Abstract
Background
Long term levodopa therapy in Parkinson's disease is associated with the development of motor complications including abnormal involuntary movements and a shortening response to each dose (wearing off phenomenon). It is thought that dopamine agonists can reduce the duration of immobile off periods and the need for levodopa therapy whilst maintaining or improving motor impairments and only minimally increasing dopaminergic adverse events.
Objectives
To compare the efficacy and safety of adjuvant cabergoline therapy versus bromocriptine in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Search methods
Electronic searches of MEDLINE, EMBASE and the Cochrane Controlled Trials Register. Handsearching of the neurology literature as part of the Cochrane Movement Disorders Group's strategy. Examination of the reference lists of identified studies and other reviews. Contact with Pharmacia Upjohn Limited.
Selection criteria
Randomised controlled trials of cabergoline versus bromocriptine in patients with a clinical diagnosis of idiopathic Parkinson's disease and long‐term complications of levodopa therapy.
Data collection and analysis
Data were abstracted independently by the authors and differences settled by discussion. The outcome measures used included Parkinson's disease rating scales, levodopa dosage, off time measurements and the frequency of withdrawals and adverse events.
Main results
Cabergoline has been compared with bromocriptine in five randomised, double‐blind, parallel group studies including 1071 patients. Only one of the phase II studies was medium term (36 weeks), the others all being short term (12 ‐15 weeks). The non‐significant difference in off time reduction produced by cabergoline compared with bromocriptine was 0.29 hours/day in favour of the former (weighted mean difference; 95% CI ‐0.10, 0.68; p = 0.15). Dyskinesia reported as an adverse event was significantly increased with cabergoline compared with bromocriptine (Peto odds ratio 1.57; 95% CI 1.05, 2.35; p = 0.03). Motor impairment and disability were measured in four of the studies using the UPDRS rating scale but the small differences in UPDRS ADL (part II) and motor (part III) scores were not statistically significant in any study. Similarly, no significant difference in Schwab and England score was seen. The number of patients rated as much or very much improved on a clinician's global impression scale was similar with both agonists. Levodopa dose reduction was no different between cabergoline and bromocriptine. There was more confusion with cabergoline (Peto odds ratio 2.02; 95% CI 1.09, 3.76; p = 0.03). Otherwise, dopaminergic adverse events were comparable with these agonists and no significant difference in all cause withdrawal rate was found.
Authors' conclusions
Cabergoline produces similar benefits to bromocriptine in off time reduction, motor impairment and disability ratings, and levodopa dose reduction over the first three months of therapy. Dyskinesia and confusion were increased with cabergoline but otherwise the frequency of adverse events and withdrawals from treatment were similar with the two agonists.
Plain language summary
Cabergoline versus bromocriptine for levodopa‐induced complications in Parkinson's disease
In the later stages of Parkinson's disease, side effects occur because of the use of levodopa treatment. These consist of involuntary writhing movements (dyskinesia), painful cramps in the legs (dystonia) and a shortened response to each dose referred to as 'end‐of‐dose deterioration' or the 'wearing‐off effect'. Dopamine agonist drugs act by mimicking levodopa in the brain, but they do not cause these long‐term treatment complications when used as initial therapy. For this reason, dopamine agonists have for some years been added once these problems develop in the hope of improving them. Cabergoline is a new dopamine agonist recently licensed in the UK for the treatment of later Parkinson's disease. In this review, we will examine the trials performed with this drug to see how effective it is compared with the older drug bromocriptine and what side effects it causes.
Cabergoline has been compared with the older agonist bromocriptine in five studies including 1071 patients. Only one of the smaller studies was medium term (36 weeks), the others all being short term (12 ‐15 weeks). The time patients spent in the immobile off state was reduced with both agonists but slightly more by cabergoline compared with bromocriptine. This small advantage of cabergoline did not reach statistical significance. Dyskinesia reported as a side effect was significantly increased with cabergoline compared with bromocriptine. Physical impairment and disability were measured in four of the studies but no statistically significant advantage for cabergoline was found. The number of patients rated as much or very much improved on a clinician's global impression scale was similar with both agonists. Levodopa dose reduction was no different between cabergoline and bromocriptine. There was significantly more confusion with cabergoline. Otherwise, dopaminergic side effects were comparable with these agonists and no significant difference in the withdrawal rate from the trials was found.
Cabergoline produces similar benefits to bromocriptine in off time reduction, physical impairment and disability ratings, and levodopa dose reduction over the first three months of therapy. The frequency of side effects and withdrawals from treatment were similar with the two agonists apart from increased dyskinesia and confusion with cabergoline.
Background
Over 20 years after its introduction, levodopa remains the most effective therapy in Parkinson's disease. However, with long‐term treatment, patients develop side effects comprised of motor and psychiatric complications. The former consist of involuntary writhing movements of the limbs and trunk (choreoathetosis), painful cramps often affecting the feet (dystonia) and a shortened response to each dose of levodopa (end‐of‐dose deterioration). These affect 50% of patients after 6 years of therapy (Rajput 1984) and 100% of young onset patients (Quinn 1986).
An alternative treatment in Parkinson's disease is the dopamine agonist class of drug. These act directly on post‐synaptic dopamine receptors in the striatum and, unlike levodopa, they do not require conversion into dopamine. They have developed the reputation of being less effective in clinical practice than expected, although they generate fewer motor complications when used as long‐term monotherapy. The use of dopamine agonists in newly diagnosed patients will be the subject of further Cochrane reviews.
Cabergoline is an ergoline class dopamine agonist along with bromocriptine, pergolide, and lisuride. It has a long half‐life of around 65 hours compared with the other dopamine agonists and thus is administered once daily. Therefore, it is easier to titrate and for the patient to take and potentially it may reduce motor complications more by reducing the phasic stimulation of dopamine receptors.
