Abstract
Background
Ivermectin, an antiparasitic agent used to treat parasitic infestations, inhibits the replication of viruses in vitro. The molecular hypothesis of ivermectin's antiviral mode of action suggests an inhibitory effect on severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) replication in the early stages of infection. Currently, evidence on efficacy and safety of ivermectin for prevention of SARS‐CoV‐2 infection and COVID‐19 treatment is conflicting.
Objectives
To assess the efficacy and safety of ivermectin compared to no treatment, standard of care, placebo, or any other proven intervention for people with COVID‐19 receiving treatment as inpatients or outpatients, and for prevention of an infection with SARS‐CoV‐2 (postexposure prophylaxis).
Search methods
We searched the Cochrane COVID‐19 Study Register, Web of Science (Emerging Citation Index and Science Citation Index), medRxiv, and Research Square, identifying completed and ongoing studies without language restrictions to 26 May 2021.
Selection criteria
We included randomized controlled trials (RCTs) comparing ivermectin to no treatment, standard of care, placebo, or another proven intervention for treatment of people with confirmed COVID‐19 diagnosis, irrespective of disease severity, treated in inpatient or outpatient settings, and for prevention of SARS‐CoV‐2 infection.
Co‐interventions had to be the same in both study arms.
We excluded studies comparing ivermectin to other pharmacological interventions with unproven efficacy.
Data collection and analysis
We assessed RCTs for bias, using the Cochrane risk of bias 2 tool. The primary analysis excluded studies with high risk of bias. We used GRADE to rate the certainty of evidence for the following outcomes 1. to treat inpatients with moderate‐to‐severe COVID‐19: mortality, clinical worsening or improvement, adverse events, quality of life, duration of hospitalization, and viral clearance; 2. to treat outpatients with mild COVID‐19: mortality, clinical worsening or improvement, admission to hospital, adverse events, quality of life, and viral clearance; (3) to prevent SARS‐CoV‐2 infection: SARS‐CoV‐2 infection, development of COVID‐19 symptoms, adverse events, mortality, admission to hospital, and quality of life.
Main results
We found 14 studies with 1678 participants investigating ivermectin compared to no treatment, placebo, or standard of care. No study compared ivermectin to an intervention with proven efficacy. There were nine studies treating participants with moderate COVID‐19 in inpatient settings and four treating mild COVID‐19 cases in outpatient settings. One study investigated ivermectin for prevention of SARS‐CoV‐2 infection. Eight studies had an open‐label design, six were double‐blind and placebo‐controlled. Of the 41 study results contributed by included studies, about one third were at overall high risk of bias.
Ivermectin doses and treatment duration varied among included studies.
We identified 31 ongoing and 18 studies awaiting classification until publication of results or clarification of inconsistencies.
Ivermectin compared to placebo or standard of care for inpatient COVID‐19 treatment
We are uncertain whether ivermectin compared to placebo or standard of care reduces or increases mortality (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.14 to 2.51; 2 studies, 185 participants; very low‐certainty evidence) and clinical worsening up to day 28 assessed as need for invasive mechanical ventilation (IMV) (RR 0.55, 95% CI 0.11 to 2.59; 2 studies, 185 participants; very low‐certainty evidence) or need for supplemental oxygen (0 participants required supplemental oxygen; 1 study, 45 participants; very low‐certainty evidence), adverse events within 28 days (RR 1.21, 95% CI 0.50 to 2.97; 1 study, 152 participants; very low‐certainty evidence), and viral clearance at day seven (RR 1.82, 95% CI 0.51 to 6.48; 2 studies, 159 participants; very low‐certainty evidence). Ivermectin may have little or no effect compared to placebo or standard of care on clinical improvement up to 28 days (RR 1.03, 95% CI 0.78 to 1.35; 1 study; 73 participants; low‐certainty evidence) and duration of hospitalization (mean difference (MD) −0.10 days, 95% CI −2.43 to 2.23; 1 study; 45 participants; low‐certainty evidence). No study reported quality of life up to 28 days.
Ivermectin compared to placebo or standard of care for outpatient COVID‐19 treatment
We are uncertain whether ivermectin compared to placebo or standard of care reduces or increases mortality up to 28 days (RR 0.33, 95% CI 0.01 to 8.05; 2 studies, 422 participants; very low‐certainty evidence) and clinical worsening up to 14 days assessed as need for IMV (RR 2.97, 95% CI 0.12 to 72.47; 1 study, 398 participants; very low‐certainty evidence) or non‐IMV or high flow oxygen requirement (0 participants required non‐IMV or high flow; 1 study, 398 participants; very low‐certainty evidence). We are uncertain whether ivermectin compared to placebo reduces or increases viral clearance at seven days (RR 3.00, 95% CI 0.13 to 67.06; 1 study, 24 participants; low‐certainty evidence). Ivermectin may have little or no effect compared to placebo or standard of care on the number of participants with symptoms resolved up to 14 days (RR 1.04, 95% CI 0.89 to 1.21; 1 study, 398 participants; low‐certainty evidence) and adverse events within 28 days (RR 0.95, 95% CI 0.86 to 1.05; 2 studies, 422 participants; low‐certainty evidence). None of the studies reporting duration of symptoms were eligible for primary analysis. No study reported hospital admission or quality of life up to 14 days.
Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection
We found one study. Mortality up to 28 days was the only outcome eligible for primary analysis. We are uncertain whether ivermectin reduces or increases mortality compared to no treatment (0 participants died; 1 study, 304 participants; very low‐certainty evidence). The study reported results for development of COVID‐19 symptoms and adverse events up to 14 days that were included in a secondary analysis due to high risk of bias. No study reported SARS‐CoV‐2 infection, hospital admission, and quality of life up to 14 days.
Authors' conclusions
Based on the current very low‐ to low‐certainty evidence, we are uncertain about the efficacy and safety of ivermectin used to treat or prevent COVID‐19. The completed studies are small and few are considered high quality. Several studies are underway that may produce clearer answers in review updates. Overall, the reliable evidence available does not support the use of ivermectin for treatment or prevention of COVID‐19 outside of well‐designed randomized trials.
Plain language summary
Ivermectin for preventing and treating COVID‐19
Is ivermectin effective for COVID‐19?
Key messages
We found no evidence to support the use of ivermectin for treating or preventing COVID‐19 infection, but the evidence base is limited.
Evaluation of ivermectin is continuing in 31 ongoing studies, and we will update this review with their results when they become available.
What is ivermectin?
Ivermectin is a medicine used to treat parasites such as intestinal parasites in animals and scabies in humans. It is cheap and is widely used in regions of the world where parasitic infestations are common. It has few unwanted effects.
Tests in the laboratory show ivermectin can slow the reproduction of the COVID‐19 (SARS‐CoV‐2) virus but such effects would need major doses in humans. Medical regulators have not approved ivermectin for COVID‐19. It should only be used as part of well‐designed studies (called randomized controlled trials) evaluating potential effects.
What did we want to find out?
We wanted to know if ivermectin reduces death, illness, and length of infection in people with COVID‐19, or is useful in prevention of the disease. We included studies comparing the medicine to placebo (dummy treatment), no treatment, usual care, or treatments for COVID‐19 that are known to work to some extent, such as remdesivir or dexamethasone. We excluded studies that compared ivermectin to other drugs that do not work, such as hydroxychloroquine, or that are not known to be effective against COVID‐19.
We evaluated the effects of ivermectin in infected people on:
– people dying; – whether people's COVID‐19 symptoms got better or worse; – unwanted effects; – hospital admission or time in hospital; – viral clearance.
For prevention, we sought the effect on preventing COVID‐19 and SARS‐CoV‐2 infection.
What did we do?
We searched for randomized controlled trials that investigated ivermectin to prevent or treat COVID‐19 in humans. People being treated with ivermectin had to have laboratory‐test confirmed COVID‐19 and be receiving treatment in hospital or as outpatients.
We compared and summarized the results of the studies and rated our confidence in the evidence, based on common criteria as to how reliable the evidence is.
What did we find?
We found 14 studies with 1678 participants that investigated ivermectin compared to no treatment, placebo, or usual care.
For treatment, there were nine studies of people with moderate COVID‐19 in hospital and four of outpatients with mild COVID‐19. The studies used different doses of ivermectin and different durations of treatment.
One study investigated ivermectin to prevent COVID‐19.
We also found 31 ongoing studies, and there are 18 studies still requiring clarification from the authors or not yet published.
Main results
Treating people in hospital with COVID‐19
We don't know whether ivermectin compared with placebo or usual care, 28 days after treatment:
– leads to more or fewer deaths (2 studies, 185 people); – worsens or improves patients' condition assessed by need for ventilation (2 studies, 185 people) or oxygen (1 study, 45 people); – increases or reduces unwanted events (1 study, 152 people).
Seven days after treatment, we don't know if ivermectin:
– increases or reduces negative COVID‐19 tests (2 studies, 159 people).
Ivermectin compared to placebo or usual care may make little or no difference to improving patients' condition 28 days after treatment (1 study, 73 people) or to length of hospital stay (1 study, 45 people).
Treating outpatients with COVID‐19
We don't know whether ivermectin compared with placebo or usual care:
– leads to more or fewer deaths 28 days after treatment (2 studies, 422 people); – worsens or improves patients' condition 14 days after treatment assessed by need for ventilation (1 study, 398 people); – increases or reduces negative COVID‐19 tests seven days after treatment (1 study, 24 people).
Ivermectin compared to placebo or usual care may make little or no difference to improving outpatients' condition 14 days after treatment (1 study, 398 people) or to the number of unwanted events 28 days after treatment (2 studies, 422 people).
No studies looked at hospital admissions in outpatients.
Preventing COVID‐19
We don't know whether ivermectin leads to more or fewer deaths compared with no drug (1 study, 304 people); no participant died 28 days after the drug. This study reported results for development of COVID‐19 symptoms (but not confirmed SARS‐CoV‐2 infection) and unwanted events, but in a way that we could not include in our analyses. This study did not look at hospital admissions.
What are the limitations of the evidence?
Our confidence in the evidence is very low because we could only include 14 studies with few participants and few events, such as deaths or need for ventilation. The methods differed between studies, and they did not report everything we were interested in, such as quality of life.
How up to date is this evidence?
The evidence is up to date to 26 May 2021.
Summary of findings
Background
Description of the condition
COVID‐19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). On 11 March 2020, after spreading from China to more than 144 countries, the World Health Organization (WHO) declared a COVID‐19 pandemic. In July 2021, over 180 million cases have been confirmed, including over 3.9 million deaths (WHO 2020a; WHO 2020b).
Available data suggest that one‐third of SARS‐CoV‐2 infections remain asymptomatic (Oran 2021), but there is still uncertainty around this estimate. About 80% of symptomatic cases show mild symptoms, including cough, fever, myalgia, headache, dyspnoea, sore throat, diarrhoea, nausea and vomiting, and loss of smell and taste. Outpatient management is appropriate for most people with a mild course of COVID‐19. Moderate, severe, and critical cases (approximately 20%), with the need for oxygen supplementation, ventilatory support, or intensive medical care, cause a considerable burden for healthcare systems. Defined risk factors for severe disease include increasing age (over 60 years) and certain comorbidities (Huang 2020; WHO 2020a). Comorbidities such as cardiovascular disease, diabetes mellitus, chronic obstructive pulmonary disease and other lung diseases, malignancies, chronic kidney disease, solid organ or haematopoietic stem cell transplantation, and obesity are associated with severe COVID‐19 and mortality (Deng 2020; Williamson 2020).
Data on mortality substantially differ between locations, depending on the population structure, the case‐mix of infected and deceased individuals, other local factors, and changes during the ongoing outbreak. With an inhospital mortality for people receiving ventilation of over 70% (Karagiannidis 2020), the patients who survive often have considerable consequential damage (Herrmann 2020; Prescott 2020). COVID‐19 can lead to death due to a variety of causes, such as severe respiratory failure, septic shock, and multiple organ failure (WHO 2020a). The case–fatality ratio worldwide is currently estimated at 2.2% with large statistical fluctuations (less than 0.1% in Singapore up to almost 20% in Yemen; status July 2021) (Dong 2020). However, these varying rates should not be interpreted as markers for the quality of health care (Karagiannidis 2020), or the aggressiveness of different virus variants. These statistics are influenced by the mean age of a population or of those infected, the quality and extent of local test strategies, and documentation and reporting systems (Kobayashi 2020). The gold standard for confirming a SARS‐CoV‐2 infection is the reverse transcription polymerase chain reaction (RT‐PCR)‐based detection of viral ribonucleic acid (RNA) from a nasopharyngeal swab test, sputum, or tracheal secretion, with a sensitivity ranging from 70% to 98%, depending on pretest probability (Watson 2020). Offering lower sensitivity but greater practicality and accessibility, antigen tests are receiving increased attention, especially in point‐of‐care diagnostics of COVID‐19 (WHO 2020c).
Transmission is typically inferred from population‐level information. Inherent properties of virus variants of concern, and individual differences in infectiousness among individuals or groups make it difficult to contain its spread in the community (WHO 2021a). Currently, the most effective and ubiquitously available measures to control virus spreading are non‐pharmaceutical interventions, including physical distancing, wearing a facemask, especially when distancing cannot be maintained, keeping rooms well ventilated, avoiding crowds and close contact, regularly cleaning your hands, and coughing into a bent elbow or tissue. Research on prophylaxis of SARS‐CoV‐2 infection and treatment of COVID‐19 is being carried out under great pressure worldwide. Evaluating the effectiveness of repurposed drugs represents one important strand of these research efforts. In this context, ivermectin — an antiparasitic intervention — has received substantial attention, especially in South America and parts of Asia.
Description of the intervention
Ivermectin is an antiparasitic agent belonging to the group of avermectins, originally a fermentation metabolite produced by the bacterium Streptomyces avermitilis. Ivermectin was introduced for medical use in 1982 and is effective against various types of nematodes and helminths, and ectoparasites such as mites and lice. The mode of action is based on binding to specific cell membrane channels that only occur in invertebrates. Channel activation ultimately leads to blocked cell signal transmission through chloride‐induced hyperpolarization. Consequently, parasites are paralysed and die, interrupting their reproduction cycle (Campbell 1983; Dourmishev 2005; Panahi 2015). Ivermectin is on the WHO List of Essential Medicines for its high effectiveness against human ectoparasite infestations (WHO 2019).
In animals and humans, ivermectin is easily resorbed by the mucosa if taken orally or the skin if taken topically. As a lipophilic compound, it accumulates in fat and liver tissue from where it effuses and takes effect. Elimination is processed through bile and faeces. Ivermectin is widely used in veterinary medicine, but it is also approved for human parasitic diseases such as onchocerciasis, lymphatic filariasis, strongyloidiasis, and scabies in several countries (e.g. the USA, Japan, France, Germany, Australia) (González‐Canga 2008). The established dosing regimen ranges from 150 µg/kg to 200 µg/kg administered orally, with a one‐ to two‐dose administration generally being effective. Dosing is generally low because of the agent's high potency (Ashour 2019).
Adhering to recommended doses, ivermectin is generally well tolerated. Adverse effects — which seem to arise partially from the rapid death of parasites, leading to hyperinflammation and anaphylactic reactions — include weakness, drowsiness, diarrhoea, nausea, and vomiting. In addition, ivermectin can cause fever and rash. Rare serious adverse effects can occur, such as vision problems, neurotoxicity, and liver damage (González‐Canga 2008).
How the intervention might work
One in vitro study showed that ivermectin can inhibit replication of the human‐immunodeficiency‐virus 1 (HIV‐1), via inhibition of the interaction of virus proteins and a human cargo protein complex called importin (IMPα/β1) (Wagstaff 2012). Importin is used by viruses for nuclear import in order to initiate their replication process (Wagstaff 2012). Besides HIV‐1, various other RNA viruses use importin as target protein, among them dengue virus, West Nile virus, and influenza. Several research groups have investigated ivermectin's efficiency on those pathogens (Goetz 2016; Tay 2013; Yang 2020). Although ivermectin showed some inhibitory potential for virus replication in vitro, there is no evidence of clinical effectiveness to date.
Before the COVID‐19 pandemic, only two clinical trials had been registered on ClinicalTrials.gov (clinicaltrials.gov/) using ivermectin as an intervention for treatment of virus diseases. Only one of these had published results (Yamasmith 2018). In this small, single‐centre study published as a conference abstract, ivermectin showed a shorter viral protein clearance time compared to placebo in people infected with dengue virus (Yamasmith 2018).
Another member of the beta‐coronavirus family, SARS‐CoV‐1, which also causes respiratory failure, revealed similar dependence on the IMPα/β1 interaction (Wulan 2015). The pathogen causing COVID‐19, SARS‐CoV‐2, is also an RNA virus closely related to SARS‐CoV‐1. In 2020, ivermectin gained high interest as a promising therapeutic option against SARS‐CoV‐2, when Caly 2020 published their experimental study results showing that ivermectin inhibits the replication of SARS‐CoV‐2 in cell culture. So far, the only drugs shown to be clearly effective in COVID‐19 treatment are targeting the immune response to a SARS‐CoV‐2 infection; for example, dexamethasone (RECOVERY 2021). Therefore, ivermectin's potential to restrict the disease's progression, or even its outbreak, indicates that it is possibly an effective antiviral agent. However, until showing success in human clinical trials with patient‐relevant outcomes, these findings remain suggestive.
The molecular hypothesis of ivermectin's antiviral mode of action, explained above, suggests an inhibitory effect on virus replication in the early stages of the disease, indicating a benefit especially for people with mild or moderate disease. This has also led to the idea of the possible preventive potency of ivermectin on infection with SARS‐CoV‐2 in individuals after exposure to a contagious contact, called postexposure prophylaxis. In response to the early promising in vitro studies on ivermectin, mentioned above, several COVID‐19 clinical trials have been initiated to investigate the prophylactic and therapeutic effects of ivermectin.
Why it is important to do this review
Ivermectin is an inexpensive and widely used medicine, mainly in low‐ and middle‐income countries with a high burden of parasitic diseases. The recently published in vitro studies, especially the results of Caly 2020, have led to great interest in ivermectin in many countries with high numbers of SARS‐CoV‐2 infections, including the USA and countries of South America and Asia. In South America in particular, people started liberally self‐medicating with ivermectin, and the drug has become part of public health policies without reliable scientific data. For example, in May 2020, Bolivian and Peruvian health officials recommended ivermectin for the treatment of COVID‐19 without supplying evidence. In Brazil, it was promoted as a preventive measure by municipalities (Rodríguez‐Mega 2020). Due to the rapid increase in interest in ivermectin and the risk of abuse, the US Food and Drug Administration (FDA) discouraged the use of ivermectin intended for animals (FDA 2020).
The increased research interest in ivermectin has led to the registration of numerous trials in clinical trials registries worldwide. As of 2 July 2021, there were 73 trials registered on ClinicalTrials.gov (clinicaltrials.gov/) investigating ivermectin in various settings.
Several studies describe ivermectin's positive effect on resolution of mild COVID‐19 symptoms or describe a reduction of inflammatory marker levels or shorter time to viral clearance, while other studies indicate no effect or even a negative effect on disease progression. Many studies are already summarized in existing systematic reviews, meta‐analyses, and guidelines (Bryant 2021; Hill 2021; NIH 2021). It has to be kept in mind that many available meta‐analyses and reviews, as well as most of the underlying original studies, have not yet been published in peer‐reviewed journals and are only available on preprint servers without any supervising authority. Given the pace of the pandemic, it is important and welcome to make new scientific findings immediately available. But non‐peer‐reviewed results have to be handled with care and should not be used as the sole basis for clinical decisions and recommendations. Methodological limitations in the design of original studies, data integrity, and potential conflicts of interests have to be critically appraised when judging trial results. Many reviews and meta‐analyses of ivermectin for COVID‐19 are not reliable due to insufficient methodological accuracy and quality.
As of July 2021, the efficacy and safety of ivermectin for COVID‐19 treatment and prophylaxis are still subject to debate. The most recent Association of the Scientific Medical Societies in Germany (AWMF) guideline recommends against the use of ivermectin as antiviral treatment (German AWMF Guideline 2021), while in February 2021, the US National Institutes of Health (NIH) revised their COVID‐19 treatment guidelines from a recommendation 'against the use of ivermectin' to 'cannot recommend either for or against the use of ivermectin,' giving clinicians leeway in individual case decision‐making (NIH 2021). The WHO recommends that the drug only be used within clinical trials as current evidence on the use of ivermectin to treat people with COVID‐19 is inconclusive (WHO 2021b).
This review aimed to provide a complete evidence profile, based on current Cochrane standards, for ivermectin with regard to efficacy and safety for postexposure prophylaxis and treatment of COVID‐19.
Objectives
To assess the efficacy and safety of ivermectin compared to no treatment, standard of care, placebo, or any other proven intervention for people with COVID‐19 receiving treatment as inpatients or outpatients, and for prevention of an infection with SARS‐CoV‐2 (postexposure prophylaxis).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) only, as this is the best study design for evaluating the efficacy of interventions (Higgins 2020a). Non‐standard RCT designs, such as cluster‐randomized and cross‐over trials, were not eligible for the review (Higgins 2020b). These designs are not appropriate in this context, since the underlying cause of COVID‐19 is an infection with the SARS‐CoV‐2 virus and the medical condition evolves over time.
We included full‐text journal articles published in PubMed‐indexed and non‐indexed journals, preprint articles, results published in trial registers, and abstract publications. All studies, especially preprint articles that have not been peer‐reviewed, must have reported robust and valid data on study design, participants' characteristics, interventions, and outcomes, to be eligible for inclusion. We categorized studies in question as 'awaiting classification' until the authors publish further information or clarify certain questions.
We applied no restrictions on the language of publication of the articles.
Types of participants
Treatment of COVID‐19
We included studies investigating participants with confirmed SARS‐CoV‐2 infection (RT‐PCR or antigen testing), regardless of age, gender, ethnicity, disease severity, and setting (inpatients and outpatients). If studies included participants with a confirmed or suspected COVID‐19 diagnosis, we used only the data for the patient population with confirmed COVID‐19 diagnosis. In cases, where data were not reported separately for people with confirmed or suspected COVID‐19 diagnosis, we excluded the study.
Prevention of SARS‐CoV‐2 infection
We included studies investigating participants who were not infected with SARS‐CoV‐2 at enrolment, but were at high risk of developing the infection (e.g. after high‐risk exposure), regardless of age, gender, ethnicity, disease severity, and setting (inpatient and outpatients). Participants may have been hospitalized for reasons other than COVID‐19. Eligible trials must have reported the history of previous SARS‐CoV‐2 infections or serological evidence in included participants. A history of SARS‐CoV‐2 infection was not an exclusion criterion.
We excluded studies investigating ivermectin for prevention and treatment of other viral diseases.
Types of interventions
All doses and regimens of ivermectin were eligible and pooled for the primary analysis. Dosing schemes were considered and categorized into low (up to 0.2 mg/kg orally, single dose) and high doses (greater than 0.2 mg/kg orally, single dose or with higher frequency). We planned to analyse different doses in subgroup analyses, if sufficient studies are available for review updates.
We compared ivermectin to no treatment, standard of care, or placebo. Co‐interventions (standard of care) must have been comparable between the study arms, i.e. ivermectin plus standard of care versus standard of care.
We planned to compare ivermectin to any other active pharmacological comparator with proven efficacy for prevention or treatment of COVID‐19. For dexamethasone, it has been shown that mortality from COVID‐19 was lower among people who were randomized to receive dexamethasone than among those who received the usual standard of care (RECOVERY 2021; Siemieniuk 2020). Remdesivir showed some benefit for people hospitalized with COVID‐19, though to a lesser extent (Beigel 2020). Therefore, dexamethasone and remdesivir will be considered eligible active comparators for review updates. For patients that qualify for (for example) dexamethasone therapy or for another intervention that proves to be beneficial in the future, it would be unethical to further conduct trials that use placebo only. In contrast, studies using comparators (e.g. hydroxychloroquine) without proven efficacy may confound the assessment of the efficacy or safety of ivermectin and were excluded. Although those types of interventions were possibly used at a certain point of time during the pandemic with the best intentions, their use was never supported by actual evidence, and they have potential adverse effects (Singh 2021). From those comparisons, no reliable evidence can be obtained.
Studies investigating various concomitant medications (e.g. doxycycline, hydroxychloroquine, azithromycin, zinc) in addition to ivermectin or as comparator drug were not eligible for this review. Due to unproven efficacy, possible adverse effects, and drug interactions, these comparisons may confound the assessment of the efficacy or safety of ivermectin.
We created these comparisons:
ivermectin versus no treatment, placebo, or standard of care; and
ivermectin versus active pharmacological intervention with proven efficacy (no studies available for the current review version).
Types of outcome measures
We analyzed different outcomes for the use of ivermectin for treatment of people with COVID‐19 in inpatient and outpatient settings, and for the prevention of SARS‐CoV‐2 infection. If studies were eligible for inclusion regarding design, population, intervention, and comparator, but did not report outcomes of interest, they were not included for meta‐analysis. However, we summarized reported outcomes for all included studies in the Characteristics of included studies table.
Ivermectin for treating COVID‐19 in inpatient settings
All‐cause mortality up to 28 days.
-
Clinical status, assessed by need for respiratory support with standardized scales (e.g. WHO Clinical Progression Scale (Marshall 2020), hereafter referred to as the WHO scale) up to 28 days. If the study did not use a standardized scale to assess the status of the participants, we categorized their status according to the WHO scale with the information provided by the study. Clinical status is a complex outcome with substantial heterogeneity. We pooled data only if clinically reasonable (see the list of specific outcomes below). When there were only a few studies available that reported different outcomes in terms of clinical status, we describe them in the results narratively.
-
Worsening of clinical status.
Need for invasive mechanical ventilation (i.e. WHO scale 7 to 9, if 6 or less at baseline).
Need for non‐invasive mechanical ventilation or high flow (i.e. WHO scale 6, if 5 or less at baseline).
Need for oxygen by mask or nasal prongs (i.e. WHO scale 5, if 4 or less at baseline).
-
Improvement of clinical status.
Weaning or liberation from invasive mechanical ventilation in surviving participants (i.e. WHO scale 6 or less, if 7 or greater at baseline).
Ventilator‐free days (ventilator‐free defined as WHO scale 6 or less).
Duration of liberation from invasive mechanical ventilation.
Liberation from supplemental oxygen in surviving participants (i.e. WHO scale 4 or less, if 5 or greater at baseline).
Duration of liberation from supplemental oxygen.
Participants discharged without respiratory deterioration or death at 28 days.
-
Adverse events (any grade, grade 1 to 2, grade 3 to 4), defined as number of participants with at least one event within 28 days.
Ivermectin for treating COVID‐19 in outpatient settings
All‐cause mortality up to 28 days.
-
Clinical status, assessed by need for respiratory support with standardized scales (e.g. WHO scale (Marshall 2020)) up to 14 days. If the study did not use a standardized scale to assess the status of the participants, we categorized their status according to the WHO scale with the information provided by the study. Clinical status is a complex outcome with substantial heterogeneity. We pooled data only if clinically reasonable (see the list of specific outcomes below). When there were only a few studies available that reported different outcomes in terms of clinical status, we described the results narratively.
-
Development of moderate‐to‐severe clinical COVID‐19 symptoms (defined as WHO scale 6 or greater).
Need for invasive mechanical ventilation, non‐invasive mechanical ventilation or high flow (i.e. WHO scale ≥ 6, severe disease).
Need for invasive mechanical ventilation (i.e. WHO scale 7 to 9).
Need for non‐invasive mechanical ventilation or high flow (i.e. WHO scale 6).
-
Need for hospitalization with or without supplemental oxygen (i.e. WHO scale 4 to 5, moderate disease).
Need for oxygen by mask or nasal prongs (i.e. WHO scale 5).
Need for hospitalization without oxygen therapy (i.e. WHO scale 4).
-
Symptom resolution (i.e. WHO scale 1).
Number of participants with symptoms resolved.
Duration of symptom resolution.
-
Admission to hospital.
Adverse events (any grade, grade 1 to 2, grade 3 to 4), defined as number of participants with at least one event within 28 days.
Ivermectin for preventing SARS‐CoV‐2 infection
SARS‐CoV‐2 infection (confirmed by RT‐PCR or antigen testing) at 14 days.
-
Development of clinical COVID‐19 symptoms up to 14 days; assessed in accordance with individual items of the WHO scale (Marshall 2020). If the study did not use a standardized scale to assess the status of the participants, we categorized their status according to the WHO scale with the information provided by the study.
Uninfected (WHO scale 0).
Ambulatory mild disease (WHO scale 1 to 3).
