Abstract
Receptor activator of NF‐κB ligand (RANKL) is essential for osteoclast formation and bone remodeling. Nevertheless, the cellular source of RANKL for osteoclastogenesis has not been fully uncovered. Different from peripheral adipose tissue, bone marrow (BM) adipose lineage cells originate from bone marrow mesenchymal stromal cells (BMSCs). Here, we demonstrate that adiponectin promoter‐driven Cre expression (AdipoqCre ) can target bone marrow adipose lineage cells. We cross the AdipoqCre mice with ranklfl/fl mice to conditionally delete RANKL from BM adipose lineage cells. Conditional deletion of RANKL increases cancellous bone mass of long bones in mice by reducing the formation of trabecular osteoclasts and inhibiting bone resorption but does not affect cortical bone thickness or resorption of calcified cartilage. AdipoqCre; ranklfl/fl mice exhibit resistance to estrogen deficiency and rosiglitazone (ROS)‐induced trabecular bone loss but show bone loss induced by unloading. BM adipose lineage cells therefore represent an essential source of RANKL for the formation of trabecula osteoclasts and resorption of cancellous bone during remodeling under physiological and pathological conditions. Targeting bone marrow adiposity is a promising way of preventing pathological bone loss.
Keywords: bone marrow adipose lineage cell, bone remodeling, osteoclast, RANKL
Subject Categories: Molecular Biology of Disease, Musculoskeletal System, Signal Transduction
Bone marrow adipose lineage cells are an essential source of RANKL for trabecular osteoclast formation and bone resorption in the context of pathological bone loss.
Introduction
Osteoclasts are specialized multinucleated cells derived from the monocyte–macrophage hematopoietic lineage and are responsible for resorption of bone matrix (Boyle et al, 2003). During longitudinal bone growth, hypertrophic chondrocytes attract osteoclasts and blood vessels to direct the matrix mineralization (Kronenberg, 2003). Under normal conditions, trabecular bone is resorbed periodically by osteoclasts followed by osteogenesis in the cavities, in a process known as remodeling (Raggatt & Partridge, 2010). Excess bone resorption by osteoclasts results in pathological bone loss disorders, including postmenopausal osteoporosis and Paget’s disease (Manolagas & Jilka, 1995).
Receptor activator of NF‐κB ligand (RANKL) is an essential mediator of osteoclast formation during bone resorption (Sobacchi et al, 2007). Matrix‐embedded hypertrophic chondrocytes and osteocytes serve as the primary sources of RANKL for mineralized cartilage resorption in the development of growing bone and remodeling of adult bone, respectively (Xiong et al, 2011). Initially, RANKL is produced in a membranous form that can be cleaved by proteases to produce soluble RANKL (Lacey et al, 1998). Because most matrix‐embedded cells do not come in direct contact with osteoclast progenitors, these cells may control osteoclast formation through the production of soluble RANKL (Liu et al, 2001). Although it does contribute to osteoclast formation and the resorption of cancellous bone in adult bone, soluble RANKL does not affect cancellous bone mass or structure in growing bone (Xiong et al, 2018). Moreover, bone loss caused by estrogen deficiency in ovariectomized mice is not prevented or reduced by a lack of soluble RANKL (Xiong et al, 2018). These results indicate that the membrane‐bound form of RANKL is sufficient for regulating osteoclast formation in a direct contact manner. As RANKL is expressed in various cell types, the cellular source of RANKL for osteoclastogenesis during bone growth and estrogen deficiency remains unclear.
Unlike white or brown adipose tissue, bone marrow (BM) adipose lineage cells derived from BM mesenchymal stromal cells (BMSCs) by lineage tracing have been implicated in osteoclastogenesis and hematopoiesis (Zhou et al, 2014; Zhou et al, 2017). The number of BM adipose lineage cells increases with age as well as under certain pathological conditions (Duque et al, 2011). BM adipose lineage cells express RANKL, a phenotype not seen in either white or brown adipose tissue (Fan et al, 2017), and increased BM adipose tissue is associated with increased osteoclast formation (Yu et al, 2018). Bone marrow‐derived preadipose cell line MC3T3‐G2/PA6 cells support osteoclast formation in the co‐culture system in the presence of 1α,25‐(OH)2D3 and dexamethasone (Udagawa et al, 1989). Based on these observations, we hypothesized that BM adipose lineage cells could serve as a source of RANKL for osteoclast formation and pathological bone resorption. We conditionally deleted RANKL in BM adipose lineage cells by crossing the AdipoqCre mice with ranklfl/fl mice. We found that RANKL derived from BM adipose lineage cells did not affect resorption of calcified cartilage in the growth plate or cortical bone but did contribute to osteoclast formation and resorption of the cancellous bone in the longitudinal bone. Mice lacking RANKL in BM adipose lineage cells were protected from bone loss after ovariectomy or rosiglitazone administration but not from bone loss induced by unloading. BM adipose lineage cells therefore represent an essential source of RANKL for the trabecular osteoclast formation and cancellous bone resorption in physiological bone resorption and pathological bone resorption.
Results
Conditional knockout of RANKL in bone marrow adipose lineage cells
As previously reported (Ambrosi et al, 2017; Wang et al, 2020b), we used the AdipoqCre to specifically delete RANKL in BM adipose lineage cells. BMSCs consist of three sub‐populations: multi‐potent stem cell‐like population, osteochondrogenic progenitor cell (OPC), and adipogenic progenitor cell (APC), while APC will irreversibly mature toward a pre‐Ad stage: pre‐adipocyte (pre‐Ad) (Ambrosi et al, 2017). To identify the subgroup targeted by the AdipoqCre , we crossed AdipoqCre mice with Rosa26‐lsl‐tdTomato mice to generate AdipoqCre; R26tdTomato mice, which permit identification of AdipoqCre‐targeted cells. Flow cytometry analysis of the femoral bone marrow showed that CD45−CD31−Sca‐1+ cells representing APC (CD45−CD31−Sca‐1+CD24−) and multi‐potent stem cells (CD45−CD31−Sca‐1+CD24+) did not express tdTomato (Fig 1A). Among CD45−CD31−Sca‐1− cells covering pre‐Ad (CD45−CD31−Sca1−Reep2+) and OPC (CD45−CD31−Sca1−PDGFα+), tdTomato+Reep2+ pre‐Ad accounted for 16.0% and tdTomato+PDGFα+ OPC accounted for 0.186%. The flow cytometry results indicated that the AdipoqCre targeted pre‐Ad in bone marrow, which is consistent with a recent study by Qin et al, (Zhong et al, 2020). The immunofluorescence staining of Aggrecan and DAPI showed that the AdipoqCre did not elicit any reporter gene activity in osteocytes (Fig 1Bi) or chondrocytes (Fig 1Bii). To further verify the specificity of the Cre line, we sorted tdTomato+ cells from AdipoqCre; R26tdTomato mice and conducted proliferation and differentiation assays in vitro and in vivo. The Giemsa staining showed that the BMSCs from control wild‐type mice could form CFU‐F colonies in vitro, while the tdTomato+ cells attached to the culture dish but could not form CFU‐F colonies (Fig EV1A and B). Then, the adipogenic potential and osteogenic potential of BMSCs and tdTomato+ cells were assessed by oil red O staining and Alizarin red staining, respectively. The results showed that BMSCs could differentiate into osteoblasts and adipocytes in vitro, while tdTomato+ cells could only undergo adipogenesis (Fig EV1C and E). The qPCR results of peroxisome proliferator‐activated receptor γ (PPARγ), lipoprotein lipase (LPL), osteocalcin (OCN), and alkaline phosphatase (ALP) showed consistent results with differentiation assays (Fig EV1D and F). For in vivo assay, we injected BMSCs and tdTomato+ cells into mouse kidney capsules which were harvested after 4 weeks. Immunofluorescence staining of OCN, Aggrecan, and Perilipin was performed. Results indicated that BMSCs formed osteoblasts, adipocytes, and chondrocytes after transplanted and sorted tdTomato+ cells from AdipoqCre; R26tdTomato mice only formed adipocytes (Fig EV2A–C).
Figure 1. AdipoqCre targets BM adipose lineage cells.

- FACS analysis of tdTomato+ cells in the bone marrow cells (percentages represent average values).
- Immunofluorescence staining of aggrecan and DAPI of femur sections from AdipoqCre; R26tdTomato mice. Scale bar = 200/100/50 µm in left/ upper right/ bottom right images. CB: cortical bone, TB: trabecular bone.
