Abstract
Background:
Individuals with alcohol use disorder (AUD) often display compromise in emotional processing and non-affective neurocognitive functions. However, relatively little empirical work explores their intersection. In this study, we examined working memory performance when attending to and ignoring facial stimuli among adults with and without AUD. We anticipated poorer performance in the AUD group, particularly when task demands involved ignoring facial stimuli. Whether this relationship was moderated by facial emotion or participant sex were explored as empirical questions.
Methods:
Fifty-six controls (30 women) and 56 treatment-seekers with AUD (14 women) completed task conditions in which performance was advantaged by either attending to or ignoring facial stimuli, including happy, neutral, or fearful faces. Group, sex, and their interaction were independent factors in all models. Efficiency (accuracy/response time) was the primary outcome of interest.
Results:
An interaction between group and condition (F1,107 = 6.03, p < .02) was detected. Individual comparisons suggested this interaction was driven by AUD-associated performance deficits when ignoring faces, whereas performance was equivalent between groups when faces were attended. Secondary analyses suggested little influence of specific facial emotions on these effects.
Conclusions:
These data provide partial support for initial hypotheses, with the AUD group demonstrating poorer working memory performance conditioned on the inability to ignore irrelevant emotional face stimuli. The absence of group differences when scenes were to be ignored (faces remembered) suggests the AUD-associated inability to ignore irrelevance is influenced by specific stimulus qualities.
Keywords: Alcohol use disorder, Cognition, Emotion processing, Working memory, Social cognition
1. Introduction
It is widely recognized that alcohol use disorder (AUD) is associated with compromise in emotion processing (e.g., Oscar-Berman, Hancock, Mildworf, Hutner, & Weber, 1990; Monnot, Lovallo, Nixon, & Ross, 2002; Maurage et al., 2009; Le Berre, 2019) as well as other neurocognitive processes (e.g., Bates, Bowden, & Barry, 2002; Le Berre, Fama, & Sullivan, 2017; Nixon & Lewis, 2019). Most current investigations address the two domains separately. However, there is a robust literature demonstrating that emotion processing influences other aspects of neurocognition and that its role is, at least partially, determined by task demands (Driver, 2001; Vuilleumier, 2005; LaBar & Cabeza, 2006; Pool, Brosch, Delplanque, & Sander, 2016). For example, when emotionally salient stimuli are to be attended to, performance is enhanced (Driver, 2001; Vuilleumier, Armony, Driver, & Dolan, 2001; Pool et al., 2016). In contrast, when they are to be ignored (i.e., they are irrelevant), these same stimuli impede the ability to suppress attention to irrelevancy and may compromise performance (Vuilleumier et al., 2001; Compton, 2003; Keefe, Sy, Tong, & Zald, 2019). The capacity to enhance attention to relevance and suppress attention to irrelevancy is a key component of cognitive control, which in turn directs attentional allocation and modulates the influence of interfering information (Abrahamse, Braem, Notebaert, & Verguts, 2016). Importantly, AUD is often associated with compromise on neurocognitive measures reliant on cognitive control, including working memory and other tasks requiring effective inhibitory processing (e.g., Nixon & Lewis, 2019; Wilcox, Dekonenko, Mayer, Bogenschutz, & Turner, 2014).
Together, these literatures suggest that the interface of disruptions in emotion processes with that of other functions may contribute to the complexity of alcohol-associated deficits. We and others are beginning to address this question using neurobehavioral and psychosocial outcomes. (e.g., Hoffman, Lewis, & Nixon, 2019; Lewis, Price, Garcia, & Nixon, 2019; Maurage et al., 2011; Marinkovic et al., 2009; Oscar-Berman et al., 2014; Schulte, Muller-Oehring, Pfefferbaum, & Sullivan, 2010). That said, many current reports lack an overarching conceptual framework. Here, we explicitly manipulated stimulus valence (neutral vs. emotional) and task instruction (remember/ignore) to interrogate alcohol-related deficits in inhibitory control and emotion processing. We anticipated that AUD would be associated with poorer performance, regardless of instructional set. However, recognizing noted deficits in inhibitory processing, we expected greater AUD-related deficits in the condition wherein emotional stimuli were to be ignored vs. when they were to be attended (i.e., when attention to the stimuli must be suppressed to benefit performance). A follow-up analysis was conducted to interrogate emotion-specific effects when faces were to be ignored. This analysis served two purposes, aiding interpretation of the primary analysis by contrasting neutral vs. emotional faces, as well as comparing between emotionally-valent conditions (i.e., Fearful vs. Happy). Due to a mixed literature (e.g., Hoffman et al., 2019; Townshend & Duka, 2003) this latter comparison was posed without a directional hypothesis. Finally, given continuing interest in sex-contingent substance use consequences and the dearth of data regarding sex in AUD-associated emotion processing outcomes (but see Sawyer et al., 2019; Lewis, Price, et al., 2019) it was included as a factor in both models.
