Abstract
Background
Hair has traditionally been removed from the surgical site before surgery; however, some studies claim that this increases surgical site infections (SSIs) and should be avoided. This is the second update of a review published in 2006 and first updated in 2011.
Objectives
To determine whether routine preoperative hair removal (compared with no removal) and the method, timing, or setting of hair removal effect SSI rates.
Search methods
In November 2019, for this second update we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase; and EBSCO CINAHL Plus. We also searched clinical trial registries for ongoing and unpublished studies, and scanned the reference lists of included studies plus reviews to identify additional studies. We applied no date or language restrictions.
Selection criteria
We included randomised controlled trials or quasi‐randomised trials that compared:
· hair removal with no hair removal; · different methods of hair removal; and · hair removal at different times before surgery.
Data collection and analysis
Two review authors independently assessed the relevance of each study. Data were extracted independently by both review authors and cross‐checked. We carried out 'Risk of bias' assessment using the Cochrane 'Risk of bias' tool and assessed the certainty of evidence according to GRADE. Sensitivity analyses excluding studies at high risk of bias were conducted.
Main results
We included 11 new studies in this update resulting in a total of 19 randomised and 6 quasi‐randomised trials (8919 participants).
Clipping compared with no hair removal
Low certainty evidence suggests there may be little difference in risk of SSI when no hair removal is compared with hair removal using clippers (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.65 to 1.39; three studies with 1733 participants).
Shaving with a razor compared with no hair removal
Moderate certainty evidence suggests the risk of SSI is probably increased in participants who have hair removal with a razor compared with no removal (RR 1.82, 95% CI 1.05 to 3.14; seven studies with 1706 participants). In terms of absolute risk this represents 17 more SSIs per 1000 in the razor group compared with the no hair removal group (95% CI 1 more to 45 more SSI in the razor group).
Based on low‐certainty evidence, it is unclear whether there is a difference in stitch abscesses between hair removal with a razor and no hair removal (1 trial with 80 participants; RR 1.00, 95% CI 0.21 to 4.66).
Based on narrative data from one trial with 136 participants, there may be little difference in length of hospital stay between participants having hair removed with a razor compared with those having no hair removal (low‐certainty evidence).
Based on narrative data from one trial with 278 participants, it is uncertain whether there is a difference in cost between participants having hair removed by shaving with a razor compared with no hair removal (very low certainty evidence).
Depilatory cream compared with no hair removal
Low certainty evidence suggests there may be little difference in SSI risk between depilatory cream or no hair removal, although there are were wide confidence intervals around the point estimate that included benefit and harm (RR 1.02, 95% CI 0.45 to 2.31; low‐certainty evidence; 1 trial with 267 participants).
Based on narrative data from one trial with 267 participants, it is uncertain whether there is a difference in cost between participants having hair removed with depilatory cream compared with no hair removal (very low certainty evidence).
Shaving with a razor compared with clipping
Moderate‐certainty evidence from 7 studies with 3723 participants suggests the risk of SSI is probably increased by shaving with a razor compared with clipping (RR 1.64, 95% CI 1.16 to 2.33).
Moderate‐certainty evidence suggests the risk of skin injury is probably increased in people who have hair removal with a razor rather than clipping (3 trials with 1333 participants; RR 1.74, CI 95% 1.12 to 2.71).
Shaving with a razor compared with depilatory cream
Moderate‐certainty evidence from 9 studies with 1593 participants suggests there is probably more SSI risk when razors are used compared with depilatory cream (RR 2.28, 95% CI 1.12 to 4.65).
Low‐certainty evidence suggests the risk of skin injury may be increased when using a razor rather than depilatory cream for hair removal (RR 6.95, CI 95% 3.45 to 13.98; 5 trials with 937 participants).
Based on narrative data from three trials with 402 participants, it is uncertain whether depilatory cream is more expensive than shaving (very low certainty evidence).
Hair removal on the day of surgery compared with one‐day preoperatively
Low‐certainty evidence suggests that there may be a small reduction in SSI risk when hair is removed on the day of surgery compared with the day before surgery although there are were wide confidence intervals around the point estimate that included benefit and harm (one trial, 977 participants; RR 0.83, 95% CI 0.54 to 1.30).
Authors' conclusions
Compared with no hair removal, there may be little difference in risk of SSI when clippers or depilatory cream are used (low‐certainty evidence). However, there are probably fewer SSIs when hair is not removed compared with shaving with a razor (moderate‐certainty evidence). If hair has to be removed, moderate‐certainty evidence suggests using clippers or depilatory cream probably results in fewer SSIs and other complications compared with shaving using a razor. There may be a small reduction in SSIs when hair is removed on the day of, rather than the day before, surgery.
Plain language summary
Does hair removal before surgery prevent infections after surgery?
Key messages?
Compared with no hair removal:
· there are probably more surgical site infections when hair is removed by shaving with a razor;
· removing hair with clippers and cream may make little to no difference to the number of infections;
Clippers and hair removal cream probably cause fewer infections than shaving using a razor.
Removing hair on the day of, rather than the day before surgery may slightly reduce the number of infections.
Why is hair removed before surgery?
Before a surgical intervention, it is common to remove hair from the area of the body that is going to have surgery. Hair can be removed using different methods, including clippers, a razor, or hair removal cream.
Hair is removed to avoid problems during and after surgery, for example when stitching up wounds or applying dressings. However, some studies claim that removing hair could cause infections after surgery and should be avoided.
What did we want to find out?
We wanted to find out if removing hair before surgery:
· causes or prevents infections;
· prevents wound complications, such as cuts to the skin or the opening up of stitched wounds;
· has an impact on how long people stay in hospital after surgery; and
· has any cost implications.
We were also interested in whether some hair removal methods or times for hair removal are better than others.
What did we do?
First, we searched for studies that compared:
· hair removal against no removal; or
· different methods and times of hair removal.
We then compared the results and summarised the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 25 studies that involved a total of 8919 people.
Ten studies compared no hair removal against hair removal, using:
· clippers (3 studies);
· shaving with a razor (8 studies, 7 of which provided useable evidence); or
· hair removal cream (1 study).
Seven studies compared using a razor against using clippers, and 10 studies compared using a razor against using cream (nine of these 10 studies provided useable evidence).
One study compared hair removal the day before surgery versus hair removal on the day of surgery.
What does the evidence show?
Hair removal compared to no hair removal
· Hair removal with clippers and cream may make little to no difference to the number of surgical site infections.
· Hair removal with a razor probably risks more infections than no hair removal.
Whether hair is removed with a razor or not removed may make little to no difference for length of hospital stay (1 study).
Comparisons of different hair removal methods
· Clippers probably cause fewer infections and skin injuries than razors.
· Cream probably causes fewer infections, and may cause fewer skin injuries, than razors.
Time of hair removal
Whether hair is removed on the day of surgery or the day before surgery may slightly reduce infection numbers (1 study).
What do we still not know?
Due to a lack of robust studies, we do not know:
· if removing hair affects wound complications and costs when compared to not removing hair;
· if different hair removal methods have different effects on length of hospital stay, or on costs; and
· if the time of hair removal affects wound complications, length of hospital stay, or costs.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to November 2019.
Summary of findings
Background
Description of the condition
The preparation of patients for surgery has traditionally included the routine removal of body hair from the intended surgical incision site. Hair is removed because its presence can interfere with the exposure of the incision site, the suturing of the incision, and the application of adhesive drapes or wound dressings (Hallstrom 1993; Miller 2001). Hair is also perceived to be associated with a lack of cleanliness, and the removal of hair is thought to reduce the risk of surgical site infections (SSIs) (Kumar 2002). However, some studies claim that preoperative hair removal is not beneficial and perhaps even contributes to SSIs, and therefore should not be carried out (Cruse 1973; Powis 1976; Seropian 1971).
A surgical site infection is an infection that occurs in the operative site within the 30 days following surgery, or up to one year if there is an implant. It can involve skin and subcutaneous tissue (superficial incisional), and/or deep soft tissue (deep incisional), and/or any part of the anatomy (organ/space) (WHO 2018b). SSIs develop when bacteria from the person having the surgery, the staff, equipment, or the environment enter the incision site during surgery (WHO 2018b). SSIs are a global problem that is estimated to affect millions of people each year (WHO 2011) The World Health Organization (WHO) reports the incidence of SSI ranging from 1.2% to 23.6% in low‐ and middle‐income countries, and 1.2% to 5.2% in high income countries (WHO 2011). SSIs are distressing for patients and can be extremely costly to treat, resulting in delayed wound healing, unnecessary pain, and, in extreme cases, death (Adams‐Howel 2015; Awad 2012; Brown 2014; Plowman 2000).
Description of the intervention
Three main methods of hair removal are currently used in healthcare settings: shaving with a razor, clipping, and chemical depilation. Shaving uses a sharp blade, held within the head of a razor, which is drawn over the patient's skin to cut hair close to the surface of the skin. A traditional ‘safety razor’ uses a single blade which cuts hair at the level of the skin. Multiblade razors use at least two blades to give a ‘closer shave’, cutting hair below the level of the skin. The first blade catches the hair and tugs it taut, whilst the second blade slices the hair which, when it goes slack, falls back slightly below the skin surface (Huguenin 2017). Disposable razors are available.
Clippers use sharpened comb‐like blades which cut hair between the teeth of the comb in a scissor‐like action. Hair is cut close to the patient's skin, leaving a short stubble. The length of stubble depends on the cutting blade or guard used (Wahl 2005). The heads of clippers can be disposed of after use to minimise the risks of cross‐infection.
Depilatory creams contain alkaline‐based chemicals that break down the keratin within the hair. This thins and weakens the hair so that it breaks at its base just below the surface of the skin and can be wiped away. This is a slower process than either shaving or clipping, as the cream has to remain in contact with the hair for between five and 10 minutes depending on the thickness of the hair. There is a risk of irritation or allergic reactions to the cream, so patch tests should be carried out before the first time the cream is applied over a larger area (Veet 2020).
How the intervention might work
During the process of shaving with a razor, the skin may sustain microscopic cuts and abrasions. It is suggested that micro‐organisms are able to enter and colonise these cuts, thus contaminating the surgical incision site and causing SSIs (Briggs 1997). In addition, abrasions may exude tissue fluid, which provides a culture medium for micro‐organisms (Seropian 1971). As clipper blades do not come into contact with people's skin, they are postulated to reduce the risk of cuts and abrasions (Fogg 2003). Similarly, depilatory cream is not abrasive to skin. Shaving and clipping can be carried out in operating theatres, anaesthetic rooms, wards, or in patients' homes by theatre staff, ward staff, or by the patients themselves. Chemical depilation is usually carried out in patients' homes, as it usually requires pre‐application testing (Veet 2020). However, research suggests that hair removal should not take place in the operating theatre, as loose hair may contaminate the sterile surgical field (Edmiston 2016). Others have suggested that hair removal should be carried out by skilled personnel to prevent abrasion injuries (Hallstrom 1993; Small 1996).
Why it is important to do this review
Having a hairless surgical field may ease surgery and make it easier to apply adhesive drapes or dressings, but could increase infection rates. Recommendations for hair removal published by professional organisations in the late 1990s and early 2000s (which have been updated since) varied slightly, and some were based upon observational studies as well as randomised trials (Kjonniksen 2003). The initial version of this review, Tanner 2006, collated data from randomised trials identified through a systematic literature review without language restrictions to identify the benefit or harm of removing hair using different methods. Most of the evidence for outcomes for hair removal practices published in the initial review, Tanner 2006, and the previous update, Tanner 2011, were supported by low‐ or very low‐certainty evidence, and in some instances there was no supporting evidence (e.g. clippers versus cream). Since 2011, several new trials of preoperative hair removal to reduce surgical site infection have been published. An updated systematic review was required to summarise and present the best evidence.
Objectives
To determine whether routine preoperative hair removal (compared with no removal) and the method, timing, or setting of hair removal effect SSI rates.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐randomised controlled trials (QRCTs) comparing:
hair removal by any method (shaving, clipping, cream) with no hair removal;
hair removal by any method (shaving, clipping, cream) compared with hair removal by any other method (shaving, clipping, cream);
hair removal carried out at different times prior to surgery; and
hair removal carried out in different settings (e.g. operating room, anaesthetic room, ward, or the home).
We classified trials as RCTs if the authors described the trial as randomised and either provided sufficient written detail about randomisation, or did not indicate that an inappropriate method had been used. QRCTs are trials which are described as randomised but use inadequate methods of randomisation, such as date of admission.
Types of participants
Adults undergoing surgery in a designated operating theatre. We anticipated that, where appropriate, studies would be grouped and analysed by type of surgery/anatomical site of surgery.
Types of interventions
We planned to include comparisons between any of the following:
no preoperative hair removal;
hair removal with razors;
hair removal with clippers;
hair removal with depilatory creams;
hair removal in different environments;
hair removal conducted at different times preoperatively.
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of participants who developed SSIs. We accepted the authors' definitions of an SSI. Where SSIs were reported at several different time points, we recorded and used the time closest to 30 days (Centers for Disease Control and Prevention (CDC) definition of an SSI).
Secondary outcomes
Secondary outcomes included:
incidence of wound complications, such as cuts, abrasions, dehiscence (wound breakdown), and stitch abscesses;
length of hospital stay;
cost of hair removal.
Search methods for identification of studies
Electronic searches
For this second update, we searched the following electronic databases in November 2019:
Cochrane Wounds Specialised Register (searched 27 November 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 10) in the Cochrane Library (searched 27 November 2019);
Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations) (1946 to 27 November 2019);
Ovid Embase (1974 to 27 November 2019);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 27 November 2019).
Search strategies can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2019). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2019). We combined the CINAHL Plus search with the trial filter developed by Glanville 2019. There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (searched 27 November 2019);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (https://www.who.int/clinical-trials-registry-platform) (searched 27 November 2019).
Searching other resources
We sought to find other potentially relevant trials by searching the reference lists of retrieved included studies plus reviews. No additional trials were found.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Tanner 2003), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Changes from the protocol or previous published versions of this review are documented in Differences between protocol and review.
Selection of studies
For this update, both review authors (JT and KM) independently assessed the titles and abstracts of records identified by the search strategy for those that were potentially relevant, using the selection criteria listed above. We retrieved the full texts of studies that appeared to meet the inclusion criteria. Both JT and KM independently assessed the full‐text reports to identify those that were eligible for inclusion in the review. The reasons for excluding full‐text studies were recorded. Any disagreements were resolved by consensus. We completed a PRISMA flow chart summarising this process (Liberati 2009).
Data extraction and management
We extracted and summarised details of the studies using a data extraction form. If data were missing, then we attempted to contact researchers to obtain the required information. All data extraction was performed by one review author, and checked by the other review author. We extracted the following data.
Method of hair removal used
Use of additional shaving cream or fluid
Venue where hair removal was carried out
When hair removal was carried out
Type of surgery
Area of the body depilated
Role of the person removing hair
Number of SSIs
Number of wound complications
Length of postoperative stay
Financial cost of hair removal method
Number of participants in each group
Pre‐trial sample size calculations
Duration of follow‐up
Assessment of risk of bias in included studies
For this review update, two review authors independently assessed each included study without blinding to journal or authorship, using the Cochrane tool for assessing risk of bias (Higgins 2017). This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other issues (see Appendix 2 for details of criteria on which the judgements were based). We recorded any issues with unit of analysis, for example where a cluster trial was undertaken but analysed at the individual level in the study report. We assessed blinding and completeness of outcome data for each of the review outcomes separately. We note that, since SSI is a subjective clinical outcome, it can be at high risk of measurement bias when outcome assessment is not blinded. We have presented our assessment of risk of bias using two 'Risk of bias' summary figures: a summary of risk of bias for each item across all studies (Figure 1), and a summary of bias for each trial across all of the 'Risk of bias' items (Figure 2). We classified studies assessed as at high risk of bias for the randomisation sequence domain (for specified outcome) as being at overall high risk of bias (for specified outcome). For trials using cluster randomisation, or within‐patient randomisation, we would also have considered the risk of bias in terms of recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials (Higgins 2019). However, neither of these study designs was encountered in this review.
1.

Risk of bias table: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We entered data into Review Manager 5 (Review Manager 2020). Estimates for dichotomous outcomes are reported as risk ratio (RR) with 95% confidence intervals (CI), such that an RR of greater than one indicates a higher risk of infection in the first group named. Absolute risk differences are presented in the 'Summary of findings' tables. Where possible, continuous data are presented as mean difference (MD) with 95% CI. If insufficient data were available to calculate mean difference or standardised mean difference, we presented the authors' reported results narratively.
Unit of analysis issues
Where studies randomised participants and reported outcomes for incision sites, and the number of sites appeared to be equal to the number of participants, we treated the participant as the unit of analysis. Due to the nature of surgical procedures, we did not anticipate an individual participant having multiple incision sites which could be randomised. However, if this occurred, and data were presented by incision site and not by person, these clustered data would have presented a unit of analysis error that could have inflated precision. In all of the studies included in this review update the study participant was the unit of randomisation and the unit of analysis. In cases where studies contained some or only clustered data, we planned to report this alongside whether data had been incorrectly treated as independent. This would have been recorded as part of the 'Risk of bias' assessment.
Dealing with missing data
High rates of withdrawals are not common in studies of hair removal from surgical sites, as the interventions are predominately carried out in hospital as part of the surgical preparation, and incision sites are assessed as part of routine surgical follow‐up. However, it is common for outcome data to be missing from trial reports. Where data were missing, we attempted to contact the study authors. If data remained unobtainable, the relevant 'Risk of bias' domain for the study was classed as unclear. In the case of missing data, we reported the study findings narratively. Where randomised participants were not included in the analysis, we carried out a completed‐case analysis for the outcome.
Assessment of heterogeneity
We examined clinical and methodological heterogeneity, looking at the setting of the study, type of intervention, participants, outcomes, and length of follow‐up. We then considered statistical heterogeneity using the Chi2 test (a significance level of P < 0.10 was considered indicative of statistically significant heterogeneity) and I2 values (Higgins 2003). The I2 statistic examines the percentage of total variation across RCTs that is due to heterogeneity rather than to chance. In general, I2 values of 25% or less may suggest a low level of heterogeneity (Higgins 2003), whilst a value of more than 75% was taken to suggest a high level of heterogeneity (Deeks 2019). Where high I2 values existed, we investigated possible causes.
Assessment of reporting biases
Most reporting biases can be avoided by including studies published in any language and by having no date restriction. We planned to assess publication bias by conducting a funnel plot for each comparison if there was a sufficient number of studies (10 or more) to provide meaningful data. We did not conduct funnel plots, as none of the comparisons included 10 or more studies.
Data synthesis
We pooled studies following assessment of clinical and methodological heterogeneity. Studies were considered similar in terms of type of intervention and outcome. Where we perceived clinical heterogeneity, or there was evidence of statistical heterogeneity, we used a random‐effects model. We used a fixed‐effect model when clinical heterogeneity was considered to be low and statistical heterogeneity was not statistically significant for the Chi2 and I2 values (Higgins 2003). We presented pooled data on forest plots where possible using Review Manager 5 software (Review Manager 2020). Where synthesis was inappropriate, we have presented a narrative overview. We pooled data regardless of the length of follow‐up in individual studies. Follow‐up varied between the studies; this is discussed in the narrative synthesis.
Subgroup analysis and investigation of heterogeneity
There is a view that the removal of genital hair from the scrotum is different from the removal of body or scalp hair, as the skin is loose and more likely to catch in clippers (Morey 2013). We conducted a post hoc subgroup analysis for studies where hair was removed from male genitals.
