Abstract
Background
Miscarriage, defined as the spontaneous loss of a pregnancy before 24 weeks’ gestation, is common with approximately 25% of women experiencing a miscarriage in their lifetime, and 15% to 20% of pregnancies ending in a miscarriage. Progesterone has an important role in maintaining a pregnancy, and supplementation with different progestogens in early pregnancy has been attempted to rescue a pregnancy in women with early pregnancy bleeding (threatened miscarriage), and to prevent miscarriages in asymptomatic women who have a history of three or more previous miscarriages (recurrent miscarriage).
Objectives
To estimate the relative effectiveness and safety profiles for the different progestogen treatments for threatened and recurrent miscarriage, and provide rankings of the available treatments according to their effectiveness, safety, and side‐effect profile.
Search methods
We searched the following databases up to 15 December 2020: Cochrane Central Register of Controlled Trials, Ovid MEDLINE(R), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP), and reference lists of retrieved studies.
Selection criteria
We included all randomised controlled trials assessing the effectiveness or safety of progestogen treatment for the prevention of miscarriage. Cluster‐randomised trials were eligible for inclusion. Randomised trials published only as abstracts were eligible if sufficient information could be retrieved. We excluded quasi‐ and non‐randomised trials.
Data collection and analysis
At least two review authors independently assessed the trials for inclusion and risk of bias, extracted data and checked them for accuracy. We performed pairwise meta‐analyses and indirect comparisons, where possible, to determine the relative effects of all available treatments, but due to the limited number of included studies only direct or indirect comparisons were possible. We estimated the relative effects for the primary outcome of live birth and the secondary outcomes including miscarriage (< 24 weeks of gestation), preterm birth (< 37 weeks of gestation), stillbirth, ectopic pregnancy, congenital abnormalities, and adverse drug events. Relative effects for all outcomes are reported separately by the type of miscarriage (threatened and recurrent miscarriage). We used the GRADE approach to assess the certainty of evidence.
Main results
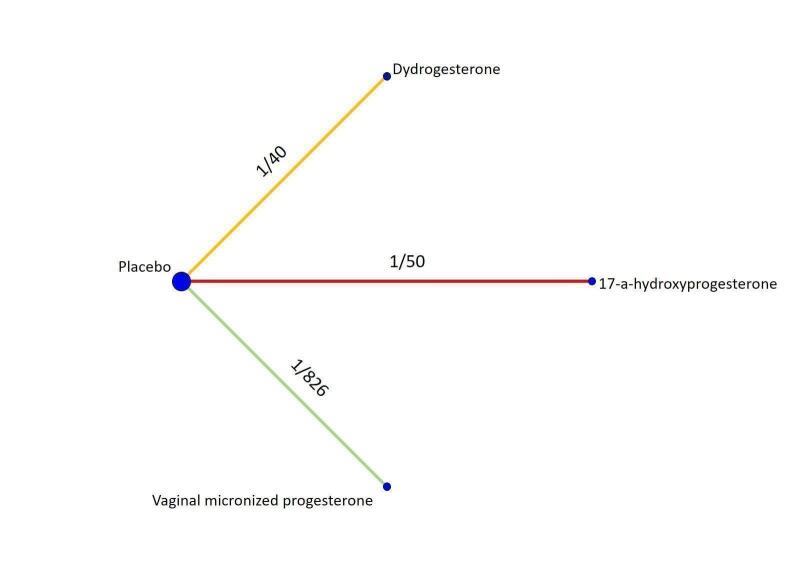
Our meta‐analysis included seven randomised trials involving 5,682 women, and all provided data for meta‐analysis. All trials were conducted in hospital settings. Across seven trials (14 treatment arms), the following treatments were used: three arms (21%) used vaginal micronized progesterone; three arms (21%) used dydrogesterone; one arm (7%) used oral micronized progesterone; one arm (7%) used 17‐α‐hydroxyprogesterone, and six arms (43%) used placebo.
Women with threatened miscarriage
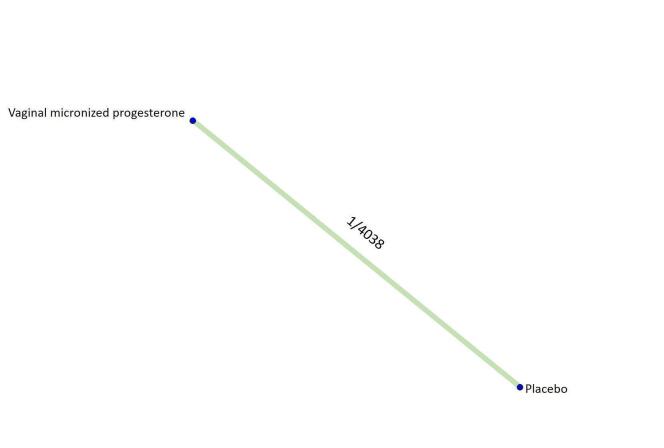
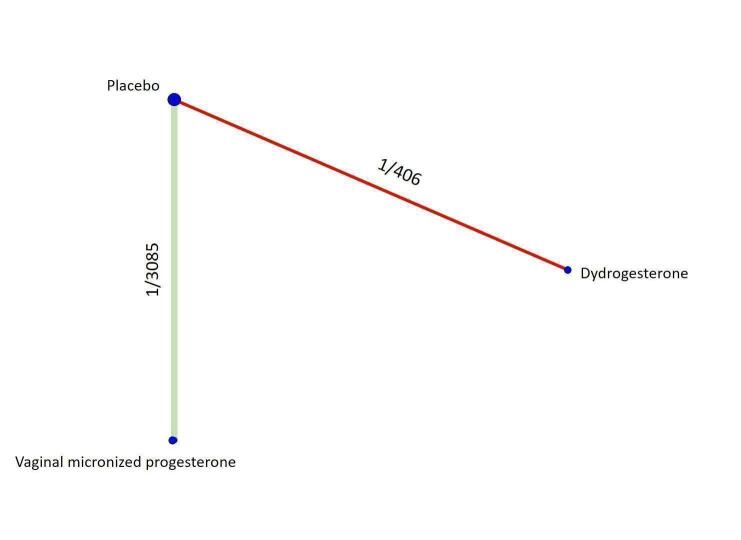
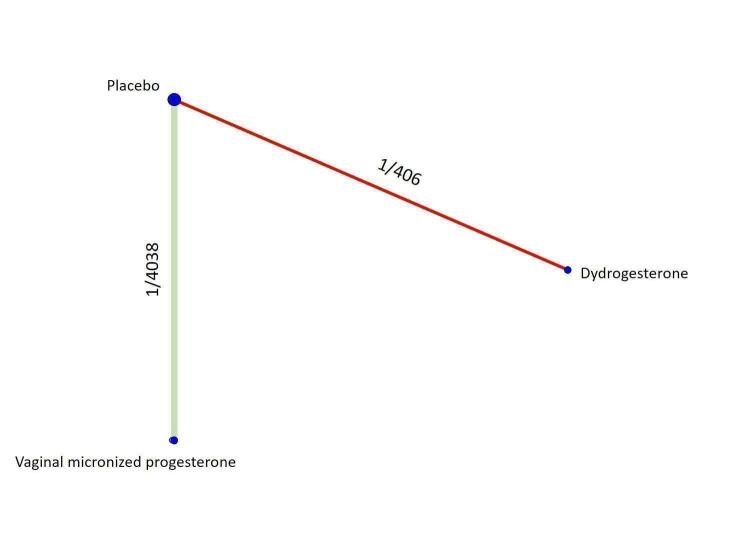
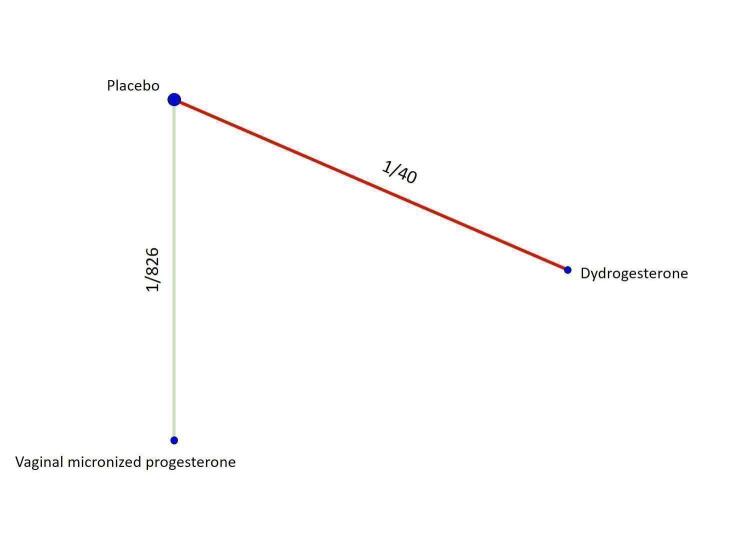
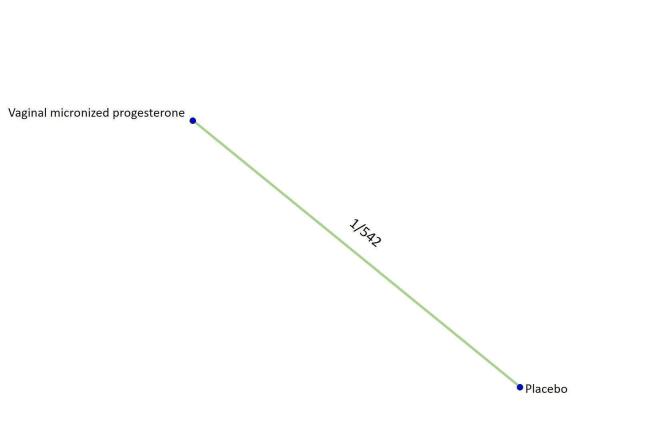
Based on the relative effects from the pairwise meta‐analysis, vaginal micronized progesterone (two trials, 4090 women, risk ratio (RR) 1.03, 95% confidence interval (CI) 1.00 to 1.07, high‐certainty evidence), and dydrogesterone (one trial, 406 women, RR 0.98, 95% CI 0.89 to 1.07, moderate‐certainty evidence) probably make little or no difference to the live birth rate when compared with placebo for women with threatened miscarriage. No data are available to assess the effectiveness of 17‐α‐hydroxyprogesterone or oral micronized progesterone for the outcome of live birth in women with threatened miscarriage.
The pre‐specified subgroup analysis by number of previous miscarriages is only possible for vaginal micronized progesterone in women with threatened miscarriage. In women with no previous miscarriages and early pregnancy bleeding, there is probably little or no improvement in the live birth rate (RR 0.99, 95% CI 0.95 to 1.04, high‐certainty evidence) when treated with vaginal micronized progesterone compared to placebo. However, for women with one or more previous miscarriages and early pregnancy bleeding, vaginal micronized progesterone increases the live birth rate compared to placebo (RR 1.08, 95% CI 1.02 to 1.15, high‐certainty evidence).
Women with recurrent miscarriage
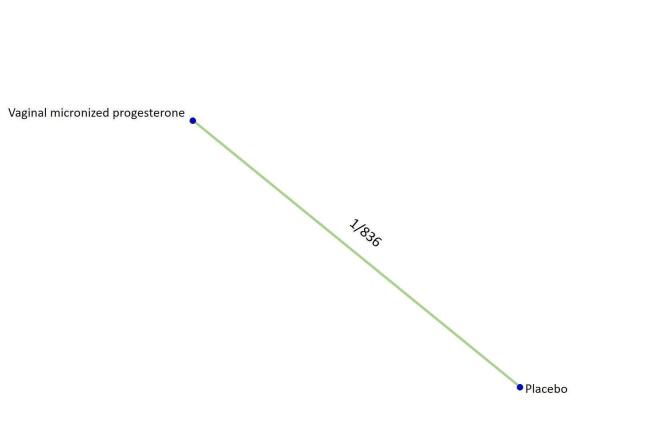
Based on the results from one trial (826 women) vaginal micronized progesterone (RR 1.04, 95% CI 0.95 to 1.15, high‐certainty evidence) probably makes little or no difference to the live birth rate when compared with placebo for women with recurrent miscarriage. The evidence for dydrogesterone compared with placebo for women with recurrent miscarriage is of very low‐certainty evidence, therefore the effects remain unclear. No data are available to assess the effectiveness of 17‐α‐hydroxyprogesterone or oral micronized progesterone for the outcome of live birth in women with recurrent miscarriage.
Additional outcomes
All progestogen treatments have a wide range of effects on the other pre‐specified outcomes (miscarriage (< 24 weeks of gestation), preterm birth (< 37 weeks of gestation), stillbirth, ectopic pregnancy) in comparison to placebo for both threatened and recurrent miscarriage. Moderate‐ and low‐certainty evidence with a wide range of effects suggests that there is probably no difference in congenital abnormalities and adverse drug events with vaginal micronized progesterone for threatened (congenital abnormalities RR 1.00, 95% CI 0.68 to 1.46, moderate‐certainty evidence; adverse drug events RR 1.07 95% CI 0.81 to 1.39, moderate‐certainty evidence) or recurrent miscarriage (congenital abnormalities 0.75, 95% CI 0.31 to 1.85, low‐certainty evidence; adverse drug events RR 1.46, 95% CI 0.93 to 2.29, moderate‐certainty evidence) compared with placebo. There are limited data and very low‐certainty evidence on congenital abnormalities and adverse drug events for the other progestogens.
Authors' conclusions
The overall available evidence suggests that progestogens probably make little or no difference to live birth rate for women with threatened or recurrent miscarriage. However, vaginal micronized progesterone may increase the live birth rate for women with a history of one or more previous miscarriages and early pregnancy bleeding, with likely no difference in adverse events. There is still uncertainty over the effectiveness and safety of alternative progestogen treatments for threatened and recurrent miscarriage.
Plain language summary
Are progestogen treatments effective in preventing miscarriage?
We set out to find out which progestogen treatment is most effective, safe, and has fewer side‐effects for preventing miscarriage in women with threatened and with recurrent miscarriage, using evidence from randomised controlled trials. We looked at the number of women who went on to have a live birth, or miscarriage.
What is the issue?
Miscarriage is the most common cause of early pregnancy loss in the first 24 weeks and one of the most common complications in early pregnancy. An estimated 15% to 20% of pregnancies will end in a miscarriage, with 25% of women experiencing a miscarriage in their lifetime. Women can be at risk of a miscarriage if they experience early pregnancy bleeding, or if they have a history of previous miscarriages.
Why is this important?
Progesterone is an important pregnancy hormone that helps to maintain a pregnancy. A variety of different progesterone‐like treatments (known as progestogens) have been used to treat women with early pregnancy bleeding. They are also used to prevent miscarriage in women with a history of previous miscarriages. There is uncertainty about the effectiveness, safety, and side‐effects of the available progestogens for preventing miscarriage in these different groups of women. We wanted to find out which, if any, of the treatments is the most effective and safest. We collected and analysed all the relevant studies to answer this question.
What evidence did we find?
We searched for evidence in December 2020 and identified seven studies involving 5,682 women. All women were managed in hospitals. Women were diagnosed with early pregnancy bleeding (known as threatened miscarriage), or had a history of three or more previous miscarriages (known as recurrent miscarriage). Four different progestogen treatments were used: vaginal micronized progesterone, oral dydrogesterone, oral micronized progesterone and 17‐α‐hydroxyprogesterone injected into muscle. In six of the studies the treatments were compared to inactive placebo.
Three studies involved 4496 women with threatened miscarriage, some of whom had previously experienced a miscarriage. Overall, vaginal micronized progesterone (high‐quality evidence) and oral dydrogesterone (moderate‐quality evidence) made little difference to the number of women who went on to have a live birth when compared with placebo. We further studied the women who had experienced a previous miscarriage, were now presenting with a threatened miscarriage, and were given vaginal micronized progesterone or placebo. For women with one or more previous miscarriages, vaginal micronized progesterone increased the live birth rate compared to placebo (high‐quality evidence). Those women who had no previous miscarriages, but were now presenting with early pregnancy bleeding showed no improvement in live birth rate (high‐certainty evidence).
For women with recurrent miscarriage, we based our findings on one study involving 826 women. Overall, vaginal micronized progesterone made little difference to the live birth rate when compared with placebo. The evidence for dydrogesterone compared with placebo for women with recurrent miscarriage is of very low‐certainty evidence, therefore the effects remain unclear. No data are available to assess the effectiveness of 17‐α‐hydroxyprogesterone or oral micronized progesterone for the outcome of live birth in women with recurrent miscarriage.
From the available data, there are likely no differences in adverse events associated with vaginal micronized progesterone. There was no difference in birth defects and side effects with vaginal micronized progesterone when compared with placebo. There was not enough information about safety and birth defects for us to analyse for all the other treatments.
What does this mean?
The overall available evidence suggests that progestogens probably make little or no difference to live birth rate for women with threatened or recurrent miscarriage. Vaginal micronized progesterone may increase the live birth rate for women who are experiencing early pregnancy bleeding and have a history of one or more previous miscarriages, with likely no difference in adverse events. There is still uncertainty over the effectiveness and safety of alternative progestogen treatments for threatened and recurrent miscarriage.
Summary of findings
Summary of findings 1. Live birth.
|
Patient or population: women with threatened miscarriage or a history of recurrent miscarriage Interventions: multiple progestogens (vaginal micronized progesterone, oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone) Comparison: placebo and dydrogesterone Outcome: live birth Settings: hospitals | |||||||
| Treatment | Direct evidence | Indirect evidence | Anticipated absolute effects for direct estimate | ||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with intervention | Risk with comparator | Risk difference with intervention | |
| Threatened miscarriage | |||||||
| Vaginal micronized progesterone versus placebo | 1.03 [1.00, 1.07] | ⊕⊕⊕⊕ HIGH |
Unavailable | ‐ | 761 per 1000 (vaginal micronized progesterone) | 725 per 1000 (placebo) |
36 more per 1000 (from 36 fewer to 123 more) |
| Subgroup analysis: number of previous miscarriages | |||||||
| No previous miscarriages and early pregnancy bleeding | 0.99 [0.95, 1.04] | ⊕⊕⊕⊕ HIGH |
Unavailable | ‐ | 739 per 1000 (vaginal micronized progesterone) | 747 per 1000 (placebo) |
7 fewer per 1000 (from 37 fewer to 30 more) |
| One or more previous miscarriages and early pregnancy bleeding | 1.08 [1.02, 1.14] | ⊕⊕⊕⊕ HIGH |
Unavailable | ‐ | 755 per 1000 (vaginal micronized progesterone) | 699 per 1000 (placebo) |
56 more per 1000 (from 14 more to 105 more) |
| Dydrogesterone versus placebo | 0.98 [0.89, 1.07] | ⊕⊕⊕⊝ MODERATEa |
Unavailable | ‐ | 816 per 1000 (dydrogesterone) | 833 per 1000 (placebo) |
17 fewer per 1000 (from 92 fewer to 58 more) |
| 17‐α‐hydroxyprogesterone versus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesterone versus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 1.07 [0.93, 1.23] | ⊕⊕⊕⊝ MODERATEb |
See comment* | See comment** | See comment*** |
| Recurrent miscarriage | |||||||
| Vaginal micronized progesterone versus placebo | 1.04 [0.94, 1.15] | ⊕⊕⊕⊕ HIGH |
Unavailable | ‐ | 659 per 1000 (vaginal micronized progesterone) | 633 per 1000 (placebo) |
25 more per 1000 (from 38 fewer to 95 more) |
| Dydrogesterone versus placebo | 1.00 [0.23, 4.37] | ⊕⊝⊝⊝ VERY LOWc |
Unavailable | ‐ | 850 per 1000 (dydrogesterone) | 850 per 1000 (placebo) |
0 fewer per 1000 (from 195 fewer to 255 more) |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 1.04 [0.79, 1.38] | ⊕⊝⊝⊝ VERY LOWd |
See comment* | See comment** | See comment*** |
| 17‐α‐hydroxyprogesterone versus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesterone versus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| *No included studies or there are no events in included studies to estimate the baseline risk. **Absolute risk with intervention cannot be estimated in the absence of absolute risk with the comparator. ***Risk difference cannot be estimated in the absence of absolute risks with intervention and the comparator. CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Direct evidence downgraded ‐1 due to serious limitations in study design.
b Indirect evidence ‐1 due to serious limitations in study design.
c Direct evidence downgraded ‐1 due to serious limitations in study design (unclear random sequence generation and allocation concealment) and ‐2 due to and severe imprecision (wide 95% CIs and small number of events).
d Indirect evidence downgraded ‐1 due to serious limitations in study design (unclear random sequence generation and allocation concealment) and ‐2 due to and severe imprecision (wide 95% CIs and small number of events).
