Abstract
Background
Systemic corticosteroids are used to treat people with COVID‐19 because they counter hyper‐inflammation. Existing evidence syntheses suggest a slight benefit on mortality. So far, systemic corticosteroids are one of the few treatment options for COVID‐19. Nonetheless, size of effect, certainty of the evidence, optimal therapy regimen, and selection of patients who are likely to benefit most are factors that remain to be evaluated.
Objectives
To assess whether systemic corticosteroids are effective and safe in the treatment of people with COVID‐19, and to keep up to date with the evolving evidence base using a living systematic review approach.
Search methods
We searched the Cochrane COVID‐19 Study Register (which includes PubMed, Embase, CENTRAL, ClinicalTrials.gov, WHO ICTRP, and medRxiv), Web of Science (Science Citation Index, Emerging Citation Index), and the WHO COVID‐19 Global literature on coronavirus disease to identify completed and ongoing studies to 16 April 2021.
Selection criteria
We included randomised controlled trials (RCTs) that evaluated systemic corticosteroids for people with COVID‐19, irrespective of disease severity, participant age, gender or ethnicity.
We included any type or dose of systemic corticosteroids. We included the following comparisons: systemic corticosteroids plus standard care versus standard care (plus/minus placebo), dose comparisons, timing comparisons (early versus late), different types of corticosteroids and systemic corticosteroids versus other active substances.
We excluded studies that included populations with other coronavirus diseases (severe acute respiratory syndrome or Middle East respiratory syndrome), corticosteroids in combination with other active substances versus standard care, topical or inhaled corticosteroids, and corticosteroids for long‐COVID treatment.
Data collection and analysis
We followed standard Cochrane methodology. To assess the risk of bias in included studies, we used the Cochrane 'Risk of bias' 2 tool for RCTs. We rated the certainty of evidence using the GRADE approach for the following outcomes: all‐cause mortality, ventilator‐free days, new need for invasive mechanical ventilation, quality of life, serious adverse events, adverse events, and hospital‐acquired infections.
Main results
We included 11 RCTs in 8075 participants, of whom 7041 (87%) originated from high‐income countries. A total of 3072 participants were randomised to corticosteroid arms and the majority received dexamethasone (n = 2322). We also identified 42 ongoing studies and 16 studies reported as being completed or terminated in a study registry, but without results yet.
Hospitalised individuals with a confirmed or suspected diagnosis of symptomatic COVID‐19
Systemic corticosteroids plus standard care versus standard care plus/minus placebo
We included 10 RCTs (7989 participants), one of which did not report any of our pre‐specified outcomes and thus our analysis included outcome data from nine studies.
All‐cause mortality (at longest follow‐up available): systemic corticosteroids plus standard care probably reduce all‐cause mortality slightly in people with COVID‐19 compared to standard care alone (median 28 days: risk difference of 30 in 1000 participants fewer than the control group rate of 275 in 1000 participants; risk ratio (RR) 0.89, 95% confidence interval (CI) 0.80 to 1.00; 9 RCTs, 7930 participants; moderate‐certainty evidence).
Ventilator‐free days: corticosteroids may increase ventilator‐free days (MD 2.6 days more than control group rate of 4 days, 95% CI 0.67 to 4.53; 1 RCT, 299 participants; low‐certainty evidence). Ventilator‐free days have inherent limitations as a composite endpoint and should be interpreted with caution.
New need for invasive ventilation: the evidence is of very low certainty. Because of high risk of bias arising from deaths that occurred before ventilation we are uncertain about the size and direction of the effects. Consequently, we did not perform analysis beyond the presentation of descriptive statistics.
Quality of life/neurological outcome: no data were available.
Serious adverse events: we included data on two RCTs (678 participants) that evaluated systemic corticosteroids compared to standard care (plus/minus placebo); for adverse events and hospital‐acquired infections, we included data on five RCTs (660 participants). Because of high risk of bias, heterogeneous definitions, and underreporting we are uncertain about the size and direction of the effects. Consequently, we did not perform analysis beyond the presentation of descriptive statistics (very low‐certainty evidence).
Different types, dosages or timing of systemic corticosteroids
We identified one study that compared methylprednisolone with dexamethasone. The evidence for mortality and new need for invasive mechanical ventilation is very low certainty due to the small number of participants (n = 86). No data were available for the other outcomes.
We did not identify comparisons of different dosages or timing.
Outpatients with asymptomatic or mild disease
Currently, there are no studies published in populations with asymptomatic infection or mild disease.
Authors' conclusions
Moderate‐certainty evidence shows that systemic corticosteroids probably slightly reduce all‐cause mortality in people hospitalised because of symptomatic COVID‐19. Low‐certainty evidence suggests that there may also be a reduction in ventilator‐free days. Since we are unable to adjust for the impact of early death on subsequent endpoints, the findings for ventilation outcomes and harms have limited applicability to inform treatment decisions. Currently, there is no evidence for asymptomatic or mild disease (non‐hospitalised participants).
There is an urgent need for good‐quality evidence for specific subgroups of disease severity, for which we propose level of respiratory support at randomisation. This applies to the comparison or subgroups of different types and doses of corticosteroids, too. Outcomes apart from mortality should be measured and analysed appropriately taking into account confounding through death if applicable.
We identified 42 ongoing and 16 completed but not published RCTs in trials registries suggesting possible changes of effect estimates and certainty of the evidence in the future. Most ongoing studies target people who need respiratory support at baseline. With the living approach of this review, we will continue to update our search and include eligible trials and published data.
Plain language summary
Are corticosteroids (anti‐inflammatory medicines) given orally or by injection an effective treatment for people with COVID‐19?
Key messages
• Corticosteroids (anti‐inflammatory medicines) given orally or by injection (systemic) are probably effective treatments for people hospitalised with COVID‐19. We don’t know whether they cause unwanted effects.
• We don’t know which systemic corticosteroid is the most effective. We found no evidence about people without symptoms or with mild COVID‐19 who were not hospitalised.
• We found 42 ongoing studies and 16 completed studies that have not published their results. We will update this review when we find new evidence.
What are corticosteroids?
Corticosteroids are anti‐inflammatory medicines that reduce redness and swelling. They also reduce the activity of the immune system, which defends the body against disease and infection. Corticosteroids are used to treat a variety of conditions, such as asthma, eczema, joint strains and rheumatoid arthritis.
Systemic corticosteroids can be swallowed or given by injection to treat the whole body. High doses of corticosteroids taken over a long time may cause unwanted effects, such as increased appetite, difficulty sleeping and mood changes.
Why are corticosteroids possible treatments for COVID‐19?
COVID‐19 affects the lungs and airways. As the immune system fights the virus, the lungs and airways become inflamed, causing breathing difficulties. Corticosteroids reduce inflammation, so may reduce the need for breathing support with a ventilator (a machine that breathes for a patient). Some patients’ immune systems overreact to the virus causing further inflammation and tissue damage; corticosteroids may help to control this response.
What did we want to find out?
We wanted to know whether systemic corticosteroids are an effective treatment for people with COVID‐19 and whether they cause unwanted effects.
We were interested in:
• deaths from any cause up to 14 days after treatment, or longer if reported; • whether people got better or worse after treatment, based on their need for breathing support; • quality of life; • unwanted effects and infections caught in hospital.
What did we do? We searched for studies that investigated systemic corticosteroids for people with mild, moderate or severe COVID‐19. People could be any age, sex or ethnicity.
Studies could compare:
• corticosteroids plus usual care versus usual care with or without placebo (sham medicine); • one corticosteroid versus another; • corticosteroids versus a different medicine; • different doses of a corticosteroid; or • early versus late treatment.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 11 studies with 8075 people. About 3000 people received corticosteroids, mostly dexamethasone (2322 people). Most studies took place in high‐income countries.
We also found 42 ongoing studies, and 16 completed studies that have not yet published their results.
Main results
Ten studies compared corticosteroids plus usual care versus usual care with or without placebo. Only one study compared two corticosteroids. The studies included only hospitalised people with confirmed or suspected COVID‐19. No studies looked at non‐hospitalised people, different doses or timing, or provided information about quality of life.
Corticosteroids plus usual care compared to usual care with or without placebo (10 studies)
• Corticosteroids probably reduce the number of deaths from any cause slightly, up to 60 days after treatment (9 studies, 7930 people). • One study (299 people) reported that people on a ventilator at the start of the study were ventilation‐free for more days with corticosteroids than with usual care, so corticosteroids may improve people’s symptoms. • Four studies (427 people) reported whether people not on a ventilator at the start of treatment later needed to be put on a ventilator, but we could not pool the studies’ results, so we are unsure if people’s symptoms get worse with corticosteroids or usual care. • We don’t know if corticosteroids increase or reduce serious unwanted effects (2 studies, 678 people), any unwanted effects (5 studies, 660 people), or infections caught in hospital (5 studies, 660 people).
Methylprednisolone versus dexamethasone (1 study, 86 people)
• We don’t know whether the corticosteroid methylprednisolone reduces the number of deaths from any cause compared to dexamethasone in the 28 days after treatment. • We don’t know if methylprednisolone worsens people’s symptoms compared to dexamethasone, based on whether they needed ventilation in the 28 days after treatment. • The study did not provide information about anything else we were interested in.
What are the limitations of the evidence?
We are moderately confident in the evidence about corticosteroids’ effect on deaths from any cause. However, our confidence in the other evidence is low to very low, because studies did not use the most robust methods, and the way results were recorded and reported differed across studies. We did not find any evidence on quality of life and there was no evidence from low‐income countries or on people with mild COVID‐19 or no symptoms, who were not hospitalised.
How up to date is this evidence?
Our evidence is up to date to 16 April 2021.
Summary of findings
Background
This work is part of a series of Cochrane Reviews investigating treatments and therapies for coronavirus disease 2019 (COVID‐19). Reviews in this series share information in the background section and methodology with the first published reviews about monoclonal antibodies (Kreuzberger 2021), and convalescent plasma (Piechotta 2021), from the German research project “CEOsys” (COVID‐19 Evidence Ecosystem).
Description of the condition
COVID‐19 is a rapidly spreading infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2; WHO 2020a). On 11 March 2020, the World Health Organization (WHO) declared the current COVID‐19 outbreak a pandemic. The severity of COVID‐19 is unprecedented in comparison to that of previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which caused 813 and 858 deaths, respectively (WHO 2007; WHO 2019). Despite intensive international efforts to contain its spread, SARS‐CoV‐2 has resulted in a continuously rising number of cases and deaths with a clearly accelerating increase in the first months of 2021 (WHO 2021a; WHO 2021b). In the meantime, the appearance of SARS‐CoV‐2 variants with higher transmissibility is further increasing infection rates (WHO 2021c).
The risk for a severe course of disease, hospitalisation and mortality is higher among individuals aged 65 years or older, smokers and those with certain underlying medical conditions such as cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart conditions, immunocompromised state, obesity, sickle cell disease or type 2 diabetes mellitus (Huang 2020; Liang 2020; WHO 2020a; Williamson 2020). COVID‐19 case fatality ratios vary widely between countries and reporting periods, from 0.0% to more than 25% (Johns Hopkins University 2021). However, these numbers may be misleading as they tend to overestimate the infection fatality ratio due to varying testing frequency, a lack of reporting dates, and variations in case definitions, especially in the beginning of the pandemic when the main focus was on severe cases (WHO 2020b).
The median incubation time is estimated to be five to six days, and 97.5% of symptomatic cases develop symptoms within 11.5 days of exposure (Lauer 2020). Sore throat, cough, fever, headache, fatigue, and myalgia or arthralgia are the most commonly reported symptoms (Struyf 2020). Other symptoms include dyspnoea, chills, nausea or vomiting, diarrhoea, and nasal congestion (WHO 2020a). The majority of infected people (approximately 80%) have mild symptoms (Wu 2020), or remain completely asymptomatic (Buitrago‐Garcia 2020). A smaller proportion (approximately 14%) are affected by severe or critical disease that requires treatment at an intensive care unit (ICU) due to respiratory failure, septic shock or multiple organ dysfunction (Wu 2020). In light of the extent of the COVID‐19 pandemic and the scarcity of effective treatments, there is an urgent need for effective therapies to save lives and to reduce the high burden on healthcare systems, especially in the face of evolving variants of the virus with the potential for increased transmissibility and the limited global availability of vaccines.
Description of the intervention
Corticosteroids are a group of stress hormones produced from the adrenal cortex. In addition to their stress‐mediated mechanisms for generating energy substrates, corticosteroids have anti‐inflammatory and immunosuppressive properties in higher doses and are applied in a wide variety of ways in almost all fields of medicine (Barnes 2006; Rhen 2005). For example, corticosteroids are used at high doses of more than 6 mg/kg up to 30 mg/kg methylprednisolone corresponding to more than 30 mg/kg up to 150 mg/kg hydrocortisone equivalents daily for short‐term, high‐dose pulse therapy against solid organ transplant rejection, or about 0.5 mg/kg hydrocortisone equivalents daily for prolonged therapy in different inflammatory lung diseases. A major representative of synthetic corticosteroids is the long‐acting compound dexamethasone. Examples of other synthetic corticosteroids with weaker and shorter activity are methylprednisolone and hydrocortisone (Bourdeau 2003). To obtain comparable effects, dosage equivalents are needed for the different corticosteroids.
How the intervention might work
It has been proposed that corticosteroids could be clinically effective against severe and critical COVID‐19. A substantial percentage of patients develop severe and critical COVID‐19 that requires hospitalisation, with dyspnoea, hypoxia, or relevant lung involvement based on imaging, as well as respiratory failure, shock, or multi‐organ dysfunction requiring ventilator support (Thibeault 2021; Wu 2020). In COVID‐19, an insufficient host defence and unbalanced inflammation is thought to play a key role in the pathophysiology of hypoxemic respiratory failure (Schulte‐Schrepping 2020). A systemic inflammatory response with the excessive release of cytokines and inflammation mediators can lead to lung injury with the development of acute respiratory distress syndrome (ARDS). The potent anti‐inflammatory effects of corticosteroids might prevent or mitigate these deleterious effects by modulating cytokine release (Villar 2020). Corticosteroids have been widely used in syndromes closely related to COVID‐19, including SARS, MERS, severe influenza, and community‐acquired pneumonia. However, the evidence to support or discourage the use of corticosteroids under these conditions has been weak. Corticosteroids can induce harm through immunosuppressive effects during the treatment of infection. In SARS‐CoV‐2 infection, viral shedding appears early in the illness and declines thereafter. The effect of corticosteroid therapy on virus clearance in COVID‐19 needs to be taken into consideration. In acutely critically ill people, dexamethasone has comparatively few side effects (Rochwerg 2018). However, patients may suffer from blood glucose problems and potential fungal infections. The therapeutic use of higher doses of corticosteroids over a longer time suppresses the hypothalamic‐pituitary‐adrenal axis such that dosage‐tapering may be needed.
Why it is important to do this review
Extensive work has been done in the field of systematic reviews regarding COVID‐19 interventions, including corticosteroids. For example, several systematic reviews investigated the association between the use of corticosteroids and COVID‐19‐related mortality based on randomised controlled trials (RCT) and non‐randomised studies (e.g. Sterne 2020; Van Paassen 2020). This Cochrane review will fill current gaps by identifying, describing, evaluating, and meta‐analysing RCTs of systemic corticosteroids in relation to clinical outcomes in COVID‐19. Unlike other systematic reviews in this field, it considers the outcome clinical improvement and worsening (defined by respiratory support) as well as subgroup analysis. The living systematic review will be updated once new evidence becomes available.
Objectives
To assess whether systemic corticosteroids are effective and safe in the treatment of people with COVID‐19, and to keep up‐to‐date with the evolving evidence base using a living systematic review approach.
Methods
Criteria for considering studies for this review
Types of studies
The main description of methods is based on Cochrane Haematology's standard template and is in line with a series of Cochrane Reviews investigating treatments and therapies against COVID‐19. We made specific adaptations related to the research question if necessary. The protocol for this review was registered with PROSPERO on 21 December 2020 (Wagner 2021).
To assess the efficacy and safety of systemic corticosteroids against COVID‐19, we included RCTs, as this study design, if performed appropriately, provides the best evidence for experimental therapies in highly controlled therapeutic settings. We used the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a).
We included the following formats, if sufficient information was available on study design, characteristics of participants, interventions and outcomes:
full‐text publications;
preprint articles.
We included preprints for a complete overview of the ongoing research activity, especially for tracking newly emerging studies about systemic corticosteroids against COVID‐19. We did not apply any limitation with respect to the length of follow‐up.
Types of participants
We included adults with a suspected or confirmed diagnosis of COVID‐19 (as described in the study) and we did not exclude any studies based on gender, ethnicity, disease severity, or setting.
We excluded studies evaluating corticosteroids against coronavirus diseases such as SARS or MERS, or other viral diseases, such as influenza. If studies enrolled populations with or exposed to mixed viral diseases, we had planned to only include these if study authors provided subgroup data for SARS‐CoV‐2 infection.
Types of interventions
We included the following interventions:
any type or dose of systemic corticosteroids;
oral or intravenous application.
We included the following comparisons:
systemic corticosteroids plus standard care versus standard care (plus/minus placebo);
dose comparisons;
timing comparisons (early versus late);
different types of corticosteroids;
systemic corticosteroid versus other active substances.
Standard care in both arms should be similar.
We excluded the following interventions:
corticosteroid plus other active substance versus standard care;
topical corticosteroids;
inhaled corticosteroids;
corticosteroids for long‐COVID treatment.
Types of outcome measures
We evaluated core outcomes in accordance with the Core Outcome Measures in Effectiveness Trials (COMET) Initiative for COVID‐19 patients (COMET 2020; Marshall 2020), and additional outcomes that have been prioritised by consumer representatives and the German guideline panel for inpatient therapy against COVID‐19.
We defined this outcome set for hospitalised individuals with a confirmed or suspected diagnosis of COVID‐19 and moderate to severe disease, according to WHO clinical progression scale stage 4 to 9 (Marshall 2020), that is, all patients who were hospitalised because of symptomatic COVID‐19 treated with all different levels of respiratory support (no additional oxygen, low‐flow oxygen prongs or mask ('low‐flow oxygen' only hereafter), high‐flow oxygen or non‐invasive ventilation, invasive mechanical ventilation including extracorporeal membrane oxygenation (ECMO)), and individuals with a confirmed or suspected diagnosis of SARS‐CoV‐2 infection and asymptomatic or mild disease, according to the WHO clinical progression scale (Marshall 2020). Of note, readers may encounter respiratory support both as a baseline characteristic and as an outcome measure ‐ in the latter case we used changes in the level of support.
Individuals with a suspected or confirmed diagnosis of COVID‐19 and moderate to severe disease
Prioritised outcomes (included in the summary of findings table)
Mortality: all‐cause mortality at day 14 or any longer observation period, in‐hospital all‐cause mortality
-
Improvement of clinical status during the longest observation period available:
ventilator‐free days
-
Deterioration of clinical status during the longest observation period available:
new need for invasive mechanical ventilation, that is, transition to WHO 7 to 9 if 6 or lower at baseline (see Figure 1). If new need was not available directly, we used death as a proxy for assumed intubation counted together with patients alive and ventilated.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) during the longest period available
Serious adverse events, defined as the number of participants with any event
Adverse events (any grade), defined as the number of participants with any event
Hospital‐acquired infections
1.
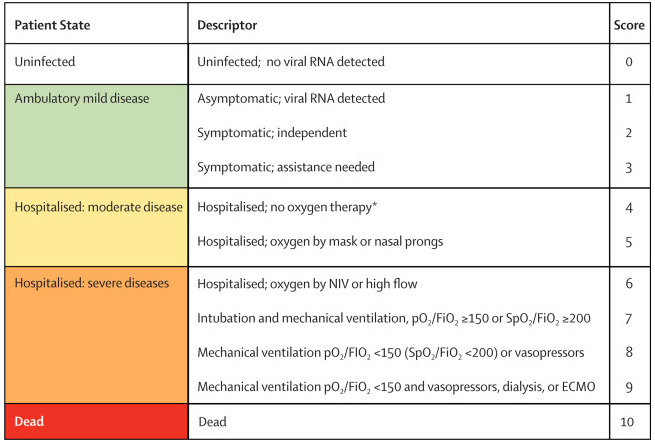
WHO Clinical Progression Scale (Marshall 2020). Copyright © 2020 Elsevier Ltd. All rights reserved: reproduced with permission.
ECMO = extracorporeal membrane oxygenation; FiO2 = fraction of inspired oxygen; NIV = non‐invasive ventilation; pO2 = partial pressure of oxygen; RNA = ribonucleic acid; SpO2 = oxygen saturation.
*If hospitalised for isolation only, record status for ambulatory patients.
Additional outcomes (not included in the summary of findings table)
Liberation from invasive mechanical ventilation in patients, that is, transition to WHO 6 or lower if 7 or higher at baseline (see Figure 1). If liberation was not available directly, we used death as a proxy for assumed non‐liberation counted together with patients alive and ventilated
Need for dialysis during the longest period available
Viral clearance, assessed with reverse transcription polymerase chain reaction (RT‐PCR) test for SARS‐CoV‐2 at baseline, up to 3, 7, and 15 days
Individuals with a suspected or confirmed diagnosis of SARS‐CoV‐2 infection and asymptomatic or mild disease
Prioritised outcomes (included in the summary of findings table)
Mortality: all‐cause mortality at day 14 or any longer observation period, in‐hospital all‐cause mortality
-
Improvement of clinical status during the longest observation period available:
ventilator‐free days
-
Deterioration of clinical status during the longest observation period available:
new need for invasive mechanical ventilation, that is, transition to WHO 7 to 9 if 6 or lower at baseline (see Figure 1). If new need was not available directly, we used death as a proxy for assumed intubation counted together with patients alive and ventilated.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) during the longest period available
Serious adverse events, defined as the number of participants with any event
Adverse events (any grade), defined as the number of participants with any event
Infections
Additional outcomes (not included in the summary of findings table)
Liberation from invasive mechanical ventilation in patients, that is, transition to WHO 6 or lower if 7 or higher at baseline (see Figure 1). If liberation was not available directly, we used death as a proxy for assumed non‐liberation counted together with patients alive and ventilated
Need for dialysis during the longest period available
Viral clearance, assessed with RT‐PCR test for SARS‐CoV‐2 at baseline, up to 3, 7, and 15 days
Timing of outcome measurement
In the case of time‐to‐event analysis, for example, for time to clinical improvement, we included the outcome measure based on the longest follow‐up time. We also collected information on outcomes from all other time points reported in the publications.
Search methods for identification of studies
Electronic searches
Our information specialist (MIM) conducted systematic searches in the following sources from the inception of each database to 16 April 2021 (search date for all databases) and did not place restrictions on the language of publication.
-
Cochrane COVID‐19 Study Register (www.covid-19.cochrane.org), comprising:
MEDLINE (PubMed), daily updates;
Embase.com, weekly updates;
ClinicalTrials.gov (www.clinicaltrials.gov), daily updates;
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch), weekly updates;
medRxiv (www.medrxiv.org), weekly updates;
Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates.
-
Web of Science Core Collection (Clarivate), from 1 January 2020 onwards:
Science Citation Index Expanded (1945 to present);
Emerging Sources Citation Index (2015 to present).
WHO COVID‐19 Global literature on coronavirus disease (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/).
Database search results for Web of Science were restricted to publications from 2020 to the present date, as no treatment studies on COVID‐19 were registered prior to January 2020. For detailed search strategies, see Appendix 1.
Searching other resources
We identified other potentially eligible studies or ancillary publications by searching the reference lists of included studies and systematic reviews.
We searched for grey literature, which we defined as searching study registries such as ClinicalTrials.gov and WHO ICTRP contained in the Cochrane COVID‐19 Study Register, as well as searching preprint servers and grey literature indexes contained in the Cochrane COVID‐19 Study Register and the WHO COVID‐10 Global literature on coronavirus disease.
Data collection and analysis
Selection of studies
Two review authors (NS, CW) independently screened the results of the search strategies for eligibility for this review by reading the titles and abstracts using EndNote Software (EndNote X9). We coded the abstracts as either 'include' or 'exclude'. In the case of disagreement or if it was unclear whether we should retrieve the abstract or not, we obtained the full‐text publication for further discussion. Two review authors assessed the full‐text articles of selected studies. If the two review authors were unable to reach a consensus, they consulted the third review author to reach a final decision.
We documented the study selection process in a flow chart, as recommended in the PRISMA statement (Moher 2009), and showed the total numbers of retrieved references and the numbers of included and excluded studies. We listed all studies that we excluded after full‐text assessment and the reasons for their exclusion in the Characteristics of excluded studies section.
Data extraction and management
We conducted data extraction according to the guidelines proposed by Cochrane (Li 2021). Two out of five review authors (AM, MG, CW, AF, KK) extracted data independently and in duplicate, using a customised data extraction form developed in Microsoft Excel (Microsoft 2018). We resolved disagreements by discussion. If we were unable to reach agreement, we involved a third review author.
Two out of three review authors (MG, MK, CW) independently assessed eligible studies obtained in the process of study selection (as described above) for methodological quality and risk of bias. If the review authors were unable to reach a consensus, they consulted a third review author.
We extracted the following information if reported.
General information: author, title, source, publication date, country, language, duplicate publications
Study characteristics: trial design, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, treatment cross‐overs, compliance with assigned treatment, length of follow‐up
Participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, number of participants with positive, negative or unknown RT‐PCR test result, additional diagnoses, severity of disease, previous treatments, concurrent treatments, co‐morbidities (e.g. diabetes, immunosuppression)
Interventions: type of corticosteroid, dose, frequency, timing, duration and route of administration, setting (e.g. inpatient, outpatient), duration of follow‐up
Control interventions: placebo, no treatment or other intervention; dose, frequency, timing, duration and route of administration, setting, duration of follow‐up
Outcomes: as specified under Types of outcome measures
Risk of bias assessment: randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome, selection of the reported results.
Assessment of risk of bias in included studies
We used the Risk of Bias 2 (RoB 2) tool (version of 22 August 2019) to analyse the risk of bias of study results (Sterne 2019). Of interest for this review is the effect of the assignment to the intervention (the intention‐to‐treat (ITT) effect), thus, we performed all assessments with RoB 2 on this effect. The outcomes that we assessed are those specified for inclusion in the summary of findings table.
Two out of three review authors (MK, MG, CW) independently assessed the risk of bias for each outcome. In case of discrepancies among their judgements and inability to reach consensus, we consulted the fourth review author to reach a final decision. We assessed the following types of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b):
bias arising from the randomisation process;
bias due to deviations from the intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
To address these types of bias we used the signalling questions recommended in RoB 2 and made a judgement using the following options.
'Yes': if there is firm evidence that the question is fulfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question).
Probably yes': a judgement has been made that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No': if there is firm evidence that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question).
'Probably no': a judgement has been made that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question).
'No information': if the study report does not provide sufficient information to allow any judgement.
We used the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias:
low risk of bias;
some concerns;
high risk of bias.
Subsequently, we derived an overall risk of bias rating for each pre‐specified outcome in each study in accordance with the following suggestions.
'Low risk of bias': we judge the trial to be at low risk of bias for all domains for this result.
'Some concerns': we judge the trial to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain.
'High risk of bias': we judge the trial to be at high risk of bias in at least one domain for the result, or we judge the trial to have some concerns for multiple domains in a way that substantially lowers confidence in the results.
We used the RoB 2 Excel tool to implement RoB 2 (available on the riskofbias.info website), stored, and presented our detailed RoB 2 assessments in the analyses section and as supplementary online material.
As we collected the data from the studies and assessed RoB 2, we noticed an issue with competing risk of death (Columbia Public Health 2021 as easily accessible introduction), which we discussed in Quality of the evidence. We dealt with this issue within domain 3 of RoB 2 (Higgins 2019).
Measures of treatment effect
For continuous outcomes, we recorded the mean, standard deviation (SD) and total number of participants in both treatment and control groups. Where continuous outcomes used the same scale, we performed analyses using the mean difference (MD) with 95% confidence intervals (CIs). For continuous outcomes measured with different scales, we performed analyses using the standardised mean difference (SMD). For interpreting SMDs, we re‐expressed SMDs in the original units of a particular scale with the most clinical relevance and impact (e.g. clinical symptoms with the WHO Clinical Progression Scale (WHO 2020c)).
For dichotomous outcomes, we recorded the number of events and total number of participants in both treatment and control groups. We reported the pooled risk ratio (RR) with a 95% CI (Deeks 2021).
If available, we planned to extract and report hazard ratios (HRs) for time‐to‐event outcomes (e.g. time to liberation from invasive ventilation). If HRs were not available, we would make every effort to estimate the HR as accurately as possible from available data using the methods proposed by Parmar and Tierney (Parmar 1998; Tierney 2007).
Unit of analysis issues
The aim of this review is to summarise studies that analyse data at the level of the individual. We would also have accepted cluster‐randomised trials for inclusion, had we found any. We collated multiple reports of one study so that the study, and not the report, is the unit of analysis.
Studies with multiple treatment groups
As recommended in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c), for studies with multiple treatment groups of the same intervention (i.e. dose, route of administration), we planned to evaluate whether study arms were sufficiently homogeneous to be combined. If arms could not be pooled, we planned to compare each arm with the common comparator separately. For pair‐wise meta‐analysis, we planned to split the ‘shared’ group into two or more groups with smaller sample size, and include two or more (reasonably independent) comparisons. For this purpose, for dichotomous outcomes, we planned to divide both the number of events and the total number of participants, and for continuous outcomes, we planned to divide the total number of participants with unchanged means and SDs.
Dealing with missing data
Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions suggests a number of potential sources for missing data, which we took into account at study level, at outcome level and at summary data level (Deeks 2021). At all levels, it is important to differentiate between data 'missing at random', which may often be unbiased, and 'not missing at random', which may bias study and thus review results.
We requested missing data for four outcomes from the corresponding authors via email, followed by a second email and a phone call attempt if necessary. The outcomes were mortality during the longest observation period, need for invasive ventilation, liberation from invasive ventilation and adverse events. We contacted authors from 10 included studies (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Farahani 2021; Horby 2021; Jamaati 2021; Jeronimo 2020; Ranjbar 2021; Tang 2021). Of these, three authors sent in the requested data (Corral‐Gudino 2021; Edalatifard 2020; Jeronimo 2020) and one (Tomazini 2020), had already provided all necessary data in the publication. We have not yet requested detailed individual time‐to‐event data from the study authors, which would be necessary to adjust for competing risks. Alternatively, data already adjusted by the study authors themselves could be presented, too, in a future version of this review.
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1. We used the I² statistic (Higgins 2003), and visual examination, to assess possible heterogeneity (I² statistic > 30% to signify moderate heterogeneity, I² statistic > 75% to signify considerable heterogeneity; Deeks 2021). If the I² statistic was above 80%, we had planned to explore potential causes through sensitivity and subgroup analyses. However, none of our analyses demonstrated I² statistic > 80%. For future updates, if we cannot identify reasons for heterogeneity in subgroup or sensitivity analysis, we will not perform a meta‐analysis but instead, provide outcome data for all studies without an overall effect estimate.
Assessment of reporting biases
As mentioned above, we searched trials registries to identify completed studies that have not been published elsewhere, to minimise or determine publication bias. We intended to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test for meta‐analyses involving at least 10 trials (Sterne 2019). We considered P < 0.1 as significant for this test.
Data synthesis
If the clinical and methodological characteristics of individual studies were sufficiently homogeneous, we pooled the data in a meta‐analysis. We performed analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We analysed studies that included different severities of disease separately, grouping them with respect to disease severity according to need for respiratory support at randomisation (see Types of outcome measures). We treated placebo and standard care as the same intervention, as well as standard care at different institutions and time points.