Hospitalized with moderate disease (WHO scale 4 to 5).
Hospitalized with severe disease (WHO scale 7 to 9).
Mortality (WHO scale 10).
Adverse events (any grade, grade 1 to 2, grade 3 to 4), defined as number of participants with at least one event within 14 days.
Timing of outcome measurement
We expected that included studies measured several outcomes — including clinical status, SARS‐CoV‐2 infection, and adverse events — at different time points. For inpatient setting outcomes, the main time point of interest was 28 days after randomization. For outpatient setting outcomes, the main time point of interest was 14 days after randomization, except for mortality and (serious) adverse events (28 days). For prevention trials, the main time point was 14 days, except for mortality only (28 days). If only a few studies had contributed data to an outcome, we pooled different time points, provided the studies had produced valid data and pooling was clinically reasonable. We reported time points of outcome measurement in the footnotes of the forest plots. If sufficient data are available for review updates, we will group the measurement time points of eligible outcomes into those measured directly after treatment (up to seven days), medium‐term outcomes (up to 14 days), and longer‐term outcomes (28 days or more).
Ivermectin for treating COVID‐19 in inpatient settings
Serious adverse events, defined as number of participants with at least one event within 28 days.
Quality of life assessed with the standardized scale, WHOQOL‐100, up to 28 days (WHO 2012).
Admission to intensive care unit (ICU).
Duration of hospitalization.
Viral clearance, assessed with RT‐PCR test for SARS‐CoV‐2 at baseline, and three, seven, and 14 days.
Ivermectin for treating COVID‐19 in outpatient settings
Serious adverse events, defined as number of participants with at least one event within 28 days.
Quality of life assessed with the standardized scale, WHOQOL‐100, up to 14 days (WHO 2012).
Viral clearance, assessed with RT‐PCR test for SARS‐CoV‐2 at baseline, and at three, seven, and 14 days.
Ivermectin for preventing SARS‐CoV‐2 infection
All‐cause mortality up to 28 days.
Admission to hospital.
Quality of life assessed with the standardized scale, WHOQOL‐100, up to 14 days (WHO 2012).
Timing of outcome measurement
We expected that included studies measured several outcomes – including serious adverse events, quality of life, and viral clearance – at different time points. We analyzed different time points for viral clearance separately due to the dynamic course of the viral load. For other inpatient setting outcomes, the main time point of interest was 28 days after randomization. For other outpatient setting trials outcomes, the main time point of interest was 14 days after randomization, except for mortality (28 days)and (serious) adverse events (28 days). For prevention trials, the main time point of interest was 14 days, except for mortality only (28 days). If only a few studies contributed data to an outcome, we pooled different time points, as long as the studies had produced valid data and pooling was clinically reasonable. We reported time points of outcome measurement in the footnotes of the forest plots. If sufficient data are available for review updates, we will group the measurement time points of eligible outcomes into those measured directly after treatment (up to seven days), medium‐term outcomes (up to 14 days), and longer‐term outcomes (28 days or more).
Search methods for identification of studies
Electronic searches
On 26 May 2021, the Information Specialist (MIM) conducted systematic searches of the following sources with no restrictions on the language of publication.
-
Cochrane COVID‐19 Study Register (CCSR) (www.covid-19.cochrane.org; from inception to 26 May 2021), comprising:
Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates;
MEDLINE (PubMed), daily updates;
Embase.com, weekly updates;
ClinicalTrials.gov (www.clinicaltrials.gov), daily updates;
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch), weekly updates; and
medRxiv (www.medrxiv.org), weekly updates.
-
Web of Science Core Collection (Clarivate; from 1 January 2020 to 26 May 2021):
Science Citation Index Expanded;
Emerging Sources Citation Index.
-
Preprint servers (from inception to 26 May 2021):
medRxiv (www.medrxiv.org/search);
Research Square (www.researchsquare.com/browse).
For detailed search strategies, see Appendix 1.
We did not conduct separate searches of the databases required by the Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards (Higgins 2021), since these databases are already regularly searched for the production of the CCSR. For greater precision, we searched the Web of Science database from 1 January 2020 onwards. We searched all other resources without date limits.
Searching other resources
We searched the reference lists of included studies, systematic reviews, and meta‐analyses to identify other potentially eligible studies or ancillary publications. We contacted the investigators of included studies to obtain additional information on the retrieved studies.
We searched for grey literature, which we defined as searching trials registries such as ClinicalTrials.gov and WHO ICTRP contained in the CCSR, as well as searching preprint servers. In addition, we screened the 'All RCTs' section on the website ivmmeta.com, which lists studies related to ivermectin and COVID‐19, and the regarding section on COVID‐NMA Working Group for eligible trials.
Data collection and analysis
Selection of studies
We performed study selection in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2020). Two review authors (SW, MP) independently screened titles and abstracts of identified records. We retrieved full‐text articles and independently assessed eligibility of the remaining records against the predefined eligibility criteria. We resolved discrepancies through discussion between the review authors. We included studies irrespective of whether measured outcome data were reported in a 'usable' way. We collated multiple reports of the same study, so that the study, rather than the report, was the unit of interest in the review.
We documented the study selection process in a PRISMA flow diagram with the total number of studies included, excluded, awaiting classification, and ongoing. We listed the reasons for exclusion and awaiting classification in the Characteristics of excluded studies and Characteristics of studies awaiting classification tables.
Data extraction and management
Two review authors (SW, MP) independently extracted data using a standardized data extraction form, including details of the study, participants, intervention, comparator, and outcomes. If necessary, we tried to obtain missing data by contacting the authors of relevant articles. At each step of data extraction, we resolved any discrepancies through discussion between the review authors.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies using the Cochrane risk of bias tool 2 (RoB 2) (Higgins 2020c; Sterne 2019). The effect of interest was the effect of assignment at baseline, regardless of whether the interventions were received as intended (the 'intention‐to‐treat effect'). We assessed the risk of bias for all results (outcomes) reported in the included studies that we specified as outcomes for the current review and that contributed to the review's summary of findings table.
Two review authors (SW, MP) independently assessed the risk of bias of all results. We resolved any disagreements through discussion with a third review author.
The RoB 2 tool considers the following domains:
bias arising from the randomization process;
bias due to deviations from the intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome; and
bias in selection of the reported result.
We assessed the RoB 2 domains using the recommended signalling questions and these response options:
yes;
probably yes;
probably no;
no; or
no information.
RoB 2 algorithms map responses to signalling questions. We used the proposed algorithm after verification to reach a risk of bias judgement, and assigned one of three levels to each domain:
low risk of bias;
some concerns; or
high risk of bias.
Similarly, we reached an overall risk of bias judgement for a specific outcome by considering all domains resulting in one of the three judgement options described above. Overall low risk of bias of the trial result was assumed when all domains were at low risk; some concerns of bias was assumed when the trial result was judged to raise some concerns in at least one domain for this result, but not at high risk of bias for any domain; overall high risk of bias of the trial result was assumed when the trial was at high risk of bias in at least one domain for this result or when it was judged to have some concerns for multiple domains in a way that substantially lowered confidence in the result (Higgins 2020c).
We used the RoB 2 Excel tool to implement RoB 2 (available at www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2). We stored the full RoB 2 data (e.g. completed Excel tool) in an online repository.
The primary analysis included only those studies that had low risk or some concerns of bias. We included studies at high risk of bias in a secondary analysis to assess the impact on the results.
Measures of treatment effect
For dichotomous outcomes, we recorded the number of events and the number of analyzed participants in the intervention and control groups. We used the risk ratio (RR) with 95% confidence interval (CI) as effect measure.
For continuous outcomes, we recorded the mean, the standard deviation (SD), and the number of analyzed participants in the intervention and control groups. If the standard deviation was not reported, we used standard errors, CIs, or P values to calculate the SD with the formulas described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020d). If studies reported data as median with interquartile range (IQR), we assumed that the median was similar to the mean when sample sizes were large and the distribution of the outcome was similar to the normal distribution. In these cases, the width of the IQR is approximately 1.35 SDs (Higgins 2020d). We used the MD with 95% CI as effect measure.
We considered effect estimates of dichotomous outcomes with the range of the 95% CIs not crossing 1 and continuous outcomes with the range of the 95% CIs not crossing 0 as statistically significant effect estimates. A statistically significant effect does not necessarily mean that the estimated effect is clinically relevant. We assessed the clinical relevance of the effect size separately and reported it transparently.
Unit of analysis issues
The unit of analysis for this review was the individually randomized participant.
In studies with multiple intervention groups, we combined groups if reasonable (e.g. study arms with different doses of ivermectin). If it had not been reasonable to pool the groups, we planned to split the 'shared' comparator group to avoid double‐counting of participants. There was no need to split shared groups for the current review.
Dealing with missing data
There are many potential sources of missing data in a systematic review or meta‐analysis, which can affect the level of studies, outcomes, summary data, individuals, or study‐level characteristics (Deeks 2020). Incomplete data can introduce bias into the meta‐analysis, if they are not missing at random. We addressed all sources of missing data. Missing studies may be the result of reporting bias, and we addressed this as described in the Assessment of reporting biases section. Missing outcomes and summary data may be the result of selective reporting bias; missing individuals may be the result of attrition from the study or lack of intention‐to‐treat analysis. We addressed these sources of missing data using the RoB 2 tool (Assessment of risk of bias in included studies). If data were incompletely reported, we contacted the study authors to request additional information.
Assessment of heterogeneity
We used the descriptive statistics reported in the Characteristics of included studies table to assess whether the studies within each pairwise comparison were homogeneous enough, with respect to study and intervention details and population baseline characteristics, that the assumption of homogeneity might be plausible. In case of excessive clinical heterogeneity, we did not pool the findings of included studies.
We measured statistical heterogeneity using the Chi2 test and the I2 statistic (Deeks 2020), and the 95% prediction interval (PI) for random‐effects meta‐analysis (IntHout 2016). The prediction interval helps in the clinical interpretation of heterogeneity by estimating what true treatment effects can be expected in future settings (IntHout 2016). We restricted calculation of a 95% PI to meta‐analyses with four or more studies (200 participants or more), since the interval would be imprecise when a summary estimate was based on only a few small studies. In the current review, there are no meta‐analyses including four or more studies. We planned to use the open‐source statistical software R package meta to calculate 95% PIs in review updates (Meta). We declared statistical heterogeneity if the P value was less than 0.1 for the Chi2 statistic, or the I2 statistic was equal to or greater than 40% (40% to 60%: moderate heterogeneity; 50% to 90%: substantial heterogeneity; 75% to 100%: considerable heterogeneity; Deeks 2020), or the range of the 95% PI revealed a different clinical interpretation of the effect estimate compared to the 95% CI.
Assessment of reporting biases
We sought to identify all research that met our predefined eligibility criteria. Missing studies can introduce bias to the analysis. We searched for completed non‐published trials in trials registers, contacted authors to seek assurance that the results will be made available, and classified them as 'awaiting classification' until the results are reported. We reported the number of completed non‐published trials.
When there are 10 or more relevant studies pooled in a meta‐analysis, we planned to investigate risk of reporting bias (publication bias) in pairwise meta‐analyses using contour‐enhanced funnel plots. In the current review, there were no meta‐analyses including 10 or more studies. For review updates, if funnel plot asymmetry is suggested by a visual assessment, we plan to perform exploratory analyses (e.g. Rücker's arcsine test for dichotomous data and Egger's linear regression test for continuous data) to further investigate funnel plot asymmetry. A P value of less than 0.1 will be considered as the level of statistical significance. In review updates, we will analyse reporting bias using the open‐source statistical software R package meta (Meta).
Data synthesis
The primary analysis included only those studies that had low risk or some concerns of bias according to the RoB 2 assessment. We included high risk of bias studies in a secondary analysis to assess the impact on the results (Sensitivity analysis).
We analyzed trials with different intentions of ivermectin use and different participant populations separately, as follows.
Treatment of COVID‐19 in an inpatient setting: participants with confirmed SARS‐CoV‐2 infection.
Treatment of COVID‐19 in an outpatient setting: participants with confirmed SARS‐CoV‐2 infection.
Prevention of SARS‐CoV‐2 infection (postexposure prophylaxis): participants at high risk of developing the infection.
We created these comparisons.
Ivermectin versus placebo or standard of care.
Ivermectin versus active pharmacological intervention with proven efficacy.
We performed meta‐analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020).
If clinical and methodological characteristics of individual studies were sufficiently homogeneous, we pooled the data in meta‐analyses. When meta‐analysis was feasible, we used the random‐effects model as we assumed that the intervention effects were related but were not the same for the included studies. For dichotomous outcomes, we performed meta‐analyses using the Mantel‐Haenszel method under a random‐effects model to calculate the summary (combined) intervention effect estimate as a weighted mean of the intervention effects estimated in the individual studies. For continuous outcomes, we used the inverse‐variance method.
We used RevMan Web software for meta‐analyses (RevMan Web 2020).
Subgroup analysis and investigation of heterogeneity
For ivermectin used as treatment for COVID‐19 in an inpatient setting, we planned to perform a subgroup analysis independent of heterogeneity for the following characteristic.
Severity of condition at baseline (moderate (WHO scale 4 to 5) versus severe disease (WHO scale 6 to 9)).
Since only one study investigated participants with moderate‐to‐severe COVID‐19, the planned subgroup analysis for severity at baseline could not be performed.
For ivermectin used to prevent SARS‐CoV‐2 infection, we planned to perform a subgroup analysis independent of heterogeneity for the following characteristic.
Studies including participants with a history of SARS‐CoV‐2 infection versus studies including only participants with no history of infection.
We investigated heterogeneity by visual inspection of the forest plot. We reported details of the intervention and age of the population for each study in the footnotes of the forest plot. We planned to investigate heterogeneity by subgroup analysis to calculate RR or MD in conjunction with the corresponding CI for each subgroup, if sufficient studies had been available (at least 10 studies per outcome). In the current review, there were not enough studies available. In review updates, we will perform subgroup analyses if statistical heterogeneity is present (P < 0.1 for the Chi2 test of heterogeneity, I2 of 50% or greater, or a different clinical conclusion of 95% CI versus 95% PI).
In review updates, we will perform subgroup analyses to investigate heterogeneity for the following characteristics.
-
Ivermectin used as treatment (inpatients and outpatients):
dose of ivermectin (low versus high);
age (children versus adults).
-
Ivermectin used for prevention:
dose of ivermectin (low versus high);
mode of exposure (e.g. working place, nursing home) and burden of exposure (e.g. living in a high‐risk area, high‐risk medical contact) in prevention studies;
confirmation of SARS‐CoV‐2 infection (RT‐PCR versus antigen testing; for the outcome 'SARS‐CoV‐2 infection').
Sensitivity analysis
We used sensitivity analyses to test the robustness of the meta‐analyses. We excluded:
non‐peer reviewed studies (including preprint articles);
studies reporting data as median instead of mean for continuous outcomes; in the current review version there were no data reported as median that were eligible for a transformation into mean.
Since high risk of bias trials were excluded from the primary analysis, we performed a secondary analysis including the studies judged as overall high risk of bias to assess the impact of those studies on the results.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in summary of findings tables, including a rating of the certainty of evidence based on the GRADE approach. We followed current GRADE guidance as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2020).
Two review authors (SW, MP) assessed the certainty of evidence, considering risk of bias, inconsistency, imprecision, indirectness, and publication bias. We used the overall RoB 2 assessment to inform the risk of bias judgement underlying the assessment of the certainty of evidence. The primary analysis including only studies at overall low risk or some concerns of bias were used as data basis for the summary of findings tables.
We created separate summary of findings tables for the use of ivermectin with different intentions (e.g. treatment of people with COVID‐19 in inpatient and outpatient settings, and prevention of SARS‐CoV‐2 infection) and for different comparisons with regard to the intervention and comparator. For the current review, we found no studies with active comparators. The summary of findings tables included the following outcomes (primary analysis).
For use of ivermectin with intention to treat COVID‐19 in an inpatient setting:
all‐cause mortality up to 28 days;
clinical worsening or improvement of symptoms up to 28 days, assessed as need for respiratory support;
adverse events (any grade) up to 28 days;
quality of life up to 28 days;
duration of hospitalization;
viral clearance at seven days.
For use of ivermectin with intention to treat COVID‐19 in an outpatient setting:
all‐cause mortality up to 28 days;
clinical worsening or improvement of symptoms up to 14 days, assessed as need for respiratory support;
admission to hospital;
adverse events (any grade) up to 28 days;
quality of life up to 14 days;
viral clearance at 7 days.
For use of ivermectin with intention to prevent SARS‐CoV‐2 infection:
SARS‐CoV‐2 infection (confirmed by RT‐PCR or antigen testing) at 14 days;
development of clinical COVID‐19 symptoms up to 14 days; assessed in accordance with the WHO scale;
adverse events (any grade) up to 14 days;
all‐cause mortality up to 28 days;
admission to hospital at day 14;
quality of life up to 14 days.
The GRADE assessment resulted in one of four levels of certainty and these express our confidence in the estimate of effect (Balshem 2011).
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We used the MAGICapp to create summary of findings tables (MAGICapp), and incorporated the results into RevMan Web manually (RevMan Web 2020).
Methods for future updates
Living systematic review considerations
Our information specialist (MIM) will provide us with new search records each week, which two review authors will screen, extract, evaluate, and integrate following the guidance for Cochrane living systematic reviews (Cochrane LSR).
We will manually check platform trials for new treatment arms investigating ivermectin.
We will wait until the accumulating evidence changes our conclusions of the implications of research and practice before republishing the review. We will consider one or more of the following components to inform this decision.
The findings of one or more prioritized outcomes.
The credibility (e.g. GRADE rating) of one or more prioritized outcomes.
New settings, populations, interventions, comparisons, or outcomes studied.
In case of emerging policy relevance because of global controversies around the intervention, we will consider republishing an updated review even though our conclusions remain unchanged. We will review the review scope and methods approximately monthly, or more frequently if appropriate, in light of potential changes in COVID‐19 research (e.g. when additional comparisons, interventions, subgroups, or outcomes, or new review methods become available).
Results
Description of studies
Results of the search
The literature search resulted in 326 records. A handsearch of reference lists identified a further 22 records, resulting in an overall 348 records. After removing duplicates, 318 records remained. During title and abstract screening, 175 records were judged as irrelevant as they did not meet the prespecified inclusion criteria. We proceeded to full‐text screening with 143 records, considering published full texts or, if these were unavailable, trial register entries. We excluded 57 records related to 38 studies after full‐text assessment. Twelve studies investigated combined treatments including ivermectin, eight studies used an active comparator without proven efficacy, and five studies analyzed inappropriate study populations including RT‐PCR‐negative participants. Furthermore, nine studies were not RCTs and four studies focused on an intervention other than ivermectin. We identified 31 ongoing studies with 37 records and 18 studies with 20 records awaiting assessment. Fourteen studies with 29 records met our eligibility criteria and were used for qualitative synthesis. One study did not report on outcome time points relevant to this review, hence, 13 studies contributed data to our meta‐analyses (quantitative syntheses). The search process is shown in Figure 1.
1.
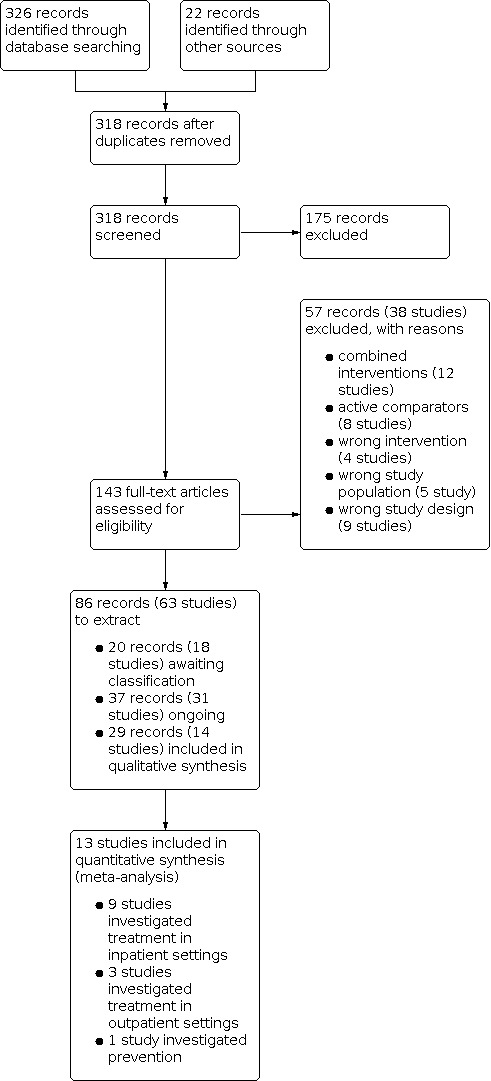
Designs of the studies and publication status
See Characteristics of included studies table.
We included 14 studies describing 1678 adults randomized to study arms relevant for the review question (Ahmed 2020; Chaccour 2021; Chachar 2020; Gonzalez 2021; Kirti 2021; Kishoria 2020; Krolewiecki 2020; López‐Medina 2021; Mohan 2021; Okumuş 2021; Podder 2020; Pott‐Junior 2021; Shah Bukhari 2021; Shoumann 2021). Eight studies had an open‐label design (Chachar 2020; Kishoria 2020; Krolewiecki 2020; Okumuş 2021; Podder 2020; Pott‐Junior 2021; Shah Bukhari 2021; Shoumann 2021), and the other six studies were double‐blind and placebo‐controlled (Ahmed 2020; Chaccour 2021; Gonzalez 2021; Kirti 2021; López‐Medina 2021; Mohan 2021). Three studies were multicentre studies in Argentina (Krolewiecki 2020), Turkey (Okumuş 2021), and Egypt (Shoumann 2021). The remaining 11 studies were single‐centre studies in Bangladesh (Ahmed 2020; Podder 2020), Brazil (Pott‐Junior 2021), Colombia (López‐Medina 2021), India (Mohan 2021; Kirti 2021; Kishoria 2020), Mexico (Gonzalez 2021), Pakistan (Chachar 2020; Shah Bukhari 2021), and Spain (Chaccour 2021).
None of the included studies had a trial size greater than 500 participants. Chaccour 2021 had the smallest sample size of 24 randomized participants. López‐Medina 2021 had the largest trial with 476 randomized participants.
Five studies were published as preprint articles by the end of May 2021 (Gonzalez 2021; Kirti 2021; Krolewiecki 2020; Mohan 2021; Shah Bukhari 2021). The remaining nine studies were available as journal publications. Three of the nine studies were published in non‐indexed journals (Chachar 2020; Kishoria 2020; Podder 2020).
Eight studies registered a study protocol prospectively (Chaccour 2021; Gonzalez 2021; Kirti 2021; Krolewiecki 2020; López‐Medina 2021; Mohan 2021; Pott‐Junior 2021; Shoumann 2021), one study registered the protocol during the recruitment period (Shah Bukhari 2021), two studies registered the study protocol retrospectively (Chachar 2020; Okumuş 2021), and three studies did not register any study protocol (Ahmed 2020; Kishoria 2020; Podder 2020).
Seven studies reported detailed information on the responsible ethics committee and approval reference number in their publication (Gonzalez 2021; López‐Medina 2021; Mohan 2021; Okumuş 2021; Pott‐Junior 2021; Shah Bukhari 2021; Shoumann 2021). Two study authors provided all the information through personal communication (Chaccour 2021; Krolewiecki 2020). Three studies only indicated the name of their ethics committee, but not the approval reference number (Ahmed 2020; Chachar 2020; Kirti 2021). Two studies gave no information on those aspects (Kishoria 2020; Podder 2020). We contacted those studies with insufficient or no information regarding details on their ethics approval.
Four studies were funded by pharmaceutical companies producing ivermectin, including Beximco Pharmaceuticals Ltd (Ahmed 2020), Laboratorio Elea Phoenix SA (Krolewiecki 2020), Windlas Biotech Ltd (Mohan 2021), and Sun Pharma Ltd (Kirti 2021). One study was funded by NeuTec Pharma (Okumuş 2021), which provided ivermectin for the study. It is unclear from the website whether NeuTec Pharma distributes ivermectin commercially. Eight studies were funded by departmental resources (Chaccour 2021; Chachar 2020; Gonzalez 2021; López‐Medina 2021; Podder 2020; Pott‐Junior 2021; Shah Bukhari 2021; Shoumann 2021). One study did not reported funding (Kishoria 2020).
Participants
One study investigated ivermectin for prevention of SARS‐CoV‐2 infections and included asymptomatic household close contacts to confirmed COVID‐19 index case (Shoumann 2021). The remaining 13 studies investigated ivermectin for treatment of COVID‐19 and included participants with SARS‐CoV‐2 infection confirmed by RT‐PCR or antigen testing. Of the 13 studies with intention to treat COVID‐19, four studies were in an outpatient setting (Chaccour 2021; Chachar 2020; López‐Medina 2021; Podder 2020), and nine studies were in an inpatient setting (Ahmed 2020; Gonzalez 2021; Kirti 2021; Kishoria 2020; Krolewiecki 2020; Mohan 2021; Okumuş 2021; Pott‐Junior 2021; Shah Bukhari 2021).
Participants included in three of the four outpatient studies had mostly mild COVID‐19 according to a patient state of 2 to 3 on the WHO scale (Chaccour 2021; Chachar 2020; Podder 2020). Participants in López‐Medina 2021 had mostly mild COVID‐19 defined as WHO scale 2 to 3, but less than 1% of participants were hospitalized with or without supplemental oxygen.
Five studies were conducted in an inpatient setting and included participants with moderate COVID‐19 and with or without supplemental oxygen according to WHO scale 4 to 5 (Kirti 2021; Krolewiecki 2020; Mohan 2021; Pott‐Junior 2021; Shah Bukhari 2021). In Ahmed 2020 and Kishoria 2020, none of the participants received supplemental oxygen. In Gonzalez 2021, all participants received supplemental oxygen. Okumuş 2021 included participants with moderate‐to‐severe COVID‐19 according to WHO scale 4 to 9.
The overall population mean age of the included studies was 43 years. Chaccour 2021 included the youngest participants with a population median age of 28 years. Okumuş 2021 included the oldest participants with a population mean age of 62 years.
The mean proportion of men in all included studies was 59%. The lowest proportions of men were included in Chachar 2020 and López‐Medina 2021 with 42% men, while Shah Bukhari 2021 included the highest proportion with 85% men.
The studies partially reported comorbidities and relevant risk factors such as obesity, diabetes, respiratory diseases, hypertension, and immunosuppression (see Characteristics of included studies table). Two studies excluded existing comorbidities and specified them in the inclusion and exclusion criteria (Chaccour 2021; Krolewiecki 2020). Four studies reported no data on risk factors in their publications or study reports (Ahmed 2020; Kishoria 2020; Podder 2020; Pott‐Junior 2021).
Interventions and comparators
All studies administered ivermectin orally. The daily dosages varied between fixed doses of 12 mg and 24 mg or weight‐adjusted doses of 100 µg/kg and 400 µg/kg. Four studies used low doses (200 µg/kg orally, single dose) in at least one study arm (Mohan 2021; Podder 2020; Pott‐Junior 2021; Shah Bukhari 2021). All other studies applied higher doses either in one single dose or multiple doses for up to five days. Participants received single‐dose ivermectin in five studies (Chaccour 2021; Kishoria 2020; Mohan 2021; Podder 2020; Shah Bukhari 2021), two doses in two studies (Kirti 2021; Shoumann 2021), three doses over 24 hours in one study (Chachar 2020), and five doses in five studies (Ahmed 2020; Gonzalez 2021; Krolewiecki 2020; López‐Medina 2021; Okumuş 2021). In one study, there was insufficient detail in the journal publication and the trial registry on whether the participants received ivermectin as a single or double dose (Pott‐Junior 2021).
We found no studies comparing ivermectin to an active comparator with proven efficacy. Six studies administered placebo tablets as the control intervention (Ahmed 2020; Chaccour 2021; Gonzalez 2021; Kirti 2021; López‐Medina 2021; Mohan 2021). The remaining seven studies administered standard of care as control intervention (Chachar 2020; Kishoria 2020; Krolewiecki 2020; Okumuş 2021; Podder 2020; Pott‐Junior 2021; Shah Bukhari 2021). Standard of care varied between studies, but was comparable between the study arms of the individual studies. Four studies did not provide details of standard of care (Ahmed 2020; Chaccour 2021; Chachar 2020; Krolewiecki 2020). Three studies used a combination of interventions including hydroxychloroquine, favipiravir, and azithromycin (Kirti 2021; Mohan 2021; Okumuş 2021). One study combined hydroxychloroquine, vitamin C, and paracetamol (Kishoria 2020). Five studies administered corticosteroids such as dexamethasone (Gonzalez 2021; Kirti 2021; López‐Medina 2021; Mohan 2021; Pott‐Junior 2021). Shah Bukhari 2021 administered vitamin C and D3 as additional standard of care. López‐Medina 2021 and Podder 2020 utilized antipyretic drugs for symptomatic treatment.
