Abstract
Background
Bilateral-Risk-Reducing-Mastectomy-(BRRM) is well described in BRCA1/2 pathogenic variant carriers. However, little is known about the relative uptake, time trends or factors influencing uptake in those at increased breast cancer risk not known to be carriers. The aim of this study is to assess these factors in both groups.
Methods
BRRM uptake was assessed from entry to the Manchester Family History Clinic or from date of personal BRCA1/2 test. Follow up was censored at BRRM, breast cancer diagnosis, death or January 01, 2020. Cumulative incidence and cause specific and competing risk regression analyses were used to assess the significance of factors associated with BRRM.
Results
Of 7195 women at ≥25% lifetime breast cancer risk followed for up to 32 years, 451 (6.2%) underwent pre-symptomatic BRRM. Of those eligible in different risk groups the 20-year uptake of BRRM was 47.7%-(95%CI = 42.4–53.2%) in 479 BRCA1/2 carriers; 9.0% (95%CI = 7.26–11.24%) in 1261 women at ≥40% lifetime risk (non-BRCA), 4.8%-(95%CI = 3.98–5.73%) in 3561 women at 30–39% risk and 2.9%-(95%CI = 2.09–4.09%) in 1783 women at 25–29% lifetime risk. In cause-specific Cox regression analysis death of a sister with breast cancer<50 (OR = 2.4; 95%CI = 1.7–3.4), mother<60 (OR = 1.9; 95%CI = 1.5–2.3), having children (OR = 1.4; 95%CI = 1.1–1.8), breast biopsy (OR = 1.4; 95%CI = 1.0–1.8) were all independently associated with BRRM uptake, while being older at assessment was less likely to be associated with BRRM (>50; OR = 0.26,95%CI = 0.17–0.41). Uptake continued to rise to 20 years from initial risk assessment.
Conclusion
We have identified several additional factors that correlate with BRRM uptake and demonstrate continued increases over time. These factors will help to tailor counselling and support for women.
Keywords: Risk reducing mastectomy, Predictors, Prevention, Breast cancer, BRCA1, BRCA2
Abbreviations: BC, Breast cancer; PV, Pathogenic variant; BRRM, Bilateral Risk Reducing Mastectomy
Highlights
-
•
BRRM continues even 20 years post original breast cancer risk assessment.
-
•
Potential triggers include death of mother/sister, children and a breast biopsy.
-
•
Uptake is clearly informed by lifetime risk of BC and higher in younger the women.
1. Introduction
The uptake of risk Bilateral-Risk-Reducing-Mastectomy-(BRRM) in women unaffected by breast cancer (BC) has been increasing, especially since the actress Angelina Jolie revealed her own decision to undergo surgery in May 2013 [1,2]. Although greater awareness of the option of surgery is a factor, there has been very little published on the predictors of uptake of BRRM in women outside of those carrying pathogenic variants (PVs) in BRCA1/BRCA2 [3]. Even in studies on uptake of BRRM in PV carriers most studies include both those undergoing pre-symptomatic BRRM and contralateral BRRM after a cancer diagnosis [4,5]. We have previously demonstrated that women at higher levels of counselled risk are more likely to undergo BRRM and that a recent false positive screen especially with a breast biopsy may also be a trigger in deciding to have surgery [6]; findings replicated by others [7]. We now update our previous work, with more than twice as many women, to assess time trends and assess factors associated with BRRM uptake in those not carrying a known BRCA1/2 PV.
2. Materials and methods
Unaffected women with a family history of BC have been referred to the Family-History-Risk-and-Prevention-Clinic (FHRPC) at the Nightingale Centre Withington/Wythenshawe Hospitals, Manchester since 1987. Lifetime and residual risk of BC was assessed using family history and standard risk factors, mainly using a manual model based on the Claus tables to 2004 [[8], [9], [10], [11]] and thereafter using the Tyrer-Cuzick model [9]. Both models were found to be equally well calibrated and show good discrimination [10,11]. Risk information was provided to women primarily by experienced clinicians (AH,SJH,DGE), with training in risk communication. Women were provided with an ‘overall’ lifetime-risk as well as a residual-risk figure although we used the overall risk figure as indicative in the study. Women with a proven familial PV in BRCA1/2 gene are offered targeted testing, which looks only for their familial PV. In contrast, unaffected women with strong family histories of BC and/or ovarian cancer-(OC) have been offered full BRCA1/2 gene PV screening if there is no affected family member available for testing and their a priori likelihood of a BRCA1/2 PV is ≥ 10% (since 2013).
Women with lifetime BC risks ≥25%, including BRCA1/2 carriers, have been offered a discussion about BRRM since 1994. Prior to this we responded to requests to discuss surgery. Risks were subdivided into: group-1 (≥40% lifetime-risk-very-high), group-2 (30-39%-lifetime-risk-high) and group-3 (25-29%-lifetime-risk-moderate) as we have previously reported these determine uptake and are based on NICE guideline categories [12]. All women at these risk levels were offered annual mammography screening from 35 to 50 years of age, with those at ≥30% lifetime risk until age 60 years.
We used a prospective cohort design to evaluate the long-term uptake of BRRM among all women with ≥25% lifetime risk including BRCA1/2 PV carriers. The individuals were identified from a prospectively maintained FHRPC database. The study was approved by the Central-Manchester-Research-Ethics-Committee-(10/H1008/24). All women considering BRRM have at least two risk sessions (DGE,AH,SJH) before a psychological assessment (JW) and then at least two surgical sessions (JH,LB,LH,AG,JM) before undertaking BRRM in line with NICE guidance [12].
