Abstract
Background:
To determine if the intersectionality of gender and poverty is associated with health disparities among adolescents with cancer. We hypothesized unobserved latent classes of patients exist with respect to cancer-related symptoms; and class classification varies by gender–poverty combinations.
Procedure:
Cross-sectional data were collected among adolescents with cancer and families (N = 126 dyads) at four tertiary pediatric hospitals. Adolescents were aged 14–21 years, English speaking, cancer diagnosis, not developmentally delayed, psychotic, homicidal, suicidal, or severely depressed. Latent class analysis and multinomial logit models were used for analysis. Patient-Reported Outcomes Measurement Information System (PROMIS) pediatric symptom measures, Short forms, evaluated anxiety, depressive symptoms, pain interference, and fatigue. Family-reported household income used 2016 Federal Poverty Level (FPL) guidelines.
Results:
Three distinct groups of patients were identified using PROMIS symptom patterns: High Distress-25%; High Physical/Low Psychological Distress-14%; and Low Distress-62%. Female adolescents living in households with incomes at or below the 2016 FPL had 30 times the odds of being classified in the High Distress class (higher probabilities of experiencing anxiety, depressive symptoms, pain interference, and fatigue) compared to those in the High Physical/Low Psychological Distress class (female and poverty: AOR = 30.27, 95% CI 1.23, 735.10), and this was statistically significant (β = 3.41, 95% CI 0.21, 6.60; p = .04) but not compared to those in Low Distress.
Conclusion:
Adolescent females with cancer with households in poverty had significantly greater odds of experiencing high symptom distress, compared to those with high physical but low psychological distress. More comprehensive screening and intervention, as needed, may decrease disparities.
Keywords: adolescents, cancer, gender, poverty, symptoms
1 |. INTRODUCTION
Adolescents diagnosed with cancer experience high symptom distress and suffering throughout the continuum of care.1–4 The Theory of Intersectionality posits that inequalities persist, because categories like gender, race, and class are overlapping and affect each other interdependently and may interact to exacerbate a health problem.5,6 We could identify no studies that examined the intersectionality of gender and poverty and how they may interact to exacerbate cancer-specific symptom distress,7 although intersectionality has been identified with some adolescent mental health outcomes.8
The impact of gender in isolation has been examined for adolescents diagnosed with cancer. Gender refers to a complex psychosocial construct that takes into account biology, but also the influences of society and environment.9 Prior cross-sectional research has examined gender differences in cancer-specific symptom experience.10 Adolescent females with cancer evidenced greater symptom distress compared to males across studies and countries.10–16 Gender disparities may be due to differences in genetics, coping skills, gender role expectations,16 or gender inequities in the social situations of women.7
The adverse impact of income on care outcomes and symptom distress has also been studied in isolation. For example, children with leukemia (N = 575) living in high-poverty areas, defined by zip code, suffered an earlier relapse compared to their wealthier counterparts.17 Similarly, in the PediQUEST trial, children with cancer (N = 78) from low-income families, defined as at 200% of the Federal Poverty Level (FPL) or <$50,000/year, experienced higher symptom distress and worse health-related quality of life.18
This prior research is limited by examining gender differences and socioeconomic status (SES) separately, as well as assumptions of data homogeneity. Each may result in misleading analysis or invalid conclusions. Heterogeneity signifies diversity within a group or sample; the opposite of homogeneity when samples are more alike than different. To increase scientific rigor, we analyzed the intersectionality of gender and poverty to determine whether adolescent subgroups were at high risk for excessive symptom distress. We hypothesized (a) distinctive latent classes/groups exist in the adolescent cancer population with respect to cancer-related symptoms; and (b) the likelihood of being classified into specific symptom classes varies by different gender and poverty combinations.
2 |. METHODS
This secondary analysis is part of the ongoing parent trial of FAmily CEntered pediatric Advance Care Planning (FACE pACP) for Teens with Cancer (FACE-TC), which is a longitudinal, intent-to-treat, single-blinded, randomized controlled trial for adolescents diagnosed with cancer and their family decision makers (R01NR015458-06, PI, Maureen Lyon). Participants were enrolled from four quaternary pediatric oncology hospital-based programs in the United States. Enrollment was from July 16, 2016 through April 30, 2019. Methods of this study and the protocol have been published elsewhere.19 Randomized intent-to-treat intervention assignment occurred post baseline assessment. All data described in this study were from the completion of baseline assessments of enrolled/eligible participants, prior to randomization. This study reports primarily on the findings from the adolescent responses to the PROMIS pediatric symptom measures.
Adolescent eligibility criteria included ever diagnosed with cancer; aged ≥14 to <21 years; knows cancer diagnosis; and English speaking. Family eligibility criteria included ≥18.0 years; provider determined not developmentally delayed; English speaking; and knows patient’s diagnosis. Secondary screening for exclusion criteria occurred after enrollment for severe depression,20 homicidality,21 suicidality,20 and psychosis21 to ensure competency to engage in shared decision making. Referrals were given as needed. Adolescents at various points in the illness trajectory were study eligible because our pilot study indicated that adolescents with cancer preferred to be involved in pediatric advance care planning (pACP) at various points in the illness trajectory.22 This finding was confirmed with the present sample: 86% of adolescents wanted early discussion of ACP, rather than waiting until hospitalized or if dying.23 Furthermore, this heterogeneity reflects the goals of the parent study, as pACP is recommended at all stages for anyone with a serious illness.24
The institutional review board at each site approved the protocol. Participants provided written informed consent/assent and were compensated for participation. Adolescents and legal guardians (for patients under the age of 18 years) or surrogates (chosen by patients aged 18 years and older) received a $25 gift card each for the baseline assessment. A Safety Monitoring Committee monitored the trial.
