Abstract
Background
Smoking is the leading preventable cause of illness and premature death worldwide. Some medications have been proven to help people to quit, with three licensed for this purpose in Europe and the USA: nicotine replacement therapy (NRT), bupropion, and varenicline. Cytisine (a treatment pharmacologically similar to varenicline) is also licensed for use in Russia and some of the former socialist economy countries. Other therapies, including nortriptyline, have also been tested for effectiveness.
Objectives
How do NRT, bupropion and varenicline compare with placebo and with each other in achieving long‐term abstinence (six months or longer)? How do the remaining treatments compare with placebo in achieving long‐term abstinence? How do the risks of adverse and serious adverse events (SAEs) compare between the treatments, and are there instances where the harms may outweigh the benefits?
Methods
The overview is restricted to Cochrane reviews, all of which include randomised trials. Participants are usually adult smokers, but we exclude reviews of smoking cessation for pregnant women and in particular disease groups or specific settings. We cover nicotine replacement therapy (NRT), antidepressants (bupropion and nortriptyline), nicotine receptor partial agonists (varenicline and cytisine), anxiolytics, selective type 1 cannabinoid receptor antagonists (rimonabant), clonidine, lobeline, dianicline, mecamylamine, Nicobrevin, opioid antagonists, nicotine vaccines, and silver acetate. Our outcome for benefit is continuous or prolonged abstinence at least six months from the start of treatment. Our outcome for harms is the incidence of serious adverse events associated with each of the treatments. We searched the Cochrane Database of Systematic Reviews (CDSR) in The Cochrane Library, for any reviews with 'smoking' in the title, abstract or keyword fields. The last search was conducted in November 2012. We assessed methodological quality using a revised version of the AMSTAR scale. For NRT, bupropion and varenicline we conducted network meta‐analyses, comparing each with the others and with placebo for benefit, and varenicline and bupropion for risks of serious adverse events.
Main results
We identified 12 treatment‐specific reviews. The analyses covered 267 studies, involving 101,804 participants. Both NRT and bupropion were superior to placebo (odds ratios (OR) 1.84; 95% credible interval (CredI) 1.71 to 1.99, and 1.82; 95% CredI 1.60 to 2.06 respectively). Varenicline increased the odds of quitting compared with placebo (OR 2.88; 95% CredI 2.40 to 3.47). Head‐to‐head comparisons between bupropion and NRT showed equal efficacy (OR 0.99; 95% CredI 0.86 to 1.13). Varenicline was superior to single forms of NRT (OR 1.57; 95% CredI 1.29 to 1.91), and to bupropion (OR 1.59; 95% CredI 1.29 to 1.96). Varenicline was more effective than nicotine patch (OR 1.51; 95% CredI 1.22 to 1.87), than nicotine gum (OR 1.72; 95% CredI 1.38 to 2.13), and than 'other' NRT (inhaler, spray, tablets, lozenges; OR 1.42; 95% CredI 1.12 to 1.79), but was not more effective than combination NRT (OR 1.06; 95% CredI 0.75 to 1.48). Combination NRT also outperformed single formulations. The four categories of NRT performed similarly against each other, apart from 'other' NRT, which was marginally more effective than NRT gum (OR 1.21; 95% CredI 1.01 to 1.46). Cytisine (a nicotine receptor partial agonist) returned positive findings (risk ratio (RR) 3.98; 95% CI 2.01 to 7.87), without significant adverse events or SAEs. Across the 82 included and excluded bupropion trials, our estimate of six seizures in the bupropion arms versus none in the placebo arms was lower than the expected rate (1:1000), at about 1:1500. SAE meta‐analysis of the bupropion studies demonstrated no excess of neuropsychiatric (RR 0.88; 95% CI 0.31 to 2.50) or cardiovascular events (RR 0.77; 95% CI 0.37 to 1.59). SAE meta‐analysis of 14 varenicline trials found no difference between the varenicline and placebo arms (RR 1.06; 95% CI 0.72 to 1.55), and subgroup analyses detected no significant excess of neuropsychiatric events (RR 0.53; 95% CI 0.17 to 1.67), or of cardiac events (RR 1.26; 95% CI 0.62 to 2.56). Nortriptyline increased the chances of quitting (RR 2.03; 95% CI 1.48 to 2.78). Neither nortriptyline nor bupropion were shown to enhance the effect of NRT compared with NRT alone. Clonidine increased the chances of quitting (RR 1.63; 95% CI 1.22 to 2.18), but this was offset by a dose‐dependent rise in adverse events. Mecamylamine in combination with NRT may increase the chances of quitting, but the current evidence is inconclusive. Other treatments failed to demonstrate a benefit compared with placebo. Nicotine vaccines are not yet licensed for use as an aid to smoking cessation or relapse prevention. Nicobrevin's UK license is now revoked, and the manufacturers of rimonabant, taranabant and dianicline are no longer supporting the development or testing of these treatments.
Authors' conclusions
NRT, bupropion, varenicline and cytisine have been shown to improve the chances of quitting. Combination NRT and varenicline are equally effective as quitting aids. Nortriptyline also improves the chances of quitting. On current evidence, none of the treatments appear to have an incidence of adverse events that would mitigate their use. Further research is warranted into the safety of varenicline and into cytisine's potential as an effective and affordable treatment, but not into the efficacy and safety of NRT.
Plain language summary
Medications to help people to stop smoking: an overview of reviews
Background Smoking is a main cause of early death throughout the world. There are a number of medications which can help people to quit smoking. Three of these, nicotine replacement therapy (NRT), bupropion and varenicline, are licensed for this purpose in the USA and Europe. Cytisine (similar to varenicline) is licensed for use in Russia and Eastern Europe. We reviewed studies of these and other treatments, including nortriptyline, to compare their benefits and risks. Methods We found 12 Cochrane reviews of different treatments. The treatments include nicotine replacement therapy (NRT); antidepressants (bupropion and nortriptyline); nicotine receptor partial agonists (varenicline and cytisine); anxiolytics; selective type 1 cannabinoid receptor antagonists (rimonabant); clonidine; lobeline; dianicline; mecamylamine; Nicobrevin; opioid antagonists; nicotine vaccines; and silver acetate. The reviews were conducted between 2008 and 2012, and analysed 267 trials, covering more than 101,000 smokers. All the reviews used randomised controlled trials, and compared the active treatment with a placebo, and sometimes with other treatments. The outcomes were measured at least six months from the start of treatment, and the results were usually checked by testing breath, blood or urine. We also assessed the risk of harms from each treatment. We then compared NRT, bupropion and varenicline with each other, using a network meta‐analysis. Results NRT and bupropion helped about 80% more people to quit than placebo; this means that for every 10 people who quit with placebo about 18 could be expected to quit with NRT or with bupropion. Varenicline more than doubled the chances of quitting compared with placebo, so that for every 10 who quit with placebo about 28 could be expected to quit with varenicline. Varenicline helped about 50% more people to quit than nicotine patch and 'other' NRT (tablets, sprays, lozenges and inhalers), and about 70% more people than nicotine gum. So for every 10 people who quit with NRT patch or with 'other' NRT, about 15 could be expected to quit with varenicline, and for every 10 who quit with NRT gum about 17 could be expected to quit with varenicline. Combining two type of NRT was as effective as using varenicline, and helped more people to quit than single types of NRT. There was little to choose between different types of NRT, apart from 'other' NRT, which helped slightly more people than nicotine gum; for every 10 people who quit with NRT gum, about 12 could be expected to quit with 'other' NRT. NRT combined with nortriptyline or with bupropion was not more effective than NRT alone. Both cytisine and nortriptyline compared with placebo improved the chances of quitting, with minimal risk of harms. Bupropion carries a known risk of seizures (about 1 per 1000 users), but we found fewer than expected in the included and excluded trials, at about 1 in 1500. Although there may be a marginal increase in the likelihood of any serious adverse event while taking bupropion, we did not find increased risks of neuropsychiatric or heart and circulatory problems in the bupropion studies. The evidence for the safety of varenicline is still under investigation; we found no evidence from the trials that it is linked to an increase in neuropsychiatric problems, or with increased heart and circulatory problems. Clonidine helped people to quit, but caused side effects. It is not clear whether or not mecamylamine used with NRT helps people to quit. Other treatments did not seem to help. So far, nicotine vaccines are not licensed for use anywhere in the world. Nicobrevin is no longer available in the UK, and rimonabant, taranabant and dianicline have all been withdrawn from the market. Conclusions NRT, bupropion and varenicline all improve the chances of quitting, with a low risk of harms. Combination use of NRT is as effective as varenicline, and more effective than single types of NRT. Cytisine has potential as a safe, effective and affordable treatment. Nortriptyline improves the chances of quitting, with little evidence of harmful events. We need continued monitoring of the safety of varenicline. More research into NRT versus placebo is unlikely to change our understanding of the treatment.
Background
Smoking remains the leading preventable cause of illness and premature death worldwide, accounting for 20% of deaths in men over 30 years of age, and 5% in women (Disease Control Priorities 2006). There are more than 435,000 smoking‐related deaths annually in the United States (Fiore 2008), and 82,900 in England (NHS 2008). Morbidity associated with tobacco use includes a broad range of cancers, respiratory and cardiovascular diseases. It has been estimated that for every death caused by smoking, approximately 20 smokers are suffering from a smoking‐related illness (MMWR 2003). In China and Russia, the prevalence of smoking among adult men exceeds 60% (Tobacco Atlas 2010). Currently in the USA and the UK, around 21% of adults continue to smoke (MMWR 2007; Fiore 2008; GLS 2009). In 2007, 70% of American smokers and 74% of British smokers reported that they wanted to quit, with most citing health and financial reasons (Fiore 2008; NHS 2008). In a survey of more than 5,000 adults in England in 2006, about half of those smoking had made at least one quit attempt in the past year, yielding an estimated permanent cessation rate of between 2 and 3% annually (West 2006).
Description of the condition
Tobacco products contain nicotine, a substance now acknowledged to be as addictive as heroin or cocaine (SCOTH 1998; RCP 2000). Nicotine triggers the release of dopamine and other neurotransmitters in the brain, which reinforce the smoker's dependence on tobacco. With long‐term habituation, smoking may become a self‐medicating behaviour, which reduces negative affect and modulates withdrawal symptoms, over and above its positive reinforcement properties (Benowitz 2008). Smokers with life‐threatening illnesses that may in part be attributable to their use of tobacco still have great difficulty in achieving permanent abstinence, with as many as 70% of those surviving a heart attack resuming smoking within a year (40% while still in hospital), and about 50% of lung cancer patients returning to smoking after surgery (Stapleton 1998).
Description of the interventions
NRT, bupropion and varenicline are widely available on prescription and in the case of NRT as an over‐the‐counter medication. They are licensed as first‐line treatments for use as smoking cessation aids in the USA and the European Union, and are widely recommended in many national guidelines. We have therefore concentrated on these three treatments in this overview. However, we also review the efficacy and safety of cytisine, a selective nicotinic receptor partial agonist of a similar type to varenicline, and the antidepressant nortriptyline.
1. Nicotine replacement therapy (NRT). This aims to reduce motivation to smoke and the physiological and psychological withdrawal symptoms often experienced during a quit attempt. It is available as patches in various dosages (absorbed slowly through the skin), and as chewing gum, lozenges, sublingual tablets, sprays and inhalers (absorbed through the oral or nasal mucosa). The treatment was first developed in the 1970s, and is widely available on prescription, or as an over‐the‐counter purchase in many countries. However, the World Health Organization currently estimates that at least 38 countries do not yet support any provision of NRT (WHO 2009).
2. Bupropion: This was developed as a non‐tricyclic antidepressant, and is sometimes preferred by smokers who do not wish to use a nicotine‐based treatment, or who have already failed to quit using NRT. The usual dose for smoking cessation is 150 mg once a day for three days increasing to 150 mg twice a day, continued for 7 to 12 weeks. The quit attempt is generally initiated a week after starting pharmacotherapy.
3. Nortriptyline: This is a tricyclic antidepressant, and is sometimes prescribed when first‐line treatments have been unsuccessful. It is licensed as a smoking cessation aid in New Zealand. The recommended regimen is a period of titration (10 ‐ 28 days) before the quit attempt, and a 12‐week therapeutic dose of 75 to 100 mg daily.
These medications are available only via prescription.
4. Varenicline: This is a selective nicotinic receptor partial agonist, licensed as a prescription‐only treatment for smoking cessation in the USA in 2006, and in Europe in 2006/2007. The standard regimen is 1mg twice a day for 12 weeks, with the first week titrated to reduce side effects, and quit date set for the second week.
5. Cystisine: This is pharmacologically similar to varenicline. Although it has been used for almost 50 years as a cessation aid, it is currently licensed only in Russia and in some former socialist economy countries, including Poland and Bulgaria. The standard regimen is a 25‐day course, gradually reducing from six 1.5 mg tablets a day to two tablets a day by the end of the treatment period, with a quit date set for day five.
Other medications which we cover in this overview include:
Antidepressants, including tricyclics, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, atypical antidepressants, and extracts of Hypericum perforatum (St John's Wort)
Anxiolytics, including buspirone, diazepam, doxepin, meprobamate, ondansetron, and the beta‐blockers metoprolol, oxprenolol and propanolol
Selective cannabinoid type 1 receptor antagonists, including rimonabant and taranabant
Clonidine
Lobeline, dianicline
Mecamylamine
Nicobrevin (a proprietary brand mixture of quinine, camphor, menthol and eucalyptus oil)
Nicotine vaccines
Opioid antagonists, including naltrexone, naloxone and buprenorphine
Silver acetate
How the intervention might work
Different treatments incorporate different mechanisms, but the underpinning principles are: (i) to mitigate the craving and withdrawal symptoms often associated with a quit attempt, and/or (ii) to reduce the reward derived from smoking by indirectly disrupting dopamine release or by desensitising receptors, and/or (iii) to deliver some positive reinforcement other than from a cigarette. It should be noted that the precise mechanisms for some therapies are still under investigation.
The major mechanisms of action, singly or in combination, are believed to be:
to block nicotine or blunt the effects of nicotine on its receptors or receptors in pharmacological pathways affected by nicotine; these include bupropion, vaccines, mecamylamine, the nicotine receptor partial agonists (varenicline, cytisine, dianicline), selective type 1 cannabinoid receptor antagonists (rimonabant, taranabant), and the opioid antagonists; *
to relieve withdrawal: these include nicotine replacement therapies, lobeline, varenicline, Nicobrevin;
to substitute for nicotine's effects: these include anxiolytics, antidepressants, clonidine, bupropion;
aversive therapy: silver nitrate;
sensory replacement: Nicobrevin.
Why it is important to do this overview
There is currently a range of pharmacological treatments to help smokers who wish to quit, and a considerable body of research which tests both their efficacy and their safety. The aim of this review is to provide relevant information to tobacco users, clinicians and policy makers, and to attempt to balance the potential benefits and harms associated with the treatments.
Objectives
To conduct an overview of Cochrane reviews which assess the efficacy and safety of pharmacological interventions designed to support smoking cessation attempts.
As part of this overview, we address the following issues:
1. How do nicotine replacement therapy, bupropion and varenicline compare with each other for efficacy, defined in this overview as the achievement of long‐term abstinence (six months or longer)? This question is explored using direct and indirect comparisons where appropriate.
2. How do the risks of adverse and serious adverse events compare between the treatments, and are there instances where the harms significantly outweigh the benefits?
3. Which of the other available pharmacological treatments might help smokers to quit?
4. Are there limitations in the current evidence base which may compromise the precision or stability of any conclusions drawn by this overview? If so, what are the implications for future research?
Methods
Criteria for considering reviews for inclusion
Types of studies
In accordance with the standard criteria for Cochrane reviews of pharmacological treatments for smoking cessation, we have restricted the included studies in this overview to randomised controlled trials for the estimation of efficacy. For an assessment of harmful effects we have also included post‐marketing surveillance data where these are available and appropriate.
Types of participants
We include all participants covered by the pharmacotherapy‐based ('primary') reviews included in this overview. These are usually adult smokers, of either gender, and of any nationality and ethnicity. We have not included all the data from those reviews which focus on particular populations of smokers, e.g. adults with mental health problems (Tsoi 2010; van der Meer 2009), smokeless tobacco users (Ebbert 2011), or pregnant women (Lumley 2009), as such reviews cover a range of interventions beyond the pharmacotherapies which are the subject of this overview. However, trials of pharmacological interventions which target specific groups of smokers, settings, intervention delivery and cessation techniques are included within the relevant sections of this overview, classified by the type of intervention.
Types of interventions
Interventions include nicotine replacement therapy (NRT), antidepressants (bupropion and nortriptyline), nicotine receptor partial agonists (varenicline and cytisine), anxiolytics, selective type 1 cannabinoid receptor antagonists (rimonabant), clonidine, lobeline, dianicline, mecamylamine, Nicobrevin, opioid antagonists, nicotine vaccines, and silver acetate. These interventions may be delivered as monotherapies or in combination.
We assess the impact of variations in the formulations (e.g. different types of NRT), and single versus combination treatments. The comparison conditions include placebo, other pharmacological treatments or combinations of treatments, and usual or standard care.
Types of outcomes
The primary beneficial outcome for this overview is sustained smoking cessation, i.e. for six months or longer. The preferred outcome is biochemically validated continuous or prolonged abstinence at the longest reported time point, and including all participants randomised in their original groups (an intention‐to‐treat analysis).
Secondary beneficial outcomes include:
reduction of withdrawal symptoms
reduction of craving
Although some clinical trials include smoking reduction as one of their target outcomes, the primary outcome of interest for this overview is limited to abstinence from smoking.
