Version Changes
Revised. Amendments from Version 1
In this version, additional details of the model have bene included in the main text, figure legends and the figures themselves, in response to the suggestions of the reviewers. Furthermore, as per the comments of reviewer 2, language has been added to emphasize the theoretical character of this work that cannot be used directly to make quantitative predictions on the course of any particular epidemic.
Abstract
Background: The start of 2021 was marked by the initiation of a global vaccination campaign against the novel coronavirus SARS-CoV-2. Formulating an optimal distribution strategy under social and economic constraints is challenging. Optimal distribution is additionally constrained by the potential emergence of vaccine resistance. Analogous to chronic low-dose antibiotic exposure, recently inoculated individuals who are not yet immune play an outsized role in the emergence of resistance. Classical epidemiological modelling is well suited to explore how the behavior of the inoculated population impacts the total number of infections over the entirety of an epidemic.
Methods: A deterministic model of epidemic evolution is analyzed, with seven compartments defined by their relationship to the emergence of vaccine-resistant mutants and representing three susceptible populations, three infected populations, and one recovered population. This minimally computationally intensive design enables simulation of epidemics across a broad parameter space. The results are used to identify conditions minimizing the cumulative number of infections.
Results: When an escape variant is only modestly less infectious than the originating strain within a naïve population, the cumulative number of infections does not monotonically decrease with the rate of vaccine distribution. Analysis of the model also demonstrates that inoculated individuals play a major role in the mitigation or exacerbation of vaccine-resistant outbreaks. Modulating the rate of host–host contact for the inoculated population by less than an order of magnitude can alter the cumulative number of infections by more than 20%.
Conclusions: Mathematical modeling shows that limiting post-vaccination contacts can perceptibly affect the course of an epidemic. The consideration of limitations on post-vaccination contacts remains relevant for the entire duration of any vaccination campaign including the current status of SARS-CoV-2 vaccination.
Keywords: Virus infection; epidemics; vaccination; escape mutants; contact limitation
Introduction
The emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the COVID-19 pandemic motivated dramatic public health intervention including recommendations for isolation and quarantine throughout most of 2020 and early 2021. 1 The beginning of 2021 was marked by widespread vaccine distribution which continues at an accelerating pace at the time of this writing. Optimizing distribution is challenging and subject to a myriad of social and economic constraints. 2 – 4 The potential emergence of vaccine-resistant variants of the virus 5 , 6 introduces additional complications. Vaccination applies new selective pressures which can lead to diverse intermediate outcomes even under conditions admitting eventual pathogen eradication. 7 – 13 The larger the size of the vaccinated population, the greater the pressure towards escape of vaccine-resistant variants.
Escape variants emerge within individual hosts after infection with the originating strain. Naïve, unvaccinated, hosts are more easily infected than vaccinated hosts but mutations conferring resistance are unlikely to provide a selective advantage in the naïve background. Thus, naïve hosts are likely to shed escape variants at low, likely, negligible rates. The reverse is true for vaccinated hosts. Recently vaccinated, inoculated, hosts that are not yet immune are a key population to consider. This population remains highly susceptible to infection with the originating strain, and in these hosts, mutations conferring resistance are more likely to provide a selective advantage. As a result, a substantial fraction or even most of the virus shed by such hosts will be resistant mutants. This situation is analogous to the administration of a low-dose antibiotic regime. 14 , 15 In both cases, the pathogen is introduced to a susceptible host and is subject to elevated selective pressure towards the emergence of resistant (escape) variants.
We sought to identify constraints on the reduction of the cumulative number of infections that might be imposed by vaccine escape and the role played by the small, but critical, population of inoculated hosts. To this end, we constructed an epidemiological compartment model to simulate vaccination campaigns over a broad parameter regime. This minimally computationally intensive approach enabled us to simulate many possible scenarios for epidemic evolution, in order to determine the optimal vaccination strategy for each condition.