We used Review Manager Web (RevMan Web) software for analyses (RevMan Web 2019). One review author entered the data into RevMan Web, and a second review author checked the data for accuracy. We used the random‐effects model for all analyses as we anticipated that true effects are related, but are not the same for included studies. If we deemed meta‐analysis inappropriate for a certain outcome because of heterogeneity of included studies both statistically or conceptually or for too high a risk of bias, we presented descriptive statistics only.
If meta‐analysis was possible, we assessed the effects of potential biases in sensitivity analyses (see Sensitivity analysis). For binary outcomes, we based the estimation of the between‐study variance using the Mantel‐Haenszel method. We planned to explore heterogeneity above 80% with subgroup analyses. If we could not find a cause for the heterogeneity, we did not perform a meta‐analysis, but commented on the results as a narrative with the results from all studies presented in tables.
Subgroup analysis and investigation of heterogeneity
Because of clinical relevance, we performed subgroup analyses of mortality for the following characteristics, irrespective of observed statistical heterogeneity.
Respiratory support at randomisation (for all comparisons planned, but currently possible only for the comparison of corticosteroids plus standard care versus standard care plus/minus placebo); respiratory support served as a baseline characteristic for the purpose of this analysis.
Type of systemic corticosteroid (for the comparison of corticosteroids plus standard care versus standard care plus/minus placebo).
For future review updates, if the I² statistic is found to be above 80% for the other outcomes, we will also conduct subgroup analyses for these outcomes (see also Assessment of heterogeneity).
Sensitivity analysis
We performed the following sensitivity analysis for all outcomes:
risk of bias assessment components (studies with a low risk of bias or some concerns versus studies with a high risk of bias).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence for the following outcomes, and prepared one summary of findings table per population.
Summary of findings
We used the GRADE pro GDT software to create summary of findings tables. For time‐to‐event outcomes, we would have calculated absolute effects at specific time points, as recommended in the GRADE guidance (Skoetz 2020).
According to Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions, the “most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes” should be included in the summary of findings table(s) (Schünemann 2021). We included outcomes prioritised according to the core outcome sets for studies for the treatment of patients with confirmed or suspected COVID‐19 (COMET 2020), and patient relevance. These outcomes were as follows.
Individuals with a suspected or confirmed diagnosis of COVID‐19 and moderate to severe disease
Mortality: all‐cause mortality at day 14 or any longer observation period, in‐hospital all‐cause mortality
-
Improvement of clinical status during the longest observation period available:
ventilator‐free days
-
Deterioration of clinical status during the longest observation period available:
new need for invasive mechanical ventilation, that is, transition to WHO 7 to 9 if 6 or lower at baseline (see Figure 1). If new need was not available directly, we used death as a proxy for assumed intubation counted together with patients alive and ventilated.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) during the longest period available
Serious adverse events
Adverse events (any grade)
Hospital‐acquired infections
Individuals with a suspected or confirmed diagnosis of SARS‐CoV‐2 infection and asymptomatic or mild disease
Mortality: all‐cause mortality at day 14 or any longer observation period, in‐hospital all‐cause mortality
-
Improvement of clinical status during the longest observation period available:
ventilator‐free days
-
Deterioration of clinical status during the longest observation period available:
new need for invasive mechanical ventilation, that is, transition to WHO 7 to 9 if 6 or lower at baseline (see Figure 1). If new need was not available directly, we used death as a proxy for assumed intubation counted together with patients alive and ventilated.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) during the longest period available
Serious adverse events
Adverse events (any grade)
Infections
Assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence for the outcomes listed in the previous section.
The GRADE approach uses five domains (risk of bias, inconsistency, imprecision, indirectness and publication bias) to assess certainty in the body of evidence for each prioritised outcome.
We downgraded our certainty of evidence for:
serious (‐1) or very serious (‐2) risk of bias;
serious (‐1) or very serious (‐2) inconsistency;
serious (‐1) or very serious (‐2) uncertainty about directness;
serious (‐1) or very serious (‐2) imprecise or sparse data;
serious (‐1) or very serious (‐2) probability of reporting bias.
The GRADE system used the following criteria for assigning grade of evidence.
'High': we are very confident that the true effect lies close to that of the estimate of the effect.
'Moderate': we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
'Low': our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
'Very low': we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We followed the current GRADE guidance for these assessments in its entirety as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 14 (Schünemann 2021).
We used the overall risk of bias judgement, derived from the RoB 2 Excel tool, to inform our decision on downgrading for risk of bias. We phrased the findings and certainty in the evidence as suggested in the informative statement guidance (Santesso 2020).
Methods for future updates
Living systematic review considerations
Our information specialist (MIM) will provide us with new search records each week, which two review authors will screen, extract, evaluate, and integrate following the guidance for Cochrane living systematic reviews (Living Evidence Network 2019).
We will manually check platform trials that were previously identified and listed as 'studies awaiting classification' for additional treatment arms.
We will wait until the accumulating evidence changes our conclusions of the implications of research and practice before republishing the review. We will consider one or more of the following components to inform this decision:
findings of one or more prioritised outcomes;
credibility (e.g. GRADE rating) of one or more prioritised outcomes;
new settings, populations, interventions, comparisons or outcomes studied.
In case of emerging policy relevance because of global controversies around the intervention, we will consider republishing an updated review even though our conclusions remain unchanged. We will review the review scope and methods approximately monthly, or more frequently if appropriate, in light of potential changes in COVID‐19 research (for example, when additional comparisons, interventions, subgroups or outcomes, or new review methods become available).
Results
Description of studies
Results of the search
We searched all databases and screened the resulting records up to 16 April 2021. We identified 1397 records. After removing duplicates, we screened 1246 records based on their titles and abstracts. We excluded 1131 records that did not meet the inclusion criteria. Of the remaining 115 records, we included 89 records:
11 RCTs (in 19 records) for inclusion in this review;
42 RCTs (in 51 records) are ongoing;
16 RCTs (in 19 records) are awaiting classification as they have been reported as being completed, but the results have not yet been published.
The study flow diagram in Figure 2 illustrates the study selection process according to PRISMA guidelines (Moher 2009).
2.

PRISMA flow diagram illustrating our study selection process.
Included studies
Design and sample size
We included 11 studies, of which two were multi‐centre platform RCTs (Horby 2021; Angus 2020), five were multi‐centre RCTs (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; Tomazini 2020), and four were single‐centre RCTs (Farahani 2021; Jamaati 2021; Jeronimo 2020; Ranjbar 2021).
Setting
Of 8075 participants in the included studies, 7041 (87%) originated from high‐income countries; there were no studies from low‐income countries (World Bank Country Groups 2021). Seven studies originated from lower‐ and upper‐middle‐income countries (Edalatifard 2020; Farahani 2021; Jamaati 2021; Jeronimo 2020; Ranjbar 2021; Tang 2021; Tomazini 2020) and four from high‐income countries (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Horby 2021).
Participants
All participants were adults hospitalised for either acute COVID‐19 or, as in the case of Angus 2020, Dequin 2020, Horby 2021, Tomazini 2020 and Jeronimo 2020, suspected acute COVID‐19. Positive RT‐PCR rates within the studies ranged from about 95% in Dequin 2020 and Tomazini 2020 to about 80% in Angus 2020. All included participants were hospitalised because of symptomatic (suspected) COVID‐19 and were treated with different levels of respiratory support (no oxygen, low‐flow oxygen, high‐flow oxygen or non‐invasive ventilation, or invasive mechanical ventilation, including ECMO). Based on the different levels of respiratory support at baseline disease severity ranged from 4 to 9 on the WHO Clinical Progression Scale (Marshall 2020).
Interventions
All the completed studies included studies compared systemic corticosteroids, that is, hydrocortisone, prednisolone and methylprednisolone, and dexamethasone to standard care (plus/minus placebo), except one that compared methylprednisolone to dexamethasone (Ranjbar 2021). Daily hydrocortisone equivalents of the initial doses ranged from 150 mg to 5000 mg and durations of treatment ranged from zero to approximately 20 days. The majority of participants (n = 2561; 83%) randomised to corticosteroids received equivalents of 200 mg/day or less, 463 (15%) received 201 mg/day to 500 mg/day, and 48 (2%) received 501 mg/day to 5000 mg/day. The route of administration was intravenous except in Horby 2021, who allowed both oral and intravenous administration, and Farahani 2021, with oral dose‐tapering after intravenous administration.
Included studies for comparison of corticosteroids plus standard care to standard care (plus/minus placebo)
We included 10 studies describing 7989 participants in this comparison, of whom 2986 were randomised to corticosteroids and 5003 to standard care (plus/minus placebo). Please see Table 3 for details, but note that no endpoint data from Farahani 2021 (29 participants) were applicable for further analysis, resulting in an analysis of nine trials only. Upon request, corresponding authors of three included studies provided us with additional data (Corral‐Gudino 2021; Edalatifard 2020; Jeronimo 2020). They sent in all‐cause mortality rates at the end of their respective observation periods stratified by respiratory support at randomisation. They also sent in definitions and rates of adverse events as well as rates of clinical improvement and deterioration based on new need for invasive ventilation on the one hand and weaning of initially invasively ventilated patients on the other.
1. Characteristics of the included studies for the comparison: corticosteroids versus placebo or standard care.
| Study ID | Intervention and regimen | Hydrocortisone equivalent of initial dose: for 80 kg bodyweight if applicable (Stoelting 2006) | Control | Randomised to corticosteroids | Randomised to control | Study design | Population/disease severity at randomisation |
| Angus 2020 | Hydrocortisone, IV, 150 mg daily for 7 days | 150 mg/d | Standard care | 143 (fixed‐dose) and 152 (shock‐dependent dose)a | 108 |
|
Severe ≥ 6 |
| Corral‐Gudino 2021 | Methylprednisolone, IV 80 mg for 3 days + 40 mg for 3 days | 400 mg/d | Standard care | 35 | 29 |
|
Moderate to severe 5‐6 |
| Dequin 2020 | Hydrocortisone, IV 200 mg for 7 days, 100 mg for 4 days + 50 mg for 3 days | 200 mg/d | Placebo | 76 | 73 |
|
Moderate to severe ≥ 5 |
| Edalatifard 2020 | Methylprednisolone, IV, 250 mg for 3 days | 1250 mg/d | Standard care | 34 | 34 |
|
Moderate to severe 5‐6 |
| Farahani 2021 | Methylprednisolone, IV 1000 mg/d for 3 days + tapering with 1 mg/kg prednisolone for 10 days | 5000 mg/d | Standard care | 14 | 15 |
|
Moderate to severe 5‐6 |
| Horby 2021 | Dexamethasone, IV or oral 6 mg daily for 10 days | 150 mg/d | Standard care | 2104 | 4321 |
|
Moderate to severe 4‐9 |
| Jamaati 2021 | Dexamethasone, IV, 20 mg for 5 days + 10 mg for 5 days | 500 mg/d | Standard care | 25 | 25 |
|
Most likely moderate 5; no IMV at randomisation |
| Jeronimo 2020 | Methylprednisolone (as sodium succinate), IV 1 mg/kg for 5 days | 400 mg/d | Placebo | 209 | 207 |
|
Moderate to severe 5‐9 |
| Tang 2021 | Methylprednisolone, IV, 1 mg/kg for 7 days | 400 mg/d | Placebo | 43 | 43 |
|
Moderate 4‐5 |
| Tomazini 2020 | Dexamethasone, IV, 20 mg for 5 days + 10 mg for 5 days | 500 mg/d | Standard care | 151 | 148 |
|
Severe 7‐9 |
| d: day;IMV: invasive mechanical ventilation; IV: intravenous | |||||||
a Shock‐dependent dose: shock‐dependent dosing strategy was that restricting hydrocortisone to the period when the patient had overt shock would maximise the risk‐benefit ratio. Shock was defined as the requirement for intravenous vasopressor infusion for the treatment of shock presumed due to COVID‐19. Hydrocortisone was discontinued in the shock‐dependent group once shock was considered to have resolved or vasopressors had been discontinued for 24 hours.
Included studies for comparison of different types of systemic corticosteroids
We included Ranjbar 2021 describing 86 participants in this comparison, of whom 44 were randomised to methylprednisolone and 42 to dexamethasone. For details please see Table 4. The corresponding study author did not reply to our data request.
2. Characteristics of the included studies for the comparison: methylprednisolone versus dexamethasone.
| Study ID | Intervention A | Prednisolone equivalent of initial dose (for 80 kg bodyweight if applicable) | Intervention B | Randomised to intervention A | Randomised to Intervention B | Study design | Population/disease severity at randomisation |
| Ranjbar 2021 | Methylprednisolone, IV 160 mg for 5 days + 80 mg for 5 days + 40 mg for 5 days + 20 mg for 5 days (approximation of tapering scheme) | 200 mg/d for methylprednisolone 40 mg/d for dexamethasone |
Dexamethasone, IV, 6 mg for 10 days | 44 | 42 |
|
Moderate 4‐5 |
| IV: intravenous | |||||||
Outcome summary
In the setting of acute COVID‐19 with immediate risk of death, we assumed in‐hospital mortality and all‐cause mortality with any observation period of 14 days and longer to be equivalent. The longest observation period was 60 days in Edalatifard 2020 and the shortest was 21 days in Dequin 2020, although most studies reported mortality at 28 days. All studies except Farahani 2021 reported utilisable dichotomous mortality data for 7930 participants overall in the comparison of corticosteroids versus standard care (plus/minus placebo) and 86 participants in the direct comparison of methylprednisolone and dexamethasone.
The reporting of adverse events, listed in Table 5, was heterogeneous among the 11 included studies. Only three studies explicitly reported adverse events regardless of their nature (Angus 2020; Edalatifard 2020; Tomazini 2020) for 618 participants. Another four studies reported specific adverse events related to the expected side effects of corticosteroids for 715 participants (Corral‐Gudino 2021; Dequin 2020; Jeronimo 2020; Tang 2021).
3. Reporting of adverse events.
| Study | Definition prespecified | Definition as published | Way of counting | Study design |
| Angus 2020 | Trial registration: not mentioned Protocol/SAP: any SAE |
Any SAE | Both available | Open‐label |
| Corral‐Gudino 2021 | Trial registration: use of biological anti‐inflammatories | Microbiology‐proven infection and hyperglycaemia | Events/patients at risk | Open‐label |
| Dequin 2020 | Trial registration: secondary infection during their ICU‐stay until day 21 after randomisation Protocol/SAP: no definition |
Nosocomial infections until day 28 defined by need for antibiotics. No other SAEs/AEs. | Events/patients at risk | Double‐blind |
| Edalatifard 2020 | Trial registration: not mentioned Protocol/SAP: not available |
All undesirable effects (adverse events) | Events/patients at risk | Single‐blind |
| Farahani 2021 | Trial registration: not mentioned Protocol/SAP: not available |
Not reported | Not applicable | Double‐blind |
| Horby 2021 | Trial registration: thrombotic events Protocol/SAP: suspected serious adverse reactions (SSARs) and suspected unexpected serious adverse reactions (SUSARs) |
Suspected drug reactions reported | Not applicable | Open‐label |
| Jamaati 2021 | Not part of trial registration, SAP not available | Not reported | Not applicable | Open‐label |
| Jeronimo 2020 | Protocol/SAP: AE: any unwanted medical occurrence SAE: 1. Results in death or puts life at risk; 2. Requires hospitalisation of the patient or extension of an existing hospitalisation; 3. Results in persistent or significant disability; 4. Results in birth defect or congenital anomaly; 5. Constitutes an important event from a clinical point of view. |
AE/SAE not explicitly reported. Positive blood culture, need for insulin therapy, sepsis reported. |
Blood culture as point prevalence on day 7 Need for insulin therapy and sepsis as patients with event any time within 28 days |
Double‐blind |
| Ranjbar 2021 | Trial registration: not mentioned Protocol/SAP: not available |
Not reported | Not applicable | Triple‐blind |
| Tang 2021 | Trial registration: not mentioned Protocol/SAP: not available |
Hyperglycaemia, ventilator‐associated pneumonia, stress ulcer, gastrointestinal haemorrhage | Events/patients at risk | Single‐blind |
| Tomazini 2020 | Trial registration: not mentioned Protocol/SAP: glycemic control until day 14, nosocomial infection until day 28, any spontaneous AEs |
Glycemic control, nosocomial infection, other AEs | Both available | Open‐label |
| AE: adverse event; ICU: intensive care unit; SAE: serious adverse event; SAP: statistical analysis plan | ||||
Apart from that, one study with 6425 participants reported safety outcomes only for the intervention arm as suspected drug reactions (Horby 2021), and two studies with 79 participants did not report safety outcomes at all (Farahani 2021; Jamaati 2021).
Other efficacy outcomes were reported heterogeneously.
Ongoing studies
We identified 42 ongoing RCTs with systemic application of steroids for acute COVID‐19 (details listed in Table 6), of which 30 were classified as ‘recruiting’ or ‘ongoing’ according to the study registrations. One was classified as ‘temporarily halted’. Eleven were classified as ‘not recruiting’. On excluding the studies that were not yet recruiting, the 31 studies that were recruiting, ongoing, and temporarily halted comprised a total of 10,083 expected participants. Most of the potentially eligible ongoing studies identified intend to recruit people who are admitted to hospital and require varying levels of respiratory support. Of the 42 ongoing studies, 16 planned to test dexamethasone, 14 methylprednisolone and three prednisolone. One study planned to compare different dexamethasone dosing regimens. Six studies planned to compare dexamethasone to methylprednisolone, and one study dexamethasone to prednisolone. One study planned to compare corticosteroids at different time points.
4. Characteristics of ongoing studies .
| Study | Sponsor/developer | Design | Population/disease severity | Setting | Drug | Route of administration | Number of participants | Status |
| ChiCTR2000029386 | Chongqing Public Health Medical Center | RCT | Severe | Inpatient | Methylprednisolone | IV | 48 | Recruiting |
| ChiCTR2000029656 | Wuhan Pulmonary Hospital | RCT | Severe | Inpatient | Methylprednisolone | IV | 100 | Not yet recruiting |
| ChiCTR2000030481 | Zhongnan Hospital of Wuhan University | RCT | Diagnosed COVID‐19 infection | Inpatient | Early corticosteroid intervention, middle‐late corticosteroid intervention | Unclear, most likely systemic | 200 | Recruiting |
| CTRI/2020/07/026608 |
Dr Ananthakumar PK, Chettinad Hospital and Research Institute Kelambakkam Kancheepuram Dist Pin 603103 | RCT | Diagnosed COVID‐infection + ARDS | Inpatient | Dexamethasone, methylprednisolone | IV | 40 | Not yet recruiting |
| CTRI/2020/10/028731 |
Professor Anders Perner, Senior Staff specialist and professor in Intensive Care Medicine Dept of Intensive Care, Rigshospitalet | RCT | IMV or NIV or continuous use of CPAP for hypoxia or oxygen supplementation with an oxygen flow of at least 10 L/min independent of delivery system | Inpatient | Dexamethasone | IV | 1500 | Recruiting |
| CTRI/2020/12/029894 | SRM Medical College Hospital and Research Centre | RCT | SpO2 < 94% under room air and requiring supplemental oxygen for hypoxemia, respiratory rate 24‐30/min | Inpatient | Dexamethasone, methylprednisolone | IV | 50 | Not yet recruiting |
| CTRI/2020/12/030143 | Maulana Azad Medical College and associated Lok Nayak Hospital | RCT | Admitted to ICU within 14 days of onset of symptoms; receiving invasive or non ‐invasive positive pressure ventilation or respiratory support through HFNC | Inpatient | Dexamethasone, methylprednisolone | IV | 500 | Not yet recruiting |
| EUCTR2020‐001413‐20‐ES |
Fundació Clínic per a la Recerca Biomèdica | RCT | Non‐critical patient with pneumonia in radiological progression and/or patient with progressive respiratory failure in the last 24‐48 h | Inpatient | Methylprednisolone | IV | 100 | Temporarily halted |
| EUCTR2020‐001457‐43‐FR |
APHP | RCT | Admitted to ICU | Inpatient | Dexamethasone | IV | 550 | Ongoing |
| EUCTR2020‐001622‐64‐ES |
Dra Ana Pueyo Bastida | RCT | Clinical diagnosis of pulmonary involvement (respiratory symptoms +/‐ pathological auscultation +/‐ O2 desaturation) + chest X‐ray with mild‐moderate or normal alterations | Outpatient | Prednisone | Oral | 200 | Ongoing |
| EUCTR2020‐001707‐16‐ES |
Iis Biodonostia | RCT | Bilateral pneumonia caused by SARS‐CoV‐2 without response to the treatment: defined as persistence of fever (above 37.5 ºC without other focus) and respiratory worsening (more dyspnoea, more cough, oxygen therapy at increasing doses, worsening of the degree of respiratory distress according to the PaO2 / FiO2 ratio in categories 'mild, moderate or serious') or absence of improvement with respect to the previous state | Inpatient | Methylprednisolone | IV | 60 | Ongoing |
| EUCTR2020‐001921‐30 | Azienda Ospedaliero‐Universitaria Policlinico di Modena | RCT | Positive pressure ventilation (either non‐invasive or invasive) from > 24 h, IMV from < 96 h, PaO2/FiO2 ratio < 150 | Inpatient | Methylprednisolone | IV | 200 | Ongoing |
| EUCTR2020‐002186‐34‐ES | Fundació Hospital Universitari Vall d'Hebron ‐ Institut de Recerca (VHIR) | RCT | Air oxygen saturation > 90 and < 94%; PaO2/FiO2 > 200 and ≤ 300 mmHg; Sa:FiO2 (O2 saturation measured with pulse oximeter / inspired O2 fraction) ≤ 350 | Inpatient | Methylprednisolone | IV | 100 | Ongoing |
| EUCTR2020‐003363‐25‐DK |
Department of Intensive Care, Rigshospitalet | RCT | Severe, IMV/NIV | Inpatient | Dexamethasone (high dose and low dose) | IV | 1000 | Ongoing |
| EUCTR2020‐004323‐16 |
Azienda Ospedaliera Arcispedale Santa Maria Nuova/IRCCS di Reggio Emilia | RCT | Need for supplemental oxygen in any delivery mode with the exception of IMV | Inpatient | Methylprednisolone | IV | 260 | Ongoing |
| NCT04329650 | Judit Pich Martínez, Fundacion Clinic per a la Recerca Biomédica | RCT | Non‐critical patient with pneumonia in radiological progression and/ or patient with progressive respiratory failure in the last 24‐48 h |
Inpatient | Methylprednisolone | IV | 200 | Recruiting |
| NCT04344730 | Assistance Publique ‐ Hôpitaux de Paris | RCT | Admitted to ICU | Inpatient | Dexamethasone | IV | Actual enrolment 550 | Not recruiting |
| NCT04345445 | University of Malaya | RCT | Excluded: receipt of mechanical ventilation | Inpatient | Methylprednisolone | IV | 310 | Not yet recruiting |
| NCT04347980 | Centre Chirurgical Marie Lannelongue | RCT | Admitted to ICU | Inpatient | Dexamethasone | IV | 122 | Recruiting |
| NCT04377503 | Hospital Sao Domingos | RCT | COVID diagnosis confirmed by real time PCR, PaO2 / FIO2 < 200, laboratory: high sensitivity CRP > 5 mg/L; LDH > 245 U/L; ferritin > 300; D‐dimer > 1500; interleukin‐6> 7.0 pg/mL | Inpatient | Methylprednisolone | Oral | 40 | Not yet recruiting |
| NCT04395105 | Centro de Educación Medica e Investigaciones Clínicas Norberto Quirno | RCT | ARDS, mechanical ventilated | Inpatient | Dexamethasone | IV | 284 | Recruiting |
| NCT04438980 | Fundacion Miguel Servet | RCT | Hospitalised; excluded: SpO2 < 90% (in air ambient) or PaO2 < 60 mmHg (in ambient air) or PaO2/FiO2 < 300 mmHg |
Inpatient | Methylprednisolone | IV | 72 | Recruiting |
| NCT04451174 | University of Chile | RCT | Excluded: requirements of mechanical ventilation (IMV/NIV) Included: oxygen requirements until 35 % by venturi mask or 5 L/min by nasal cannula |
Inpatient | Prednisone | IV | 184 | Recruiting |
| NCT04452565 | NeuroActiva, Inc. | RCT | Excluded: IMV | Inpatient | Dexamethasone | Oral | 525 | Recruiting |
| NCT04485429 | D'Or Institute for Research and Education | RCT | Excluded: imminence of orotracheal intubation Included: O2 saturation in ambient air less ≤ 93% |
Inpatient | Methylprednisolone | IV | 268 | Recruiting |
| NCT04499313 | Chattogram General Hospital | RCT | Moderate to severe COVID‐19 infection | Inpatient | Dexamethasone, methylprednisolone | IV | 60 | Recruiting |
| NCT04509973 | Scandinavian Critical Care Trials Group | RCT | IMV OR NIV or continuous use of CPAP for hypoxia OR oxygen supplementation with an oxygen flow of at least 10 L/min independent of delivery system | Inpatient | Dexamethasone | IV | 1000 | Recruiting |
| NCT04513184 | Edda Sciutto Conde | RCT | Hospitalised patients with moderate to severe respiratory complications that have not received mechanical ventilation | Inpatient | Dexamethasone | IV vs nasal | 60 | Recruiting |
| NCT04528329 | ClinAmygate | RCT | Mild to moderate severity | Unclear | Dexamethasone | Unclear, most likely systemic | 300 | Recruiting |
| NCT04528888 | Massimo Girardis, University of Modena and Reggio Emilia | RCT | Included: positive pressure ventilation (IMV/NIV) for > 24 h, IMV from < 96 h, PaO2/FiO2 ratio < 150 | Inpatient | Methylprednisolone | IV | 210 | Recruiting |
| NCT04530409 | ClinAmygate | RCT | Mild and moderate severity | Unclear | Dexamethasone | Unclear, most likely systemic | 450 | Recruiting |
| NCT04545242 |
Dr. Negrin University Hospital | RCT | Intubated and mechanically ventilated | Inpatient | Dexamethasone | IV | 980 | Not yet recruiting |
| NCT04636671 | University of Trieste | RCT | Excluded: on IMV Included: PaO2 ≤ 60 mmHg or SpO2 ≤ 90% or on HFNC, CPAP or NPPV at randomisation |
Inpatient | Dexamethasone, methylprednisolone | IV | 680 | Recruiting |
| NCT04663555 | Brno University Hospital | RCT | Intubation/mechanical ventilation or ongoing HFNC oxygen therapy; admission to ICU | Inpatient | Dexamethasone | IV | 300 | Recruiting |
| NCT04673162 | Azienda Unità Sanitaria Locale Reggio Emilia | RCT | Need for supplemental oxygen in any delivery mode with the exception of IMV | Inpatient | Methylprednisolone | IV | 260 | Not yet recruiting |
| NCT04707534 | University of Oklahoma | RCT | Positive pressure ventilation (non‐invasive or invasive) or HFNC or need supplemental oxygen with oxygen mask or nasal cannula | Inpatient | Dexamethasone | Unclear, most likely systemic | 300 | Recruiting |
| NCT04726098 | Manuel Taboada Muñiz, Hospital Clinico Universitario de Santiago | RCT | Patients requiring supplemental oxygen | Inpatient | Dexamethasone | Unclear, most likely systemic | 198 | Recruiting |
| NCT04765371 | Centre Hospitalier René Dubos | RCT | Patient with SpaO2 ≤ 94 % in room air (90% for patient with respiratory failure) and requiring an oxygen therapy | Inpatient | Dexamethasone, prednisolone | Unclear, most likely systemic | 220 | Recruiting |
| NCT04780581 | Fundación Instituto de Estudios de Ciencias de la Salud de Castilla y León | RCT | Requires supplementary oxygen due to basal saturation ≤ 93% (with ambient O2, 21%) , excluded if IMV, NIV, HFNC | Inpatient | Dexamethasone, methylprednisolone | Unclear, most likely systemic | 290 | Recruiting |
| NCT04795583 | University of Alberta | RCT | Ambulatory, confirmed SARS‐CoV‐2. Clinical symptoms compatible with COVID‐19 for ≤ 14 days before randomisation. Oxygen saturation ≥ 95% | Outpatient | Prednisone | Oral | 1526 | Not yet recruiting |
| NCT04834375 | Northwell Health | RCT | Hypoxaemia defined by an oxygen saturation < 94% or the need for supplemental oxygen | Inpatient | Dexamethasone | IV | 142 | Recruiting |
| NCT04836780 | Hospital Universitario Infanta Leonor | RCT | Peripheral capillary oxygen saturation (SpO2) ≥ 94% and < 22 breaths per minute (bpm) breathing room air. High risk of developing ARDS | Inpatient | Dexamethasone | IV |
126 | Not yet recruiting |
| ARDS: acute respiratory distress syndrome; CPAP: continuous positive airway pressure; CRP: c‐reactive protein; FiO2: fraction of inspired oxygen; HFNC: high‐flow nasal cannula; ICU: intensive care unit; IMV: invasive mechanical ventilation; IV: intravenous; LDH: lactic dehydrogenase; NIV: non‐invasive ventilation; NPPV: non‐invasive positive pressure ventilation; PaO2: partial pressure oxygen; PCR: polymerase chain reaction RCT: randomised; controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; SpO2: blood oxygen saturation | ||||||||
Studies awaiting classification
We identified 16 RCTs with systemic application of steroids for acute COVID‐19 (details listed in Table 7). Of the 16 studies comprising 4036 expected participants, nine were classified as ‘completed’, three as ‘prematurely ended’, one as ‘terminated (lack of enrolment)’, one as ‘terminated (too few patients)’ and one terminated early (external evidence indicating benefit from corticosteroids in severe COVID‐19), according to the study registrations. For one study, the preprint is available, but the methodology is unclear, so we are awaiting the publication of the full text. Three studies planned to compare dexamethasone, three methylprednisolone, two prednisolone, and one hydrocortisone or prednisone, to standard care or placebo. One study planned to compare different dexamethasone dosing regimens. Two studies planned to compare dexamethasone to methylprednisolone and one study dexamethasone to tocilizumab. Another study planned to compare dexamethasone plus hydroxychloroquine to hydroxychloroquine only. One study planned to compare methylprednisolone without specification of the control.
