Abstract
Background
People living with chronic kidney disease (CKD) experience a range of symptoms and often have complex comorbidities. Many pharmacological interventions for people with CKD have known risks of adverse events. Acupuncture is widely used for symptom management in patients with chronic diseases and in other palliative care settings. However, the safety and efficacy of acupuncture for people with CKD remains largely unknown.
Objectives
We aimed to evaluate the benefits and harms of acupuncture, electro‐acupuncture, acupressure, moxibustion and other acupuncture‐related interventions (alone or combined with other acupuncture‐related interventions) for symptoms of CKD. In particular, we planned to compare acupuncture and related interventions with conventional medicine, active non‐pharmacological interventions, and routine care for symptoms of CKD.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register up to 28 January 2016 through contact with the Information Specialist using search terms relevant to this review. We also searched Korean medical databases (including Korean Studies Information, DBPIA, Korea Institute of Science and Technology Information, Research Information Centre for Health Database, KoreaMed, the National Assembly Library) and Chinese databases (including the China Academic Journal).
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that investigated the effects of acupuncture and related point‐stimulation interventions with or without needle penetration that involved six sessions or more in adults with CKD stage 3 to 5, regardless of the language and type of publication. We excluded studies that used herbal medicine or co‐interventions administered unequally among the study groups.
Data collection and analysis
Two authors independently extracted data and assessed risk of bias. We calculated the mean difference (MD) or standardised mean difference (SMD) with 95% confidence intervals (CI) for continuous outcomes and risk ratio (RR) for dichotomous outcomes. Primary outcomes were changes in pain and depression, and occurrence of serious of adverse events.
Main results
We included 24 studies that involved a total of 1787 participants. Studies reported on various types of acupuncture and related interventions including manual acupuncture and acupressure, ear acupressure, transcutaneous electrical acupuncture point stimulation, far‐infrared radiation on acupuncture points and indirect moxibustion. CKD stages included pre‐dialysis stage 3 or 4 and end‐stage kidney disease on either haemodialysis or peritoneal dialysis.
None of the included studies assessed pain outcomes, nor formally addressed occurrence of serious adverse events, although three studies reported three participant deaths and three hospitalisations as reasons for attrition. Three studies reported minor acupuncture‐related harms; the remainder did not report if those events occurred.
All studies were assessed at high or unclear risk of bias in terms of allocation concealment. Seventeen studies reported outcomes measured for only two months.
There was very low quality of evidence that compared with routine care, manual acupressure reduced scores of the Beck Depression Inventory score (scale from 0 to 63) (3 studies, 128 participants: MD ‐4.29, 95% CI ‐7.48 to ‐1.11, I2 = 0%), the revised Piper Fatigue Scale (scale from 0 to 10) (3 studies, 128 participants: MD ‐1.19, 95% CI ‐1.77 to ‐0.60, I2 = 0%), and the Pittsburgh Sleep Quality Index (scale from 0 to 21) (4 studies, 180 participants: MD ‐2.46, 95% CI ‐4.23 to ‐0.69, I2 = 50%).
We were unable to perform further meta‐analyses because of the paucity of data and problems with clinical heterogeneity, such as different interventions, comparisons and timing of outcome measurements.
Authors' conclusions
There was very low quality of evidence of the short‐term effects of manual acupressure as an adjuvant intervention for fatigue, depression, sleep disturbance and uraemic pruritus in patients undergoing regular haemodialysis. The paucity of evidence indicates that there is little evidence of the effects of other types of acupuncture for other outcomes, including pain, in patients with other stages of CKD. Overall high or unclear risk of bias distorts the validity of the reported benefit of acupuncture and makes the estimated effects uncertain. The incomplete reporting of acupuncture‐related harm does not permit us to assess the safety of acupuncture and related interventions. Future studies should investigate the effects and safety of acupuncture for pain and other common symptoms in patients with CKD and those undergoing dialysis.
Plain language summary
Acupuncture and related interventions for the symptoms of chronic kidney disease
Background
Patients with chronic kidney disease experience various physical and psychological symptoms, but treatment options are limited because of reduced kidney function and other chronic health problems. Acupuncture is widely used to treat common symptoms such as pain, fatigue or depressive mood in patients with chronic conditions. This review aimed to investigate the current evidence and potential role for acupuncture in patients with chronic kidney disease (CKD).
Study characteristics
We searched the literature up to January 2016 and analysed 24 studies that involved 1787 participants. Of these, only seven studies provided data that could be combined for analysis. The studies reported that manual acupressure improved fatigue, depression and sleep disturbance when used as an adjunct to routine care for patients undergoing maintenance haemodialysis 4 weeks from baseline. No study assessed pain and most did not report whether adverse events of acupuncture occurred.
Key results
Overall, we found very low quality evidence about the effectiveness of acupuncture for symptoms of CKD. Manual acupressure combined with routine care may provide short‐term symptom relief from depressive mood, fatigue and sleep disturbance in patients undergoing haemodialysis. Findings from this review cannot support the benefits of other acupuncture techniques for patients with CKD because there were too few reliable studies. Pain is a common condition in patients with CKD. Thus, the potential role of acupuncture for pain control in patients with CKD deserves further research.
Clinicians should carefully monitor the safety of acupuncture in patients with CKD unless sound evidence supports the safety of these interventions for CKD patients.
Quality of the evidence
All studies were assessed at high or unclear risk of bias, especially in terms of selection of participants and selective outcome reporting, which made the validity of their results doubtful.
Summary of findings
Summary of findings for the main comparison. Manual acupressure compared with routine care for people undergoing haemodialysis.
| Manual acupressure compared with routine care for people undergoing haemodialysis | ||||||
| Patient or population: patients with end‐stage kidney disease Settings: haemodialysis centre Intervention: manual acupressure | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Manual acupressure | |||||
|
Pain1 Not measured |
See comment | See comment | Not estimable1 | ‐ | See comment | |
|
Depression
Beck's Depression Inventory Scale from: 0 to 63 Follow‐up: 4 weeks |
The mean depression ranged across control groups from 14.9 to 21.61 points | The mean depression in the intervention groups was 4.29 lower (7.48 to 1.11 lower) | 128 (3) | ⊕⊝⊝⊝ very low2,3,4,5 | ||
|
Sleep quality
Pittsburgh Sleep Quality Index6 Scale from: 0 to 21 Follow‐up: 4 weeks |
The mean sleep quality ranged across control groups from 9.36 to 10.9 points | The mean sleep quality in the intervention groups was 2.46 lower (4.23 to 0.69 lower) | 180 (4) | ⊕⊝⊝⊝ very low3,4,5,7 | ||
|
Fatigue
Revised Piper Fatigue Scale Scale from: 0 to 10 Follow‐up: mean 4 weeks |
The mean fatigue ranged across control groups from 4.7 to 5.71 points | The mean fatigue in the intervention groups was 1.19 lower (1.77 to 0.6 lower) | 128 (3) | ⊕⊝⊝⊝ very low2,3,4,5 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
1 No study measured pain 2 All study had high or unclear risk of bias in domains of random sequence generation, concealment of allocation and blinding of participants and outcome assessors, incomplete outcome data and selective outcome reporting. Therefore, quality of evidence was downgraded by two levels due to the limitations in the design and implementation 3 Collectively, quality of evidence was downgraded by three levels due to the very serious limitation in the design and implementation and the indirectness of evidence 4 The study population was restricted to the patients undergoing regular haemodialysis. Therefore, quality of evidence was downgraded by one level due to the indirectness of evidence 5 The evidence was downgraded due to small sample size of a meta‐analysis 6 Global PSQI scores were used 7 All study had high or unclear risk of bias in domains of random sequence generation, concealment of allocation and blinding of participants, incomplete outcome data and selective outcome reporting. Therefore, quality of evidence was downgraded by two levels due to the limitations in the design and implementation
Background
Description of the condition
Chronic kidney disease (CKD) is defined as the persistence of structural and/or functional abnormalities of the kidney for three or more months (NKF 2002). The prevalence of CKD is increasing, and it is acknowledged as major health issue worldwide (Imai 2008; James 2010). CKD symptoms include pain, itch (pruritus), peripheral numbness, sleep disturbances, depression, fatigue, sexual dysfunction, nausea and vomiting. Factors that contribute to these symptoms include anaemia, uraemic toxins, reduced renal capacity, chronic disease‐related inflammation, and psychological stress associated with long‐term illness. These symptoms have often been under‐treated or under‐recognised, which has contributed to an increased symptom burden and diminished quality of life among people with CKD (Abdel‐Kader 2009; Claxton 2010). Some symptoms, such as sleep disturbance and depression, lead to poor quality of life and are known to be associated with increased mortality in dialysis patients (Elder 2008; Lopes 2002; Mapes 2003). Given the overall negative impact of inappropriately treated symptoms, adequate symptom management should be an essential component for the optimal care of CKD patients (Rastogi 2008).
Current management strategies for CKD focus on controlling kidney disease, comorbidities and related symptoms. According to patients' accounts, some components of care are poorly integrated into management plans (Tong 2009). Interventions for symptom management and supportive care have often had poor levels of patient compliance. Some patients living with long‐term illness report having experienced detrimental effects from the need for therapeutic polypharmacy and a fear of drug‐related adverse events (Browne 2010; Davison 2007). Reduced renal capacity and altered pharmacokinetics in CKD patients are significant barriers to the extrapolation of the pharmacological symptom management methods used in non‐CKD populations (Verbeeck 2009). These barriers often result in insufficient symptom management and decreased quality of life among CKD patients.
Description of the intervention
Acupuncture is a complex intervention that originated from East Asian countries (MacPherson 2008). 'Acupuncture' refers to either a specific procedure involving acupuncture needling on body points or a multi‐component treatment that also involves taking patients' history, physical examination, diagnosis, and patient education based on East Asian medicine (Langevin 2011). Acupuncture has been used for pain management, supportive cancer care and other chronic illnesses to improve symptoms, quality of life and patient‐perceived well‐being (Dean‐Clower 2010; Frisk 2012; McKee 2013; Simcock 2013; Suzuki 2012). Although the frequency of acupuncture use in the general population varies, up to 82% of adults reportedly have a cumulative lifetime experience with acupuncture. The wide range may be attributable to regional, cultural and research methodology differences (Hunt 2010; KHIDI 2008; Naoto 2005). Published information about the use of acupuncture for people with CKD is sparse, but a survey of 232 Japanese haemodialysis patients revealed that approximately 30% had previously experienced acupuncture, and 35% were receptive to acupuncture for symptom management (Sakuraba 2007). Nowack 2009 investigated 180 patients with ESKD at five nephrology centres in southern Germany and found that 57% of dialysis patients and 49% of transplant patients were regular users of complementary and alternative medicine (CAM) modalities (but not acupuncture). This finding suggests a potentially high level of acupuncture and other CAM modalities use among people with end‐stage kidney disease (ESKD).
Although results from acupuncture studies are inconsistent, limited evidence of acupuncture benefits is included in the Cochrane Database of Systematic Reviews including systematic reviews of acupuncture for peripheral joint osteoarthritis (Manheimer 2010), low back pain (Furlan 2005), chemotherapy‐induced nausea and vomiting (Ezzo 2006), prevention of postoperative nausea and vomiting (Lee 2015), tension‐type headache (Linde 2009a) and migraine prophylaxis (Linde 2009b). An individual patient data meta‐analysis of acupuncture for chronic musculoskeletal pain suggested that acupuncture offered modest but significant effects compared with sham acupuncture (Vickers 2012). The results of these studies may merit considering extrapolation of such evidence to CKD and ESKD populations. The current use of acupuncture for pain, depression and other chronic conditions, which are also prevalent in people with CKD, may support a need for research in the use of acupuncture in those populations.
How the intervention might work
Garcia 2005 suggested that the possible mechanism of acupuncture‐like stimulation in CKD was associated with cholinergic anti‐inflammatory effects via parasympathetic nerve stimulation in the renal inflammation model. This effect has been proposed as a plausible concept for a number of chronic inflammatory and autoimmune diseases (Kavoussi 2007). Because chronic inflammation is prevalent, and associated with increased mortality in both non‐dialysis and dialysis CKD patients (Bergström 2000; Carrero 2008), acupuncture may help to improve medical outcomes (Carrero 2008). Other well‐known segmental and extra‐segmental analgesic effects, central regulation of endogenous opioids and serotonin might modulate symptoms and complications among CKD patients including pain, itch, and sleep disturbances (White 2008). These hypotheses require further research.
Why it is important to do this review
Previous narrative reviews have suggested acupuncture as a promising intervention for patients with CKD or ESKD (Garcia 2005; Markell 2005). We have previously conducted two systematic reviews and found that there was no evidence to inform definitive conclusions about the benefits or harms of acupuncture and acupressure for renal itch (Kim 2010a) and symptom management among people with ESKD (Kim 2010b) because of the poor methodological quality of the included studies and paucity of RCTs. Comprehensive evidence of acupuncture and related interventions for symptom management in patients with all stages of CKD remains lacking. This review aimed to systematically synthesise and critically assess the current evidence for acupuncture and related interventions for control of CKD symptoms.
Objectives
We aimed to evaluate the benefits and harms of acupuncture, electroacupuncture, acupressure, moxibustion and other acupuncture‐related interventions (alone or combined with other acupuncture‐related interventions) for symptoms of CKD. In particular, we planned to compare acupuncture and related interventions with conventional medicine, active non‐pharmacological interventions, and routine care for symptoms of CKD.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) that tested the effects of acupuncture and related interventions (hereafter, acupuncture interventions) for patients with CKD regardless of language or type of publication. Only data from the first phase of cross‐over studies were used to avoid any possible carry over effect. We excluded non‐randomised and uncontrolled studies.
Types of participants
We included adults (aged 18 years and over or as defined by authors) with CKD stage 3 to 5 or 5D, as defined by the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (NKF 2002).
Kidney damage for at least three months defined as structural or functional abnormalities of the kidney with or without decreased GFR and manifested by either pathological abnormalities or markers of kidney disease, including abnormalities in the composition of the blood or urine or abnormalities in imaging tests.
GFR < 60 mL/min/1.73 m2 for at least three months with or without kidney disease.
CKD stages
Stage 3: GFR 30 to 59 mL/min/1.73 m2
Stage 4: GFR 15 to 29 mL/min/1.73 m2
Stage 5: GFR < 15 mL/min/1.73 m2
Stage 5D: on dialysis.
We applied KDOQI criteria to define and classify CKD wherever possible. Nevertheless, we also included studies of patients with CKD defined using methods other than KDOQI because they may have been conducted in countries that do not apply the KDOQI definition (Markell 2006).
We excluded studies that involved kidney transplant recipients or patients with acute kidney injury and studies of children. We also excluded studies that lacked sufficient information to determine CKD stage (either from published reports or by contacting the authors).
Types of interventions
Included interventions
-
Acupuncture stimulates specific points with or without needle penetration to elicit a therapeutic response. We included the following forms of acupuncture using different stimulation methods.
Electrostimulation using a penetrating needle or non‐penetrating pad, laser, manual acupressure or acupressure with other devices
Moxibustion (heating the herb mugwort)
Other types of heat stimulation on acupuncture points.
Studies investigating combinations of acupuncture interventions because practitioners often use these to achieve prolonged or synergistic effects.
Comparisons of sham interventions (e.g. non‐penetrating or penetrating sham acupuncture, sham laser, sham moxibustion, sham electrostimulation, sham acupressure), as well as comparisons of routine care (e.g. treatments provided by nephrologists or physicians for CKD management) and other active comparators (e.g. exercise).
Ezzo 2000 reported that six or more sessions of acupuncture treatment were more effective than fewer sessions in patients with chronic pain. In real clinical situations, practitioners do not expect that fewer than six sessions is sufficient to manage chronic disease. Therefore, we planned to exclude studies involving fewer than six sessions of acupuncture treatments.
We excluded studies that did not provide co‐interventions equally to all randomised arms.
Excluded interventions
Acupuncture‐point injection because of the difficulty of controlling the source of any reported effect; and
Studies that compared different types of acupuncture, herbs, or other complementary medicine interventions.
Types of outcome measures
Primary outcomes
We selected two representative symptoms for primary outcomes based on the high prevalence and clinical impacts for people with CKD (Davison 2007; Finkelstein 2008; Pham 2010). The occurrence of serious adverse events was also a primary outcome for analysing harms.
Change in pain (e.g. bone and joint pain, neuropathic pain, headache or other types of bodily pain perceived by patients) measured as a dichotomous outcome (e.g. improvement: yes or no) or on a self‐rating scale such as the visual analogue scale (VAS), a numerical rating scale or other questionnaire scores
-
Change in depression symptomatology measured as a dichotomous outcome (e.g. improvement: yes or no), on a self‐rating scale such as the Beck Depression Inventory (Beck 1961), or on a clinician‐rated scale such as the Hamilton Rating Scale for Depression (Hamilton 1960).
Beck Depression Inventory: minimal depression (0‐13); mild depression (14‐19); moderate depression (20‐28); severe depression (29‐63)
Hamilton Rating Scale for Depression: normal (0‐7); mild depression (8‐13); moderate depression (14‐18); severe depression (18‐22); very severe depression (≥ 23)
The occurrence of serious adverse events, including hospitalisation, surgery other than scheduled transplantation, and death.
A subjective, non‐validated assessment tool may be a source of bias. If the same outcome was reported using multiple methods, we gave preference to validated scales.
Secondary outcomes
Changes in symptoms other than those addressed in the primary outcome measurements (e.g. fatigue, sleep disturbance, nausea/vomiting, xerostomia, restless legs syndrome) measured as a dichotomous (e.g. improvement: yes or no) or continuous (e.g. VAS or other questionnaire score) outcome
Changes in quality of life (e.g. general quality of life as measured by the Medical Outcome Study item short‐form health survey (SF‐36) and health‐related or disease‐specific quality of life (e.g. assessed by the KDQOL questionnaire)
Changes in biological or physiological parameters (e.g. blood pressure, glycaemic control, serum albumin, serum creatinine, blood urea nitrogen, calcium, phosphate, parathyroid hormone)
Changes in the occurrence of intradialytic complications (e.g. events of symptomatic intradialytic hypotension, cramps, nausea and any untoward symptoms that occurred during dialysis)
Changes in medication (e.g. analgesics, sleep medication, antiemetics or other medication) for symptom management
The occurrence of adverse events of acupuncture (any reported events during or after acupuncture such as infection, bruising or bleeding at needling sites).
We did not consider the cost of acupuncture to be an outcome.
We expected that the time course for reporting outcomes would vary among studies and grouped outcomes into four time periods (Linde 2009b).
Up to eight weeks (two months) after the start of the treatment
Three or four months after the start of the treatment
Five to six months after the start of the treatment
More than six months after the start of the treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register up to 28 January 2016 through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for the search terms used in the strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters to investigators known to be involved in previous studies seeking information about unpublished or incomplete studies.
Searches of Korean academic portal databases (i.e. NANET, RISS4U, KISS, DBpia, KMbase, KoreaMed, KISTI, NDSL, OASIS, Dlibrary, KoreanTK, and RICHIS), and Chinese databases (including the China Academic Journal) (see Appendix 2 for the search terms for Korean databases and handsearched Korean journals).
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that might include relevant data or information on studies was retained initially. Two authors independently assess retrieved abstracts, and if necessary the full text, to determine which satisfied inclusion criteria.
Data extraction and management
Data extraction was carried out independently by pairs of authors using standard data extraction forms. Studies reported in non‐English, Korean or Chinese language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancies between published versions were to be highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 3).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
We rated risk of bias for participant blinding as low only when results of sham‐credibility tests showed successful participant blinding in the study. We rated risk of bias for selective outcome reporting as low only when the study protocol was available to ensure that all planned and measured outcomes were reported.
Measures of treatment effect
For dichotomous outcomes (e.g. improved, not improved), we presented results as risk ratios (RR) with 95% confidence intervals (CI). When data were continuous (e.g. VAS, blood pressure, biochemical parameters), we used mean difference (MD) and 95% CI and the standardised mean difference (SMD).
Unit of analysis issues
For multi‐intervention groups, we aimed to combine groups into a single pair‐wise comparison where appropriate. Where studies had multiple observations for outcome variables, we grouped and then meta‐analysed according to four time periods (within two months; three to four months; five to six months; more than six months after the start of the treatment) and conducted meta‐analyses for each group of studies. If there were more than two outcome measurements in one time period, we used the time point that was measured last. We planned to assess first phase data from cross‐over studies. Where appropriate, we combined results from cross‐over studies with parallel group studies.
Dealing with missing data
We sent emails to the contact detail listed in the study to obtain incompletely reported information.
Further information required from study authors was requested by written correspondence and any relevant information obtained was to be included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. We did not impute missing data for effect estimates.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 statistic (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We planned to use funnel plots for analysing publication bias (Higgins 2011) wherever possible. There were insufficient studies per comparison to do this.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for each type of acupuncture (electrical or manual acupuncture, laser acupuncture, acupressure, moxibustion, other types of heat stimulation on acupuncture points and combinations of acupuncture‐related interventions) to identify differences in treatment effects between subgroups. We planned to conduct subgroup analysis for disease status (pre‐dialysis status in CKD stage 3 to 4 and ESKD regardless of whether patients were undergoing dialysis) to assess if treatment effects varied among subgroups. We also planned to conduct subgroup analysis by type of control (sham intervention, conventional medicine, routine care or other active non‐pharmacological intervention). Adverse effects were to be tabulated and assessed using descriptive techniques, because they were likely to differ for various interventions. Where possible, the risk difference with 95% CI was to be calculated for each adverse effect, either compared with no treatment or another agent.
