Abstract
Background
The role of vitamin D supplementation as a treatment for COVID‐19 has been a subject of considerable discussion. A thorough understanding of the current evidence regarding the effectiveness and safety of vitamin D supplementation for COVID‐19 based on randomised controlled trials is required.
Objectives
To assess whether vitamin D supplementation is effective and safe for the treatment of COVID‐19 in comparison to an active comparator, placebo, or standard of care alone, and to maintain the currency of the evidence, using a living systematic review approach.
Search methods
We searched the Cochrane COVID‐19 Study Register, Web of Science and the WHO COVID‐19 Global literature on coronavirus disease to identify completed and ongoing studies without language restrictions to 11 March 2021.
Selection criteria
We followed standard Cochrane methodology. We included randomised controlled trials (RCTs) evaluating vitamin D supplementation for people with COVID‐19, irrespective of disease severity, age, gender or ethnicity.
We excluded studies investigating preventive effects, or studies including populations with other coronavirus diseases (severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS)).
Data collection and analysis
We followed standard Cochrane methodology.
To assess bias in included studies, we used the Cochrane risk of bias tool (ROB 2) for RCTs. We rated the certainty of evidence using the GRADE approach for the following prioritised outcome categories: individuals with moderate or severe COVID‐19: all‐cause mortality, clinical status, quality of life, adverse events, serious adverse events, and for individuals with asymptomatic or mild disease: all‐cause mortality, development of severe clinical COVID‐19 symptoms, quality of life, adverse events, serious adverse events.
Main results
We identified three RCTs with 356 participants, of whom 183 received vitamin D. In accordance with the World Health Organization (WHO) clinical progression scale, two studies investigated participants with moderate or severe disease, and one study individuals with mild or asymptomatic disease. The control groups consisted of placebo treatment or standard of care alone.
Effectiveness of vitamin D supplementation for people with COVID‐19 and moderate to severe disease
We included two studies with 313 participants. Due to substantial clinical and methodological diversity of both studies, we were not able to pool data. Vitamin D status was unknown in one study, whereas the other study reported data for vitamin D deficient participants. One study administered multiple doses of oral calcifediol at days 1, 3 and 7, whereas the other study gave a single high dose of oral cholecalciferol at baseline. We assessed one study with low risk of bias for effectiveness outcomes, and the other with some concerns about randomisation and selective reporting.
All‐cause mortality at hospital discharge (313 participants)
We found two studies reporting data for this outcome. One study reported no deaths when treated with vitamin D out of 50 participants, compared to two deaths out of 26 participants in the control group (Risk ratio (RR) 0.11, 95% confidence interval (CI) 0.01 to 2.13). The other study reported nine deaths out of 119 individuals in the vitamin D group, whereas six participants out of 118 died in the placebo group (RR 1.49, 95% CI 0.55 to 4.04]. We are very uncertain whether vitamin D has an effect on all‐cause mortality at hospital discharge (very low‐certainty evidence).
Clinical status assessed by the need for invasive mechanical ventilation (237 participants)
We found one study reporting data for this outcome. Nine out of 119 participants needed invasive mechanical ventilation when treated with vitamin D, compared to 17 out of 118 participants in the placebo group (RR 0.52, 95% CI 0.24 to 1.13). Vitamin D supplementation may decrease need for invasive mechanical ventilation, but the evidence is uncertain (low‐certainty evidence).
Quality of life
We did not find data for quality of life.
Safety of vitamin D supplementation for people with COVID‐19 and moderate to severe disease
We did not include data from one study, because assessment of serious adverse events was not described and we are concerned that data might have been inconsistently measured. This study reported vomiting in one out of 119 participants immediately after vitamin D intake (RR 2.98, 95% CI 0.12 to 72.30). We are very uncertain whether vitamin D supplementation is associated with higher risk for adverse events (very low‐certainty).
Effectiveness and safety of vitamin D supplementation for people with COVID‐19 and asymptomatic or mild disease
We found one study including 40 individuals, which did not report our prioritised outcomes, but instead data for viral clearance, inflammatory markers, and vitamin D serum levels. The authors reported no events of hypercalcaemia, but recording and assessment of further adverse events remains unclear. Authors administered oral cholecalciferol in daily doses for at least 14 days, and continued with weekly doses if vitamin D blood levels were > 50 ng/mL.
Authors' conclusions
There is currently insufficient evidence to determine the benefits and harms of vitamin D supplementation as a treatment of COVID‐19. The evidence for the effectiveness of vitamin D supplementation for the treatment of COVID‐19 is very uncertain. Moreover, we found only limited safety information, and were concerned about consistency in measurement and recording of these outcomes.
There was substantial clinical and methodological heterogeneity of included studies, mainly because of different supplementation strategies, formulations, vitamin D status of participants, and reported outcomes.
There is an urgent need for well‐designed and adequately powered randomised controlled trials (RCTs) with an appropriate randomisation procedure, comparability of study arms and preferably double‐blinding. We identified 21 ongoing and three completed studies without published results, which indicates that these needs will be addressed and that our findings are subject to change in the future. Due to the living approach of this work, we will update the review periodically.
Plain language summary
Is vitamin D an effective and safe treatment for COVID‐19?
Key messages
‐ We did not find enough, good‐quality evidence to judge whether vitamin D is an effective or safe treatment for adults with COVID‐19.
‐ We need more research on this topic. Future research should focus on well‐designed studies with robust methods.
‐ We identified 21 studies on this topic that are ongoing. We will update this review when more evidence becomes available.
What is the link between vitamin D and COVID‐19?
Some studies have shown that people who are in hospital with severe COVID‐19 also have low levels of vitamin D (vitamin D deficiency). However, the risk factors for developing severe COVID‐19 are the same as those for developing vitamin D deficiency, so it is difficult to tell if vitamin D deficiency itself is a risk factor for severe COVID‐19. Risk factors include general ill‐health, a poor diet, and pre‐existing health conditions, such as diabetes, and liver and kidney disease.
Vitamin D is important for healthy bones, teeth and muscles. It helps to regulate blood sugar, the heart and blood vessels, and the lungs and airways. It also has a role in boosting the body’s immune system. These are areas affected by COVID‐19, so giving vitamin D to people with COVID‐19 might help them to recover more quickly or have the disease less severely.
What did we want to find out?
We wanted to find out the effects of giving vitamin D to adults with confirmed COVID‐19 on the following:
‐ death from any cause;
‐ improvement or worsening of the patient’s condition;
‐ unwanted effects; and
‐ quality of life.
What did we do?
We searched for studies that assessed the use of vitamin D as a treatment for adults with confirmed COVID‐19 compared with a placebo (sham treatment) or another treatment. Vitamin D could be given in any form and in any dose.
We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found three studies with 356 participants. One study took place in Brazil, and the other two in Spain. Two studies had participants with severe COVID‐19 and one had participants with mild COVID‐19 or with no symptoms. All the participants tested positive for COVID‐19 with a laboratory test called ‘PCR’, which is currently the most accurate test available.
The studies gave their participants different doses of vitamin D. They used different timings from each other, from one large dose in one study to several smaller doses over 14 days in another study. Only two studies said that their participants were vitamin D‐deficient. The other study did not say anything about their participants’ vitamin D status.
Deaths from any cause We do not know whether vitamin D helps to prevent death from COVID‐19. Two studies (in participants with severe COVID‐19) provided evidence about deaths from any cause. One reported no deaths in the 50 participants who had received vitamin D, but two deaths in the 26 participants who received the hospital’s usual COVID‐19 treatment. The other study reported nine deaths in 119 participants who had been given vitamin D and six deaths in the 118 participants given placebo. These studies were too different from each other to allow us to draw any conclusions.
Patient’s condition Vitamin D may reduce the need for patients to be put on a ventilator to help them breathe, but the evidence is uncertain. One study (in participants with severe COVID‐19) reported that nine out of 119 participants given vitamin D had to be put on a ventilator and 17 out of 118 given a placebo needed a ventilator.
Unwanted effects We do not know whether vitamin D causes unwanted effects. Only one study (in participants with severe COVID‐19) reported data on unwanted effects in a way that we could use. It found that one participant out of 119 vomited shortly after being given vitamin D.
Quality of life None of the studies reported quality of life.
What are the limitations of the evidence?
Our confidence in the evidence is very limited because the studies gave different doses of vitamin D at different times from each other, did not all report participants’ vitamin D status, and did not measure and record their results using consistent methods.
We found little evidence on unwanted effects and none on quality of life.
How up to date is this evidence?
The evidence is up to date to 11 March 2021.
Summary of findings
Summary of findings 1. Summary of Findings Table ‐ Vitamin D compared to placebo or standard care alone for individuals with moderate to severe disease.
| Vitamin D compared to placebo or standard care alone for individualswith moderate to severe disease | ||||||
| Patient or population: individualswith moderate to severe disease Setting: Inpatient Intervention: Vitamin D Comparison: placebo or standard care alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or standard care alone | Risk with Vitamin D | |||||
| All‐cause mortality at hospital discharge | Two studies reported all cause‐mortality at hospital discharge but were to heterogenous to be pooled. One study reported that 0/50 participants in the vitamin D and 2/26 participants in the control group died [RR 0.11 (95% CI 0.01 to 2.13)]. The other study reported that 9/119 participants in the vitamin D and 6/118 participants in the placebo group died [RR 1.49 (95% CI 0.55 to 4.05)]. | 313 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW a,b,c | We are uncertain whether vitamin D supplementation increases or decreases all‐cause mortality. | ||
| Improvement of clinical status assessed with: liberation from supplemental oxygen support (for the subgroup of participants requiring any supplemental oxygen or ventilator support at baseline, i.e WHO≥5), and weaning or liberation from invasive mechanical ventilation (for the subgroup of participants requiring invasive mechanical ventilationat baseline, i.e WHO≥7) | 0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | ( studies) | ‐ | No study reported this outcome. |
| Worsening of clinical status assessed with: need for invasive mechanical ventilation (for the subgroup of participants not requiring invasive mechanical ventilationat baseline, i.e WHO≤6) | 144 per 1,000 | 75 per 1,000 (35 to 163) | RR 0.52 (0.24 to 1.13) | 237 (1 RCT) | ⊕⊕⊝⊝ LOW d | Vitamin D supplementation may decrease the need for mechanical ventilation, but the evidence is uncertain. |
| Quality of life, including fatigue and neurological status assessed with: standardised scales (e.g. WHOQOL‐100) up to longest follow‐up | 0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | ( studies) | ‐ | No study reported this outcome. |
| Adverse events (any grade) | Low | RR 2.98 (0.12 to 72.30) | 237 (1 RCT) | ⊕⊝⊝⊝ VERY LOW d,e | We do not know whether vitamin D supplementation is associated with a higher risk of adverse events. | |
| 3 per 1,000 | 8 per 1,000 (0 to 204) | |||||
| Serious adverse events | 0 per 1,000 | 0 per 1,000 (0 to 0) | not estimable | ( studies) | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424522142738901140. | ||||||
a. Downgraded one level for serious study limitations, because of some concerns about risk of bias in one study. b. Downgraded two levels for very serious inconsistency, because of inconsistent directions and variations of point estimates. c. Downgraded two levels for very serious imprecision, because of wide confidence intervals, few participants, and few events. d. Downgraded two levels for very serious imprecision, because of only one study, wide confidence intervals, few participants, and few events. e. Downgraded one level for serious indirectness, because the reported outcome did not match our outcome definition.
Background
This work is part of a series of Cochrane Reviews investigating treatments and therapies for coronavirus disease 2019 (COVID‐19). Reviews of this series share information in the background section and methodology based on the first published reviews about monoclonal antibodies (Kreuzberger 2021) and convalescent plasma (Chai 2020) from the German research project “CEOsys” (COVID‐19 Evidence‐Ecosystem).
Description of the condition
COVID‐19 is a rapidly spreading infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2; WHO 2020a). On the 22 March 2020 the World Health Organization (WHO) declared the current COVID‐19 outbreak a pandemic. COVID‐19 is unprecedented in comparison to previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), with 813 and 858 deaths, respectively (WHO 2007; WHO 2019). Despite intensive international efforts to contain its spread, it has resulted in more than 120 millions confirmed cases and almost 2.7 million deaths worldwide (WHO 2021a; WHO 2021c). The emergence of SARS‐CoV‐2 variants, with potential for increased transmissibility, could result in a higher case incidence (WHO2021b).
Several vaccines against COVID‐19 have been distributed across countries and an additional hundred 100 vaccine candidates are in development (WHO 2020d). However, the process is time consuming, and challenges that go along with vaccine hesitancy and refusal have been reported. Moreover, the duration and degree to which the vaccines can protect against the disease, but also against infection and transmission is still not clear (Grubaugh 2020).
Specific risk factors for severe disease, hospitalisation and mortality have been identified: individuals aged 65 years or older, smokers and those with certain underlying medical conditions, such as cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart conditions, immunocompromised state, obesity, sickle cell disease or diabetes mellitus are more likely to have severe courses of the disease (Huang 2020; Liang 2020; WHO 2020a; Williamson 2020). COVID‐19 case fatality ratios varied widely between countries and reporting periods (from 0% to more than 25%, Johns Hopkins University & Medicine). However, these numbers may be misleading as they tend to overestimate the infection fatality ratio due to varying testing frequency, lag in reporting dates, incomplete capturing of all cases, and variations in case definitions since the beginning of the pandemic (WHO 2020b).
The median incubation period is estimated to be between five and six days; 97.5% of symptomatic cases develop symptoms within 11.5 days of exposure (Lauer 2020). Sore throat, cough, fever, headache, fatigue, and myalgia or arthralgia (joint pain) are the most commonly reported symptoms (Struyf 2020). Other symptoms include dyspnoea, chills, nausea or vomiting, diarrhoea and nasal congestion (WHO 2020a). The majority of infected people have mild symptoms (approximately 80%, Wu 2020) or remain completely asymptomatic (Buitrago‐Garcia 2020). A smaller proportion (approximately 14%) are affected by severe or critical disease with intensive care unit (ICU) admittance due to respiratory failure, septic shock or multiple organ dysfunction (Wu 2020). In light of the extent of the pandemic, including the pressure COVID‐19 puts on health systems, especially in the face of evolving variants of the virus with the potential of increased transmissibility, the ongoing scarcity of effective treatments, and the limited global availability of vaccines, there is an urgent need for effective therapies to save lives and to reduce the burden on healthcare systems.
Description of the intervention
Therapeutic interventions to treat COVID‐19 are being investigated with immense emphasis. Recently, vitamin D supplementation for treatment of COVID‐19 gained attention, since studies suggested an association between vitamin D deficiency and risk or prognosis of the disease (Grant 2020). Vitamin D3 is the precursor of a potent secosteroid hormone, intervening in several metabolic processes in the human body and showing immunomodulating (Sassi 2018) and organ protective properties (Amrein 2018; Martucci 2019; Ney 2019). Vitamin D3 (cholecalciferol) is naturally absorbed from food sources or produced by the epidermis when ultraviolet radiation is present. After being metabolised by the liver into 25‐hydroxyvitamin D [25(OH)D or calcidiol or calcifediol], and further converted into its biological active hormone 1,25‐dihydroxyvitamin D [1,25(OH)2D3 or calcitriol] by the kidneys within two or three days, it enters various cells at different organs (Lee 2009). By binding to the nuclear vitamin D receptor, a deoxyribonucleic acid (DNA) binding protein, it can regulate the transcription of genes‐ not only involved in calcium/phosphorus homeostasis and bone health (Holick 1996), but also in glycaemic control (Wu 2017), in muscle and cardiovascular function (Norman 2014), in the renin‐angiotensin system (Braun 2012), and in respiratory mechanisms (Hughes 2009). Some of these characteristics might play a role in critically ill patients and might have an influence on the course of COVID‐19 (Grant 2020).
25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D serum concentrations can be measured by radioimmunoassays and competitive protein‐binding assays, with a high risk for technical difficulties, or by liquid chromatography tandem mass spectroscopy (LC‐MS) technique (Holick 2009), or high performance liquid chromatography (HPLC) technique (Fraser 2020). The vitamin D external quality assurance scheme (DEQAS) has contributed to improve and standardise measurement procedures of serum 25(OH)D and 1,25(OH)2D.
Vitamin D deficiency is generally defined as 25‐hydroxyvitamin D values lower than 20 ng/mL (equals 50 nmol/L), while levels lower than 12 ng/mL (equals 30 nmol/L can be regarded as severely deficient (Amrein 2018). While cholecalciferol has a half life of about three months; 25‐hydroxyvitamin D's half life lies within the range of two to three weeks (Jones 2008). 1,25‐dihydroxyvitamin D is very unstable and only available and traceable for several hours (Brandi 2002; Leaf 2014). Each of the metabolites can be supplemented, however, keeping the physiology of vitamin D in mind, supplementation strategies must be well conceived and adjusted to the patient population.
Right patient population: patients at need due to 25(OH)D deficit (Amrein 2014), nutrition deficits, co‐morbidities and/or severe illness.
