Abstract
Background
Shift work is often associated with sleepiness and sleep disorders. Person‐directed, non‐pharmacological interventions may positively influence the impact of shift work on sleep, thereby improving workers’ well‐being, safety, and health.
Objectives
To assess the effects of person‐directed, non‐pharmacological interventions for reducing sleepiness at work and improving the length and quality of sleep between shifts for shift workers.
Search methods
We searched CENTRAL, MEDLINE Ovid, Embase, Web of Knowledge, ProQuest, PsycINFO, OpenGrey, and OSH‐UPDATE from inception to August 2015. We also screened reference lists and conference proceedings and searched the World Health Organization (WHO) Trial register. We contacted experts to obtain unpublished data.
Selection criteria
Randomised controlled trials (RCTs) (including cross‐over designs) that investigated the effect of any person‐directed, non‐pharmacological intervention on sleepiness on‐shift or sleep length and sleep quality off‐shift in shift workers who also work nights.
Data collection and analysis
At least two authors screened titles and abstracts for relevant studies, extracted data, and assessed risk of bias. We contacted authors to obtain missing information. We conducted meta‐analyses when pooling of studies was possible.
Main results
We included 17 relevant trials (with 556 review‐relevant participants) which we categorised into three types of interventions: (1) various exposures to bright light (n = 10); (2) various opportunities for napping (n = 4); and (3) other interventions, such as physical exercise or sleep education (n = 3). In most instances, the studies were too heterogeneous to pool. Most of the comparisons yielded low to very low quality evidence. Only one comparison provided moderate quality evidence. Overall, the included studies’ results were inconclusive. We present the results regarding sleepiness below.
Bright light
Combining two comparable studies (with 184 participants altogether) that investigated the effect of bright light during the night on sleepiness during a shift, revealed a mean reduction 0.83 score points of sleepiness (measured via the Stanford Sleepiness Scale (SSS) (95% confidence interval (CI) ‐1.3 to ‐0.36, very low quality evidence). Another trial did not find a significant difference in overall sleepiness on another sleepiness scale (16 participants, low quality evidence).
Bright light during the night plus sunglasses at dawn did not significantly influence sleepiness compared to normal light (1 study, 17 participants, assessment via reaction time, very low quality evidence).
Bright light during the day shift did not significantly reduce sleepiness during the day compared to normal light (1 trial, 61 participants, subjective assessment, low quality evidence) or compared to normal light plus placebo capsule (1 trial, 12 participants, assessment via reaction time, very low quality evidence).
Napping during the night shift
A meta‐analysis on a single nap opportunity and the effect on the mean reaction time as a surrogate for sleepiness, resulted in a 11.87 ms reduction (95% CI 31.94 to ‐8.2, very low quality evidence). Two other studies also reported statistically non‐significant decreases in reaction time (1 study seven participants; 1 study 49 participants, very low quality evidence).
A two‐nap opportunity resulted in a statistically non‐significant increase of sleepiness (subjective assessment) in one study (mean difference (MD) 2.32, 95% CI ‐24.74 to 29.38, 1 study, 15 participants, low quality evidence).
Other interventions
Physical exercise and sleep education interventions showed promise, but sufficient data to draw conclusions are lacking.
Authors' conclusions
Given the methodological diversity of the included studies, in terms of interventions, settings, and assessment tools, their limited reporting and the very low to low quality of the evidence they present, it is not possible to determine whether shift workers' sleepiness can be reduced or if their sleep length or quality can be improved with these interventions.
We need better and adequately powered RCTs of the effect of bright light, and naps, either on their own or together and other non‐pharmacological interventions that also consider shift workers’ chronobiology on the investigated sleep parameters.
Plain language summary
Non‐drug interventions for sleepiness and sleep problems for shift workers who work nights
Summary text
People who work shifts, especially night shifts, often describe being sleepy at work or having sleep problems after work. This can be bad for their well‐being, safety, and health. On the basis of a systematic literature search, we evaluated whether person‐directed, non‐drug interventions can make shift workers less sleepy during their shift, and help them sleep longer and better after their shift is over.
Studies found
We found 17 randomised controlled trials (with 556 participants) to include in this review. We rated the quality of evidence provided by most of the included studies to be between low and very low. The studies could be divided into three different types of interventions: (1) exposure to bright light; (2) a napping opportunity during the night shift; or (3) others, like physical activity or sleep education.
Key results
Bright light
Almost all of the bright light studies we looked at had some problem with the way they were designed. This problem made it difficult to know if any differences in sleepiness and sleep between those receiving bright light and those not receiving bright light were truly because of the bright light intervention. The studies were also too different in the types of bright light they used and types of light that the control groups received to compare them to one another.
Napping
The studies in the napping group did not report enough information for us to be certain whether napping helps shift workers feel more awake. The studies were very short, with each study lasting only a single night.
Others
This group of studies, which included, for example, physical exercise and sleep education, also reported too little information for us to say whether these interventions can make shift workers less sleepy on‐shift or help them sleep longer and better after their shift.
Conclusion
We conclude that there is too much uncertainty to determine whether any person‐directed, non‐drug interventions can really affect shift workers with sleepiness and sleep problems. We need studies that are better designed, report their designs and results more clearly, include more participants and last for a longer time before we can be certain. Studies also need to find out if their participants are 'morning‐types' or 'evening‐types', to be sure that the right type of shift worker gets the right type of intervention.
How up‐to‐date is this review?
We searched for studies that had been published up to August 2015.
Summary of findings
Background
Description of the condition
Shift work is common. According to the World Health Organization (WHO), 15% to 20% of employees in Europe and the USA work in some type of shift system (IARC 2010). The International Labour Organization defines working in shifts as “a method of organization of working time in which workers succeed one another at the workplace so that the establishment can operate longer than the hours of work of individual workers” (ILO 1990). There are several different definitions of night work. The ILO defines night work as “all work which is performed during a period of not less than seven consecutive hours, including the interval from midnight to 5 a.m.” (ILO 1990).
Shift work in general, and night‐shift work in particular, is known to have negative effects on personal health and well‐being. Established complaints among shift workers include difficulties in getting enough sleep and in maintaining an acceptable level of alertness while working irregular hours, both of which may lead to an increased risk of errors and accidents (Akerstedt 2011; Landrigan 2004). In the short‐term, shift workers may suffer from sleep‐related complaints, like sleepiness during or after their shift and sleep disturbances. Shift work also appears to be associated with a variety of long‐term effects on individual health, including coronary heart disease (Puttonen 2010), diabetes and metabolic syndrome (Wang 2011), gastrointestinal disorders (Knutsson 2010), and also workplace injuries (Wong 2011). Shift work that involves circadian disruption is classified as probably carcinogenic to humans (International Agency for Research on Cancer (IARC) classification 2a) (IARC 2010; Straif 2007).
Description of the intervention
A number of work‐ and worker‐directed strategies have been explored to help the individual adapt to night‐shift work. These include: (1) person‐directed, non‐pharmacological interventions (the focus of the present review), such as the use of bright light, the use of dark goggles, scheduled darkness, napping, exercise, and educational interventions; (2) shift system manipulation, whereby the actual shift system is altered, for example, by changing the speed and direction of rotation (this is the focus of the review by Erren 2013); and (3) pharmacological interventions, for instance, by the application of melatonin. This is the focus of the Liira 2014 review.
How the intervention might work
Light
One key component in the establishment of the sleep/wake rhythm in humans is melatonin secretion. This secretion peaks during the dark night, which is the habitual sleeping period. Melatonin opens the 'sleep gate' (Shochat 1998), inter alia, by inducing drowsiness and lowering body temperature, making it easier to fall and stay asleep (Burgess 2002; Cajochen 2010). Bright light exposure, predominantly blue light of 460 to 480 nanometres (nm), inhibits melatonin secretion. The application of bright light before, after, or during shifts is hypothesised to help adaptation to different shift schedules (Arendt 2010; Bougrine 1998; Burgess 2002; Costa 1993; Eastman 1991; Eastman 1994).
During the night shift, bright light is intended to increase alertness. This approach might then be supplemented by scheduled exposure to darkness at dawn/during daylight, i.e. via heavy curtains or dark goggles. This may facilitate day sleep after night shifts by preventing light from reaching the retina, thereby allowing melatonin secretions which contribute to the impending day‐sleep period (Eastman 1994; Sasseville 2006). Bright light given during the day shift, following a rotation of night shifts, is hypothesised to accelerate the individual’s readjustment to the external time environment.
Napping
Napping during a longer shift is hypothesised to increase alertness (Takeyama 2005), while napping during an overnight shift has been associated, in some studies, with lower levels of fatigue (Petrie 2004). The question of whether a single nap might impact sleepiness differently than multiple naps remains open (Banks 2015).
Other
Shift work appears to influence sleep length off‐shift. Shift workers tend to sleep less before night shifts than before day shifts, adding to sleepiness during night shifts. Sleep quality can also be influenced, since sleep episodes that occur as the melatonin level declines and body temperature rises usually are shorter and less well consolidated (Foster 2005). Educational interventions such as sleep hygiene courses address these issues, making participants aware of the physiology, and offering strategies to improve sleep off‐shift, with the aim of subsequently reducing sleepiness on‐shift. Associations between physical exercise and improvements in various sleep parameters have been observed (Kredlow 2015). Appropriately timed physical exercise is hypothesised to facilitate adaptation to night shifts and re‐adaptation to daytime schedules (Buxton 2003; Mistlberger 2005).
Why it is important to do this review
Systematic reviews exploring the effects of pharmacological interventions on problems associated with sleep‐wake disturbances have been published with increasing frequency over the past several years (Herxheimer 2008; Ker 2010; Liira 2014). While some of these interventions appear promising, pharmacological interventions may have adverse effects (Liira 2014). Until quite recently, systematic reviews on the topic of non‐pharmacological interventions to treat or prevent sleep and alertness problems were rare in the literature. To our knowledge, only two systematic reviews examining effects of person‐directed, non‐pharmacological interventions on preventing and treating sleep disturbances caused by shift work have been published (Neil‐Sztramko 2014; Ruggiero 2014). Although these reviews overlap with ours, their focus' are slightly different: Neil‐Sztramko 2014 excluded sleepiness and fatigue as outcomes in night‐shift workers, while Ruggiero 2014 examined only napping as an intervention in night‐shift workers.
Objectives
To assess the effects of person‐directed, non‐pharmacological interventions for reducing sleepiness at work abd improving the length and quality of sleep between shifts for shift workers.
Methods
Criteria for considering studies for this review
Types of studies
We included individually‐randomised and cluster‐randomised controlled trials. This includes randomised cross‐over trials, in which individuals eventually receive both interventions, but the order in which they receive these is random (counterbalancing alone was not sufficient). We included studies reported as full‐text, those published as abstract only, and unpublished data.
We also searched for laboratory trials. We defined laboratory trials as trials in which recruited individuals were exposed to the intervention in a laboratory setting that simulates shift work, and that includes night‐shift work. We present an overview of data from laboratory studies in separate tables (Table 11; Table 12; Table 13), and use the data for comparison in the Discussion section, but not for drawing conclusions on intervention effects.
1. Laboratory trials ‐ light interventions.
| Light interventions | ||||||
| Reference | Study participants | Shift system | Intervention | Key endpoints | Key results | |
| Babkoff 2002 | 11 subjects (females??) | Simulated shift‐work schedule beginning 17:30 and ending 10:00 the next morning | 1 hour bright light + placebo; exposure to 3000 lux occurred between 01:30 and 02:30 hours, placebo at 01:40 | CRT | Exposure for 1 hour to bright light combined with placebo yielded swifter CRTs immediately after the treatment, but also seemed to result in more sleepiness and greater performance deficit than when the subjects were not exposed to the bright light. | |
| Boyce 1997 | 16 subjects (all males) |
Graveyard shift (00:00‐07:59); rapidly rotating shift system, having three continuous nights' work followed by three days’ rest | 1) Low‐illuminance (250 lux, 3900 K) 2) High‐illuminance (2800 lux, 4050 K) 3) Increasing illuminance condition (200 lux to 2800 lux, 2800 K to 4050 K) 4) Decreasing illuminance condition (2800 lux to 200 lux, 4050K to 2800 K) | Sleep quality (by diary); arousal measured using the questionnaire developed by Mehrabian 1974 | High, increasing, and decreasing illuminance conditions associated with greater subjective arousal than were the low‐illuminance condition. No difference of performing simple cognitive tasks associated with the lighting conditions. | |
| Campbell 1995 | 26 subjects (7 females) |
3 consecutive night shifts between 24:00 hours until 08:00 hours the following morning | Exposed group: night 1: > 4000 lux between 00:00 hours to 04:00 hours, followed by ambient room illumination < 100 lux; night 2+3: circa 1000 lux for the duration of each shift Control group: < 100 lux (night 1‐3) |
Levels of alertness during shift assessed using the RTSW | There was little effect on measures of on‐duty alertness and performance or on off‐duty sleep. Middle‐aged subjects may be less phase‐tolerant than young subjects. |
|
| Chinoy 2015 | 8 subjects (2 females) |
4 day shifts followed by 4 night shifts | Treatment subjects (n = 4) received 2500 lux in the latter half of night shifts + a scheduled 8‐hour evening sleep episode; control subjects were in standard lighting (90 lux) | Subjective sleepiness on‐shift; PVT reaction time | For treatment subjects, by night 2, reaction time was not different from day shifts, and by night 3, subjective sleepiness was not different from day shifts. The preliminary data indicate that a combination treatment of scheduled evening sleep before night shifts and enhanced lighting during night shifts improves on‐shift sleepiness and reaction time. | |
| Czeisler 1990 | 8 subjects (all males) |
1 week of night work | Treatment study condition: circa 7000‐12,000 Iux at night and nearly complete darkness during the day (had to stay in their bedroom from 9:00‐17:00) Control study conditions: circa 150 lux; no restriction for the day |
Subjective alertness on‐shift assessed with use of a VAS; cognitive performance measured by a test involving calculations | Both alertness and cognitive performance significantly improved in the treatment group during night‐shift hours. | |
| Dawson 1991 | 13 subjects (6 females) |
3 consecutive simulated night shifts between 00:00 and 08:00 | Treatment study condition: circa 6000 Iux between 00:00 and 04:00 on the first night shift + dim light (< 200 Iux) for the remainder of the study The control group received dim light throughout |
Alertness on‐shift assessed using the RTSW; measures of sleep quality included time in bed, total sleep time, sleep efficiency, sleep onset latency, wake after sleep onset | The treatment was associated with significantly higher alertness across the night shift and improved sleep quality during the day. On‐shift alertness was improved relative to the control group. The data indicate that a single 4‐hour pulse of bright light between midnight and 04:00 is effective in ameliorating the sleep and alertness problems associated with transition to night shift. | |
| Dawson 1995 | 16 subjects (6 females) |
3 consecutive simulated night shifts between 23:00 and 07:00 | Treatment group condition: bright light (4000‐7000 Iux between 00:00 and 04:00) Control group conditions: dim red light < 50 lux |
Sleep quality measured by wrist actigraphy; cognitive performance measured using computer‐based divided attention tasks | Sleep quality and cognitive psychomotor performance was improved in the Iight‐treatment group. | |
| Eastman 1995 | 46 subjects (21 females) |
Simulated night shifts of 8 consecutive night‐work, day‐sleep days | Bright light durations of 6, 3 and 0 hours (i.e. dim light) during simulated night shifts. The bright light (circa 5,000 Iux) was used during all 8 night shifts, and dim light was < 500 Iux | Core body temperature continuously measured; sleep duration assessed by daily sleep log; mood assessed using the POMS | Substantial circadian adaptation (i.e. a large cumulative temperature rhythm phase shift) was produced in many subjects in the bright light groups, but not in the dim light group. Larger temperature rhythm phase shifts were associated with better subjective daytime sleep, less subjective fatigue and better overall mood. | |
| Englund 1990 | 22 subjects (all male?) |
1 full day shift (08:00‐16:00) + 2 night shifts (19:00‐07:00) | Four treatment groups: bright light (2000 lux) for three hours at 19:00, 22:00, 01:00 or 04:00 during the first night shift Control group: dim red light during 01:00‐04:00; 200 lux ambient lighting |
Core body temperature and wrist activity monitored by a Vitalog PMS‐8; cognitive performance and mood assessed through a battery computerised task battery. Specific measures of mood include: POMS, School of Aerospace Medicine Subjective Fatigue Checklist, SSS | Preliminary analysis indicates equal or better results across all groups on the second night shift as compared to the first night for simple reaction time, logical reasoning, addition/subtraction, sleepiness and fatigue. | |
| Foret 1998 | 8 subjects (all males) |
Simulated night shift regimen (60‐hour protocol) | Treatment group: during 20:00 to 08:00 (1st night), 4‐hour pulse of bright light (700‐1000 Iux) Control group: during 20:00 to 08:00 (1st night), dim light (circa 50 Iux); 2nd night: dim light in both groups |
Self‐rated alertness assessed using a shortened version of the Activation ‐ Deactivation Adjective Checklist; performance tests were 'search and memory' tests derived from the Memory and Search Task | Self‐assessed alertness and task performance were improved by the exposure to bright light. Subjective alertness and performance continued to show a time course during the subsequent night following exposure only to dim light. | |
| Higuchi 2011 | 11 subjects (all males) |
Simulated night work | Day 1: dim light (< 15 lux) from 20:00 to 03:00 Day 2: light for four hours from 23:00 to 03:00 with a non‐visor cap (500 lux), red‐visor cap (circa 160 lux), blue‐visor cap (circa 160 lux) |
Performance of a PVT as an index of objective sleepiness; subjective sleepiness on‐shift, fatigue, mood, visual comfort and brightness measured using a VAS | The red‐visor cap had no adverse effects on performance of the PVT, brightness and visual comfort, though it tended to increase subjective sleepiness. | |
| Hoppen 2001 | Experiment 1: Pilot study: 5 subjects (4 females) Experiment 2: 6 subjects (all females) Experiment 3: 11 subjects (all males) |
Experiment 1: two seven‐day study periods Experiment 2: 4 six‐day study periods Experiment 3: five seven‐day study periods |
Experiment 1: ambient lighting in the clinical investigation unit of 50 lux; 2 hours of 10,000 Iux or dim light during 02:00‐04:00 Experiment 2: I‐hour 10,000 Iux at 20:00 or 00:00 or 04:00, or dim light Experiment 3: 1, 2 or 4 hours of 10,000 lux or dim light in time windows 01:00‐05:00 |
Subjective fatigue measure on‐shift through Samn‐Perelli scale; alertness and performance measured using digit‐symbol substitution task, CRT, and subjective alertness ratings VAS + G15 | A series of experiments established that 2 hours of bright light (broad spectrum white, 02:00‐04:00, 10,000 lux) did improve subjective alertness and performance. Bright light given in the middle of the night (white 00:00‐01:00, 10,000 lux) was more effective than light given at 20:00 or 04:00. Light of 2 hours and 4 hours duration were more effective than 1 hour of light centred at 03:00 (white, 10,000 lux). Light of shorter wavelengths appeared to be more effective than light of Ionger wavelengths at improving nocturnal alertness and performance (01:00‐05:00, 300 lux). | |
| Kretschmer 2011 | 32 subjects (16 females) |
Three consecutive simulated night shifts between 22:00‐06:00 | Treatment group: 4‐hour pulse of bright light (3000 lux) between 22:00 and 02:00 on night 1, 1 hour later in night 2, and 2 hours later in night 3 Control group: dim light (300 Iux) |
Objective measures of working memory, selective attention, divided attention, concentration performance, and vigilance recorded by established performance tests (including working memory of the TAP, Go/No‐Go, Divided Attention of the test battery for attention testing; Konzentrations‐Leistungs‐Test; Simple Reaction Time Task) | Bright light Ieads to an improvement in working memory, divided attention and concentration performance in all three night shifts. Bright light leads to better performance for some forms of attention tasks in elderly night workers. | |
| Kretschmer 2012 | 32 subjects (16 females) |
Three consecutive simulated night shifts between 22:00‐06:00 | Treatment group: 4‐hour pulse of bright light (3000 lux) between 22:00 and 02:00 on night 1, 1 hour later in night 2, and 2 hours later in night 3 Control group: dim light (300 Iux) |
Objective measures of working memory, selective attention, divided attention, concentration performance, and vigilance recorded by established performance tests (subtest working memory of the TAP, Divided Attention of the test battery for attention testing; Konzentrations‐Leistungs‐Test (KLT‐R); PVT | Bright light exposure results in a better performance for cognitive tasks in older night workers over time. Except for high‐demand tasks, such as sustained attention tasks, bright light induces better performance in working memory and concentration tasks for older night workers. | |
| Kretschmer 2013 | 32 subjects (16 females) |
Three consecutive simulated night shifts between 22:00‐06:00 | Treatment group: 4‐hour pulse of bright light (3000 lux) between 22:00 and 02:00 on night 1, 1 hour later in night 2, and 2 hours later in night 3 Control group: dim light (300 Iux) |
Mood (Der Mehrdimensionale Befindlichkeitsfragebogen and sleepiness on‐shift (SSS) questionnaires and a concentration task, a working memory task, and a divided‐attention task by established performance tests (Konzentrations‐Leistungs‐Test (KLT‐R)); subtest working memory of the TAP; Divided Attention of the test battery for attention testing) | Results indicate that sleepiness and mood did not function as mediators in the prediction of concentration, working memory, and/or divided attention by light exposure. Bright light has a strong direct and independent effect on cognitive performance, particularly on working memory and concentration. | |
| Martin 1998 | 35 subjects (9 females) |
6 days of simulated 8‐hour night shifts | Treatment group 1: 5700 lux 3 hours/day Treatment group 2: 12:30 lux 3 hours/day Treatment group 3: < 250 lux All participants wore dark sunglasses while outside during daylight |
Core body temperature continuously measured; sleep duration via daily sleep log; mood and fatigue during day assessed using the POMS | During nights 3‐5, most subjects in the high and medium groups (100% and 85%) exhibited phase delays large enough that their body temperature minima occurred within the daytime sleep/dark period. Larger phase shifts were correlated with more sleep and less fatigue. Extremely 'bright' light may not be necessary for circadian adaptation in specific shift work situations. | |
| Rahman 2011 | 12 subjects (5 females) |
All subjects exposed to the five lighting conditions between 20:00 and 08:00 over 5 consecutive weeks | Lighting conditions: 1) complete darkness; 2) unfiltered fluorescent white light (380‐730 nm); 3) fluorescent white light with wavelengths < 480 nm filtered' 4) fluorescent white light with wavelengths < 460 nm filtered; 5) fluorescent white light with wavelengths < 480 partially filtered |
During each overnight testing session, objective and subjective neuropsychometric tests and saliva samples were collected every 2 hours. The Toronto Hospital Alertness Test, the Digit Vigilance Test, the SSS, the seven‐item Fatigue Scale self‐report questionnaire and a VAS for subjective mood were employed | Subjective alertness, mood, and errors on an objective vigilance task were significantly less impaired at 08:00 by filtering wavelengths < 480 nm compared with unfiltered nocturnal light exposure. The changes were not associated with significantly increased sleepiness or fatigue compared with unfiltered light exposure. The data suggest that spectral modulation may provide an effective method of regulating the effects of light on physiological processes | |
| Samel 1995 | 4 subject (all males) |
Two sessions of 11 days of simulated microgravity (6° head down tilt bedrest) with 6‐hour extensions of the wake period on 2 days (12‐hour phase delay) | Bright light (> 3500 Iux) for 5 hours on each of the 2 shift days and the following day at times either expected to accelerate the adjustment to the phase delay (treatment condition) or to have no phase shifting effect (control condition) | Sleep recorded polygraphically; circadian system monitored by recordings of heart rate and body temperature, and by collection of urine (electrolyte and hormone excretion); subjective sleep duration assessed via sleep log | 5‐hour exposures to bright light finishing at the time of the circadian temperature minimum were not more effective at accelerating adjustment to a 12‐hour schedule delay than exposures coinciding with the temperature maximum. We conclude that, while bright light may accelerate adjustment to work‐rest schedule delays, any such effect seems to be largely independent from the timing of the light exposure. No significant effects in polygraphically measured sleep parameters | |
| Schobersberger 2007 | 11 subjects (all males) |
Three consecutive simulated night shifts (22:00‐06:00); after a 2‐week rest, a second run of three consecutive night shifts | Treatment group: lighting environment (800 lux) with reduced short‐wavelength components Control group: unfiltered bright light (800 lux) environment |
Circadian markers (including urinary aMT6s), symptoms of lassitude, and personal mood; fatigue (and other parameters) assessed via mood rating inventory before and after the shift; Vienna Test System, including performance testing (reaction time analysis), vigilance testing and evaluation of attentiveness (Continous Attention) | Mood rating inventories did not result in differences in the subjective perception between the two lighting environments with respect to the dimensions of 'activity', 'concentration', 'deactivation', and 'fatigue'. In addition, changes in signs of vigour and weariness in the course of each night of the study were equally pronounced in test light and bright light. | |
| Sletten 2014 | 71 subjects (29 females) |
after ≥ 2 night shifts in the field, 1 simulated night shift in the laboratory | Treatment group: blue‐enriched white light (17,000 K, 150 lux) from 2300‐0700 Control group: continued background white light (4000 K, 150 lux) |
Habitual sleep‐wake patterns monitored for 1‐3 weeks via diaries and actigraphy; urine collected for aMT6s; assessments via KSS, PVT, polysomnography and mood | Exposure to blue‐enriched light was not associated with significant improvements in PVT performance, or electro‐oculogram correlates of alertness. During the biological night, however, blue‐enriched light was associated with improved subjective alertness. | |
| Thessing 1994 | 30 subjects (19 females) |
2‐night protocol | Lighting conditions: 1) bright light 00:00‐04:00; 2) dim light 00:00‐02:00 + bright light 02:00‐04:00; 3) dim light 00:00‐04:00 | Sleep estimated with actigraphy; subjective sleepiness (VAS); throughout night 2, the MSLT, SALT performance | 4‐hour exposure to bright light significantly increased MSLT scores and improved SALT performance during the early morning hours on the night following bright‐light exposure. No significant effects were noted with a 2‐hour exposure. A single exposure to bright light from 00:00 to 04:00 hours significantly decreased objectively measured sleepiness and improved performance on the subsequent night, particularly during the early morning hours. On the contrary, a 2‐hour exposure of essentially equal intensity light produced no change, relative to dim light, in any of the dependent measures. | |
| Weisgerber 2015 | 19 subjects (5 females) |
no less than 1 week between the interventions 1‐3 | Interventions: 1) No sleep deprivation (SD); 2) Overnight SD with 45 min dim light (DL+50 lux); 3) Overnight SD with 45 min BL (+5600 lux) | Body temperature and psychomotor vigilance (PVT); Saliva collected before and after light treatment for melatonin assay | Temperature, subjective alertness and PVT performance decreased significantly across the night. BL significantly suppressed melatonin, but did not improve subjective alertness or PVT performance. SD markedly increased incidents, accidents, and standard deviation of lane position. BL compared to DL did not improve performance during the first 22 min circuit, but across the 2 circuits BL significantly attenuated the effect of time on task on incidents and accidents. | |
| Light and glasses interventions | ||||||
| Reference | Study participants | Shift system | Intervention | Key endpoints | Key results | |
| Eastman 1994 | 50 subjects (19 females) | 8 consecutive simulated night shifts | Four groups in 2 x 2 design: light (bright, dim); goggles (yes, no); exposure to bright light (circa 5000 lux) for 6 hours on the first two night shifts; dim light < 500 lux | Core body temperature continuously measured; sleep duration via daily sleep log; mood and fatigue assessed using the POMS | Both bright light and goggles were significant factors for producing circadian rhythm phase shifts. The combination of bright light plus goggles was most effective; the combination of dim light and no goggles was least effective. Larger temperature‐rhythm phase shifts were associated with better subjective daytime sleep, less subjective fatigue and better mood. There was no significant main effect of goggles on sleep duration, but the main effect of light and the interaction of light and goggles were not significant. | |
| Smith 2008 | 24 subjects (14 females) |
3 simulated night shifts (2300‐0700), 2 days‐off + 4 more night shifts | Treatment group: five 15 minute bright light pulses during night shifts + sunglasses when outside + sleep in dark bedrooms at scheduled times after night shifts and on days‐off + outdoor afternoon light exposure (the “light brake”) Control group: remained in normal room light during night shifts + lighter sunglasses + unrestricted sleep and outdoor light exposure |
DLMO; daily sleep log + actigraphy, alertness on‐shift, total sleep time assessed using sleep logs and actigraphy; reaction time (SRT) test | The final DLMO of the experimental group was close to our target compromise phase position, and significantly later than the control group. Experimental subjects performed better than controls, and slept for nearly all of the allotted time in bed. Controls demonstrated pronounced performance impairments late in the night shifts, and exhibited large individual differences in sleep duration. | |
| Smith 2008a | 31 subjects (17 females) |
3 simulated night shifts (23:00‐07:00) + 2 days‐off | Two treatment groups: intermittent bright light during night shifts (75 and 120 min/night) + dark sunglasses when outside + sleep in dark bedrooms at scheduled times after night shifts and on days‐off + outdoor light exposure upon awakening from sleep Control group: dim room light during night shifts + lighter sunglasses + unrestricted sleep and outdoor light |
DLMO; daily sleep log + actigraphy; simple reaction time (SRT) test | After the days‐off, the DLMO of the experimental groups was in a good position to reach the target after subsequent night shifts with bright light. The DLMO of the control group changed little from baseline. Experimental subjects performed better than control subjects during night shifts on a reaction time task. | |
| Smith 2009 | 19 subjects (11 females) |
3 simulated night shifts (23:00‐07:00); 2 days‐off, 4 night shifts + 2 days‐off | Treatment group: four 15‐min BL pulses during night shifts + sunglasses when outside + sleep in dark bedrooms at scheduled times + outdoor afternoon light (“light brake”) Control group: remained in normal room light during night shifts + lighter sunglasses + unrestricted sleep and outdoor light |
DLMO; daily sleep log + Actiwatch‐L; Automated Neurophysiological Assessment Metrics test battery (SRT reported) | The final DLMO of the experimental group was close to the target of 03:00, and later than the control group. Subjects who phase‐delayed (whether in the experimental or control group) close to the target phase performed better during night shifts. | |
aMT6s: 6‐sulfatoxymelatonin CRT: Choice Reaction Time DLMO: Dim Light Melatonin Onset MSLT: Multiple Sleep Latency Test POMS: Profile of Mood States PVT: Psychomotor Vigilance Task RTSW: Repeated Test of Sustained Wakefulness SALT: Simulated Assembly Line Task SRT: Simple Reaction Time SSS: Stanford Sleepiness Scale TAP: Test battery for Attentional Performance VAS: Visual Analogue Scale
