Abstract
Background
Increased intestinal permeability, either due to the exposure to antigens in asthmatic patients or due to a barrier defect, plays a critical role in susceptibility to environmental allergens. House dust mite allergy occurs more commonly than any other type of allergy among Egyptian asthmatic patients.
Aim
To assess the relation between serum zonulin level as a marker of increased intestinal permeability and the severity of house dust mite allergic asthma.
Methods
A case–control study which included 48 patients with house dust mite allergic asthma and 48 healthy control subjects attending the Allergy and Immunology Unit, Microbiology and Immunology Department, Faculty of Medicine, Zagazig University.
Results
A statistically significant difference was detected between the two studied groups with respect to serum IgE and serum zonulin levels (p ˂ 0.001 and ˂ 0.001, respectively). The mean serum zonulin was equal to 258.3 ± 153.01 ng/ml in the asthmatic group and 80 ± 13 ng/ml in the control group. Serum zonulin level significantly increased with the increase of asthma severity (p ˂ 0.001). The cut off value of serum zonulin was ≥ 198 ng/ml, and the area under the curve was 0.76. It displayed sensitivity equal to 80% and specificity equal to 71.4%. Its negative predictive value was equal to 83.3%.
Conclusion
Intestinal barrier dysfunction contributes to the pathogenesis of allergic asthma. Serum zonulin level reflects an increase in intestinal permeability. Zonulin acts as prognostic factor of severity in asthma. Correction of the gut barrier defect may have a potential positive prognostic effect in asthma.
Keywords: Asthma, Zonulin, Asthma grade, Severity, House dust mites, Intestinal barrier
Introduction
Asthma is a common chronic airway disease characterized by airway inflammation, hyper-responsiveness and variable airway obstruction, which is often attributed to gene–environment interactions [1].
Epidemiologic studies have shown that sensitization to indoor allergens is an important risk factor for the occurrence of acute attacks of asthma [2].
The most prevalent indoor allergens include house dust mites (HDMs), animal dander, moulds and cockroaches. Of these, HDMs, especially D. pteronyssinus and D. farinae, are considered the major perennial indoor allergen sources inducing allergic sensitization worldwide [3].
Environmental factors, microbiome, epithelial cells and immune cells show a dynamic cross talk at the skin and mucosal barriers in the development of atopic dermatitis, allergic rhinitis, eosinophilic esophagitis and asthma [4].
Recent trends in targeting allergic disorders are directed towards personalized medicine approach which necessitates novel developments in the area of disease phenotyping and endotyping, as well as the development and application of reliable biomarkers [4].
Increased intestinal permeability due to the exposure to antigens in asthmatic patients may play a role in susceptibility to environmental allergens. Therefore, correction of the gut barrier defect may be an additional novel approach for asthma treatment [5].
More than 50 proteins act together to create effective tight junctions which are crucial to having an efficient gut mucosal barrier. A 47 kDa protein named zonulin enhances and reversibly alters intestinal permeability [6, 7]. It also shares in innate intestinal immune response. An increase in serum zonulin level has been reported in many autoimmune diseases in which a defective intestinal barrier plays a role in their pathogenesis [8]. Chronic inflammation due to environmental stimuli results in up-regulation of serum zonulin. An increase in serum zonulin was noticed to be significantly correlated to disease severity in atopic dermatitis patients [9].
Patients and methods
This is a case control study which includes 96 subjects who attended the Allergy and Immunology Unit, Microbiology and Immunology Department, Faculty of Medicine, Zagazig University.
Sample size
Assuming that serum zonulin level in case group versus control group is (11.3 ± 3.7 ng/ml) and (9.3 ± 3.3 ng/ml) respectively with confidence level 95% and power 80%, the sample size is 96 (48 in every group).
They are divided into two groups.
Group 1 (Control group) includes 48 healthy subjects who fulfill the same exclusion criteria as the patients, and have never had asthma or eczema. They were health workers recruited from the hospital staff members.
Group 2 (Asthmatic patients) includes 48 adult (≥ 18 years old) subjects with allergic asthma according to Global Initiative for Asthma consensus report (GINA), 2020 [10].
Inclusion criteria
Allergic asthma patients were selected as having:
Obstructive pattern; Low FEV1/FVC on pulmonary function test;
A positive skin prick test for house dust mites.
Exclusion criteria
Evidence of digestive disease;
Any other condition associated with increased intestinal permeability, including cystic fibrosis, intestinal parasitic infestation, inflammatory bowel diseases;
Food allergy or a positive skin test against common food allergens including, fish and shrimps;
Patient refusal to participate.
Each patient was subjected to the following:
Full detailed history and examination to exclude any comorbid condition that may affect the result of the study;
Full lab to exclude other causes that may increase the intestinal permeability, including liver function test, anti-tissue transglutaminase, stool analysis, stool culture, rheumatoid factor, Hb A1c, ESR, CBC with differential count and fecal calprotectin;
Full detailed allergic history and clinical examination of the respiratory system.