Methods
We divided the population into seven compartments ( Figure 1A). Three compartments are susceptible to infection by either the originating strain or escape mutants: naïve ( N; unvaccinated and fully susceptible to the originating strain and escape mutants), inoculated ( I; recently vaccinated and still partially susceptible to the originating strain, and fully susceptible to escape mutants), and vaccinated ( V; minimally susceptible to the originating strain, but fully susceptible to escape mutants). Two compartments represent ongoing infection with the originating strain and are distinguished by the host’s previous compartment: infected–naïve ( F, “Founder”) and infected–inoculated ( M, “Mixed”). The third infected compartment represents infection by an escape variant, infected–escape ( E). The remaining compartment, recovered ( R), contains all hosts that were previously infected and are no longer infectious. Vaccination is represented by a reduction in susceptibility to infection with the originating strain. Naïve hosts are inoculated at rate k V. Inoculated hosts do not immediately acquire immunity and mature into the vaccinated compartment at rate k M. All infected hosts recover at rate k R.
Figure 1. The model.

A. Schematic of the seven-compartment model with three susceptible, three infected, and one recovered compartments. Rates (divided by the state at the beginning of the arrow) are displayed. B. Simulated epidemics for k I = [0.15,0.175,0.2 (solid line), 0.225,0.25], α = 0.001, β = 0.01. C. The ratio of cumulative escape infections to all cumulative infections for an epidemic over a range of α. β = [0.5,0.67,0.83,1], darker color indicates higher value, k I = 0.2. The dotted line specifies the benchmark value of α = 0.001.
Within the timescale of the model, recovery is assumed to grant stable immunity, and any variation in population size due to birth/death is assumed to be negligible. It should be noted that, if recovery from the Infected-Naïve or Infected-Inoculated compartments does not confer immunity against escape infection, the key results in this work will have an even greater impact on the vaccination outcome. Hosts come into contact at rate k C. For simplicity, we assume that contact with an escape-infected host can only produce an escape infection. Also, vaccine efficacy is assumed to be perfect such that vaccinated hosts cannot be infected with the originating strain. The inoculated–infected compartment is assumed to represent a symmetric composition of escape and originating infections such that the total probability of a naïve or inoculated host being infected after contact with a naïve–infected or inoculated–infected host is the same. Finally, we assume that the probability of escape infection is the same for naïve and vaccinated hosts across all three types of infected-susceptible host interactions. This construction yields the following transition probability matrices for naïve, inoculated, and vaccinated hosts:
where k I represents the rate of infection for Naïve, Infected-Naïve host interactions which is determined both by the contact rate k C and the infectivity of the originating strain. Rows represent interaction with each of the infected compartments (infected–naïve, infected–inoculated, and infected–escape). Columns represent transitions to each of the infected compartments.
An escape mutant can emerge in an infected–naïve or infected–inoculated host. The parameter α represents the infectivity of the escape variant relative to the originating strain when a naïve host interacts with an infected–naïve host. The parameter β represents the infectivity of an escape variant when a naïve host interacts with an infected–escape host relative to the infectivity of the originating strain when a naïve host interacts with an infected–naïve host. Informally, α reflects the ratio of escape variant to originating strain shed by infected–naïve hosts, whereas β reflects the fitness of an escape variant relative to the originating strain. Finally, we introduce the parameter q to represent the impact of varying the rate of host-host contact for Inoculated hosts relative to that for the other compartments. q>1 represents increased contact, and q < 1 corresponds to decreased contact. This completes the model description and structures the differential equations:
k R = k M = 1/7 are fixed across all simulations representing a time to recovery and time between inoculation and the acquisition of immunity of one week. Reducing k I would prolong the epidemic and reducing k M would increase the size of the inoculated compartment.
k I is the principal determinant of epidemic magnitude and duration, with larger k I leading to a greater cumulative number of infections over a shorter period of time ( Figure 1B). However, feedback between the size of the infected population and the rate of host–host contact as well as spatial structure can decouple these variables. Throughout this work, k I is set to a benchmark value of 0.2 resulting in 50% of the population being infected over a period of approximately 4 months.
