Abstract
Background
Obese patients have an increased incidence of ventral hernias; in over 50% of these cases, patients are symptomatic. At the same time, morbid obesity is a disease of epidemic proportions. The combination of symptomatic hernia and obesity is a challenge for the treating surgeon, because the risk of perioperative complications and recurrence increases with increasing BMI.
Summary
This review outlines this problem and discusses interdisciplinary approaches to the management of affected patients. In emergency cases, the hernia is treated according to the surgeon's expertise. In elective cases, an individual decision must be made whether bariatric surgery is indicated before hernia repair or whether both should be performed simultaneously. After bariatric surgery a weight reduction of 25–30% of total body weight in the first year can be achieved and it is often advantageous to perform a bariatric operation prior to hernia repair. Technically, the risk of complications is lower with minimally invasive procedures than with open ones, but laparoscopy is challenging in obese patients, and meshes can only be implanted in intraperitoneal position. This mesh position has to be questioned because of adhesions, recurrence rate, and risk of contamination during re-interventions in patients who are often still relatively young.
Key Messages
Obese patients with hernia need to be approached in an interdisciplinary manner, in some patients a weight loss procedure may be advantageous before hernia repair. Recent data show the benefits of robotic hernia surgery in obese patients, as not only haptic advantages result, but especially the mesh can be implanted in a variety of extraperitoneal positions in the abdominal wall with low morbidity.
Keywords: Ventral hernia, Obesity, Robotic hernia repair, Bariatric surgery, Deferred hernia repair
Introduction
Literature Nobel laureate T.S. Eliot (1888–1965) predicted with almost prophetic brilliance 80 years ago the real challenge of our days: wisdom is needed to integrate knowledge and the flood of information into meaningful concepts (The Rock, 1934). Treating obese hernia patients is a frequent problem as both disorders are frequent and ventral hernias are even more frequent in obese patients. Obesity is epidemic in many countries; in the US it is expected to increase by 33% over the next 20 years [1]. It is likely, that depending on what door the obese patient with hernia enters a hospital (e.g., emergency, endocrinology, internal medicine, or surgery), the focus for treatment will be very different. The challenge for the hernia surgeon is to change his focus away from the hernia and look at the patient in a holistic way with their obesity and metabolic comorbidities: what is the priority? In what order should the various problems be treated? What are the risks associated with the therapeutic decision? How can surgical risks be minimized and long-term results optimized? Last but not least, it has to be considered that some of the patients will need further visceral surgery in the course of their lives (e.g., for acute cholecystitis, carcinoma surgery, bariatric surgery, etc.). This review will focus on hernia care in the context of obesity and discuss actual meaningful concepts.
Peculiarities of the Patient with Excess Body Weight and Hernia
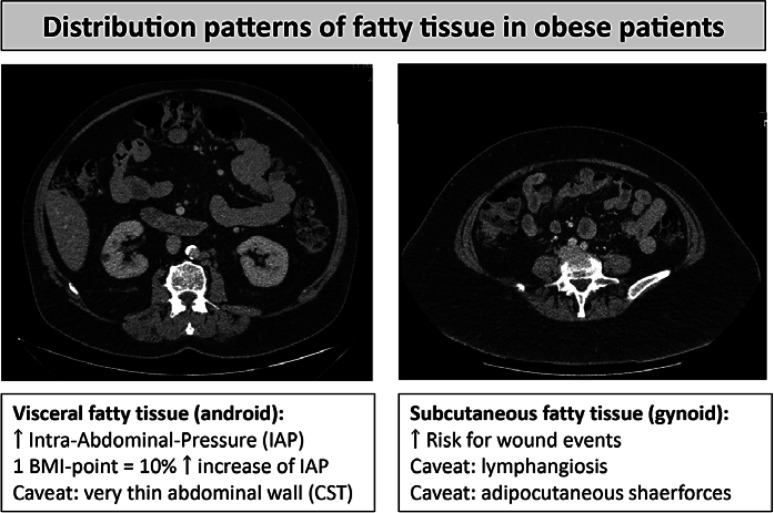
In the following, the term ventral hernia will apply to umbilical and epigastric hernias in contrast to incisional hernias, which designate hernias after a previous surgical procedure. Although multifactorial, ventral hernia is a disease directly related to overnutrition [2]. Obese patients with metabolic syndrome have several conditions that contribute to an increase in poor postoperative outcomes: arterial hypertension, diabetes, cardiac diseases, non-alcoholic fatty liver disease, carcinomas, dementia, and sarcopenia [3]. Overweight and specifically obesity with BMI above 30 doubles the probability for hernia, and the prevalence of hernia symptoms in patients with morbid obesity reaches 50% [4]. The body fat content is distributed in the subcutaneous compartment and/or the visceral compartment (Fig. 1). The visceral compartment is a risk factor for herniation and metabolic disease, while subcutaneous fat is a risk factor for surgical site infections [5]. The intraabdominal pressure is increased in patients with excess of fat in the visceral compartment: with each point of increase in BMI, there is a 10% increase in intragastric pressure [6]. In evaluation obese hernia patients the BMI is a criterion that needs to be complemented by other physical findings, since it does not differentiate between both compartments [4]: further measurements are the abdominal wall circumference (female: <88 cm; male <102 cm) and the waist-to-hip ratio (female <0.85; male <1.05); the latter correlates with increased visceral fat distribution and respective risk of metabolic syndrome. These measurements should be part of the preoperative risk assessment. Finally, obesity can mask sarcopenia, and sarcopenia correlates an increase in the recurrence rate after hernia repair. In patients with obesity and BMI between 30 and 50, preoperative weight loss should be a strategic goal to improve outcomes, since weight loss always reduces fat deposition in both the subcutaneous and the visceral compartments [7]. In general, exercise and weight loss programs lead to weight reduction of 2–3% total body weight/year, whereas bariatric procedures achieve as much as 25–30% after 1 year.
