Abstract
Background:
Organochlorine pesticides (OCPs) are lipophilic persistent organic pollutants associated with adverse health outcomes. Black women have higher body burdens compared to other U.S. populations and the research on their predictors is limited.
Methods:
Using baseline data from a prospective cohort study of Black women aged 23–35 years from the Detroit, Michigan metropolitan area (enrolled 2010–2012), we examined predictors of plasma concentrations of the following OCPs: dichlorodiphenyltrichloroethane (p,p’-DDE), hexachlorobenzene (HCB), oxychlordane, and trans-nonachlor. At enrollment, we collected non-fasting blood samples from 742 participants. We also collected demographic, behavioral, dietary, occupational, and medical history data via self-administered questionnaires, telephone interviews, and in-person clinic visits. We fit linear regression models to calculate percent (%) differences across categories of each predictor and 95% confidence intervals (CIs).
Results:
In models adjusted for all other predictors, a 5-year increase in age was associated with 24% higher oxychlordane (95% CI: 12%, 38%) and 26% higher trans-nonachlor (95% CI: 12%, 42%). Heavy alcohol use was associated with 7–9% higher plasma concentrations of p,p’-DDE, oxychlordane, and trans-nonachlor. Current smoking was associated with 10–19% higher plasma concentrations of all four OCPs. Compared with never having been breastfed during infancy, having been breastfed for >3 months was associated with 15% higher concentrations of p,p’-DDE (95% CI: 6%, 25%), 14% higher oxychlordane (95% CI=5%, 24%), and 15% higher trans-nonachlor (95% CI=5%, 27%). Consumption of ≥5 glasses of tap or bottled water vs. <2 glasses/day of tap or bottled water was associated with 8–15% higher plasma concentrations of all four OCPs, and highest for trans-nonachlor (95% CI: 6%, 26%). No other dietary predictors were appreciably associated with plasma OCP concentrations. Obesity, parity, higher birth order, and longer lactation durations were inversely associated with plasma OCP concentrations.
Conclusions:
In Black U.S. women of reproductive age, exposure to OCPs earlier in life when these persistent pesticides were at higher concentrations in the environment appears to still contribute to current blood concentrations. In addition, residual contamination of tobacco, alcohol, and drinking water may be important current sources of exposure.
Keywords: pesticides, Black women, DDT, DDE, chlordane
1. Introduction
Organochlorine pesticides (OCPs) are persistent organic pollutants associated with adverse neurodevelopmental outcomes (1), breast cancer (2, 3), metabolic dysfunction (4), and hormone disruption (5, 6). OCPs tend to accumulate in adipose tissue (7–12), especially in reproductive-aged women (13, 14). OCP concentrations in adipose tissue positively correlate with serum concentrations (15–17) although adipose concentrations may better reflect cumulative internal body burdens compared to serum (18, 19). The insecticide dichlorodiphenyltrichloroethane (p,p’-DDT) is one of the most well-known OCP, having been used extensively on crops and for vector control in the United States (U.S.) from 1945 to 1972, leading to widespread contamination of the environment (20, 21). In the U.S., racial differences in serum pesticide concentrations have been observed as far back as the 1960s (22), and concentrations are higher among Black women than White women (23). Nevertheless, there has been limited research on predictors of exposure to OCPs among reproductive-aged non-pregnant Black women.
With half-lives in humans of up to 7 years (24), many OCPs have been targeted by the Stockholm Convention on Persistent Organic Pollutants for elimination or restriction and labeled as probable carcinogens. Due to their environmental persistence, ability to bioaccumulate in humans, and adverse impacts on health, the U.S. banned the use of DDT and other OCPs for agricultural use starting in the 1970s. Uses for some extended into the late 1980’s, with Hexachlorobenzene as a fungicide until 1984 and chlordane-related pesticides, such as oxychlordane and trans-nonachlor, to control termites until 1988. Despite bans, these chemicals are still detectable in most of the U.S. population (14), the food chain (25, 26) and throughout the ecosystem (27–30). Once ingested and absorbed, OCP blood concentrations are influenced by factors related to lipid turnover and storage, including weight loss, body fat distribution, and lactation (15, 31, 32). A history of childbearing and lactation are proposed clearance routes inversely associated with OCP concentrations in blood and adipose tissue (33–44). Associations between OCPs and body mass index (BMI) are mixed (22, 39–41, 45, 46). For example, serum concentrations of p,p’-DDE have been positively (39, 46, 47) and inversely (22) associated with BMI, potentially due to the complicated nature of OCP distribution and metabolism across the body’s various adipose tissues (48).
In epidemiologic studies examining predictors of OCP concentrations, most of which were in pregnant women, determinants of OCPs vary across individual pesticides and their metabolites. The most consistent predictor of elevated OCP concentrations in blood and adipose tissue is older age (39, 41, 42, 44–46, 49, 50). Although ingestion of contaminated food sources is a putative route of exposure (8, 25, 26), associations with self-reported diet are inconsistent (13, 39–41, 43, 44, 49, 51–53), with some studies showing evidence of increased OCP concentrations with higher meat and large fish consumption (44, 51, 52, 54–56). Trace residues of OCPs are still detectable in tobacco products and in soils used to produce wine, and beer (57–62). Therefore, exposure to cigarettes and alcohol may be important sources of human exposure. Given racial disparities in exposure to environmental chemicals across the life course (63), sociodemographic and lifestyle factors are also potentially important predictors of circulating concentrations of environmental chemicals, including OCPs (40, 46).
We used cross-sectional data from a prospective cohort study of 742 Non-Hispanic Black women in the Detroit, Michigan metropolitan area to identify predictors of exposure to OCPs. We focused on demographic, behavioral, dietary, occupational, and medical history factors associated with pesticide body burdens in previous literature.
2. Subject and Methods
2.1. Study Population
The Study of Environment, Lifestyle, and Fibroids (SELF) is a prospective cohort study of 1,693 reproductive-aged Black women recruited from the Detroit, Michigan metropolitan area during 2010–2012, as described in detail elsewhere (64). Eligibility criteria included age (23–35 years) and self-identification as African American, Black, or partly African American. Exclusion criteria included prior diagnoses of uterine leiomyomata (fibroids), cancer, or autoimmune disease. Participants who were pregnant at recruitment had enrollment delayed until three months postpartum. For this sub-study of selected endocrine disrupting chemicals and leiomyomata, we used a case-cohort study design in which we randomly selected a subcohort of 639 participants from the 1,693 fibroid-free SELF participants at baseline. We then supplemented the random subcohort of 639 with and the remaining incident cases of leiomyomata through 60-months of follow-up (this included 103 cases that occurred outside of the randomly selected subcohort). This sampling strategy resulted in a total of 742 participants for the present analysis, 639 selected at baseline (183 who went on to become a case) plus the 103 supplemented cases. Demographic, behavioral, dietary, occupational, and medical history data were collected via self-administered questionnaires, telephone interviews, and in-person clinic visits. Women also completed a self-administered semi-quantitative food frequency questionnaire (65). The Institutional Review Boards of the Henry Ford Health System, National Institute of Environmental Health Sciences (NIEHS), and Boston University Medical Campus approved the study. The involvement of the Centers for Disease Control and Prevention (CDC) did not constitute engagement in human subjects’ research.
2.2. Measurement of Organochlorine Pesticides in Plasma
At baseline, enrolled participants provided a non-fasting blood sample. Collected samples were shipped to the NIEHS repository for storage at −80 degrees Celsius, and then shipped on dry ice in three batches to the CDC for analysis. We used gas chromatography/isotope dilution high-resolution mass spectrometry (66) to measure plasma concentrations of nine OCPs: 2,2-bis(4-chlorophenyl)-1,1-dichloroethene (p,p’-DDE), 2-(4-chlorophenyl)-2-(2-chlorophenyl)-1,1,1-trichloroethane (o,p’-DDT), 2,2-bis(4-chlorophenyl-1,1,1-trichloroethane (p,p’-DDT), hexachlorobenzene (HCB), β-hexachlorocyclohexane (β-HCH), γ-hexachlorocyclohexane (lindane; γ -HCH), oxychlordane, trans-nonachlor, and mirex. The choice of OCPs was informed by their prevalence of exposure in humans (14) and evidence of their effects on reproductive hormones and processes that could influence risk of uterine leiomyomata (11, 67–71). Quality control (QC) samples, reagent blanks, and blind duplicates were distributed across batches and coefficient of variation estimates for the above mentioned analytes ranged from 3.3–13.9% (72). We determined total plasma lipids using an enzymatic summation method (73) and reported all POP concentrations on a lipid-adjusted basis.