5. Characteristics of studies awaiting classification.
| Study | Sponsor/developer | Design | Population/disease severity | Setting | Drug | Route of administration | Number of participants | Status |
| EUCTR2020‐001333‐13‐FR | Groupe Hospitalier Paris Saint‐Joseph | RCT | Included: patient diagnosed COVID positive by RT‐PCR and/or scanner (patients admitted with already mechanical ventilation and sedation, or with acute respiratory failure evolving very quickly) | Inpatient | Dexamethasone | IV | 122 | Prematurely ended |
| EUCTR2020‐001307‐16‐ES |
Fundación para la Investigación Biomédica Hospital Ramón y Cajal | RCT | ARDS | Inpatient | Methylprednisolone | IV | 104 | Prematurely ended |
| EUCTR2020‐001553‐48‐FR | Hospices Civils de Lyon | RCT | Peripheral saturation by pulse oximeter SpO2 ≤ 94% in ambient air measured twice at 5‐15 min intervals, or PaO2 / FiO2 <300 mmHg | Inpatient | Prednisone | Oral | 304 | Prematurely ended |
| IRCT20081027001411N3 | Teheran University of Medical Sciences | RCT | Blood oxygen saturation < 93%; with ARDS | Inpatient | Prednisolone | Not stated | 60 | Completed |
| IRCT20100228003449N31 | Teheran University of Medical Sciences | RCT | With diagnosis of COVID‐19 according to laboratory, clinical or radiological findings; with indication for hospitalisation | Inpatient | Dexamethasone | IV | 119 | Completed |
| IRCT20120215009014N354 | Hamedan University of Medical Sciences | RCT | Hospitalised in ICU, bilateral pulmonary infiltration in chest X‐ray or CT‐scan; respiratory distress with > 24 breaths per minute | Inpatient | Hydrocortisone, methylprednisolone, dexamethasone | IV | 81 | Completed |
| IRCT20160118026097N4 | Ghoum University of Medical Sciences | RCT | Hypoxia requires supplemental oxygen to maintain oxygen saturation > 90% | Inpatient | Dexamethasone | Not stated | 64 | Completed |
| IRCT20200611047727N3 | Shahid Beheshti University of Medical Sciences | RCT | Oxygen saturation level < 93 | Inpatient | Methylprednisolone | IV | 60 | Completed |
| IRCT20201015049030N1 | Teheran University of Medical Sciences | RCT | Blood oxygen saturation between 90%‐95% | Outpatient | Dexamethasone | Not stated | 200 | Completed |
|
ISRCTN33037282 |
University of Trieste | RCT | PaO2 ≤ 60 mmHg or SpO2 ≤ 90% or on HFNC, CPAP or NPPV at randomisation Excluded: on IMV |
Inpatient | Methylprednisolone, dexamethasone | IV | 680 | Completed |
| NCT03852537 | Mayo Clinic | RCT | Acute respiratory failure SpO2/FiO2 < 315 (SpO2 < 90% on room air or < 97% on 2L NC) | Inpatient | Methylprednisolone | Not stated | 44 | Completed |
| NCT04244591 | Peking Union Medical College Hospital | RCT | PaO2/FiO2 < 200 mmHg; positive pressure ventilation (non‐invasive or invasive) or HFNC > 45 L/min for < 48 h; requiring ICU admission | Inpatient | Methylprednisolone | Not stated | 80 | Completed |
| NCT04325061 | Dr. Negrin University Hospital | RCT | Intubated and mechanically ventilated | Inpatient | Dexamethasone | IV | 19 | Terminated (lack of enrolment) |
| NCT04746430 | General Practitioners Research Institute | RCT | Exercise‐induced desaturation, defined as SpO2 < 92% (< 90% for COPD patients) and/or an absolute drop of ≥ 4% in SpO2 after a 1‐min sit‐to‐stand test or SpO2 < 92% (< 90% for COPD patients) at rest with GP's and patient's shared decision to keep patient at home despite this in itself being an indication for referral to hospital | Outpatient | Dexamethasone | Unclear, most likely systemic | 2000 | Terminated (too few patients) |
| Munch 2021 | Scandinavian Critical Care Trials Group | RCT | Severe (at least oxygen) | Inpatient | Hydrocortisone | IV | 30 | Terminated early (external evidence indicating benefit from corticosteroids in severe COVID‐19) |
| Rashad 2021 | South Valley University | RCT | Respiratory rate > 30 cycle/min, bilateral CT infiltration > 30%, PaO2/FiO2 ratio < 150 or saturation < 90 on > 6L/min | Inpatient | Dexamethasone | Unclear | 69 | Preprint published; methodology unclear; waiting for the full text |
| ARDS: acute respiratory distress syndrome; COPD: chronic obstructive pulmonary disease; CT: computed tomography; HFNC: high‐flow nasal cannula;ICU: intensive care unit; RCT: randomised controlled trial; RT‐PCR: reverse transcription polymerase chain reaction. | ||||||||
Excluded studies
We excluded 23 studies that did not meet our inclusion criteria.
Nine studies investigated corticosteroids plus other active substances versus standard care (EUCTR2020‐001445‐39‐ES; IRCT20120225009124N4; IRCT20190312043030N2; NCT04341038; NCT04411667; NCT04468646; NCT04561180; NCT04640168; NCT04826822);
Six studies examined inhaled corticosteroids (EUCTR2020‐001616‐18‐ES; EUCTR2020‐001889‐10; ISRCTN86534580; NCT04355637; NCT04381364; NCT04416399);
Four studies investigated topical corticosteroids (IRCT20200522047542N1; NCT04361474; NCT04484493; NCT04569825);
Three studies considered corticosteroids for long‐COVID treatment (NCT04551781; NCT04534478; NCT04657484);
One study stopped early before enrolling its first participant (NCT04359511).
Risk of bias in included studies
We assessed the risk of bias of results from 10 RCTs that contributed to our analyses (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Horby 2021; Jamaati 2021; Jeronimo 2020; Ranjbar 2021; Tang 2021; Tomazini 2020), using the RoB 2 tool (version 22 August 2019) recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021b). We made no assessment of bias for Farahani 2021 as it did not report any outcomes relevant to this review.
The completed RoB 2 tool with responses to all assessed signalling questions is available online at: https://zenodo.org/record/5155770.
Overall judgements for studies that included hospitalised individuals with a confirmed or suspected diagnosis of symptomatic COVID‐19
All‐cause mortality
We assessed this outcome on a study level at days 21, 28 and 60. Among the studies that reported this outcome, we considered the risk of bias to be of some concern for nine studies (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Horby 2021; Jamaati 2021; Jeronimo 2020; Ranjbar 2021; Tomazini 2020). In Tomazini 2020 (9.4 % of the participants in the control group received corticosteroids), Horby 2021 (8% of the participants in the control group received corticosteroids) and Edalatifard 2020 (17% of the participants in the control group received corticosteroids) there were deviations from the intended intervention. In Jamaati 2021, no study protocol or analysis plan was available and baseline differences between intervention groups raised some concerns for the risk of bias. Dequin 2020 reported mortality as a post‐hoc outcome, raising some concerns for risk of bias. Corral‐Gudino 2021 had different definitions of mortality in the trials register and the actual study. In Jeronimo 2020 participants were excluded from the study and from the analysis. We assessed Angus 2020 to be of some concerns because of deviations from the intended intervention (15% of the participants in the no‐hydrocortisone group received hydrocortisone) and differences in standard care given at the various hospitals.
Other outcomes
After planning the analysis and sending data requests to the corresponding authors we became increasingly aware of possible confounding through a competing risk of death in the following exploratory outcomes. The way we addressed the issue is explained in Quality of the evidence but we have also elaborated below wherever applicable.
Clinical improvement: liberation from invasive mechanical ventilation
Apart from Tomazini 2020, no study reported this outcome, so we requested the missing data from the study authors. For both Tomazini 2020 and the studies for which we received data (Corral‐Gudino 2021; Edalatifard 2020; Jeronimo 2020), we considered the risk of bias to be high because there could be relevant confounding through death as a competing risk in this analysis if no adjustment is done. We discuss this issue in Quality of the evidence.
Clinical improvement: ventilator‐free days
One study reported ventilator‐free days (Tomazini 2020). We rated the risk of bias for Tomazini 2020 to be of some concern because some participants in the control group received corticosteroids and these deviations from the intended intervention were not balanced between the two study arms. Conceptual limitations peculiar to this endpoint are discussed in Quality of the evidence.
Clinical deterioration: new need for invasive mechanical ventilation
Apart from Jamaati 2021 and Tomazini 2020, no other studies reported this outcome, so we requested missing data from the study authors. Among the studies for which we received data (Corral‐Gudino 2021; Edalatifard 2020; Jeronimo 2020) and for Jamaati 2021 and Tomazini 2020 as well, we considered the risk of bias to be high because there could be relevant confounding through death as a competing risk in this analysis if no adjustment is done.
Clinical deterioration: need for dialysis
Two studies reported this outcome (Horby 2021; Jeronimo 2020). We considered the risk of bias to be high because there could be relevant confounding through death as a competing risk in this analysis if no adjustment is performed.
Quality of life
We could not assess risk of bias for this outcome, because none of the included studies reported quality of life or neurological long‐term outcome.
Viral clearance
Jeronimo 2020 reported this outcome, which we rated high risk of bias because there could be relevant confounding through death as a competing risk in this analysis if no adjustment is done.
Serious adverse events
Two studies reported this outcome for 678 participants (Angus 2020; Tomazini 2020). Overall, we considered the risk of bias, among those studies having reported adverse events, to be high, mainly because there could be relevant confounding through death as a competing risk in this analysis if no adjustment is done.
Adverse events
Five studies reported this outcome for 660 participants (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; Tomazini 2020). Overall, we considered the risk of bias to be high for all five studies. The definition of adverse events was heterogeneous as described in Table 5 and there could be relevant confounding through death as a competing risk in this analysis if no adjustment is performed.
Hospital‐acquired infections
Five studies reported this outcome in 660 participants (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; Tomazini 2020). Overall, we considered the risk of bias to be high for all five studies mainly because there could be relevant confounding through death as a competing risk in this analysis if no adjustment is done.
Effects of interventions
Summary of findings 1. Summary of Findings Table ‐ Systemic corticosteroids plus standard care compared to standard care for adults with a suspected or confirmed diagnosis of COVID‐19.
| Systemic corticosteroids plus standard care compared to standard care for adults with a suspected or confirmed diagnosis of COVID‐19 | ||||||
| Patient or population: adults with a suspected or confirmed diagnosis of COVID‐19 Setting: inpatient, ICU Intervention: systemic corticosteroids plus standard care Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with systemic corticosteroids plus standard care | |||||
| All‐cause mortality follow up: range 21 days to 60 days | 275 per 1000 | 245 per 1000 (220 to 275) | RR 0.89 (0.80 to 1.00) | 7930 (9 RCTs) | ⊕⊕⊕⊝ MODERATE a | Systemic corticosteroids probably reduce all‐cause mortality slightly. |
| Clinical improvement: ventilator‐free days follow up: 28 days | The mean clinical improvement: ventilator‐free days was 4 days | MD 2.6 days more (0.67 more to 4.53 more) | ‐ | 299 (1 RCT) | ⊕⊕⊝⊝ LOW b | Systemic corticosteroids may increase ventilator‐free days. |
| Clinical worsening: new need for IMV follow up: 28 days | We did not perform meta‐analyses because of high risk of bias arising from deaths that occurred before ventilation. We present descriptive data with effects below 1 in favour of corticosteroids: Corral‐Gudino 2021: RR 0.98 (95% CI 0.52, 1.85); Edalatifard 2020: RR 0.14 (95% CI 0.03, 0.56); Jamaati 2021: RR 1.18 (95% CI 0.66, 2.11); Jeronimo 2020: RR 0.99 (95% CI 0.56, 1.76). | 427 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW c | |||
| Quality of life, including fatigue and neurological status ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Serious adverse events (follow up: during treatment) | We did not perform meta‐analyses because of high risk of bias, heterogeneous definitions, and underreporting. Therefore, we only present descriptive statistics with effects below 1 in favour of corticosteroids: Angus 2020 shock‐dependent hydrocortisone: RR 4.11 (95% CI 0.23, 72.98); Angus 2020 fixed‐dose hydrocortisone: RR 1.43 (95% CI 0.16, 12.49); Tomazini 2020: RR 0.54 (95% CI 0.19, 1.59). | 678 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW d | |||
| Adverse events (follow up: during treatment) | We did not perform meta‐analyses because of high risk of bias, heterogeneous definitions, and underreporting. We only present descriptive statistics with effects below 1 in favour of corticosteroids: Corral‐Gudino 2021: RR 11.60 (95% CI 1.62, 83.03); Dequin 2020: RR 0.77 (95% CI 0.59, 1.00); Edalatifard 2020: RR 0.82 (95% CI 0.12, 5.48); Tang 2021: RR 0.63 (95% CI 0.22, 1.76); Tomazini 2020: RR 0.69 (95% CI 0.50, 0.96). | 660 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW e | |||
| Hospital‐acquired infections (follow up: during treatment) | We did not perform meta‐analyses because of high risk of bias, heterogeneous definitions, and underreporting. We present descriptive statistics only: Corral‐Gudino 2021: RR 4.14 (95% CI 0.51, 33.49); Dequin 2020: RR 0.77 (95% CI 0.59, 1.00); Edalatifard 2020: RR 2.49 (95% CI 0.11, 58.74); Tang 2021: RR 2.00 (95% CI 0.19, 21.24); Tomazini 2020: RR 0.75 (95% CI 0.52, 1.09). | 660 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW f | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424096272361428897. | ||||||
a. We downgraded for risk of bias for deviation from intended interventions (Angus 2020; Edalatifard 2020; Horby 2021; Jeronimo 2020; Tomazini 2020), for selective reporting (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Jamaati 2021), for missing information about the allocation concealment (Corral‐Gudino 2021; Edalatifard 2020), for baseline differences (Jamaati 2021) and indirectness (mortality observed at 21 to 60 days, in most studies at 28 days should be seen as a proxy for long‐term survival as long as we are unsure about its predictive value; 1 point altogether). b. We downgraded because of risk of bias through deviation from intended interventions (Tomazini 2020; 1 point), and imprecision (broad confidence interval, low number of evaluated participants, 1 point). c. We downgraded because of risk of bias for mainly for problems with deviations from intended interventions (Edalatifard 2020; Jeronimo 2020), missing information about the allocation concealment ( Corral‐Gudino 2021; Edalatifard 2020), baseline differences (Jamaati 2021), missing pre‐specification (Corral‐Gudino 2021; Edalatifard 2020; Jamaati 2021; Jeronimo 2020), missing adjustment for competing risk (Corral‐Gudino 2021; Edalatifard 2020; Jamaati 2021; Jeronimo 2020) (2 points), and imprecision (fewer than 500 events, 1 point). d. We downgraded for risk of bias for deviations from intended interventions (Angus 2020; Tomazini 2020), missing adjustment for competing risk (Angus 2020; Tomazini 2020; 2 points), publication bias because 2 out 10 studies including the largest, Horby 2021, did not report this major safety outcome (downgrade 1 point), and imprecision (fewer than 500 events, downgrade 1 point). e. We downgraded because of risk of bias mainly through deviation from intended intervention (Edalatifard 2020; Tomazini 2020), missing adjustment for competing risk (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; Tomazini 2020), missing information about the allocation concealment (Corral‐Gudino 2021; Edalatifard 2020) and selection of adverse events usually associated with steroids (Corral‐Gudino 2021; Edalatifard 2020; Tang 2021, 2 points), imprecision (fewer than 500 events, 1 point), and publication bias (only 5 out of 10 reported this established safety outcome, 1 point) f. We downgraded because of risk of bias mainly from deviation from intended interventions (Edalatifard 2020; Tomazini 2020), missing adjustment for competing risk (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; Tomazini 2020), missing information about the allocation concealment (Corral‐Gudino 2021; Edalatifard 2020), missing pre‐specification of its definition (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021, 2 points), imprecision (fewer than 500 events, 1 point), and publication bias (only 5 out of 10 studies reported this outcome which represents an adverse event, 1 point).
Summary of findings 2. Summary of Findings Table ‐ Methylprednisolone compared to dexamethasone for adults with a suspected or confirmed diagnosis of COVID‐19.
| Methylprednisolone compared to dexamethasone for adults with a suspected or confirmed diagnosis of COVID‐19 | ||||||
| Patient or population: adults with a suspected or confirmed diagnosis of COVID‐19 Setting: inpatient, ICU Intervention: methylprednisolone Comparison: dexamethasone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with dexamethasone | Risk with methylprednisolone | |||||
| All‐cause mortality follow up: 28 days | 357 per 1000 | 182 per 1000 (86 to 382) | RR 0.51 (0.24 to 1.07) | 86 (1 RCT) | ⊕⊝⊝⊝ VERY LOW a | We are uncertain whether methylprednisolone can decrease all‐cause mortality directly compared to dexamethasone. Additionally, 28‐day mortality should be understood as a proxy for sustained survival. |
| Clinical improvement: ventilator‐free days ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Clinical worsening: new need for IMV follow up: 28 days | There is high risk of bias for deaths that occurred before ventilation. Therefore we only present descriptive statistics: Ranjbar 2021: RR 0.48 (95% CI 0.23, 1.00). | 86 (1 RCT) | ⊕⊝⊝⊝ VERY LOW b | |||
| Quality of life, including fatigue and neurological status ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Serious adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| Hospital‐acquired infections ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No study reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_424391243004646489. | ||||||
a. We downgraded for risk of bias for missing pre‐specification/protocol/statistical analysis plan (1 point), and serious imprecision (fewer than 50 events, 2 points). b. We downgraded for risk of bias for missing data/adjustment for competing risk and missing protocol/statistical analysis plan (1 point), and serious imprecision (fewer than 50 events, 2 points)
Hospitalised individuals with a confirmed or suspected diagnosis of symptomatic COVID‐19
From 11 RCTs in this population (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Farahani 2021; Horby 2021; Jamaati 2021; Jeronimo 2020; Ranjbar 2021; Tang 2021; Tomazini 2020), Farahani 2021 did not report any of the prioritised outcomes of interest.
Systemic corticosteroids plus standard care versus standard care (plus/minus placebo)
The evidence profile is presented in Table 1.
All‐cause mortality at day 14 or any longer observation period
Data on all‐cause mortality were available from nine studies with a total of 7930 participants (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Horby 2021; Jamaati 2021; Jeronimo 2020; Tang 2021; Tomazini 2020). Edalatifard 2020, which involved 60 participants, did not report the follow‐up time of mortality, but we found out through our author enquiry that it was 60 days. Thus, observation periods ranged from 21 to 60 days, with most studies reporting 28‐day mortality. Overall, 760 of 2955 participants in the intervention group died compared with 1367 of 4975 participants in the control group. The RR of death was 0.89 (95% CI 0.80 to 1.00; I² = 10%; random‐effects model; Analysis 1.1). We downgraded the certainty of evidence for this outcome from high to moderate due to the risk of bias.
1.1. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 1: All‐cause mortality
Subgroup analyses
Respiratory support at randomisation: we are unable to rule out differences in the effect of corticosteroids on mortality between different levels of baseline respiratory support (P = 0.08; Analysis 2.1). In contrast with the beneficial effect on mortality in all other subgroups needing respiratory support, a higher risk of death with corticosteroids was seen among symptomatic COVID‐19 participants who did not need any respiratory support (RR 1.27, 95% CI 1.0 to 1.61). The analysis of participants without respiratory support was from a single study (Horby 2021).
Dexamethasone versus methylprednisolone versus hydrocortisone indirectly compared for their effect relative to placebo/standard care: the test for subgroup differences indicated no difference (P = 0.48) between subgroups stratified by type of systemic corticosteroid (Analysis 3.1). However, the effect estimates for each agent's subgroup were in favour of corticosteroid use.
2.1. Analysis.

Comparison 2: Subgroup analysis: respiratory support for the comparison of corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 1: All‐cause mortality
3.1. Analysis.

Comparison 3: Subgroup analysis: dexamethasone versus methylprednisolone versus hydrocortisone for the comparison of corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 1: All‐cause mortality
The power to detect differences in both subgroup analyses was limited.
Sensitivity analyses
We could not perform any of our planned sensitivity analyses for the comparison of systemic corticosteroids plus standard care versus standard care (plus/minus placebo) and the outcome of all‐cause mortality.
Clinical improvement: liberation from invasive mechanical ventilation
Data on liberation from invasive mechanical ventilation can be taken from Analysis 1.2. We decided not to carry out a meta‐analysis because we are highly uncertain about the size and direction of the unadjusted effects.
1.2. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 2: Clinical improvement: Liberation from IMV
Clinical improvement: ventilator‐free days
One study with 299 participants reported ventilator‐free days (Tomazini 2020). The mean difference was 2.6 days (95% CI 0.67 to 4.53; Analysis 1.3). We downgraded the certainty of the evidence from high to low. The limitations of the endpoint are discussed in Quality of the evidence.
1.3. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 3: Clinical improvement: Ventilator‐free days
Clinical deterioration: new need for invasive mechanical ventilation
Data on the new need for invasive mechanical ventilation can be taken from Analysis 1.4. We decided not to carry out meta‐analysis because we are highly uncertain about the size and direction of the unadjusted effects.
1.4. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 4: Clinical worsening: New need for IMV
Clinical deterioration: need for dialysis
Data on the need for dialysis can be taken from Analysis 1.8. We decided not to carry out meta‐analysis because we are highly uncertain about the size and direction of the unadjusted effects.
1.8. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 8: Need for dialysis
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at longest follow‐up available
Data on quality of life were not available in any study.
Viral clearance, assessed with RT‐PCR test for SARS‐CoV‐2 at baseline, up to 3 and 7 days
Data on viral clearance can be taken from Analysis 1.9. We decided not to carry out meta‐analysis because we are highly uncertain about the size and direction of the unadjusted effects.
1.9. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 9: Viral clearance
Serious adverse events
Two studies reported serious adverse events for 678 participants. Data on serious adverse events can be taken from Analysis 1.5. We decided not to carry out a meta‐analysis because we are highly uncertain about the size and direction of the unadjusted effects. However, a cautious analysis of the descriptive statistics suggested little to no difference between the groups.
1.5. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 5: Serious adverse events
Adverse events (any grade)
Five studies reported adverse events for 660 participants with four studies focussing on a limited set of adverse events only (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021). Data on adverse events can be taken from Analysis 1.6. We decided not to carry out meta‐analysis because we are highly uncertain about size and the direction of the unadjusted effects. However, a cautious analysis of the descriptive statistics suggests little to no difference between the groups.
1.6. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 6: Adverse events
Hospital‐acquired infections
Five studies reported hospital‐acquired infections for 660 participants. Data on hospital‐acquired infections can be taken from Analysis 1.7. We decided not to carry out meta‐analysis because we are highly uncertain about size and the direction of the unadjusted effects. However, a cautious analysis of the descriptive statistics suggests little to no difference between the groups.
1.7. Analysis.

Comparison 1: Systemic corticosteroids plus standard care versus standard care (plus/minus placebo), Outcome 7: Hospital‐acquired infections
Different types of systemic corticosteroids: methylprednisolone versus dexamethasone
The evidence profile is presented in Table 2.
All‐cause mortality at day 14 or any longer observation period
Data on all‐cause mortality at 28 days was available for one trial with 86 participants (Ranjbar 2021). Eight of 44 participants in the intervention group died compared with 15 of 42 participants in the control group. The RR of death was 0.51 (95% CI 0.24 to 1.07; Analysis 4.1). We downgraded the certainty of the evidence for this outcome to very low due to risk of bias and very serious imprecision.
4.1. Analysis.

Comparison 4: Methylprednisolone versus dexamethasone, Outcome 1: All‐cause mortality
Subgroup analyses
We could not perform any of our planned subgroup analyses for the comparison of methylprednisolone versus dexamethasone and the outcome of all‐cause mortality.
Sensitivity analyses
We could not perform any of our planned sensitivity analyses for the comparison of methylprednisolone versus dexamethasone and the outcome of all‐cause mortality.
Clinical improvement: liberation from invasive mechanical ventilation
We did not identify any study reporting this outcome and also received no response to our author request.
Clinical improvement: ventilator‐free days
We did not identify any study reporting this outcome.
Clinical deterioration: new need for invasive mechanical ventilation
Data on new need for invasive mechanical ventilation can be taken from Analysis 4.2. We decided not to carry out a meta‐analysis because we are highly uncertain about the size and direction of the unadjusted effects.
4.2. Analysis.

Comparison 4: Methylprednisolone versus dexamethasone, Outcome 2: Clinical worsening: New need for IMV
Clinical deterioration: need for dialysis
We did not identify any study reporting this outcome.
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at longest follow‐up available
We did not identify any study reporting this outcome.
Viral clearance, assessed with RT‐PCR test for SARS‐CoV‐2 at baseline, up to 3, 7, and 15 days
We did not identify any study reporting this outcome.
Serious adverse events
We did not identify any study reporting this outcome.
Adverse events (any grade)
We did not identify any study reporting this outcome.
Hospital‐acquired infections
We did not identify any study reporting this outcome.
Dose comparisons
No studies provided data for this comparison.
Timing comparisons (early versus late)
No studies provided data for this comparison.
Systemic corticosteroids versus other active substances
No studies provided data for this comparison.
Outpatients with asymptomatic or mild disease
We did not identify any study that investigated the effects of systemic corticosteroids in people with asymptomatic infection or mild disease (i.e. non‐hospitalised individuals).
Discussion
Summary of main results
This review aimed to assess the efficacy and safety of systemic corticosteroids for the treatment of COVID‐19. We identified 11 RCTs (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Farahani 2021; Horby 2021; Jamaati 2021; Jeronimo 2020; Ranjbar 2021; Tang 2021; Tomazini 2020). The studies evaluated 8075 participants, of whom 3072 received corticosteroids. We identified 42 ongoing studies evaluating systemic corticosteroids and 16 completed studies lacking published results that we categorised as 'studies awaiting classification'. We checked the proportion of PCR‐positive tests in each study, as some studies included confirmed or suspected COVID‐19 infections, or both. The studies with the lowest PCR‐positive rate of approximately 80% were Angus 2020 and Jeronimo 2020.
Effects of interventions
Hospitalised individuals with a confirmed or suspected diagnosis of symptomatic COVID‐19
A total of 10 RCTs (8075 participants) evaluated systemic corticosteroids amongst hospitalised individuals: nine RCTs (7989 participants) compared corticosteroids plus standard care to standard care (plus/minus placebo), and one RCT (86 participants) compared methylprednisolone to dexamethasone.
Systemic corticosteroids plus standard care versus standard care (plus/minus placebo)
Systemic corticosteroids probably slightly decrease all‐cause mortality in hospitalised people with COVID 19. Based on the observed rate of 275 deaths per 1000 in the control arms, treatment with corticosteroids led to 30 fewer deaths (moderate‐certainty evidence). To further explore differences in the effect of systemic corticosteroids depending on the severity of COVID‐19, we performed a subgroup analysis stratified by respiratory support at randomisation, since respiratory support can be seen as a strong indicator for disease severity. Although there was no statistically significant subgroup difference, evidence of a negative effect on mortality has to be noted in the participants without respiratory support.
In addition to the slight reduction in mortality, as a further beneficial effect, corticosteroids may increase the composite outcome of ventilator‐free days (MD 2.6 days more than in the control arm with 4 days), although certain limitations of the endpoint need to be considered (see Quality of the evidence). Further outcomes in which risk of bias assessment led to the decision not to perform meta‐analysis were the need for dialysis, need for invasive ventilation, liberation from invasive ventilation, and viral clearance. Nonetheless, a cautious analysis of descriptive statistics might render the interpretation that there is no evidence to suspect further relevant effects on these outcomes for people with COVID‐19 treated with systemic corticosteroids.
Since all studies besides Tomazini 2020, among them the largest study, Horby 2021, did not report safety data (namely serious adverse events, adverse events, and hospital‐acquired infections) in detail, we see a relevant underreporting of these important parameters to characterise the safety profile of systemic corticosteroids for the treatment of COVID‐19. Due mainly to a high risk of bias and heterogeneous reporting, we deemed quantitative synthesis inappropriate. Nonetheless, an analysis of the descriptive statistics of the included studies did not generate suspicion of an exceedingly large harmful effect of systemic corticosteroid treatment.
Different types of systemic corticosteroids: methylprednisolone versus dexamethasone
We are uncertain whether methylprednisolone decreases all‐cause mortality directly compared to dexamethasone (1 RCT, 86 participants, very low‐certainty evidence).
Different dosages or timing of systemic corticosteroids
None of the included studies provided data on different dosages or timing.
Outpatients with asymptomatic or mild disease
Currently, there are no studies published in populations with asymptomatic infections or mild disease.
Overall completeness and applicability of evidence
Diagnosis of COVID‐19 was confirmed by positive SARS‐CoV‐2 PCR testing in 93.8% of the participants. In terms of virological aspects all participants included in this review were evaluated during the first months of the pandemic. Our findings might not apply to a different pathogenicity of later variants of SARS‐CoV‐2. The majority of participants also received various COVID‐19 treatment options such as antibiotics with potential antiviral and anti‐inflammatory properties (i.e. azithromycin), hydroxychloroquine, convalescent plasma, or combinations of these drugs. None of the trials evaluated participants with asymptomatic infections or mild disease (non‐hospitalised participants).
The study participants were mainly from high‐income countries. Only 13% of participants came from middle‐income countries and none from low‐income countries. This finding is even more important as the proportion of severe COVID‐19 cases can be expected to further decrease in high‐income countries with successful vaccination programmes in place in many of them. Thus, the evidence presented in this review might only partially apply to people with COVID‐19 who are treated under different circumstances to those of most studies included in our review. In low‐ and middle‐income countries, there might be a more severe shortage of hospital beds in both ordinary wards and intensive care units, shortage of oxygen and other resource constraints on the delivery of respiratory support, and other aspects of care labelled as standard care in this review.
In terms of the types of systemic corticosteroids, our included RCTs evaluated dexamethasone, methylprednisolone, and hydrocortisone. With the exception of Edalatifard 2020, the dosages of corticosteroids in the RCTs included for the comparison of systemic corticosteroids versus standard care only were significantly lower, (i.e. ≤ 500 mg/day hydrocortisone equivalent) than the usual immunosuppressive dosage of more than 1250 mg/day hydrocortisone equivalent (Angus 2020; Corral‐Gudino 2021; Dequin 2020; Horby 2021; Jamaati 2021; Jeronimo 2020; Tang 2021; Tomazini 2020). The only study with high‐dose corticosteroids was Edalatifard 2020 (1250 mg/day hydrocortisone equivalent). We have not explored in a subgroup analysis a possible different effect of relatively low‐dose strategies (≤ 500 mg/day hydrocortisone equivalent) compared to those commonly referred to as short‐term, high‐dose, ‘pulse’ regimens (> 1250 mg/day) because we had not planned it and because there were too few data for the latter. As part of our living review approach, we plan to address these knowledge gaps in subsequent updates of this review.
Different scales of disease severity and progression were used across studies, and the terms ‘mild’, ‘moderate’, ‘severe’, and ‘critical’ have been inconsistently used in the different guidelines and consensus statements of national and international organisations (Marshall 2020; WHO Living Guidance 2021 ). Hence, we decided to use the need for respiratory support (no oxygen, low‐flow oxygen support, non‐invasive ventilation/high‐flow nasal cannula, and invasive ventilation) at randomisation and its changes as a surrogate for COVID‐19 disease severity. For hospitalised patients, the need for respiratory support is a strong predictor of mortality, essentially determines the pathway within the hospital (e.g. ICU admission), and, from the individual patient's perspective, has a strong impact on acute health‐related quality of life, functional independence, and autonomy. We deem respiratory support at randomisation both a good indicator for disease severity and a surrogate for the timing of intervention. Among the 7818 participants for whom we could extract respiratory support at randomisation, the distribution was as follows: for the comparison of corticosteroids in addition to standard care and standard care only, 1560 participants (20%) did not receive any additional oxygen, 4489 (57%) received any non‐invasive respiratory support (oxygen prongs or mask, high‐flow nasal cannula, or non‐invasive ventilation), and 1769 (23%) received invasive ventilation at randomisation. For the comparison of two different types of corticosteroids, we identified one study (Ranjbar 2021). In this study, the participants probably received non‐invasive respiratory support (low‐flow oxygen, high‐flow nasal cannula, or non‐invasive ventilation) at randomisation.
We also note the lack of safety data (serious adverse events, adverse events, and hospital‐acquired infections) reported by included studies: only two evaluated serious adverse events for 678 participants, five reported partly selected adverse events for 660 participants, and five studies provided information on hospital‐acquired infections for 660 participants.
With regard to additional evidence from future publications, there are 31 ongoing, recruiting or temporarily halted studies encompassing approximately 10,000 participants, and 16 studies described as completed, prematurely ended, or terminated encompassing approximately 4000 participants. Most of the studies intend to recruit people treated with different levels of respiratory support. With the publication of even parts of these data, possible publication bias would be countered and changes to the precision and effect estimates themselves are not unlikely, although we do not expect a relevant difference or even change of direction in the effect of mortality in the first comparison but rather more precise estimates for subgroup analyses and the subordinate outcomes.
Quality of the evidence
Systemic corticosteroids plus standard care versus standard care (plus/minus placebo)
We included data from nine RCTs into the analysis of efficacy and safety of systemic corticosteroids. The population of interest was hospitalised individuals with a confirmed or suspected diagnosis of symptomatic COVID‐19 when compared to treatment with standard care (plus/minus placebo).
We had moderate to very low certainty in the identified evidence.
