Abstract
Natural Killer (NK) cells belong to the innate immune system and in part protect the host through killing of infected, foreign, stressed or transformed cells. Additionally, via cellular cross-talk, NK cells orchestrate anti-tumor immune responses. Hence, significant efforts have been undertaken to exploit the therapeutic properties of NK cells in cancer. Current strategies in preclinical and clinical development include adoptive transfer therapies, direct stimulation, recruitment of NK cells into the tumor microenvironment (TME), the blockade of inhibitory receptors that limit NK cell functions and therapeutic modulation of the TME to enhance anti-tumor NK cell function. In this review, we introduce the NK cell-cancer cycle to highlight recent advances in NK cell biology and discuss the progress and problems of NK cell-based cancer immunotherapies.
Keywords: NK cells, Cancer, Immunotherapy, Tumor microenvironment, Adoptive transfer, CAR NK cells
Natural Killer (NK) cells are effector cells of the innate immune system and belong to the family of innate lymphoid cells (ILCs). By analogy to the classification of T cells, three groups of ILCs have been defined based on cytokine production and expression of transcription factors. Group 1 ILCs include IFN-γ-producing NK cells and ILC1s, while ILC2 produce classical TH2-cytokines and ILC3 produce TH17-cytokines1,2. NK cells are professional killer cells that recognize and rapidly destroy cells dangerous to the host (e.g. stressed, foreign, infected or transformed cells) and as such contribute to transplantation rejection, viral immunity and cancer immune surveillance (particularly cancer metastasis)3,4. However, besides their ability to kill cells, it is now well-established that NK cells also play a critical role in sculpting innate and adaptive immune responses via cellular cross-talk in various disease settings5. When compared with T cells, either in a natural tumor immunity or adoptive cellular therapy (ACT) setting, NK cells display certain advantages and disadvantages (Table 1). In particular, NK cells have a more important role in elimination of early tumors and metastasis (minimal disease) and are generally found in fewer numbers in established tumors. NK cells have a broader reactivity with tumor (less specificity without TCR), equivalent effector functions, and reduced proliferative capacity and recall response. In an adoptive transfer therapeutic setting, NK cells have greater off-the-shelf utility and are safer with respect to causing fewer immune related adverse events, but they are more difficult to genetically manipulate.
Table 1.
Advantages and disadvantages of NK cells over T cells in cancer therapy
| Feature1 | NK cells | T cells | Reference |
|---|---|---|---|
| Tumor stage | Elimination and metastasis | Equilibrium and Escape | 6, 7 |
| Number in tumor | + | +++ | |
| Tumor cell recognition | +++ (NKR) | + (TCR) | 8, 9 |
| Specificity | + | +++ | 10, 8 |
| Killing capacity | +++ | ++ | 11, 12, 13, 14, 15, 16 |
| Cytokine release | ++ | +++ | 13, 8 |
| Proliferative capacity | + | +++ | 17, 18, 19 |
| Recall response | 0 | +++ | 17, 18, 8 |
| Immune related-adverse events | + | +++ | 20, 21, 22, 23, 24, 25 |
| Genetic engineering | + | ++ | 9, 26 |
| Off-the shelf utility | +++ | + | 27, 28, 29, 30 |
Feature on a scale of 0− to +++
Similar to myeloid cells, NK cells are a heterogenous and plastic population, which allows them to acquire different phenotypes dependent on the tissue context or signaling cues to which they are exposed31,32. Simply, NK cells are defined as CD3−CD56+ cells in humans and CD3−NK1.1+NKp46+ cells in mice. Further, highly cytotoxic human NK cells are defined as CD56dimCD16hi (further referred to as CD56dim) and are predominantly found in the blood, while immunomodulatory and cytokine producing NK cells are defined as CD56brightCD16lo (further referred to as CD56bright) and preferentially reside in secondary lymphoid organs e.g. lymph nodes (Figure 1)33,34,35. A large suite of additional markers can be utilized to further stratify NK cell subsets e.g. CD94/NKG2A, NKp46, CD226 and many more. Functionally similar NK cell subsets have been identified in mice, but with some different markers. For NK cell differentiation in mice NK cells are marked by tumor necrosis factor receptor superfamily member CD27 and the integrin CD11b/Mac-1. The most cytotoxic NK cells are terminally differentiated and express CD11b but little or no CD27 (i.e. are CD27−CD11b+ or “CD11b SP”), while regulatory NK cells comprise mature NK cells expressing both CD27hi and CD11b, and immature NK cells lacking CD11b (i.e. CD11b− CD27hi or “CD27 SP”) (Figure 1)36. In addition, various subsets of tissue-resident NK cells have been described, differing from conventional NK cells in terms of their origin, development, and/or function (reviewed in ref.37).
Figure 1.
Human and Mouse NK cells. Simplified human and mouse NK cells can be subdivided into three main subsets based on the expression of CD56 and CD16 in human and CD11b and CD27 in mice. Depicted are important molecules required for NK cell recruitment, activation and effector function in the TME.
The NK cell – Cancer Cycle
By analogy to the Cancer-Immunity Cycle, we here introduce the NK cell-Cancer Cycle to discuss recent advances in NK cell biology and their importance for cancer immunotherapy (Figure 2). For productive anti-tumor immune responses, the human body needs to initiate and orchestrate the activation of multiple immune cell subsets. As discussed by Chen and Mellman, the success of immune checkpoint blockade (ICB) in some cancers shows that targeting single molecules or pathways is promising, but is ultimately insufficient across the majority of cancer patients38. Thus, our current challenge is to define additional targets in the tumor microenvironment (TME) and secondary lymphatic organs to meet this need.
Figure 2.
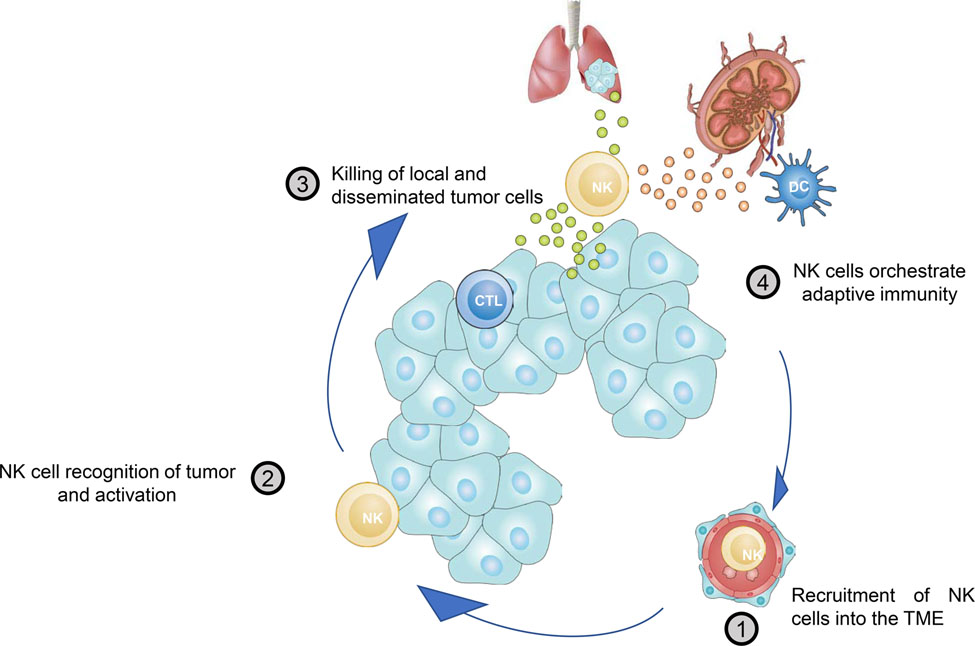
The NK cell-Cancer Cycle. To effectively eliminate cancer cells, the body needs to initiate a self-propagating anti-tumor immune response. The NK cell-cancer cycle consists of 4 steps important to initiate and maximize the efficacy of this innate response. 1. NK cells need to be recruited into the TME; 2. NK cells recognize cancer cells and undergo full activation; 3. NK cells kill cancer cells locally and systemically; 4. NK cell orchestrate innate and adaptive immunity e.g. by alerting dendritic cells. Finally, due to elimination of cancer cells locally in the TME or at distant sites antigens, chemokines and danger associated molecular patterns are liberated further fueling innate adaptive responses and allowing the cycle to begin again.
Step 1. Recruitment of NK cells to the TME.
NK cells are commonly found in human tumors, however at low frequency compared to myeloid and lymphoid cells39. Increased abundance of NK cells in the TME has been associated with increased overall survival in patients with hepatocellular carcinoma40, melanoma41,42,43,44,45, pulmonary adenocarcinoma46, gastric cancer47, squamous cell lung cancer48, non-small cell lung cancer49, breast cancer50, and renal cell carcinoma51. Recently, NK cells have been further linked to patient responsiveness to anti-PD-1 immunotherapy in metastatic melanoma43. Here, we will discuss the first step in the NK cell – cancer cycle: NK cell recruitment into the TME (Figure 2 and 3). The mechanisms controlling NK cell recruitment to the tumor can be broken down into three broad categories; chemoattractants/receptors; immunomodulation of chemokine axes, and physical barriers.
Figure 3.
