Abstract
Therapeutic apheresis (TA) is prescribed to patients that suffer from a severe progressive disease that is not sufficiently treated by conventional medications. A way to gain more knowledge about this treatment is usually by the local analysis of data. However, the use of large quality assessment registries enables analyses of even rare findings. Here, we report some of the recent data from the World Apheresis Association (WAA) registry. Data from >104,000 procedures were documented, and TA was performed on >15,000 patients. The main indication for TA was the collection of autologous stem cells (45% of patients) as part of therapy for therapy. Collection of stem cells from donors for allogeneic transplantation was performed in 11% of patients. Patients with indications such as neurological diseases underwent plasma exchange (28%). Extracorporeal photochemotherapy, lipid apheresis, and antibody removal were other indications. Side effects recorded in the registry have decreased significantly over the years, with approximately only 10/10,000 procedures being interrupted for medical reasons.
Conclusion
Collection of data from TA procedures within a multinational and multicenter concept facilitates the improvement of treatment by enabling the analysis of and feedback on indications, procedures, effects, and side effects.
Keywords: Registry, Apheresis, Adverse events, Diagnoses
Introduction
Therapeutic apheresis (TA) is performed for numerous indications in various fields of medicine with a changing panorama of indications [1, 2, 3, 4, 5, 6]. The proportions and techniques used for apheresis in different diseases also vary between countries and centers [5]. Although there are guidelines that help the clinician to motivate the inclusion of patients into the TA program, reimbursement for apheresis from insurance or hospital budgets differs between countries, which results in differences in inclusion criteria.
The results of registry data on indications, side effects, and effects of therapy for patients undergoing TA on the local, national, and international levels can guide physicians and politicians in their decisions about whether to accept or avoid therapy; these decisions must be based on the local resources, costs, and risks versus benefits for patients. At a meeting held in Paris in 2002, the World Apheresis Association (WAA) registry was developed from the concepts of the Canadian, French, and Swedish national registries already in existence [7, 8, 9, 10]. The progressive participation of centers and countries has improved the extent of available data and thereby increased statistical power. Registry reports have included data that help understand effects and side effects in the process of collecting blood and plasma for autologous or allogeneic measures [4, 5, 11, 12, 13, 14, 15, 16, 17]. This paper deals with the distribution of some diagnoses and reports recent data of side effects included in the WAA registry.
Material and Methods
Centers that have submitted data to the WAA registry applied and received access via the homepage: www.waa-registry.org. The WAA registry requests that centers include all TA procedures, even routine ones, and it is not specifically designed to assess TA procedures with side effects only. The purpose is to achieve a consecutive collection of data that enables an improved analysis. Data on patients who did not give their consent are excluded.
Data collection included variables such as gender, age, diagnosis, treatment mode, anticoagulation, and substitution fluids, reasons for treatment interruption, and the grade and type of side effect when these occur. In addition, numerous diagnoses may be graded according to their outcome over the treatment period. Grading of side effects is shown in Table 1: “Mild” (no need of medication due to side effects), “Moderate” (medication needed due to side effects), “Severe” (interruption of therapy due to side effects), and “Death” (due to the side effects of apheresis). Specific grading systems are used for different diseases.
Table 1.
Grading of side effects
| Code | Explanation/help |
|---|---|
| 1 | Mild adverse event (AE)/no medication needed |
|
| |
| 2 | Moderate adverse event (AE)/medication needed |
|
| |
| 3 | Severe adverse event (AE)/medication needed and apheresis interrupted |
|
| |
| 4 | Died due to apheresis |
The treatment is performed based on the quality of therapy assessment principles. The new European integrity regulations stipulate obtaining informed consent from the patient when they are in a state to respond, and consent from parents for children to undergo therapy. Within the WAA registry, the identity of the patient is converted into a local coding system which keeps the identity of the patient confidential. Participation by a center will enable it to download its own data for local reports and for comparison with the whole data pool, to be able to check if the local quality is above, below, or within the range at all other centers.
