y, Wen Z, Christian KM, Shi P-Y, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming G-L. 2016. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. 2013. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amin ND, Paşca SP. 2018. Building models of brain disorders with three-dimensional organoids. Neuron 100:389–405. doi: 10.1016/j.neuron.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Marton RM, Pașca SP. 2020. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol 30:133–143. doi: 10.1016/j.tcb.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran H-D, Göke J, Tan ZY, Saw TY, Tan C-P, Lokman H, Lee Y, Kim D, Ko HS, Kim S-O, Park JH, Cho N-J, Hyde TM, Kleinman JE, Shin JH, Weinberger DR, Tan EK, Je HS, Ng H-H. 2016. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krefft O, Jabali A, Iefremova V, Koch P, Ladewig J. 2018. Generation of standardized and reproducible forebrain-type cerebral organoids from human induced pluripotent stem cells. J Vis Exp 2018:56768. doi: 10.3791/56768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monzel AS, Smits LM, Hemmer K, Hachi S, Moreno EL, van Wuellen T, Jarazo J, Walter J, Brüggemann I, Boussaad I, Berger E, Fleming RMT, Bolognin S, Schwamborn JC. 2017. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep 8:1144–1154. doi: 10.1016/j.stemcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muguruma K, Nishiyama A, Ono Y, Miyawaki H, Mizuhara E, Hori S, Kakizuka A, Obata K, Yanagawa Y, Hirano T, Sasai Y. 2010. Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat Neurosci 13:1171–1180. doi: 10.1038/nn.2638. [DOI] [PubMed] [Google Scholar]
- 81.Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. 2018. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc 13:565–580. doi: 10.1038/nprot.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiang Y, Tanaka Y, Patterson B, Kang Y-J, Govindaiah G, Roselaar N, Cakir B, Kim K-Y, Lombroso AP, Hwang S-M, Zhong M, Stanley EG, Elefanty AG, Naegele JR, Lee S-H, Weissman SM, Park I-H. 2017. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21:383–398.e7. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. 2015. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 10:537–550. doi: 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 84.Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y. 2015. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 6:8896. doi: 10.1038/ncomms9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JLR, Sasai Y. 2011. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci 31:1919–1933. doi: 10.1523/JNEUROSCI.5128-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Bräuninger M, Lewitus E, Sykes A, Hevers W, Lancaster M, Knoblich JA, Lachmann R, Pääbo S, Huttner WB, Treutlein B. 2015. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 112:15672–15677. doi: 10.1073/pnas.1520760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fatehullah A, Tan SH, Barker N. 2016. Organoids as an in vitro model of human development and disease. Nat Cell Biol 18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 88.Kanton S, Boyle MJ, He Z, Santel M, Weigert A, Sanchís-Calleja F, Guijarro P, Sidow L, Fleck JS, Han D, Qian Z, Heide M, Huttner WB, Khaitovich P, Pääbo S, Treutlein B, Camp JG. 2019. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574:418–422. doi: 10.1038/s41586-019-1654-9. [DOI] [PubMed] [Google Scholar]
- 89.Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD. 2015. 3D cell culture systems: advantages and applications. J Cell Physiol 230:16–26. doi: 10.1002/jcp.24683. [DOI] [PubMed] [Google Scholar]
- 90.Langhans SA. 2018. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol 9:6. doi: 10.3389/fphar.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Costa EC, Moreira AF, de Melo-Diogo D, Gaspar VM, Carvalho MP, Correia IJ. 2016. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol Adv 34:1427–1441. doi: 10.1016/j.biotechadv.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Pontes Soares C, Midlej V, de Oliveira ME, Benchimol M, Costa ML, Mermelstein C. 2012. 2D and 3D-organized cardiac cells shows differences in cellular morphology, adhesion junctions, presence of myofibrils and protein expression. PLoS One 7:e38147. doi: 10.1371/journal.pone.0038147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pamies D, Barreras P, Block K, Makri G, Kumar A, Wiersma D, Smirnova L, Zang C, Bressler J, Christian KM, Harris G, Ming G-L, Berlinicke CJ, Kyro K, Song H, Pardo CA, Hartung T, Hogberg HT. 2017. A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34:362–376. doi: 10.14573/altex.1609122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phan DTT, Wang X, Craver BM, Sobrino A, Zhao D, Chen JC, Lee LYN, George SC, Lee AP, Hughes CCW. 2017. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 17:511–520. doi: 10.1039/c6lc01422d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lancaster MA, Knoblich JA. 2014. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc 9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. 2016. Engineering stem cell organoids. Cell Stem Cell 18:25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phelan MA, Lelkes PI, Swaroop A. 2018. Mini and customized low-cost bioreactors for optimized high-throughput generation of tissue organoids. Stem Cell Invest 5:33. doi: 10.21037/sci.2018.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forsberg SL, Ilieva M, Maria Michel T. 2018. Epigenetics and cerebral organoids: promising directions in autism spectrum disorders. Transl Psychiatry 8:14. doi: 10.1038/s41398-017-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smits LM, Reinhardt L, Reinhardt P, Glatza M, Monzel AS, Stanslowsky N, Rosato-Siri MD, Zanon A, Antony PM, Bellmann J, Nicklas SM, Hemmer K, Qing X, Berger E, Kalmbach N, Ehrlich M, Bolognin S, Hicks AA, Wegner F, Sterneckert JL, Schwamborn JC. 2019. Modeling Parkinson’s disease in midbrain-like organoids. NPJ Parkinsons Dis 5:5. doi: 10.1038/s41531-019-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gerakis Y, Hetz C. 2019. Brain organoids: a next step for humanized Alzheimer’s disease models? Mol Psychiatry 24:474–478. doi: 10.1038/s41380-018-0343-7. [DOI] [PubMed] [Google Scholar]
- 101.Ming GL, Tang H, Song H. 2016. Advances in Zika virus research: stem cell models, challenges, and opportunities. Cell Stem Cell 19:690–702. doi: 10.1016/j.stem.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zimmer B, Ewaleifoh O, Harschnitz O, Lee Y-S, Peneau C, McAlpine JL, Liu B, Tchieu J, Steinbeck JA, Lafaille F, Volpi S, Notarangelo LD, Casanova J-L, Zhang S-Y, Smith GA, Studer L. 2018. Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection. Proc Natl Acad Sci U S A 115:E8775–E8782. doi: 10.1073/pnas.1809853115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng W, Klammer AM, Naciri JN, Yeung J, Demers M, Milosevic J, Kinchington PR, Bloom DC, Nimgaonkar VL, D’Aiuto L. 2020. Patterns of herpes simplex virus 1 infection in neural progenitor cells. J Virol 94:e00994-20. doi: 10.1128/JVI.00994-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenfeld AB, Doobin DJ, Warren AL, Racaniello VR, Vallee RB. 2017. Replication of early and recent Zika virus isolates throughout mouse brain development. Proc Natl Acad Sci U S A 114:12273–12278. doi: 10.1073/pnas.1714624114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cadwell CR, Bhaduri A, Mostajo-Radji MA, Keefe MG, Nowakowski TJ. 2019. Development and arealization of the cerebral cortex. Neuron 103:980–1004. doi: 10.1016/j.neuron.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hastings AK, Yockey LJ, Jagger BW, Hwang J, Uraki R, Gaitsch HF, Parnell LA, Cao B, Mysorekar IU, Rothlin CV, Fikrig E, Diamond MS, Iwasaki A. 2017. TAM receptors are not required for Zika virus infection in mice. Cell Rep 19:558–568. doi: 10.1016/j.celrep.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lancaster MA, Knoblich JA. 2014. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 108.Gabriel E, Ramani A, Karow U, Gottardo M, Natarajan K, Gooi LM, Goranci-Buzhala G, Krut O, Peters F, Nikolic M, Kuivanen S, Korhonen E, Smura T, Vapalahti O, Papantonis A, Schmidt-Chanasit J, Riparbelli M, Callaini G, Krönke M, Utermöhlen O, Gopalakrishnan J. 2017. Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20:397–406.e5. doi: 10.1016/j.stem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. 2016. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19:258–265. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. 2016. Zika virus impairs growth in human neurospheres and brain organoids. Science 352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 111.Qian X, Nguyen HN, Jacob F, Song H, Ming GL. 2017. Using brain organoids to understand Zika virus-induced microcephaly. Development 144:952–957. doi: 10.1242/dev.140707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Flagg EW, Weinstock H. 2011. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics 127:e1–e8. doi: 10.1542/peds.2010-0134. [DOI] [PubMed] [Google Scholar]