2. Materials & methods
2.1. Participants
Participants (N = 112) included individuals with AUD recruited from treatment facilities across North Central Florida (n = 56; 14 women) and community controls (CCs; n = 56; 30 women). Participants provided written informed consent prior to data collection and were compensated for participating. All procedures were approved by the University of Florida Medical IRB.
Participants provided basic demographic information, self-reported medical histories, and completed questionnaires indexing negative affect (Beck Depressive Inventory [BDI; Beck, Steer, & Brown, 1996]; Anxiety Inventory [AI; Spielberger, 1983]). Alcohol use histories were collected, including chronicity of problem use and drinking patterns over the six months prior to treatment (i.e., quantity/frequency index [QFI; Cahalan, Cissin, & Crossley, 1969]; maximal quantity in a single day[MaxQ]).
Eligible participants were aged 25–59 years with 10–16 years of education and no significant neurologic disorder/insult, medical conditions, or medication use that would compromise neurobehavioral function (e.g. history of stroke, untreated hypertension, benzodiazepines). Participants in both groups were excluded if they met probabilistic diagnostic criteria for lifetime psychotic or bipolar disorders or current major depression, panic, or post-traumatic stress disorders. Among CCs, histories of substance use disorders (excepting nicotine) were exclusionary, as were recent drinking histories (i.e., >2/3 drink daily average for women/men, respectively) suggesting use patterns that significantly exceeded“low-risk” guidelines (≤1/2 drink daily average; Department of Health and Human Services, 2015). All individuals in the AUD group were at least 21 days abstinent and met DSM-5 criteria for moderate-to-severe AUD.
On the day of testing, negative indications for pregnancy and recent substance use were required for participation. Substance use indices included breathalyzer (Intoxylizer® 400PA; CMI, Inc., Owensboro, KY) and urinalysis (10 panel test: THC, amphetamine, methamphetamine, cocaine, opiates, methadone, PCP, benzodiazepines, barbiturates, MDMA). Current nicotine users were administered a 7 mg nicotine patch before testing to avoid withdrawal-associated cognitive effects. This dose is well-tolerated, with previous work suggesting no differential impact on performance between CC and AUD participants (Nixon, Lawton-Craddock, Tivis, & Ceballos, 2007).
2.2. Emotional attend/ignore working memory task
2.2.1. Task description
The current work used an attend/ignore working memory task modified from Gazzaley, Cooney, McEvoy, Knight, and D’Esposito (2005). We (e.g., Boissoneault, Sklar, Prather, & Nixon, 2014; Lewis, Garcia, Boissoneault, Price, & Nixon, 2019) and others (e.g., Padgaonkar, Zanto, Bollinger, & Gazzaley, 2017) have employed this task in previous work; it was adapted for the current study to incorporate emotional stimuli (see Ziaei, Samrani, & Persson, 2018 for example of similar approach). The task includes presentation of two “face” stimuli and two “scene” stimuli per trial. Presentation of the four target stimuli is followed by a brief delay period, after which a probe stimulus is presented. Participants responded to indicate whether the probe stimulus matched any of the target stimuli. Participants were instructed to either 1) Remember faces but ignore scenes (hereafter, “remember faces”); 2) Remember scenes but ignore faces (hereafter “ignore faces”); or 3) Passively view stimuli. Probe images were consistent with instructions (i.e., all face images for “remember faces” condition). For the passive viewing condition, probe images were replaced with left/right arrows and respondents indicated the direction of the arrow. The passive viewing condition was presented after remember face/scene conditions, which were counterbalanced across participants. Performance in the passive condition was utilized as a covariate in all analyses. In all conditions, participants were instructed to respond “as quickly and accurately as possible”. There were 72 trials in each of the 3 conditions. An exemplar trial is depicted in Fig. 1.
Fig. 1.

Depicts an exemplar trial from the attend/ignore working memory task in which faces are task-irrelevant. Sex and emotion of face stimuli (here female, surprised faces) were kept consistent within trials.