Results and Discussion
An increasing number of centers are participating in the WAA registry. Data are entered consecutively, and no selection is made of specific procedures with or without deviations. A total of 104,000 procedures were performed in 15,651 patients. Women represented 42% of all patients and 42.5% of treatments. The median age of patients at the start of therapy was 55 years. During the last 5 years, a total of 7,770 patients underwent 49,450 procedures. The type of apheresis procedures performed within the frame of the registry from 2014 up to and including 2018 are displayed in Table 2. The most common devices used in the procedures are displayed in Table 3.
Table 2.
Proportion of patients undergoing the most frequent specific apheresis procedures (2014–2018)
| % | |
|---|---|
| Procedure | |
| Plasma exchange (centrifugation) | 28.0 |
| Extracorporeal photochemotherapy (ECPT) | 3.6 |
| Erythrapheresis | 2.7 |
| Cascade filtration | 1.7 |
| Leukapheresis by filtration/adsorption | 1.7 |
| Exchange of erythrocytes | 1.0 |
| Depletion of erythrocytes | 0.8 |
| Plasma exchange (filtration) | 0.8 |
| Absorption: ABO-mismatch | 0.7 |
| Platelet apheresis | 0.6 |
| IgG adsorption globaffin column | 0.2 |
| Filtration, free light chain removal | 0.2 |
| LDL-removal | 0.2 |
| Protein A adsorber | 0.2 |
| IgE adsorption | 0.1 |
| Rheopheresis | 0.1 |
| IgG adsorption, sheep antibody | 0.1 |
| Plasma and lymphapheresis | <0.1 |
|
| |
| Preparative procedure | |
| Peripheral blood stem cell collection | |
| Autologous | 45.4 |
| Allogeneic | 11.0 |
Table 3.
The most frequently used devices for performing apheresis in 2014–2018
| Company, device |
|---|
| TerumoBCT, Optia |
| Haemonetics, MCS -kit |
| Theracos, cellex |
| Fresenius, comtec |
| Terumo BCT, Cobe Spectra |
| Fresenius, Dali |
| Fresenius, MONET |
| Kaneka, DX-21 |
| DiaMed, Octa Nova |
| Other systems |
| Kaneka, MA-03 (whole blood) |
| Kaneka, DL 100 |
| Asahi KASEI, Immusorba TR 350 |
| Fresenius Kabi, Amicus |
| Otsuka, Adacolumn |
| Medicap, ADAsorb Immunadsorption |
| Asahi KASEI, Immusorba PH 350 |
| BBraun, Plasmat Futura |
| Fresenius, Multifiltrate device |
| Terumo BCT, TRIMA |
| Miltenyi Biotec, LIFE 18 & 21 |
| Therakos, Uvar |
| Gambro, Prismaflex |
| Kaneka, MA-03 (La-15DL) |
| Fresenius, Art -Universal |
| Nikisso, Immunopure LPM-01 |
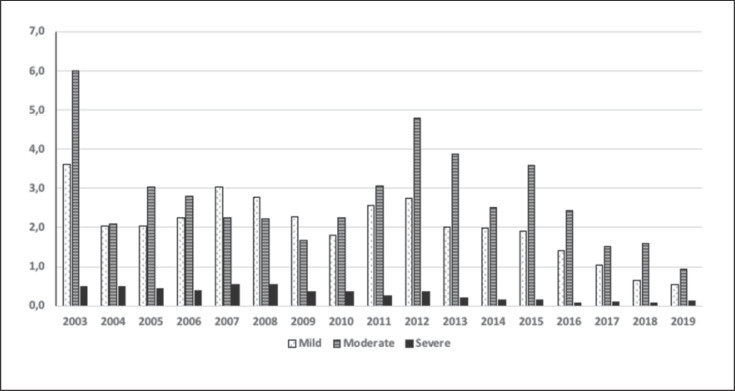
The proportions of different degrees of side effects are illustrated in Figure 1. The occurrence of side effects (including severe ones) has decreased significantly over time, from 11% of apheresis procedures in 2003 to approximately 2.3% in 2018 (p < 0.001). Of these, 0.6% procedures resulted in mild side effects, 1.6% in moderate side effects, and 0.1% in severe side effects that caused interruption of the apheresis. One death that may have been related to apheresis was reported in the total of 104,000 treatments. This case was an elderly, severely ill patient who died from myocardial infarction during the procedure. During the 49,400 procedures performed in 2014–2018, 46 severe side effects related to the apheresis itself caused interruption of the procedure. Of these, 5 were due to access problems. The severe side effects of TA during the last 5 years are given in Table 4. The WAA registry allows interaction regarding information recorded in the registry on an immediate basis. This enables users to get fast responses to questions that may arise.
