Abstract
Background
Long term levodopa therapy in Parkinson's disease is associated with the development of motor complications including abnormal involuntary movements and a shortening response to each dose (wearing off phenomenon). It is thought that dopamine agonists can reduce the duration of immobile off periods and the need for levodopa therapy whilst maintaining or improving motor impairments and only minimally increasing dopaminergic adverse events.
Objectives
To compare the efficacy and safety of adjuvant cabergoline therapy versus placebo in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Search methods
Electronic searches of MEDLINE, EMBASE and the Cochrane Controlled Trials Register. Handsearching of the neurology literature as part of the Cochrane Movement Disorders Group's strategy. Examination of the reference lists of identified studies and other reviews. Contact with Pharmacia Upjohn Limited.
Selection criteria
Randomised controlled trials of cabergoline versus placebo in patients with a clinical diagnosis of idiopathic Parkinson's disease and long‐term complications of levodopa therapy.
Data collection and analysis
Data was abstracted independently by the authors and differences settled by discussion. The outcome measures used included Parkinson's disease rating scales, levodopa dosage, off time measurements and the frequency of withdrawals and adverse events.
Main results
Cabergoline has been compared with placebo in two phase II (6 ‐ 12 weeks) and one phase III randomised controlled trials (24 weeks). These were double‐blind, parallel group, multicentre studies including 268 patients with Parkinson's disease and motor complications. The reduction of 1.14 hours (WMD; 95% CI ‐0.06, 2.33; p = 0.06) in off time in favour of cabergoline was not statistically significant. Inadequate data on dyskinesia was collected either on rating scales or as adverse event reporting to allow a conclusion to be drawn. A small but statistically significant advantage of cabergoline over placebo was seen in one study for UPDRS ADL (part II) score and UPDRS motor score. No such advantage was seen in one other study due to small numbers of patients and the comparatively low doses of cabergoline used. No significant differences in Schwab and England scale were seen in two studies. Levodopa dose reduction was significantly greater with cabergoline (WMD 149.6 mg/d; 95% CI 94.1, 205.1; p < 0.00001). There was a trend towards more dopaminergic adverse events with cabergoline but this did not reach statistical significance at the p < 0.01 level. However, there was a trend towards fewer withdrawals from cabergoline.
Authors' conclusions
In the management of the motor complications seen in Parkinson's disease, cabergoline can be used to reduce levodopa dose and modestly improve motor impairment and disability with an acceptable adverse event profile. These conclusions are based on, at best, medium term evidence.
Plain language summary
Cabergoline for levodopa‐induced complications in Parkinson's disease
In the later stages of Parkinson's disease, side effects occur because of the use of levodopa treatment. These consist of involuntary writhing movements (choreoathetosis), painful cramps in the legs (dystonia) and a shortened response to each dose referred to as 'end‐of‐dose deterioration' or the 'wearing‐off effect'. Dopamine agonist drugs act by mimicking levodopa in the brain, but they do not cause these long‐term treatment complications when used as initial therapy. For this reason, dopamine agonists have for some years been added once these problems develop in the hope of improving them. Cabergoline is a new dopamine agonist recently licensed in the UK for the treatment of later Parkinson's disease. In this review, we will examine the trials performed with this drug to see how effective it is and what side effects it causes.
Cabergoline has been compared with inactive placebo in two smaller and shorter (6 ‐ 12 weeks) studies and one larger, medium term trial (24 weeks). These trials included 268 patients with Parkinson's disease and motor complications. The average reduction in the time patients spent in the immobile off state was 1.1 hours greater with cabergoline compared with placebo, although this was not statistically significant. Inadequate data on dyskinesia was collected to allow a conclusion to be drawn. A small but significant advantage of cabergoline over placebo was seen in one study for activities of daily living and physical functioning. No such advantage was seen in one other study due to small numbers of patients and the comparatively low doses of cabergoline used. Levodopa dose reduction was greater with cabergoline by 145 mg per day. There was a trend towards more side effects with cabergoline but towards fewer withdrawals from cabergoline treatment.
In the management of the motor complications seen in Parkinson's disease, cabergoline can be used to reduce levodopa dose and modestly improve motor function and activities of daily living with an acceptable side effect profile. This is based on, at best, medium term evidence. Further long term trials are required to compare the newer with the older dopamine agonists, particularly in terms of quality of life and cost.