- Immunofluorescence staining of RANKL and Hoechst in bone marrow cells from AdipoqCre; R26tdTomato mice. Scale bar = 50 µm.
- qPCR results of Rankl in BMAT and peripheral adipose tissues, including epididymal (eWAT), inguinal (iWAT), and interscapular (iBAT) (n = 3 independent biological replicates). Data were compared using one‐way ANOVA (** indicates P < 0.01), and error bars are standard deviations.
- Immunofluorescence staining of RANKL during in vitro adipogenesis of BMSCs from AdipoqCre; ranklfl/fl mice (Rankl −/−) and ranklfl/fl mice (Rankl +/+). Scale bar = 50 μm.
- Western blotting analyses of RANKL in BMSCs during in vitro BMSCs adipogenesis (n = 3 independent biological replicates). Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
Figure EV1. CFU‐F, adipogenic, and osteogenic differentiation analysis in vitro .

-
A, BGiemsa staining and statistical analysis of CFU‐F number of BMSCs from WT mice and tdTomato+ cells from AdipoqCre; R26tdTomato mice. Scale bar = 50 µm. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations. n = 5 independent microscopic vision fields in (B).
-
C, DAdipogenic potential of BMSCs and tdTomato+ cells was assessed by oil red O staining, and PPARγ and Lpl were detected by qPCR. Scale bar = 50 µm. Data were compared using an unpaired t‐test, and error bars are standard deviations. n = 5 independent microscopic vision fields in (D).
-
E, FOsteogenic potential of BMSCs and tdTomato+ cells was assessed by the alizarin red staining, and Alp and Ocn were detected by qPCR. Scale bar = 50 µm. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations. n = 5 independent microscopic vision fields in (F).
Figure EV2. Adipogenic, osteogenic, and chondrogenic differentiation analysis in vivo .
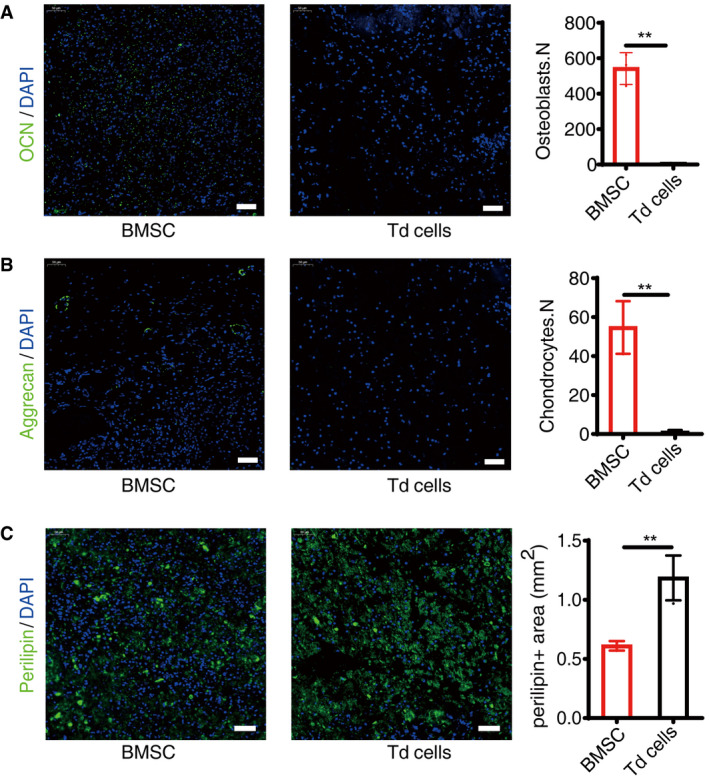
- Immunofluorescence staining of OCN and statistical analyses of BMSCs and tdTomato+ cells (Td cells), scale bar = 50 µm. Images are representative of five independent biological replicates, n = 6 independent microscopic vision fields in the right panel. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
- Immunofluorescence staining of Aggrecan and statistical analyses of positive cells, scale bar = 50 µm. Images are representative of five independent biological replicates, n = 6 independent microscopic vision fields in the right panel. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
- Immunofluorescence staining of Perilipin and statistical analyses of positive cells, scale bar = 50 µm. Images are representative of five independent biological replicates, n = 6 independent microscopic vision fields in the right panel. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
In isolated bone marrow cells from AdipoqCre; R26tdTomato mice, immunofluorescent staining showed that RANKL colocalized with all tdTomato+ cells, which indicated that all BM adipose lineage cells expressed RANKL (Fig 1C). To verify the specificity of RANKL expression in BM adipose tissue, we examined the rankl gene transcription in epididymal adipose tissue (eWAT), inguinal adipose tissue (iWAT), interscapular brown adipose tissue (iBAT), and BM adipose tissue (BMAT) from wild‐type C57BJ/6L mice by qPCR as previous reported (Fan et al, 2017). The results showed that different from peripheral adipocytes, the BM adipose lineage cells highly expressed rankl (Fig 1D). Then, we isolated BMSCs from AdipoqCre; ranklfl/fl mice and induced adipogenic differentiation. During adipogenesis, the expression of RANKL in BMSCs decreased markedly in AdipoqCre; ranklfl/fl mice but remained unchanged in ranklfl/fl mice (Fig 1E and F). These results demonstrate that the AdipoqCre efficiently deletes RANKL in bone marrow adipose lineage cells and but not in chondrocytes, osteocytes, or mesenchymal progenitors.
RANKL knockout from BM adipose lineage cells increases trabecular bone mass
To assess the role of BM adipocyte RANKL in bone mass, we established BM adipocyte RANKL knockout mice by crossing the AdipoqCre mice with ranklfl/fl mice (Appendix Fig S1A). Both male and female mice were used in the study. Conclusions are based on data analyses from both sexes, although only data from females are presented here. For both male and female mice, the body weight and body length were recorded from birth to 8 weeks (Appendix Fig S1B). And AdipoqCre; ranklfl/fl mice showed a slightly increase in body weight compared with their control littermates. RANKL deletion in BM adipose lineage cells did not affect RANKL expression in spleen (Appendix Fig S1C) by immunohistochemistry, spleen (Appendix Fig S1D), and lymph node (Appendix Fig S1E) development by HE staining, or differentiation of T and B cells by flow cytometry (Appendix Fig S1F and G).
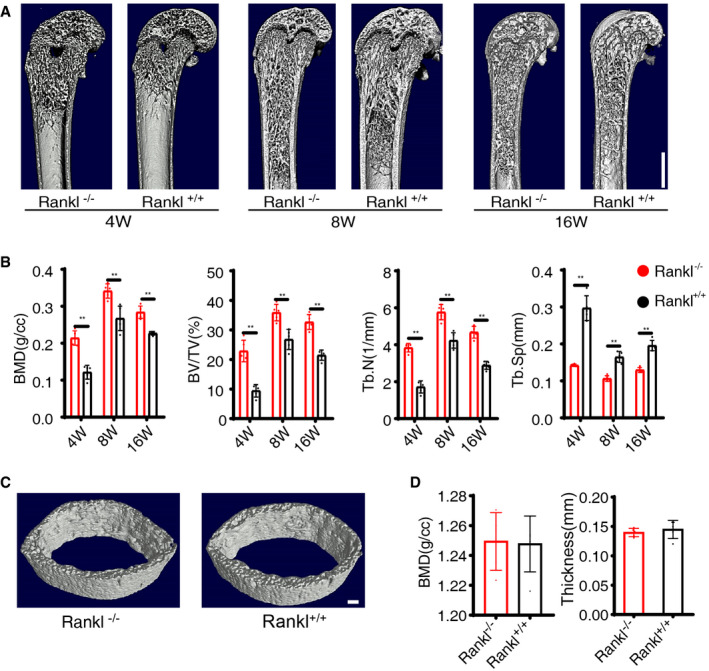
We harvested the femurs of AdipoqCre; ranklfl/fl and ranklfl/fl mice at 4, 8, and 16 weeks. Quantitative computed tomography (μ‐QCT) analyses revealed significant increases in trabecular bone mass of AdipoqCre; ranklfl/fl mice compared with ranklfl/fl mice as confirmed by increased bone mineral density (BMD), trabecular bone volume (BV/TV), trabecular number (Tb.N), and decreased trabecular spacing (Tb.Sp) (Fig 2A and B). No differences in cortical bone thickness or BMD were found between the two groups at 8 weeks (Fig 2C and D). Taken together, these results indicate that deleting RANKL from BM adipose lineage cells increases trabecular bone mass.
