Abstract
Purpose
A need exists for reduced toxicity conditioning regimens that offer less toxicity while maintaining myeloablation, especially for primary immune deficiencies where myeloablation or high donor myeloid chimerism is required to achieve cure. We adapted a busulfan and fludarabine regimen by Gungor et al. for children and young adults undergoing allogeneic HCT for non-CGD primary immune deficiencies requiring myeloablation or high donor myeloid chimerism, and herein report our experience.
Methods
We retrospectively reviewed records of 41 consecutive patients who underwent allogeneic HCT for Wiskott-Aldrich syndrome (n = 12), primary HLH/XLP (n = 10), CD40L deficiency (n = 7), or other (n = 12) primary immune deficiencies with a conditioning regimen containing pharmacokinetic-guided busulfan dosing which achieved a cumulative AUC between 57 and 74 mg/L × h (65–80% of conventional myeloablative exposure), along with fludarabine and alemtuzumab or anti-thymocyte globulin at 3 transplant centers between 2014 and 2019.
Results
Forty-one patients underwent a first (n = 33) or second (n = 8) allogeneic HCT. Median age was 2.3 years (range, 0.3 years–19.8 years). All but one patient (97.5%) achieved neutrophil recovery at a median of 14 days (range, 11–34 days). One patient developed sinusoidal obstruction syndrome and two patients developed diffuse alveolar hemorrhage. Four patients developed grades II–IV acute GVHD. Three patients developed chronic GVHD. One-year overall survival was 90% (95% confidence interval [CI] 81–99%) and event-free survival was 83% (95% CI 71–94%).
Conclusions
Our experience suggests that a reduced toxicity busulfan-fludarabine regimen offers low toxicity, low incidence of grades 2–4 GVHD, durable myeloid engraftment, and excellent survival, and may be considered for a variety of primary immune deficiencies where myeloablative HCT is desired.
Keywords: Reduced toxicity regimen, allogeneic HCT, myeloablative HCT, primary immune deficiencies, Wiskott-Aldrich syndrome, CD40 ligand deficiency, primary HLH/XLP
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a treatment option for children and young adults with severe primary immune deficiencies and primary immune regulatory disorders. Conditioning regimens for allogeneic HCT are broadly categorized into myeloablative conditioning (MAC), non-myeloablative conditioning, and reduced intensity conditioning (RIC) regimens [1]. Conventional MAC regimens typically include myeloablative dosing of busulfan along with cyclophosphamide. MAC regimens lead to durable high-level donor chimerism, but are often associated with significant acute toxicities, transplant-related mortality (TRM), and long-term sequelae in primary immune deficiencies [2, 3]. In contrast, RIC regimens incorporating melphalan and fludarabine are associated with improved survival and less toxicity, especially in high-risk disorders such as primary hemophagocytic lymphohistiocytosis (HLH), but a high incidence of mixed chimerism [4–7]. In addition, mixed chimerism following RIC is often associated with lower myeloid chimerism relative to T cell chimerism due to inadequate myeloablation [8]. Although this may be acceptable for T cell deficiencies such as severe combined immune deficiency (SCID) where donor-derived T cells can have a survival advantage, it may not be sufficient to cure primary immune deficiencies with defective hematopoiesis or phagocyte defects where myeloablation or high-level donor myeloid chimerism is desirable. For example, patients with Wiskott-Aldrich syndrome (WAS) with donor myeloid chimerism < 50% are at higher risk of persistent thrombocytopenia [9]. Mixed chimerism in WAS patients is also associated with an increased rate of incomplete immune reconstitution and post-transplant autoimmunity [9]. Additionally, declining donor chimerism following a RIC regimen often necessitates a secondary intervention such as CD34+ stem cell boost, donor lymphocyte infusion, or need for a second HCT [5, 10], and increased graft rejection has been observed with RIC regimens in patients with CD40 ligand deficiency [11]. Collectively, it is clear that there is a need for alternative regimens with less toxicity while maintaining myeloablation especially for disorders where high donor myeloid chimerism is desirable to achieve cure.
Gungor et al. reported excellent outcomes with a novel reduced toxicity regimen for patients with chronic granulomatous disease (CGD) [12, 13]. The regimen included busulfan dosed to achieve a cumulative area under the curve (AUC) of 45–65 mg/L × h, equivalent to 55–75% of a fully myeloablative cumulative AUC. Cyclophosphamide was replaced with fludarabine, a nucleoside analog with potent immunosuppressive properties and serotherapy included alemtuzumab or anti-thymocyte globulin (ATG). The regimen was well tolerated and none of the patients developed sinusoidal obstruction syndrome (SOS), interstitial pneumonitis, or severe mucositis. The 2-year probability of overall survival (OS) was 96% and event-free survival (EFS) 91% (event defined as death, engraftment failure, or rejection). Stable myeloid donor chimerism (> 90%) was achieved in 93% of surviving patients. The incidence of GVHD was low; grades II–IV acute GVHD occurred in 11% of patients and chronic GVHD occurred in 7% of patients. Graft failure was observed in 5% of patients.
We hypothesized that this reduced toxicity regimen would be efficacious in other primary immune deficiencies, especially where myeloablation or high donor myeloid chimerism is desirable, or for patients who failed transplant with reduced intensity or other conditioning. We adapted the regimen as part of clinical protocols to target a narrow but higher busulfan AUC range equivalent to 65–80% of the fully myeloablative cumulative AUC in an effort to reduce the risk of graft rejection. In addition to busulfan, the regimen included fludarabine and alemtuzumab or anti-thymocyte globulin for serotherapy. We report our experience with this approach for patients with non-CGD primary immune deficiencies requiring myeloablation or high donor myeloid chimerism.
