Abstract
Background
Alpha‐glucosidase inhibitors such as acarbose or miglitol, have the potential to improve glycemic control in type 2 diabetes mellitus. The true value of these agents, especially in relation to diabetes related mortality and morbidity, has never been investigated in a systematic literature review and meta‐analysis.
Objectives
To assess the effects of alpha‐glucosidase inhibitors in patients with type 2 diabetes mellitus.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, Current Contents, LILACS, databases of ongoing trials, reference lists of reviews on the topic of alpha‐glucosidase inhibitors and we contacted experts and manufacturers for additional trials.
Selection criteria
Randomised controlled trials of at least 12 weeks duration comparing alpha‐glucosidase inhibitor monotherapy in patients with type 2 diabetes with any other intervention and that included at least one of the following outcomes: mortality, morbidity, quality of life, glycemic control, lipids, insulin levels, body weight, adverse events.
Data collection and analysis
Two reviewers read all abstracts, assessed quality and extracted data independently. Discrepancies were resolved by consensus or by the judgement of a third reviewer. A statistician checked all extracted data entrance in the database. We attempted to contact all authors for data clarification.
Main results
We included 41 trials (8130 participants), 30 investigated acarbose, seven miglitol, one trial voglibose and three trials compared different alpha‐glucosidase inhibitors. Study duration was 24 weeks in most cases and only two studies lasted amply longer than one year. We found only few data on mortality, morbidity and quality of life. Acarbose had a clear effect on glycemic control compared to placebo: glycated haemoglobin ‐0.8% (95% confidence interval ‐0.9 to ‐0.7), fasting blood glucose ‐1.1 mmol/L (95% confidence interval ‐1.4 to ‐0.9), post‐load blood glucose ‐2.3 mmol/L (95% confidence interval ‐2.7 to ‐1.9). The effect on glycated haemoglobin by acarbose was not dose‐dependent. We found a decreasing effect on post‐load insulin and no clinically relevant effects on lipids or body weight. Adverse effects were mostly of gastro‐intestinal origin and dose dependent. Compared to sulphonylurea, acarbose decreased fasting and post‐load insulin levels by ‐24.8 pmol/L (95% confidence interval ‐43.3 to ‐6.3) and ‐133.2 pmol/L (95% confidence interval ‐184.5 to ‐81.8) respectively and acarbose caused more adverse effects.
Authors' conclusions
It remains unclear whether alpha‐glucosidase inhibitors influence mortality or morbidity in patients with type 2 diabetes. Conversely, they have a significant effect on glycemic control and insulin levels, but no statistically significant effect on lipids and body weight. These effects are less sure when alpha‐glucosidase inhibitors are used for a longer duration. Acarbose dosages higher than 50 mg TID offer no additional effect on glycated hemoglobin but more adverse effects instead. Compared to sulphonylurea, alpha‐glucosidase inhibitors lower fasting and post‐load insulin levels and have an inferior profile regarding glycemic control and adverse effects.
Keywords: Humans; Glycoside Hydrolase Inhibitors; 1‐Deoxynojirimycin; 1‐Deoxynojirimycin/analogs & derivatives; Acarbose; Acarbose/therapeutic use; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/drug therapy; Enzyme Inhibitors; Enzyme Inhibitors/therapeutic use; Glucosamine; Glucosamine/analogs & derivatives; Glucosamine/therapeutic use; Hypoglycemic Agents; Hypoglycemic Agents/therapeutic use; Imino Pyranoses; Inositol; Inositol/analogs & derivatives; Inositol/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Alpha‐glucosidase inhibitors for type 2 diabetes mellitus
Alpha‐glucosidase inhibitors may be used for patients with type 2 diabetes. They delay the absorbance of carbohydrates ('complex form of sugar') in the gut. In this review we present data from meta‐analyses that show (among other things) a decrease in glycated haemoglobin, fasting and post‐load blood glucose and post‐load insulin. But we found no evidence for an effect on mortality or morbidity. We found clues that with higher dosages the effect on glycated haemoglobin, in contrast to post‐load blood glucose, remains the same. This might be because a lower compliance due to increasing side‐effects.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. As a result there is a disturbance of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy, neuropathy and increased risk of cardiovascular disease. For a detailed overview of diabetes mellitus, please see under 'Additional information' of the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups', 'Cochrane Metabolic and Endocrine Disorders Group'). For an explanation of methodological terms, see the main Glossary in The Cochrane Library.
Description of the intervention
Currently, four alpha‐glucosidase inhibitors exist: acarbose, miglitol, voglibose and emiglitate. Of these, acarbose is by far the most prescribed drug. In most guidelines it is not a drug of first choice but used as an addition to other drugs for type 2 diabetes when treatment goals are not met, or in case of contra‐indications for other medications (EDPG 1999; Rutten 2000). The price of acarbose and miglitol is approximately $72 per month for 100 mg tablets, three times daily. Because of its lowering effect on the postprandial elevation of insulin levels, a beneficial effect on body weight is to be expected. Further, a positive effect on hypertriglyceridaemia has been reported (Reaven 1990).
Recently, alpha‐glucosidase inhibitors have been put in a new light as a result of a study on the efficacy of acarbose in patients with impaired glucose tolerance (IGT) (Chiasson 2002; Chiasson 2003). This study showed that acarbose could prevent or delay the development of IGT into type 2 diabetes. Moreover, it showed a reduced risk of cardiovascular disease and hypertension in the acarbose treated group, but the conclusions of this study are heavily debated (Kaiser 2004).
Adverse effects of the intervention
Abdominal discomfort like flatulence, diarrhoea and stomachache are the most frequently occurring adverse effects of alpha‐glucosidase inhibitors. Because of their specific working mechanism hypoglycaemic adverse events do not occur. They do not increase insulin output potentially leading to hypoglycaemia.
Existing evidence
Systematic reviews
Some reviews have been published recently on the topic of acarbose (Breuer 2003; Laube 2002) and miglitol (Campbell 2000; Scott 2000), these reviews were not performed systematically with respect to one or more of the following items: literature search, inclusion criteria of studies and quality assessment. In none of these reviews a meta‐analysis was performed. A recent meta‐analysis of seven trials with acarbose in the treatment of type 2 diabetes suggested a significant decrease in the occurrence of myocardial infarction (Hazard ratio 0.32, 95% CI 0.14 to 0.80) (Hanefeld 2004). However, we do not support the conclusions of this meta‐analysis because the study was subject to publication bias, heterogeneity, detection bias and confounding (Van de Laar 2004b).
RCTs
Several randomised clinical trials evaluating the efficacy of alpha‐glucosidase inhibitors as monotherapy or as a combination with other agents have been published. Most of these evaluated the efficacy of acarbose. One major trial reported a decrease in glycated haemoglobin of 0.6% when acarbose was given as sole therapy and compared to placebo (Coniff 1995).
Another large (n = 1946) randomised clinical trial, performed within the United Kingdom Prospective Diabetes Study (UKPDS), investigated acarbose versus placebo given in addition to diet, (combined) oral antidiabetic medication or insulin therapy (Holman 1999). At the three‐years endpoint, 39% of the patients in the acarbose group and 58% in the placebo group were still taking the study medication. The intention‐to‐treat analysis showed, that compared with placebo during three years, acarbose lowered glycated haemoglobin by 0.2% (P = 0.003). When only the proportion of patients that continued to take the study medication was considered, this difference was 0.5%. The clinical relevance of this finding remains unclear, especially when considering that even in the per‐protocol analysis for most patients using acarbose glycated haemoglobin remained higher than 8.0%. Further, data on other important outcomes like morbidity and mortality are not available from this study. Adverse effects were mostly of gastro‐intestinal origin (flatulence, stomachache) and were reported to resolve after a short while.
How the intervention might work
Alpha‐glucosidase inhibitors are reversible inhibitors of alpha‐glucosidase, an enzyme present in the brush border of the small intestine. Alpha‐glucosidase inhibitors delay absorption of complex carbohydrates and thus inhibit postprandial glucose peaks thereby leading to decreased postprandial insulin levels.
Why it is important to do this review
The scope of the current review was to assess the value of monotherapy with alpha‐glucosidase inhibitors in the treatment of type 2 diabetes mellitus with respect to patient‐oriented outcomes such as morbidity, mortality and quality of life. Further we investigated the value of alpha‐glucosidase inhibitors with respect to parameters related to glucose and lipid metabolism, body weight and adverse events. We sought studies that compared alpha‐glucosidase inhibitors with placebo or any other intervention. In the future, the review will be regularly updated to include relevant new trials.
Objectives
To assess the effects of alpha‐glucosidase inhibitors primarily on mortality, morbidity and quality of life in patients with type 2 diabetes mellitus, and secondly, the effects on parameters representing glucose and lipid metabolism (that is glycated haemoglobin, glucose, insulin and cholesterol).
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials with a minimum duration of three months were eligible for inclusion in this review. Because the common adverse effects of alpha‐glucosidase inhibitors make true blinding difficult, both blinded and non‐blinded studies were included. We included studies published in any language and all identified trials, published or unpublished, were investigated.
Types of participants
Patients with existing or newly diagnosed type 2 diabetes mellitus. Changes in diagnostic criteria (ADA 1997; ADA 1999; NDDG 1979; WHO 1980; WHO 1985; WHO 1998) may have produced variability in the clinical characteristics of the patients included as well as in the results obtained. These differences will be considered and explored in a sensitivity analysis.
Types of interventions
Monotherapy with alpha‐glucosidase inhibitors (acarbose, miglitol, voglibose, emiglitate) compared with any other intervention:
placebo;
sulphonylurea (for example, glibenclamide);
thiazolidinedione (for example, pioglitazone);
meglitinide (for example, nateglinide);
biguanide (for example, metformin);
insulin;
any other pharmacological intervention;
a non‐pharmacological intervention (for example, diet therapy).
Types of outcome measures
Primary outcomes
mortality: diabetes‐related mortality (death from myocardial infarction, stroke, renal disease, or sudden death, death from hyperosmolar nonketotic coma), total mortality;
diabetes‐related complications: vascular complications (angina pectoris, myocardial infarction, stroke, peripheral vascular disease, amputation), neuropathy, retinopathy, nephropathy, erectile dysfunction, hyperosmolar nonketotic dysregulation;
quality of life, assessed with a validated instrument.
Secondary outcomes
glycaemic control: glycated haemoglobin levels, fasting and post‐load blood glucose levels;
plasma lipids (triglycerides, total‐, high‐density lipoprotein (HDL)‐ and low‐density lipoprotein (LDL)‐cholesterol);
fasting and post‐load insulin and C‐peptide levels;
body weight (or body mass index);
adverse effects (i.e. diarrhoea, stomachache, flatulence).
Specific patient co‐variates thought to be effect modifiers
compliance
Timing of outcome measurement
We assessed a possible influence of treatment duration in a sensitivity analysis.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Central Register of Controlled Trials (CENTRAL) (2003, issue 3);
MEDLINE (up to April 2003) using the search terms listed below and combined with the MEDLINE search strategy for randomised controlled trials from the Cochrane Metabolic and Endocrine Disorders Group (see review group search strategy), without language restriction;
EMBASE (up to April 2003);
LILACS (www.bireme.br/bvs/I/ibd.htm) from up to April 2003;
Current Contents (up to December 2003).
Handsearching: checking references of existing reviews, checking abstract books and poster displays on congresses or meetings attended by the first author. The Internet was searches non‐systematically by using different combinations of (brand)names for alpha‐glucosidase inhibitors.
Databases of ongoing trials (latest access April 2003):
Current Controlled Trials (http://www.controlled‐trials.com ‐ with links to other databases of ongoing trials);
UK National Research Register (http://www.update‐software.com/National/nrr‐frame.html);
USA ‐ CenterWatch Clinical Trials Listing Service (http://www.CenterWatch.com/);
USA ‐ National Institutes of Health (http://clinicalstudies.info.nih.gov/).
All records from each database that seemed eligible after assessing the title and/or abstract were imported to a bibliographic database, Reference Manager (Version 10, ISI ResearchSoft), checked for duplicates and merged into one core database.
The described search strategy has been used for MEDLINE. For use with EMBASE and Current Contents this strategy was slightly adapted because these databases were only available with different browsers. The necessary alterations in search string were done in such a way that the search became more sensitive (that is yielded a higher number of 'hits'). In CENTRAL, LILACS and the databases of ongoing trials we searched with the various text words for the alpha‐glucosidase inhibitors and their brand names. For the detailed search strategy see Appendix 1.
Searching other resources
Authors of relevant identified studies and other experts were contacted by mail in order to obtain additional references, unpublished trials, and ongoing trials or to obtain missing data not reported in the original trials. Similarly, manufacturers and patent holders (Bayer AG, Sanofi‐Synthelabo, Pfizer, Takeda) were contacted in order to retrieve information on alpha‐glucosidase inhibitors trials, published and unpublished.
We searched reference lists of relevant trials and alpha‐glucosidase inhibitor reviews and selected possible references that were not already in our database.
Data collection and analysis
Selection of studies
Two reviewers (FVDL and PL) independently checked the titles, abstract sections and keywords of every record retrieved. Full articles were retrieved for further assessment when the information given suggested that the study: 1) included patients with diabetes mellitus, 2) compared alpha‐glucosidase inhibitors with placebo or any other active intervention, 3) assessed one or more relevant predefined clinical outcome measure, 4) used random allocation to the comparison groups. In case of any doubt regarding these criteria from the information given in the title and abstract, the full article was retrieved for clarification. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). Differences in opinion were resolved by a third party (EVDL) and when resolving the disagreement was not possible, the article was added to those 'awaiting assessment' and the authors were contacted for clarification. If the authors provided no clarification, the review group editorial base was consulted.
Data extraction and management
Two reviewers extracted data on intervention and outcomes independently, using a pre‐tested data extraction form that was adapted from a standard form provided by the review group. The data extraction form included the following items:
general information: author, type of publication (including the existence of duplicate or multiple publications), year of publication, language, country were the study was conducted, setting (general practice, hospital or outpatient / rural, city, developed / developing world / single or multi‐centre), the stated aim of the study published, sponsor(s), ethics approval;
study characteristics: parallel or cross‐over, type of control groups (placebo, other medication etc.), existence of run‐in and/or wash‐out period, description of possible carry‐over effect (for cross‐over studies), method, type and quality of randomisation, method and quality of allocation concealment, method and quality of blinding, information about handling of drop‐outs, withdrawals and losses to follow‐up, numbers of and reasons for drop‐out, existence of possible sub‐groups, method of assessment of compliance;
participants: description of diagnostic criteria for type 2 diabetes mellitus, inclusion and exclusion criteria,
interventions: specification of a possible reinforcement of diet therapy, the nature, dose and regimen (including: fixed or titrated dose, step‐up dosage scheme) of alpha‐glucosidase inhibitor(s) and control interventions, duration of intervention and follow‐up;
baseline characteristics and measurements: numbers of patients, sex, age, ethnicity, socio‐economic status and duration of diabetes, existence of significant differences at baseline, baseline glycated haemoglobin, fasting and post‐load blood glucose, plasma lipids (triglycerides, total‐, HDL‐ and LDL‐cholesterol), height, weight and body mass index (BMI), fasting and post‐load insulin and C‐peptide (standard deviations if applicable), specifications (including reference ranges) of all laboratory measurements, type of post‐load test, time between fasting and post‐load measurements, centralisation of laboratory measurements;
outcomes: total and disease specific deaths and morbidity, quality of life (including method of assessment), mean changes (standard deviation, SD) of the following values: glycated haemoglobin, fasting and post‐load blood glucose, lipids, fasting and post‐load insulin / C‐peptide, body weight, BMI, occurrence of adverse events (total and gastro‐intestinal), compliance.
When more than one publication was available from a study, all articles were abstracted and scores separately and the collected data was synthesized. In case of contradictorily findings, the author was contacted for clarification. Differences in data extraction were resolved by consensus, referring back to the original article. If necessary, information was sought from the authors of the original studies. If necessary, data were also extracted from graphical figures: two reviewers (FVDL and PL) calculated the data independently and if both outcomes were not similar, a third reviewer (EVDL) recalculated the data. A statistician checked all extracted data for errors, after transfer to the database.
Assessment of risk of bias in included studies
The two reviewers assessed each trial independently. Possible disagreement was resolved with consensus, or with consultation of a third reviewer (EVDL) in case of disagreement. In particular, the following quality criteria were assessed:
Minimisation of selection bias
Randomisation procedure: the randomisation procedures were scored adequate if the resulting sequences were unpredictable (that is computer generated schemes, tables of random numbers, coin tossing).
Allocation concealment: allocation concealment was scored adequate if participating patients and investigators could not foresee the assignment (that is by central randomisation remote from trial site, sequentially numbered and sealed radio‐opaque envelopes).
Minimisation of performance bias
Method of blinding: blinding was considered adequate if the two (or more) interventions were similar in size, colour and shape or when a double‐dummy method was applied. Because of the sometimes‐obvious adverse effects of alpha‐glucosidase inhibitors, true blinding was difficult. For trials that reported blinding of patients for medications, we also investigated whether blinding was checked; for example by asking patient and investigator afterwards about the medication they suspected to be supplied.
Minimisation of attrition bias
Handling of drop‐outs: handling of drop‐outs was considered adequate if studies gave a complete description of all patients failing to participate until the end of the trial and if the data were analysed on intention‐to‐treat (ITT) basis, that means with all randomised patients included.
Quantity of dropouts: overall dropout rate less than 15% was considered adequate.
Selective dropout: a difference in dropout rate the in main treatment groups less than 10% was considered adequate.
Minimisation of detection bias
Method of blinding outcome‐assessment: this item was considered less relevant for studies with laboratory data or death as main outcomes or if the (blinded) investigator was also outcome assessor. If applicable, outcome assessment was considered adequate if the outcome assessors were completely blind for the intervention.
We explored the influence of individual quality criteria in a sensitivity analysis (see under 'sensitivity analyses').
Based on these criteria, studies were broadly subdivided into the following three categories adapted from the Cochrane Handbook criteria (see Cochrane Handbook): A ‐ All quality criteria met (1. adequate randomisation and allocation concealment, 2. adequate blinding, 3. adequate ITT analysis and/or both drop‐out rate less than 15% and selective drop‐out less than 10%): low risk of bias. B ‐ One or more quality criteria only partially met (1. adequate randomisation or adequate allocation concealment, 2. mentioning of blinding but exact method unclear, 3. inadequate/unclear ITT analysis but drop‐out less than 15% or selective drop‐out less than 10%): moderate risk of bias. C ‐ One or more quality criteria not met (1. inadequate randomisation and allocation concealment, 2. inadequate or no blinding, 3. inadequate ITT and drop‐out rate equal to or more than 15% and selective drop‐out equal to or more than 10%): high risk of bias.
This adapted classification was also used as the basis of a sensitivity analysis.
Data synthesis
Data were summarised statistically if available and of sufficient quality. The table of comparison was first divided in all possible comparisons (that is acarbose versus placebo / voglibose versus sulphonylurea), then sub‐divided into all possible outcomes (that is death, glycated haemoglobin adverse events) and finally, within the outcomes sub‐groups were made for the different dosages. Outcomes were calculated per sub‐group and for all sub‐groups together. Dichotomous data were expressed as odds ratios (OR), but in some cases the relative risk (RR) was also calculated in addition to the OR since its interpretation is easier, especially if the outcome was a negative event, for example death. We calculated the risk difference (RD) and we converted the RD into the number needed to treat (NNT) or the number needed to harm (NNH) taking into account the time of follow‐up.
Continuous data were expressed as weighted mean differences (WMD) and an overall WMD was calculated. The actual measure of effect of all continuous variables were the differences from baseline to endpoint. The standard deviations of these differences were essential for the data to be included in the meta‐analysis. When the standard deviation (SD) of the difference was not reported we first asked the authors to provide these data. If the SDs were not provided we estimated the SD of the difference with the following formula:
SDpaireddifference = ??(SD1)2 + (SD2)2 ‐ 2 x r x SD1 x SD2].
SDpaireddifference = standard deviation of the difference (pre‐ / post‐treatment) SD1 = Standard deviation of the pre‐treatment value, SD2 = Standard deviation of the post‐treatment value, r = correlation coefficient. We used a conservative correlation coefficient of 0.4.
Overall results were calculated based on the random effects model. Heterogeneity was statistically tested by using the Z score and the Chi square statistic with significance set at P < 0.10. Possible sources of heterogeneity were assessed by subgroup, sensitivity and meta‐regression analyses as described below. Small study bias was tested for using the funnel plot or other corrective analytical methods depending on the number of clinical trials included in the systematic review (Begg 1994; Egger 1997; Hedges 1992). Quantification of the effect of heterogeneity will be assessed by means of I squared, ranging from 0‐100% including its 95% confidence interval (Higgins 2002). I squared demonstrates the percentage of total variation across studies due to heterogeneity and will be used to judge the consistency of evidence.
The analyses were done with the computer program RevMan Analyses 1.0.2 in Review Manager 4.2.3 (2003, The Cochrane Collaboration).
Subgroup analysis and investigation of heterogeneity
Significant main outcome measures were explored by subgroup analyses in order to explore differences in effect as follows:
glycated haemoglobin level at baseline (subdividing into three groups: less than 7%, 7 to 9%, more than 9%);
age (based on mean age of total randomised group);
gender (subdivided in two groups, based on data: less than 45% female, equal or more than 45% female);
body mass index (BMI) (Normal: male less than 27, female less than 25; overweight: male 27 to 30, female 25 to 30; obese: more than 30);
different kind of diets or exercise schedules used;
duration of intervention (less than 24 weeks, 24 weeks, more than 24 weeks);
Sensitivity analysis
The sensitivity of the analysis for a number of factors was determined by comparing the results of the meta‐analysis for studies with and without certain characteristics. Data from a minimum of five studies had to be available for both groups to be considered. The following factors were investigated:
comparing published and unpublished studies;
comparing studies with and without (or with unknown) quality characteristics: adequate randomisation, adequate allocation concealment, adequate method of blinding, adequate ITT analyses. Further, comparing studies with an overall drop‐out rate equal to or more than 15% and less than 15%, difference of drop‐out rates less than 10% and equal to or more than 10% between the main treatment groups. In addition, the overall score for quality based on the adapted Cochrane criteria was used so that studies with score A and B were compared with studies with C;
repeating the analysis excluding trials using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other or no sponsoring) or country;
repeating the analyses using different measures of effect size (relative risk, risk difference) and different statistical models (fixed and random effects models);
Meta‐regression analyses
We used meta‐regression analyses (in SAS proc MIXED, version 8.0) to explore the influence of characteristics of study population and study design on the outcomes. We studied the dependent variables glycated haemoglobin, fasting and post‐load glucose, fasting and post‐load insulin, total cholesterol, triglycerides and adverse effects. The independent variables were similar to the pre‐defined sub‐groups (baseline glycated haemoglobin, age, gender, baseline BMI, and duration of treatment). In addition we studied duration of diabetes at baseline, the use of a fixed dose and the use of a step‐up dosage regimen. The weight of each trial was equal to the inverse sum of the within trial variance and the residual between trial variance, in order to perform a random effects analysis. To gain sufficient power, data from at least 10 studies had to be available to calculate results from the meta‐regression.
Results
Description of studies
Results of the search
Trials identified
For details see Figure 1
1.

