ABSTRACT
The recent emergence of multiple variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a significant concern for public health worldwide. New variants have been classified either as variants of concern (VOCs) or variants of interest (VOIs) by the CDC (USA) and WHO. The VOCs include lineages such as B.1.1.7 (20I/501Y.V1 variant), P.1 (20J/501Y.V3 variant), B.1.351 (20H/501Y.V2 variant), and B.1.617.2. In contrast, the VOI category includes B.1.525, B.1.526, P.2, and B.1.427/B.1.429. The WHO provided the alert for last two variants (P.2 and B.1.427/B.1.429) and labeled them for further monitoring. As per the WHO, these variants can be reclassified due to their status at a particular time. At the same time, the CDC (USA) has marked these two variants as VOIs up through today. This article analyzes the evolutionary patterns of all these emerging variants, as well as their geographical distributions and transmission patterns, including the circulating frequency, entropy diversity, and mutational event diversity throughout the genomes of all SARS-CoV-2 lineages. The transmission pattern was observed highest in the B.1.1.7 lineage. Our frequency evaluation found that this lineage achieved 100% frequency in early October 2020. We also critically evaluated the above emerging variants mutational landscape and significant spike protein mutations (E484K, K417T/N, N501Y, and D614G) impacting public health. Finally, the effectiveness of vaccines against newly SARS-CoV-2 variants was also analyzed.
KEYWORDS: emerging variants, transmission pattern, mutational landscape, effect on vaccines
INTRODUCTION
The calamitous effects of COVID-19 are having a significant impact on existing health care systems. The pandemic has forced the world toward an economic recession. Even academic institutions have been shut down to contain the pandemic, while there has been an immense impact on communities. The industrial and the associated development sectors have plunged drastically, affecting agriculture, petroleum, manufacturing, education, information technology, research, and societal development (1). Most of the affected countries have failed to stop the pandemic. As a result, the year 2020 was the most challenging year for the world. Therefore, governments worldwide have sourced a safe and effective COVID-19 vaccine program to stop the pandemic, leading to a vaccine development race (2, 3). Global efforts by scientists led to the timely development of the COVID-19 vaccine. This was partly due to the significant efforts given by vaccine researchers as they worked around the clock to create the vaccine in 2020. At the same time, scientists from academia, pharmaceutical companies, biotechnology companies, and military organizations joined hands in developing vaccines (4), and the vaccine candidates were put into clinical trials within a span of just 6 months. The first conditional approval of the COVID-19 vaccine was given within 10 months of the COVID pandemic (5). In December 2020, the two mRNA-based COVID-19 vaccines received emergency approval, with remarkable efficacy (94 to 95%) (6, 7).
By the middle of 2020, researchers reported mutations of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome. Simultaneously several new variants came into existence and were identified through genomic data analysis. Koyama et al. reported several variants of SARS-CoV-2 in July 2020 from 10,022 genomes, obtained from four different databases. The genomes were collected and sequenced from patients from 68 countries. The study found 5,775 distinct genome variants, which included 2,969 missense mutations (8). This study affirmed that the virus was acquiring several mutations in its genome. Surprisingly, in December 2020, researchers reported a sudden rise in COVID-19 cases associated with some significant mutations, and a few of these crucial mutations were observed in the spike protein (S-protein) regions. These mutations have created several variants of SARS-CoV-2 that are more infectious among all the diversified variants (9). Reports also showed that some crucial mutations, such as D614G in some variants, might be responsible for enhanced infectivity (9, 10). Afterwards, the CDC (USA) and WHO, depending on the severity of the variants, categorized the newly emerging significant variants either as variants of concern (VOCs), variants of high consequences, or variants of interest (VOIs) (11). Three considerable VOCs that were recorded from three different regions of the world are the B.1.1.7 lineage (20I/501Y.V1 variant) from the United Kingdom, the P.1 lineage (20J/501Y.V3 variant) from Brazil, and the B.1.351 lineage (20H/501Y.V2 variant) from South Africa (12, 13). Another VOI was identified in the USA (California) and was designated B.1.427/B.1.429 (a further monitoring tag has been provided by the WHO). Consequently, a new VOC has been identified from India, which is B.1.617.2 (Delta), and this variant is now spreading worldwide. Presently, the scientific community is trying to focus on analyzing this variant and its symptomatic consequences.
Additionally, three variants are regarded as VOIs and have been identified in different parts of the world. These variants are B.1.525 (New York, USA), B.1.526 (New York, USA), and P.2 (Brazil) (Table 1). A further monitoring tag has been provided to the P.2 variant by the WHO.
TABLE 1.
Significant SARS-CoV-2 lineages, associated variants, and their countries of origin
| Serial no. | New significant SARS-CoV-2 lineage | Variant name | Variant as labeled by WHO | Country of origin | Note(s) |
|---|---|---|---|---|---|
| 1 | B.1.1.7 | 20I/501Y.V1 | Alpha | United Kingdom | Emerging variant, CDC and WHO reported as VOC |
| 2 | P.1 | 20J/501Y.V3 | Gamma | Brazil | Emerging variant, CDC and WHO declared as VOC |
| 3 | B.1.351 | 20H/501Y.V2 | Beta | South Africa | Emerging variant, CDC and WHO stated as VOC |
| 4 | B.1.427/B.1.429 | Nextstrain described as 20C/S:452R | Epsilon | USA (California) | Emerging variant, CDC and WHO reported as VOI |
| 5 | B.1.617.2 | Nextstrain described as 20A/S:478K | Delta | India | Emerging variant, CDC and WHO declared as VOC |
| 6 | B.1.525 | Nextstrain described as 20C | Eta | USA (New York) | CDC and WHO stated as VOI |
| 7 | B.1.526 | Nextstrain described as20C | Iota | USA (New York) | CDC and WHO called as VOI |
| 8 | P.2 | 20J | Zeta | Brazil | CDC and WHO stated as VOI |
Some crucial apprehensions have arisen regarding the variants’ infectivity, which is alarming for public health. On the other hand, another critical issue is the efficacy of the approved COVID-19 vaccines against the new variants. Some studies show that mutations in the variants may create a more infective virus. So, the question is: will such variants make a second wave or another wave of COVID-19 for different countries, creating even more new infections and triggering a more considerable death toll? Though vaccines have just been rolled out, studies have shown that variants are resistant to neutralizing antibodies, which is of considerable concern for the development of vaccines (14, 15). Furthermore, this year (2021) might be crucial due to the emergence of significant variants affecting public health and producing mutated viral antigens. These mutated antigens may affect the vaccine-generated antibodies and thus protection.
Here, we have analyzed the critical questions about the SARS-CoV-2 variants in three directions, or sections. A summary of the study methodology is provided as a flowchart in Fig. 1. In the first section, we studied the evolutionary patterns of the newly emerging variants, their geographical distributions and transmission patterns, circulating frequencies, entropy diversity, and mutational event diversity throughout the genomes of all circulating lineages of this virus and significant lineages, such as B.1.1.7, P.1, B.1.351, B.1.427/B.1.429, B.1.617.2, B.1.525, B.1.526, and P.2. In the second section, we critically evaluated the mutational landscape of all of the above emerging variants and significant spike protein mutations. We also assessed the significant mutations (E484K, K417T/N, N501Y, and D614G) in emerging variants that may concern public health. Finally, in the third section, we analyzed the efficacy of vaccines against the new SARS-CoV-2 variants. Our analysis will help design the future direction for pandemic control, next-generation vaccine development using alternative viral antigens, and planning for an effective vaccination program.