The primary harmful outcome is any serious or life‐threatening adverse event which may, in the trialists' opinion, be attributable to the pharmacological treatment. These may include psychological disorders, such as depression, anxiety, suicidal ideation or suicidal behaviour, and neurological events such as seizures.
Secondary harmful outcomes will vary between treatments, but may include:
psychiatric disorders
gastrointestinal disorders
cardiovascular problems
insomnia and other sleep disorders
skin disorders
allergic or hypersensitive reactions
drop‐outs due to adverse events
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews (CDSR) in The Cochrane Library, for any reviews with "smoking" in the title, abstract or keyword fields. The search was conducted in November 2012. We then identified reviews of pharmacological treatments for smoking cessation, for possible inclusion in this overview. Since Cochrane reviews strive for methodological rigour and are regularly updated, we have not sought non‐Cochrane reviews for inclusion within this overview.
Data collection and analysis
Selection of reviews
Two authors (KC and RP) independently assessed all potentially eligible reviews identified by the search strategy.
Data extraction and management
We extracted data from each included review. The data extraction form summarises key information from each review, including details of the participants, the interventions, the comparisons and the outcomes. Outcomes wherever possible include both beneficial and harmful effects of the treatments. One author (KC) extracted the data, and a second author (RP) verified the information extracted. Any persistent disagreement would have been referred to the fourth author (TL) for discussion and resolution.
Assessment of methodological quality of included reviews
We used the AMSTAR measurement tool (adapted from Shea 2007; Evans 2009) to assess the quality of the included reviews. This modified instrument comprises the following 11 items:
1. Was an 'a priori' design provided?. 2. Was there duplicate study selection and data extraction? 3. Was a comprehensive literature search performed? 4. Were published and unpublished studies eligible, irrespective of language of publication? 5. Was a list of studies (included and excluded) provided? 6. Were the characteristics of the included studies provided? 7. Was the scientific quality of the included studies assessed and documented? 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? 9. Were the methods used to combine the findings of studies appropriate? 10. Was the likelihood of publication bias assessed? 11. Was the conflict of interest stated?
Each criterion is rated as 'Yes' (definitely done), 'No' (definitely not done), 'Can't answer' (status unclear) or 'Not applicable'. A 'Yes' rating is taken to indicate adequate quality. Criteria rated as 'Not applicable' (e.g. legitimacy of methods for combining studies where included studies were absent or could not be combined) are not counted against the review, but are removed from the denominator with appropriate adjustment to the ranking (Shea 2011).
We have ranked the included reviews as being of high quality (scoring 8‐11), of medium quality (scoring 4‐7), or of low quality (scoring 0‐3). We have not excluded reviews on the basis of AMSTAR rankings, but have conducted sensitivity analyses where applicable to explore the consequences of synthesising reviews of differing quality.
We have evaluated the overall quality of the evidence for each outcome using the GRADE system (Atkins 2004). This approach identifies four elements which influence the quality of the evidence: these are study design, study quality, consistency (between estimates of effect across studies) and directness (i.e. applicability of participants, interventions and outcomes to the clinical question under consideration). Assessing and combining these components determine the initial grade of the evidence as:
High: Further research is very unlikely to change our confidence in the estimate of effect
Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: Any estimate of effect is very uncertain.
The initial assessment is determined by the study design: randomised trial = high Observational study = low Any other evidence = very low. The grade is then decreased if:
Serious (‐1) or very serious (‐2) limitation to study quality
Important inconsistency (‐1)
Some (‐1) or major (‐2) uncertainty about directness
Imprecise or sparse data (‐1)
High probability of reporting bias (‐1)
The grade is increased if:
Strong evidence of association ‐ significant risk ratio of >2 (<0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1)
Very strong evidence of association ‐ significant risk ratio of >5 (<0.2) based on direct evidence with no major threats to validity (+2)
Some (‐1) or major (‐2) uncertainty about directness
Evidence of a dose‐response gradient (+1)
All plausible confounders would have reduced the effect (+2)
Data synthesis
To assess the efficacy of the target treatments, we have as far as possible conducted this overview at review level, and have not re‐analysed the included studies within the candidate reviews, but have used the existing point estimates and pooled analyses. We present direct comparisons wherever possible, but where head‐to‐head comparisons of adequate quality were not available (e.g. NRT versus varenicline) we have undertaken indirect comparisons.
Where the source reviews include meta‐analyses, we conducted comparisons of the pooled estimates of efficacy for each treatment versus placebo, taking account of the definition of abstinence (continuous, prolonged, point prevalence) and the length of follow‐up (six or twelve months). Where the source reviews do not include meta‐analyses [lobeline, mecamylamine, Nicobrevin] we incorporate brief narrative assessments.
For NRT, bupropion and varenicline, we have conducted two separate network meta‐analyses using study level data and a Bayesian hierarchical model approach. The first analysis compares the efficacy of all three treatments with that of placebo and the second analysis also assessed efficacy but with NRT split by type (patch, gum, combination or 'other' (inhalers, sprays, tablets and lozenges)).
Network meta‐analyses differ from standard pairwise meta‐analyses primarily because they use information across all available comparisons to estimate indirect pairwise comparisons not previously tested. For example, in a pairwise meta‐analysis, to compare the effect of treatment A with treatment B, only trials comparing A and B directly in the same trial are included in the analysis. However, in a network meta‐analysis, it is also possible to use information from trials comparing A with C and B with C, where C is a common comparator treatment. To do this, we assume that the effect of A compared to B is given by the effect of A compared to C plus the effect of C compared to B.
We estimated log‐odds ratios from a random‐effects homogeneous variance consistency model using MCMC simulation (Lu 2004; Lu 2006: van Valkenhoef 2012a) and non‐informative prior probabilities. For each of the three models 100,000 burn‐in iterations were performed followed by 100,000 updates, across four chains. For the estimation we used a thinning interval of 10. We assessed convergence using the Brooks‐Gelman‐Rubin diagnostic tool and visual inspection of diagnostic plots (Brooks 1998). Although the source reviews report their findings as fixed‐effect risk ratios, we have generated odds ratios for the network meta‐analyses, in accordance with the properties of the Bayesian model, i.e. relative effects between treatments are assumed additive (consistent) and approximately normally distributed, on the log‐odds scale.
To determine whether the assumption of consistency was valid, we compared the consistency model results with those from an inconsistency model. The inconsistency model relaxes the assumption that the relative effects are additive on the log‐odds scale. We also compared the deviance information criterion (DIC) statistic from both models. A difference of 3 or more between the two DIC values is thought to be meaningful and an indication that the consistency assumption may not be met (Spiegelhalter 2002). We performed consistency and inconsistency model simulations using R (2.15.1; Team 2012) and the GeMTC package (van Valkenhoef 2012b). DIC calculations were made using GeMTC 0.14 (van Valkenhoef 2012a) to generate JAGS code and the R/JAGS interface package RJAGS (Plummer 2003; Plummer 2012; Team 2012). We also include distribution of probabilities ranking plots for the efficacy of smoking cessation treatments.
In our review, the large number of studies included for each one of the head‐to‐head comparisons make the choice of priors less crucial in determining the final estimates. As a reflection of this, there is good agreement between the point estimates and the 95% confidence interval and 95% credibility interval from the direct and indirect comparisons. These are presented in Appendix 1, and the rationale for the choice of priors in Appendix 2.
To assess serious adverse events (SAEs), we have revisited where possible the individual trials and conducted additional binary (non‐network) meta‐analyses. The data were retrieved from a mixture of published trial reports and from study‐based web synopses released by Pfizer Inc (manufacturers of varenicline). NRT has not been included in these analyses, as we found little or no information about SAEs in the trial reports.
Although we intend to maintain this overview with timely updates, in accordance with Cochrane policy, these will not necessarily be triggered by updates to the included reviews. Our decision to update the overview will be influenced by the likelihood that updates to the source reviews may substantively modify the key findings of this overview.
Results
We restricted our searching to the Cochrane Database of Systematic Reviews (CDSR), and identified 60 full reviews with 'smoking cessation' in the title, abstract or keywords. Thirty‐six of these were discarded as not including pharmacotherapies among their interventions. Of the remaining 24, 12 reviews had at least one pharmacotherapy as their main or only intervention, while the other 12 reviews included one or more comparisons of pharmacological products among the range of interventions that they tested (see Figure 1, PRISMA diagram). Of the latter group 11 covered NRT, six covered bupropion, two covered varenicline, and one covered naltrexone. They focused on candidate groups of smokers (Adolescent cessation 2006; COPD patients 2001; Hospital patients 2012; Pre‐operative patients 2010; Pregnancy 2009; Schizophrenia 2010), on settings (Internet 2010; Workplace 2008), on healthcare providers (Pharmacists 2004), and on cessation techniques (Reduction vs abrupt 2010; Relapse prevention 2009; Weight gain prevention 2009). Apart from two of the bupropion trials (Levine 2010; Planer 2011) which will be considered for inclusion in the next update of Antidepressants 2007, we did not find additional data to supplement that already covered in the treatment‐based reviews. The most recent search was conducted in November 2012.
1.
Study flow diagram.
Description of included reviews
This overview covers 12 Cochrane reviews, which investigate 26 pharmacotherapies. The most prominent of these are nicotine replacement therapy (NRT 2012), bupropion (Antidepressants 2007) and varenicline (Nicotine receptor partial agonists 2012), all of which are licensed as treatments for smoking cessation in high‐income countries The key features of all the included reviews are displayed in Table 1.
1. Characteristics of included reviews.
| Review ID | Assessed as up‐to‐date | Participants | Interventions |
Comparisons |
Outcomes |
N Pts/trials |
Review limitations |
|
Antidepressants Hughes 2007 |
June 2009 | Current smokers, mostly adult but two studies of adolescents |
Bupropion | 1. vs placebo 2. + NRT vs NRT alone 3. vs patch 4. vs varenicline | 6m+ abstinence: ‐ all; ‐ by setting; ‐ by level of behavioural support; ‐ by dosage. | 1.11440/36 2. 1106/6 3. 657/3 4. 1622/3 | Biochemical validation was reported in all B trials except Swan 2003, and in all N trials except Da Costa 2002.
Four B trials included based on abstracts only.
Sensitivity analyses of each limitation made no difference to findings. |
| Nortriptyline | 1. vs placebo 2. +NRT vs NRT alone 3. vs NRT | 6‐12m | 1. 975/6 2. 1219/4 3. 417/3 | ||||
| Fluoxetine | 1. vs placebo 2. +NRT vs placebo+NRT | 6 or 12m | 1. 1236/2 2. 250/2 | ||||
| Paroxetine | vs placebo | 6m | 224/1 | ||||
| Sertraline | vs placebo | 6m | 134/1 | ||||
| MAOIs: Moclobemide Selegiline | 1. vs placebo 2. vs placebo | 12m, 6m | 1. 88/1 2. 250/3 | ||||
| Venlafaxine | +NRT vs placebo+NRT | 12m | 147/1 | ||||
|
Anxiolytics Hughes 2011 |
October 2009 | Any smokers | Buspirone | 1. vs placebo 2. vs NRT | 1. 12m 2. 6m | 409/4 | Most trials did not report methods in enough detail to assess quality of randomisation. Abstinence often not clearly defined or bio‐validated. Meprobamate outcome was reduction, not abstinence. |
| Diazepam | vs placebo | 6m | 76/1 | ||||
| Meprobamate | 1. vs placebo 2. +individual counselling vs placebo 3. +group counselling vs placebo | reduction >85% at12m | 216/1 | ||||
| Oxprenolol | vs placebo | 12m | 130/1 | ||||
| Metoprolol | |||||||
|
CB1 receptor antagonists (rimonabant) Cahill 2011 |
January 2011 | Adult smokers | Rimonabant | 1. 20mg vs placebo 2. 5mg vs placebo 3. 20mg vs 5mg | 50 weeks | 1049/2 | Study reports unpublished, not peer‐reviewed |
| Taranabant | 1. vs placebo | 8 wks | N/A | N/A | |||
| Clonidine Gourlay 2008 | June 2008 | Adult smokers (4/6 trials specified heavy smokers) | Clonidine | 1. C vs placebo | 6m or 12m | 776/6 | No details of randomisation or blinding; Abstinence not clearly defined |
| Lobeline Stead 2009 | January 2009 | Any smokers | Lobeline | 1. vs placebo 2. vs any treatment | 6m+ | 0/0 | N/A |
| Mecamylamine Lancaster 2011 | December 2010 | Healthy volunteer smokers | Mecamylamine | 1. +NRT vs placebo+NRT 2. +NRT vs M vs NRT vs placebo | 6m or 12m | 128/2 (no MA) | Studies too small to be conclusive |
| Nicobrevin Stead 2009 | January 2009 | Adult smokers | Nicobrevin | 1. vs placebo | 6m | 0/0 | N/A |
| Nicotine vaccines Hartmann‐Boyce 2012 | 2012 | Adult smokers | NicVAX |
1. vs placebo 2. High Ab vs placebo 3. 400 vs 200 ‐ 4 shots ‐ 5 shots 4. 5 vs 4 shots ‐ 200ug ‐ 400ug |
12m, 6m |
201/1 |
Hatsukami 2011 stratified active group to give the highest level of statistical significance. Nabi trials gave insufficient info to assess. |
| NIC002 | 1. vs placebo 2. High Ab vs placebo |
12m, 6m | 341/1 | Possibly post‐hoc stratification to give significant results. | |||
| NRT Stead 2013 |
October 2012 |
Adult smokers (not trials that randomised therapists) |
Nicotine Replacement Therapy |
1. Any NRT vs placebo
1. Gum
2. Patch
3. Inhaler 4. Nasal spray 5. Tabs/lozenge 6. Oral spray 7. Choice of NRT 8. Patch + Inhaler 9. Patch + lozenge |
6m+ |
51265/119
22581/56
19586/43
976/4 887/4 3405/7 479/1 2798/5 245/1 308/1 |
In 1.1, 2 trials (Shiffman 2002, Shiffman 2009) split into separate dosage comparisons 26% of studies rated as at low risk of bias for randomisation, and 5% at high risk. Excluding all but low risk made little difference. Variable definitions of abstinence, including 4 trials allowing up to 4 cpd; excluding these made no difference. 65% reported sustained abstinence, and 80% measured to 12m or more. 89% used some form of validation. One third reported some type of blinding. |
| 2. Abstinence definition: 1 Gum 2 Patch | ‐Sus 12m ‐Sus 6m ‐PP/ns 12m ‐PP/ns 6m |
22581/56
13737/32 4187/8 2501/8 2156/8 19586/43 10928/21 4640/9 2582/6 1436/7 |
|||||
| 3.Level of support: 1. Gum 2. Patch 3. Long vs short: ‐Gum ‐Patch | Gum or patch: ‐ Low ‐High individual ‐High group Long vs Short: ‐Gum ‐Patch |
21759/55
‐11257/17
‐6891/18
‐3611/20 19585/43 ‐4388/12 ‐11559/22 ‐3638/10 800/3 ‐296/2 ‐504/1 |
|||||
| 4. Setting for Recruitment/Treatment: 1. Community 1. Gum 2. Patch 3. Inhaler 4. Tab/lozenge 5. Nasal spray 6. Combo 7. Oral spray 2. SC Clinic 1. Gum 2. Inhaler 3. Nasal spray 3. Primary care 1. Gum 2. Patch 3. Choice 4. Hospitals 1. Gum 2. Patch 3. Combo 4. Choice 5. Antenatal clinic 1. Gum 2. Patch 3. Choice 6. OTC volunteers 1. Gum 2. Patch |
6m+ |
24199/66
8336/28
10816/28
443/2
3405/7 412/2 308/1 479/1 2291/10 1283/6 533/2 475/2 11705/23 7277/16 4150/6 278/1 5506/10 2194/3 1042/4 245/1 2025/2 1675/4 194/1 1300/2 181/1 5575/5 3297/2 2278/3 |
|||||
| 5. Dosage of gum 1. 4mg vs 2mg ‐High dependence ‐Low dependence | 6m+ | 856/5 618/4 238/3 | |||||
|
6. Gum: Fixed vs ad‐lib gum |
6m+ | 689/2 | |||||
| 7. 1. Patch: High vs standard dosage
1. 44mg vs 22mg 2. 42mg vs 21mg 2. 25mg vs 15mg |
6m+ |
5101/8
1188/4 467/1 3446/3 |
|||||
| 8.1. Patch: duration 1. 16hr vs placebo 2. 24hr vs placebo 3. 24hr vs 16hr | 6m+ | /42 7618/11 10820/32 106/1 | |||||
| 9. 1. Patch: Course of treatment:
1. ≤8 wks
2. >8 wks 2. Direct comparison 1. 28 wks vs 12 wks 2. 24 wks vs 8 wks 3. 12 wks vs 3 wks 4. 12 wks vs 6 wks 5. 6 wks vs 3 wks |
6m+ |
/42
6191/17
9906/26 /5 2861/1 568/1 98/1 140/1 80/1 |
|||||
| 10. 1. Indirect comparison
1. patch vs placebo; no weaning
2. patch vs placebo; with weaning 2. Direct comparison 1. patch; abrupt withdrawal vs weaning |
6m+ |
17427/41
2807/9 14620/32 264/2 |
|||||
| 11.Combinations: 1. Long‐term Smoking cessation 1. patch+gum vs patch alone 2. patch+gum vs gum alone 3. spray+patch vs patch alone 4. spray+patch vs spray or patch 5. patch+inhaler vs inhaler alone 6. patch+inhaler vs patch or inhaler 7. patch+lozenge vs patch or lozenge |
6m+ |
4664/9
395/2 300/1 237/1 1384/1 400/1 337/1 1611/2 |
|||||
| 12. Direct comparisons between NRTs 1. Smoking cessation 1. inhaler vs patch 2. spray vs patch 3. Lozenge vs patch |
6m+. |
3201/6
222/1
1272/2 1707/3 |
|||||
| 13. Prescribed NRT with physician support vs OTC NRT without support 1.1 patch 1.2 inhaler |
6m+ |
820/2 300/1 520/1 |
|||||
| 14. 1. Precessation NRT 1. Patch 2. Gum 3. Lozenge |
6m+ |
2774/8 1772/6 406/2 596/1 |
|||||
| 15.1 NRT in pregnancy 1. At end of pregnancy 2. At longest post‐partum follow‐up. |
1675/4 625/3 |
||||||
| 16. 1. NRT v bupropion
1. patch vs bup
2. Lozenge vs bup 3. Choice vs bup 2. Combination vs bupropion alone 1. Patch + bup vs bup alone 2. Gum + bup vs bup alone 3. Lozenge + bup vs bup alone 3. Combination vs placebo 1. Patch + bup vs placebo 2. Lozenge+bup vs placebo |
6m+ |
2544/5 1552/4 781/2 211/1 1991/3 489/1 452/1 526/1 1991/4 405/1 299/1 |
|||||
| 17. Palpitations with NRT vs placebo 1. Palpitations/chest pains |
|||||||
|
Nicotine receptor partial agonists Cahill 2012 |
April 2012 | Any adult smokers | Cytisine | 1. Cytisine (Tabex) 1. vs placebo | 6m or 12m | 937/2 | Well‐conducted trials |
| Dianicline | 2. Dianicline 1. vs placebo | 6m | 602/1 | Well‐conducted trial | |||
| Varenicline | 3. Varenicline 1mg bid 1. vs placebo 2. vs placebo 3. vs placebo in pts with schizophrenia | 6m+ 12+m 6m | 6166/14 378/1 127/1 | Trials were generally conducted to a high standard, with biochemically validated outcomes. | |||
| 4. Low‐dose varenicline 1. V vs placebo 2. Standard V vs low‐dose V | 12m | 1272/4 1083/3 | |||||
| 5. Varenicline 1. vs bupropion 2. vs bupropion 3. vs bupropion | 12m 3m 6m | 1622/3 1367/2 1367/2 | |||||
| 6. Varenicline 1. vs NRT (open label) | 6m | 778/2 | |||||
| 7. Varenicline as maintenance therapy 1. vs placebo 2. vs placebo at end of double‐blind phase | 12m 6m | 1208/1 1210/1 | |||||
| 8. Commonest AEs 1. Nausea 2. Insomnia 3. Abnormal dreams 4. Headache | During 3m treatment | 6619/16 6309/15 5585/12 5913/13 | |||||
| 10. SAEs 1. vs placebo | During and after treatment | 8175/17 | |||||
| Opioid antagonists David 2009 | June 2009 | Adult smokers | Naltrexone | 1. vs placebo 2. +NRT vs placebo+NRT | 6m or 12m | 582/4 | Wong reported data from only 1 of 4 centres |
| Silver acetate Lancaster 2009 | January 2009 | Adult smokers | Silver acetate | 1. vs placebo 2. vs nicotine gum | 12m | 785/2 414/1 | randomisation not described. One trial was open‐label. |
All of the reviews used a similar methodological approach, and the same primary outcome of abstinence from smoking for at least six months. We used the most rigorous definition of abstinence available, i.e. preferring prolonged, sustained or continuous abstinence over point prevalence (Hughes 2003), and favouring biochemically confirmed findings (exhaled carbon monoxide, cotinine in plasma, urine or saliva, or plasma thiocyanate) over self report (SRNT 2002). Study participants who dropped out during the trial or who were lost to follow‐up were assumed to be continuing smokers, and were included in the meta‐analysis denominator on an intention‐to‐treat basis. Opioid antagonists 2009 also included a group of short‐term trials (less than six months follow‐up) which assessed withdrawal symptoms, attenuating the reinforcing value of smoking, and reducing ad libitum smoking. A second primary outcome in all reviews was the incidence and severity of adverse and serious adverse events (SAEs).
