Abstract
Background
There is now a rising commitment to acknowledge the role patients and families play in contributing to their safety. This review focuses on one type of involvement in safety ‐ patient and family involvement in escalation of care for serious life‐threatening conditions i.e. helping secure a step‐up to urgent or emergency care ‐ which has been receiving increasing policy and practice attention. This review was concerned with the negotiation work that patient and family members undertake across the emergency care escalation pathway, once contact has been made with healthcare staff. It includes interventions aiming to improve detection of symptoms, communication of concerns and staff response to these concerns.
Objectives
To assess the effects of interventions designed to increase patient and family involvement in escalation of care for acute life‐threatening illness on patient and family outcomes, treatment outcomes, clinical outcomes, patient and family experience and adverse events.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP) ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform from 1 Jan 2000 to 24 August 2018. The search was updated on 21 October 2019.
Selection criteria
We included randomised controlled trials (RCTs) and cluster‐randomised controlled trials where the intervention focused on patients and families working with healthcare professionals to ensure care received for acute deterioration was timely and appropriate. A key criterion was to include an interactive element of rehearsal, role play, modelling, shared language, group work etc. to the intervention to help patients and families have agency in the process of escalation of care. The interventions included components such as enabling patients and families to detect changes in patients' conditions and to speak up about these changes to staff. We also included studies where the intervention included a component targeted at enabling staff response.
Data collection and analysis
Seven of the eight authors were involved in screening; two review authors independently extracted data and assessed the risk of bias of included studies, with any disagreements resolved by discussion to reach consensus. Primary outcomes included patient and family outcomes, treatment outcomes, clinical outcomes, patient and family experience and adverse events. Our advisory group (four users and four providers) ensured that the review was of relevance and could inform policy and practice.
Main results
We included nine studies involving 436,684 patients and family members and one ongoing study. The published studies focused on patients with specific conditions such as coronary artery disease, ischaemic stroke, and asthma, as well as pregnant women, inpatients on medical surgical wards, older adults and high‐risk patients with a history of poor self‐management.
While all studies tested interventions versus usual care, for four studies the usual care group also received educational or information strategies. Seven of the interventions involved face‐to‐face, interactional education/coaching sessions aimed at patients/families while two provided multi‐component education programmes which included components targeted at staff as well as patients/families. All of the interventions included: (1) an educational component about the acute condition and preparedness for future events such as stroke or change in fetal movements: (2) an engagement element (self‐monitoring, action plans); while two additionally focused on shared language or communication skills.
We had concerns about risk of bias for all but one of the included studies in respect of one or more criteria, particularly regarding blinding of participants and personnel. Our confidence in results regarding the effectiveness of interventions was moderate to low.
Low‐certainty evidence suggests that there may be moderate improvement in patients’ knowledge of acute life‐threatening conditions, danger signs, appropriate care‐seeking responses, and preparedness capacity between interactional patient‐facing interventions and multi‐component programmes and usual care at 12 months (MD 4.20, 95% CI 2.44 to 5.97, 2 studies, 687 participants). Four studies in total assessed knowledge (3,086 participants) but we were unable to include two other studies in the pooled analysis due to differences in the way outcome measures were reported. One found no improvement in knowledge but higher symptom preparedness at 12 months. The other study found an improvement in patients’ knowledge about symptoms and appropriate care‐seeking responses in the intervention group at 18 months compared with usual care.
Low‐certainty evidence from two studies, each using a different measure, meant that we were unable to determine the effects of patient‐based interventions on self‐efficacy. Self‐efficacy was higher in the intervention group in one study but there was no difference in the other compared with usual care.
We are uncertain whether interactional patient‐facing and multi‐component programmes improve time from the start of patient symptoms to treatment due to low‐certainty evidence for this outcome. We were unable to combine the data due to differences in outcome measures. Three studies found that arrival times or prehospital delay time was no different between groups. One found that delay time was shorter in the intervention group.
Moderate‐certainty evidence suggests that multi‐component interventions probably have little or no impact on mortality rates. Only one study on a pregnant population was eligible for inclusion in the review, which found no difference between groups in rates of stillbirth. In terms of unintended events, we found that interactional patient‐facing interventions to increase patient and family involvement in escalation of care probably have few adverse effects on patient's anxiety levels (moderate‐certainty evidence).
None of the studies measured or reported patient and family perceptions of involvement in escalation of care or patient and family experience of patient care. Reported outcomes related to healthcare professionals were also not reported in any studies.
Authors' conclusions
Our review identified that interactional patient‐facing interventions and multi‐component programmes (including staff) to increase patient and family involvement in escalation of care for acute life‐threatening illness may improve patient and family knowledge about danger signs and care‐seeking responses, and probably have few adverse effects on patient’s anxiety levels when compared to usual care. Multi‐component interventions probably have little impact on mortality rates. Further high‐quality trials are required using multi‐component interventions and a focus on relational elements of care. Cognitive and behavioural outcomes should be included at patient and staff level.
Keywords: Adult; Female; Humans; Male; Pregnancy; Acute Disease; Acute Disease/mortality; Acute Disease/psychology; Acute Disease/therapy; Anxiety; Anxiety/prevention & control; Communication; Consumer Health Information; Consumer Health Information/methods; Critical Illness; Critical Illness/mortality; Critical Illness/psychology; Critical Illness/therapy; Disease Progression; Emergency Treatment; Family; Health Knowledge, Attitudes, Practice; Negotiating; Negotiating/methods; Patient Acceptance of Health Care; Patient Education as Topic; Patient Education as Topic/methods; Patient Participation; Patient Participation/methods; Patient Safety; Randomized Controlled Trials as Topic; Self Efficacy; Symptom Assessment; Symptom Assessment/methods
Plain language summary
How effective are strategies to help patients and their families secure emergency medical care when a health condition becomes life‐threatening?
Medical emergencies
A life‐threatening condition is a medical emergency. The faster a person secures the right medical care, the better their chances of surviving. When patients and their families know the signs of a life‐threatening medical emergency and how best to communicate concerns around a deterioration in health, they can act quickly to seek emergency care and work with staff to ensure a timely response.
Increasing patient and family involvement
Education and coaching are available to help patients and their families, and healthcare professionals work together to make sure patients and families can secure emergency care when needed. These strategies focus on:
‐ helping patients and their families to notice changes in a patient's condition and tell healthcare staff about them;
‐ empowering patients and families to feel confident about arranging for urgent or emergency care;
‐ healthcare staff giving patients and families a chance to talk about their concerns, and actively listening to them during an emergency consultation; and
‐ training healthcare staff to respond appropriately when patients and their families raise concerns about a patient's condition.
Why we did this Cochrane Review
We wanted to find out if education and coaching strategies could help patients and families to recognise when changes in a health condition are life‑threatening and act to help secure emergency care.
What did we do?
We searched for studies that tested strategies to involve and empower patients and their families in seeking emergency care for a life‐threatening medical condition. We also included studies where the strategy included a component targeted at enabling staff response.
We looked for studies in which the strategies people received were decided at random. This type of study usually gives the most reliable evidence about the effects of a strategy.
Search date: we included evidence published up to 21 October 2019.
What we found
We found nine relevant studies in different healthcare settings in which 436,684 patients and family members took part. Seven of the strategies studied involved face‐to‐face education or coaching sessions for patients and families, and two involved education programmes aimed at healthcare staff as well as patients and their families. All strategies had an educational part and an engagement part (for example, self‐monitoring; using action plans); two strategies additionally focused on communication skills and using shared language.
All studies compared usual care against receiving strategies to increase involvement of patients and their families in seeking emergency care. In four studies, people in the usual care group also received information or educational strategies. The studies varied in design and in their assessments, making it difficult to compare all their results.
We did not find any studies that looked at patients', or their families', satisfaction with care, or what they thought of their involvement in seeking emergency care.
What are the results of our review?
Compared with usual care, strategies to improve involvement in securing emergency care:
‐ may help patients and their families to know which danger signs to look for, and to know the right action to take (4 studies; 3086 people);
‐ probably have little to no effect on stillbirth in pregnancy (1 study; 409,175 people); and
‐ probably do not increase anxiety levels in patients and their families (1 study; 2,597 people).
We are uncertain if the strategies affected:
‐ peoples' confidence in recognising and reporting worsening in a health condition (2 studies; 217 people); or
‐ the time between the start of life‐threatening symptoms and receiving emergency treatment (4 studies; 27,023 people).
Our confidence in our results
We are moderately confident about the effect of the strategies on anxiety levels and on stillbirth, although these results might change with further evidence. We are less confident about our other findings, which are likely to change with further evidence. Some of the studies we compared had small numbers of people taking part, so their results may have been unreliable.
Conclusions
Strategies to help patients and their families to secure emergency care may improve their knowledge about life‐threatening conditions, and probably don't increase their anxiety more than usual care
Summary of findings
Summary of findings 1. Summary of findings.
| Interventions to increase patient and family involvement in escalation of care compared with usual care for acute life‐threatening illness | ||||
|
Patient or population: Adults (and/or family members) with potential for acute life‐threatening illness Settings: Community health and hospital settings Intervention: Increasing patient and family involvement in escalation of care Comparison: Usual care | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Patient and family knowledge of danger signs and appropriate care‐seeking behaviours (1a) Acute Coronary Syndrome Response Index with 3 scales to measure knowledge (21 items), attitudes (5 items) and beliefs (7 items) (Buckley 2007; Dracup 2009). Total knowledge score 0‐21 (higher scores indicate improvement). Stroke knowledge survey assessed knowledge of symptoms, risk factors, acute stroke treatment; hypothetical scenarios on action during acute stroke; and on preparedness capacity (Boden Albala 2015). Total score 0‐21 (higher scores indicate improvement). A random‐digit telephone survey with two open‐ended questions about heart attack symptoms. Responses were mapped against a published list (Luepker 2000). Follow‐up: 12 and 18 months |
The results of two studies (Buckley 2007; Dracup 2009) were pooled. The mean difference in knowledge about symptoms and appropriate responses was 4.20 higher (better) in the intervention group than with usual care (95% CI 2.44 to 5.97 higher) at 12 months, a moderate effect (2 studies, 687 participants). Boden Albala 2015 found at 12 months there was no difference in knowledge (OR 1.21, 95%CI 0.87, 1.67), however there was higher preparedness capacity (OR 7.64; 2.49, 23.49) between the intervention group (educational materials and interactive sessions) and usual care group (educational materials only). Luepker 2000 found at 18 months that more people in intervention communities reported correct messages about heart attack symptoms (2.7% (n = 645) vs 1.8% (n = 561) P < 0.03) and identified appropriate actions to take in the light of danger signs related to coronary heart disease (32.6% n = 643 vs 22.8% n = 561; P < 0.006) than control communities. |
3086 4 studies | ⊕⊕⊝⊝ Lowa | Interventions to increase patient and family involvement in escalation of care may moderately improve patient and family knowledge about symptoms, appropriate responses, and preparedness capacity. |
|
Patient and family self‐efficacy (1e) 10 item Self‐Efficacy Response Scale measuring self‐ efficacy in recognising and reporting own worsening conditions; total score for each of the two subscales 6‐30 (higher scores indicate improvement) (See 2014). Perceived Efficacy in Patient‐ Physician Interactions (PEPPI) instrument which consisted of five items and a ten‐point scale to assess patients’ self‐efficacy in communicating to primary care providers (Horn 2014). Total scores were dichotomised based on whether or not the parent had the maximum score of 50. Follow‐up: 3 days and 6 months |
See 2014 reported higher (better) self‐efficacy in the intervention group at day 3 of hospitalisation. Mean scores at follow‐up for the usual care group were 17.06 (SD 3.79) and 25.03 (SD 1.85) for the intervention group for one subscale (P < 0.0001) and 18.70 (SD 3.06) for the usual care group and 26.21 (SD 1.45) for the intervention group (P < 0.0001) for the second subscale. Horn 2014 found no difference in self‐efficacy at 6 months (aOR1.4, 95% CI: 0.6 to 3.5, P 0.42), with 60.6% reporting a maximum PEPPI self‐efficacy score in the intervention group compared to 51.6% in usual care. |
217 2 studies |
⊕⊕⊝⊝ Lowb | We are uncertain whether interventions to increase patient and family involvement in escalation of care, improve patient and family self‐efficacy. The two studies reported differing effects. |
|
Time from start of symptoms to delivery of professional treatment (2a) Mean, median prehospital delay time from symptom onset of arrival at hospital/ED, proportion of participants presenting to the emergency department (ED) within 2, 3, and 4.5 hours Follow‐up: 18 months, 2 and 5 years |
Boden Albala 2015 found no difference in arrival within the 3‐hour time window between groups. In the intervention group, 40% arrived within 3 hours compared with 46% of the usual care group (P < 0.33) Mooney 2014 found median delay time was significantly lower (better) in the intervention compared to the usual care group (1.7 versus 7.1 hours). Two studies (Dracup 2009; Luepker 2000) found no difference in the median prehospital delay time between intervention and control groups. |
27,023 4 studies |
⊕⊕⊝⊝ Lowc | We are uncertain whether interventions to increase patient and family involvement in escalation of care, improve time to treatment. Whilst one study reported moderate benefits, three studies reported no difference. |
|
Mortality measured by mortality rates including failure‐to‐rescue
rates (3a) Stillbirth Follow‐up: 3 years |
One study (Norman 2018) found that the intervention did not reduce the risk of stillbirths, adjusted odds ratio [aOR] 0·90, 95% CI 0·75 to 1·07; P = 0·23; absolute effect 5 fewer still births per 10,000 pregnancies (95% CI 11 fewer to 3 more) | 409,175 1 study |
⊕⊕⊕⊝ Moderated | Interventions to increase patient and family involvement in escalation of care, probably have little or no effect on stillbirth. |
| Patient and family perceptions of involvement in escalation of care (4b) | Not measured | Not measured | No studies were found that looked at patient and family perceptions of involvement in escalation of care. | |
| Patient and family satisfaction with care received (4e) | Not measured | Not measured | No studies were found that looked at patient and family satisfaction with care. | |
|
Patient harms associated with patient and family involvement in escalation of care (5a) Multiple Affect Adjective Checklist total score for state anxiety (range 0‐21). Higher scores signify higher anxiety; a score of ≥ 11 indicates clinically significant symptoms of anxiety. Follow‐up: 12 months |
At 12 months, Dracup 2009 found higher anxiety was found in the usual care group than the intervention group (score of 6 compared to 5.5; P = 0.01). Lower anxiety in the intervention group however was only seen in men; anxiety levels remained stable in women | 2597 1 study |
⊕⊕⊕⊝ Moderatee | Interventions to increase patient and family involvement in escalation of care probably do not increase patients' anxiety levels. |
| CI: Confidence interval; OR: Odds Ratio; aOR: adjusted Odds Ratio; SD: Standard Deviation; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
a Downgraded by two levels: for indirectness (restricted population, largely male well‐educated sample, complicated comorbidities excluded) and risk of bias (unclear randomisation and allocation concealment in 2 of 4 studies; also high risk of bias due to lack of blinding of participants, incomplete study data presented, risk of selection bias)
b Downgraded by two levels: for imprecision (small sample size) and inconsistency (variation in effects)
c Downgraded by two levels: for risk of bias (unclear randomisation and allocation concealment in 2 of 4 studies; also high risk of bias due to lack of blinding of participants, incomplete study data presented), and for inconsistency (substantially different outcome measures and timing of outcome measured, and variation in effects)
d Downgraded by one level: for indirectness (pregnant population only)
e Downgraded by one level: for indirectness (patients with confirmed coronary heart disease diagnosis only)
Background
Despite the rise of the global patient safety movement which was triggered by the publication of 'To Err is Human' (Kohn 2000), two decades later, avoidable patient harm continues to be a burden on healthcare systems across the world (Landrigan 2010; Leistikow 2011; Wachter 2010). In addition to longstanding issues, new threats to patient safety are emerging. Patients are increasing in age, have more complex needs, and are often affected by multiple chronic conditions. The increased complexity of care creates new risks of error and harm to patients (Yu 2016).
While the potential role of patients to contribute to their safety was acknowledged in To Err is Human (Kohn 2000), until recently, patient safety was largely seen as a technical and professional matter (Ocloo 2016). This position is changing. There is now a rising global commitment for providers to work together with patients and families to improve the delivery of safe care (Vincent 2016; Yu 2016). The World Health Organization has advocated that patients should become active partners in improving the safety, quality and efficiency of health service delivery (WHO 2013). Contributory roles for patients have been identified in processes such as hand hygiene, hospital rapid response systems, surgical checklists, medication safety, prevention of falls, prevention of medical errors after discharge and care transitions (Berger 2013).
There is also a strengthening evidence base that interventions are needed at provider and health system level to enable healthcare staff to engage effectively with these activities (Hor 2013; Rance 2013). Patient involvement in safety can be difficult to achieve in practice, as this role challenges established hierarchies, power differentials and social and institutional norms (Draper 2015; Johnson 2015; Keogh 2013; Kirkup 2015). It can bring with it challenges such as the need to raise awareness amongst patients of potential problems without instilling anxiety and fear, and preventing a shift of responsibility for safer care and avoidance of harm from providers to families (Entwistle 2005; Lawton 2012). Some safety activities over which they have more control (e.g. medication safety) may be perceived by patients as more acceptable to participate in than others (e.g. hygiene practices). These beliefs are linked to the social meaning and value attached to these activities, and to patient and professional expectations about responsibilities for care (Entwistle 2010; Schwappach 2010).
It is clear from the literature that patient involvement in safety encompasses different models of application and mechanisms of action, and conflating these is unlikely to be helpful (Entwistle 2006; Johnstone 2009). One type of model — patient involvement in escalation of care for acute (serious) life‐threatening conditions (i.e. helping secure a step‐up to urgent or emergency care) — has been receiving increasing policy and practice attention (Albutt 2017; CRD42015015326; Vorwerk 2015). Patient involvement can be defined on the micro‐level in relation to patients, clinicians, processes, interactions and recurring patterns in practice as distinct from meso‐level (in relation to organisations) and macro‐level (in relation to the health system) (Nelson 2002; Nelson 2008). This review's focus is at the micro‐level interaction level (see Figure 1).
1.
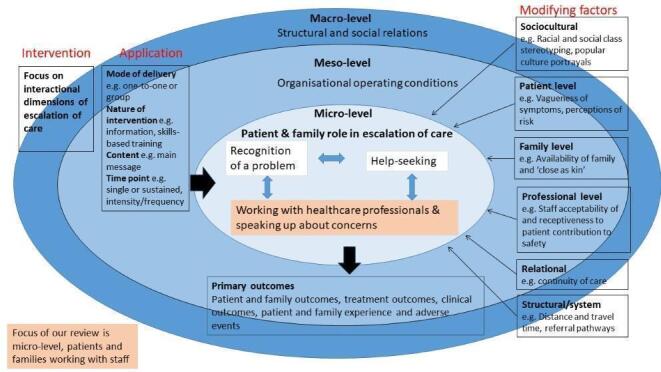
Conceptual model
Description of the condition
Patient and family involvement in escalation of care depends on a complex interplay of personal factors, lay and professional encounters, and contextual influences (Snyder 2016). Safety is an ongoing achievement which largely involves patients in interaction with family, friends and peers (Greenhalgh 2015) and healthcare staff (Hor 2013). Relationships underpin safety production, and patient involvement can be facilitated by partnership‐building and supportive communication (Snyder 2016). Trust is also linked to safety as it captures the non‐technical, interpersonal and social nature of health care. Ethnographic accounts suggest that trust is contingent on a particular context and a set of relationships, including trusting oneself, one's own body, healthcare staff and the health service (Cohn 2015).
Key requisites for patient‐initiated escalation of care, as with other safety activities, are that patients need to: (1) know how to participate (i.e. patients need to know how to recognise there is a problem, what action they can take, and why), (2) have the ability to participate which is derived not only from the patient’s knowledge, and physical and cognitive capacity, but also linked to self‐efficacy, social status and the patient’s role within the family or community, and (3) be willing to participate (Davis 2012; Schwappach 2010).
Evidence shows that there is considerable scope to improve the patient and family contributory role in detection and management of acute illness. Delayed recognition and treatment of conditions such as pneumonia and meningitis in childhood (Wolfe 2011), pre‐eclampsia and reduced fetal movements during pregnancy (Draper 2015; Warland 2015), and heart disease and stroke in adulthood (AHA 2005; ISWP 2010; Schwappach 2010), contribute significantly to the mortality and morbidity burden in low‐, middle‐ and high‐income countries. These conditions typically present with a time‐critical window for early recognition and response, and are associated with red flag signs and symptoms (such as breathlessness and pain) which can signify a serious underlying condition and act as potential markers to aid patient and family recognition of the issue and involvement in escalation of care.
Delays in recognition and receipt of appropriate treatment are linked to economic, sociocultural, healthcare system level and interpersonal factors. These factors are relevant across countries (low‐, middle‐ and high‐income) although the relative influence of each will vary (Binder 2012; Chandratheva 2010; Løvlien 2008; Mandelzweig 2006; Thaddeus 1994; Thuresson 2007). Factors affecting patients’ level of involvement include perceptions of risk and the consequences of contributing to safety as well as not participating in monitoring, seeking help and speaking up (Doherty 2012; Entwistle 2010). The local environment can hinder a patient's or family member’s ability to act (Thaddeus 1994). Some patients may choose to adopt a passive role rather than taking on explicit safety roles which may raise their anxiety and a sense of responsibility. They may therefore choose to avoid taking an active role as a means of actively protecting their personal safety (Doherty 2012). Particularly in low‐income countries (LICs), norms of passivity are underpinned by power hierarchies between patients and healthcare staff, and reinforced by broader societal and gender inequities (Béhague 2008; Grossmann‐Kendall 2001). Assumptions about personal ability to contribute to diagnosis have been shown to be significant (Entwistle 2010). The trajectory of deterioration (particularly the rapidity of onset and degree of debilitating symptoms) will influence patients’ ability to engage in the most basic of safety acts (Doherty 2012). Classic ‘red‐flag’ features of serious illness may be absent, e.g. meningococcal disease in children, making diagnosis difficult (Thompson 2006). Sociodemographic factors such as age, gender and education may also play a part although the evidence to date is inconclusive in predicting impact on patients’ willingness or ability to engage with safety (Doherty 2012). Language and health literacy will impact on patients’ and families’ contributions to their safety, as will existing or previous relationships with staff and provider organisations, perceptions of trust and safety, and knowledge and experience of navigating the organisation (Entwistle 2010; Rainey 2013; Rance 2013).
Social codes of conduct of ‘appropriate use’ of emergency services influence help‐seeking; patients and families fear making the ‘wrong’ judgement about calling for help and display uncertainty about when to seek help (Cheyne 2007; Ehrich 2003; Eri 2009; Houston 2000; Mackintosh 2012; Neill 2014). Patients' previous experiences of the health service can influence help‐seeking both positively and negatively (e.g. broken trust during a clinical encounter can contribute to subsequent delayed care‐seeking) (Binder 2012). Access barriers to help‐seeking are linked to lack of infrastructure (transport), poor signposting, gaps in the provision of services and gate‐keeping. Lack of resources and technology can lead to delays in appropriate response.
Once in receipt of care from health professionals, involvement in escalation of care necessitates vigilance from patients and family members, and may require them to take a proactive and interactive role with staff with potentially some degree of confrontation, particularly if challenging the appropriateness of decisions taken (Entwistle 2010). Helping to secure a timely response may involve speaking up about concerns about the appropriateness of care received and seeking a second tier of professional staff or a different access route to acute care. This work involves negotiating hierarchies and boundaries. Considerable cognitive and emotional resources may be required from patients and families to carry out these types of safety behaviours (Davis 2012). Differentials in social and economic capital can lead to difficulties in voicing concerns freely (Béhague 2008). Patients report wanting to be seen by staff as ‘good’ patients by not bothering, challenging or criticising them (Hrisos 2013). Patients need to defend their ‘good patient status’ in the face of a whole social structure — a powerful biomedical system, inequities in healthcare delivery and fear of differential treatment — that drives underlying debates about culpability and blame (Béhague 2008; Davis 2008; Entwistle 2005; Ocloo 2010; Schwappach 2008). The nature of professional cultures and institutional power, knowledge and politics can inhibit knowledge‐sharing (DoH 2013; Draper 2015; Johnstone 2009; Kohn 2000; Scott 2012; Waring 2009).
There are also a number of factors that moderate staff's ability to listen to patients' concerns and respond appropriately. Staff have to balance the trade‐off between inappropriate reassurance (potentially leading to catastrophic delay in diagnosis and treatment), versus creating unnecessary additional anxiety for patients (Almond 2009). Emergency departments and triage clinics are characteristically unbounded, where staff have little control over workload. Staff shortages, limited resources, overcrowding and long waiting times contribute to poor communication and diagnostic errors (Eisenberg 2005; Roscoe 2016; Wears 2003).
It is evident that there are differences in the (1) scale of avoidable morbidity and mortality between high‐ and low‐income countries, (2) timelines and presentation of trajectories of deterioration for particular conditions, and (3) facility and professional help accessibility across the emergency care escalation pathway. However, it is important to move beyond condition‐specific models and to utilise learning from both high‐ and low‐income contexts, in order to understand generic processes which influence recognition and emergency response. Conceptually we draw a distinction between (a) patient and public health behaviours which occur prior to contact with healthcare professionals which include: self‐monitoring; self‐diagnosis; the decision to seek help; and (b) the negotiation process that starts when patients (and families) come into contact with staff and start working with staff to ensure timely recognition and response. This review was concerned with this negotiation work i.e. patient and family involvement across the emergency care escalation pathway, once contact has been made with healthcare professionals. It included patients presenting with new onsets of conditions as they made contact with community health and hospital services for urgent/emergency care and timely treatment, and patients already in the healthcare system who were negotiating a step‐up in care to receive urgent/emergency treatment.
Description of the intervention
For this review, we focused on those interventions that aimed to enable interactions between patients/families and healthcare professionals in order to secure help for acute life‐threatening illness in community health and hospital settings. These interventions could be aimed at patients, families, professionals, or combinations of the three.
The interventions included one or more of these components.
Those aimed at enabling patients and families to detect changes in patients' conditions and to speak up about these changes to staff.
Those aimed at empowering patients and families to feel confident about their contribution and role in negotiating a step‐up in care.
Those aimed at enabling staff to provide opportunities for patients and families to share concerns and to listen actively to these during urgent/emergency consultations.
Those aimed at equipping staff with the skills to respond appropriately to patients and families when they raise concerns about ongoing diagnosis, treatment and management.