Methods
We retrospectively reviewed the records of 41 consecutive patients who underwent allogeneic HCT for primary immune deficiencies with a conditioning regimen containing pharmacokinetic-guided busulfan dosing, fludarabine, and alemtuzumab or rabbit ATG for serotherapy at three transplant centers between 2014 and 2019. Minimum duration of follow-up for inclusion in this study was 1-year post-HCT. Transplant centers included Cincinnati Children’s Hospital (n = 25), Children’s Hospital of Atlanta (n = 14), and Children’s National Hospital (n = 2) D.C. Data collected included patient demographics, diagnosis, donor and recipient HLA match, stem cell source, HCT-related complications including SOS, diffuse alveolar hemorrhage (DAH), thrombotic microangiopathy (TMA), viral infections or viremia, autoimmune complications, need for immunoglobulin replacement, graft versus host disease (GVHD), immune reconstitution, and donor chimerism. Survival data was collected until last follow-up. Alemtuzumab (n = 30) 0.5 mg/kg was given over 3 days from day − 8 to − 6 to patients who received bone marrow grafts, CD34+-selected peripheral blood stem cell (PBSC) grafts, and umbilical cord blood (UCB) grafts, and patients who received unmanipulated PBSC grafts received 1.0 mg/kg over 4 days from day − 9 to − 6. Rabbit ATG (thymoglobulin) 7.5 mg/kg (n = 7) was given over 3 days on days − 5 to − 3. One patient each was given alemtuzumab 0.3 mg/kg over 3 days and 1.0 mg/kg over 5 days respectively, and two patients did not receive any serotherapy per discretion of the treating physician. Fludarabine 180 mg/m2 (or 7.2 mg/kg for patients < 10 kg) was given over 6 days (n = 26) on days − 8 to − 3 or 160 mg/m2 over 4 days on days − 6 to − 3 (n = 14). One patient received 150 mg/m2 over 5 days per physician discretion. Busulfan was given over 4 days on days − 5 to − 2 either once daily (n = 15), or twice daily (n = 25) or four times daily (n = 1) to achieve a target AUC of 3500–4500 μM/min over 24 h and cumulative AUC between 57 and 74 mg/L × h (65–80% of conventional myeloablative AUC of 90 mg/L × h). Total busulfan dose was determined based on test dose busulfan (0.8 mg/kg or 1 mg/kg for > 10 kg and < 4 years) pharmacokinetic testing done prior to start of conditioning regimen or pharmacokinetic testing performed with the first dose of busulfan.
HLA typing was performed by high resolution DNA typing for HLA-A, -B, -C, DR, and DQ alleles. Patients received a graft from an HLA 10/10 matched related (n = 11) or unrelated donor (n = 23) or 1–2 allele mismatched related (n = 1) or unrelated (n = 5) donor (HLA 8/10 or 9/10). One patient received an HLA 5/10 mismatched graft from a related donor. Patients received a T-replete bone marrow (n = 36) or PBSC (N = 2) graft or CD34+-selected PBSC graft (n = 2). One patient received an umbilical cord blood graft. In patients who received a CD34+-selected graft, granulocyte colony–stimulating factor (G-CSF) mobilized PBSCs were collected from the donor and selection of CD34+ stem cells was performed using magnetic bead isolation technology (CliniMACs; Miltenyi). CD34+ cell dose was limited by the CD3+ T cell count which did not exceed 0.5 × 105 cells/kg recipient weight. GVHD prophylaxis consisted of cyclosporine and mycophenolate mofetil (n = 32), with added maraviroc (n = 4) or abatacept (n = 2), per physician discretion. One patient received prednisolone and tacrolimus for GVHD prophylaxis. Two patients did not receive any GVHD prophylaxis as they received a CD34+-selected PBSC graft. Cyclosporine was initiated on day − 3, whereas mycophenolate mofetil (MMF) was initiated on day 0 and continued until day + 21, after which it was tapered off over 4 weeks if no evidence of acute GVHD was observed. Subsequently, cyclosporine was tapered off by 6 months post-HCT or per physician discretion. In patients who received maraviroc, the drug was started on day − 3 and stopped on day + 30. In patients who received abatacept, the drug was administered intravenously on days − 1, + 5, + 14, and + 28.
Patients received acyclovir for cytomegalovirus (CMV) prophylaxis if recipient or donor were seropositive for CMV, or for herpes simplex virus (HSV) prophylaxis if recipient were seropositive for HSV. Voriconazole or micafungin were used for antifungal prophylaxis. All patients received pentamidine prophylaxis for Pneumocystis jiroveci, which was changed to cotrimoxazole after neutrophil recovery. All patients received intravenous immunoglobulin replacement. G-CSF was administered (n = 27) starting on day + 7 until neutrophil recovery. Fourteen patients did not receive GCSF per institutional preference. Acute GVHD was graded by the treating physician using the modified Glucksberg criteria [13] and chronic GVHD was graded using the 2014 National Institutes of Health chronic GVHD response criteria working group report [14]. SOS was defined as presence of abdominal pain or hepatomegaly, weight gain, and bilirubin ≥ 2.0 mg/dl with ultrasound evidence of reversal of portal venous flow. DAH was defined as acute onset of hypoxemia with presence of diffuse pulmonary infiltrates on a chest X-ray or computed tomography scan and the presence of progressively bloodier return on bronchoalveolar lavage. Serial peripheral blood monitoring of CMV, Epstein-Barr virus (EBV), BK virus, and adenovirus PCRs was performed until 6 months after HCT and subsequently per physician discretion. Neutrophil recovery was considered to be the first day of 3 consecutive days when the neutrophil count was > 0.5 × 106/L. Platelet recovery was considered to be the first day of 3 days when the platelet count was > 20 × 106/L without platelet transfusion. Primary graft failure was defined as failure to achieve neutrophil recovery and/or < 5% donor whole blood chimerism within 35 days of HCT. Secondary graft failure was defined as decline in whole blood donor chimerism to < 5% following initial neutrophil recovery or loss of a previously functioning graft by development of at least two lines of cytopenia (neutrophil count < 0.5 × 106/L, platelet count < 20 × 106/L, Hb < 7.0 g/dl), despite donor chimerism > 5%.
Serial monitoring of whole blood donor chimerism was performed from the time of neutrophil recovery. Chimerism studies were done using either XY fluorescence in situ hybridization (FISH), in the case of sex-mismatched donor, or short-term tandem repeat (STR) analysis in the case of same sex donor. Mixed chimerism was defined as whole blood donor chimerism of < 95% and onset of mixed chimerism was defined as day post-HCT when mixed chimerism was noted. Chimerism studies on magnetic bead–sorted myeloid cells (CD15 or CD33) and T cells (CD3) were performed when indicated for mixed chimerism at the discretion of the attending physician. In 14 patients, chimerism studies on myeloid cells and T cells were performed from the time of engraftment per institutional preference. In these patients, mixed chimerism was defined as myeloid or T cell subset chimerism of < 95%. All chimerism studies were performed at the clinical genetics laboratory of the treating institution.