2.2.2. Stimuli presentation/timing
The six possible sequences of target stimuli presentation (e.g., face/scene/face/scene) were pseudorandomized, with equal distribution across trials. Each target stimuli was presented for 800 ms (200 ms ISI). A 9000 ms delay period (fixation cross only) followed presentation of the last stimulus. Probe images were presented for 1500 ms. Probe stimuli matched one of the target stimuli 50% of the time. Responses were made using a two-button response pad (4000 ms response period).
2.2.3. Task stimuli
Task stimuli were grayscale images presented in the center of a 17-inch LCD monitor against a black background (E-Prime software). Scene images were retained from the original task (Gazzaley et al., 2005). Face images were selected from the FACES database (Ebner, Riediger, & Lindenberger, 2010), a validated set of adult male and female face images. Task stimuli included either happy, neutral, or fearful faces (24 trials of each). The sex and emotion of faces presented within a single trial were held constant (e.g., all images of fearful female faces). As depicted in Fig. 1, within all trials unique face models were used for each target image.
2.3. Data analysis
Statistical analyses were performed using SAS Version 9.4. Descriptive variables were subjected to t-test and/or chi square analyses, as appropriate, to ascertain differences by group and sex. Hypothesized relationships were investigated using general linear mixed models (GLMM), with performance efficiency (a ratio of percent accurate/reaction time for correct responses) as the primary outcome of interest. GLMMs were selected for their flexibility in comparing uneven sample sizes. Preliminary analyses revealed below chance (<50%) performance among two individuals (one CC; one AUD), who were excluded from the current sample.
An initial model incorporating condition effects included group, sex, and their interaction as fixed factors and condition as a repeated factor. Subsequent analyses directed to suppression of attention when face emotion was irrelevant utilized only performance in the “ignore faces” condition, and included face emotion as the repeated factor. LSMean differences were examined to aid interpretation of significant interactions. To facilitate interpretation of results, analyses of efficiency subcomponents (performance accuracy and reaction time) were conducted; given their post hoc nature, these analyses were Bonferroni corrected for familywise error rate (α ≤ 0.0125). Effect size estimates are provided as standardized regression coefficients (std.b) or Cohen’s d, as appropriate.
3. Results
3.1. Demographics, mood, and alcohol use
Participants identified as Caucasian (72.3%; n = 81), Black/African American (20.5%; n = 23), Asian American (1.8%; n = 2) or Other (5.4%; n = 6). Five participants (4.5%) endorsed Hispanic ethnicity.
Other descriptive statistics are presented in Table 1. CCs reported higher levels of education (t1,108 = 4.50, p < .001) and endorsed fewer symptoms of depression (t1,106 = 3.71, p < .001) and anxiety (t1,109 = 2.42, p = .02). Neither measure suggested significant negative affect, nor were they related to task performance for either group (rs ≤ 0.08, ps ≥ 0.52), thus they were not analyzed further. No sex-contingent differences were noted for any of the descriptive variables considered (ts ≤ 1.58 ps ≥ 0.11).
Table 1.
Demographic, Affective, and Substance Use Measures.
| CC (n = 56) M (SD) or % |
AUD (n = 56) M(SD) or % |
|
|---|---|---|
| % Women | 53.57 | 25.00 |
| Age (years) | 44.12 (12.26) | 41.37 (8.61) |
| Years of Education | 14.71 (1.53) | 13.28 (1.81) |
| Depressive Symptomatology (BDI-II) | 3.52 (4.43) | 7.89 (7.244) |
| Anxiety Symptomatology (AI) | 26.18 (7.04) | 30.18 (10.09) |
| % Current Nicotine User | 8.93 | 69.09 |
| Average Standard Drinks/Day | 0.45 (0.62) | 25.57 (18.05) |
| Maximum Standard Drinks (Single Day) | 3.68 (2.39) | 42.36 (40.00) |
| Chronicity of Alcohol Problems (years) | – | 18.05 (8.96) |
3.2. Working memory performance: condition X group X sex
As expected, efficiency was greater when faces were attended vs. ignored (F1,107 = 19.71, p < .001, std.b = 0.68), however, this relationship was qualified by an interaction with group (F1,107 = 6.03, p < .02, std.b = 0.43). LSMean contrasts indicated this interaction was consistent with hypotheses of AUD-associated deficits in suppression of attention to faces; individuals with AUD performed worse on “ignore faces” than “remember faces” (t108 = 4.56, p < .001, d = 0.50), whereas no such difference was observed for CCs (t108 = 1.52, p = .13, d = 0.14). Further, individuals with AUD displayed lower efficiency, relative to controls, during “ignore faces” trials (t108 = 2.18, p = .03, d = 0.22), whereas no difference was observed for “remember faces” (t108 = 1.29, p = .20, d = 0.13). No main effect of sex or interaction with group was observed. Efficiency by group and condition is depicted in Fig. 2.