These interventions aimed to raise patients' awareness of their role in facilitating timely emergency response and the importance of actively contributing to escalation of care. Interventions could include educational and motivational coaching programmes. These could be individualised to the patient’s specific needs to address cognitive and emotional effects impacted by involvement in escalation of care. Educational interventions could also aim to enhance patients’ and families’ self‐efficacy to contribute to recognition and response. Interventions could teach patients how to call for help while in hospital (Albutt 2017; Berger 2013; Hueckel 2012; Vorwerk 2015). Interventions could also target both patient and provider behaviours with joint training programmes (Tai‐Seale 2016; Weingart 2009).
To summarise, increasing patient and family involvement in escalation of care for acute life‐threatening illness involves a range of different approaches, which include any of the following.
Patient‐ and family‐focused interventions
One‐to‐one acute education session to increase confidence in speaking up about changes in condition and concerns using role play and motivational coaching (e.g. Mooney 2014).
Adoption of a communication tool for patients in emergency situations, providing them with guidance on what information to share with clinical staff.
Healthcare professional‐focused interventions
Team skills‐based programme providing information and training on being more open and reciprocal to enable listening and response to patients' narratives about acute life‐threatening illness.
Training on cultural competence with regards to patients and families speaking up about clinical deterioration and challenging professional diagnosis and decision‐making.
Joint interventions
Hospital‐based training to improve patients' understanding of how and why to activate a patient‐activated critical care outreach service (Vorwerk 2015), together with a staff programme to inform them of their role in encouraging patients and families to speak up about concerns.
How the intervention might work
Interventions designed at the level of individual behaviour change tend to be developed from the fields of psychology and behavioural science (Davis 2012; Schwappach 2009). Interventions draw on social cognitive theory (Bandura 1986); motivational interviewing (Miller 2012); stages of change (Prochaska 1983); the theories of reasoned action (Fishbein 1980) and planned behaviour (Ajzen 1991); and the self‐regulatory model of health and illness (Leventhal 1998). These theories focus on the importance of self‐control and empowerment. In this context, interventions aim to build on patients’ and families’ confidence and motivation to become involved, and instil new knowledge and skills for them to know how to contribute to safety (i.e. what signs and symptoms mean, how to self‐monitor, what to do when concerned, what to expect from healthcare professionals). Interventions targeted at changing behaviours of healthcare providers can also aim to address personal values, beliefs and professional goals. Behaviour change initiatives could also target both patients' and staff's communication behaviours using methods such as user‐experience design (Tai‐Seale 2016).
Why it is important to do this review
While there is increasing policy emphasis on patients as co‐producers of safety, there is a paucity of evidence regarding effectiveness of interventions to aid involvement (NPSA 2015). The research that has been conducted is generally of poor methodological quality (Berger 2013; Peat 2010). Concerns have been raised regarding the poor conceptualisation of the intended mechanisms and causal chain in many safety interventions, making it difficult to elicit how and where they are designed to act (Peat 2010).
Currently, notions of ‘expertise’, ‘involvement’ and ‘partnership’ are mostly used in the context of patients with long‐term conditions, and reflect their participation in treatment and care management decisions. It is less clear how these concepts apply to patient involvement in safety, particularly in the context of escalating care during acute life‐threatening episodes of illness. This review is distinct from others that have explored the effectiveness of chronic disease education or management programmes for patients and families (Peytremann‐Bridevaux 2015). It also adds to existing research on patient involvement in safety which has tended to be based in hospital or hospice settings, and has typically focused on error prevention (e.g. prompting staff to wash hands and detecting medication errors) (Doherty 2012).
The review is timely given concerns about poor patient experiences in securing professional response for serious safety concerns and increasing consumer interest in the potential for a greater role in being able to safely escalate care (European Patients' Forum 2017; NFWI‐NCT 2017; Scott 2012; Walton 2016). Existing research and effectiveness reviews on recognition of, and response to, acute life‐threatening illness have tended to focus on interventions for specific conditions e.g. stroke (Lecouturier 2010). This review offers the opportunity to assess commonalities and differences across conditions, settings and interventions. The focus is across the escalation of care pathway, including both community health and hospital settings, in recognition of the difficulties experienced by patients with new onset of a condition negotiating access to emergency care; and patients already in the healthcare system who require a step‐up in care to receive emergency treatment.
Research into the effectiveness of interventions aimed at patient and family involvement in safety has often focused at the patient level rather than at the point of interaction between patients and staff i.e. acknowledging that safety is co‐produced by patients and providers. This review widens the lens to include those interventions targeted at the collaborative local level of interactions between patients, families, and staff. The conceptual model (Figure 1) which underpins this review outlines the complex interactions and factors influencing escalation of care (Craig 2008; Noyes 2016). These include patient, family, professional, relational, sociocultural and system level factors. This review focuses on the micro‐level i.e. interactions between patients and staff, while also acknowledging wider contextual and organisational influences which lie outside its scope.
There is a need to assess unintended consequences of interventions. Involvement in escalation of care may heighten patient and family anxiety, and their feelings of responsibility for safety or the outcomes of treatment, or both (Davis 2012; Entwistle 2005; Warland 2013). Interventions may inappropriately burden families with responsibilities for the safe provision of care that are beyond their abilities and intentions (Johnstone 2009). There may be negative effects on patient‒provider communicative trust (Brown 2008).
This review is related to other Cochrane Reviews focusing on the provision of interventions aimed at enabling patient self‐management for long‐term conditions such as COPD and asthma (Boyd 2009; Howcroft 2016; Tapp 2007; Walters 2010). Our review is distinct in that it specifically looks at patient and family contributions to diagnosis and response once in contact with health professionals such as the GP or emergency services, for an exacerbation of the long‐term condition. It also relates to the Dwamena 2012 review which investigated the effects of interventions for healthcare providers that aimed to promote a patient‐centred approach in clinical consultations. Their review is linked in that it focused on behaviours that reflect a philosophy of care that encourages shared control of the consultation, decisions about interventions or management of the health problems with the patient.
Objectives
To assess the effects of interventions designed to increase patient and family involvement in escalation of care for acute life‐threatening illness on patient and family outcomes, treatment outcomes, clinical outcomes, patient and family experience and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐randomised controlled trials only as this is an effectiveness review and randomisation is the only way to prevent systematic differences between baseline characteristics of participants in different intervention groups in terms of both known and unknown (or unmeasured) confounders.
Types of participants
All patients (adults aged 18 or over) and family members with the potential to contribute to timely response for acute deterioration in the context of a life‐threatening illness were included. No exclusions were made based on gender, ethnicity, or specific condition.
'Family' was defined as parents, relatives, partners, friends or caregivers who were able to act as ‘close as kin’ in order to recognise changes in patients’ conditions and seek help on patients’ behalf.
We included interventions if they targeted individuals or groups e.g. ethnic minority groups or specific subcategories, e.g. parents, the elderly and pregnant women.
The review included interventions designed for patients and families in community health and hospital settings, in both low‐ and high‐income countries. This included community health centres, medical practices, emergency departments, clinics and wards.
We excluded interventions that were targeted at lay health workers (paid or voluntary) including community health workers, village health workers and birth attendants. We defined 'lay health worker' as any health worker who: (1) assists with diagnosis of, referral to and securing of professional help for patients with life‐threatening conditions; (2) is trained in some way in the context of the intervention but has received no formal professional or paraprofessional certificate or tertiary education degree.
We included interventions that were aimed at enabling professionals to engage effectively with patients and families when they sought help or spoke up about concerns. 'Professionals' were defined as those who undertake remunerated work for which formal tertiary education is required, e.g. nurse aides, medical assistants, physician assistants, paramedical workers in emergency services, and other self‐defined health professionals or health paraprofessionals. We excluded trainees of any of the professions or paraprofessions listed above.
We defined 'acute life‐threatening illnesses' as ‘time‐critical’ serious illnesses where avoidance of death is reliant on early detection and instigation of appropriate management. These conditions involve threats to a patient's life, imminent risk of clinical deterioration, or potential to progress to a serious problem. They require aggressive, rapid clinical intervention accessed via urgent or emergency care. This review focused on those physical illnesses where there is scope for patients and families to contribute to the process of securing a rapid response, for example stroke, myocardial infarction, pre‐eclampsia, reduced fetal movements, sepsis and meningitis. Interventions escalating care for seizures in epilepsy or anaphylaxis in allergy were included as well as previously undiagnosed conditions such as new‐onset asthma.
We excluded mental health conditions because of the additional problems presented by serious mental health conditions in terms of patients’ capacity to act and contribute to escalation of care
We excluded interventions that were solely aimed at enabling patients to self‐manage chronic long‐term conditions such as asthma unless the interventions included an identifiable focus on working with staff to ensure timely response to an acute life‐threatening deterioration in condition.
Types of interventions
We had originally aimed to evaluate any intervention (informative, educational, behavioural) intended to improve patients' and families’ ability to participate in escalating care for a life‐threatening illness. In the review process, we clarified distinctions regarding the interactional quality of help‐seeking and selected a focus on those interventions that aimed to affect interactions between patients/families and healthcare professionals.
We included interventions aimed at patients and families as well as those aimed at healthcare professionals. The interventions were designed at individual or group level. The interventions included access to informational resources, oral presentations, one‐on‐one or group classes or seminars, or skills‐based workshops. The interventions could take place at a single time point or involve a short series of events (e.g. a set of workshops).
We defined 'patient and family involvement in escalation of care' as working with healthcare professionals to ensure care received for acute deterioration is timely and appropriate, including raising concerns about diagnosis, treatment and management.
Studies were included if an intervention aimed to do any of the following: increase knowledge in patients, their family, or both, about what signs and symptoms of acute life‐threatening illness to report to health professionals, why and how, and what care or treatment to expect from health professionals; aid patient and/or family motivation and behavioural intent to work with health professionals; increase patient's or their family's ability to act, including speaking up about concerns about deterioration in a patient’s condition and care decisions; or to increase staff motivation, capability and ability to listen and respond to patients' and families' concerns.
We included the following comparisons.
Interventions to promote patient and family escalation of care versus no intervention.
Interventions to promote patient and family escalation of care versus standard or usual care; i.e. where active involvement of patients and families in escalation of care for acute life‐threatening conditions was not explicitly attempted.
Types of outcome measures
Outcomes related to patients and family members, healthcare professionals, and health service use. The listed outcomes were not used as criteria for including studies. From those outcomes originally listed in the protocol, we added 'attitudes and beliefs' as an extra patient and family outcome, and broadened 'behavioural intent' to 'behaviours' to include care‐seeking behaviours and behavioural intent (motivation to take on an active role in escalation of care) as our team recognised their significance for escalation of care. See Figure 1 for the conceptual model underpinning the review (showing only primary outcomes). In the case of studies that reported more than one outcome within each of the groupings (e.g. patient and family outcomes; treatment outcomes; clinical outcomes), we had originally intended for two authors to independently list the outcomes for the trial (without considering either the size of the effect or its statistical significance) and make a decision about which one was most ‘clinically’ important. We found this to be restrictive given the paucity of reported outcomes within the groupings in our included papers, so expanded this to include those outcomes deemed 'clinically important' within each grouping rather than limit ourselves to one outcome. Where we made a selection, we described the selection process clearly, including the need for involvement of a third author for further discussion and decision.
Primary outcomes
Patients or family members, or both
-
Patient and family outcomes: changes in capabilities to negotiate access to care and escalate care, measured by self‐reports or observations, captured by the following potential outcomes.
Knowledge: knowledge of danger signs and appropriate care‐seeking behaviours.
Attitudes and beliefs: attitudes and beliefs about condition and help‐seeking.
Behaviour: care‐seeking behaviours and behavioural intent (motivation to take an active role in escalation of care).
Willingness to participate: willingness to raise concerns and escalate care.
Self‐efficacy: confidence in one's own ability to self‐diagnose, seek help and work with staff to secure professional help.
Skills acquisition: skills in reporting changes in condition, asking for professional help and working with professionals.
-
Treatment outcomes: timeliness, appropriateness and effectiveness of response, measured by self‐reports or proxy reports (professionals’ or family members’) captured by the following outcomes.
Time from start of symptoms to delivery of professional treatment.
Appropriateness and effectiveness of treatment given.
-
Clinical outcomes.
Mortality, measured by mortality rates including failure‐to‐rescue rates (patient death following postoperative complications).
Morbidity, burden associated with delayed recognition and treatment of condition: measured by objective measures e.g. number of events; or presence of and severity of symptoms e.g. heart failure after acute myocardial infarction or disability after stroke.
-
Patient and family experience: measured by self‐reports captured by the following measures.
Perceptions of safety and trust in care providers.
Perceptions of involvement in escalation of care.
Perceptions of timeliness and appropriateness of healthcare professionals’ response (including being given opportunities to share concerns and help with escalation of care).
Satisfaction with healthcare professionals’ response.
Satisfaction with care received.
-
Adverse events.
Patient harms: any reports of harms or adverse events associated with patient and family involvement in escalation of care.
Patient complaints: any complaints related to delayed recognition and treatment of condition.
Secondary outcomes
Patients or family members, or both
Receptiveness to, and acceptability of, intervention to patients and families: measured by self‐reports.
Healthcare professionals
Healthcare professionals' psychological well‐being and capability/capacity to respond to patient and family concerns: measured by self‐reports (e.g. empathy, self‐compassion, self‐efficacy, communication with patients).
-
Healthcare professionals' experience of clinical encounter: measured by self‐reports captured by the following potential measures.
Healthcare professionals' experience of patient and family contribution to safety.
Healthcare professionals' satisfaction with patient and family involvement.
Receptiveness to, and acceptability of, intervention to healthcare professionals: measured by self‐reports.
Service use
Attendance and use of healthcare services: measured by call‐outs, attendance, admission and readmission rates e.g. emergency services, GP surgeries, clinics, emergency departments, critical care.
We included validated measures where possible. Non‐validated measures were recorded but excluded from the meta‐analysis.
The outcomes listed above are broad categories. Two authors independently assigned the outcomes reported in each included study to the review’s outcome categories and resolved any differences in categorisation by the involvement of a third author.
We pooled outcome data from studies examining different clinical conditions providing they considered similar constructs, e.g. changes in knowledge, even if the measures were slightly different. We reported on those constructs that were very different or measured in very different ways narratively and did not include them in the meta‐analysis.
Timing of outcome assessment
We originally intended to group the outcomes into short‐term (less than 3 months), medium‐term (3 to 12 months) and long‐term (more than one year) but, given the few included studies, we reported only the final outcome measures. Longer‐term follow‐up is more likely to be clinically relevant.
Main outcomes for summary of findings table
We prepared a 'Summary of findings' table and reported results for the following primary outcomes which we decided were the most significant for assessing patient and family involvement in escalation of care. We provided a source and rationale for each assumed risk cited in the tables, and used the GRADE system to rank the quality of the evidence (Schünemann 2011).
Patient and family knowledge of danger signs and appropriate care‐seeking behaviours (outcome 1a).
Patient and family self‐efficacy (confidence in one's own ability to self‐diagnose, seek help and work with staff to secure professional help) (outcome 1d).
Time from start of symptoms to delivery of professional treatment (outcome 2a).
Mortality, measured by mortality rates including failure‐to‐rescue rates (patient death following postoperative complications) (outcome 3a).
Patient and family perceptions of involvement in escalation of care (outcome 4b).
Patient and family satisfaction with care received (outcome 4e).
Patient harms (reports of harms or adverse events associated with patient and family involvement in escalation of care) (outcome 5a).
Search methods for identification of studies
See the Cochrane Handbook chapter 4.5 and chapter 6.
Electronic searches
The PubMed Medline search was run on 16 August 2017 for all years. This strategy was updated and translated to all the following electronic databases with more targeted strategies. The strategies were informed by the included references from the PubMed search. This second and more comprehensive update search was undertaken on 24 August 2018. All the searches were updated on 21 October 2019.
We initially searched PubMed Medline in 2017 from inception and the following electronic databases for updates in 2018 and 2019.
The Cochrane Central Register of Controlled Trials (EBM Reviews OVID)
MEDLINE (Pubmed and OvidSP) (2000 to present)
Embase (OvidSP) (2000 to present)
PsycINFO (OvidSP) (2000 to present)
ClinicalTrials.gov (2000 to present)
World Health Organization (WHO) International Clinical Trials Registry Platform (2000 to present)
World of Science: forward citations for chosen included references to present
The search strategies can be found in Appendix 1. We tailored strategies to other databases and reported them in the review. There were no language restrictions. We restricted searches from 1 Jan 2000, the year that 'To Err is Human' was published (Kohn 2000) as this marked the start of heightened awareness of patient safety in healthcare.
Searching other resources
We searched relevant grey literature sources such as the Dissertations and Theses database, OpenGREY and The Grey Literature Report as well as relevant conference proceedings.
We contacted experts in the field, our advisory group and authors of included studies for advice as to other relevant studies. We also searched reference lists of included studies and relevant systematic reviews.
We also searched online trial registers (ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform) for ongoing and recently completed studies.
Data collection and analysis
We applied the Cochrane RCT Classifier to the search results. The Classifier assigned a probability (from 0 to 100) to each citation for being a true randomised trial. Citations with the classifier scores of nine or less were excluded from further consideration. Citations that scored between 10 to 100 were reduced further by excluding from consideration those citations that had already been assessed by Cochrane Crowd as not being reports of RCTs. Two authors independently screened the remaining citations for potential inclusion.
Selection of studies
Seven of the eight authors were involved in screening (NM, RD, AE, HRJ, MA, SW, JS), ensuring that at least two authors independently screened all titles and abstracts identified from the searches to determine which met the inclusion criteria. We retrieved in full text any papers identified as potentially relevant by at least one author. Two review authors independently screened full‐text articles for inclusion or exclusion, with discrepancies resolved by discussion and by consulting other team members if necessary to reach consensus.
During the screening we further operationalised the term 'involvement in escalation of care' and refined the selection criteria to help with screening. As the focus of our review was on collaborative local level of interactions between patients, families, and staff, we chose to exclude studies that focused solely on provision of patient information about condition‐specific red flags and only included studies that specified the inclusion of a relational, dialogic element to the intervention. Therefore, for those studies that designed interventions directed at patients/families, a key criterion was to include an interactive element of rehearsal, role play, modelling, shared language, group work etc. to the intervention to help patients and families have agency in the process of escalation of care. We also included studies where the intervention included a component targeted at enabling staff response recognising this important element to the dialogic process of escalation of care.
We listed all potentially relevant papers excluded from the review as 'excluded studies', with reasons provided in the ‘Characteristics of excluded studies’ table. We also collated and reported details of duplicate publications, so that each study (rather than each report) was the unit of interest in the review. We reported the screening and selection process in an adapted PRISMA flow chart (Liberati 2009) (Figure 2).
2.
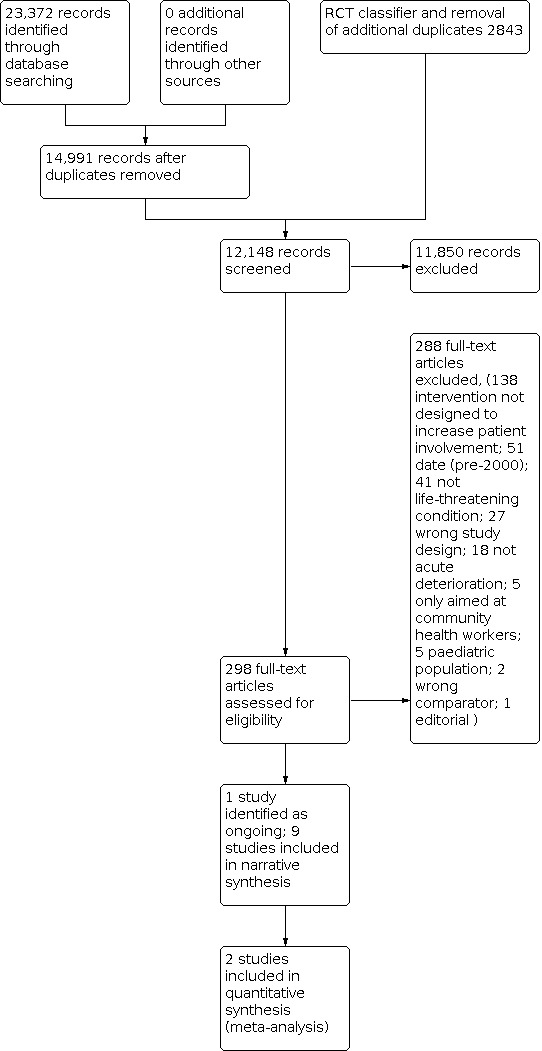
Study flow diagram.
Data extraction and management
Two review authors extracted data independently from the included studies. They resolved any discrepancies by discussion until consensus was reached, or through consultation with a third author where necessary. We developed and piloted a data extraction form using the Cochrane Consumers and Communication Group Data Extraction Template (available at cccrg.cochrane.org/author-resources).
Methods
We extracted data about the study design, the methods of recruitment of participants, the inclusion and exclusion criteria for participants, information on funding of the study, declaration of interests for the primary investigators, statistical methods used and consumer involvement. We assessed the risk of bias of included studies as described below (see Assessment of risk of bias in included studies).
Participant characteristics
From each study, we recorded the following information: description of participants (patients and/or family members), number of participants, age, gender, ethnicity and life‐threatening condition. We recorded the following information on the study: setting (community health or hospital), income of the country (high, middle or low).
Intervention
We used TIDieR (Template for Intervention DescrIption and Replication) guidelines for describing interventions in the included studies (Hoffmann 2014; Table 2). We recorded rationale and content; description of intervention and intervention components; mode of delivery; type of provider; location/context; intervention level (individual, group, patient and provider); dose; tailoring and fidelity; and description of comparison group. We reported whether the interventions and control treatments were described in sufficient detail to replicate, to investigate most relevant causal factors, and to report these factors.
1. Included studies and interventions.
| Study ID | Country | Condition | No of participants | Framing of the problem | Intervention | Measured by |
| Boden Albala 2015 | US | Stroke | 1193 patients with ischaemic stroke or TIA diagnosis | Inadequate lay knowledge and limited lay preparedness competency to respond to stroke as an emergency and recognise symptoms | Face‐to‐face, bilingual group educational sessions using interactive methods, plus a physician available for clinical queries, with stroke patients to increase preparedness for a future stroke, delivered largely on the ward but sometimes at a clinic or home compared to usual care (bilingual information only) Delivered by: Bilingual (English/Spanish) intervention team (two health educators and a physician on call) |
Proportion of acute stroke arrivals to the ED under 3 hours |
| Buckley 2007 | Australia | Coronary heart disease | 200 adult patients with diagnosis of coronary heart disease | Patient‐related delay in appraisal of symptoms of acute myocardial infarction (heart attack), decision to act and alert emergency help | Face‐to‐face, interactive educational session to prepare coronary artery disease patients for a future myocardial infarction, plus action plan, delivered in outpatients department following discharge, plus follow‐up phone call for reinforcement compared to usual care Delivered by: Researcher and cardiac nurse |
Changes in knowledge, attitudes and beliefs about AMI symptoms and appropriate responses to symptoms |
| Dracup 2009 | US, Australia, New Zealand | Coronary heart disease | 3522 adult patients with diagnosis of coronary heart disease | Patient‐related delay in appraisal of symptoms of acute coronary syndrome, decision to act and alert emergency help | Face‐to‐face individual interactive educational session for patients with coronary heart disease and family members to be better prepared for a future myocardial infarction, delivered in outpatients department, clinic or at home following discharge, plus follow‐up phone call for reinforcement compared to usual care Delivered by: Cardiology nurse |
Time from ACS symptom onset to arrival at the emergency department |
| Horn 2014 | US | Asthma | 150 caregivers of children with asthma | Open and active communication and partnerships are needed between healthcare providers and patients to reduce asthma morbidity and mortality rates. | Face‐to‐face, group educational sessions focusing on communication for parents/guardians of young children with asthma to empower them to share information with providers about the child’s asthma, using interactive methods, in outpatients or clinic, plus a follow‐up call and communication toolkit compared to a consult, asthma education but no communication education Delivered by: Asthma educator and a physician or nurse practitioner |
Greater self‐efficacy in communicating with PCPs, more reliance on PCPs for asthma care and fewer asthma‐related ED and urgent care visits relative to usual care |
| Luepker 2000 | US | Coronary heart disease | 20,364 (patients) Staff unspecified |
Patient‐related delay in appraisal of symptoms of acute myocardial infarction (AMI), decision to act and alert emergency help | Multi‐component education programme to prepare clinicians, patients and the public to respond quickly in the event of heart attacks, by: (1) Clinical staff education using various methods; (2) general public with communication via mass or local media; (3) people with coronary heart disease attending face‐to‐face, individual and group educational sessions using interactive methods in emergency departments and outpatients plus a range of impersonal communication and reminder strategies at community‐clinic level. Comparison was a matched community group. Delivered by: Physicians (patients), intervention team (staff) |
Time from symptom onset to hospital presentation and emergency medical service use |
| Mooney 2014 | US | Coronary heart disease | 1944 adult patients with diagnosis of coronary heart disease | Patient‐related delay in appraisal of symptoms of acute coronary syndrome, decision to act and alert emergency help | Face‐to‐face, educational session for individual patients with coronary artery disease (and some family members) to prepare them for the event of an acute coronary event, delivered on the ward using interactive methods, decision‐support tools, and follow‐up phone call for reinforcement compared with usual care (standard pre‐discharge education) Delivered by: Research nurse |
Time from symptom onset to hospital presentation and conformity to recommended help‐seeking behaviours |
| Norman 2018 | UK and Ireland | Fetal health during pregnancy | 409,175 pregnant women from 37 participating maternity sites Staff numbers unspecified |
Reduced fetal movement (RFM) may offer potential as an alert to prompt action and improve outcome. Women‐related delays in reporting RFM to maternity care providers and variability in staff response may increase the risk of adverse outcome. | Multi‐component education programme to reduce stillbirth by facilitating a prompt response to reduced fetal movement, aimed at pregnant women and clinicians, comprising: (1) e‐learning package for clinical staff; (2) ward‐based training; (3) leaflets for women plus posters in ward; (4) management plan and care protocols for reduced fetal movement, compared to usual care Delivered by: Midwives (women), educators (staff) |
Reduction in stillbirth |
| Schumacher 2017 | US | Mixed (chronically ill, older ED patients with limited health literacy) | 69 older, chronically ill patients with limited health literacy insured by Medicare, scheduled for ED discharge | Those with limited health literacy represent a particularly high‐risk group who are often under‐engaged in managing their health and frequently turn to the ED for care. | Post‐discharge, education/coaching session to improve patient engagement and manage discharge from emergency department to home with chronically ill, older patients with limited health literacy, delivered in their home, plus follow‐up phone calls, compared to usual care (written plus verbal discharge information) Delivered by: Community coaches |
Patient engagement in health and follow‐up ED use |
| See 2014 | Singapore | Mixed | 67 adults (over 21 years) hospitalised for one or more acute medical conditions | Although patients can help in early recognition of and response to deterioration, many patients are unlikely to verbalise their changes in condition. | Face‐to‐face interactive education plus supportive materials to enable patients to recognise and report acute deteriorating conditions, delivered bedside to patients in general medical/surgical wards compared to usual care Delivered by: Nurse researcher |
Level of self‐efficacy to recognise and report symptoms |
ACS: Acute coronary syndrome AMI: Acute myocardial infarction ED: Emergency department PCP: Primary care provider RFM: Reduced fetal movement TIA: Transient ischaemic attack
Outcomes
We listed all primary and secondary outcomes reported in each included study and described how they were assessed. We reported on the timing of follow‐up. Our analyses were confined to those outcomes selected a priori as described in Types of outcome measures.
