Abstract
Background
Traumatic brain injury is a leading cause of premature death and disability. Post‐traumatic membrane lipid peroxidation has been proposed as one mechanism leading to secondary brain damage following head injury. Aminosteroids have been shown to inhibit lipid peroxidation in laboratory animals and have the potential to improve outcome following head injury.
Objectives
To quantify the effectiveness and safety of aminosteroids in the treatment of acute traumatic brain injury.
Search methods
We searched the Cochrane Injuries Group specialised register, the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, the National Research Register, Web of Science, web‐based trials databases and conducted a general internet search. We contacted experts in the field and the company that manufactures tirilazad. The searches were last updated in March 2006.
Selection criteria
We sought to identify all randomised controlled trials of aminosteroids versus placebo in the treatment of acute traumatic brain injury. Studies using a quasi‐random form of allocation, such as alternation, were excluded from the review.
Data collection and analysis
One author examined the electronic search results for reports of possibly relevant trials for retrieval in full. Two authors (IR and PA) applied the selection criteria independently to the trial report, with no disagreement.
Main results
Two randomised controlled trials have examined the effect of the aminosteroid tirilazad mesylate on death and disability following head injury. To date, only the results of one of these trials are available for analysis. The risk of death in patients treated with tirilazad was almost identical to those given placebo RR = 1.05 (95% confidence interval 0.86 to 1.29). The risk of death and severe disability in patients treated with tirilazad was again almost identical to those given placebo RR = 1.07 (95% confidence interval 0.93 to 1.23).
Authors' conclusions
There is no evidence to support the routine use of aminosteroids in the management of traumatic head injury. On the basis of the existing evidence from randomised trials of aminosteroids in head injury, it is not possible to refute the possibility of moderate but potentially clinically important benefits or harms. A further randomised controlled trial of tirilazad mesylate with 1156 participants has been completed, the results of which should become available in the near future.
Keywords: Humans, Brain Injuries, Brain Injuries/drug therapy, Drug Evaluation, Neuroprotective Agents, Neuroprotective Agents/therapeutic use, Pregnatrienes, Pregnatrienes/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Use of aminosteroids to treat traumatic brain injury
Traumatic brain injury is a leading cause of death and disability. After the initial blow to the head, additional brain damage can occur through a reduction of oxygen to the brain tissues (cerebral hypoxia). Chemicals called aminosteroids have been shown to help stop cell membrane damage and cell death in animals.
The review author searched the medical literature to find out if aminosteroids help people with traumatic brain injury when given within seven days of the injury. The author looked for randomised controlled trials in which one group of patients received a treatment (aminosteroids) while a similar group received non‐active treatment (placebo) in addition to standard care. To reduce possible bias, each patient is randomly assigned to a group. The author found two such studies, which used the aminosteroid tirilazad mesylate, but the results of one of the studies were not available at the time of review. The completed study involved 1131 patients. The results of this study showed no benefit from the aminosteroid. The aminosteroid group did not have more side effects than the placebo group but aminosteroids are fairly new drugs that may have unknown less common side effects.
More research is needed on the use of aminosteroids to treat traumatic brain injury but currently there is no evidence to recommend their use.
Background
Traumatic brain injury is a leading cause of premature death and disability. Road crashes account for the majority of fatal head injuries (Jennet 1996). Insights from pathophysiological studies have shown that acute traumatic head injury marks only the beginning of a continuing encephalopathic process. Secondary brain damage from on‐going pathophysiological mechanisms and cerebral hypoxia is believed to be an important cause of avoidable death and long term disability. The modern management of head injury is primarily aimed at preventing secondary brain damage, which in turn requires an understanding of the pathophysiological processes involved in its development (Gentleman 1990). Demopoulos 1982 have proposed that the molecular basis for post‐traumatic neuronal degeneration is oxygen free radical induced lipid peroxidation. Lipid peroxidation once initiated is considered to be a self‐propagating process that leads to cell membrane damage and cell death. Tirilazad mesylate is a 21‐aminosteroid that has been shown to inhibit lipid peroxidation in experimental animals (Hall 1988). There is considerable debate in the basic science community about whether or not the agent crosses the blood brain barrier: if it does not then any experimental protection would be because of lipid peroxidation in the vascular space, rather than cell membranes within the brain. Tirilazad has been evaluated in a number of randomised controlled trials. To assess the safety and effectiveness of aminosteroids in patients with head injury we conducted a systematic review of randomised controlled trials.
