Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited disorder causing kidney disease. Current clinical management of ADPKD focuses primarily on symptom control and reducing associated complications, particularly hypertension. In recent years, improved understanding of molecular and cellular mechanisms involved in kidney cyst growth and disease progression has resulted in new pharmaceutical agents to target disease pathogenesis to prevent progressive disease.
Objectives
We aimed to evaluate the effects of interventions for preventing ADPKD progression on kidney function, kidney endpoints, kidney structure, patient‐centred endpoints (such as cardiovascular events, sudden death, all‐cause mortality, hospitalisations, BP control, quality of life, and kidney pain), as well as the general and specific adverse effects related to their use.
Search methods
We searched the Cochrane Renal Group's Specialised Register to 6 June 2015 using relevant search terms.
Selection criteria
Randomised controlled trials (RCTs) comparing any interventions for preventing the progression of ADPKD with other interventions or placebo were considered for inclusion without language restriction.
Data collection and analysis
Two authors independently assessed study risks of bias and extracted data. We summarised treatment effects on clinical outcomes, kidney function and structure and adverse events using random effects meta‐analysis. We assessed heterogeneity in estimated treatment effects using the Cochran Q test and I2 statistic. Summary treatment estimates were calculated as a mean difference (MD) or standardised mean difference (SMD) for continuous outcomes and a risk ratio (RR) for dichotomous outcomes together with their 95% confidence intervals.
Main results
We included 30 studies (2039 participants) that investigated 11 pharmacological interventions (angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), calcium channel blockers, beta blockers, vasopressin receptor 2 (V2R) antagonists, mammalian target of rapamycin (mTOR) inhibitors, somatostatin analogues, antiplatelet agents, eicosapentaenoic acids, statins and vitamin D compounds) in this review.
ACEi significantly reduced diastolic blood pressure (9 studies, 278 participants: MD ‐4.96 mm Hg, 95% CI ‐8.88 to ‐1.04), but had uncertain effects on kidney volumes (MD ‐42.50 mL, 95% CI ‐115.68 to 30.67), GFR (MD ‐3.41 mL/min/1.73 m2, 95% CI ‐15.83 to 9.01), and SCr (MD ‐0.02 mg/dL, 95% CI ‐0.14 to 0.09), in data largely restricted to children. ACEi did not show different effects on GFR (MD ‐8.19 mL/min/1.73 m2, 95% CI ‐29.46 to 13.07) and albuminuria (SMD ‐0.19, 95% CI ‐1.77 to 1.39) when compared with beta‐blockers, or SCr (MD 0.00 mg/dL, 95% CI ‐0.09 to 0.10) when compared with ARBs.
Data for effects of V2R antagonists on kidney function and volumes compared to placebo were limited to narrative information within a single study while these agents increased thirst (1444 participants: RR 2.70, 95% CI 2.24 to 3.24) and dry mouth (1455 participants: RR 1.33, 95% CI 1.01 to 1.76).
Compared with no treatment, mTOR inhibitors had uncertain effects on kidney function (2 studies, 115 participants: MD 4.45 mL/min/1.73 m2, 95% CI ‐3.20 to 12.11) and kidney volume (MD ‐0.08 L, 95% CI ‐0.75 to 0.59) but in three studies (560 participants) caused angioedema (RR 13.39, 95% CI 2.56 to 70.00), oral ulceration (RR 6.77, 95% CI 4.42 to 10.38), infections (RR 1.14, 95% CI 1.04 to 1.25) and diarrhoea (RR 1.70, 95% CI 1.26 to 2.29).
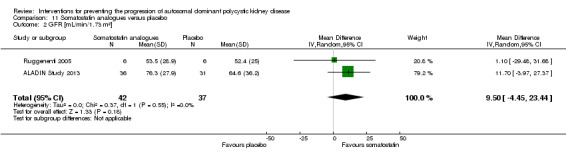
Somatostatin analogues (6 studies, 138 participants) slightly improved SCr (MD ‐0.43 mg/dL, 95% CI ‐0.86 to ‐0.01) and total kidney volume (MD ‐0.62 L, 95% CI ‐1.22 to ‐0.01) but had no definite effects on GFR (MD 9.50 mL/min, 95% CI ‐4.45 to 23.44) and caused diarrhoea (RR 3.72, 95% CI 1.43 to 9.68).
Data for calcium channel blockers, eicosapentaenoic acids, statins, vitamin D compounds and antiplatelet agents were sparse and inconclusive.
Random sequence generation was adequate in eight studies, and in almost half of the studies, blinding was not present or not specified. Most studies did not adequately report outcomes, which adversely affected our ability to assess this bias. The overall drop‐out rate was over 10% in nine studies, and few were conducted using intention‐to‐treat analyses.
Authors' conclusions
Although several interventions are available for patients with ADPKD, at present there is little or no evidence that treatment improves patient outcomes in this population and is associated with frequent adverse effects. Additional large randomised studies focused on patient‐centred outcomes are needed.
Plain language summary
Which therapies are the most effective to prevent the progression of autosomal dominant polycystic kidney disease?
Current clinical care for people who have autosomal dominant polycystic kidney disease (ADPKD) focuses on controlling future risks for need for dialysis and symptom management, mainly pain and bleeding. Newly discovered molecules that may slow kidney cyst growth has recently switched attention from care and treatment toward preventing disease progression and symptom control.
In this review, we aimed to analyse the benefits and harms of interventions directed at preventing progression of ADPKD. The literature was searched to 6 June 2015. We found 30 studies (involving 2039 participants) that tested 11 different treatments.
Reported outcomes were mostly limited to kidney function and volume. In evidence largely limited to children, it was found that ACEi (angiotensin converting enzyme inhibitor) medicines significantly reduced diastolic blood pressure but had uncertain effects on kidney volumes and how well the kidneys work (tested by measuring the glomerular filtration rate (GFR) and serum creatinine level in patients' blood). In adults, ACEi did not show different effects on GFR and the amount of a protein called albumin in the urine (albuminuria) when compared with beta blockers, or serum creatinine when compared with drugs known as ARBs (angiotensin II receptor blockers). Evidence from a single study was inconclusive concerning the effects of vasopressin receptor 2 antagonists on kidney function and volumes; however, these drugs made patients thirsty and caused dry mouth. Compared with no treatment, the group of medicines known as mTOR inhibitors (mammalian target of rapamycin inhibitors) had uncertain effects on kidney function and volume but caused soft tissue swelling, mouth ulcers, infections and diarrhoea. Drugs known as somatostatin analogues slightly improved serum creatinine and total kidney volume but had no definite effects on GFR and caused diarrhoea. Data for other drugs were sparse and inconclusive.
There is currently insufficient evidence to show that drugs used for people with ADPKD can protect kidney function to delay needing dialysis or a kidney transplant. Further evidence from large, well‐designed clinical studies is needed to inform healthcare decision making before these drugs can be chosen routinely to achieve better health outcomes for people with ADPKD.
Background
Description of the condition
ADPKD is the most common inherited disorder that affects kidney function and is a major cause of end‐stage kidney disease (ESKD). ADPKD is characterised by uncontrolled growth of kidney cysts that alter normal kidney structure and progressively impair kidney function. This means that people with ADPKD often require dialysis or kidney transplantation. Globally, over 12 million people currently live with ADPKD, of whom about 700,000 live in the US (Harris 2009). Annual incidence rates range from 4.0 to 8.7 per million people globally (Torres 2007). Recent data suggest that ADPKD accounts for about 5% of new patients commencing RRT in the US (USRDS 2008) and 3% to 10% in Europe (ERA‐EDTA 2011). By 60 years of age, about half of all people with ADPKD develop ESKD (Torres 2009).
ADPKD is a heterogeneous genetic disorder: it can evolve from mutation of the PKD1 (on chromosome 16p13.3) or PKD2 (on chromosome 4q21) genes, which encode two different polycystins. PKD1 mutations account for about 85% of all ADPKD and are usually associated with a more severe phenotype characterised by an earlier appearance, greater numbers of cysts and faster progression to ESKD. Increases in cyst numbers and size over time lead to hypertension, bleeding, infections, discomfort and pain. Cyst expansion is a major factor for the progressive loss of functional kidney tissue and function, which results from both direct (parenchymal compression) or indirect (fibrosis) mechanisms.
Description of the intervention
Healthcare for people with ADPKD principally focuses on controlling secondary conditions, particularly hypertension, to limit morbidity and mortality after the disease becomes symptomatic. Specific interventions targeting the pathogenesis of ADPKD have yet to be validated in clinical practice. Recent developments arising from better mechanistic understanding of the molecular pathways involved in cyst growth have made targeting disease pathogenesis, rather than disease complications, possible. However, although many interventions have shown promise in experimental models, few have been tested in clinical studies, and available interventions data have not been summarised previously.
How the intervention might work
Cyst growth can be targeted at different levels. Cyclic adenosine monophosphate (cAMP) plays a central role in cystogenesis (Hanaoka 2000). A hormone, arginine‐vasopressin (AVP), is the main inductor of cAMP production, working to activate an enzyme, adenylate‐cyclase, via vasopressin receptor‐2 (VR2) binding. Administration of V2R antagonists has been shown to reduce cyst and kidney volume and prevent kidney function impairment in polycystic kidney disease/vasopressin (PKD/AVP) knock‐out rats (Gattone 2003). cAMP levels can also be lowered by reducing the amount of circulating AVP by increasing water intake to reduce serum osmolality that can suppress the central release of AVP. Experimental findings confirm that chronic high fluid intake is effective in limiting cyst growth (Nagao 2006).
cAMP accumulation can be prevented by stimulating the somatostatin receptors (SRs) SST2 (Masyuk 2007). The unexpected finding that somatostatin administration was effective in stabilising cyst volume in an ADPKD patient with pituitary adenoma (a type of brain tumour) prompted interest in testing the efficacy of SR‐agonists (octreotide, lanreotide) using systematic approaches (Torres 2007).
A protein, tuberin, a regulator of mTOR kinase, is another potential target. This was initially investigated following a retrospective analysis that showed both liver and kidney volume decreased among people with ADPKD who received rapamycin therapy following kidney transplantation (Qian 2008) and confirmed by experimental models (Wahl 2006; Wu 2007) where the administration of mTOR inhibitors limited cyst enlargement and slowed progression of chronic kidney disease (CKD).
Other interventions, including dietary supplements of long‐chain omega 3 polyunsaturated (eicosapentaenoic) fatty acids (Ogborn 2000), and administration of statins (Gile 1995), have demonstrated efficacy to slow kidney impairment and contract cyst growth in different experimental models of PKD, probably as a result of a specific kidney anti‐inflammatory effect. However, it remains unclear whether other interventions broadly used to slow CKD, such as ACEis and ARBs, produce similar beneficial effects on kidney function in people with ADPKD (Schrier 2009). An ongoing clinical study, HALT‐PKD (Torres 2012), has been designed to clarify whether the combination of ACEi and ARBs could be more effective than ACEi alone to slow the decline of GFR in people with ADPKD who have stage 3 CKD and prevent CKD onset in earlier stages.
Why it is important to do this review
Kidney cyst growth usually precedes GFR decline by several years (Grantham 2006; Grantham 2008). This suggests that early approaches targeting ADPKD biology could be helpful to slow the progression of kidney disease and improve patient outcomes. However, no systematic assessments of the existing efficacy and safety evidence are yet available to inform practice or policy.
Objectives
Our objectives were to evaluate:
the effects of interventions to prevent progression of ADPKD as measured by kidney function (GFR, SCr), doubling of SCr concentration, proteinuria or urinary albumin excretion) and clinical endpoints (ESKD, need for RRT)
the effects of those interventions on kidney structure (total kidney volume, parenchymal volume, and kidney cyst volume)
the effects of those interventions on patient‐centred endpoints such as incidence of fatal and nonfatal cardiovascular events, sudden death, all‐cause mortality, hospitalisations, blood pressure control, quality of life, and kidney pain
general and specific adverse effects related to those interventions such as dizziness, diarrhoea, abdominal cramps and nausea (all treatments); hypernatraemia, thirst, dry mouth, and headache (V2R antagonists); angioedema and infections (mTOR inhibitors); alopecia (somatostatin agonists); and hyperkalaemia (ACEi and ARBs).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at interventions directed at preventing the progression of ADPKD have been included, without duration restrictions. The first period only was considered for randomised cross‐over studies. There were no language restrictions.
Types of participants
Inclusion criteria
Studies enrolling patients (adults or children) with clinical diagnosis of ADPKD (assessed by magnetic resonance imaging (magnetic nuclear imaging) or echo tomography fulfilling Ravine criteria) confirmed or unconfirmed by genetic tests, with kidney and cyst volumes of any dimension, and CKD stages 1 to 4, as defined by the by the US National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines were eligible for inclusion.
Exclusion criteria
ADPKD patients with CKD stage 5 (GFR < 15 mL/min/1.73 m2) and/or on haemodialysis and/or having undergone kidney transplantation were excluded from our analysis. Patients with autosomal recessive polycystic kidney disease (ARPKD) or other liver or kidney cystic diseases different from ADPKD were also excluded from the review.
Types of interventions
ACEi alone versus placebo, other therapy or both
ARB alone versus placebo, other therapy or both
ACEi versus ARB and standard therapy
ARB versus ACEi and standard therapy
ACEi plus ARB versus ACEi or ARB alone
VR2 antagonists (selective or nonselective) versus placebo and/or standard therapy
mTOR selective inhibitors alone or in association with other therapies versus placebo other therapy or both
Somatostatin agonists alone or in association with other therapies versus placebo and/or other therapies
Antiplatelet agents versus placebo, standard therapy or both
Eicosapentaenoic acids versus placebo, standard therapy or both
Statins versus placebo, standard therapy or both
Vitamin D or vitamin D derivatives versus other therapies
Increased versus standard fluid intake (as required).
Types of outcome measures
Outcomes were analysed at the end of treatment, and as change from beginning to end of treatment, where applicable.
Primary outcomes
Kidney function: SCr (mg/dL), measured or estimated GFR (eGFR) (mL/min or mL/min/1.73 m2), creatinine clearance (CrCl), doubling of creatinine, need for RRT or transplantation at the end of treatment.
Secondary outcomes
Total kidney volume (mL or L), total cyst volume (mL or L), total parenchymal volume (mL or L) assessed by magnetic nuclear imaging scan, echo tomography or computed tomography (CT)
Urinary protein excretion: 24 hour proteinuria or 24 hour albuminuria (mg/dL) (mg/d) or urine protein‐creatinine ratio (mg/g or g/g) or urine albumin‐creatinine ratio (mg/g or g/g)
Blood pressure (BP): systolic BP and diastolic BP (mm Hg), mean BP (mm Hg)
Fatal and nonfatal cardiovascular events including but not limited to myocardial infarction (MI), cerebrovascular accident (CVA), congestive heart failure (CHF)
All‐cause mortality
Quality of life (assessed by validated scales or any other instrument as reported by authors, such as SF‐36 or KDQOL‐SF questionnaires)
Kidney pain (rate of episodes or subjective perception as assessed by any analogue pain scale)
Any admission to hospital and duration of hospital stay (if long‐term data were available from the studies)
Adverse events: including but not limited to dizziness, diarrhoea, abdominal cramps and nausea (all treatments), hypernatraemia, thirst, dry mouth, transaminases elevation, and headache (V2R antagonists), angioedema, hyperlipidaemia, anaemia, oral ulcers and infections (mTOR inhibitors), alopecia (somatostatin agonists), hyperkalaemia (ACEi and ARBs).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register to 6 June 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from:
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register have been identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies as well as a list of hand‐searched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. Titles and abstracts were screened independently by two authors (DB, MR) who discarded studies that were not applicable; however, studies and reviews that might include relevant data or information were retained initially and reviewed in detail. The same two authors independently assessed retrieved abstracts, and if necessary the full text of these studies, to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors (DB, MR) using a standardised electronic data extraction form. Studies reported in non‐English and non‐Italian language journals were translated before assessment. Where more than one report of one study existed, reports were grouped together and the report with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier reports, these data were used. Any discrepancies between reports were highlighted.
Assessment of risk of bias in included studies
Risk of bias was independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Measures of treatment effect
For dichotomous outcomes (ESKD, need for RRT, all‐cause mortality, cardiovascular events, hospitalisations, adverse effects) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, results were reported as mean difference (MD) or standardised mean difference (SMD) if different scales were reported (SCr, GFR, proteinuria or albuminuria, BP, cyst and organ volumes, quality of life, kidney pain).
Unit of analysis issues
Data reported at the end of the first period of randomised cross‐over studies were considered.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population were carefully performed. Attrition rates, such as drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (such as last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.10 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% were considered to correspond to low, medium and high levels of heterogeneity, respectively.
Assessment of reporting biases
Although we had planned to investigate the existence possible small study bias, the overall paucity of available studies meant that it was not possible to conduct such assessment (Higgins 2011).
Data synthesis
Data for treatment effects were pooled using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We attempted to analyse where age (adults or children), stage and severity of disease (cyst and kidney dimensions at baseline, presence or absence of CKD), genetic background (mutations in PKD1 or PKD2 genes) and study follow‐up duration, were effect modifiers of the interventions studied. However, this was not possible due to the small number of included studies.
Sensitivity analysis
Sensitivity analyses were performed to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies
repeating the analysis taking account of risk of bias
repeating the analysis excluding any very long or large studies to establish how much they dominate the results
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Results
Description of studies
Results of the search
The search identified 232 records; one additional record identified from personal research was added. Full‐text assessment of 93 records resulted in the inclusion of 30 eligible studies (69 reports) that enrolled a total of 2039 participants with ADPKD (AIPRI Study 1996; ALADIN Study 2013; ELATE Study 2011; Biao 1997; Cadnapaphornchai 2005; Ecder 1999; Fassett 2010; Higashihara 2008; Hogan 2010; LOCKCYST Study 2009; Melemadathil 2013; Mora 2013; Nakamura 2001d; Nakamura 2012a; Nutahara 2005; RAPYD Study 2012; Ruggenenti 2005; SIRENA Study 2010; Soliman 2009; SUISSE ADPKD Study 2007; Temmerman 2012; TEMPO 248 & 249 2005; TEMPO 250 2011; TEMPO 3‐4 Study 2011; Ulusoy 2010; van Dijk 2001; van Dijk 2003; Walz 2010; Watson 1999; Zeltner 2008) and three ongoing studies (three reports) (DIPAK 1 Study 2014; NCT00345137; NCT01932450). Authors of some included studies were contacted for additional information with respect to study methods and/or unreported data; four investigators responded to our queries (LOCKCYST Study 2009; Soliman 2009; Temmerman 2012; Walz 2010). Figure 1 depicts the study inclusion and exclusion process.
1.
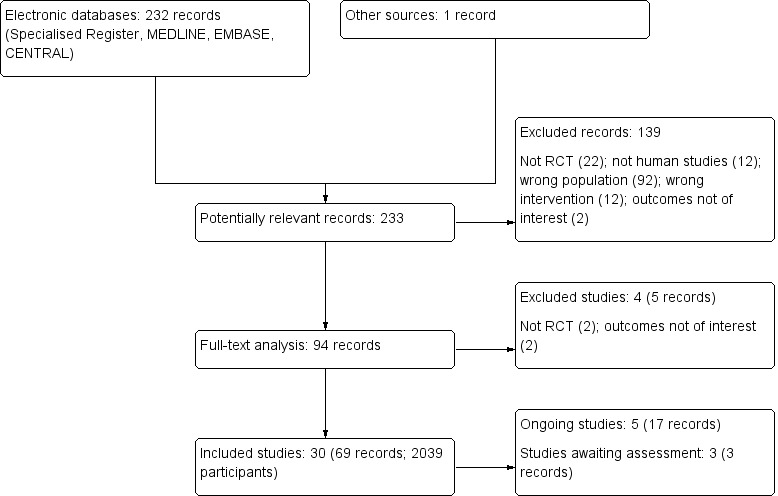
Study flow diagram
Prior to publication of this review a final search of the Specialised Register identified three new potential studies and these will be assessed for inclusion in a future update of this review (Braun 2014; NCT01233869; Vienna RAP Study 2015).Two ongoing studies have recently been completed and will be assessed in a future update of this review (Cadnapaphornchai 2011; HALT‐PKD Study 2008)
Included studies
Among the included studies, three were cross‐over studies (Ruggenenti 2005; SIRENA Study 2010; van Dijk 2001). In five studies (AIPRI Study 1996; ELATE Study 2011; Hogan 2010; LOCKCYST Study 2009; Temmerman 2012) ADPKD patients represented a subpopulation of the study cohort, but separate data for the main study outcomes were only available in two (ELATE Study 2011; LOCKCYST Study 2009). The number of participants was not specified in Watson 1999. With the exception of Cadnapaphornchai 2005 and Mora 2013, all studies were conducted in adults. Study duration ranged from five days to 60 months.