Summary of findings 2. Miscarriage (defined as delivery before 24 weeks of gestation).
|
Patient or population: women with threatened miscarriage or a history of recurrent miscarriage Interventions: multiple progestogens (vaginal micronized progesterone, oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone) Comparison: placebo and dydrogesterone Outcome: miscarriage (defined as delivery before 24 weeks of gestation) Settings: hospitals | |||||||
| Treatment | Direct evidence | Indirect evidence | Anticipated absolute effects for direct estimate | ||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with intervention | Risk with comparator | Risk difference with intervention | |
| Threatened miscarriage | |||||||
| Vaginal micronized progesterone versus placebo | 0.90 [0.80, 1.01] | ⊕⊕⊕⊕ HIGH |
Unavailable | ‐ | 201 per 1000 (vaginal micronized progesterone) | 224 per 1000 (placebo) |
22 fewer per 1000 (from 45 fewer to 2 more) |
| Dydrogesterone versus placebo | 0.90 [0.55, 1.47] | ⊕⊕⊕⊝ MODERATEa |
Unavailable | ‐ | 129 per 1000 (dydrogesterone) | 143 per 1000 (placebo) |
14 fewer per 1000 (from 64 fewer to 67 more) |
| 17‐α‐hydroxyprogesterone versus placebo | Not reported by included studies |
‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesterone versus dydrogesterone | 0.67 [0.25, 1.75] | ⊕⊝⊝⊝ VERY LOWb |
Unavailable | ‐ | 102 per 1000 (oral micronized progesterone) | 153 per 1000 (placebo) |
50 fewer per 1000 (from 114 fewer to 114 more) |
| Oral micronized progesterone versus placebo | Unavailable | ‐ | 0.74 [0.25, 2.17] | ⊕⊝⊝⊝ VERY LOWc | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 1.00 [0.60, 1.66] | ⊕⊕⊕⊝ MODERATEd |
See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus oral micronized progesterone | Unavailable | ‐ | 1.22 [0.41, 3.62] | ⊕⊝⊝⊝ VERY LOWc | See comment* | See comment** | See comment*** |
| Recurrent miscarriage | |||||||
| Vaginal micronized progesterone versus placebo | 0.96 [0.79, 1.17] | ⊕⊕⊕⊕ HIGH |
Unavailable | ‐ | 321 per 1000 (vaginal micronized progesterone) | 334 per 1000 (placebo) |
13 fewer per 1000 (from 70 fewer to 57 more) |
| Dydrogesterone versus placebo | 1.00 [0.23, 4.37] | ⊕⊝⊝⊝ VERY LOWe | Unavailable | ‐ | 150 per 1000 (dydrogesterone) | 150 per 1000 (placebo) |
0 fewer per 1000 (from 115 fewer to 505 more) |
| 17‐α‐hydroxyprogesterone versus placebo | 0.85 [0.28, 2.58] | ⊕⊝⊝⊝ VERY LOWe | Unavailable | ‐ | 185 per 1000 (17‐α‐hydroxyprogesterone) | 217 per 1000 (placebo) |
33 fewer per 1000 (from 157 fewer to 343 more) |
| Oral micronized progesterone versus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 0.96 [0.22, 4.24] | ⊕⊝⊝⊝ VERY LOWf | See comment* | See comment** | See comment*** |
| Dydrogesterone versus 17‐α‐hydroxyprogesterone | Unavailable | ‐ | 1.18 [0.19, 7.44] | ⊕⊝⊝⊝ VERY LOWf | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus 17‐α‐hydroxyprogesterone | Unavailable | ‐ | 1.13 [0.37, 3.49] | ⊕⊝⊝⊝ VERY LOWf | See comment* | See comment** | See comment*** |
| *No included studies or there are no events in included studies to estimate the baseline risk. **Absolute risk with intervention cannot be estimated in the absence of absolute risk with the comparator. ***Risk difference cannot be estimated in the absence of absolute risks with intervention and the comparator. CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Direct evidence downgraded ‐1 due to serious limitations in study design.
b Direct evidence downgraded ‐1 due to serious limitations in study design (unclear allocation concealment) and ‐2 due to and severe imprecision (wide 95% CIs and small number of events).
c Indirect evidence downgraded ‐1 due to serious limitations in study design (unclear allocation concealment) and ‐2 due to and severe imprecision (wide 95% CIs and small number of events).
d Indirect evidence downgraded ‐1 due to serious limitations in study design.
e Direct evidence downgraded ‐1 due to serious limitations in study design (unclear random sequence generation and allocation concealment) and ‐2 due to and severe imprecision (wide 95% CIs and small number of events).
f Indirect evidence downgraded ‐1 due to serious limitations in study design (unclear random sequence generation and allocation concealment) and ‐2 due to and severe imprecision (wide 95% CIs and small number of events).
Summary of findings 3. Preterm birth (defined as birth before 37 weeks of gestation).
|
Patient or population: women with threatened miscarriage or a history of recurrent miscarriage Interventions: multiple progestogens (vaginal micronized progesterone, oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone) Comparison: placebo and dydrogesterone Outcome: preterm birth (defined as birth before 37 weeks of gestation) Settings: hospitals | |||||||
| Treatment | Direct evidence | Indirect evidence | Anticipated absolute effects for direct estimate | ||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with intervention | Risk with comparator | Risk difference with intervention | |
| Threatened miscarriage | |||||||
| Vaginal micronized progesterone versus placebo | 1.08 [0.92, 1.27] | ⊕⊕⊕⊝ MODERATEa |
Unavailable | ‐ | 166 per 1000 (vaginal micronized progesterone) | 152 per 1000 (placebo) |
14 more per 1000 (from 27 fewer to 68 more) |
| Dydrogesteroneversus placebo | 0.87 [0.40, 1.88] | ⊕⊕⊝⊝ LOWb |
Unavailable | ‐ | 67 per 1000 (dydrogesterone) | 77 per 1000 (placebo) |
10 fewer per 1000 (from 46 fewer to 68 more) |
| 17‐α‐hydroxyprogesteroneversus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesterone versus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 1.25 [0.55, 2.86] | ⊕⊕⊝⊝ LOWc |
See comment* | See comment** | See comment*** |
| Recurrent miscarriage | |||||||
| Vaginal micronized progesteroneversus placebo | 1.12 [0.67, 1.87] | ⊕⊕⊕⊝ MODERATEa |
Unavailable | ‐ | 103 per 1000 (vaginal micronized progesterone) | 92 per 1000 (placebo) |
11 more per 1000 (from 30 fewer to 80 more) |
| Dydrogesteroneversus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| 17‐α‐hydroxyprogesteroneversus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesterone versus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| *No included studies or there are no events in included studies to estimate the baseline risk. **Absolute risk with intervention cannot be estimated in the absence of absolute risk with the comparator. ***Risk difference cannot be estimated in the absence of absolute risks with intervention and the comparator. CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Direct evidence downgraded ‐1 due to serious imprecision (wide 95% CIs).
b Direct evidence downgraded ‐1 due to serious limitations in study design and serious imprecision (wide 95% CIs).
c Indirect evidence downgraded ‐1 due to serious limitations in study design and serious imprecision (wide 95% CIs).
Summary of findings 4. Stillbirth.
|
Patient or population: women with threatened miscarriage or a history of recurrent miscarriage Interventions: multiple progestogens (vaginal micronized progesterone, oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone) Comparison: placebo and dydrogesterone Outcome: stillbirth Settings: hospitals | |||||||
| Treatment | Direct evidence | Indirect evidence | Anticipated absolute effects for direct estimate | ||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with intervention | Risk with comparator | Risk difference with intervention | |
| Threatened miscarriage | |||||||
| Vaginal micronized progesterone versus placebo | 0.83 [0.25, 2.71] | ⊕⊕⊝⊝ LOWa | Unavailable | ‐ | 2 per 1000 (vaginal micronized progesterone) | 3 per 1000 (placebo) |
1 fewer per 1000 (from 2 fewer to 5 more) |
| Dydrogesterone versus placebo | 0.33 [0.01, 8.13] | ⊕⊝⊝⊝ VERY LOWb | Unavailable | ‐ | 2 per 1000 (dydrogesterone) | 5 per 1000 (placebo) |
3 fewer per 1000 (from 5 fewer to 35 more) |
| 17‐α‐hydroxyprogesterone versus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesteroneversus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 2.52 [0.07, 88.08] | ⊕⊝⊝⊝ VERY LOWc |
See comment* | See comment** | See comment*** |
| Recurrent miscarriage | |||||||
| Vaginal micronized progesteroneversus placebo | 0.54 [0.05, 5.91] | ⊕⊕⊝⊝ LOWa | Unavailable | ‐ | 3 per 1000 (vaginal micronized progesterone) | 5 per 1000 (placebo) |
2 fewer per 1000 (from 4 fewer to 23 more) |
| Dydrogesterone versus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| 17‐α‐hydroxyprogesteroneversus placebo | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesteroneversus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| *No included studies or there are no events in included studies to estimate the baseline risk. **Absolute risk with intervention cannot be estimated in the absence of absolute risk with the comparator. ***Risk difference cannot be estimated in the absence of absolute risks with intervention and the comparator. CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Direct evidence downgraded ‐2 due to severe imprecision (wide 95% CIs and number of events less than 30).
b Direct evidence downgraded ‐1 due to serious limitations in study design and ‐2 for severe imprecision (wide 95% CIs and number of events less than 30).
c Indirect evidence downgraded ‐1 due to serious limitations in study design and ‐2 for severe imprecision (wide 95% CIs and number of events less than 30).
Summary of findings 5. Ectopic pregnancy.
|
Patient or population: women with threatened miscarriage or a history of recurrent miscarriage Interventions: multiple progestogens (vaginal micronized progesterone, oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone) Comparison: placebo and dydrogesterone Outcome: ectopic pregnancy Settings: hospitals | |||||||
| Treatment | Direct evidence | Indirect evidence | Anticipated absolute effects for direct estimate | ||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with intervention | Risk with comparator | Risk difference with intervention | |
| Threatened miscarriage | |||||||
| Vaginal micronized progesterone | 0.20 [0.01, 4.14] | ⊕⊕⊝⊝ LOWa | Unavailable | ‐ | 0 per 1000 (vaginal micronized progesterone) | 1 per 1000 (placebo) |
1 fewer per 1000 (from 1 fewer to 3 more) |
| Dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| 17‐α‐hydroxyprogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesteroneversus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Recurrent miscarriage | |||||||
| Vaginal micronized progesterone | 0.92 [0.31, 2.72] | ⊕⊕⊝⊝ LOWa | Unavailable | ‐ | 15 per 1000 (vaginal micronized progesterone) | 16 per 1000 (placebo) |
2 fewer per 1000 (from 4 fewer to 23 more) |
| Dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| 17‐α‐hydroxyprogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesteroneversus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| *No included studies or there are no events in included studies to estimate the baseline risk. **Absolute risk with intervention cannot be estimated in the absence of absolute risk with the comparator. ***Risk difference cannot be estimated in the absence of absolute risks with intervention and the comparator. CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Direct evidence downgraded ‐2 due to severe imprecision (wide 95% CIs and number of events less than 30).
Summary of findings 6. Congenital abnormalities.
|
Patient or population: women with threatened miscarriage or a history of recurrent miscarriage Interventions: multiple progestogens (vaginal micronized progesterone, oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone) Comparison: placebo and dydrogesterone Outcome: congenital abnormalities Settings: hospitals | |||||||
| Treatment | Direct evidence | Indirect evidence | Anticipated absolute effects for direct estimate | ||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with intervention | Risk with comparator | Risk difference with intervention | |
| Threatened miscarriage | |||||||
| Vaginal micronized progesterone | 1.00 [0.68, 1.46] | ⊕⊕⊕⊝ MODERATEa |
Unavailable | ‐ | 34 per 1000 (vaginal micronized progesterone) | 34 per 1000 (placebo) |
0 fewer per 1000 (from 11 fewer to 16 more) |
| Dydrogesterone | 0.71 [0.23, 2.21] | ⊕⊝⊝⊝ VERY LOWb |
Unavailable | ‐ | 24 per 1000 (dydrogesterone) | 34 per 1000 (placebo) |
10 fewer per 1000 (from 27 fewer to 42 more) |
| 17‐α‐hydroxyprogesterone | Unavailable | ‐ | aUnavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesterone versus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 1.41 [0.43, 4.65] | ⊕⊝⊝⊝ VERY LOWc |
See comment* | See comment** | See comment*** |
| Recurrent miscarriage | |||||||
| Vaginal micronized progesterone | 0.75 [0.31, 1.85] | ⊕⊕⊝⊝ LOWd |
Unavailable | ‐ | 30 per 1000 (vaginal micronized progesterone) | 40 per 1000 (placebo) |
10 fewer per 1000 (from 27 fewer to 34 more) |
| Dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| 17‐α‐hydroxyprogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesteroneversus dydrogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| *No included studies or there are no events in included studies to estimate the baseline risk. **Absolute risk with intervention cannot be estimated in the absence of absolute risk with the comparator. ***Risk difference cannot be estimated in the absence of absolute risks with intervention and the comparator. CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.. | |||||||
a Direct evidence downgraded ‐1 due to imprecision (wide 95% CIs).
b Direct evidence downgraded ‐1 due to serious limitations in study design and ‐2 due to severe imprecision (wide 95% CIs and number of events less than 30).
c Indirect evidence downgraded ‐1 due to serious limitations in study design and ‐2 due to severe imprecision (wide 95% CIs and number of events less than 30).
d Direct evidence downgraded ‐2 due to severe imprecision (wide 95% CIs and number of events less than 30).
Summary of findings 7. Adverse drug events.
|
Patient or population: women with threatened miscarriage or a history of recurrent miscarriage Interventions: multiple progestogens (vaginal micronized progesterone, oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone) Comparison: placebo and dydrogesterone Outcome: adverse drug events Settings: hospitals | |||||||
| Treatment | Direct evidence | Indirect evidence | Anticipated absolute effects for direct estimate | ||||
| RR (95% CI) | Certainty | RR (95% CI) | Certainty | Risk with intervention | Risk with comparator | Risk difference with intervention | |
| Threatened miscarriage | |||||||
| Vaginal micronized progesterone | 1.07 [0.81, 1.39] | ⊕⊕⊕⊝ MODERATEa |
Unavailable | ‐ | 52 per 1000 (vaginal micronized progesterone) | 49 per 1000 (placebo) |
3 more per 1000 (from 9 fewer to 19 more) |
| Dydrogesterone | 2.00 [0.18, 21.88] | ⊕⊝⊝⊝ VERY LOWb |
Unavailable | ‐ | 10 per 1000 (dydrogesterone) | 5 per 1000 (placebo) |
5 more per 1000 (from 4 fewer to 103 more) |
| 17‐α‐hydroxyprogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesteroneversus dydrogesterone | Not estimable | ‐ | Not estimable | ‐ | See comment* | See comment** | See comment*** |
| Vaginal micronized progesterone versus dydrogesterone | Unavailable | ‐ | 0.54 [0.05, 5.99] | ⊕⊝⊝⊝ VERY LOWc |
See comment* | See comment** | See comment*** |
| Recurrent miscarriage | |||||||
| Vaginal micronized progesterone | 1.46 [0.93, 2.29] | ⊕⊕⊕⊝ MODERATEa |
Unavailable | ‐ | 101 per 1000 (vaginal micronized progesterone) | 69 per 1000 (placebo) |
32 more per 1000 (from 5 fewer to 90 more) |
| Dydrogesterone | Not estimable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| 17‐α‐hydroxyprogesterone | Unavailable | ‐ | Unavailable | ‐ | See comment* | See comment** | See comment*** |
| Oral micronized progesterone versus dydrogesterone | Not estimable | ‐ | Not estimable | ‐ | See comment* | See comment** | See comment*** |
| *No included studies or there are no events in included studies to estimate the baseline risk. **Absolute risk with intervention cannot be estimated in the absence of absolute risk with the comparator. ***Risk difference cannot be estimated in the absence of absolute risks with intervention and the comparator. CI: Confidence interval; RR: Risk ratio. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||||
a Direct evidence downgraded ‐1 due to serious imprecision (wide 95% CIs).
b Direct evidence downgraded ‐1 due to serious limitations in study design and ‐2 due to severe imprecision (wide 95% CIs and number of events less than 30).
c Indirect evidence downgraded ‐1 due to serious limitations in study design and ‐2 due to severe imprecision (wide 95% CIs and number of events less than 30).
Background
Description of the condition
Miscarriage is generally defined as the spontaneous loss of a pregnancy before 24 weeks’ gestation (Shiers 2003). Approximately 15% of pregnancies end in a miscarriage (Adolfsson 2006; Linnakaari 2019; Magnus 2019; Maconochie 2007; Rossen 2018), and 25% of women experience a miscarriage in their lifetime (Alberman 1992). Miscarriage is most likely to happen during the first 12 weeks of pregnancy; the rate of pregnancy loss drops considerably after 14 weeks of gestation (Alberman 1992). Miscarriage can sometimes lead to haemorrhage and infection, and it can be an important cause of morbidity and even mortality, particularly in low‐income countries (Cantwell 2011). The psychological impact of miscarriage is substantial, and can include anxiety, depression and post‐traumatic stress disorder (Farren 2019; Murphy 2012).
Description of the intervention
Progestogens are a class of steroid hormones that bind to and activate the progesterone receptor (Evans 1988). Progesterone is the most important progestogen in the body, with an important role in maintaining pregnancy (Stephenson 2002). The physiological importance of progesterone in pregnancy has prompted researchers, physicians, and patients to consider progesterone supplementation during early pregnancy to prevent miscarriages. Progesterone supplementation in early pregnancy has been attempted in two contexts: firstly, to rescue a pregnancy in women who have started to bleed during early pregnancy (threatened miscarriage) (Sotiriadis 2004); and secondly, to prevent miscarriages in asymptomatic women who have a history of recurrent miscarriages (three or more previous pregnancy losses) (Bender Atik 2018). A range of different natural progesterones and synthetic progestogens have been tested in early pregnancy, as follows.
Micronized vaginal progesterone
Micronized oral progesterone
Oral dydrogesterone
Oral medroxyprogesterone
Oral progesterone 3‐cyclopentyl enol ether
Intramuscular 17‐OH progesterone
How the intervention might work
Progesterone in early conception is vital for a successful pregnancy: it stimulates endometrial differentiation and uterine growth (Okada 2018), modulates strong immunomodulatory effects (Polikarpova 2019), and inhibits myometrial contractions (Corner 1953). A deficiency in progesterone in early pregnancy has long been purported to be a cause of miscarriage (Palomba 2015), and numerous randomised controlled trials of different progestogens have been conducted to test this hypothesis. These trials have attempted progestogen supplementation to increase endometrial tissue concentrations during the first trimester of pregnancy in women who present with clinical signs of threatened miscarriage or with a history of recurrent miscarriage.
The type and route of administration for progestogens in early pregnancy support has long been a subject of contention. Some studies have used micronized progesterone, which has an identical molecular structure to natural progesterone. Others have used various progestogens which have a different molecular structure to natural progesterone, but are still able to exert progestogenic activity. Oral administration results in extensive first‐pass metabolism in the liver, which limits its efficacy. Administration of progesterone by intramuscular injection or the vaginal route avoids first‐pass metabolism and achieves higher concentrations in endometrial tissue, and for this reason intramuscular and vaginal routes are the primary routes of progestogen administration (Paulson 2014).
Why it is important to do this review
Two separate Cochrane Reviews have compared the different treatments in women with either threatened (Wahabi 2018) or recurrent miscarriage (Haas 2019). They used standard pairwise meta‐analyses, which can only compare a treatment with another treatment or a non‐active control; such pairwise meta‐analyses rely on head‐to‐head comparison trials (direct evidence). In the absence of head‐to‐head trials for some of the comparisons, significant uncertainty may remain about which is the most effective drug amongst multiple options.
A network meta‐analysis allows for comparisons and conclusions about which treatment is most effective amongst multiple options. A network meta‐analysis simultaneously pools all the available direct and indirect evidence on relative treatment effects, to achieve a single coherent analysis. Indirect evidence is obtained by inferring the relative effectiveness of two competing treatments through a common comparator. Thus, a network meta‐analysis produces estimates of the relative effects of each treatment compared with every other treatment in a network (even though some pairs may not have been directly compared), and has the potential to reduce the uncertainty in treatment effect estimates (Caldwell 2005). It also allows for the calculation of the probability that each treatment is the best for any given outcome. Network meta‐analysis can additionally be used to identify gaps in the evidence base.
Objectives
To estimate the relative effectiveness and safety profiles for the different progestogen treatments for threatened and recurrent miscarriage, and to provide rankings of the available treatments according to their effectiveness, safety, and side‐effect profile.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials or cluster‐randomised trials comparing the effectiveness of progestogen treatment for the prevention of miscarriage were eligible for inclusion. Quasi‐randomised, non‐randomised and cross‐over trials were excluded. Randomised trials published only as abstracts were eligible only if sufficient information could be retrieved.