The only prevention study compared ivermectin to no treatment (Shoumann 2021).
Outcome measures
One study did not report outcomes eligible for meta‐analysis due to a short follow‐up period of seven days (Chachar 2020).
In trials with intention to treat COVID‐19, the most investigated primary outcomes as defined by the study were either (time to) viral clearance or a reduction in the viral load reported in eight studies (Ahmed 2020; Chaccour 2021; Kirti 2021; Kishoria 2020; Krolewiecki 2020; Mohan 2021; Pott‐Junior 2021; Shah Bukhari 2021). Chachar 2020 and López‐Medina 2021 defined resolution of symptoms as primary outcomes, and Ahmed 2020 defined remission of fever and cough as an additional primary outcome. The primary outcomes in Gonzalez 2021 were duration of hospitalization until discharge due to clinical improvement and duration of hospitalization. The primary outcome in Okumuş 2021 was clinical response. Two studies defined safety outcomes as additional primary outcomes (Gonzalez 2021; Okumuş 2021). Podder 2020 did not define any primary outcome.
In the only prevention trial, the primary outcome was development of COVID‐19 typical symptoms (Shoumann 2021).
Primary outcomes as defined by the review for studies with intention to treat COVID‐19 were only reported by a minority of the included studies. Two studies reported all‐cause mortality up to 28 days in inpatient settings (Gonzalez 2021; Kirti 2021), and two studies in outpatient settings (Chaccour 2021; López‐Medina 2021). Three studies in an inpatient setting reported data useable to assess worsening of clinical status up to 28 days (Ahmed 2020; Gonzalez 2021; Kirti 2021), and one study in an outpatient setting reported data at 15 days (López‐Medina 2021). One study reported improvement of clinical status in an inpatient setting as "patients discharged without respiratory deterioration or death at 28 days" (Gonzalez 2021). This outcome was clinically useful and was added as new primary outcome during preparation of this review. In an outpatient setting, one study reported improvement of clinical status as participants with symptoms resolved at 15 days (López‐Medina 2021). Two studies reported duration to symptom resolution in an outpatient setting (López‐Medina 2021; Podder 2020). Four studies in an inpatient setting reported the number of participants with adverse events within 14 to 28 days (Krolewiecki 2020; Mohan 2021; Pott‐Junior 2021; Shah Bukhari 2021). Two studies in an outpatient setting reported adverse events within 21 to 28 days (Chaccour 2021; López‐Medina 2021). No studies reported data for the following primary outcomes in an inpatient setting, including all outcomes summarized as 'improvement of clinical status' and need for non‐invasive mechanical ventilation or high‐flow. For the outpatient setting, none of the included studies reported data for the primary outcomes admission to hospital and need for hospitalization with or without supplemental oxygen.
The only included study with intention to prevent SARS‐CoV‐2 infection reported two of the primary outcomes defined by the review, including development of clinical COVID‐19 symptoms at 14 days and adverse events within 14 days (Shoumann 2021). The number of participants with confirmed SARS‐CoV‐2 infection was not investigated.
Excluded studies
See Characteristics of excluded studies table.
We excluded 38 studies that did not match our inclusion criteria. Twelve studies evaluated a combination of ivermectin with other treatments that were different between groups (Chahla 2021a; Chowdhury 2021; Hashim 2020; IRCT20200408046987N2; Mahmud 2021; NCT04360356; NCT04392427; NCT04447235; NCT04482686; NCT04551755; NCT04768179; Spoorthi 2020). Eight studies investigated active comparators without proven efficacy (Babalola 2021; CTRI/2020/08/027282; CTRI/2020/08/027394; CTRI/2020/10/028335; Elgazzar 2020; Galan 2021; NCT04435587; Seet 2021). One of these studies was retracted by Research Square on 14 July 2021 due to an expression of concern (Elgazzar 2020; The Guardian 2021). Four studies investigated a wrong intervention (NCT04345419; NCT04374279; NCT04382846; NCT04723459). Five studies analyzed a wrong study population including RT‐PCR negative participants (IRCT20180922041089N4; NCT04530474; NCT04703608; Niaee 2020; Shahbaznejad 2021). Nine studies were not RCTs (Behera 2020; Cadegiani 2020; Camprubi 2020; Carvallo 2020; Chahla 2021b; Gorial 2020; Lima‐Morales 2021; Morgenstern 2020; Rajter 2021).
Studies awaiting classification
See Characteristics of studies awaiting classification table.
Eighteen studies are awaiting classification until publication of results, a protocol update, or clarification of details by the study authors. If eligible we will consider them in the next review update (2020‐001971‐33/ES; 2020‐002091‐12/BG; CTRI/2020/04/024948; CTRI/2020/06/025960; Faisal 2020; Hosseini 2021; IRCT20190602043787N3; IRCT20200408046987N3; IRCT20200422047168N2; ISRCTN90437126; NCT04351347; NCT04374019; NCT04407130; NCT04407507; NCT04716569; NCT04746365; NCT04891250; Samaha 2021).
Three studies had already been published or had results posted in the trial registry (Faisal 2020; NCT04407507; Samaha 2021). However, despite using the term 'randomized controlled trial,' there were contradictory details throughout the published text or protocol that led us to believe those studies were not fulfilling criteria of genuine RCTs. We contacted study authors for clarification but received no response at the time of review publication.
We identified five completed and potentially eligible RCTs from trial register entries, but there were no results available or published (2020‐002091‐12/BG; Hosseini 2021; IRCT20190602043787N3; IRCT20200422047168N2; NCT04407130). Three studies investigated ivermectin with standard of care versus standard of care alone in 220 participants in an inpatient setting (Hosseini 2021; IRCT20190602043787N3; IRCT20200422047168N2), another two completed studies used placebo as comparator in 192 participants (2020‐002091‐12/BG; NCT04407130). Ten studies were not explicit enough in their protocol to make a final decision on eligibility. First, none of the following eight studies reported a clear description of the type of control intervention used as comparator (2020‐001971‐33/ES; CTRI/2020/04/024948; CTRI/2020/06/025960; NCT04351347; NCT04374019; NCT04716569; NCT04746365; NCT04891250). Additionally, for two of those trials, it was unclear if an RT‐PCR‐confirmed COVID‐19 diagnosis was required for inclusion (NCT04351347; NCT04716569). Similarly, two studies investigating prevention were not well‐defined regarding the inclusion criteria of high‐risk exposure to an index patient (ISRCTN90437126; NCT04891250). Finally, for another trial, we could not evaluate the actual rationale or the considered patient population due to inconclusive PICO details (IRCT20200408046987N3).
Ongoing studies
See Characteristics of ongoing studies table.
We classified 31 studies as ongoing. With intention to treat COVID‐19, 18 studies are comparing ivermectin to placebo of which seven were to be completed by the end of May 2021, and planned to evaluate between 100 and 500 participants (ACTRN12620000982910; NCT04712279; Garcia 2021; NCT04429711; NCT04729140; NCT04834115; Vallejos 2020). However, according to the trial registry, these studies are either not yet recruiting or still recruiting at the time of review publication. Nine of 13 studies, evaluating between 60 and 2724 participants, are due to be completed by the end of 2021 (IRCT20200404046937N4; NCT04438850; NCT04472585; NCT04703205; NCT04836299; NCT04886362) or 2022 (IRCT20111224008507N4; IRCT20111224008507N5; NCT04727424). Anticipating 15,000 participants, the largest ongoing trial will be completed in 2023 (NCT04885530). One trial, which plans to evaluate 266 participants, has not reported the recruiting status or an expected completion date (2020‐001994‐66/ES). Regarding the patient setting, 13 ongoing studies described above are using ivermectin in an outpatient setting (2020‐001994‐66/ES; ACTRN12620000982910; NCT04712279; Garcia 2021;IRCT20111224008507N4; NCT04438850; NCT04703205; NCT04727424; NCT04729140; Vallejos 2020; NCT04834115; NCT04885530; NCT04886362), three in an inpatient setting (IRCT20200404046937N4; IRCT20111224008507N5; NCT04836299), and two are unclear about this issue in their protocol (NCT04429711; NCT04472585).
Ten of 31 ongoing studies are comparing ivermectin plus standard of care to standard of care alone, and plan to evaluate between 50 and 240 participants. Six of 10 studies include participants in inpatients settings (CTRI/2020/05/025068; CTRI/2020/05/025224; NCT04403555; NCT04425707; NCT04602507; PACTR202102588777597), one study includes outpatients (NCT04673214), and three studies are unclear about this issue in their protocol (IRCT20190624043993N2; NCT04445311; NCT04510233). Most of those studies were expected to be completed by May 2021, but according to the trial registry, five are still recruiting (NCT04403555; NCT04425707; NCT04445311; NCT04602507; NCT04673214), one has not started recruitment yet (NCT04510233), and one does not report the recruitment status (IRCT20190624043993N2). One trial that is currently recruiting is expected to be completed in 2022 (PACTR202102588777597). The two studies without an indicated completion date have not started recruiting yet (CTRI/2020/05/025068; CTRI/2020/05/025224).
Five studies are comparing ivermectin to placebo or no treatment with the intention to prevent SARS‐CoV‐2 infection in close contacts of COVID‐19 index cases. Two of those are planned as substudies on close contacts that also investigate treatment in 266 (2020‐001994‐66/ES) and 240 (PACTR202102588777597) participants. Three larger trials, all of which are using placebo as control, are solely focusing on the period after high‐risk exposure to COVID‐19. One trial with 750 close contacts is still recruiting, but expected to be completed during the time of review publication (NCT04894721), another one including 2000 close contacts will be completed in October 2021 (PACTR202102848675636). Finally, one study is evaluating postexposure prophylaxis in 550 healthcare workers (NCT04527211). This study was supposed to be completed in December 2020, but according to the trial registry has not started recruiting yet.
We found no studies comparing ivermectin to an active comparator eligible for this review.
Risk of bias in included studies
We assessed methodological quality and risk of bias for 13 RCTs contributing results to our prioritized outcomes using the RoB 2 tool (Ahmed 2020; Chaccour 2021; Gonzalez 2021; Kirti 2021; Kishoria 2020; Krolewiecki 2020; López‐Medina 2021; Mohan 2021; Okumuş 2021; Podder 2020; Pott‐Junior 2021; Shah Bukhari 2021; Shoumann 2021). We did not judge risk of bias for Chachar 2020, as this study reported no results eligible for any of our prioritized outcomes. In total, the 13 studies contributed 41 study results to 23 outcomes of this review that were finally assessed using RoB 2. The RoB 2 judgements for all study results per outcomes and for all domains are available in an interactive risk‐of‐bias table (Supplementary File_Ivermectin_Risk of Bias), and are briefly summarized below.
Overall risk of bias by study
Most of the 41 study results (56.1%) had some concerns for the overall risk of bias. Thirteen (31.7%) of the 41 study results were at overall high risk of bias. We excluded studies with overall high risk of bias from the primary meta‐analyses of the review. The main reasons a study result was assessed at high risk of bias were missing outcome data and measurement of the outcome. Studies with high risk of bias were included in secondary meta‐analyses. Two studies contributing five study results (12.2%) to four outcomes, including all‐cause mortality, adverse events, and viral clearance at three and seven days, were at overall low risk of bias (Chaccour 2021; Mohan 2021).
Overall risk of bias by outcome
The following section summarizes the risk of bias per outcome (primary analysis) for all outcomes included in the summary of findings tables (Table 1; Table 2; Table 3).
Summary of findings 1. Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in an inpatient setting.
| Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in an inpatient setting | ||||||
|
Patient or population: people with moderate to severe disease (WHO scale 4–9); all studies contributing results to the summary of findings table included people with moderate disease (WHO scale 4 or 5) Setting: inpatients Intervention: ivermectin Comparison: placebo or standard of care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or standard of care | Risk with ivermectin | |||||
| All‐cause mortality up to 28 days | 96 per 1000 | 58 per 1000 (13 to 241) | RR 0.60 (0.14 to 2.51) | 185 (2 RCTs) | ⨁◯◯◯ Very lowa |
We are uncertain whether ivermectin reduces or increases all‐cause mortality up to 28 days. |
| Clinical worsening: need for invasive mechanical ventilation up to 28 days | 85 per 1000 | 47 per 1000 (9 to 220) | RR 0.55 (0.11 to 2.59) | 185 (2 RCTs) | ⨁◯◯◯ Very lowa |
We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for invasive mechanical ventilation up to 28 days. |
| Clinical worsening: need for oxygen by mask or nasal prongs up to 28 days, in people with WHO scale 4 at baseline | 1 study assessed need for oxygen during the study period, but none of the participants in either group required supplemental oxygen (Ahmed 2020). | Not estimable | 45 (1 RCT) | ⨁◯◯◯ Very lowb |
We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for oxygen support up to 28 days. | |
| Clinical improvement: participants discharged without respiratory deterioration or death up to 28 days | 729 per 1000 | 752 per 1000 (569 to 1000) | RR 1.03 (0.78 to 1.35) | 73 (1 RCT) | ⨁⨁◯◯ Lowc |
Ivermectin may have little or no effect on clinical improvement assessed by the number of participants discharged without respiratory deterioration or death up to 28 days. |
| Adverse events (any grade) up to 28 days | 115 per 1000 | 139 per 1000 (58 to 342) | RR 1.21 (0.50 to 2.97) | 152 (1 RCT) | ⨁◯◯◯ Very lowd |
We are uncertain whether ivermectin may increase or reduce any adverse events up to 28 days |
| Quality of life up to 28 days | — | — | — | — | — | No studies reported quality of life up to 28 days. |
| Duration of hospitalization | The mean duration of hospitalization in the placebo group was 9.7 days | The mean duration of hospitalization in the ivermectin group was 0.1 days fewer (2.43 days fewer to 2.23 days more) | MD −0.10 (−2.43 to 2.23) | 45 (1 RCT) | ⨁⨁◯◯ Lowe |
Ivermectin may have little or no effect on duration of hospitalization. |
| Viral clearance at 7 days | 292 per 1000 | 531 per 1000 (149 to 1000) | RR 1.82 (0.51 to 6.48) | 159 (2 RCTs) | ⨁◯◯◯ Very lowf |
We are uncertain whether ivermectin increases or reduces viral clearance at 7 days. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; WHO: World Health Organization. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Explanations
aDowngraded one level for serious risk of bias and two levels for very serious imprecision due to few participants, few events, and wide CIs. bDowngraded one level for serious risk of bias and two levels for very serious imprecision due to zero events and few participants. cDowngraded one level for serious risk of bias and one level for serious imprecision due to few participants. dDowngraded one level for serious risk of bias and two levels for very serious imprecision due to few participants and wide CIs. We included studies at high risk of bias in a secondary analysis (RR 1.04, 95% CI 0.61 to 1.79). eDowngraded one level for serious risk of bias and one level for serious imprecision due to few participants. Another study reported data as medians that were not eligible for meta‐analysis. The median duration of hospitalization in the ivermectin group was six days (interquartile range (IQR) 4 to 11 days) compared to five days (IQR 4 to 7 days) in the placebo group. fDowngraded one level for serious risk of bias, one level for serious heterogeneity (I2 = 77%), and two levels for very serious imprecision due to few participants and wide CIs. We included studies at high risk of bias in a secondary analysis (RR 1.19, 95% CI 0.76 to 1.86).
Summary of findings 2. Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in an outpatient setting.
| Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in an outpatient setting | ||||||
|
Patient or population: people with mild disease (WHO scale 1–3); all studies contributing results to the summary of findings table included people with mild disease (WHO scale 2 or 3) Setting: outpatients Intervention: ivermectin Comparison: placebo or standard of care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or standard of care | Risk with ivermectin | |||||
| All‐cause mortality up to 28 days | 5 per 1000 | 2 per 1000 (1 to 40) | RR 0.33 (0.01 to 8.05) | 422 (2 RCTs) | ⨁◯◯◯ Very lowa |
We are uncertain whether ivermectin reduces or increases all‐cause mortality up to 28 days. |
| Clinical worsening: need for invasive mechanical ventilation up to 14 days | 2 per 1000 | 5 per 1000 (0 to 122) | RR 2.97 (0.12 to 72.47) | 398 (1 RCT) | ⨁◯◯◯ Very lowa |
We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for invasive mechanical ventilation up to 14 days. |
| Clinical worsening: need for non‐invasive mechanical ventilation or high flow up to 14 days | 1 study assessed need for non‐invasive mechanical ventilation or high flow at 15 days, but none of the participants in either group required non‐invasive mechanical ventilation or high flow (López‐Medina 2021). | Not estimable | 398 (1 RCT) | ⨁◯◯◯ Very lowb |
We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for non‐invasive mechanical ventilation or high flow up to 14 days | |
| Symptom resolution: participants with symptoms resolved up to 14 days | 606 per 1000 | 630 per 1000 (539 to 733) | RR 1.04 (0.89 to 1.21) | 398 (1 RCT) | ⨁⨁◯◯ Lowc |
Ivermectin may have little or no effect on clinical improvement assessed by the number of participants with symptoms resolved up to 14 days. |
| Symptom resolution: duration of symptom resolution | — | — | — | — | —d | No study with low risk or some concerns of bias reported symptom resolution. |
| Admission to hospital up to 14 days | — | — | — | — | — | No study was found that looked at admission to hospital up to 14 days |
| Adverse events (any grade) up to 28 days | 790 per 1000 | 751 per 1000 (679 to 830) | RR 0.95 (0.86 to 1.05) | 422 (2 RCTs) | ⨁⨁◯◯ Lowc |
Ivermectin may have little or no effect on any adverse events up to 28 days. |
| Quality of life up to 14 days | — | — | — | — | — | No study reported quality of life up to 14 days. |
| Viral clearance at 7 days | 28 per 1000 | 83 per 1000 (4 to 1000) | RR 3.00 (0.13 to 67.06) | 24 (1 RCT) | ⨁⨁◯◯ Lowe |
We are uncertain whether ivermectin increases or reduces viral clearance at 7 days. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio; WHO: World Health Organization. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Explanations
aDowngraded one level for serious risk of bias and two levels for very serious imprecision due to few participants, few events, and wide CIs. bDowngraded one level for serious risk of bias and two levels for very serious imprecision due to zero events and few participants. cDowngraded one level for serious risk of bias and one level for serious imprecision due to few participants. dNone of the studies were eligible for primary analysis. One study reported the median duration of symptom resolution in the placebo group was 12 days (interquartile range (IQR) 9 to 13 days) with 10 days (IQR 9 to 13 days) in the ivermectin group. We included one study at high risk of bias in a secondary analysis (mean difference −1.02, 95% CI −2.76 to 0.72). eDowngraded two levels for very serious imprecision due to few participants, few events, and wide CIs.
Summary of findings 3. Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection.
| Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection | ||||||
|
Patient or population: people who were not infected with SARS‐CoV‐2, but were at high risk of developing the infection (e.g. after high‐risk exposure) Setting: inpatient or outpatients Intervention: ivermectin Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with ivermectin | |||||
| SARS‐CoV‐2 infection (confirmed by RT‐PCR or antigen testing) at 14 days | — | — | — | — | — | No study reported SARS‐CoV‐2 infection at 14 days. |
| Development of clinical COVID‐19 symptoms up to 14 days | — | — | — | — | —a | No study with low risk or some concerns of bias reported development of clinical COVID‐19 symptoms up to 14 days. |
| Adverse events (any grade) up to 14 days | — | — | — | — | —b | No study with low risk or some concerns of bias reported adverse events up to 14 days. |
| All‐cause mortality up to 28 days | 1 study assessed all‐cause mortality during the study period, but 0 participants in either group died (Shoumann 2021). | Not estimable | 304 (1 RCT) | ⨁◯◯◯ Very lowc |
We are uncertain whether ivermectin reduces or increases all‐cause mortality up to 28 days. | |
| Admission to hospital up to 14 days | — | — | — | — | — | No study reported admission to hospital up to 14 days. |
| Quality of life up to 14 days | — | — | — | — | — | No study reported quality of life up to 14 days. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Explanations
aNo evidence available from studies with low risk or some concerns of bias. We included one study with high risk of bias in a secondary analysis (risk ratio (RR) 0.13, 95% CI 0.08 to 0.21). bNo evidence available from studies with low risk or some concerns of bias. We included one study with high risk of bias in a secondary analysis (RR 11.50, 95% CI 0.68 to 193.21). cDowngraded one level for serious risk of bias and two levels for very serious imprecision due to zero events and few participants.
Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in an inpatient setting
All‐cause mortality up to 28 days
Two studies contributed results to the primary analysis of mortality up to 28 days and risk of bias was assessed as some concerns. Gonzalez 2021 provided insufficient information on allocation concealment and blinding of healthcare providers, and did not define the time point of outcome measurement in the protocol. Kirti 2021 performed an inappropriate analysis (per‐protocol analysis).
Worsening of clinical status
Need for invasive mechanical ventilation up to 28 days
Two studies contributed results to the primary analysis of need for invasive mechanical ventilation up to 28 days and risk of bias was assessed as some concerns. Gonzalez 2021 provided insufficient information on allocation concealment and blinding of healthcare providers, and did not define the time point of outcome measurement in the protocol. Kirti 2021 performed an inappropriate analysis (per‐protocol analysis).
Need for oxygen by mask or nasal prongs up to 28 days
One study contributed results to the primary analysis of need for oxygen by mask or nasal prongs up to 28 days and risk of bias was assessed as some concerns. Ahmed 2020 provided insufficient information on randomization, allocation concealment, and blinding of participants and healthcare providers, and did not provide a prospectively registered protocol.
Improvement of clinical status
Participants discharged without respiratory deterioration or death at 28 days
One study contributed results to the primary analysis of patients discharged without respiratory deterioration or death at 28 days and risk of bias was assessed as some concerns. Gonzalez 2021 provided insufficient information on allocation concealment and blinding of healthcare providers and did not register the outcome in the protocol.
Any adverse events within 28 days
One study contributed results to the primary analysis of any adverse events within 28 days and risk of bias was assessed as some concerns. Mohan 2021 provided insufficient information on definition and measurement of the outcome, and did not prespecify the outcome in the prospectively registered protocol.
Three studies with high risk of bias were not eligible for the primary analysis (Krolewiecki 2020; Pott‐Junior 2021; Shah Bukhari 2021). All three studies provided insufficient information on definition, and measurement of the outcome and outcome assessors were not blinded. One study had missing outcome data of more than 14% (Shah Bukhari 2021). Participants withdrew against medical advice before completion of the study.
Duration of hospitalization
One study contributed results to the primary analysis of duration of hospitalization and risk of bias was assessed as some concerns. Ahmed 2020 provided insufficient information on randomization, allocation concealment, and blinding of participants and healthcare providers, and did not provide a prospectively registered protocol.
Viral clearance at seven days
Two studies contributed results to the primary analysis of viral clearance at seven days and risk of bias was assessed as some concerns. Mohan 2021 was at overall low risk of bias. Ahmed 2020 provided insufficient information on randomization, allocation concealment, blinding of participants and healthcare providers, and did not provide a prospectively registered protocol.
Two studies with high risk of bias were not eligible for the primary analysis (Kirti 2021; Pott‐Junior 2021). Kirti 2021 had more than 30% of missing data due to discharge or inconclusive results. In Pott‐Junior 2021, participants and those delivering the intervention were aware of the intervention received and 25% of participants (one of four) in the control group were excluded due to protocol violations (per‐protocol analysis). Protocol violations were not described.
Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in an outpatient setting
All‐cause mortality up to 28 days
Two studies contributed results to the primary analysis of mortality up to 28 days and risk of bias was assessed as some concerns. Chaccour 2021 was at overall low risk of bias. In López‐Medina 2021, the primary analysis was per‐protocol due to a labelling error that resulted in 16% of participants receiving the wrong intervention. Both participants and those delivering the intervention were unaware of intervention received and reported an as‐treated sensitivity analysis where results did not differ.
Development of moderate‐to‐severe clinical COVID‐19 symptoms – worsening of clinical status
Need for invasive mechanical ventilation up to 14 days
One study contributed results to the primary analysis of need for invasive mechanical ventilation at 14 days and risk of bias was assessed as some concerns. In López‐Medina 2021, the primary analysis was per‐protocol due to a labelling error that resulted in 16% of participants receiving the wrong intervention. Both participants and those delivering the intervention were unaware of intervention received and reported an as‐treated sensitivity analysis where results did not differ.
Need for non‐invasive mechanical ventilation or high flow up to 14 days
One study contributed results to the primary analysis of need for non‐invasive mechanical ventilation or high flow up to 14 days and risk of bias was assessed as some concerns. In López‐Medina 2021, the primary analysis was per‐protocol due to a labelling error that resulted in 16% of participants receiving the wrong intervention. Both participants and those delivering the intervention were unaware of intervention received and reported an as‐treated sensitivity analysis where results did not differ.
Symptom resolution
Number of participants with symptoms resolved up to 14 days
One study contributed results to the primary analysis of number of participants with symptoms resolved up to 14 days and risk of bias was assessed as some concerns. In López‐Medina 2021, the primary analysis was per‐protocol due to a labelling error that resulted in 16% of participants receiving the wrong intervention. Both participants and those delivering the intervention were unaware of intervention received and reported an as‐treated sensitivity analysis where results did not differ.
Duration of symptom resolution
No study reporting duration of symptom resolution was eligible for primary analysis.
One study reported data as median with interquartile range, which were not eligible for meta‐analysis (López‐Medina 2021). The other study was not eligible for a primary analysis because of an overall high risk of bias assessment due to inadequate randomization and lack of blinding of participants, healthcare providers, and outcome assessors, and due to missing outcome data and lack of a registered protocol (Podder 2020).
Any adverse events within 28 days
Two studies contributed results to the primary analysis of any adverse events within 28 days and risk of bias was assessed as some concerns. Chaccour 2021 was at overall low risk of bias. In López‐Medina 2021, the primary analysis was per‐protocol due to a labelling error that resulted in 16% of participants receiving the wrong intervention. Both participants and those delivering the intervention were unaware of intervention received and reported an as‐treated sensitivity analysis where results did not differ.
Viral clearance at seven days
One study contributed results to the primary analysis of viral clearance at seven days and risk of bias was assessed as low. Chaccour 2021 was at low risk of bias in all domains.
Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection
Development of clinical COVID‐19 symptoms up to 14 days
No study reporting development of clinical COVID‐19 symptoms up to 14 days was eligible for primary analysis.
One study reported results that were not eligible for the primary analysis because of an overall high risk of bias assessment due to lack of information on measurement of the outcome and lack of blinding of outcome assessors (Shoumann 2021).
Any adverse events within 14 days
No study reporting any adverse events within 14 days was eligible for primary analysis.
One study reported results that were not eligible for the primary analysis because of an overall high risk of bias assessment due to lack of information on measurement of the outcome and lack of blinding of outcome assessors (Shoumann 2021).
All‐cause mortality up to 28 days
One study contributed results to the primary analysis of mortality up to 28 days and risk of bias was assessed as some concerns. Shoumann 2021 provided insufficient information on randomization, allocation concealment, and missing outcome data, and did not prospectively register the outcome.
Effects of interventions
See: Table 1; Table 2; Table 3
We included 14 studies in the qualitative synthesis of this review (Ahmed 2020; Chaccour 2021; Chachar 2020; Gonzalez 2021; Kirti 2021; Kishoria 2020; Krolewiecki 2020; López‐Medina 2021; Mohan 2021; Okumuş 2021; Podder 2020; Pott‐Junior 2021; Shah Bukhari 2021; Shoumann 2021). With exception to one study (Chachar 2020), we included 13 studies in meta‐analyses (quantitative synthesis). All included studies compared ivermectin to no treatment, placebo, or standard of care, and we found no studies that compared ivermectin to an active comparator with proven efficacy.
Nine studies investigated ivermectin compared to placebo or standard of care for treating COVID‐19 in an inpatient setting and contributed data to meta‐analyses (Ahmed 2020; Gonzalez 2021; Kirti 2021; Kishoria 2020; Krolewiecki 2020; Mohan 2021; Okumuş 2021; Pott‐Junior 2021; Shah Bukhari 2021). Only one study investigated participants with moderate‐to‐severe COVID‐19 (Okumuş 2021). All other studies investigated only participants with moderate COVID‐19. Therefore, planned subgroup analyses for severity at baseline were not possible. The main findings are summarized in Table 1.