ADPKD assessment at baseline and end of treatment was performed by echo tomography in 12 studies (Biao 1997; Cadnapaphornchai 2005; Ecder 1999; Fassett 2010; Nakamura 2001d; Nakamura 2012a; Nutahara 2005; Ulusoy 2010; van Dijk 2001; van Dijk 2003; Watson 1999; Zeltner 2008); computed tomography in seven studies (ELATE Study 2011; Higashihara 2008; Hogan 2010; LOCKCYST Study 2009; Ruggenenti 2005; SIRENA Study 2010; Temmerman 2012); and magnetic nuclear resonance imaging in nine studies (ALADIN Study 2013; Melemadathil 2013; Mora 2013; RAPYD Study 2012; Soliman 2009; SUISSE ADPKD Study 2007; TEMPO 250 2011; TEMPO 3‐4 Study 2011; Walz 2010). Methods of assessment were not specified in two studies (AIPRI Study 1996; TEMPO 248 & 249 2005).
Genetic characterisation of PKD mutations was only made only in RAPYD Study 2012, Melemadathil 2013 (according to both study protocols only participants with the PKD1 mutation were enrolled), and Ruggenenti 2005 (patients with PKD1 and PKD2 mutations were both enrolled).
All studies excluded patients with eGFR < 15 mL/min/1.73 m2. Mean eGFR ranged from 38.2 to 124 mL/min in adult ADPKD patients and from 102 to 142 mL/min in children.
Total kidney volume was estimated in 16 studies (ALADIN Study 2013; Cadnapaphornchai 2005; ELATE Study 2011; Higashihara 2008; Hogan 2010; LOCKCYST Study 2009; Melemadathil 2013; Mora 2013; RAPYD Study 2012; Ruggenenti 2005; SIRENA Study 2010; Soliman 2009; SUISSE ADPKD Study 2007; Hogan 2010; TEMPO 3‐4 Study 2011; Walz 2010) with mean values ranging from 1000 to 2845 mL in adults and from 157 to 315 mL in children.
Total cyst volume was analysed in six studies (ALADIN Study 2013; Melemadathil 2013; RAPYD Study 2012; Ruggenenti 2005; SIRENA Study 2010; Walz 2010) with mean values ranging from 140 to 1709 mL. Total parenchymal volume was calculated in five studies (ALADIN Study 2013; Melemadathil 2013; Ruggenenti 2005; SIRENA Study 2010; Walz 2010) with values ranging from 242 to 680 mL.
Enalapril, ramipril or benazepril (ACEi) were compared to the following.
Placebo or standard therapy in three studies (Cadnapaphornchai 2005; van Dijk 2003; AIPRI Study 1996; 147 participants)
Amlodipine (calcium channel blocker) in one study (Ecder 1999; 24 participants)
Losartan or telmisartan (ARB) in two studies (Nakamura 2012a; Ulusoy 2010; 42 participants)
Atenolol or metoprolol (beta blockers) in three studies (van Dijk 2003; Watson 1999; Zeltner 2008; 65 participants).
Other comparisons were as follows.
Ramipril at a starting dose of 2.5 mg versus ramipril plus rapamycin (mTOR inhibitor) at low or high target doses (RAPYD Study 2012; 55 participants)
Telmisartan alone versus telmisartan plus sirolimus (mTOR inhibitor) (Soliman 2009; 16 participants)
Candesartan (ARB) 2 to 8 mg/d versus to amlodipine (Nutahara 2005) (49 participants)
High doses (60 + 30 mg/d) tolvaptan (selective V2R antagonist) versus low doses (45 + 15 mg/d) (TEMPO 250 2011; 46 participants)
Tolvaptan versus placebo (TEMPO 250 2011; TEMPO 3‐4 Study 2011; 1491 participants)
Rapamycin, everolimus or sirolimus (mTOR inhibitors) alone versus placebo or standard therapy in five studies (Melemadathil 2013; Mora 2013; SIRENA Study 2010; SUISSE ADPKD Study 2007; Walz 2010; 616 participants).
Octreotide or lanreotide (long‐acting somatostatin analogues) versus placebo in one parallel (ALADIN Study 2013; 79 participants) and four cross‐over studies (Ruggenenti 2005 (12 participants); LOCKCYST Study 2009 (32 participants); Hogan 2010; Temmerman 2012 (48 participants))
Octreotide alone versus octreotide plus everolimus (ELATE Study 2011; 15 participants)
Dilazep dihydrochloride (antiplatelet agent) versus placebo (Nakamura 2001d; 22 participants)
Eicosapentaenoic acids (2.4 g/d) versus standard therapy (Higashihara 2008; 41 participants)
Pravastatin or simvastatin (statins) versus placebo or standard therapy (Fassett 2010; van Dijk 2001; 69 participants)
Calcitriol (vitamin D) at 0.25 to 1 µg/d versus traditional Chinese medicine (herbs) (Biao 1997; 34 participants).
Excluded studies
After title and abstract review we excluded 139 records Figure 1. Reasons for initial exclusion were: inappropriate population (92); inappropriate intervention (12); not randomised (22); non‐clinical studies (12); outcomes not relevant to this review (2). Four studies (five reports) were excluded after full text evaluation; two studies were not RCTs (Kanno 1996; Sharma 2004); and two studies investigated outcomes that were not relevant to this review (Doulton 2006; Nakamura 2005a). One study was excluded as it was halted in 2008 due to lack of funding (ISRCTN57653760).
Risk of bias in included studies
Summaries of risk of bias in the included studies are depicted in Figure 2 and Figure 3. The overall risk of bias was highly variable since in most studies the information provided (particularly on allocation, blinding of investigators and outcome assessors and attrition) was not sufficient to permit judgment. In some cases, authors were contacted for additional information but only four investigators responded to our queries (LOCKCYST Study 2009; Soliman 2009; Temmerman 2012; Walz 2010)
2.
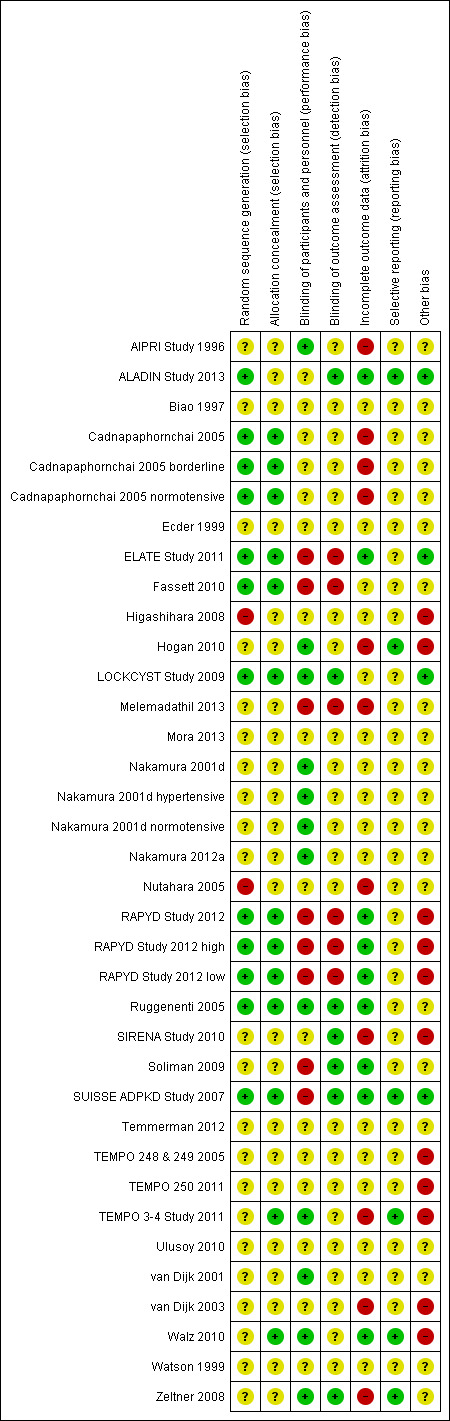
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
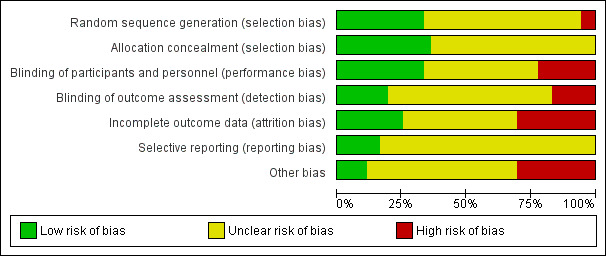
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Random sequence generation was low risk in eight studies (ALADIN Study 2013; Cadnapaphornchai 2005; ELATE Study 2011; Fassett 2010; LOCKCYST Study 2009; RAPYD Study 2012; Ruggenenti 2005; SUISSE ADPKD Study 2007), high risk in two studies (Higashihara 2008; Nutahara 2005); and there were insufficient data to inform assessment in the remaining 20 studies.
Allocation concealment was low risk in nine studies (Cadnapaphornchai 2005; ELATE Study 2011; Fassett 2010; LOCKCYST Study 2009; RAPYD Study 2012; Ruggenenti 2005; SUISSE ADPKD Study 2007; TEMPO 3‐4 Study 2011; Walz 2010) and unclear in 21 studies.
Blinding
The quality of blinding overall was variable. In most cases, blinding of investigators and outcome assessors was not specified. Participants and investigators were blinded in 10 studies (AIPRI Study 1996; Hogan 2010; LOCKCYST Study 2009; Nakamura 2001d; Nakamura 2012a; Ruggenenti 2005; TEMPO 3‐4 Study 2011; van Dijk 2001; Walz 2010; Zeltner 2008) and not blinded in six studies (ELATE Study 2011; Fassett 2010; Melemadathil 2013; RAPYD Study 2012; Soliman 2009; SUISSE ADPKD Study 2007).
In ALADIN Study 2013, participants were blinded to the treatment while investigators were aware of the allocated group. Blinding was not specified in the remainder of the studies.
Outcome assessors were blinded in seven studies (ALADIN Study 2013; LOCKCYST Study 2009; Ruggenenti 2005; SIRENA Study 2010; Soliman 2009; SUISSE ADPKD Study 2007; Zeltner 2008) whereas in four studies (ELATE Study 2011; Fassett 2010; Melemadathil 2013; RAPYD Study 2012) assessors were aware of treatment allocation. Outcome assessor blinding was unclear in the remaining 19 studies.
Incomplete outcome data
Attrition bias overall was variable; in most studies, the information provided was insufficient to permit assessment. The overall drop‐out rate ranged from 1.6% to 33% with no apparent differences among groups, with the exception of seven studies (Cadnapaphornchai 2005; ELATE Study 2011; Melemadathil 2013; Nutahara 2005; RAPYD Study 2012; TEMPO 3‐4 Study 2011; Zeltner 2008). We found that the overall drop‐out rate was greater than 10% in nine studies (AIPRI Study 1996; Cadnapaphornchai 2005; ELATE Study 2011; Hogan 2010; Nutahara 2005; SIRENA Study 2010; TEMPO 3‐4 Study 2011; van Dijk 2003; Zeltner 2008). Six studies (ALADIN Study 2013; Nutahara 2005; RAPYD Study 2012; SUISSE ADPKD Study 2007; TEMPO 3‐4 Study 2011; Walz 2010) were analysed on an intention‐to‐treat basis. ELATE Study 2011 was analysed on both per‐protocol and intention‐to‐treat bases.
Selective reporting
All predefined outcomes were reported in six studies (ALADIN Study 2013; Hogan 2010; SUISSE ADPKD Study 2007; TEMPO 3‐4 Study 2011; Walz 2010; Zeltner 2008). Selective reporting was unclear in the remaining 24 studies.
Other potential sources of bias
We found that 12 studies reported receiving funding from industry (ALADIN Study 2013; ELATE Study 2011; Higashihara 2008; Hogan 2010; LOCKCYST Study 2009; RAPYD Study 2012; SIRENA Study 2010; TEMPO 248 & 249 2005; TEMPO 250 2011; TEMPO 3‐4 Study 2011; van Dijk 2003; Walz 2010). In three studies (ALADIN Study 2013; ELATE Study 2011; LOCKCYST Study 2009) the authors specified that the sponsor was not involved in the study design, data collection, data analysis, interpretation of the study results, or writing the manuscript.
Effects of interventions
Overall, outcomes reported were mostly confined to eGFR, SCr and kidney structure (kidney and cyst volumes) while patient‐centred outcomes including RRT, mortality, and treatment‐related hazards were infrequently reported.
Kidney function
Serum creatinine
Somatostatin analogues significantly reduced SCr compared to placebo (Analysis 11.1 (ALADIN Study 2013; Ruggenenti 2005, 91 participants): MD ‐0.43 mg/dL, 95% CI ‐0.86 to ‐0.01; I2 = 0%).
11.1. Analysis.
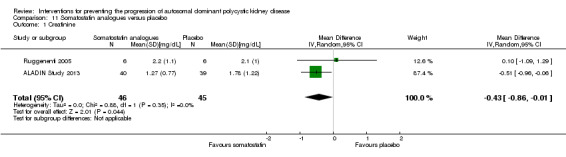
Comparison 11 Somatostatin analogues versus placebo, Outcome 1 Creatinine.
There were no significant differences in SCr for the following comparisons.
ACEi versus no treatment (Analysis 1.1 (Cadnapaphornchai 2005, 42 participants): MD ‐0.02 mg/dL, 95% CI ‐0.14 to 0.09; I2 = 23%)
ACEi versus CCB (Analysis 2.1 (Ecder 1999, 24 participants): MD 0.01 mg/dL, 95% CI ‐0.10 to 0.12)
ACEi versus ARB (Analysis 3.1 ( Nakamura 2012a; Ulusoy 2010, 52 participants): MD 0.00 mg/dL, 95% CI ‐0.09 to 0.10; I2 = 0%)
ACEi versus beta‐blockers (Analysis 4.1 (Zeltner 2008, 37 participants) MD 0.18 mg/dL, 95% CI ‐0.12 to 0.48)
ARB versus CCB (Analysis 7.1 (Nutahara 2005, 40 participants) MD ‐0.45 mg/dL, 95% CI ‐0.90 to ‐0.00)
VR2 antagonists versus placebo (Analysis 8.1 (TEMPO 3‐4 Study 2011, 1154 participants): MD ‐0.01 mg/dL, 95% CI ‐0.08 to 0.06)
High versus low dose V2R antagonists (Analysis 9.1 (TEMPO 250 2011, 46 participants): MD ‐0.12 mg/dL, 95% CI ‐0.36 to 0.12)
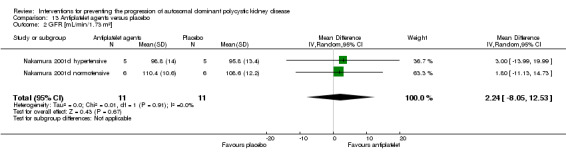
Antiplatelet agents versus placebo (Analysis 13.1 (Nakamura 2001d, 22 participants): MD ‐0.13 mg/dL, 95% CI ‐0.52 to 0.26; I2 = 69%)
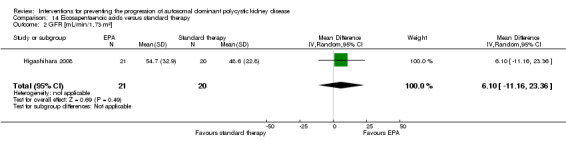
Eicosapentaenoic acid versus standard therapy (Analysis 14.1 (Higashihara 2008, 41 participants): RR 0.16, 95% CI ‐0.55 to 0.87)
1.1. Analysis.
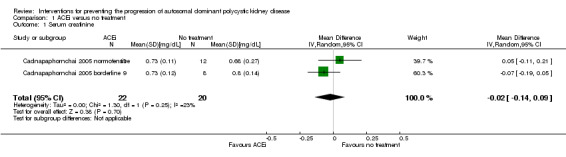
Comparison 1 ACEi versus no treatment, Outcome 1 Serum creatinine.
2.1. Analysis.
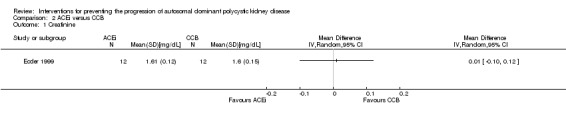
Comparison 2 ACEi versus CCB, Outcome 1 Creatinine.
3.1. Analysis.
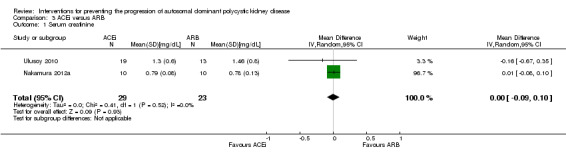
Comparison 3 ACEi versus ARB, Outcome 1 Serum creatinine.
4.1. Analysis.
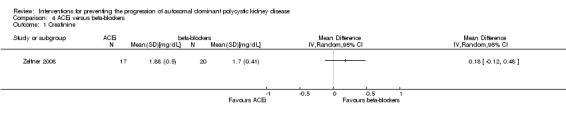
Comparison 4 ACEi versus beta‐blockers, Outcome 1 Creatinine.
7.1. Analysis.
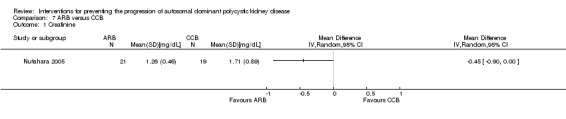
Comparison 7 ARB versus CCB, Outcome 1 Creatinine.
8.1. Analysis.
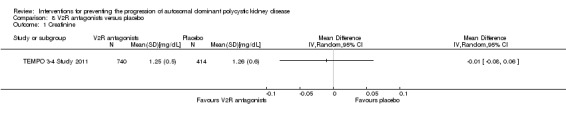
Comparison 8 V2R antagonists versus placebo, Outcome 1 Creatinine.
9.1. Analysis.
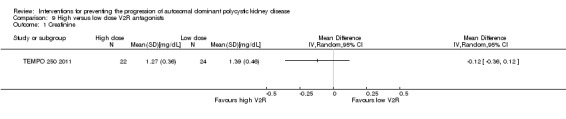
Comparison 9 High versus low dose V2R antagonists, Outcome 1 Creatinine.
13.1. Analysis.
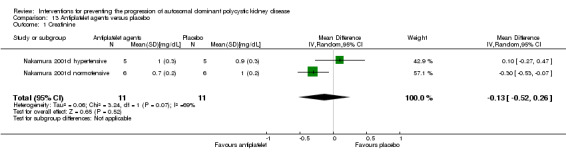
Comparison 13 Antiplatelet agents versus placebo, Outcome 1 Creatinine.
14.1. Analysis.

Comparison 14 Eicosapentaenoic acids versus standard therapy, Outcome 1 Creatinine.
Glomerular filtration rate
Ecder 1999 reported GFR was significantly lower in the ACEi group compared to the CCB group (Analysis 2.2 (24 participants): (MD ‐13.00 mL/min/1.73 m3, 95% CI ‐17.56 to ‐8.44).
2.2. Analysis.
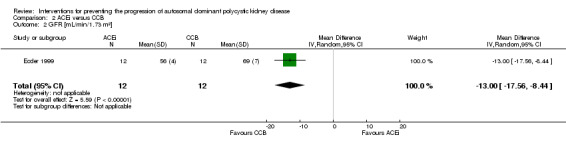
Comparison 2 ACEi versus CCB, Outcome 2 GFR [mL/min/1.73 m²].