2.1. Study population
Women unaffected by BC were eligible for follow up based on risk alone from date of entry until date of BC diagnosis, BRRM, family mutation date, death, 80th birthday, date of moving area or January 01, 2020 whichever was earliest. Those from families with BRCA1/2 PVs were eligible for uptake of BRRM from the date of personal positive PV test. Vital status was confirmed from NHS systems and BCs from the cancer registry and pathology records. As such a BRCA1/2 carrier from a family whose PV was not known at entry spent a sojourn time initially in a risk group-(1–3), and finally only after testing positive from date of genetic test. Uptake over time was assessed by modelling the cumulative incidence function (CIF) of BRRM using competing risk analysis where withdrawals due to death/BC were treated as competing risks.
2.2. Data collection
We retrieved data on date of birth, parity, date individual PV test, gene (BRCA1/BRCA2), date BRRM, date BC diagnosis and date/cause of death. In addition, evidence that mother had died aged <60 years or sister aged <50 years with BC diagnosis was recorded. These age cut-offs were used pragmatically to reflect the young ages of women at initial assessment in clinic. Details of any non-malignant breast biopsies were also noted. Women were censored for uptake of BRRM at date of death, date moved area or BC diagnosis date. We decided not to censor at other cancer diagnosis as women with ovarian cancer still undergo BRRM although we usually delay this until relapse free at least 2-years post-diagnosis. An assessment of uptake in those diagnosed with ovarian cancer can be found in supplementary-table-1.
2.3. Statistical analysis
Statistical analyses were performed using Stata version-14 (StataCorp.2015.Stata Statistical-Software:Release-14.College-Station,TX:StataCorp-LP). A Chi-squared test was used to compare categorical variables. All p values were based on two-sided tests and were considered statistically significant if < 0.05. Traditional survival analysis methods (Kaplan-Meier or Cox proportional hazards regression) assume independent or non-informative censoring, so that those individuals who are censored are still considered as being at risk of an event (i.e.BRRM), and thus will tend to overestimate the cumulative incidence of the event of interest. A cause-specific hazard Cox regression was performed to determine independent associations of first degree relative early death from BC, parity and breast biopsy on uptake of BRRM and for competing risks (BC/death). Effects of age group at entry (<30; 30–39,40-49,50+) and era (<1994; 1994–2003; 2004-Apr-2013; May-2013-) were also assessed. In addition we performed a Fine and Gray regression analysis [13] to obtain the sub hazard ratios (HR) for BRRM in the presence of competing events, with the sub-hazards presented for BRRM and for other causes (breast cancer/death) [14]. Cause-specific hazard regression is considered to be more appropriate for etiologic research, whilst sub-hazard regression for prediction research, however, the latter are more difficult to interpret and some authors recommend presenting results from both models [15].
3. Results
3.1. Study population
Of 14298 women without a previous or current BC diagnosis seen at the FHRPC from 1987 to 2019, 7195 (50.3%) were assessed as being at ≥25% residual lifetime risk of BC at clinic entry (Table 1). Within this group, 6605 women did not have a known BRCA1/2 or other high-risk gene PV in the family at entry and received a risk estimation based on family history and standard risk factors (Fig. 1). In total, 1261 women had a risk of 40–49% (Group-1), 3561 at 30–39% (Group-2) and 1783 at 26–29% (group 3). An additional 590 women were referred from families with known high risk PVs. Of the 6605 women without a known family PV at entry, 407 were subsequently censored as having a BRCA1/2 PV in the family (Supplementary table- 2.Figure 1) with 158 (39%) being found to carry this family BRCA1/2 PV (Fig. 1; supplementary-table-2). A further 317 women were censored at breast cancer diagnosis and 63 at death.
Table 1.
Demographics of each risk group follow up and BRRM.
