Abstract
The smooth muscle (SM) and nonmuscle (NM) isoforms of α-actinin are produced by mutually exclusive splicing of an upstream NM exon and a downstream SM-specific exon. A rat α-actinin genomic clone encompassing the mutually exclusive exons was isolated and sequenced. The SM exon was found to utilize two branch points located 382 and 386 nucleotides (nt) upstream of the 3′ splice site, while the NM exon used a single branch point 191 nt upstream. Mutually exclusive splicing arises from the proximity of the SM branch points to the NM 5′ splice site, and this steric repression could be relieved in part by the insertion of spacer elements. In addition, the SM exon is repressed in non-SM cells and extracts. In vitro splicing of spacer-containing transcripts could be activated by (i) truncation of the transcript between the SM polypyrimidine tract and exon, (ii) addition of competitor RNAs containing the 3′ end of the actinin intron or regulatory sequences from α-tropomyosin (TM), and (iii) depletion of the splicing extract by using biotinylated α-TM RNAs. A number of lines of evidence point to polypyrimidine tract binding protein (PTB) as the trans-acting factor responsible for repression. PTB was the only nuclear protein observed to cross-link to the actinin RNA, and the ability of various competitor RNAs to activate splicing correlated with their ability to bind PTB. Furthermore, repression of α-actinin splicing in the nuclear extracts depleted of PTB by using biotinylated RNA could be specifically restored by the addition of recombinant PTB. Thus, α-actinin mutually exclusive splicing is enforced by the unusual location of the SM branch point, while constitutive repression of the SM exon is conferred by regulatory elements between the branch point and 3′ splice site and by PTB.
Many eukaryotic genes employ alternative splicing as a means of generating protein diversity. This differential incorporation of exons into the mature RNA is often under developmental and/or tissue-specific control and enables the cell to tailor the protein to suit its own particular requirements (61, 67). The basic splicing mechanism involves a two-step process which takes place in a ribonucleoprotein complex called a spliceosome and results in adjacent exons being joined together with the intron between released in the form of a lariat (reviewed in references 1 and 57). There is a further level of complexity in alternative splicing in that different combinations of 5′ and 3′ splice sites are ligated. The mechanisms that determine which splice sites are utilized and how this is regulated in different cell types or developmental stages have still not been precisely defined. Much progress has been made in identifying the cis-acting elements involved in alternative splicing, and the roles of some general factors have been demonstrated (1, 67). cis-Acting determinants that influence competing splicing pathways include the relative strengths of the competing 5′ splice sites (e.g., 9, 78), branch point sequences (e.g., 53, 79), and polypyrimidine tracts (e.g., 45); proximity between 5′ splice sites and branch points (e.g., 12, 60); sequences between the branch point-polypyrimidine tract and the 3′ splice site (e.g., 19, 29); secondary structure (e.g., 36); and splicing enhancers which may be intronic or exonic (e.g., 30, 64, 71). Some of these elements are responsible for setting the default competition between competing splicing events, while others are necessary for the cell-specific switch to a regulated splicing pattern (e.g., 3, 19).
Less is known about the trans-acting factors involved in the regulation of tissue-specific splicing. Such factors may be tissue-specific regulators of splicing, or alternatively, tissue-specific splicing may be regulated by alterations in the concentrations or activities of general pre-mRNA splicing factors (1, 67). To date, the best understood model systems of regulated splicing are in Drosophila melanogaster where several trans-acting proteins that regulate the cascade of alternative splicing events involved in sex determination have been identified (42). In mammalian cells, the major advances in understanding trans-acting factors that regulate splicing have involved the characterization of the SR proteins, a family of splicing factors which contain arginine-serine-rich sequences (11, 39, 66). The SR proteins are required for constitutive splicing but may also regulate alternative splicing patterns, for example, by altering 5′ splice site choice in pre-mRNAs containing competing 5′ splice sites (17, 34, 75) or by promoting alternative exon inclusion (5). In 5′ splice site competition assays, different SR proteins can show distinct preferences for promoting use of distal or proximal splice sites (75, 76). In some cases, SR proteins may act instead as splicing repressors, either by binding to sites that sterically occlude spliceosome assembly (33) or by blocking the binding of more-active SR proteins (14). Thus, it is possible that the ratio of the different SR proteins contributes to splice site choice; in support of this, tissue-specific differences in the relative amounts of the individual SR proteins have been observed (14, 74, 75).
Another group of proteins with a potential role in regulating splicing are the heterogeneous nuclear ribonucleoproteins (hnRNPs). The hnRNPs bind nascent transcripts with different sequence preferences and are thought to be important in various aspects of metabolism of hnRNAs (8). hnRNP F forms part of a complex involved in activating neuron-specific splicing of the alternative c-src exon N1 (44), while hnRNP A1 is able to antagonize the actions of SR proteins in 5′ splice site selection (5, 41). It is becoming apparent that polypyrimidine tract binding protein (PTB) (16, 48), also known as hnRNP I (18), has an important role in the regulation of tissue-specific splicing (reviewed in reference 65). PTB is implicated in the regulation of several alternatively spliced genes, including α- and β-tropomyosin (α- and β-TM), c-src, and GABAA receptor γ2 subunit, and appears to regulate alternative splicing by inhibiting the splicing of exons with binding sites for PTB adjacent to or overlapping the splice sites (2, 6, 20, 23, 38, 46, 47, 50, 58). While such general factors appear to be important in splicing regulation, it is not yet clear whether a system based only on different levels of constitutive splicing factors is responsible for the full diversity of alternative splicing events observed. The possibility that tissue-specific regulators of splicing are also involved remains.
A number of pre-mRNAs are alternatively spliced in a smooth muscle (SM)-specific manner; these include α-actinin, α-TM, caldesmon, and vinculin (4, 25, 69, 72), raising the possibility that there is a general mechanism for the regulation of alternative splicing in SM cells. α-TM has a pair of mutually exclusive exons which are alternatively spliced such that exon 3 is incorporated into α-TM transcripts in all cell types except SM, where there is regulated selection of exon 2 (72) (Fig. 1). The mutually exclusive behavior of the two exons has been explained by the abnormally positioned branch point of exon 3; its proximity to the 5′ splice site of exon 2 sterically inhibits the formation of an active spliceosome complex, thus preventing splicing of exon 2 to exon 3 (60). Exon 3 is selected in most cells because the 3′ splice site elements associated with exon 3 are functionally stronger than those of exon 2 and thus outcompete exon 2 (45, 77). The SM-specific selection of exon 2 is due to an inhibition of exon 3 splicing which is mediated by at least two negative regulatory elements in the introns flanking exon 3 (19) as well as by specific PTB binding sites in the polypyrimidine tract (50). PTB has been shown to inhibit exon 3 splicing in vitro (20, 38, 58), and mutation of the PTB binding sites in the regulatory elements flanking exon 3 impairs splicing regulation in vitro and in vivo (20, 50).
FIG. 1.
Organization of the mutually exclusive exons of α-actinin and α-TM. Exons are shown as boxes, and introns are shown as lines. The sizes (in nucleotides) of the exons and introns are indicated. The α-TM exon 1 has a variable size according to the transcription start site used (72). The nonmuscle splicing pattern is shown above the gene, and the smooth muscle pattern is shown below. The relative arrangement of the mutually exclusive exons differs; in α-actinin, the SM-specific exon is the downstream exon of the pair, while in α-TM, it is the upstream exon. The C-terminal domain of α-actinin contains two EF hand calcium-binding motifs. In both the NM and SM α-actinin isoforms, the first part of the first EF hand is encoded by the EF1a exon, while the second part is encoded by the NM exon in the NM isoform and the SM exon in the SM isoform. Inclusion of the SM exon produces a nonfunctional Ca2+-binding domain. The second EF hand, which is common to the two isoforms, is encoded by the EF2 exon.
Like α-TM, α-actinin utilizes a pair of mutually exclusive exons that are alternatively spliced in a SM-specific manner (69) (Fig. 1), and there is evidence to suggest that α-TM and α-actinin alternative splicing are coregulated. The use of a splicing-dependent drug selection system to identify potential SM-specific regulators of α-TM splicing generated fibroblast cell lines in which the normal nonmuscle (NM) splicing pattern of α-TM had switched to the SM-specific pattern (54). Endogenous mRNA for α-actinin, but not caldesmon or vinculin, was also spliced with SM specificity in these cell lines, suggesting that regulation of α-TM and α-actinin pre-mRNA splicing involves common elements of control. Analysis of α-actinin-regulated splicing and comparison with α-TM might therefore be expected to reveal the general mechanistic principles underlying SM-specific mutually exclusive splicing as well as features that are gene specific. To this end, we have begun investigating the regulation of alternative splicing of α-actinin with the aim of developing the mutually exclusive α-actinin exon pair as a parallel model system of SM-specific regulated splicing alongside the well-established α-TM system.
In this article, we report the initial analysis of the mechanisms responsible for alternative splicing of the mutually exclusive exons of α-actinin. Isolation and characterization of a genomic clone encompassing the two exons indicated the presence of a number of potential regulatory elements in common with α-TM and other alternatively spliced RNAs. Mutually exclusive splicing was found to be enforced by a steric interference mechanism similar to that of TM, in which the branch points of the SM-specific exon are too close to the upstream 5′ splice site of the NM exon. Strikingly, the SM exon branch points were mapped to an unprecedented 382 and 386 nucleotides (nt) upstream of the 3′ splice site. In addition, the SM exon was found to be repressed in HeLa nuclear extracts. The repression was mediated by cis-acting elements between the upstream branch points and the exon. A number of lines of evidence pointed to PTB as the trans-acting factor responsible for this repression. In particular, depletion of PTB from the extracts by biotinylated PTB-binding RNA led to activation of SM exon splicing. This effect could be specifically reversed by the addition of recombinant PTB (rPTB).