Biao 1997 reported GFR was significantly higher in the vitamin D group compared to the Chinese herbal medicine group (Analysis 16.2 (34 participants): MD 22.60 mL/min, 95% CI 0.92 to 44.28).
16.2. Analysis.
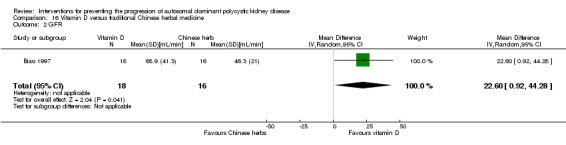
Comparison 16 Vitamin D versus traditional Chinese herbal medicine, Outcome 2 GFR.
There were no significant differences in GFR for the following comparisons.
ACEi versus not treatment (Analysis 1.2 (Cadnapaphornchai 2005; van Dijk 2003, 103 participants) MD ‐3.41 mL/min/1.73 m3, 95% CI ‐15.83 to 9.01; I2 = 46%)
ACEi versus ARB (Analysis 3.2 (Ulusoy 2010, 32 participants): MD ‐3.40 mL/min/1.73 m3, 95% CI ‐22.69 to 15.89)
ACEi versus beta‐blockers (Analysis 4.2 (van Dijk 2003; Zeltner 2008, 65 participants): MD ‐8.06 mL/min/1.73 m3, 95% CI ‐29.62 to 13.50; I2 = 95%)
ARB alone versus ARB + mTOR inhibitor (Analysis 6.1 (1 study, 16 participants): MD ‐9.60 mL/min/1.73 m3, 95% CI ‐28.18 to 8.98)
ARB versus CCB (Analysis 7.2 (Nutahara 2005, 31 participants): MD 6.30 mL/min/1.73 m3, 95% CI ‐8.49 to 21.09)
mTOR inhibitor versus no treatment (Analysis 10.1 (SIRENA Study 2010; SUISSE ADPKD Study 2007, 115 participants): MD 4.45 mL/min/1.73 m3, 95% CI ‐3.20 to 12.11; I2 = 0%)
Somatostatin analogues versus placebo (Analysis 11.2 (ALADIN Study 2013; Ruggenenti 2005, 79 participants): MD 9.50 mL/min/1.73 m3, 95% CI ‐4.45 to 23.44; I2 = 0%)
Antiplatelet agents versus placebo (Analysis 13.2 (Nakamura 2001d, 22 participants): MD 2.24 mL/min/1.73 m3, 95% CI ‐8.05 to 12.53; I2 = 0%)
Eicosapentaenoic acid versus standard therapy (Analysis 14.2 (Higashihara 2008, 41 participants): MD 6.10 mL/min/1.73 m3, 95% CI ‐11.16 to 23.36)
1.2. Analysis.
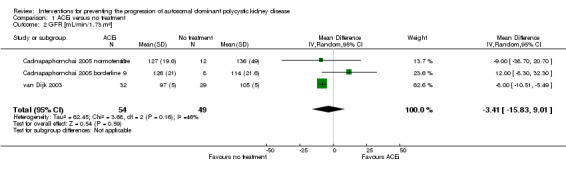
Comparison 1 ACEi versus no treatment, Outcome 2 GFR [mL/min/1.73 m²].
3.2. Analysis.
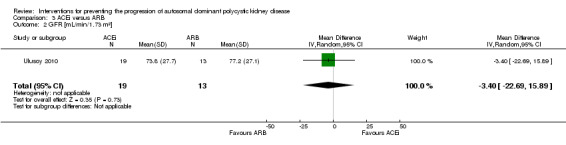
Comparison 3 ACEi versus ARB, Outcome 2 GFR [mL/min/1.73 m²].
4.2. Analysis.
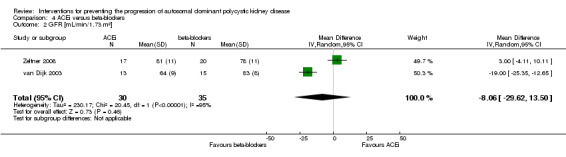
Comparison 4 ACEi versus beta‐blockers, Outcome 2 GFR [mL/min/1.73 m²].
6.1. Analysis.
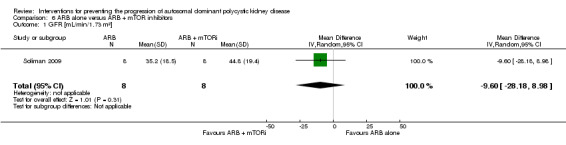
Comparison 6 ARB alone versus ARB + mTOR inhibitors, Outcome 1 GFR [mL/min/1.73 m²].
7.2. Analysis.
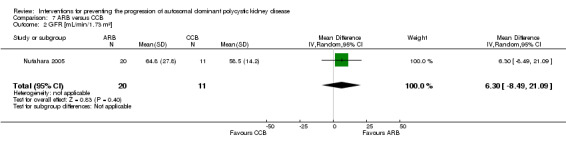
Comparison 7 ARB versus CCB, Outcome 2 GFR [mL/min/1.73 m²].
10.1. Analysis.
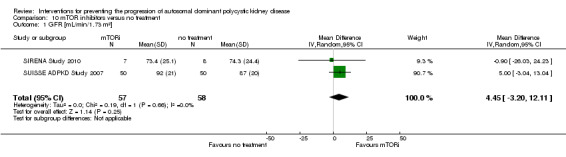
Comparison 10 mTOR inhibitors versus no treatment, Outcome 1 GFR [mL/min/1.73 m²].
11.2. Analysis.
Comparison 11 Somatostatin analogues versus placebo, Outcome 2 GFR [mL/min/1.73 m²].
13.2. Analysis.
Comparison 13 Antiplatelet agents versus placebo, Outcome 2 GFR [mL/min/1.73 m²].
14.2. Analysis.
Comparison 14 Eicosapentaenoic acids versus standard therapy, Outcome 2 GFR [mL/min/1.73 m²].
Doubling of creatinine
Fours studies reported doubling of creatinine; none reported any significant differences between the treatments studied.
ACEi versus no treatment (Analysis 1.3 (AIPRI Study 1996, 64 participants): RR 1.01, 95% CI 0.45 to 2.28)
ARB alone versus ARB + mTOR inhibitor (Analysis 6.2 (Soliman 2009, 16 participants): RR 3.00, 95% CI 0.39 to 23.07)
ARB versus CCB (Analysis 7.3 (Nutahara 2005, 49 participants): RR 0.17, 95% CI 0.02 to 1.34)
V2R antagonists versus placebo (Analysis 8.3 (TEMPO 3‐4 Study 2011, 1444 participants): RR 0.96, 95% CI 0.73 to 1.25).
1.3. Analysis.
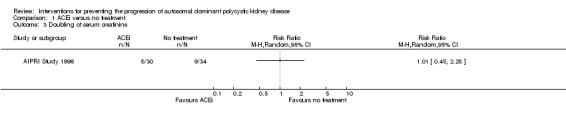
Comparison 1 ACEi versus no treatment, Outcome 3 Doubling of serum creatinine.
6.2. Analysis.

Comparison 6 ARB alone versus ARB + mTOR inhibitors, Outcome 2 Doubling of serum creatinine.
7.3. Analysis.

Comparison 7 ARB versus CCB, Outcome 3 Doubling of serum creatinine.
8.3. Analysis.

Comparison 8 V2R antagonists versus placebo, Outcome 3 Doubling of serum creatinine.
Need for renal replacement therapy or transplantation
Two studies reported need for RRT or transplantation; none reported any significant difference between the treatments studied.
ACEi versus beta‐blockers (Analysis 4.4 (Zeltner 2008, 37 participants): RR 0.39, 95% CI 0.02 to 8.97)
mTOR inhibitor versus no treatment: RRT (Analysis 10.3 (Walz 2010, 431 participants): RR 3.04, 95% CI 0.12 to 74.26); transplantation (Analysis 10.4 (Walz 2010, 431 participants): RR 1.01, 95% CI 0.06 to 16.11).
4.4. Analysis.
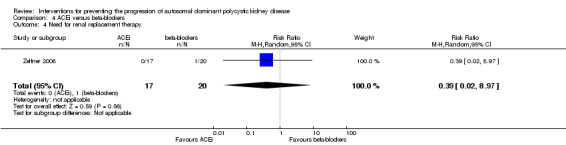
Comparison 4 ACEi versus beta‐blockers, Outcome 4 Need for renal replacement therapy.
10.3. Analysis.
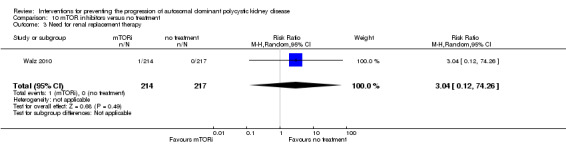
Comparison 10 mTOR inhibitors versus no treatment, Outcome 3 Need for renal replacement therapy.
10.4. Analysis.
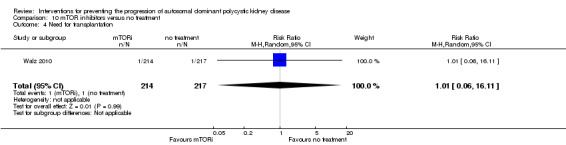
Comparison 10 mTOR inhibitors versus no treatment, Outcome 4 Need for transplantation.
Total kidney, cyst and parenchymal volume
Total kidney volume
Soliman 2009 reported a significant increase in total kidney volume with ARB alone compared to ARB + mTOR inhibitor (Analysis 6.3 (16 participants): MD 0.37 L, 95% CI 0.04 to 0.70).
6.3. Analysis.
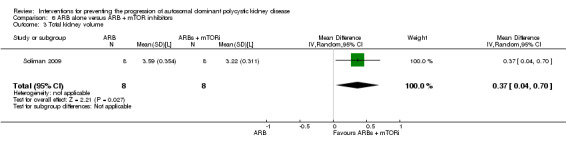
Comparison 6 ARB alone versus ARB + mTOR inhibitors, Outcome 3 Total kidney volume.
Somatostatin analogues significantly decreased total kidney volume compared to placebo (Analysis 11.3 (ALADIN Study 2013; LOCKCYST Study 2009; Ruggenenti 2005, 114 participants) MD ‐0.62 L, 95% CI ‐1.22 to ‐0.01; I2 = 11%).
11.3. Analysis.
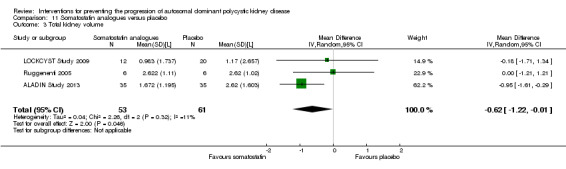
Comparison 11 Somatostatin analogues versus placebo, Outcome 3 Total kidney volume.
There were no significant differences in total kidney volume for the following comparisons.
ACEi versus no treatment (Analysis 1.4 (Cadnapaphornchai 2005, 42 participants): MD ‐42.50 mL, 95% CI ‐115.68 to 30.67; I2 = 0%)
ACEi alone versus ACEi + mTOR inhibitor (Analysis 5.2; (RAPYD Study 2012, 69 participants): MD 285.79 mL, 95% CI ‐21.92 to 593.50; I2 = 0%)
mTOR inhibitor versus no treatment Analysis 10.5; (SIRENA Study 2010; SUISSE ADPKD Study 2007, 115 participants): MD ‐0.08 L, 95% CI ‐0.75 to 0.59; I2 = 0%)
Eicosapentaenoic acid versus standard therapy Analysis 14.3 (Higashihara 2008, 41 studies): MD ‐209.00 mL, 95% CI ‐729.06 to 311.06)
1.4. Analysis.
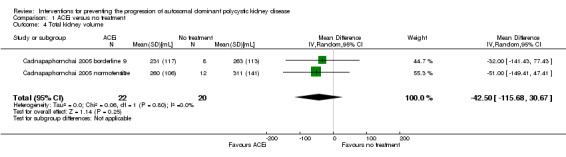
Comparison 1 ACEi versus no treatment, Outcome 4 Total kidney volume.
5.2. Analysis.
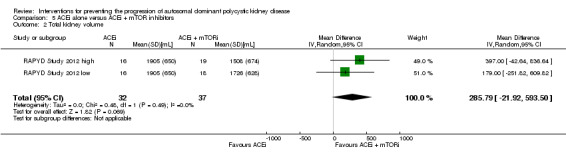
Comparison 5 ACEi alone versus ACEi + mTOR inhibitors, Outcome 2 Total kidney volume.
10.5. Analysis.
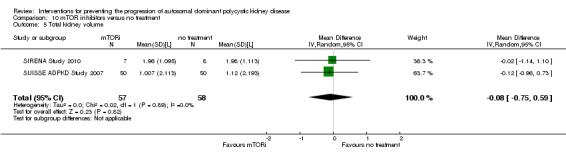
Comparison 10 mTOR inhibitors versus no treatment, Outcome 5 Total kidney volume.
14.3. Analysis.
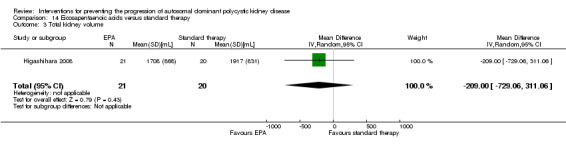
Comparison 14 Eicosapentaenoic acids versus standard therapy, Outcome 3 Total kidney volume.
Cyst volume
Four studies reported cyst volume; none reported any significant differences between the treatments studied.
ACEi alone versus ACEi + mTOR inhibitor (Analysis 5.3 (RAPYD Study 2012, 69 participants): MD 36.32 mL, 95% CI ‐6.99 to 79.64; I2 = 0%)
mTOR inhibitor versus no treatment (Analysis 10.7 (SIRENA Study 2010, 15 participants): MD ‐55.00 mL, 95% CI ‐862.98 to 752.98)
Somatostatin analogues versus placebo (Analysis 11.4 (ALADIN Study 2013; Ruggenenti 2005, 82 participants): MD ‐0.50 L, 95% CI ‐1.18 to 0.18; I2 = 37%).
5.3. Analysis.
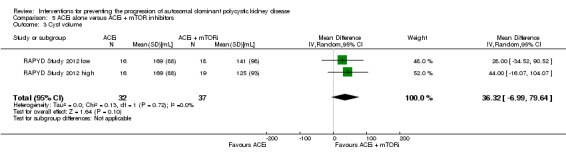
Comparison 5 ACEi alone versus ACEi + mTOR inhibitors, Outcome 3 Cyst volume.
10.7. Analysis.
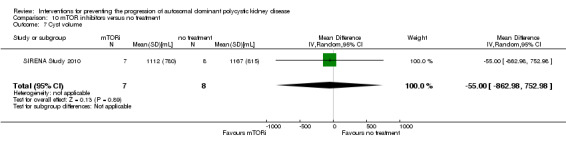
Comparison 10 mTOR inhibitors versus no treatment, Outcome 7 Cyst volume.
11.4. Analysis.
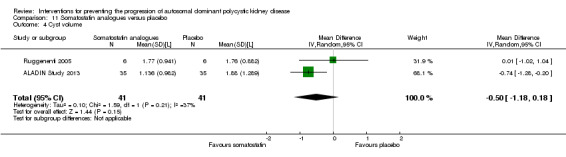
Comparison 11 Somatostatin analogues versus placebo, Outcome 4 Cyst volume.
Total parenchymal volume
Three studies reported total parenchymal volume; none reported any significant differences between the treatments studied.
mTOR inhibitor versus no treatment (Analysis 10.9 (SIRENA Study 2010, 15 participants): MD 15.00 mL, 95% CI ‐75.44 to 105.44)
Somatostatin analogues versus placebo (Analysis 11.5 (ALADIN Study 2013; Ruggenenti 2005, 82 participants): MD ‐67.67 mL, 95% CI ‐249.45 to 114.12; I2 = 78%).
10.9. Analysis.
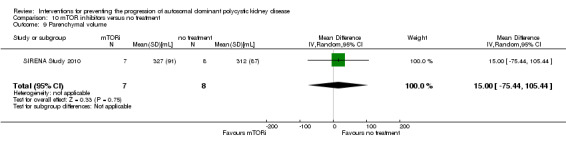
Comparison 10 mTOR inhibitors versus no treatment, Outcome 9 Parenchymal volume.
11.5. Analysis.
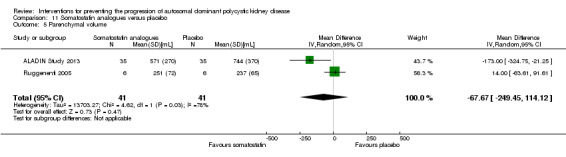
Comparison 11 Somatostatin analogues versus placebo, Outcome 5 Parenchymal volume.
Urinary protein excretion
Ecder 1999 reported a significant decrease in albuminuria with ACEi compared to CCB (Analysis 2.3 (24 participants): MD ‐134.00 mg/g, 95% CI ‐176.01 to ‐91.99).
2.3. Analysis.
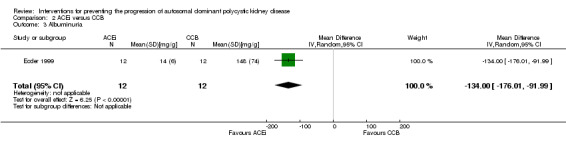
Comparison 2 ACEi versus CCB, Outcome 3 Albuminuria.
Nutahara 2005 reported ARB significantly decreased albuminuria (Analysis 7.4 (25 participants): MD ‐304.00 mg/d, 95% CI ‐578.54 to ‐29.46) and proteinuria (Analysis 7.5 (24 participants): MD ‐238.00 mg/d, 95% CI ‐394.61 to ‐81.39) compared to CCB.
7.4. Analysis.
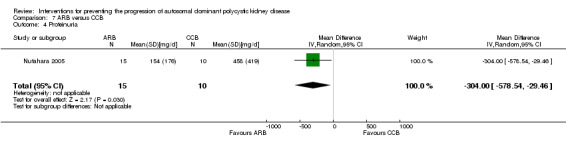
Comparison 7 ARB versus CCB, Outcome 4 Proteinuria.
7.5. Analysis.
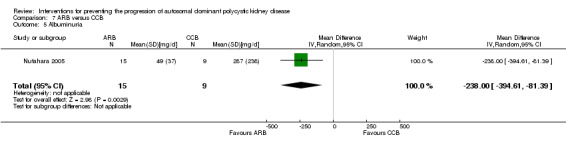
Comparison 7 ARB versus CCB, Outcome 5 Albuminuria.
There were no significant differences in either proteinuria or albuminuria for the following comparisons.
ACEi versus no treatment (Analysis 1.5 (Nakamura 2001d; van Dijk 2003, 103 participants): SMD ‐0.12, 95% CI ‐0.51 to 0.26; I2 = 0%)
ACEi versus beta‐blockers (Analysis 4.5 (van Dijk 2003; Zeltner 2008, (65 participants) SMD ‐0.19, 95% CI ‐1.77 to 1.39; I2 = 89%)
V2R antagonists versus placebo (Analysis 8.5 (TEMPO 3‐4 Study 2011, 1157 participants): MD ‐1.60 mg/mmol, 95% CI ‐3.95 to 0.75)
mTOR inhibitor versus no treatment: proteinuria (Analysis 10.11 (SIRENA Study 2010; Walz 2010, 446 participants): (SMD 0.34, 95% CI ‐0.29 to 0.98; I2 = 45%); albuminuria (Analysis 10.13 (SIRENA Study 2010; SUISSE ADPKD Study 2007, 115 participants): SMD 0.25, 95% CI ‐0.27 to 0.78; participants; I2 = 23%)
Somatostatin analogues versus placebo: proteinuria (Analysis 11.6 (ALADIN Study 2013, 79 participants): MD ‐0.05 g/24 h, 95% CI ‐0.17 to 0.07); albuminuria (Analysis 11.7, (ALADIN Study 2013; Ruggenenti 2005, 91 participants): SMD ‐0.10, 95% CI ‐0.51 to 0.31; I2 = 0%)
Antiplatelet agent versus placebo (Analysis 13.3 (Nakamura 2001d, 22 participants): MD ‐60.53 µg/min, 95% CI ‐129.06 to 8.01; I2 = 74%).
