Abstract
Background
Vascular dementia represents the second most common type of dementia after that caused by Alzheimer's disease. Particularly in older patients, the combination of vascular dementia and Alzheimer's disease is common and is referred to as mixed dementia. The classification of vascular dementia broadly follows three clinico‐pathological processes: multi‐infarct dementia, single strategic infarct dementia and subcortical dementia. Not all patients fulfil strict criteria for dementia and may be significantly cognitively impaired without memory loss and the term vascular cognitive impairment is more useful. Currently, no established standard treatment for vascular cognitive impairment exists. Reductions in acetylcholine and acetyltransferase activity are common to both Alzheimer's disease and vascular cognitive impairment raising the possibility that cholinesterase inhibitors such as galantamine may be beneficial for the latter.
Objectives
To assess the efficacy of galantamine in the treatment of people with vascular cognitive impairment or vascular dementia or mixed dementia.
Search methods
The trials were identified from a search of ALOIS: the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 12 January 2013. The register contains information on trials identified from frequent searches of a number of major healthcare and medical databases (MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS) as well as from a number of international and national trial registries and grey literature sources. The terms used were: galantamine, galanthamine, Reminyl, Razadyne, Nivalin.
Selection criteria
All unconfounded randomised double‐blind trials comparing galantamine with placebo were eligible for inclusion.
Data collection and analysis
Two review authors independently extracted the data from included studies.
Main results
Two trials, 1378 participants, employing randomised, double‐blind, parallel‐group methodology were included. Both trials were of six months duration and were testing a galantamine dose of 16‐24 mg/day in two divided doses. Both trials had an overall low risk of bias.
The GAL‐INT‐6 trial included 592 patients with vascular dementia diagnosed according to recognised criteria and patients with Alzheimer's disease and coincidental radiographic findings of cerebrovascular disease. Limited outcome data were reported for the subgroup data with vascular dementia. In the whole trial population, statistically significant treatment effects in favour of galantamine compared with placebo in cognition (ADAS‐cog, mean difference (MD) ‐2.29, 95% confidence interval (CI) ‐3.46 to ‐1.12, P = 0.0001 ), activities of daily living (DAD, MD 4.10, 95% CI 1.25 to 6.95, P = 0.005) and behaviour (NPI, MD ‐2.06, 95% CI ‐4.09 to ‐0.03, P = 0.05 ) were noted. Significantly higher numbers of patients dropped out, (102/396 galantamine, 33/196 placebo odds ratio (OR) 1.71, 95% CL 1.11 to 2.65, P = 0.02) and withdrew due to an adverse event from the group treated with galantamine compared with the placebo group (79/396 galantamine, 16/196 placebo, OR 2.80, 95% CI 1.59 to 4.95, P =0.0004).
Data were also included from a second larger trial (GAL‐INT‐26) involving 788 patients with vascular dementia diagnosed using standard criteria. Statistically significant benefits favouring galantamine over placebo in assessments of cognition (ADAS‐cog, MD ‐1.50, 95% CI ‐2.39 to ‐0.61, P = 0.0009), and favouring placebo compared with galantamine for behaviour (NPI, MD 1.80, 95% CI 0.29 to 3.31, P = 0.02) are recorded. Significantly higher numbers of patients dropped out from the group treated with galantamine compared with the placebo group (50/396 galantamine, 25/390 placebo OR 2.11, 95% CL 1.28 to 3.49, P = 0.004).
Authors' conclusions
Limited data were available when considering the impact of galantamine on vascular dementia or vascular cognitive impairment. The data available suggest some advantage over placebo in the areas of cognition and global clinical state. In both included trials galantamine produced higher rates of gastrointestinal side‐effects. More studies are needed before firm conclusions can be drawn.
Keywords: Humans; Alzheimer Disease; Alzheimer Disease/complications; Alzheimer Disease/drug therapy; Cholinesterase Inhibitors; Cholinesterase Inhibitors/therapeutic use; Cognition Disorders; Cognition Disorders/drug therapy; Cognition Disorders/etiology; Dementia, Vascular; Dementia, Vascular/drug therapy; Galantamine; Galantamine/adverse effects; Galantamine/therapeutic use; Nootropic Agents; Nootropic Agents/adverse effects; Nootropic Agents/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
No consistent evidence of efficacy of galantamine in vascular cognitive impairment
The efficacy of galantamine has been tested in two randomised controlled trials for the treatment of vascular dementia and for a mixed population of Alzheimer's disease patients with evidence of cerebrovascular disease on scanning. The rationale behind its use is to correct the cholinergic deficit seen in vascular dementia. This review found evidence of benefit for galantamine compared with placebo in measures of cognition in both studies. Both studies indicated higher rates of nausea and vomiting in patients taking galantamine compared with placebo.
Background
Vascular dementia (VaD) is the second most common subtype of dementia affecting around one quarter of all dementia sufferers. Cases of mixed vascular and Alzheimer's disease (AD) raise the proportion to around 50%. In line with demographic changes, the absolute number of patients with VaD is rising.
The diagnosis of VaD requires the presence of dementia, evidence of cerebrovascular disease on brain scanning and a likely association between the two. The requirement for memory impairment as a criterion for defining dementia excludes people who nonetheless experience significant cognitive dysfunction. The more comprehensive term "vascular cognitive impairment" (VCI) is sometimes preferred.
Several forms of vascular damage can lead to cognitive impairment. Traditional concepts based on stroke and multi‐infarct dementia are outdated as VCI arises from a broader range of pathologies. The most common form of VCI is due to the combination of vascular disease and AD (MRC CFAS 2001). The two conditions share similar risk factors and their histopathological features may overlap. It has been suggested that small vessel ischaemia may produce both Alzheimer's type pathology or white matter damage (O'Brien 2003).
Vascular cognitive impairment in the absence of AD may be secondary to large cortical infarcts at one extreme or diffuse white matter lesions at the other (O'Brien 2003). Small infarcts or lacunae may also be a feature. Unsurprisingly, VCI presents in different ways depending on the location of the neurological damage and the pathogenic mechanism. Single strategic infarcts present abruptly, while the onset of impairment due to subcortical change in the form of lacunae or white matter damage may be more insidious. Relative preservation of memory in a setting of impairment of attention and executive dysfunction is typical, and the underlying damage is to the neuronal circuitry of the prefrontal region rather than of the mesial temporal lobe (Cummings 1993; Erkinjuntti 2000). Non‐cognitive features such as depression, apathy and emotional lability may also occur (O'Brien 2000). Physical impairment such as gait disorder or imbalance may be a concomitant feature (Pohjasvaara 2003).
Currently, no standard treatment for VCI exists and clinicians must extrapolate from large primary and secondary prevention trials in ischaemic heart disease, hypertension and stroke. Cholinesterase inhibitors modestly improve a range of symptoms in some patients with AD through enhancement of cholinergic neurotransmission. Reductions in acetylcholine and acetyltransferase activity are noted to differing degrees in both AD and VCI, raising the possibility that these drugs may also be beneficial in the latter (Perry 1997; Perry 2005; Toghi 1996).
The three cholinesterase inhibitors used for the treatment of AD internationally are donepezil, rivastigmine and galantamine. Placebo‐controlled trials involving large numbers of patients have demonstrated clear cognitive benefit in AD with each drug. Additional gain has also been demonstrated in other areas such as activities of daily living (ADL), global functioning and neuropsychiatric symptoms. The efficacy of cholinesterase inhibitors in VCI is less clear. A Cochrane review of the role of donepezil in VCI noted improvements in cognitive function and activities of daily living as well as a more global measures of change (Malouf 2004). Galantamine (marketed as Reminyl by Janssen) is an alkaloid originally extracted from Amaryllidaceae (Galanthus woronowi, the Caucasian snowdrop and daffodil bulbs) but now synthesised. It is a reversible, competitive inhibitor of acetylcholinesterase with very little butyrylcholinesterase inhibitory activity (Lilienfeld 2002).
Objectives
To assess the efficacy of galantamine in the treatment of people with vascular cognitive impairment, vascular dementia, and "mixed" dementia.
Methods
Criteria for considering studies for this review
Types of studies
All unconfounded, randomised, double‐blind trials involving participants with vascular dementia or vascular cognitive impairment (VCI) or mixed dementia in which treatment with galantamine is compared with placebo.
Types of participants
Patients diagnosed as having VCI or dementia, or mixed dementia on a basis of standardised diagnostic criteria such as the ADDTC (California State Alzheimer's Disease Diagnostic and Treatment Centre) (Chui 1992), NINCDS/AIREN (National Institute of Neurological Disorders and Stroke and the Association International pour la Recherche et l' Enseignement en Neurosciences) (Roman 1993), and ICD‐10 (International Classification of Diseases of World Health Organization (WHO 1992).
In addition, diagnosis of the VCI with no dementia will be based on scores on cognitive impairment scales.
Types of interventions
Galantamine at any dose with parallel placebo control.
Types of outcome measures
The review will assess the following outcomes:
global impression;
functional performance;
behavioural disturbance;
quality of life;
cognitive function;
effect on carer;
death;
safety and adverse effects;
brain image changes;
costs.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 12 January 2013. The search terms used were: galantamine, galanthamine, Reminyl, Razadyne, Nivalin
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy people. The studies are identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and LILACS
Monthly searches of a number of trial registers: UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others)
Quarterly search of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL)
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in many of the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
The latest search (Janaury 2013) retrieved a total of 494 results. No new studies for inclusion were identified from the latest search.
Data collection and analysis
Two review authors (DC, JB) independently discarded publications deemed as irrelevant on the basis of title and abstract. Disagreements between review authors about final inclusion of trials were resolved by discussion and external expert advice.
Quality assessment
The methodological quality of each selected trial (GAL‐INT‐26; GAL‐INT‐6) was based on the 'Risk of bias' tool.
Data extraction
Data were extracted from the published reports. The summary statistics required for each trial and each outcome for continuous data are the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and the number of patients for each treatment group at each time point were extracted if available.
For binary data the numbers in each treatment group and the numbers experiencing the outcome of interest were sought.
The baseline assessment was defined as the latest available assessment prior to randomisation, but no longer than two months prior.
For each outcome measure, data were sought on every patient randomised. To allow an intention‐to‐treat analysis, the data were sought irrespective of compliance, whether or not the patient was subsequently deemed ineligible, or otherwise excluded from treatment or follow‐up. If intention‐to‐treat data were not available in the publications, "on‐treatment" or the data of those who complete the trial were sought and indicated as such.