FIG 1.
Flowchart showing the methodology of our study.
RESULTS
Phylogenetic tree of all circulating lineages, significant VOCs [lineages B.1.1.7 (Alpha), P.1 (Gamma), B.1.351 (Beta), and B.1.617.2 (Delta)] and significant VOIs [lineages B.1.525 (Eta), B.1.526 (Iota), P.2 (Zeta), and B.1.427/B.1.429 (Epsilon)].
We have developed a phylogenetic tree for all the circulating SARS-CoV-2 lineages between December 2019 and June 2021, and for this, 3,905 genomes were sampled (Fig. 2). We have analyzed molecular phylogenetics and developed a tree for lineage B.1.1.7. We found 1,182 genome samples for lineage B.1.1.7 (20I/501Y.V1) between December 2020 and June 2021 from a total of 3,905 genomes (Fig. 3A). The data from the development of the phylogenetic tree depict several significant mutations acquired by the lineage B.1.1.7 since December 2020.
FIG 2.
Phylogenetic tree of all circulating lineages of newly SARS-CoV-2 variants between December 2019 and June 2021. The phylogenetic tree of all circulating lineages was developed before 27 June 2021 through the Nextstrain server, using GISAID data.
FIG 3.
Phylogenetic trees of significant VOCs between December 2019 and June 2021. (A) Phylogenetic tree highlighting lineage B.1.1.7 (Alpha) and showing the relationship with other circulating lineages. (B) Phylogenetic tree highlighting lineage P.1 (Gamma) and showing the relationship with different circulating lineages. (C) Phylogenetic tree highlighting lineage B.1.351 (Beta) and showing the relationship with other circulating lineages. (D) Phylogenetic tree showing lineage B.1.617.2 (Delta) and showing the relationship with different circulating lineages. The phylogenetic trees of significant VOCs were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
Similarly, we have developed a phylogenetic tree of lineage P.1 and found 186 genome samples of lineage P.1 (20J/501Y.V3 variant) between December 2020 and June 2021 from the total of 3,905 genomes (Fig. 3B). The phylogenetic tree reflects that some significant mutations had been acquired by lineage P.1 (20J/501Y.V3 variant) since the middle of December 2020.
Furthermore, we developed a phylogenetic tree of lineage B.1.351 and found 231 genome samples for lineage B.1.351 (20H/501Y.V2) between December 2020 and June 2021 from the total of 3,905 genomes (Fig. 3C). Both the phylogenetic tree and the timeline indicate that lineage B.1.351 has acquired some significant mutations since September 2020.
Another phylogenetic tree of lineage B.1.617.2 (Delta) found 201 genome samples of lineage B.1.617.2 between December 2020 and June 2021 from 3,905 genomes (Fig. 3D). The lineage B.1.617.2 was first identified in India in late 2020. The phylogenetic tree indicates that a small number of significant mutations were acquired by lineage B.1.617.2 since the middle of March 2021.
In addition, we have developed a phylogenetic tree of lineage B.1.525 and found 45 genome samples of the B.1.525 lineage between December 2020 and June 2021 from the total of 3,905 genomes (Fig. 4A). The B.1.425 lineage was found in the USA. The phylogenetic tree indicates that a few mutations have been acquired by lineage B.1.525 since February 2021. It was observed that all the samples of this lineage have formed a cluster since February 2021.
FIG 4.
Phylogenetic trees of significant VOIs between December 2019 and June 2021. (A) Phylogenetic tree highlighting lineage B.1.525 (Eta) and showing the relationship with other circulating lineages. (B) Phylogenetic tree highlighting lineage B.1.526 (Iota) and showing the relationship with different circulating lineages. (C) Phylogenetic tree highlighting lineage P.2 (Zeta) and showing the relationship with other circulating lineages. (D) Phylogenetic tree highlighting lineage B.1.427/B.1.429 (Epsilon) and showing the relationship with other circulating lineages. The phylogenetic trees of significant VOIs were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
The phylogenetic tree of lineage B.1.526 found 41 genome samples of lineage B.1.526 between December 2020 and June 2021 from the 3,905 genomes (Fig. 4B). Lineage B.1.426 was also found in the USA. The phylogenetic tree shows that many mutations have been gained by lineage B.1.526 since the middle of February 2021.
Likewise, we depict the phylogenetic tree of lineage P.2, and 41 genome samples of the P.2 lineage between December 2020 and June 2021 from the 3,905 genomes were observed (Fig. 4C). It appears from the phylogenetic tree that some important mutations have been obtained by the P.2 lineage since the middle of December 2020.
Next, we also created a phylogenetic tree of lineage B.1.427/B.1.429 and found 35 genome samples for lineage B.1.427/B.1.429 (Epsilon) between December 2020 and June 2021 from 3,905 genomes (Fig. 4D). This lineage was found in the USA. The phylogenetic tree indicates there have been few significant mutations acquired by this lineage since February 2021.
Scatterplot of cluster analysis of all circulating lineages, significant VOCs [lineages B.1.1.7 (Alpha), P.1 (Gamma), B.1.351 (Beta), and B.1.617.2 (Delta)], and significant VOIs [lineages B.1.525 (Eta), B.1.526 (Iota), P.2 (Zeta), and B.1.427/B.1.429 (Epsilon)].
We performed a cluster analysis and developed scatterplots to show the clustering of the genome samples and the divergence in mutations along the regression line. Scatterplots with a linear regression line using 3,905 genomes of all circulating lineages were developed (Fig. 5). Our linear regression model shows that the sample values are distributed just alongside the regression line. The scatters in the plot describe the overall pattern of all circulating lineages through the plotted points. The pattern of the linear regression model demonstrates that this regression model follows a dense distribution pattern of samples on both sides of the line. The model also portrays that the genome samples on the upper side are more viscous than the lower ones. This regression model shows a heteroscedasticity pattern. We next developed scatterplots using 1,182 genomes of the B.1.1.7 lineage (Fig. 6A). The B.1.1.7 lineage’s scatterplot describes the B.1.17 lineage pattern through the plotted points. In the regression model of the B.1.1.7 lineage, the sample points are densely plotted on the upper side of the top portion of the regression line. Simultaneously, we also developed scatterplots using 186 genomes of the P.1 lineage (Fig. 6B). The regression model describes the pattern of the P.1 lineage through the plotted points. The scatterplot pattern of the P.1 lineage shows that the samples form a cluster on the upper side of the top portion of the regression line. This regression model is quite similar to the pattern of the B.1.1.7 lineage regression model. Next, we developed scatterplots using 231 genomes of the B.1.351 lineage (Fig. 6C). The scatterplot of the B.1.351 lineage illustrates the pattern through the plotted points. The scatterplot pattern represents that the samples form a cluster mainly on the lower side of the top portion of the regression line. This regression model shows that the data points of this cluster are dense in the upper portion. From this model, it can be inferred that most of the mutations were accumulated from March 2021 to June 2021. Likewise, a scatterplot using 201 genomes of the B.1.617.2 lineage was also plotted (Fig. 6D). The scatterplot trend demonstrated that the samples form a cluster on both the sides of the top portion of the regression line. From this regression model, it can be inferred that the Delta variant accumulated maximum mutations from April/May 2021 to June 2021.
