Abstract
Background
The impact of neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer remains uncertain.
Objectives
This review of individual patient data (IPD) aimed to assess the effect of; Neoadjuvant chemotherapy followed by radical radiotherapy compared to the same radiotherapy; and Neoadjuvant chemotherapy followed by surgery compared to radical radiotherapy.
Search methods
Searches of Medline, CancerLit and trial registers were supplemented by hand‐searching conference proceedings and contacting relevant trialists. Searches have been updated to February 2006.
Selection criteria
Trials had to be properly randomised and include patients with locally advanced cervical cancer who had received neoadjuvant chemotherapy either before radiotherapy or surgery (or both).
Data collection and analysis
We collected, validated and re‐analysed updated trial data on all randomised patients from all relevant trials. The person responsible for the trial resolved queries and verified the final data. Treatment comparisons 1 and 2 were analysed separately. For all outcomes, we obtained overall hazard ratios using the fixed‐effect model. We pre‐specified analyses that grouped trials by important aspects of their design and patients by their or their tumour characteristics, to assess whether they might influence the effect of neoadjuvant chemotherapy.
Main results
We obtained data from 18 trials and 2074 patients for the first comparison. Considering these trials together there was a high level of statistical heterogeneity, a substantial amount of which was explained by analyses of trial groups. Trials using chemotherapy cycle lengths shorter than 14 days (HR = 0.83, 95% CI = 0.69 to 1.00, p = 0.046) or cisplatin dose intensities greater than 25 mg/m2 per week (HR = 0.91, 95% CI = 0.78 to 1.05, p = 0.20) tended to show an advantage of neoadjuvant chemotherapy on survival. In contrast, trials using cycle lengths longer than 14 days (HR = 1.25, 95% CI = 1.07 to 1.46, p = 0.005) or cisplatin dose intensities lower than 25 mg/m2 per week (HR = 1.35, 95% CI = 1.11 to 1.14, p = 0.002) showed a detrimental effect of neoadjuvant chemotherapy on survival. In the second comparison, data from 5 trials and 872 patients were obtained. The combined results (HR = 0.65, 95% CI = 0.53 to 0.80, p = 0.0004) indicated a highly significant reduction in the risk of death with neoadjuvant chemotherapy, but with heterogeneity in both the design and results.
Authors' conclusions
The timing and dose intensity of cisplatin‐based neoadjuvant chemotherapy appears to have an important impact on whether or not it benefits women with locally advanced cervical cancer and warrants further exploration. Obtaining additional IPD may improve the strength of these conclusions.
Keywords: Female; Humans; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Chemotherapy, Adjuvant; Cisplatin; Cisplatin/therapeutic use; Neoadjuvant Therapy; Randomized Controlled Trials as Topic; Survival Analysis; Uterine Cervical Neoplasms; Uterine Cervical Neoplasms/drug therapy; Uterine Cervical Neoplasms/pathology
Plain language summary
Chemotherapy before surgery or radiotherapy or both for women with cervical cancer that has spread beyond the cervix to the tissues close by
Surgery, radiotherapy or sometimes both were the best treatments for locally advanced cervical cancer. This is cancer that has spread beyond the cervix (neck of the womb) into the surrounding tissues, such as the vagina, sides of the pelvis or nearby lymph nodes. The exact choice of treatment would have depended on the size and location of the tumour and the preferences of the woman and her doctor.
Nowadays, women with this type of cancer may be given combined chemotherapy (drug treatment) and radiotherapy (x‐rays that kill cancer cells) at the same time. Or, they may have surgery (to remove the womb) as well as this combined chemotherapy and radiotherapy. However, giving chemotherapy before these treatments (neoadjuvant chemotherapy) might have a similar benefit, but have less side effects than giving the treatments at the same time.
This review aimed to assess the benefits and risks of giving chemotherapy before surgery, before radiotherapy or before both treatments.
Our first comparison was based on 18 trials and 2074 women. The women who were given chemotherapy either more than a fortnight apart or with a less intense dose of cisplatin before their radiotherapy, did not live as long as those who were only given radiotherapy. However, women given chemotherapy either less than a fortnight apart or with a more intense dose of cisplatin before their radiotherapy, seemed to live longer than those who were only given radiotherapy. These second results are based on less data and are not so convincing. There were very few serious side effects that continued long after treatment and they seemed to be similar whether chemotherapy was given or not.
Our second comparison was based on 5 trials and 872 women. The women who were given chemotherapy before surgery seemed to live longer than those who were only given radiotherapy. However, there was a small amount of data, there were differences between results of trials and other treatments were used. Therefore, it is not clear if the benefit might be for reasons other than the chemotherapy.
Further assessment of neoadjuvant chemotherapy in randomised trials is required. It may be valuable to compare it to a combined chemotherapy and radiotherapy approach or even to use neoadjuvant chemotherapy together with combined chemotherapy and radiotherapy.
Background
The incidence of cervical cancer has declined in both North America and Europe, but on a global scale it is the second most common cancer in women, and is the most prevalent female malignancy in many developing countries (Parkin 1993). Most patients (in the developed world) present with early disease either confined to the cervix or with limited extension beyond it (international Federation of Gynecology and Obstetrics (FIGO) stage IB1‐IIA). In the past, standard treatment was usually radical radiotherapy or radical hysterectomy, with node dissection, each giving 5‐year survival rates of around 80 to 90% (Benedet 1998). Radical radiotherapy, comprising external beam and intracavitary treatment, tended to be the treatment of choice for locally advanced disease (IFIGO)stage IIB, III and IVA) and offered an alternative to radical surgery for patients with tumours larger than 4cms confined to the cervix ( stage IB bulky) (Eifel 2001). Pelvic radiotherapy offered a good chance of cure, but the maximum radiation dose that was given to patients was limited by normal tissue tolerance, particularly of the small bowel, rectum and bladder, and 5‐year‐survival using radiotherapy ranged from around 60% for patients with stage IIB disease to approximately 20% for patients with stage IV disease (Benedet 1998). In 1999, a National Cancer Institute Alert, based on the results of 5 randomised trials recommended that concomitant chemoradiation should be considered instead of radiotherapy alone in women with cervical cancer. A subsequent systematic review and meta‐analysis of data presented in publications suggested a large benefit of concomitant chemoradiotherapy on survival, progression‐free survival and local and distant control rates (Green 2001). Thus, for many, concomitant chemoradiotherapy has become the new standard of care for locally advanced disease.
There are, however, still potential therapeutic advantages to giving chemotherapy alongside local treatments that were standard for locally advanced disease, prior to the widespread use of concomitant chemoradiotherapy. Neoadjuvant chemotherapy given before radiotherapy may reduce tumour size and control micrometastatic disease. In addition, by giving chemotherapy prior to radiotherapy, rather than concomitantly, increased radiotherapy toxicity may be less likely. Chemotherapy given prior to surgery may render inoperable tumours (FIGO stage IIB‐IIIB) operable and treat metastases. A number of randomised trials have explored the use of neoadjuvant chemotherapy as an adjunct to either radiotherapy or surgery in comparison with radiotherapy alone and cisplatin‐based regimens have been favoured, because of impressive response rates (Eifel 2001). Most of these randomised trials have been relatively small and their results range from a significant increase in survival (Sardi 1997) to a significant reduction in survival with neoadjuvant chemotherapy (CCSG AOCOA 1995). Most of the trials however, have shown inconclusive results. Nevertheless, the combination of the results of all relevant trials in a meta‐analysis (within each treatment setting) might give sufficient statistical power to determine whether neoadjuvant chemotherapy is useful. A prior systematic review and meta‐analysis based on published summary data extracted from trial reports was of limited value, because only a subset of the trials were published at the time of the analysis and, of those, some did not report sufficient survival data to allow an appropriate analysis to be performed (Tierney 1999). As noted in the publication, the only analysis that could be carried out might be unrepresentative and potentially biased and no firm conclusions could be drawn.
We therefore initiated a systematic review and individual patient data (IPD) meta‐analysis to collect, validate and re‐analyse trial data on all randomised patients from all relevant trials. The advantages of collecting IPD over published summary data for a meta‐analysis are many (Stewart 1995). In particular, it allows us to summarise the effect of neoadjuvant chemotherapy from all, not just the published trials; it permits more sensitive time‐to‐event analysis and enables us to examine whether any effect of treatment differs between subgroups of patients. Furthermore, by seeking updated follow‐up, it provides a unique opportunity to look at long‐term survival and adverse effects in a relatively young group of women. This IPD meta‐analysis was initiated and coordinated by the Medical Research Council (UK) Clinical Trials Unit and carried out by the Neoadjuvant Chemotherapy for Cervical Cancer Meta‐analysis Collaboration.
Objectives
This meta‐analysis aimed to provide the most comprehensive and reliable summary of the effect of neoadjuvant chemotherapy and to act as a stimulus for international collaboration, debate and consensus.
Methods
Criteria for considering studies for this review
Types of studies
To be included, trials had to be properly randomised and the treatment assignment done in such a way that the allocation could not be known beforehand. Trials had to include patients with locally advanced cervical cancer who had received neoadjuvant cytotoxic chemotherapy before radiotherapy or surgery or both treatments. Concurrent chemoradiotherapy trials were not included. The comparisons had to be unconfounded by use of additional agents or interventions. The protocol specified that patient enrolment should have started after 1 January 1975 and be completed by 30 June 1998. This would have meant excluding a lot of the randomised evidence and so the protocol was subsequently amended to include all closed trials at the time of the Collaborators' Conference (September 2000). We have now extended the eligibility to include all trials closed by February 2006. Trials should have made one of the treatment comparisons described above. In comparison 2, trials that also used adjuvant radiotherapy in the neoadjuvant chemotherapy arm were included.
Types of participants
Patients with locally advanced cancer of the uterine cervix (FIGO stage 1B‐IVA)
Types of interventions
Two related, but separate treatment comparisons were considered in the systematic review:
(1) Neoadjuvant chemotherapy followed by local treatment versus the same local treatment. (2) Neoadjuvant chemotherapy followed by surgery (with or without radiotherapy) versus radiotherapy alone.
Comparison 1 investigates the effect of adding neoadjuvant chemotherapy to local treatment compared to the same local treatment. Comparison 2 investigates the effect of a combined neoadjuvant chemotherapy and surgery approach compared to what had been the more standard radiotherapy approach.
