Abstract
Background
Vitamin E supplementation may help reduce the risk of pregnancy complications involving oxidative stress, such as pre‐eclampsia. There is a need to evaluate the efficacy and safety of vitamin E supplementation in pregnancy.
Objectives
To assess the effects of vitamin E supplementation, alone or in combination with other separate supplements, on pregnancy outcomes, adverse events, side effects and use of health services.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2015) and reference lists of retrieved studies.
Selection criteria
All randomised or quasi‐randomised controlled trials evaluating vitamin E supplementation in pregnant women. We excluded interventions using a multivitamin supplement that contained vitamin E.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
Twenty‐one trials, involving 22,129 women were eligible for this review. Four trials did not contribute data. All of the remaining 17 trials assessed vitamin E in combination with vitamin C and/or other agents. Overall the risk of bias ranged from low to unclear to high; 10 trials were judged to be at low risk of bias, six trials to be at unclear risk of bias and five trials to be at high risk of bias. No clear difference was found between women supplemented with vitamin E in combination with other supplements during pregnancy compared with placebo for the risk of stillbirth (risk ratio (RR) 1.17, 95% confidence interval (CI) 0.88 to 1.56, nine studies, 19,023 participants, I² = 0%; moderate quality evidence), neonatal death (RR 0.81, 95% CI 0.58 to 1.13, nine trials, 18,617 participants, I² = 0%), pre‐eclampsia (average RR 0.91, 95% CI 0.79 to 1.06; 14 trials, 20,878 participants; I² = 48%; moderate quality evidence), preterm birth (average RR 0.98, 95% CI 0.88 to 1.09, 11 trials, 20,565 participants, I² = 52%; high quality evidence) or intrauterine growth restriction (RR 0.98, 95% CI 0.91 to 1.06, 11 trials, 20,202 participants, I² = 17%; high quality evidence). Women supplemented with vitamin E in combination with other supplements compared with placebo were at decreased risk of having a placental abruption (RR 0.64, 95% CI 0.44 to 0.93, seven trials, 14,922 participants, I² = 0%; high quality evidence). Conversely, supplementation with vitamin E was associated with an increased risk of self‐reported abdominal pain (RR 1.66, 95% CI 1.16 to 2.37, one trial, 1877 participants) and term prelabour rupture of membranes (PROM) (average RR 1.77, 95% CI 1.37 to 2.28, two trials, 2504 participants, I² = 0%); however, there was no corresponding increased risk for preterm PROM (average RR 1.27, 95% CI 0.93 to 1.75, five trials, 1999 participants, I² = 66%; low quality evidence). There were no clear differences between the vitamin E and placebo or control groups for any other maternal or infant outcomes. There were no clear differing patterns in subgroups of women based on the timing of commencement of supplementation or baseline risk of adverse pregnancy outcomes. The GRADE quality of the evidence was high for preterm birth, intrauterine growth restriction and placental abruption, moderate for stillbirth and clinical pre‐eclampsia, and low for preterm PROM.
Authors' conclusions
The data do not support routine vitamin E supplementation in combination with other supplements for the prevention of stillbirth, neonatal death, preterm birth, pre‐eclampsia, preterm or term PROM or poor fetal growth. Further research is required to elucidate the possible role of vitamin E in the prevention of placental abruption. There was no convincing evidence that vitamin E supplementation in combination with other supplements results in other important benefits or harms.
Plain language summary
Vitamin E supplementation in pregnancy
What is the issue?
Does giving vitamin E supplementation, alone or in combination with other vitamins, given to women during pregnancy improve outcomes for their babies by reducing the incidence of pre‐eclampsia and the number of babies born too early? Or does it cause harm?
Why is this important?
Although vitamin E deficiency is rarely seen in healthy adults, for pregnant women, insufficient dietary vitamin E (found in vegetable oils, nuts, cereals and some leafy green vegetables) may lead to complications such as pre‐eclampsia and the baby being born small. In addition, vitamin E deficiency can be made worse by too much iron and so it is important to investigate the optimum amounts for pregnancy.
What evidence did we find?
This review included 21 trials involving over 21,000 women. Four trials did not contribute data to the analyses. The trials were generally of variable quality. There were just three studies on vitamin E supplementation alone, but none of these studies contributed data. All other studies included vitamin C, and additional supplements or drugs.
The findings indicate that routine supplementation with vitamin E in combination with other supplements during pregnancy did not improve outcomes for babies or women. There was a reduction in the number of placentas coming away early (placental abruption) in women given vitamin E supplements in combination with other agents, which was rated as high‐quality evidence. However, it is unclear whether this finding was due to vitamin E or the other agents used in the supplement. This should be explored in further research examining the mechanisms leading to placental abruption.
The review found there may be harms associated with vitamin E supplements in pregnancy, as there was an increased risk of abdominal pain and term prelabour rupture of fetal membranes in women supplemented with vitamin E in combination with other supplements. There was no increase in preterm prelabour rupture of membranes in women supplemented with vitamin E and other agents.
What does this mean?
The large body of evidence does not support taking vitamin E supplements, alone or in combination, during pregnancy. This is because taking vitamin E in combination with other supplements during pregnancy does not help to prevent problems in pregnancy including stillbirth, baby death, preterm birth, pre‐eclampsia or low birthweight babies. In fact, it may increase abdominal pain for women and also increase the number of women having early rupture of membranes at term.
Summary of findings
Summary of findings for the main comparison. Any vitamin E supplementation versus placebo, no placebo or other supplements.
| Any vitamin E supplementation versus placebo, no placebo or other supplements | ||||||
| Population: pregnant women receiving vitamin E supplementation or control, living in areas where there is either inadequate dietary intake of vitamin E or where there is presumed adequate intake. Settings: Australia, Brazil, Canada, Holland, India, Iran, Malaysia, Mexico, Peru, South Africa, Turkey, UK, USA, Vietnam, Venezuela. Intervention: any vitamin E supplementation versus placebo, no placebo or other supplements. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any vitamin E supplementation | |||||
| Stillbirth | Study population | RR 1.17 (0.88 to 1.56) | 19023 (9 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 9 per 1000 | 11 per 1000 (8 to 14) | |||||
| Moderate | ||||||
| 14 per 1000 | 16 per 1000 (12 to 22) | |||||
| Preterm birth (less than 37 weeks' gestation) | Study population | RR 0.98 (0.88 to 1.09) | 20565 (11 studies) | ⊕⊕⊕⊕ high | ||
| 159 per 1000 | 156 per 1000 (140 to 173) | |||||
| Moderate | ||||||
| 235 per 1000 | 230 per 1000 (207 to 256) | |||||
| Clinical pre‐eclampsia (random‐effects model) | Study population | RR 0.91 (0.79 to 1.06) | 20878 (14 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 95 per 1000 | 87 per 1000 (75 to 101) | |||||
| Moderate | ||||||
| 146 per 1000 | 133 per 1000 (115 to 155) | |||||
| Intrauterine growth restriction (various definitions) | Study population | RR 0.98 (0.91 to 1.06) | 20202 (11 studies) | ⊕⊕⊕⊕ high | ||
| 106 per 1000 | 104 per 1000 (97 to 113) | |||||
| Moderate | ||||||
| 119 per 1000 | 117 per 1000 (108 to 126) | |||||
| Prelabour rupture of fetal membranes ‐ preterm | Study population | RR 1.27 (0.93 to 1.75) | 1999 (5 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 29 per 1000 | 37 per 1000 (25 to 55) | |||||
| Moderate | ||||||
| 26 per 1000 | 33 per 1000 (22 to 50) | |||||
| Bleeding episodes (placental abruption) | Study population | RR 0.64 (0.44 to 0.93) | 14922 (7 studies) | ⊕⊕⊕⊕ high | ||
| 9 per 1000 | 6 per 1000 (4 to 9) | |||||
| Moderate | ||||||
| 19 per 1000 | 12 per 1000 (8 to 18) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide confidence interval crossing the line of no effect. 2 Statistical Heterogeneity (I² > 60%).
3 Publication bias detected.
Background
Description of the condition
Vitamin E is the generic name given to eight lipid‐soluble and plant‐derived compounds; four are referred to as tocopherols (alpha, beta, gamma, delta) and four are known as tocotrienols (alpha, beta, gamma, delta) (Roberts 1990). Natural source alpha‐tocopherol is the most biologically active form of vitamin E, and consequently vitamin E activity is expressed in terms of alpha‐tocopherol equivalents (mg alpha‐TE). In foods, the main source of tocopherol is wheatgerm oil and other vegetable oils, nuts, in the fat of meat, some cereals and some leafy green vegetables (NHMRC 2006). Synthetic forms of vitamin E are also available and commonly used in vitamin preparations; however, these forms have less biological activity than their naturally occurring counterparts (IOM 2000).
Vitamin E deficiency is rarely seen in healthy adults and has primarily been characterised in preterm infants, low birthweight infants and those with fat malabsorption disorders. Reported symptoms of deficiency include haemolytic anaemia, reticulocytosis, hyperbilirubinaemia, low haemoglobin levels (Gross 1982) and peripheral neuropathy (Roberts 1990). Vitamin E deficiency is exacerbated in the presence of iron overload and a high dietary intake of polyunsaturated fatty acids (PUFAs), which is of particular relevance for preterm infants fed formula containing high levels of iron and PUFAs (Roberts 1990). Establishing a recommended dietary intake (RDI) of vitamin E has been impeded by the low observance of overt vitamin E deficiency; however, current RDI range from 7 mg to 10 mg alpha‐TE (Roberts 1990). During pregnancy, losses of vitamin E to the fetus are thought to be minimal and thus the RDI during pregnancy is often unchanged (NHMRC 2006).
Description of the intervention
Vitamin E functions as an antioxidant in the lipid phase, protecting phospholipid fatty acids from oxidation by harmful free radicals (reactive oxygen molecules) and thus stabilising cell membranes. As an antioxidant, vitamin E helps to prevent oxidative stress, which is characterised by an excess of free radicals coupled with decreased antioxidants available to quench these free radicals. Vitamin E interacts synergistically with vitamin C, a water soluble antioxidant, where vitamin C helps to convert oxidised vitamin E back into a useful form (Packer 1979). This relationship may account for the limited observation of overt vitamin E deficiency in humans, as vitamin C may aid in recycling vitamin E stores. Vitamin E and vitamin C supplements are often given concurrently to utilise this relationship and to promote antioxidant defences in both the aqueous and lipid phase. Little is known about other potential functions of vitamin E as research to date has focused on its antioxidant properties. Doses of vitamin E required to have an antioxidant effect have been reported at least to 400 international units (approximately 268 mg alpha‐TE) (Devaraj 1997).
How the intervention might work
Oxidative stress has been linked to the development of adult diseases including cardiovascular disease, cancer, chronic inflammation and neurologic disorders, resulting in many large multicentre clinical trials of vitamin E supplementation. However, the results of these large trials of vitamin E supplementation have been disappointing and in fact provide evidence of harm, including an increased risk of mortality (Bjelakovic 2012; Bjelakovic 2013). During pregnancy, oxidative stress has been implicated in the development of pre‐eclampsia (Roberts 1990), and proposed in the disease processes of intrauterine growth restriction (Kingdom 2000) and prelabour rupture of membranes(PROM) both preterm and at term (Woods 2001). Oxidative stress has also been implicated in many of the disorders common to preterm infants including chronic lung disease, intraventricular hemorrhage, periventricular leukomalacia, retinopathy of prematurity, necrotising enterocolitis and bronchopulmonary dysplasia (Saugstad 1988; Saugstad 2001). Preventing complications in pregnancy like pre‐eclampsia, growth restriction, preterm PROM and serious neonatal morbidities would represent significant cost savings in hospital and intensive care unit admissions and the use of other healthcare resources. Other Cochrane reviews are assessing 'Antioxidants for preventing pre‐eclampsia' (Rumbold 2005a) and 'Vitamin C supplementation in pregnancy' (Rumbold 2005b).
Why it is important to do this review
Vitamin E appears to have low toxicity in humans. However there is limited evidence on the safety of using vitamin E in pregnancy. Despite the lack of evidence on safety, the United States Institute of Medicine Food and Nutrition Board has set an upper tolerable limit of vitamin E ingestion in pregnancy at 1000 mg per day (IOM 2000), indicating the highest level of intake that is likely to pose no risk of adverse health effects to almost all women. In non‐pregnant adults controlled clinical trials of vitamin E supplementation in a variety of doses have failed to demonstrate any consistent side effects (Bendich 1993). Observational studies, however, have reported adverse effects including fatigue, weakness, creatinuria, dermatitis, reduced thyroid function, increased urinary androgen excretion, reduced leukocyte action and altered coagulation factors resulting in increased bleeding in vitamin K deficient individuals (Bendich 1993; Roberts 1990). The mechanisms leading to altered coagulation factors are unclear; however, vitamin E has been reported to potentiate the effect of anticoagulant therapy, such as warfarin. Newborn infants have a relative vitamin K deficiency at birth, hence vitamin E supplementation during pregnancy may influence the risk of vitamin K deficiency bleeding or haemorrhagic disease of the newborn unfavourably if vitamin K is not given at birth. In controlled trials of vitamin E supplementation in preterm infants for the treatment of retinopathy of prematurity, vitamin E supplementation has been associated with an increased risk of bacterial sepsis and necrotising enterocolitis (Johnson 1985). Given the lipid soluble nature of vitamin E, supplementation may result in increased storage of the vitamin in organs such as the liver, muscle and adipose tissue when used in high doses. The need to demonstrate the efficacy and safety of using vitamin E in pregnancy is particularly important when vitamin E is given in high doses.
The aims of this review are (i) to identify all published, unpublished randomised and quasi‐randomised controlled trials investigating vitamin E supplementation in pregnancy and (ii) to investigate the benefits and hazards of vitamin E supplementation in pregnancy.
Objectives
To assess, using the best available evidence, the effects of vitamin E supplementation, alone or in combination with other separate supplements, on pregnancy outcomes, adverse events, side effects and use of health services.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials evaluating the effect of vitamin E supplementation in pregnant women.
Types of participants
Pregnant women receiving vitamin E supplementation or control, living in areas where there is either inadequate dietary intake of vitamin E or where there is presumed adequate intake.
Women were classified into subgroups where possible, based on: (a) the dosage of the vitamin E supplement (above or equal to/below the recommended dietary intake of 7 mg alpha‐TE); (b) the gestation at trial entry (trial entry less than 20 weeks or greater than or equal to 20 weeks); (c) whether women have low or adequate dietary vitamin E intake prior to trial entry (low intake defined as intake less than the recommended dietary intake in that setting as measured by dietary questionnaire); (d) the use of vitamin E in combination with other dietary supplements; (e) women's risk status for adverse pregnancy outcomes (as defined by the trial authors).
Types of interventions
Vitamin E supplementation, alone or in combination with other separate supplements compared with placebo, no placebo or other supplements. Interventions using a multivitamin supplement (more than two vitamins or minerals combined in the one tablet preparation) that contained vitamin E were excluded.
Types of outcome measures
Primary outcomes
Maternal
Development of clinical pre‐eclampsia
Maternal haematological measures: haemolytic anaemia, reticulocytosis, hyperbilirubinaemia and haemoglobin concentrations
Preterm birth (defined as less than 37 weeks' gestation)
Neonatal
Stillbirth, neonatal death, perinatal death
Infant haematological measures: haemolytic anaemia, reticulocytosis, hyperbilirubinaemia and haemoglobin concentrations
Intrauterine growth restriction (defined as birthweight less than third centile or the most extreme centile reported)
Secondary outcomes
Maternal
Prelabour rupture of membranes (PROM), preterm and at term
Death up to six weeks postpartum
Elective delivery (induction of labour or elective caesarean section)
Caesarean section (emergency plus elective)
Bleeding episodes (such as placental abruption, antepartum hemorrhage, postpartum hemorrhage, complications of epidural anaesthesia, need for transfusion)
Measures of serious maternal morbidity (such as eclampsia, liver failure, renal failure, disseminated intravascular coagulation, pulmonary oedema), peripheral neuropathy
Adverse events related to vitamin E supplementation sufficient to stop supplementation
Side effects of vitamin E supplementation such as fatigue, weakness, altered coagulation times, immunosuppression, creatinuria, dermatitis, altered thyroid function and increased urinary androgen excretion
Maternal satisfaction with care
Neonatal
Birthweight
Infant death
Gestational age at birth
Congenital malformations
Apgar score less than seven at five minutes
Vitamin K deficiency bleeding or haemorrhagic disease of the newborn
Respiratory distress syndrome
Chronic lung disease or bronchopulmonary dysplasia
Periventricular hemorrhage
Periventricular leukomalacia
Bacterial sepsis
Necrotising enterocolitis
Retinopathy of prematurity
Peripheral neuropathy
Disability at childhood follow‐up (such as cerebral palsy, intellectual disability, hearing disability and visual impairment)
Poor childhood growth
Use of health service resources
Woman
Antenatal hospital admission
Visits to day care units
Use of intensive care
Ventilation
Dialysis
Infant
Admission to special care/intensive care nursery
Duration of mechanical ventilation
Length of stay in hospital
Development
Special needs after discharge
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 March 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[See Appendix 1 for details of additional searches carried out in the previous version of the review (Rumbold 2005).]
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeRumbold 2005.
For this update, the following methods were used for assessing the 62 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We also explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of quality of the evidence
For this update, we assessed the quality of the evidence using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons:
stillbirth;
preterm birth (defined as less than 37 weeks' gestation);
development of clinical pre‐eclampsia;
intrauterine growth restriction (defined as birthweight less than third centile or the most extreme centile reported);
preterm prelabour rupture of membranes (PROM) ;
bleeding episodes (placental abruption).
We used the GRADE profiler (GRADE 2014) to import data from Review Manager 5.3 (RevMan 2014) in order to create a 'Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials in this update. In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials,we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trial
This is not a valid study design for this review.
Other unit of analysis issues
In future updates, if we include multi‐arm studies (more than one treatment group), we will combine treatment groups if appropriate, and create a single pair‐wise comparison. We will not double count participants according to methods described in the Handbook (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots for primary outcomes. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we explored possible reasons.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses for primary outcomes:
the dosage of the vitamin E supplement (above or equal to versus below the recommended dietary intake of 7 mg alpha‐TE);
the gestation at trial entry (trial entry less than 20 weeks versus greater than or equal to 20 weeks);
whether women had low versus adequate dietary vitamin E intake prior to trial entry (low intake defined as intake less than the recommended dietary intake in that setting as measured by dietary questionnaire);
the use of vitamin E in combination with other dietary supplements versus vitamin E alone;
women's risk status for adverse pregnancy outcomes (as defined by the trial authors) versus all women.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality assessed by determining the overall risk of bias taking into consideration the method of random sequence generation and allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential bias. Studies with an overall high or unclear risk of bias were excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
We examined 62 reports of 27 trials. In this update, we included 21 trials (Anthony 1996; Beazley 2005; Borna 2005; Chappell 1999; Gulmezoglu 1997; Gungorduk 2014; Huria 2010; Kalpdev 2011; Mahdy 2004; McCance 2010; Nasrolahi 2006; Poston 2006; Pressman 2003; Rivas 2000; Roberts 2010; Rumbold 2006; Sawhney 2000; Shahraki 2006; Spinnato 2007; Villar 2009; Xu 2010), excluded five trials (Bolisetty 2002; Clark 2012; Lietz 2001; Moldenhauer 2002; Wibowo 2012) and one study (Tan 1997) is still awaiting classification because the full paper cannot be traced.
Included studies
We identified 21 trials involving 22,129 women as eligible for inclusion in the review. Of these, 14 trials assessed vitamin E supplementation for the prevention of pre‐eclampsia (Beazley 2005; Chappell 1999; Huria 2010; Kalpdev 2011; Mahdy 2004; McCance 2010; Nasrolahi 2006; Poston 2006; Rivas 2000; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009; Xu 2010). Three trials assessed vitamin E supplementation for the prevention of perinatal complications in women with established pre‐eclampsia (Anthony 1996; Gulmezoglu 1997; Sawhney 2000). Two trials assessed whether vitamin E supplementation prolonged the time to birth for women with preterm prelabour rupture of membranes (PROM) (Borna 2005; Gungorduk 2014). One trial assessed vitamin E for the treatment of leg cramps (Shahraki 2006), and one trial assessed the effect of vitamin E supplementation on concentrations of vitamin E in maternal plasma and amniotic fluid (Pressman 2003). Four studies did not report any clinically meaningful outcomes (Anthony 1996; Pressman 2003; Sawhney 2000; Shahraki 2006), therefore in the meta‐analyses data were analysed for 17 studies involving 21,856 women.
Participants
Nine trials recruited women who were at "high" or "increased" risk of pre‐eclampsia (Beazley 2005; Chappell 1999; Kalpdev 2011; McCance 2010; Poston 2006; Rivas 2000; Spinnato 2007; Villar 2009; Xu 2010). The criteria for women being at high risk varied between trials, and included: essential hypertension (Kalpdev 2011); type 1 diabetes (McCance 2010); chronic hypertension or a prior history of pre‐eclampsia in the most recent pregnancy (Spinnato 2007); previous pre‐eclampsia, chronic hypertension, insulin‐requiring diabetes mellitus or multiple gestation (Beazley 2005; Xu 2010); abnormal doppler waveform in either uterine artery at 18 to 22 weeks' gestation or a history in the preceding pregnancy of pre‐eclampsia necessitating delivery before 37 weeks' gestation, eclampsia or the syndrome of haemolysis, elevated liver enzymes, low platelets (HELLP) (Chappell 1999); chronic hypertension, renal disease, pre‐eclampsia‐eclampsia in the pregnancy preceding the index pregnancy requiring delivery before 37 weeks’ gestation, HELLP syndrome in any previous pregnancy, pregestational diabetes, primiparous with a body mass index (BMI) of ≥ 30 kg/m2, history of medically indicated preterm delivery, abnormal uterine artery Doppler waveforms and women with antiphospholipid syndrome (Villar 2009); pre‐eclampsia in the pregnancy preceding the index pregnancy, requiring delivery before 37 weeks’ gestation, diagnosis of HELLP in any previous pregnancy, eclampsia in any previous pregnancy, essential hypertension requiring medication, maternal diastolic blood pressure of 90 mm Hg or more before 20 weeks’ gestation in the current pregnancy, type 1 or type 2 diabetes, requiring insulin or oral hypoglycaemic therapy, antiphospholipid syndrome, chronic renal disease, multiple pregnancy, abnormal uterine artery doppler waveforms, and primiparity with BMI at first antenatal appointment of ≥ 30 kg/m² (Poston 2006); or nulliparity, previous pre‐eclampsia, obesity, hypertension, less than 20 years old, diabetes, nephropathy, mean arterial pressure above of 85 mmHg, positive roll‐over test, black race, family history of hypertension or pre‐eclampsia, twin pregnancy and poor socioeconomic conditions (Rivas 2000). The trial by Xu 2010 had an additional low‐risk arm which included nulliparous women, a further five trials involved women who were either primigravid or nulliparous (Huria 2010; Mahdy 2004; Nasrolahi 2006; Roberts 2010; Rumbold 2006). Three trials included women with established pre‐eclampsia (Anthony 1996; Gulmezoglu 1997; Sawhney 2000). Two trials involved women with established preterm PROM(Borna 2005; Gungorduk 2014), and one trial enrolled with pregnant women experiencing leg cramps (Shahraki 2006). The remaining trial involved women with planned caesarean section over 35 weeks of gestation (Pressman 2003).