Fig. 2.
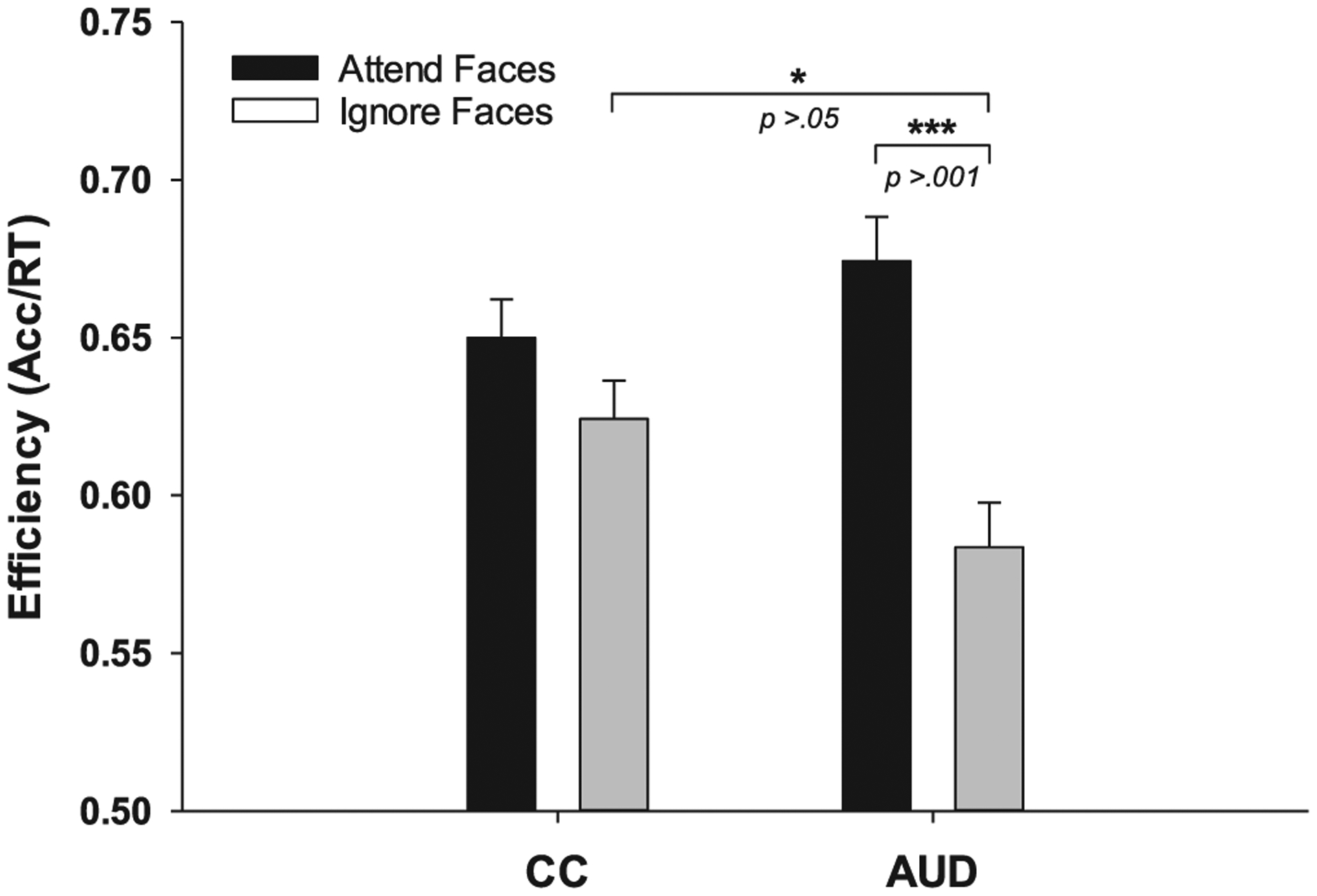
Depicts performance efficiency (LS Means ± SE) by group and task condition, for which a significant interaction was identified (F1,107 = 19.71, p < .001).
3.3. Working memory performance: group X sex X emotion in “Ignore Faces” condition
Analysis of the “ignore faces” condition confirmed an AUD-associated deficit in performance efficiency (F1,107 = 5.19, p = .02, std.b = 0.44), consistent with our hypotheses. However, no group by face emotion interaction was observed. No main effect of sex or interaction with group was detected. The directionality of emotion-specific performance across groups, while not reaching statistical significance (F2,215 = 2.31, p = .10, std.b = 0.32), appeared elevated for happy faces (M = 0.631) relative to either neutral (M = 0.589) or fearful (M = 0.595) faces.