2.3. Potential Correlates
We focused on correlates associated with persistently occurring pollutants in previous literature, including: age (years, continuous); body mass index (BMI) calculated from height and weight as measured by technicians at baseline (kg/m2, continuous); alcohol use in the past year (none, “moderate”: 1–5 drinks/day, or ≥4 drinks once per month or less, or “heavy”: ≥6 drinks/day, or ≥4 drinks twice per month or more); smoking history (never, former, or current); birth order (first, second, third or higher); parity (nulliparous or parous); cumulative duration of lactation (months, continuous, with nulliparous women coded as zero months); education (≤high school or General Education Diploma (GED), some college/Associate’s degree/technical degree, or ≥Bachelor’s degree); annual household income in U.S. dollars (<$20,000, $20,000-$50,000, or >$50,000); and cumulative residence in urban area (years, continuous). Dietary predictors of interest included frequency of consumption of the following food items: fish (grams/day, continuous), meat (grams/day, continuous), poultry (grams/day, continuous), eggs (grams/day, continuous), dairy (servings/day, continuous), vegetables (servings/day, continuous), fruit (cups/day, continuous), and tap or bottled water (glasses/day).
Measures of participants’ in utero exposures and early life experiences included: mother’s residence on a farm while pregnant with participant (yes/no), mother working on a farm while pregnant with participant (yes/no), or whether the participant lived on or visited a farm for ≥1 month before 18 years of age (yes/no). The majority of participants had the help of their mothers for questions on mother’s exposures and early life factors (85%), including whether they were breastfed as an infant (never, <3 months, or ≥3 months), both have been found to be reported with high reliability (74, 75). Measures of participant’s occupational exposure to pesticides included the following: “working on a farm or orchard” for ≥1 month (yes/no), “working with lawn care” for ≥1 month (yes/no), or “working with pesticides” ≥1 month (yes/no). We also assessed exposure to pesticides and bug repellant in the past 24 hours (yes/no).
2.4. Statistical Analysis
The median limit of detection (LOD) varied for each individual pesticide and ranged from 1.1 to 1.4 ng/g lipid (Table 2). Detection frequencies ranged from 0.3% (γ-HCH) to 100% (p,p’-DDE) and all analyses were restricted to chemicals with detection frequencies ≥60%, restricting all analyses to p,p’-DDE (100% detection), HCB (74.5% detection), oxychlordane (88.5% detection), and trans-nonachlor (91.4% detection). Values below the LOD were set to the LOD divided by the square root of two (76). We compared arithmetic means of each pesticide in the SELF cohort to the arithmetic mean of pooled serum samples representative of Non-Hispanic Black women aged 20–39 years from the National Health and Nutrition Examination Survey (NHANES) during 2009–2010 (14) (Table 2).
Table 2:
Characteristics of 742 participants in the prospective cohort Study of Environment, Lifestyle, and Fibroids (SELF) at baseline (2010–2012)
| Characteristic | Mean (SD) or N | % |
|---|---|---|
|
| ||
| Mean age in years (SD) | 28.6 (3.5) | - |
| Age, years | ||
| 23–25 | 179 | 24.1 |
| 26–28 | 182 | 24.5 |
| 29–31 | 198 | 26.7 |
| 32–35 | 183 | 24.7 |
| Mean BMI, kg/m2 (SD) | 33.7 (9.5) | - |
| BMI, kg/m2 | ||
| <25 | 139 | 18.7 |
| 25–29.9 | 157 | 21.2 |
| 30–34.9 | 147 | 19.8 |
| 35–39.9 | 124 | 16.7 |
| ≥40 | 175 | 23.6 |
| Education | ||
| ≤High school diploma/GED | 156 | 21.0 |
| Some college/Associate’s/Technical | 385 | 51.9 |
| ≥Bachelor’s degree | 201 | 27.1 |
| Annual household income, U.S. dollar | ||
| <20,000 | 340 | 45.8 |
| 20,000–50,000 | 280 | 37.7 |
| >50,000 | 122 | 16.4 |
| Urban residence in years | ||
| <5 | 70 | 9.4 |
| 5–9 | 40 | 5.4 |
| 10–19 | 155 | 20.9 |
| ≥20 | 477 | 64.3 |
| Cigarette smoking history | ||
| Never | 544 | 73.3 |
| Past | 59 | 8.0 |
| Current | 139 | 18.7 |
| Alcohol consumption, past yeara | ||
| None | 210 | 28.3 |
| Moderate | 383 | 51.6 |
| Heavy | 149 | 20.1 |
| Birth order | ||
| First | 308 | 41.5 |
| Second | 210 | 28.3 |
| Third or higher | 224 | 30.2 |
| Breastfed as an infant | ||
| Never breastfed | 521 | 70.2 |
| <3 months | 110 | 14.8 |
| ≥3 months | 111 | 15.0 |
| Parity | ||
| Nulliparous | 281 | 37.9 |
| 1 birth | 201 | 27.1 |
| 2 births | 127 | 17.1 |
| ≥3 births | 133 | 17.9 |
| Duration of lactation among parous women (n=461) | ||
| None | 159 | 34.5 |
| 1–3 months | 113 | 24.5 |
| 4–6 months | 70 | 15.2 |
| 7–12 months | 63 | 13.7 |
| >12 months | 56 | 12.2 |
| Mean dietary intake (SD) | ||
| Fish (grams/week) | 27.8 (32.6) | - |
| Meat (grams/week) | 53.5 (63.7) | - |
| Poultry (grams/week) (SD) | 35.6 (36.5) | - |
| Egg (grams/week) (SD) | 14.9 (14.4) | - |
| Dairy (servings/day) (SD) | 1.0 (0.8) | - |
| Vegetable (servings/day) (SD) | 3.0 (2.3) | - |
| Fruit (cups/day) (SD) | 1.4 (1.2) | - |
| Water intake (glasses of bottled or tap/day) | ||
| ≤2 | 215 | 29.0 |
| 3–4 | 298 | 40.2 |
| ≥5 | 229 | 30.9 |
| Occupation (≥1-month): | ||
| Farm | 4 | 0.5 |
| Lawn care | 13 | 1.8 |
| Pesticides | 5 | 0.7 |
| Participant’s mother: | ||
| Lived on a farm for ≥1-month during pregnancy | 4 | 0.5 |
| Worked on a farm for ≥1-month during pregnancy | 6 | 0.8 |
| Before the age of 18, lived on or visited a farm for ≥1-month | 29 | 3.9 |
| Used pesticides in past 24 hours | 11 | 1.5 |
| Used bug repellant in past 24 hours | 6 | 0.8 |
Abbreviations: BMI= Body mass index, GED= General education diploma, SD= standard deviation
Alcohol use in past year categories: none, “moderate”: 1–5 drinks/day, or ≥4 drinks once per month or less, or “heavy”: ≥6 drinks/day, or ≥4 drinks twice per month or more
We used Spearman correlation coefficients (rho) to evaluate correlations between individual OCPs. We fit linear regression models using log-transformed OCP concentrations as the dependent variable and each potential predictor as the independent variable, first unadjusted and then adjusted for all other predictors, as mentioned above. The percent (%) difference was calculated by exponentiating the beta coefficient comparing one category of the predictor to the reference category of that predictor, subtracting one, and then multiplying by 100 (i.e., (ebeta-1)*100). We used a Markov chain Monte Carlo method to impute missing data, which generated five imputation datasets that statistically combined estimates across the datasets (77). The percent missing for variables ranged from 0% (e.g., age) to 5% (breastfed as an infant). We imputed plasma OCP concentrations for <5% of participants: n=29 for HCB, n=4 for oxychlordane, and n=1 for trans-nonachlor.
To evaluate the extent to which inclusion of incident leiomyomata cases that were not part of the random subcohort influenced our results, we performed an additional sensitivity analysis restricted to the random subcohort of 639 women selected at baseline. Further, given the propensity for deposition of OCPs in adipose tissue, we performed analyses stratified by BMI (<30 vs. ≥30 kg/m2). Our hypothesis was that observed associations with serum OCP concentrations would be stronger in women with a BMI ≥30, assuming that they have higher cumulative body burdens due to the lipohilic nature of these chemicals.