All‐cause mortality: we downgraded for risk of bias for deviation from intended interventions (Angus 2020; Edalatifard 2020; Horby 2021; Jeronimo 2020; Tomazini 2020), for selective reporting (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Jamaati 2021), for missing information about the allocation concealment (Corral‐Gudino 2021; Edalatifard 2020), for baseline differences (Jamaati 2021) and indirectness (mortality observed at 21 to 60 days; in most studies 'at 28 days' should be seen as a proxy for long‐term survival as long as we are unsure about its predictive value; 1 point altogether).
Ventilator‐free days: we downgraded for risk of bias through deviation from intended interventions (Tomazini 2020; 1 point), and imprecision (broad confidence interval, low number of evaluated participants; 1 point).
New need for invasive ventilation: we downgraded because of risk of bias mainly for problems with deviations from intended interventions (Edalatifard 2020; Jeronimo 2020), missing information about the allocation concealment (Corral‐Gudino 2021; Edalatifard 2020), baseline differences (Jamaati 2021), missing pre‐specification (Corral‐Gudino 2021; Edalatifard 2020; Jamaati 2021; Jeronimo 2020), missing adjustment for competing risk (the term is discussed below in detail) (Corral‐Gudino 2021; Edalatifard 2020; Jamaati 2021; Jeronimo 2020; 2 points), and imprecision (fewer than 500 events; 1 point).
Serious adverse events: we downgraded for risk of bias for deviations from intended interventions (Angus 2020; Tomazini 2020), missing adjustment for competing risk (Angus 2020; Tomazini 2020; 2 points), publication bias because 2 out 10 studies including the largest, Horby 2021, did not report this major safety outcome (downgraded 1 point), and imprecision (fewer than 500 events; 1 point).
Adverse events: we downgraded because of risk of bias mainly through deviation from intended intervention (Edalatifard 2020; Tomazini 2020), missing adjustment for competing risk (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; Tomazini 2020), missing information about the allocation concealment (Corral‐Gudino 2021; Edalatifard 2020), and selection of adverse events usually associated with corticosteroids (Corral‐Gudino 2021; Edalatifard 2020; Tang 2021; 2 points), imprecision (fewer than 500 events; 1 point), and publication bias (only 5 out of 10 studies reported this established safety outcome; 1 point)
Hospital‐acquired infections: we downgraded because of risk of bias mainly from deviation from intended interventions (Edalatifard 2020; Tomazini 2020), missing adjustment for competing risk (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; Tomazini 2020), missing information about the allocation concealment (Corral‐Gudino 2021; Edalatifard 2020), missing pre‐specification of its definition (Corral‐Gudino 2021; Dequin 2020; Edalatifard 2020; Tang 2021; 2 points), imprecision (fewer than 500 events; 1 point), and publication bias (only 5 out of 10 studies reported this outcome, which represents an adverse event; 1 point).
Comparison of different types of corticosteroids
Methylprednisolone versus dexamethasone
We included the data from one RCT assessing the efficacy and safety of methylprednisolone for individuals with a confirmed or suspected diagnosis of COVID‐19 when compared to treatment with dexamethasone.
We had low to very low certainty in the identified evidence.
All‐cause mortality at 28 days: we downgraded to very low certainty due to risk of bias based on missing pre‐specification/protocol/statistical analysis plan (1 point), and serious imprecision (fewer than 50 events; 2 points).
New need for invasive ventilation: we downgraded to very low certainty due to risk of bias based on missing data/adjustment for competing risk and missing protocol/statistical analysis plan (1 point), and serious imprecision (fewer than 50 events; 2 points).
Critical appraisal of selected outcome parameters
Outcomes with death as competing risk
Death and our exploratory endpoints in the domain of clinical improvement and deterioration, as well as safety data, have to be seen as semi‐competing risks for each other: death precludes the occurrence of non‐terminal outcomes if participants die early. Consequently, the analysis of mortality using dichotomous, metric or time‐to‐event data is unaffected by this and is thus considered robust in general.
However, any non‐terminal event like the need for invasive ventilation, liberation from invasive ventilation, or even occurrence of adverse events can be severely skewed by different counts and time points of deaths in each arm. This can be illustrated with a theoretical example: if, because of a hypothetical pathophysiologic mechanism or even chance, participants in the intervention arm tend to live 10 days longer on average during an observation period of 28 days, their chance of experiencing an adverse event might be higher than in the control arm, even though death counts are equal or even lower in the intervention arm at the end of the observation period. This leads to the conclusion that a simple analysis of endpoints representing non‐terminal events regardless of the scale level might be misleading.
An inappropriate solution to this would be the exclusion of those with terminal events from the analysis because it creates new, non‐randomised samples. Likewise, in continuous outcomes, where assigning any values to those with terminal events implies the relative grading of different outcomes, representing an ethical or even economical weighting rather than a mathematically valid adjustment. In this instance, composite endpoints are often used as a compromise to both increase statistical power and address the issue of competing risks. Nonetheless, they have relevant methodological limitations mainly with regard to the possible asymmetry of the effect‐driving component in the treatment arms and the hierarchy of the components. We elaborate on that at the end of this section on the basis of our endpoint ventilator‐free days.
In order to obtain a statistically correct and practically helpful analysis of the described multi‐state model (e.g. death as a terminal state and adverse events or clinical deterioration or improvement as non‐terminal states), individual data for each study participant and each event posing competing risks as well as methodology beyond the means of the first publication of this living review would be required (Columbia Public Health 2021, Wu 2020, Brock 2011).
We addressed competing risks as an issue of bias in Domain 3 as described in the detailed ROB 2 guidance (Higgins 2019). This led to all outcomes except mortality and ventilator‐free days being assessed as having a high risk of bias. This is because measuring the respective non‐terminal outcomes on a dichotomous or even simple metric scale and without proper adjustment for competing risks can be seen as an inappropriate measurement due to missing data. Second, we decided not to perform any meta‐analysis or synthesis without meta‐analysis (SwiM) for those outcomes affected because we are uncertain as to not only the size but also the direction of the unadjusted effects. We only presented the raw numbers as extracted or sent in by the authors (Analysis 1.2; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 4.2). Finally, we would like to emphasise that, in doing so, it is our intention to make transparent our ongoing internal discussion on biostatistical aspects of the proposed Core Outcome Set for COVID‐19 (Tong 2020), which we initially adopted for our protocol. The aforementioned detailed individual time‐to‐event data have not yet been requested from the authors. Alternatively, data already adjusted by the study authors themselves could be presented.
Ventilator‐free days and other composite outcomes
Ventilator‐free days and a range of other composite endpoints come with a conceptual peculiarity: depending on the metric value that is assigned to deceased participants (e.g. zero days), they are more robust against competing risk of death than other common endpoints (e.g. simple duration of ventilation) but offer only a rather abstract measure of disease severity. Therefore, a meta‐analysis of ventilator‐free days is downgraded for imprecision due to different underlying definitions and even more difficult to interpret from the patient or economical perspective. Depending on the respective definition and distribution in the study arms, there can be an unintended mismatch in how the individual components (death, supposedly more important, and ventilation duration, supposedly less important) drive the effect of the composite outcome. Therefore, even if ventilator‐free days as a composite endpoint is more robust against competing risks, a meta‐analysis of the ventilator‐free days should thoroughly compare and evaluate the distribution of the individual components in the different study arms, consider hidden competing risks for ventilator‐free days, and might be paralleled by a competing risk regression analysis or similar procedures. Moreover, it should be plausible that both components are affected in the same direction. But, most importantly, authors should state why they chose ventilator‐free days, or any other composite outcome, to primarily measure the efficacy of a drug if in a disease death itself occurs at a sufficiently high rate to detect differences if they were relevant (Yehya 2019). Tackling these issues in detail was beyond the scope of the methodology of this first version of the review.
Potential biases in the review process
In addition to peer‐reviewed, full‐text articles, we also included preprints. We are aware of the potentially lower quality of preprint publications, and that results could change once the peer‐reviewed journal publications are available. In cases of missing data, we contacted study authors for additional data or relevant details if we needed more information. We are confident that we identified all relevant studies and will monitor ongoing studies as well as full publications of preprints closely after the publication of this review.
Agreements and disagreements with other studies or reviews
Here, we discuss four important systematic reviews with meta‐analyses including only RCTs.
First, the systematic review by Pasin 2021 had a design very similar to ours. Their analysis of mortality included 7692 participants from five RCTs with a pooled effect size and precision similar to ours. Our analysis included four further RCTs (Corral‐Gudino 2021; Edalatifard 2020; Jamaati 2021; Tang 2021), and final data from Jeronimo 2020, which had not been fully published at the time of their latest search date. Additionally, our subgroup analysis of mortality stratified by the need for respiratory support contains intermediary levels and more participants because of the aforementioned additional publications and requests to authors. As we used similar methods, the two reviews share limitations except for a smaller publication bias in our case. Nevertheless, their conclusion of a probable positive effect on mortality carried by a stronger effect among invasively ventilated participants, and even a negative effect among the moderately ill, is similar to ours.
Second, Sterne 2020 published a prospective systematic review searching three major study registries. They focussed on critically ill patients and their analysis of mortality included 1703 participants from seven RCTs with a pooled effect and precision similar to our subgroup result of invasively ventilated participants (random‐effects odds ratio (OR) 0.70 (95% CI 0.48 to 1.01)). They partly included data of trials not completed at that time, and three of the included trials have not yet published their data yet, so we could not include them at this stage. Owing to the review design and consequent homogeneity of the study populations and settings, analysis conducted by Sterne 2020 might be more robust than ours, but it did not encompass trials registered in other registries, and their last day of follow‐up on 6 July 2020 is almost one year ago now. Moreover, their chosen effect measure, that is, odds ratio, exaggerates effects when the number of participants with events is large — as in the population examined here — compared to the risk ratio we present (Ranganathan 2015). Nonetheless, the conclusion of a probable positive effect on 28‐day all‐cause mortality in participants with severe disease is similar to ours.
Third, Chaudhuri 2021 conducted a systematic review and focussed on invasively ventilated patients with ARDS, both with and without COVID‐19. Excluding the non‐COVID‐19‐related data as indirect evidence, their meta‐analysis of mortality data included 1741 participants, quasi‐resembling Sterne 2020, with the last search date of 6 September 2020, including partially unpublished data or preprints as well. They found little to no evidence of a slight beneficial effect on mortality in the above‐specified subgroups.
Fourth, the living systematic review with network meta‐analysis by Siemieniuk 2020 included published and unpublished data on 2975 participants who received corticosteroids (latest update on 6 April 2021), comparable to the 2955 participants in this review regarding the all‐cause mortality analysis. They found a probable slight beneficial effect (random‐effects model OR 0.83, 95% CI 0.69 to 0.98) with moderate certainty; this is in line with our findings but with the aforementioned limitation of the effect measure. Their result for ventilator‐free days also compares well to ours.
Of note, the above‐listed systematic reviews performed meta‐analyses to various extents for endpoints that we deemed to be at risk of bias too high for quantitative synthesis. Therefore, we decided not to repeat their findings but state that we consider none of these endpoints to be relevant in terms of effect size and quality of the evidence to remarkably change the view on corticosteroids in COVID‐19. We also decided not to further discuss systematic reviews that included observational data while comprehensive RCT‐only systematic reviews are available. Nonetheless, we would like to name Van Paassen 2020 as an example of how trialists worldwide contributed to the search for evidence in the initial phases of the pandemic.
Finally, the single study Horby 2021 must be discussed due to its huge impact. Not only was it the largest contributor to our review in terms of events and participants, it also had an immense influence on treatment guidelines and ongoing studies in 2020 (WHO Living Guideline 2021). The reported direction of the effect on mortality is congruent to our findings as well as the findings in the subgroup analysis stratified by respiratory support at randomisation. However, we observed smaller effects with similar precision after the inclusion of eight further RCTs. Without a doubt, platform trials like this one offer great chances for rapid and adaptive generation of evidence involving huge numbers of participants in the pandemic. Nevertheless, systematic appraisal of their specific sources of risk of bias is critical but not yet well‐established and beyond the scope of this first version of this living review (Park 2020).
Authors' conclusions
Implications for practice.
Based on the current evidence, we are moderately certain that systemic corticosteroids probably reduce mortality slightly amongst hospitalised, symptomatic COVID‐19 patients. Most of the participants in the studies were treated with invasive mechanical ventilation and non‐invasive ventilation/continuous positive airway pressure or high‐flow oxygen. In a subgroup analysis by baseline respiratory support, evidence of an increased risk of mortality with corticosteroids in symptomatic, hospitalised COVID‐19 patients without any need for additional oxygen, was limited by a lack of statistical significance. In a subgroup analysis of different types of systemic corticosteroids on mortality, we did not identify evidence for a subgroup difference.
There is low‐certainty evidence for a beneficial effect of corticosteroids in the observed reduction of ventilator‐free days; however, the current evidence remains uncertain due to methodological limitations.
There is very low certainty direct evidence for the comparison of methylprednisolone versus dexamethasone, results remain uncertain.
Due to the underreporting of relevant data, we have very low certainty about the safety of systemic corticosteroids as treatments for COVID‐19.
We did not identify any published study to evaluate different dosages or timing of corticosteroids in hospitalised participants. Currently, there is no evidence to characterise the benefits and harms of corticosteroids in patients with asymptomatic or mild disease (non‐hospitalised).
Implications for research.
There is an urgent need for long‐term data on survival and patient‐centred outcomes like quality of life, neurological function and independence in daily activities. To improve patient selection for treatment, good‐quality evidence is needed for specific subgroups of disease severity, for which we propose definition by level of respiratory support at randomisation. This also applies to the subgroups of different types and doses of corticosteroids. Outcomes apart from mortality should be measured and analysed appropriately, accounting for the competing risk of death. Furthermore, the existing datasets of the included studies should re‐analysed by the study authors to gain more information about the safety profile of corticosteroids in COVID‐19 patients with different disease severity, age, and co‐morbidities. The data are urgently needed by physicians and their patients to weigh up the benefits and harms of treating COVID‐19 with systemic corticosteroids in a more patient‐adapted, individualised way.
As we could not identify published RCTs on non‐hospitalised patients with mild or asymptomatic disease treated with systemic corticosteroids, strong efforts should be made to collect good‐quality data regarding these patients.
We identified 42 ongoing and 16 completed but unpublished RCTs in trials registries, which will probably increase the certainty of the evidence in the future. The studies mainly intend to recruit people with severe disease who require respiratory support. In accordance with the living approach of this review, we will continually update our search and include eligible trials.
Notes
Parts of the review's methods section and of the background were adopted from Cochrane Haematology templates (Kreuzberger 2021; Piechotta 2021).
Risk of bias
Risk of bias for analysis 1.1 All‐cause mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Angus 2020 | Low risk of bias | Participants were randomised to each locally available group using balanced assignment. Participants were randomly assigned via a computer software program to each locally available group using proportional assignment (eg, 1:1 if 2 groups available and 1:1:1 if 3 groups available) Protocol: The RAR will occur centrally as part of the computerised randomisation process. Sites will receive the allocation status and will not be informed of the randomization proportions. Each region will maintain its own computer‐based randomisation program that is accessed by sites in that region but the RAR proportions will be determined by a SAC and provided monthly to the administrator of each region's randomization program who will update the RAR proportions Baseline characteristics were similar across groups. |
Some concerns | 15% of the participants in the no hydrocortisone group received hydrocortisone | Low risk of bias | Data were available for this outcome. 238 participants were randomised and 238 were analysed. | Low risk of bias | Mortality is an observer‐reported outcome not involving judgement. | Low risk of bias | Protocol and SAP are available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention |
| Angus 2020 | Low risk of bias | Participants were randomised to each locally available group using balanced assignment. Participants were randomly assigned via a computer software program to each locally available group using proportional assignment (eg, 1:1 if 2 groups available and 1:1:1 if 3 groups available) Protocol: The RAR will occur centrally as part of the computerised randomisation process. Sites will receive the allocation status and will not be informed of the randomization proportions. Each region will maintain its own computer‐based randomisation program that is accessed by sites in that region but the RAR proportions will be determined by a SAC and provided monthly to the administrator of each region's randomization program who will update the RAR proportions Baseline characteristics were similar across groups. |
Some concerns | 15% of the participants in the no hydrocortisone group received hydrocortisone | Low risk of bias | Data were available for this outcome. 238 participants were randomised and 238 were analysed. | Low risk of bias | Mortality is an observer‐reported outcome not involving judgement. | Low risk of bias | Protocol and SAP are available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention |
| Corral‐Gudino 2021 | Some concerns | Missing information about the allocation concealment. | Low risk of bias | open‐label design (participants and clinicians were aware of the assigned treatment). ITT data presented. | Low risk of bias | No substantial data missing. | Low risk of bias | Both groups were measured at the same time and the measurements were similar between groups. | Some concerns | Outcome was registered in Eudra‐CT as in‐hospital mortality but reported as 28‐day‐mortality in the supplemental material. | Some concerns | Overall judged some concerns due to selection of reported results and missing information about the allocation concealment. |
| Dequin 2020 | Low risk of bias | Randomisation was centralised and performed electronically. Allocation sequences were generated in a 1:1 ratio by a computer‐generated random number using a blocking schema. There were no baseline differences between the groups. | Low risk of bias | Protocol: Patients, investigators and care providers will be blinded for the patient‐arm. ITT was used. | Low risk of bias | Data were available for this outcome. | Low risk of bias | The measurements were similar between groups. | Some concerns | The protocol and statistical analysis plan were available. 21‐day mortality was a post hoc outcome. | Some concerns | Overall judged some concerns due to selection of reported results. |
| Edalatifard 2020 | Some concerns | No information about the allocation concealment. | Some concerns | 6 patients in the control group received the intervention drug and were excluded from the analyses (17%). | Low risk of bias | The data were requested from the authors because the follow‐up time was not clearly visible from the publication. | Low risk of bias | The measurements were similar between groups. | Some concerns | Neither the protocol nor statistical analysis plan were available. | Some concerns | Overall judged some concerns because of the randomisation process, deviations from the intended intervention and selective reporting. |
| Horby 2021 | Low risk of bias | Randomisation was performed with the use of a web‐based system with concealment of the trial‐group assignment. No baseline differences between the groups. | Some concerns | 8% of the participants in the control group received dexamethasone. | Low risk of bias | 6425 participants were randomised and 6425 were analysed. | Low risk of bias | Both groups were measured at the same time. The measurements were similar between groups. | Low risk of bias | Protocol and statistical plan available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention in the control group. |
| Jamaati 2021 | Some concerns | The selected patients were allocated to either the dexamethasone group or the control group by block randomisation. Ten blocks were generated by the Online Randomiser website. Pulmonary disease was highly significantly more prevalent in the control group. Additionally, baseline level of respiratory support was not reported so that we can only assume the most likely circumstance, i.e. that no support beyond oxygen insufflation had been given until randomisation. |
Low risk of bias | No deviations mentioned. ITT data reported. | Low risk of bias | No missing data. | Low risk of bias | The measurements were similar between groups. | Some concerns | Trial registered after recruitment stop. No analysis plan published beyond registry entry. | Some concerns | Potential bias through probably problematic block randomisation with significant baseline imbalance for pulmonary disease, very delayed registration, and potentially not pre‐specified stop for futility raise some concerns. The issues were not discussed. Potential mild bias towards experimental. |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment Control group: 5 excluded before starting treatment, 3 excluded after starting treatment |
Low risk of bias | 416 participants randomised and 416 participants analysed. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | Protocol and statistical plan available. Data analysed and presented according to a pre‐specified plan. | Some concerns | Overall judged some concerns due to protocol deviations. |
| Tang 2021 | Low risk of bias | Randomisation was stratified by the statistician of the leading site, who produced computer‐generated block randomisation lists with a block size of 4 patients. | Low risk of bias | Single‐blind design (participants), ITT data presented. No deviations reported. | Low risk of bias | No missing data. | Low risk of bias | The data collection and end point judgement were blinded, and the statisticians were also blinded during the statistical analysis. The measurements were similar between groups | Low risk of bias | 30‐day mortality as pre‐specified in the trial registration was not explicitly reported, but could reliably derive from a synopsis of full text, figures, and supplemental material. | Low risk of bias | All domains were rated low risk of bias. |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | Low risk of bias | No missing data. | Low risk of bias | Individuals who assessed the outcomes were not blinded for the assigned treatment. Both groups were measured at the same time. The measurements were similar between groups. | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | Some concerns | Overall judged some concerns because of protocol deviations |
Risk of bias for analysis 1.2 Clinical improvement: Liberation from IMV.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment Control group: 5 excluded before starting treatment, 3 excluded after starting treatment |
High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | We requested the data from the authors. The measurement was similar between groups. | Some concerns | Data was not pre‐specified in the protocol | High risk of bias | Overall judged high due to missing outcome data. |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so that we judged this domain high. |
Risk of bias for analysis 1.3 Clinical improvement: Ventilator‐free days.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | Low risk of bias | No data missing. | Low risk of bias | Individuals who assessed the outcomes were not blinded for the assigned treatment. Both groups measured at the same time. Specified definition (alive and free from mechanical ventilation). | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | Some concerns | Overall judged some concerns due to protocol deviations. |
Risk of bias for analysis 1.4 Clinical worsening: New need for IMV.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Corral‐Gudino 2021 | Some concerns | Missing information about the allocation concealment. | Low risk of bias | ITT data presented. No protocol deviations. | High risk of bias | Unknown amount of missing data because of competing risks, and no analysis to adjust for this was made. | Low risk of bias | We requested the data from the authors. The measurements were similar between groups. | Some concerns | Data was kindly sent in upon request. Data was not pre‐specified in the protocol | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Edalatifard 2020 | Some concerns | No information about the allocation concealment. | Some concerns | 6 patients in the control group received the intervention drug and were excluded from the analyses (17%) | High risk of bias | 6 patients in the control group were not included in the analysis because of deviations from the protocol (17%). Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. |
Low risk of bias | We requested the data from the authors. The measurements were similar between groups. | Some concerns | Neither the protocol nor statistical analysis plan were available. | High risk of bias | Moreover, occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias. Further no protocol and SAP are available. |
| Jamaati 2021 | Some concerns | The selected patients were allocated to either the dexamethasone group or the control group by block randomisation. Ten blocks were generated by the Online Randomiser website. Pulmonary disease was highly significantly more prevalent in the control group. Additionally, baseline level of respiratory support was not reported so that we can only assume the most likely circumstance, i.e. that no support beyond oxygen insufflation had been given until randomisation. |
Low risk of bias | No deviations mentioned. ITT data reported. | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Some concerns | Trial registered after recruitment stop. No analysis plan published beyond registry entry. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment Control group: 5 excluded before starting treatment, 3 excluded after starting treatment |
High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | We requested the data from the authors. The measurements were similar between groups. | Some concerns | Data was kindly sent in upon request. Data was‐not pre‐specified in the protocol | High risk of bias | Overall judged high due to missing outcome data. |
Risk of bias for analysis 1.5 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Angus 2020 | Low risk of bias | Participants were randomised to each locally available group using balanced assignment. Participants were randomly assigned via a computer software program to each locally available group using proportional assignment (eg, 1:1 if 2 groups available and 1:1:1 if 3 groups available) Protocol: The RAR will occur centrally as part of the computerised randomisation process. Sites will receive the allocation status and will not be informed of the randomization proportions. Each region will maintain its own computer‐based randomisation program that is accessed by sites in that region but the RAR proportions will be determined by a SAC and provided monthly to the administrator of each region's randomization program who will update the RAR proportions Baseline characteristics were similar across groups. |
Some concerns | 15% of the participants in the no hydrocortisone group received corticosteroids | High risk of bias | Because of the issue of competing risk of death all data for this outcome might not be available. There was no adjustment in the analysis for competing risk of death. | Low risk of bias | The measurement method was appropriate. | Low risk of bias | Protocol and SAP available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention |
| Angus 2020 | Low risk of bias | Participants were randomised to each locally available group using balanced assignment. Participants were randomly assigned via a computer software program to each locally available group using proportional assignment (eg, 1:1 if 2 groups available and 1:1:1 if 3 groups available) Protocol: The RAR will occur centrally as part of the computerised randomisation process. Sites will receive the allocation status and will not be informed of the randomization proportions. Each region will maintain its own computer‐based randomisation program that is accessed by sites in that region but the RAR proportions will be determined by a SAC and provided monthly to the administrator of each region's randomization program who will update the RAR proportions Baseline characteristics were similar across groups. |
Some concerns | 15% of the participants in the no hydrocortisone group received corticosteroids | High risk of bias | Because of the issue of competing risk of death all data for this outcome might not be available. There was no adjustment in the analysis for competing risk of death. | Low risk of bias | The measurement method was appropriate. | Low risk of bias | Protocol and SAP available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
Risk of bias for analysis 1.6 Adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Corral‐Gudino 2021 | Some concerns | Missing information about the allocation concealment. | Low risk of bias | There were no deviations from intervention. An appropiate ITT analysis was used. | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Some concerns | Thresholds, definitions, and time points were called “pre‐specified” in the journal publication but not available in the Eudra‐CT registration. Statistical analysis plan not published. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Dequin 2020 | Low risk of bias | Randomisation was centralised and performed electronically. Allocation sequences were generated in a 1:1 ratio by a computer‐generated random number using a blocking schema. There were no baseline differences between the groups. | Low risk of bias | Protocol: Patients, investigators and care providers will be blinded for the patient‐arm | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups | Low risk of bias | The protocol and statistical analysis plan were available. | High risk of bias | Occurence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Edalatifard 2020 | Some concerns | No information about the allocation concealment. | Some concerns | 6 patients in the control group received the intervention drug and were excluded from the analyses (17%) | High risk of bias | 6 patients in the control group were not included in the analysis because of deviations from the protocol (17%) Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. |
Low risk of bias | The measurements were similar between groups. | Some concerns | Neither the protocol nor statistical analysis plan were available | High risk of bias | Overall judged high because 6 patients in the control group received the intervention drug and were excluded from the analyses. Moreover, occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias. |
| Tang 2021 | Low risk of bias | Randomisation was stratified by the statistician of the leading site, who produced computer‐generated block randomisation lists with a block size of 4 patients. | Low risk of bias | ITT presented. The participants were blinded and the physicians were aware of the treatment assignment. No protocol deviations. | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Some concerns | Endpoint including its subitems were not named in NCT registration. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
Risk of bias for analysis 1.7 Hospital‐acquired infections.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Corral‐Gudino 2021 | Some concerns | Missing information about the allocation concealment. | Low risk of bias | No deviations frome the procotol. | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Some concerns | Endpoint and definition is not pre‐specified in Eudra‐CT registration. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Dequin 2020 | Low risk of bias | Randomisation was centralised and performed electronically. Allocation sequences were generated in a 1:1 ratio by a computer‐generated random number using a blocking schema. There were no baseline differences between the groups. | Low risk of bias | Protocol: Patients, investigators and care providers will be blinded for the patient‐arm | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | The protocol and statistical analysis plan were available. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Edalatifard 2020 | Some concerns | No information about the allocation concealment. | Some concerns | 6 patients in the control group received the intervention drug and were excluded from the analyses (17%) | High risk of bias | 6 patients in the control group were not included in the analysis because of deviations from the protocol (17%) Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. |
Low risk of bias | The measurements were similar between groups. | Some concerns | Neither the protocol nor statistical analysis plan were available | High risk of bias | Overall judged high due to missing outcome data. |
| Tang 2021 | Low risk of bias | Randomisation was stratified by the statistician of the leading site, who produced computer‐generated block randomisation lists with a block size of 4 patients. | Low risk of bias | Single‐blind design, ITT data presented. No deviations reported. | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Some concerns | Endpoint was not named in NCT registration. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
Risk of bias for analysis 1.8 Need for dialysis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Horby 2021 | Low risk of bias | Randomisation was performed with the use of a web‐based system with concealment of the trial‐group assignment. No baseline differences between the groups. | Some concerns | 8% of the participants in the control group received dexamethasone | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | Protocol and statistical plan available. | High risk of bias | Occurrence of non‐terminal events can be precluded by death as a competing risk. Without adjustment this leads to high risk of bias, so overall we judged high risk of bias. |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment; Control group: 5 excluded before starting treatment, 3 excluded after starting treatment | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | Protocol and statistical plan available. Data analysed and presented according to a pre‐specified plan. | High risk of bias | Overall judged high due to missing outcome data. |
Risk of bias for analysis 1.9 Viral clearance.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment; Control group: 5 excluded before starting treatment, 3 excluded after starting treatment | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | Protocol and statistical plan available. Data analysed and presented according to a pre‐specified plan. | High risk of bias | Overall judged high due to missing outcome data. |
Risk of bias for analysis 2.1 All‐cause mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.1.1 Invasive ventilation | ||||||||||||
| Horby 2021 | Low risk of bias | Randomisation was performed with the use of a web‐based system with concealment of the trial‐group assignment. No baseline differences between the groups. | Some concerns | 8% of the participants in the control group received dexamethasone | Low risk of bias | 6425 randomised, 6425 analysed | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention in the control group. |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment Control group: 5 excluded before starting treatment, 3 excluded after starting treatment |
Low risk of bias | 416 participants randomised and 416 participants analysed. | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available. Data analysed and presented according to a pre‐specified plan. | Some concerns | Overall judged some concerns due to protocol deviations. |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | Low risk of bias | No missing data. | Low risk of bias | The measurements were similar between groups | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | Some concerns | Overall judged some concerns because of protocol deviations. |
| Subgroup 2.1.2 NIV or high‐flow oxygen only | ||||||||||||
| Corral‐Gudino 2021 | Some concerns | Missing information about the allocation concealment. | Low risk of bias | No protocol deviations. ITT data presented. | Low risk of bias | We requested the data from the authors, as the data was not broken down by our subgroups of interest in the publication | Low risk of bias | The measurements were similar between groups | Some concerns | Outcome was registered in Eudra‐CT as in‐hospital mortality but reported as 28‐day‐mortality in the supplemental material. | Some concerns | Overall judged some concerns due to selection of reported results. |
| Edalatifard 2020 | Some concerns | No information about the allocation concealment. | Some concerns | 6 patients in the control group received the intervention drug and were excluded from the analyses (17%) | Low risk of bias | The data were requested from the authors because the follow‐up time was not clearly visible from the publication. | Low risk of bias | Both groups were measured at the same time. The measurements were similar between groups | Some concerns | Neither the protocol nor statistical analysis plan were available | Some concerns | Overall judged some concerns because there were no information about the concealment of the allocation sequence. Further no protocol and SAP are available. |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment Control group: 5 excluded before starting treatment, 3 excluded after starting treatment |
Low risk of bias | 416 participants randomised and 416 participants analysed. | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available. Data analysed and presented according to a pre‐specified plan. | Some concerns | Overall judged some concerns due to protocol deviations. |
| Subgroup 2.1.3 NIV, high‐flow, and low‐flow oxygen combined | ||||||||||||
| Horby 2021 | Low risk of bias | Randomisation was performed with the use of a web‐based system with concealment of the trial‐group assignment. No baseline differences between the groups. | Some concerns | 8% of the participants in the control group received dexamethasone | Low risk of bias | 6425 randomised, 6425 analysed | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention in the control group. |
| Subgroup 2.1.4 Low‐flow‐oxygen only | ||||||||||||
| Corral‐Gudino 2021 | Some concerns | Missing information about the allocation concealment. | Low risk of bias | No protocol deviations. ITT data presented. | Low risk of bias | We requested the data from the authors, as the data was not broken down by our subgroups of interest in the publication | Low risk of bias | The measurements were similar between groups | Some concerns | Outcome was registered in Eudra‐CT as in‐hospital mortality but reported as 28‐day‐mortality in the supplemental material. | Some concerns | Overall judged some concerns due to selection of reported results. |
| Edalatifard 2020 | Some concerns | No information about the allocation concealment. | Some concerns | 6 patients in the control group received the intervention drug and were excluded from the analyses (17%) | Low risk of bias | The data were requested from the authors because the follow‐up time was not clearly visible from the publication. | Low risk of bias | Both groups were measured at the same time. The measurements were similar between groups | Some concerns | Neither the protocol nor statistical analysis plan were available | Some concerns | Overall judged some concerns because there were no information about the concealment of the allocation sequence. Further no protocol and SAP are available. |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment Control group: 5 excluded before starting treatment, 3 excluded after starting treatment |
Low risk of bias | 416 participants randomised and 416 participants analysed. | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available. Data analysed and presented according to a pre‐specified plan. | Some concerns | Overall judged some concerns due to protocol deviations. |
| Subgroup 2.1.5 No oxygen | ||||||||||||
| Horby 2021 | Low risk of bias | Randomisation was performed with the use of a web‐based system with concealment of the trial‐group assignment. No baseline differences between the groups. | Some concerns | 8% of the participants in the control group received dexamethasone | Low risk of bias | 6425 randomised, 6425 analysed | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available. | Some concerns | Overall judged some concerns due to deviations from the intended intervention in the control group. |
Risk of bias for analysis 3.1 All‐cause mortality.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.1.1 Dexamethasone | ||||||||||||
| Horby 2021 | Low risk of bias | Randomisation was performed with the use of a web‐based system with concealment of the trial‐group assignment. No baseline differences between the groups. | Some concerns | 8% of the participants in the control group received dexamethasone | Low risk of bias | 6425 randomised, 6425 analysed | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available | Some concerns | Overall judged some concerns due to protocol deviations. |
| Jamaati 2021 | Some concerns | The selected patients were allocated to either the dexamethasone group or the control group by block randomisation. Ten blocks were generated by the Online Randomiser website. Pulmonary disease was highly significantly more prevalent in the control group. Additionally, baseline level of respiratory support was not reported so that we can only assume the most likely circumstance, i.e. that no support beyond oxygen insufflation had been given until randomisation. |
Low risk of bias | No deviations mentioned. ITT data reported. | Low risk of bias | No missing data. | Low risk of bias | The measurements were similar between groups. | Some concerns | Trial registered after recruitment stop. No analysis plan published beyond registry entry. | Some concerns | Overall judged some concerns due to selective reporting and baseline differences. |
| Tomazini 2020 | Low risk of bias | Randomisation was performed through an online web‐based system using computer‐generated random numbers and blocks of 2 and 4, unknown to the investigators, and was stratified by centre. The group treatment was disclosed to the investigator only after all information regarding patient enrolment was recorded in the online system. Baseline characteristics were well‐balanced between groups. |
Some concerns | 25 deviations from protocol in the intervention arm (16.55%); 1 patient received a corticosteroid other than dexamethasone. In the control arm, 52 patients received corticosteroids, of which 14 were protocol deviations (9.4%). | Low risk of bias | No missing data. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | The protocol and statistical analysis plan were available. Outcomes reported as prespecified. | Some concerns | Overall judged some concerns due to protocol deviations. |
| Subgroup 3.1.2 Methylprednisolone | ||||||||||||
| Corral‐Gudino 2021 | Some concerns | Missing information about the allocation concealment. | Low risk of bias | Open‐label study. ITT data presented. | Low risk of bias | No substantial data missing. | Low risk of bias | The measurements were similar between groups | Some concerns | Endpoint was registered in Eudra‐CT as in‐hospital mortality but reported as 28d‐mortality in the supplemental material. | Some concerns | Overall judged some concerns due to selective reporting. |
| Edalatifard 2020 | Some concerns | No information about the allocation concealment. | Some concerns | 6 patients in the control group received the intervention drug and were excluded from the analyses (17%) | Low risk of bias | The data were requested from the authors because the follow‐up time was not clearly visible from the publication. | Low risk of bias | The measurements were similar between groups. | Some concerns | Neither the protocol nor statistical analysis plan were available | Some concerns | Overall judged some concerns because there were no information about the concealment of the allocation sequence. Further no protocol and SAP are available. |
| Jeronimo 2020 | Low risk of bias | An independent statistician prepared an electronically generated randomisation list with 14 blocks of 30 participants per block, generated via R software version 3.6.1 (blockrand package). The list was accessible only to non‐blinded pharmacists in the study. Participants were randomised by the study pharmacist to their designated treatment regimen at the time of inclusion and were subsequently identified throughout the study only by their allocated study number. There were no major differences in baseline characteristics between the intervention and placebo groups |
Some concerns | Intervention group: 14 excluded before starting treatment, 1 excluded after starting treatment Control group: 5 excluded before starting treatment, 3 excluded after starting treatment |
Low risk of bias | 416 participants randomised and 416 participants analysed. | Low risk of bias | The measurements were similar between groups | Low risk of bias | Protocol and statistical plan available. Data analysed and presented according to a pre‐specified plan. | Some concerns | Overall judged some concerns due to protocol deviations. |
| Tang 2021 | Low risk of bias | Randomisation was stratified by the statistician of the leading site, who produced computer‐generated block randomisation lists with a block size of 4 patients. | Low risk of bias | ITT data presented. No deviations reported. | Low risk of bias | No missing data. | Low risk of bias | The measurements were similar between groups. | Low risk of bias | 30‐day mortality as pre‐specified in the trial registration could was not explicitly reported, but could reliably derive from a synopsis of full text, figures, and supplemental material. | Low risk of bias | All domains were rated low. |
| Subgroup 3.1.3 Hydrocortisone | ||||||||||||
| Angus 2020 | Low risk of bias | Participants were randomised to each locally available group using balanced assignment. Participants were randomly assigned via a computer software program to each locally available group using proportional assignment (eg, 1:1 if 2 groups available and 1:1:1 if 3 groups available) Protocol: The RAR will occur centrally as part of the computerised randomisation process. Sites will receive the allocation status and will not be informed of the randomization proportions. Each region will maintain its own computer‐based randomisation program that is accessed by sites in that region but the RAR proportions will be determined by a SAC and provided monthly to the administrator of each region's randomization program who will update the RAR proportions Baseline characteristics were similar across groups. |
Some concerns | 15% of the participants in the no hydrocortisone group received corticosteroids | Low risk of bias | No missing data. | Low risk of bias | Mortality is an observer‐reported outcome not involving judgement. | Low risk of bias | Protocol and SAP available | Some concerns | Overall judged some concerns due to protocol deviations |
| Angus 2020 | Low risk of bias | Participants were randomised to each locally available group using balanced assignment. Participants were randomly assigned via a computer software program to each locally available group using proportional assignment (eg, 1:1 if 2 groups available and 1:1:1 if 3 groups available) Protocol: The RAR will occur centrally as part of the computerised randomisation process. Sites will receive the allocation status and will not be informed of the randomization proportions. Each region will maintain its own computer‐based randomisation program that is accessed by sites in that region but the RAR proportions will be determined by a SAC and provided monthly to the administrator of each region's randomization program who will update the RAR proportions Baseline characteristics were similar across groups. |
Some concerns | 15% of the participants in the no hydrocortisone group received corticosteroids | Low risk of bias | No missing data. | Low risk of bias | Mortality is an observer‐reported outcome not involving judgement. | Low risk of bias | Protocol and SAP available | Some concerns | Overall judged some concerns due to protocol deviations |
| Dequin 2020 | Low risk of bias | Randomisation was centralised and performed electronically. Allocation sequences were generated in a 1:1 ratio by a computer‐generated random number using a blocking schema. There were no baseline differences between the groups. | Low risk of bias | Protocol: Patients, investigators and care providers will be blinded for the patient‐arm ITT was used |
Low risk of bias | No missing data. | Low risk of bias | The measurements were similar between groups. | Some concerns | The protocol and statistical analysis plan were available. 21‐day mortality was a post hoc outcome | Some concerns | Overall judged some concerns due to selective reporting. |
Risk of bias for analysis 4.1 All‐cause mortality .
