Abstract
Background
Long‐term levodopa therapy for Parkinson's disease is complicated by the development of motor fluctuations and abnormal involuntary movements. One approach is to add a dopamine agonist at this stage of the disease to reduce the time the patient spends immobile or off and to reduce the dose of levodopa in the hope of reducing such problems in the future.
Objectives
To compare the efficacy and safety of adjuvant ropinirole therapy with bromocriptine in patients with Parkinson's disease already established on levodopa therapy and suffering from motor complications.
Search methods
Electronic searches of MEDLINE, EMBASE and the Cochrane Controlled Trials Register. Handsearching of the neurology literature as part of the Cochrane Movement Disorders Group's strategy. Examination of the reference lists of identified studies and other reviews. Contact with SmithKline Beecham.
Selection criteria
Randomised controlled trials of ropinirole versus bromocriptine in patients with a clinical diagnosis of idiopathic Parkinson's disease and long‐term complications of levodopa therapy.
Data collection and analysis
Data was abstracted independently by the authors and differences settled by discussion. The outcome measures used included Parkinson's disease rating scales, levodopa dosage, 'off' time measurements and the frequency of withdrawals and adverse events.
Main results
In the 3 trials identified, no significant differences between ropinirole and bromocriptine were found in off time reduction, dyskinesia as an adverse event, motor impairment and disability, or levodopa dose reduction. Withdrawal rates and adverse event frequency were similar with the two agents apart from significantly less nausea with ropinirole (odds ratio 0.50; 0.29, 0.84 95% CI; p =0.01).
Authors' conclusions
In patients with Parkinson's disease and motor complications, ropinirole has similar effects to bromocriptine in terms of improving off time and reducing levodopa dose, without increasing adverse events including dyskinesia. However, these comparator studies may have been underpowered to detect clinically meaningful differences between the agonists.
Keywords: Humans, Antiparkinson Agents, Antiparkinson Agents/adverse effects, Bromocriptine, Bromocriptine/therapeutic use, Dopamine Agonists, Dopamine Agonists/therapeutic use, Dyskinesias, Dyskinesias/drug therapy, Dyskinesias/etiology, Indoles, Indoles/therapeutic use, Levodopa, Levodopa/adverse effects, Parkinson Disease, Parkinson Disease/drug therapy, Randomized Controlled Trials as Topic
Plain language summary
Ropinirole versus bromocriptine for levodopa‐induced complications in Parkinson's disease
In the later stages of Parkinson's disease, side effects occur because of the use of levodopa treatment. These consist of involuntary writhing movements (dyskinesia), painful cramps in the legs (dystonia) and a shortened response to each dose referred to as 'end‐of‐dose deterioration' or the 'wearing‐off effect'. Dopamine agonist drugs act by mimicking dopamine in the brain, but they do not cause these long‐term treatment complications. For this reason, dopamine agonists have for some years been added once these problems develop in the hope of improving them. Ropinirole is a new dopamine agonist recently licensed in the UK for the treatment of early and later Parkinson's disease. In this review, we will examine the trials performed with this drug to see how it compares with one of the older agonists bromocriptine.
Three trials have compared ropinirole with bromocriptine in 482 patients in the later stages of Parkinson's disease. Two studies were conducted over the short term (8 and 16 weeks), and used relatively low doses of ropinirole (9 mg/d) and bromocriptine (17.5 and 22.5mg/d). The other study was medium term (25 weeks) and used ropinirole doses in line with the current UK licensed maximum (24 mg/d).
No significant differences were found between the agonists in the time patients spent in the immobile off state, in dyskinesia reported as a side effect, in measurements of physical difficulties and problems with activities of daily living (such as bathing, shopping, etc.), or in levodopa dose reduction. No differences in side effects or withdrawals from treatment were found apart from less nausea with ropinirole.
In patients with Parkinson's disease and motor complications, ropinirole has similar effects to bromocriptine in terms of improving off time and reducing levodopa dose, without increasing adverse events including dyskinesia. However, these comparitor studies may have been underpowered to detect clinically meaningful differences between the agonists.
Background
Over 20 years after its introduction, levodopa remains the most effective therapy in Parkinson's disease. However, with long‐term treatment, patients develop side effects comprised of motor and psychiatric complications. The former consist of involuntary writhing movements of the limbs and trunk (choreoathetosis), painful cramps often affecting the feet (dystonia) and a shortened response to each dose of levodopa (end‐of‐dose deterioration). These affect 50% of patients after 6 years of therapy (Rajput 1984) and 100% of young onset patients (Quinn 1986).