2.1 |. Procedures
The clinician at each study site generated a list of potentially eligible participants coming to the outpatient clinic that week or on the inpatient service. Through the clinician, who confirmed it was okay to approach the patient that day (e.g., no bad news being given), a trained research assistant approached potentially eligible dyads in person. After asking the adolescent, and if present the family, if they were interested in participating in research and receiving a positive response; the researcher reviewed the Institutional Review Board-approved Information Sheet with the adolescent and family and then asked if they might be interested in participating. Following informed consent/assent and prior to randomization, study measures were administered in person to adolescents and families separately in a private room. Eligible dyads (patient and family) were administered measures independently at baseline (study visit 1). Trained research assistants read study questions aloud. Responses were directly entered by the RA-Assessor into the research electronic data capture system (REDCap) database. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for reporting our study to enhance the quality and transparency of our research (https://www.equator-network.org/reporting-guidelines/strobe/).
2.2. Measures and data collection
Demographic Data Form:
It was administered by a trained research assistant at baseline to obtain participant-reported age, gender, and race/ethnicity. Families self-reported their education, employment status, household income, and number of persons residing in the household. Medical data were obtained by a trained research assistant through chart abstraction, including diagnosis, date of diagnosis, history of relapse, bone marrow transplant, and on or off active treatment.
Patient-Reported Outcomes Measurement Information System (PROMIS) pediatric symptom measures25–27
PROMIS uses a T-score metric in which 50 is the mean of a relevant reference population and 10 is the standard deviation (SD) of that population. A score of 60 is one SD higher than the mean of the reference population. To decrease subject burden (time) adolescents were administered the Short forms: Emotional Distress-Anxiety, Emotional Distress-Depressive Symptoms, Fatigue, and Pain Interference. Each form has eight items. The PROMIS Pain Interference items assess self-reported consequences of pain on relevant aspects of one’s life. The PROMIS measures are theoretically grounded and demonstrate feasibility and acceptability. Although normative data are available only for children up to age 18 years, for ease of comparison within this study, we used PROMIS pediatric symptom measures with participants ages 18 up to 21 years. The PROMIS pediatric symptom measures have been validated in pediatric oncology clinical trials,28 demonstrating changes over time and supporting construct and concurrent validity.29
Household Income:
Family participants were asked, “What is your family’s estimated annual income before taxes in US dollars?,” “How many people are living in the patient’s household? (count everyone).” The 2016 Department of Health & Human Services poverty guidelines were later used to determine the FPL, which for a household of four was $24,250.30
Time-Invariant Covariates:
Time-invariant covariates are age, gender, and race.
2.3 |. Sample size calculations
For this study, the sample size needed for latent class analysis (LCA) for adolescents only (N = 126) was calculated using the formula of Dziak et al.31, where w is an effect size, is a constant for predicting from w the required N to obtain a power of 0.80. The values of are estimated31 based on bootstrap simulations for various number of indicators used for clustering and the number of classes. Our proposed LCA is based on four indicators (four patient-reported cancer-related symptoms). According to the Simulation Experiment-2 by Dziak et al.,31 assuming a moderate effect size of w = 0.4, for identifying two to six classes based on four indicators,31 the sample size needed for our proposed LCA models is . This indicates that our baseline data with N = 126 is adequate for LCA.
2.4 |. Statistical procedures
To test Hypothesis 1 that distinctive unobserved subpopulations exist in the adolescent cancer sample with respect to cancer-related symptoms, LCA was used to identify potential latent classes/groups of patients.32–35 Each PROMIS pediatric measure has a T-score with a mean of 50 and SD of 10. We used the mean (50) as the cut-off point to generate dichotomous variables for LCA. T-score ≤50 = low symptom distress and T-score >50 = high symptom distress. Although the mean value does not have much clinical meaning, LCA enabled examination of the sample heterogeneity regarding the likelihood of experiencing higher than average PROMIS symptom scores. A series of LCA models with an increasing number of latent classes were estimated. Information criterion indices (e.g., Akaike’s information criterion [AIC], Bayesian information criterion [BIC], sample-size adjusted BIC [aBIC]) and some special LR tests that are not based on model chi-square statistics (e.g., Lo–Mendell–Rubin likelihood ratio test [LMR], adjusted Lo–Mendell–Rubin likelihood ratio test [aLMR], and Bootstrap likelihood ratio test [BLRT]) were used for model fit comparison. Lower information criterion indices indicate better model fit; statistically insignificant LR tests (p = >.05) indicate the K-class model fits data better than the (K-1)-class model. Once the optimal number of latent classes was identified, adolescent patients were classified into their most likely latent class using the estimated posterior membership probabilities. The quality of membership classification was assessed by examining average posterior probabilities and the entropy statistic. The latent classes were defined by the patterns of the probabilities of having specific patient-reported symptoms in given classes.
To test Hypothesis 2 that the likelihood of being classified into specific symptom classes will vary by gender–poverty combinations, multinomial logit model was used to examine gender and poverty effects on patient-reported symptom patterns (latent class membership). Gender–poverty interaction was included in the multinomial logit model to test whether gender effect on symptom patterns varies by poverty. Both the main effects and interaction were tested by two-sided Wald chi-squared statistic. Statistical significance level was set to α = .05. Data were analyzed using Mplus 8.4 and SAS statistical software version 9.4 (SAS Institute).