Chest x-ray to exclude other lung pathology;
Pulmonary function tests with special emphasis on FEV1and FEV1/FVC using SPIROMETRICS;
Total serum IgE for each patient using enzyme-linked immunosorbent assay (ELISA).
Skin prick testing to common allergens;
Asthma severity score, calculated according to GINA, 2020 [10].
Serum zonulin level detection by ELISA.
Asthma severity scoring
Asthma severity was classified into grades I–IV (I being the mildest and IV the most severe). Grading was based on events that occurred over the past 6 months, including day and night symptoms, effect of asthma on daily activity, use of steroids and peak expiratory flow rate as defined by GINA guidelines 2020 [10]. Pulmonary function tests were performed for all patients using a fully computerized Spirometer (Jaeger MasterScreen™ IOS, version 5.2 manufactured by VIASYS Healthcare GmbH, Hoechberg, Germany). Pulmonary functions were assessed using forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and the FEV1/FVC ratio, measured and expressed as a percentage of predicted values, with a ratio higher than 0.8 being normal.
Skin testing
Skin testing was performed according to Bernstein et al. [11].
Allergen extracts of skin testing were standardized allergen extracts provided by Hamilton (Omega, Allergy OVERSEAS consultant Inc., Canada). The test was performed using the following allergens: for aeroallergens: (house dust mites: Dermatophagoides (D.) pteronyssinus and Dermatophagoides (D.) farnae mites, grass, mixed pollens, mixed molds, tobacco, cotton, wool, cockroach and hay dust) and for food allergens: (egg, fish, shrimps, peanuts and cow milk).
Histamine dihydrochloride (10 mg/ml) was used as a positive control, while saline was used as a negative control.
Subjects were asked to stop antihistamines a week before skin testing.
The largest diameter of the wheal was measured, and it was considered positive if it was ≥ 3 mm [12].
Those with positive tests for food allergy were excluded from the study.
Sample collection
Five millilitre blood samples were collected by venipuncture under complete aseptic conditions. Samples were left to clot then centrifuged at 1000×g for 15 min. Sera were collected and stored at − 20 °C. Sera were used to measure serum levels of total IgE, and zonulin.
Serum level of total IgE
Quantitative measurement of serum level of total IgE was done using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) Kit supplied by Chemux Bioscience, Inc. (CA, USA, Cat No. 10602) according to the manufacturer’s instructions. The results were expressed in IU/ml. The total IgE level in a normal allergy-free adult is less than 150 IU/ml of serum. The minimum detectable concentration of IgE by this assay is estimated to be 5.0 IU/ml. The Absorbance of standards and samples were measured at 450 nm using a microtiter plate ELISA reader (Biotek, USA).
Measurement of serum zonulin level
Quantitative measurement of serum level of zonulin was done using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) Kit supplied by MyBiosource, Inc. (USA, Cat No: MBS774026) according to the manufacturer’s instructions. The results were expressed in ng/ml. Absorbance of standards and samples were measured at 450 nm using a microtiter plate ELISA reader (Biotek, USA). The kit detection range was 20–800 ng/ml.
Statistical analysis
The collected data were processed and coded before being analyzed using SPSS program version 23. Quantitative data were presented as minimum, maximum, mean and SD.
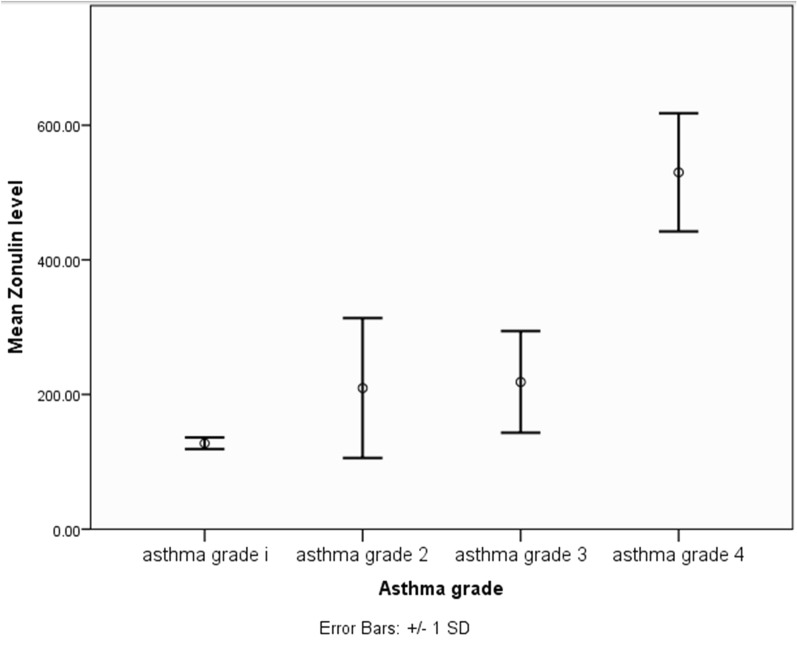
Qualitative data were presented as count and percentage. Student t test was used to compare quantitative data between two groups and One Way ANOVA test was used to compare more than two groups. Pearson’s correlation was used to measure linear relationship between two continuous variables. ROC curve was used to measure the diagnostic validity of quantitative variables and linear regression test was used to measure the independent effect of some factors on quantitative outcome variable. p value < 0.05 was considered statistically significant (Fig. 2).