Data from titration phases prior to the randomised phase were not used to assess safety or efficacy because patients were usually not randomised, nor were treatments concealed.
Data analysis
The outcomes measured in clinical trials of dementia and cognitive impairment often arise from ordinal rating scales. Where the rating scales used in the trials have a reasonably large number of ordered categories (more than 10), the data were treated as continuous outcomes arising from a normal distribution.
Summary statistics (number (n), mean and standard deviation) were required for each rating scale at each assessment time point for each treatment group in each trial for change from baseline.
When change from baseline results was not reported, the required summary statistics were calculated from the baseline and assessment time treatment group means and standard deviations. In this case a zero correlation between the measurements at baseline and assessment time was assumed. This method overestimates the standard deviation of the change from baseline, but this conservative approach is considered to be preferable in a meta‐analysis.
The meta‐analysis requires the combination of data from the trials that may not use the same rating scale to assess an outcome. The measure of the treatment difference for any outcome was the mean difference when the pooled trials used the same rating scale or test, and the standardised mean difference, which is the absolute mean difference divided by the standard deviation when they used different rating scales or tests.
The duration of the trials may vary considerably. If the range was considered too great to combine all trials into one meta‐analysis, it was divided into smaller time periods and a separate meta‐analysis conducted for each period. Some trials may contribute data to more than one time period if multiple assessments have been done.
For binary outcomes, such as clinical improvement or no clinical improvement, the odds ratio was used to measure treatment effect. A weighted estimate of the typical treatment effect across trials was calculated.
Overall estimates of the treatment difference were presented. In all cases the overall estimate from a fixed‐effect model was presented and a test for heterogeneity using a standard Chi2 statistic or the I2 statistic was performed. If, however, there was evidence of heterogeneity of the treatment effect between trials then either only homogeneous results were pooled, or a random‐effects model used (in which case the confidence intervals would be broader than those of a fixed‐effect model).
Subgroup analysis
Where relevant, and data were available, subgroup analysis included severity of impairment, and magnetic resonance imaging (MRI) lesions types.
Results
Description of studies
Two trials which met the inclusion criteria were identified, GAL‐INT‐6 and GAL‐INT‐26.
GAL‐INT‐6 is a six‐month, multicentre, randomised, double‐blind, placebo‐controlled trial of 592 patients (196 received placebo, 396 received galantamine) aged between 40 and 90 years. All patients completed a four‐week, single‐blind placebo run‐in period before randomisation to placebo or galantamine 24 mg/day. Galantamine was initiated at 4 mg/day in the first week, followed by weekly increments of 4 mg/day until they reached 24 mg/day in week six.
Eligible patients were either diagnosed with probable vascular dementia (VaD) based on NINDS‐AIREN (National Institute of Neurological Disorders of Stroke‐Association International pour la Recherche et l' Enseignement en Neurosciences) criteria (Roman 1993) or possible Alzheimer's disease (AD) based on NINCDS‐ADRDA (National Institute of Neurological and Communicative Disorders of Stroke‐Alzheimer's Disease and Related Disorders Association) criteria (McKhann 1984). They also showed significant radiological evidence of cerebrovascular disease on recent computed tomography (CT) or MR imaging. Patients had an MMSE (mini–mental state examination)score of 10 to 25 (inclusive), an ADAS‐cog/11 score of 12 or greater, and evidence from a carer on the patients ability to perform ADLs on the DAD scale. Evidence was required of relevant focal neurological signs consistent with previous stroke and cerebrovascular disease. Three diagnostic groups were identified. Where there was a history of progressive worsening of memory and other cognitive functions for at least six months combined with radiological evidence of cerebrovascular disease, patients were labelled as having AD with cerebrovascular lesions (50% of patients). Where there was dementia within three months of a stroke or a history of fluctuating or stepwise progression then patients were given the diagnosis of probable VaD (41% of patients). In cases where it was difficult to make a clear‐cut differentiation, the term 'intermediate diagnosis' was used (9% of patients). Patients were excluded if they had, other than AD, Huntington disease, other neurodegenerative dementia, any serious comorbid medical condition, or if they were using drugs for dementia.
All randomised patients who received at least one dose of trial medication and had some follow‐up data after baseline, the intention‐to‐treat (ITT) population, were included in analyses of baseline and safety data. The primary efficacy analyses were based on an observed case population, those in the ITT population with observed data at each scheduled assessment visit. In addition, last observation carried forward (LOCF) analyses were carried out on data from the ITT population with LOCF to replace missing assessments.
The primary end‐points were cognition (using the Alzheimer's Disease Assessment Scale‐cognitive subscale, ADAS‐cog) and global functioning (using the Clinician's Interview‐based Impression of Change plus caregiver input, CIBIC‐plus). Secondary efficacy measures were ADAS‐cog/13, the Neuropsychiatric Inventory (NPI), (Cummings 1994)and the disability assessment in dementia DAD (Gelinas 1999).
GAL‐INT‐26 is a 26‐week, multicentre, randomised, double‐blind, placebo‐controlled trial of 788 patients (390 received placebo and 396 received galantamine) aged between 40 and 90 years. All patients completed a four‐week, single‐blind placebo run‐in period before randomisation. Galantamine was initiated at 4 mg twice daily for four weeks, followed by 8 mg twice daily for four weeks, followed by 12 mg twice daily if the previous dose was tolerated.
The prevalence of vascular diseases was high in the selected sample (91% of the patients had cardiovascular risk factors). The diagnosis of VaD was based on NINDS‐AIREN criteria. MRI was used to confirm the clinical diagnosis of VaD. Patients had an MMSE score of 10 to 26 (inclusive), and an ADAS‐cog/11 score of 12 or greater. Patients were excluded if they had AD, Huntington disease, other neurodegenerative dementia, any serious comorbid medical condition, or if they were using drugs for dementia.
Primary outcomes were analysed via a modified ITT analysis in which galantamine (8 mg or 12 mg twice daily) was compared with placebo. The modified intent‐to‐treat (mITT) population was defined as including all participants who had both a baseline assessment and at least one post‐treatment assessment within therapeutic reach for at least one of the primary efficacy variables. Therapeutic reach was given as the maximum number of days between the last dose of the trial drug and the outcome assessment and was set at seven days, based on galantamine's pharmacokinetic and pharmacodynamic properties.
Primary outcomes were cognition (using the ADAS‐cog sum of 11 cognitive items, ADAS‐cog/11(Rosen 1984)) and activities of daily living score (Alzheimer's Disease Cooperative Study‐Activities of Daily Living inventory (ADCS‐ADL) (Galasko 1997)). Secondary objectives were to evaluate effects of galantamine on global clinical assessment (CIBIC‐plus), behaviour (NPI, (Cummings 1994)), and other cognitive scores based on the ADAS‐cog/11 (ADAS‐cog/13, ADAS‐cog/10, and ADAS‐cog/memory). The effect of galantamine on executive function was assessed using the EXIT‐25 scale (Executive Interview) (Royall 1992).
Description of the outcome measures
1. The first primary outcome test in the study GAL‐INT‐6 and GAL‐INT‐26 was the Alzheimer's Disease Assessment Scale cognitive subscale (ADAS‐cog/11) (Rosen 1984): a standard scale which assesses a set of cognitive functions including memory, language, and praxis. The battery comprises 11 individual tests, spoken language ability (0 ‐ 5), comprehension of spoken language (0 ‐ 5), recall of test instructions (0 ‐ 5), word finding difficulty (0 ‐ 5), following orders (0 ‐ 5), naming object (0 ‐ 5), construction drawing (0 ‐ 5), ideational praxis (0 ‐ 5), orientation (0 ‐ 8), word recall (0 ‐ 10) and word recognition (0 ‐ 2). The score ranges from 0 to 70 (higher the score = greater impairment). The ADAS‐cog/13 (score range 0 ‐ 85, has the additional items of concentration and distractibility and delayed word recall test), ADAS‐cog/10 (score range 0 ‐ 53, includes only non‐memory items) and ADAS‐cog/memory (score range 0 ‐ 32, includes memory items) were measures of secondary outcomes in GAL‐INT‐26, and ADAS‐cog/13 in GAL‐INT‐6.
2. The second primary outcome test in the study GAL‐INT‐26 was the ADCS‐ADL (Galasko 1997), the AD Cooperative Study Activities of Daily Living Scale, which assesses basic and instrumental ADLs. It requires an interview with a caregiver familiar with the behaviour of the patient. The items include ratings of the patient's ability to eat, dress, bathe, telephone, travel, shop, and perform other household duties. Lower scores indicate greater functional impairment. Basic ADLs (scoring range 0 ‐ 22) includes items for eating, walking, toileting, bathing, grooming, dressing, selection of clothes, and physical performance. Instrumental ADLs (scoring range 0 ‐ 56) includes items for telephone, television, conversation, dishes, managing personal belongings, obtaining beverages, making a meal or snack, disposal of garbage, travel outside home, shopping, keeping appointments, ability to be left alone, current events, reading, writing, hobbies, and household appliances.
3. The primary outcome measure of global change in GAL‐INT‐6 and a secondary outcome measure used in the study GAL‐INT‐26 was the Clinician's Interview‐Based Impression of Change‐plus care‐giver input version (CIBIC‐plus) (Knopman 1994): a global assessment instrument that provides an index of clinically important change for the assessment of dementia patients. The rating of patient function focuses on domains of concentration, orientation, memory, language, behaviour, initiative and activities of daily living. The scale rating is made on a seven‐point scale.
Very much improved: a major activity in the patient's daily routine or in mental status has been regained in addition to regaining functional independence.
Improved: in this stage some measures of functional independence‐ social, instrumental, cognitive has been regained.
Minimally improved: a noticeable change in the patient's functioning, social interactions, or mental clarity.
Indicating no change since baseline.
Minimally worsened: a marked decline in patient's functioning, social interactions, or mental clarity in any aspects of performance.
Worsened: a functional dependence on some measure.
Very much worse: there is a loss of a major activity in patient's daily activity or mental status as well as the loss of functional independence.
4. The EXIT‐25 (Royall 1992), a secondary outcome measure in GAL‐INT‐26, consists of 25 items that assess executive function including number‐letter task, verbal and design fluency, finger/nose testing, conflicting instruction and echopraxia (involuntary repetition or imitation of another person's actions). The test is scored from 0 ‐ 50 with higher scores indicating a greater executive dysfunction.