Flow chart of study selection
* CENTRAL: 262 records were retrieved and assessed on the basis of title and/or abstract (Issue 3 2003), 59 records were initially included. Ten records were excluded after the full article had been read. So 49 records were finally included in the review. * MEDLINE: 328 records found (April 2003), 43 records initially included, 34 records finally included in the review. * Embase: 567 records found (April 2003), 50 records initially included, 40 records finally included in the review. * Current Contents (December 2003): 260 records found, 27 records initially included, 23 records finally included in the review. * LILACS: 13 records found, one records initially but excluded after further scrutiny.
Experts: We obtained 14 references as a result of correspondence with experts: seven references after a general mailing to 27 experts with a request for additional references (six out of 27 forms were returned), and another seven references as a result of contacts which we established searching for missing or additional data. Two references were already in our possession (one study performed by our group but that was not published at that time (Van de Laar 2004a) and an article referring to two trials (Fölsch 1990, using data from Hoffmann 1990 and Spengler 1992). We included nine (out of these 16) references in the final review.
Manufacturers: Bayer, the developer of acarbose and miglitol, sent us 23 references, 17 were initially included and 16 were finally included in the review. The developer and patent holder of voglibose (Takeda) and the patent holders of miglitol (Pfizer and Sanofi‐Synthelabo) did not reply to our letters.
Handsearch: 22 possibly eligible references were found by handsearching (checking references of existing reviews, browsing on the internet, posters on congresses etc.). Seventeen references were initially included, of which 14 references were finally included in the review.
Databases of ongoing trials (see table Characteristics of ongoing trials): in addition three studies were identified as ongoing studies in trial registers. All attempts to retrieve reports or data from these studies, failed so far.
Interrater agreement
Interrater kappa for agreement on inclusion, calculated on basis of the first 852 titles and / or abstracts read by the two reviewers (FVDL and PL) was good: 0.74 (95% confidence interval 0.67 to 0.81). All differences in opinion were resolved by consensus.
Missing data
Because none of the articles contained all the study data we required for the quality assessment and meta‐analyses, we attempted to contact all corresponding authors. For one study we could not retrieve contact information (Hillebrand 1987). For 22 out of 41 studies we received additional data about design, quality and/or outcomes. For 12 studies the authors delegated the reply to representatives of Bayer Germany, USA or Italy because the data‐files were kept by this firm. Studies for which we received additional data are indicated in the table 'Characteristics of included studies' and the reference list (published and unpublished data).
Measurement of post‐load blood glucose, insulin and c‐peptide
There are several methods to determine the patients' response to a glucose load. The 'load' may consist of simple glucose (like in an oral Glucose Tolerance Test, oGTT), a standardised or ad libitum meal, or a standardised portion of carbohydrates. Studies may also differ in the time‐interval used for the test and if the study drug was given prior to the test. We assessed all those differences and described them in a table (Table 1). Most studies used some form of test‐meal with carbohydrates, except for two studies which used an OGTT (Hotta 1993; Van de Laar 2004a). In two studies the type of test was unclear (Hillebrand 1987; Rybka 1999). For two studies, the only post‐load measurement was at a 2‐hours interval (Hotta 1993; Pagano 1995) and six studies reported both one and two hour values (Chiasson 2001; Coniff 1994; Coniff 1995; Coniff 1995b; Kawamori 2003; Santeusanio 1993), all other studies that measured post‐load values for glucose, insulin and/or C‐peptide used an 1‐hour interval. Therefore, we chose to report the 1‐hour values for post‐load glucose, insulin and C‐peptide, and to use the 2‐hour outcomes if 1‐hour data were not available. As a sensitivity analysis, we repeated the analysis with the opposite method: using the 2‐hour values, and the 1‐hour values for studies that did not report 2‐hour measurements.
1. Methods post‐load glucose / insulin measurement.
| Study | Type of test | Interval | Data used | Medication given? |
| Braun 1996 | Breakfast ('no special meals') | 1 hour | 1 hour glucose | unclear |
| Buchanan 1988 | No post‐load test | |||
| Calle‐Pascual 1996 | No post‐load test | |||
| Campbell 1998 | No post‐load test | |||
| Chan 1998 | Individually tailored meal recommended by dietician (60% carbohydrate, <30% fat, 12‐20% protein) | 1 hour | 1 hour glucose & insulin | yes (at least at 24 weeks measurement) |
| Chiasson 1994 | Standard breakfast: 450 kcal, 55% carbohydrates, 30.5% lipids, 14.5% protein | 1, 1.5 and 2 hours measured | Data not reported | yes |
| Chiasson 2001 | Standardised liquid test breakfast (55% carbohydrate, 30% fat, and 15% protein; providing ˜450 kcal) | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | yes |
| Coniff 1994 | Breakfast, 2520 kJ, with 50% carbohydrates, 30% fat, 20% protein. | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose | yes |
| Coniff 1995 | Full‐meal tolerance test: 600 kcal breakfast (50% carbohydrate, 30% fat, 20% protein | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | yes |
| Coniff 1995b | Standardised meal tolerance test, 600‐kcal breakfast of 50% carbohydrates (75g), 30% fat (20g), 20% protein (30g) | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | yes |
| Dedov 1995 | Post‐load test performed, type of test unclear | 1 hour | 1 hour glucose | unclear |
| Delgado 2002 | Post‐load test performed, type of test unclear | Not reported | post‐load glucose | unclear |
| Drent 2002 | White bread, margarine, diet jam and cheese, 1556 kJ, 49% carbohydrate, 40% fat, 11% protein, 2,5 g fibre. | 1, 1.5 and 2 hours measured | Data not reported | yes |
| Fischer 1998 | Test meal 1562 kJ, 49% carbohydrate, 40% fat, 11% protein (80 g white bread, 10g spread, 25g diet jam, 20 g 45% fat cheese) | 1 hour measured and reported (2 hours value measured but not reported adequately) | 1 hour glucose | yes |
| Gentile 1999 | Home cooked breakfast, lunch and diner | 2 hours (after diner also after 4 hours) measured, not reported adequately | Data not reported | unclear |
| Haffner 1997 | Standardised breakfast (370 kcal; 49% carbohydrates, 40 % fat, 11% protein) | 1 hour measured and reported | 1 hour glucose & insulin | unclear |
| Hanefeld 1991 | Testmeal: 400 kcal (50% carbohydrates, 35% fat, 15% protein) | 1 hour measured and reported (2, 3, 4 and 5 hours also measured but not reported) | 1 hour glucose & insulin | yes |
| Hillebrand 1987 | Unclear | Measurement at 11 AM and 5 AM, interval not clear | Data not adequately reported | unclear |
| Hoffmann 1990 | Standard breakfast: 80 g bread, 20g low fat spread, 25g marmalade, 20 g cheese (45% fat), 1 egg | 1 hour measured and reported | 1 hour glucose | yes |
| Hoffmann 1994 | Standardised breakfast: 1,569 kJ (372 Kcal), 49% energy as (mainly complex) carbohydrates, 40% fat, 11% protein | 1 hour measured and reported | 1 hour glucose & insulin | yes |
| Hoffmann 1997 | Standardised breakfast: 1,569 kJ (372 Kcal), 49% energy as (mainly complex) carbohydrates, 40% fat, 11% protein | 1 hour measured and reported | 1 hour glucose & insulin | yes |
| Holman 1999 | No post‐load test | |||
| Holmes 2001 | No post‐load test | |||
| Hotta 1993 | 75 grams Oral Glucose Tolerance Test | 0.5, 1, 2 and 3 hours measured | 2 hours glucose, 0.5, 1 and 3 hours not reported adequately | yes |
| Johnston 1998 | Standardised test meal: 480 calories, 51% carbohydrates | 1, 1.5 and 2 hours measured | Data not reported adequately | unclear |
| Johnston 1998a | Standard 483 kcal, 51% carbohydrate mixed‐meal breakfast | 2 hours measured | Data not reported adequately | unclear |
| Johnston 1998b | Standard 438 kcal, 51% carbohydrate, 14% protein, 35% fat meal | 2 hours measured | Data not reported adequately | unclear |
| Kawamori 2003 | 'meal‐loading test' | 1 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | unclear |
| Kovacevic 1997 | Full meal tolerance test: 80 g white bread; 10 g butter, 25 g diet marmalade (with 23% fructose); 20 g cheese (45% fat); 250 ml coffee or tea | 1 hour measured and reported | 1 hour glucose & insulin | unclear |
| Meneilly 2000 | 400 ml Ensure ™ with fibre (450 kcal, 55% carbohydrate, 30% fat and 15% protein) | 1, 1.5 and 2 hours measured | Data not reported adequately | yes |
| Pagano 1995 | Standard breakfast, with 125 g fruit juice, 75 g ham and 80 g white bread (590 kcal, 44% carbohydrates, 41% lipids, 15% protein) | 0.5, 1,2 and 3 hours measured and reported, 0.5, 1, and 3 hours measured | 2 hour glucose, 0.5, 1 and 3 hours not reported adequately | yes (not with respect to glibencamide) |
| Rosenthal 2002 | Standard breakfast: 80g bread, 20 g low fat spread, 25 g marmalade, 20 g cheese (45%), 1 egg | 1 hour measured and reported | 1 hour glucose & insulin | yes |
| Rybka 1999 | Unclear | 1 hour measured | Data not reported adequately | unclear |
| Salman 2001 | Breakfast which was prepared by an experienced dietician according to individual needs | 1.5 hours measured and reported | 1.5 hours glucose, insulin & c‐peptide | no |
| Santeusanio 1993 | Mixed meal test, consisting 440 calories, as 30% protein, 20% lipid and 50% carbohydrate | 1, 2 and 3 hours measured and reported (0.5 hours not reported) | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | unclear |
| Scott 1999 | Standardised breakfast meal (1.6 MJ) | 1 and 2 hours measured | Data not reported adequately | unclear |
| Segal 1997 | Standardised breakfast test meal (372 kcal; 49% carbohydrate, 40% fat, 11% protein) | 1 and 2 hour measured | Data not reported adequately | unclear |
| Spengler 1992 | Standard breakfast: 80 g, 20 g low fat spread, 25 g marmelade, 20 g cheese, 1 egg | 1 hour measured | Data not reported adequately | yes |
| Takami 2002 | No post‐load test | |||
| Van de Laar 2004a | 75 grams Oral Glucose Tolerance Test | 1 hour mesured and reported | 1 hour glucose & insulin | no |
| Zheng 1995 | 'meal' | 1 hour measured and reported | 1 hour glucose & insulin | unclear |
Included studies
Fourty‐one studies with 8130 participants, described in 69 articles, abstracts, posters or unpublished documents were finally included in the review. Details are given in the Table of included studies. Thirty‐five studies were published as journal articles, three studies as abstract only (Campbell 1998; Hillebrand 1987; Rybka 1999) and two studies were found by their poster presentation (Holmes 2001; Kawamori 2003), one study done by our own group was accepted for publication during the review process (Van de Laar 2004a). Four studies were performed in general practice, for one study the patients were recruited in general practice but all study related activities were done in so‐called 'study‐centres' (Drent 2002), patients from 34 studies were characterised as 'outpatients' and for two studies the setting was not reported. Thirty‐nine studies had a parallel design and two were crossover studies (Gentile 1999, Hillebrand 1987). Thirty‐three studies were double‐blinded, five studies were not blinded and three studies with three treatment groups were not blinded with respect to one treatment arm (metformin and glibenclamide). Nineteen studies compared acarbose with placebo, four of which compared two or more doses with placebo. Eleven studies compared acarbose with other anti‐diabetic medication and in most cases also with placebo. Miglitol was studied in comparison with placebo in three studies, one of which with four different dosages. In four studies miglitol was compared with other anti‐diabetic medication (and placebo eventually). Two three‐arm studies compared acarbose with miglitol and placebo (one study) or glibenclamide (one study). One study compared miglitol and voglibose (and placebo) and one trial studied voglibose versus diet and glyburide (a sulphonylurea). We found no studies with emiglitate. Study duration was 24 weeks (21 studies), 16 weeks (seven studies), one year (four studies), 12 weeks (four studies), three years (two studies), 30 weeks, 36 weeks or 56 weeks (all one study). Two studies reported data on mortality (Coniff 1995; Johnston 1998) and one crossover study reported that no patients had died (Gentile 1999). Two studies reported data on morbidity (Holman 1999; Johnston 1998) and one study reported quality of life as an outcome (Meneilly 2000), but none of these data were primary efficacy measures.
Excluded studies
Fifteen studies were excluded after reading the full article (see Figure 1). The most common reason was that patients used anti‐diabetic medication in addition to the study medication. See table 'Charcteristics of excluded studies' for further details.
Risk of bias in included studies
For details on risk of bias see Figure 2.
2.

