Abstract
Background
Physical activity is associated with lower risk for endometrial cancer, but the extent to which the association is mediated by body mass index (BMI) in midlife is unclear. This study describes the physical activity–endometrial cancer association and whether BMI mediates this relationship.
Methods
Participants were 67 705 women in the National Institutes of Health-AARP Diet and Health Study (50-71 years) who recalled their physical activity patterns starting at age 15-18 years. We identified 5 long-term physical activity patterns between adolescence and cohort entry (ie, inactive, maintained low, maintained high, increasers, decreasers). We used Cox regression to assess the relationship between these patterns and midlife BMI and endometrial cancer, adjusting for covariates. Mediation analysis was used to estimate the proportion of the physical activity–endometrial cancer association that was mediated by midlife BMI.
Results
During an average 12.4 years of follow-up 1468 endometrial cancers occurred. Compared with long-term inactive women, women who maintained high or increased activity levels had a 19% to 26% lower risk for endometrial cancer (maintained high activity: hazard ratio = 0.81, 95% confidence interval [CI] = 0.67 to 0.98; increasers: hazard ratio = 0.74, 95% CI = 0.61 to 0.91). They also had a 50% to 77% lower risk for obesity in midlife (eg, maintained high activity: odds ratio for a BMI of 30-39.9 kg/m2 = 0.50, 95% CI = 0.46 to 0.55; and maintained high activity, odds ratio for a BMI of ≥40 kg/m2 = 0.32, 95% CI = 0.26 to 0.39). BMI was a statistically significant mediator accounting for 55.5% to 62.7% of the physical activity–endometrial cancer associations observed.
Conclusions
Both maintaining physical activity throughout adulthood and adopting activity later in adulthood can play a role in preventing obesity and lowering the risk for endometrial cancer.
Endometrial cancer is the fourth-most common cancer in women, and incidence rates have increased during the last 20 years (1-3). This upward trend appears to be driven, in part, by the obesity epidemic, highlighting the need for obesity prevention strategies (4-9). Engaging in moderate-to-vigorous intensity physical activity has been associated with lower risk for obesity, suggesting physical activity can be part of the solution (10).
Higher levels of leisure time physical activity (LTPA) have been associated with a 20% lower risk for endometrial cancer (11-15). However, the consistent inverse association between LTPA and endometrial cancer is often substantially attenuated after controlling for BMI (12,13,16). Consequently, this association is thought to be largely mediated by BMI (12,13,17-19), but the complex relationships between these exposures have been difficult to study. Disentangling the temporal relationships between physical activity and BMI is not always possible. Most studies measured LTPA and BMI at the same time in midlife or cohort entry and therefore cannot determine the temporality of these factors when estimating risk for endometrial cancer (12,13,16,17). Fewer studies have explored how higher levels of LTPA earlier in adulthood may prevent the development of elevated BMI in midlife and subsequently contribute to the LTPA–endometrial cancer association (20-23). LTPA participation can prevent obesity (14), which alone accounts for 40% to 60% of all endometrial cancers (4-8,14). We are unaware of studies that have conducted a formal mediation analysis, but exploring mediation in this context may help clarify the role that long-term LTPA can play in the prevention of endometrial cancer.
The goal of this study was to describe how long-term patterns of LTPA are related to midlife BMI and subsequent endometrial cancer risk. We specifically tested if midlife BMI was a mediator of the LTPA–endometrial cancer association.
Methods
Study Population
The National Institues of Health (NIH)-AARP Diet and Health Study (1995-1996) is a prospective cohort of AARP members (50-71 years) living in 6 states (CA, FL, LA, NJ, NC, PA) and 2 metropolitan areas (Atlanta, Detroit) (24). The study included a baseline questionnaire that asked about medical conditions and demographics. Approximately 6 months after, participants without renal disease, colorectal, breast, or prostate cancer received a Risk Factor Questionnaire (RFQ) that included questions about LTPA, body mass index (BMI), diet, and medical history. The NIH-AARP Diet and Health Study was approved by the Special Studies Institutional Review Board of the US National Cancer Institute, and all participants gave written informed consent.
Overall, 566 398 participants completed a baseline questionnaire and 539 213 were sent the RFQ, and 334 905 of these were returned. In this study, we included women (146 790) who completed their own questionnaires (136 407) and were not diagnosed with cancer before RFQ completion (127 336). Participants also reported no emphysema (124 462), no hysterectomy (72 762), or cessation of menstruation due to radiation, chemotherapy, or surgery (71 670). Participants also had complete long-term LTPA and midlife BMI data (67 705). The final analytical sample was similar to the original cohort of women enrolled in the NIH-AARP (Supplementary Table 1, available online).
Study Design to Evaluate Mediation
For these analyses, we used historical information about LTPA occurring between ages 15 and 18 and between 40 and 61 years, or before the baseline questionnaire. This historical information was used to maintain temporal sequence between LTPA and BMI reported on the baseline questionnaire (defined here as midlife BMI). We used this analytical design to evaluate the LTPA–endometrial cancer association and mediation by BMI at midlife. In this context, we employed BMI near the earliest LTPA age period as a covariate (BMI at age 18 years). We evaluated follow-up for endometrial cancer starting at baseline until December 2011 (Figure 1).
Figure 1.