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ranjbar 2021 | Low risk of bias | Random allocation using the block randomisation method was performed in all four branches of the strata, based on two prognostic factors such as age (< 55 and ≥55) and disease severity based on O2 saturation (<85 and ≥85). The patient, assessor, and analyser in the two groups did not have access to the randomisation list and type of administered drug (Triple blind). No major differences in the baseline characteristics. | Low risk of bias | The patient, assessor, and analyser in the two groups did not have access to the randomisation list and type of administered drug (Triple blind). ITT analysis was used. | Low risk of bias | No missing data. | Low risk of bias | The measurements were similar between groups | Some concerns | Protocol and SAP are not available. | Some concerns | Overall judged some concerns due to selective reporting. |
Risk of bias for analysis 4.2 Clinical worsening: New need for IMV.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ranjbar 2021 | Low risk of bias | Random allocation using the block randomisation method was performed in all four branches of the strata, based on two prognostic factors such as age (< 55 and ≥55) and disease severity based on O2 saturation (<85 and ≥85). The patient, assessor, and analyser in the two groups did not have access to the randomisation list and type of administered drug (Triple blind). No major differences in the baseline characteristics. | Low risk of bias | The patient, assessor, and analyser in the two groups did not have access to the randomization list and type of administered drug (Triple blind). ITT analysis was used. | High risk of bias | Unknown amount of missing data because of competing risk, and no analysis to adjust for this was made. | Low risk of bias | The measurements were similar between groups | Some concerns | Protocol and SAP are not available. | High risk of bias | Overall judged high risk of bias due to missing outcome data. |
Acknowledgements
ALF, FF, MG, and KK are thankful for the support of their colleagues with the Department of Anesthesia and Intensive Care at the University of Leipzig Medical Center while working on the manuscript of this review during the COVID‐19 pandemic.
We thank the investigators of three studies for providing us with additional information and data (Corral‐Gudino 2021; Edalatifard 2020; Jeronimo 2020). We also thank the investigators of the platform trials Angus 2020 and Horby 2021 with whom the data sharing is in progress.
Cochrane Haematology supported the authors in the development of the protocol and review. Cochrane's Central Editorial Service (https://community.cochrane.org/review-production/production-resources/cochranes-central-editorial-service) conducted the editorial and peer‐review process for this article. Contributors to the editorial process were: Toby Lasserson, Sign‐off Editor (final editorial decision), Cochrane’s Editorial and Methods Department; Joey Kwong, Managing Editor (selecting peer reviewers, collating peer‐reviewer comments, providing editorial guidance to authors, editing), Cochrane’s Editorial and Methods Department; Leticia Rodrigues, Editorial Assistant (editorial policy checks and supporting editorial team), Cochrane’s Editorial and Methods Department; Denise Mitchell, Copy Editor (copy‐editing and production), Cochrane’s Editorial and Methods Department. Peer‐reviewers (providing comments and a recommendation) were: Nuala Livingstone, Cochrane’s Editorial and Methods Department (methods); Robin Featherstone, Cochrane’s Editorial and Methods Department, (search); Tess Moore, Cochrane’s Editorial and Methods Department (methods); Kerry Dwan, Cochrane’s Editorial and Methods Department (methods); Nida Qadir, Division of Pulmonary & Critical Care Medicine, David Geffen School of Medicine at UCLA (clinical/content); Jen‐Ting Chen, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA (clinical/content); Joseph M. Vinetz, Section of Infectious Diseases, Department of Internal Medicine, Yale School of Medicine (clinical/content) and Ahlam Jamal Alhemedi (consumer).
We thank Dr. Sven Laudi and Dr. Volker Thieme at the Department of Anesthesia and Intensive Care at the Leipzig University Medical Services for their biostatistical advice.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys'). The contents of this document reflect only the authors' views and the German Ministry is not responsible for any use that may be made of the information it contains.
Appendices
Appendix 1. Search strategies
Cochrane COVID‐19 Study Register
Search string:
corticosteroid* OR corticoid* OR prednison* OR dehydrocortison* OR deltason* OR decortin* OR orasone* OR deltra* OR meticorten* OR cortancyl* OR deltacorten* OR dacortin* OR adasone* OR "delta‐cortison" OR panasol* OR decorton* OR metacortandracin* OR paracort* OR predicor* OR decortisyl* OR delta‐1‐cortison* OR "delta‐dome" OR deltadehydrocortison* OR ofisolon* OR panafcort* OR predicorten* OR predni* OR econonson* OR promifen* OR servison* OR deltison* OR lisacort* OR meproson* OR rayos OR sterapred* OR "liquid pred" OR cortan* OR rectodelt* OR predeltin* OR prednisolon* OR methylprednisolon* OR medrol OR "pred forte" OR medrone OR urbason OR wyacort OR "Delta‐F" OR duralon* OR medrate OR omnipred OR adlone OR caberdelta OR depmedalon* OR "Depo Moderin" OR "Depo‐Nisolone" OR Emmetipi OR esameton* OR firmacort OR medlon* OR "Mega‐Star" OR meprolon* OR metilbetason* OR metrocort OR metypresol OR metysolon* OR orapred OR "Predni‐M‐Tablinen" OR radilem OR sieropresol OR solpredon* OR "A‐MethaPred" OR prelone OR medrone OR aprednislon OR pediapred OR hostacortin OR "Di‐Adreson‐F" OR adnisolon* OR capsoid OR cortalon* OR cortisolon* OR deltacortril OR estilsona OR panafcortelone OR sterane OR "Delta‐Cortef" OR econopred OR dacortin OR decaprednil OR "Delta‐Diona" OR "Delta‐Phoricol" OR deltahydrocortison* OR deltasolon* OR deltidrosol OR dhasolone OR fisopred OR frisolona OR gupison* OR hydeltra OR hydeltrasol OR klismacort OR kuhlprednon OR lenisolon* OR "Lepi‐Cortinolo" OR "Linola‐H" OR longiprednil OR metacortandralon* OR "Meti Derm" OR meticortelon* OR opredsone Or precortisyl OR "Pred‐Clysma" OR predeltilon* OR prenilone OR hydrocortancyl OR "Solu Moderin" OR predonin* OR metypred OR prednisol OR dexamethason* OR "BB 1101" OR decadron OR hexadrol OR fortecortin OR dexameth OR dexone OR hexadecadrol OR desamethason* OR ozurdex OR deronil OR baycuten OR aacidexam OR spersadex OR dexacortal OR gammacorten OR visumetazon* OR adexone OR "Alba‐Dex" OR cortidexason OR decacort OR decadrol OR dectancyl OR desameton OR loverine OR millicorten OR orgadrone OR alin OR auxiloson OR cortisumman OR decalix OR decameth OR decasone OR dekacort OR deltafluorene OR "Dexa‐Mamallet" OR dexafluorene OR dexalocal OR dexamecortin OR dexamonozon OR dexapos OR dexinoral OR fluorodelta OR lokalison OR methylfluorprednisolon* OR mymethason* OR "Dexa‐Rhinosan" OR "Dexa‐Scheroson" OR "Dexa‐sine" OR dexacortin OR dexafarma OR dinormon OR baycadron OR "Aeroseb‐Dex" OR Maxidex OR Dextenza OR dexasone OR dexpak OR hydrocortison* OR cortisol OR cortef OR hydrocorton* OR cetacort OR barseb OR aeroseb OR "Cort‐Dome" OR cortenema OR cortril OR cortifan OR cortispray OR dermacort OR domolene OR eldecort OR hautosone OR "Heb‐Cort" OR hytone OR Komed OR Nutracort OR Proctocort OR Rectoid OR Hydrocort OR locoid OR Solu‐Glyc
Study characteristics: 1) "Intervention assignment": “Randomised” OR 2) "Study type": "Interventional" AND "Study design": "Parallel/Crossover" AND "Unclear"
Web of Science Core Collection (Advanced search)
#1 TI=( corticosteroid* OR corticoid* OR prednison* OR dehydrocortison* OR deltason* OR decortin* OR orasone* OR deltra* OR meticorten* OR cortancyl* OR deltacorten* OR dacortin* OR adasone* OR "delta‐cortison" OR panasol* OR decorton* OR metacortandracin* OR paracort* OR predicor* OR decortisyl* OR delta‐1‐cortison* OR "delta‐dome" OR deltadehydrocortison* OR ofisolon* OR panafcort* OR predicorten* OR predni* OR econonson* OR promifen* OR servison* OR deltison* OR lisacort* OR meproson* OR rayos OR sterapred* OR "liquid pred" OR cortan* OR rectodelt* OR predeltin* OR prednisolon* OR methylprednisolon* OR medrol OR "pred forte" OR medrone OR urbason OR wyacort OR "Delta‐F" OR duralon* OR medrate OR omnipred OR adlone OR caberdelta OR depmedalon* OR "Depo Moderin" OR "Depo‐Nisolone" OR Emmetipi OR esameton* OR firmacort OR medlon* OR "Mega‐Star" OR meprolon* OR metilbetason* OR metrocort OR metypresol OR metysolon* OR orapred OR "Predni‐M‐Tablinen" OR radilem OR sieropresol OR solpredon* OR "A‐MethaPred" OR prelone OR medrone OR aprednislon OR pediapred OR hostacortin OR "Di‐Adreson‐F" OR adnisolon* OR capsoid OR cortalon* OR cortisolon* OR deltacortril OR estilsona OR panafcortelone OR sterane OR "Delta‐Cortef" OR econopred OR dacortin OR decaprednil OR "Delta‐Diona" OR "Delta‐Phoricol" OR deltahydrocortison* OR deltasolon* OR deltidrosol OR dhasolone OR fisopred OR frisolona OR gupison* OR hydeltra OR hydeltrasol OR klismacort OR kuhlprednon OR lenisolon* OR "Lepi‐Cortinolo" OR "Linola‐H" OR longiprednil OR metacortandralon* OR "Meti Derm" OR meticortelon* OR opredsone Or precortisyl OR "Pred‐Clysma" OR predeltilon* OR prenilone OR hydrocortancyl OR "Solu Moderin" OR predonin* OR metypred OR prednisol OR dexamethason* OR "BB 1101" OR decadron OR hexadrol OR fortecortin OR dexameth OR dexone OR hexadecadrol OR desamethason* OR ozurdex OR deronil OR baycuten OR aacidexam OR spersadex OR dexacortal OR gammacorten OR visumetazon* OR adexone OR "Alba‐Dex" OR cortidexason OR decacort OR decadrol OR dectancyl OR desameton OR loverine OR millicorten OR orgadrone OR alin OR auxiloson OR cortisumman OR decalix OR decameth OR decasone OR dekacort OR deltafluorene OR "Dexa‐Mamallet" OR dexafluorene OR dexalocal OR dexamecortin OR dexamonozon OR dexapos OR dexinoral OR fluorodelta OR lokalison OR methylfluorprednisolon* OR mymethason* OR "Dexa‐Rhinosan" OR "Dexa‐Scheroson" OR "Dexa‐sine" OR dexacortin OR dexafarma OR dinormon OR baycadron OR "Aeroseb‐Dex" OR Maxidex OR Dextenza OR dexasone OR dexpak OR hydrocortison* OR cortisol OR cortef OR hydrocorton* OR cetacort OR barseb OR aeroseb OR "Cort‐Dome" OR cortenema OR cortril OR cortifan OR cortispray OR dermacort OR domolene OR eldecort OR hautosone OR "Heb‐Cort" OR hytone OR Komed OR Nutracort OR Proctocort OR Rectoid OR Hydrocort OR locoid OR Solu‐Glyc) OR AB=( corticosteroid* OR corticoid* OR prednison* OR dehydrocortison* OR deltason* OR decortin* OR orasone* OR deltra* OR meticorten* OR cortancyl* OR deltacorten* OR dacortin* OR adasone* OR "delta‐cortison" OR panasol* OR decorton* OR metacortandracin* OR paracort* OR predicor* OR decortisyl* OR delta‐1‐cortison* OR "delta‐dome" OR deltadehydrocortison* OR ofisolon* OR panafcort* OR predicorten* OR predni* OR econonson* OR promifen* OR servison* OR deltison* OR lisacort* OR meproson* OR rayos OR sterapred* OR "liquid pred" OR cortan* OR rectodelt* OR predeltin* OR prednisolon* OR methylprednisolon* OR medrol OR "pred forte" OR medrone OR urbason OR wyacort OR "Delta‐F" OR duralon* OR medrate OR omnipred OR adlone OR caberdelta OR depmedalon* OR "Depo Moderin" OR "Depo‐Nisolone" OR Emmetipi OR esameton* OR firmacort OR medlon* OR "Mega‐Star" OR meprolon* OR metilbetason* OR metrocort OR metypresol OR metysolon* OR orapred OR "Predni‐M‐Tablinen" OR radilem OR sieropresol OR solpredon* OR "A‐MethaPred" OR prelone OR medrone OR aprednislon OR pediapred OR hostacortin OR "Di‐Adreson‐F" OR adnisolon* OR capsoid OR cortalon* OR cortisolon* OR deltacortril OR estilsona OR panafcortelone OR sterane OR "Delta‐Cortef" OR econopred OR dacortin OR decaprednil OR "Delta‐Diona" OR "Delta‐Phoricol" OR deltahydrocortison* OR deltasolon* OR deltidrosol OR dhasolone OR fisopred OR frisolona OR gupison* OR hydeltra OR hydeltrasol OR klismacort OR kuhlprednon OR lenisolon* OR "Lepi‐Cortinolo" OR "Linola‐H" OR longiprednil OR metacortandralon* OR "Meti Derm" OR meticortelon* OR opredsone Or precortisyl OR "Pred‐Clysma" OR predeltilon* OR prenilone OR hydrocortancyl OR "Solu Moderin" OR predonin* OR metypred OR prednisol OR dexamethason* OR "BB 1101" OR decadron OR hexadrol OR fortecortin OR dexameth OR dexone OR hexadecadrol OR desamethason* OR ozurdex OR deronil OR baycuten OR aacidexam OR spersadex OR dexacortal OR gammacorten OR visumetazon* OR adexone OR "Alba‐Dex" OR cortidexason OR decacort OR decadrol OR dectancyl OR desameton OR loverine OR millicorten OR orgadrone OR alin OR auxiloson OR cortisumman OR decalix OR decameth OR decasone OR dekacort OR deltafluorene OR "Dexa‐Mamallet" OR dexafluorene OR dexalocal OR dexamecortin OR dexamonozon OR dexapos OR dexinoral OR fluorodelta OR lokalison OR methylfluorprednisolon* OR mymethason* OR "Dexa‐Rhinosan" OR "Dexa‐Scheroson" OR "Dexa‐sine" OR dexacortin OR dexafarma OR dinormon OR baycadron OR "Aeroseb‐Dex" OR Maxidex OR Dextenza OR dexasone OR dexpak OR hydrocortison* OR cortisol OR cortef OR hydrocorton* OR cetacort OR barseb OR aeroseb OR "Cort‐Dome" OR cortenema OR cortril OR cortifan OR cortispray OR dermacort OR domolene OR eldecort OR hautosone OR "Heb‐Cort" OR hytone OR Komed OR Nutracort OR Proctocort OR Rectoid OR Hydrocort OR locoid OR Solu‐Glyc)
#2 TI=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2") OR AB=(COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")
#3 #1 AND #2
#4 TI=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII") OR AB=(random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII")
#5 #3 AND #4
Indexes=SCI‐EXPANDED, ESCI Timespan=2020‐2021
WHO COVID‐19 Global literature on coronavirus disease
(corticosteroid* OR corticoid* OR prednis* OR hydrocorti* OR methylpredni* OR deltahydrocorti* OR dehydrocorti* OR dexameth* OR desameth*) AND (random* OR placebo OR trial OR groups OR "phase 3" or "phase3" or p3 or "pIII")
Data and analyses
Comparison 1. Systemic corticosteroids plus standard care versus standard care (plus/minus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 9 | 7930 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 1.2 Clinical improvement: Liberation from IMV | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Clinical improvement: Ventilator‐free days | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Clinical worsening: New need for IMV | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Serious adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7 Hospital‐acquired infections | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.8 Need for dialysis | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9 Viral clearance | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Subgroup analysis: respiratory support for the comparison of corticosteroids plus standard care versus standard care (plus/minus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 5 | 7199 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.04] |
| 2.1.1 Invasive ventilation | 3 | 1439 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.70, 1.04] |
| 2.1.2 NIV or high‐flow oxygen only | 3 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.20, 2.03] |
| 2.1.3 NIV, high‐flow, and low‐flow oxygen combined | 1 | 3883 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.00] |
| 2.1.4 Low‐flow‐oxygen only | 3 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.29, 1.67] |
| 2.1.5 No oxygen | 1 | 1535 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.00, 1.61] |
Comparison 3. Subgroup analysis: dexamethasone versus methylprednisolone versus hydrocortisone for the comparison of corticosteroids plus standard care versus standard care (plus/minus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality | 9 | 7930 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 3.1.1 Dexamethasone | 3 | 6774 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.83, 0.98] |
| 3.1.2 Methylprednisolone | 4 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.26, 1.54] |
| 3.1.3 Hydrocortisone | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.57, 1.05] |
Comparison 4. Methylprednisolone versus dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause mortality | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.07] |
| 4.2 Clinical worsening: New need for IMV | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angus 2020.
| Study characteristics | ||
| Methods | Trial design: multicenter, open‐label, platform RCT Type of publication: journal publication Setting: inpatient Recruitment dates: 9 March‐17 June 2020 Country: Australia, Canada, France, Ireland, the Netherlands, New Zealand, UK, USA Language: English Number of centres: 121 clinical sites Trial registration number: NCT02735707 Date first posted: 13 April 2016 |
|
| Participants | Age: mean age of
Gender:
Proportion of confirmed infections
Ethnicity
Number of participants (recruited/allocated/evaluated)
Severity of condition according to study definition
Severity of condition according to WHO score: severe ≥ 6 Co‐morbidities: diabetes, respiratory disease, kidney disease, severe cardiovascular disease, immunosuppressive disease Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses)
Treatment details of control group (e.g dose, route of administration, number of doses)
Duration of follow‐up: follow‐up ended 12 August 2020 Treatment cross‐overs: no Compliance with assigned treatment: yes |
|
| Outcomes | Primary study outcome: respiratory and cardiovascular organ support‐free days up to day 21, with subcomponents in‐hospital deaths and organ support‐free days among survivors Review outcomes: inpatient setting
Additional study outcomes: time to death, cardiovascular organ support–free days, length of ICU stay, WHO scale at day 14 |
|
| Identification | ||
| Notes | Date of publication: 2 September 2020 Sponsor/funding: Platform for European Preparedness Against (Re‐) emerging Epidemics (PREPARE) consortium by the European Union, FP7‐HEALTH‐2013‐INNOVATION‐1 (grant 602525), the Australian National Health and Medical Research Council (grant APP1101719), the New Zealand Health Research Council (grant 16/ 631), the Canadian Institute of Health Research Strategy for Patient‐Oriented Research Innovative Clinical Trials Program (grant 158584), the UK National Institute for Health Research (NIHR) and the NIHR Imperial Biomedical Research Centre, the Health Research Board of Ireland (grant CTN 2014‐012), the UPMC Learning While Doing Program, the Breast Cancer Research Foundation, the French Ministry of Health (grant PHRC‐20‐0147), and the Minderoo Foundation Risk of bias table presents two entries for analysis 1.1 All‐cause mortality and anaysis 1.5 Serious adverse events: one entry for fixed‐dose arm and one for shock‐dependent arm |
|
Corral‐Gudino 2021.
| Study characteristics | ||
| Methods | Trial design: multicentric, open‐label, RCT Type of publication: journal publication Setting: inpatient Recruitment dates: not reported Country: Spain Language: English Number of centres: 5 hospitals Trial registration number: EUCTR 2020‐001934‐37 Date of trial registration: 8 May 2020 |
|
| Participants | Age: mean age of:
Gender:
Proportion of confirmed infections: PCR positivity inclusion criterion Ethnicity: not reported Number of participants (recruited/allocated/evaluated):
Severity of condition according to study definition
Severity of condition according to WHO score: moderate to severe 5‐6 Co‐morbidities: hypertension, cardiac disease, respiratory disease, diabetes Inclusion criteria
Exclusion criteria:
|
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses)
Treatment details of control group (e.g dose, route of administration, number of doses): standard care
Duration of follow‐up: until hospital discharge or day 28 after inclusion Treatment cross‐overs: no Compliance with assigned treatment: yes |
|
| Outcomes | Primary study outcome: composite endpoint including in‐hospital all‐cause mortality, escalation to ICU admission or progression of respiratory insufficiency that required noninvasive ventilation Review outcomes: inpatient setting
Additional study outcomes: composite endpoint included in‐hospital all‐cause mortality, escalation to ICU admission, or progression of respiratory insufficiency that required noninvasive ventilation |
|
| Identification | ||
| Notes | Date of publication: 3 February 2021 Sponsor/funding: IDIVAL Instituto de Investigación Sanitaria Valdecilla |
|
Dequin 2020.
| Study characteristics | ||
| Methods | Trial design: multicenter, double‐blind randomised trial Type of publication: journal publication Setting: inpatient Recruitment dates: 7 March‐1 June 2020 Country: France Language: English Number of centres: 9 Trial registration number: NCT02517489 Date first posted: 7 August 2015 |
|
| Participants | Age:
Gender:
Proportion of PCR‐confirmed infections
Ethnicity: not reported Number of participants (recruited/allocated/evaluated):
Severity of condition according to study definition
Severity of condition according to WHO score: moderate to severe ≥ 5 Co‐morbidities: diabetes, COPD/asthma, immunosuppression Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses):
Treatment details of control group (e.g dose, route of administration, number of doses): Placebo Concomitant therapy (e.g description of standard care): not reported Duration of follow‐up: last follow‐up on 29 June 2020 Treatment cross‐overs: no Compliance with assigned treatment: yes |
|
| Outcomes | Primary study outcome: treatment failure on day 21 (death or persistent dependence of mechanical ventilation or high‐flow oxygen therapy) Review outcomes: Inpatient setting:
Additional study outcomes: endotracheal intubation (for patients noninvasively ventilated at inclusion), prone position, ECMO, inhaled nitric oxide |
|
| Identification | ||
| Notes | Date of publication: 6 October 2020 Sponsor/funding: University Hospital, Tours |
|
Edalatifard 2020.
| Study characteristics | ||
| Methods | Trial design: RCT Type of publication: journal publication Setting: inpatient Recruitment dates: 28 March‐28 May 2020 (study register entry) Country: Iran Language: English Number of centres: 4 Trial registration number: IRCT20200404046947N1 Date of trial registration: 15 April 2020 |
|
| Participants | Age
Gender:
Proportion of confirmed infections: PCR positivity inclusion criterion Ethnicity: not reported Number of participants (recruited/allocated/evaluated)
Severity of condition according to study definition
Severity of condition according to WHO score: moderate to severe 5‐6 Co‐morbidities: diabetes, hypothyroidism, cancer, respiratory disorder, renal disorder, cardiovascular disorder, hypertension, autoimmune and neurodegenerative diseases Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses):
Treatment details of control group (e.g dose, route of administration, number of doses): standard care
Duration of follow‐up: 3 days Treatment cross‐overs: no Compliance with assigned treatment: 6 patients in the control group received the intervention drug |
|
| Outcomes | Primary study outcome: time to event (discharge or death), time to improvement Review outcomes: inpatient setting
Additional study outcomes: blood SO2 level, BORG score, heart rate, temperature, respiratory rate |
|
| Identification | ||
| Notes | Date of publication: September 7, 2020 Sponsor/funding: Tehran University of Medical Sciences |
|
Farahani 2021.