Figure 2. Cancellous bone mass increases in AdipoqCre; ranklfl/fl mice.
-
A, BMicro‐CT and statistical analyses of BMD, BV/TV, Tb.N, and Tb.Sp in trabecular bone from AdipoqCre; ranklfl/fl (Rankl −/−) and ranklfl/fl (Rankl +/+) mice at 4 weeks, 8 weeks, and 16 weeks (n = 5 independent biological replicates). Scale bar = 1 mm. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
-
CMicro‐CT images of cortical bone from Rankl −/− and Rankl +/+ mice at 8 weeks. Images are representative of five independent biological replicates. Scale bar = 1 mm.
-
DMicro‐CT analyses and statistical analyses of cortical bone from Rankl −/− and Rankl +/+ mice at 8 weeks (n = 5 independent biological replicates). Data were compared using an unpaired t‐test, and error bars are standard deviations.
RANKL from BM adipose lineage cells is essential for trabecular bone remodeling
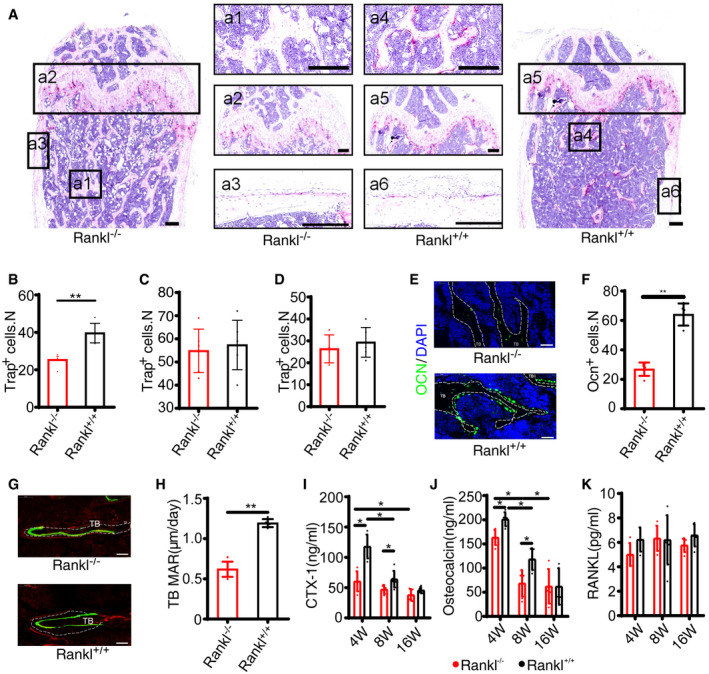
Tartrate‐resistant acid phosphatase (TRAP) staining and OCN immunofluorescence staining were performed to assess the roles of BM adipocyte‐derived RANKL in trabecular bone remodeling. TRAP staining of trabecular bone revealed a significant decrease in the number of TRAP+ cells (osteoclasts) in the bone marrow around the trabecular bones of AdipoqCre; ranklfl/fl mice compared with ranklfl/fl mice at 8 weeks (Fig 3A a1 vs. a4,B). The number of TRAP+ cells under the growth plate (Fig 3A a2 vs. a5,C) and periosteal TRAP+ cells (Fig 3A a3 vs. a6,D) did not show significant difference. The thickness of growth plate cartilage (Fig EV3A) and tooth eruption (Fig EV3B) was not significantly different between two groups.
Figure 3. RANKL knockout from BM adipose lineage cells regulates trabecular bone remodeling.
-
ARepresentative images of TRAP staining of femur from AdipoqCre; ranklfl/fl (Rankl −/−) and ranklfl/fl (Rankl +/+) mice at 8 weeks. Scale bar = 200 μm. Panels a1 and a4 indicate trabecular bone, a2 and a5 stand for growth plate, and a3 and a6 denote cortical bone.
-
BNumbers of Trap+ cells on trabecular bone (a1 vs. a4, see (A)) (n = 6 independent microscopic vision fields from random mice samples). Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
-
CNumbers of Trap+ cells under the growth plate (a2 vs. a5, see (A)) (n = 6 independent microscopic vision fields from random mice samples). Data were compared using an unpaired t‐test, and error bars are standard deviations.
-
DNumbers of periosteal Trap+ cells (a3 vs. a6, see (A)) (n = 6 independent microscopic vision fields from random mice samples). Data were compared using an unpaired t‐test, and error bars are standard deviations.
-
EImmunofluorescence staining of osteocalcin from 8‐week‐old mice (n = 6 mice in Rankl −/− group and 4 mice in Rankl +/+ group). Scale bar = 50 μm. TB: trabecular bone.
-
FStatistical analyses of Ocn+ cells (n = 6 independent microscopic vision fields from random mice samples). Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
-
GCalcein staining of trabecular bone at 8 weeks. Scale bar = 50 μm. TB: trabecular bone. Images are representative of 6 independent biological replicates in Rankl −/− and 5 replicates in Rankl +/+ group.
-
HMineral apposition rate (MAR) of trabecular bone at 8 weeks (n = 6 mice in Rankl −/− group and 5 mice in Rankl +/+ group). Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
-
I–KSerum CTX‐1, OCN, and bone marrow RANKL levels from Rankl −/− and Rankl +/+ mice at 4 weeks (n = 5 and 5), 8 weeks (n = 5 and 6), and 16 weeks (n = 5 and 5). Data were compared using an unpaired t‐test (* indicates P < 0.05, ** indicates P < 0.01), and error bars are standard deviations.
Figure EV3. RANK deletion in BM adipose lineage cells does not affect bone mass.

- Safranin O/Fast green staining of AdipoqCre; ranklfl/fl (Rankl −/−) and ranklfl/fl (Rankl +/+) mice. Scale bar = 250 µm
- X‐ray images of the head of 8‐week‐old mice. Scale bar = 2 mm.
- Calcein staining and statistical analysis from periosteal side of cortical bone (n = 6 mice in Rankl −/− group and 5 mice in Rankl +/+ group). Scale bar = 50 µm. Data were compared using an unpaired t‐test, and error bars are standard deviations.
- Calcein staining and statistical analysis from endosteal side of cortical bone (n = 6 mice in Rankl −/− group and 5 mice in Rankl +/+ group). Scale bar = 50 µm. Data were compared using an unpaired t‐test, and error bars are standard deviations.
Osteocalcin immunofluorescence staining showed fewer osteoblasts around the trabeculae in AdipoqCre; ranklfl/fl mice than in ranklfl/fl mice at 8 weeks (Fig 3E and F). Calcein staining revealed less trabecular bone formation and lower trabecular mineralization apposition rate (MAR) in AdipoqCre; ranklfl/fl mice relative to ranklfl/fl mice at 8 weeks (Fig 3G and H). By contrast, the endosteal and periosteal bone formation was not significantly different between two groups (Fig EV3C and D). The bone histomorphometric parameters of AdipoqCre; ranklfl/fl mice and ranklfl/fl mice were showed in Appendix Table S1. The above results suggested that RANKL deletion from BM adipose lineage cells impeded trabecular osteoclast formation and the following osteogenesis and bone formation, namely bone remodeling.
We isolated BMSCs and in vitro Alizarin red staining after osteogenic differentiation showed no significant difference between the two groups (Appendix Fig S2A). The expression of alkaline phosphatase (ALP) and OCN was comparable in AdipoqCre; ranklfl/fl and ranklfl/fl mice (Appendix Fig S2B and C). In vitro oil red O staining and expression of LPL and PPARγ after adipogenic differentiation of BMSCs revealed no significant difference between AdipoqCre; ranklfl/fl and ranklfl/fl mice (Appendix Fig S2D–F). The above results indicated that RANKL deletion from BM adipose lineage cells did not affect BM osteogenesis and adipogenesis.
Next, we examined serum CTX‐1, OCN levels, and bone marrow RANKL levels. Compared to ranklfl/fl mice, AdipoqCre; ranklfl/fl mice had lower serum CTX‐1 at 4, 8, and 16 weeks (Fig 3I) and lower serum OCN at 4 and 8 weeks (Fig 3J). Overall, both serum CTX‐1 and OCN decreased with age (Fig 3I and J). Bone marrow RANKL levels were not significantly different between AdipoqCre; ranklfl/fl and ranklfl/fl mice at 4, 8, and 16 weeks (Fig 3K), indicating that soluble RANKL level was not affected by RANKL knockout in BM adipose lineage cells. The above results indicated that BM adipocyte RANKL knockout decreased trabecular bone remodeling but did not affect the periosteal bone formation, calcified cartilage resorption at the growth plate.