An alternative treatment in Parkinson's disease is the dopamine agonist class of drug. These act directly on post‐synaptic dopamine receptors in the striatum and so they do not require conversion into dopamine, as does levodopa. They have developed the reputation of being less effective in clinical practice than expected, although they generate fewer motor complications when used as long‐term monotherapy (e.g. PDRG 1993). The use of dopamine agonists in newly diagnosed patients will be the subject of further Cochrane reviews.
Ropinirole is a non‐ergot based dopamine agonist like pramipexole but unlike the ergot‐based bromocriptine, pergolide, lisuride, and cabergoline. Trials in early and late Parkinson's disease have lead to it receiving a product licence in the United Kingdom for both indications. Monotherapy studies will be the subject of other Cochrane reviews.
This systematic review will examine all randomised controlled trials comparing ropinirole with bromocriptine in later Parkinson's disease with motor complications to evaluate its efficacy and safety. Another review will deal with trials comparing ropinirole with placebo.
Objectives
To compare the efficacy and safety of adjuvant ropinirole versus bromocriptine in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing adjuvant ropinirole with bromocriptine were considered for inclusion in the study.
Types of participants
Patients with a clinical diagnosis of idiopathic Parkinson's disease who had developed long‐term motor complications of dyskinesia and/or end‐of‐dose deterioration. All ages were included. Any duration of levodopa therapy was included.
Types of interventions
Oral ropinirole therapy or bromocriptine. Trial durations of greater than 4 weeks were included.
Types of outcome measures
1. Improvement in the time patients spend in the immobile 'off' state.
2. Changes in dyskinesia rating scales and the prevalence of dyskinesia.
3. Changes in parkinsonian rating scales.
4. Reduction in levodopa dose.
5. Number of withdrawals due to lack of efficacy and/or side‐effects.
Search methods for identification of studies
1. The review was based on the search strategy of the Movement Disorders Group. This included computerised searches of MEDLINE and EMBASE and hand searching of appropriate neurology journals. Relevant trials were included on the Group's specialised register of randomised controlled trials. Further details are available in the Group's module on the Cochrane Database of Systematic Reviews.
2. The Cochrane Controlled Trials Register was also searched for relevant trials.
3. The reference lists of located trials and of other ropinirole reviews were searched.
4. Additional assistance was provided by the drug manufacturer SmithKline Beecham.
Data collection and analysis
The two authors (CC, KD) independently assessed the studies identified by the search strategy. Disagreements about inclusions were resolved by discussion. The full papers were assessed for methodological quality by recording the method of randomisation and blinding, whether an intention to treat analysis was used and the number of patients lost to follow up.
Eligible data was abstracted onto standardised forms by the authors independently, checked for accuracy and amalgamated. A weighted estimate (fixed effect model) of the typical treatment effect across trials was calculated for continuous (weighted mean difference) and dichotomous (Peto odds ratio) variables such as 'off' time and prevalence of adverse events.
Results
Description of studies
See also Characteristics of Included Studies.
One study published in Japanese (Murayama 1996) and two unpublished studies have compared ropinirole with bromocriptine in patients with Parkinson's disease and motor complications. The majority of the details of this work have been provided to the authors directly by the manufacturer so it has not undergone peer review. A total of 482 patients were included with 257 receiving ropinirole. Two were randomised, double‐blind, parallel group studies (Murayama 1996; Brunt 1999) and one was a randomised, open‐label, parallel group design (Im 1999). Patients at baseline were similar in terms of age, sex, and severity of Parkinson's disease (Hoehn and Yahr scale).
Two studies were short term (16 weeks, Im 1999; 8 weeks, Murayama 1996) and one medium term (25 weeks, Brunt 1999). The maximum dose of ropinirole used in two of the studies was only 9 mg/d (Im 1999; Murayama 1996). This is well below the maximum currently licenced in the UK at 24 mg/d which was that used in the other study (Brunt 1999). Similarly, the maximum doses of bromocriptine used in two trials were lower than in the third (17.5 mg/d, Im 1999; 22.5 mg/d, Murayama 1996; 39.9 mg/d, Brunt 1999). Levodopa dose reduction was allowed in all studies.