OS and EFS probabilities were evaluated with Kaplan-Meier estimates and compared with the log-rank test. For EFS, an event was defined as death or primary or secondary graft failure. Statistical significance was considered for p < 0.05.
Results
Forty-one patients received busulfan and fludarabine with alemtuzumab or ATG for allogeneic HCT for a variety of primary immune deficiencies (first HCT in 33 patients and second HCT in 8 patients). Underlying diagnosis was genetically confirmed in all but one patient. The patient without a genetic diagnosis had early-onset inflammatory bowel disease (EOIBD). Patient characteristics are shown in Table 1. The median age was 2.3 years (range, 0.3–19.8 years). All patients receiving a second HCT had an underlying diagnosis of primary HLH/XLP and had previously experienced primary or secondary graft failure or a relapse of primary HLH following either RIC HCT with alemtuzumab, fludarabine, and melphalan (n = 6) or MAC HCT with busulfan and cyclophosphamide (n = 2). Median time to second HCT after first HCT was 22 months (range, 3 months–19 years) in the 8 patients who underwent second HCT; 6 patients underwent second HCT > 1 year following first HCT and 2 patients underwent second HCT at 3 months and 4 months after first HCT. None of the patients underwent second HCT in aplasia. All patients had evidence of adequate hematopoiesis at the time of second HCT as reflected by their normal blood cell counts, including normal hemoglobin, platelets, and absolute neutrophil count. All patients had predominantly recipient chimerism. Whole blood donor chimerism was ≤ 13% in 7 patients. In the remaining patient, whole blood donor chimerism was not available but donor myeloid chimerism was 0% and donor T cell chimerism was 21%. Genetic etiology in patients with primary HLH/XLP included pathogenic variant(s) in UNC13D (n = 3), RAB27A (n = 2), STXBP2 (n = 2), and SH2D1A (n =3). Individual transplant characteristics and outcomes post-transplant are shown in supplementary table 1.
Table 1.
Patient and transplant characteristics
| Number of patients | 41 |
| Median age (years) | 2.3 (0.3–19.8) |
| Male | 37 |
| Female | 4 |
| Diagnosis | |
| Wiskott-Aldrich syndrome | 12 |
| HLH/XLP | 10 |
| CD40 ligand deficiency | 7 |
| IPEX | 2 |
| Combined immune deficiency | 2 |
| IL-10Rb deficiency | 1 |
| Early-onset IBD | 1 |
| Lnterferon-γ receptor type 1 deficiency | 1 |
| Severe congenital neutropenia | 1 |
| X-linked SCID | 1 |
| Leukocyte adhesion deficiency type 3 | 1 |
| X-linked moesin-associated immunodeficiency | 1 |
| CTLA-4 haploinsufficiency | 1 |
| Serotherapy | |
| Alemtuzumab | 32 |
| Anti-thymocyte globulin | 7 |
| None | 2 |
| Patient/donor HLA match and relation | |
| Matched related donor | 11 |
| Matched unrelated donor | 23 |
| 1–2 allele mismatched unrelated donor | 5 |
| 2–5 allele mismatched related donor | 2 |
| Graft source | |
| Bone marrow | 36 |
| Peripheral blood stem cells | 4 |
| Cord blood | 1 |
| Graft cell dose | |
| Total nucleated cells/kg × 108 (range) | 7.7 (0.8–20.0) |
| CD34+ stem cells/kg × 106 (range) | 9.6 (1.6–100.4) |
IBD, inflammatory bowel disease; IPEX, immune dysregulation, polyendocrinopathy, enteropathy, X-linked; SCID, severe combined immune deficiency; primary HLH, hemophagocytic lymphohistiocytosis; XLP, X-linked lymphoproliferative disorder
All patients (97.5%) except one experienced neutrophil recovery at a median of 14 days post-HCT (range, 11–34 days). Platelet recovery occurred at a median of 21 days (range, 13–82 days). Post-HCT complications are shown in Table 2. One patient developed SOS that resolved with steroids and defibrotide and two patients developed DAH that required ventilatory support. All 3 patients had an underlying diagnosis of primary HLH.
Table 2.
Post-transplant complications
| Complication | Number (%) |
|---|---|
|
| |
| Toxicity | |
| Sinusoidal obstructive syndrome | 1 (2%) |
| Diffuse alveolar hemorrhage | 2 (5%) |
| Thrombotic microangiopathy | 2 (5%) |
| Graft versus host disease | |
| Grades II–IV acute GVHD | 4 (10%) |
| Grades III–IV acute GVHD | 1 (2%) |
| Chronic GVHD | 3 (7%) |
| Viral infections or viremias | |
| Cytomegalovirus | 11 (27%) |
| Epstein-Barr virus | 4 (10%) |
| Adenovirus | 3 (7%) |
| BK virus | 1 (2%) |
| Norovirus | 3 (7%) |
| Autoimmune complications | |
| Immune thrombocytopenia | 2 (5%) |
| Autoimmune hemolytic anemia | 3 (7%) |
| Guillain-Barre syndrome | 2 (5%) |
| Graft failure | |
| Primary graft failure | 1 (2%) |
| Secondary graft failure | 2 (5%) |
| Mixed chimerism | |
| Mixed chimerism | 24 (59%) |
| Donor myeloid chimerism < 90%* | 3 (9%) |
| Donor T cell chimerism < 75%* | 7 (21%) |
At 1-year post-transplant in patients with event-free survival (n = 33)
Four patients (10%) developed grades II–IV GVHD. Only one patient (2%) developed grades III–IV GVHD. Three patients (7%) developed chronic GVHD; one patient developed mild, one moderate, and one severe chronic GVHD, manifesting as skin GVHD, myositis, and bronchiolitis obliterans respectively. CMV infection or viremia occurred in 11 patients (27%), EBV infection or viremia in 4 patients (10%), adenovirus infection or viremia in 3 patients (7%), norovirus infection in 3 patients (7%), and one patient (2%) developed BK virus cystitis. None of the patients developed EBV driven post-transplant lymphoproliferative disorder (PTLD). Autoimmune complications occurred in 5 patients (WAS = 2, IPEX = 1, CD40L deficiency = 1, severe congenital neutropenia = 1); all except one patient had full donor chimerism. Immune thrombocytopenia (ITP) occurred in 2 patients (5%), autoimmune hemolytic anemia (AIHA) in 3 patients (7%), and Guillain-Barre syndrome (GBS) in 2 patients (5%). Both patients with WAS who developed autoimmune complications had full donor chimerism; both developed GBS and autoimmune cytopenia (one patient had ITP and the other AIHA). Six patients (15%) received rituximab for autoimmune complications or EBV viremia/infection.