Risk of bias data
Methodological quality
With respect to selection bias 11 studies had both an adequate randomisation and allocation concealment. The risk of attrition bias was low in 14 studies: one study had adequate ITT; one study had both adequate ITT analysis and low total / selective drop‐out (less than 15% total drop‐out, less than 10% difference between groups); 12 studies had low total / selective drop‐out. Blinding (performance bias) was adequate in 22 studies. The overall quality was roughly assessed on a three point scale according to the Cochrane handbook: five studies scored A (low risk of bias) and five studies B (moderate risk of bias). The other 31 studies scored C (high risk of bias).
Missing data
In a number of cases it was reported that certain outcomes (that is fasting blood glucose, triglycerides) were investigated, but the results were not or insufficiently reported (that is standard deviations missing). This was especially striking for a study with acarbose, that was of long duration and with a large number of participants (Campbell 1998). Data from this trial could not be used because the main outcome measure was the time until patients with good control on diet alone needed additional medication. Data from a large study of long duration investigating miglitol could not be used as no measures of variance were reported for the main outcomes (that are standard deviations) (Johnston 1998). Our written request for these data, has not been answered so far. One large study (603 participants) comparing miglitol and acarbose was published as an abstract only (Rybka 1999). Attempts to contact the author failed so far.
Effects of interventions
Heterogeneity
Statistical tests for heterogeneity yielded statistically significant results in many cases. Studies were homogenous with respect to the fact that all participants were described as having type 2 diabetes and that they used the test drug as mono therapy for at least three months. But studies could differ with respect to country (and thus dietary habits), age, severity and duration of diabetes. These possible sources for heterogeneity were investigated in the sub‐group and meta‐regression analyses.
Mortality, morbidity, quality of life
Three studies reported the occurrence of death (Coniff 1995; Holman 1999; Johnston 1998). No statistically or clinically significant differences in outcomes were found. One 3‐year study reported data on morbidity as relative risks (Holman 1999). The relative risk for acarbose users compared with placebo for "any diabetes‐related end point" was 1.0 (95% confidence interval 0.8 to 1.2) and for microvascular disease 0.9 (95% confidence interval 0.6 to 1.4). The outcome for the subgroup actually receiving acarbose monotherapy was not reported. One 56‐weeks study that compared 25 mg and 50 mg TID miglitol with glyburide and placebo, reported the number of cardiovascular events in the table of adverse effects (Johnston 1998). The percentage of occurrence of any cardiovascular event was 19%, 17%, 22% and 29% for miglitol 25 mg TID, miglitol 50 mg TID, placebo and glyburide respectively. Statistical significance was reached for the comparison miglitol 50 mg and glyburide.
Glycemic control
Glycated haemoglobin, alpha‐glucosidase inhibitors versus placebo
alpha‐glucosidase inhibitors had a clear beneficial effect on glycemic control compared to placebo. Glycated haemoglobin was considered the primary measurement in most studies. The results of the meta‐analysis for overall effect of alpha‐glucosidase inhibitor on glycated haemoglobin compared to placebo was ‐0.8% (95% confidence interval ‐0.9 to ‐0.6, 28 comparisons) for acarbose and ‐0.7% (95% confidence interval ‐0.9 to ‐0.4, seven comparisons) for miglitol. For voglibose, data from only one comparison were available: ‐0.5% (95% confidence interval ‐0.6 to ‐0.3). We did not see a clear dose dependency of the effect on glycated haemoglobin with respect to acarbose. Effect sizes for the subgroups for dosage 25 mg (n = 1 study), 50 mg (n = 2), 100 mg (n = 17), 200 mg (n = 4) and 300 mg (n = 2) TID were ‐0.5%, ‐0.9%, ‐0.8%, ‐0.8% and ‐0.8% respectively. For miglitol, there seemed to be a dose dependent effect on glycated haemoglobin, but data from only seven comparisons, of which four originating from the same multi‐arm study (Drent 2002), were available.
Fasting and post‐load blood glucose, alpha‐glucosidase inhibitors versus placebo
We also found a beneficial effect on fasting blood glucose for acarbose compared to placebo in a meta‐analysis with 28 comparisons: ‐1.1 mmol/L (95% confidence interval ‐1.4 to ‐0.8). For miglitol and voglibose two and one comparisons were available in the meta‐analysis with fasting blood glucose as outcome. These analyses resulted in a mean decrease in fasting blood glucose of ‐0.5 mmol/L (miglitol, 95% confidence interval ‐0.9 to ‐0.2) and ‐0.6 mmol/L (voglibose, 95% confidence interval ‐1.0 to ‐0.2). The influence on (1‐hour) post‐load blood glucose was more profound. Overall effect on post‐load blood glucose was ‐2.3 mmol/L (95% confidence interval ‐2.7 to ‐1.9, 22 comparisons). The sub‐groups for dosage showed a dose dependent pattern. For miglitol and voglibose only very limited data were available: miglitol ‐2.7 mmol/L 95% confidence interval ‐5.5 to 0.1, two comparisons), voglibose ‐2.4 mmol/L (95% ‐3.0 to ‐1.8, one comparison). In contrast to the effect on glycated haemoglobin, the forest plots for the comparison acarbose versus placebo and the outcome fasting and post‐load blood glucose suggested a dose dependency of the treatment effect. Because not all studies used similar methods for the measurement of post‐load blood glucose we repeated the analyses replacing 1‐hour post‐load data by 2‐hour values (if available). We found no differences in that analysis compared with the meta‐analysis in which we primarily used the 1‐hour values.
Alpha‐glucosidase inhibitors versus other medication
Studies that compared an alpha‐glucosidase inhibitor with other interventions than placebo were scarce. Pooling of results was only possible for the comparison acarbose with sulphonylurea, as data from eight comparisons were available. For other comparisons, pooling was not possible because of lack of studies (metformin and nateglinide, both one study). The overall comparison acarbose versus sulphonylureas yielded a non‐significant trend for sulphonylureas with respect to glycated haemoglobin (0.4%, 95% confidence interval ‐0.0 to 0.8). The results in the subgroup 'Acarbose 100 mg TID versus Glibenclamide 3.5 mg TID' were not consistent with the other comparisons (overall test for heterogeneity p < 0.00001). Leaving the entire sub‐group out of the analysis would give an overall effect of 0.6% (95% confidence interval 0.3 to 1.0) in favour of sulphonylurea with a non‐significant chi‐square test for heterogeneity (p = 0.15). In the comparison acarbose versus sulphonylurea one study seemed to be an outlier (Kovacevic 1997), but the results of that study were again in line with the comparisons with other sulphonylurea. For most comparisons acarbose versus sulphonylurea, acarbose was given as a fixed dose and the sulphonylurea individually adjusted, mostly sub‐maximal. The result for fasting blood glucose showed a similar pattern: superiority for sulphonylurea except for the subgroup 'Acarbose 100 mg TID vs. Glibenclamide 3.5 mg TID'. Overall effect 0.7 mmol/L (95% confidence interval 0.2 to 1.2) in favour of sulphonylurea. Without the deviating sub‐group: 1.2 mmol/L (95% confidence interval 0.6 to 1.8) in favour of sulphonylurea. The outcome post‐load blood glucose yielded no statistically significant differences between acarbose and sulphonylurea.
Results from studies not included in the meta‐analyses
In a four‐arm study comparing miglitol 25 mg TID, miglitol 50 mg TID, glyburide maximum 20 mg QD or placebo, glycated haemoglobin decreased by 0.5%, 0.4%, 0.9% and 0.0% respectively (Johnston 1998). Similarly fasting blood glucose decreased by 0.7 mmol/L, 1.1 mmol/L, 1.7 mmol/L and 0.1 mmol/L and one hour post‐load blood glucose decreased by 2.4 mmol/L, 3.2 mmol/L, 1.8 mmol/L and 0.0 mmol/L respectively. One study with 603 participants and of 24 weeks duration (Rybka 1999) reported a placebo subtracted decrease of glycated haemoglobin of 0.4%, 0.5% and 0.4% respectively for miglitol 50 mg TID, miglitol 100 mg TID and acarbose 100 mg TID.
Plasma lipids
We found no effects of acarbose compared to placebo on total, HDL‐ and LDL‐cholesterol. There was no statistically significant effect on triglycerides: ‐0.1 mmol/L (21 comparisons, 95% confidence interval ‐0.2 to 0.0). With respect to the comparison with sulphonylurea no statistically significant differences were found. Very few comparisons (arcabose versus metformin etc.) were available.
Fasting and post‐load insulin and C‐peptide
The 25 studies that assessed pancreatic function mostly used insulin levels for this purpose. We found that acarbose had no statistically significant effect on fasting insulin levels compared to placebo and a non‐statistically significant decreasing effect on post‐load insulin levels (fasting insulin: ‐1 pmol/L (15 comparisons, 95% confidence interval ‐8 to 7), post‐load insulin: ‐41 pmol/L (13 comparisons, 95% confidence interval ‐61 to ‐19)). For miglitol and voglibose only a limited number of comparisons were available and no statistically significant differences were found. Compared to sulphonylurea, acarbose had a statistically significant decreasing effect on fasting insulin (seven comparisons, ‐25 pmol/L, 95% confidence interval ‐43 to ‐6) and post‐load insulin as well (seven comparisons, ‐133 pmol/L, 95% confidence interval ‐185 to ‐82). Only one study compared miglitol with a sulphonylurea and found an opposite result: fasting insulin 28 pmol/L increase compared to sulphonylurea (Pagano 1995). Post‐load insulin was not measured in that study.
Body weight and body mass index (BMI)
Compared to placebo, alpha‐glucosidase inhibitors had minimal effects on body weight. There were no statistically significant differences for body weight in the meta‐analysis for acarbose versus placebo, but BMI decreased slightly in favour of acarbose: ‐0.2 kg/m2 (13 comparisons, 95% confidence interval ‐0.3 to ‐0.1). The reported advantage for alpha‐glucosidase inhibitors on body weight compared to sulphonylurea could not be confirmed: no significant differences were found.
Adverse events
Most studies reported the total number of adverse events and although it became clear from most reports that by far the most adverse effects were of gastro‐intestinal origin, the number of patients with gastro‐intestinal adverse effects were rarely reported exactly. Compared to placebo, patients treated with acarbose reported significantly more adverse effects: OR 3.4 (or relative risk 1.4) (23 comparisons, 95% confidence interval 3.4 to 4.4). There was a dose dependent increase in adverse effects in the range 25 mg TID to 200 mg TID. When the sub‐group for studies that applied a fixed dosage scheme (in contrast to studies with an individually titrated dose) was considered, the dose dependency was more clear: ORs for adverse events were 1.6, 2.9, 4.1, 7.0 and 8.3 for the dosages 25, 50, 100, 200 and 300 mg TID respectively. Most studies reported that the adverse events mainly consisted of gastro‐intestinal symptoms. The meta‐analysis on gastro‐intestinal adverse events yielded a similar result: OR 3.30 (or relative risk 1.8) (four comparisons, 95% confidence interval 2.2 to 4.7). The comparison miglitol versus placebo resulted in similar figures: all adverse events OR 4.0 (seven comparisons, 95% confidence interval 1.7 to 9.5). Compared to sulphonylurea, patients treated with acarbose had more adverse effects: OR 4.0 (seven comparisons, 95% confidence interval 2.0 to 7.8). Only two studies provided data for the comparison miglitol versus sulphonylurea: OR 1.3 (95% confidence interval 0.7 to 2.4).
Sensitivity analyses
We compared outcomes of meta‐analyses between studies with and without certain characteristics. The results were considered of possible interest when the 95% confidence intervals of the two groups in the analysis (for example results from studies with adequate randomisation versus inadequate randomisation) did not overlap, or when one group yielded a statistically significant result whereas the other did not. At least five studies had to be in each groups to be considered, this was only the case for the comparison acarbose versus placebo.
Unpublished versus published studies
By the time the analyses were done, one study that was initially included as unpublished study was published (Van de Laar 2004a). All other studies were published in some form. Some studies were published otherwise than as a journal article: letter‐to‐the‐editor (Calle‐Pascual 1996) or congress abstract (Campbell 1998, Hillebrand 1987, Holmes 2001, Kawamori 2003, Rybka 1999). Because data from three of these studies could not be included in the meta‐analysis, sensitivity analysis was not possible.
Methodological quality criteria
Randomisation: studies with inadequate or unclear randomisation showed a beneficial effect of acarbose on total cholesterol: ‐0.3 (95% CI ‐0.5 to ‐0.0) versus 0.0 (95% CI ‐0.1 to 0.1) for studies with adequate randomisation. No other differences between studies with adequate and inadequate/unclear randomisation were found. Allocation concealment: the studies with adequate allocation concealment showed a slightly more profound effect on glycaemic control although not statistically significant: glycated haemoglobin ‐0.8% (adequate allocation concealment) versus ‐0.7 (not adequate or unclear). Blinding: we found no differences between studies with no or inadequate blinding and studies with adequate blinding. ITT adequate: only two studies were considered to have done adequate ITT analyses, therefore sensitivity analyses were not possible. Total dropout rate: studies with a total dropout rate less than 15% showed a beneficial effect on post‐load insulin levels compared to studies with a total dropout rate equal to or more than 15%: ‐52 (95% confidence interval ‐77 to ‐29) versus ‐18 (95% confidence interval ‐55 to 19). No other differences between studies with high or low drop‐out rates were found. Selective drop‐out (difference in drop‐out between treatment groups): we found no differences between studies with selective dropout rate less than 10% or equal to or more than 10%. Overall quality: studies with a overall quality A or B (high) showed a beneficial effect on post‐load insulin levels compared to studies with an overall quality score of C (low): ‐46 (95% confidence interval ‐64 to ‐29) versus ‐8 (95% confidence interval ‐68 to 52). No other differences were found.
Other
Diagnostic criteria
Eight studies referred to the WHO criteria from 1985 (WHO 1985), three studies to the criteria from the National Diabetes Data group 1979 (NDDG 1979), two studies referred to WHO criteria of unknown data, one study referred to both ADA guidelines from 1997 (ADA 1997) and WHO guidelines from 1987 (unknown origin, no reference given), one study used the so‐called UKPDS protocol (Holman 1999) and one study referred to diagnostic criteria of the Japan Diabetes Society. Twenty‐five studies did not refer to specific diagnostic criteria of type 2 diabetes. Although most studies referred diagnostic criteria (that is fasting blood glucose more than 7.8 mmol/L), it was often not clear whether these criteria were used for the trial selection or for the original diagnosis. Sensitivity analysis was not possible with these data.
Language of publication
For most included studies the primary publication was in English, with exception of one study in Russian (Dedov 1995) and one in the Italian language (Gentile 1999). Thus, sensitivity analysis was not performed.
Source of funding
For one study the authors made clear that it was not sponsored (Calle‐Pascual 1996), two study were sponsored by fundings other than a pharmaceutical company (Gentile 1999, Haffner 1997), for five studies possible sponsoring was not specified and all other studies were sponsored by a pharmaceutical company. Accordingly, sensitivity analysis was not performed.
Country
Twenty‐five studies were conducted in Europe (including one Russian study), nine studies in the USA or Canada, six studies in Asia (including one Turkish study) and one study was performed in New Zealand and Australia. European studies versus non‐European studies: studies that were conducted in Europe showed a tendency towards a greater effect on glycated haemoglobin (‐0.9%, 95% confidence interval ‐1.0 to ‐0.7) compared to non‐European studies (‐0.7%, 95% confidence interval ‐0.8 to ‐0.5). On the other hand, the effect on post‐load blood glucose was significantly less than for the non‐European studies: ‐1.9 mmol/L (95% confidence interval ‐2.2 to ‐1.5) for the European studies versus ‐3.3 mmol/L (95% CI ‐4.2 to ‐2.3) for the non‐European studies. These differences could not be fully explained when the Asian studies were excluded from the analyses. We also compared the Asian studies with non‐Asian studies separately because of the high carbohydrate food habits in Asia. The analyses with Asian studies only yielded a lower effect on glycated haemoglobin compared with the analyses with non‐Asian studies (‐0.5% versus ‐0.8%) but in the Asian group only three comparisons were available.
Different statistical models
We repeated the analyses for all outcomes using a fixed effects model. This yielded similar results with only two exceptions: 1) the effect on fasting insulin levels in the comparison acarbose versus placebo was statistically significant with a fixed effects model (5 pmol/L in favour of placebo, 95% confidence interval 1 to 10) 2) the effect on body weight in the comparison acarbose versus sulphonylurea was statistically significant with a fixed effects model (‐1.4 in favour of acarbose, 95% confidence interval ‐1.9 to ‐0.9).
Sub‐group analyses (tables available on request)
subgroups baseline glycated haemoglobin: Subgroup 1a (acarbose ‐ placebo), Subgroup 1b (tables available on request) (acarbose ‐ sulphonylurea). The effects on glycated haemoglobin and post‐load insulin tended to be more profound with higher baseline glycated haemoglobin;
subgroups gender: Subgroup 2a, Subgroup 2b (tables available on request). No significant differences between studies with less and more or equal than 45% female participants were observed;
subgroups baseline BMI: Subgroup 3a, Subgroup 3b (tables available on request). No significant differences between studies in patients with different mean baseline BMI values were observed;
subgroups study duration: Subgroup 4a, Subgroup 4b (tables available on request). We found a tendency towards a lower effect in studies that lasted longer than 24 weeks. The effect on glycated haemoglobin was ‐0.8%, ‐0.8% and ‐0.5% for studies less than 24, 24 and more than 24 weeks respectively. However only three studies were included in the latter (more than 24 weeks) categorie.
In addition to the pre‐defined sub‐groups, we also investigated the following subgroups: different duration of diabetes (mean duration of diabetes less or equal/more than 55 months), groups with a step‐up dose regimen versus studies that administered the full dose at once and studies that used a fixed dosage scheme versus studies with an individually titrated scheme.
subgroups mean duration of diabetes: Subgroup 5a, Subgroup 5b (tables available on request). No significant differences between studies in patients with a mean duration of diabetes less or equal/more 55 months were observed;
subgroups step‐up dosage versus no step‐up dosages: Studies investigating acarbose versus placebo that used a step‐up dosing schedule, tended to result in less effect on glycated haemoglobin, fasting and post‐load blood glucose than studies that gave the full dose at once. On the other hand, the latter studies reported more adverse effects. The 95% confidence intervals for fasting blood glucose and adverse effects in both groups did not overlap indicating statistical significance (Subgroup 6a).
This effect was also found in the comparison acarbose versus sulphonylurea. (Subgroup 6b) (tables available on request)
subgroups fixed dose versus individually titrated: Subgroup 7a, Subgroup 7b (tables available on request). Studies that used a fixed dose showed more profound effect on glycated haemoglobin (‐0.8% versus ‐0.5%) with no different effect on fasting blood glucose.
Meta‐regression analyses (tables available on request)
For the comparison acarbose versus placebo, sufficient data were available to perform meta‐regression analyses. Glycated haemoglobin: regression coefficient for mean baseline glycated Hb was ‐0.12, indicating a decrease in outcome value of 0.12% per 1% increase of baseline glycated Hb. The use of a fixed dosage yielded a regression coefficient of ‐0.32 (95% CI ‐0.69 to 0.04) and a step‐up dosage scheme regression coefficient of 0.36 (95% CI 0.06 to 0.66), thus having an increasing influence on glycated haemoglobin (Metaregression 1, table available on request).
Fasting blood glucose: use of a step‐up dosages scheme had a deteriorating effect on the outcome: correlation coefficient 0.62 (95% CI 0.05 to 1.19) (Metaregression 2, table available on request).
Post‐load blood glucose: no statistically significant effects were found (Metaregression 3, table available on request). Total cholesterol: no statistically significant effects were found (Metaregression 4, table available on request). Triglycerides: no statistically significant effects were found (Metaregression 5, table available on request). Fasting insulin: no statistically significant effects were found (Metaregression 6, table available on request). Post‐load insulin: no statistically significant effects were found (Metaregression 7, table available on request) Body weight: no statistically significant effects were found (Metaregression 8, table available on request). Total adverse effects: The use of a step‐up dosing scheme had a statistically significant decreasing effect on the occurrence of adverse effects (regression coefficient 0.50, 95% CI 0.29 to 0.88) (Metaregression 9, table available on request).
Discussion
Summary of main results
In this systematic review, we found no statistically significant effect for an effect of alpha‐glucosidase inhibitors on mortality, morbidity and quality of life in patients with type 2 diabetes mellitus. Compared to placebo, alpha‐glucosidase inhibitors reduce glycated hemoglobin (0.8% acarbose, 0.7% miglitol), fasting and postprandial blood glucose (acarbose: fasting glucose 1.1 mmol/L, post‐load blood glucose 2.3 mmol/L) and post‐load insulin. We found no clinically relevant effects on plasma lipids and body weight. We found no dose dependency for the effect on glycated haemoglobin for acarbose. alpha‐glucosidase inhibitors caused significant more adverse effects, especially of gastro‐intestinal origin. It should be noted that the data of the largest and longest studies could not be used for meta‐analyses. Compared to sulphonylurea alpha‐glucosidase inhibitors were inferior with respect to glycemic control and adverse effects, the extent of this effect differed with the sulphonylurea used. On the contrary, alpha‐glucosidase inhibitors had a decreasing effect on fasting and post‐load insulin levels compared to sulphonylurea. Of the three alpha‐glucosidase inhibitors investigated, acarbose, miglitol and voglibose, most data and best outcomes were obtained for acarbose.
Overall completeness and applicability of evidence
The results from this review are relevant for physicians dealing with patients with type 2 diabetes and for the developers of treatment guidelines. Data of beneficial effects on mortality or complications from diabetes mellitus are not available at the moment. Alpha‐glucosidase‐inhibitors inhibit post‐pranidal glucose peaks thereby leading to decreased post‐load insulin levels. Further, alpha‐glucosidase inhibitors lower post‐load insulin levels, especially when compared to sulphonylurea. There are no additional advantages with respect to the lipid profile or body weight. Most evidence is available for acarbose, which has also the best results for most outcomes. The importance of these findings and the exact place of alpha‐glucosidase inhibitors in the treatment of type 2 diabetes mellitus, has to be judged in view of other evidence regarding the clinical importance of (post‐load) hyperglycaemia and hyperinsulinaemia. This review investigated alpha‐glucosidase inhibitors as monotherapy. Although, from a theoretical point of view, it seems logical that alpha‐glucosidase inhibitors offer similar potentials in addition to other antidiabetic therapies, this cannot be concluded from this review. Evidence for the possible efficacy for alpha‐glucosidase inhibitors as add‐on therapy might be derived from a systematic review that is currently going on (Navarro 2003).
Potential biases in the review process
This is the first high‐quality systematic review and meta‐analysis on the topic of alpha‐glucosidase inhibitors. It offers an up‐to‐date and most complete overview of all randomised trials concerning alpha‐glucosidase inhibitor monotherapy, because it is the result of an extensive search, including grey literature and unpublished studies. In addition, maximum efforts have been done to minimise missing or incomplete data by attempting to contact all authors. This has been successful in 22 out of 41 cases. Although we included a high number of studies, the data are remarkably consistent and heterogeneity is limited. Statistical tests for heterogeneity are less reliable when a high number of studies are involved and further scrutiny by sub‐group analysis and meta‐regression analysis yielded few possible sources for heterogeneity. The use of a fixed dose (instead of an individually titrated dosage) may cause a more profound effect with respect to glycemic control but causes also more adverse effects. The same applies to giving the full dose at once, instead of using a step‐up scheme. Although this review presents a possibly confusing amount of data and figures, we feel that completeness is one of the strengths of a Cochrane systematic review. The way we presented these data, subdivided in types of alpha‐glucosidase inhibitor, controls and outcome measures, makes it possible for the reader to find whatever specific piece of information on alpha‐glucosidase inhibitor monotherapy he or she needs. This review will be regularly updated, leaving the possibility open to add information or to correct possible errors. In fact, this is a plea for anyone who is aware of such additional data or errors in the data presented here, to report this to the authors.
Our main research question was not answered with the trials we included in this review so far. Only few studies reported data on morbidity and mortality on a reliable and consistent way. It is not likely that in the (near) future a randomised trial of long enough duration will be conducted with acarbose monotherapy to investigate mortality and morbidity. This raises the question whether our review, with its strict inclusion criteria and high demands for outcome data, overshoots the mark. Maybe with broader inclusion criteria, that is inclusion of (high quality) observational studies, we would have gained data to study a possible influence on mortality and morbidity. The use of observational data does not necessarily lead to biased outcomes (Concato 2000). Still, we feel that for the evaluation of medical interventions, well designed randomised trials are the first choice. To improve systematic reviews in the future, we strongly plea for the integration of outcome measures such as death or morbidity into all trials that evaluate medical interventions for patients with chronic diseases. Even if the trial is underpowered for that outcome, the data might always be of value for a meta‐analysis. The question of including observational studies in a future update of this review is still open to us. Despite an exhaustive and thorough search, including requests to experts and manufacturers, we still cannot rule out publication bias. For the three trials that we found in a database for ongoing trials, we were not able to reveal outcome data or additional information about the design despite the fact that one trial ended six years ago (Whitby 1998) and the others in 2003 (Holman 2003; Sa‐adu 2003). Another clue for possible publication bias was that we, despite maximum efforts to retrieve unpublished data, discovered three previously unpublished studies coincidentally (Bayer 2003; Bayer 2003a; Campbell 1998) that were used for a study on a congress poster (Hanefeld 2003). Altogether, we still think that the overall risk for publication bias is limited because the funnel plots do not point at small study bias and because of the exhaustive search. Still, we welcome unpublished data for future updates. Not all papers reported outcomes in a way that could contribute to meta‐analyses. This problem was partially solved by asking authors for additional data, imputing the standard deviation of the mean difference (see under methods, data analysis) or using data from graphical figures. As an example, data from only four of the 32 studies investigating glycated haemoglobin in relation to the use of acarbose, suited for use in the meta‐analysis directly; for twelve studies additional data had to be obtained from the authors to complete all blanks; for twelve studies we had to calculate the SD of the mean difference from the baseline and endpoint SDs and for four studies the data could not be used at all. Unfortunately, one of those four studies was of long duration (3 years) and had a high number of participants (Campbell 1998). In summary, we used the most precise data in about half of the cases (16 out of 32) and we had to use less precise figures in 12 out of 32 cases. Because we used a conservative correlation coefficient of 0.4, this will most probably have made the confidence interval larger. The influence of the missing data from the largest studies was discussed under 'existing literature'. Only nine out of the 41 studies lasted longer than 24 weeks, and only two studies were amply longer than one year (Holman 1999; Campbell 1998). For one of those two studies data could not be included in the meta‐analyses (Campbell 1998). The importance of long‐term studies is evident, especially for a chronic disease such as type 2 diabetes. In the subgroup analysed for study duration, we found clues that the effect of alpha‐glucosidase inhibitors decrease with time, This was mostly due to the UKPDS study un which a decrease of only 0.2% was found after three years of treatment (Holman 1999). Therefore, we feel that the results from our study should be interpreted with caution when applied to the long‐term treatment with alpha‐glucosidase inhibitors of patients with type 2 diabetes. Research funded by pharmaceutical companies is more likely to produce results favouring the tested drug; this is often due to inappropriate comparators or small study bias (Lexchin 2003). In this review at least 33 studies were sponsored by a pharmaceutical company, including one study in which the sponsor was the producer of the comparison drug (Holmes 2001). We suppose that this will cause a slight overestimation of the results, especially concerning the studies that compare alpha‐glucosidase inhibitors with other medication. In fact, this is probable in the comparison acarbose versus sulphonylurea (glycated haemoglobin) where acarbose is dosed in a fixed way and the comparison drugs are individually adjusted (Coniff 1995; Hoffmann 1990; Hoffmann 1994; Kovacevic 1997; Rosenthal 2002; Salman 2001) or very low dosed (Haffner 1997). In one study both treatment arms used an individually adjusted dosage scheme (Van de Laar 2004a). For the comparison with placebo the influence of this 'bias by sponsoring' is less sure as it would be similar to publication bias like we discussed before.
Agreements and disagreements with other studies or reviews
Although this is the first systematic review concerning alpha‐glucosidase inhibitor monotherapy, some reviews have been published recently about acarbose (Breuer 2003; Laube 2002) or miglitol (Campbell 2000; Scott 2000). The quality of those reviews is limited: selection criteria for the studies were insufficiently specified and there was no mention of the criteria used to assess the validity of individual trials. Further, these reviews did not present explicit methods on data extraction, assessment of heterogeneity or subgroup analyses. Both reviews on acarbose referred also to a 'meta‐analysis' of older date (Lebovitz 1998), which calculated the mean outcomes on glycemic control for 13 studies, using outcomes for single treatment arms (baseline minus endpoint) as well as placebo extracted outcomes in a non‐transparent way. Our results are roughly in line with the previous reviews with respect to the overall effect on glycemic control compared to placebo, but there are relevant differences and additional findings. First, we found no dose‐dependency of acarbose on glycated haemoglobin in the meta‐analysis. Remarkably, the effect on fasting and post‐load blood glucose appeared to be dose dependent. This discrepancy might be explained by a better compliance of patients that were using the lower dosages, because higher dosages induce more adverse effects. Prior to their visit to the study centre, it is more likely that patients took their study medication and thus achieving good fasting and post‐load glucose values. Only for glycated haemoglobin, the effect of low compliance will show up. Secondly, we could not find relevant effects on lipid levels, especially triglycerides. Thirdly, we also could not confirm the optimistic view on adverse effects reported in the previous reviews. Twenty out of 41 included studies were subject to a skewed drop‐out pattern (? 10% difference per treatment group) and 25 studies had a total drop‐out rate that was ? 15%, in most cases this was caused by adverse effects. Finally, the previous reviews are optimistic about the glucose lowering capacities of alpha‐glucosidase inhibitors compared to other agents such as sulphonylurea. We confirm a clear beneficial effect with respect to fasting and post‐load insulin levels. But overall, the effects on glycemic control are inferior to sulphonylurea. For glycated haemoglobin this is not statistically significant, but most studies that compare acarbose with sulphonylurea use inappropriate comparators (that is too low dose for sulphonylurea or using an individually titrated dosage versus a fixed dosage). Therefore, we feel that a conclusion that sulphonylurea have superior glucose lowering properties, is justified. In addition, alpha‐glucosidase inhibitors cause more adverse effects. The three‐years trial performed within the UKPDS (Holman 1999) was one of the main studies included in the review. The effects regarding glycated hemoglobin obtained in this trial alone (a decrease of 0.2%) are considerably less profound than those from the meta‐analysis. This discrepancy with the results from the meta‐analysis, point in the direction of a possible overestimation of the effect in the long (three years) term.
Authors' conclusions
Implications for practice.
In patients with type 2 diabetes, alpha‐glucosidase inhibitor monotherapy inhibit post‐prandial glucose peaks thereby leading to decreased post‐load insulin levels. There are no advantages with respect to lipid metabolism or body weight. Compared to sulphonylurea, alpha‐glucosidase inhibitors have less favourable effects with respect to glycemic control and adverse effects but they lower fasting and post‐load insulin levels compared to sulphonylurea. For all outcomes, the largest evidence base exists for acarbose.
Implications for research.
New studies that investigate alpha‐glucosidase inhibitors on proxy indicators such as glycaemic control, lipids, insulin, body weight would be redundant. Large randomised controlled trials of long duration that investigate mortality, morbidity and quality of life as primary endpoints are necessary. In addition studies comparing alpha‐glucosidase inhibitors with other glucose lowering agents (especially metformin and thiazilodines) are of use. When these trials are not available, inclusion of well‐designed observational studies in this review may be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 1 September 2004 | Amended | We received additional data for the Holman (1999) study on September 1st 2004. The information is added. (see Table of included studies, comparisons tables and study quality) |
Acknowledgements
We would like to thank the following people: All authors, investigators and manufacturers who were willing to answer our many questions and who provided us with additional data. Henk van den Hoogen (University Medical Centre Nijmegen, Department of General Practice) for his help and advice with the analyses and interpretation of the data. Shuan Wang (West China Hospital, Sichuan University, Geriatrics department. Chengdu, Sichuan, China) for her help with the protocol development. The following people for their help with translation of articles: Leon Bax (Japanese), Ka Wai Wu (Chinese), Caroline Roos (Spanish), Emile van den Hoogen and Natasja Odelevskaia (Russian). Anja van Guluck (Library of the Elkerliek Hospital, Helmond, The Netherlands) for library assistance. The members from the Brazilian Cochrane Centre for their help with retrieval of abstracts from LILACS. The members of the Editorial Base of the Cochrane Metabolic and Endocrine Disorders Group and the Dutch Cochrane Centre for their help and advice.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. 1 TYPE 2 DIABETES MELLITUS (see Metabolic and Endocrine Disorders Group search strategy) ACARBOSE 2 Acarbose [MeSH, all subheadings included] 3 acarbose OR (alph* glucos* inh*) OR (alf* glucos* inh*) OR glucobay OR precos* OR prandas* OR akarbos* 4 #2 or #3 TYPE 2 DIABETES MELLITUS AND ACARBOSE 5 #1 AND #4 CLINICAL TRIALS 6 See Metabolic and Endocrine Disorders Group search strategy TYPE 2 DIABETES AND ACARBOSE AND CLINICAL TRIALS 7 #5 AND #6 |
Appendix 2. Methods post‐load glucose / insulin measurement
| Study | Type of test | Interval | Data used | Medication given? |
| Braun 1996 | Breakfast ('no special meals') | 1 hour | 1 hour glucose | unclear |
| Buchanan 1988 | No post‐load test | |||
| Calle‐Pascual 1996 | No post‐load test | |||
| Campbell 1998 | No post‐load test | |||
| Chan 1998 | Individually tailored meal recommended by dietician (60% carbohydrate, <30% fat, 12‐20% protein) | 1 hour | 1 hour glucose & insulin | yes (at least at 24 weeks measurement) |
| Chiasson 1994 | Standard breakfast: 450 kcal, 55% carbohydrates, 30.5% lipids, 14.5% protein | 1, 1.5 and 2 hours measured | Data not reported | yes |
| Chiasson 2001 | Standardised liquid test breakfast (55% carbohydrate, 30% fat, and 15% protein; providing ˜450 kcal) | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | yes |
| Coniff 1994 | Breakfast, 2520 kJ, with 50% carbohydrates, 30% fat, 20% protein. | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose | yes |
| Coniff 1995 | Full‐meal tolerance test: 600 kcal breakfast (50% carbohydrate, 30% fat, 20% protein | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | yes |
| Coniff 1995b | Standardised meal tolerance test, 600‐kcal breakfast of 50% carbohydrates (75g), 30% fat (20g), 20% protein (30g) | 1, 1.5 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | yes |
| Dedov 1995 | Post‐load test performed, type of test unclear | 1 hour | 1 hour glucose | unclear |
| Delgado 2002 | Post‐load test performed, type of test unclear | Not reported | post‐load glucose | unclear |
| Drent 2002 | White bread, margarine, diet jam and cheese, 1556 kJ, 49% carbohydrate, 40% fat, 11% protein, 2,5 g fibre. | 1, 1.5 and 2 hours measured | Data not reported | yes |
| Fischer 1998 | Test meal 1562 kJ, 49% carbohydrate, 40% fat, 11% protein (80 g white bread, 10g spread, 25g diet jam, 20 g 45% fat cheese) | 1 hour measured and reported (2 hours value measured but not reported adequately) | 1 hour glucose | yes |
| Gentile 1999 | Home cooked breakfast, lunch and diner | 2 hours (after diner also after 4 hours) measured, not reported adequately | Data not reported | unclear |
| Haffner 1997 | Standardised breakfast (370 kcal; 49% carbohydrates, 40 % fat, 11% protein) | 1 hour measured and reported | 1 hour glucose & insulin | unclear |
| Hanefeld 1991 | Testmeal: 400 kcal (50% carbohydrates, 35% fat, 15% protein) | 1 hour measured and reported (2, 3, 4 and 5 hours also measured but not reported) | 1 hour glucose & insulin | yes |
| Hillebrand 1987 | Unclear | Measurement at 11 AM and 5 AM, interval not clear | Data not adequately reported | unclear |
| Hoffmann 1990 | Standard breakfast: 80 g bread, 20g low fat spread, 25g marmalade, 20 g cheese (45% fat), 1 egg | 1 hour measured and reported | 1 hour glucose | yes |
| Hoffmann 1994 | Standardised breakfast: 1,569 kJ (372 Kcal), 49% energy as (mainly complex) carbohydrates, 40% fat, 11% protein | 1 hour measured and reported | 1 hour glucose & insulin | yes |
| Hoffmann 1997 | Standardised breakfast: 1,569 kJ (372 Kcal), 49% energy as (mainly complex) carbohydrates, 40% fat, 11% protein | 1 hour measured and reported | 1 hour glucose & insulin | yes |
| Holman 1999 | No post‐load test | |||
| Holmes 2001 | No post‐load test | |||
| Hotta 1993 | 75 grams Oral Glucose Tolerance Test | 0.5, 1, 2 and 3 hours measured | 2 hours glucose, 0.5, 1 and 3 hours not reported adequately | yes |
| Johnston 1998 | Standardised test meal: 480 calories, 51% carbohydrates | 1, 1.5 and 2 hours measured | Data not reported adequately | unclear |
| Johnston 1998a | Standard 483 kcal, 51% carbohydrate mixed‐meal breakfast | 2 hours measured | Data not reported adequately | unclear |
| Johnston 1998b | Standard 438 kcal, 51% carbohydrate, 14% protein, 35% fat meal | 2 hours measured | Data not reported adequately | unclear |
| Kawamori 2003 | 'meal‐loading test' | 1 and 2 hours measured and reported | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | unclear |
| Kovacevic 1997 | Full meal tolerance test: 80 g white bread; 10 g butter, 25 g diet marmalade (with 23% fructose); 20 g cheese (45% fat); 250 ml coffee or tea | 1 hour measured and reported | 1 hour glucose & insulin | unclear |
| Meneilly 2000 | 400 ml Ensure ™ with fibre (450 kcal, 55% carbohydrate, 30% fat and 15% protein) | 1, 1.5 and 2 hours measured | Data not reported adequately | yes |
| Pagano 1995 | Standard breakfast, with 125 g fruit juice, 75 g ham and 80 g white bread (590 kcal, 44% carbohydrates, 41% lipids, 15% protein) | 0.5, 1,2 and 3 hours measured and reported, 0.5, 1, and 3 hours measured | 2 hour glucose, 0.5, 1 and 3 hours not reported adequately | yes (not with respect to glibencamide) |
| Rosenthal 2002 | Standard breakfast: 80g bread, 20 g low fat spread, 25 g marmalade, 20 g cheese (45%), 1 egg | 1 hour measured and reported | 1 hour glucose & insulin | yes |
| Rybka 1999 | Unclear | 1 hour measured | Data not reported adequately | unclear |
| Salman 2001 | Breakfast which was prepared by an experienced dietician according to individual needs | 1.5 hours measured and reported | 1.5 hours glucose, insulin & c‐peptide | no |
| Santeusanio 1993 | Mixed meal test, consisting 440 calories, as 30% protein, 20% lipid and 50% carbohydrate | 1, 2 and 3 hours measured and reported (0.5 hours not reported) | 1 hour (2 hours value in sensitivity analysis) glucose & insulin | unclear |
| Scott 1999 | Standardised breakfast meal (1.6 MJ) | 1 and 2 hours measured | Data not reported adequately | unclear |
| Segal 1997 | Standardised breakfast test meal (372 kcal; 49% carbohydrate, 40% fat, 11% protein) | 1 and 2 hour measured | Data not reported adequately | unclear |
| Spengler 1992 | Standard breakfast: 80 g, 20 g low fat spread, 25 g marmelade, 20 g cheese, 1 egg | 1 hour measured | Data not reported adequately | yes |
| Takami 2002 | No post‐load test | |||
| Van de Laar 2004a | 75 grams Oral Glucose Tolerance Test | 1 hour mesured and reported | 1 hour glucose & insulin | no |
| Zheng 1995 | 'meal' | 1 hour measured and reported | 1 hour glucose & insulin | unclear |
Data and analyses
Comparison 1. Acarbose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 22 | 2831 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 1.1 Acarbose 25 mg TID | 1 | 178 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.86, ‐0.10] |
| 1.2 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.1 [‐2.31, 2.11] |
| 1.3 Acarbose 50 mg TID | 2 | 217 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.20, ‐0.59] |
| 1.4 Acarbose 100 mg TID | 17 | 1615 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.95, ‐0.56] |
| 1.5 Acarbose 200‐100‐200 | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐3.75, 2.75] |
| 1.6 Acarbose 200 mg TID | 4 | 486 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.00, ‐0.53] |
| 1.7 Acarbose 300 mg TID | 2 | 298 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.18, ‐0.38] |
| 2 Change in fasting blood glucose (mmol/l) | 22 | 2838 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.36, ‐0.83] |
| 2.1 Acarbose 25 mg TID | 1 | 177 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.85, 0.27] |
| 2.2 Acarbose 50 mg BID | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐2.64, 1.18] |
| 2.3 Acarbose 50 mg TID | 1 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.51, ‐0.41] |
| 2.4 Acarbose 100 mg TID | 17 | 1632 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.41, ‐0.72] |
| 2.5 Acarbose 200‐100‐200 | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.6 [‐2.26, 5.46] |
| 2.6 Acarbose 200 mg TID | 4 | 478 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐1.92, ‐1.06] |
| 2.7 Acarbose 300 mg TID | 2 | 295 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐2.54, ‐0.27] |
| 3 Change in post‐load blood glucose (mmol/l) | 16 | 2238 | Mean Difference (IV, Random, 95% CI) | ‐2.32 [‐2.73, ‐1.92] |
| 3.1 Acarbose 25 mg TID | 1 | 176 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐2.14, ‐0.58] |
| 3.2 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐1.8 [‐3.23, ‐0.37] |
| 3.3 Acarbose 50 mg TID | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.40, ‐0.87] |
| 3.4 Acarbose 100 mg TID | 13 | 1124 | Mean Difference (IV, Random, 95% CI) | ‐2.26 [‐2.79, ‐1.73] |
| 3.5 Acarbose 200 mg TID | 3 | 411 | Mean Difference (IV, Random, 95% CI) | ‐2.78 [‐3.72, ‐1.85] |
| 3.6 Acarbose 300 mg TID | 2 | 291 | Mean Difference (IV, Random, 95% CI) | ‐3.62 [‐5.34, ‐1.89] |
| 4 Change in total cholesterol (mmol/l) | 17 | 2133 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.10, 0.09] |
| 4.1 Acarbose 25 mg TID | 1 | 179 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.16, 0.40] |
| 4.2 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.3 [‐1.39, 0.79] |
| 4.3 Acarbose 50 mg TID | 2 | 218 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.24, 0.25] |
| 4.4 Acarbose 100 mg TID | 13 | 999 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.11] |
| 4.5 Acarbose 200‐100‐200 | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.62, 1.02] |
| 4.6 Acarbose 200 mg TID | 3 | 410 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 4.7 Acarbose 300 mg TID | 2 | 290 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.16, 0.22] |
| 5 Change in HDL‐cholesterol (mmol/l) | 13 | 924 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 5.1 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.39, 0.19] |
| 5.2 Acarbose 50 mg TID | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.28, 0.10] |
| 5.3 Acarbose 100 mg TID | 10 | 608 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.06, 0.07] |
| 5.4 Acarbose 200 mg TID | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.08, 0.10] |
| 5.5 Acarbose 300 mg TID | 1 | 152 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6 Change in LDL‐cholesterol (mmol/l) | 4 | 402 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.25] |
| 6.1 Acarbose 100 mg TID | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.63, 0.80] |
| 6.2 Acarbose 200 mg TID | 1 | 93 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.12, 0.44] |
| 6.3 Acarbose 300 mg TID | 1 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.18] |
| 7 Change in triglycerides (mmol/l) | 15 | 1969 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.18, 0.00] |
| 7.1 Acarbose 25 mg TID | 1 | 179 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.22, 0.66] |
| 7.2 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.2 [‐1.96, 1.56] |
| 7.3 Acarbose 50 mg TID | 2 | 217 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.22, 0.33] |
| 7.4 Acarbose 100 mg TID | 11 | 834 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 7.5 Acarbose 200‐100‐200 | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.4 [‐0.85, 1.65] |
| 7.6 Acarbose 200 mg TID | 3 | 412 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.57, 0.02] |
| 7.7 Acarbose 300 mg TID | 2 | 290 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.25, 0.14] |
| 8 Change in fasting insulin levels (pmol/l) | 12 | 1264 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐7.90, 6.86] |
| 8.1 Acarbose 50 mg TID | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐3.50 [‐25.47, 18.47] |
| 8.2 Acarbose 100 mg TID | 11 | 882 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐8.60, 8.73] |
| 8.3 Acarbose 200 mg TID | 2 | 242 | Mean Difference (IV, Random, 95% CI) | 4.59 [‐20.63, 29.82] |
| 8.4 Acarbose 300 mg TID | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐16.35 [‐43.24, 10.54] |
| 9 Change in post‐load insulin levels (pmol/l) | 10 | 1050 | Mean Difference (IV, Random, 95% CI) | ‐40.82 [‐60.64, ‐21.01] |
| 9.1 Acarbose 50 mg TID | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 40.8 [‐90.43, 172.03] |
| 9.2 Acarbose 100 mg TID | 9 | 673 | Mean Difference (IV, Random, 95% CI) | ‐45.83 [‐71.68, ‐19.98] |
| 9.3 Acarbose 200 mg TID | 2 | 239 | Mean Difference (IV, Random, 95% CI) | ‐15.46 [‐58.62, 27.69] |
| 9.4 Acarbose 300 mg TID | 1 | 114 | Mean Difference (IV, Random, 95% CI) | ‐62.4 [‐113.24, ‐11.56] |
| 10 Change in fasting C‐peptide levels (nmol/l) | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 10.1 Acarbose 100 mg TID | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 11 Change in post‐load C‐peptide levels (nmol/l) | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 11.1 Acarbose 100 mg TID | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 12 Change in body weight (Kg) | 14 | 1451 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.46, 0.20] |
| 12.1 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐19.48, 20.08] |
| 12.2 Acarbose 100 mg TID | 10 | 864 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.61, 0.42] |
| 12.3 Acarbose 200‐100‐200 | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐9.94, 8.14] |
| 12.4 Acarbose 200 mg TID | 2 | 245 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.86, 0.39] |
| 12.5 Acarbose 300 mg TID | 2 | 305 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.67, 0.51] |
| 13 Change in body mass index (Kg/m2) | 10 | 1430 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.25, ‐0.08] |
| 13.1 Acarbose 25 mg TID | 1 | 177 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.25, 0.19] |
| 13.2 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐6.61, 6.81] |
| 13.3 Acarbose 50 mg TID | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.17] |
| 13.4 Acarbose 100 mg TID | 9 | 842 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.37, ‐0.13] |
| 13.5 Acarbose 200 mg TID | 1 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.38, 0.08] |
| 14 Total deaths | 2 | 385 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.29, 4.22] |
| 14.1 Acarbose 100 mg TID | 1 | 256 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.29, 4.22] |
| 14.2 Acarbose 200 mg TID | 1 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Disease related deaths | 1 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Acarbose 200 mg TID | 1 | 129 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Occurence of morbidity (total) | 0 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Acarbose 200 mg TID | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Occurence of morbidity (disease specific) | 0 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Acarbose 200 mg TID | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Occurence of adverse effects | 16 | 3819 | Odds Ratio (M‐H, Random, 95% CI) | 3.37 [2.60, 4.36] |
| 18.1 Acarbose 25 mg TID | 1 | 199 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.90, 2.83] |
| 18.2 Acarbose 50 mg TID | 3 | 775 | Odds Ratio (M‐H, Random, 95% CI) | 2.11 [1.29, 3.47] |
| 18.3 Acarbose 100 mg TID | 14 | 2003 | Odds Ratio (M‐H, Random, 95% CI) | 3.38 [2.53, 4.52] |
| 18.4 Acarbose 200 mg TID | 3 | 486 | Odds Ratio (M‐H, Random, 95% CI) | 6.97 [4.01, 12.12] |
| 18.5 Acarbose 300 mg TID | 2 | 356 | Odds Ratio (M‐H, Random, 95% CI) | 3.78 [1.38, 10.37] |
| 19 Occurence of gastro‐intestinal adverse effects | 3 | 1442 | Odds Ratio (M‐H, Random, 95% CI) | 3.30 [2.31, 4.71] |
| 19.1 Acarbose 50 mg TID | 1 | 522 | Odds Ratio (M‐H, Random, 95% CI) | 2.72 [1.91, 3.88] |
| 19.2 Acarbose 100 mg TID | 2 | 774 | Odds Ratio (M‐H, Random, 95% CI) | 2.82 [2.08, 3.82] |
| 19.3 Acarbose 200 mg TID | 1 | 146 | Odds Ratio (M‐H, Random, 95% CI) | 7.39 [3.51, 15.59] |
| 20 Quality of life | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Change in post‐load blood glucose (mmol/l) (2‐hours) | 16 | 2243 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐2.67, ‐1.88] |
| 21.1 Acarbose 25 mg TID | 1 | 176 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐2.14, ‐0.58] |
| 21.2 Acarbose 50 mg BID | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐1.8 [‐3.23, ‐0.37] |
| 21.3 Acarbose 50 mg TID | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.35, ‐0.84] |
| 21.4 Acarbose 100 mg TID | 13 | 1126 | Mean Difference (IV, Random, 95% CI) | ‐2.22 [‐2.75, ‐1.70] |
| 21.5 Acarbose 200 mg TID | 3 | 411 | Mean Difference (IV, Random, 95% CI) | ‐2.83 [‐3.78, ‐1.88] |
| 21.6 Acarbose 300 mg TID | 2 | 294 | Mean Difference (IV, Random, 95% CI) | ‐3.54 [‐5.12, ‐1.96] |
| 22 Change in post‐load insulin levels (pmol/l) (2‐hours) | 10 | 1057 | Mean Difference (IV, Random, 95% CI) | ‐38.83 [‐58.77, ‐18.89] |
| 22.1 Acarbose 50 mg TID | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 55.9 [‐76.79, 188.59] |
| 22.2 Acarbose 100 mg TID | 9 | 675 | Mean Difference (IV, Random, 95% CI) | ‐45.71 [‐69.57, ‐21.85] |
| 22.3 Acarbose 200 mg TID | 2 | 242 | Mean Difference (IV, Random, 95% CI) | ‐6.29 [‐61.94, 49.36] |
| 22.4 Acarbose 300 mg TID | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐39.47 [‐109.73, 30.79] |
1.1. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 1 Change in glycated haemoglobin (%).
1.2. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 2 Change in fasting blood glucose (mmol/l).
1.3. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 3 Change in post‐load blood glucose (mmol/l).
1.4. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 4 Change in total cholesterol (mmol/l).
1.5. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 5 Change in HDL‐cholesterol (mmol/l).
1.6. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 6 Change in LDL‐cholesterol (mmol/l).
1.7. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 7 Change in triglycerides (mmol/l).
1.8. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 8 Change in fasting insulin levels (pmol/l).
1.9. Analysis.
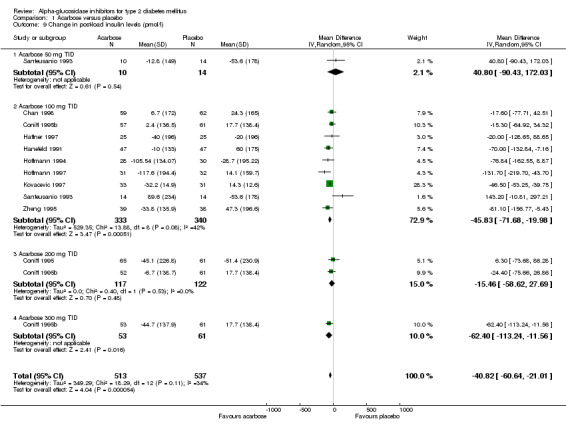
Comparison 1 Acarbose versus placebo, Outcome 9 Change in post‐load insulin levels (pmol/l).
1.10. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 10 Change in fasting C‐peptide levels (nmol/l).
1.11. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 11 Change in post‐load C‐peptide levels (nmol/l).
1.12. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 12 Change in body weight (Kg).
1.13. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 13 Change in body mass index (Kg/m2).
1.14. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 14 Total deaths.
1.15. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 15 Disease related deaths.
1.18. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 18 Occurence of adverse effects.
1.19. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 19 Occurence of gastro‐intestinal adverse effects.
1.21. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 21 Change in post‐load blood glucose (mmol/l) (2‐hours).
1.22. Analysis.