Sensitivity analysis
We planned to perform sensitivity analysis to identify the influence of methodological quality on effect estimates by excluding studies with inadequate or unclear random sequence generation, concealment of allocation and participant and assessor blinding. We also planned to conduct sensitivity analysis to assess the influence of CKD definition and classification (KDOQI criteria: yes or no) and the use of validated versus non‐validated scales on the results of our review. There were insufficient studies per comparison to undertake these sensitivity analyses.
Results
Description of studies
Results of the search
We searched English, Chinese and Korean language databases and identified 522 records. After title and abstract review 356 records were excluded and an additional 104 were excluded after translation. We screened the remaining records and identified 24 studies (30 reports) that involved a total of 1787 participants for inclusion (Figure 1).
1.
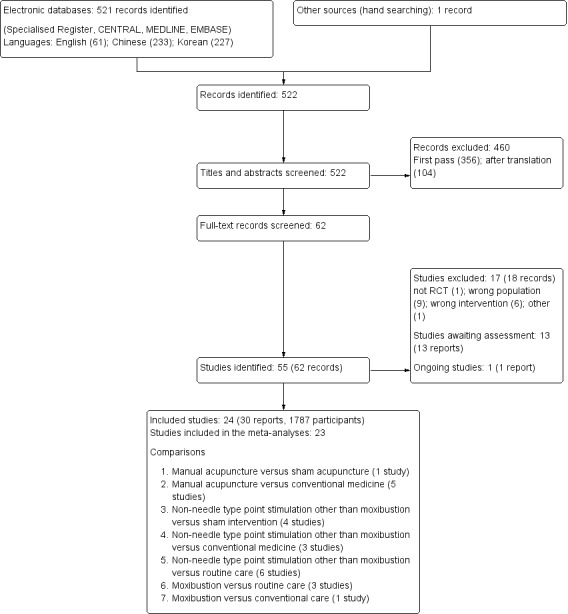
Study selection flow diagram.
Prior to publication a search of the Specialised Register (28 January 2016) identified an additional 13 potential studies (Abbasi 2015; Chen 2012a; Eglence 2013; Feng 2012; Hmwe 2015; Och 2000; Ono 2015; Sabouhi 2013; Wang 2014c; Wu 2014a; Yan 2015; Zhu 2006; Zou 2015) and one ongoing study (NCT02432508). These will be assessed in a future update of this review.
Included studies
Availability of additional information from authors
We emailed the study authors of all included studies to obtain further information because of the incomplete reporting. Three authors associated with six studies provided additional information (Che‐Yi 2005; Tsay 2003a; Tsay 2004a; Tsay 2004b; Qiu 2012; Sun 2012). For the other studies, we could not obtain additional information because there was no contact information in the study (12); the contact information was inactive (12); or there was no response to our e‐mail queries (3).
Design
We included 19 RCTs and five quasi‐RCTs (Cho 2004; Ma 2004; Rui 2002; Song 2007; Cui 2012). All included studies employed a parallel design with relatively small sample sizes (median 69; range 40 to 152). The studies were conducted in China (15), Taiwan (7), Iran (1) and Poland (1) and were published in English (12) and Chinese (12). Most of the studies enrolled participants in haemodialysis centres or community hospitals (22).
Participants
Mean ages were over 50 years (20 studies) or between 40 and 50 years (four studies). Twenty‐two studies included participants with ESKD. Two studies did not provide information on the CKD stage (Ma 2004; Song 2007). After determination of the CKD stages based on the mean serum creatinine values provided in the studies, we considered them as studies of participants of ESKD. Four studies employed K/DOQI or KDIGO guidelines for diagnosing CKD (Dai 2007a; Qiu 2012; Song 2007; Xie 2012).
In 21/22 studies involving participants with ESKD, haemodialysis or peritoneal dialysis was provided as a renal replacement therapy. One study did not specify whether the participants were undergoing any renal replacement therapy (Dai 2007a). In these studies, the haemodialysis frequency was three times/week (12 studies), two to three times/week (3), twice/week (1), twice/week or three times within two weeks (1). Three studies did not report the haemodialysis frequency (Gao 2002; Tsay 2004a; Tsay 2004b). Peritoneal dialysis frequency was four times/day in Zhao 1995; and Jedras 2003 did not report dialysis frequency.
Ten studies reported haemodialysis times of four hours (4), four to five hours (1), three to five hours (2), 3.5 to 4.5 hours (1) and 2.5 to 5.0 hours (1) per session and 15 hours a week (1). In the 14 studies that solely or partially included haemodialysis patients, only three reported baseline values of Kt/V over 1.2, representing adherence to K/DOQI guidelines for dialysis adequacy (Che‐Yi 2005; Tsay 2004a; Qiu 2012).
Further details are presented in Characteristics of included studies.
Types of interventions
Treatment interventions included manual (finger) acupressure (Cho 2004; Dai 2007a; Jedras 2003; Shariati 2012; Tsay 2003a; Tsay 2004a; Tsay 2004b), ear acupressure (Xie 2012; Zhao 2011), manual acupuncture (Che‐Yi 2005; Cui 2012; Gao 2002; Ma 2004; Rui 2002; Zhang 2011d), indirect moxibustion (Cheng 2012; Qiu 2012; Sun 2008a; Sun 2012; Zhao 1995), far infrared radiation (Hsu 2009; Lin 2011; Su 2009), adjunctive manual acupuncture plus conventional medication (Song 2007), and transcutaneous electrical acupoint stimulation (Tsay 2004b).
Interventions in the control groups varied, including routine care (12 studies) (Cheng 2012; Cho 2004; Lin 2011; Qiu 2012; Shariati 2012; Sun 2008a; Sun 2012; Tsay 2003a; Tsay 2004a; Tsay 2004b; Xie 2012; Zhao 1995), conventional medication (7) (Cui 2012; Dai 2007a; Gao 2002; Ma 2004; Rui 2002; Song 2007; Zhao 2011), sham interventions (5) (Che‐Yi 2005; Hsu 2009; Su 2009; Tsay 2003a; Tsay 2004a), haemodiafiltration (Zhang 2011d) and an unspecified control intervention (Jedras 2003).
Five of 12 studies that employed routine care as a comparison intervention provided detailed information on routine care, including blood pressure and glucose control, anaemia correction, electrolyte control and tailored dietary modifications of salt and protein (Che‐Yi 2005; Cheng 2012; Qiu 2012; Sun 2008a; Sun 2012). In five studies comparing real and sham interventions, the actual techniques of the sham intervention varied (Che‐Yi 2005; Hsu 2009; Su 2009; Tsay 2003a; Tsay 2004a). One study compared manual acupuncture with penetrating acupuncture on non‐acupuncture points (Che‐Yi 2005), and two other studies compared manual acupressure with sham acupressure on non‐acupuncture points (Tsay 2003a, Tsay 2004a). Two studies compared far infrared radiation with plain adhesive tape (Hsu 2009) or heat pad therapy (Su 2009) on the same acupuncture points that were used in the treatment intervention. These types of interventions did not appear to be identical to real treatments. After discussion between the review authors, however, we concluded that the interventions were different types of sham intervention.
Treatment lengths varied from 10 days (Ma 2004) to 24 weeks (Song 2007) (median seven weeks). In most studies, the total number of treatment sessions varied between 8 and 36. However, one study conducted acupuncture for at least 100 sessions over a 24‐week period (Song 2007).
Appendix 4 presents further summarised details of the acupuncture interventions and Appendix 5 presents Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) (www.stricta.info/) information of acupuncture interventions and comparators including sham interventions.
Outcome measures
Nine studies included at least one validated outcome (Cho 2004; Lin 2011; Qiu 2012; Shariati 2012; Su 2009; Sun 2008a; Tsay 2003a; Tsay 2004a; Tsay 2004b). The follow‐up period varied between four to 24 weeks from randomisation, although most studies reported short‐term outcomes within two months.
None of the included studies measured pain. Three studies assessed depression using Beck’s depression inventory (Cho 2004; Tsay 2004a; Tsay 2004b). Other subjective symptoms assessed in the included studies were uraemic pruritus (Che‐Yi 2005; Gao 2002; Hsu 2009; Jedras 2003; Rui 2002; Zhang 2011d), sleep quality (Dai 2007a; Tsay 2003a; Tsay 2004a; Tsay 2004b; Shariati 2012; Zhao 2011), fatigue (Cho 2004; Lin 2011; Tsay 2004a; Tsay 2004b), gastrointestinal discomfort (Cheng 2012; Cui 2012), xerostomia (Xie 2012), and symptoms related to malnutrition (Qiu 2012; Sun 2012). Three studies measured mixed symptoms as defined by traditional Chinese medicine theory (Ma 2004; Qiu 2012; Zhao 1995). One study reported a reduction of concomitant symptoms, including headache, dizziness, cognitive dysfunction, and palpitation (Dai 2007a). Four studies measured quality of life (Qiu 2012; Su 2009; Sun 2008a; Tsay 2003a).
Ten studies assessed various biochemical or physiological parameters, including blood pressure, blood and urine profile and muscle strength (Cheng 2012; Hsu 2009; Ma 2004; Qiu 2012; Song 2007; Su 2009; Sun 2012; Xie 2012; Zhang 2011d; Zhao 1995). No included study measured the occurrence of intradialytic complications or changes in medication.
Excluded studies
We excluded 18 studies. Reasons for exclusion were: use of acupuncture point injection as a treatment intervention; wrong population (no CKD patients); inclusion of kidney transplant recipients; and providing fewer than six treatment sessions. See Characteristics of excluded studies.
Risk of bias in included studies
All included studies were assessed at high or unclear risk of bias for all domains. See Figure 2 and Figure 3.
2.
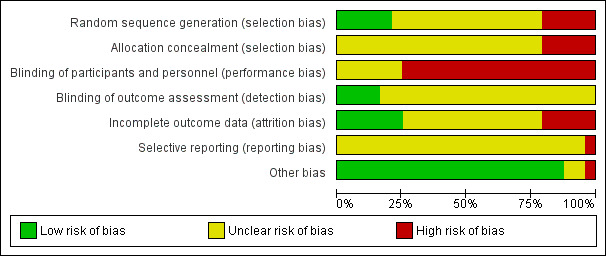
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
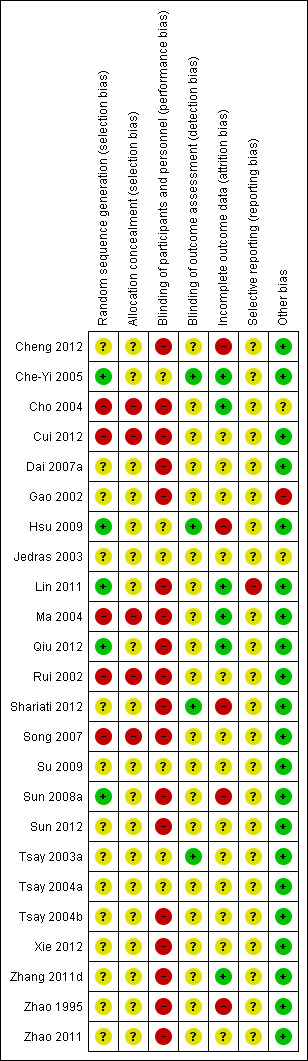
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All included studies were described as randomised, however 14 studies lacked details on randomisation methods. Five studies used adequate random sequence generation methods, including computer random number generators (Che‐Yi 2005; Lin 2011) and random number tables (Hsu 2009; Sun 2008a; Qiu 2012). Five quasi‐RCTs used inadequate allocation methods, including sequences generated by even or odd days of haemodialysis treatment (Cho 2004) or sequences generated by hospital record number (Ma 2004; Rui 2002; Song 2007; Cui 2012).
Allocation concealment
Allocation concealment was not reported by any of the included studies. In the case of the five quasi‐RCTs, we considered these to be at high risk of bias because such studies were unlikely to conceal the allocation procedure properly. Hsu 2009 reported that they contained the randomisation list in serially numbered envelopes, although they did not report whether those envelopes were opaque‐sealed. Two study authors replied to our email queries; they confused blinding processes with allocation concealment (Che‐Yi 2005; Qiu 2012). We rated all included studies other than the five quasi‐RCTs as having unclear risk of bias.
Blinding
Performance bias
For studies in which acupuncture interventions were compared with active comparators, we rated the risk of bias as high. All five sham‐controlled studies attempted patient blinding, although there was no information on its success (Che‐Yi 2005; Hsu 2009; Su 2009; Tsay 2003a; Tsay 2004a). In two studies, the sham techniques (plain adhesive tapes or heat pad therapy on the same stimulation points) were apparently not identical in shape to the treatment interventions (i.e. far infrared irradiation) (Hsu 2009; Su 2009). For one study with an unspecified control intervention, we assessed the risk of bias as unclear (Jedras 2003).
Detection bias
Because the majority of the included studies used subjective outcome assessments, we regarded the blinding of outcome assessors as the key indicator of detection bias in each study. Only four studies blinded outcome assessors and these were assessed as being at low risk of bias (Che‐Yi 2005; Hsu 2009; Tsay 2003a; Shariati 2012). The remaining studies lacked information on whether the outcome assessors were blinded and the risk of bias was judged to be unclear.
Incomplete outcome data
We rated the five studies that analysed all randomised patients as being at low risk of bias (Che‐Yi 2005; Lin 2011; Ma 2004; Qiu 2012; Zhang 2011d). In Cho 2004, the small number of drop‐outs and clear reporting of reasons of withdrawals that seemed unlikely to affect the overall study results, we rated it as having low risk of bias. We judged five studies as being at high risk of bias. Three only analysed patients who had completed the study despite the occurrence of serious adverse events as being at high risk of bias (Hsu 2009; Shariati 2012; Sun 2008a), and in two studies the total number of assessed patients erroneously exceeded the total number of included patients in each group (Cheng 2012; Zhao 1995). Su 2009 only analysed patients who had completed the study. However, we rated the study as having unclear risk of bias due to unclear reasons for withdrawal. The remaining 12 studies had unclear risk of bias because of incomplete reporting of the number of participants at the study stages of outcome measurements and incomplete reporting of reasons for attrition.
Selective reporting
The study protocol was not accessible for any of the included studies. The risk of bias was unclear in all but one study. Lin 2011 lacked any reporting of the biochemical outcomes that were measured as was judged to be at high risk of bias.
Other potential sources of bias
We rated other source of bias as high in one study (Gao 2002) because there were risk of Hawthorne effect due to longer treatment duration in the acupuncture group. Two studies were rated as being at unclear risk of bias due to insufficient information to permit judgement (Cho 2004; Jedras 2003). All other studies were judged to be at low risk of bias.
Effects of interventions
See: Table 1
Primary outcomes
Pain
None of the included studies measured pain.
Depression
Manual acupressure significantly reduced depression symptoms compared with routine care after 4 weeks of treatment on the Beck Depression Inventory (Analysis 4.1.1 (3 studies, 128 participants): MD ‐4.29, 95% CI ‐7.48 to ‐1.11; I2 = 0%) (Cho 2004; Tsay 2004a; Tsay 2004b).
4.1. Analysis.
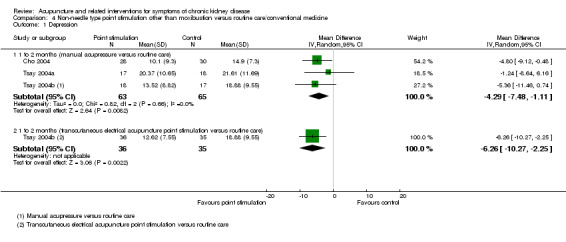
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 1 Depression.
Tsay 2004b compared transcutaneous electrical acupoint stimulation with routine care and reported a significant reduction in depression symptoms on the Beck Depression Inventory (Analysis 4.1.2 (71 participants): MD ‐6.26, 95% CI ‐10.27 to ‐2.25).
In contrast, Tsay 2004a reported manual acupressure was not superior to sham acupressure on the Beck Depression Inventory (Analysis 3.1.1 (70 participants): MD 2.17, 95% CI ‐2.90 to 7.24).
3.1. Analysis.
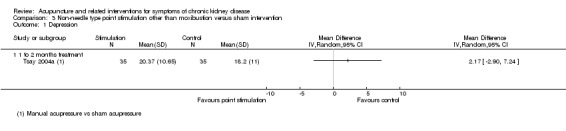
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 1 Depression.
Serious adverse events
No study formally addressed the occurrence of serious adverse events as an outcome. Death (Hsu 2009; Shariati 2012; Sun 2008a), hospitalisation (Hsu 2009; Tsay 2003a) and transfer to an intensive care unit (Shariati 2012) were reported as reasons for attrition with no further information provided.
Secondary outcomes
Uraemic pruritus
On a scale of 0 to 45, Che‐Yi 2005 reported manual acupuncture significantly improved refractory uraemic pruritus compared to sham acupuncture in haemodialysis patients at both four weeks (Analysis 1.1.1 (1 study, 40 participants): MD ‐20.20, 95% CI ‐22.99 to ‐17.41) and 16 weeks (Analysis 1.1.2 (40 participants): MD ‐20.00, 95% CI ‐22.95 to ‐17.05).
1.1. Analysis.
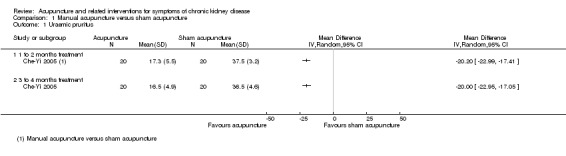
Comparison 1 Manual acupuncture versus sham acupuncture, Outcome 1 Uraemic pruritus.
Gao 2002 reported manual acupuncture was superior to first‐generation oral antihistamine and topical ointments for uraemic pruritus at four to five weeks (Analysis 2.1.1 (68 participants); RR 1.38, 95% CI 1.10 to 1.72), whereas Rui 2002 reported the effects were similar to those administered oral calcitriol 2 μg at 16 weeks (Analysis 2.2.2 (150 participants): RR 1.00, 95% CI 0.89 to 1.12).
2.1. Analysis.
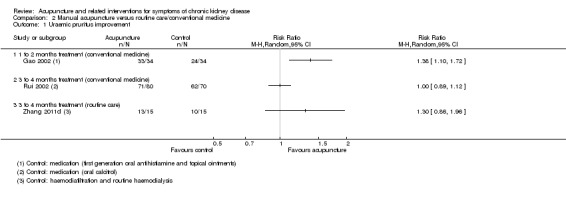
Comparison 2 Manual acupuncture versus routine care/conventional medicine, Outcome 1 Uraemic pruritus improvement.
2.2. Analysis.
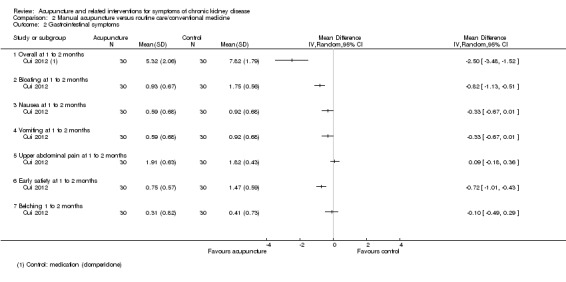
Comparison 2 Manual acupuncture versus routine care/conventional medicine, Outcome 2 Gastrointestinal symptoms.
Zhang 2011d reported that when manual acupuncture was combined with one session of haemodiafiltration per week accompanied by routine haemodialysis, there was a non‐significant improvement in uraemic pruritus when compared to compared with the same dialysis intervention alone at 12 weeks (Analysis 2.1.3 (30 participants): RR 1.30, 95% CI 0.86 to 1.96).
On a scale of 0 to 52, Hsu 2009 reported the effects of far‐infrared radiation on point SP6 were not significantly different from those of sham intervention (plain adhesive tape on the same acupoints) at eight weeks (Analysis 3.2.1 (41 participants): MD ‐2.62, 95% CI ‐6.21 to 0.97).
3.2. Analysis.
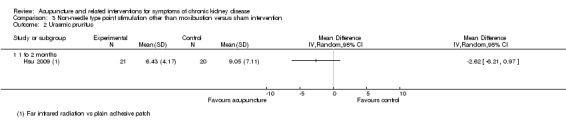
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 2 Uraemic pruritus.
On a scale of 0 to 13.5, Jedras 2003 reported manual acupressure compared to an unspecified control significantly improved uraemic pruritus at six weeks (Analysis 4.2.1 (60 participants): MD ‐6.50, 95% CI ‐7.50 to ‐5.50), 12 weeks (Analysis 4.2.2 (60 participants): MD ‐3.98, 95% CI ‐5.57 to ‐2.39), and 18 weeks (Analysis 4.2.3 (60 participants): MD ‐4.80, 95% CI ‐6.23 to ‐3.37).
4.2. Analysis.
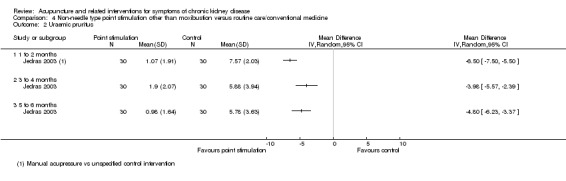
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 2 Uraemic pruritus.
Fatigue
Tsay 2004a reported no significant difference in fatigue between manual acupressure and sham acupressure at four weeks using a revised PDF scale of 0 to 10 (Analysis 3.3.1 (70 participants): MD ‐0.04, 95% CI ‐0.81 to 0.73).
3.3. Analysis.
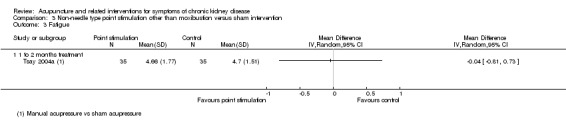
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 3 Fatigue.