- 113.Roberts S. 2009. Herpes simplex virus: incidence of neonatal herpes simplex virus, maternal screening, management during pregnancy, and HIV. Curr Opin Obstet Gynecol 21:124–130. doi: 10.1097/GCO.0b013e3283294840. [DOI] [PubMed] [Google Scholar]
- 114.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. 2003. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 289:203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 115.D’Aiuto L, Bloom DC, Naciri JN, Smith A, Edwards TG, McClain L, Callio JA, Jessup M, Wood J, Chowdari K, Demers M, Abrahamson EE, Ikonomovic MD, Viggiano L, De Zio R, Watkins S, Kinchington PR, Nimgaonkar VL. 2019. Modeling herpes simplex virus 1 infections in human central nervous system neuronal cells using two- and three-dimensional cultures derived from induced pluripotent stem cells. J Virol 93:e00111-19. doi: 10.1128/JVI.00111-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiao H, Guo M, Shang J, Zhao W, Wang Z, Liu N, Li B, Zhou Y, Wu Y, Chen P. 2020. Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLoS Pathog 16:e1008899. doi: 10.1371/journal.ppat.1008899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krenn V, Bosone C, Burkard TR, Spanier J, Kalinke U, Calistri A, Salata C, Rilo Christoff R, Pestana Garcez P, Mirazimi A, Knoblich JA. 9 April 2021. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell doi: 10.1016/j.stem.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheeran MC, Lokensgard JR, Schleiss MR. 2009. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 22:99–126. doi: 10.1128/CMR.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kawasaki H, Kosugi I, Meguro S, Iwashita T. 2017. Pathogenesis of developmental anomalies of the central nervous system induced by congenital cytomegalovirus infection. Pathol Int 67:72–82. doi: 10.1111/pin.12502. [DOI] [PubMed] [Google Scholar]
- 120.Sun G, Chiuppesi F, Chen X, Wang C, Tian E, Nguyen J, Kha M, Trinh D, Zhang H, Marchetto MC, Song H, Ming G-L, Gage FH, Diamond DJ, Wussow F, Shi Y. 2020. Modeling human cytomegalovirus-induced microcephaly in human iPSC-derived brain organoids. Cell Rep Med 1:100002. doi: 10.1016/j.xcrm.2020.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sison SL, O’Brien BS, Johnson AJ, Seminary ER, Terhune SS, Ebert AD. 2019. Human cytomegalovirus disruption of calcium signaling in neural progenitor cells and organoids. J Virol 93:e00954-19. doi: 10.1128/JVI.00954-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown RM, Rana PSJB, Jaeger HK, O’Dowd JM, Balemba OB, Fortunato EA. 2019. Human cytomegalovirus compromises development of cerebral organoids. J Virol 93:e00957-19. doi: 10.1128/JVI.00957-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. 2020. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. 2020. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, Shi C, Hu S. 2020. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res 129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang M, Li T, Qiao F, Wang L, Li C, Gong Y. 2020. Coronavirus disease 2019 associated with aggressive neurological and mental abnormalities confirmed based on cerebrospinal fluid antibodies: a case report. Medicine (Baltimore, MD) 99:e21428. doi: 10.1097/MD.0000000000021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Sabeti P. 2020. Neuropathological features of Covid-19. N Engl J Med 383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. 2020. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, Dottermusch M, Heinemann A, Pfefferle S, Schwabenland M, Sumner Magruder D, Bonn S, Prinz M, Gerloff C, Püschel K, Krasemann S, Aepfelbacher M, Glatzel M. 2020. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel H-H, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. 2021. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 131.Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, Mariappan A, Goureau O, Gruell H, Walker A, Andrée M, Hauka S, Houwaart T, Dilthey A, Wohlgemuth K, Omran H, Klein F, Wieczorek D, Adams O, Timm J, Korth C, Schaal H, Gopalakrishnan J. 2020. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J 39:e106230. doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang B-Z, Chu H, Han S, Shuai H, Deng J, Hu Y-F, Gong H-R, Lee AC-Y, Zou Z, Yau T, Wu W, Hung IF-N, Chan JF-W, Yuen K-Y, Huang J-D. 2020. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res 30:928–931. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S. 2021. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, James LC, Lancaster MA. 2020. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 27:951–961.e5. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jin Y, Ji W, Yang H, Chen S, Zhang W, Duan G. 2020. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther 5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cantó-Nogués C, Sánchez-Ramón S, Álvarez S, Lacruz C, Muñóz-Fernánde MÁ. 2005. HIV-1 infection of neurons might account for progressive HIV-1-associated encephalopathy in children. J Mol Neurosci 27:79–89. doi: 10.1385/JMN:27:1:079. [DOI] [PubMed] [Google Scholar]
- 137.Torres-Munoz J, Stockton P, Tacoronte N, Roberts B, Maronpot RR, Petito CK. 2001. Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol 60:885–892. doi: 10.1093/jnen/60.9.885. [DOI] [PubMed] [Google Scholar]
- 138.Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. 2003. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol 13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wong JK, Yukl SA. 2016. Tissue reservoirs of HIV. Curr Opin HIV AIDS 11:362–370. doi: 10.1097/COH.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, Bakiasi G, Tsai L-H, Aubourg P, Ransohoff RM, Jaenisch R. 2016. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med 22:1358–1367. doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang T, Gong N, Liu J, Kadiu I, Kraft-Terry SD, Schlautman JD, Ciborowski P, Volsky DJ, Gendelman HE. 2008. HIV-1-infected astrocytes and the microglial proteome. J Neuroimmune Pharmacol 3:173–186. doi: 10.1007/s11481-008-9110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. 2001. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol 101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- 143.Bessis A, Bechade C, Bernard D, Roumier A. 2007. Microglial control of neuronal death and synaptic properties. Glia 55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 144.de la Rosa EJ, de Pablo F. 2000. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci 23:454–458. doi: 10.1016/S0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- 145.Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. 2007. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis 26:47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 146.Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen S-EW, Ekdahl CT, Kokaia Z, Lindvall O. 2009. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia 57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 147.Katuri A, Bryant J, Heredia A, Makar TK. 2019. Role of the inflammasomes in HIV-associated neuroinflammation and neurocognitive disorders. Exp Mol Pathol 108:64–72. doi: 10.1016/j.yexmp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 148.Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, Pasterkamp RJ. 2018. Microglia innately develop within cerebral organoids. Nat Commun 9:4167. doi: 10.1038/s41467-018-06684-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Borgmann K, Gendelman HE, Ghorpade A. 2005. Isolation and HIV-1 infection of primary human microglia from fetal and adult tissue. Methods Mol Biol 304:49–70. doi: 10.1385/1-59259-907-9:049. [DOI] [PubMed] [Google Scholar]
- 150.Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. 1996. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol 70:7654–7662. doi: 10.1128/JVI.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dos Reis RS, Sant S, Keeney H, Wagner MCE, Ayyavoo V. 2020. Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Sci Rep 10:15209. doi: 10.1038/s41598-020-72214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martin E, El-Behi M, Fontaine B, Delarasse C. 2017. Analysis of microglia and monocyte-derived macrophages from the central nervous system by flow cytometry. J Vis Exp 2017:55781. doi: 10.3791/55781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brownjohn PW, Smith J, Solanki R, Lohmann E, Houlden H, Hardy J, Dietmann S, Livesey FJ. 2018. Functional studies of missense TREM2 mutations in human stem cell-derived microglia. Stem Cell Rep 10:1294–1307. doi: 10.1016/j.stemcr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin Y-T, Seo J, Gao F, Feldman HM, Wen H-L, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, Rueda R, Kritskiy O, Abdurrob F, Peng Z, Milo B, Yu CJ, Elmsaouri S, Dey D, Ko T, Yankner BA, Tsai L-H. 2018. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98:1141–1154.e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. 2017. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94:278–293.e9. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vay SU, Flitsch LJ, Rabenstein M, Rogall R, Blaschke S, Kleinhaus J, Reinert N, Bach A, Fink GR, Schroeter M, Rueger MA. 2018. The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo. J Neuroinflammation 15:226. doi: 10.1186/s12974-018-1261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Caldeira C, Oliveira AF, Cunha C, Vaz AR, Falcão AS, Fernandes A, Brites D. 2014. Microglia change from a reactive to an age-like phenotype with the time in culture. Front Cell Neurosci 8:152. doi: 10.3389/fncel.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Douvaras P, Sun B, Wang M, Kruglikov I, Lallos G, Zimmer M, Terrenoire C, Zhang B, Gandy S, Schadt E, Freytes DO, Noggle S, Fossati V. 2017. Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Rep 8:1516–1524. doi: 10.1016/j.stemcr.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haenseler W, Sansom SN, Buchrieser J, Newey SE, Moore CS, Nicholls FJ, Chintawar S, Schnell C, Antel JP, Allen ND, Cader MZ, Wade-Martins R, James WS, Cowley SA. 2017. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Rep 8:1727–1742. doi: 10.1016/j.stemcr.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pandya H, Shen MJ, Ichikawa DM, Sedlock AB, Choi Y, Johnson KR, Kim G, Brown MA, Elkahloun AG, Maric D, Sweeney CL, Gossa S, Malech HL, McGavern DB, Park JK. 2017. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat Neurosci 20:753–759. doi: 10.1038/nn.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baxter PS, Dando O, Emelianova K, He X, McKay S, Hardingham GE, Qiu J. 2021. Microglial identity and inflammatory responses are controlled by the combined effects of neurons and astrocytes. Cell Rep 34:108882. doi: 10.1016/j.celrep.2021.108882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim Y, Anderson JL, Lewin SR. 2018. Getting the “kill” into “shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe 23:14–26. doi: 10.1016/j.chom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sadowski I, Hashemi FB. 2019. Strategies to eradicate HIV from infected patients: elimination of latent provirus reservoirs. Cell Mol Life Sci 76:3583–3600. doi: 10.1007/s00018-019-03156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Spivak AM, Planelles V. 2018. Novel latency reversal agents for HIV-1 cure. Annu Rev Med 69:421–436. doi: 10.1146/annurev-med-052716-031710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haran KP, Hajduczki A, Pampusch MS, Mwakalundwa G, Vargas-Inchaustegui DA, Rakasz EG, Connick E, Berger EA, Skinner PJ. 2018. Simian immunodeficiency virus (SIV)-specific chimeric antigen receptor-T cells engineered to target B cell follicles and suppress SIV replication. Front Immunol 9:492. doi: 10.3389/fimmu.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Siliciano JD, Lai J, Callender M, Pitt E, Zhang H, Margolick JB, Gallant JE, Cofrancesco J, Moore RD, Gange SJ, Siliciano RF. 2007. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis 195:833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 167.Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, Lichterfeld M, Margolis DM, Planelles V, Bosque A. 2013. Reactivation of latent HIV-1 in central memory CD4(+) T cells through TLR-1/2 stimulation. Retrovirology 10:119. doi: 10.1186/1742-4690-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kessing CF, Nixon CC, Li C, Tsai P, Takata H, Mousseau G, Ho PT, Honeycutt JB, Fallahi M, Trautmann L, Garcia JV, Valente ST. 2017. In vivo suppression of HIV rebound by didehydro-cortistatin A, a “block-and-lock” strategy for HIV-1 treatment. Cell Rep 21:600–611. doi: 10.1016/j.celrep.2017.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mediouni S, Chinthalapudi K, Ekka MK, Usui I, Jablonski JA, Clementz MA, Mousseau G, Nowak J, Macherla VR, Beverage JN, Esquenazi E, Baran P, de Vera IMS, Kojetin D, Loret EP, Nettles K, Maiti S, Izard T, Valente ST. 2019. Didehydro-cortistatin A inhibits HIV-1 by specifically binding to the unstructured basic region of Tat. mBio 10:e02662-18. doi: 10.1128/mBio.02662-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mediouni S, Jablonski J, Paris JJ, Clementz MA, Thenin-Houssier S, McLaughlin JP, Valente ST. 2015. Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res 13:64–79. doi: 10.2174/1570162x13666150121111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mousseau G, Aneja R, Clementz MA, Mediouni S, Lima NS, Haregot A, Kessing CF, Jablonski JA, Thenin-Houssier S, Nagarsheth N, Trautmann L, Valente ST. 2019. Resistance to the Tat inhibitor didehydro-cortistatin A is mediated by heightened basal HIV-1 transcription. mBio 10:e01750-18. doi: 10.1128/mBio.01750-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang Z, Pan Q, Gendron P, Zhu W, Guo F, Cen S, Wainberg MA, Liang C. 2016. CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep 15:481–489. doi: 10.1016/j.celrep.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 173.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, Schmidt SD, Mankoff Z, Wu L, Asokan M, Beil C, Lange C, Leuschner WD, Kruip J, Sendak R, Kwon YD, Zhou T, Chen X, Bailer RT, Wang K, Choe M, Tartaglia LJ, Barouch DH, O’Dell S, Todd J-P, Burton DR, Roederer M, Connors M, Koup RA, Kwong PD, Yang Z-Y, Mascola JR, Nabel GJ. 2017. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Günthard HF. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 175.Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, McLaughlin B, Landay AL, Adeyemi O, Gilman LE, Clagett B, Rodriguez B, Martin JN, Schacker TW, Shacklett BL, Palmer S, Lederman MM, Deeks SG. 2013. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 121:4635–4646. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rubin LH, Sacktor N, Creighton J, Du Y, Endres CJ, Pomper MG, Coughlin JM. 2018. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 32:1661–1667. doi: 10.1097/QAD.0000000000001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Anderson AM, Easley KA, Kasher N, Franklin D, Heaton RK, Zetterberg H, Blennow K, Gisslen M, Letendre SL. 2018. Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J Neurovirol 24:695–701. doi: 10.1007/s13365-018-0664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gisslén M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H. 2016. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 3:135–140. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.King JE, Eugenin EA, Buckner CM, Berman JW. 2006. HIV tat and neurotoxicity. Microbes Infect 8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 180.Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M. 2010. Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol 185:4883–4895. doi: 10.4049/jimmunol.0902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chang SL, Connaghan KP, Wei Y, Li MD. 2014. NeuroHIV and use of addictive substances. Int Rev Neurobiol 118:403–440. doi: 10.1016/B978-0-12-801284-0.00013-0. [DOI] [PubMed] [Google Scholar]
- 182.Hauser KF, Knapp PE. 2014. Interactions of HIV and drugs of abuse: the importance of glia, neural progenitors, and host genetic factors. Int Rev Neurobiol 118:231–313. doi: 10.1016/B978-0-12-801284-0.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dave RS, Khalili K. 2010. Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem 110:834–845. doi: 10.1002/jcb.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reddy PV, Pilakka-Kanthikeel S, Saxena SK, Saiyed Z, Nair MP. 2012. Interactive effects of morphine on HIV infection: role in HIV-associated neurocognitive disorder. AIDS Res Treat 2012:953678. doi: 10.1155/2012/953678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. 2002. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Invest Med 50:435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu J, Xu E, Tu G, Liu H, Luo J, Xiong H. 2017. Methamphetamine potentiates HIV-1gp120-induced microglial neurotoxic activity by enhancing microglial outward K(+) current. Mol Cell Neurosci 82:167–175. doi: 10.1016/j.mcn.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Niu M, Morsey B, Lamberty BG, Emanuel K, Yu F, León-Rivera R, Berman JW, Gaskill PJ, Matt SM, Ciborowski PS, Fox HS. 2020. Methamphetamine increases the proportion of SIV-infected microglia/macrophages, alters metabolic pathways, and elevates cell death pathways: a single-cell analysis. Viruses 12:1297. doi: 10.3390/v12111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sajja RK, Rahman S, Cucullo L. 2016. Drugs of abuse and blood-brain barrier endothelial dysfunction: a focus on the role of oxidative stress. J Cereb Blood Flow Metab 36:539–554. doi: 10.1177/0271678X15616978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Williams DW, Eugenin EA, Calderon TM, Berman JW. 2012. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 91:401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Toborek M, Lee YW, Flora G, Pu H, András IE, Wylegala E, Hennig B, Nath A. 2005. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cell Mol Neurobiol 25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rahimy E, Li F-Y, Hagberg L, Fuchs D, Robertson K, Meyerhoff DJ, Zetterberg H, Price RW, Gisslén M, Spudich S. 2017. Blood-brain barrier disruption is initiated during primary HIV infection and not rapidly altered by antiretroviral therapy. J Infect Dis 215:1132–1140. doi: 10.1093/infdis/jix013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yao H, Wu W, Cerf I, Zhao HW, Wang J, Negraes PD, Muotri AR, Haddad GG. 2020. Methadone interrupts neural growth and function in human cortical organoids. Stem Cell Res 49:102065. doi: 10.1016/j.scr.2020.102065. [DOI] [PubMed] [Google Scholar]
- 193.Lee CT, Chen J, Kindberg AA, Bendriem RM, Spivak CE, Williams MP. 2017. CYP3A5 mediates effects of cocaine on human neocorticogenesis: studies using an in vitro 3D self-organized hPSC model with a single cortex-like unit. Neuropsychopharmacology 42:774–784. doi: 10.1038/npp.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, Reich B, Cohen-Gould L, Storaska A, Nakayama Y, Schenkein E, Singhania R, Cirigliano S, Magdeldin T, Lin Y, Nanjangud G, Chadalavada K, Pisapia D, Liston C, Fine HA. 2019. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep 26:3203–3211.e5. doi: 10.1016/j.celrep.2019.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tatro ET, Soontornniyomkij B, Letendre SL, Achim CL. 2014. Cytokine secretion from brain macrophages infected with human immunodeficiency virus in vitro and treated with raltegravir. BMC Infect Dis 14:386. doi: 10.1186/1471-2334-14-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Campbell LA, Richie CT, Zhang Y, Heathward EJ, Coke LM, Park EY, Harvey BK. 2017. In vitro modeling of HIV proviral activity in microglia. FEBS J 284:4096–4114. doi: 10.1111/febs.14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Terahara K, Yamamoto T, Mitsuki Y-Y, Shibusawa K, Ishige M, Mizukoshi F, Kobayashi K, Tsunetsugu-Yokota Y. 2011. Fluorescent reporter signals, EGFP, and DsRed, encoded in HIV-1 facilitate the detection of productively infected cells and cell-associated viral replication levels. Front Microbiol 2:280. doi: 10.3389/fmicb.2011.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shiri Z, Simorgh S, Naderi S, Baharvand H. 2019. Optogenetics in the era of cerebral organoids. Trends Biotechnol 37:1282–1294. doi: 10.1016/j.tibtech.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 199.Sakaguchi H, Ozaki Y, Ashida T, Matsubara T, Oishi N, Kihara S, Takahashi J. 2019. Self-organized synchronous calcium transients in a cultured human neural network derived from cerebral organoids. Stem Cell Rep 13:458–473. doi: 10.1016/j.stemcr.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, Domissy A, Vandenberghe M, Devor A, Yeo GW, Voytek B, Muotri AR. 2019. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25:558–569.e7. doi: 10.1016/j.stem.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mukhtar M, Harley S, Chen P, BouHamdan M, Patel C, Acheampong E, Pomerantz RJ. 2002. Primary isolated human brain microvascular endothelial cells express diverse HIV/SIV-associated chemokine coreceptors and DC-SIGN and L-SIGN. Virology 297:78–88. doi: 10.1006/viro.2002.1376. [DOI] [PubMed] [Google Scholar]
- 202.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. 2006. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lorin V, Danckaert A, Porrot F, Schwartz O, Afonso PV, Mouquet H. 2020. Antibody neutralization of HIV-1 crossing the blood-brain barrier. mBio 11:e02424-20. doi: 10.1128/mBio.02424-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McRae M. 2016. HIV and viral protein effects on the blood brain barrier. Tissue Barriers 4:e1143543. doi: 10.1080/21688370.2016.1143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Andersson LM, Hagberg L, Fuchs D, Svennerholm B, Gisslen M. 2001. Increased blood-brain barrier permeability in neuro-asymptomatic HIV-1-infected individuals—correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J Neurovirol 7:542–547. doi: 10.1080/135502801753248123. [DOI] [PubMed] [Google Scholar]