FIG 5.
Scatterplot showing the genome diversity cluster of all circulating lineages between December 2019 and June 2021. The scatterplot of all circulating lineages was developed before 27 June 2021 through the Nextstrain server, using GISAID data.
FIG 6.
Scatterplots showing the genome diversity clusters of significant VOCs between December 2019 and June 2021. (A) Scatterplot showing the genome samples of lineage B.1.1.7 (Alpha) with a linear regression line. (B) Scatterplot showing the genome samples of lineage P.1 (Gamma) with a linear regression line. (C) Scatterplot showing the genome samples of lineage B.1.351 (Beta) with a linear regression line (D) Scatterplot showing the genome samples of lineage B.1.617.2 (Delta) with a linear regression line. The scatterplots of significant VOCs were developed before 27 June 2021 through the Nextstrain server, which is using GISAID data.
A scatterplot using 45 genomes of the B.1.525 lineage (Fig. 7A) was plotted, and it confirmed that the B.1.425 lineage forms a cluster on the lower side of the regression line's top portion. This regression model informed us that the variant accumulated maximum mutations from February/March 2021 to June 2021. We next developed scatterplots using 41 genomes of the B.1.526 lineage (Fig. 7B). It demonstrated that the B.1.526 lineage forms a cluster mostly at the lower side of the top portion of the regression line. This regression model follows the same pattern as the B.1.425 lineage and shows that the variant accumulated maximum mutations from March 2021 to June 2021. Next, we developed a scatterplot using 41 genomes of the P.2 lineage (Fig. 7C). The scatterplot trend established that the P.2 lineage forms a cluster mostly at the lower side of the top portion of the regression line. According to this regression model, the P.2 lineage accumulated more mutations from December 2020 to May 2021 and maximum mutations during March 2021. Next a scatterplot using 35 genomes of the B.1.427/B.1.429 lineage was plotted (Fig. 7D). The scatterplot trend demonstrated that the samples form a cluster on the lower side of the top portion of the regression line. This regression model shows the same pattern as the previous regression model of the B.1.351 lineage. This model also informs us that the variant accumulated more mutations from March 2021 to June 2021. From the scatterplot and the regression model, we can observe that all VOIs show the same type of scattered plot.
FIG 7.
Scatterplots showing the genome diversity cluster of significant VOIs between December 2019 and June 2021. (A) Scatterplot showing the genome samples of the lineage B.1.525 (Eta) with a linear regression line. (B) Scatterplot showing the genome samples of the lineage B.1.526 (Iota) with a linear regression line. (C) Scatterplot showing the genome samples of the lineage P.2 (Zeta) with a linear regression line. (D) Scatterplot showing the genome samples of the lineage B.1.427/B.1.429 (Epsilon) with a linear regression line. The scatterplots of significant VOIs were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
Geographical distributions and transmission patterns from origin to other countries of all circulating lineages, significant VOCs [lineages B.1.1.7 (Alpha), P.1 (Gamma), B.1.351 (Beta), and B.1.617.2 (Delta)], and significant VOIs [lineages B.1.525 (Eta), B.1.526 (Iota), P.2 (Zeta), and B.1.427/B.1.429 (Epsilon)].
We have mapped the geographical distributions and transmission patterns of all circulating lineages at one time (Fig. 8). Genomic diversity is prominent in different regions of the world. We analyzed the geographical distribution and transmission of B.1.1.7 lineage and depict them in Fig. 9A. This lineage originated from the United Kingdom. It is very clear from the transmission pattern that the lineage got transmitted throughout Europe, the USA, and Canada, including parts of Latin America and South Africa. The geographical distribution and transmission of the P.1 lineage are recorded in Fig. 9B. The lineage originated in Brazil and got transmitted to different parts of South America. The lineage was also reported in France. The geographical distribution and transmission of B.1.351 lineage are shown in Fig. 9C. The lineage originated in South Africa and then got transmitted to different parts of Europe, Asia (India, Sri Lanka, and Malaysia), and Australia.
FIG 8.
Geographical distribution and transmission pattern from the source to other countries of all circulating lineages between December 2019 and June 2021. The geographical distribution and transmission pattern of all circulating lineages were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
FIG 9.
Geographical distributions and transmission patterns from the source to other countries of significant VOCs between December 2019 and June 2021. (A) Geographical distribution and transmission pattern of the B.1.1.7 (Alpha) lineage. (B) Geographical distribution and transmission pattern of the P.1 (Gamma) lineage. (C) Geographical distribution and transmission pattern of the B.1.351 (Beta) lineage. (D) Geographical distribution and transmission pattern of the B.1.617.2 (Delta) lineage. The geographical distributions and transmission patterns of all circulating lineages were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
Similarly, the geographical distribution and transmission of the B.1.617.2 lineage are recorded in Fig. 9D. This variant originated in the USA and then got transmitted to different parts of the United Kingdom and USA.
The geographical distribution and transmission of the B.1.525 lineage are shown in Fig. 10A. This variant originated in the USA and got transmitted to different parts of South Africa and Australia.
FIG 10.
Geographical distributions and transmission patterns from the source to other countries of significant VOIs between December 2019 and June 2021. (A) Geographical distribution and transmission pattern of the B.1.525 (Eta) lineage. (B) Geographical distribution and transmission pattern of the B.1.526 (Iota) lineage. (C) Geographical distribution and transmission pattern of the P.2 (Zeta) lineage. (D) Geographical distribution and transmission pattern of the B.1.427/B.1.429 (Epsilon) lineage. The geographical distributions and transmission patterns of all circulating lineages were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
We mapped the geographical distributions and transmission of the B.1.526 lineage and recorded them in Fig. 10B. The B.1.526 lineage originated in the USA and later got transmitted to different parts of South America. In the end, we have drawn the geographical distribution and transmission of the P.2 lineage (Fig. 10C). The lineage originated in Brazil and then got transmitted to different areas of South America, parts of the USA, and Canada.
The geographical distribution and transmission of lineage B.1.427/B.1.429 are noted in Fig. 10D. It originated in the USA and then got transmitted to different parts of Latin America, including Argentina.
Circulating frequencies of all SARS-CoV-2 lineages, significant VOCs (lineages B.1.1.7 (Alpha), P.1 (Gamma), B.1.351 (Beta), and B.1.617.2 (Delta)], and significant VOIs (lineages B.1.525 (Eta), B.1.526 (Iota), P.2 (Zeta), and B.1.427/B.1.429 (Epsilon)] over time.
We mapped the circulating frequencies of all SARS-CoV-2 lineages in a framework and recorded them in Fig. 11. The circulating frequency of the B.1.1.7 lineage is noted in Fig. 12A. The circulating frequency indicated that it originated in September, and it achieved 100% frequency in early October 2020. We next analyzed the circulating frequency of the P.1 lineage and recorded it in Fig. 12B. The data indicated that this variant achieved 100% frequency in the middle of October 2020. The circulating frequency of the B.1.351 lineage is represented in Fig. 12C. It was observed that the lineage achieved 100% frequency between June and July 2020.
FIG 11.
Frequency pattern of all circulating lineages between December 2019 and June 2021. The frequency pattern of all circulating lineages was developed before 27 June 2021 through the Nextstrain server, using GISAID data.
FIG 12.