Fig. 1.
Distribution patterns of fatty tissue in obese patients. Very thin abdominal wall may be a risk factor for complications, for example, skin necrosis after anterior component separation technique (CST). Gynoid fat deposition predisposes to lymphangiosis in cases of pendulous abdomen and may further impair the wound healing.
Treatment Strategies for Obese Patients with Ventral and Incisional Hernias
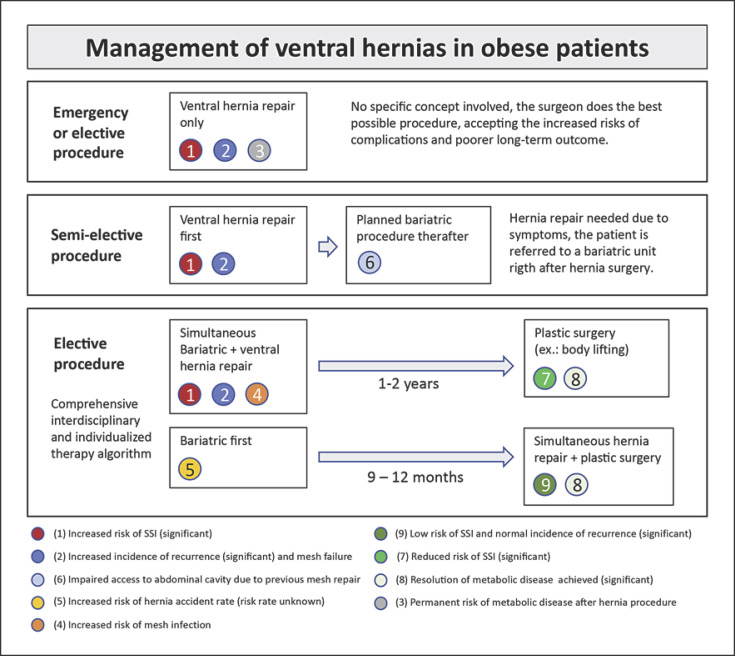
Surgical management of ventral and incisional hernias in obese patients must be approached in an interdisciplinary manner. This is especially important for elective procedures, as conservative or surgical preoperative weight reduction has been shown to successfully reduce complication and early recurrence rates [8] (Fig. 2). From a surgical point of view, multilocular hernias, irreducibility, and ileus are urgent indications; large and non-chambered hernias, small hernias filled with fatty tissue, and absence of symptoms are criteria pointing towards deferred hernia repair. In cases of incisional hernias, a CT scan is helpful to support decision-making: surrogates for adhesions, hernia gap width, thickness of the musculofascial layer, and fatty tissue distribution have to be carefully analyzed. Basically, it is always a question of weighing the risk for hernia accident without procedure against the risk of complications following the procedure.
Fig. 2.
Management of hernia in obese patients.
Elective Hernia Repair without Previous Weight Loss
Whereas the surgical indication for immediate hernia repair is clear in an emergency situation, in the case of elective surgery, the patient must be viewed holistically. If the risk of an adverse event during the period of weight loss is deemed too high, a hernia repair without prior weight loss may be appropriate. There are also patients who do not want to invest time, money, or effort in a preoperative weight reduction program. Minimally invasive mesh implantation is a good option in treating ventral hernias with small hernia gaps (<5 cm diameter) at BMI of 30–40. Using the conventional laparoscopic technique, the hernia contents are reduced, the preperitoneal fat is detached from the anterior abdominal wall, and a mesh is implanted in intraperitoneal position (IPOM). Although very popular, this procedure is known to be related to adhesions to the small bowel and may be a hindrance for bariatric surgery later on. If hernia repair is planned without prior weight loss, there are currently promising alternatives to avoid the IPOM position and to implant the mesh extraperitoneally instead, using robotic technique.