Comparison 1 Acarbose versus placebo, Outcome 22 Change in post‐load insulin levels (pmol/l) (2‐hours).
Comparison 2. Acarbose versus sulphonylurea (SU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 8 | 596 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.02, 0.77] |
| 1.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 0.7 [0.18, 1.22] |
| 1.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.39 [0.03, 0.75] |
| 1.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 1.3 [0.57, 2.03] |
| 1.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 4 | 279 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.43, 0.58] |
| 1.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.37, 1.13] |
| 2 Change in fasting blood glucose (mmol/l) | 8 | 596 | Mean Difference (IV, Random, 95% CI) | 0.69 [0.16, 1.23] |
| 2.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 1.4 [0.34, 2.46] |
| 2.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.16, 1.98] |
| 2.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 2.5 [0.69, 4.31] |
| 2.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 4 | 279 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.29, 0.69] |
| 2.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.57, 1.95] |
| 3 Change in post‐load blood glucose (mmol/l) | 8 | 591 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.43, 0.22] |
| 3.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.66, 2.66] |
| 3.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.95, 1.61] |
| 3.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐2.87, 4.47] |
| 3.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.46, 0.16] |
| 3.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐3.36, 0.22] |
| 4 Change in total cholesterol (mmol/l) | 7 | 499 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 4.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.19, 0.39] |
| 4.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.53, 0.01] |
| 4.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.40, 0.70] |
| 4.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 3 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.10] |
| 4.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.59, 0.41] |
| 5 Change in HDL‐cholesterol (mmol/l) | 7 | 485 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 5.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 5.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.08] |
| 5.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.21, 0.23] |
| 5.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 3 | 216 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| 5.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.13, 0.17] |
| 6 Change in LDL‐cholesterol (mmol/l) | 4 | 312 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.07, 0.27] |
| 6.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 0.2 [‐0.07, 0.47] |
| 6.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 95 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.26, 0.28] |
| 6.3 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 1 | 95 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.63, 0.67] |
| 6.4 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.36, 0.54] |
| 7 Change in triglycerides (mmol/l) | 8 | 591 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.18, 0.20] |
| 7.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.79, 0.99] |
| 7.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.11, 0.19] |
| 7.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.4 [‐0.20, 1.00] |
| 7.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 4 | 290 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.17, 0.29] |
| 7.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.86, 0.24] |
| 8 Change in fasting insulin levels (pmol/l) | 7 | 486 | Mean Difference (IV, Random, 95% CI) | ‐24.78 [‐43.30, ‐6.26] |
| 8.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 63 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐39.50, 36.50] |
| 8.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐25.40 [‐63.97, 13.17] |
| 8.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐54.97, 54.97] |
| 8.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 3 | 184 | Mean Difference (IV, Random, 95% CI) | ‐35.03 [‐88.53, 18.47] |
| 8.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐34.81 [‐65.98, ‐3.64] |
| 9 Change in post‐load insulin levels (pmol/l) | 7 | 483 | Mean Difference (IV, Random, 95% CI) | ‐133.17 [‐184.53, ‐81.82] |
| 9.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose (1 hour pp) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐18.9 [‐126.62, 88.82] |
| 9.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐214.1 [‐291.77, ‐136.43] |
| 9.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐180.0 [‐312.44, ‐47.56] |
| 9.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 3 | 184 | Mean Difference (IV, Random, 95% CI) | ‐100.66 [‐124.60, ‐76.72] |
| 9.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐172.38 [‐280.31, ‐64.45] |
| 10 Change in fasting C‐peptide levels (nmol/l) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.51, 0.15] |
| 10.1 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.51, 0.15] |
| 11 Change in post‐load C‐peptide levels (nmol/l) | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.94, 0.22] |
| 11.1 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.94, 0.22] |
| 12 Change in body weight (Kg) | 5 | 397 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐4.01, 0.21] |
| 12.1 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 132 | Mean Difference (IV, Random, 95% CI) | ‐3.26 [‐4.22, ‐2.30] |
| 12.2 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐3.1 [‐10.33, 4.13] |
| 12.3 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 3 | 213 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.19, 0.06] |
| 13 Change in body mass index (Kg/m2) | 4 | 230 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.83, 0.05] |
| 13.1 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.1 [‐3.23, 1.03] |
| 13.2 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 2 | 121 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.31, 0.56] |
| 13.3 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.6 [‐1.15, ‐0.05] |
| 14 Total deaths | 1 | 133 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.08] |
| 14.1 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 133 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.08] |
| 15 Disease related deaths | 1 | 133 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.08] |
| 15.1 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 133 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.08] |
| 16 Occurence of adverse effects | 7 | 607 | Odds Ratio (M‐H, Random, 95% CI) | 3.95 [2.00, 7.80] |
| 16.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 2.54 [1.07, 6.03] |
| 16.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 6.61 [2.66, 16.44] |
| 16.3 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 4 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 4.88 [1.37, 17.37] |
| 16.4 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 2.0 [0.60, 6.64] |
| 17 Occurence of gastro‐intestinal adverse effects | 1 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 7.70 [3.64, 16.31] |
| 17.1 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 145 | Odds Ratio (M‐H, Random, 95% CI) | 7.70 [3.64, 16.31] |
| 18 Change in post‐load blood glucose (mmol/l) (2 hours) | 8 | 591 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.42, 0.53] |
| 18.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.66, 2.66] |
| 18.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 1.39 [‐0.10, 2.88] |
| 18.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐2.87, 4.47] |
| 18.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.46, 0.16] |
| 18.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐3.36, 0.22] |
| 19 Change in post‐load insulin levels (pmol/l) (2 hours) | 7 | 484 | Mean Difference (IV, Random, 95% CI) | ‐115.84 [‐152.52, ‐79.15] |
| 19.1 Acarbose 100 mg TID vs Tolbutamide 2000 mg in 3 dose (1 hour pp) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐18.9 [‐126.62, 88.82] |
| 19.2 Acarbose 200 mg TID vs Tolbutamide 1000 mg TID | 1 | 131 | Mean Difference (IV, Random, 95% CI) | ‐148.0 [‐235.51, ‐60.49] |
| 19.3 Acarbose 100 mg TID vs Glibenclamide 1 mg TID | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐180.0 [‐312.44, ‐47.56] |
| 19.4 Acarbose 100 mg TID vs Glibenclamide 3,5 mg TID | 3 | 184 | Mean Difference (IV, Random, 95% CI) | ‐100.66 [‐124.60, ‐76.72] |
| 19.5 Acarbose 100 mg TID vs Gliclazide 80 mg BID | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐172.38 [‐280.31, ‐64.45] |
2.1. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 1 Change in glycated haemoglobin (%).
2.2. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 2 Change in fasting blood glucose (mmol/l).
2.3. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 3 Change in post‐load blood glucose (mmol/l).
2.4. Analysis.
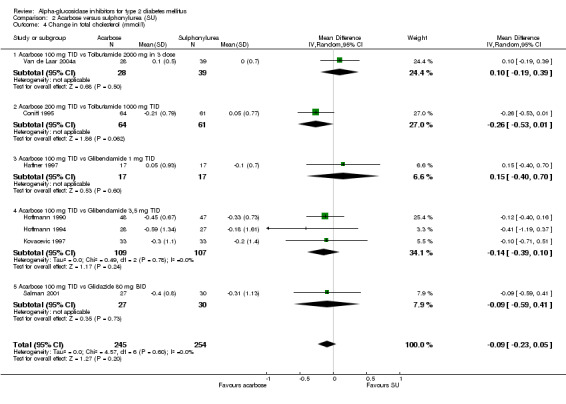
Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 4 Change in total cholesterol (mmol/l).
2.5. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 5 Change in HDL‐cholesterol (mmol/l).
2.6. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 6 Change in LDL‐cholesterol (mmol/l).
2.7. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 7 Change in triglycerides (mmol/l).
2.8. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 8 Change in fasting insulin levels (pmol/l).
2.9. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 9 Change in post‐load insulin levels (pmol/l).
2.10. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 10 Change in fasting C‐peptide levels (nmol/l).
2.11. Analysis.
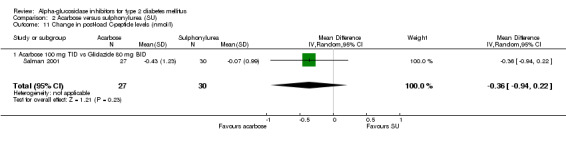
Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 11 Change in post‐load C‐peptide levels (nmol/l).
2.12. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 12 Change in body weight (Kg).
2.13. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 13 Change in body mass index (Kg/m2).
2.14. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 14 Total deaths.
2.15. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 15 Disease related deaths.
2.16. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 16 Occurence of adverse effects.
2.17. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 17 Occurence of gastro‐intestinal adverse effects.
2.18. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 18 Change in post‐load blood glucose (mmol/l) (2 hours).
2.19. Analysis.

Comparison 2 Acarbose versus sulphonylurea (SU), Outcome 19 Change in post‐load insulin levels (pmol/l) (2 hours).
Comparison 3. Acarbose versus Metformin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.61, 0.11] |
| 1.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.61, 0.11] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.74, ‐0.04] |
| 2.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.74, ‐0.04] |
| 3 Change in post‐load blood glucose (mmol/l) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.79, ‐0.05] |
| 3.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.79, ‐0.05] |
| 4 Change in total cholesterol (mmol/l) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.66, ‐0.22] |
| 4.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.66, ‐0.22] |
| 5 Change in HDL‐cholesterol (mmol/l) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.02, 0.50] |
| 5.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.02, 0.50] |
| 6 Change in LDL‐cholesterol (mmol/l) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.52, ‐0.36] |
| 6.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.52, ‐0.36] |
| 7 Change in triglycerides (mmol/l) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.80, 0.24] |
| 7.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.80, 0.24] |
| 8 Change in fasting insulin levels (pmol/l) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 33.8 [‐28.24, 95.84] |
| 8.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 33.8 [‐28.24, 95.84] |
| 9 Change in post‐load insulin levels (pmol/l) | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 115.30 [‐13.22, 243.82] |
| 9.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 115.30 [‐13.22, 243.82] |
| 10 Change in body weight (Kg) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐5.45, 4.85] |
| 10.1 Acarbose 100 mg TID versus Metformin 850 mg BID | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐5.45, 4.85] |
| 11 Occurence of adverse effects | 1 | 64 | Odds Ratio (M‐H, Random, 95% CI) | 15.0 [3.06, 73.58] |
3.1. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 1 Change in glycated haemoglobin (%).
3.2. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 2 Change in fasting blood glucose (mmol/l).
3.3. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 3 Change in post‐load blood glucose (mmol/l).
3.4. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 4 Change in total cholesterol (mmol/l).
3.5. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 5 Change in HDL‐cholesterol (mmol/l).
3.6. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 6 Change in LDL‐cholesterol (mmol/l).
3.7. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 7 Change in triglycerides (mmol/l).
3.8. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 8 Change in fasting insulin levels (pmol/l).
3.9. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 9 Change in post‐load insulin levels (pmol/l).
3.10. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 10 Change in body weight (Kg).
3.11. Analysis.

Comparison 3 Acarbose versus Metformin, Outcome 11 Occurence of adverse effects.
Comparison 4. Acarbose versus nateglinide / repaglinide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 179 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.19, 0.25] |
| 1.1 Acarbose 100 mg TID versus Nateglinide 120 mg TID | 1 | 179 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.19, 0.25] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐1.10, 1.06] |
| 2.1 Acarbose 100 mg TID versus Nateglinide 120 mg TID | 1 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐1.10, 1.06] |
| 3 Change in body weight (Kg) | 1 | 169 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.30, ‐0.06] |
| 3.1 Acarbose 100 mg TID versus Nateglinide 120 mg TID | 1 | 169 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.30, ‐0.06] |
| 4 Occurence of adverse effects | 1 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [1.05, 3.50] |
| 4.1 Acarbose 100 mg TID versus Nateglinide 120 mg TID | 1 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 1.92 [1.05, 3.50] |
| 5 Occurence of gastro‐intestinal adverse effects | 1 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 3.22 [1.66, 6.24] |
| 5.1 Acarbose 100 mg TID versus Nateglinide 120 mg TID | 1 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 3.22 [1.66, 6.24] |
4.1. Analysis.
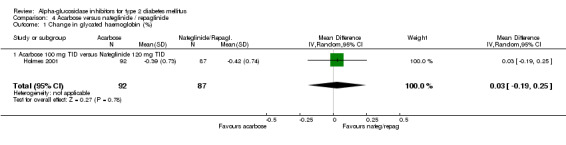
Comparison 4 Acarbose versus nateglinide / repaglinide, Outcome 1 Change in glycated haemoglobin (%).
4.2. Analysis.

Comparison 4 Acarbose versus nateglinide / repaglinide, Outcome 2 Change in fasting blood glucose (mmol/l).
4.3. Analysis.

Comparison 4 Acarbose versus nateglinide / repaglinide, Outcome 3 Change in body weight (Kg).
4.4. Analysis.