2. Laboratory trials ‐ nap interventions.
| Nap interventions | ||||||
| Reference | Study participants | Shift system | Intervention | Key endpoints | Key results | |
| Asaoka 2012 | 20 subjects (6 females) |
Subjects awakened at 07:00 of the experimental day and were prohibited from sleeping until the end of experiment except for the nap (01:00‐02:00) in the nap group Participants remained awake for 20 hours ‐ performing cognitive tasks at 21:00, 02:00 and 03:00; experimental chamber below 150 lux, 30 lux during cognitive task period, 0 lux during the nap |
Nap condition: 1‐hour nap 01:00‐02:00 Rest condition:1‐hour awake‐rest period 01:00‐02:00 |
Sleep logs + Actiwatch‐L + polysomnography + EEG; stimulus‐response compatibility (arrow‐orientation task, reaction time | Behavioural performance and amplitude of the error‐positivity declined after midnight (i.e. 02:00 and 03:00) compared with the 21:00 task period in both groups. During the task period starting at 03:00, the participants in the awake‐rest condition reported less alertness and showed fewer correct responses than those who napped. | |
| Bonnet 1994 | 12 subjects (all males) |
3 consecutive nights and 2 days in the laboratory for 2 consecutive weeks (= session 1+2) | Session 1: 4‐hour afternoon nap + caffeine at 01:30 and 07:30 Session 2: four 1‐hour naps during the night + placebo In both sessions pills (placebo or caffeine) were administered at 01:30, 07:30, 13:30, 19:30) |
MSLT, EEG; performance and mood were assessed with repeated batteries of measures (logical reasoning, WAlS, computer‐modified Williams Word Memory Test of immediate free recall, visual vigilance, subjective sleepiness/alertness, POMS, oral temperature) across the 24‐hour operation | After an afternoon nap, subjects had increased objective and subjective alertness, increased oral temperature, and increased performance on complex tasks like logical reasoning and correct additions when compared to the condition that allowed four night‐time naps. | |
| Bonnet 1994a | 24 subjects (all males) |
3 consecutive nights and 2 days in the Iaboratory | Nap condition 1: 16:00‐20:00 prior to a 24‐hour period of sleep loss Nap condition 2: as in 1 + 200 mg caffeine at 01:30 and 07:30 All subjects received pills at 01:30, 07:30, 13:30, 19:30. For all subjects, the pills received at 13:30 and 19:30 were placebos |
MSLT, EEG; visual vigilance, subjective sleepiness/alertness, POMS; performance and mood were assessed with repeated batteries of measures (logical reasoning, WAlS, computer‐modified Williams Word Memory Test of immediate free recall | Performance tests all indicated maintenance of baseline performance Ievels in the caffeine group after administration of caffeine, while performance declined in the placebo group. The combination of nap and caffeine was able to maintain alertness and performance at very close to baseline Ievels throughout a 24‐hour period without sleep. | |
| Bonnet 1995 | 140 subjects (all males) |
4 consecutive nights and 3 days in the laboratory | 1) Nap condition: nap at 12:00, 16:00, 18:00 or not at all 2) Caffeine condition: single 400 mg dose of caffeine at 01:30 each night or repeated doses of 150 mg or 300 mg every 6 hours starting at 01:30 on the 1st night of sleep loss 3) Placebo condition: no‐nap and placebo administered every 6 hours on the repeated caffeine schedule was run for 1) and 2) During the sleep‐loss period, all subjects were administered placebo capsules every 6 hours starting at 01:30 |
MSLT, EEG; performance and mood were assessed with repeated batteries of measures (logical reasoning, WAlS, computer‐modified Williams Word Memory Test of immediate free recall, visual vigilance, subjective sleepiness, POMS, oral temperature) across the 24‐hour operation | Naps provided Ionger and less graded changes in performance, mood and alertness than did caffeine, which displayed peak effectiveness and loss of effect within about 6 hours. Neither nap nor caffeine conditions could preserve performance, mood, and alertness near baseline Ievels beyond 24 hours, after which Ievels approached those of placebo. | |
| Bonnet 1995a | 12 subjects (all males) |
3 consecutive nights and 2 days in the Iaboratory | Nap condition 1: 4‐hour afternoon nap Nap condition 2: four 1‐hour naps during the night Nap condition 3: 0.125 mg of triazolam prior to a prophylactic 4‐hour nap before the 24‐hour operation |
MSLT, EEG; performance and mood were assessed with repeated batteries of measures (logical reasoning, WAlS, computer‐modified Williams Word Memory Test of immediate free recall, visual vigilance, subjective sleepiness/alertness, POMS, oral temperature) across the 24‐hour operation | When a series of 1‐hour naps was taken during the normal night period, oral temperature and psychomotor performance also declined. However, performance was relatively improved on the following evening. In contrast, with an effective 4‐hour prophylactic nap, performance remained near baseline Ievels across the night. Fatigue increased over the course of the study, the increases were similar in each group. | |
| Caldwell 1998 | 18 subjects (all males) |
3 separate 38‐hour periods of continuous wakefulness, each separated by 10 hours of recovery sleep | Nap condition 1: 2‐hour evening nap (at 21:00) induced with 10 mg zolpidem tartrate Nap condition 2: 2‐hour nap (at 21:00) with placebo; Condition 3: 2‐hour rest break with no sleep Following 1) or 2) or 3), subjects remained awake for 23 additional hours |
Sleepiness on‐shift, alertness (and others) assessed using VAS; Repeated Test of Sustained Wakefulness; polysomnography of naps; POMS; multiattribute task battery | Results indicated the effectiveness of prophylactic naps for sustaining mood, alertness, and performance throughout the final 23 hours of a 39‐hour period of sustained operations. Both napping conditions attenuated the decrements normally associated with total sleep deprivation, but the zolpidem nap was the most effective because subjects obtained the most sleep. | |
| Della Rocco 2000 | 59 subjects (31 females) |
4‐day protocol with 3 early morning shifts (07:00‐15:00) followed by a rapid rotation to the midnight shift (23:00‐07:00) | Nap condition 1: long nap of 2 hours Nap condition 2: a short nap of 45 minutes Nap condition 3: no‐nap condition |
Wrist activity monitors; sleepiness on‐shift via Stanford Sleepiness Scale; Air Traffic Scenarios Test; the Bakan, a test of vigilance | While sleepiness increased across the midnight shift for all groups, ratings were generally lower for the long nap condition and were lower for males in the short nap condition. Both cognitive performance and subjective measures of sleepiness supported the use of naps during the midnight shift. | |
| Gillberg 1984 | 12 subjects (all males) |
Sleep of 4 hours during the preceding night, work during the day and then kept awake (except for naps) in the laboratory from 17:00 to 08:00 the following morning | Nap condition 1: one‐hour nap (21:00h) Nap condition 2: one‐hour nap (04:30h) Nap condition 3: no‐nap |
EEG, EOG; self‐ratings of sleepiness on‐shift, sleep latency tests; single choice visual reaction time task | Clear positive effects of naps (especially the 04:30 nap) on performance. The sleep latency measurements showed similar, but less clear tendencies, while ratings of sleepiness did not differentiate between conditions. | |
| Hilditch 2014 | 30 subjects (18 females) |
3‐day laboratory study including one baseline sleep (22:00‐07:00) and one experimental night | Nap condition 1: total sleep deprivation (NO‐NAP) Nap condition 2: 10‐min nap (10‐NAP) Nap condition 3: 30‐min nap (30‐NAP) Nap opportunities ended at 04:00 |
Fatigue scale, sleepiness scale, and self‐rated performance scale; psychomotor vigilance test (PVT‐B), digit‐symbol substitution task | In the 30‐NAP condition, performance immediately deteriorated from pre‐nap and was still worse at 47 min postnap. A 10‐min ‐ but not a 30‐min ‐ night‐time nap had minimal sleep inertia and helped to mitigate short‐term performance impairment during a simulated night shift. | |
| Hilditch 2015 | 21 subjects (12 females) |
3‐day laboratory study; keeping subjects awake for 27 hours for 1 simulated night shift; 40‐min York highway driving task at 07:15 to simulate the commute | Nap condition 1: total sleep deprivation (NO‐NAP) Nap condition 2: 10‐min nap ending at 04:00 + a 10‐min pre‐drive nap ending at 07:10 (10‐NAP) |
Polysomnography; SP‐Fatigue; PVT‐B | In the 10‐NAP condition, PVT‐B performance was worse after the nap (07:12) compared to before the nap (06:30); no change across time was found in the NO‐NAP condition. SP‐Fatigue and driving performance did not differ significantly between conditions. | |
| Kan 2012 | 79 subjects (27 females) |
10‐day sleep restriction protocol, assignment to one of 18 sleep regimens | Nap condition 1: restricted diurnal sleep + nocturnal nap (0.4‐hour, 0.8‐hour, 1.2‐hour, 1.6‐hour, 2.0‐hour or 2.4‐hour time in bed Nap condition 2: restricted diurnal sleep + no‐nap |
Polysomnography; total sleep time (sleep duration) | Napping on the night shift does not degrade subsequent daytime SE above and beyond SE reduction associated with daytime sleep or increasing overall time in bed | |
| Kubo 2010 | 12 subjects (all males) |
3‐day experiment with 1 simulated night shift (22:00–08:00 ) and subsequent day (11:30–17:30) and night sleep (00:00–07:00) | Nap conditions: 1) 00:00–01:00 (early 60 min; E60); 2) 00:00–02:00 (E120) 3) 04:00–05:00 (late 60 min; L60) 4) 04:00–06:00 (L120) 5) no‐nap |
Polysomnography; rectal temperature; VAS for sleepiness; visual vigilance test; set of tasks, including English transcription task + a performance test battery | Posthoc analyses showed significantly longer RTs and more lapses following the L60 nap compared with no‐nap. In contrast, there was no significant difference in sleepiness between the L60, or any of the other nap conditions, and the no‐nap condition. Findings suggest the effect of sleep inertia on visual vigilance test performance was profound in the L60 condition, although no significant effects on sleepiness were self‐reported by VAS. | |
| Lovato 2009 | 22 subjects (13 females) |
Simulated night shift environment with a 2‐hour sleep in the afternoon from 15:00–17:00 hours, followed by nap condition 1 or 2 in 02:30–03:00 | Nap condition 1: 30‐min nap Nap condition 2: no‐nap |
Sleepiness on‐shift (SSS, KSS), fatigue and vigour subscales of the POMS, and the VAS for sleepiness; symbol–digit substitution task, the letter cancellation task, and the PVT | The 30‐min nap resulted in some impairment of subjective alertness for a brief period (up to 30 min) immediately following the nap when compared to the no‐nap condition. Following this brief period, alertness improved by the 30‐min nap from 04:00 until the end of the testing period at 07:00. | |
| Macchi 2002 | 8 subjects (1 female) |
Simulated night shift; alertness and performance testing sessions + 2‐hour runs in a driving simulator | From 14:00 to 17:00 Nap condition 1: sleep Nap condition 2: sedentary activities |
Polysomnography, subjective fatigue and sleepiness on‐shift via VAS, sleep quality (Sleep Quality Questionnaire); EEG; 3 computerised tests from the Walter Reed performance assessment battery | In the nap condition, the subjects showed lower subjective sleepiness and fatigue, as measured by VAS, and faster reaction times and less variability on psychomotor performance tasks. | |
| Matsumoto 1981 | 8 subjects (all male) |
5 Nap conditions between a previous full night's sleep and a day sleep on the day following the night | Nap conditions: a 2‐hour nap between: 1) 22:00‐00:00 (N1) 2) 02:00‐04:00 (N2) 3) 04:00‐06:00 (N3) 4) 06:00‐08:00 (N4) 5) no‐nap(Control group) |
EEG, EOG, EMG, ECG, respiratory movement; rectal temperature, oral temperature; flicker fusion frequency, sleepiness, fatigue complaints | Decrease in rectal temperature during the night was more marked for conditions N2, N3 and N4, with a lesser extent of individual differences, than for the Control group and N1. The self‐evaluation of the sleep depth and the rapidness of sleep onset correlated highly with sleep parameters. N3 and N4 were evaluated to have resulted in a better sleep than N1. | |
| Saito 1996 | 6 subjects (all female) |
3 x 3 days experimental conditions Day 1+2 daily activities Day 3: awake from 00:00 until 10:00 with a nap period which started at 03:00 |
On each of 3 days:
nap condition 1: no‐nap nap condition 2: 1‐hour nap nap condition 3: 2‐hour nap |
Fatigue Feelings Scale, SSS; EEG, EOG and EMG during the naps | A 1‐hour nocturnal nap gave significantly smaller scores on two subscales of Fatigue Feelings Scale during early morning hours than no napping. A 2‐hour nocturnal nap, which contained significantly Ionger duration of Slow Wave Sleep than a 1‐hour nocturnal nap, did not differ from a 1‐hour nocturnal nap in decreasing scores of fatigue feelings during these hours | |
| Salame 1995 | 24 subjects (all males) |
5 nights experimental design with one no‐nap condition before the night tests + a nap condition that comprised the 1‐hour nap followed by the test sessions | Nap condition 1: 1‐hour nap at 00:00 Nap condition 2: 1‐hour nap at 03:00 |
Sleep inertia (spatial memory) and logical reasoning tasks | No effects on accuracy, and no circadian effects of napping were found. Pooled data of intervention groups showed that the performance in the 1‐hour nap condition exhibited significant reductions of speed immediately following awakening, when compared with no‐nap, reflecting sleep inertia effects. | |
| Schweitzer 1992 | Study A: 10 subjects (7 females); Study B: 12 subjects (5 females) |
Study A&B: Two night‐time work periods separated by at least three normally timed nights of sleep | Study A ‐ Nap condition: 3‐hour nap opportunity on 1 night between 20:00 and 23:00 ; Study B ‐ Caffeine condition: 4 mg/kg caffeine on 1 night |
Polysomnography, sleepiness on‐shift SSS, VAS; alertness (computer‐driven simulated assembly line task) | Performance and subjective alertness improved after both a 2·3‐hour evening nap or ingestion of caffeine prior to the work shift. Although neither napping nor caffeine countered the strong circadian influence on performance and alertness in the early morning hours, both strategies attenuated their sharp decline. | |
| Takeyama 2002 | 13 subjects (all male) |
Simulated shift work schedules of 9 consecutive days: 2 day (08:00‐16:00) + 3 night (22:00‐08:00) + 3 day shifts; between experimenting with nap condition 1 and 2, rest period of 1 week | Nap condition 1: nap from 02:00‐04:00 Nap condition 2: no‐nap |
Fatigue and anxiety questionnaire, heart rate variability, oral temperature, salivary cortisol; two performance tasks: typing figures + performing mental arithmetic; CFF, 3‐choice reaction time | Task performances decreased and subjective fatigue and anxiety increased in proportion to the length of time worked in both M‐types (morningness) and E‐types (eveningness) who had no‐nap. In M‐types, these changes were significantly suppressed by the nap on the first night of duty. Changes for E‐types were smaller than those for M‐types in terms of task performance and psycho‐physiological parameters. | |
| Takeyama 2004 | 6 subjects (all male) |
5 nap conditions on‐shift for each participant; 3 consecutive days with one night shift (22:00‐08:00) followed by daytime sleep and night sleep; at least 5 days between the experiments | Nap conditions: 1) 00:00‐01:00 (E60) 2) 00:00‐02:00 (E120) 3) 04:00‐05:00 (L60) 4) 04:00‐06:00 (L120) 5) no‐nap (No‐nap) | Polysomnography, questionnaire on subjective fatigue, heart rate variability, rectal temperature; performance task (typing text) and tests (choice reaction time test, a logical reasoning test, a vigilance test, and a CFF test) | Sleep latency was shorter and sleep efficiency was higher in the nap in L60 and L120 than that in E60 and E120. Performance was somewhat improved by taking a 2‐hour nap later in the shift, but deteriorated after a one‐hour nap. | |
| Tremaine 2010 | 24 subjects (15 females) |
Simulated night‐shift schedules with at least one week intervening between conditions | 2‐hour afternoon sleep opportunity + one of two‐nap conditions: 1) 30‐min night‐nap; 2) no night‐nap | Polysomnography, subjective sleepiness on‐shift (SSS, KSS, VAS), objective sleepiness on‐shift (sleep latency tests); objective performance (Symbol Digit Substitution Task) + reaction time (PVT) | Subjective sleepiness was less correlated with objective sleepiness and objective performance when participants were given a 30‐min night nap. However subjective sleepiness and reaction time performance was strongly correlated in both conditions, and there was no significant difference between the nap and no‐nap conditions. | |
CFF: Critical Flicker Fusion Frequency ECG: electrocardiogram EEG: electroencephalogram EMG: electromyography EOG: electro‐oculogram KSS: Karolinska Sleepiness Scale MSLT: Multiple Sleep Latency Test POMS: Profile of Mood States PVT: Psychomotor Vigilance Task PVT‐B: brief Psychomotor Vigilance Test
RT: Reaction Time
SE: Standard Error SP‐Fatigue: Samn‐Perelli Fatigue Scale SSS: Stanford Sleepiness Scale VAS: Visual Analogue Scale
WAIS: Wechsler Adult Intelligence Scale
3. Laboratory trials ‐ other interventions.
| Other interventions | ||||||
| Reference | Study participants | Shift system | Intervention | Key endpoints | Key results | |
| Baehr 1999 | 33 subjects (17 females) |
Simulated night work study with 8 consecutive night shifts followed by daytime sleep/dark periods | 1) Intermittent bright light (6 pulses, 40‐min long each, at 5000 lux) versus dim light (< 500 lux, 20 min ) 2) Intermittent exercise (6 bouts, 15‐min long each, at 50%‐60% of maximum heart rate) versus no exercise; bright light and exercise interventions during the first 6 hours of the first 3 night shifts |
Core temperature; sleep duration (sleep log) | Intermittent bright light groups had significantly larger phase delays than dim‐light groups, and 94% of subjects who received bright light had phase shifts large enough for the individual rectal temperature minimum to reach daytime sleep. Exercise did not affect phase shifts; neither facilitating nor inhibiting phase shifts produced by bright light. During the last 4 days of the study, subjects in the bright light groups slept more (within the scheduled sleep/dark periods) and napped less than those in the dim light groups. | |
| Kelly 1994 | 43 subjects (all male) |
5‐day night work study involving a 10‐hour phase delay of the work/rest cycle | 1) Bright white light (3500‐4300 Iux) versus dim red light (200‐300 Iux) from 22:00‐02:00 each night 2) Inactive LEET versus active LEET therapy for 20 min prior to the daytime sleep periods |
Polysomnography; circadian phase shifting evaluated via core body temperature + urinary 6‐SM excretion; indirect measurement of alertness: complex reaction time, simple reaction time; subjective alertness (VAS); performance examined with a cognitive performance assessment battery | 6‐SM data indicate that bright light exposure increased the phase delay seen in this circadian rhythm in the 3 days after the work/rest schedule shift. Bright light treatment shows evidence of improving accuracy on a broad range of cognitive performance, without compensatory decreases in speed. LEET administration before the daytime sleep periods showed little evidence of affecting either performance or 6‐SM. Complex reaction time: bright light‐exposed subjects performed significantly better than did dim light‐exposed on all three postshift testing sessions, with the largest difference the second postshift night (Day 3). Bright light subjects started out slightly less alert at baseline, dropped less the first night after the shift, and rose much more the second night after the shift. On the last night both groups dropped to a similar level. | |
| Kelly 1997 | 45 subjects (all male) |
5‐day night work study with subjects working and being tested during three 9‐hour night shifts from 18:00‐03:00 | 1) Bright light (3500‐4300 lux) or dim light (200‐300 lux) from 22:00‐02:00 each night 2) LEET for 20 min prior to daytime sleep 3) Both bright light) + LEET) 4) Placebo treatments |
Polysomnography; 6‐SM, VAS of sleepiness; cognitive tests in the performance assessment battery, simple and complex reaction times (simple reaction times + complex reaction times) trials, word memory task | Bright light accelerated phase delay of the circadian melatonin rhythm after the work‐rest schedule shift. Further, subjects who received bright light had greater total sleep time and improved sleep continuity. Some minor improvements in cognitive performance were produced by light treatments but not by LEET. | |
| Neri 2002 | 28 subjects (all male) |
6‐hour night‐time flight in a flight simulator; a structured sleep/wake schedule for three nights just prior to the study; obtaining their typical amount of night‐time sleep between the hours of 22:00 and 08:00 | Treatment condition: 5 breaks spaced hourly during cruise Control condition: 1 break in the middle of cruise |
Questionnaire + interview; EEG/EOG, subjective sleepiness (KSS, VAS); vigilance performance (PVT), subjective sleepiness ratings, electrophysiological measures of drowsiness, continuous video | The treatment group showed significant reductions for 15 min postbreak in slow eye movements, theta‐band activity, and unintended sleep episodes compared with the control group. The treatment group reported significantly greater subjective alertness for up to 25 min postbreak. There was no evidence of objective vigilance performance improvement at 15‐25 min postbreak. | |
| Santhi 2008 | 35 subjects (14 females) |
10‐day shift work simulation (4 day shifts = 07:00‐15:00 and 3 night shifts = 23:00‐07:00) | 1) Morning Sleep (08:00‐16:00) + phase‐delaying light exposure (23:00‐03:00) 2) Evening Sleep (14:00‐22:00) + phase‐advancing light exposure (03:00‐07:00) |
Polysomnography; dim light salivary melatonin onset; RT in the PVT; subjective alertness via the KSS | Analysis of the dim light salivary melatonin onset indicated a modest but significant circadian realignment in both sleep groups. Daytime sleep efficiency and total sleep time did not differ between them or from their respective baseline sleep. On the final night shift, the evening sleep subjects had fewer episodes of attentional impairment and quicker responses on the PVT than their morning sleep counterparts. | |
| Sato 2010 | 8 subjects (all male) |
Simulated night work (22:00‐08:00) with an hourly exercise consisting of 30 min task, 15 min test and 15 min break | Treatment condition: hourly exercise (3 min during breaks) Control condition: no exercise during breaks |
Heart rate variability; a VAS for subjective fatigue + sleepiness, psychomotor vigilance test (PVT) | Work performance in the last 10 min of each 30‐min task was better under the treatment condition than under the control condition. During the second half of the test period, exercise showed an effect on sustained attention. Exercise was not effective in reducing subjective fatigue and sleepiness. | |
| Yamanaka 2010 | 17 subjects (all male) |
In a temporal isolation facility with dim light conditions (< 10 lux), sleep schedules were phase‐advanced by 8 hours from habitual sleep times for 4 days, followed by a free‐run session for 6 days with no time cues. During the shift schedule, the treatment and control groups performed physical exercise or not | Treatment group: physical exercise with a bicycle ergometer in the early and middle waking period for 2 hours each Control group: sat on a chair at those times |
Polysomnography, bed sensor, wrist activity + light intensity (Actiwatch); plasma melatonin, continuous rectal temperature | Sleep‐onset on the first day of free‐run in the exercise group was significantly phase‐advanced from that in the control and from the baseline. The circadian melatonin rhythm was significantly phase‐delayed in both groups, showing internal desynchronisation of the circadian rhythms. | |
EEG: electroencephalogram EOG: electro‐oculogram KSS: Karolinska Sleepiness Scale LEET: Low Energy Emission Therapy PVT: Psychomotor Vigilance Task VAS: Visual Analogue Scale 6‐SM: 6‐ sulphatoxymelatonin
Types of participants
We included studies conducted with adult workers engaged in shift work schedules that include night‐shift work, irrespective of industry, country, age or comorbidities. For inclusion in this review, we placed no restriction on the 'sleep health‐status' of the participants; we included studies examining participants who had sleep problems, studies in which participants were sleep‐disorder free, and studies in which sleep health was not assessed.
Types of interventions
We included trials comparing any person‐directed, non‐pharmacological intervention with any other intervention or no intervention.
Types of outcome measures
Primary outcomes
Sleepiness on‐shift: Measured at the beginning, middle, and end of the shift as either:
self‐rated (subjective) sleepiness, measured with a validated questionnaire such as the Karolinska Sleepiness Scale (KSS) (Akerstedt 2014), Stanford Sleepiness Scale (SSS) (Herscovitch 1981; Hoddes 1972), Epworth Sleepiness Scale (ESS) (Johns 1991), relevant questions in the Standard Shift Work Index (Barton 1995), or other visual analogue scales (VAS); or
physiological sleepiness, measured by electrophysiological methods while working (e.g. electroencephalogram or electro‐oculogram measurement) or by standardised physiological tests of sleepiness, such as, the Multiple Sleep Latency Test (Carskadon 1986), the Maintenance of Wakefulness Test (Mitler 1982), or the pupillometric assessment; or
behavioural sleepiness, measured as performance in a validated vigilance test such as the Psychomotor Vigilance Task (PVT) test (e.g. Basner 2011; Thorne 2005), the Mackworth Clock Test (Mackworth 1950), or single or multiple choice reaction time tests; or
behavioural sleepiness measured as characteristics of overt behaviour that are identified through video recording methods, such as, an Observer Rating of Drowsiness (ORD) (e.g. Wierwille 1994), or percentage of eyelid closure (PERCLOS) (Dinges 1998; Sommer 2010).
Sleep length off‐shift: Length of sleep based on the relevant questions in validated questionnaires (see examples above), sleep diaries, or wrist‐worn actigraphy.
Sleep quality off‐shift: Measured with a validated or psychometrically tested questionnaire, such as, the Bergen Insomnia Scale (Pallesen 2008), Pittsburgh Sleep Quality Index (PSQI) (Buysse 1989), Basic Nordic Sleep Questionnaire (Partinen 1995), Jenkins Sleep Questionnaire (Lallukka 2011), Karolinska Sleep Questionnaire (Akerstedt 2002; Kecklund 1992), relevant questions in the Standard Shift Work Index, and sleep diaries or wrist‐worn actigraphy‐based data.
The term 'fatigue' is usually used to describe exhaustion or tiredness due to long‐lasting exertion. Nevertheless, in some studies 'fatigue' is used as a synonym for sleepiness. Therefore, in our search we also included the term 'fatigue' as an outcome measure when it was used as a measure of sleepiness.
Secondary outcomes
In those studies that reported this review's primary outcomes, we also intended to examine the following secondary outcomes.
Costs for lighting interventions (e.g. initial and running costs of the lighting equipment).
Costs for napping interventions (e.g. number of staff and costs for covering the time when individuals sleep).
Search methods for identification of studies
Electronic searches
We searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 8), MEDLINE Ovid (1946 to 1 August 2015), Embase (1974 to 1 August 2015), Web of Knowledge (1945 to 1 August 2015), ProQuest (1970 to 1 August 2015), PsycINFO (1806 to 1 August 2015), OpenGrey (searched 1 August 2015), and OSH‐UPDATE (IOSHTIC, NIOSHTIC‐2, HSELINE, CISDOC) (1930 to 1 August 2015). We used a search strategy specifically designed for MEDLINE and subsequently adapted for other relevant databases (except for Proquest, where we searched using subject headings and keywords only) (see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6).
Because the search term 'shift' alone would have led to a very high number of citations, we combined the term 'shift' with other terms used to describe specific aspects of shift work. Examples are 'shift work', 'night shift', 'shift schedule' and 'graveyard shift'. We also accounted for terms that describe shift work, but do not use the word 'shift', such as 'duty time' or 'hours', 'rota' or 'four‐day week' or 'compressed work week' used to denote a series of 12‐hour shifts. The search was limited by terms for different outcomes or types of interventions. Due to the extreme overlap in the literature on person‐directed interventions and shift system interventions (the latter being the focus of a separate Cochrane Review (Erren 2013)), we conducted one combined search and screened for both the current review and the shift schedule review.
Searching other resources
We checked reference lists of original articles and review articles for additional references. Furthermore, we contacted experts in the field to identify additional unpublished materials. We searched the conference proceedings of the biannual symposium on night and shift work. We searched the World Health Organization (WHO) Trial Register (who.int/ictrp), as well as the most important trial registers within this register directly (clinicaltrials.gov and clinicaltrialsregister.eu).
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts of all the studies identified as a result of the search (pairs included some combination of TS, VG, MB, AD and RR). They coded these as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved all eligible or potentially eligible/unclear full‐text study reports. Two review authors independently screened these for inclusion and subsequently identified and recorded reasons for the exclusion of ineligible studies (pairs included some combination of TS, VG, MB, AD, RR and GC). We resolved any disagreement through discussion or, if required, we consulted a third review author (TE or AP).
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009). We also sought to obtain further information from field study authors when a paper was found to contain insufficient information to enable us to reach a decision on eligibility.
Data extraction and management
Two review authors independently extracted trial data (pairs included some combination of TS, VG, AP, MB, AD and RR). For field studies, extracted data included country, trial design, characteristics of the trial participants, inclusion and exclusion criteria, type of work, branch of industry, and types of interventions and outcomes. For relevant outcomes, we extracted the statistical results, such as means and standard deviations for continuous data. Disagreement was resolved by discussion with a third review author (TE). For laboratory studies, extracted data included author, year, participant number and gender, intervention details, key endpoints, and key results.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of the included field studies (TS, VG). We resolved disagreements by consensus. We contacted study authors for missing methodological information. Wherever possible we used quotes from the text to support our judgements about the individual 'Risk of bias' items. We assessed the risk of bias across the following eight domains.
Sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessors.
Incomplete outcome data.
Selective outcome reporting.
Outcome reliably or objectively measured.
Other sources of bias.
We applied a risk of bias rating of 'low', 'high' or 'unclear' to each of the eight bias domains (taken, added to, and modified where applicable from (Higgins 2011)). We judged a study to have a low risk of bias overall if we assessed all seven (or eight, for cross‐over trials) domains as having a low risk of bias. We considered a study to have a high risk of bias overall if we assessed at least one of the domains as high. We judged a study to have an unclear risk of bias overall if we assessed at least one domain as unclear (and no domain was assessed as high).
Measures of treatment effect
All relevant outcomes for this systematic review were available as continuous data (no dichotomous outcomes or measures). In trials presenting the same outcome with objective and subjective measurements, we gave preference to the objective measurements and included only those in our quantitative analyses.
For standard parallel trials, we entered the mean and standard deviation (SD) as they were reported in the publication. In cases where authors presented an effect estimate as the mean difference (MD) and 95% confidence interval (CI) (i.e. Smith‐Coggins 2006), we converted the CI to a standard error using the formula recommended in chapter 7.7.7.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and entered these data as Generic Inverse Variance data (MD and standard error).
For cross‐over trial outcomes, we intended to use the MD and its standard error based on a paired analysis.
We converted time parameters, when necessary. For the outcome total sleep time, we converted into hours those means and variance that were reported in minutes (i.e. Lowden 2004). For the outcome sleep onset latency, we converted into minutes those data reported in hours (i.e. Thorne 2010).
We reported the outcomes of studies with different study designs separately.
Unit of analysis issues
In most studies, the authors reported outcomes relevant to our review as several measurements per night, for example, at midnight, 02:00, 04:00 and 06:00. In those cases where we had several subgroups or more than two different intervention groups to combine and the data were presented separately for each group, we took the average of the measurements. We summarised the mean SDs by pooling according to the formula presented in the Cochrane Handbook for Systematic Reviews of Interventions (table 7.7.a; Higgins 2011).
For cross‐over trials, we intended to use results from paired statistical tests. Often these data were not available and we used the method described in Elbourne 2002 to perform sensitivity analyses assuming correlation coefficients of 0, 0.7, and 0.9. When authors presented analysis of variance (ANOVA) P values we reported these values for comparison with our findings (see Table 14). We transformed 95% CIs into P values using the formula recommended by Altman 2011: P = exp(−0.717 × z − 0.416 × z2), where z = the estimate of effect/the standard error.
4. Relevant trial‐reported ANOVAs compared to posthoc review author calculations.
| Cross‐over trial | Outcome | Description of ANOVA strategy | ANOVA results | Correlation coefficient (CC) | Estimates of P values by CC (see Unit of analysis issues) |
| Sadeghniiat‐Haghighi 2011 | Sleepiness on‐shift overall | "A two‐factor repeated measurement ANOVA was used. Factors: 1) treatment; 2) time (of night measurement) P values were corrected for sphericity (using the Huynh‐Feldt coefficient). Significance was defined at P < 0.05." |
Period 1 : Time of night measurement × Treatment: (F = 8.76; P < 0.001) Time of night measurement: (F = 40.98; P < 0.001) Period 2 : Time of night measurement × Treatment: (F = 5.124, P < 0.01) Time of night measurement: (F = 9.872; P < 0.001) |
0.9 0.7 0.0 |
< 0.0001 < 0.0001 < 0.0001 |
| Karchani 2011 | Sleepiness on‐shift overall | "Using the paired t‐test, we compared subjective sleepiness between two conditions (with bright light and with normal light). A repeated measure ANOVA showed interaction between independent variables in this study. The level of significance was defined at P < 0.05. (examined treatment effect, carry‐over effect, and period effect)." | The findings for treatment effect, period effect and carry‐over effect of the study population: Treatment effect: t df P value –21.95 89 0.001 |
0.9 0.7 0.0 |
< 0.0001 < 0.0001 < 0.0001 |
| Lowden 2004 | Sleep efficiency (actigraph) | "The data obtained during night work were submitted to ANOVA for repeated measures, with correction for unequal variances according to Huynh and Feldt (Huynh 1976). The two‐way ANOVA included the factors of condition (Bright light/Normal light) and day (15 examined night shifts). A third factor, time of day, was added for variables with several measures during 1 day (for melatonin and KSS). A fourth factor, week (three studied night work weeks), was added to give a more detailed analysis of KSS ratings. Posthoc mean comparisons were carried out with contrasts. KSS ratings during the night shift week (means of 3 weeks): As some workers showed missing data on Fridays, this day was omitted from the analysis. To reflect the many data points, a four‐way analysis of variance including the factors of condition, week (3 weeks), night (night 1–4 of each week) and time of day, were used." |
Condition: NS (no P value reported) Cond./Night Interaction: NS |
0.9 0.7 0.0 |
< 0.0001 0.02 0.20 |
|
Total sleep time‐main sleep (bed time; final awakening) |
Condition: NS (no P value reported) Cond./Night Interaction: NS |
0.9 0.7 0.0 |
0.01 0.14 0.43 |
||
| Total sleep time‐24‐hr sleep |
Condition: (P < 0.05) Cond./Night Interaction: NS |
0.9 0.7 0.0 |
< 0.0001 0.04 0.24 |
||
| Sleepiness on‐shift KSS (overall) | Sleepiness: "No main effects were obtained except for time of day showing an increase of sleepiness throughout the night shift (F = 36.46; P = 0.0001; df = 3/45). A significant interaction was obtained (Fig. 2) for the interaction of condition, night and time (F = 2.39; P = 0.0365; df = 9/135). Sleepiness was significantly reduced in the bright light condition at 02:00 hours on Tuesday; at 04:00 hours on Monday, Tuesday and Thursday; and at 06:00 hours on Tuesday and Thursday as shown by the posthoc mean comparisons. The reduction of sleepiness in the bright light condition was further emphasised by the significant interaction of condition and time of day (F = 3.07; P = 0.0429; df = 3/45). The interaction of week + light was insignificant." | 0.9 0.7 0.0 |
< 0.0001 0.06 0.31 |
||
| Sleepiness on‐shift KSS (postintervention) | 0.9 0.7 0.0 |
< 0.0001 0.09 0.35 |
|||
| Smith 2007 | Sleepiness on‐shift – Reaction time – postintervention (03:00 and 04:00 and 05:00 and 06:00) | "For each of the dependent variables, a set of 2 x 2 repeated measures ANOVAs were carried out. In order to control for interindividual variability in baseline performance, scores for all four dependent variables (response speed, M10%RT, lapse frequency and subjective sleepiness) were expressed relative to the baseline test score obtained at 00:00 hours, calculated by subtracting the 00:00 hours’ value from each hourly score. That is, 00:00 hours scores were zeroed and subsequent scores were relative to this point. Relative scores at each hour of shift were then averaged to obtain the mean relative performance across participants. In order to analyse specific time differences in the dependent variables after the nap, parallel ANOVAs were carried out with different levels entered for the time factor (i.e. before nap and 03:00 hours; before nap and 04:00 hours; before nap and 05:00 hours; before nap and 06:00 hours). Before‐nap mean scores were calculated from a combined average of 00:00, 01:00 and 02:00 hours data. As the aim was to compare nap and no‐nap conditions after the nap, the statistics of interest were the nap × time interactions. Significant interactions were observed for response speed at 04:00 and 06:00… and for subjective sleepiness at 03:00 and 04:00. … Participants therefore had faster reaction times, and less subjective sleepiness, after the nap." |
Before nap vs 06:00 Nap: P = 0.002 Time: P = 0.011 N x T : P = 0.012 |
0.9 0.7 0.0 |
< 0.0001 0.02 0.21 |
| Sleepiness on‐shift – Subjective sleepiness score – postintervention (03:00 and 04:00 and 05:00 and 06:00) |
Before nap vs 06:00 Nap: P = 0.16 Time: P = 0.201 N x T : P = 0.095 |
0.9 0.7 0.0 |
< 0.0001 < 0.0001 0.05 |
df: Degrees of Freedom
KSS: Karolinska Sleepiness Scale
M10%RT: Mean of the fastest 10% reaction time
NS: Not Significant
RT: Reaction Time
For studies that employed a cluster‐randomised design and reported sufficient data to be included in a meta‐analysis but did not make an allowance for the design effect, we had intended to calculate the design effect based on the methods described in chapter 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, due to the absence of any included studies using clustered data, this was not possible.
Dealing with missing data
For field studies, we contacted trial authors to obtain data not found in their reports that were needed either for the assessment of risk of bias or for outcomes relevant to this systematic review. We used all reports of trials in order to obtain missing data, including presentations, if found. We used the methods presented in chapter 7.7.3.3 in the Cochrane Handbook for Systematic Reviews of Interventions to calculate statistics (e.g. SDs or correlation coefficients) that can be calculated from other values (Higgins 2011).
Where possible, we used intention‐to‐treat analyses in randomised trials. We examined reasons for dropouts and missing data when these data were available. We recorded the methods the study authors used for dealing with missing data.
Most authors of the cross‐over trials in our review used ANOVA to analyse their data, and they did not report the standard error of the MD between the intervention and the control group postintervention. In these cases, we calculated the standard error of the MD using the formula in Elbourne 2002: SD = √ (SDi2 + SDc2 – 2 x correlation coefficient (SDi)(SDc)). However, none of the studies reported enough data to calculate a correlation coefficient.
Lacking a given correlation coefficient for a comparable analysis, we estimated the standard errors by assuming varying levels of correlation between the intervention and control groups, based on recommendations made in Elbourne 2002. Using the correlation coefficient 0 to represent no correlation, 0.7 to represent a middle level of correlation, and 0.9 to represent a strong correlation, we presented three possibilities for how the variance might behave in each cross‐over trial (see Sensitivity analysis). In the case of single trial outcomes, we present all three levels of correlation in the forest plots. For our meta‐analyses of cross‐over trials and in our 'Summary of findings' tables, we present results assuming zero correlation between cases and controls.
Assessment of heterogeneity
We assessed clinical homogeneity based on similarities of interventions, populations, shift schedules, exact outcome definitions, outcome timing and follow‐up. Subsequently, we did not combine the interventions light, napping, education or physical exercise. Within the category of light, we considered trials administering both bright light and sunglasses different from those administering bright light alone and did not combine these. For light or goggle interventions we had intended to consider the time of day, duration of light, strength, and wavelengths of light (or similar for goggles). However, due to fewer than expected studies per comparison, we were not able to do this (see Differences between protocol and review). Within the category of napping, we considered a single nap to be different from multiple naps and did not combine such trials (Banks 2015).
We had intended to consider all educational interventions to be similar enough, provided they addressed similar topics (e.g. sleep times with regard to shift, sleep conditions, exercise) and had a similar duration. In addition, we had intended to combine all exercise interventions. However, due to fewer than expected studies per comparison, we were not able to do this (see Differences between protocol and review).
Within a single comparison group, we considered studies to be similar enough to combine if they measured the same outcome variable (e.g. sleepiness on‐shift) at a similar time with regard to the shifts examined. To the extent that it was methodologically advisable, we combined different ways of measuring the same outcome variable. However, this was not always possible, as in the example of the Karolinska and Stanford sleepiness scales (see Methodological diversity and pooling).
For all intervention types, we considered interventions administered during the night shift different from those administered during the day shift or days‐off and did not combine them.
We had intended to give priority in our primary analysis to subjective measures of sleepiness, sleep quality, and sleep length, however, in order to maintain consistency with our partner publication (Liira 2014), we gave priority to objective measures (see Differences between protocol and review).