Combined Hernia Repair and Bariatric Procedure
Combined hernia repair and bariatric surgery is associated with a higher 30-day morbidity compared to bariatric surgery alone regardless of type of bariatric procedure and irrespective of the surgical approach (open or minimally invasive) [9]. This finding is also supported by a propensity-matched analysis, showing an increased 30-day morbidity of simultaneous hernia repair and bariatric surgery, with an advantage of sleeve gastrectomies over Roux-en-Y gastric bypass operations [10]. In this situation, the options for repair are limited: simple suture or IPOM mesh. The IPOM mesh seems not to be associated with increased mesh infection, but may make the approach for a revisional bariatric procedure (needed in up to 15% of patients) more difficult [11]. There is no data available on recurrence rates after synchronous bariatric and hernia operations.
Bariatric Surgery before Hernia Repair
Weight loss before elective hernia repair may offer better results concerning recurrence rates and perioperative complications due to an improved metabolic state (resolution of comorbidities as diabetes, NAFLD, etc.). Retrospective studies have shown low recurrence rates in hernia repair after prior bariatric surgery probably in part due to the decreased intraabdominal pressure [12]. Even in very large ventral hernias bariatric minimally invasive procedures are often possible, as the left upper quadrant of the abdomen is usually free of adhesions. In patients where the small bowel is affected by adhesions in the hernia sac, a sleeve gastrectomy may be the procedure of choice. Untreated hernias carry the risk of small bowel obstruction after the bariatric procedure [13]. Therefore, if there is vascularized greater omentum in the hernia gap, it should not be removed. If hernia contents are reduced during bariatric surgery, it is recommended to close the gap, either with suture (orifices <2 cm) or mesh [12, 14]. Hernia repair after bariatric operation should be postponed until the catabolic phase of weight loss is over. This is usually reached after 9–12 months and indicated by a stable weight and sufficiently substituted micronutrients. Cases of symptomatic incisional hernias after bariatric procedure complicated by peritonitis and persistent obesity need to be addressed in an individualized manner.
Hernia Repair with Simultaneous Resection of Excess Skin after Weight Loss
Since patients who undergo plastic surgery have significantly improved long-term body weight control after Roux-en-Y gastric bypass and better quality of life, a combined open ventral hernia repair and panniculectomy should be considered also in patients who undergo ventral hernia repair after previous weight loss [15]. Although combined open ventral hernia repair and panniculectomy operations have a higher risk of superficial surgical site infections (SSI), venous thromboembolism, and general morbidity, the incidence of deep SSI and the recurrence rate are similar to hernia repair alone [16]. Ventral hernia repair with simultaneous panniculectomy seems to lower the mechanical strain on the mesh repair in the early postoperative period [17].
Perioperative Measures
Due to reduced pulmonary and total chest compliance there is an increased risk of hypoxia during anesthesia in obese patients. To avoid respiratory complications, intraoperative continuous positive airway pressure (CPAP) and positive end-expiratory pressure (PEEP) are useful. Avoiding Trendelenburg position and lowering the intraabdominal pressure during laparoscopy have to be considered [18]. Obese patients have an increased risk of developing thromboembolic events and the standard dose of enoxaparin is less effective in preventing venous thromboembolism (VTE) in these patients [19]. Patients with an unimpaired renal function and a BMI higher than 40 are recommended to receive 40 mg Enoxaparin s.c. twice a day [20]. If the BMI is higher than 50 even a dose of 60 mg twice a day s.c. may be needed. This regimen was shown to be safe regarding risk of bleeding and effective in preventing VTE [21].
Laparoscopic and Robotic-Assisted Hernia Repair in Obese Patients
Ventral and Incisional Hernias
The advantages of laparoscopic approach over open procedures in obese patients in general and in hernia repair in particular have comprehensively been demonstrated and discussed in the past years [1, 22, 23]. Nevertheless, laparoscopic surgery in obese patients is particularly challenging. This stems from increased torque on instruments and trocars due to the excessive weight of the abdominal wall, which may hinder the fluidity of the surgeon's motions, which may impact precision and lead to increased tissue trauma and surgeon fatigue [24]. In the past two decades, robotic-assisted surgery has made its way into the minimally invasive arena. The robotic platform brings with it an enhanced visualization of the operative field, in terms of both its magnification and 3D visualization. Furthermore, articulated instruments provide greater degrees of freedom, which results in increased dexterity and precision needed to perform complex intracorporeal tasks [25, 26]. The robotic platform enables to place the mesh outside the abdominal cavity in several different planes, and leads to less abdominal wall trauma as well as postoperative pain since the fulcrum is at the articulation of the instruments as opposed to the abdominal wall [27]. The overarching goal of robotic hernia repair is to achieve comparable quality of repair to that of open procedures while reducing perioperative morbidity [28]. Although the availability of surgical robots and specialized surgeons is not yet ubiquitous, robotic hernia surgery is experiencing great acceptance. In the US, hernia repair is a fast-growing field, with over 30% of all robotic procedures in general surgery being hernia operations. For this reason, current results of robotic procedures are highlighted below.