Types of participants
This review included trials involving women at risk of miscarriage (e.g. threatened miscarriage, defined as early vaginal bleeding during the first trimester [12 weeks] of pregnancy; or history of recurrent miscarriage, as defined by the trialists). Threatened and recurrent miscarriage are two separate clinical populations, with their own distinct clinical treatment pathways, therefore the two populations were analysed separately in this review. The review considered studies conducted in all settings.
Types of interventions
We considered trials of progestogens for prevention of miscarriage, compared with another type of progestogen, placebo, or no treatment. The progestogens eligible for inclusion included vaginal micronized progesterone, oral micronized progesterone, dydrogesterone, and 17‐OH progesterone. Although medroxyprogesterone and progesterone 3‐cyclopentyl enol ether have been studied in the past, they have not been trialled or used for this indication for over 50 years and were therefore not included in this review. We included studies where treatment was initiated at any time during the first trimester of pregnancy. For the purposes of this review, we made the assumption that any participant that met the inclusion criteria was, in principle, equally likely to be randomised to any of the eligible interventions. Different dosages, regimens or routes of the same treatment were considered as the same node in the network. Studies comparing different dosages, regimens or routes were eligible for inclusion.
Types of outcome measures
We estimated the relative effects and rankings of the progestogens according to the following primary and secondary outcomes.
Primary outcomes
Live birth
Secondary outcomes
Miscarriage (defined as delivery before 24 weeks of gestation)
Preterm birth (defined as birth before 37 weeks of gestation)
Stillbirth
Ectopic pregnancy
Congenital abnormalities
Adverse drug events
Search methods for identification of studies
This Methods section is based on a standard template used by Cochrane Pregnancy and Childbirth and the protocol adaption for multiple interventions suggested by Chaimani and colleagues (Chaimani 2017).
We attempted to identify all relevant studies, regardless of language or publication status (published, unpublished, in press, or ongoing).
Electronic searches
We searched the following databases up to 15 December 2020, using the search terms described in Appendix 1.
Cochrane Central Register of Controlled Trials, Issue 12 of 12, December 2020
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) from 1946
ClinicalTrials.gov
WHO International Clinical Trials Registry (ICTRP) Platform (www.who.int/trialsearch)
The search methods used are detailed in Appendix 1.
Searching other resources
We retrieved additional relevant references cited in papers identified through the above search strategy. We searched for the full texts of studies identified as abstracts. We sought information from primary authors to investigate whether these studies met the eligibility criteria, and to obtain outcome and study data. Where this was not possible, abstracts were only included where we could extract sufficient information to satisfy our eligibility criteria and if the study authors report the outcomes of interest. We searched for all possible comparisons formed by the drugs of interest, which included comparisons to placebo or no treatment. We did not apply any language or date restrictions to the searches.
Data collection and analysis
Selection of studies
Two review authors retrieved and independently assessed for inclusion all the potential studies identified as a result of the search strategy (AJD, MP, AP). We resolved any disagreement through discussion or, if required, through consultation with a third person (IDG). Citations and abstracts were screened for inclusion, and if eligibility was unclear, authors were contacted for clarification where possible.
Screening eligible studies for scientific integrity/trustworthiness
All studies meeting our inclusion criteria were evaluated by two review authors against predefined criteria to select studies that, based on available information, were deemed to be sufficiently trustworthy to be included in the analysis. These criteria have been developed by Cochrane Pregnancy and Childbirth, and are as follows.
Research governance
No prospective trial registration for studies published after 2010 without plausible explanation
When requested, trial authors refuse to provide/share the protocol and/or ethics approval letter
Trial authors refuse to engage in communication with the Cochrane Review authors
Trial authors refuse to provide individual patient data (IPD) data upon request with no justifiable reason
Baseline characteristics
Characteristics of the study participants being too similar (distribution of mean (standard deviation (SD)) excessively narrow or excessively wide, as noted by Carlisle 2017).
Feasibility
Implausible numbers (e.g. 500 women with severe cholestasis of pregnancy recruited in 12 months)
(Close to) zero losses to follow‐up without plausible explanation
Results
Implausible results (e.g. massive risk reduction for main outcomes with small sample size)
Unexpectedly even numbers of women ‘randomised’ including a mismatch between the numbers and the methods, e.g. if the study authors state no blocking was used but still end up with equal numbers, or they state that blocks of four were used but the final numbers differ by six
Where a study was classified as being at ‘high risk’ for one or more of the above criteria, we attempted to contact the study authors to address any possible lack of information and concerns. If adequate information remained unavailable, the study was categorised as ‘awaiting classification’, and the concerns and communications with the author (or lack thereof) were described in detail. The process is described fully in Figure 1.
1.
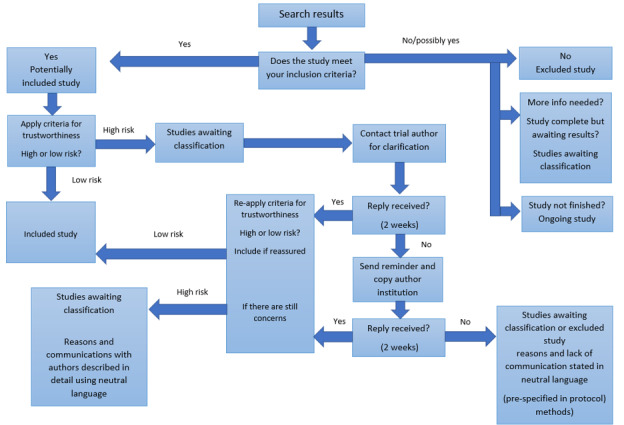
Process for using the Cochrane Pregnancy and Childbirth criteria for assessing the trustworthiness of a study
Abstracts
Data from abstracts were only included if, in addition to the trustworthiness assessment, the study authors confirmed in writing that the data included in the review have come from the final analysis and will not change. If such information was not available or provided, the study was kept in ‘awaiting classification’ (as above).
Data extraction and management
We designed a form to extract data. For eligible studies, at least two independent review authors extracted the data using the agreed form (AJD, MP, AP). We resolved discrepancies through discussion, or, if required, through consultation with a third person (IDG). For dichotomous outcomes, for each trial group, the number of patients with the event and the number analysed and randomised was extracted. We entered data into Review Manager 5 software (Review Manager 2014) and checked them for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study (AJD, MP, AP), using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or, if required, by involving a third assessor (IDG).
(1) Random sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
unclear risk of bias
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for each primary outcome. We assessed the methods as being at:
low, high, or unclear risk of bias for participants; or
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We have described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as being at:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
We have described for each included study — and for each outcome or class of outcomes — the completeness of data, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or could be supplied by the trial authors, we have re‐included missing data in the analyses which we undertook. We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data are balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data are imbalanced across groups; ‘as treated’ analysis was done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as being at:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; or the study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We have described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias, and judged each study to be at:
low risk of other bias;
high risk of other bias; or
unclear risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. Studies are ranked as 'low risk of bias’ if they are double‐blinded, and have allocation concealment with less than 10% loss to follow‐up. Studies with assessor blinding and less than 10% loss to follow‐up are ranked 'intermediate risk of bias’. Studies with no blinding or more than 10% loss to follow‐up are ranked as 'high risk of bias'. We have explored the impact of the level of bias through undertaking sensitivity analyses; see Sensitivity analysis.
Measures of treatment effect
We have presented results as summary risk ratio with 95% confidence intervals (CIs).
Relative treatment effects
We summarised the relative treatment effects of dichotomous outcomes with risk ratios (RRs).
Relative treatment ranking
We planned to estimate cumulative probabilities for each progestogen being at each possible rank and obtain a treatment hierarchy using the surface under the cumulative ranking curve (SUCRA); the larger the SUCRA the higher its rank among all available options (Salanti 2011). Uncertainty intervals (95% CIs) around the ranking of each treatment were to be reported and considered when interpreting the results. We intended to evaluate each outcome to determine confidence in the output of the network meta‐analysis, as described by Salanti and colleagues (Salanti 2014). However, due to the paucity of eligible trials and the differences in risk of bias between the studies, we decided not to estimate the cumulative probabilities of each progestogen treatment being at each possible rank and obtain a treatment hierarchy.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. We planned to adjust their sample sizes using the methods described in the Handbook (Higgins 2011), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we had used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. In cluster‐randomised trials; particular biases to consider include:
• recruitment bias; • baseline imbalance; • loss of clusters; • incorrect analysis; and • comparability with individually‐randomised trials.
We would have considered it reasonable to combine the results from both cluster‐randomised trials and individually‐randomised trials if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely. We planned to also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit. However, there were no cluster‐randomised trials included in this review.
Multi‐arm trials
We planned to include multi‐arm trials to account for the correlation between the effect sizes in the network meta‐analysis. Multi‐arm studies would have been treated as multiple independent comparisons in pairwise meta‐analyses. Multi‐arm trials that compare different dosages, regimens or routes of one drug, but also compare those versus another drug, were eligible for inclusion. We planned to merge the intervention arms of different dosages, regimens or routes of the same drug together for the global analysis of all outcomes and to treat them as separate independent comparisons only for the relevant subgroup analysis according to dosage, regimen and route of drug administration. However, there were no multi‐arm trials included in this review.
Dealing with missing data
For included studies we noted the levels of attrition (see also 'Incomplete outcome data' in Assessment of risk of bias in included studies).
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
We used the number randomised minus any participants whose outcomes were known to be missing as the denominator for each outcome in each trial.
Assessment of heterogeneity
To evaluate the presence of clinical heterogeneity, we described the study population characteristics across all included trials. We assessed the presence of clinical heterogeneity by comparing these characteristics.
Assessment of transitivity across treatment comparisons
We considered that the assumption of intransitivity for the indirect evidence is likely to hold given that: the common treatment used to compare different progestogens indirectly is likely to be similar in different trials (e.g. progestogens are administered in a similar way in studies of oral progestogens versus intramuscular progestogens as it is in studies of oral progestogens versus placebo); and pairwise comparisons are unlikely to differ in respect of the distribution of effect modifiers (e.g. all trial designs and characteristics are similar).
Assessment of reporting biases
We were not able to assess for reporting bias in view of the limited number of included trials.
Data synthesis
Methods for direct treatment comparisons
We performed standard pairwise meta‐analyses using fixed‐effect models for every treatment comparison. Treatment of threatened miscarriage and recurrent miscarriage was analysed separately, due to the known aetiology of these two patient populations.
Methods for indirect treatment comparisons
We extracted the sample size and number of outcome events per trial arm, to be used in the STATA network suite of commands (White 2015). Once extracted, we set up the data using the augmented format, where all treatments are compared with a reference treatment, and studies without the reference treatment have a reference treatment arm created with a small amount of data (White 2011). The augmentation process using arm‐based values calculated the risk estimates of the comparisons with reference treatment and their variances and covariances (White 2015). We then generated network diagrams to determine if a network meta‐analysis is feasible. We would have performed network meta‐analysis within a frequentist framework using multivariate random‐effects meta‐analysis estimated by restricted maximum likelihood. All analyses were planned using Stata statistical software, release 15 (StataCorp, College Station, TX). We would have used the network suite of Stata commands designed for this purpose (White 2015), and other STATA commands for visualising and reporting results in network meta‐analysis (Chaimani 2015). Since only indirect comparisons were possible, we used the method described by Butcher to produce indirect comparisons for the most relevant agents and outcomes (oral micronized progesterone versus placebo) (Butcher 1997). The indirect comparisons were estimated using Excel as described by Tobias (Tobias 2014).
Subgroup analysis and investigation of heterogeneity
Assumptions when estimating heterogeneity
In standard pairwise meta‐analyses we estimated the heterogeneity for each comparison. In network meta‐analyses we would assume a common estimate for heterogeneity across the different comparisons.
Measures and tests for heterogeneity
We assessed statistically the presence of heterogeneity within each pairwise comparison using the I₂ statistic and its 95% CI that measures the percentage of variability that cannot be attributed to random error. The certainty of the evidence was downgraded for inconsistency where I₂ ≥ 60%. For the network analysis, we planned to assess statistically the presence of heterogeneity in the entire network based on the magnitude of the heterogeneity variance parameter (T2) estimated from the multivariate meta‐analysis model. We planned to compare the magnitude of the heterogeneity variance with empirical distributions for dichotomous variables (Rhodes 2015; Turner 2012).
Assessment of statistical inconsistency
The statistical agreement between various sources of evidence in a network of interventions were to be evaluated by global and local approaches, in tandem with the evaluation of clinical homogeneity.
Local approaches for evaluation of inconsistency
To evaluate the presence of inconsistency locally we planned to use the node‐splitting approach. The node‐splitting technique allows two distinct components: direct evidence from direct comparisons or multi‐arm trials, and indirect evidence based on the remaining information (Dias 2010). The technique would have been applied to all comparisons in the network and would have enabled generation of graphics clearly showing the difference between combined information, direct, and indirect comparisons.
Global approaches for evaluation of inconsistency
To evaluate consistency in the entire network simultaneously we planned to use the 'design by treatment’ interaction model, as described in Higgins 2011, which was to be implemented in STATA. This method accounts for different sources of inconsistency that can occur when studies with different designs (e.g. two‐arm trials versus three‐arm trials) give different results, as well as for disagreement between direct and indirect evidence. Using this approach, we planned to infer the presence of inconsistency from any source in the entire network based on a Chi2 test.
Subgroup analysis
A subgroup analysis for the primary outcome was performed in the threatened miscarriage population by the number of previous miscarriages (no previous miscarriages and one or more previous miscarriages). A subgroup analysis by maternal age (< 35, ≥ 35 years) was performed in both the threatened and recurrent miscarriage populations.
Sensitivity analysis
For the primary outcome we planned to perform sensitivity analyses by evaluating the relative effects and assessment of model fit for the following: • overall quality of the studies (restricted to low risk of overall bias studies); • randomisation unit (restricted to individually‐randomised trials) • use of placebo (restricted to placebo‐controlled trials and removing studies with no treatment arms).
For the purpose of the sensitivity analysis, studies were ranked as having overall ’low risk of bias’ if they were double‐blinded, and had allocation concealment with less than 10% loss to follow‐up. Studies with assessor blinding and less than 10% loss to follow‐up were to be ranked ’intermediate risk of bias’. Studies with no blinding or more than 10% loss to follow‐up were to be ranked as ’high risk of bias’. We planned to assess differences by evaluating the relative effects and assessment of model fit.
Summary of findings and assessment of the certainty of the evidence
Each 'Summary of findings' table describes key features of the evidence relating to a single outcome, and there is one table for each of our most important outcomes in accordance with the GRADE approach. These include the outcome of live birth, miscarriage (< 24 weeks of gestation), preterm birth (< 37 weeks of gestation), stillbirth, ectopic pregnancy, congenital abnormalities, and adverse drug events. We used the GRADE working group's approach (Brignardello‐Petersen 2018; Puhan 2014) for rating the certainty of the analysis effect estimates for all the comparisons and all outcomes.
We assessed the certainty of the direct evidence, and rated the evidence using the standard GRADE approach based on assessment of study design limitations, inconsistency, imprecision, indirectness and publication bias (Higgins 2011). On the network diagram for all the comparisons and all outcomes we display the GRADE assessment of the direct evidence. We also rated the certainty of the indirect evidence, where available, based on the lower of the certainty ratings of the two arms forming the dominant ‘first‐order' loop in the network diagram for a specific outcome (Brignardello‐Petersen 2018; Puhan 2014).
The certainty of evidence for each outcome was rated as ‘high', ‘moderate', ‘low’ or ‘very low' in accordance with the GRADE approach: high certainty: we are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; ow certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect; and very low‐certainty evidence: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
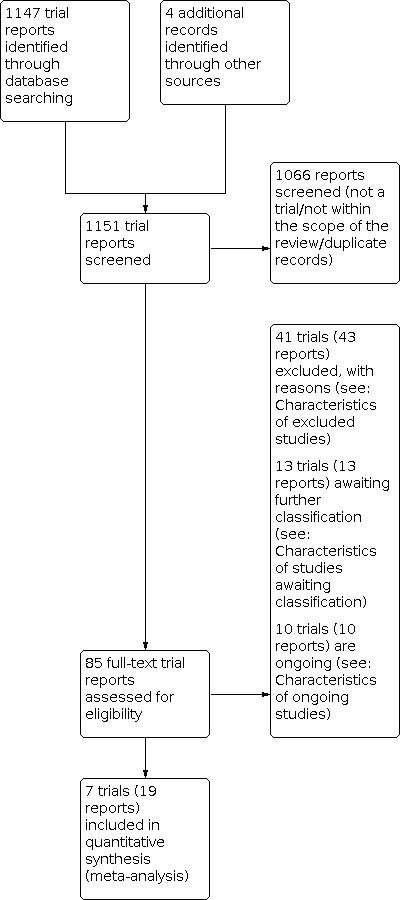
The results of the search strategy are summarised in the PRISMA (Preferred reporting Items for Systematic Reviews and Meta‐Analysis) flow diagram (Figure 2).
2.
Study flow diagram.
Our search strategy retrieved in total 1151 trial reports, from which 1066 were screened and excluded as they were not within the scope of this review. From the 85 reports remaining (71 studies), we examined the full text and included in the final analysis seven trials from 19 reports (for details see Characteristics of included studies). Forty‐one studies (43 reports) were excluded because they did not meet the inclusion criteria (for details see Characteristics of excluded studies), 10 studies were listed as ongoing (for details see Characteristics of ongoing studies) and 13 studies are awaiting classification following assessment using the Cochrane Pregnancy and Childbirth criteria for assessing the trustworthiness of a study (for details see Characteristics of studies awaiting classification).
We contacted the authors of one of the included trials for additional data and clarifications. We have also contacted the authors of two of the ongoing trials which are reported to have finished recruitment to obtain data, but no additional information was made available to us (ACTRN12611000405910; NCT02145767).
Screening eligible studies for trustworthiness
From the 20 studies that were eligible for this review, we judged that 13 did not meet our criteria for trustworthiness for the following reasons.
Six studies published since 2010 demonstrated no evidence of prospective registration (Abrar 2017; Ghosh 2014; Palagiano 2004; Pandian 2009; Turgal 2017; Yassaee 2014).
Two studies were published only as abstracts and we have not been able to confirm with the trial authors that the data were from the final analyses (Vincze 2006, Yadav 2015).
Two studies were retrospectively registered and the authors provided no reason to explain this when contacted (Agarwal 2016, Alimohamadi 2013).
Two studies had concerns about randomisation processes (Czajkowski 2007; Omar 2005).
One study had implausible results with a large risk reduction and a relatively small sample size, and the authors did not provide a justifiable reason for not sharing the individual participant data from the trial (Kumar 2014).
In all cases we made every effort to contact the authors ‐ see Characteristics of studies awaiting classification.
Included studies
This review includes seven two‐arm randomised trials, published between 1963 and 2020, involving 5,682 women. All studies were reported in English and were conducted in hospital settings across five countries: Australia, Germany, Hong Kong, United Kingdom, and Singapore. The included trials included a median of 141 participants (interquartile range (IQR) 53, 621). Two studies were funded by the NIHR Health Technology Assessment programme, UK (Coomarasamy 2015; Coomarasamy 2019), one study was funded by Health and Medical Research Fund, Hong Kong Special Administrative Region (Chan 2020), one study was funded by Khoo Student Research Award and Pitch for Grant Award, Singapore (Siew 2018), one study was funded by Schering AG (Shearman 1963) and two studies did not report a source of funding (Gerhard 1987; MacDonald 1972). None of the studies reported any declarations of interest.
Across all seven trials (14 trial treatment arms) the following progestogens were used:
three arms (21%) used vaginal micronized progesterone;
three arms (21%) used dydrogesterone;
one arm (7%) used oral micronized progesterone;
one arm (7%) used 17‐α‐hydroxyprogesterone;
six arms (43%) used a matched placebo.
Four studies were conducted on the threatened miscarriage population (Chan 2020; Coomarasamy 2019; Gerhard 1987; Siew 2018), and three studies in the recurrent miscarriage population (Coomarasamy 2015; MacDonald 1972; Shearman 1963). All studies contributed to the outcome of live birth, with the exception of Shearman 1963 and Siew 2018.
Excluded studies
We excluded 41 trials (for detail see Characteristics of excluded studies). Twenty‐six of the excluded studies had ineligible designs, 12 studies investigated ineligible interventions, two studies were terminated early due to difficulty with recruitment and therefore no data are available for analysis, and one study has been formally withdrawn by the journal following an investigation.
Risk of bias in included studies
We present summaries of the risk of bias of the included studies for each domain assessed across all studies (Figure 3) and for each included study (Figure 4).
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four trials (57%) used an adequate method to generate the random sequence and were judged to be at low risk of bias for this domain (Chan 2020; Coomarasamy 2015; Coomarasamy 2019; Siew 2018). The remaining three trials (43%) did not provide enough relevant details and thus were judged to be at unclear risk (Gerhard 1987; MacDonald 1972; Shearman 1963).