Manual acupressure significantly reduced fatigue in compared with routine care at four weeks (Analysis 4.3.1 (3 studies, 128 participants): MD ‐1.19, 95% CI ‐1.77 to ‐0.60; I2 = 0%). Tsay 2004b reported a significant reduction in fatigue with transcutaneous electrical acupoint stimulation when compared to routine care (Analysis 4.3.2 (71 participants): MD ‐1.00, 95% CI ‐1.77 to ‐0.23).
4.3. Analysis.
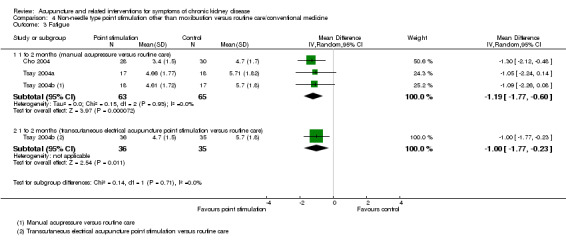
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 3 Fatigue.
Lin 2011 compared far infrared radiation with routine care and found no significant differences at eight weeks in any of the nine sub‐domain scores on the Brief Fatigue Inventory (Analysis 4.4).
4.4. Analysis.
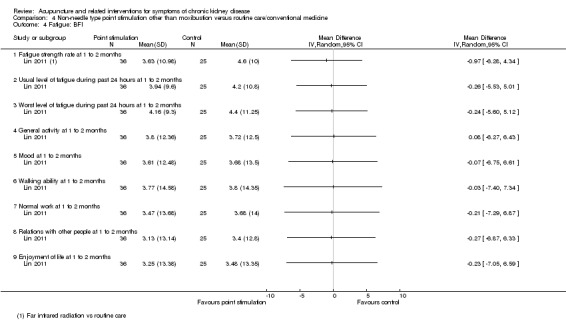
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 4 Fatigue: BFI.
Gastrointestinal symptoms
On a scale of 0 to 18, Cui 2012 reported significant improvement for manual acupuncture compared with domperidone on overall symptom scores at two weeks (Analysis 2.2.1 (60 participants): MD ‐2.50, 95% CI ‐3.48 to ‐1.52). For the subscale scores (0 to 3) there were significant improvements for bloating (Analysis 2.2.2 (60 participants): MD ‐0.82, 95% CI ‐1.13 to ‐0.51) and early satiety (Analysis 2.2.6 (60 participants): MD ‐0.72, 95% CI ‐1.01 to ‐0.43), but not for nausea, vomiting, upper abdominal pain and belching at two weeks.
Cheng 2012 reported moxibustion was not significantly different to routine care in terms of reduced appetite, nausea/vomiting, bloating, or loose stools after 10 days of treatment (Analysis 5.1).
5.1. Analysis.
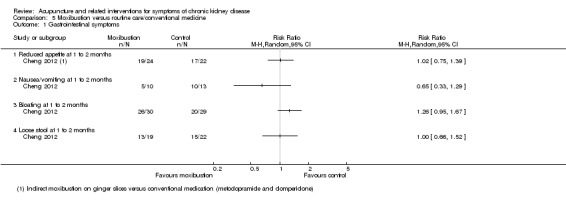
Comparison 5 Moxibustion versus routine care/conventional medicine, Outcome 1 Gastrointestinal symptoms.
Hypertension
Song 2007 compared manual acupuncture plus antihypertensive medication (oral irbesartan 150 mg/d and fosinopril 10 mg/d for 24 weeks) versus antihypertensive medication alone for the management of hypertension. No significant differences were found in either average systolic or diastolic pressure at any of the analysed time points (8, 12 and 24 weeks) (Analysis 2.3; Analysis 2.4). The number of treatment responders was, however, significantly larger in the manual acupuncture group at all of the analysed time points (Analysis 2.5, 152 participants) (8 weeks: RR 1.22, 95% CI 1.01 to 1.47; 12 weeks: RR 1.23, 95% CI 1.09 to 1.39; 24 weeks: RR 1.23, 95% CI 1.10 to 1.38).
2.3. Analysis.
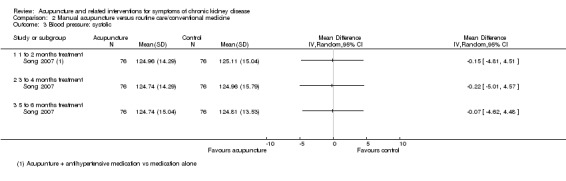
Comparison 2 Manual acupuncture versus routine care/conventional medicine, Outcome 3 Blood pressure: systolic.
2.4. Analysis.
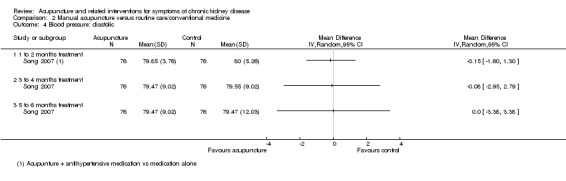
Comparison 2 Manual acupuncture versus routine care/conventional medicine, Outcome 4 Blood pressure: diastolic.
2.5. Analysis.
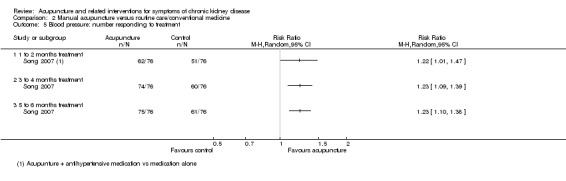
Comparison 2 Manual acupuncture versus routine care/conventional medicine, Outcome 5 Blood pressure: number responding to treatment.
Sleep quality
There was no significant difference between manual acupressure and sham acupressure in terms of global Pittsburgh Sleep Quality Index scores at four weeks (Analysis 3.4.1 (2 studies, 137 participants): MD ‐0.11, 95% CI ‐3.70 to 3.47; I2 = 83%).
3.4. Analysis.
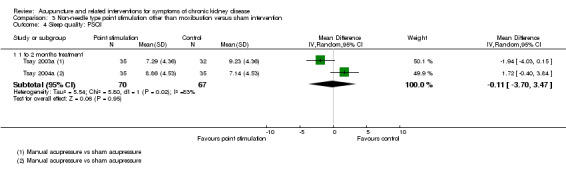
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 4 Sleep quality: PSQI.
In the pooled analysis of global Pittsburgh Sleep Quality Index scores, manual acupressure showed a significant improvement compared with routine care or conventional medicine at four weeks (Analysis 4.5.1 (4 studies, 180 participants): MD ‐2.46, 95% CI ‐4.23 to ‐0.69; I2 = 50%). Tsay 2004b reported a significant improvement in sleep quality with transcutaneous electrical acupoint stimulation when compared to routine care (Analysis 4.5.2 (71 participants): MD ‐3.43, 95% CI ‐5.57 to ‐1.29). Zhao 2011 found no significant difference between ear acupressure and conventional treatment (Analysis 4.5.3).
4.5. Analysis.
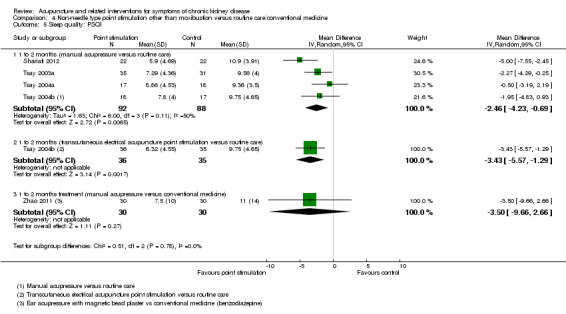
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 5 Sleep quality: PSQI.
Dai 2007a reported significant improvements in sleep disturbance with manual acupressure compared with benzodiazepine medication (1 mg of estazolam) at four weeks using the self rating scale for sleep (scale of 10 to 50) (Analysis 4.7.1 (82 participants): MD ‐6.70, 95% CI ‐8.19 to ‐5.21).
4.7. Analysis.
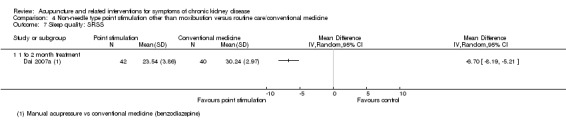
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 7 Sleep quality: SRSS.
Quality of life
Three QOL instruments, including the SF‐36 (Tsay 2003a), WHOQOL‐BREF (Su 2009) and KDQOL V 1.3 (Sun 2008a; Qiu 2012) were used (scales ranged from 0 to 100).
Tsay 2003a reported manual acupressure showed significantly less benefits compared with sham acupressure in terms of the SF‐36 physical component score at four weeks (Analysis 3.5.9 (68 participants): MD 6.45, 95% CI 2.45 to 10.45). There were no significant differences for the remaining sub‐domains.
3.5. Analysis.
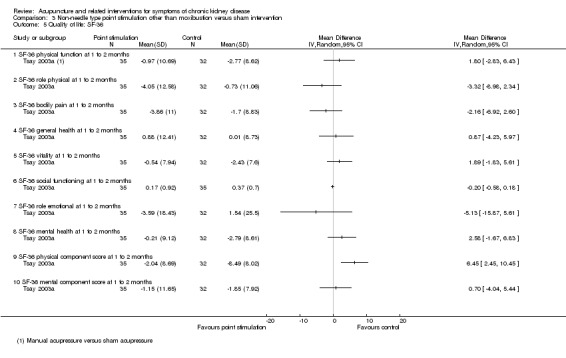
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 5 Quality of life: SF‐36.
Su 2009 reported far infrared radiation on point SP6 compared with sham intervention (i.e. a heat pad application) showed no significant effect on any all sub‐domain of the WHOQOL‐BREF at 12 weeks (Analysis 3.6).
3.6. Analysis.
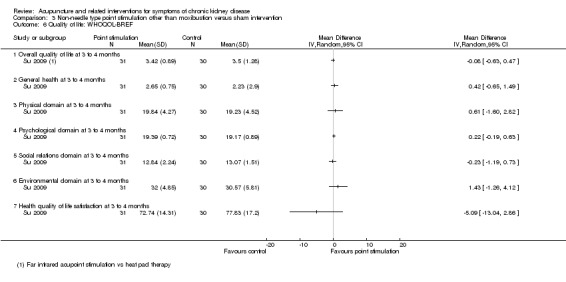
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 6 Quality of life: WHOQOL‐BREF.
Tsay 2003a reported significantly favourable effects of manual acupressure compared with routine care at four weeks on the SF‐36 physical role (Analysis 4.6.2 (66 participants): MD ‐6.05, 95% CI ‐11.94 to ‐0.16), bodily pain (Analysis 4.6.3 (66 participants): MD ‐6.28, 95% CI ‐10.45 to ‐2.11), and vitality (Analysis 4.6.5 (66 participants): MD ‐5.25, 95% CI ‐8.56 to ‐1.94) sub‐domains.
4.6. Analysis.
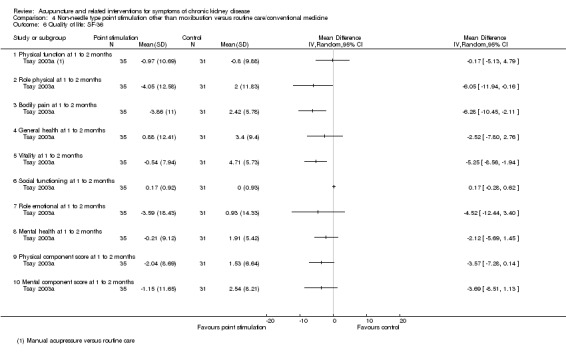
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 6 Quality of life: SF‐36.
Moxibustion showed better results than routine care on the KDQOL sub‐domains of physical functioning (Analysis 5.2.3 (2 studies, 174 participants): MD ‐9.27, 95% CI ‐15.50 to ‐3.04, I2 = 0%), vitality (Analysis 5.2.11 (2 studies, 174 participants): MD ‐6.14, 95% CI ‐11.83 to ‐0.46, I2 = 0%), and general health (Analysis 5.2.13 (2 studies, 174 participants): MD ‐10.24, 95% CI ‐16.31 to ‐4.17, I2 = 0%) three to four months from baseline.
5.2. Analysis.
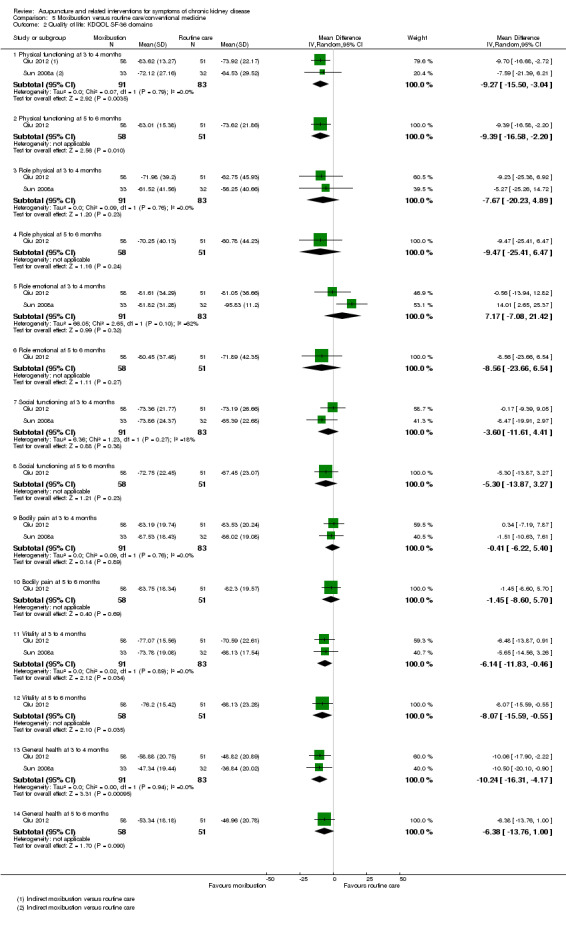
Comparison 5 Moxibustion versus routine care/conventional medicine, Outcome 2 Quality of life: KDQOL SF‐36 domains.
A six‐month follow‐up observation of moxibustion treatment (Qiu 2012) revealed favourable effects compared with routine care on the sub‐domains of physical functioning (Analysis 5.2.2 (109 participants): MD ‐9.39, 95% CI ‐16.58 to ‐2.20), vitality (Analysis 5.2.2 (109 participants): MD ‐8.07, 95% CI ‐15.59 to ‐0.55), and cognitive function (Analysis 5.3.4 (109 participants): MD ‐6.13, 95% CI ‐11.41 to ‐0.85).
5.3. Analysis.
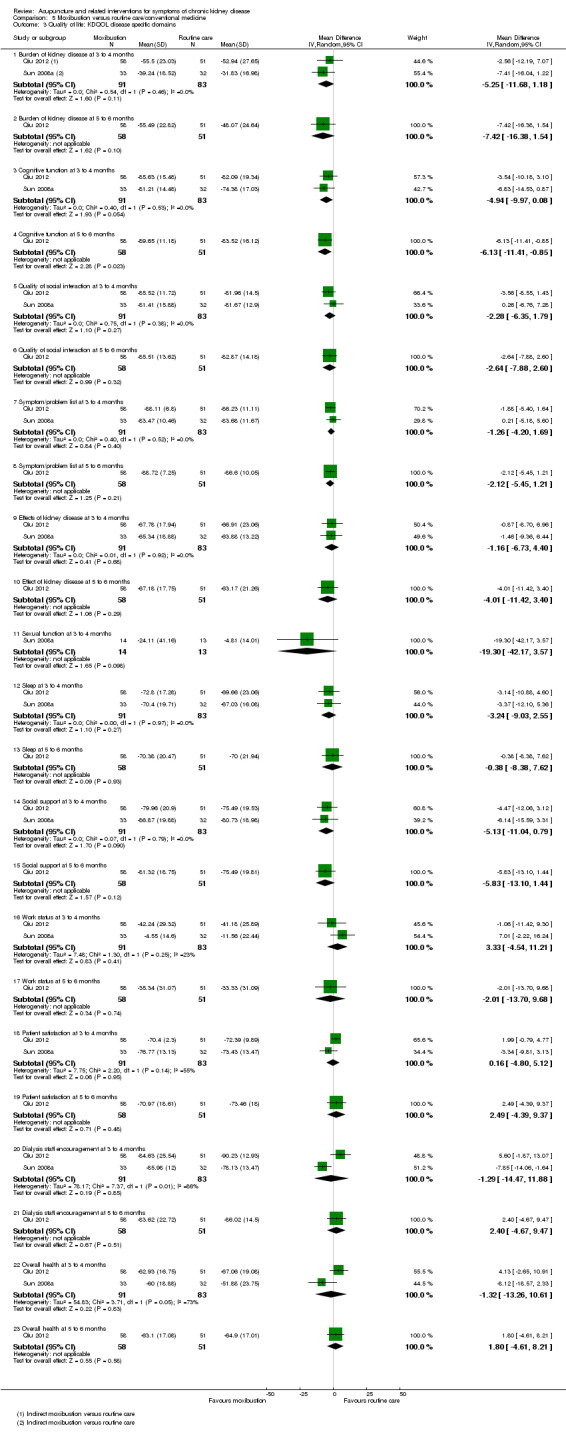
Comparison 5 Moxibustion versus routine care/conventional medicine, Outcome 3 Quality of life: KDQOL disease specific domains.
Nutritional status
Xie 2012 reported ear acupressure showed significantly less interdialytic weight gain compared to routine treatment at eight weeks (Analysis 4.8.1 (90 participants): MD ‐0.29 % dry weight/d, 95% CI ‐0.38 to ‐0.20).
4.8. Analysis.
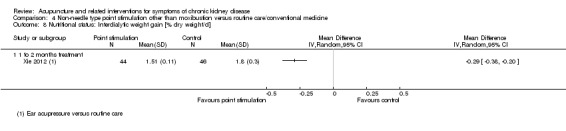
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 8 Nutritional status: Interdialytic weight gain [% dry weight/d].
Effects of moxibustion compared with routine care for nutritional improvements were assessed using modified quantitative subjective global assessment (MQSGA) scores.
Sun 2012 reported MQSGA scores at eight to nine weeks were not significantly higher in the moxibustion group compared with the routine care at eight to nine weeks (Analysis 5.5.1).
5.5. Analysis.
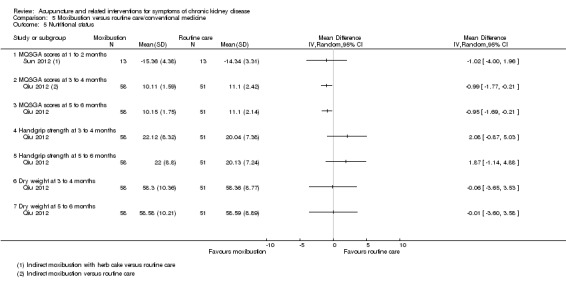
Comparison 5 Moxibustion versus routine care/conventional medicine, Outcome 5 Nutritional status.
Qiu 2012 reported MQSGA scores (scale of 7 to 35) were significantly higher in the moxibustion group at both 12 weeks (Analysis 5.5.2 (109 participants): MD ‐0.99, 95% CI ‐1.77 to ‐0.21) and 24 weeks (Analysis 5.5.3 (109 participants): MD ‐0.95, 95% CI ‐1.69 to ‐0.21) when compared to routine care.
There was no difference between the moxibustion and the routine care group for handgrip strength (Analysis 5.5.4; Analysis 5.5.5) and dry weight (Analysis 5.5.6; Analysis 5.5.6).
Overall treatment responses (symptom and kidney function improvement)
Ma 2004 and Zhao 1995 measured overall treatment responses and grouped treatment response into three (excellent, effective, failed) (Ma 2004) or four (cured, markedly effective, fair, ineffective) categories (Zhao 1995). Both studies defined the treatment response according to symptom reduction and kidney function improvements, measured by blood creatinine, uric acid, urea nitrogen and 24‐hour urinary protein excretion. Ma 2004 dichotomised outcomes by merging patients who displayed excellent and effective responses into one group and those who failed into another group, and Zhao 1995 merged patients who were cured or displayed markedly effective response into one group and the patients who displayed a fair and ineffective response into another.
Ma 2003 reported acupuncture showed significant improvement in overall symptoms and kidney function compared with conventional medication (allopurinol 200 to 300 mg/d and ibuprofen 600 mg/d) at 10 days (Analysis 2.7.1 (82 participants): RR 1.50, 95% CI 1.14 to 1.99).
2.7. Analysis.
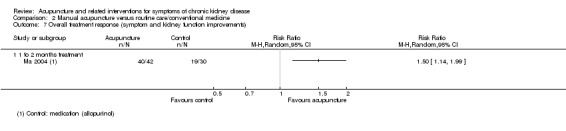
Comparison 2 Manual acupuncture versus routine care/conventional medicine, Outcome 7 Overall treatment response (symptom and kidney function improvements).
Zhao 1995 reported moxibustion showed a non‐significant improvement in overall symptoms and kidney function compared with routine care at seven weeks (Analysis 5.4.1 (152 participants): RR 2.02, 95% CI 0.99 to 4.10).
5.4. Analysis.
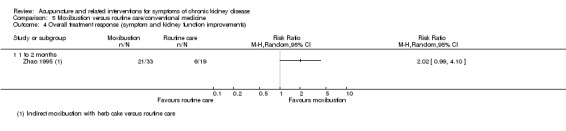
Comparison 5 Moxibustion versus routine care/conventional medicine, Outcome 4 Overall treatment response (symptom and kidney function improvements).
Xerostomia
On a scale of 0 to 100, Xie 2012 reported ear acupressure significantly improved xerostomia compared with routine care at eight weeks (Analysis 4.9.1 (90 participants): MD ‐8.01, 95% CI ‐9.21 to ‐6.81).