Right substance or right timing: either supplementing the active form calcitriol or providing enough time for the body to metabolise vitamin D3 (cholecalciferol) into its biological active form itself. Liver and kidneys must work properly in order to ensure this metabolisation process.
Right dosing: adequate dosing is necessary in order to provide sufficient 25(OH)D levels. In‐hospital, high‐dose supplementation is often required due to the great deficiency at baseline.
Right outcome: besides mortality as the overarching outcome in COVID‐19, clinical status including improvement and worsening as well as need for dialysis, and quality of life are crucial outcomes to be evaluated in the context of vitamin D supplementation for the treatment of COVID‐19.
Until now, evidence regarding specific vitamin D supplementation strategies as a treatment for individuals with COVID‐19 are sparse (NICE 2020). Recommendations were published by the European and American Societies for Clinical Nutrition and Metabolism (ESPEN and ASPEN), which focused on including malnutrition in the management of COVID‐19 patients and suggested supplementation with vitamins in these individuals if vitamin levels were deficient (Barazzoni 2020; Wells 2020). The recommendations provide guidance for those in the intensive care unit setting and align with the general principles in critical care nutrition. With this review, we aim to evaluate the role of vitamin D in the treatment of COVID‐19.
How the intervention might work
Patients at risk of developing severe COVID‐19 share many characteristics with patients at risk for vitamin D deficiency. Poor general health condition along with limited sunlight exposure, pre‐hospital malnutrition, old age, pre‐existing co‐morbidities including liver and kidney dysfunction are among the primary points of focus when analysing causes for vitamin D deficiency (Lee 2009). These characteristics also are risk factors for COVID‐19‐related hospitalisation, intensive care unit (ICU) admission and mortality (Wu 2020a). Recent observational studies showed an association between vitamin D deficiency and worse clinical outcome (including need and duration of mechanical ventilation and mortality) in COVID‐19 patients (Grant 2020; Liu 2021; Munshi 2021; Pereira 2020; Yisak 2021). These observations raise the question if vitamin D deficiency is a predictive marker or partly responsible for progression to severe COVID‐19 and accordingly, a possible target for treatment.
SARS‐CoV‐2 infection can cause severe inflammation along with serious life‐threatening complications (Wu 2020). SARS‐CoV‐2 can enter host cells by binding to human angiotensin‐converting enzyme 2 (hACE2) receptors, which are highly expressed in the upper respiratory tract of humans. After entering the host cell, viral ribonucleic acid (RNA) replication takes place, resulting in the host response including activation of alveolar macrophages and inflammatory infiltration (Cabler 2020). Early descriptions of COVID‐19 included development of a cytokine storm as a precursor for clinical instability and deterioration and still many questions related to the pathogenesis of inflammation are unanswered.
The biological active hormone 1,25‐dihydroxyvitamin D (calcitriol) shows immunomodulating properties, which might help to reduce inflammation in COVID‐19. Vitamin D receptors are present on several immunological cells such as T cells, B cells, macrophages and dendritic cells. Active immune cells can locally convert 25‐hydroxyvitamin D into the active form of vitamin D (1,25(OH)2D) via the 1‐α‐hydroxylase enzyme (CYP27B1) to provide these immunoprotective properties. In addition to immune cells, the vitamin D receptor (VDR) and the 1‐α‐hydroxylase enzyme (CYP27B1) are expressed in lung epithelial cells, which are thus also able to produce calcitriol in case of infection. 1,25(OH)2D activates several anti‐inflammatory mediators (e.g. IL‐4 and IL‐10) and inhibits pro‐inflammatory cytokines such as interferon‐γ, interleukin 2 and 6 and tumour necrosis factor‐α (Sassi 2018), which are part of the COVID‐19 induced cytokine storm. 1,25‐Dihydroxy‐vitamin‐D further triggers an upregulation of cathelicidin (LL‐37) and defensins; antimicrobial peptides with antiviral effects (Bilezikian 2020; Malaguarnera 2020). By binding to the SARS‐CoV‐2 S protein, LL‐37 inhibits the attachment of SARS‐CoV‐2 to the hACE2 receptor and thus blocks viral entry and virus replication (Roth 2020). Besides the effect on the endogenous defence system, 1,25(OH)2D influences the adaptive immune system by reducing TH1 cells and by triggering the formation of TH2 and regulatory T cells (Malaguarnera 2020). Vitamin D was shown to improve clinical outcomes in patients with acute respiratory distress syndrome (ARDS) (Martineau 2017; Martineau 2019), asthma exacerbations (Jolliffe 2017; Ramos‐Martinez 2018) and critical illness (Putzu 2017). These findings support the hypothesis that this vitamin could act as an disease‐modifying treatment for COVID‐19.
Beside its involvement in regulating the immune system, vitamin D influences arterial stiffness and is involved in the regulation of various thrombotic pathways. Calcitriol leads to an upregulation of the anticoagulants thrombomodulin and tissue factor pathway inhibitor and inhibits tissue factor mediated thrombin activation and thereby reduces hypercoagulability (Sengupta 2021). These characteristics are of particular importance for COVID‐19 patients since virus‐related injury of the vascular endothelium leads to an increased risk for coagulopathy and thrombosis (Wang 2020; Wu 2020) with life‐threatening consequences.
ACE2 receptors are also expressed by human epithelial cells that line mucosal surfaces and cover organs of liver, heart, kidney and intestine, which could explain why multiple organs can be affected during COVID‐19 (Mokhtari 2020). Rates of acute kidney injury (AKI) and the need for dialysis vary. However, AKI affects about 30% to 40% of hospitalised individuals with COVID‐19, and about 50% of people with COVID‐19 on ICU, of whom 20% require renal replacement therapy (Gupta 2021; Nadim 2020). Kidney injury can aggravate vitamin D deficiency as metabolisation of the active hormone calcitriol from vitamin D3 sources can be compromised.
Why it is important to do this review
There is a clear, urgent need for more information to guide clinical decision‐making for COVID‐19 patients. Current treatment consists of supportive care with oxygen therapy in cases with moderate disease, and with respiratory support as mechanical ventilation and extracorporeal membrane oxygenation in cases with severe disease (CDC 2020; WHO 2020f). Overall, data from randomised trials do not demonstrate a clear, major clinical benefit with most drugs evaluated so far. Data from randomized trials overall support the role of corticosteroids for severe COVID‐19 and clinical guidelines recommend the use of corticosteroids (Siemieniuk 2020). Recommendations for the use of tocilizumab and remdesivir vary to a certain extent from different panels (National COVID‐19 Clinical Evidence Taskforce 2021, Siemieniuk 2020). Other drugs, such as hydroxychloroquine, are not recommended for the treatment of COVID‐19 (Siemieniuk 2020). Moreover, existing evidence on the effectiveness of monoclonal antibodies as treatments for hospitalised individuals with COVID‐19 remains unclear.
Extensive work in the field of systematic reviews for interventions for COVID‐19 has already been undertaken, including vitamin D supplementation. For example, several systematic reviews investigated the association between vitamin D blood status, risk,and prognosis of COVID‐19, mainly based on non‐randomised studies (e.g. Liu 2021; Munshi 2021; Pereira 2020). Yisak 2021 and Shah 2021 additionally included randomised controlled trials (RCTs).
One of the most interesting sources is the regularly updated 'The National Institute for Health and Care Excellence' (NICE) guideline covering vitamin D use in the context of COVID‐19 (published in December 2020, NICE 2020). The Australian high‐priority, evidence‐based clinical COVID‐19 guidelines are continually updated with the latest research on emerging treatments, including recommendations for consideration of vitamin D analogues for people with COVID‐19 (National COVID‐19 Clinical Evidence Taskforce 2021).
This systematic review will fill current gaps by identifying, describing, evaluating and meta‐analysing RCTs for vitamin D supplementation on clinical outcomes in COVID‐19. Several clinical trials investigating the safety and effectiveness of vitamin D supplementation have been announced, and their results will need to be interpreted with care due to possible side effects of vitamin D overdoses. Thus, there needs to be a thorough understanding of the current body of evidence regarding the use of vitamin D supplementation for the treatment of COVID‐19, and an extensive review of the available literature is required.
Objectives
To assess whether vitamin D supplementation is effective and safe for the treatment of COVID‐19 in comparison to an active comparator, placebo, or standard of care alone, and to maintain the currency of the evidence, using a living systematic review. approach.
Methods
Criteria for considering studies for this review
Types of studies
The main description of methods is based on the standard template of the Cochrane Haematology review group and is in line with a series of Cochrane Reviews investigating treatments and therapies for COVID‐19. Specific adaptions related to the research question were made if necessary. The protocol for this review was registered with PROSPERO on 21 January 2021 (Stroehlein 2021a).
To assess the effectiveness and safety of vitamin D supplementation for the treatment of people with COVID‐19, we included randomised controlled trials (RCTs), as this study design, if performed appropriately, provides the best evidence for experimental therapies in highly‐controlled therapeutic settings. Cluster‐randomised and cross‐over trials were eligible for inclusion.
We excluded controlled non‐randomised studies of intervention and observational studies. We also excluded animal studies, pharmacokinetic studies, and in vitro studies.
We included the following formats, if sufficient information was available on study design, characteristics of participants, interventions and outcomes.
Full‐text publications
Preprint articles
Abstract publications
Results published in trials registries
Personal communication with investigators
We included preprints and conference abstracts to have a complete overview of the ongoing research activity, especially for tracking newly emerging studies about vitamin D supplementation in COVID‐19. We did not apply any limitation with respect to the length of follow‐up.
Types of participants
We included adults with a confirmed diagnosis of COVID‐19 (as described in the study) and we did not exclude any studies based on gender, ethnicity, disease severity, setting, or baseline vitamin D status.
We excluded studies that evaluated vitamin D supplementation for the treatment of other coronavirus diseases such as SARS or MERS, or other viral diseases, such as influenza. If studies enrolled populations with or exposed to mixed viral diseases, we had planned to only include these if trial authors provided subgroup data for SARS‐CoV‐2 infection.
Types of interventions
We included any type of vitamin D supplementation, including its active or non‐active forms, single or multiple doses, low‐ or high‐dose, and given alone or combined with individual care.
We included the following comparison.
Vitamin D supplementation (any of the above specified interventions) versus placebo or no treatment. Co‐interventions were allowed, but had to be comparable between intervention groups.
We had further planned to include the following comparisons.
Vitamin D supplementation (any of the above specified interventions) versus control intervention, for example drug treatments or micronutrient supplementation.
Low‐dose vitamin D supplementation versus high‐dose vitamin D supplementation (as defined in studies).
Single‐dose vitamin D supplementation versus multiple‐ dose Vitamin D supplementation.
We excluded studies evaluating vitamin D supplementation in combination with other active treatments, if the same treatment was not used in the control group. We also excluded studies investigating the effectiveness and safety to prevent COVID‐19.
Types of outcome measures
We evaluated core outcomes in accordance with the Core Outcome Measures in Effectiveness Trials (COMET) Initiative for COVID‐19 patients (COMET 2020; Marshall 2020), and additional outcomes that have been prioritised by consumer representatives and the German guideline panel for inpatient therapy of people with COVID‐19.
We defined outcome sets for two populations. Those are: hospitalised individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease, and ambulatory‐managed individuals with a confirmed diagnosis of SARS‐CoV‐2 infection and asymptomatic or mild disease, according to WHO clinical progression scale (WHO 2020e, see Table 2).
1. WHO clinical progression scale.
| Patient State | Descriptor | Score |
| Uninfected | Uninfected; no viral RNA detected | 0 |
| Ambulatory mild disease | Asymptomatic; viral RNA detected | 1 |
| Symptomatic; independent | 2 | |
| Symptomatic; assistance needed | 3 | |
| Hospitalised: moderate disease | Hospitalised; no oxygen therapya | 4 |
| Hospitalised; oxygen by mask or nasal prongs | 5 | |
| Hospitalised: severe disease | Hospitalised; oxygen by non‐invasive mechanical ventilation or high flow | 6 |
| Intubation and mechanical ventilation, pO2/FiO2 ≥ 150 or SpO2/FiO2 ≥ 200 | 7 | |
| Invasive mechanical ventilation pO2/FiO2 < 150 (SpO2/FiO2 < 200) or vasopressors | 8 | |
| Invasive mechanical ventilation pO2/FiO2 < 150 and vasopressors, dialysis or ECMO | 9 | |
| Dead | Dead | 10 |
WHO clinical progression scale from: WHO 2020e
aIf hospitalised for isolation only, record status as for ambulatory patient
ECMO: extracorporeal membrane oxygenation
FiO2: fraction of inspired oxygen
pO2: partial pressure of oxygen
SpO2: oxygen saturation
Individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease
Effectiveness of vitamin D supplementation
Prioritised outcomes (included in Summary of findings table)
All‐cause mortality at day 28, day 60, time‐to‐event, and at hospital discharge
-
Clinical status, assessed by need for respiratory support with standardised scales (e.g. WHO Clinical Progression Scale (WHO 2020e) (see Figure 1), WHO Ordinal Scale for Clinical Improvement (WHO 2020g) at day 28, day 60, and up to longest follow‐up); including:
-
Improvement of clinical status:
weaning or liberation from invasive mechanical ventilation in surviving patients i.e. WHO ≤ 6, if ≥ 7 at baseline;
ventilator‐free days; ventilator‐free defined as WHO ≤ 6;
duration to liberation from invasive mechanical ventilation;
liberation from supplemental oxygen in surviving patients i.e. WHO ≤ 4, if ≥ 5 at baseline;
duration to liberation from supplemental oxygen.
-
Worsening of clinical status:
need for invasive mechanical ventilation i.e. WHO 7‐9, if ≤ 6 at baseline ;
need for non‐invasive mechanical ventilation or high flow i.e. WHO=6, if ≤ 5 at baseline;
need for oxygen by mask or nasal prongs i.e. WHO = 5, if ≤ 4 at baseline
-
Need for dialysis (at up to 28 days)
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days; up to 30 days, and longest follow‐up available
1.

WHO Clinical Progression Scale (Marshall 2020)
Copyright © 2020 Elsevier Ltd. All rights reserved: reproduced with permission.
Additional outcomes (not in Summary of findings table)
Admission to the intensive care unit (ICU)
Duration of hospitalisation, or time to discharge from hospital from randomisation
Viral clearance, assessed with reverse transcription polymerase chain reaction (RT‐PCR) test for SARS‐CoV‐2 at baseline, up to 3, 7, and 15 days
Vitamin D serum levels
Safety of vitamin D supplementation
Prioritised outcomes (included in Summary of findings table)
Serious adverse events, defined as number of participants with any event
Adverse events (any grade, grade 1‐2, grade 3‐4), defined as number of participants with any event
Individuals with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease
Effectiveness of vitamin D supplementation
Prioritised outcomes (included in Summary of findings table)
All‐cause mortality at day 28, day 60, time‐to‐event, and up to longest follow‐up
Admission to hospital (WHO≥ 4)
-
Development of moderate to severe clinical COVID‐19 symptoms, defined as WHO Clinical Progression Scale ≥ 6 (WHO 2020e), up to longest follow‐up
-
Need for invasive mechanical ventilation, non‐invasive mechanical ventilation or high flow i.e. WHO ≥ 6, severe disease:
need for invasive mechanical ventilation i.e. WHO 7‐9;
need for non‐invasive mechanical ventilation or high flow i.e. WHO = 6.
-
Need for hospitalisation with or without supplemental oxygen i.e. WHO=4‐5, moderate disease:
Need for oxygen by mask or nasal prongs i.e. WHO=5;
Need for hospitalisation without oxygen therapy i.e. WHO = 4.
-
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to 7 days, up to 30 days, and longest follow‐up available
Additional outcomes (not in Summary of findings table)
Duration of hospitalisation, or time to hospital discharge from randomisation, for subgroup of participants hospitalised during course of disease
Vitamin D serum levels
Safety of vitamin D supplementation
Prioritised outcomes (included in Summary of Findings table)
Serious adverse events, defined as number of participants with any event
Adverse events (any grade, grade 1‐2, grades 3‐4), defined as number of participants with any event
Timing of outcome measurement
In case of time‐to‐event analysis, e.g. for time to discharge from hospital, we included the outcome measure based on the longest follow‐up time and measured from randomisation. We also collected information on outcomes from all other time points reported in the publications.
We included adverse events occurring during active treatment and included long‐term adverse events as well. If sufficient data were available, we grouped the measurement time points of eligible outcomes, for example, adverse events and serious adverse events, into those measured directly after treatment (up to seven days after treatment), medium‐term outcomes (up to 15 days after treatment) and longer‐term outcomes (more than 30 days after treatment).
Search methods for identification of studies
Electronic searches
On 11 March 2021 our Information Specialist (MIM) conducted systematic searches in the following sources from inception of each database to 11 March 2021 (date of last search for all databases) and did not place restrictions on the language of publication:
-
Cochrane COVID‐19 Study Register (CCSR) (www.covid-19.cochrane.org), comprising:
MEDLINE (PubMed), daily updates,
Embase.com, weekly updates,
ClinicalTrials.gov (www.clinicaltrials.gov), daily updates,
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch), weekly updates,
medRxiv (www.medrxiv.org), weekly updates,
Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates.