A recent study reported that the AdipoqCre targets PDGFR+VCAM‐1+ stromal cells (Mukohira et al, 2019), and our previous work showed that RANKL signaling in BMSCs negatively regulates osteoblastogenesis and bone formation (Chen et al, 2018a). To rule out the effects of RANKL signaling on osteoblast differentiation of BMSCs in vivo, we generated the AdipoqCre; rankfl/fl conditional knockout mice. Micro‐CT analyses revealed no significant differences between rank knockout in BM adipose lineage cells and rankfl/fl mice at any time point tested (Fig EV4A and B). Overall, these results indicate that BM adipose lineage cells are an essential source of RANKL for trabecular bone osteoclast formation and subsequent remodeling.
Figure EV4. RANK deletion in BM adipose lineage cells does not affect bone mass.

-
A, BMicro‐CT and statistical analyses of BMD, BV/TV, Tb.N, and Tb.Sp in trabecular bone from AdipoqCre; rankfl/fl (Rank −/−) and rankfl/fl (Rank +/+) mice at 4 weeks, 8 weeks, and 16 weeks. Scale bar = 1 mm. Images are representative of 5 independent biological replicates, n = 5 independent microscopic vision fields in (B). Data were compared using an unpaired t‐test, and error bars are standard deviations.
RANKL from BM adipose lineage cells does not mediate unloading‐induced bone resorption
Bone constantly adapts its structure in response to mechanical signals under the control of osteoblastic cells, which sense mechanical loading and regulate osteoclast formation and trabecular bone remodeling through RANKL (Xiong et al, 2011). To further determine whether BM adipocyte‐derived RANKL mediates skeletal mechanical loading, we carried out unloading test by tail suspension in 8‐week‐old mice for 4 weeks. In each group, micro‐CT results showed the trabecular bone BMD, BV/TV, and Tb.N were significantly decreased and the Tb. Sp was increased (Fig 4A and B). The cortical changes were consistent with trabecular bones (Fig 4C and D). The AdipoqCre; ranklfl/fl mice did not prevent bone loss induced by tail suspension. The results support that BM adipocyte RANKL does not mediate unloading‐induced bone resorption.
Figure 4. RANKL knockout from BM adipose lineage cells does not affect osteocyte regulation on osteoclasts.
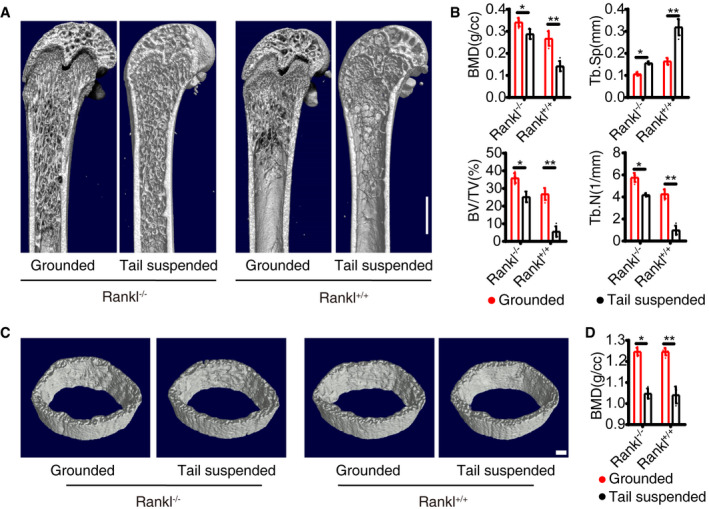
-
A, BMicro‐CT analyses of trabecular bone from Grounded and Tail suspended model (n = 5 independent biological replicates). Scale bar = 1 mm. Data were compared using an unpaired t‐test (* indicates P < 0.05, ** indicates P < 0.01), and error bars are standard deviations.
-
C, DMicro‐CT analyses of cortical bone (n = 5 independent biological replicates). Scale bar = 1 mm. Data were compared using an unpaired t‐test (* indicates P < 0.05, ** indicates P < 0.01), and error bars are standard deviations.
RANKL from BM adipose lineage cells mediates osteoclastogenesis and bone loss in ovariectomized mice
Increased BM adipose tissue is associated with increased fracture risk in postmenopausal osteoporosis (Veldhuis‐Vlug & Rosen, 2018). To examine the roles of RANKL from BM adipose lineage cells in bone resorption after estrogen withdrawal, we used a mouse model of ovariectomy (OVX)‐induced bone loss. Immunofluorescence staining of adiponectin revealed a significant increase in the number of BM adipose lineage cells following OVX in both AdipoqCre; ranklfl/fl and ranklfl/fl mice, with no statistical difference between groups (Fig EV5A and B). At 6 weeks post‐ovariectomy, trabecular bone mass was significantly decreased in ranklfl/fl mice, a phenotype not seen in AdipoqCre; ranklfl/fl mice (Fig 5A and B). Consistent results were evident via H&E staining (Fig EV5C). TRAP staining showed that in ranklfl/fl mice, after OVX the number of osteoclasts significantly increased while in AdipoqCre; ranklfl/fl mice the number did not significantly change (Fig 5C and D). The OCN staining for osteoblasts demonstrated consistent results with TRAP staining (Fig 5E and F). These results indicated that increased bone remodeling after OVX was inhibited in AdipoqCre; ranklfl/fl mice compared with their littermates. ELISA analyses revealed increased serum CTX‐1 (Fig EV5D), OCN (Fig EV5E), and bone marrow RANKL (Fig EV5F) levels in ranklfl/fl OVX mice relative to non‐OVX controls, with no significant changes in protein abundance in AdipoqCre; ranklfl/fl OVX mice. These results demonstrate that RANKL from BM adipose lineage cells is an essential mediator of excess osteoclasts formation, bone resorption, and increased bone turnover after estrogen withdrawal.
Figure EV5. RANKL knockout from BM adipose lineage cells contributes to OVX‐induced bone loss.

-
A, BImmunofluorescence staining of adiponectin and statistical analyses of the number of adipose lineage cells. Scale bar = 100 µm. Images are representative of 5 independent biological replicates, n = 5 independent microscopic vision fields in (B). Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations. CB: cortical bone, TB: trabecular bone.
-
CH&E staining of the distal femur from AdipoqCre; ranklfl/fl (Rankl −/−) and ranklfl/fl (Rankl +/+) mice treated with Sham or OVX. Scale bar = 1 mm.
-
D, FELISA analyses of serum CTX‐1, OCN, and bone marrow RANKL levels in Sham and OVX mice (n = 5 independent biological replicates). Data were compared using an unpaired t‐test (* indicates P < 0.05), and error bars are standard deviations.
Figure 5. RANKL knockout from BM adipose lineage cells contributes to excess osteoclast formation and bone resorption in OVX mice.

-
A, BMicro‐CT analyses of sham and OVX model of AdipoqCre; ranklfl/fl (Rankl −/−) and ranklfl/fl (Rankl +/+) mice (n = 5 independent biological replicates). Scale bar = 1 mm. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
-
C, DTRAP staining and statistical analyses of numbers of osteoclasts in Sham and OVX model (Images are representative of 5 independent biological replicates in (C), and n = 5 independent microscopic vision fields in (D)). Scale bar = 100 µm. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
-
E, FImmunofluorescence staining of OCN and statistical analyses of the number of osteoblasts (Images are representative of 5 independent biological replicates in (E), and n = 5 independent microscopic vision fields in (F)). Scale bar = 50 µm. TB: trabecular bone. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
RANKL from BM adipose lineage cells contributes to rosiglitazone‐induced osteoclastogenesis and bone loss
Rosiglitazone (ROS), a PPARγ activator used to treat type 2 diabetes, is associated with increased BM adipogenesis and fracture risk in both men and women (Kahn et al, 2008; Aubert et al, 2010). The mechanisms underlying these effects are still poorly understood but are likely attributable to increased adipogenesis due to PPARγ activation (Wei et al, 2010). To explore the roles of BM adipocyte RANKL in this process, we used a model of ROS‐induced bone loss. In this model, ROS (10 mg/kg) was administered for 6 weeks. Adiponectin immunofluorescent staining revealed a substantial increase in the number of BM adipose lineage cells after ROS administration in both AdipoqCre; ranklfl/fl and ranklfl/fl mice, with no significant differences between the groups (Appendix Fig S3A and B). Trabecular bone mass showed a significant decrease in ranklfl/fl mice but no decrease in AdipoqCre; ranklfl/fl mice (Fig 6A and B). H&E staining showed consistent results with CT analyses (Appendix Fig S3C). TRAP staining showed that ROS treatment significantly increased the number of osteoclasts in ranklfl/fl mice but not in AdipoqCre; ranklfl/fl mice (Fig 6C and D). OCN immunofluorescent staining showed that the number of osteoblasts was significantly increased in ranklfl/fl mice after ROS treatment but not in AdipoqCre; ranklfl/fl mice (Fig 6E and F). Serum CTX‐1 (Appendix Fig S3D) and OCN levels were increased in response to ROS in ranklfl/fl mice (Appendix Fig S3E) but not in AdipoqCre; ranklfl/fl mice. BM RANKL levels showed no significant differences in AdipoqCre; ranklfl/fl mice after treated with ROS (Appendix Fig S3F). These results indicate that increased BM adipose lineage cells are an important source of RANKL for osteoclast formation and bone loss after ROS administration.