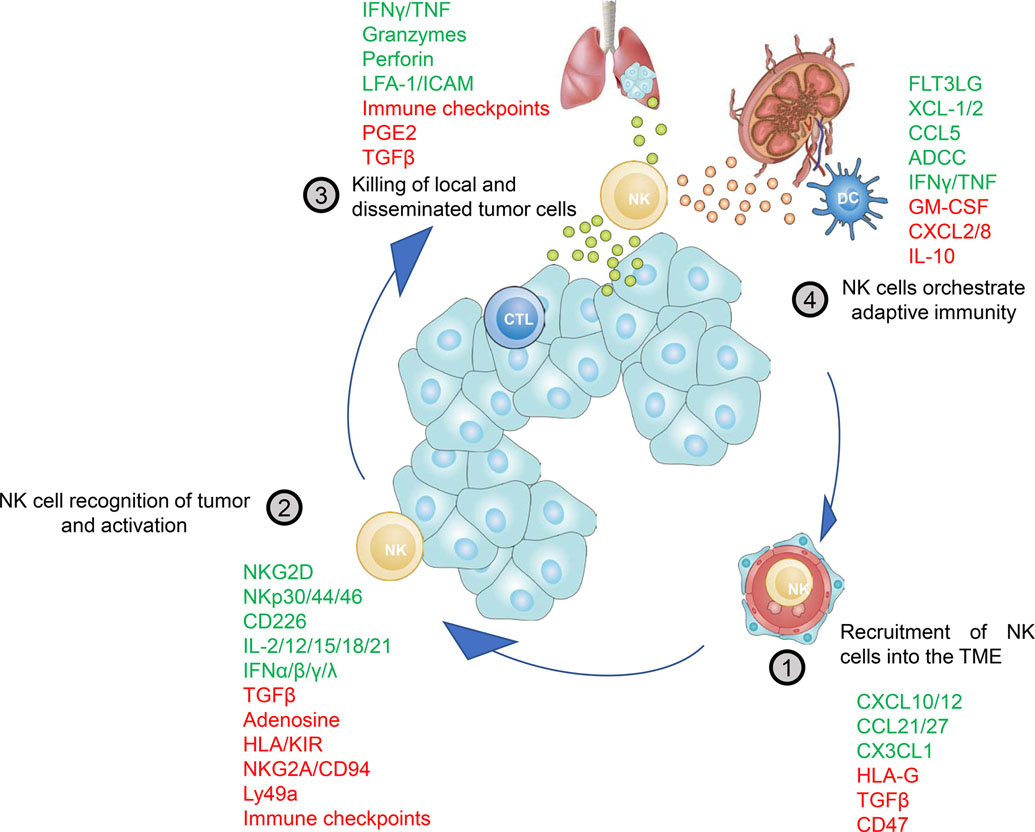
Positive and negative molecules influencing the NK cell-Cancer Cycle. Each step in the NK cell-Cancer Cycle is affected by a variety of stimulatory or inhibitory signals integrated by NK cells. Positive molecules are depicted in green and promote NK cell activity, while molecules negatively affecting NK cell activity are shown in red. These negative signals are required to prevent overshooting immune responses leading to exacerbated tissue damage. However, in the TME these pathways are hijacked by the cancer cells to escape NK cell mediated immunity and to scotch the initiation of adaptive immunity.
The two main subsets of NK cells, CD56bright and CD56dim, express unique repertoires of chemoattractant receptors, explaining the differential recruitment of NK cell subsets to various tissues (reviewed in ref.52). Peripheral blood CD56bright NK cells typically express and respond to ligands for CCR2, CCR5, CCR7, CXCR3, CXCR4 and CD62L while CD56dim NK cells express and respond to ligands to CXCR1, CXCR2, CXCR4, CX3CR1, S1P5, and ChemR2353,39,54,55,56,57,58,59,60,61 (Figure 1). As described above, mouse CD11b SP and CD27 SP NK cells are the functional equivalent to the human NK cell subsets (Figure 1). Consistent with this relationship, mouse CD11b SP and human CD56dim NK cells express similar chemokine receptors while CD27 SP NK cells have conserved expression of chemokine receptors with human CD56bright NK cells62,36 (Figure 1).
Recent single cell RNA sequencing of metastatic melanoma samples has found transcriptional heterogeneity within NK cells in the TME63. Even though there may be some transcriptional heterogeneity, CD56bright NK cells have been found to be the dominant NK cells in the TME of a number of cancers, including non-small cell lung cancer (NSCLC) and breast cancer39,64. The increased abundance of CD56bright NK cells in the TME of NSCLC and breast cancer is linked to the downregulation of the chemokine CXCL2, which signals through CXCR2, and concomitant upregulation of the chemokines CXCL9, CXCL10, and CCL19, which signal through CCR7 or CXCR3, in the TME39. Similarly, in pre-clinical mouse lymphoma models, tumor cell expression of CXCL9 and CXCL10, which signal through the chemokine receptor CXCR3 on NK cells, is important for the recruitment of NK cells into the TME65,66.
CCL5, the ligand for CCR5, which is uniquely expressed on human CD56bright and mouse CD27 SP NK cells (Figure 1), has also been implicated in NK cell recruitment to the TME. Other atypical pathways may also control NK recruitment, likely through the CCL5 axis. In one mouse model, tumor-derived progranulin (PGRN) serves to inhibit CCL5 production in an autocrine fashion, leading to reduced NK cell infiltration into the TME, loss of tumor control, and increased metastasis67. Consistent with a role for CCL5 in recruiting NK cells to the TME, in an experimental model of melanoma lung metastasis, IL-33 in the lung TME induces CCL5 production by CD8+ T cells and eosinophils, which leads to increased recruitment of NK cells and significantly reduced numbers of lung metastasis68. Other studies found that ectopic treatment or overexpression of IL-33 in transplantable melanoma models leads to increased recruitment and activation of NK cells to the TME69,68, possibly through a mechanism reliant on CCL5. Another atypical pathway shown to regulate recruitment of NK cells to the TME is controlled by the cytokine IL-17D and chemokine CCL2. In this pathway, tumor cell production of IL-17D signals to endothelial cells to induce production of CCL2 and subsequent recruitment of NK cells to the TME70.
CCL27, which signals through CCR10, is another chemokine linked to regulating NK cell recruitment to the TME. Intratumor injection of adenovirus encoding CCL27 increases recruitment of NK cells to the TME in mouse models as well as inhibit tumor growth71,72. However, one study found NK cells to be dispensable for tumor growth control making the importance of CCL27-dependent recruitment of NK cells to the TME less clear71. It is interesting to note that endometrial cancer shows a paucity of NK cells in the TME and this correlates with a reduction in CCL27, CXCL12, and CCL21 production as compared to adjacent normal tissue73. The role of CCL27 in cancer is complicated by the fact that CCR10 was also shown to have tumor cell intrinsic functions as it may enhance the growth and metastasis of melanoma and breast cancer cells74,75. Clearly, more work is needed to fully determine the relative role of CCL27 in protective immune responses and progression of cancer.
CX3CL1, also known as fractalkine, is the ligand for CX3CR1, the chemokine receptor uniquely expressed on cytotoxic CD56dim/CD11b SP NK cells. High expression of CX3CL1 is positively prognostic for patient outcome and NK cell infiltration in breast cancer 76, gastric adenocarcinoma77, colorectal cancer78, hepatocellular carcinoma79, and lung adenocarcinoma80. Furthermore, CX3CL1 is downregulated in human breast cancer tissue compared to adjacent normal, consistent with the finding that there is a large skewing of CD56bright NK cells in the TME of breast cancer39. It has also been well demonstrated that the CX3CL1/CX3CR1 is subverted by the tumor through the production of TGF-β81,82. Subsequent work showed that TGF-β1 signaling in NK cells induces the expression of microRNA miR-27a-5p which downregulates the expression of CX3CR183. It was also recently shown that CX3CL1/CX3CR1 signaling plays a role in controlling HCC metastases to the lung79. There, it was shown that tumor cells upregulate miR-561-5p which in turn inhibits the production of CX3CL1 and subsequently reduces NK cell recruitment to the tumor79.
Taken together these results suggest an important role for chemokine signaling in regulating the recruitment of NK cells into the TME and provide the rational for targeting these pathways to increase the number of NK cells in the tumor.
Disruption or modulation of chemokine signaling by two immunomodulatory molecules, HLA-G and CD47, is linked to changes in NK cell recruitment to the TME. HLA-G is a member of the nonclassical HLA-class Ib genes and has been well established to have strong immune-inhibitory functions. HLA-G is expressed in the TME but not in surrounding normal tissue and studies have demonstrated that higher HLA-G expression correlates with increased cancer stage and/or worse patient outcomes (reviewed in ref.84). HLA-G can inhibit NK cell activation, cytokine production, and cytotoxicity through the down regulation of STAT385,86 while soluble HLA-G can reduce the expression of chemokine receptors in human NK cells including, CCR2, CXCR3 and CX3CR155. These findings suggest that soluble HLA-G found in the serum of cancer patients could impair the recruitment of NK cells to the TME.
The mechanisms by which CD47 regulates NK cell recruitment to the TME remain less clear. In the TME, CD47 has an important role in inhibiting phagocytosis of cancer cells (reviewed in ref.87). However, CD47 is also expressed on NK cells where, upon binding its ligand thrombospondin-1 (TSP-1), it can inhibit NK cell activation and proliferation41. Using an anti-CD47 antibody to block TSP-1 binding to CD47 reversed TSP-1/CD47 mediated inhibition in a human NK cell line, inhibited tumor growth in B16 melanoma bearing mice, increased NK cell recruitment to the TME, and enhanced expression of granzyme B and IFN-γ in NK cells41. Further studies are needed to explore the mechanisms recruiting NK cells to the TME following anti-CD47 antibody treatment, but these findings suggest CD47 acts as a NK cell checkpoint and highlight this pathway as a potential therapeutic target to modulate NK cell numbers in the TME.
Stromal barriers may also play a role in regulating NK cell recruitment to tumors (reviewed in ref.82). In tumor regions where extracellular matrix proteins collagen type IV and laminin were high, NK cells were not seen entering the tumor, suggesting these structures around the tumor could prevent NK cell invasion into the tumor88. Consistent with this finding, in human NSCLC tissue NK cells are most commonly found in stromal regions in the tumor, not in direct contact with tumor cells64. Furthermore, it has been suggested that even in tumors where there is high expression of NK cell attracting chemokines, there is not always a concomitant recruitment of NK cells (reviewed in ref.82). These findings reinforce that more research into the physical barriers that limit intratumor NK cells are needed and suggest that emphasis should be placed on studying NK cell localization and its effect on a beneficial immune response.
Step 2. NK cell recognition of tumor and activation
In contrast to T and B lymphocytes, NK cells utilize an array of activating and inhibitory, germ-line encoded receptors, to identify foreign, stressed, infected or cancer cells and to exert destruction of the target cell after full activation. Thus, complex signals arising from multiple ligand - receptor interactions need to be integrated and form the basis of NK cell recognition and activation. In the following, we will discuss the second step in the NK cell – cancer cycle: NK cell recognition of tumor and activation by means of cell contact-dependent and cell contact independent mechanisms (Figure 2 and 3).
One, if not the most important, signal for NK cells to identify potential target cells is the loss or aberrant expression of class I Major Histocompatibility Complex (MHC-I) molecules. The recognition and elimination of MHC-I lacking cells is called “missing self-recognition”.