3 |. RESULTS
3.1 |. Participant characteristics
Three hundred sixty-six adolescent–family dyads were approached, of whom 336 dyads met initial eligibility criteria. Of these, 203 declined, three were ineligible, and 130 dyads enrolled. After enrollment four additional dyads were deemed ineligible as a result of secondary screening, leaving 126 dyads (252 participants) who met full eligibility criteria. Enrollment differed by study site: Akron Children’s Hospital (n = 80/130 dyads; 62%), St. Jude Children’s Research Hospital (n = 11/130 dyads; 8%), Children’s National Hospital (n = 4/130 dyads; 3%), and the University of Minnesota Masonic Children’s Hospital (n = 35/130 dyads; 27%). There were no statistically significant differences by study site in dyads declining to participate of those who were eligible (113/193, 59%; 23/34, 68%; 60/95, 63%; 6/10, 60%; respectively) (p = .728). The top three reasons for declining to participate in this 5-year trial were time issues, 37% (76/203); not wanting to talk about ACP by at least one member of the adolescent/family dyad, 23% (46/203); and not wanting to participate in research, 20% (41/203). Male participants (58%, 115/198) compared to female participants (44%, 57/130) were significantly more likely to decline participation in the trial (difference of 14%, 95% CI 4–25%, p = .02). Age, race, ethnicity, diagnosis, or active treatment status were not statistically significantly different between those who enrolled and those who declined participation.
Adolescents (N = 126) had a mean (SD) age of 16.9 years (1.9); 57% were female (69/126) and 79% (100/126) were White (Table 1). The four most common diagnoses were leukemia (42/126, 33%); solid tumor (34/126, 27%); brain tumor (25/126, 20%); and lymphoma (19/126 participants, 15%). The mean (SD) number of months the adolescent had known of their diagnosis was 84.2 (69); median (interquartile range) 69 (27, 148); 21% were on active treatment; 13% (17/126) had received a bone marrow transplant; and 12% (15/126) had ever relapsed.
TABLE 1.
Demographics and characteristics for adolescents with cancer at baseline (N = 126)
| Variable | Statistics |
|---|---|
| Age | |
| Mean (SD) | 16.9 (1.9) |
| Range | (14, 20) |
| Age | N (%) |
| 14–17 | 69 (54) |
| 18–20 | 57 (45) |
| Self-identified gender | |
| Male | 54 (42) |
| Female | 72 (57) |
| Self-identified race | |
| Asian | 3 (2) |
| Black or African American | 17 (13) |
| White | 100 (79) |
| More than one race | 5 (4) |
| Declined | 1 (0.8) |
| Self-identified ethnicity | |
| Not Hispanic or Latino | 116 (92) |
| Hispanic or Latino | 5 (4) |
| Declined | 5 (4) |
| Diagnosis | |
| Leukemia | 42 (34) |
| Lymphoma | 18 (14) |
| Solid tumors | 34 (27) |
| Brain tumor | 25 (20) |
| Other | 6 (5) |
| Household income by persons in family/household | |
| Equal to or below the 2016 FPL | 33 (26) |
| 101–200% of FPL | 37 (29) |
| 201–300% of FPL | 19 (15) |
| >300% of FPL | 33 (26) |
| Declined | 4 (3) |
| Household income dichotomized as | |
| Equal to or below the 2016 FPL | 33 (26) |
| ≥101% of FPL | 89 (71) |
| On active treatment | |
| Yes | 27 (21) |
| No | 99 (79) |
| Bone marrow transplant? | |
| None | 108 (86.4) |
| Once | 16 (12.8) |
| Twice | 1 (0.8) |
| Relapse ever? | |
| No | 110 (88.0) |
| Once | 13 (10.4) |
| Twice | 2 (1.6) |
3.2 |. Baseline household income
Seventy-one percent of (89/126) families reported household income greater than the 2016 FPL, while 26% (33/126) reported household income equal to or below the 2016 FPL. Three percent (4/126) declined to share this information and were not included in the multinomial logit model for the PROMIS LCA to test the interaction of gender and income. One adolescent who declined to specify race was also excluded from this model. Race was dichotomized into White versus others (see Table 1).
3.3 |. Symptom distress
No data were missing for the PROMIS symptom measures. Within the entire sample (N = 126), percentage who reported symptoms greater than the cut-off of T-score >50 were as follows: Emotional Distress-Anxiety 37% (mean = 46.7, SD = 9.4); Emotional Distress-Depressive Symptoms 33% (mean = 45.0, SD = 9.9); Pain Interference 29% (mean = 43.8, SD = 10.3); and Fatigue 34% (mean = 45.4, SD = 12.5). Table 2 presents the mean (SD) of PROMIS scores for each class.
TABLE 2.