All extracted data were entered into Review Manager 5 (Revman 5) by one review author, and were checked for accuracy against the data extraction sheets by a second review author working independently (Review Manager 2014).
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the guidelines of the Cochrane Consumers and Communication Review Group (Ryan 2011), which recommends the explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; and selective outcome reporting. We considered blinding separately for different outcomes, where appropriate (for example, blinding may have the potential to differently affect subjective versus objective outcome measures). For cluster‐RCTs, we assessed and reported the risk of bias associated with an additional domain: selective recruitment of cluster participants. Other sources of bias included baseline imbalances for both individual and cluster‐RCTs and comparability with individually randomised trials for cluster‐RCTs. We judged each item as being at high, low or unclear risk of bias as set out in the criteria provided by Higgins 2011, and provided a quote from the study report and a justification for our judgement for each item in the 'Risk of bias' table.
Studies were deemed to be at the highest risk of bias if they scored as being at unclear risk of bias for the sequence generation domain, or at high or unclear risk of bias for the allocation concealment domain, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011). We therefore excluded all studies rated at a high risk of bias for the random sequence generation item of the 'Risk of bias' tool, since these studies are categorised as quasi‐RCTs (Higgins 2011).
In all cases, two authors independently assessed the risk of bias of included studies, with any disagreements resolved by discussion to reach consensus. We contacted study authors for additional information about the included studies as required. We incorporated the results of the 'Risk of bias' assessment into the review through standard tables, and systematic narrative description and commentary about each of the elements, leading to an overall assessment of the risk of bias of included studies and a judgement about the internal validity of the review’s results.
Measures of treatment effect
Data reported in included studies that was suitable for combining in meta‐analyses were limited, but meta‐analyses were carried out for three outcomes. These outcomes were all continuous, and we analysed data based on the difference in the mean score at follow‐up, between the intervention and control groups. Where a standard deviation of the change in score from baseline to follow‐up was not reported, this was estimated as the square root of the sum of the separate variances at baseline and follow‐up, conservatively assuming no covariance (Altman 1990). Due to between study heterogeneity, random‐effects models were fitted.
Unit of analysis issues
The analysis took into account the level at which randomisation occurred. Inclusion of cluster‐randomised trials leads to potential unit of analysis problems. Whenever an adjusted (for clustering) effect was reported, we extracted this for inclusion in the review. None of our three cluster‐RCTs were included in our meta‐analyses.
Dealing with missing data
We contacted authors from one of the studies to obtain missing data (the mean scores (SD) for knowledge, attitudes and belief for both groups at each time point). Data were analysed as reported. We reported on the levels of loss to follow‐up and assessed this as a source of potential bias.
Assessment of heterogeneity
We anticipated heterogeneity in terms of intervention modalities, life‐threatening conditions, populations, settings, degree of bias, outcome measures and timing of outcome assessment. We explored qualitatively the degree of heterogeneity between the included studies. Where studies were considered sufficiently similar, based on an assessment of the above factors, to allow pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visual inspection of forest plots and using the Chi² test for heterogeneity. We quantified heterogeneity using the I² statistic, interpreting an I² value of 50% or more as representing a substantial level of heterogeneity. We interpreted the I² value in light of the size and direction of effects and the strength of evidence for heterogeneity based on the P value from the Chi² test and number of contributing studies (Higgins 2011).
We had intended, where heterogeneity was present in pooled effect estimates, to explore possible reasons for variability by conducting subgroup analysis, but this was not feasible due to small numbers of studies included in the meta‐analyses.
Assessment of reporting biases
We did not assess publication bias by use of funnel plots because we had too few studies to do so. We assessed reporting bias qualitatively based on the characteristics of the included studies (e.g. if only small studies that indicated positive findings were identified for inclusion), or where authors indicated that there were relevant unpublished studies.
Data synthesis
We decided whether to meta‐analyse data based on whether the interventions in the included trials were similar enough in terms of participants, settings, intervention, comparison and outcome measures to ensure meaningful conclusions from a statistically pooled result. Due to the anticipated variability in the populations, settings and interventions of included studies, we used a random‐effects model for meta‐analysis.
Where we were unable to pool the data statistically using meta‐analysis, we conducted a narrative synthesis of results.
Subgroup analysis and investigation of heterogeneity
We intended to conduct three subgroup analyses.
Setting (high‐income countries versus low‐ and middle‐income countries as defined by the World Bank (World Bank 2016)): due to differences in infrastructure such as transportation and health facility, and access/care pathways.
Focus of intervention (patient/family, healthcare professional, relational including both patient and staff).
Content (addressing knowledge, attitude or skills).
We were unable to carry out subgroup analyses on focus of the intervention and content due to a lack of studies. Only high‐income studies were included which is likely to reflect intervention design differences (interventions focused on low and middle‐income settings tend to be focused more at community level than at individual level and were therefore excluded).
Sensitivity analysis
We intended to conduct sensitivity analyses with studies restricted to those at low risk of bias, and grouped according to condition but this was not feasible due to small numbers of included studies. Sensitivity analysis was also planned but not done to assess the effects of any imputed data on pooled effect estimates. We assessed the impact of the inclusion of high/low‐quality studies in the review (see Risk of bias in included studies) and these judgements were used as an input to GRADE ratings of certainty of results.
Summary of findings table
We prepared a 'Summary of findings' table to present the results of analysis, based on the methods described in chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). Where possible, we presented the results of meta‐analysis for the major comparisons of the review, for each of the major primary outcomes, including potential harms, as outlined in the ‘Types of outcome measures’ section. Two members of the team used the GRADE system to rank the quality of the evidence (Ryan 2016). If meta‐analysis was not possible, we presented results in a narrative 'Summary of findings' table format.
Ensuring relevance to decisions in health care
We established an advisory group early on in the review process, to ensure that the review was of relevance and could inform policy, planners, providers and service users. We convened a group of eight stakeholders (four users and four providers). These included Carolyn Canfield (independent citizen‐patient), Helen Haskell (Mothers Against Medical Error), Tommy's baby charity, and Sands (Stillbirth And Neonatal Death charity); and four academics with expertise in patient involvement in safety (Sarah Neill, Rebecca Lawton, David Schwappach) and global health (Rohit Ramaswamy).
We held two teleconferences which were structured around 1) protocol development in terms of its scope, outcomes; and 2) findings/analysis. We circulated the draft review for comment and invited our advisory group to contribute to our key conclusions and dissemination plan.
In addition, the protocol and review received feedback from at least one consumer referee in addition to a health professional as part of Cochrane Consumers and Communication’s standard editorial processes.
Results
Description of studies
We restricted the search to randomised controlled trials and cluster‐randomised trials evaluating interventions designed to increase patient and family involvement in escalation of care for life‐threatening illness in community and hospital settings.
Results of the search
We deliberately kept our search strategy broad with our first PubMed Medline search in order to retrieve papers inclusive of different conditions, populations and settings. We then used the results of this first screening to refine subsequent searches in Medline and other databases. Our search strategy retrieved in total 23,372 references, which were reduced to 14,991 references after deduplication. Following the removal of duplicates and RCT classification, we screened a total of 12,148 abstracts for eligibility and excluded 11,850 of these. References excluded by the RCT classifier were not screened in the first search but were screened in the update search. We obtained full‐text articles for 298 abstracts and assessed these for inclusion in the review. We excluded 288 articles following full‐text analysis and team discussion. During the screening and selection process, we further developed our operational understanding of 'involvement in escalation of care'. We made the two‐way nature of involvement a key criterion for inclusion. We therefore made an interactive element of rehearsal, role play, modelling, shared language, group work etc. a key criterion for inclusion for the interventions directed at patients/families. We also looked for intervention components that reinforced and legitimised patients’ expertise in diagnosis, or provided training in communication to help patients address power differences and know how to work with staff, or provided skills training for staff to listen to patients’ concerns.
Included studies
The included studies aimed to increase patient and (sometimes) family preparedness for acute life‐threatening conditions and/or engagement in escalation of care. Some also attempted to increase patient willingness to speak up and also staff receptivity. We included nine studies and one ongoing study (see Figure 2). Of the nine published studies, six were randomised controlled trials (RCTs) (Boden Albala 2015, Buckley 2007, Dracup 2009, Horn 2014, Mooney 2014, Schumacher 2017), two were cluster trials (Luepker 2000, See 2014) and one used a stepped wedge design (Norman 2018). The ongoing study is an RCT (Mi 2018). The included studies had high heterogeneity in terms of settings, populations and life‐threatening conditions that were the focus of the interventions, the content of the interventions and the outcomes measured. Two of the studies were comparable in terms of the construct measured (knowledge and attitudes and beliefs about coronary heart disease) which enabled a meta‐analysis of the data (Buckley 2007; Dracup 2009). However, the remaining constructs measured across the studies (e.g. self‐efficacy, stroke preparedness, patient activation) were sufficiently heterogenous to prevent us combining data across studies. We therefore reported the findings narratively (descriptively) in the text of the review. Details of the studies and the interventions are provided in the table of Characteristics of included studies and Characteristics of ongoing studies and summarised in Table 2 and Table 3. Only studies looking at one of the two comparisons sought were identified (i.e. versus standard or usual care); we did not identify any studies comparing interventions against no intervention.
2. Degree of intensity and duration of the intervention.
| Study ID | Degree of intensity | Timing | Duration | Mode of delivery | Tools/techniques |
| Boden Albala 2015 | 2 group sessions | Within 3 weeks after the stroke/TIA onset, and in most cases within the initial hospitalisation stay | Not specified | Group face‐to‐face | Discussions, role play, video material, motivational interviewing |
| Buckley 2007 | 1 individual session plus 1 reinforcement phone call | Session within 6 months of discharge diagnosis Phone call within 4 weeks of session |
40‐ to 50‐min (1st session) Approx. 15 mins (phone call) |
Individual face‐to‐face and telephone | Core script, advisory sheet, action plan |
| Dracup 2009 | 1 individual session plus 1 reinforcement phone call | Sessions either organised via letters/contact with community health settings or post‐discharge Phone call within 4 weeks of session |
Approx. 40 mins (1st session) Approx. 15 mins (phone call) |
Individual face‐to‐face and telephone | Core script, advisory sheet |
| Horn 2014 | 1 individual session plus 1 booster call | Session within 2 weeks of an ED visit or hospitalisation Phone call within 4 weeks after the initial session |
30‐minute session Phone call duration not specified |
Individual face‐to‐face and telephone | Role play, visual aid, wallet sized card, communication toolkit |
| Luepker 2000 | Multi‐component including community, provider and patient education | Sequencing of components. Professional education begun early to inform providers, and to provide a basis for later patient education. Specific timings not detailed | Not specified | Individual face‐to‐face and group | Patient education (counselling, role‐modelling, behavioural rehearsal, flyers/brochures, posters, magnets and video) Staff education (academic detailing, seminars and continuing medical education programmes) |
| Mooney 2014 | 1 individual session, 1 reinforcement phone call plus reminder letter | Session during hospitalisation Phone call after 4 weeks of intervention delivery Contacted (by letter) 6 months later |
40 min (1st session) Phone call duration not specified |
Individual face‐to‐face, telephone and letter | Role play, script, action plan, symptom card, motivational coaching, fridge magnet |
| Norman 2018 | Administration of a leaflet to women; e‐learning package and training for staff |
Leaflet given to women at 20 weeks gestation A link to the staff e‐learning package was emailed about 1 month before intervention initiation. |
How leaflet incorporated into antenatal education not specified. Staff web‐based training package took approx 1 hour Duration of staff training not specified |
Leaflet (women) Individual e‐learning and group training (staff) |
Leaflet for women Staff e‐learning education package, staff training, posters, protocols, management plans |
| Schumacher 2017 | 1 session and 3 follow‐up phone calls | Session within 3 days of ED discharge Phone calls scheduled within month of discharge |
Visit 60 minutes on average Phone calls 15 minutes per call on average |
Individual face‐to‐face, telephone | Personal Health Record, transition strategies |
| See 2014 | 1 session | Within 3 days of admission (possibly on day of admission?) | 30‐minute session | Individual face‐to‐face | Mnemonic handout |
ED: Emergency department TIA: Transient ischaemic attack
Setting
Five of the included studies were conducted in the USA (Boden Albala 2015, Horn 2014, Luepker 2000, Mooney 2014, Schumacher 2017), with one study each in Australia (Buckley 2007), the UK and Ireland (Norman 2018), and Singapore (See 2014), and one based across the USA, Australia and New Zealand (Dracup 2009). With the exception of See 2014, the studies focused on enabling escalation of care from home into community health or hospital settings. The See 2014 study focused on facilitating escalation of care within hospital settings (medical and surgical wards).
Participants
The size of the studies varied, ranging from 67 (See 2014) to 409,175 participants (Norman 2018) and included 436,684 patients and family members in total. Five of the studies randomised at the patient level (Boden Albala 2015, Buckley 2007, Dracup 2009, Mooney 2014, Schumacher 2017), or at the caregiver level (Horn 2014), while one study each randomised at community level (Luepker 2000), maternity unit level (Norman 2018) or acute ward level (See 2014). Six of the studies (Boden Albala 2015, Buckley 2007, Dracup 2009, Horn 2014, Luepker 2000, Mooney 2014) targeted patients already diagnosed with specific conditions, which had the potential to be potentially life‐threatening. These included conditions such as coronary artery disease (Buckley 2007, Dracup 2009, Luepker 2000, Mooney 2014), ischaemic stroke or TIA (transient ischaemic attack) (Boden Albala 2015), and asthma (Horn 2014). The Norman 2018 study recruited pregnant women, focusing on fetal movement as a potential indicator of fetal well‐being also linked to fetal growth restriction, placental abnormalities and stillbirth. See 2014 targeted inpatients on medical‐surgical wards, recognising the potential for clinical deterioration in a variety of different patients' conditions whilst in hospital. Schumacher 2017 focused at the service engagement level and targeted high‐risk patients with a history of poor self‐management. Horn 2014 focused on parents of children aged 1 to 12 years old (57% male; 43% female) with asthma.
All the studies recruited patients already in contact with hospital services either with an established diagnosis e.g. stroke (Boden Albala 2015), coronary heart disease (Buckley 2007, Dracup 2009, Luepker 2000, Mooney 2014), or asthma (Horn 2014) or on account of being an older, chronically ill patient using emergency services (Schumacher 2017), or an inpatient on a medical/surgical ward (See 2014) or on account of being pregnant (Norman 2018). Patients were recruited in hospital (Boden Albala 2015, See 2014), after discharge (Buckley 2007), via cardiovascular, cardiac catheterisation units, and cardiac rehabilitation programmes (Dracup 2009), coronary units or cardiology wards (Mooney 2014), outpatient clinics (Dracup 2009, Horn 2014, Luepker 2000, Norman 2018), emergency departments (Luepker 2000, Schumacher 2017) and community medical practices (Dracup 2009, Luepker 2000).
Two studies had participants consisting mainly of people from low‐income groups (Horn 2014; Schumacher 2017). One study focused on low‐income, minority children in Washington, DC (Horn 2014) while the other (Schumacher 2017) focused on chronically ill older ED (emergency department) patients with limited health literacy and Medicare as a payer source.
Three studies recognised the importance of family members in escalation of care and included them in the intervention (Dracup 2009; Luepker 2000; Mooney 2014). One study also included community‐level interventions (Luepker 2000).
Two of the studies (Luepker 2000; Norman 2018) included staff as participants. Luepker 2000 included a staff education programme aimed at primary care physicians, cardiologists, and emergency medicine physicians, ED, inpatient, and outpatient nurses, pharmacists, rehabilitation staff, emergency department staff and ambulance staff. Norman 2018 included strategies at each site to encourage clinicians (doctors, midwives and ultrasonographers) to increase pregnant women’s awareness of fetal movement and to adhere to a management plan for identification and delivery of the ‘at risk’ fetus in such women. However, neither study reported implementation data on reach (how many staff received the information or attended the training).
The heterogeneity in the studies' participants demonstrates how patient and family involvement in escalation of care for life‐threatening illness has been conceptualised differently across conditions and populations (see Figure 3).
3.
Structural and social influences and discourses linked to escalation of care
Interventions
While all studies involved interventions aimed at increasing patient and family involvement in escalation of care, there was significant variation in the way this was operationalised, in terms of underpinning theories of change and in the tools and techniques adopted to support the process (Table 2 and Table 3). We present a summary of intervention characteristics, drawing on the TIDieR checklist (Hoffmann 2014), in the table Characteristics of included studies.
Theoretical basis
Six of the interventions were underpinned by behavioural theory and another two were informed by existing interventions and concepts. The majority of studies focused on enabling individual behaviour change drawing on Social Cognitive Theory (Boden Albala 2015; Horn 2014), Leventhal Self‐Regulatory Model of Illness Behaviour (Buckley 2007; Dracup 2009; Mooney 2014), and both Social Cognitive Theory and Self‐Regulatory Model (Luepker 2000). Schumacher 2017 drew on the Care Transitions Intervention (CTI) while See 2014 based their model on concepts of patient safety.
PPI involvement
Two of the studies (Boden Albala 2015; Luepker 2000) reported having patient and public involvement (PPI) in development of the intervention. Boden Albala 2015 included community input on the development of culturally tailored intervention materials. Luepker’s REACT intervention was influenced by qualitative focus groups including adults who either had experienced acute myocardial infarctions (AMI), had risk factors for AMI, or were family members of patients who had had an AMI or had risk factors; participants represented gender, age, and ethnicity groups (African‐American, Hispanic‐American, and white). The PPI group identified two major themes (symptom recognition and timely action) as a focus for patient and/or public education.
Framing of the problem: overuse versus underuse
Six of the studies framed the underlying problem they were addressing in terms of underuse of provider services, principally due to inadequate lay knowledge and preparedness competency to recognise and respond to acute events, such as stroke (Boden Albala 2015), coronary syndrome/acute myocardial infarction (Buckley 2007, Dracup 2009, Luepker 2000, Mooney 2014), or alerts such as reduced fetal movements (Norman 2018). In contrast, See 2014 framed the problem in terms of suboptimal hospital response systems which needed to structure in opportunities for patients to contribute to recognition and reporting of deterioration. The two remaining studies that focused on chronic conditions framed the problem more in terms of overuse or inappropriate use of emergency services (Horn 2014, Schumacher 2017) choosing to focus more on self‐management skills and patient‐provider communication to manage acute exacerbations. (See Figure 3).
Intervention components and strategies
Please see summary overview table (Table 4) which details the range of interventions included and their main features.
3. Summary table of interventions.
| Study | Recipient and aim | Components | Delivery, timing and setting | Comparison |
| Boden Albala 2015 | Stroke patients (to increase preparedness for a future stroke) | Bilingual education sessions (2) with interactive methods Physician for clinical queries |
Face‐to‐face Group Ward; sometimes clinic or home |
Usual care (bilingual information only) |
| Buckley 2007 | Coronary heart disease patients (to prepare for future myocardial infarction) | Interactive education session Action plan Follow‐up phone call for reinforcement |
Face‐to‐face Individual Outpatient clinic post‐discharge |
Usual care |
| Dracup 2009 | Coronary heart disease patients and family members (to prepare for future acute coronary event) | Educational/counselling session Follow‐up phone call for reinforcement |
Face‐to‐face Individual Outpatient clinic or home post‐discharge |
Usual care |
| Horn 2014 | Parents/guardians of young children with asthma (to empower them to communicate with providers about child’s asthma) | Interactive educational sessions Follow‐up call Communication toolkit |
Face‐to‐face Individual Outpatients or clinic |
Usual care (consultation plus asthma education but no communication education) |
| Luepker 2000 | Clinical staff, patients (people with coronary heart disease), public (to prepare them to respond quickly in the event of a myocardial infarction) | Multi‐component education programme Clinical staff education using interpersonal (e.g. academic detailing) and impersonal (e.g. newsletters) methods General public communication via mass or local media Interactive educational sessions (patients) |
Face‐to‐face (patients) Individual and group Emergency and outpatient departments plus impersonal communication/reminders via community‐clinic |
Matched community group |
| Mooney 2014 | Coronary heart disease patients and family members (to prepare for future acute coronary event) | Interactive educational session Decision‐support tools, action plan Follow‐up phone call for reinforcement plus letter |
Face‐to‐face Individual Wards |
Usual care (standard pre‐discharge education) |
| Norman 2018 | Clinical staff, pregnant women (to reduce stillbirth by facilitating prompt response to reduced fetal movements) | Multi‐component education programme E‐learning package for clinical staff plus unit‐based training/information sessions Leaflets for pregnant women plus posters in ward Management plan and care protocols for reduced fetal movement |
Face‐to‐face (women), online (clinical staff) Individual and group Units, wards |
Usual care |
| Schumacher 2017 | Older chronically ill patients with limited health literacy (to improve patient engagement and discharge from ED to home) | Education/coaching session Follow‐up phone calls |
Face‐to‐face Individual Post‐discharge (emergency department) in home |
Usual care (written plus verbal discharge information) |
| See 2014 | Hospitalised patients (to recognise and report acute deteriorating conditions) | Interactive education Mnemonic and use of shared language |
Face‐to‐face Individual Bedside in general medical/surgical wards |
Usual care |
ED: Emergency department
Three studies (Boden Albala 2015, Mooney 2014, See 2014) delivered the intervention on the ward whilst the patients were still inpatients. One study (Norman 2018) targeted both inpatient (for the staff component) and outpatient settings (for the women's component), three (Buckley 2007, Dracup 2009, Horn 2014) used only outpatient settings, and one (Luepker 2000) used both the emergency department and outpatient departments to deliver the intervention. One study (Schumacher 2017) delivered the interventions in patients' homes.
All of the nine studies included components designed to support individual behaviour change, but these were mostly directed at patients/family members while only two of the studies also included a staff element. This selective focus on only one side of ‘two‐way involvement’ highlights how the problem and need to improve patient and family involvement is envisaged. All studies included an explicit focus on patient monitoring of red flag signs and symptoms or in the case of the Norman 2018 study, tracking of fetal movement to enable women to detect changes in patterns. Several of the studies designed their interventions to target multiple factors that could influence patient behaviour, such as skills and competence, beliefs about capabilities, beliefs about consequences, reinforcement, intentions, goals, emotion, social influences and environmental context (e.g. Boden Albala 2015; Dracup 2009; Luepker 2000).Three of the interventions employed one or more principles of patient‐centred counselling, role‐modelling, and behavioural rehearsal (Boden Albala 2015; Dracup 2009; Luepker 2000).
Two of the studies focused on health communication with Horn 2014 providing a communication toolkit which focused on information exchange between parents and providers. See 2014 also included a communication component for patients to learn how to express concerns to staff.
Norman 2018's behaviour change strategy for women was based on a leaflet for pregnant women, distributed to women at about 20 weeks’ gestation as they attended an antenatal visit. The AFFIRM information leaflet was available in 12 languages including: Arabic, Bengali, English, Hindi, Hungarian, Latvian, Lithuanian, Mandarin, Polish, Russian and Urdu (Norman 2018). Details of how the leaflet was incorporated into antenatal visits or distributed to women was not reported.
Five of the studies (Buckley 2007; Dracup 2009, Horn 2014, Mooney 2014, Schumacher 2017) based their interventions around one individual face‐to‐face session (varying from 30 to 60 minutes duration) together with follow‐up reinforcement phone calls, about 15 minutes duration, specified by Buckley 2007, Dracup 2009 and Schumacher 2017. Four of these studies (Buckley 2007; Dracup 2009, Horn 2014, Mooney 2014) scheduled only one follow‐up call whereas Schumacher 2017 included three phone calls. Mooney 2014 also included a reminder letter. Luepker 2000's patient education component included individual and group sessions, and was supplemented by impersonal strategies (flyers/brochures, posters, magnets and other 'tokens', and video) to reach patients and their families. Boden Albala 2015 structured two group sessions (duration unspecified) while See 2014 included one 30‐minute individual face‐to‐face session.
The interventions were delivered by educators and physicians, nurses, midwives, and physicians. Some patient education in the Luepker 2000 study was conducted by REACT staff (interventionists in each community), but providers were relied on to deliver most of the patient education.
Norman 2018's staff interventions consisted of an e‐learning package, training/information sessions and posters to encourage clinicians to ask women about fetal movements in routine antenatal consultations. Luepker 2000's professional education intervention incorporated multiple interpersonal (e.g. academic detailing, continuing medical education programmes) and impersonal (e.g. newsletters, brochures) strategies to improve engagement with patients and enhance the patient‐centred nature of the dialogue.
All the interventions were compared with control or usual care. Four studies provided additional detail about usual care. Boden Albala 2015 noted that their comparison arm received a standardised packet of preparedness‐focused education materials, while usual care in Mooney 2014's study comprised predischarge patient education, and written/verbal discharge instructions and advice to follow up with a provider in Schumacher 2017. Usual care in Horn 2014's study comprised family attendance at a clinic within two weeks of an ED visit or hospitalisation for an acute asthma exacerbation, and a 90‐minute visit with an asthma educator and a physician or nurse practitioner. If randomised into ‘usual care’, participants completed the session without receiving additional education on parent‐provider communication and were discharged after reviewing their child’s individual Asthma Action Plan.
Temporal factors/deterioration trajectory
The studies varied in terms of timing of delivery of the interventions and time lag before they were able to be applied in practice. The See 2014 study scheduled the AWARE intervention soon after admission, aiming for patients to potentially benefit from it for any episodes of deterioration experienced during their hospital stay. In the Norman 2018 study, a link to the e‐learning package was emailed to all clinicians in the participating units about one month before the intended implementation of the package. Information was provided to pregnant women at 20 weeks, leaving a window of use for women during the subsequent 20 weeks of their pregnancies. A management plan for identification and delivery of babies at high risk was distributed to hospitals for management of women who presented with RFM (reduced fetal movement) from 24 weeks’ gestation (Norman 2018). Luepker 2000 employed sequencing of the different components in their intervention which took place over an 18‐month period. The professional education began early to inform providers, and to provide a basis for later patient education. Other studies scheduled their initial individual face‐to‐face sessions either before patient discharge, or within four weeks of discharge or patient attendance at the emergency department visit (Boden Albala 2015, Dracup 2009, Horn 2014, Mooney 2014, Schumacher 2017). Buckley 2007 delivered the first session within six months of discharge diagnosis. Follow‐up calls took place within four weeks of the individual sessions. Mooney 2014 reminder letter was sent out six months later.