5. The Neuropsychiatric Inventory (NPI) (Cummings 1994), a secondary outcome measure in GAL‐INT‐6 and GAL‐INT‐26, is a 12‐item, carer‐rated instrument used to evaluate behavioural and neuropsychiatric symptoms, including delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy, disinhibition, irritability, aberrant motor behaviour, night‐time behaviour, and appetite/eating disorder. A lower score indicates improvement.
6. The disability assessment in dementia (DAD, Gelinas 1999) grades the ability to execute daily activities across six domains thus: basic, initiation, instrumental, leisure, performance, and planning. Positive changes indicate improvement.
Safety for both studies was assessed by monitoring of adverse events, electrocardiograms (ECGs), physical examinations, vital signs, body weight, and laboratory tests.
Risk of bias in included studies
For GAL‐INT‐6 treatment allocation was randomised according to a code generated by the Janssen Research Foundation. Dose escalation was carried out at weekly intervals. Galantamine and placebo were administered as identical tablets taken twice daily. For GAL‐INT‐26 treatment allocation was randomised using IVRS provided by Covance. Blinding was ensured by using the same escalation schedule for both galantamine and placebo groups. The examiner who measured the drug's efficacy in a patient was not the same person who treated that patient and recorded adverse events. Overall, both trials were free from high risk of bias. See Characteristics of included studies table.
Effects of interventions
The two included trials studied different populations of patients. GAL‐INT‐6 was more inclusive, with patients diagnosed with probable VaD, or AD, both with cerebrovascular disease, whereas GAL‐INT‐26 included only the VaD patients. Therefore, results from the two trials were not pooled.
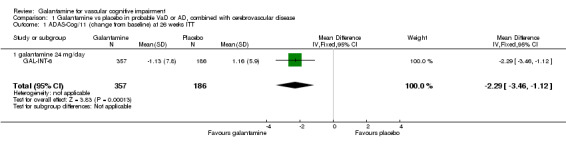
ADAS‐cog/11
There are benefits associated with galantamine 24 mg/day compared with placebo at 26 weeks (mean difference (MD) ‐2.29, 95% confidence interval (CI) ‐3.46 to ‐1.12, P = 0.0001) (ITT analysis)
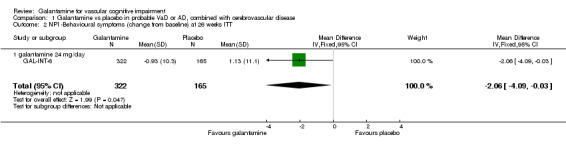
NPI‐behavioural disturbance
There are benefits associated with galantamine 24 mg/day compared with placebo at 26 weeks (MD ‐2.06, 95% CI ‐4.09 to ‐0.03, P = 0.05) (ITT analysis)
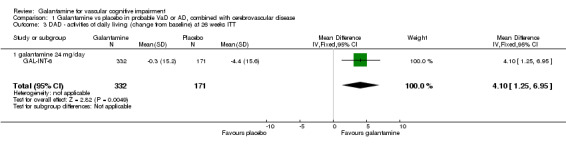
DAD‐activities of daily living
There are benefits associated with galantamine 24 mg/day compared with placebo at 26 weeks (MD 4.10, 95% CI 1.25 to 6.95, P = 0.005) (ITT analysis)
Withdrawals before end of treatment at 26 weeks
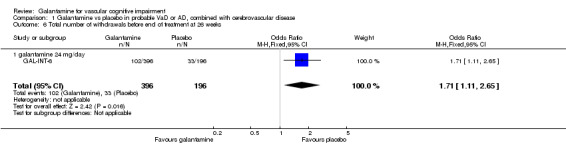
There was a significant difference, in favour of placebo, for the total number of patients who withdrew before the end of treatment at 26 weeks (102/396 galantamine, 33/196 placebo odds ratio (OR) 1.71, 95% CL 1.11 to 2.65, P = 0.02).
Withdrawals before end of treatment at 26 weeks due to an adverse event
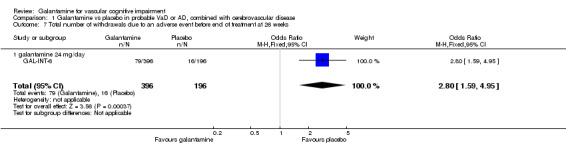
There was a significant difference, in favour of placebo, for the total number of patients who withdrew before the end of treatment at 26 weeks due to an adverse event (79/396 galantamine, 16/196 placebo OR 2.80, 95% CL 1.59 to 4.95, P = 0.0004).
Total number of patients who suffered at least one adverse event before the end of treatment at 26 weeks
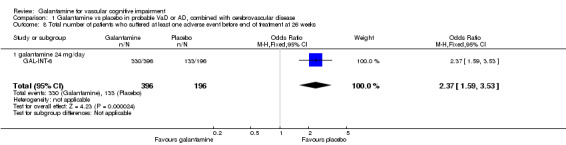
There was a significant difference, in favour of placebo, for the total number of patients who suffered at least one adverse event before the end of treatment at 26 weeks (330/396 galantamine, 133/390 placebo, OR 2.37, 95% CI 1.59 to 3.53, P < 0.0001).
Total number of patients who suffered at least one adverse event of nausea before the end of treatment at 26 weeks
There was a significant difference, in favour of placebo, for the total number of patients who suffered at least one adverse event of nausea before the end of treatment at 26 weeks (93/396 galantamine, 14/196 placebo, OR 3.99, 95% CI 2.21 to 7.21, P < 0.00001).
Total number of patients who suffered at least one adverse event of vomiting before the end of treatment at 26 weeks
There was a significant difference, in favour of placebo, for the total number of patients who suffered at least one adverse event of vomiting before the end of treatment at 26 weeks (51/396 galantamine, 11/196 placebo, OR 2.49, 95% CI 1.27 to 34.89, P = 0.008).
Total number of patients who suffered at least one adverse event of injury before the end of treatment at 26 weeks
There was a significant difference, in favour of galantamine, for the total number of patients who suffered at least one adverse event of injury before the end of treatment at 26 weeks (15/396 galantamine, 30/196 placebo, OR 0.22, 95% CI 0.11 to 0.42, P < 0.00001).
There were some data reported on the diagnostic subgroups but not in sufficient detail to be included.
ADAS‐cog/11
There are benefits associated with galantamine 16‐24 mg/day compared with placebo at 26 weeks (MD ‐1.50, 95% CI ‐2.39 to ‐0.61, P = 0.0009) (modified ITT‐LOCF analysis).
ADAS‐cog/10
There are benefits associated with galantamine 16‐24 mg/day compared with placebo at 26 weeks (MD ‐0.80, 95% CI ‐1.44 to ‐0.16, P = 0.01) (modified ITT‐LOCF analysis).
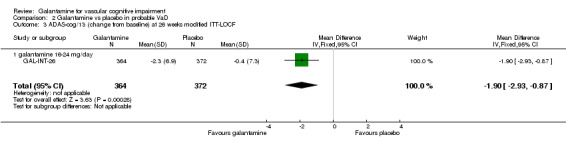
ADAS‐cog/13
There are benefits associated with galantamine 16‐24 mg/day compared with placebo at 26 weeks (MD ‐1.90, 95% CI ‐2.93 to ‐0.87, P = 0.0003) (modified ITT‐LOCF analysis).
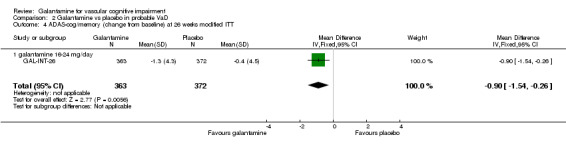
ADAS‐cog/memory
There are benefits associated with galantamine 16‐24 mg/day compared with placebo at 26 weeks (MD ‐0.90, 95% CI ‐1.54 to ‐0.26, P = 0.006) (modified ITT‐LOCF analysis).
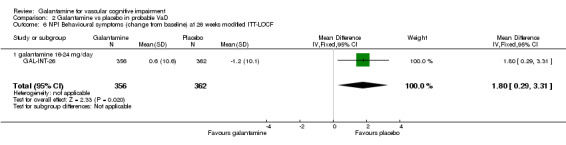
NPI‐behavioural disturbance
There are benefits associated with placebo compared with galantamine 16‐24mg/day at 26 weeks (MD 1.80, 95% CI 0.29 to 3.31, P = 0.02) (modified ITT‐LOCF analysis).
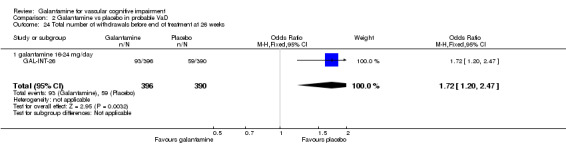
Withdrawals before end of treatment at 26 weeks
There was a significant difference, in favour of placebo, for the total number of patients who withdrew before the end of treatment at 26 weeks (93/396 galantamine, 59/390 placebo OR 1.72, 95% CL 1.20 to 2.47, P = 0.003).
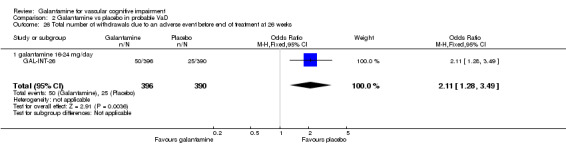
Withdrawals before end of treatment at 26 weeks due to an adverse event
There was a significant difference, in favour of placebo, for the total number of patients who withdrew before the end of treatment at 26 weeks due to an adverse event (50/396 galantamine, 25/390 placebo OR 2.11, 95% CL 1.28 to 3.49, P = 0.004).
Total number of patients who suffered at least one adverse event of nausea before the end of treatment at 26 weeks
There was a significant difference, in favour of placebo, for the total number of patients who suffered at least one adverse event of nausea before the end of treatment at 26 weeks (51/396 galantamine, 17/390 placebo, OR 3.24, 95% CI 1.84 to 5.72, P < 0.0001).
Total number of patients who suffered at least one adverse event of vomiting before the end of treatment at 26 weeks
There was a significant difference, in favour of placebo, for the total number of patients who suffered at least one adverse event of vomiting before the end of treatment at 26 weeks (31/396 galantamine, 8/390 placebo, OR 4.06, 95% CI 1.84 to 8.94, P = 0.0005).
Total number of patients who suffered at least one adverse event of dizziness before the end of treatment at 26 weeks
There was a significant difference, in favour of galantamine, for the total number of patients who suffered at least one adverse event of dizziness before the end of treatment at 26 weeks (14/396 galantamine, 26/390 placebo, OR 0.51, 95% CI 0.26 to 1.00, P = 0.05).