Types of outcome measures
Overall survival, the primary endpoint, was defined as the time from randomisation until death (from any cause). Living patients were censored on the date of last follow‐up. Loco‐regional disease‐free survival was defined as the time from randomisation until loco‐regional progression or recurrence, or death (by any cause), whichever happened first. Those alive without loco‐regional disease were censored on the date of last follow‐up. Metastases‐free survival was defined as the time from randomisation until metastases or death (by any cause), whichever happened first. Patients alive without metastases were censored on the date of last follow‐up. However, if only the first sign of recurrent disease was recorded, patients having a local progression or recurrence were censored in the analysis of metastases‐free survival and patients having metastases were censored in the analysis of loco‐regional disease‐free survival. Similarly, overall disease‐free survival was taken as the time from randomisation until any local progression or recurrence, metastases or death (by any cause), whichever happened first. Patients alive without loco‐regional disease or metastases were censored on the date of last follow‐up.
Search methods for identification of studies
To avoid publication bias, both published and unpublished trials were included in the meta‐analysis. Trials were identified by searching the bibliographic databases Medline and Cancerlit, using a version of the optimal search strategy developed by the Cochrane Collaboration (Dickersin 1995). These were supplemented by hand searching reference lists of identified trial reports and of relevant books and review articles. The National Cancer Institute PDQ (Physicians Data Query) Clinical Protocols, United Kingdom Coordinating Committee for Cancer Research and Cochrane Controlled Trial registers were also searched to identify both completed and ongoing trials. All trial investigators who took part in the meta‐analysis were asked to help add to this list of trials. Initial searches were completed for the period up to and including January 1998 and were updated regularly until December 2002, the time of the main analyses. Searches have since been updated to February 2006, to identify any additional new material that has appeared and are supplemented by searches of the Gynaecological Cancer CRG specialist register. All abstracts were downloaded and full papers obtained for all abstracts judged potentially eligible for inclusion in the meta‐analysis. Where there was uncertainty about the eligibility of a trial, this was discussed and resolved by consensus by the project secretariat and international advisory group.
See Appendix 1 for search strategy for MEDLINE and CancerLit
Data collection and analysis
Up‐to‐date information on date of randomisation, survival status, loco‐regional recurrence status (pelvic), distant recurrence status (outside pelvis) and date of last follow up was sought, as were details of treatment allocated, age, histological cell type, stage (FIGO); grade; performance status; lymph node involvement; site of lymph node involvement; treatment on recurrence; cause of death (including treatment related toxicity) and whether cervical cancer was present at death. Late chronic effects of radiotherapy on the rectum, urinary tract and vagina, whilst uncommon, can be devastating for the women who are otherwise 'cured' of their cancer. Therefore, we also aimed to collect data on late bladder, gastrointestinal and vaginal toxicity. To avoid potential bias, information was requested for all women randomised including those who had been excluded from the investigators' original analyses.
All analyses, unless otherwise stated, were pre‐specified in the protocol. Two separate sets of analyses were carried out according to the treatment comparisons (1 and 2) already described. All analyses were carried out on an intention‐to‐treat basis. Analyses of all endpoints were stratified by trial, and the log rank expected number of deaths and variance used to calculate individual trial hazard ratios (HR) and overall pooled hazard ratios using the fixed effect model (Yusuf 1985). Thus, the times to event (progression, recurrence or death) for individual patients were used within trials to calculate the HR, representing the overall risk of an event for those patients allocated to neoadjuvant chemotherapy compared to those allocated to no chemotherapy.
To explore the potential impact of trial design, we planned analyses that grouped trials by important aspects of their design that might influence the treatment effect. Therefore, these analyses focussed mainly on variations in the neoadjuvant chemotherapy used, rather than differences in the non‐randomised treatments. As specified in the protocol, trials were grouped according to frequency of chemotherapy cycles (more than 14‐day cycles, less than or equal to 14‐day cycles), cisplatin dose intensity (less than 25 mg/m2, more than or equal to 25 mg/m2), total dose of cisplatin (less than or equal to 150 mg/m2, more than 150 mg/m2) local treatment used and whether adjuvant chemotherapy was also given. For each of these analyses, a hazard ratio was calculated for each group of trials and for all trials together. A test for quantitative interaction was used to investigate whether any substantial differences in the effect of neoadjuvant treatment existed between these trial groups. Because of the unanticipated level of heterogeneity across these trials, F‐tests were also used to examine whether it was greater between groups compared to within groups. These analyses were focused on the primary endpoint of survival, but were carried out on other endpoints to help support or refute any patterns found. Owing to an insufficient number of trials and further differences between the treatment approaches in comparison 2, these analyses were necessarily confined to comparison 1. To investigate the effects of neoadjuvant chemotherapy within subgroups of patients stratified logrank analyses were done on the primary endpoint of survival. These analyses examined whether the effect of neoadjuvant chemotherapy (and not prognosis) differs by patient subgroup. They were performed for each pre‐specified subgroup, for example, separately comparing treatment versus control for patients with good performance status and treatment versus control for poor performance status within each trial. These results were then combined to give a pooled HR for good and for poor performance status patients across trials.
Results are also presented as absolute differences at 5 years, calculated using the overall HRs and the overall event rate in the control group (Parmar 1995), with confidence intervals (CI) calculated from the baseline event rate and the 95% CI around the HR. Chi‐square heterogeneity tests (ABCOC 1995) were used to test for gross statistical heterogeneity across trials, and chi‐square tests of interaction or trend were used to test for differences in outcome between subsets of trials or between subgroups of patients. Survival curves are presented as simple (non‐stratified) Kaplan‐Meier curves (Kaplan 1958). With the exception of the F‐test, all p‐values quoted are 2‐sided.
Results
Description of studies
Treatment comparison 1
Searches to December 2002 identified 24 potentially eligible trials. Three were subsequently found to be ineligible, two because chemotherapy was given concurrently with radiotherapy (Lira Puerto 1990; Lorvidhaya 1995) and one because it was ongoing at time of the original analysis (GOG 141). Twenty‐one trials were therefore eligible for inclusion. Two unpublished trials could not be included, one because the patient data could not be located (44 patients, Protocol C1, Western General Hospital, Edinburgh) and one because the data were incomplete and in a poor state, with few completed forms (less than 30 patients, Federation Nationale des Centres de Lutte Contre le Cancer Protocol COII, France). In addition, data were unavailable for one (72 patients) of two trials published (Kumar 1998b) together because the investigator was unable to comply with the data collection deadline. Only the relevant treatment arms (comparing neoadjuvant chemotherapy plus radical radiotherapy to the same radiotherapy alone) were included for the two trials with multiple treatment arms (Sardi 1996; Sardi 1998). We collected data on 83 of 92 patients who had been excluded from the investigators' analyses and they were reinstated in this meta‐analysis. We were unable to obtain the excluded patients for two trials ( Chauvergne 1993; Souhami 1991) or enough information to include another 19 patients from three trials (Chauvergne 1993; Kumar 1998a; Souhami 1991) in the analyses. As the missing patients were few and distributed evenly across treatment arms, these trials were included. Therefore, the main results for comparison 1 are based on 2074 patients from 18 trials, representing 92% of patients from eligible randomised trials. Patient accrual varied from 27 to 260 in the available trials. Cisplatin was the main drug in all the chemotherapy regimens, with a planned total dose of between 100 and 320 mg/m2 in 10 to 28 day cycles. Both the external beam radiotherapy dose and intracavitary radiotherapy dose varied (40 to 60.8 and 18 to 80 Gy respectively), with a total dose in the range 55 to 80 Gy.
The patient characteristics, which reflect the eligibility criteria of individual trials, are given in Table 1. Investigators were sometimes unable to provide all of the data we requested (Table 1), but based on the available data, the women included in comparison 1 had a median age of 48 years and good performance status. Most had moderately or poorly differentiated, stage II to III tumours of squamous histology; the largest proportion had stage III (44%) tumours.
1. Characteristics of patients included in comparison 1.
| Neo CT n (%) | No neo CT n (%) | Total (N=2074) | |
| Age (years) | |||
| <35 | 134 (13) | 139 (13) | 273 |
| 35‐50 | 491 (48) | 454 (43) | 945 |
| 51‐65 | 329 (32) | 372 (35) | 701 |
| >65 | 72 (7) | 82 (8) | 154 |
| Unknown | 0 (0) | 1 (<1) | 1 |
| Stage* | |||
| IB | 126 (12) | 130 (12) | 256 |
| II | 291 (28) | 320 (31) | 611 |
| III | 418 (41) | 418 (40) | 836 |
| IV | 27 (3) | 28 (3) | 55 |
| Unknown | 164 (16) | 152 (15) | 316 |
| Histology | |||
| Squamous | 958 (93) | 976 (93) | 1934 |
| Adenocarcinoma | 6 (1) | 9 (1) | 15 |
| Other | 54 (5) | 58 (6) | 112 |
| Unknown | 8 (1) | 5 (<1) | 13 |
| Grade† | |||
| Well differentiated | 105 (10) | 97 (9) | 202 |
| Moderately differentiated | 299 (29) | 334 (32) | 633 |
| Poorly differentiated | 302 (29) | 285 (27) | 587 |
| Unknown | 320 (31) | 332 (32) | 652 |
| Performance status‡ | |||
| 0 | 671 (65) | 656 (63) | 1327 |
| 1 | 230 (22) | 240 (23) | 470 |
| 2 | 55 (5) | 57 (5) | 112 |
| 3 | 14 (1) | 11 (1) | 25 |
| Unknown | 56 (5) | 84 (8) | 140 |
| Lymph node status§ | |||
| Uninvolved | 288 (28) | 280 (27) | 568 |
| Involved | 121 (12) | 149 (14) | 270 |
| Unknown | 617 (60) | 619 (59) | 1236 |
| N.B. Percentages may not add up to 100% because of rounding | |||
| * Data missing completely for 1 trial †Data missing completely for 3 trials and for a large proportion of patients in 1 trial ‡Data missing completely for 1 trial and for a large proportion of patients in 2 trials §Data missing completely for 8 trials and for most patients in 2 trials |
Updated searches have identified two additional trials relevant to this comparison (Napolitano 2003; Tabata 2003). These and the two other trials already identified (Kumar 1998b; GOG 141) all used cisplatin‐based chemotherapy. IPD will be sought for these trials and included in a future update of this review.
Treatment comparison 2
Searches to December 2002 identified seven trials that compared neoadjuvant chemotherapy plus surgery (with or without radiotherapy) versus radiotherapy alone, but one was subsequently found to be ineligible because neoadjuvant chemotherapy was given to all patients (Mickiewicz 1996). Therefore, six trials were eligible for inclusion. Data were not available for one trial, because we lost contact with the investigators (Park 1996). Data from all randomised patients from the remaining 5 trials were made available and so the main results for this comparison are based on 872 patients, which represent 97% of patients from known randomised trials.