Outcomes
The outcome categories in which studies reported outcomes included patient and family outcomes (knowledge of danger signs and appropriate care‐seeking behaviours; attitudes and beliefs regarding condition and appropriate care‐seeking behaviours; care‐seeking behaviours including involvement of family members; willingness to participate; self‐efficacy; receptiveness to, and acceptability of, intervention to patients and families), treatment outcomes (time from start of symptoms to delivery of professional treatment), clinical outcomes (mortality; morbidity/number of events), adverse events (patient harms), and service use (attendance and use of healthcare services). None of the studies reported outcomes related to categories focused on patient and family experience or at the level of healthcare professionals (e.g. capability to respond or experience of patient and family involvement in escalation of care). There were also no data related to acquisition of patient and family skills, or to patient complaints (as an indicator of adverse events).
Excluded studies
Our review focused on those interventions targeted at the collaborative local level of interactions between patients, families, and staff. We made the two‐way nature of involvement a key criterion for inclusion. Patients’ ‘work’ in securing response is complex whether it is in the community or into hospital. This hidden work involves decision‐making, negotiating hierarchies and boundaries. Therefore, we decided for interventions directed at patients/families, a key criterion was to include an interactive element of rehearsal, role play, modelling, shared language, group work etc. to the intervention. Of the 288 studies excluded from full‐text assessment, we reported only on those that fell just short of inclusion and required team discussion as opposed to those that were easily excluded on account of our inclusion criteria.
Risk of bias in included studies
Details of study quality are shown in the table Characteristics of included studies and the 'Risk of bias' figures (Figure 4; Figure 5).
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
5.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Seven out of the nine included studies reported an acceptable method of random sequence generation. Two did not provide an adequate description of the randomisation process, so we classified these as unclear. Allocation concealment appeared satisfactory in seven of the studies, but it was inadequately described in two studies.
Blinding
Blinding was judged as acceptable for only two studies. We assessed that both participants and personnel were aware which group they were in for five studies, and one used a stepped wedge design so blinding was not possible. One provided inadequate evidence. Detection bias was unclear in two studies and rated high for one study where both participants and personnel were aware of their group and the outcomes were subjective.
Incomplete outcome data
Two studies were rated as being at high risk in respect of attrition bias. One study was rated as high risk because the lost to follow‐up data were not reported. The other study reported a large attrition, although it was balanced across the groups.
Selective reporting
Five studies were judged as low risk. We judged studies at low risk if all outcomes were reported according to an available protocol/trial details.
Other potential sources of bias
We were unable to assess selective recruitment of clusters for two of the three studies ( Luepker 2000, See 2014). Whilst Norman 2018 was assessed as being at low risk of bias for selective recruitment of cluster participants, we were unable to assess adherence for this study as 13 maternity centres (39·4%) adhered to four or fewer of the five components of the intervention. Variability amongst those centres that reported adherence (e.g. combinations of components implemented) was unclear, and assessment of adherence was likely to be subject to recall and reporting bias by local principal investigators.
There was an additional concern in one study (Mooney 2014) that geographical factors (urban or rural residence; proximity to ED) were not reported for each participant despite prehospital delay time being tested. Horn 2014 also reported baseline imbalances between groups; families in the intervention group were more likely to be on public insurance, on lower household incomes and report exposure to smoke in the child’s home or daycare.
Effects of interventions
See: Table 1
We were able to undertake meta‐analyses on only three outcomes as opposed to the full range due to heterogeneity of outcomes across the included studies. All included studies assessed the effects of interventions versus usual care. None assessed delivery of the intervention versus none.
Primary Outcomes
Patient and family outcomes: Knowledge of danger signs and appropriate care‐seeking behaviours (1a)
Four studies (Boden Albala 2015, Buckley 2007, Dracup 2009, Luepker 2000) presented data on knowledge of danger signs and appropriate care‐seeking behaviours. We pooled results from two of the studies (Buckley 2007, Dracup 2009) as both used the Acute Coronary Syndrome Response Index to measure knowledge, attitudes and beliefs about coronary heart disease. The combined analysis from one study's participants (Buckley 2007) and a subgroup from Dracup 2009 showed a positive effect of interventions to promote patient and family escalation of care at 12 months: mean difference (MD) of 4.20 between intervention and usual care (95% confidence interval (CI) 2.44 to 5.97) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Interventions to promote patient and family escalation of care versus usual care, Outcome 1: Knowledge
We were not able to include the other two studies in our meta‐analysis due to differences in the measurement tool and differences in constructs measured. Luepker 2000 found from a random sample of community participants that a greater number of people in the intervention communities reported correct messages about heart attack symptoms (2.7% (n = 645) versus 1.8% (n = 561); P < 0.03) and identified appropriate actions to take in the light of danger signs related to coronary heart disease (32.6% (n = 643) versus 22.8% (n = 561); P < 0.006) compared to those in the control communities at 18 months. These results were also replicated in a survey of admitted patients.
Boden Albala 2015 dichotomised the 29‐item stroke knowledge (SK) scale into high SK (≥ 23 correct) and low SK (< 23 correct) to reflect an 80% knowledge cut‐point. They also included a stroke preparedness capacity (PC) assessment which was based on a complete recounting of three key preparedness skills (stroke, symptoms, time), dichotomised to reflect full competency. At 12 months, there was no difference in knowledge between the intervention group (which received educational materials and interactive sessions) and the usual care group (which received only educational materials) (OR 1.21, 95% CI 0.87, 1.67), however there was higher preparedness capacity at 12 months in the intervention group (OR 7.64; 95% CI 2.49 to 23.49).
In summary, interventions to increase patient and family involvement in escalation of care may moderately improve patient and family knowledge about symptoms, appropriate responses, and preparedness capacity. The certainty of the evidence for this outcome was rated as low. It was downgraded two levels for indirectness (restricted population, largely male well‐educated sample, complicated comorbidities excluded) and risk of bias (unclear randomisation and allocation concealment in two of four studies; also a high risk of bias due to lack of blinding of participants, incomplete study data presented, risk of selection bias).
Patient and family outcomes: Attitudes and beliefs regarding condition and appropriate care‐seeking behaviours (1b)
Two studies measured the effects of interventions to increase patient and family involvement in escalation of care on attitudes and beliefs. We were able to pool results from Buckley 2007 and a subgroup from Dracup 2009 to measure changes in attitudes and beliefs about coronary heart disease (CHD) and intended behaviour in response to symptoms. The combined results showed a slight improvement in patients' attitudes (total score 5‐20; higher scores indicate improvement) (MD 0.46 (95% CI 0.09 to 0.82) Analysis 1.2) and beliefs (based on total score 7‐28; higher scores indicate improvement) (MD 0.42 (95% CI ‐0.11 to 0.96) Analysis 1.3) about their likelihood of having another cardiac event and behaving appropriately in response to symptoms with the intervention, compared with usual care, 12 months after delivery of the intervention, but only the result for attitudes was statistically significant.
1.2. Analysis.

Comparison 1: Interventions to promote patient and family escalation of care versus usual care, Outcome 2: Attitudes
1.3. Analysis.

Comparison 1: Interventions to promote patient and family escalation of care versus usual care, Outcome 3: Beliefs
Patient and family outcomes: Care‐seeking behaviours including involvement of family members (1c)
Mooney 2014 measured patients' behavioural responses to symptoms and time to notify a significant other (a nominated person of choice) in response to symptom onset. More patients (111/177, 62.7%) in the intervention group reported their symptoms to another person within 30 minutes of onset than in the usual care group (67/137, 48.9%, P = 0.01).
Patient and family outcomes: Willingness to participate (1d)
One study specifically measured patient engagement, defined as patients’ knowledge, skills, and confidence in managing their health and healthcare and the interventions that promote healthy behaviours (Hibbard 2004). Schumacher 2017 utilised the13‐item Patient Activation Measure (PAM) (Hibbard 2004) within 31‐60 days of the ED visit to assess older ED patients' engagement. They found that while PAM scores fell in both groups (indicating lower activation) after the ED visit, the decline in PAM scores was greater in the usual care compared with the intervention group (mean decline ‐4.64 usual care versus ‐2.77 intervention group, unadjusted logistic regression beta = 1.87, P = 0.043).
Patient and family outcomes: Self‐efficacy: confidence in one’s own ability to self‐diagnose, seek help and work with staff to secure professional help (1e)
Self‐efficacy refers to how much confidence individuals have in their abilities to manage specific situations. Two studies measured self‐efficacy but the constructs measured and scores were sufficiently different to preclude pooling the data. See 2014 used a 10‐item Self‐Efficacy Response Scale (SERS), developed from the validated Health Education Impact Questionnaire (Osbourne 2007) and the General Self‐efficacy Scale (Scholz 2002) to measure self‐efficacy in (1) recognising own worsening conditions; and (2) reporting own worsening conditions. They reported that the level of reported self‐efficacy was significantly higher in the intervention group at day 3 of the participants' hospitalisation. Mean scores for the control group were 17.06 (SD 3.79) and 25.03 (SD 1.85) for the intervention group for one subscale (P < 0.0001) and 18.70 (SD 3.06) for the control group and 26.21 (SD 1.45) for the intervention group (P < 0.0001) for the second subscale. Horn 2014 used the Perceived Efficacy in Patient–Physician Interactions (PEPPI) instrument (Maly 1998) which consisted of five items to assess patients' self‐efficacy in communicating to primary care providers. The scale used in Horn 2014 had been validated for an older patients' population and was modified for use with parents. A ten‐point scale was used to measure parents’ confidence in their ability to elicit and understand information from and communicate information to their physicians, as well as confidence in their ability to get their physicians to address and act on their main medical concerns. Total scores were dichotomised based on whether or not the parent had the maximum score of 50 (Maly 2004). There were no significant differences in the proportions of parents reporting maximum PEPPI score at six‐month follow‐up (aOR (adjusted OR) 1.4, 95% CI: 0.6 to 3.5, P 0.42, n = 137).
In summary, we are uncertain whether interventions to increase patient and family involvement in escalation of care improve patient and family self‐efficacy. We did not combine the results due to substantial differences in the way the outcome was measured. The two studies reported differing effects.The certainty of the evidence for this outcome was rated as low. The evidence was downgraded by two levels; for imprecision (small sample size) and inconsistency (variation in effects).
Treatment outcomes: Time from start of symptoms to delivery of professional treatment (2a)
Four studies (Boden Albala 2015; Dracup 2009; Luepker 2000; Mooney 2014) reported on timeliness but we were unable to combine the data as the studies used different measures. Boden Albala 2015 measured time from symptom onset to triage in the emergency department for recurrent events. They found no difference in arrival within the 3‐hour time window between intervention (interactive sessions plus education) and usual care (education only) groups at 5‐year follow up. Among the intervention group, 40% arrived within three hours compared with 46% of the usual care group (P < 0.33). At two‐year follow‐up, Dracup 2009 measured mean and median prehospital delay time, and found that median prehospital delay time was no different between intervention and usual care groups (intervention 2.20 versus usual care 2.25 hours P = 0.40). Mean hospital delay time was 4.29 hours (SD ± 0.34) in the intervention group compared with 5.08 (SD ± 0.69) in the usual care group, 39 minutes shorter. Mooney 2014 measured time from symptom onset until arrival at the emergency department using median and interquartile ranges at two‐year follow‐up. The overall sample median delay time was 2.54 hours. Median delay time was significantly lower in the intervention compared to the usual care group (1.7 hours versus 7.1 hours). Luepker 2000 studied median delay time from symptom onset until arrival at the emergency department in minutes, comparing communities by month across time periods, over an 18‐month period. The median prehospital delay time was no different between communities (delay time decrease in intervention communities: ‐4.7% per year, 95% CI ‐8.6% to ‐0.6% versus reference (control): ‐6.8% per year, 95% CI ‐14.5% to 1.6%, P = 0.54).
In summary, we are uncertain whether interventions to increase patient and family involvement in escalation of care improve time to treatment. Whilst one study reported moderate benefits, three studies reported no difference between intervention and usual care/control groups. We did not combine the data due to differences in outcome measures. The certainty of the level of evidence for this outcome was rated as low. The evidence was downgraded by two levels; for risk of bias (unclear randomisation and allocation concealment in two of four studies, also a high risk of bias due to lack of blinding of participants, incomplete study data presented), and for inconsistency (substantially different outcome measures and timing of outcome measured, and variation in effects).
Clinical outcomes: Mortality (3a)
Two studies reported on mortality. One study (Norman 2018) examined the effects of interventions to increase patient and family involvement in escalation of care (alongside clinical management response) on mortality over a 3‐year period, compared with usual care. We chose their primary outcome as the most clinically significant to include in our review and reported on the impact of the reduced fetal movements (RFM) care package on stillbirth (babies delivered without signs of life after less than 24 weeks’ gestation or, if gestation was unknown, weighing 500 g or more). The RFM care package did not reduce the risk of stillbirths.The incidence of stillbirth was 4.40 per 1000 births during the control period and 4.06 per 1000 births in the intervention period (adjusted odds ratio [aOR] 0.90, 95% CI 0.75 to 1.07; P = 0.23). The second study (Luepker 2000) reported mortality in the population but, because the study was underpowered to detect a difference, we did not report this as a result.
Interventions to increase patient and family involvement in escalation of care probably have little or no effect on mortality rates, measured as stillbirth. Only one study reporting this outcome was eligible for inclusion in the review, which found no difference between intervention and usual care. The study included a large sample size and number of events in a pregnant population only. The certainty of evidence for this outcome was rated as moderate, downgraded by one level for indirectness (pregnant population only).
Clinical outcomes: Morbidity/number of events (3b)
Two studies reported on morbidity outcomes. Boden Albala 2015 measured recurrent events (stroke, TIA or stroke mimic) up to five years after intervention. They reported an increased number of detected stroke events in the interactive intervention plus education group compared with the usual care group which received only educational materials (187 versus 138; incidence rate ratio (IRR) = 1.31 (95% CI 1.05 to 1.63; intervention to control)). Because the estimates of IRR were sensitive to the assumed distribution (where one produced a significant IRR, the other did not), the authors could not conclude that the difference between groups was significant. Norman 2018 reported on a number of morbidity outcomes over a three‐year period; we selected small size for gestational age as clinically significant as it is associated with placental insufficiency and stillbirth (Flenady 2011). Norman 2018 found in babies born small for gestational age (measuring on or under 10th centile) delivered until or after 40 weeks' gestation that 2.0% (3081/157,692) were born in the control period versus 1.5% (3461/227,860) in the intervention period (adjusted OR 0.86 (0.78 to 0.95)) (P = 0.0009), a significant decrease.
In summary, we are uncertain whether interventions to increase patient and family involvement in escalation of care reduce morbidity rates. One study reported no difference in recurrent events (stroke, TIA or stroke mimic) between intervention and usual care whereas a second study found the intervention reduced morbidity (babies born small for gestational age).
Adverse events: Patient harm (5a)
Dracup 2009 measured the impact of the education intervention for patients with coronary heart disease on anxiety. Anxiety was measured using the Multiple Affect Adjective Checklist (Zuckerman 1965), which provides 132 adjectives describing anxiety, depression and hostility for respondents to select. Negative adjective responses are added while positive adjectives are subtracted to give a total score for state anxiety (range 0‐21). Higher scores signify higher levels of the emotion; a score of ≥ 11 indicates clinical symptoms of anxiety. At 12 months, higher anxiety levels were seen in the control group than the intervention group (score of 6 compared to 5.5; P = 0.01). The decrease in anxiety levels in the intervention group over time, however, was seen only in men; anxiety levels remained stable in women. It should also be noted that mean anxiety levels were below the level considered clinically meaningful in both groups.
Norman 2018 acknowledged the possible harms of a package of care which included increasing pregnant women’s awareness of the need to report early concerns about reduced fetal movement together with a management plan for identification and delivery of the ‘at risk’ fetus. They noted potential adverse effects such as increased maternal anxiety and increased intervention (including hospital use, induction of labour and caesarean section) which itself is associated with pregnancy‐related complications. However, we did not include these data as, whilst the trial authors reported on intervention rates, they did not report on the appropriateness of the increase in interventions, so it is not clear whether these constituted a patient harm (i.e. whether interventions occurred at higher rates than were clinically appropriate).
In summary, interventions to increase patient and family involvement in escalation of care probably do not increase patient's anxiety levels. Only one study reported on patient harms, which found that anxiety levels were stable in the control group but decreased slightly in the intervention group, although the clinical significance of this change is unclear. The certainty of the evidence for this outcome was rated as moderate. It was downgraded one level for indirectness (patients with confirmed coronary heart disease diagnosis only).
None of the studies reported outcomes related to the following categories: patient and family skills acquisition (1f), appropriateness and effectiveness of treatment given (2b), patient and family experience (4) and patient complaints (5b).
Secondary outcomes
Patient and family outcomes: Receptiveness to, and acceptability of, intervention to patients and families
One study (See 2014) assessed the acceptability of their intervention to increase patient and family involvement in escalation of care via a 6‐item 6‐point Likert scale administered on day three of hospitalisation. Data were only available for the intervention group (n = 34) (so it is not comparative). Scores were based on a 6‐point Likert scale, with higher mean scores correlating to greater patient satisfaction and more positive evaluation of the intervention. The mean scores indicated that the intervention was positively received by participants.
Attendance and use of healthcare services
Six studies measured the impact of the intervention on health service use but they operationalised measures of effectiveness differently depending on whether interventions to increase patient and family involvement in escalation of care were linked to conceptualisation of under‐ or over‐use of services. These differences prevented us pooling the results. Dracup 2009 assessed the number of visits to the emergency department by patients with symptoms of acute coronary syndrome following the education intervention. Over a two‐year period, the intervention did not increase emergency department utilisation (14.6% versus usual care 17.5%). Eighteen months after Luepker 2000's intervention, there was a 20% increase in use of emergency medical services in intervention communities compared with reference communities (OR 1.20, 95% CI 1.07 to 1.34, P < 0.005). The total numbers of emergency department presentations for chest pain and patients discharged from the ED with noncardiac diagnosis, as well as EMS use among patients with chest pain but noncardiac diagnosis released from the ED were not significantly different between intervention and control groups. Mooney 2014's study found that at two years, fewer in the intervention group (42/177 (23.7%)) consulted a general practitioner prior to hospital arrival than the usual care group (47/137 (36.6%) P = 0.02). There was no difference in ambulance use between the groups (69/177 (39%) in the intervention group versus 54/137 (39.4%) in the usual care group, P = 0.51).
In contrast, Horn 2014 and Schumacher 2017 used reduction in service use as a measure of the effectiveness of their interventions. Horn 2014 measured asthma‐related ED and urgent care visits. The groups did not report significantly different rates of ED visits for asthma care at six‐month follow‐up (aIRR (adjusted incidence rate ratios) = 0.6, 95% CI: 0.3 to 1.3, P = 0.23, n = 137). There was also no significant difference between groups in the parent‐reported frequencies of urgent care visits at the six‐month follow‐up (aIRR = 0.8, 95% CI: 0.4 to 1.4, P = 0.42, n = 137). Schumacher 2017 assessed self‐reports of doctor visits within 30 days of the ED visit. There was no difference in doctor visits between intervention and usual care groups (74% versus 65%, respectively, P = 0.53).
Norman 2018 measured use of neonatal services. However, we did not include these data as the clinical significance of this was unclear i.e. whether increases or decreases in use of neonatal services constituted a patient benefit or harm.
In summary, we are uncertain whether interventions to increase patient and family involvement in escalation of care have an impact on attendance and use of healthcare services. Variation in conceptualisation of benefit (seen as increased or reduced attendance/use of services) prevented us pooling the results. Mixed results were found in one study, and four studies reported no differences in service use between the intervention and usual care groups.
None of the studies reported outcomes related to healthcare professionals (psychological well‐being/capability/capacity to respond, experience of the clinical encounter).
Discussion
Summary of main results
The objective of this review was to evaluate the effectiveness of interventions to increase patient and family involvement in escalation of care for time‐critical life‐threatening conditions, when compared to usual care, control or no intervention. We deliberately acknowledged the two‐way nature of involvement, and made an interactive element of rehearsal, role play, modelling, shared language and group work a key criterion of inclusion for interventions directed at patients/families to help secure a step‐up to urgent or emergency care. We also recognised the significance of interventions enabling staff response practices to patients presenting with concerns about a deterioration in condition. We therefore excluded a number of trials where the intervention was based solely around static patient information about life‐threatening conditions such as heart attacks or stroke using written, audio or online material but did not explicitly include an interactional component or staff‐facing component.
Nine studies were included in our review, seven of which involved face‐to‐face, interactional education/coaching sessions aimed at patients/families while two provided multi‐component education programmes which included components targeted at staff as well as patients/families. All of the interventions included: (1) an educational component about the acute condition and preparedness for future events such as stroke or a change in fetal movements: (2) an engagement element (self‐monitoring, action plans); while two focused on shared language or communication skills.
The certainty of evidence, assessed using GRADE (see 'Summary of findings' table), indicated low certainty for patient and family outcomes (patient and family knowledge of danger signs and appropriate care‐seeking behaviours; patient and family self‐efficacy), low certainty for treatment outcomes (time from start of patient symptoms to delivery of professional treatment), moderate certainty for clinical outcomes (mortality) and moderate certainty for adverse events (patient harms associated with patient and family involvement in escalation of care).
The evidence to support the impact of interactional patient‐facing and multi‐component programmes on improving patients’ knowledge of acute life‐threatening conditions, danger signs, appropriate care‐seeking responses, and preparedness capacity indicates there may be some effect of the intervention. We are uncertain of the effects of patient‐based interventions on self‐efficacy.
We are also uncertain whether interactional patient‐facing and multi‐component programmes to increase patient and family involvement in escalation of care improve time from the start of patient symptoms to treatment; we were unable to combine the data due to differences in outcome measures. This evidence could usefully be strengthened by more standardised measurement. We found that a multi‐component intervention probably has little impact on mortality rates. Only one study on a pregnant population was eligible for inclusion in the review, which found no difference in stillbirth rates. In terms of unintended events, we found that interactional patient‐facing interventions to increase patient and family involvement in escalation of care probably have little adverse effect on patients' anxiety levels, although again this was measured in a single study.
None of the studies measured or reported patient and family perceptions of involvement in escalation of care or patient and family experience of patient care. Reported outcomes related to healthcare professionals (psychological well‐being, capability/capacity to respond, experience of clinical encounter) were also not reported in any studies. While several studies looked at the impact of the interventions on use of health services, findings were mixed without clear benefits or harms across studies and differences in measures used, and assumptions around use being seen as a positive or negative outcome prevented us pooling the data. This evidence could be strengthened by clarifying the conceptualisation of under‐ or over‐use of health services in relation to escalation of care strategies.
Overall completeness and applicability of evidence
Our advisory group members provided very helpful advice throughout the review process and their feedback was particularly useful for helping us reflect on the applicability of the evidence, and our conclusions. Our review deliberately moved beyond condition‐specific models, aiming to utilise learning from both high‐ and low‐income contexts, different presentations of clinical deterioration and escalation of care pathways in order to understand generic processes which influence recognition of and responses to a clinical emergency. The trials included in the review evaluated interventions focused on parents of children with asthma, adult patients with coronary heart disease and stroke, pregnant women, patients on medical‐surgical wards and patients with limited health literacy. All of the studies were conducted in high‐income countries, five of which took place in the USA with others based in Australia, New Zealand, the UK and Ireland, and Singapore (although some specifically included disadvantaged populations such as those from low‐income minority groups, or people with limited health literacy). The majority of trials focused on enabling escalation of care from home into community health or hospital settings, with only one looking at escalation of care within hospital settings. There was a high degree of heterogeneity across the studies which limited our ability to pool data and our ability to assess if the results are generalisable to other settings and other patient groups.
The included studies varied in their aims, understanding of involvement and use of theory. The review cuts across different literatures (help‐seeking and rapid response systems; self‐management and patient‐provider communication; and person‐centred care). Distinctions between effective self‐management of chronic conditions to keep patients out of hospital (reducing ED use), and effective self‐management of acute conditions to enable patients to access early treatment (encouraging ED use) linked to assumptions around service overuse and underuse. We have drawn on the candidacy framework (Dixon‐Woods 2006) and mapped the different interventional components used by our included studies to the stages involved in escalating care (Figure 6). Patients’ ‘work’ in communicating concerns about changes in condition and helping to secure a professional response involves decision‐making, negotiating hierarchies and boundaries. We also highlighted the significance of structural and social influences and discourses (Figure 3). Our review highlights how ‘fuzzy’ boundary distinctions between acute and chronic condition link to the heterogeneity in study design and outcome measures. The small number of included studies and diversity in the approaches used makes it impossible for us to assess cross‐cultural distinctions around this, linked to service demand and use.
6.
Negotiating candidacy in escalation of care
None of the trial authors specifically operationalised involvement in escalation of care in precisely the same manner that we have adopted for this review, and it was not always the primary focus of their evaluations. While all the studies included a component that focused on recognition of condition‐specific red flags and included an interactional element, they employed a variety of different intervention strategies. In two of the studies (Horn 2014, Schumacher 2017), patient and family involvement in escalation of care was packaged within broader aims around communication and engagement. The Luepker 2000 intervention was multifaceted and included community organisation and public education strategies in addition to patient and staff education. We cannot therefore assume that any observed effects in this study were due solely to the care escalation process itself.
The patient‐facing interventions were delivered by different professionals. The majority based their interventions around one individual face‐to‐face session, with some following this up with reinforcement phone calls. Only two studies included a staff component. The patient and staff‐facing components were delivered separately rather than using co‐design approaches and they focused at the individual level rather than addressing team‐based communicative dimensions of escalation of care. The staff‐facing components were timed before the patient‐facing components. Two of the studies reported having patient and public involvement (PPI) in development of the intervention. While some of the studies targeted patients with low literacy and non‐English speaking patients, others excluded those with impaired cognitive functions and communication difficulties. One study used specific strategies to promote cultural sensitivity of their materials, capitalising on intrapersonal and interpersonal relationships, as well as community engagement. This seems key, as from a patient safety perspective, linkages have been shown between culture, language and patient safety outcomes (Johnstone 2006; Johnstone 2009).
There were significant differences in timing of the intervention in relation to need. For patients already in the hospital setting, or on a maternity care pathway, timelines between the delivery of the intervention and need to use for a deterioration in condition were much shorter than those discharged who might experience a change in their condition after 12 months or more. The diversity in population and conditions included here also raises issues around contact with services and whether the intervention was a one‐off or reinforced over time e.g. in pregnancy at each antenatal visit. Studies noted that the lack of effect on delay times could be because the information presented did not address the variability of onset and the differing symptoms of an acute condition adequately (Dracup 2009, Mooney 2014). The dynamic nature of many life‐threatening conditions means that symptoms and signs may emerge at different times and in different combinations, and may not necessarily map onto those officially recognised as red flags (Mackintosh 2017; Rainey 2013).
