Abstract
Objective
Metabolic syndrome (MetS) is a cluster of cardiovascular risk factors associated with cognitive decline. We investigated the relationship between MetS and cognition in middle-aged adults. We hypothesized that higher numbers of MetS components will relate to poorer performance on executive function (EF) tasks as frontal lobe regions critical to EF are particularly vulnerable to cardiovascular disease.
Methods
197 adults (ages 40–60) participated. MetS was evaluated using established criteria. Composite scores for cognitive domains were computed as follows: Global cognitive function (subtests from the Wechsler Abbreviated Scale of Intelligence, 2nd Edition), EF (Stroop Color Word, Digit Span Backward, and Trails A and B), and memory (California Verbal Learning Test, 2 Edition).
Results
Higher number of MetS components was related to weaker EF—F(4, 191) = 3.94, p = .004, MetS components ß = −.14, p = .044. A similar relationship was detected for tests of memory—F(4, 192) = 7.86, p < .001, MetS components ß = −.15, p = .032. Diagnosis of MetS was not significantly associated with EF domain score (ß = −.05, p = .506) but was significantly associated with memory scores—F(4, 189) = 8.81, p < .001, MetS diagnosis ß = −.19, p = .006.
Conclusions
Our findings support prior research linking MetS components at midlife to executive dysfunction and demonstrate that MetS, and its components are also associated with poorer memory function. This suggests that cognitive vulnerability can be detected at midlife. Interventions for MetS at midlife could alter cognitive outcomes.
Keywords: Neuropsychological assessment, Brain, Aging, Cognitive impairment, Metabolic syndrome, Executive function
Introduction
Midlife metabolic dysfunction and cardiovascular disease have been proposed as mechanisms of degeneration of brain structure and function in middle age (Launer, 2005). Longitudinal studies examining the role of obesity on brain structure have documented late-life atrophy in older adults who were obese at midlife (Driscoll et al., 2012; Raji et al., 2010) and increased white matter lesions (WMLs) in older age for individuals with higher body mass indices (BMI) almost two decades earlier (Gustafson, Steen, & Skoog, 2004). Cross-sectional studies have reported associations in brain structure and function and in obese adults beginning at midlife quantified by neuroimaging derived brain age (Ronan et al., 2016). Midlife hypertension and diabetes have also been identified as risk factors for later life dementia (Kloppenborg, van den Berg, Kappelle, & Biessels, 2008; Whitmer, Sidney, Selby, Claiborne Johnston, & Yaffe, 2005).
Examining modifiable cardiovascular risk factors at early stages can be advantageous for developing interventions to support overall physical well-being and brain health (Barnes & Yaffe, 2011; Kivipelto, Mangialasche, & Ngandu, 2018; O’Donnell et al., 2015; Plassman, Williams, Burke, Holsinger, & Benjamin, 2010). One sensitive measure for capturing early cardiovascular disease is to use preclinical hypertension, dyslipidemia and hyperglycemia in the form of a metabolic syndrome risk score. Metabolic syndrome (MetS) is a preclinical diagnosis that identifies a cluster of risk factors for cardiovascular disease. The five key components of MetS include abdominal obesity, high triglyceride levels, low high-density lipoprotein (HDL) cholesterol, above normal blood pressure (prehypertension) and above normal blood sugar (prediabetes) (Eckel, Grundy, & Zimmet, 2005). Though many of the individual components of MetS have documented negative cognitive consequences (Arvanitakis, Bennett, Wilson, & Barnes, 2010; Kivipelto et al., 2005; Skoog et al., 1996; Whitmer et al., 2005; Yaffe et al., 2004), MetS is more than the sum of its components. MetS, defined by the co-occurrence of at least three of the five components within a single individual, has been associated with increased risk for vascular dementia (VaD) (Solfrizzi et al., 2010) and poorer current executive function (Segura et al., 2009). Additional research on cardiovascular risk and cognitive function suggests that risk factors operate in a dose-dependent manner, with likelihood of later life dementia increasing with additional diagnoses (Whitmer et al., 2005).
Frontal lobe pathology and executive dysfunction are thought to be the earliest markers for brain changes resulting from cardiovascular disease. While initial interest in cognitive impairment had focused on the temporal lobe and memory functions as the primary area of attention (Kaye et al., 1997; Visser, Verhey, Hofman, Scheltens, & Jolles, 2002), additional literature suggested the frontal lobe regions as the most susceptible early cognitive changes (MacPherson, Phillips, & Della Sala, 2002). The frontal lobe is particularly vulnerable to vascular mechanisms of cognitive decline, possibly due to higher concentrations of myelin in these regions (Bartzokis, 2004; Raz, Rodrigue, & Acker, 2003; Raz et al., 2005). White matter hyperintensities, commonly seen in older adults, are significantly associated with executive dysfunction specific to attention and speed in a clinical sample with VaD (Moser et al., 2001). Additionally, evidence that cognitive decline in aging exhibits an anterior-to-posterior gradient highlights the significance of frontal regions in early detection of this process (Head et al., 2004).