PROMIS pediatric symptom measures, Short forms mean scores for different levels of gender and poverty status
| PROMIS measures | Male with low income | Male with not low income | Female with low income | Female with not low income | |
|---|---|---|---|---|---|
| Anxiety T-score | Mean (SD) | 47.53 (6.89) | 45.49 (8.50) | 47.71 (11.13) | 47.13 (9.72) |
| Depression symptoms T-score | Mean (SD) | 43.83 (8.60) | 43.92 (9.60) | 46.80 (10.99) | 45.62 (10.23) |
| Pain interference T-score | Mean (SD) | 46.08 (12.03) | 40.42 (7.77) | 51.01 (11.29) | 43.51 (10.14) |
| Fatigue T-score | Mean (SD) | 46.73 (13.05) | 42.18 (11.85) | 50.51 (13.13) | 45.67 (12.34) |
3.4 |. Model results
The single-class model has the largest information criterion indices, and all the Likelihood Ratio tests that compare the single-subgroup with the two-class model are statistically significant (p < .05). This indicates the population is not homogeneous in terms of symptom distress, but heterogeneous with at least two unobserved subclasses, regarding the four PROMIS measures. Among the multiclass models, the four-class model has all information criterion indices larger than those of the three-class model, and all Likelihood Ratio tests cannot reject the three-class model. Comparing two-class and three-class models, the former has slightly smaller information criterion indices, but all the Likelihood Ratio tests reject the two-class model. We favored the three-class model because it has better class classification (classification probabilities for classes 1, 2, and 3 are .99, .87, and 1.00, respectively, and entropy statistic is .91) and the most useful and interpretable information from a clinical perspective.
We found three unobserved distinct classes/groups of patients. The conditional probabilities of reporting higher than average PROMIS scores are similar within class, but substantially different across classes. See Figure 1. Based on the patterns of the conditional probability (i.e., the probability of reporting higher than average PROMIS scores in given class), the classes are defined as: Class 1 (N = 78/126, 62%): Low Symptom Distress (lower probabilities of having high scores in the four PROMIS measures); Class 2 (N = 31/126, 25%): High Distress (higher probabilities of having high scores in the four PROMIS measures); and Class 3 (N = 17/126, 14%): High Physical/Low Psychological Distress (high probabilities of having high scores in only pain interference and fatigue).
FIGURE 1.
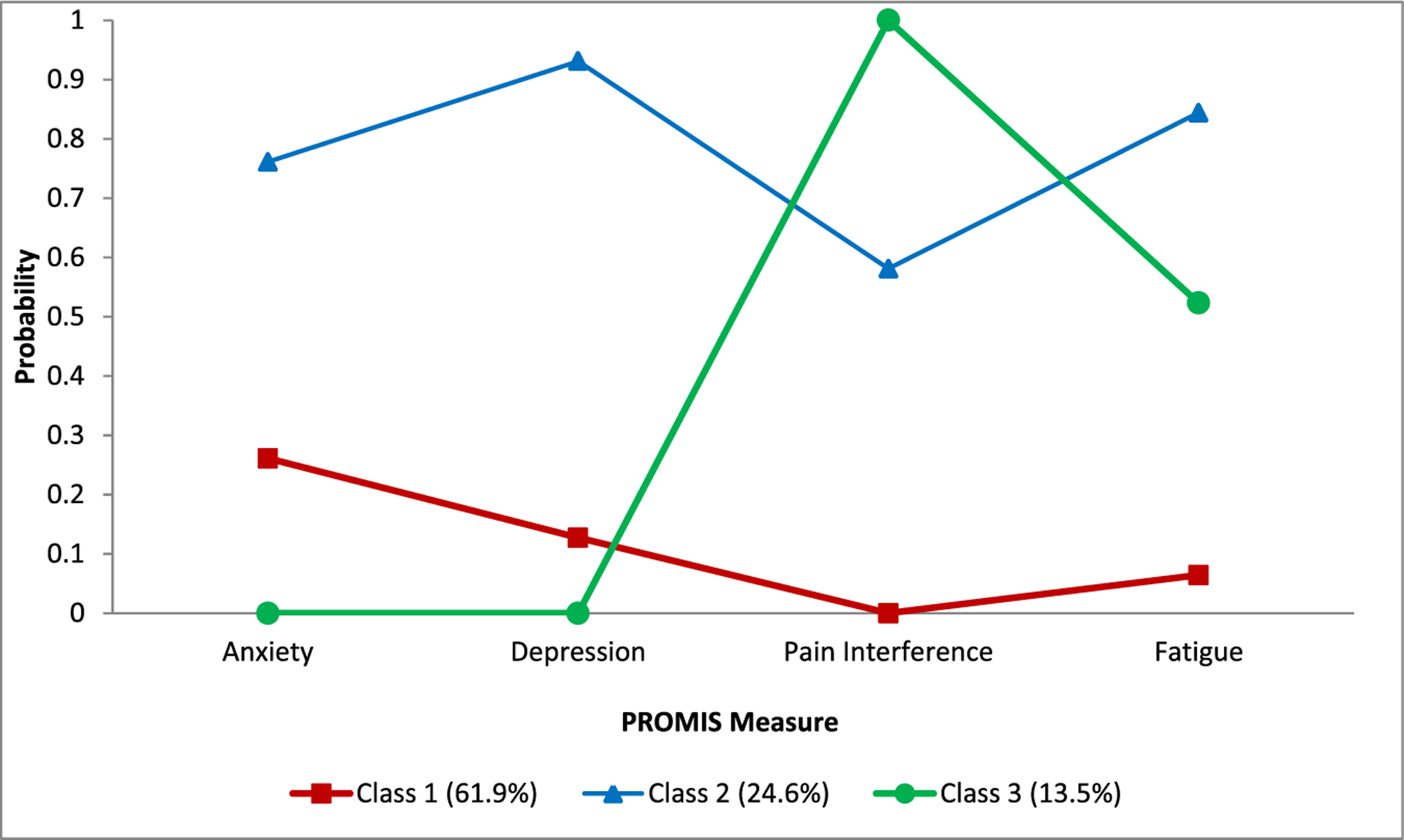
Latent class analysis of four PROMIS measures (N = 126). Four dichotomized PROMIS measures (1: T-score >50; 0: T-score ≤50) were used in latent class analysis. Definitions: Class 1, Low Distress; Class 2, High Distress; Class 3, High Physical, Low Psychological Distress
The results of the multinomial logit model are shown in Table 3 (N = 121). The model models three logits, contrasting Classes 1 versus 3, 2 versus 3, and 2 versus 1, respectively. The interaction between female and poverty is positive in the second logit, indicating female adolescents in households in poverty had 30 times the odds of being in Class 2 (High Distress) than in Class 3 (High Physical, Low Psychological Distress) (adjusted odds ratio [AOR] 30.27, 95% CI 1.23, 735.10). The confidence interval is wide indicating a level of uncertainty because of the sample size. This result is statistically significant (β = 3.41, 95% CI 0.21–6.60; p = .04) (see Table 4). See Supporting Information File for post hoc analyses of alternative models: (a) using race and poverty interaction (Tables S1 and S2); (b) adding “on active treatment,” which resulted in odds remaining high for gender–poverty interactions in Class 2 versus Class 3 (AOR 19.69, 95% CI 0.75–512.86) (Table S3), but lost statistical significance (β = 2.98, 95% CI −0.29 to 6.24; p = .07) eTable S4; and (c) latent profile analysis (LPA) in Figure S1.