The efficacy and safety of cabergoline have been examined in early and advanced Parkinson's disease. Monotherapy studies will be examined in other Cochrane reviews. Trials in later disease have lead to cabergoline being licensed in the United Kingdom for this indication in the expectation of a reduction in off time and improved motor function.
The present systematic review examines all randomised controlled trials of adjuvant cabergoline therapy compared with bromocriptine in later Parkinson's disease with motor complications to establish its efficacy and tolerability. A separate review covers the effects of adjuvant cabergoline versus placebo.
Objectives
To compare the efficacy and safety of adjuvant cabergoline versus bromocriptine in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing adjuvant cabergoline with bromocriptine were considered for inclusion in the study.
Types of participants
Patients with a clinical diagnosis of idiopathic Parkinson's disease who had developed long‐term motor complications of dyskinesia and/or end‐of‐dose deterioration. All ages were included. Any duration of levodopa therapy was included.
Types of interventions
Oral cabergoline therapy or bromocriptine. Trial durations of greater than 4 weeks were included.
Types of outcome measures
1. Improvement in the time patients spend in the immobile 'off' state.
2. Changes in dyskinesia rating scales and the prevalence of dyskinesia.
3. Changes in parkinsonian rating scales.
4. Reduction in levodopa dose.
5. Number of withdrawals due to lack of efficacy and/or side‐effects.
Search methods for identification of studies
1. The review was based on the search strategy of the Movement Disorders Group. This included computerised searches of MEDLINE and EMBASE and hand searching of appropriate neurology journals. Relevant trials were included on the Group's specialised register of randomised controlled trials. Further details are available in the Group's module on the Cochrane Database of Systematic Reviews.
2. The Cochrane Controlled Trials Register was also searched for relevant trials.
3. The reference lists of located trials and of other cabergoline reviews were searched.
4. Additional assistance was provided by the drug manufacturer Pharmacia Upjohn.
Data collection and analysis
The two authors (CC, KD) independently assessed the studies identified by the search strategy. Disagreements about inclusions were resolved by discussion. The full papers were assessed for methodological quality by recording the method of randomisation and blinding, whether an intention‐to‐treat analysis was used and the number of patients lost to follow up.
Eligible data was abstracted onto standardised forms by the authors independently, checked for accuracy and amalgamated. A weighted estimate (fixed effect model) of the typical treatment effect across trials was calculated for continuous (weighted mean difference) and dichotomous (Peto odds ratio) variables such as 'off' time and prevalence of adverse events. Since multiple comparisons of adverse events were examined statistically, the results were interpreted cautiously using 99% confidence intervals.
Results
Description of studies
See also Characteristics of Included Studies and Table 1Characteristics and Results of Included Studies.
1. Key Characteristics and Results for Included Studies.
| Study | Number of patients | Mean Hoehn & Yahr | Duration (weeks) | Mean (Maximum) dose Cabergoline / Bromocriptine (mg/d) | Mean difference (MD) L‐dopa reduction mg/d; + in favour of cabergoline) | MD off hours reduction (hours; + in favour of cabergoline) | MD UPDRS ADL (+ in favour of cabergoline) | MD UPDRS Motor (+ in favour of cabergoline) | Drop‐outs (Peto Odds Ratio < 1 in favour of cabergoline) |
| Korczyn | 42 | 2.4 | 13 | n/a (4.0) / n/a (40) | n/a | 0.7 | 1.0 (off) ‐0.5 (on) | 0 (on) | 0.49 |
| Gershanik 40 | 384 | n/a | 10 | Median 4.0 (6.0)/ 25 (40.0) | 12.6 | 0.3 | 0.8 (off) ‐0.3 (on) | ‐0.8 (off) 1.7 (on) | 0.80 |
| Gershanik 41 | 366 | 3.5 (off) 2.1 (on) | 8 | 4.4 (6.0)/ 28.7 (40.0) | ‐2.8 | 0.3 | 0.6 (off) 0.2 (on) | 1.9 (off) 0.2 (on) | 1.14 |
| Inzelberg | 44 | n/a | 36 | 3.2 (6.0)/ 22.1 (40.0) | 3.0 | 1.76 | 0 | ‐2 | 0.40 |
| Yanagisawa | 235 | 2.8 (on) | 12 | 2.7 (4.0)/ 16.4 (22.5) | 7.3 | ‐0.91 | n/a | n/a | 1.01 |
| Total/Mean | 1071 | 6.0 (WMD) | 0.38 (WMD) | 0.87 |
Five trials fulfilled the inclusion criteria (Gershanik 1994; Gershanik 1994b; Inzelberg 1996; Korczyn 1994; Yanagisawa 1996). Only two of these studies have been published (Inzelberg 1996; Yanagisawa 1996 ‐ in Japanese) but data from the other three and additional data on all trials was provided by the manufacturer. A total of 1071 patients with Parkinson's disease and motor fluctuations were included in these studies.
All five studies were randomised, double‐blind, parallel group design. Two were phase II studies (Inzelberg 1996; Korczyn 1994) and the remaining three phase III studies (Gershanik 1994; Gershanik 1994b; Yanagisawa 1996). In three trials, patients only continued in the study after titration if they had responded to the trial medication, so only the data up to the end of the titration period has been included (Gershanik 1994; Gershanik 1994b; Korczyn 1994).
Patients were well balanced across the arms of the studies in terms of age and Hoehn and Yahr score, apart from in Korczyn 1994 where the cabergoline arm has a worse Hoehn and Yahr stage at entry (see Characteristics of Included Studies).