Figure 6. RANKL knockout from BM adipose lineage cells mediates rosiglitazone‐induced bone loss.
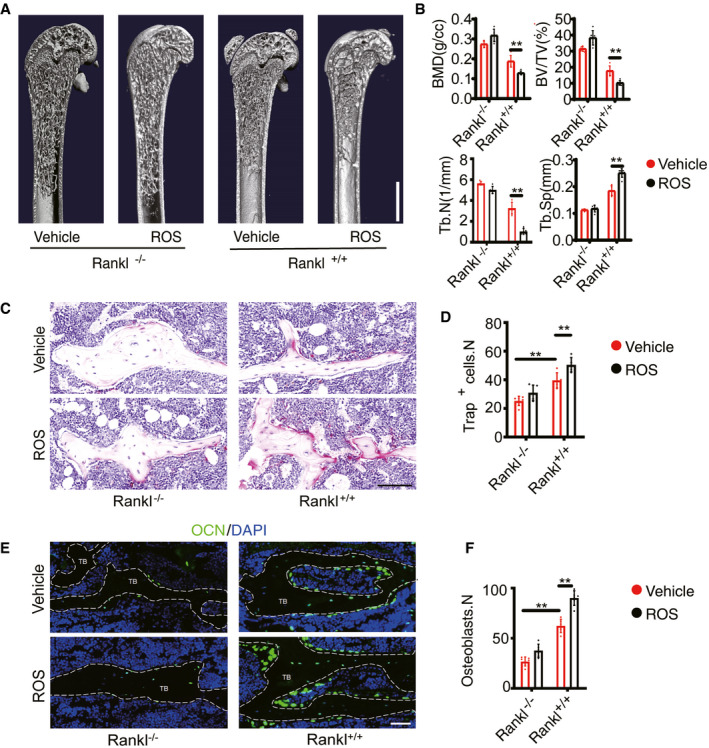
-
A, BThree‐dimensional CT analyses of BMD, BV/TV, Tb.N, and Tb.Sp in trabecular bone from Vehicle (n = 5 independent biological replicates) and ROS (n = 6 independent biological replicates) model of AdipoqCre; ranklfl/fl (Rankl −/−) and ranklfl/fl (Rankl +/+) mice. Scale bar = 1 mm. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations.
-
C, DTRAP staining and statistical analyses of numbers of osteoclasts in the trabecular bone from vehicle (n = 6 and 5 independent biological replicates) and ROS (n = 6 and 6 independent biological replicates) treatment. Scale bar = 100 µm. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations. n = 5 independent microscopic vision fields in (D).
-
E, FOCN staining and statistical analyses of the number of osteoblasts in femurs from vehicle (n = 6 and 5 independent biological replicates) and ROS (n = 6 and 6 independent biological replicates) treatment. Scale bar = 50 µm. TB: trabecular bone. Data were compared using an unpaired t‐test (** indicates P < 0.01), and error bars are standard deviations. n = 5 independent microscopic vision fields in (F).
Discussion
As an essential factor for osteoclastogenesis, RANKL is widely expressed by a variety of cell types within the bone marrow (Nakashima et al, 2011). Factors including parathyroid hormone and mechanical load regulate bone remodeling through RANKL (Fu et al, 2002). The cellular source of RANKL for osteoclast formation under physiological and some pathological conditions such as postmenopausal osteoporosis has not been fully revealed. Mice harboring a conditional knockout of RANKL using Prx1Cre demonstrate no osteoclast formation, which indicates that mesenchyme‐derived cells are an essential source of RANKL in the development of long bones (Xiong et al, 2011). Among the mesenchyme‐derived cells, matrix‐embedded hypertrophic chondrocytes and osteocytes are the major source of RANKL for osteoclastogenesis, not osteoblasts or their progenitors. Hypertrophic chondrocytes control the resorption of calcified cartilage during bone development, while osteocytes regulate bone remodeling by sensing mechanical changes (Xiong et al, 2011; Wang et al, 2020a). And osteocytes are an essential source of RANKL for cancellous bone remodeling in adult mice but not younger mice (Nakashima et al, 2011; Xiong et al, 2011).
RANKL is produced in both soluble and membrane‐bound forms. Since matrix‐embedded cells have limited direct contact with osteoclast progenitors and in postmenopausal osteoporosis plasma soluble RANKL is significantly increased (Jabbar et al, 2011), it is proposed that the soluble RANKL is a major form to regulate osteoclastogenesis. Nevertheless, growing mice lacking soluble RANKL showed no deficits in bone mass and equal amounts of bone loss after estrogen deprivation compared with controls (Xiong et al, 2018). These studies indicate that membrane‐bound RANKL is sufficient for bone growth and is responsible for bone loss induced by estrogen deficiency. Therefore, it is mysterious how matrix‐embedded cell‐derived RANKL regulates osteoclastogenesis and bone remodeling.
Although marrow adipose tissue accounts for almost 70% of the adult bone marrow volume in humans, the functions of BM adipose lineage cells are still very mysterious (Scheller & Rosen, 2014). Clinically, increased marrow adipose tissue is associated with increased fracture risk in diseases such as postmenopausal osteoporosis, anorexia nervosa, and diabetes (Veldhuis‐Vlug & Rosen, 2018). Mesenchyme‐derived BM adipose lineage cells express RANKL, which is distinctive from other adipose tissues, including both white and brown adipose (Xiong et al, 2018). Udagawa et al, first explored that bone marrow‐derived preadipose cell line MC3T3‐G2/PA6 cells support osteoclast formation in the co‐culture system (Udagawa et al, 1989). This is the first hint of the regulatory effects of bone marrow‐derived preadipocytes on osteoclastogenesis. Several studies have explored the roles of bone marrow adipose lineage cells by using the AdipoqCre (Ambrosi et al, 2017; Zhou et al, 2017). Zhou et al, reported that bone marrow adipose lineage cells promoted the regeneration of stem cells and hematopoiesis by secreting SCF (Zhou et al, 2017). The author demonstrated that four weeks after gavaging 6‐week‐old AdipoqCre/ER; R26tdTomato mice with tamoxifen, tdTomato was expressed in 93 ± 5% of perilipin+ adipocytes. The tdTomato expressing in the bone marrow besides adipocytes was a subset of LepR+ stromal cells: 5.9 ± 3.1% of LepR+ cells in AdipoqCre / ER; R26tdTomato bone marrow were tdTomato+. They concluded that AdipoqCre / ER recombines adipose lineage cells in the bone marrow. AdipoqCre / ER + cells rarely formed osteoblasts in vivo. In another study by Thomas et al, lineage tracing results showed that the AdipoqCre targeted almost all bone marrow adipose lineage cells but not matrix‐embedded cells, including hypertrophic chondrocytes or osteocytes (Ambrosi et al, 2017). A recent study by Leilei et al, (Zhong et al, 2020) identified a distinct population of non‐lipid laden adipose lineage cells targeted by the AdipoqCre by employing the single‐cell transcriptome analysis. The lineage tracing of the AdipoqCre demonstrated that at 1 month of age, Td labeled all perilipin+ adipocytes, CD45− stromal cells with a reticular shape, and pericytes, but not osteoblasts, osteocytes, periosteal surface, growth plate, or articular chondrocytes, which further confirmed that the AdipoqCre rarely labels mesenchymal progenitors that generate osteoblasts, osteocytes, and chondrocytes. In vivo transplantation under the kidney capsule showed that bone marrow Td+ cells from 1‐month‐old Col2/Td mice but not Adipoq‐Td mice form bone‐like structure. A recent study by Hisa et al, reported that the AdipoqCre could efficiently target a subset group of mesenchymal stromal cells (Mukohira et al, 2019). The AdipoqCre could efficiently target PDGFR β+VCAM‐1+ and CXCL12‐abundant reticular (CAR) stromal cells which are characterized as pericytes contributing to the vessel maintenance and hematopoietic niche. Lineage tracing showed that tdTomato expression was rarely detected in ALP+ osteoblasts of AdipoqCre; R26tdTomato mice at 8 weeks old. BMSCs are a group of heterogeneous stromal cells with various markers and functions. PDGFR β+ and CAR stromal cells represent bone marrow pericytes important for vascular functions (Hellstrom et al, 1999; Andrae et al, 2008). Interestingly, Leilei et al, reported that after adipocytes in 1‐month‐old AdipoqCre; Rosa‐Tomato/DTR (Adipoq/Td/DTR) mice were ablated via diphtheria toxin (DT) injections, BM vasculature became dilated and distorted, and the number of Emcn+CD31+ endothelial cells were decreased (Zhong et al, 2020). The results imply that the non‐lipid laden adipocyte described by Leilei et al, is probably the same subgroup of mesenchymal stromal cells characterized by Hisa et al, Given the current evidence, we used the AdipoqCre to explore the roles of BM adipocyte‐derived RANKL in osteoclastogenesis.