3. Results
The mean age of the analytic sample was 28.6 years (standard deviation=3.5) and 60.1% had a BMI ≥30 kg/m2 (Table 3). Nearly 75% of participants never smoked, and 20.1% reported heavy alcohol consumption in the past year. Approximately 62% of participants were parous, and among them 65% had breastfed at least one infant. Twenty-nine percent reported being breastfed as an infant, and 41.5% were first-born children. Few participants had potential occupational exposure to OCPs or exposure to farms or farm work (<5%). Mean total daily intakes of fish, meat, poultry, and eggs were within recommended levels from the U.S. Department of Agriculture, while intakes of dairy, fruit, and vegetable were generally below recommended levels (78). Baseline plasma OCP concentrations (ng/g lipid) in SELF were comparable to or lower than non-Hispanic Black women of similar age from NHANES in 2009–2010 (Table 3). Correlations across individual OCPs were generally moderate to high, with correlations ranging from 0.51 to 0.86 (Table 4). Chlordane and trans-nonachlor were highly correlated (rho=0.86).
Table 3:
Spearman correlations for log-transformed plasma organochlorine pesticide concentrations (ng/g lipids) among 742 Study of Environment, Lifestyle, and Fibroids (SELF) participants.
| Organochlorine pesticides | p,p’-DDE | Hexachlorobenzene | Oxychlordane | Trans-nonachlor |
|---|---|---|---|---|
|
| ||||
| p,p’-DDE | 1.0 | 0.58 | 0.70 | 0.67 |
| Hexachlorobenzene | 1.0 | 0.58 | 0.51 | |
| Oxychlordane | 1.0 | 0.86 | ||
| Trans-nonachlor | 1.0 | |||
Abbreviations: p,p’-DDE= 2,2-Bis(4-chlorophenyl)-1,1-dichloroethene
In models mutually adjusted for all other predictors, a 5-year increase in age was associated with a 8.3% increase in p,p’-DDE (95% CI: −2.7%, 20.7%), 24.3% increase in oxychlordane (95% CI: 12.4%, 37.5%), and a 26.1% increase in trans-nonachlor (95% CI: 12.0%, 41.9%) (Figure 1a). A 5-kg/m2 increase in BMI was associated with a 8.9% decrease in p,p’-DDE (95% CI: −10.3%, −7.4%), a 5.3% decrease in HCB (95% CI: −6.3%, −4.3%), a 6.8% decrease in oxychlordane (95% CI: −8.2%, −5,4%), and a 5.5% decrease in trans-nonachlor (95% CI=−7.2%, −3.8%) (Figure 1b). Compared with no alcohol use, moderate and heavy alcohol use were weakly associated with higher concentrations of plasma OCPs, with percent increases ranging from 3.7% to 8.0% (Figures 1c and 1d). Plasma OCP concentrations for never and past smokers did not differ appreciably (Figure 1e). Current smokers had higher plasma concentrations than never smokers across all pesticides, with the strongest association for trans-nonachlor (19% difference, 95% CI: 9.0%, 30.4%) (Figure 1f). We provide the percent differences and 95% confidence intervals for all predictors in the Supplemental Table.
Figure 1: Adjusted percent differences and 95% confidence intervals in organochlorine pesticides by age, body mass index (BMI), alcohol use in the past year, and cigarette smoking status.
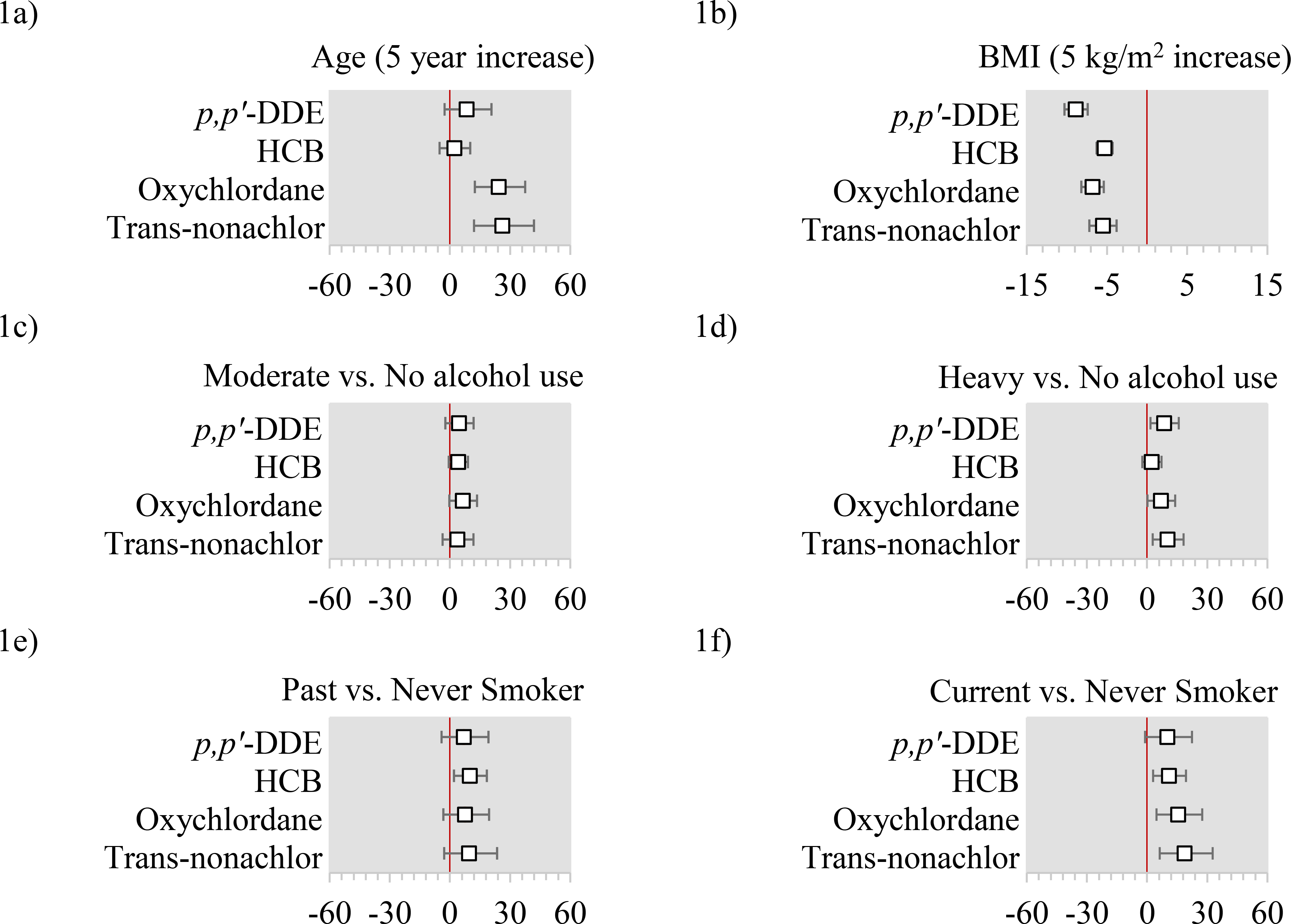
Abbreviations: p,p’-DDE= 2,2-Bis(4-chlorophenyl)-1,1-dichloroethene; HCB=Hexachlorobenzene.
All x-axis are percent differences and 95% confidence intervals. All models use the log-transformed plasma concentrations as the dependent variable and include age, education, household income, occupation (farm or orchard, lawn care, or pesticides), cigarette smoking status, alcohol use, body mass index (BMI), birth order, breastfeeding history as an infant, parity, lactation history, early exposure to pesticides (maternal farm residence or work during pregnancy, and lived on or worked on a farm before the age of 18), fish intake, meat intake, poultry intake, dairy intake, egg intake, fruit intake, vegetable intake, water intake, and total caloric intake.