Comparison 4 Acarbose versus nateglinide / repaglinide, Outcome 4 Occurence of adverse effects.
4.5. Analysis.
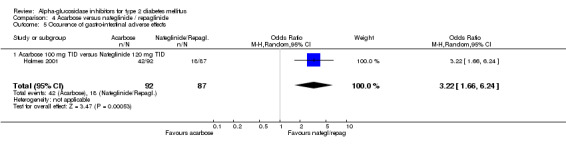
Comparison 4 Acarbose versus nateglinide / repaglinide, Outcome 5 Occurence of gastro‐intestinal adverse effects.
Comparison 5. Miglitol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 4 | 1088 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.93, ‐0.44] |
| 1.1 Miglitol 25 mg TID | 1 | 171 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.84, ‐0.08] |
| 1.2 Miglitol 50 mg TID | 2 | 413 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.72, ‐0.43] |
| 1.3 Miglitol 100 mg TID | 3 | 359 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.35, ‐0.22] |
| 1.4 Miglitol 200 mg TID | 1 | 145 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐1.67, ‐0.85] |
| 2 Change in fasting blood glucose (mmol/l) | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.88, ‐0.16] |
| 2.1 Miglitol 50 mg TID | 1 | 236 | Mean Difference (IV, Random, 95% CI) | ‐0.6 [‐0.95, ‐0.25] |
| 2.2 Miglitol 100 mg TID (max) | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐0.1 [‐0.98, 0.78] |
| 3 Change in post‐load blood glucose (mmol/l) | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.54, 0.14] |
| 3.1 Miglitol 50 mg TID | 1 | 236 | Mean Difference (IV, Random, 95% CI) | ‐4.1 [‐4.68, ‐3.52] |
| 3.2 Miglitol 100 mg TID (max) | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.2 [‐2.39, ‐0.01] |
| 4 Change in fasting insulin levels (pmol/l) | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐18.2 [‐57.01, 20.61] |
| 4.1 Miglitol 50 mg TID | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Miglitol 100 mg TID | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐18.2 [‐57.01, 20.61] |
| 5 Change in post‐load insulin levels (pmol/l) | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐16.62 [‐39.23, 6.00] |
| 5.1 Miglitol 50 mg TID | 1 | 236 | Mean Difference (IV, Random, 95% CI) | ‐15.80 [‐41.15, 9.55] |
| 5.2 Miglitol 100 mg TID | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐19.80 [‐69.83, 30.23] |
| 6 Change in body weight (Kg) | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.50, 1.04] |
| 6.1 Miglitol 100 mg TID | 1 | 162 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.50, 1.04] |
| 7 Total deaths | 1 | 408 | Odds Ratio (M‐H, Random, 95% CI) | 2.97 [0.31, 28.80] |
| 7.1 Miglitol 25 mg TID | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 2.94 [0.12, 73.07] |
| 7.2 Miglitol 50 mg TID | 1 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 74.52] |
| 8 Disease related deaths | 1 | 408 | Odds Ratio (M‐H, Random, 95% CI) | 2.94 [0.12, 73.07] |
| 8.1 Miglitol 25 mg TID | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 2.94 [0.12, 73.07] |
| 8.2 Miglitol 50 mg TID | 1 | 203 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Occurence of adverse effects | 4 | 1304 | Odds Ratio (M‐H, Random, 95% CI) | 4.01 [1.69, 9.52] |
| 9.1 Miglitol 25 mg TID | 1 | 185 | Odds Ratio (M‐H, Random, 95% CI) | 3.17 [0.62, 16.16] |
| 9.2 Miglitol 50 mg TID | 2 | 449 | Odds Ratio (M‐H, Random, 95% CI) | 1.79 [1.05, 3.03] |
| 9.3 Miglitol 100 mg TID | 3 | 484 | Odds Ratio (M‐H, Random, 95% CI) | 3.93 [0.96, 16.12] |
| 9.4 Miglitol 200 mg TID | 1 | 186 | Odds Ratio (M‐H, Random, 95% CI) | 34.34 [7.98, 147.86] |
| 10 Occurence of gastro‐intestinal adverse effects | 2 | 428 | Odds Ratio (M‐H, Random, 95% CI) | 3.12 [1.62, 6.02] |
| 10.1 Miglitol 50 mg TID | 1 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 2.30 [1.36, 3.89] |
| 10.2 Miglitol 100 mg TID | 1 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 4.5 [2.34, 8.67] |
| 11 Change in post‐load blood glucose (mmol/l) (2‐hours) | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.25, ‐1.07] |
| 11.1 Miglitol 50 mg TID | 1 | 236 | Mean Difference (IV, Random, 95% CI) | ‐1.7 [‐2.36, ‐1.04] |
| 11.2 Miglitol 100 mg TID (max) | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐2.81, ‐0.19] |
| 12 Change in post‐load insulin levels (pmol/l) (2‐hours) | 2 | 398 | Mean Difference (IV, Random, 95% CI) | ‐15.69 [‐38.62, 7.24] |
| 12.1 Miglitol 50 mg TID | 1 | 236 | Mean Difference (IV, Random, 95% CI) | ‐15.80 [‐41.15, 9.55] |
| 12.2 Miglitol 100 mg TID | 1 | 162 | Mean Difference (IV, Random, 95% CI) | ‐15.20 [‐68.99, 38.59] |
5.1. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 1 Change in glycated haemoglobin (%).
5.2. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 2 Change in fasting blood glucose (mmol/l).
5.3. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 3 Change in post‐load blood glucose (mmol/l).
5.4. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 4 Change in fasting insulin levels (pmol/l).
5.5. Analysis.
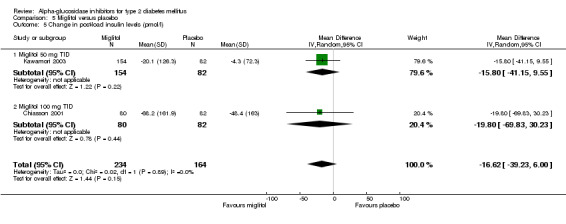
Comparison 5 Miglitol versus placebo, Outcome 5 Change in post‐load insulin levels (pmol/l).
5.6. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 6 Change in body weight (Kg).
5.7. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 7 Total deaths.
5.8. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 8 Disease related deaths.
5.9. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 9 Occurence of adverse effects.
5.10. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 10 Occurence of gastro‐intestinal adverse effects.
5.11. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 11 Change in post‐load blood glucose (mmol/l) (2‐hours).
5.12. Analysis.

Comparison 5 Miglitol versus placebo, Outcome 12 Change in post‐load insulin levels (pmol/l) (2‐hours).
Comparison 6. Miglitol versus sulphonylurea (SU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.16, 0.96] |
| 1.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.16, 0.96] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.74, 1.28] |
| 2.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.74, 1.28] |
| 3 Change in post‐load blood glucose (mmol/l) | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.43, 2.23] |
| 3.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.43, 2.23] |
| 4 Change in total cholesterol (mmol/l) | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.29, 0.45] |
| 4.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.29, 0.45] |
| 5 Change in HDL‐cholesterol (mmol/l) | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.26, 0.24] |
| 5.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.26, 0.24] |
| 6 Change in triglycerides (mmol/l) | 1 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.40, 0.32] |
| 6.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.40, 0.32] |
| 7 Change in fasting insulin levels (pmol/l) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐44.75 [‐53.72, ‐35.78] |
| 7.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐44.75 [‐53.72, ‐35.78] |
| 8 Change in body weight (Kg) | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.48, 1.40] |
| 8.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐0.48, 1.40] |
| 9 Total deaths | 1 | 414 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.09, 2.76] |
| 9.1 Miglitol 25 mg versus Glyburide 20 mg 1dd | 1 | 208 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.04, 5.55] |
| 9.2 Miglitol 50 mg versus Glyburide 20 mg 1dd | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 5.66] |
| 10 Disease related deaths | 1 | 414 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 5.14] |
| 10.1 Miglitol 25 mg versus Glyburide 20 mg 1dd | 1 | 208 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 16.20] |
| 10.2 Miglitol 50 mg versus Glyburide 20 mg 1dd | 1 | 206 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.36] |
| 11 Occurence of adverse effects | 2 | 232 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.69, 2.41] |
| 11.1 Miglitol 100 mg TID versus Glibenclamide 5 mg BID | 1 | 96 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.35, 2.54] |
| 11.2 Miglitol 100 mg TID versus Glibenclamide 3,5 mg BID | 1 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [0.70, 3.56] |
6.1. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 1 Change in glycated haemoglobin (%).
6.2. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 2 Change in fasting blood glucose (mmol/l).
6.3. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 3 Change in post‐load blood glucose (mmol/l).
6.4. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 4 Change in total cholesterol (mmol/l).
6.5. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 5 Change in HDL‐cholesterol (mmol/l).
6.6. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 6 Change in triglycerides (mmol/l).
6.7. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 7 Change in fasting insulin levels (pmol/l).
6.8. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 8 Change in body weight (Kg).
6.9. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 9 Total deaths.
6.10. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 10 Disease related deaths.
6.11. Analysis.

Comparison 6 Miglitol versus sulphonylurea (SU), Outcome 11 Occurence of adverse effects.
Comparison 7. Miglitol versus metformin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.56, 1.18] |
| 1.1 miglitol 100 mg TID vs metformin 500 TID (maximum) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.56, 1.18] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 1.0 [0.18, 1.82] |
| 2.1 Miglitol 100 mg TID vs Metformin 500 mg TID | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 1.0 [0.18, 1.82] |
| 3 Change in post‐load blood glucose (mmol/l) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.43, 1.83] |
| 3.1 Miglitol 100 mg TID vs Metformin 500 mg TID | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.43, 1.83] |
| 4 Change in fasting insulin levels (pmol/l) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐30.04, 27.84] |
| 4.1 Migitol 100 mg TID vs Metformin 500 mg TID | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐30.04, 27.84] |
| 5 Change in post‐load insulin levels (pmol/l) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐48.30 [‐94.38, ‐2.22] |
| 5.1 Miglitol 100 mg (max) TID vs Metformin 500 mg TID | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐48.30 [‐94.38, ‐2.22] |
| 6 Change in body weight (Kg) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 6.1 Miglitol 100 mg TID vs Metformin 500 mg TID | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 7 Occurence of gastro‐intestinal side‐effects | 1 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.83, 3.05] |
| 7.1 Miglitol 100 mg TID vs Metformin 500 mg TID | 1 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 1.60 [0.83, 3.05] |
| 8 Occurence of adverse effects | 1 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.39, 7.31] |
| 8.1 Miglitol 100 mg TID vs Metformin 500 mg TID, Total side effects | 1 | 165 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.39, 7.31] |
| 9 Change in post‐load blood glucose (mmol/l) (2 hours) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.8 [‐0.45, 2.05] |
| 9.1 Miglitol 100 mg TID vs Metformin 500 mg TID | 1 | 161 | Mean Difference (IV, Random, 95% CI) | 0.8 [‐0.45, 2.05] |
| 10 Change in post‐load insulin levels (pmol/l) (2‐hours) | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐67.2 [‐115.65, ‐18.75] |
| 10.1 Miglitol 100 mg (max) TID vs Metformin 500 mg TID | 1 | 161 | Mean Difference (IV, Random, 95% CI) | ‐67.2 [‐115.65, ‐18.75] |
7.1. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 1 Change in glycated haemoglobin (%).
7.2. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 2 Change in fasting blood glucose (mmol/l).
7.3. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 3 Change in post‐load blood glucose (mmol/l).
7.4. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 4 Change in fasting insulin levels (pmol/l).
7.5. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 5 Change in post‐load insulin levels (pmol/l).
7.6. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 6 Change in body weight (Kg).
7.7. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 7 Occurence of gastro‐intestinal side‐effects.
7.8. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 8 Occurence of adverse effects.
7.9. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 9 Change in post‐load blood glucose (mmol/l) (2 hours).
7.10. Analysis.

Comparison 7 Miglitol versus metformin, Outcome 10 Change in post‐load insulin levels (pmol/l) (2‐hours).
Comparison 8. Voglibose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 238 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.63, ‐0.31] |
| 1.1 Voglibose 0.2 mg TID | 1 | 238 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.63, ‐0.31] |
| 1.2 Voglibose 0,3 mg TID | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐0.6 [‐0.97, ‐0.23] |
| 2.1 Voglibose 0.2 mg TID | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐0.6 [‐0.97, ‐0.23] |
| 2.2 Voglibose 0,3 mg TID | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in post‐load blood glucose (mmol/l) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.4 [‐2.97, ‐1.83] |
| 3.1 Voglibose 0.2 mg TID | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐2.4 [‐2.97, ‐1.83] |
| 4 Change in post‐load insulin levels (pmol/l) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐12.90 [‐37.06, 11.26] |
| 4.1 Voglibose 0.2 mg TID | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐12.90 [‐37.06, 11.26] |
| 5 Occurence of adverse effects | 1 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.67, 1.97] |
| 5.1 Voglibose 0,2 mg TID | 1 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.67, 1.97] |
| 6 Occurence of gastro‐intestinal adverse effects | 1 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [0.96, 2.75] |
| 6.1 Voglibose 0,2 mg TID | 1 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [0.96, 2.75] |
| 7 Change in post‐load blood glucose (mmol/l) (2 hours) | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐1.7 [‐2.37, ‐1.03] |
| 7.1 Voglibose 0.2 mg TID | 1 | 234 | Mean Difference (IV, Random, 95% CI) | ‐1.7 [‐2.37, ‐1.03] |
8.1. Analysis.

Comparison 8 Voglibose versus placebo, Outcome 1 Change in glycated haemoglobin (%).
8.2. Analysis.

Comparison 8 Voglibose versus placebo, Outcome 2 Change in fasting blood glucose (mmol/l).
8.3. Analysis.

Comparison 8 Voglibose versus placebo, Outcome 3 Change in post‐load blood glucose (mmol/l).
8.4. Analysis.

Comparison 8 Voglibose versus placebo, Outcome 4 Change in post‐load insulin levels (pmol/l).
8.5. Analysis.

Comparison 8 Voglibose versus placebo, Outcome 5 Occurence of adverse effects.
8.6. Analysis.

Comparison 8 Voglibose versus placebo, Outcome 6 Occurence of gastro‐intestinal adverse effects.
8.7. Analysis.

Comparison 8 Voglibose versus placebo, Outcome 7 Change in post‐load blood glucose (mmol/l) (2 hours).
Comparison 9. Voglibose versus diet therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.15, 1.15] |
| 1.1 Voglibose 0,3 mg TID | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.15, 1.15] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐2.4 [‐4.58, ‐0.22] |
| 2.1 Voglibose 0,3 mg TID | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐2.4 [‐4.58, ‐0.22] |
| 3 Change in total cholesterol (mmol/l) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.64, 0.24] |
| 3.1 Voglibose 0,3 mg TID | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐1.64, 0.24] |
| 4 Change in HDL‐cholesterol (mmol/l) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.4 [‐0.81, 0.01] |
| 4.1 Voglibose 0,3 mg TID | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.4 [‐0.81, 0.01] |
| 5 Change in fasting insulin levels (pmol/l) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐19.22, 31.22] |
| 5.1 Voglibose 0,3 mg TID | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐19.22, 31.22] |
| 6 Change in body weight (Kg) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.99, 5.39] |
| 6.1 Voglibose 0,3 mg TID | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐4.99, 5.39] |
| 7 Change in body mass index (Kg/m2) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.26, 2.26] |
| 7.1 Voglibose 0,3 mg TID | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.26, 2.26] |
9.1. Analysis.

Comparison 9 Voglibose versus diet therapy, Outcome 1 Change in glycated haemoglobin (%).
9.2. Analysis.

Comparison 9 Voglibose versus diet therapy, Outcome 2 Change in fasting blood glucose (mmol/l).
9.3. Analysis.

Comparison 9 Voglibose versus diet therapy, Outcome 3 Change in total cholesterol (mmol/l).
9.4. Analysis.

Comparison 9 Voglibose versus diet therapy, Outcome 4 Change in HDL‐cholesterol (mmol/l).
9.5. Analysis.

Comparison 9 Voglibose versus diet therapy, Outcome 5 Change in fasting insulin levels (pmol/l).
9.6. Analysis.

Comparison 9 Voglibose versus diet therapy, Outcome 6 Change in body weight (Kg).
9.7. Analysis.

Comparison 9 Voglibose versus diet therapy, Outcome 7 Change in body mass index (Kg/m2).
Comparison 10. .Voglibose versus sulphonylurea (SU).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 1.3 [‐0.45, 3.05] |
| 1.1 Voglibose 0,3 mg TID vs Glyburide 1,25 mg once daily | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 1.3 [‐0.45, 3.05] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐3.15, 2.15] |
| 2.1 Voglibose 0,3 mg TID vs Glyburide 1,25 mg once daily | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐3.15, 2.15] |
| 3 Change in total cholesterol (mmol/l) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.13, 1.33] |
| 3.1 Voglibose 0,3 mg TID vs Glyburide 1,25 mg once daily | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.13, 1.33] |
| 4 Change in HDL‐cholesterol (mmol/l) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.2 [‐0.59, 0.19] |
| 4.1 Voglibose 0,3 mg TID vs Glyburide 1,25 mg once daily | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐0.2 [‐0.59, 0.19] |
| 5 Change in fasting insulin levels (pmol/l) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐11.8 [‐25.49, 1.89] |
| 5.1 Voglibose 0,3 mg TID vs Glyburide 1,25 mg once daily | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐11.8 [‐25.49, 1.89] |
| 6 Change in body weight (Kg) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐9.73, 10.93] |
| 6.1 Voglibose 0,3 mg TID vs Glyburide 1,25 mg once daily | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐9.73, 10.93] |
| 7 Change in body mass index (Kg/m2) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.40, 2.40] |
| 7.1 Voglibose 0,3 mg TID vs Glyburide 1,25 mg once daily | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.40, 2.40] |
10.1. Analysis.

Comparison 10 .Voglibose versus sulphonylurea (SU), Outcome 1 Change in glycated haemoglobin (%).
10.2. Analysis.
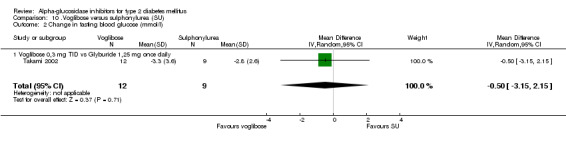
Comparison 10 .Voglibose versus sulphonylurea (SU), Outcome 2 Change in fasting blood glucose (mmol/l).
10.3. Analysis.
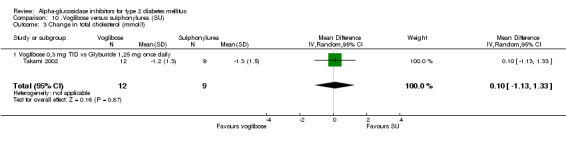
Comparison 10 .Voglibose versus sulphonylurea (SU), Outcome 3 Change in total cholesterol (mmol/l).
10.4. Analysis.

Comparison 10 .Voglibose versus sulphonylurea (SU), Outcome 4 Change in HDL‐cholesterol (mmol/l).
10.5. Analysis.
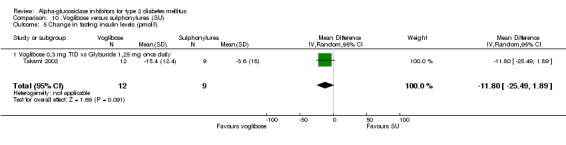
Comparison 10 .Voglibose versus sulphonylurea (SU), Outcome 5 Change in fasting insulin levels (pmol/l).
10.6. Analysis.

Comparison 10 .Voglibose versus sulphonylurea (SU), Outcome 6 Change in body weight (Kg).
10.7. Analysis.
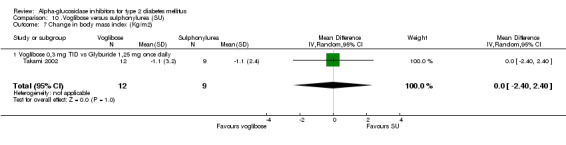
Comparison 10 .Voglibose versus sulphonylurea (SU), Outcome 7 Change in body mass index (Kg/m2).
Comparison 11. Miglitol versus voglibose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in glycated haemoglobin (%) | 1 | 312 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.24, ‐0.02] |
| 1.1 Miglitol 50 mg TID versus Voglibose 0.2 mg TID | 1 | 312 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.24, ‐0.02] |
| 2 Change in fasting blood glucose (mmol/l) | 1 | 306 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.31, 0.31] |
| 2.1 Miglitol 50 mg TID versus Voglibose 0.2 mg TID | 1 | 306 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.31, 0.31] |
| 3 Change in post‐load blood glucose (mmol/l) | 1 | 306 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.27, ‐1.13] |
| 3.1 Miglitol 50 mg TID versus Voglibose 0.2 mg TID | 1 | 306 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐2.27, ‐1.13] |
| 4 Change in post‐load insulin levels (pmol/l) | 1 | 306 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐30.04, 24.24] |
| 4.1 Miglitol 50 mg TID versus Voglibose 0.2 mg TID | 1 | 306 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐30.04, 24.24] |
| 5 Occurence of adverse effects | 1 | 348 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.96, 2.45] |
| 5.1 Miglitol 50 mg TID versus Voglibose 0.2 mg TID | 1 | 348 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.96, 2.45] |
| 6 Occurence of gastro‐intestinal adverse effects | 1 | 348 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.93, 2.16] |
| 6.1 Miglitol 50 mg TID versus Voglibose 0.2 mg TID | 1 | 348 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.93, 2.16] |
| 7 Change in post‐load blood glucose (mmol/l) (2 hours) | 1 | 312 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.61, 0.61] |
11.1. Analysis.
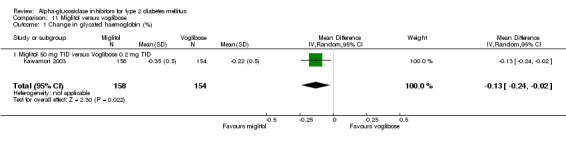
Comparison 11 Miglitol versus voglibose, Outcome 1 Change in glycated haemoglobin (%).
11.2. Analysis.

Comparison 11 Miglitol versus voglibose, Outcome 2 Change in fasting blood glucose (mmol/l).
11.3. Analysis.
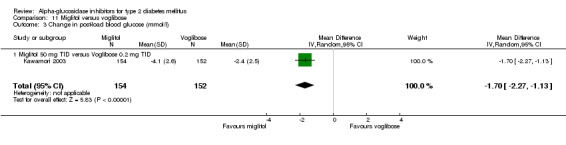
Comparison 11 Miglitol versus voglibose, Outcome 3 Change in post‐load blood glucose (mmol/l).
11.4. Analysis.

Comparison 11 Miglitol versus voglibose, Outcome 4 Change in post‐load insulin levels (pmol/l).
11.5. Analysis.

Comparison 11 Miglitol versus voglibose, Outcome 5 Occurence of adverse effects.
11.6. Analysis.

Comparison 11 Miglitol versus voglibose, Outcome 6 Occurence of gastro‐intestinal adverse effects.
11.7. Analysis.