We had intended to separately analyse studies in healthy shift workers and persons with shiftwork disorder. We had also intended to explore other differences in populations through subgroup analyses. However, due to fewer than expected studies per comparison, we were not able to do this (see Differences between protocol and review).
We tested for statistical heterogeneity by means of the Chi2 test, as implemented in the forest plot in Review Manager 5 software (RevMan 2014). We used a significance level of P < 0.10 to indicate heterogeneity. We quantified the degree of heterogeneity by using the I2 statistic, where an I2 value of 25% to 50% indicates a low degree of heterogeneity, 50% to 75% a moderate degree of heterogeneity, and > 75% a high degree of heterogeneity (Higgins 2011).
Assessment of reporting biases
We made every effort to detect duplicate studies. If multiple articles reported on the same data, we extracted data only once in order to reduce the risk of reporting bias. Location bias was prevented by searching for trials across multiple databases. To prevent language bias, we did not limit for any language.
Data synthesis
We pooled those field studies we judged to be sufficiently clinically homogeneous. For these we conducted meta‐analyses. To allow for statistical heterogeneity, we considered a random‐effects model to be appropriate for meta‐analysis.
To present the overall quality of evidence per outcome for the main findings we used the GRADE Approach, as described in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), and as implemented in the GRADEPro GDT software (GRADEproGDT). Since all included studies were RCTs, we assumed a high level of quality prior to quality assessment. Downgrading was based on five factors: (1) limitations of study; (2) indirectness of evidence; (3) inconsistency of results; (4) imprecision of results; and (5) publication bias. Thus we rated the evidence for each outcome as either, high, moderate, low, or very low.
Subgroup analysis and investigation of heterogeneity
We had intended to conduct subgroup analyses in four areas: chronotype, intervention variations, measurement variations, and age. However, due to the limited number of studies per comparison and outcome, we were not able to conduct any subgroup analyses.
Sensitivity analysis
In order to assess the impact of missing data on the cross‐over trial results, we estimated, per outcome, three separate versions of the standard error (see Dealing with missing data). We subsequently entered, for single study outcomes, all three standard errors into the forest plots, thus allowing a graphic depiction of how varying (missing) levels of correlation affect the significance of the effect estimate.
We had intended to examine the impact of missing data on effect estimates via sensitivity analysis. The total MD among studies with no or little missing data (i.e. data on loss to follow‐up) would be compared to the total MD among studies with extensive missing data. However, no sensitivity analysis was possible, due to the fact that there were never more than two to three studies per comparison, with the vast majority of comparisons containing only a single study.
Results
Description of studies
Results of the search
Figure 1 shows the PRISMA study flow diagram of included and excluded studies. The electronic database search resulted in a total of 30,202 references. We identified a further 153 studies through checking of reference lists of potentially relevant studies and reviews. After removal of duplicates, a total of 29,092 references remained. Based on a screening of titles and abstracts, we identified 2054 potentially relevant references for which we retrieved full‐text articles. Of these, a total of 72 RCTs met our inclusion criteria for this review (17 field studies and 55 laboratory studies). The 17 field study RCTs consisted of 10 bright light studies, four nap studies, and three studies with "other" types of interventions. Of these 17 field studies, 13 presented statistical data that could be included in our quantitative analysis, four of which we examined in two meta‐analyses.
1.
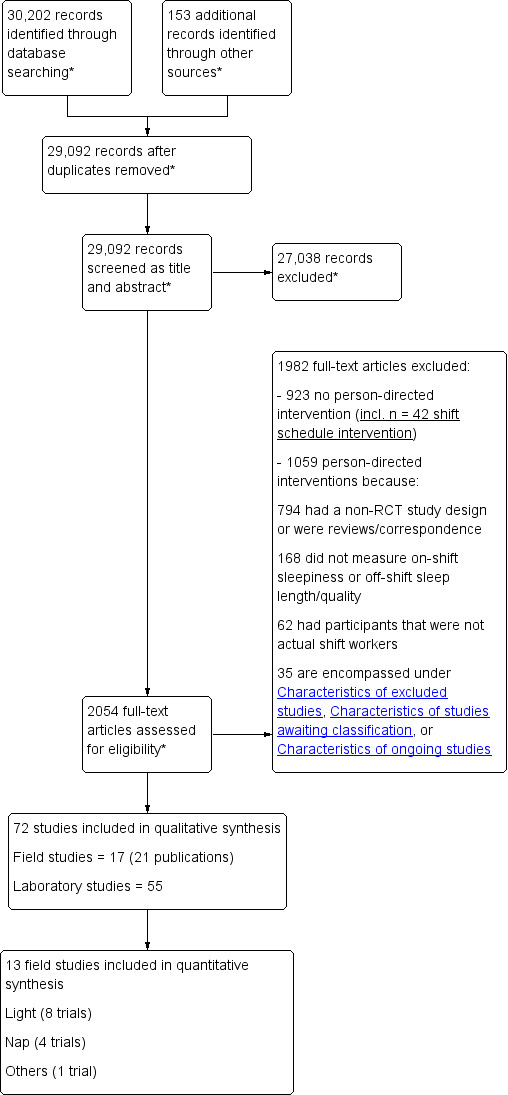
*Reflects search for both the current review and for the review "Adaptation of shift work schedules for preventing and treating sleepiness and sleep disturbances caused by shift work (028) (Erren 2013)"
Included studies
Bright light
Characteristics of the trials and participants
We included ten RCTs that investigated the effect of bright light alone (Bjorvatn 2007; Karchani 2011; Lowden 2004; Ross 1995; Sadeghniiat‐Haghighi 2011; Tanaka 2011; Boivin 2012; Huang 2013; Tapia 2011; Thorne 2010). Two trials were conducted in Iran (Karchani 2011; Sadeghniiat‐Haghighi 2011), two in Norway (Bjorvatn 2007; Thorne 2010), one in Canada (Boivin 2012), one in the USA (Tapia 2011), one in Japan (Tanaka 2011), one in Sweden (Lowden 2004), one in Taiwan (Huang 2013), and one in Antarctica (Ross 1995).
In total, the trials included 362 review‐relevant randomised participants (range: 4 to 94). The average age of the participants ranged between 21 and 46 years. Four studies explored data from men only (Karchani 2011; Ross 1995; Sadeghniiat‐Haghighi 2011; Thorne 2010) and two studies included only women (Huang 2013; Tanaka 2011). In two studies, 94% of the participants were male (Bjorvatn 2007; Lowden 2004) and in two studies women made up between 53% and 61% of the trialists (Boivin 2012; Tapia 2011).
The health status of the populations differed according to trial, with two trials including only participants with sleep problems (Bjorvatn 2007; Huang 2013), three trials including only participants who were healthy overall (Karchani 2011; Tanaka 2011; Thorne 2010), three trials with no health restrictions (Lowden 2004; Ross 1995; Sadeghniiat‐Haghighi 2011), one with untreated 'sleep disorder‐free' participants (Tapia 2011), and one trial including "drug‐free" participants (Boivin 2012).
Two of the studies explored participants' chronotype (Boivin 2012; Thorne 2010); both trials based on a Horne‐Östberg‐Score. Boivin 2012 reported the chronotype to be comparable between the study groups. However, neither analysis included a chronotype‐based subgroup analysis of our outcomes of interest.
(See also Characteristics of included studies).
Interventions
Bright light was administered via either a light box (Bjorvatn 2007; Huang 2013; Tanaka 2011; Tapia 2011; Thorne 2010), fluorescent ceiling bulbs or tubes in the break rooms (Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011), or a portable lamp (Boivin 2012). One trial described the light administered as "full‐spectrum white" (Boivin 2012), one trial simply as "white" (Tapia 2011), and one trial as "polychromatic white" (Thorne 2010). One trial described the light simply as "artificial bright light" (Huang 2013). Ross 1995 provided no information as to how the bright light was applied.
Intensity of a single dose of bright light varied between 2500 lux (Lowden 2004; Sadeghniiat‐Haghighi 2011), 2500 to 3000 lux (Karchani 2011; Ross 1995; Thorne 2010), 5444 to 8826 lux (Tanaka 2011), 7000 to 10,000 lux (Huang 2013), and 10,000 lux (Bjorvatn 2007; Tapia 2011). Boivin 2012 did not specify the light's intensity.
Six trials administered single doses of bright light (Bjorvatn 2007; Huang 2013; Ross 1995; Tanaka 2011; Tapia 2011; Thorne 2010), while four others administered more than one dose per shift (Boivin 2012; Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011). Total time exposed to bright light differed according to each trial, with a median exposure time of 30 minutes (≤ 30 minutes (Bjorvatn 2007; Lowden 2004; Tanaka 2011) and > 30 minutes (Boivin 2012; Huang 2013; Karchani 2011; Ross 1995; Sadeghniiat‐Haghighi 2011; Tapia 2011; Thorne 2010).
In six trials, bright light was administered during the night shift only (Boivin 2012; Huang 2013; Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011; Tapia 2011), and in three trials only during the day shift (Ross 1995; Tanaka 2011; Thorne 2010). A single trial administered bright light both during the night shift and during the day shift (Bjorvatn 2007). When bright light was given during the night shift only, the timing ranged from every two hours in Karchani 2011 to sometime between midnight and 06:00 in Bjorvatn 2007 and Lowden 2004 and intermittently in Boivin 2012. When bright light was administered during the day shift, it was either between 07:30 and 08:00 (Tanaka 2011), or over midday (Bjorvatn 2007; Ross 1995).
In four studies sunglasses after the night shift and prior to their day‐sleep were used (Boivin 2012; Huang 2013; Tapia 2011; Thorne 2010), with the aim of reducing or preventing chronobiologically relevant light exposure. The types of sunglasses were variously described as "orange‐tinted goggles" (Boivin 2012), "dark sunglasses (UV‐protection)" (Huang 2013), “dark goggles” (Tapia 2011), and "specialised light blocking sunglasses" (Thorne 2010). The sunglasses (or goggles) were worn from the end of shift until the beginning of sleep (Boivin 2012; Huang 2013), or during the drive home (Tapia 2011). We considered the effects of all these types of sunglasses as equally effective. In Thorne 2010, the goggles were used from waking time until the start of the bright light intervention.
In three trials, the comparison group was the same: workers exposed on the job to normal light, in which normal light was defined as 300 lux (Karchani 2011; Lowden 2004;Sadeghniiat‐Haghighi 2011). Of the three remaining trials, one defined normal light as between 530 and 648 lux (Tanaka 2011), one used red light as the control (Ross 1995), and one used normal light (300 lux) plus a placebo capsule (as part of a three‐arm study that included melatonin (Bjorvatn 2007)). For those studies investigating the effect of sunglasses, control groups were made up primarily of study participants who were neither exposed to bright light nor asked to wear glasses (Boivin 2012; Tapia 2011; Thorne 2010). In one trial, however, control participants were not exposed to bright light, but were asked to wear glasses "after work and before sleep" (Huang 2013).
Trial design and setting
Seven RCTs used a controlled cross‐over design (Bjorvatn 2007; Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011; Tanaka 2011; Tapia 2011; Thorne 2010) with the remaining study using a standard parallel design (Boivin 2012; Huang 2013; Ross 1995).
Intervention duration ranged widely between a single night in Sadeghniiat‐Haghighi 2011 and four weeks (Lowden 2004; Tanaka 2011). The cross‐over studies employed washout periods ranging from four days in Sadeghniiat‐Haghighi 2011 to three months in Lowden 2004.
Five trials were conducted in industrial settings: two on an oil rig (Bjorvatn 2007; Thorne 2010), a metallurgic production plant (Karchani 2011), a truck production plant (Lowden 2004), and a ceramic factory (Sadeghniiat‐Haghighi 2011). One trial was conducted in a hospital setting (Huang 2013; Tanaka 2011; Tapia 2011), one in a police workforce (Boivin 2012), and a further trial in a geophysical research unit (Ross 1995). All trials were single‐centre studies. Only two studies reported a power analysis (Huang 2013; Tanaka 2011).
Outcomes
Eight trials reported on the effects of bright light on sleepiness on‐shift or fatigue on‐shift (Bjorvatn 2007; Boivin 2012; Karchani 2011; Lowden 2004; Ross 1995; Sadeghniiat‐Haghighi 2011; Tanaka 2011; Tapia 2011); objectively, sleepiness was measured via reaction time test using a Palm handheld computer (Bjorvatn 2007), actigraphy (Tapia 2011), or the Psychomotor Vigilance Task (PVT) test (Boivin 2012; Tanaka 2011). Data on sleepiness and fatigue on‐shift were collected subjectively via sleepiness scales, including the Karolinska Sleepiness Scale (KSS) (Bjorvatn 2007; Lowden 2004; Tanaka 2011), the Stanford Sleepiness Scale (SSS) (Karchani 2011; Sadeghniiat‐Haghighi 2011), the Epworth Sleepiness Scale (ESS) (Tapia 2011), the Accumulated Time with Sleepiness (ATS) scale (Bjorvatn 2007), a sleep diary (Lowden 2004), and a visual analogue scale (VAS) (Boivin 2012; Ross 1995).
Sleep quality and sleep length were assessed objectively using actigraphy (Bjorvatn 2007; Lowden 2004; Tapia 2011; Thorne 2010). Subjective measurements of sleep quality and length were obtained using sleep diaries (Bjorvatn 2007; Lowden 2004; Tanaka 2011;Tapia 2011; Thorne 2010 ), sleep logs and sleep quality scales (Ross 1995), insomnia severity index (Huang 2013) and a VAS (Tanaka 2011).
Nap
Characteristics of the trials and participants
We included four trials that investigated the effect of napping on our outcomes of interest (Howard 2010; Oriyama 2014; Smith 2007; Smith‐Coggins 2006). Two studies were conducted in Australia (Howard 2010; Smith 2007), one in Japan (Oriyama 2014), and one in the USA (Smith‐Coggins 2006).
In total, the trials included 81 review‐relevant participants (range: 9 to 49). The average age of the trialists ranged between 23 and 45 years. Women made up 66% to 100% of the participants.
Trials were made up of participants who were either 'sleep disorder‐free' (Howard 2010), or for which no health‐based exclusion criteria were reported (Oriyama 2014; Smith 2007; Smith‐Coggins 2006).
One study explored participants’ preference for morning or evening work (Smith‐Coggins 2006), based on the Owl and Lark Questionnaire, and reported finding no preference.
(See also Characteristics of included studies).
Interventions
Three trials explored the effect of a single nap opportunity during the night shift (Howard 2010; Smith 2007; Smith‐Coggins 2006). The fourth study explored the effect of two naps within one night shift period (Oriyama 2014).
The single nap group offered either a 30‐minute (Howard 2010; Smith 2007), or a 40‐minute sleep opportunity (Smith‐Coggins 2006). The two‐nap study allowed for two 15‐minute nap periods (Oriyama 2014). The timing of the nap opportunities varied, with anywhere from a 02:00 to a 04:30 starting time.
The control group for all studies was no‐nap. However, only one study explicitly reported that the non‐treatment group was expected to work while the treatment group was napping (Smith‐Coggins 2006).
Trial design and setting
Two trials used a parallel design (Oriyama 2014; Smith‐Coggins 2006), and two a cross‐over design (Howard 2010; Smith 2007).
In all four trials, the intervention lasted only one night. The cross‐over trials reported washout periods between “at least” one and “at least” two weeks (Howard 2010).
All four studies were conducted in a medical workplace or research setting. The studies investigated nurses (Oriyama 2014; Smith 2007), nurses and physicians (Smith‐Coggins 2006), or persons working in a sleep disorder research unit (Howard 2010). Three studies were conducted in a single study location (Howard 2010; Smith 2007; Smith‐Coggins 2006), and one study was a multicentre study (Oriyama 2014). None of the studies presented a power calculation, although one study discussed the possibility that it “may have been inadequately powered to detect small differences” (Howard 2010), while another described their statistical power as being limited (Smith 2007).
Outcomes
All four trials examined sleepiness on‐shift.
Sleepiness was measured objectively via the PVT test (Howard 2010; Smith 2007; Smith‐Coggins 2006), and catheter simulation (Smith‐Coggins 2006). Subjectively it was measured via KSS (Howard 2010; Smith 2007; Smith‐Coggins 2006), VAS (Oriyama 2014), the Profile of Mood States (POMS), and a sleep diary (Smith‐Coggins 2006). Smith 2007 averaged together results from a VAS and a pictorial sleepiness scale score, arriving at an overall “subjective sleepiness score”.
One trial measured sleep length off‐shift objectively via actigraphy (Smith‐Coggins 2006). None of the nap studies assessed sleep quality off‐shift.
Other person‐directed interventions
Characteristics of the trials and participants
We included three trials that explored neither light nor nap, but rather physical exercise intervention programmes, with or without sleep education (Atlantis 2006; Harma 1988), and a fatigue countermeasure programme (Smith‐Coggins 1997). The studies were conducted in Australia (Atlantis 2006), Finland (Harma 1988), and the USA (Smith‐Coggins 1997).
The trials included a total of 113 review‐relevant participants (range: 6 to 75). The mean age was between 31 and 35 years. One trial included only men (Smith‐Coggins 1997), one only women (Harma 1988), and one failed to specify gender (Atlantis 2006). The health status of the participants ranged from 'overall healthy' in Atlantis 2006 to no restrictions on health status (Harma 1988; Smith‐Coggins 1997).
None of the studies reported on chronotype.
(See also Characteristics of included studies).
Interventions
One trial investigated an aerobic programme and weight‐training combined with an education/sleep hygiene programme (Atlantis 2006). The aerobic exercise and the whole body weight‐training were done over 24 weeks, at least three times per week for at least 20 minutes.
Another trial explored the effect of a physical exercise programme (Harma 1988), based on two to six training sessions per week, over four months.
The third trial offered a three‐component fatigue countermeasure programme that included education on different aspects of sleep (a two‐hour programme), improved shift schedule design, and strategies to maintain alertness and performance during work (Smith‐Coggins 1997). The follow‐up period lasted for one month.
Control groups included persons on a waiting list (Atlantis 2006), individuals not participating in a physical exercise programme (Harma 1988), and a jet lag diet as placebo (Smith‐Coggins 1997).
Trial design and setting
Two trials used a parallel design (Atlantis 2006; Harma 1988) and Smith‐Coggins 1997 used a cross‐over design with a one month washout period.
Two studies took place in a hospital setting (Harma 1988; Smith‐Coggins 1997) and Atlantis 2006 was based in a casino. Atlantis 2006 conducted a power calculation, while Smith‐Coggins 1997 acknowledged that their study may have been underpowered to detect a difference. All were single‐centre trials.
Outcomes
Harma 1988 assessed subjective sleep quality and sleep length off‐shift using a questionnaire. Atlantis 2006 assessed subjective sleep quality off‐shift using the Pittsburgh Sleep Quality Index (PSQI). Smith‐Coggins 1997 assessed subjective sleep quality and sleep length off‐shift using a diary, objective sleep quality and sleep length using polysomnography, and objective sleepiness on‐shift via the PVT test.
Excluded studies
Of the 2054 full‐texts retrieved, we excluded 923 because they did not examine the effectiveness of person‐directed interventions. We excluded the remaining 1059 full‐text articles that did examine person‐directed interventions either because they used an uncontrolled design or were reviews/correspondence (n = 794), they did not measure on‐shift sleepiness or off‐shift sleep length/quality (n = 168), their participants were not actual shift workers (n = 62), or for reasons included under either the Characteristics of excluded studies, the Characteristics of studies awaiting classification, or the Characteristics of ongoing studies table (n = 35).
Risk of bias in included studies
The 'Risk of bias' for each included trial is presented in the Characteristics of included studies tables. These assessments are further presented in Figure 2 and Figure 3.
2.
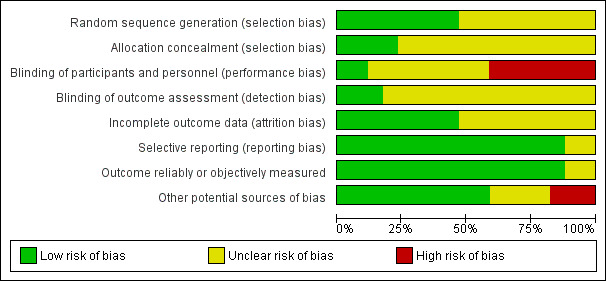
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies; The "Applicability of design" category assesses whether, in cross‐over designs, a period effect impacted the results (based on an interaction test). If no interaction test was reported, the risk of bias was considered unclear. In cases where the trial had a parallel design, the risk of bias was judged to be low.
3.
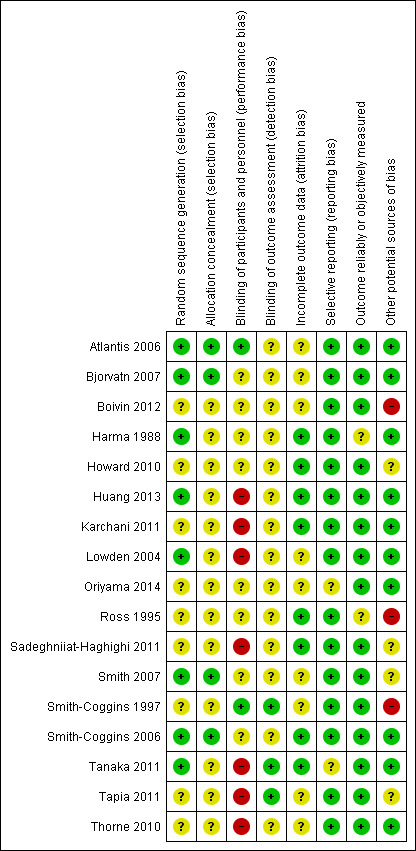
Risk of bias summary: review authors' judgements about each risk of bias item for each included study; The "Applicability of design" category assesses whether, in cross‐over designs, a period effect impacted the results (based on an interaction test). If study authors reported no interaction test, we considered the risk of bias to be unclear. In cases where the trial had a parallel design, we considered the risk of bias to be low.
Of the 17 included RCTs, we assessed seven as having an unclear risk of bias (Atlantis 2006; Bjorvatn 2007; Harma 1988; Howard 2010; Oriyama 2014; Smith 2007; Smith‐Coggins 2006).
We assessed the remaining ten trials as having a high risk of bias, due to either a lack of blinding of participants (Huang 2013; Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011; Tanaka 2011; Tapia 2011; Thorne 2010), or to a non‐appropriate study design or analysis (Boivin 2012; Ross 1995; Smith‐Coggins 1997). We judged none of the studies to have a low risk of bias.
Allocation
Eight studies reported (either in their text or through email communication) the details of their sequence generation process and we judged these studies to be at low risk of bias for random sequence generation (Atlantis 2006; Bjorvatn 2007; Harma 1988; Huang 2013; Lowden 2004; Smith 2007; Smith‐Coggins 2006; Tanaka 2011). We judged all other studies to be at unclear risk of bias for this domain.
Allocation concealment was sufficiently described in four trials and we judged these trials to be at low risk of bias for this domain (Atlantis 2006; Bjorvatn 2007; Smith 2007; Smith‐Coggins 2006); we judged all other trials to be at unclear risk of bias.
Blinding
Only two trials described in detail how participants were blinded to the intervention and we judged these trials to be at low risk of bias for blinding of participants and personnel (Atlantis 2006; Smith‐Coggins 1997). Eight trials provided no information on this aspect of study design and we judged them to be at unclear risk of bias (Bjorvatn 2007; Boivin 2012; Harma 1988; Howard 2010; Oriyama 2014; Ross 1995; Smith 2007; Smith‐Coggins 2006). We judged the remaining eight studies to be at high risk of bias as they either implicitly or explicitly reported that participant blinding was not attempted (Huang 2013; Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011; Tanaka 2011; Tapia 2011; Thorne 2010) or that it was not possible (Smith 2007).
A lack of blinding of participants was the most common reason we judged a trial as having a high risk of bias. For the nap intervention cross‐over trials we chose to assess this domain as unclear (Howard 2010; Smith 2007), since blinding under such conditions is not possible.
Blinding of outcome assessors was reported in only three trials and we judged these trials to be at low risk of bias (Smith‐Coggins 1997; Tanaka 2011; Tapia 2011); we judged all others to be at unclear risk of bias.
Incomplete outcome data
Eight trials included sufficient detail to warrant a low risk of bias for incomplete outcome data (Harma 1988; Howard 2010; Huang 2013; Karchani 2011; Ross 1995; Sadeghniiat‐Haghighi 2011; Smith‐Coggins 2006; Tanaka 2011). We judged all remaining trials to have an unclear risk of bias in this domain.
Selective reporting
We judged the majority of trials (n = 15) to have a low risk of bias regarding selective reporting. Only two trials alluded to outcomes in their texts that were not included in their final analyses (Oriyama 2014; Tanaka 2011). However, it was unclear how these omissions may have impacted the results.
Outcome reliably or objectively measured
Two trials measured outcomes using tools for which no information on validation was reported and we judged these trials to be at unclear risk of bias (Harma 1988; Ross 1995). We considered all other trials to have used reliable tools to measure their outcomes and judged them to be at low risk of bias.
Other potential sources of bias
Applicability of study design
Of the 17 trials included, 10 utilised a cross‐over design (Bjorvatn 2007; Howard 2010; Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011; Smith 2007; Smith‐Coggins 1997; Tanaka 2011; Tapia 2011; Thorne 2010).
In four of the cross‐over trials (Bjorvatn 2007; Howard 2010; Lowden 2004; Thorne 2010), the washout period ranged from two weeks to three months. For the most part, we deemed this sufficiently long, since physiologically the length of time needed to wash out the effects of bright light exposure or naps is likely to be short. However, in one trial involving a fatigue countermeasure programme (Smith‐Coggins 1997), we judged the one month washout period likely insufficient for eliminating the carry‐over effects of learned strategies to combat fatigue and judged this study to be at high risk of bias. Two cross‐over trials (Karchani 2011; Tanaka 2011), despite having relatively short washout periods (six days and one week, respectively), conducted tests for interaction between order and intervention and were able to demonstrate that carry‐over effects did not impact effect estimates; we judged both studies to be at low risk of bias.
Three cross‐over trials also had relatively short washout periods (four days to one week) (Sadeghniiat‐Haghighi 2011; Smith 2007; Tapia 2011), but did not report any possible interaction between order and intervention. It remains unclear how the short washout periods may have affected their results.
Only one cross‐over trial took advantage of their within‐person design and generated, from a paired analysis, a risk estimate that included an estimate of variance (Tanaka 2011); we judged this study to be at low risk of bias. Although Karchani 2011 used a paired analysis to estimate overall treatment effect, they did not present paired analysis data that would have allowed us to estimate variance of the risk estimate (See Dealing with missing data).
Of the seven parallel design trials, two included two participants in both the intervention and control groups (essentially partial cross‐over) (Boivin 2012; Ross 1995). Requests for results based solely on the parallel design participants went unanswered; we judged both studies to be at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Summary of findings for the main comparison. Light interventions (1/7): Bright light at night versus normal light (300 lux).
| Bright light at night versus normal light (300 lux) | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Bright light at night Comparison: Normal light (300 lux) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with normal light (300 lux) | Risk with bright light ** | |||||
| Sleepiness during the night shift overall; assessed with SSS1 | The mean sleepiness during the night shift overall in the control group was 3.10 score points | The mean sleepiness during the night shift overall in the intervention group was 0.83 lower (1.31 to 0.36 lower) |
‐ | 184 (2 RCTs) | ⊕⊝⊝⊝ very low4 | Lower sleepiness score indicates less sleepiness. Sleepiness score was averaged over the entire night. Although one study actually went on for two nights (Karchani 2011), we included only the measurements from the first night here, so that it was more comparable to the other study (Sadeghniiat‐Haghighi 2011) |
| Sleepiness during the night shift overall; assessed with KSS2 | The mean sleepiness during the night shift overall in the control group was 4.33 score points |
The mean sleepiness during the night shift overall in the intervention group was 0.26 lower (0.81 lower to 0.29 higher) |
‐ | 16 (1 RCT) | ⊕⊕⊝⊝ low5 | Lower sleepiness score indicates less sleepiness. Sleepiness score was averaged over the entire night |
| Sleepiness during the night shift; postintervention measurement assessed with SSS1 | The mean sleepiness during the night shift; postintervention measurement in the control group was 4.51 score points | The mean sleepiness during the night shift; postintervention measurement in the intervention group was 2.21 lower (2.43 to 1.99 lower) |
‐ | 90 (1 RCT) | ⊕⊕⊝⊝ low5 | Lower sleepiness score indicates less sleepiness |
| Sleepiness during the night shift; postintervention measurement assessed with KSS2 | The mean sleepiness during the night shift; postintervention measurement in the control group was 5.25 score points | The mean sleepiness during the night shift; postintervention measurement in the intervention group was 0.25 lower (0.76 lower to 0.26 higher) |
‐ | 16 (1 RCT) | ⊕⊕⊝⊝ low5 | Lower sleepiness score indicates less sleepiness |
| Total sleep time, next day ‐ main sleep time only;
assessed with actigraph |
The mean total sleep time, next day ‐ main sleep period only in the control group was 6.53 hours |
The mean total sleep time, next day ‐ main sleep time only in the intervention group was 0.25 hours longer (0.36 shorter to 0.86 longer) |
‐ | 15 (1 RCT) | ⊕⊕⊝⊝ low5 | Longer sleep time indicates more hours slept during the main sleep period |
| Total sleep time, next day ‐ 24‐hr sleep time, including naps; assessed with actigraph | The mean total sleep time, next day ‐ 24‐hour sleep period (including naps) in the control group was 5.92 hours |
The mean total sleep time, next day ‐ 24‐hour sleep period (including naps) in the intervention group was 0.63 hours longer (0.43 shorter to 1.69 longer) |
‐ | 15 (1 RCT) | ⊕⊕⊝⊝ low5 | Longer sleep time indicates more hours slept over 24‐hours, including both the main sleep period and naps |
| Sleep efficiency, next day; assessed with actigraph3 | The mean sleep efficiency, next day, in the control group was 89.5% |
The mean sleep efficiency, next day, in the intervention group was 0.9% higher (0.49 lower to 2.29 higher) |
‐ | 15 (1 RCT) | ⊕⊕⊝⊝ low5 | Higher sleep efficiency indicates that a greater part of the time spent lying in bed was actually spent sleeping |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ** In the case of cross‐over trials, the 95% CI reported here is based on an assumed correlation coefficient of 0. CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1SSS: Stanford Sleepiness Scale, a 7‐point scale with verbal anchors ranging from 1: 'feeling active, vital, alert, or wide awake' to 7: 'no longer fighting sleep, sleep onset soon, having dream‐like thoughts'. 2KSS: Karolinska Sleepiness Scale, a 9‐point scale with verbal anchors ranging from 1: 'extremely alert' to 9: 'very sleepy, great effort to keep awake, fighting sleep'. 3Sleep efficiency = ratio of amount of sleep from bedtime to final awakening/total time in bed. 4Downgraded one level due to imprecision (wide confidence intervals), one level due to risk of bias, and one level due to inconsistency (heterogeneity in study designs). 5Downgraded one level due to risk of bias (single trial only), and one level due to serious imprecision (small sample size).
Summary of findings 2. Light interventions (2/7): Bright light alone at night versus normal light (300 lux) plus placebo capsule.
| Bright light alone at night versus normal light (300 lux) plus placebo capsule | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Bright light at night Comparison: Normal light (300 lux) plus placebo capsule | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with normal light (300 lux) plus placebo capsule | Risk with bright light** | |||||
| Sleepiness during the night shift; assessed with 5‐min Reaction Time Test | The mean reaction time during the night shift in the control group was 325.19 ms | The mean reaction time during the night shift in the intervention group was 14.61 ms faster (68.10 faster to 38.88 slower) |
‐ | 14 (1 RCT) | ⊕⊝⊝⊝ very low2 | Faster reaction time suggests less sleepiness |
| Total sleep time, next day; assessed with Actiwatch | The mean total sleep time, next day, in the control group was 6.72 hours |
The mean total sleep time, next day, in the intervention group was 0.26 hours longer (0.47 shorter to 0.99 longer) |
‐ | 15 (1 RCT) | ⊕⊝⊝⊝ very low3 | Longer sleep time indicates more hours slept during the main sleep period |
| Sleep onset latency, next day; assessed with Actiwatch | The mean sleep onset latency, next day, in the control group was 6 minutes |
The mean sleep onset latency, next day, in the intervention group was 0 minutes (neither shorter nor longer) (5.08 shorter to 5.08 longer) |
‐ | 15 (1 RCT) | ⊕⊝⊝⊝ very low3 | Shorter sleep onset latency indicates fewer minutes needed to fall asleep when lying in bed |
| Sleep efficiency, next day; assessed with Actiwatch1 | The mean sleep efficiency, next day, in the control group was 86% |
The mean sleep efficiency, next day, in the intervention group was 2% higher (4.10 lower to 8.10 higher) |
‐ | 15 (1 RCT) | ⊕⊝⊝⊝ very low3 | Higher sleep efficiency indicates that a greater part of the time spent lying in bed was actually spent sleeping |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ** In the case of cross‐over trials, the 95% CI reported here is based on an assumed correlation coefficient of 0. CI: confidence interval; ms: milliseconds; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Sleep efficiency = total sleep time as the percentage of time in bed. 2Downgraded two levels due to indirectness (indirect measurement of sleepiness; single study that was primarily designed to assess the effect of melatonin tablets versus placebo tablets. We do not consider tablets to be an appropriate placebo for the study of the effects of bright light), one level due to imprecision (small sample size). 3Downgraded two levels due to indirectness (single study that was primarily designed to assess the effect of melatonin tablets versus placebo tablets. We do not consider tablets to be an appropriate placebo for the study of the effects of bright light) and one level due to imprecision (small sample size).
Summary of findings 3. Light intervention (3/7): Bright light during day versus normal light (530 to 648 lux).
| Bright light during day versus normal light (530 to 648 lux) | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Bright light during day Comparison: Normal light (530 to 648 lux) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with normal light (530 to 648 lux) | Risk with bright light | |||||
| Sleepiness during the day shift assessed with KSS1 14:00 | The mean sleepiness during the day shift in the control group was 4.28 score points |
The mean sleepiness during the day shift in the intervention group was 0.35 lower (0.72 lower to 0.02 higher) |
‐ | 61 (1 RCT) | ⊕⊕⊝⊝ low3 | Lower sleepiness score indicates less sleepiness |
| Sleep quality, next night; assessed with VAS2 | The mean sleep quality, next night, in the control group was 5.94 score points | The mean sleep quality, next night, in the intervention group was 0.37 higher (0.04 to 0.7 higher) |
‐ | 61 (1 RCT) | ⊕⊕⊝⊝ low3 | Higher sleep quality indicates a better main sleep period |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1KSS: Karolinska Sleepiness Scale, a 9‐point scale with verbal anchors ranging from 1: 'extremely alert' to 9: 'very sleepy, great effort to keep awake, fighting sleep'. 2 VAS: visual analogue scale, ranging from 0: 'unable to sleep at all' to 10: 'able to sleep very well'. 3 Downgraded one level due to risk of bias and one level due to imprecision (wide confidence intervals).
Summary of findings 4. Light interventions (4/7): Bright light during day versus dim red light.
| Bright light during day versus dim red light | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Bright light during day Comparison: Dim red light | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dim red light | Risk with bright light | |||||
| Total sleep time, next night; assessed with sleep log | The mean total sleep time, next night, in the control group was 7 hours | The mean total sleep time, next night, in the intervention group was 0.1 hours longer (1.09 shorter to 1.29 longer) |
‐ | 16 (1 RCT) | ⊕⊕⊝⊝ low1 | Longer sleep time indicates more hours slept during the main sleep period |
| Sleep onset latency, next night assessed with sleep log | The mean sleep onset latency, next night, in the control group was 16.6 minutes |
The mean sleep onset latency, next night, in the intervention group was 2.6 minutes shorter (10.72 shorter to 5.52 longer) |
‐ | 16 (1 RCT) | ⊕⊕⊝⊝ low1 | Shorter sleep onset latency indicates fewer minutes needed to fall asleep when lying in bed |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level due to risk of bias and one level due to imprecision (wide confidence intervals).