The sum total of participants across 267 studies within the included reviews is 101,804. However, this computation may include a measure of double‐counting, as some placebo participants were compared, but not pooled, across multiple arms of several studies. Counts by treatment, together with estimates of efficacy within the source reviews (risk ratio and 95% confidence interval) are given in Appendix 3.
We briefly describe the individual reviews included in this overview.
1. NRT 2012
The aim of nicotine replacement therapy (NRT) is to temporarily replace some of the nicotine from cigarettes to reduce motivation to smoke and nicotine withdrawal symptoms, thus easing the transition from cigarette smoking to complete abstinence. The authors searched the Cochrane Central Register of Controlled Trials and online registers of ongoing and completed studies (e.g. UK Clinical Trials; US Clinical Trials; WHO trials registry platform). The most recent search for this review was July 2012. The authors identified 150 included trials, with 117 (more than 51,000 participants across 122 comparisons) contributing to the primary effect measure comparing any type of NRT to a placebo or non‐NRT control group. These represented 55 trials of nicotine gum, 43 of transdermal nicotine patch, six of an oral nicotine tablet or lozenge, five offering a choice of products, four of intranasal nicotine spray, four of nicotine inhaler, one of oral spray, one providing patch plus inhaler and one providing patch plus lozenge.
Participants Adult smokers, motivated to quit, apart from one trial which recruited adolescents. Most trials recruited men and women, but one recruited only men in a workplace setting. Four trials recruited only women, and four more recruited pregnant women. Two trials recruited African‐American smokers.
Interventions and comparisons As well as different types of NRT (gum, patches, lozenges or tablets, sprays and inhalers) versus placebo, the trials covered different doses of NRT, comparing combination use of NRT to a single type, comparing NRT to bupropion and combinations of the two, and comparing the use of NRT pre‐quit date as opposed to post‐quit date only. Some analyses also stratified on the level of behavioural support provided, i.e. low intensity (<30 minutes) or high intensity (>30 minutes or multi‐session counselling). Most trials comparing nicotine gum to control provided the 2 mg dose. A few provided 4 mg gum to more highly addicted smokers, and two used only the 4 mg dose. Five trials included a comparison of 2 mg and 4 mg doses. The treatment period was typically two to three months, but ranged from three weeks to 12 months. For the patch trials, the usual maximum daily dose was 15 mg for a 16‐hour patch, or 21 mg for a 24‐hour patch. Forty‐two studies used a 24‐hour formulation, 11 a 16‐hour product, and one a 52.5 mg/24 hour patch. The minimum duration of therapy ranged from three weeks to three months, with a tapering period, if required, in 38 of the trials. Six studies tested nicotine sublingual tablets or lozenges, four tested intranasal nicotine spray, one tested oral nicotine spray and four tested nicotine inhaler. Nine trials compared combinations of two forms of nicotine therapy to one form only. Seven trials tested the use of NRT compared to placebo or control prior to quit date, with all study arms receiving NRT from the quit date onwards. Three of the trials also included a mecamylamine arm. Five trials directly compared nicotine to bupropion, with three of them also comparing nicotine‐plus‐bupropion to nicotine alone.
Outcomes One hundred and five trials (70%) reported some measure of sustained abstinence, which included continuous abstinence with not even a slip since quit day, repeated point prevalence abstinence (with or without biochemical validation) at multiple follow‐ups, or self‐reported abstinence for a prolonged period. Forty trials (27%) reported only point prevalence abstinence at the longest follow‐up. In five studies it was unclear exactly how abstinence was defined. The definition of abstinence in four studies permitted the smoking of two to three cigarettes a week. Most studies reported follow‐up at least 12 months from start of treatment, but 33 reported only to six months. Four trials in pregnant women reported follow‐up in relation to gestation and delivery date.
2. Antidepressants 2007
This review covers a group of medications, including nortriptyline; doxepin; fluoxetine; imipramine; moclobemide; paroxetine; selegiline; sertraline, tryptophan, venlafaxine and St. John's wort, but we focus here on bupropion, a widely‐used smoking cessation therapy, and also on nortriptyline. Bupropion was first approved as a treatment for depression in 1985, and was subsequently licensed as an aid for smoking cessation in 1997. It has both dopaminergic and adrenergic actions, and appears to be an antagonist at the nicotinic acetylcholinergic receptor. It may work by blocking nicotine effects, relieving withdrawal or reducing depressed mood. The authors searched the Cochrane Central Register of Controlled Trials, as well as online registers of ongoing and completed clinical trials. The searches for the current review were conducted in June 2009. There were 49 included trials of bupropion, covering more than 14,000 participants; four of the study reports were based on conference abstracts or pharmaceutical company data. There were also nine trials of nortriptyline, six of selective serotonin reuptake inhibitors (SSRIs), four of monoamine oxidase inhibitors (MAOIs), and one of venlafaxine.
Participants Most trials recruited adult current smokers, with one trial confined to men only. For the bupropion trials, special populations recruited include smokers with the following conditions: chronic obstructive pulmonary disease (three trials); schizophrenia (five trials); post traumatic stress disorder (one trial); alcoholism (one trial); and cardiovascular disease (three trials). Other populations included adolescents (two trials) and one trial each in smokers awaiting surgery, hospital staff, healthcare workers, African‐Americans, and Maori. Two studies recruited smokers who had previously failed to quit smoking using bupropion, and one included smokers who had recently failed to quit using NRT.
Interventions and comparisons Thirty‐six of the trials used bupropion as the only intervention versus placebo, covering more than 11,000 participants. Three trials compared it as the sole intervention to nicotine patch, and three more compared it to varenicline. Three of the bupropion/placebo trials included a nortriptyline arm. Six trials compared bupropion combined with NRT to NRTalone. Six nortriptyline trials compared it to placebo, and four combined nortriptyline with NRT versus NRT alone. Six trials tested SSRIs: these were two trials of fluoxetine versus placebo, two of fluoxetine plus NRT versus placebo plus NRT, and one each of paroxetine and sertraline. Four trials tested MAOIs, i.e. one trial of moclobemide versus placebo, and three of selegiline versus placebo. One trial compared venlafaxine to placebo.
Outcomes Twenty‐two of the bupropion versus placebo studies followed participants for at least 12 months from the start of treatment or the target quit day. Eighteen studies (37%) had only six months follow up. The majority of studies reported an outcome of sustained abstinence. In 12 (24%) only point prevalence rates were given, or the definition of abstinence was unclear. In all but one of the bupropion studies and all but one of the nortriptyline studies biochemical verification was used for most self‐reported quitters at some assessment points.
3. Nicotine receptor partial agonists 2012
Nicotine receptor partial agonists, including varenicline, cytisine and dianicline, may help people to stop smoking by a combination of maintaining moderate levels of dopamine to counteract withdrawal symptoms (acting as an agonist) and reducing smoking satisfaction (acting as an antagonist). The authors searched the Tobacco Addiction Group's specialised register, in the Cochrane Central Register of Controlled Trials, as well as online registers of ongoing and completed clinical trials. The searches for the current review were conducted in December 2011. The authors identified 24 included studies, i.e. 20 for varenicline, three for cytisine and one for dianicline. Fourteen of the varenicline trials were included in the main meta‐analysis, covering more than 6000 participants. Two of the varenicline trials were based on pre‐publication data, acquired from the authors or from results posted on the online clinical trials registers.
Participants Adult smokers, motivated to quit. Apart from one trial, all were multi‐centre; while most were set wholly or partly in the USA, three were conducted in Asian populations, and one across Latin America, Africa and the Middle East. Five trials studied specific patient populations: schizophrenia or schizoaffective disorders; cardiovascular diseases; acute smoking‐related illnesses; hospital inpatients; chronic obstructive pulmonary disease. The three cytisine trials were set in the former German Democratic Republic, in Kyrgyzstan and in Poland. The dianicline trial was set in six European countries.
Interventions and comparisons Fifteen randomised controlled trials of varenicline compared it to placebo. Three of these also included a direct comparison to bupropion. One trial compared varenicline plus counselling to counselling alone, and another tested varenicline against placebo, as maintenance therapy for those who had already quit with varenicline. Two open‐label trials compared varenicline to nicotine patches. The standard regimen was 1.0 mg twice a day for 12 weeks, but three trials included lower dosage arms, versus standard dosage and placebo regimens, and two trials evaluated flexible dosing schedules or flexible quit dates. All trials delivered brief behavioural support (10 minutes or less) to all participants during treatment and follow‐up phases. Three trials compared cytisine to placebo, and one compared dianicline to placebo.
Outcomes All but three of the included studies reported prolonged, sustained or continuous abstinence. They all measured abstinence at 24 to 26 weeks, and again at 52 weeks in all bar eight. All the varenicline trial outcomes were biochemically verified by expired carbon monoxide, apart from two which relied upon self‐report where biochemically verified data were not available, and one which verified outcomes at 12 but not at 24 weeks. All three cytisine trials assessed abstinence at six months, and conducted final follow‐up at two years. The dianicline trial followed its participants for 26 weeks.
4. Anxiolytics 2010
This review covers any drug with anxiolytic properties, including beta‐blockers. It has been proposed that anxiolytics may help people to stop smoking, on the basis that anxiety can be a symptom of nicotine withdrawal, and that smoking could be associated with an attempt to self‐medicate an anxiety problem. These treatments may also appeal to smokers who do not wish to use nicotine‐based medications to make a quit attempt, or who have previously tried unsuccessfully to quit with NRT. The authors searched the Tobacco Addiction Group's specialised register, in the Cochrane Central Register of Controlled Trials, in October 2009. They identified one trial each of diazepam, meprobamate, metoprolol and oxprenolol, and two trials of buspirone (covering 201 participants). A third buspirone trial was not included in meta‐analysis since the comparators were fixed dose or tapered NRT, rather than placebo.
Participants Adult smokers wanting to quit. One buspirone trial stratified participants by high or low anxiety levels, and the authors treated these separately in the meta‐analyses. Apart from the diazepam trial set in China and the beta‐blockers trial set in Scotland, all the trials were based in the USA.
Interventions and comparisons The buspirone trials compared treatment to placebo; both used low‐dose treatment for 3/4 weeks prior to quit date, and then 60 mg for eight weeks or six weeks. One trial compared four weeks of diazepam to clonidine and to placebo. One study compared 40 days of oxprenolol to metoprolol and to placebo, and another tested meprobamate against placebo, with and without different types of counselling. All the trials included a counselling component.
Outcomes One trial measured abstinence at six months, three trials at 12 months, and one defined success as a reduction of more than 85% from baseline smoking rate at 18 months longest follow‐up. Two trials used biochemical validation of abstinence, one confirmed self‐report by checking with family and co‐workers, one gave no information on abstinence criteria and did not validate, and one measured reduction rather than complete abstinence. None of the trials gave adequate details of randomisation procedures, but simply described their trial as "randomised"; one reported assigning to balance sex ratio and cotinine level, i.e. amount smoked, and another stratified by social class but gave no further information.
5. Clonidine 2008
Clonidine was originally used to lower blood pressure. It acts on the central nervous system and may reduce withdrawal symptoms in various addictive behaviours, including tobacco use. The authors identified six trials, covering more than 700 participants, which met the inclusion criteria from the Tobacco Addiction Group's specialised register, in the Cochrane Central Register of Controlled Trials, in June 2008. One study was based on abstracts only, and two study authors provided supplementary unpublished data.
Participants: Five trials were set in the community, and one in a hospital clinic. Four trials targeted heavy smokers, i.e. >20 per day, while one included moderate smokers (>10 per day), and one gave no participant details. One trial stratified allocation by gender and a history of depression. Two trials required that participants reduce their baseline smoking by at least 50% at quit date to be admitted to the study. Five studies were set in the USA, and one in China.
Interventions and comparisons: Three trials provided transdermal clonidine in dosages ranging from 0.1 to 0.3 mg per day, and three provided oral clonidine with doses ranging from 0.15 to 0.45 mg per day. All the trials compared clonidine to placebo, and one also included a diazepam arm. Treatment lasted for four weeks (three trials), six, ten or 12 weeks (one trial each). All the trials used some form of behavioural support, with four delivering individual counselling sessions for all participants and one a standard counselling message; one trial randomised half of the clonidine and control participants to receive group counselling, but this separation was dropped for the meta‐analysis.
Outcomes: The outcome in each trial was abstinence at least 12 weeks from the end of treatment. Three trials followed up for 12 months, and the other three for six months. In the three trials which defined abstinence, one selected self‐reported 7‐day point prevalence, one relied up self‐report through smoking diaries, and one identified three levels of abstinence, i.e. self‐report alone, self‐report verified by plasma cotinine but allowing one or two minor lapses in the final week, and lapse‐free self‐report verified by plasma cotinine. Biochemical validation was used systematically by three trials, and partially by two more. One trial use no biochemical validation, but sometimes cross‐checked with family or co‐workers.
6. Lobeline 2009
Lobeline is an alkaloid derived from the leaves of an Indian tobacco plant, and has been widely used in commercial smoking remedies. Other nicotinic receptor partial agonist compounds have been shown to be effective aids for smoking cessation (Nicotine receptor partial agonists 2012). The authors searched the Tobacco Addiction Group's specialised register in March 2011, but identified no trials which met the inclusion criteria. Studies generally did not include a control group, and those that did employed a cross‐over design to measure smoking over days or weeks rather than months, and/or did not follow up participants beyond the end of treatment. Smoking reduction rather than complete abstinence was the more commonly used primary outcome.
One multicentre study of sublingual lobeline sulfate tablets with 750 subjects was conducted by Dynagen in 1997. Lobeline does not contribute to any meta‐analyses in this overview review.