Patient accrual varied from 50 to 441. Cisplatin was the main drug in all the chemotherapy regimens, with a planned total of dose between 100 and 300 mg/m2 in 10 to 21 day cycles. One trial gave chemotherapy intra‐arterially (Kigawa 1996). External beam radiotherapy and intracavitary radiotherapy doses in the control arm were very similar across trials (45 to 60 and 25 to 40 Gy respectively). More than 90% of patients in two trials (Sardi 1996; Sardi 1998) received adjuvant radiotherapy as well as surgery in the neoadjuvant treatment arm and in two other trials (Chang 2000; Kigawa 1996) a smaller proportion of patients received additional radiotherapy (28% and 32% respectively). Thus, in some cases a triple modality is being compared with radiotherapy.
We obtained most of the baseline data requested. Information on age, histology, stage, grade, performance status and lymph node status was provided for all trials, although, particularly for lymph node status, data are missing for some patients (Table 2). Overall, the patient characteristics are broadly similar to those in comparison 1 (Table 2), with women of median age of 49 years, with good performance status and moderately or poorly differentiated, stage II to III tumours of squamous histology. However, there was a greater proportion of stage IB to II patients (74%) than in comparison 1.
2. Characteristics of patients included in comparison 2.
| Neo CT n (%) | No Neo CT n (%) | Total (N=872) | |
| Age (years) | |||
| <35 | 37 (8) | 44 (11) | 81 |
| 35‐50 | 234 (52) | 176 (42) | 410 |
| 51‐65 | 145 (32) | 158 (38) | 303 |
| >65 | 36 (8) | 37 (9) | 73 |
| Unknown | 1 (<1) | 4 (1) | 5 |
| Stage | |||
| IB/IIA | 164 (36) | 145 (35) | 309 |
| IIB | 169 (37) | 165 (39) | 334 |
| III | 120 (26) | 109 (26) | 229 |
| Unknown | 0 (0) | 0 (0) | 0 |
| Histology | |||
| Squamous | 427 (94) | 396 (95) | 823 |
| Adenocarcinoma | 9 (2) | 8 (2) | 17 |
| Other | 17 (4) | 15 (4) | 32 |
| Unknown | 0 (0) | 0 (0) | 0 |
| Grade | |||
| Well differentiated | 35 (8) | 44 (11) | 79 |
| Moderately differentiated | 222 (49) | 183 (44) | 405 |
| Poorly differentiated | 186 (41) | 164 (39) | 350 |
| Unknown | 10 (2) | 28 (7) | 38 |
| Performance status* | |||
| 0 | 324 (72) | 300 (72) | 624 |
| 1 | 57 (13) | 64 (15) | 121 |
| 2 | 4 (1) | 3 (1) | 7 |
| Unknown | 68 (15) | 52 (12) | 120 |
| Lymph node status† | |||
| Uninvolved | 301 (66) | 172 (41) | 473 |
| Involved | 114 (25) | 50 (12) | 164 |
| Unknown | 38 (8) | 197 (47) | 235 |
| N.B. Percentages may not add up to 100% because of rounding | |||
| * Data missing completely for 1 trial †Data missing completely on the radiotherapy arm for 3 trials and for a large proportion of patients on the radiotherapy arm in 1 trial |
Updated searches failed to identify any new trials relevant to this comparison.
Risk of bias in included studies
All data were thoroughly checked for validity, consistency, plausibility, and integrity of randomisation and follow up (Stewart 1995). Any queries were resolved and the final database entries verified by the responsible trial investigator, data manager or statistician.
Effects of interventions
Treatment comparison 1
For survival, data were available for 18 trials, comprising 2074 patients and 1084 deaths. The median follow‐up across all trials is 5.7 years for surviving patients, ranging from 1.5 to 9.0 in individual trials. The low median follow‐up results from an older trial where the follow‐up could not be updated (CCSG AOCOA 1995). Causes of death were provided for 17 trials. The results for the other endpoints are based on 16 trials and 1724 patients, because recurrence data was not supplied for two trials (Herod 2000; Kumar 1998a). In the analysis of overall disease‐free survival, there were 938 events and 780 (83%) of these first events were progressions or recurrences. For loco‐regional disease‐free survival, 911 events were recorded and of these 573 (63%) were local progressions or recurrences, and so this endpoint more evenly consists of progressions and recurrences or deaths. For metastases‐free survival, there were 899 patients who had an event, 306 of whom (34%) developed metastases as their first event. Therefore, many patients died without metastases and these deaths contribute most to this endpoint.
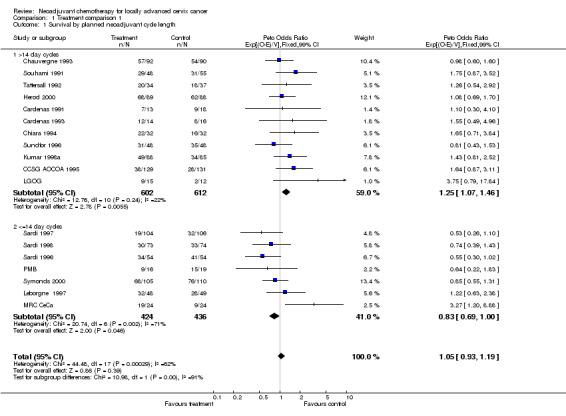
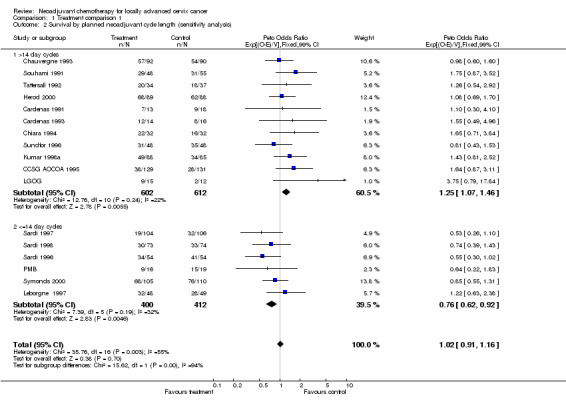
For each of the outcomes measured, when all trials were combined, a highly significant level of statistical heterogeneity was evident, such that it is inappropriate to combine the trials in this way, and in fact suggests that these trials may not be addressing exactly the same question (Table 3). For survival, heterogeneity is best accounted for by the pre‐specified grouping of trials by chemotherapy cycle length or planned cisplatin dose intensity, as shown by the F‐test (Table 4). Grouping trials by cycle length resulted in a significant variability in the direction of effect (p = 0.0009). Trials giving chemotherapy in cycles lasting longer than 14 days had a pooled HR of 1.25, representing a significant (p = 0.005) 25% increase in the risk of death with neoadjuvant chemotherapy (Table 4). With shorter chemotherapy cycle lengths, the HR of 0.83 (p = 0.046) suggests a significant 17% decrease in the risk of death (Table 4). These HRs translate into an absolute reduction in 5‐year survival of 8% (from 45% to 37%) with long cycles lengths, and a 7% absolute improvement in 5‐year survival (from 45% to 52%) with shorter cycles (Additional Fig. 1). However, heterogeneity was still evident in this latter group (p = 0.002). A sensitivity analysis excluding one very small trial (48 patients) with an extreme HR of 3.37 (MRC CeCa) reduces the heterogeneity substantially (p = 0.193) and with the weight of evidence (HR = 0.76, 95% CI = 0.62 to 0.92, p = 0.005, Table 4) still favouring the short cycle intensive administration of neoadjuvant chemotherapy.
3. All endpoints in treatment comparison 1.
| Endpoint | No. events/patients | HR (95% CI), p | Heterogeneity p |
| Survival | 1084/2074 | 1.05 (0.94‐1.19), 0.393 | 0.0003 |
| Disease‐free survival | 938/1724 | 1.00 (0.88‐1.14), 1.000 | 0.001 |
| Loco‐regional disease‐free survival | 911/1724 | 1.03 (0.90‐1.17), 0.654 | 0.0002 |
| Metastases‐free survival | 899/1724 | 1.00 (0.88‐1.14), 1.000 | 0.002 |
4. Overall survival by group of trials in comparison 1.
| Trial grouping | No. trials | No. events/patients | HR (95% CI) , p | Heterogeneity p | F‐test p | Interaction p |
| Chemotherapy cycle frequency | ||||||
| > 14 day cycles | 11 | 639/1214 | 1.25 (1.07‐1.46), 0.005 | 0.238 | ||
| <= 14 day cycles | 7 | 445/860 | 0.83 (0.69‐1.00), 0.046 | 0.002 | 0.036 | 0.0009 |
| <= 14 days cycles (‐MRC CeCa) | 6 | 417/812 | 0.76 (0.62‐0.92), 0.005 | 0.193 | 0.00045 | 0.00008 |
| Neoadjuvant cisplatin dose intensity | ||||||
| <25mg/m2 | 7 | 413/845 | 1.35 (1.11‐1.64), 0.002 | 0.746 | ||
| >= 25mg/m2 | 11 | 671/1229 | 0.91 (0.78‐1.05), 0.200 | 0.001 | 0.046 | 0.002 |
| Neoadjuvant cisplatin total dose | ||||||
| <= 150mg/m2 | 11 | 770/1413 | 1.03 (0.90‐1.19), 0.646 | 0.0002 | ||
| > 150mg/m2 | 7 | 314/661 | 1.10 (0.88‐1.38), 0.386 | 0.090 | 0.775 | 0.629 |
| Local treatment | ||||||
| Radiotherapy | 17 | 1033/1864 | 1.09 (0.96‐1.23), 0.169 | 0.001 | ||
| ± Surgery + radiotherapy | 1 | 51/210 | 0.53 (0.31‐0.93), 0.025 | NA | 0.128 | 0.01 |
| Chemotherapy scheduling | ||||||
| Neoadjuvant chemotherapy | 17 | 1046/2010 | 1.04 (0.92‐1.17), 0.563 | 0.0003 | ||
| Neoadjuvant + adjuvant chemotherapy | 1 | 38/64 | 1.65 (0.87‐3.14), 0.125 | NA | 0.404 | 0.162 |
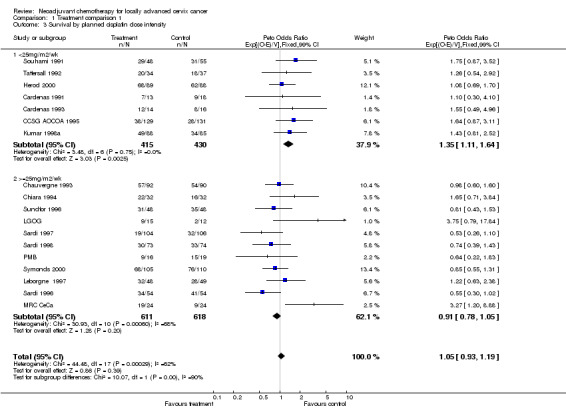
Grouping trials by planned cisplatin dose intensity shows a similar difference in the direction of effect for survival (p = 0.002). The HR of 1.35 (p = 0.002), for those trials using less than 25 mg/m2 per week, indicates a significant 35% increase in the risk of death with neoadjuvant chemotherapy. In contrast, the HR for trials using a planned dose intensity of greater than or equal to 25 mg/m2 per week is 0.91 (p = 0.200), which suggests a potential 9% decrease in the risk of death with neoadjuvant chemotherapy. These HRs translate into an 11% absolute reduction in 5‐year survival of 11% (from 45% to 34%) with lower dose intensities and a 3% absolute improvement in 5‐year survival (from 45% to 48%) with higher dose intensities. Considerable heterogeneity remains in the higher dose intensity group (p = 0.001). Unlike above, exclusion of any one trial is insufficient to explain this residual heterogeneity. For the other endpoints, grouping the trials by cycle length and cisplatin dose intensity produced patterns of results that are consistent with those for survival.