-
Web of Science Core Collection (from 1 January 2020 onwards):
Science Citation Index Expanded (1945‐present),
Emerging Sources Citation Index (2015‐present).
WHO COVID‐19 Global literature on coronavirus disease (https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/).
Database search results for Web of Science were restricted to publications from 2020 to current, as no treatment trials on COVID‐19 were registered prior to January 2020. For detailed search strategies, see Appendix 1.
Searching other resources
We identified other potentially eligible studies by searching the reference lists of included studies, systematic reviews and meta‐analyses. In addition, we contacted the investigators of included studies to obtain additional information on the retrieved studies.
We searched for grey literature, which we defined as searching study registries such as ClinicalTrials.gov and WHO ICTRP contained in the CCSR, as well as searching preprint servers and grey literature indexes contained in CCSR and WHO COVID‐10 Global Literature database. Once we established our set of included studies, we searched for preprints via Europe PMC, to check if any preprints for included studies were published since our database search.
Data collection and analysis
Selection of studies
Two out of three review authors (JS, CI, NS) independently screened the results of the search strategies for eligibility for this review by reading the titles and abstracts using EndNote Software (EndNote X9). We coded the abstracts as either 'include' or 'exclude'. In the case of disagreement or if it was unclear whether we should retrieve the abstract or not, we obtained the full‐text publication for further discussion. Both review authors assessed the full‐text articles of selected studies. If the two review authors were unable to reach a consensus, they consulted the third review author to reach a final decision.
We documented the study selection process in a flow chart, as recommended in the PRISMA statement (Moher 2009), and show the total numbers of retrieved references and the numbers of included and excluded studies. We listed all studies that we excluded after full‐text assessment and the reasons for their exclusion in the 'Characteristics of excluded studies' section.
Data extraction and management
We conducted data extraction according to the guidelines proposed by Cochrane (Li 2020). Two out of three review authors (JS, VP, CI) extracted data independently and in duplicate, using a customised data extraction form developed in Microsoft Excel (Microsoft 2018). We resolved disagreements by discussion. If no agreement was obtained, a third review author was involved to resolve the disagreement.
Two out of three review authors (JS, VP, CI) independently assessed eligible studies obtained in the process of study selection (as described above) for methodological quality and risk of bias. If the review authors were unable to reach a consensus, a third review author was consulted.
We extracted the following information, if reported.
General information: author, title, source, publication date, country, language, duplicate publications.
Study characteristics: trial design, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, treatment cross‐overs, compliance with assigned treatment, length of follow‐up.
Participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, additional diagnoses, severity of disease, previous treatments, concurrent treatments, complementary medicine (e.g. quercetin, elderberry, zinc), co‐morbidities (e.g. diabetes, immunosuppression).
Interventions: type of vitamin D, dose, frequency, timing, duration and route of administration, setting (e.g. inpatient, ambulant, prevention), duration of follow‐up.
Control interventions: placebo, no treatment, or other intervention; dose, frequency, timing, duration and route of administration, setting, duration of follow‐up.
Outcomes: as specified under Types of outcome measures.
'Risk of bias' assessment: randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported result.
Assessment of risk of bias in included studies
We used the risk of bias 2.0tool (RoB 2) to analyse the risk of bias of study results (Sterne 2019). Of interest for this review is the effect of the assignment to the intervention (the intention‐to‐treat (ITT) effect), thus, we performed all assessments with RoB 2 on this effect. The outcomes that we assessed are those specified for inclusion in the 'Summary of findings' table.
Two out of three review authors (JS, VP, CI) independently assessed the risk of bias for each outcome. In case of discrepancies among their judgements and inability to reach consensus, we consulted the third review author to reach a final decision. We assessed the following types of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020c).
Bias arising from the randomisation process
Bias due to deviations from the intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
For cluster‐RCTs, we had planned to add an additional domain to assess bias arising from the timing of identification and recruitment of participants in relation to timing of randomisation as recommended in the archived RoB 2 guidance for cluster‐randomised trials (Eldridge 2016), and in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a).
To address these types of bias we used the signalling questions recommended in RoB 2 and make a judgement using the following options.
'Yes': if there is firm evidence that the question is fulfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question).
Probably yes': a judgement has been made that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No': if there is firm evidence that the question is unfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question).
'Probably no': a judgement has been made that the question is unfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No information': if the study report does not provide sufficient information to allow any judgement.
We used the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias.
Low risk of bias
Some concerns
High risk of bias
Subsequently, we derived an overall 'Risk of bias' rating for each pre‐specified outcome in each study in accordance with the following suggestions.
'Low risk of bias': we judge the trial to be at low risk of bias for all domains for this result.
'Some concerns': we judge the trial to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain.
'High risk of bias': we judge the trial to be at high risk of bias in at least one domain for the result or we judge the trial to have some concerns for multiple domains in a way that substantially lowers confidence in the results.
We used the RoB 2 Excel tool to implement RoB 2 (available from riskofbias.info), added our judgements to the analysis for each assessed study and outcome, and stored our detailed RoB 2 assessments as supplementary online material (Stroehlein 2021b).
Measures of treatment effect
For continuous outcomes, we recorded the mean, standard deviation and total number of participants in both treatment and control groups. Where continuous outcomes used the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CIs). For continuous outcomes measured with different scales, we performed analyses using the standardised mean difference (SMD). For interpreting SMDs, we re‐expressed SMDs in the original units of a particular scale with the most clinical relevance and impact (e.g. clinical symptoms with the WHO Clinical Progression Scale (WHO 2020e)).
For dichotomous outcomes, we recorded the number of events and total number of participants in both treatment and control groups. We reported the pooled risk ratio (RR) with a 95% CI (Deeks 2020).
If available, we extracted and report hazard ratios (HRs) for time‐to‐event outcomes (e.g. time to hospital discharge). If HRs were not available, we would have made every effort to estimate the HR as accurately as possible from available data using the methods proposed by Parmar and Tierney (Parmar 1998; Tierney 2007). Had sufficient studies provided HRs, we planned to use HRs rather than RRs or MDs in a meta‐analysis, as they provide more information.
Unit of analysis issues
The aim of this review was to summarise trials that analysed data at the level of the individual. We planned to follow methods as recommended in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions for incorporating data from cluster‐RCTs and cross‐over trials (Higgins 2020a). For cross‐over trials we would have only considered results from the first period before cross‐over because COVID‐19 is not a chronic condition and its exact course and long‐term effects are yet to be defined.
Studies with multiple treatment groups
As recommended in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020b), for studies with multiple treatment groups of the same intervention (i.e. dose, route of administration), we planned to evaluate if study arms are sufficiently homogeneous to be combined. If arms could not be pooled, we had planned to compare each arm with the common comparator separately. For pairwise meta‐analysis, we planned to split the ‘shared’ group into two or more groups with smaller sample size, and include two or more (reasonably independent) comparisons. For dichotomous outcomes both the number of events and the total number of participants would have been divided, and for continuous outcomes the total number of participants would have been divided with unchanged means and SDs.
Dealing with missing data
Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions suggests a number of potential sources for missing data, which we took into account: at study level, at outcome level and at summary data level (Deeks 2020). At all levels, it is important to differentiate between data 'missing at random', which may often be unbiased, and 'not missing at random', which may bias study and thus review results.
Where data were missing, we requested these data from the principal investigators of two studies (Entrenas Castillo 2020; Murai 2021). We requested additional data for our prioritised outcomes from all authors. In addition, we requested information from Rastogi 2020 regarding clinical status of participants, and from Murai 2021 and Entrenas Castillo 2020 regarding evaluation and assessment of (serious) adverse events. We received a response from Rastogi 2020 and classified participants as ambulatory managed with mild or asymptomatic disease based on their answer. We did not receive a response from the other two authors (Entrenas Castillo 2020; Murai 2021. Where data were still missing, we had to make explicit assumptions of any methods the included studies used. For example, we assumed that the data were missing at random or we assumed that missing values had a particular value, such as a poor outcome.
Assessment of heterogeneity
Because of substantial clinical heterogeneity, we did not perform a meta‐analysis, but commented on results per study.
We would have assessed statistical heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1. We would have used the I² statistic (Higgins 2003) and visual examination, to assess possible heterogeneity (I² statistic > 30% to signify moderate heterogeneity, I² statistic > 75% to signify considerable heterogeneity; Deeks 2020). If heterogeneity had been above 80%, we planned to explore potential causes through sensitivity and subgroup analyses.
Assessment of reporting biases
As mentioned above, we searched trials registries to identify completed trials that have not been published elsewhere, to minimise or determine publication bias.
We had planned to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test for meta‐analyses involving at least 10 trials (Sterne 2019). We would have considered P < 0.1 as significant for this test.
Data synthesis
If the clinical and methodological characteristics of individual studies were sufficiently homogeneous, we would have pooled the data in meta‐analysis. We would have performed analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020). We would have analysed trials including different severities of disease separately, grouping them into mild, and moderate to severely ill, as these are different populations in different settings, resulting in differing outcomes (see Types of outcome measures). We had planned to treat placebo and no treatment as the same intervention, as well as standard of care at different institutions and time points.
We used the Review Manager Web (RevMan Web) software for analyses (RevMan Web 2019). One review author entered the data into the software, and a second review author checked the data for accuracy. We used the random‐effects model for all analyses as we anticipated that true effects are related, but are not the same for included studies. Because we could not perform a meta‐analysis, we commented on the results, with the results presented per study. For binary outcomes, we would have based the estimation of the between‐study variance using the Mantel‐Haenszel method. We would have used the inverse variance method for continuous outcomes, outcomes that included data from cluster‐RCTs, or outcomes where HRs were available. We would have explored heterogeneity above 80% with sensitivity analyses. If we could not find a cause for the heterogeneity, we had planned to not perform a meta‐analysis, but to comment on the results as a narrative with the results from all studies presented in tables.
Subgroup analysis and investigation of heterogeneity
To explore heterogeneity, we performed subgroup analyses of the following characteristics for our prioritised outcomes.
Vitamin D status at baseline: sufficiency being defined as 25(OH)D levels ≥20 ng/mL (equals 50 nmol/L), 25(OH)D deficiency <20 ng/mL and severe 25(OH)D deficiency <12ng/mL (equals 30nmol/L) (Amrein 2020; Holick 2011)
We used the tests for interaction to test for differences between subgroup results.
We had planned to perform additional subgroup analyses of the following characteristics.
Age of participants (divided into applicable age groups, e.g. children; 18 to 65 years, 65 years and older)
Pre‐existing conditions (diabetes, respiratory disease, hypertension, immunosuppression)
Severity of the disease (moderate (WHO 4‐5) vs. severe disease (WHO 6‐9), according to WHO clinical progression scale (WHO 2020c); if meaningful further divided into moderate disease without oxygen support (WHO = 4) versus moderate disease with low‐flow oxygen (WHO = 5) versus severe disease with high‐flow oxygen or non‐invasive mechanical ventilation (WHO = 6) versus severe disease with invasive mechanical ventilation (WHO 7‐9))
Duration since symptom onset
Formulation of vitamin D (active or non‐active forms)
Doses of vitamin D (single or multiple doses)
Administration of vitamin D (oral or intravenous)
Co‐treatments (e.g. steroids used as co‐treatment in up to 20% of participants vs. used in at least 80% of participants)
Sensitivity analysis
We had planned to perform sensitivity analysis of the following characteristics for our prioritised outcomes.
'Risk of bias' assessment components (studies with a low risk of bias or some concerns versus studies with a high risk of bias)
Comparison of preprints of COVID‐19 interventions versus peer‐reviewed articles
Comparison of premature termination of studies with completed studies
Summary of findings and assessment of the certainty of the evidence
We created one 'Summary of findings' table and evaluated the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for interventions evaluated in RCTs.
Summary of findings
We used the MAGICapp software to create the 'Summary of findings' table (MAGICapp). For time‐to‐event outcomes, we planned to calculate absolute effects at specific time points, as recommended in the GRADE guidance 27 (Skoetz 2020).
According to Chapter 14 of the updated Cochrane Handbook for Systematic Reviews of Interventions, the “most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes” should be included in the 'Summary of findings' table(s) (Schünemann 2020). We included outcomes prioritised according to the following Core Outcome Set for intervention studies (COMET 2020), and patient‐relevance.
Hospitalised individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease
All‐cause mortality; all‐cause mortality at hospital discharge most favourable; if not reported all‐cause mortality day 60, followed by day 28, or time‐to‐event estimate will be included in SoF table
-
Improvement of clinical status, assessed with liberation from supplemental oxygen support or invasive mechanical ventilation, in accordance with WHO Clinical Progression Scale (WHO 2020e) at longest follow‐up available
For all hospitalised individuals with oxygen support (WHO ≥5 at baseline on the WHO Clinical Progression Scale (WHO 2020e)): Liberation from supplemental oxygen in surviving patients most favourable, if not reported duration to liberation from supplemental oxygen will be included in SoF
For subgroup of severely ill individuals (WHO ≥ 7 at baseline on the WHO Clinical Progression Scale (WHO 2020e)): Liberation from invasive mechanical ventilation in surviving patients most favourable, if not reported ventilator‐free days, followed by duration to liberation from invasive mechanical ventilation will be included in SoF
Worsening of clinical status assessed by need for invasive mechanical ventilation i.e. WHO 7‐9 (if ≤ 6 at baseline) on the WHO Clinical Progression Scale (WHO 2020e) at longest follow‐up available
Quality of life, including fatigue and functional independence; assessed with standardised scales (e.g. WHOQOL‐100) at longest follow‐up available
Adverse events
Serious adverse events
Ambulatory managed individuals with a confirmed diagnosis of SARS‐CoV‐2 infection and asymptomatic or mild disease
All‐cause mortality; all‐cause mortality at longest follow‐up and > 60 days most favourable; if not reported all‐cause mortality day 60, followed by day 28, or time‐to‐event estimate, will be included in SoF table
Development of severe clinical COVID‐19 symptoms, defined as WHO Clinical Progression Scale ≥ 6 (WHO 2020e) at longest follow‐up available
Quality of life, including fatigue and functional independence; assessed with standardised scales (e.g. WHOQOL‐100) at longest follow‐up available
Adverse events
Serious adverse events
Assessment of the certainty in the evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the certainty in the evidence for the outcomes listed in the previous section.
The GRADE approach uses five domains (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty in the body of evidence for each prioritised outcome.
We downgraded our certainty of evidence for:
serious (‐1) or very serious (‐ 2) risk of bias;
serious (‐1) or very serious (‐ 2) inconsistency;
serious (‐1) or very serious (‐ 2) uncertainty about directness;
serious (‐1) or very serious (‐ 2) imprecise or sparse data;
serious (‐1) or very serious (‐ 2) probability of reporting bias.
The GRADE system used the following criteria for assigning grade of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We followed the current GRADE guidance for these assessments in its entirety as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 14 (Schünemann 2020).
We used the overall 'Risk of bias' judgement, derived from the RoB 2 Excel tool, to inform our decision on downgrading for risk of bias. We phrased the findings and certainty in the evidence as suggested in the informative statement guidance (Santesso 2020).
Methods for future updates
Living systematic review considerations
Our information specialist (MIM) will provide us with new search records each week, which two review authors will screen, extract, evaluate, and integrate following the guidance for Cochrane living systematic reviews (Cochrane LSR).
We will manually check platform trials that were previously identified and listed as 'studies awaiting classification' for additional treatment arms.
We will wait until the accumulating evidence changes our conclusions of the implications of research and practice before republishing the review. We will consider one or more of the following components to inform this decision.
The findings of one or more prioritised outcomes.
The credibility (e.g. GRADE rating) of one or more prioritised outcomes.
New settings, populations, interventions, comparisons or outcomes studied.
In case of emerging policy relevance because of global controversies around the intervention, we will consider republishing an updated review even though our conclusions remain unchanged. We will review the review scope and methods approximately monthly, or more frequently if appropriate, in light of potential changes in COVID‐19 research (for example, when additional comparisons, interventions, subgroups or outcomes, or new review methods become available).
Results
Description of studies
Results of the search
The literature search resulted in 245 records. One record was additionally identified via handsearching reference lists, resulting in overall 246 records. After removing duplicates, 244 records remained and were screened based on their titles and abstracts. 189 records did not meet the prespecified inclusion criteria and were excluded. We screened the full texts, or, if these were not available, the trial register entries, of the remaining 56 references. 20 records were excluded after full‐text assessment. Thirteen studies investigated a combined treatment including vitamin D, five studies investigated preventive effects, and two studies were no RCTs. We identified 24 ongoing records (21 studies) and three studies awaiting assessment. Finally, we included seven records (three studies) in our narrative synthesis. The search process is visualised in Figure 2 (Moher 2009).
2.