7. Mecamylamine 2011
Mecamylamine, originally marketed for lowering blood pressure, is a nicotine antagonist, which may block the rewarding effect of nicotine and thus reduce the urge to smoke. The authors searched the Tobacco Addiction Group's specialised register in October 2010, and identified two small trials, with a total of 128 participants, which met their inclusion criteria. Both trials investigated mecamylamine in combination and in comparison with NRT.
Participants: Adult smokers, smoking >1 pack per day. One trial set an upper age limit of 40, while a second version extended this to 54.
Interventions and comparisons: The first trial (48 participants) compared mecamylamine capsules plus nicotine patch to placebo capsules plus nicotine patch. Each group was further divided to begin the patches either two weeks before the quit date or coincident with the quit date. The later trial randomised 80 participants to (1) nicotine patch plus mecamylamine, or (2) nicotine patch alone, or (3) mecamylamine alone, or (4) two placebos, no active drug. These regimens applied for four weeks up to the quit date, after which all groups received nicotine patch plus mecamylamine for six weeks. Nicotine patch treatment was faded from 21 mg to 7 mg over the course of treatment, while mecamylamine, after initial titration, was administered at 5 mg twice a day, but reducible if not well tolerated.
Outcomes: The first trial measured continuous abstinence to 12 months, and the second continuous abstinence to six months. Validation in both trials was by expired carbon monoxide.
8. Nicobrevin 2009
Nicobrevin is a proprietary (off‐prescription) product containing 15 mg of quinine, 100 mg of menthyl valerate, 10 mg each of camphor and eucalyptus oil. It is marketed as an aid to smoking cessation, by reducing urges to smoke, withdrawal symptoms and cravings. The authors searched the Tobacco Addiction Group's specialised register in January 2009, but identified no trials which met the inclusion criteria.
Two trials of Nicobrevin were identified, and treated as excluded studies. One followed up only for four weeks, and did not require smokers to quit, but used the number of cigarettes smoked at the final assessment as the primary outcome. The other trial followed up for three months, and tested a number of anti‐smoking products in a single trial. Participants received their allocated therapy by post, with the placebo group receiving a placebo matched to the characteristics of another active therapy rather than to Nicobrevin, and without validation of self‐reported quit rates. Nicobrevin does not contribute to any meta‐analyses in this overview review.
9. Nicotine vaccines 2012
Nicotine vaccines are not yet licensed anywhere for use as an aid to smoking cessation or for relapse prevention. The vaccines are designed to work by blocking nicotine's access to the brain, resulting in the smoker deriving less satisfaction when they smoke a cigarette. It is hypothesised that vaccines may help smokers to quit, and may help former smokers not to relapse. The authors searched the Tobacco Addiction Group's specialised register, online clinical trials registers and company websites in March 2012, and identified four trials (2642 participants) which appeared to meet the inclusion criteria. Only two of the trials, a Swiss study testing NIC002 and a USA one testing NicVAX, reported their findings in sufficient detail to contribute to meta‐analyses. The two remaining trials, conducted in the USA, also tested NicVAX, but the manufacturers, Nabi Biopharmaceuticals, have reported no findings beyond a summary quit rate and the lack of a statistically or clinically significant difference between the performance of the active and placebo treatments.
Participants: Adults, motivated to quit, smoking moderately to heavily (15+ per day for NicVAX and 10 ‐ 40 per day for NIC002). These two trials covered 642 participants. Information was sparse for the two Nabi NicVAX trials, which each included 1000 participants.
Interventions and comparisons: One trial assigned participants to placebo or to a NicVAX regimen of 200ųg or 400ųg, and then split each group into a 4‐injection or 5‐injection schedule over 26 weeks, i.e. four experimental and two placebo groups. The two Nabi NicVAX trials delivered six injections of 400ųg over six months. The Swiss trial assigned participants to a 5‐injection schedule of 100ųg NIC002 over four months. All the trials provided behavioural counselling throughout treatment and follow‐up stages.
Outcomes: Continuous abstinence was assessed at 26 and 52 weeks for both the fully reported trials, and at 52 weeks for the two Nabi trials. Abstinence was validated by expired CO in all trials, and also by urinary cotinine in one trial. The Swiss trial and the full USA trial stratified post‐hoc by antibody titer levels, and compared abstinence and adverse events between the two Ab groups.
10. Opioid antagonists 2009
Opioid antagonists, including naltrexone, naloxone and buprenorphine, are long‐acting drugs which blunt the effects of narcotics such as heroin and morphine, and might help reduce nicotine addiction by blocking some of the rewarding effects of smoking. The authors searched the Tobacco Addiction Group's specialised register in June 2009, and identified two groups of trials for inclusion: (1) Four randomised controlled trials, covering 582 participants, with a minimum follow‐up of six months, assessing efficacy for long‐term smoking cessation, and (2) 26 randomised controlled trials with short‐term follow up that report withdrawal, reinforcing properties of smoking, or ad libitum smoking. Fourteen of the 19 naltrexone trials and five small naloxone trials were laboratory‐based. For buprenorphine, one was laboratory‐based and the other was set in a clinic.
Participants: For the four cessation trials, three targeted heavy smokers (a pack or more a day). One trial aimed at moderate smokers (10 or more cigarettes a day) reports findings from only one of the four centres that took part in the trial, with the manufacturers failing to supply the remaining data. Three trials recruited from their communities, while one recruited from healthcare facilities. All four trials were conducted in the USA.
The trials delivering short‐term findings were all community‐based, apart from those recruiting hospital employees (two trials), clinic patients (five trials), and heavy drinkers or alcohol‐dependent smokers (two trials). All the trials were set in the USA, apart from one each in France, Canada, South Korea and the UK.
Interventions and comparisons: One trial compared four weeks of naltrexone (50‐75 mg) to placebo. The remaining three cessation trials used naltrexone in combination with nicotine patch. One tested 50 mg of naltrexone for two months against placebo, with all participants using nicotine patch for one month. Another supplied all participants with nicotine patch added to varying doses (100, 50 and 25 mg) of naltrexone, compared to placebo for six weeks. The third tested naltrexone alone (50 mg for 12 weeks) against nicotine patch with placebo pill, naltrexone with nicotine patch, and placebo pills alone. All four trials included a counselling component.
Outcomes: All four cessation trials measured continuous abstinence at six months, with one also measuring point prevalence abstinence at 12 months. Three of the four validated outcomes by expired carbon monoxide, while one verified by testing plasma cotinine. Short‐term outcomes included number of cigarettes smoked per day, withdrawal symptoms, positive and negative affect, nicotine dependence, ad libitum smoking, cortisol levels and cravings to smoke.
11. Rimonabant 2011
Selective type 1 cannabinoid (CB1) receptor antagonists, including rimonabant and taranabant, may assist smoking cessation by restoring the balance of the endocannabinoid system, which can be disrupted by prolonged use of nicotine. They may also address many smokers’ reluctance to persist with a quit attempt because of concerns about weight gain. The authors searched the Tobacco Addiction Group's specialised register in January 2011, and also contacted the manufacturers of rimonabant. Three trials of rimonabant were identified for inclusion, covering more than 1700 participants: two measured smoking cessation and one tested relapse prevention. One trial of taranabant for smoking cessation could not be included as it did not assess outcomes beyond eight weeks. Rimonabant was not licensed for use as a treatment for smoking, and production of rimonabant (Sanofi‐Aventis) and taranabant (Merck) was suspended in 2008, because of concerns about the type and incidence of adverse events.
Participants: Adults, smoking at least 10 cigarettes per day. One trial was set in the USA, one in Belgium, Denmark, France, Spain, Sweden, Switzerland and the UK, and the relapse prevention trial in Australia, Canada and the USA.
Interventions and comparisons: In the two cessation trials, participants were randomised to receive 5 mg, 20 mg or placebo. Treatment was for 12 weeks, and included regular behavioural support. In the relapse prevention trial, participants were initially randomised either to 5 mg or 20 mg for ten weeks to achieve cessation. For phase 2 of the trial, successful quitters in the 5 mg group were then randomised to a further 42 weeks of either 5 mg or placebo regimens; successful quitters in the 20 mg group were randomised to 42 weeks of 5 mg, or 20 mg, or placebo. This study did not report on the provision or level of behavioural support.
Outcomes: The two cessation trials measured prolonged abstinence at 50 weeks, validated by expired carbon monoxide and cotinine testing. The relapse prevention trial scheduled measures of time to relapse up to 52 and 104 weeks, although the two‐year outcome was not been reported. One‐year outcomes were validated by expired carbon monoxide. Weight change was also assessed throughout the study period in all three trials.
12. Silver acetate 2009
Silver acetate, in gum, lozenge, and spray formulations, creates an unpleasant metallic taste when combined with cigarettes, thereby producing an aversive stimulus. It has been marketed in various forms with the aim of extinguishing the urge to smoke, by pairing the urge with an unpleasant stimulus. The authors searched the Tobacco Addiction Group's specialised register in January 2009, and identified two trials of silver acetate for long‐term smoking cessation, covering almost 1000 participants.
Participants: Adults smoking more than 10 cigarettes a day, motivated to quit. One trial was set in the USA, and the other in Denmark.
Interventions and comparisons: The USA trial supplied participants with 2.5 mg lozenges, to be taken six times a day for three weeks, versus placebo lozenges. Successful quitters were given further supplies to assist with relapse prevention. The Danish trial compared six weeks of 6 mg silver acetate gum (up to six pieces a day) to 2 mg nicotine chewing gum or ordinary chewing gum (placebo). This trial was not blinded.
Outcomes: Both trials tested sustained abstinence at 12 months, validated by expired carbon monoxide; one also used urinary cotinine testing to validate outcomes.
Methodological quality of included reviews
AMSTAR ratings for the included reviews are summarised in Table 2 (NRT, bupropion, nortriptyline, varenicline and cytisine) and in Table 3 (other pharmacotherapy reviews). All 12 reviews were classified as being of high quality, i.e. failing to score in only two or fewer of the 11 domains. Two reviews (Lobeline 2009; Nicobrevin 2009) did not have any included studies, and a third review (Mecamylamine 2011) did not conduct meta‐analyses, and therefore could not be assessed for the relevant domains. Eight reviews combined the publication of the protocol and the full review as a single document, and therefore did not present the 'a priori' design in advance of the review findings. All eight were originally published between 1996 and 1998, apart from Nicobrevin 2009, which first appeared in 2006. The other consistent shortfall was in the domain of publication bias. Two reviews did not include any eligible studies (Lobeline 2009; Nicobrevin 2009), and one had included studies but without peer‐reviewed or published data (Rimonabant 2011). Five reviews did not generate funnel plots or address the likelihood of publication bias in the text. Three of the five (Anxiolytics 2010; Clonidine 2008; Opioid antagonists 2009) had too few included studies to support a formal assessment of publication bias. Ad hoc generation of funnel plots for the remaining two reviews indicated a broadly symmetrical distribution for Antidepressants 2007 (i.e. no clear evidence of publication bias), and a lack of published studies with negative findings for Nicotine receptor partial agonists 2012. However, as the varenicline studies are routinely included in online clinical trials registers before the trials begin, in the interests of reporting transparency, consistent direction of effect may be as plausible an interpretation as failure to disclose negative or unfavourable study findings.
2. AMSTAR scores: NRT, antidepressants and nicotine receptor partial agonists.
| Question | Nicotine Replacement Therapy | Bupropion | Nicotine receptor partial agonists |
| 1.A priori design provided? | CAN'T ANSWER | CAN'T ANSWER | YES |
| 2. Duplicate study selection and data extraction? | YES | YES | YES |
| 3. Comprehensive literature search performed? | YES | YES | YES |
| 4. Published and unpublished studies included? | YES | YES | YES |
| 5. List of included and excluded studies? | YES | YES | YES |
| 6. Characteristics of included studies provided? | YES | YES | YES |
| 7. Scientific quality of included studies assessed? | YES | YES | YES |
| 8. Scientific quality of included studies applied to conclusions? | YES | YES | YES |
| 9. Appropriate methods for combining studies? | YES | YES | YES |
| 10. Likelihood of publication bias? | YES | NO | NO |
| 11. Conflict of interest stated? | YES | YES | YES |
| SCORE | 10/11 | 9/11 | 10/11 |
3. AMSTAR scores: other pharmacotherapies.
| Question | Anxiolytics | CB1 receptor antagonists | Clonidine | Lobeline | Mecamylamine | Nicobrevin | Vaccines | Opioid antagonists | Silver acetate |
| 1.A priori design provided? | CAN'T ANSWER | YES | CANT ANSWER | CAN'T ANSWER | CAN'T ANSWER | CAN'T ANSWER | YES | YES | CAN'T ANSWER |
| 2. Duplicate study selection and data extraction? | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 3. Comprehensive literature search performed? | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 4. Published and unpublished studies included? | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 5. List of included and excluded studies? | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 6. Characteristics of included studies provided? | YES | YES | YES | N/A | YES | N/A | YES | YES | YES |
| 7. Scientific quality of included studies assessed? | YES | YES | YES | N/A | YES | N/A | YES | YES | YES |
| 8. Scientific quality of included studies applied to conclusions? | YES | YES | N/A | YES | N/A | YES | YES | YES | |
| 9. Appropriate methods for combining studies? | YES | YES | YES | N/A | N/A | N/A | YES | YES | YES |
| 10. Likelihood of publication bias? | NO | N/A | NO | N/A | YES | N/A | YES | NO | N/A |
| 11. Conflict of interest stated? | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| SCORE | 9/11 | 10/10 | 9/11 | 5/6 | 9/10 | 5/6 | 11/11 | 10/11 | 9/10 |
One estimate of the quality of the included studies in the 12 reviews, measured by their risks of bias, is briefly summarised in Table 4. Newer studies are more likely to be conducted in accordance with CONSORT guidelines and to report their methodology more rigorously than earlier trials, but there remains great variation between trial methods and standards of reporting.
4. Risk of bias summary for trials in the included reviews.
| Review | N trials | randomisation % | Sustained abstinence % | Biochemical validation % |
Blinding % |
||
| Low risk | Unclear risk |
High risk |
Low risk | ||||
| NRT | 147 | 25 | 72 | 3 | 70 | 86 | 33 |
| Antidepressants | 60 | 48 | 50 | 2 | 85 | 85 | 50 |
| Nic recep partial ags | 24 | 66 | 33 | ‐ | 75 | 84 | 58 |
| Anxiolytics | 7 | ‐ | 100 | ‐ | 29 | 29 | 0 |
| Clonidine | 6 | ‐ | 100 | ‐ | 0 | 67 | 0 |
| Lobeline | 0 | No included studies | |||||
| Mecamylamine | 2 | 100 | ‐ | ‐ | 100 | 100 | 100 |
| Nicobrevin | 0 | No included studies | |||||
| Nicotine vaccines | 4 | 25 | 75 | ‐ | 50 | 50 | 50 |
| Opioid antagonists | 4 | 50 | 50 | ‐ | 50 | 100 | 100 |
| Rimonabant | 3 | ‐ | 100 | ‐ | 100 | 100 | 0 |
| Silver acetate | 2 | ‐ | 100 | ‐ | 100 | 100 | 50 |
Numbers from columns 3 to 8 are percentages of the total number of trials for each review.
Effect of interventions
Efficacy network meta‐analyses (NRT, bupropion, varenicline)
The reviews covering NRT, bupropion and varenicline showed them all to increase the chances of quitting compared with placebo. The estimated effect sizes are reported in the source reviews as risk ratios, and are given for reference in Appendix 3. Appendix 1 tabulates the binary meta‐analyses from the relevant reviews (converted to odds ratios) against the corresponding network meta‐analyses. The findings reported below are based on network meta‐analyses using the same data sets, and estimate the effect sizes as odds ratios, in accordance with the Bayesian model used in the analyses.
The first network meta‐analysis for smoking cessation shows that the odds of quitting are significantly increased for those taking NRT or bupropion over those taking placebo. The odds ratio (OR) for NRT versus placebo is 1.84; 95% credible interval (CredI) 1.71 to 1.99, and for bupropion versus placebo 1.82; 95% CredI 1.60 to 2.06. Varenicline was shown to further increase the odds of quitting compared with placebo, with an OR of 2.88; 95% CredI 2.40 to 3.47.
The comparison between bupropion and NRT suggests no advantage for either treatment, with an OR of 0.99; 95% CredI 0.86 to 1.13. Varenicline is shown to be superior both to NRT(OR 1.57; 95% CredI 1.29 to 1.91), and to bupropion (OR 1.59; 95% CredI 1.29 to 1.96). We found no evidence that the consistency assumption was not met. (Figure 2).
2.
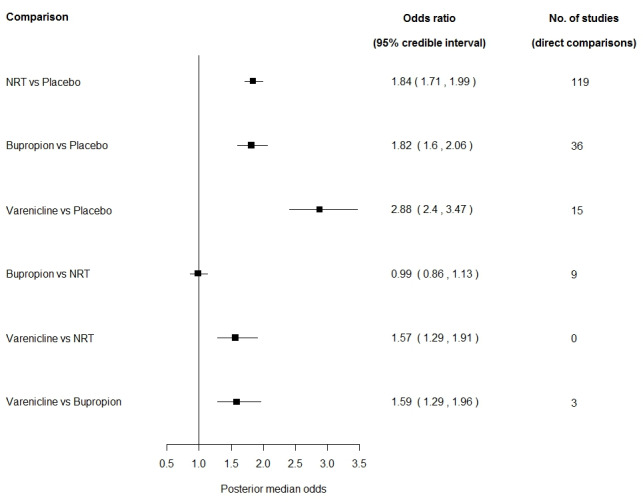
Network meta‐analysis of smoking cessation with each first‐line pharmacotherapy versus placebo and versus each other
The probability of treatment ranking plot gives an indication of how the treatments are ranked in terms of efficacy. Varenicline has a probability of being ranked first equal to 1, meaning that in the 40,000 MCMC samples, varenicline was the most effective treatment in all 40,000 samples. NRT was estimated to be the second best treatment in around 23,200 (58%) of the samples, and hence has a probability of being ranked second of 0.58. Bupropion was the second best treatment in the remaining 16,800 (42%) samples and hence has a probability of being ranked second of 0.42. Placebo was estimated to be the least effective in all of the 40,000 samples, hence it is ranked fourth with probability equal to 1 (Figure 3).