4.9. Analysis.
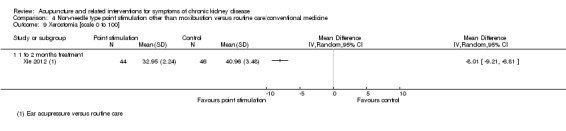
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 9 Xerostomia [scale 0 to 100].
Overall comfort scales
On a scale of 0 to 100, Zhao 2011 reported ear acupressure showed significant improvement in overall comfort compared with routine care at eight weeks (Analysis 4.10.1 (60 participants): MD 5.80, 95% CI 2.76 to 8.84).
4.10. Analysis.

Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 10 Overall comfort scores.
Biochemical parameters
The biochemical parameters assessed in the eligible studies included parathyroid hormone, serum phosphorus, serum uric acid, serum creatinine, blood urea nitrogen, 24‐hour urinary protein excretion, serum calcium, serum albumin, serum prealbumin, serum alkaline phosphatase, urea reduction ratio, serum haemoglobin, serum calcium‐phosphorus ratio, plasma sodium concentration, amount of ultrafiltration, high‐sensitivity C‐reactive protein, interleukin‐6, serum middle molecule and serum potassium level.
Ma 2004 reported manual acupuncture produced significantly lower serum uric acid (Analysis 2.6.3 (72 participants): SMD ‐1.24, 95% CI‐1.75 to ‐0.73), serum creatinine (Analysis 2.6.4 (72 participants): SMD ‐2.42, 95% CI ‐3.04 to ‐1.80), blood urea nitrogen (Analysis 2.6.5 (72 participants): SMD ‐3.45, 95% CI ‐4.20 to ‐2.70) and 24‐hour urine protein (Analysis 2.6.6 (72 participants): SMD ‐1.57, 95% CI ‐2.10 to ‐1.03) levels compared with conventional medication (allopurinol 200 to 300 mg/d and ibuprofen 600 mg/d) at 10 days in patients with gouty kidney disease.
2.6. Analysis.
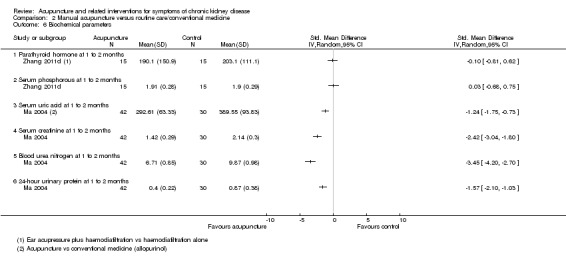
Comparison 2 Manual acupuncture versus routine care/conventional medicine, Outcome 6 Biochemical parameters.
Xie 2012 reported ear acupressure plus haemodiafiltration versus haemodiafiltration alone showed no difference in terms of parathyroid hormone, serum phosphorus, and plasma sodium concentration levels. Similarly, Hsu 2009 reported there was no difference for far infrared radiation on the acupuncture points versus sham intervention in terms of serum calcium, phosphorus, serum albumin, serum alkaline phosphatase, urea reduction ratio, parathyroid hormone level and calcium‐phosphorus ratio.
Sun 2012 reported moxibustion resulted in a higher amount of ultrafiltration compared with routine care at 60 days (Analysis 5.6.3 (26 participants): SMD 1.14, 95% CI 0.30 to 1.97), improved serum prealbumin (SMD 1.86, 95% CI 0.91 to 2.80), and reduced the levels of inflammation‐related parameters such as high‐sensitivity C‐reactive protein (Analysis 5.6.13 (26 participants): SMD ‐1.91, 95% CI ‐2.86 to ‐0.95) and interleukin‐6 (Analysis 5.6.14 (26 participants): SMD ‐1.42, 95% CI ‐2.30 to ‐0.54).
5.6. Analysis.
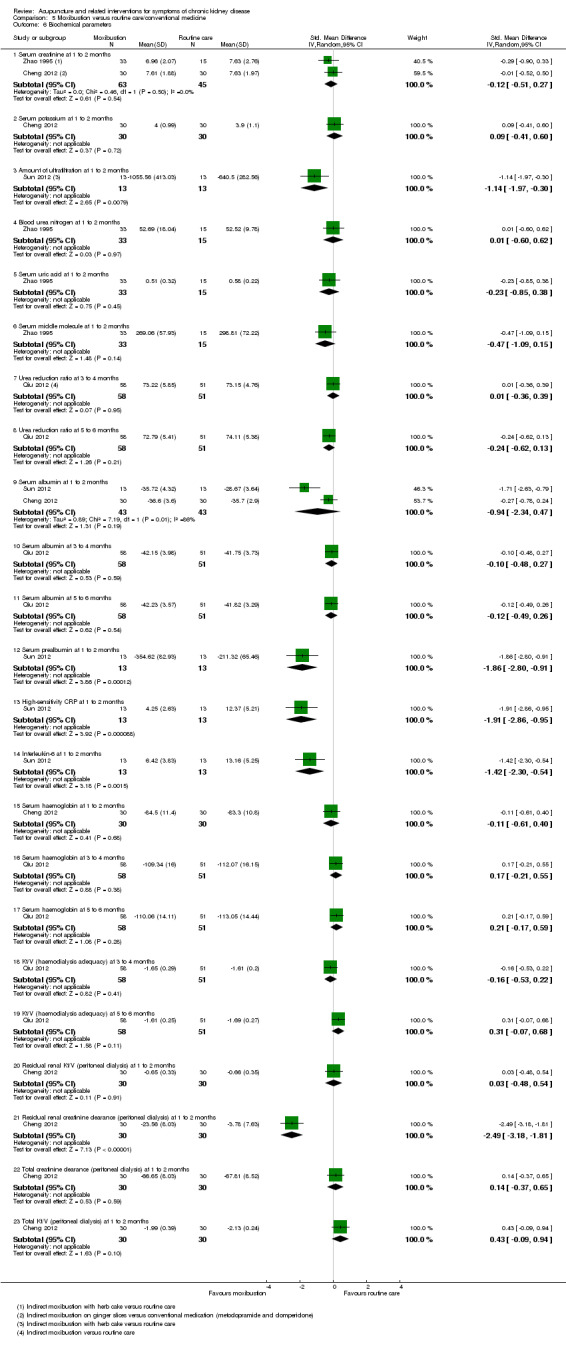
Comparison 5 Moxibustion versus routine care/conventional medicine, Outcome 6 Biochemical parameters.
Xie 2012 and Qiu 2012 assessed Kt/V in haemodialysis patients and found no significant difference between ear acupressure or moxibustion and routine care (Analysis 5.6.18; Analysis 5.6.19). Cheng 2012 measured residual kidney Kt/V, total Kt/V, residual kidney creatinine clearance and total creatinine clearance in peritoneal dialysis patients. There was a significant benefit with moxibustion compared to conventional medicine only for residual renal creatinine clearance (Analysis 5.6.22 (60 participants): SMD 2.49, 95% CI 1.81 to 3.18).
Adverse events
Che‐Yi 2005 reported adverse events included elbow soreness (two patients in the manual acupuncture and one in the sham acupuncture group) and minimal bleeding (three in the sham acupuncture group. Two studies reported that no adverse events had occurred (Rui 2002; Hsu 2009). None of the other included studies reported whether adverse events had occurred. Some practitioners injured their hand during finger acupressure interventions (Tsay 2004a).
Subgroup analysis
We initially intended to conduct subgroup analyses to explore the potential factors related to the heterogeneity of the study results (i.e. type of acupuncture interventions, stage of CKD and type of control intervention). There were insufficient studies per comparison to investigate these subgroups.
Sensitivity analysis
We intended to perform sensitivity analyses based on methodological quality assessment in terms of random sequence generation, allocation concealment and participant and assessor blinding; the methods of CKD definition and classification; or the use of validated scales for outcome measures. However, these analyses were not conducted because most of the studies had unclear or high risk of bias; did not describe their CKD definition; or did not use validated outcomes.
Assessment of reporting bias
We initially proposed to assess publication bias if there were sufficient numbers of studies. Paucity of data meant that we were unable to assess for publication bias on funnel plots.
Discussion
Summary of main results
This review included 24 studies that involved 1787 participants. We could not perform meta‐analyses of results for most outcomes because of considerable clinical heterogeneity in terms of the acupuncture interventions, comparators and conditions tested. Most studies measured the short‐term effects of acupuncture interventions at time periods equal to or less than two months. No study measured pain, which was one of the primary outcomes in this review.
Several single studies reported significant findings. In three small studies, manual acupressure reduced the severity of depression compared with routine care at four weeks (MD ‐4.29, 95% CI ‐7.48 to ‐1.11, I2 = 0%), although this effect was relatively modest compared with that of other non‐pharmacological interventions, such as cognitive behavioural group therapy versus routine care in dialysis patients (MD ‐7.10, 95% CI ‐10.88 to ‐3.32) (Duarte 2009). One study reported that transcutaneous electrical acupoint stimulation showed significant benefit compared with routine care (MD ‐6.26, 95% CI ‐10.27 to ‐2.25). There was improvement in uraemic pruritus for haemodialysis patients receiving manual acupuncture compared with sham acupuncture (MD ‐20.20, 95% CI ‐22.99 to ‐17.41) and an oral antihistamine (RR 1.38, 95% CI 1.10 to 1.72). Manual acupuncture was comparable with oral calcitriol (RR 1.00, 95% CI 0.89 to 1.12). One study in which manual acupressure was compared with an unspecified control intervention found a reduction of uraemic pruritus at six weeks (MD ‐6.50, 95% CI ‐7.50 to ‐5.50), which decreased over time but remained significant at 18 weeks (MD ‐4.80, 95% CI ‐6.23 to ‐3.37).
Three small studies reported a reduction in the severity of fatigue for patients receiving manual acupressure compared with routine care at four weeks (MD ‐1.19, 95% CI ‐1.77 to ‐0.60). One study of manual acupuncture compared with domperidone found favourable effects in overall gastrointestinal symptoms (MD ‐2.50, 95% CI ‐3.48 to ‐1.52), bloating (MD ‐0.82, 95% CI ‐1.13 to ‐0.51), and early satiety (MD ‐0.72, 95 CI ‐1.01 to ‐0.43). One study comparing manual acupuncture combined with antihypertensive medication with medication alone for pre‐dialysis CKD patients reported a significantly larger number of treatment responders, which was sustained at six months (RR 1.23, 95% CI 1.10 to 1.38). In four studies, manual acupressure improved sleep quality compared with routine care (MD ‐2.46, 95% CI ‐4.23 to ‐0.69). Transcutaneous electrical acupoint stimulation significantly improved sleep quality compared with routine care in one study (MD ‐3.43, 95% CI ‐5.57 to ‐1.29). One study comparing manual acupuncture with benzodiazepine reported significant sleep improvement (MD ‐6.70, 95% CI ‐8.19 to ‐5.21).
One study comparing acupressure with routine care, showed Improvements in quality of life, with improvements to the SF‐36 domains of role physical, bodily pain and vitality. Two studies in which moxibustion was compared with routine care reported improvements in the KDQOL domains of physical functioning, vitality and general health at three to four months, and physical functioning, vitality and cognitive function at six months. There was no significant difference between acupuncture interventions and comparators in many of the other domains of quality of life. Reasons for inconsistent results may include a possibility of effectiveness/ineffectiveness of acupuncture interventions on selective aspects of quality of life, inadequate power of studies to detect statistically meaningful differences in each subdomain of instrument, or a lack of instrument (i.e. generic questionnaires such as the SF‐36) responsiveness in patients undergoing dialysis (Patrick 2008).
Subjective assessments of nutritional status measured by MQSGA scores showed an improvement in haemodialysis patients receiving moxibustion compared with routine care at 12 weeks (MD ‐0.99, 95% CI ‐1.77 to ‐0.21) and 24 weeks (MD ‐0.95, 95% CI ‐1.69 to ‐0.21), but not at eight to nine weeks. One small study of manual acupuncture versus allopurinol and ibuprofen reported a short‐term benefit in the overall treatment responses, defined by symptom and kidney function improvement (RR 1.50, 95% CI 1.14 to 1.99). One study in which ear acupressure was compared with routine care found reductions in interdialytic weight gain (MD ‐0.29 % dry weight/d, 95% CI ‐0.38 to ‐0.20) and xerostomia (MD ‐8.01, 95% CI ‐9.21 to ‐6.81). In another small study there was an improvement in overall subjective comfort (MD 5.80, 95% CI 2.76 to 8.84). There were some significant changes in biochemical parameters after acupuncture interventions, including serum uric acid, serum creatinine, blood urea nitrogen, 24‐hour urine protein, amounts of ultrafiltration, serum prealbumin, high‐sensitivity C‐reactive protein, interleukin‐6, and residual kidney creatinine clearance.
The majority of studies comparing acupuncture interventions with sham intervention found no evidence of between‐group difference in the reduction of symptoms. Most studies lacked reports on whether adverse events occurred, which makes an assessment of the safety of acupuncture difficult. Some major events, such as death and hospitalisation, occurred during the study process, but there was no description of a possible association with the intervention. One study reported minor adverse events, such as soreness and minimal bleeding at needled points. Most studies suffered from high or unclear risk of bias, which render the reported benefits in the review questionable. Overall, there is currently little evidence that acupuncture interventions were more effective than sham interventions or were safe when used in CKD patients.
Overall completeness and applicability of evidence
The majority of the studies were conducted in China and Taiwan, which may result in the limited generalisability of the study findings in countries that have different health care systems and cultural backgrounds. For many of the studies, the participants were enrolled in haemodialysis centres or hospitals and were inpatients. Thus, the clinical contexts in included studies may be different from those in community or primary care settings. The stages of CKD were diverse and included pre‐dialysis CKD, maintenance haemodialysis, and peritoneal dialysis. Analyses based on stages of CKD were not possible because of the paucity of data. A wide range of acupuncture interventions and comparators was used, showing considerable clinical heterogeneity. We could not perform subgroup analyses to investigate this clinical heterogeneity because of the small number of studies within each subgroup; thus, the influence of clinical heterogeneity on the review results remains unclear. There was no evidence to suggest that particular types of acupuncture interventions, such as acupuncture, acupressure, moxibustion and far‐infrared radiation, are more effective. Most studies involving haemodialysis patients lacked reports on when the acupuncture treatment was provided (i.e. before, during or after a dialysis session). Because maintenance haemodialysis requires regular access to dialysis facilities, the allocation of additional patient time and resources for acupuncture treatments may be challenging. Additional visits or off‐site acupuncture may not be affordable to haemodialysis patients who have a reduced level of daily activity (Stener‐Victorin 2011). The safety of intradialytic or post‐dialysis acupuncture remains unclear, especially for patients experiencing adverse events because of haemodynamic instability or post‐dialysis fatigue. Acupuncture interventions before the start of the dialysis session may avoid such problems, but the time and space allocation required for the treatments may not be available in a busy dialysis schedule. Overall, the acceptability of acupuncture to patients and dialysis staff and the optimal delivery process in a given clinical context remains unclear. None of the included studies comparing acupuncture interventions with active controls, such as conventional medication, mentioned whether the aim of the research was to investigate the equivalence or non‐inferiority of acupuncture to an active comparator.
Quality of the evidence
Most studies had unclear or high risk of bias, especially in the domains of allocation concealment, blinding of participants, incomplete outcome reporting and selective outcome reporting (Figure 2; Figure 3). None of the included studies reported on allocation concealment which may make studies susceptible to significant risk of selection bias (Schulz 2002). Many studies compared acupuncture interventions with routine care or other active comparators, which made the blinding of patients and practitioners difficult and inevitably increased the risk of performance bias. In such cases, the appropriate blinding of outcome assessors can reduce risk of detection bias. However, for many studies, the blinding of outcome assessors was unclear. Most studies did not transparently report the number of participants for each outcome assessment, which increased the risk of bias based on incomplete outcome reporting. All included studies lacked information about where the study protocol can be accessed; thus, whether the studies selectively reported outcomes was unclear. Authors from only six studies replied to our requests for information; thus, the additional information was insufficient to determine whether those uncertainties come from a lack of reporting or from inadequate study design. Overall, the quality of current evidence is seriously impeded by these unclear or high risk of bias. The reported benefits should be interpreted with caution.
Potential biases in the review process
Five of the 24 included studies were quasi‐randomised studies, which calls for the careful interpretation of their findings. This review investigated symptom management. Thus, other important outcomes, such as mortality, hospitalisation, and preventing the progress of CKD were not analysed. We could not examine whether publication bias existed because of the insufficient number of studies. However, there is a possibility of publication bias because most of the studies reported positive outcomes. Pooled effect estimates were possible only for a few outcomes, such as depression, fatigue and sleep quality because of the paucity of data. Thus, the review largely relied on the results from single studies. Participants in most studies were those undergoing haemodialysis. The review could not explore the role of acupuncture interventions for patients with pre‐dialysis stage 3 to 4 CKD, those undergoing peritoneal dialysis or those without any renal replacement therapy. Most of the studies measured only short‐term outcomes (within one to two months); the long‐term effectiveness and safety of acupuncture interventions remain largely unknown in this review.
Agreements and disagreements with other studies or reviews
We have performed systematic reviews of the effects and the safety of acupuncture and acupressure for symptom management in patients with ESKD (Kim 2010a; Kim 2010b). Most of the analysed studies were also included in this review. There was little difference in the overall study findings between those two reviews and this review, except for the wider scope of population (patients with CKD stage 3 to 5) and intervention (diverse types of acupuncture interventions).
Authors' conclusions
Implications for practice.
We cannot make any solid recommendations unless further high‐quality randomised studies provide sufficient information. Clinicians and acupuncture practitioners should inform CKD patients who want to receive acupuncture interventions about the insufficient evidence of their effectiveness and safety. Manual acupressure may be a feasible add‐on treatment option for short‐term relief of depression, fatigue, and sleep disturbance in patients undergoing regular haemodialysis. Further well‐designed research should confirm the reported benefits and safety of such interventions before they are recommended in clinical practice. Indirect moxibustion may be used as complementary intervention for patients suffering from diminished quality of life in domains of physical function and perceived general health, although very low quality of evidence from two randomised studies supports such use. For other non‐needle forms of acupuncture‐related interventions such as ear acupressure, far infrared irradiation and transcutaneous electrical acupoint stimulation, we cannot make any clinical implication due to the paucity of data from reliable studies. Acupuncture interventions should be provided as a complementary, not a completely alternative, intervention in line with conventional managements because current evidence is mainly based on adjunctive uses of acupuncture to routine care and not on adequately designed equivalence or non‐inferiority testing studies. Qualified practitioners with appropriate clinical experience should implement acupuncture with concerns about any harm related to intervention. We discourage the long‐term application of acupuncture for more than three months without periodical screening for safety and effectiveness because of the lack of relevant information to justify such use.
Implications for research.
Future randomised controlled studies comparing acupuncture and related interventions with placebo, no treatments and active comparators such as medications, educational interventions, routine care, exercises or their combination are needed before making any conclusion about the role of acupuncture interventions for the symptom management in people with CKD. We suggest needle acupuncture with manual or electrical stimulation and manual acupressure as prioritised treatment interventions in future studies based on our review and body of evidence in non‐CKD population. Components of comparator interventions such as routine management should be transparently reported. For the studies comparing penetrating acupuncture with sham needling, non‐penetrating type of sham acupuncture (e.g. Park's sham needle) may also be a reasonable option as a control intervention. Outcomes for future research should include pain management, relief of symptoms that are known to be associated with increased mortality and poor illness trajectory (such as depression and sleep disturbance) and improvement of quality of life (Elder 2008; Lopes 2002; Mapes 2003). These outcomes should be measured through at least six months from the study, since current evidence provides only short‐term results of acupuncture interventions. Future studies should include participants with pre‐dialysis stage of CKD or patients with no renal replacement therapy, since substantial studies in this review studied patients undergoing dialysis. Studies that are conducted in various countries are required to explore the feasibility and generalisability of acupuncture interventions in different cultural backgrounds and medical systems. Whether short‐term intensive sessions of acupuncture interventions or ongoing but less‐frequent treatments are beneficial for patients was unclear in this review and may be of a topic of future research.
Further high‐quality RCTs should achieve methodological rigour including true randomisation methods, successful concealment of allocation, blinding of study participants (for sham‐controlled studies), personnel and outcome assessors (for all studies), follow‐up of all study participants, analyses of effectiveness based on data from all randomised patients, and complete and transparent reporting of pre‐defined outcomes. Prospective study registration should be normal practice to reduce publication bias and selective outcome reporting in future research. Authors should use the CONSORT and STRICTA statement as a guide for designing and reporting studies (Schulz 2010; MacPherson 2010). In future studies in which acupuncture interventions are compared with active comparators, research should employ appropriate equivalence or non‐inferiority tests for valid analysis of study findings. Authors should completely report the harms of acupuncture to enable a systematic investigation of the safety of acupuncture interventions in CKD patients. For instance, pre‐specifying potential adverse events and methods for collecting information from heterogeneous sources (e.g. medical records, researcher/personnel reporting or self‐reporting of participants/caregivers) as recommended by CONSORT for harm extension (Ioannidis 2004) will be essential for generating reliable evidence on the safety of acupuncture in CKD patients. Association between given interventions and occurrence of serious adverse events such as death or hospitalisation should be investigated and transparently reported. Both closed‐end questions such as pre‐defined checklists or structured questionnaires and patient‐reported narratives can be used to solicit the reporting of patient‐perceived adverse events (King 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2016 | Amended | Contact details updated. |
Acknowledgements
We would like to thank Gail Higgins for performing the searches for this manuscript and Narelle Willis and Leslee Edwards (Cochrane Kidney and Transplant) for helping the authors complete the review. We are also thankful to the authors of the included studies who provided additional information for this review and to the editors and referees who contributed their invaluable comments to improve the manuscript.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Search strategies for Korean and Chinese databases
We conducted comprehensive searches of Korean databases to identity Korean RCTs of acupuncture from the time of their inception to July 2011. We also searched unpublished theses and dissertations. We selected simple search terms because most Korean electronic databases supported only simple Boolean searches. We also searched studies recorded electronically on the websites of seven acupuncture‐related journals to ensure completeness of the search process.