TABLE 3.
Selected results of multinomial logit model: Testing effect of gender and poverty interaction on latent class classification (N = 121)a
| Predictor | PROMIS latent class | |||||
|---|---|---|---|---|---|---|
| Class 1 versus Class 3 | Class 2 versus Class 3 | Class 2 versus Class 1 | ||||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| Female | 0.24 | 0.05, 1.26 | 0.28 | 0.05, 1.77 | 1.17 | 0.40, 3.42 |
| Poverty | 0.21 | 0.03, 1.49 | 0.11 | 0.01, 1.73 | 0.52 | 0.05, 4.85 |
| Female and poverty | 2.83 | 0.24, 33.45 | 30.27 | 1.23, 735.10 | 10.70 | 0.82, 138.38 |
| Young (14–17 years) | 0.88 | 0.28, 2.77 | 0.36 | 0.10, 1.35 | 0.41 | 0.16, 1.06 |
| White | 2.14 | 0.63, 7.32 | 2.56 | 0.58, 11.13 | 1.19 | 0.35, 4.06 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; Class 1, Low Distress; Class 2, High Distress; Class 3, High Physical/Low Psychological Distress.
Participants with missing information on race and household income were excluded.
TABLE 4.
Multinomial logit model for PROMIS pediatric symptom measures, Short forms, latent class analysis to test the interaction of gender and poverty (N = 121)a
| Predictor | PROMIS Latent Class | |||||
|---|---|---|---|---|---|---|
| Class 1 versus Class 3 | Class 2 versus Class 3 | Class 2 versus Class 1 | ||||
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Female | −1.41 (−3.05, 0.23) | .09 | −1.26 (−3.09, 0.57) | .18 | .16 (−0.92, 1.23) | .78 |
| Poverty | −1.58 (−3.56, 0.40) | .12 | −2.24 (−5.03, 0.55) | .12 | −.66 (−2.91, 1.58) | .56 |
| Female and poverty | 1.04 (−1.42, 3.51) | .41 | 3.41 (0.21, 6.60) | .04 | 2.37 (−0.20, 4.93) | .07 |
| Young (14–17 years) | −.13 (−1.29, 1.02) | .82 | −1.02 (−2.34, 0.30) | .13 | −.89 (−1.84, 0.06) | .07 |
| White | .76 (−0.47, 1.99) | .22 | .94 (−0.54, 2.41) | .21 | .17 (−1.05, 1.40) | .78 |
Abbreviations: CI, confidence interval; Class 1, Low Distress; Class 2, High Distress; Class 3, High Physical/Low Psychological Distress.
One adolescent declined to report race and four families declined to report household income and were excluded.
4 |. DISCUSSION
Our hypothesis that unobserved classes of patients exist with respect to cancer-related symptoms was confirmed. LCA revealed that adolescents were classified into three previously unidentified groups: High Distress-25%; High Physical/Low Psychological Distress-14%; and Low Distress-62%. As hypothesized, class classification varied by gender–poverty combinations. Female adolescents living in households with incomes at or below the 2016 FPL had 30 times the odds of being classified in the High Distress class, compared to those in the High Physical/Low Psychological Distress class. This finding was also statistically significant, but not compared to those in Low Distress.