The timing of commencement of supplementation differed widely, however, most started supplementation in the second trimester. The range in gestational ages at commencement included: eight to 22 weeks' (McCance 2010), nine to 16 weeks' (Roberts 2010), 12 weeks' (Huria 2010), 12 to 18 weeks' (Xu 2010), 12 to 19 weeks' (Spinnato 2007), 13 to 19 weeks' (Kalpdev 2011), 14 to 20 weeks' (Beazley 2005), 14 to 21 weeks' (Poston 2006), 14 to 22 weeks' (Rumbold 2006; Villar 2009),16 to 22 weeks' (Chappell 1999), 24 to 32 weeks' (Gulmezoglu 1997), 24 to 34 weeks' (Gungorduk 2014), 24 to 34 weeks' (Borna 2005), 25 to 28 weeks' (Shahraki 2006), less than 29 weeks' (Rivas 2000) and 35 weeks' or more (Pressman 2003). For four trials, the commencement of supplementation was unknown (Anthony 1996; Mahdy 2004; Nasrolahi 2006; Sawhney 2000).
Interventions
Three trials supplemented women with vitamin E alone (Anthony 1996; Sawhney 2000; Shahraki 2006). Seventeen trials gave women supplements with vitamin E in addition to vitamin C (Beazley 2005; Borna 2005; Chappell 1999; Gulmezoglu 1997; Gungorduk 2014; Huria 2010; Kalpdev 2011; McCance 2010; Nasrolahi 2006; Poston 2006; Pressman 2003; Rivas 2000; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009; Xu 2010). Of these, two trials supplemented women with additional supplements to vitamin E and vitamin C, either allopurinol (Gulmezoglu 1997) or aspirin and fish oil (Rivas 2000). A further trial supplemented women with a vitamin E rich fraction of palm oil, however no further information was provided (Mahdy 2004). Fifteen trials used the same dose of daily 400 international units (IU) vitamin E (Beazley 2005; Borna 2005; Chappell 1999; Gungorduk 2014; Kalpdev 2011; McCance 2010; Nasrolahi 2006; Poston 2006; Pressman 2003; Rivas 2000; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009; Xu 2010). Three trials gave women either daily 100 mg (Shahraki 2006), 200 IU (Huria 2010) or 800 IU vitamin E (Gulmezoglu 1997). The dose of vitamin E was unknown for three trials (Anthony 1996; Mahdy 2004; Sawhney 2000).
Outcomes
For maternal primary outcomes, development of clinical pre‐eclampsia was reported in 14 trials, preterm births was reported in 11 trials, bleeding episodes was reported in seven trials. For neonatal primary outcomes, stillbirth was reported in nine trials, neonatal deaths for nine trials, perinatal deaths for six trials and intrauterine growth restriction for 11 trials. For secondary outcomes, birthweight was reported in 10 trials, PROM was reported in five trials and maternal death was reported in seven trials.
Settings
The 21 trials were from 15 countries including low‐ to high‐income countries such as Australia, Brazil, Canada, Holland, India, Iran, Malaysia, Mexico, Peru, South Africa, Turkey, UK, USA, Vietnam, and Venezuela. One trial was undertaken in populations with 'evidence of overall low nutritional status' (Villar 2009).
Excluded studies
Five studies were excluded (see Characteristics of excluded studies). Three studies were excluded because they were non‐randomised (Bolisetty 2002; Lietz 2001; Moldenhauer 2002). One study was excluded due to intervention was not supplementation but dietary advice to optimise vitamin E intake (Clark 2012). The other study was excluded because the intervention included more than 14 different vitamins (Wibowo 2012).
Risk of bias in included studies
Overall, we judged 10 trials to be at low risk of bias, six trials to be at unclear risk of bias and five trials to be at high risk of bias (Figure 1; Figure 2).
1.
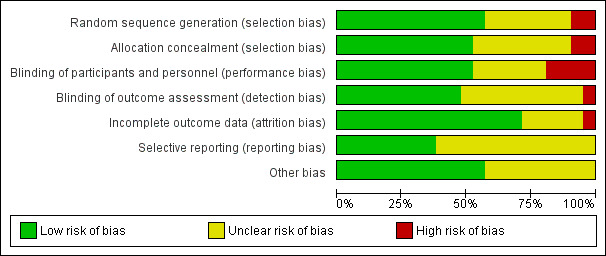
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
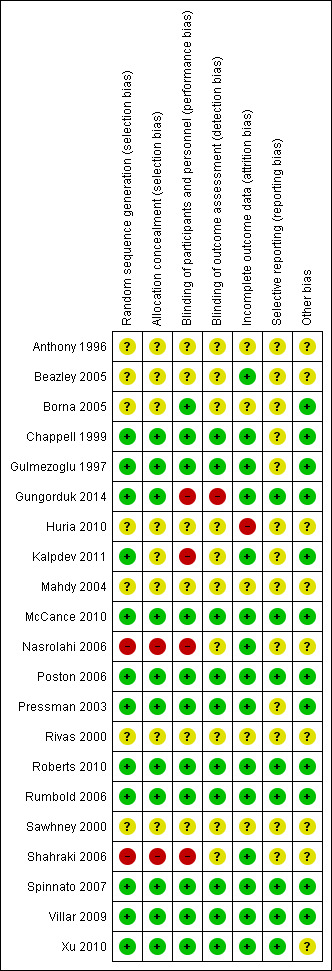
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eleven trials were judged to have used adequate methods to generate their random sequence and to conceal allocation (Chappell 1999; Gulmezoglu 1997; Gungorduk 2014; McCance 2010; Poston 2006; Pressman 2003; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009; Xu 2010), and therefore judged to be at low risk of selection bias. One trial (Kalpdev 2011) used adequate methods for sequence generation but provided insufficient detail about allocation concealment and was judged to be at unclear risk of selection bias. Two trials (Nasrolahi 2006; Shahraki 2006) had inadequate methods of both sequence generation and allocation concealment, and were judged to be at high risk of selection bias. The remaining seven trials (Anthony 1996; Beazley 2005; Borna 2005; Huria 2010; Mahdy 2004; Rivas 2000; Sawhney 2000) were judged to be at unclear risk of selection bias as there was insufficient information reported about their methods to permit a judgement.
Blinding
Ten trials undertook adequate blinding of participants, caregivers and outcome assessors and were therefore judged to be at low risk of both performance bias and detection bias (Chappell 1999; Gulmezoglu 1997; McCance 2010; Poston 2006; Pressman 2003; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009; Xu 2010). One study (Borna 2005) had adequate blinding of participants but provided no detail on blinding of outcome assessors, and was therefore judged as having an unclear risk of detection bias. Three trials used no placebo control and were judged to be at high risk of performance bias (Kalpdev 2011; Nasrolahi 2006; Shahraki 2006). Another trial (Gungorduk 2014) used a placebo control, however the tablet was not identical to the vitamin supplement which led to a lack of blinding; this trial was judged to be at high risk of performance and detection bias. Six trials were judged as having an unclear risk of both performance and detection bias due to insufficient information about any methods of blinding (Anthony 1996; Beazley 2005; Huria 2010; Mahdy 2004; Rivas 2000; Sawhney 2000). Four trials were judged as having an unclear risk of detection bias due to lack of information about blinding of outcome assessment (Borna 2005; Kalpdev 2011; Nasrolahi 2006; Shahraki 2006).
Incomplete outcome data
Sixteen trials reported information on attrition and exclusion of participants. Fifteen were judged to have a low risk of attrition bias (Beazley 2005; Chappell 1999; Gulmezoglu 1997; Gungorduk 2014; Kalpdev 2011; McCance 2010; Nasrolahi 2006; Poston 2006; Pressman 2003; Roberts 2010; Rumbold 2006; Shahraki 2006; Spinnato 2007; Villar 2009; Xu 2010), and one was judged to be at high risk (Huria 2010). Five trials provided insufficient information to assess the risk of attrition bias (Anthony 1996; Borna 2005; Mahdy 2004; Rivas 2000; Sawhney 2000).
Selective reporting
Eight trials were judged to be at low risk of reporting bias as they reported data for all expected outcomes (Gungorduk 2014; McCance 2010; Poston 2006; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009; Xu 2010). Thirteen trials were judged to be at unclear risk as there was insufficient information to assess selective reporting (Anthony 1996; Beazley 2005; Borna 2005; Chappell 1999; Gulmezoglu 1997; Huria 2010; Kalpdev 2011; Mahdy 2004; Nasrolahi 2006; Pressman 2003; Rivas 2000; Sawhney 2000; Shahraki 2006).
Other potential sources of bias
Twelve trials were judged to be at low risk of other potential sources of bias (Borna 2005; Chappell 1999; Gulmezoglu 1997; Gungorduk 2014; Kalpdev 2011; McCance 2010; Poston 2006; Pressman 2003; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009). For the other nine trials, there was insufficient information to confidently assess the risk of other sources of bias (Anthony 1996; Beazley 2005; Huria 2010; Mahdy 2004; Nasrolahi 2006; Rivas 2000; Sawhney 2000; Shahraki 2006; Xu 2010). For further details see the Characteristics of included studies tables.
Effects of interventions
See: Table 1
Twenty‐one trials involving 22,129 women were identified and of these, 17 trials involving 21,856 women reported data available for analysis. Of the 17 trials included in the analyses, all supplemented women with vitamin E in combination with other supplements (vitamins or other agents).
Vitamin E in combination with other supplements compared with placebo or no control
Primary outcomes
No clear difference was found between women supplemented with vitamin E in combination with other supplements compared with placebo for the risk of stillbirth (risk ratio (RR) 1.17, 95% confidence intervals (CI) 0.88 to 1.56, nine trials, 19,023 participants, Analysis 1.1), neonatal death (RR 0.81, 95% CI 0.58 to 1.13, nine trials, 18,617 participants, Analysis 1.2), infant death (RR 3.02, 95% CI 0.12 to 74.12, one trial, 2694 participants, Analysis 1.4) or infant hyperbilirubinaemia (RR 0.78, 95% CI 0.59 to 1.04, one trial, 725 participants, Analysis 1.7), using fixed‐effect models. Substantial heterogeneity was detected for perinatal death (I² = 43%). When using a random‐effects model, there was no clear difference in the risk of perinatal death between treatment groups (average RR 1.09, 95% CI 0.77 to 1.54,six trials, 16,923 participants, Analysis 1.3). No trials reported the outcomes haemolytic anaemia, reticulocytosis or maternal or infant haemoglobin concentrations.
1.1. Analysis.
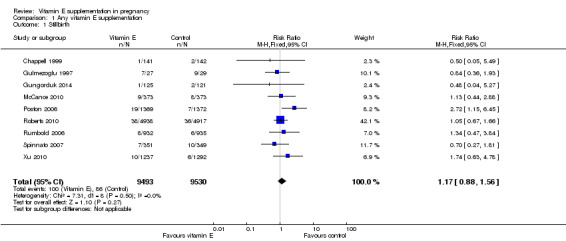
Comparison 1 Any vitamin E supplementation, Outcome 1 Stillbirth.
1.2. Analysis.
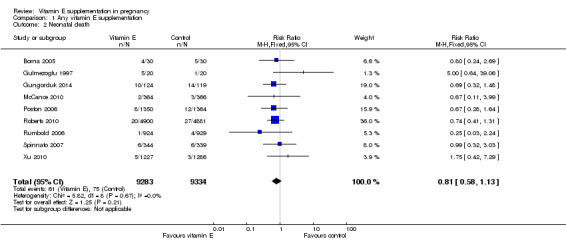
Comparison 1 Any vitamin E supplementation, Outcome 2 Neonatal death.
1.4. Analysis.
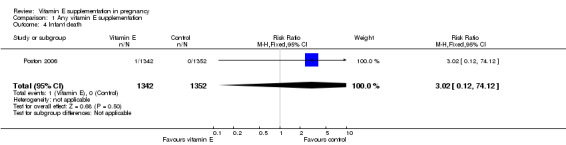
Comparison 1 Any vitamin E supplementation, Outcome 4 Infant death.
1.7. Analysis.
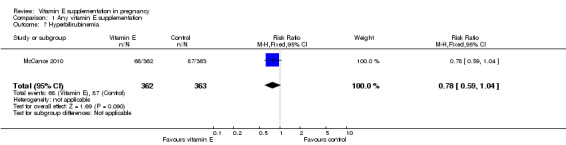
Comparison 1 Any vitamin E supplementation, Outcome 7 Hyperbilirubinemia.
1.3. Analysis.
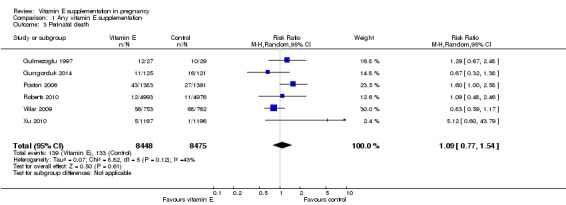
Comparison 1 Any vitamin E supplementation, Outcome 3 Perinatal death.
Substantial heterogeneity was identified for the outcomes preterm birth (I² = 52%) and clinical pre‐eclampsia (I² = 48%). When using a random‐effects model, no clear difference was found between women supplemented with vitamin E in combination with other supplements compared with placebo or no control for the risk of preterm birth (average RR 0.98, 95% CI 0.88 to 1.09, 11 trials, 20,565 participants, Analysis 1.9) or clinical pre‐eclampsia (average RR 0.91, 95% CI 0.79 to 1.06, 14 trials, 20,878 participants, Analysis 1.10). The funnel plot of preterm birth did not show any publication bias, however, the plot for clinical pre‐eclampsia was visually asymmetric (Figure 3, Figure 4, respectively).
1.9. Analysis.
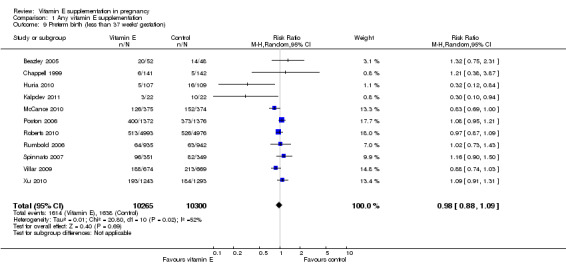
Comparison 1 Any vitamin E supplementation, Outcome 9 Preterm birth (less than 37 weeks' gestation).
1.10. Analysis.
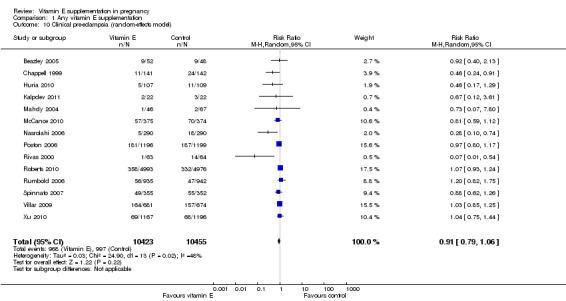
Comparison 1 Any vitamin E supplementation, Outcome 10 Clinical pre‐eclampsia (random‐effects model).
3.
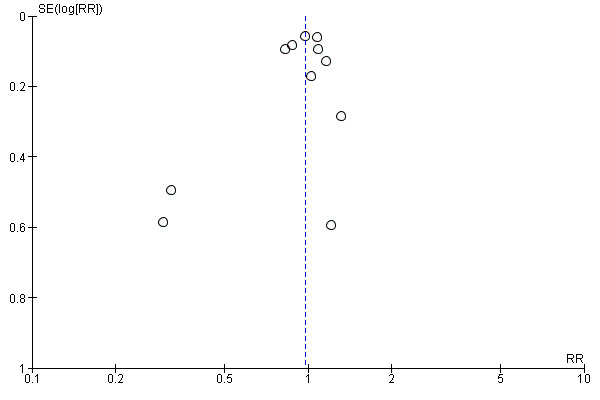
Funnel plot of comparison: 1 Any vitamin E supplementation, outcome: 1.9 Preterm birth (less than 37 weeks' gestation).
4.
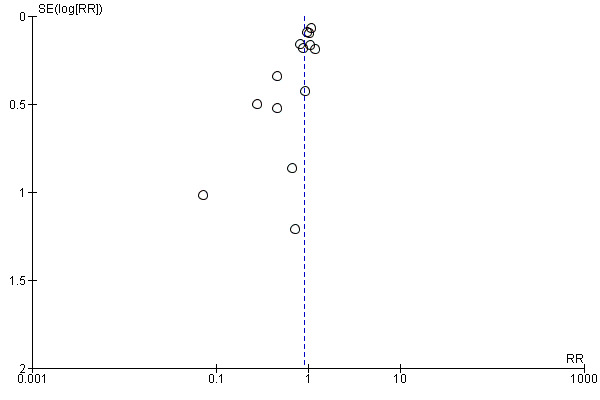
Funnel plot of comparison: 1 Any vitamin E supplementation, outcome: 1.10 Clinical pre‐eclampsia (random‐effects model).
No clear difference was found for the risk of intrauterine growth restriction (RR 0.98, 95% CI 0.91 to 1.06, 11 trials, 20,202 participants, Analysis 1.11) between women supplemented with vitamin E in combination with other supplements compared with placebo or no control. Substantial heterogeneity was detected for birthweight (I² = 68%). When using a random‐effects model, there was no clear difference between women supplemented with vitamin E in combination with other supplements compared with placebo or no control for birthweight (mean difference (MD) 22.17, 95% CI ‐23.01 to 67.36, 10 trials, 16,888 participants, Analysis 1.12). The funnel plots for intrauterine growth restriction did not show any publication bias (Figure 5).
1.11. Analysis.
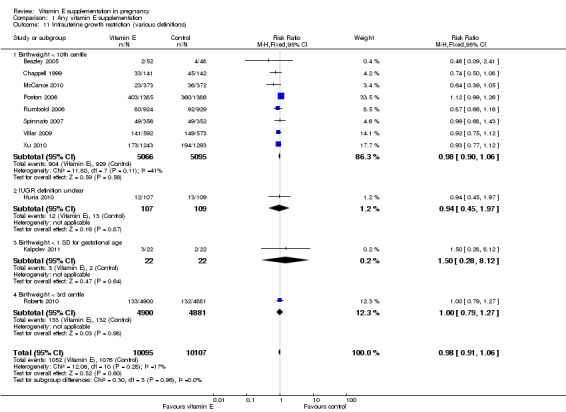
Comparison 1 Any vitamin E supplementation, Outcome 11 Intrauterine growth restriction (various definitions).
1.12. Analysis.
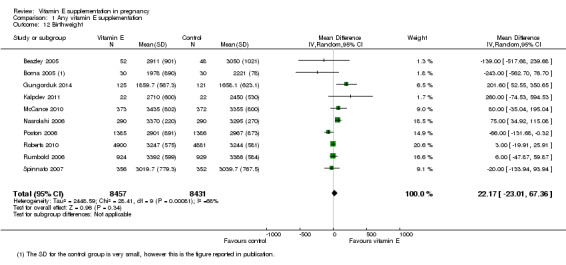
Comparison 1 Any vitamin E supplementation, Outcome 12 Birthweight.
5.
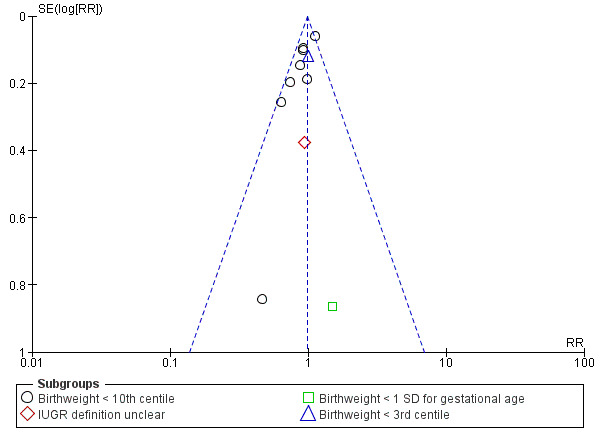
Funnel plot of comparison: 1 Any vitamin E supplementation, outcome: 1.11 Intrauterine growth restriction (various definitions).
Secondary outcomes
Women supplemented with vitamin E in combination with other supplements compared with placebo had a reduced risk of placental abruption (RR 0.64, 95% CI 0.44 to 0.93, seven trials, 14,922 participants, Analysis 1.16), however there was no difference in the risk of antepartum hemorrhage (RR 1.25, 95% CI 0.85 to 1.82, two trials, 12,256 participants, Analysis 1.16).
1.16. Analysis.
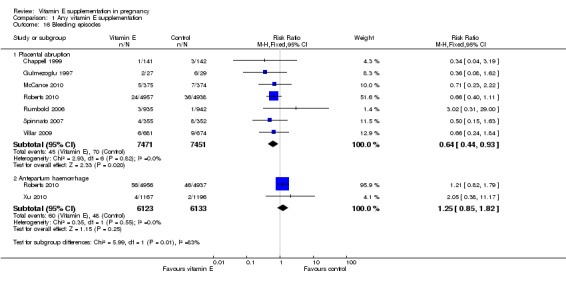
Comparison 1 Any vitamin E supplementation, Outcome 16 Bleeding episodes.
Substantial heterogeneity was detected for preterm PROM (I² = 66%). When using a random‐effects model, there was no clear difference between women supplemented with vitamin E in combination with other supplements compared with placebo for preterm PROM (average RR 1.27, 95% CI 0.93 to 1.75, five trials, 1999 participants). Conversely, women supplemented with vitamin E alone or in combination with other supplements had an increased risk of term PROM when compared with women given a placebo control (average RR 1.77, 95% CI 1.37 to 2.28, two trials, 2504 participants, Analysis 1.13).
1.13. Analysis.
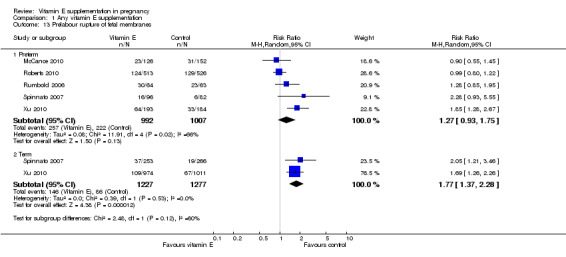
Comparison 1 Any vitamin E supplementation, Outcome 13 Prelabour rupture of fetal membranes.
There were no other differences in any maternal secondary outcomes between women supplemented with vitamin E in combination with other supplements compared with placebo or no control including maternal death (RR 0.60, 95% CI 0.14 to 2.51, seven trials, 17,120 participants, Analysis 1.14), any caesarean section (RR 1.02, 95% CI 0.97 to 1.07, six trials, 15,297 participants, Analysis 1.15), prelabour caesarean section (RR 1.15, 95% CI 0.85 to 1.56, two trials, 1932 participants, Analysis 1.15), induction of labour (RR 1.11, 95% CI 0.97 to 1.26, one trial, 1877 participants, Analysis 1.15), eclampsia (RR 1.67, 95% CI 0.82 to 3.41, eight trials, 19,471 participants, Analysis 1.17), renal failure or renal insufficiency (RR 1.49, 95% CI 0.55 to 4.02, two trials, 1933 participants, Analysis 1.17), disseminated intravascular coagulation (RR 0.36, 95% CI 0.02 to 8.41, one trial, 56 participants, Analysis 1.17), or pulmonary oedema (RR 0.40, 95% CI 0.16 to 1.03. four trials, 12,569 participants, Analysis 1.17).