The maximum dose of cabergoline used in the trials was 4.0 ‐ 6.0 mg/d which is broadly comparable with the present licensed limit of 6.0 mg/d. The maximum dose of bromocriptine ranged between 22.5 md/d in one trial (Yanagisawa 1996) and 40.0 mg/d in the others, the latter being comparable to clinically used doses.
Levodopa dose reduction was allowed in all of the trials.
Risk of bias in included studies
See also Characteristics of Included Studies and Table 1Key Characteristics and Results of Included Studies.
Details on randomisation and concealment of allocation were described in four of the trial reports and were found to be adequate. However, information on the other study was not available (Inzelberg 1996).
The double‐blind design of all of the trials should exclude performance and attrition bias. Detection bias is unlikely in view of the double‐blind design and the use of a pre‐specified statistical analysis for all studies.
One of the phase II studies was short term (Korczyn 1994, 13 weeks) whereas the other was medium term (Inzelberg 1996; 36 weeks). All of the phase III studies were short term (12 ‐15 weeks).
Sample size calculations were not included in the small phase II trials which is standard practice. Such calculations were provided in all phase III study reports (Gershanik 1994; Gershanik 1994b; Yanagisawa 1996).
Effects of interventions
See also Table 1Characteristics and Results of Included Studies and Table 2 Adverse Events for Included Studies.
2. Adverse Events for Included Studies (Peto Odds Ratio < 1 favours cabergoline).
| Study (number) | Nausea | Postural Hypotension | Hallucinations | Confusion | Dyskinesia | Insomnia | Sleep disorder | Vivid Dreams | Somnolence |
| Korczyn (42) | 0.88 | 0.89 | 1.83 | 1.83 | 1.40 | 0.45 | 1.00 | n/a | 0.64 |
| Gershanik 40 (384) | 0.98 | 1.07 | 1.36 | 1.69 | 1.93 | 1.38 | 2.06 | n/a | 2.24 |
| Gershanik 41 (366) | 0.83 | 0.45 | 1.43 | 2.38 | 1.48 | 0.79 | 1.02 | n/a | 0.56 |
| Inzelberg (44) | 2.01 | 1.82 | 0.12 | 2.01 | 1.00 | 0.30 | n/a | 1.00 | n/a |
| Yanagisawa (235) | 1.28 | 0.53 | 1.86 | n/a | 1.05 | 7.75 | n/a | n/a | 1.05 |
| Total/Mean (1071) | 0.99 | 0.74 | 1.34 | 2.02 | 1.57 | 0.95 | 1.35 | 1.00 | 0.97 |
| P value (Test for overall effect) | 1.00 | 0.15 | 0.17 | 0.03 | 0.03 | 0.80 | 0.20 | null | 0.90 |
Cabergoline has been compared with bromocriptine in two phase II (Inzelberg 1996; Korczyn 1994) and three phase III randomised controlled trials (Gershanik 1994; Gershanik 1994b; Yanagisawa 1996). These were double‐blind, parallel group studies including 1071 patients with Parkinson's disease and motor complications. Only one of the phase II studies was medium term (36 weeks), the others all being short term (12 ‐15 weeks).
Information on off time reduction was available expressed as hours per day in only four of the five trials (Table 7). The difference in the off time reduction produced by cabergoline compared with bromocriptine was 0.29 hours/day (weighted mean difference; 95% CI ‐0.10, 0.68; p = 0.15; Table 7). The difference between the agonists in off time reduction was only statistically significant for one of the small phase II studies (Inzelberg 1996).
None of the studies used a dyskinesia rating scale. Dyskinesia reported as an adverse event was significantly increased with cabergoline compared with bromocriptine (Peto odds ratio 1.57; 95% CI 1.05, 2.35; p = 0.03; Table 12).
Motor impairment and disability were measured in four of the studies using the UPDRS rating scale (Tables 1 and 2). The small differences in UPDRS ADL (part II) and motor (part III) scores were not statistically significant in any study. Similarly, no significant difference in Schwab and England score was seen (Table 4). The number of patients rated as much or very much improved on a clinician's global impression scale was similar with both agonists (Table 5).
Levodopa dose reduction was no different between cabergoline and bromocriptine (Table 6).
There was significantly more confusion with cabergoline (Peto odds ratio 2.02; 95% CI 1.09, 3.76; p = 0.03; Table 11). Otherwise, dopaminergic adverse events were comparable with these agonists (Tables 8 ‐ 10 and 12 ‐ 16). The all cause withdrawal rate was also similar (Table 17).
Discussion
Cabergoline has been compared with bromocriptine in five randomised controlled trials involving 1071 patients with Parkinson's disease and motor complications. This is the largest number of patients involved in any of the placebo‐ or bromocriptine‐controlled adjuvant therapy programmes according to previous Cochrane reviews. In spite of this, all bar one small phase II study were short term lasting for just 12 to 15 weeks. Thus, the conclusions of this review should not be extrapolated beyond this period.
Reducing the time patients spend in the off phase is a major goal of adjuvant therapy at this stage of the disease. Cabergoline and bromocriptine both reduced off time in these studies. Although there was a trend towards more reduction in off time with cabergoline, this did not reach statistical significance (0.29 hours/day; 95% CI ‐0.10, 0.68; p = 0.15). In contrast, there was increased dyskinesia reported as an adverse event in the cabergoline treated patients. No significant benefit of cabergoline over bromocriptine was found in the improvement in motor impairments and disability as measured with the UPDRS ADL and motor scales and the Schwab and England score. Similarly, levodopa dose reduction was comparable with the two agonists. There was more confusion with cabergoline but adverse events and withdrawals were otherwise similar with both agonists.