The diversity in populations and approaches made it impossible for us to make any assessment about different effects in population subgroups.
For some of the studies the usual care group received additional strategies (e.g. the control group in Boden Albala 2015 received enhanced educational materials and in Horn 2014 a visit with an asthma educator or clinician). This may help to explain why some of the interventions did not have much of an effect as there was less of a difference between what the intervention group received and what constituted usual care.
There was wide diversity of outcome measures used which impacted on our ability to aggregate the data. Some studies focused largely on clinical outcomes while others included only cognitive or behavioural outcomes. The cognitive or behavioural outcomes were at the patient level rather than related to staff response or staff experience. There was little acknowledgement of the dialogic nature of escalation of care, and only two studies focused on relational elements of care. Only one study captured potential harms of the intervention. It was difficult to interpret the data on morbidity and potential harms in Norman 2018 as the clinical significance of increased interventions and use of neonatal services was unclear.
Quality of the evidence
The majority of the studies (seven out of nine) were assessed as having a low risk of bias in relation to random sequence generation, but two studies provided unclear information. Two studies were also unclear with regard to allocation concealment. Performance bias was judged as acceptable for only two studies. We assessed that both participants and personnel were aware which group they were in for five studies; one used a stepped wedge design so blinding was not possible, and one provided inadequate evidence. Detection bias was unclear in two studies and rated as high for one study. Two studies were rated as being at high risk in respect of attrition bias (one because of a large attrition and one because attrition was not reported), and five were assessed as being at low risk for selective reporting bias; protocols were not available for four studies.
In terms of certainty of the evidence, three of the key outcomes (selected for our 'Summary of findings' table) were rated as having low‐certainty evidence and two outcomes were assessed as having moderate‐certainty evidence. Outcomes with low‐certainty evidence were assessed as such due to restricted population, methodological limitations (risk of bias), small sample size or inconsistency in the measures used or in the effects. Mortality and patient harms were assessed as having a moderate certainty of evidence to support the conclusions. No outcomes were considered to have a high certainty of evidence.
Continuity of carers appeared significant particularly for some populations (and distinguished those who had only sporadic contact with health services from those in regular contact) yet this was not assessed, although one study (Horn 2014) focused on connectedness to primary care providers. No outcomes for healthcare professionals were reported, despite their involvement as intervention recipients in two studies.
Few of the studies included implementation outcomes and issues around fidelity and local customisation were difficult to assess. Given the lack of implementation or fidelity information, it was hard to assess the role that this may have had on the effectiveness (or lack thereof) of the interventions evaluated in this review.
Potential biases in the review process
We clarified our interpretation of involvement in escalation of care to include an interactive element of rehearsal, role play, modelling, shared language, or group work for the interventions directed at patients/families. We acknowledge that patient education plays an important role in enabling escalation of care. Indeed, the majority of studies included in our review provided supplementary information in addition to the interactive coaching/skills‐based sessions. However, our review excluded those studies which focused solely on patient education material (e.g. leaflets and videos), limiting our assessment of their stand‐alone value. We decided a focus on the dialogic element was important, including preparation for patients' and staff to share and respond to concerns. We had anticipated our included interventions would include components that reinforced and legitimised patients’ expertise in diagnosis, or provided training in communication to help patients address power differences and know how to work with staff, or provided skills training for staff to listen to patients’ concerns. However, our included studies were predominantly directed at individual behaviour change (mostly at the level of patients and families) rather than at enabling dialogic reciprocal relationships during escalation of care.
Two review authors, working independently, carried out study identification and data extraction. We met as a team to discuss any disagreements and used consensus to enable resolution. Studies that focused on person‐centred care and personalised care‐planning, while sharing our interest on the dialogic collaborative element of patient‐provider relationships, tended to focus on self‐management rather than explicitly including reference to warning signs of deterioration or escalation of care, so were excluded. For the same reasons, our review only included two studies (Horn 2014 and Schumacher 2017) and one ongoing study (Mi 2018) from the wider evidence base on care transitions.
Agreements and disagreements with other studies or reviews
Several reviews have focused on patient and family involvement in safety (Doherty 2012; Hall 2010; Schwappach 2009) and noted the limited evidence base. Our review focused specifically on one aspect of safety, notably escalation of care in community health and hospital settings. Schwappach 2009 noted that involvement in safety may be successful if interventions promote complex behavioural change and are sensitively implemented in healthcare settings. Doherty 2012 also found that when clinicians encourage patients’ involvement in safety then patients are generally willing to participate, and there was a need for more effective clear written or verbal information for patients to enable involvement. Our review builds on these findings as interventions utilising oral, written information and group discussions may improve patient knowledge of danger signs and care‐seeking behaviour, and may improve patient attitudes regarding the acute condition and appropriate care‐seeking. Nurses and physicians were amongst the personnel delivering the intervention which may have helped enable impact. Doherty 2012 and King 2019 also noted that further exploration of the clinicians’ role towards patients’ engagement in safety was required to enhance our understanding of how to improve staff responsiveness.
Our review highlights the point that interventions studied to date are mainly focused at the patient level rather than at the communication level between clinician and patient, or wider organisational barriers to patient‐clinician collaboration on safety issues (e.g. models of care and care pathways) (Entwistle 2010; Gill 2018; King 2019). We drew on the candidacy framework (Dixon‐Woods 2006) as a means of usefully widening our conceptual lens (Figure 1). The candidacy framework enabled us to expand on patients’ micro‐level hidden ‘work’ in communicating concerns about changes in condition and helping to secure a professional response (see Figure 6), adding to previous research in this area (Llanwarne 2017). We also highlight the significance of structural and social influences and discourses linked to escalation of care (Figure 3).
Reviews focused on patient and family involvement in escalation of care in hospital using direct access to critical care outreach services (Albutt 2017; King 2019; Vorwerk 2015) have highlighted an evidence gap regarding effectiveness. Our review included one trial but effectiveness was limited to considering self‐efficacy in recognising and reporting symptoms as opposed to measuring changes in behavioural and clinical outcomes.
Other reviews have examined interventions designed to train health professionals to promote a patient‐centred approach (Dwamena 2012). They found that there is some indication that complex interventions directed at providers and patients that include condition‐specific educational materials have beneficial effects on health behaviour and health status. The evidence from the two studies that included patient and provider elements in our review is difficult to interpret given the lack of implementation and staff level outcome data.
We included a trial (Norman 2018) focusing on a reduced fetal movement care package which was based on a large observational quality improvement Norwegian study that showed improvements in rates of stillbirth after providing written information to women combined with consensus clinical guidance on management (Tveit 2009). In the Norwegian study, there was no increase in the proportion of women who presented with RFM when rates were compared before and after the intervention, but women with RFM presented significantly earlier to hospital (Tveit 2009). Norman 2018 failed to show any reduction in stillbirth rates in their study, and while they reported several other outcomes related to morbidity, these were difficult to interpret. Several other similar trials are now underway, including the My Baby’s Movements trial in Australia and New Zealand, and the Mindfetalness study (Radestad 2016) in Sweden.
Authors' conclusions
Implications for practice.
Our review identified some positive indications; notably that interactional patient‐facing interventions and multi‐component programmes to increase patient and family involvement in escalation of care for acute life‐threatening illness may improve patient and family knowledge about danger signs and care‐seeking responses, and probably have little adverse effect on patient’s anxiety levels when compared to usual care. We were pleased to see the lack of adverse effects of patients being proactively and interactionally involved in escalating health concerns. We note that the sparsity of evidence may certainly be a factor in this finding, which needs to be confirmed further by studies addressing it as an explicit endpoint and also by further qualitative research. Patient‐focused interventions that facilitate the patient to be proactively engaged with healthcare providers and/or services at the very least appear to result in better informed patients – which, from an ethical and health policy perspective, is a positive aspect of the reviewed interventions.
There were also many outcomes for which there was no indication of an effect and/or the evidence was too sparse or of low quality, meaning that effects are uncertain. Changes in knowledge and preparedness were not translated into effects on time to treatment or clinical outcomes (morbidity; mortality) when compared to usual care. The evidence is insufficient for us to attribute effects to particular strategies, intensity and duration of the intervention, or timing of delivery in relation to application when an episode of patient deterioration in condition occurs. The evidence on treatment outcomes, and clinical outcomes is limited and uncertain. While the interventions may be beneficial for a wide range of acute life‐threatening illnesses, the evidence identified in this review was too limited to recommend any change in current practice.
From our included studies, it is clear that interventions to improve escalation of care are complex interventions which need to include a number of different components. Our review highlights the need for policy makers and practitioners to understand escalation of care as a form of negotiated boundary work within the complex social system of healthcare (Shojania 2019). The reframing of this concept has potential implications for the organisation and delivery of care, in terms of understanding how escalation of care operates within the wider context of services, pathways and routes into urgent care (see Figure 3). The interventions emphasised personal deficits of patients and families (e.g. lack of knowledge about signs and symptoms, lack of engagement with services or inappropriate use of services), and staff (e.g. lack of person‐centred care). Organisational and service limitations to the exercise of individual (patient, family or staff) knowledge and behaviours were not acknowledged, which we believe needs addressing. Our review also found insufficient information about the different communication elements of escalation of care strategies. Such evidence is needed to guide the levels of training or support required to meet the different needs of diverse populations to produce patient benefit.
Implications for research.
Our review highlights the finding that escalation of care involves a number of different components, but further research is required to establish fidelity of form (i.e. which of these components are required to have impact at patient, staff and service level) and the significance of relationships between the components. Further research broadening the focus of interventions beyond individual behaviour change to dialogic reciprocal relationships is required. This could usefully examine use of cognitive aids (such as mnemonics) and communication strategies (Denham 2008; Mackintosh 2010) and how these might contribute to improvements e.g. in patients’ self‐efficacy, as noted by See 2014. Future research is also needed to understand the influence of diverse populations, conditions and connectedness to services with regard to escalation of care. A focus on generic self‐management and communication skills may be more important in some populations than others; similarly provision of technical warning signs may have greater impact in some conditions (e.g. stroke) than others (e.g. childhood illness). This may be linked to the degree of uncertainty (e.g. the indeterminacy of future outcomes associated with fetal movement, compared to chest pain, and symptoms of a stroke).
Further studies are needed to establish effectiveness around time of delivery of the intervention and its application with regard to recognition and response behaviours to deterioration. The follow‐up periods for those patient educational interventions focusing on stroke and acute coronary events were relatively short (maximum of two years). We need to better understand the significance of different levels of engagement with services as a mediator of escalation of care, and the role of continuity of care models in this process.
There are also implications for future researchers in terms of using consistent terminology and thinking about the same outcome categories and outcome measures to enable further comparison of results. Our review highlights the need to include cognitive, behavioural and clinical outcomes. The danger with relying solely on cognitive measures such as knowledge is that this does not necessarily translate into change in behaviours. Similarly, deaths and delays may not accurately reflect any underlying behaviour change and subsequent intervention (based on escalation of care) because the course of the illness may be fundamentally difficult to predict. We believe there is a greater need to highlight and measure staff‐based and relational elements in escalation of care (Bosk 2019), together with measurement of patient access to and use of response pathways. Studies should report adverse effects of the intervention, for both patients (and family members) and staff, with effects on health systems and what any changes in usage mean clearly defined. Lastly, we would encourage researchers to include implementation outcomes when assessing the effects of these outcomes. These need to include data on the fidelity of the interventions, their reach and uptake, and their acceptability to patients, families and staff.
What's new
| Date | Event | Description |
|---|---|---|
| 18 October 2017 | Amended | Inserted correct search strategy and acknowledgement amended. |
History
Protocol first published: Issue 10, 2017 Review first published: Issue 12, 2020
Notes
This protocol is based on standard text and guidance provided by Cochrane Consumers and Communication (CCCRG 2014).
Acknowledgements
We thank our advisory group members (Carolyn Canfield, Helen Haskell, Tommy's baby charity, Sands, Sarah Neill, Rebecca Lawton, David Schwappach and Rohit Ramaswamy) for their valuable help with the protocol development. We thank the Cochrane Consumers and Communication editors and staff, particularly the managing editor, Bronwen Merner, contact editor, Anneliese Synnot and information specialist, Steve McDonald, for their input. We are grateful to Clare Gillies who provided us with statistical assistance.
Appendices
Appendix 1. Search strategy
CENTRAL: Search Name: Mackintosh _December 2019_update
Last Saved: 27/11/2019 14:45:55
Comment:
ID Search
#1 MeSH descriptor: [Decision Making] this term only
#2 MeSH descriptor: [Decision Support Techniques] this term only
#3 MeSH descriptor: [Health Education] explode all trees
#4 MeSH descriptor: [Patient Acceptance of Health Care] this term only
#5 MeSH descriptor: [Patient Care Team] explode all trees
#6 MeSH descriptor: [Family] this term only
#7 MeSH descriptor: [Patients] this term only
#8 MeSH descriptor: [Interpersonal Relations] explode all trees
#9 MeSH descriptor: [Health Facilities] explode all trees
#10 MeSH descriptor: [Critical Care] this term only
#11 MeSH descriptor: [Critical Illness] this term only
#12 MeSH descriptor: [undefined] explode all trees
#13 {OR #1‐#12}
#14 (((patient* or consumer* or family or families or relative* or parent* or child* or partner* or women* or carer* or caregiver* or advocate*) N5 (activat* or involv* or initiat* or engag* or participat* or contribut* or collaborat* or role or cooperat* or assist* or champion* or advoc* or help‐seek*) N5 (deteriorat* or escalat* or "life threatening" or life‐threatening or critical or emergenc* or complication* or "warning signs" or "danger signs" or adverse))):ti,ab,kw (Word variations have been searched)
#15 ("escalation of care" or "failure to rescue" or "rapid response" or "rapid‐response" or "critical incident" or "early warning score" or "critical care outreach" or "calling for help" or "patient deteriorat*" or "deteriorating patient" or "medical emergency team" or "failure to escalate"):ti,ab,kw (Word variations have been searched)
#16 {OR #13‐#15}
#17 MeSH descriptor: [Palliative Care] explode all trees
#18 MeSH descriptor: [Rehabilitation] explode all trees
#19 MeSH descriptor: [Psychology] explode all trees
#20 {OR #17‐#19}
#21 #16 NOT #20 with Cochrane Library publication date Between Aug 2018 and Nov 2019
Mackintosh_Embase_Update
December 2019
1. community participation/
2. stakeholder engagement/
3. patient counseling/ or patient education/ or patient attitude/
4. ((share or shared or sharing or support* or inform* or making or behavior* or aid*) adj2 (decision* or deciding or choice*)).ti,ab,kw.
5. ((patient* or consumer* or user* or carer* or caregiver* or client* or famil* or lay* communit*) adj3 (partner* or participat* or centre* or center* or communicat* or consult* or decision* or deliberation* or co#design* or involv* or contribut* or role* or empower* or engag* or collab* or advoca* or organi#ation* or respons* or question* or educat* or inform* or train* or shar* or joint or choice* or preference* or interven* or mobili*)).tw.
6. ((communicat* or community or counsel*) adj3 intervention*).tw.
7. or/1‐6
8. exp primary health care/
9. exp general practice/
10. general practitioner/
11. exp home care/
12. private practice/
13. community care/
14. general practice/
15. exp community health nursing/
16. pharmacy/
17. health auxiliary/
18. exp preventive health service/
19. community medicine/
20. health center/
21. exp health promotion/
22. health promotion.ti,ab,kw.
23. ((home* or visit* or preventive* or general or family or primary or community) adj3 (health or practice* or medicine or physician* or nursing or pharmacy or program* or service* or care)).ti,ab,kw.
24. ((family or primary or general or community) adj2 (pharmacist* or physician* or doctor* or practitioner* or healthcare*)).ti,ab,kw.
25. ((nurse* or nursing) adj2 (practice* or practitioner* or prescriber*)).ti,ab,kw.
26. (GPs or GPSI or GPwSI).ti,ab,kw.
27. "emergency ward"/
28. or/8‐27
29. randomized controlled trial/
30. controlled clinical trial/
31. single blind procedure/ or double blind procedure/
32. crossover procedure/
33. random*.tw.
34. placebo*.tw.
35. ((singl* or doubl*) adj (blind* or mask*)).tw.
36. (crossover or cross over or factorial* or latin square).tw.
37. (assign* or allocat* or volunteer*).tw.
38. or/29‐37
39. (escalat* or failure to rescue or danger sign* or response or rapid‐response or critical incident or warning* or critical care outreach or calling for help or (patient adj2 deteriorat*) or medical emergency team or failure to escalate or high‐risk or highrisk or (significant adj3 report*) or (immediate adj3 care) or prepare* or warning* or delay* or mobilisation or mobilization or prolong* or life threatening or life‐threatening or adverse).ti,ab,kw.
40. (pallia* or rehab* or psycholog*).mp.
41. and/7,28,38‐39
42. 41 not 40
43. exp hospital care/
44. exp maternal care/
45. exp intensive care/
46. pediatrics/
47. ((hospital or maternal or intensive or critical or paediatric or pediatric) adj3 (care or caring)).ti,ab,kw.
48. 43 or 44 or 45 or 46 or 47
49. 28 or 48
50. and/7,38‐39,49
51. 50 not 40
52. 51 not 42
53. limit 52 to yr="2018 ‐Current"
Mackintosh_Medline_Steve_Update
December 2019
1. (Decision Making/ or Decision support techniques/ or exp Health Education/ or "Patient Acceptance of Health Care"/ or exp Patient Care Team/ or Family/ or Patients/ or exp Interpersonal Relations/) and (exp Health Facilities/ or Critical care/ or Critical Illness/ or exp Pregnancy/)
2. ((patient$ or consumer$ or family or families or relative$ or parent$ or child* or partner$ or women$ or carer$ or caregiver$ or advocate$) adj5 (activat$ or involv$ or initiat$ or engag$ or participat$ or contribut$ or collaborat$ or role or cooperat$ or assist$ or champion$ or advoc$ or help‐seek$) adj5 (deteriorat$ or escalat$ or "life threatening" or life‐threatening or critical or emergenc$ or complication$ or "warning signs" or "danger signs" or adverse)).tw.
3. ("escalation of care" or "failure to rescue" or "rapid response" or "rapid‐response" or "critical incident" or "early warning score" or "critical care outreach" or "calling for help" or "patient deteriorat$" or "deteriorating patient" or "medical emergency team" or "failure to escalate").tw.
4. 1 or 2 or 3
5. (randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or randomly.ab. or trial.ti,ab.
6. 4 and 5
7. limit 6 to ed=20180821‐20191201
Mackintosh_PsycInfo_Update
December 2019
1. client participation/
2. exp client attitudes/
3. exp cooperation/
4. self‐efficacy/
5. right to treatment/
6. exp Advocacy/
7. exp Group Discussion/
8. ((patient$ or consumer$ or family or families or relative$ or parent$ or child$ or partner$ or women$ or carer$ or caregiver$ or advocate$) adj5 (activat$ or involv$ or initiat$ or engag$ or participat$ or contribut$ or collaborat$ or role or cooperat$ or assist$ or champion$ or advoc$ or help‐seek$ or understand*)).ti,ab,tw.
9. or/1‐8
10. ("escalation of care" or "failure to rescue" or "rapid response" or "rapid‐response" or "critical incident" or "early warning score" or "critical care outreach" or "calling for help" or "patient deteriorat$" or "deteriorating patient" or "medical emergency team" or "failure to escalate").ti,ab,id.
11. (deteriorat$ or escalat$ or "life threatening" or life‐threatening or critical or emergenc$ or complication$ or "warning signs" or "danger signs" or adverse).ti,ab,id.
12. or/10‐11
13. exp decision making/
14. exp intervention/
15. health education/
16. exp intensive care/
17. exp community services/
18. (interven* or educat* or counsel*).ti,ab,id.
19. or/13‐18
20. control:.tw.
21. random:.tw.
22. exp treatment/
23. or/20‐22
24. and/9,12,19,23
25. (pallia* or rehab* or psycholog*).mp.
26. 24 not 25
27. limit 26 to yr="2018 ‐Current"
Data and analyses
Comparison 1. Interventions to promote patient and family escalation of care versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Knowledge | 2 | 687 | Mean Difference (IV, Random, 95% CI) | 4.20 [2.44, 5.97] |
| 1.2 Attitudes | 2 | 686 | Mean Difference (IV, Random, 95% CI) | 0.46 [0.09, 0.82] |
| 1.3 Beliefs | 2 | 688 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.11, 0.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boden Albala 2015.
| Study characteristics | ||
| Methods |
Study design: randomised trial Unit of allocation: patient Unit of analysis: patient Funding source: this work was supported by the National Institute of Health National Institute of Neurological Disorders and Stroke (NINDS) through the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) Network, P50 NS049060 P. 3, and the Robert Wood Johnson Health and Society Scholars Pilot Funds Confict of interest: none declared |
|
| Participants |
Country: US Setting: Columbia University Medical Center (CUMC) Numbers/conditions: 1193 patients recruited (601 to interactive intervention (II) group, 592 to enhanced educational (EE) materials) ‐ patients with an initial diagnosis of ischaemic stroke or trans‐ischaemic attack (TIA) |
|
| Interventions |
Theoretical framework: Social Cognitive Theory (SCT) Rationale: increasing knowledge about stroke and preparedness competency to respond to stroke symptoms as an emergency will increase acute stroke arrivals to hospital. Targeted at: patients with initial diagnosis of ischaemic stroke or TIA Delivered by: a bilingual intervention team consisting of two health educators and a physician on call to handle clinical focused questions Materials: intervention script, video material. Materials had been culturally developed using participatory methods. These included "a) bilingual materials with translations by community health worker; b) visuals integrating community places and promoting recognition of familiar surroundings into intervention; c) film footage of community stroke survivors recalling stroke experiences in their own language; and d) integration and instructions for current community resources into intervention" (Boden Albala 2010, p 238). Procedures: during the group sessions, individuals engaged in discussions and role play to describe stroke symptoms and interact with ED staff, and viewed video material about stroke, stroke symptoms, early medical treatment and emergency medical procedures. The FAST mnemonic (Face Droop, Arm Weakness, Speech Affected/Slurred and Time to call 911) was also included. Motivational interviewing techniques were employed. Participants were taught to navigate the emergency health system, and feel comfortable taking action in the event of a stroke, taking into account barriers such as mistrust of the healthcare system, fear of a recurrent stroke event, or frustration in communication with health providers. At the end of each session, a verbal competency evaluation was completed. Modes of delivery: an interactive multi‐media educational/behavioural intervention strategy within a group setting Where: largely during hospitalisation. Patients transferred to rehabilitation or discharged home early were brought in for the sessions. If needed, the interventions were conducted at home. When and how much: two intervention sessions were scheduled to occur within 3 weeks after the stroke/TIA onset, and in most cases within the initial hospitalisation stay. Fidelity/Tailoring: every effort was made to be flexible in scheduling in order to complete intervention within 3 weeks of event. Attrition: low (6 too sick to complete, 2 died before intervention, 2 discharged before intervention) Comparison: usual care comprised bilingual stroke preparedness materials, without in‐hospital sessions. |
|
| Outcomes |
Recurrent stroke arrival times (arrival to emergency department < 3 hours, pre‐post‐intervention arrival < 3 hours) Tool: hospital medical record, self‐report by patients/family members, 24‐hour hotline number set up for identification and tracking of acute stroke among participants Review category: Treatment outcomes/timeliness Patients followed up for 5 years Incidence of identified stroke event Tool: stroke screening instrument; stroke surveillance system, hospital medical record Review category: Clinical outcomes/morbidity Measured at 1, 12 months and annually for 5 years Stroke knowledge and preparedness capacity Tool: Stroke knowledge survey (National Stroke Association 1996) Review category: Patient and family outcomes/knowledge Measured at baseline, 1 and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An 8‐cell randomisation scheme with stratification by language (English versus Spanish) was used. |
| Allocation concealment (selection bias) | Unclear risk | An 8‐cell computerised randomisation scheme, but not clear how patients were allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the interactive intervention (II) and enhanced educational (EE) groups were given a standardised packet of preparedness focused education materials and received a medical alert bracelet. However, participants randomised to the intervention group were scheduled to participate in group sessions carried out while in hospital. Patients transferred to rehabilitation or those discharged home early were brought in for the group sessions. It is likely that both participants and personnel were aware which group they were in. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants blinded to intervention status responded to any remaining participant questions following stroke knowledge assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the II group, 1.7% did not receive the intervention as assigned (6 too sick to complete, 2 died and 2 discharged before intervention) and 1.5% of participants were lost to follow‐up; in the EE group, all received the intervention as assigned and 1.9% were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes listed on www.clinicaltrials.gov; results published for all outcomes |
| Other bias | Low risk | The study's exclusions may affect generalisability of the study's results (participants were excluded if not an English or Spanish speaker or without a household telephone). Those with cognitive, comprehension or communication difficulties (e.g. severe aphasia limiting comprehension; pre‐stroke dementia history) or in long‐term nursing home or requiring 24‐h care; or end stage disease were also excluded. The groups did not differ at baseline except those in the control group were more likely to arrive by ambulance. |
Buckley 2007.
| Study characteristics | ||
| Methods |
Study design: randomised trial Unit of allocation: patient Unit of analysis: patient Funding source: National Institutes of Health Confict of interest: none declared |
|
| Participants |
Country: Australia Setting: a metropolitan tertiary referral hospital in Sydney Numbers/conditions: 200 patients (95 control and 105 intervention) ‐ adults with a hospital discharge diagnosis indicating coronary artery disease (CAD) in the previous 6 months. No upper age limit was set. Participants were excluded if they were not proficient in English language or were unwilling to give their telephone number. |
|
| Interventions |
Theoretical framework: Leventhal Self‐Regulatory Model of Illness Behaviour Rationale: increasing knowledge about cardiac symptoms and improving attitudes and beliefs about seeking care will decrease time from symptom onset to hospital arrival. The problem of delayed presentation is multifactorial, influenced by disease characteristics, treatment plan and setting, as well as personality factors and emotional/social states when the person is experiencing symptoms. Targeted at: patients with coronary artery disease Delivered by: the researcher and another experienced cardiac nurse Materials: the three essential components to the intervention were: information provision (on the common symptoms of AMI and the appropriate actions to take in the event of experiencing these symptoms), discussion of emotional and social factors associated with delay, and action plan rehearsal Procedures: the providers followed a core script. A flip chart with basic pictures and keywords enabled participants to focus on key points and concepts. Modes of delivery: individual face‐to‐face and telephone f/up Where: first session in an outpatient clinic, follow‐up reinforcement phone call When and how much: 40‐ to 50‐min for first session, phone call within 4 weeks Fidelity/Tailoring: the core script allowed for tailoring of the content to differing levels of knowledge, clinical and sociodemographic characteristics. Attrition: Low ‐ 6 lost to follow‐up Comparison: usual care |
|
| Outcomes |
Knowledge, attitudes and beliefs about CHD and symptoms, intended behaviour in response to evolving AMI symptoms Tool: ACS Response Questionnaire, modified from the REACT study (Simons‐Morton 1998) Review category: Patient and family outcomes/knowledge Measured at baseline, 3 and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | Participants were given a study number that had been previously allocated by computer to either the intervention or control group. Allocation was concealed in a sealed opaque envelope until informed consent was obtained. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details of blinding with regards to care delivery, ancillary tests etc. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Three‐ and twelve‐month data were collected by research assistants blinded to group allocation by telephone. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition of eligible participants randomised and consistent between group (2 lost to follow‐up and 2 withdrew control/6 lost to follow‐up intervention) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | The study's exclusions may affect generalisability of the study's results (i.e. participants were excluded if not proficient in English language or were unwilling to give their telephone number). The authors noted "A proportion of eligible patients were unable to be contacted and many declined to participate" (Buckley 2007 p 110). Groups were comparable at baseline (except for gender). |
Dracup 2009.