1.14. Analysis.
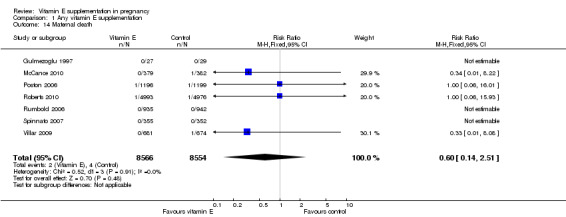
Comparison 1 Any vitamin E supplementation, Outcome 14 Maternal death.
1.15. Analysis.
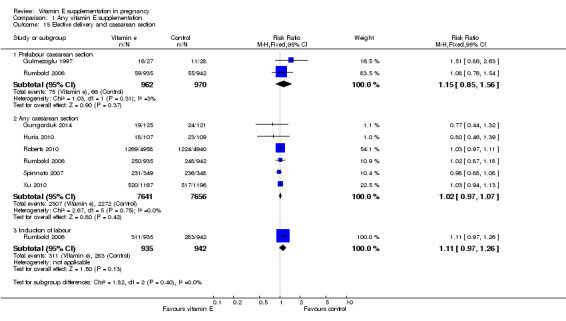
Comparison 1 Any vitamin E supplementation, Outcome 15 Elective delivery and caesarean section.
1.17. Analysis.
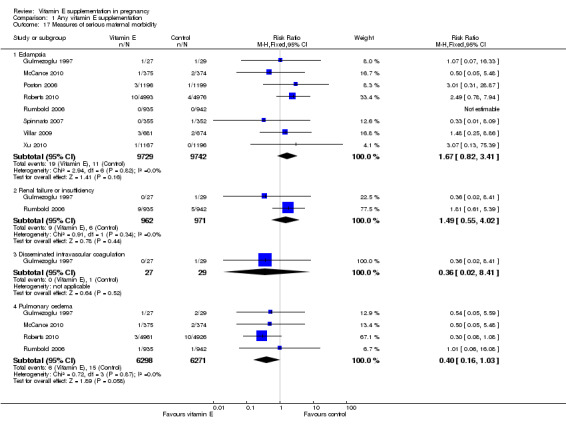
Comparison 1 Any vitamin E supplementation, Outcome 17 Measures of serious maternal morbidity.
For the infant, substantial heterogeneity was detected for gestational age at birth, bacterial sepsis, necrotising enterocolitis and chronic lung disease or bronchopulmonary dysplasia. When using a random‐effects models, there were no clear differences between treatment groups in gestational age at birth (MD 0.15, 95% CI ‐0.12 to 0.43, seven trials, 13,783 participants, I² = 81%; Analysis 1.20), or the risk of bacterial sepsis (average RR 1.10, 95% CI 0.73 to 1.67, five trials, 13,324 participants; I² = 40%), necrotising enterocolitis (average RR 0.74, 95% CI 0.36 to 1.55, seven trials, 18,514 participants; I² = 45%, Analysis 1.28), or chronic lung disease/bronchopulmonary dysplasia (average RR 0.69, 95% CI 0.10 to 4.69, three trials, 3262 participants; I² = 57%, Analysis 1.25). There were no clear differences between treatment groups for any other infant outcomes including: congenital malformations (RR 1.16, 95% CI 0.83 to 1.63, four trials, 5511 participants, Analysis 1.21), or Apgar score less than seven at five minutes (RR 0.73, 95% CI 0.42 to 1.27, three trials, 3531 participants, Analysis 1.22), respiratory distress syndrome (RR 0.98, 95% CI 0.89 to 1.08, eight trials, 18,574 participants, Analysis 1.24), periventricular hemorrhage and periventricular leukomalacia (RR 0.91, 95% CI 0.58 to 1.42, six trials, 17,787 participants, Analysis 1.26) or retinopathy of prematurity (RR 1.18, 95% CI 0.72 to 1.93, six trials, 18,270 participants, Analysis 1.29). No trials reported maternal or infant peripheral neuropathy, maternal satisfaction with care, vitamin K deficiency bleeding or haemorrhagic disease of the newborn, disability at childhood follow‐up, poor childhood growth or any adverse events related to vitamin E supplementation.
1.20. Analysis.
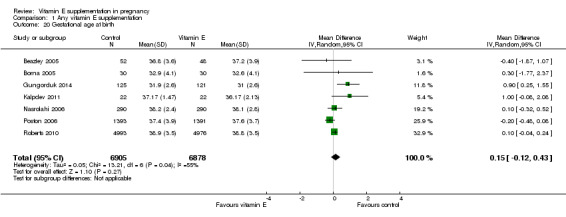
Comparison 1 Any vitamin E supplementation, Outcome 20 Gestational age at birth.
1.28. Analysis.
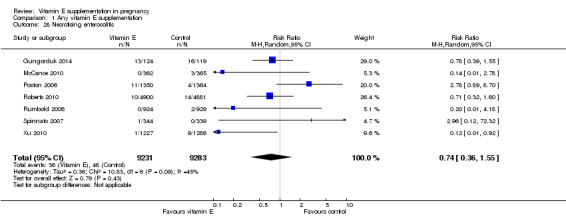
Comparison 1 Any vitamin E supplementation, Outcome 28 Necrotising enterocolitis.
1.25. Analysis.
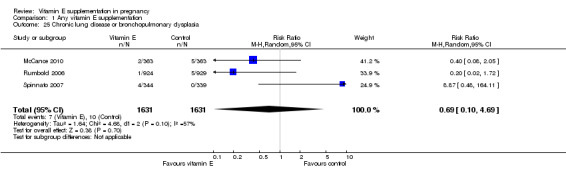
Comparison 1 Any vitamin E supplementation, Outcome 25 Chronic lung disease or bronchopulmonary dysplasia.
1.21. Analysis.
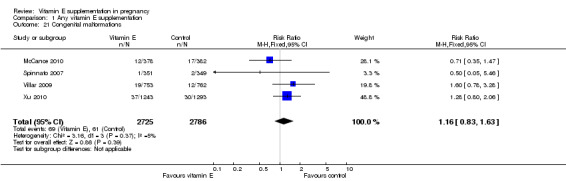
Comparison 1 Any vitamin E supplementation, Outcome 21 Congenital malformations.
1.22. Analysis.
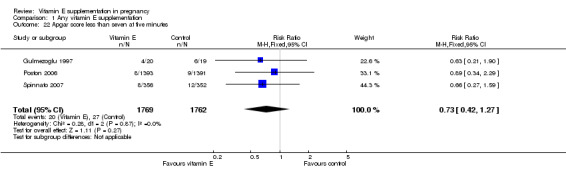
Comparison 1 Any vitamin E supplementation, Outcome 22 Apgar score less than seven at five minutes.
1.24. Analysis.
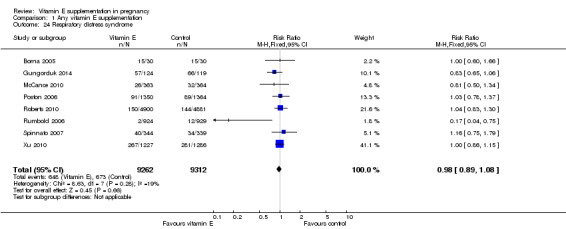
Comparison 1 Any vitamin E supplementation, Outcome 24 Respiratory distress syndrome.
1.26. Analysis.
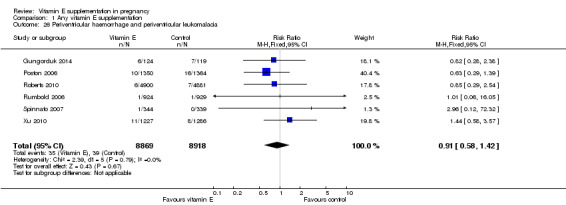
Comparison 1 Any vitamin E supplementation, Outcome 26 Periventricular haemorrhage and periventricular leukomalacia.
1.29. Analysis.
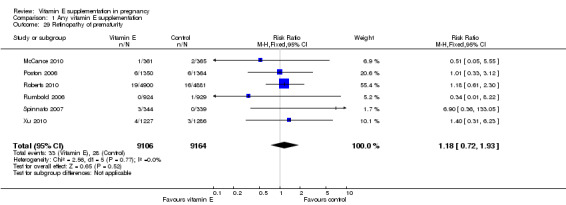
Comparison 1 Any vitamin E supplementation, Outcome 29 Retinopathy of prematurity.
None of the studies reported on adverse events that were sufficient to stop supplementation. Possible side effects of supplementation were poorly reported (Analysis 1.33). Three trials (Roberts 2010; Rumbold 2006; Xu 2010) reported on the presence of elevated liver enzymes, and there was overall no clear difference in the risk of this outcome between treatment groups (RR 1.00, 95% CI 0.71 to 1.41, three studies, 14,209 participants). An additional study (Poston 2006), reported that there was no clear difference in liver enzymes between treatment groups, however the data could not be included in the meta‐analysis. One trial reported an increased risk of abdominal pain in women supplemented with vitamin E in combination with other supplements (RR 1.66, 95% CI 1.16 to 2.37, 1877 participants). However, there were no clear differences in the risk of developing other side effects including acne (RR 3.21, 95% CI 0.14 to 75.68, one trial, 56 participants), transient weakness (RR 5.36, 95% CI 0.27 to 106.78, one trial, 56 participants), or skin rash (RR 3.21, 95% CI 0.14 to 75.68, one trial, 56 participants), or any side effect (symptoms combined) (RR 1.16, 95% CI 0.39 to 3.41, one trial, 707 participants) between treatment groups.
1.33. Analysis.
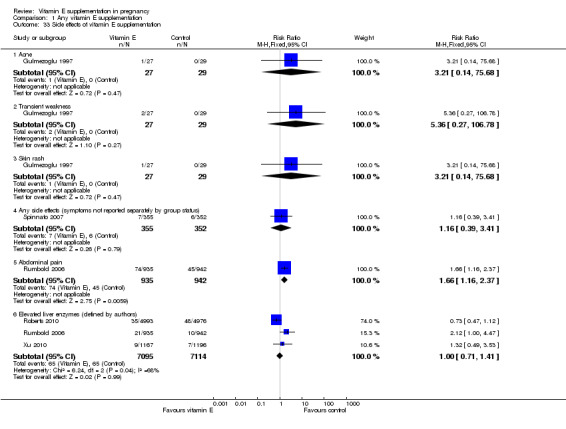
Comparison 1 Any vitamin E supplementation, Outcome 33 Side effects of vitamin E supplementation.
Furthemore, one study (McCance 2010), stated in the text that there were "no adverse events or side effects attributable to supplementation."
Substantial heterogeneity for found for outcomes related to use of health service resources for the mother (Analysis 1.34).There was no clear difference between women supplemented with vitamin E in combination with other supplements compared with placebo in the risk of admission to the adult intensive care unit (average RR 0.60, 95% CI 0.16 to 2.30; two trials, 3718, participants; I² = 45%), or hospitalisations in pregnancy (average RR 0.74, 95% CI 0.30 to 1.80, two trials, 2407 participants; I² = 61%), when using a random‐effects model. There were no clear differences between treatment groups for any of the outcomes related to use of health service resources for the infant (Analysis 1.35), including: admission to the intensive care unit (RR 1.01, 95% CI 0.95 to 1.08, eight trials, 17,594 participants) and use of mechanical ventilation (RR 1.02, 95% CI 0.84 to 1.25, six trials, 8531 participants).
1.34. Analysis.
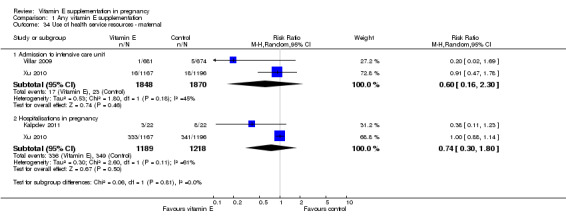
Comparison 1 Any vitamin E supplementation, Outcome 34 Use of health service resources ‐ maternal.
1.35. Analysis.
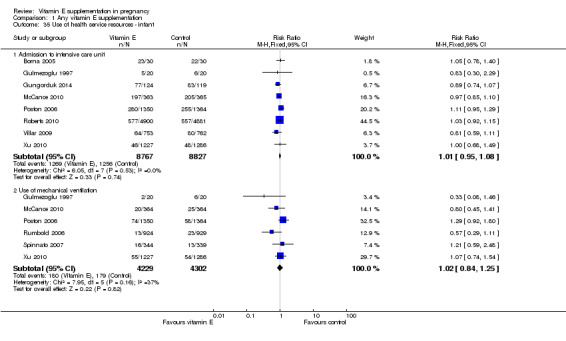
Comparison 1 Any vitamin E supplementation, Outcome 35 Use of health service resources ‐ infant.
Sensitivity analyses by trial quality
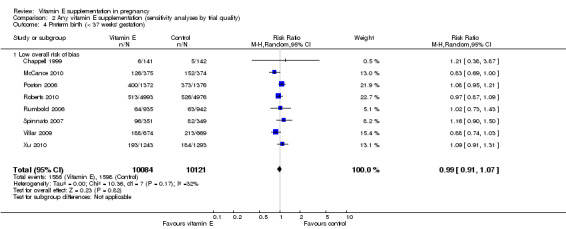
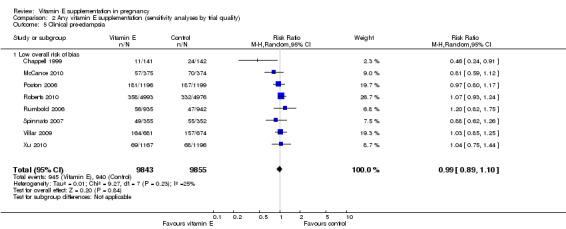
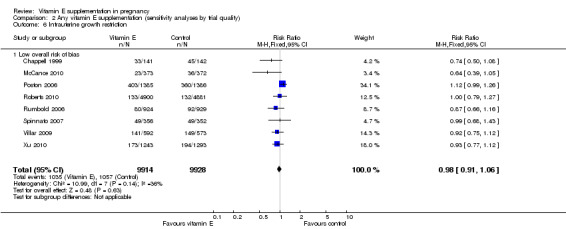
Assessments of the treatment effects were made for the primary outcomes based on trial quality. Ten trials were judged to have a low overall risk of bias (Chappell 1999; Gulmezoglu 1997; McCance 2010; Poston 2006; Pressman 2003; Roberts 2010; Rumbold 2006; Spinnato 2007; Villar 2009; Xu 2010), for six trials the overall risk was unclear (Anthony 1996; Beazley 2005; Borna 2005; Mahdy 2004; Rivas 2000; Sawhney 2000), and five trials had a high overall risk of bias (Gungorduk 2014; Huria 2010; Kalpdev 2011; Nasrolahi 2006; Shahraki 2006). When the analyses were restricted to studies at low overall risk of bias, the risks of stillbirth, neonatal death, perinatal death, preterm birth, pre‐eclampsia and intrauterine growth restriction did not change substantively to the analyses which included all trials (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6). However, for the outcomes preterm birth and clinical pre‐eclampsia (Analysis 2.4; Analysis 2.5), restricting the analyses to studies at low risk of bias reduced the heterogeneity, from 52% to 32% and 48% to 25%, respectively, and there was a small reduction in the effect sizes (although both remained not statistically significant), suggesting that variation in trial quality explains some of the heterogeneity detected for these outcomes.
2.1. Analysis.
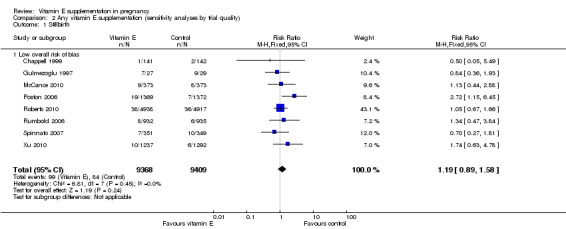
Comparison 2 Any vitamin E supplementation (sensitivity analyses by trial quality), Outcome 1 Stillbirth.
2.2. Analysis.
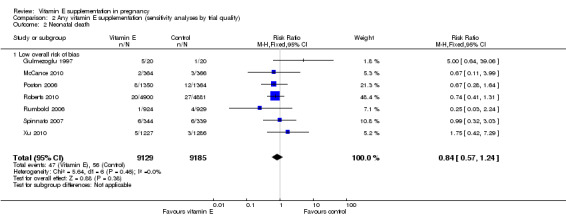
Comparison 2 Any vitamin E supplementation (sensitivity analyses by trial quality), Outcome 2 Neonatal death.
2.3. Analysis.
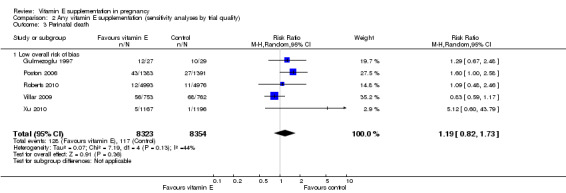
Comparison 2 Any vitamin E supplementation (sensitivity analyses by trial quality), Outcome 3 Perinatal death.
2.4. Analysis.
Comparison 2 Any vitamin E supplementation (sensitivity analyses by trial quality), Outcome 4 Preterm birth (< 37 weeks' gestation).
2.5. Analysis.
Comparison 2 Any vitamin E supplementation (sensitivity analyses by trial quality), Outcome 5 Clinical pre‐eclampsia.
2.6. Analysis.
Comparison 2 Any vitamin E supplementation (sensitivity analyses by trial quality), Outcome 6 Intrauterine growth restriction.
Subgroup analyses
Dosage of the vitamin E supplement (above or equal to/below the recommended dietary intake of 7 mg alpha‐TE)
All of the included studies supplemented women with vitamin E in a dosage above the recommended dietary intake (RDI). Therefore, subgroup analyses based on dosage were not performed. Furthermore, there was limited variation in the dosages used above the RDI. For example, 15 trials used the same dose of daily 400 international units (IU) vitamin E, a further three trials gave women either daily 100 mg, 200 IU or 800 IU vitamin E, and the dose was unknown for a further three trials.
Gestation at trial entry (less than 20 weeks or greater than or equal to 20 weeks)
Five trials ( Huria 2010; Kalpdev 2011; Roberts 2010; Spinnato 2007; Xu 2010) enrolled women from less than 20 weeks' gestation; five trials (Borna 2005; Gulmezoglu 1997; Gungorduk 2014; Pressman 2003; Shahraki 2006) enrolled women after 20 weeks' gestation; and the other seven trials (Beazley 2005:Chappell 1999; McCance 2010; Poston 2006; Rivas 2000; Rumbold 2006; Villar 2009) enrolled women both before and after 20 weeks' gestation. For a further four trials (Anthony 1996; Mahdy 2004; Nasrolahi 2006; Sawhney 2000), the gestation at trial entry was unknown.
When the analyses were stratified across these groups, the test for subgroup differences was not significant for stillbirth, preterm birth, neonatal death, perinatal death, preterm birth and intrauterine growth restriction (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6), and the findings in the subgroups were not substantively different to the main analyses. Subgroup differences were apparent for the outcome pre‐eclampsia (Chi² = 7.11, P = 0.03, I² = 71.9%), indeed the risk of pre‐eclampsia was reduced in the two trials that reported pre‐eclampsia and where gestation at trial entry was unknown (RR 0.32, 95% CI 0.13 to 0.79, two trials, 693 participants, I² = 0%). However, there was no significant difference in risk of pre‐eclampsia in the trials that enrolled women prior to 20 weeks' (RR 1.03, 95% CI 0.91 to 1.16,five trials, 13,299 participants; I² = 0%), or those that enrolled both women before and after 20 weeks' (RR 0.90, 95% CI 0.73 to 1.12, seven trials, 6886 participants; I² = 57%). As the only significant difference was detected in trials with an unknown gestation at trial entry, the subgroup findings for pre‐eclampsia are likely to reflect characteristics related to the quality of the trial, not the timing of commencement of supplementation. Collectively, these findings suggest that the treatment effect does not vary substantively by the gestation at trial entry and that differences in this characteristic do not contribute significantly to the observed heterogeneity.
3.1. Analysis.
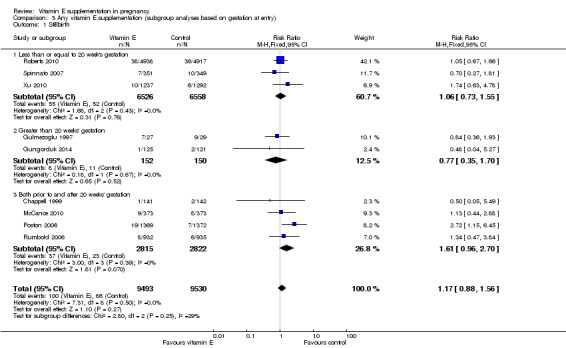
Comparison 3 Any vitamin E supplementation (subgroup analyses based on gestation at entry), Outcome 1 Stillbirth.
3.2. Analysis.
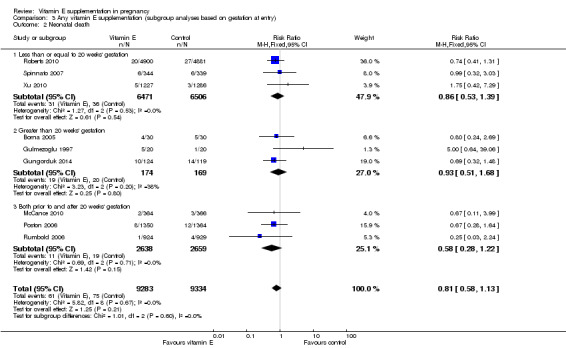
Comparison 3 Any vitamin E supplementation (subgroup analyses based on gestation at entry), Outcome 2 Neonatal death.
3.3. Analysis.
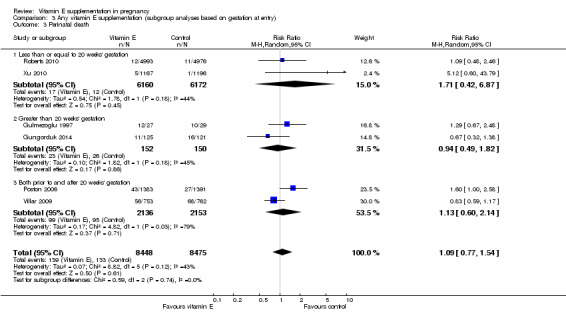
Comparison 3 Any vitamin E supplementation (subgroup analyses based on gestation at entry), Outcome 3 Perinatal death.