Sensitivity analysis
Where there were sufficient studies, we carried out sensitivity analyses to explore the effects of study design on the primary outcomes. For the sensitivity analysis, we excluded studies that were quasi‐randomised or had at least one 'Risk of bias' domain rated as high. The domain 'blinding of participants' was excluded, as it is not possible for a person having surgery to be unaware if they have had hair removed or by what method.
Summary of findings and assessment of the certainty of the evidence
'Summary of findings' tables and GRADE assessment are new to this update, therefore outcomes were not prespecified. We have presented the primary and secondary outcomes of the review in 'Summary of findings' tables (Schünemann 2019):
surgical site infection;
wound complications;
length of stay;
cost.
These tables present key information concerning the quality of the data, the magnitude of the effects of the interventions examined, and the sum of available data. The 'Summary of findings' tables also include an overall grading of the evidence. The GRADE approach, as outlined in the GRADE Handbook (GRADE 2013), provides a summary of the intervention effect and a measure of the certainty of evidence for each outcome. It uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the body of evidence for each outcome.
Evidence can be downgraded by one level for serious (or by two levels for very serious) limitations. We used the following decision rules for downgrading the evidence:
if no serious concern existed, no downgrade;
if serious concern existed, we downgraded the evidence by one level;
if very serious concern existed, we downgraded the evidence by two levels.
In terms of the GRADE assessment, outcomes were downgraded by one level for the following reasons: serious risk of bias where studies were QRCTs and considered to lower confidence in the estimate of effect; serious imprecision where confidence intervals from the majority of studies were wide and crossed the line of no effect, or the sample size was small, or the event number was small. Outcomes were downgraded by two levels for very serious imprecision where there were both low event numbers or small samples, plus wide confidence intervals which crossed the line of no effect.
The certainty of evidence is described as follows.
High certainty: very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect (Ryan 2016).
Results
Description of studies
For details of individual studies, see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The initial version of this review included 11 studies (Tanner 2006). For the first update (Tanner 2011), four studies were added (Abouzari 2009; Celik 2007; Ilankovan 1992; Nascimento 1991), and one previously included study was excluded (Ko 1992), resulting in 14 included studies. The literature search for this 2019 second update yielded 342 abstracts, which were screened for eligibility. We obtained the full‐text papers for 35 studies. We included a total of 11 new studies: 10 from the search strategy (Adisa 2011; Domes 2011; Grober 2013; Karegoudar 2012; Kattipattanapong 2013; Kowalski 2016; Lu 2002; Sun 2014; Suvera 2013; Taylor 2005), and following further assessment, Ko 1992 was reinstated. We therefore included a total of 25 studies in this second update, of which 23 provided sufficient data for inclusion in a quantitative meta‐analysis. Four trials that had been awaiting classification at the time of the previous update were excluded, as they involved endoscopic transurethral surgery, Fraser 1978; Menéndez 2004; Menendez Lopez 2004, or perineal hair removal (hair cutting), Kovavisarach 2005, where there were no skin incision sites. The abstract from one study with a total of 34 participants comparing hair removal on the day before surgery with hair removal on the day of surgery is currently awaiting assessment until the full text can be obtained (Pascual 1994). The flow of studies is outlined in a PRISMA diagram (Figure 3). Throughout the initial review and subsequent updates, a total of 14 studies required translation into English, six of which have been included in the review (Breiting 1981; Goëau‐Brissonnière 1987; Lu 2002; Nascimento 1991; Sun 2014; Thorup 1985). The clinical trials registries identified one relevant study, which was scheduled to run from 2009 to 2015. The data from this study have been published and are included in the review (Kowalski 2016). We identified no ongoing studies.
3.
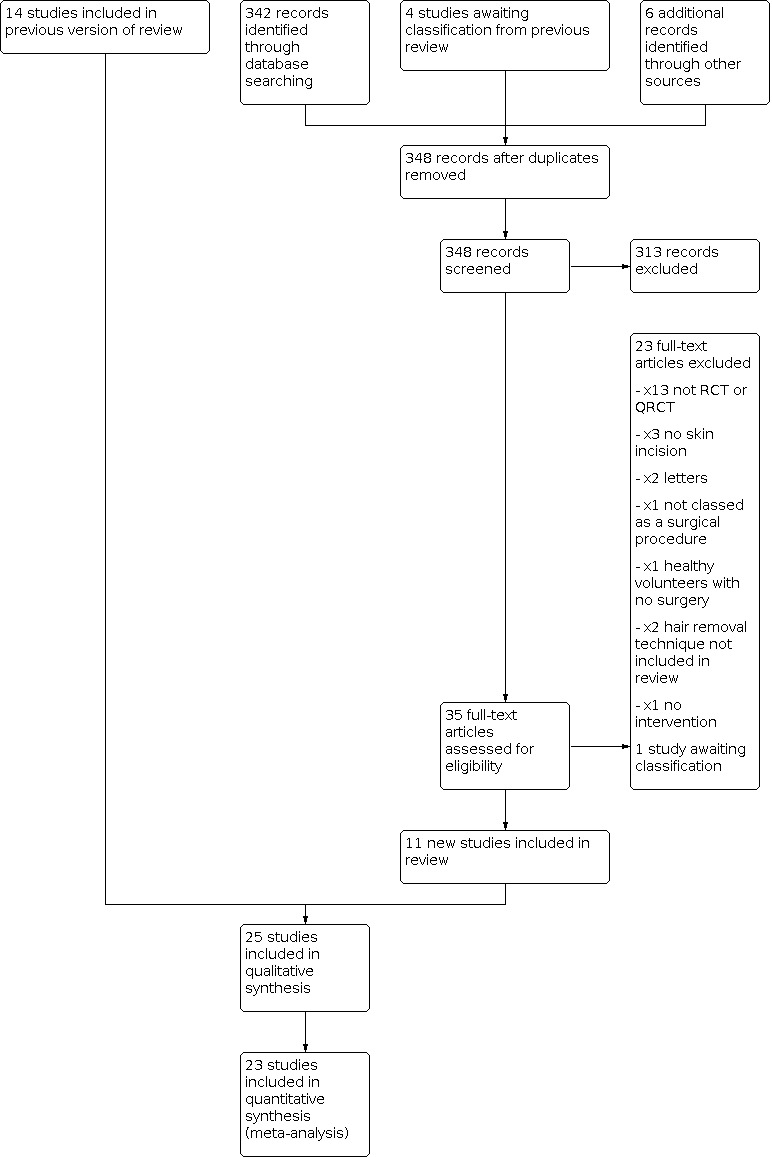
Study flow diagram.
Included studies
Design
This 2019 update includes 19 RCTs and 6 QRCTs with a total of 8919 participants. Most of the included studies had two arms, except for Abouzari 2009 and Court‐Brown 1981, which had three arms; and Alexander 1983, which had four arms.
Sample sizes
Sample sizes varied widely. Eight studies included between 50 and 100 participants (Domes 2011; Goëau‐Brissonnière 1987; Ilankovan 1992; Lu 2002; Nascimento 1991; Powis 1976; Rojanapirom 1992; Thorup 1985), and 11 studies had between 101 and 253 participants (Abouzari 2009; Adisa 2011; Balthazar 1983; Breiting 1981; Grober 2013; Karegoudar 2012; Kattipattanapong 2013; Sun 2014; Suvera 2013; Taylor 2005; Thur de Koos 1983). Three studies had between 400 and 800 participants (Celik 2007; Court‐Brown 1981; Seropian 1971). The three largest studies included 1013 participants (Alexander 1983), 1678 participants (Kowalski 2016), and 1980 participants (Ko 1992). Kowalski 2016 was the only study that reported calculating a required sample size a priori on the basis of a clinically significant effect.
Interventions
Nine studies compared hair removal versus no hair removal: three using clippers (Abouzari 2009; Kowalski 2016; Lu 2002), eight using razors (Abouzari 2009; Celik 2007; Court‐Brown 1981; Ilankovan 1992; Kattipattanapong 2013; Nascimento 1991; Rojanapirom 1992; Sun 2014), and one using cream (Court‐Brown 1981). Seven studies compared razors versus clippers (Abouzari 2009; Alexander 1983; Balthazar 1983; Domes 2011; Grober 2013; Ko 1992; Taylor 2005), and 10 studies compared razors with cream (Adisa 2011; Breiting 1981; Court‐Brown 1981; Goëau‐Brissonnière 1987; Karegoudar 2012; Powis 1976; Seropian 1971; Suvera 2013; Thorup 1985; Thur de Koos 1983). One study compared hair removal on the day of surgery with hair removal the day before surgery (Alexander 1983). Court‐Brown 1981 presented data for hair removal six hours before surgery compared with hair removal 18 hours before surgery; however, these groups were divided by patients having elective or emergency surgery and were not randomised.
Timing of hair removal
Hair removal was carried out immediately before surgery in five studies (Balthazar 1983; Grober 2013; Ilankovan 1992; Kowalski 2016; Nascimento 1991), the morning of surgery in one study (Sun 2014); and on the day of surgery in three studies (Adisa 2011; Suvera 2013; Taylor 2005). Hair was removed the day before surgery in three studies (Goëau‐Brissonnière 1987; Lu 2002; Thorup 1985). Thur de Koos 1983 removed hair with a razor immediately before surgery and used cream the evening before. Powis 1976 allowed hair to be removed the day of surgery or the day before depending on the surgeon's preference, and Court‐Brown 1981 and Ko 1992 shaved the evening before for participants having elective (scheduled) surgery, and the day of surgery for participants having emergency (unscheduled) surgery. With the exception of Alexander 1983, who randomised time of hair removal, the remaining eight studies did not report hair removal times.
Person conducting hair removal
Hair removal was carried out by nursing staff (Adisa 2011; Breiting 1981; Goëau‐Brissonnière 1987; Sun 2014; Suvera 2013), perioperative staff (Celik 2007; Taylor 2005), and by the participants themselves (Thorup 1985). The remaining 17 studies did not specify who conducted hair removal.
Venue for hair removal
Six studies stated that hair removal took place within the operating department (Adisa 2011; Celik 2007; Grober 2013; Ilankovan 1992; Ko 1992: Thur de Koos 1983). One study stated that hair removal took place on the ward (Breiting 1981), and another on the day surgery unit (Taylor 2005). Only one study stated that hair removal with cream took place in participants' homes (Thorup 1985).
Type of surgery
Most studies (18) undertook surgical procedures which required the removal of body hair (Adisa 2011; Balthazar 1983; Breiting 1981; Celik 2007; Court‐Brown 1981; Goëau‐Brissonnière 1987; Karegoudar 2012; Ko 1992; Kowalski 2016; Lu 2002; Nascimento 1991; Powis 1976; Rojanapirom 1992; Seropian 1971; Sun 2014; Suvera 2013; Taylor 2005; Thorup 1985). Three studies undertook surgery which required the removal of scalp hair (Abouzari 2009; Ilankovan 1992; Kattipattanapong 2013). Two studies included surgical procedures which required the removal of body or scalp hair (Alexander 1983; Thur de Koos 1983), and two studies undertook surgery on male genitalia which required the removal of pubic hair (Domes 2011; Grober 2013).
Outcome measures
Surgical site infection was the primary outcome in 17 studies (Abouzari 2009; Adisa 2011; Alexander 1983; Balthazar 1983; Celik 2007; Court‐Brown 1981; Goëau‐Brissonnière 1987; Karegoudar 2012; Kattipattanapong 2013; Ko 1992; Kowalski 2016; Nascimento 1991; Powis 1976; Rojanapirom 1992; Seropian 1971; Sun 2014; Thur de Koos 1983), and the secondary outcome in eight studies (Breiting 1981; Domes 2011; Grober 2013; Ilankovan 1992; Lu 2002; Suvera 2013; Taylor 2005; Thorup 1985). A definition of infection was provided for all but six studies (Breiting 1981; Domes 2011; Karegoudar 2012; Lu 2002; Rojanapirom 1992; Thur de Koos 1983). Infections were assessed within one week (Goëau‐Brissonnière 1987; Ilankovan 1992; Powis 1976), within two weeks (Balthazar 1983; Nascimento 1991; Rojanapirom 1992; Taylor 2005; Thorup 1985), within one month (Adisa 2011; Alexander 1983; Court‐Brown 1981; Kattipattanapong 2013), within six weeks (Suvera 2013), or within three months (Celik 2007; Domes 2011; Grober 2013). Abouzari 2009 and Seropian 1971 stated that participants were followed up at weekly intervals, but did not say for how long. Ko 1992, Breiting 1981, and Kowalski 2016 stated that participants were assessed at the postoperative visit, but did not say when this was conducted, although Kowalski 2016 also stated that attempts were made to contact participants at 30 days. Karegoudar 2012, Lu 2002, Sun 2014, and Thur de Koos 1983 did not provide any details as to when infections were assessed.
Other outcomes were adequacy of hair removal (Adisa 2011; Balthazar 1983; Breiting 1981; Domes 2011; Goëau‐Brissonnière 1987; Grober 2013; Suvera 2013; Thorup 1985), injury or skin condition (Adisa 2011; Balthazar 1983; Domes 2011; Grober 2013; Powis 1976; Suvera 2013; Taylor 2005), satisfaction or preference (Breiting 1981; Court‐Brown 1981; Ilankovan 1992; Sun 2014; Taylor 2005; Thorup 1985), time taken to remove hair (Sun 2014), cost (Alexander 1983; Court‐Brown 1981), skin bacterial culture (Powis 1976), impact on ease of surgery (Kowalski 2016), length of stay (Alexander 1983), and meningitis (Celik 2007).
Country of origin
The included studies were carried out in a wide range of countries: six in the USA (Alexander 1983; Balthazar 1983; Ko 1992; Kowalski 2016; Seropian 1971; Thur de Koos 1983), four in the UK (Court‐Brown 1981; Ilankovan 1992; Powis 1976; Taylor 2005), two in Thailand (Kattipattanapong 2013; Rojanapirom 1992), two in India (Karegoudar 2012; Suvera 2013), two in Denmark (Breiting 1981; Thorup 1985), two in China (Lu 2002; Sun 2014), plus single studies in Iran (Abouzari 2009), Nigeria (Adisa 2011), Turkey (Celik 2007), France (Goëau‐Brissonnière 1987), Canada (Grober 2013), and Brazil (Nascimento 1991). The limited information from Domes 2011 does not state in which country the study was conducted, although it could be assumed to be Canada.
Excluded studies
We excluded 66 studies after full‐text assessment (see Characteristics of excluded studies). The reasons for exclusion were as follows.
Fifty‐four studies were not RCTs or QRCTs (Adeleye 2008; Bekar 2001; Bird 1984; Braun 1995; Breuer 2012; Broekman 2011; Chen 2002; Clarke 1983; Corcoran 2013; Cruse 1973; Dizer 2009; Faheem 2012; Finkelstein 2005; Gil 2003; Hallstrom 1993; Horgan 1997; Howell 1988; Huber 2016; Idali 2004; Kapadia 2012; Kapadia 2013; Korfali 1994; Kretschmer 2000; Kumar 2002; Le Roux 1975; Li 2013; Liu 2008; Maksimovic 2008; Masterson 1984; McIntyre 1994; Mehta 1988; Miller 2001; Mishriki 1990; Moro 1996; Parizh 2016; Ratanalert 1999; Ratanalert 2005; Scherpereel 1993; Sellick 1991; Sheinberg 1999; Siddique 1998; Small 1996; Stephens 1966; Tang 2001; Tokimura 2009; Vestal 1952; Viney 1992; Waddington 2008; Wang 1990; Wang 1999; Westermann 1979; Winfield 1986; Winston 1992; Zentner 1987).
Four studies had intervention groups that were not listed in our inclusion criteria: Hoe 1985 (hair removal around the immediate incision site versus hair removal hair from a large area of surrounding skin); Kose 2016 (a 2‐centimetre strip of cranial hair removed compared with a 5‐centimetre strip of cranial hair removed); Kovavisarach 2005 (hair cutting); and Lui 1984 (hair cropping).
Three studies were of transurethral surgery and had no surgical incision site (Fraser 1978; Menéndez 2004; Menendez Lopez 2004).
One study was of episiotomies (Meiland 1986), which are not carried out in an operating room and therefore not classed as a surgical procedure (WHO 2018a).
One study was of healthy volunteers (Marecek 2015).
Three studies were letters commenting on published studies (Morey 2013; Penson 2017; Wang 2017).
Risk of bias in included studies
See the 'Risk of bias' graph (Figure 1) and 'Risk of bias' summary (Figure 2).
It was not possible for a study to be at low risk of performance bias (blinding of participants), as it would have been obvious to participants if they had had hair removed, and also by which method if they were conscious at the time of removal. This risk of bias was therefore unavoidable, but we judge that the primary or secondary outcomes were unlikely to have been influenced by lack of blinding. Similarly, the blinding status of caregivers was also considered unlikely to influence primary or secondary outcomes, as hair removal would take place in pre‐ or intraoperative settings, which are likely staffed by a different set of staff from the postoperative setting. Additionally, hair would grow back within a few days, and caregivers would be unable to tell whether hair was removed or by which method. We considered one trial to be at low risk of bias (Kowalski 2016). Six QRCTs, Breiting 1981; Ko 1992; Lu 2002; Powis 1976; Seropian 1971; Thur de Koos 1983, and five RCTs, Celik 2007; Domes 2011; Grober 2013; Karegoudar 2012; Taylor 2005, were considered to be at high risk of bias and were excluded from sensitivity analyses.
Allocation
Method of randomisation
All 25 included studies were described as randomised; however, only 12 studies provided details of adequate generation of a randomisation sequence and were assessed as at low risk of bias (Adisa 2011; Alexander 1983; Balthazar 1983; Celik 2007; Goëau‐Brissonnière 1987; Ilankovan 1992; Kattipattanapong 2013; Kowalski 2016; Nascimento 1991; Sun 2014; Suvera 2013; Taylor 2005). Six studies described randomisation sequences which were inadequate; we assessed these studies as at high risk of bias (Breiting 1981; Ko 1992; Lu 2002: Powis 1976; Seropian 1971; Thur de Koos 1983). Seven studies described their participant allocation to groups as being randomised, but did not provide sufficient detail to permit a valid judgement as to whether adequate methods had been used (Abouzari 2009; Court‐Brown 1981; Domes 2011; Grober 2013; Karegoudar 2012: Rojanapirom 1992; Thorup 1985); we assessed these studies as at unclear risk of bias regarding their randomisation process. We did not identify any cluster‐RCTs.
Allocation concealment
Five studies described adequate allocation concealment and were therefore assessed as at low risk of bias (Adisa 2011; Alexander 1983: Kowalski 2016; Suvera 2013; Taylor 2005). Six studies described approaches in which allocation was not concealed, such as randomisation by date of admission or hospital number, where pre‐allocation predictability is possible; we assessed these studies as at high risk of bias (Breiting 1981; Ko 1992; Lu 2002; Powis 1976; Seropian 1971; Thur de Koos 1983). The remaining 14 studies did not provide any details regarding allocation concealment and were assessed as at unclear risk of bias (Abouzari 2009; Balthazar 1983; Celik 2007; Court‐Brown 1981; Domes 2011; Goëau‐Brissonnière 1987; Grober 2013; Ilankovan 1992; Karegoudar 2012; Kattipattanapong 2013; Nascimento 1991; Rojanapirom 1992; Sun 2014; Thorup 1985).
Blinding
In one study (Grober 2013), the treatment providers (the surgical team) also conducted the hair removal intervention; we assessed this study as at high risk of bias. None of the remaining 24 studies stated whether the treatment providers were aware of the intervention allocation; we therefore assessed these studies as at unclear risk of bias.