Three trials (43%) reported adequate methods for allocation concealment and were found to be at low risk of bias (Chan 2020; Coomarasamy 2015; Coomarasamy 2019). Three more trials (43%) did not provide enough information on methods to conceal the allocated interventions and were judged to be at unclear risk for this domain (Gerhard 1987; MacDonald 1972; Shearman 1963). In one trial (14%) authors reported the use of small blocked randomisation, which may have enabled personnel to predict the assignments. Therefore, this trial was judged to have an unclear risk for allocation concealment (Siew 2018).
Blinding
In total, six out of the seven included trials (86%) reported adequate methods for blinding both participants and personnel to treatment allocation and were judged to be at a low risk of performance bias. One trial (14%) was an open‐label, randomised controlled trial in which study participants and caregivers were not masked to treatment allocations. This study was judged to be a high risk of performance bias (Siew 2018). However, all trials reported adequate methods for blinding outcome assessors and were judged to be at a low risk of detection bias.
Incomplete outcome data
Six trials (86%) were judged to be at a low risk of attrition bias, since missing data were balanced across study arms and did not exceed 10%. One trial (14%) reported an attrition rate greater than 10% and was judged to be a high risk for incomplete outcome (Gerhard 1987).
Selective reporting
Only two out of the seven included trials (28%) pre‐specified all outcomes of interest in prospectively registered, publicly available protocols and were judged to be at a low risk of reporting bias (Coomarasamy 2015; Coomarasamy 2019). One trial (14%) reported all outcomes as specified in the published protocol, which was, however, retrospectively registered. This trial was judged to be at an unclear risk of bias (Siew 2018). In one prospectively registered trial (14%), authors did not report results for one of the pre‐specified secondary outcomes, and thus this trial was judged to be at high risk of reporting bias (Chan 2020). For the remaining three trials (43%), the protocol was unavailable for verification, and these were found to be at unclear risk of bias (Gerhard 1987; MacDonald 1972; Shearman 1963).
Other potential sources of bias
Two trials (Gerhard 1987; MacDonald 1972) were assessed as having an unlear risk of other bias because sources of trial funding were not reported. All other included trials had no other potential sources of bias and were assessed as low risk of other bias.
Overall risk of bias
All seven were judged to have a low overall risk of bias, therefore a sensitivity analysis was not performed.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
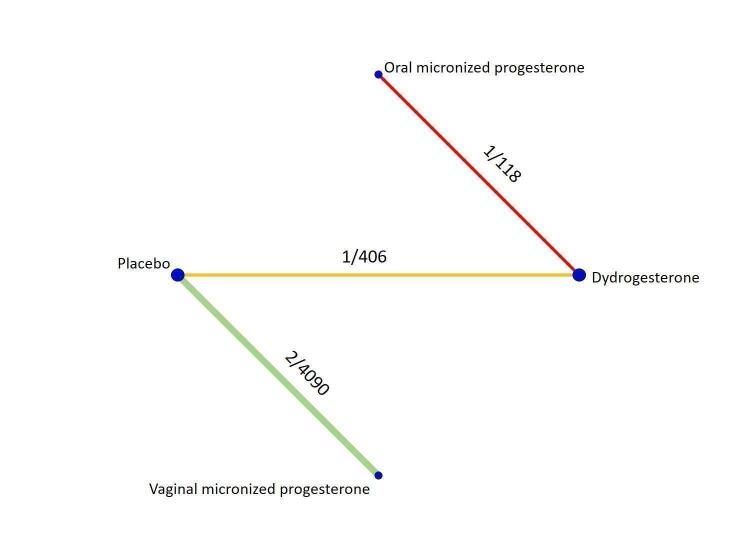
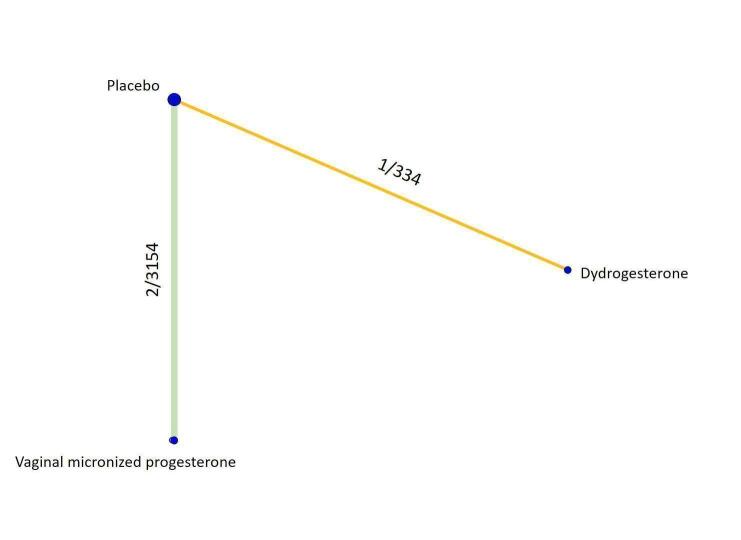
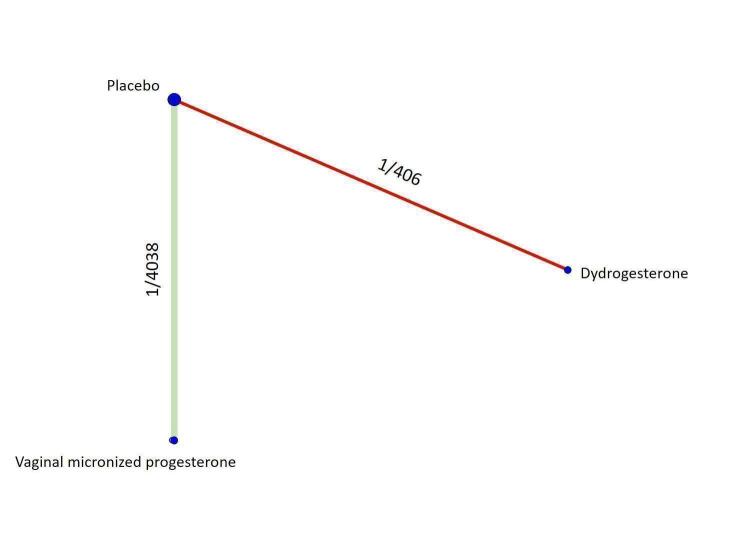
All the analyses presented in the Data and analyses section relate to the 'direct evidence' and were used to grade the certainty of the generated evidence. The analyses for the only possible indirect comparison of oral micronized progesterone versus placebo are described narratively and are included in the 'Summary of findings' tables, where available. For each outcome we analysed the evidence for threatened and recurrent miscarriage separately, and presented the network diagrams displaying the available comparisons and the grading of the direct evidence.
Threatened miscarriage
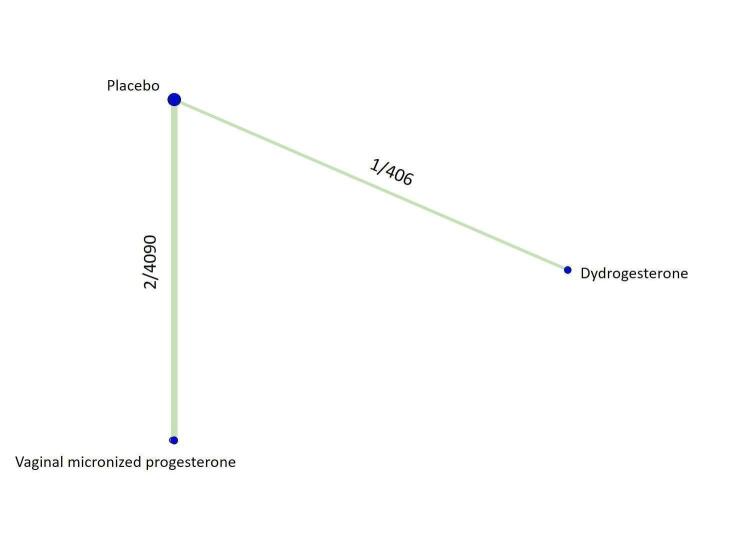
Primary outcome
Live birth
The network diagram for live birth in women with threatened miscarriage is presented in Figure 5. There were two available comparisons for this outcome. Vaginal micronized progesterone compared with placebo (2 trials, 4090 women), and dydrogesterone compared with placebo (1 trial, 406 women). Based on the relative effects from the pairwise meta‐analysis, the administration of vaginal micronized progesterone makes little or no difference to live birth rate when compared with placebo (risk ratio (RR) 1.03, 95% confidence interval (CI) 1.00 to 1.07, high‐certainty evidence, Table 1).
5.
Network diagram for the outcome of live birth in women with threatened miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence.
A subgroup analysis by number of previous miscarriages was only possible for vaginal micronized progesterone in women with threatened miscarriage. In women with no previous miscarriages and early pregnancy bleeding, there is probably no improvement in live birth rate when treated with vaginal micronized progesterone compared to placebo (RR 0.99, 95% CI 0.95 to 1.04, high‐certainty evidence, Table 1). However, for women with one or more previous miscarriages and early pregnancy bleeding, vaginal micronized progesterone increases the live birth rate compared to placebo (RR 1.08, 95% CI 1.02 to 1.14, high‐certainty evidence, Table 1). For the comparison of vaginal micronized progesterone against placebo, the subgroup analysis by maternal age did not reveal any substantial differences (Analysis 1.9).
1.9. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 9: Live birth (subgrouped by maternal age)
For the second comparison, we found that dydrogesterone probably makes little or no difference to live birth rate when compared with placebo (RR 0.98, 95% CI 0.89 to 1.07, moderate‐certainty evidence, Table 1). Subgroup analyses by number of previous miscarriages and maternal age were not possible from the evidence for dydrogesterone treatment of threatened miscarriage. An indirect comparison found that vaginal micronized progesterone probably makes little or no difference to live birth rate when compared with dydrogesterone in women with threatened miscarriage (RR 1.07, 95% CI 0.93 to 1.23, moderate‐certainty evidence, Table 1).
Secondary outcomes
Miscarriage (defined as delivery before 24 weeks of gestation)
The network diagram for miscarriage in women with threatened miscarriage is presented in Figure 6. There were three available comparisons for this outcome. Vaginal micronized progesterone compared with placebo (2 trials, 4090 women), dydrogesterone compared with placebo (1 trial, 406 women), and oral micronized progesterone compared with dydrogesterone (1 study, 118 women). Based on the relative effects from the pairwise meta‐analysis, we cannot rule out a substantial reduction in the miscarriage rate with vaginal micronized progesterone compared to placebo (RR 0.90, 95% CI 0.80 to 1.01, high‐certainty evidence, Table 2).
6.
Network diagram for the outcome of miscarriage in women with threatened miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence, orange for low‐certainty evidence, and red for very low‐certainty evidence.
We found that dydrogesterone is compatible with a wide range of treatment effects for miscarriage compared with placebo (RR 0.90, 95% CI 0.55 to 1.47, moderate‐certainty evidence, Table 2). An indirect comparison found that vaginal micronized progesterone probably makes little or no difference to miscarriage rate when compared with dydrogesterone in women with threatened miscarriage (RR 1.00, 95% CI 0.60 to 1.66, moderate‐certainty evidence, Table 2). For the direct comparison between oral micronized progesterone and dydrogesterone, and the indirect comparison of oral micronized progesterone against placebo and vaginal micronized progesterone versus oral micronized progesterone, the generated evidence was of very low‐certainty evidence. Therefore, these effects remain unclear (Table 2).
Preterm birth (defined as birth before 37 weeks of gestation)
The network diagram for preterm birth in women with threatened miscarriage is presented in Figure 7. There were two available comparisons for this outcome. Both vaginal micronized progesterone (2 trials, 3154 women), and dydrogesterone (1 trial, 334 women) were compared with placebo. Vaginal micronized progesterone (RR 1.08, 95% CI 0.92 to 1.27, moderate‐certainty evidence, Table 3) and dydrogesterone (RR 0.87, 95% CI 0.40 to 1.88, low‐certainty evidence, Table 3) are compatible with a wide range of treatment effects for the outcome of preterm birth when compared with placebo. An indirect comparison found that vaginal micronized progesterone is also compatible with a wide range of treatment effects to this outcome when compared with dydrogesterone in women with threatened miscarriage (RR 1.25, 95% CI 0.55 to 2.86, low‐certainty evidence, Table 3).
7.
Network diagram for the outcome of preterm birth in women with threatened miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence, and orange for low‐certainty evidence.
Stillbirth
The network diagram for stillbirth in women with threatened miscarriage is presented in Figure 8. Vaginal micronized progesterone was compared with placebo (1 trial, 4038 women), and dydrogesterone was also compared with placebo (1 trial, 406 women). Based on the results from the single study, for the outcome of stillbirth, vaginal micronized progesterone is compatible with a wide range of effects, when compared to placebo (RR 0.83, 95% CI 0.25, 2.71, low‐certainty evidence, Table 4). For the comparison of dydrogesterone against placebo, the generated evidence was of very low‐certainty evidence, and thus these effects remain unclear (Table 4). For the indirect comparison of vaginal micronized progesterone versus dydrogesterone, the generated evidence was of very low‐certainty evidence. Therefore, these effects remain unclear (Table 4).
8.
Network diagram for the outcome of stillbirth in women with threatened miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence, and red for very low‐certainty evidence.
Ectopic pregnancy
The network diagram for ectopic pregnancy in threatened miscarriage is presented in Figure 9. There was only one available comparison for this outcome. Vaginal micronized progesterone was compared with placebo (1 trial, 4038 women). Based on the relative effects from the single study, for the outcome of ectopic pregnancy, vaginal micronized progesterone is compatible with a wide range of effects, when compared to placebo (RR 0.20, 95% CI 0.01 to 4.14, low‐certainty evidence, Table 5).
9.
Network diagram for the outcome of ectopic pregnancy in women with threatened miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence.
Congenital abnormalities
The network diagram for congenital abnormalities in women with threatened miscarriage is presented in Figure 10. There were two available comparisons for this outcome. Vaginal micronized progesterone was compared with placebo (1 trial, 3085 women), and dydrogesterone was also compared with placebo (1 trial, 406 women). Based on the results from the single study, vaginal micronized progesterone probably makes no difference to the congenital abnormality rate in comparison to placebo, but the CIs are wide and compatible with a wide range of effects (RR 1.00, 95% CI 0.68 to 1.46, moderate‐certainty evidence, Table 6). For the second comparison, the generated evidence was of very low‐certainty evidence, and thus the relative effects of dydrogesterone compared to placebo remain unclear (Table 6). For the indirect comparison of vaginal micronized progesterone versus dydrogesterone, the generated evidence was of very low‐certainty evidence. Therefore, these effects remain unclear (Table 6).
10.
Network diagram for the outcome of congenital abnormalities in women with threatened miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence, and red for very low‐certainty evidence.
Adverse drug events
The network diagram for adverse drug events in women with threatened miscarriage is presented in Figure 11. There were two available comparisons for this outcome. Vaginal micronized progesterone was compared with placebo (1 trial, 4038 women), and dydrogesterone was also compared with placebo (1 trial, 406 women). Based on the results from the single study, vaginal micronized progesterone probably makes little or no difference to this outcome in comparison to placebo (RR 1.07, 95% CI 0.81 to 1.39, moderate‐certainty evidence, Table 7). For the comparison of dydrogesterone against placebo, the generated evidence was of very low‐certainty evidence. Therefore, these effects remain unclear (Table 7). For the indirect comparison of vaginal micronized progesterone versus dydrogesterone, the generated evidence was of very low‐certainty evidence. Therefore, these effects remain unclear (Table 7).
11.
Network diagram for the outcome of adverse drug events in women with threatened miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence, and red for very low‐certainty evidence.
Recurrent miscarriage
Primary outcome
Live birth
The network diagram for live birth in women with recurrent miscarriage is presented in Figure 12. There were two available comparisons for this outcome. Vaginal micronized progesterone compared with placebo (1 trial, 826 women), and dydrogesterone compared with placebo (1 trial, 40 women). Based on the relative effects from the single study, vaginal micronized progesterone probably makes little or no difference in live birth rate in comparison to placebo (RR 1.04, 95% CI 0.94 to 1.15, high‐certainty evidence, Table 1). A subgroup analysis for vaginal micronized progesterone by maternal age demonstrated that there is probably no difference in effectiveness when compared to the pooled analysis: women aged < 35 years (RR 1.04, 95% CI 0.92 to 1.18, high‐certainty evidence), women aged ≥ 35 years (RR 1.04, 95% CI 0.87 to 1.25, high‐certainty evidence). For the direct comparison of dydrogesterone with placebo, the generated evidence was of very low‐certainty evidence, therefore the effects remain unclear (Table 1). For the indirect comparison of vaginal micronized progesterone with dydrogesterone, the generated evidence was of very low‐certainty evidence. Therefore, these effects remain unclear (Table 1).
12.
Network diagram for the outcome of live birth in women with recurrent miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence, and red for very low‐certainty evidence.
Secondary outcomes
Miscarriage (defined as delivery before 24 weeks of gestation)
The network diagram for miscarriage in women with recurrent miscarriage is presented in Figure 13. There were three available comparisons for this outcome. Vaginal micronized progesterone was compared with placebo (1 trial, 826 women), dydrogesterone was compared with placebo (1 trial, 40 women) and 17‐α‐hydroxyprogesterone was compared with placebo (1 study, 50 women). Based on the relative effects from the single study, vaginal micronized progesterone probably makes little or no difference to the miscarriage rate in comparison to placebo (RR 0.96, 95% CI 0.79 to 1.17, high‐certainty evidence, Table 2). For the direct comparisons of dydrogesterone with placebo and 17‐α‐hydroxyprogesterone with placebo, and the indirect comparisons of vaginal micronized progesterone with dydrogesterone, dydrogesterone with 17‐α‐hydroxyprogesterone and vaginal micronized progesterone with 17‐α‐hydroxyprogesterone the generated evidence was of very low‐certainty evidence, therefore the effects remain unclear (Table 2).
13.
Network diagram for the outcome of miscarriage in women with recurrent miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence, orange for low‐certainty evidence, and red for very low‐certainty evidence.
Preterm birth (defined as birth before 37 weeks of gestation)
The network diagram for preterm birth in recurrent miscarriage is presented in Figure 14. There was one available comparison for this outcome where vaginal micronized progesterone was compared with placebo (1 trial, 533 women). Based on the relative effects from the single study, vaginal micronized progesterone is compatible with a wide range of treatment effects for preterm birth in comparison to placebo (RR 1.12, 95% CI 0.67 to 1.87, moderate‐certainty evidence, Table 3).
14.
Network diagram for the outcome of preterm birth in women with recurrent miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence.
Stillbirth
The network diagram for stillbirth in recurrent miscarriage is presented in Figure 15. There was one available comparison for this outcome where vaginal micronized progesterone was compared with placebo (1 trial, 826 women). Based on the relative effects from the single study, vaginal micronized progesterone is compatible with a wide range of treatment effects for stillbirth in comparison to placebo (RR 0.54, 95% CI 0.05 to 5.91, low‐certainty evidence, Table 4).
15.
Network diagram for the outcome of stillbirth in women with recurrent miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence.
Ectopic pregnancy
The network diagram for ectopic pregnancy in recurrent miscarriage is presented in Figure 16. There was one available comparison for this outcome where vaginal micronized progesterone was compared with placebo (1 trial, 826 women). Based on the relative effects from the single study, vaginal micronized progesterone is compatible with a wide range of treatment effects for ectopic pregnancy in comparison to placebo (RR 0.92, 95% CI 0.31 to 2.72, low‐certainty evidence, Table 5).
16.
Network diagram for the outcome of ectopic pregnancy in women with recurrent miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence.
Congenital abnormalities
The network diagram for congenital abnormalities in recurrent miscarriage is presented in Figure 17. There was one available comparison for this outcome where vaginal micronized progesterone was compared with placebo (1 trial, 542 women). Based on the relative effects from the single study, vaginal micronized progesterone may make little to no difference to the congenital abnormality rate in comparison to placebo (RR 0.75, 95% CI 0.31 to 1.85, low‐certainty evidence, Table 6).
17.
Network diagram for the outcome of congenital abnormalities in women with recurrent miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence.
Adverse drug events
The network diagram for adverse drug events in recurrent miscarriage is presented in Figure 18. There was one available comparison for this outcome where vaginal micronized progesterone was compared with placebo (1 trial, 836 women). Based on the relative effects from the single study, vaginal micronized progesterone is compatible with a wide range of treatment effects for adverse drug events in comparison to placebo (RR 1.46, 95% CI 0.93 to 2.29, moderate‐certainty evidence, Table 7).
18.
Network diagram for the outcome of adverse drug events in women with recurrent miscarriage. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is light green for moderate‐certainty evidence.