3.4. Analysis.
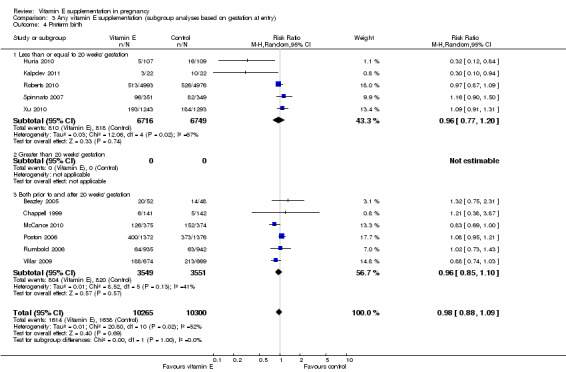
Comparison 3 Any vitamin E supplementation (subgroup analyses based on gestation at entry), Outcome 4 Preterm birth.
3.5. Analysis.
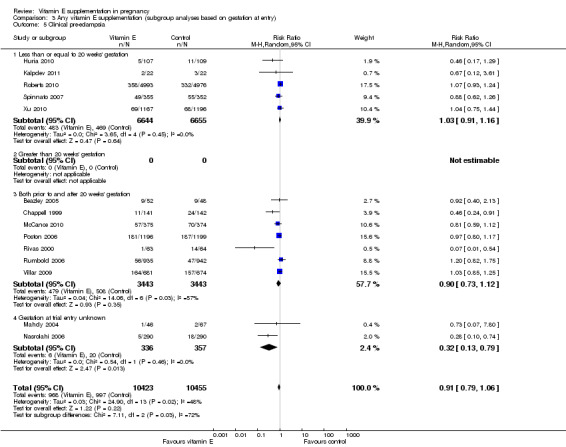
Comparison 3 Any vitamin E supplementation (subgroup analyses based on gestation at entry), Outcome 5 Clinical pre‐eclampsia.
3.6. Analysis.
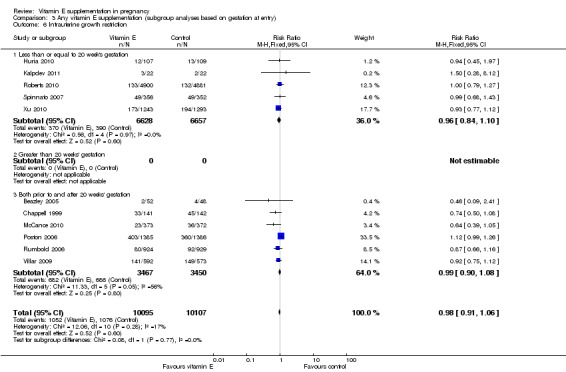
Comparison 3 Any vitamin E supplementation (subgroup analyses based on gestation at entry), Outcome 6 Intrauterine growth restriction.
Low or adequate dietary vitamin E intake prior to trial entry (low intake defined as intake less than the recommended dietary intake in that setting as measured by dietary questionnaire)
Two trials (Roberts 2010; Rumbold 2006) reported on dietary vitamin E intake of participants at trial entry. One study (Roberts 2010), was classified as including participants with "adequate intake", as the median intake in the treatment and control groups was above the RDI. The other study (Rumbold 2006), was classified as including women with "low intake" as less than half of all participants met the RDI at trial entry (43% and 42% in the treatment and control groups, respectively. One trial (Villar 2009), was designed specifically to assess the effect of vitamin E in combination with vitamin C in populations with poor nutrition, however the dietary intake of vitamin E and other micronutrients of participants was not assessed. This trial was classified as including participants with "low nutritional status", the remaining 18 trials were classified as "dietary intake unclear". Three studies (Chappell 1999; McCance 2010; Poston 2006), assessed plasma concentrations of vitamin E at baseline, and one of these trials (McCance 2010), reported information about pre‐eclampsia according to baseline vitamin E status.
There were no clear differences in the risks of stillbirth, neonatal death, perinatal death, preterm birth or intrauterine growth restriction between women supplemented with vitamin E in combination with other supplements compared with placebo or no control, in any of the subgroups based on dietary intake of vitamin E (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.6). ) F Furthermore, the test for subgroup differences were not significant for any of these outcomes, suggesting that the treatment effects due not differ substantively between trials in these subgroups.
4.1. Analysis.
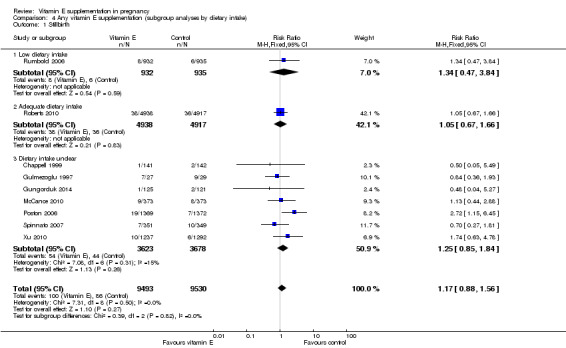
Comparison 4 Any vitamin E supplementation (subgroup analyses by dietary intake), Outcome 1 Stillbirth.
4.2. Analysis.
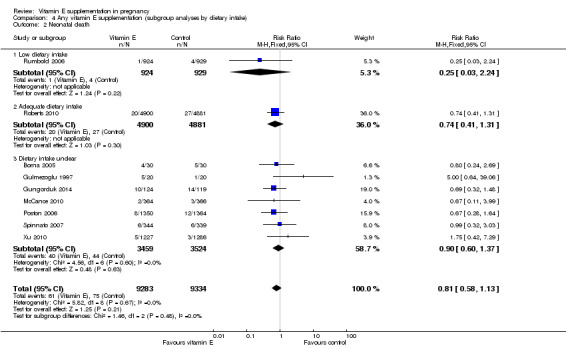
Comparison 4 Any vitamin E supplementation (subgroup analyses by dietary intake), Outcome 2 Neonatal death.
4.3. Analysis.
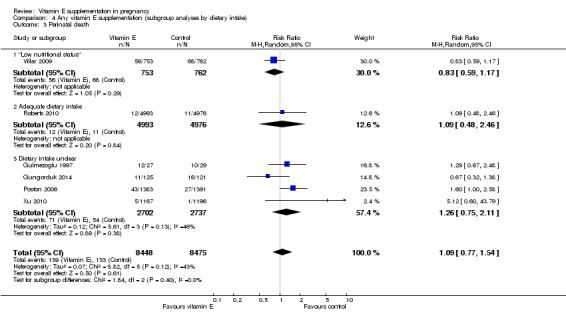
Comparison 4 Any vitamin E supplementation (subgroup analyses by dietary intake), Outcome 3 Perinatal death.
4.4. Analysis.
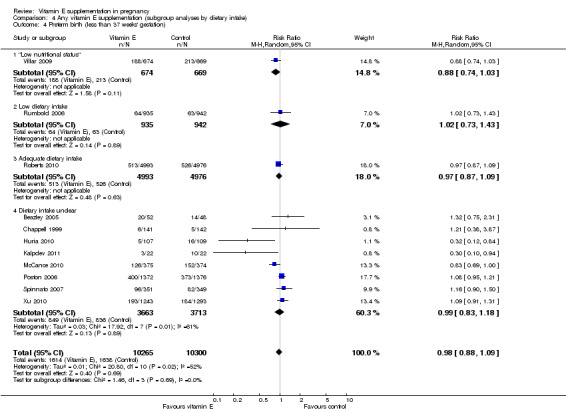
Comparison 4 Any vitamin E supplementation (subgroup analyses by dietary intake), Outcome 4 Preterm birth (less than 37 weeks' gestation).
4.6. Analysis.
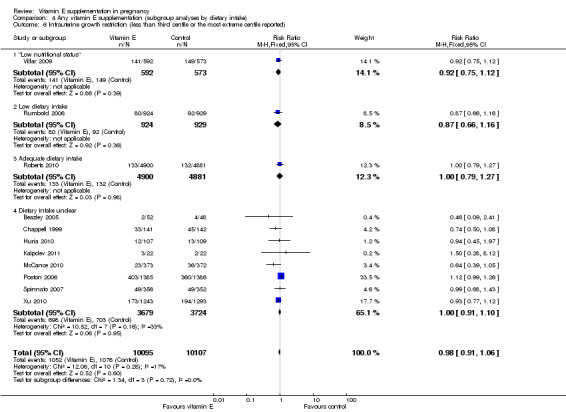
Comparison 4 Any vitamin E supplementation (subgroup analyses by dietary intake), Outcome 6 Intrauterine growth restriction (less than third centile or the most extreme centile reported).
For the outcome pre‐eclampsia (Analysis 4.5), in a subgroup group of women with low baseline vitamin E status, the risk was reduced in women supplemented with vitamin E in combination with other supplements compared with placebo, although the result did not reach statistical significance (average RR 0.35, 95% CI 0.12 to 1.02; one trial, 95 participants). A reduced risk of pre‐eclampsia was also observed among women supplemented with vitamin E in the trials where the baseline dietary intake was unclear (average RR 0.74, 95% CI 0.56 to 0.98, 10 trials, 6928 participants; I² = 52%). However, there was no observed reduction in the risk of pre‐eclampsia in vitamin E supplemented women with low intake (average RR 1.20, 95% CI 0.82 to 1.75, one trial, 1877 participants), or from populations with low nutritional status (average RR 1.03, 95% CI 0.85 to 1.25, one trial, 1355 participants). There was no clear difference in the risk of pre‐eclampsia between treatment groups in women with adequate intake (RR 1.07, 95% CI 0.93 to 1.24, one trial, 9969 participants), or moderate/high baseline vitamin E status (average RR 0.77, 95% CI 0.53 to 1.12, one trial, 572 participants). The test of subgroup differences was significant for this outcome (Chi² = 11.63, P = 0.02, I² = 65.6%).
4.5. Analysis.
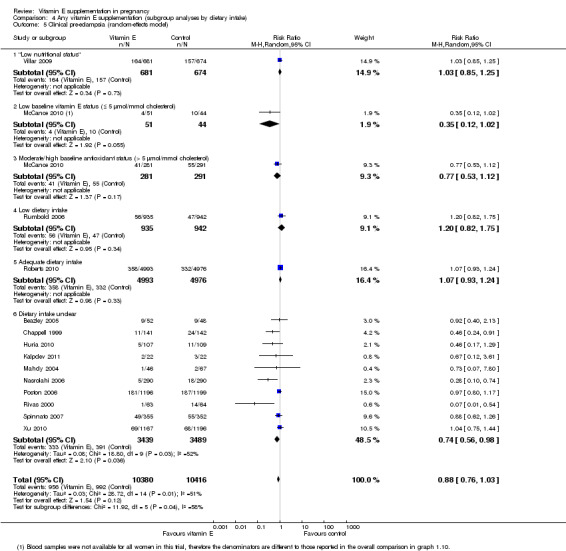
Comparison 4 Any vitamin E supplementation (subgroup analyses by dietary intake), Outcome 5 Clinical pre‐eclampsia (random‐effects model).
The use of vitamin E in combination with other dietary supplements
Three trials supplemented women with vitamin E alone (Anthony 1996; Sawhney 2000; Shahraki 2006), however none of these trials reported any clinically meaningful information. Seventeen trials gave women vitamin E in combination with vitamin C, and two of these trials also supplemented women additionally with either allopurinol (Gulmezoglu 1997), or aspirin and fish oil (Rivas 2000). The remaining trial supplemented women with a vitamin E rich fraction of palm oil, which is likely to have contained other nutritional agents, however, no further information was provided about the content of the supplement. Therefore, as there were no trials available to assess the effect of vitamin E supplementation alone, subgroup analyses were not performed.
Women's risk status for adverse pregnancy outcomes (as defined by the authors)
Nine trials supplemented women who were at increased risk or high risk of pre‐eclampsia (Beazley 2005; Chappell 1999; Kalpdev 2011; McCance 2010; Poston 2006; Rivas 2000; Spinnato 2007; Villar 2009; Xu 2010), three trials supplemented women with established pre‐eclampsia (Anthony 1996; Gulmezoglu 1997; Sawhney 2000), and two trials supplemented women with established preterm PROM (Borna 2005; Gungorduk 2014). For this subgroup analysis, all 14 of these studies were classified as including women at 'high/increased risk' of adverse pregnancy outcomes. Five trials supplemented nulliparous or primiparous women (Huria 2010; Mahdy 2004; Nasrolahi 2006; Roberts 2010; Rumbold 2006), one trial supplemented pregnant women with leg cramps (Shahraki 2006), and a further trial supplemented with planned caesarean section (Pressman 2003). These remaining seven trials were classified as including women at 'low/moderate risk' of adverse pregnancy outcomes.
For the outcomes stillbirth, neonatal death, perinatal death, preterm birth, clinical pre‐eclampsia and intrauterine growth restriction, there were no clear differences in the effects of vitamin E supplementation in combination with other supplements versus placebo or no control for women classified as 'high/increased risk' and for those classified as 'low/moderate' risk (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6). Furthermore, the tests for subgroup differences were not significant for any of these outcomes. For the outcomes pre‐eclampsia and preterm birth, substantial heterogeneity was present in the analyses of both of these subgroups, suggesting that heterogeneity between included studies may be due to other factors rather than just differences in baseline risk of adverse pregnancy outcomes.
5.1. Analysis.
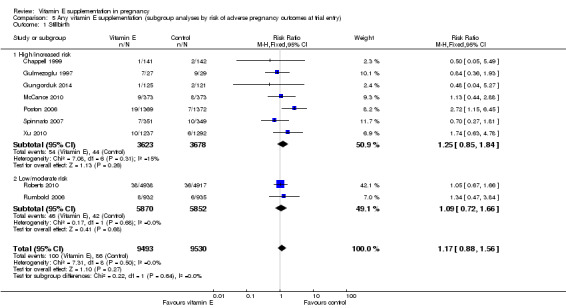
Comparison 5 Any vitamin E supplementation (subgroup analyses by risk of adverse pregnancy outcomes at trial entry), Outcome 1 Stillbirth.
5.2. Analysis.
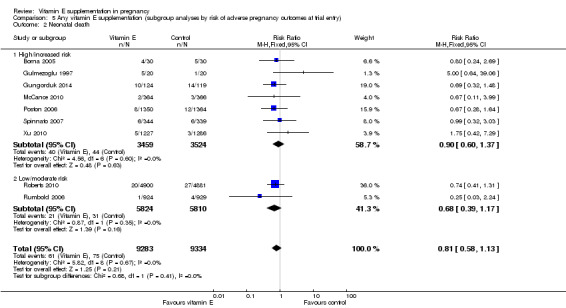
Comparison 5 Any vitamin E supplementation (subgroup analyses by risk of adverse pregnancy outcomes at trial entry), Outcome 2 Neonatal death.
5.3. Analysis.
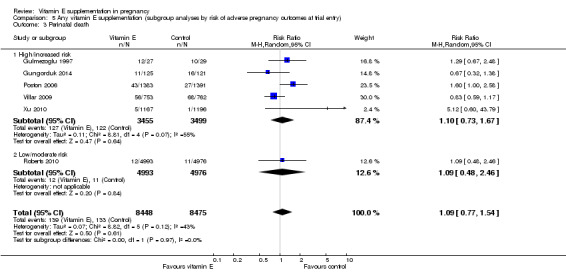
Comparison 5 Any vitamin E supplementation (subgroup analyses by risk of adverse pregnancy outcomes at trial entry), Outcome 3 Perinatal death.
5.4. Analysis.
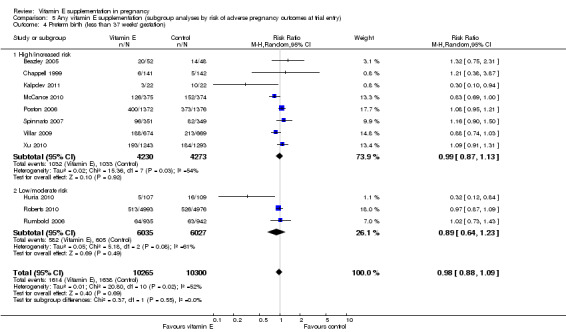
Comparison 5 Any vitamin E supplementation (subgroup analyses by risk of adverse pregnancy outcomes at trial entry), Outcome 4 Preterm birth (less than 37 weeks' gestation).
5.5. Analysis.
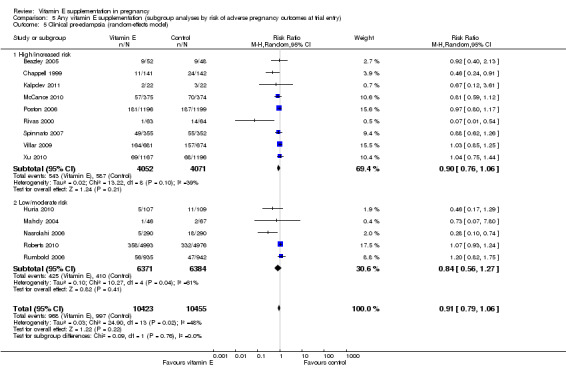
Comparison 5 Any vitamin E supplementation (subgroup analyses by risk of adverse pregnancy outcomes at trial entry), Outcome 5 Clinical pre‐eclampsia (random‐effects model).
5.6. Analysis.
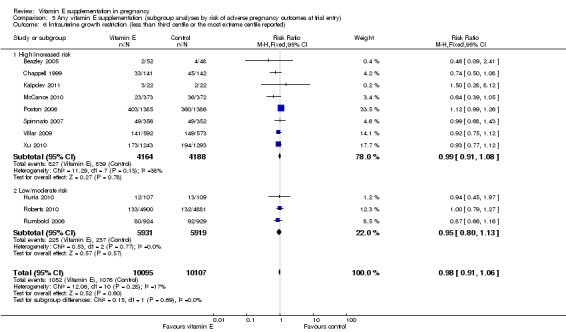
Comparison 5 Any vitamin E supplementation (subgroup analyses by risk of adverse pregnancy outcomes at trial entry), Outcome 6 Intrauterine growth restriction (less than third centile or the most extreme centile reported).
Discussion
Summary of main results
The results of this review, which included 21 trials involving over 21,000 women and their babies, do not support routine vitamin E supplementation in combination with other supplements in pregnancy. We found no clear differences between women supplemented with vitamin E compared with placebo or control for the risk of any primary maternal or infant outcomes including fetal, perinatal or neonatal death, preterm birth, pre‐eclampsia or intrauterine growth restriction. Supplementation was associated with a reduced risk of placental abruption, which warrants further investigation. However, there was also some evidence of harm, as supplementation appeared to increase the risk of term prelabour rupture of membranes (PROM) as well as self‐reported abdominal pain. There was no convincing evidence that vitamin E supplementation in combination with other supplements results in any other important benefits or harms.
Overall completeness and applicability of evidence
In this review, three trials assessed vitamin E supplementation alone in pregnancy; however, the data from these trials could not be used in the meta‐analysis. Seventeen trials assessed vitamin E in conjunction with vitamin C, and one further trial gave with a vitamin E rich fraction of palm oil. Therefore, there was no information available to assess whether vitamin E supplementation alone may be beneficial or harmful for women, hence any treatment effects seen here may not be directly attributable to vitamin E.
This review provides reliable information about the impact of vitamin E supplementation in combination with other supplements on a range of maternal, perinatal and infant health outcomes. Pre‐eclampsia was the most commonly reported outcome among included studies (14 trials, 20,878 participants). This is not surprising as 14 trials assessed vitamin E supplementation in combination with other supplements for the prevention of pre‐eclampsia. Other commonly reported outcomes were preterm birth (11 trials, 20,565 participants), intrauterine growth restriction (11 trials, 20,202 participants), birthweight (10 trials, 16,888 participants), fetal and neonatal death (nine trials, 19,023 participants), eclampsia (eight trials, 19,471 participants), neonatal respiratory distress syndrome (eight trials, 18,574 women) and admission to the neonatal intensive care unit (eight trials, 17,782 participants).
However, no trials reported any maternal and infant haematological measures (except hyperbilirubinaemia reported by one trial), vitamin K deficiency bleeding, peripheral neuropathy, maternal satisfaction with care or any possible long‐term benefits or harms of supplementation for the mother. One trial (Poston 2006) reported on various measures of respiratory function (wheezing, asthma) in children aged up to two years, however, whether antenatal supplementation influences the health of children beyond two years of age is unknown .There were also scarce available data on side effects of vitamin E supplementation. One trial reported an increased risk of low abdominal pain in the vitamin supplemented group compared with a placebo control. There were no other clear differences between women supplemented with vitamin E compared with placebo or no control for any other potential side effects assessed, including elevated liver enzymes, acne, transient weakness and skin rash.
We detected substantial heterogeneity for the outcomes perinatal death, preterm birth, pre‐eclampsia, birthweight, and preterm PROM. For perinatal death, excluding the trial by Villar 2009 reduced the heterogeneity to zero. Therefore, the observed heterogeneity may reflect different characteristics of participating women or differential access to maternity care, as the Villar 2009 trial involved women of low income in developing countries at risk of poor nutritional status, who may have a generally higher risk of perinatal mortality than women in the other trials who were predominantly from Western, developed, countries.
For preterm birth and pre‐eclampsia, the identified heterogeneity appears to be explained in part by variation in the quality of included studies. For example, in the sensitivity analyses, when studies at high or unclear risk of bias were excluded the magnitude of the heterogeneity was reduced markedly. Furthermore, for preterm birth, removing the trial by McCance 2010 further reduced the heterogeneity (down to I2 = 5%). The heterogeneity that appears to be associated with McCance 2010 may reflect a higher baseline risk of preterm birth among participants in this trial compared with other trials, as McCance 2010 included women with type 1 diabetes, a known risk factor for preterm birth (Hanson 1993). Indeed the rate of preterm birth in the control group of this trial was 41%, the rate in the control group of other included studies ranged from 4% to 45% (median 23%).
In the sensitivity analyses for pre‐eclampsia, removing the trial by Chappell 1999 reduced the heterogeneity to zero. It is possible that heterogeneity associated with Chappell 1999 may reflect differences in underlying risk, however the rate of pre‐eclampsia in the control group (17%) was similar to that observed in other included trials (range 3% to 25%, median 15%). As Chappell 1999 was a small, positive trial, we explored the possibility of reporting bias for this outcome using a funnel plot (see Figure 4). The distribution of the results in Figure 4 are skewed, indicating that small studies reporting negative findings may be missing which could indicate reporting bias.
For preterm PROM, removing the trials by Spinnato 2007 and Xu 2010 reduced the heterogeneity to zero and the point estimate changed from RR = 1.27 to 1.02. The cause of the heterogeneity associated with these two studies is unclear. Both were judged to be at low overall risk of bias, and participants did not appear to be at high risk of preterm PROM, as the rate in the control group of both trials was lower than the rate observed in other included trials (7% to 18% versus 20% to 37%). One possibility is that there was a greater proportion of women with a history of PROM (either preterm or term) in the vitamin E group; however, this cannot be examined as information about the history of PROM among multiparous participants is not available. As these two trials are the only studies that contribute data for the outcome term PROM, the finding of an increased risk of term PROM among vitamin E supplemented women should be interpreted with caution. It should be noted that another included trial (Poston 2006), reported that there was no difference in the risk of either preterm or term PROM between treatment groups, however, individual data were not reported so this trial did not contribute to the meta‐analysis for those outcomes. Further observational research investigating the role of vitamin E in the development of preterm and term PROM is warranted.