Total number of patients who suffered a possible trial drug‐related adverse event before the end of treatment at 26 weeks
There was a significant difference, in favour of placebo, for the total number of patients who suffered a possible trial drug related adverse event before the end of treatment at 26 weeks (140/396 galantamine, 103/390 placebo, OR 1.52, 95% CI 1.12 to 2.07, P = 0.007).
There were some data reported on the subgroups defined by type of lesions but not in sufficient detail to be included.
Discussion
In this review we examined the effect of galantamine in patients with vascular dementia (VaD)/cognitive impairment. The only clinical trial identified that focused specifically on VaD, GAL‐INT‐26, showed statistically significant benefits in favour of galantamine in cognition, and in favour of placebo in behavioural symptoms over a short period of 26 weeks. Adverse effects were noted to be higher in the galantamine‐treated group and twice the number of participants (13% versus 6%) had more than one adverse events leading to withdrawal from medication.
It is difficult to reach firm conclusions regarding the other included trial, GAL‐INT‐6. The trial population was composed of patients with VaD diagnosed according to recognised criteria (41% of patients), most of whom had stroke, plus patients with Alzheimer's disease (AD) and radiographic evidence of cerebrovascular disease (50% of patients). In this population benefits across a range of domains (cognition, activities of daily living, and behaviour ) favouring galantamine were noted but these should be viewed in the context of significantly higher rates of adverse events and significantly higher withdrawal rates in the galantamine‐treated group.
A report of the six month open‐label extension of GAL‐INT‐6 (Small 2003) demonstrated that patients treated with galantamine were able to maintain their cognitive scores throughout the total duration of this study. Interpretation of results is difficult when both VaD and AD plus cerebrovascular disease are analysed collectively in this way as the response to galantamine may be due to the drug effect on the AD component. There is limited utility in including AD patients with concomitant cerebrovascular disease in clinical trials until more is understood regarding the symptomatology and natural history of what might be considered 'mixed' dementia. In general, trying to define treatment response prior to defining the natural history of the condition one is hoping to improve is potentially frustrating and undermines conclusions from treatment trials such as GAL‐INT‐6. Both GAL‐INT‐26 and GAL‐INT‐6 relied on the NINDS‐AIREN (National Institute of Neurological Disorders and Stroke; Association Internationale pour la Recherche et l'Enseigement en Neurosciences) criteria (Roman 1993) for possible or probable VaD. These criteria, which capture VaD in all its forms, may not be optimal for interpreting the results of large randomised controlled trials when distinct pathologies are likely to influence clinical response.
Authors' conclusions
Implications for practice.
There are some weak indications that galantamine is useful in improving cognition in dementia secondary to vascular damage. However, the possible benefit must be balanced against the potential to harm as active treatment was associated with higher rates of adverse event (nausea and vomiting) and withdrawal. These patients desperately need better treatment options and clearer management strategies. Before recommending galantamine for the treatment of VaD, well‐designed and adequately powered trials of sufficient duration which clearly define the subgroup of VaD under investigation are needed.
Implications for research.
Further research into the aetiological mechanisms and clinico‐pathological subtypes of VaD is needed as more accurate division of patient groups within the context of clinical trials may better demonstrate useful treatment effects. Further exploration of the cholinergic deficiencies in the brain of patients with dementia and vascular pathology would strengthen the rationale for using this potentially helpful group of drugs.
What's new
| Date | Event | Description |
|---|---|---|
| 28 March 2013 | New search has been performed | New lead author; an update search was performed for this review on 12 January 2013 |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 28 July 2010 | New search has been performed | An update search was performed for this review on 28 July 2010. The search identified new studies for consideration by the authors |
| 26 August 2008 | Amended | Converted to new review format. |
| 13 December 2007 | New search has been performed | December 2007: update search revealed new studies for consideration. Full data from the published report of GAL‐INT‐26 (Auchus 2007) has been added to the review. |
| 16 November 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We gratefully acknowledge the contributions of the consumer editor U Hla Htay.
Appendices
Appendix 1. Update search: January 2013
| Source | Strategy | Hits |
| ALOIS www.medicine.ox.ac.uk/alois |
Advanced search: (galantamin* OR galanthamin* OR reminyl* OR razadyne* OR nivalin*) AND date imported to ALOIS between 2010‐2013 | 6 |
| MEDLINE | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. "cognit* impair*".mp. 22. exp *Cognition Disorders/ 23. MCI.ti,ab. 24. ACMI.ti,ab. 25. ARCD.ti,ab. 26. SMC.ti,ab. 27. CIND.ti,ab. 28. BSF.ti,ab. 29. AAMI.ti,ab. 30. MD.ti,ab. 31. LCD.ti,ab. 32. QD.ti,ab. 33. AACD.ti,ab. 34. MNCD.ti,ab. 35. MCD.ti,ab. 36. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 37. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 38. "preclinical AD".mp. 39. "pre‐clinical AD".mp. 40. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 41. (aMCI or MCIa).ti,ab. 42. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 43. ("GDS 3" or "stage 3 GDS").ti,ab. 44. ("global deterioration scale" and "stage 3").mp. 45. "Benign senescent forgetfulness".ti,ab. 46. "mild neurocognit* disorder*".ti,ab. 47. (prodrom* adj2 dement*).ti,ab. 48. (episodic* adj2 memory).mp. 49. ("preclinical dementia" or "pre‐clinical dementia").mp. 50. or/21‐49 51. Galantamine/ 52. galanthamin*.ti,ab. 53. galantamin*.ti,ab. 54. reminyl*.ti,ab. 55. razadyne*.ti,ab. 56. nivalin*.ti,ab. 57. or/51‐56 58. 20 or 50 59. 57 and 58 60. randomized controlled trial.pt. 61. controlled clinical trial.pt. 62. randomized.ab. 63. placebo.ab. 64. drug therapy.fs. 65. randomly.ab. 66. trial.ab. 67. groups.ab. 68. or/60‐67 69. (animals not (humans and animals)).sh. 70. 68 not 69 71. 59 and 70 72. (2010* or 2011* or 2012* or 2013*).ed. 73. 71 and 72 |
148 |
| EMBASE | 1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. "cognit* impair*".mp. 25. exp cognitive defect/ 26. exp mild cognitive impairment/ 27. MCI.ti,ab. 28. ACMI.ti,ab. 29. ARCD.ti,ab. 30. SMC.ti,ab. 31. CIND.ti,ab. 32. BSF.ti,ab. 33. AAMI.ti,ab. 34. MD.ti,ab. 35. LCD.ti,ab. 36. QD.ti,ab. 37. AACD.ti,ab. 38. MNCD.ti,ab. 39. MCD.ti,ab. 40. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 41. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 42. "preclinical AD".mp. 43. "pre‐clinical AD".mp. 44. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 45. (aMCI or MCIa).ti,ab. 46. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 47. ("GDS 3" or "stage 3 GDS").ti,ab. 48. ("global deterioration scale" and "stage 3").mp. 49. "Benign senescent forgetfulness".ti,ab. 50. "mild neurocognit* disorder*".ti,ab. 51. (prodrom* adj2 dement*).ti,ab. 52. "age‐related symptom*".mp. 53. (episodic adj2 memory).mp. 54. ("pre‐clinical dementia" or "preclinical dementia").mp. 55. or/24‐54 56. 23 or 55 57. GALANTAMINE/ 58. galanthamin*.ti,ab. 59. galantamin*.ti,ab. 60. reminyl*.ti,ab. 61. razadyne*.ti,ab. 62. nivalin*.ti,ab. 63. or/57‐62 64. 56 and 63 65. "randomized‐controlled‐trial"/ 66. "double‐blind‐procedure"/ 67. placebo.ti,ab. 68. (randomly or randomi?ed).ab. 69. trial.ti,ab. 70. or/65‐69 71. 64 and 70 72. (2010* or 2011* or 2012* or 2013*).em. 73. 71 and 72 |
49 |
| PsycINFO | 1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. "cognit* impair*".mp. 26. exp Cognitive Impairment/ 27. MCI.ti,ab. 28. ACMI.ti,ab. 29. ARCD.ti,ab. 30. SMC.ti,ab. 31. CIND.ti,ab. 32. BSF.ti,ab. 33. AAMI.ti,ab. 34. MD.ti,ab. 35. LCD.ti,ab. 36. QD.ti,ab. 37. AACD.ti,ab. 38. MNCD.ti,ab. 39. MCD.ti,ab. 40. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 41. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 42. "preclinical AD".mp. 43. "pre‐clinical AD".mp. 44. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 45. (aMCI or MCIa).ti,ab. 46. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 47. ("GDS 3" or "stage 3 GDS").ti,ab. 48. ("global deterioration scale" and "stage 3").mp. 49. "Benign senescent forgetfulness".ti,ab. 50. "mild neurocognit* disorder*".ti,ab. 51. (prodrom* adj2 dement*).ti,ab. 52. "age‐related symptom*".mp. 53. (episodic adj2 memory).mp. 54. ("pre‐clinical dementia" or "preclinical dementia").mp. 55. or/25‐54 56. 24 or 55 57. Galanthamine/ 58. galanthamin*.ti,ab. 59. galantamin*.ti,ab. 60. reminyl*.ti,ab. 61. razadyne*.ti,ab. 62. nivalin*.ti,ab. 63. or/57‐62 64. 56 and 63 65. Clinical Trials/ 66. random*.ti,ab. 67. placebo*.ti,ab. 68. ("double‐blind*" or "double‐masked").ti,ab. 69. trial.ab. 70. or/64‐69 71. 64 and 70 72. (2010* or 2011* or 2012* or 2013*).up. 73. 71 and 72 |
110 |
| ISI Web of Knowledge – all databases | Topic=(dement* OR alzheimer* OR "cognit* impair*" OR Lewy OR VaD OR AD) AND Topic=(galantamin* OR galanthamin* OR reminyl* OR razadyne* OR nivalin*) AND Topic=(rct OR random* OR placebo OR blind* OR "control group" OR trial) AND Year Published=(2010‐2013) Timespan=All Years. Search language=English Lemmatization=On |
153 |
| CENTRAL | #1 MeSH descriptor: [Dementia] explode all trees #2 MeSH descriptor: [Delirium] this term only #3 MeSH descriptor: [Wernicke Encephalopathy] this term only #4 MeSH descriptor: [Delirium, Dementia, Amnestic, Cognitive Disorders] this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 "cognit* impair*" #22 MCI or ACMI or ARCD or SMC or CIND or BSF or AAMI or AACD or MNCD or MCD #23 "preclinical AD" or "pre‐clinical AD" #24 "mild neurocognit* disorder*" #25 episodic* near/2 memory #26 "preclinical dementia" or "pre‐clinical dementia" #27 #21 or #22 or #23 or #24 or #25 or #26 #28 #20 or #27 #29 MeSH descriptor: [Galantamine] this term only #30 galanthamin* #31 galantamin* #32 reminyl* #33 razadyne* #34 nivalin* #35 #29 or #30 or #31 or #32 or #33 or #34 #36 #28 and #35 from 2010 to 2013, in Trials |
9 |
| ClinicalTrials.gov | Interventional Studies | dementia OR alzheimer OR alzheimer's OR cognitive OR cognition | galantamine OR galanthamine OR reminyl OR razadyne OR nivalin | received from 01/01/2010 to 01/14/2013 | 9 |