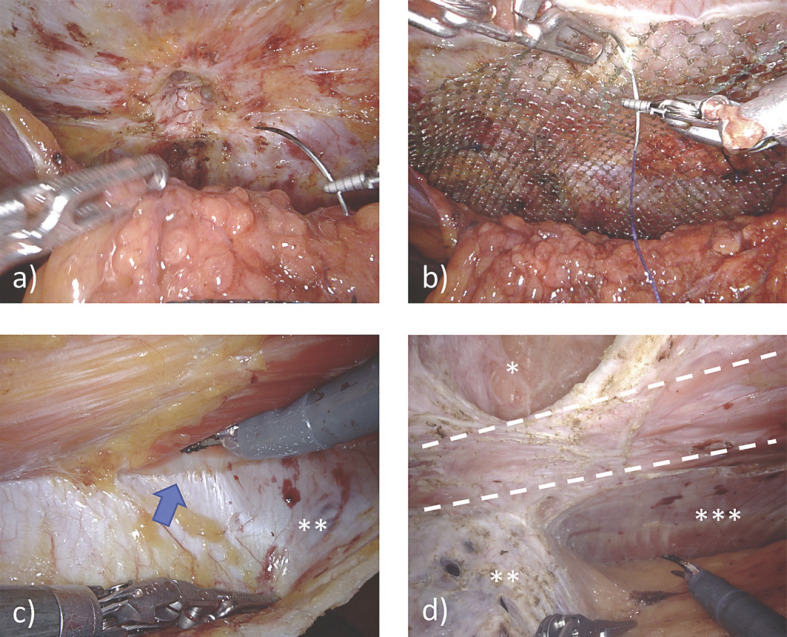
One multi-institutional study evaluated 368 robotic ventral and incisional hernia repairs in a majority of the obese and morbidly obese population (47.8 and 20.9%, respectively). Defect closure was achieved in 69.3% of patients, with only 1.6% of repairs incorporating transfascial mesh fixation [29]. This relative ease with intracorporeal suturing afforded by the robotic platform was further emphasized in a study of 134 patients undergoing VHR, half of whom were obese [30]. Due to a concern for visceral adhesions, possible mesh migration, and other complications with IPOM repair, there has been an increasing trend among hernia surgeons for extraperitoneal mesh placement [31]. Using the peritoneal layer as a barrier between the mesh and visceral contents minimizes adhesion formation, fistula development, and the need for using more costly coated mesh (Level 4) [22]. Robotic technology enables surgeons with precise preperitoneal dissection, thereby facilitating transabdominal preperitoneal (r-ventral TAPP) repair. Peritoneal flap dissection is initiated 5 cm from the defect on the ipsilateral trocar side. The IEHS guidelines recommend this technique for smaller ventral hernias (Level 4) as a safe and feasible alternative to r-IPOM (Grade D) [22]. Panteleimonitis et al. [32] reported early experience with r-ventral TAPP repair, demonstrating the safety and feasibility of this technique; out of 54 patients, 64.8% were obese or morbidly obese; during peritoneal dissection, peritoneal integrity was maintained in 85.2% of patients. Another study examined the operative time-based learning curve of r-TAPP across 105 patients with a mean BMI of 31.5. As a measure of repair quality, peritoneal flap completeness was used to adjust for repair quality; the authors suggest that an accumulated experience with 61 cases is sufficient to achieve good-quality repairs in a timely fashion [33] (Fig. 3).
Fig. 3.
Robotic ventral hernia repair with extraperitoneal mesh placement, a r-ventral TAPP in umbilical hernia: the peritoneum was mobilized, the fatty tissue retrieved from de hernia orifice, and the orifice is being closed. b A large mesh overlap is reached, the last step will be the suture of the peritoneum. c r-retromuscular approach (r-RM): **posterior rectus sheet, arrow = convergence of posterior and anterior rectus sheets at lina alba. d r-transversus abdominis release (r-TAR): between dotted lines = rectus abdominis muscle, *hernia gap, **posterior rectus sheet, ***transverse abdominal muscle.