In summary, cabergoline produces similar benefits and hazards to bromocriptine, although these conclusions must be limited to the first three months of therapy in view of the short duration of most of the studies. Caution should be exercised in interpreting the results of this review as small but clinically relevant differences between the agonists may have been disclosed in larger trials with appropriate statistical power to avoid a false negative conclusion. Also, if larger doses of bromocriptine had been used, any differences between the agonists may have disappeared. Further trials are necessary before it can be concluded that one agonist is superior to another. In the meantime, other characteristics of these agents should be considered such as the ease of titration and administration of cabergoline versus the lower cost of bromocriptine.
Regarding the conduct of these five trials, a number of comments on their design and reporting should be made in the hope of improving the quality of similar work in the future:‐
Only one of these studies has been published and it seems unlikely to the authors that the others will ever enter the public domain. This potentially may give rise to publication bias, so manufacturers should be encouraged to publish all such work in some form.
The standard of reporting of methods and results was better in these more recent studies than with some of the earlier agonist development programmes judging from previous Cochrane reviews. However, most were internal industry reports which are necessarily more detailed. On publication, the CONSORT reporting guidelines should be followed (CONSORT 1996).
The trials with cabergoline were similar to those with all of the other dopamine agonists in using outcomes which measured motor impairments and disability. No quality of life measures or health economics outcomes were included. Therefore, no conclusions regarding the effectiveness of these agents can be drawn. Further studies are required to examine these issues.
Further studies of adjuvant therapy in Parkinson's disease should have sufficient power to examine whether the new agonists are superior to the less expensive bromocriptine.
Authors' conclusions
Implications for practice.
Cabergoline produces similar benefits to bromocriptine in off time reduction, motor impairment and disability ratings, and levodopa dose reduction over the first three months of therapy. Dyskinesia and confusion were greater with cabergoline but otherwise the frequency of adverse events and withdrawals from treatment were similar with the two agonists. In view of the low power of these studies to detect small but clinically significant differences between the agonists, these results should be treated with caution. Further work is required before one agonist can be prefered over another, particularly in view of the ease of titration and administration of cabergoline and the lower cost of bromocriptine.
Implications for research.
Incomplete Reporting
Only one of the five studies included here has been published in a peer reviewed journal. Some mechanism must be found to ensure that such information enters the public domain to reduce the potential for publication bias.
All publications stemming from randomised controlled trials should conform to the CONSORT reporting guidelines (CONSORT 1996).
Further Trials
A further paper publication will summarise the results of all of the adjuvant therapy Cochrane reviews, but it is apparent that no one of the more recently introduced dopamine agonists is clearly superior to another or the older bromocriptine. Further large pragmatic trials are required to provide data on the comparative efficacy, effectiveness and safety of the dopamine agonists as adjuvant therapy in Parkinson's disease.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2015 | Amended | PLS correction |
| 13 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 17 November 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors thank Pharmacia Upjohn for their assistance in performing this review.
Data and analyses
Comparison 1. Cabergoline versus Bromocriptine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 UPDRS ADL scores (part II) | Other data | No numeric data | ||
| 2 UPDRS motor scores (part III) | Other data | No numeric data | ||
| 3 Hoehn and Yahr stage | Other data | No numeric data | ||
| 4 Schwab and England scale | Other data | No numeric data | ||
| 5 Clinicians global impression (number much or very much improved) | 3 | 784 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.76, 1.33] |
| 6 Levodopa dose reduction (mg) | 4 | 909 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐21.79, 33.79] |
| 7 Off time reduction (hours) | 4 | 612 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.10, 0.68] |
| 8 Adverse events ‐ Nausea | 5 | 1048 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.73, 1.35] |
| 9 Adverse events ‐ Postural hypotension | 5 | 1048 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.49, 1.11] |
| 10 Adverse events ‐ Hallucinations | 5 | 1048 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.88, 2.06] |
| 11 Adverse events ‐ Confusion | 4 | 835 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [1.09, 3.76] |
| 12 Adverse event ‐ Dyskinesia | 5 | 1048 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [1.05, 2.35] |
| 13 Adverse events ‐ Insomnia | 5 | 1048 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.58, 1.54] |
| 14 Adverse events ‐ Sleep disorder | 3 | 793 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.82, 2.21] |
| 15 Adverse events ‐ Vivid dreams | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.06, 16.52] |
| 16 Adverse events ‐ Somnolence | 4 | 1006 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.55, 1.73] |
| 17 All cause withdrawal rate | 5 | 1071 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.63, 1.20] |
1.1. Analysis.
Comparison 1 Cabergoline versus Bromocriptine, Outcome 1 UPDRS ADL scores (part II).
| UPDRS ADL scores (part II) | |
|---|---|
| Study | |
| Gershanik 1994 | 'Off' state: Improvement on cabergoline ‐6.1 (SD5.3) v bromocriptine ‐5.3 (SD5.6) p > 0.05; NS. 'On' state: Improvement on cabergoline ‐2.9 (SD3.9) v bromocriptine ‐3.2 (SD3.9) p > 0.05; NS. |
| Gershanik 1994b | 'Off' state: Improvement on cabergoline ‐4.7 (SD5.4) v bromocriptine ‐4.1 (SD4.5) p > 0.05; NS. 'On' state: Improvement on cabergoline ‐2.9 (SD3.9) v bromocriptine ‐2.7 (SD3.8) p > 0.05; NS. |
| Inzelberg 1996 | Improvement on Cabergoline (n=22) ‐2 (SD 5.1) v bromocriptine (n=22) ‐2 (SD 5.0) p > 0.05; NS. |
| Korczyn 1994 | 'Off' state: Improvement on cabergoline (n=10) ‐3.9 (SD 7.9) v bromocriptine (n=10) ‐2.9 (SD 5.0) p > 0.05; NS. 'On' state: Improvement on cabergoline (n=16) ‐0.5 (SD 4.4) v bromocriptine (n=15) ‐1.0 (SD 3.4) p > 0.05; NS. |
1.2. Analysis.
Comparison 1 Cabergoline versus Bromocriptine, Outcome 2 UPDRS motor scores (part III).