Comparison 11 Miglitol versus voglibose, Outcome 7 Change in post‐load blood glucose (mmol/l) (2 hours).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braun 1996.
| Methods | DESIGN: karallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Germany SETTING: general practice NUMBER: randomised: AGI 80, CONTROL 72, analysed: AGI 42, CONTROL 44 SEX (F/M): AGI 16/26, CONTROL 20/24 AGE (YEARS (MEAN)): analysed patients: AGI 60, CONTROL 61 DURATION OF DIABETES (MONTHS (MEAN)): analysed patients: AGI 16, CONTROL 17 | |
| Interventions | Dietary reinforcement: unclear AGI: acarbose, week 1‐2 50 mg TID, week 3‐24 100 mg TID CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: total cholesterol, HDL‐cholesterol, triglycerides 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: oharmaceutical Author contacted: chief of department replied, data not in file, original authors were no longer working there Study retrieved: CENTRAL, EMBASE, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Buchanan 1988.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 16 weeks | |
| Participants | COUNTRY: Scotland SETTING: outpatient NUMBER: randomised 28, analysed 20 (AGI 9, CONTROL 11) SEX (F/M): AGI 3/6, CONTROL 3/8 AGE (YEARS (MEAN, SD)): analysed patients: AGI 60,1 (6,8), CONTROL 57,6 (8,2) DURATION OF DIABETES (MONTHS (MEAN, SD)): analysed patients: AGI 44,9 (28,6), CONTROL 50,6 (30,1) | |
| Interventions | Dietary reinforcement: unclear; high complex carbohydrates / low‐fat diet generally advised. AGI: acarbose, week 0‐2 50 mg TID, week 3‐8 100 mg TID, week 9‐12: 200‐100‐100 mg, week 13‐16 200‐100‐200 mg, in case of adverse effects patients were instructed to reduce the dosage of acarbose to that which could be tolerated. CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1), fasting blood glucose 5. Lipids: total cholesterol, triglycerides 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: co‐author replied but could not give detailed answers Study retrieved: CENTRAL, MEDLINE, EMBASE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Calle‐Pascual 1996.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 16 weeks | |
| Participants | COUNTRY: Spain SETTING: outpatient NUMBER: randomised AGI 20, control 20; dropout AGI 3/20, control 4/20 SEX: data missing AGE: data missing DURATION OF DIABETES: data missing | |
| Interventions | Dietary reinforcement: yes, patients included in a behaviour modification program. AGI: acarbose, week 1‐4 50 mg TID, week 5‐16 100 mg TID CONTROL: placebo |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting blood glucose 5. Lipids: total‐ and HDL‐cholesterol, triglycerides 6. Insulin levels: fasting insulin 7. Weight: bodyweight, BMI 8. Adverse effects: yes | |
| Notes | Sponsor: not sponsored Author contacted: additional data on design, quality and outcomes send by author Study retrieved: CENTRAL, MEDLINE, EMBASE Short report, published as letter to the editor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Campbell 1998.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 3 years | |
| Participants | COUNTRY: UK SETTING: general practice NUMBER: randomised: 789 (baseline data: AGI 236, CONTROL1 254, CONTROL2 243) SEX (F/M): AGI 87/150, CONTROL1 98/156, CONTROL2 71/172 AGE (YEARS (MEAN)): AGI 62, CONTROL1 62, CONTROL2 62 DURATION OF DIABETES (MONTHS (MEAN)): AGI 34.7, CONTROL1 37.8, CONTROL2 41.6 | |
| Interventions | Dietary reinforcement: unclear AGI: acarbose 100 MG TID CONTROL1: placebo CONTROL2: acarbose 50 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c) 5. Lipids: ND 6. Insulin levels: ND 7. Weight: ND 8. Adverse effects: yes | |
| Notes | Sponsor: Pharmaceutical Author contacted: addtional data on design, quality and outcomes via manufacturer. The sparse outcome data of insufficient quality to be included in meta‐analysis Study retrieved: handsearch Published as an abstract only. Patients were followed‐up and an interim analysis was planned when the HbA1c progressed to >= 8.0 on two consecutive visits or > 10.6% at any time. Therefore the results are not suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chan 1998.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRIES: China, Taiwan, Hong Kong, Philippines, Korea, Singapore, Malaysia SETTING: outpatient NUMBER: randomised AGI 63, CONTROL 63, analysed AGI 59, CONTROL 62 SEX (F/M): AGI 31/32, CONTROL 31/32 AGE (YEARS (MEAN, SD)): randomised patients: AGI 52,8 (10,2), CONTROL 54,0 (10,0) DURATION OF DIABETES (MONTHS (MEAN, SD)): randomised patients: AGI 32,4 (42), CONTROL 25,2 (40,8) | |
| Interventions | Dietary reinforcement: unclear AGI: acarbose, week 1‐4 50 mg TID, week 5‐24 100 mg TID CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: total‐, HDL‐ & LDL‐cholesterol, triglycerides 6. Insulin levels: fasting & post‐load insulin 7. Weight: body weight, BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: no reply Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chiasson 1994.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 1 year | |
| Participants | COUNTRY: Canada SETTING: outpatient NUMBER: 354 patients randomised, 77 treated with diet alone; 67 (of 77) analysed SEX (F/M): 29/48 AGE (YEARS (MEAN, SD)): all randomised patients in diet‐only group 57,2 (9.7) DURATION OF DIABETES (MONTHS (MEAN, SD)): all randomised patients in diet‐only group 62,4 (63,6) | |
| Interventions | Dietary reinforcement: yes, according to Canadian Association Nutritional guidelines (1993). AGI: acarbose 50, 100 or 200 mg TID, dose adjusted according to blood glucose values and / or tolerance, main target to achieve a postprandial blood glucose < 12 mmol/l CONTROL: placebo |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & 90 minutes post‐load blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: ND 8. Adverse effects: ND | |
| Notes | Sponsor: pharmaceutical Author contacted: author requested us to send questions again, no reply since Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, manufacturer, handsearch For this review the reported data from the 'diet only' subgroup is used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chiasson 2001.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 36 weeks | |
| Participants | COUNTRY: Canada SETTING: outpatient NUMBER: total: randomised 324, analysed 318; AGI 82, CONTROL1 83, CONTROL2 83, CONTROL3 76 SEX (F/M): AGI 18/64, CONTROL1 27/56, CONTROL2 22/61, CONTROL3 17/59 AGE (YEARS (MEAN, SD)): AGI 57,3 (9,0), CONTROL1 57,7 (9,9), CONTROLl2 57,9 (8,6), CONTROL3 58,9 (7,9) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 62,4 (56,4), CONTROL1 61,2 (58,8), CONTROL2 90,0 (88,8), CONTROL3 73,2 (66,0) | |
| Interventions | Dietary reinforcement: yes, 'well‐balanced weight‐reducing diet' (reference Diabetes Care 1994, 17(5) 490‐519). AGI: miglitol, week 1‐4 25 mg TID, week 5‐12 50 mg TID, week 13‐36 100 mg TID CONTROL1: placebo CONTROL2: metformin 500 mg TID CONTROL4: combination of miglitol 100 mg TID and metformin 500 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: fasting & post‐load insulin 7. Weight: body weight 8. Adverse effects: any AE, gastrointestinal AE | |
| Notes | Sponsor: pharmaceutical Author contacted: author requested us to send questions again, no reply since (4 months) Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Coniff 1994.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: USA SETTING: outpatient NUMBER: randomised: AGI 105, CONTROL 107; analysed: AGI 91, CONTROL 98 SEX (F/M): analysed group: AGI 50/41, CONTROL 45/53 AGE (YEARS (MEAN, SD)): analysed group: AGI 56,0 (9,5), CONTROL 55,6 (9,9) DURATION OF DIABETES (MONTHS (MEDIAN, RANGE)): analysed group: AGI 48 (6‐396), CONTROL 36 (6‐252) | |
| Interventions | Dietary reinforcement: yes, standard diabetic diet containing at least 50% carbohydrates. AGI: acarbose titrated to a maximum of 300 mg TID: dose in‐ or decreased according to fasting blood glucose and tolerance (cut‐off point fasting blood glucose > 11.1 mmol/l) CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of Life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: triglycerides, total‐, HDL‐ & LDL‐cholesterol 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, manufacturer, handsearch | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Coniff 1995.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: USA SETTING: outpatient NUMBER: randomised: AGI 76, CONTROL1 72, CONTROL2 72, CONTROL3 70; analysed: AGI 67, CONTROL1 62, CONTROL2 66, CONTROL3 60 SEX (F/M): analysed group: AGI 41/26, CONTROL1 30/32, CONTROL2 29/37, CONTROL3 29/31 AGE (YEARS (MEAN)): analysed group: AGI 56,2, CONTROL1 56,3, CONTROL2 55,4, CONTROL3 55,7 DURATION OF DIABETES (MONTHS (MEAN, SD)): | |
| Interventions | Dietary reinforcement: yes, standard diabetic diet with 50% energy as carbohydrates. AGI: acarbose 200 mg TID CONTROL1: placebo CONTROL2: tolbutamide, individually adjusted in steps of 250 mg TID, maximum dose unclear CONTROL4: acarbose & tolbutamide combination (data not used in this review) |
|
| Outcomes | 1. Mortality: yes 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: triglycerides, total‐, HDL‐ & LDL‐cholesterol 6. Insulin levels: fasting & post‐load insulin 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CCRCT, Medline, Embase, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Coniff 1995b.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 16 weeks | |
| Participants | COUNTRY: USA SETTING: outpatient NUMBER: randomised: AGI 73, CONTROL1 73, CONTROL2 72, CONTROL3 72; analysed: AGI 58, CONTROL1 64, CONTROL2 54, CONTROL3 53 SEX (F/M): analysed group: AGI 28/30, CONTROL1 27/37, CONTROL2 22/32, CONTROL3 22/31 AGE (YEARS (MEAN)): analysed group: AGI 55, CONTROL1 54, CONTROL2 56, CONTROL3 54 DURATION OF DIABETES (MONTHS (MEAN)): analysed group: AGI 72, CONTROL1 60, CONTROL2 60, CONTROL3 60 | |
| Interventions | Dietary reinforcement: yes, weight stable ADA diet (1979): 50% carbohydrate, 30% fat, 20% protein. AGI: acarbose 100 mg TID CONTROL1: placebo TID CONTROL2: acarbose, week 1‐2 100 mg TID, week 3‐16 200 mg TID CONTROL3: acarbose, week 1‐2 100 mg TID, week 3‐4 200 mg TID, week 5‐16 300 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes Related Complications: ND 3. Quality of Life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: total cholesterol, triglycerides 6. Insulin levels: fasting & post‐load insulin levels 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dedov 1995.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Russia SETTING: outpatient NUMBER: randomised 180 patients, analysed 155 (AGI 82, CONTROL 73). Baseline values are given for 161 patients SEX (F/M): baseline group AGI 50/33, CONTROL 50/28 AGE (YEARS (MEAN, SD)): baseline group AGI 52,6 (9,5), CONTROL 49,2 (9,5) DURATION OF DIABETES: ND | |
| Interventions | Dietary reinforcement: unclear AGI: acarbose, week 1‐2 50 mg TID, week 3‐24 wk 100 mg TID CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: not specified Author contacted: no reply Study retrieved: CENTRAL, EMBASE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Delgado 2002.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 16 weeks | |
| Participants | COUNTRY: Switzerland SETTING: outpatient NUMBER: AGI 9, CONTROL 8 SEX (F/M): AGI 3/6, CONTROL 3/5 AGE: ND DURATION OF DIABETES (MONTHS (MEAN, SD)): all patients 26 (6) | |
| Interventions | Dietary reinforcement: yes, for details article referred to article in French (Journeés de diabétologie Hôtel Dieu 1998: 51‐69). AGI: acarbose, week 1‐2 50 mg once daily, week 3‐16 50 mg BID CONTROL1: placebo BID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: total cholesterol, HDL‐cholesterol, triglycerides 6. Insulin levels: Reaven's triple test 7. Weight: body weight, BMI 8. Adverse effects: ND | |
| Notes | Sponsor: Not specified Author contacted: no reply Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, handsearch Study mainly about insulin insulin resistance & secretion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Drent 2002.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: The Netherlands SETTING: patients recruited in general practice, study performed in 'study centres' NUMBER: 599 enrolled, 468 randomised, 384 analysed (AGI 71, CONTROL1 87, CONTROL2 84, CONTROL3 58, CONTROL4 84) SEX (F/M): AGI 34/37, CONTROL1 38/49, CONTROL2 37/47, CONTROL3 21/37, CONTROL4 43/41 AGI (YEARS (MEAN, SD)): AGI 63 (11), CONTROL1 63 (11), CONTROL2 63 (9), CONTROL3 64 (10), CONTROL4 64 (10) DURATION OF DIABETES (MONTHS (MEAN)): AGI 36, CONTROL1 30, CONTROL2 48, CONTROL3 46, CONTROL4 41.5 | |
| Interventions | Dietary reinforcement: when patients were not using diet, advice was given during screening period, ADA/EASD guidelines, at least 40% carbohydrates . AGI: miglitol, week 1‐2 50 mg TID, week 3‐24 100 mg TID CONTROL1: placebo TID CONTROL2: miglitol 50 mg TID CONTROL3: miglitol, week 1‐2 100 mg TID, week 3‐24 200 mg TID CONTROL4: miglitol 25 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: "blood lipids" 6. Insulin levels: fasting & post‐load insulin 7. Weight: weight & BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes send by author Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents (2nd reference via author) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fischer 1998.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Germany, Austria, Croatia, Hungary, Italy SETTING: outpatient NUMBER: randomised 495, analysed 420 (AGI 25 mg 86, AGI 50 mg 88, AGI 100 mg 78, AGI 200 mg 87, CONTROL 81) SEX (F/M): AGI 25 mg 40/46, AGI 50 mg 45/43, AGI 100 mg 32/46, AGI 200 mg 43/44, CONTROL 38/43 AGE (YEARS (MEAN, SD)): analysed group: AGI 25 mg 58,5 (8,4), AGI 50 mg 55,5 (9,6), AGI 100 mg 56,8 (9,4), AGI 200 mg 59,4 (8,6), CONTROL 52,7 (8,7) DURATION OF DIABETES (MONTHS (MEDIAN)): AGI 25 mg 26, AGI 50 mg 20, AGI 100 mg 17, AGI 200 mg 21, CONTROL 24 | |
| Interventions | Dietary reinforcement: yes, ADA nutritional recommendations 1986 AGI: acarbose divided in 4 groups: 25 mg, 50 mg, 100 mg (week 1‐2 50 mg TID) and 200 mg TID (week 1‐2 100 mg TID) CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gentile 1999.
| Methods | DESIGN: cross‐over study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 2 x 12 weeks | |
| Participants | COUNTRY: Italy SETTING: outpatient NUMBER: 76 SEX (F/M): 33/43 AGE: ND DURATION OF DIABETES (MONTHS (MEAN, SD)): 110,4 (49,2) | |
| Interventions | Dietary reinforcement: unclear, general advice 60% carbohydrates, 20‐22% fat, 18‐20% protein. AGI: acarbose, week 1 50 mg TID, week 2‐12 100 mg TID CONTROL: placebo |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin, fasting blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: ND 8. Adverse effects: yes | |
| Notes | Sponsor: "Fundi MURST", not clear whether this is a pharmaceutical sponsor Author contacted: no reply Study retrieved: CENTRAL, MEDLINE, EMBASE This study is done with patients suffering from non‐alcoholic liver cirrhosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Haffner 1997.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 16 weeks | |
| Participants | COUNTRY: Germany SETTING: outpatient NUMBER: 77 patients randomised and analysed (AGI 25, CONTROL1 25, CONTROL2 27) SEX (F/M): AGI 6/19, CONTROL1 8/17, CONTROL2 11/16 AGI (YEARS (MEAN, SD)): AGI 59.4 (28), CONTROL1 58.6 (31.5), CONTROL2 58.1 (36.4) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 94.0 (59.9), CONTROL1 77.3 (53.5), CONTROL2 69.5 (49.9) | |
| Interventions | Dietary reinforcement: yes, body weight stable, 15% protein, 35% fat, 50% carbohydrates AGI: acarbose 100 mg TID CONTROL1: placebo TID CONTROL2: glibenclamide 1 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: triglycerides, total & HDL‐cholesterol 6. Insulin levels: fasting & post‐load insulin 7. Weight: weight & BMI 8. Adverse effects: ND | |
| Notes | Sponsor: non‐industry (National Heart Lung and Blood Institute) Author contacted: no reply Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, handsearch | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hanefeld 1991.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Germany SETTING: outpatient NUMBER: randomised 100, analysed 94; AGI 47, CONTROL 47 SEX (F/M): AGI 24/23, CONTROL 22/25 AGE (YEARS (MEAN)): analysed patients AGI 60, CONTROL 59 DURATION OF DIABETES (MONTHS (MEAN)): analysed patients AGI 70, CONTROL 49 | |
| Interventions | Dietary reinforcement: yes, specification diet unclear. AGI: acarbose 100 mg TID CONTROL: placebo |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related Complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1), fasting & 1 hour post‐load blood glucose 5. Lipids: triglycerides, total‐ and HDL‐cholesterol 6. Insulin levels: fasting & 1 hour post‐load insulin 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hillebrand 1987.
| Methods | DESIGN: cross‐over study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: treatment periods of 12 weeks | |
| Participants | COUNTRY: Germany SETTING: outpatient NUMBER: 76 SEX (F/M): 33/43 AGE: ND DURATION OF DIABETES (MONTHS (MEAN, SD)): 110,4 (49,2) | |
| Interventions | Dietary reinforcement: unclear AGI: acarbose 200 mg BID CONTROL1: miglitol 200 mg BID CONTROL2: glibenclamide 7 mg once daily |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: ND 8. Adverse effects: yes | |
| Notes | Sponsor: not specified Author contacted: authors could not be retrieved Study retrieved: handsearch Published as abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hoffmann 1990.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: no blinding DURATION: 24 weeks | |
| Participants | COUNTRY: Germany SETTING: outpatient NUMBER: 95 patients included; AGI 48, CONTROL 47 SEX (F/M): AGI 30/18, CONTROL 26/21 AGE (YEARS (MEAN, SD)): AGI 61.8 (5.6), CONTROL 61.2 (5.5) DURATION OF DIABETES (MONTHS (MEAN (SD)): AGI 22.4 (16.2), CONTROL 30.7 (29.2) | |
| Interventions | Dietary reinforcement: yes, normocaloric diet of 1500 kcal with 120 g carbohydrates, 50 g protein, 55 g fat AGI: acarbose, week 1‐4 50 mg TID, week 5‐25 100 mg TID (for one patient dose reduced to 100 mg BID) CONTROL: glibenclamide 3,5 mg administered individually 1‐3 times per day |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1), fasting & post‐load blood glucose 5. Lipids: triglycerides, total‐, HDL and LDL‐cholesterol 6. Insulin levels: ND 7. Weight: body weight, Broca index 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, experts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hoffmann 1994.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind regarding comparison acarbose / placebo, glibenclamide single‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Germany SETTING: outpatient NUMBER: 96 patients randomised, 85 analysed for efficacy (AGI 28, control1 30, control2 27) SEX (F/M): AGI 15/13, CONTROL1 18/12, CONTROL2 14/13 AGE (YEARS (MEAN, SD)): analysed patients: AGI 58,8 (6,9), CONTROL1 56,9 (6,7), CONTROL2 59,9 (5,7) DURATION OF DIABETES (MONTHS (MEAN, SD)): analysed patients: AGI 12,7 (10,8), CONTROL1 12,1 (10,8), CONTROL2 17,6 (13,1) | |
| Interventions | Dietary reinforcement: yes, 50% carbohydrates, 35% fat, 15% protein. AGI: acarbose 100 mg TID CONTROL1: placebo TID CONTROL2: glibenclamide 3,5 mg administered individually 1‐3 times per day |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: triglycerides, total‐ and HDL‐cholesterol 6. Insulin levels: fasting & post‐load insulin 7. Weight: body weight, BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hoffmann 1997.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double blind regarding comparison acarbose / placebo, metformin single‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Germany SETTING: outpatient NUMBER: 96 patients randomised; 94 analysed for efficacy (AGI 31, CONTROL1 32, CONTROL2 31) SEX (F/M): AGI 25/6, CONTROL1 20/12, CONTROL2 17/14 AGE (YEARS (MEAN, SD)): analysed patients: AGI 58,9 (9,4), CONTROL1 60,2 (8,6), CONTROL2 55,9 (7,8) DURATION OF DIABETES (MONTHS (MEAN, SD)): analysed patients: AGI 36,9 (27,2), CONTROL1 43,2 (33,9), CONTROL2 25,0 (17,4) | |
| Interventions | Dietary reinforcement: yes, 50% carbohydrates, 35% fat, 15% protein AGI: acarbose 100 mg TID CONTROL1: placebo TID CONTROL2: metformin 850 mg BID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting post‐load blood glucose 5. Lipids: triglycerides, total‐, HDL‐ & LDL‐cholesterol 6. Insulin levels: fasting & post‐load insulin 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Holman 1999.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 3 years | |
| Participants | COUNTRY: England SETTING: outpatient, part of the United Kingdom Prospective Diabetes Study NUMBER: 1946 patients randomised, total 1624 analysed (intention‐to‐treat): diet only group randomised 256, diet only group analysed (HbA1c) AGI 83, CONTROL 107. SEX (F/M): AGI 36/84, CONTROL 38/98 AGE (YEARS (MEAN, SD)): AGI 60.0 (8.2), CONTROL 60.9 (9.0) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 82.6 (33.3), CONTROL 91.3 (34.9) | |
| Interventions | Dietary reinforcement: no (dietary advice according to UKPDS protocol) AGI: acarbose, 50 mg once, BID & TID at two‐week intervals; 4 months after start dosage increased in 3 weeks period with 50 mg per step to 100 mg TID. In case of side effects patients were allowed to reduce the dose. CONTROL: placebo |
|
| Outcomes | 1. Mortality: yes 2. Diabetes related complications: yes 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c) 5. Lipids: ND 6. Insulin levels: ND 7. Weight: body weight, BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes send by authors Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, Manufacturer For this review the reported data from the 'diet only' subgroup is used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Holmes 2001.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Germany, France and Spain SETTING: outpatient NUMBER: 260 patients entered run‐in period, 179 randomised (AGI 92, CONTROL 87). analysed (for HbA1c) AGI 90, CONTROL 85 SEX (F/M): randomised group AGI 33/59; CONTROL 30/57 AGE (YEARS (MEAN, SD)): randomised patients AGI 60,6 (10.2); CONTROL 64.3 (10.4) DURATION OF DIABETES (MONTHS (MEAN (SD)): randomised patients AGI 53.9 (62.4 or 64.4 ); CONTROL 63.4 (66.5) | |
| Interventions | Dietary reinforcement: no (''patients continued with their normal dietary habits'). AGI: acarbose, week 0‐4 50 mg TID, week 4‐8 100 mg TID, in case of side‐effects to be reduced to 50 mg CONTROL: nateglinide 120 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes send by author Study retrieved: handsearch | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hotta 1993.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Japan SETTING: outpatient NUMBER: randomised: AGI 20, CONTROL 20, analysed: AGI 16, CONTROL 15, (baseline values given for 37 patients) SEX (F/M): AGI 5/14, CONTROL 4/14 AGE (YEARS (MEAN)): AGI 49,8, CONTROL 47,9 DURATION OF DIABETES (MONTHS (MEAN)): AGI 55,2, CONTROL 57,6 | |
| Interventions | Dietary reinforcement: yes, specification unclear AGI: acarbose 100 mg TID CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: total‐ & HDL‐cholesterol, triglycerides 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes send by author Study retrieved: CENTRAL, MEDLINE, EMBASE, manufacturer, (2nd reference via author) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Johnston 1998.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 56 weeks | |
| Participants | COUNTRY: USA SETTING: outpatient NUMBER: randomised: AGI 102, CONTROL1 104, CONTROL2 104, CONTROL3 101, analysed: AGI 85, CONTROL1 95, CONTROL2 92, CONTROL3 92 SEX (F/M): analysed patients: AGIN24/61, CONTROL1 35/60, CONTROL2 33/59, CONTROL3 26/66 AGE (YEARS (MEAN, SD)): analysed group: AGI 67,8 (5,5), CONTROL1 67,2 (5,8), CONTROL2 67,7 (5,8), CONTROL3 68,5 (5,8) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 81,6 (88,8), CONTROL1 90 (93,6), CONTROL2 86,4 (92,4), CONTROL3 84 (92,4) | |
| Interventions | Dietary reinforcement: yes, ADA approved diet >= 50% carbohydrates AGI: miglitol 50 mg TID CONTROL1: miglitol 25 mg TID CONTROL2: glyburide 20 mg once daily, step up & individually titrated: every 2 weeks increase: 2,5/5/7,5/10/15/20 mg CONTROL4: placebo TID and once daily |
|
| Outcomes | 1. Mortality: yes 2. Diabetes related complications: yes 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: triglycerides 6. Insulin levels: fasting & post‐load insulin 7. Weight: BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: Bayer replied that the data from this study was transferred to Pfizer. Pfizer didn't reply to our requests do far. Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Johnston 1998a.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 52 weeks, main outcomes measured at 26 weeks | |
| Participants | COUNTRY: USA SETTING: outpatient NUMBER: total randomised: AGI 254, CONTROL 131, diet only group 55 (AGI), 14 (CONTROL); analysed: AGI 19, CONTROL 10 SEX: no data for diet only group AGE: no data for diet only group DURATION OF DIABETES: no data for diet only group | |
| Interventions | Dietary reinforcement: yes, at least 50% carbohydrates, intended to maintain weight. AGI: miglitol 50 mg: when tolerant the patient increased the dose to 100/150/200 TID at wk 13/26 and 39 respectively. Backtitration allowed (in case of intolerance). CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c) 5. Lipids: no data for diet only group 6. Insulin levels: no data for diet only group 7. Weight: no data for diet only group 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: Bayer replied that the data from this study was transferred to Pfizer. Pfizer didn't reply to our requests so far. Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents Both patients using diet only and patients receiving additional sulphonylurea therapy were included in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Johnston 1998b.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 52 weeks, primary efficacy criterion measured at 28 weeks | |
| Participants | COUNTRY: USA SETTING: outpatient NUMBER: total randomised: AGI 229, CONTROL 116; valid for efficacy diet only group: AGI 32, CONTROL 13; analysed for HbA1c: AGI 30, CONTROL 9 SEX (F/M): diet only group valid for efficacy: AGI 12/20, CONTROL 7/6 AGE (YEARS (MEAN, SD)): diet only group valid for efficacy: AGI 57,3 (10,2), CONTROL 54,9 (12,6) DURATION OF DIABETES (MONTHS (MEAN, SD)): diet only group valid for efficacy: AGI 57,6 (95,0), CONTROL 30 (38,9) | |