NK cells constitutively express a variety of inhibitory receptors of the Ly49-family in mouse and Killer Immunoglobulin-like Receptors (KIRs) in human as well as the CD94-NKG2A heterodimer in both species89,90. Inhibitory KIR and Ly49 receptors are critical for the education of NK cells during development as these receptors recognize classical polymorphic self-MHC-I molecules and thus allow NK cells to distinguish between healthy self-tissue and stressed, infected, foreign or transformed cells91 (reviewed in ref.92). To evade adaptive immunity, cancer cells frequently downregulate classical MHC-I molecules which in turn renders them susceptible to NK cell-mediated control. In addition, CD94-NKG2A recognizes less polymorphic non-classical MHC-I molecules e.g. HLA-E in human and Q-1 in mice93,94. But beside inhibitory receptors, NK cells need to receive activating signals to exert their effector function. Here, below we will discuss contact-dependent NK cell activation in the TME (Figure 2 and 3).
NK cells are equipped with an armory of activating receptors, which are thought to recognize stress-induced ligands on cancer cells. Natural cytotoxicity receptors (NCRs), namely NKp46 (NCR1/CD335), NKp44 (NCR2/CD336) and NKp30 (NCR3/CD337) belong to the immunoglobulin (Ig) superfamily and are associated with various ITAM-containing adaptor proteins to recruit and activate downstream kinases (e.g. Lck, Fyn, Syk and ZAP-70) to fully activate NK cells (reviewed in ref.95). The identification of cancer cell ligands for NCRs is still a matter of ongoing research. While some ligands for NKp30 have been identified, e.g. B7-H6 and BCL-2-associated athanogene 6 (Bag-6), the ligands for NKp46 remain unknown96, 97. NKp80 which has activating properties in NK cells binds to activation-induced C-type lectin (AICL, encoded by CLEC2B), which is upregulated by TLR stimulation on myeloid cells98. Recently, Barrow et al. identified the platelet-derived growth factor (PDGF)-DD as a ligand for NKp44 using a secretome library screen99. NK cells activated with PDGF-DD secreted IFNγ and TNF, leading to cell cycle arrest of melanoma, ovarian and breast cancer cells in vitro. Importantly, increased PDGF-DD gene expression correlated with NCR2 and effector cytokine expression and was associated with a favourable survival in glioblastoma patients99. In line with the idea that NCRs can sense soluble mediators Nidogen-1, an extracellular matrix protein, was recently described to bind NKp44100. Together these data suggest that NK cells can be activated or inhibited by secreted molecules engaging with NCRs. This novel concept opens up a new avenue of research with potential therapeutic value. Beside NCRs, the lectin-like type 2 transmembrane receptor NKG2D plays a crucial role in NK cell-mediated tumor cell killing. NKG2D is expressed on the majority of NK cells in humans and mice and recognizes a variety of MHC-related ligands that are poorly expressed in healthy tissues but strongly expressed in cancer cells101, 102. In mice retinoic acid early inducible-1 (RAE-1), murine UL16-binding protein like transcript-1 (MULT-1) and H60 proteins are ligands for NKG2D, while the ligands for human NKG2D are UL16-binding proteins and MHC class I-chain-related proteins (MICA/MICB)103, 104.
Adhesion molecules have also been shown to promote NK cell activation. Lymphocyte function-associated antigen-1 (LFA-1) is expressed on NK cells and interacts with intercellular adhesion molecules (ICAMs) on target cells. Binding of LFA-1 to ICAM-1 can enhance NK cell-mediated cytotoxicity through enhanced polarization of the cytoskeleton machinery, which is required for effective delivery of cytotoxic granules105. DNAX accessory molecule-1 (CD226/DNAM-1) also contributes to NK cell adhesion, migration and function106. Upon binding its ligand CD155 or CD112, both frequently expressed on cancer cells, CD226 promotes NK cell activation and cytotoxicity107.
NK cells are also regulated by many soluble extracellular factors in the TME. Notably, it has become increasingly clear that tumor cells and associated myeloid cells and fibroblasts, secrete a number of environmental factors such as cytokines, growth factors, exosomes, and microRNAs impacting the NK cell response. These have been extensively reviewed elsewhere 108,109,110,111 and to name a few key ones include TGF-β1 and associated family members, IL-10, extracellular adenosine, prostaglandin E2, and nitric oxide. Additionally, hypoxia and metabolic reprogramming impacts NK cell responsiveness 112,113,114. These represent non-receptor immune checkpoints for NK cells that now shape many of the new therapeutic approaches to maintain and boost NK cell effector functions in tumors (see below).
Another important axis of NK cell activation in the TME is driven by proinflammatory cytokines and danger associated molecular patterns (DAMPs). Cytokines, upon binding their cognate receptor, augment activation, survival, proliferation and maturation of NK cells. Cytokines with NK cell stimulatory capacities are IL-2, IL-12, IL-15, IL-18 and IL-21. While IL-2 and IL-15, either alone or in combination with other cytokines promote survival and proliferation, IL-12 and IL-18 mainly stimulate IFN-γ production and cytotoxicity in NK cells. IL-21 can enhance NK cell-mediated cytotoxicity by upregulating granzymes and perforin, and exhibited synergistic effect with IL-2 for NK cell activation115. Additionally, type I and III interferons are important for NK cell homeostasis and activation116,117. Soluble ligands also have a strong impact on NK cell activation. One example is soluble HLA-G, which is able to activate human NK cells via KIR2DL4 leading to the production of cytokines and chemokines 118. Soluble NKG2D ligands can inhibit NK cell function by downregulation of NKG2D119. In contrast, Deng et al. showed that soluble forms of high-affinity NKG2D ligands led to NK cell activation120. Thus, it remains unclear what role soluble NKG2D ligands play in NK cell activation.
Step 3. NK cell killing of tumor cells
NK cells are able to kill local and disseminated tumor cells (Figure 2 and 3, step 3). Furthermore, an eleven-year follow-up study found that reduced NK cell killing capacity in the peripheral blood is correlated with tumor development121. Thus, NK cell killing of local and disseminated tumor cells is an important effector function that can help control tumorigenesis. The mechanisms used by NK cells to kill cancer cells have been extensively discussed and are summarized in Box 1. Paradoxically, NK cell killing and abundance in the tumor correlate with better patient outcomes (Reviewed in 122,40, 41,42,43,44,45, 46, 47, 48, 49, 50, 51, but these cells are found at relatively low levels in tumors40, 41,42,43,44,45, 46, 47, 48, 49, 50, 51. This finding has led many to ponder how this rare cell type can be integral for protecting against cancer. While NK cell killing is clearly protective, NK cells have a number of functions, such as cytokine and chemokine production, that shape the immune response to cancer (discussed in Step 5 of the NK cell – Cancer cycle) that could amplify their importance. As such, in addition to the obvious anti-tumor activity of direct cytotoxicity, NK cell-mediated killing of cancer cells also has important impacts on the availability of antigen for presentation, both in the context of normal immune responses as well as in more clinical settings where monoclonal antibodies are used to induce antibody-dependent cellular cytotoxicity (ADCC) (reviewed in ref.123). NK cell induced tumor cell death can lead to increased release of tumor antigen, which, if phagocytosed and processed by DCs, can increase the amount of tumor antigen presented to T cells, thus acting as a possible mechanism to boost T cell responses to cancer. Thus, NK cell killing of tumor cells can occur in the primary or disseminated tumor and can lead to a release of tumor antigens to prime an adaptive immune response, but this likely does not explain all of the protection afforded by the presence of NK cells in solid tumors.
Box 1: NK cell-mediated killing.
Cytotoxic granules contain a number of cytotoxic proteins, including the pore-forming proteins perforin and/or granulysin and effector proteases called granzymes201,202, 203. A recent study using mass cytometry to profile the expression of cytotoxic molecules in PBMCs found that human NK cell subsets have differential expression of cytotoxic molecules; with CD56bright NK cells showing low GzmB and perforin expression and high GzmK expression, while CD56dim NK cells showed high GzmB and perforin expression, with low GzmK expression204. These findings are consistent with the different cytotoxic activities of both NK cell subsets. In the context of tumor immunity this is also interesting given the clear bias towards recruitment of CD56bright NK cells to the tumor (as described in section: NK cell recruitment). Granule exocytosis normally accounts for antibody-mediated cellular cytotoxicity (ADCC), a mechanism through which Fc receptor-bearing NK cells can recognize and kill antibody-coated target cells expressing tumor-derived antigens on their surface. This is a particularly relevant consideration for antibody-based therapies. Death receptor signaling: In addition to NK cell-mediated killing by cytotoxic granules, activated NK cells are capable of inducing apoptosis in target cells through the interaction of Fas ligand (FasL/CD95L) and/or TNF-related apoptosis-inducing ligand (TRAIL) with death receptors on the surface of target cells. TRAIL and FasL have been shown to play an important role in controlling immune responses to cancer205. In particular these mechanisms appear important in NK cell-mediated control of liver metastasis206,207, NK cell-mediated killing of immature DC208, and prenatally in the function of fetal NK cells206. In the context of the NK cell cancer cycle, tumor necrosis factor alpha (TNF-α) likely plays an important role in modulating the TME and inducing direct cytotoxicity of tumor cells209. However, studies have shown that TNF-α alone is weakly cytotoxic or cytostatic and that TNF-α induces apoptosis only when metabolic inhibitors are present (Reviewed in ref.210). Thus, it remains unclear whether NK cell-derived TNF-α controls tumors directly by cytotoxicity of tumor cells, or indirectly on the immune microenvironment, and on the tumor vasculature209.
Step 4. NK cell orchestration of adaptive immune responses
The most common ways that NK cells exert their effect on the adaptive immune response to cancer is through the production of cytokines and modulating dendritic cell (DC) responses (Figure 2 and 3, step 4).
Activated NK cells produce a variety of cytokines, including IFN-γ, GM-CSF, G-CSF, M-CSF, TNF, IL-5, IL-10, IL-13, and others124. IFN-γ is one of the best studied cytokines in the context of anti-tumor immunity and is a major factor in regulating positive and negative anti-tumor immunity. As the details of the mechanisms by which IFN-γ regulates immune responses have recently been discussed in great detail (reviewed in ref.125,126), we will focus our discussion on how NK cell production of IFN-γ is linked to changes in the adaptive immune response to cancer.