| UPDRS motor scores (part III) | |
|---|---|
| Study | |
| Gershanik 1994 | 'Off' state: Improvement on cabergoline ‐12.5 (SD 11) v bromocriptine ‐13.3 (SD10.1) p > 0.05; NS. 'On' state: Improvement on cabergoline ‐9.0 (SD8.9) v bromocriptine ‐7.3 (SD8.4) p > 0.05; NS. |
| Gershanik 1994b | 'Off' state: Improvement on cabergoline ‐11.1 (SD11.3) v bromocriptine ‐9.2 (SD8.5) p > 0.05; NS. 'On' state: Improvement on cabergoline ‐6.1 (SD8.7) v bromocriptine ‐5.9 (SD7.9) p > 0.05; NS. |
| Inzelberg 1996 | Improvement on cabergoline (n=22) ‐7 (SD 12.6) v bromocriptine (n=22) ‐9 (SD 12.7) p > 0.05; NS. |
| Korczyn 1994 | 'On' state: Improvement on cabergoline (n=16) ‐6.0 (SD 10.8) v bromocriptine (n=14) ‐6.0 (SD 7.9) p > 0.05; NS. |
1.3. Analysis.
Comparison 1 Cabergoline versus Bromocriptine, Outcome 3 Hoehn and Yahr stage.
| Hoehn and Yahr stage | |
|---|---|
| Study | |
| Gershanik 1994 | 'Off' state: Improvement on cabergoline ‐0.5 (SD 0.6) v bromocriptine ‐0.6 (SD 0.7) 'On' state: Improvement on cabergoline ‐0.2 (SD 0.4) v bromocriptine ‐0.3 (SD 0.5) |
| Gershanik 1994b | 'Off' state: Improvement on cabergoline ‐0.5 (SD 0.7) v bromocriptine ‐0.4 (SD 0.6) 'On' state: Improvement on cabergoline ‐0.1 (SD 0.5) v bromocriptine ‐0.2 (SD 0.5) |
| Inzelberg 1996 | Not available |
| Korczyn 1994 | 'Off' state: Improvement on cabergoline (n=17) ‐0.38 (SD 0.81) v bromocriptine (n=15) ‐0.23 (SD 1.02). 'On' state: Improvement on cabergoline (n=19) ‐0.16 (SD 0.55) v bromocriptine (n=15) ‐0.30 (SD 0.72). |
1.4. Analysis.
Comparison 1 Cabergoline versus Bromocriptine, Outcome 4 Schwab and England scale.
| Schwab and England scale | |
|---|---|
| Study | |
| Gershanik 1994 | 'Off' state: Improvement on cabergoline 11.7 (SD 13.6) v bromocriptine 12.8 (SD 14.5) p > 0.05; NS. 'On' state: Improvement on cabergoline 5.7 (SD 7.5) v bromocriptine 6.1 (SD 9.5) p > 0.05; NS. |
| Gershanik 1994b | 'Off' state: Improvement on cabergoline 9.9 (SD 14.4) v bromocriptine 8.5 (SD 12.9) p > 0.05; NS. 'On' state: Improvement on cabergoline 3.0 (SD 9.7) v bromocriptine 3.5 (SD 8.6) p > 0.05; NS. |
| Inzelberg 1996 | Improvement on cabergoline (n=22) 1 (SD 10.2) v bromocriptine (n=22) ‐2 (SD 9.5) p > 0.05; NS. |
| Korczyn 1994 | 'Off' state: Improvement on cabergoline (n=18) 7.78 (SD 19.29) v bromocriptine (n=15) 6.67 (SD 22.72) p > 0.05; NS. 'On' state: Improvement on cabergoline (n=18) ‐0.55 (SD 11.83) v bromocriptine (n=15) 3.33 (SD 12.55) p > 0.05; NS. |
1.5. Analysis.
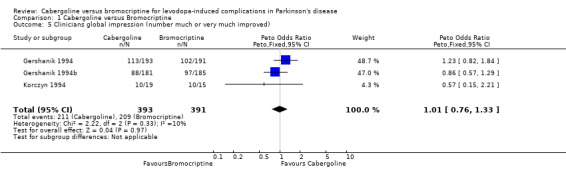
Comparison 1 Cabergoline versus Bromocriptine, Outcome 5 Clinicians global impression (number much or very much improved).
1.6. Analysis.
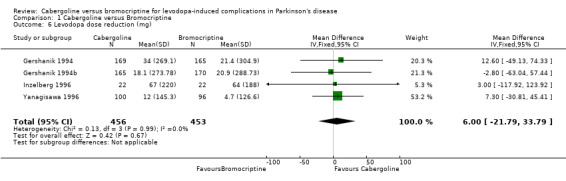
Comparison 1 Cabergoline versus Bromocriptine, Outcome 6 Levodopa dose reduction (mg).
1.7. Analysis.
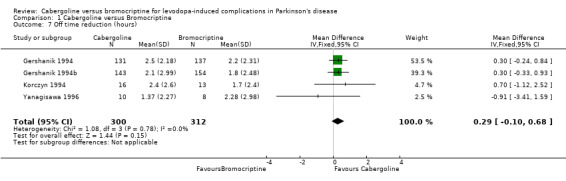
Comparison 1 Cabergoline versus Bromocriptine, Outcome 7 Off time reduction (hours).