In models mutually adjusted for all other predictors, relative to never having been breastfed in infancy, having been breastfed for <3 months was not materially associated with plasma OCP concentrations (Figure 2a), while having been breastfed for ≥3 months was associated with 15.2% higher p,p’-DDE (95% CI: 6.0%, 25.2%), 13.8% higher oxychlordane (95% CI: 4.9%, 23.6%), and 15.1% higher trans-nonachlor (95% CI: 4.7%, 26.6%) concentrations (Figure 2b). Compared to nulliparous women, parous women had suggestively lower HCB, oxychlordane, and trans-nonachlor, although estimates were not statistically significant (Figure 2c). Among parous women, a 3-month increase in lactation was associated with 1–4% lower plasma concentrations of OCPs (Figure 2d). We observed 2–9% lower plasma concentrations of all OCPs comparing participants who were third born or higher to those who were first-born (Figure 2f). Greater plasma concentrations of all OCPs were observed comparing the highest (≥5 glasses/day) with the lowest water consumers (≤2 glasses/day), with percent increases ranging from 4–15% (Figure 3b). Farm work or residence were rare (<4%) and observed associations with plasma OCP concentrations were imprecise. For example, residence on a farm or farm work prior to the age of 18 was associated with 31.6% higher p,p’-DDE (95% CI: 12.9%, 53.0%) (Figure 3c).
Figure 2: Adjusted percent differences and 95% confidence intervals in organochlorine pesticides by history of breastfeeding in infancy, parity, lactation history, and order of birth.
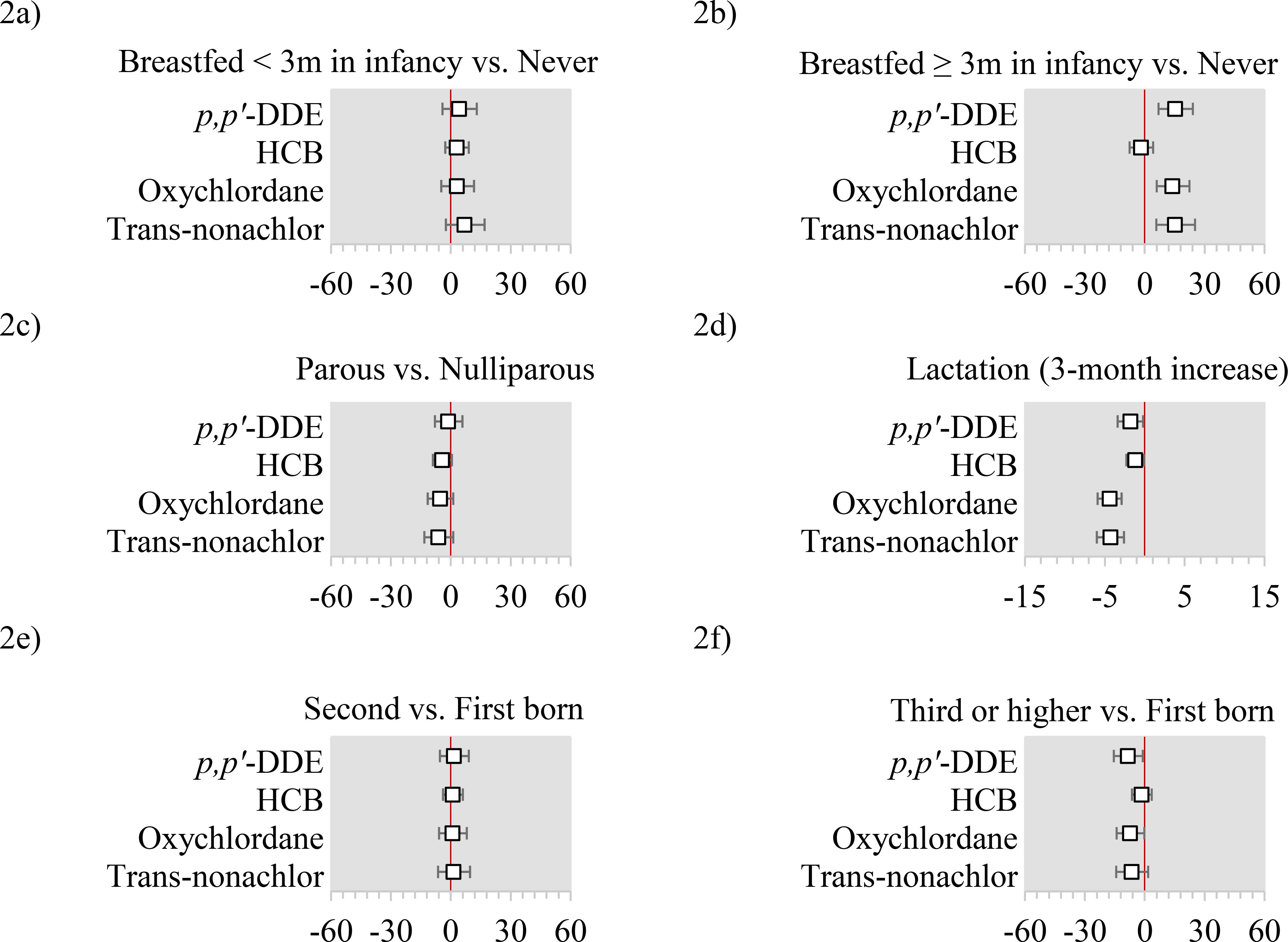
Abbreviations: p,p’-DDE= 2,2-Bis(4-chlorophenyl)-1,1-dichloroethene; HCB=Hexachlorobenzene.
All x-axis are percent differences and 95% confidence intervals. All models use the log-transformed plasma concentrations as the dependent variable and include age, education, household income, occupation (farm or orchard, lawn care, or pesticides), cigarette smoking status, alcohol use, body mass index (BMI), birth order, breastfeeding history as an infant, parity, lactation history, early exposure to pesticides (maternal farm residence or work during pregnancy, and lived on or worked on a farm before the age of 18), fish intake, meat intake, poultry intake, dairy intake, egg intake, fruit intake, vegetable intake, water intake, and total caloric intake.
Figure 3: Adjusted percent differences and 95% confidence intervals in organochlorine pesticides by water intake (tap or bottled), and farm exposure in early life.
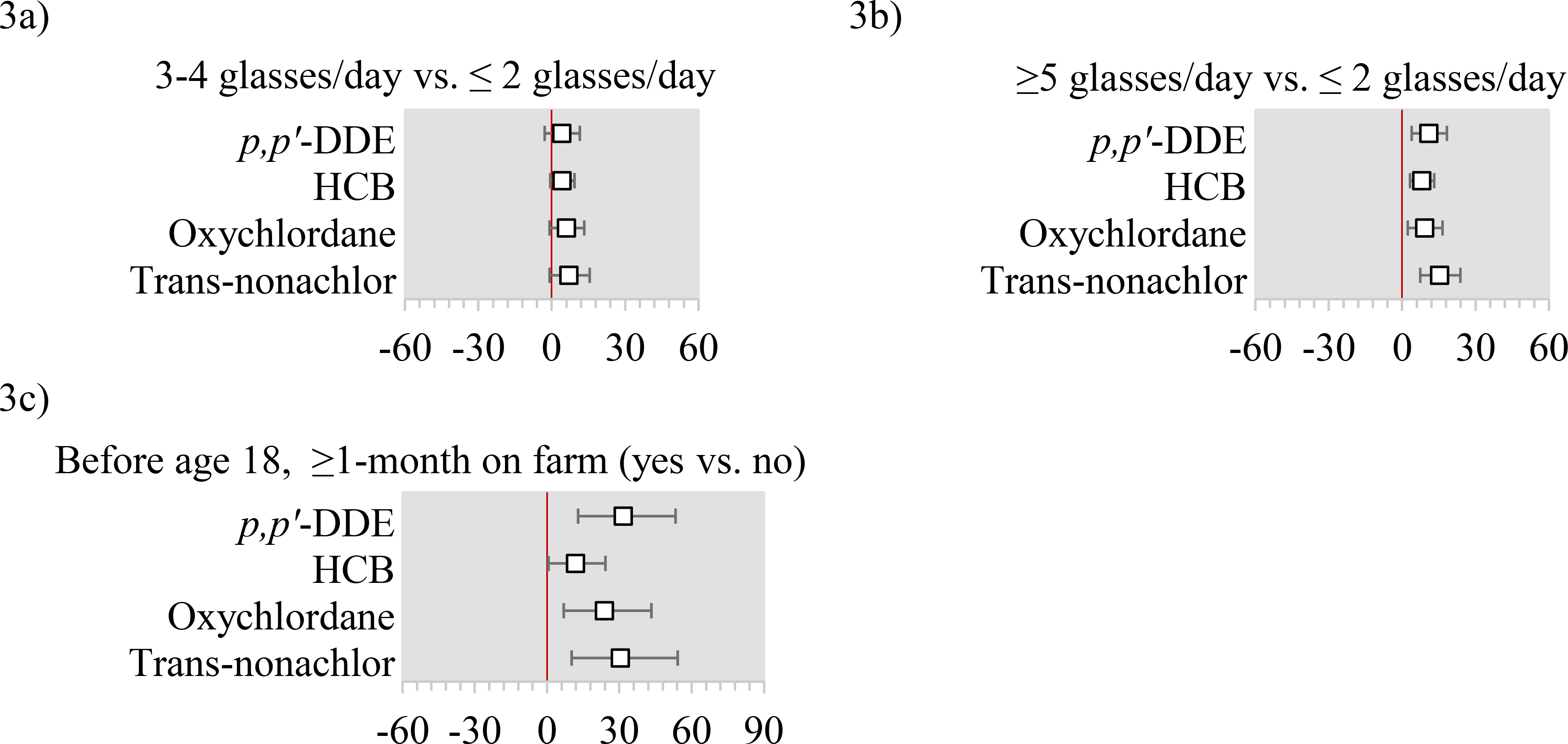
Abbreviations: p,p’-DDE= 2,2-Bis(4-chlorophenyl)-1,1-dichloroethene; HCB=Hexachlorobenzene.