| Study characteristics | ||
| Methods |
Study design: randomised trial Unit of allocation: patient Unit of analysis: patient Funding source: National Institute of Health, National Institute of Nursing Research Confict of interest: none |
|
| Participants |
Country: US, Australia, New Zealand Setting: In‐hospital cardiovascular and cardiac catheterisation units and from a variety of outpatient clinics, cardiac rehabilitation programmes and community medical practices in six sites: Los Angeles, California; Lexington, Kentucky; San Diego, California; Seattle, Washington; Sydney, Australia; and Auckland, New Zealand Numbers/conditions: participants (n = 3522) ‐ (from US (n = 1985), Australia or New Zealand (n = 1537) ‐ with documented coronary heart disease confirmed by their physician or hospital medical record, and if they lived independently (i.e. not in an institutional setting) were randomised to experimental (n = 1777) or control (n = 1745) groups. |
|
| Interventions |
Theoretical framework: Leventhal’s self‐regulatory model of illness behaviour Rationale: using an education and counselling intervention to decrease prehospital delay by increasing knowledge and patients' ability to identify cardiac symptoms and seek care immediately Targeted at: patients with coronary heart disease Delivered by: delivered by nurse with expertise in cardiology, follow‐up call delivered by the same nurse Materials: the intervention addresses 3 areas for patients at High Risk for Acute Myocardial Infarction: (1) information, (2) emotional issues, and (3) social factors. Using a script, the nurse used a flip chart with the main points listed and pictures illustrating the process of coronary occlusion and how reperfusion therapies restore blood flow to the myocardium. In addition, an information sheet (the National Heart Attack Alert Program advisory form), personalised for the patient’s use and completed at the end of the intervention session, was provided, including symptoms and drug use etc. to post at home, a space to write in the location of the nearest emergency department with appropriate cardiovascular services. Patients were asked to put the advisory form in a prominent place in the house (e.g. on the refrigerator or by the phone). Procedures: information: patients were given information about typical symptoms and the fact that onset may be gradual and intermittent, rather than stereotypical sudden crushing chest pain. They were advised to take appropriate actions, (e.g. taking nitroglycerin tablets (if prescribed) and calling an ambulance immediately). Patients were encouraged to identify the emergency facility closest to their home/work. Patients were asked to anticipate the emotional responses to ACS symptoms that might lead to delay, and to reflect on previous experiences accessing the medical system using standardised role playing scenarios. Social: patients were asked to bring their spouse, another family member or friend to the intervention session to act as deputy decision‐maker if the patient hesitated to call EMS. Potential emotional responses of the family member were discussed (e.g. denial, fear, ambivalence, etc.) and rewards for quick action were emphasised. Modes of delivery: individual face‐to‐face and telephone f/up Where: the information was delivered in a quiet, private outpatient setting e.g. clinic office or the patient’s home. When and how much: approx. 40 mins; one month after a nurse telephones the patient to review the main points – approx. 15 mins Fidelity/Tailoring: the intervention was standardised with a flip chart so that patients and family members received the same information components, but it was tailored to the patient’s own past medical experience and living situation. Attrition: completed 24 month follow‐up: n = 1580 (89%). Lost to follow‐up: 89 (5%). Withdrawn: 41 (2.3%). Deceased: 67 (3.8%) Comparison: usual care |
|
| Outcomes |
Prehospital delay (time from symptom onset to hospital presentation) Tool: hospital medical record or emergency medical services prehospital medical reports Review category: Treatment outcomes/timeliness Patients followed up for 2 years Mode of transportation Tool: emergency department records, emergency medical services prehospital medical reports Patients followed up for 2 years Aspirin use Tool: emergency department records, self‐report by patient Patients followed up for 2 years Knowledge, attitudes and beliefs about acute coronary syndrome (ACS) Tool: ACS Response Index (Riegel 2007) Review category: Patient and family outcomes/knowledge Measured at baseline, 3 and 12 months Perceived control Tool: Control Attitudes Scale‐Revised (Moser 2009) Measured at baseline, 3 months and 12 months Anxiety Tool: Multiple Affect Adjective Checklist (Zuckerman 1965) Review category: Adverse events/patient harms Measured at baseline, 3 months and 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Series of sequential study numbers were assigned to study groups by computer randomisation in blocks of 100 by site. |
| Allocation concealment (selection bias) | Low risk | The assignment of each study number was revealed only after consent was given. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians caring for patients and nurses collecting follow‐up data were blinded to study assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel involved in follow‐up data collection were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rates (lost to follow‐up, withdrawn and deceased) which were comparable across groups (16% in control group versus 11% in intervention group). For the linked study, data from 2597 patients with anxiety data at all time points were included from the total of 3522. Baseline characteristics were similar between groups. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes listed on www.clinicaltrials.gov. All outcomes reported |
| Other bias | Low risk | No significant differences between groups apart from body mass index, gender and insurance for ambulance use. Exclusion criteria (complicating serious comorbidity e.g. psychiatric illness or untreated malignancy, neurological disorder with impaired cognition, or inability to read or understand English) may limit generalisability. |
Horn 2014.
| Study characteristics | ||
| Methods |
Study design: randomised trial Unit of allocation: caregiver Unit of analysis: caregiver Funding source: National Center for Research Resources/Verizon Foundation/Novartis/Kellogg Foundation Confict of interest: none |
|
| Participants |
Country: US Setting: the IMPACT DC Asthma Clinic operates within two emergency departments affiliated with Children’s National Medical Center (an urban, tertiary care, academic paediatric medical centre) in Washington, DC Numbers/conditions: 150 (77 intervention group/73 control group) caregivers. The parent/guardian was the child’s primary asthma caregiver and could identify a specific primary care provider (PCP) for the child. The child was between 12 months and 12 years old and had prior physician‐diagnosed asthma. |
|
| Interventions |
Theoretical framework: Social Cognitive Theory Rationale: facilitating African‐American parents’ open communication with healthcare providers and enhancing connectedness to child’s primary care provider (PCPs) will lead to improved health outcomes and reduced asthma‐related healthcare costs. Targeted at: parents/guardians Delivered by: an asthma educator and a physician or nurse practitioner Materials: the research assistant (RA) used a large visual aid and provided the parent with a wallet‐sized card that listed communication components and included space to enter the child’s PCP name and contact information and current medication plan. Procedures: usual care focused on three key elements of asthma management: (1) medical care; (2) trigger identification and control; and (3) care co‐ordination. The enhanced care condition also received a health communication intervention ‐ (‘‘Parent Empowerment Program for Asthma Care’’ [PEPAC]). This focused on the information exchange components of an asthma care visit with the child’s primary care practitioner, using a communication toolkit comprising ‘‘Three Ss’’: (1) Sharing (parents encouraged to ask questions, share concerns regarding their child’s asthma needs and identify goals for the visit) (2) Seeking (parents encouraged to seek information from the PCP about diagnosis, and care plan) (3) Saying it back (parents encouraged to say the plan of care back in their own words, to enable shared goal‐setting During a follow‐up booster call, parents were given the opportunity to role play an asthma care visit over the phone. Modes of delivery: face‐to‐face and f/up telephone call Where: face‐to‐face delivered in clinic When and how much: 90‐minute visit within two weeks of an ED visit or hospitalisation for an acute asthma exacerbation. The intervention added an extra communication component which lasted an additional 30 minutes. Four weeks after the initial session, intervention families received a booster call. Fidelity/Tailoring: space on the card to enter the child’s PCP name and contact information and current medication plan Attrition: low attrition (2 withdrew and 9 lost to follow‐up) Comparison: usual care comprised family attendance at a clinic within two weeks of an ED visit or hospitalisation for an acute asthma exacerbation, and a 90‐minute visit with an asthma educator and a physician or nurse practitioner. If randomised into ‘usual care’, participants completed the session without receiving additional education on parent‐provider communication and were discharged after reviewing their child’s individual Asthma Action Plan. |
|
| Outcomes |
Number of scheduled asthma care visits with child's primary care provider during the study follow‐up period Tool: patient self‐report via questionnaires Measured at baseline, 2 and 6 months Identification of child’s primary care provider as well as child's primary asthma care provider Tool: patient self‐report via questionnaires Measured at baseline, 2 and 6 months Parents' self‐efficacy in communicating with their child’s primary care provider Tool: Perceived Efficacy in Patient–Physician Interactions (PEPPI) instrument (Maly 1998) Review category: Patient and family outcomes/self‐efficacy Measured at baseline, 2 and 6 months Healthcare Utilisation Tool: National survey (Akinbami 2012) Review category: Service use Measured at baseline, 2 and 6 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred in blocks of 20 families, using sealed and shuffled opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Staff unconnected with the trial were involved with allocation using envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Usual care in the IMPACT DC clinic served as the control to ensure that validated and effective asthma care and education was provided to all families and to control for nonspecific intervention features (e.g. time and attention). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study personnel involved in follow‐up data collection were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition low and comparable across groups at follow‐up (between 3‐8 participants unable to reach/1‐2 participants withdrew) |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported in the trial protocol but not all (e.g. with regards to the primary outcomes, only one of the 3 indicators of parent‐perceived connectedness to ongoing primary care were listed in the trial protocol). |
| Other bias | High risk | There were baseline imbalances between groups; families in the intervention group were more likely to be on public insurance, on lower household incomes and report exposure to smoke in the child’s home or daycare. The study's exclusions may affect generalisability of the study's results (families were excluded if the child had significant medical comorbidities, was being seen by a pulmonary or allergy specialist, was enrolled in another asthma research study and/or if the caregiver's primary language was not English). |
Luepker 2000.
| Study characteristics | ||
| Methods |
Study design: cluster‐randomised trial Unit of allocation: community level Unit of analysis: patient Funding source: The Rapid Early Action for Coronary Treatment (REACT) trial was supported by cooperative agreements U01‐HL‐53141, U01‐HL‐53142, U01‐ HL‐53149, U01‐HL‐53155, U01‐HL‐53211, U01‐HL‐53135 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland. Additional support for reproduction of intervention materials was provided by Genentech, Inc. Confict of interest: None reported |
|
| Participants |
Country: US, HIC Setting: communities recruited by five REACT field centres at the University of Alabama at Birmingham; University of Minnesota; University of Massachusetts Medical School; University of Texas Health Science Center at Houston; and University of Washington, in collaboration with Oregon Health Sciences University Numbers/conditions: 10 matched pairs from 20 US cities (1 for intervention, 1 for control). The study communities ranged in size from approximately 56,000 to 240,000 persons. Of the numbers recruited (36,151), only 20,364 met the criteria of suspected acute coronary heart disease (CHD) on admission and discharged with a CHD‐related diagnosis (10,563 intervention, 9,801 control). The eligible diagnoses were suspected acute CHD and discharged with a CHD‐related diagnosis (ICD 410‐414,427‐429, 440, 786.9). |
|
| Interventions |
Theoretical framework: Social Cognitive Theory and Self‐Regulatory Theory Rationale: to decrease patient delay from symptom onset to hospital admission, and to increase emergency services use by increasing knowledge about cardiac symptoms and improving attitudes and beliefs about seeking care immediately. The multi‐component educational programme targeted the cognitive, environmental and behavioural factors influencing individuals’ healthcare‐seeking behaviour. The four intervention components were:1) community organisation; 2) professional education; 3) patient education; 4) public education Targeted at: (1) general public, (2) those at high risk of ischaemic heart disease such as the elderly, persons with risk factors for coronary heart disease (CHD) (e.g. hypertension, dyslipidaemia, or diabetes), or with a history of CHD and (3) healthcare providers, including physicians, nurses, and emergency staff, in an effort to promote their participation and increase office or hospital‐based patient education and counselling activities related to the REACT message Delivered by: some patient education was conducted by REACT staff, but providers were relied on to deliver most of the patient education. Emergency services staff volunteered as speakers at REACT community events and were heavily featured in the REACT media. Healthcare organisations, including hospitals and health maintenance organisations (HMOs) also were involved. Materials: the patient education component included interpersonal (individual and group) as well as impersonal (flyers/brochures, posters, magnets and other 'tokens', and video) strategies to reach high‐risk patients and their families. Procedures: the key message delivered to the public and patients was the need to recognise the signs and symptoms of acute cardiac ischaemia and to seek emergency medical care promptly if such symptoms persisted for at least 15 minutes, preferably by calling 911 to summon local emergency services. Professional education intervention components were designed to: (1) improve understanding of factors related to patient delay, (2) enhance motivation to learn skills and intervene with patients, (3) enhance patient‐centred counselling, and (4) impact clinical practice. Modes of delivery: community education targeted the general public through three approaches: mass media, such as radio, newspaper, and television; local media, such as newsletters, billboards, and brochures; and events conducted in group settings. Principles of patient‐centred counselling, role‐modelling, and behavioural rehearsal were employed by healthcare providers and EMS personnel in the community. Where: community, hospital settings When and how much: the intervention was delivered over an 18‐month period. The community organisation component was begun first to promote community support. Professional education was also begun early to inform providers, develop programme support, and provide a platform for subsequent patient education. The community education component was divided into six phases, each lasting three months. During each phase, a distinct theme was emphasised, the last of which emphasised the benefits of using emergency services when an acute cardiac event was suspected. Fidelity/Tailoring: aimed to create a uniform, but flexible, intervention by creating minimum core exposure standards and detailing activities in community through Site Action Plans. Intervention intensity (numbers of high risk patients reached, distribution of print and video materials to patient groups) was measured. Attrition: 5% (n = 1900) of patients were admitted and discharged with noncardiac diagnoses and were excluded from these analyses. Patients with missing data on insurance (2.2%), marital status (2.7%), or blood pressure (0.6%) were also excluded from these analyses, leaving a sample of 22,701 patients in the primary population. Comparison: matched community group, comparable on selected sociodemographic characteristics and population size, acted as control. |
|
| Outcomes |
Time from symptom onset to emergency department arrival Tool: hospital medical record (emergency department staff received training in recording nature and time of onset of symptoms) Review category: Treatment outcomes/timeliness Measured at baseline (3 months) and 18 months Use of emergency medical services Tool: hospital medical record Review category: Service use Measured at baseline (3 months) and 18 months Knowledge, attitudes and behaviours relevant to care‐seeking Tool: random digit dial telephone survey delivered by co‐ordinating centre which asked questions about signs and symptoms of a heart attack and appropriate actions. Responses were coded to predetermined categories. Review category: Patient and family outcomes/knowledge Measured at 4 time points (baseline, early, mid and late in the study) Mortality Tool: hospital medical record Measured at baseline (3 months) and 18 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | One city in each pair was assigned to intervention, the other to control, but no further details of randomisation were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As there was multimedia and community involvement, the participants and personnel were aware. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in any detail |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | No available protocol |
| Other bias | Unclear risk | Unable to assess selective recruitment of cluster participants as details not reported. Demographic characteristics of the matched pairs of intervention and control cities were similar. Exclusion criteria: (patients who were living in an institutional setting at the time of hospital presentation, had chest pain due to trauma, or had been transferred from a nonparticipating hospital) may affect generalisability. |
Mooney 2014.
| Study characteristics | ||
| Methods |
Study design: randomised trial Unit of allocation: patient Unit of analysis: patient Funding source: Health Research Board, Ireland Confict of interest: none declared |
|
| Participants |
Country: Republic of Ireland Setting: 5 large tertiary hospitals Numbers/conditions: patients hospitalised with Acute Coronary Syndrome (ACS) diagnosis (n = 1944; control: 972, intervention: 972). |
|
| Interventions |
Theoretical framework: Leventhal Self‐Regulatory Model of illness behaviour Rationale: education on prehospital delay can reduce decision delay, physician delay, and transport delay. Delivered by: delivered by research nurse Materials: take home action plan (reminders of what to do if symptoms arise; list of emergency numbers and location of nearest ED) and card (lists typical and atypical ACS symptoms and reminder of key intervention messages) Procedures: face‐to‐face individualised intervention using flip charts and prescriptive scripts as educational aides. Motivational coaching by research nurse of patient (and sometimes family member); patient completion of take‐home action plan (x 1). Education on decision delay was individualised to patient’s specific needs and illness experiences and included: symptom recognition and variability (and actual and potential responses to them); when to alert someone else; when to use prescribed nitrites; positive messages to reinforce early treatment; use of patient’s own study to enhance information impact; and role play to assist development of action plan. Motivational coaching helped to challenge the cognitive and emotional responses that delay treatment seeking. Education on physician and transport delay included a focus on the need to access emergency services (not GP), and on the importance of ambulance use. Modes of delivery: face‐to‐face, telephone and letter follow‐up Where: first session in hospital, follow‐up reinforcement phone call When and how much: 40 min to deliver face‐to‐face component. Contacted (by telephone) after 4 weeks of intervention delivery (to support and reinforce key messages); contacted (by letter) 6 months later to reinforce intervention and key messages Fidelity/Tailoring: fidelity to the trial was optimised through the delivery of identical education and training to each research nurse with respect to the purpose and design of the trial, data collection skills, ethical requirements, and the delivery of the educational intervention. The intervention was individualised to patient’s specific needs and illness. Attrition: 14 withdrew, 21 died Comparison: usual care which comprised predischarge patient education (ACS symptoms, medications, modifiable risk factors, and advice about lifestyle adjustments) |
|
| Outcomes |
Prehospital delay time Tool: ACS Response Index (Riegel 2007), hospital medical record, patient self‐report Review category: Treatment outcomes/timeliness Measured at 3, 12 and 24 months Ambulance use Tool: ACS Response Index (Riegel 2007), hospital medical record, patient self‐report Review category: Service use Measured at 3, 12 and 24 months Nitrate use Tool: ACS Response Index (Riegel 2007), hospital medical record Measured at 3, 12 and 24 months Time to disclosure of symptoms to someone else within 30 mins of onset Tool: ACS Response Index (Riegel 2007), hospital medical record Measured at 3, 12 and 24 months Review category: Patient and family outcomes/care‐seeking behaviours Consultation with a GP prior to attending the ED Tool: ACS Response Index (Riegel 2007), hospital medical record Measured at 3, 12 and 24 months Review category: Service use |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator used to devise random sequences for the five hospitals. Random numbers divided into blocks of 20 for each site (50% control and 50% intervention for each block). |
| Allocation concealment (selection bias) | Low risk | Group assignment was concealed until after baseline data was collected. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No intended blinding. Not possible to blind personnel or participants to intervention. However ‘usual care’ was not completely standardised between the research sites (except no site delivered extensive information). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported and similar across groups (10 withdrew and 17 died in each group) |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available |
| Other bias | High risk | Additional concern that listed participant characteristics did not include geographical factors (notably, urban or rural residence; proximity to ED) even though prehospital delay time being tested. Geographical variation between urban and rural hospitals noted in study limitations (Pg. 503) however recruitment and training was through tertiary (not urban) hospitals. Exclusion criteria (those with cognitive impairment (e.g. severe learning disability or due to neurological disorder), or living in an institutional setting or with complicating serious comorbidity e.g. psychiatric illness or untreated malignancy) may limit generalisability. |
Norman 2018.
| Study characteristics | ||
| Methods |
Study design: stepped wedge, cluster‐randomised trial Unit of allocation: maternity unit level Unit of analysis: patient Funding source: Chief Scientist Office, the Scottish Government (CZH/4/882); Tommy’s Charity and Sands (Stillbirth and Neonatal Death charity) Confict of interest: JEN reports grants from Chief Scientist Office, Tommy’s, and Sands during the conduct of the study, and grants from NIHR and the Medical Research Council and consultancy payments to the University of Edinburgh from GlaxoSmithKline and Dilafor, outside the submitted work. CJW reports grants from the Chief Scientist Office, the Scottish Government (CZH/4/882), Tommy’s, and Sands, and salary support from NHS Lothian (via the Edinburgh Clinical Trials Unit) during the conduct of the study. AR reports grants from the Chief Scientist Office, the Scottish Government (CZH/4/882), Tommy’s, and Sands during the conduct of the study. AEPH reports grants from NIHR during the conduct of the study. AH was appointed as a trustee of Sands (which part‐funded AFFIRM) after AFFIRM was funded. All other authors declared no competing interests. |
|
| Participants |
Country: UK and Ireland Setting: 37 public maternity hospitals were recruited. All women delivering at the maternity hospitals were enrolled. Whilst all maternity hospitals in Scotland were expected to join those in England, Ireland, and Wales joined voluntarily. Limited information was collected on characteristics of the participating hospitals (e.g. how many district and specialist units were included in the study), how many had home birth centres) versus non‐participating hospitals and units. Numbers/conditions: data were collected from 409,175 pregnancies (157,692 deliveries during the control period, 23,623 deliveries in the washout period, and 227,860 deliveries in the intervention period). |
|
| Interventions |
Theoretical framework: not reported Rationale: the intervention was based on a large observational study conducted in Norway (Tveit 2009) that found a significant fall in rates of stillbirth after the introduction of an intervention package which included information for women and a standardised management protocol. The study rationalised that a reduced fetal movement (RFM) care package for pregnant women and clinicians that increased women’s awareness of the need for prompt reporting of RFM, and a standardised clinical management, including timely delivery, would alter the incidence of stillbirth. Targeted at: pregnant women and clinicians (doctors, midwives and ultrasonographers) Delivered by: midwives to women, educators to staff Materials: 1) an e‐learning education package for clinical staff about the importance of a recent change in the frequency of fetal movements and how to manage RFM; 2) training/information sessions in each unit; 3) a leaflet for pregnant women which described the importance of fetal movements, the need to get to know normal fetal activity, how fetal movements change in late pregnancy and who to contact if the mother perceives RFM. This AFFIRM information leaflet was available in 12 languages including: Arabic, Bengali, English, Hindi, Hungarian, Latvian, Lithuanian, Mandarin, Polish, Russian and Urdu; 4) a clinical management plan for identification and delivery of babies at high risk; 5) changes to local protocol for managing RFM; 6) posters in each unit to describe the practice change Procedures: a link to the e‐learning package was emailed to all clinicians in the participating unit one month before implementation of the package to ensure that staff reinforced messaging at antenatal contacts. The leaflet for pregnant women was distributed to women at about 20 weeks’ gestation. A management plan for identification and delivery of babies at high risk was distributed to hospitals for management of women who presented with RFM from 24 weeks’ gestation which included cardiotocography, measurement of liquor volume, and a growth scan to estimate fetal weight and abdominal circumference. Maternity units were encouraged to use umbilical artery Doppler in addition to the growth scan if the facilities were available. Delivery (with senior clinician input into decision‐making) was recommended for women who were at or after 37 weeks’ gestation who presented with specific criteria, and management of other scenarios was also indicated in the protocol. Modes of delivery: multi‐method, posters, face‐to‐face, online Where: in hospital When and how much: the short web‐based training package took approximately 1 hour to complete for all clinicians in each centre. No detail provided about local training sessions Fidelity/Tailoring: in the leaflet for women, one page was blank and invited local stickers denoting named people "who to contact if you are concerned”. The sites were asked to report if they had implemented the e‐learning education package for staff, issued RFM leaflets to pregnant women, and implemented any of the other three specific aspects of the management plan in line with the protocol. Sites that largely implemented at least four of five of these aspects of the AFFIRM intervention were categorised as adherent, and those sites that had implemented less than four of the aspects were categorised as non‐adherent. 13/33 maternity centres (39·4%) adhered to four or fewer of the five components of the intervention. There was no specification about 'core' essential components of the intervention which guided fidelity of function. Attrition: none reported. 4 withdrew before study control period ‐ reasons for withdrawal: lack of staff (largely sonographer time); cost implications (largely ultrasound scanning costs) Comparison: usual care |
|
| Outcomes |
Stillbirth (babies delivered without signs of life after less than 24 weeks’ gestation, or, if gestation was unknown, weighing 500 g or more) Tool: Scottish Birth record (Scotland), National Perinatal Reporting System (Ireland), Northern Ireland Maternity Statistics database (Northern Ireland), Office of National Statistics (England and Wales) Review category: Clinical outcomes/mortality Measured annually for 2 years Babies born small for gestational age (measuring on or under 10th centile) delivered until or after 40 weeks' gestation Tool: Scottish Birth record (Scotland), National Perinatal Reporting System (Ireland), Northern Ireland Maternity Statistics database (Northern Ireland), Office of National Statistics (England and Wales) Review category: Clinical outcomes/morbidity Measured annually for 2 years Other outcomes from the trial but not in our review included: stillbirth at 22, 24, 28 and 37 weeks’ gestation and above; perinatal mortality; number of caesarean sections; rates of admission to the neonatal unit; induction of labour; number of elective deliveries; number of spontaneous vaginal deliveries; gestation at birth; proportion of babies born preterm (< 37 weeks’ gestation); sex of the baby; birthweight of the baby; Apgar scores; and resuscitation required at birth Tool: Scottish Birth record (Scotland), National Perinatal Reporting System (Ireland), Northern Ireland Maternity Statistics database (Northern Ireland), Office of National Statistics (England and Wales) Measured annually for 2 years |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clusters were randomised before the beginning of the trial, using a computer‐generated allocation scheme, to one of nine intervention implementation dates. |
| Allocation concealment (selection bias) | Low risk | Participating maternity hospitals were grouped and randomised, using a computer‐generated allocation scheme, to one of nine intervention implementation dates (at 3‐month intervals). This date was concealed from clusters and the trial team until 3 months before the implementation date. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unblinded ‐ stepped wedge design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data, potential confounders, and effect modifiers were derived from routinely collected hospital data (database codes were described in the appendix). Data were anonymised at the source and transferred at yearly intervals by secure file transfer to a dedicated AFFIRM project area in the NHS Scotland electronic Data Research and Innovation Service (eDRIS); there were 18 where statistical analysis was done at the end of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 37 hospitals were enrolled in the study, but 4 withdrew and provided no data before the study control period because of lack of staff (mostly sonographer time) and cost implications (largely ultrasound scanning costs). Data from all 409,175 women from the remaining 33 hospitals were included in the primary intention‐to‐treat analysis. 87 of 409,175 datapoints (0.02%) were missing from the primary outcome analysis. 409,175 women delivered during the study (157,692 deliveries during the control period, 23,623 deliveries in the washout period, and 227,860 deliveries in the intervention period). There were no obvious differences reported between groups. |
| Selective reporting (reporting bias) | Low risk | Protocol available. All outcomes reported with one difference (proportion of infants with birthweight less than the fifth centile reported in protocol, proportion of infants with birthweight less than the tenth centile reported in trial) |
| Other bias | High risk | There were no obvious differences in characteristics between the intervention groups. Low risk of bias for selective recruitment of cluster participants. This was a step‐wedge design and all eligible clusters, and all eligible women within clusters, received the intervention at some point during the trial. However, adherence to the intervention was a potential effect modifier, assessed on the basis of results of a questionnaire sent to the lead investigator. Sites that reported implementing at least four of five components of the AFFIRM intervention (staff e‐learning education package; RFM leaflets given to pregnant women; implementation of any of the other three specific aspects of the management plan) were categorised as adherent. The level of variability amongst sites assessed as adherent on the basis of implementation of different components was unclear. The assessment of adherence was also subject to recall and reporting bias. |
Schumacher 2017.