Figure 37 shows the search strategy for the Korean databases
4.

A list of seven acupuncture‐related journals
Journal of Korean Acupuncture and Moxibustion Society
Korean Journal of Acupuncture (formerly the Journal of Korean AM‐Meridian & Pointology Society)
Journal of Pharmacopuncture
Journal of Oriental Rehabilitation Medicine
Journal of Korea CHUNA Manual Medicine for Spine & Nerves
Journal of Korean Oriental Medicine
Journal of Korean Oriental Internal Medicine
In the Chinese database (China Academy Journal), following Chinese search terms were used.
1. 针
2. 针刺
3. 针灸
4. 电针
5. 耳针
6. 头针
7. #1‐6 (these correspond to the English term "acupuncture")
8. 灸
9. 艾灸
10. 灸法
11. 艾条
12. 隔灸
13. #8‐12 (corresponding to the term "moxibustion")
14. 穴位按压
15. 按摩
16. 指压
17. 推拿
18. #14‐17 (corresponding to the term "acupressure" or "Tui Na")
19. 经络
20. 经线
21. 穴位
22. #19‐21 (corresponding to the term "meridian" or "acupuncture point")
23. #7 or #13 or #18 or #22
24. 慢性肾
25. 慢性肾脏病
26. 慢性肾脏疾病
27. 慢性肾病
28. #24 ‐ 27 (corresponding to the term "chronic kidney disease")
29. 终末期肾病
30. 终末期肾脏疾病
31. 终末期肾衰竭
32. 终末期肾衰
33. #29‐32 (corresponding to the term "end‐stage renal disease")
34. 尿毒
35. 尿毒症
36. #34‐35 (corresponding to the term "uremia")
37. 血液透析
38. 血液渗析
39. 腹膜透析
40. #37‐39 (corresponding to the term "hemodialysis" or "peritoneal dialysis")
41. #28 or #33 or #36 or #40
42. #23 and #41
Appendix 3. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 4. Summarized details of acupuncture interventions
The acupuncture points used were classic points (20), non‐classical extra points (Song 2007), ear points (Xie 2012; Zhao 2011) and undefined body points (Jedras 2003). No study employed myofascial trigger points. The number of acupuncture points used was less than 10 in all studies, except for one study where the number of used points was 140 (Jedras 2003).
The formulation of acupuncture points was either standardised (all mandatory points in all participants) (20) or semi‐standardised (some mandatory points plus predefined point selections depending on concomitant conditions) (3) (Ma 2004; Xie 2012; Zhao 2011). One study used 140 points, but whether a standardised or flexible regimen was used was unclear (Jedras 2003). No study used fully individualised point selection.
De‐qi sensation (i.e. a unique feeling of patients following successful acupuncture point stimulation) was reported in 11 studies (Cho 2004; Che‐Yi 2005; Dai 2007a; Ma 2004; Song 2007; Tsay 2003a; Tsay 2004a; Tsay 2004b; Shariati 2012; Xie 2012; Zhao 2011). Three studies reported skin redness and/or warm feeling after indirect moxibustion (Sun 2012; Qiu 2012; Sun 2008a). All studies conducted treatment intervention based on traditional acupuncture theory.
No information about the possible therapeutic relationship between participants and acupuncture practitioner was reported in most of included studies. However, one study author (Che‐Yi 2005) provided additional information by author contact indicating that there was minimal participant‐practitioner relationship in the experimental sham‐controlled study. Another study reported that the participants were allowed to ask any questions about the study at any stage, although it remains unclear whether therapeutic interaction occurred (Lin 2011).
Among 19 studies involving patients with HD, nine studies conducted treatments during HD (Cho 2004; Che‐Yi 2005; Rui 2002; Su 2009; Sun 2008a; Tsay 2003a; Lin 2011; Shariati 2012; Qiu 2012). One study provided acupressure treatments immediately before or after HD (Jedras 2003). One study conducted indirect moxibustion treatments when blood pressure was stabilised after initiation of dialysis treatments at each HD session only in case of patients without any intradialytic complication (Sun 2008a). Two studies employed the seed or magnetic‐attachment technique on ear acupuncture points to provide constant point‐stimulation over the study period (Xie 2012; Zhao 2011).The remaining eight studies did not report when the acupuncture treatments were delivered (Gao 2002; Zhao 1995; Hsu 2009; Tsay 2004a; Tsay 2004b; Cui 2012; Zhang 2011d).
Appendix 5. STRICTA1 information of acupuncture interventions
| Study | Treatment | Control |
| Che‐Yi 2005 | Manual acupuncture (20) Theoretical basis: Traditional acupuncture theory Style of acupuncture: fixed formula Number of points selected: 1 Points stimulated: LI11 (unilateral) Depth of insertion: 1.5 inch (author contact) Needle sensation: usually patient felt soreness or light electric shock sensation (author contact) Methods of point stimulation: no stimulation was used (author contact) Needle retention time: 1 hour Needle type: 1‐inch 34‐gauge acupuncture needle. (manufacturer and material information not reported) Delivery time of acupuncture: during HD (author contact) Practitioner information: "All the acupuncture was performed by one physician who practiced acupuncture for more than 5 years." (author contact) Information on treatment context: "The physician performed the acupuncture and left the bedside. There was not much interaction between the physician and patients. A haemodialysis physician always stayed in the dialysis room who was responsible for caring any complain." (author contact) |
Sham acupuncture (20) Style of acupuncture: fixed formula Number of points selected: 1 Points stimulated: 2 cm lateral to LI11 (unilateral) Depth of insertion: 1.5 inch (author contact) Needle sensation: "Some patients still felt light electric shock sensation for the sham acupuncture. However, the needle sensation was still different from the treatment group." (author contact) Methods of point stimulation: no stimulation (author contact) Needle retention time: 1 hour Needle type: 1‐inch 34‐gauge acupuncture needle (manufacturer and material information not reported) Delivery time of acupuncture: during HD (author contact) Practitioner information: all the acupuncture was performed by one physician who practiced acupuncture for more than 5 years (author contact) Concomitant treatments: patients in both groups continued previous medications during the study, including antihistamines and phosphate binders |
| Cheng 2012 | Indirect moxibustion on ginger slices (30) Style of moxibustion: TCM style Rationale of moxibustion: not reported Extent to which treatment was varied: fixed Number of moxibustion points: 2 Names (or location if no standard name) of points used: CV8, CV12 Stimulated uni/bilateral: not applicable (the point was located centrally) Response sought: not reported Moxibustion stimulation methods: 3 zhuang (application of 3 moxibustion constituting a single session) moxibustion on the sliced ginger pieces (2 cm of diameter and 3mm of thickness) Moxibustion retention time: flexible (when patients felt uncomfortable burning sensation on moxibustion points during the treatment, the moxibustion was removed and the rest phase was allowed to cool down the skin. When this was achieved, treatments were continued) Moxibustion type: Indirect moxibustion on ginger slices (2 cm of diameter and 3 mm of thickness) Number of treatment sessions: 7 to 10 sessions Frequency and duration of treatment sessions: daily for 7 to 10 days Details of other interventions administered to the moxibustion group: a close attention of practitioners was paid not to make patients being burned by moxibustion. Setting and context of treatment: not reported Delivery time of moxibustion: not reported Description of participating acupuncturists: not reported |
Western conventional care (30) Precise description of the control or comparator: metoclopramide 10 mg (IM injection once/day) and domperidone 10mg (oral administration 3 times/day) Any co‐interventions in all groups: CAPD and other routine care including blood pressure and glucose control, anaemia correction, symptom managements and dietary modification such as low salt, low fat and low protein meals |
| Cho 2004 | Manual acupressure (28) Theoretical basis: traditional acupuncture theory (Yang Xu and Qi Xu) Style of acupressure: fixed formula Number of points selected: 4 Points stimulated: ST36, SP6, KI3, KI1 (unilateral or bilateral not reported) Acupressure sensation: sore, numb, heavy, distended and/or warm during the acupoint massage Methods of point stimulation: finger acupressure (manual) Duration of acupressure treatment per session: acupressure stimulation for 12 min (3 min for each point) and lower extremity massage for 3 min (totally 15 min) Delivery time of acupressure: during HD Practitioner information: one registered nurse (the first author of this article). No information about the period of relevant clinical experience for acupressure treatment was not provided. Information on treatment context: not reported |
Routine care (30) Details of routine care: routine care from the HD unit. Other informations were not reported Practitioner information: not reported Concomitant treatments: not reported |
| Cui 2012 | Acupuncture plus oral administration of domperidone (30) Style of acupuncture: TCM style Rationale of acupuncture: not reported Extent to which treatment was varied: fixed Number of acupuncture points: 8 points Names (or location if no standard name) of points used: ST36, PC6, CV12, LR3, KI3, SP9, CV6, GV20 Needled uni/bilateral: not reported Depth of insertion: not reported Response sought: not reported Needle stimulation methods: manual manipulation of needle using plain tonification‐reduction technique Needle retention time: 30 min Needle type (length and diameter): piriform needle with 40 mm of length and 0.40 mm of diameter Number of treatment sessions: 14 Frequency and duration of treatment sessions: daily administration of acupuncture during 2 weeks Details of other interventions administered to the acupuncture group: not reported Setting and context of treatment: not reported Delivery time of acupuncture: not reported Description of participating acupuncturists: not reported |
Domperidone alone (30) Rationale for the control or comparator in the context of the research question: not reported Precise description of the control or comparator: domperidone 10 mg, 3 times/day Any co‐interventions in all groups: Western conventional care |
| Dai 2007a | Manual acupressure (42) Theoretical basis: traditional acupuncture theory Style of acupuncture: fixed formula Number of points selected: 4 Points stimulated: ST36, SP6, KI3, KI1 (unilateral or bilateral not reported) Acupressure sensation: patients' feelings of sore, numb, heavy, distended and/or warm during the acupoint massage Methods of point stimulation: finger acupressure (manual) Duration of acupressure treatment per session: 20 to 30 min/session Delivery time of acupressure: not reported Practitioner information: not reported Information on treatment context: not reported |
Conventional medicine (40) Details of drug treatment: 1 mg of estazolam, orally intake before sleep once daily for 4 weeks Provider information: not reported Concomitant treatments: not reported |
| Gao 2002 | Manual acupuncture (34) Style of acupuncture: TCM style Rationale of acupuncture: TCM theory and Western medicine theory Extent to which treatment was varied: fixed Number of needle insertions: 2 Names (or location if no standard name) of points used: LI11, ST36 Needled uni/bilateral: not reported Depth of insertion: not reported Response sought: not reported Needle stimulation methods: lifting‐thrusting reducing method for LI11, lifting‐thrusting reinforcing method for ST36 Needle retention time: 30 min Needle type (length and diameter): not reported Number of treatment sessions: 8 Frequency and duration of treatment sessions: 2 times/week for 4 weeks Details of other interventions administered to the acupuncture group: not reported Setting and context of treatment: not reported Delivery time of acupuncture: During HD Description of participating acupuncturists: not reported |
Conventional medicine (34) Rationale for the control or comparator in the context of the research question: not reported Precise description of the control or comparator: Chlor‐trimeton 4mg 3 times/d (first‐generation H1‐receptor antagonist) and topical ointment for dermatitis (Sanjiu Medical Company). For 2 weeks. No other information available. Any co‐interventions in all groups: not reported |
| Hsu 2009 | Far infrared radiation (FIR) (24) Theoretical basis: traditional acupuncture theory and conventional theory (sympathetic nervous system stimulation via higher temperature far infrared stimulation) Style of FIR: fixed formula Number of points selected: 1 or 2 Points stimulated: SP6 (unilateral or bilateral not reported) Depth of insertion: N/A (non‐penetrating stimulation) Sensation of point stimulation: not reported Methods of point stimulation: far infrared radiation with a constant temperature of 40°C on SP6 Total number of treatment session: 18 Frequency and duration of treatment: 2 times/week for 2 months (8 weeks) Duration of FIR treatment per session: 15 min/session Delivery time of FIR: not reported Practitioner information: One principal investigator who had experience in caring for HD patients. The principal investigator (PI) underwent training to determine the acupoint, and the accuracy of acupoint selection was confirmed by TCM doctor. However, no information was available how long PI has been trained for acupoint determination. Information on treatment context: Information on practitioner‐patient relationship was not reported. In a quiet and warm room with a FIR device, participants were placed in a supine position on a bed for 15 min and kept warm by being wrapped in a blanket. Participants remained on bed rest with the blanket for an additional 30 min after the thermal therapy in each session. |
Plain adhesive patch (25) Details of control group intervention: Plain adhesive patch was placed on the same acupoint. Other information were lacked Provider information: not reported. The PI stayed with participants in control group for the same duration as the thermal therapy group Concomitant treatments: Folic acid for health problems (38), calcium (27) |
| Jedras 2003 | Manual acupressure (30) Style of acupressure: not reported, but probably traditional Chinese or Mongolian styles Rationale of acupressure: not reported Extent to which treatment was varied: unclear Names (or location if no standard name) of points used: 140 pressure points on the entire body surface (20 points each on the head, both hands, anterior and posterior trunk, and both legs) Uni/bilateral: not reported, but probably bilateral Response sought: not reported Stimulation methods: massage, compression and slapping on acupressure points Whole time of treatment: 15 to 20 min of total acupressure time per session Number of treatment sessions: 15 sessions Frequency and duration of treatment sessions: 3 sessions a week for 5 consecutive weeks Treatment delivery time: immediately before or after the dialysis (for HD patients). Not reported about peritoneal dialysis patients Details of other interventions administered to the acupressure group: not reported Setting and context of treatment: not reported Description of participating treatment providers: an experienced Mongolian doctor |
Unspecified control intervention (30) Rationale for the control or comparator in the context of the research question: no information on the control intervention other than that the control intervention is not a placebo treatment Any co‐interventions in all groups: not reported |
| Lin 2011 | FIR on acupoints (36) Style of acupuncture: TCM style Rationale of acupuncture: previous study (reference provided) Extent to which treatment was varied: fixed Number of point stimulation: 4 Names (or location if no standard name) of points used: CV3, CV4, CV6, ST25 Stimulated Uni/bilateral: unclear Response sought: not reported Stimulation methods: FIR Number of treatment sessions: 24 Frequency and duration of treatment sessions: 3 times/week and 30 min of FIR per session Details of other interventions administered to the acupuncture group: not reported Setting and context of treatment: Patients were trained to administer FIR acupoint treatments. A brief explanatory note for administering FIR acupoints were also provided to the patients Delivery time of acupuncture: not reported (self‐administration of FIR acupoints) Description of participating acupuncturists: self‐administration by trained patients |
No intervention (25) Rationale for the control or comparator in the context of the research question: not reported Precise description of the control or comparator: no intervention Any co‐interventions in all groups: not reported |
| Ma 2004 | Manual acupuncture (42) Style of acupuncture: TCM style Rationale of acupuncture: not reported Extent to which treatment was varied: semi‐standardised Number of needle insertions: not exactly reported Names (or location if no standard name) of points used: 1) main points: BL23, BL22, CV3, CV4, SP10, SP6, KI3 (these points were divided into two groups for alternating application). 2) supplement points: if acute arthritis, LI11, LI4, ST44 added. If dizziness, GB20, LR3 added. If oppressed feeling in the chest and palpitation, PC6, HT7 used Needled uni/bilateral: not reported Depth of insertion: not reported Response sought: Yes. (arrival of qi) Needle stimulation methods: manual stimulation (uniform reinforcing‐reducing manipulation) Needle retention time: 30 min Needle type (length and diameter): not reported Number of treatment sessions: 10 Frequency and duration of treatment sessions: once/day with 10 sessions as a course of treatment (10 days) Details of other interventions administered to the acupuncture group: not reported Setting and context of treatment: not reported Delivery time of acupuncture: outpatient visit Description of participating acupuncturists: not reported |
Conventional medicine (30) Rationale for the control or comparator in the context of the research question: not reported Precise description of the control or comparator: orally given allopurinol, 100 mg each time, 2 to 3 times/day. Ibuprofen was added for those with joint swelling, 200 mg per each time, 3 times daily. Any co‐interventions in all groups: not reported |
| Qiu 2012 | Moxibustion (58) Style of moxibustion: TCM style Rationale of moxibustion: not reported Extent to which treatment was varied: fixed Number of moxibustion points: 2 points Names (or location if no standard name) of points used: ST36, SP6 (ST36 and SP6 alternated by each day) Moxibustion uni/bilateral: unilateral Response sought: yes (feeling of local skin flushing by patients) Moxibustion stimulation methods: smokeless indirect moxibustion per point 1 to 2 zhuang. According to the environmental requirement of blood purification centre, smokeless moxibustions were used Moxibustion retention time: not reported Moxibustion type (length and diameter): smokeless moxibustion (with use of paper tube production Acupoint moxibustion device, which were made by Taizhou City, Jiangsu Province, Moxibustion Research Institute) Number of treatment sessions: 24 to 36 Frequency and duration of treatment sessions: 2 to 3 times/week for 12 weeks in every HD session Delivery time of acupuncture: during HD Details of other interventions administered to the acupuncture group: not reported Setting and context of treatment: not reported Description of participating acupuncturists: research staff (details of qualification were not reported) |
Routine care (51) Rationale for the control or comparator in the context of the research question: “Routine haemodialysis and medicine treatment” Precise description of the control or comparator: standard bicarbonate HD, routine HD therapy including erythropoietin, iron for renal anaemia, phosphorus binding (calcium carbonate), calcitriol or alfacalcidol alcohol treatment of secondary hyperparathyroidism). Other details for medication (dose, time) were not reported Any co‐interventions in all groups: Western conventional care |
| Rui 2002 | Manual acupuncture (80) Style of acupuncture: TCM style Rationale of acupuncture: not reported Extent to which treatment was varied: fixed Number of needle insertions: 4 Names (or location if no standard name) of points used: LI11, ST36, ST35, SP6 Needled uni/bilateral: unclear Depth of insertion: not reported Response sought: not reported Needle stimulation methods: Manual stimulation (plain tonification and reduction technique) Needle retention time: 20 min Needle type (length and diameter): not reported Number of treatment sessions: 24 or 32 sessions Frequency and duration of treatment sessions: 2 times/week or 3 times within 2 weeks Delivery time of acupuncture: during HD Details of other interventions administered to the acupuncture group: not reported Setting and context of treatment: not reported Description of participating acupuncturists: not reported |
Conventional medicine (70) Rationale for the control or comparator in the context of the research question: not reported Precise description of the control or comparator: Calcitriol 2 μg just after the end of HD on dialysis day per one intake. Two times a week or three times within 2 weeks for 16 weeks. Calcium carbonate (CaCO3) as phosphate binder to manage serum phosphorus level when necessary Any co‐interventions in all groups: not reported |
| Shariati 2012 | Acupressure (22) Style of acupressure: TCM style Rationale of acupressure: literature search and experts consensus Extent to which treatment was varied: fixed Names (or location if no standard name) of points used: ST36, LI4, HT7 Uni/bilateral: not reported Response sought: feeling of sore, numb, heavy, distended and/or warm during the acupoint massage Stimulation methods: finger acupressure Treatment time: acupressure stimulation for 12 min (3 min for each point) and lower extremity massage for 3 min Number of treatment sessions: 12 Frequency and duration of treatment sessions: 3 times/week for 4 weeks Details of other interventions administered to the acupuncture group: not reported Setting and context of treatment: not reported Delivery time of acupuncture: Acupressure was delivered during HD (one hour after beginning of HD because patients were in better emotional status at that time) Description of participating acupuncturists: trained investigator and assistant for applying acupressure massage under supervision of a specialist |
Routine care (22) Control group received only routine care from the HD unit. No further explanation for components of routine care was provided Any co‐interventions in all groups: routine care |
| Song 2007 | Acupuncture and antihypertensive medication (76) Style of acupuncture: TCM style Rationale of acupuncture: two previous studies (traditional theory) Extent to which treatment was varied: fixed Number of moxibustion points: 2 points Names (or location if no standard name) of points used: antihypertension point (located on the middle point of plantar arch), kidney disease point (8 cm upper the prominence of lateral malleus, medial border of fibula, i.e. one third from the fibula head to the prominence of lateral malleus) (left and right side alternated) Needled uni/bilateral: unilateral (side alternated) Depth of insertion: not reported Response sought: yes Needle stimulation methods: moderate to strong stimulation by thrusting‐lifting technique until arrival of the de‐qi response Needle retention time: not reported Needle type (length and diameter): not reported Number of treatment sessions: not clearly reported but probably more than 100 sessions (daily acupuncture treatment for 2 weeks as one treatment course with 3‐day between‐course interval. 24 weeks as a total treatment period) Frequency and duration of treatment sessions: daily treatments for 2 weeks; duration of sessions was not reported Details of other interventions administered to the acupuncture group: no information on the use of acupuncture‐specific co‐intervention Setting and context of treatment: not reported Delivery time of acupuncture: not reported Description of participating acupuncturists: not reported |
Antihypertensive medication (76) Rationale for the control or comparator in the context of the research question: not reported Precise description of the control or comparator: oral irbesartan 150mg once daily and fosinopril (ACEi) 10 mg once daily for 24 weeks Any co‐interventions in all groups: oral irbesartan (ARB) 75 mg once/day. if the target DBP (< 80 mm Hg) were not achieved, additional oral intake of hydrochlorothiazide 12.5 to 25 mg was provided (dose and frequency was not reported) |
| Su 2009 | FIR stimulation (34) Rationale of acupoint stimulation: both TCM and contemporary (FIR stimulation can increase the excretion rate of bodily wastes) rationale of point selection and stimulation were suggested with references Extent to which treatment was varied: fixed Number of point stimulation: 3 Names (or location if no standard name) of points used: CV6, CV4, CV3 Stimulated uni/bilateral: not applicable (all points locate on the midline of anterior trunk) Response sought: not reported Stimulation methods: a constant temperature of 40 degrees celsius FIR on acupoints FIR retention time: 30 min Information on point stimulator: not reported Number of treatment sessions: 36 Frequency and duration of treatment sessions: 3 times/week for 12 weeks Details of other interventions administered to the FIR group: not reported Setting and context of treatment: not reported Delivery time of acupoint stimulation: during the HD session Description of participating acupuncturists: not reported |