Thirteen patients (32%) maintained complete donor chimerism through 1-year post-HCT and remain free of their underlying disease at last follow-up. In these patients, median donor chimerism was 100% (range, 97–100%) at time of engraftment, 100% (range, 96–100%) at 100 days, 100% (range, 95–100) at 6 months, and 100% (range, 95–100%) at 12 months post-HCT.
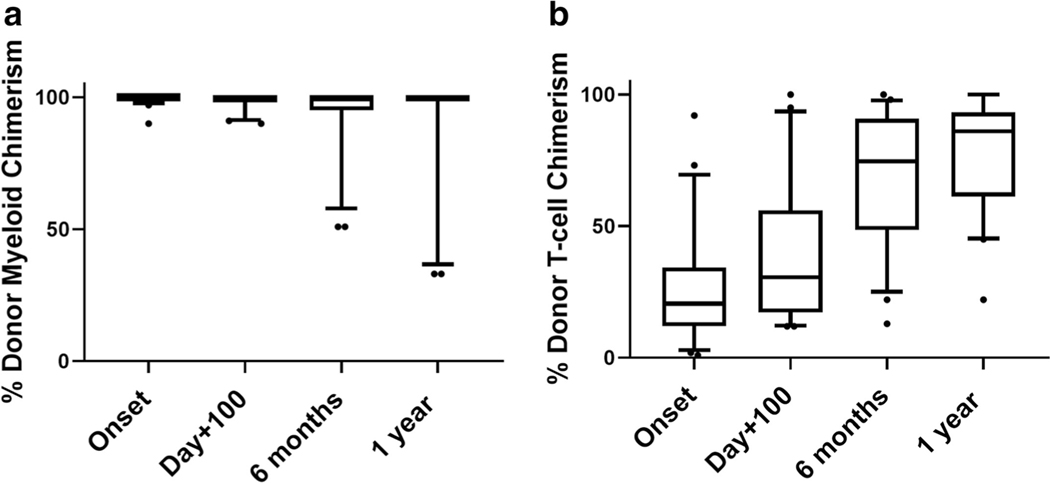
Twenty-four patients (59%) developed mixed chimerism post-HCT at a median of 22 days post-HCT (range, 12–118 days) but 19/23 (83%) maintained donor myeloid chimerism > 90% through 1-year post-HCT. In one patient with mixed chimerism, donor myeloid or T cell chimerism was not performed. Whole blood donor chimerism for this patient was 96%, 89% and 95% at 100 days, 6 months, and 1 year post-HCT, respectively. In the remaining 23 patients with mixed chimerism, median donor myeloid chimerism was 100% (range, 90–100%) at the onset of mixed chimerism, 100% (range 90–100%) at 100 days, 100% (range, 51–100%) at 6 months, and 100% (range, 33–100%) at 12 months post-HCT as shown in Fig. 1 a. Unlike the predominantly high donor myeloid chimerism, donor T cell chimerism was generally low at the onset of mixed chimerism and progressively increased with time. Median donor T cell chimerism was 21% (range, 1–92%) at the onset of mixed chimerism, 31% (range 12–100%) at 100 days, 69% (range, 13–100%) at 6 months, and 80% (range, 22–100%) at 12 months post-HCT as shown in Fig. 1 b. One patient (with IL10Rb deficiency) received a CD34+-selected stem cell boost at 6 months post-HCT for donor myeloid chimerism of 50% without any response, and donor myeloid chimerism decreased to 33% at 1-year post-HCT. No other patients received any hematopoietic cell product interventions. Twenty-two of 24 patients (92%) with mixed chimerism remain free of their underlying disease at last follow-up.
Fig. 1.