All x-axis are percent differences and 95% confidence intervals. All models use the log-transformed plasma concentrations as the dependent variable and include age, education, household income, occupation (farm or orchard, lawn care, or pesticides), cigarette smoking status, alcohol use, body mass index (BMI), birth order, breastfeeding history as an infant, parity, lactation history, early exposure to pesticides (maternal farm residence or work during pregnancy, and lived on or worked on a farm before the age of 18), fish intake, meat intake, poultry intake, dairy intake, egg intake, fruit intake, vegetable intake, water intake, and total caloric intake.
Education, income, and all other dietary variables were not meaningfully associated with plasma OCP concentrations (Supplemental figures a–k). Mother’s exposure to farm work or farm residence was not associated with participant’s plasma OCP concentrations (Supplemental figures l and m). Exposure to pesticides, farm work, lawn care, and bug repellant were also rare (< 2%). Occupations were not associated with plasma OCP concentrations (Supplemental figures m–p), while pesticide exposure in the past 24 hours was associated with 35% higher plasma concentrations of p,p’-DDE (95% CI=6.7%, 70.7%) (Supplemental figure q). In sensitivity analyses restricted to the random subcohort selected at baseline, heavy alcohol use was no longer associated with elevated levels of HCB. All other associations were similar (data not shown). Predictors of p,p’-DDE were suggestively stronger among women with BMI ≥30 kg/m2 (relative to BMI <30 kg/m2) for heavy alcohol use (8.3% higher vs. 3.7% higher) and having been breastfed for ≥3 months in infancy (19.9% higher vs. 10.6% higher).
4. Discussion
Four out of the nine pesticides selected for analysis were detectable in ≥60% of SELF participants, and p,p’-DDT and β-HCH were detectable in 50% and 36% of participants, respectively. Lindane (γ-HCH), mirex, and o,p’-DDT were detectable in <10% of SELF-participants. Chlordane-related OCPs (oxychlordane and trans-nonachlor) were highly correlated with one another. In models adjusted for all potential predictors, older age, heavy alcohol use, current cigarette smoking, having been breastfed ≥3 months in infancy, and greater water intake were associated with higher plasma OCP concentrations. BMI, parity, longer lactation, and higher birth order were inversely associated with plasma OCP concentrations. Education, income, maternal exposures, and other dietary factors were not meaningfully associated with elevated plasma concentrations.
Among our study population of 23–35 year-old Black U.S. women, plasma concentrations of the selected OCPs were either comparable to or lower than those from a NHANES sample of Non-Hispanic Black U.S. women of a similar age range (14). With the exception of oxychlordane, pesticides that were not estimable by NHANES due to insufficient detection levels were also present at low frequencies of detection in our sample (Table 1). In contrast, oxychlordane was not estimable by NHANES yet it was highly detectable among SELF-participants (88.5%). Trans-nonachlor, another chlordane-related compound, was also commonly detected (91.4%). We may have expected to find higher chlordane residues among SELF-participants because participants resided in Michigan, where chlordane-related compounds have been detected in the nearby environment (79, 80). In contrast, NHANES participants were sampled from across the U.S. HCB and p,p’-DDE were also highly prevalent in our participants (74.5% and 100%, respectively) and observed in women globally (31, 81, 82).
Table 1:
Distribution of plasma organochlorine pesticides (ng/g lipid) in the Study of Environment, Lifestyle, and Fibroids (SELF) cohort (N=742) and NHANES (2009–2010)
| SELF Participants (2010–2012) 23–35 year old Black Women |
NHANES 2009–2010 20–39 year old Non-Hispanic Black Women |
||||
|---|---|---|---|---|---|
|
| |||||
| Organochlorine Pesticide | Median LOD | % Detected (>LOD) | Median (25th, 75th percentiles) | Mean | Mean |
| p,p’-DDT | 1.4 | 50.1 | >60% below the LOD | - | 3.0 |
| o,p’-DDT | 1.1 | 3.0 | >60% below the LOD | - | NC |
| p,p’-DDE | 1.4 | 100.0 | 51.2 (38.6, 69.3) | 57.7 | 110.0 |
|
| |||||
| Hexachlorobenzene | 1.4 | 74.5 | 5.9 (5.0, 7.2) | 6.3 | 6.0 |
|
| |||||
| β-HCH | 1.1 | 36.3 | >60% below the LOD | - | NC |
| γ-HCH (Lindane) | 1.1 | 0.3 | >60% below the LOD | - | NC |
|
| |||||
| Oxychlordane | 1.1 | 88.5 | 2.1 (1.6, 2.8) | 2.3 | NC |
| Trans-nonachlor | 1.2 | 91.4 | 2.4 (1.8, 3.4) | 2.8 | 6.8 |
|
| |||||
| Mirex | 1.1 | 8.1 | >60% below the LOD | - | NC |
Abbreviations: LOD=Limit of detection, NC= Not calculated “the proportion of results below LOD for that congener was too high to provide a valid result”, p,p’-DDT= 2,2-Bis(4-chlorophenyl-1,1,1-trichloroethane, HCH= Hexachlorocyclohexane
Higher plasma concentrations of p,p’-DDE and chlordanes, in particular, are consistent with their reported persistence and biochemistry. p,p’-DDE is the main bioaccumulative metabolite of DDT and, when compared with other DDT metabolites, is detectable at higher concentrations in human blood and adipose tissue. Positive associations between OCPs and age in our study of non-pregnant Black U.S. women of reproductive-age are consistent with previous studies (15, 32, 39, 41, 42, 44–46, 49, 50), albeit most were of predominantly White populations. Given that older participants were more likely to be exposed to OCPs earlier in life and continue to have higher plasma OCP concentrations compared with younger participants, these findings suggest that exposure to OCPs earlier in life may continue to contribute to current blood concentrations.
We observed inverse associations between increasing BMI and plasma OCP concentrations. Storage of OCPs in fat has been called “protective,” as it decreases the amount of bioavailable pesticides for biological activity (83). In line with this hypothesis, inverse associations between BMI and blood OCP concentrations are plausible and previously reported (22). A nine percent decrease in p,p’-DDE per 5-kg/m2 increase in BMI in our study (Supplemental Table) is nearly identical to estimates reported by James and colleagues (22) using pre-pregnancy BMI and serum collected from U.S. pregnant women in the 1960s. Lactation mobilizes fat and is a major route of excretion of OCPs and other persistently occurring pollutants (39, 49, 84), and is inversely associated with p,p’-DDE and HCB concentrations in adipose tissue and positively associated with concentrations in milk (15, 31). Higher plasma concentrations of p,p’-DDE and chlordane-related pesticides among participants who themselves were exposed to prolonged breastfeeding in infancy are also consistent with this hypothesis, and may suggest that inherited body burdens from early life may persist into adulthood.