- 206.Leibrand CR, Paris JJ, Ghandour MS, Knapp PE, Kim W-K, Hauser KF, McRae M. 2017. HIV-1 Tat disrupts blood-brain barrier integrity and increases phagocytic perivascular macrophages and microglia in the dorsal striatum of transgenic mice. Neurosci Lett 640:136–143. doi: 10.1016/j.neulet.2016.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge W-P, Wu Q, Wang X. 2020. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol 18:e3000705. doi: 10.1371/journal.pbio.3000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. 2018. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36:432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang Y-J, Chapeton K, Patterson B, Yuan Y, He C-S, Raredon MSB, Dengelegi J, Kim K-Y, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee S-H, Yoon Y-S, Park I-H. 2019. Engineering of human brain organoids with a functional vascular-like system. Nat Methods 16:1169–1175. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B. 2018. Generation of human vascularized brain organoids. Neuroreport 29:588–593. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Coban H, Robertson K, Smurzynski M, Krishnan S, Wu K, Bosch RJ, Collier AC, Ellis RJ. 2017. Impact of aging on neurocognitive performance in previously antiretroviral-naive HIV-infected individuals on their first suppressive regimen. AIDS 31:1565–1571. doi: 10.1097/QAD.0000000000001523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. 2004. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 18(Suppl 1):S11–S18. [PubMed] [Google Scholar]
- 213.Mahy M, Autenrieth CS, Stanecki K, Wynd S. 2014. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS 28(Suppl 4):S453–S459. doi: 10.1097/QAD.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cohen RA, Seider TR, Navia B. 2015. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther 7:37. doi: 10.1186/s13195-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sanford SL, Welfer GA, Freudenthal BD, Opresko PL. 2020. Mechanisms of telomerase inhibition by oxidized and therapeutic dNTPs. Nat Commun 11:5288. doi: 10.1038/s41467-020-19115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Torres RA, Lewis W. 2014. Aging and HIV/AIDS: pathogenetic role of therapeutic side effects. Lab Invest 94:120–128. doi: 10.1038/labinvest.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chai Q, Jovasevic V, Malikov V, Sabo Y, Morham S, Walsh D, Naghavi MH. 2017. HIV-1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nat Commun 8:1522. doi: 10.1038/s41467-017-01795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hategan A, Bianchet MA, Steiner J, Karnaukhova E, Masliah E, Fields A, Lee M-H, Dickens AM, Haughey N, Dimitriadis EK, Nath A. 2017. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol 24:379–386. doi: 10.1038/nsmb.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cornacchia D, Studer L. 2017. Back and forth in time: directing age in iPSC-derived lineages. Brain Res 1656:14–26. doi: 10.1016/j.brainres.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nasto LA, Robinson AR, Ngo K, Clauson CL, Dong Q, St Croix C, Sowa G, Pola E, Robbins PD, Kang J, Niedernhofer LJ, Wipf P, Vo NV. 2013. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res 31:1150–1157. doi: 10.1002/jor.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim J-w, Kriks S, Taldone T, Fusaki N, Tomishima MJ, Krainc D, Milner TA, Rossi DJ, Studer L. 2013. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13:691–705. doi: 10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu G-H, Barkho BZ, Ruiz S, Diep D, Qu J, Yang S-L, Panopoulos AD, Suzuki K, Kurian L, Walsh C, Thompson J, Boue S, Fung HL, Sancho-Martinez I, Zhang K, Yates J, Izpisua Belmonte JC. 2011. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature 472:221–225. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Camacho-Gonzalez AF, Kingbo MH, Boylan A, Eckard AR, Chahroudi A, Chakraborty R. 2015. Missed opportunities for prevention of mother-to-child transmission in the United States. AIDS 29:1511–1515. doi: 10.1097/QAD.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.American Academy of Pediatrics Committee on Pediatric AIDS. 2008. HIV testing and prophylaxis to prevent mother-to-child transmission in the United States. Pediatrics 122:1127–1134. doi: 10.1542/peds.2008-2175. [DOI] [PubMed] [Google Scholar]