Background
Over 20 years after its introduction, levodopa remains the most effective therapy in Parkinson's disease. However, with long‐term treatment, patients develop side effects comprised of motor and psychiatric complications. The former consist of involuntary writhing movements of the limbs and trunk (choreoathetosis), painful cramps often affecting the feet (dystonia) and a shortened response to each dose of levodopa (end‐of‐dose deterioration). These affect 50% of patients after 6 years of therapy (Rajput 1984) and 100% of young onset patients (Quinn 1986).
An alternative treatment in Parkinson's disease is the dopamine agonist class of drug. These act directly on post‐synaptic dopamine receptors in the striatum and so they do not require conversion into dopamine, as does levodopa. They have developed the reputation of being less effective in clinical practice than expected, although they generate fewer motor complications when used as long‐term monotherapy. The use of dopamine agonists in newly diagnosed patients will be the subject of further Cochrane reviews.
Cabergoline is an ergoline class dopamine agonist along with bromocriptine, pergolide, and lisuride. It has a long half‐life of around 65 hours compared with the other dopamine agonists and thus is administered once daily. Therefore, it is easier to titrate and for the patient to take and potentially it may reduce motor complications more by reducing the phasic stimulation of dopamine receptors.
Its efficacy and safety have been examined in early and advanced Parkinson's disease. Monotherapy studies will be examined in other Cochrane reviews. Trials in later disease have led to cabergoline being licensed in the United Kingdom for this indication in the expectation of a reduction in off time and improved motor function.
The present systematic review examines all randomised controlled trials of adjuvant cabergoline therapy compared with placebo in later Parkinson's disease with motor complications to establish its efficacy and tolerability. A separate review covers the effects of adjuvant cabergoline versus bromocriptine.
Objectives
To compare the efficacy and safety of adjuvant cabergoline therapy versus placebo in patients with Parkinson's disease, already established on levodopa and suffering from motor complications.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing adjuvant cabergoline with placebo were considered for inclusion in the study.
Types of participants
Patients with a clinical diagnosis of idiopathic Parkinson's disease who had developed long‐term motor complications of dyskinesia and/or end‐of‐dose deterioration. All ages were included. Any duration of levodopa therapy was included.
Types of interventions
Oral cabergoline therapy or placebo. Trial durations of greater than 4 weeks were included.
Types of outcome measures
1. Improvement in the time patients spend in the immobile 'off' state.
2. Changes in dyskinesia rating scales and the prevalence of dyskinesia.
3. Changes in parkinsonian rating scales.
4. Reduction in levodopa dose.
5. Number of withdrawals due to lack of efficacy and/or side‐effects.
Search methods for identification of studies
1. The review was based on the search strategy of the Movement Disorders Group. This included computerised searches of MEDLINE and EMBASE and hand searching of appropriate neurology journals. Relevant trials were included on the Group's specialised register of randomised controlled trials. Further details are available in the Group's module on the Cochrane Database of Systematic Reviews.
2. The Cochrane Controlled Trials Register was also searched for relevant trials.
3. The reference lists of located trials and of other cabergoline reviews were searched.
4. Additional assistance was provided by the drug manufacturer Pharmacia Upjohn.
Data collection and analysis
The two authors (CC, KD) independently assessed the studies identified by the search strategy. Disagreements about inclusions were resolved by discussion. The full papers were assessed for methodological quality by recording the method of randomisation and blinding, whether an intention‐to‐treat analysis was used and the number of patients lost to follow up.
Eligible data was abstracted onto standardised forms by the authors independently, checked for accuracy and amalgamated. A weighted estimate (fixed effect model) of the typical treatment effect across trials was calculated for continuous (weighted mean difference) and dichotomous (Peto odds ratio) variables such as 'off' time and prevalence of adverse events. Since multiple comparisons of adverse events were examined statistically, the results were interpreted cautiously using 99% confidence intervals.
Results
Description of studies
See also Characteristics of Included Studies and Table 1Key Characteristics and Results of Included Studies.
1. Key Characteristics and Results for Included Studies.
| Study | Number of patients | Mean Hoehn & Yahr | Duration (weeks) | Mean (Maximum) Cabergoline dose (mg/d) | Mean difference (MD) L‐dopa reduction (mg/d; + in favour of cabergoline) | MD off hours reduction (hours; + in favour of cabergoline) | MD improvement in UPDRS ADL score (+ in favour of cabergoline) | MD Improvement in UPDRS Motor score (+ in favour of cabergoline) |
| Hutton | 188 | 2.0 (median) | 24 | 3.66 (5.0) | 149.6 | 1.32 | 2.3 | 1.6 |
| Miguel | 43 | n/a | 6‐10 | 2.64 (3.0) | n/a | 0.86 | 0.41 (on) 0.17 (off) | 1.93 (on) 9.97 (off) |
| Steiger | 37 | 3.5 | 12 | 5.4 (10.0) | 10.0 | 1.30 | n/a | n/a |
| Total or Mean | 268 | 145.0 (WMD) | 1.22 (WMD) |
Three trials fulfilling the inclusion criteria were found (Steiger 1996; Miguel 1993; Hutton 1996), one of which was unpublished but details were provided by the manufacturer (Miguel 1993). Ahlskog 1996 was excluded after contact with the author as these patients were included in Hutton 1996.