MATERIALS AND METHODS
Library screening.
Oligonucleotide primers directed to the EF1a and EF2 exons (ACT5′1 [5′-CGAGAGGGCTGGGAGCAGCT-3′] and ACT3′1 [5′-ACATGAAGTCAATGAAGGCYTG-3′]) were used for reverse transcriptase PCR (RT-PCR) of rat SM RNA to obtain two cDNA products encoding the EF hand region of the NM and SM α-actinin isoforms (54). A rat genomic library in λGEM11 (Promega) was screened by using a mixture of the two cDNAs labeled with [α-32P]dCTP by the random priming method. Hybridization was performed overnight at 65°C in a solution containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA, and 5× Denhardt’s solution, following which the filters were washed twice for 1 h at 65°C in 1× SSC–0.1% SDS before autoradiography. A positive plaque was identified and purified to homogeneity. λ DNA was isolated by CsCl centrifugation and subjected to restriction enzyme and Southern blot analysis. A 6-kb BamHI fragment was subcloned into pGEM4Z, and the entire region between the EF1a and EF2 exons was sequenced.
Construct preparation.
Constructs for in vitro transcription and transient transfections were prepared by standard cloning techniques (56). The NM and SM exons and intron between the exons were amplified from rat SM genomic DNA by using the primers NM5′ (5′-GATCACTCCGGCACGT-3′) and SM3′ (5′-CATGTTGTAACCCATGGAGATA-3′) and cloned into the HincII site of pGEM3Z (Promega). Sequencing of this PCR-generated fragment agreed exactly with that of the genomic clone. A 30-bp spacer consisting of two annealed oligonucleotides, (PL/S [5′-GGCATGCATCGATCCGCGGCCGGCATGCTG-3′] and PL/AS [5′-CATGCCGGCCGCGGATCGATGCATGCCCAG-3′]) was inserted into the BglI site downstream of the NM 5′ splice site (nt 1218 to 1228 [see Fig. 2]). For both constructs, templates for transcription were produced by digestion with BamHI for full-length transcripts or with AflIII, NcoI, EcoRI, or ClaI for truncated transcripts. Transcripts containing the EF1a and NM exons and intron between the two exons were generated from a template extending from the EF1a exon to the BglI site downstream of the NM exon. The template for the α-actinin competitor RNA was made by cloning the (blunt-ended) NcoI-BanI fragment (nt 1300 to 1606 [see Fig. 2]) into the HincII site of pGEM3Z. The templates for the α-TM DY and DYΔPC competitor RNAs were prepared as described previously (20).
FIG. 2.
Sequence of the rat α-actinin gene in the region encoding the two EF hand calcium-binding motifs. The exons are shown in uppercase, and the introns are in lowercase. The putative branch point adenosines utilized in splicing of the NM and SM exons are shown in boldface uppercase and underlined (nt 911 for the NM exon and nt 1237 and 1241 for the SM exon). The adjacent polypyrimidine tracts are shown in italics. Clusters of TGC motifs, similar to those of the URE and DRE of α-TM (19, 20), are shown in italic boldface (nt 701 to 725 and 1319 to 1335). Optimal PTB binding TCTT motifs (50) in the vicinity of the two alternative exons are underlined, and GCATG motifs (30) are shown in boldface.
For the transfection experiments, the NspBII fragment (nt 25 to 2951 [see Fig. 2]) of the actinin genomic clone was cloned into the (blunt-ended) HindIII site of the expression vector pSVpA (60). The constructs with the 30-bp spacer element and/or the EcoRI-AflIII deletion were derived from this construct.
In vitro transcription and splicing reactions.
32P-labeled RNA transcripts for splicing were transcribed from pGEM vectors with SP6 or T7 polymerase, as described previously (60). Competitor RNAs were produced by scaled-up reaction mixtures containing a small trace of radiolabel to allow quantitation. In vitro splicing reaction mixtures typically contained 20 to 50 fmol of 32P-labeled RNA transcript, 2.6% polyvinyl alcohol, 2 mM MgCl2, 500 μM ATP, 20 mM creatine phosphate, 20 U of RNasin, and 20 to 60% HeLa nuclear extract. The reactions were performed at 30°C for the times indicated in the figures, followed by proteinase K digestion, phenol extraction, and ethanol precipitation. For reaction mixtures containing RNA competitors, the competitor was preincubated with the nuclear extract prior to addition of the labeled substrate RNA, unless otherwise stated. Splicing reaction intermediates and products were debranched in HeLa cell cytoplasmic fraction S-100 for 30 min at 30°C. Reaction products were analyzed on 4, 6, 8, or 12% polyacrylamide gels containing 8 M urea followed by autoradiography. SR proteins were prepared from sheep uterus tissue as previously described (74). Recombinant His-tagged PTB was prepared as previously described (38, 50) with minor modifications. The concentration of PTB in undiluted nuclear extracts was estimated to be ∼0.9 μM by Western blotting with known amounts of His-tagged PTB (20).
Branch point mapping.
The lariat branch points were identified by RT extension of a 32P-end-labeled oligonucleotide primer complementary to sequences in the intron between the NM and SM exons for the SM branch point (5′-AGGGAGAATTCAGACA-3′ or 5′-CTGTGGGCAGTGGTG-3′) or in the intron between the EF1a and NM exons for the NM branch point (5′-GGGGAAGGGGGGGGGAGA-3′ or 5′-CTGGGGCCGTGGTGC-3′). RNA (100 fmol) was spliced and then hybridized with the 5′-end-labeled primer (1 pmol). After annealing, extension was carried out in the presence of 0.2 mM deoxynucleoside triphosphates and 10 U of avian myeloblastosis virus RT at 42°C for 1 h. Labeled RNA was then hydrolyzed by the addition of NaOH to 200 mM at room temperature for 1 h, followed by neutralization with HCl, phenol-chloroform extraction, and ethanol precipitation. The extended products were analyzed on 16% denaturing polyacrylamide gels alongside a sequencing reaction with the same primer and an appropriate plasmid template.
Cell culture and transfections.
HeLa cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum. Transient transfection was carried out by calcium phosphate coprecipitation followed by glycerol shock as described previously (60).
Isolation and analysis of cellular RNA.
Total RNA was isolated from transfected cells with TriReagent (Sigma). For RT-PCR, the RNA was denatured at 80°C for 5 min, placed on ice, and then incubated with 50 ng of primer (SV3′3 [5′-ACCTGTGGCTGAGTTTGC-3′]), 2 mM deoxynucleoside triphosphates, and RT buffer at 42°C for 45 min followed by the addition of 5 U of avian myeloblastosis virus RT (Promega) and incubation for a further 45 min at 42°C. For PCR, 1 μl of the RT reaction mixture was used as a template. The first round of PCR consisted of 30 cycles, with 1 cycle being 30 s at 94°C, 1 min at 60°C, and 1 min at 72°C, with primers to the simian virus 40 (SV40) sequences, SV5′1 (5′-GAGCTATTCCAGAAGTAGTGAGGAG-3′) and SV3′1 (5′-ACTCACTGCGTTCCAGGCAATGCT-3′). One microliter of this reaction mixture was diluted 1:100 and used as the template for a second round of PCR, using the same conditions, with the SV40 primer SV5′2 (5′-GGAGGCCTAGGCTTTTGCAAAAAG-3′) and the actinin primer ACT3′1, which was 32P end labeled. The labeled PCR products were resolved on a 4% polyacrylamide gel.
UV cross-linking and immunoprecipitation.
High-specific-activity [α-32P]UTP-labeled RNA probes were incubated in reaction mixtures containing 20% HeLa nuclear extract, binding buffer (10 mM HEPES [pH 7.2], 3 mM MgCl2, 5% glycerol, 1 mM dithiothreitol), and 0.05 mg of Escherichia coli rRNA per ml at 30°C for 30 min. Heparin was added to a concentration of 1.25 mg/ml 5 min before the end of the reaction. The samples were then irradiated with 254-nm-wavelength light in a cross-linker (Spectronic) and received 1.92 J of energy per cm2 from the light. The probe was digested with RNase T1 (0.8 U/μl) and RNase A (0.4 U/μl), and the labeled cross-linked proteins were resolved by SDS-polyacrylamide gel electrophoresis followed by autoradiography. For immunoprecipitation, following RNase digestion, the UV cross-linking samples were incubated with either anti-PTB antiserum or preimmune serum for 1 h at 4°C. Protein A Sepharose beads (Pharmacia) were then added and incubated for a further hour at 4°C. The beads were then washed three times in NETS buffer (150 mM NaCl, 50 mM Tris [pH 7.5], 5 mM EDTA, 0.05% Nonidet P-40) and boiled in loading buffer for 5 min, and the proteins were resolved by SDS-polyacrylamide gel electrophoresis.
Depletion of PTB from HeLa nuclear extract.