The values of α and β impact the size of the infected–escape compartment. Even in the absence of vaccination, large α/ β results in the emergence of would-be resistant variants ( Figure 1C). In all analyses in this work, α is fixed at the benchmark value of 0.001 resulting in a modest number of would-be resistant infections for β close to 1 in the absence of vaccination. Although a larger α would result in a greater total number of escape infections, the fraction of those infections attributable to contact with inoculated hosts would be smaller.
The solutions of the ordinary differential equations (ODEs) were obtained using the MATLAB ode45 method. 16 ode45 is based on the Dormand-Prince pair, 17 an explicit Runge-Kutta formula which could be implemented in a variety of open-access alternatives. Epidemics are simulated until the size of the Recovery compartment at arbitrarily long times is approached. The principal quantity of interest is the cumulative number of infections. When k V is selected to minimize this value, minima are found through explicit simulation over a range of rates. In the subsequent analysis, some values are expressed relative to the cumulative number of infections in the absence of vaccination, R Null ~ 50%.
Results
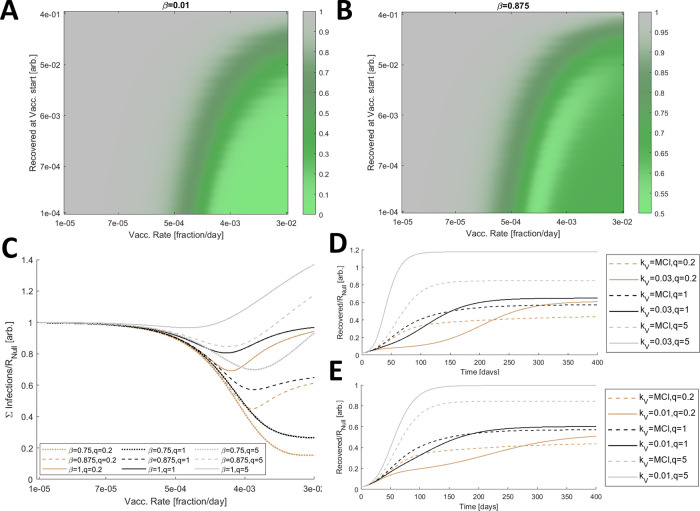
In addition to the rate of vaccination, the outcome of a vaccination campaign depends on how far the epidemic has progressed before vaccination begins, which can be measured by the relative size of the recovered compartment. The results also depend on β, informally, the fitness of an escape variant relative to the originating strain. We considered two values for β (low: 0.01; high: 0.875) and varied both the start and the rate of vaccination. When β is low, that is, the escape mutant is much less fit than the originating strain ( Figure 2A), vaccinating earlier and distributing the vaccine faster decreases the cumulative number of infections. If distribution is sufficiently prompt, the cumulative number of infections becomes negligible.
Figure 2. Optimal vaccine distribution.
A. The cumulative number of infections relative to no vaccination, R Null , for a range of vaccine initiations and distribution rates. Here β is low, 0.01. B. The cumulative number of infections relative to no vaccination, R Null , for a range of vaccine initiations and distribution rates and a large β (0.875). A/B. 3840 values were computed for each panel and 4× by 4× bilinearly interpolated points are displayed. Note that the color axis differs between A and B. C. The cumulative number of infections relative to no vaccination, R Null , for β = [0.75 (dotted), 0.875 (dashed), 1 (solid)] and three relative contact rates, q = [0.2 (brown), 1 (black), 5 (gray)]. D. Simulated epidemics comparing a high fixed rate of vaccination, k V = 0.03 (solid) representing the inoculation of 3% of naïve hosts per day, and the vaccination rate that minimizes the cumulative number of infections, MCI, each condition (dashed). β = 0.875 is fixed and q = [0.2 (brown), 1 (black), 5 (gray)]. E. Same as D. with a low fixed rate of vaccination, k V = 0.01. C/D/E. The minima for the dashed lines in C correspond to the dashed lines in D/E.