3.4. Post hoc decomposition of efficiency
To further characterize performance efficiency under conditions in which faces were irrelevant, models were reiterated using reaction time and accuracy as separate DVs. Reaction time analyses revealed no differences by group or sex (Fs ≤ 1.65, ps ≥ 0.20). Accuracy analyses suggested a group difference (F1,107 = 7.55, p < .01, std.b = 0.59) consistent with that observed for efficiency. In contrast with efficiency analyses, a sex effect (F1,107 = 11.55, p < .01, std.b = 0.66) was observed. The group by sex interaction (F1,107 = 5.87, p = .02, std.b = 0.55) failed to reach significance after Bonferroni correction. Performance efficiency, accuracy, and reaction time are depicted by group and sex in Fig. 3.
Fig. 3.
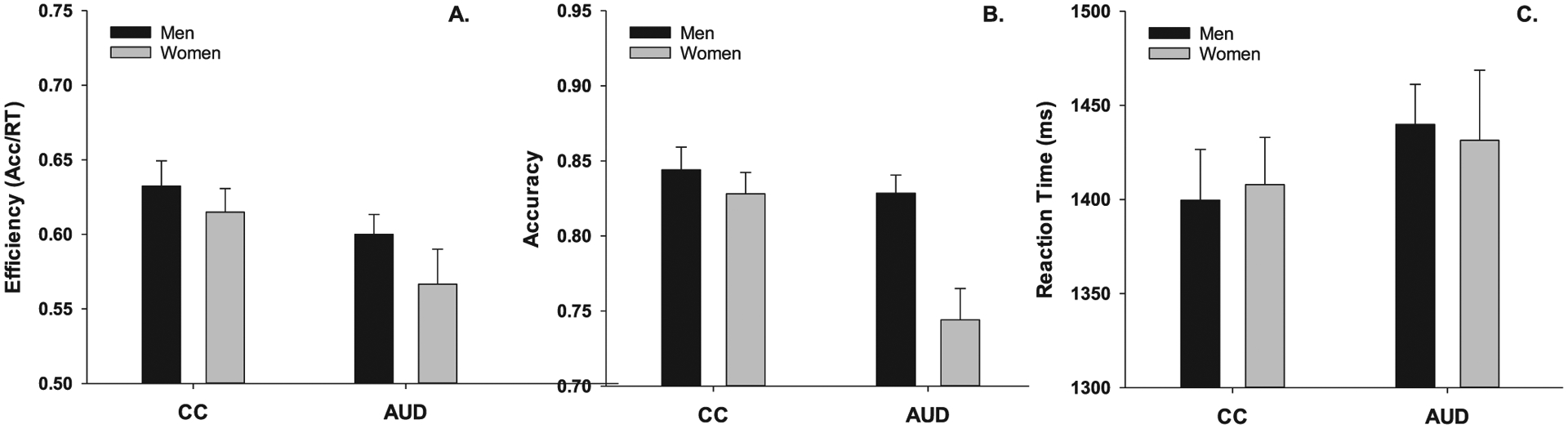
Depicts task performance (LS Means ± SE) by group and sex. Fig. 3a depicts performance efficiency (group effect only; p < .01). Fig. 3b depicts performance accuracy, for which significant group (p < .01) and sex (p < .01) effects were observed, although their interaction (p = .02) failed to reach significance after Bonferroni correction. Fig. 3c depicts reaction time (no significant effects).
4. Discussion
AUD-associated perturbations in both cognitive control and the processing of interpersonal affective cues are recognized by substantive literatures, however their intersection remains relatively unexamined. The conceptual framework for this experiment relied on previous findings suggesting individuals with AUD ascribe greater emotional intensity to facial stimuli (e.g., Kornreich et al., 2001) and display difficulties with top-down control of attention (e.g., Wilcox et al., 2014). Given these findings, and the intrinsic nature of facial stimuli to capture attention, we hypothesized that inhibiting attention to emotional facial stimuli would be particularly challenging. We examined this hypothesis using a behavioral task in which inappropriate attention to irrelevant stimuli commonly results in performance deficits. Analyses focused on contrasts between task conditions in which faces were relevant vs. irrelevant, and within the latter condition, contrasts between emotionality of the facial stimuli.