Summary of findings 5. Light interventions (5/7): Bright light alone during day versus normal light (300 lux) plus placebo capsule.
| Bright light alone during day versus normal light (300 lux) plus placebo capsule | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Bright light during day Comparison: Normal light (300 lux) plus placebo capsule | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with normal light (300 lux) plus placebo capsule | Risk with bright light** | |||||
| Sleepiness during the day shift; days assessed with 5‐min Reaction Time Test | The mean reaction time, during the day‐shift days, in the control group was 296.16 ms | The mean reaction time, during the day‐shift days, in the intervention group was 14.05 ms slower (17.37 faster to 45.47 slower) |
‐ | 12 (1 RCT) | ⊕⊝⊝⊝ very low2 | Slower reaction time suggests more sleepiness |
| Total sleep time, next night; assessed with Actiwatch | The mean total sleep time, next night, in the control group was 5.8 hours |
The mean total sleep time next night, in the intervention group was 0.32 hours longer (0.35 shorter to 0.99 longer) |
‐ | 13 (1 RCT) | ⊕⊝⊝⊝ very low3 | Longer sleep time indicates more hours slept during the main sleep period |
| Sleep onset latency, next night; assessed with Actiwatch | The mean sleep onset latency, next night, in the control group was 6 minutes |
The mean sleep onset latency, next night, in the intervention group was 1 minute longer (4.47 shorter to 6.47 longer) |
‐ | 13 (1 RCT) | ⊕⊝⊝⊝ very low3 | Longer sleep onset latency indicates more minutes needed to fall asleep when lying in bed |
| Sleep efficiency, next night; assessed with Actiwatch1 | The mean sleep efficiency, next night, in the control group was 85% |
The mean sleep efficiency, next night, in the intervention group was 2% higher (5.19 lower to 9.19 higher) |
‐ | 13 (1 RCT) | ⊕⊝⊝⊝ very low3 | Higher sleep efficiency indicates that a greater part of the time spent lying in bed was actually spent sleeping |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ** In the case of cross‐over trials, the 95% confidence interval reported here is based on an assumed correlation coefficient of 0. CI: confidence interval; ms: milliseconds; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Sleep efficiency = total sleep time as the percentage of time in bed. 2Downgraded two levels due to indirectness (indirect measurement of sleepiness; single study that was primarily designed to assess the effect of melatonin tablets versus placebo tablets. We do not consider tablets to be an appropriate placebo for the study of the effects of bright light), and one level due to imprecision (small sample size). 3Downgraded two levels due to indirectness (single study that was primarily designed to assess the effect of melatonin tablets versus placebo tablets. We do not consider tablets to be an appropriate placebo for the study of the effects of bright light), and one level due to imprecision (small sample size).
Summary of findings 6. Light interventions (6/7): Bright light at night plus glasses versus normal light (unclear lux) and no glasses.
| Bright light at night plus glasses versus normal light (unclear lux) and no glasses | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Bright light at night plus glasses Comparison: Normal light (unclear lux) and no glasses | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with normal light (unclear lux) and no glasses | Risk with bright light at night plus glasses | |||||
| Sleepiness during the night shift assessed with Psychomotor Vigilance Task and Median Reaction Time tests | The median reaction time during the night shift in the control group was 68.29 ms | The median reaction time during the night shift in the intervention group was 0.11 ms slower (20.83 faster to 21.05 slower) |
‐ | 17 (1 RCT) | ⊕⊝⊝⊝ very low1 | Slower reaction time suggests more sleepiness |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ms: milliseconds; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded one level due to risk of bias, one level due to imprecision (wide confidence intervals, single trial only), and one level due to indirectness (indirect measurement of sleepiness).
Summary of findings 7. Light interventions (7/7): Bright light plus glasses during day versus normal light and no glasses.
| Bright light plus glasses during day versus normal light and no glasses | ||||||
| Patient or population: Shift workers Setting: Individual workplace, offshore Intervention: Bright light plus glasses during day Comparison: Normal light and no glasses | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with normal light and no glasses | Risk with bright light plus glasses** | |||||
| Total sleep time, next night; assessed with actigraph | The mean total sleep time, next night, in the control group was 6.25 hours |
The mean total sleep time, next night, in the intervention group was 0.32 hours longer (0.39 shorter to 1.03 longer) |
‐ | 3 (1 RCT) | ⊕⊕⊝⊝ low2 | Longer sleep time indicates more hours slept during the main sleep period |
| Sleep onset latency, next night; assessed with actigraph | The mean sleep onset latency, next night, in the control group was 18 minutes |
The mean sleep onset latency, next night, in the intervention group was 2.4 minutes longer (13.08 shorter to 17.88 longer) |
‐ | 3 (1 RCT) | ⊕⊕⊝⊝ low2 | Longer sleep onset latency indicates more minutes needed to fall asleep when lying in bed |
| Sleep efficiency, next night; assessed with Actiwatch1 | The mean sleep efficiency, next night, in the control group was 76.18% |
The mean sleep efficiency, next night, in the intervention group was 6.59% higher (4.35 lower to 17.53 higher) |
‐ | 3 (1 RCT) | ⊕⊕⊝⊝ low2 | Higher sleep efficiency indicates that a greater part of the time spent lying in bed was actually spent sleeping |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ** In the case of cross‐over trials, the 95% confidence interval reported here is based on an assumed correlation coefficient of 0. CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Sleep efficiency = total sleep time as the percentage of time in bed. 2Downgraded one level due to risk of bias and one level due to imprecision (wide confidence intervals).
Summary of findings 8. Nap interventions (1/2): Nap at night (single nap opportunity) versus no‐nap.
| Nap at night (single nap opportunity) versus no‐nap | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Nap at night (single nap opportunity) Comparison: No‐nap | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no‐nap | Risk with nap (single nap opportunity)** | |||||
| Sleepiness during the night shift, postintervention; assessed with Psychomotor Vigilance Task test and Mean Reaction Time test | The mean reaction time during the night shift, postintervention, in the control group was 180.50 ms |
The mean reaction time during the night shift, postintervention, in the intervention group was 11.87 ms faster (31.94 faster to 8.20 slower) |
‐ | 16 (2 RCTs) | ⊕⊝⊝⊝ very low3 | Faster reaction time suggests less sleepiness |
| Sleepiness during the night shift, postintervention; assessed with KSS1 | The mean sleepiness during the night shift, postintervention, in the control group was 6.63 score points |
The mean sleepiness during the night shift, postintervention in the intervention group was 0.13 higher (0.46 lower to 0.72 higher) |
‐ | 8 (1 RCT) | ⊕⊕⊝⊝ low4 | Higher sleepiness score indicates more sleepiness; study design: cross‐over |
| Sleepiness during the night shift, postintervention; assessed with: KSS1 | The mean sleepiness during the night shift, postintervention, in the control group was 6.48 score points |
The mean sleepiness during the night shift, postintervention, in the intervention group was 1.12 lower (1.83 to 0.41 lower) |
‐ | 49 (1 RCT) | ⊕⊕⊝⊝ low4 | Higher sleepiness score indicates more sleepiness; study design: parallel |
| Sleepiness during the night shift, postintervention; assessed with Psychomotor Vigilance Task Test (slowest 10% reciprocal reaction time) | The mean slowest 10% reciprocal reaction time during the night shift, postintervention, in the control group was 2.46 ms |
The mean slowest 10% reciprocal reaction time during the night shift, postintervention, in the intervention group was 0.19 ms faster (0.67 slower to 1.05 faster) |
‐ | 7 (1 RCT) | ⊕⊝⊝⊝ very low5 | Faster reaction time suggests less sleepiness |
| Sleepiness during the night shift, postintervention; assessed with Psychomotor Vigilance Task Test (slowest 10% reciprocal reaction time) | The mean slowest 10% reciprocal reaction time during the night shift, postintervention, in the control group was 2.13 ms |
The mean slowest 10% reciprocal reaction time during the night shift, postintervention, in the intervention group was 0.32 ms faster (0.21 slower to 0.85 faster) |
‐ | 49 (1 RCT) | ⊕⊝⊝⊝ very low5 | Faster reaction time suggests less sleepiness |
| Sleepiness during the night shift, postintervention;
assessed with subjective sleepiness score2 |
The mean sleepiness during the night shift, postintervention, in the control group was 52.02 score points |
The mean sleepiness during the night shift, postintervention, in the intervention group was 16.14 lower (31.37 to 0.91 lower) |
‐ | 9 (1 RCT) | ⊕⊝⊝⊝ very low4 | Lower sleepiness score indicates less sleepiness |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); ** In the case of cross‐over trials, the 95% confidence interval reported here is based on an assumed correlation coefficient of 0. CI: confidence interval; ms: milliseconds; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 KSS: Karolinska Sleepiness Scale, a 9‐point scale with verbal anchors ranging from 1: 'extremely alert' to 9: 'very sleepy, great effort to keep awake, fighting sleep'. 2Subjective sleepiness score: VAS and pictorial sleepiness score were significantly correlated and were averaged to create an overall sleepiness score out of 100. 3Downgraded one level due to imprecision (wide confidence intervals), one level due to indirectness (indirect measurement of sleepiness), and one level due to inconsistency (study results inconsistent). 4Downgraded two levels due to imprecision (wide confidence intervals, short observation period). 5Downgraded two levels due to imprecision (wide confidence intervals, short observation period), and one level due to indirectness (indirect measurement of sleep).
Summary of findings 9. Nap intervention (2/2): Nap at night (two‐nap opportunities) versus no‐nap.
| Nap at night (two nap opportunities) versus no‐nap | ||||||
| Patient or population: Shift workers Setting: Individual workplace Intervention: Nap at night (two‐nap opportunities) Comparison: No‐nap | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no‐nap | Risk with Nap (two‐nap opportunities) | |||||
| Sleepiness during the night shift, postintervention; assessed with VAS1 | The mean sleepiness during the night shift postintervention, in the control group was 40.21 score points | The mean sleepiness during the night shift, postintervention, in the intervention group was 2.32 higher (24.74 lower to 29.38 higher) |
‐ | 15 (1 RCT) | ⊕⊕⊝⊝ low2 | Higher sleepiness score indicates more sleepiness |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1VAS: visual analogue scale, 100 millimetre scale ranging from 0 mm: not at all sleepy/tired to 100 mm: extremely sleepy/tired. 2Downgraded one level due to imprecision (wide confidence intervals) and one level due to indirectness.
Summary of findings 10. Other interventions (1/1): Physical exercise and sleep hygiene education versus wait‐list for sleepiness and sleep disturbances caused by shift work.
| Physical exercise and sleep hygiene education versus wait‐list for sleepiness and sleep disturbances caused by shift work | ||||||
|
Patient or population: Shift workers Settings: Individual workplace Intervention: Physical exercise and sleep hygiene education Comparison: Wait‐list | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with wait‐list | Risk with physical exercise and sleep hygiene education | |||||
| Sleep quality, postintervention, assessed with PSQI1 | The mean sleep quality, postintervention, over previous one‐month period in the control group was 5.6 score points |
The mean sleep quality, postintervention, over previous one‐month period in the intervention group was 1.4 lower (3.10 lower to 0.30 higher) |
32 (1 RCT) | ⊕⊕⊕⊝ moderate2 | Higher sleep quality indicates a better main sleep period | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1PSQI: Pittsburgh Sleep Quality Index, 0 to 21 point index, lower score = higher sleep quality. 2Downgraded one level due to imprecision (small sample size).
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10.
1 Bright light administered during the night shift versus normal light (300 lux)
1.1 Sleepiness during the night shift
1.1.1 Sleepiness during the night shift, overall
Three trials examined the effects of bright light administered during the night shift on on‐shift sleepiness (Karchani 2011; Lowden 2004; Sadeghniiat‐Haghighi 2011).
Two studies used the Stanford Sleepiness Scale (SSS = a 7‐point verbal scale from 1: 'feeling active, vital, alert, or wide awake' to 7: 'no longer fighting sleep, sleep onset soon, having dream‐like thoughts') to explore sleepiness (Karchani 2011; Sadeghniiat‐Haghighi 2011). In both trials, the bright light group was less sleepy than the control group (1.19 and 0.67 fewer degrees of sleepiness, respectively); a meta‐analysis showed that the bright light group was 1.03 scale points less sleepy than the control group, even when 0 correlation was assumed (mean difference (MD) ‐0.83 scale points, 95% confidence interval (CI) ‐1.31 to ‐0.36; 2 trials, 184 participants, very low quality evidence; Analysis 1.1; Table 1). This analysis was based on a single night of bright light intervention and no assessment of chronotype.
1.1. Analysis.
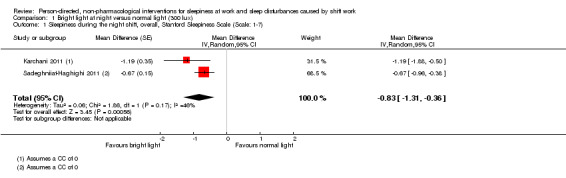
Comparison 1 Bright light at night versus normal light (300 lux), Outcome 1 Sleepiness during the night shift, overall, Stanford Sleepiness Scale (Scale: 1‐7).
Lowden 2004 also examined sleepiness, but using the Karolinska Sleepiness Scale (KSS = a 9‐point scale with verbal anchors ranging from 1: 'extremely alert' to 9: 'very sleepy, great effort to keep awake, fighting sleep'). The bright light group was less sleepy than the control group, but significance depended on the correlation coefficient (CC) (MD ‐0.26 scale points, 95% CI ‐0.42 to ‐0.10 (CC = 0.9); ‐0.53 to 0.01 (CC = 0.7); ‐0.81 to 0.29 (CC = 0); 1 trial, 16 participants, low quality evidence; Analysis 1.2; Table 1). This analysis is based on four weeks of bright light intervention.
1.2. Analysis.
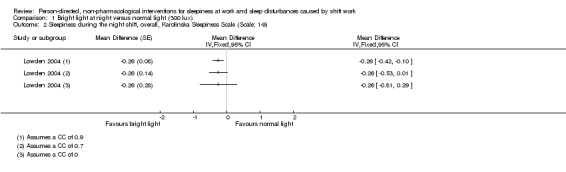
Comparison 1 Bright light at night versus normal light (300 lux), Outcome 2 Sleepiness during the night shift, overall, Karolinska Sleepiness Scale (Scale: 1‐9).
1.1.2 Sleepiness during the night shift, postintervention measurements only
Two trials compared on‐shift sleepiness during night shift and reported hourly measurements allowing for a postintervention‐only analysis (Karchani 2011; Lowden 2004). One study used the SSS to measure sleepiness (Karchani 2011). Here, the bright light group was less sleepy postintervention compared to the control group, and the finding remained statistically significant no matter the CC (MD ‐2.21 scale points, 95% CI ‐2.27 to ‐2.15 (CC = 0.9); ‐2.33 to ‐2.09 (CC = 0.7); ‐2.43 to ‐1.99 (CC = 0); 1 trial, 90 participants, low quality evidence; Analysis 1.3; Table 1). This analysis was based on two nights of bright light intervention.
1.3. Analysis.
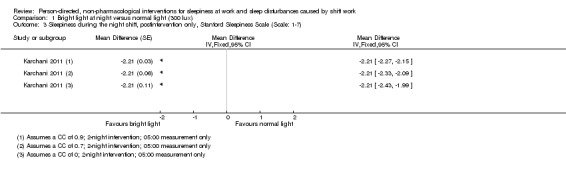
Comparison 1 Bright light at night versus normal light (300 lux), Outcome 3 Sleepiness during the night shift, postintervention only, Stanford Sleepiness Scale (Scale: 1‐7).
The other study measured sleepiness using the KSS (Lowden 2004). These results showed that the bright light group was also less sleepy postintervention compared to the control group, but that the statistical significance of the finding depended on the CC (MD ‐0.25 scale points, 95% CI ‐0.43 to ‐0.07 (CC = 0.9); ‐0.54 to 0.04 (CC = 0.7); ‐0.76 to 0.26 (CC = 0); 1 trial, 16 participants, low quality evidence; Analysis 1.4; Table 1).
1.4. Analysis.
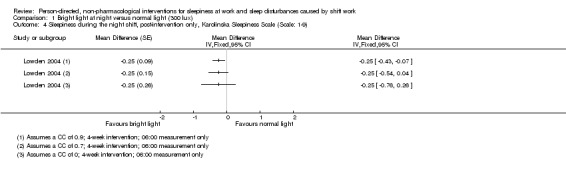
Comparison 1 Bright light at night versus normal light (300 lux), Outcome 4 Sleepiness during the night shift, post‐intervention only, Karolinska Sleepiness Scale (Scale: 1‐9).
1.2 Sleep quality and sleep length after the night shift
1.2.1 Total sleep time, next day
One trial compared the effect of bright light at night on total sleep time the next day, separately for the main sleep period and for the 24‐hour period following night shift (Lowden 2004). The bright light group slept longer than the control group during the main sleep period, but the difference was statistically significant only when a very high correlation was assumed (MD 0.25 hours, 95% CI: 0.05, 0.45 (CC = 0.9); ‐0.08, 0.58 (CC = 0.7); ‐0.36, 0.86 (CC = 0); 1 trial, 15 participants, low quality evidence; Analysis 1.5; Table 1). When totaling the sleep time from regular sleep and naps over the 24‐hour period following night shift, the bright light group slept longer than the control group. However, statistical significance depended on the level of the assumed correlation (MD 0.63 hours, 95% CI 0.24 to 1.02 (CC = 0.9); 0.02 to 1.24 (CC = 0.7); ‐0.43 to 1.69 (CC = 0); 1 trial, 14 participants, low quality evidence; Analysis 1.5; Table 1).
1.5. Analysis.
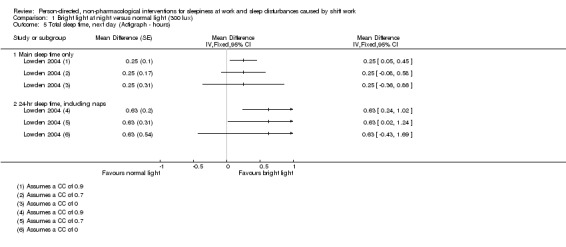
Comparison 1 Bright light at night versus normal light (300 lux), Outcome 5 Total sleep time, next day (Actigraph ‐ hours).
1.2.2 Sleep efficiency, next day
One trial compared the effect of bright light at night on sleep efficiency the next day (Lowden 2004), using actigraphy‐based data (sleep efficiency = ratio of amount of sleep from bedtime to final awakening/total time in bed). Objectively measured, the bright light group slept more efficiently than the control group, however, the level of significance varied depending upon the assumed extent of correlation between the intervention and control measurements (MD 0.90%, 95% CI 0.47 to 1.33 (CC = 0.9); 0.14 to 1.66 (CC = 0.7); ‐0.49 to 2.29 (CC = 0); 1 trial, 15 participants, low quality evidence; Analysis 1.6; Table 1). The study authors themselves reported no significance, possibly suggesting that their analysis was based on a very low CC.
1.6. Analysis.
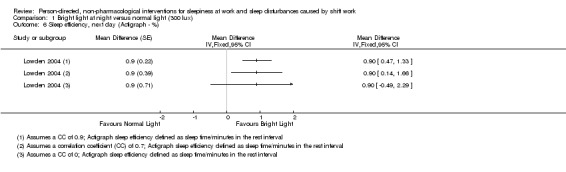
Comparison 1 Bright light at night versus normal light (300 lux), Outcome 6 Sleep efficiency, next day (Actigraph ‐ %).
2 Bright light alone administered during the night shift versus normal light (300 lux) plus placebo capsule
2.1 Sleepiness during the night shift
2.1.1 Sleepiness during the night shift, overall
One study compared the effect of bright light on sleepiness during the night shift to normal light plus the administration of a placebo capsule (Bjorvatn 2007). Results from a 5‐minute reaction time test showed that the bright light group reacted more quickly than the control group (which may suggest less sleepiness), but the results were not statistically significant (MD ‐14.61 milliseconds, 95% CI ‐39.05 to 9.83 (CC = 0.9); ‐47.79 to 18.57 (CC = 0.7); ‐68.10 to 38.88 (CC = 0); 1 trial, 14 participants, very low quality evidence; Analysis 2.1; Table 2).
2.1. Analysis.
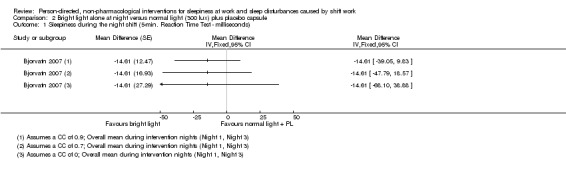
Comparison 2 Bright light alone at night versus normal light (300 lux) plus placebo capsule, Outcome 1 Sleepiness during the night shift (5‐min. Reaction Time Test ‐ milliseconds).
2.2 Sleep quality and sleep length after the night shift
2.2.1 Total sleep time, next day
One study compared the effect of bright light at night on total sleep time the next day to normal light plus the administration of a placebo capsule (Bjorvatn 2007). These Actiwatch data showed that the bright light group slept longer than the control group, but statistical significance was reached only when very high correlation was assumed (MD 0.26 hours, 95% CI 0.02 to 0.50 (CC = 0.9); ‐0.15 to 0.67 (CC = 0.7); ‐0.47 to 0.99 (CC = 0); 1 trial, 15 participants, very low quality evidence; Analysis 2.2; Table 2).
2.2. Analysis.
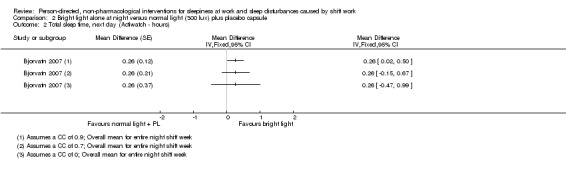
Comparison 2 Bright light alone at night versus normal light (300 lux) plus placebo capsule, Outcome 2 Total sleep time, next day (Actiwatch ‐ hours).
2.2.2 Sleep onset latency, next day
One study compared the effect of bright light at night on sleep onset latency the next day to normal light plus the administration of a placebo capsule (Bjorvatn 2007). These Actiwatch data showed that bright light had no effect on sleep onset latency and that for no assumed level of correlation was statistical significance reached (MD 0 minutes, 95% CI ‐1.88 to 1.88 (CC = 0.9); ‐2.90 to 2.90 (CC = 0.7); ‐5.08 to 5.08 (CC = 0); 1 trial, 15 participants, very low quality evidence; Analysis 2.3; Table 2).
2.3. Analysis.
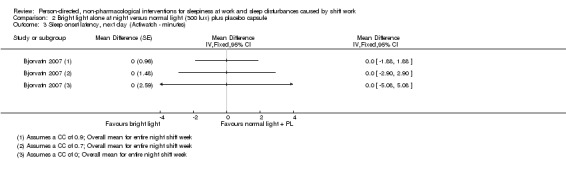
Comparison 2 Bright light alone at night versus normal light (300 lux) plus placebo capsule, Outcome 3 Sleep onset latency, next day (Actiwatch ‐ minutes).
2.2.3 Sleep efficiency, next day
One study compared the effect of bright light at night on sleep efficiency the next day to normal light plus the administration of a placebo capsule (Bjorvatn 2007). These Actiwatch data showed that the bright light group slept more efficiently than the control group, however statistical significance depended on the CC (MD 2%, 95% CI 0.02 to 3.98 (CC = 0.9); ‐1.37 to 5.37 (CC = 0.7); ‐4.10 to 8.10 (CC = 0); 1 trial, 15 participants, very low quality evidence; Analysis 2.4; Table 2).
2.4. Analysis.
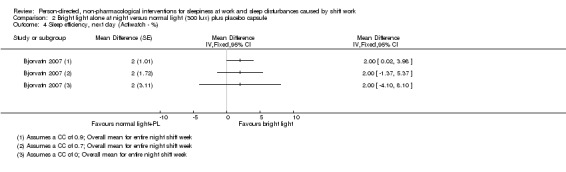
Comparison 2 Bright light alone at night versus normal light (300 lux) plus placebo capsule, Outcome 4 Sleep efficiency, next day (Actiwatch ‐ %).
3 Bright light administered at the beginning of the day shift versus normal light (530 to 648 lux)
3.1 Sleepiness during the day shift
One study compared the effect of bright light administered in the mornings before day shift with normal light on sleepiness on‐shift. Based on the KSS, the bright light group was statistically significantly less sleepy than the control group, at both 10:00 (MD ‐0.55 scale points, 95% CI ‐0.90 to ‐0.20) and at 14:00 (MD ‐0.35 scale points, 95% CI ‐0.72 to ‐0.02) (1 trial, 61 participants, low quality evidence; Analysis 3.1; Table 3).
3.1. Analysis.
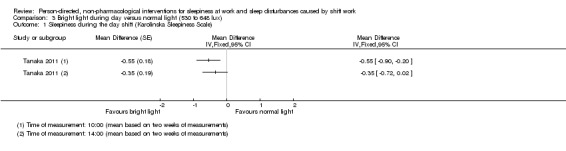
Comparison 3 Bright light during day versus normal light (530 to 648 lux), Outcome 1 Sleepiness during the day shift (Karolinska Sleepiness Scale).
3.2 Sleep quality and sleep length after the day shift
The same study compared the effect of bright light administered in the mornings before day shift with normal light on sleep quality the next night (Tanaka 2011). Based on data from a visual analogue scale (VAS: from 0 = unable to sleep at all, to 10 = slept very well) the bright light group slept better the next night than did the control group, and the difference was statistically significant (MD 0.37 scale points, 95% CI 0.04 to 0.70; 1 trial, 61 participants, low quality evidence; Analysis 3.2; Table 3).
3.2. Analysis.

Comparison 3 Bright light during day versus normal light (530 to 648 lux), Outcome 2 Sleep quality, next night (Visual Analogue Scale ‐ 0 to 10).
4 Bright light administered during the day shift versus dim red light
4.1 Sleep quality and sleep length after the day shift
4.1.1 Total sleep time, next night
One study compared the administration of bright light during the day shift compared to dim red light on the effects of total sleep time measured via sleep log (Ross 1995). They found that the bright light group slept longer than the control group, but this difference was not statistically significant (MD 0.10 hours, 95% CI ‐1.09 to 1.29; 1 trial, 16 participants, low quality evidence; Analysis 4.1; Table 4).
4.1. Analysis.

Comparison 4 Bright light during day versus dim red light, Outcome 1 Total sleep time, next night (sleep log ‐ hours).
4.1.2 Sleep onset latency, next night
The same study compared the administration of bright light during the day shift compared to dim red light on the effects of sleep onset latency measured via sleep log (Ross 1995). They found that the bright light group fell asleep more quickly than the red light group, but the difference was not statistically significant (MD ‐2.60 minutes, 95% CI ‐10.72 to 5.52; 1 trial, 16 participants, low quality evidence; Analysis 4.2; Table 4).
4.2. Analysis.
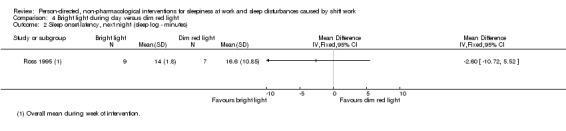
Comparison 4 Bright light during day versus dim red light, Outcome 2 Sleep onset latency, next night (sleep log ‐ minutes).
5 Bright light alone administered during the day shift versus normal light (300 lux) plus placebo capsule
5.1 Sleepiness during the day shift
5.1.1 Sleepiness during the day shift, over entire day
Bjorvatn 2007 also compared the effect of bright light during the day shift to normal light plus the administration of a placebo capsule on sleepiness during the day shift. Results from a 5‐minute reaction time test showed that the bright light group was slightly sleepier than the control group, but statistical significance was reached only when very high correlation was assumed (MD 14.05 milliseconds, 95% CI 0.57 to 27.53 (CC = 0.9); ‐4.94 to 33.04 (CC = 0.7); ‐17.37 to 45.47 (CC = 0); 1 trial, 12 participants, very low quality evidence; Analysis 5.1; Table 5).
5.1. Analysis.
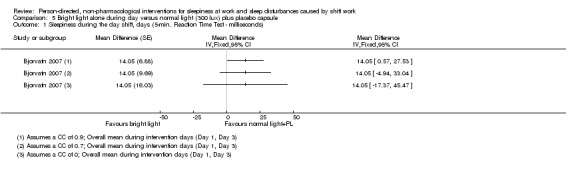
Comparison 5 Bright light alone during day versus normal light (300 lux) plus placebo capsule, Outcome 1 Sleepiness during the day shift, days (5‐min. Reaction Time Test ‐ milliseconds).
5.2 Sleep quality and sleep length after the day shift
5.2.1 Total sleep time, next night
The effect of bright light during the day shift on total sleep time the next night compared to normal light plus the administration of a placebo capsule was assessed as well (Bjorvatn 2007). These Actiwatch data showed that the bright light group slept longer than the control group, but statistical significance was reached only when very high correlation was assumed (MD 0.32 hours, 95% CI 0.08 to 0.56 (CC = 0.9); ‐0.05 to 0.69 (CC = 0.7); ‐0.35 to 0.99 (CC = 0); 1 trial, 13 participants, very low quality evidence; Analysis 5.2; Table 5).
5.2. Analysis.
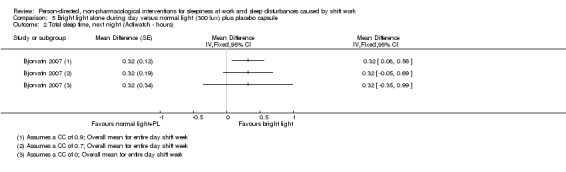
Comparison 5 Bright light alone during day versus normal light (300 lux) plus placebo capsule, Outcome 2 Total sleep time, next night (Actiwatch ‐ hours).
5.2.2 Sleep onset latency, next night
The same study compared the effect of bright light during the day shift to normal light plus the administration of a placebo capsule on sleep onset latency the next night (Bjorvatn 2007). These Actiwatch data found that the bright light group took one minute longer to fall asleep, but the results was not statistically significant (MD 1 minute, 95% CI ‐1.25 to 3.25 (CC = 0.9); ‐2.25 to 4.25 (CC = 0.7); ‐4.47 to 6.47 (CC = 0); 1 trial, 13 participants, very low quality evidence; Analysis 5.3; Table 5).
5.3. Analysis.
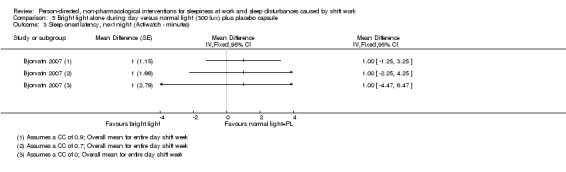
Comparison 5 Bright light alone during day versus normal light (300 lux) plus placebo capsule, Outcome 3 Sleep onset latency, next night (Actiwatch ‐ minutes).
5.2.3 Sleep efficiency, next night
One study compared the effect of bright light during the day shift to normal light plus the administration of a placebo capsule on sleep efficiency the next night (Bjorvatn 2007). These Actiwatch data found that the bright light group slept more efficiently than the control group, but findings were not statistically significant (MD 2%, 95% CI ‐0.47 to 4.47 (CC = 0.9); ‐2.04 to 6.04 (CC = 0.7); ‐5.19 to 9.19 (CC = 0); 1 trial, 13 participants, very low quality evidence; Analysis 5.4; Table 5).
5.4. Analysis.
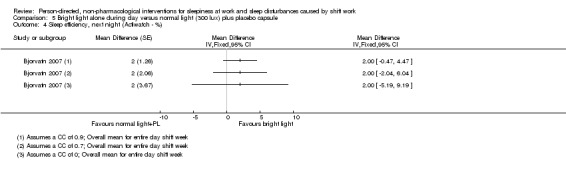
Comparison 5 Bright light alone during day versus normal light (300 lux) plus placebo capsule, Outcome 4 Sleep efficiency, next night (Actiwatch ‐ %).
6 Bright light administered during the night shift plus glasses at dawn versus normal light (unclear lux) and no glasses
6.1 Sleepiness during the night shift, overall
One study examined the effect of bright light at night plus sunglasses at dawn compared to normal light and no sunglasses on sleepiness on‐shift (Boivin 2012). Based on a Psychomotor Vigilance Task (PVT) ‐Median Reaction Time test, the authors found the bright light plus sunglasses group was slightly sleepier than the control group, but the difference was not statistically significant (MD 0.11 milliseconds, 95% CI ‐20.83 to 21.05; 1 trial, 17 participants, very low quality evidence; Analysis 6.1; Table 6). This analysis was based on seven nights of bright light intervention.
6.1. Analysis.
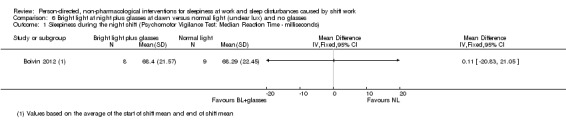
Comparison 6 Bright light at night plus glasses at dawn versus normal light (unclear lux) and no glasses, Outcome 1 Sleepiness during the night shift (Psychomotor Vigilance Test: Median Reaction Time ‐ milliseconds).
An additional study assessing sleepiness on‐shift between a bright light at night plus sunglasses group and a normal light and no glasses group found no statistically significant difference (for either the Epworth Sleepiness Scale (ESS) or VAS) (Tapia 2011). However, significant sleepiness was reported not to have been present at baseline (ESS 4.2 ± 1.9, VAS 26.3 ± 21.7 mm). The authors did not present detailed statistical data and a request for more precise data went unanswered. We did not include this trial in any statistical analyses.
One study looked at the same intervention (bright light at night and sunglasses), but included a control group that also wore sunglasses (Huang 2013). As we were unable to obtain data for the subgroup of night shift workers alone, we lacked sufficient data to draw any conclusions about the effect of the intervention. We did not include this trial in any statistical analyses.
7 Bright light plus glasses administered during the day versus normal light and no glasses
7.1 Sleep quality and sleep length, next night following off‐duty bright light intervention
7.1.1 Total sleep time, next night
Thorne 2010 compared bright light during the day plus sunglasses with normal light and no sunglasses on total sleep time the next night. The actigraphic data showed that the bright light plus sunglasses group slept longer than the control group, but statistical significance depended on the assumed CC (MD 0.32 hours, 95% CI 0.08 to 0.56 (CC = 0.9); ‐0.07 to 0.71 (CC = 0.7); ‐0.39 to 1.03 (CC = 0); 1 trial, 3 participants, low quality evidence; Analysis 7.1; Table 7).
7.1. Analysis.
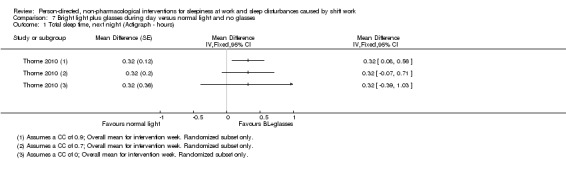
Comparison 7 Bright light plus glasses during day versus normal light and no glasses, Outcome 1 Total sleep time, next night (Actigraph ‐ hours).
7.1.2 Sleep onset latency, next night
Thorne 2010 also compared bright light during the day plus sunglasses with normal light and no sunglasses on sleep onset latency the next night. These actigraphic data found that the bright light group lay awake longer than the control group, but the findings did not reach statistical significance (MD 2.4 minutes, 95% CI ‐9.01 to 13.81 (CC = 0.9); ‐10.03 to 14.83 (CC = 0.7); ‐13.08 to 17.88 (CC = 0); 1 trial, 3 participants, low quality evidence; Analysis 7.2; Table 7).
