Abstract
Background
Determining factors affecting the transmission of rifampicin (RR) and multidrug-resistant (MDR) Mycobacterium tuberculosis complex strains under standardized tuberculosis (TB) treatment is key to control TB and prevent the evolution of drug resistance.
Methods
We combined bacterial whole genome sequencing (WGS) and epidemiological investigations for 37% (n = 195) of all RR/MDR-TB patients in Cameroon (2012–2015) to identify factors associated with recent transmission.
Results
Patients infected with a strain resistant to high-dose isoniazid, and ethambutol had 7.4 (95% CI 2.6–21.4), and 2.4 (95% CI 1.2–4.8) times increased odds of being in a WGS-cluster, a surrogate for recent transmission. Furthermore, age between 30 and 50 was positively correlated with recent transmission (adjusted OR 3.8, 95% CI 1.3–11.4). We found high drug-resistance proportions against three drugs used in the short standardized MDR-TB regimen in Cameroon, i.e. high-dose isoniazid (77.4%), ethambutol (56.9%), and pyrazinamide (43.1%). Virtually all strains were susceptible to fluoroquinolones, kanamycin, and clofazimine, and treatment outcomes were mostly favourable (87.5%).
Conclusion
Pre-existing resistance to high-dose isoniazid, and ethambutol is associated with recent transmission of RR/MDR strains in our study. A possible contributing factor for this observation is the absence of universal drug susceptibility testing in Cameroon, likely resulting in prolonged exposure of new RR/MDR-TB patients to sub-optimal or failing first-line drug regimens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06593-8.
Keywords: MDR-TB, Mycobacterium tuberculosis, Cameroon, Transmission
Background
An estimated half a million tuberculosis (TB) patients are eligible for a rifampicin resistant or multidrug resistant (additional resistance to isoniazid) tuberculosis (RR/MDR-TB) treatment regimen each year [1]. To reduce the exceptionally long MDR-TB treatment period (minimum 18 months), high treatment costs, severe drug-related side effects and thus suboptimal adherence and low cure rates in many settings worldwide, in 2016 the WHO endorsed a shorter (9–12 months), standardized MDR-TB treatment regimen, including seven antibiotics. In the intensive phase (4–6 months) patients receive high dose isoniazid, ethambutol, pyrazinamide, prothionamide, clofazimine, kanamycin, and a fluoroquinolone. The 5 month continuation phase includes ethambutol, pyrazinamide, a fluoroquinolone, and clofazimine [2, 3]. The short MDR-TB regimen is recommend for patients infected with a RR/MDR Mycobacterium tuberculosis complex (MTBC) strain who have not been treated for more than 1 month with the above mentioned antibiotics and for whom resistance to fluoroquinolones and second-line injectable drugs has been excluded [4]. The most recent guidance is for a similar shorter, all-oral 9–12 month regimen with bedaquiline replacing the second-line injectable [5].
Notably, the short course, standardized MDR-TB regimen achieved high cure rates (> 85%) in Bangladesh, Cameroon, and Niger, however, its implementation in settings with high pyrazinamide and ethambutol resistance rates, e.g. in Eastern Europe, was criticized [6–9]. Prudent usage with careful follow-up has been recommended when resistance to isoniazid, pyrazinamide or second-line injectable drugs is present at the initiation of the short MDR-TB therapy [10–12]. Recent studies have shown that resistance to individual drugs, except fluoroquinolones, at the start of the therapy can be tolerated and did not compromise treatment outcomes [2, 10, 11]. More pronounced effects on treatment outcomes were observed when resistance to both a fluoroquinolone and pyrazinamide were diagnosed at baseline [2]. Besides the elevated risks of treatment failure under standardized treatment regimens, pre-existing drug resistances have been linked to increased transmission rates of MDR MTBC strains globally [13–15].
In Cameroon, a previous observational prospective study of patients treated between 2008 and 2011 reported 134/150 (89%) patients with successful outcomes on the short MDR-TB regimen [16]. Here, we analysed RR/MDR strains consecutively sampled from 195 patients between 2012 and 2015 at the Tuberculosis Reference Laboratory Bamenda (TBRL), representing 37.0% (195/527) of all laboratory confirmed MDR-TB cases in Cameroon during the study period (WHO data repository). We employed whole genome sequencing (WGS) and epidemiological analysis to define drug resistance profiles prior to the initiation of the short MDR-TB therapy, to investigate drug resistance proportions over time, and to identify factors associated with recent transmission of MDR-MTBC strains in Cameroon.
Methods
Study design and study population
We performed a retrospective genomic epidemiological study to analyse transmission patterns and risk factors for the transmission of RR/MDR MTBC strains in Cameroon. Specimens from consecutive TB-patients with bacteriologically-confirmed RR/MDR-TB received between December 31, 2011 and June 26, 2015 at the Tuberculosis Reference Laboratory Bamenda (TBRL) were included in the study. This laboratory serves as the reference laboratory for four geographical regions of Cameroon, covering an estimated population of approximately 7.8 million people and including 40% of the notified TB cases in the country from 2012 to 2015 (WHO data). Following the National TB Program guidelines, TB culture and drug susceptibility testing is performed systematically for previously treated TB patients, prisoners and known contacts of people with MDR-TB. Initial detection of TB disease in Cameroon was performed primarily with acid fast bacilli smear microscopy during the study period. For people initiating TB treatment with smear-positive TB, cure was defined as a negative smear at 5 and/or 6 months on treatment, following the NTP guidance. Further details on the data collection and contact tracing questionnaires are provided in the Additional file 1.