However, the outcome substantially differs for high β ( Figure 2B). In this case, the cumulative number of infections does not monotonically decrease with increasing vaccination rate due to the enhanced selective pressure for the emergence of escape variants, and the minimum cumulative number of infections is still substantial. In all subsequent analyses, the vaccination rate is varied but vaccination is fixed to begin when 1% of the population has recovered from infection.
Infections can be mitigated by reducing contacts among the hosts. We sought to determine how perturbing the contact rates for hosts in the inoculated compartment relative to that of all other compartments, q, affects the outcome. For β ranging between 0.75 and 1, we considered three relative contact rates, q = [0.2,1,5] ( Figure 2C). Increasing the rate of host–host contact only within this compartment has a significant impact on the cumulative number of infections. The rate, at which the minimum cumulative number of infections, is achieved, is also perturbed. Furthermore, if k V exceeds MCI, reducing q below 1 slows the accumulation of infections ( Figure 2D). The converse is true for increasing q, and the landscape is similar when k V is less than MCI ( Figure 2E).
Having demonstrated how q perturbs MCI and how reducing q below 1 can mitigate or delay the accumulation of infections even if this rate is not met or, conversely, is exceeded, we sought to establish the impact of q on the cumulative number of infections across a wide range of β given k V = MCI for each condition ( Figure 3A). The cumulative number of infections is sensitive to q across the entire range of β. Varying q within an order of magnitude can substantially aid or hinder the vaccination campaign, and when q>>1, k V = MCI = 0. Note that the maximum vaccination rate considered is k V=0.03 representing the inoculation of 3% of naïve hosts per day. This rate is sufficiently high that the cumulative number of infections at this rate ( Figure 3B) is similar or higher compared with the hypothetical case where the entire naïve population is immediately inoculated ( Figure 3C). Some of these effects are not specific to the inoculated compartment. Increasing the rate of contact for any arbitrary subpopulation can increase the cumulative number of infections. We define the effective k I , k Ieff, such that the cumulative number of infections for k I = k Ieff and q = 1 is equal to the cumulative number of infections for k I = 0.2 (the benchmark) and arbitrary q. Increasing q within an order of magnitude is equivalent to substantially increasing k I for the entire population for the entirety of the epidemic ( Figure 3D).
Figure 3. Post-vaccination contacts.
A. The cumulative number of infections relative to no vaccination, R Null , for a range of β and q, where k V = MCI minimizes the cumulative number of infections for each condition. B. Same as in A. for fixed k V = 0.03 representing the inoculation of 3% of naïve hosts per day. C. Same as in A. for the case where all naïve hosts are immediately inoculated. D. Log of the effective k I , k Ieff, relative to k I = 0.2, the benchmark, versus log of q for b = [0.5 (dotted line), 0.7 (dashed line), 1 (solid line)]. E. The cumulative number of infections relative to R Null and q = 1 scaled by the fraction of infections due to vaccine escape, ∑Escape( q)/∑Infections( q)(∑Infections( q)-∑Infections ( q = 1))/ R Null , for a range of β and q, where k V = MCI for each condition. F. Same as in E for fixed k V = 0.03. A-C/E/F. 961 values were computed for each panel and 4× by 4× bilinearly interpolated points are displayed.
We additionally consider the cumulative infections added or subtracted relative to the outcome corresponding to q = 1 and scaled by the fraction of infections due to vaccine escape ( Figure 3E). Varying q within an order of magnitude alters the cumulative number of infections added or subtracted by more than 20% of the cumulative number of infections in the absence of vaccination, R Null , again demonstrating the critical role played by inoculated hosts with respect to vaccine escape. The landscape is similar when then rate of vaccination is fixed ( Figure 3F).