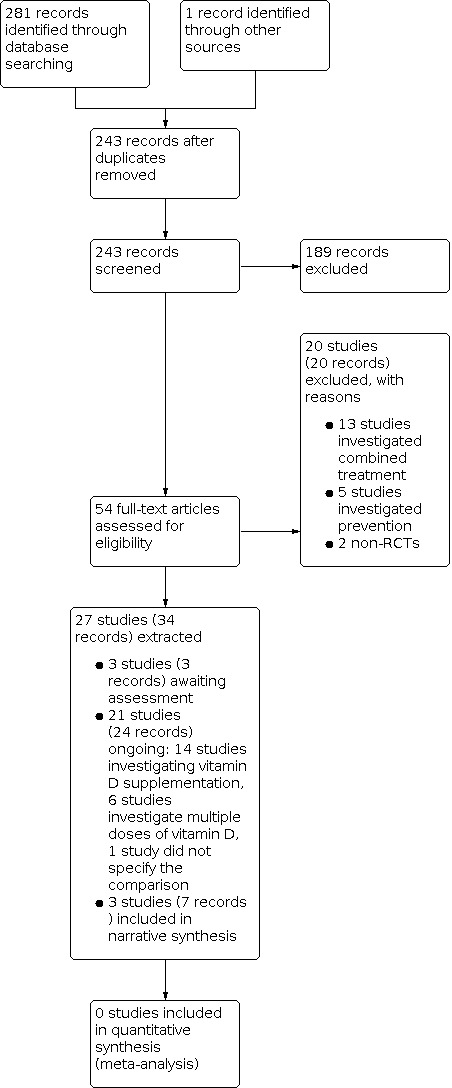
Included studies
Designs of the studies and involved study‐centres
We included three randomised controlled trials (RCTs) describing 356 adult participants (Entrenas Castillo 2020; Murai 2021; Rastogi 2020). One of the studies (Entrenas Castillo 2020) had an open‐label design; the other two studies (Murai 2021; Rastogi 2020) were double‐blinded and placebo‐controlled. Murai 2021 performed a multicentre study in two sites in Brazil. The other two studies (Entrenas Castillo 2020; Rastogi 2020) were performed each in one centre, in Spain (Entrenas Castillo 2020) and in India (Rastogi 2020).
Participants
Two of the three studies were conducted with hospitalised participants with COVID‐19 (Entrenas Castillo 2020; Murai 2021). In Entrenas Castillo 2020, all participants had both a polymerase chain reaction (PCR)‐confirmed diagnosis of COVID‐19, and radiological signs of pneumonia. At least one of these criteria (PCR or radiological signs) was necessary for inclusion in the study in Murai 2021 and 61.2% of participants had a positive PCR‐result for COVID‐19. Rastogi 2020 investigated outpatients with mild or asymptomatic disease, and all randomised participants were positive for COVID‐19. The other studies (Entrenas Castillo 2020; Murai 2021) included participants with clinical signs of an acute respiratory infection with moderate to severe illness. In Entrenas Castillo 2020, all participants had a Confusion‐Respiratory rate‐Blood pressure (CRB)‐65‐score > 1, independently of the components of the score. There were no details on oxygen supplementation and the only detail regarding the extent of respiratory insufficiency is the mean of partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio at baseline. However, the participants did not need intensive care unit (ICU) treatment at baseline. In Murai 2021, all participants had moderate or severe COVID‐19 as defined by respiratory rate greater than 24/min, saturation less than 93% while breathing room air or risk factors for complications. The vast majority of participants in this study needed oxygen supplementation (71.7% of patients in the intervention arm versus 80.8% of patients in the control arm), while non‐invasive ventilation was necessary in 15% and 11.7% of participants in each group. Participants requiring invasive ventilation before randomisation were excluded.
In Rastogi 2020, the participants were slightly younger than in the other two studies (between 36 and 51 years in the intervention arm and 39 and 49 years in the control arm), while in Entrenas Castillo 2020 and Murai 2021, the approximate mean age of participants was 53.14 +/‐ 10.77 years (intervention group) and 52.77 +/‐ 9.35 years (control group) or 56.8 +/‐ 14.2 years (intervention group) and 55.8 +/‐ 15.0 years (control group), respectively. In Entrenas Castillo 2020, the risk factors arterial hypertension and diabetes mellitus were less common in the intervention group (in the case of arterial hypertension this difference was statistically significant), while these risk factors were balanced between intervention and control group in Murai 2021. Comparing these two studies, diabetes mellitus and arterial hypertension were more frequent in Murai 2021 than in Entrenas Castillo 2020, in particular in the intervention arm (diabetes mellitus 40.8% versus 6% and arterial hypertension 56.7% versus 24.19%, respectively). In Murai 2021, more participants in both study arms had cardiovascular disease compared with those in Entrenas Castillo 2020 (13%versus 4%, respectively). Body mass index (BMI) and percentage of participants with obesity were reported only in Murai 2021. Rastogi 2020 did not report details on comorbidities of participants.
Only one study (Murai 2021) provided information on mean 25‐hydroxyvitamin D level at baseline as well as rough information on previous vitamin D supplementation, since supplementation > 1000 IU/day was one of the exclusion criteria. In Rastogi 2020, all participants randomised had 25(OH)Vitamin D3 level <= 20 ng/mL.
Interventions and comparators
Participants in the intervention arm of Entrenas Castillo 2020 received calcifediol (25(OH)Vitamin D3) at day 1 (0.532 mg) and continued with 0.266 mg on days 3 and 7, and then weekly until discharge or ICU admission. In Murai 2021 and Rastogi 2020, participants in the intervention arm received cholecalciferol (vitamin D3) as a single, oral dose of 200, 000 IU (Murai 2021) or in multiple, daily doses of 60, 000 IU over seven days (Rastogi 2020). Rastogi 2020 continued daily supplementation until day 14, if 25‐hydroxyvitamin D levels at day 7 achieved < 50 ng/mL. If 25‐hydroxyvitamin D levels at day 7 achieved > 50 ng/mL, participants continued with weekly supplementation of 60, 000 IU. Murai 2021 and Rastogi 2020 were placebo‐controlled. In Murai 2021, participants in the control arm received a single, oral dose of placebo (a peanut oil solution). In Rastogi 2020, participants received 5 mL distilled water as placebo up to day 7.
In Entrenas Castillo 2020, all participants received "best available therapy", which consisted of a combination of hydroxychloroquine (400 mg every 12 h on the first day, and 200 mg every 12 h for the following 5 days), azithromycin (500 mg orally for 5 days) and for participants with pneumonia and National Early Warning Score (NEWS) ≥ 5, a broad‐spectrum antibiotic (ceftriaxone 2 g intravenously every 24 h for 5 days). Standard care therapy was not prespecified in Murai 2021 and Rastogi 2020. However, in the study by Murai 2021 the majority of participants in both intervention and control arm received a concomitant corticosteroid therapy (64.7% vs. 61.9%, respectively).
Outcome measures
Primary outcomes were different in all three studies, including rate of ICU admissions and 28‐day mortality (Entrenas Castillo 2020), length of hospital stay (Murai 2021) and viral clearance before week three (Rastogi 2020). Overall mortality and rate of ICU admissions were secondary outcomes in Murai 2021. Other secondary outcomes in this study were number of participants requiring mechanical ventilation, as well as duration of mechanical ventilation and 25‐hydroxyvitamin D levels, calcium, creatinine, C‐reactive protein (CRP) and D‐dimer. Other outcomes, like differences in length of hospital stay, mortality, admission to ICU, mechanical ventilation requirement and duration, were reported in post‐hoc analysis for subgroups with and without vitamin D deficiency. The change of vitamin D serum levels and inflammatory markers (procalcitonin, CRP, fibrinogen, D‐dimer) were secondary outcomes in Rastogi 2020.
Ongoing studies
Of the 21 ongoing studies, 14 RCTs are comparing the effects of vitamin D supplementation with either placebo or no intervention, (CTRI/2020/12/030083; EUCTR2020‐001717‐20‐ES; EUCTR2020‐001960‐28‐ES; NCT04334005; NCT04363840; NCT04386850; NCT04411446; NCT04489628; NCT04502667; NCT04536298; NCT04552951; NCT04621058; NCT04636086; NCT04641195).
Of these, three studies were expected to be completed in 2020 and planned to evaluate between 30 and 1080 participants, but according to the trial registry, two are not yet recruiting (NCT04334005; NCT04363840) and one is still recruiting (NCT04552951). Six studies are expected to be completed in 2021, and plan to evaluate between 40 and 2700 participants (CTRI/2020/12/030083; NCT04411446; NCT04489628; NCT04502667; NCT04536298; NCT04621058) and two studies were scheduled to be completed by the time of writing and planned to evaluate between 100 and 1500 participants (NCT04386850; NCT04636086). One study is expected to be completed in 2022, and plans to evaluate 700 participants (NCT04641195). Two studies are not indicating an expected completion date, and plan to evaluate 108 and 1008 participants (EUCTR2020‐001717‐20‐ES; EUCTR2020‐001960‐28‐ES).
Six of the 21 ongoing studies investigate different doses of vitamin D supplementation, of which all are RCTs (CTRI/2020/06/026189; EUCTRCT2020‐002312‐43‐ES; NCT04344041; NCT04385940; NCT04482673; NCT04525820 and plan to evaluate between 64 and 210 participants. Of these, one RCT was expected to be completed in 2020, but according to the trial registry, is not yet recruiting (NCT04385940), two RCTs are expected to be completed in 2021 (NCT04482673; NCT04525820), one RCT is expected to be completed in 2022 (CTRI/2020/06/026189), and one RCT is not indicating any completion date (EUCTRCT2020‐002312‐43‐ES). Further, one RCT (NCT04344041) has two different trial registrations, one in ClinicalTrials.gov and one in the EU Clinical Trial Registry. In the first registry, the study is expected to be completed in 2021 and in the second registry, the study is indicated to be completed already, but no results are available yet.
One of the ongoing studies is an RCT, but it is unclear what comparison the study performs with vitamin D (EUCTR2020‐002274‐28‐ES).
Studies awaiting classification
We identified three completed RCTs from trial register entries, but no results are available or have been published yet. Of these, one RCT investigates different doses of vitamin D supplementation (IRCT20110726007117N11), whereas the other two RCTs investigate vitamin D treatment versus placebo (IRCT20200324046850N1; NCT04733625 ).
Excluded studies
We excluded 20 references (20 studies) that did not match our inclusion criteria.
Five studies investigated the effect of Vitamin D on prevention: NCT04535791; NCT04579640; NCT04476680; NCT04483635; NCT04596657.
13 studies evaluated a combination of vitamin D with other treatments: NCT04507867; IRCT20200319046819N1; NCT04565392; Euctr2020‐001903‐17; NCT04399746; IRCT20200705048013N1; NCT04395768; Euctr2020‐001363‐85‐dk; NCT04482686; CTRI/2020/06/026191; IRCT20200319046819N1; NCT04780061; NCT04351490.
Two studies were non‐RCTs. Nogues 2021 investigated the potential role of vitamin on mortality and intensive care treatment in hospitalised patients, but we had serious concerns about adequacy of the randomisation procedure, as participants were randomised to hospitals and not to treatments. the other study was a single‐arm trial (NCT04407286).
Risk of bias in included studies
Risk of bias in randomised controlled trials
We assessed methodological quality and risk of bias for two RCTs (Entrenas Castillo 2020; Murai 2021) using RoB 2 tool to analyse the risk of bias of our prioritised outcomes (Sterne 2019), recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020c). We did not judge risk of bias for Rastogi 2020, as they reported none of our prioritised outcomes. The completed RoB 2 tool with responses to all assessed signalling questions is available online at https://zenodo.org/record/4771734#.YKTEFKj7Ryw (Stroehlein 2021b).
Overall judgement for studies including individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease
Overall, we rated the risk of bias, among those studies having reported a mortality outcome, to be of some concerns in Entrenas Castillo 2020 and to be low in Murai 2021. For the reported outcome worsening of clinical status, we judged the risk of bias to be low for Murai 2021.
All‐cause mortality
We assessed this outcome on a study level at day 28, day 60, time‐to‐event, and at hospital discharge. For Entrenas Castillo 2020, there were some concerns arising form the randomisation process, as the randomisation list was accessible to "study specialists" and some concerns for bias arising from the selection of reported mortality results. The definition of mortality differed between the trial registry and the journal publication, making it unclear how long participants were followed and how the outcome was defined at protocol stage. For Murai 2021, we did not identify any concerns for the mortality outcome, and judged the risk of bias to be low (Table 29).
Risk of bias for analysis 1.1 All‐cause mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Entrenas Castillo 2020 | Some concerns | Participants were randomize in a 2:1 ratio through an electronically‐generated list, to receive standard treatment coupled with vitamin D or standard treatment alone and the allocation sequence was probably not concealed, as this list was accessible to "study specialists". There are no baseline differences that would suggest a problem with randomization. | Low risk of bias | Both participants and those delivering the intervention were aware of intervention received, but there were no deviations from intended interventions and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for all 76 participants randomized. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were probably unaware of the intervention received. | Some concerns | For this outcome, there was no information on whether the data that produced this result was analysed in accordance with the predefined protocol, as it was unclear how long patients were followed and how outcome was defined and the protocol was not available. | Some concerns | For this outcome there is a low risk of bias due to deviations from intended interventions, due to missing outcome data and in measurement of the outcome. However, there are some concerns for bias from the randomization process and in selection of the reported result. |
| Murai 2021 | Low risk of bias | Participants were randomized via computer‐generated random numbering in a 1:1 ratio to receive either a single dose of vitamin D3 or placebo and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomization. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for all 240 participants randomized. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified outcomes reported in the trial registry. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Clinical status
We assessed this outcome on a study level, by the need for respiratory support in accordance with standardised scales (WHO 2020e), and included both clinical improvement and clinical worsening in our assessment.
Improvement of clinical status
We could not assess the risk of bias for this outcome, because none of the studies reported measures of improvement.
Worsening of clinical status: assessed with need for invasive mechanical ventilation
The outcome was only reported in one study (Murai 2021). We did not identify any concerns that could have biased the reported outcome, and therefore judged the risk of bias to be low (Table 30).
Risk of bias for analysis 1.2 Worsening of clinical status: need for mechanical ventilation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Murai 2021 | Low risk of bias | Participants were randomized via computer‐generated random numbering in a 1:1 ratio to receive either a single dose of vitamin D3 or placebo and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomization. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for all 240 participants randomized. | Low risk of bias | The measurement of the outcome was appropriate and it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Low risk of bias | The data that produced this result was analysed in accordance with the pre‐specified outcomes reported in the trial registry. | Low risk of bias | For this outcome, there is a low risk of bias for all the domains. |
Quality of life
We could not assess the risk of bias for this outcome, because none of the studies reported quality of life.
Serious adverse events
We could not assess the risk of bias for this outcome, because none of the studies reported serious adverse events.
Adverse events (any grade)
Adverse events of any grade were reported in one study Murai 2021. We had concerns about selection bias due to insufficient information about recording and reporting of adverse events, and judged risk of bias with some concerns (Table 31).
Risk of bias for analysis 1.6 Adverse events (any grade).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Murai 2021 | Low risk of bias | Participants were randomized via computer‐generated random numbering in a 1:1 ratio to receive either a single dose of vitamin D3 or placebo and the allocation sequence was concealed. There are no baseline differences that would suggest a problem with randomization. | Low risk of bias | Both participants and those delivering the intervention were unaware of the assigned intervention received and the analysis was appropriate. | Low risk of bias | Data for this outcome was available for nearly all participants randomized. | Low risk of bias | There was no information provided on the measurement of the outcome, but it is unlikely that it differed between intervention groups. The outcome assessors were unaware of the intervention received. | Some concerns | For this outcome, there was no information on whether the data that produced this result was analysed in accordance with the predefined protocol, as it was unclear how the outcome was defined and the protocol was not available. | Some concerns | For this outcome, there is a low risk of bias from the randomization process, due to deviations from intended interventions, due to missing outcome data and in measurement of the outcome. However, there are some concerns for bias in selection of the reported result. |
Overall judgement for studies including individuals with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease
Overall, the one study (Rastogi 2020) including individuals with asymptomatic and mild disease did not report our prioritised outcomes, and thus risk of bias could not be formally assessed for this study using RoB 2.0.
Effects of interventions
See: Table 1
We identified three studies and included them in this review (Entrenas Castillo 2020; Murai 2021; Rastogi 2020). We requested disease status of participants in Rastogi 2020. Based on their reply, we included the study for our population with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease. We also investigated additional outcomes for effectiveness of vitamin D supplementation which were not included in the summary of findings table.
Individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease
The evidence profile for vitamin D supplementation for individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease is presented in Summary of findings table 1. Two studies were included (Entrenas Castillo 2020; Murai 2021).