Fig. 1.
Distribution of the different grades of adverse events considered to be due to the apheresis procedure (from 2003 to June 2019).
Table 4.
Severe side effects causing interruption of the apheresis procedure (a total of 41 symptomatic episodes appeared out of 49,400 procedures representing 8 severe events/10,000 procedures)
| Finding | n (%) |
|---|---|
| Hypotension | 11 (26.8) |
| Urticaria, conjunctivitis | 8 (19.5) |
| Abdominal pain | 6 (14.6) |
| Cardiac arrest (resuscitated) | 2 (4.9) |
| Bronchospasm | 2 (4.9) |
| Nausea and/or vomiting | 2 (4.9) |
| Tingling, stitching | 2 (4.9) |
| Chills and fever (>38° C) | 2 (4.9) |
| Anaphylactic shock | 1 (2.4) |
| Arrhythmia | 1 (2.4) |
| Hypertension | 1 (2.4) |
| Back pain related to apheresis | 1 (2.4) |
| Flush | 1 (2.4) |
| Late complication, other | 1 (2.4) |
The most common procedure is apheresis for stem cell collection. Stem cells are mainly used by cancer patients with cells being reinfused after oncologic therapy. The cells collected are for autologous or allogeneic transplantation.
Differentiation of analyses of apheresis is made possible by separating the indications according to diagnosis (ICD-10 code). This enables specific analysis of the treatment of a disease, e.g., sickle cell disease. The side effects differ depending on the mode of therapy but also on the replacement fluid used; for instance, hypotension was found to be more common if albumin only (and not plasma) was used as replacement, while urticaria was mainly related to replacement with plasma [4]. As shown by Norda et al. [18], hypotension was more frequent if the albumin concentration was 3.5% instead of 5% (risk ratio 4.0; 95% confidence interval [CI] 1.5–10.2; p < 0.001). This knowledge can help to guide patients and clinicians to prepare for the therapy.
During recent years, indications for TA for neurological diseases have increased. Table 5 displays some common neurological indications for apheresis in 644 patients who suffered from 66 different diagnoses. The large number of patients with new neurological diagnoses that are offered TA is in parallel with the increased knowledge of antibody-mediated diseases aside from inflammation and infection [19, 20, 21, 22, 23, 24, 25, 26, 27]. This stresses the need for developing new methods to detect previously unknown antibodies.
Table 5.
Distribution of diagnoses (given as ICD-10 codes) as a percentage of a total of 644 patients treated for neurological diseases in 2014–2018 (≥0.3% of neurological therapeutic indications are displayed; 31 other diagnoses are less represented in the WAA registry)
| % | ICD-10 | Diagnosis |
|---|---|---|
| 29.0 | G700 | Myasthenia gravis |
| 19 | G35 | Multiple sclerosis |
| 18.8 | G610 | Guillain-Barré syndrome |
| 5.7 | G619B | Inflammatory polyneuropathy, unspecified |
| 4.0 | G99A | Autonomic neuropathy related to endocrine and metabolic diseases |
| 2.6 | G049 | Encephalitis, myelitis, and encephalomyelitis, unspecified |
| 2.3 | G360 | Neuromyelitis optica |
| 2.2 | G629 | Polyneuropathy, unspecified |
| 1.4 | G618 | Other specified polyneuropathies |
| 1.1 | G0481 | Encephalomyelitis |
| 0.8 | G040 | Acute disseminated encephalomyelitis |
| 0.8 | G6181 | CIDP (chronic inflammatory demyelinating polyneuropathy) |
| 0.8 | G98 | Other diseases of the nervous system not specified in another location |
| 0.6 | G0481 | Limbic encephalitis |
| 0.5 | I677 | Susac syndrome |
| 0.5 | G049A | Encephalitis, unspecified |
| 0.5 | G2582 | Stiff-man syndrome |
| 0.5 | G600 | Sensory polyneuropathy |
| 0.5 | G731 | Lambert-Eaton syndrome |
| 0.5 | G934 | Encephalopathy, unspecified |
| 0.3 | G318 | ANEC (acute necrotizing encephalopathy of childhood) |
| 0.3 | G049B | Myelitis, unspecified |
| 0.3 | G373 | Acute transversal myelitis |
| 0.3 | G379 | Demyelinating disease, unspecified |
| 0.3 | G409 | Epilepsy |
| 0.3 | G611 | Serum neuropathy |
| 0.3 | G6181 | Optic neuritis |
| 0.3 | G728 | Myopathies and rhabdomyolysis |
| 0.3 | G804 | Ataxic cerebral palsy |
| 0.3 | G99A | Paraneoplastic syndromes |
A limitation of the WAA registry is that only a few centers enter outcome data, although the registry allows such entries. One reason may be that the clinician responsible does not actually write up the TA report submitted to the registry. In addition, the physician at the apheresis unit may be unaware of the various stages, i.e., improvement or impairment, that might have developed at the specific ward. The local staff at the apheresis unit may communicate with the patient and enter data on a rough scale that estimates the patient's functional capacity (e.g., from being unconscious to performing athletic competition exercise) by asking the patient questions. These data can also be relevant and are currently being analyzed.