Results suggested equivalent working memory performance between CC and AUD groups when attention was directed to faces. In contrast, when faces were task-irrelevant (i.e., to be ignored) performance in the AUD group was disproportionally affected, consistent with hypotheses of AUD-associated deficits in the capacity to appropriately ignore emotionally-valent stimuli. Disrupted inhibitory control processes are well appreciated as both risk factors for, and neurobehavioral sequela of AUD. The current data contribute to the growing appreciation for how such disruption can impact social cognition, potentially contributing to interpersonal problems across AUD development and recovery.
Results revealed only a trend toward emotion-specific impacts on performance, with no group-contingent interaction observed. An effect of stimulus valence was anticipated based on studies reporting AUD-associated deficits in emotion identification (see Donadon & Osorio, 2014 for review). While the lack of similar investigations utilizing attend/ignore paradigms highlights the novelty of the current work, it limits opportunities for comparison/contrast when considering alternative interpretations of results. One possibility for the absence of emotion-specific effects is that emotional stimuli were not sufficiently distinct. However, validation studies with the stimulus set (Ebner et al., 2010) indicate this explanation is unlikely.
A second possibility is that these results reflect AUD-associated differences in general face processing. Effectively ignoring irrelevancy requires sufficient processing of the stimulus to identify its irrevelance (e.g., recognition that a target stimulus is a face in “ignore faces” trials). Thus, the observed results may be driven by less efficient face processing during this early stimulus discrimination phase, potentially disrupting working memory maintenance processes. However, weakening this argument are numerous studies finding little support for AUD-associated deficits in generalized face-processing (i.e., discriminating sex of neutral faces; Maurage, Campanella, Philippot, Martin, & de Timary, 2008; Lewis, Price, et al., 2019).
A third possibility is that, at least in some contexts, neutral faces communicate sufficient emotion as to serve the same function as more robustly defined faces. More specifically, it may be that individuals with AUD are more susceptible to failures in inhibitory functions when confronted with high attention capture stimuli conferring emotional information such as faces (e.g., Theeuwes & Van der Stigchel, 2006; Ro, Russell, & Lavie, 2001), regardless of specific emotion or intensity. We should note that in the current context, the utility of the neutral faces lies primarily in providing a comparator for evaluating emotion-specific effects. In validating the stimuli set employed in this study (Ebner et al., 2010), neutral expressions were commonly judged as non-neutral (~14% misattribution frequency). Moreover, neutral expressions consistently receive non-zero ratings with regard to emotional intensity (e.g., mean of ~3.5 for neutral vs. ~5.0 for happy or angry stimuli; Garrido & Prada, 2017). These findings argue strongly against interpreting neutral expressions as an absence of emotion. Thus, the lack of a group by emotion interaction is not sufficient support for the fourth possibility: that AUD-associated deficits in attentional control are insensitive to emotional face content. While further work is necessary to disambiguate these issues, we speculate that inappropriate attention to emotional faces may extend to faces with ambiguous emotional content (i.e., neutral faces).
Given evidence for susceptibility to emotion processing deficits among women with AUD (Lewis, Price, et al., 2019) and ongoing interest in sex differences among treatment seekers with AUD, potential interactions with sex were considered. While women displayed reduced task accuracy when ignorning faces, sex was not a significant moderator of AUD effects.
4.1. Limitations
Several limitations bear consideration. Facial stimuli were limited to Caucasian individuals. Well-validated sets with greater diversity among face models have become available [e.g. RADIATE (Conley et al., 2018), Chicago Face Database (Ma, Correll, & Wittenbrink, 2015)] and may enhance the generalizability of future work. While the cross-sectional nature of the study facilitates interrogation of emotion processing in recently detoxified individuals, it limits generalization to pre- or post-treatment functioning. Similarly, the design precludes characterization of disrupted social cognitive processes as either cause or consequence of AUD. This distinction requires longitudinal investigation, which would also serve to improve generalizability and clarify the role of emotion processing in sustained recovery. Although we included analyses of sex effects, the relatively small sample of treatment-seeking women constrained statistical power. That a trend-level interaction with sex was nonetheless detected for accuracy suggests the import of its consideration in future analyses. The current report focused on behavioral outcomes. Additional studies using neurophysiological and/or neuroimaging methods will expand understanding of neural mechanisms underlying these effects.