1.5. Analysis.
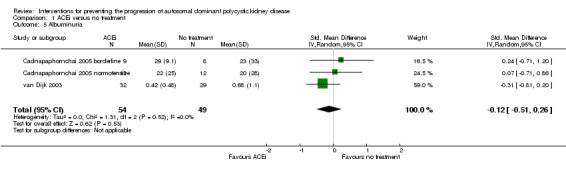
Comparison 1 ACEi versus no treatment, Outcome 5 Albuminuria.
4.5. Analysis.
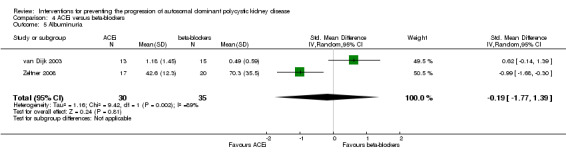
Comparison 4 ACEi versus beta‐blockers, Outcome 5 Albuminuria.
8.5. Analysis.
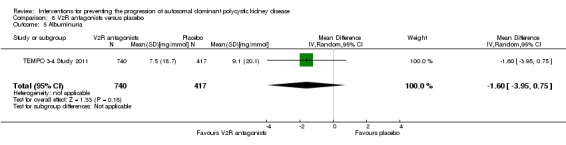
Comparison 8 V2R antagonists versus placebo, Outcome 5 Albuminuria.
10.11. Analysis.
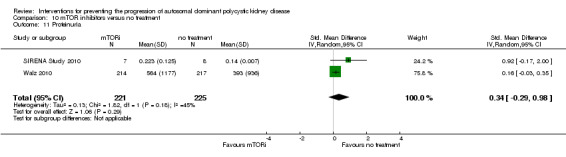
Comparison 10 mTOR inhibitors versus no treatment, Outcome 11 Proteinuria.
10.13. Analysis.
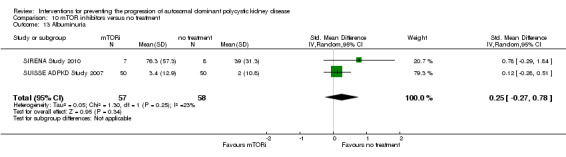
Comparison 10 mTOR inhibitors versus no treatment, Outcome 13 Albuminuria.
11.6. Analysis.
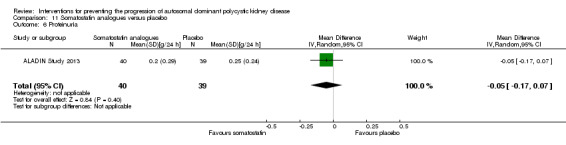
Comparison 11 Somatostatin analogues versus placebo, Outcome 6 Proteinuria.
11.7. Analysis.
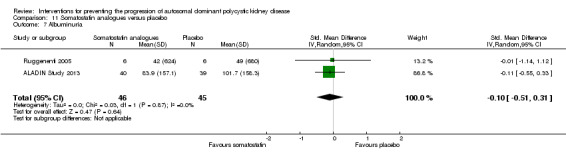
Comparison 11 Somatostatin analogues versus placebo, Outcome 7 Albuminuria.
13.3. Analysis.
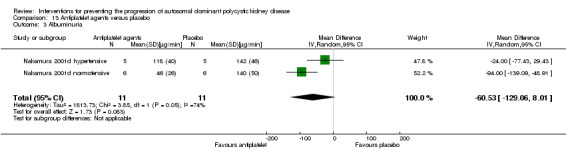
Comparison 13 Antiplatelet agents versus placebo, Outcome 3 Albuminuria.
Blood pressure
Systolic blood pressure
Ecder 1999 reported ACEi significantly decreased systolic BP compared to CCB (Analysis 2.4 (24 participants): MD ‐5.00 mm Hg, 95% CI ‐8.62 to ‐1.38).
2.4. Analysis.
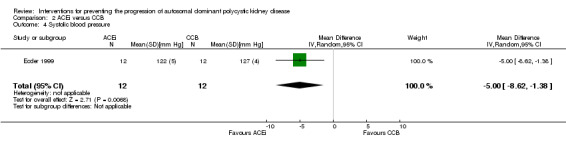
Comparison 2 ACEi versus CCB, Outcome 4 Systolic blood pressure.
TEMPO 250 2011 reported high dose V2R antagonists significantly reduced systolic BP compared to low dose V2R antagonists (Analysis 9.2 ( 46 participants): MD ‐9.00 mm Hg, 95% CI ‐16.98 to ‐1.02).
9.2. Analysis.
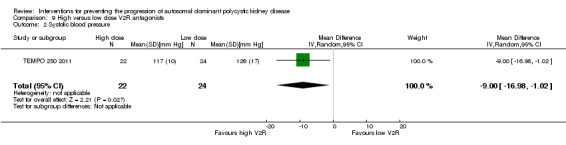
Comparison 9 High versus low dose V2R antagonists, Outcome 2 Systolic blood pressure.
There were no significant differences in systolic BP for the following comparisons.
ACEi versus no treatment (Analysis 1.6 (Cadnapaphornchai 2005, 42 participants): MD ‐5.44 mm Hg, 95% CI ‐14.26 to 3.38; I2 = 96%)
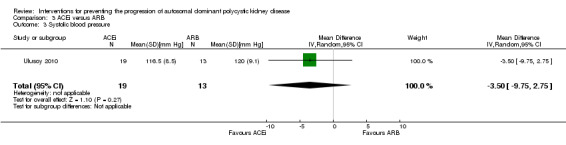
ACEi versus ARB (Analysis 3.3 (Ulusoy 2010, 32 participants): MD ‐3.50 mm Hg, 95% CI ‐9.75 to 2.75)
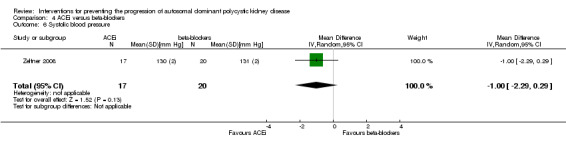
ACEi versus beta‐blocker (Analysis 4.6 (Zeltner 2008, 37 participants): MD ‐1.00 mm Hg, 95% CI ‐2.29 to 0.29)
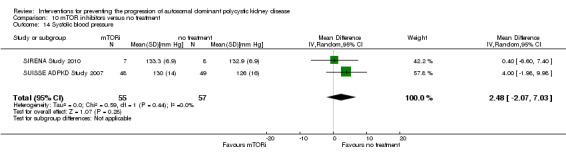
mTOR inhibitor versus no treatment (Analysis 10.14 (SIRENA Study 2010; SUISSE ADPKD Study 2007, 112 participants): MD 2.48 mm Hg, 95% CI ‐2.07 to 7.03; I2 = 0%)
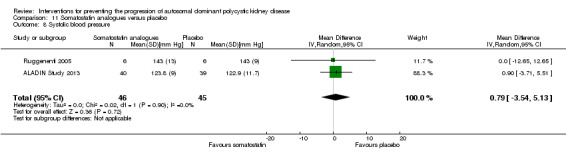
Somatostatin analogues versus placebo (Analysis 11.8 (ALADIN Study 2013; Ruggenenti 2005, 91 participants): MD 0.79 mm Hg, 95% CI ‐3.54 to 5.13; I2 = 0%)
Antiplatelet agent versus placebo (Analysis 13.4 (Nakamura 2001d, 22 participants): MD 5.04 mm Hg, 95% CI ‐7.34 to 17.43; I2 = 0%)
Statins versus no treatment (Analysis 15.4 (Fassett 2010, 49 participants): MD 1.70 mm Hg, 95% CI ‐6.39 to 9.79).
1.6. Analysis.
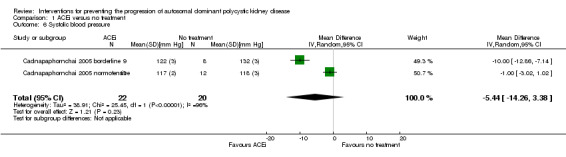
Comparison 1 ACEi versus no treatment, Outcome 6 Systolic blood pressure.
3.3. Analysis.
Comparison 3 ACEi versus ARB, Outcome 3 Systolic blood pressure.
4.6. Analysis.
Comparison 4 ACEi versus beta‐blockers, Outcome 6 Systolic blood pressure.
10.14. Analysis.
Comparison 10 mTOR inhibitors versus no treatment, Outcome 14 Systolic blood pressure.
11.8. Analysis.
Comparison 11 Somatostatin analogues versus placebo, Outcome 8 Systolic blood pressure.
13.4. Analysis.
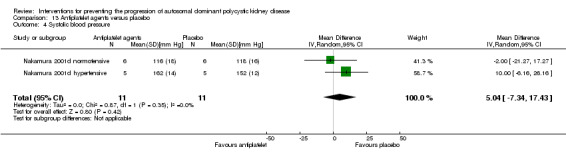
Comparison 13 Antiplatelet agents versus placebo, Outcome 4 Systolic blood pressure.
15.4. Analysis.
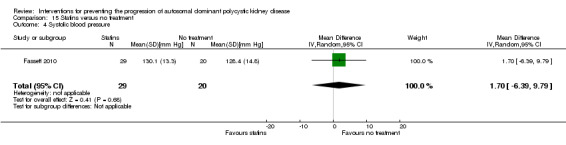
Comparison 15 Statins versus no treatment, Outcome 4 Systolic blood pressure.
Diastolic blood pressure
Cadnapaphornchai 2005 reported ACEi significantly reduce diastolic BP compared to no treatment (Analysis 1.7 (42 participants): MD ‐4.96 mm Hg, 95% CI ‐8.88 to ‐1.04; I2 = 90%)
1.7. Analysis.
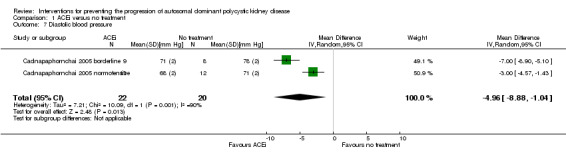
Comparison 1 ACEi versus no treatment, Outcome 7 Diastolic blood pressure.
Ecder 1999 reported ACEi significantly decreased diastolic BP compared to CCB (Analysis 2.5 (24 participants): MD ‐3.00 mm Hg, 95% CI ‐5.40 to ‐0.60)
2.5. Analysis.
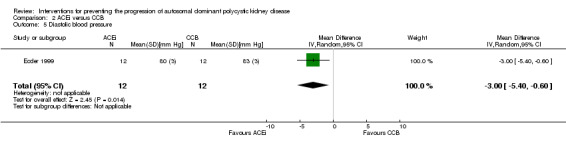
Comparison 2 ACEi versus CCB, Outcome 5 Diastolic blood pressure.
Zeltner 2008 reported beta‐blockers significantly decrease diastolic BP compared to ACEi (Analysis 4.7 (37 participants): MD 1.00 mm Hg, 95% CI 0.35 to 1.65)
4.7. Analysis.
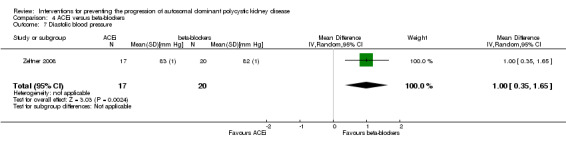
Comparison 4 ACEi versus beta‐blockers, Outcome 7 Diastolic blood pressure.
TEMPO 250 2011 reported high dose V2R antagonists significantly reduced diastolic BP compared to low dose V2R antagonists (Analysis 9.3 (46 participants): MD ‐6.00 mm Hg, 95% CI ‐11.21 to ‐0.79)
9.3. Analysis.
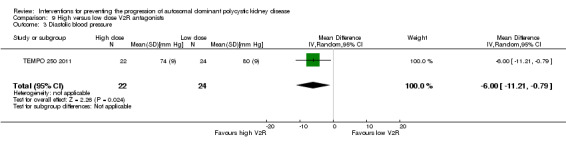
Comparison 9 High versus low dose V2R antagonists, Outcome 3 Diastolic blood pressure.
There were no significant differences in diastolic BP for the following comparisons.
ACEi versus ARB (Analysis 3.4 (Ulusoy 2010, 32 participants): MD ‐1.80 mm Hg, 95% CI ‐5.23 to 1.63)
mTOR inhibitor versus no treatment (Analysis 10.15 (SIRENA Study 2010; SUISSE ADPKD Study 2007, 112 participants): MD 0.27 mm Hg, 95% CI ‐3.30 to 3.85; I2 = 0%)
Somatostatin analogues versus placebo (Analysis 11.9 (ALADIN Study 2013; Ruggenenti 2005, 91 participants): MD ‐0.38 mm Hg, 95% CI ‐3.68 to 2.92; I2 = 0%)
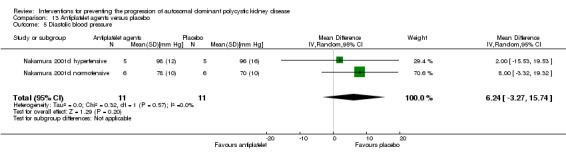
Antiplatelet agent versus placebo (Analysis 13.5 (Nakamura 2001d, 22 participants): MD 6.24 mm Hg, 95% CI ‐3.27 to 15.74; I2 = 0%)
Statins versus no treatment (Analysis 15.5 (Fassett 2010, 49 participants): MD ‐1.40 mm Hg, 95% CI ‐5.54 to 2.74).
3.4. Analysis.
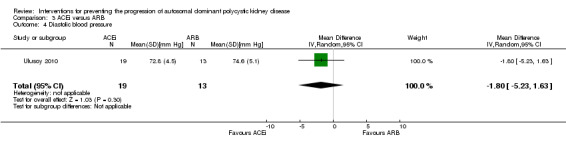
Comparison 3 ACEi versus ARB, Outcome 4 Diastolic blood pressure.
10.15. Analysis.
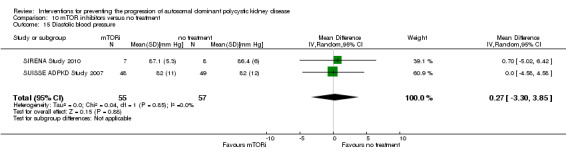
Comparison 10 mTOR inhibitors versus no treatment, Outcome 15 Diastolic blood pressure.
11.9. Analysis.
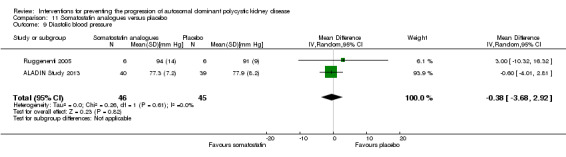
Comparison 11 Somatostatin analogues versus placebo, Outcome 9 Diastolic blood pressure.
13.5. Analysis.
Comparison 13 Antiplatelet agents versus placebo, Outcome 5 Diastolic blood pressure.
15.5. Analysis.
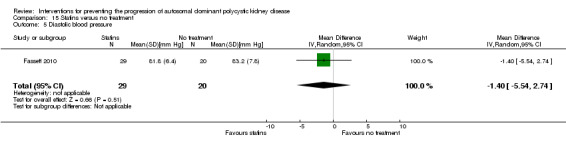
Comparison 15 Statins versus no treatment, Outcome 5 Diastolic blood pressure.
Mean arterial pressure
van Dijk 2003 reported ACEi significantly decreased MAP compared to no treatment (Analysis 1.8 (61 participants): MD ‐5.00 mm Hg, 95% CI ‐6.29 to ‐3.71).
1.8. Analysis.
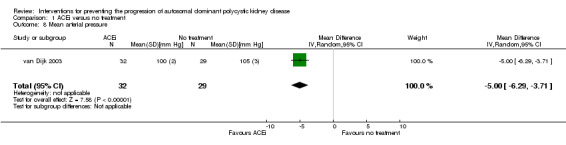
Comparison 1 ACEi versus no treatment, Outcome 8 Mean arterial pressure.
Ecder 1999 reported ACEi significantly decreased MAP compared to CCB (Analysis 2.6 (24 participants): MD ‐3.00 mm Hg, 95% CI ‐5.40 to ‐0.60).
2.6. Analysis.
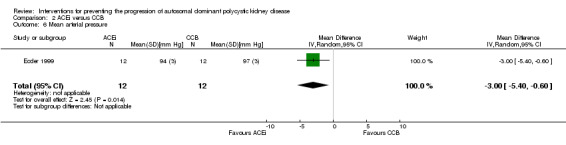
Comparison 2 ACEi versus CCB, Outcome 6 Mean arterial pressure.
van Dijk 2003 reported ACEi significantly decreased MAP compared to beta‐blockers (Analysis 4.8 (28 participants): MD ‐3.00 mm Hg, 95% CI ‐4.92 to ‐1.08).
4.8. Analysis.
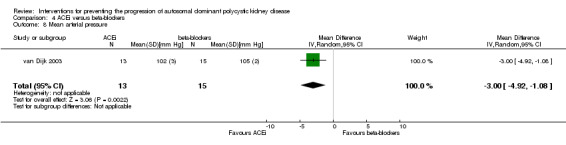
Comparison 4 ACEi versus beta‐blockers, Outcome 8 Mean arterial pressure.
There were no significant differences in MAP for the following comparisons.
ACEi versus ARB (Analysis 3.5 (Ulusoy 2010, 32 participants): MD ‐2.20 mm Hg, 95% CI ‐6.41 to 2.01)
ACEi alone versus ACEi plus mTOR inhibitors (Analysis 5.5 (RAPYD Study 2012, 69 participants): MD 0.64 mm Hg, 95% CI ‐6.21 to 7.50)
Somatostatin analogues versus placebo (Analysis 11.10 (ALADIN Study 2013, 79 participants): MD ‐0.10 mm Hg, 95% CI ‐3.66 to 3.46).
3.5. Analysis.
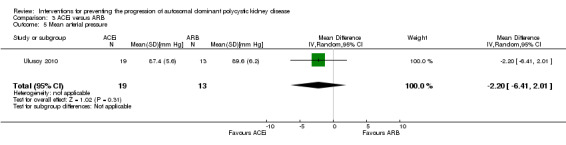
Comparison 3 ACEi versus ARB, Outcome 5 Mean arterial pressure.
5.5. Analysis.
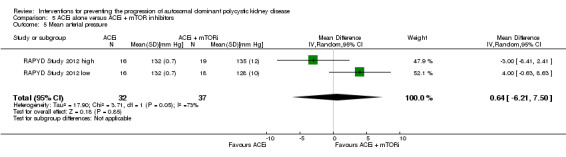
Comparison 5 ACEi alone versus ACEi + mTOR inhibitors, Outcome 5 Mean arterial pressure.
11.10. Analysis.
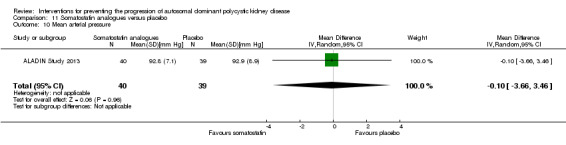
Comparison 11 Somatostatin analogues versus placebo, Outcome 10 Mean arterial pressure.
Cardiovascular events
Cardiovascular events were only reported in Zeltner 2008. There was no significant difference in the number of cardiovascular events between ACEi and beta‐blockers (Analysis 4.10 (37 participants): (RR 1.18, 95% CI 0.08 to 17.42).
4.10. Analysis.
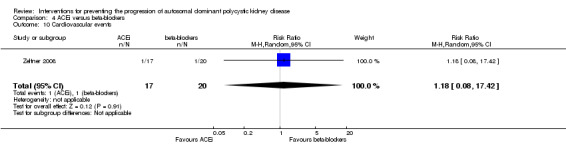
Comparison 4 ACEi versus beta‐blockers, Outcome 10 Cardiovascular events.
All‐cause mortality
Death was only reported in Walz 2010. There was no significant difference in the number of deaths between mTOR inhibitors and no treatment (Analysis 10.17 (431 participants): RR 2.03, 95% CI 0.19 to 22.20).