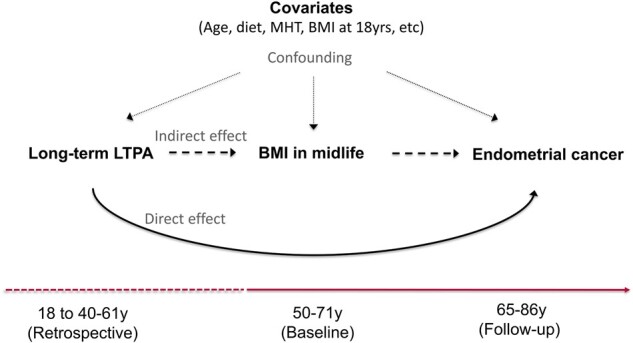
Directed acyclic graph for the physical activity–endometrial cancer association and mediation through body mass index (BMI) in midlife. The dashed and solid line at the bottom illustrates the timing of the measures. Women aged 50-71 years reported their BMI at baseline (ie, BMI in midlife) and provided information on several other risk factors for endometrial cancer risk (ie, confounders), including age (years), race-ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other, or missing), education (less than high school, high school, post high-school or some college, bachelor degree or more, missing), smoking status and dose (never smoker, former smoker and ≤20 cigarettes per day, former smoker and >20 cigarettes per day, current smoker and ≤20 cigarettes pe day, current smoker and >20 cigarettes per day, missing), diet quality (2015 Healthy Eating Index; 0-100 points), total energy intake (kilocalories per day), alcohol consumption (grams per day), parity (number of births), use of oral contraceptives (never or <1 year, 1-4 years, 5-9 years, ≥10 years, missing), menopausal hormone therapy use (never, continuous estrogen plus progestin [EPT] use [15+ d/mo progestin], sequential EPT [<15 d/mo progestin], estrogen only, missing), and BMI at age 18 years (normal weight [<25.0 kg/m2], overweight [25.0-29.9 kg/m2], obese class I [30.0-34.9 kg/m2], obese class II [35.0-39.9 kg/m2], and obese class III [≥40.0 kg/m2]). At baseline, women retrospectively reported their participation in leisure time physical activity (LTPA) from ages 18 years through 40-61 years (ie, long-term LTPA). Incident endometrial cancer was ascertained during follow-up until women were 65-86 years. The solid line indicates the direct effect of long-term LTPA through adulthood on endometrial cancer risk and the long dashed line indicates the indirect effect of long-term LTPA on endometrial cancer that goes through BMI in midlife. The small dashed lines indicate the effects of confounders on these associations. MHT = menopausal hormone therapy use.
Long-Term LTPA and BMI Measures
Participants were asked to report LTPA duration (hours per week) at ages 15-18 years, 19-29 years, and 30-35 years, and the 10 years before cohort entry (ie, equivalent to approximately 40-61 years age range based on participant age at baseline; Supplementary Figure 1, available online). The reported LTPA duration for each age period was coded as being 0 (rarely or never), 0.5 (weekly but <1 hour), 2.0 (1-3 h/wk), 5.5 (4-7 h/wk), and 7 (7+ h/wk) hours per week. These data were used to create LTPA participation trajectories. Previous assessments of reliability of the LTPA items among AARP members (50-74 years) showed that 6-month test-retest intraclass correlations for these items ranged from 0.52 to 0.55 (25). Women also reported body weight at age 18, 35, and 50 years and their current body weight and height at baseline. BMI was calculated as weight (kg) divided by height squared (m2) and examined using BMI classifications: normal weight (<25.0 kg/m2), overweight (25.0-29.9 kg/m2), obese class I (30.0-34.9 kg/m2), obese class II (35.0-39.9 kg/m2), and obese class III (≥40.0 kg/m2) (26). Body mass in midlife was defined as BMI classification status at baseline (aged 50-71 years).
Covariate Assessment
Covariates included age, race-ethnicity, education, smoking status and dose, diet quality, total energy intake, alcohol consumption, parity, use of oral contraceptives, and menopausal hormone therapy use. Again, we also assumed that BMI at age 18 years generally preceded our long-term trajectories of LTPA and could have affected our LTPA trajectories. In preliminary analysis, we found that adults with higher BMI at age 18 years were less likely to be classified as LTPA maintainers or increasers (Supplementary Table 2, available online). Hence, BMI at age 18 years was also included as a covariate.
Ascertainment of Endometrial Cancer
Incident endometrial cancer was determined through probabilistic linkage with state registries. This process is expected to identify 90% of all cancer cases (27). Vital status was determined using the National Death Index, and residency was confirmed through the US Postal Office National Change of Address database. Endometrial cancer, confirmed by the cancer registry, was classified using the International Classification of Diseases of Oncology (third edition) codes C540–C549 and C559 (28). We defined “type I” tumors as endometrioid, adenocarcinoma, and mucinous tumors: 8140, 8210, 8260, 8262, 8380, 8382, 8383, 8480–8482, 8560, and 8570. Nontype I tumors included those with a serous, clear cell; other; or with unknown histology. Follow-up was calculated from date of the physical activity assessment at baseline to the date of cancer diagnosis, death, or end of follow-up (December 31, 2011), whichever came first.
Statistical Analysis
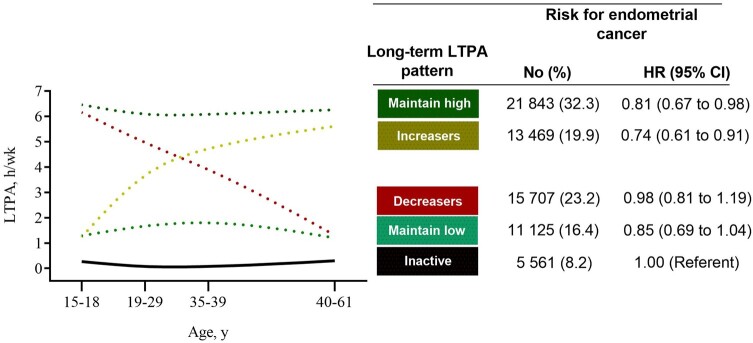
Each pattern of LTPA was determined based on membership probability using maximum likelihood estimates obtained from semi-parametric group-based mixture models (25,29,30). Membership probability indicates the extent that assigned long-term LTPA patterns match observed LTPA values and in this study was high, exceeding 0.9 for all groups (Table 1). Women’s LTPA were categorized into 5 groups based on their long-term (eg, 15-18 years to 40-61 years) patterns of physical activity. We identified women that maintained: 1) no LTPA at all time points—inactive (n = 5561); 2) low amounts (approximately 1-2 h/wk) of LTPA—maintaining low (n = 11 125); or 3) high amounts (approximately 6-7 h/wk) of LTPA—maintaining high (n = 21 843). Women were also classified as: 4) increasers (n = 13 469); or 5) decreasers (n = 15 707) if their LTPA at 40-61 years was higher or lower, respectively, compared with LTPA at 15-18 years.
Table 1.