3.
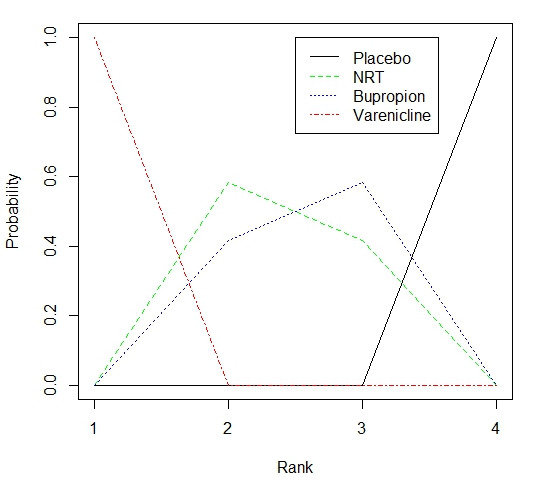
Distribution of probabilities of each treatment being ranked at each of the four possible positions for smoking cessation
The second network meta‐analysis split NRT into four sub‐groups: patch, gum, combination NRT and "other" NRT (i.e. inhalers, sprays, tablets and lozenges). We excluded trials that gave patients a choice of NRT either in the intervention or control arm. The four NRT categories, bupropion and varenicline were all compared with placebo and with each other (Figure 4). All six treatments significantly increased the odds of quitting when compared to placebo. Varenicline significantly increased the odds of quitting compared with NRT patch (OR 1.51; 95% CredI 1.22 to 1.87), compared with NRT gum (OR 1.72; 95% CredI 1.38 to 2.13), and compared with 'other' NRT (OR 1.42; 95% CredI 1.12 to 1.79), but was not more effective than combination NRT (OR 1.06; 95% CredI 0.75 to 1.48). The four types of NRT performed similarly against each other, apart from 'other' NRT, which was marginally more effective than NRT gum (OR 1.21; 95% CredI 1.01 to 1.46). As with the first meta‐analysis, we found no evidence of inconsistency.
4.
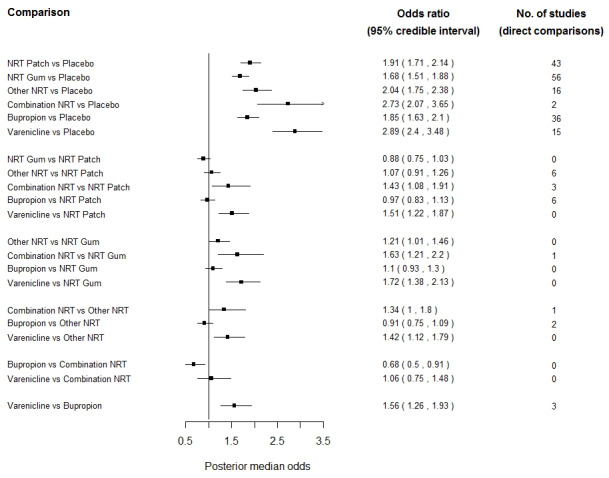
Network meta‐analysis of first‐line pharmacotherapies versus placebo and versus each other, with NRT split by type
From the Antidepressants 2007 binary meta‐analyses, and not included in the network meta‐analysis, bupropion combined with NRT was not shown to be more effective than NRT alone (RR 1.23, 95% CI 0.67 to 2.26; six trials).
Adverse events and Serious Adverse Events (SAEs)
For the purposes of the source reviews, adverse events that were life‐threatening or resulted in death, hospitalisation, significant disability or birth defect were considered to be SAEs, whether or not the trialists attributed them to use of the medication (Tonstad 2010). We have not included NRT in our analyses, as coverage of SAEs is either sparse or entirely absent from the trial reports.
NRT Adverse events There was extensive variation in reporting the nature, timing and duration of symptoms. The major side effects usually reported with nicotine gum include hiccoughs, gastrointestinal disturbances, jaw pain, and orodental problems (Fiore 1992; Palmer 1992). The only side effect that appears to interfere with use of the patch is skin sensitivity and irritation; this may affect up to 54% of patch users, but it is usually mild and rarely leads to withdrawal of patch use (Fiore 1992). Nicotine inhalers and nasal and oral sprays may lead to mild or moderate local irritation at the site of administration . For nasal spray, nasal irritation and runny nose are the most commonly reported side effects. In the study of oral spray, hiccoughs and throat irritation were the most commonly reported adverse events (Tønnesen 2012). Nicotine sublingual tablets have been reported to cause hiccoughs, burning and smarting sensation in the mouth, sore throat, coughing, dry lips and mouth ulcers (Wallstrom 1999).
Reviews and trials exploring the incidence of adverse events among people with cardiac disease have found no excess or increased risks (Greenland 1998; TNWG 1994; Joseph 1996; Joseph 2003; Meine 2005). The four trials assessing NRT use in pregnant women did not detect significant increases in serious adverse events amongst the treatment groups. Recruitment for Pollak 2007 was suspended early when interim analysis found a higher rate of negative birth outcomes in the NRT arm; however, when adjusted for previous birth outcomes the adverse event rate between the two groups was not significantly different in final analysis.
Despite NRT's safety record, a recent meta‐analysis of adverse events associated with it (Mills 2010) across 92 RCTs and 28 observational studies has raised questions about a possible excess of chest pains and heart palpitations among users of NRT compared with placebo groups. The authors calculate an OR of 2.06; 95% CI 1.51 to 2.82 across 12 studies. We replicated this data collection exercise and analysis across 260 included and excluded studies in the NRT review, where such data were reported. We calculated a similar but slightly lower estimate across 15 studies, OR 1.88; 95% CI 1.37 to 2.57 (expressed as an odds ratio for comparability with the Mills point estimate). In our estimation, this is potentially the only clinically significant adverse event to emerge from the trials, and constitutes an extremely rare event, occurring at a rate of 2.5% in the NRT group compared with 1.4% in the control group in the 15 trials in which it was reported at all. This finding should be treated with caution, because of wide disparities in the scale and type of reporting of adverse events across the trials.
Serious Adverse Events Little or no information on SAEs was provided in the trial reports.
Bupropion Adverse events The most common adverse events reported for bupropion in the trials were insomnia, occurring in 30% to 40% of patients, dry mouth (10%) and nausea. Typical drop‐out rates due to adverse events ranged from 7% to 12%. The trials also report the occurrence of allergic reactions, including pruritus, hives, angioedema and dyspnoea, at rates of about 1 to 3 per 1000, an incidence rate in line with post‐marketing surveillance data (GlaxoSmithKline). National surveillance schemes also contain case reports of arthralgia, myalgia, fever with rash, and other symptoms suggestive of delayed hypersensitivity linked to bupropion use, but it is not possible to determine frequency rates from these sources.
Serious Adverse Events: The main serious adverse event (SAE) for bupropion was seizures, which may occur at a rate of around 1:1000 users. This risk appeared to be reduced for the sustained‐release formulation, given at dosages of 300 mg or less a day, and excluding those with a history of seizures, with eating disorders, or with a personal or family history of epilepsy. This incidence rate is reflected in prescription‐event monitoring studies, observational studies and national surveillance databases in the UK and Canada (Dunner 1998, Boshier 2003, Hubbard 2005). A report to the US Toxic Exposure Surveillance System (1998‐9), covering accidental and intentional overdoses and adverse effects of bupropion, noted that 6% of exposure events led to a seizure, with highest rates associated with Welbutrin (immediate release formulation), and lowest with Zyban (sustained release). Bupropion is not recommended for people with a history of seizures.
In the UK, France and Australia (countries in which bupropion is licensed only for smoking cessation), it has been implicated in reports of suicidal ideation and suicidal behaviour, with reported event rates in the order of 1:10,000 (MHRA 2004, TGA 2004, Beyens 2008). A review of bupropion's safety, undertaken by the European Agency for the Evaluation of Medicines for Human Use (EMEA 2002) identified six cases of suicidal ideation among 4067 trial participants, and commented that the rate was lower than that found in the general population, but without providing supporting data. The committee stated that they found no pharmacological or clinical reason for suspecting that bupropion could be causally associated with depression or suicide. However, they recommended strengthening warnings to clinicians on the possibility of hypersensitivity and of depression in patients taking bupropion for smoking cessation.
A follow‐up study of 136 women who had taken bupropion during the first trimester of pregnancy detected no increase in major malformations, but reported a significant increase in spontaneous abortions (Chun‐Fai‐Chan 2005). Bupropion is also an inhibitor of CYP2D6, so care is needed when starting or stopping treatment for patients taking other medication metabolised by this route (Kotlyar 2005).
In 2004, the US Food and Drug Administration (FDA) issued warnings for several antidepressants, including bupropion, when used to treat depression. In 2009 this warning was extended to the use of bupropion for smoking cessation, suggesting that it might be associated with serious neuropsychiatric symptoms (USFDA 2009a). This followed 46 reports of suicidal ideation and 29 of suicidal behaviour from 1997 to November 2007. It remains unclear what relationship, if any, exists between these events and concomitant use of bupropion.
For this overview, we have conducted meta‐analyses of SAEs reported in the 49 included and 33 excluded trials of bupropion, based on all participants randomised. Meta‐analyses of 21 bupropion trials (included and excluded) which reported any SAEs while on treatment found a marginal but statistically non‐significant excess of events in the bupropion groups compared with the placebo groups (RR 1.29; 95% CI 0.99 to 1.69; 7859 participants). The event rates for any SAE were 2.5% for bupropion and 2.2% for placebo users. Subgroup analysis of neuropsychiatric SAEs detected no difference between the bupropion and placebo arms, with an RR of 0.88, 95% CI 0.31 to 2.50 (six trials). The event rates were 0.8% and 0.9% respectively. Subgroup analysis of cardiovascular events detected no difference between the two groups, with an RR of 0.77; 95% CI 0.37 to 1.59 (ten trials) and event rates of 0.3% for bupropion and 0.5% for placebo.
Although we tallied reports of seizures across the trials, there were too few to support a meaningful meta‐analysis. Four trials reported six seizures in the bupropion arms, and a further two reported one seizure each in the open‐label pre‐randomised populations, all of whom at that stage were either taking bupropion alone or bupropion plus NRT. No seizures were reported for any of the placebo participants. Twenty‐five bupropion trials gave sufficient detail on SAEs to be included in the meta‐analyses (including seizures data), two reported total SAEs across all participants (data not usable), 15 trials reported that no SAEs occurred, while the remaining trials provided no information.
Varenicline Adverse events The main adverse event for varenicline was nausea, generally at mild to moderate levels and subsiding over time. Titration, self‐regulation of dosage and lower dosages tended to reduce the incidence. Attributable discontinuation rates ranged from 0.6% to 7.6%. Participants also experienced raised levels of insomnia, abnormal dreams and headache. In the two seminal Phase III trials (Gonzales 2006; Jorenby 2006), an average of 9.5% in the varenicline groups discontinued treatment but remained in the trial for follow‐up, compared with an average of 14% in the bupropion groups and 8% in the placebo groups. Discontinuation rates for any adverse event were highest in Williams 2007, where participants took the trial medication for a year, at 28.3% in the varenicline group and 10.3% in the control group.
Serious Adverse Events Meta‐analyses of any SAE while on or immediately after treatment (usually within one week or one month) in those who took at least one dose of varenicline compared to those on placebo did not detect any excess of events: RR 1.06 (95% CI 0.72 to 1.55; 14 trials, 6333 participants). The event rates for any SAE were 2.1% in the varenicline arms and 2.0% in the placebo arms. Subgroup analysis restricted to neuropsychiatric events found no difference between varenicline and placebo users: RR 0.53 (95% CI 0.17 to 1.67), with event rates of 0.15% for varenicline and 0.21% for placebo arms respectively. The subgroup analysis for cardiovascular events identified no difference between the two arms: RR 1.26 (95% CI 0.62 to 2.56), with event rates of 0.6% and 0.5% for varenicline and placebo respectively. Sensitivity analyses excluding from the denominator those who had not completed treatment made no difference to our findings.
Post‐marketing surveillance has raised concerns about possible links between varenicline use and neuropsychiatric or cardiac events. In 2008, the FDA required the manufacturers of varenicline to include a boxed warning on the packaging, to alert users and clinicians to the possibility of increased risks of behaviour change, agitation, depressed mood, and suicidal ideation and behaviour. Tonstad 2010, a meta‐analysis of the incidence of psychiatric adverse events in ten completed RCTs of varenicline, found no excess of events apart from sleep disorders, with an RR of 1.02 (95% CI 0.86 to 1.22). A UK cohort study (Gunnell 2009) and prescription‐event monitoring studies in the UK (Kasliwal 2009) and in New Zealand (Harrison‐Woolrych 2011) have not detected significantly raised incidence rates of depression or of suicidal ideation and behaviour.
The FDA has sponsored two retrospective cohort studies, to explore possible relationships between varenicline and neuropsychiatric events (FDA 2011). One study, conducted by the Department of Veterans' Affairs, examined the incidence of psychiatric hospitalisations in 14,131 varenicline users versus an equal number of NRT users; at 30 days post‐prescription, they found no statistically significant difference in the risk of this outcome, with a Cox proportional hazard ratio for varenicline/NRT of 0.76 (95% CI 0.40 to 1.46). The second FDA‐sponsored study was conducted by the Department of Defence, in a propensity‐matched cohort of 10,814 varenicline users and the same number of NRT users. This study also found no excess of psychiatric hospitalisations at 30 or 60 days for either group; the hazard ratio for varenicline/NRT was 1.14 (95% CI 0.56 to 2.34) (Meyer 2013). While these studies cannot be assumed to have captured all relevant data, e.g. adverse events that did not lead to an inpatient episode, they nonetheless represent substantial cohorts of people in a real‐world setting with a range of comorbidities, and with findings consistent between the two studies.
The FDA issued a Drug Safety Communication warning in 2011, following publication of a trial of varenicline in patients with stable cardiovascular disease (Rigotti 2010), although the trialists themselves concluded that varenicline was well tolerated and was not associated with increases in cardiovascular events, deaths, blood pressure, or heart rate. A meta‐analysis of 14 trials (Singh 2011) detected an increased risk of serious cardiovascular events for varenicline users, with a Peto odds ratio of 1.72; 95% CI 1.09 to 2.71; however, this analysis may have some methodological flaws and limitations which could weaken the conclusions that the authors draw from their findings. A subsequent systematic review and meta‐analysis of 22 varenicline trials (Prochaska 2012), restricted to events within the period of drug treatment plus 30 days and presenting four summary measures (risk difference, risk ratio, Mantel‐Haenszel and Peto odds ratios), detected neither a clinically nor a statistically significant excess for the varenicline users. A prescription‐event monitoring study of almost 16,000 people prescribed varenicline in New Zealand between April 2007 and November 2010 (Harrison‐Woolrych 2012) identified 172 cardiovascular adverse events. Among these cases 48 were classified as myocardial Ischaemic events, and 50 as hypotensive events. Within each of these subgroups, two cases were considered by the investigators to have possibly been triggered by varenicline use. However, despite these areas of uncertainty, the FDA concludes that "the Agency continues to believe that the drug's benefits outweigh the risks and the current warnings in the Chantix drug label are appropriate" (FDA 2011).
Cytisine: efficacy, adverse events and serious adverse events
The two recent cytisine trials measured continuous abstinence, biochemically confirmed at longest follow‐up. The RR was 3.98; 95% CI 2.01 to 7.87. The 1971 cytisine trial used self‐reported point prevalence abstinence at two years, yielding an RR of 1.61; 95% CI 1.24 to 2.08.
None of the trials reported any serious adverse events associated with cytisine. A recent trial reported 10 events in eight participants (four from each group), including dyspepsia, nausea and headache, while the other reported gastrointestinal disorders at higher rates in the cytisine than in the placebo group (13.8% vs 8.1%, P = 0.02)
Nortriptyline: efficacy, adverse events and serious adverse events
Six trials comparing nortriptyline with placebo detected a risk ratio (RR) of 2.03; 95% confidence interval (CI) 1.48 to 2.78, in favour of the treatment; however, four trials testing it as an adjunct to NRT did not demonstrate an unequivocal benefit for the addition of nortriptyline (RR 1.29; 95% CI 0.97 to 1.72).
Adverse events for nortriptyline included dry mouth, drowsiness, light‐headedness and constipation, observed in studies treating depression in which doses were often > 150 mg. Nortriptyline for smoking cessation was generally prescribed at lower doses, with drop‐out rates ranging from 4% to 12%, which is similar to that for bupropion and NRT. The only serious adverse event in someone treated with nortriptyline was collapse/palpitations, thought possibly to have been caused by treatment.
Efficacy, adverse events and serious adverse events of other treatments:
The efficacy, adverse events and serious adverse events for bupropion and nortriptyline are covered in the sections above.
Among the SSRIs, there was no evidence of clinically significant benefit of using fluoxetine (four trials; RR 0.92; 95% CI 0.68 to 1.24), or for paroxetine (one trial: RR 1.08; 95% CI 0.64 to 1.82), or for sertraline (one trial; RR 0.71; 95% CI 0.30 to 1.64).