In Brunt 1999, three different types of patients were recruited: Group A on low‐dose levodopa with no motor complications but needing more therapy; Group B on high‐dose levodopa with complications; Group C on levodopa with complications and already on a dopamine agonist. Only Group B patients fulfilled the inclusion criteria so the other patients were excluded from this review.
In Murayama 1996, untreated de novo patients were recruited as well as levodopa‐treated patients. Only the latter have been included in the review. It is not clear from the trial report how many of these levodopa‐treated patients had motor complications.
Risk of bias in included studies
See also Characteristics of Included Studies.
Details of the method of randomisation and concealment of allocation were poorly described in the reports sent to us. Further enquiry with the manufacturer has clarified that all three studies were randomised by computer generated random numbers and allocation concealed from investigators by sending coded supplies to them.
One study (Im 1999) was open‐label which raises the potential for performance bias (bias in favour or against a treatment by clinician or patient who is aware of allocation) and attrition bias (withdrawal from the trial because of knowledge of allocated treatment). Im 1999 was also analysed on a per protocol basis so attrition bias cannot be excluded.
Detection bias was unlikely by virtue of the statisticians working to analysis plans which had been agreed before the blind was broken.
Two studies were only short term (16 weeks, Im 1999; 8 weeks, Murayama 1996) and one medium term (25 weeks, Brunt 1999).
Sample size calculation was based on the adverse events of confusion and/or hallucinations in Brunt 1999 but this was for the whole study with all three Groups, not Group B alone as included here. The sample size calculation in Murayama 1996 also applied to the combination of levodopa‐treated and untreated patients, not just the treated ones included in this review. A sample size calculation was not available for Im 1999.
Effects of interventions
See also Table 1Key Characteristics of Included Studies and Table 2 Adverse Events for Included Studies.
1. Key Characteristics and Results for Included Studies.
| Study | Number of patients | Mean Hoehn & Yahr | Duration (weeks) | Mean (Max) Ropinirole dose / Mean (Max) Bromocriptine dose (mg/d) | Mean difference (MD) L‐dopa reduction (mg/d; + in favour of ropinirole) | MD off hours reduction (hours; + in favour of ropinirole) | MD UPDRS Motor (+ in favour of ropinirole) | Drop‐outs (Peto odds ratio < 1 favours ropinirole) |
| Brunt | 139 | 2.9 | 25 | R:10.0 (24) B: 18 (39.9) | ‐0.34 | 0.72 | 0.4 | n/a |
| Im | 76 | 2.5 | 16 | R:? (9) B: ? (17.5) | 101.3 | 0.96 | ‐1.3 | 0.86 |
| Murayama | 267 | 3 (median) | 8 | R:4.0 (9) B: 9.2 (22.5) | n/a | n/a | n/a | 0.74 |
| Total | 482 | 49.52 (WMD) | 0.80 (WMD) | 0.76 |
2. Adverse Events for Included Studies (Peto Odds Ratio < 1 favours ropinirole).
| Study (Number) | Nausea | Postural Hypotension | Hallucinations | Confusion | Dyskinesia | Sleep Disorder | Somnolence | Vivid Dreams | Insomnia |
| Brunt (139) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Im (76) | 0.43 | 1.05 | 7.80 | 7.80 | 2.32 | n/a | n/a | 1.48 | 1.61 |
| Murayama (267) | 0.51 | 1.54 | 0.63 | 7.62 | 1.02 | 7.56 | 0.52 | n/a | n/a |
| Total (482) | 0.50 | 1.38 | 0.76 | 7.68 | 1.51 | 7.56 | 0.52 | 1.48 | 1.61 |
| P value (Test for overall effect) | 0.01 | 0.7 | 0.6 | 0.08 | 0.3 | 0.3 | 0.6 | 0.7 | 0.6 |
One published Japanese trial (Murayama 1996) and two unpublished Korean and European randomised controlled trials have compared ropinirole with bromocriptine in a total of 482 patients with Parkinson's disease and motor fluctuations. Two were randomised, double‐blind, parallel group studies (Murayama 1996; Brunt 1999) and one was a randomised, open‐label, parallel group design (Im 1999). Two studies were short term (16 weeks, Im 1999; 8 weeks, Murayama 1996) and one medium term (25 weeks, Brunt 1999). In Brunt 1999, only sub‐group B fulfilled the inclusion criteria for this review. Similarly, only levodopa‐treated patients in Murayama 1996 were included.