Risk of bias from participants being aware of allocation status was unavoidable, but we judged that the primary or secondary outcomes were unlikely to be influenced by lack of blinding. We assessed all 25 studies as at high risk of bias related to participants not being blinded to intervention allocation. Grober 2013 specifically stated that participants were not blinded, and in the Thorup 1985 study, participants carried out the hair removal. For the remaining 23 studies, participants would have either witnessed the hair removal method, or would be able to tell if hair had been removed or not (Abouzari 2009; Adisa 2011; Alexander 1983; Balthazar 1983; Breiting 1981; Celik 2007; Court‐Brown 1981; Domes 2011; Goëau‐Brissonnière 1987; Ilankovan 1992; Karegoudar 2012; Kattipattanapong 2013; Ko 1992; Kowalski 2016; Lu 2002; Nascimento 1991; Powis 1976; Rojanapirom 1992; Seropian 1971; Sun 2014; Suvera 2013; Taylor 2005; Thur de Koos 1983).
Assessors were blinded to intervention allocation in nine studies, which we judged as at low risk of bias (Adisa 2011; Domes 2011; Goëau‐Brissonnière 1987; Grober 2013; Kowalski 2016; Lu 2002; Powis 1976; Suvera 2013; Thorup 1985). We assessed two studies as at high risk of bias: one where the assessors also conducted the intervention (Breiting 1981), and another where the participants assessed their own wounds (Taylor 2005). The remaining 14 studies did not report on blinding of assessors and were assessed as at unclear risk of bias for this domain (Abouzari 2009; Alexander 1983; Balthazar 1983; Celik 2007; Court‐Brown 1981; Ilankovan 1992; Karegoudar 2012; Kattipattanapong 2013; Ko 1992; Nascimento 1991; Rojanapirom 1992; Seropian 1971; Sun 2014; Thur de Koos 1983).
Incomplete outcome data
We considered 15 studies to be at low risk of attrition bias based on their loss to follow‐up. In eight of these studies, the numbers of participants lost to both groups were similar and valid reasons were given (Abouzari 2009; Adisa 2011; Alexander 1983; Court‐Brown 1981; Kattipattanapong 2013; Kowalski 2016; Suvera 2013; Taylor 2005), whilst in the remaining seven studies no participants dropped out or were lost to follow‐up (Balthazar 1983; Breiting 1981; Goëau‐Brissonnière 1987; Lu 2002; Nascimento 1991; Powis 1976; Thorup 1985). Six studies did not provide sufficient data or information on attrition and were assessed as at unclear risk of bias for this domain (Grober 2013; Ilankovan 1992; Karegoudar 2012; Ko 1992; Rojanapirom 1992; Sun 2014). We assessed the following four studies as at high risk of attrition bias. Seropian 1971 states that "406 cases were obtained for analysis", and this excluded 155 participants who had not received the intervention plus five participants who had received two interventions, which resulted in unequal groups (157 and 249). Celik 2007 reported the loss of 47 participants from the intervention group through incomplete follow‐up, but did not report any losses in the control group. When we contacted Celik 2007 for further information, the study author replied that participants from the non‐shaved group had also been lost to follow‐up, but was not able to say how many participants had been originally recruited. In the Thur de Koos 1983 study, 253 of the 302 potential participants were considered suitable; however, 137 were randomised to one group, and 116 to the other group. Although the group sizes were unequal, no dropouts were reported. Similarly, Domes 2011 did not provide details of any dropouts; however, the groups appear to be unequally distributed (37 and 28).
One study undertook an intention‐to‐treat analysis and was assessed as at low risk of bias (Kowalski 2016). The remaining 24 studies did not report attrition or dropout data sufficiently to permit a judgement regarding risk of bias; we rated these studies as at unclear risk of bias (Abouzari 2009; Adisa 2011; Alexander 1983; Balthazar 1983; Breiting 1981; Celik 2007; Court‐Brown 1981; Domes 2011; Goëau‐Brissonnière 1987; Grober 2013; Ilankovan 1992; Karegoudar 2012; Kattipattanapong 2013; Ko 1992; Lu 2002; Nascimento 1991; Powis 1976; Rojanapirom 1992; Seropian 1971; Sun 2014; Suvera 2013; Taylor 2005; Thorup 1985; Thur de Koos 1983).
Selective reporting
One study referred to a protocol and was considered to be at low risk of reporting bias (Kowalski 2016). One study did not report on any prespecified outcomes and was assessed as at high risk of bias (Karegoudar 2012). Ilankovan 1992 did not provide the raw data for their secondary outcome (SSI), but reported that there was no difference in SSI between the two groups. Study protocols were not available for any of the remaining 23 studies; however, as all the key outcome measures stated in the methods section were reported in the results, we judged these studies to be at low risk of reporting bias (Abouzari 2009; Adisa 2011; Alexander 1983; Balthazar 1983; Breiting 1981; Celik 2007; Court‐Brown 1981; Domes 2011; Goëau‐Brissonnière 1987; Grober 2013; Ilankovan 1992; Kattipattanapong 2013; Ko 1992; Lu 2002; Nascimento 1991; Powis 1976; Rojanapirom 1992; Seropian 1971; Sun 2014; Suvera 2013; Taylor 2005; Thorup 1985; Thur de Koos 1983).
Other potential sources of bias
Participant groups were equal or similar in 18 studies (Abouzari 2009; Adisa 2011; Alexander 1983; Balthazar 1983; Breiting 1981; Court‐Brown 1981; Goëau‐Brissonnière 1987; Ilankovan 1992; Kattipattanapong 2013; Ko 1992; Kowalski 2016; Lu 2002; Nascimento 1991; Powis 1976; Rojanapirom 1992; Seropian 1971; Sun 2014; Thorup 1985), which we assessed as at low risk of other bias. Celik 2007 reported a significantly higher number of complex operations in one group, and six studies did not comment on the similarity of groups (Domes 2011; Grober 2013; Karegoudar 2012; Suvera 2013; Taylor 2005; Thur de Koos 1983).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Clipping compared with no hair removal for participants undergoing surgery.
| Clipping compared with no hair removal for participants undergoing surgery | ||||||
| Patient or population: people undergoing surgery Setting: hospital Intervention: clipping Comparison: no hair removal | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no hair removal | Risk with clipping | |||||
| Surgical site infection (SSIs) assessed with: proportion of SSIs follow‐up: 4 weeks | Study population | RR 0.95 (0.65 to 1.39) | 1733 (3 RCTs) | ⊕⊕⊝⊝ Low 1 | Pooled meta‐analysis suggests there may be little difference in SSIs when preoperative hair removal with clippers is compared with no hair removal. | |
| 60 per 1000 | 3 fewer SSIs per 1000 (21 fewer to 23 more) | |||||
| Wound complications | No data were reported for this outcome. | |||||
| Length of stay | No data were reported for this outcome. | |||||
| Cost of care | No data were reported for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels for very serious imprecision because of wide confidence intervals across the three included studies.
Summary of findings 2. Shaving compared with no hair removal for participants undergoing surgery.
| Shaving compared with no hair removal for participants undergoing surgery | ||||||
| Patient or population: people undergoing surgery Setting: hospital Intervention: shaving Comparison: no hair removal | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no hair removal | Risk with shaving | |||||
| Surgical site infection (SSIs) assessed with: proportion of SSIs follow‐up: range 10 days to 3 months | Study population | RR 1.82 (1.05 to 3.14) | 1706 (7 RCTs) | ⊕⊕⊕⊝ Moderate 1 | Pooled meta‐analysis suggests the risk of SSI is probably lower in people who do not have hair removed than in those who have hair removed with a razor. | |
| 21 per 1000 | 17 more SSIs per 1000 (1 more to 45 more) | |||||
| Wound complications assessed with: proportion of stitch abscesses follow‐up: range 7 to 10 days | Study population | RR 1.00 (0.21 to 4.66) | 80 (1 RCT) | ⊕⊕⊝⊝ Low 2 | It is unclear whether there is a difference in the incidence of stitch abscesses between no hair removal and hair removal with a razor. | |
| 75 per 1000 | 0 difference in complications per 1000 (59 fewer to 275 more) | |||||
| Length of stay assessed with: number of days follow‐up: 1 month | The mean length of stay for 66 participants who had hair removed with a razor was 4.6 days, and the mean length of stay for 70 participants who had no hair removed was 4.3 days. | ‐ | 136 (1 RCT) | ⊕⊕⊝⊝ Low 3 | There may be little difference in length of stay between people who have hair removed with a razor and those who do not have hair removed. | |
| Cost follow‐up: mean 1 month | The cost of razors for 100 people was GBP 14. | ‐ | 278 (1 RCT) | ⊕⊝⊝⊝ Very low 4 | It is uncertain whether there is a difference in cost between people who have had hair removed with a razor and those who have not had hair removed. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for serious imprecision because of wide confidence intervals. 2Downgraded two levels for very serious imprecision due to small sample size and wide confidence intervals. 3Downgraded two levels for serious imprecision due to small sample size. 4Downgraded one level for serious imprecision due to small sample size and two levels for very serious indirectness, as only one aspect of treatment costs was assessed.
Summary of findings 3. Depilatory cream compared with no hair removal for participants undergoing surgery.
| Depilatory cream compared with no hair removal for participants undergoing surgery | ||||||
| Patient or population: people undergoing surgery Setting: hospital Intervention: depilatory cream Comparison: no hair removal | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no hair removal | Risk with depilatory cream | |||||
| Surgical site infection (SSI) assessed with: proportion of SSIs follow‐up: mean 1 month | Study population | RR 1.02 (0.45 to 2.31) | 267 (1 RCT) | ⊕⊕⊝⊝ Low 1 | There may be little difference in SSIs when hair is removed with depilatory cream versus no hair removal. | |
| 78 per 1000 | 2 more SSIs per 1000 (43 fewer to 102 more) | |||||
| Wound complications | No data were reported for this outcome. | |||||
| Length of stay | No data were reported for this outcome. | |||||
| Cost follow‐up: 1 month | 1 trial estimated the cost of providing depilatory cream for 100 people was GBP 22. | ‐ | 267 (1 RCT) | ⊕⊝⊝⊝ Very low 2 | It is uncertain whether there is a difference in cost between people who have had hair removed using depilatory cream and people who have had no hair removed. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels for very serious imprecision due to small sample size and wide confidence interval. 2Downgraded one level for serious imprecision due to small sample size and two levels for very serious indirectness, as only one aspect of treatment costs was assessed.
Summary of findings 4. Shaving compared with clipping for participants undergoing surgery.
| Shaving with a razor compared with clipping for participants undergoing surgery | ||||||
| Patient or population: people undergoing surgery Setting: hospital Intervention: shaving Comparison: clipping | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with clipping | Risk with shaving | |||||
| Surgical site infection (SSI) assessed with: proportion of SSIs follow‐up: range 2 weeks to 3 months | Study population | RR 1.64 (1.16 to 2.33) | 3723 (7 RCTs) | ⊕⊕⊕⊝ Moderate1 | Pooled meta‐analysis suggests the risk of SSI is probably lower in people who have hair removed with clippers than in those who have hair removed with a razor. | |
| 25 per 1000 | 16 more SSIs per 1000 (4 more to 33 more) | |||||
| Wound complications follow‐up: range 2 weeks to 1 month | Study population | RR 1.74 (1.12 to 2.71) | 1333 (3 RCTs) | ⊕⊕⊕⊝ Moderate 2 | Pooled meta‐analysis suggests the risk of skin injury is probably lower in people who have hair removed from their body with clippers rather than with razors. | |
| 44 per 1000 | 33 more complications per 1000 (5 more to 75 more) | |||||
| Length of stay follow‐up: 1 month | No data were reported for this outcome. | |||||
| Cost follow‐up: 1 month | No data were reported for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for serious risk of bias, as one large study was quasi‐randomised. 2Downgraded one level for serious imprecision due to wide confidence intervals.
Summary of findings 5. Shaving compared with cream for participants undergoing surgery.
| Shaving compared with depilatory cream for participants undergoing surgery | ||||||
| Patient or population: people undergoing surgery Setting: hospital Intervention: shaving Comparison: depilatory cream | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with cream | Risk with shaving | |||||
| Surgical site infection (SSI) assessed with: proportion of SSI follow‐up: range 5 days to 6 weeks | Study population | RR 2.28 (1.12 to 4.65) | 1593 (9 RCTs) | ⊕⊕⊕⊝ Moderate 1 | Pooled meta‐analysis suggests the risk of SSI is probably higher when hair is removed with a razor than with depilatory cream. | |
| 36 per 1000 | 46 more SSIs per 1000 (4 more to 131 more) | |||||
| Wound complications follow‐up: range 10 days to 6 weeks | Study population | RR 6.95 (3.45 to 13.98) | 937 (5 RCTs) | ⊕⊕⊝⊝ Low 2 | Pooled meta‐analysis suggests the risk of skin injury may be lower in people who have hair removed with depilatory cream than in those who have hair removed with razors. | |
| 17 per 1000 | 101 more complications per 1000 (42 more to 221 more) | |||||
| Length of stay | No data were reported for this outcome. | |||||
| Cost follow‐up: range 5 to 28 days | 3 studies reported on cost. 1 trial reported the approximate costs per 100 people were GBP 14 for razors and GBP 22 for depilatory cream. 1 trial found the cost of the cream was GBP 0.25 compared with GBP 0.80 for the average cost of a shave. 1 trial stated the cost of 1 tube of cream was GBP 0.90, and the cost of 1 razor was GBP 0.08. | ‐ | 402 (3 RCTs) | ⊕⊝⊝⊝ Very low 3 | Narratives from 3 studies report mixed findings for the cost of a tube of depilatory cream compared with a disposable razor. We have very little confidence in this effect. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for serious risk of bias, as several studies were quasi‐randomised. 2Downgraded one level for serious risk of bias, as two studies were quasi‐randomised, and one level for serious imprecision due to wide confidence intervals. 3Downgraded one level for serious imprecision due to small sample size and two levels for very serious indirectness, as only one aspect of treatment costs was assessed.
Summary of findings 6. Hair removal the day before surgery compared with hair removal on the day of surgery for participants undergoing surgery.
| Hair removal the day before surgery compared with hair removal on the day of surgery for participants undergoing surgery | ||||||
| Patient or population: people undergoing surgery Setting: hospitals Intervention: hair removal the day before surgery Comparison: hair removal on the day of surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hair removal on the day of surgery | Risk with hair removal the day before surgery | |||||
| Surgical site infection (SSIs) assessed with: proportion of SSIs follow‐up: 30 days | Study population | RR 0.83 (0.54 to 1.30) | 977 (1 RCT) | ⊕⊕⊝⊝ Low 1,2 | The findings from 1 study suggest there may be a small reduction in SSI if hair is removed on the day of surgery compared with hair removal on the day before surgery. | |
| 69 per 1000 | 12 fewer SSIs per 1000 (32 fewer to 20 more) | |||||
| Wound complications | No data were reported for this outcome. | |||||
| Length of stay follow‐up: 30 days | No data were reported for this outcome. | |||||
| Cost follow‐up: 30 days | No data were reported for this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for serious risk of imprecision due to low event numbers and wide confidence intervals.
2 Downgraded due to inconsistency in the types of hair removal used.
See: 'Summary of findings' tables for the following comparisons; clipping versus no hair removal (Table 1), shaving with a razor versus no hair removal (Table 2), depilatory cream versus no hair removal (Table 3), shaving with a razor versus clipping (Table 4), shaving with a razor versus cream (Table 5), and hair removal on the day before surgery versus hair removal on the day of surgery (Table 6). One trial, Ilankovan 1992, did not provide sufficient outcome data to be included in a meta‐analysis, and Karegoudar 2012 did not provide sufficient outcome data to be included in either a narrative synthesis or meta‐analysis. We note that where data were pooled we used random effects models, meaning the estimates presented are average effect estimates and should be interpreted as such.
Comparison 1: clipping compared with no hair removal (3 trials, 1733 participants)
Three studies with a total of 1733 randomised participants compared clipping with no hair removal (Abouzari 2009; Kowalski 2016; Lu 2002).
1.1 Primary outcome: surgical site infection
All three studies reported surgical site infection as an outcome. Infections were measured at four weeks (Abouzari 2009), "follow up" or at 30 days (Kowalski 2016), or no details were given (Lu 2002). Results are presented as risk ratio (RR), where the risk ratio is the risk of infection in the clipping group divided by the risk of infection in the no hair removal group. A risk ratio of less than one indicates fewer infections in the clipping group. We pooled all trials using a random‐effects model. There may be little difference in the risk of SSI when hair is removed by clippers compared with no hair removal (RR 0.95, 95% confidence interval (CI) 0.65 to 1.39; 3 trials, n = 1733) (Analysis 1.1). This corresponds to an absolute effect of 3 fewer surgical site infections per 1000 people in the clipping group (95% CIs 21 fewer infections to 23 more infections per 1000 people). The evidence is of low certainty, downgraded twice for very serious imprecision (Table 1). We carried out a sensitivity analysis to exclude any QRCTs (Lu 2002), or studies at high risk of bias from the meta‐analysis. This showed no change in effect, resulting in a pooled RR of 0.97 (95% CI 0.66 to 1.42; 2 trials, n = 1673) (Analysis 1.2). The certainty of evidence for the sensitivity analysis is low, downgraded twice for very serious imprecision due to wide confidence intervals and a relatively small number of events.
1.1. Analysis.

Comparison 1: Clipping compared with no hair removal, Outcome 1: Surgical site infection
1.2. Analysis.

Comparison 1: Clipping compared with no hair removal, Outcome 2: Surgical site infection ‐ sensitivity analysis
1.2 Secondary outcomes
No secondary outcomes were reported.
Comparison 2: shaving with a razor compared with no hair removal (8 trials, 1756 participants)
Eight studies with a total of 1756 randomised participants compared shaving with a razor with no hair removal (Abouzari 2009; Celik 2007; Court‐Brown 1981; Ilankovan 1992; Kattipattanapong 2013; Nascimento 1991; Rojanapirom 1992; Sun 2014).
2.1 Primary outcome: surgical site infection
All eight studies reported surgical site infection as an outcome; however, Ilankovan 1992 did not provide any raw data and is reported separately as a narrative. We pooled the remaining seven studies in a meta‐analysis with 1706 participants. Infections in the seven studies were measured at 7 to 10 days (Rojanapirom 1992), discharge or 8 to 10 days (Nascimento 1991), 28 days (Court‐Brown 1981), 30 days (Abouzari 2009; Kattipattanapong 2013), and 1 and 3 months (Celik 2007). Sun 2014 did not state when infections were assessed. Results are presented as risk ratio (RR), where the risk ratio is the risk of infection in the shaving group divided by the risk of infection in the no hair removal group. A risk ratio of more than one indicates more risk of infection in the shaving (with a razor) group. We pooled all trials using a random‐effects model. The evidence shows the risk of surgical site infection is probably increased in people who have hair removed with a razor compared with no hair removal (RR 1.82, 95% CI 1.05 to 3.14; 7 trials, n = 1706) (Analysis 2.1). This corresponds to an absolute increase in surgical site infections in the razor group of 17 more surgical site infections per 1000 (95% CIs 1 more to 45 more infections per 1000 people). The evidence is of moderate certainty, downgraded once for serious imprecision (Table 2). We carried out a sensitivity analysis to exclude any QRCTs or other studies at high risk of bias, Celik 2007, from the meta‐analysis. In this sensitivity analysis the confidence intervals were wider and included no effect and the possibility of harm; pooled RR of 1.71 (95% CI 0.97 to 3.01; 6 trials, n = 917) (Analysis 2.2). The certainty of evidence for the sensitivity analysis is moderate, downgraded once for serious imprecision. Ilankovan 1992 included 50 participants and reported no difference in the incidence of infection between groups.