IFN-γ acts directly on a variety of immune cells including macrophages, DCs, B cells, T cells, and even NK cells themselves. IFN-γ signaling in macrophages activates these cells leading to increased inflammatory cytokine production, increased phagocytosis and antigen presentation, and enhanced nonspecific cytocidal activity toward microbial pathogens and tumors127. Additionally, IFN-γ induces DC maturation, which induces an increase in MHC-I and II expression, upregulation of co-stimulatory molecules, and upregulation of the cellular machinery needed for processing antigens to present to T cells128. In addition to upregulating antigen presentation machinery, IFN-γ activation of DCs has been shown to induce expression of the cytokines IL-12 and IL-15 in DCs which can play an important role in inducing anti-tumor Th1 CD4 T cell and CD8 cytotoxic T cell immune responses129,104,130,131,132,133. IFN-γ signaling also affects T cell function directly. IFN-γ signaling in CD4+ T cells can push them into an anti-tumor Th1 phenotype and induces an upregulation of granzyme and IL-2 receptor on CD8+ T cells, licensing these cells to their full cytotoxic potential (reviewed in ref.125,126). Furthermore, IFN-γ can directly increase antigen presentation in tumor cells, leading to increased tumor immunogenicity134,135,136,137,138,139.
It is important to note that while IFN-γ is a strong driver of anti-tumor immunity it also contributes to immune evasion through increased expression of immune suppressive molecules such as programmed death-ligand 1 (PD-L1) on tumor and myeloid cells in the TME. Thus, this cytokine can act as a double-edged sword and its function may depend on how it is spatially distributed. More work is required to fully understand the role of IFN-γ in tumor progression and anti-tumor immunity; however, there is clear evidence that IFNγ production by NK cells is a major factor that allows NK cells to be integral players in shaping the adaptive immune responses to cancer and disease.
It is well established that there is cross-talk between NK cells and DCs and that the interaction between these two innate immune cell types leads to profound adaptive immune responses to disease and cancer (reviewed in ref.140,141). DCs are key players in the induction of T cell immune responses and, as antigen presenting cells (APCs), bridge the gap between the innate and adaptive immune system, making them an important partner in NK cell regulation of adaptive immune responses. Developmental, phenotypical and functional criteria distinguish DCs into two broad classes, the conventional DCs type 1 (cDC1s) and conventional DCs type 2 (cDC2s) in humans and mice. cDC1s are classically described as mediators of cellular immunity against intracellular pathogens and cancer, at least partially due to their specialization to cross-present antigens to CD8+ T cells, while cDC2s are more heterogenous and thought to be more efficient at inducing CD4+ T cell responses in cancer (reviewed in ref.142). There is a rich literature describing the relationship between NK cells and DCs. Initial studies showed that NK cells likely play an important role in shaping DC responses through editing DCs by directly killing immature DCs or by inducing maturation of DCs 143,144,145,146,147,148,131,149. Thus, as is a common theme in immunology, the NK – DC interaction may act to induce proper and full DC function, but in certain settings can also negatively regulate adaptive immune responses. However, all of these data together clearly provide evidence of a functional link between NK cell activation and anti-tumor adaptive immune responses.
In addition to the important role of NK cells in regulating DC maturation, recent studies have found that NK cells play a role upstream of DC maturation and are also key regulators of DC recruitment, retention, and/or survival in the TME43,44. In a transplantable BRAFV600E mouse model of melanoma it was shown that NK cells are key in producing the chemokines CCL5 and XCL1/2 to recruit cDC1 into the tumor44. Importantly, the NK cell-dependent recruitment of cDC1 to the tumor is only seen in the absence of tumor-produced prostaglandin E2 (Ptgs1/Ptgs2−/−), suggesting that NK cell production of CCL5 and XCL1/2 and recruitment of cDC1 into the tumor is acutely sensitive to the immune-suppressive prostaglandin E2 (PGE2). NK cells in the TME also make the cytokine FLT3LG, the formative cytokine for cDC1150,151, and NK cell levels and FLT3LG expression in the tumor correlates with increased cDC1 levels in the TME43. FLT3LG production by NK cells may be regulating cDC1 levels in the TME by increasing differentiation of precursor DCs (pre-DCs) in the TME or by increasing the survival of cDC1 within the TME43,152. Together, these findings suggest that NK cells, in addition to regulating DC maturation and subsequent T cell priming, are integral in recruiting and supporting cDC1 levels in the TME, an important function given the role cDC1 in the TME play in supporting protective immune responses to cancer43,152,142,153,154,155.
In this step of the NK cell – cancer cycle, it is clear that NK cells directly modulate T cell activity through the production of IFNγ while also shaping the DC response to cancer through induction of DC maturation and the recruitment and maintenance of DCs in the TME. The findings presented here suggest that NK cells play an integral role in coordinating and initiating the adaptive immune response to cancer.
Targeting NK cells in cancer – progress and challenges
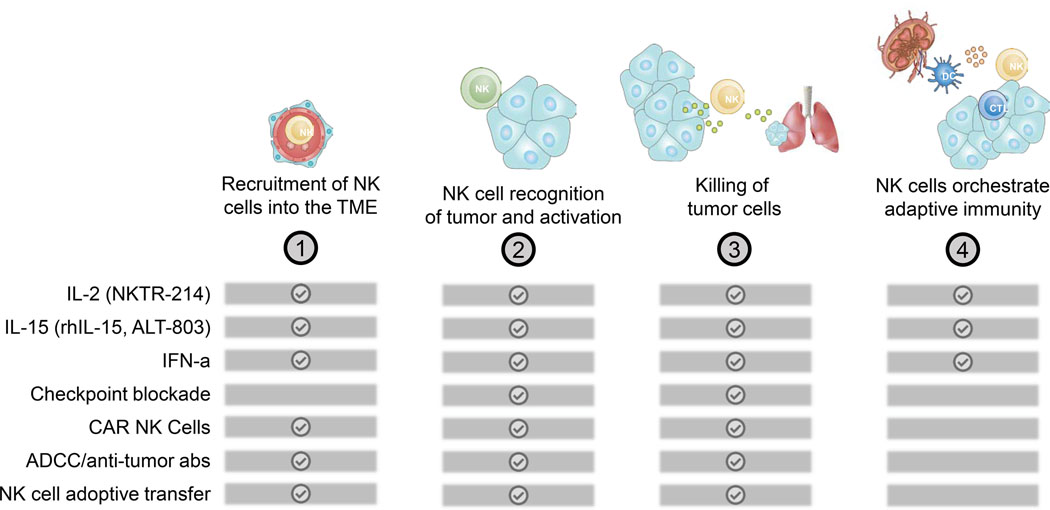
In the previous sections, we discussed individual steps required for NK cell recruitment, activation and effector function in the TME. The knowledge gained from basic and preclinical research over the last 40 years lay the foundation for the development of NK cell-based cancer immunotherapies. In the next section, we will discuss encouraging results and limitations of NK cell-based therapeutic approaches currently under preclinical and clinical evaluation (Figure 4).
Figure 4.
Therapies affecting individual steps of the NK cell-Cancer cycle.
The ideal therapeutic approach should aim to improve NK cells at every step in the NK cell-cancer cycle. A major problem for targeting NK cells in cancer patients is that many tumors are sparsely infiltrated with NK cells. Thus, a leading approach to boost NK cell-mediated tumor immunity is the adoptive transfer of ex vivo activated autologous (from the same patient) or allogeneic (from a healthy donor) NK cells. While adoptively transferred T cells can cause many severe side effects (e.g. cytokine release syndrome, graft-versus-host disease, etc.), the transfer of NK cells is comparatively safe (reviewed in ref.10). For example, two clinical trials showed efficacy of adoptively transferred haploidentical NK cells in non-Hodgkin’s lymphoma and refractory or relapsed myelodysplastic syndrome as well as secondary AML and de novo AML patients, respectively156,157. Although encouraging results have been achieved in patients with liquid cancers, response rates in patients with solid cancers remain unsatisfying. Genetic engineering of NK cell products is a promising approach to improve the efficacy of NK cell transfer therapies (Box 2). One strategy currently under preclinical evaluation is to overexpress activating molecules including NKG2D, CXCR2 or membrane bound IL-15 in NK cells158,159,160,161, or alternatively, to reduce the expression of inhibitory receptors like NKG2A162. Kamiya et al. elegantly showed that expression of a single-chain variable fragment binding NKG2A fused with an endoplasmic reticulum-retention domain in NK cells prevents the shuttling of NKG2A to the cell surface162. NKG2A-modified NK cells showed superior killing against HLA-E expressing target cells162. The discovery and development of the CRISPR/Cas9 genome editing technology, further opens up a future avenue to enhance NK cell products by modifying the expression levels of activating or inhibitory molecules (reviewed in ref.163).
Box 2: CAR NK cells harness the power of innate lymphocytes.
Expression of chimeric antigen receptors (CAR) in T cells represents an effective strategy to redirect the specificity of effector cells. Adoptive transfer of CAR T cells induces significant and durable responses averaging at around 60–70 % across multiple cancer types. Thus, two anti-CD19 CAR T cell products are approved by the FDA and currently in clinical use. However, beside logistical challenges (e.g. sophisticated and expensive production) the biggest problem of CAR T cells is the severe toxicities observed in patients including cytokine release syndrome, neurotoxicity and Graft-versus-host-disease211. A recent clinical trial using cord blood-derived HLA-mismatched anti-CD19 CAR NK cells showed a 73% response rate in non-Hodgkin’s lymphoma and chronic lymphocytic leukemia patients. Most importantly, the treatment was not associated with adverse immune-related events166. This very promising study highlights the safety and therapeutic potential of “off-the-shelf” CAR NK cells. There are a number of properties of NK cells that need to be considered with respect to CAR NK cell development. NK cells are more difficult to expand in large numbers than T cells and their persistence in vivo after infusion is limited. NK cells are also educated continually by molecules such as MHC class I212 and their functions are highly regulated by a balance of inhibition and activation signals, meaning that their action may be short-lived. NK cells cryopreserve quite well like T cells, but NK cells are more difficult to transfect and genetically engineer than T cells. CAR have higher affinity for tumor antigen than either endogenous NK cell receptors or TCR, but synthetic immunology is very effective for NK cells in the context of ADCC. Many questions remain open, such as what is the best target and would additional genetic manipulation of NK cells create superior products? Furthermore, the question of whether CAR NK cells are superior over CAR T cells needs to be addressed in prospective clinical trials.