We also undertook pre‐specified subgroup analyses exploring the effect of variation in baseline risk of adverse pregnancy outcomes. Approximately two‐thirds of all trials included women considered to be at high or increased risk of adverse outcomes (mostly pre‐eclampsia). For all of the primary outcomes, the effect sizes did not vary substantially between women at high/increased risk and women at low/moderate risk, suggesting that there are no benefits of supplementation in particular subgroups based on underlying risk.
We also undertook other subgroup analyses to explore the impact of variation in timing of commencement of supplementation (i.e. gestation at trial entry) and adequacy of dietary intake of vitamin E prior to trial entry. While there was substantial variation in the gestation at trial entry across all of the included studies, the subgroup analyses did not reveal any substantial differences in the effect size between trials enrolling women prior to 20 weeks’, after 20 weeks’ or that included women both prior to and after 20 weeks’ gestation. This suggests that there is no benefit of commencing supplementation either earlier or later in pregnancy.
Whether vitamin E supplementation is beneficial for women with low or inadequate vitamin E intake is unclear. Only two studies reported information specifically about women's dietary intake of vitamin E at trial entry, and in the subgroup analyses including these trials, there were no apparent benefits of vitamin E supplementation either in women with low or adequate intake. One study (Villar 2009) was undertaken in women from populations with a presumed low dietary intake of vitamin E, based on dietary information collected in previous studies of clinic attendees. There was no clear difference in the pattern of risks of adverse outcomes in these women either. One trial (McCance 2010), reported information about pre‐eclampsia according to baseline plasma vitamin E status. In this trial, there appeared to be a reduced risk of pre‐eclampsia in women with a low baseline vitamin E intake, although the result was of borderline statistical significance and should be interpreted with caution due to the small number of women in this subgroup (n = 95).
Subgroup analyses exploring the impact of vitamin E dosage and use of vitamin E combined with other supplements were planned but could not be undertaken due to insufficient data in each subgroup.
Quality of the evidence
The overall risk of bias is low to unclear for most of the studies, however, there were some high quality, large trials that were heavily weighted in the analysis. Five trials were judged to be at high risk of bias.
The quality of the evidence using GRADE was high for preterm birth, intrauterine growth restriction and placental abruption, moderate for stillbirth and clinical pre‐eclampsia, and low for preterm PROM (Table 1). The outcomes were downgraded due to wide confidence interval crossing the line of no effect, statistical heterogeneity, and publication bias.
Potential biases in the review process
We followed the Cochrane Pregnancy and Childbirth Group search strategies, which includes a search of the Trials Register that is updated weekly to monthly from a range of databases as well as handsearches from selected journals and proceedings of major conferences. Therefore, it is unlikely that studies that have been missed, although it is possible that studies that have not been published could be missing. Should any further studies be identified, we will include them in future updates of the review. We also followed the Cochrane Pregnancy and Childbirth Group recommended review process to reduce potential biases, which included having at least two review authors independently assessing identified studies, extracting data and evaluating risk of bias.
Agreements and disagreements with other studies or reviews
The findings of this review are in agreement with several meta‐analyses examining the effects of vitamin C and E supplementation for the prevention of pre‐eclampsia and other maternal and perinatal complications (Basaran 2010; Conde‐Agudelo 2011).
The finding of a 36% reduction in the relative risk of placental abruption among vitamin E supplemented women compared with placebo or no control warrants further investigation. There is some limited evidence of serum markers of oxidative stress as well as low serum levels of vitamin E in women with placental abruption (Incebiyik 2015; Sharma 1986), suggesting a biologically plausible role for vitamin E supplementation. However, lower serum levels of other antioxidants including vitamin C have also been demonstrated (Sharma 1985), therefore, it is unclear whether the result observed in this review is due to vitamin E or C or the combination of both agents. Further observational research examining the underlying pathways to placental abruption is required, before any firm conclusions can be drawn about this finding.
Authors' conclusions
Implications for practice.
There is now a large body of evidence from randomised trials involving over 21,000 women assessing the effects of vitamin E supplementation in combination with other supplements including vitamin C in pregnancy. The available data do not support routine vitamin E supplementation in combination with other supplements for the prevention of fetal or neonatal death, preterm birth, pre‐eclampsia, or intrauterine growth restriction for all women or for women at high risk of adverse pregnancy outcomes. Supplementation was associated with a reduced risk of placental abruption, this should be explored in further research examining the specific role of vitamin E in the etiology of placental abruption. There was some evidence of harm, as vitamin E supplementation appeared to increase the risk of term PROM and self‐reported abdominal pain.
Implications for research.
The reduction in placental abruption requires further assessment. Future research should be conducted to examine whether the observed effects for placental abruption are due to vitamin E or the influence of other agents including vitamin C. Long‐term follow‐up studies of women and children enrolled in the current trials are required to determine whether there are any longer term benefits or harms of vitamin E supplementation. Further research is also required to examine the effect of supplementation in women with a low or inadequate intake of vitamin E prior to and in early pregnancy.
What's new
| Date | Event | Description |
|---|---|---|
| 22 March 2016 | Amended | Added external source of support for Erika Ota (the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization). |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 8 April 2015 | New citation required and conclusions have changed | There are now data available for over 21,000 women assessing the effects of vitamin E supplementation in combination with other supplements including vitamin C in pregnancy. The new data do not support routine vitamin E supplementation in combination with other agents for the prevention of stillbirth, neonatal death, preterm birth, pre‐eclampsia, prelabour rupture of membranes or poor fetal growth. Supplementation was associated with a reduced risk of placental abruption. There was some evidence of harm, as vitamin E supplementation appeared to increase the risk of term prelabour rupture of membranes and self‐reported abdominal pain. There were no clear differing patterns in the effects of vitamin E supplementation in subgroups of women based on the timing of commencement of supplementation or baseline risk of adverse pregnancy outcomes. |
| 8 April 2015 | New search has been performed | Search updated and 27 trials identified, of these, 21 were eligible for inclusion. Four trials did not report any clinical outcomes and therefore do not contribute data to the review (in the previous version of the review, three of these trials were excluded).The methods, results and discussion have been updated, new subgroup analyses were undertaken and a 'Summary of findings' table added. |
| 29 August 2011 | New search has been performed | Data on stillbirth and perinatal death added for 5 new eligible studies, in order to be used in the review "Interventions for preventing stillbirth during pregnancy: an overview of Cochrane systematic reviews". |
| 7 May 2010 | Amended | Search updated. Twenty‐three new reports added to Studies awaiting classification. |
| 7 July 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Philippa Middleton for her advice and comments regarding the format of the review.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategy
In the previous version of the review (Rumbold 2005), authors searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2004, Issue 2), MEDLINE (1966 to May 2004), Current Contents (1998 to May 2004) and EMBASE (1980 to May 2004) for potentially eligible studies:
CENTRAL (The Cochrane Library 2004, Issue 2)
pregnan*, vitamin*, tocopher*.
MEDLINE (1966 to May 2004), Current Contents (1998 to May 2004) and EMBASE (1980 to May 2004):
vitam*
tocopherol*
alpha‐tocopherol*
pregnan*
#4 and (#1 or #2 or #3)
random*
controlled‐clinical‐trial
#6 or #7
#5 and #8
Data and analyses
Comparison 1. Any vitamin E supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 9 | 19023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.88, 1.56] |
| 2 Neonatal death | 9 | 18617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.13] |
| 3 Perinatal death | 6 | 16923 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.77, 1.54] |
| 4 Infant death | 1 | 2694 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 74.12] |
| 5 Haemolytic anemia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Reticulocytosis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Hyperbilirubinemia | 1 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 8 Haemoglobin levels | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Maternal | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Infant | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Preterm birth (less than 37 weeks' gestation) | 11 | 20565 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 10 Clinical pre‐eclampsia (random‐effects model) | 14 | 20878 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 11 Intrauterine growth restriction (various definitions) | 11 | 20202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 11.1 Birthweight < 10th centile | 8 | 10161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| 11.2 IUGR definition unclear | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.97] |
| 11.3 Birthweight < 1 SD for gestational age | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.28, 8.12] |
| 11.4 Birthweight < 3rd centile | 1 | 9781 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.27] |
| 12 Birthweight | 10 | 16888 | Mean Difference (IV, Random, 95% CI) | 22.17 [‐23.01, 67.36] |
| 13 Prelabour rupture of fetal membranes | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Preterm | 5 | 1999 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.93, 1.75] |
| 13.2 Term | 2 | 2504 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.37, 2.28] |
| 14 Maternal death | 7 | 17120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.14, 2.51] |
| 15 Elective delivery and caesarean section | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Prelabour caesarean section | 2 | 1932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.85, 1.56] |
| 15.2 Any caesarean section | 6 | 15297 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.07] |
| 15.3 Induction of labour | 1 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.26] |
| 16 Bleeding episodes | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Placental abruption | 7 | 14922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.93] |
| 16.2 Antepartum haemorrhage | 2 | 12256 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.82] |
| 17 Measures of serious maternal morbidity | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Eclampsia | 8 | 19471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.82, 3.41] |
| 17.2 Renal failure or insufficiency | 2 | 1933 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.55, 4.02] |
| 17.3 Disseminated intravascular coagulation | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.41] |
| 17.4 Pulmonary oedema | 4 | 12569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.16, 1.03] |
| 18 Peripheral neuropathy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 Maternal | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Infant | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal satisfaction with care | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Gestational age at birth | 7 | 13783 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.12, 0.43] |
| 21 Congenital malformations | 4 | 5511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.63] |
| 22 Apgar score less than seven at five minutes | 3 | 3531 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.42, 1.27] |
| 23 Vitamin K deficiency bleeding or haemorrhagic disease of the newborn | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Respiratory distress syndrome | 8 | 18574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.08] |
| 25 Chronic lung disease or bronchopulmonary dysplasia | 3 | 3262 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.69] |
| 26 Periventricular haemorrhage and periventricular leukomalacia | 6 | 17787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.58, 1.42] |
| 27 Bacterial sepsis | 5 | 13324 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.73, 1.67] |
| 28 Necrotising enterocolitis | 7 | 18514 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.36, 1.55] |
| 29 Retinopathy of prematurity | 6 | 18270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.72, 1.93] |
| 30 Disability at childhood follow‐up | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Poor childhood growth | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32 Adverse events related to vitamin E supplementation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 33 Side effects of vitamin E supplementation | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 Acne | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.14, 75.68] |
| 33.2 Transient weakness | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.36 [0.27, 106.78] |
| 33.3 Skin rash | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.14, 75.68] |
| 33.4 Any side effects (symptoms not reported separately by group status) | 1 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.41] |
| 33.5 Abdominal pain | 1 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.16, 2.37] |
| 33.6 Elevated liver enzymes (defined by authors) | 3 | 14209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.71, 1.41] |
| 34 Use of health service resources ‐ maternal | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 34.1 Admission to intensive care unit | 2 | 3718 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.16, 2.30] |
| 34.2 Hospitalisations in pregnancy | 2 | 2407 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.30, 1.80] |
| 35 Use of health service resources ‐ infant | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 Admission to intensive care unit | 8 | 17594 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.08] |
| 35.2 Use of mechanical ventilation | 6 | 8531 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.84, 1.25] |
1.27. Analysis.
Comparison 1 Any vitamin E supplementation, Outcome 27 Bacterial sepsis.
Comparison 2. Any vitamin E supplementation (sensitivity analyses by trial quality).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 8 | 18777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.89, 1.58] |
| 1.1 Low overall risk of bias | 8 | 18777 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.89, 1.58] |
| 2 Neonatal death | 7 | 18314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.24] |
| 2.1 Low overall risk of bias | 7 | 18314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.57, 1.24] |
| 3 Perinatal death | 5 | 16677 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.82, 1.73] |
| 3.1 Low overall risk of bias | 5 | 16677 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.82, 1.73] |
| 4 Preterm birth (< 37 weeks' gestation) | 8 | 20205 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.91, 1.07] |
| 4.1 Low overall risk of bias | 8 | 20205 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.91, 1.07] |
| 5 Clinical pre‐eclampsia | 8 | 19698 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.10] |
| 5.1 Low overall risk of bias | 8 | 19698 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.10] |
| 6 Intrauterine growth restriction | 8 | 19842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 6.1 Low overall risk of bias | 8 | 19842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
Comparison 3. Any vitamin E supplementation (subgroup analyses based on gestation at entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 9 | 19023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.88, 1.56] |
| 1.1 Less than or equal to 20 week's gestation | 3 | 13084 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.73, 1.55] |
| 1.2 Greater than 20 weeks' gestation | 2 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.35, 1.70] |
| 1.3 Both prior to and after 20 weeks' gestation | 4 | 5637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.96, 2.70] |
| 2 Neonatal death | 9 | 18617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.13] |
| 2.1 Less than or equal to 20 weeks' gestation | 3 | 12977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.53, 1.39] |
| 2.2 Greater than 20 weeks' gestation | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.68] |
| 2.3 Both prior to and after 20 weeks' gestation | 3 | 5297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.28, 1.22] |
| 3 Perinatal death | 6 | 16923 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.77, 1.54] |
| 3.1 Less than or equal to 20 weeks' gestation | 2 | 12332 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.42, 6.87] |
| 3.2 Greater than 20 weeks' gestation | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.49, 1.82] |
| 3.3 Both prior to and after 20 weeks' gestation | 2 | 4289 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.60, 2.14] |
| 4 Preterm birth | 11 | 20565 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 4.1 Less than or equal to 20 weeks' gestation | 5 | 13465 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.20] |
| 4.2 Greater than 20 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Both prior to and after 20 weeks' gestation | 6 | 7100 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.10] |
| 5 Clinical pre‐eclampsia | 14 | 20878 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 5.1 Less than or equal to 20 weeks' gestation | 5 | 13299 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.16] |
| 5.2 Greater than 20 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Both prior to and after 20 weeks' gestation | 7 | 6886 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.12] |
| 5.4 Gestation at trial entry unknown | 2 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.13, 0.79] |
| 6 Intrauterine growth restriction | 11 | 20202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 6.1 Less than or equal to 20 week's gestation | 5 | 13285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.10] |
| 6.2 Greater than 20 weeks' gestation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Both prior to and after 20 weeks' gestation | 6 | 6917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.08] |
Comparison 4. Any vitamin E supplementation (subgroup analyses by dietary intake).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 9 | 19023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.88, 1.56] |
| 1.1 Low dietary intake | 1 | 1867 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.47, 3.84] |
| 1.2 Adequate dietary intake | 1 | 9855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.67, 1.66] |
| 1.3 Dietary intake unclear | 7 | 7301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.84] |
| 2 Neonatal death | 9 | 18617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.13] |
| 2.1 Low dietary intake | 1 | 1853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.24] |
| 2.2 Adequate dietary intake | 1 | 9781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.31] |
| 2.3 Dietary intake unclear | 7 | 6983 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.60, 1.37] |
| 3 Perinatal death | 6 | 16923 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.77, 1.54] |
| 3.1 "Low nutritional status" | 1 | 1515 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.17] |
| 3.2 Adequate dietary intake | 1 | 9969 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.48, 2.46] |
| 3.3 Dietary intake unclear | 4 | 5439 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.75, 2.11] |
| 4 Preterm birth (less than 37 weeks' gestation) | 11 | 20565 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 4.1 "Low nutritional status" | 1 | 1343 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.74, 1.03] |
| 4.2 Low dietary intake | 1 | 1877 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.73, 1.43] |
| 4.3 Adequate dietary intake | 1 | 9969 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.09] |
| 4.4 Dietary intake unclear | 8 | 7376 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.83, 1.18] |
| 5 Clinical pre‐eclampsia (random‐effects model) | 14 | 20796 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.03] |
| 5.1 "Low nutritional status" | 1 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 5.2 Low baseline vitamin E status (≤ 5 µmol/mmol cholesterol) | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.12, 1.02] |
| 5.3 Moderate/high baseline antioxidant status (> 5 µmol/mmol cholesterol) | 1 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.53, 1.12] |
| 5.4 Low dietary intake | 1 | 1877 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.82, 1.75] |
| 5.5 Adequate dietary intake | 1 | 9969 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.24] |
| 5.6 Dietary intake unclear | 10 | 6928 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.56, 0.98] |
| 6 Intrauterine growth restriction (less than third centile or the most extreme centile reported) | 11 | 20202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 6.1 "Low nutritional status" | 1 | 1165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.75, 1.12] |
| 6.2 Low dietary intake | 1 | 1853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.66, 1.16] |
| 6.3 Adequate dietary intake | 1 | 9781 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.27] |
| 6.4 Dietary intake unclear | 8 | 7403 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
Comparison 5. Any vitamin E supplementation (subgroup analyses by risk of adverse pregnancy outcomes at trial entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 9 | 19023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.88, 1.56] |
| 1.1 High/increased risk | 7 | 7301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.84] |
| 1.2 Low/moderate risk | 2 | 11722 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.72, 1.66] |
| 2 Neonatal death | 9 | 18617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.58, 1.13] |
| 2.1 High/increased risk | 7 | 6983 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.60, 1.37] |
| 2.2 Low/moderate risk | 2 | 11634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.39, 1.17] |
| 3 Perinatal death | 6 | 16923 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.77, 1.54] |
| 3.1 High/increased risk | 5 | 6954 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.73, 1.67] |
| 3.2 Low/moderate risk | 1 | 9969 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.48, 2.46] |
| 4 Preterm birth (less than 37 weeks' gestation) | 11 | 20565 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 4.1 High/increased risk | 8 | 8503 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.13] |
| 4.2 Low/moderate risk | 3 | 12062 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.23] |
| 5 Clinical pre‐eclampsia (random‐effects model) | 14 | 20878 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 5.1 High/increased risk | 9 | 8123 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.06] |
| 5.2 Low/moderate risk | 5 | 12755 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.56, 1.27] |
| 6 Intrauterine growth restriction (less than third centile or the most extreme centile reported) | 11 | 20202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 6.1 High/increased risk | 8 | 8352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.08] |
| 6.2 Low/moderate risk | 3 | 11850 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anthony 1996.
| Methods | A double‐blind, placebo‐controlled trial of vitamin E supplementation was conducted. All patients were managed in the same unit by the same team using standardised protocols over the last 3 years. | |
| Participants | First recruited 73 women undergoing conservative antenatal management of pre‐eclampsia. | |
| Interventions | Vitamin E supplementation versus placebo. | |
| Outcomes | 1. Duration of conservative management 2. Indications for delivery 3. Renal function, protein excretion and platelet levels |
|
| Notes | Recruitment was still open at the time of publishing the abstract, and final analysis was presented on the day of poster session. No data incorporated in the analysis of this review. Setting: South Africa. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind" stated in abstract, no further details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statistically significant differences between the groups but did not identify any details and the number of patients in each comparison groups. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was used but no data given in this abstract. |
| Other bias | Unclear risk | Only abstract available. |
Beazley 2005.
| Methods | Treatment allocation: unclear, women were "randomised". Blinding of outcome assessment: "double blind" stated. Documentation of exclusion: 9 (8%) women were lost to follow‐up. Use of placebo control: placebo control. |
|
| Participants | 109 women were recruited into the study. Inclusion criteria: women at "high risk of pre‐eclampsia" including those with previous pre‐eclampsia, chronic hypertension, pregestational diabetes and multiple pregnancy. Nil exclusion criteria stated. Women were randomised at 14‐20 weeks' gestation to receive either daily vitamin E and C (n = 54) or placebo (n = 55). | |
| Interventions | Women randomised to the treatment group received daily 400 IU vitamin E in addition to 1000 mg vitamin C. No details on the content of the placebo were given. | |
| Outcomes | 1. Pre‐eclampsia (not defined) 2. GA at delivery (weeks) 3. Preterm birth (< 37 weeks' gestation) 4. Birthweight 5. Birthweight < 10 centile 6. Total antioxidant status and 8‐isoprostane | |
| Notes | Dosage: daily 400 IU vitamin E, above RDI. GA at trial entry: <= 20 weeks' gestation. Dietary vitamin E intake before trial entry: unclear, no dietary information reported. Type of supplement: vitamin E given in addition to vitamin C. Women's risk status: women were at high risk of pre‐eclampsia. Intention‐to‐treat analyses: stated that analyses were intention‐to‐treat, however losses to follow‐up were not included in the totals. Available case analysis. Sample size calculation: none reported. Compliance: unclear, no details given. Location: United States of America. Timeframe: unclear. The trial was stopped early after cessation of funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blind, randomized clinical trial" stated, but no details about sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | As above, no details provided about method of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind" and "placebo" stated but no further details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 (8%) women were lost to follow‐up or withdrew, 6 (11%) in control group, and 3 (5%) in the vitamin group. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | No details provided about the baseline characteristics of each group. |
Borna 2005.
| Methods | A randomised controlled trial conducted from September 2002 to September 2003 at a teaching hospital in Tehran, Iran. Block randomisation method was used for allocation of participants. |
|
| Participants | 60 women with singleton pregnancy who had preterm premature rupture of membrane at 26 to 34 weeks' gestation were enrolled to the study. Exclusion criteria included chorioamnionitis, non‐reassuring fetal status on admission, obstetric indication for immediate delivery, delivery within 24 hour of admission, major congenital anomalies, and fetal growth restriction. |
|
| Interventions | Intervention group (n = 30) received tablets containing 500 mg of vitamin C and 400 IU of vitamin E daily. Control group (n = 30) received placebo tablets similar to the intervention group. All women received prophylactic antibiotics (ampicillin 2 g and erythromycin 250 mg div q6h for first 48 hours, followed by amoxicillin 250 mg and erythromycin base 333 mg po tid for 5 days). All women received 2 injections of 12 mg betamethasone during first 24 hours of admission. |
|
| Outcomes | Study protocol was not available and following outcomes were reported: 1. Latency (mean, SD) 2. Birthweight (mean, SD) 3. GA at delivery (mean, SD) 4. Amniotic fluid index <= 5 cm at admission (number, percentage) 5. Amniotic fluid index <= 5 cm after the beginning of labour (number, percentage) 6. Caesarean section due to fetal distress (number, percentage) 7. Chorioamnionitis (number, percentage) 8. Postpartum endometritis (number, percentage) 9. RDS (number, percentage) 10. NICU admission (number, percentage) 11. Neonatal sepsis (number, percentage) 12. Neonatal mortality (number, percentage) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation methods used, but no details of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details provided about any method of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Randomization was blinded to both patients and caregivers". Quote: "The control group received placebo tablets similar to those of vitamins C and E at the same frequency"; therefore, the review authors believe blinding of patients and caregivers was probably maintained. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No specific information provided about blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided about attrition. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Chappell 1999.