Transcription of α-TM DY RNA was done in the presence of 100 μM biotin-14-CTP and trace labeled to allow quantitation. The biotinylated DY RNA was then bound to streptavidin magnetic beads (Dynabeads; 100 pmol of RNA/50 μl of beads) in 2× BW buffer (10 mM Tris [pH 7.5], 1 mM EDTA, 2 M NaCl). One hundred microliters of HeLa nuclear extract was preincubated with 0.5 μl of 100 mM dithiothreitol and 34 U of RNasin for 15 min at room temperature, followed by incubation with the DY RNA-streptavidin beads for 2 min, using 1,440 fmol of RNA/μl of extract. The beads were removed from the extract by using a magnetic particle concentrator. A second round of depletion was done with 2,400 fmol of RNA/μl of extract. The beads were washed twice in Dignam buffer E, and then the proteins bound to the beads were eluted in Dignam buffer E containing 1 M KCl and dialyzed on a filter against 50 ml of Dignam buffer E for 30 to 60 min at 4°C. The protein concentration of the complete and depleted extracts was determined by the Bradford method (3a). Western blot analysis with anti-PTB antibodies was performed as described previously (54).
RESULTS
Isolation and analysis of rat α-actinin genomic clone.
In order to investigate alternative splicing of α-actinin, it was first necessary to obtain a genomic clone encompassing the alternatively spliced region. Oligonucleotide primers directed to the EF1a and EF2 exons, based on the chicken and human α-actinin sequences (43, 69), were used for RT-PCR of rat SM RNA to obtain two cDNA products encoding the EF hand region of the NM and SM α-actinin isoforms (54). The two cDNAs were used to make probes labeled with [α-32P]dCTP to screen a rat genomic library in λGEM11. A positive plaque was obtained, and the region containing the EF1a, NM, SM, and EF2 exons and the intervening introns was sequenced and is shown in Fig. 2. The exon sequence of the rat NM isoform (i.e., EF1a-NM-EF2) shows approximately 85% identity to the chicken sequence and 95% identity to the human sequence (69). The rat SM isoform (EF1a-SM-EF2) shows approximately 85% identity to the chicken sequence (the sequence of the human SM isoform has not yet been published).
The arrangement of the rat α-actinin genomic clone was broadly similar to that of the chicken gene (69) with the NM exon upstream of the SM exon. The introns between the EF1a, NM, SM, and EF2 exons are 954, 440, and 1,127 nt, respectively. Each intron has canonical GU and AG terminal dinucleotides, and the three 5′ splice sites show six of nine matches to the consensus sequence. The EF2 exon has a short pyrimidine tract adjacent to the 3′ splice site UAG, a short G/U tract, and a probable branch point in a reasonable consensus context 32 nt upstream of the exon. Sequence inspection showed that both the NM and SM exons had potential branch points within a good consensus context and with a 3′ adjacent pyrimidine tract a large distance upstream of the associated exon. For the NM exon, the identified probable branch point was 191 nt upstream of the exon at nt 911, while for the SM exon it was 386 nt upstream of the exon at nt 1237. In both cases, there were no AG dinucleotides between the potential branch point and the 3′ splice site, which has also been observed for other distant branch points (21, 27, 60). Despite the different 5′-to-3′ ordering of the NM and SM exons in the α-actinin and α-TM genes (Fig. 1), the apparent coregulation of alternative splicing of α-actinin and α-TM raises the possibility that the two genes share common regulatory elements. Consistent with this speculation, comparison of the rat α-actinin and α-TM gene sequences in the region of the mutually exclusive exons revealed some similarities. The repression of α-TM exon 3 in SM cells requires two negative regulatory elements in the introns flanking exon 3: an upstream regulatory element (URE), consisting of a short stretch containing three UGC motifs and a pyrimidine tract, and a downstream regulatory element (DRE) containing four UGC motifs and a pyrimidine tract (DY) (19, 20). The α-actinin NM exon is also flanked by two regions of UGC clusters, one approximately 380 nt upstream of the exon (nt 701 to 729) and the other approximately 150 nt downstream (nt 1319 to 1334 [Fig. 2]). Regulated splicing of α-TM exon 3 involves the binding of PTB to sites in the exon 3 polypyrimidine tract and the pyrimidine-rich stretch of the DRE (20, 50). Both of these elements contain copies of the optimal PTB binding site, which is UCUU within a pyrimidine-rich context (50). There are nine copies of the UCUU motif in the intron between the NM and SM exons, although some are in a more-favorable pyrimidine context than others (Fig. 2). This suggests the possibility that PTB may be involved in controlling selection of the actinin SM and NM exons. Also of interest is the presence of a number of GCAUG motifs, particularly the three copies lying between the putative branch point and the 3′ splice site of the NM exon (nt 968 to 972, 1018 to 1022, and 1068 to 1072). The repeated motif UGCAUG has been found to be involved in the activation of the rat fibronectin alternative exon EIIIB, and UGCAUG (or GCAUG) motifs have also been identified in the cis-acting elements involved in the regulation of the alternatively spliced genes c-src and calcitonin/CGRP (26, 30, 37). No such motifs are found in the region of the mutually exclusive exons of α-TM.
Steric interference enforces mutually exclusive splicing.
The identification of the potential branch point of the SM exon 386 nt upstream of the 3′ splice site suggested a simple explanation for the mutually exclusive behavior of the two exons. This branch point would be only 55 nt from the NM 5′ splice site, which is very close to the minimal distance required for spliceosome assembly (12, 55, 60, 73). Thus, mutually exclusive behavior could be due to a mechanism similar to that observed for the α-TM gene. Steric interference between the NM 5′ splice site and SM branch point could prevent spliceosome assembly between the two sites. In order to determine whether the NM and SM exons are absolutely mutually exclusive or whether in the absence of other splice sites they are able to splice together, the two exons and the intron between the exons were cloned into pGEM3Z to allow transcription in vitro. The plasmid was linearized at the BamHI site to provide a template for transcription. Following incubation in HeLa nuclear extract for 3 h, the α-actinin transcripts were not detectably spliced, indicating that splicing of the NM 5′ splice site to the SM 3′ splice site is extremely inefficient, even in the absence of other splice sites (Fig. 3, lane 2). To investigate the possibility that the distance between the NM 5′ splice site and SM branch points is responsible for this lack of splicing, the separation of the two sites was increased via the insertion of a 30-bp spacer sequence into the BglI site downstream of the NM 5′ splice site (nt 1224 [Fig. 2]). A small proportion of the full-length transcripts containing the spacer underwent 5′ exon cleavage (Fig. 3, lane 3), suggesting that the spacer had relieved the block to splicing and therefore that the proximity of the branch point to the NM 5′ splice site is at least partially responsible for the mutually exclusive nature of the two exons.
FIG. 3.
Increasing the distance between the 5′ splice site and the branch point and the length of truncation activate α-actinin NM-SM splicing. Full-length α-actinin transcripts (BamHI [B]) or transcripts truncated at the AflIII (A), NcoI (N), or EcoRI (E) site, with (+) or without (−) a 30-nt spacer between the NM 5′ splice site and putative branch point, were incubated with HeLa nuclear extract for 3 h, and the RNA species were resolved on an 8% polyacrylamide gel. The initial transcripts and splicing intermediates are indicated to the sides of the gel. The lariats for the BamHI and AflIII transcripts are not well resolved on gels with this percentage of polyacrylamide; the 5′ exon is the clearest diagnostic band for processing. The insertion of the spacer between the 5′ splice site and branch point activates splicing, particularly for transcripts truncated at the NcoI and EcoRI sites.
The fact that the spacer did not fully activate α-actinin splicing suggested that there may be additional mechanisms operating to suppress splicing of the NM to the SM exon in non-SM cells. In the cases of mutually exclusive splicing of the β-TM (13, 24, 29, 35) and αSTM exons (22), the downstream skeletal muscle-specific exon is repressed in NM cells, and in both cases, elements between the exon and the branch point mediate this default repression. The inability of the spacer to activate NM-SM splicing may indicate that the actinin SM exon is subject to similar repression in HeLa nuclear extracts. The possibility of repressor elements in the intron was investigated by examining the splicing of transcripts truncated at various points in the intron between the proposed branch point and SM exon in an attempt to remove potential repressor sequences. Templates for the truncated transcripts were produced by digesting the plasmid with AflIII (terminating 243 nt downstream of the putative branch point; nt 1476 [Fig. 2]), NcoI (terminating 67 nt downstream of the putative branch point; nt 1300 [Fig. 2]), or EcoRI (terminating 39 nt downstream of the putative branch point; nt 1272 [Fig. 2]). All of these transcripts retained the putative branch point and adjacent pyrimidine tract and so should be able to go through step 1 of splicing (29, 52, 62). Since the truncated transcripts lack a 3′ splice site, the 5′ exon and lariat intron should accumulate. As predicted, the truncations improved the efficiency of processing of the transcripts containing the spacer, consistent with the removal of repressor elements between the exon and the upstream branch point and pyrimidine tract (Fig. 3, lanes 5, 7, and 9). Nevertheless, the truncated transcripts lacking the spacer element were still not processed, confirming the importance of the location of the SM branch point with respect to the NM 5′ splice site (Fig. 3, lanes 4 to 9). Of the four spacer-containing transcripts, those truncated at the AflIII site (lane 5) appeared to undergo 5′ exon cleavage slightly more efficiently than the full-length transcripts, while transcripts truncated at the NcoI and EcoRI sites were much more efficiently processed (lanes 7 and 9). This implies that there are sequences between the polypyrimidine tract and SM exon which mediate repression of the SM exon in non-SM cells. Moreover, these data demonstrate that the region upstream of the EcoRI site contains a functional branch point and polypyrimidine tract and suggest that steric interference between this branch point and the 5′ splice site of the NM exon is responsible for the mutually exclusive nature of the exons.
Branch points hundreds of nucleotides upstream of NM and SM exons.