Grouping trials according to total cisplatin dose, whether surgery was used as part of the local treatment or whether additional adjuvant chemotherapy was given did not explain the heterogeneity in the survival results (F‐test, Table 4). Furthermore, the groups did not appear to differ significantly in terms of the size and direction of effect (Table 4) and this was also the case for the other endpoints.
As there were already substantial differences in the direction of effect by trial group, it did not seem appropriate to carry out analyses to assess any differential effect of neoadjuvant chemotherapy by patient subgroup across all the trials. Instead we carried out subgroup analyses within trial groups defined by the chemotherapy cycle length, where heterogeneity was less. Together with missing baseline data, this limits the power of these analyses. Nevertheless, there was no evidence to suggest that chemotherapy was differentially effective in groups of patients defined by age (trend, p = 0.384, p = 0.762), stage (trend p = 0.365, p = 0.533), histology (interaction p = 0.332, p = 0.611), grade (trend, p = 0.757, p = 0.779) or performance status (interaction p = 0.647, p = 0.660) for trials with chemotherapy cycles lasting more than 14 days or 14 days or less, respectively. Data on nodal involvement were too sparse to warrant subgroup analyses.
Although late toxicity data were provided for more than half the trials, only a relatively small number of late effects on the bladder (91), intestine (117) and vagina (79) were recorded. Of these, there were 14 serious late events associated with the bladder, 21 associated with the gastrointestinal tract and 3 associated with the vagina. Although these data were not sufficient to warrant formal analysis, there is little to suggest that serious late toxicity is a greater problem in the neoadjuvant chemotherapy arm compared with the control arm (5 versus 9, 10 versus 11 and 2 versus 1, respectively).
One trial previously identified (Kumar 1998b) and two trials from updated searches (Napolitano 2003; Tabata 2003) are eligible for inclusion in this comparison. All gave cisplatin‐based chemotherapy in cycles of less than 14 days and at a dose intensity of less than 25mg/m2 of cisplatin per week. None reported survival results that were significantly in favour of neoadjuvant chemotherapy or control. GOG 141, which was identified by the original searches, used a higher dose intensity of cisplatin, in cycles longer than 14 days. The results of this trial have still not been published. Therefore, without seeking IPD for these trials, it is difficult to assess their impact on the results of the meta‐analysis.
Treatment comparison 2
Information on all endpoints and cause of death was provided for the 5 trials and 872 patients. The median follow‐up across all trials is 5 years for surviving patients, ranging from 3.9 to 9.0 in individual trials. For the primary endpoint of survival, there were 368 deaths. For overall disease‐free survival, there were 415 events and 368 (89%) of these first events were progressions or recurrences. There were 403 events in the analysis of loco‐regional disease‐free survival and, of these, 290 patients (72%) experienced a local progression or recurrence as the first event. Thus, both of these endpoints are dominated by progressions and recurrences. For metastases‐free survival there were 381 events, but metastases only accounted for 120 (31%) of them and so this endpoint mainly comprises deaths.
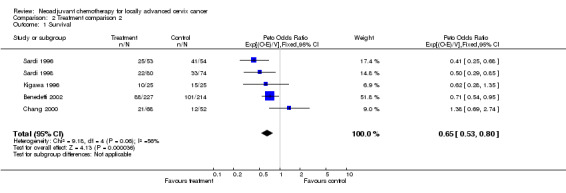
For survival, 3 trials had results that were significantly in favour of neoadjuvant chemotherapy (Benedetti 2002; Sardi 1996; Sardi 1998), but the CIs for all individual trials are wide. Combining the results of all trials gives an overall HR of 0.65 and a narrower overall CI, indicating a highly significant (p = 0.0004) 35% reduction in the risk of death with neoadjuvant chemotherapy (Table 5). The HR translates into a 14% absolute improvement in survival at 5 years, increasing it from 50 to 64% (Additional Fig. 2). There is some indication of heterogeneity in the individual trial results (p = 0.06), which seems to be due to one trial that has a result quite different from the others (Chang 2000). The results for overall disease‐free survival and loco‐regional disease‐free survival are very similar to those for survival, although there is a greater degree of heterogeneity (Table 5). The HR for both is 0.68 (p = 0.001), a statistically significant 32% reduction in the risk of progression, recurrence or death with neoadjuvant chemotherapy. This is equivalent to a 13% absolute improvement in both endpoints from 45 to 58%. For metastases‐free survival, the HR is 0.63 (p = 0.0001), equivalent to a significant 37% reduction in the risk of metastases or death (Table 5). In this case there was no obvious statistical heterogeneity across trials (p = 0.217) and the HR is equivalent to an absolute benefit of 15% at 5 years, increasing metastases‐free survival from 45 to 60%.
5. All endpoints in comparison 2.
| Endpoint | No. events/patients | HR (95% CI), p | Heterogeneity p |
| Survival | 368/872 | 0.65 (0.53‐0.80), 0.00004 | 0.06 |
| Disease‐free survival | 414/872 | 0.68 (0.56‐0.82), 0.0001 | 0.02 |
| Loco‐regional disease‐free survival | 402/872 | 0.68 (0.56‐0.82), 0.0001 | 0.005 |
| Metastases‐free survival | 381/872 | 0.63 (0.52‐0.78), 0.00001 | 0.217 |
There were fewer deaths (368) in this comparison and in some cases missing baseline data. Therefore, the power to determine whether there was evidence of a differential effect of neoadjuvant chemotherapy by pre‐defined patient subgroups was very limited. Nevertheless, based on the available data, there was no evidence to suggest that the effect of neoadjuvant chemotherapy on survival varied across groups of patients defined by age (trend p = 0.363), stage (trend p = 0.258), histology (interaction p = 0.082), grade (trend, p = 0.781) or performance status (interaction p = 0.713). Determination of nodal status was largely confined to the neoadjuvant chemotherapy plus surgery arms of these trials (Table 2) and so any subgroup analysis by nodal status would lack power and be difficult to interpret.
Discussion
At the outset of this project, despite the enrolment of more than 3000 women in randomised trials, it was not clear whether neoadjuvant chemotherapy was effective in the treatment of locally advanced cervical cancer. In the interim, concomitant chemotherapy and radiotherapy has probably become a "standard of care" for women with locally advanced disease. This was in response to a National Cancer Institute Alert based on the results of five randomised trials stating "strong consideration should be given to the incorporation of chemotherapy with radiotherapy in women who require radiotherapy for the treatment of cervical cancer". A subsequent systematic review and meta‐analysis of available published and unpublished summary data from 19 randomised trials of concomitant chemoradiotherapy versus radiotherapy, showed a 29% reduction in the risk of death which translated into an overall survival benefit of 12% at 5 years for this treatment (Green 2001) and it has become a standard against which new treatments are being tested. However, meta‐analyses of published or other summary data may over‐estimate the treatment effect (Stewart 1993). Also, there are still a number of issues regarding the use of chemoradiotherapy, including which chemotherapy regimens and doses are optimal, precisely which patients benefit and whether post‐radiation adjuvant chemotherapy provides additional therapeutic gain (Rose 2002). Therefore, a similar systematic review and meta‐analysis of individual patient data from the concomitant chemoradiotherapy trials is planned. As neoadjuvant chemotherapy may offer benefits similar to chemoradiotherapy when compared to local treatment, in a similar group of women, this current systematic review and meta‐analysis aimed to provide a comprehensive, reliable and up‐to‐date estimate of the average effect of this treatment.
Treatment comparison 1
At the time of the main analysis, ninety‐three percent of the known randomised evidence comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy for locally advanced cervical cancer (comparison 1) was collected and re‐analysed. Considering all trials together, there was no evidence of an effect of neoadjuvant chemotherapy on survival, or any of the other endpoints. However, there was a very high level of statistical heterogeneity, suggesting strongly that the results should not be combined in this way. A substantial proportion of heterogeneity is explained by pre‐specified analyses grouping trials according to the way they delivered the chemotherapy. Such analyses yielded the intriguing observation that trials which gave more intensive chemotherapy in terms of a shorter cycle length and/or a higher dose intensity tended to show an advantage for neoadjuvant chemotherapy, whereas those that delivered chemotherapy in a less intensive and more prolonged manner, with a longer cycle length or a lower dose intensity tended to show a detrimental effect of chemotherapy.
Among the planned analyses, grouping trials by the length of the chemotherapy cycle is the best explanation for the heterogeneity seen between trials. For trials giving cycles greater than 14 days, the pooled HR was 1.25, equivalent to an absolute detriment of 8% in 5‐year survival. A detriment was also observed in overall and loco‐regional disease‐free survival and metastases‐free survival. In contrast, trials using shorter cycle lengths gave a pooled HR of 0.83, equivalent to a 7% absolute improvement in 5‐year survival. Results for overall and loco‐regional disease‐free survival and metastases‐free survival similarly suggested a benefit of short cycle chemotherapy. A comparable, but somewhat less persuasive pattern was observed when trials were split according to planned cisplatin dose intensity. The pooled HR of 1.35 from trials using less than 25 mg/m2 per week suggests a significant 11% reduction in 5‐year survival, with comparable reductions in overall and loco‐regional disease‐free survival and metastases‐free survival. These results were homogeneous for each endpoint and significant for survival and loco‐regional disease‐free survival. On the other hand, the HR of 0.91 for trials using dose intensities of cisplatin greater than 25 mg/m2 per week suggests a potential 3% absolute improvement in 5‐year survival, but this is not conventionally statistically significant. Similar absolute improvements in overall, loco‐regional disease‐free and metastases‐free survival were demonstrated, but again these results were not conventionally significant.