Three studies investigated ivermectin compared to placebo or standard of care for treating COVID‐19 in an outpatient setting and contributed data to meta‐analyses (Chaccour 2021; López‐Medina 2021; Podder 2020). The main findings are summarized in Table 2.
One study investigated ivermectin compared to no treatment for preventing SARS‐CoV‐2 infection and contributed data to meta‐analyses (Shoumann 2021). The main findings are summarized in Table 3.
Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in an inpatient setting
All‐cause mortality up to 28 days
Two studies comparing ivermectin to placebo reported data on mortality at 28 days for 185 participants with moderate disease (Gonzalez 2021; Kirti 2021). Both studies were included in the primary meta‐analysis due to the overall risk of bias assessment (Analysis 1.1). In the meta‐analysis, five participants died in the ivermectin group and nine participants in the placebo group. We are uncertain whether ivermectin reduces or increases all‐cause mortality up to 28 days compared to placebo (RR 0.60, 95% CI 0.14 to 2.51; 2 RCTs, 185 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to few participants, few events, and wide CIs. Both studies were published as preprint articles.
1.1. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 1: All‐cause mortality up to 28 days (primary analysis)
Two studies in an inpatient setting reported mortality at time points not eligible for meta‐analysis. Mohan 2021 reported mortality at 14 days, which is too short, and Okumuş 2021 reported an unclear time frame of follow‐up. In both cases, data were not comparable with studies reporting our predefined time point of 28 days.
Worsening of clinical status
Need for invasive mechanical ventilation up to 28 days
Two studies comparing ivermectin to placebo reported data on clinical worsening assessed by need for invasive mechanical ventilation at 28 days for 185 participants with moderate disease (Gonzalez 2021; Kirti 2021). Both studies were included in the primary meta‐analysis due to the overall risk of bias assessment (Analysis 1.2). In the meta‐analysis, four participants in the ivermectin group and eight participants in the placebo group showed clinical worsening. We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for invasive mechanical ventilation compared to placebo up to 28 days (RR 0.55, 95% CI 0.11 to 2.59; 2 studies, 185 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to few participants, few events, and wide CIs. Both studies were published as preprint articles.
1.2. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 2: Worsening of clinical status – need for invasive mechanical ventilation up to 28 days (primary analysis)
Two studies in an inpatient setting reported worsening of clinical status at seven days (Krolewiecki 2020) and 14 days (Mohan 2021), which was clinically not comparable with studies reporting our predefined time point of 28 days. Therefore, those studies were not eligible for meta‐analysis.
Need for non‐invasive mechanical ventilation or high flow up to 28 days
No study reported data for need for non‐invasive mechanical ventilation or high flow up to 28 days.
Need for oxygen by mask or nasal prongs up to 28 days
One study comparing ivermectin to placebo assessed need for oxygen by mask or nasal prongs during the study period (14 days) for 45 participants without supplemental oxygen at baseline, but none of the participants in either group required supplemental oxygen during the study (Ahmed 2020). We accepted the shorter time point of 14 days for the outcome as there are no other data available that would cause clinical incompatibility. The study was included in the primary meta‐analysis due to the overall risk of bias assessment (Analysis 1.3). We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for oxygen support up to 28 days compared to placebo (RR not estimable; 1 study, 45 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to zero events and few participants. Ahmed 2020 was published as a journal article.
1.3. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 3: Worsening of clinical status – need for oxygen up to 28 days (primary analysis)
Improvement of clinical status
Weaning or liberation from invasive mechanical ventilation in surviving participants up to 28 days
No study reported data for weaning or liberation from invasive mechanical ventilation in surviving participants up to 28 days.
Ventilator‐free days
No study reported data for ventilator‐free days.
Duration of liberation from invasive mechanical ventilation
No study reported data for duration of liberation from invasive mechanical ventilation.
Liberation from supplemental oxygen in surviving participants up to 28 days
No study reported data for liberation from supplemental oxygen in surviving participants up to 28 days.
Duration of liberation from supplemental oxygen
No study reported data for duration of liberation from supplemental oxygen.
Participants discharged without respiratory deterioration or death at 28 days
One study comparing ivermectin to placebo in 73 participants with moderate disease reported patients discharged without respiratory deterioration or death at 28 days (Gonzalez 2021). The study was included in the primary meta‐analysis due to the overall risk of bias assessment (Analysis 1.4). In both groups, 27 participants were discharged without respiratory deterioration or death at 28 days. Ivermectin may have little or no effect on clinical improvement assessed by the number of participants discharged without respiratory deterioration or death up to 28 days compared to placebo (RR 1.03, 95% CI 0.78 to 1.35; 1 study, 73 participants; low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and one level for serious imprecision due to few participants. Gonzalez 2021 was published as a preprint article.
1.4. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 4: Improvement of clinical status – participants discharged without respiratory deterioration or death at 28 days (primary analysis)
Any adverse events within 28 days
Four studies comparing ivermectin to placebo or standard of care reported any adverse events within 14 days (Mohan 2021), 28 days (Pott‐Junior 2021; Shah Bukhari 2021), and one month (Krolewiecki 2020) in 314 participants with moderate disease. One of these studies was included in the primary analysis due the overall risk of bias assessment (Analysis 1.5) (Mohan 2021). We are uncertain whether ivermectin may increase or reduce any adverse events up to 28 days compared to placebo (RR 1.21, 95% CI 0.50 to 2.97; 1 study, 152 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to few participants and wide CIs. The other three studies with high risk of bias were included in a secondary analysis (Analysis 1.6) (Krolewiecki 2020; Pott‐Junior 2021; Shah Bukhari 2021). The effect estimate of the secondary analysis was comparable to the primary analysis (RR 1.04, 95% CI 0.61 to 1.79; 4 studies, 314 participants). Only the study included in the primary analysis was published as a preprint article (Mohan 2021).
1.5. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 5: Any adverse events within 28 days (primary analysis)
1.6. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 6: Any adverse events within 28 days (secondary analysis)
Okumuş 2021 reported adverse events within five days and was not eligible for meta‐analysis on adverse events within 28 days due to the short follow‐up period.
Serious adverse events within 28 days
Three studies comparing ivermectin to placebo or standard of care reported serious adverse events within 14 days (Ahmed 2020; Mohan 2021) and 30 days (Krolewiecki 2020) in 242 participants with moderate disease. Two of these studies with 197 participants were included in the primary analysis due the overall risk of bias assessment (Analysis 1.7) (Krolewiecki 2020; Mohan 2021). We are uncertain whether ivermectin increases or reduces serious adverse events up to 28 days compared to placebo or standard of care (RR 1.55, 95% CI 0.07 to 35.89; 2 studies, 197 participants). The other study with high risk of bias was included in a secondary analysis (Analysis 1.8) (Ahmed 2020). The effect estimate of the secondary analysis is identical to the primary analysis as the study contributed no events (RR 1.55, 95% CI 0.07 to 35.89; 3 studies, 242 participants). The two studies included in the primary analysis were published as preprint articles (Krolewiecki 2020; Mohan 2021).
1.7. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 7: Serious adverse events within 28 days (primary analysis)
1.8. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 8: Serious adverse events within 28 days (secondary analysis)
Quality of life up to 28 days
No study reported data for quality of life up to 28 days.
Admission to intensive care unit
Two studies comparing ivermectin to placebo or standard of care reported the number of participants who were admitted to ICU at 28 days for participants with moderate disease (Kirti 2021; Pott‐Junior 2021). Both studies with 143 participants were included in the primary analysis due the overall risk of bias assessment (Analysis 1.9). We are uncertain whether ivermectin increases or reduces the number of participants who were admitted to the ICU at 28 days compared to placebo or standard of care (RR 0.53, 95% CI 0.11 to 2.51; 2 studies, 143 participants). One study was published as a preprint article (Kirti 2021), the other was published as journal publication (Pott‐Junior 2021). The sensitivity analysis including only the study published in a journal estimated the intervention effect at RR 0.15 (95% CI 0.01 to 1.93; 1 study, 31 participants).
1.9. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 9: Admission to intensive care unit (primary analysis)
Duration of hospitalization
Two studies comparing ivermectin to placebo reported duration of hospitalization (Ahmed 2020; Gonzalez 2021). One of these studies reported data as median with IQR on duration of hospitalization in 73 participants with moderate disease (Gonzalez 2021). The median duration of hospitalization in the ivermectin group was six days (IQR 4 to 11 days) compared to five days (IQR 4 to 7 days) in the placebo group. Ahmed 2020, investigating 45 participants with moderate disease, was included in the primary meta‐analysis due to the overall risk of bias assessment and reporting data as means with SDs (Analysis 1.10). Ivermectin may have little or no effect on duration of hospitalization compared to placebo (MD −0.10 days, 95% CI −2.43 to 2.23; 1 study, 45 participants; low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and one level for serious imprecision due to few participants. Ahmed 2020 was published as a journal article and Gonzalez 2021 was published as a preprint article.
1.10. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 10: Duration of hospitalization (primary analysis)
Viral clearance at three days
Four studies comparing ivermectin to placebo or standard of care reported viral clearance at three days in 288 participants with moderate disease (Ahmed 2020; Kishoria 2020; Mohan 2021; Shah Bukhari 2021). Two of the studies with 170 participants were included in the primary analysis due to the overall risk of bias assessment (Analysis 1.11) (Ahmed 2020; Mohan 2021). We are uncertain whether ivermectin increases or reduces viral clearance at three days compared to placebo (RR 1.02, 95% CI 0.45 to 2.32; 2 studies, 170 participants). The other two studies with high risk of bias were included in a secondary analysis (Analysis 1.12) (Kishoria 2020; Shah Bukhari 2021). The point estimate of the secondary analysis favoured ivermectin, but the 95% CI was wide including 1 and heterogeneity was high (RR 1.73, 95% CI 0.59 to 5.04; I2 = 73%; 4 studies, 288 participants). One study included in the primary analysis was published as a preprint article (Mohan 2021), the other study was published as a journal article (Ahmed 2020). The sensitivity analysis including only the study published in a journal estimated the intervention effect at RR 2.09 (95% CI 0.42 to 10.29; 1 study, 45 participants).
1.11. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 11: Viral clearance at 3 days (primary analysis)
1.12. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 12: Viral clearance at 3 days (secondary analysis)
Viral clearance at seven days
Four studies comparing ivermectin to placebo or standard of care reported viral clearance at seven days in 265 participants with moderate disease (Ahmed 2020; Kirti 2021; Mohan 2021; Pott‐Junior 2021). Two of the studies with 159 participants were included in the primary analysis due the overall risk of bias assessment (Analysis 1.13) (Ahmed 2020; Mohan 2021). We are uncertain whether ivermectin increases or reduces viral clearance at seven days compared to placebo (RR 1.82, 95% CI 0.51 to 6.48; 2 studies, 159 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias, one level for serious heterogeneity (I2 = 77%), and two levels for very serious imprecision due to few participants and wide CIs. The other two studies with high risk of bias were included in a secondary analysis (Analysis 1.14) (Kirti 2021; Pott‐Junior 2021). The point estimate of the secondary analysis lay closer to 1 and the 95% CI was wide and included 1 (RR 1.19, 95% CI 0.76 to 1.86; 4 studies, 265 participants). One study included in the primary analysis was published as a preprint article (Mohan 2021), the other study was published as a journal article (Ahmed 2020). The sensitivity analysis including only the study published in a journal favoured ivermectin compared to placebo (RR 3.83, 95% CI 1.23 to 11.93; 1 study, 45 participants).
1.13. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 13: Viral clearance at 7 days (primary analysis)
1.14. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 14: Viral clearance at 7 days (secondary analysis)
Viral clearance at 14 days
Two studies comparing ivermectin to placebo or standard of care reported viral clearance at 14 days in 69 participants with moderate‐to‐severe disease (Ahmed 2020; Okumuş 2021). One of the studies with 45 participants was included in the primary analysis due the overall risk of bias assessment (Analysis 1.15) (Ahmed 2020). Ivermectin may increase viral clearance at 14 days compared to placebo (RR 1.97, 95% CI 1.13 to 3.45; 1 study, 45 participants). The other study with high risk of bias was included in a secondary analysis (Analysis 1.16) (Okumuş 2021). The effect estimate of the secondary analysis was comparable to the primary analysis (RR 2.07, 95% CI 1.28 to 3.33; 2 studies, 69 participants). The study included in the primary analysis was published as a journal article (Ahmed 2020).
1.15. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 15: Viral clearance at 14 days (primary analysis)
1.16. Analysis.

Comparison 1: Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting, Outcome 16: Viral clearance at 14 days (secondary analysis)
Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in an outpatient setting
All‐cause mortality up to 28 days
Two studies comparing ivermectin to placebo reported data on mortality at 21 days (López‐Medina 2021) and at 28 days (Chaccour 2021) for 422 participants with mild disease. Both studies were included in the primary meta‐analysis due to the overall risk of bias assessment (Analysis 2.1). In the meta‐analysis, none of the participants died in the ivermectin group and one participant died in the placebo group. We are uncertain whether ivermectin reduces or increases all‐cause mortality up to 28 days compared to placebo (RR 0.33, 95% CI 0.01 to 8.05; 2 studies, 422 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to few participants, few events, and wide CIs. Both studies were published as journal articles.
2.1. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 1: All‐cause mortality up to 28 days (primary analysis)
Development of moderate‐to‐severe clinical COVID‐19 symptoms – worsening of clinical status
Need for invasive mechanical ventilation, or non‐invasive mechanical ventilation or high flow
Need for invasive mechanical ventilation up to 14 days
One study comparing ivermectin to placebo reported data on clinical worsening assessed by need for invasive mechanical ventilation at 15 days in 398 participants with mild disease (López‐Medina 2021). The study was eligible for the primary meta‐analysis due to the overall risk of bias assessment (Analysis 2.2). One participant in the ivermectin group and no participants in the placebo group showed clinical worsening. We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for invasive mechanical ventilation compared to placebo up to 14 days (RR 2.97, 95% CI 0.12 to 72.47; 1 study, 398 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to few participants, few events, and wide CIs. The study was published as a journal article.
2.2. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 2: Worsening of clinical status – need for invasive mechanical ventilation up to 14 days (primary analysis)
Need for non‐invasive mechanical ventilation or high flow up to 14 days
One study comparing ivermectin to placebo reported the need for non‐invasive mechanical ventilation or high flow at 15 days in 398 participants with mild disease (López‐Medina 2021). None of the participants in either group required non‐invasive mechanical ventilation or high flow at 15 days. The study was eligible for the primary analysis due to the overall risk of bias assessment (Analysis 2.3). We are uncertain whether ivermectin reduces or increases clinical worsening assessed by the need for non‐invasive mechanical ventilation or high flow at 15 days compared to placebo (RR not estimable; 1 study, 398 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to zero events and few participants. López‐Medina 2021 was published as a journal article.
2.3. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 3: Worsening of clinical status – need for non‐invasive mechanical ventilation or high flow up to 14 days (primary analysis)
Need for hospitalization with or without supplemental oxygen
López‐Medina 2021 reported hospitalization with or without supplemental oxygen. The data were not eligible for meta‐analysis as less than 1% of the participants already had a WHO status of 4 and 5 at baseline. Therefore, these data were not useful to judge clinical worsening.
Need for oxygen by mask or nasal prongs up to 14 days
No study reported data for need for oxygen by mask or nasal prongs up to 14 days.
Need for hospitalization without oxygen therapy up to 14 days
No study reported data for need for hospitalization without oxygen therapy up to 14 days.
Symptom resolution
Number of participants with symptoms resolved up to 14 days
One study comparing ivermectin to placebo reported data on symptom resolution at 15 days in 398 participants with mild disease (López‐Medina 2021). The study was eligible for the primary meta‐analysis due to the overall risk of bias assessment (Analysis 2.4). Ivermectin may have little or no effect on clinical improvement assessed by the number of participants with symptoms resolved up to 14 days (RR 1.04, 95% CI 0.89 to 1.21; 1 study, 398 participants; low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and one level for serious imprecision due to few participants. The study was published as a journal article.
2.4. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 4: Symptom resolution – number of participants with symptoms resolved up to 14 days (primary analysis)
Chachar 2020 reported symptom resolution within seven days, which was too early and clinically not comparable to our predefined time point of 14 days.
Duration of symptom resolution
Two studies reported data on duration of symptom resolution (López‐Medina 2021; Podder 2020). One studies comparing ivermectin to placebo reported data as median with IQR on duration of symptom resolution in 398 participants with mild disease (López‐Medina 2021). The median duration of symptom resolution in the ivermectin group was 10 days (IQR 9 to 13 days) compared to 12 days (IQR 9 to 13 days) in the placebo group. The study was not eligible for meta‐analysis due to an asymmetric distribution of the data. The other study comparing ivermectin to standard of care reported data as means with SDs on duration to symptom resolution in 62 participants with mild disease (Podder 2020). The study was not eligible for a primary analysis due to the overall risk of bias assessment. This study with high risk of bias was analyzed in a secondary analysis (Analysis 2.5). The effect estimate of the secondary analysis showed no clinically relevant difference in the duration to symptom resolution (MD −1.02 days, 95% CI −2.76 to 0.72; 1 study, 62 participants). Both studies were published as journal articles.
2.5. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 5: Duration to symptom resolution (secondary analysis)
Admission to hospital
No study reported data for admission to hospital.
Any adverse events within 28 days
Two studies comparing ivermectin to placebo reported any adverse events within 21 days (López‐Medina 2021) and 28 days (Chaccour 2021) in 422 participants with mild disease. Both studies were included in the primary analysis due the overall risk of bias assessment (Analysis 2.6). Ivermectin may have little or no effect on any adverse events compared to placebo (RR 0.95, 95% CI 0.86 to 1.05; 2 studies, 422 participants; low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and one level for serious imprecision due to few participants. Both studies were published as journal articles.
2.6. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 6: Any adverse events within 28 days (primary analysis)
Chachar 2020 reported adverse events within seven days, which was not eligible for meta‐analysis due to the short follow‐up period.
Serious adverse events within 28 days
Two studies comparing ivermectin to placebo reported serious adverse events within 21 days (López‐Medina 2021) and 28 days (Chaccour 2021) in 422 participants with mild disease. Both studies were included in the primary analysis due the overall risk of bias assessment (Analysis 2.7). Chaccour 2021 reported zero events in both groups. We are uncertain whether ivermectin increases or reduce serious adverse events within 28 days compared to placebo (RR 0.99, 95% CI 0.14 to 6.96; 2 studies, 422 participants). Both studies were published as journal articles.
2.7. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 7: Serious adverse events within 28 days (primary analysis)
Quality of life up to 14 days
No study reported data for quality of life up to 14 days.
Viral clearance at three days
No study reported data for viral clearance at three days.
Viral clearance at seven days
One study comparing ivermectin to placebo reported viral clearance at seven days in 24 participants with mild disease (Chaccour 2021). The study was eligible for the primary analysis due the overall risk of bias assessment (Analysis 2.8). We are uncertain whether ivermectin increases or reduces viral clearance at seven days compared to placebo (RR 3.00, 95% CI 0.13 to 67.06; 1 study, 24 participants; low‐certainty evidence). We downgraded the certainty of evidence two levels for very serious imprecision due to few participants, few events, and wide CIs. The study was published as a journal article.
2.8. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 8: Viral clearance at 7 days (primary analysis)
Viral clearance at 14 days
One study comparing ivermectin to standard of care reported viral clearance at 14 days in 40 participants with mild disease (Podder 2020). The study was not eligible for the primary analysis due the overall risk of bias assessment. The data of Podder 2020 with high risk of bias were included in a secondary analysis (Analysis 2.9). Ivermectin may have no effect on viral clearance at 14 days compared to standard of care (RR 0.95, 95% CI 0.79 to 1.13; 1 study, 40 participants). The study was published as a journal article.
2.9. Analysis.

Comparison 2: Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting, Outcome 9: Viral clearance at 14 days (secondary analysis)
Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection
SARS‐CoV‐2 infection (confirmed by RT‐PCR or antigen testing) at 14 days
No study reported data for SARS‐CoV‐2 infection (confirmed by RT‐PCR or antigen testing) at 14 days.
Development of clinical COVID‐19 symptoms up to 14 days
One study comparing ivermectin to no treatment reported the development of clinical COVID‐19 symptoms at 14 days in 304 asymptomatic participants with household close contacts to confirmed COVID‐19 index case (Shoumann 2021). The study result was not eligible for the primary analysis due to the overall risk of bias assessment. The data of Shoumann 2021 with high risk of bias were included in a secondary analysis (Analysis 3.1). Ivermectin may reduce the development of clinical COVID‐19 symptoms in participants in contact with confirmed COVID‐19 index cases compared to no treatment (RR 0.13, 95% CI 0.08 to 0.21; 1 study, 304 participants). The study was published as a journal article.
3.1. Analysis.

Comparison 3: Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection, Outcome 1: Development of clinical COVID‐19 symptoms (secondary analysis)
Any adverse events within 14 days
One study comparing ivermectin to no treatment reported any adverse events within 14 days in 304 asymptomatic participants with household close contacts to confirmed COVID‐19 index case n (Shoumann 2021). The study result was not eligible for the primary analysis due to the overall risk of bias assessment. The data of Shoumann 2021 with high risk of bias were included in a secondary analysis (Analysis 3.2). We are uncertain whether ivermectin increases or reduces any adverse events in participants in contact with confirmed COVID‐19 index cases compared to no treatment (RR 11.50, 95% CI 0.68 to 193.21; 1 study, 304 participants). The study was published as a journal article.
3.2. Analysis.

Comparison 3: Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection, Outcome 2: Any adverse events within 14 days (secondary analysis)
All‐cause mortality up to 28 days
One study comparing ivermectin to no treatment reported mortality within 14 days in 304 asymptomatic participants with household close contacts to confirmed COVID‐19 index case (Shoumann 2021). The study result was included in the primary meta‐analysis due to the overall risk of bias assessment (Analysis 3.3). We are uncertain whether ivermectin reduces or increases mortality up to 28 days compared to no treatment as none of the participants in either group died (RR not estimable; 1 study, 304 participants; very low‐certainty evidence). We downgraded the certainty of evidence one level for serious risk of bias and two levels for very serious imprecision due to zero events and few participants. The study was published as a journal article.
3.3. Analysis.

Comparison 3: Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection, Outcome 3: All‐cause mortality up to 28 days (primary analysis)
Admission to hospital
No study reported data for admission to hospital.
Quality of life up to 14 days
No study reported data for quality of life up to 14 days.
Discussion
Summary of main results
This review included 14 studies with 1678 participants investigating ivermectin compared to placebo or standard of care. With intention to treat COVID‐19, nine studies were conducted in inpatient settings with mainly moderate COVID‐19 (WHO 4 to 5) and four studies in outpatient settings with mild COVID‐19 (WHO 2 to 3). One study investigated ivermectin for the prevention of SARS‐CoV‐2 infection. The included studies contributed 41 study results to the review of which about one third were assessed at overall high risk of bias. The main findings of this review are summarized in Table 1 (treatment; inpatients), Table 2 (treatment; outpatients), and Table 3 (prevention). The number of studies per outcome was low. Only one or two studies per outcome provided useful data for our prioritized outcomes included in the summary of findings tables.
Ivermectin showed no evidence of an effect on increasing or decreasing mortality at 28 days, the most important outcome during this pandemic, neither in inpatients (two studies), outpatients (two studies), or the preventive setting (one study). The certainty for this finding was very low. The same accounts for clinical worsening up to 28 days in an inpatient setting and up to 14 days in an outpatient setting.
With regard to clinical improvement, ivermectin may have little or no effect compared to placebo or standard of care on clinical improvement up to 28 days and duration of hospitalizations in an inpatient setting. For outpatients, ivermectin may have little or no effect on the number of participants with symptoms resolved up to 14 days. Based on very low certainty evidence, there was no significant increase in viral clearance at seven days in participants treated with ivermectin in inpatient settings and based on low‐certainty evidence for outpatient settings. For adverse events, ivermectin may have little or no difference on occurrence of adverse events within 14 days in an outpatient setting, while we are uncertain about the effect of ivermectin on adverse events within 28 days in an inpatient setting.
The most significant outcome for participants not infected but at high risk of developing the infection following high‐risk exposure was development of confirmed SARS‐CoV‐2 infection, which no studies reported. One study reported development of clinical COVID‐19 symptoms, which we could not include in the primary analysis due to high risk of bias. The same accounted for adverse events within 14 days.
Overall completeness and applicability of evidence
Nine of 14 included studies were conducted in inpatient settings. However, participants mostly had moderate‐severity COVID‐19. Only one study included participants with severe COVID‐19 requiring mechanical ventilation at baseline (Okumuş 2021). Therefore, the findings of this review are transferable to inpatients with moderate COVID‐19 only. Four of 14 included studies were conducted in an outpatient setting with mild COVID‐19 symptoms, though only two studies reported relevant outcomes and also had overall low risk or some concerns of bias (Chaccour 2021; López‐Medina 2021). Based on the eligible study pool, there is currently no evidence available for the use of ivermectin in severely ill people with COVID‐19 or those at high risk of disease progression. Most studies reported a mean age far below 60 years (overall mean age was 43 years with a mean range from 28 to 62 years) and included people with very few or no comorbidities (e.g. obesity). This major risk factor for severe COVID‐19, was only reported in three studies (Chachar 2020; Krolewiecki 2020; López‐Medina 2021). Considering age and pre‐existing conditions as the most important risk factors for developing severe COVID‐19, the current evidence is not applicable to patients who are at most risk of death from COVID‐19.
Hence, for outpatients as well as for inpatients we are still in need of good‐quality trials in relevant populations to obtain evidence that would justify the use of ivermectin in regular patient care. In June 2021, the PRINCIPLE trialists announced inclusion of ivermectin in their platform trial, which might provide us with applicable data that helps to complete evidence for outpatient treatment (PRINCIPLE trial).
Only 1 of 14 included studies investigated the potential of ivermectin for prevention of SARS‐CoV‐2 infection in people after high‐risk exposure. The study did not report results free of high risk of bias for one of the primary outcomes of interest for this review. Therefore, it is currently unclear whether ivermectin can prevent SARS‐CoV‐2 infection in people who have had a high‐risk contact.
Seven studies were conducted in Asia, four studies in South America, two in Europe, and one in Africa. In some countries where studies were conducted, uncontrolled ivermectin use is making it difficult to test the effectiveness of the antiparasite drug against SARS‐CoV‐2 (Rodríguez‐Mega 2020). With Chaccour 2021, only one study was conducted in a country with high healthcare expenditure.
All studies administered ivermectin per mouth, but the doses and durations of administration varied. We set 200 µg/kg orally per day as the low dose based on the dosing recommendation for strongyloidiasis (WHO 2019). Four of the 14 studies used low doses in at least one study arm. All other studies utilized higher doses either in a single dose or over two to five days. Due to the small number of studies per outcome, we did not perform any subgroup analyses with low versus high doses and no evidence or clinical implication can be obtained regarding a certain dosing regimen.
We found no studies that compared ivermectin to an active comparator with confirmed efficacy such as dexamethasone. Eight of the 14 studies had an open‐label design and used no treatment or standard of care as comparators. Six studies were placebo‐controlled studies. Standard of care must be comparable between the studies' arms. There are several studies circulating that investigate various concomitant medications (e.g. doxycycline, hydroxychloroquine, azithromycin, zinc) in addition to ivermectin. Due to unproven efficacy and possible adverse effects, these comparisons may confound the assessment of the efficacy or safety of ivermectin, and we considered the inclusion of such combination therapies inappropriate. The same accounts for the comparison of ivermectin with an active comparator that has no proven efficacy in COVID‐19. Although those types of interventions (e.g. hydroxychloroquine) were possibly used at a certain point of the pandemic with the best intentions, their use was never supported by actual evidence, and they have potential adverse effects (Singh 2021). As we do not know the effect of many of those experimental comparators in people with COVID‐19, consequently no reliable evidence for ivermectin can be obtained from those comparisons either.
Although 14 studies were eligible for the review questions, primary outcomes for studies with intention to treat COVID‐19, as defined by the review, were only reported by a minority of studies. For some outcomes, different time points of outcome assessment or different outcome definitions prevented clinically useful pooling of the study results. Clinical worsening and improvement were heterogeneously reported, including outcomes that represent competing risks. Few studies followed the WHO Clinical Progression Scale (Marshall 2020). One study reported improvement and worsening of clinical status in an inpatient setting as the number of 'patients discharged without respiratory deterioration or death at 28 days' and as 'patients with respiratory deterioration or death at 28 days' (Gonzalez 2021). If reported at the same day, both outcomes give useful information on the clinical status of the study population in both directions – improvement and worsening – without containing competing risks. The outcome 'patients discharged without respiratory deterioration or death at 28 days' was deemed to be clinically useful and was added as a new primary outcome during preparation of this review. With further studies reporting these two very precise and unambiguous endpoints, evidence on ivermectin becomes more complete and patient‐relevant.