Phenotypic and molecular drug susceptibility tests
Routine diagnostics were performed at the TBRL Bamenda, a laboratory accredited in accordance with the recognized International Standard ISO 15189:2012 (SANAS Accredited Medical Laboratory, No. M0593). Molecular tests for rifampicin resistance were performed either by Xpert MTB/RIF (Cepheid, U.S.) or Genotype MTBDRplus (Hain Life Science, Germany) according to the manufacturer’s instructions. The proportion method on Löwenstein–Jensen media with WHO recommended critical concentrations was employed for phenotypic drug susceptibility tests. Further details are provided in the Additional file 1.
Next generation sequencing
WGS was performed on an Illumina NextSeq 500 instrument using Nextera XT library preparation kit according to manufacturer’s instructions (Illumina, USA). Raw read data (fastq files) were deposited in the European Nucleotide Archive under the accession number PRJEB40777, and processed with the MTBseq pipeline as described previously [17]. Details on the phylogenetic analysis, molecular cluster definitions, and genotypic drug resistance prediction can be found in the Additional file 1.
Statistics
A variance analysis to compare proportions of drug resistances from 2012 to 2015 was performed with a Kruskal–Wallis test and a pairwise Dunn’s post-hoc test, considering a significance level of 0.05. Proportions of resistance to individual drugs and 95% confidence intervals (CI) were calculated with the Wilson Score interval. We employed univariate and multiple logistic regression models to obtain odds ratios for factors associated with recent transmission, i.e. molecular clusters, by using R version 4.0.2 and the glm function. Response variables were transformed to 0 (ungrouped) and 1 (clustered). As predictors for an increased transmission likelihood, we analyzed the variables MTBC lineage, number of genotypic resistances, age, gender, HIV status, and genotypic resistance to isoniazid, ethambutol, pyrazinamide, and prothionamide. Other drug resistances were found at very low prevalence throughout the study period and were not considered as predictor variables. Most people included in this analysis had a history of TB treatment (84%), so treatment history was also not included as a predictor variable. Missing data (NA) was kept as a separate category, and categorical data was transformed to factors. Factors with P < 0.1 in the univariate model were included in the multiple logistic regression. The best model was determined with a backwards exclusion approach aiming for the best statistical support, i.e. lowest Akaike information criterion (AIC) value. The resulting model was supported by a forward selection approach. Differences between patient characteristics (included versus excluded patients) were determined with chi-squared tests for categorical variables, and with a Mann Whitney U test for age. For chi-squared tests, we treated missing data as its own category, and in addition performed pairwise Fisher exact tests, excluding missing data.
Results
Patient characteristics and treatment outcomes
Specimens from 261 patients with RR/MDR-TB were received at the laboratory during the study period. Out of these 195 (75%) were included in the current analysis. For 66 patients, WGS data was not available because specimens could not be either cultured or sub-cultured, or extracted DNA was not suitable for WGS. These 66 excluded patients had similar age, sex, and geographic distribution as those included in the analysis; there were more excluded patients with an unknown HIV status and history of TB treatment relapse (Additional file 4: Table S3). Of the 195 patients with specimens included in the analysis, the median age was 34 years (IQR 27–43 years), 78 (40%) were female, and 163 (84%) had a known history of TB treatment. In total, 149/195 (76.4%) patients started MDR-TB treatment in the study period, including 126/149 (84.6%) patients with a good MDR-TB therapy outcome, i.e. cured or treatment completed, 15/149 (10.1%) patients who died after starting MDR-TB treatment, 5/149 (3.4%) patients who were lost to follow-up, and 3/149 (2.0%) patients who failed the treatment. The remaining 46/195 (23.6%) patients did not start MDR-TB treatment and were lost to follow-up.
MTBC population structure in Cameroon
Based on 10,838 SNPs, we calculated a maximum likelihood phylogeny using a general time reversible substitution model, and further classified MTBC strains into different phylogenetic lineages according to a recently proposed SNP barcode from Coll et al. [18] (Fig. 1).
Fig. 1.