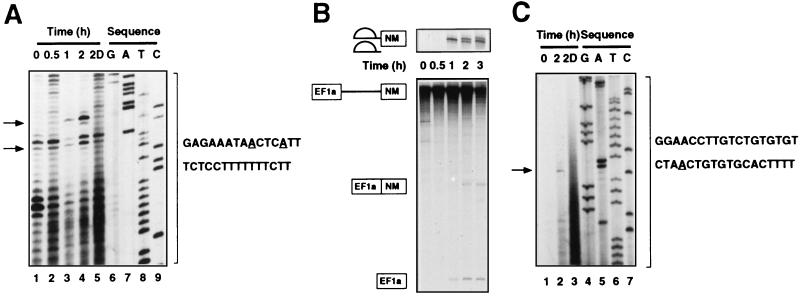
The branch point of the SM exon was mapped by RT primer extension analysis, using an antisense oligonucleotide directed to a region of the intron downstream of the putative branch points (nt 1267 to 1282 [Fig. 2]) and spacer-containing transcripts truncated at the NcoI site (Fig. 4A). The major extension product that accumulated in parallel with the splicing time course and which disappeared upon debranching prior to primer extension corresponded to arrest of RT at the branch point suggested by sequence analysis (nt 1237 [Fig. 2]). In addition, the adenosine at nt 1241, 4 nt further downstream, was also used as a branch point. Primer extension analysis on spliced full-length transcripts containing the spacer (using conditions in which splicing was activated [see below]), with a primer complementary to the 3′ end of the intron (nt 1608 to 1612 [Fig. 2]), failed to detect the use of any of the adenosines close to the 3′ splice site as a branch point (data not shown). Thus, the SM exon utilizes two branch points, located 386 and 382 nt upstream of the 3′ splice site (Fig. 2), which is well beyond the usual 18 to 40 nt. These branch points are only 55 and 59 nt downstream of the NM 5′ splice site, so activation of splicing by the spacer element is likely to be due to simple relief of steric interference between these branch points and the NM 5′ splice site.
FIG. 4.
Mapping the SM and NM branch points. (A) NcoI truncated α-actinin NM-SM transcripts, containing the spacer, were not processed or were spliced for the times indicated over the gels, followed by primer extension with an oligonucleotide complementary to nt 1267 to 1282. In addition, RNA from a 2-h splicing reaction was debranched (2D) prior to primer extension. The samples were run alongside a sequencing reaction with the same primer. The extended products arising from arrest of the RT at the branch points at A1237 and A1241 are indicated by the arrows, and the corresponding branch points in the sequence are underlined. Use of the minor branch point at nt 1241 is indicated by the increased proportion of the lower of the two bands that are present as background in the unprocessed RNA sample. The 1-h timepoint in this experiment was loaded incorrectly, resulting in the anomalously low signal. (B) Splicing of transcripts containing EF1a and NM exons and intron between these two exons. Transcripts were not processed or were incubated in HeLa nuclear extract for the times indicated. The upper gel has a lower percentage of polyacrylamide to facilitate resolution of the lariats. (C) Transcripts containing the EF1a and NM exons were not processed, spliced for 2 h, or spliced for 2 h and debranched (2D [lane 3]), followed by primer extension with an oligonucleotide complementary to nt 934 to 951. The extended product is indicated by an arrow, and the corresponding branch point at A911 is underlined.
The NM branch point was mapped using transcripts containing the EF1a and NM exons and intron between the two exons. Figure 4B shows a time course of splicing of this transcript, with the appearance first of the 5′ exon and lariat intermediate after 30 min of splicing, followed by the spliced product and lariat intron from 1 h. The NM branch point was mapped by primer extension analysis, using a primer complementary to the last 15 nt of the intron between the EF1a and NM exons (nt 1087 to 1101 [Fig. 2]). Although there is a potential branch point adenosine, followed by a stretch of 15 pyrimidine residues, located 59 nt upstream of the NM 3′ splice site, no extension products corresponding to the use of this adenosine (or any other adenosine in this region) as a branch point were detected. However, an extension product of approximately 190 nt was observed (data not shown). To more precisely map this branch point, an antisense oligonucleotide directed to a sequence in the intron further upstream from the 3′ splice site (nt 934 to 951 [Fig. 2]) was used (Fig. 4C). This indicated that the adenosine 191 nt upstream of the 3′ splice site, which was suggested first by sequence analysis, was indeed the NM branch point (nt 911 [Fig. 2]). Thus, both of the mutually exclusive exons of α-actinin utilize distant branch points. Unlike the SM branch point, the distant location of the NM branch point is presumably not required to enforce the mutually exclusive splicing pattern. However, it seems likely that the extensive region between the NM branch point and 3′ splice site AG contains elements involved in regulation of tissue-specific splicing.
Increasing the distance between the 5′ splice site and the branch point overcomes mutually exclusive splicing in vivo.
In order to analyze α-actinin splicing patterns in vivo, HeLa cells were transiently transfected with an α-actinin construct extending from the EF1a exon to the EF2 exon. RT-PCR analysis showed that virtually all of the RNA derived from the α-actinin construct contained the NM exon (Fig. 5A, lane 1), with only a very small proportion of RNA containing the SM exon instead (approximately 2%, as determined by PhosphorImager analysis [Fig. 5B]). Transfection of a construct containing the 30-nt spacer between the NM 5′ splice site and the SM branch point resulted in substantial amounts of RNA containing both the NM and SM exon sequences (Fig. 5A, lane 2). This confirms the results obtained in vitro that the mutually exclusive behavior of these two exons could be at least partially overcome by increasing the distance between the NM 5′ splice site and the branch point. Approximately 40% of the transcripts with the spacer contained both exons, with the remainder mostly containing the NM exon (Fig. 5B), suggesting that the SM exon is still subject to some degree of repression in HeLa cells. The experiments with the truncated α-actinin transcripts suggested that repressor elements lie in the region of intron between the EcoRI and AflIII sites (Fig. 3). Deletion of this region had little effect on its own in vivo (Fig. 5A, lane 3), but in combination with the spacer, there was a small, but consistently observed, increase in the proportion of RNA containing both the NM and SM exons (Fig. 5A, lane 4; Fig. 5B). These results are qualitatively consistent with the in vitro data. The quantitative differences in the effects of the intron truncations in vivo and in vitro may reflect the differences between the reporter constructs—single intron in vitro and three introns in vivo.
FIG. 5.
Increasing the distance between the 5′ splice site and the branch point activates α-actinin NM-SM splicing in vivo. (A) HeLa cells were transiently transfected with α-actinin constructs. An α-actinin construct extending from the EF1a exon to the EF2 exon (lane 1), a construct containing a 30-bp spacer element between the NM 5′ splice site and SM branch point (lane 2), a construct containing an EcoRI-AflIII (E-A) deletion in the intron between the NM and SM exons (lane 3), or a construct with both the spacer element and deletion (lane 4). (B) PhosphorImager analysis was performed, the intensity of each of the three products was expressed as a percentage of the total signal, and the mean and standard deviation of the results of five experiments were calculated. Increasing the distance between the 5′ splice site and the branch point activates α-actinin NM-SM splicing in vivo.
Derepression of the α-actinin SM exon.
The preceding data suggested that NM-SM splicing in HeLa extract was prevented not only by steric interference but also by repression mediated by sequences between the SM exon and upstream pyrimidine tract. Relieving steric interference by the spacer element in vitro could therefore be observed only in transcripts that lacked the SM exon and could undergo only step 1 of splicing. We next attempted to demonstrate relief of steric interference in full-length NM-SM transcripts by using alternative approaches to antagonize the repression of splicing. The first such approach was to preincubate transcripts with SR proteins, which promote early steps in spliceosome assembly (reviewed in references 11, 39, and 66). Preincubation with SR proteins can commit pre-mRNAs to splicing in nuclear extracts that have been challenged with excess splicing substrates (10). Preincubation of SR proteins with full-length and NcoI-truncated actinin transcripts, with and without the spacer element, led to only a modest increase in the efficiency of processing of each type of transcript (data not shown).
The increased efficiency of processing of the truncated transcripts observed in Fig. 3 and 5 implied that the α-actinin transcripts may bind factors in the HeLa nuclear extract that repress splicing of the SM exon. Our second approach to antagonize the repression of α-actinin splicing was to supplement the HeLa extract with competitor RNAs containing the sequences that had been removed in the truncated RNAs. If these sequences bind factors that cause repression of splicing, then addition of the competitor RNAs should sequester repressors in the nuclear extract and thus allow splicing to occur (2, 6, 20). An unlabeled α-actinin competitor RNA consisting of the 3′ portion of the intron (nt 1302 to 1610 [Fig. 2]) was used in splicing reaction mixtures containing the full-length transcripts with the spacer. This competitor RNA caused an activation of α-actinin splicing (Fig. 6, lanes 3 to 6), consistent with titration of repressors by the competitor. However, step 2 of splicing was still relatively inefficient, as indicated by the low levels of spliced product. This is perhaps not unexpected, as longer distances between the branch point and 3′ splice site are associated with slower kinetics of step 2 of splicing in vitro (7). The α-actinin competitor RNA contains six optimal PTB binding UCUU motifs. We have previously shown that the DY negative element in the α-TM gene, which contains two overlapping UCUU motifs, can alter α-TM in vitro splicing patterns by binding and sequestering PTB (20). We therefore tested the ability of the DY RNA to activate splicing of the α-actinin transcript. The DY competitor also activated α-actinin splicing (Fig. 6, lanes 7 to 10) over a similar concentration range (22.5 to 225 nM) (Fig. 6). The estimated concentration of PTB in the diluted nuclear extracts is ∼180 nM (20). Therefore, the activity of the competitor RNAs is compatible with their binding of PTB, especially since it is likely that both RNAs would be able to bind more than one molecule of PTB. The α-actinin RNA competitor can also affect the α-TM splicing pattern (data not shown), suggesting that the α-actinin and α-TM competitor RNAs bind similar regulatory factors. A mutant α-TM DY competitor which does not bind PTB, DYΔPC (20), was virtually ineffective in activating splicing (Fig. 6, lanes 11 and 12), as was an α-actinin competitor RNA truncated at the ClaI site in the spacer and which therefore lacks UCUU motifs (lanes 13 and 14). These data suggest that PTB is a candidate for the activity that represses SM exon splicing in HeLa nuclear extract.