Discussion
Epidemics can be mitigated through the reduction of contact among the hosts via quarantines and other similar measures, and vaccination. A reduction in contact carries non-negligible social and economic burdens so that, when vaccination becomes possible, the continuation of such interventions might appear unnecessarily costly. Formulating the optimal public health response to balance these pressures is challenging and is further complicated by the possibility of vaccine escape. Escape variants emerging as a result of vaccination are likely to be less infectious than the originating strain. More infectious variants, which are also capable of vaccine escape, would likely already be in circulation. Indeed, multiple variants of SARS-CoV-2 capable of antibody evasion emerged prior to the onset of mass vaccination, 18 – 23 indicating that this virus has a large mutational repertoire for evading antibodies while maintaining host receptor binding.
However, newly emergent variants after the onset of mass vaccination can still be substantially infectious and result in non-negligible disease incidence. 24 , 25 Here, we demonstrate that, even when escape variants are modestly less infectious than the originating strain, the cumulative number of infections does not monotonically decrease with the rate of vaccine distribution. This rate minimizing the cumulative number of infections, k V = MCI, depends on the infectivity of the escape variants. To our knowledge this phenomenon, analogous to the evolution of antibiotic resistance, is not widely appreciated and, as such, seems to warrant consideration. However, minimizing the cumulative number of infections does not necessarily constitute the “optimal” vaccine distribution regime (see Limitations).
Of more practical concern is the role of inoculated hosts in the emergence of escape variants. Within low-dose antibiotic regimes, 14 , 15 hosts are susceptible to infection with the originating variant, and in such hosts, the pathogen is subjected to elevated selective pressures towards the emergence of resistance. Similarly, within inoculated hosts, the virus is subjected to gradually increasing selective pressures towards the emergence of resistance while the intra-host population remains sufficiently large to explore a substantial fraction of the mutation space. We demonstrate that moderately increasing or decreasing the host-host contact rates for inoculated hosts only can substantially aid or hinder the vaccination campaign. The time between vaccination and the acquisition of immunity can be readily approximated from clinical endpoints 26 , 27 and is likely to be short enough that the societal costs of limiting post-vaccination contact would be outweighed by these benefits.
Limitations
In this study, we leveraged classical modelling techniques to elucidate the factors that could substantially impact the outcome of any vaccination campaign; however the model presented here is not designed to forecast long-term outcome, a topic that has been thoroughly addressed for the case of SARS-Cov-2 and more generally. 7 – 13 , 28 – 30 Furthermore, it is important to emphasize that this is a theoretical analysis. Although we believe that our results highlight under appreciated aspects of vaccine distribution and that a thorough understanding of the features discussed here is critical for optimizing vaccine distribution, this work cannot be directly leveraged to make quantitative predictions about the ongoing SARS-CoV-2 pandemic.
Conclusions
The cumulative number of infections does not monotonically decrease with the rate of vaccine distribution. Contact rates for recently vaccinated and not yet fully immune (inoculated) hosts can have a substantial impact on the outcomes of vaccination campaigns. Even a brief and moderate limitation of contacts in this well-defined population can potentially mitigate epidemics.
The results presented here appear to be of immediate interest in relation to the vaccination campaign against SARS-CoV-2, which is ongoing at the time of this writing. Diversification of the virus is apparent 31 – 33 and, as discussed above, antibody evasion had been investigated early on and demonstrated prior to mass vaccination. 5 , 6 , 18 – 23 , 34 , 35 The existence of these variants indicates that evolution of the SARS-CoV-2 antigen is not subject to constraints that would prohibit reduction in antibody affinity to achieve immune evasion, while maintaining host receptor affinity sufficient for infection. In other words, the emergence of novel, infectious, vaccine-resistant variants remains possible if not probable for the duration of the ongoing vaccination campaign and beyond.