Reported associations between OCPs and tobacco smoking are mixed (40, 85). Elevated concentrations of serum HCB, β-HCH, and p,p’-DDE concentrations have been associated with smoking habits among pregnant U.S. women (40) and in Finnish samples (50% males) (86). Pesticides have also been detected in tobacco smoke (87) and tobacco leaves (88, 89). Our study suggests that trace residues in the soil may still be an important source of human exposure. However, in contrast, other studies report no associations with smoking habits (85). Pesticides have also been detected in vineyard soils and wines (58, 59), as well as in hops used in beer brewing (60, 61). Trace levels of OCPs in the soils of these agricultural products may also help to explain elevated plasma concentrations in heavy drinkers in our cohort. Ingestion of contaminated food sources is a putative route of OCP exposure (8, 25, 26). However, associations with self-reported diet are inconsistent across the literature (13, 39–41, 43, 44, 49, 51–53). In our study, water consumption was the most important dietary predictor. Although pesticides from runoff and residential uses can be detected in water sources (90) and public water contamination of chlordane has been reported in the U.S. (91), this finding should be interpreted with caution. We did not distinguish tap from bottled water consumption on our questionnaire, and we could not eliminate the possibility of unmeasured or residual confounding from factors associated with water consumption and potential exposure to OCPs (e.g. gardening). Further studies are needed to determine whether these findings are robust and what water sources, if any, may be implicated.
Identified differences in predictors of exposure across OCPs are likely due to differences in chemical properties related to absorption, distribution, metabolism, excretion, and toxicity (92). For example, the chlordane-related compounds (oxychlordane and trans-nonachlor) have similar chemical structures and effect estimates were similar for most predictors. Chlordanes and p,p’-DDE were also strongly positively correlated with one another and have long environmental half-lives in soil (5–15 years) (82). At a constant rate of intake, the concentration of DDT in adipose tissue is known to reach a steady-state value and remain relatively constant (83). Therefore, elevated plasma levels of p,p’-DDE and chlordane isomers may reflect both early-life exposures (e.g., having been breastfed in infancy) as well as prolonged exposure to residues in agricultural and food products (e.g., tobacco, alcohol, and water).
Our study has some limitations. First, the study analysis was cross-sectional in that predictors and plasma concentrations were assessed at baseline only. Reporting of some predictors, such as dietary intake in the last year, was retrospective. Dietary measurement error can lead to underestimated effect estimates and reduced precision (93). However, we do not believe that reporting of most correlates would be differential with respect to OCP plasma concentrations as women are not usually aware of their exposure. Second, we did not have information on water sources, making conclusions with water consumption tenuous and requiring additional research. Lastly, findings may not be generalizable to all U.S. women, which is especially the case for chlordane-related pesticides, where the historic sources of exposure in Michigan differed from those of other states (79, 80). Our study has a number of strengths. We collected data on a wide range of potential predictors and confounders, including sociodemographic, educational, behavioral, dietary, early-life, occupational, and medical history factors. These factors were mutually controlled for in all analyses. Furthermore, we used state-of-the-art detection methods for measuring OCP concentrations in plasma which are positively correlated with adipose concentrations (15, 16). Lastly, SELF fills an important gap in the environmental health literature by focusing on an understudied population.
5. Conclusions
Older age, heavy alcohol use, current cigarette smoking, having been breastfed ≥3 months in infancy, and greater water intake were associated with higher plasma OCP concentrations in our study. Our findings suggest that exposure to OCPs earlier in life may directly or indirectly contribute to current blood concentrations, and that agricultural and water sources may be sources of pesticide exposure in U.S. Black women of reproductive age.
Supplementary Material
Highlights.
Older age was associated with higher plasma pesticide concentrations
Contamination of tobacco, alcohol and water may be important sources of pesticides
Diet was not associated with plasma pesticide concentrations
6. Acknowledgments:
This research was funded primarily by the extramural program of the National Institute of Environmental Health Services (R01-ES024749). The funding sources had no role in the study design, collection, analysis, and interpretation of the data; writing of the report; or in the decision to submit the article for publication. The research was supported in part by the Intramural Research Program of the National Institute of the Environmental Health Sciences and in part by funds allocated for health research by the American Recovery and Reinvestment Act.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Declaration of Interests: None
References
- 1.Saeedi Saravi SS, Dehpour AR. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: A review. Life Sci. 2016;145:255–64. [DOI] [PubMed] [Google Scholar]
- 2.Dorgan JF, Brock JW, Rothman N, Needham LL, Miller R, Stephenson HE Jr., et al. Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes Control. 1999;10(1):1–11. [DOI] [PubMed] [Google Scholar]
- 3.Snedeker SM. Pesticides and breast cancer risk: a review of DDT, DDE, and dieldrin. Environmental health perspectives. 2001;109Suppl 1:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mustieles V, Fernandez MF, Martin-Olmedo P, Gonzalez-Alzaga B, Fontalba-Navas A, Hauser R, et al. Human adipose tissue levels of persistent organic pollutants and metabolic syndrome components: Combining a cross-sectional with a 10-year longitudinal study using a multi-pollutant approach. Environment international. 2017;104:48–57. [DOI] [PubMed] [Google Scholar]
- 5.Karami-Mohajeri S, Abdollahi M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a systematic review. Hum Exp Toxicol. 2011;30(9): 1119–40. [DOI] [PubMed] [Google Scholar]
- 6.Tiemann U In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: a review. Reprod Toxicol. 2008;25(3):316–26. [DOI] [PubMed] [Google Scholar]
- 7.Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev Environ Contam Toxicol. 1991;120:1–82. [DOI] [PubMed] [Google Scholar]
- 8.Nakata H, Kawazoe M, Arizono K, Abe S, Kitano T, Shimada H, et al. Organochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissues from china: status of contamination, historical trend, and human dietary exposure. Arch Environ Contam Toxicol. 2002;43(4):473–80. [DOI] [PubMed] [Google Scholar]
- 9.Stellman SD, Djordjevic MV, Muscat JE, Gong L, Bernstein D, Citron ML, et al. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York. Cancer Epidemiol Biomarkers Prev. 1998;7(6):489–96. [PubMed] [Google Scholar]
- 10.Archibeque-Engle SL, Tessari JD, Winn DT, Keefe TJ, Nett TM, Zheng T. Comparison of organochlorine pesticide and polychlorinated biphenyl residues in human breast adipose tissue and serum. J Toxicol Environ Health. 1997;52(4):285–93. [DOI] [PubMed] [Google Scholar]
- 11.Trabert B, Chen Z, Kannan K, Peterson CM, Pollack AZ, Sun L, et al. Persistent organic pollutants (POPs) and fibroids: results from the ENDO study. J Expo Sci Environ Epidemiol. 2015;25(3):278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arrebola JP, Cuellar M, Claure E, Quevedo M, Antelo SR, Mutch E, et al. Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and adipose tissue from Bolivia. Environmental research. 2012;112:40–7. [DOI] [PubMed] [Google Scholar]
- 13.Arrebola JP, Martin-Olmedo P, Fernandez MF, Sanchez-Cantalejo E, Jimenez-Rios JA, Torne P, et al. Predictors of concentrations of hexachlorobenzene in human adipose tissue: a multivariate analysis by gender in Southern Spain. Environment international. 2009;35(1):27–32. [DOI] [PubMed] [Google Scholar]
- 14.CDC US. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2018March2018. [Google Scholar]
- 15.Artacho-Cordon F, Fernandez-Rodriguez M, Garde C, Salamanca E, Iribarne-Duran LM, Torne P, et al. Serum and adipose tissue as matrices for assessment of exposure to persistent organic pollutants in breast cancer patients. Environmental research. 2015;142:633–43. [DOI] [PubMed] [Google Scholar]
- 16.Hagmar L, Wallin E, Vessby B, Jonsson BA, Bergman A, Rylander L. Intra-individual variations and time trends 1991–2001 in human serum levels of PCB, DDE and hexachlorobenzene. Chemosphere. 2006;64(9):1507–13. [DOI] [PubMed] [Google Scholar]
- 17.Ploteau S, Antignac JP, Volteau C, Marchand P, Venisseau A, Vacher V, et al. Distribution of persistent organic pollutants in serum, omental, and parietal adipose tissue of French women with deep infiltrating endometriosis and circulating versus stored ratio as new marker of exposure. Environment international. 2016;97:125–36. [DOI] [PubMed] [Google Scholar]
- 18.Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, Lickley LA, et al. Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9(1):55–63. [PubMed] [Google Scholar]
- 19.Whitcomb BW, Schisterman EF, Buck GM, Weiner JM, Greizerstein H, Kostyniak PJ. Relative concentrations of organochlorines in adipose tissue and serum among reproductive age women. Environ Toxicol Pharmacol. 2005;19(2):203–13. [DOI] [PubMed] [Google Scholar]
- 20.Agency USEP. Persistent Organic Pollutants: A Global Issue, A Global Response 2002[updated December 2009. Available from: https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response.