Frequency patterns of significant VOCs between December 2019 and June 2021. (A) Frequency pattern of the B.1.1.7 (Alpha) lineage. (B) Frequency pattern of the P.1 (Gamma) lineage. (C) Frequency pattern of the B.1.351 (Beta) lineage. (D) Frequency pattern of the B.1.617.2 (Delta) lineage. The frequency patterns of significant VOCs were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
Similarly, we evaluated the circulating frequencies of the B.1.617.2 lineage, as shown in Fig. 12D. It was noted that the lineage achieved 100% frequency during the last week of January 2021 and early February 2021.
Furthermore, the circulating frequency of B.1.525 lineage is noted in Fig. 13A. It achieved 100% frequency during November 2020. At the same time, we mapped the circulating frequency of the B.1.526 lineage (Fig. 13B). This variant achieved 100% frequency during the first week of December 2020. Finally, we have drawn the circulating frequency pattern of the P.2 lineage (Fig. 13C). The frequency was recorded as 100% at the end of September 2020 or early October 2020. The circulating frequency of lineage B.1.427/B.1.429 is noted in Fig. 13D. The lineage achieved 100% frequency during early December 2020.
FIG 13.
Frequency patterns of significant VOIs between December 2019 and June 2021. (A) Frequency pattern of the B.1.525 (Eta) lineage. (B) Frequency pattern of the B.1.526 (Iota) lineage. (C) Frequency pattern of the P.2 (Zeta) lineage. (D) Frequency pattern of the B.1.427/B.1.429 (Epsilon) lineage. The frequency patterns of significant VOIs were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
Entropy diversity and mutational event diversity throughout the genomes of all circulating lineages, significant VOCs [lineages B.1.1.7 (Alpha), P.1 (Gamma), B.1.351 (Beta), and B.1.617.2 (Delta)], and significant VOIs [lineages B.1.525 (Eta), B.1.526 (Iota), P.2 (Zeta), and B.1.427/B.1.429 (Epsilon)].
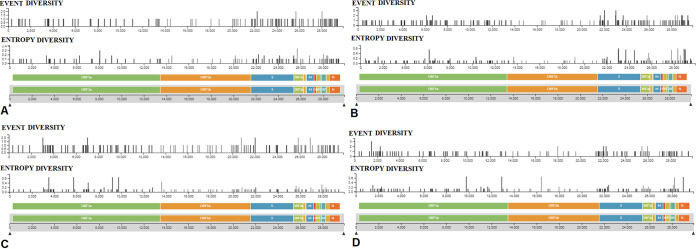
Entropy diversity is a measure to understand the pattern of mutational changes in a particular position in the genome. At the same time, it also helps us to understand the tendency of amino acids to swap from wild type to mutant (16). Similarly, mutational event diversity or mutational profiling studies inform us about the mutational events throughout the genome or at a specific position. Such studies can assist us in understanding the mechanisms that cause the SARS-CoV-2 evolution (17). Thus, we have depicted the entropy diversity and mutational event pattern throughout the genomes of all circulating lineages in a frame (Fig. 14). Based on the entropy pattern and mutational event points throughout the genome of the B.1.1.7 lineage (Fig. 15A), there was a maximum entropy of 0.8, and in two positions in ORF1b, the entropy was noted nearby as 0.6. The entropy diversity and event diversity points throughout the genome of P.1 lineage are depicted in Fig. 15B. In this case, maximum entropy was noted as 0.4, but it was pointed out in a position in ORF1a. The entropy diversity and mutational event plots throughout the genome of the B.1.351 lineage are depicted in Fig. 15C. Maximum entropy in this case was noted between 0.6 and 0.8, but it was pointed out in a position in S-protein. Similarly, we evaluated the entropy diversity and mutational event plot throughout the genome of the B.1.617.2 lineage (Fig. 15D). In this case, also, maximum entropy was noted at about 0.8, but it was noted at different positions (nine positions in ORF1a, one position in ORF1b, one position in S-protein, and one position in N-protein).
FIG 14.
Entropy diversity and event diversity of all circulating lineages between December 2019 and June 2021. The entropy diversity and event diversity of all circulating lineages were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
FIG 15.
Entropy diversities and event diversities of significant VOCs between December 2019 and June 2021. (A) Entropy diversity and event diversity of the B.1.1.7 (Alpha) lineage. (B) Entropy diversity and event diversity of the P.1 (Gamma) lineage. (C) Entropy diversity and event diversity of the B.1.351 (Beta) lineage. (D) Entropy diversity and event diversity of the B.1.617.2 (Delta) lineage. Entropy diversity and event diversity of significant VOCs were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
Again, we have depicted the entropy diversity and mutational event pattern plot throughout the genome of the B.1.525 lineage (Fig. 16A). The maximum entropy was noted at about 0.4 in ORF3a. At two positions in ORF1a, the entropy observed was about 0.3.
FIG 16.
Entropy diversity and event diversity of significant VOIs between December 2019 and June 2021. (A) Entropy diversity and event diversity of the B.1.525 (Eta) lineage. (B) Entropy diversity and event diversity of the B.1.526 (Iota) lineage. (C) Frequency pattern of the P.2 (Zeta) lineage. (D) Entropy diversity and event diversity of the B.1.427/B.1.429 (Epsilon) lineage. The entropy diversities and event diversities of significant VOIs were developed before 27 June 2021 through the Nextstrain server, using GISAID data.
At the same time, we mapped the entropy diversity and mutational events throughout the genome of the B.1.526 lineage (Fig. 16B). In this case, the maximum entropy noted was about 0.6 in different positions.
Furthermore, we have drawn the entropy diversity and mutational event pattern throughout the genome of the P.2 lineage (Fig. 16C). In this case, the maximum entropy noted was about 0.6 in four different positions in ORF1a. Finally, we recorded the entropy diversity and mutational event plots throughout the genome of lineage B.1.427/B.1.429 (Fig. 16D). Here, the maximum entropy noted was about 0.6 in five different positions (two positions in ORF1a, one position in ORF1b, and one position in N-protein).
Viral mutational landscapes of all major VOCs and VOIs, their significant mutations, and significant mutations in spike protein.
The mutational landscapes of all emerging variants (VOC and VOI), as reported by the CDC and WHO, are shown in Tables 2 and 3. The mutational landscapes and the significant mutational positions are described through the schematic illustration for all major VOCs, such as lineages B.1.1.7 (Fig. 17A), P.1 (Fig. 17B), B.1.351 (Fig. 17C), and B.1.617.2 (Fig. 17D). At the same time, the mutational landscapes and the significant mutational positions are described through the schematic illustration for all major VOIs, such as lineages B.1.525 (Fig. 18A), B.1.526 (Fig. 18B), P.2 (Fig. 18C), and B.1.427/B.1.429 (Fig. 18D).
TABLE 2.