Obviously, virus evolution during a pandemic is a fast-moving target, so that some aspects of this analysis unavoidably will be outdated by the time of publication. In particular, vaccine efficiency is assumed to be perfect within this model, reflecting the expectation as of December 2020. As of March 2021, this is no longer the case. In this work, we emphasize the role played by individuals who recently received their vaccination. The selective environment within such a partially susceptible host in the days immediately following the administration of a perfect vaccine is not completely equivalent to that within a host that remains partially susceptible in the months following the administration of an imperfect vaccine. Nonetheless, we believe that this and related work 36 , 37 unequivocally demonstrates the continued importance of reducing host-host contact well after the onset of mass vaccination.
Author contributions
Conceptualization and Formal Analysis: NDR, YIW, and EVK
Writing – Original Draft Preparation: NDR and EVK
Writing – Review & Editing: NDR, YIW, and EVK
Data availability
Underlying data
No data are associated with this article.
Acknowledgements
The authors thank Koonin group members for helpful discussions.
Funding Statement
U.S. Department of Health and Human Services. NDR, YIW, and EVK are supported by the Intramural Research Program of the National Institutes of Health (National Library of Medicine).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1.CDC: Public Health Guidance for Potential COVID-19 Exposure Associated with Travel. 2020.
- 2.Neumann-Böhme S, Varghese NE, Sabat I, et al. : Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. The European Journal of Health Economics. Springer;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn SC, Kumar S, Freimuth VS, et al. : Public willingness to take a vaccine or drug under Emergency Use Authorization during the 2009 H1N1 pandemic. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science. 2009;7(3):275–290. 10.1089/bsp.2009.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills MC, Salisbury D: The challenges of distributing COVID-19 vaccinations. EClinicalMedicine. 2020. 10.1016/j.eclinm.2020.100674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Wu J, Nie J, et al. : The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 2020;182(5):1284–94. e9. 10.1016/j.cell.2020.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy DA, Read AF: Monitor for COVID-19 vaccine resistance evolution during clinical trials. PLoS Biol. 2020;18(11):e3001000. 10.1371/journal.pbio.3001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magpantay F, King A, Rohani P: Age-structure and transient dynamics in epidemiological systems. Journal of the Royal Society Interface. 2019;16(156):20190151. 10.1098/rsif.2019.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Restif O, Grenfell BT: Integrating life history and cross-immunity into the evolutionary dynamics of pathogens. Proceedings of the Royal Society B: Biological Sciences. 2006;273(1585):409–416. 10.1098/rspb.2005.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues HS, Monteiro MTT, Torres DF: Vaccination models and optimal control strategies to dengue. Math Biosci. 2014;247:1–12. 10.1016/j.mbs.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 10.Safan M, Kretzschmar M, Hadeler KP: Vaccination based control of infections in SIRS models with reinfection: special reference to pertussis. J Math Biol. 2013;67(5):1083–1110. 10.1007/s00285-012-0582-1 [DOI] [PubMed] [Google Scholar]
- 11.Scherer A, McLean A: Mathematical models of vaccination. British Medical Bulletin. 2002;62(1):187–199. 10.1093/bmb/62.1.187 [DOI] [PubMed] [Google Scholar]
- 12.van Boven M, Mooi FR, Schellekens JF, Melker HE, Kretzschmar M: Pathogen adaptation under imperfect vaccination: implications for pertussis. Proceedings of the Royal Society B: Biological Sciences 2005;272(1572):1617–1624. 10.1098/rspb.2005.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandon S, Day T: The evolutionary epidemiology of vaccination. Journal of the Royal Society Interface. 2007;4(16):803–817. 10.1098/rsif.2006.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livermore DM: Minimising antibiotic resistance. The Lancet Infectious Diseases. 2005;5(7):450–459. 10.1016/S1473-3099(05)70166-3 [DOI] [PubMed] [Google Scholar]
- 15.Roberts JA, Kruger P, Paterson DL, et al. : Antibiotic resistance—what’s dosing got to do with it? Crit Care Med. 2008;36(8):2433–2440. 10.1097/CCM.0b013e318180fe62 [DOI] [PubMed] [Google Scholar]
- 16.Shampine LF, Reichelt MW: The matlab ode suite. SIAM Journal on Scientific Computing. 1997;18(1):1–22. 10.1137/S1064827594276424 [DOI] [Google Scholar]
- 17.Dormand JR, Prince PJ: A family of embedded Runge-Kutta formulae. Journal of Computational and Applied Mathematics. 1980;6(1):19–26. 10.1016/0771-050X(80)90013-3 [DOI] [Google Scholar]
- 18.Faria NR, Claro IM, Candido D, et al. : Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. Virological. 10.1126/science.abh2644 [DOI] [Google Scholar]
- 19.Tegally H, Wilkinson E, Giovanetti M, et al. : Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020. 10.1101/2020.12.21.20248640 [DOI] [Google Scholar]
- 20.Voloch CM, Ronaldo da Silva F, Almeida LG, et al. : Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. medRxiv 2020. 10.1128/JVI.00119-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Davis BD, Chen SS, et al. : Emergence of a Novel SARS-CoV-2 Variant in Southern California. JAMA. 2021. 10.1001/jama.2021.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.England PH: Variants: distribution of cases data.