All three studies were randomised, double‐blind, parallel group design and included 268 patients. The two phase II studies selected patients to continue in the study after titration only if they had responded to the trial medication, so only the data up to the end of the titration period has been included (Miguel 1993; Steiger 1996).
The patients entering the two arms of each trial were well balanced according to age, sex, and Hoehn and Yahr score. However, the mean Hoehn and Yahr score in Steiger 1996 was 3.5 compared to the median in Hutton 1996 of 2.0, so the latter patients had relatively mild disease compared with the former.
The maximum dose of cabergoline used in two trials was comparable with the present licensed limit of 6.0 mg/d (Hutton 1996, 5.0; Steiger 1996, 10.0). The other phase II study used a maximum of only 3.0 mg/d (Miguel 1993). This is reflected in the mean doses used in the studies (Hutton 1996, 3.66 mg/d; Steiger 1996, 5.4 mg/d; Miguel 1993, 2.64 mg/d).
Levodopa dose reduction was allowed in Miguel 1993 and Hutton 1996 but not Steiger 1996.
Risk of bias in included studies
See also Characteristics of Included Studies and Table 1 Key Characteristics and Results of Included Studies.
Details on randomisation and concealment of allocation were described in two trial reports (Hutton 1996; Miguel 1993) and information on the third was obtained from one of the investigators (Steiger 1996). There was no suggestion of selection bias in these studies.
The double‐blind design of all of the trials should exclude performance and attrition bias. Detection bias is unlikely in view of the double‐blind design of all three trials and the use of blinded statisticians in two studies (Miguel 1993; Hutton 1996).
The two phase II studies were short term (Steiger 1996, 12 weeks; Miguel 1993, 6‐10 weeks) compared with the phase III study which was medium term (Hutton 1996, 24 weeks).
Sample size calculations were not included in the phase II trial reports which is standard practice, but the phase III study failed to report such a calculation (Hutton 1996).
Effects of interventions
See also Table 1Key Characteristics and Results of Included Studies and Table 2 Adverse Events for Included Studies.
2. Adverse Events for Included Studies (Peto Odds Ratio < 1 favours cabergoline).
| Study (number) | Nausea | Postural hypotension | Hallucinations | Confusion | Dyskinesia | Insomnia | Sleep Disorder | Somnolence | All cause withdrawals |
| Hutton (188) | 1.64 | 2.11 | 2.48 | 2.26 | n/a | 1.00 | 4.61 | 1.41 | 0.57 |
| Miguel (43) | 7.49 | 6.49 | 0.87 | 6.49 | 6.49 | n/a | n/a | 6.49 | 0.65 |
| Steiger (37) | 1.32 | 7.01 | 1.91 | 7.89 | n/a | 0.94 | n/a | n/a | 0.47 |
| Total (268) | 1.84 | 2.28 | 2.08 | 3.73 | 6.49 | 1.00 | 4.61 | 1.63 | 0.58 |
| P value (Test for overall effect) | 0.06 | 0.03 | 0.15 | 0.05 | 0.40 | 1.00 | 0.50 | 0.40 | 0.13 |
Cabergoline has been compared with placebo in two phase II (Steiger 1996; Miguel 1993) and one phase III randomised controlled trials (Hutton 1996). These were double‐blind, parallel group, multicentre studies including 268 patients with Parkinson's disease and motor complications. The phase II studies were short term (6 ‐ 12 weeks) and the phase III study medium term (24 weeks).
In spite of obtaining further information from the manufacturer, it proved impossible to obtain data on the reduction in off time in hours for all of the studies. The reduction in off time of 1.14 hours/day more with cabergoline than placebo (WMD; 95% CI ‐0.06, 2.33; p = 0.06; Table 7) was not statistically significant.
Although a dyskinesia rating scale was used in Steiger 1996, no difference was seen between the two arms of the study and no data was reported. Dyskinesia reported as an adverse event was not available for the larger phase III study but there was a trend towards an increase with cabergoline in one of the small phase II studies (Table 13).