Another extraperitoneal repair technique is robotic retromuscular (r-RM) repair with or without transversus abdominis release (r-TAR). r-RM repair can be performed using either transabdominal (r-TA) or totally extraperitoneal (r-TEP) access (Fig. 3). Much like the r-ventral TAPP method, r-RM repair involves extraperitoneal mesh placement, thus avoiding contact of the mesh with the abdominal viscera. Furthermore, placement of the mesh between the rectus muscle and its posterior sheath may eliminate the need for mesh fixation (Level 3) [22]. Patients with incisional hernias and larger defects require larger mesh sizes. The retromuscular space can accommodate large meshes to patch large defects in order to obtain adequate mesh-to-defect (M/D) ratios, a necessary factor for minimizing recurrence rates (Level 3) [22]. Obese patients are not only associated with larger defects and higher recurrence rates, but also have increased tension on the abdominal wall layers, making it difficult to ensure quality repairs. In such cases, dissecting laterally beyond the linea semilunaris and performing TAR may help overcome these obstacles. r-RM repair with or without TAR has established benefits over its open counterpart in terms of LOS and incidence of surgical site infections (Level 2C and 4) [22]. Belyansky et al. [34] reported their experience with the retromusular approach (r-eTEP) in a small group of 37 patients with an average BMI of 36; among all patients, only two clinically relevant seromas were reported as well as one readmission for PO intolerance; with no sign of early hernia recurrences (30 days), the authors support the safety and feasibility of this technique. Similarly, Gokcal et al. [35] studied this technique among a total of 101 patients, 54 of whom underwent concomitant r-TAR and 47 of whom did not; both groups had a mean BMI >30; they found that although the r-TAR+ group experienced a higher rate of complications, this difference between groups did not persist at follow-up; furthermore, the only factor associated with the development of complications in these patients was the presence of an incarcerated hernia. Thus, the safety and feasibility of this approach was supported by the study results.
In parallel to the increased number of publications, the guidelines for the laparoscopic treatment of ventral and incisional abdominal wall hernias by the IEHS have incorporated some recommendations for robotic repair [36]. Despite this, specific recommendations for the obese population have yet to be included. Kudsi et al. [37] first reported the perioperative and mid-term outcomes in morbidly obese (BMI ≥40) patients who underwent rVHR within a 5-year period. Fifty patients with a median BMI of 42.9 (min.-max.: 40.2–59.2) were included in the study. Across various techniques, including r-IPOM, r-TAPP, and r-RM with/without TAR, the median skin-to-skin time was 71 min, with no intraoperative complications and a mean length of stay (LOS) of 0.34 days for the entire cohort. While minor complications (Clavien-Dindo/CD grade I and II) were seen in 40% of patients, major complications (CD grade III and IV) were seen in 6%. In the regression analysis, BMI (p = 0.037; OR 1.172, 95% CI 1.010–1.361), adhesiolysis (p = 0.005; OR 16.055, 95% CI 2.270–113.574), IPOM mesh (p = 0.049; OR 4.625, 95% CI 1.006–21.262), and off-console time (p = 0.033; OR 1.139, 95% CI 1.010–1.285) were found as predictors of postoperative complications. Hernia recurrence was seen in one (2%) patient within a mean follow-up period of 22.7 months, and the mean freedom-of-recurrence was 57.4 months (95% CI 54.6–60.2). Kudsi et al. [38] compared perioperative outcomes after robotic-assisted VHR between patients with a BMI ≥35 (class II or class III obesity) and BMI <35 (non-obese, class I obesity). A 1:1 propensity score matching (PSM) analysis was run to obtain balanced groups. The unmatched sample included 526 patients with an average BMI of 31.2. After PSM, 142 patients were allocated to each group. According to univariate analyses, there was no statistical difference between the two groups regarding 90-day complications. Authors concluded that a BMI ≥35 did not significantly affect the postoperative short-term outcomes of rVHR.
Groin Hernia
While a higher BMI is an established risk factor for both primary ventral and incisional hernias, several studies indicate that a higher BMI is inversely related to inguinal hernia incidence. However, the unique physiological considerations of the obese population still place them at risk of perioperative adverse events after inguinal hernia repair (IHR). A cohort study including 12,697 patients undergoing IHR confirms this, with findings showing an association between increasing BMI and risk of postoperative complications as well as length of stay (LOS) [39]. Here, the robotic platform may have significant advantages over other surgical modalities in terms of reducing these risks. r-IHR is typically performed using the r-TAPP technique. In contrast to laparoscopic repair, flap suturing with this technique is facilitated by the robotic platform, much like in VHR. One of the only investigations into the role of r-IHR in obesity is a multicenter study by Kolachalam et al. [40], where 651 r-TAPP repairs were compared with 593 open IHRs (plug-and-patch, Lichtenstein, or the Prolene hernia system) in a propensity-matched analysis of an obese population; after matching, 96 patients were allocated to the r-IHR group with a mean BMI of 33.6 and 93 patients were allocated to the o-IHR group with a mean BMI of 34.2; a significantly higher proportion of postoperative complications were seen in the o-IHR group as compared to the r-IHR group (10.8 vs. 3.2%, respectively); the authors' conclusions support the efficacy of this technique and help encourage the adoption of minimally invasive hernia repair with its associated clinical benefits. Literature surrounding r-IHR, particularly in obesity, is still in its early stages. Further study of robotics with this patient population is warranted to establish clear guidelines and benefits of this technique.