10.17. Analysis.
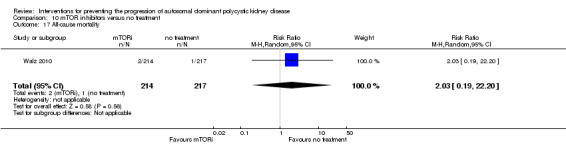
Comparison 10 mTOR inhibitors versus no treatment, Outcome 17 All‐cause mortality.
Quality of life
Quality of life was not reported in any of the included studies.
Kidney pain
Kidney pain was only reported in TEMPO 3‐4 Study 2011. There was no significant difference in the number with kidney between V2R antagonists and placebo (Analysis 8.6 (1444 participants); (RR 0.77, 95% CI 0.66 to 0.90).
Admission to hospital
Admission to hospital was not reported in any of the included studies.
Adverse events
RAPYD Study 2012 reported no significant differences between ACEi alone and ACEi plus mTOR inhibitors in anaemia (Analysis 5.6.1 (53 participants): RR 0.45, 95% CI 0.02 to 8.82), hyperlipidaemia (Analysis 5.6.2 (53 participants): RR 0.10, 95% CI 0.01 to 1.56), infection (Analysis 5.6.3 (53 participants): RR 0.45, 95% CI 0.02 to 8.82), or oral ulcers (Analysis 5.6.4 (53 participants): RR 0.13, 95% CI 0.01 to 2.15).
5.6. Analysis.
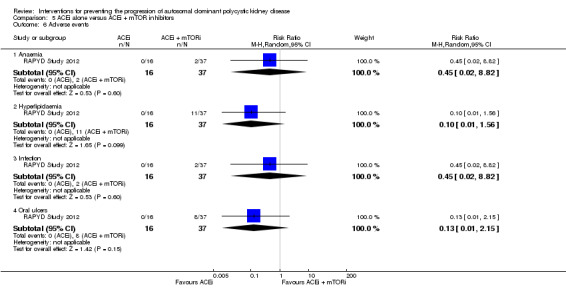
Comparison 5 ACEi alone versus ACEi + mTOR inhibitors, Outcome 6 Adverse events.
Soliman 2009 reported no significant difference between ARB alone and ARB plus mTOR inhibitors for infection (Analysis 6.5 (16 participants): RR 0.50, 95% CI 0.13 to 2.00).
6.5. Analysis.
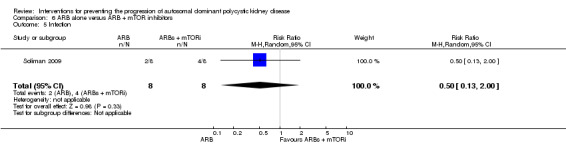
Comparison 6 ARB alone versus ARB + mTOR inhibitors, Outcome 5 Infection.
Compared to placebo, V2R antagonists significantly increased dry mouth (Analysis 8.7.4 (2 studies, 1455 participants): RR 1.33, 95% CI 1.01 to 1.76; I2 = 0%) and thirst (Analysis 8.7.6 (1 study, 1444 participants): RR 2.70, 95% CI 2.24 to 3.24). There were no significant differences in headache (Analysis 8.7.1 (2 studies,1455 participants): RR 1.03, 95% CI 0.85 to 1.25; I2 = 0%), diarrhoea (Analysis 8.7.2 (1 study, 1444 participants): RR 1.21, 95% CI 0.90 to 1.64), dizziness (Analysis 8.7.3 1 study, 1444 participants): RR 1.30, 95% CI 0.93 to 1.83), nausea (Analysis 8.7.5 (1 study, 1444 participants): RR 0.86, 95% CI 0.64 to 1.18), or liver enzyme elevation (Analysis 8.7.7 (1 study, 1444 participants): RR 2.26, 95% CI 0.49 to 10.43).
8.7. Analysis.
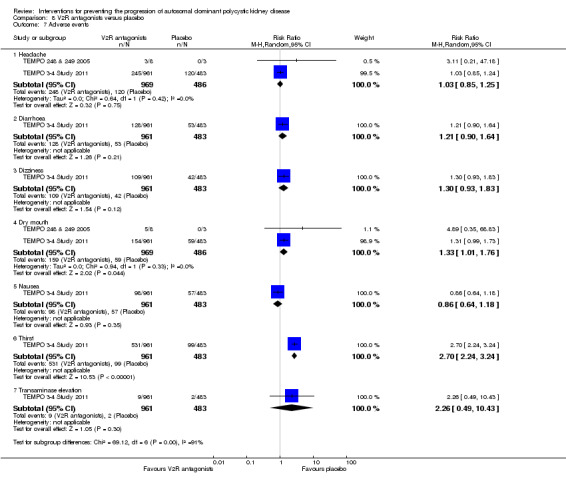
Comparison 8 V2R antagonists versus placebo, Outcome 7 Adverse events.
Compared with no treatment, mTOR inhibitors were associated with significant increases in anaemia (Analysis 10.18.1 (1 study, 431 participants): RR 3.41, 95% CI 1.79 to 6.51), angioedema (Analysis 10.18.2 (3 studies, 560 participants): RR 13.39, 95% CI 2.56 to 70.00; I2 = 0%), diarrhoea (Analysis 10.18.3 (3 studies 560 participants): RR 1.70, 95% CI 1.26 to 2.29; I2 = 0%); hyperlipidaemia (Analysis 10.18.4 (1 study, 431 participants): RR 5.68, 95% CI 2.23 to 14.43), infection (Analysis 10.18.5 (3 studies, 560 participants): RR 1.14, 95% CI 1.04 to 1.25; I2 = 0%), and oral ulcers (Analysis 10.18.7 (3 studies, 560 participants): RR 6.77, 95% CI 4.42 to 10.38; I2 = 0%), but not nausea (Analysis 10.18.6 (1 study, 431 participants): RR 1.69, 95% CI 0.85 to 3.37).
10.18. Analysis.
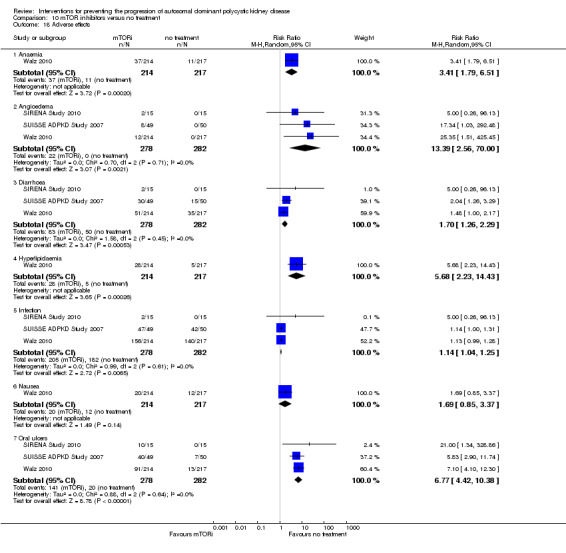
Comparison 10 mTOR inhibitors versus no treatment, Outcome 18 Adverse effects.
Somatostatin analogues were associated with significant risk of diarrhoea compared to placebo (Analysis 11.11.3 (2 studies, 91 participants): RR 3.72, 95% CI 1.43 to 9.68; I2 = 0%) but not alopecia (Analysis 11.11.1 (1 study, 79 participants): RR 4.88, 95% CI 0.24 to 98.47), anaemia (Analysis 11.11.2 (1 study, 79 participants): RR 1.30, 95% CI 0.50 to 3.40), dizziness (Analysis 11.11.4 (1 study, 79 participants): RR 0.97, 95% CI 0.06 to 15.05), or infection (Analysis 11.11.5 (1 study, 79 participants): RR 1.24, 95% CI 0.64 to 2.39).
11.11. Analysis.
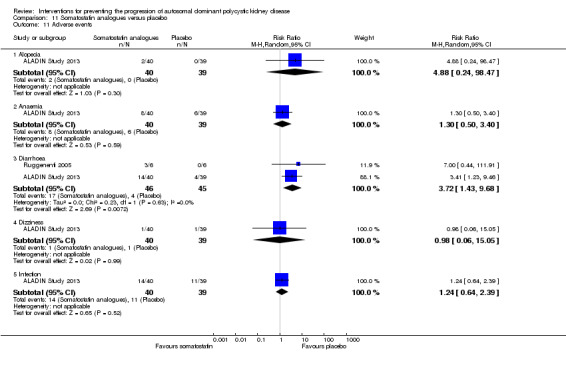
Comparison 11 Somatostatin analogues versus placebo, Outcome 11 Adverse events.
Discussion
Summary of main results
In this systematic review we could include 30 randomised studies (2039 adults with ADPKD) evaluating 11 interventions (ACEi, ARBs, calcium channel blockers, beta blockers, V2R antagonists, mTOR inhibitors, somatostatin analogues, antiplatelet agents, eicosapentaenoic acids, statins and vitamin D). For most interventions, data were available only from single studies and provided information for surrogate outcomes such as GFR, blood pressure and kidney and cyst volumes, leading to low confidence in estimated treatment effects. Overall, there was little or no evidence that currently available interventions improve patient‐related or kidney health outcomes while evidence for adverse events was sparse and showed potential for increased harm.
ACEi significantly reduced diastolic BP but had uncertain effects on mortality, ESKD, kidney volumes, GFR, creatinine levels and albuminuria. ACEi did not produce different effects on kidney function when compared with beta blockers or ARBs.
In meta‐analyses of data pooled from two studies, V2R antagonists increased thirst and dry mouth. In addition, data from a single RCT (TEMPO 3‐4 Study 2011) showed a greater proportion of patients treated with these drugs had elevations of liver‐enzyme levels. V2R antagonists showed apparent benefits on kidney function and volumes but confident interferences about their impact on the progression to ESKD could not be drawn as such benefits were only shown by a single study (TEMPO 3‐4 Study 2011). Furthermore, in this study data were analysed on an intention‐to‐treat basis. A higher percentage of patients in the intervention than in the placebo arm (22.9% versus 13.8%) discontinued the study, mostly due to low compliance to the treatment. This may introduce a significant attrition bias limiting the confidence and the overall applicability of findings.
mTOR inhibitors had uncertain effects on GFR, total kidney volume, BP and other secondary outcomes (albuminuria, proteinuria) but caused oral ulceration, infection, and diarrhoea. Of note the use of these drugs was associated with a remarkable increase in the risk of angioedema (RR 13.39), although the clinical reliability of this point estimate might be questioned due to the very wide confidence interval observed (2.56 to 70.00). Few data were available on mortality and RRT outcomes and treatment effects were accordingly absent.
When compared with placebo, somatostatin analogues reduced SCr and total kidney volume, but had uncertain benefits on GFR and other secondary outcomes, while causing diarrhoea. As shown in a three‐year duration study (ALADIN Study 2013), the benefits of these drugs on kidney outcomes (particularly, kidney volumes) seemed to be more evident in the early treatment phase (one year) while they tended to dilute at later stages (three years).
Little or no evidence was found to exist for the impact of calcium channel blockers, eicosapentaenoic acids, statins, vitamin D compounds and antiplatelet agents on disease progression and patient outcomes. These treatments were associated with undefined benefits in terms of kidney function and other secondary endpoints, such as BP or proteinuria.
Overall completeness and applicability of evidence
The evidence on available interventions for slowing progression of ADPKD was sparse in both adults and children. Information on ADPKD progression, both in terms of cyst/kidney volumes and deteriorating kidney function was limited, restricted to few interventions and mostly inconclusive because of the availability of single studies only. Most key mortality and cardiovascular outcomes were only marginally addressed. There were few or no data on major patient‐centred outcomes, such as CKD progression, mortality, major morbid events, quality of life and disease‐related symptoms. Conversely, the vast majority of studies only demonstrated sparse benefits in surrogate endpoints (e.g. change in total kidney or cyst volumes, blood pressure control, proteinuria, albuminuria) without evidence of clear benefits on outcomes of CKD progression (e.g. RRT). Surrogate outcomes are indeed useful as proxies for patient‐centred outcomes, particularly in slow‐progressing diseases such as ADPKD. However, the main disadvantage of using surrogate outcomes is that favourable effects of interventions do not always translate into clear benefits to harder endpoints. In some cases, surrogate outcomes may even be "hypothesis‐generating" at best. Although GFR remains the preferable outcome measure for treatment effectiveness, in the majority of ADPKD patients this parameter remains relatively steady until late in the disease. Total kidney volume has been extensively adopted as surrogate outcome measure in ADPKD studies. However, whether a reduced rate of kidney enlargement effectively translates into slowed kidney function deterioration is still object of debate. Accordingly, recently the FDA did not consider the observed improvement in kidney volumes in the TEMPO studies as enough to justify lifelong therapy with the V2R‐antagonist Tolvaptan.
The extreme heterogeneity in study length (ranging from five days to 60 months) also deserves mentioning. Many studies were indeed designed to assess treatment effects in very short time as per their exploratory nature (e.g. pilot or small cross‐over studies: Biao 1997; TEMPO 248 & 249 2005; van Dijk 2003). Short‐time studies preclude interpretations on hard outcomes (death, dialysis), particularly in slowly progressing chronic diseases. In addition, short‐term studies can be powered to investigate the effect of treatments on surrogate endpoints only, showing no proof of significant clinical changes in the long‐term. Performing cumulative outcome analyses with such study heterogeneity in follow‐up duration is potentially unreliable.
All studies were pilot or cross‐over studies conducted on very small populations, with the exception of two multicentre studies (TEMPO 3‐4 Study 2011; Walz 2010). Results from small studies are inconclusive in nature and probably more useful to set the stage for larger confirmation studies rather than for providing definite indications for clinical practice.
The applicability of findings is also limited by the large number of drop‐out found in most studies. Although the overall drop‐out rate varied widely across the studies (1.6% to 33%), this was greater than 10% in nine studies which included all the largest studies conducted on ADPKD patients. Furthermore, in most cases drop‐outs were unbalanced among the study groups, being more frequently observed in the active rather than in the control arm (Cadnapaphornchai 2005; ELATE Study 2011; Melemadathil 2013; Nutahara 2005; RAPYD Study 2012; TEMPO 3‐4 Study 2011; Zeltner 2008). High dropout rates may introduce important attrition bias and limit the internal validity of findings. Per‐protocol analyses can be useful to bypass limitations related with high dropout rates. However, such approaches convey a high risk of bias due to selection of patients and may provide clinically dubious information as they may over‐estimate the benefit or underestimate the harm of an intervention.
Quality of the evidence
Three of the included studies were cross‐over studies (Ruggenenti 2005; SIRENA Study 2010; van Dijk 2001). Most studies focused on small cohorts, were not powered to observe differences in patient‐centred outcomes and did not provide adequate study reporting or information on blinding of patients or investigators or both to assess risks of bias properly.
Limitations in study reporting and design markedly reduced confidence in the results. Actual treatment effects may differ significantly from those calculated from existing studies.
Random sequence generation was adequate in only eight studies (ALADIN Study 2013; Cadnapaphornchai 2005; ELATE Study 2011; Fassett 2010; LOCKCYST Study 2009; RAPYD Study 2012; Ruggenenti 2005; SUISSE ADPKD Study 2007), and in almost half the studies, blinding was not present or not specified.
The overall drop‐out rate was over 10% in nine studies (AIPRI Study 1996; Cadnapaphornchai 2005; ELATE Study 2011; Hogan 2010; Nutahara 2005; SIRENA Study 2010; TEMPO 3‐4 Study 2011; van Dijk 2003; Zeltner 2008) and only six were conducted using intention‐to‐treat analyses (ALADIN Study 2013; Nutahara 2005; RAPYD Study 2012; SUISSE ADPKD Study 2007; TEMPO 3‐4 Study 2011; Walz 2010).
Potential biases in the review process
Despite being the first overall summary of treatment for ADPKD based on a peer‐reviewed protocol, a systematic search of electronic databases including the Cochrane Renal Group’s specialised register of studies, and applying a standardised procedure for data extraction and analysis incorporating assessment of study methodology, the findings of our review should be interpreted with caution. The lack of data in the available studies represents the key limitation. In most cases, the effect of a given intervention was addressed by single studies, which prevented meta‐analyses with sufficient power to draw definitive conclusions on relevant outcomes. Furthermore, data on patient‐level outcomes (such as ESKD, mortality and cardiovascular events and adverse effects) were collectively scarce or absent. Study design was heterogeneous with marked differences among studies with respect to follow up duration, baseline kidney function and methods of assessment of ADPKD severity. Finally, in most cases, ADPKD assessment was made by echo tomography, a technique widely recognised to be inaccurate for identifying small changes in kidney volumes and poorly suited for very expanded kidneys.
Agreements and disagreements with other studies or reviews
After decades of symptomatic treatment for ADPKD, we now have several novel interventions targeting ADPKD biology arising from a wealth of experimental and non‐randomised studies (Chang 2012). Unfortunately, despite great optimism based on preliminary results, to date there has not been sufficient evidence from the available RCTs to demonstrate clear therapeutic benefits that outweigh treatment hazards. In addition, large RCTs are needed before these interventions could be considered as effective treatments to improve outcomes in ADPKD.
The Consortium for Radiologic Imaging for the Study of Polycystic Kidney Disease (Rule 2006) demonstrated that in people with ADPKD, baseline kidney volume values predicted the rate of increase in kidney volumes regardless of age, and accordingly, higher rates of kidney enlargement reflected a faster decline in kidney function. Kidney volumes have therefore been proposed and extensively used in studies as surrogate endpoints of disease progression to overcome the difficulty of following kidney function slope for very long periods of time in RCTs. Despite this, the benefits of some interventions (e.g. mTOR inhibitors) on kidney volumes in ADPKD have not corresponded with substantial changes in kidney function decline. This raises the question as to whether these biomarkers are appropriate as outcomes for assessing treatment effectiveness of novel interventions in ADPKD when used in isolation (Grantham 2011) and whether other surrogates of kidney function or damage would be more appropriate in studies of ADPKD patients (Helai 2012). In this regard, negative results of mTOR inhibitors studies were largely disappointing. These drugs have been demonstrated to be powerful inhibitors of cyst growth in experimental models (Wu 2007) and retrospective observations clearly showed a reduced cystic phenotype in the livers of kidney transplant recipients undergoing immunosuppression with mTOR inhibitors (Qian 2008). Future directions have been hypothesised for exploring whether there is room for mTOR inhibitors in the pathogenetic treatment of ADPKD, including higher doses or longer regimens of treatment, lower doses in combination with other therapeutic approaches (to minimise adverse events) or the use of analogues with better side‐effects profiles or improved kidney penetration (Wüthrich 2009). We suggest that any future study of higher dose mTOR inhibition requires careful systematic measurement of adverse effects and is based on patient‐relevant outcomes.
Although preliminary findings in animal and human studies have suggested that V2R antagonists and somatostatin analogues can be efficacious in slowing cyst growth (Harris 2009), our analyses demonstrated no conclusive effects for V2R and only small effects for somatostatin analogues on kidney function or kidney volumes. On the other hand, concerns might arise concerning the safety profile of these agents because their use is associated with thirst and dry mouth (V2R antagonists) and diarrhoea (somatostatin analogues). Future studies focusing on the effects on kidney function decline rather than kidney volume surrogates are eagerly awaited to confirm and generalise the benefits of these agents in retarding ADPKD progression.
Blood pressure control is currently one of the mainstays of ADPKD management in clinical practice. Hypertensive ADPKD patients have greater and faster annual rates of kidney volumes growth and an increased prevalence of cardiovascular comorbidities and complications with respect to normotensive people (Ecder 2013). Since hypertensive ADPKD patients are at higher risk of kidney disease progression, these people might represent a higher risk population for future studies which would then be powered to capture patient‐centred kidney and mortality outcomes.