So far, only aggregated data have been reported. Retrospective follow-up of outcome data has been performed for thrombotic microangiopathy, and this is underway for some other conditions. No interventional studies have been included thus far, but it is possible to enter these into the frame of the electronic system.
One benefit offered by this multicenter registry is that the participating centers can compare their data with other centers (aggregate data). The system does not involve extra costs for the users. Approximately 40 variables can be analyzed. Each center may analyze their own data whenever necessary. Longitudinal analyses can be performed of outcomes of multiple variables over time. Separate diagnoses have been given various outcome criteria.
A risk of selection bias exists, mainly due to some patients not consenting to their data being used for quality assessment and improvement. It is rare that units omit entering data about a procedure. The collection of outcome data is limited, however, mainly due to poor feedback from the clinician to the person who submits the data to the registry.
Most patients treated by extracorporeal photopheresis suffer from graft versus host disease [5]. Stem cell collection is mainly performed by autologous means for cancer treatment [5]. Notably, approximately 10% of cell collection procedures are performed on donors.
The decrease in side effects over time is most likely due to the increased awareness of the causes of problems that can arise, e.g., a low concentration of albumin in the return fluid will increase the risk for hypotension. Awareness obtained from data analyses and access to other publications increase the knowledge of how to prevent side effects at all participating centers. Analysis of data from the Swedish registry showed that side effects during TA differed for various diseases [18, 28, 29, 30, 31, 32]. Replacement and processing techniques are other markers to refer to [33]. The standardization of stem cell collection may be responsible for the small number of adverse events in this population compared to in patients performing direct therapeutic plasma exchange [4]. Differences between centers also are present, and this encourages specific statistical analyses and interpretations [34].
In conclusion, this report on the WAA registry shows that the combined use and interactive communications between participating centers enable the analysis of large amounts of data. The feedback within centers may be one reason for the significant reduction of especially severe side effects. Although many patients suffer from life-threatening diseases, when treatment is performed, the addition of this extracorporeal therapeutic approach seems fairly well tolerated. We invite more centers to apply for participation in the registry to enter data to enable further knowledge of therapeutic measures and side effects. The application can be made at www.waa-registry.org.
Conflict of Interest Statement
None of the authors have a conflict of interest.
Funding Sources
We received funds from the Swedish National Registry, Njurföreningen Västerbotten, and Sweden's municipalities and regions.
Acknowledgement
Our thanks for funding go to the Swedish National Registry authority that enabled establishment of the Web-based internationally available registry. Njurföreningen Västerbotten and Sweden's municipalities and regions also gave their support. The World Apheresis Association supported coordinating meetings.