Neuropsychological assessments of executive functioning (EF), which measures abilities such as working memory, cognitive flexibility and inhibitory control, are frequently used as a proxy for frontal lobe function, though there is a lack of support for using these terms synonymously (Alvarez & Emory, 2006). EF is a complex cognitive construct with variable definitions in the literature; while some research refers to EF broadly as a grouping of mental control processes, other descriptions of EF include sustained and selective attention and verbal fluency (Denckla, 1996). Activity in the dorsolateral prefrontal cortex is often highlighted as a specific neural correlate of these functions (Baggetta & Alexander, 2016). EF performance has also been shown to predict overall social or global functioning (Bell-McGinty, Podell, Franzen, Baird, & Williams, 2002; Insel, Morrow, Brewer, & Figueredo, 2006; Mirelman et al., 2012) and lesions to ventromedial circuitry and the orbitofrontal cortex are linked to impaired social behaviors (Alvarez & Emory, 2006; Cicerone & Tanenbaum, 1997).
Additional literature suggests cognitive processing speed contributes to deficits in EF performance in older individuals with cardiovascular disease (Liebel et al., 2017) and that reduced blood flow in CVD patients impacts EF performance (Jefferson, Poppas, Paul, & Cohen, 2007) through disruption of networks critical to processing speed (Turken et al., 2008). Though processing speed and EF are often considered separate cognitive constructs, timed EF assessments in particular are highly dependent on processing speed abilities (Demakis, 2004). Executive functions also support multiple types of behavioral outcomes, particularly health-related behaviors (Hofmann, Schmeichel, & Baddeley, 2012). Thus, EF is a key subcomponent of cognitive function for examining early signs of cardiovascular disease burden on brain health.
In this study, we set out to test if differences in cognitive test performance in relation to number of components of MetS within a single individual can be detected with conventional neuropsychological tests in middle-aged adults without clinically significant cognitive impairment. We hypothesized that the number of MetS components present will be associated with poorer performance on tests of executive function as frontal lobe regions critical to EF are particularly vulnerable to the effects of cardiovascular disease (Bartzokis, 2004).
Method
Participants
A total of 409 right-handed adults between the ages of 40 and 60 with normal or corrected to normal vision were recruited for the study through flyering on bulletin boards on the University of Texas at Austin campus, grocery stores and bus stops in the Austin, Texas area. Bus ads, Craigslist ads and newspaper advertisements in the Austin American-Statesman were also utilized. Two hundred seventy-four individuals were enrolled and cognitive and metabolic demographic data were available on 197 participants. Exclusions from the study included history of neurological disease (e.g. large vessel stroke, seizure disorder, Parkinson’s disease, clinically significant traumatic brain injury, multiple sclerosis, or brain infection/meningitis), major psychiatric illness (e.g. schizophrenia, bipolar disorder), diagnosis of or previous hospitalization of substance abuse, or MRI contraindication. Approximately, 73 participants were excluded from analysis for failing to complete all study visits. The health assessment was completed on first visit and cognitive testing, and MRI data were collected at the second visit. Mean duration between health assessment visit and cognitive testing session was 4.58 ± 4.50 weeks. Time between visits ranged from 0 to 39.43 weeks. For two participants, the visits were completed in the reverse order. Four participants were removed from analysis for presenting with diminished global cognitive function (Full Scale Intelligence Quotient, FSIQ scores <80, outside the normal range). Participant characteristics are provided by Table 1. Cognitive Testing data provided in Table 2. Analyses of variance were performed to determine if participant characteristics differed significantly between MetS component groups and no significant differences were observed for age—F(5, 191) = 0.73, p = .602, IQ—F(5, 191) = 0.91, p = .474, and education—F(5, 191) = 0.61, p = .691. Sex significantly differed across MetS component groupings—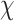 2(5, N = 197) = 15.6, p = .008. Sex characteristics by MetS component groupings provided in Table 3.
2(5, N = 197) = 15.6, p = .008. Sex characteristics by MetS component groupings provided in Table 3.
Table 1.
Participant characteristics (n = 197)
| Participant characteristics | Mean ± SD |
|---|---|
| Age, years | 49 ± 6 |
| Sex (male/female) | 83/114 |
| Education, y | 16 ± 3 |
| Race, n (%) | |
| Non-Hispanic white | 122, 62% |
| Hispanic | 43, 22% |
| Asian American | 6, 3% |
| African American | 21, 11% |
| Unknown | 5, 3% |
| Health measures | |
| Body weight, kg | 84 ± 21 |
| BMI, kg/m2 | 29 ± 7 |
| Systolic blood pressure, mm Hg | 121 ± 13 |
| Diastolic blood pressure, mm Hg | 73 ± 9 |
| Total-C, mg/dL | 200 ± 43 |
| HDL-C, mg/dL | 51 ± 17 |
| LDL-C, mg/dL | 128 ± 38 |
| Triglyceride, mg/dL | 124 ± 84 |
| Glucose, mg/dL | 99 ± 30 |
| Waist circumference, cm | 97 ± 16 |
Notes: Data are means ± SD. MetS = metabolic syndrome; BMI = body mass index; BP = blood pressure; C = cholesterol.
Table 2.
Participant characteristics, cognitive data (n = 197)
| Participant characteristics | Mean ± SD |
|---|---|
| Global cognitive function WASI FSIQ—2 subtest, score | 113 ± 15 |
| Executive function, z-score | −0.01 ± 0.69 |
| Trails A, time | 29 ± 9 |
| Trails B, time | 64 ± 32 |
| Digit span, backward | 9 ± 2 |
| Stroop test, color word | 43 ± 11 |
| Memory, z-score | 0.00 ± 0.88 |
| CVLT-II, short delay free recall | 11 ± 3 |
| CVLT-II, long delay free recall | 12 ± 3 |
| CVLT-II, recognition (yes/no) | 3 ± 1 |
Notes: Data are means ± SD. WASI = Wechsler Abbreviated Scale of Intelligence; CVLT-II = California Verbal Learning Test, 2nd edition.