Statistical inconsistency and heterogeneity
Assessment of statistical inconsistency was not possible due to an absence of closed loops with the network. The pairwise meta‐analyses of two studies for the threatened miscarriage population comparing vaginal micronized progesterone and placebo showed low levels of heterogeneity: live birth, I2 = 24%; miscarriage, I2 = 0%; preterm birth, I2 = 1%. All other comparisons and interventions only included one study.
Discussion
Summary of main results
In summary, we reviewed seven trials, involving 5682 women in five countries. All trials were conducted in hospital settings and randomised women with threatened miscarriage in their current pregnancy, or a history of recurrent miscarriage. The following agents were used in the trial: vaginal micronized progesterone; dydrogesterone; oral micronized progesterone, and 17‐α‐hydroxyprogesterone. It was not possible to perform a network meta‐analysis and rank the available progestogens, because of the limited number of trials. Indirect comparison were possible for vaginal micronized progesterone versus dydrogesterone with the common comparator being placebo; oral micronized progesterone versus placebo with the common comparator being dydrogesterone; vaginal micronized progesterone versus oral micronized progesterone with the common comparators being placebo and dydrogesterone; dydrogesterone versus 17‐α‐hydroxyprogesterone with the common comparators being placebo; and vaginal micronized progesterone versus 17‐α‐hydroxyprogesterone with the common comparators being placebo.
The pooled analyses found that the available progestogen treatments overall make little to no difference in live birth and miscarriage rates for women with threatened miscarriage. Vaginal micronized progesterone is the only treatment that shows it may improve the live birth rates in comparison to placebo; however, this improvement in live birth is only observed in women with early pregnancy bleeding and previous history of at least one miscarriage. There is also evidence of a biological gradient of effect, with the improvement in live birth rate greatest in women with three or more previous miscarriages.
For the live birth and miscarriage outcomes, the evidence on vaginal micronized progesterone versus placebo was of high certainty, providing confidence that the true effect lies close to that of the effect estimate. For the other progestogens, the evidence is of moderate, low or very low‐certainty for all outcomes, indicating a high degree of uncertainty in the findings. An indirect comparison between vaginal micronized progesterone and dydrogesterone, oral micronized progesterone and placebo, vaginal micronized progesterone and oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone, and vaginal micronized progesterone and 17‐α‐hydroxyprogesterone could be made, but the available evidence is also of very low‐certainty evidence.
Vaginal micronized progesterone makes little or no difference to live birth rate when compared with placebo for women with recurrent miscarriage. The evidence for dydrogesterone compared with placebo for women with recurrent miscarriage is of very low certainty, therefore the effects remain unclear. No data are available to assess the effectiveness of 17‐α‐hydroxyprogesterone or oral micronized progesterone for the outcome of live birth in women with recurrent miscarriage.
All progestogen treatments have a wide range of effects on the other pre‐specified outcomes (miscarriage [< 24 weeks of gestation], preterm birth [< 37 weeks of gestation], stillbirth, ectopic pregnancy) in comparison to placebo for both threatened and recurrent miscarriage. Moderate‐certainty evidence with a wide range of effects suggests that probably there is no difference in congenital abnormalities and adverse drug events with vaginal micronized progesterone for threatened or recurrent miscarriage compared with placebo. There are limited data and very low‐certainty evidence on congenital abnormalities and adverse drug events for the other progestogens.
Overall completeness and applicability of evidence
This review set out to find the most effective progestogen treatment for the prevention of miscarriage. Seven trials met the inclusion criteria and reported results for our primary and secondary outcomes. Two included studies did not report the primary outcome for this review of live birth (Shearman 1963; Siew 2018); all studies reported the miscarriage outcome. Trials recruited women who were pregnant and experiencing early pregnancy bleeding (threatened miscarriage) or had a history of three or more previous miscarriages (recurrent miscarriage). Women with significant co‐morbidities were largely excluded from all trials. The most frequent intervention reported was vaginal micronized progesterone compared with placebo. The dosage and route of administration for each progestogen also varied by trial (Characteristics of included studies). A sensitivity analysis and the planned network meta‐analysis were not performed given the paucity of trials. Further trials are yet to report, which should allow for a more complete set of available comparisons in the future (see Characteristics of ongoing studies).
Quality of the evidence
We applied the appraising method proposed by the GRADE Working Group. Our confidence in the effect estimates of this review ranged from very low to high with the majority of the available evidence being of moderate certainty. See Table 1, Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7. Downgrading decisions related mainly to limitations in imprecision and study design. For the primary outcome of live birth, we have a range in the confidence of where the true effect estimates might lie, depending on the intervention, ranging from low to high levels of confidence. Overall, only some of the comparisons involving vaginal micronized progesterone generated evidence of high certainty. In all cases, the evidence from the direct comparison between dydrogesterone and placebo was of low or very low certainty. The single trial that provided data on the direct comparison of 17‐α‐hydroxyprogesterone and placebo is of very low certainty. For our indirect comparisons of vaginal micronized progesterone and dydrogesterone, oral micronized progesterone and placebo, vaginal micronized progesterone and oral micronized progesterone, dydrogesterone and 17‐α‐hydroxyprogesterone, and vaginal micronized progesterone and 17‐α‐hydroxyprogesterone, the evidence is of very low certainty. One out of the seven included trials (Figure 4) comparing two oral active treatments was judged to be at high risk of bias regarding the blinding of participants and personnel. One other trial (Figure 4) was judged to be at high risk of bias regarding incomplete outcome data since the lost to follow‐up rate was greater than 10%.
Potential biases in the review process
Three review authors have been involved in two of the included trials. For the purpose of this review, tasks such as assessment for inclusion/exclusion, trial quality, data extraction, risk of bias and grade assessment were carried out by other members of the team who were not directly involved in these two protocols (AP, MP). For all other trials, all these tasks were carried out by two review authors independently (AJD, AP) and checked where necessary by a third independent author (IDG). The literature search was conducted by a specialist, independent member of the Cochrane Pregnancy and Childbirth group.
Agreements and disagreements with other studies or reviews
Our results are consistent with the existing Cochrane Reviews (Haas 2019; Wahabi 2018); however this review has identified that for women with one or more previous miscarriages and early pregnancy bleeding, treatment with vaginal micronized progesterone is of particular benefit. The review has reclassified some of the existing trials into trials awaiting classification as more evidence of trustworthiness is pending from the trial authors.
Authors' conclusions
Implications for practice.
The results of this review suggest that vaginal micronized progesterone may be effective in the treatment of threatened miscarriage for women with a history of one or more previous miscarriages. The current evidence suggests that no other types of progestogen are effective at treating women with either threatened or recurrent miscarriage. There was no difference in congenital abnormalities and adverse drug events with vaginal micronized progesterone for threatened or recurrent miscarriage.
Implications for research.
A uniform core outcome set would aid future evidence synthesis, with particular focus on the number of previous miscarriages that participants have experienced, and establishing a pre‐specified subgroup analysis of this key risk factor. More high‐quality data comparing the different types of progestogens would also be beneficial since a network meta‐analysis was not possible due to a paucity of trials.
History
Protocol first published: Issue 11, 2020 Review first published: Issue 4, 2021
Acknowledgements
As part of the pre‐publication editorial process, this protocol has been commented on by five peers (an editor and four referees who are external to the editorial team), a member of our international panel of consumers, and our Group's Statistical Adviser. The review authors are grateful to the following peer reviewers for their time and comments: Dr LC Morley (MRC Clinical Research Fellow, Leeds Institute of Cardiovascular and Metabolic Medicine, School of Medicine, University of Leeds, UK); Dr Nigel AB Simpson (Senior Lecturer in Obstetrics and Gynaecology, Division of Women's and Children's Health, School of Medicine, University of Leeds, UK); and to two referees who wish to remain anonymous.
This project was supported by the National Institute for Health Research (NIHR), via Evidence Synthesis Programme funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
This project is funded by the National Institute for Health Research (NIHR) [NIHR Review Incentive Award number (NIHR133289). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods for Cochrane Central Register of Controlled Trials, Ovid MEDLINE(R), ClinicalTrials.gov and WHO International Clinical Trials Registry (ICTRP) Platform
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to December 15, 2020>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Abortion, Spontaneous/
2 Abortion, Habitual/
3 Abortion, Threatened/
4 Embryo Loss/
5 (pregnan* adj3 (failure* or loss or losses)).mp.
6 (abortion adj2 (habitual or recurrent or threaten* or spontaneous)).tw.
7 (bleeding adj4 ("early pregnancy" or "first trimester" or "1st trimester" or vaginal*)).tw.
8 miscarriage*.tw.
9 Fetal Death/
10 f?tal loss*.tw.
11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
12 exp Progesterone/ or Progestins/ or progest*.mp.
13 Dydrogesterone/ or dydrogesterone.mp.
14 17‐alpha‐Hydroxyprogesterone/ or 17 alpha‐Hydroxyprogesterone Caproate/ or ("17 alpha‐Hydroxyprogesterone" or "17 alpha‐Hydroxy‐progesterone" or "17 α‐Hydroxyprogesterone" or "17α‐Hydroxy‐progesterone" or "17alpha‐Hydroxyprogesterone" or "17alpha‐Hydroxy‐progesterone" or "17α‐Hydroxyprogesterone" or "17α‐Hydroxy‐progesterone" or "17‐OH" or "17‐OHPC").mp.
15 Medroxyprogesterone/ or medroxyprogesterone.mp.
16 12 or 13 or 14 or 15
17 11 and 16
18 (Controlled Clinical Trial or Randomized Controlled Trial).pt.
19 (randomi?ed or placebo or randomly or trial or groups).ab.
20 drug therapy.fs.
21 18 or 19 or 20
22 (exp Animals/ not Humans/) or (animal or animals or (assisted adj3 reproduct*) or bitches or bovine or canine or cattle or climacteric or contraception or contraceptive or contraceptives or cow or cows or dog or dogs or (embryo* adj3 (frozen or transfer)) or ewe or ewes or heifers or "hormone replacement" or HRT or "in vitro" or IVF or "intraturine devices" or IUD or IUDs or mares or menopaus* or mice or mouse or ovine or postmenopaus* or post‐menopaus* or rat or rats or rattus).ti.
23 21 not 22
24 17 and 23
Cochrane Central Register of Controlled Trials
Issue 12 of 12, December 2020
#1 MeSH descriptor: [Abortion, Spontaneous] explode all trees
#2 MeSH descriptor: [Abortion, Habitual] explode all trees
#3 MeSH descriptor: [Abortion, Threatened] explode all trees
#4 MeSH descriptor: [Embryo Loss] explode all trees
#5 pregnan* NEAR/3 (failure* or loss or losses)
#6 abortion NEAR/2 (habitual or recurrent or threaten* or spontaneous)
#7 bleeding NEAR/4 ("early pregnancy" or "first trimester" or "1st trimester" or vaginal*)
#8 miscarriage
#9 MeSH descriptor: [Fetal Death] explode all trees
#10 "fetal loss*" or "foetal loss*"
#11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
#12 MeSH descriptor: [Progesterone] explode all trees
#13 MeSH descriptor: [Progestins] explode all trees
#14 progest*
#15 dydrogesterone
#16 ("17 alpha‐Hydroxyprogesterone" or "17 alpha‐Hydroxy‐progesterone" or "17 α‐Hydroxyprogesterone" or "17α‐Hydroxy‐progesterone" or "17alpha‐Hydroxyprogesterone" or "17alpha‐Hydroxy‐progesterone" or "17α‐Hydroxyprogesterone" or "17α‐Hydroxy‐progesterone" or "17‐OH" or "17‐OHPC")
#18 MeSH descriptor: [17‐alpha‐Hydroxyprogesterone] explode all trees
#19 MeSH descriptor: [17 alpha‐Hydroxyprogesterone Caproate] explode all trees
#20 medroxyprogesterone.
#21 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19
#22 #11 and #21
#23 ((assisted NEAR/3 reproduct*) or climacteric or contraception or contraceptive or contraceptives or (embryo* NEAR/3 (frozen or transfer)) or "hormone replacement" or HRT or "in vitro" or IVF or "intraturine devices" or IUD or IUDs or menopaus* or postmenopaus* or post‐menopaus*):ti (Word variations have been searched)
#24 #107 not #108
Clinicaltrials.gov
Terms and Synonyms
Progesterone, Utrogestan, crinone, Progesteron, prometrium, BHR‐100, Corpus Luteum Hormones,
Cyclogest, Endometrin, Pregnenediones, prochieve, progest, Progesterona, Proluton, Pregnancy Loss, Abortion, Pregnancy with abortive outcome, Miscarriage, Spontaneous Abortions, Fetal Death,
Fetal Demise, fetus death, foetal death, Intrauterine death.
WHO ICTRP:
miscarriage and progesterone*
Miscarriage and dydrogesteron*
Data and analyses
Comparison 1. Threatened miscarriage: Vaginal micronized progesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Live birth | 2 | 4090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [1.00, 1.07] |
| 1.2 Miscarriage (defined as delivery before 24 weeks of gestation) | 2 | 4090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.80, 1.01] |
| 1.3 Preterm birth (defined as birth before 37 weeks of gestation) | 2 | 3154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.27] |
| 1.4 Stillbirth | 1 | 4038 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.71] |
| 1.5 Ectopic pregnancy | 1 | 4038 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.14] |
| 1.6 Congenital abnormalities | 1 | 3085 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.68, 1.46] |
| 1.7 Adverse drug events | 1 | 4038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.81, 1.39] |
| 1.8 Live birth (subgrouped by no previous miscarriages and one or more previous miscarriages) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 No previous miscarriages and early pregnancy bleeding | 2 | 2261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.04] |
| 1.8.2 One or more previous miscarriages and early pregnancy bleeding | 2 | 1829 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.02, 1.14] |
| 1.9 Live birth (subgrouped by maternal age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 Maternal age < 35 years | 1 | 3113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.99, 1.07] |
| 1.9.2 Maternal age ≥ 35 years | 1 | 925 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.95, 1.13] |
1.1. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 1: Live birth
1.2. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 2: Miscarriage (defined as delivery before 24 weeks of gestation)
1.3. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 3: Preterm birth (defined as birth before 37 weeks of gestation)
1.4. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 4: Stillbirth
1.5. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 5: Ectopic pregnancy
1.6. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 6: Congenital abnormalities
1.7. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 7: Adverse drug events
1.8. Analysis.

Comparison 1: Threatened miscarriage: Vaginal micronized progesterone versus placebo, Outcome 8: Live birth (subgrouped by no previous miscarriages and one or more previous miscarriages)
Comparison 2. Threatened miscarriage: Dydrogesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Live birth | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.07] |
| 2.2 Miscarriage (defined as delivery before 24 weeks of gestation) | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.55, 1.47] |
| 2.3 Preterm birth (defined as birth before 37 weeks of gestation) | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.40, 1.88] |
| 2.4 Stillbirth | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.13] |
| 2.5 Congenital abnormalities | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.21] |
| 2.6 Adverse drug events | 1 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 21.88] |
2.1. Analysis.

Comparison 2: Threatened miscarriage: Dydrogesterone versus placebo, Outcome 1: Live birth
2.2. Analysis.

Comparison 2: Threatened miscarriage: Dydrogesterone versus placebo, Outcome 2: Miscarriage (defined as delivery before 24 weeks of gestation)
2.3. Analysis.

Comparison 2: Threatened miscarriage: Dydrogesterone versus placebo, Outcome 3: Preterm birth (defined as birth before 37 weeks of gestation)
2.4. Analysis.

Comparison 2: Threatened miscarriage: Dydrogesterone versus placebo, Outcome 4: Stillbirth
2.5. Analysis.

Comparison 2: Threatened miscarriage: Dydrogesterone versus placebo, Outcome 5: Congenital abnormalities
2.6. Analysis.

Comparison 2: Threatened miscarriage: Dydrogesterone versus placebo, Outcome 6: Adverse drug events
Comparison 3. Threatened miscarriage: Oral micronized progesterone versus dydrogesterone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Miscarriage (defined as delivery before 24 weeks of gestation) | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.75] |
| 3.2 Adverse drug events | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
3.1. Analysis.

Comparison 3: Threatened miscarriage: Oral micronized progesterone versus dydrogesterone, Outcome 1: Miscarriage (defined as delivery before 24 weeks of gestation)
3.2. Analysis.

Comparison 3: Threatened miscarriage: Oral micronized progesterone versus dydrogesterone, Outcome 2: Adverse drug events
Comparison 4. Recurrent miscarriage: Vaginal micronized progesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Live birth | 1 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.15] |
| 4.2 Miscarriage (defined as delivery before 24 weeks of gestation) | 1 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.17] |
| 4.3 Preterm birth (defined as birth before 37 weeks of gestation) | 1 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.67, 1.87] |
| 4.4 Stillbirth | 1 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.05, 5.91] |
| 4.5 Ectopic pregnancy | 1 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.31, 2.72] |
| 4.6 Congenital abnormalities | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.31, 1.85] |
| 4.7 Adverse drug events | 1 | 836 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.93, 2.29] |
| 4.8 Live birth (subgrouped by maternal age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.8.1 Maternal age < 35 years | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.18] |
| 4.8.2 Maternal age ≥ 35 years | 1 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.25] |
4.1. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 1: Live birth
4.2. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 2: Miscarriage (defined as delivery before 24 weeks of gestation)
4.3. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 3: Preterm birth (defined as birth before 37 weeks of gestation)
4.4. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 4: Stillbirth
4.5. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 5: Ectopic pregnancy
4.6. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 6: Congenital abnormalities
4.7. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 7: Adverse drug events
4.8. Analysis.

Comparison 4: Recurrent miscarriage: Vaginal micronized progesterone versus placebo, Outcome 8: Live birth (subgrouped by maternal age)
Comparison 5. Recurrent miscarriage: Dydrogesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Live birth | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 5.2 Miscarriage (defined as delivery before 24 weeks of gestation) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.23, 4.37] |
5.1. Analysis.

Comparison 5: Recurrent miscarriage: Dydrogesterone versus placebo, Outcome 1: Live birth
5.2. Analysis.

Comparison 5: Recurrent miscarriage: Dydrogesterone versus placebo, Outcome 2: Miscarriage (defined as delivery before 24 weeks of gestation)
Comparison 6. Recurrent miscarriage: 17‐α‐hydroxyprogesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Miscarriage (defined as delivery before 24 weeks of gestation) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.28, 2.58] |
6.1. Analysis.

Comparison 6: Recurrent miscarriage: 17‐α‐hydroxyprogesterone versus placebo, Outcome 1: Miscarriage (defined as delivery before 24 weeks of gestation)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chan 2020.
| Study characteristics | ||
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 406 women were randomised in a hospital setting in Hong Kong from March 2016 to May 2018. The population comprised women aged 18 to 40 years of age, presence of intrauterine gestational sac(s) only if a urine pregnancy test was first positive within the past 2 weeks or presence of intrauterine fetus(es) with positive fetal heart pulsations or presence of intrauterine fetus(es) with crown‐rump length of < 7 mm and no fetal pulsation on pelvic scanning; and absence of fever (temperature 38.5o C). Exclusion criteria comprised women that history of recurrent miscarriage defined as 3 or more consecutive spontaneous miscarriages; history of known parental chromosomal abnormalities; heavy vaginal bleeding or severe abdominal pain requiring surgical intervention; absence of cardiac pulsation in a fetal pole with crown‐rump length of 7 mm on transvaginal scanning; use of hCG or progestogen for threatened miscarriage prior to recruitment; or women with current or suspected breast or genital cancers, hepatic disease or tumours. | |
| Interventions | 40 mg dydrogesterone orally, followed by 30 mg dydrogesterone 3 times a day versus placebo. | |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth, miscarriage (< 20 weeks)*, preterm birth, stillbirth, congenital abnormalities and adverse drug events. *different time point to that specified in the protocol (miscarriage defined as < 24 weeks). |
|
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. This study was funded by the Health and Medical Research Fund, HKSAR (reference number 12132341). The authors declare that they have no competing interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated in 1:1 ratio in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition bias was < 10%. |
| Selective reporting (reporting bias) | High risk | The study report matches the study protocol that was registered prospectively (NCT02128685). However, results for 1 of the prespecified outcomes, i.e. proportion of heavy vaginal bleeding or severe abdominal pain requiring surgical intervention (< 20 weeks), were not reported. |
| Other bias | Low risk | This study was funded by the Health and Medical Research Fund, HKSAR (reference number 12132341). |
Coomarasamy 2015.