Conclusion
The treatment of hernia in patients with excess body weight needs an interdisciplinary approach. The perioperative morbidity and the incidence of recurrence are significantly higher in obese patients. For patients with low risk of hernia accident, a bariatric procedure before hernia repair may be considered. The results of laparoscopic hernia repair in patients with excess body weight have been further improved by the robotic approach, with high rate of gap closure and extraperitoneal mesh implantation. Further interdisciplinary algorithms need to be matured to guide tailored approach. Future studies will further refine the benefits of robotic surgery and provide guidance for optimal tailoring.
Statement of Ethics
The use of the images of Figures 1 and 3 is used according the terms of use of the Ethic Commission for the Northwest and Central Switzerland (2019-02046). No human or animal studies were conducted by the authors for this paper. For the studies listed, the ethical guidelines stated therein apply in each case.
Conflict of Interest Statement
U.A.D. and O.Y.K. are proctors for robotic hernia surgery (Intuitive); all the other authors have nothing to disclose. soH (U.A.D.) has received teaching course fees from Intuitive Surgical. O.Y.K. has received teaching course and/or consultancy fees from Intuitive Surgical, Bard-Davol, and W.L. Gore & Associates outside the submitted work.
Funding Sources
This is a review text that has not been funded by any third party.
Author Contributions
U.A.D. and A.W. designed the review. U.P., J.B., and G.R. contributed with the bariatric approaches and the risk. O.Y.K., F.G., and N.B.-A. contributed with the topics of robotic repair. U.A.D. and U.P. created the figures and algorithm. U.A.D., U.P., and A.W. drafted the final manuscript.
References
- 1.Pearson DG, Carbonell AM. Obesity and abdominal wall reconstruction: outcomes, implications, and optimization. Plast Reconstr Surg. 2018 Sep;142((3 Suppl)):30S–5S. doi: 10.1097/PRS.0000000000004845. [DOI] [PubMed] [Google Scholar]
- 2.Liang MK, Bernardi K, Holihan JL, Cherla DV, Escamilla R, Lew DF, et al. Modifying Risks in Ventral Hernia Patients With Prehabilitation: A Randomized Controlled Trial. Ann Surg. 2018 Oct;268((4)):674–80. doi: 10.1097/SLA.0000000000002961. [DOI] [PubMed] [Google Scholar]
- 3.Zavlin D, Jubbal KT, Van Eps JL, Bass BL, Ellsworth WA, 4th, Echo A, et al. Safety of open ventral hernia repair in high-risk patients with metabolic syndrome: a multi-institutional analysis of 39,118 cases. Surg Obes Relat Dis. 2018 Feb;14((2)):206–13. doi: 10.1016/j.soard.2017.09.521. [DOI] [PubMed] [Google Scholar]
- 4.Menzo EL, Hinojosa M, Carbonell A, Krpata D, Carter J, Rogers AM. American Society for Metabolic and Bariatric Surgery and American Hernia Society consensus guideline on bariatric surgery and hernia surgery. Surg Obes Relat Dis. 2018 Sep;14((9)):1221–32. doi: 10.1016/j.soard.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Winters H, Knaapen L, Buyne OR, Hummelink S, Ulrich DJ, van Goor H, et al. Pre-operative CT scan measurements for predicting complications in patients undergoing complex ventral hernia repair using the component separation technique. Hernia. 2019 Apr;23((2)):347–54. doi: 10.1007/s10029-019-01899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadaleto BF, Herbella FA, Patti MG. Gastroesophageal reflux disease in the obese: pathophysiology and treatment. Surgery. 2016 Feb;159((2)):475–86. doi: 10.1016/j.surg.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Weiss R, Appelbaum L, Schweiger C, Matot I, Constantini N, Idan A, et al. Short-term dynamics and metabolic impact of abdominal fat depots after bariatric surgery. Diabetes Care. 2009 Oct;32((10)):1910–5. doi: 10.2337/dc09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang MK, Holihan JL, Itani K, Alawadi ZM, Gonzalez JR, Askenasy EP, et al. Ventral Hernia Management: Expert Consensus Guided by Systematic Review. Ann Surg. 2017 Jan;265((1)):80–9. doi: 10.1097/SLA.0000000000001701. [DOI] [PubMed] [Google Scholar]