Baseline characteristics by LTPA pattern, National Institues of Health-AARP Diet and Health Study, 1995-2011
| Trajectorya | Inactive (referent) | Maintain low | Maintain high | Increasers | Decreasers |
|---|---|---|---|---|---|
| Total No. (%) | 5561 (8.2) | 11 125 (16.4) | 21 843 (32.3) | 13 469 (19.9) | 15 707 (23.2) |
| LTPA group probability, mean (SD) | 0.93 (0.1) | 0.95 (0.1) | 0.99 (0.0) | 0.97 (0.1) | 0.98 (0.1) |
| Endometrial cancers, No. (%) | 149 (2.7) | 245 (2.2) | 439 (2.0) | 259 (1.9) | 376 (2.4) |
| Incidence rate, No. per 100 000 person-yb | 219.1 | 177.4 | 161.1 | 152.4 | 196.1 |
| Mean age (SD), y | 62.3 (5.5) | 61.9 (5.5) | 62.6 (5.4) | 62.4 (5.5) | 62.0 (5.5) |
| Non-Hispanic White, No. (%) | 5009 (90.1) | 10 230 (92.0) | 20 430 (93.5) | 12 576 (93.4) | 14 443 (92.0) |
| Less than high school, No. (%) | 292 (5.3) | 417 (3.8) | 904 (4.2) | 509 (3.8) | 503 (3.2) |
| Smoker: >20 cig/d, No. (%) | 176 (3.2) | 396 (3.6) | 764 (3.5) | 318 (2.4) | 785 (5.0) |
| LTPA at age group, mean (SD), h/wk | |||||
| 15-18 y | 0.3 (0.6) | 1.3 (0.9) | 6.5 (0.7) | 1.2 (0.9) | 6.2 (1.0) |
| 19-29 y | 0.1 (0.2) | 1.7 (1.3) | 6.1 (1.6) | 3.7 (2.5) | 5.0 (2.1) |
| 35-39 y | 0.1 (0.3) | 1.8 (1.5) | 6.1 (1.5) | 4.7 (2.3) | 3.9 (2.3) |
| 40-61 y | 0.3 (0.7) | 1.2 (0.9) | 6.3 (0.8) | 5.6 (1.6) | 1.3 (0.9) |
| Obese BMI, No. (%) | |||||
| At 18 y ≥ 30.0 kg/m2 | 169 (3.0) | 200 (1.8) | 212 (1.0) | 227 (1.7) | 197 (1.3) |
| Current ≥30.0 kg/m2 | 1571 (28.3) | 2649 (23.8) | 3352 (15.4) | 1952 (14.5) | 4585 (29.2) |
| Healthy Eating Index, mean (SD), 0-100c | 66.8 (9.9) | 68.2 (9.4) | 70.3 (9.1) | 70.3 (9.0) | 68.3 (9.4) |
| Energy intake, mean (SD), kcal/dd | 1.5 (0.8) | 1.5 (0.7) | 1.6 (0.7) | 1.5 (0.6) | 1.6 (0.7) |
| Alcohol, mean (SD), g/d | 5.5 (16.6) | 6.0 (17.0) | 7.0 (17.8) | 6.7 (16.2) | 6.7 (20.1) |
| Nulliparous, No. (%) | 1441 (25.9) | 2328 (20.9) | 3205 (14.7) | 2246 (16.7) | 2883 (18.4) |
| Use of oral contraceptives, never or <1 y, No. (%) | 3478 (62.5) | 6322 (56.8) | 12 992 (59.5) | 7810 (58.0) | 8931 (56.9) |
| Menopausal hormone therapy use, estrogen only, No. (%) | 338 (6.5) | 669 (6.0) | 1389 (6.4) | 844 (6.5) | 1009 (6.4) |
Participants with little or no physical activity (<1 h/wk) at each age period were classified as Inactive; those maintaining low levels of activity over time were classified as maintaining low activity; those maintaining high levels of activity over time were classified as maintaining high activity; those that increased their activity over time were classified as increasers; and those that decreased their activity over time were classified as decreasers. BMI = body mass index; LTPA = leisure-time physical activity.
Unadjusted endometrial cancer rates.
2015 Healthy Eating Index scores range from 0 (least healthy) to a 100 (most healthy) and describe diet quality as recommended by the 2015-2020 Dietary Guidelines for Americans.
Indicates kcal/day per 1000.
Our first set of analyses examined the separate links between long-term LTPA, BMI at midlife, and endometrial cancer to establish whether mediation by midlife BMI was plausible. We first examined the association between long-term LTPA and incident endometrial cancer using Cox proportional hazard models, adjusting for the covariates. Women who reported no LTPA (inactive) were set as the referent group for all analyses. Next, we assessed if midlife BMI was associated with endometrial cancer risk by modeling the association between midlife BMI and endometrial cancer, adjusting for covariates, using Cox proportional hazard models and with normal-weight BMI in midlife as the referent group. Finally, we determined whether LTPA was associated with midlife BMI by modeling the association between long-term LTPA and midlife BMI using logistic regression, with the midlife BMI outcome categorized as normal weight, overweight, obese-class I/II, or obese-class III and adjusting for covariates. The proportional hazards assumption was tested and confirmed for our main exposures, long-term LTPA, and midlife BMI.
We then conducted formal mediation analysis to determine the extent to whether midlife BMI was a mediator of the relationship between long-term LTPA and endometrial cancer. We specifically assessed mediation using counter-factual reasoning and the mediation framework for estimating natural effects proposed by Lange and colleagues (31), including covariates and models with and without additional adjustment for BMI at age 18 years. Here, the “total” effect of long-term LTPA on endometrial cancer can be decomposed into an “indirect” effect mediated by midlife BMI and a “direct” effect (see Figure 1). The interpretation of the total effect is consistent with the standard analysis described above for the long-term LTPA-endometrial cancer association, without adjustment for midlife BMI. To clarify the direct and indirect effects, we describe an example comparing the effects of being in the inactive vs the maintaining high-LTPA groups. The direct effect would be the effect of changing to the maintaining high LTPA category if an individual’s midlife BMI status remained unchanged from what it would be with inactivity. The indirect effect would be the effect of changing an individual's midlife BMI status to the level that would be expected had they been in the maintaining high-LTPA group (eg, holding all other factors as if they were still in the inactive category). We can then compare these effects to estimate the proportion of the long-term LTPA–endometrial cancer association that is likely attributable to the effects of long-term LTPA on midlife BMI. Using standard counterfactual notation (31), let denote an individual’s survival time (ie, time until cancer diagnosis) if the exposure (eg, indicator for inactive vs maintaining high LTPA) is set to and the mediator,, is set to the value it would have taken had the exposure been set to . Then, we assume the log-hazard can be modeled as:
| (1) |
.
We define the natural indirect effect by , the natural direct effect by , and the proportion mediated by ; we estimate these quantities and their 95% confidence intervals using the simulation approach developed by Lange (31). A more detailed description of our approach can be found in the Supplementary Methods (available online).