1.8. Analysis.
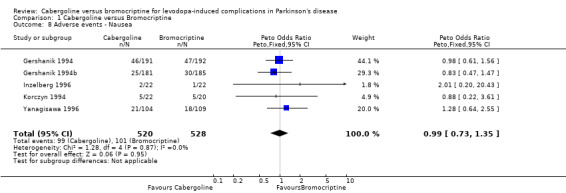
Comparison 1 Cabergoline versus Bromocriptine, Outcome 8 Adverse events ‐ Nausea.
1.9. Analysis.
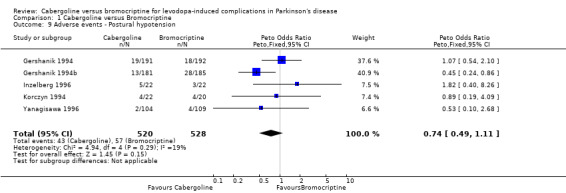
Comparison 1 Cabergoline versus Bromocriptine, Outcome 9 Adverse events ‐ Postural hypotension.
1.10. Analysis.
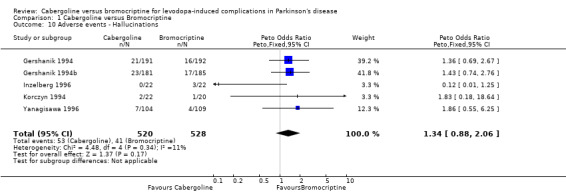
Comparison 1 Cabergoline versus Bromocriptine, Outcome 10 Adverse events ‐ Hallucinations.
1.11. Analysis.
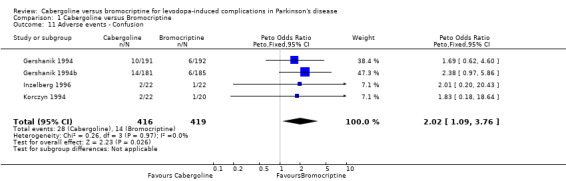
Comparison 1 Cabergoline versus Bromocriptine, Outcome 11 Adverse events ‐ Confusion.
1.12. Analysis.
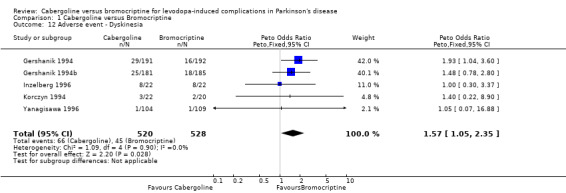
Comparison 1 Cabergoline versus Bromocriptine, Outcome 12 Adverse event ‐ Dyskinesia.
1.13. Analysis.
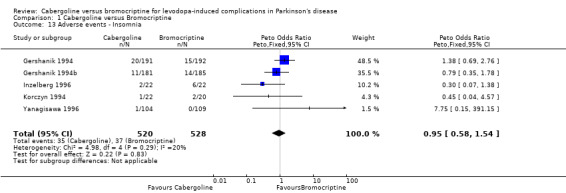
Comparison 1 Cabergoline versus Bromocriptine, Outcome 13 Adverse events ‐ Insomnia.
1.14. Analysis.
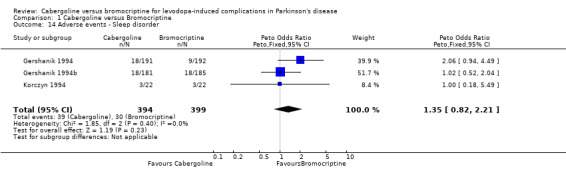
Comparison 1 Cabergoline versus Bromocriptine, Outcome 14 Adverse events ‐ Sleep disorder.
1.15. Analysis.
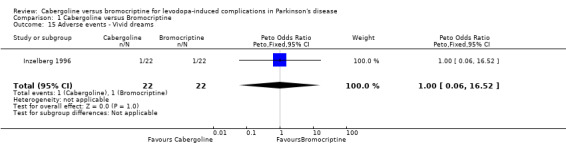
Comparison 1 Cabergoline versus Bromocriptine, Outcome 15 Adverse events ‐ Vivid dreams.
1.16. Analysis.
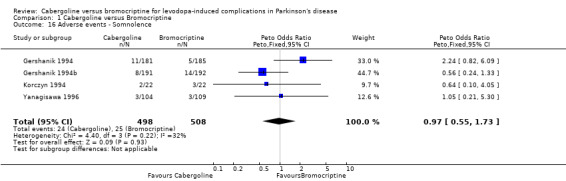
Comparison 1 Cabergoline versus Bromocriptine, Outcome 16 Adverse events ‐ Somnolence.
1.17. Analysis.
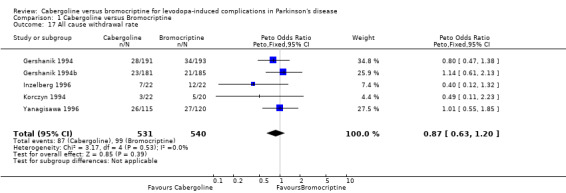
Comparison 1 Cabergoline versus Bromocriptine, Outcome 17 All cause withdrawal rate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gershanik 1994.