In the MAOI group of drugs, one trial of moclobemide demonstrated efficacy at the six‐month assessment, but this had dissipated by 12 months (RR 1.57; 95% CI 0.67 to 3.68). Three trials of selegiline did not detect a benefit of treatment (RR 1.45; 95% CI 0.81 to 2.61), and demonstrated significant heterogeneity (I² = 55%).
Anxiolytics 2010 Two trials comparing buspirone with placebo yielded a RR of 0.76; 95% CI 0.42 to 1.37. The point estimate does not establish effectiveness, but the confidence intervals do not rule out a clinically useful effect. One of the trials found a significant benefit of buspirone among the high anxiety subjects at the end of drug therapy (88% versus 61%, P < 0.01). However, the other trial did not replicate this effect.
One trial each of diazepam and meprobamate found no statistically or clinically significant effect of either drug. The meprobamate trial took an 85% or greater reduction as its outcome rather than complete abstinence, and found that the placebo groups produced higher quit rates than the intervention groups. One beta‐blocker trial found a cessation rate at 12‐month follow‐up of 17% for oxprenolol, 24% for metoprolol and 3% for placebo. The difference was statistically significant for metoprolol but not for oxprenolol. However the marked difference between the groups on active drug and placebo developed after the end of drug treatment, which is surprising.
The review notes that many of the anxiolytics have significant side effects such as a risk of abuse or dependence, and sedation. In view of uncertain efficacy and the side effects of the drugs, there is little justification for using them.
Clonidine 2008 Six studies meeting the inclusion criteria favoured clonidine treatment, although only one reached a statistically significant conclusion. Combining the results of the six studies gives a pooled RR of 1.63; 95% CI 1.22 to 2.18, suggesting that clonidine is effective. This equates to an absolute increase in the likelihood of quitting using clonidine of about 9%, given the quit rate amongst the pooled control groups of 14%.
Clonidine has clinically significant symptoms of sedation and postural hypotension occurring in a dose‐dependent manner in parallel with efficacy. it is reasonable to consider oral or transdermal clonidine as a second‐line pharmacotherapy for smoking cessation, but close medical supervision is essential to titrate the dose appropriately and monitor for potentially severe adverse effects.
Lobeline 2009 There were no studies which met the inclusion criteria. At six‐week follow‐up in the Dynagen 1997 study, there was no statistically significant difference in quitting between placebo (15% abstinent) and lobeline (17%).
Adverse events for lobeline include dizziness, nausea and vomiting,and tablets and pastilles containing lobeline may cause throat irritation.
The review authors considered that the data from two small trials were insufficient to support a meta‐analysis, and relied upon reporting quit rates and P values. In the first included study, the combination of mecamylamine capsules and nicotine patches compared to nicotine patches and placebo capsules led to a statistically significant difference in rates of sustained abstinence at six months (37.5% versus 12.5%, P = 0.046) and at 12 months (37.5% versus 4.2%, P = 0.004). In the second study, the reported rates of sustained abstinence at six months were 40% in the group pre‐treated with nicotine + mecamylamine, 20% in the group treated with nicotine alone, and 15% in the groups treated with mecamylamine alone, and with no drug treatment. The higher rate of abstinence in the group pre‐treated with nicotine and mecamylamine was not statistically significant. The authors detected a significant benefit for the two groups receiving mecamylamine prior to cessation compared to the groups which did not.
Mecamylamine can have significant adverse effects, including drowsiness, hypotension and constipation. In the first study, 70% of subjects treated with mecamylamine reported constipation compared to 30% treated with placebo, and two subjects required a dose reduction. In the second study, 40% of subjects required a reduction in dose of mecamylamine.
Nicobrevin 2009 Neither of the identified trials met the inclusion criteria, and both would have had some methodological concerns. In one, the smokers were not asked to try to quit, and numbers of cigarettes smoked on the last day of therapy was the primary outcome. Levels of blood carbon monoxide were measured, but not related to smoking status so could not be used to validate self‐reported quitting. In the second study, a variety of smoking cessation products were tested in a single trial. Participants received their allocated therapy by post. The placebo group received a placebo matched to the characteristics of another active therapy rather than to Nicobrevin, and there was no validation of self‐reported quit rates.
In April 2011, the Medicines and Healthcare products Regulatory Agency (MHRA) published a public assessment report on Nicobrevin. They determined that efficacy is unproven, that none of the UK smoking cessation agencies recommend its use, and that the possibility of side effects associated with the quinine and camphor components had led them to the view that the risks of Nicobrevin outweighed its benefits. They therefore withdrew its UK license from January 31st 2011 (MHRA 2011). This decision post‐dates the latest update of the Nicobrevin review.
Nicotine receptor partial agonists 2012 The efficacy, adverse events and serious adverse events for varenicline and cytisine are covered in the sections above.
The dianicline trial did not demonstrate a significant effect, measuring continuous abstinence biochemically confirmed at six months, with an RR of 1.20; 95% CI 0.82 to 1.75. This trial, EURODIAN, based in six European countries, was one of two conducted by its manufacturers, Sanofi‐Aventis. We have been unable to obtain trial information or results for the USA‐based companion trial, AMERIDIAN. The drug is no longer in development.
Nicotine vaccines 2012 None of the four included studies detected a statistically significant difference in long‐term cessation between participants receiving vaccine and those receiving placebo. For the NIC002 trial, the RR was 1.35 (95% CI 0.82 to 2.22), and for the NicVAX trial the RR was1.74; 95% CI 0.73 to 4.18. The two Phase III NicVAX trials, for which full results were not available, reported similar quit rates of approximately 11% in both intervention and placebo groups in the first trial, and results that were "not different" in the second.
In the two studies with full results available, post hoc analyses detected higher cessation rates in participants with higher levels of nicotine antibodies, but these findings are not readily generalisable. At six months, subgroups with high levels of antibody (Ab) titer in both studies demonstrated statistically significantly higher continuous abstinence rates than groups with lower Ab levels, with an RR of 1.76 in the NIC002 trial (95% CI 1.23 to 2.54, high Ab vs low + medium Ab groups combined) and an RR of 2.65 in the NicVAX trial (95% CI 1.34 to 5.22). However, at 12 months the difference in abstinence between low and high Ab groups in the NicVAX trial was no longer statistically significant (RR 1.80; 95% CI 0.81 to 3.99).
The two studies with full results showed nicotine vaccines to be well tolerated, with the majority of adverse events classified as mild or moderate. In the NIC002 trial, participants receiving the vaccine were more likely to report mild to moderate adverse events, most commonly flu‐like symptoms, whereas in the NicVAX trial there was no significant difference between the two arms. Findings on adverse events were not available for the two large Phase III trials of NicVAX, although the treatment was described in a Nabi press release as " well‐tolerated with a clinically acceptable safety and tolerability profile".
Opioid antagonists 2009 Four trials of naltrexone did not detect a significant effect of the intervention over placebo for long‐term abstinence, with or without NRT supplementation. The RR was 1.21; 95% CI 0.83 to 1.77. The review includes a further 24 trials, which assessed withdrawal symptoms, ad libitum smoking and hedonic effects, without contributing to the abstinence data.
The review did not report the incidence or severity of any adverse events.
Rimonabant 2011 The two cessation trials detected an RR of 1.50; 95% CI 1.10 to 2.05, favouring rimonabant 20 mg over placebo. No significant benefit was demonstrated for rimonabant at 5 mg dosage. In the relapse prevention trial, smokers who had quit on the 20 mg regimen were more likely to remain abstinent on either of the active regimens than on placebo; the RR for the 20 mg maintenance group was 1.29; 95% CI 1.06 to 1.57, and for the 5 mg maintenance group 1.30; 95% CI 1.06 to 1.59. There appeared to be no significant benefit of maintenance treatment for those who had quit on 5 mg.
Adverse events included nausea and upper respiratory tract infections, but little information was provided.
Silver acetate 2009 The first trial, comparing silver acetate gum with nicotine gum or with ordinary gum, found no significant differences in smoking status between the silver acetate and NRT arms (RR 0.98; 95% CI 0.69 to 1.39). The second trial, comparing silver acetate with placebo lozenges also failed to detect a significant effect, with an RR of 1.04; 95% CI 0.69 to 1.57.
In both trials, the total dose of silver acetate was restricted to reduce the chance of developing the rare outcome of argyrism (silver deposition in body tissues), and no subject suffered this side‐effect. The main adverse effects reported were those expected from this aversive stimulus; unpleasant tastes and sensations in the mouth, and gastrointestinal disturbances.
Discussion
We have placed particular emphasis in this overview upon the three most widely used pharmacotherapies. These are also the most comprehensive of the pharmacotherapy reviews in this area; the nicotine replacement therapy (NRT) review covers 150 included studies, the antidepressants review includes 49 studies of bupropion and nine of nortriptyline, and the nicotine receptor partial agonists review covers 20 included studies of varenicline and three of cytisine. We have conducted network meta‐analyses of the efficacy of NRT, bupropion and varenicline, versus placebo and versus each other, and have also used conventional meta‐analyses to explore the serious adverse event profiles of bupropion and varenicline. The remaining treatments are examined through a combination of narrative and statistical evaluations.
Summary of main results
This overview identified 12 Cochrane reviews of pharmacotherapies used to assist smoking cessation. Three treatments (NRT, bupropion and varenicline) are licensed as aids for smoking cessation in high‐income countries and recommended by many national guidelines, and we have concentrated on these interventions for this overview.
Both NRT and bupropion are similarly effective compared with placebo in helping people to quit. Varenicline is more effective than NRT or bupropion, when compared with placebo.
Direct and indirect comparisons between the three treatments demonstrate no advantage for NRT over bupropion. Varenicline is shown to be superior to any single type of NRT and to bupropion. Different types of NRT are generally equally effective. Combinations of NRT outperform single formulations, and may be as effective as varenicline. None of the network meta‐analysis findings show evidence of inconsistency.
Cytisine (pharmacologically similar to varenicline) also increases the chances of quitting compared with placebo.
Nortriptyline increases the chances of quitting compared with placebo. NRT combined with nortriptyline or with bupropion is not shown to be more effective than NRT alone.
There is a known risk of seizures in people taking bupropion, at a rate of about 1:1000, and probably lower than this for the slow‐release formulation. Our estimate of six seizures across all the bupropion arms is lower than the expected rate, at about 1:1500. Our meta‐analyses of serious adverse events (SAEs) in the bupropion studies demonstrate no excess of neuropsychiatric or cardiovascular events for bupropion users. These findings, while reassuring, may not be as robust as the efficacy results, since data on SAEs may have been under‐reported, especially in earlier trials.
Varenicline's superior efficacy must be tempered by current questions about its safety. Meta‐analysis of any SAE while on varenicline compared with placebo finds no difference between them, and subgroup analyses detect no significant excess of neuropsychiatric or cardiovascular events. While this finding is supported by a number of observational studies and challenged by others, the evidence is inconclusive at present, and long‐term post‐marketing surveillance will continue to inform the debate.
Among other treatments, clonidine also appears to be effective, but this is offset by a dose‐dependent rise in adverse events. Mecamylamine in combination with NRT may increase the chances of quitting, but the current evidence is inconclusive. Cytisine returns positive findings, without significant adverse events or SAEs. Other treatments subjected to meta‐analysis within the reviews fail to demonstrate a statistically or clinically significant benefit compared with placebo. Nicotine vaccines are not yet licensed for use as an aid to smoking cessation or relapse prevention. Nicobrevin's UK license is now revoked, and the development of rimonabant, taranabant and dianicline have all been suspended by their manufacturers.
Overall completeness and applicability of evidence
The completeness of an overview is necessarily limited by the recency of the source reviews. For NRT, bupropion and varenicline, while NRT 2012 and Nicotine receptor partial agonists 2012 are currently up to date, Antidepressants 2007 (covering bupropion and nortriptyline) has not been updated since 2009. Two bupropion trials derived from the excluded reviews which are not covered in Antidepressants 2007 have both been published since that review was last updated. This means that the contributing evidence for bupropion is out of date compared with the other two interventions. A further four reviews (Clonidine 2008; Lobeline 2009; Opioid antagonists 2009; Silver acetate 2009) are at least one year beyond the optimum 2½‐year update cycle. Opioid antagonists 2009 is currently being updated, and we are not aware of any randomised controlled trials awaiting evaluation for the other three reviews. However, there remains a possibility that out‐of‐date reviews may compromise the stability and completeness of the overview findings.
The NRT and bupropion trials in this overview span a longer period (from 1979 and 1992 respectively) than the varenicline studies (2006 onwards), and they include a higher proportion of non‐industry studies (Etter 2007). One factor in the apparently lower efficacy of NRT and bupropion compared with varenicline may be associated with the mix of smaller trials of lower quality, and the more varied settings and populations typical of older trials, which could be expected to reduce the point estimate. However, as pragmatic trials of community‐based or disease‐ or age‐specific populations have accrued to the varenicline review, the point estimate has remained relatively stable, maintaining a two‐ to three‐fold increase in the chances of quitting. The robustness of effect as the evidence base expands lends further support to varenicline's advantage as an aid to cessation.
We report on two recent trials of cytisine, which are covered in the Nicotine receptor partial agonists 2012 review. The success of varenicline has focused attention on its predecessor, Tabex [cytisine], which was developed in Bulgaria during the 1960s, and is widely available in Russia and the former socialist economy countries, and as an internet commodity. Although it is not licensed for use within the European Union or the USA, its potential as an affordable and effective smoking cessation treatment has sparked interest in further testing and development (Hajek 2013). A trial of Tabex versus NRT was registered in 2010, aiming to recruit 1310 smokers in New Zealand, but we are not aware of any other ongoing research in this area, so the gaps are likely to be in the funded research agenda rather than in the overview's coverage of this treatment.
While the evidence base for the efficacy of treatments may be considered adequate, information on adverse and serious adverse events is generally less well reported. We have not included NRT in our meta‐analyses of SAEs, since coverage of this information was patchy or absent from many trial reports. It was often not clear whether this could be ascribed to the lack of such events or to under‐reporting and/or misattribution. Recent studies have tended to report SAEs more comprehensively, to the benefit of the bupropion and varenicline evidence bases. However, much of the information that has caused concern over the safety profile of these treatments has emerged from monitoring and surveillance programmes rather than from trial reports. An evaluation of safety based on trial reports would require substantial sample sizes of real‐world heterogenous populations, perhaps supplemented by analyses of individual patient data and expert clinical assessment.
Quality of the evidence
Although the quality of the included reviews in this overview is considered to be high (based on AMSTAR ratings), the quality of the trials covered within individual reviews is variable. Across the board, older trials tended to under‐report their methodology. While increased online publication and the CONSORT 1996 statement have partially addressed this situation, the conduct of the trials may not necessarily have improved in line with reporting standards.
For the three network meta‐analysis treatments, the newest, varenicline, maintained the highest study quality, with 81% of trials estimated to be at low risk of bias for their randomisation procedures (sequence generation and allocation concealment). This compares with 21% of the NRT trials, and 46% of the bupropion trials. About 85% of trial outcomes in all three treatments were biochemically confirmed. Four NRT trials and four bupropion trials were based on conference abstracts only, while all the varenicline trials used for the meta‐analysis were from published reports. Although abstracts are compromised by a lack of detail and of peer‐review, their inclusion in Cochrane reviews can be justified by the contribution they make to the evidence base. It has been estimated that more than 50% of trials fail to translate to full publication, and that those which do include disproportionately positive findings (Scherer 2007). Approximately 10% of trials referenced in Cochrane reviews are taken from conference proceedings and other grey literature sources (Mallett 2002).
The remaining treatments were generally limited to small studies, frequently with inadequate or no description of the methodological features used to estimate study quality. Only five treatments within this category assessed populations of more than 1,000 participants. Fluoxetine (1236 participants in two trials) was not shown to improve cessation rates, and nortriptyline (1219 participants in four trials) did not enhance the performance of NRT. The efficacy of rimonabant (1049 participants across two trials) and NicVAX (2000 participants across two trials) could not be conclusively demonstrated, since the findings came from abstracts and company reports rather than from published papers. Cytisine (2151 participants in three trials) demonstrated a significant effect, although the study which contributed more than half the participants was the oldest and the least adequately conducted and reported.
Potential biases in the overview process
There are a number of potential sources of bias within the overview process. We have confined ourselves to Cochrane reviews, on the assumption that they represent the most comprehensive and coherent body of evidence available. An associated potential weakness is that three of the authors of this overview (KC, RP, TL) have contributed to most of the included reviews, either explicitly as authors or implicitly during the editorial processes. To counterbalance this, we have attempted to assess the quality and limitations of the included reviews as rigorously as possible, using independent and objective criteria, including the modified AMSTAR scale; but it is still possible that we have introduced biases by being insufficiently distanced from the review/overview process.
Our preference for intention‐to‐treat analyses may have introduced bias into our methods for handling missing data. The serious adverse events analyses for varenicline and bupropion include all participants randomised who were known to have received at least one dose of treatment, whether or not we had follow‐up data for them on these events. Our assumption has been that, provided there are no great disparities between rates of attrition from the intervention and control groups, missing data are unlikely to misrepresent the relative incidence of such events. We have also conducted sensitivity analyses, excluding participants known to have discontinued treatment or to have incomplete data, and found no substantive differences in the results. Our approach may nonetheless be viewed by some as limiting the findings of this overview. An intrinsic limitation of conducting an overview is that not every component review will be as up to date as we might wish. The bupropion review only covers trials available to 2009, and is due to be updated. We decided against conducting a 'quick and dirty' update of the bupropion trials, so as not to compromise the editorial quality of the contributing reviews, but this means accepting that the key reviews are not chronologically aligned.