- 9.Khorgami Z, Haskins IN, Aminian A. Concurrent ventral hernia repair in patients undergoing laparoscopic bariatric surgery: a case-matched study using the National Surgical Quality Improvement Program Database Surg Obes Relat Dis. 2017;13:997–1002. doi: 10.1016/j.soard.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Moolla M, Dang J, Modasi A, Byrns S, Switzer N, Birch DW, et al. Concurrent Laparoscopic Ventral Hernia Repair with Bariatric Surgery: a Propensity-Matched Analysis. J Gastrointest Surg. 2020 Jan;24((1)):58–66. doi: 10.1007/s11605-019-04291-0. [DOI] [PubMed] [Google Scholar]
- 11.Praveen Raj P, Bhattacharya S, Saravana Kumar S, Parthasarathi R, Cumar B, Palanivelu C. Morbid obesity with ventral hernia: is concomitant bariatric surgery with laparoscopic ventral hernia mesh repair the best approach? An experience of over 150 cases. Surg Obes Relat Dis. 2019 Jul;15((7)):1098–103. doi: 10.1016/j.soard.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Newcomb WL, Polhill JL, Chen AY, Kuwada TS, Gersin KS, Getz SB, et al. Staged hernia repair preceded by gastric bypass for the treatment of morbidly obese patients with complex ventral hernias. Hernia. 2008 Oct;12((5)):465–9. doi: 10.1007/s10029-008-0381-1. [DOI] [PubMed] [Google Scholar]
- 13.Eid GM, Wikiel KJ, Entabi F, Saleem M. Ventral hernias in morbidly obese patients: a suggested algorithm for operative repair. Obes Surg. 2013 May;23((5)):703–9. doi: 10.1007/s11695-013-0883-5. [DOI] [PubMed] [Google Scholar]
- 14.Olmi S, Uccelli M, Cesana GC, Ciccarese F, Oldani A, Giorgi R, et al. Laparoscopic Ventral Hernia Repair in Bariatric Patients: the Role of Defect Size and Deferred Repair. Obes Surg. 2020 Oct;30((10)):3905–11. doi: 10.1007/s11695-020-04747-2. [DOI] [PubMed] [Google Scholar]
- 15.Toma T, Harling L, Athanasiou T, Darzi A, Ashrafian H. Does Body Contouring After Bariatric Weight Loss Enhance Quality of Life? A Systematic Review of QOL Studies. Obes Surg. 2018 Oct;28((10)):3333–41. doi: 10.1007/s11695-018-3323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer JP, Tuggle CT, Wes AM, Kovach SJ. Concurrent panniculectomy with open ventral hernia repair has added risk versus ventral hernia repair: an analysis of the ACS-NSQIP database. J Plast Reconstr Aesthet Surg. 2014 May;67((5)):693–701. doi: 10.1016/j.bjps.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Saxe A, Schwartz S, Gallardo L, Yassa E, Alghanem A. Simultaneous panniculectomy and ventral hernia repair following weight reduction after gastric bypass surgery: is it safe? Obes Surg. 2008 Feb;18((2)):192–5. doi: 10.1007/s11695-007-9344-3. [DOI] [PubMed] [Google Scholar]
- 18.Domi R, Laho H. Anesthetic challenges in the obese patient. J Anesth. 2012 Oct;26((5)):758–65. doi: 10.1007/s00540-012-1408-4. [DOI] [PubMed] [Google Scholar]
- 19.Lock JF, Wagner J, Luber V, Dietz UA, Lichthardt S, Matthes N, et al. [Perioperative handling of anticoagulation] Chirurg. 2018 Feb;89((2)):95–102. doi: 10.1007/s00104-017-0526-9. [DOI] [PubMed] [Google Scholar]
- 20.Sebaaly J, Covert K. Enoxaparin Dosing at Extremes of Weight: Literature Review and Dosing Recommendations. Ann Pharmacother. 2018 Sep;52((9)):898–909. doi: 10.1177/1060028018768449. [DOI] [PubMed] [Google Scholar]
- 21.Borkgren-Okonek MJ, Hart RW, Pantano JE, Rantis PC, Jr, Guske PJ, Kane JM, Jr, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008 Sep-Oct;4((5)):625–31. doi: 10.1016/j.soard.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Bittner R, Bain K, Bansal VK, Berrevoet F, Bingener-Casey J, Chen D, et al. Update of Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS))-Part A. Surg Endosc. 2019 Oct;33((10)):3069–139. doi: 10.1007/s00464-019-06907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietz UA, Fleischhacker A, Menzel S, Klinge U, Jurowich C, Haas K, et al. Risk-adjusted procedure tailoring leads to uniformly low complication rates in ventral and incisional hernia repair: a propensity score analysis and internal validation of classification criteria. Hernia. 2017 Aug;21((4)):569–82. doi: 10.1007/s10029-017-1622-y. [DOI] [PubMed] [Google Scholar]
- 24.Alfalah H, Philippe B, Ghazal F, Jany T, Arnalsteen L, Romon M, et al. Intragastric balloon for preoperative weight reduction in candidates for laparoscopic gastric bypass with massive obesity. Obes Surg. 2006 Feb;16((2)):147–50. doi: 10.1381/096089206775565104. [DOI] [PubMed] [Google Scholar]