| Study characteristics | ||
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 836 women were randomised in a hospital setting in the UK from June 2010 to October 2013. The population comprised women aged 18‐39 years with a history of 3 or more unexplained miscarriages and conceived naturally within 1 year of being approached about the trial. Exclusion criteria comprised women that were unable to conceive naturally within 1 year after recruitment; had the antiphospholipid syndrome or other recognized thrombophilic conditions; had uterine cavity abnormalities (as assessed with the use of ultrasonography, hysterosonography, hysterosalpingogram, or hysteroscopy), an abnormal parental karyotype, or other identifiable cause of recurrent miscarriage such as diabetes, thyroid disease, or systemic lupus erythematosus (tests were initiated only if clinically indicated); were currently receiving heparin therapy; or had contraindications to progesterone use. | |
| Interventions | Twice daily vaginal suppositories containing 400 mg of micronized progesterone versus placebo. | |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth, miscarriage (< 24 weeks), preterm birth (< 37 weeks), stillbirth, ectopic pregnancy, congenital abnormalities and adverse drug events. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. Funded by the United Kingdom NIHR Health Technology Assessment program (project number HTA 08/38/01). The authors declare no relevant conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated in 1:1 ratio with the use of minimisation. |
| Allocation concealment (selection bias) | Low risk | Assignment through secure Internet facility. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition bias was < 10% and balanced across study arms. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol (ISRCTN92644181) that was registered prospectively. |
| Other bias | Low risk | Funded by the United Kingdom NIHR Health Technology Assessment program (project number HTA 08/38/01). |
Coomarasamy 2019.
| Study characteristics | ||
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 4153 women were randomised in a hospital setting in the UK from May 2015 to June 2017. The population comprised women aged 16 to 39 years of age, if they had completed less than 12 weeks of pregnancy, if they presented with vaginal bleeding, and if they had an intrauterine gestational sac that was visible on ultrasonography. Exclusion criteria comprised women that if at the time of presentation the fetal crown–rump length was 7 mm or longer with no visible heartbeat; if the gestational sac was a mean of 25 mm or greater in diameter with no visible fetal pole on ultrasonography; if they had evidence of ectopic pregnancy; if they had life‐threatening bleeding; if they had current or recent use of progesterone supplementation; if they had contraindications to progesterone therapy (i.e. a history of liver tumours; current genital or breast cancer, severe arterial disease, or acute porphyria; or a history during pregnancy of idiopathic jaundice, severe pruritus, or pemphigoid gestationis); or if they were participating in any other blinded, placebo controlled trials of medicinal products in pregnancy. | |
| Interventions | Twice daily vaginal suppositories containing 400 mg of micronized progesterone versus placebo. | |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth, miscarriage (< 24 weeks), preterm birth (< 37 weeks), stillbirth, ectopic pregnancy, congenital abnormalities and adverse drug events. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. Funded by the United Kingdom NIHR Health Technology Assessment program (project number HTA 12/167/26). The authors declare no relevant conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated in 1:1 ratio with the use of minimization. |
| Allocation concealment (selection bias) | Low risk | Through a secure, centralised Internet facility. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition bias was < 10% and balanced across study arms. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol (ISRCTN14163439) that was registered prospectively. |
| Other bias | Low risk | Funded by the United Kingdom NIHR Health Technology Assessment programme (project number HTA 12/167/26). |
Gerhard 1987.
| Study characteristics | ||
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | 52 women were randomised in a hospital setting in Germany between 1983 and 1984. The population comprised women that presented with vaginal bleeding and a closed internal cervical os. | |
| Interventions | 1 vaginal suppository twice daily, containing 25 mg progesterone versus placebo. | |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth, miscarriage (< 24 weeks) and preterm birth (< 37 weeks). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. Source(s) of funding were not reported, declarations of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition bias was > 10% balanced across study arms. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. However, this study was conducted before protocol registration became mandatory. |
| Other bias | Unclear risk | Source(s) of funding were not reported. |
MacDonald 1972.
| Study characteristics | ||
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | 40 women were randomised in a hospital setting in the UK (date range not specified). The population comprised women with a history of 2 or more consecutive previous miscarriages and then subsequent pregnancy with cervical mucus ferning present. | |
| Interventions | 2 x 5 mg tablets of dydrogesterone tablets 3 times daily, increased to 4 tablets 3 times daily if ferning persisted, no duration specified versus placebo. | |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth and miscarriage (< 24 weeks). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. Source(s) of funding were not reported, declarations of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. However, this study was conducted before protocol registration became mandatory. |
| Other bias | Unclear risk | Source(s) of funding were not reported. |
Shearman 1963.
| Study characteristics | ||
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | 50 women were randomised in a hospital setting in Australia (date range not specified). The population comprised women with a history of 2 or more previous miscarriages. Exclusion criteria comprised women with persistently normal levels of pregnanediol. | |
| Interventions | Up to 8 weeks' gestation: 250 mL/week IM hydroxyprogesterone; 8‐11 weeks' gestation: 375 mL/week IM of 17‐a‐hydroxyprogesterone; 12‐16 weeks' gestation: 500 mL/week IM of 17‐a‐hydroxyprogesterone; 17‐20 weeks' gestation: 375 mg/week IM of 17‐a‐hydroxyprogesterone; 21‐24 weeks' gestation: 250 mg/week IM of 17‐a‐hydroxyprogesterone versus placebo. | |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. The study was funded by Schering AG. Declarations of interest were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. However, this study was conducted before protocol registration became mandatory. |
| Other bias | Low risk | No other bias noted. |
Siew 2018.
| Study characteristics | ||
| Methods | 2‐arm active‐controlled randomised trial | |
| Participants | 141 women were randomised in a hospital setting in Singapore between January 2014 and February 2017. The population comprised women aged 21 to 45 years with a single intrauterine pregnancy, gestational age of 6‐10 weeks, and presented with pregnancy‐related vaginal bleeding. Exclusion criteria comprised women that had previously used a progestogen in the current pregnancy, women with inevitable miscarriage, planning to terminate their pregnancy, women who conceived using assisted reproductive technology, women known to have recurrent miscarriages, and women who have pre‐existing luteal phase deficiency or other forms of diagnosed progesterone deficiency. | |
| Interventions | 200 mg oral micronized progesterone twice daily versus 10 mg oral dydrogesterone twice daily. | |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage (≤ 16 weeks)*, adverse drug events. *different time point to that specified in the protocol (miscarriage defined as < 24 weeks). |
|
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. Funded by the Khoo Student Research Award and Pitch for Grant Award. The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Participants were randomised in blocks of size 4 using a pre‐determined randomisation schedule generated by a biostatistician.'' |
| Allocation concealment (selection bias) | Unclear risk | Small block randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was an open‐label study. It is highly unlikely that the outcome was influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition bias was < 10% and balanced across study arms. |
| Selective reporting (reporting bias) | Unclear risk | This study report matches the study protocol (ChiCTR‐IOR‐17011593) that was registered retrospectively. |
| Other bias | Low risk | This study was funded by the Khoo Student Research Award and Pitch for Grant Award. |
hCG: Human chorionic gonadotropin; IM: intramuscular.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2020 | Not eligible population. |
| Beigi 2016 | Trial includes both 1st and 2nd trimester treatment and does not subgroup the effects, therefore data cannot be included. |
| Berle 1977 | Combination therapy of progesterone and oestrogen was used in this study. |
| Berle 1980 | Quasi‐randomised trial, study type which is excluded from this review. |
| Check 1985 | No method of randomisation was used for this trial. |
| Check 1987 | It is unclear as to whether there was any randomisation. The authors of this review attempted, but failed, to contact the trial authors. |
| Check 1987a | It is unclear as to whether there was any randomisation. The authors of this review attempted, but failed, to contact the trial authors. |
| Check 1987b | It is unclear as to whether there was any randomisation. The authors of this review attempted, but failed, to contact the trial authors. |
| Check 1995 | The intervention considered in this trial is progesterone in association with immunotherapy rather than progesterone alone. |
| Chi 2019 | Progestogen supplementation for luteal phase support. |
| Clifford 1996 | Not an eligible population. |
| Corrado 2002 | Not an eligible population. |
| Costantino 2016 | The intervention is not eligible for inclusion in this review. |
| Daya 1988 | This trial was not a randomised controlled trial. |
| Devine 2018 | Not an eligible population. |
| El‐Zibdeh 2005 | Quasi‐randomised trial, which are excluded from this review. |
| El‐Zibdeh 2009 | Quasi‐randomised trial, which are excluded from this review. |
| EUCTR2016‐002777‐35‐IT | This trial was terminated early due to difficulty in recruitment. Therefore, no data are available for analysis. |
| Fuchs 1966 | This trial was terminated before the results were of sufficient size to statistically analyse. Therefore, data are incomplete. |
| Goldzieher 1964 | Trials using medroxyprogesterone are excluded from this review. |
| Govaerts‐Videtzky 1965 | No method of randomisation was used for this trial. |
| Ismail 2018 | This publication has been formally withdrawn by the journal. |
| Johnson 1975 | It is not clear from the manuscript when progestogen treatment was commenced. Since the outcome measure reported in this trial was preterm delivery and not miscarriage, treatment was most probably commenced in the 2nd trimester. |
| Klopper 1965 | Trials using progesterone 3‐cyclopentyl enol ether are excluded from this review. |
| LeVine 1964 | Quasi‐randomised trial, which are excluded from this review. |
| Moller 1966 | The intervention is not eligible for inclusion in this review. |
| Nyboe Andersen 2002 | Not an eligible population. |
| Ozer 2020 | Not eligible population. |
| Porcaro 2015 | The intervention is not eligible for inclusion in this review. |
| Prietl 1992 | The active intervention given in this trial was a combination of progestogen and oestrogen. |
| Pustotina 2018 | Progestogen supplementation in 2nd trimester, therefore excluded from this review. |
| Rehal 2020 | Progestogen supplementation in 2nd trimester, therefore excluded from this review. |
| Reijnders 1988 | The trial population is not eligible for inclusion in this review. |
| Shu 2002 | Intervention group administered Chinese herbal medicine (CHM) plus human chorionic gonadotropin and progesterone, compared with multi‐vitamin. |
| Sondergaard 1985 | This trial was conducted to ascertain the efficacy of progesterone to prevent preterm birth rather than miscarriage. |
| Song 2007 | Combination progestogen with vitamin E, therefore excluded from this review. |
| Souka 1980 | Trial includes both 1st and 2nd trimester treatment and does not subgroup the effects, therefore data cannot be included. |
| Swyer 1953 | Quasi‐randomised trial, which are excluded from this review. |
| Tognoni 1980 | No method of randomisation was used for this trial. |
| Tomic 2015 | Progestogen supplementation for luteal phase support, therefore excluded from this review. |
| Zhang 2000 | Combination progestogen with vitamin E, therefore excluded from this review. |
Characteristics of studies awaiting classification [ordered by study ID]
Abrar 2017.
| Methods | 2‐arm active‐controlled randomised trial. |
| Participants | 98 women were randomised in a hospital setting in Pakistan. The population comprised women aged 15‐45 years with threatened miscarriage in their first trimester (up to 12 weeks of gestation). Exclusion criteria comprised women with history of trauma during pregnancy or a history of bleeding disorders. |
| Interventions | 10 mg oral progesterone, twice daily for 1 week versus 400 mg vaginal progesterone, once daily for 1 week. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Agarwal 2016.
| Methods | 2‐arm no treatment‐controlled randomised trial. |
| Participants | Women with a history of 2 or more consecutive miscarriages of unknown cause, aged between 21 and 40 years with euthyroid state and normal thyroid function tests. Women with a history of repeated miscarriage of known cause will be excluded. |
| Interventions | 200 mg oral micronized progesterone, given twice daily until 16 weeks of gestation versus no treatment |
| Outcomes | Miscarriage |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Alimohamadi 2013.
| Methods | 2‐arm placebo‐controlled randomised trial. |
| Participants | 160 women were randomised in a hospital setting in Iran. The population comprised women with clinical symptoms of threatened abortion (bleeding, spotting, and uterine cramps before the 20th week of pregnancy) and singleton pregnancy. Exclusion criteria comprised women with systemic diseases, maternal hypertension before or during pregnancy, uterine tenderness, genetic or anatomical defects of the fetus, renal or cardiac diseases, genital tract anomalies of the mother and diabetes and those patients who had used a progestational drug during pregnancy, prior to being recruited into the study. |
| Interventions | 200 mg vaginal progesterone, twice daily for 1 week versus placebo. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage and preterm birth. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, who replied but did not answer all of our queries or provide the requested documentation or data. The trial was retrospectively registered and no explanation for this was provided. |
Czajkowski 2007.
| Methods | 2‐arm active‐controlled randomised trial |
| Participants | 53 women were randomised in a hospital setting in Poland. The population comprised women with signs and symptoms of threatened abortion (vaginal bleeding usually accompanied by abdominal pains), live singleton intrauterine pregnancy confirmed by ultrasound examination, and gestational age of 12 weeks. Exclusion criteria comprised women with a history of hypertension, diabetes mellitus, severe liver disorders (i.e. Rotor syndrome, Dubin‐Johnson syndrome, jaundice, or liver failure), drug or alcohol addiction, uterine anomalies, cervical disease, cerebral apoplexy, and allergy to any component of the drugs that would be administered in the study. Women who had received any hormonal drug during the last 3 weeks preceding the study or had participated any other clinical trial during the last 3 months were also excluded. |
| Interventions | 300 mg micronized vaginal progesterone daily plus oral placebo versus 30 mg of oral dydrogesterone daily plus vaginal placebo for 6 weeks. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Ghosh 2014.
| Methods | 2‐arm active‐controlled randomised trial, plus a non‐intervention control group. |
| Participants | 101 women were randomised in a hospital setting in India. The population comprised women with 3 or more consecutive miscarriages within the first trimester (up to 12 weeks of gestation) followed by spontaneous conception that was euthyroid, normoprolactinemic. Only women who had not received any medication in the last 3 months were considered. Exclusion criteria comprised women who had a cause for their recurrent miscarriages identified using 1 or more of the following tests: thyroid‐stimulating hormone and antithyroid antibody tests, antiphospholipid antibodies test (anticardiolipin antibodies and lupus anticoagulants immunoglobulin G and M), TORCH (toxoplasmosis, rubella, cytomegalovirus and herpes) tests, paternal and maternal chromosomal analysis, hysterosalpingography, and hysteroscopy to rule out uterine defects, abnormal fasting level of homocysteine, exclusion of diabetes mellitus, and estimation of midluteal serum progesterone to exclude luteal phase defect. |
| Interventions | 10 mg oral dydrogesterone, twice daily versus 100 mg micronized vaginal progesterone, thrice daily. Women without history of recurrent miscarriage in the control group did not receive any progestogen. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Kumar 2014.
| Methods | 2‐arm placebo‐controlled randomised trial, plus a non‐intervention control group. |
| Participants | 360 women were randomised in a hospital setting in India. The population comprised women aged 18‐35 years with a history of idiopathic, ≥ 3 first‐trimester pregnancy losses and currently in the first trimester with a live pregnancy (preferably 2‐8 weeks' gestation). Exclusion criteria comprised women with a known cause of recurrent miscarriage, women who had taken an injection of hCG or hydroxyprogesterone. |
| Interventions | 20 mg oral dydrogesterone (taken as 2 x 10 mg tablets) from time of enrolment to 20 weeks versus placebo. The trial also included an additional control group of women without a history of miscarriage who were age‐matched, healthy, pregnant women with at least 1 live birth. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage and preterm birth. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, who replied but did not answer all of our queries or provide the requested data by the time that this review was published. |
Omar 2005.
| Methods | 2‐arm no treatment‐controlled randomised trial. |
| Participants | 154 women were randomised in a hospital setting in Malaysia. The population comprised women with mild or moderate vaginal bleeding, no history of loss of conception material, absence of systemic illness or fever, normal size and shape gestation sac at 5 weeks, presence of yolk sac at 5–6 weeks, presence of fetal heart at 7 weeks and a gestational age less than 13 weeks. Women were excluded from the study if they had either an empty sac of more than 26 mm or history of recurrent miscarriage. |
| Interventions | Dydrogesterone 40 mg stat, followed by 10 mg twice a day until the bleeding stopped versus no treatment. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Palagiano 2004.
| Methods | 2‐arm placebo‐controlled randomised trial. |
| Participants | 50 women were randomised in a hospital setting in Italy. The population comprised women with threatened abortion, an ongoing, viable pregnancy, amenorrhoea between 6 and 12 weeks' gestation, and a closed uterine cervix. Exclusion criteria comprised women with a previous adequate luteal phase, women who were using hormonal treatment or other drugs affecting uterine contractility, women with vaginal infection, absence of embryo’s heartbeat, open cervix (> 2 cm measured by ultrasound), and embryo’s size 1 week more than the corresponding amenorrhoea. |
| Interventions | 90 mg micronized vaginal progesterone once daily for 5 days versus placebo. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Pandian 2009.
| Methods | 2‐arm no treatment‐controlled randomised trial. |
| Participants | 191 women were randomised in a hospital setting in Malaysia. The population comprised women with threatened abortion, no systematic illness or fever and no loss of conception tissue. Viability of the fetus was confirmed by ultrasound. Exclusion criteria comprised women with recurrent miscarriage (> 3), heavy bleeding, cervical polyp, multiple gestation, empty sac > 26 mm. |
| Interventions | 40 mg oral dydrogesterone followed by 10 mg twice daily versus conservative treatment with bed rest only. |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth, miscarriage, preterm birth and congenital abnormalities. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Turgal 2017.
| Methods | 2‐arm placebo‐controlled randomised trial. |
| Participants | 83 women were randomised in a hospital setting in Turkey. The population comprised women with threatened abortion and with presence singleton pregnancy and live embryo, before 9 weeks of gestation. Exclusion criteria comprised women with a non‐viable fetus, twin pregnancy, presence of subchorionic haematoma and history of hypertension, diabetes mellitus, severe hepatic disorders, uterine leiomyoma, congential uterine anomaly and recurrent pregnancy loss. |
| Interventions | 400 mg micronized oral progesterone daily for 4 weeks versus placebo. |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth and miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Vincze 2006.
| Methods | 2‐arm active‐controlled randomised trial. |
| Participants | 156 women were randomised in a hospital setting. Inclusion and exclusion criteria not specified. |
| Interventions | Micronized vaginal progesterone versus oral dydrogesterone (doses and duration not specified). |
| Outcomes | Outcomes not specified. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Yadav 2015.
| Methods | 2‐arm no treatment‐controlled randomised trial. |
| Participants | 100 women were randomised in a hospital setting in India. The population comprised women with threatened abortion. No details are provided on exclusion criteria. |
| Interventions | 30 mg oral dydrogesterone daily versus no treatment. |
| Outcomes | The study recorded the following outcomes relevant for this review: miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, but no response was received by the time of publication of this review. |
Yassaee 2014.
| Methods | 2‐arm no treatment‐controlled randomised trial. |
| Participants | 60 women were randomised in a hospital setting in Iran. The population comprised pregnant women with threatened abortion. The presence of singleton pregnancy and detection of fetal heart activity, besides gestational age of < 20 weeks, was verified by ultrasound. Exclusion criteria comprised women that had reaction to Cyclogest, repeated abortions, multiple gestation, absence of fetus or fetal heart tone, uterine anomaly or fetal anomaly. |
| Interventions | 400 mg micronized vaginal progesterone daily until their bleeding stopped in less that 1 week versus no treatment. |
| Outcomes | The study recorded the following outcomes relevant for this review: live birth and miscarriage. |
| Notes | Evidence of trial documentation and data requested from the corresponding author, who replied but did not answer all of our queries or provide the requested documentation or data. |
hCG: Human chorionic gonadotropin.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611000405910.
| Study name | Supporting Threatened Outcomes with Progesterone (STOP trial) |
| Methods | 2‐arm placebo‐controlled randomised trial. |
| Participants | Women aged 18 years or older presenting with threatened miscarriage and a live intrauterine pregnancy with a gestational age less than 10 weeks. Women with a pregnancy as a result of Assisted Reproductive Technologies will be excluded. |
| Interventions | Progesterone pessary 400 mg nightly, until 12 weeks' gestation versus placebo. |
| Outcomes | Live birth, complete miscarriage, gestation at birth, birthweight, congenital abnormality, antepartum haemorrhage. |
| Starting date | 30‐Jan‐2012 |
| Contact information | lukemclindon@bigpond.com |
| Notes | The trial has been completed and the findings are currently being prepared for publication. |
ChiCTR‐IOR‐15007526.