| Methods | Treatment allocation: a computer‐generated randomisation list using blocks of 10 was given to the hospital pharmacy departments. Researchers allocated the next available number to participants and women collected the trial tablets from the pharmacy department. Blinding of outcome assessment: women, caregivers and researchers were blinded to the treatment allocation until recruitment, data collection and laboratory analyses were complete. Documentation of exclusion: pregnancy outcome data were reported according to treatment allocation for all women randomised. Use of placebo control: placebo control. |
|
| Participants | 283 women were recruited into the study. Inclusion criteria: abnormal Doppler waveform in either uterine artery at 18‐22 weeks' gestation or a history in the preceding pregnancy of pre‐eclampsia necessitating delivery before 37 weeks' gestation, eclampsia or the syndrome of HELLP. Exclusion criteria: heparin or warfarin treatment, abnormal fetal‐anomaly scan or multiple pregnancy. Women were randomised at 18‐22 weeks' gestation; however, women with a previous history who were identified at an earlier stage were randomised at 16 weeks' gestation. Women with abnormal Doppler waveform analysis returned for a second scan at 24 weeks' gestation, those with a normal waveform at this time stopped treatment and were withdrawn from the study. The remaining women who had persistently abnormal waveforms and those with a previous history or pre‐eclampsia remained in the study and were seen every 4 weeks through the rest of pregnancy. 1512 women underwent Doppler screening, 273 women had abnormal waveforms and, of these, 242 women consented to the study. An additional 41 women who had a history of pre‐eclampsia consented. 283 women were randomised to either the vitamin C and E group (n = 141) or the placebo group (n = 142), 72 women had normal Doppler scans at 24 weeks' gestation and 24 women did not return for a second scan and were withdrawn. A further 27 women withdrew from the trial after 24 week's gestation for various reasons. In total, 160 women completed the trial protocol until delivery, 79 in the vitamin C and E group and 81 in the placebo group. Pregnancy outcome data were presented for all women randomised (n = 283) as well as only for those women completing the trial protocol (n = 160). | |
| Interventions | Women randomised to the vitamin E and C group received capsules containing 400 IU natural source vitamin E daily and tablets containing 1000 mg vitamin C daily. Women randomised to the placebo group received capsules containing soya bean oil and tablets containing microcrystalline cellulose that were identical in appearance to the vitamin E capsules and vitamin C tablets. After 24 weeks' gestation women were seen every 4 weeks, and blood samples were taken at each visit. | |
| Outcomes | 1. Ratio of PAI‐1 to PAI‐2 2. Pre‐eclampsia (defined according to the International Society for the Study of Hypertension in Pregnancy guidelines) 3. Placental abruption 4. Spontaneous preterm delivery (< 37 weeks' gestation) 5. Intrauterine death 6. SGA infants (on or below the 10th centile) 7. Mean systolic and diastolic blood pressure before delivery 8. GA at delivery (median, IQR) 9. Birthweight (median, IQR) 10. Birthweight centile (median, IQR) 11. Mean plasma ascorbic acid and alpha‐tocopherol concentrations during gestation 12. Biochemical indices of oxidative stress and placental function | |
| Notes | Dosage: 400 IU natural source vitamin E daily, above RDI. GA at trial entry: between 16 to 22 weeks' gestation. Dietary vitamin E intake before trial entry: unknown, not assessed. Type of supplement: vitamin E given in addition to vitamin C. Women's risk status: women were at "high risk for pre‐eclampsia". Intention‐to‐treat analyses: performed, pregnancy outcome data were available for all women randomised, and results were presented according to initial treatment allocation. Sample size calculation: the study had 80% power to detect a 30% reduction in PAI‐1. Compliance: not specifically reported. "Within the treated group, plasma ascorbic acid concentration increased by 32% from baseline values and plasma alpha‐tocopherol increased by 54%." Location: London, United Kingdom. Timeframe: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomisation list was drawn up by the statistician, with randomisation in blocks of ten". |
| Allocation concealment (selection bias) | Low risk | Researchers allocated the next available number to participants and women collected the trial tablets directly from the pharmacy department. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, caregivers and researchers were blinded to the treatment allocation until recruitment, data collection and laboratory analyses were complete. Placebo control used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, caregivers and researchers were blinded to the treatment allocation until recruitment, data collection and laboratory analyses were complete. Placebo control used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Gulmezoglu 1997.
| Methods | Treatment allocation: "The treatment packs were randomised by computer generated random numbers in blocks of ten". Randomisation was carried out by an independent researcher who was not involved in the study, and medications were placed in consecutively numbered sealed opaque bags. Blinding of outcome assessment: women, caregivers and researchers were blinded to the treatment allocation. Documentation of exclusion: no exclusions documented. Use of placebo control: placebo control. |
|
| Participants | 56 women were recruited into the study. Inclusion criteria: women who were admitted to the antenatal wards with a diagnosis of severe pre‐eclampsia, as defined by 2+ proteinuria on urine dipstix testing (in at least 2 consecutive tests 4 to 6 hours apart), with a blood pressure of 160/110 mmHg, or 3+ proteinuria with blood pressure >= 150/100 mmHg; between 24 and 32 weeks' gestation; with a single live fetus; with no systemic disorder (such as diabetes or systemic lupus erythematosus) and no allergy to study medications. Women were approached when they were eligible for conservative management, as defined by an absence of significant renal impairment, the HELLP syndrome or thrombocytopenia alone. Conservative management consisted of advising women to stay in hospital until delivery, with weekly betamethasone injections up to 32‐34 weeks' gestation, and with frequent fetal and maternal monitoring. Exclusion criteria: none stated. 59 women were approached and counselled about the study, of which 56 women gave informed written consent, and allocated to either the vitamin group (n = 27) or placebo (n = 29). | |
| Interventions | Women randomised to the vitamin group received twice daily 400 IU vitamin E (800 IU daily total), 500 mg vitamin C (1000 mg daily total) and 100 mg allopurinol (200 mg daily total). Women randomised to the placebo group received the same number of tablets that were identical to the vitamin C and allopurinol tablets. Vitamin C placebos were used as placebos for vitamin E because it was not possible to obtain 2 separate sets of placebos from the supplier; however, the vitamin E tablets and their placebos were slightly different. To preserve blinding all medications were placed in dark brown coloured bottles and sealed opaque paper bags. | |
| Outcomes | 1. Delivery within 14 days 2. Maternal deaths 3. Serious maternal complications (pulmonary oedema, eclampsia, HELLP syndrome, DIC, renal failure) 4. Placental abruption 5. Prelabour caesarean section 6. Use of antihypertensives 7. Stillbirth 8. Apgar score < 7 at 1 minute and < 7 at 5 minutes 9. Umbilical artery pH < 7.2 10. Admission to intensive care unit 11. Mechanical ventilation 12. Neonatal death 13. Perinatal death 14. Birthweight (median, range) 15. Lipid peroxide and vitamin E levels 16. Haematological and renal function parameters 17. Placental lipid peroxide and glutathione levels | |
| Notes | Dosage: 800 IU vitamin E, above RDI. GA at trial entry: > 20 weeks' gestation. Dietary vitamin E intake before trial entry: unclear, no dietary information reported. Type of supplement: vitamin E given in addition to vitamin C and allopurinol. Women's risk status: women had established early onset severe pre‐eclampsia. Intention‐to‐treat analyses: all data were reported according to women's treatment allocation, and were available for all women for the primary outcome. There were missing data for the outcomes Apgar score < 7 at 1 minute, Apgar score < 7 at 5 minutes, umbilical artery pH < 7.2, and the lipid peroxide and vitamin E levels and haematological and renal function parameters. Sample size calculation: a sample size of 54 women had 80% power to detect a halving in the number of women needing delivery within 14 days, from 80% to 40%. Compliance: compliance in the vitamin group was estimated at 84%, 89%, 93% for the vitamin E, vitamin C and allopurinol tablets. For the placebo group compliance was 75%, 100% and 86% for the vitamin E, vitamin C and allopurinol placebo tablets. Location: Johannesburg, South Africa. Timeframe: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment packs were randomised by computer generated random numbers in blocks of ten". |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was done by an independent researcher who was not involved in the study" and "medications (active or placebo) were placed in consecutively numbered sealed opaque paper bags". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, caregivers and researchers were blinded to the treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, caregivers and researchers were blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data were reported according to women's treatment allocation, and were available for all women for the primary outcome. Some missing data for secondary outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Gungorduk 2014.
| Methods | A randomised controlled trial conducted between January 2011 and November 2011 at a teaching hospital in Istanbul, Turkey. Prior to the recruitment, code was generated with computer and stored in a sealed, consecutively numbered opaque envelope. Stratification of randomisation by GA (24.0‐25.9, 26.0‐27.9, 28.0‐29.9, 30.0‐31.9, and 32.0‐33.9 weeks) was carried out. Both participants and caregivers were not blinded. |
|
| Participants | 246 women with singleton pregnancy who had non‐anomalous fetus and preterm premature rupture of membranes at 24 to 34 weeks' gestation were enrolled to the study. All patients were admitted to the hospital. Exclusion criteria included fetus anomalies, chorioamnionitis, within 14 days of amniocentesis or cervical cerclage placement, multiple gestation, obstetric indication for immediate delivery, intrauterine fetal death at the time of presentation. Patients who had active preterm labour were also excluded. |
|
| Interventions | Intervention group received 1000 mg of vitamin C (ascorbic acid) and 400 IU of vitamin E (RRR α‐tocopherol acetate). Control group received placebo. Intervention was initiated within 1 hour after the diagnosis of rupture of membrane. Amoxicillin 2 g/d for 7 days was administered as prophylaxis. In case of allergy to amoxicillin, erythromycin 1 g/d for 7 days was used. 2 doses of 12 mg IM betamethasone injection were administered at interval of 24 hours. |
|
| Outcomes | Study protocol was not available and following outcomes were reported. Primary outcome Latency period until delivery Secondary outcomes 1. Birthweight 2. Mode of delivery 3. Occurrence of clinical chorioamnionitis 4. Postpartum endometritis 5. Early onset neonatal sepsis 6. Grade 3 to 4 IVH 7. Stage 2 to 3 NEC 8. Admission to the NICU 9. RDS 10. Birth within 48 hours of randomisation 11. Birth within 7 days of randomisation 12. Composite perinatal morbidity/mortality (defined as the occurrence of RDS, grade III or IV IVH, NEC, neonatal sepsis, or perinatal death |
|
| Notes | Sample size was calculated based on hospital experience; 6 days latency period with standard deviation of 3 days. 112 participants needed to be recruited to detect 50% increase in latency period (type I error 5% and power 80%). Intention‐to‐treat analysis was carried out. Figure in paper states that 229 women were randomised, however, the sum of the 2 groups analysed is 246. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated code prepared prior to recruitment". |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed, consecutively numbered, opaque envelope" used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "identical supplements could not be used for the control and experimental groups; therefore the patients and researchers were not blinded to the conditions". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors was not stated but is unlikely to have occurred (see above quote). The primary outcome was latency to delivery, which could be influenced by biased clinical decisions about timing of delivery based on knowledge of treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (1%) patients were lost to follow‐up, and the number (and reason) for losses to follow‐up was balanced between the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and all outcomes reported. |
| Other bias | Low risk | Baseline characteristics similar between groups. |
Huria 2010.
| Methods | A randomised controlled trial conducted during June 2006 to August 2007 at a medical college hospital in Chandigarh, India. | |
| Participants | 285 women attending antenatal clinic were enrolled to the study. Inclusion criteria: women who gave consent to participate in the study, primigravida, singleton pregnancy, willing to deliver at the study hospital. Exclusion criteria: women with blood pressure higher than 130/85 mmHg, using antihypertensive medication, proteinuria, intention to deliver at other hospital, known complication such as diabetes and hypothyroidism, known fetal malformations, using more than 150 mg of vitamin C or more than 75 IU of vitamin E, using NSAID. 145 were allocated to the intervention group and 140 to the control group. |
|
| Interventions | Intervention group received 1000 mg vitamin C and 200 mg vitamin E. Control group received placebo. All participants received 100 doses of iron and folic acid from 12 weeks of pregnancy. |
|
| Outcomes | A study protocol was not available and following outcomes were reported:. 1. Gestational hypertension or pre‐eclampsia 2. IUGR 3. Preterm birth 4. Caesarean section Preterm birth defined as "birthweight less than 2.5kg" in text, but in one table is reported according to gestation at delivery. |
|
| Notes | Inconsistent description of vitamin E dose; both “mg” and “IU” were used. Although the report claimed that “women identified at the risk of preeclampsia” were studied, no such description could be found in the method section. There are discrepancies between the reported numbers lost to follow‐up and the final numbers included in the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random allocation of study subjects" stated, but no details on sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided about any method of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Double blinding was ensured by random allocation of study subjects and coding of drugs". No further details were provided to permit an assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double blinding was ensured by random allocation of study subjects and coding of drugs". No further details were provided to permit an assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data for 72 (25%) participants missing, 34 (23%) in vitamin group and 38 (27%) in control group. Of the 34 women in the vitamin group, 18 were "lost to follow up" and 16 delivered elsewhere. Of the 38 women in the control group, 16 were "lost to follow up" and 22 delivered elsewhere. The reasons for delivery elsewhere were not provided. Although the number of women lost to follow‐up in each group was similar, given the magnitude of the effect size for some outcomes (e.g. preterm birth), the review authors suspect that there may be clinically relevant bias. For example, the risk of preterm birth was 14.7% in control and 4.7% in the vitamin group, which could reflect women in the vitamin group who are in preterm labour being transferred to another hospital for delivery. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. |
| Other bias | Unclear risk | Information about baseline characteristics was not available. |
Kalpdev 2011.
| Methods | A randomised controlled trial conducted between June 2005 to June 2007 in Chandigarh, India. Computer‐generated number was used for randomisation. |
|
| Participants | 50 women between 13 and 19 weeks of gestation were enrolled to the study. 25 were allocated to intervention group and 25 to control group. Inclusion criteria: women with essential hypertension who booked in the Hypertensive Disorders of Pregnancy Clinic, singleton pregnancy at GA between 16 to 22 weeks, giving consent for participation. Exclusion criteria: multifetal pregnancy, known fetal abnormalities, use of illicit drug or alcohol during pregnancy, intention to deliver outside of study site, renal hypertension, proteinuria, already taking vitamin C and E. |
|
| Interventions | Intervention group received 500 mg vitamin C twice daily (1000 mg/day) and 400 IU natural vitamin E once daily. Control group did not receive vitamins (not placebo‐controlled trial). All women received iron, folic acid and calcium. |
|
| Outcomes | Study protocol was not available but following outcomes were evaluated. Primary outcome Development of superimposed pre‐eclampsia Secondary outcomes Maternal 1. Aggravation of hypertension 2. Need for admission 3. Need to increase antihypertensive drugs 4. Incidence of HELLP 5. Low platelet count 6. Liver enzyme equal or greater than 5 times in absence of obstetric cholestasis Fetal/neonatal 1. Incidence of growth retardation 2. Gestation at delivery 3. Birthweight 4. Stillbirth 5. Apgar score at birth |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women were randomized using computer‐generated numbers". |
| Allocation concealment (selection bias) | Unclear risk | No details provided about method of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo control and no details of blinding provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details of blinding provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 (12%) participants were not included in analyses, 3 in each treatment group, but no details about the reasons for missing data for these women. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. |
| Other bias | Low risk | No reported differences between groups at baseline. |
Mahdy 2004.
| Methods | A randomised placebo‐controlled trial. | |
| Participants | Interim analysis was performed on 113 primigravida women, 46 were allocated to intervention group, 67 were in control group. | |
| Interventions | Intervention group: tocotrienol‐rich fraction (TRF) of palm oil. Control group: placebo. |
|
| Outcomes |
Primary outcomes
1. Pregnancy‐induced hypertension 2. Pre‐eclampsia |
|
| Notes | Result of interim analysis. Poster session abstract only. Setting: Malaysia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, double blind placebo‐controlled clinical trial"; no further details given (only abstract available). |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind" stated in abstract, no further details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind" stated in abstract, no further details given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to assess attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess reporting bias. |
| Other bias | Unclear risk | Insufficient information to assess other bias. |
McCance 2010.
| Methods | A randomised placebo‐controlled trial conducted in 25 UK antenatal metabolic clinics. Enrolment was carried out between April 2003 and June 2008. A randomisation sequence was generated by Victoria Pharmaceuticals using computer software (PRISYM ID, version 1.0009). The sequence was stratified by clinic with balanced block size of 8. Both participants and study personnel were blinded to the allocation status until the completion of the trial. |
|
| Participants | Inclusion criteria: pre‐existing type 1 diabetes before pregnancy, GA between 8 and 22 weeks at presentation, singleton pregnancy, age 16 years or older. Exclusion criteria: those who did not give consent, already enrolled in other study, warfarin treatment, known history of drug misuse, taking vitamin supplements containing daily dose of more than 500 mg vitamin C or more than 200 IU vitamin E. 1621 women were assessed for eligibility, 859 were excluded, and 762 were randomised into 2 groups; 379 in intervention group and 383 in control group. 1 participant in control group was lost to follow‐up with consent withdrawal. |
|
| Interventions | Intervention group received 1000 mg vitamin C and 400 IU vitamin E daily from recruitment until delivery. Control group received matched placebo daily. |
|
| Outcomes |
Primary outcome Pre‐eclampsia Secondary outcomes 1. Placental and endothelial activation 2. Birthweight centile Postnatal follow‐up ‐ weight, length and head circumference are reported as SD scores, assessed between 6 to 12 weeks of age, however the data were not reported in a suitable format to be included in the meta‐analysis. Results include the following statement: "We noted no adverse events or side effects attributable to supplementation with vitamin C or E in mothers or infants." |
|
| Notes | ISRCTN27214045 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation sequence generated in advance by Victoria. Pharmaceuticals using PRISYM ID software". |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation sequence was stratified by centre with balanced blocks of eight patients, and was held by Victoria Pharmaceuticals. Individual sealed envelopes containing treatment allocations were given to trial pharmacists in every centre, allowing treatment group to be revealed in a clinical emergency". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treatment allocation was masked from all trial personnel and participants until trial completion", and identical looking placebo tablets were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data missing for 1 participant (placebo group) who withdrew consent. Quote: "749 women were assessed for pre‐eclampsia, by original assigned group (375 vitamin, 374 placebo). There were 12 deviations from the inclusion and exclusion criteria‐‐eight women were enrolled outside the 22‐week cutoff for gestation (all were within 4 days of this threshold) and 4 patients were later reclassified as having type 2 diabetes. All 12 women were included in the analysis". |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes in the study protocol were reported. |
| Other bias | Low risk | Quote: "Although most maternal baseline characteristics did not differ between groups, history of pre‐eclampsia, hypertension, antihypertensive treatment, and microalbuminuria were more common in the placebo group than in the vitamin group". Although these factors were not adjusted for in the analyses, the possible impact of this would be to overestimate the treatment effect, however, no differences were found between the treatment groups, therefore the risk of likely to be low. |
Nasrolahi 2006.
| Methods | Randomised controlled trial conducted from March 2003 to March 2004 in Hamadan, Iran. Participants were divided into 2 groups based on the first day of visit to the prenatal care. Women visited on even numbered days were put into the treatment group, whereas those visited on odd days were put into the control group. |
|
| Participants | 580 women were enrolled, 290 in intervention group and 290 in control group. Inclusion criteria Primiparous women Singleton pregnancy Exclusion criteria History of underlying hypertension Obese with BMI greater than 35 Smoker Multifetal pregnancy Molar pregnancy History of previous abortion |
|
| Interventions | Intervention group received daily dose of 400 unit vitamin E and 1 g vitamin C until the end of pregnancies. Control group received ferrous sulphate during pregnancy. |
|
| Outcomes | 1. Pre‐eclampsia 2. Gestational age at birth 3. Birthweight |
|
| Notes | Translated from Persian to English by Ross C Poletti in December 2014. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: participants were "divided into two groups based on the first day of prenatal admission". |
| Allocation concealment (selection bias) | High risk | Quote: participants were "divided into two groups based on the first day of prenatal admission". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo control, and no further details of blinding, therefore, the review authors believe blinding is unlikely to have occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data presented for all participants. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Treatment groups were similar for maternal age at baseline, no other characteristics were presented. |
Poston 2006.
| Methods | A randomised controlled trial (the Vitamins in Pre‐eclampsia [VIP] trial) was conduced between Aug 6, 2003, and June 27, 2005 to which we enrolled women with clinical risk factors for pre‐eclampsia from 25 UK hospitals in 10 geographical areas. The last baby was delivered on Dec 3, 2005. Eligible women could be referred to trial centres from any location in the UK. 13 women were recruited in Amsterdam, Holland. | |
| Participants | 2410 women in GA 14–21 weeks plus 1 or more of the following risk factors: pre‐eclampsia in the pregnancy preceding the index pregnancy, requiring delivery before 37 completed weeks’ gestation at increased risk of pre‐eclampsia from 25 UK hospitals in 10 geographical areas. Text states that 2404 women were "validly randomised". |
|
| Interventions | 1 tablet and 1 capsule daily of vitamin C and vitamin E and placebo from the second trimester of pregnancy until delivery. Custom Pharmaceuticals manufactured the vitamin C (1000 mg) and identical placebo tablets (microcrystalline cellulose with addition of tartaric and citric acid to provide similar acidic taste), and Banner Pharmacaps provided identical gelatin capsules containing 400 IU natural source vitamin E (RRR α tocopherol) or placebo (sunflower seed oil). DHP Investigational Medicinal Products Clinical Trial Supplies (Crickhowell, Powys, Wales, UK) packaged the tablets and capsules sealed in blister strips each with 1 week’s supply, according to the randomisation sequence provided. Each pack contained a 7‐month supply of trial medication. The midwives told women to take 1 tablet and 1 capsule daily, and asked participants to leave unused tablets or capsules in the blister strip. Postage prepaid envelopes were provided for return of blister strips to the research midwife at intervals of 4 weeks. If not received, the computer program generated a prompt to remind the women (by telephone) to return that month’s packs. Participants were also given a postage pre‐paid postcard to notify the research midwife of delivery. |
|
| Outcomes |
Primary outcome Pre‐eclampsia Secondary outcomes Severe pre‐eclampsia, gestational hypertension, severe gestational hypertension, antenatal onset of pre‐eclampsia, postpartum onset of pre‐eclampsia, delivery for pre‐eclampsia before 37 weeks’ gestation, delivery for pre‐eclampsia before 34 weeks’ gestation, HELLP syndrome, eclampsia, severe proteinuria (> 5 g in 24 hours), magnesium sulphate for pre‐eclampsia, intravenous anti‐hypertensive therapy, antenatal steroids, maternal death, antenatal inpatient nights (mean, SD), LBW (< 2·5 kg) and small size for GA (< 5th centile). The study also reported on preterm and term PROM (no differences observed between groups), however no raw data were provided so the study did not contribute to the meta‐analysis for those outcomes. The study also reported outcomes related to respiratory function in children at 2 years of age. |
|
| Notes | Additional analyses were carried out that were not in the predefined analysis plan with selecting patients with similar rates of pre‐eclampsia.This trial was registered an International Standard Randomised Controlled Trial, number ISRCTN 62368611. Location: UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was blocked..by centre in groups of two to ten individuals... The trial statistician (PTS) wrote the computer program that generated the sequence and a statistician not involved with the trial ran it with a new random number sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was undertaken online and the participant allocated a locally stored pack of trial medication identified by a centre specific and participant‐specific number". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All women and trial staff blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All women and trial staff blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 (0.4%) participants were lost to follow‐up and the proportion of missing data was similar between groups. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. Study protocol was available (ISRCTN 62368611). |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Pressman 2003.