Effectiveness of vitamin D supplementation
All‐cause mortality
Both studies reported data for mortality at hospital discharge for 313 participants (Entrenas Castillo 2020; Murai 2021). Entrenas Castillo 2020 reported that none of the 50 participants treated with vitamin D died, compared to two of 26 participants in the control group (risk ratio (RR) 0.11, 95% confidence interval (CI) 0.01 to 2.13). Murai 2021 reported nine deaths out of 119 individuals treated with vitamin D, and six deaths out of 118 participants in the placebo group (RR 1.49, 95% CI 0.55 to 4.05). The evidence is very uncertain whether vitamin D supplementation has an effect on all‐cause mortality at hospital discharge (very low‐certainty evidence, see Analysis 1.1).
Our main reasons for downgrading were serious study limitations due to unclear risk of bias in Entrenas Castillo 2020, very serious inconsistency due to inconsistent directions of point estimates, as well as due to very serious imprecision because of wide confidence intervals along with a low number of participants and events.
We did not find any data for mortality up to day 28, day 60 or time‐to‐event.
Subgroup analysis
As we are comparing two studies with substantial clinical and methodological diversity, subgroup analyses regarding dose, administration, formulation and other subgroups will rather reflect effects of these differences. Therefore, we conducted subgroup analysis for vitamin D status only, but not for other subgroups. Vitamin D status at baseline was unknown for participants in Entrenas Castillo 2020 and we therefore did not include the study for subgroup analyses.
Murai 2021 reported data for 115 participants with vitamin D deficiency at baseline. We did not find evidence for subgroup differences between vitamin D deficient and non‐deficient individuals for all‐cause mortality at hospital discharge (see Analysis 2.1).
2.1. Analysis.

Comparison 2: Subgroup analysis (vitamin D baseline status): Vitamin D supplementation vs. placebo or standard care alone in individuals with moderate to severe disease, Outcome 1: All‐cause mortality
Clinical Status (assessed by need for respiratory support with standardised scales)
We did not find data for outcomes for improvement of clinical status (weaning or liberation from invasive mechanical ventilation in surviving patients, ventilator‐free days, duration to liberation from invasive mechanical ventilation, liberation from supplemental oxygen in surviving patients, duration to liberation from supplemental oxygen).
One study reported data for worsening of clinical status for 237 participants, measured as need for mechanical invasive ventilation (Murai 2021). Nine out of 119 participants treated with vitamin D needed invasive mechanical ventilation compared to 17 out of 118 participants in the control group (RR 0.52, 95% CI 0.24, 1.13). Vitamin D supplementation may decrease need for invasive mechanical ventilation, but the evidence is uncertain (low‐certainty evidence, see Analysis 1.2). We graded down due to very serious imprecision because of the low number of participants and events as well as because of data from only one study.
1.2. Analysis.

Comparison 1: Vitamin D supplementation vs. placebo or standard care alone for individuals with moderate to severe disease, Outcome 2: Worsening of clinical status: need for mechanical ventilation
We did not find any data for other outcomes regarding worsening of clinical status (need for non‐invasive mechanical ventilation or high flow, need for oxygen by mask or nasal prongs).
Subgroup analysis
115 participants with vitamin D deficiency at baseline were included (Murai 2021). We did not find evidence for subgroup differences between vitamin D deficient and non‐deficient individuals for invasive mechanical ventilation (see Analysis 2.2), and confidence intervals were overlapping.
2.2. Analysis.

Comparison 2: Subgroup analysis (vitamin D baseline status): Vitamin D supplementation vs. placebo or standard care alone in individuals with moderate to severe disease, Outcome 2: Need for mechanical ventilation
Need for dialysis (at up to 28 days)
We did not find any data for this outcome.
Quality of life (including fatigue and neurological status)
We did not find any data for this outcome (at up to seven days, up to 30 days, longest follow‐up available).
Additional outcomes for effectiveness of vitamin D supplementation (not included in summary of findings table)
Admission to intensive care unit (ICU)
Both studies reported the outcome for 313 participants (Entrenas Castillo 2020; Murai 2021). Entrenas Castillo 2020 reported one participant out of 50 treated with vitamin D that was referred to ICU, compared to 13 out of 26 participants in the control group (RR 0.04, 95% CI 0.01 to 0.29). 19 out of 119 participants in the vitamin D group were referred to ICU, and 25 out of 118 in the control group in Murai 2021 (RR 0.75, 95% CI 0.44 to 1.29), see Analysis 1.5].
1.5. Analysis.

Comparison 1: Vitamin D supplementation vs. placebo or standard care alone for individuals with moderate to severe disease, Outcome 5: Admission to intensive care unit
Subgroup analysis
Murai 2021 reported data for 115 participants with vitamin D deficiency. We did not find evidence for subgroup differences between vitamin D deficient and non‐deficient individuals for admission to ICU (see Analysis 2.3).
2.3. Analysis.

Comparison 2: Subgroup analysis (vitamin D baseline status): Vitamin D supplementation vs. placebo or standard care alone in individuals with moderate to severe disease, Outcome 3: Admission to intensive care unit
Duration of hospitalisation
One study reported data for this outcome for 237 participants (Murai 2021). The median duration of hospitalisation was 7 (interquartile range (IQR) 4‐10) days in the vitamin D group and 7 (IQR 5‐13) days in the control group (Hazard ratio (HR) 1.07, 95% CI 0.81 to 1.41), see Analysis 1.3].
1.3. Analysis.

Comparison 1: Vitamin D supplementation vs. placebo or standard care alone for individuals with moderate to severe disease, Outcome 3: Duration of hospitalisation
Viral clearance (assessed with RT‐PCR test for SARS‐CoV‐2)
We did not find any data for this outcome (at baseline, up to 3, 7, and 15 days).
Vitamin D serum levels
Only Murai 2021 reported data for this outcome for 237 participants. 25‐hydroxyvitamin D levels increased in individuals treated with vitamin D (119 participants, baseline: 21.2 (standard deviation (SD) 10.1) ng/mL to 44.4 (SD 15) ng/mL), but not in the placebo group (118 participants, baseline: 20.6 (SD 8.1) ng/mL to 19.8 (SD 10.5) ng/mL [mean difference after intervention 24.70 ng/mL (95% CI 21.41 to 27.99), see Analysis 1.4].
1.4. Analysis.

Comparison 1: Vitamin D supplementation vs. placebo or standard care alone for individuals with moderate to severe disease, Outcome 4: Vitamin D serum levels
Safety of vitamin D supplementation
Serious adverse events
The authors reported that no serious adverse events were observed throughout the trial. There was insufficient information provided to assess whether serious adverse reactions have been assessed in a consistent manner for all participants.
Adverse events (any grade, grade 1‐2, grade 3‐4)
One study reported adverse events (any grade) for 237 participants. One out of 119 participants treated with vitamin D had an adverse event (vomiting) compared to none participant out of 118 control group (RR 2.98, 95% CI 0.12 to 72.30). We are very uncertain whether vitamin D supplementation is associated with a higher risk of adverse events (very low‐certainty evidence, see Analysis 1.6). We graded down due to very serious imprecision because of the low number of participants and events, data from only one study as well as due to serious indirectness, as outcome definition did not match our definition.
1.6. Analysis.

Comparison 1: Vitamin D supplementation vs. placebo or standard care alone for individuals with moderate to severe disease, Outcome 6: Adverse events (any grade)
Individuals with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease
Rastogi 2020 investigated individuals with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease. However, we could not find any data for our prioritised outcomes. Instead, authors reported data for viral clearance, vitamin D serum levels and inflammatory markers.
Effectiveness of vitamin D supplementation
All‐cause mortality
We could not find any data for mortality at day 28, at day 60, time‐to‐event, and up to longest follow‐up.
Admission to hospital (WHO ≥ 4)
We could not find any data for this outcome.
Development of moderate to severe clinical COVID‐19 symptoms
We did not find data for need for invasive mechanical ventilation, need for non‐invasive mechanical ventilation or high flow, need for hospitalisation, with or without supplemental oxygen (need for oxygen by mask or nasal prongs, need for hospitalisation without oxygen therapy).
Quality of life (including fatigue and neurological status)
We could not find any data for this outcome (at up to seven days, 30 days, and longest follow‐up available).
Additional outcomes for effectiveness of vitamin D supplementation (not included in summary of findings table)
Duration of hospitalisation (for subgroup of participants hospitalised during course of disease)
We could not find any data for this outcome.
Time to hospital discharge (for subgroup of participants hospitalised during course of disease)
We could not find any data for this outcome.
Vitamin D serum levels
The outcome was reported in Rastogi 2020 for 40 individuals with vitamin D deficiency at baseline (defined as < 20 ng/mL). Baseline 25‐hydroxyvitamin D levels were comparable between groups [median 8.6 (IQR 7.1‐13.2) for vitamin D group and 9.54 (IQR 8.1‐12.5) for placebo group]. 25‐hydroxyvitamin D levels increased in individuals treated with vitamin D (16 participants, median increase 42.4 (IQR 39 to 48.8) ng/mL), but not in the placebo group (24 participants, median increase of 5.1 (IQR 0‐12.3) ng/mL). The median 25‐hydroxyvitamin D levels at day 14 were 51.7 (IQR 48.9‐59.9) ng/mL in the vitamin D group and 15.2 (IQR 12.7‐19.5) ng/mL in the placebo group.
Safety of vitamin D supplementation
Serious adverse events
We did not find data for this outcome.
Adverse events (any grade, grade 1‐2, grade 3‐4)
Rastogi 2020 reported that no events of hypercalcaemia were observed during the intervention in both groups. Information about other adverse events was missing. We did not include data of this study for this outcome as we found only insufficient information provided to assess whether adverse reactions have been assessed in a consistent manner for all participants.
Discussion
Summary of main results
The aim of this review was to investigate the effectiveness and safety of vitamin D supplementation in individuals with confirmed COVID‐19 and moderate to severe disease or asymptomatic to mild disease. We identified three randomised controlled trials (RCTs) (Entrenas Castillo 2020; Murai 2021; Rastogi 2020, n = 356), of which two reported all‐cause mortality (Entrenas Castillo 2020; Murai 2021, n = 313), and one reported the need for invasive mechanical ventilation and adverse events (Murai 2021, n = 237) in individuals with COVID‐19 and moderate to severe disease. We could not find data for any other of our prioritised outcomes. We found one RCT with participants with confirmed COVID‐19 and asymptomatic or mild disease (Rastogi 2020), which did not report data for our prioritised outcomes.
We identified a further 21 ongoing studies evaluating the effectiveness and safety of vitamin D supplementation and three completed studies, but without any results published yet that are awaiting classification.
Risk of bias
We judged risk of bias with some concerns for Entrenas Castillo 2020, and as low for Murai 2021 for mortality outcomes. Moreover, we judged risk of bias for outcomes regarding worsening of clinical status (need for invasive mechanical ventilation) as low‐certainty evidence for one study (Murai 2021). We were not able to conduct bias assessment for Rastogi 2020, as the authors did not report our prioritised outcomes.
Effectiveness of vitamin D supplementation for hospitalised individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease
We identified two RCTs that evaluated effectiveness of vitamin D supplementation in hospitalised individuals with confirmed COVID‐19 and moderate to severe disease. Due to substantial clinical and methodological diversity between Entrenas Castillo 2020 and Murai 2021, we were unable to calculate pooled effect estimates. Therefore, results are presented individually for each study.
All‐cause mortality
Both included studies evaluated all‐cause mortality at hospital discharge. We are very uncertain whether vitamin D supplementation has an effect on this outcome.
Subgroup analysis
Murai 2021 reported data for 115 participants with vitamin D deficiency at baseline. We did not find evidence for subgroup differences for all‐cause mortality at hospital discharge
Clinical status
We did not find data for improvement of clinical status.
Murai 2021 reported data for worsening of clinical status (outcome need for invasive mechanical ventilation) for 237 participants. Vitamin D supplementation may decrease need for invasive mechanical ventilation, but the evidence is uncertain.
Subgroup analysis
Murai 2021 reported data for 115 participants with vitamin D deficiency at baseline. We did not find evidence for subgroup differences for invasive mechanical ventilation.
Quality of life (including fatigue and neurological status)
We could not find data for this outcome.
Safety of vitamin D supplementation for hospitalised individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease
We did not include data because insufficient information provided in Murai 2021 about evaluation and assessment of serious adverse events. Murai 2021 reported one adverse event (any grade) for 237 participants. We are very uncertain whether vitamin D supplementation is associated with a higher risk of adverse events.
Effectiveness of vitamin D supplementation for individuals with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease
We identified one RCT which investigated outpatients with COVID‐19 and asymptomatic or mild disease (Rastogi 2020). However, we did not find data for our prioritised outcomes (all‐cause mortality, development of severe clinical COVID‐19 symptoms, quality of life).
Safety of vitamin D supplementation for individuals with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease
We did not include data for safety outcomes due to insufficient information provided in Rastogi 2020 about evaluation and assessment of adverse events or serious adverse events. We did not identify other RCTs that reported these outcomes (serious adverse events, adverse events).
Overall completeness and applicability of evidence
We identified three RCTs with 356 participants, either with hospitalised participants with moderate to severe disease (Entrenas Castillo 2020; Murai 2021), or with ambulatory‐managed individuals with mild or asymptomatic disease (Rastogi 2020).
Hospitalised participants with moderate to severe disease
Two studies investigated vitamin D supplementation in 316 hospitalised participants with moderate or severe COVID‐19 (Entrenas Castillo 2020; Murai 2021), of whom 170 received vitamin D supplementation.
Most of the hospitalised participants with moderate or severe disease received concomitant medication, either solely or in combination. These included corticosteroids, hydroxychloroquine, and antibiotics, as azithromycin. Moreover, most participants received respiratory support (i.e. oxygen therapy or noninvasive ventilation).
In both studies, some relevant confounding factors, like age, gender, severity of disease, and co‐morbidities, were considered by authors in the analyses. Entrenas Castillo 2020 reported that more participants in the placebo control group had diabetes and hypertension, which are risk factors for developing severe disease. This may have confounded the results of the study.
Murai 2021 reported baseline 25‐hydroxyvitamin D levels for all participants, as well as baseline demographics and clinical characteristics from participants with vitamin D deficiency. Post hoc analysis revealed that patients with vitamin D deficiency and no treatment had the best clinical outcome questioning the applicability of these study results. Entrenas Castillo 2020 did not report vitamin D status of participants. In addition, Murai 2021 also excluded participants who had received previous vitamin D supplementation (> 1000 international units (IU)/day), which was not reported in Entrenas Castillo 2020.
Outpatients with mild or asymptomatic disease
Rastogi 2020 reported data for 40 outpatients with COVID‐19 and mild or asymptomatic disease, of whom 16 were treated with vitamin D, but not for our prioritised outcomes. Moreover, only participants with vitamin D deficiency (defined as < 20 ng/mL) were included in the study. Despite age and gender, authors did not report important prognostic factors of COVID‐19 (e.g. co‐morbidities, obesity) of participants at baseline, thus comparability of both groups remains uncertain. Despite this trial with a small number of participants, no other studies were identified.
Ongoing studies
We identified 21 ongoing studies investigating either effectiveness of vitamin D supplementation versus placebo or no treatment, or different doses of vitamin D (CTRI/2020/06/026189; CTRI/2020/12/030083; EUCTR2020‐001717‐20‐ES; EUCTR2020‐001960‐28‐ES; EUCTRCT2020‐002312‐43‐ES; NCT04334005; NCT04344041; NCT04363840; NCT04385940; NCT04386850; NCT04411446; NCT04482673; NCT04489628; NCT04502667; NCT04525820; NCT04536298; NCT04552951; NCT04621058; NCT04636086; NCT04641195; EUCTR2020‐002274‐28‐ES). Of these, three studies were expected to be completed in 2020, but according to the trial registry, two are not yet recruiting (NCT04334005; NCT04363840), and one is still recruiting (NCT04552951). Six studies are expected to be completed in 2021, and plan to evaluate between 40 and 2700 participants (CTRI/2020/12/030083; NCT04411446; NCT04489628; NCT04502667; NCT04536298; NCT04621058). Two studies were scheduled to be completed by the time of writing and planned to evaluate between 100 and 1500 participants (NCT04386850; NCT04636086).
Three studies are reported to be completed, but no results are available or have been published yet. Of these, one RCT investigates different doses of vitamin D supplementation (IRCT20110726007117N11), whereas the other two RCTs investigate vitamin D treatment versus placebo (NCT04733625; IRCT20200324046850N1).
Currently, there is only low‐certainty evidence regarding the effects and safety of vitamin D supplementation. Publication of the identified ongoing RCTs will necessitate an update of this review. The conclusions of the updated review could differ from those of the present review, and may allow for a better judgement regarding the effectiveness and safety of vitamin D supplementation for the treatment of COVID‐19.
Quality of the evidence
We assessed two studies for risk of bias (Entrenas Castillo 2020; Murai 2021), as Rastogi 2020 did not report our prioritised outcomes. Risk of bias was in general low for Murai 2021, except for adverse events, which we judged with some concerns due to selective reporting. We had concerns about randomisation in Entrenas Castillo 2020, as the list was accessible to "study specialists". Moreover, we observed differences between regarding outcome definition in the trial registry and the journal article.