Objectives
To quantify the effectiveness and safety of aminosteroids in the treatment of acute traumatic brain injury.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of aminosteroids versus placebo (or no aminosteroid) for the treatment of acute traumatic brain injury.
Types of participants
People of all ages with clinically diagnosed acute traumatic brain injury secondary to head injury treated with aminosteroids within seven days of the injury. All severities of head injury were eligible. Trials in people with non‐traumatic subarachnoid haemorrhage were excluded.
Types of interventions
Any regimen of any aminosteroid versus placebo, if started within seven days of head injury.
Types of outcome measures
All‐cause fatality.
Any valid and reliable measure of neurological functioning.
Quality of life measures.
Side effects.
Economic outcomes were considered relevant if available.
Search methods for identification of studies
There was no language restriction applied to the search.
An updated search was conducted in March 2006.
Electronic searches
We searched:
the Cochrane Injuries Group's specialised register,
the Cochrane Central Register of Controlled Trials,
MEDLINE,
EMBASE,
the National Research Register.
Search strategies for each database are given in Appendix 1.
Searching other resources
We searched relevant websites, and reference lists of all potentially eligible studies were examined for other relevant articles.
We also contacted an expert in the field and the company that manufactures tirilazad.
Data collection and analysis
Selection of studies
One author examined the electronic search results for reports of possibly relevant trials for retrieval in full and applied the selection criteria to the trial report.
Data extraction and management
Information on the following was extracted: method of allocation concealment, number of randomised patients, type of participants and the interventions. The outcome data sought were as listed in the selection criteria. The reviewer was not blinded to the authors or journal when doing this, as evidence for the value of this is far from conclusive (Berlin 1997).
Where there was insufficient information in the published report, the authors were contacted for clarification.
Disability was assessed using the Glasgow Outcome Scale, with a favourable outcome defined as good recovery (e.g. return to work) or moderate disability.
Assessment of risk of bias in included studies
Since there is evidence that the quality of allocation concealment particularly affects the results of studies (Schulz 1995), the author scored this quality on the scale used by Schulz 1995 as shown below, assigning C to poorest quality and A to best quality:
C=trials in which concealment was inadequate (such as alternation or reference to case record numbers or to dates of birth)
B=trials in which the authors either did not report an allocation concealment approach at all or reported an approach that did not fall into one of the other categories
A=trials deemed to have taken adequate measures to conceal allocation (that is, central randomisation; numbered or coded bottles or containers; drugs prepared by the pharmacy; serially numbered, opaque, sealed envelopes; or other description that contained elements convincing of concealment)
Where the method used to conceal allocation was not clearly reported, the reviewer planned to contact the author, but this was not necessary.
Information on blinding and the degree of loss to follow up, was also collected but a score was not assigned to this.
Data synthesis
The author planned to calculate summary statistics for the trial using intention to treat.
The author planned to examine trials for statistical evidence of heterogeneity using a chi squared test. If there was no obvious heterogeneity on visual inspection or statistical testing, pooled relative risks and 95% confidence intervals would be calculated using a fixed effects model. For continuous data, we would examine the mean and standard deviation to look for evidence of skewness as described by Altman (Altman 1996) and, if not present, calculate pooled weighted mean differences.
The effect of excluding trials judged to have inadequate (scoring C) allocation concealment was planned as a sensitivity analysis.
The author also intended to prepare funnel plots if there were sufficient trials to look for evidence of publication bias (Egger 1997).