Donor myeloid and T cell chimerism in patients with mixed chimerism. a Donor myeloid chimerism (%) in patients with mixed chimerism (n = 23) at onset of mixed chimerism (range, 12–73 days), day + 100, 6 months, and 1-year post-transplant. Data represented as Tukey box-and-whisker plots. Each box is defined by the 25th and 75th percentiles of the data and represents the inter-quartile range (IQR). The median is represented by a solid horizontal line. Whiskers are extended from the box to the last value at < 1.5 IQR, towards the maximum and the minimum and outliers represented as round dots. b Donor T cell chimerism (%) in patients with mixed chimerism (n = 23) at onset of mixed chimerism (range, 12–73 days), day + 100, 6 months, and 1-year post-transplant. Data represented as Tukey box-and-whisker plots. Each box is defined by the 25th and 75th percentiles of the data and represents the inter-quartile range (IQR). The median is represented by a solid horizontal line. Whiskers are extended from the box to the last value at < 1.5 IQR, towards the maximum and the minimum and outliers represented as round dots
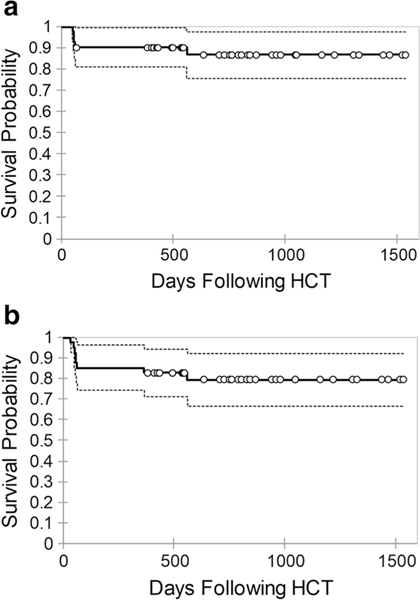
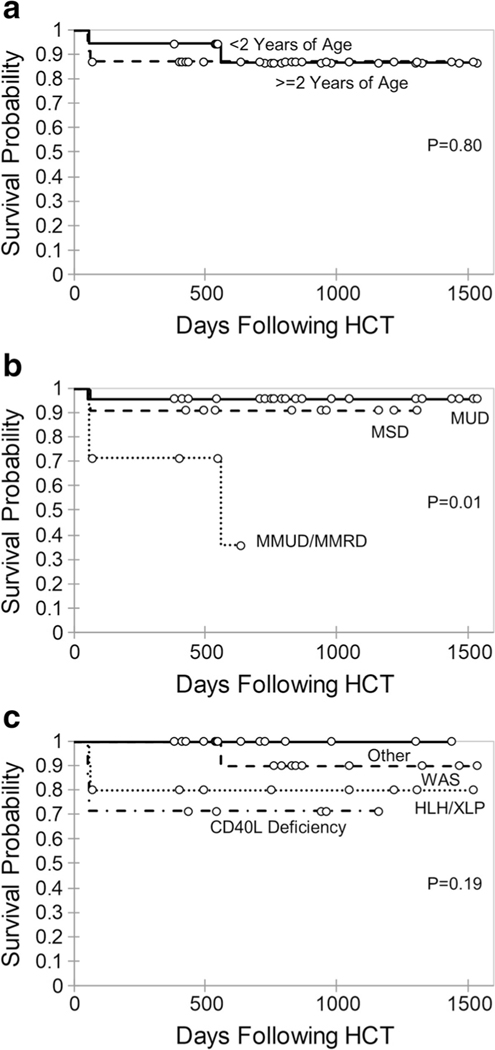
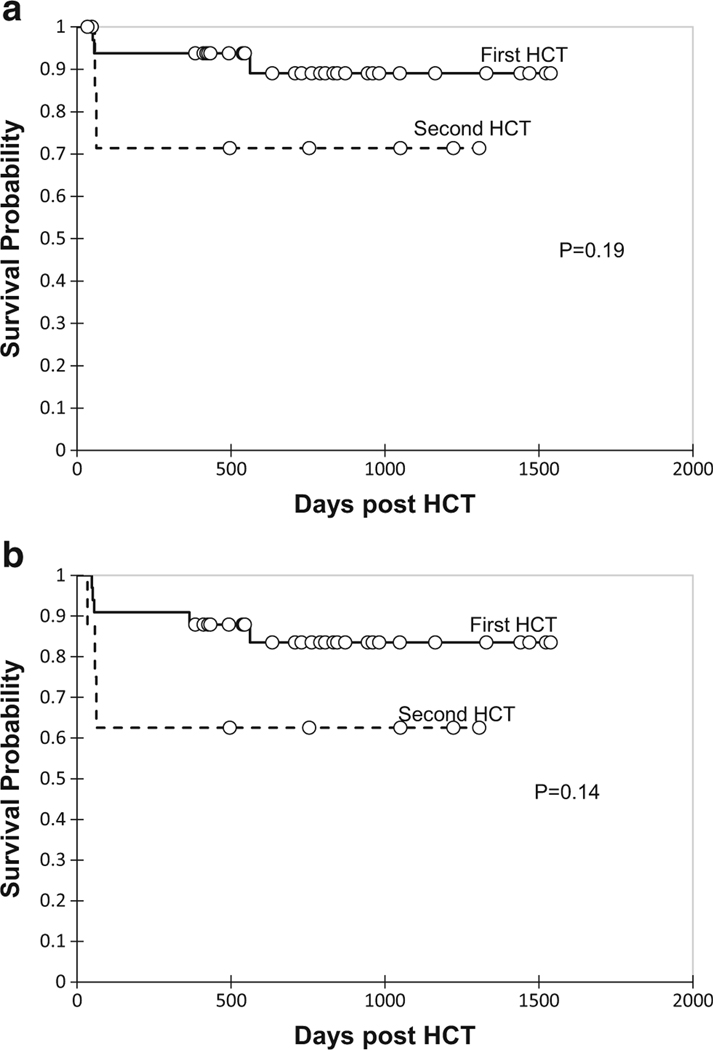
One-year OS was 90% (95% confidence interval [CI] 81–99%) and EFS was 83% (95% CI 71–94%). Survival probabilities are shown in Fig. 2. One patient developed primary graft failure and two patients developed secondary graft failure. Five patients died in our cohort at a median of 58 days (range, 51–562 days). Cause of death included diffuse alveolar hemorrhage (n = 2), grade III acute GVHD and bronchiolitis obliterans (n = 1), idiopathic pneumonia syndrome (n = 1), and progression of pre-existing CNS neurodegeneration complicated by adenovirus encephalitis (n = 1). Survival outcomes were compared in children < 2 years and > 2 years as the median age of the cohort was 2.3 years. Probability of OS did not differ by age (< 2 years vs. > 2 years, P = 0.80) as shown in Fig. 3 a. However, patients undergoing HCT with a mismatched related or unrelated donor graft had inferior outcomes compared to matched sibling or unrelated donor grafts (P = 0.01) as shown in Fig. 3 b. Survival outcomes were compared between patients with WAS, primary HLH, CD40 ligand deficiency, and other primary immune deficiencies. There was no significant difference in survival based on underlying diagnosis (P = 0.19) as shown in Fig. 3 c. Probability of OS and EFS in patients who underwent second HCT was not significantly different compared to patients who underwent first HCT (Fig. 4).
Fig. 2.
Probability of overall survival (a) and event-free survival (b) for all patients
Fig. 3.
Probability of overall survival based on age (a), donor and recipient HLA match (b), and underlying diagnosis (c). MSD, matched sibling donor; MRD, matched related donor; MMRD, mismatched related donor; MMUD, mismatched unrelated donor; primary HLH, hemophagocytic lymphohistiocytosis; XLP, X-linked lymphoproliferative disorder; WAS, Wiskott-Aldrich syndrome
Fig. 4.