Appendix 2. Update search: July 2010
| Source | Search strategy | Hits |
| ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search: (Study design: RCT OR Unclear) AND (Intervention: galantamine) [which will capture: galantamine OR galanthamine OR reminyl OR razadyne OR nivalin] | 39 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. "cognit* impair*".mp. 22. exp *Cognition Disorders/ 23. MCI.ti,ab. 24. ACMI.ti,ab. 25. ARCD.ti,ab. 26. SMC.ti,ab. 27. CIND.ti,ab. 28. BSF.ti,ab. 29. AAMI.ti,ab. 30. MD.ti,ab. 31. LCD.ti,ab. 32. QD.ti,ab. 33. AACD.ti,ab. 34. MNCD.ti,ab. 35. MCD.ti,ab. 36. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 37. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 38. "preclinical AD".mp. 39. "pre‐clinical AD".mp. 40. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 41. (aMCI or MCIa).ti,ab. 42. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 43. ("GDS 3" or "stage 3 GDS").ti,ab. 44. ("global deterioration scale" and "stage 3").mp. 45. "Benign senescent forgetfulness".ti,ab. 46. "mild neurocognit* disorder*".ti,ab. 47. (prodrom* adj2 dement*).ti,ab. 48. (episodic* adj2 memory).mp. 49. ("preclinical dementia" or "pre‐clinical dementia").mp. 50. or/21‐49 51. Galantamine/ 52. galanthamin*.ti,ab. 53. galantamin*.ti,ab. 54. reminyl*.ti,ab. 55. razadyne*.ti,ab. 56. nivalin*.ti,ab. 57. or/51‐56 58. 20 or 50 59. 57 and 58 60. randomized controlled trial.pt. 61. controlled clinical trial.pt. 62. randomized.ab. 63. placebo.ab. 64. drug therapy.fs. 65. randomly.ab. 66. trial.ab. 67. groups.ab. 68. or/60‐67 69. (animals not (humans and animals)).sh. 70. 68 not 69 71. 59 and 70 72. (2008* or 2009* or 2010*).ed. 73. (200711* or 200712*).ed. 74. 72 or 73 75. 71 and 74 |
173 |
| EMBASE 1980‐2010 week 29 (Ovid SP) |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. "cognit* impair*".mp. 25. exp cognitive defect/ 26. exp mild cognitive impairment/ 27. MCI.ti,ab. 28. ACMI.ti,ab. 29. ARCD.ti,ab. 30. SMC.ti,ab. 31. CIND.ti,ab. 32. BSF.ti,ab. 33. AAMI.ti,ab. 34. MD.ti,ab. 35. LCD.ti,ab. 36. QD.ti,ab. 37. AACD.ti,ab. 38. MNCD.ti,ab. 39. MCD.ti,ab. 40. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 41. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 42. "preclinical AD".mp. 43. "pre‐clinical AD".mp. 44. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 45. (aMCI or MCIa).ti,ab. 46. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 47. ("GDS 3" or "stage 3 GDS").ti,ab. 48. ("global deterioration scale" and "stage 3").mp. 49. "Benign senescent forgetfulness".ti,ab. 50. "mild neurocognit* disorder*".ti,ab. 51. (prodrom* adj2 dement*).ti,ab. 52. "age‐related symptom*".mp. 53. (episodic adj2 memory).mp. 54. ("pre‐clinical dementia" or "preclinical dementia").mp. 55. or/24‐54 56. 23 or 55 57. GALANTAMINE/ 58. galanthamin*.ti,ab. 59. galantamin*.ti,ab. 60. reminyl*.ti,ab. 61. razadyne*.ti,ab. 62. nivalin*.ti,ab. 63. or/57‐62 64. 56 and 63 65. "randomized‐controlled‐trial"/ 66. "double‐blind‐procedure"/ 67. placebo.ti,ab. 68. (randomly or randomi?ed).ab. 69. trial.ti,ab. 70. or/65‐69 71. 64 and 70 72. (2007* or 2008* or 2009* or 2010*).em. 73. 71 and 72 |
248 |
| PSYCINFO 1806‐July week 3 2010 (Ovid SP) |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. "cognit* impair*".mp. 26. exp Cognitive Impairment/ 27. MCI.ti,ab. 28. ACMI.ti,ab. 29. ARCD.ti,ab. 30. SMC.ti,ab. 31. CIND.ti,ab. 32. BSF.ti,ab. 33. AAMI.ti,ab. 34. MD.ti,ab. 35. LCD.ti,ab. 36. QD.ti,ab. 37. AACD.ti,ab. 38. MNCD.ti,ab. 39. MCD.ti,ab. 40. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 41. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 42. "preclinical AD".mp. 43. "pre‐clinical AD".mp. 44. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 45. (aMCI or MCIa).ti,ab. 46. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 47. ("GDS 3" or "stage 3 GDS").ti,ab. 48. ("global deterioration scale" and "stage 3").mp. 49. "Benign senescent forgetfulness".ti,ab. 50. "mild neurocognit* disorder*".ti,ab. 51. (prodrom* adj2 dement*).ti,ab. 52. "age‐related symptom*".mp. 53. (episodic adj2 memory).mp. 54. ("pre‐clinical dementia" or "preclinical dementia").mp. 55. or/25‐54 56. 24 or 55 57. Galanthamine/ 58. galanthamin*.ti,ab. 59. galantamin*.ti,ab. 60. reminyl*.ti,ab. 61. razadyne*.ti,ab. 62. nivalin*.ti,ab. 63. or/57‐62 64. 56 and 63 65. Clinical Trials/ 66. random*.ti,ab. 67. placebo*.ti,ab. 68. ("double‐blind*" or "double‐masked").ti,ab. 69. trial.ab. 70. or/64‐69 71. 64 and 70 72. (2007* or 2008* or 2009* or 2010*).up. 73. 71 and 72 |
142 |
| CINAHL (EBSCOhost) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "cognit* impair*" S21 TX "cognit* defect*" S22 (MH "Cognition Disorders+") S23 TX MCI S24 TX ACMI S25 TX ARCD S26 TX SMC S27 TX CIND S28 TX BSF S29 TX AAMI S30 AB MD S31 AB LCD S32 AB QD OR "questionable dementia" S33 TX AACD S34 TX MNCD S35 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S36 TX "preclinical AD" S37 TX "pre‐clinical AD" S38 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S39 TX aMCI OR MCIa S40 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S41 TX "GDS 3" OR "stage 3 GDS" S42 TX "global deterioration scale" AND "stage 3" S43 TX "Benign senescent forgetfulness" S44 TX "mild neurocognit* disorder*" S45 TX prodrom* N2 dement* S46 TX "age‐related symptom*" S47 TX cognit* N2 deficit* S48 TX cognit* N2 deteriorat* S49 TX cognit* N2 declin* S50 TX cognit* N2 degenerat* S51 TX cognit* N2 complain* S52 TX cognit* N2 disturb* S53 TX cognit* N2 disorder* S54 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S55 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S56 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S57 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S58 TX "pre‐clinical dementia" or TX "preclinical dementia" S59 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 S60 S19 or S59 S61 (MM "Galanthamine") S62 TX galanthamin* S63 TX galantamin* S64 TX reminyl* S65 TX razadyne* S66 S61 or S62 or S63 or S64 or S65 S67 S60 and S66 S68 EM 2007 S69 EM 2008 S70 EM 2009 S71 EM 2010 S72 S68 or S69 or S70 or S71 S73 S67 and S72 S74 (MH "Clinical Trials") S75 TX placebo S76 TX "double‐blind*" S77 TX random* S78 S74 or S75 or S76 or S77 S79 S73 and S78 |
61 |
| LILACS (BIREME) | galanthamin$ OR galantamin$ OR reminyl$ OR razadyne$ OR nivalin$ [Words] | 5 |
| CENTRAL (The Cochrane Library) | #1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 "cognit* impair*" #22 MCI OR ACMI OR ARCD OR SMC OR CIND OR BSF OR AAMI OR AACD OR MNCD OR MCD #23 "preclinical AD" OR "pre‐clinical AD" #24 "mild neurocognit* disorder*" #25 episodic* NEAR/2 memory #26 "preclinical dementia" OR "pre‐clinical dementia" #27 (#21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 (#20 OR #27) #29 MeSH descriptor Galantamine, this term only #30 galanthamin* #31 galantamin* #32 reminyl* #33 razadyne* #34 nivalin* #35 (#29 OR #30 OR #31 OR #32 OR #33 OR #34) #36 (#28 AND #35), from 2007 to 2010 |
38 |
| Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | dementia OR alzheimer OR alzheimer's OR cognitive OR cognition | galantamine OR galanthamine OR reminyl OR razadyne OR nivalin | received from 11/01/2007 to 07/29/2010 | 16 |
| ICTRP Search Portal (WHO Portal) (http://apps.who.int/trialsearch/) | Interventional Studies | dementia OR alzheimer OR alzheimer's OR cognitive OR cognition | galantamine OR galanthamine OR reminyl OR razadyne OR nivalin | received from 01/11/2007 to 29/07/2010 | 12 |
| Web of Science with Conference Proceedings (1945 to present) (ISI Web of Knowledge) | Topic=(dement* OR alzheimer* OR "cognit* impair*" OR Lewy OR VaD OR AD) AND Topic=(galantamin* OR galanthamin* OR reminyl* OR razadyne* OR nivalin*) AND Topic=(rct OR random* OR placebo OR blind* OR "control group" OR trial) Timespan=2008‐2010. Databases=SCI‐EXPANDED, CPCI‐S |
128 |
| TOTAL before de‐duplication and first‐assess | 862 | |
| TOTAL after de‐dupe and first‐assess | 32 | |
Data and analyses
Comparison 1. Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog/11 (change from baseline) at 26 weeks ITT | 1 | 543 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐3.46, ‐1.12] |
| 1.1 galantamine 24 mg/day | 1 | 543 | Mean Difference (IV, Fixed, 95% CI) | ‐2.29 [‐3.46, ‐1.12] |
| 2 NPI ‐Behavioural symptoms (change from baseline) at 26 weeks ITT | 1 | 487 | Mean Difference (IV, Fixed, 95% CI) | ‐2.06 [‐4.09, ‐0.03] |
| 2.1 galantamine 24 mg/day | 1 | 487 | Mean Difference (IV, Fixed, 95% CI) | ‐2.06 [‐4.09, ‐0.03] |
| 3 DAD ‐ activities of daily living (change from baseline) at 26 weeks ITT | 1 | 503 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [1.25, 6.95] |
| 3.1 galantamine 24 mg/day | 1 | 503 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [1.25, 6.95] |
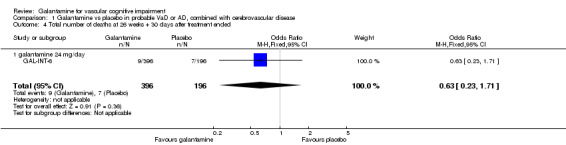
| 4 Total number of deaths at 26 weeks + 30 days after treatment ended | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.23, 1.71] |
| 4.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.23, 1.71] |
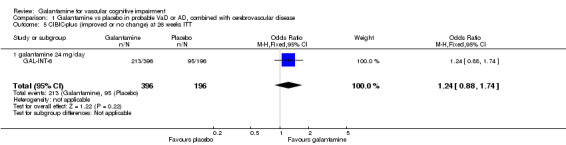
| 5 CIBIC‐plus (improved or no change) at 26 weeks ITT | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.88, 1.74] |
| 5.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.88, 1.74] |
| 6 Total number of withdrawals before end of treatment at 26 weeks | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.11, 2.65] |
| 6.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.11, 2.65] |
| 7 Total number of withdrawals due to an adverse event before end of treatment at 26 weeks | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.59, 4.95] |
| 7.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.59, 4.95] |
| 8 Total number of patients who suffered at least one adverse event before end of treatment at 26 weeks | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.59, 3.53] |
| 8.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.59, 3.53] |
| 9 Total number of patients who suffered from at least one adverse event of nausea by end of treatment at 26 weeks | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.99 [2.21, 7.21] |
| 10 Total number of patients who suffered from at least one adverse event of vomiting before end of treatment at 26 weeks | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.27, 4.89] |
| 10.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.27, 4.89] |
| 11 Total number of patients who suffered at least one adverse event of injury before end of treatment at 26 weeks | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.11, 0.42] |
| 11.1 galantamine 24 mg/day | 1 | 592 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.11, 0.42] |
1.1. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 1 ADAS‐Cog/11 (change from baseline) at 26 weeks ITT.