Due to substantial clinical and methodological diversity of the studies, we did not conduct meta‐analysis. We included data for all‐cause mortality (at hospital discharge), worsening of clinical status (need for mechanical ventilation) and adverse events (any grade). All other prioritised outcomes of this review were not reported by trial authors.
In individuals with a confirmed diagnosis of COVID‐19 and moderate to severe disease, we graded the certainty of the evidence for all‐cause mortality (at hospital discharge) as very low due to serious risk of bias (1 point, unclear risk of bias because of randomisation process in Entrenas Castillo 2020), due to very serious imprecision (2 points, inconsistent directions and variations of point estimates), and due to very serious inconsistency (2 points, wide confidence intervals along with a low number of participants and events). We graded the certainty of the evidence for worsening of clinical status (need for mechanical ventilation) as low due to very serious imprecision (low number of participants and events). For adverse events (any grade), we graded certainty in the evidence as very low due to serious indirectness and very serious imprecision.
Regarding individuals with a confirmed diagnosis of COVID‐19 and asymptomatic or mild disease, we identified one RCT Rastogi 2020, but trial authors did not report any of our prioritised outcomes.
Potential biases in the review process
To avoid potential bias in the review process, we were committed at all times to conduct a systematic review that followed published guidance provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020d).
Experienced medical information specialists of the CEOsys consortium developed an all‐encompassing search strategy to identify available evidence to answer our research question. We aimed at identifying all completed, but also ongoing studies for inclusion in our review. The sensitive search included relevant electronic databases as well as clinical trial registries. As a supplement, we screened reference lists of included studies. In addition to peer‐reviewed full‐text articles, we also included preprints. We are aware of the potentially lower quality of preprint publications, and that results could change once the peer‐reviewed journal publications are available. In cases of missing data, we contacted study authors for additional data or relevant details if we needed more information, details are provided in the 'Characteristics of included studies' tables. We are confident that we identified all relevant studies and will monitor ongoing studies as well as full publication of preprints closely after the publication of this review.
We outlined the study selection process comprehensively and in full detail (Figure 2). Two out of three review authors performed all levels of the selection process (judgement on eligibility, data extraction and judgement on risk of bias) independently and in duplicate; analyses were conducted by one review author and checked by a colleague. We provide reasons for the exclusion of studies from this systematic review and describe each included study in full detail and made explicit judgements on individual risk of bias. We identified no other potential sources of bias in our review process.
Agreements and disagreements with other studies or reviews
We identified (very) lo‐ certainty evidence for vitamin D supplementation as a treatment for COVID‐19, which is similar to findings of the rapid guideline by NICE 2020 published in December 2020. Authors included only one RCT, which we also identified in this review (Entrenas Castillo 2020). Based on this RCT, they reported that people treated with vitamin D are less likely to be admitted to intensive care unit or to die, but certainty in the evidence was very low for both outcomes due to very serious risk of bias and the low number of participants in the study. Author's recommendations for future research were the investigation of vitamin D supplementation in randomised controlled designs, including subgroup analyses with a focus on age (older than 75 years), ethnicity, and co‐morbidities (obesity) and with a minimum eight‐week follow‐up.
Liu 2021 investigated the association of vitamin D status with risk of COVID‐19 infection, which was not the scope of this review. However, we identified several systematic reviews and meta‐analyses which studied the association of vitamin D levels and prognosis of COVID‐19 based mainly on non‐randomised trials (Munshi 2021; Pereira 2020; Shah 2021; Yisak 2021). Pereira 2020 included data for 8176 individuals with COVID‐19 and suggests a positive association of vitamin D deficiency (25‐hydroxyvitamin D levels < 50 nmol/L) and COVID‐19 severity. Additionally, authors reported that participants with vitamin D levels < 75 nmol/L had a higher likelihood for hospitalisation and mortality (Pereira 2020). Similar results were found by Munshi 2021, who investigated prognosis of COVID‐19 in 1368 individuals. In this study, a poor prognosis was associated with lower vitamin D levels and vice versa. Yisak 2021 and colleagues included nine studies and reported an association between vitamin D deficiency (not defined) and hospitalisation, admission to intensive care unit (ICU) and severity of COVID‐19. Shah 2021 included data from 532 hospitalised participants based on two RCTs and one retrospective study. They reported a significant effect on admission to ICU, and a comparable risk for mortality.
Authors of all relevant reviews reported significant heterogeneity and high risk of bias for included studies, and results should therefore be interpreted with caution. Despite Shah 2021, who included two RCTs, none of these reviews or studies were able to show causality of findings because of the design of the included studies. For example, retrospective and cross‐sectional analyses (Pereira 2020; Munshi 2021; Yisak 2021) do not provide information whether vitamin D deficiency was present before diagnosis of COVID‐19 or if it was rather a consequence of the disease. In addition, risk factors for severe COVID‐19, such as older age, obesity, hypertension, diabetes mellitus and others, often overlap with risk factors for vitamin D deficiency. This exacerbates interpretation and the assumption of causality.
We further identified a non‐randomised controlled study with 930 participants (Nogues 2021), suggesting that vitamin D supplementation decreases mortality and admission to ICU. However, the study was removed from the preprint server due to serious concerns about the methodological proceeding.
Currently, there is a lack of adequately powered randomised controlled trials investigating the effectiveness of vitamin D supplementation on COVID‐19. It also remains unclear which populations (e.g. critical ill individuals with vitamin D deficiency) benefit from vitamin D supplementation. Therefore, based on the limited evidence, neither this work nor other published reviews or studies can draw conclusions about the therapeutic effect and safety of vitamin D supplementation for individuals with COVID‐19.
Authors' conclusions
Implications for practice.
Based on the current evidence, we are very uncertain about the effectiveness of vitamin D supplementation for participants with COVID‐19. Moreover, inconsistency in the reporting of adverse and serious adverse events impeded evaluation of safety of vitamin D supplementation. Therefore, we cannot draw conclusions about vitamin D supplementation as a treatment for individuals with COVID‐19. With respect to the identified studies in trial registries, our results are subject to change in the future.
Implications for research.
To elucidate the effectiveness and safety of vitamin D supplementation for individuals with COVID‐19, more randomised controlled trials (RCTs) are necessary. Heterogeneity of studies was problematic in this review and impeded conduction of meta‐analysis. Studies were mainly heterogenous because of different supplementation strategies, formulations, vitamin D statuses of participants, and reported outcomes. Moreover, measurement and reporting of serious adverse events was inconsistent across included studies as well, which exacerbate the evaluation of safety.
Currently, there is an urgent need for good‐quality evidence, ideally based on RCTs with appropriate randomisation procedures, comparability of study arms and a preferably double‐blinded design. We identified 21 ongoing RCTs in trial registries, which might increase the evidence about vitamin D supplementation as a treatment for COVID‐19 in the future. In accordance with the living approach of this review, we will continually update our search and include eligible trials.
Notes
Parts of the review's methods section is adopted from templates of Cochrane Haematology and a similar protocol published by Piechotta 2020, and the corresponding reviews (Chai 2020).
Risk of bias
Acknowledgements
This work is part of a series of reviews investigating treatments and therapies for COVID‐19 as part of the project CEOsys. Text passages in the background section (e.g. "description of the condition" and "why it is important to do this review") are shared between reviews of this series. We thank the authors of the first published reviews of this series for providing and sharing this information. Moreover, we thank the Cochrane Haematology working group for using the template for the description of methods.
This review was published in collaboration with the Cochrane Editorial and Methods Department. We particularly thank Liz Bickerdike (Managing Editor), Robin Featherstone (Information Specialist), Tess Moore (Methods Editor), and Heather Maxwell (Copy Editor) for their excellent support, their valuable comments on the review and timely management of the editorial process. We thank Robert Walton of the Analysis of Review Group Output (ARGO) for his comments on the abstract, and Denise Mitchell for writing the Plain Language Summary.
We thank all external peer reviewers who read and commented on this review. We thank Saurabh Mehta (Division of Nutritional Sciences, Cornell University, Ithaca and Samantha Huey, Division of Nutritional Sciences, Cornell University, Ithaca), John White (Department of Physiology, McGill University), and our consumer referee Abhijit Dutta (PDMJ, Research Scientist, Office of Medical Research &Data Management, Sanjiban Hospital, Howrah, India), who greatly helped to improve this review.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "CEOSys"). The contents of this document reflect only the authors' views and the German Ministry is not responsible for any use that may be made of the information it contains.
Appendices
Appendix 1. Search strategies
Cochrane COVID‐19 Study Register
Search string: "vitamin d" OR "vitamind" OR "vitamin d3" OR "vitamin d2" OR "hydroxyvitamin d" OR "dihydroxyvitamin d" OR cholecalciferol* OR colecalcifer* OR calciferol* OR calciol* OR calcidiol* OR calcitriol* OR calcifediol* OR calciferol* OR ercalcidiol* OR ercalcitriol* OR ergocalciferol* OR doxercalciferol* OR colecalciferol* OR paricalcitol* OR alphacalcidol* OR dihydrotachysterol*
Study characteristics: 1) "Intervention assignment": “Randomised” OR 2) "Study type": "Interventional" AND "Study design": "Parallel/Crossover" OR "Unclear" OR "Other"
= 62 studies (75 references)
Web of Science Core Collection (Advanced search)
#1
TI=("vitamin d" OR "vitamind" OR "vitamin d3" OR "vitamin d2" OR "hydroxyvitamin d" OR "dihydroxyvitamin d" OR cholecalciferol* OR colecalcifer* OR calciferol* OR calciol* OR calcidiol* OR calcitriol* OR calcifediol* OR calciferol* OR ercalcidiol* OR ercalcitriol* OR ergocalciferol* OR doxercalciferol* OR colecalciferol* OR paricalcitol* OR alphacalcidol* OR dihydrotachysterol*) OR AB=("vitamin d" OR "vitamind" OR "vitamin d3" OR "vitamin d2" OR "hydroxyvitamin d" OR "dihydroxyvitamin d" OR cholecalciferol* OR colecalcifer* OR calciferol* OR calciol* OR calcidiol* OR calcitriol* OR calcifediol* OR calciferol* OR ercalcidiol* OR ercalcitriol* OR ergocalciferol* OR doxercalciferol* OR colecalciferol* OR paricalcitol* OR alphacalcidol* OR dihydrotachysterol*)
#2
TI=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2") OR AB=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")
#3
#1 AND #2
#4
TI=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII") OR AB=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII")
#5
#3 AND #4 Indexes=SCI‐EXPANDED, ESCI Timespan=2020‐2021
= 100
WHO COVID‐19 Global literature on coronavirus disease
("vitamin d" OR "vitamind" OR "vitamin d3" OR "vitamin d2" OR "hydroxyvitamin d" OR "dihydroxyvitamin d" OR cholecalciferol* OR colecalcifer* OR calciferol* OR calciol* OR calcidiol* OR calcitriol* OR calcifediol* OR calciferol* OR ercalcidiol* OR ercalcitriol* OR ergocalciferol* OR doxercalciferol* OR colecalciferol* OR paricalcitol* OR alphacalcidol* OR dihydrotachysterol*) AND (random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII") ‐ without MEDLINE and PubMed = 106
Data and analyses
Comparison 1. Vitamin D supplementation vs. placebo or standard care alone for individuals with moderate to severe disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Worsening of clinical status: need for mechanical ventilation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Duration of hospitalisation | 1 | 237 | Hazard Ratio (IV, Fixed, 95% CI) | 1.07 [0.81, 1.41] |
| 1.4 Vitamin D serum levels | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | 24.70 [21.41, 27.99] |
| 1.5 Admission to intensive care unit | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Adverse events (any grade) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: Vitamin D supplementation vs. placebo or standard care alone for individuals with moderate to severe disease, Outcome 1: All‐cause mortality
Comparison 2. Subgroup analysis (vitamin D baseline status): Vitamin D supplementation vs. placebo or standard care alone in individuals with moderate to severe disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Vitamin D non‐deficient | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.30, 3.17] |
| 2.1.2 Vitamin D deficient | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 4.07 [0.47, 35.31] |
| 2.2 Need for mechanical ventilation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Vitamin D non‐deficient | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.15, 1.08] |
| 2.2.2 Vitamin D deficient | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.23, 2.88] |
| 2.3 Admission to intensive care unit | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Vitamin D non‐deficient | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.48, 3.50] |
| 2.3.2 Vitamin D deficient | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.56, 2.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Entrenas Castillo 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary study outcome: admission to ICU, mortality at hospital discharge Review outcomes
Additional study outcomes: ‐ |
|
| Notes |
|
|
Murai 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary study outcome: Hospital length of stay Review outcomes
Additional study outcomes: serum levels of calcium, C‐reactive protein and D‐dimer, duration of mechanical ventilation |
|
| Notes |
|
|
Rastogi 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
Additional study outcomes: the change in the level of inflammatory markers with treatment |
|
| Notes |
|
|
ICU: intensive care unit; NA: not applicable; NR: not reported; PCR: polymerase chain reaction; RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beigmohammadi 2020 | combination of vitamin D with other treatments |
| CTRI/2020/06/026191 | prevention |
| Euctr2020‐001363‐85‐dk | prevention |
| Euctr2020‐001903‐17 | combination of vitamin D with other treatments |
| IRCT20200319046819N1 | combination of vitamin D with other treatments |
| IRCT20200705048013N1 | combination of vitamin D with other treatments |
| NCT04351490 | study withdrawn: the study stopped early, before enrolling its first participant. Combination of vitamin D with other treatments |
| NCT04395768 | combination of vitamin D with other treatments |
| NCT04399746 | combination of vitamin D with other treatments |
| NCT04407286 | single‐arm study |
| NCT04476680 | prevention |
| NCT04482686 | combination of vitamin D with other treatments |
| NCT04483635 | prevention |
| NCT04507867 | combination of vitamin D with other treatments |
| NCT04535791 | prevention |
| NCT04565392 | combination of vitamin D with other treatments |
| NCT04579640 | prevention |
| NCT04596657 | prevention |
| NCT04780061 | combination of vitamin D with other treatments |
| Nogues 2021 | non‐randomised study; paper removed from preprint server quote: "due to concerns about the description of the research in this paper" |
Characteristics of studies awaiting classification [ordered by study ID]
IRCT20110726007117N11.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes:
|
| Notes |
|
IRCT20200324046850N1.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes (add for each outcome if planned or not planned)
Additional study outcomes:
|
| Notes |
|
NCT04733625.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes: no |
| Notes |
|
NA: not applicable; NI: no information; NP: not planned; NR: not reported; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
CTRI/2020/06/026189.
| Study name | Randomized, double blind, parallel group study of vitamin D3 & magnesium in Covid 19 infection |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes:
|
| Starting date | 01/08/2020 |
| Contact information | AVN Sridhar suraksha pharma 8‐3‐898/5, suraksha towers, Ameerpet, Hyderabad TELANGANA 500073 India svp@surakshapharma.com |
| Notes |
|
CTRI/2020/12/030083.
| Study name | To study the role of vitamin D in the treatment of confirmed COVID‐19 infection |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 31/12/2020 |
| Contact information | Dr I Shyam Sundar Varaprasad Raju Gandhi Medical College and Hospital Musheerabad Padmarao Nagar Secunderabad Hyderabad TELANGANA 500003 India 9848152349 drgsgmbbs@gmail.com |
| Notes |
|
EUCTR2020‐001717‐20‐ES.
| Study name | Prevention and treatment with Calcifediol of Coronavirus COVID‐19‐induced acute respiratory syndrome (SARS) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 20/04/2020 |
| Contact information | Antonio Luque Edificio IMIBIC. Avenida Menéndez Pidal s/n 14004 Córdoba Spain 0034671596070 0034957736571 uicec@imibic.org |
| Notes |
|
EUCTR2020‐001960‐28‐ES.
| Study name | Efficacy of vitamin D treatment in patients diagnosed with pneumonia who require hospital admission and have vitamin D deficiency and a positive diagnosis for SARS‐Cov‐2 (COVID‐19) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 26/05/2020 |
| Contact information | Investigation Institute Bioaraba Inés Pérez Francisco c/José Atxotegui s/n Vitoria‐Gasteiz 01009 Spain INES.PEREZFRANCISCO@osakidetza.eus |
| Notes |
|
EUCTR2020‐002274‐28‐ES.
| Study name | Usefulness of vitamin D on morbidity and mortality of SARS‐COV‐2 virus infection (Covid‐19) at the Central University Hospital of Asturias |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome: (14 and 21 days or until a negative SARS‐CoV‐2 test every 7 days)
Review outcomes
Additional study outcomes: |
| Starting date | 2020‐05‐21 |
| Contact information | Avenida Hospital Universitario s/n: enrique.caso@gmail.com |
| Notes |
|
EUCTRCT2020‐002312‐43‐ES.
| Study name | Clinical trial, PHASE III, randomized, open‐label, to evaluate the efficacy of administering high‐dose cholecalciferol orally alongside standard therapy in patients with COVID‐19 pneumonia (COVID‐19 HUSO). |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 31/05/2020 |
| Contact information | Miguel Cervero Avenida Orellana S/N Leganés 28911 Spain 00349148180008338 mcerveroj@gmail.com |
| Notes |
|
NCT04334005.