Fig. 2.
Correlation between asthma grade of severity and serum zonulin level
Results
The current study is a case–control study which includes 96 subjects who attended the Allergy and Immunology Unit, Microbiology and Immunology Department, Faculty of Medicine, Zagazig University during the year 2021.They were divided into two groups, group 1 included healthy controls with a median age of 28 [n = 48] and group 2 included patients with allergic asthma according to Global Initiative for asthma consensus report (GINA), 2020 with a median age of 30 [n = 48].
Regarding the asthmatic patients, the mean value of age is 30.67 ± 15.609, and most of the patients are female (62.5%) the majority of whom live in rural areas (54.16%). 39 patients (81.3%) have positive family history of atopy. All patients displayed positive skin prick test results to house dust mites, with most of them (70.8%) sensitive to D. pteronyssinus, while 60.4% sensitive to D. farina. 14 patients were mono sensitized to a single type of house dust mites and 34 patients were polysensitized to both types. As for the grade of asthma severity, 4 patients (8.3%) had grade 1 severity, 24 patients (50%) had grade 2 asthma severity, 12 (25%) patients had grade 3 severity, and 8 patients (16.7%) had grade 4 asthma severity (Table 1).
Table 1.
Description of studied subjects
| Variable | Control group (n = 48) | Percentage % | Asthmatic patients (n = 48) | Percentage % |
|---|---|---|---|---|
| Sex | ||||
| Male | 28 | 85.3% | 18 | 37.5 |
| Female | 20 | 14.7% | 30 | 62.5 |
| Age (years) | ||||
| X ± SD | 32.7 ± 11.2 | 30.67 ± 15.609 | ||
| Median | 28 | 30 | ||
| Residence | ||||
| Rural | 34 | 70.8% | 26 | 54.16 |
| Urban | 14 | 29.2% | 22 | 45.84 |
| Family history of atopy | ||||
| Positive | 11 | 22.9% | 39 | 81.3 |
| Negative | 37 | 77% | 9 | 18.8 |
| Skin prick | ||||
| Negative | 48 | 100% | 0 | 0 |
| Positive | 0 | 0% | 48 | 100 |
| House dust mites | 48 | 100 | ||
| D. pteronyssinus | – | – | 34 | 70.8 |
| D. farina | 29 | 60.4 | ||
| Both | 34 | 70.8 | ||
| Asthma grade | Asthma grade 1 | 4 | 8.3 | |
| Asthma grade 2 | 24 | 50.0 | ||
| Asthma grade 3 | 12 | 25.0 | ||
| Asthma grade 4 | 8 | 16.7 | ||
Concerning the control group, the mean value of age was 32.7 ± 11.2. Most patients were males (85.3%) and 70.8% of them lived in rural areas. As for the family history of atopy, only 11 (22.9%) patients had positive family history of atopy and all of them displayed negative skin prick test results.
Upon comparing serum total immunoglobulin E (IgE) level between asthmatic patients and control subjects, there was a highly significant statistical difference between both groups (p = 0.000). Asthmatic patients displayed a higher statistically significant level of serum total IgE. The mean serum IgE was equal to 233.3 ± 103.4 IU/ml in the asthmatic group and 84.4 ± 18.6 IU/ml in the control group (Table 2).
Table 2.
Comparison between total IgE levels and zonulin levels among both control and case groups
| Variable | ControlGroup 1 (n = 48) | Asthmatic patientsGroup 2 (n = 48) | F | P |
|---|---|---|---|---|
| Total IgE level (IU/ml) | ||||
| Mean ± SD | 84.4 ± 18.6 | 223.3 ± 103.4 | 4.8 | 0.000HS |
| Zounlin (ng/ml) | ||||
| Mean ± SD | 80 ± 13 | 258.3 ± 153.01 | 6.12 | 0.000HS |
NS, Non-significant; S, Significant; HS, Highly significant
Similarly, there was a highly significant statistical difference between both groups as regards serum zonulin level. Asthmatic patients displayed a higher statistically significant level of serum zonulin than control subjects. The mean serum zonulin was equal to 258.3 ± 153.01 ng/ml in the asthmatic group and 80 ± 13 ng/ml in the control group (Table 2).