1.2. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 2 NPI ‐Behavioural symptoms (change from baseline) at 26 weeks ITT.
1.3. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 3 DAD ‐ activities of daily living (change from baseline) at 26 weeks ITT.
1.4. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 4 Total number of deaths at 26 weeks + 30 days after treatment ended.
1.5. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 5 CIBIC‐plus (improved or no change) at 26 weeks ITT.
1.6. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 6 Total number of withdrawals before end of treatment at 26 weeks.
1.7. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 7 Total number of withdrawals due to an adverse event before end of treatment at 26 weeks.
1.8. Analysis.
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 8 Total number of patients who suffered at least one adverse event before end of treatment at 26 weeks.
1.9. Analysis.
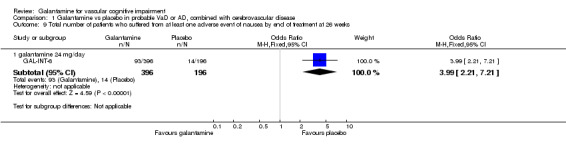
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 9 Total number of patients who suffered from at least one adverse event of nausea by end of treatment at 26 weeks.
1.10. Analysis.

Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 10 Total number of patients who suffered from at least one adverse event of vomiting before end of treatment at 26 weeks.
1.11. Analysis.
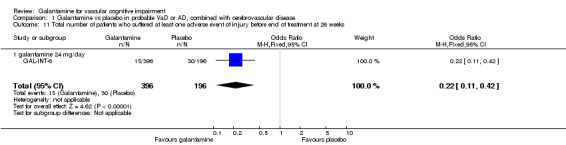
Comparison 1 Galantamine vs placebo in probable VaD or AD, combined with cerebrovascular disease, Outcome 11 Total number of patients who suffered at least one adverse event of injury before end of treatment at 26 weeks.
Comparison 2. Galantamine vs placebo in probable VaD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐cog/11 (change from baseline) at 26 weeks modified ITT‐LOCF | 1 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.39, ‐0.61] |
| 1.1 galantamine 16‐24 mg/day | 1 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.39, ‐0.61] |
| 2 ADAS‐cog/10 (change from baseline) at 26 weeks modified ITT‐LOCF | 1 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.44, ‐0.16] |
| 2.1 galantamine 16‐24 mg/day | 1 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.44, ‐0.16] |
| 3 ADAS‐cog/13 (change from baseline) at 26 weeks modified ITT‐LOCF | 1 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐2.93, ‐0.87] |
| 3.1 galantamine 16‐24 mg/day | 1 | 736 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐2.93, ‐0.87] |
| 4 ADAS‐cog/memory (change from baseline) at 26 weeks modified ITT | 1 | 735 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.54, ‐0.26] |
| 4.1 galantamine 16‐24 mg/day | 1 | 735 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.54, ‐0.26] |
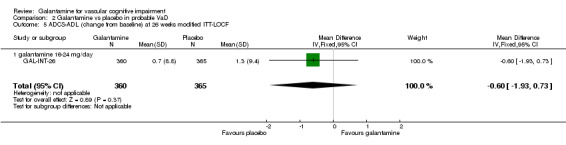
| 5 ADCS‐ADL (change from baseline) at 26 weeks modified ITT‐LOCF | 1 | 725 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.93, 0.73] |
| 5.1 galantamine 16‐24 mg/day | 1 | 725 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.93, 0.73] |
| 6 NPI Behavioural symptoms (change from baseline) at 26 weeks modified ITT‐LOCF | 1 | 718 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.29, 3.31] |
| 6.1 galantamine 16‐24 mg/day | 1 | 718 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.29, 3.31] |
| 7 CIBIC‐plus (improved or no change) at 26 weeks modified ITT‐LOCF | 1 | 734 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 7.1 galantamine 16‐24 mg/day | 1 | 734 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 8 EXIT‐25 executive function (change from baseline) at 26 weeks modified ITT‐LOCF | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.24, 0.24] |
| 8.1 galantamine 16‐24 mg/day | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.24, 0.24] |
| 9 Total number of patients who suffered at least one adverse event before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.93, 1.75] |
| 9.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.93, 1.75] |
| 10 Total number of patients who suffered from at least one adverse event of nausea by end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [1.84, 5.72] |
| 10.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [1.84, 5.72] |
| 11 Total number of patients who suffered from at least one adverse event of vomiting before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.06 [1.84, 8.94] |
| 11.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.06 [1.84, 8.94] |
| 12 Total number of patients who suffered at least one adverse event of injury before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.62, 2.04] |
| 12.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.62, 2.04] |
| 13 Total number of patients who suffered at least one adverse event of diarrhoea before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.92, 2.68] |
| 13.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.92, 2.68] |
| 14 Total number of patients who suffered at least one adverse event of dizzines before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.26, 1.00] |
| 14.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.26, 1.00] |
| 15 Total number of patients who suffered at least one adverse event of anorexia before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.32 [2.49, 27.87] |
| 15.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.32 [2.49, 27.87] |
| 16 Total number of patients who suffered at least one adverse event of urinary tract infection before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.52] |
| 16.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.52] |
| 17 Total number of patients who suffered at least one adverse event of a fall before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.47, 1.29] |
| 17.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.47, 1.29] |
| 18 Total number of patients who suffered a possible trial drug related adverse event before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.12, 2.07] |
| 18.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.12, 2.07] |
| 19 Total number of patients who suffered at least one serious adverse event before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.78, 1.59] |
| 19.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.78, 1.59] |
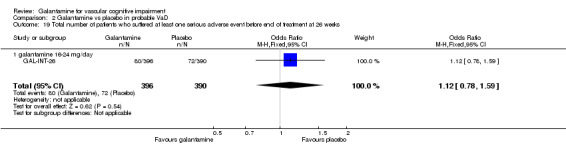
| 20 Total number of patients who suffered at least one serious adverse event (cerebrovascular disorder) before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.59] |
| 20.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.59] |
| 21 Total number of patients who suffered at least one serious adverse event of urinary tract infection before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.16, 1.92] |
| 21.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.16, 1.92] |
| 22 Total number of patients who suffered at least one serious adverse event of myocardial infarction before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.25, 2.70] |
| 22.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.25, 2.70] |
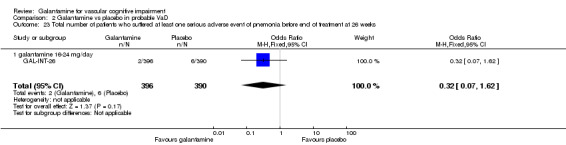
| 23 Total number of patients who suffered at least one serious adverse event of pnemonia before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.62] |
| 23.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.62] |
| 24 Total number of withdrawals before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.20, 2.47] |
| 24.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.20, 2.47] |
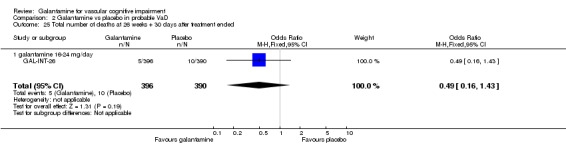
| 25 Total number of deaths at 26 weeks + 30 days after treatment ended | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.16, 1.43] |
| 25.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.16, 1.43] |
| 26 Total number of withdrawals due to an adverse event before end of treatment at 26 weeks | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.28, 3.49] |
| 26.1 galantamine 16‐24 mg/day | 1 | 786 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.28, 3.49] |
2.1. Analysis.
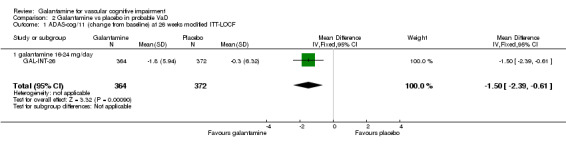
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 1 ADAS‐cog/11 (change from baseline) at 26 weeks modified ITT‐LOCF.