In our study, we found that loss of RANKL production by BM adipose lineage cells did not alter osteogenic or adipogenic differentiation of bone marrow mesenchymal stromal progenitors. Lineage tracing results showed the AdipoqCre efficiently targeted BM adipose lineage cells and unlike the BMSCs, the tdTomato+ cells from AdipoqCre; R26tdTomato mice mainly formed adipocytes and could not proliferate and undergo osteogenesis and chondrogenesis differentiation in vitro and in vivo. Based on the previous studies and our findings, we conclude that the AdipoqCre could efficiently target the BM adipose lineage cells.
AdipoqCre; ranklfl/fl mice showed increased numbers of trabeculae, elevated BMD, decreased osteoclast formation in the marrow, and decreased resorption of cancellous bone. The number of osteoblasts and bone formation in the distal femur was also reduced. The reduced osteogenesis was not due to the decreased BMSC differentiation capacity, but secondary to decreased osteoclastogenesis and bone turnover rate. These results demonstrate that RANKL from BM adipose lineage cells contributes to trabecular bone remodeling in growing and adult mice. The difference in BMD between AdipoqCre; ranklfl/fl and ranklfl/fl mice is smaller in adult mice than in growing mice. This is probably due to the increasing impact of RANKL from osteocytes on trabecular bone remodeling in adult mice.
TRAP+ cells in different areas could possess distinct functions. Resorption of calcified cartilage in the growth plates is essential for bone elongation (Lewinson & Silbermann, 1992). In growing mice, loss of BM adipocyte RANKL does not affect resorption of calcified cartilage. TRAP staining demonstrated the number of TRAP+ osteoclasts under the growth plate, identified as a non‐bone‐resorbing osteoclast subtype called vessel‐associated osteoclast (VAO)(Romeo et al, 2019), and was not altered after the deletion of RANKL. Periosteal TRAP+ cells could regulate cortical bone formation (Gao et al, 2019). Macrophage‐lineage TRAP+ cells induced transcriptional expression of periostin and recruitment of periosteum‐derived cells (PDCs) to the periosteal surface through secretion of PDGF‐BB, where the recruited PDCs underwent osteoblast differentiation coupled with type H vessel formation, which is different from the trabecular bone remodeling. Loss of RANKL in BM adipose lineage cells did not affect cortical bone thickness or the number of periosteal TRAP+ cells.
The function of soluble RANKL on osteoclast formation and bone resorption has recently been identified. Mice lacking soluble RANKL show normal tooth eruption and bone development. Soluble RANKL contributes to the formation of osteoclasts in cancellous bone in adult mice, with membrane‐bound RANKL proving sufficient for normal growth and skeletal development (Xiong et al, 2018). Our findings showed that the bone marrow soluble RANKL levels were not significantly different between AdipoqCre; ranklfl/fl and ranklfl/fl mice, which indicated that BM adipose lineage cells are not an important source of sRANKL production. In some pathological conditions, including PMOP, periodontitis, and inflammatory joint disease, soluble RANKL levels are elevated (Geusens et al, 2006; Chen et al, 2010; Nile et al, 2013), which indicate that soluble RANKL is functionally involved in these conditions. However, mice lacking soluble RANKL showed a similar amount of bone loss after estrogen withdrawal (Xiong et al, 2018), which implies that soluble RANKL is not essential for osteoclast formation and bone resorption after estrogen withdrawal. In our study, no bone loss was observed in AdipoqCre; ranklfl/fl mice after estrogen withdrawal. Osteoclast formation and bone resorption were significantly inhibited following the deletion of RANKL in BM adipose lineage cells. Furthermore, the bone turnover rate was significantly lower in AdipoqCre; ranklfl/fl mice compared with their littermate controls. These results suggest that RANKL from BM adipose lineage cells contributes to excess osteoclast formation and bone resorption in pathological bone loss diseases with increased BM adiposity.
Rosiglitazone is widely clinically used to lower blood glucose levels in diabetic patients via the activation of PPARγ. However, the use of ROS is associated with an increased risk of fractures as well as an increase in BM adipocyte counts due to the activation of PPARγ, as essential mediator of adipocyte differentiation and function (Kahn et al, 2008; Aubert et al, 2010). Although the role of PPARγ in osteoclast differentiation is still a topic of considerable debate (Mbalaviele et al, 2000; Wan et al, 2007; Wei et al, 2010; Zou et al, 2016), our findings showed that administering ROS to AdipoqCre; ranklfl/fl mice failed to increase osteoclast formation and bone resorption or induce bone loss, as observed in ranklfl/fl mice. These results indicate that ROS increases osteoclast formation and bone resorption at least in part by increasing the number of BM adipose lineage cells.
Osteocytes sense mechanical loading and secrete RANKL to control osteoclast formation and bone remodeling in adult mice and unloading‐induced bone loss (Xiong et al, 2011; Wang et al, 2020a). Since the AdipoqCre does not target osteocyte, we further testify whether adipocyte RANKL mediates skeletal mechanical loading. Tail suspension did induce a significant loss of cancellous bone volume in both AdipoqCre; ranklfl/fl and ranklfl/fl mice, indicating adipocyte RANKL did not mediate unloading‐induced bone resorption.
Taken together, our study demonstrates that the RANKL from BM adipose lineage cells serves as an essential source of trabecular bone remodeling in both physiological and pathological conditions but does not mediate resorption of calcified cartilage in growth plate or regulate cortical bone remodeling.
Materials and Methods
Mice and study design
Wild‐type (WT) C57BL/6J mice were purchased from Vital River Company (Beijing, China). The AdipoqCre mice were obtained as a generous gift from Prof. Ma Xinran from the East China Normal University. Ranklfl/fl and rankfl/fl mice were purchased from the Jackson Laboratory (JAX stocks No. 018978 and 027495). Rosa26‐lsl‐tdTomato mice were provided by the Shanghai Model Organisms (Shanghai, China). Cross‐fertilization, breeding, and feeding of all mice were performed at Shanghai Model Organisms (SCXK [Shanghai] 2017‐0010 and SYXK [Shanghai] 2017‐0012). The mice were kept in SPF facilities with no more than 5 in a cage with food and water available ad libitum. The protocols are in compliance with the regulations of the ethical committee of Shanghai Changhai Hospital. Mice were anesthetized at defined intervals (4, 8, and 16 weeks) by 5% chloral hydrate to collect femur, blood, centrum, bone marrow, and tooth samples. Samples were divided into different groups randomly, and littermates were used as controls. Sample sizes were calculated on the assumption that a 30% difference in the parameters measured would be considered biologically significant with an estimate of sigma of 10–20% of the expected mean.
In vivo pathological bone loss models
Female mice of 8 weeks age were anesthetized with 5% chloral hydrate and then ovariectomized via transection to establish the postmenopausal osteoporosis (PMOP) model. After 6 weeks, the mice were anesthetized by 5% chloral hydrate to collect femur and blood.