If, as the results of this comparison suggest, short cycle, dose intensive chemotherapy is beneficial and longer less intensive schedules are detrimental, then they may be informative about the biology of cervical cancer and the effects of drug treatment. Cervical tumours are rapidly proliferating with a median potential doubling time (Tpot) of only 4 to 4.5 days (Rew 2000) and a relatively high growth fraction (Bolger 1996). Following effective chemotherapy, tumours shrink, but tumour re‐growth may be accelerated, particularly if the drug and dose schedule is not optimal. After a few cell divisions the tumour volume may be restored, but the tumour cells may be less sensitive to chemotherapy and potentially less sensitive to conventionally fractionated radiotherapy, due to the changed growth kinetics. In this scenario, the scheduling of chemotherapy may be important, with short cycle, high dose‐intensity chemotherapy being optimal to minimise the repopulation growth perturbations, which undermine radiation cytotoxicity. Conversely, long cycle and low dose‐intensity chemotherapy would have a lesser effect in reducing clonogenic cells, consequently enhancing their re‐growth perturbations.
Whilst all the analyses by groups of trials were planned prospectively, chemotherapy cycle length and cisplatin dose intensity may not provide the correct or most important explanation for the differences in the effect of neoadjuvant chemotherapy across trials. Furthermore, these analyses are correlated, such that the some combination of cycle length and dose intensity may better discriminate between trials. Other factors relating to scheduling of the treatment may have a role. By similar mechanisms to those described above, the hypothesis that prolongation of the duration of radiotherapy reduces local control by allowing extra time for repopulation with resistant cells, has been supported by a number of retrospective studies (Perez 1995; Petereit 1995), but potential clinical and methodological biases have been highlighted in these analyses (Eifel 1995). Nevertheless, variation in the duration of radiotherapy amongst the trials in this meta‐analysis could contribute to the differences seen in local control and survival. Furthermore, if there is combined chemotherapy and radiotherapy cross‐resistance, the duration of chemotherapy, the delay to radiotherapy and the duration of radiotherapy making up the overall treatment time, could each have an impact on prognosis. Interestingly, those trials giving more prolonged chemotherapy tended to be those with longer delays to radiotherapy and longer durations of radiotherapy and vice versa.
As they were based only on the planned dose intensity of cisplatin, our analyses ignore the potential impact of other drugs in the regimens. Whilst an analysis by composite dose intensity may offer an alternative way of grouping these trials, many untestable assumptions would have to be made. In addition, the chemotherapy regimens appear more homogeneous within the subset of trials using higher dose intensities (Table of included studies). Alternatively, differences in the patient characteristics within trial groupings could lead to some differences in the effect of neoadjuvant chemotherapy. The eligibility criteria of the individual trials do indeed lead to some differences in the types of patients included in the trials using short chemotherapy cycle lengths, compared to those using longer cycle lengths. However, the control group survival of these two groups of patients is very similar (Additional Fig. 1) and within these groups, there is no evidence that particular types of patients benefit more or less from chemotherapy.
Although, it is difficult to conduct comprehensive literature searches across cancer sites, we have found no other reports of qualitative differences in effect resulting from modest variations in the delivery of neoadjuvant chemotherapy. Of course this may mean that: the potential impact of chemotherapy dose and scheduling on the effects of neoadjuvant chemotherapy have not been formally explored; or it has been explored, but no such association has been noted or that no real variation in delivery exists between trials. For example, in head and neck cancer, another rapidly‐proliferating, radio‐responsive tumour, most randomised trials of neoadjuvant chemotherapy used similar 3‐weekly cycles and a meta‐analysis found a trend towards a survival benefit with no clear heterogeneity (Pignon 2000). Alternatively, the heterogeneity in results we have identified may reflect known or unknown treatment‐related, design, patient or indeed other factors that are peculiar to particular trials and that we could not take account of in the meta‐analyses. In addition, we cannot rule out the possibility that our results in cervix cancer are chance findings.
Treatment comparison 2
The meta‐analysis was successful in collecting and re‐analysing 97% of the known randomised evidence from trials comparing neoadjuvant chemotherapy plus surgery with radiotherapy for cervical cancer. However, the number of patients (872) and events (368) is not large and the results need to be interpreted with caution. Also, some of the patients included in these trials would be considered as having localised disease (FIGO stage IB‐IIA) and others would be considered locally advanced (FIGO stage IB bulky, IIB‐IIIB) and as such, clinically they would not often be considered together. This comparison is further complicated by the fact that intra‐arterial chemotherapy was used in one trial [24] and by the use of pelvic radiotherapy in the neoadjuvant chemotherapy plus surgery arm. In two trials (Sardi 1996; Sardi 1998), almost all patients received pelvic radiotherapy and in another two trials (Chang 2000; Kigawa 1996), it was given to around of 30% of patients. Therefore, there a number of possible confounding factors that we have been unable to tease out in the analysis. Nevertheless, the results are fairly consistent from trial to trial and suggest a highly significant effect of neoadjuvant chemotherapy. Although the HR of 0.65 indicates a 14% absolute overall improvement in 5‐year survival, because baseline survival differs considerably by stage, this relative benefit translates into absolute improvements ranging from 8 to 14% at 2 years and 12 to 16% at 5 years. It is noteworthy that generally chemotherapy was intense and of short duration. Of course in this comparison, with surgery as the definitive local treatment, any potential radio‐resistance with more prolonged or less dose intensive regimens, is probably inconsequential.
Authors' conclusions
Implications for practice.
Although, the overall results do not support the use of cisplatin‐based neoadjuvant chemotherapy prior to radiotherapy for women with locally advanced cervical cancer, the more detailed pre‐planned analyses are emphasised.These imply that the timing and dose intensity of neoadjuvant chemotherapy has an important impact on whether it is beneficial. Prolonged or less dose‐intensive chemotherapy seems to have a detrimental effect on women. Despite some remaining unexplained heterogeneity, they may also indicate that there are two alternative neoadjuvant strategies to improve long‐term outcomes. One is a short cycle, dose‐intensive course of cisplatin‐based chemotherapy prior to radiotherapy. The second is similar chemotherapy given prior to surgery (with or without radiotherapy), which could provide a reasonable alternative to radical radiotherapy for earlier stage tumours and perhaps, more contentiously, for more advanced stages. However, given the problem of heterogeneity in comparison 1 and potential confounding factors and the small quantity of data available for comparison 2, further assessment of these approaches in randomised trials is probably required. Updating the follow‐up of included trials and obtaining IPD from additional trials may improve the strengh of these conclusions.
Implications for research.
For those planning to assess further the value of neoadjuvant chemotherapy, it may be valuable to compare the policies of neoadjuvant chemotherapy and concomitant chemoradiotherapy in terms of efficacy and toxicity or even to combine them in a single treatment approach. Our results suggest that in any such comparison, particularly where radiotherapy is the definitive treatment, it would be prudent to avoid potentially detrimental long cycles or low doses of chemotherapy. Rather, short cycle, dose intensive neoadjuvant chemotherapy, perhaps similar to that commonly given concurrently with radiation, would be preferable. Such a trial could be of considerable value, because there may be reasons why one or other approach would be preferable to individual women, clinicians, or health care systems. For example, it might enable clinicians to take the pragmatic decision to use short cycle, dose‐intensive neoadjuvant chemotherapy when there is an unavoidable delay in being able to give radical radiotherapy or perhaps offer greater choice to women who have concerns about receiving chemotherapy and radiotherapy at the same time.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
| 22 April 2013 | Review declared as stable | IPD data review |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
| 21 July 2009 | Amended | New search date added |
| 20 October 2008 | Amended | Converted to new review format. |
| 15 June 2006 | Amended | Minor update: 15/05/06 New studies found but not yet included or excluded: 28/02/06 Conclusions changed: 28/02/06 Searches have been updated and new trials identified, but they will only be included in the analysis when individual patient data has been obtained. |
| 25 November 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
*Neoadjuvant Chemotherapy for Cervical Cancer Meta‐analysis Collaboration (NACCCMA Collaboration) All aspects of this systematic review and meta‐analysis were carried out under the auspices of the NACCCMA Collaboration. Pierluigi Benedetti‐Panici (Libera Università "Campus Bio‐Medico" di Roma, Rome, Italy), Adriana Bermudez (Buenos Aires University, Buenos Aires, Argentina), Peter Blake (Royal Marsden Hospital, London, England), Jesus Cárdenas (Centro Estatal de Cancerologia, Colima, Mexico), Ting‐Chang Chang (Chang Gung Memorial Hospital, Linkou, Taiwan), Silvana Chiara (Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy), G Di Paola (Buenos Aires University, Buenos Aires, Argentina), Anne Floquet (Institut Bergonié, Bordeaux, France), David Guthrie (Derbyshire Royal Infirmary, Derby, England), Junzo Kigawa (Tottori University School of Medicine, Yonago, Japan), Lalit Kumar (All India Institute of Medical Sciences, New Delhi, India), Felix Leborgne (Hospital Pereira Rossell, Montevideo, Uruguay), Nick Lodge (Royal Marsden Hospital, London, England), Chris Poole (City Hospital Birmingham, Birmingham, England), Juan Sardi (Buenos Aires University, Buenos Aires, Argentina), Luis Souhami (Hôpital Général de Montréal, Montréal, Canada) , Kolbein Sundfør (The Norwegian Radium Hospital, Oslo, Norway), Paul Symonds (Leicester Royal Infirmary, Leicester, England) and Martin Tattersall (The University of Sydney, Sydney, Australia) collated and supplied the individual patient data from their trials, contributed to the discussion and interpretation of the results and commented on drafts of the report. The project was organised by the Advisory Group, Stefano Greggi, (Istituto Nazionale Tumori "Fondazione G. Pascale", Naples, Italy) David Guthrie, Vicky Parker (COU‐RAGE UK, Manchester, England), Mahesh K B Parmar (MRC Clinical Trials Unit, London, England), Juan Sardi, Lesley A Stewart (MRC Clinical Trials Unit, London, England), Jayne F Tierney (MRC Clinical Trials Unit, London, England), who were responsible for formulating the questions, developing the protocol and discussing the preliminary results. The secretariat, Mahesh K B Parmar, Lesley A Stewart and Jayne F Tierney, were responsible for receiving, checking and analysing data. Jayne F Tierney managed the project and Jayne F Tierney drafted the report, with detailed input from Lesley A Stewart and Mahesh K B Parmar.
The British Medical Research Council funded the coordination of the meta‐analysis and the collaborators' meeting. We would like to thank all those women who took part in the trials and contributed to this research. The meta‐analysis would not have been possible without them or without the help of collaborating institutions that kindly collated and supplied their trial data. We are particularly grateful to Paul Symonds for his very helpful advice and input into early stages of the manuscript.