FIG. 6.
Derepression of the α-actinin SM exon by RNA competitors. Full-length α-actinin transcripts, containing the spacer, were not processed (lane 1) or were spliced for 3 h in the absence (−) (lane 2) or presence of a 10-, 25-, 50-, or 100-fold molar excess over the pre-mRNA (22.5, 56, 113, or 225 nM) of α-actinin competitor RNA (lanes 3 to 6, respectively); 10-, 25-, 50-, or 100-fold molar excess of α-TM DY competitor RNA (lanes 7 to 10); 50- or 100-fold molar excess of α-TM DYΔPC competitor RNA (lanes 11 and 12); or 50- or 100-fold molar excess of the α-actinin ClaI competitor RNA (lanes 13 and 14). The RNA species were resolved on a 12% polyacrylamide gel so that the lariats migrated above the pre-mRNA. α-Actinin NM-SM splicing was activated by both the α-actinin and α-TM DY competitors but not the truncated actinin or mutant TM competitors which lack UCUU motifs.
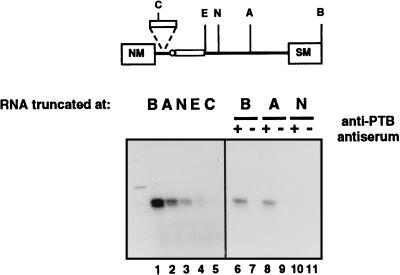
To directly test whether PTB binds to the actinin RNA, UV cross-linking of HeLa extract proteins was carried out with transcripts containing the spacer, i.e., full-length (BamHI), or truncated at the AflIII, NcoI, or EcoRI site or at the ClaI site in the spacer (Fig. 7). The full-length transcript contains nine UCUU motifs, the AflIII transcript contains five, and the NcoI and EcoRI transcripts contain two motifs each, while the ClaI transcript does not contain any. A prominent doublet at approximately 58 kDa, the expected mobility for PTB (16, 48), was observed for the full-length transcripts (lane 1). Under various conditions, virtually no other proteins were observed to cross-link to these RNAs. The intensity of this doublet decreased with the progressive truncations and appeared to relate to the number of UCUU motifs remaining in the transcript. Immunoprecipitation experiments were performed to test whether the cross-linked bands were PTB. The cross-linked doublet was immunoprecipitated by anti-PTB serum (Fig. 7, lanes 6, 8, and 10) but not by preimmune serum (lanes 7, 9, and 11), confirming that PTB binds to the α-actinin transcripts. Although this technique is not strictly quantitative, it is interesting that the amount of cross-linked PTB was much less for the NcoI and EcoRI transcripts which were more active in splicing assays (compare Fig. 7 and 3).
FIG. 7.
UV cross-linking of HeLa extract proteins with α-actinin transcripts. Cross-linking reactions of α-actinin transcripts containing the spacer i.e., full-length (BamHI [B]), or truncated at the AflIII (A), NcoI (N), or EcoRI (E) site or at the ClaI (C) site in the spacer were done in HeLa nuclear extract (lanes 1 to 5). Cross-linking reactions of the full-length and AflIII and NcoI truncated transcripts were immunoprecipitated with anti-PTB antiserum (+) or preimmune serum (−) (lanes 6 to 11). PTB cross-links to α-actinin sequences, and this cross-link decreases with progressive truncations of the α-actinin intron.
rPTB restores repression to PTB-depleted extracts.
To further investigate the role of PTB in the repression of the α-actinin SM exon, we attempted to deplete HeLa nuclear extract of PTB, using the α-TM DY RNA which binds PTB (20). Biotinylated α-TM DY RNA was bound to streptavidin magnetic beads and incubated in the nuclear extract, and the resulting RNA-protein complexes were removed. Following two rounds of depletion, Western blot analysis using an anti-PTB antibody showed that most of the PTB had been removed from the extract by this method (Fig. 8A, lanes 7 and 8). Approximately 15% of the PTB remained in the extract, as estimated by comparison with serial dilutions of complete extract (lanes 2 to 6), and this residual pool of PTB remained resistant to further rounds of depletion. The proteins retained on the streptavidin beads were eluted with 1 M KCl and shown to contain PTB (lane 9). If PTB were responsible for repression of the α-actinin SM exon in HeLa extract, then its removal from the extract should allow splicing of the spacer-containing α-actinin transcripts. As observed previously, this transcript was not spliced in the complete extract (Fig. 8B, lane 2), but splicing could be activated by 240 nM α-TM DY RNA (lane 4). Splicing of the actinin transcript was also activated in the PTB-depleted extract (lane 5) but not in a mock-depleted extract (lane 3). The activation of splicing was usually not as strong as that observed with the competitor RNA in complete extract, probably due to the residual PTB in the extract. Adding the eluted proteins back into the depleted extract inhibited splicing (Fig. 8B, lanes 6 and 7), as expected. The addition of rPTB (86, 172, and 345 nM [lanes 8 to 10]) also restored the inhibition, confirming that PTB is able to repress splicing of the α-actinin SM exon. Note that the middle concentration of rPTB is equivalent to the original concentration of total PTB in the extract and that this concentration causes complete repression. A number of control experiments were carried out to ascertain the specificity of this repression by PTB. First, to determine whether the repression was specific to PTB, two other RNA binding proteins, sex-lethal (sxl) and unr, were tested. sxl is a negative regulator of splicing that binds pyrimidine-rich sequences but with a distinct optimal binding site from PTB (58). unr is a protein with five cold shock RNA binding domains that binds single-stranded RNA with high affinity (31). Neither protein had an effect on α-actinin splicing comparable to PTB, although unr had a slight effect at the very highest concentration (Fig. 8B, lanes 11 to 13 and 14 to 16). Next we tested the effects of deleting PTB and then adding it back upon an unrelated pre-mRNA under the same conditions. β-Globin transcripts were efficiently spliced in both the complete HeLa nuclear extract (Fig. 8C, lane 2) and the PTB-depleted extract (lane 3), and the further addition of PTB had no detectable effect upon splicing of the transcripts (lanes 4 to 6). Taken together, these experiments demonstrate that PTB represses splicing of the α-actinin SM exon in HeLa extracts.
FIG. 8.
Depletion of PTB from HeLa nuclear extract activates α-actinin splicing. (A) PTB was depleted from HeLa nuclear extract by using biotinylated α-TM DY RNA bound to streptavidin magnetic beads. Western blot analysis with an anti-PTB antibody was performed on recombinant His-tagged PTB (His-PTB) (25 ng) (lane 1), serial dilutions of undepleted HeLa nuclear extract (NE) (0.25 to 2 μl) (lanes 2 to 6), equal protein loads (18 μg) of complete nuclear extract (NE) and depleted extract (DEP) (lanes 7 & 8), and on an equivalent volume of the proteins eluted from the beads (EP) (1 μl) (lane 9). The majority of the PTB was removed from the extract (80 to 90% as estimated by comparison with serial dilutions of undepleted extract). (B) Full-length α-actinin transcripts, containing the spacer, were not processed (lane 1) or were spliced for 3 h in the complete nuclear extract (NE) (lane 2), mock-depleted (MOCK DEP) extract (lane 3), complete extract in the presence of 240 nM of α-TM DY competitor RNA (lane 4) or PTB-depleted extract (−) (lane 5). The proteins eluted from the streptavidin beads (1 and 2 μl) were included in splicing reactions with the depleted extract (lanes 6 and 7, respectively), or recombinant His-tagged PTB (lanes 8 to 10), sxl (lanes 11 to 13), or unr (lanes 14 to 16) was added to the reaction mixtures to a final concentration of 86, 172, or 345 nM, respectively. α-Actinin splicing is activated in PTB-depleted HeLa nuclear extract. This activation can be reversed by the addition of rPTB but not by the RNA binding proteins sxl and unr. (C) β-Globin transcripts were not processed (lane 1) or were incubated with complete HeLa nuclear extract (NE) (lane 2), depleted extract (lane 3), or depleted extract with rPTB at a concentration of 86, 172, or 345 nM (lanes 4 to 6, respectively).
DISCUSSION
In this study, we have begun to analyze the mutually exclusive splicing of α-actinin pre-mRNA, with a view to comparing its regulation with that of α-TM. We anticipated that this should allow us to distinguish which features of control are generally essential for SM-specific splicing and which are specific for each pre-mRNA.
Mutually exclusive splicing of α-actinin caused by steric hindrance.