Agreements and disagreements with other studies or reviews
Five other systematic overviews of smoking cessation pharmacotherapies have been published since the introduction of varenicline in 2006. Two were restricted to trials in pregnant women (Coleman 2012; Myung 2012), with neither identifying any randomised controlled trials of bupropion or varenicline in these populations. We note them here, but do not discuss them further, other than to observe their findings that the evidence on the safety and efficacy of NRT for smoking cessation in pregnancy is currently inconclusive.
Our findings are broadly consistent with those of the remaining three reviews (Eisenberg 2008a; Mills 2012; Wu 2006). Appendix 4 reports the individual findings from each review. The Mills and Wu reviews were both partially funded by Pfizer Inc (manufacturers of varenicline). The 2006 review by Wu and colleagues, although based on fewer trials than our reviews (70 NRT trials versus 122; 12 bupropion trials versus 36; 4 varenicline trials versus 15), calculated odds ratios and confidence intervals across all comparisons similar to those reported in this overview. Outcomes were biochemically confirmed and assessed at one year or longer. In the absence of head‐to‐head data, they conducted indirect comparisons between NRT and varenicline, using methods described in Bucher 1997, which preserve the integrity of the randomisation in individual trials.
Mills 2012, with two authors in common with the Wu review, revisits much of the same data. However, the later review stratifies outcomes by time of assessment (short‐term, three, six and twelve months), and draws comparisons between standard and high‐dose NRT, and between combination and single NRT formulations, as well as direct comparisons between the three different treatments. They conduct a series of pairwise meta‐analyses, and a network meta‐analysis using a Bayesian random‐effects multiple treatment comparison. Splitting outcomes by time point produced the occasional anomalous result, e.g. combination NRT versus placebo/control at three months and at one year did not achieve statistically significant effects, and varenicline at six months did not outperform high‐dose nicotine patch, although it was significantly more effective at all other time points across all other comparisons. As we prefer longest follow‐up for this overview and for the contributing reviews, we have compared their twelve‐month (longest) results with our own outcomes, and found them to be broadly consistent, allowing for their preference for risk ratios rather than odds ratios.
Eisenberg 2008a included trials with biochemically validated outcomes at six and 12 months, and used the most rigorous definition of abstinence available, i.e. preferring continuous to point prevalence abstinence. They explored NRT efficacy across five different formulations (gum, patch, spray, inhaler and tablet), comparing each of them with placebo. They also compared bupropion and varenicline with placebo, and with each other, but did not conduct indirect comparisons between active treatments. They found all treatments to be more effective than placebo, although nicotine inhaler (four trials) did not achieve a statistically significant result compared with placebo. Their direct comparison between varenicline and bupropion detected a lower OR (1.40; 95% CredI 0.75 to 2.66; Eisenberg 2008b) than did the other three overviews, which all found a statistically significant effect in favour of varenicline. The authors commented on this disparity as follows: "The wider interval obtained in our analysis ... is a reflection of how we handled the Nides study, as well as our use of Bayesian techniques, which produce wider intervals than their frequentist equivalents as they incorporate greater uncertainty (particularly compared with fixed effects models)" (Eisenberg 2013.
We include the Cochrane Tobacco Addiction Group's glossary of smoking‐related terms Appendix 5.
Authors' conclusions
Efficacy for NRT, bupropion and varenicline is well‐established across a strong evidence base. There are fewer trials of cytisine and nortriptyline, but current findings indicate that they also improve the chances of quitting. The adverse and serious adverse event profiles are less clearly defined, and rely more heavily upon monitoring and surveillance systems than on data from trials.
Implications for practice.
Both nicotine replacement therapy (NRT) and bupropion perform similarly compared with placebo in helping people to quit (odds ratios (ORs) of 1.84 (95% CredI 1.71 to 1.99) and 1.82 (95% CredI 1.60 to 2.06) respectively).
Different types of NRT are generally equally effective.
Combinations of NRT outperform single formulations (versus patch: OR 1.43 (95% CredI 1.08 to 1.91); versus gum: OR 1.63 (95% CredI1.21 to 2.20); versus 'other': OR 1.34 (95% CredI 1.00 to 1.80)).
Varenicline is more effective than NRT or bupropion, when each is compared with placebo (varenicline vs placebo OR 2.88 (95% CredI 2.40 to 3.47)).
Varenicline is superior to any single type of NRT, and is as effective as combinations of NRT (OR 1.06 (95% CredI 0.75 to 1.48)).
Varenicline outperforms bupropion in head‐to‐head comparisons (OR 1.59 (95% CredI 1.29 to 1.96)).
NRT combined with nortriptyline or with bupropion is not shown to be more effective than NRT alone.
Cytisine increases the chances of quitting compared with placebo, without significant adverse or serious adverse events (RR 3.98 (95% CI 2.01 to 7.87))
Nortriptyline approximately doubles the chances of quitting (RR 2.03 (95% CI 1.48 to 2.78)).
Bupropion demonstrates no excess of neuropsychiatric events (RR 0.88 (95% CI 0.31 to 2.50)) or of cardiovascular events (RR 0.77 (95% CI 0.37 to 1.59)).
Varenicline demonstrates no excess of neuropsychiatric events (RR 0.53 (95% CI 0.17 to 1.67)), and a marginal but non‐significant increase in cardiovascular events (RR 1.26 (95% CI 0.62 to 2.56)).
Implications for research.
Randomised controlled trials comparing varenicline with NRT (single and combination formulations) would address current uncertainties about their respective efficacy and safety.
Long‐term post‐marketing surveillance should continue for varenicline, to determine the likelihood of its implication in neuropsychiatric and/or cardiac events.
Cytisine's potential as an effective and affordable therapy should be explored through additional large‐scale trials.
Further trials of NRT versus placebo are unlikely to modify the known benefits and risks of this treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2015 | Amended | Citation of Mills 2010 corrected |
Acknowledgements
We thank Paul Aveyard, Linda Bauld and Neal Benowitz for reading and commenting on the protocol in draft. We thank Edward Mills and Robert Gibbons for reading and commenting on the full review in draft, and the Group's external editorial team (Paul Aveyard, John Hughes and Robert West) for advice and comments at the review stage. We also thank Beverley Shea for discussion and advice on revisions to the AMSTAR scale. We thank Monika Epler for interpretation of a Polish trial report. We thank Natalie Walker for advice about her ongoing trial of cytisine, and Mark Eisenberg for clarification of data. We thank Robert Gibbons for sight of unpublished data on varenicline's safety profile. We thank Jamie Hartmann‐Boyce for assistance with serious adverse events data.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Tobacco Addiction Group. The views and opinions expressed in the Group's reviews are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Individual review meta‐analyses vs network meta‐analyses
| Comparison | Review OR | 95% CI | Network OR | 95% CI | Notes |
| NRT vs placebo | 1.78 | 1.68 to 1.88 | 1.84 | 1.71 to 1.99 | 119 comparisons |
| Bupropion vs placebo | 1.86 | 1.66 to 2.08 | 1.82 | 1.60 to 2.06 | 36 comparisons |
| Varenicline vs placebo | 2.83 | 2.45 to 3.26 | 2.88 | 2.40 to 3.47 | 15 comparisons |
| Bupropion vs NRT; | 1.02 | 0.84 to 1.24 | 0.99 | 0.86 to 1.13 | Data from NRT review, 7 trials. Two trials missing from NRT review. Including them drops I² from 41 to 23%, and raises OR from 1.02 to 1.03 (95% CI 0.85 to 1.24) |
| Varenicline vs bupropion | 1.66 | 1.28 to 2.16 | 1.59 | 1.29 to 1.96 | 3 trials (NRT and bupropion reviews agree) |
| Varenicline vs NRT | N/A | N/A | 1.57 | 1.29 to 1.91 |
Appendix 2. Rationale for the choice of priors
Our network meta‐analyses are based on trial level data, using non‐informative priors.The choice of priors remains a contentious issue within any Bayesian framework. It is well recorded that even choosing so‐called non‐informative priors will have an impact on the findings. However, it is also well understood that this impact reduces as the sample size increases. Results from a recent paper (Thorlund 2013) are entirely consistent with this, where, in the first example, the number of trials included in the model is sparse and the choice of priors has a considerable impact on the estimates (less so in the second example). In our review, the large number of studies included for each one of the head‐to‐head comparisons makes the choice of priors less crucial in determining the final estimates. As a reflection of this, there is good agreement between the point estimates and the 95% confidence interval and 95% credible interval from the direct and indirect comparisons (Appendix 1).
Appendix 3. Trials, participants and point estimates in the included reviews
| DRUG | versus | TRIALS | PTS | RR (95% CI) |
| Bupropion | placebo | 36 | 11440 | 1.69 (1.53 to 1.85) |
| Bupropion | NRT | 3 | 657 | 1.26 (0.73 to 2.18) |
| Bupropion + NRT | NRT alone | 6 | 1106 | 1.23 (0.67 to 2.26) |
| Nortriptyline | placebo | 6 | 975 | 2.03 (1.48 to 2.78) |
| Nortriptyline + NRT | NRT alone | 4 | 1219 | 1.29 (0.97 to 1.72) |
| Fluoxetine | placebo | 2 | 1236 | 0.92 (0.65 to 1.30) |
| Fluoxetine + NRT | placebo+NRT | 2 | 250 | 0.92 (0.53 to 1.61) |
| Paroxetine | placebo | 1 | 224 | 1.08 (0.64 to 1.82) |
| Sertraline | placebo | 1 | 134 | 0.71 (0.30 to 1.64) |
| Moclobemide | placebo | 1 | 88 | 1.57 (0.67 to 3.68) |
| Selegiline | placebo | 3 | 250 | 1.45 (0.81 to 2.61) |
| Venlafaxine | placebo | 1 | 147 | 1.22 (0.64 to 2.32) |
| 66 | 17726 | |||
| Varenicline | placebo | 14 | 6166 | 2.27 (2.02 to 2.55) |
| Varenicline low‐dose | placebo | 4 | 1272 | 2.09 (1.56 to 2.78) |
| Varenicline | bupropion | 3 | 1622 | 1.52 (1.22 to 1.88) |
| Varenicline | placebo (maintenance) | 1 | 1208 | 1.19 (1.03 to 1.36) |
| Cytisine | placebo | 2 | 937 | 3.98 (2.01 to 7.87) |
| 1 | 1214 | 1.61 (1.24 to 2.08) | ||
| Dianicline | placebo | 1 | 602 | 1.20 (0.82 to 1.75) |
| 26 | 13021 | |||
| NRT | placebo | 119 | 51265 | 1.60 (1.53 to 1.68) |
| Combination NRT | Other forms or mixes of NRT | 9 | 4664 | 1.34 (1.18 to 1.51) |
| Physician + NRT | OTC NRT | 2 | 820 | 4.58 (1.18 to 17.88) |
| Patch before TQD | patch from TQD | 8 | 2774 | 1.18 (0.98 to 1.41) |
| At end of pregnancy | placebo | 4 | 1675 | 1.30 (1.00 to 1.68) |
| NRT +/‐ bupropion | bupropion, placebo or patch | 5 | 2544 | 1.01 (0.87 to 1.18) |
| 147 | 63742 | |||
| Rimonabant | placebo | 2 | 1049 | 1.50 (1.10 to 2.05) |
| 2 | 1049 | |||
| Buspirone | placebo | 3 | 201 | 0.76 (0.42 to 1.37) |
| Buspirone | NRT patch | 1 | 208 | 1.08 (0.70 to 1.65) |
| Diazepam | placebo | 1 | 76 | 1.00 (0.56 to 1.80) |
| Meprobamate | placebo | 1 | 324 | 0.45 (0.18 to 1.18) |
| Oxprenolol | placebo | 1 | 66 | 5.31 (0.68 to 41.74) |
| Metoprolol | placebo | 1 | 64 | 7.52 (1.00 to 56.66) |
| 8 | 939 | |||
| Clonidine | placebo | 6 | 776 | 1.63 (1.22 to 2.18) |
| 6 | 776 | |||
| Mecamylamine/+‐NRT | placebo/+‐NRT | 2 | 128 | No MA data |
| 2 | 128 | |||
| Vaccines | placebo | 2 | 642 | 1.35 (0.82 to 2.22) |
| 2 | 2000 | No MA data | ||
| 4 | 2642 | |||
| Naltrexone | placebo | 2 | 129 | 1.34 (0.49 to 3.69) |
| Naltrexone + NRT | placebo + NRT | 3 | 453 | 1.24 (0.74 to 2.09) |
| 4 | 582 | |||
| Silver acetate | placebo | 2 | 785 | 1.04 (0.69 to 1.57) |
| Silver acetate | NRT gum | 1 | 414 | 0.98 (0.69 to 1.39) |
| 2 | 1199 | |||
| TOTALS | 267 | 101,804 |
Appendix 4. Comparison with other overviews: OR and (95% CI)
| NRT vs placebo | Bup vs placebo | V vs placebo | Bup vs NRT | NRT vs V OR | V vs bup OR | |
| Cahill 2013 | 1.84 (1.71 to 1.99) 122 trials | 1.82 (1.6 to 2.06) 36 trials | 2.88 (2.4 to 3.47) 15 trials | 0.99 (0.86 – 1.13) 9 trials |
1.57 (1.29 to 1.91) Indirect | 1.59 (1.29 to 1.96) 3 trials |
| Wu 2006 | 1.71 (1.55 to 1.88) 70 trials | 1.56 (1.10 to 2.21) 12 trials | 2.96 (2.12 to 4.12) 4 trials | 1.14 (0.20 to 6.42) 2 trials | 1.66 (1.17 to 2.36) Indirect | 1.58 (1.22 to 2.05) 3 trials |
| Mills 2012 (RRs) | 1.48 (1.30 to 1.69) 32 trials | 1.40 (1.22 to 1.60) 27 trials | 2.39 (1.96 to 2.88) 7 trials | 0.94 (0.77 to 1.15) Not stated, not included in MTC | 1.65 (1.29 to 2.07) 1 trial (Aubin) | 1.61 (1.32 to 193) 3 trials |
| Eisenberg 2008 | 1.71 (1.35 to 2.21) 22 gum trials 2.07 (1.69 to 2.62) 30 patch trials 2.17 (0.95 to 5.43) 4 inhaler trials 2.37 (1.12 to 5.13) 4 spray trials 2.06 (1.12 to 5.13) 6 tablet trials |
2.07 (1.73 to 2.55) 16 trials |
2.41 (1.91 to 3.12) 6 trials/13 groups |
1.40 (0.75 to 2.66) 3 trials/5 groups |
Appendix 5. Tobacco Addiction Group Glossary
This is a brief topic‐specific glossary of terms used in studies of tobacco use and cessation, and in our Group's reviews. For methodological and statistical terminology used in systematic reviews, see the Glossary of Terms in the Cochrane Collaboration: http://www3.interscience.wiley.com/homepages/106568753/glossary.pdf
Abstinence A period of being quit, i.e. stopping the use of cigarettes or other tobacco products. May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence
Biochemical verification Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide (CO) in exhaled breath or in blood. See: SRNT Subcommittee on Biochemical Verificationl 'Biochemical verification of tobacco use and cessation'; Nicotine & Tobacco Research, 2002: 4 (2); 149‐59
Bupropion A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant).
Carbon monoxide (CO) A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence.
Cessation Also called 'quitting' . The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour
Continuous abstinence Also called 'sustained abstinence'; cf 'prolonged abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence
'Cold Turkey' Quitting abruptly, and/or quitting without behavioural or pharmaceutical support
Craving A very intense urge or desire [to smoke] See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614
Dopamine A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement
Efficacy Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups
Harm reduction Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco.
Lapse/ slip Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse.
nAChR [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine
Nicotine An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking.
Nicotine Replacement Therapy (NRT) A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges.
Outcome Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial
Pharmacotherapy A treatment using pharmaceutical drugs, e.g. NRT, bupropion, varenicline
Point prevalence abstinence (PPA) A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence
Prolonged abstinence A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13‐25
Relapse A return to regular smoking after a period of abstinence
Second‐hand smoke Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins.
Self‐efficacy The belief that one will be able to change one's behaviour, e.g. to quit smoking
SPC [Summary of Product Characteristics] Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively.
Tapering A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment
Tar The toxic chemicals found in cigarettes. In solid form, it is the brown, tacky residue visible in a cigarette filter and deposited in the lungs of smokers.
Titration A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects.
Withdrawal A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614
Contributions of authors
KC and TL developed the concept of this overview. RP was responsible for the statistical approach and the methodology, and SS conducted the network meta‐analyses. KC conducted the search and wrote the review, and all authors contributed to the final version.
Sources of support
Internal sources
Department of Primary Care Health Sciences, University of Oxford, UK.
National School for Health Research School for Primary Care Research, UK.
External sources
National Institute for Health Research (NIHR), UK.
Declarations of interest
Three authors of this overview (KC, RP, TL) have contributed to many of the included reviews.