Appendices
Appendix 1. Search Strategy for MEDLINE and CancerLit
1 randomized controlled trial.pt. 2 Randomized Controlled Trials/ 3 Random Allocation/ 4 Double‐Blind Method/ 5 Single‐Blind Method/ 6 1 or 2 or 3 or 4 or 5 7 clinical trial.pt. 8 exp Clinical Trials/ 9 (clin$ adj5 trial$).ab,ti. 10 ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).ab,ti. 11 Placebos/ 12 placebo$.ab,ti. 13 random$.ab,ti. 14 Research Design/ 15 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16 6 or 15 17 cervical carcinoma$.ab,ti. 18 cervical cancer$.ab,ti. 19 cervix carcinoma$.ab,ti. 20 cervix cancer$.ab,ti. 21 (carcinoma adj3 cervix).ab,ti. 22 (cancer adj3 cervix).ab,ti. 23 exp Uterine Cervical Neoplasms/ 24 17 or 18 or 19 or 20 or 21 or 22 or 23 25 chemotherapy.ab,ti. 26 dt.fs. 27 radiotherapy.ab,ti. 28 rt.fs. 29 25 or 26 or 27 or 28 30 16 and 24 and 29 (856) Please note: Format shown is for Ovid $ is a truncation symbol
Data and analyses
Comparison 1. Treatment comparison 1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival by planned neoadjuvant cycle length | 18 | 2074 | Peto Odds Ratio (99% CI) | 1.05 [0.93, 1.19] |
| 1.1 >14 day cycles | 11 | 1214 | Peto Odds Ratio (99% CI) | 1.25 [1.07, 1.46] |
| 1.2 <=14 day cycles | 7 | 860 | Peto Odds Ratio (99% CI) | 0.83 [0.69, 1.00] |
| 2 Survival by planned neoadjuvant cycle length (sensitivity analysis) | 17 | 2026 | Peto Odds Ratio (99% CI) | 1.02 [0.91, 1.16] |
| 2.1 >14 day cycles | 11 | 1214 | Peto Odds Ratio (99% CI) | 1.25 [1.07, 1.46] |
| 2.2 <=14 day cycles | 6 | 812 | Peto Odds Ratio (99% CI) | 0.76 [0.62, 0.92] |
| 3 Survival by planned cisplatin dose intensity | 18 | 2074 | Peto Odds Ratio (99% CI) | 1.05 [0.93, 1.19] |
| 3.1 <25mg/m2/wk | 7 | 845 | Peto Odds Ratio (99% CI) | 1.35 [1.11, 1.64] |
| 3.2 >=25mg/m2/wk | 11 | 1229 | Peto Odds Ratio (99% CI) | 0.91 [0.78, 1.05] |
1.1. Analysis.
Comparison 1 Treatment comparison 1, Outcome 1 Survival by planned neoadjuvant cycle length.
1.2. Analysis.
Comparison 1 Treatment comparison 1, Outcome 2 Survival by planned neoadjuvant cycle length (sensitivity analysis).
1.3. Analysis.
Comparison 1 Treatment comparison 1, Outcome 3 Survival by planned cisplatin dose intensity.
Comparison 2. Treatment comparison 2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 5 | 872 | Peto Odds Ratio (95% CI) | 0.65 [0.53, 0.80] |
2.1. Analysis.
Comparison 2 Treatment comparison 2, Outcome 1 Survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benedetti 2002.
| Methods | RCT 1990‐1996 | |
| Participants | 441 (0*) FIGO stage IB2‐IIA>+4cm, IIB, III | |
| Interventions | CT + S or RT vs S CT: CDDP 80mg/m2 BLM 15mg/m2. Every 21 days for 2 cycles Or CDDP 50mg/m2 VCR 1mg/m2 BLM 30mg/m2. Every 7 days for 6 cycles Or CDDP 43mg/m2 IFOS 3.5mg/m2. Every 7 days for 7 cycles (IFOS cycles 1, 4, 7 only) Or CDDP 40mg/m2. Every 7 days for 6 cycles. RT: External beam 45‐50 Gy over 35‐42 days. Intracavitary 20‐30Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Cardenas 1991.
| Methods | RCT 1988‐1992 | |
| Participants | 31 (0*) FIGO stage IIB | |
| Interventions | CT + RT vs RT CT: CDDP 50 mg/m2 EPI 75 mg/m2 CTX 500mg/m2. Every 21 days for 4 cycles. RT: External beam 50Gy over 42‐45 days. Intracavitary 33.2Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival Late toxicity | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cardenas 1993.
| Methods | RCT 1987‐1992 | |
| Participants | 30 (0*) FIGO stage IIIB | |
| Interventions | CT + RT vs RT CT: CDDP 50 mg/m2 EPI 75 mg/m2 CTX 500mg/m2. Every 21 days for 4 cycles. RT: External beam 50Gy over 42‐45 days. Intracavitary 33Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival Late toxicity | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
CCSG AOCOA 1995.
| Methods | RCT 1989‐1993 | |
| Participants | 260 (0*) FIGO stage IIB‐IVA | |
| Interventions | CT + RT vs RT CT: CDDP 60 mg/m2 EPI 110 mg/m2. Every 21 days for 2‐3 cycles. RT: External beam 40‐50Gy over 28‐35 days. Intracavitary 30‐35Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Chang 2000.
| Methods | RCT 1992‐1999 | |
| Participants | 124 (0*) FIGO stage Bulky IB, IIA | |
| Interventions | CT + S vs RT CT: CDDP 50mg/m2 VCR 1mg/m2 BLM 25mg/m2. Every 10 days for 3 cycles. RT: External beam 50Gy in 25Fover 35 days. Intracavitary 25.8Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Chauvergne 1993.
| Methods | RCT 1982‐1987 | |
| Participants | 182 (13*) IIB‐N1, III, M0 | |
| Interventions | CT + RT vs RT CT: CDDP 80 mg/m2 MTX 30 mg/m2 CLB 20 mg/m2 VCR 0.7 mg/m2. Every 21 days for 2 cycles and further 2 to responders. RT: External beam 50Gy. Intracavitary 18 y |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Chiara 1994.
| Methods | RCT 1988‐1992 | |
| Participants | 64 (0*) FIGO stage IIB‐III | |
| Interventions | CT + RT + CT vs RT CT: CDDP 60 mg/m2. Every 15 days for 2 cycles before RT (and for 4 cycles after RT). RT: External beam 60Gy in 30F. Intracavitary 40Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Herod 2000.
| Methods | RCT 1986‐1995 | |
| Participants | 177 (0*) FIGO stage IIB‐IVA, IIA inoperable | |
| Interventions | CT + RT vs RT CT: CDDP 50 mg/m2 BLM 30 mg/m2 IFOS 5g/m2. Every 28 days for 2‐3 cycles. RT: External beam according to institutional policy. Intracavitary according to institutional policy |
|
| Outcomes | Survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kigawa 1996.
| Methods | RCT 1989‐1991 | |
| Participants | 50 (0*) FIGO stage IIB‐IIIB | |
| Interventions | Intra‐arterial CT +/‐ S or +/‐ RT vs RT CT: CDDP 50mg/m2 BLM 30mg/m2. Every 21 days 2‐3 cycles. RT: External beam 50Gy in 25F over 35 days. Intracavitary 24‐38Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kumar 1998a.
| Methods | RCT 1990‐1993 | |
| Participants | 173 (11*) FIGO stage IIB‐IVA | |
| Interventions | CT + RT vs RT CT: CDDP 50mg/m2 BLM 15 mg/m2 IFOS 5g/m2 MESNA 3g/m2. Every 21 days for 2 cycles. RT: External beam 40Gy in 22F + 10Gy in 5Fover 35 days. Intracavitary 30Gy |
|
| Outcomes | Survival Late toxicity | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Leborgne 1997.
| Methods | RCT 1990‐1993 | |
| Participants | 97 (0*) FIGO stage IB‐IVA (IB >4cm) | |
| Interventions | CT + RT vs RT CT: CDDP 50mg/m2 VCR 3mg/m2 BLM 75mg/m2. Every 10 days for 3 cycles. RT: External beam 20‐60Gy in 10‐30F. Intracavitary 30Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival Late toxicity | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
LGOG.
| Methods | RCT 1991‐1995 | |
| Participants | 27 FIGO stage IIB‐IVA, bulky IB, IIA | |
| Interventions | CT + RT vs RT CT: CDDP 60mg/m2 MTX 300 mg/m2 BLM 30 mg. Every 14 days for 3 cycles. RT: External beam 50Gy over 39 days. Intracavitary 28Gy |
|
| Outcomes | Survival Disease‐free survival Locor‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
MRC CeCa.
| Methods | RCT 1991‐1995 | |
| Participants | 48 (0*) FIGO stage IB‐IVA | |
| Interventions | CT RT or S vs RT or S CT: CDDP 50mg/m2 MTX 100 mg/m2 Folinic acid 15mg po/6h 30 after start of CDDP and MTX. Every 14 days for 3 cycles. RT: External beam 46Gy in 23F over 32 days. Intracavitary 20Gy |
|
| Outcomes | Survival Disease‐free survival Locor‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
PMB.
| Methods | RCT 1989‐1991 | |
| Participants | 35 (0*) FIGO stage IIB‐IVA, bulky IB, IIA | |
| Interventions | CT + RT vs RT CT: CDDP 60mg/m2 MTX 300 mg/m2 BLM 30 mg. Every 14 days for 3 cycles. RT: External beam 42.5 Gy in 20F over 28 days. Intracavitary 76‐80Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sardi 1996.
| Methods | RCT (3 arms) 1988‐1993 | |
| Participants | 108 (0*)** FIGO stage IIIB 108 (0*)** FIGO stage IIIB |
|
| Interventions | CT + RT vs RT arms CT: CDDP 50mg/m2 VCR 1mg/m2 BLM 25mg/m2. Every 10 days for 3 cycles. RT: External beam 50‐60Gy over 45‐50 days. Intracavitary 25‐35Gy CT: As above. RT: As above. |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival Late toxicity | |
| Notes | Treatment comparison 1 Treatment comparison 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sardi 1997.
| Methods | RCT 1987‐1993 | |
| Participants | 145 (0*)** FIGO stage IIB patients | |
| Interventions | CT + RT (± S) vs RT (± S) CT: CDDP 50mg/m2 VCR 1mg/m2 BLM 25mg/m2. Every 10 days for 3 cycles. RT: External beam 50‐60Gy. Intracavitary 25‐35Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival Late toxicity | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sardi 1998.