Finally, 31 studies are ongoing and 18 studies are awaiting classification due to an unpublished status or requiring clarification due to inconsistencies. When the studies are published or inconsistencies are clarified by study authors via personal communication, we will include them in a review update and conclusions of this review may change. Especially the most recently registered trials are proposing much larger numbers of participants than those of published trials so far. With group sizes ranging within the thousands, those trials may help to increase the certainty of evidence on the efficacy and safety of ivermectin. So far, we included only one study with intention to prevent SARS‐CoV‐2 infection. We identified several ongoing and unpublished studies focusing on this rationale. We are awaiting publication of those results to close the current gaps in the evidence on ivermectin used in postexposure prophylaxis.
Quality of the evidence
The certainty of evidence for prioritized outcomes presented in the summary of findings tables ranged from very low to low (Table 1; Table 2; Table 3).
For the summary of findings and assessment of the certainty of the evidence according to Schünemann 2020, we used the results from the primary meta‐analyses. Primary meta‐analyses included only studies with low risk or some concerns of bias. We excluded studies at high risk of bias for their respective outcome and only analyzed them in secondary analyses to test the robustness of the results. One third of the study results were at overall high risk of bias. Most study results had some concerns for risk of bias. Two studies contributing five study results to four outcomes, including all‐cause mortality, adverse events, and viral clearance at three and seven days, were at overall low risk of bias (Chaccour 2021; Mohan 2021). For the summary of findings, the certainty of evidence was downgraded one level due to serious risk of bias because most of the results were assessed as 'some concerns' of bias. Details of the risk of bias assessments per outcome are reported in Risk of bias in included studies. We could only include one study with overall low risk of bias on viral clearance at day seven in an outpatient setting in the primary analysis (Chaccour 2021). The certainty of evidence was not downgraded for study limitations. Nevertheless, this effect estimate was associated with high uncertainty based on the low number of participants and few events.
Another limitation for the certainty of evidence was the low number of participants, or events, or both, leading to wide CIs and high uncertainty of the estimated effects. All outcomes included in the summary of findings tables were downgraded one or two levels for imprecision.
Heterogeneity was rarely a reason to downgrade the certainty of evidence. This is mainly due to the small number of studies per meta‐analysis. The only outcome with high heterogeneity (I2 = 77%) included in the summary of findings tables was viral clearance at day seven in an inpatient setting. Two studies with conflicting results, one favouring ivermectin (Ahmed 2020), and one showing no important difference between ivermectin and placebo (Mohan 2021), caused the high statistical heterogeneity.
We did not downgrade any of the outcomes included in the summary of findings tables for indirectness. In all cases, the effect estimates were based on comparisons of interest, on the population of interest, and on outcomes of interest.
In the current phase of the pandemic, it is impossible to reliably assess the risk of publication bias. Most of the registered studies are still ongoing or, in the case of a completed study status, their results have not yet been published. We will follow the publication and trial history of each ongoing study and study awaiting classification. Currently, we did not suspect publication bias for any outcome included in this review. However, this may change in updates of this review.
Potential biases in the review process
This review aimed to provide a complete evidence profile for ivermectin with regard to efficacy and safety for postexposure prophylaxis and treatment of COVID‐19 based on current Cochrane standards (Higgins 2020a).
The review team is part of the German research project 'CEOsys' (COVID‐19 Evidence‐Ecosystem). CEOsys is a consortium of clinical and methodological experts supported by the German Federal Ministry of Education and Research to synthesize clinical evidence during this global pandemic. The involved medical information specialists of this consortium carried out a rigorous search of electronic databases including preprint servers and clinical trial registries to identify the complete extent of published and ongoing trials on this topic. Additionally, we compared our search results with those from 'living' meta‐analysis and reviews (COVID‐NMA Working Group; ivmmeta.com). Therefore, we are confident that we identified all relevant studies and are monitoring ongoing studies as well as full publication of preprints closely after the publication of this review.
Five studies were preprint articles. We are aware that articles may change following peer‐review. Nevertheless, we are convinced that including all eligible data in a highly dynamic situation such as the COVID‐19 pandemic is crucial to be up‐to‐date and to provide timely information on potentially promising treatment options. Journal publications and corresponding preprint articles were compared in terms of consistency and all study results were assessed for their risk of bias.
The immense amount of ongoing RCTs reflects the persistent lack of clarity on this intervention and the need for an update of this review. It should be considered that conclusions of the updated version differ from those of the present review. Review updates may allow for a more concise judgement of the effectiveness and the safety of ivermectin for treatment and prevention of COVID‐19.
To minimise errors in screening, data extraction, and risk of bias assessment, two review authors independently conducted all processes. Analyses were conducted by one review author and checked by a second review author. We provided reasons for the exclusion of studies from this systematic review and described each included study in full detail and made explicit judgements on individual risk of bias.
We contacted study authors if the publication included unclear or inconclusive information or in case of missing information. Unfortunately, not all attempts of gathering data were successful. Details of the communication with authors are provided in the Characteristics of included studies table.
For three studies that had already published results, we could not finally judge eligibility due to inconsistencies in their study design description (Faisal 2020; NCT04407507; Samaha 2021). We contacted the corresponding authors to clarify those questions, though we have not received any satisfying response at the time of review publication. Another 15 trials classified as awaiting classification have not yet published results appropriately. We will monitor trials that have completed recruitment according to the trial registry closely for publication in the near future.
None of the members of the review author team has any affiliation with any stakeholder group who favours or disapproves of ivermectin or the comparators used in relevant studies.
Agreements and disagreements with other studies or reviews
Numerous reviews have been conducted investigating the efficacy of ivermectin for the treatment and prophylaxis of COVID‐19 with inconsistent results in meta‐analyses and conclusions, in many cases conflicting with our findings. Conflicts are mainly due to inclusion of studies investigating active comparators with unproven efficacy (e.g. hydroxychloroquine), pooling of studies with active and inactive comparators, different definitions of outcomes or outcomes assessment times, and different interpretations of the certainty of evidence.
In this context, two groups are especially worth a mention, the Front Line COVID‐19 Critical Care Alliance (FLCCC) and the British Ivermectin Recommendation Development (BIRD) group, which were, to some extent, founded and supported by the same scientists. Both groups and individual group associates conducted various systematic reviews and meta‐analyses, all with conclusions strongly in favour of the effectiveness of ivermectin for treatment and prevention of COVID‐19 (BIRD 2021; Bryant 2021; Kory 2021). Additionally, there is an online and regularly updated analysis of published and emerging trials available (ivmmeta.com), postulating a strong beneficial effect of ivermectin for people with COVID‐19. The website does not provide authorship details, though states the FLCCC and BIRD as its resources. Hill and colleagues published another large systematic review in favour of ivermectin (Hill 2021). Main findings of the reviews and disagreements to our findings are briefly summarized in the following paragraphs.
Hill 2021 identified 18 RCTs up to December 2020 and was a preprint article. The author team used the Cochrane Risk of Bias tool 1 for critical appraisal. However, the certainty of evidence was not assessed. Six RCTs investigating people with moderate‐to‐severe COVID‐19 were pooled for the meta‐analysis on mortality with a benefit of 75% for ivermectin (RR 0.25, 95% CI 0.12 to 0.52). Hill 2021 compared ivermectin alone or in combination with doxycycline to control interventions, including placebo, standard of care, or hydroxychloroquine, and pooled all in one comparison. We did not include five of the six studies in our meta‐analysis on mortality of people with moderate COVID‐19, including Elgazzar 2020, Hashim 2020, Mahmud 2021, Niaee 2020, and Okumuş 2021. Hashim 2020 and Mahmud 2021 combined ivermectin with doxycycline, which makes it impossible to isolate any potential effect to the individual drugs used. Elgazzar 2020 compared ivermectin to hydroxychloroquine. The latter is not effective for the treatment of COVID‐19 and has resulted in clinical adverse effects (Singh 2021). We did not consider hydroxychloroquine an eligible comparator to investigate the efficacy and safety profile of ivermectin for the treatment of COVID‐19. Niaee 2020 included a population of about 30% of people who were SARS‐CoV‐2‐negative, which we did not consider appropriate to investigate SARS‐CoV‐2‐specific antiviral effect of ivermectin. Okumuş 2021 compared ivermectin to an eligible comparator and investigated an eligible population, but reported mortality at an ineligible time point (e.g. on average three months). Finally, there is only one small study with broad CIs and high uncertainty for a mortality benefit (RR 0.12, 95% CI 0.01 to 2.09), which is also included in our meta‐analysis on mortality of people with moderate COVID‐19 (Kirti 2021). However, we included mortality at day 28 rather than in‐hospital mortality, which was different in one participant who died in the control group.
Kory 2021 identified seven RCTs on the efficacy of ivermectin in outpatients with mild COVID‐19 and six RCTs in hospitalized people with COVID‐19. The review was published in the American Journal of Therapeutics and did not provide any search date or other methodological details used for meta‐analyses. Kory 2021 concluded there was a mortality benefit based on the inclusion of six of the 13 studies (odds ratio (OR) 0.13, 95% CI 0.07 to 0.28), which was not a valid inclusion because Elgazzar 2020, Hashim 2020, Mahmud 2021, and Niaee 2020 were not eligible for the reasons described above, and Cadegiani 2020 was not an RCT. As described for Hill 2021, there remains only one small study (Kirti 2021), and a high degree of uncertainty for a mortality benefit.
The most recent systematic review with meta‐analysis was published by Bryant 2021 in the American Journal of Therapeutics (same journal as Kory 2021). The review stated they followed the Cochrane's rapid review template and had a review protocol that was not registered on an appropriate register (e.g. PROSPERO). The author team used the Cochrane Risk of Bias tool 1 for critical appraisal and GRADE to assess the certainty of evidence. A meta‐analysis of 15 trials found that ivermectin reduced the risk of death by an average of 62% compared with no ivermectin treatment (RR 0.38, 95% CI 0.19 to 0.73). The certainty of evidence was moderate due to study design limitations. The author team conducted several sensitivity analyses excluding outlier studies, studies at high risk of bias, and studies with active comparators. The effect estimates remained robust. However, even the sensitivity analysis excluding studies with active comparators, which was the most comparable analysis to our analysis on mortality, based their conclusion on studies that did not meet eligibility criteria for this current Cochrane Review (RR 0.41, 95% CI 0.23 to 0.74). Hashim 2020 and Mahmud 2021 combined ivermectin with doxycycline. Niaee 2020 and Rezai 2020 (Shahbaznejad 2021) included a mixed population with about 30% (Niaee 2020) and 75% (Rezai 2020 (Shahbaznejad 2021)) of participants with negative SARS‐CoV‐2 PCR tests. The registry entry of Petkov 2020 (2020‐002091‐12/BG) was eligible for our review, though there is no scientific publication of results except a press release on the manufacturer's website. Okumuş 2021 and Mohan 2021 reported mortality at an ineligible time point and Ahmed 2020 did not report mortality in the journal publication. Finally, the four remaining studies were included in our meta‐analyses for outpatients (Chaccour 2021; López‐Medina 2021) and inpatients (Gonzalez 2021; Kirti 2021) with COVID‐19. All studies had broad CIs with a high uncertainty for a mortality benefit. Moreover, Bryant 2021 used a trial sequential analysis to test whether there was sufficient evidence to detect or reject intervention effects and to address imprecision of the effect estimates in this way. They concluded that there may have been sufficient evidence accrued before the end of 2020 to show a significant benefit of ivermectin over control for all‐cause mortality. However, trial sequential analysis cannot adjust for risk of bias or wrong comparators. Therefore, inclusion of all trials in this context into the analysis does not yield reliable results.
Even if not eligible for the Cochrane Review, two studies in Bryant 2021 and all the other meta‐analyses were notable because of the size of the effect reported and the narrow CIs: Elgazzar 2020 and Niaee 2020 help drive the large effects seen in the random‐effects analysis. Elgazzar 2020, for example, reported among people with severe disease two deaths out of 100 in the ivermectin group and 20 deaths out of 100 in the chloroquine group; and Niaee 2020 reported two deaths out of 100 in the ivermectin group and 11 deaths out of 60 in the control group. These effect sizes are extreme. A recent press release claimed that the large trial by Elgazzar 2020 showed clear signs of fraudulence and should be withdrawn over ethical concerns (The Guardian 2021). Research Square withdrew this preprint on 14 July 2021 due to an expression of concern (Elgazzar 2020).
The website ivmmeta.com provides several meta‐analyses of pooled effects including up to 60 studies. This website shows pooled estimates suggesting significant benefits with ivermectin, which has resulted in confusion for clinicians, patients, and decision‐makers (Garegnani 2021). The analyses are misleading and have several limitations. As described for the other reviews, several ineligible interventions and comparators were pooled. Additionally, different outcomes were pooled and reported as percentage improvement with ivermectin studied in RCTs ranging from 40% improvement when used as late treatment to 83% improvement when used as prophylaxis. However, there is no full prospective protocol available describing the relevant review methodology, and there is no assessment of the risk of bias or the certainty of evidence.
National and international guidelines regarding the use of ivermectin for the treatment or prevention of COVID‐19 have been developed over the past 12 months. Recommendations from the WHO, updated 31 March 2021 (WHO 2021b); European Medicines Agency, updated 22 March 2021 (EMA 2021); Infectious Diseases Society of America, updated 13 February 2021 (IDSA 2021); and the COVID Management Guidelines India Group, updated 15 May 2021 (COVID Guidelines India 2021), concur that ivermectin should only be used for treatment of COVID‐19 in the context of clinical trials. The EMA additionally advises against the use of ivermectin for prophylaxis outside RCTs. (EMA 2021). The US NIH guidance updated on 11 February 2021 describes 'insufficient data' to permit a recommendation for or against the use of ivermectin for the treatment of COVID‐19 (NIH 2021). One statement in February 2021 by Merck, a manufacturer of ivermectin, describes the conclusions of their review of the evidence as providing "no meaningful evidence for clinical activity or efficacy in patients with COVID‐19" (Merck 2021).
Authors' conclusions
Implications for practice.
Based on the current very low‐ to low‐certainty evidence, we are uncertain about the efficacy and safety of ivermectin used to treat people with COVID‐19 in the inpatient and outpatient settings and to prevent a SARS‐CoV‐2 infection in people after having high‐risk exposure. There is also no evidence available from the study pool as to which is the best dose and regimen of ivermectin. Overall, the reliable evidence available does not support the use of ivermectin for treatment or prevention of COVID‐19 outside of well‐designed randomized controlled trials (RCTs). With respect to the number of identified studies in trial registries and with accordance to the living approach of this review, we will continually update our search and include eligible trials.
Implications for research.
There remains insufficient evidence regarding the efficacy and safety of ivermectin used for the treatment of people with COVID‐19 in the inpatient and outpatient settings and to prevent SARS‐CoV‐2 infection in people after high‐risk exposure. Based on our review, we define the following gaps in the evidence.
High‐quality RCTs: double‐blind, placebo‐controlled, randomized studies with sufficient power and conducted in accordance to the CONSORT 2010 Statement.
Reporting of patient‐relevant outcomes with clear definition and relevant time points of outcome measurement (see Types of outcome measures).
Complete and transparent reporting of participants' characteristics and patient status according to World Health Organization Clinical Progression Scale (Marshall 2020).
Studies including people with severe COVID‐19.
Dose‐finding studies.
Currently, there is an urgent need for good‐quality evidence, based on RCTs with appropriate randomization procedures, comparability of study arms, and a preferably double‐blind design. We identified 31 ongoing RCTs in trial registries and another 18 studies which are potentially eligible but have either not published their results yet or where we require additional clarifications from the study investigators. The findings from these studies may help to answer more clearly the question of ivermectin and its effects in treating and preventing COVID‐19 in the future. In accordance with the living approach of this review, we will continually update our search and include eligible trials.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2021 | Amended | Minor edit to review group order |
History
Protocol first published: Issue 4, 2021 Review first published: Issue 7, 2021
| Date | Event | Description |
|---|---|---|
| 17 August 2021 | Amended | Corrected minor typographical errors; the minor corrections have not changed the review findings |
Risk of bias
Risk of bias for analysis 1.1 All‐cause mortality up to 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.1.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Gonzalez 2021 | Some concerns | Computer‐generated randomization. There was no information on allocation concealment. Baseline details differed between groups. Due to small group sizes and stratification according to QT prolongation the deviations may be caused by chance. | Some concerns | Participants were not aware of the intervention received. No information on those delivering the intervention. No information reported whether there were deviations from the intended interventions or not. 2/108 participants were excluded from analysis due to transfer to another hospital (mITT). | Low risk of bias | Most people were followed up >95%. 2/108 participants were excluded from analysis due to transfer to another hospital. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. But knowledge of intervention received could not have affected outcome measurement. | Some concerns | The protocol was prospectively registered and the outcome was registered. The time point of outcome measurement was not defined. | Some concerns | Due to insufficient information on allocation concealment and blinding of health care providers. Due to lack of defining the time point of outcome measurement in the protocol. |
| Kirti 2021 | Low risk of bias | Randomization was performed by an independent person not part of the investigating team using a computer‐based program. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. 1/57 participants in the intervention group and 1/58 participants in the control group received ivermectin by the treating team. Both participants were excluded (per protocol analysis). The analysis was not appropriate, but it is unlikely to have an impact on the result. | Low risk of bias | Most people were followed up >95%. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 1.2 Worsening of clinical status – need for invasive mechanical ventilation up to 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.2.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Gonzalez 2021 | Some concerns | Computer‐generated randomization. There was no information on allocation concealment. Baseline details differed between groups. Due to small group sizes and stratification according to QT prolongation the deviations may be caused by chance. | Some concerns | Participants were not aware of the intervention received. No information on those delivering the intervention. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate (mITT). | Low risk of bias | Most people were followed up >95%. 2/108 participants were excluded from analysis due to transfer to another hospital. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Some concerns | The protocol was prospectively registered and the outcome was registered. The time point of outcome measurement was not defined. | Some concerns | Due to insufficient information on allocation concealment and blinding of health care providers. Due to lack of defining the time point of outcome measurement in the protocol. |
| Kirti 2021 | Low risk of bias | Randomization was performed by an independent person not part of the investigating team using a computer‐based program. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. 1/57 participants in the intervention group and 1/58 participants in the control group received ivermectin by the treating team. Both participants were excluded (per protocol analysis). The analysis was not appropriate, but it is unlikely to have an impact on the result. | Low risk of bias | Most people were followed up >95%. | Low risk of bias | Outcome assessors were not aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 1.3 Worsening of clinical status – need for oxygen up to 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.3.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
Risk of bias for analysis 1.4 Improvement of clinical status – participants discharged without respiratory deterioration or death at 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.4.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Gonzalez 2021 | Some concerns | Computer‐generated randomization. There was no information on allocation concealment. Baseline details differed between groups. Due to small group sizes and stratification according to QT prolongation the deviations may be caused by chance. | Some concerns | Participants were not aware of the intervention received. No information on those delivering the intervention. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate (mITT). | Low risk of bias | Most people were followed up >95%. 2/108 participants were excluded from analysis due to transfer to another hospital. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified in the study protocol. | Some concerns | Due to insufficient information on allocation concealment and blinding of health care providers. Due to lack of registering the outcome in the protocol. |
Risk of bias for analysis 1.5 Any adverse events within 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.5.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Most people were followed up >95%. Five participants withdrew consent. | Some concerns | Outcome assessors were not aware of the intervention received. There was insufficient information on definition and measurement of the outcome. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | Some concerns | Due to insufficient information on outcome definition and measurement of the outcome, and lack of prospectively registering the outcome. |
Risk of bias for analysis 1.6 Any adverse events within 28 days (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.6.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Krolewiecki 2020 | Low risk of bias | Centralized web‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. The reason for withdrawals and changing treatments/ withdrawal are consistent with routine care. The analysis was appropriate (ITT). | Low risk of bias | Data for this outcome were available for all randomized participants. | High risk of bias | Outcome assessors were aware of the intervention received. There was insufficient information on definition and measurement of the outcome. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | High risk of bias | Due to lack of information on definition and measurement of the outcome and lack of blinding of outcome assessors. |
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Most people were followed up >95%. Five participants withdrew consent. | Some concerns | Outcome assessors were not aware of the intervention received. There was insufficient information on definition and measurement of the outcome. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | Some concerns | Due to insufficient information on outcome definition and measurement of the outcome, and lack of prospectively registering the outcome. |
| Pott‐Junior 2021 | Low risk of bias | Random sequence was generated by a computer‐based program. Allocation assignment was concealed from investigators and patients using sequentially numbered sealed opaque envelopes. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. None of the patients discontinued the assigned intervention. The analysis was appropriate (mITT). | Low risk of bias | Most people were followed up >90%. One participant dropped out. | High risk of bias | Outcome assessors were aware of the intervention received. There was insufficient information on measurement of the outcome. Knowledge of intervention received could have affected outcome measurement. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | High risk of bias | Due to lack of information on definition and measurement of the outcome and lack of blinding of outcome assessors. Due to lack of prospectively registering the outcome. |
| Shah Bukhari 2021 | Some concerns | There was no information on allocation concealment. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | High risk of bias | 14% of participants dropped out (9/50 intervention and 5/50 control). Some participants left against medical advice before completion of the study. Reasons for dropping out could be related to participants' health status. There is no analysis to look at the effect of the missing data. | High risk of bias | Outcome assessors were aware of the intervention received. There was no information on definition and measurement of adverse events. Knowledge of intervention received could have affected outcome measurement. | Some concerns | Protocol was registered during the recruitment period. The outcome was not pre‐specified. | High risk of bias | Due to missing outcome data, lack of information on definition and measurement of the outcome, and lack of blinding of participants, health care providers, and outcome assessors. Due to insufficient information on allocation concealment and lack of preregistering the outcome. |
Risk of bias for analysis 1.7 Serious adverse events within 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.7.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Krolewiecki 2020 | Low risk of bias | Centralized web‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. The reason for withdrawals and changing treatments/ withdrawal are consistent with routine care. The analysis was appropriate (ITT). | Low risk of bias | Data for this outcome were available for all randomized participants. | Some concerns | Outcome assessors were aware of the intervention received. There was insufficient information on definition and measurement of the outcome. Judgement of severity of symptoms could have been influenced by knowledge of the intervention, regarding the results it seems not likely. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | Some concerns | Due to lack of information on definition and measurement of the outcome, lack of blinding of outcome assessors, and lack of prospectively registering the outcome. |
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Most people were followed up >95%. Five participants withdrew consent. | Some concerns | Outcome assessors were not aware of the intervention received. There was insufficient information on definition and measurement of the outcome. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Some concerns | Due to insufficient information on outcome definition and measurement of the outcome. |
Risk of bias for analysis 1.8 Serious adverse events within 28 days (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.8.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | High risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. There was no information on definition and measurement of serious adverse events. | Some concerns | There was no trial register entry and no published protocol. | High risk of bias | Due to lack of information on measurement of the outcome and blinding of outcome assessors. Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
| Krolewiecki 2020 | Low risk of bias | Centralized web‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. 2/30 participants in the intervention group withdrew consent due to adverse events and 1/15 participants in the control group was withdrawn due to initiation of lopinavir. The analysis was appropriate (ITT). | Low risk of bias | Data for this outcome were available for all randomized participants. | Some concerns | Outcome assessors were aware of the intervention received. There was insufficient information on definition and measurement of the outcome. Judgement of severity of symptoms could have been influenced by knowledge of the intervention, regarding the results it seems not likely. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | Some concerns | Due to lack of information on definition and measurement of the outcome, lack of blinding of participants, health care providers, and outcome assessors, and lack of prospectively registering the outcome. |
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Most people were followed up >95%. Five participants withdrew consent. | Some concerns | Outcome assessors were not aware of the intervention received. There was insufficient information on definition and measurement of the outcome. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Some concerns | Due to insufficient information on outcome definition and measurement of the outcome. |
Risk of bias for analysis 1.9 Admission to intensive care unit (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.9.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Kirti 2021 | Low risk of bias | Randomization was performed by an independent person not part of the investigating team using a computer‐based program. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. 1/57 participants in the intervention group and 1/58 participants in the control group received ivermectin by the treating team. Both participants were excluded (per protocol analysis). The analysis was not appropriate, but it is unlikely to have an impact on the result. | Low risk of bias | Most people were followed up >95%. | Low risk of bias | Outcome assessors were not aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
| Pott‐Junior 2021 | Low risk of bias | Random sequence was generated by a computer‐based program. Allocation assignment was concealed from investigators and patients using sequentially numbered sealed opaque envelopes. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received. One participant in the ivermectin group dropped out before receiving the intervention. The analysis was appropriate (mITT). | Low risk of bias | Most people were followed up >90%. One participant dropped out. | Low risk of bias | Outcome assessors were aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | Some concerns | Due to lack of prospectively registering the outcome. |
Risk of bias for analysis 1.10 Duration of hospitalization (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.10.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
Risk of bias for analysis 1.11 Viral clearance at 3 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.11.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Reasons for missing outcome data were described and appropriate in the context of this outcome (only RT‐PCR positive people at baseline). 11/125 participants were no longer hospitalized at day 7 and missing for analysis. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Low risk of bias | Due to low risk of bias in all domains. |
Risk of bias for analysis 1.12 Viral clearance at 3 days (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.12.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
| Kishoria 2020 | Some concerns | There was insufficient information on randomisation and allocation concealment. Baseline details were insufficiently reported. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | High risk of bias | Missing data not balanced between groups and no reasons provided for missing data. There is no analysis to look at the effect of the missing data. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | High risk of bias | Due to missing outcome data. Due to insufficient information on allocation concealment and lack of blinding of participants and health care providers, and lack of a prospectively registered protocol. |
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Reasons for missing outcome data were described and appropriate in the context of this outcome (only RT‐PCR positive people at baseline). 11/125 participants were no longer hospitalized at day 7 and missing for analysis. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Low risk of bias | Due to low risk of bias in all domains. |
| Shah Bukhari 2021 | Some concerns | There was no information on allocation concealment. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | High risk of bias | 14% of participants dropped out (9/50 intervention and 5/50 control). Some participants left against medical advice before completion of the study. Reasons for dropping out could be related to participants' health status. There is no analysis to look at the effect of the missing data. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | High risk of bias | Protocol was registered during the recruitment period. There were different outcome time points reported in the protocol and the journal publication. The analysis was pre‐specified. | High risk of bias | Due to missing outcome data and potential bias in the selection of the reported result. Due to insufficient information on allocation concealment and lack of blinding of participants and health care providers. |
Risk of bias for analysis 1.13 Viral clearance at 7 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.13.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Reasons for missing outcome data were described and appropriate in the context of this outcome (only RT‐PCR positive people at baseline). 11/125 participants were no longer hospitalized at day 7 and missing for analysis. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Low risk of bias | Due to low risk of bias in all domains. |
Risk of bias for analysis 1.14 Viral clearance at 7 days (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.14.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
| Kirti 2021 | Low risk of bias | Randomization was performed by an independent person not part of the investigating team using a computer‐based program. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. 1/57 participants in the intervention group and 1/58 participants in the control group received ivermectin by the treating team. Both participants were excluded (per protocol analysis). The analysis was not appropriate, but it is unlikely to have an impact on the result. | High risk of bias | More than 30% of participants were missing due to discharge or inconclusive results. There is no analysis to look at the effect of the missing data. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | High risk of bias | Due to inappropriate analysis (per protocol analysis) and missing outcome data. |
| Mohan 2021 | Low risk of bias | Centralized telephone‐based randomization. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received and the analysis was appropriate. | Low risk of bias | Reasons for missing outcome data were described and appropriate in the context of this outcome (only RT‐PCR positive people at baseline). 11/125 participants were no longer hospitalized at day 7 and missing for analysis. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Low risk of bias | Due to low risk of bias in all domains. |
| Pott‐Junior 2021 | Low risk of bias | Random sequence was generated by a computer‐based program. Allocation assignment was concealed from investigators and patients using sequentially numbered sealed opaque envelopes. There was no baseline imbalance that would suggest a problem with randomisation. | High risk of bias | Both participants and those delivering the intervention were aware of intervention received. 1/4 participants in the control group was excluded due to protocol violation (per protocol analysis). Protocol violation was not described. The analysis was not appropriate and due to the small number of participants in the control group (n = 4) the excluded participant may have changed the result. | Low risk of bias | Most people were followed up >90%. Two patients were missing (one drop‐out and one protocol violation). | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | The protocol was prospectively registered. The outcome was not measured as registered. The change may have been due to varying recommendations of PCR testing during the pandemic. | High risk of bias | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 1.15 Viral clearance at 14 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.15.1 Moderate disease (WHO 4 to 5) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