Results
Description of studies
Marshall 1998: The trial randomised 1131 patients to receive tirilazad mesylate or placebo. Eleven patients were excluded from the analysis because they did not complete their assigned protocol. Efforts to obtain these data from the study authors have so far been unsuccessful. A further 55 participants were lost before the six month follow up (38 in the tirilazad group and 28 in the placebo group). Data on side effects were collected from 1016 participants. Despite the use of random allocation, there were substantial pre‐treatment imbalances in hypotension and hypoxia.
Bleck (ongoing): A second randomised controlled trial of tirilazad mesylate in the treatment of head injury in 1156 patients has been conducted but the results are not yet available for inclusion in this review.
Bleck 1995 (unpublished): Randomised double‐blind vehicle controlled study of tirilazad mesylate at three escalating dosage tiers (0.6, 2.0, 6.0 mg/kg/day) in a total of 181 adults patients with Glasgow Coma Scores of 4 to 12 within four hours of closed head injury. Data were collected on mortality, GOS, and side effects. The study author was contacted to obtain the unpublished data and has agreed to discuss provision of the data with Pharmacia and Upjohn, as the material is covered by a confidentiality agreement. However, data on mortality were presented at conference and are in the public domain. These data have therefore been included.
Mathew 1993 (unpublished): Thirty moderately head‐injured patients shown by CT scan to have a focal intracranial lesion allocated to treatment with tirilazad or placebo in a randomised, double‐blind protocol. Treatment commenced within 24 hours of injury and continued for four days. MR imaging with Gadolinium was performed at five days and the presence and extent of enhancement in the region of the lesion was measured. Also late MRI scans (six months) are being performed to detect if the ultimate resolution of a lesion is influenced by the therapy. The code was broken in the spring of 1993. The authors have been contacted for the unpublished data but, as yet, no data are available for inclusion.
Risk of bias in included studies
Marshall 1998: Allocation concealment was by the use of matching placebo. There were 66 participants excluded from the final analysis (about 6% of those randomised) ‐ eleven due to protocol violation and 55 others lost to follow up. All personnel involved in the study were blinded to whether the patient received drug or placebo.
Effects of interventions
Two randomised controlled trials have examined the effect of the aminosteroid tirilazad mesylate on death and disability following head injury. To date, only the results of one of these trials are available for analysis. The risk of death in patients treated with tirilazad was almost identical to those given placebo RR=1.05 (95% confidence interval 0.86 to 1.29). The risk of death and disability in patients treated with tirilazad was again almost identical to those given placebo RR = 1.07 (95% confidence interval 0.93 to 1.23).
Because aminosteroids are new drugs, there was little information that could be used to predict the side effect profile. As a result, the side effects reported in this review were those reported in the trial itself. The criterion for reporting side effects in the trial was events that occurred in more than 2% of cases. There was no clear evidence of increased side effects in the aminosteroid treated group. However, the precision of the point estimates for many of the side effects was low and it is not possible to refute the possibility of clinically important adverse effects.
Discussion
There is no evidence that aminosteroids reduce the risk of death or disability following head injury. However, on the basis of the existing evidence it is not possible to refute the possibility of moderate but potentially clinically important benefits or harms.
The published trial (Marshall 1998) pointed out that there were imbalances in some prognostic factors between treatment and control groups (pretreatment hypotension and hypoxia). It is not possible to tell what effect this confounding had on the overall result but it would be unlikely to make a major difference to the point estimate.
Authors' conclusions
Implications for practice.
There is no evidence to support the routine use of aminosteroids in the management of traumatic head injury.
Implications for research.
On the basis of the existing evidence from randomised trials of aminosteroids in head injury it is not possible to refute the possibility of moderate but potentially clinically important benefits or harms. A further randomised controlled trial of tirilazad mesylate with 1156 participants has been completed, the results of which should become available in the near future.
What's new
| Date | Event | Description |
|---|---|---|
| 6 May 2010 | Review declared as stable | Aminosteroids are no longer produced. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 17 July 2008 | Amended | Converted to new review format. |
| 1 March 2006 | New search has been performed | An updated search for new trials was done in March 2006. No new trials for inclusion have been identified. |
Acknowledgements
Thanks to Reinhard Wentz, Fiona Renton and Karen Blackhall for their help with searching.