Heat pad therapy (35) Rationale of control stimulation: not reported Precise description of the control or comparator: heat pad therapy application on the same acupoints Any co‐interventions in all groups: not reported |
| Sun 2008a | Moxibustion (37) Style of moxibustion: TCM style Rationale of moxibustion: not reported Extent to which treatment was varied: fixed Number of stimulated points: 3 points Names (or location if no standard name) of points used: CV4, ST36, SP6 (ST36 and SP6 alternated by each day) Stimulated uni/bilateral: unilateral Response sought: yes (skin colour turning red) Stimulation methods: smokeless indirect moxibustion Moxibustion retention time: 6 min (2 unit of moxibustion/each point) Type of moxibustion (length and diameter): not reported Number of treatment sessions: 24 to 36 Frequency and duration of treatment sessions: 2 to 3 times for 12 weeks in every HD session Details of other interventions administered to the moxibustion group: not reported Setting and context of treatment: not reported Delivery time of moxibustion: After blood pressure is stabilized during HD, moxibustion was performed. Patients who showed adverse reaction during HD were excluded from moxibustion treatments Description of participating acupuncturists: research staffs. (details of qualification was not reported) |
Routine care (34) Rationale for the control or comparator in the context of the research question: Western routine practice Precise description of the control or comparator: conventional medication for symptom management and supportive care (details not reported) Any co‐interventions in all groups: not reported |
| Sun 2012 | Indirect moxibustion with herb cake (13) Style of moxibustion: TCM style Rationale of moxibustion: not reported Extent to which treatment was varied: not reported Number of moxibustion points: 1 Names (or location if no standard name) of points used: CV8 Stimulated uni/bilateral: not applicable (the point was located centrally) Response sought: not reported Stimulation methods: 10 zhuang (application of 10 moxibustion constituting a single session) moxibustion on the herb cake (consisted of Cnidium officinale, Asarum sieboldii, synthetic musk, Lumbricidae, Epimedium koreanum Nakai, Poria Cocos) attached at the umbilicus (CV8) Moxibustion retention time: not applicable Moxibustion type (length and diameter): herb‐cake moxibustion Number of treatment sessions: 120 sessions Frequency and duration of treatment sessions: 2 times/day for 60 days Details of other interventions administered to the moxibustion group: not reported Setting and context of treatment: not reported Delivery time of moxibustion: not reported Description of participating acupuncturists: not reported |
Western routine care (13) Rationale for the control or comparator in the context of the research question: CAPD Precise description of the control or comparator: CAPD and other routine care including blood pressure and glucose control, anaemia correction, electrolyte control and tailored dietary modification such as high protein meals” Any co‐interventions in all groups: Western routine care |
| Tsay 2003a | Manual acupressure plus routine care (35) Style of acupressure: TCM style Rationale of acupressure: literature review and consensus of 5 licensed TCM practitioners Extent to which treatment was varied: fixed Number of acupressure points: 3 or 6 points (depending on the uni‐ or bi‐lateral selection of acupressure points) Names (or location if no standard name) of points used: KI1, HT7, ear Shenmen Stimulated uni/bilateral: not reported Response sought: de‐qi response ("subject felt sore, numb, heavy, distended, and/or warm sensation.") Stimulation methods: finger pressure on acupuncture points with the force of 3 to 4 kg Stimulation time: totally 14 min (5 min of massage to relax the person and 3 min of acupoints massage; 3 min/acupoints) Number of treatment sessions: 12 sessions Frequency and duration of treatment sessions: 3 times/week for 4 consecutive weeks Details of other interventions administered to the acupressure group: not reported Setting and context of treatment: not reported Delivery time of acupressure: during HD Description of participating acupuncturists: research investigators and assistants specifically trained two months for the study by an acupressure treatment expert The reliability and validity of the acupressure treatment was assessed by applying consistent pressure on the correct acupoints to the same patient, and using a scale (20 g to 6 kg) to measure the force of finger pressure between 3 to 4 kg. Two experts evaluated the accuracy of acupoints selected for this study and confirmed this with 100% agreement |
Control group 2 Sham acupressure and Routine care (32) Precise description of the control or comparator: non‐acupoints, 1 cm (corresponding to body unit) away from meridian. Otherwise, the same treatment protocol with the manual acupressure group was applied |
| Control group 2 Routine care alone (31) Any co‐interventions in all groups: not reported | ||
| Tsay 2004a | Manual acupressure plus routine care (35) Style of acupressure: TCM style Rationale of acupressure: literature review and consensus of 5 licensed TCM practitioners Extent to which treatment was varied: fixed Number of acupressure points: 8 points Names (or location if no standard name) of points used: KI1, SP6, ST36, GB34 Stimulated uni/bilateral: bilateral Response sought: de‐qi response ("subject felt sore, numb, heavy, distended, and/or warm sensation.") Stimulation methods: finger pressure on acupuncture points with the force of 3 to 4 kg Stimulation time: totally totally 15 min (3 min of massage to relax the person and 12 min of acupoints massage; 3 min/acupoints) Number of treatment sessions: 12 sessions Frequency and duration of treatment sessions: 3 times/week for 4 consecutive weeks Details of other interventions administered to the acupressure group: not reported Setting and context of treatment: not reported Delivery time of acupressure: during HD (not clearly reported but probably, compared to other studies conducted by the same authors) Description of participating acupuncturists: research investigators and assistants specifically trained one month for the study by an acupressure treatment expert The reliability and validity of the acupressure treatment was assessed by applying consistent pressure on the correct acupoints to the same patient, and using a scale (20 g to 6 kg) to measure the force of finger pressure between 3 to 4 kg. Two experts evaluated the accuracy of acupoints selected for this study and confirmed this with 100% agreement |
Control group 1 Sham acupressure and routine care (35) Precise description of the control or comparator: non‐acupoints. Acupuncture points, sessions and stimulation duration was identical with the manual acupressure group. For the sham acupressure intervention, pressure intensity and obtaining de‐qi response were not reported. |
| Control group 2 Routine care alone (36) Any co‐interventions in all groups: not reported | ||
| Tsay 2004b | manual acupressure plus routine care (36) Style of acupressure: TCM style Rationale of acupressure: literature review and consensus of 5 licensed TCM practitioners Extent to which treatment was varied: fixed Number of acupressure points: 8 points Names (or location if no standard name) of points used: KI1, SP6, ST36, GB34 Stimulated uni/bilateral: bilateral Response sought: de‐qi response ("subject felt sore, numb, heavy, distended, and/or warm sensation.") Stimulation methods: finger pressure on acupuncture points with the force of 3 to 4 kg Stimulation time: totally totally 15 mins (3 min of massage to relax the person and 12 min of acupoints massage; 3 min/acupoints) Number of treatment sessions: 12 sessions Frequency and duration of treatment sessions: 3 times/week for 4 consecutive weeks Details of other interventions administered to the acupressure group: not reported Setting and context of treatment: not reported Delivery time of acupressure: during HD (not clearly reported but probably, compared to other studies conducted by the same authors) Description of participating acupuncturists: research investigators and assistants specifically trained one month for the study by an acupressure treatment expert The reliability and validity of the acupressure treatment was assessed by applying consistent pressure on the correct acupoints to the same patient, and using a scale (20 g to 6 kg) to measure the force of finger pressure between 3 to 4 kg. Two experts evaluated the accuracy of acupoints selected for this study and confirmed this with 100% agreement |
Control group 1 TEAS plus routine care (36) Precise description of the control or comparator: The TEAS was standardised before each treatment and was set at 2Hz alternating with 100Hz, each lasting for 3 seconds, which has been documented as the most effective frequency. A TEAS was used. Dr. Han, a physiologist at Peking medical University, Peking, China, developed and tested the TEAS (HANS LH202H). Points, stimulation duration and sessions were identical to the acupressure group Control group 2 Routine care alone (36) Any co‐interventions in all groups: not reported |
| Xie 2012 | Ear acupressure plus routine care (44) Style of acupressure: TCM style Rationale of acupressure: previous research literature Extent to which treatment was varied: semi‐standardised Number of acupressure points: 6 core points plus 1 to 3 additional points based on TCM diagnosis were used (totally 6 to 9 points) Names (or location if no standard name) of points used: core points (upper tragus, lower tragus, mouth, sympathetic, ear shenmen, spleen), additional points (kidney for yan deficiency; external ear for yin deficiency; kidney and external ear for yin and yang deficiency; heart, liver and lung for blood stasis; ear Lánwĕixué, subcortical and large intestine for dampness) Stimulated uni/bilateral: unilateral (ear points on both sides were used alternatively) Depth of insertion: not applicable (non‐penetrating pressure) Response sought: de‐qi response (Aching, soreness, distension, heaviness, warmth and dull pain evoked by ear acupressure) Stimulation methods: manual pressure of Vaccaria seeds attached on ear points by using tonification (mild intensity pressure), plain tonification‐reduction (moderate intensity pressure) and reduction (heavy pressure) technique. Pressure was applied for one minute, three to five times daily. Ear points were alternated on contralateral side per three to seven days. Ear acupressure retention time: Vaccaria seeds were attached on ear points during the study process. Number of treatment sessions: almost 56 sessions (one day was regarded as one treatment session) Frequency and duration of treatment sessions: daily administration of acupressure during eight weeks Details of other interventions administered to the acupressure group: not reported Setting and context of treatment: not reported Delivery time of acupressure: not reported Description of participating acupuncturists: not reported |
Routine care alone (46) Precise description of the control or comparator: symptom management and maintenance dialysis (details were not reported) Any co‐interventions in all groups: routine care |
| Zhang 2011d | Acupuncture and moxibustion plus HDF (15) Style of acupuncture: TCM style Rationale of acupuncture: TCM theory and Western medicine theory Extent to which treatment was varied: fixed Number of needle insertions: 6 Names (or location if no standard name) of points used: LU5, LI11, LI4, ST36, SP10, BL17 Needled uni/bilateral: unclear Depth of insertion: not reported Response sought: not reported Needle stimulation methods: reduction technique, manipulated every 10 min Needle retention time: 30 min Needle type (length and diameter): not reported (piriform needles used) Number of treatment sessions: 10 or 20 Frequency and duration of treatment sessions: once daily for 10 or 20 days Details of other interventions administered to the acupuncture group: not reported Moxibustion: no information on the use of moxibustion was reported Setting and context of treatment: not reported Delivery time of acupuncture: not reported Description of participating acupuncturists: not reported |
Control group 1 HDF group (15) Precise description of the control or comparator: one HDF and two HD sessions/week during the study period |
| Control group 2 HD group (16) Precise description of the control or comparator: 3 HD sessions/week during the study period Any co‐interventions in all groups: not reported | ||
| Zhao 1995 | Indirect moxibustion with herb cake (33) Herb cakes were made of Astragalus membranaceus, Angelica sinensis, Psoralea corylifolia L., Curculigo orchioides Gaertner that were passed through a sieve; a diameter of 3 cm and a thickness of 0.8 cm. A diameter of 2 cm, a height of 1.5 cm, a weight of 1.5 g of Mugwort moxa on the herb cakes on each points were burnt. Three moxa were burnt on each points in each session Theoretical basis: traditional acupuncture theory Style of moxibustion: fixed formula Number of points selected: 11 (5 or 6 points used in each sessions) Points stimulated: totally 11 points were divided by 2 groups (group 1: GV14, BL20, BL23 (bilateral), group 2: CV17, CV22, CV8, CV4, ST36 (bilateral)) and employed in an alternating manner. Moxibustion sensation: not reported Methods of point stimulation: Indirect moxibustion with herb cake Duration of moxibustion treatment per session: not reported Duration of intervention: 7 weeks Moxibustion type: not reported Delivery time of moxibustion: not reported Practitioner information: not reported Information on treatment context: not reported |
Routine care (15) Precise description of the control or comparator: ‐ Symptomatic management: electrolytic imbalance correction, anti‐infective care, protein restriction, water restriction. ‐ Negative nitrogen balance, amino‐acid supplementation, sodium polystyrene sulfonate (Kayexalate), muscular injection of compound danshe, loop diuretics (furosemide) (for non‐dialytic ESKD patients) Any co‐interventions in all groups: routine care |
| Zhao 2011 | Ear acupressure using magnetic bead plaster (30) Style of acupuncture: TCM style Rationale of acupressure: not reported Extent to which treatment was varied: not reported Number of auricular point magnetic bead plaster points: 4 Names (or location if no standard name) of points used: ear point Fixed points: Shenmen, subcortex, sympathetic, heart; individual points: liver, bile, kidney, spleen, Stomach Stimulated uni/bilateral: take the side of each ear, 2 days later using another ear Response sought: not reported Stimulation methods: daily self‐pressure of 4 times (3 meals/day before meals 30 min and before at bedtime 30 min, total 4 times), take the side of each ear, 2 days later using another ear Attached plaster retention time: 48 hours Stimulator type (length and diameter): magnetic bead Number of treatment sessions: 24 sessions Frequency and duration of treatment sessions: twice daily for 56 days Details of other interventions administered to the acupressure group: not reported Setting and context of treatment: not reported Delivery time of acupressure: not reported Description of participating acupuncturists: not reported |
Routine care (30) Precise description of the control or comparator: management of sleep disorders and supporting comfort status in maintenance HD patients Any co‐interventions in all groups: none |
Abbreviations
ACEi ‐ angiotensin converting enzyme inhibitor; ARB ‐ angiotensin receptor blocker, CAPD ‐ continuous ambulatory peritoneal dialysis; DBP ‐ diastolic blood pressure; FIR ‐ far infrared radiation; HD ‐ haemodialysis; HDF ‐ haemodiafiltration; IM ‐ intramuscular; TCM ‐ traditional Chinese medicine; TEAS ‐ transcutaneous electrical acupuncture point stimulation
1 Standards for Reporting Interventions in Clinical Trials of Acupuncture (www.stricta.info/)
Data and analyses
Comparison 1. Manual acupuncture versus sham acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uraemic pruritus | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 3 to 4 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Manual acupuncture versus routine care/conventional medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uraemic pruritus improvement | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 1 to 2 months treatment (conventional medicine) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 3 to 4 months treatment (conventional medicine) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 3 to 4 months treatment (routine care) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Gastrointestinal symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Overall at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Bloating at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Nausea at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Vomiting at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Upper abdominal pain at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Early satiety at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Belching 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Blood pressure: systolic | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3 to 4 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 5 to 6 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Blood pressure: diastolic | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 3 to 4 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 5 to 6 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Blood pressure: number responding to treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 1 to 2 months treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 3 to 4 months treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 5 to 6 months treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Biochemical parameters | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Parathyroid hormone at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Serum phosphorous at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Serum uric acid at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Serum creatinine at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Blood urea nitrogen at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 24‐hour urinary protein at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Overall treatment response (symptom and kidney function improvements) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 1 to 2 months treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Non‐needle type point stimulation other than moxibustion versus sham intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Uraemic pruritus | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fatigue | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Sleep quality: PSQI | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 1 to 2 months treatment | 2 | 137 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐3.70, 3.47] |
| 5 Quality of life: SF‐36 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 SF‐36 physical function at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 SF‐36 role physical at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 SF‐36 bodily pain at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 SF‐36 general health at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 SF‐36 vitality at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 SF‐36 social functioning at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 SF‐36 role emotional at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 SF‐36 mental health at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.9 SF‐36 physical component score at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.10 SF‐36 mental component score at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of life: WHOQOL‐BREF | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Overall quality of life at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 General health at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Physical domain at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Psychological domain at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Social relations domain at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Environmental domain at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Health quality of life satisfaction at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Biochemical parameters | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Serum phosphorus at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Serum calcium at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Serum albumin at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Serum alkaline phosphatase at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Blood urea nitrogen at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Urea reduction ratio at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 Serum haemoglobin at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 Serum intact parathyroid hormone at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.9 Serum calcium‐phosphorus ratio at 1 to 2 months | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Heart rate variability: SDNN | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.7. Analysis.
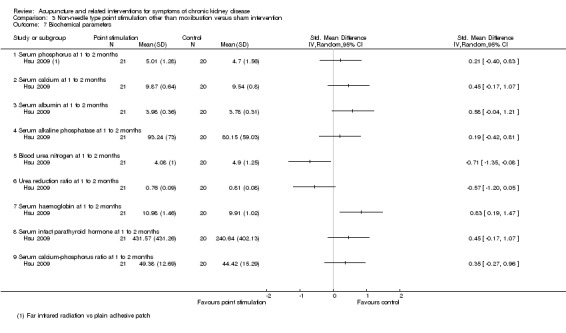
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 7 Biochemical parameters.
3.8. Analysis.
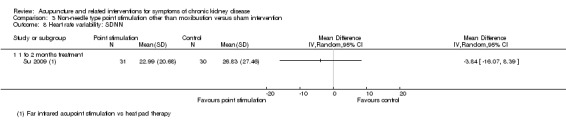
Comparison 3 Non‐needle type point stimulation other than moxibustion versus sham intervention, Outcome 8 Heart rate variability: SDNN.
Comparison 4. Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 1 to 2 months (manual acupressure versus routine care) | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐4.29 [‐7.48, ‐1.11] |
| 1.2 1 to 2 months (transcutaneous electrical acupuncture point stimulation versus routine care) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐6.26 [‐10.27, ‐2.25] |
| 2 Uraemic pruritus | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 5 to 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fatigue | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 1 to 2 months (manual acupressure versus routine care) | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐1.77, ‐0.60] |
| 3.2 1 to 2 months (transcutaneous electrical acupuncture point stimulation versus routine care) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.77, ‐0.23] |
| 4 Fatigue: BFI | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Fatigue strength rate at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Usual level of fatigue during past 24 hours at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Worst level of fatigue during past 24 hours at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 General activity at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Mood at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Walking ability at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Normal work at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Relations with other people at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Enjoyment of life at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Sleep quality: PSQI | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 1 to 2 months (manual acupressure versus routine care) | 4 | 180 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐4.23, ‐0.69] |
| 5.2 1 to 2 months (transcutaneous electrical acupuncture point stimulation versus routine care) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐5.57, ‐1.29] |
| 5.3 1 to 2 months treatment (manual acupressure versus conventional medicine) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐9.66, 2.66] |
| 6 Quality of life: SF‐36 | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Physical function at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Role physical at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Bodily pain at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 General health at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Vitality at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Social functioning at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Role emotional at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Mental health at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.9 Physical component score at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.10 Mental component score at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Sleep quality: SRSS | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 1 to 2 month treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Nutritional status: Interdialytic weight gain [% dry weight/d] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Xerostomia [scale 0 to 100] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Overall comfort scores | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 1 to 2 months treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Biochemical parameters | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Plasma sodium concentration at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.11. Analysis.
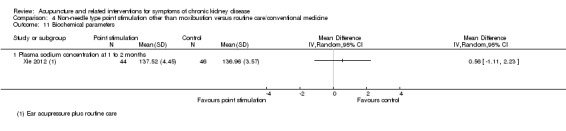
Comparison 4 Non‐needle type point stimulation other than moxibustion versus routine care/conventional medicine, Outcome 11 Biochemical parameters.