- 23.Brejová B, Hodorová V, Boršová K, et al. : B. 1.258_Delta, a SARS-CoV-2 variant with Delta_H69, Delta_V70 in the Spike protein circulating in the Czech Republic and Slovakia. arXiv preprint arXiv:210204689 2021.
- 24.Carman WF, Karayiannis P, Waters J, et al. : Vaccine-induced escape mutant of hepatitis B virus. The Lancet. 1990;336(8711):325–329. 10.1016/0140-6736(90)91874-a [DOI] [PubMed] [Google Scholar]
- 25.Brueggemann AB, Pai R, Crook DW, et al. : Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 2007;3(11):e168. 10.1371/journal.ppat.0030168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polack FP, Thomas SJ, Kitchin N, et al. : Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Zeng G, Pan H, et al. : Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases. 2020. 10.1016/S1473-3099(20)30831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saad-Roy CM, Wagner CE, Baker RE, et al. : Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020;370(6518):811–818. 10.1126/science.abd7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day T, Gandon S, Lion S, et al. : On the evolutionary epidemiology of SARS-CoV-2. Curr Biol. 2020. 10.1016/j.cub.2020.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochman ND, Wolf YI, Koonin EV: Evolution of Human Respiratory Virus Epidemics. medRxiv 2020. 10.1101/2020.11.23.20237503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Tao Q, Weaver S, et al. : An evolutionary portrait of the progenitor SARS-CoV-2 and its dominant offshoots in COVID-19 pandemic. bioRxiv 2020. [DOI] [PMC free article] [PubMed]
- 32.Rochman ND, Wolf YI, Faure G, Zhang F, Koonin EV: Ongoing Global and Regional Adaptive Evolution of SARS-CoV-2. bioRxiv 2020. [DOI] [PMC free article] [PubMed]
- 33.Dorp L, Acman M, Richard D, et al. : Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infection, Genetics, Evolution 2020;104351. 10.1016/j.meegid.2020.104351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrett ME, Galloway J, Chu HY, et al. : High resolution profiling of pathways of escape for SARS-CoV-2 spike-binding antibodies. bioRxiv 2020. [DOI] [PMC free article] [PubMed]
- 35.Starr TN, Greaney AJ, Hilton SK, et al. : Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell 2020;182(5):1295–310. e20. 10.1016/j.cell.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makhoul M, Ayoub HH, Chemaitelly H, et al. : Epidemiological impact of SARS-CoV-2 vaccination: mathematical modeling analyses. Vaccines. 2020;8(4):668. 10.3390/vaccines8040668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rella S, Kulikova Y, Dermitzakis E, Kondrashov F: SARS-CoV-2 transmission, vaccination rate and the fate of resistant strains. medRxiv 2021. [DOI] [PMC free article] [PubMed]