| Study characteristics | ||
| Methods |
Study design: randomised trial Unit of allocation: patient level Unit of analysis: patient level Funding source: Health Policy Grant from the Emergency Medicine Foundation and supported by the UF Clinical and Translational Science Institute, the NIH National Center for Advancing Translational Sciences (UL1 TR000064), and the Patient‐Centered Outcomes Research Institute (IHS‐1306‐01451) Confict of interest: none declared |
|
| Participants |
Country: US, HIC Setting: two community settings: Site 1 ‐ a tertiary referral centre serving a community of 250,000 (62% white; 28% African‐American) with various payers (40% public, 36% private); Site 2 ‐ a tertiary referral centre serving a metropolitan area of one million and African‐American (59%), white (33%), publicly insured (44%) and uninsured (24%) patients Numbers/conditions: 69 chronically ill, older patients who had attended the emergency department with limited health literacy (35 randomised to intervention, and 34 to control) |
|
| Interventions |
Theoretical framework: Not reported. Rationale: the intervention was based on the Care Transitions Intervention (CTI) (Parry 2003, Coleman 2004), an evidence‐based programme to engage older adults, with limited health literacy who frequently turn to the emergency department (ED) for care, to help them maintain engagement, better manage their health and avert future health crises. Targeted at: chronically ill, ED patients (60 years or older) with limited health literacy Delivered by: trained coaches from community area agencies on ageing administered the intervention. Materials: a Personal Health Record Procedures: coaches helped patients 1) schedule follow‐up doctor visits; 2) recognise disease worsening; 3) reconcile medications; and 4) communicate with providers Modes of delivery: face‐to‐face and via telephone When and how much: 1 face‐to‐face visit (60 minutes on average) by coach who visited patients’ homes within three days of ED discharge and 3 follow‐up phone calls (15 minutes per call on average) Fidelity/Tailoring: coaches worked with patients to set individualized goals Attrition: 4 withdrawals/exclusions (1 death, 1 hospice, 2 withdrew). 25 lost to follow‐up Comparison: usual care which included written and verbal discharge instructions and advice to follow‐up with a provider |
|
| Outcomes |
Between‐group differences in Patient Activation Measure scores Tool: Patient Activation Measure Hibbard 2004 Review category: Patient and family outcomes/willingness to participate Measured at baseline and 31‐60 days post‐ED visit Self‐reported doctor visits Tool: Medicare Current Beneficiary Access‐to‐Care Survey items Review category: Service use Measured at 30 days post‐ED visit. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment using a random number generator was provided to research associates (RAs) who determined patient eligibility by screening the ED electronic health record (EHR). RAs were blinded to assignment. |
| Allocation concealment (selection bias) | Low risk | Research Assistants were blinded to assignment until baseline survey completion. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Likely that participants aware, unclear about personnel as the intervention involved coaches. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research Assistants were blinded to assignment until baseline survey completion. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition: only 62% (40/65) completed the follow‐up telephone survey. 25 lost to follow‐up. However, balanced across groups |
| Selective reporting (reporting bias) | Low risk | Outcomes listed on www.clinicaltrials.gov; results published for all outcomes. |
| Other bias | Low risk | No differences in participant characteristics at baseline. Exclusion criteria (dementia, psychosis, active substance use or cancer treatment, dialysis, organ transplantation, living in an institutional setting or hospice care) may affect generalisability. |
See 2014.
| Study characteristics | ||
| Methods |
Study design: Cluster‐randomised trial Unit of allocation: Cluster – two wards Unit of analysis: patient Funding source: not reported Confict of interest: none declared |
|
| Participants |
Country: Singapore Setting: general medical–surgical wards at an acute tertiary hospital Numbers/conditions: 2 wards recruited, 67 individual patients with mixed conditions (34 to intervention, 33 to control) |
|
| Interventions |
Theoretical framework: Not reported. Rationale: by speaking up about changes in their condition, patients can quickly alert the medical team to their deterioration and lead to earlier intervention. The AWARE intervention was developed by a multidisciplinary healthcare team from a Rapid Improvement Escalation workgroup based on concepts of patient safety. Targeted at: patients on general medical–surgical wards Delivered by: a single nurse researcher Materials: a mnemonic handout was attached at the foot of the patients’ beds. Procedures: the intervention focused on three areas: (1) be alert, (2) recognise worsening conditions and (3) report early. The educational contents in the AWARE intervention were validated by four content experts (a nurse educator, an advanced practice nurse, an associate consultant who headed a specialised team involved in acute care initiatives and an academic nurse researcher) for appropriateness and clarity in the presentation style. Be alert: the patients were informed about the importance of early recognition and reporting of deteriorating conditions to prevent delays in treatment, and their contributory role was emphasised. Recognize worsening conditions: common signs and symptoms of deteriorating conditions were presented using the ABCDE (Airway blocked, Breathlessness, Cold hands and feet, Dizziness, Extreme pain, and Expel and Excrete blood) mnemonic together with information on possible causes. Report early: using role play and rehearsal, the patients were taught to practice alerting staff e.g. using the phrase ‘‘I am worried about my condition. I feel. . . . . .’’ followed by their experiencing symptoms. Modes of delivery: multiple learning modalities, face‐to‐face interactive teaching, role play and written instructional materials Where: at the patient’s bedside in hospital When and how much: one‐on‐one session that lasted 30 minutes Fidelity/Tailoring: intervention fidelity was assessed during a training session by an expert supervisor and maintained through self‐monitoring in actual implementation using an intervention checklist but results were not reported. Attrition: no attrition Comparison: usual care |
|
| Outcomes |
Levels of self‐efficacy to recognise and report symptoms Tool: Self‐Efficacy Response Scale (Osbourne 2007; Scholz 2002) Review category: Patient and family outcomes/self‐efficacy Measured at baseline and day 3 of hospitalisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The two wards were randomised, using a coin tossing method. |
| Allocation concealment (selection bias) | Low risk | The assignment of the intervention or control revealed only after consent was given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients aware of intervention through nature of the programme (e.g. participation or standard care) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The post‐test assessment using SERS was conducted on the third day of the participants’ hospitalisation. In addition to the SERS, the experimental group was given a questionnaire to evaluate their perception of the AWARE intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported loss to follow‐up in either group |
| Selective reporting (reporting bias) | Unclear risk | No available trial protocol |
| Other bias | Unclear risk | Demographic and clinical characteristics were similar between groups. Unable to assess selective recruitment of cluster participants as details not reported |
ABCDE: Airway blocked, Breathlessness, Cold hands and feet, Dizziness, Extreme pain, and Expel and Excrete blood ACS: Acute coronary syndrome AFFIRM: Awareness of fetal movements and care package to reduce fetal mortality AMI: Acute myocardial infarction AWARE: Alert Worsening conditions And Report Early CAD: Coronary artery disease CHD: Coronary heart disease CTI: Care Transitions Intervention CUMC: Columbia University Medical Center DC: District of Columbia ED: Emergency department eDRIS: electronic Data Research and Innovation Service EE: Enhanced educational EHR: Electronic health record EMS: Emergency medical services FAST: Face Droop, Arm Weakness, Speech Affected/Slurred and Time to call 911 f/up: Follow‐up GP: General practitioner HIC: High income country HMO: Health maintenance organisations IMPACT: Improving Paediatric Asthma Care in the District of Columbia PCP: Primary care provider PEPAC: Parent Empowerment Program for Asthma Care PEPPI: Perceived Efficacy in Patient‐ Physician Interactions RA: Research assistant REACT: Rapid Early Action for Coronary Treatment RFM: Reduced fetal movement SCT: Social Cognitive Theory SERS:Self‐Efficacy Response Scale TIA: Transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12617001075370 | Interactive element was on self‐management, rather than escalation of care |
| Arnold 2009 | Insufficient information about intervention, appeared to be based on information provision |
| Barbanel 2003 | Lack of explicit partnership working in the intervention |
| Benzo 2016 | Lack of explicit partnership working in the intervention |
| Blank 2002 | Lack of explicit partnership working in the intervention |
| Boone 2007 | Community‐based system‐level initiative |
| Botngård 2013 | Based on multimedia information provision |
| Community 2016 | No interactive element to the intervention |
| Grzeskowiak 2014 | Focused on health management |
| Jennings 2010 | Based on information provision |
| Jennings 2015 | Based on information provision |
| Kumar 2012 | The intervention was community‐based |
| Müller‐Nordhorn 2009 | No interactive element to the intervention |
| Ong 2016 | Lack of explicit partnership working in the intervention |
| Tongpeth 2020 | Although there was a level of personalisation via the App, the element of interactivity was limited/no coaching |
| Tripathy 2016 | The intervention was almost exclusively based at community level |
| Tsuyuki 2004 | Focus on patient education, self‐care and compliance |
| Williams 2018 | Paediatric population, school‐based education package |
Characteristics of ongoing studies [ordered by study ID]
Mi 2018.
| Study name | A randomized controlled trial testing the effectiveness of a paramedic‐delivered care transitions intervention to reduce emergency department revisits |
| Methods |
Study design: randomised trial Unit of allocation: patient level Unit of analysis: patient level Funding source: The National Institute on Aging of the National Institutes of Health (Award Number R01AG050504) and NIH National Center for Advancing Translational Sciences award (Award Number UL1TR000427) Conflict of interest: none declared |
| Participants |
Country: US Setting: emergency departments (EDs) of three hospitals in Rochester (New York) and Madison (Wisconsin) Numbers/conditions: aiming for a final sample size of 1200 subjects in each group, total target of 2400 |
| Interventions |
Theoretical framework: not reported Rationale: the intervention aimed to enable transition from emergency department (ED) to home, including recognition of red flags or warning signs needing prompt medical attention. Targeted at: patients over 60 years discharged from the ED (including ED observation), within 24 hrs of arrival, and their caregivers. Patients and caregivers must speak English, and have a telephone. Patients must live in independent home dwellings within the specified two US settings and linked to the designated health systems. Delivered by: paramedics from the ambulance‐based emergency medical services (EMS) system Materials: use of a Personal Health Record Procedures: based on the Care Transitions Intervention (Coleman 2004; Parry 2003), which consists of a structured programme. The coach (paramedic) uses motivational interviewing techniques, behaviour modelling, skill transfer, and role playing Modes of delivery: face‐to‐face and via telephone When and now much: home visit following ED discharge, ideally within 24–48 h, and up to three phone calls over 4‐week programme Comparison: usual care |
| Outcomes |
Understanding of red flags Tool: Patient survey Review category: Patient and family outcomes/knowledge Measured at baseline, 4 days post‐discharge Family caregiver activation scores Tool: Family Caregiver Activation in Transitions tool (Coleman 2015) Review category: Patient and family outcomes/willingness to participate Measured at baseline and 30 days post‐ED visit Programme satisfaction Tool: Patient/Caregiver survey Review category: Patient and family outcomes: Receptiveness to, and acceptability of, intervention to patients and families Measured at 30 days post‐ED visit Healthcare use Tool: Patient/Caregiver survey Review category: Service use Measured at 30 days post‐ED visit Other outcomes to be included in the trial but will not be included in our review include: follow‐up with primary care physicians, specialists, urgent care; implementation of medication changes; health competency assessment using Wallston’s Perceived Health Competence Scale (PHCS) (Smith 1995); mortality; patient experiences of continuity, cost of healthcare and programme |
| Starting date | The trial was registered at ClinicalTrials.gov (NCT02520661), registration date: August 13, 2015 |
| Contact information | |
| Notes |
ED: Emergency department EMS: Emergency medical services PHCS: Perceived Health Competence Scale
Differences between protocol and review
During the screening, we further operationalised the term 'involvement in escalation of care' and refined the selection criteria to help with screening. Given the paucity of reported outcomes in our included papers, we expanded our list of outcomes to include all those outcomes within each grouping rather than limit ourselves to only one outcome within each of the groupings (e.g. patient and family outcomes; treatment outcomes; clinical outcomes). We added 'attitudes and beliefs' as an additional patient and family outcome, and broadened 'behavioural intent' to 'behaviours' to include care‐seeking behaviours and behavioural intent (motivation to take on an active role in escalation of care). We also added to our conceptual diagram using a Candidacy Framework to map the interventions in our included papers.
Contributions of authors
NM is the review’s guarantor.
NM conceived the review question, and has led the review process. All authors have contributed to the writing and editing of the protocol for publication.
Responsibilities for the full review are as follows:
| Develop and run searches | NM |
| Obtain studies | NM |
| Select which studies to include (minimum of 2 people) | NM, JS, NS, RD, SW, AE, MA, HR‐J |
| Extract data from studies (minimum of 2 people) | NM, RD, SW, AE, MA, HR‐J |
| Enter data into Review Manager 5 | NM |
| Analysis | NM, JS, NS, RD, SW, HR‐J, AE, MA, |
| Prep review report | NM, JS, NS, RD, SW, HR‐J, AE, MA |
| Update review | NM, JS, NS, RD, SW, HR‐J, AE, MA |
Sources of support
Internal sources
-
Author sources of support, UK
Nick Sevdalis and Jane Sandall’s research is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) South London at King’s College Hospital NHS Foundation Trust. Nick Sevdalis is also a member of King’s Improvement Science, which offers co‐funding to the NIHR ARC South London and comprises a specialist team of improvement scientists and senior researchers based at King’s College London. Its work is funded by King’s Health Partners (Guy’s and St Thomas’ NHS Foundation Trust, King’s College Hospital NHS Foundation Trust, King’s College London and South London and Maudsley NHS Foundation Trust), Guy’s and St Thomas’ Charity and the Maudsley Charity.
External sources
No sources of support supplied
Declarations of interest
Nicola Mackintosh: none known
Jane Sandall has a long standing interest in this topic from a patient and family, and professional and policy response viewpoint. She was a member of the WHO Expert advisory group member on patient participation in reducing healthcare‐related safety risks, WHO Regional Office for Europe. She is a member of the National Childbirth Trust Research Advisory Group and RCOG SANDS Stillbirth Study Group Member and Obstetric Anaesthetists' Association Maternal Critical Care Sub Committee.
Dr Nick Sevdalis is the director of London Safety & Training Solutions Ltd, which delivers team interventions, assessments and training to hospitals in the UK and internationally on a consultancy basis.
Rachel Davis: none known
Hannah Rayment‐Jones: none known
Abigail Easter: none known
Sophie Williams: none known
Mary Adams: none known
New
References
References to studies included in this review
Boden Albala 2015 {published data only}
- Boden-Albala B, Stillman J, Perez T, Evensen L, Moats H, Wright C, et al. A stroke preparedness RCT in a multi-ethnic cohort: design and methods. Contemporary Clinical Trials 2010;31:235-41. [DOI] [PubMed] [Google Scholar]
- *.Boden-Albala B, Stillman J, Roberts ET, Quarles LW, Glymour MM, Chong J, et al. Comparison of acute stroke preparedness strategies to decrease emergency department arrival time in a multi-ethnic cohort. The Stroke Warning Information and Faster Treatment study. Stroke 2015;46(7):1806-12. [DOI] [PubMed] [Google Scholar]
Buckley 2007 {published data only}
- *.Buckley T, McKinlay S, Gallagher R, Dracup K, Moser DK, Aitken LM. The effect of education and counselling on knowledge, attitudes and beliefs about responses to acute myocardial infarction symptoms. European Journal of Cardiovascular Nursing 2007;6(2):105-11. [DOI] [PubMed] [Google Scholar]
Dracup 2009 {published data only}
- Dracup K, McKinley S, Riegel B, Mieschke H, Doering LV, Moser DK. A nursing intervention to reduce prehospital delay in acute coronary syndrome: a randomized clinical trial. Journal of Cardiovascular Nursing 2006;21(3):186-93. [DOI] [PubMed] [Google Scholar]
- *.Dracup K, McKinley S, Riegel B, Moser DK, Meischke H, Doering LV, et al. A randomized clinical trial to reduce patient prehospital delay to treatment in acute coronary syndrome. Circulation: Cardiovascular Quality and Outcomes 2009;2(6):524-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser DK, McKinley S, Riegel B, Doering LV, Meischke H, Pelter M, et al. The impact on anxiety and perceived control of a short one-on-one nursing intervention designed to decrease treatment seeking delay in people with coronary heart disease. European Journal of Cardiovascular Nursing 2012;11(2):160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel B, Elmi A, Moser DK, McKinley S, Meischke H, Doering LV, et al. Who listens to our advice? A secondary analysis of data from a clinical trial testing an intervention designed to decrease delay in seeking treatment for acute coronary syndrome. Patient Education and Counseling 2011;85(2):e33-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horn 2014 {published data only}
- *.Horn IB, Mitchell SJ, Gillespie CW, Burke KM, Godoy L, Teach SJ. Randomized trial of a health communication intervention for parents of children with asthma. Journal of Asthma 2014;51(9):989-95. [DOI] [PubMed] [Google Scholar]
Luepker 2000 {published data only}
- Feldman HA, Proschan MA, Murray DM, Goff DC, Stylianou M, Dulberg E, et al. Statistical design of REACT (Rapid Early Action for Coronary Treatment), a multisite community trial with continual data collection. Controlled Clinical Trials 1998;19(4):391-403. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sellers DE, McGovern PG, Meischke H, Goldberg RJ, Bittner V, et al. Knowledge of heart attack symptoms in a population survey in the United States: the REACT trial. Archives of Internal Medicine 1998;158(21):2329-38. [DOI] [PubMed] [Google Scholar]
- *.Luepker RV, Raczynski JM, Osganian S, Goldberg RJ, Finnegan Jr JR, Hedges JR, et al. Effect of a community intervention on patient delay and emergency medical service use in acute coronary heart disease: the Rapid Early Action for Coronary Treatment (REACT) trial. JAMA 2000;284(1):60-7. [DOI] [PubMed] [Google Scholar]
- Osganian SK, Zapka JG, Feldman HA, Goldberg RJ, Hedges JR, Eisenberg MS, et al. Use of emergency medical services for suspected acute cardiac ischemia among demographic and clinical patient subgroups: the REACT trial. Prehospital Emergency Care 2002;6(2):175-85. [DOI] [PubMed] [Google Scholar]
- Raczynski JM, Finnegan Jr JR, Zapka JG, Meischke H, Meshack A, Stone EJ, et al. REACT theory-based intervention to reduce treatment-seeking delay for acute myocardial infarction. American Journal of Preventive Medicine 1999;16(4):325-34. [DOI] [PubMed] [Google Scholar]
Mooney 2014 {published data only}
- *.Mooney M, McKee G, Fealy G, O'Brien F, O'Donnell S, Moser D. A randomized controlled trial to reduce prehospital delay time in patients with acute coronary syndrome (ACS). Journal of Emergency Medicine 2014;46(4):495-506. [DOI] [PubMed] [Google Scholar]
Norman 2018 {published data only}
- Heazell AE, Weir CJ, Stock SJ, Calderwood CJ, Burley SC, Froen JF, et al. Can promoting awareness of fetal movements and focusing interventions reduce fetal mortality? A stepped wedge cluster randomised trial (AFFIRM). BMJ Open 2017;7(8):p.e014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Norman JE, Heazell AE, Rodriguez A, Weir CJ, Stock SJ, Calderwood CJ, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge, cluster-randomised trial. Lancet 2018;392:1629-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2017 {published data only}
- *.Schumacher JR, Lutz BJ, Hall AG, Pines JM, Jones AL, Hendry P, et al. Feasibility of an ED-to-home intervention to engage patients: a mixed-methods investigation. Western Journal of Emergency Medicine 2017;18(4):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
See 2014 {published data only}
- *.See MTA, Chan WCS, Huggan PJ, Tay YK, Liaw SY. Effectiveness of a patient education intervention in enhancing the self-efficacy of hospitalized patients to recognize and report acute deteriorating conditions. Patient Education and Counseling 2014;97(1):122-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12617001075370 {published data only}
- ACTRN12617001075370. Chronic Obstructive Pulmonary Disease (COPD) management at home to reduce emergency department presentations: a randomised controlled, feasibility trial. ACTRN12617001075370 (first received 25 July 2017).
Arnold 2009 {published data only}
- Arnold J, Goodacre S, Bath P, Price J. Information sheets for patients with acute chest pain: randomised controlled trial. British Medical Journal 2009;338:b541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbanel 2003 {published data only}
- Barbanel D, Eldridge S, Griffiths C. Can a self-management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 2003;58(10):851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzo 2016 {published data only}
- Benzo R, Vickers K, Novotny PJ, Tucker S, Hoult J, Neuenfeldt P, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization. A randomized study. American Journal of Respiratory and Critical Care Medicine 2016;194(6):672-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blank 2002 {published data only}
- Blank FS, Smithline HA. Evaluation of an educational video for cardiac patients. Clinical Nursing Research 2002;11(4):403-16. [DOI] [PubMed] [Google Scholar]
Boone 2007 {published data only}
- Boone P, Mann V, Eble A, Mendiratta T, Mukherjee R, Figueiredo R, et al. Community health and medical provision: impact on neonates (the CHAMPION trial). BMC Pediatrics 2007;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Botngård 2013 {published data only}
- Botngård A, Skranes LP, Skranes J, Døllner H. Multimedia based health information to parents in a pediatric acute ward: a randomized controlled trial. Patient Education and Counseling 2013;93(3):389-93. [DOI] [PubMed] [Google Scholar]
Community 2016 {published data only}
- *.TEC4Home Healthcare Innovation Community. Supporting heart failure patient transitions from acute to community care with home telemonitoring technology: A protocol for a provincial randomized controlled trial (TEC4Home). JMIR Research Protocols 2016;5(4):e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grzeskowiak 2014 {published data only}
- Grzeskowiak LE, Dekker G, Rivers K, Roberts-Thomson K, Roy A, Smith B, et al. A randomized controlled trial to assess the clinical and cost effectiveness of a nurse-led Antenatal Asthma Management Service in South Australia (AAMS study). BMC Pregnancy and Childbirth 2014;14(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2010 {published data only}
- Jennings L, Yebadokpo AS, Affo J, Agbogbe M. Antenatal counseling in maternal and newborn care: use of job aids to improve health worker performance and maternal understanding in Benin. BMC Pregnancy and Childbirth 2010;10(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2015 {published data only}
- Jennings L, Yebadokpo A, Affo J, Agbogbe M. Use of job aids to improve facility-based postnatal counseling and care in rural Benin. Maternal and Child Health Journal 2015;19(3):557-65. [DOI] [PubMed] [Google Scholar]
Kumar 2012 {published data only}
- Kumar V, Kumar A, Das V, Srivastava NM, Baqui AH, Santosham M, et al, Saksham Study Group. Community‐driven impact of a newborn‐focused behavioral intervention on maternal health in Shivgarh, India. International Journal of Gynecology & Obstetrics 2012;117(1):48-55. [DOI] [PubMed] [Google Scholar]
Müller‐Nordhorn 2009 {published data only}
- Müller-Nordhorn J, Wegscheider K, Nolte CH, Jungehülsing GJ, Rossnagel K, Reich A, et al. Population-based intervention to reduce prehospital delays in patients with cerebrovascular events. Archives of Internal Medicine 2009;169(16):1484-90. [DOI] [PubMed] [Google Scholar]
Ong 2016 {published data only}
- Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition–heart failure (BEAT-HF) randomized clinical trial.. JAMA Internal Medicine 2016;176(3):310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tongpeth 2020 {published data only}
- Tongpeth J, Du H, Barry T, Clark RA. Effectiveness of an Avatar application for teaching heart attack recognition and response: a pragmatic randomized control trial. Journal of Advanced Nursing 2020;76:297–311. [DOI] [PubMed] [Google Scholar]
Tripathy 2016 {published data only}
- Tripathy P, Nair N, Sinha R, Rath S, Gope RK, Rath S, et al. Effect of participatory women's groups facilitated by accredited social health activists on birth outcomes in rural eastern India: a cluster-randomised controlled trial. Lancet Global Health 2016;4(2):e119-e28. [DOI] [PubMed] [Google Scholar]
Tsuyuki 2004 {published data only}
- Tsuyuki RT, Fradette M, Johnson JA, Bungard TJ, Eurich DT, Ashton T, et al. A multicenter disease management program for hospitalized patients with heart failure. Journal of Cardiac Failure 2004;10(6):473‐80. [DOI] [PubMed] [Google Scholar]
Williams 2018 {published data only}
- Williams O, Leighton-Herrmann QE, Teresi J, Eimicke JP, Kong J, Ogedegbe G, et al. Improving community stroke preparedness in the HHS (Hip-Hop Stroke) randomized clinical trial. Stroke 2018;49(4):972‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Mi 2018 {published data only}
- *.Mi R, Hollander MM, Jones CM, DuGoff EH, Caprio TV, Cushman JT, et al. A randomized controlled trial testing the effectiveness of a paramedic-delivered care transitions intervention to reduce emergency department revisits. BMC Geriatrics 2018;18(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AHA 2005
- American Heart Association. Heart Disease and Stroke Statistics Y2005 Update. Dallas, Texas: American Heart Association, 2005. [Google Scholar]
Ajzen 1991
- Ajzen I. The theory of planned behaviour. Organizational Behavior and Human Decision Processes 1991;50:179-211. [Google Scholar]
Akinbami 2012
- Akinbami LJ, Sullivan SD, Campbell JD, Grundmeier RW, Hartert TV, Lee TA, et al. Asthma outcomes: healthcare utilization and costs. Journal of Allergy and Clinical Immunology 2012;129:S49–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albutt 2017
- Albutt AK, O'Hara JK, Conner MT, Fletcher SJ, Lawton RJ. Is there a role for patients and their relatives in escalating clinical deterioration in hospital? A systematic review. Health Expectations 2017;20(5):818-25. [DOI: 10.1111/hex.12496] [DOI] [PMC free article] [PubMed]
Almond 2009
- Almond S, Mant D, Thompson M. Diagnostic safety-netting. British Journal of General Practice 2009;59(568):872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1990
- Altman DG. Practical Statistics for Medical Research. CRC press, 1990. [Google Scholar]
Bandura 1986
- Bandura A. Social Foundations of Thought and Action: a Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, 1986. [Google Scholar]
Béhague 2008
- Béhague DP, Kanhonou LG, Filippi V, Legonou S, Ronsmans C. Pierre Bourdieu and transformative agency: a study of how patients in Benin negotiate blame and accountability in the context of severe obstetric events. Sociology of Health & Illness 2008;30(4):489-510. [DOI] [PubMed] [Google Scholar]
Berger 2013
- Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Quality & Safety 2014;23(7):548-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Binder 2012
- Binder P, Johnsdotter S, Essén B. Conceptualising the prevention of adverse obstetric outcomes among immigrants using the ‘three delays’ framework in a high-income context. Social Science & Medicine 2012;75(11):2028-36. [DOI] [PubMed] [Google Scholar]
Bosk 2019
- Bosk CL, Pedersen KZ. Blind spots in the science of safety. Lancet 2019;9(10175):978. [DOI] [PubMed] [Google Scholar]
Boyd 2009
- Boyd M, Lasserson TJ, McKean MC, Gibson PG, Ducharme FM, Haby M. Interventions for educating children who are at risk of asthma-related emergency department attendance. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD001290. [DOI: 10.1002/14651858.CD001290.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2008
- Brown PR. Trusting in the new NHS: instrumental versus communicative action. Sociology of Health & Illness 2008;30(3):349-63. [DOI] [PubMed] [Google Scholar]
CCCRG 2014
- Cochrane Consumers and Communication Review Group. Standard protocol text and additional guidance for review authors, (cccrg.cochrane.org) (accessed prior to 12 November 2020).