Risk of bias for analysis 1.16 Viral clearance at 14 days (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.16.1 Moderate to severe disease (WHO 4 to 9) | ||||||||||||
| Ahmed 2020 | Some concerns | There was no information on randomisation or allocation concealment. Baseline details were not reported. The summary statement on baseline details suggests that there are no baseline differences that would suggest a problem with randomisation. | Some concerns | There was insufficient information on blinding provided. No information reported whether there were deviations from the intended interventions or not. The analysis was appropriate. | Low risk of bias | Most people were followed up >90% and reasons for missing outcome data were described. | Low risk of bias | There was insufficient information on whether the outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | Some concerns | Due to insufficient information on randomisation, allocation concealment, blinding of participants and health care providers, and lack of a prospectively registered protocol. |
| Okumuş 2021 | High risk of bias | The method used to generate the randomization sequence was predictable (alternation; even‐odd numbers). There are no baseline differences that would suggest a problem with randomisation. | High risk of bias | Both participants and those delivering the intervention were aware of the intervention received. Participants in the ivermectin group (> 15%) with a specific mutation in the ivermectin metabolism were excluded and the intervention was not continued. The analysis was not appropriate (per‐protocol). | High risk of bias | More than 50% of participants in the intervention group and more than 25% in the control group were missing. Reasons for dropping out/missing data could be related to participants' health status. There is no analysis to look at the effect of the missing data. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | The protocol was retrospectively registered. | High risk of bias | Due to inadequate randomization, lack of blinding of participant and health care providers, and missing outcome data. Due to retrospectively registered protocol. |
Risk of bias for analysis 2.1 All‐cause mortality up to 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chaccour 2021 | Low risk of bias | Randomization was performed by an independent trial statistician generating a list of random numbers. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received, there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | Data for this outcome were available for all randomized participants. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered. The outcome was not registered, but results were in full detail reported in the trial register. Due to relevance of this outcome data in this context of this trial we did not assume that the results have been selected. | Low risk of bias | Due to low risk of bias in all domains. |
| López‐Medina 2021 | Low risk of bias | Random sequence was generated by an independent pharmacist using a computer‐based program. Allocation assignment was concealed from investigators and patients. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. The primary analysis is a per‐protocol analysis. 76 (16%) participants receiving the wrong intervention due to a labelling error in the early study phase were excluded. The analysis was not appropriate, but an as‐treated sensitivity analysis was reported and results did not differ. | Low risk of bias | Data for this outcome were available for all participants included in the per protocol population. Reasons for missing outcome data are reported and are unrelated to the outcome (labelling error). | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 2.2 Worsening of clinical status – need for invasive mechanical ventilation up to 14 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| López‐Medina 2021 | Low risk of bias | Random sequence was generated by an independent pharmacist using a computer‐based program. Allocation assignment was concealed from investigators and patients. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. The primary analysis is a per‐protocol analysis. 76 (16%) participants receiving the wrong intervention due to a labelling error in the early study phase were excluded. The analysis was not appropriate, but an as‐treated sensitivity analysis was reported and results did not differ. | Low risk of bias | Data for this outcome were available for all participants included in the per protocol population. Reasons for missing outcome data reported an unrelated to the outcome (labelling error). | Low risk of bias | Outcome assessors were not aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Low risk of bias | The protocol was prospectively registered. Results were not selected from multiple outcome measurements or analyses of the data. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 2.3 Worsening of clinical status – need for non‐invasive mechanical ventilation or high flow up to 14 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| López‐Medina 2021 | Low risk of bias | Random sequence was generated by an independent pharmacist using a computer‐based program. Allocation assignment was concealed from investigators and patients. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. The primary analysis is a per‐protocol analysis. 76 (16%) participants receiving the wrong intervention due to a labelling error in the early study phase were excluded. The analysis was not appropriate, but an as‐treated sensitivity analysis was reported and results did not differ. | Low risk of bias | Data for this outcome were available for all participants included in the per protocol population. Reasons for missing outcome data reported an unrelated to the outcome (labelling error). | Low risk of bias | Outcome assessors were not aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Low risk of bias | The protocol was prospectively registered. Results were not selected from multiple outcome measurements or analyses of the data. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 2.4 Symptom resolution – number of participants with symptoms resolved up to 14 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| López‐Medina 2021 | Low risk of bias | Random sequence was generated by an independent pharmacist using a computer‐based program. Allocation assignment was concealed from investigators and patients. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. The primary analysis is a per‐protocol analysis. 76 (16%) participants receiving the wrong intervention due to a labelling error in the early study phase were excluded. The analysis was not appropriate, but an as‐treated sensitivity analysis was reported and results did not differ. | Low risk of bias | Data for this outcome were available for all participants included in the per protocol population. Reasons for missing outcome data reported an unrelated to the outcome (labelling error). | Low risk of bias | Outcome assessors were not aware of the intervention received. By following a clinical protocol, knowledge of the intervention received could only minimally affect the outcome measurement. | Low risk of bias | The protocol was prospectively registered. The primary outcome was changed 6 weeks after start of recruitment and the outcome symptom resolution was added as a new primary outcome. The originally registered outcome was time until clinical deterioriation. Since the incidence of clinical deterioration was below 3% after 6 weeks the original planned analysis was futile. Results per group were not known at that time point. Results were not selected from multiple outcome measurements or analyses of the data. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 2.5 Duration to symptom resolution (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Podder 2020 | High risk of bias | The method used to generate the randomization sequence was predictable (alternation; even‐odd numbers). There are no baseline differences that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. No information reported whether there were deviations from the intended interventions or not. Group allocation of excluded participants was not described. The analysis was probably appropriate (mITT). | High risk of bias | Eighteen patients (22%) with symptoms for more than seven days at the time of enrolment were excluded after randomisation. Group allocation not reported. Reasons for dropping out could be related to participants' health status. There is no analysis to look at the effect of the missing data. | High risk of bias | Outcome assessors were aware of the intervention received. There was insufficient information on measurement of the outcome. Knowledge of intervention received could have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | High risk of bias | Due to inadequate randomization and lack of blinding of participants, health care providers, and outcome assessors. Due to missing outcome data and lack of a registered protocol. |
Risk of bias for analysis 2.6 Any adverse events within 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chaccour 2021 | Low risk of bias | Randomization was performed by an independent trial statistician generating a list of random numbers. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received, there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | Data for this outcome were available for all randomized participants. | Low risk of bias | Knowledge of intervention received could have affected outcome measurement. But outcome assessors were not aware of the intervention received. | Low risk of bias | The protocol was prospectively registered and the outcome drug‐related adverse events (7 days) in the journal publication was reported as registered. Any adverse events within 28 days was not registered but results were in full detail reported in the trial register. Due to relevance of this outcome data in this context of this trial we did not assume that the results have been selected. | Low risk of bias | Due to low risk of bias in all domains. |
| López‐Medina 2021 | Low risk of bias | Random sequence was generated by an independent pharmacist using a computer‐based program. Allocation assignment was concealed from investigators and patients. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. The primary analysis is a per‐protocol analysis. 76 (16%) participants receiving the wrong intervention due to a labelling error in the early study phase were excluded. The analysis was not appropriate, but an as‐treated sensitivity analysis was reported and results did not differ. | Low risk of bias | Data for this outcome were available for all participants included in the per protocol population. Reasons for missing outcome data are reported and are unrelated to the outcome (labelling error). | Low risk of bias | Knowledge of intervention received could have affected outcome measurement. But outcome assessors were not aware of the intervention received. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 2.7 Serious adverse events within 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chaccour 2021 | Low risk of bias | Randomization was performed by an independent trial statistician generating a list of random numbers. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received, there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | Data for this outcome were available for all randomized participants. | Some concerns | Outcome assessors were not aware of the intervention received. There was insufficient information on definition and measurement of the outcome. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | Some concerns | Due to lack of information on definition and measurement of the outcome. Due to lack of prospectively registering the outcome. |
| López‐Medina 2021 | Low risk of bias | Random sequence was generated by an independent pharmacist using a computer‐based program. Allocation assignment was concealed from investigators and patients. There was no baseline imbalance that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were not aware of intervention received. The primary analysis is a per‐protocol analysis. 76 (16%) participants receiving the wrong intervention due to a labelling error in the early study phase were excluded. The analysis was not appropriate, but an as‐treated sensitivity analysis was reported and results did not differ. | Low risk of bias | Data for this outcome were available for all participants included in the per protocol population. Reasons for missing outcome data are reported and are unrelated to the outcome (labelling error). | Low risk of bias | Knowledge of intervention received could have affected outcome measurement. But outcome assessors were not aware of the intervention received. | Low risk of bias | The protocol was prospectively registered. The outcome of the journal publication was not registered. Due to relevance of this outcome data in this context of this trial we did not assume that the results have been selected. | Some concerns | Due to inappropriate analysis (per protocol analysis). |
Risk of bias for analysis 2.8 Viral clearance at 7 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Chaccour 2021 | Low risk of bias | Randomization was performed by an independent trial statistician generating a list of random numbers. There was no baseline imbalance that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were not aware of the intervention received, there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | Data for this outcome were available for all randomized participants. | Low risk of bias | Outcome assessors were not aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | Low risk of bias | Due to low risk of bias in all domains. |
Risk of bias for analysis 2.9 Viral clearance at 14 days (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Podder 2020 | High risk of bias | The method used to generate the randomization sequence was predictable (alternation; even‐odd numbers). There are no baseline differences that would suggest a problem with randomisation. | Some concerns | Both participants and those delivering the intervention were aware of intervention received. No information reported whether there were deviations from the intended interventions or not. Group allocation of excluded participants was not described. The analysis was probably appropriate (mITT). | High risk of bias | Eighteen patients (22%) with symptoms for more than seven days at the time of enrolment were excluded after randomisation. Group allocation not reported. Reasons for dropping out could be related to participants' health status. There is no analysis to look at the effect of the missing data. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | There was no trial register entry and no published protocol. | High risk of bias | Due to inadequate randomization and lack of blinding of participants and health care providers. Due to missing outcome data and lack of a registered protocol. |
Risk of bias for analysis 3.1 Development of clinical COVID‐19 symptoms (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Shoumann 2021 | Some concerns | There was no information on randomisation or allocation concealment. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of the intervention received, there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | About 10% of outcome data were missing (participants were lost to follow‐up). Missing data were balanced between groups. Reasons for loss of follow‐up were not described. It is unlikely that missingness depended on its true value. | High risk of bias | Outcome assessors were aware of the intervention received. There was insufficient information on measurement of the outcome. Knowledge of intervention received could have affected outcome measurement. | Low risk of bias | The protocol was prospectively registered and the outcome in the journal publication was reported as registered. | High risk of bias | Due to lack of information on measurement of the outcome and lack of blinding of outcome assessors. Due to insufficient information on randomisation, allocation concealment, and missing outcome data. |
Risk of bias for analysis 3.2 Any adverse events within 14 days (secondary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Shoumann 2021 | Some concerns | There was no information on randomisation or allocation concealment. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of the intervention received, there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | About 10% of outcome data were missing (participants were lost to follow‐up). Missing data were balanced between groups. Reasons for loss of follow‐up were not described. It is unlikely that missingness depended on its true value. | High risk of bias | Outcome assessors were aware of the intervention received. There was insufficient information on measurement of the outcome. Knowledge of intervention received could have affected outcome measurement. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | High risk of bias | Due to lack of information on measurement of the outcome and lack of blinding of outcome assessors. Due to insufficient information on randomisation, allocation concealment, and missing outcome data. Due to lack of prospectively registering the outcome. |
Risk of bias for analysis 3.3 All‐cause mortality up to 28 days (primary analysis).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Shoumann 2021 | Some concerns | There was no information on randomisation or allocation concealment. There are no baseline differences that would suggest a problem with randomisation. | Low risk of bias | Both participants and those delivering the intervention were aware of the intervention received, there were no deviations from intended interventions and the analysis was appropriate. | Some concerns | About 10% of outcome data were missing (participants were lost to follow‐up). Missing data were balanced between groups. Reasons for loss of follow‐up were not described. It is unlikely that missingness depended on its true value. | Low risk of bias | Outcome assessors were aware of the intervention received. Knowledge of intervention received could not have affected outcome measurement. | Some concerns | The protocol was prospectively registered. The outcome was not prespecified. | Some concerns | Due to insufficient information on randomisation, allocation concealment, and missing outcome data. Due to lack of prospectively registering the outcome. |
Acknowledgements
The Academic Editor is Dr Hellen Gelband, and Sign‐off Editor is Professor Lisa Bero.
We thank Paul Garner (Cochrane Infectious Diseases Group (CIDG) Co‐ordinating Editor), Kerry Dwan (Statistical Editor), Maria Rosaria Cozzolino (Consumer Peer Reviewer), Robin Featherstone (Information Specialist), Jennifer Hilgart (Associate Editor), and clinical peer reviewers: Tom Fletcher (Senior Clinical Lecturer) (protocol stage), Paul Hine (CIDG Editor) (Department of Clinical Sciences, LSTM) (protocol and review stages), and Rachel Pringle, Pennine Acute Hospitals NHS Trust (review stage). We thank Anne Lawson (Cochrane Copy Editor) for copy editing the review.
This review was partly developed in the framework of the CEOsys (COVID‐19 Evidence Ecosystem) project. We thank those involved at CEOsys for their support (covid-evidenz.de/). Moreover, we thank the Cochrane Haematology working group for contributing to this review.
The research was part of a project supported by the Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project). The contents of this document reflect only the review authors' views and the German Ministry is not responsible for any use that may be made of the information it contains.
The CIDG editorial base is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK Government's official policies.
Susan Gould is partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK Government; however, the views expressed do not necessarily reflect the UK Government's official policies.
Appendices
Appendix 1. Search strategies
Cochrane COVID‐19 Study Register
Search string: ivermectin* OR stromectol* OR mectizan* OR "MK 933" OR MK933 OR eqvalan* OR soolantra* OR sklice* OR stromectal* OR ivomec*
Study characteristics: 1) "Intervention assignment": “Randomised” OR 2) "Study type": "Interventional" AND "Study design": "Parallel/Crossover" AND "Unclear" = 119 references
Web of Science Clarivate (Advanced search)
#1. TI=(ivermectin* OR stromectol* OR mectizan* OR "MK 933" OR MK933 OR eqvalan* OR soolantra* OR sklice* OR stromectal* OR ivomec*) OR AB=(ivermectin* OR stromectol* OR mectizan* OR "MK 933" OR MK933 OR eqvalan* OR soolantra* OR sklice* OR stromectal* OR ivomec*)
#2. TI=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR"SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2") OR AB=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR"SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")
#3. #1 AND #2 Indexes=SCI‐EXPANDED, ESCI Timespan=2020‐2021 = 160 references
medRxiv (Advanced search)
for abstract or title "ivermectin AND randomized" (match all words) for abstract or title "ivermectin AND randomised" (match all words) for abstract or title "ivermectin AND randomly" (match all words) for abstract or title "ivermectin AND groups" (match all words) = 35 references
Research Square
Abstract: ivermectin Manually selected relevant references on ResearchSquare based on Title/Abstract = 12 references
Data and analyses
Comparison 1. Ivermectin compared to placebo or standard of care for people with moderate‐to‐severe COVID‐19 treated in the inpatient setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality up to 28 days (primary analysis) | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.14, 2.51] |
| 1.1.1 Moderate disease (WHO 4 to 5) | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.14, 2.51] |
| 1.2 Worsening of clinical status – need for invasive mechanical ventilation up to 28 days (primary analysis) | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.11, 2.59] |
| 1.2.1 Moderate disease (WHO 4 to 5) | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.11, 2.59] |
| 1.3 Worsening of clinical status – need for oxygen up to 28 days (primary analysis) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3.1 Moderate disease (WHO 4 to 5) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4 Improvement of clinical status – participants discharged without respiratory deterioration or death at 28 days (primary analysis) | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.35] |
| 1.4.1 Moderate disease (WHO 4 to 5) | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.35] |
| 1.5 Any adverse events within 28 days (primary analysis) | 1 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.50, 2.97] |
| 1.5.1 Moderate disease (WHO 4 to 5) | 1 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.50, 2.97] |
| 1.6 Any adverse events within 28 days (secondary analysis) | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.61, 1.79] |
| 1.6.1 Moderate disease (WHO 4 to 5) | 4 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.61, 1.79] |
| 1.7 Serious adverse events within 28 days (primary analysis) | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.07, 35.89] |
| 1.7.1 Moderate disease (WHO 4 to 5) | 2 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.07, 35.89] |
| 1.8 Serious adverse events within 28 days (secondary analysis) | 3 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.07, 35.89] |
| 1.8.1 Moderate disease (WHO 4 to 5) | 3 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.07, 35.89] |
| 1.9 Admission to intensive care unit (primary analysis) | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.51] |
| 1.9.1 Moderate disease (WHO 4 to 5) | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.51] |
| 1.10 Duration of hospitalization (primary analysis) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.43, 2.23] |
| 1.10.1 Moderate disease (WHO 4 to 5) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.43, 2.23] |
| 1.11 Viral clearance at 3 days (primary analysis) | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.45, 2.32] |
| 1.11.1 Moderate disease (WHO 4 to 5) | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.45, 2.32] |
| 1.12 Viral clearance at 3 days (secondary analysis) | 4 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.59, 5.04] |
| 1.12.1 Moderate disease (WHO 4 to 5) | 4 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.59, 5.04] |
| 1.13 Viral clearance at 7 days (primary analysis) | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.51, 6.48] |
| 1.13.1 Moderate disease (WHO 4 to 5) | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.51, 6.48] |
| 1.14 Viral clearance at 7 days (secondary analysis) | 4 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.86] |
| 1.14.1 Moderate disease (WHO 4 to 5) | 4 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.86] |
| 1.15 Viral clearance at 14 days (primary analysis) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.13, 3.45] |
| 1.15.1 Moderate disease (WHO 4 to 5) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.13, 3.45] |
| 1.16 Viral clearance at 14 days (secondary analysis) | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.28, 3.33] |
| 1.16.1 Moderate to severe disease (WHO 4 to 9) | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.28, 3.33] |
Comparison 2. Ivermectin compared to placebo or standard of care for people with mild COVID‐19 treated in the outpatient setting.
Comparison 3. Ivermectin compared to no treatment for prevention of SARS‐CoV‐2 infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Development of clinical COVID‐19 symptoms (secondary analysis) | 1 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.08, 0.21] |
| 3.2 Any adverse events within 14 days (secondary analysis) | 1 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 11.50 [0.68, 193.21] |
| 3.3 All‐cause mortality up to 28 days (primary analysis) | 1 | 304 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmed 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Chaccour 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Chachar 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Gonzalez 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Kirti 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Kishoria 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Krolewiecki 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
López‐Medina 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Mohan 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Okumuş 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Podder 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Pott‐Junior 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Shah Bukhari 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
Shoumann 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
ALT: alanine transaminase; AST: aspartate aminotransferase; CRP: C‐reactive protein; CT: computer tomography; ECG: electrocardiograph; ICU: intensive care unit; IgG: immunoglobulin G; LDH: lactose dehydrogenase; PaO2/FiO2: partial pressure of oxygen/fraction of inspired oxygen; PCR: polymerase chain reaction; n: number; NA: not available; NIH: National Institutes of Health; NR: not reported; NSAID: non‐steroidal anti‐inflammatory drug; RCT: randomized controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; rRT‐PCR: real‐time reverse transcription polymerase chain reaction; SD: standard deviation; SpO2: oxygen saturation; WHO World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babalola 2021 | Active comparator: ivermectin compared to a control (lopinavir/ritonavir) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| Behera 2020 | Wrong study design: case‐control study. |
| Cadegiani 2020 | Wrong study design: historical control group, i.e. no RCT. |
| Camprubi 2020 | Wrong study design: retrospective study. |
| Carvallo 2020 | Wrong study design: prospective cohort study; additionally ivermectin was administered in combination with other active drugs with unknown influence on COVID‐19. |
| Chahla 2021a | Combined intervention: ivermectin administered in combination with another active substance (iota‐carrageenan) with unknown influence on prevention of COVID‐1,9 which we did not consider eligible to determine ivermectin's true effect. |
| Chahla 2021b | Wrong study design: cluster‐randomised trial. |
| Chowdhury 2021 | Combined intervention: ivermectin administered in combination with another active drug (doxycycline) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. Additionally, the study used an active comparator with unproven efficacy (hydroxychloroquine + azithromycin). |
| CTRI/2020/08/027282 | Active comparator: ivermectin compared to a control (vitamin supplements) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| CTRI/2020/08/027394 | Active comparator: ivermectin compared to a control (chloroquine/azithromycin/vitamin supplements) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| CTRI/2020/10/028335 | Active comparator: ivermectin was compared to a control (tinefcon) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. Additionally, ivermectin was administered in combination with another active drug (hydroxychloroquine) with unknown influence on COVID‐19. |
| Elgazzar 2020 |
Active comparator (treatment arm): ivermectin was compared to a control (hydroxychloroquine) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. Wrong population (prevention arm): participants investigated formed a distinguishable group with both pre‐exposure and postexposure risk. No examination on possible infection that had already taken place at randomization. Study retracted due to ethical concerns on 14 July 2021. |
| Galan 2021 | Active comparator: ivermectin compared to control arms (hydroxychloroquine/chloroquine) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| Gorial 2020 | Wrong study design: historical control group, i.e. no RCT. |
| Hashim 2020 | Combined intervention: ivermectin administered in combination with another active drug (doxycycline) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| IRCT20180922041089N4 | Wrong population: study plans to also include participants with diagnosis of COVID‐19 based on suspect CT scan without PCR or antigen test confirmation. |
| IRCT20200408046987N2 | Combined intervention: ivermectin administered in combination with another active drug (sofosbuvir/daclatasvir) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| Lima‐Morales 2021 | Wrong study design: prospective cohort study; additionally ivermectin was administered in combination with other active drugs (azithromycin, montelukast, aspirin) with unknown influence on COVID‐19. |
| Mahmud 2021 | Combined intervention: ivermectin administered in combination with another active drug (doxycycline) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| Morgenstern 2020 | Wrong study design: retrospective study. |
| NCT04345419 | Wrong intervention: registry entry changed investigated intervention from ivermectin to remdesivir. |
| NCT04360356 | Combined intervention: ivermectin administered in combination with another active drug (nitazoxanide) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| NCT04374279 | Wrong intervention: registry entry changed investigated intervention from ivermectin to only bicalutamide. |
| NCT04382846 | Wrong intervention: registry entry changed investigated intervention from ivermectin to only nitazoxanide. |
| NCT04392427 | Combined intervention: ivermectin administered in combination with another active drug (nitazoxanide/ribavirin) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| NCT04435587 | Active comparator: ivermectin was compared to a control (darunavir/ritonavir/hydroxychloroquine) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| NCT04447235 | Combined intervention: ivermectin administered in combination with another active drug (losartan) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| NCT04482686 | Combined intervention: ivermectin administered in combination with another active drug (doxycycline) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| NCT04530474 | Wrong population: study plans to include participants with diagnosis of COVID‐19 only based on suspect symptoms without PCR or antigen test confirmation. |
| NCT04551755 | Combined intervention: ivermectin administered in combination with another active drug (doxycycline) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| NCT04703608 | Wrong population: study plans to also include participants with diagnosis of COVID‐19 based on suspect clinical or radiological symptoms without PCR or antigen test confirmation. |
| NCT04723459 | Wrong intervention: study plans to investigate ivermectin in impregnated masks, not its systemic effect in the human body. |
| NCT04768179 | Combined intervention: ivermectin administered in combination with another active drug (aspirin) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| Niaee 2020 | Wrong population: study included around 30% of SARS‐CoV‐2‐negative participants, which we did not consider appropriate to include into evidence regarding treatment of COVID‐19. |
| Rajter 2021 | Wrong study design: retrospective study. |
| Seet 2021 | Active comparator: ivermectin compared to control arms (hydroxychloroquine/povidone‐iodine/vitamin supplements) with unknown influence on prevention of COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
| Shahbaznejad 2021 | Wrong population: study included 76.8% participants with unknown or negative SARS‐CoV‐2 status, which we did not consider appropriate to include into evidence regarding treatment of COVID‐19. |
| Spoorthi 2020 | Combined intervention: ivermectin administered in combination with another active drug (doxycycline) with unknown influence on COVID‐19, which we did not consider eligible to determine ivermectin's true effect. |
CT: computer tomography; PCR: polymerase chain reaction; RCT: randomized controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
2020‐001971‐33/ES.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
2020‐002091‐12/BG.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
CTRI/2020/04/024948.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
CTRI/2020/06/025960.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
Faisal 2020.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
Hosseini 2021.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
IRCT20190602043787N3.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
IRCT20200408046987N3.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
IRCT20200422047168N2.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
ISRCTN90437126.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT04351347.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT04374019.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT04407130.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT04407507.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT04716569.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT04746365.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
NCT04891250.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
Samaha 2021.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Notes |
|
ALT: alanine aminotransferase; AST: aspartate aminotransferase; COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; CT: computer tomography; ESR: erythrocyte sedimentation rate; GGT: gamma glutamyl transferase; ICU: intensive care unit; IgA: immunoglobulin A; IgM: immunoglobulin M; NR: not reported; PaO2: partial pressure of oxygen; PCR: polymerase chain reaction; RCT: randomized controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; rRT‐PCR: real‐time reverse transcription polymerase chain reaction; SpO2: oxygen saturation by pulse oximetry; ULN: upper limit of normal; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
2020‐001994‐66/ES.
| Study name | Randomised clinical trial of ivermectin for treatment and prophylaxis of COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | NR |
| Contact information | Fundació Assistencial Mútua Terrassa Passeig Olabarria s/n Valldoreix 08197 Spain tomas.perez.porcuna@gmail.com |
| Notes |
|
ACTRN12620000982910.
| Study name | A randomized double‐blind placebo‐controlled trial of oral ivermectin for outpatient treatment of those at high risk for hospitalization due to COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 15 February 2021 |
| Contact information | Dr Mark Stein Department of Diabetes and Endocrinology Royal Melbourne Hospital 300 Grattan Street Victoria, 3050 Australia msteintpep1@florey.edu.au |
| Notes |
|
CTRI/2020/05/025068.
| Study name | A phase IIB open label randomized controlled trial to evaluate the efficacy and safety of Ivermectin in reducing viral loads in patients with hematological disorders who are admitted with COVID 19 infection |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 27 May 2020 |
| Contact information | Biju George, Professor Christian Medical College Vellore Department of Haematology Vellore Tamil Nadu 632004 India biju@cmcvellore.ac.in |
| Notes |
|
CTRI/2020/05/025224.
| Study name | Study to efficacy of Ivermectin in patients of COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 24 May 2020 |
| Contact information | Dr Ashish Pathak R. D. Gardi Medical College Department of Pediatrics Agar Road, Surasa Ujjain MADHYA PRADESH 456006 India drashish.jpathak@gmail.com |
| Notes |
|
Garcia 2021.
| Study name | Randomized clinical trial to compare the efficacy of ivermectin versus placebo to negativize nasopharyngeal PCR in patients with early COVID‐19 in Peru (SAINT‐Peru): a structured summary of a study protocol for randomized controlled trial |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 29 August 2020 |
| Contact information | Hansel Mundaca, MD Hospital Nacional Cayetano Heredia Lima, Peru hansel.mundaca@upch.pe |
| Notes |
|
IRCT20111224008507N4.
| Study name | Double‐blind placebo‐controlled clinical trial of evaluating the effectiveness of Ivermectin in treatment of outpatients with COVID‐19 in 2021 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 19 February 2021 |
| Contact information | Dr Mohammad Sadegh Rezai Mazandaran University of Medical Sciences Boali Hospital, Pasdaran Blv. 485838477 Sari, Mazandaran Iran drmsrezaii@yahoo.com |
| Notes |
|
IRCT20111224008507N5.
| Study name | Double‐blind placebo‐controlled clinical trial of evaluating the effectiveness of Ivermectin in treatment of patients admitted with COVID‐19 in 2021 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 19 February 2021 |
| Contact information | Dr Mohammad Sadegh Rezai Mazandaran University of Medical Sciences Boali Hospital, Pasdaran Blv. 485838477 Sari, Mazandaran Iran drmsrezaii@yahoo.com |
| Notes |
|
IRCT20190624043993N2.