Table 3.
Participant characteristics by MetS components (n = 197)
| Participant characteristics | MetS = 0 | MetS = 1 | MetS = 2 | MetS = 3 | MetS = 4 | MetS = 5 |
|---|---|---|---|---|---|---|
| Mean ± SD | ||||||
| Participants, n | 48 | 60 | 27 | 33 | 20 | 9 |
| Sex (male/female) | 21/27 | 14/46 | 15/12 | 16/17 | 13/7 | 4/5 |
| Age | 49 ± 6 | 49 ± 6 | 49 ± 6 | 49 ± 6 | 50 ± 7 | 52 ± 7 |
| Education, y | 16 ± 2 | 16 ± 2 | 16 ± 3 | 16 ± 3 | 16 ± 3 | 15 ± 2 |
| WASI FSIQ—2 subtest, score | 114 ± 13 | 114 ± 14 | 110 ± 16 | 113 ± 14 | 112 ± 20 | 105 ± 9 |
| Executive function, z-score | 0.11 ± 0.63 | −0.02 ± 0.58 | −0.04 ± 0.71 | 0.12 ± 0.75 | 0.02 ± 0.48 | −0.94 ± 1.15 |
| Memory, z-score | 0.00 ± 0.96 | 0.26 ± 0.81 | 0.14 ± 0.74 | −0.23 ± 0.91 | −0.30 ± 0.80 | −0.61 ± 1.02 |
Notes: Data are means ± SD. MetS = metabolic syndrome; WASI = Wechsler Abbreviated Scale of Intelligence.
Measures
Global cognitive function
The Matrix Reasoning and Vocabulary subtests of the Wechsler Abbreviated Scale of Intelligence, 2nd edition (WASI-II) were administered to provide an estimated Full-Scale Intelligence Quotient (FSIQ) (McCrimmon & Smith, 2013).
Executive function
The Adult Version of the Stroop Color and Word Test was administered to participants to measure inhibitory control, in particular the ability to inhibit an automatic verbal response (Jensen & Rohwer, 1966). The assessment is administered in three consecutive 45-s portions. The first portion requires the participant to read printed words describing a color such as red, blue, green aloud within the allotted time. The second portion or condition requires the participant to name the ink color of a neutral stimulus. The third condition, which is the primary measure considered in determinations of executive function, asks participants to name the ink color of a printed word that does not correspond to the color of the ink, such as a red word printed in green. The assessment is widely used in assessing frontal lobe function due to its relationship to cognitive control abilities (Bench et al., 1993; Stuss, Floden, Alexander, Levine, & Katz, 2001).
The Trail Making Test is a timed graphomotor assessment that consists of two conditions. The first or “A” condition involves connecting numerical dots. The “B” condition involves connecting dots composed of both numerical and alphabetical cues, requiring the participant to alternate between numbers and letters. The utilization of set shifting in this task is thought to assess executive control (Arbuthnott & Frank, 2000; Moll, De Oliveira-Souza, Moll, Bramati, & Andreiuolo, 2002). Since “A” is primarily a measure of psychomotor speed, subtracting the “A” task time from “B” isolates the executive control component of the task from psychomotor and graphomotor abilities.
Though executive function measures are often dependent on processing speed (Demakis, 2004), decline in executive function operates independently of age-related changes in psychomotor speed (Keys & White, 2000). Since both Trails B and Stroop Color and Word are timed assessments, our executive component z-scores are dependent on processing speed, even with the subtraction of Trails A from the B condition, which is often thought of as a baseline for motor control (Arbuthnott & Frank, 2000). Additionally, research examining the relationship between Trails A and B have shown that shown that the “A” condition has stronger associations with speed overall than the “B” condition but that the “B minus A” calculation is still related to speed (Salthouse, 2011).
The Digit Span assessment from the Weschler Adult Intelligence Scale—Fourth edition (WAIS-IV) requires participants to repeat back a series of numbers dictated verbally by the assessor both forward and backward. The task gradually loads on more digits as the task progresses to increase intensity. Only the backward condition was included in the calculation of executive functioning as research suggests that the forward condition is more of an attentional measure and is less directly related to working memory and executive functioning than the backward measure (Hale, Hoeppner, & Fiorello, 2002; Lefebvre, Marchand, Eskes, & Connolly, 2005).
Memory function
The immediate free recall, delayed free recall and Recognition Discriminability scores were selected from the California Verbal Learning Test, 2nd edition (CVLT-II, 2020). The task requires participants to attempt to recall a list of sixteen words, administered over five practice trials followed by recall of a distractor list. This is followed by an Immediate Recall trial and a 20-min delayed recall trial (Delis, Kramer, Kaplan, & Ober, 2000).
Health/Risk Factor Assessment
Following an 8 hr fast, blood samples were collected by venipuncture through the antecubital vein. Resting blood pressure was measured using a semiautomated device (VP-200, Omron Healthcare, Bannockburn, IL). Participants rested in a supine position for 15 min prior to this measure. A tape measure was used for participant waist and hip circumference while a beam balance scale was used for measuring height and weight to calculate body mass index (BMI). Glucose, triglycerides, total cholesterol and HDL-cholesterol concentrations were measured using a standard enzymatic technique. Categorical cut points for the 5 MetS components were determined by Alberti and colleagues (2009) consensus criteria: fasting glucose ≥100 mg/dL or drug treatment for hyperglycemia, triglycerides ≥150 mg/dL, HDL-C ≤40 mg/dL in males and ≤50 mg/dL in females or drug treatment for dyslipidemia, systolic blood pressure ≥130 mm Hg or diastolic ≥85 mm Hg or antihypertensive medication, and waist circumference ≥102 cm for males and ≥88 cm for females. If someone fulfilled criteria for three or more components, they were categorized as having an MetS diagnosis (Alberti et al., 2009). Three individuals in the sample did not have sufficient information to indicate a diagnosis of MetS but met criteria for at least one MetS component.