| Study name | Vitamin D on prevention and treatment of COVID‐19 (COVITD‐19) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
Time Frame: through study completion, an average of 10 weeks |
| Starting date | 10/04/2020 |
| Contact information | Manuel J Castillo, MD, PhD +34 649440850 mcgarzon@ugr.es |
| Notes |
|
NCT04344041.
| Study name | COvid‐19 and Vitamin D supplementation: a multicenter randomizedcControlled trial of high dose versus standard dose vitamin D3 in high‐risk COVID‐19 Patients (CoVitTrial) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes Inpatient setting
Outpatient setting
Additional study outcomes:
|
| Starting date | 15/04/2020 |
| Contact information | Cédric ANNWEILER 241354725 ext +33 cedric.annweiler@chu-angers.fr |
| Notes |
|
NCT04363840.
| Study name | The LEAD COVID‐19 Trial: Low‐risk, Early Aspirin and vitamin D to reduce COVID‐19 hospitalizations (LEAD COVID‐19) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes:
|
| Starting date | 05/2020 |
| Contact information | Frank H Lau, MD 504 412 1240 flau@lsuhsc.edu |
| Notes |
|
NCT04385940.
| Study name | Vitamin D and COVID‐19 management |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes Outpatient setting
Inpatient setting
Additional study outcomes
|
| Starting date | 06/2020 |
| Contact information | Aldo Montano‐Loza Associate Professor of Medicine, Program Director of Hepatology University of Alberta |
| Notes |
|
NCT04386850.
| Study name | Oral 25‐hydroxyvitamin D3 and COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes:
|
| Starting date | 14/04/2020 |
| Contact information | Zhila Maghbooli, PhD +98 21 6670 6142 zhilayas@gmail.com |
| Notes |
|
NCT04411446.
| Study name | Randomized controlled trial of high dose of vitamin D as compared with placebo to prevent complications among COVID‐19 patients |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 11/08/2020 |
| Contact information | Javier Mariani, MD +541142109000 ext 1523 ja_mariani@hotmail.com |
| Notes |
|
NCT04482673.
| Study name | The role of vitamin D in mitigating COVID‐19 infection severity: focusing on reducing health disparities in South Carolina |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 31/07/2020 |
| Contact information | Carol L Wagner, MD 843‐792‐8829 washinro@musc.edu Medical University of South Carolina |
| Notes |
|
NCT04489628.
| Study name | Tele‐health enabled clinical trial for COVID‐19: vitamin D as aniImmunomodulator to prevent complications and reduce resource utilization in Ootpatients |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes: none |
| Starting date | 01/08/2020 |
| Contact information | Kevin Cooper, MD 216‐844‐5197 UHDermatologyClinicalTrials@UHhospitals.org |
| Notes |
|
NCT04502667.
| Study name | Efficacy of vitamin D treatment in pediatric Patients hospitalized by COVID‐19: open controlled clinical trial |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes:
|
| Starting date | 15/07/2020 |
| Contact information | Hospital Centro Medico Nacional Siglo XXI Carla Castuera Martinez 56276900 ext 21218 carla_martinez@imss.gob.mx |
| Notes |
|
NCT04525820.
| Study name | High dose vitamin‐D substitution in patients With COVID‐19: a randomized controlled, multicCenter study (VitCov) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 15/12/2020 |
| Contact information | Jörg D Leuppi, Professor +41 61 925 2181 joerg.leuppi@ksbl.ch |
| Notes |
|
NCT04536298.
| Study name | A cluster‐randomized, double‐blind, placebo‐controlled study to evaluate the efficacy of vitamin D3 supplementation to reduce disease severity in persons with newly diagnosed COVID‐19 infection and to prevent infection in household members |
| Methods |
|
| Participants | Inclusion/exclusion criteria for INDEX CASES
Inclusion/exclusion criteria for HOUSEHOLD CONTACTS
|
| Interventions |
|
| Outcomes |
|
| Starting date | 28/12/2020 |
| Contact information | Trisha Copeland, MS, RD1‐877‐517‐2555 pcopeland2@bwh.harvard.edu |
| Notes |
|
NCT04552951.
| Study name | Effect of vitamin D on morbidity and mortality of the COVID‐19 (COVID‐VIT‐D) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
Additional study outcomes
|
| Starting date | 04/04/2020 |
| Contact information | Jorge B Cannata‐Andía, MD PhD Hospital Universitario Central de Asturias *34 985 106137 cannata@hca.es |
| Notes |
|
NCT04621058.
| Study name | Efficacy of vitamin D treatment in mortality reduction due to COVID‐19 |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 09/11/2020 |
| Contact information | Joaquín Durán Cantolla Vitoria‐Gasteiz, Alava, Spain, 01002 +34945207925 joaquin.durancantolla@gmail.com |
| Notes |
|
NCT04636086.
| Study name | Vitamin D supplementation and Covid‐19: a randomised, double‐ blind, controlled study |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome
Review outcomes
|
| Starting date | November 12, 2020 |
| Contact information | Contact: Anne‐Françoise Rousseau, MD, PhD +3243667495 afrousseau@chuliege.be |
| Notes |
|
NCT04641195.
| Study name | Vitamin D and zinc supplementation for improving treatment outcomes among COVID‐19 patients in India |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | 01/03/2021 |
| Contact information | Wafaie W Fawzi, MBBS, MPH, MS, DrPH 617 432 2086 mina@hsph.harvard.edu |
| Notes |
|
CRP: C‐reactive protein; HDL: high‐density lipoprotein; ICU: intensive care unit; IV: intravenous; LDL: low‐density lipoprotein; NA: not applicable; NI: no information; NP: not planned; NR: not reported; TAU: treatment as usual
Differences between protocol and review
The following differences exist between protocol (Stroehlein 2021a) and review:
Objectives
We also included studies with an active comparator, such as another treatment, if these were comparable between groups.
Types of outcome measures
We specified outcomes regarding effectiveness and safety of vitamin D supplementation for individuals with COVID‐19 and either moderate to severe or mild to asymptomatic disease after a guideline consortium (CEOSys) that took place after protocol registration. We created outcome categories and added/specified the following outcomes for hospitalised participants with moderate or severe COVID‐19:
All‐cause mortality at day 60, time‐to‐event, and at hospital discharge
Need for dialysis (at up to 28 days)
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to seven days; up to 30 days, and longest follow‐up available
Admission to ICU
Duration of hospitalisation
Time to discharge from hospital
Adverse events (any grade, grade 1‐2, grade 3‐4), defined as number of participants with event
For ambulatory managed individuals with mild to asymptomatic disease, we added and specified the following outcomes:
All‐cause mortality at day 60, time‐to‐event, and up to longest follow‐up
Admission to hospital (WHO≥ 4)
-
Need for hospitalisation with or without supplemental oxygen i.e. WHO=4‐5, moderate disease;
Need for hospitalisation without oxygen therapy i.e. WHO=4.
Need for oxygen by mask or nasal prongs i.e. WHO=5;
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at up to 7 days, up to 30 days, and longest follow‐up available
Adverse events (any grade, grade 1‐2, grade 3‐4), defined as number of participants with event
Vitamin D serum levels
Types of subgroup analyses
We expanded subgroup analysis and additionally plan to conduct separate analysis in the next updates of this review for
age (< 18 years, 18‐65 years, and > 65 years)
pre‐existing conditions
duration since symptom onset (below or above seven days)
formulation of vitamin D (active, non‐active)
doses of vitamin D (single or multiple)
administration of vitamin D
co‐treatments
Summary of findings and assessment of the certainty of the evidence
After guideline consortium (CEOSys), we prioritised the following outcome categories for hospitalised individuals with COVID‐19 and moderate or severe disease:
All‐cause mortality
-
Improvement of clinical status, assessed with liberation from supplemental oxygen support or invasive mechanical ventilation, in accordance with WHO Clinical Progression Scale (WHO 2020c) at longest follow‐up available
Liberation from supplemental oxygen
Liberation from invasive mechanical ventilation
Worsening of clinical status assessed by need for invasive mechanical ventilation
Quality of life
Adverse events
Serious adverse events
For ambulatory managed individuals with COVID‐19 and mild or asymptomatic disease, we prioritised the outcome categories:
All‐cause mortality
Development of severe clinical COVID‐19 symptoms
Quality of life
Adverse events
Serious adverse events
Contributions of authors
JS: methodological expertise, study selection, data extraction and assessment, conception and writing of the manuscript
JW: clinical expertise, conception and writing of the manuscript
CI: methodological expertise, study selection, data extraction and assessment, writing of the manuscript
AM: clinical expertise, conception and writing of the manuscript
MS: clinical expertise, conception and writing of the manuscript
CB: methodological expertise, conception and writing of the manuscript
MIM: information specialist, development of the search strategy, writing of the manuscript
PM: clinical expertise and advice, proof‐reading of the manuscript
MB: data extraction
NS: methodological expertise and advice, study selection, conception, writing and proof‐reading of the manuscript
VP: methodological expertise and advice, data extraction and assessment, conception, writing and proof‐reading of the manuscript
Sources of support
Internal sources
-
University Hospital of Cologne, Germany
Cochrane Cancer, Department I of Internal Medicine
External sources
-
Federal Ministry of Education and Research, Germany
This review is part of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021.
Declarations of interest
JS: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "CEOSys", which was paid to the institution).
JW: none known
CI: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "CEOSys", which was paid to the institution).
AM: none known
CB: none known
MIM: is member of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021, paid to the institution.
PM: institution received grants by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF), grant number 01KG1815, for the VITDALIZE‐Study.
MB: none known
NS: none known
MS: none known
VP: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project "CEOSys", which was paid to the institution).
contributed equally
contributed equally
New
References
References to studies included in this review
Entrenas Castillo 2020 {published data only}
- Entrenas Castillo E, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Diaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus bestavailable therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. Journal of Steroid Biochemistry and Molecular Biology 2020;203:105-751. [DOI: 10.1016/j.jsbmb.2020.105751] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murai 2021 {published data only}
- Murai IH, Fernandes AL, Sales LP, Pinto AJ, Giessler KF, Curan CS, et al. Effect of vitamin D 3 supplementation vs placebo on hospital length of stay in patients with severe COVID-19: a multicenter, double-blind, randomized controlled trial (preprint). Medrxiv. [DOI: ]
- Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CS, et al. Effect of a single high dose of vitamin D 3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA 2021;Published online February 17, 2021:E1-8. [DOI: 10.1001/jama.2020.26848] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rastogi 2020 {published data only}
- Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgraduate Medical Journal 2020;0:1-4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beigmohammadi 2020 {published data only}
- Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Mahmoodi Ali Abadi M, et al. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):614. [PMID: 10.1186/s13063-020-04547-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2020/06/026191 {published data only}
- CTRI/2020/06/026191. To study the safety and efficacy of vitamin D3,vitamin K2-7 & magnesium, in prevention of COVID 19 infection in health care professional (HCP). www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45075 2020.
Euctr2020‐001363‐85‐dk {published data only}
- Odense University, Hospital. Medication for prevention of Corona-virus for Danish nursing home residents. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001363-85.
Euctr2020‐001903‐17 {unpublished data only}
- 2020-001903-17. A randomized clinical trial (IIIb) of eficacy of a single dose of Tocilizumab or a combination of Tocilizumab plus vitamin D (single i.m. dose) for the treatment of the COVID-19 hyperimmune complication. Assessment of IL-6. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001903-17/ES.
IRCT20200319046819N1 {published data only}
- Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Mahmoodi Ali Abadi M, et al. Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):614. [DOI: 10.1186/s13063-020-04547-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT20200319046819N1. Impact of vitamin B, A, D, E, C supplementation on improvement and mortality rate in patients with COVID-19 admitted in intensive care unit. https://www.irct.ir/trial/46838 (first received April 4, 2020).
IRCT20200705048013N1 {published data only}
- Karaj University of Medical Sciences. The effect of Pentoxifylline in patients with Covid-19. http://en.irct.ir/trial/49525.
NCT04351490 {published data only}
- NCT04351490. Influence of zinc and vitamin D3 supplementation on survival in elderly institutionalized individuals with CoV-2 SARS infection. https://clinicaltrials.gov/ct2/show/NCT04351490 (first received April 17, 2020).
NCT04395768 {published data only}
- National Institute of Integrative Medicine, Australia. Therapies to Prevent Progression of COVID-19, Including Hydroxychloroquine, Azithromycin, Zinc, vitamin D, vitamin B12 with or without vitamin C, a multi-centre, international, randomized trial: the International ALLIANCE Study. https://clinicaltrials.gov/show/NCT04395768.
NCT04399746 {published data only}
- NCT04399746. Ivermectin-Azithromycin-Cholecalciferol (IvAzCol) combination therapy for COVID-19. https://clinicaltrials.gov/show/NCT04399746.
NCT04407286 {published data only}
- NCT04407286. Vitamin D testing and treatment for COVID 19. https://clinicaltrials.gov/ct2/show/NCT04407286 (First received: May 29, 2020).
NCT04476680 {published data only}
- NCT04476680. Reducing asymptomatic infection with vitamin D in coronavirus disease. https://clinicaltrials.gov/ct2/show/NCT04476680 (first received July 20, 2020).
NCT04482686 {published data only}
- ProgenaBiome. Trial of combination therapy to treat COVID-19 infection. https://clinicaltrials.gov/show/NCT04482686.
NCT04483635 {published data only}
- NCT04483635. Preventing COVID-19 with high-dose vitamin D supplements. https://clinicaltrials.gov/ct2/show/NCT04483635 (first received July 23, 2020).
NCT04507867 {published data only}
- NCT04507867. Effect of a Nss to reduce complications in patients with Covid-19 and comorbidities in stage III. https://clinicaltrials.gov/ct2/show/NCT04507867 (first received 11 August 2020).
NCT04535791 {published data only}
- NCT04535791. Efficacy of vitamin D supplementation to prevent the risk of acquiring COVID-19 in healthcare workers (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04535791 (first received September 2, 2020).
NCT04565392 {published data only}
- drpykessupplementscom. Proof-of-concept randomized placebo-controlled trial of Famotidine for outpatients With COVID-19. https://clinicaltrials.gov/show/NCT04565392.
NCT04579640 {published data only}
- NCT04579640. Trial of vitamin D to reduce risk and severity of COVID-19 and other acute respiratory infections (CORONAVIT). https://clinicaltrials.gov/ct2/show/NCT04579640 (first received October 8, 2020).
NCT04596657 {published data only}
- NCT04596657. Vitamin D3 supplementation to prevent respiratory tract infections. https://clinicaltrials.gov/ct2/show/NCT04596657 (first received October 22, 2020).
NCT04780061 {published data only}
- The Canadian College of Naturopathic, Medicine. Dietary supplements for COVID-19. https://clinicaltrials.gov/show/NCT04780061.
Nogues 2021 {published data only}
- Nogues X, Ovejero D, Quesada-Gomez JM, Bouillon R, Arenas D, Pasqual J, et al. Calcifediol treatment and COVID-19-related outcomes (preprint). SSRN. [DOI: ]
References to studies awaiting assessment
IRCT20110726007117N11 {published data only}
- IRCT20110726007117N11. Effect of vitamin D supplementation in novel corona virus 2019. https://en.irct.ir/trial/48287 (first received 2020-07-05).
IRCT20200324046850N1 {published data only}
- IRCT20200324046850N1. Comparison of vitamin D3 and N-acetylcysteine prescription in COVID-19 patients and their effect on recovery process. https://en.irct.ir/trial/46732 (first received 29 March 2020).
NCT04733625 {published data only}
- Kasr El Aini Hospital. TheeEffect of vitamin D tn morbidity and moratlity in patients with SARS-CoV 2 infection. https://clinicaltrials.gov/show/NCT04733625.
References to ongoing studies
CTRI/2020/06/026189 {published data only}
- CTRI/2020/06/026189. To compare the safety and efficacy of vitamin D, with magnesium in mild to moderate Covid 19 patients. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45029 (first received 26/06/2020).
CTRI/2020/12/030083 {published data only}
- CTRI/2020/12/030083. To study the role of vitamin D in the treatment of confirmed COVID-19 infection. ctri.nic.in/Clinicaltrials/showallp.php?mid1=46899&EncHid=&userName=CTRI/2020/12/030083 (first received 29 December 2020).
EUCTR2020‐001717‐20‐ES {published data only}
- EUCTR2020-001717-20-ES. Prevention and treatment with Calcifediol of respiratory problems caused by COVID-19. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001717-20 (first received 20 April 2020).
EUCTR2020‐001960‐28‐ES {published data only}
- EUCTR2020-001960-28-ES. Efficacy of vitamin D treatment in patients diagnosed with pneumonia who require hospital admission and have vitamin D deficiency and a positive diagnosis for SARS-Cov-2 (COVID-19). https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001960-28/ES (first received 26 Mai 2020).