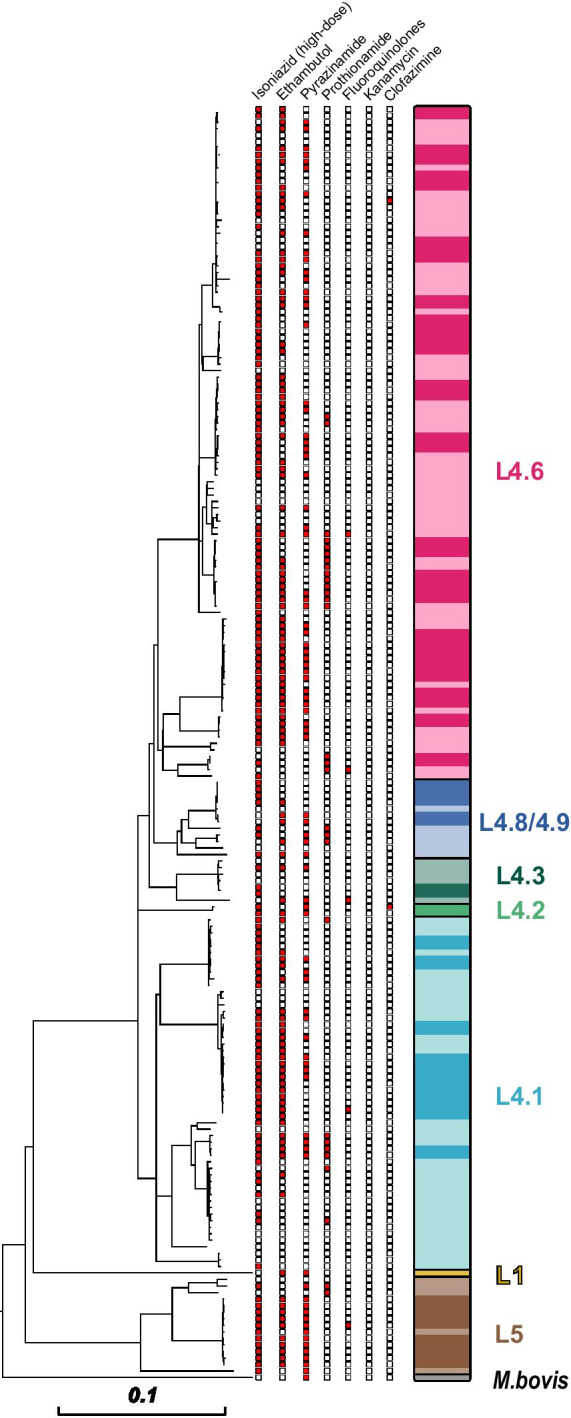
Drug resistance and molecular clusters of multidrug resistant (MDR) Mycobacterium tuberculosis complex (MTBC) strains in Cameroon. Maximum likelihood phylogeny of 195 rifampicin resistant and MDR-MTBC strains from Cameroon (2012–2015). MTBC lineages are color coded, stronger colours denote closely related strains (≤ 5 SNP pairwise distance) as surrogate for recently transmitted strains. Genotypic resistances to individual drugs are represented by red squares
In total, 15/195 (7.7%) MTBC strains belonged to lineage 5/M. africanum, one strain (0.5%) was classified as lineage 1, one strain (0.5%) was identified as M. bovis. The majority, i.e. 178/195 (91.3%), of patients were infected with a lineage 4 MTBC strain. Within lineage 4, we further stratified the strains to particular sub-lineages as follows: 4.1 (54/195, 27.7%), 4.2 (2/195, 1.0%), 4.3 (7/195, 3.6%), 4.6 (103/195, 52.8%), 4.8 (11/195, 5.6%), and 4.9 (1/195, 0.5%) (Fig. 1).
Genotypic drug resistance profiles
In total, we investigated 27 genes associated with resistance to any drug in the short MDR-TB regimen (Additional file 2: Table S1). With regard to rifampicin resistance, we identified 29 different mutations or combination of mutations mainly in the rifampicin resistance determining region of rpoB (Additional file 2: Table S1). Three strains harboured the mutation rpoB V170F outside the rifampicin resistance determining region described before as rifampicin resistance marker [19]. Moreover, 57/195 (29.2%) rifampicin resistant strains also had an additional putative compensatory mutation in genes coding for the adjacent RNA-polymerase subunits RpoA or RpoC. We assumed that MTBC strains were resistant to high-dose isoniazid when they harboured a mutation in the catalase gene katG at position 315, or a pre-mature stop codon, or a frame shift mutation. Based on this classification, we observed high-dose isoniazid resistance in 77.4% (151/195, 95% CI 71.1–82.7%) of all strains (Table 1).
Table 1.
Univariate logistic regression analysis to identify factors associated with molecular clusters, i.e. as a surrogate for recent transmission
| Ungrouped (n = 110) | % | Clustered (n = 85) | % | Univariate logistic regression | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% lower | 95% upper | P value | |||||
| Age | ||||||||
| > 50 | 18 | 16.4 | 6 | 7.1 | REF | |||
| 30–50 | 51 | 46.4 | 44 | 51.8 | 2.6 | 0.9 | 7.1 | 0.06 |
| < 30 | 34 | 30.9 | 30 | 35.3 | 2.6 | 0.9 | 7.5 | 0.07 |
| Unknown | 7 | 6.4 | 5 | 5.9 | 2.1 | 0.5 | 9.4 | 0.31 |
| Gender | ||||||||
| Female | 41 | 37.3 | 37 | 43.5 | REF | |||
| Male | 66 | 60 | 46 | 54.1 | 0.8 | 0.4 | 1.4 | 0.39 |
| Unknown | 3 | 2.7 | 2 | 2.4 | 0.7 | 0.1 | 4.7 | 0.75 |