In our review, the use of ACEi was associated with significant improvement in BP control. Unfortunately, this benefit was mostly confined to children, and meta‐analyses were underpowered to detect differences in treatment effects on disease progression. Potentially, results from the ongoing HALT‐PKD Study 2008a testing the efficacy of RAAS‐blockade on the progression of cystic disease and decline in kidney function, will clarify whether an intensive (≤ 110/75 mm Hg) versus standard (≤ 130/80 mm Hg) BP control might produce different effects on the disease course in ADPKD patients with both early (GFR > 60 mL/min/1.73 m2) and advanced (GFR 25 to 60 mL/min/1.73 m2) kidney impairment.
Authors' conclusions
Implications for practice.
Despite preliminary observations, no hard evidence was found to support the introduction of any of these interventions in clinical practice because treatment effects on patient‐centred endpoints are lacking, and although sparse, adverse event data indicate harm. Findings from single studies need to be confirmed by other studies evaluating the long‐term impact of these therapies on primary kidney outcomes such as GFR decline and ESKD.
Implications for research.
Future studies designed to observe differences in tangible outcomes, such as progression to ESKD, need for transplantation, mortality, hospital admissions, major morbidities and quality of life would be informative. However, clinical studies looking at some of these hard endpoints (e.g. mortality of ESKD) may be problematic in slowly progressing diseases. Alternative strategies should therefore be implemented, mostly focusing on the identification and validation of new endpoints (such as thresholds for clinically meaningful changes in kidney function or the assessment of patient‐reported outcomes) and the exact definition of ADPKD patients to be studied in clinical studies as more likely to benefit from early intervention (e.g. in relation to kidney volumes, range of kidney function, tendency to rapid disease progression). Given the high number of interventions tested so far, research efforts should also prioritise a smaller number of drugs and focus on agreed core outcomes to improve generalisability of findings.
More conclusive data on the safety profile of some agents and long‐term effects on kidney function as primary outcomes are needed. Studies that include patients at higher risk of clinical outcomes, such as hypertension, might be better placed to indicate treatment effects.
Until then, patient and policy decisions in ADPKD are unsupported by robust study evidence.
Feedback
Ongoing studies now complete
Summary
For the review, 'Interventions for preventing the progression of autosomal dominant polycystic kidney disease' I was just extracting the research recommendations at the end of the review so they can be promoted for research funding. Part of extracting the research uncertainties or recommendations is to list any on‐going studies which might address the uncertainty, so that research funders know to wait for any on‐going research to complete. Going form this review, it lists several ongoing studies which are completed. Shouldn't these now be listed in the awaiting assessment section of the review
Reply
Thank you for your feedback. The ongoing studies have now been moved to "Studies awaiting classification" and the authors will assess these studies in a future update of this review.
Contributors
Mark Fenton ‐ Database of Uncertainties about the Effects of Treatments (DUETs); National Institute for Health and Clinical Excellence
Narelle Willis ‐ Managing Editor, Cochrane Kidney and Transplant
What's new
| Date | Event | Description |
|---|---|---|
| 3 September 2015 | Amended | Two ongoing studies moved to studies awaiting assessment; one ongoing study move to excluded studies |
| 3 September 2015 | Feedback has been incorporated | Ongoing studies now completed |
History
Protocol first published: Issue 1, 2013 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 31 August 2015 | Amended | Correction of search dates |
Acknowledgements
We would like to thank the Cochrane Renal Group for their valued support and the referees for their feedback and advice during the preparation of the review. We also thank Drs JP Drenth and A Soliman for providing additional details about their studies which were included in this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. ACEi versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum creatinine | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.14, 0.09] |
| 2 GFR [mL/min/1.73 m²] | 3 | 103 | Mean Difference (IV, Random, 95% CI) | ‐3.41 [‐15.83, 9.01] |
| 3 Doubling of serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Total kidney volume | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐42.50 [‐115.68, 30.67] |
| 5 Albuminuria | 3 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.51, 0.26] |
| 6 Systolic blood pressure | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐5.44 [‐14.26, 3.38] |
| 7 Diastolic blood pressure | 2 | 42 | Mean Difference (IV, Random, 95% CI) | ‐4.96 [‐8.88, ‐1.04] |
| 8 Mean arterial pressure | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐6.29, ‐3.71] |
Comparison 2. ACEi versus CCB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 GFR [mL/min/1.73 m²] | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐13.00 [‐17.56, ‐8.44] |
| 3 Albuminuria | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐134.0 [‐176.01, ‐91.99] |
| 4 Systolic blood pressure | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐8.62, ‐1.38] |
| 5 Diastolic blood pressure | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐5.40, ‐0.60] |
| 6 Mean arterial pressure | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐5.40, ‐0.60] |
Comparison 3. ACEi versus ARB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum creatinine | 2 | 52 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.09, 0.10] |
| 2 GFR [mL/min/1.73 m²] | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐22.69, 15.89] |
| 3 Systolic blood pressure | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐9.75, 2.75] |
| 4 Diastolic blood pressure | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐5.23, 1.63] |
| 5 Mean arterial pressure | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐6.41, 2.01] |
Comparison 4. ACEi versus beta‐blockers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 GFR [mL/min/1.73 m²] | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐8.06 [‐29.62, 13.50] |
| 3 GFR descriptive data | Other data | No numeric data | ||
| 4 Need for renal replacement therapy | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.02, 8.97] |
| 5 Albuminuria | 2 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.77, 1.39] |
| 6 Systolic blood pressure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.29, 0.29] |
| 7 Diastolic blood pressure | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 1.0 [0.35, 1.65] |
| 8 Mean arterial pressure | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐4.92, ‐1.08] |
| 9 Blood pressure descriptive data | Other data | No numeric data | ||
| 10 Cardiovascular events | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.08, 17.42] |
4.3. Analysis.
Comparison 4 ACEi versus beta‐blockers, Outcome 3 GFR descriptive data.
| GFR descriptive data | |
|---|---|
| Study | |
| Watson 1999 | eGFR (Cockcroft‐Gault formula) significantly decreased in both groups over the 3 year period (ACEi: 19.3 mL/min/1.73 m2; beta‐blockers: 14.3 mL/min/1.73 m2) but there was no difference in the rate of decline between groups. |
4.9. Analysis.
Comparison 4 ACEi versus beta‐blockers, Outcome 9 Blood pressure descriptive data.
| Blood pressure descriptive data | |
|---|---|
| Study | |
| Watson 1999 | Good blood pressure control was achieved in both groups (ACEi: 132.6/84.6 mm Hg; beta‐blockers: 130.9/84.5 mm Hg) |
Comparison 5. ACEi alone versus ACEi + mTOR inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 GFR [mL/min/1.73 m²] | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐15.04, 4.20] |
| 2 Total kidney volume | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 285.79 [‐21.92, 593.50] |
| 3 Cyst volume | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 36.32 [‐6.99, 79.64] |
| 4 Proteinuria | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.65, 0.12] |
| 5 Mean arterial pressure | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 0.64 [‐6.21, 7.50] |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Anaemia | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.02, 8.82] |
| 6.2 Hyperlipidaemia | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.56] |
| 6.3 Infection | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.02, 8.82] |
| 6.4 Oral ulcers | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.15] |
5.1. Analysis.
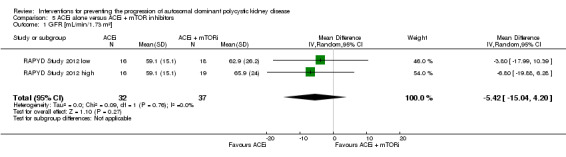
Comparison 5 ACEi alone versus ACEi + mTOR inhibitors, Outcome 1 GFR [mL/min/1.73 m²].
5.4. Analysis.
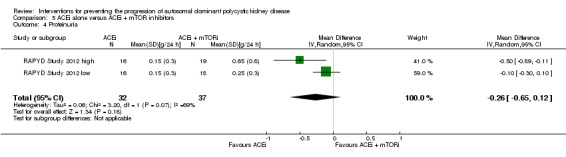
Comparison 5 ACEi alone versus ACEi + mTOR inhibitors, Outcome 4 Proteinuria.
Comparison 6. ARB alone versus ARB + mTOR inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 GFR [mL/min/1.73 m²] | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐9.60 [‐28.18, 8.98] |
| 2 Doubling of serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Total kidney volume | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.37 [0.04, 0.70] |
| 4 Blood pressure descriptive data | Other data | No numeric data | ||
| 5 Infection | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.13, 2.00] |
6.4. Analysis.
Comparison 6 ARB alone versus ARB + mTOR inhibitors, Outcome 4 Blood pressure descriptive data.
| Blood pressure descriptive data | |
|---|---|
| Study | |
| Soliman 2009 | The mean diastolic pressure decreased by 2.5 to 4.0 mm Hg in the ARB + mTOR group and increased by 0.5 to 1.5 mm Hg in the ARB alone group The mean systolic pressure decreased by 2.5 to 5.0 mm Hg in the ARB + mTOR group and increased by 1.0 to 2.5 mm Hg in the ARB alone group |
Comparison 7. ARB versus CCB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 GFR [mL/min/1.73 m²] | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 6.30 [‐8.49, 21.09] |
| 3 Doubling of serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Proteinuria | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐304.0 [‐578.54, ‐29.46] |
| 5 Albuminuria | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐238.0 [‐394.61, ‐81.39] |
Comparison 8. V2R antagonists versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 GFR descriptive data | Other data | No numeric data | ||
| 3 Doubling of serum creatinine | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Total kidney volume descriptive data | Other data | No numeric data | ||
| 5 Albuminuria | 1 | 1157 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐3.95, 0.75] |
| 6 Kidney pain | 1 | 1444 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.90] |
| 7 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Headache | 2 | 1455 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 7.2 Diarrhoea | 1 | 1444 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.90, 1.64] |
| 7.3 Dizziness | 1 | 1444 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.93, 1.83] |
| 7.4 Dry mouth | 2 | 1455 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.01, 1.76] |
| 7.5 Nausea | 1 | 1444 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.64, 1.18] |
| 7.6 Thirst | 1 | 1444 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [2.24, 3.24] |
| 7.7 Transaminase elevation | 1 | 1444 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.49, 10.43] |
8.2. Analysis.
Comparison 8 V2R antagonists versus placebo, Outcome 2 GFR descriptive data.
| GFR descriptive data | |
|---|---|
| Study | |
| TEMPO 3‐4 Study 2011 | The slope of kidney function (as assessed by means of the reciprocal of the SCr level) from the end of dose escalation to month 36, favoured V2R‐antagonists, with a slope of −2.61 (mg/mL)−1 per year, as compared with −3.81 (mg/mL)−1 per year with placebo; the treatment effect was an increase of 1.20 (mg/mL)−1 per year (95% CI 0.62 to 1.78; P < 0.001) |
8.4. Analysis.
Comparison 8 V2R antagonists versus placebo, Outcome 4 Total kidney volume descriptive data.
| Total kidney volume descriptive data | |
|---|---|
| Study | |
| TEMPO 3‐4 Study 2011 | quote: "Over the 3‐year period, total kidney volume increased by 2.8% per year (95% confidence interval [CI], 2.5 to 3.1) with V2R‐antagonists versus 5.5% per year (95% CI, 5.1 to 6.0) with placebo" |
8.6. Analysis.
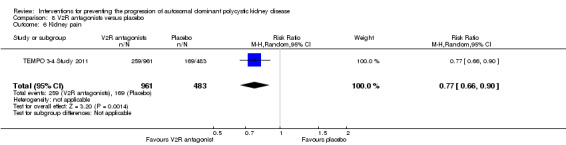
Comparison 8 V2R antagonists versus placebo, Outcome 6 Kidney pain.
Comparison 9. High versus low dose V2R antagonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Systolic blood pressure | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐16.98, ‐1.02] |
| 3 Diastolic blood pressure | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐11.21, ‐0.79] |
Comparison 10. mTOR inhibitors versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 GFR [mL/min/1.73 m²] | 2 | 115 | Mean Difference (IV, Random, 95% CI) | 4.45 [‐3.20, 12.11] |
| 2 GFR descriptive data | Other data | No numeric data | ||
| 3 Need for renal replacement therapy | 1 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [0.12, 74.26] |
| 4 Need for transplantation | 1 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.06, 16.11] |
| 5 Total kidney volume | 2 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.75, 0.59] |
| 6 Total kidney volume descriptive data | Other data | No numeric data | ||
| 7 Cyst volume | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐55.0 [‐862.98, 752.98] |
| 8 Cyst volume descriptive data | Other data | No numeric data | ||
| 9 Parenchymal volume | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 15.0 [‐75.44, 105.44] |
| 10 Parenchymal volume descriptive data | Other data | No numeric data | ||
| 11 Proteinuria | 2 | 446 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.29, 0.98] |
| 12 Proteinuria descriptive data | Other data | No numeric data | ||
| 13 Albuminuria | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.27, 0.78] |
| 14 Systolic blood pressure | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 2.48 [‐2.07, 7.03] |
| 15 Diastolic blood pressure | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐3.30, 3.85] |
| 16 Blood pressure descriptive data | Other data | No numeric data | ||
| 17 All‐cause mortality | 1 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.19, 22.20] |
| 18 Adverse effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Anaemia | 1 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 3.41 [1.79, 6.51] |
| 18.2 Angioedema | 3 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 13.39 [2.56, 70.00] |
| 18.3 Diarrhoea | 3 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.26, 2.29] |
| 18.4 Hyperlipidaemia | 1 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 5.68 [2.23, 14.43] |
| 18.5 Infection | 3 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.04, 1.25] |
| 18.6 Nausea | 1 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.85, 3.37] |
| 18.7 Oral ulcers | 3 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 6.77 [4.42, 10.38] |
10.2. Analysis.
Comparison 10 mTOR inhibitors versus no treatment, Outcome 2 GFR descriptive data.
| GFR descriptive data | |
|---|---|
| Study | |
| Walz 2010 | quote: "The estimated GFR decreased by 8.9 ml per minute in the mTOR‐inhibitors group and 7.7 ml per minute in the placebo group (P = 0.15) over the 2‐year study period" |
10.6. Analysis.
Comparison 10 mTOR inhibitors versus no treatment, Outcome 6 Total kidney volume descriptive data.
| Total kidney volume descriptive data | |
|---|---|
| Study | |
| Melemadathil 2013 | quote: "there was a statistically significant reduction in total kidney volume when mTOR treatment was extended for 1 year" |
| Mora 2013 | quote: "the mTOR group showed a kidney volume growth of 9,4 ±1,2mL/year compared with 11 ± 1.4 mL/year in control group" |
| Walz 2010 | quote: "among patients receiving mTOR‐inhibitors, the mean total kidney volume increased from 2028 ml to 2063 ml at 1 year and to 2176 ml at 2 years, and among those receiving placebo, it increased from 1911 ml to 2061 ml and to 2287 ml, respectively" |
10.8. Analysis.
Comparison 10 mTOR inhibitors versus no treatment, Outcome 8 Cyst volume descriptive data.
| Cyst volume descriptive data | |
|---|---|
| Study | |
| Melemadathil 2013 | quote: "there was a statistically significant reduction in total cyst volume when mTOR treatment was extended for 1 year" |
| Walz 2010 | quote: "The cyst volume increased by 76 ml at 1 year and 181 ml at 2 years in the mTOR‐inhibitors group and by 98 ml and 215 ml, respectively, in the placebo group" |
10.10. Analysis.
Comparison 10 mTOR inhibitors versus no treatment, Outcome 10 Parenchymal volume descriptive data.
| Parenchymal volume descriptive data | |
|---|---|
| Study | |
| Melemadathil 2013 | quote: "there was a small but significant increase in renal parenchymal volume in patients receiving mTOR" |
| Walz 2010 | quote: "The parenchymal volume increased by 26 ml at 1 year and by 56 ml at 2 years in the mTOR‐inhibitors group; the corresponding changes in the placebo group were 62 and 93 ml" |
10.12. Analysis.
Comparison 10 mTOR inhibitors versus no treatment, Outcome 12 Proteinuria descriptive data.
| Proteinuria descriptive data | |
|---|---|
| Study | |
| Melemadathil 2013 | quote: "there was a statistically significant increase in proteinuria in the mTOR arm as compared to the standard treatment group at the end of 6 months" |
10.16. Analysis.
Comparison 10 mTOR inhibitors versus no treatment, Outcome 16 Blood pressure descriptive data.
| Blood pressure descriptive data | |
|---|---|
| Study | |
| Walz 2010 | quote: "The change from baseline in the systolic blood pressure at 24 months was −2.0 mm Hg in the mTOR‐inhibitors group and −1.5 mm Hg in the placebo group (P = 0.76); the corresponding changes in diastolic blood pressure were −2.7 mm Hg and −2.6 mm Hg (P = 0.89)" |
Comparison 11. Somatostatin analogues versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.86, ‐0.01] |
| 2 GFR [mL/min/1.73 m²] | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 9.50 [‐4.45, 23.44] |
| 3 Total kidney volume | 3 | 114 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.22, ‐0.01] |
| 4 Cyst volume | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.18, 0.18] |
| 5 Parenchymal volume | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐67.67 [‐249.45, 114.12] |
| 6 Proteinuria | 1 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 7 Albuminuria | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.51, 0.31] |
| 8 Systolic blood pressure | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐3.54, 5.13] |
| 9 Diastolic blood pressure | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐3.68, 2.92] |
| 10 Mean arterial pressure | 1 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.66, 3.46] |
| 11 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Alopecia | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 4.88 [0.24, 98.47] |
| 11.2 Anaemia | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.3 [0.50, 3.40] |
| 11.3 Diarrhoea | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [1.43, 9.68] |
| 11.4 Dizziness | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.06, 15.05] |
| 11.5 Infection | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.64, 2.39] |
Comparison 12. Somatostatin analogues + mTOR inhibitors versus somatostatin analogues alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total kidney volume descriptive data | Other data | No numeric data |
12.1. Analysis.
Comparison 12 Somatostatin analogues + mTOR inhibitors versus somatostatin analogues alone, Outcome 1 Total kidney volume descriptive data.
| Total kidney volume descriptive data | |
|---|---|
| Study | |
| ELATE Study 2011 | quote: "The median kidney volume was not affected by octreotide and did not change significantly in the 6 patients through the course of the trial (from 798 mL (IQR 675–1960 mL) at baseline to 811 mL (IQR 653–1960 mL) after 48 weeks, p=0.75). Likewise, octreotide‐everolimus combination treatment (n=6) did not affect kidney volume over the course of 48 weeks (from 623 mL (IQR 483–1110 ml) to 602 mL (IQR 493–1259 mL), p=0.75). Change in kidney volume did not differ between treatment arms (p=1.00)" |
Comparison 13. Antiplatelet agents versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 2 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.52, 0.26] |
| 2 GFR [mL/min/1.73 m²] | 2 | 22 | Mean Difference (IV, Random, 95% CI) | 2.24 [‐8.05, 12.53] |
| 3 Albuminuria | 2 | 22 | Mean Difference (IV, Random, 95% CI) | ‐60.53 [‐129.06, 8.01] |
| 4 Systolic blood pressure | 2 | 22 | Mean Difference (IV, Random, 95% CI) | 5.04 [‐7.34, 17.43] |
| 5 Diastolic blood pressure | 2 | 22 | Mean Difference (IV, Random, 95% CI) | 6.24 [‐3.27, 15.74] |
Comparison 14. Eicosapentaenoic acids versus standard therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 GFR [mL/min/1.73 m²] | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 6.10 [‐11.16, 23.36] |
| 3 Total kidney volume | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐209.0 [‐729.06, 311.06] |
| 4 Albuminuria | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 82.40 [‐162.09, 326.89] |
14.4. Analysis.
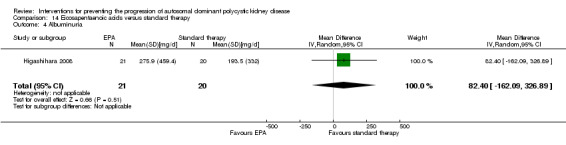
Comparison 14 Eicosapentaenoic acids versus standard therapy, Outcome 4 Albuminuria.