| Grp 1 v high 40–49% | Grp 2 high 30–39% | Grp 3 high moderate 25–29% | High risk mutation known in family at entry | Grp1-3a post fam BRCA result | Total | BRCA1/2 carriers from Individual test | untested high risk gene | Negative for BRCA in family | non BRCA carrier | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 1261 | 3561 | 1783 | 590 | 407 | 7195 | 479b | 191 | 289+ | 16 |
| age range at entry+ (years) | 16.0–70.1 | 16.9–72.0 | 16.7–70.8 | 18.3–77.4 | 22.2–78.2 | 16–77.4 | 18.6–75.7+ | 19.7–69 | 18.8–68.3+ | 30.3–48.6+ |
| Median | 38.0 | 38.9 | 40.2 | 36.1 | 42.4 | 38.9 | 38.4 | 37.7 | 42.1 | 35.8 |
| age range at censor (years) | 16.0–85.2 | 17.8–89.2 | 18.9–98.8 | 24.3–88.0 | 27.6–91.2 | 16.0–98.8 | 24.3–88.0 | 25.2–91.2 | 25.0–81.9 | 35.0–72.1 |
| Median | 52.4 | 51.6 | 53.0 | 44.6 | 54.3 | 51.6 | 44.0 | 50.41 | 53.3 | 47.7 |
| Parous | 915 | 2455 | 1203 | 390 | 331 | 4963 | 355 | 125 | 218 | 10 |
| % parous | 72.56% | 68.94% | 67.47% | 66.10% | 81.33% | 68.98% | 74.11% | 65.45% | 75.43% | 66.67% |
| non malignant biopsy | 106 | 290 | 139 | 44 | 51 | 579 | 40 | 22 | 29 | 3 |
| % | 8.41% | 8.14% | 7.80% | 7.46% | 12.53% | 8.05% | 8.35% | 11.52% | 10.03% | 20.00% |
| Sister/mother death BC | 310 | 666 | 358 | 83 | 126 | 1417 | 108 | 37 | 55 | 3 |
| % | 24.58% | 18.70% | 20.08% | 14.07% | 30.96% | 19.69% | 22.55% | 19.37% | 19.03% | 20.00% |
| BRRM | 87 | 127 | 36 | 146 | 55 | 451 | 193 | 2 | 1 | 4 |
| % | 6.90% | 3.57% | 2.02% | 24.75% | 13.51% | 6.27% | 40.29% | 1.05% | 0.34% | 26.67% |
| median to BRRM years | 2.8 | 3.5 | 4.3 | 1.7 | 1.9 | 1.5 | 2.98 | |||
| years fu | 15017.09 | 40595.32 | 22243.15 | 4400.15 | 4530.445 | 86786.16 | 2866.48 | 2404.97 | 2977.84 | 177.62 |
| BC in total population | 76 | 165 | 76 | 33 | 33 | 383 | 42 | 4 | 4 | 1 |
| BC rate per 1000/year | 5.06 | 4.06 | 3.42 | 7.50 | 7.73 | 4.41 | 14.65 | 1.66 | 1.34 | 5.63 |
| b82 censored before test | +20 censored before test |
Numbers counted in this group are already counted in Groups 1–3 but breast cancers and BRRM occurred after censoring for identification of a family PV, group excludes those who were censored before PV (55 BC, 29 BRRM, 2 died, 5 moved area); BC = breast cancer.
or from genetic test in later columns.
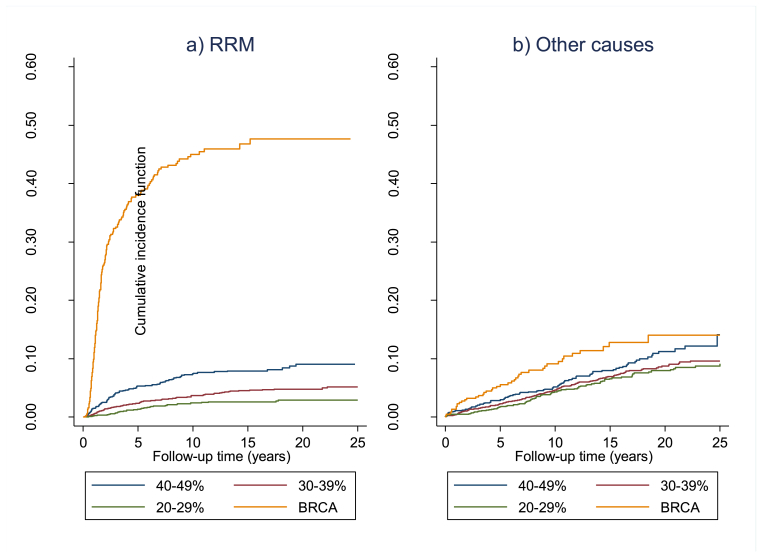
Fig. 1.
CONSORT diagram for all 7195 women showing BRRM and breast cancers. BRRM figures in red contribute to prospective BRRM in BRCA carriers with in blue those that were based on risk. In green are additional BRRMs in families with PVs excluding BRCA carriers).
A further 321 women were referred to the FHRPC as either known PV BRCA1/2 carriers (n = 192) or from families with a known PV who were subsequently tested positive (n = 129; Fig. 1). Thus 479 BRCA1/2 carriers were eligible for BRRM assessment as a living PV carrier unaffected by BC (Fig. 1). A further 16 women with PVs in other high-risk genes have been referred (4xTP53,4xPTEN,5xPALB2,3xCDH1) of which four have undergone BRRM (2xPALB2,1xPTEN,1xCDH1). This group was considered too small for analysis and was not investigated further. A further seven were referred untested for other high-risk genes. There have been 196 deaths overall, 107 after a BC diagnosis (BC-65) 40 in BRCA PV carriers (BC = 23) with six dying who underwent BRRM (BC = 2 occult at surgery).
3.2. Follow-up time
Follow up comprised/totalled 86786.16 women years. BC was diagnosed in 383 women in the entire cohort giving an annual incidence rate of 4.4 per 1000 (excluding seven occult cancers found at BRRM). One-hundred-and-sixty-three women were censored at date of moving area, 80 at 80th birthday and 65 at date of death. Four-hundred and fifty-one women (6.3%) have undergone pre-symptomatic BRRM, 193 with a BRCA PV (15 identified after BRRM) and 258 without a known PV at surgery. Seven occult BCs (1.75%) were diagnosed at surgery, 3 in BRCA PV carriers. Median time to BRRM was 2.97 years (range 0.14–22.3years; IQR = 1.3–6.7years) from clinic entry with 154 (34.1%) having surgery >5 years after clinic entry, 47 (10.4%) >10 years and three more than 20 years after entry. Uptake was clearly related to risk with only 1.95% of those at 26–29% risk, 3.6% of those at 30–39% risk and 6.9% of those at 40–49% risk having undergone BRRM (Fig. 1; p < 0.0001), whilst in that risk group. Four carriers of PVs in other high-risk genes and four untested women also underwent BRRM. Uptake at 10 and 20years (by CIF) was 45.0% and 47.7% for BRCA1/2 PV carriers (Table 2) compared to 7.4% and 9.0% for group 1, 3.7% and 4.8% for group 2 and 2.4% and 2.9% for group 3 (p < 0.0001). Median time to uptake of BRRM was also risk dependent with BRCA1/2 PV carriers opting for surgery much sooner than other risk categories (Table 1,Fig. 2).