Factors affecting serum total IgE level were male sex, asthma grade, and positive skin prick test. Male patients displayed a statistically significant higher serum total IgE level (p = 0.03). The mean value of serum total IgE was 272.89 ± 141.33 among male patients and 193.53 ± 56.38 IU/ml among female patients. Besides, post hoc test showed a significant difference between grade 1 vs grade 2 as regards serum total IgE. As the asthma severity increased, there was a significant corresponding increase in the serum total IgE (p = 0.02). Moreover, there was a highly significant statistical difference as regards the mean value of serum total IgE between patients displaying positive skin prick test results and those displaying negative skin prick test results (p = 0.002). The mean value of serum total IgE among patients displaying positive skin prick test results was 244.41 ± 114.85 IU/ml (Table 3).
Table 3.
Factors affecting total IgE level
| Variables | Total IgE | Test value | p value | |
|---|---|---|---|---|
| Mean | SD | |||
| Sex | ||||
| Male | 272.89 | 141.33 | 2.29* | 0.03 S |
| Female | 193.53 | 56.38 | ||
| Residence | ||||
| Urban | 219.27 | 93.30 | 0.25* | 0.81 NS |
| Rural | 226.69 | 112.99 | ||
| Age (years) | ||||
| < 30 | 240.00 | 120.07 | 1.12* | 0.27 NS |
| > 30 | 206.58 | 82.78 | ||
| Asthma grade | ||||
| Asthma grade 1 | 154.50 | .58 | 5.77** | 0.02 S |
| Asthma grade 2 | 205.87 | 88.23 | ||
| Asthma grade 3 | 241.08 | 113.93 | ||
| Asthma grade 4 | 283.25 | 132.03 | ||
| Skin prick | ||||
| Negative | 172.00 | 34.37 | 3.33* | 0.002 HS |
| Positive | 244.41 | 114.85 | ||
*Student t test
**One Way ANOVA test (post hoc test shows significant difference between grade 1 vs grade 2)
NS, Non-significant; S, Significant; HS, Highly significant
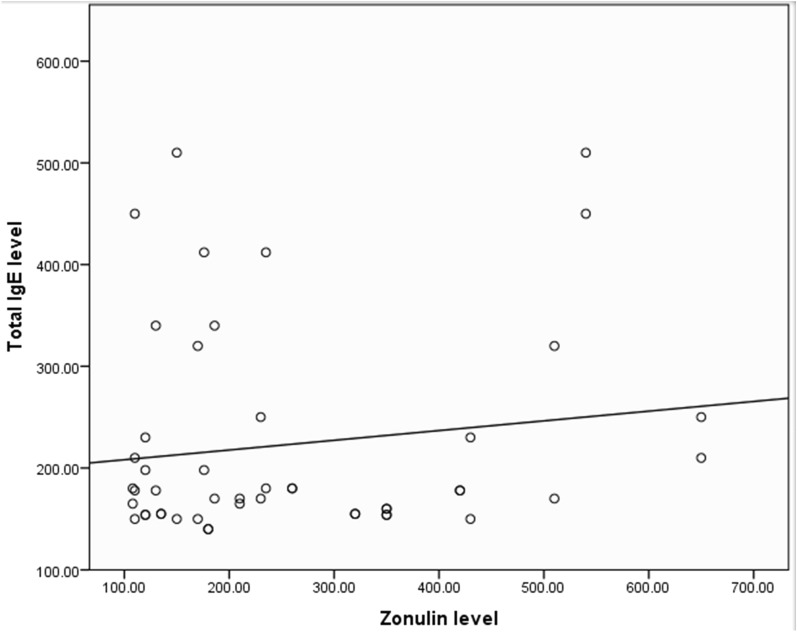
With regard to the correlation between serum zonulin level and serum total IgE, it did not reach a level of statistical significance (p = 0.34) (Table 4 and Fig. 1)
Table 4.