NK cell expression of chimeric antigen receptors (CARs), directed against surface antigens expressed by tumor cells, is another encouraging approach. For example, NK cells expressing anti-CD19 CARs can efficiently kill autologous acute lymphoblastic leukemia (ALL) cells, which are resistant to CAR-negative NK cell-mediated killing164. Similarly, Li et al. recently showed that anti-mesothelin CAR expressing NK cells, derived from induced pluripotent stem cells (iPSCs) significantly impaired the growth of ovarian cancer in a xenograft model165. There, not only were they able to generate CAR NK cells from iPSCs providing another resource for an “off-the-shelf” NK cell product, but they also designed next-generation NK cell-specific CAR constructs. After screening multiple CAR-variants, NK cells expressing a CAR containing the transmembrane domain of NKG2D, the co-stimulatory domain of 2B4 as well as the signaling domain of CD3ζ showed the strongest efficacy165. Importantly, a landmark study by Liu et al. demonstrated that adoptive transfer of allogenic anti-CD19 CAR NK cells was safe and effective in high-risk CD19+ chronic lymphocytic leukemia and non-Hodgkin lymphoma patients166. This clinically relevant study, is encouraging and highlights the potential for future CAR NK-based therapies.
NK cells express or upregulate a variety of inhibitory receptors such as KIRs, NKG2A/CD94, programmed death-1 (PD-1), T cell immunoglobulin- and mucin-domain-containing molecule 3 (TIM-3), cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), lymphocyte activation gene 3 (LAG-3), T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibition motif domains (TIGIT), and CD96 (reviewed in ref.167,168, 169,170). Recent reviews have thoroughly discussed our understanding of these inhibitory pathways and NK cells in cancer (reviewed in ref.167,168, 169,170). In many cases, the underlying biological mechanisms of these inhibitory pathways, as well as the clinical benefit of targeting the majority of these inhibitory receptors, need to be further explored. Here, we will focus our discussion on inhibitory pathways that have recently been studied in a clinical setting.
The first strategy tested to enhance NK cell function aimed to block HLA-inhibitory receptor interactions. The engagement of inhibitory receptors with HLA-molecules is considered a major impediment to NK cell activation. IPH2101 (1-7F9, human IgG4) mAb binds with high affinity to the inhibitory receptors KIR2DL-1,−2 and −3 and thereby prevents inhibitory signalling mediated by HLA-C molecule allotypes171. Phase I clinical trials showed that IPH2101 enhanced NK cell activation and ex vivo cytotoxicity and is safe and well tolerated by cancer patients172, 173. Pre-clinical studies supporting the combination of KIR blocking with lenalidomide or with rituximab for the treatment of multiple myeloma and lymphoma, respectively, have been previously reviewed174. Although in vitro studies had suggested IPH2101 induced KIR-ligand mismatched tumor killing by NK cells, a phase II clinical trial in patients with smoldering MM (KIRMONO) was terminated early due to a lack of clinical efficacy175. Subsequent investigations revealed that KIR2D molecules were removed from the surface of IPH2101-treated NK cells by trogocytosis, with reductions in NK cell function directly correlating with loss of free KIR2D surface molecules176. These data raise concerns that the unexpected biological events could compromise some antibody-based strategies designed at augmenting NK cell tumor killing177. In spite of its limited clinical benefits as a single agent, future studies will need to assess the potential of IPH2101 in combination therapies.
In addition to KIRs, the heterodimer NKG2A-CD94 has also received great attention. The heterodimer NKG2A-CD94 binds to the non-classical HLA class I molecule HLA-E (Qa1b in mice), which is often up-regulated on cancer cells. Recently, it has been shown that NKG2A inhibits NK activation and target cell killing123. Monalizumab (IPH2201), a blocking humanized anti-NKG2A antibody, has been tested in a number of clinical trials as a single agent or in combination across different cancers, however many open questions remain (Box 3 and extensively reviewed in178). Two recent studies have further shed light on NKG2A as a cancer immunotherapeutic target (reviewed in ref.179). Van Montfoort et al. demonstrated in preclinical solid tumor models that peptide vaccination combined with antibody blockade of NKG2A on CD8+ T cells improved response rate and survival of mice over peptide vaccination alone180. While, André et al. demonstrated activity of NKG2A inhibition in combination with anti-PD-1/PD-L1 blockade in mouse lymphoma models and in human in vitro experiments181. In addition, the interim results of a clinical trial in head and neck squamous cell carcinoma patients of combination of monalizumab and cetuximab, a clinically approved anti-EGFR antibody, led to a 30% objective response rate in immunotherapy refractory patients. Although these results are very promising and provide hope for future improvement of immunotherapies for cancer patients, many open questions remain and need to be addressed in preclinical and clinical studies.
Box 3: NKG2A one of the most promising new targets in cancer immunotherapy?
The inhibitory receptor complex NKG24/CD94 is an attractive and promising target213,214. Currently, six Phase I and II clinical trials using monalizumab as single agent or in combination are recruiting patients across different solid and hematological malignancies including Head and Neck Squamous Cell Carcinoma and Non-Small Cell Lung Cancer (NSCLC) (NCT02671435, NCT02921685, NCT02643550, NCT03822351, NCT03794544, NCT03088059). Furthermore, two phase II trials in microsatellite stable colorectal cancer and immunotherapy resistant NSCLC patients are about to start (NCT04145193, NCT03833440). Despite these clinical efforts, translational research will need to address pressing questions around the NKG2A-pathway. Little is known about the expression of NKG2A/CD94 in tumor infiltrating CD8+ and CD4+ T cells. Additionally, we need to understand the regulation and expression of HLA-E in healthy and malignant tissue to potentially be able to stratify patients based on the expression of the NKG2A Ligand. The work by André et al. and van Montfoort et al. also raise the question about the relative contribution of NKG2A for the function of NK and T cells181,180. Overall, blocking or genetic deletion of NKG2A in effector cells seems to be a rational strategy and future studies will shed further light on the underlying biology and clinical relevance of this molecule.
As described in step 3 of the NK cell cancer cycle, cytokines play an important role in NK cell activation and function (Figure 2 and 3). Consistent with preclinical studies clinical work has focused on using NK cell stimulatory cytokines to increase NK cell activity and abundance in the TME, with some of these immunotherapies having beneficial effects on patient survival and disease outcome182. In this review we will focus on three NK cell stimulatory molecules being explored in the clinic: IL-2, IL-15, and IFN-α.
IL-2 activates NK cells by binding to the heterotrimeric IL-2 receptor (IL-2Rα/CD25, IL-2Rβ/CD122, and IL-2Rγ/CD132). Early studies found that recombinant IL-2 expands effector T cells and NK cells, but also induces a robust Treg cell expansion in patients. Thus, a number of drugs have been developed that lead to preferential activation and expansion of CD8+ T cells and NK cells. The preferential targeting of CD8+ T cells and NK cells has been accomplished by skewing binding of IL-2 away from the IL2Rα subunit, which is more abundant on Treg cells182,183. One such drug, NKTR-214 (Bempegaldesleukin), is a modified recombinant IL-2 protein that is well tolerated by patients and is capable of inducing a robust activation and increase of CD8+ T cells and NK cells within the TME without changing Treg cell numbers184. Monotherapy with NKTR-214 to heavily pre-treated, non-responsive patients with solid tumors led to 9 of 26 patients showing some level of stable disease, although no objective responses were measured by RECIST criteria184. These findings led to the combination of NKTR-214 with anti-PD-1 immunotherapy in patients with advanced solid tumors and preliminary results presented at the 2019 Society for Immunotherapy of Cancer (SITC) Annual Meeting, suggest that response to this combination therapy were durable and increased over time, with evaluable patients showing an objective response rate (ORR) of 53% (20/38) and 34% (13/38) of patients achieving complete responses (CR) at a median time of follow-up of 18.6 months (NCT02983045). Phase three trials are currently underway using NKTR-214 in combination with Nivolumab (anti-PD-1) in melanoma (NCT03635983), muscle invasive bladder cancer (NCT04209114), and renal cell carcinoma (NCT03729245). Other IL-2 combination therapies have been less successful, as bevacizumab, an inhibitor of VEGF and an antibody thought to induce ADCC, showed no benefit above IL-2 therapy alone185.
IL-15 also has stimulatory capacity for NK cells, but does not induce Treg expansion. Intravenous infusion or subcutaneous injection of recombinant human IL-15 (rhIL-15) led to expansion and activation of NK cells and CD8+ T cells in patients with solid tumors186,187. While there were hints to clinical benefit in patients treated with rhIL-15, no responses were detected based on RECIST criteria186,187. Larger studies will be needed to fully elucidate the clinical benefit of rhIL-15 monotherapy. rhIL-15 has also been combined with haploidentical NK cell infusions in refractory acute myeloid leukemia (AML) and this treatment led to remission in 35% of patients and better rates of in vivo NK cell expansion and remission compared to previous trials with IL-2188. While rhIL-15 holds therapeutic promise, animal studies have found that the IL-15 superagonist, IL-15 pre-associated with its soluble receptor IL-15Rα, can lead to a large increase in biological activity and enhanced activity as a cancer immunotherapeutic189. ALT-803 is a pharmacological grade IL-15 superagonist that promotes expansion and activation of NK cells and CD8+ T cells in patients with post-relapse hematologic malignancies or solid tumors190,165. ALT-803 has shown some clinical response as a monotherapy in hematologic malignancies and solid tumors and can also induce signs of clinical response in combination with anit-PD-1 immunotherapy in patients with non-small cell lung cancer (NSCLC) that had refractory or relapsed disease from previous anti-PD-1 treatment190,165,191. These early clinical studies highlight an important role for NK cell expansion by recombinant IL-15 or IL-15 superagonists and have led to the initiation of a number of active Phase 2 or 3 studies across different cancer indications and in combination with other therapies (including: NCT02989844, NCT03586869, NCT03387098, NCT03329248, NCT03228667, NCT03136406, NCT02523469, NCT02384954, NCT01885897, NCT03022825, NCT02138734).
IFN-α treatment can activate NK cells and T cells to kill cancer cells in acute myeloid leukemia patients192. A major issue facing patients receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) to treat acute leukemia is post-transplant relapse. Patients with minimal residual disease following allo-HSCT were found to have lower relapse rates if they were treated with IFN-α193,194. This finding was attributed to immunomodulation of NK cells and T cells, or alternatively, through direct inhibitory effects of IFN-α on blast cells193,194. Future multicenter clinical studies will be necessary to confirm the efficacy of IFNα treatment to protect against relapse in leukemia patients with minimal residual disease.