| HIV status | ||||||||
| Negative | 50 | 45.5 | 51 | 60 | REF | |||
| Positive | 33 | 30 | 20 | 23.5 | 0.6 | 0.3 | 1.2 | 0.13 |
| Unknown | 27 | 24.5 | 14 | 16.5 | 0.5 | 0.2 | 1.1 | 0.08 |
| Lineage | ||||||||
| Others | 7 | 6.4 | 10 | 11.8 | REF | |||
| L4.X | 49 | 44.5 | 26 | 30.6 | 0.4 | 0.1 | 1.1 | 0.07 |
| L4.6 | 54 | 49.1 | 49 | 57.6 | 0.6 | 0.2 | 1.8 | 0.39 |
| Genotypic resistances (n) | 1.7 (mean) | 2.4 (mean) | 1.7 | 1.3 | 2.3 | 6.64E−05 | ||
| (per unit increase) | ||||||||
| Compensatory mutation | ||||||||
| No | 85 | 77.3 | 53 | 62.4 | REF | |||
| Yes | 25 | 22.7 | 32 | 37.6 | 2.1 | 1.1 | 3.8 | 0.02 |
| High dose isoniazid | ||||||||
| Susceptible | 39 | 35.5 | 5 | 5.9 | REF | |||
| Resistant | 71 | 64.5 | 80 | 94.1 | 8.8 | 3.3 | 23.5 | 1.53E−05 |
| Ethambutol | ||||||||
| Susceptible | 62 | 56.4 | 22 | 25.9 | REF | |||
| Resistant | 48 | 43.6 | 63 | 74.1 | 3.7 | 2 | 6.8 | 3.02E−05 |
| Pyrazinamide | ||||||||
| Susceptible | 70 | 63.6 | 41 | 48.2 | REF | |||
| Resistant | 40 | 36.4 | 44 | 51.8 | 1.8 | 1.1 | 3.3 | 0.03 |
| Prothionamide | ||||||||
| Susceptible | 87 | 79.1 | 69 | 81.2 | REF | |||
| Resistant | 23 | 20.9 | 16 | 18.8 | 0.9 | 0.4 | 1.8 | 0.72 |
P-values < 0.05 indicated in bold text
Factors with P ≤ 0.1 were included in backwards exclusion/forward selection procedures to obtain the best supported multiple logistic regression model, which results are reported in the main text only
OR odds ratio
Genotypic drug resistance rates to drugs other than isoniazid across all patient were as follows: ethambutol 56.9% (111/195, 95% CI 49.9–63.7%), pyrazinamide 43.1% (84/195, 95% CI 36.3–50.1%), prothionamide 20.0% (39/195, 95% CI 15.0–26.2%), kanamycin 0% (0/195, 95% CI 0.0–1.9%), fluoroquinolones 2.6% (5/195, 95% CI 1.1–5.9%), and clofazimine 1.0% (5/195, 95% CI 0.3–3.7%). We observed overall no differences (0.21 < P < 0.92, Kruskal–Wallis test) between individual drug resistance proportions over the 3.5 years study period (Fig. 2).
Fig. 2.
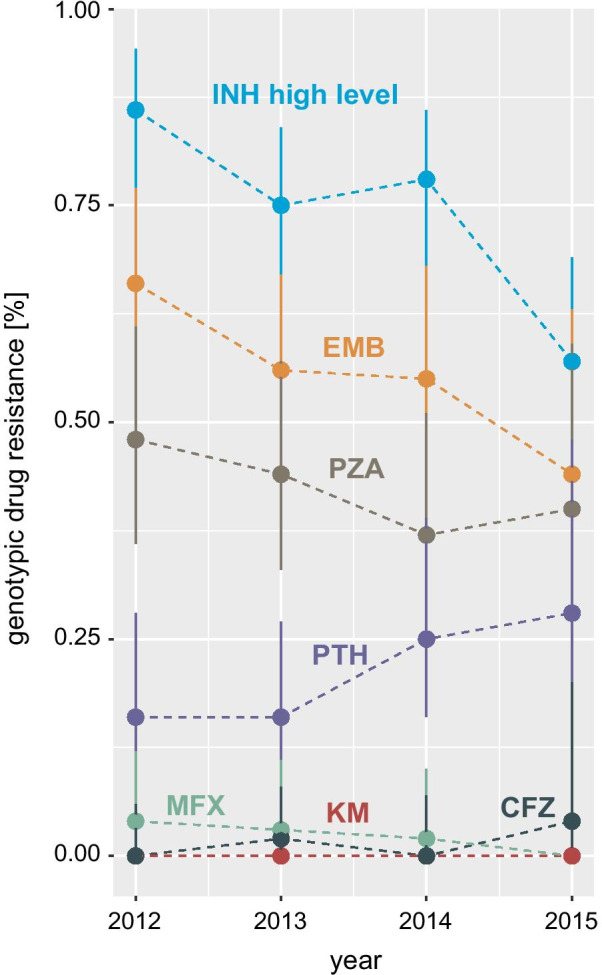
Drug resistance proportions among multidrug resistant (MDR) tuberculosis patients in Cameroon (2012–2015). Annual changes of genotypic drug resistance proportions of 195 rifampicin resistant and MDR Mycobacterium tuberculosis complex strains in Bamenda, Cameroon. Colours code for individual drugs, vertical lines represent 95% confidence interval. INH = isoniazid, EMB = ethambutol, PZA = pyrazinamide, PTH = prothionamide, MFX = moxifloxacin, KM = kanamycin, CFZ = clofazimine
MTBC transmission networks
To allocate patients to recent transmission networks, we defined clusters based on a strain-to-strain genetic distance measurement, i.e. a maximum distance of 5 SNPs between at least two strains [20–22].