Although the intron between the mutually exclusive exons of α-actinin is 440 nt long, the two SM branch points are located only 55 and 59 nt downstream of the NM 5′ splice site, suggesting that the mutually exclusive splicing of α-actinin is enforced by a steric hindrance mechanism, as is the case for α-TM (60). The insertion of a 30-nt spacer to increase the distance between the NM 5′ splice site and SM branch points activated NM-SM splicing, as long as measures had been taken to alleviate the constitutive repression of the SM exon. The minimal distances between the 5′ splice site and the branch point determined for other introns are 51 to 59 nt for the intron between exons 2 and 3 of α-TM (60), 47 to 54 nt for the human β-globin first intron (55), 43 to 55 nt for the rabbit β-globin second intron (73), and 46 to 48 nt for the small t intron (12). The actinin SM branch points are not below the absolute threshold distance from the 5′ splice site, and indeed, there does not appear to be an absolute block to splicing, since a small amount of NM-SM splicing could be observed in HeLa cells transfected with the native actinin construct (Fig. 5). This is similar to the SV40 small t intron where the distance between the 5′ splice site and the branch point is very slightly above the minimum, and the disadvantage of the steric effect may be overcome by the addition of high concentrations of the SR protein ASF/SF2 (12, 17). However, a complete block to splicing may not be necessary to maintain mutually exclusive splicing of α-actinin in vivo. Splicing of the NM 5′ splice site to the SM 3′ splice site may be so inefficient that each splice site is likely to interact more efficiently with the flanking constitutive splice sites, which would be sufficient to enforce mutually exclusive behavior. Furthermore, steric interference may be reinforced by the mechanisms regulating tissue-specific splicing, for example, if the SM exon is repressed in NM tissue, then only the NM exon may be selected in such cell types. α-Actinin is only the second identified example of mutually exclusive splicing imposed by a steric hindrance mechanism. While the downstream exon of the rat and chicken β-TM mutually exclusive exon pairs also utilize distant branch points, they are not so close to the 5′ splice site to prevent splicing, and it is the mechanisms involved in the regulation of β-TM tissue-specific splicing that maintain the mutually exclusive behavior (21, 27–29, 35). The MLC1/3 gene also has a pair of internal mutually exclusive exons (51, 63), but in this case the mutually exclusive behavior is due to a hierarchy of compatibilities between specific pairs of 5′ and 3′ splice sites (15).
Given that the overwhelming majority of branch points are located 18 to 40 nt upstream of the 3′ splice site, the locations of the α-actinin SM branch points, 382 and 386 nt upstream from the 3′ splice site, are most unusual. The downstream exons of the α- and β-TM mutually exclusive exon pairs also utilize distant branch points, and this use may be a feature of mutually exclusive exons. Such an arrangement may not only enforce mutually exclusive behavior in some cases but more generally may provide space for regulatory sequences between the branch point and 3′ splice site, as in the α-, β- and αSTM genes (19, 22, 29). The accommodation of regulatory elements is a possible explanation for the location of the NM branch point 191 nt from its 3′ splice site, since this cannot be involved in a steric hindrance mechanism. Despite the extreme distance between the branch points and SM exon, the SM 3′ splice site is at the first AG dinucleotide downstream from the branch points, as is also the case for the distant branch points of the α-actinin NM exon and α- and β-TM (21, 27, 60). This is consistent with some form of scanning mechanism during step two of splicing that recognizes the first AG dinucleotide downstream of the branch point-polypyrimidine tract (59, 62).
Repression of the SM exon in non-SM cells by PTB.
Although the proximity of the SM branch points to the NM 5′ splice site was an important factor in preventing NM-SM splicing, increasing the distance between the 5′ splice site and the branch point did not fully activate splicing, suggesting that the SM exon is normally repressed in NM cells. Splicing could be activated by truncation of the transcripts between the polypyrimidine tract and SM 3′ splice site, presumably due to the removal of repressor sequences in this region. Splicing could also be activated by α-actinin and α-TM competitor RNAs, which suggests that the competitor RNAs bind and titrate away factors in the nuclear extract that usually inhibit use of the SM exon. This repressor activity appears to be due at least in part, and perhaps solely, to PTB. The competitor RNAs contain optimal PTB binding sites, and UV cross-linking and immunoprecipitation studies indicated that PTB binds to both the α-actinin transcripts and to the α-TM DY competitor RNA (20). It is of note that the truncations of the α-actinin intron which activated splicing (NcoI and EcoRI) removed all but two of the nine UCUU motifs and significantly reduced the amount of PTB cross-link observed. Furthermore, a deletion in the DY element that impaired its binding to PTB also impaired its ability to activate α-actinin splicing. However, the strongest evidence for a repressive role for PTB in α-actinin splicing was provided by the experiments in which PTB was deleted and then added back (Fig. 8). Removal of more than 80% of PTB from the HeLa extract resulted in activation of actinin splicing. Inhibition could be restored by the addition of rPTB but not other RNA binding proteins. This is the first demonstration of depletion of PTB from nuclear extract without an accompanying loss of splicing activity. Depletion of PTB from nuclear extracts using antibodies results in loss of splicing activity, most likely due to the removal of other proteins, such as PSF (48, 49). Depletion of PTB from rabbit reticulocyte lysates has been achieved by passing extracts over an RNA affinity column containing a PTB binding domain from encephalomyocarditis virus (32). However, we found that this method was not effective for our experiments, partly due to release of RNA from the column by nucleases in the extract (20a). Only by carefully titrating the amount of RNA required to activate actinin splicing and then rapidly removing the PTB-RNA complexes with magnetic streptavidin beads were we able to deplete the majority (80 to 90%) of the PTB from the extract without loss of splicing activity and with minimal competitor RNA remaining in the extract. The reason for the inability to deplete the remaining 10 to 20% of PTB in the extracts remains unclear. Successive rounds of depletion with biotinylated RNA eventually led to inhibition of splicing without removing the residual pool of PTB. Electrophoretic mobility shift assays and supershifts using anti-PTB antiserum show that the remaining PTB is still competent for RNA binding. The possibility that there may be an authentic functional difference between the two pools of PTB (as defined by their ability to be depleted) was suggested by the effect of the depleted extract on in vitro splicing of TM RNAs. Despite the fact that addition of TM DY competitor RNA to complete extract alters the balance of TM splicing away from exon 2-4 and towards exon 3-4 splicing (20), TM splicing is the same in depleted and complete extracts. However, smaller amounts of the competitor RNA are required to alter the TM splicing pattern in the depleted extract (20a). This suggests that alternative splicing of TM and actinin is affected by different pools of PTB. We are currently attempting to develop conditions that will allow complete depletion of PTB and to reveal the basis of the different behaviors of the two pools. Nevertheless, our data are clear that rPTB can confer repression upon splicing of actinin SM exon. The approach in which PTB is deleted and then added back provides more-rigorous evidence than previous experiments in which PTB has been added back to extracts that still contain the PTB binding competitor RNA (2, 6, 20). In the latter type of experiment, it is possible for the recombinant protein to displace the authentic repressors from the competitor RNA, thus allowing them to restore inhibition of splicing. For instance, we found that the unr protein, which was much less potent than PTB at restoring inhibition to depleted extracts (Fig. 8), was as effective as PTB when it was added into extracts that still contained the competitor RNA (data not shown).
PTB is emerging as a regulator of alternative splicing in a number of systems (reviewed in reference 65). In addition to a role in the regulation of α-TM splicing, PTB has been implicated in the regulation of alternative splicing of β-TM, c-src, and γ2 pre-mRNAs (2, 6, 23, 46, 58). Repression of the skeletal muscle-specific exon 7 of rat β-TM in NM tissue requires sequences between the branch points and the 3′ splice site (29). PTB has been found to bind in this region, and mutations that result in the use of exon 7 in NM cells in vivo also disrupt binding of PTB in vitro, suggesting that the interaction of PTB with the intron regulatory sequences upstream of exon 7 may play a role in its repression in NM tissues (46). c-src and γ2 pre-mRNAs have cassette exons that are included in the mature mRNA in neurons but skipped in other cell types (40, 70). In both cases, a short RNA segment containing the 3′ splice site region upstream of the cassette exon was able to titrate away factors in HeLa extract to allow splicing of the neuron-specific exon in a nonneuronal environment. The RNA competitors were shown to bind PTB, and exon skipping could be restored by the addition of rPTB in the presence of the competitor (2, 6). In this respect, α-actinin splicing resembles these three systems rather than α-TM in that PTB enforces the default splicing pattern through repression of the tissue-specific exon. In contrast, in α-TM splicing, PTB is involved in repression of the default exon which then allows selection of the SM-specific exon, i.e., the tissue-specific splicing pathway. In each case, however, PTB is acting as an inhibitory factor in splicing. Experiments with α- and β-TM suggested that PTB may simply compete with U2AF for binding to specific regulated polypyrimidine tracts (38, 58) in a manner analogous to the way in which sex-lethal protein regulates the splicing of transformer pre-mRNA (68). In support of this view, optimal PTB binding sites are present in the appropriate polypyrimidine tracts of α- and β-TM (50). However, even in these cases, the inhibition of splicing also requires additional regulatory elements, some of which also bind PTB, such as the DY element downstream of TM exon 3 (19, 20, 50). In the c-src gene, PTB binding sites on both sides of the N1 exon collaborate to inhibit the 5′ splice site of the exon (6), and in the γ2 gene, PTB binding sites are present on either side of the branch point, which would be consistent with direct inhibition not only of U2AF binding but also of U2 small nuclear RNP (2). The common themes in these examples is that more than one PTB binding site is required for repression of splicing and that, depending upon the locations of the sites, splicing can be inhibited in a number of ways. Although we have not dissected the precise binding sites for PTB, the presence of multiple UCUU motifs (50) in the region of the actinin SM exon suggests that a large inhibitory PTB-containing complex may be involved in this case too.