2.1. Analysis.

Comparison 2: Shaving with a razor compared with no hair removal, Outcome 1: Surgical site infection
2.2. Analysis.

Comparison 2: Shaving with a razor compared with no hair removal, Outcome 2: Surgical site infection ‐ sensitivity analysis
2.2.1 Wound complications
Two studies reported on wound complications (Celik 2007; Rojanapirom 1992). Celik 2007 reported that of the four participants with an SSI who had hair removed with a razor, three participants required surgical debridement, and the fourth required reoperation. Rojanapirom 1992 found three stitch abscesses in 40 participants who were shaved with a razor, and three stitch abscesses in 40 participants who did not have hair removed. It is unclear whether there is a difference in the incidence of stitch abscesses between no hair removal and hair removal with a razor (RR 1.00, 95% CI 0.21 to 4.66; 1 trial, n = 80) (Analysis 2.3). The evidence is of low certainty, downgraded twice for very serious imprecision (Table 2).
2.3. Analysis.

Comparison 2: Shaving with a razor compared with no hair removal, Outcome 3: Wound complication ‐ stitch abscess
2.2.2 Length of stay
One study reported on length of stay. Data were insufficient to conduct statistical analysis, so the study author's findings are reported as a narrative. Kattipattanapong 2013 found that the mean length of stay for 66 participants who had hair shaved with a razor was 4.6 days, and the mean length of stay for 70 participants who had no hair removed was 4.3 days. There may be little difference in length of stay between people who have had hair removed with a razor and people who have had no hair removed. The evidence is of low certainty, downgraded twice for very serious imprecision (Table 2).
2.2.3 Cost
One study reported on cost of the equipment. Data were insufficient to conduct statistical analysis, so the study author's findings are reported as a narrative. Court‐Brown 1981 estimated the cost of shaving with razors for 100 participants was GBP 14. The evidence is of very low certainty, downgraded once for serious imprecision and twice for very serious indirectness (Table 2), and we have little confidence in the effect.
Comparison 3: depilatory cream compared with no hair removal (1 trial, 267 participants)
One study with 267 randomised participants compared depilatory cream with no hair removal (Court‐Brown 1981).
3.1 Primary outcome: surgical site infection
One study reported surgical site infection as an outcome (Court‐Brown 1981). Court‐Brown 1981 included 267 randomised participants, and infections were measured at 28 days. Results are presented as risk ratio (RR), where the risk ratio is the risk of infection in the depilation group divided by the risk of infection in the no hair removal group. A risk ratio of more than one indicates more infections in the depilation group. The evidence shows there may be little difference in surgical site infections when hair is removed with a depilatory cream or is not removed (RR 1.02, 95% CI 0.45 to 2.31; 1 trial, n = 267) (Analysis 3.1). This corresponds to an absolute increase in surgical site infections in the cream group of 2 more infections per 1000 people (95% CIs 43 fewer infections to 102 more infections per 1000 people). The evidence is of low certainty, downgraded twice for very serious imprecision (Table 3).
3.1. Analysis.

Comparison 3: Cream compared with no hair removal, Outcome 1: Surgical site infection
3.2.1 Wound complications
Data were not reported for this outcome.
3.2.2 Length of stay
Data were not reported for this outcome.
3.2.3 Cost
One study reported on cost of the equipment (Court‐Brown 1981). Data were insufficient to conduct statistical analysis, so the study author's findings are reported as a narrative. The estimated cost of depilatory cream for 100 participants was GBP 22. The evidence is of very low certainty, downgraded once for serious imprecision and twice for very serious indirectness (Table 3), and we have little confidence in the effect.
Comparison 4: shaving with a razor compared with clipping (7 trials, 3723 participants)
Seven studies with a total of 3723 randomised participants compared hair removal with razors or with clippers (Abouzari 2009; Alexander 1983; Balthazar 1983; Domes 2011; Grober 2013; Ko 1992; Taylor 2005).
4.1 Primary outcome: surgical site infection
All seven studies reported surgical site infection as an outcome. Infections were assessed at two weeks (Balthazar 1983; Taylor 2005), 30 days (Abouzari 2009; Alexander 1983; Ko 1992), and three months (Domes 2011; Grober 2013). Results are presented as risk ratio (RR), where the risk ratio is the risk of infection in the shaving with razor group divided by the risk of infection in the clipping group. A risk ratio of more than one indicates more infections in the shaving (with a razor) group. We pooled all trials using a random‐effects model. The evidence shows the risk of surgical site infection is probably higher in participants who had hair removed with a razor compared with those who had hair removed with clippers (RR 1.64, 95% CI 1.16 to 2.33; 7 trials, n = 3723) (Analysis 4.1). The evidence is of moderate certainty, downgraded once for serious risk of bias (Table 4). This corresponds to an absolute increase in surgical site infection risk in the razor group of 16 more surgical site infections per 1000 people (95% CIs 4 more to 33 more infections per 1000 people). We carried out a sensitivity analysis to exclude any QRCTs or studies at high risk of bias, as well as studies on scrotal hair from the meta‐analysis (Domes 2011; Grober 2013; Ko 1992). This demonstrated no change in effect, resulting in a pooled RR of 1.55 (95% CI 1.07 to 2.25; 4 trials, n = 1457) (Analysis 4.2). The certainty of evidence for the sensitivity analysis is moderate, downgraded once for serious imprecision.
4.1. Analysis.

Comparison 4: Shaving with a razor compared with clipping, Outcome 1: Surgical site infection
4.2. Analysis.

Comparison 4: Shaving with a razor compared with clipping, Outcome 2: Surgical site infection ‐ sensitivity analysis
We conducted a post hoc subgroup analysis to address the concern that clippers are not suited to hair removal from male genitalia because of the possibility of catching skin folds in the clipper (Morey 2013). Results from two studies comparing hair removal from male scrotums showed that it is unclear whether there is a difference in risk of surgical site infection when hair was removed with razors or clippers (RR 0.99, 95% CI 0.14 to 6.91; 2 trials, n = 280) (Analysis 4.1) (Domes 2011; Grober 2013). The evidence is of very low certainty, downgraded once for serious risk of bias and twice for serious imprecision.
4.2.1 Wound complications
Three studies of body hair removal reported sufficient data on this outcome to be pooled in a meta‐analysis (Alexander 1983; Balthazar 1983; Taylor 2005). Skin condition was assessed at two weeks, Balthazar 1983; Taylor 2005, and 30 days (Alexander 1983). Results are presented as risk ratio (RR), where the risk ratio is the risk of skin injury in the shaving group divided by the risk of skin injury in the clipping group. A risk ratio of more than one indicates more injuries in the shaving with a razor group. We pooled data from all three trials using a random‐effects model. The evidence shows the risk of skin injury is probably increased in people who have hair removed by shaving with a razor compared with clippers (RR 1.74, 95% CI 1.12 to 2.71; 3 trials, n = 1333) (Analysis 4.3). The evidence is of moderate certainty, downgraded once for serious imprecision (Table 4). Additionally, Alexander 1983 found six stitch abscesses in the razor group (n = 520) compared with one stitch abscess in the clipper group (n = 457).
4.3. Analysis.

Comparison 4: Shaving with a razor compared with clipping, Outcome 3: Skin injury
Two studies of hair removal from male genitals did not provide sufficient detail to be included in a meta‐analysis and are presented separately (Domes 2011; Grober 2013). In the study by Grober 2013 of scrotal hair removal in 215 participants, the average score for skin trauma in participants who had hair removed with clippers was 2.82 compared with 1.91 for participants who were shaved with a razor (1 = no evidence of trauma, 5 = significant trauma). Grober 2013 claims razors cause significantly less skin trauma to male genitalia than clippers (P = 2.5). Conducting a similar study with 65 participants, Domes 2011 also reported significantly less trauma to the scrotal skin when razors were used instead of clippers (P < 0.001) (raw data are not presented). Both studies reported that razors cause less skin trauma than clippers; however, this is low‐certainty evidence, downgraded for serious imprecision from low sample sizes and insufficient raw data.
4.2.2 Length of stay
Data were not reported for this outcome.
4.2.3 Cost
Data were not reported for this outcome.
Comparison 5: shaving with a razor compared with depilatory cream (10 trials, 1793 participants)
Ten studies with a total of 1793 randomised participants compared the effects of shaving with a razor with use of a depilatory cream (Adisa 2011; Breiting 1981; Court‐Brown 1981; Goëau‐Brissonnière 1987; Karegoudar 2012; Powis 1976; Seropian 1971; Suvera 2013; Thorup 1985; Thur de Koos 1983).
5.1 Primary outcome: surgical site infection
Nine studies reported surgical site infection as an outcome assessed at 10 days (Thorup 1985), 28 days (Court‐Brown 1981), five weeks (Adisa 2011), six weeks (Suvera 2013), weekly review (Seropian 1971), and at discharge and outpatient visit (Breiting 1981). Thur de Koos 1983 did not state when infections were assessed. One study stated that they would report surgical site infections (Karegoudar 2012); however, as no details were provided, it was not possible to present this study as a narrative or in a meta‐analysis. We combined the remaining nine studies in a meta‐analysis, and presented results as risk ratio (RR), where the risk ratio is the risk of infection in the shaving group divided by the risk of infection in the depilatory cream group. A risk ratio of more than one indicates more infections in the shaving with a razor group. We pooled all trials using a random‐effects model. The risk of surgical site infections is probably higher in participants who have hair removed by shaving with a razor compared with removal by depilatory cream (RR 2.28, 95% CI 1.12 to 4.65; 9 trials, n = 1593) (Analysis 5.1). This corresponds to an absolute increase in surgical site infection of 46 more SSIs per 1000 people in the razor group (95% CI 4 to 131 more infections per 1000 people). The evidence is of moderate certainty, downgraded once for serious risk of bias (Table 5). The direction of the evidence was the same in a sensitivity analysis excluding QRCTs and studies at high risk of bias (RR 2.80, 95% CI 1.22 to 6.46; 5 trials, n = 790) (Analysis 5.2). The certainty of evidence for the sensitivity analysis is moderate, downgraded once for serious imprecision.
5.1. Analysis.

Comparison 5: Shaving with a razor compared with cream, Outcome 1: Surgical site infection
5.2. Analysis.

Comparison 5: Shaving with a razor compared with cream, Outcome 2: Surgical site infection ‐ sensitivity analysis
5.2.1 Wound complications
Five studies reported data for wound complications and are pooled in a meta‐analysis (Adisa 2011; Breiting 1981; Seropian 1971; Suvera 2013; Thorup 1985). Powis 1976 also explored wound complications but did not provide raw data, stating instead that "several patients in (the shaved group) had obvious skin damage". The results for the five studies are presented as risk ratio (RR), where the risk ratio is the risk of skin injury in the shaving group divided by the risk of skin injury in the depilatory cream group. A risk ratio of more than one indicates more injuries in the shaving with a razor group. We pooled all trials using a random‐effects model. Pooled meta‐analysis suggests the risk of skin injury may be higher in participants who have hair removed with a razor rather than with depilatory cream (RR 6.95, 95% CI 3.45 to 13.98; 5 trials, n = 937) (Analysis 5.3). The evidence is of low certainty, downgraded once for serious risk of bias and once for serious imprecision (Table 5).
5.3. Analysis.

Comparison 5: Shaving with a razor compared with cream, Outcome 3: Skin injury
5.2.2 Length of stay
Data were not reported for this outcome.
5.2.3 Cost
Three studies reported on cost (Court‐Brown 1981; Powis 1976; Thorup 1985). The variations in which data were reported meant that it was not possible to pool data, and data are therefore presented as a narrative. Court‐Brown 1981 reported that the approximate costs per 100 participants were GBP 14 for a shave preparation and GBP 22 for depilatory cream. Powis 1976 found the cream "at a cost of 25p compared with 80p for the average cost of a shave, taking into account the time of staff and the disposable equipment used". Thorup 1985 states that the cost of one tube of depilatory cream was DKK 9.80 (GBP 0.90), and one disposable razor DKK 0.85 (GBP 0.08). The evidence is of very low certainty evidence, downgraded once for serious imprecision and twice for very serious indirectness (Table 5), and we have very little confidence in the effect.
Comparison 6: hair removal on the day of surgery compared with one‐day preoperatively (1 trial, 977 participants)
One study with 977 randomised participants compared shaving the day before surgery with shaving on the day of surgery, and also compared clipping the night before surgery with clipping on the day of surgery (Alexander 1983).
6.1 Primary outcome: surgical site infection
Alexander 1983 reported surgical site infection as an outcome. Infections were assessed 30 days after discharge. The results are presented as risk ratio (RR), where the risk ratio is the risk of infection from removing hair the day before surgery is divided by the risk of infection from removing hair the day of surgery. A risk ratio of less than one indicates fewer infections when removing hair the day of surgery. There may be a small reduction in surgical site infections when hair is removed on the day of surgery compared with the day before surgery (RR 0.83, 95% CI 0.54 to 1.30; 1 trial, n = 977) (Analysis 6.1). This corresponds to an absolute decrease in surgical site infection risk of 12 fewer infections per 1000 people on the day of removal group (95% CI 32 fewer to 20 more infections per 1000 people). The evidence is of low certainty, downgraded once for serious imprecision and once for inconsistency given the different types of hair removal approaches used (Table 6). Subgroup analysis for hair removal with either razors or clippers showed some heterogeneity in findings.
6.1. Analysis.

Comparison 6: Hair removal day of surgery versus day before surgery, Outcome 1: Surgical site infection
6.2.1 Wound complications
Data were not reported for this outcome.
6.2.2 Length of stay
Data were not reported for this outcome.
6.2.3 Cost
Data were not reported for this outcome.
Discussion
Summary of main results
The objectives of this review were to determine whether routine preoperative hair removal (compared with no removal) and the method, timing, or setting of hair removal influence rates of SSI.
There may be little to no difference in SSIs whether hair is not removed or removed with clippers (low‐certainty evidence). A sensitivity analysis of this comparison also suggests there may be little to no difference in SSIs (low‐certainty evidence). SSIs are probably lower when hair is not removed rather than removed with a razor (moderate‐certainty evidence), although in a sensitivity analysis the confidence intervals narrowly include the possibility of no effect or harm (moderate‐certainty evidence). Low‐certainty evidence for the comparison of no hair removal with hair removal with depilatory cream suggests there may be little difference in SSIs.
When comparing different methods of hair removal, there is moderate‐certainty evidence that SSIs are probably lower when clippers or depilatory creams are used rather than shaving with razors. A subgroup analysis comparing clippers with razors on male genitalia found that it was unclear whether there was a difference in SSIs in this specific anatomical region, based on very low‐certainty evidence.
There may be a small reduction in SSIs when hair is removed on the day of surgery compared with the day before surgery (low‐certainty evidence).
Based on low‐certainty evidence, it is unclear whether there is a difference in the incidence of wound complications between no hair removal and hair removal with a razor, although moderate‐certainty evidence suggests the risk of skin injury is probably lower in participants who have hair removed (from their body as opposed to male genitalia) with clippers and may be lower (low‐certainty evidence) when using cream compared with razors. Low‐certainty evidence suggests that razors may result in fewer injuries to scrotal skin than clippers.
There may be little difference in length of hospital stay between participants who have hair removed with a razor versus those with no hair removal (low‐certainty evidence). It is uncertain whether there is a difference in cost when hair is removed with either a razor or with cream.
We identified no studies that compared clipping with depilatory cream, or that investigated the application of depilatory cream at different preoperative time points, or hair removal in different settings (e.g. ward, anaesthetic room).
Overall completeness and applicability of evidence
Surgical site infection was the primary outcome in 17 of the included studies and a secondary outcome in 8 included studies. The primary outcome in studies where SSI was a secondary outcome focused mainly on skin damage, patient preference, and adequacy of the hair removal. Most surgical specialty groups (except children) were represented in the included studies, and the studies were set in 12 different countries, therefore the results should apply to a wide range of people undergoing surgery.
Thirteen of the 25 included studies were published between 20 and 50 years ago, which will have an effect on the applicability of the findings of this review. Whilst the definition of a surgical site infection has not changed, many of the practices surrounding surgery, hair removal, and infections have changed. For example, the design and quality of razors, clippers, and depilatory cream have improved; antibiotic usage has changed greatly; the length of hospital stay has reduced, thereby affecting SSI surveillance and costs; pre‐hospital admissions have greatly reduced, affecting hair removal practices and costs; and orderlies who shaved patients in advance of surgery are no longer employed. Future updates and reviews may consider excluding trials over a specified age.
Very few studies explored treatment costs, and any exploration was very limited. As already mentioned, data from older studies regarding costs and length of stay may no longer be relevant.
Recent searches identified an increasing number of studies where hair removal protocols were part of a care bundle, though these studies were usually pre‐ and postintervention studies rather than RCTs. More recent studies seem more likely to use recognised definitions of SSIs with longer follow‐up periods.
The previous update of this review (Tanner 2011), which included studies where body hair or scalp hair was removed, suggested that clippers were associated with fewer SSIs than razors. Since that publication, the appropriateness of using clippers on scrotal hair has been questioned (Morey 2013), and studies have been carried out focusing on SSI and skin injury when using clippers or razors on male genitalia.
Quality of the evidence
We used the GRADE approach to assess all outcomes. For the primary outcome, surgical site infection, the certainty of evidence was assessed as moderate for five comparisons and low for one comparison. We downgraded the evidence for serious risk of bias (a substantial amount of data came QRCTs and were considered to be at high risk of bias), serious imprecision with wide confidence intervals, and very serious imprecision with a small sample size and wide confidence intervals. The certainty of evidence contributing to the secondary outcomes, wound complications, length of stay, and cost, was mainly assessed as low or very low, with one outcome rated as moderate certainty. In addition to serious risk of bias, serious imprecision due to either wide confidence intervals or small sample size, very serious imprecision due to small sample and wide confidence intervals, plus small sample size and small number of events, we considered studies of cost to have very serious indirectness, as only one aspect of treatment costs was included. We did not downgrade for inconsistency, as statistical heterogeneity was either not present or moderate. Three sensitivity analyses were conducted excluding QRCTs; the certainty of the evidence for all of these analyses was moderate due to serious imprecision (wide confidence intervals). The effect did not change in any of the sensitivity analyses.
The study setting and timing of hair removal relative to surgery were often poorly reported. Often the identity of the outcome assessor and the timing of assessment were not clear. Most of the trials provided information on the sex and age of study participants and the surgical procedure carried out, which permitted baseline comparisons.
Potential biases in the review process
We did not conduct funnel plots, as there were fewer than 10 studies in each comparison. All studies included in this review have been published in full, with the exception of Domes 2011, for which only an abstract has been published. We attempted to contact four study authors for additional information (Domes 2011; Ilankovan 1992; Lu 2002; Shi 2016), but these attempts were unsuccessful.
We identified 14 articles in languages other than English that required translation. Six of these studies were subsequently included in the review, and were published in Danish (Breiting 1981; Thorup 1985), French (Goëau‐Brissonnière 1987), Portuguese (Nascimento 1991), and Chinese (Lu 2002; Sun 2014). Only one potentially relevant study was identified through the clinical trials registries, and the findings from this study had already been published (Kowalski 2016). A systematic review, published in English by a researcher in China (Shi 2016), included four Chinese studies that were not identified through our search strategy (Li 2013; Liu 2008; Lu 2002; Sun 2014). We were able to obtain the full text for three of these studies (Liu 2008; Lu 2002; Sun 2014), and the abstract for the remaining study (Li 2013), and have these translated. Following translation, Lu 2002 and Sun 2014 were included in the review, and Li 2013 and Liu 2008 were excluded for not being randomised studies.