Analyses were done using SAS version 9.4 and the R software version 3.5.3. Results were statistically significant when P values were less than .05. All tests were 2-sided.
Sensitivity Analyses
We also examined mediation results separately by the dualistic type of endometrial cancer (type I vs nontype I tumors) because type I cancers typically have the strongest associations with BMI (32). We assessed effect modification and reverse causality bias by comparing the inactive vs maintaining high-LTPA risk for endometrial cancer. Effect modification was examined by conducting stratified analyses by age group (<60 vs 60+ years), BMI (normal weight vs overweight/obese), diet quality, total energy intake, alcohol consumption, parity, oral contraceptive use, menopausal hormonal therapy use, and prevalent vs no diagnosis of diabetes and cardiovascular disease. Because latent disease may influence BMI at baseline (ie, midlife BMI), we examined potential reverse causality by excluding the first 2, 4, and 6 years of follow-up and excluding participants that reported losing weight in the 1-10 years preceding the baseline assessment.
Results
Descriptive Characteristics
During an average 12.4 years (SD = 4.4 years) of follow-up in 67 705 women, there were 1468 incident endometrial cancers. Women were on average 62.3 years (SD = 5.5 years) at baseline, and the majority were non-Hispanic White (92.6%), with 12 years or more of formal education (96.1%), and postmenopausal (93.3%) (Table 1).
Long-Term LTPA and Endometrial Cancer
Compared with women who were inactive across adulthood, those maintaining high amounts of LTPA (ie, maintaining high; 6-7 h/wk) had a 19% lower risk for endometrial cancer (hazard ratio [HR] = 0.81, 95% confidence interval [CI] = 0.67 to 0.98) after adjusting for BMI at age 18 years and other covariates (Figure 2; Supplementary Table 3, available online). Women maintaining lower amounts of LTPA (ie, maintaining low; 1-2 h/wk) had lower risk for endometrial cancer risk (HR = 0.85, 95% CI = 0.69 to 1.04) that did not reach statistical significance. Women who increased their LTPA (ie, increasers) had a 26% lower risk for endometrial cancer compared with inactive women (HR = 0.74, 95% CI = 0.61 to 0.91). Women who decreased their LTPA (ie, decreasers) retained no protection against endometrial cancer compared with inactive women (HR = 0.98, 95% CI = 0.81 to 1.19; Figure 2).
Figure 2.
Long-term leisure time physical activity (LTPA) patterns and endometrial cancer risk. Hazard ratios were adjusted for age (years), race-ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Other or missing), education (less than high school, high school, post high-school or some college, bachelor degree or more, missing), smoking status/dose (never smoker, former smoker and ≤20 cigarettes per day, former smoker and >20 cigarettes per day, current smoker and ≤20 cigarettes per day, current smoker and >20 cigarettes per day, missing), diet quality (2015 Healthy Eating Index; 0-100 points), total energy intake (kilocalories per day), alcohol consumption (grams per day), parity (number of births), use of oral contraceptives (never or <1 year, 1-4 years, 5-9 years, ≥10 years, missing), menopausal hormone therapy use (never, continuous estrogen plus progestin [EPT] use [15+ d/mo progestin], sequential EPT [<15 d/mo progestin], estrogen only, missing), and body mass index (BMI) at age 18 years (normal weight [<25.0 kg/m2], overweight [25.0-29.9 kg/m2], obese class I [30.0-34.9 kg/m2], obese class II [35.0-39.9 kg/m2], and obese class III [≥40.0 kg/m2]). CI = confidence interval; HR= hazard ratio.
BMI at Midlife and Endometrial Cancer
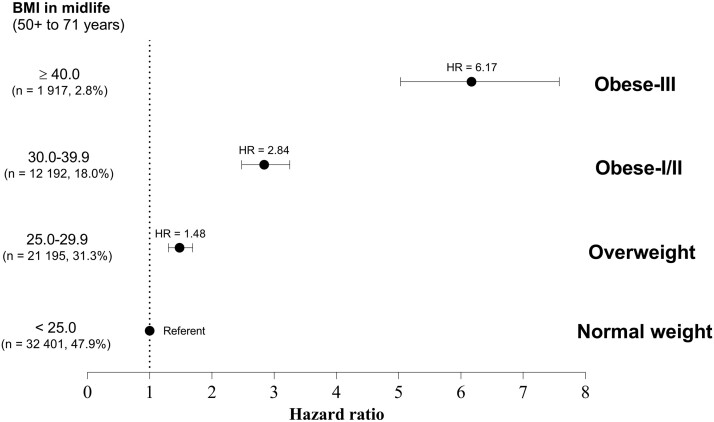
BMI in midlife was strongly associated with endometrial cancer after adjusting for covariates and BMI at age 18 years. Compared with normal-weight women, women who were overweight at midlife had an approximately 50% greater risk for endometrial cancer (HR = 1.48, 95% CI = 1.30 to 1.69), and those who were obese class I/II (BMI = 30.0-39.9 kg/m2) had a 2.8 times greater risk (HR = 2.84, 95% CI = 2.48 to 3.25) (Figure 3). Women who were obese class III (BMI ≥ 40.0 kg/m2) had a more than sixfold greater risk (HR = 6.17, 95% CI = 5.03 to 7.58).
Figure 3.
Body mass index (BMI) at midlife (50-71 years) and risk for endometrial cancer. Hazard ratios (HRs) were adjusted for age (years), race-ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other or missing), education (less than high school, high school, post high-school or some college, bachelor degree or more, missing), smoking status and dose (never smoker, former smoker and ≤20 cigarettes per day, former smoker and >20 cigarettes per day, current smoker and ≤20 cigarettes per day, current smoker and >20 cigarettes per day, missing), diet quality (2015 Healthy Eating Index; 0-100 points), total energy intake (kilocalories per day), alcohol consumption (grams per day), parity (number of births), use of oral contraceptives (never or <1 year, 1-4 years, 5-9 years, ≥10 years, missing), menopausal hormone therapy use (never, continuous estrogen plus progestin [EPT] use [15+ d/mo progestin], sequential EPT [<15 d/mo progestin], estrogen only, missing), and BMI at age 18 years (normal weight [<25.0 kg/m2], overweight [25.0-29.9 kg/m2], obese class I [30.0-34.9 kg/m2], obese class II [35.0-39.9 kg/m2], and obese class III [≥40.0 kg/m2]). Error bars represent the 95% confidence intervals.