| Study name | Evaluation effects of the standard regimen along with ivermectin on treatment of corona virus type 2 pneumonia |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 22 August 2020 |
| Contact information | Foroud Shahbazi Kermanshah University of Medical Sciences Parastar Blve 1673‐67145 Kermanshah Iran Foroud08@gmail.com |
| Notes |
|
IRCT20200404046937N4.
| Study name | Evaluating the efficacy and safety of Ivermectin in the treatment of COVID‐19 patients: a double‐blind randomized controlled trial, phase II |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 30 July 2020 |
| Contact information | Mehran Varnasseri Ahvaz University of Medical Sciences Razi hospital Felestin Ave, Amanieh Ave Ahvaz, Khouzestan Iran drvarnasei.m@gmail.com |
| Notes |
|
NCT04403555.
| Study name | Ivermectin as a Novel Therapy in COVID‐19 Treatment |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 1 June 2020 |
| Contact information | Sherief Abd‐Elsalam Tanta University Tanta 35111 Egypt sheriefabdelsalam@yahoo.com |
| Notes |
|
NCT04425707.
| Study name | Ivermectin In treatment of COVID 19 patients |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 9 June 2020 |
| Contact information | Dr Ehab Kamal General Director of Fever Hospitals Ministry of Health and Population Cairo Egypt |
| Notes |
|
NCT04429711.
| Study name | Ivermectin vs. placebo for the treatment of patients with mild to moderate COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 12 May 2020 |
| Contact information | Eli Schwartz, Prof Sheba Medical Center Ramat‐Gan Israel Eli.schwartz@sheba.health.gov.il |
| Notes |
|
NCT04438850.
| Study name | COVidIVERmectin: ivermectin for treatment of COVID‐19 (COVER) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 31 July 2020 |
| Contact information | Zeno Bisoffi, PhD IRCCS Sacro Cuore Don Calabria hospital Negrar, Verona 37024 Italy zeno.bisoffi@sacrocuore.it |
| Notes |
|
NCT04445311.
| Study name | Ivermectin in treatment of COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 31 May 2020 |
| Contact information | Waheed Shouman, MD Zagazig University Sharkia 44519 Egypt shouman66@gmail.com |
| Notes |
|
NCT04472585.
| Study name | Efficacy of subcutaneous ivermectin with or without zinc in COVID‐19 patients (SIZI‐COVID‐PK) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 14 November 2020 |
| Contact information | Shoaib Ashraf, PhD Harvard University Boston USA sashraf@mgh.harvard.edu |
| Notes |
|
NCT04510233.
| Study name | Ivermectin nasal spray for COVID19 patients |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2020 |
| Contact information | Kamal Okasha, PhD Tanta University Tanta 35111 Egypt okasha70@yahoo.com |
| Notes |
|
NCT04527211.
| Study name | Effectiveness and safety of ivermectin for the prevention of COVID‐19 infection in Colombian health personnel (IveprofCovid19) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 7 September 2020 |
| Contact information | Dr Eduar D Echeverri Pontificia Universidad Javeriana Valle Del Cauca 760501 Cali Colombia dr.echeverri@gmail.com |
| Notes |
|
NCT04602507.
| Study name | Ivermectin in adults with severe COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 7 December 2020 |
| Contact information | Federico Rodríguez‐Vega, MD Clinica CES Medellín, Antioquia 050001 Colombia federicorodriguez@clinicaces.edu.co |
| Notes |
|
NCT04673214.
| Study name | Evaluation of prognostic modification in COVID‐19 patients in early intervention treatment |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 16 December 2020 |
| Contact information | Gilberto Cruz Arteaga Coordinación de Investigación en Salud Distrito Federal 02000 Mexico gilberto.cruz@imss.gob.mx |
| Notes |
|
NCT04703205.
| Study name | Study in COvid‐19 Patients With iveRmectin (CORVETTE‐01) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 16 September 2020 |
| Contact information | Kunihiro K Yamaoka, PhD Kitasato University Sagamihara Kanagawa Japan yamaoka@med.kitasato‐u.ac.jp |
| Notes |
|
NCT04712279.
| Study name | The (HD)IVACOV Trial (The High‐Dose IVermectin Against COVID‐19 Trial) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 25 January 2021 |
| Contact information | Flavio A Cadegiani, MD, PhD Corpometria Institute +55 61 99650.6111 flavio.cadegiani@gmail.com |
| Notes |
|
NCT04727424.
| Study name | Repurposed approved therapies for outpatient treatment of patients with early‐onset COVID‐19 and mild symptoms |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 19 January 2021 CARDRESEARCH – Cardiologia Assistencial e de Pesquisa Belo Horizonte, Minas Gerais 30150240 Brazil |
| Contact information | Gilmar Reis, MD, PhD |
| Notes |
|
NCT04729140.
| Study name | An outpatient clinical trial using ivermectin and doxycycline in COVID‐19 positive patients at high risk to prevent COVID‐19 related hospitalization |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 28 December 2020 |
| Contact information | Werther Marciales, MD MAX HEALTH, Subsero Health 2055 Wood Street, Suite 100 Sarasota, Florida, 34237 US werther40@msn.com |
| Notes |
|
NCT04834115.
| Study name | Efficacy of ivermectin in outpatients with non‐severe COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 17 November 2020 |
| Contact information | Gabriela Avila, MD, MSc, PhD Facultad de Ciencias Médicas ‐ Universidad Nacional de Asunción Asunción 111421 Paraguay mavila@med.una.py |
| Notes |
|
NCT04836299.
| Study name | Clinical trial to "Study the Efficacy and Therapeutic Safety of Ivermectin: (SAINTBO)" |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 8 May 2021 |
| Contact information | Jorge L Aviles, MPH Universidad Mayor de San Simón Cochabamba Bolivia |
| Notes |
|
NCT04885530.
| Study name | ACTIV‐6: COVID‐19 study of repurposed medications |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2021 |
| Contact information | Allison DeLong Duke Clinical Research Institute Durham 27701 North Carolina USA allison.hayes@duke.edu |
| Notes |
|
NCT04886362.
| Study name | Ivermectina Colombia (IVERCOL) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | July 2021 |
| Contact information | Juan Carlos Chacón Jimenez, MD ceivercol@suramericana.com.co |
| Notes |
|
NCT04894721.
| Study name | Prophylaxis for COVID‐19: ivermectin in close contacts of COVID‐19 cases (IVERNEX‐TUC) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 20 March 2021 |
| Contact information | Maria Peral, PhD SI.PRO.SA, Ministerio de Salud Pública Tucumán 4000 Argentina mperal@fm.unt.edu.ar |
| Notes |
|
PACTR202102588777597.
| Study name | Ivermectin Treatment Trial (ITT) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 3 February 2021 |
| Contact information | Akin Osibogun Lagos Nigeria akinosibogun@yahoo.co.uk |
| Notes |
|
PACTR202102848675636.
| Study name | Double blind, community‐based, randomized controlled trial on the use of ivermectin as post exposure chemo‐prophylaxis for COVID‐19 among high risk individuals in Lagos (IVERPEPCOV) COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 1 March 2021 |
| Contact information | Olufemi Babalola Department of Surgery Bingham University Karu Lagos Nigeria bablo57@gmail.com |
| Notes |
|
Vallejos 2020.
| Study name | Ivermectin to prevent hospitalizations in COVID‐19 (IVERCORCOVID19) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 19 August 2020 |
| Contact information | Dr Julio Vallejos Ministry of Public Health of the Province of Corrientes Corrientes 3400 Argentina juliovallejos@funcacorr.org.ar |
| Notes |
|
ALT: alanine aminotransferase; AST: aspartate aminotransferase; COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; CT: computer tomography; ICU: intensive care unit; IgA: immunoglobulin A; IgG: immunoglobulin G; IgM: immunoglobulin M; NA: not available; NAAT: nucleic acid amplification test; NR: not reported; PaO2/FIO2: partial pressure of oxygen/fraction of inspired oxygen; PCR: polymerase chain reaction; RCT: randomized controlled trial; RNA: ribonucleic acid; RT‐PCR: reverse transcription polymerase chain reaction; RT‐qPCR: reverse transcription quantitative polymerase chain reaction; SaO2: oxygen saturation; SD: standard deviation; WHO: World Health Organization.
Differences between protocol and review
The review differs from the protocol for the following aspects (Popp 2021).
We introduced 'patients discharged without respiratory deterioration or death at 28 days' that was reported by one study as a new primary outcome in the category 'improvement of clinical status' (inpatient setting) (Gonzalez 2021). This outcome was considered clinically useful.
We added the following paragraph to the 'type of participants section' to clarify how we handled studies including a mixed population with confirmed and suspected COVID‐19 diagnosis: "If studies included participants with a confirmed or suspected COVID‐19 diagnosis, we used only the data for the patient population with confirmed COVID‐19 diagnosis. In cases, where data were not reported separately for people with confirmed or suspected COVID‐19 diagnosis, we excluded the study." We considered this specification clinically relevant since it would be a loss of evidence to exclude data on people positive for SARS‐CoV‐2.
Since we found no eligible trials that reported on 'serious adverse events' and 'adverse events' in an outpatient setting within 14 days, we changed the eligible time point for both of those outcomes from 'within 14 days' to 'within 28 days,' which two studies provided and was considered clinically useful (Chaccour 2021; López‐Medina 2021).
We added a paragraph to the methods section 'Methods for future updates.' The living systematic review approach was included in this review from the beginning as part of the CEOsys project.
Contributions of authors
MP: conception of the review; design of the review; search and selection of studies for inclusion in the review; collection of data for the review; assessment of the risk of bias in the included studies; analysis of data; assessment of the certainty in the body of evidence; interpretation of data, and writing of the review.
MS: conception of the review; design of the review; interpretation of data; writing and proofreading of the review.
MIM: search strategy design and writing of the review.
SG: interpretation of data; writing and proofreading of the review.
PK: conception of the review; design of the review; interpretation of data; proofreading of the review.
PM: conception of the review; design of the review; interpretation of data; proofreading of the review.
NS: conception of the review; design of the review; interpretation of data; proofreading of the review.
SW: conception of the review; design of the review; co‐ordination of the review; search and selection of studies for inclusion in the review; collection of data for the review; assessment of the risk of bias in the included studies; analysis of data; assessment of the certainty in the body of evidence; interpretation of data, and writing of the review.
Sources of support
Internal sources
-
University Hospital Wuerzburg, Germany
Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg
Liverpool School of Tropical Medicine, UK
External sources
-
Federal Ministry of Education and Research, Germany
NaFoUniMedCovid19 (funding number: 01KX2021); part of the project "CEOsys"
-
Foreign, Commonwealth, and Development Office (FCDO), UK
Project number 300342‐104
Declarations of interest
MP: funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project, which was paid to the institution).
MS: none.
MIM: none.
SG: none.
PK: none.
PM: none.
NS: none.
SW: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project, which was paid to the institution).
Edited (no change to conclusions)
References
References to studies included in this review
Ahmed 2020 {published data only}
- Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. International Journal of Infectious Diseases 2020;103:214-6. [DOI: 10.1016/j.ijid.2020.11.191] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaccour 2021 {published data only}
- Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with mild COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. researchsquare.com/article/rs-116547/v1 (first received 7 December 2020). [DOI: 10.21203/rs.3.rs-116547/v1] [DOI] [PMC free article] [PubMed]
- Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021;32:100720. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaccour C, Ruiz-Castillo P, Richardson MA, Moncunill G, Casellas A, Carmona-Torre F, et al. The SARS-CoV-2 ivermectin Navarra-ISGlobal Trial (SAINT) to evaluate the potential of ivermectin to reduce COVID-19 transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: a structured summary of a study protocol for a randomized control pilot trial. Trials 2020;21(1):498. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2020-001474-29-ES. Pilot study to evaluate the potential of ivermectin to reduce COVID-19 transmission. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001474-29/ES (first received 8 May 2020).
- NCT04390022. Sars-CoV-2/COVID-19 ivermectin Navarra-ISGlobal Trial (SAINT). clinicaltrials.gov/ct2/show/NCT04390022 (first received 17 December 2020).
Chachar 2020 {published data only}
- Chachar AZ, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. International Journal of Sciences 2020;9:31-5. [DOI: 10.18483/ijSci.2378] [DOI] [Google Scholar]
- NCT04739410. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04739410 (first received 4 February 2021).
Gonzalez 2021 {published data only}
- Gonzalez BJ, González Gámez M, Enciso EA, Maldonado RJ, Palacios HP, Dueñas Campos S, et al. Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial. medrxiv.org/content/early/2021/02/23/2021.02.18.21252037 (first received 23 February 2021). [DOI: 10.1101/2021.02.18.21252037] [DOI]
- NCT04391127. Hydroxychloroquine and ivermectin for the treatment of COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04391127 (first received 18 May 2020).
Kirti 2021 {published data only}
- CTRI/2020/08/027225. Ivermectin as a possible treatment for COVID-19. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=46660&EncHid=&modid=&compid=','46660det' (first received 18 August 2020).
- Kirti R, Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, et al. Ivermectin as a potential treatment for mild to moderate COVID-19 – a double blind randomized placebo-controlled trial. medrxiv.org/content/10.1101/2021.01.05.21249310v1 (first received 9 January 2021). [DOI: 10.1101/2021.01.05.21249310] [DOI] [PubMed]
Kishoria 2020 {published data only}
- Kishoria N, Mathur SL, Parmar V, Kaur RJ, Agarwal H, Verma S. Ivermectin as adjuvant to hydroxychlorquine in patients resistant to standard treatment for SARS-CoV-2: results of an open-label randomized clinical study. Paripex — Indian Journal of Research 2020;9(8):4801859. [DOI: 10.36106/paripex/4801859] [DOI] [Google Scholar]
Krolewiecki 2020 {published data only}
- Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso D, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: a pilot randomised, controlled, open label, multicentre trial. ssrn.com/abstract=3714649 (first received 11 November 2020). [DOI: 10.2139/ssrn.3714649] [DOI]
- NCT04381884. Ivermectin effect on SARS-CoV-2 replication in patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04381884 (first received 11 May 2020).
López‐Medina 2021 {published data only}
- López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 2021;325(14):1426-35. [DOI: 10.1001/jama.2021.3071] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04405843. Efficacy of ivermectin in adult patients with early stages of COVID-19 (EPIC Trial) (EPIC). clinicaltrials.gov/ct2/show/NCT04405843 (first received 28 May 2020).
Mohan 2021 {published data only}
- CTRI/2020/06/026001. Ivermectin in COVID. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=44196&EncHid=&modid=&compid=%27,%2744196det%27 (first received 21 June 2020).
- Mohan A, Tiwari P, Suri T, Mittal S, Patel A, Jain A, et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. researchsquare.com/article/rs-191648/v1 (first received 2 February 2021). [DOI: 10.21203/rs.3.rs-191648/v1] [DOI] [PMC free article] [PubMed]
Okumuş 2021 {published data only}
- NCT04646109. Ivermectin for severe COVID-19 management. clinicaltrials.gov/ct2/show/NCT04646109 (first received 27 November 2020).
- Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı IY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infectious Diseases 2021;21:411. [DOI: 10.1186/s12879-021-06104-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı IY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. researchsquare.com/article/rs-224203/v1 (first received 24 February 2021). [DOI: 10.21203/rs.3.rs-224203/v1] [DOI] [PMC free article] [PubMed]
Podder 2020 {published data only}
- Podder CS, Chowdhury N, Sina MI, Haque WM. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC Journal of Medical Science 2020;14(2):11-8. [DOI: ] [Google Scholar]
Pott‐Junior 2021 {published data only}
- NCT04431466. A study to compare the efficacy and safety of different doses of ivermectin for COVID-19. clinicaltrials.gov/ct2/show/NCT04431466 (first received 16 June 2020).
- Pott-Junior H, Bastos Paoliello MM, Miguel AQ, da Cunha AF, Melo Freire CC, Neves FF, et al. Use of ivermectin in the treatment of Covid-19: a pilot trial. Toxicology Reports 2021;8:505-10. [DOI: 10.1016/j.toxrep.2021.03.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Shah Bukhari 2021 {published data only}
- NCT04392713. Efficacy of ivermectin in COVID-19. clinicaltrials.gov/ct2/show/NCT04392713 (first received 19 May 2020).
- Shah Bukhari KH, Asghar A, Perveen N, Hayat A, Mangat SA, Butt KR, et al. Efficacy of ivermectin in COVID-19 patients with mild to moderate disease. medrxiv.org/content/early/2021/02/05/2021.02.02.21250840 (first received 5 February 2021). [DOI: 10.1101/2021.02.02.21250840] [DOI]
Shoumann 2021 {published data only}
- NCT04422561. Prophylactic ivermectin in COVID-19 contacts. clinicaltrials.gov/ct2/show/NCT04422561 (first received 9 June 2020).
- Shoumann WM, Hegazy AA, Nafae RM, Ragab MI, Samra SR, Ibrahim DA, et al. Use of ivermectin as a potential chemoprophylaxis for COVID-19 in Egypt: a randomized clinical trial. Journal of Clinical and Diagnostic Research 2021;15(2):27-32. [DOI: 10.7860/JCDR/2021/46795.14529] [DOI] [Google Scholar]
References to studies excluded from this review
Babalola 2021 {published data only}
- Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomised controlled double-blind, dose-response study in Lagos. Quarterly Journal of Medicine 2021 Feb 18 [Epub ahead of print]. [DOI: 10.1093/qjmed/hcab035] [DOI] [PMC free article] [PubMed]
- Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomised controlled double blind dose response study in Lagos. medrxiv.org/content/10.1101/2021.01.05.21249131v1 (first received 6 January 2021). [DOI: 10.1101/2021.01.05.21249131] [DOI] [PMC free article] [PubMed]
- ISRCTN40302986. Does ivermectin cure and/or prevent COVID-19? isrctn.com/ISRCTN40302986 (first received 23 April 2020). [DOI: 10.1186/ISRCTN40302986] [DOI]
Behera 2020 {published data only}
- Behera P, Patro BK, Singh AK, Chandanshive PD, Ravi K, Pradhan SK, et al. Role of ivermectin in the prevention of COVID-19 infection among healthcare workers in India: a matched case-control study. 10.1101/2020.10.29.20222661 (first received 3 November 2020). [DOI: 10.1101/2020.10.29.20222661] [DOI]
Cadegiani 2020 {published data only}
- Cadegiani F, Goren A, Wambier CG, McCoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly reduced symptoms compared to known outcomes in untreated patients. medrxiv.org/content/10.1101/2020.10.31.20223883v1 (first received 4 November 2020). [DOI: 10.1101/2020.10.31.20223883] [DOI] [PMC free article] [PubMed]
- Cadegiani F, Wambier CG, Goren A, McCoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly reduced symptoms compared to known outcomes in untreated patients. researchsquare.com/article/rs-100994/v1 (first received 3 November 2020). [DOI: 10.21203/rs.3.rs-100994/v1] [DOI]
- Cadegiani F, Goren A, McCoy J, Wambier CG. Hydroxychloroquine, nitazoxanide and ivermectin have similar effects in early COVID-19: a head-to-head comparison of the Pre-AndroCoV Trial. researchsquare.com/article/rs-98106/v1 (first received 29 October 2020). [DOI: 10.21203/rs.3.rs-98106/v1] [DOI]
- Cadegiani F, Goren A, Wambier CG, McCoy J. An open-label prospective observational study of antiandrogen and non-antiandrogen early pharmacological approaches in females with mild-to-moderate COVID-19. The Pre-AndroCoV Female Trial. medrxiv.org/content/10.1101/2020.10.05.20206870v1 (first received 6 October 2020). [DOI: 10.1101/2020.10.05.20206870] [DOI]
Camprubi 2020 {published data only}
- Camprubi D, Almuedo-Riera A, Marti-Soler H, Soriano A, Hurtado JC, Subira C, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PloS One 2020;15(11):e024218. [DOI: 10.1371/journal.pone.0242184] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carvallo 2020 {published data only}
- Carvallo H, Hirsch R, Farinella EM. Safety and efficacy of the combined use of ivermectin, dexamethasone, enoxaparin and aspirin against COVID 19. medrxiv.org/content/10.1101/2020.09.10.20191619v1 (first received 15 September 2020). [DOI: 10.1101/2020.09.10.20191619] [DOI]
- NCT04425863. Ivermectin, aspirin, dexamethasone and enoxaparin as treatment of Covid 19 (IDEA). clinicaltrials.gov/ct2/show/NCT04425863 (first received 11 June 2020).
Chahla 2021a {published data only}
- Chahla RE, Medina Ruiz L, Mena T, Brepe Y, Terranova P, Ortega ES, et al. A randomized trial – intensive treatment based in ivermectin and iota-carrageenan as pre-exposure prophylaxis for COVID-19 in healthcare agents. medrxiv.org/content/10.1101/2021.03.26.21254398v1 (first received 30 March 2021). [DOI: 10.1101/2021.03.26.21254398v1] [DOI]
- NCT04701710. Prophylaxis Covid-19 in healthcare agents by intensive treatment with ivermectin and Iota-carrageenan (Ivercar-Tuc). clinicaltrials.gov/ct2/show/NCT04701710 (first received 8 January 2021).
Chahla 2021b {published data only}
- Chahla RE, Medina Ruiz L, Mena T, Brepe Y, Terranova P, Ortega ES, et al. Cluster randomised trials – ivermectin repurposing for COVID-19 treatment of outpatients with mild disease In primary health care centers. researchsquare.com/article/rs-495945/v1 (first received 6 May 2021). [DOI: 10.21203/rs.3.rs-495945/v1] [DOI]
- Chahla RE, Medina Ruiz L, Mena T, Brepe Y, Terranova P, Ortega ES, et al. Ivermectin reproposing for COVID-19 treatment outpatients in mild stage in primary health care centers. medrxiv.org/content/10.1101/2021.03.29.21254554v1 (first received 20 March 2021). [DOI: 10.1101/2021.03.29.21254554v1] [DOI]
Chowdhury 2021 {published data only}
- Chowdhury AT, Shahbaz M, Karim R, Islam J, Dan G, Shuixiang H. A comparative study on ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID-19 patients. Eurasian Journal of Medicine and Oncology 2021;5(1):63-70. [DOI: 10.14744/ejmo.2021.16263] [DOI] [Google Scholar]
- Chowdhury AT, Shahbaz M, Karim R, Islam J, Dan G, Shuixiang H. A randomized trial of ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID19 patients. researchsquare.com/article/rs-38896/v1 (first received 14 July 2020). [DOI: 10.21203/rs.3.rs-38896/v1] [DOI]
- NCT04434144. A comparative study on ivermectin and hydroxychloroquine on the COVID19 patients in Bangladesh. clinicaltrials.gov/ct2/show/NCT04434144 (first received 16 June 2020).
CTRI/2020/08/027282 {published data only}
- CTRI/2020/08/027282. Prophylactic ivermectin in COVID 19 contacts. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=46676&EncHid=&modid=&compid=%27,%2746676det%27 (first received 20 August 2020).
CTRI/2020/08/027394 {published data only}
- CTRI/2020/08/027394. Assessment of response of ivermectin on virological clearance in COVID-19 patients. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=46873&EncHid=&modid=&compid=%27,%2746873det%27 (first received 26 August 2020).
CTRI/2020/10/028335 {published data only}
- CTRI/2020/10/028335. A clinical study to assess the efficacy and safety of Tinefcon in patients with moderate COVID-19 infection. cochranelibrary.com/es/central/doi/10.1002/central/CN-02186249/full (first received 30 November 2020).
Elgazzar 2020 {published data only}
- Elgazzar A, Eltaweel A, Youssef SA, Hany B, Hafez M, Moussa H. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic (preprint). researchsquare.com/article/rs-100956/v3 (first received 28 December 2020). [DOI: 10.21203/rs.3.rs-100956/v3] [DOI]
- NCT04668469. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic. clinicaltrials.gov/ct2/show/NCT04668469 (first received 16 December 2020).
Galan 2021 {published data only}
- Galan LE, Melo dos Santos N, Asato MS, Araújo JV, Moreira A, Marques-Araújo AM. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathology Global Health 2021;115(4):235-42. [DOI: 10.1080/20477724.2021.1890887] [DOI] [PMC free article] [PubMed]
- RBR-8h7q82. The effect of chloroquine, hydroxychloroquine or ivermectin in patients with severe manifestations of coronavirus. ensaiosclinicos.gov.br/rg/RBR-8h7q82/ (first received 2 October 2020).
Gorial 2020 {published data only}
- Gorial FI, Mashhadani S, Sayaly HM, Dakhil BD, AlMashhadani MM, Aljabory AM, et al. Effectiveness of ivermectin as add-on therapy in COVID-19 management (pilot trial). medrxiv.org/content/10.1101/2020.07.07.20145979v1 (first received 8 July 2020). [DOI: 10.1101/2020.07.07.20145979] [DOI]
- NCT04343092. Ivermectin adjuvant to hydroxychloroquin in COVID19 patients. clinicaltrials.gov/ct2/show/NCT04343092 (first received 4 November 2020).
Hashim 2020 {published data only}
- Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medrxiv.org/content/10.1101/2020.10.26.20219345v1 (first received 27 October 2020). [DOI: 10.1101/2020.10.26.20219345] [DOI]
- NCT04591600. Effectiveness of ivermectin and doxycycline on COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04591600 (first received 19 October 2020).
IRCT20180922041089N4 {published data only}
- IRCT20180922041089N4. Evaluation of the effect of oral Ivermectin on the outcome of patients with COVID-19 and compare it with the effect of conjunctional therapies in patients admitted to Ziaeian, Baharloo, Imam Khomeini in the spring and summer 2020. en.irct.ir/trial/50305 (first received 23 August 2020).
IRCT20200408046987N2 {published data only}
- IRCT20200408046987N2. Determination the therapeutic effect of Ivermectin and Sovodak on patients infected with COVID-19: a clinical trial. en.irct.ir/trial/51007 (first received 7 November 2020).
Lima‐Morales 2021 {published data only}
- Lima-Morales R, Méndez-Hernández P, Flores YN, Osorno-Romero P, Sancho-Hernándezk CR, Cuecuecha-Rugerio E. Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast, and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico. International Journal of Infectious Diseases 2021;105:598-605. [DOI: 10.1016/j.ijid.2021.02.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmud 2021 {published data only}
- Mahmud R, Rahman M, Alam I, Ahmed KG, Humayon Kabir AK, Jakaria Been Sayeed SK, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. Journal of International Medical Research 2021;49(5):1-14. [DOI: 10.1177/03000605211013550] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04523831. Clinical trial of ivermectin plus doxycycline for the treatment of confirmed Covid-19 infection. clinicaltrials.gov/ct2/show/results/NCT04523831 (first received 24 August 2020).
Morgenstern 2020 {published data only}
- Morgenstern J, Redondo JN, De León A, Canela JM, Torres N, Tavares J, et al. The use of compassionate Ivermectin in the management of symptomatic outpatients and hospitalized patients with clinical diagnosis of COVID-19 at the Medical Center Bournigal and the Medical Center Punta Cana, Rescue Group, Dominican Republic, from May 1 to August 10, 2020. medrxiv.org/content/10.1101/2020.10.29.20222505v1 (first received 3 November 2020). [DOI: 10.1101/2020.10.29.20222505] [DOI]
NCT04345419 {published data only}
- NCT04345419. Remdesivir efficacy in coronavirus disease. clinicaltrials.gov/ct2/show/NCT04345419 (first received 14 April 2020).
NCT04360356 {published data only}
- NCT04360356. Ivermectin and nitazoxanide combination therapy for COVID-19. clinicaltrials.gov/ct2/show/NCT04360356 (first received 24 April 2020).
NCT04374279 {published data only}
- NCT04374279. Trial to promote recovery from COVID-19 with ivermectin or endocrine therapy. clinicaltrials.gov/ct2/show/NCT04374279 (first received 5 May 2020).
NCT04382846 {published data only}
- NCT04382846. Novel regimens in COVID-19 treatment. clinicaltrials.gov/ct2/show/NCT04382846 (first received 11 May 2020).
NCT04392427 {published data only}
- NCT04392427. New antiviral drugs for treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04392427 (first received 18 May 2020).
NCT04435587 {published data only}
- NCT04435587. Ivermectin vs combined hydroxychloroquine and antiretroviral drugs (ART) among asymptomatic COVID-19 infection (IDRA-COVID19). clinicaltrials.gov/ct2/show/NCT04435587 (first received 17 June 2020).
NCT04447235 {published data only}
- NCT04447235. Early treatment with ivermectin and losartan for cancer patients with COVID-19 Infection. clinicaltrials.gov/ct2/show/NCT04447235 (first received 25 June 2020).