| Study characteristics | ||
| Methods | Trial design: open‐label, single‐centre RCT Type of publication: preprint Setting: inpatient Recruitment dates: 30 March‐18 May 2020 (only estimated dates from registry entry) Country: Iran Language: English Number of centres: 1 Trial registration number: IRCT20200406046963N1 Date of trial registration: 22 April 2020 |
|
| Participants | Age: 18‐90 years Gender: no sexes excluded Proportion of confirmed infections: PCR positivity inclusion criterion Ethnicity: no ethnicities excluded Number of participants (recruited/allocated/evaluated): 14 intervention group, 15 control group Severity of condition according to study definition:
Severity of condition according to WHO score: moderate‐severe 5‐6 Co‐morbidities: not reported Inclusion criteria
Exclusion criteria
Previous treatments: not specified |
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses): 1000 mg methylprednisolone IV for 3 days followed by 1 mg/kg oral prednisolone with dose tapering for 7 days + standard care Treatment details of control group (e.g dose, route of administration, number of doses): standard care Concomitant therapy (e.g. description of standard care):
Duration of follow‐up: not specified Treatment cross‐overs: none reported Compliance with assigned treatment: no deviations reported |
|
| Outcomes | Primary study outcome: mortality rate, blood O2 saturation and need for further oxygen therapy Review outcomes: inpatient setting
Additional study outcomes
|
|
| Identification | ||
| Notes | Date of publication: 9 September 2020 Sponsor/funding: Artesh University of Medical Sciences |
|
Horby 2021.
| Study characteristics | ||
| Methods | Trial design: open‐label RCT Type of publication: journal publication Setting: inpatient Recruitment dates: recruitment ended on 8 June 2020 Country: UK Language: English Number of centres: 176 Trial registration number: NCT04381936 Date of trial registration: 11 May 2020 |
|
| Participants | Age: mean age
Gender
Proportion of PCR test results
Ethnicity: not reported Number of participants (recruited/allocated/evaluated):
Severity of condition according to study definition
Severity of condition according to WHO score: moderate to severe 4‐9 Co‐morbidities: diabetes, heart disease, chronic lung disease, tuberculosis, HIV infection, severe liver disease, severe kidney impairment Inclusion criteria
Exclusion criteria
Previous treatments: not reported |
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses)
Treatment details of control group (e.g dose, route of administration, number of doses)
Concomitant therapy (e.g description of standard care): none Duration of follow‐up: until discharge or death, or 28 days after randomisation Treatment cross‐overs: no Compliance with assigned treatment: 8% in the control group received intervention drug |
|
| Outcomes | Primary study outcome: 28‐day mortality Review outcomes: inpatient setting
Additional study outcomes: composite outcome IMV or death |
|
| Identification | ||
| Notes | Date of publication: 17 July 2020 Sponsor/funding: University of Oxford |
|
Jamaati 2021.
| Study characteristics | ||
| Methods | Trial design: RCT Type of publication: journal publication Setting: inpatient Recruitment dates: March 2020 Country: Iran Language: English Number of centres: 1 Trial registration number: IRCT20151227025726N17 Date of trial registration: 31 May 2020 |
|
| Participants | Age: median age
Gender
Proportion of confirmed infections: PCR positivity inclusion criterion Ethnicity: not reported Number of participants (recruited/allocated/evaluated):
Severity of condition according to study definition: PaO2/FiO2 between 100 and 300 mmHg Severity of condition according to WHO score: most likely 5, no invasive ventilation at randomisation Co‐morbidities: diabetes, hypertension, cardiovascular disease Inclusion criteria
Exclusion criteria
Previous treatments: not reported |
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses)
Treatment details of control group (e.g dose, route of administration, number of doses)
Concomitant therapy (e.g description of standard care): oxygen support, fluid support, lopinavir/ritonavir (200/50 mg, 2 tablets twice a day) Duration of follow‐up: 28 days Treatment cross‐overs: no Compliance with assigned treatment: yes |
|
| Outcomes | Primary study outcome: need for IMV, death rate Review outcomes: inpatient setting
Additional study outcomes: duration of clinical improvement, radiological changes in the CT scan |
|
| Identification | ||
| Notes | Date of publication: 16 February 2021 Sponsor/funding: Shahid Beheshti University of Medical Sciences |
|
Jeronimo 2020.
| Study characteristics | ||
| Methods | Trial design: double‐blind RCT Type of publication: journal publication Setting: inpatient Recruitment dates: not reported Country: Brazil Language: English Number of centres: 1 Trial registration number: NCT04343729 Date of trial registration: 13 April 2020 |
|
| Participants | Age: mean age
Gender
Proportion of PCR test results
Ethnicity: white, black, admixed, Asian, Amerindian Number of participants (recruited/allocated/evaluated)
Severity of condition according to study definition
Severity of condition according to WHO score: moderate to severe: 5‐9 Co‐morbidities: diabetes, hypertension, alcohol use disorder, heart disease, asthma, rheumatic disease, liver disease, previous tuberculosis, COPD Inclusion criteria
Exclusion criteria
Previous treatments: not reported |
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses):
|
|
| Outcomes | Primary study outcome: 28‐day mortality Review outcomes: inpatient setting
Additional study outcomes: |
|
| Identification | ||
| Notes | Date of publication: 12 August 2020 Sponsor/funding: Fundação de Medicina Tropical Dr. Heitor Vieira Dourado |
|
Ranjbar 2021.
| Study characteristics | ||
| Methods | Trial design: triple‐blind RCT Type of publication: preprint Setting: inpatient Recruitment dates: 10 August 2020‐15 November 2020 Country: Iran Language: English Number of centres: 1 Trial registration number: IRCT20200204046369N1 Date of trial registration: 8 April 2020 |
|
| Participants | Age: mean age
Gender
Proportion of confirmed infections: PCR positivity inclusion criterion Ethnicity: not reported Number of participants (recruited/allocated/evaluated):
Severity of condition according to study definition: patients with SpO2 < 92 in room air Severity of condition according to WHO score: moderate 4‐5 Co‐morbidities: diabetes, cardiovascular disease, hypertension, renal diseases, liver diseases Inclusion criteria
Exclusion criteria: Pregnancy
Previous treatments: not reported |
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses)
Treatment details of control group (e.g dose, route of administration, number of doses): 6 mg of dexamethasone IV daily for 10 days Concomitant therapy (e.g description of standard care): no Duration of follow‐up: 28 days Treatment cross‐overs: no Compliance with assigned treatment: yes |
|
| Outcomes | Primary study outcome: all‐cause mortality in 28 days, clinical status after 5 and 10 days after enrolment with 9‐point WHO scale Review outcomes: inpatient setting:
|
|
| Identification | ||
| Notes | Date of publication: 1 February 2021 Sponsor/funding: Shiraz University of Medical Sciences Dr. Mohsen Moghadami |
|
Tang 2021.
| Study characteristics | ||
| Methods | Trial design: prospective, multicenter, single‐blind RCT Type of publication: journal publication Setting: inpatient Recruitment dates: 19 February 2020‐31 March 2020 Country: China Language: English Number of centres: 7 Trial registration number: NCT04273321 Date of trial registration: 15 February 2020 |
|
| Participants | Age: median age
Gender
Proportion of confirmed infections: PCR positivity inclusion criterion Ethnicity: not reported Number of participants (recruited/allocated/evaluated):
Severity of condition according to study definition:
Severity of condition according to WHO score: moderate to severe 4‐6 Co‐morbidities: COPD, asthma, hypertension, coronary heart disease, diabetes, chronic renal failure Inclusion criteria
Exclusion criteria
Previous treatments: not reported |
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses)
Treatment details of control group (e.g dose, route of administration, number of doses)
Concomitant therapy (e.g. description of standard care): standard therapy of COVID‐19: according to the Chinese Diagnosis and Treatment Plan for COVID‐19 (trial version 6); antivirals: 67 (77.9%) of patients, antibiotics: 61 (70.9%) of patients Duration of follow‐up: at least 14 days after randomisation or until hospital discharge Treatment cross‐overs: none documented Compliance with assigned treatment: yes |
|
| Outcomes | Primary study outcome: clinical deterioration 14 days after randomisation Review outcomes: inpatient setting
Additional study outcomes
|
|
| Identification | ||
| Notes | Date of publication: 22 January 2021 Sponsor/funding: Beijing Chao Yang Hospital |
|
Tomazini 2020.
| Study characteristics | ||
| Methods | Trial design: multicenter, open‐label RCT Type of publication: journal publication Setting: inpatient Recruitment dates: 17 April‐23 June 2020 Country: Brazil Language: English Number of centres: 41 Trial registration number: NCT04327401 Date of trial registration: 31 March 2020 |
|
| Participants |
|
|
| Interventions | Treatment details of intervention group (e.g dose, route of administration, number of doses):
Treatment details of control group (e.g dose, route of administration, number of doses): standard care Concomitant therapy (e.g description of standard care): hydroxychloroquine, azithromycin, other antibiotics, oseltamivir Duration of follow‐up: 28 days Treatment cross‐overs: no Compliance with assigned treatment
|
|
| Outcomes | Primary study outcome: number of days alive and free from mechanical ventilation for at least 48 consecutive hours Review outcomes: Inpatient setting
Additional study outcomes: Sequential Organ Failure Assessment (SOFA) scores |
|
| Identification | ||
| Notes | Date of publication: 2 September 2020 Sponsor/funding: this trial was funded and supported by the Coalition COVID‐19 Brazil. The Laboratórios Farmacêuticos provided the study drug, distribution logistics, and insurance for the study patients |
|
AE: adverse event; ARDS: acute respiratory distress syndrome; COPD: chronic obstructive pulmonary disease; CPK: creatine phosphokinase; CT: computed tomography; ECMO: extracorporeal membrane oxygenation; FiO2: fraction of inspired oxygen ICU: intensive care unit; IMV: invasive mechanical ventilation; IQR: interquartile range: IV: intravenous; LDH: lactate dehydrogenase; NIV: non‐invasive ventilation; PaO2: partial pressure of oxygen; PEEP: positive end‐expiratory pressure; RCT: randomised controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; SAE: serious adverse event; SaO2: arterial oxygen saturation; SpO2: blood oxygen saturation; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| EUCTR2020‐001445‐39‐ES | Corticosteroid plus other active substances versus standard care |
| EUCTR2020‐001616‐18‐ES | Inhaled corticosteroids |
| EUCTR2020‐001889‐10 | Inhaled corticosteroids |
| IRCT20120225009124N4 | Corticosteroid plus other active substances versus standard care |
| IRCT20190312043030N2 | Corticosteroid plus other active substances versus standard care |
| IRCT20200522047542N1 | Topical corticosteroids |
| ISRCTN86534580 | Inhaled corticosteroids |
| NCT04341038 | Corticosteroid plus other active substances versus standard care |
| NCT04355637 | Inhaled corticosteroids |
| NCT04359511 | Withdrawn (competitor test RECOVERY) |
| NCT04361474 | Topical corticosteroids |
| NCT04381364 | Inhaled corticosteroids |
| NCT04411667 | Corticosteroid plus other active substances versus standard care |
| NCT04416399 | Inhaled corticosteroids |
| NCT04468646 | Corticosteroid plus other active substances versus standard care |
| NCT04484493 | Topical corticosteroids |
| NCT04534478 | Corticosteroids for long‐COVID treatment |
| NCT04551781 | Corticosteroids for long‐COVID treatment |
| NCT04561180 | Corticosteroid plus other active substances versus standard care |
| NCT04569825 | Topical corticosteroids |
| NCT04640168 | Corticosteroid plus other active substances versus standard care |
| NCT04657484 | Corticosteroids for long‐COVID treatment |
| NCT04826822 | Corticosteroid plus other active substance versus standard care |
Characteristics of studies awaiting classification [ordered by study ID]
EUCTR2020‐001307‐16‐ES.
| Methods | Trial design: open RCT Sample size: 104 Setting: inpatient Language: Spanish, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: methylprednisolone
Treatment details of control group (e.g dose, route of administration): no information Concomitant therapy: no information |
| Outcomes | Primary study outcome: death for any cause in the first 28 days after randomisation |
| Notes | Recruitment status: prematurely ended Prospective completion date: 6‐month duration Date last update was posted: unclear Sponsor/funding: Fundación para la Investigación Biomédica Hospital Ramón y Cajal |
EUCTR2020‐001333‐13‐FR.
| Methods | Trial design: open RCT Sample size: 122 Setting: inpatient Language: French, English Number of centres: 18 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Concomitant therapy: no information |
| Outcomes | Primary study outcome: mortality on day 28 |
| Notes | Recruitment status: prematurely ended Date of the global end of the trial: 7 August 2020 Date last update was posted: unclear Sponsor/funding: Groupe Hospitalier Paris Saint‐Joseph |
EUCTR2020‐001553‐48‐FR.
| Methods | Trial design: open RCT Sample size: 304 Setting: inpatient Language: French Number of centres: 17 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): standard care Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Notes | Recruitment status: prematurely ended Prospective completion date: 7 month predicted Date last update was posted: unclear Sponsor/funding: Hospices Civils de Lyon |
IRCT20081027001411N3.
| Methods | Trial design: single‐blinded RCT Sample size: 60 Setting: inpatient Language: English Number of centres: 4 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): control group receives standard treatment for COVID‐19 disease Concomitant therapy: not stated |
| Outcomes | Primary study outcome
|
| Notes | Recruitment status: completed Prospective completion date: 30 June 2020 Date last update was posted: 1 June 2020 Sponsor/funding: Teheran University of Medical Sciences |
IRCT20100228003449N31.
| Methods | Trial design: open‐label, randomised clinical trial Sample size: 119 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | 3 groups comparing different doses of dexamethasone Details of intervention:
Treatment details of control groups (e.g dose, route of administration)
Concomitant therapy: no |
| Outcomes | Primary study outcome
|
| Notes | Recruitment status: completed Prospective completion date: no information Date last update was posted: 8 October 2020 Sponsor/funding: Teheran University of Medical Sciences |
IRCT20120215009014N354.
| Methods | Trial design: double‐ blind, phase II RCT Sample size: 81 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention group 1
Details of intervention group 2
Details of intervention group 3
Treatment details of control group (e.g dose, route of administration): Concomitant therapy: routine care |
| Outcomes | Primary study outcome
|
| Notes | Recruitment status: completed Prospective completion date: 5 August 2020 Date last update was posted: 1 May 2020 Sponsor/funding: Hamedan University of Medical Sciences |
IRCT20160118026097N4.
| Methods | Trial design: unblinded, RCT Sample size: 60 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): treatment according to Ministry of Health's protocol Concomitant therapy: no information |
| Outcomes | Primary study outcome: mortality rate or recovery within 30 days after hospitalisation |
| Notes | Recruitment status: completed Prospective completion date: no information Date last update was posted: 13 September 2020 Sponsor/funding: Ghoum University of Medical Sciences |
IRCT20200611047727N3.
| Methods | Trial design: single blinded, RCT Sample size: 60 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): standard care Concomitant therapy: no |
| Outcomes | Primary study outcome: radiological changes (before the intervention and 6 weeks later) |
| Notes | Recruitment status: completed Prospective completion date: no information Date last update was posted: 3 January 2021 Sponsor/funding: Shahid Beheshti University of Medical Sciences |
IRCT20201015049030N1.
| Methods | Trial design: single‐blind, RCT Sample size: 200 Setting: outpatient Language: English Number of centres: 4 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): standard care Concomitant therapy: no |
| Outcomes | Primary study outcome
|
| Notes | Recruitment status: completed Prospective completion date: no information Date last update was posted: 7 November 2020 Sponsor/funding: Teheran University of Medical Sciences |
ISRCTN33037282.
| Methods | Trial design: open‐label, RCT Sample size: 680 Setting: inpatient Language: English Number of centres: 3 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: per‐protocol methylprednisolone administration and tapering
Treatment details of control group: per‐protocol dexamethasone administration
Concomitant therapy: no |
| Outcomes | Primary study outcome
|
| Notes | Recruitment status: completed Prospective completion date: 30 April 2021 Date last update was posted: 19 November 2020 Sponsor/funding: University of Trieste |
Munch 2021.
| Methods | Trial design: quadruple blinded, multi‐centre RCT Sample size: 1000 Setting: inpatient Language: English Number of centres: 14 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration):
Concomitant therapy: no information |
| Outcomes | Primary study outcome: days alive without life support (i.e. IMV, circulatory support or renal replacement therapy) from randomisation to day 28 |
| Notes | Recruitment status: active, not recruiting Prospective completion date: 8 June 2021 Date last update was posted: 9 March 2021 Sponsor/funding: Scandinavian Critical Care Trials Group |
NCT03852537.
| Methods | Trial design: double‐blinded RCT Sample size: 44 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): usual care as determined by the patients treatment team. Concomitant therapy: no information |
| Outcomes | Primary study outcome: fasibility of the timely initiation of corticosteroids and implementation of biomarker‐titrated corticosteroid dosing: percentage of eligible patients adhered to the timely initiation (time frame: within 30 days of enrolment in study) |
| Notes | Recruitment status: completed Prospective completion date: 15 March 2021 Date last update was posted: 13 April 2021 Sponsor/funding: Mayo Clinic |
NCT04244591.
| Methods | Trial design: randomised, open‐label Sample size: 80 Setting: inpatient Language: English Number of centres: multicenter Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria:
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): standard care Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Notes | Recruitment status: completed Prospective completion date: 13 April 2020 Date last update was posted: 13 April 2020 Sponsor/funding: Peking Union Medical College Hospital |
NCT04325061.
| Methods | Trial design: open‐label RCT Sample size: 19 Setting: inpatient Language: English Number of centres: multicenter Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): patients will be treated with standard intensive care Concomitant therapy: no information |
| Outcomes | Primary study outcome: all‐cause mortality at 60 days after enrolment |
| Notes | Recruitment status: terminated (lack of enrolment) Prospective completion date: June 2020 Date last update was posted: February 2021 Sponsor/funding: Dr. Negrin University Hospital |
NCT04746430.
| Methods | Trial design: open‐label RCT Sample size: 2000 Setting: outpatient Language: Dutch, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): only remote monitoring Concomitant therapy: no information |
| Outcomes | Primary outcome: time to first hospital admission or death (time frame: 28 days) |
| Notes | Recruitment status: terminated (too few patients) Prospective completion date: March 2022 Date last update was posted: 5 April 2021 Sponsor/funding: General Practitioners Research Institute |
Rashad 2021.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary study outcome: proportion of participants with Overall Survival at 14 days |
| Notes | Recruitment status: preprint published; unclear methodology; waiting for published fulltext Prospective completion date: August 2020 Date last update was posted: August 2020 Sponsor/funding: South Valley University |
ALT: alanine transaminase; ARDS: acute respiratory distress syndrome; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; CPK: creatine phsphokinase; CRP: C‐reactive protein; CT: computed tomography; FiO2: fraction of inspired oxygen; HFNC: high‐flow nasal cannula; ICU: intensive care unit; IMV: invasive mechanical ventilation; IQR: interquartile range: IV: intravenous; LDH: lactate dehydrogenase; NPPV: non‐invase positive pressure ventilation; PaO2: partial pressure of oxygen; PEEP: positive end‐expiratory pressure; RCT: randomised controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; SpO2: blood oxygen saturation
Characteristics of ongoing studies [ordered by study ID]
ChiCTR2000029386.
| Study name | Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomised controlled trial |
| Methods | Trial design: open‐label RCT Sample size: 48 Setting: inpatient Language: Chinese, English Number of centres: single centre Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): no glucocorticoid use Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Starting date | No information |
| Contact information | Dr. Yao‐Kai Chen, Division of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing 400036, China E‐Mail: yaokaichen@hotmail.com |
| Notes | Recruitment status: recruiting Prospective completion date: unclear Date last update was posted: 5 May 2020 Sponsor/funding: Chongqing Special Research Project for Prevention and Control of Novel Coronavirus Pneumonia |
ChiCTR2000029656.
| Study name | A randomised, open‐label study to evaluate the efficacy and safety of low‐dose corticosteroids in hospitalised patients with novel coronavirus pneumonia (COVID‐19) |
| Methods | Trial design: randomised, open‐label Sample size: 100 Setting: inpatient Language: Chinese, English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): standard treatment Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Starting date | 14 February 2020 |
| Contact information | Ronghui Du, +86 15337110926, bluesearh006@sina.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: 14 April 2020 Date last update was posted: 12 February 2020 Sponsor/funding: Wuhan Pulmonary Hospital |
ChiCTR2000030481.
| Study name | The clinical value of corticosteroid therapy timing in the treatment of novel coronavirus pneumonia (COVID‐19): a prospective randomised controlled trial |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): no corticosteroid Concomitant therapy: no information |
| Outcomes | Primary study outcome: the time of duration of COVID‐19 nucleic acid RT‐PCR test results of respiratory specimens (such as throat swabs) or blood specimens change to negative. Among secondary outcomes: 21‐day all‐cause mortality |
| Starting date | 1 March 2020 |
| Contact information | Chen Zhenshun, +86 13627288300, chzs1990@163.com |
| Notes | Recruitment status: recruiting Prospective completion date: 30 April 2020 Date last update was posted: 3 March 2020 Sponsor/funding: Science and Technology Department of Hubei Province |
CTRI/2020/07/026608.
| Study name | A clinical trial to study the effects of two drugs methylprednisolone and dexamethasone in patients with severe COVID‐19 |
| Methods | Trial design: randomised, parallel group trial Sample size: 40 Setting: inpatient Language: English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): injection dexamethasone 6 mg IV once a day for 3 days Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Starting date | 27 July 2020 |
| Contact information | Prof V R Mohan Rao , India, 9841210011, medicinehod@chettinadhealthcity.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: estimated duration of trial 3 months Date last update was posted: 15 July 2020 Sponsor/funding: Chettinad Hospital and Research Institute Kelambakkam, Dr Ananthakumar PK |
CTRI/2020/10/028731.
| Study name | Higher vs. lower doses of steroids in patients with COVID‐19 |
| Methods | Trial design: randomised, parallel group trial Sample size: 1500 Setting: inpatient Language: English Number of centres: 3 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: dexamethasone
Treatment details of control group (e.g dose, route of administration): dexamethasone 6 mg, IV, up to 10 days from randomisation or until hospital discharge or death, frequency: once daily Concomitant therapy: no |
| Outcomes | Primary outcome: days alive without life support (i.e. IMV, circulatory support or renal replacement therapy) from randomisation (day 28) |
| Starting date | 1 November 2020 |
| Contact information | Dr Vivekanand Jha; vjha@georgeinstitute.org.in |
| Notes | Recruitment status: recruiting Prospective completion date: estimated duration of trial 1 year Date last update was posted: no information Sponsor/funding: Professor Anders Perner, Senior Staff specialist and professor in Intensive Care Medicine Dept of Intensive Care, Rigshospitalet |
CTRI/2020/12/029894.
| Study name | Comparing the effectiveness of dexamethasone versus methylprednisolone in patients with moderate COVID 19 ‐ a randomised controlled trial |
| Methods | Trial design: open‐label, RCT Sample size: 50 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: dexamethasone
Treatment details of control group (e.g dose, route of administration): methylprednisolone 32 mg/60 mg intravenous, 6 days Concomitant therapy: no |
| Outcomes | Primary outcome: mortality during hospital stay (day 1‐day 7) |
| Starting date | No information |
| Contact information | DR R Nivetha, Department of General Medicine 1st floor SRM Medical College Hospital and Research Centre SRM University Potheri Kattankulathur, India nivethamdr@gmail.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: estimated duration 6 months Date last update was posted: no information Sponsor/funding: SRM Medical College Hospital and Research Centre |
CTRI/2020/12/030143.
| Study name | Evaluation of different steroid regimes in critically ill adult patients of COVID‐19 admitted to intensive care units |
| Methods | Trial design: Sample size: 500 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: dexamethasone
Treatment details of control group (e.g dose, route of administration): methylprednisolone, 1 to 2 mg/kg body weight, IV, frequency: daily for 10 days Concomitant therapy: no |
| Outcomes | Primary outcome: 28‐day mortality |
| Starting date | No information |
| Contact information | Dr Sukhyanti Kerai, Maulana Azad Medical College New Delhi, India drsukhi25@gmail.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: estimated duration 6 months Date last update was posted: no information Sponsor/funding: Maulana Azad Medical College and associated Lok Nayak Hospital |
EUCTR2020‐001413‐20‐ES.
| Study name | Efficacy and safety of siltuximab vs. corticosteroids in hospitalised patients with COVID‐19 pneumonia |
| Methods | Trial design: phase 2, randomised, open‐label Sample size: 100 Setting: inpatient Language: Spanish, English Number of centres: single‐centre Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: siltuximab
Treatment details of control group (e.g dose, route of administration): methylprednisolone 250 mg/kg Concomitant therapy: no information |
| Outcomes | Primary study outcome: proportion of patients requiring ICU admission at any time within the study period (time frame 29 days). |
| Starting date | No information |
| Contact information | Felipe García, +349322754002884, fgarcia@clinic.cat |
| Notes | Recruitment status: temporarily halted Prospective completion date: prospective duration of trial 45 days Date last update was posted: no information Sponsor/funding: Fundació Clínic per a la Recerca Biomèdica |
EUCTR2020‐001457‐43‐FR.
| Study name | Dexamethasone and oxygen support strategies in ICU patients with COVID‐19 pneumonia |
| Methods | Trial design: double‐blind (Phase III) RCT Sample size: 550 Setting: inpatient Language: French, English Number of centres: 12 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
For patients without mechanical ventilation other exclusion criteria are:
|
| Interventions | Details of intervention: dexamethasone
Treatment details of control group (e.g dose, route of administration): placebo, IV Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Starting date | No information |
| Contact information | fadila.amerali@aphp.fr |
| Notes | Recruitment status: ongoing Prospective completion date: October 2020 Date last update was posted: 2 April 2020 Sponsor/funding: APHP (An ancillary study CACAO (Covidicus air contamination) will be performed in 4 centers aiming at assessing the environmental contamination by SARS‐CoV‐2 according to the oxygen support modality. Additional funding will be searched for these analyses (submitted for ANR call) |
EUCTR2020‐001622‐64‐ES.
| Study name | Outpatient treatment of COVID‐19 with early pulmonary corticosteroids as an opportunity to modify the course of the disease (TAC‐COVID‐19) |
| Methods | Trial design: open (phase IV) RCT Sample size: 200 Setting: outpatient Language: Spanish, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: prednisone
Treatment details of control group (e.g dose, route of administration): concomitant therapy Concomitant therapy: symptomatic treatment + hydroxychloroquine + azithromycin |
| Outcomes | Primary study outcome: the aim of this study is to explore the effectiveness and safety of oral corticosteroids (prednisone) in the treatment of early stage SARS‐Cov‐2 pneumonia in patients who do not yet meet hospital admission criteria: admission after 30 days. |
| Starting date | 19 April 2020 |
| Contact information | María Jesús Coma 0034610620180, mjcoma@hubu.es |
| Notes | Recruitment status: ongoing Prospective completion date: 3‐month estimated duration of trial Date last update was posted: 20 April 2020 Sponsor/funding: Dra Ana Pueyo Bastida, Hospital Universitario de Burgos |
EUCTR2020‐001707‐16‐ES.
| Study name | Outpatient treatment of COVID‐19 with early pulmonary corticosteroids as an opportunity to modify the course of the disease (TOCICOVID) |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: tocilizumab
Treatment details of control group (e.g dose, route of administration): methylprednisolone, 331 mg, IV Concomitant therapy: no information |
| Outcomes | Primary study outcome: respiratory situation at 24 hours, 3 and 7 days based on PaO2 / FiO2 ratio that graduates respiratory distress from mild (200‐300), moderate (100‐200) and severe (< 100). In addition, it will include: presence of dyspnea and grade according to the New York Health Association (NYHA) scale, presence of respiratory work and respiratory rate (FR) |
| Starting date | 22 July 2020 |
| Contact information | IIS BIODONOSTIA 943006288 |
| Notes | Recruitment status: ongoing Prospective completion date: 20‐month estimated duration of trial Date last update was posted: 20 April 2020 Sponsor/funding: IIS BIODONOSTIA |
EUCTR2020‐001921‐30.
| Study name | Steroids and unfractionated heparin in critically‐ill patients with pneumonia from COVID‐19 infection. A multicenter, interventional, randomised, three arms study design |
| Methods | Trial design: open RCT Sample size: 200 Setting: inpatient Language: Italian, English Number of centres: 9 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: methylprednisolone + unfractionated heparin (Eparina)
Treatment details of control group (e.g dose, route of administration): heparin subcutaneous (25,000 international units) Concomitant therapy: no information |
| Outcomes | Primary study outcome: all‐cause mortality at day 28 |
| Starting date | 14 May 2020 |
| Contact information | Clinical Trials Quality Team, 0594225868, mighali.pasquale@aou.mo.it |
| Notes | Recruitment status: ongoing Prospective completion date: 1 year later, so May 2021 Date last update was posted: 26 June 2020 Sponsor/funding: AZIENDA OSPEDALIERO‐UNIVERSITARIA POLICLINICO DI MODENA |
EUCTR2020‐002186‐34‐ES.
| Study name | Efficacy of the early use of corticotherapy in CoV‐2 infection to prevent the progression of acute respiratory distress syndrome (ARDS) in COVID‐19 |
| Methods | Trial design: open‐label, randomised trial Sample size: 100 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention: methylprednisolone
Treatment details of control group (e.g dose, route of administration): standard care Concomitant therapy: no |
| Outcomes | Primary outcome: incidence of a combined variable made up of the variables death, ICU admission, non‐IMV or need for high‐flow oxygen therapy (defined as SaFi <200 with FiO2 ≥ 50%) (day 90) |
| Starting date | No information |
| Contact information | Ferran Martínez Valle, Fundació Hospital Universitari Vall d'Hebron ‐ Institut de Recerca (VHIR) |
| Notes | Recruitment status: ongoing Prospective completion date: estimated duration 1 year Date last update was posted: no information Sponsor/funding: Fundació Hospital Universitari Vall d'Hebron ‐ Institut de Recerca (VHIR) |
EUCTR2020‐003363‐25‐DK.
| Study name | Higher vs. lower doses of dexamethasone in patients with COVID‐19 and severe hypoxia: the COVID STEROID 2 trial |
| Methods | Trial design: double‐blinded RCT Sample size: 1000 Setting: inpatient Language: English Number of centres: 36 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): dexamethasone 6 mg, IV Concomitant therapy: no information |
| Outcomes | Primary study outcome: days alive without life support (i.e.IMV, circulatory support or renal replacement therapy) from randomisation to day 28 |
| Starting date | 18 August 2020 |
| Contact information | Department of intensive care, Ringshospitalet +4535457237, covid‐steroid@cric.nu |
| Notes | Recruitment status: ongoing Prospective completion date: 18‐month duration planned Date last update was posted: 18 August 2020 Sponsor/funding: Department of Intensive Care, Rigshospitalet, Novo Nordisk Foundation |
EUCTR2020‐004323‐16.
| Study name | A randomised, multi‐centre, double‐blind study to evaluate the efficacy of high‐dose administration of methylprednisolone in addition to standard treatment, in SARS‐CoV2 (COVID‐19) pneumonia patients |
| Methods | Trial design: randomised, multi‐centre, double‐blind study Sample size: 260 Setting: inpatient Language: Italian, English Number of centres: 5 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): placebo, IV Concomitant therapy: no information |
| Outcomes | Primary study outcome: length of hospitalisation, calculated as the interval between randomisation and discharge from the hospital without the need for supplemental oxygen |
| Starting date | 25 November 2020 |
| Contact information | Massimo Costantini, 3355477208, massimo.costantini@ausl.re.it |
| Notes | Recruitment status: ongoing Prospective completion date: 4‐month trial duration planned Date last update was posted: 25 November 2020 Sponsor/funding: AZIENDA OSPEDALIERA ARCISPEDALE SANTA MARIA NUOVA/IRCCS DI REGGIO EMILIA |
NCT04329650.
| Study name | Efficacy and safety of siltuximab vs. corticosteroids in hospitalised patients with COVID‐19 pneumonia |
| Methods | Trial design: phase 2, randomised, open‐label Sample size: 200 Setting: inpatient Language: Spanish, English Number of centres: 4 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration)
Concomitant therapy: no information |
| Outcomes | Primary study outcome: proportion of patients requiring ICU admission at any time within the study period (time frame: 29 days) |
| Starting date | |
| Contact information | Contact: Felipe García, MD+34932275400 ext 2884 NCT04329650, SILCOR‐COVID‐19, Efficacy and Safety of Siltuximab vs. Corticosteroids in Hospitalized Patients With COVID‐19 Pneumonia" type="EXTERNAL">fgarcia@clinic.ca |
| Notes | Recruitment status: recruiting Prospective completion date: 20 May 2020 Date last update was posted: 17 April 2020 Sponsor/funding: Judit Pich Martínez |
NCT04344730.