7.2. Analysis.
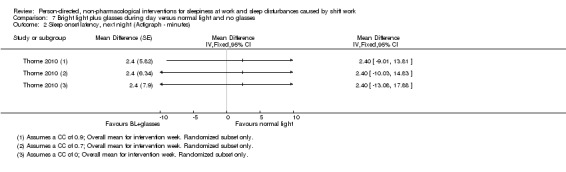
Comparison 7 Bright light plus glasses during day versus normal light and no glasses, Outcome 2 Sleep onset latency, next night (Actigraph ‐ minutes).
7.1.3 Sleep efficiency, next night
One study compared bright light during the day plus sunglasses with normal light and no sunglasses on sleep efficiency the next night (Thorne 2010). Actigraphy showed that the bright light plus sunglasses group slept more efficiently than the control group, but that the statistical significance depended upon the assumed CC (MD 6.59%, 95% CI 2.69 to 10.49 (CC = 0.9); 0.40 to 12.78 (CC = 0.7); ‐4.35 to 17.53 (CC = 0); 1 trial, 3 participants, low quality evidence; Analysis 7.3; Table 7).
7.3. Analysis.
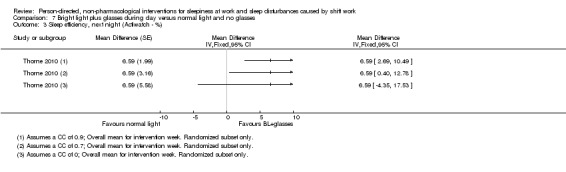
Comparison 7 Bright light plus glasses during day versus normal light and no glasses, Outcome 3 Sleep efficiency, next night (Actiwatch ‐ %).
8 Nap during the night shift (single nap opportunity) versus no‐nap
8.1 Sleepiness during the night shift
8.1.1 Sleepiness during the night shift, postintervention measurement only
Two studies used PVT test data to compare the effect of a single nap opportunity during the night shift on sleepiness on‐shift. One trial found that the nap group was slightly sleepier, while the other trial found that the no‐nap group was sleepier. When we combined these trials for a meta‐analysis, the nap group was less sleepy, but the results were not statistically significant (MD ‐11.87 milliseconds, 95% CI ‐31.94 to 8.20; 2 trials, 16 participants, very low quality evidence; Howard 2010; Smith 2007; Analysis 8.1; Table 8). Neither trial assessed chronotype.
8.1. Analysis.
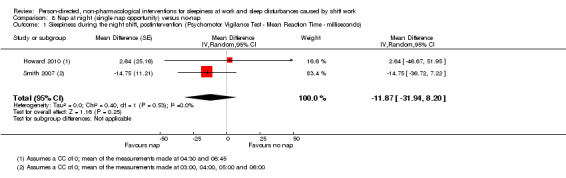
Comparison 8 Nap at night (single nap opportunity) versus no‐nap, Outcome 1 Sleepiness during the night shift, postintervention (Psychomotor Vigilance Test ‐ Mean Reaction Time ‐ milliseconds).
In an assessment of the same outcome, two studies of differing study designs used KSS data to examine a single nap opportunity on sleepiness on‐shift (Howard 2010; Smith‐Coggins 2006). The cross‐over designed study found that the nap group was sleepier than the no‐nap group (Howard 2010), but not statistically significantly (MD 0.13 scale points, 95% CI ‐0.40 to 0.66 (CC = 0.9); ‐0.75 to 1.01 (CC = 0.7); ‐1.46 to 1.72 (CC = 0); 1 trial, 8 participants, low quality evidence; Analysis 8.2; Table 8). The parallel designed study on the other hand (Smith‐Coggins 2006), found the nap group to be less sleepy than the no‐nap group, and the findings were statistically significant (MD ‐1.12 scale points, 95% CI ‐1.83 to ‐0.41; 1 trial, 49 participants, low quality evidence; Analysis 8.2; Table 8).
8.2. Analysis.
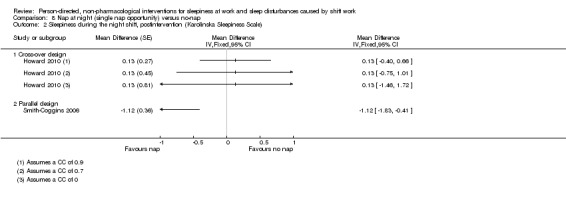
Comparison 8 Nap at night (single nap opportunity) versus no‐nap, Outcome 2 Sleepiness during the night shift, postintervention (Karolinska Sleepiness Scale).
The same two studies compared the effect of a single nap opportunity during the night shift on sleepiness on‐shift using the PVT test parameter's slowest 10% reciprocal reaction time (Howard 2010; Smith‐Coggins 2006). Both trials found the nap group to be less sleepy than the no‐nap group, but neither finding was statistically significant (MD 0.19 milliseconds, 95% CI ‐0.28 to 0.66 (CC = 0.9); ‐0.40 to 0.78 (CC = 0.7); ‐0.67 to 1.05 (CC = 0); 1 trial, 7 participants, very low quality evidence; Howard 2010; Analysis 8.3; Table 8) and MD 0.32 milliseconds, 95% CI ‐0.21 to 0.85; 1 trial, 49 participants, very low quality evidence; Smith‐Coggins 2006; Analysis 8.3; Table 8).
8.3. Analysis.
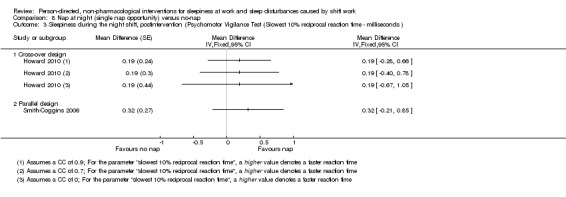
Comparison 8 Nap at night (single nap opportunity) versus no‐nap, Outcome 3 Sleepiness during the night shift, postintervention (Psychomotor Vigilance Test (Slowest 10% reciprocal reaction time ‐ milliseconds ).
Smith 2007 compared the effect of a single nap opportunity during the night shift on sleepiness on‐shift using a subjective sleepiness scale (in which a lower score denoted less sleepiness and a higher score more sleepiness (out of 100)). The nap group was statistically significantly less sleepy than the no‐nap group, irrespective of which CC was used (MD ‐16.14 scale points, 95% CI ‐22.10 to ‐10.18 (CC = 0.9); ‐25.04 to ‐7.24 (CC = 0.7); ‐31.37 to ‐0.91 (CC = 0); 1 trial, 9 participants, very low quality evidence; Analysis 8.4; Table 8).
8.4. Analysis.
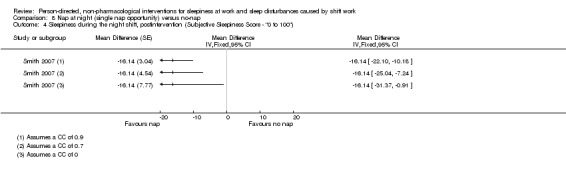
Comparison 8 Nap at night (single nap opportunity) versus no‐nap, Outcome 4 Sleepiness during the night shift, postintervention (Subjective Sleepiness Score ‐ "0 to 100").
9 Naps during the night shift (two‐nap opportunities) versus no‐naps
9.1 Sleepiness during the night shift
9.1.1 Sleepiness during the night shift, postintervention measurement only
Oriyama 2014 compared the effect of two‐nap opportunities in one night on sleepiness on‐shift.
Data from a VAS showed that the nap group was actually sleepier than the no‐nap group, but that the difference was not statistically significant (MD 2.32 scale points, 95% CI ‐24.74 to 29.38; 1 trial, 15 participants, low quality evidence; Analysis 9.1; Table 9).
9.1. Analysis.

Comparison 9 Naps at night (two‐nap opportunities) versus no‐naps, Outcome 1 Sleepiness during the night shift, postintervention (Visual Analogue Scale ‐ 0 mm to 100 mm).
10 Physical exercise plus sleep education versus wait‐list
10.1 Sleep quality and sleep length off‐shift
10.1.1 Sleep quality off‐shift, postintervention measurement only
In a standard parallel design, Atlantis 2006 examined sleep quality off‐shift (baseline and follow‐up, sleep quality for the preceding month) using physical exercise plus sleep education versus a wait‐list only group. Using the Pittsburgh Sleep Quality Index (PSQI) (0 to 21, a lower score = less sleepiness, a higher score = more), and looking at study completers, the authors reported a P value of 0.001 for the shift worker subgroup, postintervention, wherein the intervention group reported better quality sleep than the control group. However, we found the difference to be statistically non‐significant (MD ‐1.40 index points, 95% CI ‐3.10 to 0.30; 1 trial, 32 participants, moderate quality evidence; Analysis 10.1; Table 10).
10.1. Analysis.
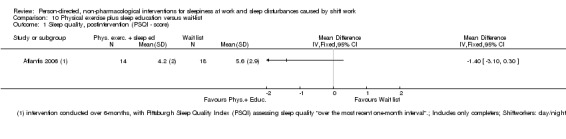
Comparison 10 Physical exercise plus sleep education versus wait‐list, Outcome 1 Sleep quality, postintervention (PSQI ‐ score).
The authors also reported that a subgroup of shift workers, "the poor sleepers", showed significant improvement in the PSQI postintervention (P = 0.04). Although the overall PSQI findings remained significant when data were analysed on an intention‐to‐treat (ITT) basis, the same cannot be reported with certainty for the shift work subgroup. We were unable to obtain ITT shift worker‐only data.
Harma 1988 examined both sleep length and sleep quality off‐shift following the night shift (baseline and follow‐up, sleep parameters over the preceding three weeks). They reported no significant difference between the group receiving the physical exercise programme and the group not receiving the programme: sleep length off‐shift (intervention: percentage change +6.9, control: +1.9; not significant); sleep quality off‐shift (intervention: percentage change ‐6.2, control: ‐8.3; not significant). Although the authors reported in the publication percentage change and positive significance levels based on their questionnaire, they did not report levels of variance. A request for data went unanswered. We therefore could not include these data in our quantitative analysis.
Smith‐Coggins 1997 concluded that the intervention (intensive sleep education plus improved shift schedule) did not significantly improve sleep and sleepiness compared to the placebo diet. While the majority of the analyses were day versus night, there was insufficient reporting of data (no reported variance) for those analyses that met our inclusion criteria. We did not include these data in our quantitative analysis.
Subgroup analysis
The included studies did not allow us to conduct subgroup analyses. See Differences between protocol and review.
Publication bias and quality of the evidence
None of our comparisons contained a sufficient number of trials to assess publication bias.
We assessed 31 outcomes in our quantitative analysis. We downgraded outcomes one level (i.e. from high quality to moderate quality) if the trials from which they came had an overall high risk of bias. We downgraded both of our two meta‐analyses for inconsistency, once for varying lengths of study duration and once for inconsistency of results. We downgraded all 31 of our outcomes at least once for imprecision due to small sample size (but considered the assessment 'small sample size/wide confidence interval' to be a single category and cause for only a single downgrade). In a number of cases we downgraded a second time for imprecision if the observation period was very short (a single day or night). Downgrading for indirectness occurred most often for the outcome 'sleepiness on‐shift'. This is because we consistently gave priority to objective measurements of our outcomes of interest. Objective sleepiness, however, is a variable that currently can only be measured indirectly (e.g. pupillometric assessment). This needs to be considered in future GRADE assessments of such variables.
Discussion
Summary of main results
In view of the large number of individuals working in shift work and 25% of all workers engaged in shift work described as suffering from sleep related problems (Liira 2014), the number of studies that met the criteria for inclusion in the present review is small (n = 17). The included studies explored person‐directed non‐pharmacological interventions with bright light, naps, or other interventions, such as education or physical exercise.
Bright light
The ten included trials present very low to low quality evidence, with no clear indication as to whether bright light might improve sleep parameters among shift workers.
The included studies administered bright light of wide‐ranging intensities and doses (from 2500 lux for 20 minutes up to 10,000 lux for 180 minutes) during shifts, with or without the additional use of light‐blocking goggles whilst off‐shift. They included diverse control conditions (300 lux normal light; normal light plus placebo medication; red light) and, even for the same outcome measure, a variety of measurement tools (e.g. sleepiness on‐shift: Stanford Sleepiness Scale (SSS), Karolinska Sleepiness Scale (KSS), visual analogue scale (VAS), Psychomotor Vigilance Task (PVT) test, Epworth Sleepiness Scale (ESS), actigraphy). Their population inclusion criteria ranged from workers with sleep problems to those who were sleep‐disorder free, and those who were healthy overall, and finally to those with no specific criteria given. Duration of the interventions ranged from one night or day to four weeks. The shift schedules in which the workers were involved also varied significantly.
The single meta‐analysis we were able to conduct in this category found, albeit based on only two trials at high risk of bias, that a bright light intervention at night, over the span of a single night, reduced sleepiness on‐shift overall by one SSS scale point (Analysis 1.3, very low quality evidence). Whether the observed effect is truly due to the bright light treatment is uncertain. Extremely short‐term interventions can be vulnerable to observer effects (for example, the Hawthorne effect (Zhong 2012)) and to placebo effects.
The extensive clinical heterogeneity makes both quantitative pooling and an overall summary statement about the effects of bright light intervention on sleep quality and sleep length off‐shift, and sleepiness on‐shift impossible. Adding to this, missing information around the correlation coefficients in the cross‐over trials leaves uncertainty around the statistical significance of a majority of our outcomes of interest. Finally, the lack of blinding, particularly among the cross‐over trials, introduces a level of bias that potentially undermines any statistically significant findings.
Napping
Based on the small number of trials available for pooling and the limited quality of reporting, we conclude that the data are currently insufficient to draw conclusions regarding the effectiveness of napping during the night shift on sleep parameters.
We rated the quality of the evidence, based on four trials, that napping reduces sleepiness on‐shift as very low to low.
The largest trial in this category with 49 participants (parallel design) found that the nap group reported being one scale score less sleepy than the no‐nap group and that this finding was statistically significant. However, the finding was not confirmed using the same measurement tool in a smaller cross‐over study, regardless of the assumptions we made regarding missing information (Analysis 8.2). A further trial found that the nap group reported being less sleepy than the no‐nap group, and the estimates remained stable regardless of assumptions of correlation (Analysis 8.4).
All of the studies lasted a single night only. Information on the ambient setting for the nap opportunity or opportunities in regard to the perceived light intensity ('darkness'), room temperature, comfort, and noise was seldom reported.
Other person‐directed interventions
Based on the limited data provided in the reports and the substantial clinical heterogeneity, we do not have sufficient information to conclude whether and to what extent physical exercise or educational programmes impact sleep quality or sleep length off‐shift or sleepiness on‐shift.
Of the three included trials, we judged one as having a high risk of bias and two as having an unclear risk of bias.
Only one trial offered sufficient information for us to conduct our own quantitative analysis, and our results ended up differing from those of the study authors’. The one trial in which the exercise programme included outdoor sports did not address the possibility that light exposure during outdoor sports activities likely acted as an effect modifier of their positive findings.
Methodological diversity and pooling
The methodological diversity of the included studies, in terms of interventions, settings, and assessment tools precluded ‐ with two exceptions – pooling study results. The following example may explain the 'pooling dilemma' among relatively very similar trials: Should results from three studies be statistically pooled if one measured their outcome using the KSS (Lowden 2004), and the other two measured the same outcome using the SSS (Karchani 2011; Sadeghniiat‐Haghighi 2011)? The three studies gave each test every two hours throughout the night shift. On the one hand, all measured subjective sleepiness and in a very similar manner, and, in general, the results obtained by using these two methods seem to correlate (Tremaine 2010). These facts would appear to support pooling the data from the above studies. On the other hand, the scales and verbal anchors are quite different and, indeed, the KSS was developed to replace the SSS, as many researchers had found the SSS to be too multidimensional, that is, it measures more dimensions than simply the difficulty of staying awake. We were unable to identify examples in the literature of successful pooling of KSS and SSS results. Therefore, we did not pool the data, even if the results obtained by using the two scales could have been standardised.
An overview of laboratory studies
As indicated in our protocol (Herbst 2013), we included randomised laboratory studies in our literature search and now present those that met our inclusion criteria in a separate table from the field studies. It is important to point out three caveats to this overview: (1) all trial information is taken verbatim from either the publication (when available), from a published abstract or from a thesis; (2) while we excluded laboratory studies in which randomisation was clearly not done, we did not contact the authors of laboratory studies to settle questions of randomisation; and, perhaps most importantly, (3) we did not assess the risk of bias for any of the laboratory studies.
This overview may be summarised in the following way: 22 experimental studies examined 'light'; four examined 'light and glasses'; 21 examined 'nap'; and seven examined 'other interventions'. The laboratory studies – singly and as a whole – suggest that different interventions can have effects on the outcomes of our review. For instance, one may posit that a certain amount of bright light exposure (hypothetically more than 1000 lux for at least two hours beginning at midnight) during consecutive nights may reduce sleepiness on these shifts. Indications of intervention effects in laboratory settings are important because, if no effects were detected under such controlled conditions, one would not expect less controlled field studies to generate significant results. However, it remains conceivable that differences between laboratory and field studies such as sample size or simulated versus real shift work or study populations in the laboratory (risk of selection bias) versus field setting or other codeterminants of interest may disallow the detection of intervention effects in a laboratory study which may be detected in the field investigation or vice versa.
Simulations of shift work conditions as well as interventions and endpoint assessments differed considerably across studies. With regard to results, while quantitative results were often found in the full‐text, almost none of the 54 investigations provided a quantitative result of intervention effects in their abstract, which is where one would expect to find the most important and defensible results. Overall, the laboratory studies provide no clear‐cut findings which could readily be used to augment what we observe in – and interpret from – the field studies.
Taken together, the scientific literature lacks published systematic reviews, including meta‐analyses, of laboratory studies related to the interventions evaluated in this Cochrane review. Our overview shows that empirical evidence in laboratory trials is inconsistent, fragmentary, and, across studies, no significant effects emerge consistently, even under optimised laboratory conditions. We conclude that, based on this overview, it is not possible to predict with much likelihood, which – if any – of the interventions investigated in laboratory studies will be effective – and detected – under naturalistic conditions in the field. Subject to the provision that a future Cochrane Review of laboratory studies were to identify patterns of interest which may be masked in Table 11, Table 12 and Table 13, the laboratory studies – as presented here – do not support evidence‐based recommendations. Future standards of how to simulate shift work, how to design, conduct and report interventions and how to measure effectiveness appear imperative to allow comparisons across laboratory and field studies.
The possible caveat of biological time not being equal to civil time
Individual chronobiology provides key information regarding the impact of interventions, such as (1) light, (2) napping or (3) physical activity, over 24 hours. Empirically, individuals vary in their chronobiological propensity for when – over 24 hours – physiology, endocrinology, metabolism, and behaviour render them more awake and active ('biological day') or passive or asleep ('biological night') (Erren 2013a; Erren 2014).
With few exceptions, the included studies define 'night' and 'day' according to measures of 'civil' time (Erren 2015), and they do not take latitude into account. Thus, information on critical determinants of the chronobiologically relevant biological day and biological night is lacking in the studies. From a chronobiological point of view, it would appear impossible, in these studies, to temporally attribute observed effects of interventions such as bright light on sleepiness and other outcomes to meaningful estimates of the study participants' biological night and biological day. Using 'artificial' time windows over 24 hours of 'civil night' and 'civil day' which do not correspond with the critical chronobiological timescale of 'biological night' and 'biological day' may blur the chronobiological basis and may mask relevant effects observed in study individuals who must be expected to vary significantly in their chronotype. In principle, this caveat may impede interpretation of single studies. Moreover, it may render grouping, for instance, light studies according to 'civil day' and 'civil night' as chronobiologically uninformative and possibly misleading.
Overall completeness and applicability of evidence
The literature search we conducted for the present review was extensive. We included multiple databases, used a broad range of search terms, and did not limit our search by date or language. We contacted field experts and authors to obtain additional data and information. We are therefore confident that we have identified all relevant and accessible studies pertaining to our study questions.
It is important to note that in several publications the study authors did not present all the relevant data. We attempted to contact study authors to ask for missing information. While a number of study authors responded, many could not be reached or failed to respond. In the latter case, depending on the parameters in question, we indicated in our assessments that the information was unclear or we discussed the studies in only a qualitative way.
Because the term 'shift work' refers not only to night‐shift work but also to differing day shifts (morning shift, afternoon shift, evening shift), it is important to note that our review was limited to the effect of person‐directed interventions on sleep parameters among shift workers involved in night work. Thus, readily extrapolating the effect of these same interventions to day‐shift workers is not possible.
The included studies observed workplaces that do not seem to be generalisable to shift work settings in general. Nine of the 17 studies observed workers in a hospital. Two of these studies investigated workers in a sleep laboratory setting. It can be assumed that persons working in medical settings may differ from individuals in other occupational settings. They may be more familiar with adverse health effects associated with shift work, behavioural options to mitigate the negative effects of shift work, and have knowledge concerning possible effects of interventions like exposure to light or napping. Furthermore, two included studies presented data from oil rigs as a unique workplace environment with very specific tasks and settings. Only three studies explored workers in a 'typical' industrial setting. Hence, the overall study population covered by this Cochrane review does not represent the overall shift work population in modern societies.
We defined sleepiness and sleep‐related parameters (e.g. sleep quality and sleep length) as our outcomes. These outcomes can be measured in different ways, such as directly via the Stanford Sleepiness Scale (SSS) or the Karolinska Sleepiness Scale (KSS), or indirectly via measurement of reaction times. When possible, we gave preference to objective measurements. It is thus possible that we may have missed relevant subjective results. Furthermore, lapses or accidents during shift work (or after work) could also indicate sleepiness. However, we did not include such outcomes and may therefore have missed studies with other indirect measurements of sleepiness. However, we expect that the outcomes we have chosen do cover the most important aspects and facets of sleepiness and sleep.
We had intended to examine as secondary outcomes the cost of the interventions in those studies that reported this review's primary outcomes. As the included studies did not report any information on such specific expenses, this review does not include data in this regard.
Finally, possible adverse effects of some of the included interventions, such as safety risks from wearing sunglasses after the night shift on the way home, or a temporarily decreased alertness in the first minutes following naps during the night shift (inertia), were beyond the scope of our review but should not be ignored.
Quality of the evidence
Although we limited our review to randomised controlled trials (RCTs), the quality of the evidence was limited. Due to the extreme diversity of the interventions and controls, nearly all of our results are based on single trials.
The sample size of each trial was relatively small (mean: 36, median: 18; minimum: 4; maximum: 94), and their risk of bias was unclear or high. Despite the fact that blinding would have been theoretically possible, we found no indication that blinding of participants to the bright light interventions was undertaken. All nap studies lasted only a single night.
In some cases, a cross‐over design was used even when not appropriate (i.e. when the intervention cannot be 'unlearned', such as sleep education; or when participants cannot be blinded to the intervention, such as with naps).
When discussing the quality of our evidence we believe it is important to differentiate between evidence that is based on poorly executed studies and evidence that is based on poorly reported studies. The majority of the studies we identified fall into the latter category.
Misclassification bias and missing information on effect modifiers also impair the quality of the evidence. In the case of the former, some studies investigating light interventions acknowledged that, as field studies, light exposure in the surrounding environment is difficult to control. Other trials, however did not address this factor and how it may have impacted their effect estimates. We found limited information about normal light conditions at the workplaces, about the precise latitudes of the trials’ settings, and on the season when the trials were conducted.
With few exceptions, the studies did not explore their findings in the context of individual chronotypes, a factor that could be a relevant effect modifier of the adjustment to shift work conditions and of the outcomes of the investigated interventions (see Assessment of heterogeneity).
Potential biases in the review process
For our assessment of study quality, we followed the guidelines of The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Overall, our included studies provided sufficient information for us to assess the domains for reporting bias, attrition bias, and other biases. However, the study authors did not sufficiently report the domains for random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. Only two of the 17 included studies reported that participants were blinded to the intervention, and both were 'other intervention' studies with parallel groups. Information on blinding in bright light studies and nap studies was either non‐existent or studies directly acknowledged not having blinded. We contacted all study authors when information for this domain was missing. Responses either never arrived or they confirmed that blinding was not performed. For our final assessment of that domain, we judged the study authors’ acknowledgement of no blinding to indicate a potentially high risk of bias, whereas we judged a lack of information to indicate an unclear risk of bias. We understand that this approach is not ideal, and that 'rewarding' a lack of information over full reporting is problematic. However, the approach never transformed a potentially low risk study to high risk. Even if we had instead left blinding unclear for all bright light and nap studies, none would have been assessed as low risk. At most, these high risk of bias trials would have converted to unclear.
For the outcome 'on‐shift sleepiness for nap versus no‐nap', we reported separately RCTs with different study designs (cross‐over or parallel). An alternative approach to present these data would have been to combine the different trial types and then conduct a sensitivity analysis.
As described, we used sleepiness on‐shift and sleep length and sleep quality off‐shift as primary outcomes. We did not include all possible objective measurements of sleepiness and thus may have missed some studies and relevant data. As we gave preference to objective measurements over subjective ones, we may have missed significant findings in the subjective data. Nonetheless, we believe that the outcomes we have chosen capture the most important aspects of shift work sleep problems.
We included studies in which all sleep health groups were represented. The sleep health‐status of a population can impact the effects of person‐directed interventions on sleep parameters. For example, people reporting sleep problems might respond differently to a bright light intervention than those not reporting sleep problems. While it would have been interesting to conduct a sensitivity analysis examining the potential modifying effects of sleep health‐status, our pooling options were too limited to do so.
The missing correlation coefficient information in the cross‐over trials made it necessary for us to assume varying levels of correlation between the intervention and control groups, in order to estimate standard errors of the effect estimates (Elbourne 2002). While such data imputation can lead to bias, we presented the results for each of the assumed levels graphically or in the text or both. In the case of our meta‐analyses (n = 2), we assumed zero correlation between the intervention and control groups, thus minimising the risk of presenting findings as statistically significant when this, in fact, may not be the case. There was no clinical justification for a specific correlation coefficient. None of the studies we found described a correlation coefficient or sufficient data to calculate one (e.g. the overall standard deviation (SD) as well as the SD for intervention and control). Hence, we decided to present three different coefficients using a wide range (0 to 0.9) of assumed correlations to investigate its influence on the results.
Finally, it is possible that an assessment of the quality of evidence for the outcome 'sleepiness on‐shift' based solely on subjective (but direct) measurements might have resulted in less of a downgrading for that outcome overall. However, in no case would such an assessment have resulted in an outcome reaching a high level quality of evidence.
Agreements and disagreements with other studies or reviews
Neil‐Sztramko 2014 conducted a comprehensive review on several aspects of night‐shift work and health‐related interventions. Their review and our review both examined controlled light interventions and interventions based on behaviour modification (i.e. physical activity or rest periods). Because the inclusion criteria of their review and our review differed (most notably, they excluded sleepiness and fatigue as outcomes, and included biological markers of chronic disease, laboratory trials and non‐randomised studies), Neil‐Sztramko 2014 assessed a number of light studies that we had excluded. Conversely, because they excluded sleepiness, they did not include any of our nap studies. However, we identified in our search every study in their included studies lists for light and behavioural interventions and screened them closely for inclusion in our review.
In contrast to our study, Neil‐Sztramko 2014 judged the reporting quality of the light studies to be high. Although they report the summary scores in an appendix, we were unable to find a specific breakdown of these scores, but we assumed that higher scores indicated better quality reporting. Our risk of bias judgement differed mainly in our assessment of one study (Tanaka 2011), likely because of the assessment issue for blinding mentioned above. However, overall, the authors of the Neil‐Sztramko 2014 review described the ‘substantial heterogeneity’ of the studies and concluded, as do we, that meta‐analyses are not possible with the current evidence available.
A narrative systematic review by Ruggiero 2014 specifically investigated napping on sleepiness and sleep‐related performance. The review authors accepted pseudo‐randomised studies and therefore ended up including several more than the four nap trials we included. Most of the remaining studies on their list are listed in our review under Characteristics of excluded studies or Table 11, Table 12 and Table 13. According to Ruggiero 2014 nap interventions appear to “hold promise” in improving sleepiness and performance among shift workers, but that the small sample size and high heterogeneity make conclusions difficult. These are in agreement with our findings. We include one further trial that was likely published too late for the Ruggiero 2014 review (Oriyama 2014). This additional trial found no statistically significant difference between the nap and no‐nap groups. With 15 participants, the trial is also small. The fact that the intervention involves two‐nap opportunities adds to the heterogeneity of the body of evidence for napping, ultimately supporting the findings of both Ruggiero 2014 and our own.
Authors' conclusions
Implications for practice.
Based on the current evidence base it is not possible to determine whether person‐directed, non‐pharmacological interventions including bright light, naps, physical exercise, or sleep education have any influence on outcomes including sleepiness, sleep length, and quality. All results from the pooled analyses, as well as results from the single trials should be interpreted with caution as we graded the quality of the evidence provided by most of the included studies as low to very low.
Implications for research.
Overall, there is a noticeable lack of interpretable evidence and study evidence of links between the interventions and outcomes investigated in this review. Our extensive literature searches and analyses culminate in the following guidelines for future studies in this field.
Most studies appeared considerably underpowered to adequately identify or exclude practically and clinically important effects. Power and sample size calculations should be calculated before a study is conducted and systematically reported. The actual number of participants to make the study adequately powered will depend on the study specifics.
Wherever possible, blinding should be applied.
Future research must consider chronobiological aspects, such as the chronotype, to take note of the individual biological night and day and consider this information as a possible effect modifier. The tools chosen to assess chronotype should assess chronobiological propensity accurately. Unfortunately, to date there has been no consensus or standardisation regarding how chronotype should be most appropriately assessed in practice.
Questionnaires which can be used to assess chronobiological information include the Morningness‐Eveningness Questionnaire (MEQ) (Horne 1976), cited 1843 times as of 28 April 28 2016; the Munich Chronotype Questionnaire (MCTQ) (Roenneberg 2003), cited 429 times as of 28 April 28 2016; and the recently proposed MCTQshift (Juda 2013). Moreover, in place of – or to complement – questionnaires such as the MEQ or MCTQ, laboratory measurements such as the dim light melatonin onset (DLMO) tool could be employed as it is considered the most reliable measure of central circadian timing in humans (Kantermann 2015).
One example may illustrate challenges regarding the issue of how to consider chronotype in practice. When investigating possible relationships between shift work involving circadian disruption and cancer (IARC 2010), the assessment of chronobiological propensity has been based on answers to as little as one single question. In the relevant Hansen 2012 study, the authors referred to the Roenneberg 2007 study where the authors wrote in 2007, “It is remarkable that an introduction combined with a single question of self‐assessing one’s chronotype gives almost the same results as a questionnaire consisting of 19 items.” Importantly, the 'introduction + single question' reads: “Self‐assessment: After you have answered the preceding questions, you should have a feeling to which chronotype (time‐of‐day‐type) you belong to. …. Please tick only one possibility” (information added: out of seven categories) (MCTQ; Roenneberg 2003); p. 82).
To date, the extent to which the answer to the 'single question' is influenced by the answers to the preceding 19 questions is not clear. How well a single question without such preceding questions into chronobiological information can capture chronotype appears therefore open.
Reports of studies should have comprehensive descriptions of the studies' methods (e.g. randomisation), data, and analyses. Furthermore, study location and the time period when a study was conducted should be reported.
A general approach to make future research more informative could be to convene a panel of knowledgeable scientists from the different fields concerned (International Agency for Research on Cancer (IARC) example in Stevens 2011). Candidates for such a panel would include scientists from occupational medicine, chronobiology, and sleep medicine who could identify – at least a convincing minimum of – uniform study requirements. Such a panel could also attempt to resolve methodological controversies, e.g. which questionnaire(s) should be used and when it (they) should be used, or whether to use the DLMO tool in addition to, or instead of, other measures.
Acknowledgements
We thank Dr. Kathrin Bauer for preparatory work for Cochrane Reviews within the Institute and Policlinic for Occupational Medicine, Environmental Medicine, and Prevention Research. We thank Dr. Christine Herbst for contributing Cochrane Review work up to 2014. We thank Melissa Koch for contributing to the Cochrane Review protocol design. We thank Ingrid Vukadinovic for retrieving full‐texts from the farthest‐flung corners of the earth (and beyond). We thank Leena Isotalo and Kaisa Neuvonen from the Cochrane Work Review Group for advice and help with the search strategy. We also thank the Managing Editor, Jani Ruotsalainen and Co‐ordinating Editor, Jos Verbeek from the Cochrane Work Review Group for their continuous help. We thank trial authors who provided additional information about their publications. We thank Clare Dooley for copy editing the text of our review.