Clearly, the modulation of NK cells through the treatment of patients with cytokines is an important area of clinical research. The potential benefits of cytokine therapies are clear, but these therapies are better understood in hematologic malignancies than in solid tumors. The large number of ongoing clinical trials in hematologic malignancies and solid tumors are going to lead to exciting findings and novel treatments that will undoubtedly target NK cells at every step of the NK cell – cancer cycle.
Bi-specific or tri-specific killer cell engagers (BiKEs or TriKEs) represent an alternative strategy to efficiently engage CD16 and induce ADCC-like responses (reviewed in ref.174, 195, 196, 197). Initial BiKE and TriKE constructs fused a single-chain variable fragment (Fv) against CD16 with, in the case of BiKEs, a single-chain Fv against a tumor antigen, or in the case of TrIKEs, two tumor antigens. Strategies for BiKEs and TriKEs are many, but include: single tumor antigen targeting (e.g. CD19, CD20, CD30, CD133, PMSA, BCMA, Her2, CEA, EGFR, etc.), dual tumor antigen targeting (allowing for avidity-tuned binding to two cancer antigens which increases the range of targetable tumors), dual TME targeting (allowing for avidity-tuned binding of two antigens on tumor-promoting cells occurs without affecting cells in healthy tissues), and dual targeting of both tumor and TME antigens (combining the prior two approaches). Some molecules like PD-L1, CD155, CD47, and CD38 may serve the purpose of acting as tumor antigens or immunosuppressive immune cell surface antigens elevated in the TME. The Fc itself when required may be afucosylated to enhance ADCC or Fc mutated to silence that function. Nanobodies (Nbs) derived from camelid animals are emerging as a new force in antibody therapy.
Killer engagers are designed by fusing Fv domains that recognize tumor cell antigens with Fv domains binding CD16 and other relatively NK cell-specific surface activation molecules (eg. NKp30, NKp44, NKp46, NKG2D). There are very few of these other NK cell-specific molecules of choice at this stage, but molecules like NKp30 appear to be stably expressed by all NK cells and its ligation can trigger strong degranulation and cytokine release from NK cells. In the absence of cytokines, resting NK cells can be stimulated by combining the activity of a hierarchy of several activating receptors. CD16 is unique in its ability to mediate ADCC and to activate significant cytotoxicity and cytokine secretion when triggered alone. Many of the ligands recognized by NK cell receptors represent the body’s method to detect altered or defective cells and support immune activation. The amount of ligand expression on the cell surface can also be modulated by ligand shedding, secretion of ligands, or excretion in macrovesicles. The degree that such ligand regulation affects immune targeting remains to be determined. Recently, Ferrari de Andrade et al. generated an antibody against the human NKG2D ligand MICA/B (Major Histocompatibility Complex Class-I chain related gene A/B), which prevented proteolytic shedding of the extracellular domains of MICA and MICB and stimulated antitumor immunity by activation of NKG2D and CD16 on NK cells. In a metastasis model pre-treated with human NK cells, this antibody effectively inhibited tumor growth in vivo198.
A number of Important considerations need to be considered in this area are. First, how does the multivalent therapeutic overcome tumor escape from NK cells? Second, is the engager regulated by the TME (e.g. by molecules such as TGF-β)? For TriKE constructs it remains to be seen if the engagement of the NK cell-specific engager (eg. NKp30) is sufficient or if CD16 signalling is also required? It also remains to be seen if all (or a subset of) NK cell effector functions and proliferation are enhanced by these molecular designs. Another important consideration is if the activation by these therapeutics is too effective, will this lead to NK cell exhaustion? Further, more work is needed to determine what combination therapies would make a good partner with multivalent therapeutics to improve NK cell numbers, recruitment to and survival in the TME? Lastly, there is a risk that antigen negative variants of tumors will selectively grow out over time.
Regarding the TME, tailored additional modules (like TGF-β traps) and survival promoting cytokines (like IL-15 and IL-2) remain attractive strategies built into NK cell engagers (e.g. BiKEs, TriKEs, and killer enagers). An interesting strategy of simultaneous blockade of the immune checkpoint ligand PD-L1 and TGF-β, using a bifunctional antibody-ligand trap was recently reported199. But overall, these multivalent drugs are currently being investigated in preclinical studies and safety remains a concern with the potential to trigger cytokine cascades. With these future therapeutics different constructs have been engineered and indeed hundreds of possible formats may be tested pre-clinically, while some subset of these will reach the clinic in the future. We believe this whittling process should be guided by the rational provided by the NK cell – cancer cycle presented herein.
Outlook
The therapeutic potential of NK cells is incredibly high and the recent findings that CAR-transduced NK cells have low toxicity and are associated with high response rates drive home the importance of NK cells as potential immunotherapies to treat patients166. NK cell-based immunotherapies have been more efficacious in hematologic cancers, but progress is also being made developing these therapies for solid tumors (reviewed in ref. 200). There is a growing excitement about the use of these cells to target cancer and our understanding of the NK cell – cancer cycle is constantly evolving. While NK cell cytotoxicity is an obvious effector function that plays important roles in controlling cancer, an equally important role of NK cells is to shape the TME and to modulate adaptive immune responses to cancer. Given the diverse roles NK cells play in shaping the immune response to cancer, described here in the NK cell – cancer cycle, as we develop and design novel therapies it will be important to try to target pathways that will hit all, or the majority, of the steps in the NK cell – cancer cycle with a focus not just on inducing NK cell cytotoxicity, but also to harness the immunomodulatory effects of NK cells.
Acknowledgements
T.B. was supported by a National Health and Medical Research Council (NH&MRC) Early Career Research Fellowship (1138757) and Project Grant (1124690). M.J.S. was supported by a NH&MRC Investigator Award (1173958) and Program Grant (1132519), a Cancer Research Institute CLIP grant, and a Cancer Council of Queensland Project Grant (1140251).
Footnotes
Competing interest statement
T. Bald has research agreements with ENA Therapeutics and Bristol Myers Squibb and is on the scientific advisory board of Oncomyx. M.F. Krummel is a founder and shareholder of Pionyr Immunotherapeutics and has research agreements with Bristol Myers Squib, Eli Lilly, Pfizer, Amgen, Abbvie and Genentech. M.J. Smyth has research agreements with Bristol Myers Squibb and Tizona Therapeutics and is on the scientific advisory board of Tizona Therapeutics and Compass Therapeutics. K.C. Barry declares no conflict of interest.
References
- 1.Vivier E et al. Innate Lymphoid Cells: 10 Years On. Cell 174, 1054–1066 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Colonna M Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity 48, 1104–1117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiossone L, Dumas PY, Vienne M & Vivier E Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18, 671–688 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Soto A, Gonzalez S, Smyth MJ & Galluzzi L Control of Metastasis by NK Cells. Cancer Cell 32, 135–154 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Gasteiger G & Rudensky AY Interactions between innate and adaptive lymphocytes. Nat Rev Immunol 14, 631–639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber RD, Old LJ & Smyth MJ Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Vesely MD, Kershaw MH, Schreiber RD & Smyth MJ Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29, 235–271 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg J & Huang J CD8(+) T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy. Curr Opin Chem Eng 19, 9–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingegnere T et al. Human CAR NK Cells: A New Non-viral Method Allowing High Efficient Transfection and Strong Tumor Cell Killing. Front Immunol 10, 957 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimasaki N, Jain A & Campana D NK cells for cancer immunotherapy. Nat Rev Drug Discov (2020). [DOI] [PubMed] [Google Scholar]
- 11.Deguine J, Breart B, Lemaitre F, Di Santo JP & Bousso P Intravital imaging reveals distinct dynamics for natural killer and CD8(+) T cells during tumor regression. Immunity 33, 632–644 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Halle S, Halle O & Forster R Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol 38, 432–443 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Chiang SC et al. Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood 121, 1345–1356 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Vanherberghen B et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood 121, 1326–1334 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Choi PJ & Mitchison TJ Imaging burst kinetics and spatial coordination during serial killing by single natural killer cells. Proc Natl Acad Sci U S A 110, 6488–6493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasconcelos Z et al. Individual Human Cytotoxic T Lymphocytes Exhibit Intraclonal Heterogeneity during Sustained Killing. Cell Rep 11, 1474–1485 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Sun JC & Lanier LL NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol 11, 645–657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun JC, Beilke JN & Lanier LL Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dokun AO et al. Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2, 951–956 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Lupo KB & Matosevic S Natural Killer Cells as Allogeneic Effectors in Adoptive Cancer Immunotherapy. Cancers (Basel) 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JS et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Rubnitz JE et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 28, 955–959 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barkholt L et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy 1, 753–764 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Vacca P et al. Exploiting Human NK Cells in Tumor Therapy. Front Immunol 10, 3013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woan KV & Miller JS Harnessing Natural Killer Cell Antitumor Immunity: From the Bench to Bedside. Cancer Immunol Res 7, 1742–1747 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezvani K, Rouce R, Liu E & Shpall E Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther 25, 1769–1781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonifant CL, Jackson HJ, Brentjens RJ & Curran KJ Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 3, 16011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu S, Yi M, Qin S & Wu K Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer 18, 125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimabukuro-Vornhagen A et al. Cytokine release syndrome. J Immunother Cancer 6, 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santomasso BD et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov 8, 958–971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crinier A et al. High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity 49, 971–986 e975 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins PL et al. Gene Regulatory Programs Conferring Phenotypic Identities to Human NK Cells. Cell 176, 348–360 e312 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frey M et al. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol 161, 400–408 (1998). [PubMed] [Google Scholar]
- 34.Sedlmayr P et al. Differential phenotypic properties of human peripheral blood CD56dim+ and CD56bright+ natural killer cell subpopulations. Int Arch Allergy Immunol 110, 308–313 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa Y, Huntington ND, Nutt SL & Smyth MJ Functional subsets of mouse natural killer cells. Immunol Rev 214, 47–55 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa Y & Smyth MJ CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 176, 1517–1524 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Peng H & Tian Z Diversity of tissue-resident NK cells. Semin Immunol 31, 3–10 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Chen DS & Mellman I Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Carrega P et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol 192, 3805–3815 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Wu M, Mei F, Liu W & Jiang J Comprehensive characterization of tumor infiltrating natural killer cells and clinical significance in hepatocellular carcinoma based on gene expression profiles. Biomed Pharmacother 121, 109637 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Nath PR et al. Natural Killer Cell Recruitment and Activation Are Regulated by CD47 Expression in the Tumor Microenvironment. Cancer Immunol Res 7, 1547–1561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cursons J et al. A Gene Signature Predicting Natural Killer Cell Infiltration and Improved Survival in Melanoma Patients. Cancer Immunol Res 7, 1162–1174 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Barry KC et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med 24, 1178–1191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bottcher JP et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 172, 1022–1037 e1014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali TH et al. Enrichment of CD56(dim)KIR + CD57 + highly cytotoxic NK cells in tumour-infiltrated lymph nodes of melanoma patients. Nat Commun 5, 5639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takanami I, Takeuchi K & Giga M The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 121, 1058–1063 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Ishigami S et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 88, 577–583 (2000). [PubMed] [Google Scholar]