| Study name | Dexamethasone and oxygen support strategies in ICU patients with COVID‐19 pneumonia (COVIDICUS) |
| Methods | Trial design: quadruple‐masked RCT Sample size: 550 Setting: inpatient Language: French, English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): placebo Concomitant therapy: study stratified according to subgroups (only oxygen therapy, CPAP, HFNC, IMV) |
| Outcomes | Primary study outcome
|
| Starting date | 10 April 2020 |
| Contact information | Jean François TIMSIT, Pr |
| Notes | Recruitment status: active, not recruiting Prospective completion date: 31 December 2021 Date last update was posted: 9 February 2021 Sponsor/funding: Assistance Publique ‐ Hôpitaux de Paris |
NCT04345445.
| Study name | Study to evaluate the efficacy and safety of tocilizumab versus corticosteroids in hospitalised COVID‐19 patients with high risk of progression |
| Methods | Trial design: open‐label, randomised, cross‐over interventional study Sample size: 310 Setting: inpatient Language: English Number of centres: 4 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): methylprednisolone 120 mg/day for 3 days IV Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Starting date | 14 April 2020 |
| Contact information | Adeeba Kamarulzaman, MBBS+603‐79492050 NCT04345445, TVCS‐COVID19, Study to Evaluate the Efficacy and Safety of Tocilizumab Versus Corticosteroids in Hospitalised COVID‐19 Patients With High Risk of Progression" type="EXTERNAL">adeeba@um.edu.my |
| Notes | Recruitment status: not yet recruiting Prospective completion date: 31 October 2020 Date last update was posted: 14 April 2020 Sponsor/funding: University of Malaya |
NCT04347980.
| Study name | Dexamethasone treatment for severe acute respiratory distress syndrome induced by COVID‐19 (DHYSCO) |
| Methods | Trial design: single‐blinded (participants) RCT Sample size: 122 Setting: inpatient Language: French, English Number of centres: multi‐centre, no concrete information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): 200 mg x 3/d enterally from J1 of the hydroxychloroquine for 10 days. If the patient is extubated before the 10th day, he will receive his last dose of hydroxychloroquine before. Concomitant therapy: no information |
| Outcomes | Primary study outcome: day‐28 mortality |
| Starting date | April 2020 |
| Contact information | Francois STEPHAN, MD, PhD33140948580 NCT04347980, 2037815010, Dexamethasone Treatment for Severe Acute Respiratory Distress Syndrome Induced by COVID‐19" type="EXTERNAL">f.stephan@hml.fr |
| Notes | Recruitment status: recruiting Prospective completion date: August 2020 Date last update was posted: 17 April 2020 Sponsor/funding: Centre Chirurgical Marie Lannelongue |
NCT04377503.
| Study name | Tocilizumab versus methylprednisolone in the cytokine release syndrome of patients with COVID‐19 |
| Methods | Trial design: phase II trial (open‐label) RCT Sample size: 40 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): methylprednisolone at a dose of 1.5 mg/kg/d divided into 2 daily doses for 7 days. Then 1 mg/kg/day for another 7 days in 2 daily doses. Finally 0.5 mg/kg/d for another 7 days Concomitant therapy: no information |
| Outcomes | Primary outcome: patient clinical status 15 days after randomisation on a 7‐category ordinal scale (time frame: 15 days after randomisation) |
| Starting date | No information |
| Contact information | Jose A Azevedo, MD, PhD+559832168110 NCT04377503, covid‐19 hsd, Tocilizumab Versus Methylprednisolone in the Cytokine Release Syndrome of Patients With COVID‐19" type="EXTERNAL">jrazevedo47@gmail.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: no information Date last update was posted: 6 May 020 Sponsor/funding: Hospital Sao Domingos |
NCT04395105.
| Study name | Dexamethasone for COVID‐19 related ARDS: a multicenter, randomised clinical trial |
| Methods | Trial design: multi‐centre, open‐label RCT Sample size: 284 Setting: inpatient Language: Spanish, English Number of centres: 3 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): usual treatment without using up to 6 mg /d of dexamethasone for 10 days Concomitant therapy: no information |
| Outcomes | Primary outcome
|
| Starting date | 21 May 2020 |
| Contact information | Contact: Pablo O Rodriguez, MD+541152990100 ext 4307 NCT04395105, 1264, Dexamethasone for COVID‐19 Related ARDS: a Multicenter, Randomized Clinical Trial" type="EXTERNAL">prodriguez@cemic.edu.ar |
| Notes | Recruitment status: recruiting Prospective completion date: 31 January 2021 Date last update was posted: 20 July 2020 Sponsor/funding: Centro de Educación Medica e Investigaciones Clínicas Norberto Quirno |
NCT04438980.
| Study name | Glucocorticoids in COVID‐19 (CORTIVID) |
| Methods | Trial design: double‐blinded RCT Sample size: 72 Setting: inpatient Language: Spanish, English Number of centres: 2 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
|
| Outcomes | Primary outcome
|
| Starting date | 28 May 2020 recruitment start |
| Contact information | Iñigo Les Bujanda, PhD0034636346833 NCT04438980, CORTIVID, Glucocorticoids in COVID‐19 (CORTIVID)" type="EXTERNAL">ilesbujanda@gmail.com |
| Notes | Recruitment status: recruiting Prospective completion date: February 2021 Date last update was posted: 22 July 2020 Sponsor/funding: Fundacion Miguel Servet |
NCT04451174.
| Study name | Early use of corticosteroids in non‐critical patients with COVID‐19 pneumonia (PREDCOVID) |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): no intervention Concomitant therapy: no information |
| Outcomes | Primary outcome
|
| Starting date | 23 June 2020 |
| Contact information | Mauricio Salinas, MD+56 9 98254095 NCT04451174, 2092, Early Use of Corticosteroids in Non‐critical Patients With COVID‐19 Pneumonia" type="EXTERNAL">mrsalinas@uchile.cl |
| Notes | Recruitment status: recruiting Prospective completion date: 3 December 2020 Date last update was posted: 20 October 2020 Sponsor/funding: University of Chile |
NCT04452565.
| Study name | NA‐831, atazanavir and dexamethasone combination therapy for the treatment of COVID‐19 infection (NATADEX) |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
|
| Starting date | First Posted: 30 June 2020 |
| Contact information | Brian Tran, MD1‐415‐941‐3133 NCT04452565, NATADEX, NA‐831, Atazanavir and Dexamethasone Combination Therapy for the Treatment of COVID‐19 Infection" type="EXTERNAL">BTran@neuroactiva.com |
| Notes | Recruitment status: recruiting Prospective completion date: 15 February 2021 Date last update was posted: 7 September 2020 Sponsor/funding: NeuroActiva, Inc. |
NCT04485429.
| Study name | Efficacy assessment of methylprednisolone and heparin in patients with COVID‐19 pneumonia |
| Methods | Trial design: open‐label RCT Sample size: 268 Setting: inpatient Language: English Number of centres: 1 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome: rate of IMV (time Frame: 28 days) |
| Starting date | |
| Contact information | Eduardo M Rego, MD, PhD55 16 981110090 NCT04485429, 31180820600005249, Efficacy Assessment of Methylprednisolone and Heparin in Patients With COVID‐19 Pneumonia" type="EXTERNAL">edumrego@hotmail.com |
| Notes | Recruitment status: recruiting Prospective completion date: 13 December 2020 Date last update was posted: 24 July 2020 Sponsor/funding: D'Or Institute for Research and Education |
NCT04499313.
| Study name | Dexamethasone versus methylprednisolone for the treatment of patients with ARDS caused by COVID‐19 |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): methylprednisolone sodium succinate at a dose of 0.5 mg/kg (injectable solution) Concomitant therapy: no information |
| Outcomes | Primary outcome
|
| Starting date | 2 August 2020 |
| Contact information | Abu Taiub Mohammed Mohiuddin Chowdhury, MBBS, MD008801817711079 NCT04499313, 10000753, Dexamethasone Vs Methylprednisolone for the Treatment of Patients With ARDS Caused by COVID‐19" type="EXTERNAL">dr_mohiuddinchy@yahoo.com |
| Notes | Recruitment status: recruiting Prospective completion date: 30 November 2020 Date last update was posted: 18 August 2020 Sponsor/funding: Chattogram General Hospital |
NCT04509973.
| Study name | Higher vs. lower doses of dexamethasone for COVID‐19 and severe hypoxia (COVIDSTEROID2) |
| Methods | Trial design: quadruple‐blinded, multi‐centre, clinical RCT Sample size: 1000 Setting: inpatient Language: Swedish, Danish, English Number of centres: 53 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): dexamethasone 6 mg once daily in addition to standard care for up to 10 days. We will allow the use of betamethasone 6 mg at sites, where dexamethasone is not available. Concomitant therapy: no information |
| Outcomes | Primary outcome: days alive without life support (i.e. IMV, circulatory support or renal replacement therapy) from randomisation to day 28 |
| Starting date | 27 August 2020 |
| Contact information | Anders Perner, MD, PhD, Professor+4535458333 NCT04509973, RH‐ITA‐009, Higher vs. Lower Doses of Dexamethasone for COVID‐19 and Severe Hypoxia" type="EXTERNAL">anders.perner@regionh.dk |
| Notes | Recruitment status: recruiting Prospective completion date: 17 February 2022 Date last update was posted: 1 September 2020 Sponsor/funding: Scandinavian Critical Care Trials Group |
NCT04513184.
| Study name | Randomised clinical trial of intranasal dexamethasone as an adjuvant in patients with COVID‐19 |
| Methods | Trial design: multicenter, double‐masked (participant, care provider), RCT Sample size: 60 Setting: inpatient Language: Spanish, English Number of centres: 3 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): nasal dexamethasone 0.12 mg/kg/daily for 3 days from day 1, followed by 0.06 mg/kg/daily from day 4‐10 after randomisation Concomitant therapy: no information |
| Outcomes | Primary outcome
|
| Starting date | 14 July 2020 |
| Contact information | Graciela A Cárdenas‐Hernández, PhD+525556063822 ext 2012 NCT04513184, DI/20/407/04/36, Randomized Clinical Trial of Intranasal Dexamethasone as an Adjuvant in Patients With COVID‐19" type="EXTERNAL">gracielacardenas@yahoo.com.mx |
| Notes | Recruitment status: recruiting Prospective completion date: primary completion date 30 March 2021 estimated study completion date 31 July 2021 Date last update was posted: 12 November 2020 Sponsor/funding: Edda Sciutto Conde |
NCT04528329.
| Study name | Anosmia and / or ageusia in COVID‐19: timeline, treatment with early corticosteroid and recovery |
| Methods | Trial design: open‐label RCT Sample size: 300 Setting: no information Language: Arabic, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): dexamethasone is to be used lately upon the deterioration of cases Concomitant therapy: no information |
| Outcomes | Time to recovery (time frame: 1‐6 weeks) from anosmia and/or ageusia |
| Starting date | 30 August 2020 |
| Contact information | Emad R Issak, MD01272228989 NCT04528329, PR0013, Anosmia and / or Ageusia and Early Corticosteroid Use" type="EXTERNAL">dr.emad.r.h.issak@gmail.com |
| Notes | Recruitment status: recruiting Prospective completion date: 15 April 2021 Date last update was posted: 29 March 2021 Sponsor/funding: ClinAmygate |
NCT04528888.
| Study name | Steroids and unfractionated heparin in critically ill patients with pneumonia from COVID‐19 infection (STAUNCH‐19) |
| Methods | Trial design: multicenter, national, interventional, randomised, open‐label Sample size: 210 Setting: inpatient Language: Italian, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome: all‐cause mortality at day 28, defined as the comparison of proportions of patients' death for any cause at day 28 from randomisation |
| Starting date | 1 September 2020 |
| Contact information | Massimo Girardis, PD0594225878 ext 0039 NCT04528888, Staunch‐19‐1.1‐26‐04‐20, Steroids and Unfractionated Heparin in Critically Ill Patients With Pneumonia From COVID‐19 Infection" type="EXTERNAL">massimo.girardis@unimore.it |
| Notes | Recruitment status: recruiting Prospective completion date: 30 July 2021 Date last update was posted: 27 August 2020 Sponsor/funding: Massimo Girardis, University of Modena and Reggio Emilia |
NCT04530409.
| Study name | Timing of corticosteroids in COVID‐19 |
| Methods | Trial design: open‐label RCT Sample size: 450 Setting: no information Language: Arabic, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): dexamethasone is to be used upon the deterioration of cases Concomitant therapy: no information |
| Outcomes | Primary outcome
|
| Starting date | |
| Contact information | Emad R Issak, MD01272228989NCT04530409, PR0012, Timing of Corticosteroids in COVID‐19" type="EXTERNAL">dr.emad.r.h.issak@gmail.com |
| Notes | Recruitment status: recruiting Prospective completion date: estimated primary completion date 1 April 2021; Estimated study completion date: 1 May 2021 Date last update was posted: 16 February 2021 Sponsor/funding: ClinAmygate |
NCT04545242.
| Study name | Efficacy of dexamethasone in patients with acute hypoxemic respiratory failure caused by infections (DEXA‐REFINE) |
| Methods | Trial design: multicenter, open‐label, clinical RCT Sample size: 980 Setting: inpatient Language: English Number of centres: 40 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): dexamethasone: 20 mg/IV/ daily from day of randomisation (day 1) during 5 days, followed by 10 mg/IV/ daily from day 6‐10 of randomisation Concomitant therapy: no information |
| Outcomes | Primary outcome: all‐cause mortality at 60 days after randomisation |
| Starting date | 8 February 2021 |
| Contact information | Jesús Villar, MD+34606860027 NCT04545242, ICI20‐00062, Efficacy of DEXamethasone in Patients With Acute Hypoxemic REspiratory Failure Caused by INfEctions" type="EXTERNAL">jesus.villar54@gmail.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: 30 December 2023 Date last update was posted: 22 January 2021 Sponsor/funding: Dr. Negrin University Hospital |
NCT04636671.
| Study name | Methylprednisolone vs. dexamethasone in COVID‐19 pneumonia (MEDEAS RCT) (MEDEAS) |
| Methods | Trial design: open‐label RCT Sample size: 680 Setting: inpatient Language: Italian, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): A. dexamethasone 6 mg IV in 30 min or orally from day 1‐10 or until hospital discharge (if sooner). B. After day 10 study treatment is interrupted. Concomitant therapy: no information |
| Outcomes | Primary outcome: survival proportion at 28 days in both arms |
| Starting date | 25 November 2020 |
| Contact information | Marco Confalonieri, MD+390403994667 NCT04636671, MEDEAS1, Methylprednisolone vs. Dexamethasone in COVID‐19 Pneumonia (MEDEAS RCT)" type="EXTERNAL">mconfalonieri@units.it |
| Notes | Recruitment status: recruiting Prospective completion date: estimated primary completion date 31 March 2021; estimated study completion date 30 April 2021 Date last update was posted: 19 November 2020 Sponsor/funding: University of Trieste and Centro di Riferimento Oncologico ‐ Aviano and National Institute for the Infectious Diseases (L. Spallanzani) ‐ Rome |
NCT04663555.
| Study name | Effect of two different doses of dexamethasone in patients with ARDS and COVID‐19 (REMED) |
| Methods | Trial design: prospective, phase II, open‐label, RCT Sample size: 300 Setting: inpatient Language: Czech, English Number of centres: 11 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): dexamethasone 6 mg day 1‐10 Concomitant therapy: no information |
| Outcomes | Primary outcome: number of ventilator‐free days at 28 days after randomisation, defined as being alive and free from mechanical ventilation (> 48 h) |
| Starting date | |
| Contact information | Jan Maláska, MD, PhD, EDIC+420723784101 NCT04663555, CZECRIN No. 2020/47, Effect of Two Different Doses of Dexamethasone in Patients With ARDS and COVID‐19" type="EXTERNAL">jan.malaska@gmail.com |
| Notes | Recruitment status: recruiting Prospective completion date: 31 March 2021 Date last update was posted: 4 February 2021 Sponsor/funding: Brno University Hospital |
NCT04673162.
| Study name | Evaluation of the efficacy of high doses of methylprednisolone in SARS‐CoV2 (COVID‐19) pneumonia patients |
| Methods | Trial design: quadruple‐blind, multicentric, randomised study Sample size: 260 Setting: inpatient Language: Italian, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): standard treatment (currently dexamethasone 6 mg/daily for 10 days) plus placebo Concomitant therapy: no information |
| Outcomes | Primary study outcome
|
| Starting date | |
| Contact information | Massimo Costantini, MD+390522296986 NCT04673162, RCT‐MP‐COVID‐19, Evaluation of the Efficacy of High Doses of Methylprednisolone in SARS‐CoV2 ( COVID‐19) Pneumonia Patients" type="EXTERNAL">massimo.costantini@ausl.re.it |
| Notes | Recruitment status: not yet recruiting Prospective completion date: estimated primary completion date April 2021; estimated study completion date June 2021 Date last update was posted: 17 December 2020 Sponsor/funding: Azienda Unità Sanitaria Locale Reggio Emilia |
NCT04707534.
| Study name | Dexamethasone for COVID‐19 |
| Methods | Trial design: open‐label RCT Sample size: 300 Setting: inpatient Language: English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): dexamethasone 6 mg daily for 10 days Concomitant therapy: no information |
| Outcomes | Primary outcome: 8‐scale World Health Organisation ordinal scale at day 28 |
| Starting date | 21 January 2021 |
| Contact information | Huimin Wu, MD MPH(405) 271‐6173 NCT04707534, 12927, Dexamethasone for COVID‐19" type="EXTERNAL">Huimin‐Wu@ouhsc.edu |
| Notes | Recruitment status: recruiting Prospective completion date: 21 June 2021 Date last update was posted: 8 January 2021 Sponsor/funding: University of Oklahoma |
NCT04726098.
| Study name | Low or high dose of dexamethasone in patients with respiratory failure by COVID‐19 (HIGHLOWDEXA) |
| Methods | Trial design: open‐label RCT Sample size: 198 Setting: inpatient Language: Spanish, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): dexamethasone 20 mg/day for 5 days + dexamethasone 10 mg/day for 5 days (total 10 days) Concomitant therapy: no information |
| Outcomes | Primary outcome: percentage of patients with treatment failure at day 11 defined as death, need of ICU and ECMO, need of NIV or nasal high‐flow oxygen therapy, or worsening of the clinical condition of the patient during treatment (2 of these: need to increase: fraction of inspired oxygen inspired > 20%, need for fraction inspired oxygenation > 50%, increase in respiratory rate > 25, increase in inflammatory markers) |
| Starting date | 15 January 2021 |
| Contact information | Manuel Taboada Muñiz, Ph.D.+34678195618 NCT04726098, HIGHLOWDEXA‐COVID, Low or High Dose of Dexamethasone in Patients With Respiratory Failure by COVID‐19" type="EXTERNAL">manutabo@yahoo.es |
| Notes | Recruitment status: recruiting Prospective completion date: estimated primary completion date 30 June 2021; estimated study completion date 31 December 2022 Date last update was posted: 27 January 2021 Sponsor/funding: Manuel Taboada Muñiz |
NCT04765371.
| Study name | Comparison between prednisolone and dexamethasone on mortality in patients on oxygen therapy, with COVID‐19 (COPreDex) |
| Methods | Trial design: open‐label RCT Sample size: 220 Setting: inpatients Language: French, English Number of centres: 6 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): 60 mg/d of prednisolone during 10 days Concomitant therapy: no information |
| Outcomes | Primary outcome: mortality assessment at day 28 |
| Starting date | March 2021 |
| Contact information | Maryline Delattre 0033130754131 NCT04765371, CHRD1520, Comparison Between Prednisolone and Dexamethasone on Mortality in Patients on Oxygen Therapy, With CoViD‐19" type="EXTERNAL">maryline.delattre@ght‐novo.fr |
| Notes | Recruitment status: recruiting Prospective completion date: October 2021 Date last update was posted: 1 March 2021 Sponsor/funding: Centre Hospitalier René Dubos |
NCT04780581.
| Study name | Glucocorticoid therapy in coronavirus disease COVID‐19 patients |
| Methods | Trial design: open‐label RCT Sample size: 290 Setting: inpatient Language: Spanish, English Number of centres: 5 Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): high‐dose methylprednisolone bolus (250 mg/4 h ‐ 3 days) Concomitant therapy: no information |
| Outcomes | Primary outcome: mortality rate in COVID‐19 patients after high‐dose methylprednisolone bolus administration versus mortality rate intermediate‐dose dexamethasone pattern (time frame: 28 days) |
| Starting date | 1 February 2021 |
| Contact information | Luis Corral Gudino983 420400 NCT04780581, MP3‐pulses‐COVID‐19, Glucocorticoid Therapy in Coronavirus Disease COVID‐19 Patients" type="EXTERNAL">lcorral@saludcastillayleon.es |
| Notes | Recruitment status: recruiting Prospective completion date: 31 December 2021 Date last update was posted: 3 March 2021 Sponsor/funding: Fundación Instituto de Estudios de Ciencias de la Salud de Castilla y León |
NCT04795583.
| Study name | Corticosteroids for COVID‐19 (CORE‐COVID) |
| Methods | Trial design: interventional, randomised, placebo‐controlled, triple‐blinded, adaptive clinical trial Sample size: 1526 Setting: outpatient Language: English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): capsules with the same appearance as prednisone Concomitant therapy: no information |
| Outcomes | Primary outcome
|
| Starting date | April 2021 |
| Contact information | Carlos Cervera, MD, PhD780‐492‐5346 NCT04795583, 00001, Corticosteroids for COVID‐19" type="EXTERNAL">cerveraa@ualberta.ca |
| Notes | Recruitment status: not yet recruiting Prospective completion date: August 2022 Date last update was posted: 18 March 2021 Sponsor/funding: University of Alberta |
NCT04834375.
| Study name | Randomised open investigation determining steroid dose (ROIDS‐Dose) |
| Methods | Trial design: randomised, open‐label trial Sample size: 142 Setting: probably inpatient Language: English Number of centres: single centre Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g dose, route of administration): dexamethasone 0.2 mg/kg/d IV (maximum 20 mg daily) for 10 days Concomitant therapy: no information |
| Outcomes | Primary outcome: all‐cause mortality at 28 days |
| Starting date | 19 March 2021 |
| Contact information | Carlos X Rabascall, MD5164655400 NCT04834375, 21‐0171, Randomized Open Investigation Determining Steroid Dose" type="EXTERNAL">crabascallay@northwell.edu |
| Notes | Recruitment status: recruiting Prospective completion date: 19 April 2022 Date last update was posted: 8 April 2021 Sponsor/funding: Northwell Health |
NCT04836780.
| Study name | Dexamethasone early administration in hospitalised patients with COVID‐19 pneumonia (EARLYDEXCoV2) |
| Methods | Trial design: prospective, multicenter, phase‐4, parallel‐group, open‐label RCT Sample size: 126 Setting: inpatient Language: Spanish, English Number of centres: no information Type of intervention (treatment/prevention): treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention:
Treatment details of control group (e.g dose, route of administration): standard care therapy Concomitant therapy: no information |
| Outcomes | Primary outcome
|
| Starting date | Nno information |
| Contact information | Anabel Franco Moreno, MD, PhD +34 911 91 80 00 NCT04836780, EARLY‐DEX Covid‐19, DEXamethasone EARLY Administration in Hospitalized Patients With Covid‐19 Pneumonia" type="EXTERNAL">afranco278@hotmail.com |
| Notes | Recruitment status: not yet recruiting Prospective completion date: 30 June 2021 Date last update was posted: 8 April 2021 Sponsor/funding: Hospital Universitario Infanta Leonor |
AHRF: acute hypercapnic respiratory failure; ALT: alanine transaminase; APPT: activated partial thromboplastin time; ARDS: acute respiratory distress syndrome; AST: aspartate transaminase; CT: computed tomography; ECG: echocardiogram; ECMO: extracorporeal membrane oxygenation; FiO2: fraction of inspired oxygen; HFNC: high‐flow nasal cannula; HFNO: high‐flow nasal oxygen; ICU: intensive care unit; IMV: invasive mechanical ventilation; IV: intravenous; LMWH: low molecular weight heparin; NLR: neutrophil‐lymphocyte ratio; NPPV: non‐invase positive pressure ventilation; PCR: polymerase chain reaction; PEEP: positive end‐expiratory pressure; RCT: randomised controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; SC: subcutaneously; SpO2: blood oxygen saturation; ULN: upper limit of normal
Differences between protocol and review
Types of participants
For the different endpoints of interest and treatment settings, we excluded participants treated for symptoms of long‐COVID. Still, treatments for long‐COVID should soon be addressed outside this review.
Types of outcome measures
For heterogeneous reporting and high bias through death as competing risk, we omitted most endpoints with regard to clinical improvement and worsening, and length of hospital stay. As a compromise and because of their importance for the patients, or a rather easy ascertainment or strong implication for resource usage, we kept in the first publication of this living review: new need for invasive ventilation, liberation from invasive ventilation, ventilator‐free days, need for dialysis, viral clearance, quality of life/neurological outcome.
We counted adverse events regardless of their grades because the included studies did not report grades of adverse events.
If not specified otherwise, the observation period for outcomes other than mortality was the longest period available. Where this differed between arms of a trial, risk of bias assessment would be adjusted.
Types of intervention
After discussion with clinical experts we added comparisons to meet questions arising in daily routine:
dose comparisons
time comparisons (early versus late) but indirectly in terms of disease severity as described below
two different types of corticosteroids
corticosteroid versus another active substance (e.g. remdesivir, tocilizumab)
On the other hand, we excluded topical and inhaled steroids from this review because of inherently different pharmacokinetics and treatment settings. Still, their role is critical and should soon be addressed in another review.
Analysis
We made calculations with RevMan Web instead of RevMan 5.4 software.
We omitted subgroup analysis for treatment settings, that is, outpatient, inpatient, and intensive care unit, because triage criteria, definition of intensive care or high‐dependency units, and available resources were deemed too heterogeneous. Taking away a degree of indirectness, we instead performed subgroup analysis stratified by the level of respiratory support needed at randomisation. This allowed for the fact that levels of respiratory support can at least partially be delivered independent of the treatment setting and hence rendered a more valid conclusion about disease severity.
Contributions of authors
CW: screening, data extraction, risk of bias assessment, meta‐analysis, writing of the review, consulting with Cochrane Methodological Support, taking responsibility for reading and checking the review before submission
MG: data extraction, risk of bias assessment, meta‐analysis, conception and writing of the review, contacting corresponding authors for additional information, consulting with Cochrane Methodological Support, taking responsibility for reading and checking the review before submission
AM: extraction, clinical expertise, writing of the review, taking responsibility for reading and checking the review before submission
AMu: clinical expertise, writing of the review, taking responsibility for reading and checking the review before submission
MIM: design and conduct of searches, drafting of search methods section, taking responsibility for reading and checking the review before submission
ALF: extraction, characteristics of ongoing studies, writing of the review, taking responsibility for reading and checking the review before submission
MK: risk of bias assessment, writing of the review, taking responsibility for reading and checking the review before submission
MN: characteristics of studies awaiting classification, writing of the review, taking responsibility for reading and checking the review before submission
MS: clinical expertise, writing of the review, securing the funding, taking responsibility for reading and checking the review before submission
KK: extraction, taking responsibility for reading and checking the review before submission
NS: screening, methodological expertise and advice, conception and writing of the review, securing the funding, characteristics of studies awaiting classification, taking responsibility for reading and checking the review before submission
FF: clinical expertise, writing of the review, meta‐analysis, securing the funding, taking responsibility for reading and checking the review before submission
Sources of support
Internal sources
-
University Hospital of Cologne, Germany
Cochrane Cancer, Department I of Internal Medicine
-
Leipzig University Hospital, Germany
Department of Anaesthesiology and Intensive Care
External sources
-
Federal Ministry of Education and Research, Germany
NaFoUniMedCovid19“ (funding number: 01KX2021) part of the project „CEO‐Sys“
Declarations of interest
CW: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution).
MG: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution); works as a resident with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center; is member of the German Society for Anaesthesia and Intensive Care.
AM: works as a physician at the Department of Infectious Diseases and Respiratory Medicine at Charité University medicine Berlin; is a member of the German Society for Infectious Diseases; works in the office of STAKOB at Robert Koch‐Institut; coordinates the work of the specialist group COVRIIN.
AMu: none known.
MM: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution).
AF: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution) and works as a resident with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center.
MK: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution).
MN: works as a health professional; Leadership or other fiduciary role in other board, society, committee, or advocacy group; Published opinions in medical journals, the public press, broadcast and social media relevant to the interventions in the work.
MS: none known.
KK: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the project 'CEOSys', which was paid to the institution); works as an intensive care specialist with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center; is a member of the German Society for Anaesthesia and Intensive Care.
NS: none known.
FF: works as an intensive care consultant with the Department of Anaesthesiology and Intensive Care at the University of Leipzig Medical Center and is a member of the CEOsys project (no direct funding), the German Society for Anaesthesia and Intensive Care (DGAI), and the German Interdisciplinary Association for Intensive and Emergency Medicine (DIVI). Leading role in German guideline on respiratory failure and invasive mechanical ventilation.
contributed equally (first author)
contributed equally (first author)
contributed equally (last author)
contributed equally (last author)
New
References
References to studies included in this review
Angus 2020 {published data only}
- Angus DC, Berry S, Lewis RJ, Al-Beidh F, Arabi Y, Van Bentum-Puijk W, et al. The randomised embedded multifactorial adaptive platform for community-acquired pneumonia (REMAP-CAP) study: rationale and design. Annals of the American Thoracic Society 2020;17(7):879-91. [DOI: 10.1513/AnnalsATS.202003-192SD] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomised clinical trial. JAMA 2020;324(13):1317-29. [DOI: 10.1001/jama.2020.17022] [NCT02735707] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corral‐Gudino 2021 {published data only}
- Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, et al. Methylprednisolone in adults hospitalised with COVID-19 pneumonia: an open-label randomised trial (GLUCOCOVID). Wiener Klinische Wochenschrift 2021;133(7-8):303-11. [DOI: 10.1007/s00508-020-01805-8] [EUCTR2020-001934-37-ES] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral L, Bahamonde A, Arnaiz delas Revillas F, Gomez-Barquero J, Abadia-Otero J, Garcia-Ibarbia C, et al. GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalised with COVID-19 pneumonia. medRxiv [Preprint]. [DOI: 10.1101/2020.06.17.20133579] [EUCTR2020-001934-37-ES] [DOI]
Dequin 2020 {published data only}
- Dequin PF, Gouge AL, Tavernier E, Giraudeau B, Zohar S. Embedding a COVID-19 group sequential clinical trial within an ongoing trial: lessons from an unusual experience. Statistics in Biopharmaceutical Research 2020:1-5. [EMBASE: https://www.embase.com/search/results?subaction=viewrecord&id=L2005906375&from=export] [DOI] [PMC free article] [PubMed]
- Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomised clinical trial. JAMA 2020;324(13):1298-306. [DOI: 10.1001/jama.2020.16761] [NCT02517489] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edalatifard 2020 {published data only}
- Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. European Respiratory Journal 2020;56(6):1-13. [DOI: 10.1183/13993003.02808-2020] [IRCT20200404046947N1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farahani 2021 {published data only}
- Farahani RH, Mosaed R, Nezami-Asl A, Chamanara M, Soleiman-Meigooni S, Kalantar S, et al. Evaluation of the efficacy of methylprednisolone pulse therapy in treatment of COVID-19 adult patients with severe respiratory failure: randomised, clinical trial. Research Square 2021;Version 1:1-19. [DOI: 10.21203/rs.3.rs-66909/v1] [IRCT20200406046963N1] [DOI] [Google Scholar]
Horby 2021 {published data only}
- Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalised patients with COVID-19: preliminary report. medRxiv [Preprint] 2020. [DOI: ]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalised patients with COVID-19. New England Journal of Medicine 2021;384(8):693-704. [DOI: 10.1056/NEJMoa2021436] [NCT04381936] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford University. Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. University of Oxford 2020.