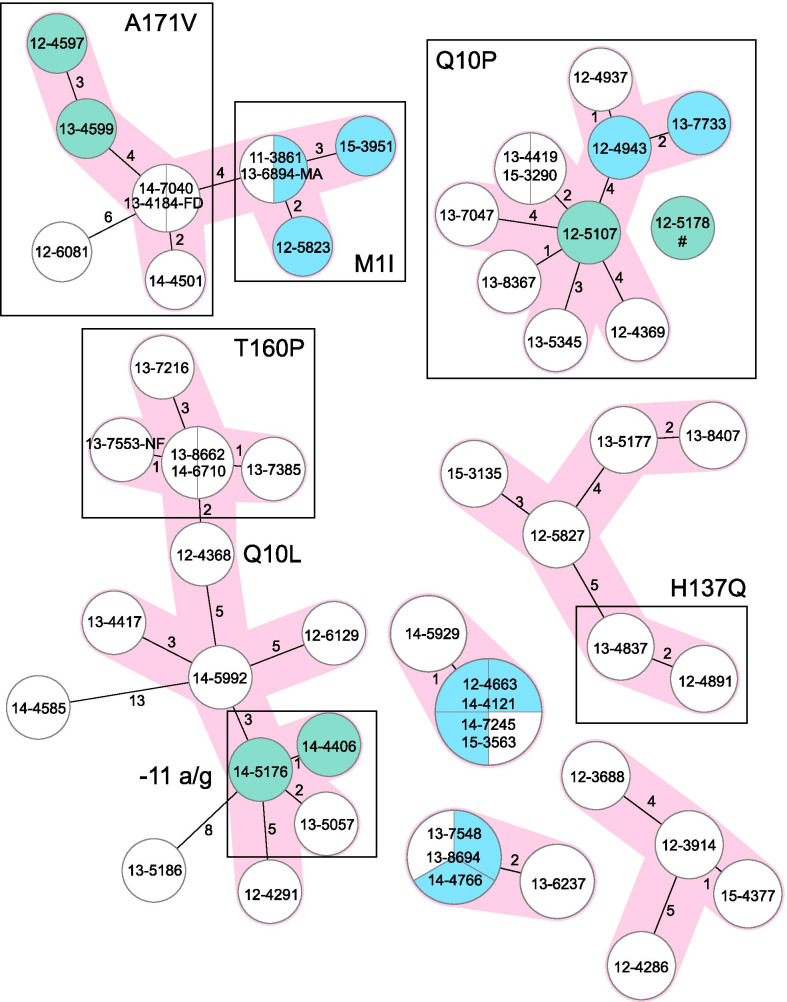
Overall, 85/195 (43.6%) strains were grouped in 29 different SNP-based clusters each comprising 2–10 strains. As another measurement of strain relatedness, we performed a core genome multi locus sequence type (cgMLST) analysis based on a gene-to-gene (allele) difference [23]. In total, 89/187 (47.6%) MTBC strains were assigned to a cgMLST clonal complex with a maximum pairwise distance of 5 alleles, mainly confirming the previously defined SNP-based clusters and proportions of strains in clusters. For the cgMLST analysis, 8 strains were excluded with ≥ 10% bad quality alleles (Additional file 2: Table S1). Retrospective contact tracing confirmed that 21/85 (24.7%) patients with strains in SNP-based clusters had an epidemiological link, mainly between household contacts (Additional file 3: Table S2). Confirmed transmission events often involved MTBC strains with identical pncA mutations that confer pyrazinamide resistance (Fig. 3, Additional file 3: Table S2). Notably, some SNP-based clusters and cgMLST clonal complexes were further differentiated by distinct pncA mutations suggesting recent acquisition of pyrazinamide resistance and continued transmission of pyrazinamide resistant strains (Fig. 3, Additional file 3: Table S2).
Fig. 3.
Transmission networks among multidrug resistant (MDR) tuberculosis patients in Cameroon. Seven largest molecular clusters of rifampicin resistant and MDR Mycobacterium tuberculosis complex strains in Bamenda, Cameroon. Numbers on connecting lines indicate alelle differences; pink branches connect strains with a maximum distance of five alleles. Boxes indicate strains with identical pncA mutations, and identical node colors represent patients with confirmed epidemiological links
Factors associated with recent transmission of MDR-MTBC strains
We employed logistic regression models to identify bacterial genetic and patient demographic factors associated with recent transmission, using molecular clusters (SNP-based) as a surrogate. In the univariate logistic regression analysis we did not find associations of MTBC lineage, age, gender, or HIV status with recent transmission (Table 1). However, RR/MDR strains with a putative compensatory mutation in genes coding for RNA-polymerase subunits rpoA, and rpoC were associated with recent transmission (OR 2.1, 95% CI 1.1–3.8, P = 0.02). Furthermore, an increasing number of pre-existing resistances against drugs used in the short MDR-TB regimen was associated with an increased odds of patients being recently infected (OR 1.7 per unit increase, i.e. for any additional drug resistance, 95% CI 1.3–2.3, P < 0.001). Strains that were considered resistant against high-dose isoniazid (OR 8.8, 95% CI 3.3–23.5, P < 0.001), ethambutol (OR 3.7, 95% CI 2.0–6.8, P < 0.001), or pyrazinamide (OR 1.8, 95% CI 1.1–3.3, P = 0.03) were associated with recent transmission (Table 1).
Next, we considered all predictors with P ≤ 0.1 in a multiple logistic regression model and performed a step-wise backwards exclusion of factors aiming for the best supported model, i.e. lowest AIC value. The predictor “number of genotypic drug resistances” showed high collinearity (variance inflation factor 43) and was not considered in the multiple logistic regression model. The best supported model included to against high-dose isoniazid (aOR 7.4, 95% CI 2.6–2.4, P < 0.001), resistance to ethambutol (aOR 2.4, 95% CI 1.2–4.8, P = 0.014), and an age between 30 and 50 (aOR 3.8, 95% CI 1.3–11.4, P = 0.016) as relevant predictors for recent transmission of MDR-MTBC strains. Resistance to pyrazinamide, HIV status, and the presence of a putative compensatory mutation were not found to be strong predictors for recent transmission in the multiple logistic regression analysis. This was confirmed in a forward selection approach.