| Methods | In a randomised, double‐blind study conducted in 20 women scheduled to undergo planned caesarean delivery at term who met specified inclusion and exclusion criteria randomly received a daily prenatal vitamin with or without 400 IU of vitamin E and 500 mg of vitamin C, starting at 35 weeks' gestation. | |
| Participants | 20 women included planned caesarean section at term (38 weeks' or later) at Strong Memorial Hospital, USA. Exclusion criteria included maternal complications requiring delivery before scheduled caesarean delivery, onset of labour before caesarean section, rupture of membranes before caesarean section, known fetal anomalies, known maternal collagen vascular disease, maternal diabetes mellitus, lactose intolerance, and maternal age younger than 18 years. | |
| Interventions | Supplement group of 10 women received a standard prenatal vitamin (containing 120 mg of vitamin C and 30 IU of vitamin E) plus (400 IU of vitamin E and 500 mg of vitamin C). Control group of 10 women received a standard daily prenatal vitamin (containing 120 mg of vitamin C and 30 IU of vitamin E). Commercially obtained 200‐IU vitamin E capsules were enclosed in opaque gelatin capsules by the research pharmacy. The placebos of vitamin E consisted of identical opaque capsules enclosing lactose‐containing capsules of identical weight. Powdered vitamin C (250 mg) capsules and matched lactose capsules were also prepared by the research pharmacy. All patients were instructed to take 1 vitamin E/placebo capsule and 1 vitamin C/placebo capsule twice a day in addition to their regular prenatal vitamin. Compliance was assessed by pill counting at delivery. |
|
| Outcomes |
Concentrations of: 1. Maternal plasma vitamin E 2. Maternal RBC vitamin E 3. Maternal plasma vitamin C 4. Cord plasma vitamin E 5. Cord RBC vitamin E 6. Cord plasma vitamin C 7. Chorioamnion vitamin E 8. Amniotic fluid vitamin C |
|
| Notes | This trial did not report any clinical outcomes of interest to the review, and therefore does not contribute any data to the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation was performed in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by the research pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to the supplementation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded to the supplementation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 women from the control group did not complete the protocol because of preterm labour and delivery and samples from her delivery were not available for analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. |
| Other bias | Low risk | The baseline is comparable between groups. |
Rivas 2000.
| Methods | Treatment allocation: unclear, women were "randomly divided into two sub‐groups". Blinding of outcome assessment: "triple blind" stated. Documentation of exclusion: none stated. Use of placebo control: placebo control. |
|
| Participants | 127 women were recruited into the study. Inclusion criteria: women less than 29 weeks' gestation and with "high risk for pre‐eclampsia", including any of the following factors: nulliparity, previous pre‐eclampsia, obesity, hypertension, less than 20 years old, diabetes, nephropathy, mean arterial pressure above of 85 mmHg, positive roll‐over test, black race, family history of hypertension or pre‐eclampsia, twin pregnancy and poor socioeconomic conditions. Exclusion criteria: unclear, none stated. 127 women were allocated to vitamins C and E, aspirin and fish oil (n = 63) or placebo (n = 64). | |
| Interventions | Women allocated to the treatment group received 400 IU vitamin E per day, 500 mg vitamin C per day, 100 mg aspirin 3 times a week and 1 g fish oil 3 times a day. Women allocated to the placebo group, received placebo "at the same posology and presentation". | |
| Outcomes | 1. Pre‐eclampsia (not defined) 2. The authors report that "no serious maternal and neonatal side effects of treatment occurred in either group", no other details were given | |
| Notes | Dosage: daily 400 IU vitamin E, above RDI. GA at trial entry: unclear, "less than 29 weeks". Dietary vitamin E intake before trial entry: unclear, no dietary information reported. Type of supplement: vitamin E in addition to vitamin C, aspirin and fish oil. Women's risk status: women were at "high risk for pre‐eclampsia". Intention‐to‐treat analyses: unclear, no details given. Sample size calculation: unclear, reported as an abstract only. Compliance: no details given. Location: Merida, Venezuela. Timeframe: unclear, no details given. Published in abstract format only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, multicentric, randomised, triple‐blind, placebo‐controlled clinical trial" stated, but no other details provided (only abstract available). |
| Allocation concealment (selection bias) | Unclear risk | Quote: "prospective, multicentric, randomised, triple‐blind, placebo‐controlled clinical trial" stated, but no other details provided (only abstract available), no details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Abstract states that the study was "triple blind" and used a placebo control, but no further details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Abstract states that the study was "triple blind" and used a placebo control, but no further details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to assess attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Insufficient information to assess the potential for other sources of bias. |
Roberts 2010.
| Methods | Randomised, double‐blind trial was conducted from July 2003 through February 2008 at the 16 clinical centres and the independent data co‐ordinating centre of the MFMU Network (USA). | |
| Participants | 10,154 pregnant women at 6 clinical centres who had a singleton fetus with a GA of less than 16 weeks 0 days at the time of screening, or who had not had a previous pregnancy that lasted beyond 19 weeks 6 days. Their GA at randomisation was between 9 weeks 0 days and 16 weeks 6 days. Eligible women who were no more than 15 weeks pregnant and who consented to participate in the study were given a supply of placebo and asked to return within 2 weeks. | |
| Interventions | Women were to take daily supplementation with 1000 mg of vitamin C and 400 IU of vitamin E between 9th and 16th weeks of pregnancy or matching placebo (mineral oil). Women were instructed to take the study drug each day until they delivered their babies. The study participants returned on a monthly basis to return any unused study drug from the previous month, receive a new supply of the study drug for the coming month, report on side effects, and have their blood pressure and urine protein level (as assessed on dipstick testing) measured. |
|
| Outcomes |
Primary outcomes 1. Severe pregnancy‐associated hypertension alone 2. Severe or mild hypertension with: elevated liver‐enzyme levels, thrombocytopenia, elevated serum creatinine levels, eclamptic seizure, indicated preterm birth, fetal‐growth restriction, or perinatal death Secondary outcomes were other maternal and neonatal outcomes. 1. Mild pre‐eclampsia 2. Severe pre‐eclampsia 3. Proteinuria 4. Pulmonary edema 5. Thrombocytopenia 6. HELLP syndrome 7. Preterm birth 8. Fetal or neonatal death 9. SGA 10. Birth weight < 2500 g 11. Admission to NICU 12. RDS |
|
| Notes | ClinicalTrials.gov number, NCT00135707. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The simple urn method, with stratification according to clinical centre, was used by the data coordinating centre to create a randomization sequence". |
| Allocation concealment (selection bias) | Low risk | Boxes containing medications were packaged according to the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the participants nor the investigators were aware of the treatment assignments" and matching placebo tables used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "identified medical charts of all women with pregnancy‐associated hypertension were reviewed centrally by at least 3 reviewers who were unaware of the treatment assignments" and all data was "managed by an independent data coordinating centre". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 183 (1.8%) participants were lost to follow‐up, 94 in vitamin group and 89 in control group. A further 2 patients (one in each group) had information removed either at their request or by an institutional review board. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported. (ClinicalTrials.gov number, NCT00135707). |
| Other bias | Low risk | Baseline characteristics were similar between the 2 groups. |
Rumbold 2006.
| Methods | A multicentre, randomised trial was conducted involving nulliparous women with a singleton pregnancy between 14 and 22 weeks of gestation. The protocol approved by the research and ethics committees at the 9 collaborating hospitals and all women provided written informed consent. | |
| Participants | 1877 nulliparous women with a singleton pregnancy between 14 and 22 weeks of gestation. Eligible women had normal blood pressure at the first measurement in pregnancy and again at trial entry. Women with any of the following were ineligible: known multiple pregnancy, known potentially lethal fetal anomaly, known thrombophilia, chronic renal failure, antihypertensive therapy, or specific contraindications to vitamin C or E therapy such as haemochromatosis or anticoagulant therapy. Women were advised not to take any other supplements although a multivitamin that provided a daily intake of no more than 200 mg vitamin C or 50 IU vitamin was permitted. |
|
| Interventions | Women assigned to the vitamin group were advised to take 4 coated tablets of a combination of 250 mg of vitamin C (as ascorbic acid) and 100 IU of vitamin E (as d‐alpha‐tocopherol succinate) each day from trial entry until they gave birth. The total daily dose of vitamin C was 1000 mg, and that of vitamin E, 400 IU. Women assigned to placebo were advised to take 4 tablets daily containing microcrystalline cellulose, which were similarly coated and identical in appearance to the vitamin tablets. Women were asked to swallow the tablets whole without crushing or chewing them and were advised to take 2 tablets in the morning and 2 tablets in the evening. They were advised not to take any other antioxidant supplements, although a multivitamin preparation that provided a daily intake of no more than 200 mg of vitamin C or 50 IU of vitamin E was permitted. All infants in the study were recommended to receive IM vitamin K after birth. |
|
| Outcomes |
Primary outcomes 1. Pre‐eclampsia 2. A composite measure of death or serious outcomes in the infant 3. Small for gestational age Secondary outcomes Infants Serious complications occurring before hospital discharge Women A composite of any of the following until 6 weeks postpartum: death, pulmonary oedema, eclampsia, stroke, thrombocytopenia, renal insufficiency, RDS, cardiac arrest, respiratory arrest, placental abruption, abnormal liver function, preterm PROM , major postpartum haemorrhage, postpartum pyrexia, pneumonia, deep‐vein thrombosis, or pulmonary embolus requiring anticoagulant therapy Other secondary outcomes Antenatal, intrapartum, and postnatal end points, the need for antenatal hospitalisation, antenatal care during the day for hypertension, need for induction of labour for hypertension, use of antihypertensive agents, and use of magnesium sulphate |
|
| Notes | The Australian Collaborative Trial of Supplements (ACTS). Location: Australia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule used balanced variable blocks, with stratification by collaborating centre and GA. The schedule was prepared by an investigator not involved in group allocation. |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment packs contained four sealed, opaque, white plastic bottles of either the antioxidants vitamin C and vitamin E or the placebo and were prepared by a researcher not involved in recruitment or clinical care". Randomisation was undertaken using a central telephone service. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, caregivers and researchers were blinded to group allocation until after completion of the study and matching placebo tables were used in the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above, the treatment allocations were revealed after the analyses were completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Sawhney 2000.
| Methods | 60 women with pre‐eclampsia were included in the study and were randomly allocated to treatment (study) and no treatment (control) group. | |
| Participants | 60 pregnant women with pre‐eclampsia participated in the study on the effect of vitamin E supplementation on lipid peroxide levels in pre‐eclampsia. | |
| Interventions | Vitamin E supplementation versus no treatment. | |
| Outcomes | Lipid peroxide levels were measured by MDA‐TBAR assay and alpha‐tocopherol levels were measured by HPLC. | |
| Notes | Only conference abstract available. No data incorporated in the analysis of this review. Setting: India. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants were randomly allocated to treatment (study) and no treatment (control) group. No detail was provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of pregnant women in each comparison group was not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Unclear risk | Insufficient information to assess the potential for other sources of bias. |
Shahraki 2006.
| Methods | Quasi‐randomised trial. Sampling was randomised non presumptive and minimum volume of sample according to minimum volume of sample needed for biological studies (cross‐sectional interventional study). A 3‐arm comparison and have no control group. |
|
| Participants | 120 multiparous pregnant women age between 25 to 35 years old being in 25th‐28th weeks of pregnancy suffering from leg cramps with normal pregnancy pattern, absence of twin pregnancy, over weight gain and homodynamic background disease, unused medications causing cramp, patients satisfaction conditions of exit from the study were including at the gynaecologic clinic in Shahrekord between September 2004 and July 2005. | |
| Interventions | A form containing demographic information. age of pregnancy, number severity and duration of muscular cramps were registered for each women and then a written prescription was given to each of them in order to provide and take the prescription. First group was given 100 mg vitamin E oral pill a day. Second group was given 8 cc milk of magnesium suspension 8%, oral 3 times a day before meal. Third group was given a 500 mg calcium carbonate oral pill a day. The duration of intervention was 45 days and after 45 days, the women were visited again and information about the number, severity and duration of cramps was registered in the form. After 90 days from beginning the treatment, women were visited again and some information about cramps was gathered and registered. |
|
| Outcomes | 1. Number of cramps during each 24 hours 2. Cramp pain duration 3. Cramp severity on a scale of 1‐10 (1‐5 as low pain rate, 6‐10 as high pain rate) |
|
| Notes | Location: Iran. (Paper was presented with inadequate English proficiency.) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised trial with unclear details; however, women appeared to be randomised based on the day or time of presentation to the clinic. |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised, details are unclear, however women appeared to be randomised based on the day or time of presentation to the clinic. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided about outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total of 120 women with 40 in each arm. First group 4 (3.3%), second group 7 (5.8%), and third group 7 (5.8%) lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. |
| Other bias | Unclear risk | No details provided about baseline characteristics of treatment groups. |
Spinnato 2007.
| Methods | A placebo‐controlled, double‐blind trial was conducted within the National Institute of Child Health and Human Development (NICHD) Global Network for Women’s and Children’s Health Research. | |
| Participants | Women seeking prenatal care who were 12(0/7) to 19(6/7) weeks pregnant and diagnosed with non‐proteinuric chronic hypertension or a prior history of pre‐eclampsia in their most recent pregnancy that progressed beyond 20 weeks’ gestation from 4 clinical centre (serves a primarily urban low‐income population) at different sites. | |
| Interventions | 739 women were assigned randomly to receive daily vitamin C 1000 mg and vitamin E 400 IU or placebo. The medications were manufactured as soft gel capsules and each active treatment gel cap contained 500 mg of ascorbic acid, 100 IU of d‐alpha tocopherol, 100 IU of d‐alpha tocopherol acetate, and excipients (gelatin, soybean oil, glycerin, water lecithin, and caramel colour). The placebo gel caps contained excipients only and were externally identical to the active drug. Participants were instructed to ingest 2 gel caps daily from enrolment until delivery or until the diagnosis of pre‐eclampsia. Compliance with treatment was assessed by counting residual pills at monthly return visits. A TrackCap recording was used to motivate optimal compliance and the percentage of women judged by returned pill counts as having received at least 80% of the intended doses was substantial. | |
| Outcomes |
Primary outcome The development of pre‐eclampsia Planned secondary outcomes Preterm and term PROM Additional secondary outcomes 1. Abruptio placentae 2. Preterm birth 3. SGA 4. LBW infants |
|
| Notes | Denominators for some outcomes vary due to missing responses, and some vary between publications which separately report outcomes for pre‐eclampsia, PROM and chronic hypertension. Setting: Brazil. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was constructed by the data coordinating centre as permuted blocks of random size, stratified by clinical centre". |
| Allocation concealment (selection bias) | Low risk | Quote: Randomisation was "implemented via a program residing on the clinical centre’s study computer". "Correct supplier randomization assignment was verified by the data coordinating centre". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All women, caregivers and clinical investigators were blinded, and matching placebo tablets were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical investigators who assessed the data were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 (4.3%) participants lost to follow‐up, 16 in each group. Number of participants were similar between groups and reasons for missing data were described. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported. |
| Other bias | Low risk | Baseline characteristics similar between groups. |
Villar 2009.
| Methods | A multicentre, randomised, placebo‐controlled, double‐blinded trial was performed between October 2004 and December 2006, at antenatal clinics that served populations with low socioeconomic status and had evidence of overall low nutritional status from a previous WHO survey. These clinics, located in Nagpur, India; Lima and Trujillo, Peru; Cape Town, South Africa; and Ho Chi Minh City, Viet Nam form part of the WHO Maternal and Perinatal Research Network––all having extensive experience in conducting large multicentre randomised trials. The trial followed the research protocol used in the recently completed United Kingdom based multicentre trial of vitamins C and E (the VIP trial) 5 with only minor adaptations to accommodate local resources. | |
| Participants | 1365 pregnant women between14‐22 GA with high risk for pre‐eclampsia (chronic hypertension, renal disease, pre‐eclampsia‐eclampsia in the pregnancy preceding the index pregnancy requiring delivery before 37 weeks’ gestation, HELLP syndrome in any previous pregnancy, pregestational diabetes, primiparous with a BMI ≥ 30 kg/m2, history of medically indicated preterm delivery, abnormal uterine artery Doppler waveforms and women with antiphospholipid syndrome) were considered eligible. Exclusion criteria: women ingesting vitamin supplements that contained ≥ 200 mg of vitamin C and/or ≥ 50 IU of vitamin E and women receiving warfarin. |
|
| Interventions | Women were assigned randomly to receive vitamin C and E tablets or identical placebos from enrolment (between14‐22 GA to delivery), the assigned prescription continued even after pre‐eclampsia or hypertension was diagnosed. They were instructed to take 1 tablet and 1 capsule daily and to leave unused tablets or capsules in the blister and to return the blisters at the subsequent trial visit, regardless of whether all tablets and capsules had been taken. The active and placebo tablets for each vitamin were identical in form, colour and taste and were provided in boxes containing 4 blister packs, each marked Monday to Sunday. Custom Pharmaceuticals prepared vitamin C (1000 mg) and identical placebo tablets (microcrystalline cellulose) with addition of tartaric and citric acid to provide similar acidic taste and Banner Pharmacaps prepared identical gelatin capsules containing 400 IU natural source vitamin E (RRR‐a‐tocopherol) or a placebo (sunflower seed oil) and the tablets and capsules sealed in blister strips, each with a 1‐week supply were packaged. For compliance: median compliance was 87%, and was similar between the treatment groups. | |
| Outcomes |
Primary outcomes 1. Pre‐eclampsia 2. Gestational hypertension 3. Proteinuria 4. Severe gestational hypertension 5. Severe pre‐eclampsia 6. Pre‐eclampsia and eclampsia 7. LBW (< 2500 g) 8. SGA (< 10th centile of the WHO recommended standard) 9. Intrauterine or neonatal death before hospital discharge Secondary outcomes 1. Placental abruption 2. Pre‐eclampsia 3. eclampsia 4. Preterm delivery (< 37 weeks) 5. Early preterm delivery (< 34 weeks) 6. Very LBW (< 1500 g) 7. ≥ 7 days in the NICU 8. Congenital malformations. Pre‐eclampsia information was unavailable for 14 women in the vitamins and 9 in the placebo group. There were data from 81 supplemented (11.8%) and 100 placebo‐treated (14.7%) women with multiple pregnancies, for whom newborn outcomes were considered separately. |
|
| Notes | Women ingesting medications with aspirin‐like compounds were not excluded. Intention‐to‐treat analyses performed. Location: antenatal clinics in India, Peru, South Africa and Viet Nam. Reported that "Adverse event rates were 4.9 and 4.3% in the vitamins and placebo groups respectively", no further details given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation sequence was blocked by centre in groups of two to ten individuals". |
| Allocation concealment (selection bias) | Low risk | Central allocation, quote: "randomisation was performed by the statisticians of the British VIP Trial". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators and local research staff were blinded to the allocation, until all analyses were completed. Quote: "The active and placebo tablets for each vitamin were identical in form, colour and taste and were provided in boxes containing four blister packs". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators and local research staff were blinded to the allocation, until all analyses were completed. Quote: "The active and placebo tablets for each vitamin were identical in form, colour and taste and were provided in boxes containing four blister packs". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were small losses to follow‐up , but the number and reasons were similar across the treatment groups. Quote: "Pre‐eclampsia information was unavailable for 14 women (2%) in the vitamins and 9 (1.3%) in the placebo group, but the remainder of the data from these women was included in the analyses". |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported except neonatal death but perinatal death was reported instead; therefore, there is a low chance of reporting bias. |
| Other bias | Low risk | Baseline characteristics were similar between the groups. |
Xu 2010.
| Methods | A double‐blinded, multicentre trial in Canada (17 centres) and Mexico (10 centres) was conducted from January 2004 through March 2006. Randomisation was performed through an electronic data management platform, which enabled randomisation and data entry over the Internet through a secured and restricted‐access web site and stored the data in a centralised database. | |
| Participants | Women were eligible for the trial if they were between 12 and 18 completed weeks of pregnancy on the basis of last menstrual period and confirmed by early ultrasound examination. | |
| Interventions | Women were provided either with the vitamins C and E or placebo with the total daily dose of vitamin C was 1000 mg, and that of vitamin E was 400 IU. Women assigned to the vitamin group were advised to take 2 soft gel capsules, each containing 500 mg of vitamin C (ascorbic acid) and 200 IU of vitamin E (100 IU d‐alpha‐tocopherol, 100 IU d‐alpha‐tocopheryl acetate). Women in the placebo group were advised to take capsules that were identical in appearance to the active treatment capsules. Women were asked to swallow the capsules whole without crushing or chewing them and were advised not to take other antioxidant supplements. At the time of randomisation and at 26 weeks of gestation, participants compliance was calculated as the proportion of tablets not returned in the bottles over the total number of tablets given to each woman and defined as compliant to treatment if > 80% of tablets were used. |
|
| Outcomes |
Primary outcomes 1. Gestational hypertension 2. Adverse conditions: diastolic pressure or systolic pressure; proteinuria; eclampsia; thrombocytopenia; elevated liver enzyme levels; haematocrit or blood transfusion; IUGR birthweight < 3rd centile for GA; and perinatal death (fetal death > 20 weeks or neonatal death within 7 days). |
|
| Notes | They planned to recruit 5000 women per group in stratum I (low risk) for a total of 10,000 women and 1250 women per group in stratum II (high risk) for a total of 2500 women to detect 30% reduction of pre‐eclampsia, with a power of 90% and alpha error of 5%. The trial was prematurely stopped with a total of 2640 eligible pregnant women included in the final analysis due to adverse outcome. Location: Canada and Mexico. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation performed through an electronic data management platform", with stratification for centre and maternal risk status. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation performed through an electronic data management platform", with stratification for centre and maternal risk status. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women, trial staff and caregivers were blinded to the treatment allocation until the analyses had been completed. In additional matching placebo tablets were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, trial staff and caregivers were blinded to the treatment allocation until the analyses had been completed. In additional matching placebo tablets were used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 277 (10.5%) women lost to follow‐up, 148 (11%) in the vitamin group and 129 (10%) in the placebo groups, therefore, the proportion of missing data was similar across treatment groups. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Unclear risk | Baseline characteristics were similar across groups, however, the trial was terminated early once the results of Poston 2006 and Rumbold 2005 were published. A total of 2640 participants were assessed (planned to recruit 10,000). |
BMI: body mass index DIC: disseminated intravascular coagulation GA: gestational age HELLP: haemolysis, elevated liver enzymes, low platelets HPLC: high performance liquid chromatography IM: intramuscular IQR: interquartile range IU: international units IUGR: Intrauterine growth retardation IVH: intraventricular haemorrhage LBW: low birthweight MDA‐TBAR: malondialdehyde‐thiobarbituric acid reactive NEC: necrotising enterocolitis NICU: neonatal intensive care unit NSAID: non‐steroidal anti‐inflammatories PAI‐1: plasminogen activator inhibitor‐1 PAI‐2: plasminogen activator inhibitor‐2 PROM: prelabour rupture of membranes RDI: recommended dietary intake RDS: respiratory distress syndrome SD: standard deviation SGA: small‐for‐gestational age WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bolisetty 2002 | Not a randomised trial, pilot case‐control study. 12 women at risk of preterm birth and between 30 and 36 weeks' gestation were given daily 20 mg beta‐carotene, 167.8 mg vitamin E and 1000 mg vitamin C or acted as controls. Biochemical assessments of oxidative stress and maternal plasma concentrations of beta‐carotene, vitamin E and vitamin C were reported. |
| Clark 2012 | The intervention was dietary advice to optimise vitamin E intake to 15 mg/day, not specifically vitamin E supplementation. |
| Lietz 2001 | Not a randomised trial. Women were supplemented with either red palm oil (a source of vitamin A), sunflower oil (a source of vitamin E) or acted as control. Allocation to treatment groups was "based on practicality". Reported outcomes included measures of plasma and breast milk vitamin A status, maternal haemoglobin and maternal weight. |
| Moldenhauer 2002 | Women were not randomised to vitamin E supplementation. Women in this study were participating in a randomised placebo‐controlled trial of calcium supplementation, and completed a dietary assessment at 12 to 21 weeks' gestation and 29 to 31 weeks' gestation. Unclear whether all women took a standard prenatal multivitamin or just women in the placebo group. Results are presented according to "teens", "twins" and "singleton" pregnancies, not according to whether women took the supplement or not. Outcomes reported included dietary intakes of vitamin C and E (with and without the contribution of the prenatal vitamin supplement). Published in abstract form only. |
| Wibowo 2012 | The Intervention was a multivitamin supplement including vitamin E, more than 14 other vitamins were also included in the supplement. |
Characteristics of studies awaiting assessment [ordered by study ID]
Tan 1997.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Unable to trace full paper. |
Differences between protocol and review
Methods updated to current standard text for Cochrane Pregnancy and Childbirth. A 'Summary of findings' table has been incorporated.