Regarding motor impairment and disability, a statistically significant advantage of cabergoline over placebo was seen in Hutton 1996 for UPDRS ADL (part II) score (Table 1) and UPDRS motor score (Table 2). It is presumed these were measured in the on phase. No such advantage was seen in Miguel 1993 but this may be attributable to the small numbers of patients and the lower doses of cabergoline used in this trial. No significant differences in Schwab and England scale were seen in Miguel 1993 and Steiger 1996 (Table 4), but the number of patients rated as much or very much improved in Steiger 1996 was significantly greater than with cabergoline than placebo (Table 5).
Levodopa dose reduction was significantly greater with cabergoline (WMD 149.6 mg/d; 95% CI 94.1, 205.1; p < 0.00001; Table 6). This was based entirely on data from Hutton 1996 and may have been greater if dose changes had not been prevented in Steiger 1996 and data had been available for Miguel 1993.
There was a trend towards more dopaminergic adverse events with cabergoline but this did not reach statistical significance at the p < 0.01 level (Tables 9 to 16). There was a trend towards fewer withdrawals from cabergoline (Table 17).
Discussion
Only three randomised controlled trials have attempted to establish the efficacy and safety of cabergoline versus placebo in Parkinson's disease with motor complications. Two of these were small, short term, phase II studies (Miguel 1993; Steiger 1996) and only one phase III study over 24 weeks has been performed (Hutton 1996). The total number of randomised patients was 268.
The principal aim of dopamine agonist adjuvant therapy is to reduce the time patients spend in the relatively immobile off phase. Cabergoline provided a non‐significantly greater reduction in off time than placebo by a weighted mean difference of 1.14 hours/day (WMD; 95% CI ‐0.06, 2.33; p = 0.06; Table 7). Agonists often produce this benefit at the expense of increased dyskinesia. Unfortunately, insufficient information was available on dyskinesia, either from rating scales or as an adverse event, to draw any conclusions about this for cabergoline.
It is also hoped that adjuvant agonist therapy can improve motor impairments and disability. The larger phase III trial (Hutton 1996) did demonstrate greater improvements in UPDRS ADL and motor scores with cabergoline. This was probably not seen in the phase II trial reporting these outcomes as the numbers were too small and lower doses of cabergoline were used (Miguel 1993). The smaller studies also failed to show any difference in Schwab and England scale, possibly due to the small numbers. There was a significant benefit in clinicians global impression scale in Steiger 1996 in favour of cabergoline. In summary, although the evidence is scant, it seems likely that cabergoline produces a clinically small improvement in motor impairment and disability in this type of patient.
The significant superiority of cabergoline over placebo in terms of levodopa dose reduction was based on the evidence from the larger phase III study (Hutton 1996) which used comparable doses of cabergoline to those now used in clinical practice.
Any trends towards more adverse events with cabergoline failed to reach statistical significance. In contrast, the withdrawal rate showed a trend in favour of cabergoline, presumably due to its efficacy.
In summary, the reduction in levodopa dose produced by cabergoline was accompanied by a small benefit in motor impairment and disability. No significant differences in adverse events, including dyskinesia, were found but this may be due to the small sample size.
Valuable lessons can be learned from the problems with these studies:‐
It is important that all studies (including Miguel 1993) are published in some format to avoid publication bias.
The issue that sample size calculations are often restricted to efficacy outcomes and not safety reporting needs to be addressed. In this meta‐analysis, efficacy would appear to be reasonable but no clear comment on safety can be made as the studies were underpowered even after quantitative review.
The practice of excluding patients from further analysis if they have not achieved a predefined degree of improvement can be questioned. Full intention‐to‐treat analysis is more appropriate.
Reporting standards must be improved by the adoption of the CONSORT guidelines (CONSORT 1996).
Authors' conclusions
Implications for practice.
In the management of the motor complications seen in Parkinson's disease, cabergoline can be used to reduce levodopa dose and modestly improve motor impairment and disability. This is based on, at best, medium term evidence and no clear comments on safety can be made as the trials were probably underpowered in this regard.
Implications for research.
Incomplete Reporting In the future, adjuvant therapy trials in Parkinson's disease should:‐
All be published to avoid publication bias.
Should be reported using the CONSORT guidelines (CONSORT 1996).
Include valid sample size calculations which also take into account safety issues.
Provide full data on outcome measures including mean and standard deviation/error.
Express results in the original unit of measurement (hours rather than percentage off time).