Appendices
Appendix 1. MEDLINE search strategy
1. exp Chronobiology Disorders/ 2. exp Sleep Disorders/ 3. exp Circadian Rhythm/ 4. exp "wounds and injuries"/ OR occupational injuries/ 5. (errors OR incidents OR accidents OR mistakes OR safety).tw. 6. Death, Sudden, Cardiac/ OR Death, Sudden/ OR death?.tw. OR Death/ 7. exp "costs and cost analysis"/ 8. (econom$ OR cost OR costs).tw. 9. (chronotherapy OR light OR daylight OR dark OR darkness).tw. 10. exp sleep disorders, intrinsic/ OR exp "sleep initiation and maintenance disorders"/ 11. (sleep OR sleepiness OR circadian OR vigilance OR altertness OR alert OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence OR dyssomnia OR eveningness OR morningness OR "concentration difficulties" OR attentiveness OR arousal OR performance OR vigilance OR vigilant).tw. 12. (nap OR napping OR rest OR resting).tw. 13. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 14. ((shift OR shifts) adj1 (rota OR system OR systems OR schedul* OR hours OR time OR pattern$ OR cycle OR extend$ OR evening OR late OR roster OR early OR weekend OR twilight OR graveyard OR night$ OR split OR non‐standard OR "non standard" OR flex$ OR turnaround OR continuous OR rotat$)).tw. 15. (day adj2 schedule?).tw. 16. (rota OR roster OR 'day week' OR flexitime OR 'hours of work' OR nightshift* OR shiftwork*).tw. 17. ((work$ OR duty) adj1 (shift OR shifts OR rota OR system OR systems OR schedul* OR hours OR time OR pattern$ OR cycle OR extend$ OR evening OR late OR roster OR early OR weekend OR twilight OR graveyard OR night* OR split OR non‐standard OR "non standard" OR flex$ OR turnaround OR continuous OR rotation$)).tw. 18. ((backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying) adj1 (rotation OR rotate OR rotating)).tw. 19. (rota OR roster OR duty OR shift OR shifts OR shiftwork OR hours OR week OR work).mp. 20. 18 and 19 21. 14 OR 15 OR 16 OR 17 OR 20 22. (randomized controlled trial OR controlled clinical trial).pt. OR randomized.ab. OR placebo.ab. OR drug therapy.fs. OR randomly.ab. OR trial.ab. OR groups.ab. 23. (effect* OR controll* OR control OR controls* OR controli* OR controle* OR controla* OR evaluation* OR program*).tw. 24. (work OR works* OR work* OR worka* OR worke* OR workg* OR worki* OR workl* OR occupation* or prevention* OR protect*).tw. 25. 24 and 23 26. (cohort OR cross sectional OR study OR survey OR questionnaire? OR diary OR diaries).tw. 27. Case‐control studies/ OR cohort studies/ OR evaluation studies/ OR feasibility studies/ OR longitudinal studies/ OR program evaluation/ OR prospective studies/ OR retrospective studies/ OR exp follow‐up studies/ OR exp risk Factors/ OR exp evaluation studies/ OR exp retrospective Studies OR exp chi‐square distribution/ OR logistic models/ OR exp treatment outcome/ OR exp comparative studies OR cross‐sectional studies/ OR multivariate analysis/ 28. 22 OR 25 OR 26 OR 27 29. exp animals/ not humans.sh. 30. 28 NOT 29 31. 13 AND 21 AND 30
Appendix 2. Embase search strategy
1. ((work NEAR/2 hour*) OR (shift NEAR/2 work*) OR (work* NEAR/2 week) OR nightshift* OR shiftwork* OR (day NEAR/2 schedule)) 2. ((rotat* NEAR/2 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying)) AND (shift* OR work* OR schedule OR time OR duty OR hours OR rota OR roster)) 3. (shift$ NEAR/1 (rota OR system$ OR schedul* OR hours OR time OR pattern* OR cycle OR extend* OR evening OR late OR roster OR early OR weekend OR twilight OR graveyard OR night$ OR split OR non‐standard OR "non standard" OR flex$ OR turnaround OR continuous OR rotat*) 4. (shift* NEAR/2 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying OR roster OR rota OR "day week")) 5. (nightshift* OR shiftwork*).tw. OR rota*?.tw. OR roster*.tw. OR 'day week'.tw. 6.exp Sleep Disorders/ OR 3. exp Circadian Rhythm/ OR exp "wounds and injuries"/ OR occupational injuries/ OR exp Death, Sudden, Cardiac/ OR Death, Sudden/ OR Death/ OR exp "costs and cost analysis"/ OR exp Chronobiology Disorders/ 7. (sleep OR sleepiness OR circadian OR vigilance OR altertness OR alert OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence OR dyssomnia OR eveningness OR morningness OR "concentration difficulties" OR attentiveness OR arousal OR performance OR vigilant OR nap OR napping OR rest OR resting OR errors OR incidents OR accidents OR mistakes OR safety OR deaths OR death OR mortality OR injury OR injuries OR chronotherapy OR light OR daylight OR dark OR darkness OR econom$ OR cost OR costs).tw. 8. treatment outcome/ OR intermethod comparison/ OR major clinical study/ OR controlled study/ OR prospective study/ OR case‐control study/ OR clinical article/ OR controlled study/ OR risk factor/ OR exp Follow Up/ OR outcomes research/ OR multivariate analysis/ OR retrospective study/ OR cohort analysis/ OR comparative study/ OR population research/ OR risk factors/ 9. (cross adj1 sectional).tw OR compared.tw OR compares.tw. OR (cohort OR cross‐sectional OR case‐control OR study OR survey OR surveys OR diary OR diaries OR questionnaire? OR groups OR comparison$ OR multivariate OR risk factor$ OR effectiveness).mp. 10. 1 OR 2 OR 3 OR 4 OR 5 11. 6 OR 7 12. 8 OR 9 13. 10 AND 11 AND 12
Appendix 3. OPEN GREY
(((work NEAR/2 hour*) OR (shift NEAR/2 work*) OR (work* NEAR/2 week) OR nightshift* OR shiftwork* OR (day NEAR/2 schedule) OR ((rotat* NEAR/1 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying) AND (shift* OR work* OR schedule OR time OR duty OR hours OR rota OR roster)) OR (shift$ NEAR/1 (rota OR system$ OR schedul* OR hours OR time OR pattern* OR cycle OR extend* OR evening OR late OR roster OR early OR weekend OR twilight OR graveyard OR night$ OR split OR non‐standard OR "non standard" OR flex$ OR turnaround OR continuous OR rotat*)) OR (shift* NEAR/2 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying OR roster OR rota OR “day week” )) AND (sleep OR sleepiness OR circadian OR vigilance OR altertness OR alert OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence OR dyssomnia OR eveningness OR morningness OR "neurocognitive performance" OR "concentration difficulties" OR attentiveness OR arousal OR performance OR vigilant OR nap OR napping OR rest OR resting OR errors OR incidents OR accidents OR mistakes OR safety OR deaths OR death OR mortality OR injury OR injuries OR chronotherapy OR light OR daylight OR dark OR darkness OR econom$ OR cost OR costs OR light OR dark OR darkness OR goggles OR exercise))
Appendix 4. PsycINFO
S1 TX ((work N2 hour*) OR (shift N2 work*) OR (work* N2 week) OR nightshift* OR shiftwork* OR (day N2 schedule)) S2 TX ((rotat* N2 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying)) AND (shift* OR work* OR schedule OR time OR duty OR hours OR rota OR roster)) S3 TX (shift$ N2 (rota OR system$ OR schedul* OR hours OR time OR pattern* OR cycle OR extend* OR evening OR late OR roster OR early OR weekend OR twilight OR graveyard OR night$ OR split OR non‐standard OR "non standard" OR flex$ OR turnaround OR continuous OR rotat*)) S4 TX (shift* N2 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying) OR (roster OR rota) OR "day week") S5 TX (sleep OR sleepiness OR circadian OR vigilance OR altertness OR alert OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence OR dyssomnia OR eveningness OR morningness OR "neurocognitive performance" OR "concentration difficulties" OR attentiveness OR arousal OR performance OR vigilant OR nap OR napping OR rest OR resting OR errors OR incidents OR accidents OR mistakes OR safety OR deaths OR death OR mortality OR injury OR injuries OR chronotherapy OR light OR daylight OR dark OR darkness OR econom$ OR cost OR costs) S6 DE workday shifts S7 DE human biological rhythms S8 DE sleepiness S9 DE sleep deprivation S10 DE sleep disorders S11 DE sleep S12 DE physiological arousal S13 DE fatigue S14 DE workday shifts S15 DE work scheduling S16 DE performance S17 DE occupational safety S18 DE napping S19 DE job performance S20 DE wakefulness S21 DE sleep onset S22 DE mortality rate S23 DE trends S24 DE risk factors S25 DE longitudinal studies S26 DE follow up studies S27 DE retrospective studies S28 TX control OR (cross N1 sectional) OR compared OR compares OR cohort OR cross‐sectional OR (case N1 control) OR study OR survey OR surveys OR diary OR diaries OR questionnaire? OR evaluation OR evaluate OR groups OR comparison$ OR multivariate OR risk factor$ OR effectiveness OR random* OR allocation OR allocate OR allocated S29 S28 OR S27 OR S26 OR S25 OR S24 OR S23 S30 S22 OR S21 OR S20 OR S19 OR S18 OR S17 OR S16 OR S13 OR S12 OR S11 OR S10 OR S9 OR S8 OR S7 OR S5 S31 S1 OR S2 OR S3 OR S4 OR S6 OR S15 S32 S31 AND S30 AND S29
Appendix 5. Web of Knowledge
TS=(work NEAR/2 hour*)
TS=(shift NEAR/2 work*)
TS=(work* NEAR/2 week)
TS=(nightshift* OR shiftwork*)
TS=(day NEAR/2 schedule*)
TS=(rotat* NEAR/1 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying))
TS=(shift* OR work* OR schedule OR time OR duty OR hours OR rota OR roster)
#6 AND #7
TS=(shift$ NEAR/1 (rota OR system$ OR schedul* OR hours OR time OR pattern* OR cycle OR extend* OR evening OR late OR roster OR early OR weekend OR twilight OR graveyard OR night$ OR split OR non‐standard OR "non standard" OR flex$ OR turnaround OR continuous OR rotat*))
TS=(roster OR rota)
TS=("day week")
TS=(shift* NEAR/1 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying))
TS=(sleep OR sleepiness OR circadian OR vigilance OR altertness OR alert OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence OR dyssomnia OR eveningness OR morningness OR "neurocognitive performance" OR "concentration difficulties" OR attentiveness OR arousal OR performance OR vigilant OR nap OR napping OR rest OR resting OR errors OR incidents OR accidents OR mistakes OR safety OR deaths OR death OR mortality OR injury OR injuries OR chronotherapy OR light OR daylight OR dark OR darkness OR econom$ OR cost OR costs)
TS=(effect* OR controll* OR control OR controls* OR controli* OR controle* OR controla* OR evaluation* OR program* OR cohort OR cross sectional OR study OR survey OR questionnaire? OR diary OR diaries OR placebo OR random* OR trial OR groups OR multivariate OR compare? OR comparison* OR risk factor?)
#1 OR #2 OR #3 OR #4 OR #5 OR #8 OR #9 OR #10 OR #11 OR #12#15 AND #14 AND #13
TI=(mice OR rats)
#15 NOT #16
Appendix 6. Cochrane CENTRAL
#1 ((work NEAR/2 hour*) OR (shift NEAR/2 work*) OR (work* NEAR/2 week) OR nightshift* OR shiftwork* OR (day NEAR/2 schedule)):kw #2 ((rotat* NEAR/2 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying)) AND (shift* OR work* OR schedule OR time OR duty OR hours OR rota OR roster)):kw #3 (shift$ NEAR/2 (rota OR system$ OR schedul* OR hours OR time OR pattern* OR cycle OR extend* OR evening OR late OR roster OR early OR weekend OR twilight OR graveyard OR night$ OR split OR non‐standard OR "non standard" OR flex$ OR turnaround OR continuous OR rotat*)):kw #4 (shift* NEAR/2 (backward OR forward OR rapid OR slow OR rapidly OR slowly OR advancing OR delaying) OR (roster OR rota) OR "day week"):kw #5 MeSH descriptOR Work Schedule Tolerance explode all trees #6 (sleep OR sleepiness OR circadian OR vigilance OR altertness OR alert OR wakefulness OR drowsiness OR fatigue OR insomnia OR hypersomnolence OR dyssomnia OR eveningness OR morningness OR "neurocognitive performance" OR "concentration difficulties" OR attentiveness OR arousal OR performance OR vigilant OR nap OR napping OR rest OR resting OR errors OR incidents OR accidents OR mistakes OR safety OR deaths OR death OR mortality OR injury OR injuries OR chronotherapy OR light OR daylight OR dark OR darkness OR econom$ OR cost OR costs):kw #7 MeSH descriptor Sleep Phase Chronotherapy explode all trees #8 MeSH descriptor Chronotherapy explode all trees #9 MeSH descriptor Chronobiology Disorders explode all trees #10 MeSH descriptor Sleep Disorders, Circadian Rhythm explode all trees #11 MeSH descriptor Dyssomnias explode all tree #12 MeSH descriptor Sleep Disorders, Circadian Rhythm explode all trees #13 MeSH descriptor Sleep Initiation and Maintenance Disorders explode all trees #14 MeSH descriptor Sleep Deprivation explode all trees #15 MeSH descriptor Sleep Disorders, Intrinsic explode all trees #16 MeSH descriptor Sleep Disorders explode all trees #17 MeSH descriptor Sleep explode all trees #18 MeSH descriptor Psychomotor Performance explode all trees #19 MeSH descriptor Medical Errors explode all trees #20 MeSH descriptor Mortality explode all trees #21 MeSH descriptor Death explode all trees #22 MeSH descriptor Wounds and Injuries explode all trees #23 MeSH descriptor Fatigue explode all trees #24 MeSH descriptor Economics explode all trees #25 MeSH descriptor Cost of Illness explode all trees #26 (#1 OR #2 OR #3 OR #4 OR #5) #27 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) #28 (#26 AND #27)
Data and analyses
Comparison 1. Bright light at night versus normal light (300 lux).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleepiness during the night shift, overall, Stanford Sleepiness Scale (Scale: 1‐7) | 2 | Mean Difference (Random, 95% CI) | ‐0.83 [‐1.31, ‐0.36] | |
| 2 Sleepiness during the night shift, overall, Karolinska Sleepiness Scale (Scale: 1‐9) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Sleepiness during the night shift, postintervention only, Stanford Sleepiness Scale (Scale: 1‐7) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Sleepiness during the night shift, post‐intervention only, Karolinska Sleepiness Scale (Scale: 1‐9) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Total sleep time, next day (Actigraph ‐ hours) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5.1 Main sleep time only | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 24‐hr sleep time, including naps | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Sleep efficiency, next day (Actigraph ‐ %) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 2. Bright light alone at night versus normal light (300 lux) plus placebo capsule.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleepiness during the night shift (5‐min. Reaction Time Test ‐ milliseconds) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Total sleep time, next day (Actiwatch ‐ hours) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Sleep onset latency, next day (Actiwatch ‐ minutes) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Sleep efficiency, next day (Actiwatch ‐ %) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 3. Bright light during day versus normal light (530 to 648 lux).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleepiness during the day shift (Karolinska Sleepiness Scale) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Sleep quality, next night (Visual Analogue Scale ‐ 0 to 10) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 4. Bright light during day versus dim red light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total sleep time, next night (sleep log ‐ hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Sleep onset latency, next night (sleep log ‐ minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Bright light alone during day versus normal light (300 lux) plus placebo capsule.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleepiness during the day shift, days (5‐min. Reaction Time Test ‐ milliseconds) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Total sleep time, next night (Actiwatch ‐ hours) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Sleep onset latency, next night (Actiwatch ‐ minutes) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Sleep efficiency, next night (Actiwatch ‐ %) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 6. Bright light at night plus glasses at dawn versus normal light (unclear lux) and no glasses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleepiness during the night shift (Psychomotor Vigilance Test: Median Reaction Time ‐ milliseconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Bright light plus glasses during day versus normal light and no glasses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total sleep time, next night (Actigraph ‐ hours) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Sleep onset latency, next night (Actigraph ‐ minutes) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Sleep efficiency, next night (Actiwatch ‐ %) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 8. Nap at night (single nap opportunity) versus no‐nap.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleepiness during the night shift, postintervention (Psychomotor Vigilance Test ‐ Mean Reaction Time ‐ milliseconds) | 2 | Mean Difference (Random, 95% CI) | ‐11.87 [‐31.94, 8.20] | |
| 2 Sleepiness during the night shift, postintervention (Karolinska Sleepiness Scale) | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2.1 Cross‐over design | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Parallel design | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sleepiness during the night shift, postintervention (Psychomotor Vigilance Test (Slowest 10% reciprocal reaction time ‐ milliseconds ) | 2 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3.1 Cross‐over design | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Parallel design | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Sleepiness during the night shift, postintervention (Subjective Sleepiness Score ‐ "0 to 100") | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 9. Naps at night (two‐nap opportunities) versus no‐naps.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleepiness during the night shift, postintervention (Visual Analogue Scale ‐ 0 mm to 100 mm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 10. Physical exercise plus sleep education versus wait‐list.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleep quality, postintervention (PSQI ‐ score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Atlantis 2006.
| Methods |
Trial design: Randomised controlled trial Intervention setting: Gambling casino Shift system: Permanent days, permanent nights, and varied (rotating) shifts. Varied shift work schedules entailed a rotation of two months of: daytime work (12:00‐20:00), night‐time work (20:00‐04:00), and morning time work (04:00‐12:00) Follow‐up period (intervention plus follow‐up): 24 weeks (Washout period): Not relevant |
|
| Participants |
Inclusion criteria: "Healthy but sedentary". Had not participated in regular exercise (at least 20 minutes of aerobic or weight‐training exercise, ≥ 2 days/week) within previous 3 months; able to produce a doctor’s clearance to commence an exercise regime; able to attend the fitness centre at least 3 days/week for 60 minutes, and physiological data collection on 3 occasions, over 24‐week study; willingness to be randomised to either treatment or wait‐list control; shift workers defined as those working non‐daytime hours, which includes those working both day/night shifts and permanent night shifts Exclusion criteria: Clinically diagnosed with a medical (e.g. HIV) or psychiatric condition (e.g. depression) to preclude those receiving medical treatment; classified as a “workers compensation” case; pregnant Number screened: n = 3800 Number eligible: n = 73 (but unclear what % of these were shift workers) Number included in our analysis: n = 32 shift workers (out of n = 44 overall) Industry: Gambling casino service Age in years: "There were no significant between‐group differences in baseline characteristics in the shift worker subgroup" Gender: See "Age in years" Country: Austrialia Month(s) study conducted: Unclear "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Physical education (PhysEd) programme: aerobic and weight‐training plus health education/sleep hygiene (HealthSleep) programme Shift‐based timing: Day (off‐shift) Hours of intervention: PhysEd: between 07:00 and 11:00 or 13:00 and 15:00 or 17:00 and 19:00; SleepEd: unclear Dose/frequency/duration: PhysEd: (1) moderate‐to‐high intensity aerobic exercise, at least 3 exposures per week, 20 minutes per exposure for 24 weeks; (2) moderate‐to‐high intensity whole body weight‐training exercise, average of 3 exposures per week, 30 minutes per exposure for 24 weeks; HealthSleep: unclear. One‐on‐one health counselling sessions offered (60 minutes per month per subject) Control/comparison intervention: Wait‐list |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: Outcome not examined Sleep quality off‐shift:
Sleepiness on‐shift: Outcome not examined |
|
| Notes | Funding: Support from the casino for use of employees and their time reported. No other funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation into either treatment (24 weeks) or wait‐list control (control 24 weeks, then treatment for 24 weeks) groups were stratified by gender, and by normal or abnormal scores for any one of three psychological constructs using the Depression, Anxiety, and Stress Scales (DASS) (Lovibond 1995), via computer‐generated permuted blocks |
| Allocation concealment (selection bias) | Low risk | Although there was no concealment, at this early stage of the study participants were not known to the research team and could not be identified by name |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants: Employees were not informed of the research hypothesis regarding sleep disturbance, rather, an overall change in health was explained as the main outcome under investigation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Out of the 73 subjects recruited: Intervention: 44.4% dropout Control: 35.1% dropout Numbers for the subgroup of shift workers not given. Intention‐to‐treat analysis presented for all n = 73 participants, but not for the subgroup of shift work participants |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Pittsburgh Sleep Quality Index: has been validated against objective sleep measures and is sensitive to change after weight‐training exercise treatment of depression (Singh 1997), as well as following aerobic exercise for insomnia (King 1997). |
| Other potential sources of bias | Low risk | Not relevant |
Bjorvatn 2007.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Offshore oilrig Shift system: Swing shift (two‐week tour during which employees work 12‐hour nights (18:30‐06:30) the first seven days and 12‐hour days (06:30‐18:30) the second seven days, with a "swing day" (04:00‐10:00) bridging the two weeks. The two weeks are followed by 3‐4 weeks off, then the schedule is repeated) Follow‐up period (intervention plus follow‐up): 9‐10 weeks (2 weeks of intervention x 3 groups plus 3‐4 week washout) (Washout period): 3‐4 weeks |
|
| Participants |
Inclusion criteria: Problems adjusting to shift work (needing > 3 days to (re)adapt), or more than moderate sleep problems (based on authors’ questionnaire) Exclusion criteria: None reported Number screened: n = 109 Number eligible: n = 38 Number included in our analysis: n = 17 Industry: Offshore oil industry Age in years (mean (range)): 42 (29‐55) Gender: 94% male Country: Norway (North Sea) Month(s) study conducted: April 2002 to April 2003 "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to bright light via a light box Shift‐based timing: Light: On‐shift (night) and on‐shift (day) Hours of intervention: Individualised timing starting two hours before the assumed nadir of the circadian phase and moved backward by one hour every day (during night shift: between 00:00 and 05:00; during day shift: between 12:00 and 14:30) Dose/duration/frequency: 10,000 lux, 30 minutes per exposure, 1 exposure per day/night for first 4 days of both night‐shift week and day‐shift week (8 exposures in total) Control/comparison intervention: Placebo (of a 3 mg melatonin capsule) taken off‐shift, 1 hour before bedtime (Part of a three‐armed trial, with the other arm of the intervention being a 3 mg melatonin capsule. This aspect of intervention reported in Cochrane Review Liira 2014 |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift:
|
|
| Notes | Funding: Unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated method" (according to author email) |
| Allocation concealment (selection bias) | Low risk | "The randomization code was kept in sealed envelopes" (according to author email) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subjects blinded to medication (melatonin or placebo), but no information available on light treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information regarding light intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of the 38 included persons, 17 completed the study (45%). The others did not participate or did not complete the study for the following reasons: (i) did not want to participate (8 persons), (ii) on sick leave (3 persons), (iii) stopped working this shift schedule (5 persons), (iv) quit or on leave (3 persons), or dropped out (2 persons, 1 claiming the study protocol took too much time and 1 wanting to take melatonin regularly during the work periods)" "In order to retain as many participants as possible in the analysis, we replaced missing data with careful estimates. If data from, for example, night 3 were missing, an average of night 2 and 4 was inserted. If night 7 or day 7 was missing, night or day 6 was inserted. If night 1 or day 1 was missing, night 2 or day 2 was inserted. The total number of missing data that were corrected varied between 1.1% and 3.6%, except for the recorded intake of coffee and tea, for which 8.0% of the data were missing" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | KSS; ATS; RT; Sleep diary; Actiwatch |
| Other potential sources of bias | Low risk | No test for interaction of order reported. We consider a 3 to 4 week washout period to be adequate |
Boivin 2012.
| Methods |
Trial design: Randomised controlled trial Intervention setting: Police patrol cars Shift system: 35‐day roster. Succession of 8‐8.5 hr shifts according to a predetermined sequence: 3 evening shifts; 2 rest days; 4 day shifts; 2 rest days 7 night shifts (starting at 22:00, 22:30, 23:00, or 23:30 and lasted 8 to 8.5 hr) 6 rest days; 4 evening shifts; 2 rest days; 3 day shifts; 2 rest days Follow‐up period (intervention plus follow‐up): approx. 18 days (5‐7 day preparatory phase; first 48‐hour in‐laboratory assessment; 7 nights of intervention/control; second 48‐hour in‐laboratory assessment) (Washout period): Not relevant* |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: None reported Number screened: Not reported Number eligible: Not reported Number included in our analysis: n = 17 (this includes both RCT participants (n = 15) and RXO participants (n = 2)) Industry: Police officers on patrol Age in years (mean ± SD): 29.8 ± 6.5 (intervention); 30.3 ± 4.1 (control) (n = 17*) Gender: 53% female Country: Canada Month(s) study conducted: not reported, but "season of study...comparable between groups" "Chronotype" score: 49 ± 9 (intervention); 47 ± 12 (control) (mean ± SD; n = 17) *data analysis includes two male police officers who completed both intervention and control conditions, at a 1‐year interval |
|
| Interventions |
Intervention: Exposure to full‐spectrum bright, white light via a portable lamp; wearing of orange‐tinted goggles; instructed to keep stable 8 hour daytime sleep episode from 2 hours after the end of the night shift; darkened bedroom windows Shift‐based timing: Light: Night (on‐shift) Goggles: Morning, from sunrise until beginning of daytime sleep episode at home Hours of intervention: Between 22:00 and 05:30 Dose/frequency/duration: Unclear lux, "intermittently" over 8 to 8.5 hour period, "intermittent" exposure per night for 7 nights Control/comparison intervention: No light; no goggles; no sleep instructions (but darkened bedroom windows) |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: Outcome not examined Sleep quality off‐shift: Outcome not examined Sleepiness on‐shift:
|
|
| Notes | Funding: Institute de recherche Robert‐Sauvé en santé et en sécurité du travail; Canadian Institutes for Health Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sleepiness: PVT data: intervention group (n = 8), control group (n = 9). "Nine missing data points were estimated in order to include all police officers in the statistical analysis" "Light exposure during the ambulatory period was analysed independently based on data collected from the actiwatch and the light sensor. Periods during which the device was removed when data were lost due to technical problems for ≥ 3.5 consecutive hours were discarded. For the actiwatch, all data were retained except for two participants (1i, a period of 5 hr and 7i, a period of 5 and 9 hr). For the light sensor, data from eight participants were discarded, for periods ranging from 3.5 to 39 consecutive hours, leaving ≥ 72.9% of data for each participant" |
| Selective reporting (reporting bias) | Low risk | P values given for all statistically significant outcomes but not all of those without statistical significance; detailed data not presented |
| Outcome reliably or objectively measured | Low risk | Sleepiness measured through Psychomotor Vigilance Task and through a VAS |
| Other potential sources of bias | High risk | Mixed study type ‐ 2 participants participated in both groups, in a cross‐over fashion, with a one‐year interval between the two conditions |
Harma 1988.
| Methods |
Trial design: Randomised controlled trial Intervention setting: Hospital ward Shift system: Three‐week shift cycle: day, evening, and night shifts were irregularly placed, allowing the direction of rotation to vary, and the same shift could occur either once or several times in succession. Shift length was 8 hours in the morning and evening shifts and 10 hours in night shifts. On average there were seven day shifts, five evening shifts, and three night shifts in a shift cycle (the number of different shifts in a shift cycle was similar in the training and control groups and the irregularity of the schedules was kept constant during the intervention) Follow‐up period (intervention plus follow‐up): 4 months (Washout period): Not relevant |
|
| Participants |
Inclusion criteria: At least 1.5 years' experience in shift work; age 20‐49 years; working as a nurse or nursing aide in the University Hospital of Kuopio, Finland Exclusion criteria: None reported Number screened: n = 428 Number eligible: n = 151 ("n = 119 volunteered...training and control groups were formed...") Number included in our analysis: n = 75 Industry: Hospital Age in years (mean ± SD): 34.6 ± 6.8 (intervention); 35.7 ± 6.5 (control) Gender: 100% women Country: Finland Month(s) study conducted: Not reported "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Physical exercise programme (individualised training according to submaximal ergometer test, age and sport habits of the individual. Individuals jogged, ran, swam, skied, and walked and or did gymnastics at 60% of heart rate max and increased to 70% heart rate max during the last month) Shift‐based timing: Not reported Hours of intervention: Not reported Dose/frequency/duration: 2‐6 training sessions per week for 4 months Control/comparison intervention: No physical exercise programme |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift: Relevant outcome not measured (assessed retrospectively, not during shifts) |
|
| Notes | Funding: The Finnish Work Environment Fund and the Finnish Medical Board | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participation in the training or the control groups was randomised in every separate group of three similar sets, with two subjects joining the training and one subject joining the control group. If there were only two similar sets, one joined the training and the other the control group |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 dropouts in the training group were accounted for by sickness (n = 5), pregnancy (n = 3), and unwillingness to continue (n = 6) 12 dropouts in the control group were sickness (n = 1), pregnancy (n = 1), absence from work (n = 3), and unwillingness to continue (n = 6) The characteristics of the final groups (training and control) were similar |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Unclear risk | All outcomes based on “a questionnaire”, with no indication of its/their validity Authors do write:"The quality and length of sleep were investigated using, as the basis, Kleitman's (1963) theory that the main components of sleep quality are difficulty in falling asleep, interrupted sleep, and the refreshing, restorative effect after waking." |
| Other potential sources of bias | Low risk | Not relevant |
Howard 2010.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Sleep disorders research unit Shift system: At least one night shift per fortnight during the six months preceding the study (21:00‐07:00) Follow‐up period (intervention plus follow‐up): 1 night (Washout period): Minimum of 2 weeks |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: Visual impairment that did not correct with eye‐glasses; regularly used sedative medications; history of sleep apnoea or clinical features of sleep apnoea; chronic sleepiness (score greater than 10 on the Epworth Sleepiness Scale) Number screened: Not reported Number eligible: Not reported Number included in our analysis: n = 8 Industry: Sleep research Age in years: (mean ± SD): 31 ± 9.6 Gender: 75% female Country: Australia "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to a nap Shift‐based timing: Night (on‐shift) Hours of intervention: 04:00 Dose/frequency/duration: 30 minutes per exposure,1 exposure per night for 1 night Control/comparison intervention: No‐nap
|
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: Outcome not examined Sleep quality off‐shift: Outcome not examined Sleepiness on‐shift:
|
|
| Notes | Funding: VicRoads | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "alternating sequence" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not advised in advance which condition they would be participating in on a given night (of 3 possibilities) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information (see Applicability of design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants completed all experimental conditions |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Karolinska Sleepiness Scale; Psychomotor Vigilance Task Test |
| Other potential sources of bias | Unclear risk | We deem a 2‐week washout period sufficient to avoid a possible carry‐over effect. However, no main effect testing was reported for period effect Notable: "...sleep inertia...may have played a role in the lack of significant performance improvement following the morning nap in the current study" |
Huang 2013.
| Methods |
Trial design: Randomised controlled trial Intervention setting: Hospital Shift system: Three‐shift rotation (evening/night shift) Follow‐up period (intervention plus follow‐up): approx. 14 days (Washout period): Not relevant |
|
| Participants |
Inclusion criteria: Rotating‐shift female nurses working the evening/night shift; 3‐shift rotation including day, evening, and night shifts in the most recent 6 months; pre‐treatment Insomnia Severity Index score > 14 (so, having clinical insomnia) Exclusion criteria: None reported Number screened: n = 102 Number eligible (% of screened): n = 92 (90.2%) Number included in our analysis: n = 30 night‐shift workers (out of n = 92 night‐shift and evening‐shift workers) Industry: Nursing Age in years (mean ± SD): 30.2 ± 4.5 (intervention); 30.0 ± 4.7 (control) (n = 92) Gender: 100% female Country: Taiwan Month(s) study conducted: 1 May, 2009 to 31 March, 2010 "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to bright light via a light box; dark sunglasses with ultraviolet (UV) protection Shift‐based timing: Light: night (on‐shift); glasses: morning after work and before sleep, including days‐off Hours of intervention: Between 23:00 and 00:00 Dose/duration/frequency: 7000‐10,000 lux, ≥ 30 minutes per exposure, 1 exposure per night for 10‐14 nights Control/comparison intervention: No bright light (but did wear glasses) |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: Outcome not examined Sleep quality off‐shift:
Sleepiness on‐shift: Outcome not examined |
|
| Notes | Funding: Chang Gung Memorial Hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random digit table |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This study was not a double‐blind study. The subjects in both groups might work in the same unit, and the use of a sham light box (a light box of a much lower intensity or red light) in the control group would be able to be detected by the controls, who would discern the difference" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sleep quality: “A total of 92 rotating‐shift female hospital nurses ...were recruited...forty‐six subjects were in the treatment group, and the remainder were in the control group. All subjects completed the study procedure reported by themselves” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Insomnia Severity Index (ISI)...developed by Morin, is a 7‐item self‐rated scale designed to assess subjective perception of the severity of insomnia (Morin 1993) |
| Other potential sources of bias | Low risk | Not relevant |
Karchani 2011.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Metal production plant Shift system: 2 morning shifts, 2 evening shifts, 2 night (22:00‐06:00) shifts, 2 days‐off; repeat Follow‐up period (intervention plus follow‐up): 2 nights intervention, 2 nights control (Washout period): 6 days |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: Disease and long‐term drug use Number screened: Not reported (but "93 shift workers...volunteered to participate...") Number eligible: n = 90 Number included in our analysis: n = 90 Industry: Metal production operation Age in years (mean ± SD): 30.34 ± 6.34 (Group 1); 30.49 ± 5.81 (Group 2) Gender: 100% male Country: Iran Month(s) study conducted: Not reported "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to bright white light during work break via fluorescent ceiling bulbs Shift‐based timing: Night (on‐shift) Hours of intervention: 22:00, 00:00, 02:00, 04:00 Dose/duration/frequency: 2500‐3000 lux, 15 minutes per exposure, 4 exposures per night for two nights Control/comparison intervention: Normal light |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: Outcome not examined Sleep quality off‐shift: Outcome not examined Sleepiness on‐shift:
|
|
| Notes |
Funding: The Research Department of Tehran University of Medical Sciences Karchani and Sadeghniiat‐Haghighi share a co‐author (Karchani) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Consenting participants were randomized into two groups..." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "One limitation of our study was that the participants were completely informed about the study’s goals and procedures which resulted in the lack of any real placebo effect. It is possible that this may have had an effect on the results" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The study population was 93 shift workers who volunteered to participate in the investigation...Ninety of the workers were included in the ultimate analysis and three subjects were excluded from the study because of disease and long‐term drug use." "All of the workers participated in both stages" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Stanford Sleepiness Scale |
| Other potential sources of bias | Low risk | "There was no significant difference in period effect and carry‐over effect, which shows that primacy or subsequence of light encounter, has no effect on the final results in both groups" |
Lowden 2004.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Truck production plant Shift system: Four consecutive 5‐day weeks of night shifts (00:00‐06:30). Weekends‐off. Night shifts 6.5 hours long except for the first shift of each week, which started at 21:45 hours (Sunday evening) and lasted 8.75 hours Follow‐up period (intervention plus follow‐up): Intervention: 15 days (washout unclear) (Washout period): Unclear, possibly 3 months ("One group obtained...bright light...in the spring...a similar treatment in autumn") |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: None reported Number screened: n = 24 Number eligible: Not reported Number included in our analysis: n = 18 (n = 15‐18, depending on outcome) Industry: Truck production operation Age in years: (mean (range)): 36.2 (24‐56) Gender: 94% male Country: Sweden Month(s) study conducted: "spring" and "autumn" "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to bright light via fluorescent ceiling tubes in break room Shift‐based timing: On‐shift (night) Hours of intervention: During break. Workers were permitted 2 short breaks at night (2 x 10 min. (plus an additional 10 min. on Mondays)), but were also allowed to leave workstation for shorter periods. The timing of breaks was self‐chosen Dose/duration/frequency: 2500 lux, 10 minutes per exposure, 2 exposures per night for 15 nights Control/comparison intervention: Normal light during break |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift:
|
|
| Notes | Funding: The Swedish Work Environment Fund and the Volvo Powertrain Co‐operation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The workers "were assigned to two groups (blocked randomisation using cards) for the order of treatment presentation" (according to author email) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The subjects in the present study were aware of the two conditions and thus the study lacked a true placebo condition. It is likely that this could have influenced the results" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors note that, " (of the original 24 volunteers) six workers had to be excluded in the final analysis because of sickness (1 worker), change of work (1 worker), change of individual work schedule (3 workers) and personal reasons (1 worker). Thus, 18 workers remained for analysis." However, authors also report (via email) that, "We...tried to obtain the following: equal overall number of subjects starting with each condition, equal subjects measured at the same period in both conditions to control for climate, time of year, etc., also maintaining similar design balance within each of the three teams" In addition, authors report, "As some workers showed missing data on Fridays, this day was omitted from the analysis." No further mention of how differential or non‐differential this omission might have been |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Karolinska Sleepiness Scale; Karolinska Sleepiness Diary; actigraphy |
| Other potential sources of bias | Low risk | Could not identify an interaction test based on order and an outcome, however text suggests that washout period was at least 3 months: "Workers were randomly assigned to two groups in a cross‐over design. One group obtained BL in the spring and the other group received normal indoor light (NL). A similar treatment was undertaken in the autumn" |
Oriyama 2014.
| Methods |
Trial design: Randomised controlled trial Intervention setting: General hospital ward Shift system: Three‐shift system, with 7‐8 night shifts every month Night shift “8‐h”: either 00:00‐08:45 or 00:30‐09:15. The day before the night shift was a day‐off. Study carried out on the first “day” of the night shift. Break of 60 minutes allowed between 01:00 and 06:00, either all at once or divided up Follow‐up period (intervention plus follow‐up): 1 night |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: None reported Number screened: Not reported Number eligible: Not reported Number included in our analysis: n = 15 Industry: Nursing Age in years (mean ± SD): 23.00 ± .92 (intervention); 23.71 ± 1.88 (control) (P = 0.46) Gender: 100% female Country: Japan "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to a nap Shift‐based timing: Night (on‐shift) Hours of intervention: between 02:30 and 03:30 and between 04:30 and 05:45 Dose/frequency/duration: 15 minutes per exposure/2 exposures per night for 1 night Control/comparison intervention: No‐nap |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: See "Selective reporting" in 'Risk of bias' table below Sleep quality off‐shift: Outcome not examined Sleepiness on‐shift:
|
|
| Notes | Funding: Ministry of Education, Culture, Sports, Science, and Technology of Japan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The nurses were randomly allocated to the two (Nap and No‐nap condition) groups |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N = 15 included in all analyses. No mention of any (relevant) missings or exclusions, but number screened and eligible not reported |
| Selective reporting (reporting bias) | Unclear risk | "The researcher also noted and recorded...sleeping time in the daytime following the night shift." We understand this outcome to refer to sleep duration. It is not clear which instrument(s) was/were used to measure this outcome. Outcome not reported in analysis |
| Outcome reliably or objectively measured | Low risk | VAS |
| Other potential sources of bias | Low risk | Not relevant |
Ross 1995.