Statistical Analyses
To limit the number of comparisons, we created composite cognitive domain scores. Though there is no current standard in the field and some research utilizes factor modeling to derive distinct constructs of cognitive functioning, composite scores have demonstrated higher sensitivity to neuropathology and change in cognition over time over the use of individual subtest scores, particularly in Alzheimer’s and MCI populations (Gibbons et al., 2012; Lim et al., 2016). Raw scores for each cognitive test were converted to z-scores using the study sample mean and SD. Z-scores for Trail Making Test B minus A, Stroop Word-Color, and Digit Span Backwards were averaged to form the executive domain score. Because the outcome measure of Trail Making test is time, with lower times indicating a better performance, scores were multiplied by −1 so that higher scores indicate a superior performance for consistency with the other assessments. Z-scores for CVLT-II short delay free recall, long delay free recall, and recognition discriminability were averaged to form the memory domain z-score.
The association between executive and memory domain scores and number of MetS components or MetS diagnosis were assessed using multiple regression, adjusting for age, sex, and years of education. Since only four analyses were planned, the level of statistical significance was set at alpha <0.05. Regression residuals were examined using the Kolmogorov–Smirnov test of normality. Residuals for the analysis of the memory domain were deemed normally distributed for MetS components and MetS diagnosis (p = .20, p = .20). Residuals for the analyses of the executive function domain were deemed normally distributed for MetS components (p = .05) but were not normally distributed for MetS diagnosis (p = .03). Therefore, the results from that analysis should be interpreted with caution.
Results
Selected participant characteristics are reported in Table 1. Raw cognitive test scores are reported in Table 2. Descriptive statistical analyses revealed a cognitively normal, ethnically diverse, middle-aged sample, well representative of the population of the state of Texas. The mean executive function domain z-score for the sample was −0.01 ± 0.67. The mean memory function domain z-score for the sample was 0.00 ± 0.88. The linear regression models revealed that executive domain scores were significantly predicted by age, sex, education, and number of MetS components—F(4, 191) = 3.94, p = .004, MetS components ß = −.14, p = .044, Fig. 1, Table 3. The independent effects of age (ß = −.07, p = .301) and sex (ß = −.05, p = .423) did not account for any unique variance in executive function scores. The effect of education on executive function was significant (ß = .20, p = .004). Additionally, the linear regression models showed that memory domain z-scores were also significantly predicted by age, sex, education and number of MetS components—F(4, 192) = 7.86, p < .001, MetS components ß = −.15, p = .032, Fig. 2, Table 4. Age (ß = −.18, p = .010) and education (ß = .22, p = .001) also had significant independent effects on memory.
Fig. 1.

Executive function and MetS score. Number of MetS components is described on the x-axis. Executive function performance, calculated with a domain z-score, is described on the y-axis. Bars represent standard error of the mean.
Fig. 2.

Memory and MetS score. Number of MetS components is described on the x-axis. Memory performance, calculated with a domain z-score, is described on the y-axis. Bars represent standard error of the mean.
Table 4.
Results (n = 197)
| Results | Estimate | SE | t Value | Pr (|t|) |
|---|---|---|---|---|
| Executive function and MetS components, n = 196 | ||||
| MetS components | −0.07 | 0.03 | −2.03 | 0.044 |
| Age | −0.01 | 0.01 | −1.04 | 0.301 |
| Sex | −0.08 | 0.10 | −0.80 | 0.423 |
| Education | 0.06 | 0.02 | 2.92 | 0.004 |
| Memory and MetS components, n = 197 | ||||
| MetS components | −0.09 | 0.04 | −2.16 | 0.032 |
| Age | −0.03 | 0.01 | −2.60 | 0.010 |
| Sex | 0.29 | 0.12 | 0.16 | 0.017 |
| Education | 0.08 | 0.02 | 3.25 | <.001 |
| Executive function and MetS Dx, n = 193 | ||||
| MetS | −0.07 | 0.11 | −0.67 | 0.506 |
| Age | −0.01 | 0.01 | −1.27 | 0.206 |
| Sex | −0.08 | 0.10 | −0.75 | 0.456 |
| Education | 0.06 | 0.02 | 2.87 | 0.005 |
| Memory and MetS Dx, n = 194 | ||||
| MetS | −0.36 | 0.13 | −2.80 | 0.006 |
| Age | −0.03 | 0.01 | −2.63 | 0.009 |
| Sex | 0.27 | 0.12 | 2.26 | 0.025 |
| Education | 0.07 | 0.02 | 3.14 | 0.002 |
Notes: Executive function and MetS components F-statistic: 3.94 on 4 and 191 DF, p-value = .004; memory and MetS components F-statistic: 7.96 on 4 and 192 DF, p-value < .001; executive function and MetS F-statistic: 2.97 on 4 and 188 DF, p-value = .021; memory and MetS F-statistic: 7.96 on 4 and 189 DF, p-value < .001.