Further Trials A summary systematic review is in preparation to draw together the results of the Cochrane reviews of lisuride, pergolide, pramipexole, ropinirole, and cabergoline versus placebo and the same agents versus bromocriptine. However, it is unlikely that robust conclusions on comparative efficacy and safety can be made based on the trials performed to date. Further much larger studies would be required to allow direct comparison of these agents in terms of efficacy, effectiveness, and safety.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2015 | Amended | PLS correction |
| 13 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 17 November 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors thank Pharmacia Upjohn for their assistance in performing this review.
Data and analyses
Comparison 1. Cabergoline versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 UPDRS ADL scores (part II) | Other data | No numeric data | ||
| 2 UPDRS motor score (part III) | Other data | No numeric data | ||
| 3 Hoehn and Yahr stage | Other data | No numeric data | ||
| 4 Schwab and England scale | Other data | No numeric data | ||
| 5 Clinicians global impression scale | Other data | No numeric data | ||
| 6 Levodopa dose reduction (mg) | 1 | 188 | Mean Difference (IV, Fixed, 95% CI) | 149.6 [94.12, 205.08] |
| 7 Off time reduction (hours) | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 1.14 [‐0.06, 2.33] |
| 8 Dyskinesia rating scale | Other data | No numeric data | ||
| 9 Adverse events ‐ Nausea | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.84 [0.97, 3.49] |
| 10 Adverse events ‐ Postural hypotension | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [1.11, 4.69] |
| 11 Adverse events ‐ Hallucinations | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [0.77, 5.57] |
| 12 Adverse events ‐ Confusion | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.73 [1.02, 13.67] |
| 13 Adverse events ‐ Dyskinesia | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.49 [0.13, 329.99] |
| 14 Adverse events ‐ Insomnia | 2 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.46, 2.15] |
| 15 Adverse events ‐ Sleep disorder | 1 | 188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.61 [0.07, 284.12] |
| 16 Adverse events ‐ Somnolence | 2 | 231 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [0.48, 5.48] |
| 17 All cause withdrawal rate | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.28, 1.18] |
1.1. Analysis.
Comparison 1 Cabergoline versus Placebo, Outcome 1 UPDRS ADL scores (part II).
| UPDRS ADL scores (part II) | |
|---|---|
| Study | |
| Hutton 1996 | Improvement on Cabergoline ‐2.9 (SD 7.6) v placebo ‐0.6 (SD 6.6). p=0.032. |
| Miguel 1993 | 'On' state: Improvement on cabergoline ‐1.7 (SD 2.83) (n=10) v placebo ‐1.29 (SD 4.39) (n=7) p > 0.05; NS. 'Off' state: Improvement on cabergoline ‐1.57 (SD 2.7) (n=7) v placebo ‐1.4 (SD 10.94) (n=10). p > 0.05; NS. |
| Steiger 1996 | Not available |
1.2. Analysis.
Comparison 1 Cabergoline versus Placebo, Outcome 2 UPDRS motor score (part III).
| UPDRS motor score (part III) | |
|---|---|
| Study | |
| Hutton 1996 | Improvement on Cabergoline ‐2.7 (SD 8.5) v placebo ‐1.1 (SD 8.0). p=0.031. |
| Miguel 1993 | 'On' state: Improvement on cabergoline ‐4.47 (SD 5.14) (n=17) v placebo ‐2.54 (SD 3.6) (n=13). p > 0.05; NS. 'Off' state: Improvement on cabergoline ‐10.88 (SD 6.42) (n=8) v placebo ‐0.91 (SD 17.57) (n=11). p > 0.05; NS. |
| Steiger 1996 | Not available |
1.3. Analysis.
Comparison 1 Cabergoline versus Placebo, Outcome 3 Hoehn and Yahr stage.
| Hoehn and Yahr stage | |
|---|---|
| Study | |
| Hutton 1996 | Improvement on Cabergoline (n=109) ‐0.18 (SD 0.76) v Placebo (n=54) ‐0.08 (SD 0.74) |
| Miguel 1993 | Not available |
| Steiger 1996 | 'On' phase: Improvement on Cabergoline ‐0.4 (SD 1.1) v placebo ‐0.2 (SD 1.1). 'Off' phase: Improvement on Cabergoline ‐0.3 (SD 1.0) v placebo 0.1 (SD 1.4). |
1.4. Analysis.
Comparison 1 Cabergoline versus Placebo, Outcome 4 Schwab and England scale.