| Methods | Randomised, double blind, parallel group design. Computerised randomisation list generated. Treatments were balanced within blocks of 4 patients. Intention to treat analysis only for clinicians global impression score, all other data analysed on a per protocol basis. Location: 69 sites in 13 countries Duration: Titration phase of 15 weeks maximum (mean 10 weeks), if the patients showed minimal improvement or better there followed a 3 month stable dose phase. Patients in whom improvement was maintained entered a follow‐up treatment period (mean 10 months) carried out in double‐blind conditions until completion of last patient in each country, and subsequently in open conditions. Median duration, cabergoline = 444 days, bromocriptine = 455 days. | |
| Participants | Cabergoline: 191 patients with 28 drop‐outs (15%) at the end of the titration phase. Bromocriptine: 193 patients with 34 drop‐outs (18%) at the end of the titration phase. Age: Cabergoline = 62.3 years (SD9.1), Bromocriptine = 61.7 years (SD8.4) Hoehn and Yahr scale at baseline: 2 or 2.5 in 111/191 of the patients in the cabergoline group, 123/193 in the bromocriptine group. Inclusion criteria: IPD with motor fluctuations Exclusion criteria: history of intolerance of dopamine agonists, severe depression, other CNS disorders, serious cardiac disease, kidney or liver impairment. | |
| Interventions | Drugs titrated over 15 weeks, 8 dose levels, increments applied at weekly/biweekly intervals. Cabergoline: initial dose = 0.5mg/d, maximum = 6mg/d, median = 4mg/d. Bromocriptine: initial dose = 5mg/d, maximum = 40mg/d, median = 25mg/d. Levodopa could be reduced. | |
| Outcomes | Primary: Clinicians global impression scale (7 points). Secondary: Off hours UPDRS Hoehn and Yahr Schwab and England Adverse Events | |
| Notes | Only data from the end of titration phase was used in this review as patients were subsequently selected for on the basis of their response to the drugs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Gershanik 1994b.
| Methods | Randomised, double blind, parallel group design. Intention to treat analysis only for clinicians global impression score, all other data analysed on a per protocol basis. Location: 67 sites throughout Europe, Israel and Latin America. Duration: Titration phase 13 weeks maximum (mean 2 months), if the patients showed minimal improvement or better there followed a 3 month stable dose phase. Patients in whom improvement was maintained entered a follow‐up treatment period carried out in double‐blind conditions until completion of last patient in each country, and subsequently in open conditions. | |
| Participants | Cabergoline: 181 patients with 23 drop‐outs (13%) after titration phase. Bromocriptine: 185 patients with 21 drop‐outs (11%) after titration phase. Age: Cabergoline = 61.0 years (SD9.8), Bromocriptine = 60.9 years (SD9.3). Hoehn and Yahr score at baseline, 'On' state: cabergoline 2.1, bromocriptine 2.1; 'Off' state: cabergoline 3.4, bromocriptine 3.5. Inclusion criteria: IPD with motor fluctuations Exclusion criteria: History of intolerance of dopamine agonists, severe depression, other CNS disorders, serious cardiac disease, liver or renal impairment. | |
| Interventions | Drugs titrated over 15 weeks, 8 dose levels, increments applied at weekly/biweekly intervals. Cabergoline: initial dose = 0.5mg/d, maximum = 6mg/d, mean 4.4mg/d. Bromocriptine: initial dose = 5mg/d, maximum = 40mg/d, mean 28.7 mg/d Levodopa could be reduced. | |
| Outcomes | Primary: Clinicians global impression score (7 points) Secondary: Off/On diaries UPDRS Hoehn and Yahr Schwab and England Adverse Events | |
| Notes | Only data from the end of titration phase was used in this review as patients were subsequently selected for on the basis of their response to the drugs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Inzelberg 1996.
| Methods | Randomised, double blind, parallel group design. Randomisation method not stated. Analysis not intention to treat. All follow‐up visit results included in calculating 'mean' response to treatment then this was compared with 'baseline' values. Location: 1 site in Israel. Duration: Variable titration phase followed by 6‐12 month stable dose phase. Mean duration 9 months (SD5). | |
| Participants | Cabergoline: 22 patients with 7 drop‐outs (32%). Bromocriptine: 22 patients with 12 drop‐outs (55%). Details of terminations given. Withdrawals allowed due to lack of efficacy. Mean age 71 years (SD8). Hoehn and Yahr score at baseline: Stage II or III when 'on'. No means given. Inclusion criteria: IPD with motor complications. Exclusion criteria: history of severe psychiatric disturbances with dopamine agonists, severe depression, serious heart disease, renal or liver impairment. | |
| Interventions | Cabergoline: Initial dose 0.5mg/d, increased every two weeks by 0.25mg/d or 0.5mg/d, maximum dose 6mg/d, mean dose 3.2mg/d. Bromocriptine: Initial dose 5mg/d, increased every two weeks by 2.5mg/d or 5mg/d, maximum dose 40mg, mean dose 22.1mg/d Levodopa could be reduced | |
| Outcomes | UPDRS ADL subsection UPDRS motor subsection Items of motor UPDRS (tremor, rigidity etc) Dyskinesia (UPDRS item 32) Schwab and England On/Off charts Adverse Events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Korczyn 1994.
| Methods | Randomised, double‐blind, parallel group design. A computerized randomisation list was generated. Treatments were balanced within blocks of 4 cases. The study medication was prepared and labelled with corresponding patient numbers. In each centre patients were to recieve progressive randomisation numbers according to theri temporal entry into the study. Data analysed on a per protocol basis. Location: 12 sites. Duration: Titration phase of 13 weeks, if the patients showed minimal improvement or better there followed a 3 month stable dose phase. | |
| Participants | Cabergoline: 22 patients with 3 drop‐outs (14%) at end of titration phase. Bromocriptine: 20 patients with 5 drop‐outs (25%) at end of titration phase. Age: Cabergoline = 61.4 years (SD 6.4), Bromocriptine = 59.6 years (SD 9.6) Hoehn and Yahr at baseline, 'On' state: Cabergoline 2.3, Bromocriptine 2.5, 'Off' state: Cabergoline 3.2, bromocriptine 3.4. Inclusion criteria: IPD with motor fluctuations. Other dopamine agonists stopped 2 weeks prior to trial. Exclusion criteria: Other CNS degenerative disorders, severe depression or dementia, cardiopathies, history of severe psychiatric disorder with previous dopamine agonists, renal or hepatic impairment, child bearing potential. | |
| Interventions | Drugs titrated over 13 weeks, increments applied at weekly/fortnightly intervals. Cabergoline: initial dose = 0.5mg/d, maximum = 4.0mg/d Bromocriptine: initial dose = 5.0mg/d, maximum = 40mg/d Levodopa could be reduced. | |
| Outcomes | Primary: Clinicians Global Impression score (7 points) Secondary: Off hours UPDRS parts II and III Hoehn and Yahr Swab and England Adverse Events | |
| Notes | Only data from the end of the titration phase was used in this review as patients were subsequently selected on the basis of their response to the drugs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Yanagisawa 1996.