Overall diagnosis of MetS was not significantly associated with executive function domain score (ß = −.05, p = .506, Table 4, Fig. 3) but was significantly associated with memory scores—F(4, 189) = 8.81, p < .001, MetS diagnosis ß = −.19, p = .006, Table 4, Fig. 4. Age (ß = −.18, p = .009), sex (ß = .15, p = .025) and education (ß = .21, p = .002) also had significant independent effects on memory.
Fig. 3.

Executive function and MetS. MetS diagnosis is described on the x-axis. Executive function performance, calculated with a domain z-score, is described on the y-axis. Bars represent standard error of the mean.
Fig. 4.

Memory and MetS. MetS diagnosis is described on the x-axis. Memory performance, calculated with a domain z-score, is described on the y-axis. Bars represent standard error of the mean.
Follow-up Analyses
Contrary to our expectations, the association between executive function and MetS components in our sample did not appear to operate in the hypothesized dose dependent manner, where increasing number of components results in a gradual decrease in executive function performance (Table 3, Fig. 1). Therefore, we performed an exploratory analyses of covariance of executive test performance among the MetS components groups, adjusting for age, education, sex and FSIQ and including post hoc pairwise comparisons. Scores were significantly lower in the five MetS components group, compared to all the other groups (p = .003–.044). Performance was not significantly different among the other groups (p = .847–1.000).
Discussion
The aim of the present study was to determine the relationship between MetS factors at midlife and current cognitive function. The principle findings from the study are as follows: First, in a sample of 197 middle aged adults with and without cardiovascular risk, we found a significant association between the number of MetS components and scores on assessments of EF. We also found a significant relationship between number of MetS components and scores on assessments of memory. Though we did not find a significant relationship between MetS diagnosis and executive function, the relationship between MetS diagnosis and memory domain score was significant. All analyses were adjusted for age, sex, and education.
We predicted that MetS components and executive function would be related but did not anticipate a relationship between MetS components and memory (Fig. 2). Since we recruited a middle-aged sample without severe cognitive dysfunction, we expected to see only the most subtle of cognitive vulnerabilities. Age-related changes in cognition, particularly in the presence of cardiovascular disease, typically follow an anterior to posterior gradient, where the fronto-parietal network and executive functions supported by this region demonstrate changes prior to onset of difficulties with memory (Head et al., 2004; MacPherson et al., 2002). Therefore, we expected to see only changes in executive function. However, age-related memory decline is not an undocumented phenomenon (Kaye et al., 1997; Visser et al., 2002). Research on clinical populations with depression and cognitive decline has shown relationships between verbal memory and executive function (Brooks, Weaver, & Scialfa, 2006; Fossati, Coyette, Ergis, & Allilaire, 2002). The PROOF study of non-demented elderly community residents reported a link between the MetS and executive function as well as memory (Rouch et al., 2014). In another study, individuals with MetS exhibited lower memory performance than a non-MetS group, mediated by levels of cerebral blood flow (CBF), where CBF levels were strongly associated with abdominal obesity and elevated triglycerides (Birdsill et al., 2013). Studies have also linked individual MetS components to memory. Higher levels of visceral adipose tissue have been associated with lower hippocampal volumes, larger ventricular volumes and poorer verbal memory in the elderly (Isaac et al., 2011). In midlife, higher BMI has been indirectly related to poorer memory performance through elevated cerebral myo-inositol levels (Gonzales et al., 2012). Type 2 diabetes has been linked to poorer memory performance in adults at cross section (McCrimmon, Ryan, & Frier, 2012), while higher HDL cholesterol levels have been associated with better performance on working memory (Crichton, Elias, Davey, Sullivan, & Robbins, 2014), verbal and episodic memory tasks (Leritz, McGlinchey, Salat, & Milberg, 2016).
Contrary to our expectations, the association between executive function and MetS components in our sample did not appear to operate in the hypothesized dose-dependent manner, where increasing number of components results in a gradual decrease in executive function performance. Based on the MetS literature suggesting that a minimum threshold of three components has an effect beyond that of the individual components (Solfrizzi et al., 2010) and that there was no added effect of additional components (Falkowski, Atchison, Debutte-Smith, Weiner, & O’Bryant, 2014), we also would have anticipated that MetS diagnosis might be significantly related to executive function performance. Interestingly, in our sample, executive function weaknesses were not detected in relation to number of MetS components at the standard clinical cutoff point of three or more components and Fig. 1 appears to indicate that in our sample, individuals with five components demonstrated the greatest weakness in executive function. Notably, this group has the lowest average IQ score, though these scores are still within the normal range and the difference is not statistically significant. These findings suggest that individuals’ executive function performance remained intact until a relatively high degree of cardiovascular risk was reached. One noted limitation of our study is that our sample is highly educated (16 ± 3 years of education) with mean IQ scores in the high average range (113 ± 15). Additionally, we did not control for race in the analyses.
We interpret the relationship between MetS components and poorer cognitive function in midlife to indicate that individuals with poorer executive function in the presence of cardiovascular risk factors warrant additional attention, treatment for cardiovascular disease, and tracking of cognitive function over time. The presence of MetS components in midlife may indicate brain vulnerability to later cognitive decline and some researchers have gone as far as to suggest that MetS may be a prodromal stage for vascular cognitive impairment (Segura et al., 2009). However, this is a cross-sectional examination, so we cannot be certain about the direction of the association found. Research on EF across the lifespan has shown that EF can impact health behaviors that lead to cardiovascular disease (Houben, Nederkoorn, & Jansen, 2014; McAuley et al., 2011). Certainly, EF supports the planning and performance monitoring necessary for individuals to participate in healthy behaviors, such as exercise or obtaining adequate sleep and inhibit desires to partake in unhealthy behaviors (Hofmann et al., 2012). The most probable scenario incorporates all of these ideas, where predisposition to cardiovascular risk factors impacts cognitive function, EF in particular, which in turn fosters accumulation of cardiovascular risk and related neuropathology. Given the ultimate aim of using modification of these risk factors to alter disease trajectories, it might be useful to consider how cognitive abilities could be impacting an individual’s likelihood to respond to a lifestyle or health behavior intervention.