Factors affecting zonulin level
| Zonulin level | Test value | p value | ||
|---|---|---|---|---|
| Mean | SD | |||
| Sex | ||||
| Male | 290.11 | 163.89 | 1.12* | 0.27 NS |
| Female | 239.27 | 145.59 | ||
| Residence | ||||
| Urban | 315.55 | 157.34 | 2.51* | 0.02 S |
| Rural | 209.92 | 133.81 | ||
| Age (years) | ||||
| < 30 | 261.67 | 152.41 | 0.15* | 0.88 NS |
| > 30 | 255.00 | 156.83 | ||
| Asthma grade | ||||
| Asthma grade 1 | 127.50 | 8.66 | 64.54** | < 0.001 HS |
| Asthma grade 2 | 209.50 | 103.91 | ||
| Asthma grade 3 | 218.50 | 75.50 | ||
| Asthma grade 4 | 530.00 | 87.83 | ||
| Skin prick | ||||
| Negative | 299.29 | 182.05 | 1.20* | 0.24 NS |
| Positive | 241.47 | 138.88 | ||
| Zonulin level | ||||
| Total IgE | ||||
| Pearson correlation | 0.14 | |||
| p value | 0.34NS | |||
*Student t test
**One Way ANOVA test (post hoc test shows significant difference between grade 1 vs grade 2, grade 1 vs grade 3, grade 1 vs grade 4, grade 2 vs grade 4 and grade 3 vs grade 4)
NS, Non-significant; S, Significant; HS, Highly significant
Fig. 1.
Correlation between serum zonulin level and total serum IgE level
As for the factors affecting serum zonulin level, they were (the place of) residence and asthma grade. There was a statistically significant difference in the serum zonulin level between patients living in urban areas and those living in rural areas (p = 0.002). The mean value of serum zonulin was significantly higher in patients living in urban areas as it equaled 315.55 ± 157.34 ng/ml. Post hoc test showed a highly significant statistical difference between grade 1 vs grade 2, grade 1 vs grade 3, grade 1 vs grade 4, grade 2 vs grade 4 and grade 3 vs grade 4 as regards serum zonulin level (p ˂ 0.001) (Table 4 and Fig. 2)
Grade of asthma had an independent significant effect on zonulin level (p < 0.001), while serum total IgE had no significant effect on zonulin level via linear regression analysis (p = 0.684 NS) (Table 5).
Table 5.
Linear regression analysis for factors affecting zonulin level
| Unstandardized coefficients | Standardized coefficients | Sig | 95.0% confidence interval for B | |||
|---|---|---|---|---|---|---|
| B | Std. error | Beta | Lower bound | Upper bound | ||
| Grade 3 or 4 | 167.317 | 29.962 | 0.838 | < 0.001 HS | 107.007 | 227.628 |
| Total IgE | 0.075 | 0.183 | 0.062 | 0.684 NS | − 0.293 | 0.443 |
NS, Non-significant; S, Significant; HS, Highly significant
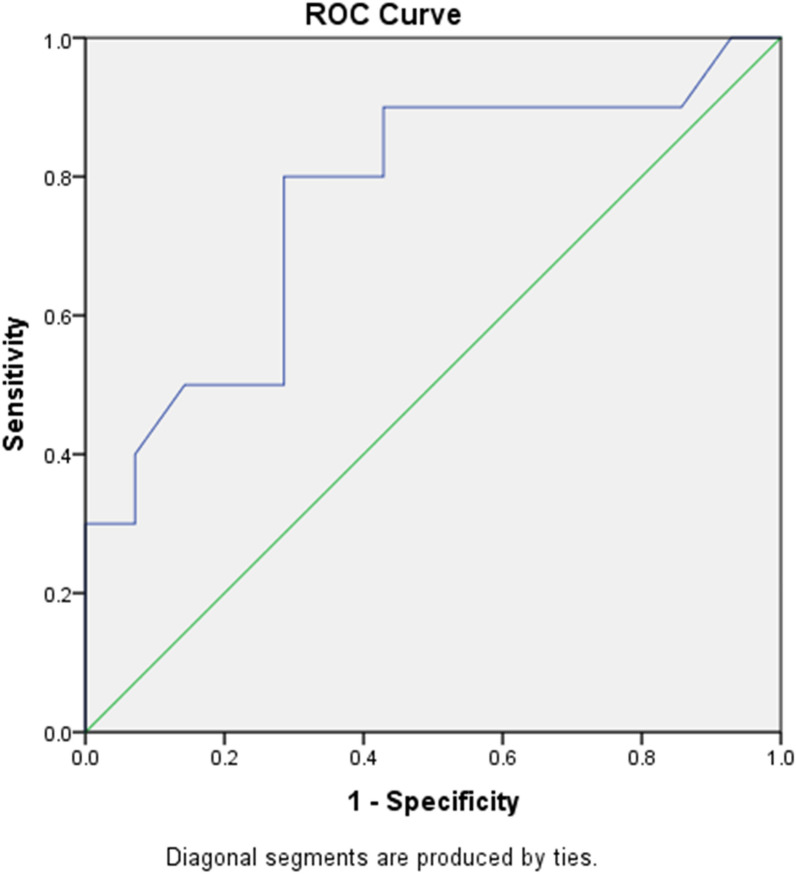
Roc curve was done to assess the validity of zonulin level for differentiation between (grade 1 or 2) vs (grade 3 or 4) (p = 0.002). It displayed sensitivity equal to 80% and specificity equal to 71.4%. Its positive predictive value was equal to 66.7% and negative predictive value was equal to 83.3%. The cut off value of serum zonulin was ≥ 198 ng/ml. The area under the curve was 0.76 (Table 6 and Fig. 3)
Table 6.