Regulation of α-actinin tissue-specific splicing.
For α-TM, the NM exon (exon 3) is the default choice due to its functionally stronger splice site elements which outcompete those of the SM-specific exon (exon 2). In SM cells, exon 3 is specifically inhibited, allowing selection of exon 2 instead. For α-actinin, the in vitro data suggest that the SM exon is repressed in NM tissues, raising the questions of how it is activated in SM and whether the NM exon is specifically repressed in SM or simply outcompeted by the activated SM exon. The coregulation of α-actinin with α-TM in fibroblasts which have been selected for URE- and DRE-mediated repression of α-TM exon 3 splicing (54) suggests that the α-actinin NM exon should also be repressed in SM cells. As for α-TM, this repression could be mediated by the flanking UGC motifs, possibly via interaction with a SM-specific factor(s) (19, 20, 54). However, the UGC clusters are not as closely associated with the actinin NM exon as they are with TM exon 3, and whether this difference in location reflects a different role in the regulation of actinin splicing remains to be determined. We plan to address the regulation of α-actinin tissue-specific splicing by transient transfections of SM and NM cell lines.
Our results suggest that the regulation of α-actinin alternative splicing has features in common with both α-TM and β-TM as well as some novel characteristics. The basis for the mutually exclusive behavior and the presence of UGC clusters flanking the NM exon are reminiscent of α-TM, although the role of the actinin UGC clusters has yet to be determined. On the other hand, the constitutive repression of the downstream exon of the two mutually exclusive exons mediated by sequences between the pyrimidine tract and 3′ splice site resembles β- and αSTM. This initial report on the regulation of α-actinin alternative splicing opens up many avenues for further investigation and validates the choice of α-actinin for study, not only for comparison with α-TM splicing but also as an interesting example of alternative splicing in its own right.
ACKNOWLEDGMENTS
We thank Paul Kemp for the genomic library, Jim Patton for the recombinant His-tagged PTB clone, and Sarah Hunt and Richard Jackson for the PTB antibodies, preimmune serum, and recombinant unr. We also thank Gavin Roberts for critically reading the manuscript.
This work was supported by a Wellcome Travelling Research Fellowship to J.S. and grants from the Wellcome Trust (052968) and Medical Research Council (G9417692) to C.W.J.S.
REFERENCES
- 1.Adams M D, Rudner D Z, Rio D C. Biochemistry and regulation of pre-mRNA splicing. Curr Opin Cell Biol. 1996;8:331–339. doi: 10.1016/s0955-0674(96)80006-8. [DOI] [PubMed] [Google Scholar]
- 2.Ashiya M, Grabowski P J. A neuron-specific splicing switch mediated by an array of pre-mRNA repressor sites: evidence of a regulatory role for the polypyrimidine tract binding protein and a brain-specific counterpart. RNA. 1997;3:1–20. [PMC free article] [PubMed] [Google Scholar]
- 3.Black D L. Activation of c-src neuron-specific splicing by an unusual RNA element in vivo and in vitro. Cell. 1992;69:795–807. doi: 10.1016/0092-8674(92)90291-j. [DOI] [PubMed] [Google Scholar]
- 3a.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Byrne B J, Kaczorowski Y J, Coutu M D, Craig S W. Chicken vinculin and meta-vinculin are derived from a single gene by alternative splicing of a 207 base pair exon unique to meta-vinculin. J Biol Chem. 1992;267:12845–12850. [PubMed] [Google Scholar]
- 5.Caceres J F, Stamm S, Helfman D M, Krainer A R. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- 6.Chan R C, Black D L. The polypyrimidine tract binding protein binds upstream of the neural-specific c-src exon N1 to repress splicing of the intron downstream. Mol Cell Biol. 1997;17:4667–4676. doi: 10.1128/mcb.17.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiara M D, Palandjian L, Kramer R F, Reed R. Evidence that U5 snRNP recognizes the 3′ splice site for catalytic step II in mammals. EMBO J. 1997;15:4746–4759. doi: 10.1093/emboj/16.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyfuss G, Matunis M J, Pinol Roma S, Burd C G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 9.Eperon L P, Estibeiro J P, Eperon I C. The role of nucleotide sequences in splice site selection in eukaryotic pre-messenger RNA. Nature. 1986;324:280–282. doi: 10.1038/324280a0. [DOI] [PubMed] [Google Scholar]
- 10.Fu X D. Specific commitment of different pre-mRNAs to splicing by single SR proteins. Nature. 1993;365:82–85. doi: 10.1038/365082a0. [DOI] [PubMed] [Google Scholar]
- 11.Fu X D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 12.Fu X-Y, Colgan J D, Manley J L. Multiple cis-acting sequence elements are required for efficient splicing of simian virus 40 small-t antigen pre-mRNA. Mol Cell Biol. 1988;8:3582–3590. doi: 10.1128/mcb.8.9.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallego M E, Balvay L, Brody E. cis-Acting sequences involved in exon selection in the chicken beta-tropomyosin gene. Mol Cell Biol. 1992;12:5415–5425. doi: 10.1128/mcb.12.12.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego M E, Gattoni R, Stevenin J, Marie J, Expert-Bezancon A. The SR splicing factors ASF/SF2 and SC35 have antagonistic effects on intronic enhancer-dependent splicing of the β-tropomyosin alternative exon 6A. EMBO J. 1997;16:1772–1784. doi: 10.1093/emboj/16.7.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallego M E, Nadal-Ginard B. Myosin light-chain 1/3 gene alternative splicing: cis regulation is based upon a hierarchical compatibility between splice sites. Mol Cell Biol. 1990;10:2133–2144. doi: 10.1128/mcb.10.5.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Blanco M A, Jamison S F, Sharp P A. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 1989;3:1874–1886. doi: 10.1101/gad.3.12a.1874. [DOI] [PubMed] [Google Scholar]
- 17.Ge H, Manley J L. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990;62:25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- 18.Ghetti A, Pinol Roma S, Michael W M, Morandi C, Dreyfuss G. hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 1992;20:3671–3678. doi: 10.1093/nar/20.14.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooding C, Roberts G C, Moreau G, Nadal Ginard B, Smith C W J. Smooth muscle-specific switching of alpha-tropomyosin mutually exclusive exon selection by specific inhibition of the strong default exon. EMBO J. 1994;13:3861–3872. doi: 10.1002/j.1460-2075.1994.tb06697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooding C G, Roberts G C, Smith C W J. Role of an inhibitory pyrimidine-element and general pyrimidine-tract binding proteins in regulation of α-tropomyosin alternative splicing. RNA. 1998;4:85–100. [PMC free article] [PubMed] [Google Scholar]
- 20a.Gooding, C., and C. W. J. Smith. Unpublished observations.