- 21.Porter RD, Wiemeyer SN. Dieldrin and DDT: effects on sparrow hawk eggshells and reproduction. Science. 1969;165(3889):199–200. [DOI] [PubMed] [Google Scholar]
- 22.James RA, Hertz-Picciotto I, Willman E, Keller JA, Charles MJ. Determinants of serum polychlorinated biphenyls and organochlorine pesticides measured in women from the child health and development study cohort, 1963–1967. Environmental health perspectives. 2002;110(7):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J, Orentreich N. Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. J Natl Cancer Inst. 1994;86(8):589–99. [DOI] [PubMed] [Google Scholar]
- 24.Woodruff T, Wolff MS, Davis DL, Hayward D. Organochlorine exposure estimation in the study of cancer etiology. Environmental research. 1994;65(1):132–44. [DOI] [PubMed] [Google Scholar]
- 25.Li YF, Macdonald RW. Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: a review. The Science of the total environment. 2005;342(1–3):87–106. [DOI] [PubMed] [Google Scholar]
- 26.Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, et al. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environmental health perspectives. 2010;118(6):796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cromartie E, Reichel WL, Locke LN, Belisle AA, Kaiser TE, Lamont TG, et al. Residues of organochlorine pesticides and polychlorinated biphenyls and autopsy data for bald eagles, 1971–72. Pestic Monit J. 1975;9(1):11–4. [PubMed] [Google Scholar]
- 28.Harner T, Wideman JL, Jantunen LM, Bidleman TF, Parkhurst WJ. Residues of organochlorine pesticides in Alabama soils. Environ Pollut. 1999;106(3):323–32. [DOI] [PubMed] [Google Scholar]
- 29.Li XH, Ma LL, Liu XF, Fu S, Cheng HX, Xu XB. Distribution of organochlorine pesticides in urban soil from Beijing, People’s Republic of China. Bull Environ Contam Toxicol. 2005;74(5):938–45. [DOI] [PubMed] [Google Scholar]
- 30.Shen L, Wania F, Lei YD, Teixeira C, Muir DC, Bidleman TF. Atmospheric distribution and long-range transport behavior of organochlorine pesticides in North America. Environmental science & technology. 2005;39(2):409–20. [DOI] [PubMed] [Google Scholar]
- 31.Artacho-Cordon F, Belhassen H, Arrebola JP, Ghali R, Amira D, Jimenez-Diaz I, et al. Serum levels of persistent organic pollutants and predictors of exposure in Tunisian women. The Science of the total environment. 2015;511:530–4. [DOI] [PubMed] [Google Scholar]
- 32.Fruge AD, Cases MG, Schildkraut JM, Demark-Wahnefried W. Associations between Obesity, Body Fat Distribution, Weight Loss and Weight Cycling on Serum Pesticide Concentrations. J Food Nutr Disord. 2016;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schade G, Heinzow B. Organochlorine pesticides and polychlorinated biphenyls in human milk of mothers living in northern Germany: current extent of contamination, time trend from 1986 to 1997 and factors that influence the levels of contamination. The Science of the total environment. 1998;215(1–2):31–9. [DOI] [PubMed] [Google Scholar]
- 34.Waliszewski SM, Aguirre AA, Infanzon RM, Silva CS, Siliceo J. Organochlorine pesticide levels in maternal adipose tissue, maternal blood serum, umbilical blood serum, and milk from inhabitants of Veracruz, Mexico. Arch Environ Contam Toxicol. 2001;40(3):432–8. [DOI] [PubMed] [Google Scholar]
- 35.Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, et al. Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. The Science of the total environment. 2006;372(1):20–31. [DOI] [PubMed] [Google Scholar]
- 36.Brevik EM. Gas chromatograhic method for the determination of organochlorine pesticides in human milk. Bull Environ Contam Toxicol. 1978;19(3):281–6. [DOI] [PubMed] [Google Scholar]
- 37.Skaare JU, Tuveng JM, Sande HA. Organochlorine pesticides and polychlorinated biphenyls in maternal adipose tissue, blood, milk, and cord blood from mothers and their infants living in Norway. Arch Environ Contam Toxicol. 1988;17(1):55–63. [DOI] [PubMed] [Google Scholar]
- 38.Newsome WH, Davies D, Doucet J. PCB and organochlorine pesticides in Canadian human milk−−1992. Chemosphere. 1995;30(11):2143–53. [DOI] [PubMed] [Google Scholar]
- 39.Ibarluzea J, Alvarez-Pedrerol M, Guxens M, Marina LS, Basterrechea M, Lertxundi A, et al. Sociodemographic, reproductive and dietary predictors of organochlorine compounds levels in pregnant women in Spain. Chemosphere. 2011;82(1):114–20. [DOI] [PubMed] [Google Scholar]
- 40.Bradman AS, Schwartz JM, Fenster L, Barr DB, Holland NT, Eskenazi B. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Expo Sci Environ Epidemiol. 2007;17(4):388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaclavik E, Tjonneland A, Stripp C, Overvad K, Weber JP, Raaschou-Nielsen O. Organochlorines in Danish women: Predictors of adipose tissue concentrations. Environmental research. 2006;100(3):362–70. [DOI] [PubMed] [Google Scholar]
- 42.Caspersen IH, Kvalem HE, Haugen M, Brantsaeter AL, Meltzer HM, Alexander J, et al. Determinants of plasma PCB, brominated flame retardants, and organochlorine pesticides in pregnant women and 3 year old children in The Norwegian Mother and Child Cohort Study. Environmental research. 2016;146:136–44. [DOI] [PubMed] [Google Scholar]
- 43.Sarcinelli PN, Pereira AC, Mesquita SA, Oliveira-Silva JJ, Meyer A, Menezes MA, et al. Dietary and reproductive determinants of plasma organochlorine levels in pregnant women in Rio de Janeiro. Environmental research. 2003;91(3):143–50. [DOI] [PubMed] [Google Scholar]
- 44.Moysich KB, Ambrosone CB, Mendola P, Kostyniak PJ, Greizerstein HB, Vena JE, et al. Exposures associated with serum organochlorine levels among postmenopausal women from western New York State. Am J Ind Med. 2002;41(2):102–10. [DOI] [PubMed] [Google Scholar]
- 45.Brauner EV, Raaschou-Nielsen O, Gaudreau E, LeBlanc A, Tjonneland A, Overvad K, et al. Predictors of polychlorinated biphenyl concentrations in adipose tissue in a general Danish population. Environmental science & technology. 2011;45(2):679–85. [DOI] [PubMed] [Google Scholar]
- 46.Cao LL, Yan CH, Yu XD, Tian Y, Zhao L, Liu JX, et al. Relationship between serum concentrations of polychlorinated biphenyls and organochlorine pesticides and dietary habits of pregnant women in Shanghai. Science of the Total Environment. 2011;409(16):2997–3002. [DOI] [PubMed] [Google Scholar]
- 47.Glynn AW, Granath F, Aune M, Atuma S, Darnerud PO, Bjerselius R, et al. Organochlorines in Swedish women: determinants of serum concentrations. Environmental health perspectives. 2003;111(3):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu GW, Laseter J, Mylander C. Persistent organic pollutants in serum and several different fat compartments in humans. J Environ Public Health. 2011;2011:417980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laden F, Neas LM, Spiegelman D, Hankinson SE, Willett WC, Ireland K, et al. Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Environmental health perspectives. 1999;107(1):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolff MS, Deych E, Ojo F, Berkowitz GS. Predictors of organochlorines in New York City pregnant women, 1998–2001. Environmental research. 2005;97(2):170–7. [DOI] [PubMed] [Google Scholar]
- 51.Lee SA, Dai Q, Zheng W, Gao YT, Blair A, Tessari JD, et al. Association of serum concentration of organochlorine pesticides with dietary intake and other lifestyle factors among urban Chinese women. Environment international. 2007;33(2):157–63. [DOI] [PubMed] [Google Scholar]
- 52.DeVoto E, Kohlmeier L, Heeschen W. Some dietary predictors of plasma organochlorine concentrations in an elderly German population. Archives of environmental health. 1998;53(2):147–55. [DOI] [PubMed] [Google Scholar]
- 53.Rylander C, Lund E, Froyland L, Sandanger TM. Predictors of PCP, OH-PCBs, PCBs and chlorinated pesticides in a general female Norwegian population. Environment international. 2012;43:13–20. [DOI] [PubMed] [Google Scholar]