Table 2.
BRRM uptake at 10 and 20 years by risk group and additional factors.
| BRRM |
Competing risks (breast cancer/death) |
||||
|---|---|---|---|---|---|
| 10 year % (95%CI) | 20 year % (95%CI) | 10 year % (95%CI) | 20 year % (95%CI) | ||
| Risk group | 25–29% | 2.39 (1.69–3.38) | 2.92 (2.09–4.09) | 4.33 (3.31–5.65) | 7.92 (6.37–9.84) |
| 30–39% | 3.66 (3.03–4.41) | 4.78 (3.98–5.73) | 4.58 (3.85–5.44) | 8.73 (7.48–10.18) | |
| 40–49% | 7.39 (5.96–9.14) | 9.04 (7.26–11.24) | 5.13 (3.95–6.67) | 11.25 (9.05–13.96) | |
| BRCA1/2 | 45.00 (40.18–50.11) | 47.67 (42.40–53.24) | 9.11 (6.55–12.59) | 14.05 (10.08–19.41) | |
| Mother BC | No FDR died BC | 5.67 (5.05–6.37) | 6.73 (5.98–7.58) | 4.55 (3.96–5.22) | 8.72 (7.70–9.88) |
| Mother BC death | 10.22 (8.52–12.25) | 11.68 (9.78–13.93) | 5.53 (4.27–7.16) | 10.72 (8.71–13.17) | |
| Sister BC | Sister death | 10.49 (7.60–14.38) | 11.66 (8.57–15.77) | 6.06 (3.90–9.35) | 11.19 (1.93–15.64) |
| Age entry | <30 | 8.77 (6.89–11.13) | 11.93 (9.44–15.03) | 1.50 (0.90–2.80) | 5.05 (3.29–7.71) |
| 30–39 | 8.46 (7.46–9.59) | 9.42 (8.32–10.67) | 3.93 (3.22–4.78) | 8.36 (7.05–9.89) | |
| 40–49 | 4.41 (3.59–5.41) | 5.33 (4.34–6.54) | 5.67 (4.70–6.83) | 10.07 (8.53–11.87) | |
| 50+ | 3.79 (2.64–5.43) | 3.79 (2.64–5.43) | 8.88 (6.91–11.39) | 13.78 (10.95–17.28) | |
| Biopsy | No | 6.34 (5.73–7.02) | 7.40 (6.69–8.19) | 5.22 (4.64–5.88) | 9.93 (8.95–11.02) |
| Yes | 9.24 (7.05–12.07) | 10.88 (8.37–14.09) | 0.40 (0.10–1.61) | 2.09 (0.10–4.43) | |
| Parity | Nulliparous | 5.21 (4.20–6.45) | 7.34 (6.60–8.16) | 4.48 (3.51–5.70) | 7.42 (5.93–9.27) |
| Parous | 6.32 (5.07–7.87) | 8.49 (7.64–9.42) | 4.97 (4.35–5.69) | 9.82 (8.75–11.01) | |
| Era | <1994 | 4.50 (3.04–6.65) | 5.79 (4.08–8.18) | 6.23 (4.47–8.66) | 15.96 (12.98–19.55) |
| 1994–2003 | 5.89 (5.00–6.92) | 6.97 (5.98–8.11) | 5.57 (4.71–6.59) | 9.39 (8.22–10.73) | |
| 2003-04/2013 | 7.86 (6.82–9.04) | 9.13 (7.77–10.79) | 4.48 (3.69–5.43) | 5.46 (4.47–6.67) | |
| ≥ May 2013 | 14.17 (4.82–37.7) | 14.17 (4.82–37.7) | 0.87 (0.05–1.57) | 0.87 (0.05–1.57) | |
RRM – Risk reducing mastectomy, BC – breast cancer, FDR – first degree relative.
Fig. 2.
Cumulative incidence function (CIF) for a) RRM and b) other causes in BRCA1/2 (BRCA carriers from individual test) from date of testing with risk groups from date of entry to clinic.