- 48.Villegas FR et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 35, 23–28 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Soo RA et al. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget 9, 24801–24820 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muntasell A et al. NK Cell Infiltrates and HLA Class I Expression in Primary HER2(+) Breast Cancer Predict and Uncouple Pathological Response and Disease-free Survival. Clin Cancer Res 25, 1535–1545 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Remark R et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res 19, 4079–4091 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Melsen JE, Lugthart G, Lankester AC & Schilham MW Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front Immunol 7, 262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernardini G, Antonangeli F, Bonanni V & Santoni A Dysregulation of Chemokine/Chemokine Receptor Axes and NK Cell Tissue Localization during Diseases. Front Immunol 7, 402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima M et al. Chemokine Receptor Expression on Normal Blood CD56(+) NK-Cells Elucidates Cell Partners That Comigrate during the Innate and Adaptive Immune Responses and Identifies a Transitional NK-Cell Population. J Immunol Res 2015, 839684 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morandi F et al. Soluble HLA-G dampens CD94/NKG2A expression and function and differentially modulates chemotaxis and cytokine and chemokine secretion in CD56bright and CD56dim NK cells. Blood 118, 5840–5850 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Gregoire C et al. The trafficking of natural killer cells. Immunol Rev 220, 169–182 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parolini S et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 109, 3625–3632 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Campbell JJ et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol 166, 6477–6482 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Polentarutti N et al. IL-2-regulated expression of the monocyte chemotactic protein-1 receptor (CCR2) in human NK cells: characterization of a predominant 3.4-kilobase transcript containing CCR2B and CCR2A sequences. J Immunol 158, 2689–2694 (1997). [PubMed] [Google Scholar]
- 60.Allavena P et al. Induction of natural killer cell migration by monocyte chemotactic protein-1, −2 and −3. Eur J Immunol 24, 3233–3236 (1994). [DOI] [PubMed] [Google Scholar]
- 61.Ponzetta A et al. Multiple Myeloma Impairs Bone Marrow Localization of Effector Natural Killer Cells by Altering the Chemokine Microenvironment. Cancer Res 75, 4766–4777 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Marquardt N, Wilk E, Pokoyski C, Schmidt RE & Jacobs R Murine CXCR3+CD27bright NK cells resemble the human CD56bright NK-cell population. Eur J Immunol 40, 1428–1439 (2010). [DOI] [PubMed] [Google Scholar]
- 63.de Andrade LF et al. Discovery of specialized NK cell populations infiltrating human melanoma metastases. JCI Insight 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carrega P et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 112, 863–875 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Przewoznik M et al. Recruitment of natural killer cells in advanced stages of endogenously arising B-cell lymphoma: implications for therapeutic cell transfer. J Immunother 35, 217–222 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Wendel M, Galani IE, Suri-Payer E & Cerwenka A Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res 68, 8437–8445 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Voshtani R et al. Progranulin promotes melanoma progression by inhibiting natural killer cell recruitment to the tumor microenvironment. Cancer Lett 465, 24–35 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi L et al. Interleukin-33 activates and recruits natural killer cells to inhibit pulmonary metastatic cancer development. Int J Cancer 146, 1421–1434 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Gao X et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol 194, 438–445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Sullivan T et al. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep 7, 989–998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao JQ et al. Antitumor effect by interleukin-11 receptor alpha-locus chemokine/CCL27, introduced into tumor cells through a recombinant adenovirus vector. Cancer Res 63, 4420–4425 (2003). [PubMed] [Google Scholar]
- 72.Gao JQ et al. NK cells are migrated and indispensable in the anti-tumor activity induced by CCL27 gene therapy. Cancer Immunol Immunother 58, 291–299 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Degos C et al. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front Immunol 10, 877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin HY et al. CCR10 activation stimulates the invasion and migration of breast cancer cells through the ERK1/2/MMP-7 signaling pathway. Int Immunopharmacol 51, 124–130 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Simonetti O et al. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur J Cancer 42, 1181–1187 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Park MH, Lee JS & Yoon JH High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J Surg Oncol 106, 386–392 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Hyakudomi M et al. Increased expression of fractalkine is correlated with a better prognosis and an increased number of both CD8+ T cells and natural killer cells in gastric adenocarcinoma. Ann Surg Oncol 15, 1775–1782 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Ohta M et al. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol 26, 41–47 (2005). [PubMed] [Google Scholar]
- 79.Chen EB et al. The miR-561-5p/CX3CL1 Signaling Axis Regulates Pulmonary Metastasis in Hepatocellular Carcinoma Involving CX3CR1(+) Natural Killer Cells Infiltration. Theranostics 9, 4779–4794 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J et al. Increased CX3CL1 mRNA expression level is a positive prognostic factor in patients with lung adenocarcinoma. Oncol Lett 17, 4877–4890 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castriconi R et al. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol 190, 5321–5328 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Castriconi R et al. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front Immunol 9, 2324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Regis S et al. TGF-beta1 Downregulates the Expression of CX3CR1 by Inducing miR-27a-5p in Primary Human NK Cells. Front Immunol 8, 868 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carosella ED, Rouas-Freiss N, Tronik-Le Roux D, Moreau P & LeMaoult J HLA-G: An Immune Checkpoint Molecule. Adv Immunol 127, 33–144 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Lindaman A, Dowden A & Zavazava N Soluble HLA-G molecules induce apoptosis in natural killer cells. Am J Reprod Immunol 56, 68–76 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Lesport E et al. Human melanoma cell secreting human leukocyte antigen-G5 inhibit natural killer cell cytotoxicity by impairing lytic granules polarization toward target cell. Hum Immunol 70, 1000–1005 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Feng M et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer 19, 568–586 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hagenaars M et al. Characteristics of tumor infiltration by adoptively transferred and endogenous natural-killer cells in a syngeneic rat model: implications for the mechanism behind anti-tumor responses. Int J Cancer 78, 783–789 (1998). [DOI] [PubMed] [Google Scholar]
- 89.Morvan MG & Lanier LL NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16, 7–19 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Shifrin N, Raulet DH & Ardolino M NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol 26, 138–144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raulet DH, Vance RE & McMahon CW Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol 19, 291–330 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Vivier E, Nunes JA & Vely F Natural killer cell signaling pathways. Science 306, 1517–1519 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Braud VM et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 (1998). [DOI] [PubMed] [Google Scholar]
- 94.Vance RE, Kraft JR, Altman JD, Jensen PE & Raulet DH Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b). J Exp Med 188, 1841–1848 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barrow AD, Martin CJ & Colonna M The Natural Cytotoxicity Receptors in Health and Disease. Front Immunol 10, 909 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brandt CS et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med 206, 1495–1503 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pogge von Strandmann E et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27, 965–974 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Welte S, Kuttruff S, Waldhauer I & Steinle A Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol 7, 1334–1342 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Barrow AD et al. Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell 172, 534–548 e519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gaggero S et al. Nidogen-1 is a novel extracellular ligand for the NKp44 activating receptor. Oncoimmunology 7, e1470730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diefenbach A et al. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol 3, 1142–1149 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Gilfillan S, Ho EL, Cella M, Yokoyama WM & Colonna M NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol 3, 1150–1155 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Cerwenka A et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 12, 721–727 (2000). [DOI] [PubMed] [Google Scholar]
- 104.Diefenbach A, Jensen ER, Jamieson AM & Raulet DH Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413, 165–171 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Urlaub D, Hofer K, Muller ML & Watzl C LFA-1 Activation in NK Cells and Their Subsets: Influence of Receptors, Maturation, and Cytokine Stimulation. J Immunol 198, 1944–1951 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Martinet L et al. DNAM-1 expression marks an alternative program of NK cell maturation. Cell Rep 11, 85–97 (2015). [DOI] [PubMed] [Google Scholar]
- 107.Chan CJ et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 15, 431–438 (2014). [DOI] [PubMed] [Google Scholar]
- 108.Baginska J et al. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol 4, 490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stiff A et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin Cancer Res 24, 1891–1904 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Viel S et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal 9, ra19 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Gao Y et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 18, 1004–1015 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Krzywinska E et al. Loss of HIF-1alpha in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nat Commun 8, 1597 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Michelet X et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 19, 1330–1340 (2018). [DOI] [PubMed] [Google Scholar]
- 114.O’Brien KL & Finlay DK Immunometabolism and natural killer cell responses. Nat Rev Immunol 19, 282–290 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Skak K, Frederiksen KS & Lundsgaard D Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 123, 575–583 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Swann JB et al. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol 178, 7540–7549 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Souza-Fonseca-Guimaraes F et al. NK cells require IL-28R for optimal in vivo activity. Proc Natl Acad Sci U S A 112, E2376–2384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajagopalan S HLA-G-mediated NK cell senescence promotes vascular remodeling: implications for reproduction. Cell Mol Immunol 11, 460–466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Molfetta R et al. c-Cbl regulates MICA- but not ULBP2-induced NKG2D down-modulation in human NK cells. Eur J Immunol 44, 2761–2770 (2014). [DOI] [PubMed] [Google Scholar]
- 120.Deng W et al. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 348, 136–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imai K, Matsuyama S, Miyake S, Suga K & Nakachi K Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356, 1795–1799 (2000). [DOI] [PubMed] [Google Scholar]
- 122.Melaiu O, Lucarini V, Cifaldi L & Fruci D Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front Immunol 10, 3038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S & Ferris RL Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res 50, 248–254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peritt D et al. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol 161, 5821–5824 (1998). [PubMed] [Google Scholar]