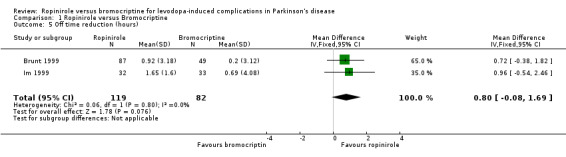
The amount of time patients spent in the relatively immobile off state improved on both ropinirole and bromocriptine in the two trials that evaluated this, although these differences were not statistically significant (Table 5; Brunt 1999; Im 1999). There was a trend for a greater improvement in off time with ropinirole compared with bromocriptine (weighted mean difference [WMD] 0.80 hours; ‐0.08, 1.69 95%CI; Table 5).
Dyskinesia rating scales were not used in these trials. However, dyskinesia reported as an adverse event occurred with a similar frequency with ropinirole and bromocriptine (Table 10).
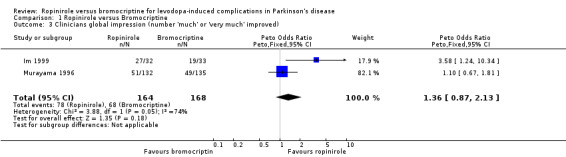
Regarding motor impairments and disability rating, the clinicians global impression scale showed no significant advantage for ropinirole in terms of the number of patients who were 'much' or 'very much' improved (Table 3). UPDRS motor scores (part III) improved with both agents in Brunt 1999 and Im 1999 but this was not significantly different between the agonists in the latter trial and standard deviations of means were not available in the former (Table 1). The percentage improvement in the Hoehn and Yahr scale in Murayama 1996 was similar with both agonists (Table 2).
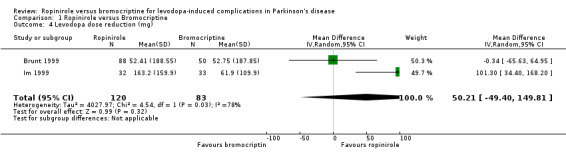
Data on levodopa dose reduction was available for two studies. Superficially, meta‐analysis shows a significant advantage for ropinirole over bromocriptine in levodopa dose reduction (WMD 49.52 mg/d; 2.52, 95.97 95% CI; Table 4). However, there was significant heterogeneity using a fixed effects model because the larger trial (Brunt 1999) showed no difference, whereas the smaller Im 1999 study showed a large advantage in favour of ropinirole. Using a random effects model, no significant difference was found.
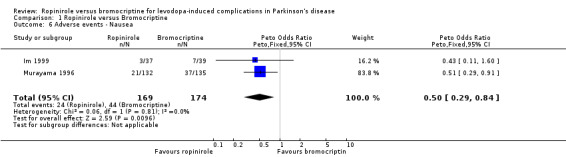
The only significant difference between the agonists regarding adverse events (Tables 6 to 14) was less nausea in those given ropinirole (odds ratio 0.50; 0.29, 0.84 95% CI; p =0.01; Table 6). Withdrawal rates were comparable with the two agonists (Table 15).
Discussion
A number of significant criticisms can be levelled at the trials included in this review:‐
Two of the three studies comparing ropinirole with bromocriptine in later Parkinson's disease with motor fluctuations remain unpublished and thus have not undergone peer review.
The studies were short to medium term (8 to 25 weeks).
Two studies used relatively low maximum doses of ropinirole (9 mg/d) and bromocriptine (17.5 and 22.5mg/d).
Details of randomisation and concealment of allocation were poorly reported. Additional data from the manufacturer on methodology and outcomes was necessary before the review could be performed.
Murayama 1996 probably included levodopa‐treated patients without motor complications.
In terms of motor complications, reduction in off time and the incidence of dyskinesia as an adverse event were similar with ropinirole and bromocriptine, with no clear advantage of one over another. Motor impairments and disability were poorly evaluated in these studies but again no significant advantage was seen with ropinirole over bromocriptine in UPDRS motor score, Hoehn and Yahr scale, or clinicians global impression score. Levodopa dose reduction was not significantly different between the two agonists. The only significant difference in adverse event reporting was less nausea with ropinirole (odds ratio 0.50; 0.29, 0.84 95% CI; p =0.01; Table 6), but unfortunately domperidone usage was not recorded. Withdrawal rates were also similar.