3.3. Associated factors for uptake of BRRM
Factors associated with BRRM in unadjusted analyses were, having had children (HR = 1.38,95% confidence interval-(CI) = 1.09–1.73,p = 0.007), a sister die with BC < 50 (HR 1.82,95%CI = 1.30–2.57,p = 0.001) or a mother die with BC < 60 (HR = 1.85,95%CI = 1.50–2.29,p < 0.0001) or a non-malignant breast biopsy (HR = 1.39,95%CI1.04–1.84, p = 0.024) (Supplementary-table-3). A cause-specific Cox regression (Table 3) showed these factors were still independently associated with BRRM uptake with hazards ratios for a sister dying with BC < 50 of 2.36 (95%CI = 1.66–3.35), mother<60 (HR = 1.86; 95%CI = 1.49–2.31), having children (OR = 1.43; 95%CI = 1.13–1.81) and a benign breast biopsy (OR1.36; 95%CI = 1.02–1.82) in those who were still alive and did not have a competing risk (death or breast cancer). There was also evidence for a stepwise decrease in uptake by age at entry with those aged <30 years at entry being more than 3.5 times more likely to undergo BRRM than those aged >50 (Table 3). Further a stepwise increase in uptake was seen by risk category compared to the 25–29% group: 30–39% risk (OR = 1.8; 95%CI = 1.2–2.6), 40–49% (OR = 3.2; 95%CI = 2.2–4.8) and BRCA1/2 group (OR = 36.5; 95%CI = 25.2–52.8). Overall, there was a substantial jump in BRRM following the May-2013 revelation of Angelina Jolie's surgery (supplementary Figure- 1) and those seen from May-2013 had a higher rate of BRRM (OR-1.68; 95%CI = 1.05–2.69). For BRCA1/2 carriers uptake was higher in those with known PVs in their family at entry, 138/316 (43.7%) versus 55/163 (33.7%)-p = 0.04 those found later: 10-year uptake 47.5% (95%CI = 41.7–53.8) versus 39.4% (95%CI = 31.4–48.6) in those with PVs found later (OR = 1.2; 95%CI = 0.9–1.6)-Supplementary-table-4, this was of borderline significance for BRCA1 (OR = 1.6; 95%CI = 0.99–2.4); p = 0.077 supplementary-figure-2). Uptake and cause-specific HRs for competing risks are shown in Table 2, Table 3 The HR for competing risks (death/BC) were also significant for those with 30–39%, 40–49% and the BRCA1/2 groups compared to the 25–29% group. Older age was associated with increased HRs of competing risks, while for time of assessment the converse was true. In contrast to BRRM a benign breast biopsy was associated with a decreased HR for competing risks. Results for the Fine and Gray regression models were broadly similar to those for the cause-specific regression since the proportion of those with competing risks was relatively low.
Table 3.
Multivariate cause-specific Cox regression and Fine and Gray regression model: All variables adjusted for other factors in the model.
| Cause specific - RRM | Cause specific - other | SHR - RRM | SHR - other | ||
|---|---|---|---|---|---|
| Risk group | Group 3 25–29% | Ref | Ref | ||
| Group 2 30–39% | 1.78 (1.22–2.58) | 1.31 (1.01–1.70) | 1.76 (1.21–2.56) | 1.31 (1.01–1.70) | |
| Group 1 ≥ 40% | 3.22 (2.17–4.77) | 1.70 (1.25–2.29) | 3.17 (2.14–4.71) | 1.64 (1.21–2.20) | |
| BRCA1/2 | 36.46 (25.18–52.79) | 5.71 (3.97–8.20) | 34.39 (23.68–49.93) | 3.84 (2.63–5.60) | |
| High risk non-BRCA | 1.57 (0.69–3.54) | 0.54 (0.20–1.48) | 1.57 (0.70–3.55) | 0.54 (0.20–1.50) | |
| Mother died BC | No | Ref | Ref | ||
| Yes | 1.86 (1.49–2.31) | 1.23 (0.96–1.55) | 1.86 (1.51–2.30) | 1.17 (0.93–1.48) | |
| Sister died BC | No | Ref | Ref | ||
| Yes | 2.36 (1.66–3.35) | 1.04 (0.73–1.50) | 2.33 (1.62–3.33) | 0.99 (0.69–1.42) | |
| Age entry | <30 | Ref | Ref | Ref | |
| 30–39 | 0.80 (0.61–1.04) | 1.90 (1.24–2.92) | 0.78 (0.60–1.08) | 1.94 (1.27–2.95) | |
| 40–49 | 0.48 (0.35–0.65) | 2.47 (1.61–3.80) | 0.46 (0.34–0.63) | 2.63 (1.72–4.03) | |
| 50+ | 0.26 (0.17–0.41) | 3.45 (2.18–5.46) | 0.26 (0.16–0.40) | 3.78 (2.40–5.96) | |
| Biopsy | No | Ref | Ref | ||
| Yes | 1.36 (1.02–1.82) | 0.12 (0.06–0.26) | 1.42 (1.06–1.91) | 0.12 (0.06–0.26) | |
| Parity | Nulliparous | Ref | Ref | ||
| Parous | 1.43 (1.13–1.81) | 1.09 (0.85–1.38) | 1.44 (1.13–1.82) | 1.07 (0.84–1.36) | |
| Era | <1994 | Ref | Ref | ||
| 1994–2003 | 1.03 (0.69–1.53) | 0.49 (0.38–0.64) | 1.01 (0.68–1.49) | 0.48 (0.37–0.62) | |
| 2003-04/2013 | 1.02 (0.67–1.55) | 0.34 (0.25–0.46) | 1.00 (0.66–1.50) | 0.31 (0.23–0.43) | |
| > May 2013 | 1.68 (1.05–2.69) | 0.17 (0.09–0.33) | 1.66 (1.06–2.61) | 0.14 (0.07–0.25) |
(n.b. sister BC death and mother BC deaths entered rather than mother or sister vs no FDR BC death – as this excludes those that have a mother or sister with BC death due to missing values). RRM – risk reducing mastectomy, HR – hazard ratio, SHR – subhazard ratio, CI – confidence interval.