Appendices
Appendix 1. Search strategy
CENTRAL, MEDLINE, National Research Register #1 TIRILAZAD in MeSH #2 PREGNATRIENES in MeSH #3 AMINOSTEROIDS in MeSH #4 lazaroid* OR antioxidan* OR aminosteroid* #5 #1 OR #2 OR #3 OR #4 #6 CRANIOCEREBRAL TRAUMA in MeSH #7 (head OR brain) AND (injur* OR trauma*) #8 #6 OR #7 #9 #8 AND #5 #10 RCT filter (Clarke 2001)
EMBASE #1 TIRILAZAD/ #2 PREGNATRIENES/ #3 AMINOSTEROIDS/ #4 lazaroid$ OR antioxidan$ OR aminosteroid$ #5 #1 OR #2 OR #3 OR #4 #6 HEAD INJURY/ #7 (head OR brain) AND (injur$ OR trauma$) #8 #6 OR #7 #9 #8 AND #5
Data and analyses
Comparison 1. Any aminosteroid at any dose versus no aminosteroid or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 1240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.28] |
| 2 Death or disability (GOS) | 1 | 1065 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.23] |
| 3 Sepsis | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.35] |
| 4 Cardiac arrest | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.06] |
| 5 hypotension | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.54, 2.06] |
| 6 multiple organ failure | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.84, 4.16] |
| 7 brain edema | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.46] |
| 8 intraparenchymal haemorrhage | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.58, 1.70] |
| 9 epidural haematoma | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.48, 2.43] |
| 10 cerebral infarction | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.60, 2.41] |
| 11 brain herniation | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.10] |
| 12 intracranial hypertension | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.21] |
| 13 pneumonia | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.52, 1.58] |
| 14 ARDS | 1 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.67, 3.53] |
1.1. Analysis.
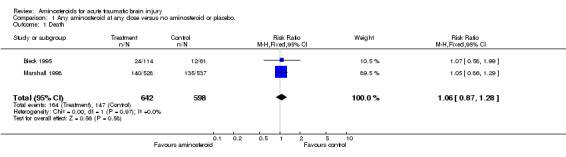
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 1 Death.
1.2. Analysis.
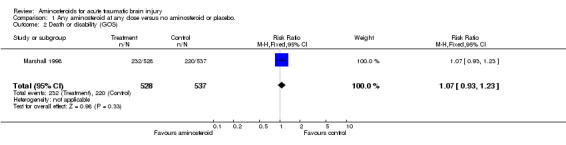
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 2 Death or disability (GOS).
1.3. Analysis.
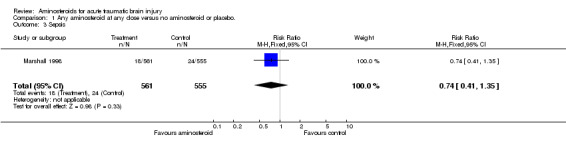
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 3 Sepsis.
1.4. Analysis.
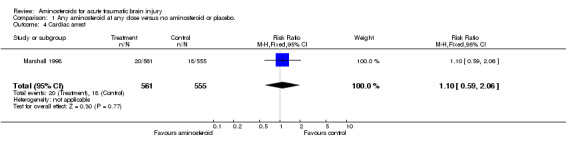
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 4 Cardiac arrest.
1.5. Analysis.
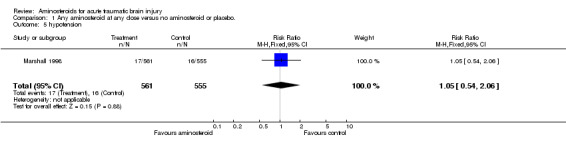
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 5 hypotension.
1.6. Analysis.
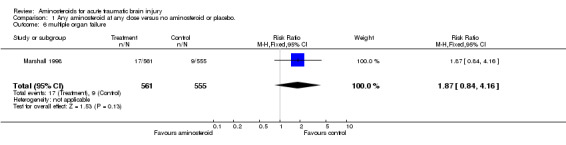
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 6 multiple organ failure.