It is tempting to conclude that ropinirole is at least as good as bromocriptine in patients with Parkinson's disease and motor complications. It is on this basis that ropinirole will have received its product licence. However in clinical practice, since bromocriptine is less expensive than ropinirole in most countries, if ropinirole shows no significant advantages then the less costly bromocriptine should be preferred. Unfortunately, the absence of significant differences between the two agents in these trials does not exclude such differences. The trials may have had insufficient power to detect significant changes. There were quite large trends in favour of ropinirole in terms of off time reduction and withdrawal rate which may have become significant with larger numbers of patients. Underpowering of bromocriptine comparator trials is a common problem with the newer dopamine agonists and has been shown with the equivalent pramipexole Cochrane review.
Authors' conclusions
Implications for practice.
No significant differences between ropinirole and bromocriptine were found in off time reduction, dyskinesia as an adverse event, motor impairment and disability, or levodopa dose reduction. Withdrawal rates and adverse event frequency were similar with the two agents apart from significantly less nausea with ropinirole. However, these studies are likely to be underpowered to detect clinically relevant differences between the agonists so caution should be exercised in the interpretation of these results.
Implications for research.
In common with previous Cochrane adjuvant agonist reviews, this study of ropinirole has shown major deficiencies in the performance and reporting of clinical trials. It is suggested that in future studies:‐
All randomised controlled trials must be published or made available in some way to avoid publication bias.
Reporting standards must be lifted by adopting the CONSORT guidelines (CONSORT 1996).
Adjuvant therapy trials must provide more long‐term results over 12 months or more.
Doses of bromocriptine must be increased in such trials to allow a fair comparison; 40 mg/d or more should be used.
Sample size calculations mean that comparisons between two active agents require large numbers of patients to avoid false negative conclusions. Rather than split phase III adjuvant therapy trials across North America, Europe, and Japan for licensing reasons, it may be better to mount one or two international trials which are much larger and more likely to produce a significant advantage in favour of the new agent.
It would be of considerable value to clinicians to know whether one or more of the available dopamine agonists is significantly better than any other. This would require a direct head‐to‐head comparison trial which would need to be extremely large to find meaningful differences. Such a study is nevertheless desirable and should include quality of life measures and health economics data.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2015 | Amended | PLS correction |
| 13 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 13 November 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors wish to thank SmithKline Beecham for their assistance in performing this review.
Data and analyses
Comparison 1. Ropinirole versus Bromocriptine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 UPDRS motor scores (Part III) | Other data | No numeric data | ||
| 2 Hoehn and Yahr stage | Other data | No numeric data | ||
| 3 Clinicians global impression (number 'much' or 'very much' improved) | 2 | 332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.87, 2.13] |
| 4 Levodopa dose reduction (mg) | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 50.21 [‐49.40, 149.81] |
| 5 Off time reduction (hours) | 2 | 201 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.08, 1.69] |
| 6 Adverse events ‐ Nausea | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.29, 0.84] |
| 7 Adverse events ‐ Postural hypotension | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.31, 6.14] |
| 8 Adverse events ‐ Hallucinations | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.26, 2.22] |
| 9 Adverse events ‐ Confusion | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.68 [0.80, 74.04] |
| 10 Adverse events ‐ Dyskinesia | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [0.65, 3.49] |
| 11 Adverse events ‐ Sleep disorder | 1 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.56 [0.15, 381.04] |
| 12 Adverse events ‐ Somnolence | 1 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 5.07] |
| 13 Adverse event ‐ Vivid dreams | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.19, 11.42] |
| 14 Adverse event ‐ Insomnia | 1 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.61 [0.27, 9.78] |
| 15 All cause withdrawal rate | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.45, 1.27] |
1.1. Analysis.
Comparison 1 Ropinirole versus Bromocriptine, Outcome 1 UPDRS motor scores (Part III).
| UPDRS motor scores (Part III) | |
|---|---|
| Study | |
| Brunt 1999 | Improvement on ropinirole ‐5.9 (n=76) v bromocriptine ‐6.3 (n=42). (Standard deviations not available; significance testing not given). |
| Im 1999 | Improvement on ropinirole ‐5.9 (SD 5.9) (n=32) v bromocriptine ‐4.6 (SD 9.1) (n=33). NS at p < 0.05. |
| Murayama 1996 | Not available |
1.2. Analysis.
Comparison 1 Ropinirole versus Bromocriptine, Outcome 2 Hoehn and Yahr stage.
| Hoehn and Yahr stage | |
|---|---|
| Study | |
| Brunt 1999 | Not available |
| Murayama 1996 | % improvement on ropinirole 7.7% (95% CI 3.6˜14.1) v bromocriptine 6.6% (95% CI 2.7˜13.1). Significance testing not given. |
1.3. Analysis.
Comparison 1 Ropinirole versus Bromocriptine, Outcome 3 Clinicians global impression (number 'much' or 'very much' improved).