4. Discussion
To our knowledge, this is the largest study of uptake of BRRM in unaffected women at high-risk of BC. This updates our previous report from 2009 in 211 known unaffected BRCA1/2 PV carriers and 3515 women at ≥25% lifetime-risk [6]. As such we have more than doubled the BRCA1/2 population and doubled the non-BRCA PV carriers with almost twice the follow up. We have shown a clear impact of counselled risk on BRRM uptake over a more than 30-year period. Uptake continues to rise up to the 20-year point and three BRRM were undertaken >20 years post initial risk assessment. We were unable to find any other studies assessing uptake of BRRM in unaffected women who were not known to be carriers of a PV in a high-risk gene. A number of studies have assessed uptake of BRRM in unaffected BRCA1/2 PV carriers [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. In a Canadian study 537 women without prior BC, 30.8% BRCA1 carriers and 28.3% BRCA2 underwent BRRM after at least 1-year from genetic testing [16]. In a Welsh study 280 women underwent pre-symptomatic BRCA1/2 PV testing (median-age = 36) of whom 34% underwent BRRM (median-age = 37) [17]. In a retrospective US study 87 evaluable female BRCA1/2 PV without a cancer diagnosis at genetic testing, 51-(59%) undertook BRRM [18]. The only study, to our knowledge, to assess longitudinal uptake of BRRM using a Kaplan-Meier analysis from a multi-centre study assessed lifetime (to age 70years) reported uptake of 46% for BRRM in BRCA1/2 PV carriers [19]. However, the study did not assess uptake from date of testing and involved a very heterogeneous population with BRRM only assessed as the first risk reducing surgery option and not if undertaken after oophorectomy. Another US study of 305 eligible women, (BRCA1 = 170 positive) reported that 44% underwent BRRM in a median time from testing to surgery of 6 months [20], with a smaller US study of 136 unaffected women showing BRRM uptake of 42% [21]. Another West coast US study of 272 female carriers, followed for a median of 3.7 years showed a lower 23% uptake of BRRM [4]. Two Dutch studies reported cumulative uptakes of 35.6% of BRRM within the first five years after disclosure of DNA test results [5] and the earliest uptake study we could find showed 51% of 68 carriers opted for BRRM [22]. A Danish study showed that of 306 healthy BRCA1/2 carriers without a personal history of ovarian or BC, 50% opted for BRRM [23]. An Australian study of 325 unaffected female BRCA1/2 carriers reported that 21% underwent BRRM [24]. Differences in uptake by country were reported by one multicentre study with low uptake (≤10%) in Israel, Poland and Italy and higher uptake (>30%) in the Netherlands and North America [25]. A further study of 170 Israeli women without a cancer history, reported that only 22 (13%) had a risk-reducing mastectomy [26]. It is likely that these international differences reflect not only cultural differences, but also clinician attitudes and potentially the way in which BRRM is discussed, if at all, as a preventive option [27]. Our uptake of 47.7% for BRCA1/2 PV carriers is similar to the ∼40–50% uptake in many other North American and Northern European centres. However, this study has demonstrated a much longer follow up. On the basis of our uptake curves, follow up of <2 years, and even <5 years, is insufficient to assess the likely long-term uptake figures.
Women attending familial breast screening are likely to opt for pre-symptomatic testing with over 80% of eligible women in the Manchester FHRPC opting for this when available. Furthermore, women who have undergone BRRM when a test was not available were at least, if not more likely, to opt for testing when this became available (96.4%-p = 0.04).
A number of studies have also assessed factors that may predict which women choose to undergo BRRM [4,5,21,23,28,29]. A study from Los Angeles [21] showed that family history of first- and second-degree relatives having died from BC was predictive of uptake of BRRM (OR = 11.0; P = 0.005). Our data support this association beyond BRCA1/2 PV carriers to those at increased risk due to FH and standard risk factors, with deaths in first degree relatives. They also showed that parity predicted BRRM uptake (OR = 4.2; 95%CI = 1.8–10.0) [21], which was again corroborated by our analysis. A study from San Francisco [4] demonstrated that age below 60 years predicted uptake of BRRM (OR = 1.8,p = 0.04). One Dutch study [5] revealed that women were more likely to opt for BRRM if they were younger than 50 years of age HR = 2.67,95%CI = 1.30–5.48) or had a mother who had had BC (HR = 1.5195%CI = 1.04–2.18). A Canadian study also showed that women with a sister with BC were more likely to have a BRRM than those without a sister with BC (OR = 2.36,95%CI = 1.30–3.86) [29]. Another small study assessed the effect of false positive mammographic screening in 170 BRCA1/2 carriers and reported an increase in BRRM (OR = 4.2; 95%CI = 1.35–4.12) as well as cancer anxiety [28]. Our multivariate cause-specific Cox regression analysis confirmed that losing a mother or sister to BC at younger ages, having children or a breast biopsy after a false positive screen were all independently associated with uptake of BRRM in BRCA1/2 carriers and non-carriers. We also saw a higher uptake in women who underwent risk assessment from May-2013, and have continued to undertake larger numbers of BRRM since the Angelina Jolie news article [30] as seen in supplementary figure- 1. We also saw a lower uptake of BRRM in BRCA1/2 carriers whose family PV was found after clinic entry. It is possible that having been ‘reassured’ by years of screening these women were less likely to consider BRRM than those who were referred having without previous breast screening.