Intersectionality may prove important for understanding the specific challenges facing economically vulnerable adolescent females with cancer. However, our small sample size created a level of uncertainty about this finding, as evidenced by the wide confidence interval in the odds ratio. Similarly, in post hoc analysis when “on active treatment” was added to the model, female adolescents living in households below the 2016 FPL had 20 times the odds of High Distress compared to those in the High Physical, Low Psychological Distress class, but the confidence interval was wide and statistical significance disappeared. Thus, this study needs to be replicated with a larger sample. Future research should also examine the possible reasons for this finding. For example, are adolescent females living in poverty and not on active treatment, more likely than males in this situation, to provide family caregiving (e.g., taking care of siblings or ill family members, cooking), particularly considering symptoms of fatigue and pain interference, as has been found among adults?7 If family caregiving is present, what may be beneficial or burdensome?36
Prior research on disparities in pediatric cancer outcomes indicated SES was a robust predictor of access to and quality of health care.37 SES has also been shown to mediate the association between race/ethnicity and adolescent cancer survival.38 Adolescents with cancer living in poverty are less likely to receive end-of-life cancer treatment than peers with higher incomes, but the reasons are unknown.39 In a registry-based study disparities by cancer stage and SES worsened over time.40
This study offers methods for how to study gender and poverty interactions in cancer care delivery research. Findings may move the field of symptom management and patient-reported symptom science forward in two ways: (a) with respect to research, by highlighting the importance of LCA so that assumptions of homogeneity are tested to ensure valid interpretation of results; and (b) with respect to clinical care, by identifying “hidden” groups who may be experiencing the greatest symptom distress in need of palliation. Here, we tested the assumption that the study sample is homogenous, as traditional statistics make this assumption. LCA is a person-centered approach to the data rather than a variable-centered approach. Traditional moderator analysis of intervention results are variable-centered approaches. However, variable-centered and person-centered analytical approaches are often integrated in a more generalized analytical framework. As such, examining the influence of time insensitive variables such as gender, race, and income on symptom distress can be readily done after the unobserved latent classes/groups are identified.
Limitations of this study include its cross-sectional design. We were limited in our application of Intersectionality Theory with respect to the overlapping identities of race, class, and gender because of the lack of racial diversity in the sample. Results may not generalize beyond well-resourced tertiary pediatric hospitals with palliative care and psychosocial supports. We do not have data on household income for those who declined participation. Fewer males agreed to participate than females. Males who declined could have been more symptomatic or more at risk for psychological distress significantly biasing study outcomes. This was also true for capturing a sample of patients whose gender falls outside the gender binary who may experience poverty41 and symptom distress differently. Males are less likely to participate in clinical trials of ACP generally.42 Our enrollment rate may bias generalizability, but is comparable to adult longitudinal clinical trials involving palliative care.43,44 Most of the study population was not diagnosed during adolescence. Applying the 2016 FPL to household income data collected in 2019 likely underestimated poverty. Social desirability bias could have occurred with face-to-face administration of study questionnaires. We chose this approach to enable monitoring of emotional reactions and to control for issues of literacy, impaired or uncorrected vision, item comprehension, and questionnaire completeness. There is no validation of the PROMIS Pediatric measures for cancer patients aged 18–20 years old. Approximately one-fourth of potentially eligible dyads declined to participate, because they did not want to talk about pACP. This is true of adults as well.45 Talking about death and dying is taboo.46 Some adolescents prefer to defer to their family or doctor. Some families believe it is their role alone to make these decisions. Other families believe pACP is against their religion or cultural norms,47 “shocking” as one elder put it. Post hoc analysis has limitations, as discussed in Supporting Information.48
Future research needs to independently validate the three latent classes that emerged from these data in other adolescent samples, and to replicate the finding of health disparities with respect to gender and poverty interactions. Findings support policy recommendations for routine screening of patient-reported symptoms and psychosocial aspects of care to improve health equity and increase longevity.49–58 Some systems are effectively doing this already.59 The American Academy of Pediatrics has campaigned to encourage physicians to screen patients for social needs and has developed tools to help physicians.60 The Centers for Medicare and Medicaid Services has prioritized screening for five social needs associated with medical outcomes and has also created a free screening tool.61 In conclusion, adolescent females with cancer with households below the 2016 FPL had significantly greater odds of experiencing high symptom distress, compared to those with high physical but low psychological distress. More comprehensive screening and intervention, as needed, may decrease disparities.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health, Health of Women of Understudied, Underrepresented and Underreported (U3) Populations 2018 Administrative Supplement from the Office of Research on Women’s Health (ORWH FY) to the United States National Institute of Nursing Research (NINR), Award Number R01NR015458-04S1. This content is solely the responsibility of the authors and does not necessarily represent the official views of the NINR or the NIH. The funders were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The authors gratefully acknowledge the participating adolescents and their families, and the following study staff who assisted with data collection: Jessica Thompkins, Elaine Churney, Kristine Allmendinger-Goertz, Jody Chrastek, Rachel Jenkins, Karuna Ramcharran, Alaina Martinez, Jessica Livingston, Sue Flesch, Robin Wilcox, Jennifer Zabrowski, and Melanie Gattas.
CONFLICT OF INTEREST
Maureen E. Lyon received funding for the research on the parent study from the National Institutes of Health. Maureen E. Lyon is also receiving funding from the American Cancer Society to adapt/translate this protocol into Spanish. There are no other conflicts of interest to disclose.
Abbreviations:
- ACP
advance care planning
- FACE pACP
FAmily CEntered pediatric Advance Care Planning
- FPL
Federal Poverty Level
- LCA
latent class analysis
- PROMIS
Patient-Reported Outcomes Measurement Information System
- SES
socioeconomic status
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
DATA AVAILABILITY STATEMENT
Deidentified data that support the findings of this study are available upon request from the corresponding author.