Probability of overall survival (a) and event-free survival (b) for patients undergoing first versus second HCT
Overall, among 33 survivors without graft failure, 28 patients (85%) have discontinued immunoglobulin replacement. Three of the patients on immunoglobulin replacement had received rituximab following HCT. All except three patients are off immune suppression at last follow-up. Details of immune reconstitution in survivors without graft failure at last follow-up are shown in Table 3. All except three patients achieved normal mitogen responses as determined by PHA (PHA data were not available for three patients including two patients who also lacked lymphocyte subset data). CD4 naïve counts were not available for three additional patients, who had normal lymphocyte subset counts and normal mitogen (PHA) response. Notably, all surviving WAS patients achieved a normal platelet count.
Table 3.
Blood counts and immune reconstitution at last follow-up
| Median (range) | |
|---|---|
|
| |
| Last follow-up | 27 months (13–51 months) |
| Hemoglobin | 12.4 g/dl (8.6–16.6 g/dl) |
| Platelet count | 301 × 103 mcL (50–496 × 103 mcL) |
| Absolute lymphocyte count | 2442× 103 mcL (945–9260 × 103 mcL) |
| Absolute CD3 T cell count | 1998 cells/mcL (582–5378 cells/mcL) |
| Absolute CD4 T cell count | 1082 cells/mcL (318–2879 cells/mcL) |
| Absolute CD4 naïve T cell count | 530 cells/mcL (13–2297 cells/mcL) |
| Absolute CD8 T cell count | 688 cells/mcL (124–3513 cells/mcL) |
| Absolute B cell count | 716 cells/mcL (128–2289 cells/mcL) |
| Absolute NK cell count | 193 cells/mcL (44–1226 cells/mcL) |
| IgG level | 790 g/dl (275–1520 g/dl) |
| IgM level | 70 g/dl (7–224 g/dl) |
| IgA level | 121 g/dl (<6–208 g/dl) |
At last follow-up in patients with event-free survival (n = 33)
The only patient with primary graft failure in our cohort received an HLA 9/10 mismatched unrelated donor (MMUD) bone marrow graft. This was the patient’s second HCT for XLP. Notably, the patient had also developed primary graft failure following first HCT with the same donor and recovered with host hematopoiesis before proceeding to second HCT at 4 months. Following primary graft failure after second HCT, the patient remained aplastic and successfully underwent a third HCT on day + 34 with a haploidentical maternal donor (5/10 HLA match) CD34+ selected PBSC graft. Both patients with secondary graft failure also underwent a subsequent HCT. One patient received a 10/10 MUD bone marrow graft for severe congenital neutropenia and engrafted with 100% donor myeloid chimerism. The patient developed poor graft function with pancytopenia and bone marrow aplasia despite 99% donor myeloid chimerism at 1-year post-HCT. At 14 months post-HCT, the patient successfully underwent a second HCT with a haploidentical paternal donor (5/10 HLA match). The other patient with secondary graft failure received an 8/10 MMUD cord blood graft for first transplant. Notably, viability of CD34+ stem cells in the graft was only 55%. After accounting for viability, CD34+ stem cell dose was only 2.3 × 105 cells/kg. The patient engrafted with 35% donor chimerism and subsequently developed secondary graft failure on day + 48. The patient underwent a second HCT at a separate institution and succumbed to complications post-HCT, details of which are not available.
Discussion
This study evaluates the outcomes of a reduced toxicity busulfan-fludarabine regimen with alemtuzumab, or ATG serotherapy for children and young adults undergoing allogeneic HCT for WAS, primary HLH/XLP, CD40L deficiency, and a variety of other non-CGD primary immune deficiencies. Specifically, busulfan exposure was targeted to achieve a narrow range of exposure that is 65–80% of conventional myeloablative busulfan exposure. Our data show excellent 1-year overall survival (90%) with low toxicity, durable myeloid engraftment, and low incidence of grades II–IV GVHD.
OS in our cohort is superior to conventional myeloablative regimens that include busulfan and cyclophosphamide. Rao et al. reported an overall survival of 53% in 19 patients with primary immunodeficiencies who received conventional myeloablative HCT [15]. Antoine et al. reported a 3-year survival of 71% and 59% in matched related and unrelated donor HCT, respectively, after conventional myeloablative HCT for non-SCID primary immune deficiencies [3]. In the same study, a 3-year OS for primary HLH, WAS, and T cell deficiencies was 59%, 62%, and 43%, respectively. TRM was a major cause of death in all both studies in addition to infection. We observed low toxicity in our cohort based on observed frequencies of SOS, DAH, and TMA. Lower systemic exposure to busulfan compared to conventional myeloablative regimens and, replacing cyclophosphamide with fludarabine are likely explanations for the lower toxicity observed in our cohort. It is important to note that the reports by Rao et al. and Antoine et al. are from 2005 and 2003, respectively, and improvement in HCT supportive measures in the current era could also have contributed to improved outcomes. More recently, Burroughs et al. reported OS of 91% in 129 patients with WAS who underwent HCT; 68% received a myeloablative regimen and 30% received a RIC regimen [23]. Despite excellent OS, it is noteworthy that in this report 16 patients (12%) developed one or more severe organ toxicity within the first 3 months after HCT. Nine patients required mechanical ventilation, 5 patients developed SOS, 3 patients developed cardiac complications, and 2 patients required dialysis. Almost all patients received conventional myeloablative busulfan/cyclophosphamide (n = 10) or reduced intensity busulfan/cyclophosphamide or busulfan/fludarabine (n = 4). Notably, none of our patients with WAS developed severe organ toxicity during the first 3 months after HCT. Contreras et al. recently reported low toxicity and excellent overall survival in patients who received a busulfan, fludarabine, and alemtuzumab regimen with busulfan exposure similar to our cohort in a wide variety of non-malignant disorders [16]. Excellent overall survival and low toxicity observed in our study is comparable to a treosulfan, fludarabinebased regimen that is more widely used in Europe than in North America due to limited availability. Slatter et al. reported an overall survival of 83% with low toxicity in 160 patients with primary immune deficiencies who received treosulfan, fludarabine with alemtuzumab, or ATG for serotherapy [17]. Notably, none of their patients developed SOS. Incidence of acute GVHD and grades III–IV GVHD was 46% and 9% respectively in their cohort. In contrast, we observed low incidence of GVHD, 10% grades II–IV GVHD, and 2% grades III–IV GVHD, which also likely contributed to overall favorable outcomes in our study. The low incidence of GVHD is likely due to effective in vivo T cell depletion of the graft with alemtuzumab, which was the predominant serotherapy in our cohort. Low rates of GVHD have been observed in other alemtuzumab-containing regimens [18–20]. It is possible that in addition to improved short-term toxicity, lower busulfan exposure could also have a beneficial role in reducing late effects of the conditioning regimen such as growth retardation, endocrine deficiencies, and infertility. Future studies exploring late toxicity can help determine the impact of reducing busulfan exposure on late effects.