| Methods |
Trial design: Randomised controlled trial Intervention setting: Antarctic research base Shift system: One week of night shift, usually twice during the year Follow‐up period (intervention plus follow‐up): 5 weeks (1 week prior to night shift, 1 week of night shift and the first, second, and third weeks after night shift) |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: None reported Number screened: Not reported Number eligible: Not reported Number included in our analysis: n = 13 (out of n = 14 overall) Industry: Geophysical research Age in years: 21‐35 Gender: 100% male Country: Antarctica Months study conducted: Late March to mid‐September "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to full‐spectrum white light Shift‐based timing: On‐shift (day) Hours of intervention: 11:00‐13:00 Dose/duration/frequency: 2500‐3000 lux, 2 hours per exposure, 1 exposure per day for 7 days Control/comparison intervention: Exposure to dim red light ‐ lux unclear (Abstract and text report different lux values: "> 500 lux", Fig 1a: "> 300 lux", Table 1: "< 300 lux") |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift:
|
|
| Notes | Funding: Supported by the British Antarctic Survey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. It is unclear whether the participants knew that the light intensity differed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One (subject) did not complete any sleep logs, but otherwise participated fully White light group n = 8, red light group n = 7, except week 1 where incomplete data were obtained from two subjects (one in each group) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Unclear risk | Sleep log; VAS; Mood scale (used to measure sleepiness on‐shift) ‐ no indication that this is a validated tool |
| Other potential sources of bias | High risk | "Two subjects appear twice in the study, each time in a different treatment group, due to the nature of the base rota. They are treated as separate subject‐period data in the data analysis" |
Sadeghniiat‐Haghighi 2011.
| Methods |
Trial design: Randomised cross‐over trial (randomisation confirmed through contact with author) Intervention setting: Ceramic factory Shift system: Two 12‐hour day shifts (06:00–18:00) followed by two days off‐work, and then two 12‐hour night shifts (18:00–06:00); the schedule was then repeated. Average working time per month was 220 hours Follow‐up period (intervention plus follow‐up): Not entirely clear if intervention lasted only one night (of the two night shifts) or both nights (Washout period): 4 days |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: None reported Number screened: n = 97 Number eligible: n = 97 Number included in our analysis: n = 94 Industry: Ceramic production plant operation Age in years (mean (range)): 33 (21‐45) Gender: 100% male Country: Iran Month(s) study conducted: Not reported "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to full‐spectrum white light via fluorescent ceiling tubes in break room Shift‐based timing: Night (on‐shift) Hours of intervention: 00:30 and 02:30 Dose/duration/frequency: 2500 lux, 20 minutes per exposure, 2 exposures per night for 1 night Control/comparison intervention: Normal light (300 lux) during breaks. Break room similar with respect to temperature, colour, and general ambience |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: Outcome not examined Sleep quality off‐shift: Outcome not examined Sleepiness on‐shift:
|
|
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors additional information indicates that study was random |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “One limitation of this study was that the participants were aware of the two conditions and thus the study lacked a true placebo condition. It is possible that this may have had an effect on the results” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three workers had to be excluded from the final analysis due to personal reasons |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Stanford Sleepiness Scale |
| Other potential sources of bias | Unclear risk | No information on interaction The washout period of 4 days is relatively short, but unlikely to have had a physiological carry‐over effect |
Smith 2007.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Hospital Shift system: Blocks of night shifts (20:30‐07:00) over 1‐3 consecutive days Follow‐up period (intervention plus follow‐up): Minimum 16 days (Washout period): Minimum of 1 week |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: None reported Number screened: Not reported Number eligible: Not reported Number included in our analysis: n = 9 Industry: Nursing and medical science Age in years) (mean ± SD): 45.7 ± 13.2 Gender: 66% female Country: Australia Month(s) study conducted: Not reported "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to a nap Shift‐based timing: Night (on‐shift) ‐ first night of (potential 3‐night block) Hours of intervention: Between 02:00 and 03:00 Dose/duration/frequency: 30 minutes per exposure, 1 exposure per night for 1 night Control/comparison intervention: No‐nap (and no corresponding break) |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift: Outcome not examined Sleep quality off‐shift: Outcome not examined Sleepiness on‐shift:
|
|
| Notes | Funding: The School of Psychology, The University of Queensland, and the Sleep Disorders Centre, The Prince Charles Hospital, Brisbane, Queensland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The experiment used a randomized, controlled, crossover design." "The order of the conditions was randomized ahead of the experiment – this was done using a random number generator function in Excel in blocks of 4 to counterbalance the order across participants (e.g. the order could have been 1100, 0011, 1010, 0101 in each block). The allocation sequence was known to one investigator (not at the hospital site and never meeting the participants)" |
| Allocation concealment (selection bias) | Low risk | "The allocations were put in sealed and numbered envelopes, in a box kept at the study site. Another investigator (at the hospital site and conducting the study) opened the envelope on the day prior to each participant’s first condition" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Participants were unaware of which condition (Nap or No‐nap) that they were undergoing until the night of testing." But they eventually did know. No testing was reported for a possible period effect. Since napping impossible to blind in a cross‐over design, this is the only way to check for the impact of participants knowing about the nap they are not receiving on the effect estimate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of relevant missing data (electroencephalogram (EEG) during nap missing, but not relevant for this review). Numbers screened and eligible not reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | "The VAS and pictorial sleepiness scale scores were significantly correlated (r = 0.84, P < 0.01) and were averaged to create an overall subjective sleepiness score out of 100" |
| Other potential sources of bias | Unclear risk | We deem a washout of at least one week sufficient to avoid physiological carry‐over effect of nap. However, although the authors report that, "The order of the conditions was randomized, and counterbalanced across participants", no period effect testing was reported (see Blinding of participants and personnel) |
Smith‐Coggins 1997.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Hospital emergency department Shift system: "Each subject had 10‐16 8‐ or 9‐hour shifts per month with 4‐5 of those being night shifts. The nights were lumped in blocks of 3 and 2, although 2 of the physicians preferred their nights in 1 block of 5. No attempts were made to control the pattern of shifts for baseline and active placebo evaluations because the random nature of shifts vs strict adherence to chronobiologic scheduling was one aspect that was being tested" Follow‐up period (intervention plus follow‐up): Time between baseline data collection and experiment begin: not reported; 1 month experimental intervention and 1 month placebo control (Washout period): 1 month |
|
| Participants |
Inclusion criteria: None reported Exclusion criteria: None reported Number screened: Not reported Number eligible: n = 8 (assumed from "Six faculty members out of a clinical faculty of 8 participated") Number included in our analysis: n = 6 Industry: Hospital emergency care Age in years (mean ± SD): 34 ± 2.0 Gender: 100% male Country: USA Month(s) study conducted: Not reported "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to a 3‐component fatigue countermeasure programme: (1) educational session with information on sleep physiology, circadian rhythms, good sleep hygiene, chonobiologic principles of scheduling; (2) improved shift schedule design*; and (3) 31 countermeasure strategies to maintain alertness and performance during work Shift‐based timing: Not reported Hours of intervention: Not reported Dose/frequency/duration: 2‐hour education session Control/comparison intervention: "Jet lag diet" (considered active placebo); 2‐hour general information on normal sleep physiology and circadian rhythms *this study also included in forthcoming Shift‐Schedule Review |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift:
|
|
| Notes | Funding: Emergency Medicine Foundation, the Society for Academic Emergency Medicine, NIH Grant MH44193 and the Institute for Experimental Psychiatry Research Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All the subjects …were randomly assigned to either group A or B to do experimental intervention or active placebo intervention, respectively" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The subjects were blinded to the fact that the diet was an active placebo." "...performance tests done by persons blinded to group" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Polysomnographic data: Due to a technical problem, 25% of the baseline polysomnographic data were lost. Analysis was completed with the remaining baseline data and complete postintervention data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Psychomotor Vigilance Test Polysomnograph |
| Other potential sources of bias | High risk | "The question of order effect was addressed by the use of counterbalancing in the within‐subjects design" "Since the subjects had been in medicine for a decade, they had learned many of the suggested countermeasures by trial and error and had already incorporated these principles into their daily habits. It may have been difficult for the subjects to give up the strategies during the active placebo and this may have decreased the difference between the 2 conditions" |
Smith‐Coggins 2006.
| Methods |
Trial design: Randomised controlled trial Intervention setting: Hospital emergency department Shift system: 3 consecutive 12‐hour night shifts Follow‐up period (intervention plus follow‐up): 11 days |
|
| Participants |
Inclusion criteria: Resident physicians and nurses working at least 3 consecutive 12‐hour night shifts in the emergency department Exclusion criteria: None reported Number screened: Not reported Number eligible: n = 53 Number included in our analysis: n = 49 Industry: Emergency room health care Age in years (mean ± SD): 30 ± 5.5 (intervention); 30 ± 4.3 (control) Gender: 81% female (intervention); 52% female (control); Country: USA Month(s) study conducted: June 2001 to June 2002 "Chronotype" or morningness/eveningness score: No preference for morning or evening work (Owl and Lark Questionnaire) |
|
| Interventions |
Intervention: Exposure to a nap Shift‐based timing: Night (on‐shift) Hours of intervention: Between 03:00 and 04:00 Dose/frequency/duration: 40 minute exposure, 1 exposure per night for 1 night Control/comparison intervention: No‐nap |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift: outcome not examined Sleepiness on‐shift:
|
|
| Notes | Funding: None reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | …subjects were randomised into “nap” or “no‐nap” groups, using a 50:50 randomisation allocation ratio (assuming that this means blocked randomisation done) |
| Allocation concealment (selection bias) | Low risk | Investigators created sealed envelopes containing concealed assignment codes given sequentially to eligible subjects by a research associate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subjects and researchers were blinded as to group assignment until 11 p.m. of night 3 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Polysomnographic data were analysed by an experienced technologist blinded to the protocol, but for remainder of outcomes, no information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure E1: N = 53 eligible ‐4 who withdrew n = 49 randomised n = 26 nap; n = 23 no‐nap n = 0 lost to follow‐up n = 0 excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Polysomnography; Psychomotor Vigilance Task; Profile of Mood States; Karolinska Sleepiness Scale; Daily sleep/wake diary; Actiwatch; CathSim (authors note "the construct and content validity of CathSim intravenous insertion virtual reality simulation have been established") |
| Other potential sources of bias | Low risk | Not relevant |
Tanaka 2011.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Hospital ward Shift system: Rapidly rotating cycle: (2‐3 consecutive day shifts, 1 day‐off, 1‐2 consecutive night shift(s) (16:30‐08:30), 1 day‐off Follow‐up period (intervention plus follow‐up): 2 months plus one week (Washout period): 1 week |
|
| Participants |
Inclusion criteria: Age 20‐60 yrs; working a two‐shift system Exclusion criteria: Individuals with sensitivity to bright light, eye disorders including asthenopia or who reported headaches or mood disorders; senior nursing officers Number screened: n = 276 Number eligible: Not reported Number included in our analysis: n = 61 Industry: Nursing Age in years (mean ± SD): 29.7± 8.6 Gender: 100% Female Country: Japan Month(s) study conducted: Beginning of June to beginning of August 2006 "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to bright light via a light box Shift‐based timing: Day (on‐shift) Hours of intervention: 07:30‐08:00 Dose/duration/frequency: 5444‐8826 lux (with illumination at 40‐30 cm from the light source), 10 minutes per exposure, 1 exposure each day‐shift workday for one month Control/comparison intervention: Normal light (530 and 648 lux, based on measured values in a windowless nurses' station room) |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift:
|
|
| Notes | Funding: Japan Society for the Promotion of Science | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomly assigned to one of two groups: a group for BL exposure in the first half of the study and a group for BL exposure in the second half. Random assignment was performed using a permuted block method with a block size of four. A random number sequence was generated by a computer. A research assistant with not direct contact with participants was responsible for generating the random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Self‐reported limitations: "open‐label trials involve potential biases resulting from difference in management, intervention, or assessment of participants that may arise due to participants or investigators knowing about the assigned intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Evaluators were masked to allocation" (Email from author) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between‐group analysis with regard to BL exposure was performed based on intention‐to‐treat “The PVT values were excluded from the analysis since the PVT was administered to only 11 participants” |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported except for sleep diary outcomes, all P values of analyses (except sleep diary) given. However, sleep diary was not listed among the primary or secondary outcomes. Authors write: "In addition to the above items, participants were asked to keep a sleep diary...Sleep diary entries confirmed that actual waking times among most participants were between 05:30‐06:30." Otherwise, no mention of sleep diaries |
| Outcome reliably or objectively measured | Low risk | Karolinska Sleepiness Scale; Psychomotor Vigilance Task Test; VAS; Sleep diary |
| Other potential sources of bias | Low risk | “No significant main effect of order or interaction between BL and order were found for any items” |
Tapia 2011.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Sleep technology research laboratory Shift system: Only night shifts but not necessarily on consecutive nights. Typical night shift 18:00‐06:00 Follow‐up period (intervention plus follow‐up): 6 weeks (1 week prior to study entry, 2 weeks Intervention/control, 1 week washout, 2 weeks intervention/control) (Washout period): 1 week |
|
| Participants |
Inclusion criteria: Sleep technologists working night shifts at the Children’s Hospital of Philadelphia and the University of Pennsylvania Sleep Centers Exclusion criteria: Known bipolar disease, serious ocular disease (e.g. glaucoma, cataracts), current use of photosensitising medication, and untreated serious sleep disorders (e.g. narcolepsy, obstructive sleep apnoea) Number screened: Not reported Number eligible: Not reported Number included in our analysis: n = 18 Industry: Sleep laboratory technology Age in years (mean ± SD or SE (unclear)): 32.6 ± 8 Gender: 61% female Country: USA Month(s) study conducted: Not reported "Chronotype" or morningness/eveningness score: Not reported |
|
| Interventions |
Intervention: Exposure to bright white light via a light box; dark goggles when driving home; information (handout) on good sleep hygiene measures; dark plastic film to cover bedroom windows Shift‐based timing: Light: night (on‐shift); goggles: morning, following night shift Hours of intervention: "Night" Dose/ duration/ frequency: 10,000 lux, 180 minutes per exposure, 1 exposure per night for "2 weeks" Control/comparison intervention: "Normal light" |
|
| Outcomes |
Outcomes (measurement tool and timing) relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift:
|
|
| Notes | Funding: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomized using a computer system" (according to author email) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigators were blinded to the light exposure order." "Investigators were blinded to the randomization block" (according to author email) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sleep length/sleep quality: "…many (participants) were non‐adherent with wearing the actigraph devices and/or completing the sleep diaries as instructed. Therefore, we were unable to assess the effects of BL in sleep consolidation. Data that were collected, which possibly were not representative of the group, showed very irregular sleep wake patterns" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | ESS VAS |
| Other potential sources of bias | Unclear risk | No information on interaction The washout period of one week is relatively short, but unlikely to have had a physiological carry‐over effect |
Thorne 2010.
| Methods |
Trial design: Randomised cross‐over trial Intervention setting: Onshore at home following offshore oilrig platform work Shift system: Two weeks of night shift, followed by two weeks at home, two weeks of day shift, followed by two weeks at home. Repeat Follow‐up period (intervention plus follow‐up): 21 days (last 7 days of a 2‐ or 3‐week night‐shift schedule, 14 days at home after completion of the night shift) (Washout period): 6‐8 weeks |
|
| Participants |
Inclusion criteria: Working a 2–3 week night shift Exclusion criteria: On any medication known to affect the melatonin rhythm (b‐blockers, a‐blockers, calcium channel blockers, antipsychotics, benzodiazepines, antidepressants, barbiturates, and antiepileptic drugs) Number screened: Not reported Number eligible: n = 8 Number included in our analysis: n = 4 (n = 3‐4, depending on outcome) Industry: Offshore oilrig (drilling) Age in years: (mean ± SD): 46 ± 11 years (n = 8 from randomised subgroup before exclusions) Gender: 100% male Country: Norway Month(s) study conducted: May‐August ("were recruited") "Chronotype" or morningness/eveningness score: Horne‐Östberg Questionnaire score: 57 ± 8 |
|
| Interventions |
Intervention: Light: exposure to white polychromatic light via a light box; glasses: specialised light blocking sunglasses Shift‐based timing: Light: Day (off‐shift) Sunglasses: Morning (off‐shift) Hours of intervention: Light: Treatment 1 (T1): 13:00; T2: 12:00; T3: 11:00; T4: 10:00 Sunglasses: from end of last night shift until T1, then each morning from wake‐up until T2/T3/T4 Dose/duration/frequency: Light: 3000 lux, 60 minutes per exposure, 1 exposure per day; sunglasses: from wake‐up until light treatment Control/comparison intervention: No bright light, no sunglasses |
|
| Outcomes |
Outcomes (measurement tool and timing), relevant to current review: Sleep length off‐shift:
Sleep quality off‐shift:
Sleepiness on‐shift: Outcome not examined |
|
| Notes | For the purposes of this review, we included only data from the subgroup of n = 8 participants who were randomly allocated (these data obtained through contacting the author). A number of subjects recruited in the winter months took part in a non‐randomised version of the protocol and were not included here Funding: Japan Society for the Promotion of Science |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was a randomised cross‐over design. "First subject started the light treatment leg first, second subject started the no light treatment leg first, third subject started the light treatment first and so forth" (according to author email) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Subject motivation may also be very important given that it is virtually impossible to blind such light experiments. The subjects recruited in this field study were motivated to try out the light treatment hoping that it would reduce their complaints of feeling “jet‐lagged” upon returning home from night shift. This may have provoked them to provide more positive subjective sleep scores following the bright light treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sleep length/sleep quality: Out of n = 8 randomised participants, n = 6 were excluded (did not adapt to night shift (n = 2); completed only one leg of cross‐over study (n = 2); no actigraphy data obtained (n = 1). No sensitivity analysis of excluded participants |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Outcome reliably or objectively measured | Low risk | Sleep diary; actigraphy |
| Other potential sources of bias | Low risk | Washout period 6‐8 weeks |
ATS: Accumulated Time with Sleepiness BL: Bright Light KSS: Karolinska Sleepiness Scale PVT: Psychomotor Vigilance Task RT: Reaction Time RXO: Randomised crossover design SD: Standard Deviation VAS: Visual Analogue Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bjorvatn 1999 | Not a RCT. Comparison group was studied at a different time period than the treatment group. |
| Boivin 2002 | Participants were not randomised. |
| Boivin 2012a | Participants were not randomised. |
| Budnick 1995 | Participants were not randomised. |
| Costa1993 | Participants were not randomised. |
| Figueiro 2001 | Participants were not randomised. |
| Frey 2002 | There was an insufficient number of measurements for our inclusion criteria (sleepiness was not measured after shift ‐ only before and during shift). The authors note: "Repeated testing during and after a night on duty would have been of interest". |
| Hauck 2011 | Participants were not randomised. |
| Holbrook 1994 | No control group. |
| Jung 1987 | Participants were not randomised. |
| Järnefelt 2012 | Participants were not randomised. |
| Kerin 2005 | Participants were not randomised. |
| Lowden 2012 | Participants were not randomised. |
| Matsumoto 1994 | Not a RCT. |
| Morgan 2012 | No night‐shift group reported. |
| Purnell 2002 | Participants were not randomised. |
| Rahman2013 | Not a RCT. Comparison group was studied at a different time period than the treatment group. |
| Schweitzer 2006 | Wrong design. A nap versus no‐nap comparison was not possible in the field study. |
| Signal 2009 | Participants were not randomised. |
| Smith 2015 | Not a RCT. |
| Takahashi 1999 | No intervention. |
| Wilson 2007 | Participants were not randomised. |
| Youngstrom 2014 | Wrong population. Not shift workers, but rather people with jet lag. |
RCT: Randomised Controlled Trial
Characteristics of studies awaiting assessment [ordered by study ID]
Anglade 1994.
| Methods | Possibly RCT but unclear |
| Participants | 18 nurses |
| Interventions | Information on polyphasic sleep |
| Outcomes | Sleep quality |
| Notes | Publication is in French. Unclear whether or not participants were randomised |
Arora 2007.
| Methods | Prospective cohort study; pre‐call/post‐call analysis |
| Participants | Interns from a university inpatient medicine service, n = 58 |
| Interventions | SAFER programme intervention (60‐90 min lecture; SAFER: Sleep, Alertness, and Fatigue Education in Residency) |
| Outcomes | Sleep loss, recovery sleep (wristwatch activity monitors) |
| Notes | Unclear whether or not participants were randomised. Author did not respond to email requesting information about randomisation |
Campos 2010.
| Methods | Pre‐test/post‐test design |
| Participants | Professionals of urgency and emergency services, n = 12 |
| Interventions | 8 x 15 minute on‐site chair massage |
| Outcomes | Sleep quality (Pittsburgh Sleep Quality Index (PSQI)) |
| Notes | Methods and inclusion criteria unclear. Unable to locate either author with certainty |
Carlson 1991.
| Methods | Treatment group versus delayed‐treatment group study |
| Participants | Female shift workers from Chicago medical centres, n = 20 |
| Interventions | Education (sleep hygiene, relaxation techniques, stimulus control principles) |
| Outcomes | Sleep length |
| Notes | Unable to obtain full‐text. Awaiting full copy of dissertation promised via email by author |
Chang 2015.
| Methods | RCT |
| Participants | Nurses, n = 63 |
| Interventions | Nap 30 minutes |
| Outcomes | Sleepiness, sleep length |
| Notes | Full‐text arrived too late for review |
Kakooei 2010.
| Methods | Possibly a cross‐over design, but unclear |
| Participants | Shift work nurses at university hospital, n = 34 |
| Interventions | Bright light (4500 lux) during two breaks (21:15‐22:00 and 03:15‐04:00) or dim light (300 lux) |
| Outcomes | Subjective alertness (Karolinska Sleepiness Scale (KSS)) |
| Notes | Unclear whether or not participants were randomised, or precisely what the study design was. Author did not respond to email requesting information about randomisation |
Kamei 1994.
| Methods | Unclear |
| Participants | Healthy nurses of a psychiatric unit, n = 11 |
| Interventions | Bright light (> 2,500 lux at least for 30 min, 0:00‐1:30) |
| Outcomes | Self‐evaluated sleep (Oguri, Shirakawa and Azumi’s sleep inventory), sleep duration (sleep log) |
| Notes | Unclear whether or not participants were randomised. Unable to find contact information for either first or last author |
Pialot 2015.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | No abstract. Unable to locate author |
Shochat 2015.
| Methods | The study is a repeated measures design. Each participant took part in 2 nap nights and two non‐naps nights, in randomised orders (information based on email exchange with author) |
| Participants | Female and male nurses, n = 122 |
| Interventions | Nap 30‐40 minutes long, at 04:00 |
| Outcomes | Sleepiness, vigour |
| Notes | Publication expected soon |
van Drongelen 2014.
| Methods | RCT |
| Participants | Airline pilots n = 502 |
| Interventions | Electronic app of "tailored advice regarding exposure to daylight, sleep, physical activity, and nutrition, and aiming to improve health‐related behavior" |
| Outcomes | Sleep, fatigue |
| Notes | Full‐text arrived too late for review |
Yoon 2002.
| Methods | Cross‐over design |
| Participants | Night shift nurses, n = 12 |
| Interventions | Room light: light exposure during night, followed by 1 hour exposure to sunlight or 10,000 lux light the next morning Bright light: 4 hour nocturnal light exposure of 4,000‐6,000 lux (from 1:00 to 5:00), followed by 1 hour exposure to sunlight or 10,000 lux light the next morning Bright light with sunglasses: 4 hour nocturnal light exposure of 4,000‐6,000 lux (from 1:00 to 5:00), followed by light attenuation in the morning |
| Outcomes | Nocturnal alertness (VAS), daytime sleep (actigraphy) |
| Notes | Unclear whether or not participants were randomised. Author did not respond to email requesting information about randomisation |
RCT: Randomised Controlled Trial VAS: Visual Analogue Scale
Characteristics of ongoing studies [ordered by study ID]
Patterson 2014.
| Trial name or title | Mobile phone text messaging intervention to improve alertness and reduce sleepiness and fatigue during shiftwork among emergency medicine clinicians: study protocol for the SleepTrackTXT pilot randomised controlled trial |
| Methods | Single‐centre, two‐arm, parallel, single‐blind, randomised controlled trial |
| Participants | Adult emergency medical service workers, n = 100 |
| Interventions | Text‐message‐based intervention prompting behaviour change |
| Outcomes | Sleepiness, or fatigue, or both |
| Starting date | Unclear, protocol registered 10 January 2014 |
| Contact information | pattersond@upmc.edu |
| Notes | A study protocol |
Differences between protocol and review
We had intended to use the Stata Software (STATA 2011) for calculations not possible with Review Manager 5 (RevMan 2014), but instead were able to conduct our calculations with Microsoft Excel (2011).
We stated in our protocol that we would give preference to subjective measures of sleepiness, sleep quality, and sleep length (Herbst 2013). However, in order to be consistent with our partner publication on pharmacological interventions (Liira 2014), we gave priority to objective measures of these outcome variables. To this end, when a study reported an outcome in more than one way (e.g. sleep diary and actigraphy), we used the objective measure (actigraphy) in our main analysis.
We had intended to conduct sensitivity analyses according to a number of different factors, including 'Risk of bias' domains and model effects (random versus fixed). We had also intended to conduct subgroup analyses according to various factors, including chronotype, sleep health‐status, intervention details, age, and light characteristics (i.e. wavelength and intensity). Both the sensitivity and the subgroup analyses were precluded, however, due to too few trials per outcome. Due to lack of data, we did not conduct analyses of cost‐effectiveness or secondary outcomes. For the same reason, we did not analyse different ways of measuring the same outcome (subjective versus objective measures), as laid out in the protocol.
There were some changes in our author team between the publication of our protocol and this full Cochrane Review. Christine Herbst and Melissa Koch departed the team whilst we added Tracy Slanger, J. Valérie Gross, Andreas Pinger, Peter Morfeld, Miriam Bellinger, Anna Duhme and Rosalinde Reichardt.
Contributions of authors
Juha Liira (JL) and Thomas Erren (TE) conceived the protocol. TE co‐ordinated the protocol. TE wrote the protocol, together with an earlier member of the department. Mikael Sallinen (MS) and Lin Fritschi (LF) supported the team by defining the outcomes. LF and Tim Driscoll (TD) gave methodological advice. TE, MS, LF, TD, JL, Giovanni Costa (GC), and Russel Foster (RF) commented on and contributed to all protocol drafts.
Tracy Slanger (TS), J. Valérie Groß (VG), Miriam Bellinger (MB), Anna Duhme (AD), and Rosalinde Amancay Reichardt Ortega (RR) undertook searches and identified potentially relevant trials.
TS, VG, MB, AD, RR, MS, and GC screened retrieved papers against the eligibility criteria. TE and Andreas Pinger (AP) helped when authors could not agree.
TS, VG, AP, MB, AD, and RR extracted and synthesised the data from the trials. TS wrote to authors for additional information. TS and VG assessed study quality (‘Risk of bias’ and GRADE). Peter Morfeld (PM), TS, and VG analysed and interpreted the data.
TS, VG, and TE wrote the systematic review. RF, AP, PM, MS, LF, GC, TD, and JL commented on the systematic review text.
Sources of support
Internal sources
-
Institute and Policlinic for Occupational Medicine, Environmental Medicine and Preventive Research, University of Cologne, Cologne, Germany, Germany.
Institutional support, in the form of salaries to Thomas Erren, Valérie Groß, Andreas Pinger and Tracy Slanger
-
Finnish Institute of Occupational Health, Finland.
Salary for Mikael Sallinen and Juha Liira
External sources
No sources of support supplied
Declarations of interest
Tracy Slanger: **
J. Valérie Gross: **
Andreas Pinger: **
Peter Morfeld: **
Miriam Bellinger: **
Anna Duhme: **
Rosalinde Reichardt: **
Mikael Sallinen: I have received a total of EUR 12,000 for five lectures on well‐being at work from the insurance company Ilmarinen. I have also received a total of EUR 2,000 for a review of applications submitted to the Petromaks Research Programme from the Research Council of Norway.
Lin Fritschi: none known.
Giovanni Costa: none known.
Tim Driscoll: none known.
Russell Foster: none known.
Juha Liira: none known.
Thomas Erren: **
** Shift work is regularly conducted at Evonik Industries and at the University Hospital of Cologne University. Since 1988, the University of Cologne and Evonik Industries (and their corporate predecessor, RAG) have had a public‐private partnership regarding research. The contributing research institutes, The Institute and Policlinic for Occupational and Environmental Medicine and Prevention Research (IPOEP) in Cologne and The Institute for Occupational Epidemiology and Risk Assessment (IERA) in Essen, select and conduct their research independently of one another. In Germany, scientific freedom in research, teaching and instruction, and study is constitutionally guaranteed. The right to the Freedom of Research and protection thereof are inseparably coupled with responsibility. The public‐private partnership contract between the University of Cologne and Evonik Industries explicitly emphasises the freedom in research and scientific publication of collaborating institutions.
New
References
References to studies included in this review
Atlantis 2006 {published data only}
- Atlantis E, Chow CM, Kirby A, Fiatarone Singh MA. Worksite intervention effects on physical health: a randomized controlled trial. Health Promotion International 2006;21(3):191‐200. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Chow CM, Kirby A, Fiatrone Singh M. Am effective exercise‐based intervention for improving mental health and quality of life measures: a randomised controlled trial. Preventive Medicine 2004;39:424‐34. [DOI] [PubMed] [Google Scholar]
- Atlantis E, Chow CM, Kirby A, Singh MA. Worksite intervention effects on sleep quality: a randomized controlled trial. Journal of Occupational Health Psychology 2006;11(4):291‐304. [DOI] [PubMed] [Google Scholar]
Bjorvatn 2007 {published data only}
- Bjorvatn B, Sangenes K, Oyane N, Forberg K, Lowden A, Holsten F, et al. Randomized placebo‐controlled field study of the effects of bright light and melatonin in adaptation to night work. Scandinavian Journal of Work, Environment & Health 2007;33(3):204‐14. [DOI] [PubMed] [Google Scholar]
Boivin 2012 {published data only}
- Boivin DB, Boudreau P, Tremblay GM. Phototherapy and orange‐tinted goggles for night‐shift adaptation of police officers on patrol. Chronobiology International 2012;29(5):629‐40. [DOI] [PubMed] [Google Scholar]
Harma 1988 {published data only}
- Harma M. Effects of improved physical‐fitness on perceived quality of sleep and wakefulness in shift‐work. In: Horne JA editor(s). Sleep '88. New York: Gustav Fischer Verlag, 1989:36‐8. [Google Scholar]
- Harma MI, Ilmarinen J, Knauth P, Rutenfranz J, Hanninen O. Physical training intervention in female shift workers: I. The effects of intervention on fitness, fatigue, sleep, and psychosomatic symptoms. Ergonomics 1988;31(1):39‐50. [DOI] [PubMed] [Google Scholar]
- Harma MI, Ilmarinen J, Knauth P, Rutenfranz J, Hanninen O. Physical training intervention in female shift workers: II. The effects of intervention on the circadian rhythms of alertness, short‐term memory, and body temperature. Ergonomics 1988;31(1):51‐63. [DOI] [PubMed] [Google Scholar]
Howard 2010 {published data only}
- Howard ME, Radford L, Jackson ML, Swann P, Kennedy GA. The effects of a 30‐minute napping opportunity during an actual night shift on performance and sleepiness in shift workers. Biological Rhythm Research 2010;41(2):137‐48. [Google Scholar]
Huang 2013 {published data only}
- Huang LB, Tsai MC, Chen CY, Hsu SC. The effectiveness of light/dark exposure to treat insomnia in female nurses undertaking shift work during the evening/night shift. Journal of Clinical Sleep Medicine 2013;9(7):641‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karchani 2011 {published data only}
- Karchani M, Kakooei H, Yazdi Z, Zare M. Do bright‐light shock exposures during breaks reduce subjective sleepiness in night workers?. Sleep and Biological Rhythms 2011;9:95‐102. [Google Scholar]
Lowden 2004 {published data only}
- Lowden A, Akerstedt T, Wibom R. Suppression of sleepiness and melatonin by bright light exposure during breaks in night work. Journal of Sleep Research 2004;13:37‐43. [DOI] [PubMed] [Google Scholar]
Oriyama 2014 {published data only}
- Oriyama S, Miyakoshi Y, Kobayashi T. Effects of two 15‐min naps on the subjective sleepiness, fatigue and heart rate variability of night shift nurses. Industrial Health 2014;52:25‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 1995 {published data only}
- Ross JK, Arendt J, Horne J, Haston W. Night‐shift work in Antarctica: Sleep characteristics and bright light treatment. Physiology & Behavior 1995;57(6):1169‐74. [DOI] [PubMed] [Google Scholar]
Sadeghniiat‐Haghighi 2011 {published data only}
- Sadeghniiat‐Haghighi K, Yazdi Z, Jahanihashemi H, Aminian O. The effect of bright light on sleepiness among rapid‐rotating 12‐hour shift workers. Scandinavian Journal of Work, Environment & Health 2011;37(1):77‐9. [DOI] [PubMed] [Google Scholar]
Smith 2007 {published data only}
- Smith A, Kilby S, Jorgensen G, Douglas J. Napping and nightshift work: Effects of a short nap on psychomotor vigilance and subjective sleepiness in health workers. Sleep and Biological Rhythms 2007;5:117‐25. [Google Scholar]
Smith‐Coggins 1997 {published data only}
- Smith‐Coggins R, Rosekind MR, Buccino KR, Dinges DF, Moser RP. Rotating shiftwork schedules: can we enhance physician adaptation to night shifts?. Academic Emergency Medicine 1997;4:951‐61. [DOI] [PubMed] [Google Scholar]
Smith‐Coggins 2006 {published data only}
- Smith‐Coggins R, Howard SK, Mac DT, Wang C, Kwan S, Rosekind MR, et al. Improving alertness and performance in emergency department physicians and nurses: the use of planned naps. Annals of Emergency Medicine 2006;48(5):596‐604. [DOI] [PubMed] [Google Scholar]
Tanaka 2011 {published data only}
- Tanaka K, Takahashi M, Tanaka M, Takanao T, Nishinoue N, Kaku A, et al. Brief morning exposure to bright light improves subjective symptoms and performance in nurses with rapidly rotating shifts. Journal of Occupational Health 2011;53:258‐66. [DOI] [PubMed] [Google Scholar]
Tapia 2011 {published data only}
- Tapia IE, Walsh CM, Meltzer LJ, Ndicu G, Karamessinis L, Cucchiara AJ, et al. Effect of light therapy on sleep technologist functioning. Sleep 2011;34:A167. [Google Scholar]
Thorne 2010 {published data only}
- Thorne HC, Hampton SM, Morgan LM, Skene DJ, Arendt J. Returning from night shift to day life: Beneficial effects of light on sleep. Sleep and Biological Rhythms 2010;8:212‐21. [Google Scholar]
References to studies excluded from this review
Bjorvatn 1999 {published data only}
- Bjorvatn B, Kecklund G, Åkerstedt T. Bright light treatment used for adaptation to night work and re‐adaptation back to day life. A field study at an oil platform in the North Sea. Journal of Sleep Research 1999;8:105‐12. [DOI] [PubMed] [Google Scholar]
Boivin 2002 {published data only}
- Boivin DB, James FO. Circadian adaptation to night‐shift work by judicious light and darkness exposure. Journal of Biological Rhythms 2002;17(6):556‐67. [DOI] [PubMed] [Google Scholar]
Boivin 2012a {published data only}
- Boivin DB, Boudreau P, James FO, Ng Ying Kin NMK. Photic resetting in night‐shift work: impact on nurses’ sleep. Chronobiology International 2012;29(5):619‐28. [DOI] [PubMed] [Google Scholar]
Budnick 1995 {published data only}
- Budnick LD, Lerman SE, Nicolich MJ. An evaluation of scheduled bright light and darkness on rotating shiftworkers:trial and limitations. American Journal of Industrial Medicine 1995;27:771‐82. [DOI] [PubMed] [Google Scholar]
Costa1993 {published data only}
- Costa G, Ghirlanda G, Minors D, Waterhouse J. Effect of bright light on tolerance to night work. Scandinavian Journal of Work, Environment & Health 1993;19:414‐20. [DOI] [PubMed] [Google Scholar]
Figueiro 2001 {published data only}
- Figueiro MG, Rea MS, Boyce P, White R, Kolberg K. The effects of bright light on day and night shift nurses' performance and well‐being in the NICU. Neonatal Intensive Care 2001;14(1):29‐32. [Google Scholar]
Frey 2002 {published data only}
Hauck 2011 {published data only}
- Hauck EL. Development and evaluation of a fatigue countermeasure training program for shiftworkers. Dissertation Abstracts International: Section B: The Sciences and Engineering. US: ProQuest Information & Learning, 2011; Vol. 72, issue 3‐B.