- 25.Schluender S, Conrad J, Divino CM, Gurland B. Robot-assisted laparoscopic repair of ventral hernia with intracorporeal suturing. Surg Endosc. 2003 Sep;17((9)):1391–5. doi: 10.1007/s00464-002-8795-9. [DOI] [PubMed] [Google Scholar]
- 26.Warren JA, Love M. Incisional Hernia Repair: Minimally Invasive Approaches. Surg Clin North Am. 2018 Jun;98((3)):537–59. doi: 10.1016/j.suc.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Parker SG, Halligan S, Liang MK, et al. An International Classification for Abdominal Wall Planes (ICAP): A Delphi Consensus of Expert Hernia Surgeons. Br J Surg. 2020a;107:209–17. doi: 10.1002/bjs.11400. [DOI] [PubMed] [Google Scholar]
- 28.Donkor C, Gonzalez A, Gallas MR, Helbig M, Weinstein C, Rodriguez J. Current perspectives in robotic hernia repair. Robot Surg. 2017 May;4:57–67. doi: 10.2147/RSRR.S101809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez A, Escobar E, Romero R, Walker G, Mejias J, Gallas M, et al. Robotic-assisted ventral hernia repair: a multicenter evaluation of clinical outcomes. Surg Endosc. 2017 Mar;31((3)):1342–9. doi: 10.1007/s00464-016-5118-0. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez AM, Romero RJ, Seetharamaiah R, Gallas M, Lamoureux J, Rabaza JR. Laparoscopic ventral hernia repair with primary closure versus no primary closure of the defect: potential benefits of the robotic technology. Int J Med Robot. 2015 Jun;11((2)):120–5. doi: 10.1002/rcs.1605. [DOI] [PubMed] [Google Scholar]
- 31.Gokcal F, Morrison S, Kudsi OY. Short-term comparison between preperitoneal and intraperitoneal onlay mesh placement in robotic ventral hernia repair. Hernia. 2019a Oct;23((5)):957–67. doi: 10.1007/s10029-019-01946-4. [DOI] [PubMed] [Google Scholar]
- 32.Panteleimonitis S, Pickering O, Abbas H, Harper M, Kandala N, Figueiredo N, et al. Robotic rectal cancer surgery in obese patients may lead to better short-term outcomes when compared to laparoscopy: a comparative propensity scored match study. Int J Colorectal Dis. 2018 Aug;33((8)):1079–86. doi: 10.1007/s00384-018-3030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudsi OY, Gokcal F, Bou-Ayash N, Crawford AS, Chung SK, Chang K, et al. Learning curve in robotic transabdominal preperitoneal (rTAPP) ventral hernia repair: a cumulative sum (CUSUM) analysis. Hernia. 2020 Jun; doi: 10.1007/s10029-020-02228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belyansky I, Reza Zahiri H, Sanford Z, Weltz AS, Park A. Early operative outcomes of endoscopic (eTEP access) robotic-assisted retromuscular abdominal wall hernia repair. Hernia. 2018 Oct;22((5)):837–47. doi: 10.1007/s10029-018-1795-z. [DOI] [PubMed] [Google Scholar]
- 35.Gokcal F, Morrison S, Kudsi OY. Robotic retromuscular ventral hernia repair and transversus abdominis release: short-term outcomes and risk factors associated with perioperative complications. Hernia. 2019b Apr;23((2)):375–85. doi: 10.1007/s10029-019-01911-1. [DOI] [PubMed] [Google Scholar]
- 36.Bittner R, Bain K, Bansal VK, Berrevoet F, Bingener-Casey J, Chen D, et al. Update of Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS)): part B. Surg Endosc. 2019 Nov;33((11)):3511–49. doi: 10.1007/s00464-019-06908-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudsi OY, Gokcal F, La Grange S, Bou-Ayash N, Chang K. Are elderly patients at high risk for postoperative complications after robotic ventral hernia repair? A propensity score matching analysis. Int J Med Robot. 2020 Jun;16((3)):e2095. doi: 10.1002/rcs.2095. [DOI] [PubMed] [Google Scholar]
- 38.Kudsi OY, Gokcal F, Chang K. Propensity score matching analysis of short-term outcomes in robotic ventral hernia repair for patients with a body mass index above and below 35 kg/m(2) Hernia. 2019 doi: 10.1007/s10029-019-02108-2. [DOI] [PubMed] [Google Scholar]
- 39.Lindström D, Sadr Azodi O, Bellocco R, Wladis A, Linder S, Adami J. The effect of tobacco consumption and body mass index on complications and hospital stay after inguinal hernia surgery. Hernia. 2007 Apr;11((2)):117–23. doi: 10.1007/s10029-006-0173-4. [DOI] [PubMed] [Google Scholar]
- 40.Kolachalam R, Dickens E, D'Amico L, Richardson C, Rabaza J, Gamagami R, et al. Early outcomes of robotic-assisted inguinal hernia repair in obese patients: a multi-institutional, retrospective study. Surg Endosc. 2018 Jan;32((1)):229–35. doi: 10.1007/s00464-017-5665-z. [DOI] [PubMed] [Google Scholar]