5. Summary
The current study identifies AUD-associated deficits in the capacity to appropriately ignore emotionally-valent stimuli. Implications of these results are potentially far-reaching. As noted above, emotion processing deficits are thought to contribute to interpersonal difficulties and appear to predict early cessation of treatment. Difficulty classifying emotions and judging their intensity are recognized as core components of these deficits; the current work suggests inappropriate attention to emotionality may play a contributory (and potentially compounding) role. Further, our results suggest a novel avenue by which to enhance emotion processing. In contrast to the challenges associated with improving accuracy in emotion identification, protocols for retraining attentional biases among individuals with AUDs are established and appear efficacious (e.g., Wiers et al., 2015). Should future extension/replication of the current results support their utility, this method could be modified to remediate inappropriate attention to emotion. Taken together, this work highlights the complexity and breadth of AUD-associated disruptions in social cognition, meaningfully extends this literature beyond examinations of emotion identification or intensity ratings, and contributes to studies exploring the intersection of cognitive and emotional functions.
Acknowledgements
The authors thank Robert Prather, Lauren Hoffman, Ian Frazier for their contributions to data collection and/or task design. The authors thank the participating treatment facilities and all study volunteers.
7. Statement 1: Role of funding sources
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Grant Number R01 AA022456 (Sara Jo Nixon, PI). Further support was provided by K01 AA026893 (Ben Lewis, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author agreement
All authors have seen and approved the final version of the submitted manuscript. The manuscript is the authors’ original work, hasn’t received prior publication, and isn’t under consideration for publication elsewhere.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abrahamse E, Braem S, Notebaert W, & Verguts T (2016). Grounding cognitive control in associative learning. Psychological Bulletin, 142(7), 693–728. 10.1037/bul0000047. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, & Barry D (2002). Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Experimental and Clinical Psychopharmacology, 10(3), 193–212. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory (2nd Ed.). San Antonio: The Psychological Corporation. [Google Scholar]
- Boissoneault J, Sklar A, Prather R, & Nixon SJ (2014). Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. Journal of Studies on Alcohol and Drugs, 75(5), 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cissin L, & Crossley H (1969). American Drinking Practices: A National Study of Drinking Behaviors and Attitudes (Monograph No. 6). New Brunswick, NJ: Rutgers Center of Alcohol Studies. [Google Scholar]
- Compton RJ (2003). The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behavioral and Cognitive Neuroscience Reviews, 2(2), 115–129. 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Conley MI, Dellarco DV, Rubien-Thomas E, Cohen AO, Cervera A, Tottenham N, & Casey BJ (2018). The racially diverse affective expression (RADIATE) face stimulus set. Psychiatry Research, 270, 1059–1067. 10.1016/j.psychres.2018.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health & Human Services (2015). Dietary Guidelines for Americans. Office of Disease Prevention & Health Promotion. https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/ (Accessed 12 November 2020). [Google Scholar]
- Donadon MF, & Osorio FDL (2014). Recognition of facial expressions by alcoholic patients: A systematic literature review. Neuropsychiatric Disease and Treatment, 10, 1655–1663. 10.2147/NDT.S65376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J (2001). A selective review of selective attention research from the past century. British Journal of Psychology, 92(Part 1), 53–78. [PubMed] [Google Scholar]
- Ebner NC, Riediger M, & Lindenberger U (2010). FACES—A database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods, 42(1), 351–362. 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Garrido MV, & Prada M (2017). KDEF-PT: Valence, emotional intensity, familiarity and attractiveness ratings of angry, neutral, and happy faces. Frontiers in Psychology, 8, 2181. 10.3389/fpsyg.2017.02181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, & D’Esposito M (2005). Top-down enhancement and suppression of the magnitude and speed of neural activity. Journal of Cognitive Neuroscience, 17(3), 507–517. [DOI] [PubMed] [Google Scholar]
- Hoffman LA, Lewis B, & Nixon SJ (2019). Neurophysiological and interpersonal correlates of emotional face processing in alcohol use disorder. Alcoholism, Clinical and Experimental Research, 43(9), 1928–1936. 10.1111/acer.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe JM, Sy JL, Tong F, & Zald DH (2019). The emotional attentional blink is robust to divided attention. Atten Percept Psychophys, 81(1), 205–216. 10.3758/s13414-018-1601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Nöel X, Streel E, … Verbanck. (2001). Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. Journal of Studies on Alcohol, 62(4), 533–542. [DOI] [PubMed] [Google Scholar]