| Schwab and England scale | |
|---|---|
| Study | |
| Hutton 1996 | Not available |
| Miguel 1993 | 'On' state: Improvement on cabergoline 1.76 (SD9.51) (n=17) v placebo ‐2.14 (SD6.99) (n=14) p > 0.05; NS. 'Off' state: Improvement on cabergoline 10 (SD24.25) (n=18) v placebo 6.67 (SD19.52) (n=15) p > 0.05; NS. |
| Steiger 1996 | 'On' phase: Improvement on Cabergoline 6 (SD 17) v placebo 1 (SD 23). p>0.05; NS. 'Off' phase: Improvement on Cabergoline 6 (SD 21) v placebo 2 (SD 29). p>0.05; NS. |
1.5. Analysis.
Comparison 1 Cabergoline versus Placebo, Outcome 5 Clinicians global impression scale.
| Clinicians global impression scale | |
|---|---|
| Study | |
| Hutton 1996 | Not available |
| Miguel 1993 | Not available |
| Steiger 1996 | Mean score on Cabergoline 1.5 (SD 1.1) v placebo 0.6 (SD 1.2). p<0.05. |
1.6. Analysis.
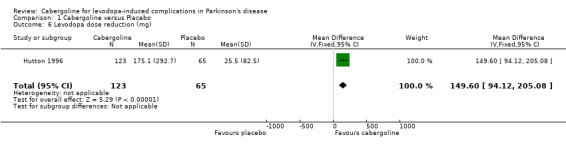
Comparison 1 Cabergoline versus Placebo, Outcome 6 Levodopa dose reduction (mg).
1.7. Analysis.
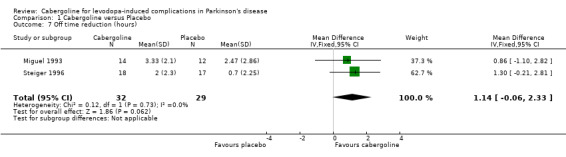
Comparison 1 Cabergoline versus Placebo, Outcome 7 Off time reduction (hours).
1.8. Analysis.
Comparison 1 Cabergoline versus Placebo, Outcome 8 Dyskinesia rating scale.
| Dyskinesia rating scale | |
|---|---|
| Study | |
| Hutton 1996 | Not available |
| Miguel 1993 | Not available |
| Steiger 1996 | Dyskinesia rating scale data not available but no significant difference was found between cabergoline and placebo. |
1.9. Analysis.
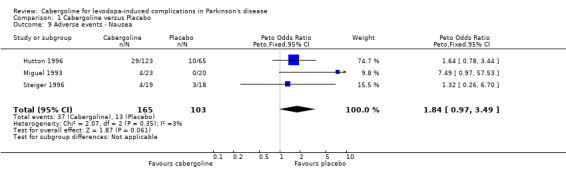
Comparison 1 Cabergoline versus Placebo, Outcome 9 Adverse events ‐ Nausea.
1.10. Analysis.
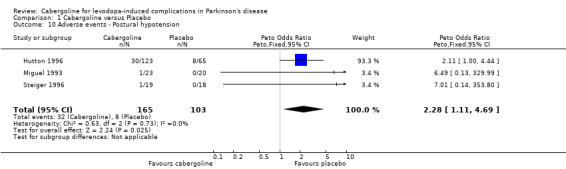
Comparison 1 Cabergoline versus Placebo, Outcome 10 Adverse events ‐ Postural hypotension.
1.11. Analysis.

Comparison 1 Cabergoline versus Placebo, Outcome 11 Adverse events ‐ Hallucinations.
1.12. Analysis.
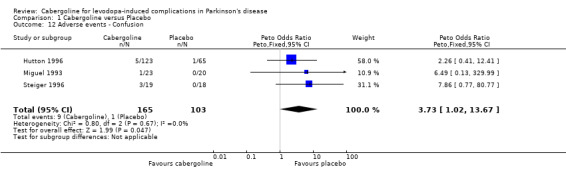
Comparison 1 Cabergoline versus Placebo, Outcome 12 Adverse events ‐ Confusion.
1.13. Analysis.
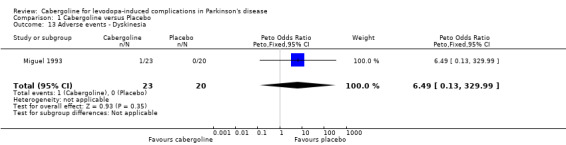
Comparison 1 Cabergoline versus Placebo, Outcome 13 Adverse events ‐ Dyskinesia.