In conclusion, our results show a subtle but significant relationship between cardiovascular risk factors and current cognitive function as measured by both EF and memory at midlife. Since MetS and its components are modifiable risk factors for later, more severe forms of cognitive decline, they should be targeted with interventions. The bidirectional relationships between EF and some of the MetS components suggest that premorbid executive control might be a barrier to intervention strategies and should be measured at baseline when developing a treatment plan.
Funding
This work was supported by the National Institutes of Health (R01NS75565, R21AG050898).
Conflict of Interest
None declared.
Supplementary Material
Contributor Information
Janelle T Foret, Department of Psychology, The University of Texas at Austin, Austin, TX, USA.
Stephanie Oleson, Department of Psychiatry and Biobehavioral Sciences, Jane and Terry Semel Institute for Neuroscience and Human Behavior at UCLA, Los Angeles, CA, USA.
Brennan Hickson, Department of Kinesiology and Health Education, The University of Texas at Austin, Austin, TX, USA.
Stephanie Valek, McGovern School of Medicine, University of Texas Health Science Center at Houston, Houston, TX, USA.
Hirofumi Tanaka, Department of Kinesiology and Health Education, The University of Texas at Austin, Austin, TX, USA.
Andreana P Haley, Department of Psychology, The University of Texas at Austin, Austin, TX, USA; Biomedical Imaging Center, The University of Texas at Austin, Austin, TX, USA.
References
- Alberti, K. G. M. M., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A. et al. (2009). Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation, 120(16), 1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Alvarez, J. A., & Emory, E. (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Arbuthnott, K., & Frank, J. (2000). Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. Journal of Clinical and Experimental Neuropsychology, 22(4), 518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Arvanitakis, Z., Bennett, D. A., Wilson, R. S., & Barnes, L. L. (2010). Diabetes and cognitive systems in older black and white persons. Alzheimer Disease and Associated Disorders, 24(1), 37–42. doi: 10.1097/WAD.0b013e3181a6bed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggetta, P., & Alexander, P. A. (2016). Conceptualization and operationalization of executive function. Mind, Brain and Education: The Official Journal of the International Mind, Brain, and Education Society, 10(1), 10–33. doi: 10.1111/mbe.12100. [DOI] [Google Scholar]
- Barnes, D. E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet Neurology.. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis, G. (2004). Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging, 25(1), 5–18author reply 49–62. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty, S., Podell, K., Franzen, M., Baird, A. D., & Williams, M. J. (2002). Standard measures of executive function in predicting instrumental activities of daily living in older adults. International Journal of Geriatric Psychiatry, 17(9), 828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- Bench, C. J., Frith, C. D., Grasby, P. M., Friston, K. J., Paulesu, E., Frackowiak, R. S. et al. (1993). Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia, 31(9), 907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Birdsill, A. C., Carlsson, C. M., Willette, A. A., Okonkwo, O. C., Johnson, S. C., Xu, G. et al. (2013). Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity, 21(7), 1313–1320. doi: 10.1002/oby.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, B. L., Weaver, L. E., & Scialfa, C. T. (2006). Does impaired executive functioning differentially impact verbal memory measures in older adults with suspected dementia? The Clinical Neuropsychologist, 20(2), 230–242. doi: 10.1080/13854040590947461. [DOI] [PubMed] [Google Scholar]
- Cicerone, K. D., & Tanenbaum, L. N. (1997). Disturbance of social cognition after traumatic orbitofrontal brain injury. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 12(2), 173–188. https://www.ncbi.nlm.nih.gov/pubmed/14588429. [PubMed] [Google Scholar]
- Crichton, G. E., Elias, M. F., Davey, A., Sullivan, K. J., & Robbins, M. A. (2014). Higher HDL cholesterol is associated with better cognitive function: The Maine-Syracuse study. Journal of the International Neuropsychological Society: JINS, 20(10), 961–970. doi: 10.1017/S1355617714000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis, D. C., Kramer, J. H., Kaplan, E., & Ober, B. A. (2000). California Verbal Learning Test, 2nd edition (CVLT-II). The Psychological Corporation. [Google Scholar]
- Demakis, G. J. (2004). Frontal lobe damage and tests of executive processing: A meta-analysis of the category test, stroop test, and trail-making test. Journal of Clinical and Experimental Neuropsychology, 26(3), 441–450. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- Denckla, M. B. (1996). A theory and model of executive function: A neuropsychological perspective. In Attention, memory, and executive function.. [Google Scholar]
- Driscoll, I., Beydoun, M. A., An, Y., Davatzikos, C., Ferrucci, L., Zonderman, A. B. et al. (2012). Midlife obesity and trajectories of brain volume changes in older adults. Human Brain Mapping, 33(9), 2204–2210. doi: 10.1002/hbm.21353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel, R. H., Grundy, S. M., & Zimmet, P. Z. (2005). The metabolic syndrome. The Lancet, 365(9468), 1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Falkowski, J., Atchison, T., Debutte-Smith, M., Weiner, M. F., & O’Bryant, S. (2014). Executive functioning and the metabolic syndrome: A project FRONTIER study. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 29(1), 47–53. doi: 10.1093/arclin/act078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati, P., Coyette, F., Ergis, A.-M., & Allilaire, J.-F. (2002). Influence of age and executive functioning on verbal memory of inpatients with depression. Journal of Affective Disorders, 68(2–3), 261–271. doi: 10.1016/s0165-0327(00)00362-1. [DOI] [PubMed] [Google Scholar]