Agreements and disagreements with other studies or reviews
Compared with previous updates
The previous update of this systematic review included studies published up to 2011 (Tanner 2011). This second update includes studies published up to 2019. The differences in outcomes and certainty of evidence between the 2011 update and this 2019 update are as follows.
The outcome for the comparison clippers versus no hair removal remains unchanged: there may be little difference in the risk of SSI when hair is removed by clippers compared with no hair removal. However, the certainty of the evidence has increased.
Additional data for the comparison shaving with a razor versus no hair removal has changed the outcome to show that the risk of SSI is probably lower in participants who do not have hair removed compared with having hair removed with a razor, although in a sensitivity analysis the confidence intervals narrowly include the possibility of no effect or harm.
Additional data for the comparison razors versus cream has changed the outcome to show that there are probably more SSIs when hair is removed with razors rather than with cream.
For this update, the previous separate comparisons of clipping and shaving on the day of surgery versus the day before surgery have been merged to compare hair removal on the day of surgery versus hair removal the day before. There may be a small reduction in the risk of SSI when hair is removed on the day of surgery compared with the day before.
A narrative summary reports that clippers cause more skin damage to male genitalia than razors; however, the evidence is of low certainty.
Compared with other reviews
The findings from this update review are similar to the findings from some recently published systematic reviews. A systematic review of preoperative hair removal was published in 2015 (Lefebvre 2015). The review by Lefebvre 2015 includes 19 studies and reports the same findings as this update review. In contrast to this update, Lefebvre 2015 does not include Domes 2011, Ilankovan 1992, Karegoudar 2012, Lu 2002, Sun 2014, and Suvera 2013, or Kowalski 2016, which was published after the Lefebvre 2015 review. Instead, Lefebvre 2015 includes Meiland 1986, which was excluded from this review as episiotomies do not meet the definition of a 'surgical procedure' (WHO 2018a).
One systematic review was published by researchers in China in 2016 (Shi 2016). This review included 14 RCTs and controlled clinical trials. The Shi 2016 review and this update have 11 studies in common. Shi 2016 compared shaving versus no hair removal, clipping versus no hair removal, shaving versus clipping, and shaving versus depilatory cream, and found no difference in SSIs in any of the comparisons.
The World Health Organization (WHO) published recommendations for the prevention of surgical site infection in 2018 (WHO 2018b). This included 15 RCTs and QRCTs, all of which are included in this update, with the exception of Horgan 1997, which is considered to be a 'historical control' study and not an RCT. WHO 2018b reports no difference between shaving and no hair removal, clipping and no hair removal, and cream and no hair removal. WHO 2018b also found that clipping resulted in fewer SSIs than razors, but found little difference between depilatory cream and razors. Based on what they consider to be moderate‐quality evidence, WHO 2018b strongly recommends that hair should not be removed, and if it is absolutely necessary to remove hair then it should be removed with clippers only. Razors are strongly discouraged. These findings are similar to those of this update, although we found there are probably more SSIs when hair is removed with a razor compared with no hair removal, and when razors are used instead of cream. This update found there may be a small reduction in the risk of SSIs when hair is removed on the day of surgery compared with the day before.
Authors' conclusions
Implications for practice.
Guidelines recommend that hair surrounding the operative site should not be routinely removed, and if hair has to be removed, then this should be done using clippers only in most cases (WHO 2018b). Review findings map to these recommendations, suggesting hair removal with a razor probably increases risk of SSI compared with no removal, and there may be little or no difference in SSI risk between no hair removal and use of clippers. Hair removal with razors probably increases the risk of SSI compared with clippers or cream. Regarding hair removal from male scrotal skin, very low‐certainty evidence means it is unclear whether razors or clippers cause more SSIs, but low‐certainty evidence suggests that razors may result in less trauma than clippers.
Implications for research.
There is the potential for further research in this area but trials would need to focus on areas of priority for patients and health care staff, this may include the impact of approaches to hair removal on specific anatomical regions, the timing of removal and the setting. Where trials are conducted:
best practice in key methodological domains should be adopted;
sample sizes need to allow clinically important differences to be detected;
details of randomisation, allocation concealment, and blinding must be provided;
an internationally accepted definition of surgical site infections should be used, such as that of the Centers for Disease Control and Prevention (CDC) (CDC 2008);
trial participants need to be followed up for recognised surgical site infections follow‐up times (see CDC 2008);
other wound complications should be included as outcomes.
It may be beneficial to conduct a network meta‐analysis. A network meta‐analysis is a single review that includes all relevant interventions and presents their comparative effectiveness and potential for harm.
What's new
| Date | Event | Description |
|---|---|---|
| 25 August 2021 | New search has been performed | Second update, new search, 11 new trials included (Adisa 2011; Domes 2011; Grober 2013; Karegoudar 2012; Kattipattanapong 2013; Ko 1992; Kowalski 2016; Lu 2002; Sun 2014; Suvera 2013; Taylor 2005). Two trials that were awaiting classification have now been excluded (Fraser 1978; Menéndez 2004), and one additional trial is awaiting classification (Pascual 1994). Two findings within this update have changed from the previously updated version of this review. Previously, there was little difference between not removing hair and removing hair with a razor. Now, moderate‐certainty evidence shows that surgical site infections (SSIs) are probably lower when hair is not removed, although a sensitivity analysis found confidence intervals which narrowly included the possibilities of no effect or harm. Previously, there appeared to be no difference in SSIs between whether hair was removed by a razor or by cream. Moderate‐certainty evidence now shows that hair removal with cream probably results in fewer SSIs than hair removal with a razor. Two new findings show that more skin injury is probably sustained when hair is removed with razors compared with clippers (moderate‐certainty evidence) or with depilatory cream (low‐certainty evidence). A new subgroup has been created for the removal of hair from the male genital area. A narrative summary reports that clippers may cause more skin damage to male genitalia than razors (low‐certainty evidence), and that it is unclear whether there is a difference in SSIs between using razors or clippers on male genitalia (very low‐certainty evidence). |
| 25 August 2021 | New citation required and conclusions have changed | Updated. Conclusions changed. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 14 September 2011 | New citation required but conclusions have not changed | New author added to the review team. |
| 12 August 2011 | New search has been performed | First update new search, three new trials included (Abouzari 2009; Celik 2007; Nascimento 1991); as a result of further assessment one trial which was previously excluded has been included in this update (Ilankovan 1992), and one trial which was previously included has now been excluded (Ko 1992). The conclusions of this update remain unchanged. |
| 21 September 2010 | New search has been performed | Converted to new review format |
| 21 April 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to acknowledge the contribution Diane Woodings, Peter Norrie, and Wendy Padley made to the original review and previous update. They would also like to thank David Margolis, Andrea Nelson, Sally Stapley, Marie Westwood, Rachel Richardson and Vicky Whittaker for comments on previous versions of this review, and Gill Norman, Sarah Rhodes, and Oren Lapid for their comments on this version. Thanks are also due to Pia Brandt Danborg for her translation services and to Elizabeth Royle and Lisa Winer for copy editing previous versions of this review and the current version.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Hair Removal EXPLODE ALL AND INREGISTER
2 (hair near3 remov*) AND INREGISTER
3 shav* AND INREGISTER
4 clip* AND INREGISTER
5 depilat* AND INREGISTER
6 #1 OR #2 OR #3 OR #4 OR #5 AND INREGISTER
7 MESH DESCRIPTOR Surgical Wound Infection EXPLODE ALL AND INREGISTER
8 MESH DESCRIPTOR Surgical Wound Dehiscence EXPLODE ALL AND INREGISTER
9 surg* near5 infection* AND INREGISTER
10 surg* near5 wound* AND INREGISTER
11 wound* near5 infection* AND INREGISTER
12 (postoperative or post‐operative) near5 infection* AND INREGISTER
13 #7 OR #8 OR #9 OR #10 OR #11 OR #12 AND INREGISTER
14 #6 AND #13 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Hair Removal] explode all trees
#2 (hair near/3 remov*):ti,ab,kw
#3 shav*:ti,ab,kw
#4 clip*:ti,ab,kw
#5 depilat*:ti,ab,kw
#6 {or #1‐#5}
#7 MeSH descriptor: [Surgical Wound Infection] explode all trees
#8 MeSH descriptor: [Surgical Wound Dehiscence] explode all trees
#9 surg* near/5 infection*:ti,ab,kw
#10 surg* near/5 wound*:ti,ab,kw
#11 wound* near/5 infection*:ti,ab,kw
#12 ((postoperative or post‐operative) near/5 infection*):ti,ab,kw
#13 {or #7‐#12}
#14 {and #6, #13}
Ovid MEDLINE
1 exp Hair Removal/
2 (hair adj3 remov$).ti,ab.
3 (shav$ or clip$ or depilat$).ti,ab.
4 or/1‐3
5 exp Surgical Wound Infection/
6 exp Surgical Wound Dehiscence/
7 (surg$ adj5 infection$).ti,ab.
8 (surg$ adj5 wound$).ti,ab.
9 (wound$ adj5 infection$).ti,ab.
10 ((postoperative or post‐operative) adj5 infection$).ti,ab.
11 or/5‐10
12 and/4,11
13 randomized controlled trial.pt.
14 controlled clinical trial.pt.
15 randomi?ed.ab.
16 placebo.ab.
17 clinical trials as topic.sh.
18 randomly.ab.
19 trial.ti.
20 or/13‐19
21 exp animals/ not humans.sh.
22 20 not 21
23 12 and 22
Ovid Embase
1 exp Hair Removal/
2 (hair adj3 remov$).ti,ab.
3 (shav$ or clip$ or depilat$).ti,ab.
4 or/1‐3
5 exp Surgical Infection/
6 exp Wound Dehiscence/
7 (surg$ adj5 infection$).ti,ab.
8 (surg$ adj5 wound$).ti,ab.
9 (wound$ adj5 infection$).ti,ab.
10 ((postoperative or post‐operative) adj5 infection$).ti,ab.
11 or/5‐10
12 and/4,11
13 Randomized controlled trials/
14 Single‐Blind Method/
15 Double‐Blind Method/
16 Crossover Procedure/
17 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
18 (doubl* adj blind*).ti,ab.
19 (singl* adj blind*).ti,ab.
20 or/13‐19
21 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
22 human/ or human cell/
23 and/21‐22
24 21 not 23
25 20 not 24
26 12 and 25
EBSCO CINAHL Plus
S36 S12 AND S35
S35 S34 NOT S33
S34 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27
S33 S31 NOT S32
S32 MH (human)
S31 S28 OR S29 OR S30
S30 TI (animal model*)
S29 MH (animal studies)
S28 MH animals+
S27 AB (cluster W3 RCT)
S26 MH (crossover design) OR MH (comparative studies)
S25 AB (control W5 group)
S24 PT (randomized controlled trial)
S23 MH (placebos)
S22 MH (sample size) AND AB (assigned OR allocated OR control)
S21 TI (trial)
S20 AB (random*)
S19 TI (randomised OR randomized)
S18 MH cluster sample
S17 MH pretest‐posttest design
S16 MH random assignment
S15 MH single‐blind studies
S14 MH double‐blind studies
S13 MH randomized controlled trials
S12 S4 and S11
S11 S5 or S6 or S7 or S8 or S9 or S10
S10 TI ( postoperative infection* or post operative infection* ) or AB ( postoperative infection* or post operative infection* )
S9 TI wound* N5 infection* or AB wound* N5 infection*
S8 TI surg* N5 wound* or AB surg* N5 wound*
S7 TI surg* N5 infection* or AB surg* N5 infection*
S6 (MH "Wound Infection")
S5 (MH "Surgical Wound Infection")
S4 S1 or S2 or S3
S3 TI ( shav* or clip* or depilat* ) or AB ( shav* or clip* or depilat* )
S2 TI hair N3 remov* or AB hair N3 remov*
S1 (MH "Hair Removal")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
hair OR shave OR clip OR depilat | Surgical Site Infection
hair OR shave OR clip OR depilat | Surgical Wound Infection
hair OR shave OR clip OR depilat | Surgical Wound Dehiscence
hair OR shave OR clip OR depilat | surgical wound
hair OR shave OR clip OR depilat | surgical infection
World Health Organization International Clinical Trials Registry Platform
hair OR shave OR clip OR depilate [intervention] | Surgical Site Infection [Title]
hair OR shave OR clip OR depilate [intervention] | Surgical Site Infection [Condition]
hair OR shave OR clip OR depilate [intervention] | surgical wound infection [Title]
hair OR shave OR clip OR depilate [intervention] | surgical wound infection [Condition]
hair OR shave OR clip OR depilate [intervention] surgical wound dehiscence [Title]
hair OR shave OR clip OR depilate [intervention] surgical wound dehiscence [Condition]
Appendix 2. Criteria for judgement of risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, nonopaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definitive judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded, and the non‐blinding of others is unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but it is likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to introduce bias.
Unclear
Either of the following.
Insufficient information provided to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) amongst missing outcomes is not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data is likely to be related to true outcome, with an imbalance in either numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardized difference in means) amongst missing outcomes is enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address incomplete outcome data.
5. Are reports of the study free of the suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Clipping compared with no hair removal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Surgical site infection | 3 | 1733 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.65, 1.39] |
| 1.2 Surgical site infection ‐ sensitivity analysis | 2 | 1673 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.66, 1.42] |
Comparison 2. Shaving with a razor compared with no hair removal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Surgical site infection | 7 | 1706 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.05, 3.14] |
| 2.2 Surgical site infection ‐ sensitivity analysis | 6 | 917 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.97, 3.01] |
| 2.3 Wound complication ‐ stitch abscess | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.21, 4.66] |
Comparison 3. Cream compared with no hair removal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Surgical site infection | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.45, 2.31] |
Comparison 4. Shaving with a razor compared with clipping.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Surgical site infection | 7 | 3723 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.16, 2.33] |
| 4.1.1 Body and scalp hair | 5 | 3443 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.17, 2.38] |
| 4.1.2 Genital hair | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.91] |
| 4.2 Surgical site infection ‐ sensitivity analysis | 4 | 1457 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.07, 2.25] |
| 4.3 Skin injury | 3 | 1333 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.12, 2.71] |
Comparison 5. Shaving with a razor compared with cream.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Surgical site infection | 9 | 1593 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [1.12, 4.65] |
| 5.2 Surgical site infection ‐ sensitivity analysis | 5 | 790 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [1.22, 6.46] |
| 5.3 Skin injury | 5 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 6.95 [3.45, 13.98] |
Comparison 6. Hair removal day of surgery versus day before surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Surgical site infection | 1 | 977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.30] |
| 6.1.1 Removal with a razor | 1 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.66, 1.93] |
| 6.1.2 Removal with clippers | 1 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abouzari 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 195 people undergoing elective cranial surgery in Iran between March 2005 and December 2007 | |
| Interventions | 3 preoperative surgical site treatments involving removal, or no removal, of scalp hair:
Group 1: removal with a razor (n = 65)
Group 2: removal with hair clippers (n = 65)
Group 3: no hair removal (n = 65) Product details: no details are given for the razor, the clippers were "barbers clippers". Time of hair removal: no details given. Hair removed by: not specified. Venue for hair removal: not reported. |
|
| Outcomes | Outcome: infection. Defined as including presence of pus, bacterial culture, development of postoperative meningitis and microbiology. Participants were followed up at 3 or 4 weekly intervals until complete wound healing or the development of an infection. | |
| Notes | No statistical test of significance used. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated" Comment: no further details given. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. Comment: not clear whether the person allocating people to groups would have been able to predict the group to which a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | No details reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | No details reported. Comment: participants would be aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | No details reported. Comment: no information provided regarding whether participants were analysed in the groups to which they were allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants are distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Adisa 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 165 people undergoing clean general surgery in Nigeria between August 2007 and July 2008 | |
| Interventions | Group 1: removal with a razor (n = 86) Group 2: removal with cream (n = 79) Product details: no details are given for the razor, the cream was Veet. Timing of hair removal: the morning of the operation. Hair removed by: nursing staff. Venue for hair removal: the operating theatre. |
|
| Outcomes | Primary outcome: wound infection. Southampton wound infection scoring system used. Secondary outcomes: presence of injury, presence of skin reactions, and adequacy of hair removal. Wounds assessed on days 3, 5, and 7 postoperatively by an independent "senior resident". All participants were followed up for at least 5 weeks. |
|
| Notes | No funding sources mentioned. No details of conflict of interest. This paper is an abridged version of an unpublished dissertation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomised into two groups using a balloting method"; "Consecutive patients were asked to pick one of two sealed envelopes containing a folded paper on which one of the two methods was written" Comment: done. |
| Allocation concealment (selection bias) | Low risk | Quote: "patients asked to pick one of two sealed envelopes" Comment: done. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "wounds were inspected by senior resident who had not participated in the surgery" Comment: probably done. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no information provided regarding whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants are distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Alexander 1983.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 1013 people having elective, clean surgery in the USA between July 1979 and July 1981. Includes vascular, thoracic, abdominal, gynaecological, and neurosurgical. | |
| Interventions | Group 1: clipping day before surgery (n = 249)
Group 2: clipping on the morning of surgery (n = 226)
Group 3: shaving with a razor the day before surgery (n = 271)
Group 4: shaving with a razor on the morning of surgery (n = 266) Product details: no details are given for the razor or the clippers. Timing of hair removal: either the day of surgery or the day before surgery according to randomisation. Hair removed by: not specified. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: wound infection defined as "discharge of pus"; assessed by a research nurse at discharge and with follow‐up 30 days after discharge. Secondary outcomes: length of stay and cost. |
|
| Notes | No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by draw of a card from a sealed envelope" Comment: random sequence generation not described in adequate detail. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised by draw of a card from a sealed envelope" Comment: done |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | No details reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | No details reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | No details reported. Comment: no information provided regarding whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants are distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Balthazar 1983.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 200 people having elective inguinal hernia repair in the USA between 1978 and 1981 | |
| Interventions | Group 1: preoperative hair removal with a razor (n = 100)
Group 2: preoperative hair removal with clippers (n = 100) Product details: razors were "safety razors" with a wet shave which were washed between cases, the clippers were "ordinary barbers electric clippers". Timing of hair removal: immediately before surgery. Hair removed by: not specified. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: wound infection defined as "discharge of purulent exudate", assessed by infection control nurse daily for 5 days postoperatively and by unspecified practitioners at 2 weeks postoperatively. Secondary outcome: adequacy of hair removal, skin trauma judged by attending surgeon. |
|
| Notes | All recruited participants were male; no statistical test of significance. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients randomised using standard table of random numbers" Comment: done. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no information provided regarding whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | It appears that no participants dropped out from the study. Comment: 200 participants reported in the results section. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Breiting 1981.