References
- 1.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016 Jun;31((3)):149–62. doi: 10.1002/jca.21470. [DOI] [PubMed] [Google Scholar]
- 2.De Silvestro G. The Italian registry of therapeutic apheresis − 2015. Transfus Apheresis Sci. 2017 Feb;56((1)):75–81. doi: 10.1016/j.transci.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 3.De Silvestro G, Tison T. Italian Society of A, Cell M. Italian Registry of Therapeutic Apheresis. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2018;57((2)):143–147. doi: 10.1016/j.transci.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Mörtzell Henriksson M, Newman E, Witt V, Derfler K, Leitner G, Eloot S, et al. Adverse events in apheresis: an update of the WAA registry data. Transfus Apheresis Sci. 2016 Feb;54((1)):2–15. doi: 10.1016/j.transci.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Stegmayr B, Mortzell Henriksson M, Newman E, Witt V, Derfler K, Leitner G, et al. Distribution of indications and procedures within the framework of centers participating in the WAA apheresis registry. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2017 doi: 10.1016/j.transci.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019 Jun;34((3)):171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 7.Stegmayr BG, Ivanovich P, Korach JM, Rock G, Norda R, Ramlow W. World apheresis registry. J Clin Apher. 2005 Jul;20((2)):126–7. doi: 10.1002/jca.20044. [DOI] [PubMed] [Google Scholar]
- 8.Stegmayr BG, Ivanovich P, Korach JM, Rock G, Norda R, Ramlow W. World apheresis association—world apheresis registry. Transfus Apheresis Sci. 2005 Apr;32((2)):205–7. doi: 10.1016/j.transci.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Stegmayr B, Korach JM, Norda R, Rock G, Fadel F. Is there a need for a national or a global apheresis registry? Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2003;29((2)):179–85. doi: 10.1016/S1473-0502(03)00145-9. [DOI] [PubMed] [Google Scholar]
- 10.Stegmayr B. The World Apheresis Association Registry. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2017 doi: 10.1016/j.transci.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Stegmayr B, Ptak J, Wikstrom B. World apheresis registry report. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2007;36((1)):13–6. doi: 10.1016/j.transci.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Stegmayr B, Ptak J, Wikström B, Berlin G, Axelsson CG, Griskevicius A, et al. World apheresis registry 2003-2007 data. Transfus Apheresis Sci. 2008 Dec;39((3)):247–54. doi: 10.1016/j.transci.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Witt V, Stegmayr B, Ptak J, Wikström B, Berlin G, Axelsson CG, et al. World apheresis registry data from 2003 to 2007, the pediatric and adolescent side of the registry. Transfus Apheresis Sci. 2008 Dec;39((3)):255–60. doi: 10.1016/j.transci.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Bláha M, Pták J, Cáp J, Ceeová V, Masín V, Filip S, et al. WAA apheresis registry in the Czech Republic: two centers experience. Transfus Apheresis Sci. 2009 Aug;41((1)):27–31. doi: 10.1016/j.transci.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Blaha M, Ptak J, Cap J, Ceeova V, Masin V, Filip S, et al. WAA apheresis registry in the Czech Republic: two centers experience. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2009;41((1)):27–31. doi: 10.1016/j.transci.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Mortzell M, Berlin G, Nilsson T, Axelsson CG, Efvergren M, Audzijoni J, et al. Analyses of data of patients with Thrombotic Microangiopathy in the WAA registry. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2011;45((2)):125–31. doi: 10.1016/j.transci.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Stegmayr B, Ptak J, Nilsson T, Berlin G, Mirea V, Axelsson CG, et al. Panorama of adverse events during cytapheresis. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2013;48((2)):155–6. doi: 10.1016/j.transci.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Norda R, Axelsson CG, Axdorph U, Berlin G, Wikström B, Stegmayr B, Swedish Apheresis Group Recognition of intercenter differences may help develop best practice. Ther Apher Dial. 2008 Oct;12((5)):347–54. doi: 10.1111/j.1744-9987.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 19.Bing-Lei W, Jia-Hua Z, Yan L, Zan Y, Xin B, Jian-Hua S, et al. Three cases of antibody-LGI1 limbic encephalitis and review of literature. Int J Neurosci. 2019 Jul;129((7)):642–8. doi: 10.1080/00207454.2018.1512985. [DOI] [PubMed] [Google Scholar]