- Gibbons, L. E., Carle, A. C., Mackin, R. S., Harvey, D., Mukherjee, S., Insel, P. et al. (2012). A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging and Behavior, 6(4), 517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, M. M., Tarumi, T., Eagan, D. E., Tanaka, H., Vaghasia, M., & Haley, A. P. (2012). Indirect effects of elevated body mass index on memory performance through altered cerebral metabolite concentrations. Psychosomatic Medicine, 74(7), 691–698. doi: 10.1097/PSY.0b013e31825ff1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson, D. R., Steen, B., & Skoog, I. (2004). Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. International Psychogeriatrics/IPA, 16(3), 327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- Hale, J. B., Hoeppner, J. A. B., & Fiorello, C. A. (2002). Analyzing digit span components for assessment of attention processes. Journal of Psychoeducational Assessment. doi: 10.1177/073428290202000202. [DOI] [Google Scholar]
- Head, D., Buckner, R. L., Shimony, J. S., Williams, L. E., Akbudak, E., Conturo, T. E. et al. (2004). Differential vulnerability of anterior White matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hofmann, W., Schmeichel, B. J., & Baddeley, A. D. (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Houben, K., Nederkoorn, C., & Jansen, A. (2014). Eating on impulse: The relation between overweight and food-specific inhibitory control. Obesity, 22(5), E6–E8. doi: 10.1002/oby.20670. [DOI] [PubMed] [Google Scholar]
- Insel, K., Morrow, D., Brewer, B., & Figueredo, A. (2006). Executive function, working memory, and medication adherence among older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 61(2), P102–P107. doi: 10.1093/geronb/61.2.p102. [DOI] [PubMed] [Google Scholar]
- Isaac, V., Sim, S., Zheng, H., Zagorodnov, V., Tai, E. S., & Chee, M. (2011). Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Frontiers in Aging Neuroscience, 3, 12. doi: 10.3389/fnagi.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, A. L., Poppas, A., Paul, R. H., & Cohen, R. A. (2007). Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of Aging, 28(3), 477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, A. R., & Rohwer, W. D., Jr. (1966). The Stroop color-word test: A review. Acta Psychologica, 25(1), 36–93. doi: 10.1016/0001-6918(66)90004-7. [DOI] [PubMed] [Google Scholar]
- Kaye, J. A., Swihart, T., Howieson, D., Dame, A., Moore, M. M., Karnos, T. et al. (1997). Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology, 48(5), 1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- Kivipelto, M., Mangialasche, F., & Ngandu, T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nature Reviews. Neurology, 14(11), 653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- Kivipelto, M., Ngandu, T., Fratiglioni, L., Viitanen, M., Kåreholt, I., Winblad, B. et al. (2005). Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology, 62(10), 1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Kloppenborg, R. P., van den Berg, E., Kappelle, L. J., & Biessels, G. J. (2008). Diabetes and other vascular risk factors for dementia: Which factor matters most? A systematic review. European Journal of Pharmacology, 585(1), 97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- Launer, L. J. (2005). The epidemiologic study of dementia: A life-long quest? Neurobiology of Aging, 26(3), 335–340. doi: 10.1016/j.neurobiolaging.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Lefebvre, C. D., Marchand, Y., Eskes, G. A., & Connolly, J. F. (2005). Assessment of working memory abilities using an event-related brain potential (ERP)-compatible digit span backward task. Clinical Neurophysiology, 116(7), 1665–1680. doi: 10.1016/j.clinph.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Leritz, E. C., McGlinchey, R. E., Salat, D. H., & Milberg, W. P. (2016). Elevated levels of serum cholesterol are associated with better performance on tasks of episodic memory. Metabolic Brain Disease, 31(2), 465–473. doi: 10.1007/s11011-016-9797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebel, S. W., Jones, E. C., Oshri, A., Hallowell, E. S., Jerskey, B. A., Gunstad, J. et al. (2017). Cognitive processing speed mediates the effects of cardiovascular disease on executive functioning. Neuropsychology, 31(1), 44–51. doi: 10.1037/neu0000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Y. Y., Snyder, P. J., Pietrzak, R. H., Ukiqi, A., Villemagne, V. L., Ames, D. et al. (2016). Sensitivity of composite scores to amyloid burden in preclinical Alzheimer’s disease: Introducing the Z-scores of attention, verbal fluency, and episodic memory for nondemented older adults composite score. Alzheimer’s & Dementia: The Journal of the Alzheimer's Association, 2, 19–26. doi: 10.1016/j.dadm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson, S. E., Phillips, L. H., & Della Sala, S. (2002). Age, executive function, and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychology and Aging, 17(4), 598–609. doi: 10.1037/0882-7974.17.4.598. [DOI] [PubMed] [Google Scholar]