| Study characteristics | ||
| Methods | QRCT | |
| Participants | 104 adult men having elective surgery on lower legs in Denmark (dates not given, though study published in 1981). Procedures included knee arthrotomy, plastic surgery to knee ligaments, foot joints and tibial osteotomy. | |
| Interventions | Group 1: preoperative hair removal with a razor (n = 52)
Group 2: preoperative hair removal with depilatory cream (n = 52) Product details: the razor was disposable, the cream was Prelprep. Timing of hair removal: not reported. Hair removed by: razors ‐ nurses, cream ‐ study authors. Venue for hair removal: razors on the ward, cream not reported. |
|
| Outcomes | Primary outcome: effectiveness of hair removal and patient preference, both assessed by participants Secondary outcome: clinical evidence of superficial and deep infections assessed at discharge and outpatient visit by surgical staff. Definition of infection was not given. |
|
| Notes | Paper published in Danish. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Allocated on the date of admission to hospital" Comment: inadequate randomisation technique. |
| Allocation concealment (selection bias) | High risk | Quote: "Allocated on the date of admission to hospital" Comment: care‐provider could predict allocation by reference to date of admission to hospital. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Interventions and assessments were carried out by study authors. Comment: outcome assessors were not blinded, and it is likely that knowledge of the group the participant was in could have influenced their judgement. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no information provided regarding whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | No participants appeared to have dropped out from the study. Comment: data presented for 104 participants. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Celik 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 789 people undergoing spinal surgery in Turkey between January 2000 and September 2004 | |
| Interventions | Group 1: surgical site shaved with a razor immediately prior to surgery (n = 371)
Group 2: no hair removal (n = 418) Product details: no details are given for the razor. Timing of hair removal: immediately before surgery. Hair removed by: theatre staff. Venue for hair removal: on the operating table. |
|
| Outcomes | Primary outcome: SSI defined by pus, pain, tenderness or redness, plus haematological evidence. Secondary outcome: meningitis and abscess defined by haematological evidence. Data regarding the wound and presence of infection were collected continuously (wounds were assessed at 1‐ and 3‐month follow‐up visits, though it is not known who carried out the follow‐up ‐ personal communication). |
|
| Notes | No funds were received to support the study. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated on a 1:1 ratio on a randomisation sheet" Comment: method of generation of randomisation sequence unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: insufficient detail provided to enable a judgement regarding whether there was full analysis of all participants in their randomised groups. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | High risk | Personal communication with the author revealed that the dropout rate for the non‐shaved group was unknown, participants not evenly distributed across groups. Comment: participants were not evenly distributed across groups. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | High risk | Comment: a higher number of complex operations were reported for 1 group. |
Court‐Brown 1981.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 418 people undergoing abdominal surgery (except colostomy) in Scotland between November 1977 and October 1978 | |
| Interventions | Group 1: hair removal with a razor (n = 137)
Group 2: hair removal with depilatory cream (n = 126)
Group 3: no hair removal (n = 141) Product details: the razors were disposable safety razors with a wet shave, the cream was Veeto. Timing of hair removal: 18 to 24 hours before elective surgery and within 6 hours before emergency surgery. Hair removed by: not specified. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: wound infection defined by presence of pus. Wounds were assessed daily whilst in hospital, and at 28 days postoperatively, unclear by whom. Secondary outcome: acceptability of each method based on "information from staff and patients", cost, and infections when hair removed within 6 hours versus 18 to 24 hours. |
|
| Notes | No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients were randomly allocated" Comment: no description provided of generation of randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no information provided regarding whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants are distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Domes 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 65 men having surgery on male genitalia. Country in which study was conducted and dates of the study are not given. | |
| Interventions | Group 1: preoperative hair removal with a razor (n = 28) Group 2: preoperative hair removal with clippers (n = 37) Product details: no details are given on the razor or the clippers. Timing of hair removal: not reported. Hair removed by: not reported. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: skin trauma and shave quality. Photographs taken immediately after hair removal and assessed in a blinded fashion by 5 surgeons and 15 nurses. Secondary outcome: surgical site infection. Definition not given. Assessed at 3 months, no details regarding who conducted assessment. |
|
| Notes | Abstract from poster presentation. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Patients were randomised" Comment: no description provided of generation of randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded. Comment: blinded assessment undertaken . |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no information provided regarding whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | High risk | Dropouts for SSI were not reported, but group sizes appeared unequal. Comment: participants were not evenly distributed across groups. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Unclear risk | Comment: no information on the similarity of groups. |
Goëau‐Brissonnière 1987.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 100 people undergoing elective surgery, excluding amputation, vaginal, proctological, urological, and gynaecological procedures, in France between January and July 1986 | |
| Interventions | Group 1: preoperative hair removal with a razor (n = 51)
Group 2: preoperative hair removal with depilatory cream (n = 49) Product details: the razor was used with a wet shave, the cream was Immac. Timing of hair removal: the evening before surgery. Hair removed by: nursing staff. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: clinical evidence of wound infection assessed by a doctor at day 2 and day 5 postoperatively. Infection defined as redness, swelling, pus, and positive swab culture. Secondary outcome: quality of skin preparation assessed by the surgeon, the theatre nurse, and the participant. |
|
| Notes | Paper published in French. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were taken at random using a table of random numbers" Comment: probably suitable randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: unclear whether allocation was concealed. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Infection was noticed. . . by a doctor who was not aware of which type of skin preparation was used" Comment: blinding of assessors reported. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | No participants appeared to have dropped out from the study. Comment: 100 participants reported in results. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Grober 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 215 adult men having surgery involving their genitalia in Canada (dates not given). Included vasectomy reversal, penile prosthesis insertion, testis biopsy, orchidectomy, and scrotal skin surgery. | |
| Interventions | Group 1: hair removal with clippers (n = 107) Group 2: hair removal with razor (n = 108) Product details: razors were Gillette 2 blade disposable, clippers were 3M surgical clippers. Timing of hair removal: after anaesthesia had commenced. Hair removed by: not specified. Venue for hair removal: the operating department. |
|
| Outcomes | Primary outcome: effectiveness of method and skin trauma. Photographs taken immediately after hair removal and assessed in a blinded fashion by "groups of urologic surgeons and surgical nursing staff". Secondary outcome: SSIs defined by evidence of increasing cellulitis or pus, or both, within 3 months of surgery, monitored throughout the duration of study. Participants assessed at 1‐ and 3‐month follow‐up visits, no details regarding who performed the assessment. |
|
| Notes | No funding sources mentioned. Conflict of interest status: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomised" Comment: method used for random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | High risk | Quote: "Members of the surgical team ... perform the hair removal on the scrotal skin within the surgical field ..." Comment: care providers were involved in hair removal. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Quote: "Participants not blinded" Comment: it is possible that participants were aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "photographs were reviewed in a blinded fashion" Comment: assessors were blinded to intervention. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Unclear risk | Not reported. Comment: insufficient details to permit a judgement. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Unclear risk | Comment: no information on the similarity of groups. |
Ilankovan 1992.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 50 people (age 15 to 60) requiring zygomatic arch repair in England. Dates of study not given. | |
| Interventions | Group 1: hair removal with a razor (n = 25)
Group 2: no hair removal (n = 25) Product details: no details are given for the razor. Timing of hair removal: after anaesthesia had started. Hair removal carried out by: not specified. Venue for hair removal: the operating table. |
|
| Outcomes | Primary outcome: participant and surgeon preferences. Participants were asked to assess their appearance (scalp hair removal) immediately after surgery. Surgeons were asked to assess surgical difficulty. Secondary outcome: signs of local infection, including pus in conjunction with erythema associated with tenderness and wound breakdown. Assessed 1 week after surgery, no details as to who conducted the assessment. |
|
| Notes | Numbers of infections were not reported, though "there was no difference in the incidence of infection between the two groups". No funding sources mentioned. No details on conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by a random number sequence into two groups" Comment: adequate approach. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Quote: "Group 1 underwent conventional temporal shaving, while group 2 had no preoperative hair removal" Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Unclear risk | Dropouts were not reported. Comment: insufficient details to permit a judgement. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: raw data for study secondary outcome (SSI) are not presented. The author states no difference between groups in SSIs. Unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Karegoudar 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 200 people (older than 15 years) having "all elective surgeries", "except neck, face and thyroid" in India. Dates not given. | |
| Interventions | Group 1: hair removal with depilatory cream (n = 100) Group 2: hair removal with razor (n = 100) Product details: no details are given on the razor or the cream. Timing of hair removal: not reported. Hair removed by: not reported. Venue for hair removal: not reported. |
|
| Outcomes | Outcome: "the effect of preoperative shaving with chemical depilation on wound infection". No definition of infection is given, nor how data were assessed or by whom. | |
| Notes | No data presented. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The prospective randomised study" Comment: no details on randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware if hair had been removed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Unclear risk | Not reported. Comment: insufficient details to permit a judgement. |
| Selective reporting (reporting bias) | High risk | Outcomes are not reported. Comment: possible selective reporting. |
| Other bias | Unclear risk | Comment: no information given on the similarity of groups. |
Kattipattanapong 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 133 adults who underwent ear surgery with a post‐auricular approach in Thailand between May 2010 and May 2011 | |
| Interventions | Group 1: hair removal with razor (n = 66) Group 2: no hair removal (n = 70) Product details: a razor was used with a dry shave. Timing of hair removal: not reported. Hair removed by: not reported. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: SSIs as defined by CDC criteria. SSIs within 30 days were recorded, but no details provided as to when they were assessed or by whom. Secondary outcome: factors associated with SSI ‐ demographic data. |
|
| Notes | Competing interests: none. Sponsorship: none. Funding sources: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomization using random allocation software" Comment: consider adequate approach. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants were distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Ko 1992.
| Study characteristics | ||
| Methods | QRCT | |
| Participants | 1980 adults having cardiopulmonary bypass surgery in the USA between July 1987 and June 1989 | |
| Interventions | Group 1: hair removal with razor (n = 990) Group 2: hair removal with a clipper (n = 990) Product details: no details are given for the razor, the clippers were Remington, 3M. Timing of hair removal: the night before surgery for elective cases, and immediately before surgery for emergency cases. Hair removed by: not specified. Venue for hair removal: not reported for elective surgery, the operating table for emergency cases. |
|
| Outcomes | Outcome: suppurative mediastinitis (the authors use this term interchangeably with sternal wound infection). Definition included; pain, fever, erythema, purulent drainage, sternal instability, tenderness, and leukocytosis. Needle aspiration sometimes used to assist diagnosis. Wounds were examined by the surgical team at least twice a day. 1 infection was diagnosed at the 30‐day postoperative follow‐up visit. No other details are given regarding assessment. |
|
| Notes | Participants were also randomised to receive either saline or povidone iodine intraoperative irrigation. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "according to the last two digits of their hospital numbers" Comment: inadequate randomisation technique. |
| Allocation concealment (selection bias) | High risk | Quote: "according to the last two digits of their hospital numbers" Comment: care provider could predict allocation by reference to hospital number. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Wounds were examined by the surgical team twice a day. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Unclear risk | Not reported. Comment: insufficient details to permit a judgement. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Kowalski 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 1678 people having elective general surgery in the USA between October 2009 and February 2015 who were considered to have "sufficient androgenic hair" | |
| Interventions | Group 1: hair removed with clippers (n = 834) Group 2: no hair removed (n = 844) Product details: clippers were single‐use disposable electric clippers. Timing of hair removal: immediately before surgery. Hair removed by: not specified. Venue for hair removal: not specified. |
|
| Outcomes | Primary outcome: SSI defined by the CDC criteria. SSI was assessed by an independent research nurse at the participant's follow‐up visit. Research nurses also attempted to contact participants at 30 days postoperatively. Secondary outcomes: classification of SSI using demographic, clinical, and surgical data collected by study nurses. Surgeons were asked to comment, via a questionnaire, on the impact of hair removal on ease of surgery. |
|
| Notes | Disclosure: authors have nothing to disclose. Portions of the study were funded by Gundersen Health Systems and the Gundersen Medical Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block design with a block size of 100. Comment: consider adequate approach. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. Comment: consider adequate approach. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: SSI was assessed by an independent nurse not directly involved with the patients' care. Comment: consider adequate approach. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Low risk | ITT analysis was conducted. Comment: consider adequate approach. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants were distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Complied with protocol, supported by funding but not from a pharmaceutical company. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Lu 2002.
| Study characteristics | ||
| Methods | QRCT | |
| Participants | 60 people (from 6 years of age) having elective thoracic surgery in China between August and December 2001 | |
| Interventions | Group 1: hair removal with clipper (n = 30) Group 2: no hair removal (n = 30) Product details: no details are given for the clipper. Timing of hair removal: the morning of the day before surgery. Hair removed by: not reported. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: skin bacterial culture taken on the operating table prior to skin preparation and 16 hours after surgery. Secondary outcome: surgical site infection. Assessed by a trained person. Definition of infection or time when assessed not provided. |
|
| Notes | No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation by odd and even numbers. Comment: inadequate randomisation technique. |
| Allocation concealment (selection bias) | High risk | Randomisation by odd and even numbers. Comment: care provider could predict allocation by reference to hospital number. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment used a "double blind method". Comment: achieved. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | No participants appeared to have dropped out from the study. Comment: unclear whether participants failed to complete. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Nascimento 1991.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 88 people (from 14 years of age) undergoing clean elective surgery in Brazil between June 1985 and January 1986. Includes hernia, vagotomy, and thyroidectomy plus other. | |
| Interventions | Group 1: hair removal with a razor (n = 44)
Group 2: no hair removal (n = 43) Product details: razors were used after the skin had been "slightly scrubbed with water and soap". Timing of hair removal: within 2 hours before transfer to the operating theatre. Hair removed by: not specified. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: wound infection defined as discharge of pus. Assessed daily until discharge or until removal of sutures on days 8 to 10 postoperatively. Assessed by the surgical team. | |
| Notes | Paper published in Portuguese. No funding sources mentioned. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to a random draw. Comment: method of generation of randomisation clear. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | No participants appeared to have dropped out from the study. Comment: dropout rate not reported. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Powis 1976.
| Study characteristics | ||
| Methods | QRCT | |
| Participants | 92 people undergoing general surgery in England. Dates not given. Operations include; cholecystectomy, varicose veins, mastectomy, appendicectomy, laparotomy. | |
| Interventions | Group 1: hair removal with a razor (n = 46)
Group 2: hair removal with depilatory cream (n = 46) Product details: a disposable razor or a safety razor with disposable blades with a wet shave, the cream was Ipso. Timing of hair removal: either on the day of surgery or the day before surgery depending on surgeon preference. Hair removed by: not reported. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: clinical evidence of wound infection assessed at day 2 and day 5 by an independent observer. Infections were classified by redness, swelling, exudate, and pus. Wound swabs were also taken at the end of the operation. Secondary outcomes: skin bacteria were assessed at the start and at the end of the operation using agar plates. Skin condition was assessed on day 2 and day 5 by an independent observer, and "spontaneous observations by the patients concerning the preparation were encouraged". |
|
| Notes | The cream used in the study was supplied by Knox Laboratories Ltd. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Allocated randomly depending on the last digit of their hospital registration number." Comment: non‐random approach. |
| Allocation concealment (selection bias) | High risk | Quote: "Allocated randomly depending on the last digit of their hospital registration number." Comment: the person allocating participants to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Quote: "Patients allocated to group 1 . . . were then shaved with either a disposable razor or a safety razor. Patients allocated to group 2 received an application of Ipso. . . in accordance with the manufacturer's instructions" Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Independant observer who was unaware of the method of preparation" Comment: consider adequate approach. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Quote: "A prospective randomised survey was performed in 92 patients" Comment: 92 analysed for wound infection, no dropouts reported. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Rojanapirom 1992.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 80 acute patients (from 12 years of age) undergoing appendicectomy in Thailand between May and August 1988 | |
| Interventions | Group 1: hair removal with a razor (n = 40)
Group 2: no hair removal (n = 40) Product details: skin was shaved with a razor on the ward following a scrub with antiseptic solution by hospital staff. Timing of hair removal: not reported. Hair removed by: not reported. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: wound infection. Wounds were examined on days 2 and 3 postoperatively and until the stitches were removed (days 7 to 10). Definition of an infection and details of who assessed the wound not provided. Wound swabs were also taken intraoperatively. | |
| Notes | No details of any funding. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each pair was randomly divided into control (shaved skin) and experiment (unshaved skin) groups." Comment: process of randomisation not given. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Quote: "Skin preparation in the control group. . . skin shaving with a razor. In the experiment group skin shaving was omitted." Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Unclear risk | Not reported. Comment: insufficient details to permit a judgement. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Seropian 1971.
| Study characteristics | ||
| Methods | QRCT | |
| Participants | 406 people undergoing surgery, excluding endoscopy, fractures, burns, oral surgery, abscesses, proctological and vaginal surgery, in the USA between June 1968 and February 1969 | |
| Interventions | Group 1: hair removal with a razor (n = 249)
Group 2: hair removal with depilatory cream (n = 157) Product details: no details are given for the razor, the cream consisted of calcium thioglycollate, calcium hydroxide, and strontium hydroxide. Timing of hair removal: not reported. Hair removed by: not reported. Venue for hair removal: not reported. |
|
| Outcomes | Outcome: evidence of wound infection as recorded by wound infection control office through case follow‐up and weekly review; no further details given. | |
| Notes | The study was supported by a grant from CIBA Pharmaceutical Company. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: the method used was "determined arbitrarily by the last digit of the patient's hospital number". Comment: non‐random approach. |
| Allocation concealment (selection bias) | High risk | Quote: the method used was "determined arbitrarily by the last digit of the patient's hospital number". Comment: the person allocating participants to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | High risk | Reasons for dropouts were given, but group sizes appeared to be unequal. Comment: participants were not evenly distributed across groups. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Sun 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 206 adults undergoing cholecystectomy in China | |
| Interventions | Group 1: hair removal with a razor (n = 98)
Group 2: no hair removal (n = 110) Product details: no details are given for the razor. Timing of hair removal: not reported. Hair removed by: trained nurses. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: surgical site infection assessed using Ministry of Health definition. No further details given. | |
| Notes | Published in Chinese. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated using random number tables. Comment: method of generation of randomisation clear. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware if hair had been removed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Unclear risk | Not reported. Comment: insufficient details to permit a judgement. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Suvera 2013.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 215 adults having general surgery in India between April 2010 and March 2012 | |
| Interventions | Group 1: hair removal with a razor (112 participants) Group 2: hair removal with depilatory cream (103 participants) Product details: no details are given for the razor, the cream consisted of potassium thioglycolate. Timing of hair removal: on the day of operation. Hair removed by: nursing staff. Venue for hair removal: not reported. |
|
| Outcomes | Primary outcome: adequacy of method, skin trauma. Skin was assessed preoperatively by a surgical resident and postoperatively by a senior resident on days 3, 5, and 7. Secondary outcome: SSIs were recorded using a "modification of the Southampton scoring system". All participants were followed up for at least 6 weeks. No further details provided. |
|
| Notes | Sources of support: none. Conflict of interest: none declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "consecutive patients were asked to pick one of two sealed envelopes ... on which one of the two methods was written" Comment: randomised. |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutive patients were asked to pick one of two sealed envelopes containing a folded paper on which one of the two methods was written" Comment: allocation concealed. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff who assessed wounds did not participate in the surgery and were unaware of participants' group allocation status. Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants are distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Unclear risk | Comment: no information on the similarity of groups. |
Taylor 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 156 people having day surgery for "a range of procedures including hernia and varicose veins removal" in England. Dates not given. | |
| Interventions | Group 1: hair removal with a razor (n = 78) Group 2: hair removal with clippers (n = 78) Product details: razors were "standard hospital supply disposable razors", clippers were 3M with disposable heads. Timing of hair removal: the day of surgery. Hair removed by: perioperative staff. Venue for hair removal: day surgery unit. |
|
| Outcomes | Primary outcome: participant preferences and skin trauma, identified through interview immediately after hair removal by hospital staff and 2 weeks after surgery by the research team. Secondary outcome: SSI described by participant during follow‐up phone call with research study staff 2 weeks after surgery. Defined as red, pain, swelling, and discharge. |
|
| Notes | Study supported by an award from the NATN/3M Clinical Fellowship. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number tables" Comment: consider adequate approach. |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation cards placed inside sequentially numbered envelopes" Comment: consider adequate approach. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Comment: interviews were carried out by perioperative staff and 1 of the research team who may have known participant allocation groups, plus participants self‐assessed. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants are distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Unclear risk | Comment: no information on the similarity of groups. |
Thorup 1985.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 50 people (from 13 years of age) undergoing inguinal hernia repair in Denmark between June and November 1983 | |
| Interventions | Group 1: hair removal with a razor (n = 24)
Group 2: hair removal with depilatory cream (n = 26) Product details: the razors were disposable, the cream was Pilidan. Timing of hair removal: the day before surgery. Hair removed by: participants. Venue for hair removal: participants' own homes. |
|
| Outcomes | Primary outcome: participant preferences and efficiency of hair removal. Information was obtained from participants after hair removal but before surgery. Efficiency of hair removal was assessed on the operating table. No further details. Secondary outcome: wound infection assessed immediately postoperatively and on day of suture removal on day 10. No definition given for infection. No further details. |
|
| Notes | Paper published in Danish. No details of any funding. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised" Comment: insufficient information provided about the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Comment: not clear whether the person responsible for allocation to groups would have been able to predict to which group a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: not feasible due to nature of intervention ‐ use of cream was conducted by participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examinations on the day of operation and when the sutures were removed were carried out without any knowledge of the nature of depilation used." Comment: blinding of outcome assessment reported. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | Low risk | Participants who dropped out are accounted for, and participants are distributed evenly across groups. Comment: the number of dropouts was judged to be unlikely to have altered the result, even in a worst‐case scenario (i.e. assuming that those that dropped out developed an SSI). |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Low risk | Comment: participant groups were equal or similar. |
Thur de Koos 1983.