| Methods | RCT (4 arms) 1988‐1993 | |
| Participants | 147 (0*)** FIGO stage IIB patients 154 (0*)** FIGO stage IIB patients |
|
| Interventions | CT + RT vs RT arms CT: CDDP 50mg/m2 VCR 1mg/m2BLM 25mg/m2. Every 10 days for 3 cycles. RT: External beam 50Gy 25‐28F. Intracavitary 35‐40Gy CT + S vs RT arms CT: As above RT: As above |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival Late toxicity | |
| Notes | Treatment comparison 1 Treatment comparison 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Souhami 1991.
| Methods | RCT 1984‐1986 | |
| Participants | 103 (4*) FIGO stage IIIB patients | |
| Interventions | CT + RT vs RT CT: CDDP 50mg/m2 BLM 120U VCR 1mg/m2 MMC 10mg/m2. Every 21 days for 3 cycles. RT: 50Gy in 25F over 35 days. Intracavitary 40Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional diease‐free survival Metastases‐free survival Late toxicity | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sundfor 1996.
| Methods | RCT 1989‐1992 | |
| Participants | 96 (0*) FIGO stage IIIB‐IVA | |
| Interventions | CT + RT vs RT CT: CDDP 100mg/m2 5‐FU 5000mg/m2 . Every 21 days for 3 cycles. RT: External beam 64.8Gy in 36F over 50 days |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Symonds 2000.
| Methods | RCT 1990‐1995 | |
| Participants | 215 (0*) FIGO stage II, III, IVA | |
| Interventions | CT + RT vs RT CT: CDDP 50 mg/m2 MTX 100 mg/m2. Every 14 days for 3 cycles. RT: External beam. 40‐45Gy in 20F over 28 days. Intracavitary 24‐33.75Gy |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Tattersall 1992.
| Methods | RCT 1985‐1990 | |
| Participants | 71 (0*) FIGO stage IIB‐IVA patients | |
| Interventions | CT + RT vs RT CT: CDDP 50 mg/m2 VBL 4 mg/m2 BLM 45mg. Every 21 days for 3 cycles. RT: External beam 40‐55Gy in 20‐25F over 28‐35 days |
|
| Outcomes | Survival Disease‐free survival Loco‐regional disease‐free survival Metastases‐free survival | |
| Notes | Treatment comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
*Missing data **Only relevant treatment arms included Abbreviations used: PMB ‐ after 'PMB' regimen; CCSG AOCOA ‐ Cervical Cancer Study Group of the Asian Oceanian Clinical Oncology Association; LGOG ‐ London Gynaecological Oncology Group; CT ‐ chemotherapy; RT‐ radiotherapy; S ‐ surgery; CDDP ‐ cisplatin; MTX ‐ methotrexate; CLB ‐ chlorambucil; VCR ‐ vincristine; BLM ‐ bleomycin; MMC ‐ mitomycin; VBL ‐vinblastine; IFOS ‐ ifosfamide; EPI ‐ epirubicin; CTX ‐ cyclophosphamide; 5‐FU ‐ 5‐fluorouracil; F ‐ fractions
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| FNCLCC COII | Data incomplete and in a poor state. Less than 30 patients. |
| GOG 141 | Ongoing at the time of analysis. |
| Kumar 1998b | Investigator unable to make repeated data collection deadlines. |
| Lira Puerto 1990 | Ineligible. Neoadjuvant CT given during RT. |
| Lorvidhaya 1995 | Ineligible. Neoadjuvant CT given during RT. |
| Mickiewicz 1996 | All patients received neoadjuvant chemotherapy. |
| Napolitano 2003 | |
| Park 1996 | Lost contact with investigators. |
| Tabata 2003 | |
| WGH | Data could not be located. 44 patients. |
Contributions of authors
All aspects of this systematic review and meta‐analysis were carried out under the auspices of the NACCCMA Collaboration. Pierluigi Benedetti‐Panici (Libera Università "Campus Bio‐Medico" di Roma, Rome, Italy), Adriana Bermudez (Buenos Aires University, Buenos Aires, Argentina), Peter Blake (Royal Marsden Hospital, London, England), Jesus Cárdenas (Centro Estatal de Cancerologia, Colima, Mexico), Ting‐Chang Chang (Chang Gung Memorial Hospital, Linkou, Taiwan), Silvana Chiara (Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy), G Di Paola (Buenos Aires University, Buenos Aires, Argentina), Anne Floquet (Institut Bergonié, Bordeaux, France), David Guthrie (Derbyshire Royal Infirmary, Derby, England), Junzo Kigawa (Tottori University School of Medicine, Yonago, Japan), Lalit Kumar (All India Institute of Medical Sciences, New Delhi, India), Felix Leborgne (Hospital Pereira Rossell, Montevideo, Uruguay), Nick Lodge (Royal Marsden Hospital, London, England), Chris Poole (City Hospital Birmingham, Birmingham, England), Juan Sardi (Buenos Aires University, Buenos Aires, Argentina), Luis Souhami (Hôpital Général de Montréal, Montréal, Canada) , Kolbein Sundfør (The Norwegian Radium Hospital, Oslo, Norway), Paul Symonds (Leicester Royal Infirmary, Leicester, England) and Martin Tattersall (The University of Sydney, Sydney, Australia) collated and supplied the individual patient data from their trials, contributed to the discussion and interpretation of the results and commented on drafts of the report. The project was organised by the Advisory Group, Stefano Greggi, (Istituto Nazionale Tumori "Fondazione G. Pascale", Naples, Italy) David Guthrie, Vicky Parker (COU‐RAGE UK, Manchester, England), Mahesh K B Parmar (MRC Clinical Trials Unit, London, England), Juan Sardi, Lesley A Stewart (MRC Clinical Trials Unit, London, England), Jayne F Tierney (MRC Clinical Trials Unit, London, England), who were responsible for formulating the questions, developing the protocol and discussing the preliminary results. The secretariat, Mahesh K B Parmar, Lesley A Stewart and Jayne F Tierney, were responsible for receiving, checking and analysing data. Jayne F Tierney managed the project and Jayne F Tierney drafted the report, with detailed input from Lesley A Stewart and Mahesh K B Parmar.
Sources of support
Internal sources
Medical Research Council, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Benedetti 2002 {published and unpublished data}
- Benedetti‐Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Gianarelli D, et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced cervical cancer: Results from the Italian multicenter randomised study. Journal of Clinical Oncology 2002;20(1):179‐88. [DOI] [PubMed] [Google Scholar]
Cardenas 1991 {published and unpublished data}
- Cárdenas J, Olguín A, Figueroa F, Beccerra F, Huizar R. Randomized neoadjuvant chemotherapy in cervical carcinoma stage IIb. PEC+ RT vs RT. Proceedings of the American Society of Clinical Oncology 1991;10:189, A620. [Google Scholar]
Cardenas 1993 {published and unpublished data}
- Cárdenas J, Olguín A, Figueroa F, Peña J, Beccerra F, Huizar R. A randomized trial of chemotherapy (CT) followed by radiotherapy (RT) vs radiotherapy alone in Stage IIIb cervical carcinoma: preliminary results. Fourth International Congress on Anti‐Cancer Chemotherapy; 1993. 1993:87.
CCSG AOCOA 1995 {published and unpublished data}
- Tattersall MHN, Lorvidhaya V, Vootiprux V, Cheirsilpa A, Wong F, Azhar T, et al. for the Cervical Cancer Study Group of the Asian Oceanian Clinical Oncology Association. Randomized trial of epirubicin and cisplatin chemotherapy followed by pelvic radiation in locally advanced cervical cancer. Journal of Clinical Oncology 1995;13(2):444‐51. [DOI] [PubMed] [Google Scholar]
Chang 2000 {published and unpublished data}
- Chang T‐C, Lai C‐H, Hong J‐H, Hsueh S, Huang K‐G, Chou H‐H, et al. Randomized trial of neaodjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage Ib and IIA cervical cancer. Journal of Clinical Oncology 2000;18(8):1740‐47. [DOI] [PubMed] [Google Scholar]
Chauvergne 1993 {published and unpublished data}
- Chauvergne J, Lhomme C, Rohart J, Heron JF, Ayme Y, Goupil A, et al. Neoadjuvant chemotherapy of stage IIb or III cancers of the uterine cervix. Long‐term results of a multicenter randomized trial of 151 patients. [Chimiothérapie néoadjuvante des cancer du col utérin aux stades IIb e III. Résultats éloignés d'un essai randomisé pluricentrique portant sur 151 patients]. Bulletin du Cancer 1993;80:1069‐79. [PubMed] [Google Scholar]
Chiara 1994 {published and unpublished data}
- Chiara S, Bruzzone M, Merlini L, Bruzzi P, Rosso R, Franzone P, et al. Randomized study comparing chemotherapy plus radiotherapy versus radiotherapy alone in FIGO stage IIB‐III cervical carcinoma. American Journal of Clinical Oncology 1994;17(4):294‐7. [DOI] [PubMed] [Google Scholar]
Herod 2000 {published and unpublished data}
- Herod J, Burton A, Bixton J, Tobias J, Luesley D, Jordan S, et al. A randomised prospective phase III clinical trial of primary bleomycin, ifosfamide and cisplatin (BIP) chemotherapy followed by radiotherapy versus radiotherapy alone in operable cancer of the cervix. Annals of Oncology 2000;11:1175‐81. [DOI] [PubMed] [Google Scholar]
Kigawa 1996 {published and unpublished data}
- Kigawa J, Minagawa Y, Ishihara H, Itamochi H, Kanamori Y, Terawaka N. The role of neoadjuvant intra‐arterial infusion chemotherapy with cisplatin and bleomycin for locally advanced cervical cancer. American Journal of Clinical Oncology 1996;19(3):255‐9. [DOI] [PubMed] [Google Scholar]
Kumar 1998a {published and unpublished data}
- Kumar L, Grover R, Pokharel YH, Chander S, Kumar S, Singh R, et al. Neoadjuvant chemotherapy in locally advanced cervical cancer: two randomised studies. Australian and New Zealand Journal of Medicine 1998;28:387‐90. [DOI] [PubMed] [Google Scholar]
Leborgne 1997 {published and unpublished data}
- Leborgne F, Leborgne JH, Doldán R, Zubizarreta E, Ortega B, Maisonneuve J, et al. Induction chemotherapy and radiotherapy of advanced cancer of the cervix: a pilot study and phase III randomized trial. International Journal of Radiation Oncology, Biology, Physics 1997;37(2):343‐50. [DOI] [PubMed] [Google Scholar]
LGOG {unpublished data only}
- LGOG. Neo‐adjuvant chemotherapy in locally advanced carcinoma of the cervix. Unpublished.
MRC CeCa {unpublished data only}
- Medical Research Council. A study of potentially curative therapy alone or preceded by chemotherapy in the treatment of Stage Ib‐IVa cervical carcinoma. Unpublished.