1.7. Analysis.
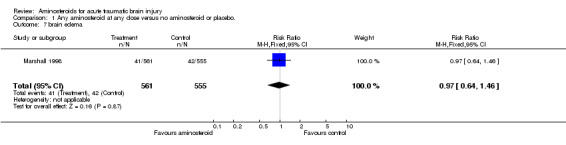
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 7 brain edema.
1.8. Analysis.
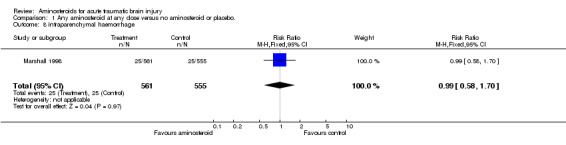
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 8 intraparenchymal haemorrhage.
1.9. Analysis.
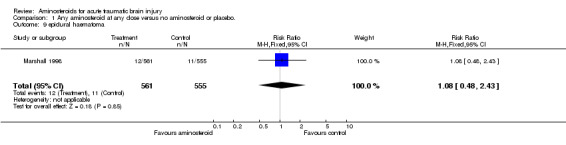
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 9 epidural haematoma.
1.10. Analysis.
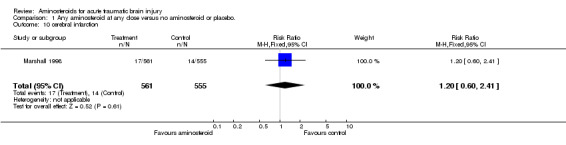
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 10 cerebral infarction.
1.11. Analysis.
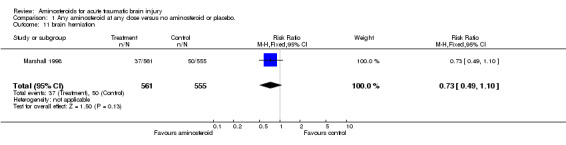
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 11 brain herniation.
1.12. Analysis.
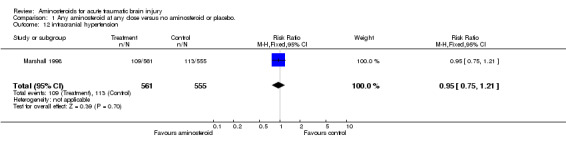
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 12 intracranial hypertension.
1.13. Analysis.
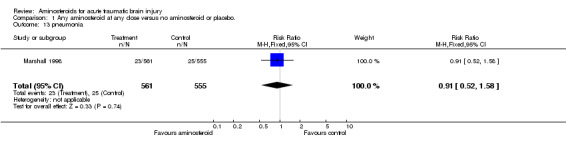
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 13 pneumonia.
1.14. Analysis.
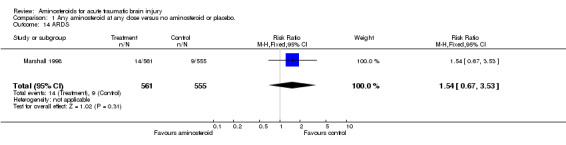
Comparison 1 Any aminosteroid at any dose versus no aminosteroid or placebo., Outcome 14 ARDS.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bleck 1995.
| Methods | Randomised double‐blind controlled study. | |
| Participants | Adult patients with GCS 4‐12 within 4 hours after closed head injury. | |
| Interventions | Tirilazad mesylate at three escalating dosage tiers (0.6, 2.0, 6.0 mg/kg/day). | |
| Outcomes | Death at the end of the study period. Unpublished data on non‐fatal outcome is being sought. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Marshall 1998.