- LaBar KS, & Cabeza R (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7(1), 54–64. 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Le Berre AP (2019). Emotional processing and social cognition in alcohol use disorder. Neuropsychology, 33(6), 808–821. 10.1037/neu0000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A-P, Fama R, & Sullivan EV (2017). Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcoholism, Clinical and Experimental Research, 41(8), 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Garcia CC, Boissoneault J, Price JL, & Nixon SJ (2019). Working memory performance following acute alcohol: replication and extension of dose by age interactions. Journal of Studies on Alcohol and Drugs, 80(1), 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Price JL, Garcia CC, & Nixon SJ (2019). Emotional face processing among treatment-seeking individuals with alcohol use disorders: Investigating sex differences and relationships with interpersonal functioning. Alcohol and Alcoholism. 10.1093/alcalc/agz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DS, Correll J, & Wittenbrink B (2015). The Chicago face database: A free stimulus set of faces and norming data. Behavior Research, 47(4), 1122–1135. 10.3758/s13428-014-0532-5. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, & Harris GJ (2009). Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism, Clinical and Experimental Research, 33(11), 1880–1892. 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Charest I, Martin S, & de Timary P (2009). Impaired emotional facial expression decoding in alcoholism is also present for emotional prosody and body postures. Alcohol and Alcoholism, 44(5), 476–485. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Martin S, & de Timary P (2008). Face processing in chronic alcoholism: A specific deficit for emotional features. Alcoholism Clin Exp Res, 32(4), 600–606. [DOI] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noel X, Joassin F, Philippot P, Hanak C, … Campanella S (2011). Dissociation between affective and cognitive empathy in alcoholism: a specific deficit for the emotional dimension. Alcoholism, Clinical and Experimental Research, 35(9), 1662–1668. [DOI] [PubMed] [Google Scholar]
- Monnot M, Lovallo WR, Nixon SJ, & Ross E (2002). Neurological basis of deficits in affective prosody comprehension among alcoholics and fetal alcohol–exposed adults. Journal of Neuropsychiatry and Clinical Neurosciences, 14(3), 321–328. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Lawton-Craddock A, Tivis R, & Ceballos N (2007). Nicotine’s effects on attentional efficiency in alcoholics. Alcoholism Clin Exp Res, 31(12), 2083–2091. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, & Lewis B (2019). Cognitive training as a component of treatment of alcohol use disorder: A review. Neuropsychology, 33(6), 822–841. 10.1037/neu0000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, & Gravitz ZR (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handbook of Clinical Neurology (Vol. 125,, 183–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, Hutner N, & Weber DA (1990). Emotional perception and memory in alcoholism and aging. Alcoholism Clin Exp Res, 14(3), 383–393. [DOI] [PubMed] [Google Scholar]
- Padgaonkar NA, Zanto TP, Bollinger J, & Gazzaley A (2017). Predictive cues and age-related declines in working memory performance. Neurobiology of Aging, 49, 31–39. 10.1016/j.neurobiolaging.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool E, Brosch T, Delplanque S, & Sander D (2016). Attentional bias for positive emotional stimuli: A meta-analytic investigation. Psychological Bulletin, 142(1), 79–106. 10.1037/bul0000026. [DOI] [PubMed] [Google Scholar]
- Ro T, Russell C, & Lavie N (2001). Changing faces: A detection advantage in the flicker paradigm. Psychological Science, 12(1), 94–99. [DOI] [PubMed] [Google Scholar]
- Sawyer KS, Maleki N, Urban T, Marinkovic K, Karson S, Ruiz SM, … Oscar-Berman M (2019). Alcoholism gender differences in brain responsivity to emotional stimuli. Elife, 8. 10.7554/eLife.41723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, & Sullivan EV (2010). Neurocircuitry of emotion and cognition in alcoholism: Contributions from white matter fiber tractography. Dialogues in Clinical Neuroscience, 12(4), 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Theeuwes J, & Van der Stigchel S (2006). Faces capture attention: Evidence from inhibition of return. Visual Cognition, 13(6), 657–665. [Google Scholar]
- Townshend JM, & Duka T (2003). Mixed emotions: Alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia, 41(7), 773–782. 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585–594. 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, & Dolan RJ (2001). Effects of attention and emotion on face processing in the human Brain. Neuron, 30(3), 829–841. 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Houben K, Fadardi JS, van Beek P, Rhemtulla M, & Cox MS (2015). Alcohol cognitive bias modification training for problem drinkers over the web. Addictive Behaviors, (40), 21–26. 10.1016/j.addbeh.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, & Turner JA (2014). Cognitive control in alcohol use disorder: Deficits and clinical relevance. Reviews in the Neurosciences, 25(1), 1–24. 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei M, Samrani G, & Persson J (2018). Age differences in the neural response to emotional distraction during working memory encoding. Cognitive, Affective & Behavioral Neuroscience, 18(5), 869–883. 10.3758/s13415-018-0610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