| Interventions | Dietary reinforcement: yes, overweight patients received counselling to produce gradual (1 lb./week) weight loss. AGI: miglitol, week 1‐12 50 mg TID, week 12‐52 100 mg TID. In case of intolerance to be decreased to 50 mg CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c) 5. Lipids: no data for diet only group 6. Insulin levels: no data for diet only group 7. Weight: no data for diet only group 8. Adverse effects: no data for diet only group | |
| Notes | Sponsor: pharmaceutical Author contacted: Bayer replied that the data from this study was transferred to Pfizer. Pfizer didn't reply to our requests so far. Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents Study among African‐American patients. Both patients using diet only and patients receiving additional sulphonylurea therapy were included in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kawamori 2003.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 12 weeks | |
| Participants | COUNTRY: Japan SETTING: unclear NUMBER: 445 patients enrolled, efficacy data for 396 patients (AGI1 158, AGI2 154, CONTROL 84) SEX: Data missing AGE: Data missing DURATION OF DIABETES: Data missing | |
| Interventions | Dietary reinforcement: unclear AGI1: miglitol 50 mg TID AGI2: voglibose 0.2 mg TID CONTROL: placebo |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: post‐load insulin 7. Weight: ND 8. Adverse effects: yes | |
| Notes | Sponsor: not specified Author contacted: no additional data before study was published as journal article Study retrieved: handsearch Data extracted from a congress abstract and a copy of a poster presentation. Authors refused to give more data before this study was published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kovacevic 1997.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind with respect to acarbose and placebo, single blind with respect to glibenclamide DURATION: 24 weeks | |
| Participants | COUNTRY: Croatia SETTING: outpatient NUMBER: randomised: AGI 34, CONTROL1 34, CONTROL2 34; analysed AGI 33, CONTROL1 31, CONTROL2 33 SEX (F/M): total group 55/47; analysed AGI 16/17, CONTROL1 18/13, CONTROL2 20/13 AGE (YEARS (MEAN, SD)): total group 57,5 (8,1), analysed AGI 58.42 (7.76), CONTROL1 59.35 (8.61), CONTROL2 54.73 (7.80) DURATION OF DIABETES (MONTHS (MEAN)): total group 54 | |
| Interventions | Dietary reinforcement: yes, 40‐50% carbohydrates, 35‐40% fat, 15% protein AGI: acarbose 100 mg TID CONTROL1: placebo TID CONTROL2: glibenclamide 3.5 mg adjusted individually, maximum TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood‐glucose 5. Lipids: tot cholesterol, HDL and triglycerides 6. Insulin levels: fasting & post‐load insulin 7. Weight: BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes send by author Study retrieved: CENTRAL, EMBASE, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Meneilly 2000.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 12 months | |
| Participants | COUNTRY: Canada SETTING: outpatient NUMBER: AGI 93, CONTROL 99 SEX (F/M): AGI 28/65, CONTROL 39/60 AGE (YEARS (MEAN, SD)): AGI 69.7 (4,8), CONTROL 70.3 (5,0) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 69,6 (81,6), CONTROL 57,6 (60) | |
| Interventions | Dietary reinforcement: yes, advised to maintain diet to ensure that calorie intake was consistent throughout the study. AGI: acarbose, week 1: 50 mg once daily, week 2: 50 mg BID, week 3: 50 mg TID, week 4‐52 titrated upward to 100 mg TID when post‐load blood glucose > 12 mmol/l, downtitrated in case of intolerance. CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: SF 36 & Boyer quality of life rating instrument 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: fasting & post‐load insulin 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: no reply Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, manufacturer, handsearch Study conducted in older patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pagano 1995.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Italy SETTING: outpatient NUMBER: 100 patients randomised, 96 patients completed study: AGI 49, CONTROL 47. Primary efficacy data for 90 patients SEX (F/M): AGI 16/33, CONTROL 23/24 AGE (YEARS (MEAN, SD)): patients that completed study: AGI 57 (8.4), CONTROL 59 (7.5) DURATION OF DIABETES (MONTHS (MEAN, SD)): patients that completed study: AGI 60 (48.3), CONTROL 84 (64.4) | |
| Interventions | Dietary reinforcement: yes, 30 kcal per Kg of ideal body weight per day (60% carbohydrates, 25% fat, 15% protein, 30g dietary fibres). AGI: miglitol, week 1‐6 50 mg TID, week 7‐24 100 mg TID CONTROL: glibenclamide week 1‐6 2,5 mg BID, week 7‐24 5 mg BID, 1 placebo tablet to ensure blinding. |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: total‐, HDL‐cholesterol, triglycerides 6. Insulin levels: fasting insulin 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes send by author Study retrieved: CENTRAL, MEDLINE, EMBASE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rosenthal 2002.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: no blinding DURATION: 24 weeks | |
| Participants | COUNTRY: Germany SETTING: general practice NUMBER: selected: AGI 39, CONTROL 37, analysed: AGI 32, CONTROL 31 SEX: data missing AGE (YEARS (MEAN, SD)): AGI 57.4 (8.6), CONTROL 57.7 (10.5) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 20.2 (31.2), CONTROL 35.6 (44.8) | |
| Interventions | Dietary reinforcement: no AGI: acarbose, 50 mg TID, uptitrated to 100 mg TID (exact scheme not reported) CONTROL: glibenclamide, maximum 10.5 mg daily (7 mg ‐ 0 ‐ 3.5 mg), step‐up scheme as long as fasting blood glucose remained > 8.9 mmol/l |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood‐glucose 5. Lipids: total cholesterol, HDL, triglycerides 6. Insulin levels: fasting & post‐load insulin 7. Weight: body weight, BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: EMBASE, Current Contents, manufacturer, (2 additional references via authors) Main outcome is blood pressure. According to the statistical report, the changes for lipids are calculated with standardised values (using a linear transformation to the interval [0,1] with respect to normal range), and therefore cannot be used for the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rybka 1999.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: multiple European countries, not further specification SETTING: unclear NUMBER: 603 patients included SEX: data missing AGE: data missing DURATION OF DIABETES: data missing | |
| Interventions | Dietary reinforcement: yes, specifications unclear AGI: acarbose 100 mg TID CONTROL1: placebo CONTROL2: miglitol 50 mg TID CONTROL3: miglitol 100 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: no reply Study retrieved: handsearch Published as an abstract. A non‐systematic review on miglitol cited this study also as an unpublished document (Scott 2000). Bayer referred to Pfizer being the current owner of this data, but wen received no reply from Pfizer so far. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Salman 2001.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: no blinding DURATION: 24 weeks | |
| Participants | COUNTRY: Turkey SETTING: outpatient NUMBER: randomised 72; analysed: AGI 27, CONTROL 30 SEX (F/M): analysed patients: AGI 10/17, CONTROL 14/16 AGE (YEARS (MEAN, SD)): analysed group: AGI 52,6 (9,1), CONTROL 56,1 (8,7) DURATION OF DIABETES (MONTHS (MEAN, SD)): analysed group: AGI 50,4 (40,8), CONTROL 56,4 (67,2) | |
| Interventions | Dietary reinforcement: patients under dietary recommendations for at least 3 months, controlled for diet compliance before study inclusion. AGI: acarbose, week 1 to 4 every week 50 mg increase to 100 mg BID, week 4‐24 100 mg TID, dose reduced to 100 mg BID in case of adverse events CONTROL: gliclazide maximum 80 mg BID, depending on degree of glycemic control; in general maximum dose was not recommended |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic Control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: triglycerides, total‐, HDL‐ & LDL‐cholesterol 6. Insulin levels: fasting & post‐load insulin, fasting & post‐load C‐peptide 7. Weight: body weight, BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes send by author Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Santeusanio 1993.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: adequate BLINDING: double‐blind DURATION: 16 weeks | |
| Participants | COUNTRY: Italy SETTING: outpatient NUMBER: randomised: AGI 27, CONTROL1 29, CONTROL2 28; evaluated in ITT‐analysis: AGI 23, CONTROL1 23, CONTROL2 18 SEX (F/M): ITT: AGI 8/15, CONTROL1 7/16, CONTROL2 8/10 AGE (YEARS (MEAN, SD)): ITT: AGI 53,8 (11,0), CONTROL1 55,5 (11,5), CONTROL2 58,9 (9,8) DURATION OF DIABETES (MONTHS (MEAN, SD)): ITT: AGI 60,6 (57,6), CONTROL1 46,4 (51,6), CONTROL2 46,4 (36,0) | |
| Interventions | Dietary reinforcement: yes, iso‐caloric diet to maintain stable body weight (50‐55% carbohydrates, <30% lipids, 15‐20% protein and <10 g/1000 kcal as fibre). AGI: acarbose m100 mg TID CONTROL1: placebo TID CONTROL2: acarbose 50 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: fasting & post‐load insulin 7. Weight: ND 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents, manufacturer, handsearch | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Scott 1999.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 16 weeks | |
| Participants | COUNTRY: New Zealand / Australia SETTING: outpatient NUMBER: AGI 53, CONTROL 52 SEX (F/M): AGI 20/33, CONTROL 18/34 AGE (YEARS (MEAN, SD)): AGI 56 (9), CONTROL 57 (8) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 21 (15), CONTROL 26 (17) | |
| Interventions | Dietary reinforcement: yes, 'conforming to current recommendations for type 2 diabetes' AGI: acarbose, week 1‐2 50 mg TID, wk 3‐16 100 mg TID, dose reduced to 50 mg TID in case of adverse events CONTROL: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting blood glucose 5. Lipids: triglycerides, total‐ and HDL‐cholesterol 6. Insulin levels: fasting insulin 7. Weight: ND 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: author replied that he passed our queries through to Bayer Australia, but we received no reply from Bayer Australia since. Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Segal 1997.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: Germany, Austria, Israel, Czech Republic SETTING: outpatient NUMBER: randomised 201, ITT 186, PP 119 (AGI 40, CONTROL 37, CONTROL2 42) SEX (F/M): PP: AGI 18/22, CONTROL1 14/23, CONTROL2 18/24 AGE (YEARS (MEAN)): PP: AGI 61, CONTROL1 56, CONTROL2 59 DURATION OF DIABETES (MONTHS (MEAN, SD)): ND | |
| Interventions | Dietary reinforcement: no AGI: miglitol, week 1‐4 50 mg TID, week 5‐25 100 mg TID CONTROL1: glibenclamide 3,5 mg once or twice daily CONTROL2: placebo TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: no reply Study retrieved: CENTRAL, MEDLINE, EMBASE, Current Contents | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Spengler 1992.
| Methods | DESIGN: Parallel study RANDOMISATION PROCEDURE: adequate BLINDING: no blinding DURATION: 24 weeks | |
| Participants | COUNTRY: Germany SETTING: outpatient NUMBER: randomised 72, analysed: AGI 26, CONTROL 29 SEX (F/M): AGI 15/11, CONTROL 18/11 AGE (YEARS (MEAN, SD)): analysed: AGI 59 (5), CONTROL 60 (7) DURATION OF DIABETES (MONTHS (MEDIAN)): analysed: AGI 12.0, CONTROL 8.4 | |
| Interventions | Dietary reinforcement: unclear AGI: acarbose, week 1‐2 50 mg TID, week 3‐24 100 mg TID CONTROL: glibenclamide maximum 3,5 mg TID |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: ND 6. Insulin levels: ND 7. Weight: body weight 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: additional data on design, quality and outcomes via manufacturer Study retrieved: CENTRAL, MEDLINE, EMBASE, experts (1 additional reference via author) For all outcomes except body weight, geometric means are reported; true means not available from articles and statistical reports. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Takami 2002.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: no blinding DURATION: 3 months | |
| Participants | COUNTRY: Japan SETTING: outpatient NUMBER: Analysed: AGI 12, CONTROL1 11, CONTROL2 9 SEX (F/M): AGI 3/9, CONTROL1 4/7, CONTROL2 3/10 AGE (YEARS (MEAN, SD)): total group (n=36!) men 48,7 (8,3), women 55,0 (7,8) DURATION OF DIABETES: Newly diagnosed patients | |
| Interventions | Dietary reinforcement: yes, 30 kcal/Kg of ideal body weight per day, 60% carbohydrate, 20% fat, 20% protein. AGI: voglibose 0,3 mg TID CONTROL1: diet therapy CONTROL2: glyburide 1,25 mg once daily |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting bloodglucose 5. Lipids: Total & HDL‐cholesterol, triglycerides 6. Insulin levels: fasting insulin 7. Weight: weight & BMI 8. Adverse effects: ND | |
| Notes | Sponsor: not specified Author contacted: no reply Study retrieved: CENTRAL, MEDLINE, Current Contents, handsearch 36 'study subjects', 32 randomised and 4 patients assigned to diet group after random phase to 'facilitate analysis of correlations between the changes in abdominal adipose tissue and glycemic control with diet'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Van de Laar 2004a.
| Methods | DESIGN: Parallel studyRANDOMISATION PROCEDURE: adequateBLINDING: double‐blindDURATION: 30 weeks | |
| Participants | COUNTRY: The NetherlandsSETTING: general practiceNUMBER: randomised: AGI 48, CONTROL 48, ITT: AGI 32, CONTROL 43SEX (F/M): ITT: AGI 16/16, CONTROL 20/23AGE (YEARS (MEAN, SD)): ITT: AGI 58.6 (7.7), CONTROL 58.6 (7.1)DURATION OF DIABETES (MONTHS (MEDIAN)): analysed: AGI 12.0, CONTROL 8.4 | |
| Interventions | Dietary reinforcement: yes, advice tailored to individual food habits by dietician with access to current recommendationsAGI: acarbose, maximum dosage schedule at week 0, 2, 4 and 6‐30 was (mg): 50 ‐ 0 ‐ 0, 50 ‐ 0 ‐ 50, 50 ‐ 50 ‐ 50 and 100 ‐ 100 ‐ 100 respectivelyCONTROL: tolbutamide, maximum dosage schedule at week 0, 2, 4 and 6‐30 (mg) was 500 ‐ 0 ‐ 0, 500 ‐ 0 ‐ 500, 500 ‐ 500 ‐ 500 and 1000 ‐ 500 ‐ 500 respectively. | |
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of Life: ND4. Glycaemic Control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose5. Lipids: triglycerides, total‐, LDL‐ & HDL‐cholesterol6. Insulin levels: fasting & post‐load insulin 7. Weight: BMI8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceuticalAuthor contacted: data possessed by authors reviewStudy retrieved: expertsEquivalence study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zheng 1995.
| Methods | DESIGN: parallel study RANDOMISATION PROCEDURE: unclear BLINDING: double‐blind DURATION: 24 weeks | |
| Participants | COUNTRY: China SETTING: outpatient NUMBER: AGI 39, CONTROL 38 SEX (F/M): AGI 19/20, CONTROL 18/20 AGE (YEARS (MEAN, SD)): AGI 49.6 (6.9), CONTROL 49.0 (6.6) DURATION OF DIABETES (MONTHS (MEAN, SD)): AGI 49.2 (33.6), CONTROL 50.4 (43.2) | |
| Interventions | Dietary reinforcement: unclear ('diet and level of activity had to remain stable) AGI: acarbose, week 1‐3 50 mg TID, wk 4‐24 100 mg TID CONTROL: placebo |
|
| Outcomes | 1. Mortality: ND 2. Diabetes related complications: ND 3. Quality of life: ND 4. Glycaemic control: glycated haemoglobin (HbA1c), fasting & post‐load blood glucose 5. Lipids: triglycerides, total‐ and HDL‐cholesterol 6. Insulin levels: fasting & post‐load insulin 7. Weight: BMI 8. Adverse effects: yes | |
| Notes | Sponsor: pharmaceutical Author contacted: no reply Study retrieved: CENTRAL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BID = two times per day; BMI = body mass index; CENTRAL = Cochrane Central Register of Controlled Trials; HDL = high‐density lipoprotein; ITT = intention‐to‐treat analysis; LDL = low‐density lipoprotein; ND = no reported data; PP = per protocol analysis; TID = three times per day, For interventions the maximum dosage is given For outcomes: Outome measures that are reported are given
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bachmann 2003 | Use of additional anti‐diabetic medication |
| Bayer 2003 | Use of additional anti‐diabetic medication |
| Bayer 2003a | Use of additional anti‐diabetic medication, included patients with type 1 and type 2 diabetes |
| Coniff 1995a | Falsely included on basis of Embase search (excluded from Medline search) acarbose given as additional therapy (added to insulin therapy) |
| De Leiva 1993 | Use of additional anti‐diabetic medication, reported data does not allow subgroup analysis of AGI only group |
| Escobar‐Jimenez 1995 | Use of additional anti‐diabetic medication, reported data does not allow subgroup analysis of AGI only group |
| Fujita 2001 | Use of additional anti‐diabetic medication, reported data does not allow subgroup analysis of AGI only group |
| Hasche 1999 | Use of additional medication, reported data does not allow subgroup analysis of AGI only group |
| Holman 1991 | Duration of AGI treatment < 12 wk (4 wk) |
| Ikeda 1998 | Use of additional anti‐diabetic medication, reported data does not allow subgroup analysis of AGI only group |
| Jenney 1993 | No randomisation; Acarbose not given as monotherapy |
| Rosak 2002 | Study duration < 12 wk (1 day) |
| Rosenbaum 2002 | Us of additional anti‐diabetic medication, reported data does not allow subgroup analysis of AGI only group |
| Soonthornpun 1998 | Use of additional anti‐diabetic medication |
| Wang 2000 | Patients with impaired glucose tolerance (in stead of type 2 diabetes mellitus) |
Characteristics of ongoing studies [ordered by study ID]
Holman 2003.
| Trial name or title | Early Diabetes Intervention Study (EDIT) |
| Methods | |
| Participants | Subjects were selected on the basis of two consecutive fasting plasma glucose values of 5.5 to 7.7 mmol/l. They all underwent OGTTs at entry into the study but if the 2‐h glucose was found to be in the diabetic range (i.e. 11.1 or above) they were not excluded, provided that the fasting remained below 7.8 mmol/l. |
| Interventions | Acarbose (50mg TID), metformin (500mg TID) and placebo; Design: prospective, parallel group, double blind, double dummy, randomised, factorial design, multicentre study; Duration 6 years |
| Outcomes | Progression to frank diabetes; Glycaemic reduction |
| Starting date | 01 / 04 / 1998; end date: 30 / 04 / 2003 |
| Contact information | Dr Rury Holman Diabetes Research Laboratories Radcliffe Infirmary Woodstock Rd Oxford OX2 6HE UK rury.holman@dtu.ox.ac.uk |
| Notes | A subgroup of 106 patients had postprandial blood glucose in the diabetic range (> 11.1 mmol/l, but fasting blood glucose < 7.8 mmol/l). Data from this sub‐group might be possible included in the review |
Sa‐adu 2003.
| Trial name or title | A one‐year multicentre, international, randomised, double‐blind comparison of Mitiglinide (10to40mgTID) and Acarbose (50mgODto100mgTID) administered orally for the treatment of elderly type 2 diabetic patients |
| Methods | |
| Participants | Elderly type 2 diabetic patients suboptimally controlled with diet alone. |
| Interventions | Mitiglinide (10 to 40 mg TID) and Acarbose (50 mg OD to 100 mg TID); Design: comparative, randomised, double blind, parallel group phase III |
| Outcomes | HbA1c |
| Starting date | 01 /12 / 2‐‐1; end date: 01/ 06 / 2003 |
| Contact information | Prof Alan Sinclair, The University of Warwick; Dr Alfa Sa‐adu Care of the Elderly Watford General Hospital Vicarage Road Watford Herts WD18 0HB UK Telephone: 01923 217227 E‐mail: a.saadu.btinternet.com |
| Notes | Two e‐mails to prof. Sinclair were not answered. Dr Sa‐adu replied that he was not a contributor to this study and that recruitment was taken to East European Countries. |
Whitby 1998.
| Trial name or title | A long‐term study to investigate the effects of acarbose (glucobay) in preventing or delaying deterioration in glycaemic status in non‐insulin diabetes will controlled on diet alone. |
| Methods | |
| Participants | Non‐insulin dependent diabetics, either newly diagnosed or well controlled on diet alone. |
| Interventions | Acarbose versus placebo |
| Outcomes | Not specified |
| Starting date | 28 / 09 / 1993; end date: 31 / 07 / 1996 |
| Contact information | Dr Robert E J Ryder Department of Diabetes City Hospital Dudley Road Birmingham West Midlands B18 7QH England Telephone: 0121 554 3801 Dr R J Whitby Linden Medical Centre Linden Ave Kettering NN15 7NX Northants |
| Notes | Dr Ryder and dr. Whitby were contacted. Dr Ryder referred to prof. Holman as leading investigator, but Professor Holman did not reply to our e‐mails regarding questions about this study. |
TID = three times per day
Contributions of authors
FLORIS VAN DE LAAR: Protocol development, searching for trials, abstract assessment for eligibility, quality assessment of trials, data extraction, data entry, data analysis, review development
PETER LUCASSEN: Protocol development, abstract assessment for eligibility, quality assessment of trials, data extraction, data analysis, review development
REINIER AKKERMANS: (double) data entry, data analysis, review development
ELOY VAN DE LISDONK: Quality assessment of trials (referee), data analysis, translation Italian articles, review development
GUY RUTTEN: Protocol development, data analysis (advisor), review development
CHRIS VAN WEEL: Protocol development, data analysis (advisor), review development
Sources of support
Internal sources
Radboud University Nijmegen Medical Centre, Netherlands.
Julius Centre for for Health Sciences and Primary Care, Netherlands.
External sources
No sources of support supplied
Declarations of interest
FvdL, PL, EvdL, GR and CvW conducted and published a trial that was sponsored by Bayer (Van de Laar 2004a).
Edited (no change to conclusions)
References
References to studies included in this review
Braun 1996 {published data only}
- Braun D, Schonherr U, Mitzkat H‐J. Efficacy of acarbose monotherapy in patients with type 2 diabetes: A double‐blind study conducted in general practice. Endocrinology & Metabolism 1996;3(4):275‐280. [MEDLINE: ] [Google Scholar]
Buchanan 1988 {published data only}
- Buchanan DR, Collier A, Rodrigues E, Millar AM, Gray RS, Clarke BF. Effectiveness of acarbose, an alpha‐glucosidase inhibitor, in uncontrolled non‐obese non‐insulin dependent diabetes. European Journal of Clinical Pharmacology 1988;34(1):51‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Calle‐Pascual 1996 {published and unpublished data}
- Calle‐Pascual A, Garcia‐Honduvilla J, Martin‐Alvarez PJ, Calle JR, Maranes JP. Influence of 16‐week monotherapy with acarbose on cardiovascular risk factors in obese subjects with non‐insulin‐dependent diabetes mellitus: a controlled, double‐blind comparison study with placebo [letter]. Diabetes & Metabolism 1996;22(3):201‐202. [MEDLINE: ] [PubMed] [Google Scholar]
Campbell 1998 {published and unpublished data}
- Campbell I, Robertson‐Mackay F, Streets E, Gibbons F, Holman RR. Maintenance of glycaemic control with acarbose in diet treated Type 2 diabetic patients. Diabetic Medicine 1998;15(Suppl 2):S29‐30. [MEDLINE: ] [Google Scholar]
- Holman RR, Robertson‐Mackay F, Gibbons F, Montegriffo E, Campbell I. Acarbose maintains glycaemic control in diet treated type 2 diabetes. Clinical Science 1999;96:7p. [MEDLINE: ] [Google Scholar]
Chan 1998 {published data only}
- Chan JC, Chan KW, Ho LL, Fuh MM, Horn LC, Sheaves R, et al. An Asian multicenter clinical trial to assess the efficacy and tolerability of acarbose compared with placebo in type 2 diabetic patients previously treated with diet. Asian Acarbose Study Group. Diabetes Care 1998;21(7):1058‐1061. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chiasson 1994 {published data only}
- Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, et al. The efficacy of acarbose in the treatment of patients with non‐insulin‐dependent diabetes mellitus. A multicenter controlled clinical trial [see comments]. Annals of Internal Medicine 1994;121(12):928‐935. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Josse RG. Acarbose for the treatment of type II diabetes: the results of a Canadian multi‐centre trial [published erratum appears in Diabetes Res Clin Pract 1995 Sep;29(3):215]. Diabetes Research and Clinical Practice 1995;28 Suppl:S167‐S172. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rodger NW, Chiasson JL, Josse RG, Hunt JA, Palmason C, Ross SA, et al. Clinical experience with acarbose: results of a Canadian multicentre study. Clinical and investigative medicine. Medecine clinique et experimentale 1995;18(4):318‐324. [MEDLINE: ] [PubMed] [Google Scholar]
- Ross S, Hunt J, Josse R, Mukherjee J, Palmason C, Rodger W, et al. Acarbose significantly improves glucose control in non‐insulin‐dependent diabetes mellitus subjects (NIDDM): results of the mult‐centre Canadian trial. Diabetes 1992;41(Suppl. 1):193A. [MEDLINE: ] [Google Scholar]
- Wolever TM, Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, et al. No relationship between carbohydrate intake and effect of acarbose on HbA1c or gastrointestinal symptoms in type 2 diabetic subjects consuming 30‐60% of energy from carbohydrate. Diabetes Care 1998;21(10):1612‐1618. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wolever TM, Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, et al. Small weight loss on long‐term acarbose therapy with no change in dietary pattern or nutrient intake of individuals with non‐insulin‐dependent diabetes. International Journal of Obesity and Related Metabolic Disorders 1997;21(9):756‐763. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chiasson 2001 {published data only}
- Chiasson JL, Naditch L. The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care 2001;24(6):989‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wolever TMS, Assiff L, Basu T, Chiasson J‐L, Boctor M, Gerstein HC, et al. Miglitol, an alpha‐glucosidase inhibitor, prevents the metformin‐induced fall in serum folate and vitamin B12 in subjects with type 2 diabetes. Nutrition Research 2000;20(10):1447‐1456. [MEDLINE: ] [Google Scholar]
Coniff 1994 {published and unpublished data}
- Coniff RF, Shapiro JA, Seaton TB. Long‐term efficacy and safety of acarbose in the treatment of obese subjects with non‐insulin‐dependent diabetes mellitus. Archives of Internal Medicine 1994;154(21):2442‐2448. [MEDLINE: ] [PubMed] [Google Scholar]
- Innerfield RJ, Coniff RF. A multi‐center, double‐blind, placebo‐controlled study of the long‐term efficacy and safety of acarbose (Bay g 5421) in the Rx of obese patients with NIDDM Rxed by diet alone. Diabetes 1990;39(Suppl. 1):211A. [MEDLINE: ] [Google Scholar]
Coniff 1995 {published and unpublished data}