Mutational landscape of different emerging variants of concern
| Variant name | Mutation | Mutation position | Amino acid change |
|---|---|---|---|
| B.1.1.7 | N501Y | Receptor binding domain of spike protein | Asn501→Tyr |
| H69–V70 | Spike protein | Deletion mutation | |
| P681H | S1/S2 furin cleavage site of spike protein | Pro681→His | |
| Y144/145 | Spike deletion | Deletion mutation | |
| A570D | Spike glycoprotein subunit1 | Ala570→Asp | |
| T716I | Spike glycoprotein chain A | Thr7161→Ile | |
| S982A | Spike glycoprotein chain A | Ser982→Ala | |
| D1118H | Spike glycoprotein chain A | Asp1118→His | |
| Q27 | ORF3a | Stop codon generated due to mutation in 27th position | |
| T1001I | ORF1a | Thr1001→Ile | |
| A1708D | ORF1a | Ala1708→Asp | |
| I2230T | ORF1a | Ile12230→Thr | |
| R52I | ORF3a | Arg521→Ile | |
| D3L | ORF3a | Asp3→Leu | |
| S235F | Nucleoprotein | Ser235→Phe | |
| SGF3675–3677 | ORF1a | Deletion mutation | |
| Y73C | ORF3a | Tyr73→Cys | |
| D614G | Spike glycoprotein | Asp614→Gly | |
| P.1 | L18F | Spike glycoprotein | Leu18→Phe |
| T20N | Spike glycoprotein | Thr20→Asn | |
| P26S | Spike glycoprotein | Pro26→Ser | |
| D138Y | Spike glycoprotein | Asp138→Tyr | |
| R190S | Spike glycoprotein | Arg190→Ser | |
| K417T | Spike glycoprotein | Lys417→Thr | |
| H655Y | Spike glycoprotein | His655→Tyr | |
| N501Y | Spike glycoprotein | Asn501→Tyr | |
| E484K | Spike glycoprotein | Glu484→Lys | |
| T1027I | Spike glycoprotein | Thr1027→Ile | |
| E92K | ORF3a | Glu92→Lys | |
| P80R | Nucleoprotein | Pro80→Arg | |
| S1188L | ORF1a | Ser1188→Leu | |
| K1795Q | ORF1a | Lys1795→Gln | |
| SGF3675–3677 | ORF1a | Deletion mutation | |
| E5665D | ORF1b | Glu5665→Asp | |
| D614G | Spike glycoprotein | Asp614→Gly | |
| B.1.351 | L18F | Spike glycoprotein | Leu18→Phe |
| D80A | Spike glycoprotein | Asp80→Ala | |
| D215G | Spike glycoprotein | Asp215→Gly | |
| R246I | Spike glycoprotein | Arg2461→Ile | |
| K417N | Spike glycoprotein | Lys417→Asn | |
| E484K | Spike glycoprotein | Glu484→Lys | |
| N501Y | Spike glycoprotein | Asn501→Tyr | |
| A701V | Spike glycoprotein | Ala701→Val | |
| P71L | Envelope protein | Pro71→Leu | |
| T205I | Nucleoprotein | Thr205→Ile | |
| K1655N | ORF1a | Lys1655→Asn | |
| SGF3675–3677 | ORF1a | Deletion mutation | |
| D614G | Spike glycoprotein | Asp614→Gly | |
| B.1.617.2 | T19R | Spike glycoprotein | Thr19→Arg |
| G142D | Spike glycoprotein | Gly142→Asp | |
| 156del | Spike glycoprotein | Deletion mutation | |
| R158G | Spike glycoprotein | Arg158→Gly | |
| L452R | Spike glycoprotein | Leu452→Arg | |
| T478K | Spike glycoprotein | Thr478→Lys | |
| D614G | Spike glycoprotein | Asp614→Gly | |
| P681R | Spike glycoprotein | Pro681→Arg | |
| D950N | Spike glycoprotein | Asp950→Asn | |
| 157del | Spike glycoprotein | Deletion mutation | |
TABLE 3.
Mutational landscape of different emerging variants of interest
| Variant name | Mutation | Mutation position | Amino acid change |
|---|---|---|---|
| B.1.525 | Q677H | Spike glycoprotein | Gln677→His |
| A67V | Spike glycoprotein | Ala67→Val | |
| E484K | Spike glycoprotein | Glu484→Lys | |
| D614G | Spike glycoprotein | Asp614→Gly | |
| F888L | Spike glycoprotein | Phe888→Leu | |
| T2007I | ORF1a | Thr2007→Ile | |
| P314F | ORF1b | Pro314→Phe | |
| I82T | Membrane protein | Ile82→Thr | |
| A12G | Nucleoprotein | Ala12→Gly | |
| T205I | Nucleoprotein | Thr205→Ile | |
| R81C | 5′ UTR | Arg81→Cys | |
| B.1.526 | L5F | Spike glycoprotein | Leu5→Phe |
| T95I | Spike glycoprotein | Thr95→Ile | |
| D253G | Spike glycoprotein | Asp253→Gly | |
| E484K | Spike glycoprotein | Glu484→Lys | |
| D614G | Spike glycoprotein | Asp614→Gly | |
| A701V | Spike glycoprotein | Ala701→Val | |
| L3201P | ORF1a | Leu3201→Pro | |
| T265I | ORF1a | Thr265→Ile | |
| P314L | ORF1b | Pro314→Leu | |
| Q1011H | ORF1b | Gln1011→His | |
| P42L | ORF3a | Pro42→Leu | |
| Q57H | ORF3a | Gln57→His | |
| T11I | ORF8 | Thr11→Ile | |
| R81C | 5′ UTR | Arg81→Cys | |
| P.2 | E484K | Spike glycoprotein | Glu484→Lys |
| D614G | Spike glycoprotein | Asp614→Gly | |
| V1176F | Spike glycoprotein | Val1176→Phe | |
| L3468V | ORF1a | Leu3468→Val | |
| L3930F | ORF1a | Leu3930→Phe | |
| P314L | ORF1b | Pro314→Leu | |
| A119S | Nucleoprotein | Ala119→Ser | |
| R203K | Nucleoprotein | Arg203→Lys | |
| G204R | Nucleoprotein | Gly204→Arg | |
| M234I | Nucleoprotein | Met234→Ile | |
| R81C | 5′ UTR | Arg81→Cys | |
| B.1.427/B.1.429 | L452R | Spike glycoprotein | Leu452→Arg |
| D614G | Spike glycoprotein | Asp614→Gly | |
FIG 17.
Mutational landscapes throughout the genomes of significant VOCs between December 2019 and June 2021. (A) Significant mutational landscape throughout the genome of the B.1.1.7 (Alpha) lineage. (B) Significant mutational landscape throughout the genome of the P.1 (Gamma) lineage. (C) Significant mutational landscape throughout the genome of the B.1.351 (Beta) lineage. (D) Significant mutational landscape throughout the genome of the B.1.617.2 (Delta) lineage.
FIG 18.
Mutational landscapes throughout the genomes of significant VOIs between December 2019 and June 2021. (A) Significant mutational landscape throughout the genome of the B.1.525 (Eta) lineage. (B) Significant mutational landscape throughout the genome of the B.1.526 (Iota) lineage. (C) Significant mutational landscape throughout the genome of the P.2 (Zeta) lineage. (D) Significant mutational landscape throughout the genome of the B.1.427/B.1.429 (Epsilon) lineage.
The significant mutational positions in the S-protein are illustrated through the diagrammatic representation of lineages B.1.1.7 (Fig. 19A and B), P.1 (Fig. 19C), B.1.351 (Fig. 19D), B.1.617.2 (Fig. 19E), B.1.525 (Fig. 20A), B.1.526 (Fig. 20B), P.2 (Fig. 20C), and B.1.427/B.1.429 (Fig. 20D).
FIG 19.