- 125.Alspach E, Lussier DM & Schreiber RD Interferon gamma and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb Perspect Biol 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burke JD & Young HA IFN-gamma: A cytokine at the right time, is in the right place. Semin Immunol 43, 101280 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schreiber RD, Hicks LJ, Celada A, Buchmeier NA & Gray PW Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol 134, 1609–1618 (1985). [PubMed] [Google Scholar]
- 128.Pan J et al. Interferon-gamma is an autocrine mediator for dendritic cell maturation. Immunol Lett 94, 141–151 (2004). [DOI] [PubMed] [Google Scholar]
- 129.Kelly JM et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol 3, 83–90 (2002). [DOI] [PubMed] [Google Scholar]
- 130.Cerwenka A, Baron JL & Lanier LL Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A 98, 11521–11526 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mocikat R et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19, 561–569 (2003). [DOI] [PubMed] [Google Scholar]
- 132.Morandi B et al. NK cells provide helper signal for CD8+ T cells by inducing the expression of membrane-bound IL-15 on DCs. Int Immunol 21, 599–606 (2009). [DOI] [PubMed] [Google Scholar]
- 133.Walzer T, Dalod M, Robbins SH, Zitvogel L & Vivier E Natural-killer cells and dendritic cells: “l’union fait la force”. Blood 106, 2252–2258 (2005). [DOI] [PubMed] [Google Scholar]
- 134.Shankaran V et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001). [DOI] [PubMed] [Google Scholar]
- 135.Kaplan DH et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 95, 7556–7561 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bander NH et al. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate 33, 233–239 (1997). [DOI] [PubMed] [Google Scholar]
- 137.Seliger B et al. IFN-gamma-mediated coordinated transcriptional regulation of the human TAP-1 and LMP-2 genes in human renal cell carcinoma. Clin Cancer Res 3, 573–578 (1997). [PubMed] [Google Scholar]
- 138.Hisamatsu H et al. Newly identified pair of proteasomal subunits regulated reciprocally by interferon gamma. J Exp Med 183, 1807–1816 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Castro F, Cardoso AP, Goncalves RM, Serre K & Oliveira MJ Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol 9, 847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reschner A, Hubert P, Delvenne P, Boniver J & Jacobs N Innate lymphocyte and dendritic cell cross-talk: a key factor in the regulation of the immune response. Clin Exp Immunol 152, 219–226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wallace ME & Smyth MJ The role of natural killer cells in tumor control--effectors and regulators of adaptive immunity. Springer Semin Immunopathol 27, 49–64 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Wculek SK et al. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20, 7–24 (2020). [DOI] [PubMed] [Google Scholar]
- 143.Carbone E et al. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol 29, 4022–4029 (1999). [DOI] [PubMed] [Google Scholar]
- 144.Wilson JL et al. Targeting of human dendritic cells by autologous NK cells. J Immunol 163, 6365–6370 (1999). [PubMed] [Google Scholar]
- 145.Fernandez NC et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 5, 405–411 (1999). [DOI] [PubMed] [Google Scholar]
- 146.Della Chiesa M et al. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol 33, 1657–1666 (2003). [DOI] [PubMed] [Google Scholar]
- 147.Ferlazzo G et al. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol 33, 306–313 (2003). [DOI] [PubMed] [Google Scholar]
- 148.Piccioli D, Sbrana S, Melandri E & Valiante NM Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med 195, 335–341 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adam C et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood 106, 338–344 (2005). [DOI] [PubMed] [Google Scholar]
- 150.Broz ML et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu K & Nussenzweig MC Origin and development of dendritic cells. Immunol Rev 234, 45–54 (2010). [DOI] [PubMed] [Google Scholar]
- 152.Pulendran B et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A 96, 1036–1041 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jahchan NS et al. Tuning the Tumor Myeloid Microenvironment to Fight Cancer. Front Immunol 10, 1611 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bottcher JP & Reis ESC The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 4, 784–792 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Broz ML & Krummel MF The emerging understanding of myeloid cells as partners and targets in tumor rejection. Cancer Immunol Res 3, 313–319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bachanova V et al. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother 67, 483–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bjorklund AT et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clin Cancer Res 24, 1834–1844 (2018). [DOI] [PubMed] [Google Scholar]
- 158.Parihar R et al. NK Cells Expressing a Chimeric Activating Receptor Eliminate MDSCs and Rescue Impaired CAR-T Cell Activity against Solid Tumors. Cancer Immunol Res 7, 363–375 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chang YH et al. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res 73, 1777–1786 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Kremer V et al. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer 5, 73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Imamura M et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood 124, 1081–1088 (2014). [DOI] [PubMed] [Google Scholar]
- 162.Kamiya T, Seow SV, Wong D, Robinson M & Campana D Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest 129, 2094–2106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Afolabi LO, Adeshakin AO, Sani MM, Bi J & Wan X Genetic reprogramming for NK cell cancer immunotherapy with CRISPR/Cas9. Immunology 158, 63–69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Imai C, Iwamoto S & Campana D Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106, 376–383 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Margolin K et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin Cancer Res 24, 5552–5561 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu E et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med 382, 545–553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.O’Donnell JS, Teng MWL & Smyth MJ Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 16, 151–167 (2019). [DOI] [PubMed] [Google Scholar]
- 168.Bi J & Tian Z NK Cell Dysfunction and Checkpoint Immunotherapy. Front Immunol 10, 1999 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Minetto P et al. Harnessing NK Cells for Cancer Treatment. Front Immunol 10, 2836 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beldi-Ferchiou A & Caillat-Zucman S Control of NK Cell Activation by Immune Checkpoint Molecules. Int J Mol Sci 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Romagne F et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–2677 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Benson DM Jr. et al. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clin Cancer Res 21, 4055–4061 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Benson DM Jr. et al. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood 120, 4324–4333 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guillerey C, Huntington ND & Smyth MJ Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17, 1025–1036 (2016). [DOI] [PubMed] [Google Scholar]
- 175.Korde N et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica 99, e81–83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carlsten M et al. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin Cancer Res 22, 5211–5222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Felices M & Miller JS Targeting KIR Blockade in Multiple Myeloma: Trouble in Checkpoint Paradise? Clin Cancer Res 22, 5161–5163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.van Hall T et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J Immunother Cancer 7, 263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Haanen JB & Cerundolo V NKG2A, a New Kid on the Immune Checkpoint Block. Cell 175, 1720–1722 (2018). [DOI] [PubMed] [Google Scholar]
- 180.van Montfoort N et al. NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines. Cell 175, 1744–1755 e1715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Andre P et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 175, 1731–1743 e1713 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Z, Yang Y, Liu LL & Lundqvist A Strategies to Augment Natural Killer (NK) Cell Activity against Solid Tumors. Cancers (Basel) 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Doberstein SK Bempegaldesleukin (NKTR-214): a CD-122-biased IL-2 receptor agonist for cancer immunotherapy. Expert Opin Biol Ther 19, 1223–1228 (2019). [DOI] [PubMed] [Google Scholar]
- 184.Bentebibel SE et al. A First-in-Human Study and Biomarker Analysis of NKTR-214, a Novel IL2Rbetagamma-Biased Cytokine, in Patients with Advanced or Metastatic Solid Tumors. Cancer Discov 9, 711–721 (2019). [DOI] [PubMed] [Google Scholar]
- 185.Donskov F, Jensen NV, Smidt-Hansen T, Brondum L & Geertsen P A randomized phase II trial of interleukin-2 and interferon-alpha plus bevacizumab versus interleukin-2 and interferon-alpha in metastatic renal-cell carcinoma (mRCC): results from the Danish Renal Cancer Group (DaRenCa) study-1. Acta Oncol 57, 589–594 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Conlon KC et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 33, 74–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miller JS et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin Cancer Res 24, 1525–1535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cooley S et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv 3, 1970–1980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo Y, Luan L, Patil NK & Sherwood ER Immunobiology of the IL-15/IL-15Ralpha complex as an antitumor and antiviral agent. Cytokine Growth Factor Rev 38, 10–21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Romee R et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wrangle JM et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol 19, 694–704 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smits EL, Anguille S & Berneman ZN Interferon alpha may be back on track to treat acute myeloid leukemia. Oncoimmunology 2, e23619 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mo XD et al. IFN-alpha Is Effective for Treatment of Minimal Residual Disease in Patients with Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant 23, 1303–1310 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Mo X et al. Minimal residual disease-directed immunotherapy for high-risk myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Front Med 13, 354–364 (2019). [DOI] [PubMed] [Google Scholar]
- 195.Felices M, Lenvik TR, Davis ZB, Miller JS & Vallera DA Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol Biol 1441, 333–346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Runcie K, Budman DR, John V & Seetharamu N Bi-specific and tri-specific antibodies- the next big thing in solid tumor therapeutics. Mol Med 24, 50 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Labrijn AF, Janmaat ML, Reichert JM & Parren P Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 18, 585–608 (2019). [DOI] [PubMed] [Google Scholar]
- 198.Ferrari de Andrade L et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 359, 1537–1542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ravi R et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat Commun 9, 741 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nayyar G, Chu Y & Cairo MS Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front Oncol 9, 51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sparrow E & Bodman-Smith MD Granulysin: The attractive side of a natural born killer. Immunol Lett 217, 126–132 (2020). [DOI] [PubMed] [Google Scholar]
- 202.Kumar S Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 154, 383–393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Orange JS Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 8, 713–725 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bengsch B et al. Deep immune profiling by mass cytometry links human T and NK cell differentiation and cytotoxic molecule expression patterns. J Immunol Methods 453, 3–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Smyth MJ, Street SE & Trapani JA Cutting edge: granzymes A and B are not essential for perforin-mediated tumor rejection. J Immunol 171, 515–518 (2003). [DOI] [PubMed] [Google Scholar]
- 206.Takeda K et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood 105, 2082–2089 (2005). [DOI] [PubMed] [Google Scholar]
- 207.Smyth MJ et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med 193, 661–670 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hayakawa Y et al. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol 172, 123–129 (2004). [DOI] [PubMed] [Google Scholar]
- 209.Josephs SF et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med 16, 242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Balkwill F Tumour necrosis factor and cancer. Nat Rev Cancer 9, 361–371 (2009). [DOI] [PubMed] [Google Scholar]
- 211.Rafiq S, Hackett CS & Brentjens RJ Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 17, 147–167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Long EO, Kim HS, Liu D, Peterson ME & Rajagopalan S Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 31, 227–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li F et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenteirology 144, 392–401 (2013). [DOI] [PubMed] [Google Scholar]
- 214.Sun C et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 6, e1264562 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]