1.14. Analysis.
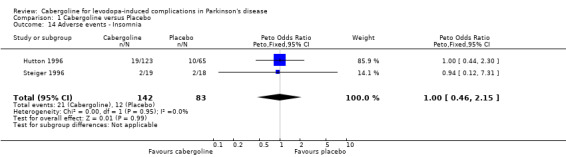
Comparison 1 Cabergoline versus Placebo, Outcome 14 Adverse events ‐ Insomnia.
1.15. Analysis.
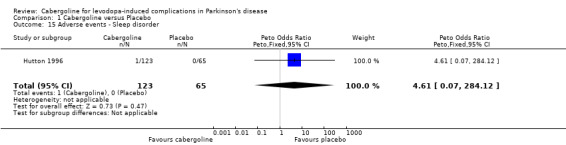
Comparison 1 Cabergoline versus Placebo, Outcome 15 Adverse events ‐ Sleep disorder.
1.16. Analysis.
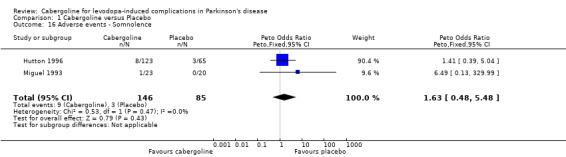
Comparison 1 Cabergoline versus Placebo, Outcome 16 Adverse events ‐ Somnolence.
1.17. Analysis.
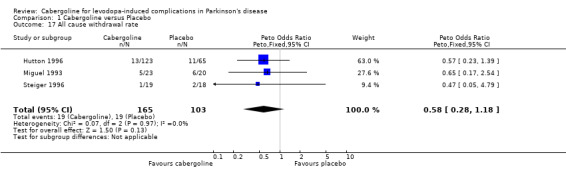
Comparison 1 Cabergoline versus Placebo, Outcome 17 All cause withdrawal rate.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hutton 1996.
| Methods | Randomised, double‐blind, parallel group design The sequence of study drug code numbers was generated prospectively, separately for each site, using 2:1 (cabergoline to placebo) randomisation schedule. All assignments were made under double blind conditions. Analysed on a per protocol basis Location: 10 sites in the USA Duration: 24 weeks | |
| Participants | Cabergoline: 123 patients with 13 drop‐outs (11%) Placebo: 65 patients with 11 drop‐outs (17%) Details of terminations given Mean age: Cabergoline = 63.4 (SD10.0) years, placebo 62.8 (SD8.9) years Hoehn and Yahr at baseline: Median 2.0 in both groups (no SD given) Inclusion criteria: IPD with end‐of‐dose wearing off or motor complications Exclusion criteria: On dopamine agonists within 1 month of trial | |
| Interventions | Cabergoline 0.5mg/day initially, increasing by 0.5mg increments every week to a maximum of 5.0mg/day. Mean dose of 3.66mg/day. Levodopa stable for 4 weeks. | |
| Outcomes | Primary: UPDRS ADL and motor subscales Secondary: On‐Off diaries (60 min epochs) Levodopa dose UPDRS parts I and IV Swab and England Hoehn and Yahr Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Miguel 1993.
| Methods | Randomised double‐blind parallel group design. Randomised according to a computer generated randomisation list. Patients received progressive randomistion numbers according to their temporal entry into the study. Method of data analysis unclear. Location: 5 sites, Spain. Duration: 6‐10 week titration period followed by a 4 week stable dose period (on 3‐4mg/day) if the patient had a >30% reduction in off hours. | |
| Participants | Cabergoline: 23 patients with 5 drop‐outs (22%) Placebo: 20 patients with 6 drop‐outs (30%) Details of terminations given. Mean age of patients, cabergoline 60 (SD8.9), placebo 62 (SD10.2). Inclusion criteria: IPD with motor fluctuations on l‐dopa, l‐dopa stable for 4 weeks, no other dopamine agonist for 4 weeks. Exclusion criteria: Other CNS or degenerative disorders, severe depression or dementia, cardiopathies, history of psychiatric disturbances on other dopamine agonists, renal or hepatic impairment, child‐bearing potetial. | |
| Interventions | Cabergoline initial dose 0.75mg/d. For 6 Cabergoline & 9 placebo; titrated over 6 weeks to a maximum of 2mg/d. For 12 cabergoline & 5 placebo; titrated over 10 weeks to maximum of 3mg/d. L‐dopa reduction allowed but only occured in 3 cabergoline and 1 placebo patient | |
| Outcomes | Primary: >30% reduction in off hours Secondary: UPDRS, all subsections Schwab & England Hoehn & Yahr Adverse events | |
| Notes | Only data to end of titration phase used as after that the population was selected for those who responded to the Cabergoline. Allowed withdrawals due to lack of efficacy after titration phase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Steiger 1996.