Long-Term LTPA and BMI at Midlife
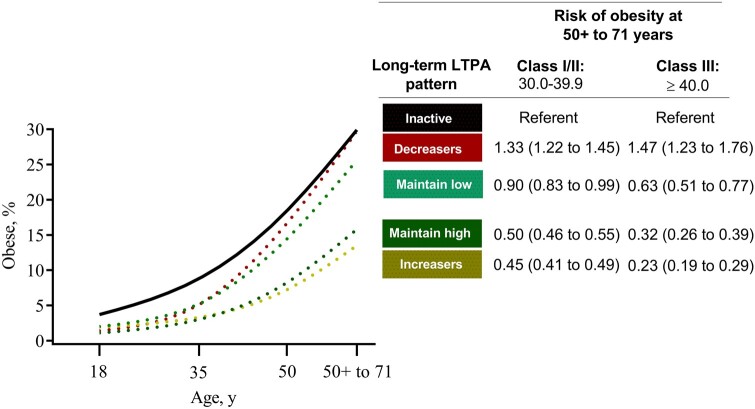
Although the prevalence of obesity increased with age in all activity groups, maintaining high LTPA was associated with a 50% lower risk for class I/II obesity in midlife (OR = 0.50, 95% CI = 0.46 to 0.55) and a 68% lower risk for class III obesity (OR = 0.32, 95% CI = 0.26 to 0.39) compared with inactive women (Figure 4; Supplementary Table 4, available online). Women who increased their LTPA (ie, increasers) had a 55% lower risk for class I/II obesity (OR = 0.45, 95% CI = 0.41 to 0.49) and a 77% lower risk for class III obesity (OR = 0.23, 95% CI = 0.19 to 0.29). Women who decreased their LTPA (ie, decreasers) were at statistically significantly higher risk for obesity.
Figure 4.
Risk of being obese in midlife (50-71 years) by long-term leisure time physical activity (LTPA) patterns . Odds ratios (ORs; with 95% confidence intervals in parentheses) were adjusted for age (years), race-ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other, or missing), education (less than high school, high school, post high-school or some college, bachelor degree or more, missing), smoking status and dose (never smoker, former smoker and ≤20 cigarettes per day, former smoker and >20 cigarettes per day, current smoker and ≤20 cigarettes per day, current smoker and >20 cigarettes per day, missing), diet quality (2015 Healthy Eating Index; 0-100 points), total energy intake (kilocalories per day), alcohol consumption (grams per day), parity (number of births), use of oral contraceptives (never or <1 year, 1-4 years, 5-9 years, ≥10 years, missing), menopausal hormone therapy use (never, continuous estrogen plus progestin [EPT] use [15+ d/mo progestin], sequential EPT [<15 d/mo progestin], estrogen only, missing), and BMI at age 18 years (normal weight [<25.0 kg/m2], overweight [25.0-29.9 kg/m2], obese class I [30.0-34.9 kg/m2], obese class II [35.0-39.9 kg/m2], and obese class III [≥40.0 kg/m2]).
Long-Term LTPA, BMI at Midlife, and Endometrial Cancer: Mediation Results
The association between long-term LTPA and endometrial cancer appeared to be mediated substantially by midlife BMI. The total effect for the long-term LTPA–endometrial cancer association was statistically significant for women who maintained high LTPA (HR = 0.80, 95% CI = 0.68 to 0.95) and women who increased their LTPA (HR = 0.73, 95% CI = 0.59 to 0.94) (Table 2). The indirect effect between LTPA and endometrial cancer through BMI in midlife was also statistically significant among women who maintained high LTPA (HR = 0.87, 95% CI = 0.84 to 0.90) and who increased LTPA by midlife (HR = 0.84, 95% CI = 0.81 to 0.89). There were no statistically significant direct effects for any LTPA groups. This corresponded to a substantial amount of the total effects for either maintaining high LTPA or increasing LTPA on endometrial cancer that were mediated by the effects of long-term LTPA on midlife BMI (proportion mediated ranged from 56% to 63%) (Table 2). Results remained similar when mediation models were unadjusted for BMI at age 18 years (Supplementary Table 5, available online).
Table 2.
Summary of mediation analysis: long-term LTPA patterns, BMI, and risk for endometrial cancer
| Effect | Long-term LTPA patternsa |
||||
|---|---|---|---|---|---|
| Inactive | Maintain low | Maintain high | Increasers | Decreasers | |
| (n = 5561) | (n = 11 125) | (n = 21 843) | (n = 13 469) | (n = 15 707) | |
| Endometrial cancers, No. (%) | 149 (2.7) | 245 (2.2) | 439 (2.0) | 259 (1.9) | 376 (2.4) |
| Total effectb, HR (95% CI)c | Referent | 0.84 (0.66 to 1.12) | 0.80 (0.68 to 0.95) | 0.73 (0.59 to 0.94) | 0.98 (0.83 to 1.17) |
| Indirect effect (through midlife BMI), HR (95% CI)c | Referent | 0.97 (0.93 to 1.01) | 0.87 (0.84 to 0.90) | 0.84 (0.81 to 0.89) | 0.98 (0.83 to 1.17) |
| Direct effect, HR (95% CI)c | Referent | 0.87 (0.71 to 1.13) | 0.92 (0.79 to 1.09) | 0.87 (0.70 to 1.11) | 0.92 (0.77 to 1.08) |
| Proportion of LTPA through BMI at midlife, % (95% CI) | Referent | NE | 62.7 (30.6 to 100.0) | 55.5 (31.4 to 100.0) | NE |
Participants with little or no physical activity (<1 h/wk) at each age period were classified as Inactive; those maintaining low levels of activity over time were classified as maintaining low activity; those maintaining high levels of activity over time were classified as maintaining high activity; those that increased their activity over time were classified as increasers; and those that decreased their activity over time were classified as decreasers. BMI = body mass index; CI = confidence intervals; HR = hazard ratio; LTPA = leisure time physical activity; NE = not estimated (ie, the denominator for the proportion was approximately 0 and the estimation was not stable).
The total effect presented in this table can differ from the effects provided in Figure 2 by a residual amount.