- 20.Esposito S, Principi N, Calabresi P, Rigante D. An evolving redefinition of autoimmune encephalitis. Autoimmun Rev. 2019 Feb;18((2)):155–63. doi: 10.1016/j.autrev.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Jones RB, Hiemstra TF, Ballarin J, Blockmans DE, Brogan P, Bruchfeld A, et al. European Vasculitis Study Group (EUVAS) Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis. 2019 Mar;78((3)):399–405. doi: 10.1136/annrheumdis-2018-214245. [DOI] [PubMed] [Google Scholar]
- 22.McAdoo SP, Medjeral-Thomas N, Gopaluni S, Tanna A, Mansfield N, Galliford J, et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant. 2019 Jan;34((1)):63–73. doi: 10.1093/ndt/gfx378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson K, Iglesias E, Nasrallah M, González-Álvarez V, Suñol M, Anton J, et al. Anti-MOG encephalitis mimicking small vessel CNS vasculitis. Neurol Neuroimmunol Neuroinflamm. 2019 Feb;6((2)):e538. doi: 10.1212/NXI.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepper RJ, McAdoo SP, Moran SM, Kelly D, Scott J, Hamour S, et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford) 2019 Feb;58((2)):260–8. doi: 10.1093/rheumatology/key288. [DOI] [PubMed] [Google Scholar]
- 25.Si Z, Wang A, Liu J, Zhang Z, Hu K. Typical clinical and imaging manifestations of encephalitis with anti-γ-aminobutyric acid B receptor antibodies: clinical experience and a literature review. Neurol Sci. 2019 Apr;40((4)):769–77. doi: 10.1007/s10072-018-3679-5. [DOI] [PubMed] [Google Scholar]
- 26.Strunk D, Schmidt-Pogoda A, Beuker C, Milles LS, Korsukewitz C, Meuth SG, et al. Biomarkers in Vasculitides of the Nervous System. Front Neurol. 2019 Jun;10:591. doi: 10.3389/fneur.2019.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuhlmüller B, Schneider U, González-González JB, Feist E. Disease Specific Autoantibodies in Idiopathic Inflammatory Myopathies. Front Neurol. 2019 May;10:438. doi: 10.3389/fneur.2019.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norda R, Berséus O, Stegmayr B. Adverse events and problems in therapeutic hemapheresis. A report from the Swedish registry. Transfus Apheresis Sci. 2001 Aug;25((1)):33–41. doi: 10.1016/s1473-0502(01)00079-9. [DOI] [PubMed] [Google Scholar]
- 29.Norda R, Knutson F, Berseus O, Akerblom O, Nilsson-Ekdahl K, Stegmayr B, et al. Unexpected effects of donor gender on the storage of liquid plasma. Vox Sang. 2007 Oct;93((3)):223–8. doi: 10.1111/j.1423-0410.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 30.Norda R, Schött U, Berséus O, Akerblom O, Nilsson B, Ekdahl KN, et al. Complement activation products in liquid stored plasma and C3a kinetics after transfusion of autologous plasma. Vox Sang. 2012 Feb;102((2)):125–33. doi: 10.1111/j.1423-0410.2011.01522.x. [DOI] [PubMed] [Google Scholar]
- 31.Norda R, Stegmayr BG, Swedish Apheresis Group Therapeutic apheresis in Sweden: update of epidemiology and adverse events. Transfus Apheresis Sci. 2003 Oct;29((2)):159–66. doi: 10.1016/S1473-0502(03)00121-6. [DOI] [PubMed] [Google Scholar]
- 32.Norda R, Stegmayr BG, Swedish Apheresis Study Group Apheresis registry in Sweden: scope, techniques and indications for treatment. A report from the Swedish apheresis study group. Transfus Apheresis Sci. 2001 Feb;24((1)):49–55. doi: 10.1016/s0955-3886(00)00132-6. [DOI] [PubMed] [Google Scholar]
- 33.Stegmayr B, Tärnvik A. Complement activation in plasma exchange by single filtration and centrifugation and in cascade filtration. Blood Purif. 1989;7((1)):10–5. doi: 10.1159/000169568. [DOI] [PubMed] [Google Scholar]
- 34.Toss F, Edgren G, Berlin G, Stegmayr B, Witt V. Does prophylactic calcium in apheresis cause more harm than good? - Centre heterogeneity within the World Apheresis Association Register prevents firm conclusions. Vox Sang. 2018 Oct;113((7)):632–8. doi: 10.1111/vox.12698. [DOI] [PubMed] [Google Scholar]