- 54.Asplund L, Svensson BG, Nilsson A, Eriksson U, Jansson B, Jensen S, et al. Polychlorinated biphenyls, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane (p,p’-DDT) and 1,1-dichloro-2,2-bis(p-chlorophenyl)-ethylene (p,p’-DDE) in human plasma related to fish consumption. Archives of environmental health. 1994;49(6):477–86. [DOI] [PubMed] [Google Scholar]
- 55.McGraw JE, Waller DP. Fish ingestion and congener specific polychlorinated biphenyl and p,p’-dichlorodiphenyldichloroethylene serum concentrations in a great lakes cohort of pregnant African American women. Environment international. 2009;35(3):557–65. [DOI] [PubMed] [Google Scholar]
- 56.Hanrahan LP, Falk C, Anderson HA, Draheim L, Kanarek MS, Olson J. Serum PCB and DDE levels of frequent Great Lakes sport fish consumers-a first look. The Great Lakes Consortium. Environmental research. 1999;80(2 Pt 2):S26–S37. [DOI] [PubMed] [Google Scholar]
- 57.McDaniel PA, Solomon G, Malone RE. The tobacco industry and pesticide regulations: case studies from tobacco industry archives. Environmental health perspectives. 2005;113(12):1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pose-Juan E, Sanchez-Martin MJ, Andrades MS, Rodriguez-Cruz MS, Herrero-Hernandez E. Pesticide residues in vineyard soils from Spain: Spatial and temporal distributions. The Science of the total environment. 2015;514:351–8. [DOI] [PubMed] [Google Scholar]
- 59.Vitali Cepo D, Pelajic M, Vinkovic Vrcek I, Krivohlavek A, Zuntar I, Karoglan M. Differences in the levels of pesticides, metals, sulphites and ochratoxin A between organically and conventionally produced wines. Food Chem. 2018;246:394–403. [DOI] [PubMed] [Google Scholar]
- 60.Hengel MJ, Shibamoto T. Method development and fate determination of pesticide-treated hops and their subsequent usage in the production of beer. J Agric Food Chem. 2002;50(12):3412–8. [DOI] [PubMed] [Google Scholar]
- 61.Inoue T, Nagatomi Y, Suga K, Uyama A, Mochizuki N. Fate of pesticides during beer brewing. J Agric Food Chem. 2011;59(8):3857–68. [DOI] [PubMed] [Google Scholar]
- 62.Quadroni S, Bettinetti R. An unnoticed issue: Organochlorine pesticides in tobacco products around the world. Chemosphere. 2019;219:54–7. [DOI] [PubMed] [Google Scholar]
- 63.James-Todd TM, Chiu YH, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baird DD, Harmon QE, Upson K, Moore KR, Barker-Cummings C, Baker S, et al. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. J Womens Health (Larchmt). 2015;24(11):907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. American journal of epidemiology. 1986;124(3):453–69. [DOI] [PubMed] [Google Scholar]
- 66.Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE 3rd, Patterson DG Jr. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Analytical chemistry. 2004;76(7):1921–7. [DOI] [PubMed] [Google Scholar]
- 67.Hodges LC, Bergerson JS, Hunter DS, Walker CL. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 2000;54(2):355–64. [DOI] [PubMed] [Google Scholar]
- 68.Hunter DS, Hodges LC, Eagon PK, Vonier PM, Fuchs-Young R, Bergerson JS, et al. Influence of exogenous estrogen receptor ligands on uterine leiomyoma: evidence from an in vitro/in vivo animal model for uterine fibroids. Environmental health perspectives. 2000;108Suppl 5:829–34. [DOI] [PubMed] [Google Scholar]
- 69.Saxena SP, Khare C, Farooq A, Murugesan K, Buckshee K, Chandra J. DDT and its metabolites in leiomyomatous and normal human uterine tissue. Arch Toxicol. 1987;59(6):453–5. [DOI] [PubMed] [Google Scholar]
- 70.Scsukova S, Rollerova E, Bujnakova Mlynarcikova A. Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone-related cancer. Reprod Biol. 2016;16(4):243–54. [DOI] [PubMed] [Google Scholar]
- 71.Qin YY, Leung CKM, Leung AOW, Wu SC, Zheng JS, Wong MH. Persistent organic pollutants and heavy metals in adipose tissues of patients with uterine leiomyomas and the association of these pollutants with seafood diet, BMI, and age. Environ Sci Pollut R. 2010;17(1):229–40. [Google Scholar]
- 72.Health USCE. Laboratory Procedure Manual: Polybrominated diphenyl ethers (PBDEs), Polybrominated Biphenyls (PBBs), Polychlorinated biphenyls, and Persistent Pesticides (PPs). 2016.
- 73.Bernert JT, Turner WE, Patterson DG Jr., Needham LL. Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere. 2007;68(5):824–31. [DOI] [PubMed] [Google Scholar]
- 74.Li R, Scanlon KS, Serdula MK. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev. 2005;63(4):103–10. [DOI] [PubMed] [Google Scholar]
- 75.Troy LM, Michels KB, Hunter DJ, Spiegelman D, Manson JE, Colditz GA, et al. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int J Epidemiol. 1996;25(1):122–7. [DOI] [PubMed] [Google Scholar]
- 76.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 77.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med. 2001;20(9–10):1541–9. [DOI] [PubMed] [Google Scholar]
- 78.Kantor LS. A dietary assessment of the U.S. food supply : comparing per capita food consumption with food guide pyramid serving recommendations. Washington, D.C.: U.S. Dept. of Agriculture, Economic Research Service; 1998. iii, 52 p. p. [Google Scholar]
- 79.Gooch JW, Matsumura F, Zabik MJ. Chlordane Residues in Great-Lakes Lake Trout - Acute Toxicity and Interaction at the Gaba Receptor of Rat and Lake Trout Brain. Chemosphere. 1990;21(3):393–406. [Google Scholar]
- 80.Agency USEP. Results of the Lake Michigan Mass Balance Study: Polychlorinated Biphenyls and trans-Nonachlor Data Report. 2004.
- 81.Namulanda G, Maisonet M, Taylor E, Flanders WD, Olson D, Sjodin A, et al. In utero exposure to organochlorine pesticides and early menarche in the Avon Longitudinal Study of Parents and Children. Environment international. 2016;94:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dearth MA, Hites RA. Chlordane accumulation in people. Environmental science & technology. 1991;25(7):1279–85. [Google Scholar]
- 83.Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Goodman AG. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill; 1996. [Google Scholar]
- 84.Weldon RH, Webster M, Harley KG, Bradman A, Fenster L, Davis MD, et al. Serum persistent organic pollutants and duration of lactation among Mexican-American women. J Environ Public Health. 2010;2010:861757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Achour A, Derouiche A, Barhoumi B, Kort B, Cherif D, Bouabdallah S, et al. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue from northern Tunisia: Current extent of contamination and contributions of socio-demographic characteristics and dietary habits. Environmental research. 2017;156:635–43. [DOI] [PubMed] [Google Scholar]
- 86.Weisskopf MG, Knekt P, O’Reilly EJ, Lyytinen J, Reunanen A, Laden F, et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74(13):1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dane AJ, Havey CD, Voorhees KJ. The detection of nitro pesticides in mainstream and sidestream cigarette smoke using electron monochromator-mass spectrometry. Analytical chemistry. 2006;78(10):3227–33. [DOI] [PubMed] [Google Scholar]
- 88.Mussalo-Rauhamaa H, Leppanen A, Salmela SS, Pyysalo H. Cigarettes as a source of some trace and heavy metals and pesticides in man. Archives of environmental health. 1986;41(1):49–55. [DOI] [PubMed] [Google Scholar]
- 89.Guthrie FE, Bowery TG. Pesticide residues on tobacco. Residue Rev. 1967;19:31–56. [DOI] [PubMed] [Google Scholar]
- 90.Detroit Water & Sewage Department Water Quality Report. 2017.
- 91.Harrington JM, Baker EL Jr., Folland DS, Saucier JW, Sandifer SH. Chlordane contamination of a municipal water system. Environmental research. 1978; 15(1):155–9. [DOI] [PubMed] [Google Scholar]
- 92.Genuis SJ, Lane K, Birkholz D. Human Elimination of Organochlorine Pesticides: Blood, Urine, and Sweat Study. Biomed Res Int. 2016;2016:1624643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 2011;103(14):1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