Hazard ratios were adjusted for: age (years), race-ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, other, or missing), education (less than high school, high school, post high-school or some college, bachelor degree or more, missing), smoking status/dose (never smoker, former smoker and ≤20 cigarettes/day, former smoker and >20 cigarettes/day, current smoker and ≤20 cigarettes/day, current smoker and >20 cigarettes/day, missing), diet quality (2015 Healthy Eating Index; 0-100 points), total energy intake (kcal/day), alcohol consumption (grams/day), parity (number of births), use of oral contraceptives (never or <1 year, 1-4 years, 5-9 years, ≥10 years, missing), menopausal hormone therapy use (never, continuous estrogen plus progestin [EPT] use [15+ days progestin/month], sequential EPT [<15 days progestin/month], estrogen only, missing) and, BMI at age 18 years (normal weight [<25.0 kg/m2], overweight [25.0-29.9 kg/m2], obese class I [30.0-34.9 kg/m2], obese class II [35.0-39.9 kg/m2], and obese class III [≥40.0 kg/m2]). Models also included BMI in midlife (normal weight [<25.0 kg/m2], overweight [25.0-29.9 kg/m2], obese class I [30.0-34.9 kg/m2], obese class II [35.0-39.9 kg/m2], and obese class III [≥40.0 kg/m2]) as a mediator of the LTPA-endometrial cancer association.
Sensitivity Analyses
The mediation results were similar for type I endometrial cancers, but associations were weaker and imprecise for nontype I tumors (Table 3). We found no evidence of effect modification of the long-term LTPA–endometrial cancer association except for energy intake (Pinteraction= .02) (Supplementary Table 6, available online). LTPA–endometrial cancer associations did not change excluding the first 2-6 years of follow-up or women who reported losing weight (Supplementary Table 7, available online).
Table 3.
Summary of mediation analysis: long-term LTPA patterns, BMI, and risk for type I vs nontype I cancers
| Histology | Long-term LTPA patternsa |
||||
|---|---|---|---|---|---|
| Inactive | Maintain low | Maintain high | Increasers | Decreasers | |
| (n = 5561) | (n = 11 125) | (n = 21 843) | (n = 13 469) | (n = 15 707) | |
| Type I cancersb, No. (%) | 124 (2.2) | 199 (1.8) | 355 (1.6) | 205 (1.5) | 313 (2.0) |
| Total effect of LTPA on endometrial cancer, HR (95% CI)c | Referent | 0.82 (0.66 to 1.06) | 0.77 (0.63 to 0.97) | 0.70 (0.56 to 0.85) | 0.98 (0.83 to 1.23) |
| Natural indirect effect, through BMI, HR (95% CI)c | Referent | 0.96 (0.92 to 0.99) | 0.86 (0.83 to 0.90) | 0.84 (0.80 to 0.87) | 1.06 (1.03 to 1.12) |
| Natural direct effect, independent of BMI, HR (95% CI)c | Referent | 0.85 (0.68 to 1.08) | 0.90 (0.73 to 1.12) | 0.83 (0.67 to 1.05) | 0.92 (0.76 to 1.17) |
| Proportion mediated by BMI, % (95% CI) | Referent | NE | 59.4 (29.4 to 100.0) | 47.4 (28.5 to 100.0) | NE |
| Nontype I cancers, No. (%)d | 25 (0.4) | 46 (0.4) | 84 (0.4) | 54 (0.4) | 63 (0.4) |
| Total effect of LTPA on endometrial cancer, HR (95% CI)c | Referent | 0.90 (0.10 to 1.43) | 0.90 (0.09 to 1.36) | 0.92 (0.19 to 1.49) | 0.93 (0.09 to 1.62) |
| Natural indirect effect, through BMI, HR (95% CI)c | Referent | 0.96 (0.10 to 1.43) | 0.93 (0.85 to 2.51) | 0.91 (0.82 to 1.34) | 0.95 (0.97 to 1.84) |
| Natural direct effect, independent of BMI, HR (95% CI)c | Referent | 0.93 (0.07 to 1.42) | 0.97 (0.04 to 1.42) | 1.01 (0.20 to 1.72) | 0.89 (0.05 to 1.48) |
| Proportion mediated by BMI, % (95% CI) | Referent | NE | NE | NE | NE |
Participants with little or no physical activity (<1 h/wk) at each age period were classified as inactive; those maintaining low levels of activity over time were classified as maintaining low activity; those maintaining high levels of activity over time were classified as maintaining high activity; those that increased their activity over time were classified as increasers; and those that decreased their activity over time were classified as decreasers. BMI = body mass index; CI = confidence interval; HR = hazard ratio; LTPA = leisure time physical activity; NE = not estimated (ie, the denominator for the proportion was approximately 0 and the estimation was not stable).
Hazard ratios were adjusted for: age (years), race-ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other, or missing), education (less than high school, high school, post high-school or some college, bachelor degree or more, missing), smoking status/dose (never smoker, former smoker and ≤20 cigarettes/day, former smoker and >20 cigarettes/day, current smoker and ≤20 cigarettes/day, current smoker and >20 cigarettes/day, missing), diet quality (2015 Healthy Eating Index; 0-100 points), total energy intake (kcal/day), alcohol consumption (grams/day), parity (number of births), use of oral contraceptives (never or <1 year, 1-4 years, 5-9 years, ≥10 years, missing), menopausal hormone therapy use (never, continuous estrogen plus progestin [EPT] use [15+ days progestin/month], sequential EPT [<15 days progestin/month], estrogen only, missing), and BMI at age 18 years (normal weight [<25.0 kg/m2], overweight [25.0-29.9 kg/m2], obese class I [30.0-34.9 kg/m2], obese class II [35.0-39.9 kg/m2], and obese class III [≥40.0 kg/m2]).
Type I tumor types with histologic codes 8140, 8210, 8260, 8262, 8380, 8382, 8383, 8480, 8481, 8482, 8560, and 8570.
Non-type I cancers include those designated as Type II, other, and unknown histology.
Discussion
Our study indicates that higher levels of physical activity across adulthood were associated with a lower risk for endometrial cancer. Women who were inactive late in adolescence but increased their LTPA by 40-61 years also had a lower risk for endometrial cancer compared with women that were consistently inactive. We found that the benefits of long-term LTPA for endometrial cancer prevention may be primarily explained by the association between higher levels of physical activity and lower risk for obesity (BMI ≥ 30.0 kg/m2) in midlife. Compared with women who were inactive throughout their adult years, women who increased or maintained high amounts of LTPA (6-7 h/wk) had a lower risk for obesity and were less likely to develop endometrial cancer.