| Study name | Chinese herbal medicine and micronized progesterone for threatened miscarriage: an International Cooperation multicenter randomized controlled trial |
| Methods | 2x2 factorial randomised placebo‐controlled trial. |
| Participants | Women aged 18 to 37 years that are currently pregnant (as confirmed by positive urinary pregnancy tests) at 5‐10 weeks of gestation, presenting with vaginal bleeding with or without abdominal pain, while the cervix is closed by visual exam and the fetus is viable inside the uterine cavity during early pregnancy. Women will have had no previous treatment for miscarriage. Exclusion criteria include women with a pregnancy of unknown location; ectopic pregnancy; multiple pregnancy; a non‐viable pregnancy; serial serum hCG levels which show a decline or a plateau, intrauterine abnormalities and fibroids distorting uterine cavity (as assessed by ultrasound); known abnormal parental karyotype; bleeding attributed to a vulvar, vaginal, or cervical source unrelated to the pregnancy; presence of a congenital or acquired bleeding diathesis; presence of contributing major medical disorders (regardless of severity). These include poorly controlled diabetes, uncontrolled hypertension, systemic lupus erythematosus (SLE), untreated or active cancer (any cancer in remission or non‐melanoma skin cancer is not included in the exclusion criteria), liver disease, renal disease, rheumatoid arthritis, cardiac disease, pulmonary disease other than mild asthma, neurological disease requiring medical treatment, uncontrolled hypothyroidism, uncontrolled seizure disorder. Untreated vitamin B12 deficiency, severe anaemia (Hct < 30%), haemophilia, gout, nasal polyps, among others; use of agents that may contribute to bleeding such as aspirin, NSAIDs; current use of Chinese Herbal Medicines (CHM) within 2 months; current use of progestins‐ oral, intramuscular, vaginal, etc within 2 months; known current or recent alcohol abuse or illicit drug use; current diagnosis of sexually transmitted infection (STI) (temporary exclusion). |
| Interventions | Chinese herbal medicine + micronized progesterone placebo versus Chinese herbal medicine placebo + micronized progesterone versus Chinese herbal medicine + micronized progesterone versus Chinese herbal medicine placebo + micronized progesterone placebo. |
| Outcomes | Live birth rate (> 37 weeks of gestation); early abortion; intrauterine pregnancy abortion before 20 weeks of pregnancy; intrauterine pregnancy abortion after 20 weeks of pregnancy; continuous pregnancy rate beyond 12 weeks of pregnancy; persistent pregnancy rate over 20 weeks of pregnancy; preterm birth rate (pregnancy > 24 weeks, < 37 weeks); maternal hypertension; maternal diabetes; prenatal haemorrhage; premature delivery; late pregnancy; preeclampsia; abnormal fetal testing; intrauterine growth restriction; low birthweight; stillbirth; neonatal death; congenital abnormalities; adverse reactions. |
| Starting date | 30‐Nov‐2015 |
| Contact information | xiaokewu2002@vip.sina.com |
| Notes |
CTRI/2020/10/028244.
| Study name | A clinical trial to study the effects of two drugs dydrogesterone and micronized progesterone in pregnant women with threatened abortion |
| Methods | 2‐arm active‐controlled randomised trial |
| Participants | Women aged from 20 to 35 years, with a singleton intrauterine live pregnancy, gestational age less than 12 weeks and presenting with threatened abortion. The exclusion criteria includes history of recurrent miscarriage (defined as at least 3 consecutive spontaneous miscarriages), history of cervical surgery, heavy vaginal bleeding requiring surgical intervention, any uterine anomalies, and any associated medical and surgical illness. |
| Interventions | 40 mg dydrogesterone stat followed by 10 mg BD till 14 weeks of gestation versus 200 mg progesterone twice daily till 14 weeks of gestation |
| Outcomes | Miscarriage before 20 weeks of gestation; live birth rate; period of gestational age at delivery; birthweight at delivery; antepartum haemorrhage; pre‐eclampsia |
| Starting date | 12‐Oct‐2020 |
| Contact information | saicharishma1544@gmail.com |
| Notes |
IRCT20120104008611N10.
| Study name | The effect of dydrogesterone and micronized progesterone on threatened abortion |
| Methods | 2‐arm active‐controlled randomised trial. |
| Participants | Pregnant women with a gestational age 6 to 13 weeks and observed fetal heart rate, vaginal bleeding, absence of uterine and fetal anomalies. Exclusion criteria include breast carcinoma, severe liver problems, genital carcinoma, thromboembolic disorders, epilepsy, diabetes, hypertension. |
| Interventions | 10 mg dydrogesterone, twice daily until 1 week after bleeding versus 100 mg progesterone, twice daily until 1 week after bleeding. |
| Outcomes | Preeclampsia; gestational diabetes; preterm labor; low birthweight; abortion under 20 weeks of pregnancy. |
| Starting date | 22‐Oct‐2019 |
| Contact information | pakniat110@yahoo.com |
| Notes |
IRCT20120104008611N8.
| Study name | The Comparison between the effect of dyrogesterone and Cyclogest intake on pregnancy in case of threatened abortion |
| Methods | 2‐arm active‐controlled randomised trial. |
| Participants | Inclusion criteria: gestational age 6 to 13 weeks; observation of fetal heart rate; vaginal bleeding; uterine closure; uterine or fetal anomalies. Exclusion criteria: unwillingness of the patient; lack of fetal heart rate; severe liver problems; epilepsy; diabetes; hypertension. |
| Interventions | 400 mg vaginal micronized progesterone daily versus 20 mg dydrogesterone daily up to the end of 16 weeks of pregnancy. |
| Outcomes | Threatened abortion; gestational diabetes; preterm labor; low birthweight; abortion under 20 weeks of pregnancy |
| Starting date | 22‐May‐2017 |
| Contact information | pakniat110@yahoo.com |
| Notes |
IRCT20120104008611N9.
| Study name | Comparison of the effect of two progesteron drugs in protection of abortion |
| Methods | 2‐arm active‐controlled randomised trial. |
| Participants | Pregnant women with gestational age between 6 and 13 weeks, presence of a fetal heart beat, uterine bleeding and a closed uterine orifice. Exclusion criteria include embryonic or uterine anomalies identified by ultrasound, multi‐fetal hydatidiform mole, known underlying disease in mother, women who have been treated by a specific medication to treat abortion. |
| Interventions | 400 mg daily vaginal progesterone versus 100 mg daily oral progesterone |
| Outcomes | Abortion, pre‐eclampsia, gestational diabetes |
| Starting date | 21‐Apr‐2018 |
| Contact information | hpakniat@qums.ac.ir |
| Notes |
IRCT20120918010876N5.
| Study name | Evaluation of the effect of oral dydrogesterone (Duphaston) in threatened abortion at first trimester |
| Methods | 2‐arm no treatment‐controlled randomised trial. |
| Participants | Pregnant women aged between 18 and 35 years in the first trimester, presenting with symptoms and signs of threatened abortion. Women will have a singleton pregnancy and a viable fetus will be identified by ultrasound. |
| Interventions | 40 mg dydrogesterone followed by 10 mg every 8 hours until bleeding was discontinued versus no treatment |
| Outcomes | Abortion; vaginal bleeding; |
| Starting date | 21‐Nov‐2017 |
| Contact information | drmoradi000@yahoo.com |
| Notes |
NCT02145767.
| Study name | Progesterone for the prevention of miscarriage and preterm birth in women with first trimester bleeding: PREEMPT Trial |
| Methods | 2‐arm placebo‐controlled randomised trial. |
| Participants | Women aged between 18 and 45 years with a live intrauterine singleton pregnancy of <14 weeks by crown‐rump length on ultrasound with documented fetal cardiac activity, and presence of a perigestational (subchorionic) haemorrhage on ultrasound. Exclusion criteria include contraindication to progesterone and any indication for progesterone. |
| Interventions | Progesterone 200 mg suppository administered vaginally at bedtime until 34 completed weeks of pregnancy versus placebo |
| Outcomes | Miscarriage (< 20 weeks); preterm birth (< 37 weeks); maternal outcomes; neonatal outcomes; healthcare outcomes |
| Starting date | December 2014 |
| Contact information | preempttrial@gmail.com |
| Notes |
NCT02690129.
| Study name | Vaginal progesterone for treatment of threatened miscarriage; randomized clinical trial |
| Methods | 2‐arm no treatment‐controlled randomised trial. |
| Participants | Women aged between 20 and 35 years, who are pregnant with a gestational age less than 24 weeks, presenting with bleeding with or without pain, a single viable fetus (confirmed by ultrasound examination. Exclusion criteria include currently under medication for any chronic diseases (DM, thyroid, liver, renal, cardiac and autoimmune disease); hypersensitivity to progesterone; any documented congenital fetal anomaly in the current pregnancy; women receiving hormonal treatment in the current pregnancy; women that conceived via ART. |
| Interventions | 200 mg vaginal micronized progesterone for 15 days versus no treatment |
| Outcomes | Miscarriage rate up to 28 weeks of gestation; gestational age at delivery or termination of pregnancy |
| Starting date | February 2016 |
| Contact information | omshaaban2000@yahoo.com |
| Notes |
NCT03930212.
| Study name | Progesterone supplementation in threatened abortion (Prothreat) |
| Methods | 2‐arm placebo‐controlled randomised trial. |
| Participants | Women presenting with threatened abortion diagnosed by history and ultrasound examination, a singleton viable fetus with gestational age < 20 weeks and a closed normal length cervix. Exclusion criteria include short cervix < 2 cm; multiple pregnancy; dead fetus; open cervix ≥ 2 cm; history of cervical surgery. |
| Interventions | 400 mg rectal progesterone suppositories once daily versus placebo |
| Outcomes | Relief of pain; completion of pregnancy beyond 20 weeks; stoppage of bleeding; abortion less than 20 weeks |
| Starting date | 01‐Jan‐2018 |
| Contact information | ayman.dawood@med.tanta.edu.eg |
| Notes |
DM: diabetes mellitus;hCG: Human chorionic gonadotropin;NSAIDs: non steroidal anti‐inflammatory drugs.
Differences between protocol and review
There are some differences between this review and our published protocol (Devall 2020) ‐ these are listed below.
Miscarriage (defined as delivery before 24 weeks of gestation) was mistakenly listed as a primary outcome in our protocol. This is a secondary outcome. Our intention was always to have live birth as the single, primary outcome for this review since live birth has been used as the primary outcome in all the large trials on this subject and is the most clinically important outcome.
The search methods for the review were conducted differently to those proposed in the protocol. In the protocol, we had planned to use the standard Cochrane Pregnancy and Childbirth search methods, which are carried out by their Information Specialist. However, due to unforeseen circumstances, we commissioned a review‐based search, as detailed in Search methods for identification of studies, and Appendix 1.
The threatened and recurrent miscarriage populations have been analysed independently since they are two clinically different populations. Presentation of the data in this way is consistent with the two previous Cochrane Reviews that examined the evidence of threatened (Wahabi 2018) and recurrent (Haas 2019) miscarriage.
The pre‐specified subgroup analysis in the study protocol for number of previous miscarriages were no previous miscarriages, one or more previous miscarriages and three or more previous miscarriages. However, to avoid the issue of overlapping between the subgroups, we have now only focused on no previous miscarriages or one or more previous miscarriages for this subgroup analysis, following discussion with the Editor.
In the protocol, we had planned to use a random‐effects model for all pairwise analyses in the review. However, a fixed‐effect model has been applied for all pairwise analyses because of the small number of included studies and lack of heterogeneity.
Contributions of authors
Adam J Devall (AJD), Ioannis D Gallos (IDG), and Arri Coomarasamy (AC) conceived the idea for this review. AJD, IDG, AC, and Malcolm J Price (MJP) designed the meta‐analysis. AJD designed the electronic data collection forms. AJD, Argyro Papadopoulou (AP), Marcelina Podesek (MP) and IDG performed study selection. AJD, AP, MP and IDG performed data extraction. MJP provided statistical support and analysis. AJD, AP, MP and IDG graded the evidence and AJD created the 'summary of findings' tables. AJD and IDG created the protocol. AJD drafted this review. IDG, AD, MJP, AC and David M Haas (DMH) edited and revised the review. AD and IDG are the guarantors for this review.
Sources of support
Internal sources
Tommy's National Centre for Miscarriage Research, University of Birmingham, UK
External sources
-
Tommy's Charity, UK
Tommy's Charity provide financial support to the Tommy's National Centre for Miscarriage Research
-
National Institute for Health Research, UK
NIHR Review Incentive Award number NIHR133289
Declarations of interest
Adam J Devall (AJD): was the trial manager for the UK National Institute for Health Research HTA Project Award 12/167/26 entitled 'Effectiveness of progesterone to prevent miscarriage in women with early pregnancy bleeding: A randomised placebo‐controlled trial (PRISM Trial: PRogesterone In Spontaneous Miscarriage Trial)'. AJD did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates; these tasks have been carried out by other members of the team who were not directly involved in the trial. This work is supported by Tommy's Charity who fund the Tommy's National Centre for Miscarriage Research, which is held by Prof Arri Coomarasamy (AC). This review is supported by an award from the NIHR Incentive Awards Scheme 2020, NIHR133289.
Argyro Papadopoulou (AP): is undertaking a PhD studentship supported by Tommy's Charity who fund the Tommy's National Centre for Miscarriage Research, which is held by Prof Arri Coomarasamy. This review is supported by an award from the NIHR Incentive Awards Scheme 2020, NIHR133289.
Marcelina Podesek (MP): is supported by Tommy's Charity who fund the Tommy's National Centre for Miscarriage Research, which is held by Prof Arri Coomarasamy (AC). This review is supported by an award from the NIHR Incentive Awards Scheme 2020, NIHR133289.
David M Haas (DMH): holds National Institutes of Health (NIH) research grants that are unrelated to this work. DMH was the lead author on a Cochrane review on progestogens for recurrent miscarriage and attends to patients with recurrent miscarriage.
Malcolm J Price (MJP): none known.
Arri Coomarasamy (AC): was the National Clinical Co‐ordinator for the UK National Institute for Health Research HTA Project Award 08/38/01 entitled 'First trimester progesterone therapy in women with a history of unexplained recurrent miscarriages: A randomised, double‐blind, placebo‐controlled, multi‐centre trial [The PROMISE (PROgesterone in recurrent MIScarriage) Trial]'. AC was also the Chief Investigator for the UK National Institute for Health Research HTA Project Award 12/167/26 entitled 'Effectiveness of progesterone to prevent miscarriage in women with early pregnancy bleeding: A randomised placebo‐controlled trial (PRISM Trial: PRogesterone In Spontaneous Miscarriage Trial)'. AC did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates; these tasks have been carried out by other members of the team who were not directly involved in the trial. This work is supported by Tommy's Charity who fund the Tommy's National Centre for Miscarriage Research, which is held by AC. This review is supported by an award from the NIHR Incentive Awards Scheme 2020, NIHR133289.
Ioannis D Gallos (IDG): was an author for the UK National Institute for Health Research HTA Project Award 12/167/26 entitled 'Effectiveness of progesterone to prevent miscarriage in women with early pregnancy bleeding: A randomised placebo‐controlled trial (PRISM Trial: PRogesterone In Spontaneous Miscarriage Trial)'. IDG did not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates; these tasks have been carried out by other members of the team who were not directly involved in the trial. This work is supported by Tommy's Charity who fund the Tommy's National Centre for Miscarriage Research, which is held by Prof Arri Coomarasamy (AC). This review is supported by an award from the NIHR Incentive Awards Scheme 2020, NIHR133289.
New
References
References to studies included in this review
Chan 2020 {published data only}
- Chan DM, Cheung KW, Ko JK, Yung SS, Lai SF, Lam MT, et al. Use of oral progestogen in women with threatened miscarriage in the first trimester: a randomized double-blind controlled trial. Human Reproduction 2020;Online ahead of print:DOI: 10.1093/humrep/deaa327. [DOI] [PubMed] [Google Scholar]
- Chan DM, Cheung KW, Yung SS, Lee VCY, Li R, Ng EH. A randomized double-blind controlled trial of the use of dydrogesterone in women with threatened miscarriage in the first trimester: study protocol for a randomized controlled trial. Trials 2016;17(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DM. NCT02128685: Randomized double-blind controlled trial of use of dydrogesterone in threatened miscarriage. https://clinicaltrials.gov/ct2/show/NCT02128685. [DOI] [PMC free article] [PubMed]
Coomarasamy 2015 {published data only}
- Coomarasamy A, Devall AJ, Brosens JJ, Rai R, Regan L, Gallos ID, et al. Micronized vaginal progesterone to prevent miscarriage: a critical evaluation of randomized evidence. American Journal of Obstetrics and Gynecology 2020;223(2):167-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. New England Journal of Medicine 2015;373(22):2141-8. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby, S, et al. PROMISE: first-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages - a randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technology Assessment (Winchester, England) 2016;20(41):1-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomarasamy A. ISRCTN92644181: Progesterone in recurrent miscarriages(PROMISE) study. https://doi.org/10.1186/ISRCTN92644181.
Coomarasamy 2019 {published data only}
- Coomarasamy A, Devall AJ, Cheed V, Harb H, Middleton LJ, Gallos ID, et al. A randomized trial of progesterone in women with bleeding in early pregnancy. New England Journal of Medicine 2015;380(19):1815-24. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Harb HM, Devall AJ, Cheed V, Roberts, TE, Goranitis I, et al. Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT. Health Technology Assessment (Winchester, England) 2020;24(33):1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomarasamy A. ISRCTN14163439: PRISM: Progesterone in spontaneous miscarriage. https://doi.org/10.1186/ISRCTN14163439.
Gerhard 1987 {published data only}
- Gerhard I, Gwinner B, Eggert-Kruse W, Runnebaum B. Double-blind controlled trial of progesterone substitution in threatened abortion. Biological Research in Pregnancy & Perinatology 1987;8(1):26-34. [PubMed] [Google Scholar]
MacDonald 1972 {published data only}
- MacDonald RR, Goulden R, Oakey RE. Cervical mucus, vaginal cytology and steroid excretion in recurrent abortion. Obstetrics & Gynecology 1972;40(3):394-402. [PubMed] [Google Scholar]
Shearman 1963 {published data only}
- Shearman RP, Garrett WJ. Double-blind study of effect of 17-hydroxyprogesterone caproate on abortion rate. BMJ 1963;1(5326):292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siew 2018 {published data only}
- Chye TT. ChiCTR-IOR-17011593. The randomised controlled trial of micronised progesterone and dydrogesterone (TRoMaD) for threatened miscarriage. chictr.org.cn/showproj.aspx?proj=19668 (first received 6 July 2017).
- Chye TT. ChiCTR-IPR-14005251. Micronised progesterone versus dydrogesterone: a randomised controlled trial. chictr.org.cn/showproj.aspx?proj=9539 (first received 21 September 2014).