| Methods | Randomised double blind parallel group design. A computerized randomisation list was generated. Treatments were balanced within blocks of 4 cases. Data analysed on a per protocol basis. Intention to treat analysis claimed for primary outcomes but 2 patients in bromocriptine group that withdrew consent before the start of the therapy were excluded. Location: Multicentre, Japan. Duration: 12 weeks (including 8 week titration phase). | |
| Participants | Cabergoline: 115 patients with 26 drop‐outs (23%). Bromocriptine: 120 patients with 27 drop‐outs (23%). Age: Cabergoline = 65.8 years (SD8.9), bromocriptine = 64.2 years (SD8.7). Hoehn & Yahr at baseline, 'On' state: Cabergoline 2.7, bromocriptine 2.8, (32 drop‐outs not characterised). Inclusion criteria: PD, over 20 years old, on l‐dopa with motor fluctuations or on l‐dopa & obtaining therapeutic effect but desiring further improvement. Exclusion criteria: Ever recieved cabergoline, on other ergot derivatives (but could be included after 1 month wash‐out period). Hypersensitive to ergot derivative. Patients with complications of PD other than IPD. Patients with conciousness disturbance, severe hypotension or organ dysfunction. Peripheral vascular lesions, active peptic ulcer, childbearing potential. | |
| Interventions | Cabergoline: Initial dose 0.25mg/d, increased weekly to maximum 4.0mg/d. Mean dose 2.74 mg/d. Bromocriptine: Initial dose 1.25mg/d increased weekly to maximum 22.5mg/d. Mean dose 16.44mg/d. Levodopa could be reduced. | |
| Outcomes | Primary: Improvement of neurological symptoms Final Global Improvement Overall safety Usefulness Secondary: Levodopa reduction Off hours reduction Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Declarations of interest
CEC has received payment from Pharmacia Upjohn for lectures and attending meetings.
Edited (no change to conclusions)
References
References to studies included in this review
Gershanik 1994 {unpublished data only}
- Destee A, Schneider E, Gershanik O, et al. Efficacy and tolerability of cabergoline compared to bromocriptine in patients suffering from levodopa associated motor complications (not on treatment with DA‐agents). Movement Disorders 1996;11(Supplement 1):269. [Google Scholar]
- Gershanik O, Dom R, Tichy J, Ziegler M, Schneider E, Korczyn AD. Multicenter, multinational double‐blind study of the activity and tolerability of cabergoline vs bromocriptine in parkinsonian patients suffering from L‐dopa associated motor complications, not on treatment with dopamine agonist agents. Farmitalia Carlo Erba; Clinical Reference 40 1994.
Gershanik 1994b {unpublished data only}
- Gershnik O, Dom R, Ziegler M, Schneider E, Korczyn AD, Nappi G. Multicenter, multinational double‐blind study of the activity and tolerability of cabergoline vs bromocriptine in parkinsonian patients suffering from L‐dopa associated motor complications on treatment with dopamine agonist agents. Farmitalia Carlo Erba; Clincal Reference 41 1994.
- Schneider E, Gershanik O, Dom R, et al. Efficacy and tolerability of cabergoline compared to bromocriptine in patients suffering from levodopa associated motor complications (on treatment with DA‐agents). Movement Disorders 1996;11(Supplement 1):269. [Google Scholar]
Inzelberg 1996 {published data only}
- Inzelberg R, Nisipeanu P, Rabey JM, et al. Double‐blind comparison of cabergoline and bromocriptine in Parkinson's disease patients with motor fluctuations. Neurology 1996;47:785‐788. [DOI] [PubMed] [Google Scholar]
Korczyn 1994 {unpublished data only}
- Korczyn A, Stern G, Parkes D, et al. Multicentre, multinational double blind study of the activity and tolerability of cabergoline vs bromocriptine in Parkinsonian patients suffering fromL‐dopa associated motor complications. FCE Report 21336/736i 1994; Vol. Clinical Reference 37.
Yanagisawa 1996 {published and unpublished data}
- Yanagisawa N, Kowa H, Mizuno Y, Kanazawa I, Ogawa N, Nakashima M. The clinical evaluation of CG‐101 (Cabergoline) in Parkinson's disease patients with L‐DOPA ‐ A multi‐centred phase III double‐blind comparative study VS. bromocriptine mesilate ‐. Journal of Clinical Therapeutics and Medicines 1996;12(17):12. [Google Scholar]
Additional references
CONSORT 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials: the CONSORT statement.. Journal of the American Medical Association 1996;276(8):637‐639. [DOI] [PubMed] [Google Scholar]
Quinn 1986
- Quinn N, Critchley P, Parkes D, Marsden CD. When should levodopa be started?. Lancet 1986;ii:985‐986. [DOI] [PubMed] [Google Scholar]
Rajput 1984
- Rajput AH, Stern W, Laverty WH. Chronic low‐dose levodopa therapy in Parkinson's disease. Neurology 1984;34:991‐996. [DOI] [PubMed] [Google Scholar]