Discussion
Our genomic epidemiological study of RR/MDR MTBC strains in Cameroon showed that, besides age of 30–50 years, genotypic resistance to high-dose isoniazid, and ethambutol is a strong predictor for molecular clusters, which is a surrogate for recent transmission. This may be at least partially due to current TB diagnostics and TB patient management in the country. Drug susceptibility tests are prioritized for patients with a high risk of RR/MDR-TB, including patients with a history of TB treatment, prisoners, and known contacts of people with MDR-TB. As a result of this prioritization, RR/MDR-TB patients without these risk factors often start on a first-line drug regimen comprising isoniazid, rifampicin, ethambutol, and pyrazinamide. In 2019 for instance, only an estimated 24% of patients with bacteriologically-confirmed TB in Cameroon had a test for RR/MDR-TB, as compared to the global average of 61% [1]. In fact, undiagnosed RR/MDR-TB has been associated with transmission, as well as selection of additional drug resistances [24], and likely contributes to the association between first-line drug resistances, such as isoniazid and ethambutol, and transmission in our study. Thus, access to universal drug susceptibility testing for all TB patients, as recommended by the WHO [1], is urgently needed in Cameroon to maintain the effectivity of the current MDR-TB treatment regimen, and to curb the evolution of drug resistance.
Although drug resistance proportions remained stable over the relatively short 3.5 year study period, it remains to be determined if pre-existing drug resistances may impact on outcomes of the standardized short MDR-TB regimen in the next years. The very low prevalence of fluoroquinolone, kanamycin and clofazimine resistance likely explains the generally favourable treatment outcomes [16]. However, containing and reducing the risk of RR/MDR-MTBC transmission in general will be crucial to preserve the overall effectiveness of the short MDR-TB regimen and to prevent selection of drug resistances in the region.
There is mounting evidence that globally increasing MDR-TB rates are largely attributable to recent transmission of RR/MDR-MTBC strains, rather than to acquired resistance while on TB treatment [13–15, 25]. Our results suggest that pre-existing drug resistance is an important factor that is associated with recent transmission of RR/MDR MTBC strains in Cameroon, and it should be considered with other factors such as diagnostic delays in many other world settings as it renders initial TB treatment regimens less effective [24, 26]. Patients, especially those with undiagnosed RR/MDR-TB and infected with MTBC strains with additional resistances against ethambutol and pyrazinamide, are more prone to fail initial first-line TB therapies [27]. Likewise, suboptimal therapies and delayed diagnosis increase the time of infectiousness and the likelihood of transmission [27–30].
In addition, bacterial genetic factors such as compensatory evolution, which counteracts the fitness costs of drug-resistance mediating mutations, has also been shown to drive the transmission of MDR-MTBC strains in some settings [14, 24, 31, 32]. In a univariate analysis we found an association of putative compensatory mutations with recent transmission (i.e. molecular clusters), however, including the presence of such mutations did not improve the predictability of recent transmission in the multiple logistic regression analysis.
The observed overall favourable treatment outcomes in our study are in line with a recent phase 3 non-inferiority trial in Bangladesh in patients infected with rifampicin resistant strains but susceptible to fluoroquinolones and aminoglycosides [11].
On the other hand, the short MDR-TB regimen also generated concerns about its efficacy in settings with high resistance rates to ethambutol and pyrazinamide, e.g. Eastern Europe, but also Sub-Saharan Africa, exposing patients to drugs with a high likelihood of pre-existing resistance [6–9, 33]. Thus, decision makers in many settings are confronted with the dilemma that the short MDR-TB regimen is at least non-inferior to a long-term conventional individualized therapy with high cure rates, but it may increase the risk of drug resistance evolution over time. In fact, using drugs despite proven resistance is highly associated with poor treatment outcomes for conventional MDR-TB regimens [24]. Critically, the loss of fluoroquinolones as an effective second-line TB drug would jeopardize the efficacy of both short and conventional MDR-TB therapies [34–36].
Our genomic epidemiological study has particular limitations. The large number of patients lost to follow-up prevented the analysis of factors associated with treatment outcomes. As mentioned before, previously treated patients are prioritized for drug susceptibility testing, and thus are overrepresented in our patient cohort (84% of those studied here had a history of TB treatment). The proportions of pre-existing drug resistances and their implication on the transmission of MTBC strains may not be representative for undiagnosed RR/MDR-TB patients in Cameroon. Furthermore, transmission events that occurred before the study and transmission events during the study including patients that were diagnosed after the study period could not be captured with our analysis. Lastly, the impact of socioeconomic factors on the transmission of MTBC strains could only be partially investigated through retrospective tracing of epidemiological links in molecular clusters, and other relevant factors such as time to diagnosis and detailed treatment histories were not available for inclusion in the analysis.
Conclusions
We show that resistance to high-dose isoniazid, and ethambutol is associated with recent transmission of MDR-MTBC in Cameroon. Our findings highlight the importance of universal drug susceptibility testing for the early identification of patients who need RR/MDR-TB treatment. The rapid initiation of appropriate treatment regimens will be crucial for both improving treatment outcomes and minimizing transmission of MDR-TB strains as a basis for future MDR-TB control.
Supplementary Information
Additional file 1. Supplementary Methods.
Additional file 2: Table S1. Molecular and phenotypic data of MTBC isolates from 195 RR/MDR-TB patients in Cameroon (2013–2015) including patient characteristics.