- Coniff RF, Shapiro JA, Seaton TB, Bray GA. Multicenter, placebo‐controlled trial comparing acarbose (BAY g 5421) with placebo, tolbutamide, and tolbutamide‐plus‐acarbose in non‐insulin‐dependent diabetes mellitus. The American Journal of Medicine 1995;98(5):443‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coniff 1995b {published and unpublished data}
- Coniff RF, Shapiro JA, Robbins D, Kleinfield R, Seaton TB, Beisswenger P, et al. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM. A placebo‐controlled dose‐comparison study. Diabetes Care 1995;18(6):817‐824. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dedov 1995 {published data only}
- Dedov II, Balabolkin MI, Mkrtumyan AM, Ametov AS, Kakhnovsky IM, Chazova TE, et al. Glucobai therapy of diabetes mellitus. [Russian]. Problemy Endokrinologii 1995;41(3):11‐13. [MEDLINE: ] [Google Scholar]
Delgado 2002 {published data only}
- Delgado H, Lehmann T, Bobbioni‐Harsch E, Ybarra J, Golay A. Acarbose improves indirectly both insulin resistance and secretion in obese type 2 diabetic patients. Diabetes & Metabolism 2002;28(3):195‐200. [MEDLINE: ] [PubMed] [Google Scholar]
Drent 2002 {published and unpublished data}
- Drent ML, The Dutch Miglitol Investigators Group. Miglitol as single oral hypoglycemic agent in type 2 diabetes. Diabetologia 1994;37(Suppl. 1):A211. [MEDLINE: ] [Google Scholar]
- Drent ML, Tollefsen AT, Heusden FH, Hoenderdos EB, Jonker JJ, Veen EA. Dose‐dependent efficacy of miglitol, an alpha‐glucosidase inhibitor, in type 2 diabetic patients on diet alone: results of a 24‐week double‐blind placebo‐controlled study. Diabetes, Nutrition & Metabolism 2002;15(3):152‐159. [MEDLINE: ] [PubMed] [Google Scholar]
Fischer 1998 {published and unpublished data}
- Fischer S, Hanefeld M, Spengler M, Boehme K, Temelkova‐Kurktschiev T. European study on dose‐response relationship of acarbose as a first‐line drug in non‐insulin‐dependent diabetes mellitus: efficacy and safety of low and high doses. Acta Diabetologica 1998;35(1):34‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gentile 1999 {published data only}
- Gentile S, Turco S, Guarino G, Oliviero B, Rustici A, Torella R. Non‐insulin‐dependent diabetes mellitus associated with nonalcoholic liver cirrhosis: an evaluation of treatment with the intestinal alpha‐glucosidase inhibitor acarbose [Diabete mellito non insulino‐dipendente associato a cirrosi epatica non etilica: valutazione del trattamento con un inhibitore delle alpha‐glucosidasi intestinali, acarbose]. Annali Italiani di Medicina Interna 1999;14(1):7‐14. [MEDLINE: ] [PubMed] [Google Scholar]
- Gentile S, Turco S, Persico M, Pananello A, Conte S, Gesuè L, et al. Efficacy of acarbose in the control of diabetes mellitus associated with liver cirrhosis. Journal of Hepatology 1997;26(Suppl 1):S101. [MEDLINE: ] [Google Scholar]
- Gentile S, Turco S, Persico M, Panariello A, Conte S, Seta M, Gesuè L, et al. The acarbose tretament of type 2 diabetes mellitus associated with liver cirrhosis. Diabetologia 1997;40:A306. [MEDLINE: ] [Google Scholar]
Haffner 1997 {published data only}
- Fischer S, Patzak A, Rietzsch H, Schwanebeck U, Kohler C, Wildbrett J, et al. Influence of treatment with acarbose or glibenclamide on insulin sensitivity in type 2 diabetic patients. Diabetes, Obesity and Metabolism 2003;5(1):38‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haffner SM, Hanefeld M, Fischer S, Fucker K, Leonhardt W. Glibenclamide, but not acarbose, increases leptin concentrations parallel to changes in insulin in subjects with NIDDM. Diabetes Care 1997;20(9):1430‐1434. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hanefeld M, Haffner SM, Menschikowski M, Koehler C, Temelkova‐Kurktschiev T, Wildbrett J, Fischer S. Different effects of acarbose and glibenclamide on proinsulin and insulin profiles in people with Type 2 diabetes. Diabetes Research and Clinical Practice 2002;55(3):221‐227. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hanefeld 1991 {published and unpublished data}
- Hanefeld M, Fischer S, Schulze J, Spengler M, Wargenau M, Schollberg K, et al. Therapeutic potentials of acarbose as first‐line drug in NIDDM insufficiently treated with diet alone. Diabetes Care 1991;14(8):732‐737. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leonhardt W, Hanefeld M, Fischer S, Schulze J, Spengler M. Beneficial effects on serum lipids in noninsulin dependent diabetics by acarbose treatment. Arzneimittelforschung 1991;41(7):735‐738. [MEDLINE: ] [PubMed] [Google Scholar]
Hillebrand 1987 {published data only}
- Hillebrand I, Englert R. Efficacy and tolerability of a 12‐week treatment with acarbose (BAY g5421), miglitol (BAY m1099) and glibenclamid. Diabetes 1987;26:134A. [MEDLINE: ] [Google Scholar]
Hoffmann 1990 {published and unpublished data}
- Fölsch UR, Spengler M, Boehme K, Sommerauer B. Efficacy of glucosidase inhibitors compared to sulphonylureas in the treatment and metabolic control of diet treated Type II diabetic subjects: Two long‐term comparative studies. Diabetes, Nutrition & Metabolism 1990;3(Suppl. 1):63‐68. [MEDLINE: ] [Google Scholar]
- Hoffmann J. Acarbose and Glibenclamid in Type‐II Diabetes. A Comparative Study on Efficacy and Side Effects [Acarbose und Glibenclamid bei Typ‐II‐Diabetes. Eine Vergleichsstudie zu Wirksamkeit und Nebenwirkungen]. Münchener Medizinische Wochenschrift 1990;132(31‐32):487‐490. [MEDLINE: ] [Google Scholar]
- Hoffmann, J. Adjustment of metabolism and eating behaviour of type II diabetics. Results of a six‐months treatment with glibenclamide, respectively with acarbose [Stoffwechseleinstellung und Essverhalten von Typ‐II‐Diabetikern. Ergebnisse einer sechsmonatigen Behandlung mit Glibenclamid bzw. mit Acarbose]. Zeitschrift für Allgemeinmedizin 1992;68(29):970‐977. [MEDLINE: ] [Google Scholar]
Hoffmann 1994 {published and unpublished data}
- Hoffmann J, Spengler M. Efficacy of 24‐week monotherapy with acarbose, glibenclamide, or placebo in NIDDM patients. The Essen Study. Diabetes Care 1994;17(6):561‐566. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hoffmann 1997 {published and unpublished data}
- Hoffmann J, Spengler M. Efficacy of 24‐week monotherapy with acarbose, metformin, or placebo in dietary‐treated NIDDM patients: the Essen‐II Study. American Journal of Medicine 1997;103(6):483‐490. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holman 1999 {published and unpublished data}
- Holman RR, Cull CA, Turner RC. A randomized double‐blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44) [see comments] [published erratum appears in Diabetes Care 1999 Nov;22(11):1922]. Diabetes Care 1999;22(6):960‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holmes 2001 {published and unpublished data}
- Holmes D, Raccah D, Escobar‐Jimenez F, Standl E. Targeting postprandial hyperglycemia in patients with type 2 diabetes: nateglinide vs acarbose. Diabetologia 2001;44(Suppl 1):A215. [MEDLINE: ] [Google Scholar]
- Holmes D, Raccah D, Escobar‐Jimenez F, Standl E. Targeting postprandial hyperglycemia to achieve glycemic control in patients with type 2 diabetes: a comparison of nateglinide and acarbose [Poster presentation]. EASD Congress 9‐13 september 2001, Glasgow (UK). [MEDLINE: ]
- Raccah D, Escobar‐Jimenez F, Gomis R, Holmes D, Standl E. Targeting prandial glucose with nateglinide and acarbose in the treatment of type 2 diabetes: a double‐blind clinical comparison. Unpublished Document. [MEDLINE: ]
Hotta 1993 {published and unpublished data}
- Hotta N, Kakuta H, Sano T, Matsumae H, Yamada H, Kitazawa S, et al. Long‐term effect of acarbose on glycaemic control in non‐insulin‐dependent diabetes mellitus: a placebo‐controlled double‐blind study. Diabetic Medicine 1993;10(2):134‐138. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Hotta N, Kakuta H, Sano T, Yamada H, Matsumae H, et al. An investigation into the efficacy of long term usage of Bay g 5421 (acarbose) for non‐insulin dependent diabetes. A placebo controlled, double blind trial [japanese]. Rinshou to Kenkyu 1990;67(1):219‐233. [MEDLINE: ] [Google Scholar]
Johnston 1998 {published data only}
- Johnston PS, Lebovitz HE, Coniff RF, Simonson DC, Raskin P, Munera CL. Advantages of alpha‐glucosidase inhibition as monotherapy in elderly type 2 diabetic patients. The Journal of Clinical Endocrinology and Metabolism 1998;83(5):1515‐1522. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnston 1998a {published data only}
- Johnston PS, Feig PU, Coniff RF, Krol A, Davidson JA, Haffner SM. Long‐term titrated‐dose alpha‐glucosidase inhibition in non‐insulin‐requiring Hispanic NIDDM patients. Diabetes Care 1998;21(3):409‐415. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnston 1998b {published data only}
- Johnston PS, Feig PU, Coniff RF, Krol A, Kelley DE, Mooradian AD. Chronic treatment of African‐American type 2 diabetic patients with alpha‐glucosidase inhibition. Diabetes Care 1998;21(3):416‐422. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kawamori 2003 {published data only}
- Kawamori R, Toyota T, Oka Y, Yamada A, Iwamoto Y, Tajima N, et al. Improvement of glycaemic control following 12‐week treatment with miglitol in Japanese type 2 diabetics: a double‐blind, randomized, placebo‐ and voglibose‐controlled trial. Poster display, IDF Congress Paris august 25 2003. [MEDLINE: ]
Kovacevic 1997 {published and unpublished data}
- Kovacevic I, Profozic V, Skrabalo Z, Cabrijan T, Zjacic‐Rotkvic V, Goldoni V, et al. Multicentric clinical trial to assess efficacy and tolerability of acarbose (BAY G 5421) in comparison to glibenclamide and placebo. Diabetologia Croatica 1997;26(2):83‐89. [MEDLINE: ] [Google Scholar]
Meneilly 2000 {published data only}
- Josse RG, Chiasson JL, Ryan EA, Lau DC, Ross SA, Yale JF, et al. Acarbose in the treatment of elderly patients with type 2 diabetes. Diabetes Research and Clinical Practice 2003;59(1):37‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, et al. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care 2000;23(8):1162‐1167. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pagano 1995 {published and unpublished data}
- Marena S, Pagani A, Montegrosso G, Boella G, Pagano A, Michieli F, et al. Comparison of miglitol and glibenclamide in non insulin‐dependent diabetic patients. [Italian]. Giornale Italiano di Diabetologia 1993;13(4):383‐388. [MEDLINE: ] [Google Scholar]
- Marena S, Pagani A, Montegrosso G, Boella G, Pagano A, Michieli F, et al. Miglitol vs Glibenclamide in non‐insulin dependent diabete mellitus. European Journal of Clinical Investigation 1993;23(Suppl 1):A43. [MEDLINE: ] [Google Scholar]
- Pagano G, Marena S, Corgiat‐Mansin L, Cravero F, Giorda C, Bozza M, et al. Comparison of miglitol and glibenclamide in diet‐treated type 2 diabetic patients. Diabete & Metabolisme 1995;21(3):162‐167. [MEDLINE: ] [PubMed] [Google Scholar]
Rosenthal 2002 {published and unpublished data}
- Mauersberger H, Rosenthal JH. Wirkung von endogenem Insulin sowie Acarbose oder Glibenclamide in der Therapie der Hypertonie bei Typ‐2‐Diabetikern. CF‐Journal 2001, (1):26‐28. [MEDLINE: ] [Google Scholar]
- Rosenthal JH, Mauersberger H. Effects on blood pressure of the alpha‐glucosidase inhibitor acarbose compared with the insulin enhancer glibenclamide in patients with hypertension and type 2 diabetes mellitus. Clinical Drug Investigation 2002;22(10):695‐701. [MEDLINE: ] [Google Scholar]
- Rosenthal JH, Mauersberger H. Hypertension in type 2‐diabetic patients ‐ Effects of endogenous insulin and antidiabetic therapies. American Journal of Hypertension 2000;13(4 Suppl 1):S81. [MEDLINE: ] [Google Scholar]
Rybka 1999 {published data only}
- Bayer Vital GmbH, Company K.H. Efficacy and tolerability of miglitol (Bay m 1099) and acarbose (Bay g 5421) in type II diabetes, Report no: R6245 (Data on File). 1994. [MEDLINE: ]
- Rybka J, Goke B, Sissmann J. European comparative study of 2 alpha‐glucosidase inhibitors, miglitol and acarbose. Diabetes 1999;48(Suppl. 1):101. [MEDLINE: ] [Google Scholar]
Salman 2001 {published and unpublished data}
- Salman S, Salman F, Satman I, Yilmaz Y, Ozer E, Sengul A, et al. Comparison of acarbose and gliclazide as first‐line agents in patients with type 2 diabetes. Current Medical Research and Opinion. 2001;16(4):296‐306. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Santeusanio 1993 {published and unpublished data}
- Santeusanio F, Ventura MM, Contadini S, Compagnucci P, Moriconi V, Zaccarini P, et al. Efficacy and safety of two different dosages of acarbose in non‐insulin dependent diabetic patients treated by diet alone. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 1993;6(3):147‐154. [MEDLINE: ] [Google Scholar]
Scott 1999 {published and unpublished data}
- Scott R, Lintott CJ, Zimmet P, Campbell L, Bowen K, Welborn T. Will acarbose improve the metabolic abnormalities of insulin‐resistant type 2 diabetes mellitus?. Diabetes Research and Clinical Practice 1999;43(3):179‐185. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Segal 1997 {published data only}
- Segal P, Feig PU, Schernthaner G, Ratzmann KP, Rybka J, Petzinna D, et al. The efficacy and safety of miglitol therapy compared with glibenclamide in patients with NIDDM inadequately controlled by diet alone. Diabetes Care 1997;20(5):687‐691. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spengler 1992 {published and unpublished data}
- Fölsch UR, Spengler M, Boehme K, Sommerauer B. Efficacy of glucosidase inhibitors compared to sulphonylureas in the treatment and metabolic control of diet treated Type II diabetic subjects: Two long‐term comparative studies. Diabetes, Nutrition & Metabolism 1990;3(Suppl. 1):63‐68. [MEDLINE: ] [Google Scholar]
- Spengler M, Hansel G, Boehme K. 6 Months monotherapy of NIDDM with acarbose or glibenclamide. New Aspects in Diabetes. Treatment Strategies with Alpha‐Glucosidase Inhibitors. Third International Symposium on Acarbose. Berlin ‐ New York: Walter de Gruyter, 1992:243‐6. [MEDLINE: ]
- Spengler M, Hansel G, Boehme K. Efficacy of 6 months monotherapy with glucosidase inhibitor acarbose versus sulphonylurea glibenclamide on metabolic control of dietary treated type II diabetics (NIDDM). Hormone & Metabolic Research 1992, (Supp 26):50‐51. [MEDLINE: ] [PubMed] [Google Scholar]
- Spengler M, Hänsel G, Boehme K. Efficacy of 6 months monotherapy with glucosidase inhibitor acarbose versus sulphonylurea glibenclamid on metabolic control of dietary treated type II diabetics. European Journal of Clinical Investigation. 1989; Vol. 19, issue 2 part II:A71. [MEDLINE: ] [PubMed]
- Spengler M, Hänsel, M, Boehme, K. Acarbose and glibenclamide in type II diabetes [Acarbose und Glibenclamid bei Typ‐II‐Diabetes]. Zeitschrift für Allgemeinmedizin 1990;65(22):606‐610. [MEDLINE: ] [Google Scholar]
Takami 2002 {published data only}
- Takami K, Takeda N, Nakashima K, Takami R, Hayashi M, Ozeki S, et al. Effects of dietary treatment alone or diet with voglibose or glyburide on abdominal adipose tissue and metabolic abnormalities in patients with newly diagnosed type 2 diabetes. Diabetes Care 2002;25(4):658‐662. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van de Laar 2004a {published and unpublished data}
- Laar FA, Lucassen PLBJ, Kemp J, Lisdonk EH, Weel C, Rutten GEHM. Is acarbose equivalent to tolbutamide as first treatment for newly diagnosed diabetes in general practice? A randomised controlled trial. Diabetes Research and Clinical Practice 2004;63(1):57‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zheng 1995 {published data only}
- Zheng GF, Wang JP, Zhang H, Hu ZX, Liu J, Xiao JZ, et al. Clinical observation on glucobay treatment for NIDDM. [Chinese]. Chinese Journal of Endocrinology 1995;11(3):163‐164. [MEDLINE: ] [Google Scholar]
References to studies excluded from this review
Bachmann 2003 {published data only}
- Bachmann W, Petzinna D, Sotiros A, Wascher T. Long‐Term Improvement of Metabolic Control by Acarbose in Type 2 Diabetes Patients Poorly Controlled with Maximum Sulfonylurea Therapy. Clinical Drug Investigation 2003;23(10):679‐686. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bayer 2003 {unpublished data only}
- Bayer AG. Study No. 541. Data on file, Bayer. [MEDLINE: ]
Bayer 2003a {unpublished data only}
- Bayer AG. Study No. 656. Data on file, Bayer. [MEDLINE: ]
Coniff 1995a {published data only}
- Coniff RF, Shapiro JA, Seaton TB, Hoogwerf BJ, Hunt JA. A double‐blind placebo‐controlled trial evaluating the safety and efficacy of acarbose for the treatment of patients with insulin‐requiring type II diabetes. Diabetes Care 1995;18(7):928‐932. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Leiva 1993 {published data only}
- Leiva A, Piñón F, Tébar J, Escobar‐Jiménez F, Calle H, Herrera‐Pombo JL, et al. Clinical efficacy and tolerance to acarbose in the treatment of non‐insulin‐dependent diabetic patients [Eficacia clínica y tolerancia de la acarbosa en el tratamiento de pacientes diabéticos no dependientes de la insulina (tipo II)]. Medicina clinica 1993;100(10):368‐371. [MEDLINE: ] [PubMed] [Google Scholar]
Escobar‐Jimenez 1995 {published data only}
- Escobar‐Jimenez F, Barajas C, Leiva A, Cano FJ, Masoliver R, Herrera‐Pombo JL, et al. Efficacy and tolerability of miglitol in the treatment of patients with non‐insulin‐dependent diabetes mellitus. Current Therapeutic Research, Clinical & Experimental 1995;56(3):258‐268. [MEDLINE: ] [Google Scholar]
Fujita 2001 {published data only}
- Fujita H, Yamagamu T, Ahshima K. Long‐term ingestion of a fermented soybean‐derived Touchi‐extract with alpha‐glucosidase inhibitory activity is safe and effective in humans with borderline and mild type‐2 diabetes. Journal of Nutrition 2001;131(8):2105‐2108. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hasche 1999 {published data only}
- Hasche H, Mertes G, Bruns C, Englert R, Genthner P, Heim D, et al. Effects of acarbose treatment in Type 2 diabetic patients under dietary training: a multicentre, double‐blind, placebo‐controlled, 2‐year study. Diabetes, Nutrition & Metabolism 1999;12(4):277‐285. [MEDLINE: ] [PubMed] [Google Scholar]
Holman 1991 {published data only}
- Holman RR, Steemson J, Turner RC. Post‐prandial glycaemic reduction by an alpha‐glucosidase inhibitor in type 2 diabetic patients with therapeutically attained basal normoglycaemia. Diabetes Research (Edinburgh, Lothian) 1991;18(4):149‐153. [MEDLINE: ] [PubMed] [Google Scholar]
Ikeda 1998 {published data only}
- Ikeda T, Murao A, Santou Y, Murakami H, Yamamoto R. Comparison of the clinical effect of acarbose and voglibose on blood glucose in non‐obese, non‐insulin dependent diabetes mellitus [Japanese]. Therapeutic Research 1998;19(9):271‐278. [MEDLINE: ] [Google Scholar]
Jenney 1993 {published data only}
- Jenney A, Proietto J, O'Dea K, Nankervis A, Traianedes K, D'Embden H. Low‐dose acarbose improves glycemic control in NIDDM patients without changes in insulin sensitivity. Diabetes Care 1993;16(2):499‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rosak 2002 {published data only}
- Rosak C, Haupt E, Walter T, Werner J. The effect of combination treatment with acarbose and glibenclamide on postprandial glucose and insulin profiles: additive blood glucose lowering effect and decreased hypoglycaemia. Diabetes, Nutrition & Metabolism 2002;15(3):143‐151. [MEDLINE: ] [PubMed] [Google Scholar]
Rosenbaum 2002 {published data only}
- Rosenbaum P, Peres RB, Zanella MT, Ferreira SRG. Improved glycemic control by acarbose therapy in hypertensive diabetic patients: effects on blood pressure and hormonal parameters. Brazilian Journal of Medical and Biological Research 2002;35(8):877‐884. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Soonthornpun 1998 {published data only}
- Soonthornpun S, Rattarasarn C, Thamprasit A, Leetanaporn K. Effect of acarbose in treatment of type II diabetes mellitus: a double‐blind, crossover, placebo‐controlled trial. Journal of the Medical Association of Thailand 1998;81(3):195‐200. [MEDLINE: ] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang H, Xu WH, Wang GY. An evalualion on efficacy of acarbose interfering treatment on IGT. Shanxi Clinical Medicine Journal 2000;9(2):116‐117. [MEDLINE: ] [Google Scholar]
References to ongoing studies
Holman 2003 {published data only}
- Holman R. Early Diabetes Intervention Study (EDIT). The National Research Register 2003, issue 1. [N0162013485]
Sa‐adu 2003 {published data only}
- Sa‐adu A. A one‐year multicentre, international, randomised, double‐blind comparison of Mitiglinide (10to40mgTID) and Acarbose (50mgODto100mgTID) administered orally for the treatment of elderly type 2 diabetic patients. The National Research Register 2003, issue 1. [N0235102775]
Whitby 1998 {published data only}
- Ryder REJ. BIOS ‐ A long‐term study to investigate the effects of acarbose (Glucobay) in preventing or delaying deterioration in glycaemic status in non‐insulin dependent diabetes well controlled on diet alone. The National Research Register 2003, issue 1. [N0064018154]
- Whitby RJ. A long‐term study to investigate the effects of acarbose (glucobay) in preventing or delaying deterioration in glycaemic status in non‐insulin diabetes well controlled on diet alone. The National Research Register 2003, issue 1:Publication ID: B0201167. [Publication ID: B0201167]
Additional references
ADA 1997
- American Diabetic Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183‐97. [DOI] [PubMed] [Google Scholar]
ADA 1999
- American Diabetic Association. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1999;22(Suppl 1):S1‐114. [Google Scholar]
Begg 1994
- Begg CB, Mazumbar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐1101. [PubMed] [Google Scholar]
Breuer 2003
- Breuer HW. Review of acarbose therapeutic strategies in the long‐term treatment and in the prevention of type 2 diabetes. International Journal of Clinical Pharmacology and Therapeutics 2003;41(10):421‐440. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Campbell 1996
- Campbell LK, White JR, Campbell RK. Acarbose: its role in the treatment of diabetes mellitus [see comments]. The Annals of Pharmacotherapy 1996;30(11):1255‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell LK, Baker DE, Campbell RK. Miglitol: assessment of its role in the treatment of patients with diabetes mellitus. The Annals of Pharmacotherapy 2000;34(11):1291‐1301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chiasson 2002
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP‐NIDDM randomised trial. Lancet 2002;359(9323):2072‐2077. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chiasson 2003
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP‐NIDDM trial. JAMA : the journal of the American Medical Association 2003;290(4):486‐494. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Concato 2000
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. The New England Journal of Medicine 2000;342(25):1887‐1892. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EDPG 1999
- Anonymous. A desktop guide to Type 2 diabetes mellitus. European Diabetes Policy Group 1999. Diabetic Medicine 1999;16(9):716‐730. [MEDLINE: ] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fölsch 1990
- Fölsch UR, Spengler M, Boehme K, Sommerauer B. Efficacy of glucosidase inhibitors compared to sulphonylureas in the treatment and metabolic control of diet treated Type II diabetic subjects: Two long‐term comparative studies. Diabetes, Nutrition & Metabolism 1990;3(Suppl. 1):63‐68. [MEDLINE: ] [Google Scholar]
Hanefeld 2003
- Hanefeld M, Petzinna D, Cagatay M. MeRIA Study: Acarbose Reduces the Incidence of Infarction in Patients with Type 2 Diabetes ‐ Metaanalysis of Placebo‐Controlled Long‐Term Studies. Poster presentation, IDF Paris August 25th. IDF Paris August 25th 2003, 2003. [MEDLINE: ]
Hanefeld 2004
- Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta‐analyses of seven long‐term studies. European Heart Journal 2004;25:10‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hedges 1992
- Hedges LV. Modeling publication selection effects in meta‐analysis. Statistical Science 1992;7:246‐255. [Google Scholar]
Kaiser 2004
- Kaiser T, Sawicki PT. Acarbose for prevention of diabetes, hypertension and cardiovascular events? A critical analysis of the STOP‐NIDDM data. Diabetologia 2004. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Laube 2002
- Laube H. Acarbose. An Update of Its Therapeutic Use in Diabetes Treatment. Clinical Drug Investigation 2002;22(3):141‐156. [MEDLINE: ] [Google Scholar]
Lebovitz 1998
- Lebovitz HE. Alpha‐Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Reviews 1998;6(2):132‐145. [MEDLINE: ] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. British Medical Journal 2003;326(7400):1167‐1170. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1996
- Martin AE, Montgomery PA. Acarbose: an alpha‐glucosidase inhibitor. American Journal of Health‐system Pharmacy 1996;53(19):2277‐2290. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navarro 2003
- Navarro J, Valdivieso M, Bonet A, Navarro A, Gosalbes V. Oral combination therapy for type 2 diabetes mellitus (Protocol for a Cochrane Review). The Cochrane Library Issue 4. [MEDLINE: ] [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
Reaven 1990
- Reaven GM, Lardinois CK, Greenfield MS, Schwartz HC, Vreman HJ. Effect of acarbose on carbohydrate and lipid metabolism in NIDDM patients poorly controlled by sulfonylureas. Diabetes Care 1990;13 Suppl 3:32‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rutten 2000
- Rutten GEHM, Verhoeven S, Heine RJ, Grauw WJC, Cromme PVM, Reenders K, et al. Dutch College of General Practitioners.Guidelines on Type 2 Diabetes [NHG Standaard diabetes mellitus type 2]. Huisarts en Wetenschap 2000;42(2):67‐84. [MEDLINE: ] [Google Scholar]
Scott 2000
- Scott LJ, Spencer CM. Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs 2000;59(3):521‐549. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van de Laar 2004b
- Laar FA, Lucassen PLBJ. No evidence for a reduction of myocardial infarctions by acarbose. European Heart Journal 2004;25(13):1179. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. World Health Organisation, 1980. [PubMed]
WHO 1985
- World Health Organisation. Diabetes Mellitus: Report of a WHO Study Group. Technical Report Series No. 727. World Health Organisation, 1985. [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van de Laar 2005
- Laar FA, Lucassen PL, Akkermans RP, Lisdonk EH, Rutten GE, Weel C. Alpha‐glucosidase inhibitors for patients with type 2 diabetes. Results from a Cochrane systematic review and meta‐analysis.. Diabetes Care 2005;28(1):154‐163. [DOI] [PubMed] [Google Scholar]