Comparison 15. Statins versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 GFR descriptive data | Other data | No numeric data | ||
| 2 GFR descriptive data from cross‐over studies | Other data | No numeric data | ||
| 3 Proteinuria descriptive data | Other data | No numeric data | ||
| 4 Systolic blood pressure | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐6.39, 9.79] |
| 5 Diastolic blood pressure | 1 | 49 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐5.54, 2.74] |
15.1. Analysis.
Comparison 15 Statins versus no treatment, Outcome 1 GFR descriptive data.
| GFR descriptive data | |
|---|---|
| Study | |
| Fassett 2010 | There was a 23% reduction in the rate of GFR change in statins‐treated patients compared with controls, although not statistically significant |
15.2. Analysis.
Comparison 15 Statins versus no treatment, Outcome 2 GFR descriptive data from cross‐over studies.
| GFR descriptive data from cross‐over studies | |
|---|---|
| Study | |
| van Dijk 2001 | Compared to placebo, treatment with statins significantly increased GFR from 124 ± 4 mL/min to 132 ± 6 mL/min (p < 0.05) |
15.3. Analysis.
Comparison 15 Statins versus no treatment, Outcome 3 Proteinuria descriptive data.
| Proteinuria descriptive data | |
|---|---|
| Study | |
| Fassett 2010 | Urinary protein excretion decreased by 2.8% in statins‐treated patients and increased by 21.2% in controls |
Comparison 16. Vitamin D versus traditional Chinese herbal medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 GFR | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 22.60 [0.92, 44.28] |
16.1. Analysis.
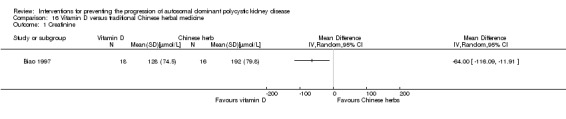
Comparison 16 Vitamin D versus traditional Chinese herbal medicine, Outcome 1 Creatinine.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AIPRI Study 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Sixty‐eight patients in the benazepril group and 61 in the placebo group did not complete the study be cause of death, other adverse events, lack of cooperation, or protocol violations" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ALADIN Study 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation according to a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded to treatment but study physicians and nurses were aware of the allocated group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/79 (7.5%) patients did not complete the study. Data were analysed on a modified ITT basis |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported |
| Other bias | Low risk | The study was partly funded by Novartis; however, the authors state that "....the sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication" |
Biao 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cadnapaphornchai 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Block randomisation using a sealed, numbered envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22/85 (26%) patients withdrew. Data were not analysed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cadnapaphornchai 2005 borderline.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Block randomisation using a sealed, numbered envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22/85 (26%) patients withdrew. Data were not analysed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cadnapaphornchai 2005 normotensive.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Block randomisation using a sealed, numbered envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22/85 (26%) patients withdrew. Data were not analysed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Ecder 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ELATE Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised generated randomisation list |
| Allocation concealment (selection bias) | Low risk | "A computer generated randomisation list is made by an independent biostatistics unit using a permuted block design with a random block size of 4 to guarantee a balanced allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/39 (11%) patients dropped from the study. Unclear how many were ADPKD. The authors performed both ITT and PP analyses on the primary outcome measure |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | "Novartis provided the drug everolimus and partially funded the study. They did not have any influence on the execution of the trial or the preparation of the manuscript, since this was an investigator‐initiated trial" |
Fassett 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Repeating blocks of 10 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Higashihara 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "...using the dynamic balancing method to ensure equal distributions" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement, presumably open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement, presumably open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Sponsored by Mochida Pharmaceutical Co. Ltd |
Hogan 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization assignment to octreotide or matching placebo treatment was independently managed by the research pharmacy" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All patients completed the study but 13 were excluded from kidney outcomes (volume and function) assessment |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported |
| Other bias | High risk | Novartis supported the study |
LOCKCYST Study 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by an un‐blinded investigational pharmacist in blocks of 4, and the 2 treatment arms were allocated in a 1:1 ratio within each block" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All CT scans were blinded to patient identity and date of birth as well as date of scan" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analyses were performed on an ITT basis. Unclear whether all the 32 ADPKD patients completed the established follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Computer‐generated random number list |
| Other bias | Low risk | The study was sponsored by Ipsen. The authors state that "The sponsor of the study had no role in the study design, data collection, data analysis, interpretation of the study results, or writing of the manuscript" |
Melemadathil 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 2:1. Sequence generation not defined |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/40 (15%) patients in the mTOR group dropped or were lost to follow up. Unclear whether the study was analysed on ITT or PP basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Mora 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nakamura 2001d.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nakamura 2001d hypertensive.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nakamura 2001d normotensive.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nakamura 2012a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nutahara 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "...using the dynamic balancing method to ensure equal distributions" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/49 (24.4%) patients analysed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
RAPYD Study 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by random number tables |
| Allocation concealment (selection bias) | Low risk | Block randomisation land adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/55 (3.6%) patients analysed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Sponsored by Wyeth and Pfizer |
RAPYD Study 2012 high.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Block randomisation and adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/55 (3.6%) patients were analysed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Sponsored by Wyeth and Pfizer |
RAPYD Study 2012 low.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using random number tables |
| Allocation concealment (selection bias) | Low risk | Block randomisation and adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/55 (3.6%) patients analysed on ITT basis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Sponsored by Wyeth and Pfizer |
Ruggenenti 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Blocks of four using a 1:1 allocation ratio |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors analysing liver and kidney volumes were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All subjects completed the study |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
SIRENA Study 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Kidneys were first manually outlined on all acquired digital images by a trained operator (AC), who was blind to the treatment phase" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/21 patients withdrew. These patients were not included in final analyses |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Wyeth‐Lederle S.p.A. supplied the study drug |
Soliman 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...observers were blinded to all clinical and radiologic data, as well as their first measurements and the results of the other observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
SUISSE ADPKD Study 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by biostatistics unit independent of study team |
| Allocation concealment (selection bias) | Low risk | Sealed sequentially numbered opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Each observer was unaware of all clinical data and the findings of the other observer, and the measurements were performed in random order" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/100 (4%) patients withdrew. These patients were analysed on ITT basis |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported |
| Other bias | Low risk | "Wyeth Switzerland (now Pfizer), provided the study drug and an unrestricted research grant. The company had no role in the design of the trial or in the collection, analysis, or interpretation of the data or the writing of the manuscript" |
Temmerman 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
TEMPO 248 & 249 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Sponsored by Otsuka pharmaceutical |
TEMPO 250 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Sponsored by Otsuka pharmaceutical |
TEMPO 3‐4 Study 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Allocation was performed in a 2:1 ratio to receive tolvaptan or placebo, and with stratification |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data analysed on ITT basis. 221/961 (22.9%) and 67/483 (13.8%) patients, in the intervention and control group respectively, discontinued the study |
| Selective reporting (reporting bias) | Low risk | All selected outcomes were reported |
| Other bias | High risk | Supported by Otsuka Pharmaceuticals and Otsuka Pharmaceutical Development and Commercialization |
Ulusoy 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
van Dijk 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
van Dijk 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed for each patient in the pharmacy of our hospital" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The normotensive group (72) participated in a randomised double‐blind placebo‐controlled study while the hypertensive group (35) was randomised for open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/72 normotensive and 7/35 hypertensive patients did not complete the 36 months follow‐up and were not included in the final analysis. Complete data were available in 89/106 (83.9%) patients |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | "Enalapril and placebo were provided by Merck, Sharp and Dohme" |
Walz 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | 1:1 ratio |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/213 and 6/216 patients in the intervention and control groups respectively withdrew |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported |
| Other bias | High risk | "Data collection and management were the responsibility of the sponsor" |
Watson 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Zeltner 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (echo‐data) were blinded to patients |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/23 (30.4%) and 2/23 (8.6%) of patients in the intervention and control group respectively withdrew |
| Selective reporting (reporting bias) | Low risk | All defined outcomes were reported |
| Other bias | Unclear risk | "This research was supported by Astra‐Zeneca who provided the study medication" |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ADPKD ‐ autosomal dominant polycystic kidney disease; AST ‐ aminotransferase; AVP ‐ arginine vasopressin; BP ‐ blood pressure; CHF ‐ congestive heart failure; CrCl ‐ creatinine clearance; CVA ‐ cerebrovascular accident; DBP ‐ DBP; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; eGFR ‐ estimated glomerular filtration rate; GFR ‐ glomerular filtration rate; HD ‐ haemodialysis; IHD ‐ Ischaemic heart disease; IM ‐ intramuscular; ITT ‐ intention‐to‐treat; LVMI ‐ left ventricular mass index; M/F ‐ male/female; MDRD ‐ Modification of Diet in Renal Disease; mGFR ‐ measured glomerular filtration rate; MI ‐ myocardial infarction; magnetic nuclear imaging ‐ magnetic resonance imaging; mTOR ‐ mammalian target of rapamycin; NSAID ‐ nonsteroidal anti‐inflammatory drug; NYHA ‐ New York Heart Association; PLD ‐ polycystic liver disease; PP ‐ per protocol; PVD ‐ peripheral vascular disease; RCT ‐ randomised control trial; RRT ‐ renal replacement therapy; SBP ‐ systolic blood pressure; SCr ‐ serum creatinine; SD ‐ standard deviation; UAE ‐ urinary albumin excretion; UPE ‐ urinary protein excretion
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Doulton 2006 | Outcome not relevant |
| ISRCTN57653760 | Halted in 2008 due to lack of funding; no results published |
| Kanno 1996 | Not RCT |
| Nakamura 2005a | Wrong outcome |
| Sharma 2004 | Not RCT |
Characteristics of studies awaiting assessment [ordered by study ID]
Braun 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Cadnapaphornchai 2011.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
Duration of intervention
Co‐interventions
|
| Outcomes | Combined endpoint of 20% or greater change in:
Overall change in:
|
| Notes |
HALT‐PKD Study 2008.
| Methods |
|
| Participants |
|
| Interventions | Study A
Study B
Co‐interventions
|
| Outcomes | Study A
Study B
|
| Notes |
NCT01233869.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Vienna RAP Study 2015.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
DIPAK 1 Study 2014.
| Trial name or title | Study of lanreotide to treat polycystic kidney disease (DIPAK1) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | June 2012 |
| Contact information | Dr Esther Meijer, Dr Ron Gansevoort; University Medical Centre Groningen, Netherlands |
| Notes | This study is ongoing, but not recruiting participants |
NCT00345137.
| Trial name or title | Effects of systemic NO‐inhibition on renal hemodynamics in patients with polycystic kidney disease and chronic glomerulonephritis |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control
|
| Outcomes |
|
| Starting date | 2006 |
| Contact information | Prof Erling B Pedersen, Dept. of Medicine, Holstebro Hospital, 7500 Holstebro, Denmark |
| Notes | This study is ongoing, but not recruiting participants |
NCT01932450.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | This study is currently recruiting patients |
ACEi ‐ angiotensin‐converting enzyme inhibitors; ACR ‐ albumin creatinine ratio; ADPKD ‐ autosomal dominant polycystic kidney disease; ARB ‐ angiotensin receptor blocker; BP ‐ blood pressure; eGFR ‐ estimated glomerular filtration rate; LVMI ‐ left ventricular mass index; MI ‐ myocardial infarction; NSAID ‐ nonsteroidal anti‐inflammatory drug; RCT ‐ randomised controlled trial; SCR ‐ serum creatinine; UAE ‐ urinary albumin excretion
Contributions of authors
Draft the protocol: DB, JC, GS
Study selection: DB, MR
Extract data from studies: DB, MR
Enter data into RevMan: DB, MR
Carry out the analysis: DB, MR, SP, GS
Interpret the analysis: DB, SP, GS
Draft the final review: DB, CZ, JC, SP, GS
Disagreement resolution: SP
Update the review: DB, MR, SP, GS
Declarations of interest
Davide Bolignano: none known
Suetonia C Palmer: none known
Marinella Ruospo: none known
Carmine Zoccali: none known
Jonathan C Craig: none known
Giovanni FM Strippoli: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
AIPRI Study 1996 {published data only}
- Apperloo AJ, Rensma PL. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curren CG. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Hogan TJ, Elliott WJ, Seto AH, Bakris GL. Antihypertensive treatment with and without benazepril in patients with chronic renal insufficiency: a US economic evaluation. Pharmacoeconomics 2002;20(1):37‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Locatelli F, Carbarns IR, Maschio G, Mann JF, Ponticelli C, Ritz E, et al. Long‐term progression of chronic renal insufficiency in the AIPRI Extension Study. The Angiotensin‐Converting‐Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Kidney International ‐ Supplement 1997;63:S63‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Locatelli F, Vecchio L, Andrulli S, Marai P, Tentori F. The role of underlying nephropathy in the progression of renal disease. Kidney International ‐ Supplement 2000;75:S49‐55. [MEDLINE: ] [PubMed] [Google Scholar]
- Mann JF, Maschio G, Alberti D, AIPRI investigators. Perspectives of ACE inhibition in progressive renal failure [abstract]. Nephrology 1997;3(Suppl 1):S39. [CENTRAL: CN‐00461248] [Google Scholar]
- Maschio G, Alberti D, Janin G, Locatelli F, Mann J, Motolese M, et al. The use of benazepril in the treatment of progressive renal disease [abstract]. 13th International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:65.
- Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese F, et al. Effect of the angiotensin‐converting‐enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin‐Converting‐Enzyme Inhibition in Progressive Renal Insufficiency Study Group. New England Journal of Medicine 1996;334(15):939‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maschio G, Alberti D, Locatelli F, Mann JF, Motolese M, Ponticelli C, et al. Angiotensin‐converting enzyme inhibitors and kidney protection: the AIPRI trial. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study Group. Journal of Cardiovascular Pharmacology 1999;33 Suppl 1:S16‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maschio, G. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596‐7. [DOI] [PubMed] [Google Scholar]
- Hout BA, Simeon GP, McDonnell J, Mann JF. Economic evaluation of benazepril in chronic renal insufficiency. Kidney International ‐ Supplement 1997;52(Suppl 63):S159‐62. [MEDLINE: ] [PubMed] [Google Scholar]
ALADIN Study 2013 {published data only}
- Caroli A, Perico N, Perna A, Antiga L, Brambilla P, Pisani A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo‐controlled, multicentre trial. Lancet 2013;382(9903):1485‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Biao 1997 {published data only}
- Biao S. Clinical observation on adult polycystic kidney disease treated with calcitriol and 'qijudihuang mixt' [abstract no: P1057]. Nephrology 1997;3(Suppl 1):S338. [CENTRAL: CN‐00460393] [Google Scholar]
Cadnapaphornchai 2005 {published data only}
- Cadnapaphornchai MA, Fick‐Brosnahan GM, Duley I, Johnson AM, Strain JD, DeGroff CG, et al. Design and baseline characteristics of participants in the study of antihypertensive therapy in children and adolescents with autosomal dominant polycystic kidney disease (ADPKD). Contemporary Clinical Trials 2005;26(2):211‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Prospective change in renal volume and function in children with ADPKD. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(4):820‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadnapaphornchai 2005 borderline {published data only}
- Cadnapaphornchai MA, Fick‐Brosnahan GM, Duley I, Johnson AM, Strain JD, DeGroff CG, et al. Design and baseline characteristics of participants in the study of antihypertensive therapy in children and adolescents with autosomal dominant polycystic kidney disease (ADPKD). Contemporary Clinical Trials 2005;26(2):211‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Prospective change in renal volume and function in children with ADPKD. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(4):820‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadnapaphornchai 2005 normotensive {published data only}
- Cadnapaphornchai MA, Fick‐Brosnahan GM, Duley I, Johnson AM, Strain JD, DeGroff CG, et al. Design and baseline characteristics of participants in the study of antihypertensive therapy in children and adolescents with autosomal dominant polycystic kidney disease (ADPKD). Contemporary Clinical Trials 2005;26(2):211‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Prospective change in renal volume and function in children with ADPKD. Clinical Journal of the American Society of Nephrology: CJASN 2009;4(4):820‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ecder 1999 {published data only}
- Ecder T, Chapman AB, Brosnahan GM, Edelstein CL, Johnson AM, Schrier RW. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. American Journal of Kidney Diseases 2000;35(3):427‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ecder T, Edelstein CL, Chapman AB, Johnson AM, Tison L, Gill EA, et al. Reversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 1999;14(5):1113‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ecder T, McFann KK, Johnson AM, Chapman CB, Edelstein CL, Tison M, et al. Reversal of left ventricular hypertrophy in autosomal dominant polycystic kidney disease (ADPKD) patients with rigorous blood pressure (BP) control [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):534A. [CENTRAL: CN‐00550560] [Google Scholar]
- Schrier R, McFann K, Johnson A, Chapman A, Edelstein C, Brosnahan G, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal‐dominant polycystic kidney disease: results of a seven‐year prospective randomized study. Journal of the American Society of Nephrology 2002;13(7):1733‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ELATE Study 2011 {published data only}
- Chrispijn M, Drenth JP. Everolimus and long acting octreotide as a volume reducing treatment of polycystic livers (ELATE): study protocol for a randomized controlled trial. Trials [Electronic Resource] 2011;12:246. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: Results from a randomized controlled trial in polycystic liver disease patients [abstract]. Journal of Hepatology 2013;58(Suppl 2):S557‐8. [EMBASE: 71055656] [DOI] [PubMed] [Google Scholar]
- Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. Journal of Hepatology 2013;59(1):153‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fassett 2010 {published data only}
- Fassett RG, Coombes JS, Packham D, Fairley KF, Kincaid‐Smith P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scandinavian Journal of Urology & Nephrology 2010;44(1):56‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kincaid‐Smith P, Vincent J, Fairley K. Randomised controlled trial of HMG CO A reductase inhibitor in polycystic renal disease in man [abstract no: A1733]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):375A. [CENTRAL: CN‐00446097] [Google Scholar]
Higashihara 2008 {published data only}
- Higashihara E, Nutahara K, Horie S, Muto S, Hosoya T, Hanaoka K, et al. The effect of eicosapentaenoic acid on renal function and volume in patients with ADPKD. Nephrology Dialysis Transplantation 2008;23(9):2847‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hogan 2010 {published data only}
- Hogan M, Masyuk TV, Torres V, King BF, Kim BF, LaRusso NF. OctreotideLAR inhibits hepatorenal cystogenesis in the human polycystic liver diseases. Hepatology 2009;50(Suppl 4):328A. [EMBASE: 70073470] [Google Scholar]
- Hogan MC, Masyuk TV, Page L, Holmes DR 3rd, Li X, Bergstralh EJ, et al. Somatostatin analog therapy for severe polycystic liver disease: Results after 2 years. Nephrology Dialysis Transplantation 2012;27(9):3532‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, et al. Randomized clinical trial of long‐acting somatostatin for autosomal dominant polycystic kidney and liver disease. Journal of the American Society of Nephrology 2010;21(6):1052‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
LOCKCYST Study 2009 {published data only}
- Chrispijn M, Keimpema L, Nevens F, Vanslembrouck R, Oijen MG, Hoffmann AD, et al. Growth of liver volume stops after one year of lanreotide in patients with polycystic livers. Journal of Hepatology 2010;52(Suppl 1):S31. [EMBASE: 70130918] [Google Scholar]