1.4. Analysis.
Comparison 1 Ropinirole versus Bromocriptine, Outcome 4 Levodopa dose reduction (mg).
1.5. Analysis.
Comparison 1 Ropinirole versus Bromocriptine, Outcome 5 Off time reduction (hours).
1.6. Analysis.
Comparison 1 Ropinirole versus Bromocriptine, Outcome 6 Adverse events ‐ Nausea.
1.7. Analysis.
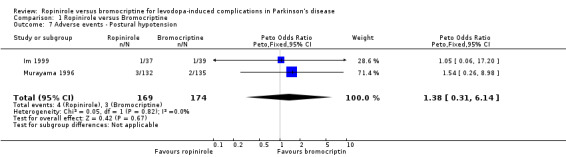
Comparison 1 Ropinirole versus Bromocriptine, Outcome 7 Adverse events ‐ Postural hypotension.
1.8. Analysis.
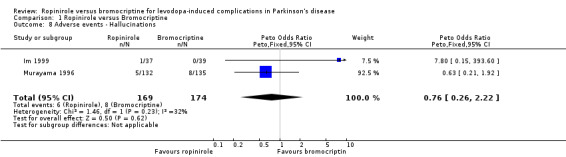
Comparison 1 Ropinirole versus Bromocriptine, Outcome 8 Adverse events ‐ Hallucinations.
1.9. Analysis.
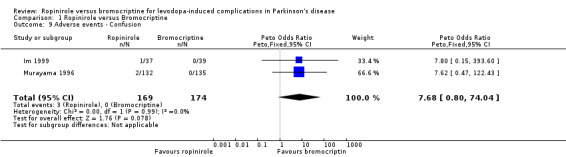
Comparison 1 Ropinirole versus Bromocriptine, Outcome 9 Adverse events ‐ Confusion.
1.10. Analysis.
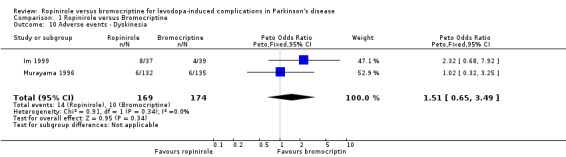
Comparison 1 Ropinirole versus Bromocriptine, Outcome 10 Adverse events ‐ Dyskinesia.
1.11. Analysis.
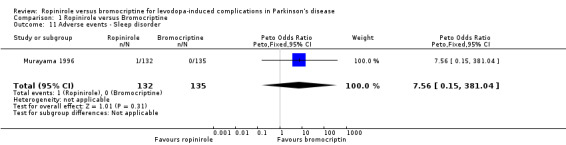
Comparison 1 Ropinirole versus Bromocriptine, Outcome 11 Adverse events ‐ Sleep disorder.
1.12. Analysis.
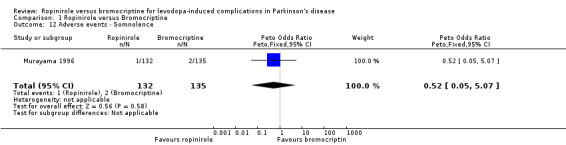
Comparison 1 Ropinirole versus Bromocriptine, Outcome 12 Adverse events ‐ Somnolence.
1.13. Analysis.
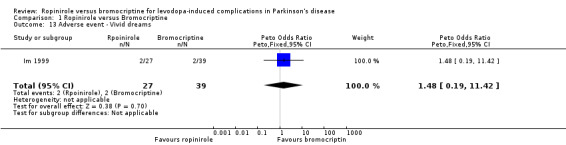
Comparison 1 Ropinirole versus Bromocriptine, Outcome 13 Adverse event ‐ Vivid dreams.
1.14. Analysis.
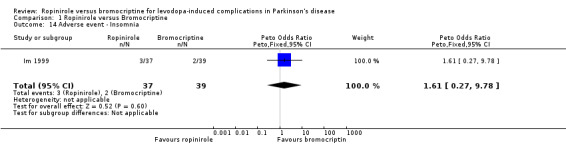
Comparison 1 Ropinirole versus Bromocriptine, Outcome 14 Adverse event ‐ Insomnia.