Validity of zonulin level for differentiation between (grade 1 or 2) vs (grade 3 or 4)
| Parameters | Cut off point | AUC | Sig | Sensitivity (%) | Specificity (%) | +PV (%) | −PV (%) | 95% confidence interval | |
|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||||
| Zonulin | ≥ 198 | 0.764 | 0.002 HS | 80 | 71.4 | 66.7 | 83.3 | 0.622 | 0.906 |
Fig. 3.
Roc curve of zonulin level for differentiation between (grade 1 or 2) vs (grade 3 or 4)
Discussion
Asthma is a chronic inflammatory lung disease which is characterized by airway inflammation, intermittent airflow obstruction, and bronchial hyper-responsiveness [13]. Allergic asthma can be sparked by allergen sensitization in which house dust mites (HDM) are among the most prevalent indoor allergens [14]. Exposure to dust mite allergens is a risk factor for clinical asthma in sensitized subjects [15].
A detailed clinical history and physical examination followed by the detection of total serum IgE level and IgE immunoreactivity against specific allergens still represents the cornerstone in approaching allergic disorders [4].
The relationship between allergic asthma and intestinal permeability is a subject of research interest. An increase in the intestinal permeability may result in facilitating the entry of allergenic proteins from the intestinal lumen into the systemic circulation, thereby causing activation of the adaptive immune system, allergen sensitization and/or extra-intestinal inflammation [16].
In the current study the mean value of age is 30.67 ± 15.609, and most of the patients are female (62.5%) the majority of whom live in rural areas (54.16%). 39 patients (81.3%) have positive family history of atopy. All patients displayed positive skin prick test results to house dust mites, with most of them (70.8%) sensitive to D. pteronyssinus, while 60.4% sensitive to D. farina. 14 patients were mono sensitized to a single type of house dust mites and 34 patients were polysensitized to both types. As for the grade of asthma severity, 4 patients (8.3%) had grade 1 severity, 24 patients (50%) had grade 2 asthma severity, 12 (25%) patients had grade 3 severity, and 8 patients (16.7%) had grade 4 asthma severity. Asthma patients were more commonly living in rural areas (54% rural vs 45.84% urban asthmatics) and all of them had positive skin prick test response to house dust mites, DP and Df (70% and 60%, respectively). When serum total immunoglobulin E (IgE) level was compared in asthmatic patients and control subjects in the present study, it was found that asthmatic patients displayed a higher statistically significant level of serum total IgE. It was statistically significantly higher among male asthmatic patients. There was a positive correlation between total serum IgE level and asthma severity grade and skin prick test results.
The disparity in the development of total IgE between males and females is the consequence of the fact that the levels of total IgE depend on many other factors, such as parasitic infestations, smoking, pollution, local diet and different genetic background in which males are at greater risk of exposure [17].
The impairment of the epithelial barrier is a corner stone in the development of allergic diseases. Increased intestinal permeability, either due to the exposure to antigens in asthmatic patients or due to a barrier defect, play a critical role in susceptibility to environmental allergens [18]. The process responsible for enhanced intestinal permeability in asthma is still indeterminate. There is an association between enhanced intestinal permeability and the severity of the asthma, which suggests that it is a bronchus to gut disease [19]. This could be attributed to the fact that mucosal defects can exist simultaneously in many organs and antigenic stimulation as well as environmental factors can result in its clinical expression in a single organ [20]. For instance, identical histological mucosal changes have been observed in both duodenal and bronchial mucosa simultaneously [8].
Therefore, correction of the gastrointestinal barrier defect is possibly an additional alternative approach for controlling asthma severity [5–21] as it leads to reduction of epithelial liability to damage and resultant inflammatory and remodeling reaction. In addition to growth factors, several peptides, such as AT-1001, a peptide inhibitor of zonulin, can repair barrier function [22]. It is, therefore, essential to study serum zonulin level and its regulation in patients with asthma.
The present study revealed that asthmatic patients displayed a higher statistically significant level of serum zonulin than control subjects. Patients living in rural areas had a significantly higher level on serum zonulin. Furthermore, there was a significant correlation between zonulin level and asthma grade. The higher the zonulin level, the greater the grade of asthma severity. Nevertheless, we found that there was no difference in serum levels of zonulin subjects regarding gender and age. The cut off value of serum zonulin level to differentiate between grade 1–2 asthma severity and grade 3–4 asthma severity was ≥ 198 ng/ml. In addition, serum zonulin showed a sensitivity equal to 80%, a specificity equal to 71.4%, a positive predictive value equal to 66.7% and a negative predictive value equal to 83.3%. There was no statistically significant correlation to serum total IgE concentration. Grade of asthma had an independent significant effect on zonulin level while serum total IgE had no significant effect on zonulin level via linear regression analysis.