REFERENCES
- 1.Bona K, Wolfe J. Disparities in pediatric palliative care: an opportunity to strive for equity. Pediatrics. 2017;140(4):e20171662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(5):326–333. [DOI] [PubMed] [Google Scholar]
- 3.Levine DR, Mandrell BN, Sykes A, et al. Patients’ and parents’ needs, attitudes, and perceptions about early palliative care integration in pediatric oncology. JAMA Oncol. 2017;3(9):1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics. Committee on Bioethics and Committee on Hospital Care. Palliative care for children. Pediatrics. 2000;106(2, pt1):351–357. [PubMed] [Google Scholar]
- 5.Cho S, Crenshaw KW, McCall L. Toward a field of intersectionality studies: theory, applications, and praxis. Signs. 2013;38(4):785–810. [Google Scholar]
- 6.Singer M, Clair S. Syndymics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17(4):423–441. [DOI] [PubMed] [Google Scholar]
- 7.Gott M, Morgan T, Williams L. Gender and palliative care: a call to arms. Palliat Care Soc Pract. 2020;14:2632352420957997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern MR, Duinhof EL, Walsh SD, et al. Intersectionality and adolescent mental well-being: a cross-nationally comparative analysis of the interplay between immigration background, socioeconomic status and gender. J Adolesc Health. 2020;66(6S):S12–S20. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Exploring the Biological Contributions to Human Health: Does Sex Matter?. Washington, DC: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 10.Geue K, Sender A, Schmidt R, et al. Gender-specific quality of life after cancer in young adulthood: a comparison with the general population. Qual Life Res. 2014;23:1377–1386. [DOI] [PubMed] [Google Scholar]
- 11.Nowe E, Stobel-Richter Y, Sneder A, Leuteritz K, Friedrich M, Geue K. Cancer-related fatigue in adolescents and young adults: a systematic review of the literature. Crit Rev Oncol Hematol. 2017;118:63–69. [DOI] [PubMed] [Google Scholar]
- 12.Spathis A, Hatcher H, Booth S, et al. Cancer-related fatigue in adolescents and young adults after cancer treatment: persistent and poorly managed. J Adolesc Young Adult Oncol. 2017;6(3):489–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poort H, Kaal SEJ, Knoop H, et al. Prevalence and impact of severe fatigue in adolescent and young adult cancer patients in comparison with population-based controls. Support Care Cancer. 2017;25:2911–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant J, Cranston A, Horsman J, et al. Health status and health-related quality of life in adolescent survivors of cancer in childhood. J Adolesc Health. 2006;38:504–510. [DOI] [PubMed] [Google Scholar]
- 15.Vlachioti E, Matziou V, Perdikaris P, et al. Assessment of quality of life of children and adolescents with cancer during their treatment. Jpn J Clin Oncol. 2016;46(5):453–461. [DOI] [PubMed] [Google Scholar]
- 16.Hechler T, Chalkiadis GA, Hasan C, et al. Sex differences in pain intensity in adolescents suffering from cancer: differences in pain memories? J Pain. 2009;10:586–593. [DOI] [PubMed] [Google Scholar]
- 17.Bona K, Blonquist TM, Neubert DS, Silverman LB, Wolfe J. Impact of socioeconomic status on timing of relapse and overall survival for children treated on Dana-Farber Cancer Institute ALL consortium protocols (2000–2010). Pediatr Blood Cancer. 2016;63(6):1012–1018. [DOI] [PubMed] [Google Scholar]
- 18.Ilowite MF, Al-Sayegh H, Ma C, et al. The relationship between household income and patient-reported symptom distress and quality of life in children with advanced cancer: a report from the PediQUEST study. Cancer. 2018;124(19):3934–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtin KB, Watson AE, Wang J, Okonkwo OC, Lyon ME. Pediatric advance care planning (pACP) for teens with cancer and their families: design of a dyadic, longitudinal RCCT. Contemp Clin Trials. 2017;62:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 21.Shaffer D, Fisher P, Dulcan MK, et al. The NIMH Diagnostic Interview Schedule for Children, Version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA study. J Am Acad Child Adolesc Psychiatry. 1996;35(7):865–877. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs S, Perez J, Cheng YI, Sill A, Wang J, Lyon ME. Teen end of life preferences and congruence with their parents’ wishes: results of a survey of teens with cancer. Pediatr Blood Cancer. 2015;62(4): 710–714. [DOI] [PubMed] [Google Scholar]
- 23.Friebert S, Grossoehme DH, Baker JN, et al. Congruence gaps between adolescents with cancer and their families regarding, values, goals, and beliefs in end-of-life care. JAMA Netw Open. 2020;3(5):e205424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rietjens JAC, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543–e551. [DOI] [PubMed] [Google Scholar]
- 25.Hinds PS, Nuss SL, Ruccione K, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatr Blood Cancer. 2013;60(3):402–408. [DOI] [PubMed] [Google Scholar]
- 26.Menard JC, Hinds PS, Jacobs SS, et al. Feasibility and acceptability of the patient-reported outcomes measurement information system measures in children and adolescents in active cancer treatment and survivorship. Cancer Nurs. 2014;37(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeWitt EM, Stucky BD, Thissen D, et al. Construction of the eight item PROMIS Pediatric Physical Function Scales: built using item response theory. J Clin Epidemiol. 2011;64(7):794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinds PS, Wang J, Stern ED, et al. Voices of children and adolescents on phase 1 or phase 2 cancer trials: a new trial endpoint? Cancer. 2017;123(19):3799–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinds PS, Wang J, Cheng YI, et al. PROMIS pediatric measures validated in a longitudinal study design in pediatric oncology. Pediatr Blood Cancer. 2019;66(5):e27606. [DOI] [PubMed] [Google Scholar]
- 30.Department of Health and Human Services. Federal Register. January25, 2016. http://www.parkviewmc.com/app/files/public/a65ca87d-685d-423e-ba6f-125cac7b88b5/2016-Poverty-Level-Chart.pdf.AccessedDecember 21, 2020.