EUCTR2020‐002274‐28‐ES {published data only}
- EUCTR2020-002274-28-ES. Usefulness of vitamin D on morbidity and mortality of SARS-COV-2 virus infection (Covid-19) at the Central University Hospital of Asturias. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-002274-28/ES (first received 2020-05-21).
EUCTRCT2020‐002312‐43‐ES {published data only}
- EUCTR2020-002312-43-ES. Clinical trial, randomized, open-label, to evaluate the efficacy of high-dose vitamin D in patients with COVID-19 pneumonia. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-002312-43/ES (first received 2020-05-31).
NCT04334005 {published data only}
- NCT04334005. Vitamin D on prevention and treatment of COVID-19. https://clinicaltrials.gov/ct2/show/NCT04334005 (first received April 3, 2020).
NCT04344041 {published data only}
- Annweiler C, Beaudenon M, Gautier J, Simon R, Dubee V, Gonsard J, et al. COvid-19 and high-dose VITamin D supplementation trial in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials 2020;21(1):1031. [DOI: 10.1186/s13063-020-04928-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2020-001602-34-FR. COvid-19 and Vitamin D supplementation: a multicenter randomized controlled Trial of high dose versus standard dose vitamin D3 in high-risk COVID-19 patients. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001602-34/FR (first received on 3 April 2020).
- NCT04344041. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT04344041 (first received 14 April 2020). [DOI] [PMC free article] [PubMed]
NCT04363840 {published data only}
- NCT04363840. The LEAD COVID-19 trial: Low-risk, early aspirin and Vitamin D to reduce COVID-19 hospitalizations. https://clinicaltrials.gov/ct2/show/NCT04363840 (first received April 27, 2020).
NCT04385940 {published data only}
- NCT04385940. Vitamin D and COVID-19 management. https://clinicaltrials.gov/ct2/show/NCT04385940 (First posted: 13 May 2020).
NCT04386850 {published data only}
- NCT04386850. Oral 25-hydroxyvitamin D3 and COVID-19. https://clinicaltrials.gov/ct2/show/NCT04386850 (first received May 13, 2020).
NCT04411446 {published data only}
- Mariani J, Tajer C, Antonietti L, Inserra F, Ferder L, Manucha W. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: a structured summary of a study protocol for a randomised controlled trial (CARED-TRIAL). Trials 2021;22(1):111. [DOI: 10.1186/s13063-021-05073-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04411446. Cholecalciferol to improve the outcomes of COVID-19 patients (CARED). https://clinicaltrials.gov/ct2/show/NCT04411446 (First posted June 2, 2020).
NCT04482673 {published data only}
- NCT04482673. Vitamin D supplementation in the prevention and mitigation of COVID-19 infection (VitD-COVID19). https://clinicaltrials.gov/ct2/show/NCT04482673 (first received July 22, 2020).
NCT04489628 {published data only}
- NCT04489628. Tele-health enabled clinical trial for COVID-19. https://clinicaltrials.gov/ct2/show/NCT04489628 (First posted 28 July 2020).
NCT04502667 {published data only}
- NCT04502667. Efficacy of vitamin D treatment in pediatric patients hospitalized by COVID-19 (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04502667 (first received August 6, 2020).
NCT04525820 {published data only}
- NCT04525820. High dose vitamin-D substitution in patients with COVID-19: a randomized controlled, multi center study. https://clinicaltrials.gov/ct2/show/NCT04525820 (first received August 25, 2020).
NCT04536298 {published data only}
- NCT04536298. Vitamin D and COVID-19 trial (VIVID). https://clinicaltrials.gov/ct2/show/NCT04536298 (First posted 2 September 2020).
- Wang R, DeGruttola V, Lei Q, Mayer KH, Redline S, Hazra A, et al. The vitamin D for COVID-19 (VIVID) trial: a pragmatic cluster-randomized design. Contemporary clinical trials January 2021;100:106176. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04552951 {published data only}
- NCT04552951. Effect of vitamin D on morbidity and mortality of the COVID-19 (COVID-VIT-D). https://clinicaltrials.gov/ct2/show/NCT04552951 (first received September 17, 2020).
NCT04621058 {published data only}
- NCT04621058. Efficacy of vitamin D treatment in mortality reduction due to COVID-19. https://clinicaltrials.gov/ct2/show/NCT04621058 (first received November 9, 2020).
NCT04636086 {published data only}
- NCT04636086. Effect of vitamin D on hospitalized adults with COVID-19 infection. https://clinicaltrials.gov/ct2/show/NCT04636086 (First posted 19 November 2020).
NCT04641195 {published data only}
- NCT04641195. Vitamin D and zinc supplementation for improving treatment outcomes among COVID-19 patients in India. https://clinicaltrials.gov/ct2/show/NCT04641195 (first received November 23, 2020).
Additional references
Amrein 2014
- Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. Jama 2014;312(15):1520-30. [DOI] [PubMed] [Google Scholar]
Amrein 2018
- Amrein K, Papinutti A, Mathew E, Vila G, Parekh D. Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocrine Connections 2018;7(12):304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amrein 2020
- Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Kostenberger M, Tmava Berisha A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. European Journal of Clinical Nutrition 2020;74(11):1498-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barazzoni 2020
- Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clinical Nutrition 2020;39(6):1631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilezikian 2020
- Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in endocrinology: Vitamin D and COVID-19. European Journal of Endocrinology 2020;183(5):R133-R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandi 2002
- Brandi L, Egfjord M, Olgaard K. Pharmacokinetics of 1,25(OH)(2)D(3) and 1alpha(OH)D(3) in normal and uraemic men. Nephrology Dialysis Transplantation 2002;17(5):829-42. [DOI] [PubMed] [Google Scholar]
Braun 2012
- Braun AB, Litonjua AA, Moromizato T, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and acute kidney injury in the critically ill. Critical Care Medicine 2012;40(12):3170-9. [DOI] [PubMed] [Google Scholar]
Buitrago‐Garcia 2020
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLOS Medicine 2020;17(9):e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cabler 2020
- Cabler SS, French AR, Orvedahl A. A cytokine circus with a viral ringleader: SARS-CoV-2-associated cytokine storm syndromes. Trends in Molecular Medicine 2020;26(12):1078-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2020
- Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed 22 March 2021).
Chai 2020
- Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane LSR
- Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode. Available from community.cochrane.org/review-production/production-resources/living-systematic-reviews (accessed 22 March 2021).
COMET 2020
- Core outcome set developers’ response to COVID-19. Available from www.comet-initiative.org/Studies/Details/1538 (accessed 22 March 2021).
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventionsversion 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Eldridge 2016
- Eldridge S, Campbell M, Campbell M, Dahota A, Giraudeau B, Higgins JT, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). Additional considerations for cluster-randomized trials. www.riskofbias.info/welcome/rob-2-0-tool/archive-rob-2-0-cluster-randomized-trials-2016 (accessed 22 March 2021).
EndNote X9 [Computer program]
- EndNote X9. The EndNote Team. Clarivate, 2013.
Fraser 2020
- Fraser WD, Tang JC, Dutton JJ, Schoenmakers I. Vitamin D measurement, the debates continue, new analytes have emerged, developments have variable outcomes. Calcified Tissue International 2020;106(1):3-13. [DOI] [PubMed] [Google Scholar]
Grant 2020
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020;12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grubaugh 2020
- Grubaugh ND, Hanage WP, Rasmussen AL. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell 2020;182(4):794-95. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2021
- Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. Journal of the American Society of Nephrology 2021;32(1):161-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020a
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2020b
- Higgins JP, Tianging L, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2020c
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Higgins 2020d
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Available from: training.cochrane.org/handbook 2020.
Holick 1996
- Holick MF. Vitamin D and bone health. Journal of Nutrition 1996;126(4 Suppl):1159s-64s. [DOI] [PubMed] [Google Scholar]
Holick 2009
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Annals of Epidemiology 2009;19(2):73-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holick 2011
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2011;96(7):1911-30. [DOI] [PubMed] [Google Scholar]
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2009
- Hughes DA, Norton R. Vitamin D and respiratory health. Clinical and Experimental Immunology 2009;158(1):20-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johns Hopkins University & Medicine
- Johns Hopkins University & Medicine Coronavirus Resource Center. Mortality analyses. Available from https://coronavirus.jhu.edu/data/mortality (accessed at 22 March 2021).
Jolliffe 2017
- Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA, Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. The Lancet Respiratory Medicine 2017;5(11):881-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2008
- Jones G. Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition 2008;88(2):582S-586S. [DOI] [PubMed] [Google Scholar]
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Chai KL, Piechotta V, Valk SJ, Estcourt LJ, et al. SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauer 2020
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of Internal Medicine 2020;172(9):577–82. [DOI: 10.7326/M20-0504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leaf 2014
- Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. American Journal of Respiratory and Critical Care Medicine 2014;190(5):533-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2009
- Lee P, Eisman JA, Center JR . Vitamin D deficiency in critically ill patients. New England Journal of Medicine 2009;360(18):1912-4. [DOI] [PubMed] [Google Scholar]
Li 2020
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Liang 2020
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncology 2020;21(3):335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2006
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311(5768):1770-3. [DOI] [PubMed] [Google Scholar]
Liu 2021
- Liu N, Sun J, Wang X, Zhang T, Zhao M, Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. International Journal of Infectious Diseases 2021;104:58-64. [DOI: 10.1016/j.ijid.2020.12.077] [DOI] [PMC free article] [PubMed] [Google Scholar]
MAGICapp [Computer program]
- Norwegian MAGIC Evidence Ecosystem Foundation (powered by UserVoice Inc.) MAGICapp. Brønnøysund (NOR): Norwegian MAGIC Evidence Ecosystem Foundation (powered by UserVoice Inc.), accessed 25 February 2021. Available at magicapp.org.
Malaguarnera 2020
- Malaguarnera L. Vitamin D3 as potential treatment adjuncts for COVID-19. Nutrients 2020;12(11):3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2020
- Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI: 10.1016/S1473-3099(20)30483-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martineau 2017
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martineau 2019
- Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technology Assessment 2019;23(2):1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martucci 2019
- Martucci G, Amrein K, Ney J. Vitamin D deficiency in ICU patients. ICU Management & Practice 2019;19(2):114-6. [Google Scholar]
Microsoft 2018 [Computer program]
- Mircosoft Excel. Microsoft Corporation. Microsoft Corporation, 2018. office.microsoft.com/excel.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Mokhtari 2020
- Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. Journal of Molecular Histology 2020;51(6):613-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munshi 2021
- Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. Journal of Medical Virology 2021;93(2):733-40. [DOI] [PubMed] [Google Scholar]
Nadim 2020
- Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nature Reviews Nephrology 2020;16(12):747-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
National COVID‐19 Clinical Evidence Taskforce 2021
- National COVID-19 Clinical Evidence Taskforce. Caring for people with COVID-19: supporting Australia’s healthcare professionals with continually updated, evidence-based clinical guidelines. Available from covid19evidence.net.au/#living-guidelines (accessed 22 March 2021).
Ney 2019
- Ney J, Heyland DK, Amrein K, Marx G, Grottke O, Choudrakis M, et al. The relevance of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentration for postoperative infections and postoperative organ dysfunctions in cardiac surgery patients: The eVIDenCe study. Clinical Nutrition 2019;38(6):2756-2762. [DOI] [PubMed] [Google Scholar]
NICE 2020
- National Institute for Health and Care Excellence. COVID-19 rapid guideline: vitamin D. Available from https://www.nice.org.uk/guidance/ng187/evidence (assessed 22 March 2021). [PubMed]
Nogues 2021
- Nogues X, Ovejero D, Quesada-Gomez JM, Bouillon R, Arenas D, Pascual J et al. Calcifediol treatment and COVID-19 related outcomes. Available from https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3771318 (assessed 22 March 2021). [DOI] [PMC free article] [PubMed]
Norman 2014
- Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circulation Research 2014;114(2):379-93. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pereira 2020
- Pereira M, Dantas Damascena A, Galvao Azevedo LM, Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Critical Reviews in Food Science and Nutrition 2020;1-9:Epub ahead of print.. [DOI: 10.1080/10408398.2020.1841090.] [DOI] [PubMed] [Google Scholar]
Piechotta 2020
- Piechotta V, Valk SJ, Chai KL, Wood EM, Lamikanra A, Kimber C, et al. Safety and effectiveness of convalescent plasma or hyperimmune globulin for people with COVID-19: a rapid review. osf.io/dwf53 2020. [DOI: 10.17605/OSF.IO/DWF53] [DOI] [PMC free article] [PubMed]
Putzu 2017
- Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, et al. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. Journal of Critical Care 2017;38:109-14. [DOI] [PubMed] [Google Scholar]
Ramos‐Martinez 2018
- Ramos-Martinez E, Lopez-Vancell MR, Fernandez de Cordova-Aguirre JC, Rojas-Serrano J, Chavarria A, Velasco-Medina A, et al. Reduction of respiratory infections in asthma patients supplemented with vitamin D is related to increased serum IL-10 and IFNgamma levels and cathelicidin expression. Cytokine 2018;108:239-46. [DOI] [PubMed] [Google Scholar]
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. revman.cochrane.org.
Roth 2020
- Roth A, Lütke S, Meinberger D, Hermes G, Sengle G, Koch M, et al. LL-37 fights SARS-CoV-2: The vitamin D-inducible peptide LL-37 inhibits binding of SARS-CoV-2 spike protein to its cellular receptor angiotensin converting enzyme 2 in vitro. bioRxiv 2020/01/01. [DOI: ]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Sassi 2018
- Sassi F, Tamone C, D'Amelio P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018;10(11):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Sengupta 2021
- Sengupta T, Majumder R, Majumder S. Role of vitamin D in treating COVID-19-associated coagulopathy: problems and perspectives. Molecular and Cellular Biochemistry 2021;(no indication):(no indication). [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2021
- Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM: An International Journal of Medicine 24 January 2021;0:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siemieniuk 2020
- Siemieniuk R, Rochwerg B, Agoritsas T, Lamontagne F, Leo Y S, Macdonald H, et al. A living WHO guideline on drugs for Covid-19. BMJ 2020;370: m3379:1-14. [DOI: ] [DOI] [PubMed] [Google Scholar]
Skoetz 2020
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020
- Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020;181(4):894-904 e9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2020
- Wells Mulherin D, Walker R, Holcombe B, Guenter P. ASPEN report on nutrition support practice processes with COVID-19: The first response. Nutrition in Clinical Practice 2020;35(5):783-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Cumulative number of reported probable cases of SARS. Available from www.who.int/csr/sars/country/2003_07_11/en (accessed 22 March 2021).
WHO 2019
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Available from www.who.int/emergencies/mers-cov/en (accessed 22 March 2021).
WHO 2020a
- World Health Organization. Report of the WHO‐China Joint Mission on coronavirus disease 2019 (COVID‐19). Available from www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report (accessed 22 March 2021).
WHO 2020b
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. Available from www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 22 March 2021).
WHO 2020c
- World Health Organization. Corticosteroids for COVID-19 - living guidance 2 September 2020. Available from www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed 22 March 2021).
WHO 2020d
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. Available from www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed 22 March 2021).
WHO 2020e
- WHO working group on the clinical characterisation and management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI] [PMC free article] [PubMed]
WHO 2020f
- World Health Organization (WHO). Clinical management of COVID-19 - interim guidance, 27 May 2020. WHO/2019-nCoV/clinical/2020.4.
WHO 2020g
- World Health Organization. COVID-19 Therapeutic Trial Synopsis. Available from www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf (assessed 27 April 2021).
WHO 2021a
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 22 March 2021).
WHO2021b
- World Health Organization. SARS-CoV-2 Variants. Available from https://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed 22 March 2021).
WHO 2021c
- World Health Organization. Weekly epidemiological update - 23 February 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---23-february-2021 (accessed 22 March 2021).
Williamson 2020
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [DOI: 10.1038/s41586-020-2521-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2017
- Wu C, Qiu S, Zhu X, Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism 2017;73:67-76. [DOI] [PubMed] [Google Scholar]
Wu 2020
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI: 10.1001/jama.2020.2648] [DOI] [PubMed] [Google Scholar]
Wu 2020a
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI] [PubMed] [Google Scholar]
Yisak 2021
- Yisak H, Ewunetei A, Kefale B, Mamuye M, Teshome F, Ambaw B, et al. Effects of vitamin D on COVID-19 infection and prognosis: A systematic review. Risk management and healthcare policy 2021;14:31-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Stroehlein 2021a
- Stroehlein JK, Wallqyist J, Iannizzi C, Benstoem C, Meybohm P, Mikolajewska A, et al. Effects of vitamin D supplementation on clinical outcomes of COVID-19 patients (part of German Evidence Ecosystem CEO-sys). PROSPERO 21 January 2021 (available from https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021232283). [PROSPERO: CRD42021232283]
Stroehlein 2021b
- Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf MI, Benstoem C, et al. Risk of bias assessments for the Cochrane review 'Vitamin D supplementation for the treatment of COVID-19: a living systematic review'. https://zenodo.org/record/4771734#.YKTEFKj7Ryw 2021. [DOI: 10.5281/zenodo.4771734] [DOI] [PMC free article] [PubMed]