A study conducted by Benard and colleagues to assess the intestinal permeability in asthmatics via administering oral radioactive CrEDTA and estimating its urinary recovery revealed that asthmatic patients had increased intestinal permeability in comparison. Contrary to the current results, it was found not to be correlated to the severity of asthma as deduced by pulmonary function test measurements or to the use of steroid treatment [23]. This may be due to the difference in the method used to assess intestinal permeability.
Furthermore, a study performed by Cervantes-García and collaborators aiming at assessing the outcome of oral Lactococcus lactis NZ9000 use on airway inflammation and lung remodeling in asthmatic rats and its relation to the preservation of the intestinal barrier, concluded that oral L. lactis could be used for asthma prevention through its maintenance of an adequately functioning intestinal barrier [24].
Initial findings by Fasano A imply that a group of asthmatic patients has both high levels of serum zonulin and enhanced intestinal permeability [25]. These findings indicate that antigens presentation and subsequent lung inflammation occur as result of the passage of antigens through lung and intestinal mucosa and submucosa and the subsequent stimulation of the immune system resulting in lung inflammation [26].
Moreover, a study performed by Yamaide et al. 2020 to examine the differences between serum zonulin level among allergic children and non-allergic children concluded that serum zonulin was significantly greater in children with allergy (Food allergy, Bronchial Asthma). In addition, it was significantly higher in patients with food allergy than in bronchial asthma patients. However, their results are not completely reliable as food allergy can be the main reason for increased intestinal permeability and this explains the higher increase in zonulin level among food allergy patients than among asthmatic ones [18].
Up to the present moment, no studies have been conducted to assess the relationship between serum zonulin level and asthma severity or to assess its correlation to different residential distribution. A study was performed by Sheen et al. to assess serum zonulin level in atopic dermatitis (AD) and its correlation to disease severity. They found that AD group had a greater median serum zonulin level than the control group and that serum zonulin level had significantly positive correlations with age and the SCORAD index, but not with total IgE, total eosinophil count (TEC), or the number of allergens to which a child is sensitized. They concluded that each 1 ng/ml increase in serum zonulin was associated with a 15% greater risk of moderate–severe AD. [9] Their results agree with the current results as regards the correlation between serum zonulin level and total serum IgE level. Both revealed no statistically significant correlation.
The current study did not assess whether the increased permeability was the cause or the consequence of bronchial asthma. It disclosed the significant positive correlation between zonulin level and asthma severity. Hence, this suggests that increased intestinal permeability has a deleterious impact on asthma severity. Further studies are needed to assess its role in the pathogenesis of asthma.
Conclusion
Intestinal barrier dysfunction contributes to the pathogenesis of allergic asthma. Serum zonulin level reflects an increase in intestinal permeability. Zonulin acts as prognostic factor of severity in asthma. Correction of the gut barrier defect may have a potential positive prognostic effect in asthma.
Acknowledgements
None.
Abbreviations
- AD
Atopic dermatitis
- ANOVA test
Analysis of variance test
- CBC
Complete blood count
- CrEDTA
Chromium ethylenediaminetetraacetic acid
- COX
Cyclooxygenase
- Ig E
Immunogloulin E
- D
Dermatophagoides
- Df
Dermatophagoides farnae
- DP
Dermatophagoides pteronyssinus
- ELISA
Enzyme-linked immunosorbent assay
- ERs
Estrogen receptors
- ESR
Erythrocyte sedimentation rate
- Fev1
Forced expiratory flow volume in 1 s
- FVC
Forced vital capacity
- GINA
Global Initiative for Asthma
- Hb A1c
Glycosylated hemoglobin type A1C
- HDMs
House dust mites
- IRB
International Review Board
- kDa
Kilo daltons
- L. lactis
Lactococcus lactis
- P-value
Probability value
- ROC curve
Receiver operating characteristic curve
- SCORAD index
Scoring of Atopic Dermatitis index
- SD
Standard deviation
- SPSS Program
Statistical Package for the Social Sciences Program
- TEC
Total eosinophil count
Authors’ contributions