| Study characteristics | ||
| Methods | QRCT | |
| Participants | 253 adult males undergoing thoracic, abdominal, vascular, and head and neck surgery in the USA between January and October 1981 | |
| Interventions | Group 1: hair removal with a razor (n = 137)
Group 2: hair removal with depilatory cream (n = 116) Product details: razors were used with a wet shave, the cream was Neet. Timing of hair removal: shaving was carried out 30 minutes before surgery, cream was applied the night before surgery. Hair removed by: not specified. Venue for hair removal: razors ‐ the theatre department, cream ‐ the ward. |
|
| Outcomes | Primary outcome: evidence of SSI, but no definition given. Details or time of assessment not given. | |
| Notes | Sampling exclusion criteria unclear. The study was supported in part by Whitehall Laboratories, New York. No details of conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients in beds with even numbers were prepared with depilatory cream ... patients in odd numbered beds had a wet razor shave." Comment: non‐random approach. |
| Allocation concealment (selection bias) | High risk | Quote: "Patients in beds with even numbers were prepared with depilatory cream ... patients in odd numbered beds had a wet razor shave." Comment: the person allocating participants to groups would have been able to predict the group to which a potential participant would be allocated. |
| Blinding (performance bias and detection bias) Care providers blinded | Unclear risk | Not reported. Comment: unclear whether care providers were blinded to intervention allocation. |
| Blinding (performance bias and detection bias) Participants blinded | High risk | Not reported. Comment: participants would have been aware of hair removal method. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Comment: unclear whether assessors were blinded to intervention allocation. |
| Incomplete outcome data (attrition bias) ITT analysis undertaken | Unclear risk | Not reported. Comment: no discussion of whether participants were analysed in the groups to which they had been allocated. |
| Incomplete outcome data (attrition bias) Drop out rate acceptable | High risk | Dropouts were not reported, but group sizes appeared to be unequal. Comment: participants were not evenly distributed across groups. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Comment: unlikely to be affected by reporting bias. |
| Other bias | Unclear risk | Comment: no information on the similarity of groups. |
Abbreviations
CDC = Centers for Disease Control and Prevention ITT = intention‐to‐treat analysis QRCT = quasi‐randomised controlled trial RCT = randomised controlled trial SSI = surgical site infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adeleye 2008 | Not an RCT or QRCT |
| Bekar 2001 | Not an RCT or QRCT |
| Bird 1984 | Not an RCT or QRCT |
| Braun 1995 | Not an RCT or QRCT |
| Breuer 2012 | Not an RCT or QRCT |
| Broekman 2011 | Not an RCT or QRCT |
| Chen 2002 | Not an RCT or QRCT |
| Clarke 1983 | Not an RCT or QRCT |
| Corcoran 2013 | Not an RCT or QRCT |
| Cruse 1973 | Not an RCT or QRCT |
| Dizer 2009 | Not an RCT or QRCT ‐ control and intervention groups ran at different time periods |
| Faheem 2012 | Not an RCT or QRCT |
| Finkelstein 2005 | Not an RCT or QRCT |
| Fraser 1978 | There was no skin incision ‐ transurethral surgery. |
| Gil 2003 | Not an RCT or QRCT ‐ retrospective study |
| Hallstrom 1993 | Not an RCT or QRCT |
| Hoe 1985 | Comparison (shaving surrounding surgical site versus shaving large area of skin) is not included in the review inclusion criteria. |
| Horgan 1997 | Not an RCT or QRCT |
| Howell 1988 | Not an RCT or QRCT |
| Huber 2016 | Not an intervention study |
| Idali 2004 | Not an RCT or QRCT |
| Kapadia 2012 | Not an RCT or QRCT |
| Kapadia 2013 | Not an RCT or QRCT |
| Korfali 1994 | Not an RCT or QRCT |
| Kose 2016 | Compared a 2‐centimetre strip of head hair removed by clippers against a 5‐centimetre strip of head hair removed by clippers ‐ this comparison is not included in the review inclusion criteria |
| Kovavisarach 2005 | Intervention group (cutting) not included in review inclusion criteria, and no surgical site skin incision. |
| Kretschmer 2000 | Not an RCT or QRCT |
| Kumar 2002 | Not an RCT or QRCT |
| Le Roux 1975 | Not an RCT or QRCT |
| Li 2013 | Not an RCT or QRCT |
| Liu 2008 | Not an RCT or QRCT |
| Lui 1984 | Intervention group (cropping) is not included in the review inclusion criteria. |
| Maksimovic 2008 | Not an RCT or QRCT |
| Marecek 2015 | Participants were healthy volunteers and not patients undergoing surgical procedures. |
| Masterson 1984 | Not an RCT or QRCT |
| McIntyre 1994 | Not an RCT or QRCT |
| Mehta 1988 | Not an RCT or QRCT |
| Meiland 1986 | Not classed as a surgical procedure |
| Menéndez 2004 | There was no skin incision ‐ transurethral surgery. |
| Menendez Lopez 2004 | There was no skin incision ‐ transurethral surgery |
| Miller 2001 | Not an RCT or QRCT |
| Mishriki 1990 | Not an RCT or QRCT |
| Morey 2013 | A letter in response to a trial by Grober 2013 |
| Moro 1996 | Not an RCT or QRCT |
| Parizh 2016 | Not an RCT or QRCT. Intervention was care bundle. |
| Penson 2017 | Letter in response to article |
| Ratanalert 1999 | Not an RCT or QRCT |
| Ratanalert 2005 | Not an RCT or QRCT |
| Scherpereel 1993 | Not an RCT or QRCT |
| Sellick 1991 | Not an RCT or QRCT |
| Sheinberg 1999 | Not an RCT or QRCT |
| Siddique 1998 | Not an RCT or QRCT |
| Small 1996 | Not an RCT or QRCT |
| Stephens 1966 | Not an RCT or QRCT |
| Tang 2001 | Not an RCT or QRCT |
| Tokimura 2009 | Not an RCT or QRCT |
| Vestal 1952 | Not an RCT or QRCT |
| Viney 1992 | Not an RCT or QRCT |
| Waddington 2008 | Not an RCT or QRCT |
| Wang 1990 | Not an RCT or QRCT |
| Wang 1999 | Not an RCT or QRCT |
| Wang 2017 | A letter, not an RCT |
| Westermann 1979 | Not an RCT or QRCT |
| Winfield 1986 | Not an RCT or QRCT |
| Winston 1992 | Not an RCT or QRCT |
| Zentner 1987 | Not an RCT or QRCT |
Abbreviations
QRCT = quasi‐randomised controlled trial RCT = randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Pascual 1994.
| Methods | Not known. |
| Participants | 34 people having abdominal surgery. Country and dates of study are not known. |
| Interventions | Group 1: "The surgical field was prepared the day prior to surgery." (n = 17) Group 2: "The surgical field was prepared 2 hours prior to surgery." (n = 17) No details are available regarding product, venue, or who removed the hair. |
| Outcomes | Results obtained were analysed in each group with regard to the germs isolated, age of the participants, base disease, postoperative infections, and length of postoperative stay. |
| Notes | We have as yet been unable to obtain a copy of the full text of this paper. |
Differences between protocol and review
Where surgical site infections were reported at several different time points, the time closest to 30 days (Centers for Disease Control and Prevention (CDC) definition of a surgical site infection) was used.
We carried out a sensitivity analysis to explore the effects of study design on the primary outcome. For the sensitivity analysis, we excluded studies that were quasi‐randomised studies (the inclusion of this type of study was not prespecified in the protocol for this review) and studies at high risk of selection bias.
We carried out a post hoc subgroup analysis for studies where hair was removed from male genitalia due to the differences in the surface of the skin (loose and likely to catch in hair removal devices).
The comparisons 'clipping the day before surgery versus the day of surgery' and 'shaving the day before surgery versus the day of surgery' have been merged into the following single comparison: hair removal the day before surgery versus hair removal on the day of surgery.
Estimates for dichotomous outcomes are presented as risk ratio. Where possible, continuous data are presented as mean difference with 95% confidence interval.
Contributions of authors
Judith Tanner: conceived the review; designed the review update; coordinated the review update; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; produced the first draft of the review update; contributed to writing or editing the review update; advised on the review update; performed previous work that was the foundation of the current review update; wrote to study authors/experts/companies; approved final review update prior to publication; is guarantor of the review update.
Kate Melen: conceived the review; designed the review update; checked quality of data extraction; undertook quality assessment; produced the first draft of the review update; advised on the review update; performed previous work that was the foundation of the current review update; wrote to study authors/experts/companies; approved final review update prior to publication.
Contributions of editorial base
Nicky Cullum and Jo Dumville (Joint Co‐ordinating Editors): edited the previous and current versions of this review respectively, advised on methodology, interpretation, and review content. Jo Dumville approved this update prior to publication.
Sally Bell‐Syer (Managing Editor): co‐ordinated the editorial process for previous versions of this review and advised on methodology, interpretation and content.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process for this update, advised on content and edited the update.
Ruth Foxlee, Reetu Child and Sophie Bishop (Information Specialists): designed the search strategy, ran the searches, and edited the search methods section for previous and current versions of this review.
Tom Patterson (Editorial Assistant): edited the reference sections for this update.
Sources of support
Internal sources
De Montfort University, UK
University of Nottingham, UK
External sources
-
The National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
The Theatre Nurses' Trust Fund, UK
The Association for Perioperative Practice, UK
Declarations of interest
Judith Tanner: none known. Kate Melen: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abouzari 2009 {published data only}
- Abouzari M, Sodagari N, Hasibi M, Behzadi M, Rashidi A. Effect of hair on surgical wound infection after cranial surgery: a 3-armed randomized clinical trial. Surgical Neurology 2009;71(2):261-2. [DOI] [PubMed] [Google Scholar]
Adisa 2011 {published data only}
- Adisa AO, Lawal OO, Adejuyigbe O. Evaluation of two methods of preoperative hair removal and their relationship to postoperative wound infection. Journal of Infection in Developing Countries 2011;5(10):717-22. [DOI] [PubMed] [Google Scholar]
Alexander 1983 {published data only}
- Alexander WJ, Fischer JE, Boyajian M, Palmquist J, Morris MJ. The influence of hair removal methods on wound infections. Archives of Surgery 1983;118:347-52. [DOI] [PubMed] [Google Scholar]
Balthazar 1983 {published data only}
- Balthazar ER, Colt JD, Nichlos R. Preoperative hair removal: a random prospective study of shaving versus clipping. Southern Medical Journal 1982;75:799-801. [PubMed] [Google Scholar]
Breiting 1981 {published data only}
- Breiting V, Hellberg S. Chemical depilation as an alternative to shaving. Ugeskrift fur Laeger 1981;143:1646-7. [PubMed] [Google Scholar]
Celik 2007 {published and unpublished data}
- Celik SE, Kara A. Does shaving the incision site increase the infection rate after spinal surgery? Spine 2007;32(15):1575-7. [DOI] [PubMed] [Google Scholar]
Court‐Brown 1981 {published data only}
- Court-Brown CM. Preoperative skin depilation and its effect on postoperative wound infections. Journal of the Royal College of Surgeons of Edinburgh 1981;26:238-41. [PubMed] [Google Scholar]
Domes 2011 {published data only}
- Domes T, Grober E. Pre-operative hair removal on the male genitalia - clippers versus razors. Journal of Sexual Medicine 2011;8 Suppl 1:42. [DOI] [PubMed] [Google Scholar]
Goëau‐Brissonnière 1987 {published data only}
- Goëau-Brissonnière O, Coignard S, Merào AP, Haicault G, Sasako M, Patel JC. Pre-operative skin preparation. A study comparing a depilatory agent in shaving [Préparation préopératoire de peau. Une étude prospective comparant un agent dépilatoire à raser]. Presse Médicale 1987;16(31):1517-9. [PubMed] [Google Scholar]
Grober 2013 {published data only}
- Grober ED, Domes T, Fanipour M, Copp JE. Preoperative hair removal on the male genitalia: clippers vs razors. Journal of Sexual Medicine 2013;10(2):580-94. [DOI] [PubMed] [Google Scholar]
Ilankovan 1992 {published data only}
- Ilankovan V, Starr DG. Preoperative shaving: patient and surgeon preferences and complications for the Gillies incision. Journal of the Royal College of Surgeons of Edinburgh 1992;37:399-401. [PubMed] [Google Scholar]
Karegoudar 2012 {published data only}
- Karegoudar JS, Prabhakar PJ, Vijayanath V, Anitha MR, Supur RR, Patil VM. Shaving versus depilation cream for pre-operative skin preparation. Indian Journal of Surgery 2012;74(4):294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kattipattanapong 2013 {published data only}
- Kattipattanapong W, Isaradisaikul S, Hanprasertpong C. Surgical site infection in ear surgery: hair removal effect; a preliminary, randomized trial study. Otolaryngology - Head and Neck Surgery 2013;148(3):469-74. [DOI] [PubMed] [Google Scholar]
- Kattipattanapong W, Isaradisaikul S. Surgical site infections in ear surgery: hair removal effect. Otolaryngology - Head and Neck Surgery 2012;147 (2 Suppl):222. [DOI] [PubMed] [Google Scholar]
Ko 1992 {published data only}
- Ko W, Lazenby WD, Zelano JA, Isam OW, Krieger KH. Effects of shaving methods and intraoperative irrigation on suppurative mediastinitis after bypass operations. Annals of Thoracic Surgery 1992;53:301-5. [DOI] [PubMed] [Google Scholar]
Kowalski 2016 {published data only}
- Kothari SN, Bogart AJ, Kowalski TJ. Impact of clipping vs no hair removal on surgical site infections: a prospective randomised trial. Journal of the American College of Surgeons 2016;223 (4 Suppl 2):e19-20. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Kothari SN, Mathiason MA, Borgert AJ. Impact of hair removal on surgical site infection rates: a prospective randomised noninferiority trial. Journal of the American College of Surgeons 2016;223:704-11. [DOI] [PubMed] [Google Scholar]
Lu 2002 {published data only}
- Lu XY, Zhao JJ, Zhou Y, Cao J, Qin SH. Influence of hair removal on postoperative wound infection in thoracic surgery. Nursing Journal of Chinese People's Liberation Army 2002;19:12-3. [Google Scholar]
Nascimento 1991 {published data only}
- Nascimento JE, Caporossi C, Marra JG, Botosso R. Influence of preoperative shaving in wound infections of clean operations. Arquivos Brasileiros de Medicina 1991;65(2):157-9. [Google Scholar]
Powis 1976 {published data only}
- Powis SJ, Waterworth T, Arkell D. Preoperative skin preparation: clinical evaluation of depilatory cream. British Medical Journal 1976;2:1166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rojanapirom 1992 {published data only}
- Rojanapirom S, Danchaivijitr S. Pre-operative shaving and wound infection in appendectomy. Journal of the Medical Association of Thailand 1992;75:20-3. [PubMed] [Google Scholar]
Seropian 1971 {published data only}
- Seropian R, Reynolds B. Wound infections after preoperative depilatory versus razor preparation. American Journal of Surgery 1971;121:253-4. [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun J, Kou JL. Two methods of skin preparation before laparoscopic cholecystectomy. Chinese Journal of Modern Nursing 2014;20:1559-61. [Google Scholar]
Suvera 2013 {published data only}
- Suvera M, Vyas P, Patel M, Varghese V, Ahmed A, Kashyap R, et al. Two methods of pre-operative hair removal and their effect on post-operative period. International Journal of Medical Science and Public Health 2013;2(4):885-8. [Google Scholar]
Taylor 2005 {published data only}
- Taylor T, Tanner J. Razors versus clippers. British Journal of Perioperative Nursing 2005;15(12):518-23. [DOI] [PubMed] [Google Scholar]
Thorup 1985 {published data only}
- Thorup J, Fischer A, Lindenberg S, Schjorring-Thyssen U, Jenson J, Burcharth F. Chemical depilation versus shaving. Ugeskrift fur Laeger 1985;25(13):1108-10. [PubMed] [Google Scholar]
Thur de Koos 1983 {published data only}
- Thur de Koos P, McComas B. Shaving versus skin depilatory cream for preoperative skin preparation. American Journal of Surgery 1983;145:377-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adeleye 2008 {published data only}
- Adeleye AO, Olowookere KG. Nonshaved cranial surgery in black Africans: a short term prospective preliminary study. Surgical Neurology 2008;69:69-72. [DOI] [PubMed] [Google Scholar]
Bekar 2001 {published and unpublished data}
- Bekar A, Korfali S, Dogan S, Yilmazlar Z, Aksoy K. The effect of hair on infection after cranial surgery. Acta Neurochirurgica 2001;143:533-7. [DOI] [PubMed] [Google Scholar]
Bird 1984 {published data only}
- Bird BJ, Chrisp DB, Scimegeour G. Extensive preoperative shaving: a costly exercise. New Zealand Medical Journal 1984;97:727-9. [PubMed] [Google Scholar]
Braun 1995 {published data only}
- Braun VM, Richter HP. Shaving the hair - is it always necessary for cranial neurosurgical procedures? Acta Neurochirurgica 1995;135:84-6. [DOI] [PubMed] [Google Scholar]
Breuer 2012 {published data only}
- Breuer J, Ivanovic N, Panfil EM. Prevention of postoperative infection by preoperative hair removal. Pflege Zeitschrift [Care Journal] 2012;65(6):356-7. [PubMed] [Google Scholar]
Broekman 2011 {published data only}
- Broekman ML. Neurosurgery and shaving: what's the evidence? A review. Journal of Neurosurgery 2011;115(4):670-8. [DOI] [PubMed] [Google Scholar]
Chen 2002 {published data only}
- Chen W, Lu H, Huang H. Impacts of preserved skin without hair-razing on puncturing site infection in patients who underwent cardiac intervention therapy. Chinese Nursing Research 2002;16(8):459-60. [Google Scholar]
Clarke 1983 {published data only}
- Clarke J. Theatre nursing. The effectiveness of surgical skin preparations. Nursing Times 1983;79(39 Suppl):8-17. [PubMed] [Google Scholar]
Corcoran 2013 {published data only}
- Corcoran S, Jackson V, Coulter-Smith S, Loughrey J, McKenna P, Cafferkey M. Surgical site infection after cesarean section: implementing 3 changes to improve the quality of patient care. American Journal of Infection Control 2013;41(12):1258-63. [DOI] [PubMed] [Google Scholar]