Holbrook 1994 {published data only}
- Holbrook MI, White MH, Hutt MJ. Increasing awareness of sleep hygiene in rotating shift workers: Arming law‐enforcement officers against impaired performance. Perceptual and Motor Skills 1994;79:520‐2. [DOI] [PubMed] [Google Scholar]
Järnefelt 2012 {published data only}
- Järnefelt H, Lagerstedt R, Kajaste S, Sallinen M, Savolainen A, Hublin C. Cognitive behavioral therapy for shift workers with chronic insomnia. Sleep Medicine 2012;13:1238‐46. [DOI] [PubMed] [Google Scholar]
Jung 1987 {published data only}
- Jung FD. The effects of a night shiftworker adjustment program on the sleeping patterns, sleep quality, health, and job performance of permanent night shiftworkers. www.search.proquest.com/docview/303439143?accountid=10218 (accessed 19 July 2016).
Kerin 2005 {published data only}
- Kerin A, Aguirre A. Improving health, safety, and profits in extended hours operations (shiftwork). Industrial Health 2005;43(1):201‐8. [DOI] [PubMed] [Google Scholar]
Lowden 2012 {published data only}
- Lowden A, Åkerstedt T. Assessment of a new dynamic light regimen in a nuclear power control room without windows on quickly rotating shiftworkers ‐ effects on health, wakefulness, and circadian alignment: A pilot study. Chronobiology International 2012;29(5):641‐49. [DOI] [PubMed] [Google Scholar]
Matsumoto 1994 {published data only}
- Matsumoto K, Harada M. The effect of night‐time naps on recovery from fatigue following night work. Ergonomics 1994;37(5):899‐907. [DOI] [PubMed] [Google Scholar]
Morgan 2012 {published data only}
- Morgan PJ, Collins CE, Plotnikoff RC, Cook AT, Berthon B, Mitchell S, et al. The Impact of a workplace‐based weight loss program on work‐related outcomes in overweight male shift workers. Journal of Occupational and Environmental Medicine 2012;54(2):122‐7. [DOI] [PubMed] [Google Scholar]
Purnell 2002 {published data only}
- Purnell MT, Feyer AM, Herbison GP. The impact of a nap opportunity during the night shift on the performance and alertness of 12‐h shift workers. Journal of Sleep Research 2002;11:219‐27. [DOI] [PubMed] [Google Scholar]
Rahman2013 {published data only}
- Rahman SA, Shapiro CM, Wang F, Ainlay H, Kazmi S, Brown TJ, et al. Effects of filtering visual short wavelengths during nocturnal shiftwork on sleep and performance. Chronobiology International 2013;30(8):951‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schweitzer 2006 {published data only}
- Schweitzer PK, Randazzo AC, Stone K, Erman M, Walsh JK. Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work. Sleep 2006;29(1):39‐50. [DOI] [PubMed] [Google Scholar]
Signal 2009 {published data only}
- Signal TL, Gander PH, Anderson H, Brash S. Scheduled napping as a countermeasure to sleepiness in air traffic controllers. Journal of Sleep Research 2009;18(1):11‐9. [DOI] [PubMed] [Google Scholar]
Smith 2015 {published data only}
- Smith K.C, Wallace DP, Gwin C, Maliszewski G. Improving the sleep of hospital‐based healthcare workers through an email‐delivered sleep wellness program. Sleep 2015;38:A226. [Google Scholar]
Takahashi 1999 {published data only}
- Takahashi M, Arito H, Fukuda H. Nurses' workload associated with 16‐h night shifts. II: Effects of a nap taken during the shifts. Psychiatry and Clinical Neurosciences 1999;53(2):223‐5. [DOI] [PubMed] [Google Scholar]
Wilson 2007 {published data only}
- Wilson MG, Polzer‐Debruyne A, Chen S, Fernandes S. Shift work interventions for reduced work‐family conflict. Employee Relations 2007; Vol. 29, issue 2:162‐77.
Youngstrom 2014 {published data only}
- Youngstrom E. Preliminary test of amber glasses as a way of resetting circadian melatonin release: Randomized trial during travel from Asia. Neuropsychopharmacology 2014;39:S562. [Google Scholar]
References to studies awaiting assessment
Anglade 1994 {published data only}
- Anglade J, Badet E, Becque G, Brugne JF, Burr C, Fernandez J, et al. Vigilance and quality of sleep in night nurses [Vigilance et qualité de sommeil des soignants de nuit]. Revue de L'Infirmiere 1994;17:37‐40. [PubMed] [Google Scholar]
Arora 2007 {published data only}
- Arora VM, Georgitis E, Woodruff JN, Humphrey HJ, Meltzer D. Improving sleep hygiene of medical interns: can the sleep, alertness, and fatigue education in residency program help?. Archives of Internal Medicine 2007;167(16):1738‐44. [DOI] [PubMed] [Google Scholar]
Campos 2010 {published data only}
- Campos E, Duque Neto S. The effect of chair massage on sleep quality of professionals in an urgency and emergency service. Journal of Sleep Research 2010;19:218‐9. [Google Scholar]
Carlson 1991 {published data only}
- Carlson Martha L. Sleep‐management training: An intervention program to improve the sleep of shift workers. Dissertation Abstracts International. US: ProQuest Information & Learning, 1991; Vol. 52, issue 1‐B.
Chang 2015 {published data only}
- Chang Yu San, Wu Yu Hsuan, Lu Mei Rou, Hsu Chung Yao, Liu Ching Kuan, Hsu Chin. Did a brief nap break have positive benefits on information processing among nurses working on the first 8‐h night shift?. Applied Ergonomics 2015;48:104‐8. [DOI] [PubMed] [Google Scholar]
Kakooei 2010 {published data only}
- Kakooei H, Ardakani ZZ, Ayattollahi MT, Karimian M, Saraji GN, Owji AA. The effect of bright light on physiological circadian rhythms and subjective alertness of shift work nurses in Iran. International Journal of Occupational Safety and Ergonomics 2010;16(4):477‐85. [DOI] [PubMed] [Google Scholar]
Kamei 1994 {published data only}
- Kamei Y, Ishizuka Y, Usui A, Watanabe T, Okado T, Nagasaka A, et al. The effect of bright light on self‐evaluation for sleep after night work. Japanese Journal of Psychiatry and Neurology 1994;48(2):496‐7. [Google Scholar]
Pialot 2015 {published data only}
- Pialot V. A new study on night‐shift work. Soins; La Revue de Reference Infirmiere 2015;793(Suppl):S3. [PubMed] [Google Scholar]
Shochat 2015 {published data only}
- Shochat T, Zion N. The effects of a scheduled nap during the nightshift on performance, sleepiness and vigor in nurses. Sleep Medicine 2013;14(S1):e266. [Google Scholar]
- Shochat T, Zion N. The effects of a scheduled nap on self‐reported sleepiness and vigor during the nightshift in female nurses working rotating 8‐HR shifts: A prospective field study. Sleep 2015;38:A207. [Google Scholar]
van Drongelen 2014 {published data only}
- Drongelen A, Boot CRL, Hlobil H, Twisk JWR, Smid T, Beek AJ. Evaluation of an mHealth intervention aiming to improve health‐related behavior and sleep and reduce fatigue among airline pilots. Scandinavian Journal of Work Environment & Health 2014;40(6):557‐68. [DOI] [PubMed] [Google Scholar]
Yoon 2002 {published data only}
- Yoon IY, Jeong DU, Kwon KB, Kang SB, Song BG. Bright light exposure at night and light attenuation in the morning improve adaptation of night shift workers. Sleep 2002;25(3):351‐6. [PubMed] [Google Scholar]
References to ongoing studies
Patterson 2014 {published data only}
- Patterson PD, Moore CG, Weaver MD, Buysse DJ, Suffoletto BP, Callaway CW, et al. Mobile phone text messaging intervention to improve alertness and reduce sleepiness and fatigue during shiftwork among emergency medicine clinicians: study protocol for the SleepTrackTXT pilot randomized controlled trial. Trials 2014;15:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Akerstedt 2002
- Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress, and work hour ‐ cross sectional study. Journal of Psychosomatic Research 2002;53(3):741‐8. [DOI] [PubMed] [Google Scholar]
Akerstedt 2011
Akerstedt 2014
- Akerstedt T, Anund A, Axelsson J, Kecklund G. Subjective sleepiness is a sensitive indicator of insufficient sleep and impaired waking function. Journal of Sleep Research 2014;23(3):240‐52. [DOI] [PubMed] [Google Scholar]
Altman 2011
Arendt 2010
- Arendt J. Shift work: coping with the biological clock. Occupational Medicine 2010;60(1):10‐20. [DOI] [PubMed] [Google Scholar]
Asaoka 2012
- Asaoka K, Fukuda T, Murphy TI, Abe T, Inoue Y. The effects of a nighttime nap on the error‐monitoring functions during extended wakefulness. Sleep 2012;35(6):871‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babkoff 2002
- Babkoff H, French J, Whitmore J, Sutherlin R. Single‐dose bright light and/or caffeine effect on nocturnal performance. Aviation, Space, and Environmental Medicine 2002;73(4):341‐50. [PubMed] [Google Scholar]
Baehr 1999
- Baehr EK, Fogg LF, Eastman CI. Intermittent bright light and exercise to entrain human circadian rhythms to night work. American Journal of Physiology 1999;277(6 Pt 2):R1598‐604. [DOI] [PubMed] [Google Scholar]
Banks 2015
- Banks S, Hilditch C, Centofanti S, Dorrian J. Napping on night shift: Powerful tool or hazard?. Eat, Sleep, Work 2015;1:pre‐press proof. [Google Scholar]
Barton 1995
- Barton J, Spelten E, Totterdell P, Smith L, Folkard S, Costa G. The Standard Shiftwork Index ‐ a battery of questionnaires for assessing shiftwork‐related problems. Work and Stress 1995;9(1):4‐30. [Google Scholar]
Basner 2011
- Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a Brief Psychomotor Vigilance Test (PVT‐B) to total and partial sleep deprivation. Acta Astronautica 2011;69:949‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonnet 1994
- Bonnet MH, Arand DL. Impact of naps and caffeine on extended nocturnal performance. Physiology & Behavior 1994;56(1):103‐9. [DOI] [PubMed] [Google Scholar]
Bonnet 1994a
- Bonnet MH, Arand DL. The use of prophylactic naps and caffeine to maintain performance during a continuous operation. Ergonomics 1994;37(6):1009‐20. [DOI] [PubMed] [Google Scholar]
Bonnet 1995
- Bonnet MH, Gomez S, Wirth O, Arand DL. The use of caffeine versus prophylactic naps in sustained performance. Sleep 1995;18(2):97‐104. [DOI] [PubMed] [Google Scholar]
Bonnet 1995a
- Bonnet MH, Arand DL. Consolidated and distributed nap schedules and performance. Journal of Sleep Research 1995;4(2):71‐7. [DOI] [PubMed] [Google Scholar]
Bougrine 1998
- Bougrine S, Mollard R, Ignazi G, Coblentz A. Days off and bright light: effects on adaptation to night work. International Journal of Industrial Ergonomics 1998;21(3‐4):187‐98. [Google Scholar]
Boyce 1997
- Boyce P, Beckstead JW, Eklund NH, Strobel RW, Rea MS. Lighting the graveyard shift: The influence of a daylight‐simulating skylight on the task performance and mood of night‐shift workers. Lighting Research and Technology 1997;29(3):105‐18. [Google Scholar]
Burgess 2002
- Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Medicine Reviews 2002;6(5):407‐20. [PubMed] [Google Scholar]
Buxton 2003
- Buxton OM, Lee CW, L’Hermite‐Baleriaux M, Turek FW, Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. American Journal of Physiology 2003;284:R714‐R24. [DOI] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193‐213. [DOI] [PubMed] [Google Scholar]
Cajochen 2010
- Cajochen C, Chellappa S, Schmidt C. What keeps us awake? The role of clocks and hourglasses, light, and melatonin. International Review of Neurobiology 2010;93:57‐90. [DOI] [PubMed] [Google Scholar]
Caldwell 1998
- Caldwell JA Jr, Caldwell JL. Comparison of the effects of zolpidem‐induced prophylactic naps to placebo naps and forced rest periods in prolonged work schedules. Sleep 1998;21(1):79‐90. [DOI] [PubMed] [Google Scholar]
Campbell 1995
- Campbell SS. Effects of timed bright‐light exposure on shift‐work adaptation in middle‐aged subjects. Sleep 1995;18(6):408‐16. [DOI] [PubMed] [Google Scholar]
Carskadon 1986
- Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep 1986;9(4):519‐24. [DOI] [PubMed] [Google Scholar]
Chinoy 2015
- Chinoy ED, Kim M, Hong JY, Wang W, Duffy JF. Effects of scheduled sleep and enhanced lighting during simulated shift work on circadian phase, performance, and subjective sleepiness in older adults. Sleep 2015;38:A75. [Google Scholar]
Costa 1993
- Costa G, Ghirlanda G, Minors DS, Waterhouse JM. Effect of bright light on tolerance to night work. Scandinavian Journal of Work, Environment & Health 1993;19(6):414‐20. [DOI] [PubMed] [Google Scholar]
Czeisler 1990
- Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. New England Journal of Medicine 1990;322(18):1253‐9. [DOI] [PubMed] [Google Scholar]
Dawson 1991
- Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep 1991;14(6):511‐5. [DOI] [PubMed] [Google Scholar]
Dawson 1995
- Dawson D, Encel N, Lushington K. Improving adaptation to simulated night shift: timed exposure to bright light versus daytime melatonin administration. Sleep 1991;18(1):511‐6. [DOI] [PubMed] [Google Scholar]
Della Rocco 2000
- Della Rocco P, Comperatore C, Caldwell L, Cruz C. The effects of napping on night shift performance. FAA Office of Aviation Medicine Reports 2000;DOT‐FAA‐AM‐00‐10:1‐33. [Google Scholar]
Dinges 1998
- Dinges DF, Mallis M, Maislin G, Powell JW. Evaluation of techniques for ocular measurement as an index of fatigue and as the basis for alertness management. National Highway Traffic Safety Administration 1998.
Eastman 1991
- Eastman CI. Squashing versus nudging circadian rhythms with artificial bright light: solutions for shift work?. Perspectives in Biology and Medicine 1991;34(2):181‐95. [DOI] [PubMed] [Google Scholar]
Eastman 1994
- Eastman CI, Stewart KT, Mahoney MP, Liu L, Fogg LF. Dark goggles and bright light improve circadian rhythm adaptation to night‐shift work. Sleep 1994;17(6):535‐43. [DOI] [PubMed] [Google Scholar]
Eastman 1995
- Eastman CI, Liu L, Fogg LF. Circadian rhythm adaptation to simulated night shift work: effect of nocturnal bright‐light duration. Sleep 1995;18(6):399‐407. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Englund 1990
- Englund C, Loving R, Kripke DF. Bright light amelioration of shift work. Annual Review of Chronopharmacology 1990;7:33‐6. [Google Scholar]
Erren 2013
- Erren TC, Herbst C, Koch MS, Fritschi L, Foster RG, Driscoll TR, et al. Adaptation of shift work schedules for preventing and treating sleepiness and sleep disturbances caused by shift work. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010639] [DOI] [Google Scholar]
Erren 2013a
- Erren TC, Morfeld P. Shift work and cancer research: a thought experiment into a potential chronobiological fallacy of past and perspectives for future epidemiological studies. Neuroendocrinology Letters 2013;34(4):282‐6. [PubMed] [Google Scholar]
Erren 2014
- Erren TC, Morfeld P. Computing chronodisruption: how to avoid potential chronobiological errors in epidemiological studies of shift work and cancer. Chronobiology International 2014;31(4):589‐9. [DOI] [PubMed] [Google Scholar]
Erren 2015
- Erren TC, Gross JV. Civil time ≠ biological time: Recent options for empirically testing possible effects of chronodisruption. Chronobiology International 2015;32(5):697‐8. [DOI] [PubMed] [Google Scholar]
Foret 1998
- Foret J, Daurat A, Tirilly G. Effect of bright light at night on core temperature, subjective alertness and performance as a function of exposure time. Scandinavian Journal of Work, Environment & Health 1998;24(3):115‐20. [PubMed] [Google Scholar]
Foster 2005
- Foster RG, Wulff K. The rhythm of rest and excess. Nature Reviews Neuroscience 2005;6:407‐14. [DOI] [PubMed] [Google Scholar]
Gillberg 1984
- Gillberg M. The effects of two alternative timings of a one‐hour nap on early morning performance. Biological Psychology 1984;19(1):45‐54. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University. Version 3.2 for Windows. Hamilton: Evidence Prime, Inc, 2015.
Hansen 2012
- Hansen J, Lassen CF. Nested case‐control study of night shift work and breast cancer risk among women in the Danish military. Occupational and Environmental Medicine 2012;69(8):551‐6. [DOI] [PubMed] [Google Scholar]
Herscovitch 1981
- Herscovitch J, Broughton R. Sensitivity of the Stanford Sleepiness Scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep 1981;4(1):83‐92. [DOI] [PubMed] [Google Scholar]
Herxheimer 2008
- Herxheimer A. Jet lag. Systematic review 2303. www.clinicalevidence.bmj.com/x/systematic‐review/2303/overview.html (accessed 10 April 2014).
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Available from www.cochrane‐handbook.org: The Cochrane Collaboration.
Higuchi 2011
- Higuchi S, Fukuda T, Kozaki T, Takahashi M, Miura N. Effectiveness of a red‐visor cap for preventing light‐induced melatonin suppression during simulated night work. Journal of Physiological Anthropology 2011;30(6):251‐8. [DOI] [PubMed] [Google Scholar]
Hilditch 2014
- Hilditch C, Centofanti S, Dorrian J, Dongen H, Banks S. Performance and fatigue after waking from 10 min and 30 min night‐time naps. Sleep and Biological Rhythms 2014;12:2‐3. [Google Scholar]
Hilditch 2015
- Hilditch C, Centofanti S, Dorrian J, Dongen H, Banks S. Do 10 min naps before the commute home from a night shift cause sleep inertia?. Sleep 2015;38:A129‐A130. [Google Scholar]
Hoddes 1972
- Hoddes E, Zarcone V, Dement W. Development and use of Stanford Sleepiness Scale (SSS). Psychophysiology 1972; Vol. 9, issue 1:150.
Hoppen 2001
- Hoppen KE. The effects of light on alertness and performance in relation to melatonin secretion. British Library Document Supply Centre 2001.
Horne 1976
- Horne JA, Ostberg O. A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. International Journal of Chronobiology 1976;4(2):97‐110. [PubMed] [Google Scholar]
Huynh 1976
- Huynh H, Feldt LS. Estimation at the box correction for degrees of freedom for sample data in randomised block and split‐plot designs. Journal of Educational Statistics 1976(1):69‐82. [Google Scholar]
IARC 2010
- International Agency for Research on Cancer. Painting, firefighting, and shiftwork. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 98, Lyon, France: WHO/IARC, 2010. [PMC free article] [PubMed] [Google Scholar]
ILO 1990
- International Labour Organization. Night Work Convention. C171, Geneva, ILO. www.ilo.org/dyn/normlex/en/f?p=NORMLEXPUB:12100:0::NO::P12100_INSTRUMENT_ID:312316 (accessed 12 August 2016).
Johns 1991
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540‐5. [DOI] [PubMed] [Google Scholar]
Juda 2013
- Juda M, Vetter C, Roenneberg T. The Munich ChronoType Questionnaire for Shift‐Workers (MCTQShift). Journal of Biological Rhythms 2013;28(2):130‐40. [DOI] [PubMed] [Google Scholar]
Kan 2012
- Kan K, Mollicone D, Van DH, Basner M, Dinges DF. Does napping on the night shift affect the efficiency of daytime recovery sleep?. Sleep 2012;35:A119. [Google Scholar]
Kantermann 2015
- Kantermann T, Sung H, Burgess HJ. Comparing the Morningness‐Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. Journal of Biological Rhythms 2015;30(5):449‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kecklund 1992
- Kecklund G, Akerstedt T. The psychometric properties of the Karolinska Sleep Questionnaire. Journal of Sleep Research 1992;6:221‐9. [Google Scholar]
Kelly 1994
- Kelly TL, Ryman DH, Hayduk R, Kripke DK. The effects of bright light and LEET on 6‐Sulphatoxymelatonin, core temperature, and cognitive performance after a 10‐hour phase delay. Report Naval Health Research Center 1994;95(12):27. [Google Scholar]
Kelly 1997
- Kelly TL, Kripke DF, Hayduk R, Ryman D, Pasche B, Barbault A. Bright light and LEET effects on circadian rhythms, sleep and cognitive performance. Stress Medicine 1997;13(4):251‐8. [DOI] [PubMed] [Google Scholar]
Ker 2010
- Ker K, Edwards PJ, Felix LM, Blackhall K, Roberts I. Caffeine for the prevention of injuries and errors in shift workers. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD008508] [DOI] [PMC free article] [PubMed] [Google Scholar]
King 1997
- King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate‐intensity exercise and self‐rated quality of sleep in older adults. A randomized controlled trial. JAMA 1997;277(1):32‐7. [PubMed] [Google Scholar]
Knutsson 2010
- Knutsson A, Boggild H. Gastrointestinal disorders among shift workers. Scandinavian Journal of Work, Environment & Health 2010;36(2):85‐95. [DOI] [PubMed] [Google Scholar]
Kredlow 2015
- Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta‐analytic review. Journal of Behavioral Medicine 2015;38(3):427‐49. [DOI] [PubMed] [Google Scholar]
Kretschmer 2011
- Kretschmer V, Griefahn B, Schmidt KH. Light‐induced adaptation to night work: Effects on cognitive performance in elderly persons. Journal of Psychophysiology 2011;25:20. [Google Scholar]
Kretschmer 2012
- Kretschmer V, Griefahn B, Schmidt KH. Bright light effects on working memory, sustained attention and concentration of elderly night shift workers. Lighting Research and Technology 2012;44:316‐33. [Google Scholar]
Kretschmer 2013
- Kretschmer V, Griefahn B, Schmidt KH. Bright‐light effects on cognitive performance in elderly persons working simulated night shifts: psychological well‐being as a mediator?. International Archives of Occupational and Environmental Health 2013;86(8):901‐14. [DOI] [PubMed] [Google Scholar]
Kubo 2010
- Kubo T, Takahashi M, Takeyama H, Matsumoto S, Ebara T, Murata K, et al. How do the timing and length of a night‐shift nap affect sleep inertia?. Chronobiology International 2010;27(5):1031‐44. [DOI] [PubMed] [Google Scholar]
Lallukka 2011
- Lallukka T, Dregan A, Armstrong D. Comparison of a sleep item from the General Health Questionnaire‐12 with the Jenkins Sleep Questionnaire as measures of sleep disturbance. Journal of Epidemiology 2011;21(6):474‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Landrigan 2004
- Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. New England Journal of Medicine 2004;351(18):1838‐48. [DOI] [PubMed] [Google Scholar]
Liira 2014
- Liira J, Verbeek JH, Costa G, Driscoll TR, Sallinen M, Isotalo LK, et al. Pharmacological interventions for sleepiness and sleep disturbances caused by shift work. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009776.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovato 2009
- Lovato N, Lack L, Ferguson S, Tremaine R. The effects of a 30‐min nap during night shift following a prophylactic sleep in the afternoon. Sleep and Biological Rhythms 2009;7(1):34‐42. [Google Scholar]
Lovibond 1995
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2. Sydney: Psychology Foundation, 1995. [Google Scholar]
Macchi 2002
- Macchi MM, Boulos Z, Ranney T, Simmons L, Campbell SS. Effects of an afternoon nap on nighttime alertness and performance in long‐haul drivers. Accident; Analysis and Prevention 2002;34(6):825‐34. [DOI] [PubMed] [Google Scholar]
Mackworth 1950
- Mackworth NH. Researches on the measurement of human performance. Medical Research Council Special Report Series 1950;268:156. [Google Scholar]
Martin 1998
- Martin SK, Eastman CI. Medium‐intensity light produces circadian rhythm adaptation to simulated night‐shift work. Sleep 1998;21(2):154‐65. [PubMed] [Google Scholar]
Matsumoto 1981
- Matsumoto K. Effects of nighttime naps on body temperature changes, sleep patterns, and self‐evaluation of sleep. Journal of Human Ergology 1981;10(2):173‐84. [PubMed] [Google Scholar]
Mehrabian 1974
- Mehrabian A, Russell JA. An approach to environmental psychology. Cambridge, MA 1974. [Google Scholar]
Mistlberger 2005
- Mistlberger RE, Skene DJ. Nonphotic entrainment in humans?. Journal of Biological Rhythms 2005;20:339‐52. [DOI] [PubMed] [Google Scholar]
Mitler 1982
- Mitler MM, Gujavarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluation treatment efficacy in patients with excessive somnolence. Electroencephalography and Clinical Neurophysiology 1982;53(6):658‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Morin 1993
- Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford Press, 1993. [Google Scholar]
Neil‐Sztramko 2014
- Neil‐Sztramko SE, Pahwa M, Demers PA, Gotay CC. Health‐related interventions among night shift workers: a critical review of the literature. Scandanavian Journal of Work, Environment & Health 2014;40(6):543‐56. [DOI] [PubMed] [Google Scholar]
Neri 2002
- Neri DF, Oyung RL, Colletti LM, Mallis MM, Tam PY, Dinges DF. Controlled breaks as a fatigue countermeasure on the flight deck. Aviation, Space, and Environmental Medicine 2002;73(7):654‐64. [PubMed] [Google Scholar]
Pallesen 2008
- Pallesen S, Bjorvatn B. A new scale for measuring insomnia: the Bergen insomnia scale. Perceptual and Motor Skills 2008;107:691‐701. [DOI] [PubMed] [Google Scholar]
Partinen 1995
- Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ) ‐ a quantitated measure of subjective sleep complaints. Journal of Sleep Research 1995;4:150‐5. [DOI] [PubMed] [Google Scholar]
Petrie 2004
- Petrie KJ, Powell D, Broadbent E. Fatigue self‐management strategies and reported fatigue in international pilots. Ergonomics 2004;47(5):461‐8. [DOI] [PubMed] [Google Scholar]
Puttonen 2010
- Puttonen S, Harma M, Hublin C. Shift work and cardiovascular disease ‐ pathways from circadian stress to morbidity. Scandinavian Journal of Work, Environment & Health 2010;36(2):96‐108. [DOI] [PubMed] [Google Scholar]
Rahman 2011
- Rahman S, Marcu S, Shapiro CM, Brown TJ, Casper RF. Spectral modulation attenuates molecular, endocrine, and neurobehavioral disruption induced by nocturnal light exposure. American Journal of Physiology‐Endocrinology and Metabolism 2011;300(3):E518‐E527. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roenneberg 2003
- Roenneberg T, Wirz‐Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms 2003;18(1):80‐90. [DOI] [PubMed] [Google Scholar]
Roenneberg 2007
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Medicine Reviews 2007;11(6):429‐38. [DOI] [PubMed] [Google Scholar]
Ruggiero 2014
- Ruggiero JS, Redeker NS. Effects of napping on sleepiness and sleep‐related performance deficits in night‐shift workers: a systematic review. Biological Research for Nursing 2014;16(2):134‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saito 1996
- Saito Y, Sasaki T. The effect of length of a nocturnal nap on fatigue feelings during subsequent early morning hours. The Journal of Science of Labour 1996;72(1):15‐23. [Google Scholar]
Salame 1995
- Salame P, Otzenberger H, Ehrhart J, Dewasmes G, Nicolas A, Tassi P, et al. Effects of sleep inertia on cognitive performance following a 1‐hour nap. Work and Stress 1995;9(4):528‐39. [Google Scholar]
Samel 1995
- Samel A, Gander P. Bright light as a chronobiological countermeasure for shiftwork in space. Acta Astronautica 1995;36(8‐12):669‐83. [DOI] [PubMed] [Google Scholar]
Santhi 2008
- Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. Journal of Biological Rhythms 2008;23(4):341‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sasseville 2006
- Sasseville A, Paquet N, Sevigny J, Hebert M. Blue blocker glasses impede the capacity of bright light to suppress melatonin production. Journal of Pineal Research 2006;41(1):73‐8. [DOI] [PubMed] [Google Scholar]
Sato 2010
- Sato T, Kubo T, Ebara T, Takeyama H, Inoue T, Iwanishi M, et al. Brief hourly exercise during night work can help maintain workers' performance. Industrial Health 2010;48(4):470‐7. [DOI] [PubMed] [Google Scholar]
Schobersberger 2007
- Schobersberger W, Gufler V, Griesmacher A, Bartenbach C, Canazel M, Staggl S, et al. Effect of two different lighting environments on urinary neopterin and sulphatoxymelatonin in an experimental night shift model. Pteridines 2007;18(3):69‐78. [Google Scholar]
Schweitzer 1992
- Schweitzer PK, Muehlbach MJ, Walsh JK. Countermeasures for night work performance deficits: The effect of napping or caffeine on continuous performance at night. Work and Stress 1992;6(4):355‐65. [Google Scholar]
Shochat 1998
- Shochat T, Haimov I, Lavie P. Melatonin ‐ the key to the gate of sleep. Annals of Medicine 1998;30(1):109‐14. [DOI] [PubMed] [Google Scholar]
Singh 1997
- Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of the effect of exercise on sleep. Sleep 1997;20(2):95‐101. [DOI] [PubMed] [Google Scholar]
Sletten 2014
- SlettenTL, Ftouni S, Nicholas CL, Magee M, Grunstein R, Ferguson S, et al. Randomised controlled trial of the efficacy of a blue‐enriched light intervention to improve alertness and neurobehavioural performance in night shiftworkers. Sleep and Biological Rhythms 2014;12:55. [Google Scholar]
Smith 2008
- Smith MR, Eastman CI. Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off. Sleep 2008;31(12):1639‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2008a
- Smith MR, Cullnan EE, Eastman CI. Shaping the light/dark pattern for circadian adaptation to night shift work. Physiology & Behavior 2008;95(3):449‐56. [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: study 4. Journal of Biological Rhythms 2009;24(2):161‐72. [DOI] [PubMed] [Google Scholar]
Sommer 2010
STATA 2011 [Computer program]
- College Station, TX: StataCorp LP. Stata Statistical Software. Release 12. College Station, TX: StataCorp LP, 2011.
Stevens 2011
- Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, et al. Considerations of circadian impact for defining 'shift work' in cancer studies: IARC Working Group Report. Occupational and Environmental Medicine 2011;68(2):154‐62. [DOI] [PubMed] [Google Scholar]
Straif 2007
- Straif K, Baan R, Grosse Y, Secretan B, Ghissassi F, Bouvard V, et al. Carcinogenicity of shift‐work, painting, and fire‐fighting. Lancet Oncology 2007;8(12):1065‐6. [DOI] [PubMed] [Google Scholar]
Takeyama 2002
- Takeyama H, Itani T, Tachi N, Sakamura O, Suzumura H. Psycho‐physiological effects of naps during night shifts on morning types and evening types. Journal of Occupational Health 2002;44(2):89‐98. [Google Scholar]
Takeyama 2004
- Takeyama H, Matsumoto S, Murata K, Ebara T, Kubo T, Tachi N, et al. Effects of the length and timing of nighttime naps on task performance and physiological function. Revista de Saude Publica 2004;38(Suppl):32‐7. [DOI] [PubMed] [Google Scholar]
Takeyama 2005
- Takeyama H, Kubo T, Itani T. The nighttime nap strategies for improving night shift work in workplace. Industrial Health 2005;43:24‐9. [DOI] [PubMed] [Google Scholar]
Thessing 1994
- Thessing VC, Anch AM, Muehlbach MJ, Schweitzer PK, Walsh JK. Two‐ and 4‐hour bright‐light exposures differentially effect sleepiness and performance the subsequent night. Sleep 1994;17(2):140‐5. [DOI] [PubMed] [Google Scholar]
Thorne 2005
- Thorne DR, Johnson DE, Redmond DP, Sing HC, Belenky G, Shapiro JM. The Walter Reed palm‐held psychomotor vigilance test. Behavior Research Methods 2005;37:111‐8. [DOI] [PubMed] [Google Scholar]
Tremaine 2010
- Tremaine R, Dorrian J, Lack L, Lovato N, Ferguson S, Zhou X, et al. The relationship between subjective and objective sleepiness and performance during a simulated night‐shift with a nap countermeasure. Applied Ergonomics 2010;42(1):52‐61. [DOI] [PubMed] [Google Scholar]
Wang 2011
- Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occupational Medicine 2011;61(2):78‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisgerber 2015
- Weisgerber DM, Mistlberger R. Driving home from the night shift: A bright light intervention study. Sleep 2015;38:A131. [DOI] [PubMed] [Google Scholar]
Wierwille 1994
- Wierwille WW, Wreggit SS, Kirn CL, Ellsworth LA, Fairbanks RJ. Research on vehicle‐based driver status/performance monitoring; development, validation, and refinement of algorithms for detection of driver drowsiness. http://ntl.bts.gov/lib/jpodocs/repts_te/9006.pdf (accessed 12 August 2016).
Wong 2011
- Wong IS, McLeod CB, Demers PA. Shift work trends and risk of work injury among Canadian workers. Scandinavian Journal of Work, Environment & Health 2011;37(1):54‐61. [DOI] [PubMed] [Google Scholar]
Yamanaka 2010
- Yamanaka Y, Hashimoto S, Tanahashi Y, Nishide SY, Honma S, Honma K. Physical exercise accelerates reentrainment of human sleep‐wake cycle but not of plasma melatonin rhythm to 8‐h phase‐advanced sleep schedule. American Journal of Physiology. Regulatory, Integrative & Comparative Physiology 2010;298(3):R681‐R91. [DOI] [PubMed] [Google Scholar]
Zhong 2012
- Zhong CB, House, J. Hawthorne revisited: Organizational implications of the physical work environment. Research in Organizational Behavior 2012;32:3‐22. [Google Scholar]
References to other published versions of this review
Herbst 2013
- Herbst C, Erren TC, Sallinen M, Fritschi L, Costa G, Driscoll TR, et al. Person‐directed non‐pharmacological interventions for preventing and treating sleepiness and sleep disturbances caused by shift work. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010641] [DOI] [Google Scholar]