- 21.Goux-Pelletan M, Libri D, d’Aubenton-Carafa Y, Fiszman M, Brody E, Marie J. In vitro splicing of mutually exclusive exons from the chicken β-tropomyosin gene: role of the branch point and very long pyrimidine stretch. EMBO J. 1990;9:241–249. doi: 10.1002/j.1460-2075.1990.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham I R, Hamshere M, Eperon I C. Alternative splicing of a human alpha-tropomyosin muscle-specific exon: identification of determining sequences. Mol Cell Biol. 1992;12:3872–3882. doi: 10.1128/mcb.12.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman J S, Meyer M I, Wang Y-C, Mulligan G J, Kobayashi R, Helfman D M. The use of antibodies to the polypyrimidine tract binding protein (PTB) to analyze the protein components that assemble on alternatively spliced pre-mRNAs that use distant branch points. RNA. 1998;4:613–625. doi: 10.1017/s1355838298971448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo W, Mulligan G J, Wormsley S, Helfman D M. Alternative splicing of beta-tropomyosin pre-mRNA: cis-acting elements and cellular factors that block the use of a skeletal muscle exon in nonmuscle cells. Genes Dev. 1991;5:2096–2107. doi: 10.1101/gad.5.11.2096. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K, Yano H, Hashida T, Takeuchi R, Takeda O, Asada K, Takahashi E, Kato I, Sobue K. Genomic structure of the human caldesmon gene. Proc Natl Acad Sci USA. 1992;89:12122–12126. doi: 10.1073/pnas.89.24.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedjran F, Yeakley J M, Huh G S, Hynes R O, Rosenfeld M G. Control of alternative pre-mRNA splicing by distributed pentameric repeats. Proc Natl Acad Sci USA. 1997;94:12343–12347. doi: 10.1073/pnas.94.23.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helfman D M, Ricci W M. Branch point selection in alternative splicing of tropomyosin pre-messenger RNAs. Nucleic Acids Res. 1989;17:5633–5650. doi: 10.1093/nar/17.14.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helfman D M, Ricci W M, Finn L A. Alternative splicing of tropomyosin pre-mRNAs in vitro and in vivo. Genes Dev. 1988;2:1627–1638. doi: 10.1101/gad.2.12a.1627. [DOI] [PubMed] [Google Scholar]
- 29.Helfman D M, Roscigno R F, Mulligan G J, Finn L A, Weber K S. Identification of two distinct intron elements involved in alternative splicing of beta-tropomyosin pre-mRNA. Genes Dev. 1990;4:98–110. doi: 10.1101/gad.4.1.98. [DOI] [PubMed] [Google Scholar]
- 30.Huh G S, Hynes R O. Regulation of alternative pre-mRNA splicing by a novel repeated hexanucleotide element. Genes Dev. 1994;8:1561–1574. doi: 10.1101/gad.8.13.1561. [DOI] [PubMed] [Google Scholar]
- 31.Hunt, S. L., J. J. Hsuan, N. Totty, and R. J. Jackson. unr, a cellular cytoplasmic RNA-binding protein with five cold shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev., in press. [DOI] [PMC free article] [PubMed]
- 32.Kaminski A, Hunt S L, Patton J G, Jackson R J. Direct evidence that polypyrimidine tract binding protein (PTB) is essential for internal initiation of translation of encephalomyocarditis virus RNA. RNA. 1995;1:924–938. [PMC free article] [PubMed] [Google Scholar]
- 33.Kanopka A, Muhlemann O, Akusjarvi G. Inhibition by SR proteins of splicing of a regulated adenovirus pre-mRNA. Nature. 1996;381:535–538. doi: 10.1038/381535a0. [DOI] [PubMed] [Google Scholar]
- 34.Krainer A R, Conway G C, Kozak D. The essential pre-mRNA splicing factor SF2 influences 5′ splice site selection by activating proximal sites. Cell. 1990;62:35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- 35.Libri D, Balvay L, Fiszman M Y. In vivo splicing of the beta tropomyosin pre-mRNA: a role for branch point and donor site competition. Mol Cell Biol. 1992;12:3204–3215. doi: 10.1128/mcb.12.7.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libri D, Piseri A, Fiszman M Y. Tissue-specific splicing in vivo of the beta-tropomyosin gene: dependence on an RNA secondary structure. Science. 1991;252:1842–1845. doi: 10.1126/science.2063196. [DOI] [PubMed] [Google Scholar]
- 37.Lim L P, Sharp P A. Alternative splicing of the fibronectin EIIIB exon depends on specific TGCATG repeats. Mol Cell Biol. 1998;18:3900–3906. doi: 10.1128/mcb.18.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin C H, Patton J G. Regulation of alternative 3′ splice site selection by constitutive splicing factors. RNA. 1995;1:234–245. [PMC free article] [PubMed] [Google Scholar]
- 39.Manley J L, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 40.Martinez R, Mathey Prevot B, Bernards A, Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987;237:411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- 41.Mayeda A, Krainer A R. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992;68:365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- 42.McKeown M. Sex differentiation: the role of alternative splicing. Curr Opin Genet Dev. 1992;2:299–303. doi: 10.1016/s0959-437x(05)80288-6. [DOI] [PubMed] [Google Scholar]
- 43.Millake D B, Blanchard A D, Patel B, Critchley D R. The cDNA sequence of a human placental alpha-actinin. Nucleic Acids Res. 1989;17:6725. doi: 10.1093/nar/17.16.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Min H, Chan R C, Black D L. The generally expressed hnRNP F is involved in a neural-specific pre-mRNA splicing event. Genes Dev. 1995;9:2659–2671. doi: 10.1101/gad.9.21.2659. [DOI] [PubMed] [Google Scholar]
- 45.Mullen M P, Smith C W J, Patton J G, Nadal Ginard B. Alpha-tropomyosin mutually exclusive exon selection: competition between branchpoint/polypyrimidine tracts determines default exon choice. Genes Dev. 1991;5:642–655. doi: 10.1101/gad.5.4.642. [DOI] [PubMed] [Google Scholar]
- 46.Mulligan G J, Guo W, Wormsley S, Helfman D M. Polypyrimidine tract binding protein interacts with sequences involved in alternative splicing of beta-tropomyosin pre-mRNA. J Biol Chem. 1992;267:25480–25487. [PubMed] [Google Scholar]
- 47.Norton P A. Polypyrimidine tract sequences direct selection of alternative branch sites and influence protein binding. Nucleic Acids Res. 1994;22:3854–3860. doi: 10.1093/nar/22.19.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patton J G, Mayer S A, Tempst P, Nadal Ginard B. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev. 1991;5:1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- 49.Patton J G, Porro E B, Galceran J, Tempst P, Nadal Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 1993;7:393–406. doi: 10.1101/gad.7.3.393. [DOI] [PubMed] [Google Scholar]
- 50.Perez I, Lin C-H, McAfee J G, Patton J G. Mutation of PTB binding sites causes misregulation of alternative 3′ splice site selection in vivo. RNA. 1997;3:764–778. [PMC free article] [PubMed] [Google Scholar]
- 51.Periasamy M, Strehler E E, Garfinkel L I, Gubits R M, Ruiz Opazo N, Nadal Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984;259:13595–13604. [PubMed] [Google Scholar]
- 52.Reed R. The organization of 3′ splice-site sequences in mammalian introns. Genes Dev. 1989;3:2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- 53.Reed R, Maniatis T. The role of the mammalian branchpoint sequence in pre-mRNA splicing. Genes Dev. 1988;2:1268–1276. doi: 10.1101/gad.2.10.1268. [DOI] [PubMed] [Google Scholar]
- 54.Roberts G C, Gooding C, Smith C W J. Smooth muscle alternative splicing induced in fibroblasts by heterologous expression of a regulatory gene. EMBO J. 1996;15:6301–6310. [PMC free article] [PubMed] [Google Scholar]
- 55.Ruskin B, Greene J M, Green M R. Cryptic branch point activation allows accurate in vitro splicing of human beta-globin intron mutants. Cell. 1985;41:833–844. doi: 10.1016/s0092-8674(85)80064-7. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 57.Sharp P A. Split genes and RNA splicing. Cell. 1994;77:805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 58.Singh R, Valcarcel J, Green M R. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 59.Smith C W J, Chu T T, Nadal Ginard B. Scanning and competition between AGs are involved in 3′ splice site selection in mammalian introns. Mol Cell Biol. 1993;13:4939–4952. doi: 10.1128/mcb.13.8.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith C W J, Nadal Ginard B. Mutually exclusive splicing of alpha-tropomyosin exons enforced by an unusual lariat branch point location: implications for constitutive splicing. Cell. 1989;56:749–758. doi: 10.1016/0092-8674(89)90678-8. [DOI] [PubMed] [Google Scholar]
- 61.Smith C W J, Patton J G, Nadal Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- 62.Smith C W J, Porro E B, Patton J G, Nadal Ginard B. Scanning from an independently specified branch point defines the 3′ splice site of mammalian introns. Nature. 1989;342:243–247. doi: 10.1038/342243a0. [DOI] [PubMed] [Google Scholar]
- 63.Strehler E E, Periasamy M, Strehler-Page M-A, Nadal-Ginard B. Myosin light-chain 1 and 3 gene has two structurally distinct and differentially regulated promoters evolving at different rates. Mol Cell Biol. 1985;5:3168–3182. doi: 10.1128/mcb.5.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian M, Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- 65.Valcarcel J, Gebauer F. Post-transcriptional regulation: the dawn of PTB. Curr Biol. 1997;7:R705–R708. doi: 10.1016/s0960-9822(06)00361-7. [DOI] [PubMed] [Google Scholar]
- 66.Valcarcel J, Green M R. The SR protein family: pleiotropic functions in pre-mRNA splicing. Trends Biochem Sci. 1996;21:296–301. [PubMed] [Google Scholar]
- 67.Valcarcel J, Singh R, Green M R. Mechanisms of regulated pre-mRNA splicing. In: Lamond A I, editor. Pre-mRNA processing. R. G. New York, N.Y: Landes Company; 1995. pp. 97–112. [Google Scholar]
- 68.Valcarcel J, Singh R, Zamore P D, Green M R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature. 1993;362:171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- 69.Waites G T, Graham I R, Jackson P, Millake D B, Patel B, Blanchard A D, Weller P A, Eperon I C, Critchley D R. Mutually exclusive splicing of calcium-binding domain exons in chick alpha-actinin. J Biol Chem. 1992;267:6263–6271. [PubMed] [Google Scholar]
- 70.Wang Z, Grabowski P J. Cell- and stage-specific splicing events resolved in specialised neurons of the rat cerebellum. RNA. 1996;2:1241–1253. [PMC free article] [PubMed] [Google Scholar]
- 71.Watakabe A, Tanaka K, Shimura Y. The role of exon sequences in splice site selection. Genes Dev. 1993;7:407–418. doi: 10.1101/gad.7.3.407. [DOI] [PubMed] [Google Scholar]
- 72.Wieczorek D F, Smith C W J, Nadal Ginard B. The rat alpha-tropomyosin gene generates a minimum of six different mRNAs coding for striated, smooth, and nonmuscle isoforms by alternative splicing. Mol Cell Biol. 1988;8:679–694. doi: 10.1128/mcb.8.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wierenga B, Hofer E, Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit β-globin intron. Cell. 1984;37:915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- 74.Zahler A M, Lane W S, Stolk J A, Roth M B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- 75.Zahler A M, Neugebauer K M, Lane W S, Roth M B. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993;260:219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- 76.Zahler A M, Roth M B. Distinct functions of SR proteins in recruitment of U1 small nuclear ribonucleoprotein to alternative 5′ splice sites. Proc Natl Acad Sci USA. 1995;92:2642–2646. doi: 10.1073/pnas.92.7.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zamore P D, Patton J G, Green M R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355:609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- 78.Zhuang Y, Leung H, Weiner A M. The natural 5′ splice site of simian virus 40 large T antigen can be improved by increasing the base complementarity to U1 RNA. Mol Cell Biol. 1987;7:3018–3020. doi: 10.1128/mcb.7.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhuang Y A, Goldstein A M, Weiner A M. UACUAAC is the preferred branch site for mammalian mRNA splicing. Proc Natl Acad Sci USA. 1989;86:2752–2756. doi: 10.1073/pnas.86.8.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]