1.15. Analysis.
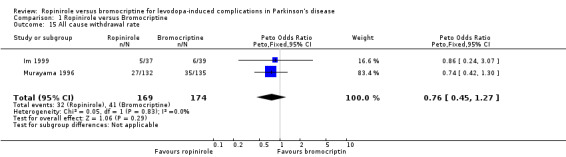
Comparison 1 Ropinirole versus Bromocriptine, Outcome 15 All cause withdrawal rate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brunt 1999.
| Methods | Randomised, double‐blind, parallel group design. Randomisation by computer generated random numbers. Patients randomised 2:1, ropinirole: bromocriptine. Intention‐to‐treat analysis. Location: 66 sites, 11 European countries, Israel, South Africa, and Canada. Duration: 25 weeks, titration phase maximum 13 weeks. | |
| Participants | Ropinirole: 367 patients with 68 drop‐outs (19%). Bromocriptine: 188 patients with 36 drop‐outs (19%) No details of terminations given. Group B patients: Ropinirole: 88 Bromocriptine: 51 Age (group B): Ropinirole 63.5 years Bromocriptine 65.1 years. Hoehn and Yahr at baseline: Ropinirole = 2.8 Bromocriptine = 2.9. Inclusion criteria: IPD then divided into 3 groups: Group A: low dose L‐dopa Group B: high dose L‐dopa with motor complications Group C: L‐dopa and dopamine agonists with motor complications. Age over 30 years. Exclusion criteria: Psychosis, dementia, severe systemic disease, history of hallucinations. | |
| Interventions | Therapy titrated over a maximum of 13 weeks, then optimal therapeutic dose maintained to end of study. Ropinirole titrated to a maximum of 24mg/d, mean 10 mg/d. Bromocriptine titrated to a maximum of 39.9mg/d, mean 18 mg/d. Levodopa could be reduced. | |
| Outcomes | UPDRS motor score Levodopa dose On/Off charts Clinicians global impression score (7 points) Responders: Group A: 20% reduction in L‐dopa and 20% reduction in UPDRS. Group B: 20% reduction in L‐dopa and 20% reduction in off time. Group C: 20% reduction in L‐dopa and improved CGI. Adverse events | |
| Notes | Only data from Group B (IPD with motor complications on high dose of l‐dopa) used in comparison. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Im 1999.
| Methods | Randomised open parallel group design. Method of randomisation not stated. Data analysed on a per protocol basis. Location: 1 site, South Korea. Duration: 16 weeks. | |
| Participants | Ropinirole: 37 patients with 5 drop‐outs (14%). Bromocriptine: 39 patients with 6 drop‐outs (15%). Details of terminations given. Age: Ropinirole 63.5 (SD 10.8); Bromocriptine 60.0 (SD 8.3). Hoehn and Yahr score at baseline: Ropinirole 2.5; Bromocriptine 2.4. Inclusion criteria: IPD >40 years old, Hoehn & Yahr stage 2‐4, on l‐dopa with motor complications or on a dopamine agonist (not bromocripitine) as an adjunct to l‐dopa. Exclusion criteria: Late stage advance patients, severe orthostatic hypotension, severe systemic disease, arthritis, dementia, neurosis, psychosis, history of alcoholism or drug dependancy, on > 5mg/day pergolide or lisuride, hypersensitivity or contraindications to ergot alkaloids including bromocriptine. | |
| Interventions | Therapy titrated over 8 weeks, then optimal therapeutic dose continued until end of study. Ropinirole: Initial dose 0.75 mg/d, minimum dose 4.5 mg/d, maximum dose 9mg/d. Bromocriptine: Initial dose 1.25mg/d, minimum dose 10mg/d, maximum dose 17.5mg/d. L‐dopa reduction allowed after optimal dose of study drug achieved. | |
| Outcomes | Primary: >20% reduction in l‐dopa. Secondary: reduction in l‐dopa. >20% increase in UPDRS motor score. >20% reduction in off hours. Improvement in CGI Adverse events. | |
| Notes | 1 drop‐out in bromocriptine group due to lack of efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Murayama 1996.