- Chrispijn M, Nevens F, Gevers TJ, Vanslembrouck R, Oijen MG, Coudyzer W, et al. The long‐term outcome of patients with polycystic liver disease treated with lanreotide. Alimentary Pharmacology & Therapeutics 2012;35(2):266‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keimpema L, Nevens F, Vanslembrouck R, Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: A randomized, double‐blind, placebo‐controlled trial [abstract]. Hepatology 2009;50(Suppl 4):328A. [DOI] [PubMed] [Google Scholar]
- Keimpema L, Nevens F, Vanslembrouck R, Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double‐blind, placebo‐controlled trial. Gastroenterology 2009;137(5):1661‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Melemadathil 2013 {published data only}
- Melemadathil S, Kamal M. Efficacy and safety of sirolimus in reducing cyst volume in patients with autosomal dominant polycystic kidney disease [abstract]. Nephrology Dialysis Transplantation 2013;28:i81‐2. [EMBASE: 71075211] [Google Scholar]
Mora 2013 {published data only}
- Mora FP, Codianni P, Liern M, Grammatico D, Vallejo G. Use of rapamycin to reduce the pathologic kidney volume growth in autosomal polycystic kidney disease [abstract]. Pediatric Nephrology 2013;28(8):1492. [EMBASE: 71127367] [Google Scholar]
Nakamura 2001d {published data only}
- Nakamura T, Ushiyama C, Takahashi Y, Tanaka A, Shimada N, Ebihara I, et al. Effect of dilazep dihydrochloride on urinary albumin excretion in patients with autosomal dominant polycystic kidney disease. Nephron 2001;88(1):80‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2001d hypertensive {published data only}
- Nakamura T, Ushiyama C, Takahashi Y, Tanaka A, Shimada N, Ebihara I, et al. Effect of dilazep dihydrochloride on urinary albumin excretion in patients with autosomal dominant polycystic kidney disease. Nephron 2001;88(1):80‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2001d normotensive {published data only}
- Nakamura T, Ushiyama C, Takahashi Y, Tanaka A, Shimada N, Ebihara I, et al. Effect of dilazep dihydrochloride on urinary albumin excretion in patients with autosomal dominant polycystic kidney disease. Nephron 2001;88(1):80‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2012a {published data only}
- Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Yamada S, Ueda Y, et al. Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. American Journal of the Medical Sciences 2012;343(1):46‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nutahara 2005 {published data only}
- Nutahara K, Higashihara E, Horie S, Kamura K, Tsuchiya K, Mochizuki T, et al. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron 2005;99(1):c18‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RAPYD Study 2012 {published data only}
- Stallone G, Infante B, Bruno F, Bristogiannis C, Grandaliano G, Macarini L, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (ADPKD) study: a randomized, controlled study [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii46‐7. [EMBASE: 70765435] [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD‐study): a randomized, controlled study. Nephrology Dialysis Transplantation 2012;27(9):3560‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RAPYD Study 2012 high {published data only}
- Stallone G, Infante B, Bruno F, Bristogiannis C, Grandaliano G, Macarini L, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (ADPKD) study: a randomized, controlled study [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii46‐7. [EMBASE: 70765435] [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD‐study): a randomized, controlled study. Nephrology Dialysis Transplantation 2012;27(9):3560‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RAPYD Study 2012 low {published data only}
- Stallone G, Infante B, Bruno F, Bristogiannis C, Grandaliano G, Macarini L, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (ADPKD) study: a randomized, controlled study [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii46‐7. [EMBASE: 70765435] [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD‐study): a randomized, controlled study. Nephrology Dialysis Transplantation 2012;27(9):3560‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ruggenenti 2005 {published data only}
- Caroli A, Antiga L, Cafaro M, Fasolini G, Remuzzi A, Remuzzi G, et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(5):783‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene‐Iordache B, et al. Safety and efficacy of long‐acting somatostatin treatment in autosomal‐dominant polycystic kidney disease. Kidney International 2005;68(1):206‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SIRENA Study 2010 {published data only}
- Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, et al. Sirolimus therapy to halt the progression of ADPKD. Journal of the American Society of Nephrology 2010;21(6):1031‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soliman 2009 {published data only}
- Soliman A, Zamil S, Lotfy A, Ismail E. Sirolimus produced S‐shaped effect on adult polycystic kidneys after 2‐year treatment. Transplantation Proceedings 2012;44(10):2936–9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Soliman AR, Ismail E. Sirolimus therapy for patients with adult polycystic kidney disease ‐ a pilot study [abstract no: TH‐PO053]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):123A. [CENTRAL: CN‐00716073] [DOI] [PubMed] [Google Scholar]
- Soliman AR, Ismail E, Zamil S, Lotfy A. Sirolimus therapy for patients with adult polycystic kidney disease: a pilot study. Transplantation Proceedings 2009;41(9):3639–41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SUISSE ADPKD Study 2007 {published data only}
- Braun M, Young J, Reiner CS, Poster D, Krauer F, Kistler AD, et al. Low‐dose oral sirolimus and the risk of menstrual‐cycle disturbances and ovarian cysts: analysis of the randomized controlled SUISSE ADPKD Trial. PLoS ONE [Electronic Resource] 2012;7(10):e45868. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Young J, Reiner CS, Poster D, Wuthrich RP, Serra AL. Ovarian toxicity from sirolimus. New England Journal of Medicine 2012;366(11):1062‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serra A, Poster D, Kistler AD, Krauer F, Raina F, Voneckardstein A, et al. Safety, tolerability and adherence of sirolimus in autosomal dominant polycystic kidney disease [abstract no: 2.5]. Swiss Medical Weekly 2008;138(Suppl 167):4S. [Google Scholar]
- Serra AL, Kistler AD, Poster D, Krauer F, Senn O, Raina S, et al. Safety and tolerability of sirolimus treatment in patients with autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2009;24(11):3334‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serra AL, Kistler AD, Poster D, Struker M, Wuthrich RP, Weishaupt D, et al. Clinical proof‐of‐concept trial to assess the therapeutic effect of sirolimus in patients with autosomal dominant polycystic kidney disease: SUISSE ADPKD study. BMC Nephrology 2007;8:13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. New England Journal of Medicine 2010;363(9):820‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serra AL, Poster D, Kistler AD, Wuthrich RP. Interim analysis of efficacy, safety and tolerability of sirolimus in patients with autosomal dominant polycystic kidney disease (ADPKD) [abstract no: SA064]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
Temmerman 2012 {published data only}
- Temmerman F, Vanslembrouck R, Coudyzer W, Bammens B, Laleman W, Cassiman D, et al. The reduction in liver volume in polycystic liver disease with lanreotide is dose dependent and is most pronounced in patients with the highest liver volume [abstract]. Journal of Hepatology 2012;56:S547. [EMBASE: 70749518] [Google Scholar]
TEMPO 248 & 249 2005 {published data only}
- Chapman AB, Torres VE, Grantham JJ, Shoaf SS, Ouyang JJ, Czerwiec FS. A phase IIB pilot study of the safety and efficacy of tolvaptan, a vasopressin V2 receptor antagonist (V2RA), in patients with ADPKD [abstract no: F‐FC139]. Journal of the American Society of Nephrology 2005;16:68A. [CENTRAL: CN‐00653783] [Google Scholar]
- Grantham JJ, Chapman AB, Torres VE, Ouyang JJ, Shoaf SE, Czerwiec FS. Acute and chronic osmostasis after vasopressin V2 receptor inhibition with tolvaptan in ADPKD [abstract no: F‐PO106]. Journal of the American Society of Nephrology 2005;16(October):361A. [CENTRAL: CN‐00653784] [Google Scholar]
- Torres VE, Wang X, Ward CJ, Grantham JJ, Chapman AB, Ouyang JJ, et al. Urine aquaporin 2 and cyclic AMP responses to tolvaptan administration in autosomal dominant polycystic kidney disease [abstract no: F‐PO108]. Journal of the American Society of Nephrology 2005;16(October):361A. [CENTRAL: CN‐00653785] [Google Scholar]
TEMPO 250 2011 {published data only}
- Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, et al. Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(10):2499‐507. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Grantham JJ, Chapman AB, Watnick T, Kedzierski K, Ouyang JJ, et al. Phase 2 open‐label study to determine safety, tolerability and efficacy of split‐dose tolvaptan in ADPKD [abstract no: SA‐PO077]. Journal of the American Society of Nephrology 2007;18:361A‐2A. [CENTRAL: CN‐00653786] [Google Scholar]
TEMPO 3‐4 Study 2011 {published data only}
- Devuyst O, Chapman AB, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, et al. Urine osmolality and outcome in ADPKD: Results from the TEMPO 3:4 trial [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii6. [EMBASE: 71491481] [Google Scholar]
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. New England Journal of Medicine 2012;367(25):2407‐18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Higashihara E, Perrone RD, et al. Tolvaptan‐treatment of ADPKD confers persistent EGFR improvement: Results from the TEMPO 4:4 extension trial [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii6. [EMBASE: 71491483] [Google Scholar]
- Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3‐4 Study. American Journal of Kidney Diseases 2011;57(5):692‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ulusoy 2010 {published data only}
- Ulusoy S, Ozkan G, Orem C, Kaynar K, Kosucu P, Kiris A. A comparison of the effects of ramipril and losartan on blood pressure control and left ventricle hypertrophy in patients with autosomal dominant polycystic kidney disease. Renal Failure 2010;32(8):913‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Dijk 2001 {published data only}
- Dijk MA, Kamper AM, Veen S, Souverijn JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2001;16(11):2152‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Dijk 2003 {published data only}
- Dijk MA, Breuning MH, Duiser R, Es LA, Westendorp RG. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2003;18(11):2314‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walz 2010 {published data only}
- Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. New England Journal of Medicine 2010;363(9):830‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watson 1999 {published data only}
- Watson ML, Macnicol AM, Borg‐Costanzi J, Vareesanghip K, Chauveau D, Cohen G, et al. A long‐term comparison of the effects of renal function of BP control with either atenolol (A) or enalapril (E) in polycystic kidney disease (PKD) [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):428A. [Google Scholar]
Zeltner 2008 {published data only}
- Mueller H, Schmieder RE, Zeltner R, Poliak R, Graf S, Schulze BD. Determinants for the treatment of hypertensive patients with autosomal dominant polycystic kidney disease (ADPKD): choice of drug versus blood pressure (BP) control [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):109A. [CENTRAL: CN‐00653777] [Google Scholar]
- Zeltner R, Poliak R, Stiasny B, Schmieder RE, Schulze BD. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2008;23(2):573‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Doulton 2006 {published data only}
- Doulton TW, Saggar‐Malik AK, He FJ, Carney C, Markandu ND, Sagnella GA, et al. The effect of sodium and angiotensin‐converting enzyme inhibition on the classic circulating renin‐angiotensin system in autosomal‐dominant polycystic kidney disease patients. Journal of Hypertension 2006;24(5):939‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ISRCTN57653760 {published data only}
- O'Shaugnessy K. A rotation study through the main therapeutic classes of antihypertensive in patients with polycystic kidney disease and hypertension. controlled‐trials.com/ISRCTN57653760 (accessed 1 June 2015).
Kanno 1996 {published data only}
- Kanno Y, Suzuki H, Okada H, Takenaka T, Saruta T. Calcium channel blockers versus ACE inhibitors as antihypertensives in polycystic kidney disease. Qjm 1996;89(1):65‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kanno Y, Okada H, Konishi K, Nakazato Y, Okamiya Y, et al. Renal protective effects of calcium channel blocker on hypertensive patients with autosomal dominant polycystic kidney disease [abstract]. Journal of the American Society of Nephrology 1994;5(3):568. [Google Scholar]
Nakamura 2005a {published data only}
- Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Candesartan reduces urinary fatty acid‐binding protein excretion in patients with autosomal dominant polycystic kidney disease. American Journal of the Medical Sciences 2005;330(4):161‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharma 2004 {published data only}
- Sharma RK, Kohli R, Rathore D, Gupta A, Gupta RK. Magnetic resonance based studies as a marker of disease progression in autosomal‐dominant polycystic kidney disease and the effect of simvastatin on disease progression [abstract]. Indian Journal of Nephrology 2004;14:126. [Google Scholar]
References to studies awaiting assessment
Braun 2014 {published data only}
- Braun WE, Schold JD, Stephany BR, Spirko RA, Herts BR. Low‐dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open‐label randomized controlled pilot study. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):881‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadnapaphornchai 2011 {published data only}
- Cadnapaphornchai MA, George DM, Masoumi A, McFann K, Strain JD, Schrier RW. Effect of statin therapy on disease progression in pediatric ADPKD: design and baseline characteristics of participants. Contemporary Clinical Trials 2011;32(3):437‐45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):889‐96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
HALT‐PKD Study 2008 {published data only}
- Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT‐PKD studies. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(4):1197‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chapman AB, Torres VE, Perrone RD, Steinman TI, Bae KT, Philip MJ, et al. The HALT polycystic kidney disease trials: design and implementation. Clinical Journal of The American Society of Nephrology: CJASN 2010;5(1):102‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskulin D, Chapman A, Steinman T, Schrier R, Torres V, Perrone R, et al. Impact of autosomal dominant polycystic kidney disease on quality of life: results from the HALT‐PKD study [abstract no: TH‐PO052]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):123A. [CENTRAL: CN‐00756923] [Google Scholar]
- Miskulin DC, Abebe KZ, Chapman AB, Perrone RD, Steinman TI, Torres VE, et al. Health‐related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1‐4: a cross‐sectional study. American Journal of Kidney Diseases 2014;63(2):214‐26. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone RD, Abebe KZ, Schrier RW, Chapman AB, Torres VE, Bost J, et al. Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(10):2508‐15. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari‐Oskoui FF, Miskulin DC, Hogan MC, Fielder O, Torres VE, Bost JE, et al. Short‐term reproducibility of ambulatory blood pressure monitoring in autosomal dominant polycystic kidney disease. Blood Pressure Monitoring 2011;16(2):47‐54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R, Torres V, Chapman A, Perrone R, Miller JP, Meyers C, et al. Design of the HALT‐PKD studies [abstract no: F‐PO105]. Journal of the American Society of Nephrology 2005;16:361A. [CENTRAL: CN‐00792560] [Google Scholar]
- Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, et al. Blood pressure in early autosomal dominant polycystic kidney disease. New England Journal of Medicine 2014;371(24):2255‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman TI, Schrier RW, Torres VE, Chapman AB, Perrone RD, Grantham JJ, et al. The HALT‐PKD trials: selection of novel endpoints and study update [abstract no: PUB023]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):837A. [CENTRAL: CN‐00691816] [Google Scholar]
- Torres VE, Abebe KZ, Chapman AB, Schrier RW, Braun WE, Steinman TI, et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. New England Journal of Medicine 2014;371(24):2267‐76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Chapman AB, Perrone RD, Bae KT, Abebe KZ, Bost JE, et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney International 2012;81(6):577‐85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Schrier RW, Chapman AB, Perrone RD, Miskulin D, Steinman T, et al. Achievement and maintenance of blood pressure targets in HALT‐PKD [abstract no: TH‐PO051]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):123A. [CENTRAL: CN‐00755104] [Google Scholar]
NCT01233869 {published data only}
- NCT01233869. A phase 2, multicenter, randomized, double‐blind, placebo‐controlled study of the safety, clinical activity and pharmacokinetics of bosutinib (PF‐05208763) versus placebo in subjects with autosomal dominant polycystic kidney disease (ADPKD). www.clinicaltrials.gov/ct2/show/NCT01233869 2014. [MEDLINE: ]
Vienna RAP Study 2015 {published data only}
- Riegersperger M, Herkner H, Sunder‐Plassmann G. Pulsed oral sirolimus in advanced autosomal‐dominant polycystic kidney disease (Vienna RAP Study): study protocol for a randomized controlled trial. Trials 2015;16(1):182. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
DIPAK 1 Study 2014 {published data only}
- Meijer E, Drenth JP, d'Agnolo H, Casteleijn NF, Fijter JW, Gevers TJ, et al. Rationale and design of the DIPAK 1 Study: a randomized controlled clinical trial assessing the efficacy of lanreotide to halt disease progression in autosomal dominant polycystic kidney disease. American Journal of Kidney Diseases 2014;63(3):446‐55. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00345137 {published data only}
- NCT00345137. Phase 1 study of systemic effects of Ng‐monomethyl‐L‐arginine on renal hemodynamics in patients with polycystic kidney disease and chronic glomerulonephritis. www.clinicaltrials.gov/ct2/show/NCT00345137 (accessed 1 June 2015).
NCT01932450 {published data only}
- NCT01932450. A randomized, open‐label study investigating the effect of bilateral renal artery sympathetic denervation by catheter‐based radiofrequency ablation on blood pressure and disease progression in autosomal dominant polycystic kidney disease. www.clinicaltrials.gov/ct2/show/NCT01932450 2013. [CENTRAL: CN‐00874871]
Additional references
Chang 2012
- Chang MY, Ong AC. Mechanism‐based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clinical Practice 2012;120(1):c25‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ecder 2013
- Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Current Hypertension Reviews 2013;9(1):2‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ERA‐EDTA 2011
- ERA‐EDTA Registry. ERA‐EDTA Registry Annual Report 2011. Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands, 2011. www.era‐edta‐reg.org/files/annualreports/pdf/AnnRep2011.pdf (accessed 1 June 2015).
Gattone 2003
- Gattone VH 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nature Medicine 2003;9(10):1323–6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gile 1995
- Gile RD, Cowley BD Jr, Gattone VH 2nd, O'Donnell MP, Swan SK, Grantham JJ. Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. American Journal of Kidney Diseases 1995;26(3):501‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grantham 2006
- Grantham JJ, Torres VE, Chapman AB, Guay‐Woodford LM, Bae KT, King BF Jr, et al. Volume progression in polycystic kidney disease. New England Journal of Medicine 2006;354(20):2122–30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grantham 2008
- Grantham JJ, Cook LT, Torres VE, Bost JE, Chapman AB, Harris PC, et al. Determinants of renal volume in autosomal‐dominant polycystic kidney disease. Kidney International 2008;73(1):108–16. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grantham 2011
- Grantham JJ, Bennett WM, Perrone RD. mTOR inhibitors and autosomal dominant polycystic kidney disease. New England Journal of Medicine 2011;364(3):286‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hanaoka 2000
- Hanaoka K, Guggino W. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. Journal of the American Society of Nephrology 2000;11(7):1179‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harris 2009
- Harris PC, Torres VE. Polycystic kidney disease. Annual Review of Medicine 2009;60:321‐37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Helai 2012
- Helal I, Reed B, Schrier RW. Emergent early markers of renal progression in autosomal‐dominant polycystic kidney disease patients: implications for prevention and treatment. American Journal of Nephrology 2012;36(2):162‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Masyuk 2007
- Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3’,5’‐cyclic monophosphate. Gastroenterology 2007;132(3):1104‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nagao 2006
- Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. Journal of the American Society of Nephrology 2006;17(8):2220‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ogborn 2000
- Ogborn MR, Nitschmann E, Weiler HA, Bankovic‐Calic N. Modification of polycystic kidney disease and fatty acid status by soy protein diet. Kidney International 2000;57(1):159‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Qian 2008
- Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, et al. Sirolimus reduces polycystic liver volume in ADPKD patients. Journal of the American Society of Nephrology 2008;19(3):631‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rule 2006
- Rule AD, Torres VE, Chapman AB, Grantham JJ, Guay‐Woodford LM, Bae KT, et al. Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: the consortium of radiologic imaging studies of polycystic kidney disease cohort. Journal of the American Society of Nephrology 2006;17(3):854‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schrier 2009
- Schrier RW. Renal volume, renin‐angiotensin‐aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. Journal of the American Society of Nephrology 2009;20(9):1888‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Torres 2007
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007;369(9569):1287‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Torres 2009
- Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney International 2009;76(2):149‐68. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres 2012
- Torres VE, Chapman AB, Perrone RD, Bae KT, Abebe KZ, Bost JE, et al. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney International 2012;81(6):577‐85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
USRDS 2008
- Table A.1.7 Incident counts of reported ESRD: all patients by age, gender, race, ethnicity, & primary diagnosis. IN: US Renal Data Services. Table A.1, Incident counts of reported ESRD: all patients. www.usrds.org/2008/ref/A_incidence_08.pdf (accessed 1 June 2015).
Wahl 2006
- Wahl PR, Serra AL, Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrology Dialysis Transplantation 2006;21(3):598‐604. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu M, Wahl PR, Hir M, Wackerle‐Men Y, Wüthrich RP, Serra AL. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney & Blood Pressure Research 2007;30(4):253‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wüthrich 2009
- Wüthrich RP, Serra AL, Kistler AD. Autosomal dominant polycystic kidney disease: new treatment options and how to test their efficacy. Kidney & Blood Pressure Research 2009;32(5):380‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bolignano 2013
- Bolignano D, Ruospo M, Zoccali C, Craig JC, Strippoli GF. Interventions for preventing the progression of autosomal dominant polycystic kidney disease. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010294] [DOI] [PMC free article] [PubMed] [Google Scholar]