2.2. Analysis.
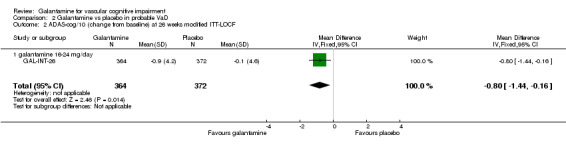
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 2 ADAS‐cog/10 (change from baseline) at 26 weeks modified ITT‐LOCF.
2.3. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 3 ADAS‐cog/13 (change from baseline) at 26 weeks modified ITT‐LOCF.
2.4. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 4 ADAS‐cog/memory (change from baseline) at 26 weeks modified ITT.
2.5. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 5 ADCS‐ADL (change from baseline) at 26 weeks modified ITT‐LOCF.
2.6. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 6 NPI Behavioural symptoms (change from baseline) at 26 weeks modified ITT‐LOCF.
2.7. Analysis.
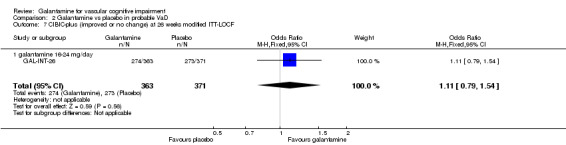
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 7 CIBIC‐plus (improved or no change) at 26 weeks modified ITT‐LOCF.
2.8. Analysis.
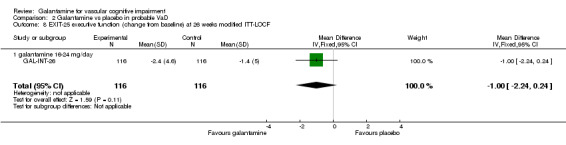
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 8 EXIT‐25 executive function (change from baseline) at 26 weeks modified ITT‐LOCF.
2.9. Analysis.
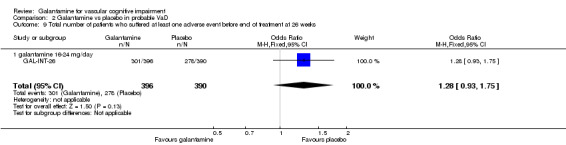
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 9 Total number of patients who suffered at least one adverse event before end of treatment at 26 weeks.
2.10. Analysis.
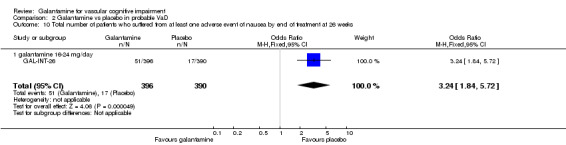
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 10 Total number of patients who suffered from at least one adverse event of nausea by end of treatment at 26 weeks.
2.11. Analysis.
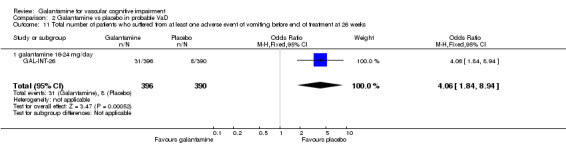
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 11 Total number of patients who suffered from at least one adverse event of vomiting before end of treatment at 26 weeks.
2.12. Analysis.
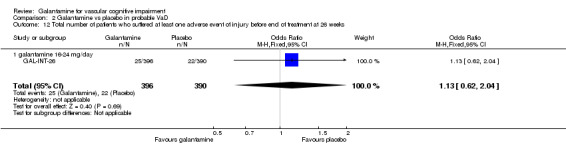
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 12 Total number of patients who suffered at least one adverse event of injury before end of treatment at 26 weeks.
2.13. Analysis.
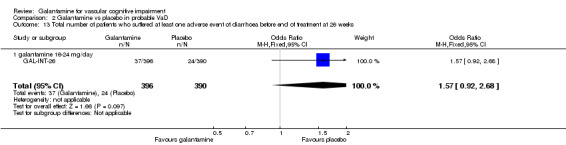
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 13 Total number of patients who suffered at least one adverse event of diarrhoea before end of treatment at 26 weeks.
2.14. Analysis.
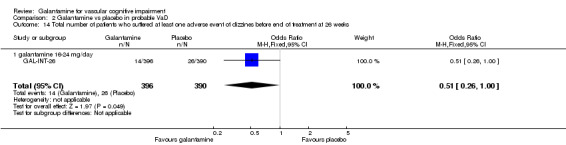
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 14 Total number of patients who suffered at least one adverse event of dizzines before end of treatment at 26 weeks.
2.15. Analysis.
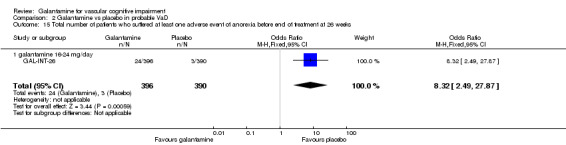
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 15 Total number of patients who suffered at least one adverse event of anorexia before end of treatment at 26 weeks.
2.16. Analysis.
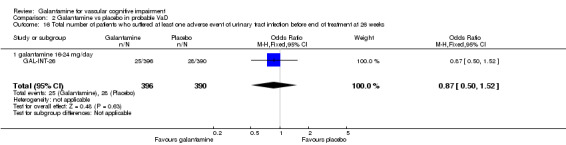
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 16 Total number of patients who suffered at least one adverse event of urinary tract infection before end of treatment at 26 weeks.
2.17. Analysis.
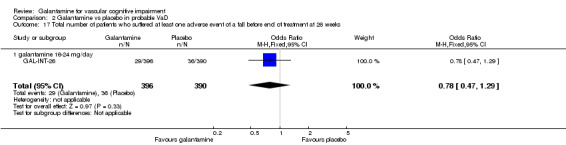
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 17 Total number of patients who suffered at least one adverse event of a fall before end of treatment at 26 weeks.
2.18. Analysis.
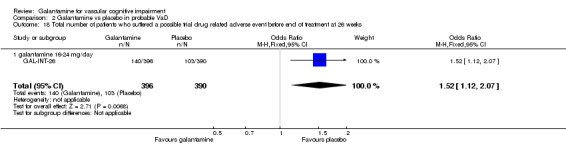
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 18 Total number of patients who suffered a possible trial drug related adverse event before end of treatment at 26 weeks.
2.19. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 19 Total number of patients who suffered at least one serious adverse event before end of treatment at 26 weeks.
2.20. Analysis.
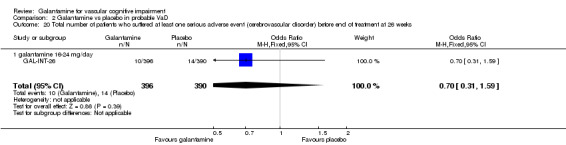
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 20 Total number of patients who suffered at least one serious adverse event (cerebrovascular disorder) before end of treatment at 26 weeks.
2.21. Analysis.
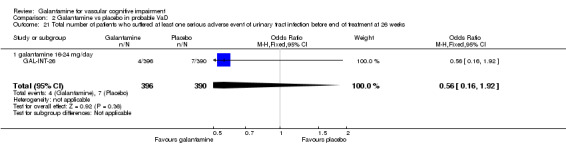
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 21 Total number of patients who suffered at least one serious adverse event of urinary tract infection before end of treatment at 26 weeks.
2.22. Analysis.
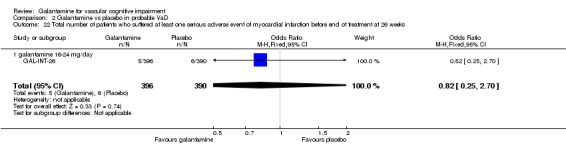
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 22 Total number of patients who suffered at least one serious adverse event of myocardial infarction before end of treatment at 26 weeks.
2.23. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 23 Total number of patients who suffered at least one serious adverse event of pnemonia before end of treatment at 26 weeks.
2.24. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 24 Total number of withdrawals before end of treatment at 26 weeks.
2.25. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 25 Total number of deaths at 26 weeks + 30 days after treatment ended.
2.26. Analysis.
Comparison 2 Galantamine vs placebo in probable VaD, Outcome 26 Total number of withdrawals due to an adverse event before end of treatment at 26 weeks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
GAL‐INT‐26.
| Methods | Randomised, placebo‐controlled, multicentre, double‐blind trial of 6 months duration. | |
| Participants | 788 participants with probable vascular dementia (AIREN criteria, Clinical confirmation on MRI scan) mean age 72.3 ± 8.9 years, 64% men. | |
| Interventions | Galantamine (8 or 12 mg b.i.d.) versus placebo for 6 months. | |
| Outcomes | ADAS‐Cog/11, ADCS‐ADL, CIBIC‐plus, ADAS‐Cog/13, ADAS‐Cog/10, NPI, EXIT‐25 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred immediately before double‐blind trial medication administered. The participant's number and treatment code were assigned after phoning into IVRS provided by Covance, which is a remote data entry system used to collect and retrieve standard information such as the participant's drug treatment assignment and number. The caller used their own user ID and PIN, and then gave the requested participant details, e.g., participant's initials, date of birth. Based on this information, IVRS assigned a unique participant number and treatment code, which dictated the treatment assignment for that participant. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo tablets were escalated in the same escalation schedule as the galantamine tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the data for the clarified outcome measures in the study method were reported, completers' data and ITT data. |
| Selective reporting (reporting bias) | Low risk | All positive and negative data for both groups were reported. |
| Other bias | Low risk | The trial was overall free from any other biases. |
GAL‐INT‐6.