Chan 2011
- Chan RJ, Webster J, Marquart L. Information interventions for orienting patients and their carers to cancer care facilities. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD008273. [DOI: 10.1002/14651858.CD008273.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandratheva 2010
- Chandratheva A, Lasserson DS, Geraghty OC, Rothwell PM. Population-based study of behavior immediately after transient ischemic attack and minor stroke in 1000 consecutive patients: lessons for public education. Stroke 2010;41(6):1108-14. [DOI] [PubMed] [Google Scholar]
Cheyne 2007
- Cheyne H, Terry R, Niven C, Dowding D, Hundley V, McNamee P. 'Should I come in now?’ A qualitative investigation of how women’s experiences in early labour influence their decision of when to go to hospital. British Journal of Midwifery 2007;15(10):604-9. [Google Scholar]
Cohn 2015
- Cohn S. ‘Trust my doctor, trust my pancreas’: trust as an emergent quality of social practice. Philosophy, Ethics, and Humanities in Medicine 2015;10(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2004
- Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. Journal of the American Geriatrics Society 2004;52(11):1817-25. [DOI] [PubMed] [Google Scholar]
Coleman 2015
- Coleman EA, Ground KL, Maul A. The family caregiver activation in transitions (FCAT) tool: a new measure of family caregiver self-efficacy. Joint Commission Journal on Quality and Patient Safety 2015;41(11):502-7. [DOI] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
CRD42015015326
- CRD42015015326. Review of Paediatric Early Warning Systems (PEWS) and scores for clinical deterioration of children in hospital: their development and validation, effectiveness and factors associated with implementation and generative mechanisms. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015015326 (accessed prior to 8 August 2017). [DOI: 10.15124/CRD42015015326] [DOI]
Davis 2008
- Davis RE, Koutantji M, Vincent CA. How willing are patients to question healthcare staff on issues related to the quality and safety of their healthcare? An exploratory study. Quality & Safety in Health Care 2008;17(2):90-6. [DOI] [PubMed] [Google Scholar]
Davis 2012
- Davis RE, Sevdalis N, Jacklin R, Vincent CA. An examination of opportunities for the active patient in improving patient safety. Journal of Patient Safety 2012;8(1):36-43. [DOI] [PubMed] [Google Scholar]
Denham 2008
- Denham C. SBAR for Patients. Journal of Patient Safety 2008;4(1):38-48. [Google Scholar]
Dixon‐Woods 2006
- Dixon-Woods M, Cavers D, Agarwal S, Annandale E, Arthur A, Harvey J, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Medical Research Methodology 2006;6(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
DoH 2013
- Department of Health. Report of the Mid Staffordshire NHS Foundation Trust Public Inquiry Volumes 1-3. London: The Stationery Office, 2013. [Google Scholar]
Doherty 2012
- Doherty C, Stavropoulou C. Patients' willingness and ability to participate actively in the reduction of clinical errors: a systematic literature review. Social Science & Medicine 2012;75(2):257-63. [DOI] [PubMed] [Google Scholar]
Draper 2015
- Draper ES, Kurinczuk JJ, Kenyon S (Eds), MBRRACE-UK. MBRRACE-UK Perinatal Confidential Enquiry: Term, Singleton, Normally Formed, Antepartum Stillbirth. University of Leicester, Leicester: Infant Mortality and Morbidity Studies, 2015. [Google Scholar]
Dwamena 2012
- Dwamena F, Holmes-Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD003267. [DOI: 10.1002/14651858.CD003267.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrich 2003
- Ehrich K. Reconceptualizing 'Inappropriateness': researching multiple moral positions in demand for primary healthcare. Health 2003;7(1):109-26. [Google Scholar]
Eisenberg 2005
- Eisenberg EM, Murphy AG, Sutcliffe K, Wears R, Schenkel S, Perry S, et al. Communication in emergency medicine: implications for patient safety 1. Communication Monographs 2005;72(4):390-413. [Google Scholar]
Entwistle 2005
- Entwistle VA, Mello MM, Brennan TA. Advising patients about patient safety: current initiatives risk shifting responsibility. Joint Commission Journal on Quality and Patient Safety/Joint Commission Resources 2005;31(9):483-94. [DOI] [PubMed] [Google Scholar]
Entwistle 2006
- Entwistle VA, Watt IS. Patient involvement in treatment decision-making: the case for a broader conceptual framework. Patient Education and Counselling 2006;63(3):268-78. [DOI] [PubMed] [Google Scholar]
Entwistle 2010
- Entwistle VA, McCaughan D, Watt IS, Birks Y, Hall J, Peat M, et al. Speaking up about safety concerns: multi-setting qualitative study of patients' views and experiences. Quality and Safety in Health Care 2010;19(6):1-7. [DOI] [PubMed] [Google Scholar]
Eri 2009
- Eri TS, Blystad A, Gjengedal E, Blaaka G. Negotiating credibility: first-time mothers' experiences of contact with the labour ward before hospitalisation. Midwifery 2009;26(6):e25-e3. [DOI] [PubMed] [Google Scholar]
European Patients' Forum 2017
- European Patients' Forum. In: Patient & family empowerment for better patient safety. EPF Conference 2016. www.eu-patient.eu/globalassets/events/2016/2016-ps-conference/2017_patients_family_empowerment_for_better_safety_conference_report.pdf, 2017.
Fishbein 1980
- Fishbein M. A theory of reasoned action: some applications and implications. Nebraska Symposium on Motivation 1980;27:65-116. [PubMed] [Google Scholar]
Flenady 2011
- Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 2011;377(9774):1331-40. [DOI] [PubMed] [Google Scholar]
Gill 2018
- Gill FJ, Leslie GD, Marshall AP. Barriers and facilitators to implementing a process to enable parent escalation of care for the deteriorating child in hospital. Health Expectations 2018;21(6):1095-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenhalgh 2015
- Greenhalgh T, Clinch M, Afsar N, Choudhury Y, Sudra R, Campbell-Richards D, et al. Socio-cultural influences on the behaviour of South Asian women with diabetes in pregnancy: qualitative study using a multi-level theoretical approach. BMC Medicine 2015;13(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grossmann‐Kendall 2001
- Grossmann-Kendall F, Filippi V, De Koninck M, Kanhonou L. Giving birth in maternity hospitals in Benin: testimonies of women. Reproductive Health Matters 2001;9(18):90–8. [DOI] [PubMed] [Google Scholar]
Hall 2010
- Hall J, Peat M, Birks Y, Golder S, Entwistle V, Gilbody S, et al. Effectiveness of interventions designed to promote patient involvement to enhance safety: a systematic review. Quality and Safety in Health Care 2010;19(5):e10. [DOI] [PubMed] [Google Scholar]
Hibbard 2004
- Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Services Research 2004;39 (4p1):1005-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
Hor 2013
- Hor SY, Godbold N, Collier A, Iedema R. Finding the patient in patient safety. Health 2013;17(6):567-83. [DOI] [PubMed] [Google Scholar]
Houston 2000
- Houston AM, Pickering AJ. 'Do I or don’t I call the doctor’: a qualitative study of parental perceptions of calling the GP out-of-hours. Health Expectations 2000;3(4):234-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howcroft 2016
- Howcroft M, Walters EH, Wood-Baker R, Walters JAE. Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005074. [DOI: 10.1002/14651858.CD005074.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrisos 2013
- Hrisos S, Thomson RG. Seeing it from both sides: do approaches to involving patients in improving their safety risk damaging the trust between patients and healthcare professionals? An interview study. PLOS One 2013;8(11):e80759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hueckel 2012
- Hueckel RM, Mericle JM, Frush K, Martin PL, Champagne MT. Implementation of condition help: family teaching and evaluation of family understanding. Journal of Nursing Care Quality 2012;27(2):176-81. [DOI] [PubMed] [Google Scholar]
ISWP 2010
- Intercollegiate Stroke Working Party. National Sentinel Stroke Audit Report 2009 London. London: Royal College of Physicians, 2010. [Google Scholar]
Johnson 2015
- Johnson JH, Haskell H, Barach P. Case Studies in Patient Safety: Foundations for Core Competencies. Burlington, MA: Jones & Bartlett Learning, 2015. [Google Scholar]
Johnstone 2006
- Johnstone M-J, Kanitsaki O. Culture, language, and patient safety: making the link. International Journal for Quality in Health Care 2006;18(5):383-8. [DOI] [PubMed] [Google Scholar]
Johnstone 2009
- Johnstone MJ, Kanitsaki O. Engaging patients as safety partners: some considerations for ensuring a culturally and linguistically appropriate approach. Health Policy 2009;90(1):1-7. [DOI] [PubMed] [Google Scholar]
Keogh 2013
- Keogh B. Review into the Quality of Care and Treatment Provided by 14 Hospital Trusts in England: Overview Report. London: NHS, 2013. [Google Scholar]
King 2019
- King L, Peacock G, Crotty M, Clark R. Consumers’ perspectives on their involvement in recognizing and responding to patient deterioration — developing a model for consumer reporting. Health Expectations 2019;22(3):385-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkup 2015
- Kirkup B. The Report of the Morecambe Bay Investigation. An independent investigation into the management, delivery and outcomes of care provided by the maternity and neonatal services at the University Hospitals of Morecambe Bay NHS Foundation Trust from January 2004 to June 2013. London: Stationery Office, 2015. [Google Scholar]
Kohn 2000
- Kohn LT, Corrigan JM, Donaldson MS (editors). To Err Is Human: Building a Safer Health Care System. Washington, DC: National Academy Press, 2000. [PubMed] [Google Scholar]
Landrigan 2010
- Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. New England Journal of Medicine 2010;363(22):2124-34. [DOI] [PubMed] [Google Scholar]
Lawton 2012
- Lawton R, Armitage G. The role of the patient in clinical safety. www.health.org.uk/sites/health/files/TheRoleOfThePatientInClinicalSafety.pdf (accessed prior to 8 August 2017).
Lecouturier 2010
- Lecouturier J, Rodgers H, Murtagh MJ, White M, Ford GA, Thomson RG. Systematic review of mass media interventions designed to improve public recognition of stroke symptoms, emergency response and early treatment. BMC Public Health 2010;10(1):784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leistikow 2011
- Leistikow IP, Kalkman CJ, De Bruijn H. Why patient safety is such a tough nut to crack. BMJ 2011;342:d3447. [DOI] [PubMed] [Google Scholar]
Leventhal 1998
- Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: a perceptual-cognitive approach. Psychology & Health 1998;13:717-33. [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llanwarne 2017
- Llanwarne N, Newbould J, Burt J, Campbell JL, Roland M. Wasting the doctor's time? A video-elicitation interview study with patients in primary care. Social Science & Medicine 2017;176:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Løvlien 2008
- Løvlien M, Schei B, Hole T. Myocardial infarction: psychosocial aspects, gender differences and impact on pre-hospital delay. Journal of Advanced Nursing 2008;63(2):148-54. [DOI] [PubMed] [Google Scholar]
Mackintosh 2010
- Mackintosh N, Sandall J. Overcoming gendered and professional hierarchies in order to facilitate escalation of care in emergency situations: the role of standardised communication protocols. Social Science & Medicine 2010;71(9):1683-6. [DOI] [PubMed] [Google Scholar]
Mackintosh 2012
- Mackintosh JE, Murtagh MJ, Rodgers H, Thomson RG, Ford GA, White M. Why people do, or do not, immediately contact emergency medical services following the onset of acute stroke: qualitative interview study. PLOS One 2012;7(10):e46124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackintosh 2017
- Mackintosh N, Rance S, Carter W, Sandall J. Working for patient safety: a qualitative study of women’s help-seeking during acute perinatal events. BMC Pregnancy and Childbirth 2017;17(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maly 1998
- Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient-physician interactions (PEPPI): validationof an instrument in older persons. Journal of the American Geriatrics Society 1998;46:889–94. [DOI] [PubMed] [Google Scholar]
Maly 2004
- Maly RC, Umezawa Y, Leake B, Silliman RA. Determinants of participation in treatment decision-making by older breast cancer patients. Breast Cancer Research and Treatment 2004;85:201–9. [DOI] [PubMed] [Google Scholar]
Mandelzweig 2006
- Mandelzweig L, Goldbourt U, Boyko V, Tanne D. Perceptual, social, and behavioral factors associated with delays in seeking medical care in patients with symptoms of acute stroke. Stroke 2006;37(5):1248-53. [DOI] [PubMed] [Google Scholar]
Miller 2012
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. New York: Guilford Press, 2012. [Google Scholar]
Moser 2009
- Moser DK, Riegel B, McKinley S, Doering LV, Meischke H, Heo S, et al. The control attitudes scale-revised: psychometric evaluation in three groups of patients with cardiac illness. Nursing Research 2009;58:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
National Stroke Association 1996
- National Stroke Association. Awareness and knowledge of stroke prevention: a study of adults 50 years of age and over. National Stroke Association 1996.
Neill 2014
- Neill SJ, Cowley S, Williams C. The role of felt or enacted criticism in understanding parent's help seeking in acute childhood illness at home: a grounded theory study. International Journal of Nursing Studies 2013;50(6):757-67. [DOI] [PubMed] [Google Scholar]
Nelson 2002
- Nelson EC, Batalden PB, Huber TP, Mohr JJ, Godfrey MM, Headrick LA, et al. Microsystems in health care: part 1. Learning from high-performing front-line clinical units. Joint Commission Journal on Quality Improvement 2002;28(9):472-93. [DOI] [PubMed] [Google Scholar]
Nelson 2008
- Nelson EC, Godfrey MM, Batalden PB, Berry SA, Bothe Jr AE, McKinley KE, et al. Clinical microsystems, part 1. The building blocks of health systems. Joint Commission Journal on Quality and Patient Safety 2008;34(7):367-78. [DOI] [PubMed] [Google Scholar]
NFWI‐NCT 2017
- National Federation of Women’s Institutes and NCT . ‘Support overdue’: women’s experiences of maternity services. www.thewi.org.uk/__data/assets/pdf_file/0009/187965/NCT-nct-WI-report-72dpi.pdf (accessed prior to 8 August 2017).
Noyes 2016
- Noyes J, Hendry M, Booth A, Lewin S, Glenton C, Garside R, et al. Current use was established and Cochrane guidance on selection of social theories for systematic reviews of complex interventions was developed. www.sciencedirect.com/science/article/pii/S0895435616000056 (accessed prior to 8 August 2017). [DOI] [PubMed]
NPSA 2015
- National Patient Safety Foundation. Free from Harm: Accelerating Patient Safety Improvement Fifteen Years after To Err Is Human. Boston, MA: National Patient Safety Foundation, 2015. [Google Scholar]
Ocloo 2010
- Ocloo JE. Harmed patients gaining voice: challenging dominant perspectives in the construction of medical harm and patient safety reforms. Social Science & Medicine 2010;71(3):510-6. [DOI] [PubMed] [Google Scholar]
Ocloo 2016
- Ocloo J, Matthews R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Quality & Safety 2016;25(8):626-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osbourne 2007
- Osborne RH, Elsworth GR, Whitfield K. The Health Education Impact Questionnaire (HEIQ): an outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Education and Counseling 2007;66:192–201. [DOI] [PubMed] [Google Scholar]
Parry 2003
- Parry C, Coleman EA, Smith JD, Frank J, Kramer AM. The care transitions intervention: a patient-centered approach to ensuring effective transfers between sites of geriatric care. Home Health Care Services Quarterly 2003;22(3):1-7. [DOI] [PubMed] [Google Scholar]
Peat 2010
- Peat M, Entwistle V, Hall J, Birks Y, Golder S. Scoping review and approach to appraisal of interventions intended to involve patients in patient safety. Journal of Health Services Research & Policy 2010;15(suppl 1):17-25. [DOI] [PubMed] [Google Scholar]
Peytremann‐Bridevaux 2015
- Peytremann-Bridevaux I, Arditi C, Gex G, Bridevaux PO, Burnand B. Chronic disease management programmes for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD007988. [DOI: 10.1002/14651858.CD007988.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 1983
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. Journal of Consulting and Clinical Psychology 1983;51(3):390–5. [DOI] [PubMed] [Google Scholar]
Radestad 2016
- Rådestad I, Akselsson A, Georgsson S, Lindgren H, Pettersson K, Steineck G. Rationale, study protocol and the cluster randomization process in a controlled trial including 40,000 women investigating the effects of mindfetalness. Sexual and Reproductive Health 2016;10:56–61. [DOI] [PubMed] [Google Scholar]
Rainey 2013
- Rainey H, Ehrich K, Mackintosh N, Sandall J. The role of patients and their relatives in ‘speaking up’ about their own safety – a qualitative study of acute illness. Health Expectations 2013;18(3):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rance 2013
- Rance S, McCourt C, Rayment J, Mackintosh N, Carter W, Watson K, et al. Women's safety alerts in maternity care: is speaking up enough? BMJ Quality & Safety 2013;22(4):348-55. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riegel 2007
- Riegel B, McKinley S, Moser DK, Meischke H, Doering L, Dracup K. Psychometric evaluation of the acute coronary syndrome (ACS) response index. Research in Nursing and Health 2007;30:584–94. [DOI] [PubMed] [Google Scholar]
Roscoe 2016
- Roscoe LA, Eisenberg EM, Forde C. The role of patients’ stories in emergency medicine triage. Health Communication 2016 Feb 16 [Epub ahead of print]. [DOI: 10.1080/10410236.2015.1046020] [DOI] [PubMed] [Google Scholar]
Ryan 2011
- Ryan R, Hill S, Prictor M, McKenzie J. Cochrane Consumers and Communication Review Group. Study Quality Guide. (www.latrobe.edu.au/chcp/cochrane/resources) (accessed prior to 12 November 2020).
Ryan 2016
- Ryan R, Hill S. Cochrane Consumers and Communication Group. How to GRADE the quality of the evidence. (cccrg.cochrane.org/author-resources) (accessed prior to 12 November 2020).
Scholz 2002
- Scholz U, Dona BG, Sud S, Schwarzer R. Is general self-efficacy a universal construct? Psychometric findings from 25 countries. European Journal of Psychological Assessment 2002;18:242–51. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ’Summary of findings’ tables. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Schwappach 2008
- Schwappach D. 'Against the silence': development and first results of a patient survey to assess experiences of safety-related events in hospital. BMC Health Services Research 2008;8(59):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwappach 2009
- Schwappach DL. Engaging patients as vigilant partners in safety: a systematic review. Medical Care Research and Review 2009;67(2):119-48. [DOI] [PubMed] [Google Scholar]
Schwappach 2010
- Schwappach DL, Wernli M. Am I (un) safe here? Chemotherapy patients' perspectives towards engaging in their safety. Quality and Safety in Health Care 2010;19(5):e9-e. [DOI] [PubMed] [Google Scholar]
Scott 2012
- Scott J, Bevan C. Preventing Babies' Deaths: What Needs To Be Done. London: Sands, 2012. [Google Scholar]
Shojania 2019
- Shojania KG. Are increases in emergency use and hospitalisation always a bad thing? Reflections on unintended consequences and apparent backfires. BMJ Quality and Safety 2019;28:687-92. [bmjqs-2019-009406] [DOI] [PubMed] [Google Scholar]
Simons‐Morton 1998
- Simons-Morton DG, Goff DC, Osganian S, Goldberg RJ, Raczynski JM, Finnegan JR, et al. Rapid early action for coronary treatment: rationale, design and baseline characteristics. Academic Emergency Medicine 1998;5(7):726–38. [DOI] [PubMed] [Google Scholar]
Smith 1995
- Smith MS, Wallston KA, Smith CA. The development and validation of the Perceived Health Competence Scale. Health Education Research 1995;10(1):51-64. [DOI] [PubMed] [Google Scholar]
Snyder 2016
- Snyder H, Engström J. The antecedents, forms and consequences of patient involvement: a narrative review of the literature. International Journal of Nursing Studies 2016;53:351-78. [DOI] [PubMed] [Google Scholar]
Tai‐Seale 2016
- Tai-Seale M, Elwyn G, Wilson CJ, Stults C, Dillon EC, Li M, et al. Enhancing shared decision making through carefully designed interventions that target patient and provider behavior. Health Affairs 2016;35(4):605-12. [DOI] [PubMed] [Google Scholar]
Tapp 2007
- Tapp S, Lasserson TJ, Rowe BH. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD003000. [DOI: 10.1002/14651858.CD003000.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thaddeus 1994
- Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Social Science & Medicine 1994;38(8):1091-110. [DOI] [PubMed] [Google Scholar]
Thompson 2006
- Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006;367(9508):397-403. [DOI] [PubMed] [Google Scholar]
Thuresson 2007
- Thuresson M, Jarlöv MB, Lindahl B, Svensson L, Zedigh C, Herlitz J. Thoughts, actions, and factors associated with prehospital delay in patients with acute coronary syndrome. Heart & Lung: Journal of Acute and Critical Care 2007;36(6):398-409. [DOI] [PubMed] [Google Scholar]
Tveit 2009
- Tveit JV, Saastad E, Stray-Pedersen B, Børdahl PE, Flenady V, Fretts R, et al. Reduction of late stillbirth with the introduction of fetal movement information and guidelines – a clinical quality improvement. BMC Pregnancy and Childbirth 2009;1(32):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vincent 2016
- Vincent C, Amalberti R. Safer Healthcare. Strategies for the Real World. Heidelberg: Springer Open, 2016. [PubMed] [Google Scholar]
Vorwerk 2015
- Vorwerk J, King L. Consumer participation in early detection of the deteriorating patient and call activation to rapid response systems: a literature review. Journal of Clinical Nursing 2015;25(1-2):38-52. [DOI] [PubMed] [Google Scholar]
Wachter 2010
- Wachter 2010. Patient safety at ten: unmistakable progress, troubling gaps. Health Affairs 2010;29(1):165-73. [DOI] [PubMed] [Google Scholar]
Walters 2010
- Walters JAE, Turnock AC, Walters EH, Wood-Baker R. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD005074. [DOI: 10.1002/14651858.CD005074.pub3] [DOI] [PubMed] [Google Scholar]
Walton 2016
Waring 2009
- Waring JJ. Constructing and re-constructing narratives of patient safety. Social Science & Medicine 2009;69(12):1722-31. [DOI] [PubMed] [Google Scholar]
Warland 2013
- Warland J. Keeping baby SAFE in pregnancy: evaluating the brochure. Midwifery 2013;29(2):174-9. [DOI] [PubMed] [Google Scholar]
Warland 2015
- Warland J, O’Brien LM, Heazell AEP, Mitchell EA. An international internet survey of the experiences of 1,714 mothers with a late stillbirth: the STARS cohort study. BMC Pregnancy and Childbirth 2015;15(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wears 2003
- Wears RL, Sutcliffe KM, Perry SJ, Eisenberg EM, Murphy AG, Vanderhoef M. Understanding Errors in Emergency Departments: A Convergence Approach. McClean, VA: National Patient Safety Foundation, 2003. [Google Scholar]
Weingart 2009
- Weingart SN, Simchowitz B, Kahlert E, Morway L, Spencer J, Zhu J, et al. The You CAN campaign: teamwork training for patients and families in ambulatory oncology. Joint Commission Journal on Quality and Patient Safety/Joint Commission Resources 2009;35(2):63-71. [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organisation. Exploring patient participation in reducing health-care-related safety risks. www.euro.who.int/__data/assets/pdf_file/0010/185779/e96814.pdf (accessed prior to 8 August 2017).
Wolfe 2011
- Wolfe I, Cass H, Thompson MJ, Craft A, Peile E, Wiegersma PA, et al. Improving child health services in the UK: insights from Europe and their implications for the NHS reforms. BMJ 2011;342:d1277. [DOI] [PubMed] [Google Scholar]
World Bank 2016
- World Bank. New country classifications by income level. blogs.worldbank.org/opendata/new-country-classifications-2016 (accessed prior to 8 August 2017).
Yu 2016
- Yu A, Flott K, Chainani N, Fontana G, Darzi A. Patient Safety 2030. London, UK: NIHR Imperial Patient Safety Translational Research Centre, 2016. [Google Scholar]
Zuckerman 1965
- Zuckerman M, Lubin B, Robins S. Validation of the multiple affect adjective check list in clinical situations. Journal of Consulting Psychology 1965;29:594. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mackintosh 2017b
- Mackintosh NJ, Davis RE, Easter A, Rayment‐Jones H, Sevdalis N, Wilson S, et al. Interventions to increase patient and family involvement in escalation of care for acute life‐threatening illness in community health and hospital settings. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]