- 31.Dziak JJ, Lanza ST, Tan X. Effect size, statistical power, and sample size requirements for the bootstrap likelihood ratio test in latent class analysis. Struct Equ Modeling. 2014;21(4):534–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: with Applications in the Social Behavioral, and Health Sciences. Hoboken: NJ: Wiley; 2010. [Google Scholar]
- 33.Lanza ST, Tan X, Bray BC. Latent class analysis with distal outcomes: a flexible model-based approach. Struct Equ Modeling. 2013;20:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthén BO, Beyond SEM. General latent variable modeling. Behaviormetrika. 2002;29:81–117. [Google Scholar]
- 35.Wang J, Wang X. Structural Equation Modeling: Applications Using Mplus. 2nd ed. New York: John Wiley; 2020. [Google Scholar]
- 36.East PL. Children’s provision of family caregiving: benefit or burden? Child Dev Perspect. 2010;4(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia S Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56(6):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osypuk TL. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018;124(20):4090–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delaver A, Barnes JM, Wang X, Johnson KJ. Associations between race/ethnicity and US childhood and adolescent cancer survival by treatment amenability. JAMA Pediatr. 2020;174(5):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moke DJ, Tsai K, Hamilton AS, et al. Emerging cancer survival trends, disparities, and priorities in adolescents and young adults: a California cancer registry-based study. JNCI Cancer Spectr. 2019;3(2):pkz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee-Badgett M, Choi SK, Wilson BDM. LGBT Poverty in the United States: a Study of Differences Between Sexual Orientation and Gender Identity Groups. Los Angeles, CA: UCLA Williams Institute; 2019. [Google Scholar]
- 42.Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc. 2013;61(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirchhoff KT, Kehl KA. Recruiting participants in end-of-life research. Am J Hosp Palliat Care. 2007;24(6):515–521. [DOI] [PubMed] [Google Scholar]
- 44.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 46.Walter JK, Rosenberg AR, Feudtner C. Tackling taboo topics: how to have effective advanced care planning discussions with adolescents and young adults with cancer. JAMA Pediatr. 2013;167(5):489–490. [DOI] [PubMed] [Google Scholar]
- 47.Field MJ, Behrman RE. When Children Die: Improving Palliative and End-of-Life Care for Children and Their Families. Washington, DC: Institute of Medicine; 2002. [PubMed] [Google Scholar]
- 48.Peto R Current misconception 3: that subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer. 2011;104(7):1057–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbera LC, Sutradhar R, Earle C, et al. The impact of routine ESAS use on overall survival: results of a population-based retrospective matched cohort analysis. J Clin Oncol. 2019;37(15):S6509–S6509. [Google Scholar]
- 50.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denis F, Basch E, Septans A, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA. 2019;321(3):306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottlieb LM, Hessler D, Long D, et al. Effects of social needs screening and in-person service navigation on child health: a randomized clinical trial. JAMA Pediatr. 2016;170(11):e162521. [DOI] [PubMed] [Google Scholar]
- 53.Wiener L, Kazak AE, Noll RB, Patenaude AF, Kupst MJ. Standards for the psychosocial care of children with cancer and their families: an introduction to the special issue. Pediatr Blood Cancer. 2015;62(S5):S419–S424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kazak A, Abrams A, Banks J, et al. Psychoscocial assessment as a standard of care in pediatric care. Pediatr Blood Cancer. 2015;62:S426–S459. [DOI] [PubMed] [Google Scholar]
- 55.Gitterman BA, Flanagan PJ, Cotton WH, et al. Poverty and child health in the United States. Pediatrics. 2016;137(4):e20160339. [DOI] [PubMed] [Google Scholar]
- 56.Zheng DJ, Shyr D, Ma C, Muriel A, Wolfe J, Bona K. Feasibility of systematic poverty screening in a pediatric oncology referral center. Pediatr Blood Cancer. 2018;65(12):e27380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson KW, McGinn T. Screening for social determinants of health: the known and the unknown. JAMA. 2019;322(11):1037–1038. [DOI] [PubMed] [Google Scholar]
- 58.Pelletier W, Bona K. Assessment of financial burden as standard of care in pediatric oncology. Pediatr Blood Cancer. 2015;62(5):S619–S631. [DOI] [PubMed] [Google Scholar]
- 59.Fraze TK, Brewster AL, Lewis VA, Beidler LB, Murray GF, Colla CH. Prevalence of screening for food insecurity, housing instability, utility needs, transportation needs, and interpersonal violence by US physician practices and hospitals. JAMA Netw Open. 2019;2(9): e1911514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagan JF, Shaw JS, Duncan PM. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017. [Google Scholar]
- 61.Billioux A, Verlander K, Anthony S, Alley D. Standardized Screening for Health-Related Social Needs in Clinical Settings: the Accountable Health Communities Screening Tool. National Academy of Medicine. PublishedMay20, 2017. https://www.ihconline.org/filesimages/Tools/Pop%20Health/SIM/SDOH%20Toolkit/Accountable%20Health.pdf.AccessedJanuary 31, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data that support the findings of this study are available upon request from the corresponding author.