PMB {unpublished data only}
- PMB Group. Neo‐adjuvant chemotherapy in locally advanced carcinoma of the cervix. Unpublished.
Sardi 1996 {published and unpublished data}
- Sardi J, Giaroli A, Sananes C, Rueda NG, Vighi S, Ferreira M, et al. Randomized trial with neoadjuvant chemotherapy in stage IIIB squamous carcinoma cervix uteri: an unexpected therapeutic management. International Journal of Gynecological Cancer 1996;6:85‐93. [Google Scholar]
Sardi 1997 {published and unpublished data}
- Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, et al. Long‐term follow‐up of the first randomized trial using neoadjuvant chemotherapy in Stage Ib squamous carcinoma of the cervix. The final results. 1997. Gynecologic Oncology 1997;67:61‐9. [DOI] [PubMed] [Google Scholar]
Sardi 1998 {published and unpublished data}
- Sardi JE, Sananes CE, Giaroli AA, Bermudez A, Ferreira M, Soderini AH, et al. Neoadjuvant chemotherapy in cervical carcinoma stage IIB: a randomized controlled trial. International Journal of Gynecological Cancer 1998;8:441‐50. [Google Scholar]
Souhami 1991 {published and unpublished data}
- Souhami L, Gil RA, Allan SE, Canary PC, Araujo CM, Pinto LH, et al. A randomized trial of chemotherapy followed by pelvic radiation therapy in Stage IIIB carcinoma of the cervix. Journal of Clinical Oncology 1991;9(6):970‐7. [DOI] [PubMed] [Google Scholar]
Sundfor 1996 {published and unpublished data}
- Sundfor K, Tropé CG, Högberg T, Onsrud M, Koern J, Simonsen E, et al. Radiotherapy and neoadjuvant chemotherapy for cervical carcinoma. A randomized multicenter study of sequential cisplatin and 5‐fluoracil and radiotherapy in advanced cervical carcinoma Stage 3B and 4A. Cancer 1996;77:2371‐8. [DOI] [PubMed] [Google Scholar]
Symonds 2000 {published and unpublished data}
- Symonds RP, Habeshaw T, Reed NS, Paul J, Pyper E, Yosef H, et al. The Scottish and Manchester randomised trial of neo‐adjuvant chemotherapy for advanced cervical cancer. European Journal of Cancer 2000;36:994‐1001. [DOI] [PubMed] [Google Scholar]
Tattersall 1992 {published and unpublished data}
- Tattersall MHN, Ramirez C, Coppleson M. A randomized trial comparing platinum‐based chemotherapy followed by radiotherapy alone in patients with locally advanced cervical cancer. International Journal of Gynecological Cancer 1992;2:244‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
FNCLCC COII {unpublished data only}
- Groupe cooeperateur de gynecologie de la FNCLCC. Phase III randomized study of radiotherapy with vs without BLEO/MITO/CDDP/VP‐16 for locally advanced squamous cell carcinoma of the cervix. Unpublished.
GOG 141 {unpublished data only}
- GOG 141. Phase III study in the treatment of bulky Stage IB carcinoma of the cervix: neoadjuvant CDDP/VCR vs no neoadjuvant therapy followed by radical hysterectomy and pelvic/para‐aortic node dissection. Unpublished neoadjuvant CDDP/VCR vs no neoadjuvant therapy followed by radical hysterectomy and pelvic/para‐aortic node dissection. Unpublished.
Kumar 1998b {published data only}
- Kumar L, Grover R, Pokharel YH, Chander S, Kumar S, Singh R, et al. Neoadjuvant chemotherapy in locally advanced cervical cancer: two randomised studies. Australian and New Zealand Journal of Medicine 1998;28:387‐90. [DOI] [PubMed] [Google Scholar]
Lira Puerto 1990 {published data only}
- Lira Puerto V, Huerta R, Cortes H, et al. Ciplatin (CDDP) plus radiotherapy (RT) vs radiotherapy alone in locally advanced cervical cancer. Proceedings of the Annual Meeting of the American Society of Clinical Oncology 1990;9:abstract 633. [Google Scholar]
Lorvidhaya 1995 {published data only}
- Lorvidhaya V, Tonusin A, Sukthomya W, Changwiwit W, Nimmolrat A. Induction chemotherapy and irradiation in advanced carcinoma of the cervix. Gan To Kagaku Ryoho 1995;22 Suppl 3:244‐51. [PubMed] [Google Scholar]
Mickiewicz 1996 {published data only}
- Mickiewicz E, Porcella H, Roth B, et al. Do patients with locally advanced cervical carcinoma responding to adjuvant chemotherapy benefit from a surgical approach? Preliminary report of a prospective randomised trial. Proceedings of the Annual Meeting of the American Society of Clinical Oncology 1996;15:abstract 823. [Google Scholar]
Napolitano 2003 {published data only}
- Napolitano U, Imperato F, Mossa B, Framarino ML, Marziani R, Marzetti L. The role of neoadjuvant chemotherapy for squamous cell cervical cancer (Ib‐IIIb): a long‐term randomized trial. European Journal of Gynaecological Oncology 2003;24(1):51‐9. [PubMed] [Google Scholar]
Park 1996 {published and unpublished data}
- Park SY, Kim JH, Kim BG, et al. Phase III randomized study of MVC intraarterial (arm 1), or systemic (arm 2) neoadjuvant chemotherapy versus radiation therapy only (arm3) for stage IIb bulky carcinoma of uterine cervix: preliminary report. Proceedings of the Annual Meeting of the American Society of Clinical Oncology 1996;15:302, abstract 840. [Google Scholar]
Tabata 2003 {published data only}
- Tabata T, Takeshima N, Nishida H, Hirai Y, Hasumi K. A randomized study of primary bleomycin, vincristine, mitomycin and cisplatin (BOMP) chemotherapy followed by radiotherapy versus radiotherapy alone in stage IIIB and IVA squamous cell carcinoma of the cervix. Anticancer Research 2003;23(3C):2885‐90. [PubMed] [Google Scholar]
WGH {unpublished data only}
- Western General Hospital Edinburgh. Treatment of advanced FIGO stage IIB or IIIB carcinoma of the cervix. Unpublished.
Additional references
ABCOC 1995
- Advanced Bladder Cancer Overview Collaboration. Does neo‐adjuvant cisplatin‐based chemotherapy improve the survival of patients with locally advanced bladder cancer: a meta‐analysis of individual patient data from randomised clinical trials. British Journal of Urology 1995;75:206‐13. [DOI] [PubMed] [Google Scholar]
Benedet 1998
- Benedet J, Odicino F, Maisonneuve P, et al. Carcinoma of the cervix uteri. Journal of Epidemiology and Biostatistics 1998;3:5‐34. [PubMed] [Google Scholar]
Bolger 1996
- Bolger BS, Symonds RP, Stanton PD, MacLean AB, Burnett R, Kelly P, et al. Prediction of radiotherapy response of cervical carcinoma through measurement of proliferation rate. British Journal of Cancer 1996;74:1223‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickersin 1995
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. In: Chalmers I, Altman DG editor(s). Systematic Reviews. London: BMJ Publishing Group, 1995:17‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eifel 1995
- Eifel PJ, Thames HD. Has the influence of treatment duration on local control of carcinoma of the cervix been defined?. International Journal of Radiation Oncology, Biology, Physics 1995;32(5):1527‐9. [DOI] [PubMed] [Google Scholar]
Eifel 2001
- Eifel PJ, Berek JS, Thigpen JT. Gynecologic Cancers. Section 2. Cancer of the cervix, vagina, vulva. In: DeVita VT, Jr, Hellman S, Rosenberg SA editor(s). Cancer: Principles & Practice of Oncology. 6th Edition. Vol. 2, Philadelphia: Lippincott‐Raven, 2001:1526‐56. [Google Scholar]
Green 2001
- Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant cehmotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta‐analysis. Lancet 2001;358:781‐6. [DOI] [PubMed] [Google Scholar]
Kaplan 1958
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statistical Association 1958;53:457‐81. [Google Scholar]
Parkin 1993
- Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. International Journal of Cancer 1993;54:594‐606. [DOI] [PubMed] [Google Scholar]
Parmar 1995
- Parmar MKB, Machin D. Survival analysis: a practical approach. John Wiley & Sons Ltd, 1995. [Google Scholar]
Perez 1995
- Perez CA, Grigsby PW, Castro‐Vita H, Lockett MA. Carcinoma of the cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on the outcome of radiation therapy. International Journal of Radiation Oncology Biology Physics 1995;32(5):1275‐88. [DOI] [PubMed] [Google Scholar]
Petereit 1995
- Petereit DG, Sarkaria JN, Chappell R, Fowler JF, Hartmann TJ, Kinsella, et al. The adverse effect of treatment prolongation in cervical carcinoma. International Journal of Radiation Oncology, Biology, Physics 1995;32(5):1301‐7. [DOI] [PubMed] [Google Scholar]
Pignon 2000
- Pignon JP, Bourhis J, Domenge C, Designé L, on behalf of the MACH‐NC Collaborative Group. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: three meta‐analyses of updated individual data. Lancet 2000;355:949‐55. [PubMed] [Google Scholar]
Rew 2000
- Rew DA, Wilson GD. Cell production rates in human tissues and tumours and their significance. Part II: clinical data. European Journal of Surgical Oncology 2000;26:405‐417. [DOI] [PubMed] [Google Scholar]
Rose 2002
- Rose PG. Chemoradiotherapy for cervical cancer. European Journal of Cancer 2002;38:270‐8. [DOI] [PubMed] [Google Scholar]
Stewart 1993
- Stewart LA, Parmar MKB. Meta‐analysis of the literature or of individual patient data: is there a difference?. Lancet 1993;341:418‐22. [DOI] [PubMed] [Google Scholar]
Stewart 1995
- Stewart LA, Clarke MJ, on behalf of the Cochrane Working Party Group on Meta‐analysis using Individual Patient Data. Practical methodology of meta‐analyses (overviews) using updated individual patient data. Statistics in Medicine 1995;14:2057‐79. [DOI] [PubMed] [Google Scholar]
Tierney 1999
- Tierney JF, Stewart LA, Parmar MKB. Can the published data tell us about the effectiveness of neoadjuvant chemotherapy for locally advanced cancer of the uterine cervix?. European Journal of Cancer 1999;35(3):406‐9. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta‐blockade during and after myocardial infarction: An overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
NACCCMA 2003
- Neoadjuvant Chemotherapy for Cervical Cancer Meta‐analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta‐analysis of individual patient data from 21 randomised trials. European Journal of Cancer 2003;39(17):2470‐86. [DOI] [PubMed] [Google Scholar]