| Methods | Randomised, double‐blind, parellel group design. Method of randomisation not given. Per protocol analysis. Location ‐ Two centres UK Duration of therapy ‐Titration 12 weeks, then if patient achieved minimal benefit or greater, stable dose for 3 months | |
| Participants | Cabergoline: 19 patients with 1 drop‐out (5%) Placebo: 18 patients with 2 drop‐outs (11%) Details of terminations given Mean age of patients, cabergoline 60.8 years (SD9.1), placebo 63.4 years (SD7.2) Hoehn and Yahr scale at baseline, cabergoline = 3.5 (SD1.0), placebo = 3.5 (SD1.4) Inclusion criteria: IPD with motor complications Exclusion criteria: history of neuropsychiatric side effects with dopamine agonist therapy | |
| Interventions | Cabergoline; initial dose of 0.5mg/day. After 1 week the dose was increased fortnightly by 0.5mg/d increments up to 3mg/d. Then the dose was increased fortnightly by 1mg/d to 5mg/d. If patients reported continuing improvement with the 5mg/d dose compared to the 4mg/d, further dosage increments were allowed up to 10mg/d. Titration 12 weeks, then if patient achieved minimal benifit or greater, stable dose for 3 months Mean dose = 5.4 (SD1.9) mg/day. Levodopa dose and intervals constant. | |
| Outcomes | Primary: none given Secondary: Clinicians Global Improvement Scale Hoehn and Yahr Swab and England On‐Off diary (60 min epochs) Dyskinesia scale (ad hoc 5 point scale ‐ no reference given) Levodopa dose Adverse events | |
| Notes | Only data up to end of titration phase used as after that the population was selected for those that responded to the Cabergoline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahlskog 1994 | Randomised to 5 different doses |
| Ahlskog 1996 | Included in Hutton 96 |
Declarations of interest
CEC has received payment from Pharmacia Upjohn for lectures and attending meetings.
Edited (no change to conclusions)
References
References to studies included in this review
Hutton 1996 {published and unpublished data}
- Ahlskog JE, Wright KF, Muenter MD, Adler CH. Adjunctive cabergoline therapy of Parkinson's disease: comparison with placebo and assessment of dose responses and duration of effect. Clinical Neuropharmacology 1996;19:202‐212. [DOI] [PubMed] [Google Scholar]
- Hutton JT, Koller WC, Ahlskog JE, et al. Multicentre, placebo‐controlled trial of cabergoline taken once daily in the treatment of Parkinson's disease. Neurology 1996;46:1062‐1065. [DOI] [PubMed] [Google Scholar]
Miguel 1993 {unpublished data only}
- Miguel F, Obeso JA, Olive Plana JM, Tolosa E, Villaueva Eusa JA, Dubini A, et al. Double‐blind parallel group study of the efficacy and tolerability of the cabergoline vs placebo in Parkinsonian patients with motor fluctuations. FCE Report: 21336/722i, Clinical Reference 29 1993.
Steiger 1996 {published data only}
- Steiger MJ, El‐Debas T, Anderson T, Findley LJ, Marsden CD. Double‐blind study of the activity and tolerability of cabergoline versus placebo in parkinsonains with motor fluctuations. Journal of Neurology 1996;243:68‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahlskog 1994 {published data only}
- Ahlskog JE, Muenter MD, Maraganore DM, et al. Fluctuating Parkinson's disease. Treatment with the long‐acting dopamine agonist cabergoline. Archives of Neurology 1994;51:1236‐1241. [DOI] [PubMed] [Google Scholar]
Ahlskog 1996 {published data only}
- Ahlskog EJ, Wright KF, Muenter MD, Adler CH. Adjunctive cabergoline therapy of Parkinson's disease: comparison with placebo and assessment of dose responses and duration of effect. Clinical Neuropharmacology 1996;19:202‐212. [DOI] [PubMed] [Google Scholar]
Additional references
CONSORT 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials: the CONSORT statement.. Journal of the American Medical Association 1996;276(8):637‐639. [DOI] [PubMed] [Google Scholar]
Quinn 1986
- Quinn N, Critchley P, Parkes D, Marsden CD. When should levodopa be started?. Lancet 1986;ii:985‐986. [DOI] [PubMed] [Google Scholar]
Rajput 1984
- Rajput AH, Stern W, Laverty WH. Chronic low‐dose levodopa therapy in Parkinson's disease. Neurology 1984;34:991‐996. [DOI] [PubMed] [Google Scholar]