Significant mutational landscapes in spike protein of significant VOCs between December 2019 and June 2021. (A) Mutational landscape in the spike protein of the B.1.1.7 (Alpha) lineage (closed form). (B) Mutational landscape in the spike protein of the B.1.1.7 (Alpha) lineage (closed form with 90° rotation) (C) Mutational landscape in the spike protein of the P.1 (Gamma) lineage. (D) Mutational landscape in the spike protein of the B.1.351 (Beta) lineage. (E) Mutational landscape in the spike protein of the B.1.617.2 (Delta) lineage.
FIG 20.
Significant mutational landscapes in spike protein of significant VOIs between December 2019 and June 2021. (A) Mutational landscape in the spike protein of the B.1.525 (Eta) lineage. (B) Mutational landscape in the spike protein of the B.1.526 (Iota) lineage. (C) Mutational landscape in spike protein of the P.2 (Zeta) lineage. (D) Mutational landscape in the spike protein of the B.1.427/B.1.429 (Epsilon) lineage.
It has been reported that there are three significant mutations in the P.1 lineage. These are present in spike receptor binding domain (RBD) (E484K, K417T/N, and N501Y) (7, 18). Simultaneously, it was reported that the mutation D614G can augment the capability to spread compared to the wild type (10, 18). Another mutation (nonsynonymous mutation P681H) was observed in the S protein of the B.1.1.7 lineage.
Some significant mutations (E484K, K417T/N, N501Y, and D614G) found in emerging variants and their structural landscapes.
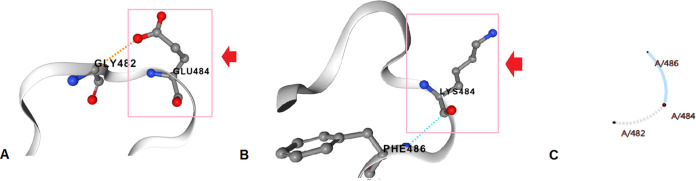
We performed structural landscape analysis of some significant mutations, such as E484K, K417T/N, N501Y, and D614G, which are frequently reported in emerging variants. We have analyzed the E484K mutation. Here, the structure of E484K changes due to the replacement Glu484→Lys. The structural analysis of E484K is shown in different forms, such as the interaction abilities of the wild-type residues (Fig. 21A) and the interaction abilities of the mutant protein structure (Fig. 21B), and in addition, a snapshot during the toggle of the molecular interaction is included to understand the interactions between the wild-type (dashed lines) and mutant (straight lines) residues (Fig. 21C). The wild-type amino acid configuration shows the interaction of Glu484 with Gly482. In contrast, the mutant amino acid configuration shows the interaction of Lys484 with Phe486.
FIG 21.
Structural landscape of the E484K mutation. (A) Contacts of the wild type (Glu). (B) Interactions of the mutant type (Lys). (C) Interactions of the wild type (Glu) with the mutant (Lys).
We next analyzed the K417T mutation, and the structure of K417T is altered due to the replacement Lys417→Thr. The structural analysis of K417T is shown in different forms, such as interaction abilities of the wild-type residues (Fig. 22A), interaction abilities of the mutant residues (Fig. 22B), and interactions of wild-type with the mutant residues (Fig. 22C). The wild-type amino acid configuration shows the interaction of Lys417 with Leu455, Tyr421, and Asn370. In contrast, the mutant amino acid configuration shows the interaction of Thr417 with Tyr421, Leu455, and Asp420.
FIG 22.
Structural landscape of the K417T mutation. (A) Contacts of the wild type (Lys). (B) Interactions of the mutant type (Thr). (C) Interactions of the wild type (Lys) with the mutant (Thr).
Furthermore, we analyzed the K417N mutation. Here, the structure of K417N is changed due to the replacement Lys417→Asn. The structural analysis of K417N is shown in different forms, such as interaction abilities of the wild-type residues (Fig. 23A), interaction abilities of the mutant residues (Fig. 23B), and interactions of the wild-type and mutant residues (Fig. 23C). The wild-type amino acid shows the interaction of Lys417 with Leu455, Tyr421, and Asn370. In contrast, the mutant amino acid configuration shows the interaction of Asn417 with Tyr421, Leu455, and Asp420.
FIG 23.
Structural landscape of the K417N mutation. (A) Contacts of the wild type (Lys). (B) Interactions of the mutant (Asn). (C) Interactions of the wild type (Lys) with the mutant (Asn).
Simultaneously, we also analyzed the N501Y mutation. Here, the structure of N501Y was altered due to the replacement Asn501→Tyr. The structural analysis of N501Y is shown in different forms, such as interaction abilities of the wild-type residues (Fig. 24A), interaction abilities of the mutant residues (Fig. 24B), and interaction of the wild-type and mutant residues (Fig. 24C). The wild-type amino acid configuration shows the interaction of Asn501 with Gln506 and Pro499. In contrast, the mutant amino acid configuration shows the interaction of Tyr501 with Gln498 and Gln506. The wild-type and mutant residues also show some other interactions.
FIG 24.
Structural landscape of the N501Y mutation. (A) Contacts of the wild type (Asn). (B) Interactions of the mutant (Tyr). (C) Interactions of the wild type (Asn) with the mutant (Tyr).
Finally, the D614G mutation was analyzed. Here, the structure of D614G was changed due to the replacement of Asp614→Gly. The structural analysis of D614G is shown in different forms, such as interaction abilities of the wild-type residues (Fig. 25A), interaction abilities of the mutant residues (Fig. 25B), and interactions of wild-type and mutant residues (Fig. 25C). The wild-type amino acid configuration shows the interaction of Asp614 with Ala647. In contrast, the mutant amino acid configuration shows the interaction of Gly614 with Ala647. The interaction pattern between the residues of the wild type and mutant did not show any changes.
FIG 25.
Structural landscape of the D614G mutation. (A) Contacts of the wild type (Asp). (B) Interactions of the mutant (Gly). (C) Interactions of the wild type (Asp) with the mutant (Gly).
The new SARS-CoV-2 variants and efficacy of vaccines against these variants.
We have tried to understand the new SARS-CoV-2 variants and their recognition by neutralizing antibodies produced by different vaccines. The data were obtained from the various available data in the literature (Table 4). The effectiveness of vaccines against newly emerging SARS-CoV-2 variants is noted from different reports in the literature and is summarized in Table 5. These two tables help us to evaluate the reduction in vaccine efficacy by the newly developed virus variants.
TABLE 4.
Major approved COVID-19 vaccines and their reported efficacy against SARS-CoV-2
| Sl no. | Vaccine | Efficacy of COVID-19 vaccine against SARS-CoV-2 (%) | Reference |
|---|---|---|---|
| 1 | Ad26.CoV2.S (Johnson & Johnson) | 85 | 35 |
| 2 | BNT162b2 mRNA COVID-19 (Pfizer) | 95 | 36 |
| 3 | mRNA-1273 (Moderna) | 94.1 | 37 |
| 4 | Sputnik V COVID-19 (Gamaleya) | 91.6 | 38 |
| 5 | AZD1222 COVID-19 (AstraZeneca) | 70.4 | 39 |
| 6 | NVX-CoV2373 (Novavax) | 96.4 | 40 |
| 7 | CoronaVac COVID-19 (Sinovac) | 79 | 41 |
| 8 | BBIBP-CorV (Sinopharm) | 79 | 12 |
TABLE 5.