Interestingly, we observed a striking incidence of mixed donor and recipient T cell chimerism early following transplant, while donor myeloid chimerism remained high in most patients. Our experience suggests that high donor myeloid chimerism is durable with this regimen, despite mixed chimerism in the T cell compartment in the early months following HCT. Similar findings were observed in children with CGD who mostly received a reduced toxicity regimen for allogeneic HCT [21]. Stable and sustained myeloid chimerism is particularly important for primary immune deficiencies with dysfunctional hematopoiesis or phagocytic disorders. Notably, none of our surviving patients with WAS, including those who developed mixed chimerism, had recurrence of thrombocytopenia. While conventional MAC regimens are associated with a lower incidence of mixed chimerism, this benefit is offset by the increased incidence of toxicity and TRM. Treosulfan-based regimens offer less toxicity but are associated with a high incidence of mixed myeloid chimerism, which can be problematic. Slatter et al. reported donor myeloid chimerism < 50% at 1 year in 35% of patients with bone marrow grafts and 20% of patients with PBSC grafts in patients with primary immune deficiencies who received a treosulfan-based regimen [17]. Therefore, a higher proportion of patients achieved myeloablation or high myeloid chimerism with our regimen when compared to a treosulfan-based regimen. Our data suggest that busulfan exposure in this regimen in combination with fludarabine strikes the optimal balance by achieving durable myeloablation/high donor myeloid chimerism while limiting toxicity. Furthermore, our data suggest while donor T cell chimerism is low early post-HCT, T cell chimerism progressively increases with duration post-HCT. Importantly, low donor T cell chimerism early post-HCT was not associated with secondary graft loss or need for a secondary intervention. In contrast, donor chimerism can continue to decline following RIC regimens containing alemtuzumab, fludarabine, and melphalan and often necessitates a secondary intervention such as donor lymphocyte infusion, CD34+ stem cell boost, or second HCT for secondary graft failure [5, 10]. Decline in chimerism occurs due to recovery of host hematopoiesis following inadequate myeloablation with a RIC regimen, which then becomes dominant over time. We believe that myeloablation achieved with the regimen used in this study is sufficient to prevent recovery of host hematopoiesis thereby ensuring durable donor hematopoiesis.
Limited data exists on outcomes following second HCT for HLH. We observed favorable outcomes for patients undergoing second HCT for primary HLH/XLP with 1-year OS of 75%. In contrast, OS can be as low as 43% with conventional MAC regimens for primary HLH due to high rates of toxicity, which are a predominant contributor to early mortality [6]. OS observed in our cohort for second HCT is comparable to that of first HCT using a RIC regimen. Allen et al. reported OS of 80% following an alemtuzumab, fludarabine, and melphalan RIC regimen in patients with primary HLH [5]. However, these authors observed high rates of mixed chimerism and graft failure. At 1-year post-HCT, the proportion of patients alive with sustained engraftment without donor lymphocyte infusion or second HCT was only 39.1%, and probability of being alive and engrafted (with or without DLI) was 61%. In contrast, all patients with EFS had durable myeloid chimerism at 1-year post-HCT and did not require additional cellular therapy. Felber et al. recently reported an overall survival of 100% for first HCT with a similar busulfan-fludarabine regimen with comparable busulfan exposure [22]. However, the incidence of moderate/severe SOS was 32%. Although only one patient developed SOS in our cohort, the combined incidence of SOS and DAH was similar (33%). While we did not observe excess toxicity for second HCT, it is notable that the only patients in our entire cohort (first and second HCT) who developed SOS or DAH were primary HLH patients, suggesting that these patients remain at increased risk of toxicity. Given that donor myeloid chimerism is durable, it may be feasible to reduce target busulfan exposure in this group to decrease risk of toxicity.
Limitations of our study include relatively small patient size and wide disease heterogeneity which make it difficult to make disease-specific recommendations. However, our experience suggests that a reduced toxicity busulfan-fludarabine regimen with alemtuzumab or ATG as serotherapy offers a promising approach with low toxicity, durable myeloid engraftment, low incidence of grades 2–4 GVHD, and excellent survival. Until more definitive disease-specific regimen-related recommendations are available, this approach may be considered for a variety of primary immune deficiencies, especially where myeloablation or high myeloid chimerism is desirable or for patients who experienced a failed transplant with reduced intensity or other conditioning. Considering our cohort included only a limited number of patients who received a graft from a haploidentical donor, caution should be exercised when considering a haploidentical donor for patients receiving allogeneic HCT with this regimen.
Supplementary Material
Acknowledgments
We thank the patients and their families for their support of our work.