Female mice of 8‐week age were treated with rosiglitazone (10 mg/kg/day, intragastric administration). After 6 weeks, the mice were anesthetized by 5% chloral hydrate to collect femur and blood.
For the unloading model, we performed the tail suspension. The tail of mice was tied to the rearing cage. After 4 weeks, the femur was collected and scanned by micro‐CT.
Cell culture
To verify the proliferation capacity of tdTomato+ cells of AdipoqCre; R26tdTomato mice, tdTomato+ cells were sorted by flow cytometry. And bone marrow adipose lineage cells were collected following previously reported (Fan et al, 2017). The tdTomato+ cells were cultured in DMEM medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (HyClone), and the colony‐forming unit‐fibroblast (CFU‐F) was analyzed by the Giemsa (Beyotime, Shanghai, China) staining after 7 days of culture. Bone marrow mesenchymal stromal cells (BMSCs) were seeded onto 96‐well plates and treated with dexamethasone (10−8 mol/l), β‐glycerophosphate (10 mM), and ascorbic acid (50 mg/ml) for 3 days to induce osteogenesis. After 21 days, the alizarin red staining was performed to detect osteogenesis. BMSCs were induced by β‐sodium glycerophosphate (10 mmol/l), dexamethasone (10−8 mol/l), and vitamin C (10 mmol/l) for adipogenesis (Xin et al, 2018). Adipocyte formation was analyzed via oil red O staining.
ELISA
Commercially available ELISA kits were used to determine concentrations of active CTX‐1 (Novus, catalog no. NBP2‐69074), OCN (Novus, catalog no. NBP2‐68151) and RANKL (Abcam, catalog no. ab100749) in bone marrow according to the manufacturer’s instructions.
Real‐time PCR
The total RNA of cells was collected by RNA fast 200 kit (catalog no. 220010, Feijie bio company, Shanghai, China). We amplified and detected β‐actin (mouse: 5′‐TCTGCTGGAAGGTGGACAGT‐3′ [forward], 5′‐CCTCTATGCCAACACAGTGC‐3′ [reverse]), Rankl (mouse: 5′‐TGTACTTTCGAGCGCAGATG‐3′ [forward], 5′‐AGGCTTGTTTCATCCTCCTG‐3′ [reverse]), PPARγ (mouse: 5′‐GGAAGACCACTCGCATTCCTT‐3′ [forward], 5′‐GTAATCAGCAACCATTGGGTCA‐3′ [reverse]), Lpl (mouse: 5′‐ATGGATGGACGGTAACGGGAA‐3′ [forward], 5′‐CCCGATACAACCAGTCTACTACA‐3′ [reverse]), Alp (mouse: 5′‐CCAACTCTTTTGTGCCAGAGA‐3′ [forward], 5′‐GGCTACATTGGTGTTGAGCTTTT‐3′ [reverse]), and Ocn (mouse: 5′‐GGACCATCTTTCTGCTCACTCTGC‐3′ [forward], 5′‐TGTTCACTACCTTATTGCCCTCCTG‐3′ [reverse]) using a real‐time PCR system (analytic Jena), as described previously (Chen et al, 2018b).
Western blotting
Protein expression was determined by Western blotting using standard methods. Blots were probed with anti‐LPL (ab91600, 1:500), anti‐PPARγ (ab59256, 1:800), anti‐OCN (ab93876, 1:500), anti‐ALP (ab229126, 1:1,000), and anti‐RANKL (ab45039, 1:300) antibodies (Abcam, Cambridge, Britain), as described previously (Luo et al, 2016; Ma et al, 2017).
Flow cytometry
Cells were collected from femurs of ranklfl/fl mice, AdipoqCre; ranklfl/fl mice, AdipoqCre; R26tdTomato mice, after which bone marrow cells were collected and labeled with monoclonal anti‐mouse CD3 (Sigma, catalog no. SAB4700050, 1: 1,000), CD19 (Sigma, catalog no. SAB4700108, 1: 1,000) antibodies, CD45 (eBioscience, catalog no. 11‐0451, 1: 500), CD31 (BD Bioscience, catalog no. 558738, 1: 500), Sca‐1 (eBioscience, catalog no. 56‐5981, 1: 500), PDGFα (BioLegend, catalog no. 135907, 1: 500), and Reep2 (Invitrogen, catalog no. MA5‐25878, 1: 500 and Abcam, catalog no. ab6563, 1: 1,000). Cells were analyzed on an LSRII flow cytometer system (BD Biosciences) using FlowJo software (Tree Star), as described previously (Chen et al, 2018a). tdTomato+ cells were sorted and collected from the femur of AdipoqCre; R26tdTomato mice.
In vivo heterotopic bone formation assay
The sorted tdTomato+ cells from AdipoqCre; R26tdTomato mice and BMSCs were collected and 0.5 × 106 cells mixed with 40 mg hydroxyapatite in 100 μl α‐MEM and incubated overnight. Then, the cells were implanted under the kidney capsules of BALB/c nude mouse for 4 weeks. After retrieval, it was paraformaldehyde‐fixed and paraffin‐embedded. The slice was stained with OCN, Aggrecan, and Perilipin, and positive cells were counted.
Micro‐CT and X‐ray
Bone was fixed with 4% paraformaldehyde for 24 h and analyzed by Skyscan 1172 high‐resolution micro‐CT (Skyscan, Antwerp, Belgium). Scan conditions were set at 8 µm per pixel resolution, 80 kV voltage, and 124 μA current. Using these images, we constructed three‐dimensional models and analyzed images by CTAn and CTVol, including bone mineral density (BMD), bone volume/tissue volume (BV/TV), trabecular number (Tb.N), trabecular separation (Tb.Sp), and cortical thickness. And the tooth eruption was evaluated by the X‐ray.
Histochemistry, immunohistochemistry, and immunofluorescent staining
Femurs were fixed in 4% paraformaldehyde for 24 h and decalcified in 10% EDTA (room temperature) for 2 weeks. After dehydration in an alcohol gradient (50, 75, 85, 95, or 100%), femurs were embedded in paraffin, cut into 4 µm thick sections using a Thermo Scientific Microm HM 325 Rotary Microtome, and processed for hematoxylin‐eosin (H&E) staining and TRAP staining according to the manufacturer’s instructions (Collins et al, 2017). For immunohistochemistry staining, sections were incubated with primary antibodies against RANKL (Abcam; catalog no. ab216484, 1:100) with a horseradish peroxidase‐conjugated anti‐rabbit secondary antibody used to detect immunoactivity. For immunofluorescence staining, frozen sections were incubated with primary antibodies overnight to osteocalcin (Biocompare; catalog no. bs‐4917R; 1:100), adiponectin (Abcam; catalog no. ab181281; 1:200), aggrecan (ProteinTech; catalog no. 13880‐1‐AP; 1:200), and perilipin (Invitrogen; catalog no. PA1‐1051; 1:100). Then, the sections were incubated with corresponding fluorescence‐conjugated secondary antibodies (1:200) and DAPI (1:500) for 1 h at room temperature. Then, the samples were scanned on either a NanoZoomer or 3D Histech system, with exported images analyzed using Case Viewer and NDP view software.
Calcein staining
To detect mineral deposition, we injected mice with alizarin red S (Sigma; 40 mg/kg body weight) 10 days and calcein (Sigma; 20 mg/kg body weight) 3 days before euthanasia. We performed undecalcified bone slicing and calculated the mineral apposition rate (MAR) of trabecular bone and cortical bone.
Statistics
All of the analyses were performed by an independent investigator blinded to the genotypes of the animals under analysis. Data are presented as means ± SDs. Independent‐samples t‐tests and one‐way ANOVAs were used to assess statistical significance. All analyses were performed using SPSS 21.0 (IBM, Chicago, IL, USA). Statistical significance was set at P < 0.05.
Author contributions
XC, YH, XZ, and JCS designed the study. XZ, WC, HWC, XQL, YJW, LPW, JC, YH, LHC, WC, YHL, YL, JWG, SCW, and WZW collected the data. XZ, CF, QRZ, YJW, BTH, and XQL analyzed the data. XC and JCS interpreted the data. XZ collected and prepared the figures. XC and XZ drafted the manuscript. XC and JCS revised the manuscript content. JCS involved in approving final version of manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
We thank Prof. Ma Xinran from the East China Normal University for giving us the AdipoqCre mice as a generous gift. All the transgenic mice in the study were bred and housed in Shanghai Model Organisms Center Inc. We thank the Textcheck for language polishing. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/zWIVZE. This work was supported by the National Key R&D Program of China (2018YFC2001500); National Natural Science Foundation (NNSF) Key Research Program in Aging (91749204); National Natural Science Foundation of China (81871099, 81771491); Municipal Human Resources Development Program for Outstanding Leaders in Medical Disciplines in Shanghai (2017BR011); and Shanghai Rising‐Star Program (21QA1412000).