Efficacy of some approved significant vaccines against new SARS-CoV-2 variants
| Sl no. | New significant SARS-CoV-2 lineage | SARS-CoV-2 vaccine efficacya |
|||
|---|---|---|---|---|---|
| Ad26.COV2.S (Johnson & Johnson) | BNT162b2 (Pfizer) | mRNA-1273 (Moderna) | NVX-CoV2373 (Novavax) | ||
| 1 | B.1.1.7 | NA | Reduced neutralizing activity | Decreased neutralizing antibodies | 85.6% efficacy in United Kingdom population; 60% efficacy in South African population |
| 2 | P.1 | NA | Significant reduction in neutralization efficacy | Decreased neutralizing antibodies | NA |
| 3 | B.1.351 | 64.0% efficacy in Brazilian population; 52% efficacy in South African population | Reduced neutralizing activity | Reduced neutralizing activity | 49% efficacy in South African population |
DISCUSSION
The first section of the data analysis has shown a depiction of the current scenario of the evolution of emerging variants of SARS-CoV-2 virus, the cluster of genome samples and their divergence, and the geographical distribution and transmission pattern of the variants. Presently, the transmission of the virus has become uncontrolled in different parts of the world. According to published reports, some varieties have shown higher transmission potential, such as the B.1.1.7 lineage (19). Similarly, we have found a higher transmission pattern in the B.1.1.7 lineage. Therefore, the transmission pattern analyzed for these variants is highly significant in the present perspective when the transmission continues to increase in several countries. We have also described the circulating frequencies of all the lineages and all the newly emerging lineages. From the analysis, we found that 100% frequency was achieved by lineage B.1.1.7 in early October 2020. We also found entropy diversity and mutational event diversity throughout the genomes of all newly emerging lines.
We have critically evaluated the mutational landscape of all the above emerging variants. The data analysis has shown significant mutations throughout the genomes of all newly emerging lineages. The important mutations were recorded in the spike protein, especially in the RBD of all newly emerging lineages. There is already recorded evidence that the mutations in spike protein, especially in the RBD, can influence the transmission pattern of this virus (10). We have also evaluated the structural landscape of several significant mutations (E484K, K417T/N, N501Y, and D614G) in the emerging variants. The structural analysis will help us understand the structural contacts of the wild-type protein, structural connections of the mutant protein, and wild-type and mutant interactions, assisting in understanding the mutational landscape. These significant mutations are related to more infectivity in most emerging lineages and death tolls (7, 10, 18).
It is a known fact that the E484K mutation affects neutralization by convalescent-phase sera or monoclonal antibodies (MAbs) (19, 20). Similarly, the combination mutation of K417N and N501Y affects neutralization by MAbs and convalescent-phase sera (19–21). Therefore, our analysis confirms this observed phenomenon and is very noteworthy for now. Finally, we analyzed the vaccines’ efficacy against the new variants of this virus and compared them with the vaccines’ reported effectiveness. We found a reduction in vaccine efficacy for the new variants. However, the efficacy of all approved vaccines has not been analyzed against all newly emerging SARS-CoV-2 variants. Therefore, further evaluation is urgently needed.
Furthermore, the year 2021 will be more challenging due to the emergence of numerous variants. Therefore, we raise the following queries. How will the recently emerged variants affect people's health? Will the COVID-19 vaccines protect people against the recently emerged variants? Can the COVID-19 vaccination program be successfully implemented across the developing world?
Conclusion.
The emergence of several new lineages of SARS-CoV-2 due to viral mutation is crucial as vaccination programs have started throughout the globe. Scientists have started research to protect against all new significant mutant variants. They have already designed next-generation vaccines against this pandemic virus using different epitopes from all important mutant variants, including the Wuhan variant (22). However, our analysis will help to design future countrywide pandemic planning, focusing on emerging variants, as well as next-generation vaccine development using alternative wild-type antigens and significant viral antigens, and immediate planning for ongoing vaccination programs worldwide.
MATERIALS AND METHODS
Data collection.
We retrieved or collected different data sets for new SARS-CoV-2 variants from the WHO (23) and CDC (24). We searched for different keywords in databases such as Web of Science (25), PubMed (26, 27), and Google Scholar. For the database search, we used keywords such as “SARS-CoV-2 variants,” “variants of consequence,” “VOI,” “VOC,” and “variants and vaccines,” etc. We also searched for the new variants with keywords such as “B.1.1.7 lineage,” “P.1 lineage,” and “B.1.351 lineage.” A search for two other variant names with the keywords “B.1.427/B.1.429 variant” and “B.1.617.2 variant” was also performed. Similarly, we searched for three other different variant names as keywords: “B.1.526 variant,” “B.1.525 variant,” and “P.2 variant.”
We have tried to collect data from different sources, such as The New York Times (coronavirus-variant-tracker) (28) and various other resources. For further data collection, we used several databases and servers, such as Nextstrain (SARS-CoV-2 resources) (28, 29), Pango lineages (30), Pango lineages on GitHub (31), and COVID-3D (32, 33). Nextstrain uses the data from GISAID. We analyzed and retrieved the data from the Nextstrain server in April 2021.
We have followed the nomenclature for lineages of this virus proposed by Rambaut et al. (34).
Data analysis and interpretation.
For data analysis, we used several servers, such as Nextstrain (SARS-CoV-2 resources) (26, 27), Pango lineages (30), Pango lineages on GitHub (31), and COVID-3D (32). We also used COVID-3D for the structural analysis of significant mutations (E484K, K417T/N, N501Y, and D614G) in emerging variants (18). We have depicted the study methodology through a flowchart summarizing the overall process in Fig. 1.
ACKNOWLEDGMENTS
This study was supported by Hallym University Research Fund and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1C1C1008694 and NRF-2020R1I1A3074575).
We used different webservers and databases (e.g., Nextstrain SARS-CoV-2 resources, Pango lineages, GitHub, COVID-3D, WHO, CDC, Web of Science, PubMed, and Google Scholar) in this study. We are thankful to the researchers who developed these webservers and databases.
The authors have no conflict of interests to declare.
Footnotes
Citation Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee S-S. 2021. Evolution, mode of transmission, and mutational landscape of newly emerging SARS-CoV-2 variants. mBio 12:e01140-21. https://doi.org/10.1128/mBio.01140-21.
Contributor Information
Chiranjib Chakraborty, Email: drchiranjib@yahoo.com.
Sang-Soo Lee, Email: 123sslee@gmail.com.