There are some limitations to the present study. We did not collect information on marital/partner status nor on education level or socio-economic class which may also have influenced uptake. Also we have not censored at other cancer diagnosis although we do not believe this would have affected the results. The women with BRCA1/2 path-variants have all initially opted-in to screening and the cohort therefore did not include those women referred directly from the Manchester Clinical Genetics Department to the protocol for BRRM. As such the uptake may underestimate uptake of BRRM in all BRCA carriers. We have not taken into account risk reassessments, for example due to additional relatives diagnosed with BC, other than for those identified as PV carriers, but have included all follow up, even of those eventually identified as a carrier until a PV was identified in their family. However, reassessment that changed risk category was infrequent. In the future, risk assessment is likely to be far more comprehensive, including testing of multiple common variants in a polygenic risk score, as moderate and high risk genes and non-familial risk factors such as mammographic density, are now incorporated into risk models such as Tyrer-Cuzick and CanRisk [31]. The current research suggests that if BC risk falls below 50%, even in BRCA PV carriers, due to favourable factors such as PRS, then the uptake of BRRM is likely to fall substantially.
In conclusion, we have shown that women continue to undertake BRRM even 20 years post their original BC risk assessment. Potential triggers for this include death of a mother or sister, having children and a breast biopsy after a false positive screen. Uptake is clearly informed by lifetime risk of BC and is more likely the younger the woman is at initial assessment.
Acknowledgements
DGE, EFH, SJH and AH are supported by the National Institute for Health Research (NIHR) BRC Manchester (Grant Reference Number 1215-200074). This work was also supported by Prevent Breast Cancer.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.08.015.
Ethics declaration
PROCAS was approved in 2009 by the Central Manchester Research Ethics Committee (reference: 09/H1008/81).
Author contribution
DGE and AH conceived the study; Data curation DGE, EFH data analysis DGE, EFH; manuscript writing all; approval of final version all.
Potential conflicts
DGE has received consultancy fees from Astrazeneca, Cerexis and Springworks.
Groups 1–3 - follow-up from date of entry to date of family mutation; High risk PV known at entry - follow-up from date of entry to date of censor; Group 1–3 post family BRCA mutation – follow-up from date of family mutation to date of censor; BRCA carriers from individual test – follow-up from date of individual test to date of censor; untested high risk gene – follow-up from date of family mutation to date of censor; Negative for BRCA family – follow-up from date of individual test to date of censor; non-BRCA carriers – follow up from date of entry to date of censor.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Evans D.G., Wisely J., Clancy T., et al. Longer term effects of the Angelina Jolie effect: increased risk-reducing mastectomy rates in BRCA carriers and other high-risk women. Breast Cancer Res. 2015 Nov 25;17(1):143. doi: 10.1186/s13058-015-0650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liede A., Cai M., Crouter T.F., Niepel D., Callaghan F., Evans D.G. Risk-reducing mastectomy rates in the US: a closer examination of the Angelina Jolie effect. Breast Canc Res Treat. 2018 Sep;171(2):435–442. doi: 10.1007/s10549-018-4824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ladd M.K., Peshkin B.N., Senter L., et al. Predictors of risk-reducing surgery intentions following genetic counseling for hereditary breast and ovarian cancer. Transl Behav Med. 2018 Nov 10 doi: 10.1093/tbm/iby101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beattie M.S., Crawford B., Lin F., Vittinghoff E., Ziegler J. Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genet Test Mol Biomarkers. 2009 Feb;13(1):51–56. doi: 10.1089/gtmb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Driel C.M., Eltahir Y., de Vries J., et al. Risk-reducing mastectomy in BRCA1/2 mutation carriers: factors influencing uptake and timing. Maturitas. 2014 Feb;77(2):180–184. doi: 10.1016/j.maturitas.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Evans D.G., Lalloo F., Ashcroft L., et al. Uptake of risk reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age and time dependent. Canc Epidemiol Biomarkers Prev. 2009;18(8):2318–2324. doi: 10.1158/1055-9965.EPI-09-0171. [DOI] [PubMed] [Google Scholar]
- 7.Portnoy D.B., Loud J.T., Han P.K., Mai P.L., Greene M.H. Effects of false-positive cancer screenings and cancer worry on risk-reducing surgery among BRCA1/2 carriers. Health Psychol. 2015 Jul;34(7):709–717. doi: 10.1037/hea0000156. [DOI] [PubMed] [Google Scholar]
- 8.Lalloo F., Evans D.G. Familial breast cancer. Clin Genet. 2012;82(2):105–114. doi: 10.1111/j.1399-0004.2012.01859.x. [DOI] [PubMed] [Google Scholar]
- 9.Claus E.B., Risch N., Thompson W.D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- 10.Amir E., Evans D.G., Shenton A., et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003;40(11):807–814. doi: 10.1136/jmg.40.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D.G., Ingham S., Dawe S., et al. Breast cancer risk assessment in 8,824 women attending a family history evaluation and screening programme. Fam Cancer. 2014;13(2):189–196. doi: 10.1007/s10689-013-9694-z. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh A, Shaw C, Evans G, Turnbull N, Bahar N, Barclay M, Easton D, Emery J, Gray J, Halpin J, Hopwood P, McKay J, Sheppard C, Sibbering M, Watson W, Wailoo A, Hutchinson A (2004 updated 2006 and 2013) Clinical guidelines and evidence review for the classification and care of women at risk of familial breast cancer, London: National Collaborating Centre for Primary Care/University of Sheffield. NICE guideline CG164. https://www.nice.org.uk/Guidance/CG164.