Cruse 1973 {published data only}
- Cruse PJ, Foord R. A five year prospective study of 23,649 surgical wounds. Archives of Surgery 1973;107:206-9. [DOI] [PubMed] [Google Scholar]
Dizer 2009 {published data only}
- Dizer B, Hatipoglu S, Kaymakcioglu N, Tufan T, Yava A, Iyigun E, et al. The effect of nurse-performed preoperative skin preparation on postoperative surgical site infections in abdominal surgery. Journal of Clinical Nursing 2009;18(23):3325-32. [DOI] [PubMed] [Google Scholar]
Faheem 2012 {published data only}
- Faheem M, Rafay Zafar M, Othman H. Cranial access procedures without shaving scalp hair. Pan Arab Journal of Neurosurgery 2012;15(2):1-4. [Google Scholar]
Finkelstein 2005 {published data only}
- Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, et al. Surgical site infection rates following cardiac surgery: the impact of a 6-year infection control program. American Journal of Infection Control 2005;33:450-4. [DOI] [PubMed] [Google Scholar]
Fraser 1978 {published data only}
- Fraser I, MacPherson S, Panagakis A. Should patients be shaved prior to transurethral surgery? British Journal of Urology 1978;50:109-10. [DOI] [PubMed] [Google Scholar]
Gil 2003 {published data only}
- Gil Z, Cohen JT, Spektor S, Fliss DM. The role of hair shaving in skull base surgery. Otolaryngology - Head and Neck Surgery 2003;128(1):43-7. [DOI] [PubMed] [Google Scholar]
Hallstrom 1993 {published data only}
- Hallstrom R, Beck S. Implementation of the AORN skin shaving standard. AORN Journal 1993;58(3):498-506. [DOI] [PubMed] [Google Scholar]
Hoe 1985 {published data only}
- Hoe NY, Nambiar R. Is preoperative shaving really necessary? Annals of the Academy of Medicine 1985;14(4):700-4. [PubMed] [Google Scholar]
Horgan 1997 {published data only}
- Horgan MA, Piatt JH. Shaving the scalp may increase the rate of infection in CSF shunt surgery. Pediatric Neurosurgery 1997;26:180-4. [DOI] [PubMed] [Google Scholar]
Howell 1988 {published data only}
- Howell JM, Morgan JA. Scalp laceration repair without prior hair removal. American Journal of Emergency Medicine 1988;6(1):7-10. [DOI] [PubMed] [Google Scholar]
Huber 2016 {published data only}
- Huber K, Quinones-Hinojosa A, Huang J, Sood G. A reduction in craniotomy surgical site infections at an academic teaching facility. Open Forum Infectious Diseases 2016;3(1):1460. [Google Scholar]
Idali 2004 {published data only}
- Idali B, Lahyat B, Khaleq K, Ibahioin K, El Azahari A, Barrou L. Postoperative infection following craniotomy in adults [L'infection postopératoire après craniotomie chez l'adulte]. Médecine et Maladies Infectieuses 2004;34(5):221-4. [DOI] [PubMed] [Google Scholar]
Kapadia 2012 {published data only}
- Kapadia BH, Johnson AJ, Issa K, Naziri Q, Daley JA, Mont MA. Prevention methodologies against infection after total joint arthroplasty. Current Orthopaedic Practice 2012;23(6):533-9. [Google Scholar]
Kapadia 2013 {published data only}
- Kapadia BH, Pivec R, Johnson AJ, Issa K, Naziri Q, Daley JA, et al. Infection prevention methodologies for lower extremity total joint arthroplasty. Expert Review of Medical Devices 2013;10(2):215-24. [DOI] [PubMed] [Google Scholar]
Korfali 1994 {published data only}
- Korfali E, Bekar A, Boyaci S, Celik S, Ipekoglu Z, Doygun M. The effects of hair on infection in craniotomy. Turkish Neurosurgery 1994;4:120-2. [Google Scholar]
Kose 2016 {published data only}
- Kose G, Tastan S, Kutlay M, Bedir O. The effects of different types of hair shaving on body image and surgical site infection in elective cranial surgery. Journal of Clinical Nursing 2016;25(13):1876-85. [DOI] [PubMed] [Google Scholar]
Kovavisarach 2005 {published data only}
- Kovavisarach E, Jirasettasiri P. Randomised controlled trial of perineal shaving versus hair cutting in parturients on admission in labor. Journal of the Medical Association of Thailand 2005;88(9):1167-71. [PubMed] [Google Scholar]
Kretschmer 2000 {published data only}
- Kretschmer T, Braun V, Richter HP. Neurosurgery without shaving: indications and results. British Journal of Neurosurgery 2000;14(4):341-4. [DOI] [PubMed] [Google Scholar]
Kumar 2002 {published data only}
- Kumar K, Thomas J, Chan C. Cosmesis in neurosurgery: is the bald head necessary to avoid post operative infection? Annals of the Academy of Medicine, Singapore 2002;31:150-4. [PubMed] [Google Scholar]
Le Roux 1975 {published data only}
- Le Roux BT, Lowther CE, Mukheibir SC. Pre operative preparation of the skin with a depilatory. South African Medical Journal 1975;49:1761-3. [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li XF, Wu XJ, Cao J, Zhao H. Preoperative skin preparation with a depilatory cream in orthopaedic patients. Journal of Nursing Studies 2013;16:1-3. [Google Scholar]
Liu 2008 {published data only}
- Liu S, Yu L, Huang D, Peng S. Comparison between infection rates of incisional wound in patients administered different skin preparation methods before operation. Chinese Journal of Infection Control 2008;4:262-3. [Google Scholar]
Lui 1984 {published data only}
- Lui PS, Ching KC, Salmon YM, Choo HT, Yeo GC, Sng EH. Post operative wound infection following gynaecological operations. Singapore Medical Journal 1984;25(1):46-7. [PubMed] [Google Scholar]
Maksimovic 2008 {published data only}
- Maksimovic J, Markovic-Denic L, Bumbasirevic M, Marinkovic J, Vlajinac H. Surgical site infections in orthopedic patients: prospective cohort study. Croatian Medical Journal 2008;49:58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marecek 2015 {published data only}
- Marecek GS, Weatherford BM, Fuller EB, Saltzman MD. The effect of axillary hair on surgical antisepsis around the shoulder. Journal of Shoulder and Elbow Surgery 2015;24(5):804-8. [DOI] [PubMed] [Google Scholar]
Masterson 1984 {published data only}
- Masterson TM, Rodeheaver GT, Morgan RF, Edlich RF. Bacteriologic evaluation of electric clippers for surgical hair removal. American Journal of Surgery 1984;148:301-2. [DOI] [PubMed] [Google Scholar]
McIntyre 1994 {published data only}
- McIntyre FJ. Shaving patients before operation; a dangerous myth? Annals of the Royal College of Surgeons 1994;76:3-4. [PMC free article] [PubMed] [Google Scholar]
Mehta 1988 {published data only}
- Mehta G, Prakash B, Karmoker S. Computer assisted analysis of wound infection in neurosurgery. Journal of Hospital Infection 1988;11:244-52. [DOI] [PubMed] [Google Scholar]
Meiland 1986 {published data only}
- Meiland HT, Feder E, Roseno H. Episiotomy after shaving or cutting [Episiotomi-infektion efter rasering eller klipning]. Ugeskr Laeger 1986;148:2481-2. [PubMed] [Google Scholar]
Menéndez 2004 {published data only}
- Menéndez V, Galán JA, Elia M, Collado A, Lloréns F, Fernández C. Is it necessary to shave the pubic and genital regions of patients undergoing endoscopic urological surgery? Infection Control and Hospital Epidemiology 2004;25:6. [DOI] [PubMed] [Google Scholar]
Menendez Lopez 2004 {published data only}
- Menéndez Lopez V, Galán Llopis JA, Elía López M, Carro Rubias C, Paz Cruz L, Royo García G, et al. On the need of pubic region shaving in patients undergoing endoscopic urologic surgery [Sobre la necesidad del rasuro de la region pubica en los pacientes que van a ser sometidos a cirugia urologica endoscopica]. Actas Urologicas Espanolas 2004;28(10):761-5. [DOI] [PubMed] [Google Scholar]
Miller 2001 {published data only}
- Miller JJ, Weber PC, Patel S, Ramey J. Intracranial surgery: to shave or not to shave? Otology and Neurotology 2001;22:908-11. [DOI] [PubMed] [Google Scholar]
Mishriki 1990 {published data only}
- Mishriki SF, Law DJF, Jeffery PJ. Factors affecting the incidence of postoperative wound infection. Journal of Hospital Infection 1990;16:223-30. [DOI] [PubMed] [Google Scholar]
Morey 2013 {published data only}
- Morey A. Re: Preoperative hair removal on the male genitalia: clippers vs razors. Journal of Urology 2013;190:2144. [DOI] [PubMed] [Google Scholar]
Moro 1996 {published data only}
- Moro ML, Carrieri MP, Tozzi AE, Lana S, Greco D. Risk factors for surgical wound infections in clean surgery: a multi center study. Annali Italiani di Chirurgia 1996;1:13-7. [PubMed] [Google Scholar]
Parizh 2016 {published data only}
- Parizh D, Ascher E, Rizvi SA, Hingorani A, Amaturo M, Johnson E. Quality improvement initiative: preventative surgical site infection protocol in vascular surgery. Vascular 2018;26(1):47-53. [DOI] [PubMed] [Google Scholar]
Penson 2017 {published data only}
- Penson D. RE: Impact of hair removal on surgical site infection rates: a prospective randomised noninferiority trial. Journal of Urology 2017;197(6):1462-3. [DOI] [PubMed] [Google Scholar]
Ratanalert 1999 {published data only}
- Ratanalert S, Saehaeng S, Sripairojkul B, Liewchanpattana K, Phuenpathom N. Non shaved cranial neurosurgery. Surgical Neurology 1999;51(4):458-63. [DOI] [PubMed] [Google Scholar]
Ratanalert 2005 {published data only}
- Ratanalert S, Musikawat P, Oearsakul T, Saeheng S. Nonshaved ventriculoperitoneal shunt in Thailand. Journal of Clinical Neuroscience 2005;12(2):147-9. [DOI] [PubMed] [Google Scholar]
Scherpereel 1993 {published data only}
- Scherpereel B. No hair shaving [Le non rasage]. Neurochirurgie 1993;39:374-5. [PubMed] [Google Scholar]
Sellick 1991 {published data only}
- Sellick J, Stelmach M, Mylotte JM. Surveillance of surgical wound infections following open heart surgery. Infection Control and Hospital Epidemiology 1991;12(10):591-6. [DOI] [PubMed] [Google Scholar]
Sheinberg 1999 {published data only}
- Sheinberg MA, Ross DA. Cranial procedures without hair removal. Neurosurgery 1999;44(6):1263-7. [DOI] [PubMed] [Google Scholar]
Siddique 1998 {published data only}
- Siddique MS, Matai V, Sutcliffe JC. The pre operative skin shave in neurosurgery: is it justified? British Journal of Neurosurgery 1998;12:131-5. [DOI] [PubMed] [Google Scholar]
Small 1996 {published data only}
- Small S. Preoperative hair removal: a case report with implications for nursing. Journal of Clinical Nursing 1996;5:79-84. [DOI] [PubMed] [Google Scholar]
Stephens 1966 {published data only}
- Stephens FO, Conolly WB. The use of depilatory cream in surgery. Medical Journal of Australia 1966;2(19):886-8. [DOI] [PubMed] [Google Scholar]
Tang 2001 {published data only}
- Tang K, Yeh JS, Sgouros S. The influence of hair shave on the infection rate in neurosurgery. Pediatric Neurosurgery 2001;35(1):13-7. [DOI] [PubMed] [Google Scholar]
Tokimura 2009 {published data only}
- Tokimura H, Taitsu K, Yamahata H, Taniguchi A, Takayama K, Kaji M, et al. Cranial surgery without head shaving. Journal of Craniomaxillofacial Surgery 2009;37(8):477-80. [DOI] [PubMed] [Google Scholar]
Vestal 1952 {published data only}
- Vestal P. Preoperative preparation of the skin with a depilatory cream and a detergent. American Journal of Surgery 1952;83(3):398-403. [DOI] [PubMed] [Google Scholar]
Viney 1992 {published data only}
- Viney C. Pre operative shaving in gynaecology. Nursing Standard 1992;7(8):25-7. [DOI] [PubMed] [Google Scholar]
Waddington 2008 {published data only}
- Waddington C. Changing behaviour: evidence based practice supporting hair removal with clippers. ORL Head and Neck Nursing 2008;26(4):8-12. [PubMed] [Google Scholar]
Wang 1990 {published data only}
- Wang XY, Yang JM, Li LC. The comparison on preoperative preparation of skin with or without preserved skin shaving in gynaecological abdominal operations. Chung-Hua Hu Li Tsa Chih [Chinese Journal of Nursing] 1990;25(9):451-3. [Google Scholar]
Wang 1999 {published data only}
- Wang JW, Chen AC, Shen YJ. Study on the effects of preserved skin shaving in preoperative skin preparation. Chung-Hua Hu Li Tsa Chih [Chinese Journal of Nursing] 1999;34(2):83. [Google Scholar]
Wang 2017 {published data only}
- Wang DS. Re: Impact of hair removal on surgical site infection rates: a prospective randomised noninferiority trial. Journal of Urology 2017;197(5):1278. [DOI] [PubMed] [Google Scholar]
Westermann 1979 {published data only}
- Westermann K, Malkotte R. Does preoperative shaving cause disturbance of wound healing. Unfallheilkunde 1979;82:200-5. [PubMed] [Google Scholar]
Winfield 1986 {published data only}
- Winfield U. Too close a shave. Nursing Times 1986;82(10):64-8. [PubMed] [Google Scholar]
Winston 1992 {published data only}
- Winston KR. Hair and neurosurgery. Neurosurgery 1992;31:320-9. [DOI] [PubMed] [Google Scholar]
Zentner 1987 {published data only}
- Zentner J, Gilsbach J, Daschner F. Incidence of wound infection in patients undergoing craniotomy. Acta Neurochirurgica 1987;86:79-82. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pascual 1994 {published data only}
- Pascual MC. Contamination of the surgical field related to shaving. Enfermeria Clinica 1994;4(4):156-61. [Google Scholar]
Additional references
Adams‐Howel 2015
- Adams-Howel P, Bhabra M, Enright M, Kiernan M, Kolvekar S, Trueman P. Under the knife: taking a zero tolerance approach to preventable surgical site infections in UK hospitals. www.carefusion.co.uk/documents/international/continuing-education/infection-prevention/IP_Under-the-Knife_CE_EN.pdf (accessed 29 April 2020).
Awad 2012
- Awad SS. Adherance to surgical care improvement project measures and post operative surgical site infections. Surgical Infections 2012;13(4):234-7. [DOI] [PubMed] [Google Scholar]
Briggs 1997
- Briggs M. Principles of closed surgical wound care. Journal of Wound Care 1997;6(6):288-92. [DOI] [PubMed] [Google Scholar]
Brown 2014
- Brown B, Tanner J, Padley W. This wound has spoiled everything: emotional capital and the experience of surgical site infections. Sociology of Health and Illness 2014;36(8):1171-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2008
- Centers for Disease Control and Prevention (CDC). Surgical site infection (SSI). www.cdc.gov/HAI/ssi/ssi.html (accessed 29 April 2020).
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Crumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available at training.cochrane.org/handbook/archive/v6.
Edmiston 2016
- Edmiston C, Griggs RK, Tanner J, Spencer M, Seabrook GR, Leaper D. Perioperative hair removal in the 21st century: utilizing an innovative vacuum-assisted technology to safely expedite hair removal before surgery. American Journal of Infection Control 2016;1(12):1639-44. [DOI] [PubMed] [Google Scholar]
Fogg 2003
- Fogg D. Infection control. In: Meeker MH, Rothrock JC, editors(s). Alexander's Care of the Patient in Surgery. 12th edition. St Louis (MO): Mosby, 2003:134-47. [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Higgins 2003
- Higgins JP, Thompson S, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available at training.cochrane.org/handbook/archive/v5.2.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Crumpston M, LiT, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Huguenin 2017
- Huguenin P. I switched to a single blade razor. Here's what happened. www.menshealth.com/style/a19526899/single-blade-razor-shave/ (accessed 29 April 2020).
Kjonniksen 2003
- Kjonniksen I, Andersen BM, Sondenaa VG, Segedal L. Preoperative hair removal - a systematic literature review. AORN Journal 2003;75(5):928-38. [DOI] [PubMed] [Google Scholar]
Lefebvre 2015
- Lefebvre A, Saliou P, Lucet JC, Mimoz O, Keita-Perse O, Grandbastien B, Bruyere F, Boirenoult P, Lepelletier D, Aho-Glele LS. Preoperative hair removal and surgical site infections: network meta-analysis of randomised controlled trials. Journal of Hospital Infection 2015;91:100-8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Crumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available at training.cochrane.org/handbook/archive/v6.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ionnidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plowman 2000
- Plowman R, Graves N, Griffin M. The Socio-economic Burden of Hospital Acquired Infection. London (UK): Public Health Laboratory Service, 2000. [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group. Version 3.0. cccrg.cochrane.org/author-resources (accessed September 2020).
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Crumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available at training.cochrane.org/handbook/archive/v6.
Shi 2016
- Shi D, Yao Y, Yu W. Comparison of preoperative hair removal methods for the reduction of surgical site infections: a meta-analysis. Journal of Clinical Nursing 2016;26:2907-14. [DOI] [PubMed] [Google Scholar]
Veet 2020
- Veet. What are depilatory creams and how do they work. www.veet.co.uk/blogs/tips/what-are-depilatory-creams-and-how-do-they-work (accessed 29 April 2020).
Wahl 2005
- Wahl J. Wahl, History of Wahl Clipper. Sterling (IL): John F Wahl, 2005. [Google Scholar]
WHO 2011
- World Health Organization. Report on the burden of endemic health care associated infection worldwide. apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf?sequence=1 (accessed 30 April 2020).
WHO 2018a
- World Health Organization. Protocol for surgical site infection surveillance with a focus on settings with limited resources. www.who.int/infection-prevention/tools/surgical/SSI-surveillance-protocol.pdf (accessed 29 April 2020).
WHO 2018b
- World Health Organization. Global guidelines for the prevention of surgical site infection, second edition. www.who.int/gpsc/ssi-prevention-guidelines/en/ (accessed 15 May 2021).
References to other published versions of this review
Tanner 2003
- Tanner J, Woodings D, Moncaster K. Pre-operative hair removal to reduce surgical site infection. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD004122. [DOI: 10.1002/14651858.CD004122] [DOI] [Google Scholar]
Tanner 2006
- Tanner J, Moncaster K, Woodings D. Preoperative hair removal: a systematic review. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD004122. [DOI: 10.1002/14651858.CD004122.pub3] [DOI] [PubMed] [Google Scholar]
Tanner 2007
- Tanner J, Moncaster K, Woodings D. Preoperative hair removal: a systematic review. Journal of Perioperative Practice 2007;17(3):118-21. [DOI] [PubMed] [Google Scholar]
Tanner 2011
- Tanner J, Norrie P, Melen K. Preoperative hair removal to reduce surgical site infection. Cochrane Database of Systematic Reviews 2011, Issue 11. Art. No: CD004122. [DOI: 10.1002/14651858.CD004122.pub4] [DOI] [PubMed] [Google Scholar]