Additional file 3: Table S2. Contact tracing data of patients in molecular clusters and pyrazinamide resistance conferring mutations in pncA.
Additional file 4: Table S3. Comparison of patient characteristics, included versus excluded RR/MDR-TB patients.
Acknowledgements
We thank Angela Neh, Vanessa Mohr, Fenja Boysen, Tanja Niemann, Carina Hahn, Tanja Struve-Sonnenschein, and Anja Lüdemann for excellent technical assistance.
Matthias Merker
is an Assistant Professor at the Research Center Borstel. His research is focused on the evolution of drug resistances, M. tuberculosis population genomics and the application and translation of next generation sequencing techniques into clinical practice.
Abbreviations
- MTBC
Mycobacterium tuberculosis complex
- RR
Rifampicin resistance
- MDR
Multidrug resistance
- TB
Tuberculosis
- SNP
Single nucleotide polymnorphism
- HIV
Human immunodeficiency virus
- cgMLST
Core genome multi locus sequence typing
Authors’ contributions
MM, MSS conceived the study. MM, SN, MSS drafted the manuscript. NFE and VC performed strain cultivation, DNA extraction, and drug susceptibility tests. YN performed cluster investigations to identify epidemiological links. TAK, VD performed whole genome sequencing and molecular drug resistance predictions. MM performed statistics and phylogenetic analysis. CK, JN, SN gave substantial intellectual input and helped with the interpretation of the data. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work performed in Germany was funded by the German Center for Infection Research (DZIF), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germanys Excellence Strategy—EXC 2167 Precision Medicine in Chronic Inflammation, and the Leibniz Science Campus Evolutionary Medicine of the LUNG (EvoLUNG). The funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Availability of data and materials
Raw sequencing data (fastq files) of Mycobacterium tuberculosis complex strains were deposited in the European Nucleotide Archive under the Accession Number PRJEB40777.
Declarations
Ethics approval and consent to participate
The study was approved by the National Ethics Committee of Cameroon. We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stefan Niemann and Melissa S. Sander have contributed equally to this article
References
- 1.WHO | Global tuberculosis report 2020. WHO. http://www.who.int/tb/publications/global_report/en/. Accessed 23 Oct 2020.
- 2.Aung KJM, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, et al. Successful “9-month Bangladesh regimen” for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18:1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 3.Moodley R, Godec TR. Short-course treatment for multidrug-resistant tuberculosis: the STREAM trials. Eur Respir Rev. 2016;25:29–35. doi: 10.1183/16000617.0080-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO | WHO consolidated guidelines on drug-resistant tuberculosis treatment. WHO. http://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/. Accessed 6 Aug 2020.
- 5.WHO Consolidated Guidelines on Tuberculosis, Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. https://www.who.int/publications-detail-redirect/9789240007048. Accessed 4 Oct 2020. [PubMed]
- 6.Van Deun A, Maug AKJ, Salim MAH, Das PK, Sarker MR, Daru P, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 7.Kuaban C, Noeske J, Rieder HL, Aït-Khaled N, Abena Foe JL, Trébucq A. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis. 2015;19:517–524. doi: 10.5588/ijtld.14.0535. [DOI] [PubMed] [Google Scholar]
- 8.Piubello A, Harouna SH, Souleymane MB, Boukary I, Morou S, Daouda M, et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis. 2014;18:1188–1194. doi: 10.5588/ijtld.13.0075. [DOI] [PubMed] [Google Scholar]
- 9.Lange C, Duarte R, Fréchet-Jachym M, Guenther G, Guglielmetti L, Olaru ID, et al. Limited benefit of the new shorter multidrug-resistant tuberculosis regimen in Europe. Am J Respir Crit Care Med. 2016;194:1029–1031. doi: 10.1164/rccm.201606-1097LE. [DOI] [PubMed] [Google Scholar]
- 10.Schwœbel V, Trébucq A, Kashongwe Z, Bakayoko AS, Kuaban C, Noeske J, et al. Outcomes of a nine-month regimen for rifampicin-resistant tuberculosis up to 24 months after treatment completion in nine African countries. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunn AJ, Phillips PPJ, Meredith SK, Chiang C-Y, Conradie F, Dalai D, et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019;380:1201–1213. doi: 10.1056/NEJMoa1811867. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad Khan F, Salim MAH, du Cros P, Casas EC, Khamraev A, Sikhondze W, et al. Effectiveness and safety of standardised shorter regimens for multidrug-resistant tuberculosis: individual patient data and aggregate data meta-analyses. Eur Respir J. 2017;50(1):1700061. doi: 10.1183/13993003.00061-2017. [DOI] [PubMed] [Google Scholar]
- 13.Kendall EA, Fofana MO, Dowdy DW. Burden of transmitted multidrug resistance in epidemics of tuberculosis: a transmission modelling analysis. Lancet Respir Med. 2015;3:963–972. doi: 10.1016/S2213-2600(15)00458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merker M, Barbier M, Cox H, Rasigade J-P, Feuerriegel S, Kohl TA, et al. Compensatory evolution drives multidrug-resistant tuberculosis in Central Asia. eLife. 2018 doi: 10.7554/eLife.38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah NS, Auld SC, Brust JCM, Mathema B, Ismail N, Moodley P, et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N Engl J Med. 2017;376:243–253. doi: 10.1056/NEJMoa1604544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trébucq A, Schwoebel V, Kashongwe Z, Bakayoko A, Kuaban C, Noeske J, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis. 2018;22:17–25. doi: 10.5588/ijtld.17.0498. [DOI] [PubMed] [Google Scholar]