| Methods | Randomised double‐blind placebo controlled trial. Randomisation stratified by severity of head injury as assessed on the Glasgow Coma Scale (4‐8 severe, 9‐12 moderate). About 1% of participants excluded due to protocol violations and about a further 5% were lost to follow up before the 6‐month evaluation. | |
| Participants | 1131 randomised patients with moderate and severe head injury. Patients were eligible for inclusion in the trial if the following criteria were met: Glasgow Coma Score 4‐12, age between 15 and 65 years, abnormalities could be observed on computerised tomography scanning, treatment could be initiated within four hours from the time of injury. Exclusion criteria: pregnancy, other investigational drugs taken within 30 days, any disease expected to make outcome meaurement difficult. | |
| Interventions | 1) Tirilazad mesylate 10 mg/kg by i.v. infusion every 6 hours for 5 days. (n=562, excluding protocol violators) 2) Placebo. (n=558, excluding protocol violators) | |
| Outcomes | Death. Favourable outcome (good recovery and moderate disability) at six months as determined by the Glasgow Outcome Scale. | |
| Notes | Other treatment by 'standardised protocols'. Patients not allowed to be given calcium antagonists or megadose steroids. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of ongoing studies [ordered by study ID]
Bleck.
| Trial name or title | North American phase III trial of tirilazad mesylate |
| Methods | |
| Participants | Patients with moderate and severe head injury |
| Interventions | Tirilazad mesylate |
| Outcomes | Death and disability |
| Starting date | Recruitment now complete (1156 randomised participants) |
| Contact information | Thomas Bleck Professor of Neurological Surgery and Internal Medicine The University of Virginia USA |
| Notes |
Contributions of authors
IR examined the search results and selected relevant trials, extracted data, contacted trialists and wrote the review. PA selected relevant trials, extracted data and helped to write the review.
Sources of support
Internal sources
Institute of Child Health, University of London, UK.
External sources
NHS Research and Development, UK.
Declarations of interest
Ian Roberts is a principal investigator in the MRC CRASH trial, a large simple randomised controlled trial of 48 hours of methylprednisolone in adults with head injury. Pharmacia Corporation are donating drug and placebo for the MRC CRASH trial but the design, management and finance of the study are entirely independent of them. Pharmacia Corporation also manufacture tirilazad.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bleck 1995 {published data only (unpublished sought but not used)}
- Bleck TP, Germanson TP, Jane JA and the participants in the Tirilazad Head Trauma Trial, phase II. Tirilazad mesylate is safe in patients with moderate or severe head injury. Neurology 1995;45(suppl 4):A345. [Google Scholar]
Marshall 1998 {published data only}
- Marshall LF, Maas AIR, Bowers Marshall S, Bricolo A, Fearnside M, Iannotti F, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. Journal of Neurosurgery 1998;89:519‐25. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mathew 1993 {published data only (unpublished sought but not used)}
- Mathew P, Hadley D, Condon B, Teasdale GM. Blood Brain Barrier Permeability in head injured patients‐effect of tirilazad mesylate. 2nd International Neurotrauma symposium. 1993:80.
References to ongoing studies
Bleck {published data only (unpublished sought but not used)}
- Bleck T. Tirilazad mesylate in the treatment of head injury.
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlin 1997
- Berlin JA for the University of Pennsylvania Meta‐analysis blinding study. Does blinding of readers affect the results of meta‐analyses?. Lancet 1997;350:185‐6. [DOI] [PubMed] [Google Scholar]
Demopoulos 1982
- Demopoulos HB, Flamm ES, Seligman ML. Further studies on free radical pathology in the major central nervous system disorders: effects of very high doses of methylprednisolone on the functional outcome, morphology, and chemistry of experimental spinal cord impact injury. Canadian Journal of Physiology & Pharmacology 1982;60:1415‐24. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analyses detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gentleman 1990
- Gentleman D. Preventing secondary brain damage after head injury: a multidisciplinary challenge. Injury 1990;21:305‐8. [DOI] [PubMed] [Google Scholar]
Hall 1988
- Hall ED, Yonkers PA, McCall JM, et al. Effects of the 21 aminosteroid U‐74006F on experimental head injury in mice. Journal of Neurosurgery 1988;68:456‐61. [DOI] [PubMed] [Google Scholar]
Jennet 1996
- Jennett B. Epidemiology of head injury. Journal of Neurology, Neurosurgery, and Psychiatry 1996;60(4):362‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]