- McAuley, E., Mullen, S. P., Szabo, A. N., White, S. M., Wójcicki, T. R., Mailey, E. L. et al. (2011). Self-regulatory processes and exercise adherence in older adults: Executive function and self-efficacy effects. American Journal of Preventive Medicine, 41(3), 284–290. doi: 10.1016/j.amepre.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon, A. W., & Smith, A. D. (2013). Review of the Wechsler abbreviated scale of intelligence, second edition (WASI-II). Journal of Psychoeducational Assessment, 31(3), 337–341. doi: 10.1177/0734282912467756. [DOI] [Google Scholar]
- McCrimmon, R. J., Ryan, C. M., & Frier, B. M. (2012). Diabetes and cognitive dysfunction. The Lancet, 379(9833), 2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- Mirelman, A., Herman, T., Brozgol, M., Dorfman, M., Sprecher, E., Schweiger, A. et al. (2012). Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PloS One, 7(6), e40297. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll, J., de Oliveira-Souza, R., Moll, F. T., Bramati, I. E., & Andreiuolo, P. A. (2002). The cerebral correlates of set-shifting: An fMRI study of the trail making test. Arquivos de Neuro-Psiquiatria, 60(4), 900–905. doi: 10.1590/s0004-282x2002000600002. [DOI] [PubMed] [Google Scholar]
- Moser, D. J., Cohen, R. A., Paul, R. H., Paulsen, J. S., Ott, B. R., Gordon, N. M. et al. (2001). Executive function and magnetic resonance imaging subcortical hyperintensities in vascular dementia. Neuropsychiatry, Neuropsychology and Behavioral Neurology, 14(2), 89–92. [PubMed] [Google Scholar]
- O’Donnell, C. A., Browne, S., Pierce, M., McConnachie, A., Deckers, K., van Boxtel, M. P. J. et al. (2015). Reducing dementia risk by targeting modifiable risk factors in mid-life: Study protocol for the innovative midlife intervention for dementia deterrence (in-MINDD) randomised controlled feasibility trial. Pilot and Feasibility Studies, 1(1), 40. doi: 10.1186/s40814-015-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman, B. L., Williams, J. W., Burke, J. R., Holsinger, T., & Benjamin, S. (2010). Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. 153(3), 182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Raji, C. A., Ho, A. J., Parikshak, N. N., Becker, J. T., Lopez, O. L., Kuller, L. H. et al. (2010). Brain structure and obesity. Human Brain Mapping, 31(3), 353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz, N., Lindenberger, U., Rodrigue, K. M., Kennedy, K. M., Head, D., Williamson, A. et al. (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15(11), 1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz, N., Rodrigue, K. M., & Acker, J. D. (2003). Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience, 117(6), 1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Ronan, L., Alexander-Bloch, A. F., Wagstyl, K., Farooqi, S., Brayne, C., Tyler, L. K., Cam-CAN, & Fletcher, P. C. (2016). Obesity associated with increased brain age from midlife. Neurobiology of Aging, 47, 63–70. doi: 10.1016/j.neurobiolaging.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch, I., Trombert, B., Kossowsky, M. P., Laurent, B., Celle, S., Ntougou Assoumou, G. et al. (2014). Metabolic syndrome is associated with poor memory and executive performance in elderly community residents: The PROOF study. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 22(11), 1096–1104. doi: 10.1016/j.jagp.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (2011). What cognitive abilities are involved in trail-making performance? Intelligence, 39(4), 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura, B., Jurado, M. A., Freixenet, N., Albuin, C., Muniesa, J., & Junqué, C. (2009). Mental slowness and executive dysfunctions in patients with metabolic syndrome. Neuroscience Letters, 462(1), 49–53. doi: 10.1016/j.neulet.2009.06.071. [DOI] [PubMed] [Google Scholar]
- Skoog, I., Lernfelt, B., Landahl, S., Palmertz, B., Andreasson, L. A., Nilsson, L. et al. (1996). 15-year longitudinal study of blood pressure and dementia. The Lancet, 347(9009), 1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Solfrizzi, V., Scafato, E., Capurso, C., D’Introno, A., Colacicco, A. M., Frisardi, V. et al. (2010). Metabolic syndrome and the risk of vascular dementia: The Italian longitudinal study on ageing. Journal of Neurology, Neurosurgery and Psychiatry. doi: 10.1136/jnnp.2009.181743. [DOI] [PubMed] [Google Scholar]
- Stuss, D. T., Floden, D., Alexander, M. P., Levine, B., & Katz, D. (2001). Stroop performance in focal lesion patients: Dissociation of processes and frontal lobe lesion location. Neuropsychologia, 39(8), 771–786. doi: 10.1016/s0028-3932(01)00013-6. [DOI] [PubMed] [Google Scholar]
- CVLT-II (2020). Test–Second Edition (CVLT-II). San Antonio, TX, The Psychological Corporation. [Google Scholar]
- Thompson, P. M. (2010). Brain structure and obesity. Human Brain Mapping. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken, A., Whitfield-Gabrieli, S., Bammer, R., Baldo, J. V., Dronkers, N. F., & Gabrieli, J. D. E. (2008). Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage, 42(2), 1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, P. J., Verhey, F. R. J., Hofman, P. A. M., Scheltens, P., & Jolles, J. (2002). Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. Journal of Neurology, Neurosurgery, and Psychiatry, 72(4), 491–497. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer, R. A., Sidney, S., Selby, J., Johnston, S. C., & Yaffe, K. (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 64(2), 277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Yaffe, K., Kanaya, A., Lindquist, K., Simonsick, E. M., Harris, T., Shorr, R. I. et al. (2004). The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA: The Journal of the American Medical Association, 292(18), 2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