- 17.MTBseq: a comprehensive pipeline for whole genome sequence analysis of Mycobacterium tuberculosis complex isolates [PeerJ]. https://peerj.com/articles/5895/. Accessed 21 Jul 2020. [DOI] [PMC free article] [PubMed]
- 18.Coll F, McNerney R, Guerra-Assunção JA, Glynn JR, Perdigão J, Viveiros M, et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun. 2014;5:4812. doi: 10.1038/ncomms5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heep M, Brandstätter B, Rieger U, Lehn N, Richter E, Rüsch-Gerdes S, et al. Frequency of rpoB mutations inside and outside the cluster I region in rifampin-resistant clinical Mycobacterium tuberculosis isolates. J Clin Microbiol. 2001;39:107–110. doi: 10.1128/JCM.39.1.107-110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker TM, Ip CL, Harrell RH, Evans JT, Kapatai G, Dedicoat MJ, et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis. 2013;13:137–146. doi: 10.1016/S1473-3099(12)70277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jajou R, Kohl TA, Walker T, Norman A, Cirillo DM, Tagliani E, et al. Towards standardisation: comparison of five whole genome sequencing (WGS) analysis pipelines for detection of epidemiologically linked tuberculosis cases. Euro Surveill. 2019 doi: 10.2807/1560-7917.ES.2019.24.50.1900130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diel R, Kohl TA, Maurer FP, Merker M, Meywald Walter K, Hannemann J, et al. Accuracy of whole-genome sequencing to determine recent tuberculosis transmission: an 11-year population-based study in Hamburg, Germany. Eur Respir J. 2019 doi: 10.1183/13993003.01154-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohl TA, Harmsen D, Rothgänger J, Walker T, Diel R, Niemann S. Harmonized genome wide typing of tubercle bacilli using a web-based gene-by-gene nomenclature system. EBioMedicine. 2018;34:131–138. doi: 10.1016/j.ebiom.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5:291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Luo T, Shen X, Wu J, Gan M, Xu P, et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: a retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect Dis. 2017;17:275–284. doi: 10.1016/S1473-3099(16)30418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lew W, Pai M, Oxlade O, Martin D, Menzies D. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med. 2008;149:123–134. doi: 10.7326/0003-4819-149-2-200807150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Hoek KGP, Schaaf HS, Gey van Pittius NC, van Helden PD, Warren RM. Resistance to pyrazinamide and ethambutol compromises MDR/XDR-TB treatment. S Afr Med J. 2009;99:785–7. [PubMed] [Google Scholar]
- 28.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 29.Fitzwater SP, Caviedes L, Gilman RH, Coronel J, LaChira D, Salazar C, et al. Prolonged infectiousness of tuberculosis patients in a directly observed therapy short-course program with standardized therapy. Clin Infect Dis. 2010;51:371–378. doi: 10.1086/655127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escombe AR, Moore DAJ, Gilman RH, Pan W, Navincopa M, Ticona E, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLOS Med. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kodama C, Lange B, Olaru ID, Khan P, Lipman M, Seddon JA, et al. Mycobacterium tuberculosis transmission from patients with drug-resistant compared to drug-susceptible TB: a systematic review and meta-analysis. Eur Respir J. 2017 doi: 10.1183/13993003.01044-2017. [DOI] [PubMed] [Google Scholar]
- 32.Comas I, Borrell S, Roetzer A, Rose G, Malla B, Kato-Maeda M, et al. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat Genet. 2011;44:106–110. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngabonziza JCS, Diallo AB, Tagliani E, Diarra B, Kadanga AE, Togo ACG, et al. Half of rifampicin-resistant Mycobacterium tuberculosis complex isolated from tuberculosis patients in Sub-Saharan Africa have concomitant resistance to pyrazinamide. PLoS ONE. 2017;12:e0187211. doi: 10.1371/journal.pone.0187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox HS, Sibilia C, Feuerriegel S, Kalon S, Polonsky J, Khamraev AK, et al. Emergence of extensive drug resistance during treatment for multidrug-resistant tuberculosis. N Engl J Med. 2008;359:2398–2400. doi: 10.1056/NEJMc0805644. [DOI] [PubMed] [Google Scholar]
- 35.Trébucq A, Decroo T, Van Deun A, Piubello A, Chiang C-Y, Koura KG, et al. Short-course regimen for multidrug-resistant tuberculosis: a decade of evidence. J Clin Med. 2020;9:55. doi: 10.3390/jcm9010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad N, Ahuja SD, Akkerman OW, Alffenaar J-WC, Anderson LF, et al. Collaborative group for the meta-analysis of individual patient Data in MDR-TB treatment–2017. Lancet. 2018;392:821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Methods.
Additional file 2: Table S1. Molecular and phenotypic data of MTBC isolates from 195 RR/MDR-TB patients in Cameroon (2013–2015) including patient characteristics.
Additional file 3: Table S2. Contact tracing data of patients in molecular clusters and pyrazinamide resistance conferring mutations in pncA.
Additional file 4: Table S3. Comparison of patient characteristics, included versus excluded RR/MDR-TB patients.
Data Availability Statement
Raw sequencing data (fastq files) of Mycobacterium tuberculosis complex strains were deposited in the European Nucleotide Archive under the Accession Number PRJEB40777.