Comparison 5. Moxibustion versus routine care/conventional medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Reduced appetite at 1 to 2 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Nausea/vomiting at 1 to 2 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Bloating at 1 to 2 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Loose stool at 1 to 2 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Quality of life: KDQOL SF‐36 domains | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Physical functioning at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐9.27 [‐15.50, ‐3.04] |
| 2.2 Physical functioning at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐9.39 [‐16.58, ‐2.20] |
| 2.3 Role physical at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐7.67 [‐20.23, 4.89] |
| 2.4 Role physical at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐9.47 [‐25.41, 6.47] |
| 2.5 Role emotional at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 7.17 [‐7.08, 21.42] |
| 2.6 Role emotional at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐8.56 [‐23.66, 6.54] |
| 2.7 Social functioning at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐11.61, 4.41] |
| 2.8 Social functioning at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐13.87, 3.27] |
| 2.9 Bodily pain at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐6.22, 5.40] |
| 2.10 Bodily pain at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐8.60, 5.70] |
| 2.11 Vitality at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐6.14 [‐11.83, ‐0.46] |
| 2.12 Vitality at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐8.07 [‐15.59, ‐0.55] |
| 2.13 General health at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐10.24 [‐16.31, ‐4.17] |
| 2.14 General health at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐6.38 [‐13.76, 1.00] |
| 3 Quality of life: KDQOL disease specific domains | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Burden of kidney disease at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐11.68, 1.18] |
| 3.2 Burden of kidney disease at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐7.42 [‐16.38, 1.54] |
| 3.3 Cognitive function at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐4.94 [‐9.97, 0.08] |
| 3.4 Cognitive function at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐6.13 [‐11.41, ‐0.85] |
| 3.5 Quality of social interaction at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐6.35, 1.79] |
| 3.6 Quality of social interaction at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐7.88, 2.60] |
| 3.7 Symptom/problem list at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐4.20, 1.69] |
| 3.8 Symptom/problem list at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐5.45, 1.21] |
| 3.9 Effects of kidney disease at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐6.73, 4.40] |
| 3.10 Effect of kidney disease at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐4.01 [‐11.42, 3.40] |
| 3.11 Sexual function at 3 to 4 months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐19.3 [‐42.17, 3.57] |
| 3.12 Sleep at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐9.03, 2.55] |
| 3.13 Sleep at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐8.38, 7.62] |
| 3.14 Social support at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐5.13 [‐11.04, 0.79] |
| 3.15 Social support at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐5.83 [‐13.10, 1.44] |
| 3.16 Work status at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 3.33 [‐4.54, 11.21] |
| 3.17 Work status at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐13.70, 9.68] |
| 3.18 Patient satisfaction at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐4.80, 5.12] |
| 3.19 Patient satisfaction at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 2.49 [‐4.39, 9.37] |
| 3.20 Dialysis staff encouragement at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐14.47, 11.88] |
| 3.21 Dialysis staff encouragement at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐4.67, 9.47] |
| 3.22 Overall health at 3 to 4 months | 2 | 174 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐13.26, 10.61] |
| 3.23 Overall health at 5 to 6 months | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐4.61, 8.21] |
| 4 Overall treatment response (symptom and kidney function improvements) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 1 to 2 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Nutritional status | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 MQSGA scores at 1 to 2 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 MQSGA scores at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 MQSGA scores at 5 to 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Handgrip strength at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Handgrip strength at 5 to 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Dry weight at 3 to 4 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Dry weight at 5 to 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Biochemical parameters | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Serum creatinine at 1 to 2 months | 2 | 108 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.51, 0.27] |
| 6.2 Serum potassium at 1 to 2 months | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.41, 0.60] |
| 6.3 Amount of ultrafiltration at 1 to 2 months | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.97, ‐0.30] |
| 6.4 Blood urea nitrogen at 1 to 2 months | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.60, 0.62] |
| 6.5 Serum uric acid at 1 to 2 months | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.85, 0.38] |
| 6.6 Serum middle molecule at 1 to 2 months | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.09, 0.15] |
| 6.7 Urea reduction ratio at 3 to 4 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.36, 0.39] |
| 6.8 Urea reduction ratio at 5 to 6 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.62, 0.13] |
| 6.9 Serum albumin at 1 to 2 months | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.34, 0.47] |
| 6.10 Serum albumin at 3 to 4 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.48, 0.27] |
| 6.11 Serum albumin at 5 to 6 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.49, 0.26] |
| 6.12 Serum prealbumin at 1 to 2 months | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐2.80, ‐0.91] |
| 6.13 High‐sensitivity CRP at 1 to 2 months | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐2.86, ‐0.95] |
| 6.14 Interleukin‐6 at 1 to 2 months | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐2.30, ‐0.54] |
| 6.15 Serum haemoglobin at 1 to 2 months | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 6.16 Serum haemoglobin at 3 to 4 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.21, 0.55] |
| 6.17 Serum haemoglobin at 5 to 6 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.17, 0.59] |
| 6.18 Kt/V (haemodialysis adequacy) at 3 to 4 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.53, 0.22] |
| 6.19 Kt/V (haemodialysis adequacy) at 5 to 6 months | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.07, 0.68] |
| 6.20 Residual renal Kt/V (peritoneal dialysis) at 1 to 2 months | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.48, 0.54] |
| 6.21 Residual renal creatinine clearance (peritoneal dialysis) at 1 to 2 months | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.49 [‐3.18, ‐1.81] |
| 6.22 Total creatinine clearance (peritoneal dialysis) at 1 to 2 months | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.37, 0.65] |
| 6.23 Total Kt/V (peritoneal dialysis) at 1 to 2 months | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.09, 0.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Che‐Yi 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 4 weeks |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used SPSS build in function selection, random sampling to select patients for intervention and sham intervention group." (author contact) |
| Allocation concealment (selection bias) | Unclear risk | Not reported. The author regarded allocation concealment process as blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The author reported that participants were blinded. However, credibility test of the sham acupuncture was not performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To avoid personal bias, two different nurses administered the same questionnaire to the same patient... Because the two needling points (one for real the other for sham) are close, patients and nurses could not recognise the difference." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Every enrolled patient tolerated the procedure and finished the 1 month course of treatment." The author confirmed that all randomised patients completed the outcome assessment at post‐treatment evaluation and at 3 months from the end of treatments. (author contact) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not accessible |
| Other bias | Low risk | Baseline data were comparable. Participants in both groups continued to their prior medication for uraemic pruritus |
Cheng 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 7 to 10 days |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (10 days) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total number of assessed patients exceeds the total number of included patients in each group |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Cho 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 4 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (4 weeks) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocated by the time of dialysis treatment (even or odd days) |
| Allocation concealment (selection bias) | High risk | Allocated by the time of dialysis treatment (even or odd days) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “The data collection to examine the effectiveness was completed by two trained research assistants” “Two trained assistants collected post‐test data” These assistants were separated from treatment process. However, no information is available to judge whether they were blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients in the treatment group and one patient in the control group was dropped out and excluded from analysis "The reasons for dropping out were relocation or being transferred to other dialysis centres." The number of patients excluded from analysis is small and reasons of dropout are unlikely to affect the outcome |
| Selective reporting (reporting bias) | Unclear risk | No protocol information available |
| Other bias | Unclear risk | No details for concomitant medication |
Cui 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 2 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (2‐week from baseline) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Hospital record number was used |
| Allocation concealment (selection bias) | High risk | Unlikely to be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of patients at week 2 was not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Dai 2007a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 4 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: 4 weeks |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information about the number of participants who have been analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol information available |
| Other bias | Low risk | Study appears free of other biases |
Gao 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 4 weeks (acupuncture); 2 weeks (conventional medicine) |
|
| Outcomes |
Time points measured (or reported) in the study: 5 and 16 weeks (acupuncture group); 4 weeks (conventional medicine) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information about the number of participants who have been analysed. |
| Selective reporting (reporting bias) | Unclear risk | No protocol information available |
| Other bias | High risk | Different treatment duration and different time‐point for post‐treatment evaluation (Four weeks treatments and follow‐up at 5 weeks in the acupuncture group; two weeks medication and follow‐up at 4 weeks in the medication group). Risk of Hawthorne effect due to longer treatment duration in the acupuncture group could not be excluded |
Hsu 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 8 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: 4 and 8 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table was used |
| Allocation concealment (selection bias) | Unclear risk | "A staff team serially numbered envelops." However, whether opaque envelopes were used was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors insisted that patient blinding were maintained. However, no credibility tests for successful blinding and apparently different shapes of two interventions makes patient blinding doubtful. Authors also insisted that clinical staff at the dialysis centre were remained blinded to the group allocation. However, the possibility of unblinding clinical staff could not be excluded due to apparently different shapes of two interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment conducted by research assistants who had no knowledge of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis based on participants who have completed the whole study process, not on those randomised (3 in the treatment group and 5 in the control group withdrew the study after randomisation). Reasons for withdrawal included serious adverse events including hospitalisation in the treatment group (1) and death in the control group (2). |
| Selective reporting (reporting bias) | Unclear risk | Protocol information not available |
| Other bias | Low risk | Study appears free of other biases |
Jedras 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 5 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: 6, 12 and 18 weeks |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lin 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 8 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (8 weeks) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors reported that the study was conducted as a quasi‐randomised study in the abstract and the methods section. However, the authors subsequently revealed in the methods section that the study was randomised as follows "Prior to the intervention process, the selected patients were randomly divided by computer into two groups: an experimental group (n = 36) and a control group (n = 25)...". No answer from the authors was available. We judged that this study was randomised by computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study (FIR acupoint therapy versus no treatment) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether outcome assessors for primary outcome (BFI‐T) were blinded was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Serum blood chemistry results were not reported |
| Other bias | Low risk | Study appears free of other biases |
Ma 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 10 days |
|
| Outcomes |
Time points measured (or reported) in the study: 4 weeks |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocated by the hospital record number |
| Allocation concealment (selection bias) | High risk | Allocation results are unlikely to be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants (open label) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Qiu 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 12 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (12 weeks), follow‐up (24 weeks) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was used |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Rui 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 2 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (16 weeks), unknown follow‐up time point (up to one year) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Hospital record number |
| Allocation concealment (selection bias) | High risk | Allocation results were unlikely to be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Shariati 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 4 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (4 weeks) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported that "Subjects were randomly divided into two groups using simple randomisation method." However, the exact method of simple randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the interviewer and care providers did not know the allocation result." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Among 48 initially included patients, 4 dropped out due to various reason and 44 completed the study. Only those who completed the study were analysed. The reason of dropout included death, transplantation, transfer to ICU and disagreement with participation. Although major adverse outcome (death) were occurred, types and the number of adverse events has not been reported per each group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Song 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 24 weeks |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Hospital record number |
| Allocation concealment (selection bias) | High risk | Unlikely to be concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Su 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 12 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: 4, 8, 12 weeks for ANS activity; 12 weeks for QOL |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The heat pad control intervention was regarded as sham intervention of FIR therapy, because of its heat property similar to FIR therapy. However, appearances of two interventions were not identical. Results of credibility test of the control intervention were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only participants who had completed the study were analysed (3 from FIR group and 5 in control group were withdrawn from 69 randomised patients for unknown reasons) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Sun 2008a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 12 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (12 weeks) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was used. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely to be blinded (open‐label study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor: Research staffs (whether these staffs were blinded was not reported) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients dropped out due to adverse clinical outcomes were not incorporated in the analyses (4 in the treatment group and 2 in the control group from 71 randomised patients withdrew the study for the reason of the long study duration, aggravation of conditions, deaths or transfer to other hospital. There were serious adverse events such as deaths that may have affected the analysis. Therefore, high risk of attrition bias was given) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Sun 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 60 days |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (60 days) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of patients at post‐treatment was not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Tsay 2003a.
| Methods |
|
|
| Participants | Country: Taiwan
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Duration of intervention: 4 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (4 weeks) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, simply described as "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors reported that “The interviewer, care provider, acupressure practitioner and participants did not know what types of treatment patients received.” However, reviewers thought acupressure practitioners could not be blinded to the group allocation. No blinding credibility test was performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors reported that “.. interviewer… did not know what types of treatment patients received” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate was 6.7% with reasons of hospitalisation (1) and transfer to other dialysis centres (6). This was occurred in the sham acupressure group (3) and the control group (4). It was unclear whether those reasons were associated with adverse events during the study |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Other bias | Low risk | Study appears free of other biases |
Tsay 2004a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Duration of intervention: 4 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (4 weeks) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported. Simply noted as "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Sham acupressure (no blinding credibility test) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of enrolled participants were not provided, but only the completed was reported (no flowchart provided). |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Other bias | Low risk | Study appears free of other biases |
Tsay 2004b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Duration of intervention: 4 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (4 weeks) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate was 1.9% (106 of 108 patients completed the study). Reasons included medical reason (n = 1) and relocation (n = 1). It seems unclear whether dropout due to medical reason might influence the effect estimates |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Other bias | Low risk | Study appears free of other biases |
Xie 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 8 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: 4 weeks, 8 weeks |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Detailed randomisation method was not reported. Simply noted as "randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of patients at week 4 and 8 was not reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Other bias | Low risk | Study appears free of other biases |
Zhang 2011d.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Duration of intervention: 10 or 20 days |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (10 days or 20 days depending on the provided treatment sessions) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were analysed for the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Zhao 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 7 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (7 weeks) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details whether dropout, withdrawal and implementation of missing occurred. The number of participants in the control group at post‐treatment evaluation exceeded those at baseline (15 participants in the control group at baseline versus those of 19 at post‐treatment evaluation) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Zhao 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention: 8 weeks |
|
| Outcomes |
Time points measured (or reported) in the study: post‐treatment (8 weeks) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Detailed randomisation method was not reported (only 'randomly divided') |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of patients at post‐treatment was not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Low risk | Study appears free of other biases |
Alb ‐ albumin; ANS ‐ Autonomic nervous system; BDI ‐ Beck depression inventory; BFI‐T ‐ Brief Fatigue Inventory‐Taiwan Form; BUN ‐ blood urea nitrogen; CAPD ‐ continuous ambulatory peritoneal dialysis; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; DSM IV ‐ Diagnostic and Statistical Manual of Mental Disorders‐Fourth Edition; ESKD ‐ end‐stage kidney disease; FIR ‐ far infrared radiation; GI ‐ gastrointestinal; Hb ‐ haemoglobin; HD ‐ haemodialysis; HDF ‐ haemodiafiltration; IM ‐ intramuscular; iPTH ‐ intact parathyroid hormone; K+ ‐ potassium; Kt/V ‐ treatment adequacy; M/F ‐ male/female; MQSGA ‐ Modified Quantitative Subjective Global Assessment; PD ‐ peritoneal dialysis; PO4‐3 ‐ phosphate; PSQI ‐ Pittsburgh Sleep Quality Index; QOL ‐ quality of life; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; SE ‐ standard error; SRSS ‐ self‐rating scale for sleep; TCM ‐ traditional Chinese medicine; TEAS ‐ transcutaneous electrical acupoint stimulation; URR ‐ urea reduction ratio; VAS ‐ visual analogue scale; WHOQOL‐BREF ‐ World Health Organization Quality of Life instrument
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cao 2010 | Wrong intervention: acupoint injection was studied |
| Chen 2001b | The study compared acupuncture with traditional herbal medicine plus western drug |
| Chen 2007 | The study did not involve participants with CKD |
| Cheng 1999 | The study employed the acupuncture‐point injection intervention |
| Chu 2007 | Patients with only diabetic nephropathy were included |
| Fan 2012 | Unit of analysis was event, not people |
| Ge 2008 | Patients after kidney transplantation were studied |
| Gong 1993 | The study did not involve participants with CKD |
| Jiang 2001 | Total treatment sessions were fewer than 6 |
| Jin 2009 | The study did not involve participants with CKD |
| Lan 2012 | The study did not involve participants with CKD |
| Liang 1992 | The study did not involve participants with CKD |
| Pang 2003a | The study did not involve participants with CKD |
| Peng 1999 | The study employed acupuncture plus traditional herbal medicine as treatment intervention |
| Qiu 2012a | Not an acupuncture study |
| Rao 2013 | Unclear CKD stage |
| Yang 2010a | Not RCT |
CKD ‐ chronic kidney disease; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02432508.
| Trial name or title | Efficacy of laser acupuncture on pruritus in patients with chronic kidney disease undergoing hemodialysis: a multiple centers, randomized, assessor‐ and participant‐blind, controlled, cross‐over clinical trial |
| Methods | We plan to enroll 200 volunteer patients with uremic pruritus. After the waiting period (as the waiting list group), you will be randomized to laser acupuncture and sham laser acupuncture group. Each group will include 100 patients and given intervention according to their hemodialysis frequency, i.e., BIW or TIW. The intervention will then be crossed over to the other one after 4 week of wash‐our period. Outcome measurement includes questionnaires, biochemistry analysis, instrumental analysis and medication score. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Active Comparator
Sham Comparator
|
| Outcomes | Primary Outcome Measures
Secondary Outcome Measures
|
| Starting date | October 2014 |
| Contact information | Chang Chiz‐Tzung, Ph.D China Medical University Hospital Taichung, Taiwan, 420 |
| Notes |
Contributions of authors
Draft the protocol: KKH, LMS, KTH, KJW, CTY, LJD
Study selection: KKH, CTY
Extract data from studies: KKH, KTH
Obtain additional data from study authors: KJW, CTY
Enter data into RevMan: KKH, KTH
Carry out the analysis: KKH, LMS, KTH, KJW
Interpret the analysis: KKH, LMS, KJW
Draft the final review: KKH
Disagreement resolution: LMS
Update the review: KKH
Sources of support
Internal sources
-
National Research Foundation of Korean Government, Korea, South.
This work was supported by a Grant to Korean Medical Science Research Center for Healthy Aging from the National Research Foundation of Korean Government (2014R1A5A2009936).
-
Korea Institute of Oriental Medicine, Korea, South.
Lee MS and Choi T‐Y were supported by Korea Institute of Oriental Medicine (K16111 and K16121).
External sources
No sources of support supplied
Declarations of interest
-
Non‐financial conflict of interest:
-
Financial conflict of interest
Tae‐Young Choi: none known
Jung Won Kang: none known
Kun Hyung Kim: none known
Tae‐Hun Kim: none known
Jae Dong Lee: none known
Myeong Soo Lee: none known
Edited (no change to conclusions)
References
References to studies included in this review
Cheng 2012 {published data only}
- Cheng Li. The clinical observation of ginger separated moxibustion treat gastrointestinal disorders of peritoneal dialysis [dissertation]. Wuhan City, Hubei, China: Hubei University of Chinese Medicine, 2012. [Google Scholar]
Che‐Yi 2005 {published data only}
- Che‐Yi C, Wen CY, Min‐Tsung K, Chiu‐Ching H. Acupuncture in haemodialysis patients at the Quchi (LI11) acupoint for refractory uraemic pruritus. Nephrology Dialysis Transplantation 2005;20(9):1912‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cho 2004 {published data only}
- Cho YC, Tsay SL. The effect of acupressure with massage on fatigue and depression in patients with end‐stage renal disease. Journal of Nursing Research 2004;12(1):51‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cui 2012 {published data only}
- Cui YC. Efficacy of acupuncture in the treatment of chronic renal failure in patients with digestive disorders: an analysis of 30 cases. Journal of Chinese General Practice 2012;15(18):2088‐90. [1007‐9572] [Google Scholar]
Dai 2007a {published data only}
- Dai XJ, Xing XY, Shi Y, Jiang WX, Zhou ME. Lower extremity point massage for improving quality of sleep in patients with end‐stage renal disease: a clinical study of 42 cases. Journal of Traditional Chinese Medicine 2007;48(1):44‐6. [Google Scholar]
Gao 2002 {published data only}
- Gao H, Zhang W, Wang Y. Acupuncture treatment for 34 cases of uremic cutaneous pruritus. Journal of Traditional Chinese Medicine 2002;22(1):29‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Hsu 2009 {published data only}
- Hsu MC, Chen HW, Hwu YJ, Chanc CM, Liu CF. Effects of thermal therapy on uremic pruritus and biochemical parameters in patients having haemodialysis. Journal of Advanced Nursing 2009;65(11):2397‐408. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jedras 2003 {published data only}
- Jedras M, Bataa O, Gellert R, Ostrowski G, Wojtaszek E, Lange J, et al. Acupressure in the treatment of uremic pruritus. Dialysis & Transplantation 2003;32(1):8‐10. [EMBASE: 2003030067] [Google Scholar]
Lin 2011 {published data only}
- Lin CH, Lee LS, Su LH, Huang TC, Liu CF. Thermal therapy in dialysis patients ‐ a randomized trial. American Journal of Chinese Medicine 2011;39(5):839‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ma 2004 {published data only}
- Ma X. Clinical analysis for the acupuncture treatment in 42 cases of gouty renal damage. Journal of Traditional Chinese Medicine 2004;24(3):185‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Ma XP. Clinical analysis on 42 cases of gouty renal injury treated with acupuncture. Journal of Traditional Chinese Medicine 2003;44(5):350‐1. [PubMed] [Google Scholar]
Qiu 2012 {published data only}
- Li N, Qiu MY, Hao JR, Zhang QM, Wang SH, Liang F, et al. Randomized controlled trial on moxibustion for maintenance hemodialysis patients in deficiency syndrome. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2011;31(1):15‐8. [CENTRAL: CN‐00778767] [PubMed] [Google Scholar]
- Qiu MY, LI N, Wang SH, Luan J, Li BQ, Liu XL, et al. Moxibustion on spleen and stomach meridian to improve the nutritional status of hemodialysis patients in multi‐center clinical study. World Federation of Acupuncture‐Moxibustion Societies Proceedings; 2010 Nov 6‐7; San Francisco, CL. 2010:465‐73.