Stephen P. Goff, Columbia University/HHMI
REFERENCES
- 1.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. 2020. The socio-economic implications of the coronavirus and COVID-19 pandemic: a review. Int J Surg 78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2020. Race for a COVID-19 vaccine. EBioMedicine 55:102817. doi: 10.1016/j.ebiom.2020.102817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Peng Y, Xu H, Cui Z, Williams RO. 2020. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech 21:225. doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J. 2020. Vaccine designers take first shots at COVID-19. Science 368:14–16. doi: 10.1126/science.368.6486.14. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Tenchov R, Smoot J, Liu C, Watkins S, Zhou Q. 2021. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci 7:512–533. doi: 10.1021/acscentsci.1c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olliaro P. 2021. What does 95% COVID-19 vaccine efficacy really mean? Lancet Infect Dis 21:769. doi: 10.1016/S1473-3099(21)00075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. 2021. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet 397:952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama T, Platt D, Parida L. 2020. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ 98:495–504. doi: 10.2471/BLT.20.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauring AS, Hodcroft EB. 2021. Genetic variants of SARS-CoV-2—what do they mean? JAMA 325:529–531. doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- 10.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Hengartner N, Giorgi EE, Bhattacharya T, Foley B, Hastie KM, Parker MD, Partridge DG, Evans CM, Freeman TM, de Silva TI, McDanal C, Perez LG, Tang H, Moon-Walker A, Whelan SP, LaBranche CC, Saphire EO, Montefiori DC, Sheffield COVID-19 Genomics Group. 2020. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182:812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty C, Bhattacharya M, Sharma AR. 2021. Present variants of concern (VOC) and variants of interest (VOI) of SARS-CoV-2: their significant mutations in S-glycoprotein, infectivity, re-infectivity, immune escape, and vaccines activity. Rev Med Virol doi: 10.1002/rmv.2270. [DOI] [Google Scholar]
- 12.Abdool Karim SS, de Oliveira T. 2021. New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N Engl J Med 384:1866–1868. doi: 10.1056/NEJMc2100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty C, Bhattacharya M, Sharma AR, Lee SS, Agoramoorthy G. 2021. SARS-CoV-2 Brazil variant in Latin America: more serious research urgently needed on public health and vaccine protection. Ann Med Surg (Lond) 66:102428. doi: 10.1016/j.amsu.2021.102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore JP, Offit PA. 2021. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA 325:821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 15.Golob JL, Lugogo N, Lauring AS, Lok AS. 2021. SARS-CoV-2 vaccines: a triumph of science and collaboration. JCI Insight 6:e149187. doi: 10.1172/jci.insight.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomaszewski T, DeVries RS, Dong M, Bhatia G, Norsworthy MD, Zheng X, Caetano-Anollés G. 2020. New pathways of mutational change in SARS-CoV-2 proteomes involve regions of intrinsic disorder important for virus replication and release. Evol Bioinform Online 16:1176934320965149. doi: 10.1177/1176934320965149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azgari C, Kilinc Z, Turhan B, Circi D, Adebali O. 2021. The mutation profile of SARS-CoV-2 is primarily shaped by the host antiviral defense. Viruses 13:394. doi: 10.3390/v13030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soriano V, Fernández-Montero JV. 2021. New SARS-CoV-2 variants challenge vaccines protection. AIDS Rev 23:57–58. doi: 10.24875/AIDSRev.M21000040. [DOI] [PubMed] [Google Scholar]
- 19.Collier DA, De Marco A, Ferreira IATM, Meng B, Datir RP, Walls AC, Kemp SA, Bassi J, Pinto D, Silacci-Fregni C, Bianchi S, Tortorici MA, Bowen J, Culap K, Jaconi S, Cameroni E, Snell G, Pizzuto MS, Pellanda AF, Garzoni C, Riva A, Elmer A, Kingston N, Graves B, McCoy LE, Smith KGC, Bradley JR, Temperton N, Ceron-Gutierrez L, Barcenas-Morales G, Harvey W, Virgin HW, Lanzavecchia A, Piccoli L, Doffinger R, Wills M, Veesler D, Corti D, Gupta RK, COVID-19 Genomics UK (COG-UK) Consortium. 2021. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 593:136–141. doi: 10.1038/s41586-021-03412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greaney A, Loes A, Crawford K, Starr T, Malone K, Chu H, Bloom J. 2021. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. Cell Host Microbe 29:463–476. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, Muecksch F, Rutkowska M, Hoffmann H-H, Michailidis E, Gaebler C, Agudelo M, Cho A, Wang Z, Gazumyan A, Cipolla M, Luchsinger L, Hillyer CD, Caskey M, Robbiani DF, Rice CM, Nussenzweig MC, Hatziioannou T, Bieniasz PD. 2020. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya M, Sharma AR, Ghosh P, Lee SS, Chakraborty C. 2021. A next-generation vaccine candidate using alternative epitopes to protect against Wuhan and all significant mutant variants of SARS-CoV-2: an immunoinformatics approach. Aging Dis doi: 10.14336/AD.2021.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. 2020. SARS-CoV-2 variants. https://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/. Accessed 14 April 2021.
- 24.CDC. 2021. SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html. Accessed April 14, 2021.
- 25.Farooq RK, Rehman SU, Ashiq M, Siddique N, Ahmad S. 2021. Bibliometric analysis of coronavirus disease (COVID-19) literature published in Web of Science 2019–2020. J Family Community Med 28:1–7. doi: 10.4103/jfcm.JFCM_332_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo X, Chen Y, Ohno-Machado L, Xu H. 2021. How do we share data in COVID-19 research? A systematic review of COVID-19 datasets in PubMed Central articles. Brief Bioinform 22:800–811. doi: 10.1093/bib/bbaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmer JCaC. 2021. Coronavirus variants and mutations, on The New York Times. https://www.nytimes.com/interactive/2021/health/coronavirus-variant-tracker.html. Accessed 14 April 2021.
- 28.Hadfield J, Megill C, Bell SM, Huddleston J, Potter B, Callender C, Sagulenko P, Bedford T, Neher RA. 2018. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nextstrain. 2021. Nextstrain SARS-CoV-2 resources, on Nextstrain. https://nextstrain.org/sars-cov-2/. Accessed 14 April 2021.
- 30.Banchich A, O’Toole Á. 2021. PANGO lineages. https://cov-lineages.org/index.html. Accessed 14 April 2021.
- 31.GitHub. 2021. cov-lineages/pangolin. https://github.com/cov-lineages/pangolin. Accessed 14 April 2021
- 32.Portelli S, Olshansky M, Rodrigues CHM, D'Souza EN, Myung Y, Silk M, Alavi A, Pires DEV, Ascher DB. 2020. Exploring the structural distribution of genetic variation in SARS-CoV-2 with the COVID-3D online resource. Nat Genet 52:999–1001. doi: 10.1038/s41588-020-0693-3. [DOI] [PubMed] [Google Scholar]
- 33.Portelli S, Olshansky M, Rodrigues CHM, D'Souza EN, Myung Y, Silk M, Alavi A, Pires DEV, Ascher DB. 2021. Author correction: exploring the structural distribution of genetic variation in SARS-CoV-2 with the COVID-3D online resource. Nat Genet 53:254–254. doi: 10.1038/s41588-020-00775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rambaut A, Holmes EC, O'Toole Á, Hill V, McCrone JT, Ruis C, Du Plessis L, Pybus OG. 2020. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M, ENSEMBLE Study Group. 2021. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones I, Roy P. 2021. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 397:642–643. doi: 10.1016/S0140-6736(21)00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, Oxford COVID Vaccine Trial Group, et al. 2021. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alderson J, Batchelor V, O’Hanlon M, Cifuentes L, Richter FC, Kopycinski J, Oxford-Cardiff COVID-19 Literature Consortium. 2021. Overview of approved and upcoming vaccines for SARS-CoV-2: a living review. Oxf Open Immunol 2:iqaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallapaty S. 2021. WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature 594:161–162. doi: 10.1038/d41586-021-01497-8. [DOI] [PubMed] [Google Scholar]
- 42.Gómez CE, Perdiguero B, Esteban M. 2021. Emerging SARS-CoV-2 variants and impact in global vaccination programs against SARS-CoV-2/COVID-19. Vaccines 9:243. doi: 10.3390/vaccines9030243. [DOI] [PMC free article] [PubMed] [Google Scholar]