EMBO reports (2021) 22: e52481.
See also: M Onji et al (July 2021)
Contributor Information
Sicheng Wang, Email: w.s.c@sina.com.
Xiao Chen, Email: sirchenxiao@126.com.
Jiacan Su, Email: drsujiacan@163.com.
Data availability
This study includes no data deposited in external repositories.
References
- Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank A‐M, Bocian C, Woelk L, Fan H, Logan DW, Schürmann Aet al (2017) Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell‐Based Hematopoietic and Bone Regeneration. Cell Stem Cell 20: 771–784.e776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C (2008) Role of platelet‐derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M (2010) Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab 12: 716–721 [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337–342 [DOI] [PubMed] [Google Scholar]
- Chen CH, Chen HA, Liao HT, Liu CH, Tsai CY, Chou CT (2010) Soluble receptor activator of nuclear factor‐kappaB ligand (RANKL) and osteoprotegerin in ankylosing spondylitis: OPG is associated with poor physical mobility and reflects systemic inflammation. Clin Rheumatol 29: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Chen X, Zhi X, Wang J, Su J (2018a) RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res 6: 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhi X, Yin Z, Li X, Qin L, Qiu Z, Su J (2018b) 18beta‐glycyrrhetinic acid inhibits osteoclastogenesis in vivo and in vitro by blocking RANKL‐mediated RANK‐TRAF6 interactions and NF‐kappaB and MAPK signaling pathways. Front Pharmacol 9: 647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FL, Williams JO, Bloom AC, Singh RK, Jordan L, Stone MD, McCabe LR, Wang ECY, Williams AS (2017) CCL3 and MMP‐9 are induced by TL1A during death receptor 3 (TNFRSF25)‐dependent osteoclast function and systemic bone loss. Bone 97: 94–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque G, Li W, Adams M, Xu S, Phipps R (2011) Effects of risedronate on bone marrow adipocytes in postmenopausal women. Osteoporosis Int 22: 1547–1553 [DOI] [PubMed] [Google Scholar]
- Fan Y, Hanai J‐I, Le PT, Bi R, Maridas D, DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt Met al (2017) Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab 25: 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Jilka RL, Manolagas SC, O'Brien CA (2002) Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP‐response element‐binding protein. J Biol Chem 277: 48868–48875 [DOI] [PubMed] [Google Scholar]
- Gao B, Deng R, Chai Y, Chen H, Hu B, Wang X, Zhu S, Cao Y, Ni S, Wan Met al (2019) Macrophage‐lineage TRAP+ cells recruit periosteum‐derived cells for periosteal osteogenesis and regeneration. J Clin Invest 129: 2578–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geusens PP, Landewe RB, Garnero P, Chen D, Dunstan CR, Lems WF, Stinissen P, van der Heijde DM, van der Linden S, Boers M (2006) The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum 54: 1772–1777 [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF‐B and PDGFR‐beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055 [DOI] [PubMed] [Google Scholar]
- Jabbar S, Drury J, Fordham JN, Datta HK, Francis RM, Tuck SP (2011) Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis. J Clin Pathol 64: 354–357 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RPet al (2008) Rosiglitazone‐associated fractures in type 2 diabetes: an Analysis from a Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 31: 845–851 [DOI] [PubMed] [Google Scholar]
- Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423: 332–336 [DOI] [PubMed] [Google Scholar]
- Lacey Dl, Timms E, Tan H‐l, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully Set al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176 [DOI] [PubMed] [Google Scholar]
- Lewinson D, Silbermann M (1992) Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anatomical Rec 233: 504–514 [DOI] [PubMed] [Google Scholar]
- Liu WG, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T (2001) Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol 155: 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G, Huang J, Dai W, Li C, Zheng Cet al (2016) LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 22: 539–546 [DOI] [PubMed] [Google Scholar]
- Ma YM, Fu ST, Lu L, Wang XH (2017) Role of androgen receptor on cyclic mechanical stretch‐regulated proliferation of C2C12 myoblasts and its upstream signals: IGF‐1‐mediated PI3K/Akt and MAPKs pathways. Mol Cell Endocrinol 450: 83–93 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332: 305–311 [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Abu‐Amer Y, Meng A, Jaiswal R, Beck S, Pittenger MF, Thiede MA, Marshak DR (2000) Activation of peroxisome proliferator‐activated receptor‐gamma pathway inhibits osteoclast differentiation. J Biol Chem 275: 14388–14393 [DOI] [PubMed] [Google Scholar]
- Mukohira H, Hara T, Abe S, Tani‐Ichi S, Sehara‐Fujisawa A, Nagasawa T, Tobe K, Ikuta K (2019) Mesenchymal stromal cells in bone marrow express adiponectin and are efficiently targeted by an adiponectin promoter‐driven Cre transgene. Int Immunol 31: 729–742 [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh‐hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EFet al (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17: 1231–1234 [DOI] [PubMed] [Google Scholar]
- Nile CJ, Sherrabeh S, Ramage G, Lappin DF (2013) Comparison of circulating tumour necrosis factor superfamily cytokines in periodontitis patients undergoing supportive therapy: a case‐controlled cross‐sectional study comparing smokers and non‐smokers in health and disease. J Clin Periodontol 40: 875–882 [DOI] [PubMed] [Google Scholar]
- Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285: 25103–25108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo SG, Alawi KM, Rodrigues J, Singh A, Kusumbe AP, Ramasamy SK (2019) Endothelial proteolytic activity and interaction with non‐resorbing osteoclasts mediate bone elongation. Nat Cell Biol 21: 430–441 [DOI] [PubMed] [Google Scholar]
- Scheller EL, Rosen CJ (2014) What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci 1311: 14–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop Net al (2007) Osteoclast‐poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39: 960–962 [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T (1989) The bone marrow‐derived stromal cell lines MC3T3‐G2/PA6 and ST2 support osteoclast‐like cell differentiation in cocultures with mouse spleen cells. Endocrinology 125: 1805–1813 [DOI] [PubMed] [Google Scholar]
- Veldhuis‐Vlug AG, Rosen CJ (2018) Clinical implications of bone marrow adiposity. J Intern Med 283: 121–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM (2007) PPAR‐gamma regulates osteoclastogenesis in mice. Nat Med 13: 1496–1503 [DOI] [PubMed] [Google Scholar]
- Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W (2020a) Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast‐osteoclast crosstalk. Nat Commun 11: 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Chen Y, Qin Q (2020b) Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol 92: 568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y (2010) PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone‐induced bone loss. Cell Metab 11: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Jin C, Chao L, Zheng Z, Liehu C, Panpan P, Weizong W, Xiao Z, Qingjie Z, Honggang Het al (2018) A matrine derivative M54 suppresses osteoclastogenesis and prevents ovariectomy‐induced bone loss by targeting ribosomal protein S5. Front Pharmacol 9: 22 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xiong J, Cawley K, Piemontese M, Fujiwara Y, Zhao H, Goellner JJ, O'Brien CA (2018) Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy‐induced bone loss. Nat Commun 9: 2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA (2011) Matrix‐embedded cells control osteoclast formation. Nat Med 17: 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Huo L, Liu Y, Deng P, Szymanski J, Li J, Luo X, Hong C, Lin J, Wang CY (2018) PGC‐1alpha controls skeletal stem cell fate and bone‐fat balance in osteoporosis and skeletal aging by inducing TAZ. Cell Stem Cell 23: 615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Yao L, Tower RJ, Wei Y, Miao Z, Park J, Shrestha R, Wang L, Yu W, Holdreith Net al (2020) Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. eLife 9: e54695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, Morrison SJ (2017) Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol 19: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ (2014) Leptin‐receptor‐expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15: 154–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Rohatgi N, Chen TH, Schilling J, Abu‐Amer Y, Teitelbaum SL (2016) PPAR‐gamma regulates pharmacological but not physiological or pathological osteoclast formation. Nat Med 22: 1203–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File
Data Availability Statement
This study includes no data deposited in external repositories.