| Methods | Randomised, placebo‐controlled, multicentre, double‐blind trial. | |
| Participants | 592 patients with probable vascular dementia or Alzheimer's disease combined with CVD (NINDS‐AIREN or NINCDS‐ADRDA criteria plus significant radiological evidence of CVD on CT or MRI). mean age 75.1 ± 7.1 years, 53% men. | |
| Interventions | Galantamine (24 mg/day) versus placebo for 6 months. | |
| Outcomes | ADAS‐Cog/11, CIBIC‐plus, ADAS‐Cog‐13, DAD, NPI. | |
| Notes | Subgroup analysis of VaD plus AD with CVD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation method was applied according to a code generated by the Janssen Research Foundation. |
| Allocation concealment (selection bias) | Low risk | Independent clinician, masked to other components of the study, undertook CIBIC assessment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical appearance placebo and galantamine were used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Observed cases and ITT analyses reported. |
| Selective reporting (reporting bias) | Low risk | Data reported on all outcomes. |
| Other bias | Low risk | |
AD: Alzheimer's disease ADAS: Alzheimers Disease Assessment Scale ADL: activities off daily living ADRDA: Alzheimer's Disease and Related Disorders Association AIREN: Association International pour la Recherche et l' Enseignement en Neurosciences b.i.d.: twice daily CIBIC: Clinician's Interview‐based Impression of Change plus caregiver input CT: computed tomography CVD: cerebrovascular disease DAD: disability assessment for dementia EXIT: Executive Interview ITT: intention‐to‐treat MRI: magnetic resonance imagingVaD: vascular dementia NINCDS: National Institute of Neurological and Communicative Disorders of Stroke NINDS: National Institute of Neurological Disorders of Stroke NPI: Neuropsychiatric Inventory
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Senanarong 2004 | Thai patients had AD with/without CVD in an open‐label study with no placebo group. |
| Small 2003 | Open label extension (GAL‐INT‐6). |
AD: Alzheimer's disease CVD: cerebrovascular disease
Contributions of authors
DC ‐ drafting protocol versions, all correspondence, updating of review in 2008. RM ‐ updating of review in 2008. JB ‐ drafting protocol versions. JB ‐ updating the review in 2013. Consumer editor: U Hla Htay. Contact editor: Lon Schneider.
This review was peer reviewed in November 2005 and February 2009.
Sources of support
Internal sources
No sources of support supplied
External sources
The Health Research Board, Dublin and the R&D Office for Health and Personal Social Services, Belfast, Ireland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
GAL‐INT‐26 {published and unpublished data}
- Anon. GAL‐INT‐26. Johnson and Johnson Pharmceutical Research and Development 26 February 2004.
- Auchus AP, Brashear HR, Salloway S, Korczyn AD, Deyn PP, Gassmann‐Mayer C, GAL‐INT‐26 Study Group. Galantamine treatment of vascular dementia: a randomized trial. Neurology 2007;69(5):448‐58. [DOI] [PubMed] [Google Scholar]
- Gassmann‐Mayer C. Email author. E‐mail correspondence from Dr Cristina Gassmann‐Mayer. Email to: R Malouf. Email recipient 24 October 2008.
GAL‐INT‐6 {published data only}
- Bullock R, Erkinjuntti T, Lilienfeld S, GAL INT 6 Study Group. Management of patients with Alzheimer's disease plus cerebrovascular disease: 12‐month treatment with galantamine. Dementia and Geriatric Cognitive Disorders 2004;17(1‐2):29‐34. [DOI] [PubMed] [Google Scholar]
- Bullock R, Lilienfeld S. Galantamine shows promising results in Alzheimer's disease with cerebrovascular components and probable vascular dementia (preliminary results). Journal of Neuroscience 2001;187(Suppl 1):S59. [Google Scholar]
- Burke W, Lilienfeld S. Galantamine improves behaviour and relieves caregiver distress in Alzheimer's disease (AD), vascular dementia and AD with cerebrovascular disease. Proceedings of the 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20‐25, Stockholm, Sweden 2002:Abstract No 428. 2002.
- Erkinjuntti T. Broad therapeutic benefits in patients with probable vascular dementia or Alzheimer's disease with cerebrovascular disease after treatment with galantamine. European Journal of Neurology 2002;9(5):545. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Gauthier S, Bullock R, Kurz A, Hammond G, Schwalen S, et al. Galantamine treatment in Alzheimer's disease with cerebrovascular disease: Responder analyses from a randomized, controlled trial (GAL‐INT‐6). Journal of Psychopharmacology 2008;22(7):761‐8. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet 2002;359(9314):1283‐90. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Kurz A, Small GW, Bullock R, Lilienfeld S, Damaraju CV. An open‐label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clinical Therapeutics 2003;25(6):1765‐82. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Lilienfed S. Cognitive benefits with 12 months of galantamine treatment in patients with Alzheimer's disease and concomitant cerebrovascular disease (mixed dementia). Proceedings of the 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20‐25, Stockholm, Sweden 2002:Abstract No 446. 2002.
- Erkinjuntti T, Lilienfeld S, Damaraju CV. Long‐term treatment with galantamine is effective in slowing cognitive decline in patients with probable vascular dementia. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, Apr 3‐6, Geneva 2002:181. 2002.
- Kertesz A. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomized trial. Current Neurology & Neuroscience Reports 2002;2(6):503‐4. [DOI] [PubMed] [Google Scholar]
- Kurz A. Non‐cognitive benefits of galantamine (Reminyl(R)) treatment in vascular dementia. Acta Neurologica Scandinavica 2002;106(178 Suppl):19‐24. [DOI] [PubMed] [Google Scholar]
- Kurz A, Lilienfeld S, Damaraju CV. Galantamine is safe and effective for the long‐term treatment of cognitive decline in patients with Alzheimer disease with cerebrovascular components or probable vascular dementia. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, Apr 3‐6, Geneva 2002:206. 2002.
- Kurz AF, Erkinjuntti T, Small GW, Lilienfeld S, Damaraju CR. Long‐term safety and cognitive effects of galantamine in the treatment of probable vascular dementia or Alzheimer's disease with cerebrovascular disease. European Journal of Neurology the official journal of the European Federation of Neurological Societies 2003;10(6):633‐40. [DOI] [PubMed] [Google Scholar]
- Small G, Erkinjuntti T, Kurz A, Lilienfeld S. Galantamine in the treatment of cognitive decline in patients with vascular dementia or Alzheimer's disease with cerebrovascular disease. CNS drugs 2003;17(12):905‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Senanarong 2004 {published data only}
- Senanarong V, Poungvarin N, Phanthumchinda K, Tavichachart N, Chankrachang S, Praditsuwan R, et al. Tolerability study of galantamine in possible Alzheimer's disease with or without cerebrovascular disease; a slow‐titration regimen in Thai patients. 8th Congress of the European Federation of the Neurological Sciences. Paris, France. September 4‐7. 2004.
- Thavichachart N, Phanthumchinda K, Chankrachang S, Praditsuwan R, Nidhinandana S, Senanarong V, et al. Efficacy study of galantamine in possible Alzheimer's disease with or without cerebrovascular disease; a slow‐titration regimen in Thai patients. 8th Congress of the European Federation of the Neurological Sciences. Paris, France. September 4‐7. 2004.
Small 2003 {published data only}
- Small GW, Ekinjuntti T, Kurz A, Lilienfeld S. Galantamine in the treatment of cognitive decline in patients with vascular dementia or Alzheimer's disease with cerebrovascular disease. CNS Drugs 2003;17:905‐14. [DOI] [PubMed] [Google Scholar]
Additional references
Chui 1992
- Chui H, Victoroff J, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 1992;42(3 Part 1):473‐80. [DOI] [PubMed] [Google Scholar]
Cummings 1993
- Cummings JL. Frontal‐subcortical circuits and human behavior. Archives of Neurology 1993;50(8):873‐80. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐14. [DOI] [PubMed] [Google Scholar]
Erkinjuntti 2000
- Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. Journal of Neural Transmission 2000;59(Supplement):23‐30. [DOI] [PubMed] [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer's Disease and Associated Disorders 1997;11 Suppl 2:S33‐9. [PubMed] [Google Scholar]
Gelinas 1999
- Gelinas I, Gauthier L, McIntyre, M. Development of a functional measure for persons with Alzheimer's disease: the Disability Assessment for Dementia. American Journal of Occupational Therapy 1999;53:471‐81. [DOI] [PubMed] [Google Scholar]
Knopman 1994
- Knopman DS, Knapp MJ, Gracon SI, Davis CS. The Clinician Interview Based Impression (CIBI): a clinician's global change rating scale in Alzheimer's disease. Neurology 1994;44:2315‐21. [DOI] [PubMed] [Google Scholar]
Lilienfeld 2002
- Lilienfeld S. Galantamine: a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer's disease. CNS Drug Reviews 2002;8(2):159‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malouf 2004
- Malouf R, Birks J. Donepezil for vascular cognitive impairment. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004395.pub2] [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Sciences Task Force on Alzheimer's disease. Neurology 1984;34:939‐44. [DOI] [PubMed] [Google Scholar]
MRC CFAS 2001
- MRC CFAS, Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Pathological correlates of late‐onset dementia in a multicentre, community‐based population in England and Wales. The Lancet 2001;357:169‐75. [DOI] [PubMed] [Google Scholar]
O'Brien 2000
- O'Brien J, Perry R, Barber R, Gholkar A, Thomas A. The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Annals of the New York Academy of Sciences 2000;903:482‐9. [DOI] [PubMed] [Google Scholar]
O'Brien 2003
- O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. The Lancet Neurology 2003;2:89‐98. [DOI] [PubMed] [Google Scholar]
Perry 1997
- Perry E, Kay DW. Some developments in brain ageing and dementia. British Journal of Biomedical Science 1997;54(3):201‐15. [PubMed] [Google Scholar]
Perry 2005
- Perry E, Ziabreva I, Perry R, Aarsland D, Ballard C. Absence of cholinergic deficits in "pure" vascular dementia. Neurology 2005;64:132‐3. [DOI] [PubMed] [Google Scholar]
Pohjasvaara 2003
- Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Clinical features of MRI‐defined subcortical vascular disease. Alzheimer's Disease and Associated Disorders 2003;17(4):236‐42. [DOI] [PubMed] [Google Scholar]
Roman 1993
- Roman G, Tatemichi T, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. [DOI] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141(11):1356‐64. [DOI] [PubMed] [Google Scholar]
Royall 1992
- Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. Journal of the American Geriatrics Society 1992;40(12):1221‐6. [DOI] [PubMed] [Google Scholar]
Toghi 1996
- Toghi H, Abe T, Kimura M, Saheki M, Takahashi S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarcts types as compared with Alzheimer‐type dementia. Journal of Neural Transmission 1996;103:1211‐20. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International classification of diseases, 10th revision. Geneva: World Health Organization, 1992. [Google Scholar]
References to other published versions of this review
Craig 2006
- Craig D, Birks J. Galantamine for vascular cognitive impairment. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004746.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