Edited (no change to conclusions)
References
References to included reviews
Antidepressants 2007
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD000031.pub3] [DOI] [PubMed] [Google Scholar]
Anxiolytics 2010
- Hughes JR, Stead LF, Lancaster T. Anxiolytics for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD002849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clonidine 2008
- Gourlay SG, Stead LF, Benowitz N. Clonidine for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000058.pub2] [DOI] [Google Scholar]
Lobeline 2009
- Stead LF, Hughes JR. Lobeline for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000124] [DOI] [PubMed] [Google Scholar]
Mecamylamine 2011
- Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicobrevin 2009
- Stead LF, Lancaster T. Nicobrevin for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicotine receptor partial agonists 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub5] [DOI] [PubMed] [Google Scholar]
Nicotine vaccines 2012
- Hartmann‐Boyce J, Cahill K, Hatsukami D, Cornuz J. Nicotine vaccines for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD007072] [DOI] [PMC free article] [PubMed] [Google Scholar]
NRT 2012
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD000146.pub3] [DOI] [PubMed] [Google Scholar]
Opioid antagonists 2009
- David SP, Stead LF, Lancaster T, Evins AE. Opioid antagonists for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003086.pub2] [DOI] [PubMed] [Google Scholar]
Rimonabant 2011
- Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD005353.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silver acetate 2009
- Lancaster T, Stead LF. Silver acetate for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000191] [DOI] [PubMed] [Google Scholar]
References to excluded reviews
Adolescent cessation 2006
- Grimshaw G, Stanton A. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003289] [DOI] [PubMed] [Google Scholar]
COPD patients 2001
- Meer RM, Wagena E, Ostelo RWJG, Jacobs AJE, Schayck OCP. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002999] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hospital patients 2012
- Rigotti N, Munafó MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001837.pub2] [DOI] [PubMed] [Google Scholar]
Internet 2010
- Civljak M, Sheikh A, Stead LF, Car J. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007078.pub3] [DOI] [PubMed] [Google Scholar]
Pharmacists 2004
- Sinclair HK, Bond C, Stead LF. Community pharmacy personnel interventions for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003698.pub2] [DOI] [PubMed] [Google Scholar]
Pre‐operative patients 2010
- Thomsen T, Villebro N, Møller A. Interventions for preoperative smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD002294.pub3] [DOI] [PubMed] [Google Scholar]
Pregnancy 2009
- Lumley J, Chamberlain C, Dodswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001055.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reduction vs abrupt 2010
- Lindson N, Aveyard P, Hughes JR. Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008033.pub2] [DOI] [PubMed] [Google Scholar]
Relapse prevention 2009
- Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003999.pub3] [DOI] [PubMed] [Google Scholar]
Schizophrenia 2010
- Tsoi DT, Porwal M, Webstre AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD007253.pub2] [DOI] [PubMed] [Google Scholar]
Weight gain prevention 2009
- Parsons A, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006219.pub2] [DOI] [PubMed] [Google Scholar]
Workplace 2008
- Cahill K, Moher M, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003440.pub3] [DOI] [PubMed] [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S et al [GRADE Working Group]. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benowitz 2008
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clinical Pharmacology and Therapeutics 2008;83(4):531‐41. [DOI] [PubMed] [Google Scholar]
Beyens 2008
- Beyens MN, Guy C, Mounier G, Laporte S, Ollagnier M. Serious Adverse Reactions of Bupropion for Smoking Cessation Analysis of the French Pharmacovigilance Database from 2001 to 2004. Drug Safety 2008;31:1017‐26. [DOI] [PubMed] [Google Scholar]
Boshier 2003
- Boshier A, Wilton LV, Shakir SA. Evaluation of the safety of bupropion (Zyban) for smoking cessation from experience gained in general practice use in England in 2000. European Journal of Clinical Pharmacology 2003;59(10):767‐73. [DOI] [PubMed] [Google Scholar]
Brooks 1998
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics 1998;7(4):434‐45. [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Chun‐Fai‐Chan 2005
- Chun‐Fai‐Chan B, Koren G, Fayez I, Kalra S, Voyer‐Lavigne S, Boshier A, Shakir S, Einarson A. Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. Am J Obstet Gynecol 2005;192(3):932‐936. [DOI] [PubMed] [Google Scholar]
Coleman 2012
- Coleman T, Chamberlain C, Davey M‐A, Cooper SE, Leonardi‐Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010078] [DOI] [PubMed] [Google Scholar]
CONSORT 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials: The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Disease Control Priorities 2006
- Disease Control Priorities Project. Tobacco Addiction. http://www.dcp2.org/file/52/DCPP‐Tobacco.pdf (accessed 24th March 2013).
Dunner 1998
- Dunner DL, Zisook S, Billow AA, Batey SR, Johnston JA, Ascher JA. A prospective safety surveillance study for bupropion sustained‐release in the treatment of depression. Journal of Clinical Psychiatry 1998;59:366‐73. [DOI] [PubMed] [Google Scholar]
Ebbert 2011
- Ebbert J, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD004306.pub4] [DOI] [PubMed] [Google Scholar]
Eisenberg 2008a
- Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, et al. Pharmacotherapies for smoking cessation: a meta‐analysis of randomized controlled trials. Canadian Medical Association Journal 2008;179(2):135‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenberg 2008b
- Eisenberg MJ, Filion KB. Correction. Canadian Medical Association Journal 2008;179(8):802. [Google Scholar]
Eisenberg 2013
- Filion K, Eisenberg M. Personal communication 6th January 2013.
EMEA 2002
- European Agency for the Evaluation of Medicines for Human Use. Committee for Proprietary Medicinal Products. Opinion following an article 36 referral. Bupropion hydrochloride. http://www.emea.eu.int/pdfs/human/referral/2761002en.pdf Accessed 29 April 2004.
Etter 2007
- Etter JF, Burri M, Stapleton J. The impact of pharmaceutical company funding on results of randomized trials of nicotine replacement therapy for smoking cessation: a meta‐analysis. Addiction 2007;102:815‐22. [DOI] [PubMed] [Google Scholar]
Evans 2009
- Evans JR, Virgili G, Gordon I, Bunce C, Chakravarthy U, Desai P, et al. Interventions for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007650] [DOI] [Google Scholar]
FDA 2011
- U. S. Food, Drug Administration. FDA drug safety communication: safety review update of Chantix (varenicline) and risk of neuropsychiatric adverse events. http://www.fda.gov/Drugs/DrugSafety/ucm276737.htm (accessed 19/1/2012) October 2011.
Fiore 1992
- Fiore MC, Jorenby DE, Baker TB, Kenford SL. Tobacco dependence and the nicotine patch. Clinical guidelines for effective use. JAMA 1992;268:2687‐94. [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Treating tobacco use and dependence: 2008 Update. Clinical Practice Guideline. Rockville MD: US Department of Health and Human Services, 2008. [Google Scholar]
GlaxoSmithKline
- GlaxoSmithKline. Zyban(R) bupropion hydrochloride Sustained‐Release Tablets. Product Information. www.gsk.com/products/assets/us_zyban.pdf (accessed 15/11/2006).
GLS 2009
- Office for National Statistics. Smoking and drinking among adults, 2009. A report on the 2009 General Lifestyle Survey. ONS, 2011. [Google Scholar]
Gonzales 2006
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4ß2 nicotinic acetylcholine receptor partial agonist, vs sustained‐release bupropion and placebo for smoking cessation. JAMA 2006;296(1):47‐55. [clinicaltrials.gov ID: NCT00141206] [DOI] [PubMed] [Google Scholar]
Greenland 1998
- Greenland S, Satterfield MH, Lanes SF. A meta‐analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Safety 1998;18:297‐308. [DOI] [PubMed] [Google Scholar]
Gunnell 2009
- Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ 2009;339:b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2013
Harrison‐Woolrych 2011
- Harrison‐Woolrych M, Ashton J. Psychiatric adverse events associated with varenicline: an intensive postmarketing prospective cohort study in New Zealand. Drug Safety 2011;34(9):763‐72. [DOI] [PubMed] [Google Scholar]
Harrison‐Woolrych 2012
- Harrison‐Woolrych M, Maggo S, Tan M, Savage R, Ashton J. Cardiovascular events in patients taking varenicline: a case series from intensive postmarketing surveillance in New Zealand. Drug Safety 2012;35(1):33‐43. [DOI] [PubMed] [Google Scholar]
Hubbard 2005
- Hubbard R, Lewis S, West J, Smith C, Godfrey C, Smeeth L, et al. Bupropion and the risk of sudden death: a self‐controlled case‐series analysis using The Health Improvement Network. Thorax 2005;60(10):848‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2003
- Hughes JR, Keely JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research 2003;5(1):13‐25. [PubMed] [Google Scholar]
Jorenby 2006
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4ß2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained‐release bupropion for smoking cessation. JAMA 2006;296(1):56‐63. [clinicaltrials.gov ID NCT00143364] [DOI] [PubMed] [Google Scholar]
Joseph 1996
- Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. New England Journal of Medicine 1996;335:1792‐8. [DOI] [PubMed] [Google Scholar]
Joseph 2003
- Joseph AM, Fu SS. Safety issues in pharmacotherapy for smoking in patients with cardiovascular disease. Progress in Cardiovascular Diseases 2003;45:429‐41. [DOI] [PubMed] [Google Scholar]
Kasliwal 2009
- Kasliwal R, Wilton LV, Shakir SA. Safety and drug utilization profile of varenicline as used in general practice in England: interim results from a prescription‐event monitoring study. Drug Safety 2009;32(6):499‐507. [DOI] [PubMed] [Google Scholar]
Kotlyar 2005
- Kotlyar M, Brauer LH, Tracy TS, Hatsukami DK, Harris J, Bronars CA, Adson DE. Inhibition of CYP2D6 activity by bupropion. Journal of Clinical Psychopharmacology 2005;25:226‐9. [DOI] [PubMed] [Google Scholar]
Levine 2010
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, Marcus MD. Bupropion and cognitive behavioural therapy for weight‐concerned women smokers. Archives of Internal Medicine 2010;170(6):543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2004
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23(20):3105‐24. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59. [Google Scholar]
Lumley 2009
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001055.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mallett 2002
- Mallett S, Hopewell S, Clarke M. Grey literature in systematic reviews: The first 1000 Cochrane systematic reviews. Fourth symposium on systematic reviews: Pushing the boundaries. Oxford, 2002.
Meine 2005
- Meine TJ, Patel MR, Washam JB, Pappas PA, Jollis JG. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. American Journal of Cardiology 2005;95:976‐8. [DOI] [PubMed] [Google Scholar]
Meyer 2013
- Meyer TE, Taylor LG, Xie S, Graham DJ, Mosholder AD, Williams JR, et al. Neuropsychiatric events in varenicline and nicotine replacement patch users in the Military Health System. Addiction 2013;108(1):203‐10. [DOI] [PubMed] [Google Scholar]
MHRA 2004
- Pharmacovigilance Information Unit, Medicines and Healthcare products Regulatory Agency (UK). Personal Communication 29 April 2004.
MHRA 2011
- Medicines and Healthcare products Regulatory Agency. MHRA UK Public Assessment Report|: Nicobrevin: withdrawn from UK market as risks outweigh benefits. http://www.mhra.gov.uk/home/groups/pl‐p/documents/websiteresources/con114612.pdf (accessed 20/11/2012) April 2011.
Mills 2010
- Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta‐analysis of one hundred and twenty studies involving 177,390 individuals. Tobacco Induced Diseases 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high‐dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta‐analysis. Annals of Medicine 2012;44(6):588‐97. [DOI] [PubMed] [Google Scholar]
MMWR 2003
- Centers for Disease Control and Prevention. Cigarette smoking‐attributable morbidity ‐ United States 2000. MMWR Sep 2003.
MMWR 2007
- Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2006. MMWR 2007;56:1157‐61. [PubMed] [Google Scholar]
Myung 2012
- Myung S‐K, Ju W, Jung H‐S, Park C‐H, Oh S‐W, Seo HG, et al. Efficacy and safety of pharmacotherapy for smoking cessation among pregnant smokers: a meta‐analysis. British Journal of Obstetrics and Gynaecology 2012;119(9):1029‐39. [DOI] [PubMed] [Google Scholar]
NHS 2008
- The Information Centre for Health and Social Care. Statistics on smoking: England. National Health Service, 2008. [Google Scholar]
Palmer 1992
- Palmer KJ, Buckley MM, Faulds D. Transdermal Nicotine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an aid to smoking cessation. Drugs 1992;44:498‐529. [DOI] [PubMed] [Google Scholar]
Planer 2011
- Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T, et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Archives of Internal Medicine 2011;171(12):1055‐60. [DOI] [PubMed] [Google Scholar]
Plummer 2003 [Computer program]
- Plummer M. JAGS: a program for analysis of bayesian graphical models using gibbs sampling. Sourceforge ( https://sourceforge.net/projects/mcmc‐jags), 2003.
Plummer 2012 [Computer program]
- Plummer M. RJAGS: Bayesian graphical models using MCMC (http://CRAN.R‐project.org/package=rjags). Sourceforge (http://mcmc‐jags.sourceforge.net), 2012.
Pollak 2007
- Pollak KI, Oncken CA, Lipkus IM, Lyna P, Swamy GK, Pletsch PK, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. American Journal of Preventive Medicine 2007;33:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 2012
- Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta‐analysis. BMJ 2012;344:e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
RCP 2000
- Royal College of Physicians. Tobacco Addiction in Britain: a report of the Tobacco Advisory Group of the Royal College of Physicians. http://old.rcplondon.ac.uk/pubs/books/nicotine/index.htm (accessed 19th April 2011) 2000.
Rigotti 2010
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease. Circulation 2010;121(2):221‐9. [clinicaltrials.gov ID: NCT00282984] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherer 2007
- Scherer RW, Langenberg P, Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000005.pub3] [DOI] [PubMed] [Google Scholar]
SCOTH 1998
- Department of Health. Report of the Scientific Committee on Tobacco and Health. http://www.archive.official‐documents.co.uk/document/doh/tobacco/report.htm (accessed 19th April 2011) 1998.
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI: 10.1186/1471-2288-7-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shea 2011
- Shea B. Personal communication August 2011.
Singh 2011
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta‐analysis. Canadian Medical Association Journal 2011;183(12):1359‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spiegelhalter 2002
- Spiegelhalter DJ, Best NG, Carlin BP, Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B: Statistical Methodlogy 2002;64(4):583‐616. [Google Scholar]
SRNT 2002
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research 2002;4(2):149‐59. [DOI] [PubMed] [Google Scholar]
Stapleton 1998
- Stapleton J. Cigarette smoking prevalence, cessation and relapse. Statistical Methods in Medical Research 1998;7(2):187‐203. [DOI] [PubMed] [Google Scholar]
Team 2012
- Team RC. A language and environment for statistical computing. http://www.R‐project.org (accessed 20/11/2012) 2012.
TGA 2004
- Adverse Drugs Reactions Unit, Therapeutic Goods Administration (Australia). Personal Communication 31 March 2004.
Thorlund 2013
- Thorlund K, Thabane L, Mills EJ. Modelling heterogeneity variances in multiple treatment comparison meta‐analysis ‐ Are informative priors the better solution?. Medical Research Methodology 2013 11 January [Epub ahead of print]; Vol. 13, issue 2. [DOI: 10.1186/1471-2288-13-2] [DOI] [PMC free article] [PubMed]
TNWG 1994
- Transdermal Nicotine Working Group. Nicotine replacement therapy for patients with coronary artery disease. Working Group for the Study of Transdermal Nicotine in Patients with Coronary Artery Disease. Archives of Internal Medicine 1994;154:989‐95. [PubMed] [Google Scholar]
Tobacco Atlas 2010
- Shafey O, Eriksen M, Ross H, Mackay J. The Tobacco Atlas. The Tobacco Atlas. 3rd Edition. World Health Organization, 2010. [Google Scholar]
Tonstad 2010
- Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double‐blind, placebo‐controlled clinical trials of varenicline: a pooled analysis. Drug Safety 2010;33(4):289‐301. [DOI] [PubMed] [Google Scholar]
Tsoi 2010
- Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD007253.pub2] [DOI] [PubMed] [Google Scholar]
Tønnesen 2012
- Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double blind trial. European Respiratory Journal 2012;40(3):548‐54. [DOI: 10.1183/09031936.00155811] [DOI] [PMC free article] [PubMed] [Google Scholar]
UK Clinical Trials [Computer program]
- Current Controlled Trials. metaRegister of Controlled Trials (mRCT). Springer Science+Business Media, http://www.controlled‐trials.com/mrct/ (accessed 23rd May 2012).
US Clinical Trials [Computer program]
- United States National Institutes of Health. ClinicalTrials.gov. USNIH, http://clinicaltrials.gov/ct2/home (accessed 23rd May 2012).
USFDA 2009a
- Anon. Varenicline and bupropion. Reports of suicidality associated with used of varenicline (marketed as CHANTIX) and bupropion (marketed as Zyban and generics). FDA Drug Safety Newsletter 2009;2(1):1‐4. [Google Scholar]
van der Meer 2009
- Meer RM, Willemsen MC, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006102] [DOI] [PubMed] [Google Scholar]
van Valkenhoef 2012a
van Valkenhoef 2012b
- Valkenhoef G, Kuiper J. GeMTC: GeMTC network meta‐analysis. http://CRAN.R‐project.org/package=gemtc (accessed 20/11/2012) 2012.
Wallstrom 1999
- Wallstrom M, Sand L, Nilsson F, Hirsch JM. The long‐term effect of nicotine on the oral mucosa. Addiction 1999;94:417‐23. [DOI] [PubMed] [Google Scholar]
West 2006
- West R. Background smoking cessation rates in England. http://www.smokinginengland.info/Ref/paper2.pdf (accessed 19th April 2011) 2006.
WHO 2009
- World Health Organization. 17th expert committee on the selection and use of essential medicines. http://www.who.int/selection_medicines/committees/expert/17/en/index.html (accessed 19th April 2011) 2009.
WHO trials registry platform [Computer program]
- World Health Organization. Internation Clinical Trials Registry Platform Search Portal. WHO, http://apps.who.int/trialsearch/Default.aspx (accessed May 23rd 2012).
Williams 2007
- Williams KE, Reeves KR, Billing CB, Pennington AM, Gong J. A double‐blind study evaluating the long‐term safety of varenicline for smoking cessation. Current Medical Research and Opinion 2007;23(4):793‐801. [clinical trials.gov ID: 00143299] [DOI] [PubMed] [Google Scholar]
Wu 2006
- Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis. BMC Public Health 2006;6:300. [DOI: 10.1186/1471-2458-6-300] [DOI] [PMC free article] [PubMed] [Google Scholar]