| Methods | Randomised, double‐blind, parallel group design. Randomisation method not given. Randomised in blocks of 4, (2 ropinirole, 2 bromocriptine). Intention to treat and per protocol analysis. Location: 105 sites, Japan. Duration: 8 weeks. | |
| Participants | On L‐dopa group: Ropinirole: 132 patients with 27 drop‐outs (20%). Bromocriptine: 135 patients with 35 drop‐outs (26%). Details of terminations given. Age: ropinirole 65.8 years (SD 8.5), bromocriptine 64.2 years (SD 9.1). Hoehn and Yahr at baseline: both groups median stage III. Inclusion criteria: IPD ‐ 2 groups: de novo and L‐dopa treated patients. Some of the latter may not have suffered motor complications. Age 20‐80 years. Exclusion criteria: Severe psychiatric symptoms, severe cardiac, hepatic or renal disease. | |
| Interventions | Ropinirole: Initial dose 0.5mg/d increased by 1mg/d increments weekly to a maximum of 9mg/d, mean 4mg/d. Bromocriptine: Initial dose 1.25mg/d increased by 2.5mg/d increments weekly to a maximum of 22.5mg/d, mean 9.2mg/d. Levodopa could be reduced. | |
| Outcomes | Hoehn and Yahr Individual symptoms of Parkinson's Adverse events | |
| Notes | Only data from the l‐dopa treated group used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Contributions of authors
CEC instigated and arranged funding for the review. Both authors performed the search strategy, the data abstraction, and the analysis of the data. Both authors wrote the review and will act as guarantors for the accuracy of the review.
Declarations of interest
CEC has taken part in clinical trials with ropinirole. He has received payment from SmithKline Beecham for lectures, for attending meetings, and for serving on advisory boards.
Edited (no change to conclusions)
References
References to studies included in this review
Brunt 1999 {unpublished data only}
- Brunt C, Aitken C. A double‐blind comparitive study of ropinirole versus bromocriptine in the treatment of parkinsonian patients not optimally controlled on l‐dopa. Journal of Neurology 1996;243(Supplement 2):S38. [Google Scholar]
- Brunt ER, Brooks DJ, Korczyn AD, Montastruc J‐L, Poewe WH, Stocchi F. A six‐month multicentre, double‐blind, bromocriptine‐controlled study of the efficacy and saftey of ropinirole in the treatment of parkinsonian patients not optimally controlled on L‐dopa. SB Pharmaceuticals 1999:11‐35. [DOI] [PubMed]
- Group TRS. A double‐blind comparative study of ropinirole vs bromocriptine in the treatment of parkinsonian patients not optimally controlled on l‐dopa. Movement Disorders 1996;11(Supplement 1):188. [Google Scholar]
Im 1999 {published and unpublished data}
- Im JH, Ha JH, Cho IS, Lee MC. The efficacy and saftey of ropinirole as an adjunct to levodopa in Korean patients with Parkinson's disease. Parkinsonism and Related Disorders. 1999; Vol. 5:S75.
Murayama 1996 {published and unpublished data}
- Murayama S, Narabayashi H, Kowa H, et al. Clinical evaluation of ropinirole hydrochloridein patients with Parkinson's disease: a double‐blind comparative study versus bromocriptine mesylate. Japanese Pharmacological Therapeutics 1996;24:1939‐2007. [Google Scholar]
- Murayama S, Narabayashi H, Kowa H, et al. Clinical evaluation of ropinirole hydrochloride (SK&F 101468) in patients with Parkinson's disease ‐ A double‐blind comparitive study vs. bromocriptine mesilate ‐. SmithKline Beecham Pharmaceuticals, CPMS 080.
Additional references
CONSORT 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA 1996;276(8):637‐639. [DOI] [PubMed] [Google Scholar]
PDRG 1993
- Parkinson's Disease Study Group in the United Kingdom. Comparison of the therapeutic effects of levodopa, levodopa and selegiline, and bromocriptine in patients with early, mild Parkinson's disease: three year interim report. British Medical Journal 1993;307:469‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 1986
- Quinn N, Critchley P, Parkes D, Marsden CD. When should levodopa be started?. Lancet 1986;ii:985‐986. [DOI] [PubMed] [Google Scholar]
Rajput 1984
- Rajput AH, Stern W, Laverty WH. Chronic low‐dose levodopa therapy in Parkinson's disease. Neurology 1984;34:991‐996. [DOI] [PubMed] [Google Scholar]