- Qiu, MY. A clinical trial of moxibustion for nourishing treatment of spleen‐stomach to improve quality of life in uremic hemodialysis patients [PhD thesis]. Beijing, China: China Academy of Chinese Medical Sciences, 2012. [Google Scholar]
- Sun H, Qiu M, Hao J, Zhang Q, Li N, Wang S, et al. Multi‐central clinical study of regulating spleen‐stomach moxibustion for improving nutritional status in patients accepted hemodialysis. Modern Journal of Integrated Traditional Chinese & Western Medicine 2012;21(7):685‐7. [Google Scholar]
Rui 2002 {published data only}
- Rui H, Lin W, Sha J. Observation on therapeutic effect of 80 cases of uremic cutaneous pruritus treated with acupuncture. Zhongguo Zhenjiu [Chinese Acupuncture & Moxibustion] 2002;22(4):235‐6. [Google Scholar]
Shariati 2012 {published data only}
- Shariati A, Jahani S, Hooshmand M, Khalili N. The effect of acupressure on sleep quality in hemodialysis patients. Complementary Therapies in Medicine 2012;20(6):417‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2007 {published data only}
- Song YH. Observation on therapeutic effects of combined acupuncture and medicine therapy and simple medication on renal hypertension of chronic kidney disease. Zhongguo Zhenjiu [Chinese Acupuncture & Moxibustion] 2007;27(9):641‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Su 2009 {published data only}
- Su LH, Wu KD, Lee LS, Wang H, Liu CF. Effects of far infrared acupoint stimulation on autonomic activity and quality of life in hemodialysis patients. American Journal of Chinese Medicine 2009;37(2):215‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sun 2008a {published data only}
- Sun H, Qiu MY, Li BQ, Wang SH, Chen ZY, Jiang Y, et al. Effect of moxibustion on quality of life in hemodialysis patients. Zhongguo Zhenjiu [Chinese Acupuncture & Moxibustion] 2008;28(5):321‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Sun 2012 {published data only}
- Sun H, Liang Q, Sun YS, Liu XY, Huang KQ, Wang LH. Effects of moxibustion on peritoneal dialysis malnutrition in hyper‐transport CAPD patients. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2012;13(9):791‐3. [Google Scholar]
Tsay 2003a {published data only}
- Tsay SL, Chen ML. Acupressure and quality of sleep in patients with end‐stage renal disease‐‐a randomized controlled trial. International Journal of Nursing Studies 2003;40(1):1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tsay SL, Rong JR, Lin PF. Acupoints massage in improving the quality of sleep and quality of life in patients with end‐stage renal disease.[Retraction in J Adv Nurs. 2011 Apr;67(4):923; PMID: 21410508]. Journal of Advanced Nursing 2003;42(2):134‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2004a {published data only}
- Tsay SL. Acupressure and fatigue in patients with end‐stage renal disease‐a randomized controlled trial. International Journal of Nursing Studies 2004;41(1):99‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsay 2004b {published data only}
- Tsay SL, Cho YC, Chen ML. Acupressure and transcutaneous electrical acupoint stimulation in improving fatigue, sleep quality and depression in hemodialysis patients. American Journal of Chinese Medicine 2004;32(3):407‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xie 2012 {published data only}
- Xie M, Hu ZF. An experimental study of ear acupressure for xerostomia in 90 hemodialysis patients. Chinese Journal of Traditional Medical Science & Technology 2012;19(6):538‐539. [1005‐7072] [Google Scholar]
Zhang 2011d {published data only}
- Zhang F, Qiu ZL, Huang HX, Fang XX, Shwm Y. The efficacy of acupuncture plus hemodiafiltration treatment of uremic with cutaneous pruritus. Journal of Practical Medicine 2011; Vol. 27, issue 9:1687‐9. [1006‐5725]
Zhao 1995 {published data only}
- Cuiying Z. A clinical research in treatment of chronic renal failure by indirect moxibustion with herb cake. Shanghai Zhenjiu Zazhi [Shanghai Journal of Acupuncture & Moxibustion] 1995;14(3):101. [Google Scholar]
- Zhao CY, Chen HP, Xie XZ, Shen Y, Zhang XY, Hong X. A clinical research in treatment of chronic renal failure by indirect moxibustion with herb cake. Shanghai Zhongyiyao Zazhi [Shanghai Journal of Traditional Chinese Medicine] 1995;14(3):101‐3. [CENTRAL: CN‐00672060] [Google Scholar]
Zhao 2011 {published data only}
- Zhao T. The effect study of auricular point magnetic bead plaster therapy on sleep disorders and comfort status in maintenance hemodialysis patients [dissertation]. Fuzhou, Fujian, China: Fujian University of Traditional Chinese Medicine, 2011. [Google Scholar]
References to studies excluded from this review
Cao 2010 {published data only}
- Cao W, Liu JH, Zhang H, Zhang L, Zhang LY, Pan MM. Effect of acupoint injection on erythropoietin resistance in patients with chronic renal failure. Zhongguo Zhenjiu 2010;30(11):891‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Chen 2001b {published data only}
- Chen J. Chronic glomerulonephritis treated by acupuncture. International Journal of Clinical Acupuncture 2001;12(4):319‐21. [CENTRAL: CN‐00633842] [Google Scholar]
Chen 2007 {published data only}
- Chen J, Liu JH. Acupuncture for treatment of kinetic insufficiency of kidney‐qi and study on the mechanism. Zhongguo Zhenjiu 2007;27(7):479‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Cheng 1999 {published data only}
- Cheng S, Zhou H, Wu Y. Clinical observation of glomerulonephritis treated by acupoint‐injection. Shanghai Zhenjiu Zazhi [Shanghai Journal of Acupuncture & Moxibustion] 1999;18(5):8‐9. [CENTRAL: CN‐00632131] [Google Scholar]
Chu 2007 {published data only}
- Chu Q, Wang L, Liu GZ. Clinical observation on acupuncture for treatment of diabetic nephropathy. Zhongguo Zhenjiu 2007;27(7):488‐90. [MEDLINE: ] [PubMed] [Google Scholar]
- Chu Q, Wang L, Liu GZ. Effect of acupuncture on hemorheology in patients with diabetic nephropathy. Chen Tzu Yen Chiu [Acupuncture Research] 2007;32(5):335‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Fan 2012 {published data only}
- Fan XH, Huang KQ, Yang XM, Ke H. Acupressure concurrent adjuvant therapy of muscle cramps in hemodialysis patients. Nursing and Rehabilitation Journal 2012;11(2):184‐185. [1671‐9875] [Google Scholar]
Ge 2008 {published data only}
- Ge HY, Chen B, Li YT. Effects of acupuncture on gastrointestinal responses after renal transplantation. Zhongguo Zhenjiu 2008;28(3):177‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Gong 1993 {published data only}
- Gong DF, Liang CL, Lai XS, Lai XL. Effects of different acupuncture manipulation on plasma estradiol, testosterone and cortisol in patients with kidney deficiency. Chen Tzu Yen Chiu [Acupuncture Research] 1993;18(4):253‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Jiang 2001 {published data only}
- Jiang Q. Clinical observation of acupuncture therapy for hypertension in hemodialysis. Beijing Zhongyi [Beijing Journal of Traditional Chinese Medicine] 2001;20(5):52. [CENTRAL: CN‐00632326] [Google Scholar]
Jin 2009 {published data only}
- Jin KK, Chen L, Pan JY, Li JM, Wang Y, Wang FY. Acupressure therapy inhibits the development of diabetic complications in Chinese patients with type 2 diabetes. Journal of Alternative & Complementary Medicine 2009;15(9):1027‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lan 2012 {published data only}
- Lan XM. Clinical research on dong’s acupuncture to uroschesis caused by postoperative hemorrhoids [PhD thesis]. Guangzhou, China: Guangzhou University of Chinese Medicine, 2012. [Google Scholar]
Liang 1992 {published data only}
- Liang Z, Liang C, Liu I, Lin J. The influence of different acupuncture manipulations on the plethysmogram of the patient with kidney deficiency. Chen Tzu Yen Chiu [Acupuncture Research] 1992;17(1):61‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Pang 2003a {published data only}
- Pang Y. Clinical observation on acupuncture treatment of ischemic apoplexy by nourishing the kidney and regulating the du channel. Journal of Traditional Chinese Medicine 2003;23(4):286‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Peng 1999 {published data only}
- Peng YH, Fan GX, Zhang L. Clinical study on the treatment of early diabetic nephropathy by western medicine combined with acupuncture‐moxibustion and Chinese herbs. Shijie Zhenjiu Zazhi (Yingwenban) [World Journal of Acupuncture‐Moxibustion] 1999;9(1):8‐14. [CENTRAL: CN‐00678062] [Google Scholar]
Qiu 2012a {published data only}
- Qiu H, Jia M, Xie YX, Qiu YJ, Wang HX. Efficacy massage combined with lower dose urokinase for arteriovenous fistula thrombosis in hemodialysis patients. Clinical Focus 2012;27(22):1989‐91. [1004‐583X] [Google Scholar]
Rao 2013 {published data only}
- Rao JZ, Zhang Y0W. Acupuncture treatment for lower limb edema 74 in chronic renal failure patients. Journal of North Pharmacy 2013;10(2):57. [1672‐8351] [Google Scholar]
Yang 2010a {published data only}
- Yang LY, Yates P, Chin CC, Kao TK. Effect of acupressure on thirst in hemodialysis patients. Kidney & Blood Pressure Research 2010;33(4):260‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abbasi 2015 {published data only}
- Abbasi P, Mojalli M, Kianmehr M. The effect of acupressure on constipation in patients with chronic renal failure undergoing hemodialysis [abstract]. Avicenna Journal of Phytomedicine 2015;5:97‐8. [EMBASE: 72156805] [PMC free article] [PubMed] [Google Scholar]
Chen 2012a {published data only}
- Chen YB, Chen RN, Li YL. Observation on therapeutic effect of type II early diabetic nephropathies intervened by acupoint thread embedding. Zhongguo Zhenjiu 2012;32(5):390‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Eglence 2013 {published data only}
- Eglence R, Karatas N, Tasci S. The effect of acupressure on the level of fatigue in hemodialysis patients. Alternative Therapies in Health & Medicine 2013;19(6):23‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Feng 2012 {published data only}
- Feng SK. Observation on curative effect of glomerular pathological proteinuria treated with heat‐producing needling. Zhongguo Zhenjiu 2012;32(1):8‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Hmwe 2015 {published data only}
- Hmwe NT, Subramanian P, Tan LP, Chong WK. The effects of acupressure on depression, anxiety and stress in patients with hemodialysis: a randomized controlled trial. International Journal of Nursing Studies 2015;52(2):509‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Och 2000 {published data only}
- Och B, Jedras M, Gellert R. Is acupressure effective in the treatment of uremic pruritus? [abstract]. 37th Congress. European Renal Association. European Dialysis and Transplantation Association; 2000 Sep 17‐20; Nice, France. 2000:157. [CENTRAL: CN‐00461428]
Ono 2015 {published data only}
- Ono S, Mukaino Y. Efficacy and cost effectiveness of the acupuncture treatment using a new skin stimulus tool called M‐test which is a measure based on symptoms accompanied with body movements: a pragmatic RCT targeting hemodialysis patients. Evidence‐Based Complementary & Alternative Medicine: eCAM 2015;2015:802846. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabouhi 2013 {published data only}
- Sabouhi F, Kalani L, Valiani M, Mortazavi M, Bemanian M. Effect of acupressure on fatigue in patients on hemodialysis. Iranian Journal of Nursing & Midwifery Research 2013;18(6):429‐34. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Wang 2014c {published data only}
- Wang S, Chen Z, Fu P, Zang L, Wang L, Zhai X, et al. Use of auricular acupressure to improve the quality of life in diabetic patients with chronic kidney diseases: a prospective randomized controlled trial. Evidence‐Based Complementary & Alternative Medicine: eCAM 2014;2014:343608. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014a {published data only}
- Wu Y, Zou C, Liu X, Wu X, Lin Q. Auricular acupressure helps improve sleep quality for severe insomnia in maintenance hemodialysis patients: a pilot study. Journal of Alternative & Complementary Medicine 2014;20(5):356‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yan 2015 {published data only}
- Yan CN, Yao WG, Bao YJ, Shi XJ, Yu H, Yin PH, et al. Effect of auricular acupressure on uremic pruritus in patients receiving hemodialysis treatment: a randomized controlled trial. Evidence‐based Complementary & Alternative Medicine: eCAM 2015;2015:593196. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2006 {published data only}
- Zhu LF, Pan XH, Zhou C, Huang KQ. Effects of adjunctive acupressure for muscle cramps of lower extremities in patients undergoing hemodialysis. Xiandai Zhongxiyijiehe Zazhi [Modern Journal of Integrated Traditional Chinese & Western Medicine] 2006;17:2347‐8. [Google Scholar]
Zou 2015 {published data only}
- Zou C, Yang L, Wu Y, Su G, Chen S, Guo X, et al. Auricular acupressure on specific points for hemodialysis patients with insomnia: a pilot randomized controlled trial. PLoS ONE [Electronic Resource] 2015;10(4):e0122724. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02432508 {published data only}
- Chang CT. Efficacy of laser acupuncture on pruritus in patients with chronic kidney disease undergoing hemodialysis. www.clinicaltrials.gov/ct2/show/NCT02432508 (accessed 28 January 2016).
Additional references
Abdel‐Kader 2009
- Abdel‐Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end‐stage kidney disease. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(6):1057‐64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Medelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4(6):561‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bergström 2000
- Bergstrom J, Lindholm B, Lacson E, Owen W, Lowrie EG, Glassock RJ, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients?. Seminars in Dialysis 2000;13(3):163‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Browne 2010
- Browne T, Merighi JR. Barriers to adult hemodialysis patients' self‐management of oral medications. American Journal of Kidney Diseases 2010;56(3):547‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carrero 2008
- Carrero JJ, Yilmaz MI, Lindholm B, Stenvinkel P. Cytokine dysregulation in chronic kidney disease: how can we treat it?. Blood Purification 2008;26(3):291‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Claxton 2010
- Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemodialysis. Journal of Pain & Symptom Management 2010;39(2):211‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davison 2007
- Davison SN. The prevalence and management of chronic pain in end‐stage renal disease. Journal of Palliative Medicine 2007;10(6):1277‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dean‐Clower 2010
- Dean‐Clower E, Doherty‐Gilman AM, Keshaviah A, Baker F, Kaw C, Lu W, et al. Acupuncture as palliative therapy for physical symptoms and quality of life for advanced cancer patients. Integrative Cancer Therapies 2010;9(2):158‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duarte 2009
- Duarte PS, Miyazaki MC, Blay SL, Sesso R. Cognitive‐behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney International 2009;76(4):414‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elder 2008
- Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology Dialysis Transplantation 2008;23(3):998‐1004. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ezzo 2000
- Ezzo J, Berman B, Hadhazy VA, Jadad AR, Lao L, Singh BB. Is acupuncture effective for the treatment of chronic pain? A systematic review. Pain 2000;86(3):217‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ezzo 2006
- Ezzo J, Richardson MA, Vickers A, Allen C, Dibble S, Issell BF, et al. Acupuncture‐point stimulation for chemotherapy‐induced nausea or vomiting. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD002285.pub2] [DOI] [PubMed] [Google Scholar]
Finkelstein 2008
- Finkelstein FO, Wuerth D, Troidle LK, Finkelstein SH. Depression and end‐stage renal disease: a therapeutic challenge. Kidney international 2008;74(7):843‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frisk 2012
- Frisk J, Kallstrom AC, Wall N, Fredrikson M, Hammar M. Acupuncture improves health‐related quality‐of‐life (HRQoL) and sleep in women with breast cancer and hot flushes. Supportive Care in Cancer 2012;20(4):715‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furlan 2005
- Furlan AD, Tulder MW, Cherkin D, Tsukayama H, Lao L, Koes BW, et al. Acupuncture and dry‐needling for low back pain. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001351.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2005
- Garcia GE, Ma SX, Feng L. Acupuncture and kidney disease. Advances in Chronic Kidney Disease 2005;12(3):282‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry 1960;23:56‐62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunt 2010
- Hunt KJ, Coelho HF, Wider B, Perry R, Hung SK, Terry R, et al. Complementary and alternative medicine use in England: results from a national survey. International Journal of Clinical Practice 2010;64(11):1496‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Imai 2008
- Imai E, Matsuo S. Chronic kidney disease in Asia. Lancet 2008;371(9631):2147‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of internal medicine 2004;141(10):781‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
James 2010
- James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010;375(9722):1296‐309. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kavoussi 2007
- Kavoussi B, Ross BE. The neuroimmune basis of anti‐inflammatory acupuncture. Integrative Cancer Therapies 2007;6(3):251‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KHIDI 2008
- Korea Health Industry Development Institute. National survey on the current status of practice of traditional Korean medicine and demands of research and development for traditional Korean medicine. Internal reports 2008.
Kim 2010a
- Kim KH, Lee MS, Choi SM, Ernst E. Acupuncture for treating uremic pruritus in patients with end‐stage renal disease: a systematic review. Journal of Pain & Symptom Management 2010;40(1):117‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2010b
- Kim KH, Lee MS, Kang KW, Choi SM. Role of acupressure in symptom management in patients with end‐stage renal disease: a systematic review. Journal of Palliative Medicine 2010;13(7):885‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
King 2010
- King A, Daniels J, Lim J, Cochrane DD, Taylor A, Ansermino JM. Time to listen: a review of methods to solicit patient reports of adverse events. Quality & Safety in Health Care 2010;19(2):148‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Langevin 2011
- Langevin HM, Wayne PM, Macpherson H, Schnyer R, Milley RM, Napadow V, et al. Paradoxes in acupuncture research: strategies for moving forward. Evidence‐Based Complementary & Alternative Medicine: eCAM 2011;2011:180805. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015
- Lee A, Chan SK, Fan LT. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD003281.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2009a
- Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR. Acupuncture for migraine prophylaxis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001218.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2009b
- Linde K, Allais G, Brinkhaus B, Manheimer E, Vickers A, White AR. Acupuncture for tension‐type headache. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007587] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopes 2002
- Lopes AA, Bragg J, Young E, Goodkin D, Mapes D, Combe C, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney international 2002;62(1):199‐207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacPherson 2008
- MacPherson H, Thomas K. Introduction: acupuncture and the emerging evidence mosaic. In: MacPherson H, Hammerschlag T, Lewith G, Schnyer R editor(s). Acupuncture research: strategies for establishing an evidence base. 1st Edition. London: Churchill Livingstone, 2008:1‐14. [Google Scholar]
MacPherson 2010
- MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. Journal of Evidence‐based Medicine 2010;3(3):140‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manheimer 2010
- Manheimer E, Cheng K, Linde K, Lao L, Yoo J, Wieland S, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001977.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mapes 2003
- Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, et al. Health‐related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney international 2003;64(1):339‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Markell 2005
- Markell MS. Potential benefits of complementary medicine modalities in patients with chronic kidney disease. Advances in Chronic Kidney Disease 2005;12(3):292‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Markell 2006
- Markell MS. Complementary and alternative medicine: an overlooked adjunct for the care of patients with kidney disease. Dialysis & Transplantation 2006;35(11):724‐6. [EMBASE: 2008501348] [Google Scholar]
McKee 2013
- McKee MD, Kligler B, Fletcher J, Biryukov F, Casalaina W, Anderson B, et al. Outcomes of acupuncture for chronic pain in urban primary care. Journal of the American Board of Family Medicine: JABFM 2013;26(6):692‐700. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naoto 2005
- Naoto I, Masahiro W, Tadashi Y, Naoya O, Shuzo N, Kenji K, et al. Survey research on prevalence, aim and image of acupuncture and moxibustion in Japan (1): prevalence and aim of acupuncture and moxibustion. Journal of the Japan Society of Acupuncture & Moxibustion 2005;55(5):697‐705. [DOI: 10.3777/jjsam.55.697] [DOI] [Google Scholar]
NKF 2002
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. American Journal of Kidney Diseases 2002;39(2 Suppl 1):S1‐S266. [MEDLINE: ] [PubMed] [Google Scholar]
Nowack 2009
- Nowack R, Balle C, Birnkammer F, Koch W, Sessler R, Birck R. Complementary and alternative medications consumed by renal patients in southern Germany. Journal of Renal Nutrition 2009;19(3):211‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patrick 2008
- Patrick D, Guyatt HV, Acquadro C. Chapter 17: Patient‐reported outcomes. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Pham 2010
- Pham PC, Dewar K, Hashmi S, Toscano E, Pham PM, Pham PA, et al. Pain prevalence in patients with chronic kidney disease. Clinical Nephrology 2010;73(4):294‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Rastogi 2008
- Rastogi A, Linden A, Nissenson AR. Disease management in chronic kidney disease. Advances in Chronic Kidney Disease 2008;15(1):19‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sakuraba 2007
- Sakuraba H, Takeuchi H, Takeuchi M, Syoji M, Moriyama T. Questionnaire survey of complaints and acupuncture treatment in maintenance hemodialysis patients. Nihon Toseki Igakkai Zasshi 2007;40(6):513‐6. [DOI: 10.4009/jsdt.40.513] [DOI] [Google Scholar]
Schulz 2002
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359(9306):614‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):726‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Simcock 2013
- Simcock R, Fallowfield L, Monson K, Solis‐Trapala I, Parlour L, Langridge C, et al. ARIX: a randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Annals of Oncology 2013;24(3):776‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stener‐Victorin 2011
- Stener‐Victorin E, Manheimer E. Commentary on the Cochrane Review of acupuncture and assisted conception. Explore: The Journal of Science & Healing 2011;7(2):120‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2012
- Suzuki M, Muro S, Ando Y, Omori T, Shiota T, Endo K, et al. A randomized, placebo‐controlled trial of acupuncture in patients with chronic obstructive pulmonary disease (COPD): the COPD‐acupuncture trial (CAT). Archives of Internal Medicine 2012;172(11):878‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tong 2009
- Tong A, Sainsbury P, Chadban S, Walker RG, Harris DC, Carter SM, et al. Patients' experiences and perspectives of living with CKD. American Journal of Kidney Diseases 2009;53(4):689‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Verbeeck 2009
- Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. European Journal of Clinical Pharmacology 2009;65(8):757‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vickers 2012
- Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Foster NE, et al. Acupuncture for chronic pain: individual patient data meta‐analysis. Archives of Internal Medicine 2012;172(19):1444‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2008
- White A, Cummings M, Filshie J. An introduction to western medical acupuncture. China: Churchill Livingstone Elsevier, 2008. [Google Scholar]
References to other published versions of this review
Kim 2011
- Kim KH, Lee MS, Kim TH, Kang JW, Choi TY, Lee JD. Acupuncture and related interventions for the symptoms of chronic kidney disease. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009440] [DOI] [PMC free article] [PubMed] [Google Scholar]