Consistent with many previous studies, we found that higher amounts of LTPA were associated with a lower risk for endometrial cancer. Uniquely, we were able to investigate the relation between long-term patterns of physical activity (ie, from adolescence to 40-61 years) and midlife BMI and found that most of the association between long-term LTPA and endometrial cancer was mediated by BMI in midlife. In our study, women accumulating 6-7 h/wk of LTPA by 40-61 years had an approximately 70% lower risk for obesity. Evidence that physical activity can attenuate weight gain among women is compelling. A study of approximately 5000 women, aged 18-23 years, with a healthy BMI at baseline found that after 10 years, 40% had transitioned to the overweight/obese BMI category. However, women doing 3-6 h/wk of LTPA were 20% to 40% more likely to maintain a healthy BMI (33). In the Coronary Artery Risk Development in Young Adults (CARDIA) longitudinal study of 3500 adults aged 25 years at baseline, women reporting the most LTPA (2.5+ h/wk) gained on average 6.1 kg less than their inactive counterparts by midlife (34). This evidence shows that accumulating moderate-high amounts of LTPA prevents weight gain during adulthood and midlife and is aligned with the general recommendations for weight gain prevention issued by the US Department of Health and Human Services and others (10,14,15,35). Our study confirmed these findings and adds new insights by examining physical activity over a 40-year period and its associations with class I-III obesity in midlife, which in turn increased endometrial cancer risk by three- to sixfold in our analysis.
We also found that the timing of women’s activity from late adolescence throughout adulthood may have important implications for endometrial cancer risk. In our study, maintaining high amounts of LTPA across adulthood was associated with a 19% lower risk for endometrial cancer. Women who were inactive late in adolescence but increased their LTPA by 40-61 years also had a 26% lower risk for endometrial cancer. The finding that increasing LTPA in adulthood was associated with lower risk is aligned with the 1 study that examined longitudinal patterns of LTPA and endometrial cancer (23). In a study of 71 570 female nurses aged approximately 50 years, increased LTPA during adulthood was associated with a 37% lower risk for endometrial cancer before adjustment for BMI at midlife (23). Another important finding in our study was that there was little evidence of a benefit of higher activity levels solely in adolescence and early adulthood on later endometrial cancer risk. Women who did not maintain or increase their activity by 40-61 years had a similar risk as women who were consistently inactive. This finding is consistent with previous studies (23). A few other studies have assessed both earlier adulthood and current or baseline physical activity, but these were either limited to short-term changes in LTPA during midlife (22) or did not assess if the association between long-term physical activity and endometrial cancer varied across activity patterns (eg, increasers vs maintainers) (20,21). Our study adds additional evidence that promoting physical activity among inactive middle-aged women may be an important strategy for preventing endometrial cancer.
Our study has limitations that should be considered. First, we used self-reported measures of long-term LTPA and BMI, which added measurement error that could have resulted in attenuation of the associations reported in our study (36). Based on previous examinations, we expect that LTPA items used in this study have adequate reliability (r = 0.5-0.6) (25), similar to other questionnaires of historical physical activity (r = 0.4-0.8) (37–39). Previous studies have also shown that women tend to underreport their BMI (by approximately 1.0 kg/m2) but that self-reported BMI is highly correlated with measured BMI (r = approximately 0.90) (40,41). Second, our results are limited to women who were enrolled in the NIH-AARP study and may not be generalizable to women with different demographics and health backgrounds. Third, although we controlled for dietary intake at cohort entry or baseline, we cannot rule out the contribution of diet earlier in life on BMI in midlife (42–46). Fourth, we were also limited in our ability to adjust for potential confounders that could have affected both early adulthood and midlife BMI. To minimize these concerns, we: 1) established a 40-year temporal sequence between long-term activity and BMI in midlife, 2) adjusted for BMI at 18 years or early in adulthood, and 3) examined our associations across patterns of long-term activity by endometrial tumor dualistic subtype and stratified by BMI at age 18 years. Finally, our mediation results may also reflect the complex cumulative effect that long-term physical activity has on BMI across the adult life course and not only at midlife. A longitudinal mediation design with multiple measures of LTPA and BMI can potentially disentangle some of the LTPA-BMI relationships during adulthood; however, in our study, we were limited by the lower number of endometrial cancers as demonstrated by the imprecise estimates (ie, wide confidence intervals) in some of our mediation estimates.
These findings support previous evidence of the benefits of physical activity for cancer prevention (14,47,48) and highlight the importance of maintaining regular participation in physical activity throughout adulthood. Among women who are inactive, adopting LTPA later in adulthood (40-61 years) may still confer important health benefits. These findings provide encouragement to the 51% of adult women in the United States who are currently inactive (49) that is not too late to start an exercise program or routine. Further, given the age-related increases in body weight often observed, LTPA can be prescribed to attenuate unhealthy weight gain, a risk factor for endometrial cancer. Our study adds important information for clinicians, suggesting they should promote LTPA to prevent weight gain and reduce the risk for endometrial cancer.
Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors report no conflicts of interest.
Author contributions: PSM handled data curation, formal analysis, and wrote the original version of the manuscript. JNS, BT and CEM assisted with conceptualization, formal analysis, writing the original draft of the manuscript, and supervised the work. KAM, SCM, and EL, participated in the conceptualization of the study, methodology, and reviewing/editing the manuscript. KC and MBC contributed to the methodology used in the study and reviewing/editing the manuscript.
Disclaimers: The views expressed herein are solely those of the authors and do not necessarily reflect those of the Florida Cancer Data System or the Florida Department of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Acknowledgements: Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under contract with the Florida Department of Health, Tallahassee, Florida. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, The Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, Nevada.
Data Availability
Data are maintained by the National Cancer Institute, Division of Cancer Epidemiology and Genetics and are available upon submitting a proposal to be approved by the NIH-AARP Steering Committee. For more information visit https://www.nihaarpstars.com/.