Abbreviations
- HCT
Hematopoietic cell transplant
- CGD
Chronic granulomatous disease
- WAS
Wiskott-Aldrich syndrome
- HLH
Hemophagocytic lymphohistiocytosis
- XLP
X-linked lymphoproliferative disease
- AUC
Area under the curve
- GVHD
Graft versus host disease
- MAC
Myeloablative conditioning
- RIC
Reduced intensity conditioning
- TRM
Transplant-related mortality
- SCID
Severe combined immune deficiency
- ATG
Anti-thymocyte globulin
- SOS
Sinusoidal obstruction syndrome
- OS
Overall survival
- EFS
Event-free survival
- HLA
Human leukocyte antigen
- DAH
Diffuse alveolar hemorrhage
- TMA
Thrombotic microangiopathy
- PBSC
Peripheral blood stem cells
- G-CSF
Granulocyte colony–stimulating factor
- MMF
Mycophenolate
- CMV
Cytomegalovirus infection
- HSV
Herpes simplex virus
- FISH
Fluorescent in situ hybridization
- STR
Short tandem repeats
- EOIBD
Early-onset inflammatory bowel disease
- IPEX
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked
- PTLD
Post-transplant lymphoproliferative disorder
- GBS
Guillain-Barre syndrome
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflicts of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10875-020-00888-2) contains supplementary material, which is available to authorized users.
References
- 1.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer A, Landais P, Friedrich W, Gerritsen B, Fasth A, Porta F, et al. Bone marrow transplantation (BMT) in Europe for primary immunodeficiencies other than severe combined immunodeficiency: a report from the European Group for BMT and the European Group for Immunodeficiency. Blood. 1994;83(4):1149–54. [PubMed] [Google Scholar]
- 3.Antoine C, Müller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361(9357):553–60. [DOI] [PubMed] [Google Scholar]
- 4.Chiesa R, Veys P. Reduced-intensity conditioning for allogeneic stem cell transplant in primary immune deficiencies. Expert Rev Clin Immunol. 2012;8(3):255–66quiz 67. [DOI] [PubMed] [Google Scholar]
- 5.Allen CE, Marsh R, Dawson P, Bollard CM, Shenoy S, Roehrs P, et al. Reduced-intensity conditioning for hematopoietic cell transplant for HLH and primary immune deficiencies. Blood. 2018;132(13):1438–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsh RA, Vaughn G, Kim MO, Li D, Jodele S, Joshi S, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116(26): 5824–31. [DOI] [PubMed] [Google Scholar]
- 7.Marsh RA, Rao MB, Gefen A, Bellman D, Mehta PA, Khandelwal P, et al. Experience with alemtuzumab, fludarabine, and melphalan reduced-intensity conditioning hematopoietic cell transplantation in patients with nonmalignant diseases reveals good outcomes and that the risk of mixed chimerism depends on underlying disease, stem cell source, and alemtuzumab regimen. Biol Blood Marrow Transplant. 2015;21(8):1460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao K, Adams S, Qasim W, Allwood Z, Worth A, Silva J, et al. Effect of stem cell source on long-term chimerism and event-free survival in children with primary immunodeficiency disorders after fludarabine and melphalan conditioning regimen. J Allergy Clin Immunol. 2016;138(4):1152–60. [DOI] [PubMed] [Google Scholar]
- 9.Moratto D, Giliani S, Bonfim C, Mazzolari E, Fischer A, Ochs HD, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980–2009: an international collaborative study. Blood. 2011;118(6):1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra S, Bleesing JJ, Jordan MB, Grimley MS, Khandelwal P, Davies SM, et al. Post-transplant CD34+selected stem cell “boost” as intervention for mixed chimerism following reduced intensity conditioning HSCT in children and young adults with primary immune deficiencies. Biology of Blood and Marrow Transplant. 2018;24(3):S406–S7. [DOI] [PubMed] [Google Scholar]
- 11.Ferrua F, Galimberti S, Courteille V, Slatter MA, Booth C, Moshous D, et al. Hematopoietic stem cell transplantation for CD40 ligand deficiency: results from an EBMT/ESID-IEWP-SCETIDE-PIDTC study. J Allergy Clin Immunol. 2019;143(6):2238–53. [DOI] [PubMed] [Google Scholar]
- 12.Güngör T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383(9915):436–48. [DOI] [PubMed] [Google Scholar]
- 13.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21(6):984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao K, Amrolia PJ, Jones A, Cale CM, Naik P, King D, et al. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood. 2005;105(2):879–85. [DOI] [PubMed] [Google Scholar]
- 16.Contreras CF, Long-Boyle JR, Shimano KA, Melton A, Kharbanda S, Dara J, et al. Reduced toxicity conditioning for nonmalignant hematopoietic cell transplants. Biol Blood Marrow Transplant. 2020. [DOI] [PubMed]
- 17.Slatter MA, Rao K, Abd Hamid IJ, Nademi Z, Chiesa R, Elfeky R, et al. Treosulfan and fludarabine conditioning for hematopoietic stem cell transplantation in children with primary immunodeficiency: UK experience. Biol Blood Marrow Transplant. 2018;24(3): 529–36. [DOI] [PubMed] [Google Scholar]
- 18.Kottaridis PD, Milligan DW, Chopra R, Chakraverty RK, Chakrabarti S, Robinson S, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96(7):2419–25. [PubMed] [Google Scholar]
- 19.Green K, Pearce K, Sellar RS, Jardine L, Nicolson PLR, Nagra S, et al. Impact of alemtuzumab scheduling on graft-versus-host disease after unrelated donor fludarabine and melphalan allografts. Biol Blood Marrow Transplant. 2017;23(5):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robin M, Raj K, Chevret S, Gauthier J, de Lavallade H, Michonneau D, et al. Alemtuzumab vs anti-thymocyte globulin in patients transplanted from an unrelated donor after a reduced intensity conditioning. Eur J Haematol. 2018;101(4): 466–74. [DOI] [PubMed] [Google Scholar]
- 21.Marsh RA, Leiding JW, Logan BR, Griffith LM, Arnold DE, Haddad E, et al. Chronic granulomatous disease-associated IBD resolves and does not adversely impact survival following allogeneic HCT. J Clin Immunol. 2019;39(7):653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felber M, Steward CG, Kentouche K, Fasth A, Wynn RF, Zeilhofer U, et al. Targeted busulfan-based reduced-intensity conditioning and HLA-matched HSCT cure hemophagocytic lymphohistiocytosis. Blood Adv. 2020;4(9):1998–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burroughs LM, Petrovic A, Brazauskas R, Liu X, Griffith LM, Ochs HD. Excellent outcomes following hematopoietic cell transplantation for Wiskott-Aldrich syndrome: a PIDTC report. Blood. 2020;135(23):2094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.