Jamaati 2021 {published data only}
- Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomised clinical trial. European Journal of Pharmacology 2021;897:173947. [DOI: 10.1016/j.ejphar.2021.173947] [IRCT20151227025726N17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeronimo 2020 {published data only}
- Jeronimo CM, Farias ME, Val FF, Sampaio VS, Alexandre MA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalised with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clinical Infectious Diseases 2020;72(9):e373–e81. [DOI: 10.1093/cid/ciaa1177] [NCT04343729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranjbar 2021 {published data only}
- Ranjbar K, Shahriarirad R, Erfani A, Khodamoradi Z, Saadi MHG, et al. Methylprednisolone or dexamethasone, which one is the superior corticosteroid in the treatment of hospitalised COVID-19 Patients: a triple-blinded randomised controlled trial. Research Square (Preprint). [DOI: ] [IRCT20200204046369N1]
Tang 2021 {published data only}
- Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomised control trial. Respiration 2021;100(2):116-26. [DOI: 10.1159/000512063] [NCT04273321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomazini 2020 {published data only}
- Tomazini BM, Maia IS, Bueno FR, Silva Mvao Baldassare FP, Costa EL, Moura RA, et al. COVID-19-associated ARDS treated with DEXamethasone (CoDEX): study design and rationale for a randomised trial. medRxiv [Preprint] 2020. [DOI: ] [DOI] [PMC free article] [PubMed]
- Tomazini BM, Maia IS, Bueno FR, Silva Mvao Baldassare FP, Costa EL, Moura RA, et al. COVID-19-associated ARDS treated with dexamethasone (CoDEX): study design and rationale for a randomised trial. Revista Brasileira de Terapia Intensiva 2020;32(3):354-62. [DOI: 10.5935/0103-507X.20200063] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomised clinical trial. JAMA 2020;324(13):1307-16. [DOI: 10.1001/jama.2020.17021] [NCT04327401] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
EUCTR2020‐001445‐39‐ES {published data only}
- EUCTR2020-001445-39-ES. Clinical trial to evaluate methylprednisolone pulses and tacrolimus in hospitalized patients with severe pneumonia secondary to COVID-19 (tacrovid). www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001445-39 (first received 31 March 2020).
EUCTR2020‐001616‐18‐ES {published data only}
- TACTIC-COVID. www.clinicaltrialsregister.eu/ctr-search/search?query=2020-001616-18.
- Treatment with inhaled corticoids in patients with COVID-19 and pneumonia. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-001616-18-ES.
EUCTR2020‐001889‐10 {published data only}
- EUCTR2020-001889-10. Use of inhaled corticosteroids as treatment of early COVID-19 infection to prevent clinical deterioration and hospitalisation. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001889-10/GB (first received 15 May 2020).
IRCT20120225009124N4 {published data only}
- IRCT20120225009124N4. Letter to the editor: efficacy of different methods of combination regimen administrations including dexamethasone, intravenous immunoglobulin, and interferon-beta to treat critically ill COVID-19 patients: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):549. [DOI: 10.1186/s13063-020-04499-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20190312043030N2 {published data only}
- IRCT20190312043030N2. The effect of selenium, vitamin C and methylprednisolone combination on mortality and morbidity of COVID-19 patients. en.irct.ir/trial/49508 (first received 19 August 2020).
IRCT20200522047542N1 {published data only}
- IRCT20200522047542N1. Effect of corton on olfactory dysfunction in COVID-19 patients. en.irct.ir/trial/48379 (first received 4 August 2020).
ISRCTN86534580 {published data only}
- ISRCTN86534580. PRINCIPLE: a trial evaluating treatments for suspected COVID-19 in people aged 50 years and above with pre-existing conditions and those aged 65 years and above. www.isrctn.com/ISRCTN86534580 (first received 20 March 2020).
NCT04341038 {published data only}
- NCT04341038. Clinical trial to evaluate methylprednisolone pulses and tacrolimus in patients with COVID-19 lung injury. clinicaltrials.gov/show/NCT04341038 (first received 10 April 2020).
- NCT04341038. Pragmatic, open-label, single-center, randomized, phase II clinical trial to evaluate the efficacy and safety of methylprednisolone pulses and tacrolimus in patients with severe pneumonia secondary to COVID-19: the TACROVID trial protocol. medRxiv [Preprint] 2021;21(100716):1-8. [DOI: 10.1016/j.conctc.2021.100716] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04355637 {published data only}
- NCT04355637. Inhaled corticosteroid treatment of COVID-19 patients with pneumonia. clinicaltrials.gov/show/NCT04355637 (first received 21 April 2020).
NCT04359511 {published data only}
- NCT04359511. Efficacy and safety of corticosteroids in oxygen-dependent patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04359511 (first received 24 April 2020).
NCT04361474 {published data only}
- Daval M, Corre A, Palpacuer C, Houssette J, Poillon G, Eliezer M, et al. Efficacy of local budesonide therapy in the management of persistent hyposmia in COVID-19 patients without signs of severity: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(666):1-3. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04361474. Trial evaluating the efficacy of local budesonide therapy in the management of hyposmia in COVID-19 patients without signs of severity. clinicaltrials.gov/show/NCT04361474 (first received 24 April 2020).
NCT04381364 {published data only}
- NCT04381364. Inhalation of ciclesonide for patients with COVID-19: a randomised open treatment study (HALT COVID-19). clinicaltrials.gov/show/NCT04381364 (first received 8 May 2020).
NCT04411667 {published data only}
- Sakoulas G, Geriak M, Kullar R, Greenwood KL, Habib M, Vyas A, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Critical Care Explorations 2020;2(11):e0280. [DOI: 10.1097/CCE.0000000000000280] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04416399 {published data only}
- Ramakrishnan S, Nicolau DV Jr, Langford B, Mahdi M, Jeffers H, Mwasuku C, et al. Inhaled budesonide in the treatment of early COVID-19 illness: a randomised controlled trial. medRxiv [Preprint]. [DOI: ] [DOI] [PMC free article] [PubMed]
NCT04468646 {published data only}
- Riffat M, Fridoon A, Ahad Q, Muhammad AR, Syed AG, Muhammad AT, et al. Aprepitant as a combinant with dexamethasone reduces the inflammation via neurokinin 1 receptor antagonism in severe to critical COVID-19 patients and potentiates respiratory recovery: a novel therapeutic approach. medRxiv [Preprint]. [DOI: ]
NCT04484493 {published data only}
- Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomised controlled trial. American Journal of Otolaryngology 2021;42(2):1-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04534478 {published data only}
- NCT04534478. Oral prednisone regimens to optimise the therapeutic strategy in patients with organising pneumonia post-COVID-19. clinicaltrials.gov/show/NCT04534478 (first received 1 September 2020).
NCT04551781 {unpublished data only}
- NCT04551781. Short term low dose corticosteroids for management of post COVID19 pulmonary fibrosis. clinicaltrials.gov/show/NCT04551781 (first received 16 September 2020).
NCT04561180 {published data only}
- NCT04561180. Study to evaluate the efficacy and safety of EG-HPCP-03a compared to DEX in patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04561180 (first received 23 September 2020).
NCT04569825 {published data only}
- NCT04569825. Effect of nasal steroid in the treatment of anosmia due to COVID-19 disease. clinicaltrials.gov/ct2/show/NCT04569825 (first received 30 September 2020).
NCT04640168 {published data only}
- NCT04640168. Adaptive COVID-19 treatment trial 4 (ACTT-4). clinicaltrials.gov/show/NCT04640168 (first received 23 November 2020).
NCT04657484 {published data only}
- NCT04657484. Comparison of two corticosteroid regimens for post-COVID diffuse lung disease. clinicaltrials.gov/show/NCT04657484 (first received 8 December 2020).
NCT04826822 {published data only}
- NCT04826822. Spironolactone and dexamethasone in patients hospitalised with COVID-19 (SPIDEX-II). clinicaltrials.gov/show/NCT04826822 (first received 1 April 2021).
References to studies awaiting assessment
EUCTR2020‐001307‐16‐ES {published data only}
- EUCTR2020-001307-16-ES. Efficacy and safety of corticoids in patients with adult respiratory distress syndrome (ARDS) secondary to coronavirus infection. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001307-16 (first received 1 April 2020).
EUCTR2020‐001333‐13‐FR {published data only}
- EUCTR2020-001333-13-FR. Dexamethasone associated with hydroxychloroquine vs. hydroxychloroquine alone for the early treatment of severe ARDS caused by COVID-19: a randomised controlled trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001333-13 (first received 9 April 2020).
EUCTR2020‐001553‐48‐FR {published data only}
- EUCTR2020-001553-48-FR. Corticoids in COVID-19 viral pneumonia in infection with SARS-CoV-2 (translation by the review authors) [Corticoides au cours de la pneumonie virale COVID-19 liee a linfection par le SARS-CoV-2]. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001553-48 (first received 13 April 2020).
IRCT20081027001411N3 {published data only}
- IRCT20081027001411N3. Effect of prednisolone on treatment of COVID-19. irct.ir/trial/46975 (first received 6 January 2020).
IRCT20100228003449N31 {published data only}
- IRCT20100228003449N31. Corticosteroids in COVID-19. irct.ir/trial/51163 (first received 8 October 2020).
IRCT20120215009014N354 {published data only}
- IRCT20120215009014N354. Evaluating the effect of intravenous hydrocortisone, methylprednisolone, and dexamethasone in treatment of patients with moderate to severe acute respiratory distress syndrome caused by COVID-19. irct.ir/trial/48043 (first received 12 May 2020).
IRCT20160118026097N4 {published data only}
- IRCT20160118026097N4. The effect of dexamethasone in the treatment of high-risk COVID-19 patients. irct.ir/trial/48310 (first received 13 September 2020).
IRCT20200611047727N3 {published data only}
- IRCT20200611047727N3. Evaluation of efficacy and safety of low dose corticosteroid with severe pneumonia COVID-19. irct.ir/trial/49116 (first received 3 January 2021).
IRCT20201015049030N1 {published data only}
- IRCT20201015049030N1. Effect of dexamethasone on treatment of COVID-19. www.irct.ir/trial/51736 (first received 7 November 2020).
ISRCTN33037282 {published data only}
- ISRCTN33037282. Comparing two medications (dexamethasone and methylprednisolone high dose) for the treatment of pneumonia in patients with COVID-19. www.isrctn.com/ISRCTN33037282 (first received 26 November 2020).
Munch 2021 {published data only}
- Munch MW, Meyhoff TS, Helleberg M, Kjær M-BN, Granholm A, Hjortsø CJ, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiologica Scandinavica 2021 Jun 17 [Epub ahead of print]. [DOI: 10.1111/aas.13941] [DOI] [PMC free article] [PubMed]
- NCT04348305. Hydrocortisone for COVID-19 and severe hypoxia. clinicaltrials.gov/show/NCT04348305 (first received 16 April 2020).
- Petersen MW, Meyhoff TS, Helleberg M, Nørregaard Kjær MB, Granholm A, Steensen Hjortsø CJ, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia (COVID STEROID) trial-protocol and statistical analysis plan. Acta Anaesthesiologica Scandinavica 2020;64(9):1365-75. [DOI: 10.1111/aas.13673] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03852537 {published data only}
- NCT03852537. Steroid dosing by biomarker guided titration in critically ill patients with pneumonia. clinicaltrials.gov/show/NCT03852537 (first received 25 February 2019).
NCT04244591 {published data only}
- NCT04244591. Glucocorticoid therapy for novel coronavirus critically ill patients with severe acute respiratory failure. www.clinicaltrials.gov/ct2/show/NCT04244591 (first received 28 January 2020).
NCT04325061 {published data only}
- NCT04325061. Efficacy of dexamethasone treatment for patients with ARDS caused by COVID-19. clinicaltrials.gov/ct2/show/NCT04325061 (first received 27 March 2020).
NCT04746430 {published data only}
- NCT04746430. COVID-19 primary care platform for early treatment and recovery (COPPER) study. clinicaltrials.gov/show/NCT04746430 (first received 9 February 2021).
Rashad 2021 {unpublished data only}
- NCT04519385. Tocilizumab versus dexamethasone in severe COVID19 cases. clinicaltrials.gov/show/NCT04519385 (first received 19 August 2020).
- Rashad A, Mousa S, Nafady‑Hego H, Nafady A, Elgendy H. Short term survival of critically ill COVID‑19 Egyptian patients on assisted ventilation treated by either dexamethasone or tocilizumab. Scientific Reports 2021;11(8816):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR2000029386 {published data only}
- Qin YY, Zhou YH, Lu YQ, Sun F, Yang S, Harypursat V, et al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomised controlled trial. Chinese Medical Journal 2020;133(9):1080-6. [DOI: 10.1097/CM9.0000000000000791] [ChiCTR2000029386] [DOI] [PMC free article] [PubMed]
ChiCTR2000029656 {published data only}
- ChiCTR2000029656. A randomised, open-label study to evaluate the efficacy and safety of low-dose corticosteroids in hospitalised patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=49086 (first received 9 February 2020).
ChiCTR2000030481 {published data only}
- ChiCTR2000030481. The clinical value of corticosteroid therapy timing in the treatment of novel coronavirus pneumonia (COVID-19): a prospective randomised controlled trial. www.chictr.org.cn/showprojen.aspx?proj=50453 (first received 3 March 2020).
CTRI/2020/07/026608 {published data only}
- CTRI/2020/07/026608. A clinical trial to study the effects of two drugs methylprednisolone and dexamethasone in patients with severe COVID-19. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45638 (first received 15 July 2020).
CTRI/2020/10/028731 {published data only}
- CTRI/2020/10/028731. Higher vs. lower doses of dexamethasone in patients with COVID-19 and severe hypoxia. ctri.nic.in/Clinicaltrials/showallp.php?mid1=46442&EncHid=&userName=28731 (first received 29 October 2020).
- CTRI/2020/10/028731. Higher vs. lower doses of steroids in patients with COVID-19. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/10/028731 (first received 29 October 2020).
CTRI/2020/12/029894 {published data only}
- Comparing the effectiveness of dexamethasone versus methylprednisolone in patients with moderate COVID 19 - a randomised controlled trial. ctri.nic.in/Clinicaltrials/showallp.php?mid1=49273&EncHid=&userName=029894 (first received 18 December 2020).
- CTRI/2020/12/029894. A study to compare the effectiveness of two drugs, dexamethasone versus methylprednisolone in the treatment of moderate Covid 19 patients. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/029894.
CTRI/2020/12/030143 {published data only}
- CTRI/2020/12/030143. Comparison of different steroid regimes in critically ill adult patients of COVID-19. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2020/12/030143.
- Evaluation of different steroid regimes in critically ill adult patients of COVID-19 admitted to intensive care units. ctri.nic.in/Clinicaltrials/showallp.php?mid1=50886&EncHid=&userName=30143 (first received 31 December 2020).
EUCTR2020‐001413‐20‐ES {published data only}
- EUCTR2020-001413-20-ES. Efficacy and safety of siltuximab vs. corticosteroids in hospitalized patients with COVID-19 pneumonia. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001413-20 (first received 7 April 2020).
EUCTR2020‐001457‐43‐FR {published data only}
- EUCTR2020-001457-43-FR. Dexamethasone and oxygen support strategies in ICU patients with COVID-19 pneumonia. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001457-43 (first received 10 April 2020).
EUCTR2020‐001622‐64‐ES {published data only}
- EUCTR2020-001622-64-ES. Outpatient treatment of COVID-19 with early pulmonary corticosteroids as an opportunity to modify the course of the disease. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001622-64 (first received 19 April 2020).
- Saiz-Rodríguez M, Peña T, Lázaro L, González A, Martínez A, Cordero JA, et al. Outpatient treatment of COVID-19 with steroids in the phase of mild pneumonia without the need for admission as an opportunity to modify the course of the disease: a structured summary of a randomised controlled trial. Trials 2020;21(632):1-3. [DOI: 10.1186/s13063-020-04575-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2020‐001707‐16‐ES {published data only}
- EUCTR2020-001707-16-ES. Phase III randomised, unicentric open, controlled clinical trial to demonstrate the effectiveness of tocilizumab against systemic corticotherapy in patients entered by COVID-19 with bilateral pneumonia and bad evolution. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001707-16/ES (first received 25 June 2020).
EUCTR2020‐001921‐30 {published data only}
- Busani S, Tosi M, Mighali P, Vandelli P, D'Amico R, Marietta M, et al. Multi-centre, three arm, randomised controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):724. [DOI: 10.1186/s13063-020-04645-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2020-001921-30. Steroids and unfractionated heparin in critically-ill patients with pneumonia from COVID-19 infection. A multicenter, interventional, randomised, three arms study design. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001921-30 (first received 26 June 2020).
EUCTR2020‐002186‐34‐ES {published data only}
- EUCTR2020-002186-34-ES. Efficacy of the early use of corticotherapy in CoV-2 infection to prevent the progression of acute respiratory distress syndrome (ARDS). www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-002186-34 (first received 22 July 2020).
EUCTR2020‐003363‐25‐DK {published data only}
- EUCTR2020-003363-25-DK. Higher vs. lower doses of dexamethasone in patients with COVID-19 and severe oxygen deficiency: the COVID STEROID 2 trial. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-003363-25 (first received 18 August 2020).
EUCTR2020‐004323‐16 {published data only}
- EUCTR2020-004323-16. Evaluation of the efficacy of high doses of methylprednisolone in SARS-CoV2 pneumonia patients. www.clinicaltrialsregister.eu/ctr-search/trial/2020-004323-16/IT (first received 23 November 2020).
NCT04329650 {published data only}
- NCT04329650. Efficacy and safety of siltuximab vs. corticosteroids in hospitalised patients with COVID19 pneumonia. clinicaltrials.gov/show/NCT04329650 (first received 1 April 2020).
NCT04344730 {published data only}
- NCT04344730. Dexamethasone and oxygen support strategies in ICU patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04344730 (first received 14 April 2020).
NCT04345445 {published data only}
- NCT04345445. Study to evaluate the efficacy and safety of tocilizumab versus corticosteroids in hospitalised COVID-19 patients with high risk of progression. clinicaltrials.gov/show/NCT04345445 (first received 14 April 2020).
NCT04347980 {published data only}
- NCT04347980. Dexamethasone treatment for severe acute respiratory distress syndrome. clinicaltrials.gov/show/NCT04347980 (first received 15 April 2020).
NCT04377503 {published data only}
- NCT04377503. Tocilizumab versus methylprednisolone in the cytokine release syndrome of patients with COVID-19. clinicaltrials.gov/show/NCT04377503 (first received 6 May 2020).
NCT04395105 {published data only}
- Maskin LP, Olarte GL, Palizas F Jr, Velo AE, Lurbet MF, Bonelli I, et al. High dose dexamethasone treatment for acute respiratory distress syndrome secondary to COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):743. [DOI: 10.1186/s13063-020-04646-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04395105. Dexamethasone for COVID-19 related ARDS: a multicenter, randomised clinical trial. clinicaltrials.gov/show/NCT04395105 (first received 20 May 2020).
NCT04438980 {published data only}
- Les Bujanda I, Loureiro-Amigo J, Capdevila Bastons F, Elejalde Guerra I, Anniccherico Sánchez J, Murgadella-Sancho A, et al. Treatment of COVID-19 pneumonia with glucocorticoids (CORTIVID): a structured summary of a study protocol for a randomised controlled trial. Trials 2021;22(1):43. [DOI: 10.1186/s13063-020-04999-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04438980. Glucocorticoids in COVID-19 (CORTIVID). clinicaltrials.gov/show/NCT04438980 (first received 19 June 2020).
NCT04451174 {published data only}
- NCT04451174. Early use of corticosteroids in hospitalised patients with moderate COVID19 pneumonia. clinicaltrials.gov/show/NCT04451174 (first received 30 June 2020).
- Salinas M, Andino P, Palma L, Valencia J, Figueroa E, Ortega J. Early use of corticosteroids in non-critical patients with COVID-19 pneumonia (PREDCOVID): a structured summary of a study protocol for a randomised controlled trial. Trials 2021;22(1):92. [DOI: 10.1186/s13063-021-05046-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04452565 {published data only}
- NCT04452565. NA-831, atazanavir and dexamethasone in the treatment of SARSCov-2 infection (NATADEX). clinicaltrials.gov/show/NCT04452565 (first received 30 June 2020).
NCT04485429 {published data only}
- NCT04485429. Efficacy assessment of methylprednisolone and heparin in patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04485429 (first received 24 July 2020).
NCT04499313 {published data only}
- NCT04499313. Dexamethasone versus methylprednisolone for the treatment of patients with ARDS caused by COVID-19. clinicaltrials.gov/show/NCT04499313 (first received 5 August 2020).
NCT04509973 {published data only}
- Munch MW, Granholm A, Myatra SN, Vijayaraghavan BK, Cronhjort M, Wahlin RR, et al. Higher vs. lower doses of dexamethasone in patients with COVID-19 and severe hypoxia (COVID STEROID 2) trial: protocol and statistical analysis plan. Acta Anaesthesiologica Scandinavica 2021;00:1-12. [DOI: 10.1111/aas.13795] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04509973. Higher vs. lower doses of dexamethasone for COVID-19 and severe hypoxia. clinicaltrials.gov/show/NCT04509973 (first received 12 August 2020).
NCT04513184 {published data only}
- NCT04513184. Randomised clinical trial of nasal dexamethasone as an adjuvant in patients with COVID-19. clinicaltrials.gov/show/NCT04513184 (first received 14 August 2020).
NCT04528329 {published data only}
- NCT04528329. Anosmia and / or ageusia and early corticosteroid use. clinicaltrials.gov/show/NCT04528329 (first received 27 August 2020).
NCT04528888 {published data only}
- NCT04528888. Steroids and unfractionated heparin in critically ill patients with pneumonia from COVID-19 infection. clinicaltrials.gov/show/NCT04528888 (first received 27 August 2020). [EUDRA-CT: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001921-30/IT] [NCT: https://clinicaltrials.gov/ct2/show/study/NCT04528888]
NCT04530409 {published data only}
- NCT04530409. Timing of corticosteroids in COVID-19. clinicaltrials.gov/show/NCT04530409 (first received 28 August 2020).
NCT04545242 {published data only}
- NCT04545242. Efficacy of dexamethasone in patients with acute hypoxemic respiratory failure caused by infections. clinicaltrials.gov/show/NCT04545242 (first received 10 September 2020).
NCT04636671 {published data only}
- NCT04636671. Methylprednisolone vs. dexamethasone in COVID-19 pneumonia (MEDEAS RCT). clinicaltrials.gov/show/NCT04636671 (first received 19 November 2020).
NCT04663555 {published data only}
- NCT04663555. Effect of two different doses of dexamethasone in patients with ARDS and COVID-19. clinicaltrials.gov/show/NCT04663555 (first received 11 December 2020).
NCT04673162 {published data only}
- NCT04673162. Evaluation of the efficacy of high doses of methylprednisolone in SARS-CoV2 ( COVID-19) pneumonia patients. clinicaltrials.gov/show/NCT04673162 (first received 17 December 2020).
NCT04707534 {published data only}
- NCT04707534. Dexamethasone for COVID-19. clinicaltrials.gov/show/NCT04707534 (first received 13 January 2021).
NCT04726098 {published data only}
- NCT04726098. Low or high dose of dexamethasone in patients with respiratory failure by COVID-19. clinicaltrials.gov/ct2/show/record/NCT04726098 (first received 27 January 2021).
NCT04765371 {published data only}
- NCT04765371. Comparison between prednisolone and dexamethasone on mortality in patients on oxygen therapy, with COVID-19. clinicaltrials.gov/ct2/show/NCT04765371 (first received 21 February 2021).
NCT04780581 {published data only}
- NCT04780581. Glucocorticoid therapy in coronavirus disease COVID-19 patients. clinicaltrials.gov/show/NCT04780581 (first received 3 March 2021).
NCT04795583 {published data only}
- NCT04795583. Corticosteroids for COVID-19. clinicaltrials.gov/show/NCT04795583 (first received 12 March 2021).
NCT04834375 {published data only}
- NCT04834375. Randomised open investigation determining steroid dose. clinicaltrials.gov/show/NCT04834375 (first received 8 April 2021).
NCT04836780 {published data only}
- NCT04836780. Dexamethasone early administration in hospitalised patients with COVID-19 pneumonia. clinicaltrials.gov/show/NCT04836780 (first received 8 April 2021).
Additional references
Barnes 2006
- Barnes PJ. How corticosteroids control inflammation: quintiles prize lecture. British Journal of Pharmacology 2005;148(3):245-54. [DOI: 10.1038/sj.bjp.0706736] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bourdeau 2003
- Bourdeau I, Stratakis CA. Glucocorticoids, Pharmacology of. In: Henry H, Norman A, editors(s). Encyclopedia of Hormones. Vol. -. Cambridge, MA: Academic Press, 2003:142-50. [DOI: ] [Google Scholar]
Brock 2011
- Brock GN, Barnes C, Ramirez JA, Myers J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Medical Research Methodology 2011;11:144. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buitrago‐Garcia 2020
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Medicine 2020;17(9):e1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaudhuri 2021
- Chaudhuri D, Sasaki K, Karkar A, Sharif S, Lewis K, Mammen MJ, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Medicine 2021;47:521–37. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Columbia Public Health 2021
- Competing Risk Analysis. Columbia University Mailman School of Public Health Website. Available from www.publichealth.columbia.edu/research/population-health-methods/competing-risk-analysis (accessed 13 July 2021). [ACCESSED JULY 13 2021: https://www.publichealth.columbia.edu/research/population-health-methods/competing-risk-analysis]
COMET 2020
- Core outcome set developers’ response to COVID-19. www.comet-initiative.org/Studies/Details/1538 (accessed 2 November 2020).
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
EndNote X9 [Computer program]
- EndNote X9. Clarivate, 2020.
GRADE pro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Savovic J, Page MJ, Sterne JA. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed 7 July 2021).
Higgins 2021a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version version 6.2 (updated February 2021). Cochrane, 2021. Available from: training.cochrane.org/handbook 2020.
Higgins 2021b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021c
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johns Hopkins University 2021
- Johns Hopkins University. Mortality analyses. coronavirus.jhu.edu/data/mortality (accessed 11 March 2021).
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Chai KL, Piechotta V, Valk SJ, Estcourt LJ, et al. SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauer 2020
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of Internal Medicine 2020;172(9):577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2021
- Li T, Higgins JP, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.1 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liang 2020
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncology 2020;21(3):335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Living Evidence Network 2019
- Living Evidence Network. Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode (Version December 2019). Available from community.cochrane.org/review-production/production-resources/living-systematic-reviews (accessed 22 March 2021).
Marshall 2020
- Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Microsoft 2018 [Computer program]
- Microsoft Excel. Microsoft Corporation. Microsoft Corporation, 2018. office.microsoft.com/excel.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Park 2020
- Park JJ, Harari O, Dron L, Lester RT, Thorlund K, Mills EJ. An overview of platform trials with a checklist for clinical readers. Journal of Clinical Epidemiology 2020;125:1-8. [DOI: 10.1016/j.jclinepi.2020.04.025] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pasin 2021
- Pasin L, Navalesi P, Zangrillo A, Kuzovlev A, Likhvantsev V, Hajjar LA, et al. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized clinical trials. Journal of Cardiothoracic and Vascular Anesthesia 2021;35(2):578-84. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piechotta 2021
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranganathan 2015
- Ranganathan P, Aggarwal R, Pramesh CS. Common pitfalls in statistical analysis: odds versus risk. Perspectives in Clinical Research 2015;6(4):222–4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. revman.cochrane.org.
Rhen 2005
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids - new mechanisms for old drugs. New England Journal of Medicine 2005 ;353(16):1711-23. [DOI: 10.1056/NEJMra050541] [DOI] [PubMed] [Google Scholar]
Rochwerg 2018
- Rochwerg B, Oczkowski SJ, Siemieniuk RA, Agoritsas T, Belley-Cote E, D'Aragon F, et al. Corticosteroids in sepsis: an updated systematic review and meta-analysis. Critical Care Medicine 2018;46(9):1411-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI] [PubMed] [Google Scholar]
Schulte‐Schrepping 2020
- Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020;182(6):1419-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Siemieniuk 2020
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ 2020;370:m2980. [BMJ: https://www.bmj.com/content/370/bmj.m2980] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skoetz 2020
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and evidence profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Sterne 2020
- Sterne JA. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324(13):1330-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoelting 2006
- Stoelting RK, Hiller SC (editors). Chapter Pharmacology. In: Pharmacology & Physiology in Anesthetic Practice. 4 edition. Philadelphia: Lippincott Williams & Wilkins, 2006:462. [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thibeault 2021
- Thibeault C, Mühlemann B, Helbig ET, Mittermaier M, Lingscheid T, Tober-Lau P, et al. Clinical and virological characteristics of hospitalised COVID-19 patients in a German tertiary care centre during the first wave of the SARS-CoV-2 pandemic: a prospective observational study. Infection 2021;-:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tong 2020
- Tong A, Elliott JH, Azevedo LC, Baumgart A, Bersten A, Cervantes L, et al. Core outcomes set for trials in people with coronavirus disease 2019. Critical Care Medicine 2020;48(11):1622–35. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Paassen 2020
- Van Paassen J, Vos JS, Hoekstra EM, Neumann KM, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Critical Care 2020;24(696):1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villar 2020
- Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respiratory Medicine 2020;8(3):267-76. [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Cumulative number of reported probable cases of SARS. www.who.int/csr/sars/country/2003_07_11/en (accessed 22 March 2021).
WHO 2019
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/emergencies/mers-cov/en (accessed 22 March 2021).
WHO 2020a
- World Health Organization. Report of the WHO‐China joint mission on coronavirus disease 2019 (COVID‐19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report (accessed 22 March 2021).
WHO 2020b
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 22 March 2021).
WHO 2020c
- WHO working group on the clinical characterisation and management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI] [PMC free article] [PubMed]
WHO 2021a
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. covid19.who.int (accessed 1 March 2021).
WHO 2021b
- World Health Organization. Weekly epidemiological update - 23 February 2021. www.who.int/publications/m/item/weekly-epidemiological-update---23-february-2021 (accessed 1 March 2021).
WHO 2021c
- World Health Organization. SARS-CoV-2 variants. Available from www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed 22 March 2021).
WHO Living Guidance 2021
- World Health Organization 2021. COVID-19 Clinical management: living guidance. 6. Who do the recommendations apply to? www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 2021;(3):14. [WHO WEBSITE: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1]
WHO Living Guideline 2021
- WHO. Therapeutics and COVID-19: living guideline. Systemic corticosteroids. www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2;(3):37-50. [WHO WEBSITE: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1]
Williamson 2020
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [DOI: 10.1038/s41586-020-2521-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank Country Groups 2021
- World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 15 April 2021). [WEBSITE: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups]
Wu 2020
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI: 10.1001/jama.2020.2648] [DOI] [PubMed] [Google Scholar]
Yehya 2019
- Yehya, Harhay, Curely et al. Reappraisal of ventilator-free days in critical care research. American Journal of Respiratory and Critical Care Medicine 2019;200(7):828-36. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wagner 2021
- Wagner C, Griesel M, Micholajewska A, Fichtner F, Skoetz N. Corticosteroids for the treatment of COVID-19 (part of German Ecosystem CEO-Sys). PROSPERO 1 February 2021 (available from www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=227450). [PROSPERO: CRD42020227450]