Supplementary Material
Contributor Information
Pedro F Saint-Maurice, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Joshua N Sampson, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Kara A Michels, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Steven C Moore, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Erikka Loftfield, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Kathleen McClain, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Michael B Cook, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Britton Trabert, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Charles E Matthews, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Henley SJ, Ward EM, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2020;126(10):2225–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook LS, Meisner ALW, Weiss NS, eds. Endometrial cancer. In: Cancer Epidemiology and Prevention. 4th ed. New York, NY: Oxford University Press; 2018:909–923. [Google Scholar]
- 4.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L.. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet (London, England). 2014;384(9945):755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Kaaks R.. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. [DOI] [PubMed] [Google Scholar]
- 6.Onstad MA, Schmandt RE, Lu KH.. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34(35):4225–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felix AS, Brinton LA.. Cancer progress and priorities: uterine cancer. Cancer Epidemiol Biomark Prev. 2018;27(9):985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL.. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL.. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22(1):39–47. [DOI] [PubMed] [Google Scholar]
- 10.Saris WH, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4(2):101–114. [DOI] [PubMed] [Google Scholar]
- 11.Matthews CE, Moore SC, Arem H, et al. Amount and intensity of leisure-time physical activity and lower cancer risk. J Clin Oncol. 2020;38(7):686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M.. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30(5):397–412. [DOI] [PubMed] [Google Scholar]
- 14.Physical Activity Guidelines for Americans Committee. Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- 15.Jakicic JM, Powell KE, Campbell WW, et al. Physical activity and the prevention of weight gain in adults: a systematic review. Med Sci Sports Exer. 2019;51(6):1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maliniak ML, Gapstur SM, McCullough LE, et al. Joint associations of physical activity and body mass index with the risk of established excess body fatness-related cancers among postmenopausal women. Cancer Causes Control. 2021;32(2):127–138. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E.An integrative approach for deciphering the causal associations of physical activity and cancer risk: the role of adiposity. J Natl Cancer Inst. 2018;110(9):935–941. [DOI] [PubMed] [Google Scholar]
- 18.Keum N, Ju W, Lee DH, et al. Leisure-time physical activity and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2014;135(3):682–694. [DOI] [PubMed] [Google Scholar]
- 19.McTiernan A, Wu L, Chen C, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring, MD). 2006;14(9):1662–1677. [DOI] [PubMed] [Google Scholar]
- 20.Dieli-Conwright CM, Ma H, Lacey JV Jr, et al. Long-term and baseline recreational physical activity and risk of endometrial cancer: the California Teachers Study. Br J Cancer. 2013;109(3):761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gierach GL, Chang SC, Brinton LA, et al. Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2009;124(9):2139–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel AV, Feigelson HS, Talbot JT, et al. The role of body weight in the relationship between physical activity and endometrial cancer: results from a large cohort of US women. Int J Cancer. 2008;123(8):1877–1882. [DOI] [PubMed] [Google Scholar]
- 23.Du M, Kraft P, Eliassen AH, Giovannucci E, Hankinson SE, De Vivo I.. Physical activity and risk of endometrial adenocarcinoma in the Nurses' Health Study. Int J Cancer. 2014;134(11):2707–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: The National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. [DOI] [PubMed] [Google Scholar]
- 25.Saint-Maurice PF, Coughlan D, Kelly SP, et al. Association of leisure-time physical activity across the adult life course with all-cause and cause-specific mortality. JAMA Netw Open. 2019;2(3):e190355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: Report of a WHO consultation; 2000. https://apps.who.int/iris/handle/10665/42330. Accessed August 21, 2020. [PubMed]
- 27.Michaud DS, Midtune D, Hermansen S, et al. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Regist Manag. 2005;32(2):70–75. [Google Scholar]
- 28.World Health Organization. International Classification of Disease for Oncology (ICD-O). Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 29.Jones BL, Nagin DS, Roeder K.. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393. [Google Scholar]
- 30.Arem H, Loftfield E, Saint-Maurice PF, Freedman ND, Matthews CE.. Physical activity across the lifespan and liver cancer incidence in the NIH-AARP Diet and Health Study cohort. Cancer Med. 2018;7(4):1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lange T, Hansen KW, Sorensen R, Galatius S.. Applied mediation analyses: a review and tutorial. Epidemiol Health. 2017;39:e2017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setiawan VW, Yang HP, Pike MC, et al. ; The Australian National Endometrial Cancer Study Group. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown WJ, Kabir E, Clark BK, Gomersall SR.. Maintaining a healthy BMI: data from a 16-year study of young Australian women. Am J Prev Med. 2016;51(6):e165–e178. [DOI] [PubMed] [Google Scholar]
- 34.Hankinson AL, Daviglus ML, Bouchard C, et al. Maintaining a high physical activity level over 20 years and weight gain. JAMA. 2010;304(23):2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.United States Department of Health and Human Services. Physical Activity Guidelines for Americans. Washington, DC; 2018. [Google Scholar]
- 36.Hutcheon JA, Chiolero A, Hanley JA.. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. [DOI] [PubMed] [Google Scholar]
- 37.Smith AW, Cronin KA, Bowles H, et al. Reproducibility of physical activity recall over fifteen years: longitudinal evidence from the CARDIA Study. BMC Public Health. 2013;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS.. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155(3):282–289. [DOI] [PubMed] [Google Scholar]
- 39.Evenson KR, Wilcox S, Pettinger M, et al. ; Women's Health Initiative Observational Cohort Study. Vigorous leisure activity through women's adult life: the Women's Health Initiative Observational Cohort Study. Am J Epidemiol. 2002;156(10):945–953. [DOI] [PubMed] [Google Scholar]
- 40.McAdams MA, Van Dam RM, Hu FB.. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring). 2007;15(1):188–196. [DOI] [PubMed] [Google Scholar]
- 41.Willett W, Hu F.. Anthropometric measures and body composition. In: Willett W, ed. Nutritional Epidemiology. New York: Oxford University Press; 2013:213–259. [Google Scholar]
- 42.Hu FB.Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14(8):606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenheck R.Fast food consumption and increased caloric intake: a systematic review of a trajectory towards weight gain and obesity risk. Obes Rev. 2008;9(6):535–547. [DOI] [PubMed] [Google Scholar]
- 44.Ello-Martin JA, Ledikwe JH, Rolls BJ.. The influence of food portion size and energy density on energy intake: implications for weight management. Am J Clin Nutr. 2005;82(suppl 1):236S–241S. [DOI] [PubMed] [Google Scholar]
- 45.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB.. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendonca RD, Pimenta AM, Gea A, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) Cohort Study. Am J Clin Nutr. 2016;104(5):1433–1440. [DOI] [PubMed] [Google Scholar]
- 47.Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Cancer Research Fund. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. London, UK; 2018.
- 49.Centers for Disease Control and Prevention. Fastats: Exercise or Physical Activity. 2018. https://www.cdc.gov/nchs/fastats/exercise.htm. Accessed December 8, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are maintained by the National Cancer Institute, Division of Cancer Epidemiology and Genetics and are available upon submitting a proposal to be approved by the NIH-AARP Steering Committee. For more information visit https://www.nihaarpstars.com/.