SAB, SMI, AE and SF designed the study. SAB and SHF shared in sample collection. SMI and AE shared in sample collection and did the statistical analysis. SHF and SIT wrote the draft. SAB, SIT and AE performed the critical review of the manuscript. All authors reviewed and approved the final version. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All the data needed to support the current findings could be found in a supporting sheet.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (IRB) No #6856/14-12-2020, Faculty of Medicine, Zagazig University. An informed written consent was obtained from all parents at the time of recruitment. This study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu X, Cui J, Yi L, Qin J, et al. The role of T cells and macrophages in asthma pathogenesis: a new perspective on mutual crosstalk. Mediat Inflamm. 2020;2020:7835284. doi: 10.1155/2020/7835284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trompette A, Divanovic S, Visintin A, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taha A, Etewa S, Abdel-Rahman S, et al. House dust mites among allergic patients at the Allergy and Immunology Unit, Zagazig University: an immunologic and serologic study. J Parasit Dis. 2018;42(3):405–415. doi: 10.1007/s12639-018-1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breiteneder H, Peng YQ, Agache I, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. 2020;75(12):3039–3068. doi: 10.1111/all.14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hijazi Z, Molla AM, Al-Habashi H, et al. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89:227–229. doi: 10.1136/adc.2003.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4(4):e1251384. doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohlsson B, Orho-Melander M, Nilsson PM. Higher levels of serum zonulin may rather be associated with increased risk of obesity and hyperlipidemia, than with gastrointestinal symptoms or disease manifestations. Int J Mol Sci. 2017;18(3):582. doi: 10.3390/ijms18030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheen YH, Jee HM, Kim DH, et al. Serum zonulin is associated with presence and severity of atopic dermatitis in children, independent of total IgE and eosinophil. Clin Exp Allergy. 2018;48:1059–1062. doi: 10.1111/cea.13158. [DOI] [PubMed] [Google Scholar]
- 10.National Institutes of Health. Global initiative for asthma. Global strategy for Asthma Management and Prevention Revision. 2020. http://www.ginaasthma.org.
- 11.Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100:S1–S148. doi: 10.1016/S1081-1206(10)60480-2. [DOI] [PubMed] [Google Scholar]
- 12.Australasian Society of Clinical Immunology and Allergy (ASCIA) Skin Prick testing guide for diagnosis of allergic disease the ASCIA website. 2020. www.allergy.org.au/members/committees#wpim.
- 13.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 14.Dhaliwal AK, Singh D, Randhawa RK, et al. Sensitivity in allergic asthmatic subjects towards house dust mite allergens. Syst Appl Acarol. 2021;26(1):75–84. [Google Scholar]
- 15.Chu HT, Tran TN, Doyen V, et al. The protective effect of rural life on mite sensitization disappears among urban migrants in the South of Vietnam. World Allergy Organ J. 2019;12:100085. doi: 10.1016/j.waojou.2019.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker J, Dieleman L, Mah D, et al. High prevalence of abnormal gastrointestinal permeability in moderate-severe asthma. Clin Invest Med. 2014;1:E53–E57. doi: 10.25011/cim.v37i2.21086. [DOI] [PubMed] [Google Scholar]
- 17.Fereidouni M, Hossini RF, Azad FJ, et al. Skin prick test reactivity to common aeroallergens among allergic rhinitis patients in Iran. Allergol Immunopathol. 2009;37(2):73–79. doi: 10.1016/S0301-0546(09)71108-5. [DOI] [PubMed] [Google Scholar]
- 18.Yamaide F, Fikri B, Nakano T, et al. Serum zonulin levels are higher in pediatric allergic patients than those in healthy children. Authorea Preprints. 2020.
- 19.Djukanovic R, Roche WR, Wilson JW, et al. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990;142:434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- 20.Knutson TW, Bengtsson U, Dannaeus A, et al. Intestinal reactivity in allergic and nonallergic patients: an approach to determine the complexity of the mucosal reaction. J Allergy Clin Immunol. 1993;91(2):553–559. doi: 10.1016/0091-6749(93)90261-D. [DOI] [PubMed] [Google Scholar]
- 21.Paterson BM, Lammers KM, Arrieta MC, et al. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–766. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 22.Swindle EJ, Collins JE, Davies DE. Breakdown in epithelial barrier function in patients with asthma: identification of novel therapeutic approaches. J Allergy Clin Immunol. 2009;124:23–34. doi: 10.1016/j.jaci.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Benard A, Desreumeaux P, Huglo D, et al. Clinical aspects of allergic disease increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol. 1996;97(6):1173–1178. doi: 10.1016/S0091-6749(96)70181-1. [DOI] [PubMed] [Google Scholar]
- 24.Cervantes-García D, Jiménez M, Rivas-Santiago CE, et al. Lactococcus lactis NZ9000 prevents asthmatic airway inflammation and remodelling in rats through the improvement of intestinal barrier function and systemic TGF-β production. Int Arch Allergy Immunol. 2021;182(4):277–291. doi: 10.1159/000511146. [DOI] [PubMed] [Google Scholar]
- 25.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 26.Sturgeona C, Fasanoa A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases, review. Tissue Barriers. 2016;4(4):e1251384 (19 pages). 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data needed to support the current findings could be found in a supporting sheet.