NCT04482686 {published data only}
- NCT04482686. Trial of combination therapy to treat COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04482686 (first received 22 July 2020).
NCT04530474 {published data only}
- NCT04530474. Outpatient use of ivermectin in COVID-19. clinicaltrials.gov/ct2/show/NCT04530474 (first received 28 August 2020).
NCT04551755 {published data only}
- NCT04551755. Safety and efficacy of ivermectin and doxycycline in treatment of Covid-19. clinicaltrials.gov/ct2/show/NCT04551755 (first received 16 September 2020).
NCT04703608 {published data only}
- NCT04703608. Prevention and treatment for COVID -19 (severe acute respiratory syndrome coronavirus 2 SARS-CoV-2) associated severe pneumonia in the Gambia (PaTS-COVID). clinicaltrials.gov/ct2/show/NCT04703608 (first received 11 January 2021).
NCT04723459 {published data only}
- NCT04723459. Efficacy of nano-ivermectin impregnated masks in prevention of Covid-19 among healthy contacts and medical staff. clinicaltrials.gov/ct2/show/NCT04723459 (first received 25 January 2021).
NCT04768179 {published data only}
- NCT04768179. Safety & efficacy of low dose aspirin/ivermectin combination therapy for treatment of Covid-19 patients (IVCOM). clinicaltrials.gov/ct2/show/NCT04768179 (first received 24 February 2021).
Niaee 2020 {published data only}
- IRCT20200408046987N1. Dose-finding study of Ivermectin treatment on patients infected with Covid-19: a clinical trial. en.irct.ir/trial/47012 (first received 27 April 2020).
- Niaee MS, Gheibi N, Namdar P, Allami A, Zolghadr L, Javadi A, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial (preprint). researchsquare.com/article/rs-109670/v1 (first received 24 November 2020). [DOI: 10.21203/rs.3.rs-109670/v1] [DOI]
Rajter 2021 {published data only}
- Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19. medrxiv.org/content/10.1101/2020.06.06.20124461v2 (first received 10 June 2020). [DOI: 10.1101/2020.06.06.20124461] [DOI]
- Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest 2021;159(1):85-92. [DOI: 10.1016/j.chest.2020.10.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seet 2021 {published data only}
- NCT04446104. A preventive treatment for migrant workers at high-risk of COVID-19. clinicaltrials.gov/ct2/show/NCT04446104 (first received 24 June 2020).
- Seet RC, Lin Quek AM, Qin Ooi DS, Sengupta S, Lashminarasappa SR, Yang Koo C. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. International Journal of Infectious Diseases 2021;106:314-22. [DOI: 10.1016/j.ijid.2021.04.035] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahbaznejad 2021 {published data only}
- IRCT20111224008507N3. Effectiveness of ivermectin in the treatment of coronavirus infection in patients admitted to educational hospitals of Mazandaran in 2020. en.irct.ir/trial/49174 (first received 27 June 2020).
- Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clinical Therapeutics 6 May 2021 [Epub ahead of print]. [DOI: 10.1016/j.clinthera.2021.04.007] [DOI] [PMC free article] [PubMed]
Spoorthi 2020 {published data only}
- Spoorthi V, Sasank S. Utility of ivermectin and doxycycline combination for the treatment of SARS-CoV-2. International Archives of Integrated Medicine 2020;7(10):177-82. [iaimjournal.com/wp-content/uploads/2020/10/iaim_2020_0710_23.pdf] [Google Scholar]
References to studies awaiting assessment
2020‐001971‐33/ES {published data only}
- 2020-001971-33/ES. Pragmatic study "CORIVER": ivermectin as antiviral treatment for patients infected by SARS-COV2 (COVID19). clinicaltrialsregister.eu/ctr-search/trial/2020-001971-33/ES (first received 22 July 2020).
2020‐002091‐12/BG {published data only}
- 2020-002091-12/BG. Multicenter, randomized, double-blind, placebo-controlled study investigating efficacy, safety and tolerability of ivermectin HUVE-19 in patients with proven SARS-CoV-2 infection (COVID-19) and manifested clinical symptoms. clinicaltrialsregister.eu/ctr-search/trial/2020-002091-12/BG (first received 5 May 2020).
CTRI/2020/04/024948 {published data only}
- CTRI/2020/04/024948. A clinical trial to study the effects of hydroxychloroquine, ciclesonide and ivermectin in treatment of moderate COVID-19 illness. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=43364 (first received 30 April 2020).
CTRI/2020/06/025960 {published data only}
- CTRI/2020/06/025960. To study effect of ivermectin drug in patients infected with SARS-CoV-2 virus. ctri.nic.in/Clinicaltrials/showallp.php?mid1=44373&EncHid=&userName=CTRI/2020/06/025960 (first received 18 June 2020).
Faisal 2020 {published data only}
- Faisal R, Ali Shah SF, Hussain M. Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19. Professional Medical Journal 2020;28(5):737-41. [DOI: 10.29309/TPMJ/2021.28.05.5867] [DOI] [Google Scholar]
Hosseini 2021 {published data only}
- Hosseini FS, Malektojari A, Ghazizadeh S, Hassaniazad M, Davoodian P, Dadvand H, et al. The efficacy and safety of ivermectin in patients with mild and moderate COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2021;22(1):4. [DOI: 10.1186/s13063-020-04988-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT20200506047323N6. The efficacy and safety of Ivermectin in patients with COVID-19: a randomized clinical trial. en.irct.ir/trial/49501 (first received 17 November 2020).
IRCT20190602043787N3 {published data only}
- IRCT20190602043787N3. Evaluation of the effect of ivermectin in hospitalized patients with COVID-19 in Imam Reza Hospital in Mashhad. en.irct.ir/trial/49180 (first received 20 July 2020).
IRCT20200408046987N3 {published data only}
- IRCT20200408046987N3. Evaluation of prophylaxis induced by ivermectin in populations exposed to COVID-19 patients. www.irct.ir/trial/51999 (first received 6 December 2020).
IRCT20200422047168N2 {published data only}
- IRCT20200422047168N2. Clinical trial study of the therapeutic effect of ivermectin, besides kaletra and chloroquine in patients with coronavirus disease 2019 (COVID-19). en.irct.ir/trial/48444 (first received 30 May 2020).
ISRCTN90437126 {published data only}
- ISRCTN90437126 . Study on the effects of using ivermectin to prevent COVID-19 in an adult population in Brazil. www.isrctn.com/ISRCTN90437126 (first received 11 November 2020).
NCT04351347 {published data only}
- NCT04351347. The efficacy of ivermectin in larger doses in COVID-19 treatment. clinicaltrials.gov/ct2/show/NCT04351347 (first received 17 April 2020).
NCT04374019 {published data only}
- NCT04374019. Novel agents for treatment of high-risk COVID-19 positive patients. clinicaltrials.gov/ct2/show/NCT04374019 (first received 5 May 2020).
NCT04407130 {published data only}
- NCT04407130. Efficacy and safety of ivermectin and doxycycline in combination or IVE alone in patients with COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04407130 (first received 29 May 2020).
NCT04407507 {published data only}
- NCT04407507. Efficacy, safety and tolerability of ivermectin in subjects infected with SARS-CoV-2 with or without symptoms. clinicaltrials.gov/ct2/show/NCT04407507 (first received 29 May 2020).
NCT04716569 {published data only}
- NCT04716569. Evaluation of ivermectin mucoadhesive nanosuspension as nasal spray in management of early Covid-19. clinicaltrials.gov/ct2/show/NCT04716569 (first received 20 January 2020).
NCT04746365 {published data only}
- NCT04746365. Ivermectin Role In Covid-19 Clinical Trial (IRICT). clinicaltrials.gov/ct2/show/NCT04746365 (first received 9 February 2021).
NCT04891250 {published data only}
- NCT04891250. The Zambia Ivermectin Trial for the treatment and prevention of COVID-19 (ZIT). clinicaltrials.gov/ct2/show/NCT04891250 (first received 18 May 2021).
Samaha 2021 {published data only}
- ChiCTR2000033627. In vivo use of ivermectin (IVR) for treatment for corona virus infected patients: a randomized controlled trial. chictr.org.cn/showprojen.aspx?proj=54707 (first received 7 June 2020).
- Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, Bazzal AA, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses 2021;13(6):989. [DOI: 10.3390/v13060989] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
References to ongoing studies
2020‐001994‐66/ES {published data only}
- 2020-001994-66/ES. Study of the efficacy of ivermectin in the treatment and prevention of COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-001994-66/ES (first received 7 May 2020).
ACTRN12620000982910 {published data only}
- ACTRN12620000982910. A randomized double-blind placebo-controlled trial of oral ivermectin for outpatient treatment of those at high risk for hospitalization due to COVID-19. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000982910 (first received 14 September 2020).
CTRI/2020/05/025068 {published data only}
- CTRI/2020/05/025068. A phase IIB open label randomized controlled trial to evaluate the efficacy and safety of ivermectin in reducing viral loads in patients with hematological disorders who are admitted with COVID 19 infection. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=43449 (first received 7 May 2020).
CTRI/2020/05/025224 {published data only}
- CTRI/2020/05/025224. Study to efficacy of ivermectin in patients of COVID-19. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=43728&EncHid=&modid=&compid=%27,%2743728det%27 (first received 18 May 2020).
Garcia 2021 {published data only}
- Garcia PJ, Hurtado HM, Ugarte-Gil C, Leon P, Malaga G, Chaccour C, et al. Randomized clinical trial to compare the efficacy of ivermectin versus placebo to negativize nasopharyngeal PCR in patients with early COVID-19 in Peru (SAINT-Peru): a structured summary of a study protocol for randomized controlled trial. Trials 2021;22(1):262. [DOI: 10.1186/s13063-021-05236-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garica PJ, Huratdo HM, Urgae-Gil C, Leon P, Malaga G, Chaccour C, et al. Randomized clinical trial to compare the efficacy of ivermectin versus placebo to negativize nasopharyngeal PCR in patients with early COVID-19 in Peru (SAINT-Peru): a structured summary of a study protocol for randomized controlled trial. researchsquare.com/article/rs-345747/v1 (first received 23 May 2021). [DOI: 10.21203/rs.3.rs-345747/v1] [DOI] [PMC free article] [PubMed]
- NCT04635943. Randomized phase IIA clinical trial to evaluate the efficacy of ivermectin to obtain negative PCR results in patients with early phase COVID-19 (SAINT-PERU). clinicaltrials.gov/ct2/show/NCT04635943 (first received 19 November 2020).
- PER-034-20. Randomized phase IIa clinical trial to compare the efficacy of ivermectin versus placebo to obtain negative PCR results in patients with early phase Covid-19. www.ins.gob.pe/ensayosclinicos/rpec/recuperarECPBNuevoEN.asp?numec=034-20 (first received 17 July 2020).
IRCT20111224008507N4 {published data only}
- IRCT20111224008507N4. Double-blind placebo-controlled clinical trial of evaluating the effectiveness of ivermectin in treatment of outpatients with COVID-19 in 2021. irct.ir/trial/53949 (first received 31 January 2021).
IRCT20111224008507N5 {published data only}
- IRCT20111224008507N5. Double-blind placebo-controlled clinical trial of evaluating the effectiveness of ivermectin in treatment of patients admitted with COVID-19 in 2021. en.irct.ir/trial/54402 (first received 22 February 2021).
IRCT20190624043993N2 {published data only}
- IRCT20190624043993N2. Evaluation effects of the standard regimen along with ivermectin on treatment of corona virus type 2 pneumonia. irct.ir/trial/49280 (first received 12 July 2020).
IRCT20200404046937N4 {published data only}
- IRCT20200404046937N4. Evaluating the efficacy and safety of Ivermectin in the treatment of COVID-19 patients: a double-blind randomized controlled trial, phase II. en.irct.ir/trial/49935 (first received 6 August 2020).
NCT04403555 {published data only}
- NCT04403555. Ivermectin as a novel therapy in COVID-19 treatment. clinicaltrials.gov/ct2/show/NCT04403555 (first received 27 May 2020).
NCT04425707 {published data only}
- NCT04425707. Ivermectin in treatment of COVID 19 patients. clinicaltrials.gov/ct2/show/NCT04425707 (first received 11 June 2020).
NCT04429711 {published data only}
- NCT04429711. Ivermectin vs. placebo for the treatment of patients with mild to moderate COVID-19. clinicaltrials.gov/ct2/show/NCT04429711 (first received 12 June 2020).
NCT04438850 {published data only}
- 2020-002283-32/IT. Randomized, double-blind, multi centre phase II, proof of concept, dose finding clinical trial on ivermectin for the early treatment of COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-002283-32/IT (first received 10 August 2020).
- NCT04438850. COVidIVERmectin: ivermectin for treatment of Covid-19 (COVER). clinicaltrials.gov/ct2/show/NCT04438850 (first received 19 June 2020).
NCT04445311 {published data only}
- NCT04445311. Ivermectin in treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04445311 (first received 24 June 2020).
NCT04472585 {published data only}
- NCT04472585. Efficacy of subcutaneous ivermectin with or without zinc in COVID-19 patients (SIZI-COVID-PK). clinicaltrials.gov/ct2/show/NCT04472585 (first received 15 July 2020).
NCT04510233 {published data only}
- NCT04510233. Ivermectin nasal spray for COVID19 patients. clinicaltrials.gov/ct2/show/NCT04510233 (first received 12 August 2020).
NCT04527211 {published data only}
- NCT04527211. Effectiveness and safety of ivermectin for the prevention of Covid-19 infection in Colombian health personnel (IveprofCovid19). clinicaltrials.gov/ct2/show/NCT04527211 (first received 26 August 2020).
NCT04602507 {published data only}
- NCT04602507. Ivermectin in adults with severe COVID-19. clinicaltrials.gov/ct2/show/NCT04602507 (first received 26 October 2020).
NCT04673214 {published data only}
- NCT04673214. Evaluation of prognostic modification in COVID-19 patients in early intervention treatment, a randomized clinical trial. clinicaltrials.gov/ct2/show/NCT04673214 (first received 17 December 2020).
NCT04703205 {published data only}
- jRCT2031200120. Double-blind study in Covid-19 patients with ivermectin. jrct.niph.go.jp/en-latest-detail/jRCT2031200120 (first received 16 September 2020).
- NCT04703205. Study in Covid-19 patients with ivermectin (CORVETTE-01). clinicaltrials.gov/ct2/show/NCT04703205 (first received 11 January 2021).
NCT04712279 {published data only}
- NCT04712279. The (HD)IVACOV Trial (The High-Dose IVermectin Against COVID-19 Trial). https://clinicaltrials.gov/ct2/show/NCT04712279 (first received 15 January 2021).
NCT04727424 {published data only}
- NCT04727424. Repurposed approved therapies for outpatient treatment of patients with early-onset COVID-19 and mild symptoms. clinicaltrials.gov/ct2/show/NCT04727424 (first received 27 January 2021).
NCT04729140 {published data only}
- NCT04729140. An outpatient clinical trial using ivermectin and doxycycline in COVID-19 positive patients at high risk to prevent COVID-19 related hospitalization. clinicaltrials.gov/ct2/show/NCT04729140 (first received 28 January 2021).
NCT04834115 {published data only}
- NCT04834115. Efficacy of ivermectin in outpatients with non-severe COVID-19. clinicaltrials.gov/ct2/show/NCT04834115 (first received 6 April 2021).
NCT04836299 {published data only}
- NCT04836299. Clinical trial to "study the efficacy and therapeutic safety of ivermectin (SAINTBO). clinicaltrials.gov/ct2/show/NCT04836299 (first received 8 April 2021).
NCT04885530 {published data only}
- NCT04885530. ACTIV-6: COVID-19 study of repurposed medications. clinicaltrials.gov/ct2/show/NCT04885530 (first received 13 May 2021).
NCT04886362 {published data only}
- NCT04886362. Ivermectina Colombia (IVERCOL). clinicaltrials.gov/ct2/show/NCT04886362 (first received 14 May 2021).
NCT04894721 {published data only}
- NCT04894721. Prophylaxis for COVID-19: ivermectin in close contacts of COVID-19 cases (IVERNEX-TUC). clinicaltrials.gov/ct2/show/NCT04894721 (first received 20 May 2021).
PACTR202102588777597 {published data only}
- PACTR202102588777597. Ivermectin Treatment Trial (ITT). pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-PACTR202102588777597 (first received 12 February 2021).
PACTR202102848675636 {published data only}
- PACTR202102848675636. Double blind, community-based, randomized controlled trial on the use of ivermectin as post exposure chemo-prophylaxis for COVID-19 among high risk individuals in Lagos (IVERPEPCOV) COVID-19. pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ictrp-PACTR202102848675636 (first received 11 February 2021).
Vallejos 2020 {published data only}
- NCT04529525. Ivermectin to prevent hospitalizations in COVID-19 (IVERCORCOVID19). clinicaltrials.gov/ct2/show/NCT04529525 (first received 27 August 2020).
- Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19): a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):965. [DOI: 10.1186/s13063-020-04813-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ashour 2019
- Ashour DS. Ivermectin: from theory to clinical application. International Journal of Antimicrobial Agents 2019;54(2):134-42. [DOI: 10.1016/j.ijantimicag.2019.05.003] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines – 3: rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beigel 2020
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. New England Journal of Medicine 2020;383:1813-26. [DOI: 10.1056/NEJMoa2007764] [DOI] [PMC free article] [PubMed] [Google Scholar]
BIRD 2021
- British Ivermectin Recommendation Development panel. The BIRD recommendation on the use of ivermectin for Covid-19. francesoir.fr/sites/francesoir/files/media-icons/bird-proceedings-02-03-2021-v151.pdf (accessed prior to 19 July 2021).
Bryant 2021
- Bryant A, Lawrie T, Dowswell T, Fordham E, Mitchell S, Hill S, et al. Ivermectin for prevention and treatment of COVID-19 infection. American Journal of Therapeutics 17 June 2021 [Epub ahead of print]. [DOI: 10.1097/MJT.0000000000001402] [DOI] [PMC free article] [PubMed]
Caly 2020
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research 2020;178:e1047874. [DOI: 10.1016/j.antiviral.2020.104787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1983
- Campbell WC, Fisher MH, Stapley EO, Albers-Schoenberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science 1983;221(4613):823-8. [DOI: 10.1126/science.6308762] [DOI] [PubMed] [Google Scholar]
Cochrane LSR
- Cochrane LSR. Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Available from community.cochrane.org/review-production/production-resources/living-systematic-reviews (accessed 22 March 2021).
CONSORT 2010 Statement
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. [DOI] [PubMed] [Google Scholar]
COVID Guidelines India 2021
- Covid Management Guidelines India Group. COVID Management Guidelines India. indiacovidguidelines.org/ivermectin/ (accessed 15 May 2021).
COVID‐NMA Working Group
- COVID-NMA working group. The COVID-NMA initiative: a living mapping and living systematic review of Covid-19 trials. covid-nma.com (accessed prior to 1 July 2021).
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. Available from training.cochrane.org/handbook/archive/v6.1.
Deng 2020
- Deng G, Yin M, Chen X, Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Critical Care 2020;24:e179. [DOI: 10.1186/s13054-020-02902-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dong 2020
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infectious Diseases 2020;20(5):533-4. [DOI: 10.1016/S1473-3099(20)30120-1] [DOI] [PMC free article] [PubMed]
Dourmishev 2005
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. International Journal of Dermatology 2005;44(12):981-8. [DOI: 10.1111/j.1365-4632.2004.02253.x] [DOI] [PubMed] [Google Scholar]
EMA 2021
- European Medicines Agency. EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials. ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-covid-19-outside-randomised-clinical-trials (accessed 15 May 2021).
FDA 2020
- US Food and Drug Administration. Product safety information: COVID-19 and ivermectin intended for animals. fda.gov/animal-veterinary/product-safety-information/faq-covid-19-and-ivermectin-intended-animals (accessed 19 February 2020).
Garegnani 2021
German AWMF Guideline 2021
- Kluge S, Janssens U, Welte T, Weber-Carstens S, Schälte G, Spinner CD, et al. S3-Guideline – recommendations on Inpatient Treatment of Patients With COVID-19 [S3-Leitlinie - Empfehlungen zur stationären Therapie von Patienten mit COVID-19]. awmf.org/uploads/tx_szleitlinien/113-001l_S3_Empfehlungen-zur-stationaeren-Therapie-von-Patienten-mit-COVID-19__2021-02.pdf (accessed 25 February 2021). [DOI] [PubMed]
Goetz 2016
- Goetz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Hoeper D, et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Scientific Reports 2016;6:e23138. [DOI: 10.1038/srep23138] [DOI] [PMC free article] [PubMed] [Google Scholar]
González‐Canga 2008
- González-Canga A, Sahagún-Prieto AM, Diez-Liébana MJ, Fernández-Martínez N, Sierra-Vega M, García-Vieitez JJ. The pharmacokinetics and interactions of ivermectin in humans. Journal of the American Association of Pharmaceutical Scientists 2008;10:42-6. [DOI: 10.1208/s12248-007-9000-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrmann 2020
- Herrmann J, Adam EH, Notz Q, Helmer P, Sonntagbauer M, Ungemach-Papenberg P, et al. COVID-19 induced acute respiratory distress syndrome – a multicenter observational study. Frontiers in Medicine 2020;7:599533. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2020b
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2020c
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2020d
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2021
- Higgins JP, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version February 2021. Available from: www.community.cochrane.org/mecir-manual/ (accessed prior to 19 July 2021).
Hill 2021
- Hill A, Abdulamir A, Ahmed S, Ashgar A, Babalola OE, Basri E, et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. researchsquare.com/article/rs-148845/v1 (first received 19 January 2021). [DOI: 10.21203/rs.3.rs-148845/v1] [DOI]
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI: 10.1016/S0140-6736(20)30183-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
IDSA 2021
- Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19, version 4.3.0. idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed 15 May 2021).
IntHout 2016
- IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;12(6):e010247. [DOI: 10.1136/bmjopen-2015-010247] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ivmmeta.com
- Ivermectin for COVID-19: real-time meta analysis of 60 studies. ivmmeta.com/ (accessed prior to 1 July 2021).
Karagiannidis 2020
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respiratory Medicine 2020;9:853-62. [DOI: 10.1016/S2213-2600(20)30316-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobayashi 2020
- Kobayashi T, Jung S, Linton NM, Kinoshita R, Hayashi K, Miyama T, et al. Communicating the risk of death from novel coronavirus disease (COVID-19). Journal of Clinical Medicine 2020;9(2):580. [DOI: 10.3390/jcm9020580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kory 2021
- Kory P, Meduri GU, Iglesias J, Varon J, Berkowitz K, Kornfeld H, et al. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. osf.io/wx3zn/ (first received 16 January 2021). [DOI: 10.31219/osf.io/wx3zn] [DOI] [PMC free article] [PubMed]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from: training.cochrane.org/handbook/archive/v6.1.
MAGICapp [Computer program]
- MAGICapp. MAGIC, 2020. Available at magicevidence.org/magicapp/.
Marshall 2020
- Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI: 10.1016/S1473-3099(20)30483-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Merck 2021
- Merck. Merck statement on ivermectin use during the COVID-19 pandemic. merck.com/news/merck-statement-on-ivermectin-use-during-the-covid-19-pandemic/ (accessed 15 May 2021).
Meta [Computer program]
- cran.r-project.org/web/packages/meta Meta: general package for meta-analysis. cran.r-project.org/web/packages/meta, 2021. Available at cran.r-project.org/web/packages/meta/meta.pdf.
NIH 2021
- COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. www.covid19treatmentguidelines.nih.gov/ (accessed 19 February 2021).
Oran 2021
- Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Annals of Internal Medicine 2021;174(5):655-62. [DOI: 10.7326/M20-6976] [DOI] [PMC free article] [PubMed]
Panahi 2015
- Panahi Y, Poursaleh Z, Goldust M. The efficacy of topical and oral ivermectin in the treatment of human scabies. Annals of Parasitology 2015;61(1):11-6. [PMID: ] [PubMed] [Google Scholar]
Prescott 2020
- Prescott HC, Girard TD. Recovery from severe COVID-19. JAMA 2020;324(8):739-40. [DOI: 10.1001/jama.2020.14103] [DOI] [PubMed] [Google Scholar]
PRINCIPLE trial
- University of Oxford. Ivermectin to be investigated in adults aged 18+ as a possible treatment for COVID-19 in the PRINCIPLE trial. principletrial.org/news/ivermectin-to-be-investigated-as-a-possible-treatment-for-covid-19-in-oxford2019s-principle-trial (accessed 1 July 2021).
RECOVERY 2021
- Recovery Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. New England Journal of Medicine 2021;384:693-704. [DOI: 10.1056/NEJMoa2021436] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 2.3.0. Cochrane, 2020. Available at revman.cochrane.org.
Rodríguez‐Mega 2020
- Rodríguez-Mega E. Latin America's embrace of an unproven COVID treatment is hindering drug trials. Nature 2020;586:481-2. [DOI: 10.1038/d41586-020-02958-2] [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Siemieniuk 2020
- Siemieniuk R, Rochwerg B, Agoritsas T, Lamontagne F, Leo Y, Macdonald H, et al. A living WHO guideline on drugs for Covid-19. BMJ 2020;370:m379. [DOI: 10.1136/bmj.m3379] [DOI] [PubMed] [Google Scholar]
Singh 2021
- Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013587. [DOI: 10.1002/14651858.CD013587.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [PMID: ] [DOI] [PubMed] [Google Scholar]
Supplementary File_Ivermectin_Risk of Bias
- Weibel S, Popp M. Supplementary File_Ivermectin_Risk of Bias Excel Tool (Version 1). Zenodo 2021. [DOI: 10.5281/zenodo.5118956] [DOI]
Tay 2013
- Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Research 2013;99(3):301-6. [DOI: 10.1016/j.antiviral.2013.06.002] [DOI] [PubMed] [Google Scholar]
The Guardian 2021
- Davey M. Huge study supporting ivermectin as Covid treatment withdrawn over ethical concerns. Available at www.theguardian.com/science/2021/jul/16/huge-study-supporting-ivermectin-as-covid-treatment-withdrawn-over-ethical-concerns (accessed 15 July 2021).
Wagstaff 2012
- Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochemical Journal 2012;443(3):851-6. [DOI: 10.1042/BJ20120150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2020
- Watson J, Whiting PF, Brush JE. Interpreting a Covid-19 test result. BMJ 2020;369:m1808. [DOI: 10.1136/bmj.m1808] [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. The World Health Organization Quality of Life (WHOQOL). www.who.int/publications/i/item/WHO-HIS-HSI-Rev.2012.03 (accessed 19 March 2021).
WHO 2019
- World Health Organization. WHO model list of essential medicines, 21st list. www.who.int/medicines/publications/essentialmedicines/en (accessed 19 February 2021).
WHO 2020a
- World Health Organization. Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID-19). www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (accessed 3 June 2021).
WHO 2020b
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. covid19.who.int/ (accessed 2 July 2021).
WHO 2020c
- World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays: interim guidance, 11 September 2020. Available at https://apps.who.int/iris/handle/10665/334253.
WHO 2021a
- World Health Organization. Weekly epidemiological update – 19 January 2021. www.who.int/publications/m/item/weekly-epidemiological-update---19-january-2021 (accessed 19 February 2021).
WHO 2021b
- World Health Organization. Therapeutics and COVID-19. Living guideline. who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1 (accessed 3 June 2021).
Williamson 2020
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [DOI: 10.1038/s41586-020-2521-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wulan 2015
- Wulan WN, Heydet D, Walker EJ, Gahan ME, Ghildyal R. Nucleocytoplasmic transport of nucleocapsid proteins of enveloped RNA viruses. Frontiers in Microbiology 2015;6:e553. [DOI: 10.3389/fmicb.2015.00553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamasmith 2018
- Yamasmith E, Saleh-arong FA, Avirutnan P, Angkasekwinai N, Mairiang D, Wongsawat E, et al. Efficacy and safety of ivermectin against dengue infection: a phase III, randomized, double-blind, placebo-controlled trial. Internal Medicine and One Health. 34th Annual Meeting of the Royal College of Physicians of Thailand; 2018 April 26-28; Chonburi (THA) 2018.
Yang 2020
- Yang SN, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Research 2020;177:e104760. [DOI: 10.1016/j.antiviral.2020.104760] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Popp 2021
- Popp M, Stegemann M, Metzendorf MI, Kranke P, Meybohm P, Skoetz N, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No: CD015017. [DOI: 10.1002/14651858.CD015017] [DOI] [PMC free article] [PubMed] [Google Scholar]