- 13.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 14.Schuster N.A., Hoogendijk E.O., Kok A.A.L., Twisk J.W.R., Heymans M.W. Ignoring competing events in the analysis of survival data may lead to biased results: a nonmathematical illustration of competing risk analysis. J Clin Epidemiol. 2020 Jun;122:42–48. doi: 10.1016/j.jclinepi.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Latouche A., Allignol A., Beyersmann J., Labopin M., Fine J.P. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648–653. doi: 10.1016/j.jclinepi.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Hanley G.E., McAlpine J.N., Cheifetz R., Schrader K.A., McCullum M., Huntsman D. Selected medical interventions in women with a deleterious BRCA mutation: a population-based study in British Columbia. Curr Oncol. 2019 Feb;26(1):e17–e23. doi: 10.3747/co.26.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long J., Evans T.G., Bailey D., Lewis M.H., Gower-Thomas K., Murray A. Uptake of risk-reducing surgery in BRCA gene carriers in Wales, UK. Breast J. 2018 Jul;24(4):580–585. doi: 10.1111/tbj.12978. [DOI] [PubMed] [Google Scholar]
- 18.Flippo-Morton T., Walsh K., Chambers K., et al. Surgical decision making in the BRCA-positive population: institutional experience and comparison with recent literature. Breast J. 2016 Jan-Feb;22(1):35–44. doi: 10.1111/tbj.12521. [DOI] [PubMed] [Google Scholar]
- 19.Chai X., Friebel T.M., Singer C.F., et al. Use of risk-reducing surgeries in a prospective cohort of 1,499 BRCA1 and BRCA2 mutation carriers. Breast Canc Res Treat. 2014 Nov;148(2):397–406. doi: 10.1007/s10549-014-3134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia C., Wendt J., Lyon L., et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol. 2014 Feb;132(2):428–433. doi: 10.1016/j.ygyno.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Singh K., Lester J., Karlan B., Bresee C., Geva T., Gordon O. Impact of family history on choosing risk-reducing surgery among BRCA mutation carriers. Am J Obstet Gynecol. 2013 Apr;208(4):329.e1–329.e6. doi: 10.1016/j.ajog.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Meijers-Heijboer E.J., Verhoog L.C., Brekelmans C.T., et al. Pre-symptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet. 2000;355(9220):2015–2020. doi: 10.1016/s0140-6736(00)02347-3. [DOI] [PubMed] [Google Scholar]
- 23.Skytte A.B., Gerdes A.M., Andersen M.K., et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: uptake and timing. Clin Genet. 2010 Apr;77(4):342–349. doi: 10.1111/j.1399-0004.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 24.Collins I.M., Milne R.L., Weideman P.C., McLachlan S.A., Friedlander M.L., Kathleen Cuningham Foundation Consortium For Research Into Familial Breast Cancer. Hopper J.L., Phillips K.A. Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med J Aust. 2013 Nov 18;199(10):680–683. doi: 10.5694/mja13.10848. [DOI] [PubMed] [Google Scholar]
- 25.Metcalfe K.A., Birenbaum-Carmeli D., Lubinski J., et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Canc. 2008;122(9):2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laitman Y., Vaisman Y., Feldman D., et al. Rates of risk-reducing surgery in Israeli BRCA1 and BRCA2 mutation carriers. Clin Genet. 2014 Jan;85(1):68–71. doi: 10.1111/cge.12149. [DOI] [PubMed] [Google Scholar]
- 27.Den Heijer M., van Asperen C.J., Harris H., et al. International variation in physicians' attitudes towards prophylactic mastectomy - comparison between France, Germany, The Netherlands and the United Kingdom. Eur J Canc. 2013;49(13):2798–2805. doi: 10.1016/j.ejca.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Portnoy D.B., Loud J.T., Han P.K., Mai P.L., Greene M.H. Effects of false-positive cancer screenings and cancer worry on risk-reducing surgery among BRCA1/2 carriers. Health Psychol. 2015 Jul;34(7):709–717. doi: 10.1037/hea0000156. [DOI] [PubMed] [Google Scholar]
- 29.Metcalfe K.A., Ghadirian P., Rosen B., et al. Variation in rates of uptake of preventive options by Canadian women carrying the BRCA1 or BRCA2 genetic mutation. Open Med. 2007;1(2):e92–e98. [PMC free article] [PubMed] [Google Scholar]
- 30.Evans D.G., Wisely J., Clancy T., et al. Longer term effects of the Angelina Jolie effect: increased risk-reducing mastectomy rates in BRCA carriers and other high-risk women. Breast Cancer Res. 2015;17:143. doi: 10.1186/s13058-015-0650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archer S., Babb de Villiers C., Scheibl F., et al. Evaluating clinician acceptability of the prototype CanRisk tool for predicting risk of breast and ovarian cancer: a multi-methods study. PloS One. 2020 Mar 6;15(3) doi: 10.1371/journal.pone.0229999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.