Contributions of authors
Alice Rumbold developed and wrote the protocol, extracted data, assessed risk of bias and wrote the review. Caroline Crowther commented on and revised the various drafts of the protocol, extracted data and commented on all drafts of the review. Erika Ota, Hiroyuki Hori, and Celine Myazaki assessed eligible studies, extracted data, assessed risk of bias and developed the 'Summary of findings' table.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, The University of Adelaide, Australia.
National Centre for Child Health and Development 27B‐10, 26A‐5, Japan.
External sources
-
Japan Agency for Medical Research and development, Japan.
AMED No.27300101
-
National Health and Medical Research Council, Australia.
Funding for the PCG Australian and New Zealand Satellite
The Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Declarations of interest
Caroline Crowther and Alice Rumbold are Investigators on one of the included trials (Rumbold 2006). Decision about inclusion of this trial and extraction of data about this trial was undertaken by Erika Ota, Hiroyuki Hori and Celine Miyazaki.
Edited (no change to conclusions)
References
References to studies included in this review
Anthony 1996 {published data only}
- Anthony J, Smith P, Mantel G, Johanson R. A randomised controlled trial of vitamin E supplementation in conservatively managed pre‐eclamptic patients. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8;Seattle, Washington, USA. 1996:193.
Beazley 2005 {published data only}
- Beazley D, Ahokas R, Livingston J, Griggs M, Scroggs M, Sibai B. Effects of vitamin c and e supplementation on total antioxidant status (tas) and 8‐isoprostane (ip) levels in women at high risk for preeclampsia [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S76. [DOI] [PubMed] [Google Scholar]
- Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2005; Vol. 192:520‐1. [DOI] [PubMed]
- Beazley D, Livingston J, Kao L, Sibai B. Vitamin c and e supplementation in women at high risk for preeclampsia: a double‐blind placebo controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S216. [DOI] [PubMed] [Google Scholar]
Borna 2005 {published data only}
Chappell 1999 {published data only}
- Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, et al. Effect of antioxidants on the occurrence of pre‐eclampsia in women at increased risk: a randomised trial. Lancet 1999;354(9181):810‐6. [DOI] [PubMed] [Google Scholar]
- Chappell LC, Seed PT, Kelly FJ, Briley A, Hunt BJ, Charnock‐Jones DS, et al. Vitamin C and E supplementation in women at risk of preeclampsia is associated with changes in indices of oxidative stress and placental function. American Journal of Obstetrics and Gynecology 2002;187(3):777‐84. [DOI] [PubMed] [Google Scholar]
Gulmezoglu 1997 {published data only}
- Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MM. Antioxidants in the treatment of severe pre‐eclampsia: an explanatory randomised controlled trial. British Journal of Obstetrics and Gynaecology 1997;104(6):689‐96. [DOI] [PubMed] [Google Scholar]
- Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MM. Placental malondialdehyde and glutathione levels in a controlled trial of antioxidant treatment in severe preeclampsia. Hypertension in pregnancy 1996;15(3):287‐95. [Google Scholar]
Gungorduk 2014 {published data only}
- Gungorduk K, Asicioglu O, Gungorduk OC, Yildirim G, Besimoglu B, Ark C. Does vitamin C and vitamin E supplementation prolong the latency period before delivery following the preterm premature rupture of membranes? a randomized controlled study. American Journal of Perinatology 2014;31(3):195‐202. [DOI] [PubMed] [Google Scholar]
Huria 2010 {published data only}
- Huria A, Gupta P, Kumar D, Sharma MK. Vitamin C and vitamin E supplementation in pregnant women at risk for pre eclampsia: a randomized controlled trial. Internet Journal of Health 2010; Vol. 10, issue 2.
Kalpdev 2011 {published data only}
- Kalpdev A, Saha SC, Dhawan V. Vitamin C and E supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertension in Pregnancy 2011;30(4):447‐56. [DOI] [PubMed] [Google Scholar]
Mahdy 2004 {published data only}
- Mahdy ZA, Siraj HH, Azwar MH, Wahab MA, Khaza'ai H, Mutalib MSA, et al. The role of palm oil vitamin E in the prevention of pregnancy‐induced hypertension [abstract]. Hypertension in Pregnancy 2004; Vol. 23, issue Suppl 1:67.
McCance 2010 {published data only}
- Holmes V, Young I, Maresh M, Pearson D, Walker J, McCance D, et al. The diabetes and pre‐eclampsia intervention trial (DAPIT) [abstract]. Hypertension in Pregnancy 2004;23(Suppl 1):68. [DOI] [PubMed] [Google Scholar]
- Holmes VA, Young IS, Maresh MJA, Pearson DWM, Walker JD, McCance D and the DAPIT Study Group. The diabetes and pre‐eclampsia intervention trial. International Journal of Gynecology & Obstetrics 2004;87:66‐71. [DOI] [PubMed] [Google Scholar]
- Holmes VA, Young IS, Patterson CC, Pearson DWM, Walker JD, Maresh MJA, et al. Optimal glycemic control, pre‐eclampsia, and gestational hypertension in women with type 1 diabetes in the Diabetes and Pre‐Eclampsia Intervention Trial. Diabetes Care 2011;34(8):1683‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PC, Powell LA, McCance DR, Pogue K, McMaster C, Gilchrist S, et al. Placental protein tyrosine nitration and MAPK in type 1 diabetic pre‐eclampsia: Impact of antioxidant vitamin supplementation. Journal of Diabetes & its Complications 2013;27(4):322‐7. [DOI] [PubMed] [Google Scholar]
- McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, et al. Vitamins C and E for prevention of pre‐eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo‐controlled trial. Lancet 2010;376(9737):259‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissgerber TL, Gandley RE, Roberts JM, Patterson CC, Holmes VA, Young IS, et al. Haptoglobin phenotype, pre‐eclampsia, and response to supplementation with vitamins C and E in pregnant women with type‐1 diabetes. BJOG :an international journal of obstetrics and gynaecology 2013;120(10):1192‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasrolahi 2006 {published data only}
- Nasrolahi Sh, Alimohammady Sh, Zamani M. The effect of antioxidants (vitamin e and c) on preeclampsia in primiparous women. Journal of Gorgan University of Medical Sciences 2006; Vol. 8, issue 1:17‐21.
Poston 2006 {published data only}
- Duckworth S, Shennan A, Chappell L, Seed P, Briley A. Effect of antioxidant supplementation on pre‐labour rupture of the membranes. Archives of Disease in Childhood: Fetal and Neonatal Edition 2011;96:Fa75‐Fa76. [Google Scholar]
- Greenough A, Shaheen S, Shennan A, Seed P. Respiratory outcome in childhood of maternal vitamin C and E supplementation. American Journal of Respiratory and Critical Care Medicine 2009;179:A5975. [Google Scholar]
- Greenough A, Shaheen SO, Shennan A, Seed PT, Poston L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax 2010;65(11):998‐1003. [DOI] [PubMed] [Google Scholar]
- Manning L, Briley A, Poston L, Chappell L, Shennan A. Supplementation with vitamins C and E in pregnancy and liver function [abstract]. BJOG: an international journal of obstetrics and gynaecology 2007; Vol. 114, issue 8:1038.
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH, for the Vitamins in Pre‐eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre‐eclampsia (VIP trial): randomised placebo‐controlled trial. Lancet 2006; Vol. 367, issue 9517:1145‐54. [DOI] [PubMed]
- Poston L, Shennan A, Maternal and Fetal Research Unit. Vitamins in pre‐eclampsia (VIP). Maternal and Fetal Research Unit www.mfru.info/VIP.html (accessed 25 February 2004).
- Shennan AH, Briley AL, Seed PT, Kelly FJ, Poston L. A randomised controlled trial (RCT) of vitamin C and E supplementation in high risk women to prevent pre‐eclampsia (the VIP trial; on behalf of the VIP trial consortium) [abstract]. Journal of Obstetrics and Gynaecology 2006; Vol. 26, issue 1:S18‐20.
Pressman 2003 {published data only}
- Pressman EK, Cavanaugh JL, Mingione M, Norkus EP, Woods JR. Effects of maternal antioxidant supplementation on maternal and fetal antioxidant levels: a randomized, double‐blind study. American Journal of Obstetrics and Gynecology 2003;189:1720‐5. [DOI] [PubMed] [Google Scholar]
Rivas 2000 {published data only}
- Rivas‐Echeverria CA, Echeverria Y, Molina L, Novoa D. Synergic use of aspirin, fish oil and vitamins C and E for the prevention of preeclampsia [abstract]. Hypertension in Pregnancy 2000;19(Suppl 1):30. [Google Scholar]
Roberts 2010 {published data only}
- Gandley R, Abramovici A. Prenatal vitamin C and e supplementation is associated with a reduction in placental abruption and preterm birth in smokers. Pregnancy Hypertension 2012;2(3):331‐2. [DOI] [PubMed] [Google Scholar]
- Hauth J, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. Vitamin C and E supplementation to prevent preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 2009; Vol. 201, issue 6 Suppl 1:S175‐S176.
- Hauth JC. Maternal insulin resistance and preeclampsia. American Journal of Obstetrics & Gynecology 2011;204(1 Suppl):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauth JC, Clifton RG, Roberts JM, Myatt L, Spong CY, Leveno KJ, et al. Maternal insulin resistance and preeclampsia. American Journal of Obstetrics and Gynecology 2011;204(4):327.e1‐327.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauth JC, Clifton RG, Roberts JM, Spong CY, Myatt L, Leveno KJ, et al. Vitamin C and E supplementation to prevent spontaneous preterm birth: a randomized controlled trial. Obstetrics & Gynecology 2010;116(3):653‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHD MFMU Network. Combined antioxidants and preeclampsia prediction studies. http://www.bsc.gwu.edu/mfmu/projects/capps.cgi (accessed 1 September 2004).
- National Institute of Child Health and Human Development (NICHD). Antioxidant therapy to prevent preeclampsia. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 14 June 2005) 2005.
- Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy‐associated hypertension. New England Journal of Medicine 2010;362(14):1282‐91. [PUBMED: 20375405] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). A randomized controlled trial of antioxidant vitamins to prevent serious complications associated with pregnancy related hypertension in low risk, nulliparous women. American Journal of Obstetrics and Gynecology 2008; Vol. 199, issue 6 Suppl 1:S4.
- Weissgerber T, Gandley R. Risk of types of pregnancy associated hypertension according to haptoglobin phenotype. Reproductive Sciences 2011;18(3 Suppl 1):69A. [Google Scholar]
- Weissgerber TL, Gandley RE, McGee PL, Spong CY, Myatt L, Leveno KJ, et al. Haptoglobin phenotype, preeclampsia risk and the efficacy of vitamin C and E supplementation to prevent preeclampsia in a racially diverse population. PLoS ONE 2013;8(4):e60479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rumbold 2006 {published data only}
- Crowther CA, Dekker G, Haslam R, Robinson J. Australian collaborative trial of supplements with vitamin C and vitamin E for the prevention of pre‐eclampsia: a randomised trial. Personal Communication 2004.
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Australian collaborative trial of supplements (ACTS) with vitamin C and E for the prevention of pre‐eclampsia ‐ a randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia 2006:180.
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS, for the ACTS Study Group. Vitamins C and E and the risks of preeclampsia and perinatal complications. New England Journal of Medicine 2006; Vol. 354, issue 17:1796‐806. [DOI] [PubMed]
Sawhney 2000 {published data only}
- Sawhney H, Singhal S, Vasishta K, Majumbar S. Effect of vitamin E supplementation on lipid peroxide levels in preeclampsia [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA 2000;Book 5:31. [Google Scholar]
Shahraki 2006 {published data only}
- Shahraki AD. Effects of vitamin E, calcium carbonate and milk of magnesium on muscular cramps in pregnant women. Journal of Medical Sciences 2006; Vol. 6, issue 6:979‐83.
Spinnato 2007 {published data only}
- Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins‐Costa S, et al. The impact of prior preeclampsia on the risk of superimposed preeclampsia and other adverse pregnancy outcomes in patients with chronic hypertension. American Journal of Obstetrics and Gynecology 2011;204(4):345.e1‐345.e6. [DOI] [PubMed] [Google Scholar]
- Spinnato II J, Freire S, Pinto e Silva JLP, Rudge M, Martins‐Costa S, Koch M, et al. Antioxidant therapy and PROM. American Journal of Obstetrics and Gynecology 2007; Vol. 197, issue 6 Suppl 1:S196, Abstract no: 685.
- Spinnato II JA, Freire S, Pinto E Silva JL, Cunha Rudge MV, Martins‐Costa S, Koch MA, et al. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstetrics & Gynecology 2007;110(6):1311‐8. [DOI] [PubMed] [Google Scholar]
- Spinnato II JA, Freire S, Pinto e Silva JL, Rudge MV, Martins‐Costa S, Koch MA, et al. Antioxidant supplementation and premature rupture of the membranes: a planned secondary analysis. American Journal of Obstetrics and Gynecology 2008; Vol. 199, issue 4:433.e1‐433.e8. [DOI] [PMC free article] [PubMed]
Villar 2009 {published data only}
- Villar J, Purwar M, Merialdi M, Zavaleta N, Ngoc N, Anthony J, et al. Effect of vitamin C & E supplementation of pregnant women at risk of preeclampsia plus low nutritional status: the WHO trial. Hypertension in Pregnancy 2008; Vol. 27, issue 4:501.
- Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre‐eclampsia in populations of low nutritional status from developing countries. BJOG: an international journal of obstetrics and gynaecology 2009; Vol. 116, issue 6:780‐8. [DOI] [PubMed]
- Villar J, Purwar M, Merialdi M, Zavaleta N, Tien NN, Anthony J. WHO randomized trial of vitamin C and E supplementation among women at high risk for preeclampsia and nutritional deficiency. American Journal of Obstetrics and Gynecology 2007; Vol. 197, issue 6 Suppl 1:S4, Abstract no: 8.
Xu 2010 {published data only}
- Fraser W, Xiong X, Poston L, Shennan A. Bi‐national randomized controlled trial of vitamin c and e supplementation of pregnancy [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):43. [Google Scholar]
- Xu H, Perez‐Cuevas R, Xiong X, Reyes H, Julien P, Smith G, et al. A international trials of vitamins C and E in the prevention of preeclampsia (INTAPP trial). American Journal of Obstetrics and Gynecology 2009; Vol. 201, issue 6 Suppl 1:S2. [DOI] [PubMed]
- Xu H, Perez‐Cuevas R, Xiong X, Reyes H, Roy C, Julien P, et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). American Journal of Obstetrics and Gynecology 2010; Vol. 202, issue 3:239.e1‐239.e10. [DOI] [PubMed]
References to studies excluded from this review
Bolisetty 2002 {published data only}
- Bolisetty S, Naidoo D, Lui K, Koh TH, Watson D, Whitehall J. Antenatal supplementation of antioxidant vitamins to reduce the oxidative stress at delivery ‐ a pilot study. Early Human Development 2002;67:47‐53. [DOI] [PubMed] [Google Scholar]
Clark 2012 {published data only}
- Clark J, Craig L, McNeill G, Smith N, Norrie J, Devereux G. A novel dietary intervention to optimize vitamin E intake of pregnant women to 15 mg/day. Journal of the Academy of Nutrition and Dietetics 2012;112(2):297‐301. [DOI] [PubMed] [Google Scholar]
Lietz 2001 {published data only}
- Lietz G, Henry CJK, Mulokozi G, Mugyabuso JKL, Ballart A, Ndossi GD, et al. Comparison of the effects of supplemental red palm oil and sunflower oil on maternal vitamin A status. American Journal of Clinical Nutrition 2001;74:501‐9. [DOI] [PubMed] [Google Scholar]
Moldenhauer 2002 {published data only}
- Moldenhauer J, Guo S, Liang R, Prada J. Dietary intake levels of the antioxidants vitamin c and vitamin e are adequately achieved with standard prenatal vitamin supplementation in high risk pregnancy groups [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S99. [Google Scholar]
Wibowo 2012 {published data only}
- Sezikawa A. Antioxidant supplementation in pregnant women with low antioxidant status. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 June 2007] 2007.
- Wibowo N, Purwosunu Y, Sekizawa A, Farina A, Idriansyah L, Fitriana I. Antioxidant supplementation in pregnant women with low antioxidant status. Journal of Obstetrics and Gynaecology Research 2012;38(9):1152‐61. [PUBMED: 22563751] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Tan 1997 {published data only}
- Tan YL, Han BF, Cheng YZ, Lin ML, Huang JX, Hou SY, et al. The prevention of vitamin E in severe pregnancy induced hypertension syndrome. Journal of Practice Obstetrics and Gynecology 1997;13(6):313‐4. [Google Scholar]
Additional references
Basaran 2010
- Basaran A, Basaran M, Topatan B. Combined vitamin C and E supplementation for the prevention of preeclampsia: a systematic review and meta‐analysis. Obstetrical & Gynecological Survey 2010;65(10):653‐67. [DOI] [PubMed] [Google Scholar]
Bendich 1993
- Bendich A, Machlin LJ. The safety of oral intake of vitamin E: data from clinical studies from 1986‐1991. In: Packer L, Fuchs J editor(s). Vitamin E in Health and Disease. New York: Marcel Dekker, 1993:411‐6. [Google Scholar]
Bjelakovic 2012
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD007176] [DOI] [PubMed] [Google Scholar]
Bjelakovic 2013
- Bjelakovic G, Nikolova D, Gluud C. Meta‐regression analyses, meta‐analyses, and trial sequential analyses of the effects of supplementation with beta‐carotene, vitamin A, and vitamin E singly or in different combinations on all‐cause mortality: do we have evidence for lack of harm?. PLoS One 2013;8(9):e74558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conde‐Agudelo 2011
- Conde‐Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. American Journal of Obstetrics and Gynecology 2011;204(6):503.e1‐503.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devaraj 1997
- Devaraj S, Adams‐Huet B, Fuller CJ, Jialal I. Dose‐response comparison of RRR‐alpha‐tocopherol and all‐racemic alpha‐tocopherol on LDL oxidation. Arteriosclerosis, Thrombosis and Vascular Biology 1997;17(10):2273‐9. [DOI] [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Gross 1982
- Gross SJ, Landaw SA. The effect of vitamin E on red cell hemolysis and bilirubinaemia. Annals of the New York Academy of Science 1982;393:315‐22. [DOI] [PubMed] [Google Scholar]
Hanson 1993
- Hanson U, Persson B. Outcome of pregnancies complicated by type 1 insulin‐dependent diabetes in Sweden: acute pregnancy complications, neonatal mortality and morbidity. American Journal of Perinatology 1993;10(4):330‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Incebiyik 2015
- Incebiyik A, Vural M, Camuzcuoglu A, Camuzcuoglu H, Hilali NG, Taskin A, et al. Comparison of tissue prolidase enzyme activity and serum oxidative stress level between pregnant women with placental abruption and those with a healthy pregnancy. Archives of Gynecology and Obstetrics 2015;291(4):805‐9. [DOI] [PubMed] [Google Scholar]
IOM 2000
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academy Press, 2000. [PubMed] [Google Scholar]
Johnson 1985
- Johnson L, Bowen FW Jr, Abbasi S, Herrmann N, Weston M, Sacks L, et al. Relationship of prolonged pharmacologic serum levels of vitamin E to incidence of sepsis and necrotizing enterocolitis in infants with birthweight 1,500 grams or less. Pediatrics 1985;75:619‐38. [PubMed] [Google Scholar]
Kingdom 2000
- Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. European Journal of Obstetrics & Gynecology and Reproductive Biology 2000;92:35‐43. [DOI] [PubMed] [Google Scholar]
NHMRC 2006
- NHMRC. Nutrient Reference Values for Australia and New Zealand. Canberra, Australia: Commonwealth of Australia, 2006. [Google Scholar]
Packer 1979
- Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 1979;278:737‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1990
- Roberts DCK. Vitamin E. In: Truswell AS, Dreosti IE, English RM, Palmer N, Rutihauser IHE editor(s). Recommended Nutrient Intakes, Australian Papers. Sydney: Australian Professional Publications, 1990:158‐73. [Google Scholar]
Rumbold 2005a
- Rumbold A, Duley L, Crowther C, Haslam R. Antioxidants for preventing pre‐eclampsia. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004227.pub3] [DOI] [PubMed] [Google Scholar]
Rumbold 2005b
- Rumbold AR, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004072.pub2] [DOI] [PubMed] [Google Scholar]
Saugstad 1988
- Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatric Research 1988;23:143‐50. [DOI] [PubMed] [Google Scholar]
Saugstad 2001
- Saugstad OD. Update on oxygen radical disease in neonatology. Current Opinion in Obstetrics and Gynecology 2001;13(2):147‐53. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Sharma 1985
- Sharma SC, Walzman M, Bonnar J, Molloy A. Blood ascorbic acid and histamine levels in patients with placental bleeding. Human Nutrition. Clinical Nutrition 1985;39(3):233‐8. [PubMed] [Google Scholar]
Sharma 1986
- Sharma SC, Bonnar J, Dóstalóva L. Comparison of blood levels of vitamin A, beta‐carotene and vitamin E in abruptio placentae with normal pregnancy. International Journal for Vitamin and Nutrition Research 1986;56(1):3‐9. [PubMed] [Google Scholar]
Woods 2001
- Woods JR Jr, Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing preterm premature rupture of membranes?. American Journal of Obstetrics and Gynecology 2001;185(1):5‐10. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rumbold 2005
- Rumbold A, Crowther CA. Vitamin E supplementation in pregnancy. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004069.pub2] [DOI] [PubMed] [Google Scholar]