- Hui CY, Siew SJ, Tan TC. Biochemical and clinical outcomes following the use of micronised progesterone and dydrogesterone for threatened miscarriage - A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122:276. [Google Scholar]
- Siew JY, Allen JC, Hui CY, Ku CW, Malhotra R, Ostbye T. The randomised controlled trial of micronised progesterone and dydrogesterone (TRoMaD) for threatened miscarriage. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2018;228:319-24. [DOI] [PubMed] [Google Scholar]
- Siew JY, Allen JC, Malhotra R, Østbye T, He S, Tan TC. Progestogen for threatened miscarriage: a randomised controlled trial comparing micronised progesterone and dydrogesterone. Journal of Maternal-fetal & Neonatal Medicine 2014;27:243. [Google Scholar]
- Siew S, Yan Hui CY, Tan TC, Allen JC, Malhotra R, Østbye T. Micronized progesterone compared with dydrogesterone for threatened miscarriage: a randomized controlled trial. Obstetrics and gynecology 2015;125:104S. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2020 {published data only}
- Ali MK, Ahmed SE, Sayed GH, Badran EY, Abbas AM. Effect of adjunctive vaginal progesterone after McDonald cerclage on the rate of second-trimester abortion in singleton pregnancy: a randomized controlled trial. International Journal of Gynaecology & Obstetrics 2020;149:370-6. [DOI] [PubMed] [Google Scholar]
Beigi 2016 {published data only}
- Beigi A, Esmailzadeh A, Pirjani R. Comparison of risk of preterm labor between vaginal progesterone and 17-Alpha-Hydroxy-Progesterone Caproate in women with threatened abortion: a randomized clinical trial. International Journal of Fertility & Sterility 2016;10(2):162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berle 1977 {published data only}
- Berle P, Behnke K. [The treatment of imminent abortion with monitoring of the maternal serum HPL level (proceedings)]. Archiv fur Gynakologie 1977;224(1-4):90-1. [DOI] [PubMed] [Google Scholar]
Berle 1980 {published data only}
- Berle P, Budenz M, Michaelis J. [Is hormonal therapy still justified in imminent abortion? (author's transl)]. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184(5):353-8. [PubMed] [Google Scholar]
Check 1985 {published data only}
- Check JH, Wu CH, Adelson HG. Decreased abortions in HMG-induced pregnancies with prophylactic progesterone therapy. International Journal of Fertility 1985;30(3):45-7. [PubMed] [Google Scholar]
Check 1987 {published data only}
- Check JH, Chase JS, Wu CH, Adelson HG, Teichman M, Rankin A. The efficacy of progesterone in achieving successful pregnancy: I. Prophylactic use during luteal phase in anovulatory women. International Journal of Fertility 1987;32(2):135-8. [PubMed] [Google Scholar]
Check 1987a {published data only}
- Check JH, Adelson HG. The efficacy of progesterone in achieving successful pregnancy: II. In women with pure luteal phase defects. International Journal of Fertility 1987;32(2):139-41. [PubMed] [Google Scholar]
Check 1987b {published data only}
- Check JH, Chase JS, Nowroozi K, Wu CH, Adelson HG. Progesterone therapy to decrease first-trimester spontaneous abortions in previous aborters. International Journal of Fertility 1987;32(3):192-3. [PubMed] [Google Scholar]
Check 1995 {published data only}
- Check JH, Tarquini P, Gandy P, Lauer C. A randomized study comparing the efficacy of reducing the spontaneous abortion rate following lymphocyte immunotherapy and progesterone treatment versus progesterone alone in primary habitual aborters. Gynecologic & Obstetric Investigation 1995;39(4):257-61. [DOI] [PubMed] [Google Scholar]
Chi 2019 {published data only}
- Chi H, Li R, Qiao J, Chen X, Wang X, Hao G. Vaginal progesterone gel is non-inferior to intramuscular progesterone in efficacy with acceptable tolerability for luteal phase support: A prospective, randomized, multicenter study in China. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2019;237:100-5. [DOI] [PubMed] [Google Scholar]
Clifford 1996 {published data only}
- Clifford K, Rai R, Watson H, Franks S, Regan L. Does suppressing luteinising hormone secretion reduce the miscarriage rate? Results of a randomised controlled trial. BMJ 1996;312(7045):1508-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corrado 2002 {published data only}
- Corrado F, Dugo C, Cannata ML, Di Bartolo M, Scilipoti A, Carlo Stella N. A randomised trial of progesterone prophylaxis after midtrimester amniocentesis. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2002;100(2):196-8. [DOI] [PubMed] [Google Scholar]
Costantino 2016 {published data only}
- Costantino M, Guaraldi C, Costantino D. Resolution of subchorionic hematoma and symptoms of threatened miscarriage using vaginal alpha lipoic acid or progesterone: clinical evidences. European Review for Medical & Pharmacological Sciences 2016;20(8):1656-63. [PubMed] [Google Scholar]
Daya 1988 {published data only}
- Daya S, Ward S, Burrows E. Progesterone profiles in luteal phase defect cycles and outcome of progesterone treatment in patients with recurrent spontaneous abortion. American Journal of Obstetrics & Gynecology 1988;158(2):225-32. [DOI] [PubMed] [Google Scholar]
Devine 2018 {published data only}
- Devine K, Richter KS, Widra EA, McKeeby J. Three-arm RCT: vaginal only progesterone is inferior, but vaginal plus intramuscular (IM) progesterone every third day is equivalent, to daily IM progesterone for vitrified-warmed blastocyst transfer in terms of live birth. Fertility and Sterility. Conference: 74th annual congress of the American Society for Reproductive Medicine, ASRM 2018. Denver Colorado, United States 2018;110(4):e2. [Google Scholar]
El‐Zibdeh 2005 {published data only}
- El-Zibdeh MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):431-4. [DOI] [PubMed] [Google Scholar]
El‐Zibdeh 2009 {published data only}
- El-Zibdeh MY, Yousef LT. Dydrogesterone support in threatened miscarriage. Maturitas 2009;65 Suppl 1:S43-6. [DOI] [PubMed] [Google Scholar]
EUCTR2016‐002777‐35‐IT {published data only}
- Prospective, double-blind, randomised, placebo controlled, phase III clinical study assessing the efficacy of natural progesterone 25 mg/bid administered subcutaneously in the maintenance of early pregnancy in women with symptoms of threatened abortion. https://clinicaltrials.gov/ct2/show/NCT02950935.
- Prospective, double-blind, randomised, placebo controlled, phase III clinical study assessing the efficacy of natural progesterone 25 mg/bid administered subcutaneously in the maintenance of early pregnancy in women with symptoms of threatened abortion. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2016-002777-35.
Fuchs 1966 {published data only}
- Fuchs F, Olsen P. An attempted double-blind controlled trial of progesterone therapy in habitual abortion. Ugeskrift for Laeger 1966;128(49):1461-2. [PubMed] [Google Scholar]
Goldzieher 1964 {published data only}
- Goldzieher JW. Double-blind trial of a progestin in habitual abortion. JAMA 1964;188(7):651-4. [DOI] [PubMed] [Google Scholar]
Govaerts‐Videtzky 1965 {published data only}
- Govaerts-Videtzky M, Martin L, Hubinont PO. A double-blind study of progestogen treatment in spontaneous abortion. (Preliminary report). Journal of Obstetrics & Gynaecology of the British Commonwealth 1965;72(6):1034. [DOI] [PubMed] [Google Scholar]
Ismail 2018 {published data only}
- Ismail A, Nasr A, Amin A, Al-Inany H. Peri-conceptional progesterone treatment in women with unexplained recurrent miscarriage, a randomized double-blind controlled trial. Human Reproduction (Oxford, England) 2015;30:i194. [Google Scholar]
- Ismail AM, Abbas AM, Ali MK, Amin AF. Peri-conceptional progesterone treatment in women with unexplained recurrent miscarriage: a randomized double-blind placebo-controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(3):388-94. [DOI] [PubMed] [Google Scholar]
Johnson 1975 {published data only}
- Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. New England Journal of Medicine 1975;293(14):675-80. [DOI] [PubMed] [Google Scholar]
Klopper 1965 {published data only}
- Klopper A, MacNaughton M. Hormones in recurrent abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1022-8. [Google Scholar]
LeVine 1964 {published data only}
- LeVine L. Habitual abortion. A controlled clinical study of progestational therapy. Western Journal of Surgery 1964;72:30-6. [PubMed] [Google Scholar]
Moller 1966 {published data only}
- Moller KJ, Fuchs F. [Treatment of threatened abortion with methylprogesterone acetate]. Ugeskrift for Laeger 1966;128:1457-60. [PubMed] [Google Scholar]
Nyboe Andersen 2002 {published data only}
- Nyboe Andersen A, Popovic-Todorovic B, Schmidt KT, Loft A, Lindhard A, Højgaard A, et al. Progesterone supplementation during early gestations after IVF or ICSI has no effect on the delivery rates: a randomized controlled trial. Human Reproduction (Oxford, England) 2002;17(2):357-61. [DOI] [PubMed] [Google Scholar]
Ozer 2020 {published data only}
- Ozer G, Yuksel B, Yucel Cicek OS, Kahraman S. Oral dydrogesterone vs. micronized vaginal progesterone gel for luteal phase support in frozen-thawed single blastocyst transfer in good prognosis patients. Journal of Gynecology Obstetrics and Human Reproduction 2020;50(5):1020-30. [DOI] [PubMed] [Google Scholar]
Porcaro 2015 {published data only}
- Porcaro G, Brillo E, Giardina I, Di Iorio R. Alpha Lipoic Acid (ALA) effects on subchorionic hematoma: preliminary clinical results. European Review for Medical & Pharmacological Sciences 2015;19(18):3426-32. [PubMed] [Google Scholar]
Prietl 1992 {published data only}
- Prietl G, Diedrich K, Ven HH, Luckhaus J, Krebs D. The effect of 17 alpha-hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: a prospective and randomized controlled trial. Human Reproduction (Oxford, England) 1992;7 Suppl 1:1-5. [DOI] [PubMed] [Google Scholar]
Pustotina 2018 {published data only}
- Pustotina O. Effectiveness of dydrogesterone, 17-OH progesterone and micronized progesterone in prevention of preterm birth in women with a short cervix. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(14):1830-8. [DOI] [PubMed] [Google Scholar]
Rehal 2020 {published data only}
- Rehal A, Benko Z, De Paco Matallana C, Syngelaki A, Janga D, Cicero S. Early vaginal progesterone versus placebo in twin pregnancies for the prevention of spontaneous preterm birth: a randomized, double-blind trial. American Journal of Obstetrics & Gynecology 2020;26:26. [DOI] [PubMed] [Google Scholar]
Reijnders 1988 {published data only}
- Reijnders FJ, Thomas CM, Doesburg WH, Rolland R, Eskes TK. Endocrine effects of 17 alpha-hydroxyprogesterone caproate during early pregnancy: a double-blind clinical trial. British Journal of Obstetrics & Gynaecology 1988;95(5):462-8. [DOI] [PubMed] [Google Scholar]
Shu 2002 {published data only}
- Shu J, Miao P, Wang RJ. [Clinical observation on effect of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody-positive early recurrent spontaneous abortion]. Chinese Journal of Integrated Traditional & Western Medicine 2002;22(6):414-6. [PubMed] [Google Scholar]
Sondergaard 1985 {published data only}
- Sondergaard F, Ottesen B, Detlefsen GU. Treatment with progesterone in threatened abortion and low level of serum progesterone. A double blind randomized trial. Contraception Fertilite Sexualite 1985;13(12):1227-31. [Google Scholar]
Song 2007 {published data only}
- Song YL, Zhu LP. [The fetus protection effects of Zhixue Baotai Decoction on women of early threatened abortion with dark area surrounding pregnancy sac]. Chinese Journal of Integrated Traditional & Western Medicine 2007;27(11):1025-8. [PubMed] [Google Scholar]
Souka 1980 {published data only}
- Souka AR, Osman M, Sibaie F, Einen MA. Therapeutic value of indomethacin in threatened abortion. Prostaglandins 1980;19(3):457-60. [DOI] [PubMed] [Google Scholar]
Swyer 1953 {published data only}
- Swyer GI, Daley D. Progesterone implantation in habitual abortion. BMJ 1953;1:1073-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tognoni 1980 {published data only}
- Tognoni G, Ferrario L, Inzalaco M, Crosignani PG. Progestagens in threatened abortion. Lancet 1980;2(8206):1242-3. [DOI] [PubMed] [Google Scholar]
Tomic 2015 {published data only}
- Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K. Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2015;186:49-53. [DOI] [PubMed] [Google Scholar]
Zhang 2000 {published data only}
- Zhang J, Zhang Y, Liu G. [Clinical and experimental study on yun'an granule in treating threatened abortion]. Chinese Journal of Integrated Traditional & Western Medicine 2000;20(4):251-4. [PubMed] [Google Scholar]
References to studies awaiting assessment
Abrar 2017 {published data only}
- Abrar S, Abrar T, Tahir M, Sayyed E. Role of progesterone in the treatment of threatened miscarriage in first trimester. Journal of Medical Sciences (Peshawar) 2017;25(4):407-10. [Google Scholar]
Agarwal 2016 {published data only}
- Agarwal N. Role of inflammatory markers in recurrent pregnancy loss and effect of oral micronized therapy on these cases. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=14740.
Alimohamadi 2013 {published data only}
- Alimohamadi S, Javadian P, Gharedaghi MH, Javadian N, Alinia H, Khazardoust, S. Progesterone and threatened abortion: a randomized clinical trial on endocervical cytokine concentrations. Journal of Reproductive Immunology 2013;98(1-2):52-60. [DOI] [PubMed] [Google Scholar]
Czajkowski 2007 {published data only}
- Czajkowski K, Sienko J, Mogilinski M, Bros M, Szczecina R, Czajkowska A. Uteroplacental circulation in early pregnancy complicated by threatened abortion supplemented with vaginal micronized progesterone or oral dydrogesterone. Fertility & Sterility 2007;87(3):613-8. [DOI] [PubMed] [Google Scholar]
Ghosh 2014 {published data only}
- Ghosh S, Chattopadhyay R, Goswami S, Chaudhury K, Chakravarty B, Ganesh A. Assessment of sub-endometrial blood flow parameters following dydrogesterone and micronized vaginal progesterone administration in women with idiopathic recurrent miscarriage: a pilot study. Journal of Obstetrics & Gynaecology Research 2014;40(7):1871-6. [DOI] [PubMed] [Google Scholar]
Kumar 2014 {published data only}
- Kumar A, Begum N, Prasad S, Aggarwal S, Sharma S. Oral dydrogesterone treatment during early pregnancy to prevent recurrent pregnancy loss and its role in modulation of cytokine production: a double-blind, randomized, parallel, placebo-controlled trial. Fertility & Sterility 2014;102(5):1357-63.e3. [DOI] [PubMed] [Google Scholar]
Omar 2005 {published data only}
- Omar MH, Mashita MK, Lim PS, Jamil MA. Dydrogesterone in threatened abortion: pregnancy outcome. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):421-5. [DOI] [PubMed] [Google Scholar]
Palagiano 2004 {published data only}
- Palagiano A, Bulletti C, Pace MC, DE Ziegler D, Cicinelli E, Izzo A. Effects of vaginal progesterone on pain and uterine contractility in patients with threatened abortion before twelve weeks of pregnancy. Annals of the New York Academy of Sciences 2004;1034:200-10. [DOI] [PubMed] [Google Scholar]
Pandian 2009 {published data only}
- Pandian RU. Dydrogesterone in threatened miscarriage: a Malaysian experience. Maturitas 2009;65 Suppl 1:S47-50. [DOI] [PubMed] [Google Scholar]
Turgal 2017 {published data only}
Vincze 2006 {published data only}
- Vincze E, Molnar-GB, Foldesi I, Pal A. Treatment possibilities using progestagens and progesteron-like preparations in threatened abortion. Magyar Noorvosok Lapja 2006;69(4):281-4. [Google Scholar]
Yadav 2015 {published data only}
- Yadav R, Yadav S, Jain A. To study the impact of progesterone (dydrogesterone) on proinflammatory (IL-6 and TNF-alpha) and antiinflammatory (IL-10) cytokines in threatened abortion. International Journal of Gynaecology and Obstetrics 2015;131:E196. [Google Scholar]
Yassaee 2014 {published data only}
- Yassaee F, Shekarriz-Foumani R, Afsari S, Fallahian M. The effect of progesterone suppositories on threatened abortion: a randomized clinical trial. Journal of Reproduction & Infertility 2014;15(3):147-51. [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12611000405910 {published data only}
- ACTRN12611000405910. In women with threatened miscarriage, does progesterone supplementation increase the likelihood of live birth? https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336813.
ChiCTR‐IOR‐15007526 {published data only}
- ChiCTR-IOR-15007526. Chinese herbal medicine and micronized progesterone for threatened miscarriage: an international cooperation multicenter randomized controlled trial. http://www.chictr.org.cn/showproj.aspx?proj=12677.
CTRI/2020/10/028244 {published data only}
- CTRI/2020/10/028244. A clinical trial to study the effects of two drugs dydrogesterone and micronized progesterone in pregnant women with threatened abortion. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=38656.
IRCT20120104008611N10 {published data only}
- IRCT20120104008611N10. The effect of dydrogesterone and micronized progesterone on threatened abortion. http://en.irct.ir/trial/33884.
IRCT20120104008611N8 {published data only}
- IRCT20120104008611N8. The effect of dydrogesterone and Cyclogest on threatened abortion. http://en.irct.ir/trial/32531.
IRCT20120104008611N9 {published data only}
- IRCT20120104008611N9. Comparison of the effect of two progesteron drugs in protection of abortion. http://en.irct.ir/trial/30167.
IRCT20120918010876N5 {published data only}
- IRCT20120918010876N5. Evaluation of the effect of oral dydrogesterone (Duphaston) in threatened abortion at first trimester. http://en.irct.ir/trial/28300.
NCT02145767 {published data only}
- NCT02145767. Progesterone for the prevention of miscarriage and preterm birth in women with first trimester bleeding: PREEMPT Trial. https://ClinicalTrials.gov/show/NCT02145767.
NCT02690129 {published data only}
- NCT02690129. Vaginal progesterone for treatment of threatened miscarriage. https://ClinicalTrials.gov/show/NCT02690129.
NCT03930212 {published data only}
- NCT03930212. Progesterone supplementation in threatened abortion. https://clinicaltrials.gov/show/NCT03930212.
Additional references
Adolfsson 2006
- Adolfsson A, Larsson PG. Cumulative incidence of previous spontaneous abortion in Sweden in 1983-2003: a register study. Acta Obstetricia et Gynecologica Scandinavica 2006;85(6):741-7. [DOI] [PubMed] [Google Scholar]
Alberman 1992
- Alberman E. Spontaneous abortions: epidemiology. In: Spontaneous Abortion: Diagnosis and Treatment. London: Springer Verlag, 1992:9-20. [Google Scholar]
Bender Atik 2018
- Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Human Reproduction Open 2018;2:hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brignardello‐Petersen 2018
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, GRADE Working Group. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. [DOI] [PubMed] [Google Scholar]
Butcher 1997
- Butcher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683-91. [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331(7521):897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantwell 2011
- Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG: an international journal of obstetrics and gynaecology 2011;1:1-203. [DOI] [PubMed] [Google Scholar]
Carlisle 2017
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72:944–52. [DOI] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015;15:4. [Google Scholar]
Chaimani 2017
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. [DOI] [PubMed] [Google Scholar]
Corner 1953
- Corner GW, Csapo A. Action of the ovarian hormones on uterine muscle. British Medical Journal 1953;1(4812):687-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 2010;29:932-44. [DOI] [PubMed] [Google Scholar]
Evans 1988
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science 1988;240:889-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farren 2019
- Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, Al-Memar M, et al. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. American Journal of Obstetrics and Gynecology 2019;222(4):367.E1-367. [DOI] [PubMed] [Google Scholar]
Haas 2019
- Haas DM, Hathaway TJ, Ramsey PS. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD003511. [DOI: 10.1002/14651858.CD003511.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Linnakaari 2019
- Linnakaari R, Helle N, Mentula M, Bloigu A, Gissler M, Heikinheimo O, et al. Trends in the incidence, rate and treatment of miscarriage-nationwide register-study in Finland, 1998-2016. Human Reproduction 2019;34(11):2120-8. [DOI] [PubMed] [Google Scholar]
Maconochie 2007
- Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage—results from a UK-population-based case–control study. Epidemiology 2007;114:170-86. [DOI] [PubMed] [Google Scholar]
Magnus 2019
- Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ 2019;364:l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2012
- Murphy FA, Lipp A, Powles DL. Follow-up for improving psychological well being for women after a miscarriage. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD008679. [DOI: 10.1002/14651858.CD008679.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okada 2018
- Okada H, Tsuzuki T, Murata H. Decidualization of the human endometrium. Reproductive Medicine and Biology 2018;17(3):220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palomba 2015
- Palomba S, Santagni S, Battista La Sala, G. Progesterone administration for luteal phase deficiency in human reproduction: an old or new issue? Journal of Ovarian Research 2015;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paulson 2014
- Paulson RJ, Collins MG, Yankov VI. Progesterone pharmacokinetics and pharmacodynamics with 3 dosages and 2 regimens of an effervescent micronized progesterone vaginal insert. Journal of Clinical Endocrinology and Metabolism 2014;99(11):4241-9. [DOI] [PubMed] [Google Scholar]
Polikarpova 2019
- Polikarpova AV, Levina IS, Sigai NV, Zavarzin IV, Morozov IA, Rubtsov PM, et al. Immunomodulatory effects of progesterone and selective ligands of membrane progesterone receptors. Steroids 2019;145:5-18. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 2015
- Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. Journal of Clinical Epidemiology 2015;68(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossen 2018
- Rossen LM, Ahrens KA, Branum AM. Trends in risk of pregnancy loss among US women, 1990-2011. Paediatric and Perinatal Epidemiology 2018;32(1):19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLOS One 2014;9(7):e996882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shiers 2003
- Shiers C. Myles’ Textbook for Midwives. 14th edition. Churchill Livingstone, 2003. [Google Scholar]
Sotiriadis 2004
- Sotiriadis A, Papatheodorou S, Makrydimas G. Threatened miscarriage: evaluation and management. BMJ 2004;329(7458):152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephenson 2002
- Stephenson, MD, Awaratani, KA, Robinson, WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Human Reproduction 2002;17:446-51. [DOI] [PubMed] [Google Scholar]
Tobias 2014
- Tobias A, Catala-Lopez F, Roque M. Development of an Excel spreadsheet for meta-analysis of indirect and mixed treatment comparisons [Desarrollo de una hoja excel para metaanalisis de somparaciones inderectas y mixtas]. Revista Espanola de Salud Publica 2014;88(1):5-15. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis,using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahabi 2018
- Wahabi HA, Fayed AA, Esmaeil SA, Bahkali KH. Progestogen for treating threatened miscarriage. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD005943. [DOI: 10.1002/14651858.CD005943.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2011
- White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata Journal 2011;11(2):255-70. [Google Scholar]
White 2015
- White IR. Network meta-analysis. Stata Journal 2015;15(4):951-85. [Google Scholar]
References to other published versions of this review
Devall 2020
- Devall AJ, Papadopoulou A, Haas DM, Price MJ, Coomarasamy A, Gallos ID. Progestogens for preventing miscarriage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013792. [DOI: 10.1002/14651858.CD013792] [DOI] [PMC free article] [PubMed] [Google Scholar]